Attached files
| file | filename |
|---|---|
| EX-99.02 - EXHIBIT 99.02 - PALISADE BIO, INC. | v234738_ex99-02.htm |
| 8-K - FORM 8-K - PALISADE BIO, INC. | v234738_8k.htm |
Exhibit 99.01

Neural Stem Cell Technology Platform Cell Therapy and Pharmaceuticals NYSE Amex: CUR

2 NEURALSTEM, INC. SAFE HARBOR STATEMENT This presentation contains forward - looking statements. Neuralstem wishes to caution the readers of and listeners participating in this presentation that actual results may differ from those discussed in the forward - looking statements and may be adversely affected by, among other things, US FDA responses, and responses from other jurisdictions to various regulatory submissions; SEC responses to various registration submissions; changes in corporate strategy; the need to raise additional capital; the success or failure of other private and public organizations and/or academic and corporate institutions engaged in stem cell research and development, and the market for stem cell research in general. For further information, please review the company's filings with the Securities and Exchange Commission. For complete management biographical information and links to all SEC documents please visit the company’s Web site: neuralstem.com.
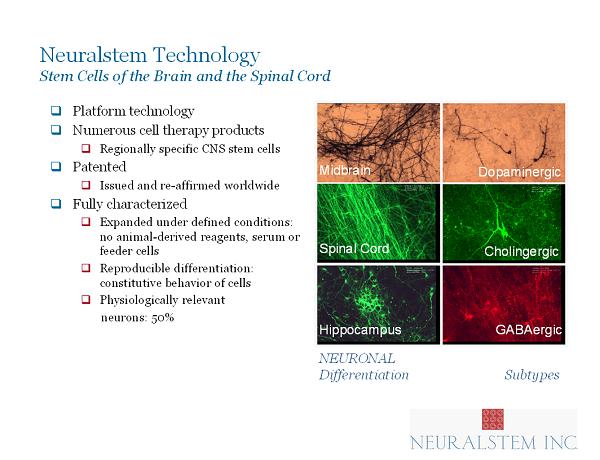
Neuralstem Technology Stem Cells of the Brain and the Spinal Cord □ Platform technology □ Numerous cell therapy products □ Regionally specific CNS stem cells □ Patented □ Issued and re - affirmed worldwide □ Fully characterized □ Expanded under defined conditions: no animal - derived reagents, serum or feeder cells □ Reproducible differentiation: constitutive behavior of cells □ Physiologically relevant neurons: 50% Midbrain Spinal Cord GABAergic Dopaminergic Cholingergic NEURONAL Differentiation Subtypes Hippocampus

Neuralstem, Inc. The Promise of Stem Cell Therapeutics 15 Years in the Making □ Cell manufacturing: cGMP □ FDA approved for human trials □ Phase I Human Trials □ Cells – Safe □ Procedure – Safe □ ALS – First 12 patients completed □ Drug screening – resulting in new class of compounds: patented and in Phase I human trials □ U.S. and Global Platform Roll - out: Cell Therapy Pharmaceuticals ALS - Amyotrophic Lateral Sclerosis Alzheimer’s disease Brain Cancer Anxiety Chronic Spinal Cord Injury Bipolar Disorder Chronic Stroke Major Depression Huntington’s Disease Schizophrenia Ischemic Spastic Paraplegia 4 Technology Platform Cell Pharmaceuticals Therapy Direct Injection/ Small molecule Transplantation Discovery/ To Spinal Cord Development Or Brain Via Proprietary Stem Cell - based Screening

Neuralstem, Inc. Global Clinical Trial and Product Status Overview □ U.S. FDA - approved Trials to Date □ Cell Therapy □ ALS: Phase I commenced Jan. 2010; 12 patients transplanted to date □ Orphan status designation awarded by the FDA in Nov. 2010 □ Pharmaceuticals □ Major Depression: Phase Ia for NSI - 189 approved Dec. 2010; first patient dosed Feb. 28, 2011; Ia completion scheduled Sept. 2011, commencement of Ib expected Fall 2011 □ U.S. Next Indication □ Chronic Spinal Cord Injury: IND filed for same product, same route of administration as ALS trial; pending approval □ International Products’ Status □ Neuralstem China: Stroke – trial expected to commence in late 2011/early 2012 □ India: Spinal Cord Injury – IND filing expected in 2011 □ Japan: NSI - 189 – retained Sumitomo’s Summit Pharmaceuticals International Corporation for Ib data licensing arrangement 5

U.S. Neuralstem ALS Trial World - Class Trial Design and Execution □ Principal Investigator: Eva L. Feldman, M.D., Ph.D. , Professor of Neurology & Director A. Alfred Taubman Medical Research Institute of the University of Michigan Medical School □ Site Principal Investigator: Jonathan D. Glass, M.D. , Professor of Neurology & Director Emory ALS Center Emory University □ Co - Investigator & Neurosurgeon: Nicholas M. Boulis M.D . , Assistant Professor Neurosurgery Emory University □ Trial Advisory Board: □ Zachary Simmons M.D., Chairman, SMB , Professor of Neurology Penn State University Hershey Medical Center and Director Neuromuscular Program and ALS Center □ Mark Bromberg M.D. , Ph.D. Professor of Neurology & Director of the Motor Neuron Disease/ALS Clinic University of Utah School of Medicine □ Lucie Bruijn, Ph.D. , Chief Scientist & Sr. VP of Research and Development ALS Association; □ Thomas Freeman M.D. , Professor, Dept of Neurosurgery & Medical Director, Center for Aging and Brain Repair University of South Florida College of Medicine □ Clifton L. Gooch, M.D. , Professor & Chairman Department of Neurology & Director Division of Neuromuscular Disease University of South Florida College of Medicine □ Hiroshi Mitsumoto M.D., D.Sc. , Professor of Neurology Columbia University & Director of the Eleanor and Lou Gehrig MDA/ALS Research Center and Neuromuscular Division at the Neurological Institute of New York □ Paul Park M.D. , Assistant Professor & Neurosurgeon University of Michigan School of Medicine □ Mike Vogelbaum M.D., Ph.D . , Associate Director, Brain Tumor and Neuro - Oncology Center Cleveland Clinic □ Safety Monitoring Board/SMB: Dr. Clifton L. Gooch ; Dr. Hiroshi Mitsumoto ; Dr. Paul Park ; Dr. Zachary Simmons ; Dr. Mike Vogelbaum ; SMB Non - Voting Monitor: Dr. Lucie Bruijn 6

U.S. Neuralstem Cell Therapeutics ALS Trial Data Collection □ Primary Endpoint □ Safety □ Secondary Endpoints □ Attenuation of motor function loss □ Maintenance of respiratory capacity □ Stabilization of ALS functional rating scale □ Reduction of spasticity/rigidity if present □ Graft survival at autopsy upon mortality 7 Group A: Nonambulatory Patients #1 - 3: Five Injections at Lumbar Cord, Unilateral Patients #4 - 6: Ten Injections at Lumbar Cord, Bilateral Commenced: January 2010 Completed: August 2010 Groups B/C: Ambulatory – Lumbar Patients #7 - 9 (B) : Five Injections at Lumbar Cord, Unilateral Patients #10 - 12 (C): Ten Injections at Lumbar Cord, Bilateral Commenced: October 2010 Completed: April 2011 Groups D/E: Ambulatory – Cervical Patients #13 - 15 (D): Five Injections at Cervical Cord, Unilateral Patients #16 - 18 (E): Ten Injections at Lumbar Cord, Bilateral - Plus Five Injections at Cervical Cord, Unilateral Group D: Fall 2011 – est. start
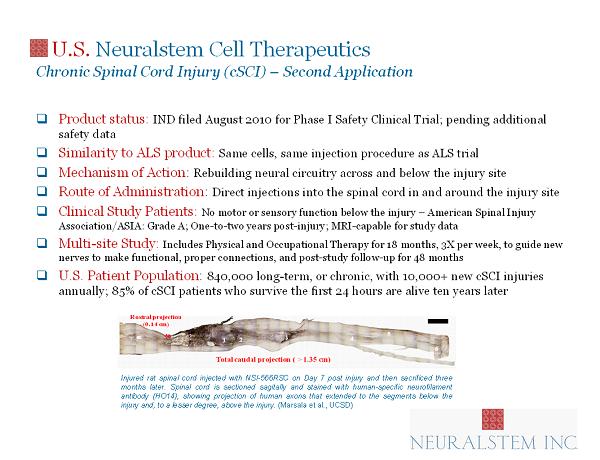
U.S. Neuralstem Cell Therapeutics Chronic Spinal Cord Injury (cSCI) – Second Application □ Product status: IND filed August 2010 for Phase I Safety Clinical Trial; pending additional safety data □ Similarity to ALS product: Same cells, same injection procedure as ALS trial □ Mechanism of Action : Rebuilding neural circuitry across and below the injury site □ Route of Administration : Direct injections into the spinal cord in and around the injury site □ Clinical Study Patients: No motor or sensory function below the injury – American Spinal Injury Association/ASIA: Grade A; One - to - two years post - injury; MRI - capable for study data □ Multi - site Study: Includes Physical and Occupational Therapy for 18 months, 3X per week, to guide new nerves to make functional, proper connections, and post - study follow - up for 48 months □ U.S. Patient Population : 840,000 long - term, or chronic, with 10,000+ new cSCI injuries annually; 85% of cSCI patients who survive the first 24 hours are alive ten years later 8 Injured rat spinal cord injected with NSI - 566 RSC on Day 7 post injury and then sacrificed three months later . Spinal cord is sectioned sagitally and stained with human - specific neurofilament antibody (HO 14 ), showing projection of human axons that extended to the segments below the injury and, to a lesser degree, above the injury . (Marsala et al . , UCSD)

U.S. Neuralstem Cell Therapeutics Chronic Motor Disorders from Stroke – Next Indication □ Product status: IND to be filed 2011 for Phase I Safety Clinical Trial □ Mechanism of Action : Neural plasticity and regeneration □ Route of Administration : Intracerebral injections at peri - infarct sites – method already shown safe in a prior university study: Meltzer et al. Neurosurgery. 2001 Sep;49(3):586 - 91 □ Clinical Study Patients : Chronic paralysis for minimum six months due to stroke, with post - study follow - up for 48 months □ U.S. Patient Population : More than 6 million people in the U.S. today have survived a stroke, with an estimated 500,000 new cases per year; 40% of stroke patients are severely disabled. Stroke is the leading cause of adult disability and third leading cause of death 9

U.S. Neuralstem Cell Therapeutics Brain Cancer – U.S. Department of Defense Contract □ Product Research status : Neuralstem’s first anti - cancer cell therapeutics program □ Product Funding: DOD contract to develop and engineer neural stem cell technology for the treatment of brain cancer □ Contract award: $1.6 million for the first year of the project, of which Neuralstem receives $625,000 □ Duration: DOD has three one - year options to continue the program based upon milestones □ Program goal: therapeutic product for the treatment of cancerous brain tumors ready to submit to the FDA by the end of the fourth year (2015) □ Mechanism of Action : Expression: 1) antibody, 2) enzyme, and 3) antiangiogenic protein □ Route of Administration : Intracerebral injections at cancerous tumor sites □ Principal Investigator : John Zhang, MD, PhD, Professor of Neurosurgery, Loma Linda University (CA) □ U.S. Patient Population : 600,000+ people in the U.S. are living with a primary brain tumor, and each year approximately 210,000 people in the U.S. will be diagnosed with a primary or metastatic brain tumor. Metastatic brain tumors occur in 20 to 40% of persons with cancer and are the most common type of brain tumor. An estimated 22,000+ people in the U.S. will be diagnosed with primary malignant brain and CNS tumors annually. An estimated 35% of adults living with a primary malignant brain or CNS tumor will live five years or longer, and glioblastoma is the most common, and most aggressive, malignant brain tumor. 10 SR19

International Neuralstem Program Advances Cell Therapeutics’ Product Status □ Neuralstem China : Stroke – Trial expected to commence in late 2011/early 2012 □ Wholly owned subsidiary: Neuralstem China 神脑生物医药公司 (Suzhou Neuralstem Biopharmaceutical Company, Ltd.) □ BaYi Brain Hospital in Beijing, collaborator □ India : Spinal Cord Injury (chronic and acute) – IND filing expected in 2011 □ Company evaluating potential trial centers and collaborators □ Czech Republic : Spinal Cord Injury – Preclinical protocol design stage □ Czech Institute of Experimental Medicine, Prague □ Germany : Huntington’s Disease – IND filing expected in early 2012 □ Albert - Ludwigs - University in Freiburg □ Korea : Partner CJ Cheil Jedang Corporation □ Exclusive option agreement for cell therapy products in South Korea, Indonesia, Malaysia, Philippines, Singapore and Vietnam 11
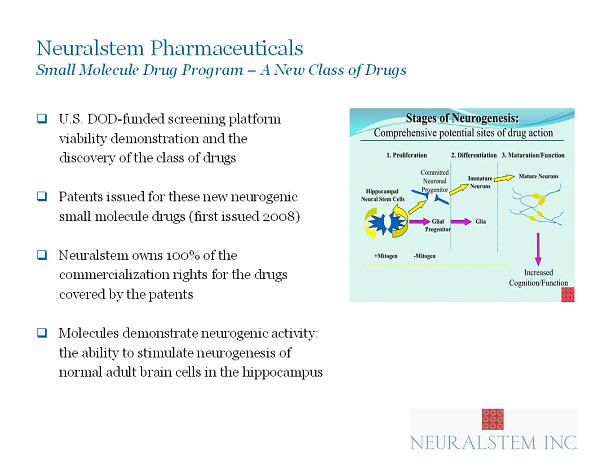
Neuralstem Pharmaceuticals Small Molecule Drug Program – A New Class of Drugs □ U.S. DOD - funded screening platform viability demonstration and the discovery of the class of drugs □ Patents issued for these new neurogenic small molecule drugs (f irst issued 2008) □ Neuralstem owns 100% of the commercialization rights for the drugs covered by the patents □ Molecules demonstrate neurogenic activity: the ability to stimulate neurogenesis of normal adult brain cells in the hippocampus 12

U.S. Neuralstem Pharmaceuticals Neurogenic S mall Molecule Drug Program □ NSI - 189 □ First in this new class of compounds □ Characteristics: molecular wt. <400, orally active, excellent brain penetration, key biological activity: enhanced neurogenesis and increased hippocampal volume □ Major Depression – leading indication □ Product Status : Phase Ia human safety trial dosing in Feb. 2011 □ Mechanism of Action : Neurogenesis □ Route of Administration : Oral □ Clinical lot : 12 kg cGMP completed □ Timeline : Phase Ia completion scheduled Sept. 2011, and Ib expected to be completed in early 2012 □ Other potential indications include: □ Alzheimer’s Disease □ Anxiety □ Bipolar Disorder □ Schizophrenia 13

Business Strategy Pharmaceuticals and Cell Therapy □ Pharmaceuticals □ Take NSI - 189 through at least Phase II human trial before licensing out – except for Japanese market □ Japan : Retained Sumitomo’s Summit Pharmaceuticals International Corporation – early stage partnership □ Ib data licensing arrangement for NSI - 189 in Japan □ Procure agreement to develop remainder of the Patent - protected compounds □ Explore collaborations with pharmaceutical industry, giving selective access to the cells for screening of libraries □ Cell Therapy □ Platform Technology – Develop myriad indications that all use the same investigational products: spinal cord stem cells □ Leverage ongoing U.S. FDA safety trials worldwide to simultaneously advance the study designs for faster proof - of - concept data and faster marketing approval □ Roll out select products ourselves in the U.S. □ Build out partnership network for worldwide distribution 14

Neuralstem, Inc. 2011 Catalysts □ Current Trials □ ALS lumbar - transplantations conclusion; cervical - transplantations commencement and completion of enrollment □ ALS Orphan Status Designation from FDA □ NSI - 189, Major Depression, Phase Ia Trial completion; approval and dosing of Ib Trial □ Trial Approvals □ cSCI Phase I Trial in U.S. □ Chronic and acute SCI Trial in India □ Stroke Trials in China □ IND Filings □ Stroke Phase I Trial in U.S. □ First collaborative small molecule drug discovery deal 15
