Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-12998_28k.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a11-12998_2ex99d2.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a11-12998_2ex99d1.htm |
Exhibit 99.3
Below is a graphical representation of the poster entitled “Long-term Treatment With Controlled-Release Phentermine/Topiramate Demonstrates Sustained Weight Loss Over 108 Weeks”:
Below is a reproduction of the contents of the poster entitled “Long-term Treatment With Controlled-Release Phentermine/Topiramate Demonstrates Sustained Weight Loss Over 108 Weeks”:
Authors: W. Timothy Garvey, MD(a); Wesley W. Day, PhD(b); Charles H. Bowden, MD(b)
(a)University of Alabama at Birmingham, Birmingham, Alabama, USA; (b)Vivus, Inc., Mountain View, California, USA
Contact details: Dr. W. T. Garvey, MD; University of Alabama at Birmingham, 1675 University Blvd, Birmingham, AL 35294-3360; Office: (205) 996-7433; Email: garveyt@uab.edu
· Introduction
· Obesity is a chronic disease that is associated with and contributes to poor outcomes with various comorbid conditions, including diabetes, cardiovascular disease, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.(1)-(4)
· As a result, excess body weight has been shown to reduce life expectancy and is the sixth most important cause of disease burden globally.(5)
· Weight loss of >5% significantly reduces the health risks associated with obesity, and greater weight loss is associated with increasing benefit on weight-related comorbidities.(6)-(8)
· Lifestyle interventions alone are not always sufficient to induce and maintain significant weight loss; therefore, an effective and well-tolerated medical therapy may be needed for the effective treatment of obesity in the longer term.(9)
· An investigational low-dose, controlled-release combination of phentermine and topiramate (PHEN/TPM CR) previously demonstrated significant weight loss compared with placebo in overweight and obese subjects through 56 weeks in the CONQUER study.(10)
· Objective
· To determine the long-term weight-loss benefits resulting from 108 weeks of treatment with PHEN/TPM CR in overweight/obese subjects with weight-related comorbidities.
· Methods
· PHEN/TPM CR was evaluated over 108 weeks in 2 consecutive clinical studies:
· CONQUER was a 56-week, double-blind, placebo-controlled, Phase 3 trial of 2487 overweight/obese adult subjects with > 2 weight-related comorbidities who were randomized to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92).(10)
· Subjects from selected sites who completed CONQUER on treatment were eligible to enter SEQUEL, a double-blind, placebo-controlled extension of the CONQUER study consisting of a further 52-week treatment period in which subjects continued on their original double-blind treatment.
· All subjects were managed to standard of care for their respective comorbidities, including medication management as needed, and received lifestyle modification counseling, including guidance on nutrition and increased physical activity, based on the LEARN program.(11)
· Assessments
· Primary efficacy endpoints for the SEQUEL trial were percent weight loss and the percentage of subjects achieving > 5% weight loss from baseline (CONQUER Week 0) to Week 108 in the intent-to-treat (ITT) population with last observation carried forward (LOCF).
· Secondary endpoints included percentage of subjects achieving > 10%, > 15%, or > 20% weight loss and change in waist circumference from baseline (CONQUER Week 0) to Week 108.
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status as fixed effects, with baseline weight as a covariate.
· Results
Baseline demographics
· In total, 866 subjects were eligible to participate in SEQUEL; 676 subjects (78%) were enrolled in the study, and the remainder (190 subjects) elected not to continue.
· A higher percentage of eligible CONQUER subjects on 15/92 (86%) chose to enrol in the extension study than in either the 7.5/46 (79%) or placebo (69%) group.
· Of the 676 enrolled subjects, 574 (84.9%) subjects completed all study visits.
· The percentages of subjects completing the study were similar between treatment groups (placebo, 86.8%; 7.5/46, 83.8%; and 15/92, 84.1%).
· One subject in the 7.5/46 group enrolled but discontinued from the study prior to receiving study drug (ITT; N=675).
· Baseline demographics were similar in each treatment group (Table 1).
Table 1. Baseline Demographics (Safety Sample).
|
|
|
Placebo |
|
PHEN/TPM CR 7.5/46 |
|
PHEN/TPM CR 15/92 |
|
|
Mean age, years |
|
52.7 |
|
52.2 |
|
51.2 |
|
|
Gender, female, % |
|
64.8 |
|
69.3 |
|
66.1 |
|
|
Race, Caucasian, % |
|
87.2 |
|
87.6 |
|
82.7 |
|
|
Mean weight, kg |
|
101.1 |
|
102.2 |
|
101.9 |
|
|
Mean body mass index, kg/m2 |
|
36.0 |
|
36.1 |
|
36.2 |
|
|
Mean waist circumference, cm |
|
113.0 |
|
112.9 |
|
112.2 |
|
Baseline was defined as the last measurement on or before the first dose of double-blind study drug in the CONQUER study (Week 0).
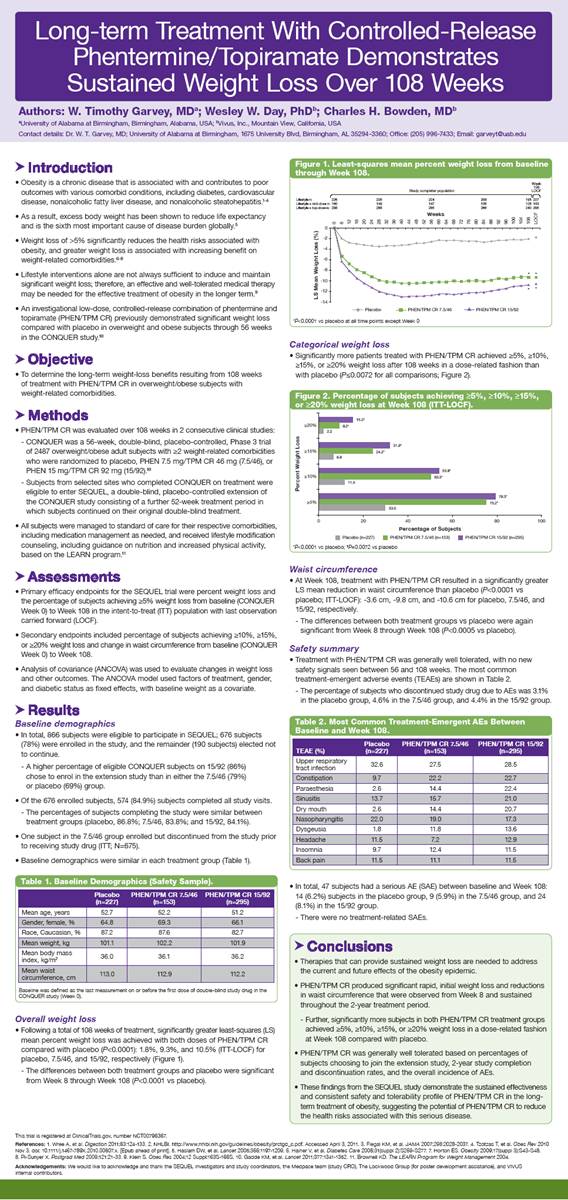
Overall weight loss
· Following a total of 108 weeks of treatment, significantly greater least-squares (LS) mean percent weight loss was achieved with both doses of PHEN/TPM CR compared with placebo (P<0.0001): 1.8%, 9.3%, and 10.5% (ITT-LOCF) for placebo, 7.5/46, and 15/92, respectively (Figure 1).
· The differences between both treatment groups and placebo were significant from Week 8 through Week 108 (P<0.0001 vs placebo).
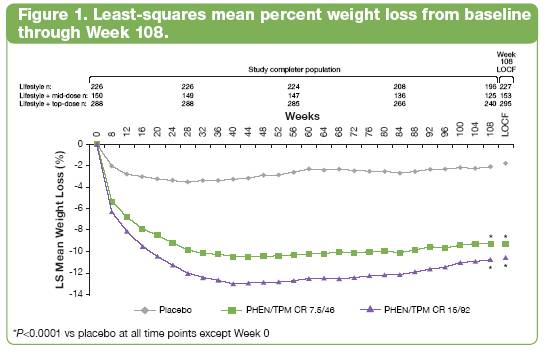
Categorical weight loss
· Significantly more patients treated with PHEN/TPM CR achieved > 5%, > 10%, > 15%, or > 20% weight loss after 108 weeks in a dose-related fashion than with placebo (P<0.0072 for all comparisons; Figure 2).
Waist circumference
· At Week 108, treatment with PHEN/TPM CR resulted in a significantly greater LS mean reduction in waist circumference than placebo (P<0.0001 vs placebo; ITT-LOCF): -3.6 cm, -9.8 cm, and -10.6 cm for placebo, 7.5/46, and 15/92, respectively.
· The differences between both treatment groups vs placebo were again significant from Week 8 through Week 108 (P<0.0005 vs placebo).
Safety summary
· Treatment with PHEN/TPM CR was generally well tolerated, with no new safety signals seen between 56 and 108 weeks. The most common treatment-emergent adverse events (TEAEs) are shown in Table 2.
· The percentage of subjects who discontinued study drug due to AEs was 3.1% in the placebo group, 4.6% in the 7.5/46 group, and 4.4% in the 15/92 group.
Table 2. Most Common Treatment-Emergent AEs Between Baseline and Week 108.
|
TEAE (%) |
|
Placebo |
|
PHEN/TPM CR 7.5/46 |
|
PHEN/TPM CR 15/92 |
|
|
Upper respiratory tract infection |
|
32.6 |
|
27.5 |
|
28.5 |
|
|
Constipation |
|
9.7 |
|
22.2 |
|
22.7 |
|
|
Paraesthesia |
|
2.6 |
|
14.4 |
|
22.4 |
|
|
Sinusitis |
|
13.7 |
|
15.7 |
|
21.0 |
|
|
Dry mouth |
|
2.6 |
|
14.4 |
|
20.7 |
|
|
Nasopharyngitis |
|
22.0 |
|
19.0 |
|
17.3 |
|
|
Dysgeusia |
|
1.8 |
|
11.8 |
|
13.6 |
|
|
Headache |
|
11.5 |
|
7.2 |
|
12.9 |
|
|
Insomnia |
|
9.7 |
|
12.4 |
|
11.5 |
|
|
Back pain |
|
11.5 |
|
11.1 |
|
11.5 |
|
· In total, 47 subjects had a serious AE (SAE) between baseline and Week 108: 14 (6.2%) subjects in the placebo group, 9 (5.9%) in the 7.5/46 group, and 24 (8.1%) in the 15/92 group.
· There were no treatment-related SAEs.
· Conclusions
· Therapies that can provide sustained weight loss are needed to address the current and future effects of the obesity epidemic.
· PHEN/TPM CR produced significant rapid, initial weight loss and reductions in waist circumference that were observed from Week 8 and sustained throughout the 2-year treatment period.
· Further, significantly more subjects in both PHEN/TPM CR treatment groups achieved > 5%, > 10%, > 15%, or > 20% weight loss in a dose-related fashion at Week 108 compared with placebo.
· PHEN/TPM CR was generally well tolerated based on percentages of subjects choosing to join the extension study, 2-year study completion and discontinuation rates, and the overall incidence of AEs.
· These findings from the SEQUEL study demonstrate the sustained effectiveness and consistent safety and tolerability profile of PHEN/TPM CR in the long-term treatment of obesity, suggesting the potential of PHEN/TPM CR to reduce the health risks associated with this serious disease.
This trial is registered at ClinicalTrials.gov, number NCT00796367.
References: (1) Wree A, et al. Digestion 2011;83:124-133. (2) NHLBI. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed April 3, 2011. (3) Flegal KM, et al. JAMA 2007;298:2028-2037. (4) Tzotzas T, et al. Obes Rev 2010 Nov 3. doi: 10.1111/j.1467-789X.2010.00807.x. [Epub ahead of print]. (5) Haslam DW, et al. Lancet 2005;366:1197-1209. (6) Hainer V, et al. Diabetes Care 2008;31(suppl 2):S269-S277. (7) Horton ES. Obesity 2009;17(suppl 3):S43-S48. (8) Pi-Sunyer X. Postgrad Med 2009;121:21-33. (9) Klein S. Obes Res 2004;12 Suppl:163S-166S. (10) Gadde KM, et al. Lancet 2011;377:1341-1352. (11) Brownell KD. The LEARN Program for Weight Management 2004.
Acknowledgements: We would like to acknowledge and thank the SEQUEL investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
