Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | c24524e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - Unilife Corp | c24524exv99w1.htm |
Exhibit 99.2

| First Quarter 2012 Earnings Conference Call |

| Cautionary Note Regarding Forward-Looking Statements This presentation contains forward looking statements under the safe harbor provisions of the US securities laws. These forward-looking statements are based on management's beliefs and assumptions and on information currently available to our management. Our management believes that these forward-looking statements are reasonable as and when made. However you should not place undue reliance on any such forward looking statements as these are subject to risks and uncertainties. Please refer to our press release and our SEC filings for more information regarding the use of forward looking statements." |

| First Quarter 2012 Overview Alan Shortall Chief Executive Officer |
| Delivering on Unilife's Strategy 1Q FY2012 milestones Commenced initial Unifill supply to pharmaceutical customers Introduction of expanded portfolio of highly differentiated, market-leading drug delivery systems: Auto-injectors Infusion and injection pumps Drug reconstitution delivery systems (including EZMix) Expanded U.S. distribution of Unitract(r) 1mL safety syringes Expanded management team to support R&D, operations and sales |
| Innovative, Differentiated Solutions for Unmet Needs Diversified Portfolio of Delivery Systems |
| Unifill Syringe Commenced initial supply of the Unifill syringe to customers in July 2011 Continuing to increase production capacity at Unilife's York, PA facility Strong customer acceptance with many opportunities advancing in commercial pipeline |

| Rita Auto-Injector Leverages design and safety features of Unifill syringe Ultra compact size relative to conventional auto-injectors True end-of-dose indicator (audible, tactile click, plus visual window) Automatic needle retraction - patient never sees needle Unilife Auto-Injectors |

| Only prefilled reconstitution syringe with integrated safety Dual drug chambers in single delivery system Leverages materials and proven technology of Unifill syringe Addresses unmet patient safety needs Easy to use, fewer steps than conventional reconstitution systems EZMix |
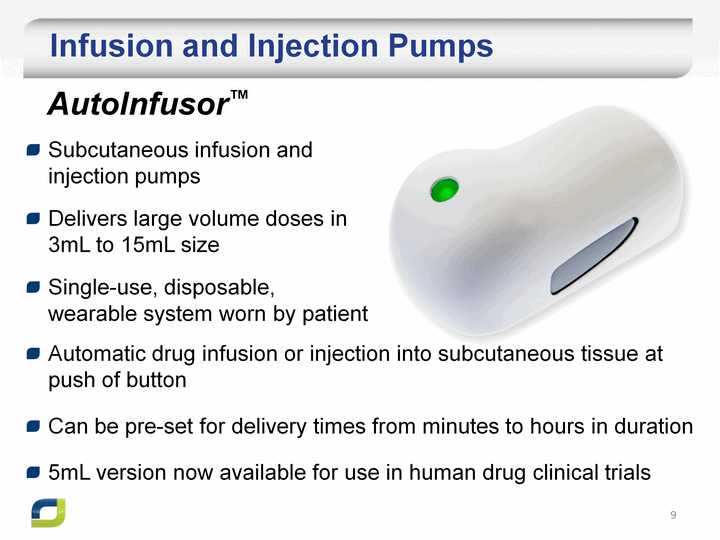
| Infusion and Injection Pumps Subcutaneous infusion and injection pumps Delivers large volume doses in 3mL to 15mL size Single-use, disposable, wearable system worn by patient AutoInfusor(tm) Automatic drug infusion or injection into subcutaneous tissue at push of button Can be pre-set for delivery times from minutes to hours in duration 5mL version now available for use in human drug clinical trials |

| Devices for Target Organ Delivery Devices delivering drug to target organ Examples of target organs include brain, ear and eye Scheduled to enter into drug clinical trials with pharmaceutical partner in early 2012 Strong financial opportunity with U.S. and international prevalence rates for the target disease state being very high U.S. treatment costs exceeding $30 billion Device helps enable drug commercialization (avoids invasive surgery) |
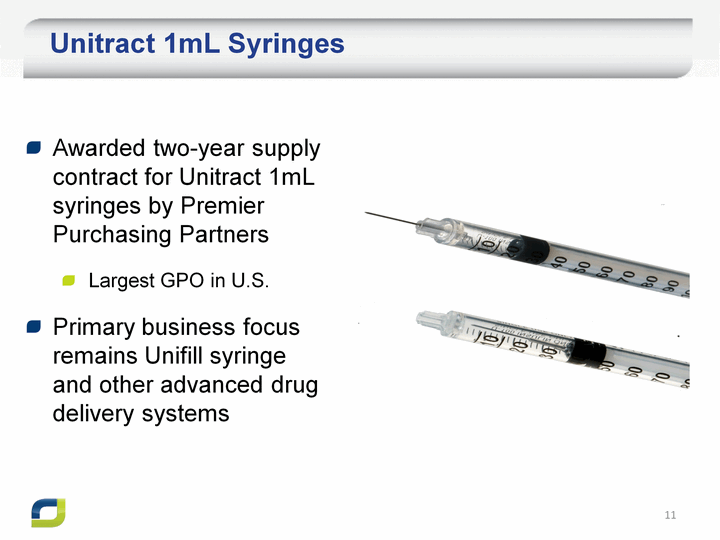
| Unitract 1mL Syringes Awarded two-year supply contract for Unitract 1mL syringes by Premier Purchasing Partners Largest GPO in U.S. Primary business focus remains Unifill syringe and other advanced drug delivery systems |

| Financial Data Overview Rich Wieland Chief Financial Officer |
| Revenues for the quarter of $2.1M, compared to $3.5M for 1Q 2011 Product sales from discontinued contract manufacturing operations decreased by $1.6M Net loss for the quarter was $9.7M, or $0.16 per diluted share, compared to a net loss of $7.2M, or $0.14 per diluted share for 1Q 2011 R&D expenses increased by $2.5M due to costs related to the development of additional advanced drug delivery devices Selling, general and administrative expenses decreased by $1.1M as a result of declines in non-cash share-based compensation expense as well as a decline in payroll and related fees Financial Data - Fiscal 1st Quarter 2012 |
| Adjusted net loss of $6.5M, or $0.11 per diluted share, compared to an adjusted net loss of $3.9M, or $0.07 per diluted share for 1Q 2011 Excludes approx. $3.2M in non-cash share-based compensation expense, depreciation and amortization and interest expense compared to $3.4M in non-cash items in 1Q 2011 Financial Data - Fiscal 1st Quarter 2012 Continued |

| Looking Ahead in FY2012 Alan Shortall Chief Executive Officer |

| Continued Unifill supplies to current and new customers Unifill placement onto stability studies Potential for additional access or royalty fees Commercial supply agreements Agreements for additional products within portfolio Develop or customize Unilife devices to address specific customer needs for pipeline and approved drugs and vaccines Clinical development and supply agreements Continuing to address unmet market needs in other areas Looking Forward |

| Questions |
