Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-12998_28k.htm |
| EX-99.3 - EX-99.3 - VIVUS INC | a11-12998_2ex99d3.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a11-12998_2ex99d1.htm |
Exhibit 99.2
Below is a graphical representation of the poster entitled “Weight Loss With Low-Dose, Controlled-Release Phentermine/Topiramate Therapy Improves Glycaemic Status and Prevents Progression to Type 2 Diabetes Mellitus”:
Below is a reproduction of the contents of the poster entitled “Weight Loss With Low-Dose, Controlled-Release Phentermine/Topiramate Therapy Improves Glycaemic Status and Prevents Progression to Type 2 Diabetes Mellitus”:
Authors: W. Timothy Garvey, MD(a); Craig A. Peterson, MS(b); Barbara Troupin, MD(b)
(a)University of Alabama at Birmingham, Birmingham, Alabama, USA; (b)Vivus, Inc., Mountain View, California, USA
Contact details: Dr. W. T. Garvey, MD; University of Alabama at Birmingham, 1675 University Blvd, Birmingham, AL 35294-3360; Office: (205) 996-7433; Email: garveyt@uab.edu
· Abstract
Introduction
Obesity is associated with increased mortality due to comorbidities, such as type 2 diabetes mellitus (T2DM). Once-daily, controlled-release phentermine/topiramate (PHEN/TPM CR) previously demonstrated significant and well-tolerated weight loss, which may mitigate the risk of the growing T2DM epidemic in obese patients.
Methods
To evaluate the effects of PHEN/TPM CR on glycaemic parameters and rates of progression to T2DM, we conducted prespecified analyses of data from the CONQUER study, a double-blind, Phase 3 trial, which randomised 2487 overweight/obese adults with >2 weight-related comorbidities to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks. Glycaemic parameters were evaluated at baseline and at Week 56.
Results
Glycated haemoglobin, fasting glucose, and fasting insulin were significantly improved at Week 56 (ITT-LOCF; P<0.005 vs placebo). Among subjects with ADA-defined Prediabetes at baseline (n=1119), normoglycaemia was achieved in 39.1% and 45.2% of subjects in the 7.5/46 and 15/92 arms, respectively. In this same sample, progression to T2DM was reported in 9.0% of subjects on placebo, 6.7% on 7.5/46, and 4.4% on 15/92 (P<0.05 vs placebo). Treatment with PHEN/TPM CR was generally well tolerated.
Conclusion
In obese patients with metabolic comorbidities, treatment with PHEN/TPM CR over 56 weeks improved glycaemic parameters. In subjects with Prediabetes at baseline, PHEN/TPM CR therapy normalized glycaemic parameters in >39% of subjects and led to significant reductions in the progression to T2DM.
Note that in the original abstract submission, the WHO criteria for Prediabetes were used to define the Prediabetes sample. In the presented abstract and poster, the current ADA definition of Prediabetes was utilized.
· Introduction
· Obesity is an independent risk factor for cardiovascular disease and also contributes to the development of weight-related comorbidities, such as type 2 diabetes mellitus (T2DM).(1)-(5)
· The presence of Prediabetes, defined as impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), increases the risk of developing T2DM.(6)
· Even modest weight loss in patients with Prediabetes has been shown to delay progression to T2DM and lead to improvements in glycaemic parameters.(6),(7)
· Controlled-release phentermine/topiramate (PHEN/TPM CR) is an investigational combination therapy that was developed to maximize weight loss and improve weight-related comorbidities.
· The CONQUER study was a double-blind, Phase 3 trial that randomised 2487 overweight/obese adults with >2 weight-related comorbidities to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks.(8)
· Objectives
· To assess the effects of PHEN/TPM CR on weight loss and glycaemic parameters over a 56-week period in the overall CONQUER sample.
· To evaluate changes in glycaemic parameters and rates of progression to T2DM over a 56-week period in a prespecified subset of patients in CONQUER with Prediabetes.
· Methods
· This 56-week, double-blind, parallel-group Phase 3 trial (CONQUER) randomised 2487 overweight/obese adult subjects with >2 weight-related comorbidities to placebo (n=994), 7.5/46 (n=498), or 15/92 (n=995).
· All subjects were managed to standard of care for their respective comorbidities, including the option to add or modify medications, and received lifestyle modification, including nutrition guidance and increased physical activity, based on the LEARN program.(9)
· Assessments
· The primary efficacy endpoint in this trial was percent weight loss at Week 56 in the intent-to-treat (ITT) population with last observation carried forward (LOCF).
· Other endpoints included change in HbA1c, fasting glucose, and fasting insulin at Week 56.
· A subanalysis assessed changes at Week 56 in glycaemic parameters and the change in diabetic status among subjects with Prediabetes (defined as IFG [5.6 to 6.9 mmol/L] or IGT [7.8 to 11.0 mmol/L as measured by oral glucose tolerance test (OGTT)]) at baseline.
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status as fixed effects, with baseline weight as a covariate.
· Results
Baseline Characteristics
· Subjects randomised in CONQUER were mostly female (70%) and Caucasian (86%) with a mean age of 51 years, mean weight of 103.1 kg, and mean body mass index of 36.6 kg/m2. Mean baseline HbA1c was 5.9%, fasting glucose was 5.9 mmol/L, and fasting insulin was 125.7 ρmol/L.
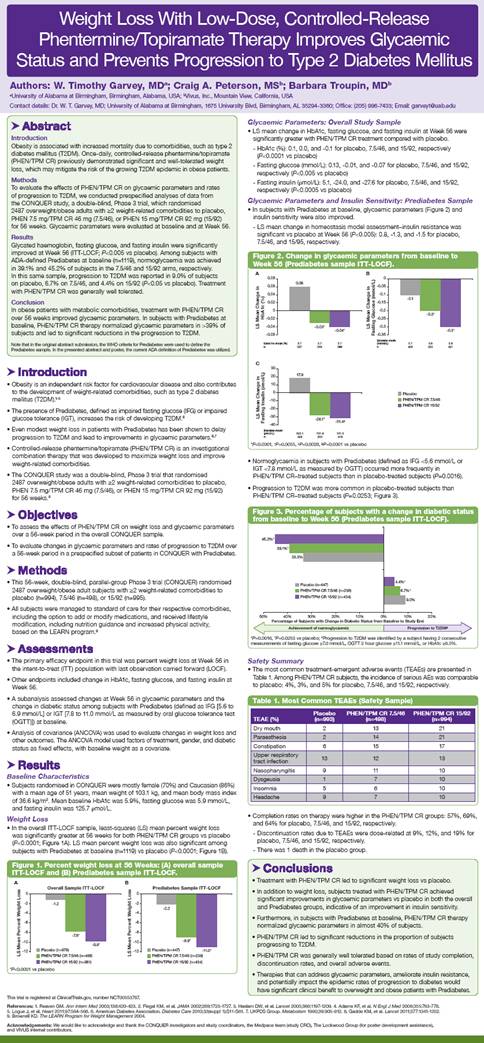
Weight Loss
· In the overall ITT-LOCF sample, least-squares (LS) mean percent weight loss was significantly greater at 56 weeks for both PHEN/TPM CR groups vs placebo (P<0.0001; Figure 1A). LS mean percent weight loss was also significant among subjects with Prediabetes at baseline (n=1119) vs placebo (P<0.0001; Figure 1B).
Glycaemic Parameters: Overall Study Sample
· LS mean change in HbA1c, fasting glucose, and fasting insulin at Week 56 were significantly greater with PHEN/TPM CR treatment compared with placebo.
· HbA1c (%): 0.1, 0.0, and -0.1 for placebo, 7.5/46, and 15/92, respectively (P<0.0001 vs placebo)
· Fasting glucose (mmol/L): 0.13, -0.01, and -0.07 for placebo, 7.5/46, and 15/92, respectively (P<0.005 vs placebo)
· Fasting insulin (ρmol/L): 5.1, -24.0, and -27.6 for placebo, 7.5/46, and 15/92, respectively (P<0.0005 vs placebo)
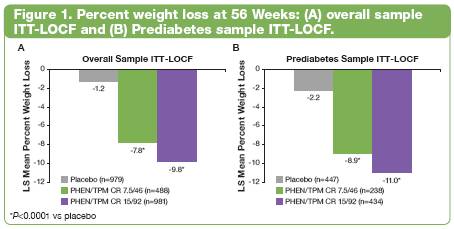
Glycaemic Parameters and Insulin Sensitivity: Prediabetes Sample
· In subjects with Prediabetes at baseline, glycaemic parameters (Figure 2) and insulin sensitivity were also improved.
· LS mean change in homeostasis model assessment—insulin resistance was significant vs placebo at Week 56 (P<0.005): 0.8, -1.3, and -1.5 for placebo, 7.5/46, and 15/95, respectively.
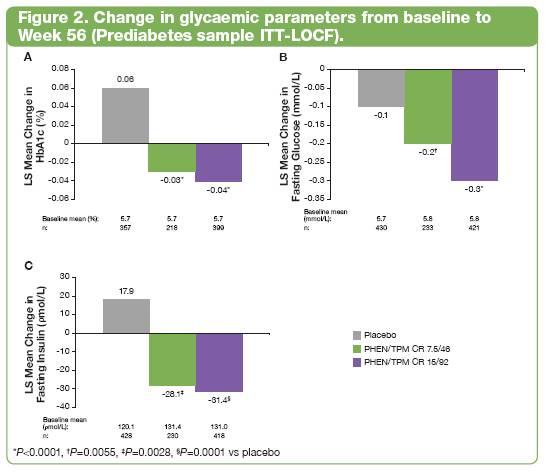
· Normoglycaemia in subjects with Prediabetes (defined as IFG <5.6 mmol/L or IGT <7.8 mmol/L as measured by OGTT) occurred more frequently in PHEN/TPM CR—treated subjects than in placebo-treated subjects (P=0.0016).
· Progression to T2DM was more common in placebo-treated subjects than PHEN/TPM CR—treated subjects (P=0.0253; Figure 3).
Safety Summary
· The most common treatment-emergent adverse events (TEAEs) are presented in Table 1. Among PHEN/TPM CR subjects, the incidence of serious AEs was comparable to placebo: 4%, 3%, and 5% for placebo, 7.5/46, and 15/92, respectively.
Table 1. Most Common TEAEs (Safety Sample)
|
|
|
Placebo |
|
PHEN/TPM CR 7.5/46 |
|
PHEN/TPM CR 15/92 |
|
|
TEAE (%) |
|
(n=993) |
|
(n=498) |
|
(n=994) |
|
|
Dry mouth |
|
2 |
|
13 |
|
21 |
|
|
Paraesthesia |
|
2 |
|
14 |
|
21 |
|
|
Constipation |
|
6 |
|
15 |
|
17 |
|
|
Upper respiratory tract infection |
|
13 |
|
12 |
|
13 |
|
|
Nasopharyngitis |
|
9 |
|
11 |
|
10 |
|
|
Dysgeusia |
|
1 |
|
7 |
|
10 |
|
|
Insomnia |
|
5 |
|
6 |
|
10 |
|
|
Headache |
|
9 |
|
7 |
|
10 |
|
· Completion rates on therapy were higher in the PHEN/TPM CR groups: 57%, 69%, and 64% for placebo, 7.5/46, and 15/92, respectively.
· Discontinuation rates due to TEAEs were dose-related at 9%, 12%, and 19% for placebo, 7.5/46, and 15/92, respectively.
· There was 1 death in the placebo group.
· Conclusions
· Treatment with PHEN/TPM CR led to significant weight loss vs placebo.
· In addition to weight loss, subjects treated with PHEN/TPM CR achieved significant improvements in glycaemic parameters vs placebo in both the overall and Prediabetes groups, indicative of an improvement in insulin sensitivity.
· Furthermore, in subjects with Prediabetes at baseline, PHEN/TPM CR therapy normalized glycaemic parameters in almost 40% of subjects.
· PHEN/TPM CR led to significant reductions in the proportion of subjects progressing to T2DM.
· PHEN/TPM CR was generally well tolerated based on rates of study completion, discontinuation rates, and overall adverse events.
· Therapies that can address glycaemic parameters, ameliorate insulin resistance, and potentially impact the epidemic rates of progression to diabetes would have significant clinical benefit to overweight and obese patients with Prediabetes.
This trial is registered at ClinicalTrials.gov, number NCT00553787.
References: (1) Reaven GM. Ann Intern Med 2003;138:420-423. (2) Flegal KM, et al. JAMA 2002;288:1723-1727. (3) Haslam DW, et al. Lancet 2005;366:1197-1209. (4) Adams KF, et al. N Engl J Med 2006;355:763-778. (5) Logue J, et al. Heart 2011;97:564-568. (6) American Diabetes Association. Diabetes Care 2010;33(suppl 1):S11-S61. (7) UKPDS Group. Metabolism 1990;39:905-912. (8) Gadde KM, et al. Lancet 2011;377:1341-1352. (9) Brownell KD. The LEARN Program for Weight Management 2004.
Acknowledgements: We would like to acknowledge and thank the CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
