Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | c23543e8vk.htm |
Exhibit 99.1


| Not an Offer for Securities This presentation has been prepared by REVA Medical, Inc. (REVA or the Company) solely for its use at presentations to be made by the Company. This presentation does not constitute an offer, invitation, solicitation or recommendation with respect to the purchase or sale of any security in the Company nor does it constitute financial product advice nor take into account your investment objectives, taxation situation, financial situation or needs. An investor must not act on the basis of any matter contained in this presentation but must make its own assessment of the Company and conduct its own investigations and analysis. Information is a synopsis only This presentation only contains a synopsis of information on the Company and accordingly no reliance may be placed for any purpose whatsoever on the sufficiency or completeness of such information The information presented in this presentation is subject to change without notice and the Company does not have any responsibility or obligation to inform you of any matter arising or coming to their notice, after the date of this presentation, which may affect any matter referred to in this presentation. Currency references Financial amounts in this presentation are expressed in US Dollars., except where specifically noted. Forward looking Statements This announcement contains or may contain forward-looking statements that are based on management's beliefs, assumptions and expectations and on information currently available to management. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation our expectations with respect to regulatory submissions and approvals; our expectations with respect to our clinical trials, including enrolment in or completion of our clinical trials; our expectations with respect to the integrity or capabilities of our intellectual property position; our ability to commercialize our existing products; our ability to develop and commercialize new products; and our estimates regarding our capital requirements and financial performance, including profitability. Management believes that these forward-looking statements are reasonable as and when made. You should not place undue reliance on forward-looking statements because they speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. REVA may not actually achieve the plans, projections or expectations disclosed in forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, described in "Risk Factors" in our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the "SEC") on March 30, 2011, as updated in our Quarterly Reports on Form 10-Q filed with the SEC for the periods ended March 31, 2011 and June 30, 2011. We may update our risk factors from time to time in our periodic reports or other current reports filed with the SEC. Disclaimer This presentation has been prepared by the Company based on information which is available to it. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure that the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness or correctness of the information and opinions contained in this presentation and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of the Company, or any of its members, directors, officers, employees or agents, advisers nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of the Company or any of its directors, officers, employees or agents. 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference "We are devoted to developing and manufacturing novel polymer devices for the treatment of vascular disease that improve health and quality of life." REVA Mission Statement |

| REVA's drug-eluting bioresorbable stent potentially offers significant benefits for patients - answers a 25-year need Allows natural healing and may reduce long-term drug therapy $5 billion* annual worldwide market awaits next major advance Abbott and Biotonik are market "bow waves" - investing heavily, demonstrating clinical success, proving safety REVA intends to commence a pilot clinical study this quarter with CE Mark application targeted by the end of 2013 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference * JP Morgan Interventional Cardiology Market Model, June 15, 2011 |
| We address two huge needs: The $5 billion stent industry in need of a growth engine Clinicians seeking improved and safer stent technology REVA's A$85 million IPO in 2010 structured to provide capital for commercialization/CE Mark Near-term milestones and path to market Clinical trial to begin this quarter; patient follow-up at 1, 6 and 12-month time points CE Mark application targeted by the end of 2013; sales in EU after approval REVA has option to accept distribution through BSC at 50% royalty to REVA Highly experienced management team, board and investors Substantial success and value creation in medical technologies Brookside Capital, Cerberus Capital Management, Domain Partners, Saints Capital, Medtronic and Boston Scientific Corporation Medtronic owns ~7%* 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference * 2010 Annual Report on Form 10-K |
| Traded on the ASX in Chess Depository Interests ("CDIs") ASX Code RVA.AX CDI Price (as of 10/14/11) A$0.58 (or A$5.80 per share) Cash and Equivalents* $72,980,000 Outstanding CDIs* 327,750,030 (or 32,775,003 shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) Outstanding Options* 34,120,000 (or 3,412,000 equivalent shares) 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference * at 30 June, 2011 |
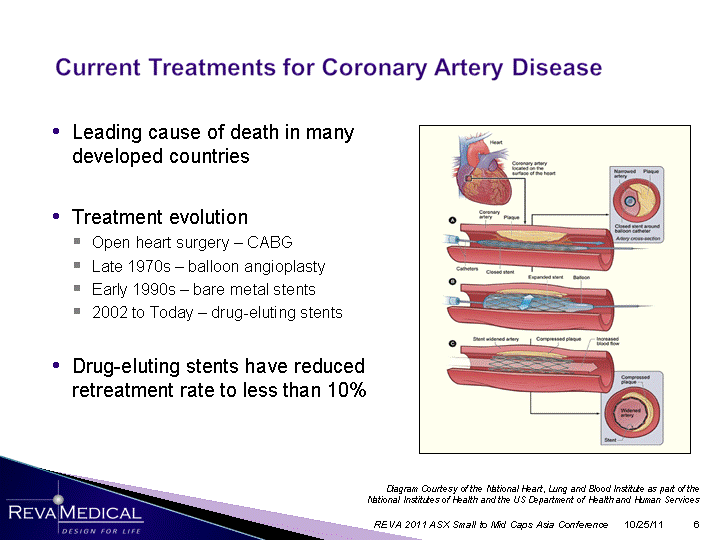
| Leading cause of death in many developed countries Treatment evolution Open heart surgery - CABG Late 1970s - balloon angioplasty Early 1990s - bare metal stents 2002 to Today - drug-eluting stents Drug-eluting stents have reduced retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Diagram Courtesy of the National Heart, Lung and Blood Institute as part of the National Institutes of Health and the US Department of Health and Human Services |

| Large worldwide market (~ $5 billion annually) Stent procedures increasing in all markets Drug-eluting stents are ~ 74% of stents implanted Drug-eluting stent products are at parity; stent prices decreasing Historically dominated by four major players (ABT, BSX, MDT, JNJ) History of rapid adoption of new technologies Bioresorbable stent poised to reinvigorate market 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference * JP Morgan Interventional Cardiology Market Model, June 15, 2011 |

| Allows artery to heal, then goes away Restores natural movement of the artery May reduce or eliminate blood clots associated with permanent stents May reduce the need for anti-clotting drugs Ideal vehicle to treat vulnerable plaque in the future Ideal vehicle to treat vulnerable plaque in the future Ideal vehicle to treat vulnerable plaque in the future Ideal vehicle to treat vulnerable plaque in the future Ideal vehicle to treat vulnerable plaque in the future Ideal vehicle to treat vulnerable plaque in the future 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Huge clinical and consumer appeal |
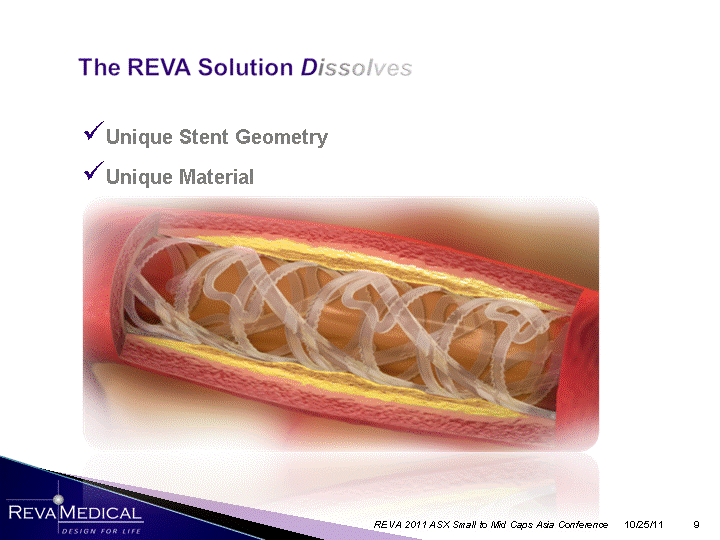
| Unique Stent Geometry Unique Material 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
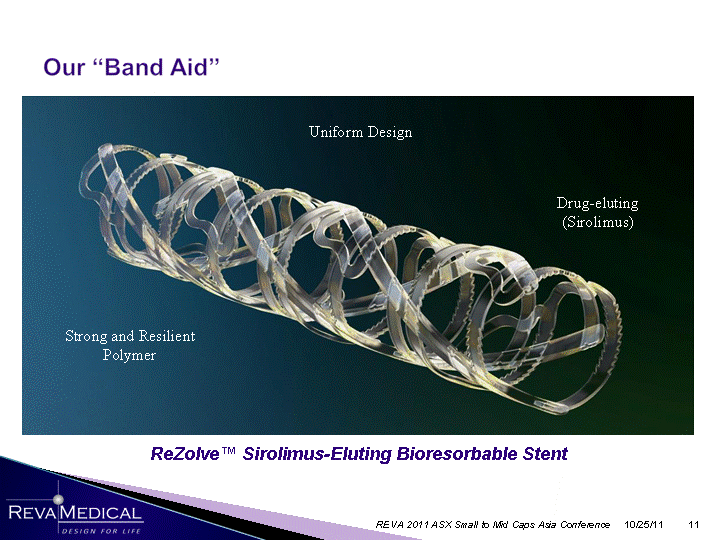
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference ReZolve(tm) Sirolimus-Eluting Bioresorbable Stent Drug-eluting (Sirolimus) Strong and Resilient Polymer Uniform Design |
| To develop a stent that provides the benefits of metal stents without their permanency Strength Maintain lumen (passage) in vessel Maintain support throughout healing process Performance Expansion range to accommodate "real world" clinical use Visibility Visualize entire stent during and after deployment Resorption Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant Dissolves once its job is done leaving the patient free of a permanent implant 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Intent is to drive meaningful clinical benefits |
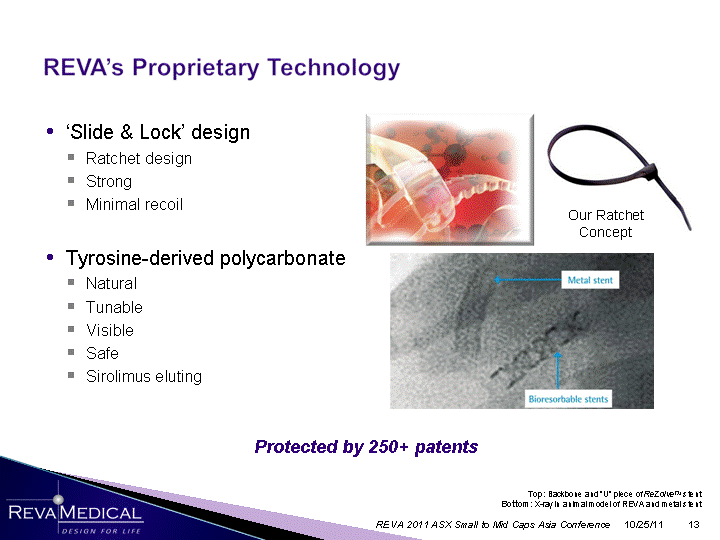
| 'Slide & Lock' design Ratchet design Strong Minimal recoil Tyrosine-derived polycarbonate Natural Tunable Visible Safe Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Sirolimus eluting Top: Backbone and "U" piece of ReZolveTM stent Bottom: X-ray in animal model of REVA and metal stent 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Our Ratchet Concept Protected by 250+ patents |
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference 80µg dose Sirolimus with abluminal delivery Delivery kinetics match Cypher Drug embedded in stent polymer on the surface Thin coating layer (^ 5µm) Reproducible coating process |
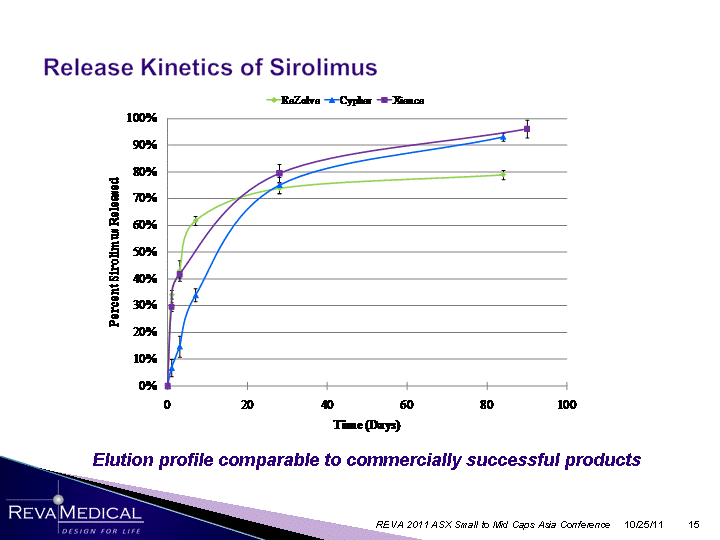
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Elution profile comparable to commercially successful products |

| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |

| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
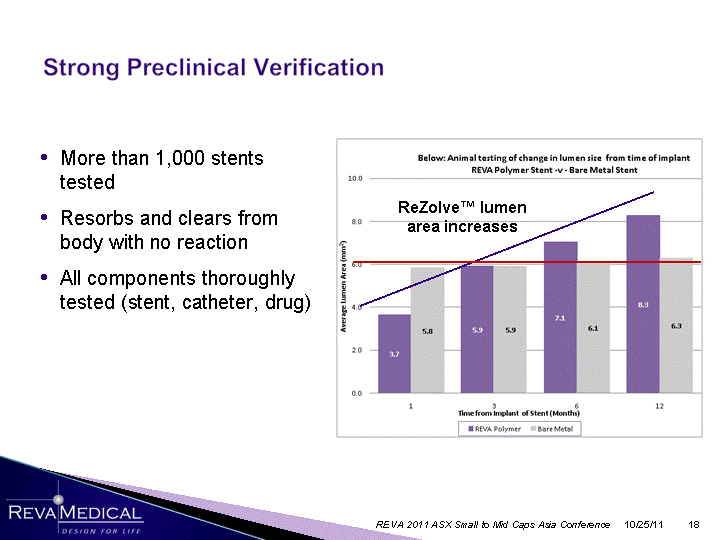
| More than 1,000 stents tested Resorbs and clears from body with no reaction All components thoroughly tested (stent, catheter, drug) tested (stent, catheter, drug) tested (stent, catheter, drug) tested (stent, catheter, drug) tested (stent, catheter, drug) tested (stent, catheter, drug) tested (stent, catheter, drug) ReZolve(tm) lumen area increases 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
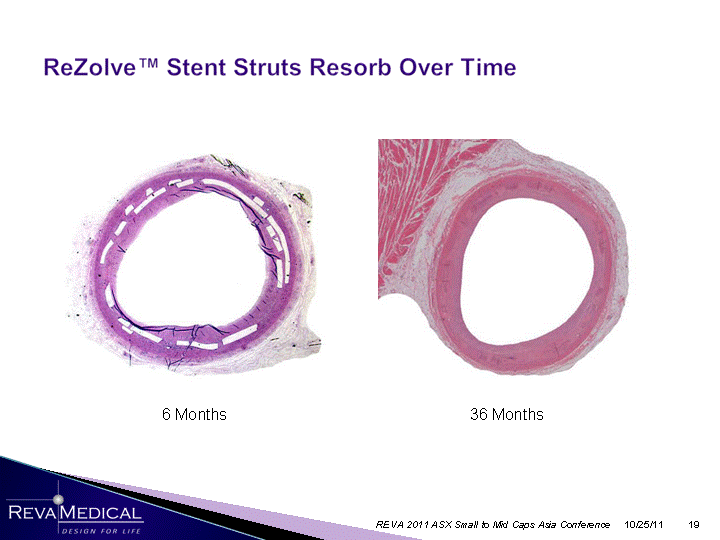
| 36 Months 6 Months 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
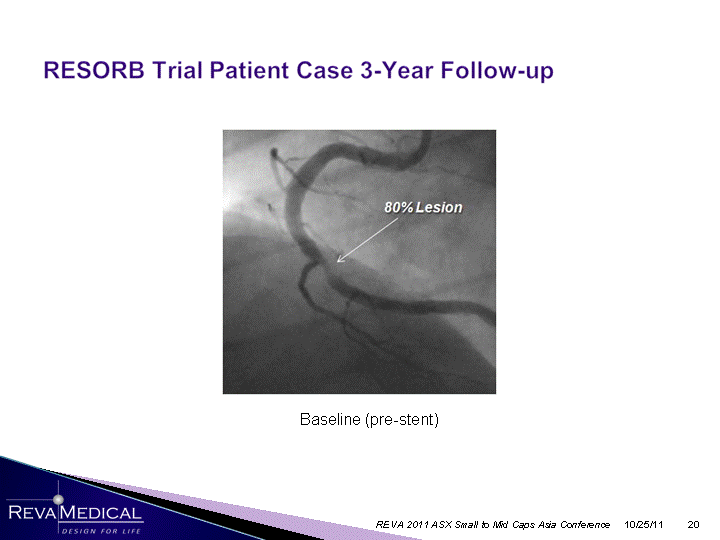
| Baseline (pre-stent) 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
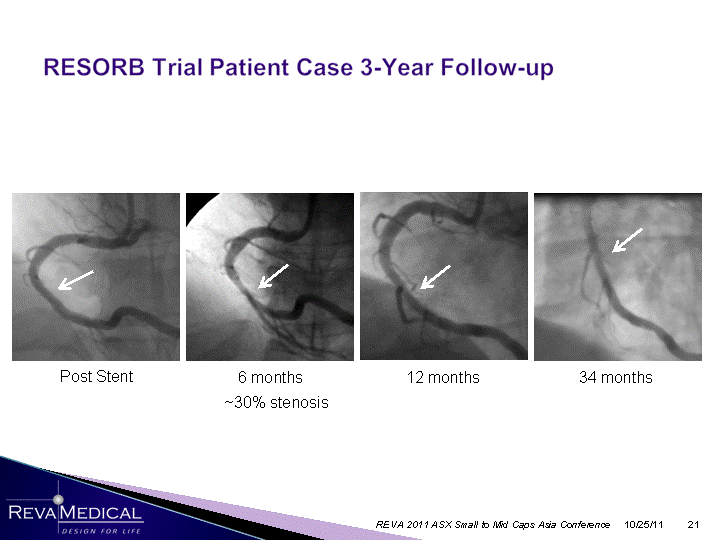
| 6 months 34 months 12 months ~30% stenosis 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Post Stent |
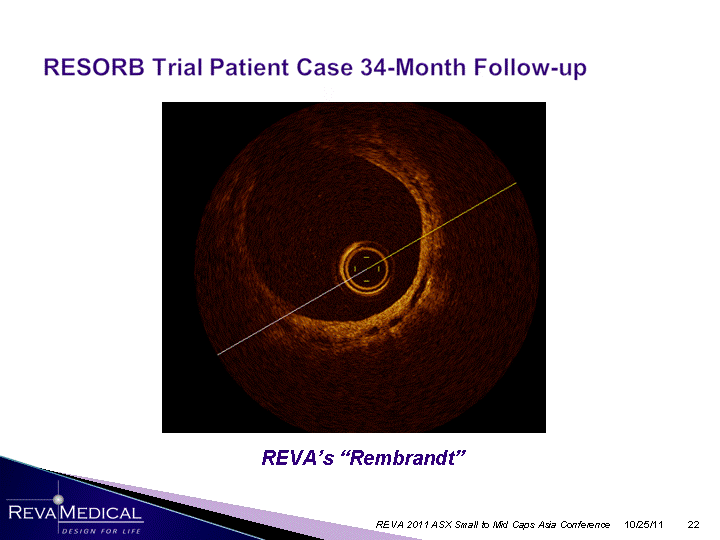
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference REVA's "Rembrandt" |
| Four large multinational device companies > 95% of stent sales Abbott Boston Scientific Medtronic Johnson & Johnson (recently announced DES exit) No coronary bioresorbable stent available for sale...yet Bioresorbable coronary stents in or approaching clinical trials Abbott's ABSORB(tm) stent - CE mark January 2011, commercialization pending Biotronik's 'Dreams' stent - magnesium based, clinical trial initiated August 2010 REVA's ReZolve(tm) stent - to enter pilot clinical trial this quarter 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
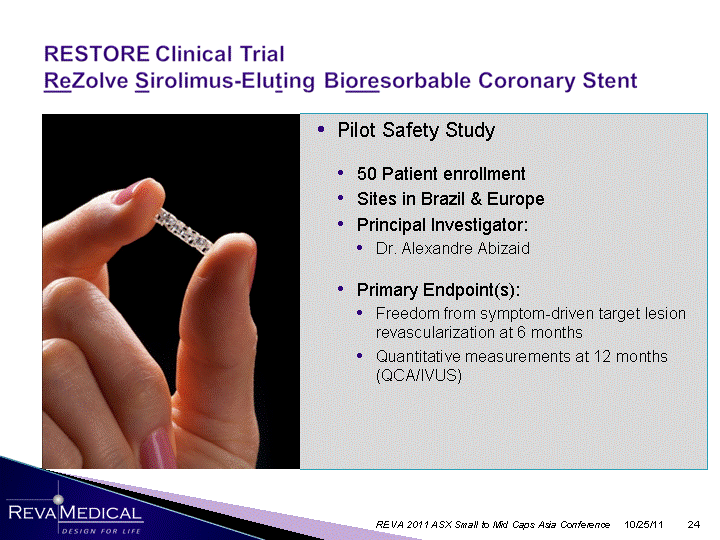
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference Pilot Safety Study 50 Patient enrollment Sites in Brazil & Europe Principal Investigator: Dr. Alexandre Abizaid Primary Endpoint(s): Freedom from symptom-driven target lesion revascularization at 6 months Quantitative measurements at 12 months (QCA/IVUS) |

| Pilot clinical trial to begin this quarter All required regulatory approvals have been received to initiate the trial in both Brazil and Germany Pursuing additional clinical sites in Europe Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals Pilot clinical trial will likely involve up to eleven hospitals 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |

| REVA is singularly-driven in the development of its unique bioresorbable stent technology REVA believes bioresorbable stents are the next transformation in the treatment of coronary artery disease REVA's technology is poised to enter an annual $5 billion market Investors in REVA include some of the most prestigious financial institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world institutions and medtech companies in the world 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
| 10/25/11 REVA 2011 ASX Small to Mid Caps Asia Conference |
