Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a11-12998_28k.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a11-12998_2ex99d2.htm |
| EX-99.3 - EX-99.3 - VIVUS INC | a11-12998_2ex99d3.htm |
Exhibit 99.1
Below is a graphical representation of the poster entitled “Efficacy and Tolerability of Topiramate Alone and in Combination with Phentermine”:

Below is a reproduction of the contents of the poster entitled “Efficacy and Tolerability of Topiramate Alone and in Combination with Phentermine”:
Authors: George A. Bray, MD(a); Louis J. Aronne, MD(b); Alok K. Gupta, MD(a); Wesley W. Day, PhD(c); Barbara Troupin, MD, MBA(c)
(a)Pennington Biomedical Research Center, Baton Rouge, LA; (b)Weill Cornell Medical Center, New York, NY; (c)Vivus, Inc., Mountain View, CA
Contact details: George A. Bray, MD; Pennington Biomedical Research Center, 6400 Perkins Road, Baton Rouge, LA 70808-4124; Office: (225) 763-3140; Email: george.bray@pbrc.edu
· Introduction
· Obesity is an independent risk factor for cardiovascular disease and contributes significantly to the development of multiple weight-related comorbidities, such as diabetes and metabolic syndrome.(1),(2)
· While lifestyle interventions are the mainstay for treating obesity, many patients require additional therapeutic intervention, such as pharmacotherapy or bariatric surgery.(3)
· One such therapy is topiramate, an agent currently approved for the treatment of seizure disorders and for migraine prophylaxis. Topiramate is a carbonic anhydrase inhibitor that modulates effects at the glutamate and γ-aminobutyric acid (GABA) receptors, blocks some sodium- and calcium-dependent ion channels, may increase neuropeptide Y (NPY), and likely also impacts glucose and lipid metabolism peripherally. The exact mechanisms that lead to weight loss are not conclusively known. Topiramate as monotherapy has been studied extensively for the treatment of obesity with and without comorbidities.(4)-(13)
· A second therapy is phentermine, a sympathomimetic agent, approved in the United States since 1959 as an adjunct to diet and exercise for short-term treatment of obesity.(14)
· As is common in the treatment of other complex diseases, such as diabetes or hypertension, combination therapy is often used initially to address the multiple and redundant mechanisms leading to obesity.(15)
· An investigational combination therapy of two approved agents, phentermine and a controlled-release topiramate (PHEN/TPM CR), has been evaluated to assess the efficacy, safety, and tolerability of these agents in combination, in low doses relative to approved doses.(16)
· Objective
· To review and contrast available data on different formulations of topiramate from published literature or clinical trials data.
· Methods
· Two randomised clinical trials using topiramate were reviewed and contrasted based on having similar designs and enrolled populations. Both studies added blinded therapy as an adjunct to a standardised lifestyle intervention.
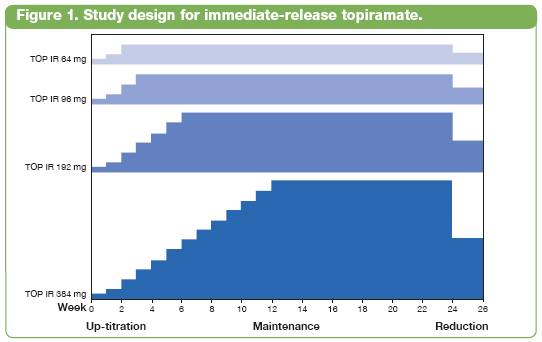
· The first (Bray 2003), a dose-ranging study of immediate-release topiramate (TOP IR), was a 24-week, placebo-controlled weight loss study of 4 doses of TOP IR (64, 96, 192, or 384 mg total daily dose) with a 2- to 12-week titration period, depending on dose, added to the Pathways to Change lifestyle-modification program (Figure 1).(8),(17)
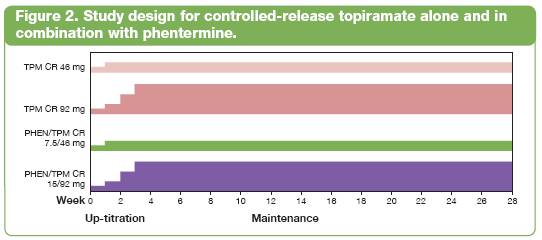
· The second, a more recent study (OB-301), assessed a controlled-release topiramate (TPM CR) alone and in combination with phentermine (PHEN/TPM CR) for weight loss. OB-301 was a 28-week, placebo-controlled study evaluating the effects of two doses each of TPM CR (46 and 92 mg) and PHEN/TPM CR (7.5/46 and 15/92 mg) with a 4-week titration, added to the LEARN lifestyle-modification program. Due to space limitations, the phentermine alone data are not presented here (Figure 2).(18)
NOTE: These were not head-to-head studies and comparisons cannot be viewed as conclusive.
· Results
Baseline demographics
· The Bray 2003 (TOP IR) and the OB-301 (TPM CR and PHEN/TPM CR) studies were of similar size and duration, and study participants had similar baseline attributes, which were balanced across treatment arms (Table 1).(8)
· 10% of subjects in the Bray 2003 study were hypertensive at baseline (systolic blood pressure [SBP] >140 mm Hg and/or diastolic BP [DBP] >90 mm Hg); 29% were hypertensive in the OB-301 study, by the same definition.(8)
Table 1. Baseline Demographics: TOP IR Monotherapy and TPM CR or PHEN/TPM CR.
|
|
|
TOP IR |
|
TPM CR or PHEN/TPM CR |
|
|
|
|
Bray 2003 |
|
OB-301 |
|
|
|
|
(n=380) |
|
(n=756) |
|
|
Mean age, years |
|
44.3 |
|
45.6 |
|
|
Female, % |
|
85.3 |
|
79.2 |
|
|
White, % |
|
76 |
|
79 |
|
|
Mean weight, kg |
|
103.7 |
|
101.3 |
|
|
Mean body mass index, kg/m2 |
|
37.4 |
|
36.3 |
|
|
Mean SBP, mm Hg |
|
121.2 |
|
122.1 |
|
|
Mean DBP, mm Hg |
|
78.6 |
|
79.0 |
|
Weight loss
· Percent weight loss: Each study showed significant mean percent weight loss in both the ITT-LOCF and completers populations (P<0.05 vs placebo after Week 4 in all treatment arms), in a mostly dose-dependent fashion (Table 2).(8)
Table 2. Percent Weight Loss at End of Treatment: TOP IR Monotherapy and TPM CR or PHEN/TPM CR.
|
|
|
TOP IR* |
|
TPM CR or PHEN/TPM CR* |
| ||||||||||||||||
|
|
|
Bray 2003 |
|
OB-301 |
| ||||||||||||||||
|
|
|
|
|
TOP |
|
TOP |
|
TOP |
|
TOP |
|
|
|
TPM CR |
|
TPM CR |
|
PHEN/TPM CR |
|
PHEN/TPM CR |
|
|
|
|
Placebo |
|
64 mg |
|
96 mg |
|
192 mg |
|
384 mg |
|
Placebo |
|
46 mg |
|
92 mg |
|
7.5/46 mg |
|
15/92 mg |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mean percent weight loss, ITT-LOCF |
|
-2.6 |
|
-5.0 |
|
-4.8 |
|
-6.3 |
|
-6.3 |
|
-1.5 |
|
-4.9 |
|
-6.1 |
|
-8.2 |
|
-9.0 |
|
|
Mean percent weight loss, completers |
|
-3.6 |
|
-5.8 |
|
-6.5 |
|
-8.2 |
|
-8.5 |
|
-2.2 |
|
-6.0 |
|
-8.5 |
|
-10.4 |
|
-11.5 |
|
*P<0.05 compared to placebo after Week 4 in all treatment arms
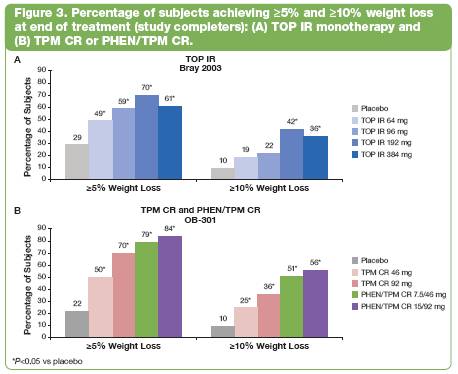
· Categorical weight loss: Both studies showed significantly more subjects in each treatment arm achieving >5% or >10% weight loss than subjects receiving placebo, except in TOP IR 64 mg and TOP IR 96 mg subjects at >10% (P<0.05 vs placebo; Figure 3).(8)
Blood pressure
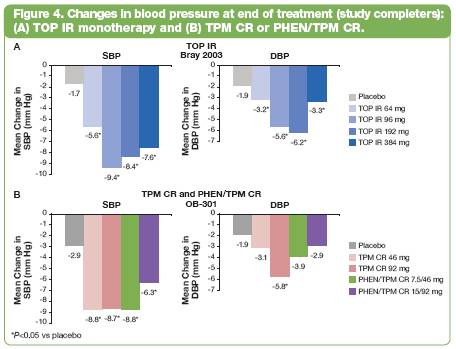
· Subjects completing treatment saw significantly greater decreases in SBP from baseline to end of treatment vs placebo (P<0.05; Figure 4).(8)
Safety summary
· In the Bray 2003 study, 385 subjects were randomised, 64% completed.(8) The OB-301 study randomised 756 subjects, 72% completed.
· In the Bray 2003 study, the most frequent adverse events (AEs) were central nervous system (CNS)-related (Table 3).(8)
· In OB-301, the most frequent AEs (across all treatment arms) were paraesthesia (see Table 3), dry mouth (7-19% in active, 0% in placebo), constipation (4-16% in active, 8% in placebo), headache (8-16% in active, 13% in placebo), upper respiratory infection (8-13% in active, 11% in placebo), insomnia (see Table 3), and nasopharyngitis (3-10% in active, 10% in placebo).
· In both studies, CNS-related AEs were most commonly reported in the first couple months of therapy (during titration), were generally mild or moderate in severity, and in most cases spontaneously resolved on treatment prior to the end of the study. Using similar coding conventions, AEs appeared to occur less frequently with TPM CR and PHEN/TPM CR than with TOP IR.(8)
· Table 3 shows CNS-related AEs (includes central and peripheral nervous systems, psychiatric, and fatigue), which occurred in more than 5% of topiramate (any formulation) subjects, and where total topiramate incidence was greater than placebo incidence. Data for treatment groups in OB-301 were provided for all terms matching those provided for TOP IR, even if values were below 5% threshold.
Table 3. CNS-Related Adverse Events.
|
|
|
TOP IR |
|
TPM CR or PHEN/TPM CR |
| ||||||||||||||||
|
|
|
Bray 2003 |
|
OB-301 |
| ||||||||||||||||
|
|
|
|
|
TOP |
|
TOP |
|
TOP |
|
TOP |
|
|
|
TPM CR |
|
TPM CR |
|
PHEN/TPM CR |
|
PHEN/TPM CR |
|
|
Preferred |
|
Placebo |
|
64 mg |
|
96 mg |
|
192 mg |
|
384 mg |
|
Placebo |
|
46 mg |
|
92 mg |
|
7.5/46 mg |
|
15/92 mg |
|
|
term (%) |
|
(n=75) |
|
(n=76) |
|
(n=75) |
|
(n=76) |
|
(n=78) |
|
(n=109) |
|
(n=106) |
|
(n=107) |
|
(n=106) |
|
(n=108) |
|
|
Paraesthesia |
|
5 |
|
36 |
|
41 |
|
42 |
|
50 |
|
4 |
|
11 |
|
22 |
|
16 |
|
23 |
|
|
Memory difficulties/ impairment |
|
8 |
|
11 |
|
23 |
|
28 |
|
21 |
|
1 |
|
0 |
|
1 |
|
0 |
|
0 |
|
|
Fatigue |
|
12 |
|
16 |
|
11 |
|
18 |
|
18 |
|
6 |
|
8 |
|
7 |
|
6 |
|
9 |
|
|
Somnolence |
|
13 |
|
9 |
|
12 |
|
13 |
|
21 |
|
2 |
|
2 |
|
2 |
|
1 |
|
2 |
|
|
Anorexia/decreased appetite |
|
3 |
|
8 |
|
9 |
|
13 |
|
13 |
|
0 |
|
0 |
|
0 |
|
1 |
|
2 |
|
|
Dizziness |
|
7 |
|
7 |
|
7 |
|
11 |
|
15 |
|
2 |
|
7 |
|
4 |
|
4 |
|
8 |
|
|
Difficulty in concentration/ disturbance in attention |
|
5 |
|
7 |
|
9 |
|
11 |
|
12 |
|
1 |
|
2 |
|
4 |
|
7 |
|
4 |
|
|
Insomnia |
|
5 |
|
8 |
|
15 |
|
7 |
|
5 |
|
6 |
|
4 |
|
5 |
|
12 |
|
10 |
|
|
Nervousness |
|
3 |
|
7 |
|
11 |
|
8 |
|
4 |
|
1 |
|
0 |
|
0 |
|
1 |
|
1 |
|
|
Hypoaesthesia |
|
1 |
|
4 |
|
7 |
|
11 |
|
4 |
|
1 |
|
3 |
|
4 |
|
3 |
|
2 |
|
|
Depression |
|
4 |
|
3 |
|
7 |
|
5 |
|
10 |
|
3 |
|
4 |
|
5 |
|
1 |
|
4 |
|
|
Anxiety |
|
0 |
|
8 |
|
3 |
|
7 |
|
5 |
|
1 |
|
2 |
|
2 |
|
0 |
|
3 |
|
|
Psychomotor slowing |
|
0 |
|
3 |
|
3 |
|
4 |
|
12 |
|
0 |
|
0 |
|
0 |
|
0 |
|
0 |
|
|
Dysgeusia, taste perversion |
|
0 |
|
9 |
|
9 |
|
16 |
|
10 |
|
0 |
|
7 |
|
2 |
|
9 |
|
15 |
|
· AEs led to discontinuation in 21% of TOP IR—treated subjects and 11% of placebo-treated subjects in the Bray 2003 study; 15% of the TPM CR— or PHEN/TPM CR—treated subjects and 7% of placebo-treated subjects in OB-301.(8)
· Serious AEs occurred in 4% of TOP IR—treated and 3% of placebo-treated subjects in the Bray 2003 study; and 1% of TPM CR— or PHEN/TPM CR—treated and 0% of placebo-treated subjects in OB-301.(8)
· Conclusions
· Due to similar design and endpoints in the 2 studies (Bray 2003 and OB-301), it is possible to contrast the efficacy and tolerability of the two topiramate formulations and its use in combination with phentermine.
· Any assessments need to be considered in the context that these were not head-to-head studies and cannot be deemed conclusive, merely informative.
· Topiramate has consistently demonstrated significant value when used to induce weight loss and has shown improvements in BP as well. Tolerability has been a limiting factor, specifically CNS-related events, but perhaps alternate formulations or combinations may help to mitigate these effects while retaining the benefits.
· A lower Cmax with TPM CR, lower absolute doses, and its use in combination with phentermine likely contribute to observed differences in efficacy and tolerability, attributes important for long-term use.
The OB-301 trial was registered at ClinicalTrials.gov, number NCT00563368.
References: (1) Logue J, et al. Heart. 2011;97(7):564-568. (2) Haslam DW, James WP. Lancet. 2005;366(9492):1197-1209. (3) NHLBI. http://www.nhlbi.nih.gov/guidelines/obesity/prctgd_c.pdf. Accessed May 17, 2011. (4) Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.;2009. (5) Rosenfeld WE. Clin Ther. 1997;19(6):1294-1308. (6) Rogawski MA, Porter RJ. Pharmacol Rev. 1990;42(3):223-286. (7) Macdonald RL, McLean MJ. Adv Neurol. 1986;44:713-736. (8) Bray GA, et al. Obes Res. 2003;11(6):722-733. (9) Wilding J, et al. Int J Obes Relat Metab Disord. 2004;28(11):1399-1410. (10) Tonstad S, et al. Am J Cardiol. 2005;96(2):243-251. (11) Stenlöf K, et al. Diabetes Obes Metab. 2007;9(3):360-368. (12) Toplak H, et al. Int J Obes (Lond). 2007;31(1):138-146. (13) Rosenstock J, et al. Diabetes Care. 2007;30(6):1480-1486. (14) Adipex-P [package insert]. Teva Pharmaceuticals USA;2005. (15) Glandt M, Raz I. J Obes. 2011;636181:1-13. (16) Gadde KM, et al. Lancet. 2011;377(9774):1341-1352. (17) Pathways to Change. Johnson & Johnson Health Care Systems: Piscataway, NJ. (18) Brownell KD. The LEARN Program for Weight Management. 2004.
Acknowledgements: The authors appreciate the contributions of the enrolled patients, the study investigators and staff, with poster graphics assistance from The Lockwood Group (funded by Vivus).
