Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d8k.htm |
 May 24, 2011
May 24, 2011
A specialty pharmaceutical company focused on the development
and commercialization of proprietary products to address important
therapeutic needs in the field of neuroscience
Exhibit 99.1 |
 Forward
looking statements Forward looking statements
2
This presentation contains forward-looking statements that involve substantial risks and
uncertainties. All statements, other than statements of historical facts, included in
this presentation are forward-looking statements. Examples of such statements include
our expectation regarding the FDA having a favorable view of the Intermezzo
®
residual effect profile; the sufficiency of the
Intermezzo
®
NDA to gain FDA approval; that Intermezzo
®
will become the first prescription sleep aid indicated for use in the
middle of the night; the potential market size for a middle of the night sleep aid;
plans to commercialize Intermezzo ® through the
Purdue collaboration and the receipt by Transcept of payments from Purdue thereunder;
reimbursement coverage for Transcept product candidates; intellectual property
protection being obtained and maintained for Transcept product candidates; and the potential for
Intermezzo
®
to reduce patient exposure to zolpidem as compared to prophylactic use of other zolpidem
products. Transcept may not actually meet these expectations and carryout these
plans. Various important factors that could cause actual events to differ materially
from such forward-looking statements include FDA deemed insufficiencies in our Intermezzo
®
NDA resubmission,
competitive product commercialization; adverse patent decisions at the USPTO or in court;
the willingness of Purdue to commercialize Intermezzo
®
and its ability to do so successfully; payer opinion of
Intermezzo ®
, if approved; commercial acceptance
of Intermezzo
®
; and variability in the business of Transcept generally. These and other risks are
described in greater detail in the "Risk Factors" section of Transcept
periodic reports filed with the Securities and Exchange Commission. Forward-looking
statements do not reflect the potential impact of any future in-licensing,
collaborations, acquisitions, mergers, dispositions, joint ventures, or investments
Transcept may enter into or make. Transcept does not assume any obligation to update any forward-
looking statements, except as may be required by law.
|
 Introduction:
Glenn A. Oclassen, CEO
Transcept Pharmaceuticals
Introduction:
Glenn A. Oclassen, CEO
Transcept Pharmaceuticals |
 Today’s
agenda Today’s agenda
Welcome and introduction
Glenn Oclassen, CEO
Insomnia overview
Andrew Krystal, MD
–
Definition, diagnosis and prevalence
–
Consequences and co-morbidities
–
Current treatment practices
Middle of the night awakenings
Thomas Roth, PhD
–
What are physicians and patients doing now?
–
Treatment rationale
Intermezzo
®
clinical overview
Thomas Roth, PhD
Concluding remarks
Glenn Oclassen, CEO
4 |
 Andrew Krystal, MD, MS: professional biography
Andrew Krystal, MD, MS: professional biography
Professor, Department of
Psychiatry
Duke University Medical Center
Director, Duke Sleep Research Laboratory, Insomnia Clinic,
Quantitative EEG Lab, Brain Stimulation Research Program
B.S. M.S. Biomedical Engineering,
MIT
M.D. and Psychiatry Residency, Duke University
5 |
 Andrew
Krystal, MD, MS: consulting relationships Andrew Krystal, MD, MS: consulting
relationships Consultant: Abbott, Actelion, Astellas, Kingsdown, Arena, AstraZeneca,
Axiom, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Jazz,
Johnson & Johnson, King, Merck, Neurocrine, Neurogen, Novartis,
Organon, Pfizer, Philips/Respironics, Sanofi-Aventis, Sunovion, Somaxon,
Takeda, Transcept Pharmaceuticals
Grant/Research Support: Abbott, Astellas, Cephalon, Evotec,
GlaxoSmithKline, Merck, Neurocrine, Neurogen, Neuronetics, NIH, Pfizer,
Sanofi-Aventis, Philips/Respironics, Kingsdown, Sunovion, Somaxon,
Takeda, Transcept Pharmaceuticals
6 |
 Thomas Roth,
PhD: professional biography Thomas Roth, PhD: professional biography
Director of Sleep Medicine Henry Ford Hospital, Detroit
Clinical Professor, Department of Psychiatry, University of
Michigan
Past President National Sleep Foundation, American
Academy of Sleep Medicine, and Sleep Research Society
Former Chair NIH National Center of Sleep Disorders
Research, and World Health Organization, Worldwide
Projects on Sleep and Health
Past Editor in Chief of the journal SLEEP
7 |
 Thomas Roth, PhD: consulting relationships
Thomas Roth, PhD: consulting relationships
Consultant: Abbott, Acadia, Acologix, Actelion, Alkermes, Alza, Ancile,
Arena, AstraZeneca, Aventis, Baxter, Bristol-Myers Squibb, Cephalon,
Cypress, Dove, Eisai, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline,
Hypnion, Intec, Jazz, Johnson & Johnson, King, Lundbeck, Ortho-McNeil,
MediciNova, Merck, Neurim, Neurocrine Biosciences, Neurogen, Novartis,
Orexo, Organon, Pfizer, Prestwick, Proctor & Gamble, Purdue, Resteva,
Roche, Sanofi, Schering-Plough, Sepracor, Servier, Shire, Somaxon,
Syrex, Takeda, Transcept Pharmaceuticals, Wyeth
Speaker's Bureau: Somaxon, Sepracor
Grant/Research Support: Aventis, Cephalon, Fund for Henry Ford
Hospital, GlaxoSmithKline, Neurocrine, NIH, Pfizer, Sanofi, Schering-
Plough, Sepracor, Somaxon, Syrex, Takeda, Transcept Pharmaceuticals,
Wyeth, XenoPort
8 |
 Therapeutic
focus Large markets,
unmet needs
Commercial
platform
U.S. primary care partnership: Purdue Pharma
Co-promote
option
1
yr
post
Intermezzo
launch
3/31/11: $63M cash, equivalents & investments
No debt
Neuroscience / psychiatry
Intermezzo
:
middle
of
the
night
awakenings
TO-2061
Transcept: preparing for Intermezzo
®
commercialization
Transcept: preparing for Intermezzo
®
commercialization
9
Strong balance
sheet
Intermezzo
PDUFA
date
July
14,
2011
TO-2061
Near term
catalysts
®
®
®
: treatment resistant OCD
Phase
2
results:
anticipated
mid
2012 |
 High
salivary pH induced by Intermezzo ®
sublingual buffer
system facilitates penetration of zolpidem through oral mucosa
High salivary pH induced by Intermezzo
®
sublingual buffer
system facilitates penetration of zolpidem through oral mucosa
Buffer combination
raises salivary pH to 9.5
Zolpidem tartrate salt form
(hydrophilic) in
sublingual tablet
Zolpidem free base
(lipophilic)
Lipid lining of the
oral mucosa
CH
3
C
H
3
O
C
H
3
C
H
3
N
N
H
N
2
OH
O
OH
OH
O
O
H
CH
3
C
H
3
O
CH
3
C
H
3
N
N
H
N |
 Intermezzo
®
3.5 mg delivered
more
zolpidem
earlier
than
a
~3x
higher
Ambien
®
dose
Intermezzo
®
3.5 mg delivered
more
zolpidem
earlier
than
a
~3x
higher
Ambien
®
dose
PK
comparison
study:
Intermezzo
®
3.5mg
vs.
Ambien
10mg
PO
(n=33)
Time (min)
9.6x
3.0x
1.5x
Intermezzo
®
3.5 mg
Ambien
®
10 mg
11
0
0
5
10
15
20
1
2
3
4 |
 Insomnia:
Definition, diagnosis, prevalence
Andrew Krystal, MD
Insomnia:
Definition, diagnosis, prevalence
Andrew Krystal, MD |
 Next-day consequences
Insomnia overview
Insomnia overview
13
Difficulty Falling
Asleep
At least 3x per week
Difficulty Staying
Asleep
(MOTN awakening)
At least 3x per week
Waking
Too
Early
At least 3x per week
Poor Quality of
Sleep
At least 3x per week
INSOMNIA
-
More than one type of disturbance may be present
-
Symptoms may vary over time
Diagnostic
and
Statistical
Manual
of
Mental
Disorders.
4
ed.1994;
The
International
Classification
of
Sleep
Disorders:
Diagnostic
&
Coding
Manual,
ICSD-2.
2
2005.
nd
th |
 Sleep
maintenance symptoms are the most commonly reported sleep problem
Sleep maintenance symptoms are the most commonly
reported sleep problem
14
Roth et al., Biological Psychiatry, 2011.
0.0
10.0
20.0
30.0
40.0
50.0
60.0
70.0
Sleep onset
Maintenance
(MOTN awakening)
Early morning
awakening
Non-restorative
Sleep only |
 Stanford
Sleep Epidemiology Research Center: Middle of the night awakening: #1 insomnia
symptom Stanford Sleep Epidemiology Research Center:
Middle of the night awakening: #1 insomnia symptom
15
Population based epidemiology study: ~9,000 subjects over 4 years
Persistence:
-
>90% report awakenings persist more than 6 months
-
50% report awakenings persist more than 5 years
(1) U.S. Census data, 2010; (2) Ohayon, et al., World Sleep Conference 2007; (3) Estimate
based on percentage applied to adults with difficulty returning to sleep, with and
without difficulty initiating sleep in Ohayon, et al., Sleep Medicine, 2010. Ohayon, et
al., Nocturnal Awakenings in the American General Population: Prevalence and
Consequences 235
M
U.S.
adults
(1)
x
Ohayon
83 M
36 M
22 M
3.6 M
MOTN sufferers at least 3x week
(2)
…with difficulty returning to sleep
(2)
…without difficulty initiating sleep
(2)
…who seek MD treatment
(3) |
 Insomnia:
consequences and co-morbidities Andrew Krystal, MD
Insomnia: consequences and co-morbidities
Andrew Krystal, MD |
 Consequences
of insomnia Consequences of insomnia
17
Walsh et al., World Association of Sleep Medicine, 2009.
Days out of work or role
>3x more for people with insomnia vs. people
with no insomnia
Prevalence of accidents or injury
>1.5x greater for people with insomnia vs.
people with no insomnia |
 > 25% of
days out of work are attributable to insomnia > 25% of days out of work are
attributable to insomnia Accidents (%)
Days out of role/30 days (%)
Any insomnia
9.8
29.1
COPD
0.5
3.5
Diabetes
0.3
-1.5
Osteoarthritis
-0.1
2.6
GERD
0.9
4.0
Sleep apnea
-0.6
1.1
Neuropathic pain
2.6
2.9
Chronic heart failure
0.3
2.3
Climacteric symptoms
0.3
2.1
Hypertension
3.9
6.2
Major depressive disorder
1.5
3.9
Rheumatoid arthritis
-0.1
0.7
All of the above disorders
18.0
52.0
Significant results given in orange (P<0.05)
18
Hajak et al., World Association of Sleep Medicine, 2009.
|
 Insomnia
symptoms in common co-morbid conditions Insomnia symptoms in common co-morbid
conditions MOTN patient demographics are similar to those of other insomnia
patients, but comorbid conditions seem to vary by presentation.
Insomnia presentation
MOTN only
Bedtime only
MOTN &
Bedtime
N
240
170
370
Comorbid conditions:
Depression
Anxiety
Pain
43.6%
21.0%
13.7%
42.5%
41.3%
7.5%
39.9%
28.2%
14.5%
19
Roth et al., International Forum on Mood and Anxiety Disorders, 2010.
|
 Insomnia:
Current treatment practices Andrew Krystal, MD
Insomnia: Current treatment practices
Andrew Krystal, MD |
 21
Ancoli-Israel et al., SLEEP, 1999.
Patients with insomnia either present this as
a secondary complaint or, not at all
Patients with insomnia either present this as
a secondary complaint or, not at all
Never
discussed
69%
Discussed
sleep problem
during visit for
other
purposes
26%
Visited
specifically to
discuss sleep
problem
5% |
 All
currently approved sleep aids are indicated for bedtime administration only
All currently approved sleep aids are indicated for
bedtime administration only
Drug (generic)
Brand name
Half-life (h)
Doses (mg)
Benzodiazepines
Estazolam
ProSom
8-24
1, 2
Flurazepam
Dalmane
48-120
15, 30
Quazepam
Doral
48-120
7.5, 15
Temazepam
Restoril
8-20
7.5, 15, 22.5, 30
Triazolam
Halcion
2-4
0.125, 0.25
Nonbenzodiazepines
Zolpidem
Ambien
1.5-2.4
5, 10
Zolpidem
extended-release
Ambien CR
2.8-2.9
6.25, 12.5
Zaleplon
Sonata
~1
5, 10
Eszopiclone
Lunesta
5-7
1, 2, 3
Melatonin receptor agonist
Ramelteon
Rozerem
1-2.6
8
H
1
antagonist
Doxepin
Silenor
15.3
3, 6
22 |
 Current
dosing strategies for MOTN insomnia may promote prophylactic (every night) use
Current dosing strategies for MOTN insomnia may
promote prophylactic (every night) use
Bedtime prophylactic dosing
–
The only current option/strategy
•
Approved hypnotics are indicated for bedtime use
–
Only Ambien CR, Lunesta and Silenor are indicated for sleep maintenance
•
MOTN awakenings generally occur 3-5x per week, not every night
•
Currently available 8 hr drugs often dosed nightly to prevent
awakenings, whether or not treatment would have been needed
•
A bedtime dosing strategy may be suited for patients who experience
MOTN awakening with difficulty returning to sleep >5 nights per week
23
only |
 Middle of
the night awakenings: what are physicians and patients doing now?
Thomas Roth, PhD
Middle of the night awakenings: what are
physicians and patients doing now?
Thomas Roth, PhD |
 No product
is currently indicated or suited for prn treatment at the time of a middle of
the night awakening No product is currently indicated or suited for prn
treatment at the time of a middle of the night awakening
MOTN awakenings, like other insomnia symptoms, typically
do not occur every night
An ideal therapeutic:
–
Used only at the time patients need help returning to sleep,
not every night in advance of a problem that may not occur
–
Rapid onset: only half the night remaining
–
Rapid offset: avoid next day residual effects despite MOTN dosing
25 |
 Physician
data: most MOTN patients do not experience awakenings every night of the week
Physician data: most MOTN patients do not experience
awakenings every night of the week
n=294
Patients selected for multiple
and prolonged awakenings
Mean of 4-5 awakenings per
week
<20% awakening every night
26
Roth et al., International Forum on Mood and Anxiety Disorders, 2010.
Insomnia patient-record survey, n=890
Intermezzo
®
outpatient study
n=232 (MOTN patients)
34.1%
5 to 6 nights
per week
35.3%
3 to 4 nights
per week
8.6%
< 3 nights
per week
22.0%
every night |
 Epidemiologic data: off-label patient use of bedtime
hypnotics in the middle of the night is common
Epidemiologic data: off-label patient use of bedtime
hypnotics in the middle of the night is common
1,927 subjects who received a hypnotic Rx in last year
Results:
–
~11% of all hypnotic users sometimes take their sleep
aid in the middle of the night in order to return to sleep
–
~50% of those hypnotic users who reported middle of
the night awakening as their most bothersome insomnia
symptom sometimes take their bedtime sleep aid in the
middle of the night
Patients allow on average ~6.6 hrs between hypnotic use
after MOTN awakening and time to get up the next morning
27
Roth, Kessler et al., Society of Biological Psychiatry, 2011. |
 Exclusively
at bedtime (se)
Exclusively
MOTN (se)
MOTN awakening: the most bothersome sleep problem (%)
28.4 (3.0)
43.0 (3.9)
When MOTN is the most bothersome problem:
Mean number of nights per week with MOTN waking
Never get back to sleep after MOTN waking (%)
Average (mean) time to get back to sleep (in minutes)
4.3 (0.3)
1.9 (1.0)
73.7 (9.9)
3.5 (0.3)
2.9 (1.7)
86.1 (6.6)
1
Exclusive bedtime users in this table represent a 20% random sub-sample of all
respondents in the main sample who reported exclusive bedtime use.
Epidemiologic data: characteristics of patients
taking hypnotics at bedtime vs MOTN
Epidemiologic data: characteristics of patients
taking hypnotics at bedtime vs MOTN
Characteristics of middle of the night sleep problems prior to beginning medication use among
respondents who take prescription sleep medication either exclusively at bedtime (n =
303) or MOTN
(but
never
twice
in
a
single
night)
(n
=
209)
¹
28
Roth, Kessler et al., Society of Biological Psychiatry, 2011. |
 Intermezzo
®
:
Clinical
Data
Thomas Roth, PhD
Intermezzo
®
:
Clinical
Data
Thomas Roth, PhD |
 Intermezzo
®
proposed indication statement
Intermezzo
®
proposed indication statement
Intermezzo
®
(zolpidem tartrate sublingual tablet)
is indicated for use as-needed for the treatment of
insomnia when a middle of the night awakening is
followed by difficulty returning to sleep
30 |
 Novel
formulation Low dose
Fast acting
Sleep lab, objective data: 14 minute sleep
onset with 3.5 mg dose
No residual next-morning effects vs. placebo in
sleep lab and outpatient studies
Driving study primary statistical analysis: no
significant next morning effect vs. placebo 4 hrs
after dosing, consistent with proposed label
Sublingual tablet dissolves in ~ 2 minutes
pH drives
zolpidem
base, rapidly absorbed
28%
of
Ambien
CR
®
dose
Intermezzo
®
: anticipated to be the first sleep aid for
use in the middle of the night at the time of awakening
Intermezzo
®
: anticipated to be the first sleep aid for
use in the middle of the night at the time of awakening
31
Favorable 4 hr
residual effects |
 PK comparison
study: Intermezzo ®
3.5mg vs. Ambien 10mg PO (n=33)
Time (min)
9.6x
3.0x
1.5x
Intermezzo
®
3.5 mg
Ambien
®
10 mg
32
Intermezzo
®
3.5 mg delivered
more
zolpidem
earlier
than
a
~3xhigher
Ambien
®
dose
Intermezzo
®
3.5 mg delivered
more
zolpidem
earlier
than
a
~3xhigher
Ambien
®
dose
0
1
2
3
4
0
5
10
15
20 |
 Intermezzo
®
offers potentially significant reduction in
patient exposure to zolpidem
Intermezzo
®
offers potentially significant reduction in
patient exposure to zolpidem
33
Nights of dosing
4 x 3.5mg=14mg
7 x 12.5mg=87.5mg
~84% reduction in
overall exposure
Hypothetical case based on 7 nights of prophylactic
bedtime dosing of Ambien CR vs. 4 nights of dosing with
Intermezzo
®
at the time a MOTN awakening occurs |
 Phase 3
sleep lab study Phase 3 sleep lab study |
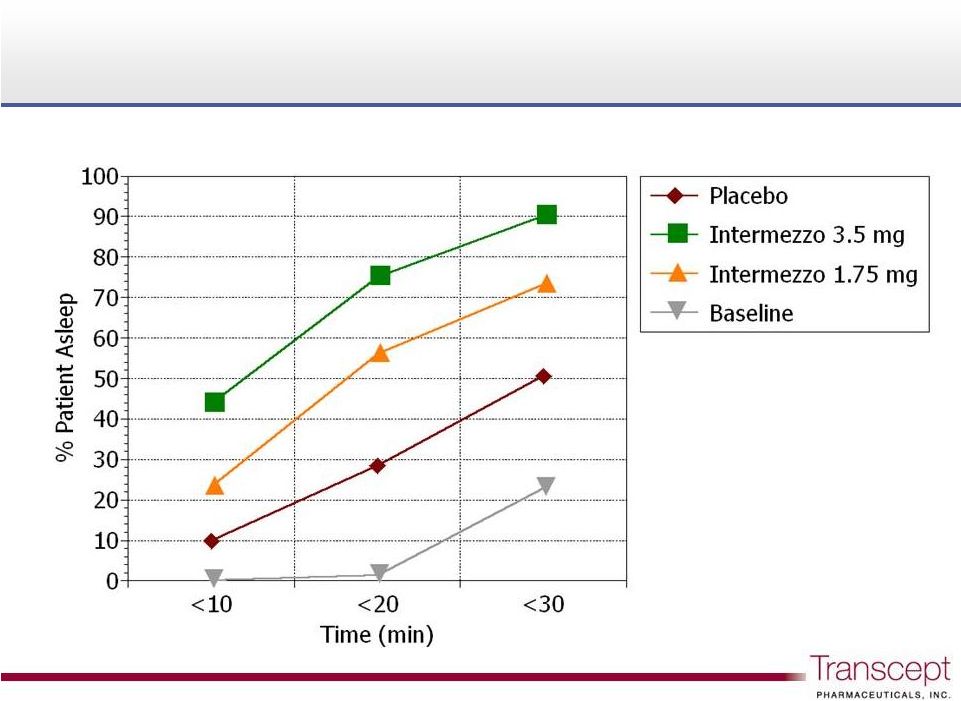 Intermezzo
®
: proportion of patients asleep following a
middle of the night awakening
Intermezzo
®
: proportion of patients asleep following a
middle of the night awakening
75%
56%
28%
Phase 3 sleep lab study n = 82
35 |
 Next-day
residual effects of Intermezzo ®
1.75 mg
and 3.5 mg vs. placebo
Next-day residual effects of Intermezzo
®
1.75 mg
and 3.5 mg vs. placebo
36
Phase 3 sleep lab study n = 82
0
10
20
30
40
50
60
70
Objective (DSST)
61.88
61.59
60.57
Placebo
Intermezzo®
1.75 mg
Intermezzo®
3.5 mg
Intermezzo
®
1.75mg
p<0.62
vs.
Placebo
p<0.44
vs.
Placebo
p<0.15
vs.
Placebo
p<0.91
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
0
10
20
30
40
50
60
70
Subjective (VAS)
62.35
64.25
63.56 |
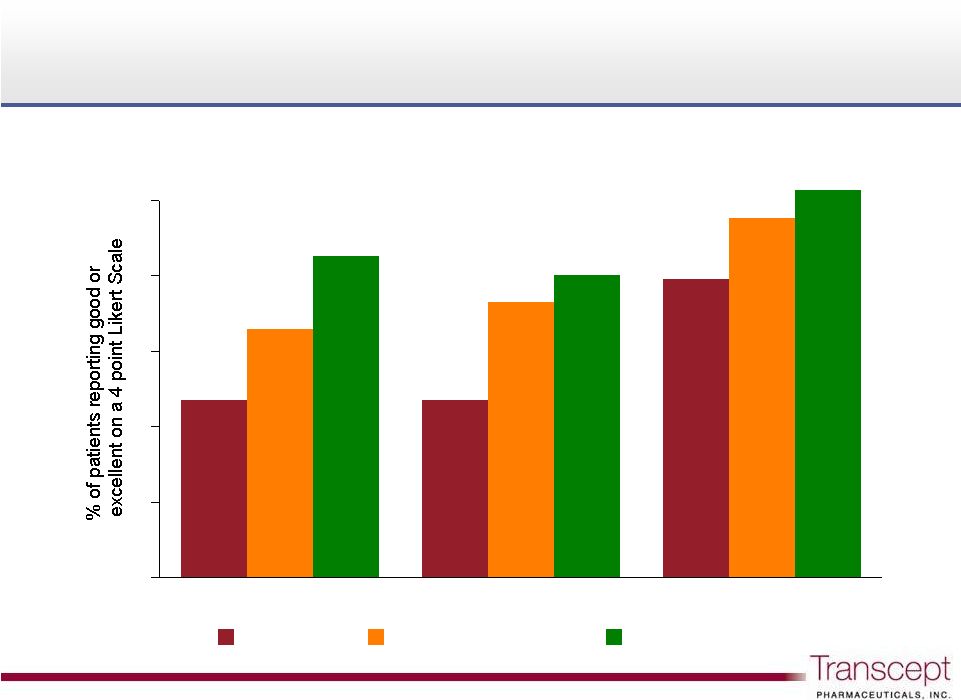 Intermezzo
®
: “Good”
or “Excellent”
next day ratings
Intermezzo
®
: “Good”
or “Excellent”
next day ratings
Phase 3 sleep lab study n = 82
37
Sleep Quality
Refreshing Sleep
Ability to Function
0
10
20
30
40
50
23.5
32.9
42.6
23.5
36.5
40.0
39.5
47.6
51.3
Placebo
Intermezzo®
1.75 mg
Intermezzo®
3.5 mg
Intermezzo
®
1.75mg
NS
p<0.017
vs.
Placebo
p<0.001
vs.
Placebo
p<0.001
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
p<0.024
vs.
Placebo
p<0.009
vs.
Placebo |
 Intermezzo
®
outpatient study
Intermezzo
®
outpatient study |
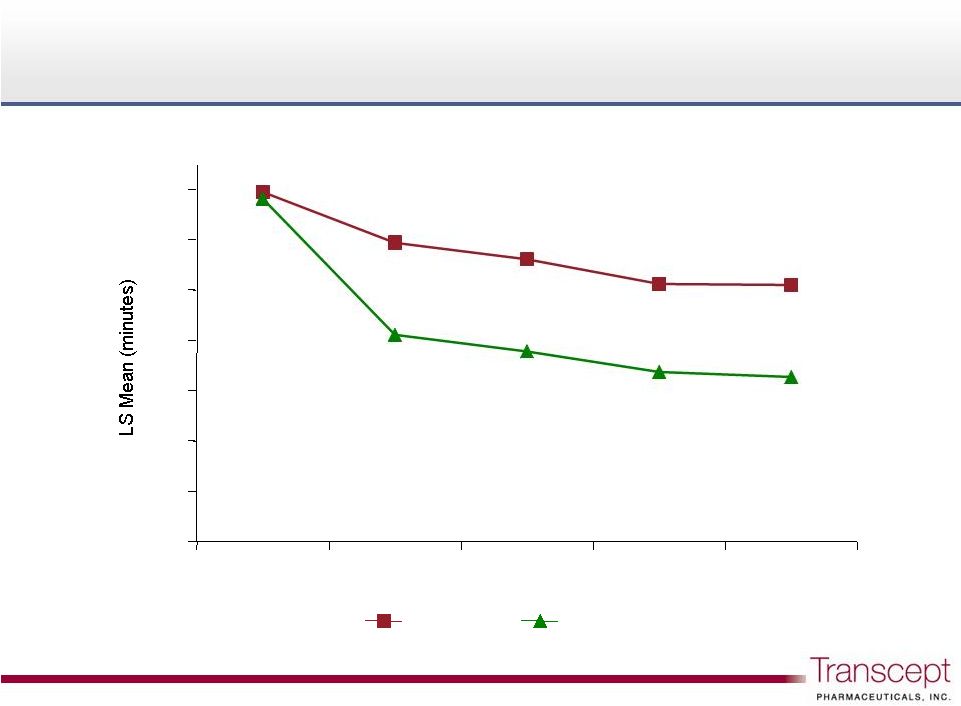 Intermezzo
®
: Latency to Sleep Onset, weekly
Intermezzo
®
: Latency to Sleep Onset, weekly
Phase 3 outpatient study n = 294
39
Baseline
week 1
week 2
week 3
week 4
0
10
20
30
40
50
60
70
Placebo
Intermezzo®
3.5 mg
Intermezzo
®
3.5mg
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo |
 Intermezzo
®
: average weekly treatment exposure
Intermezzo
®
: average weekly treatment exposure
40
Phase 3 outpatient study n = 294
Placebo
wk 1
Placebo
wk 2
week 1
week 2
week 3
week 4
0
1
2
3
4
5
Placebo
Intermezzo®
3.5 mg
Intermezzo
®
3.5mg
Placebo |
 Intermezzo
®
highway driving study
Intermezzo
®
highway driving study |
 Intermezzo
®
highway driving study overview
Intermezzo
®
highway driving study overview
Approximately 40 healthy adult volunteers
Highway driving over a one-hour period
Single-center (Maastricht),
double-blind,
randomized,
placebo-controlled
crossover design
Key comparisons:
–
Intermezzo
3.5mg vs. placebo, dosed 4 hours prior to driving
–
Intermezzo
3.5mg vs. placebo, dosed 3 hours prior to driving
–
Zopiclone 7.5mg vs. placebo (positive control)
Key measure of driving performance: standard
deviation of lateral position (variability in lane position)
as compared to placebo
42
®
® |
 Intermezzo
®
highway driving study results
Intermezzo
®
highway driving study results
4 hrs after MOTN Intermezzo
dosing
–
Test condition consistent with proposed label
–
Primary analysis (symmetry): no statistically significant effect
–
Secondary analysis (mean): statistically significant but small
effect of 0.8 cm
SDLP
3 hrs after MOTN Intermezzo
dosing
–
Test condition included to characterize risk profile of
Intermezzo
when not used according to label
–
Primary analysis (symmetry): statistically significant effect up
to
4.0
cm
SDLP
–
Secondary analysis (mean): statistically significant but small
effect
of
1.5
cm
SDLP
–
One drive discontinued at 3 hour condition due to excessive
drowsiness
43
®
®
® |
 44
Mean change in SDLP (cm) and 95% confidence intervals (cross-study comparison)
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
2.5
4.0
0
5
10
15
Mean change in SDLP (cm)
(1)
Mean change in SDLP produced
by 0.05% blood alcohol is 2.4 cm
(EU DUI concentration limit). 2.5
cm was used as primary cutpoint
in
TSPT driving study.
(2)
Mean change in SDLP produced
by 0.08% blood alcohol is 4.2 cm
(USA DUI concentration limit). 4.0
cm was used as a secondary
cutpoint
in TSPT driving study.
Flurazepam
30mg
10-11hrs
Temazepam
20mg
10-11hrs
Zaleplon
20mg
4-5 hrs
Lormetazepam
1mg
10-11hrs
Intermezzo
®
3.5mg
4-5 hrs
Nitrazepam
10mg
10-11hrs
Loprazolam
1mg
10-11hrs
Intermezzo
®
3.5mg
3-4 hrs
Lormetazepam
1mg 10-11hrs
Flunitrazepam
2mg
10-11hrs
Lormetazepam
2mg 10-11hrs
Zopiclone
7.5mg
10-11hrs
Zolpidem
20mg
4-5 hrs
Flurazepam
30mg 10-11hrs
Loprazolam
2mg
10-11hrs
Zolpidem
10mg
4-5 hrs
Flurazepam
15mg 10-11hrs
Oxazepam
50mg
10-11hrs
Zopiclone
7.5mg
9-10 hrs
Drug & dose Drive time
I
I
I
Zaleplon
10mg
4-5 hrs
Zolpidem
10mg
5-6 hrs
0.08%
(2)
0.05%
(1)
~ SDLP produced by BAC of |
 Estimated
probability of exceeding SDLP 2.5 cm threshold for various hypnotics
and 95% confidence intervals (cross-study comparison)
45
Zaleplon 10mg
4-5 hrs
Zaleplon 20mg
4-5 hrs
Intermezzo
®
3.5mg
4-5 hrs
Lormetazepam 1mg 10-11hrs
Intermezzo
®
3.5mg
3-4 hrs
Lormetazepam 1mg
10-11hrs
Temazepam 20mg
10-11hrs
Nitrazepam 10mg
10-11hrs
Zolpidem 10mg
4-5 hrs
Zopiclone 7.5mg
9-10 hrs
Flurazepam 15mg
10-11hrs
Flunitrazepam 2mg
10-11hrs
Loprazolam 1mg
10-11hrs
Lormetazepam 2mg 10-11hrs
Flurazepam 30mg
10-11hrs
Flurazepam 30mg
10-11hrs
Oxazepam 50mg
10-11hrs
Loprazolam 2mg
10-11hrs
Zolpidem 20mg
4-5 hrs
Drug & dose Drive time
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
SDLP 2.4 cm
driving impairment
associated with 0.05% blood alcohol
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
0 |
 Estimated
probability of exceeding SDLP 4.0 cm threshold for various hypnotics and 95% confidence
intervals (cross-study comparison) 46
Intermezzo
®
3.5mg
4-5 hrs
Zaleplon 10mg
4-5 hrs
Zaleplon 20mg
4-5 hrs
Nitrazepam 10mg
10-11hrs
Lormetazepam 1mg
10-11hrs
Lormetazepam 1mg 10-11hrs
Intermezzo
®
3.5mg
3-4 hrs
Temazepam 20mg
10-11hrs
Flunitrazepam 2mg
10-11hrs
Lormetazepam 2mg 10-11hrs
Loprazolam 1mg
10-11hrs
Oxazepam 50mg
10-11hrs
Zopiclone 7.5mg
9-10 hrs
Zolpidem 10mg
4-5 hrs
Flurazepam 15mg 10-11hrs
Flurazepam 30mg 10-11hrs
Flurazepam 30mg 10-11hrs
Loprazolam 2mg
10-11hrs
Zolpidem 20mg
4-5 hrs
Drug & dose Drive time
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
I
SDLP 4.2 cm
driving impairment
associated with 0.08% blood alcohol
0
10%
20%
30%
40%
50%
60%
70%
80%
90%
100% |
 Driving
study results - labeling vs. approvability:
HORIZANT
™
Prescribing Information
Driving study results -
labeling vs. approvability:
HORIZANT
™
Prescribing Information
Prescribing Information: Warnings and Precautions
“HORIZANT causes significant driving impairment.”
“Patients being treated with HORIZANT should not drive until they
have gained sufficient experience to assess whether HORIZANT
impairs their ability to drive.”
“However, prescribers and patients should be aware that patients’
ability to assess their own driving competence…can be imperfect.”
47
HORIZANT
™
(gabapentin enacarbil) NDA approval for the
treatment of Restless Legs Syndrome: April 6, 2011
A nightly 1,200 mg dose of HORIZANT
™
produced significant
impairment 14 hours after dosing in a driving simulator study
FDA approval letter contains agreements to conduct a further
post-marketing study of driving impairment |
 Question
& answer Question & answer |
 Concluding
remarks: Glenn A. Oclassen, CEO
Concluding remarks:
Glenn A. Oclassen, CEO |
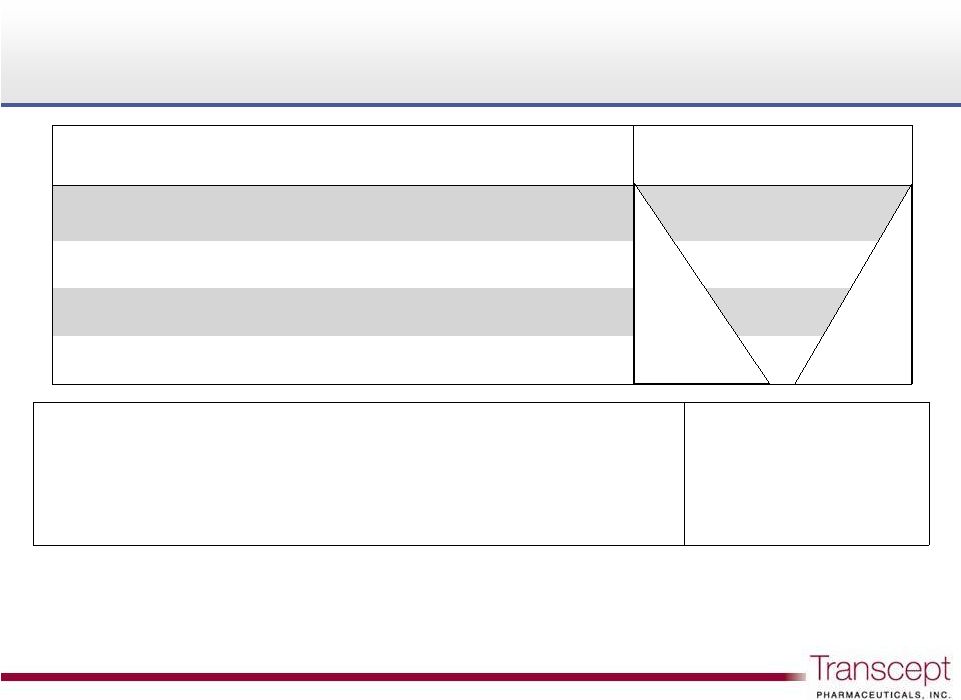 Estimating
the MOTN market size at branded prices Estimating the MOTN market size at branded
prices x mean length of therapy, currently marketed Rx sleep aids
108 to 169 days
(4)
x per tablet branded pricing, currently marketed Rx sleep aids
$5.05 to $5.67
(5)
= 100% market estimate: treatment seeking MOTN patients
$1.9 B to $3.4 B
(1) U.S. Census data, 2010; (2) Ohayon, et al., World Sleep Conference 2007; (3) Estimate
based on percentage applied to adults with difficulty returning
to
sleep,
with
and
without
difficulty
initiating
sleep
in
Ohayon,
et
al.,
Sleep
Medicine
(2010).
(4)
IMS:
Length
of
therapy
in
the
sleep
disorder
market,
March
2007.
Products
evaluated
are
indicated
for
bedtime
use:
Ambien
®
,
Ambien
CR
®
,
Lunesta
®
,
Restoril
®
,
Rozerem
®
,
Sonata
®
,
Desyrel
®
;
(5) Wolters
Kluwer
WAC
pricing
(branded)
Jan
2011
(products
evaluated:
Ambien
®
,
Ambien
CR
®
, Lunesta
®
, Silenor
®
; Ambien
®
and Ambien CR
®
are currently available in generic form).
50
Ohayon, et al., Nocturnal Awakenings in the American
General Population: Prevalence and Consequences
235 M U.S. adults
(1)
x Ohayon
MOTN sufferers at least 3x week
(2)
83 M
…with difficulty returning to sleep
(2)
36 M
…without difficulty initiating sleep
(2)
22 M
…who seek MD treatment
(3)
3.6M |
 Leading
pain therapeutic franchise 2010 net revenue: ~$2.4 billion
Branded products include:
OxyContin
®
, MSContin
®
, Dilaudid
®
, Butrans
®
Sales force of >500 field reps calling on primary care
physicians and high prescribing specialists
High value pain prescribers tend to be significant insomnia
prescribers
Multi-year sales and marketing agreement with psychiatry
co-promote option, royalties and milestone payments
Commercialization partnership with Purdue Pharma
Commercialization partnership with Purdue Pharma
51 |
 Commercialization agreement: key Transcept
benefits
Commercialization agreement: key Transcept
benefits
Co-promote option: foundation for a commercial future
–
Transcept
option: co-promote to psychiatrists as early as the first
anniversary after Purdue launch
Milestone payments
–
Upfront license fee: $25M received August 2009
–
FDA approval milestone of $30M, reduced by $2M each 30-day period
after June 30, 2010 ($6M if approved on July 14, 2011 PDUFA date)
–
Up to $90M additional upon the achievement of certain patent
milestones and net sales targets, including $10M for first formulation
patent listed in Orange Book
Royalty structure
–
Base royalty: mid-teens up to mid 20% level on net sales
–
Co-promote royalty: additional double digit royalty on psychiatrist Rx
net sales
52 |
 U.S.
primary care partnership: Purdue Pharma Co-promote
option
1
yr
post
®
launch
3/31/11: $63M cash, equivalents & investments
No debt
Neuroscience / psychiatry
Intermezzo
: middle of the night awakenings
Transcept: preparing for Intermezzo
®
commercialization
Transcept: preparing for Intermezzo
®
commercialization
53
Intermezzo
PDUFA date July 14, 2011
Large markets,
unmet needs
Therapeutic focus
Commercial
platform
Strong balance
sheet
Near term
catalysts
TO-2061
: treatment resistant OCD
TO-2061
Phase
2
results:
anticipated
mid
2012
®
®
Intermezzo |
 Intermezzo
®
is a registered trademark of Transcept Pharmaceuticals, Inc.
Ambien
®
and Ambien CR
®
are registered trademarks of sanofi-aventis
Lunesta
®
is a registered trademark of Sunovion Pharmaceuticals Inc.
Zofran
®
and
Paxil
®
are registered trademarks of The GlaxoSmithKline Group of Companies
Prozac
®
is a registered trademark of Eli Lilly & Co.
Luvox
®
is a registered trademark of Solvay Pharmaceuticals, Inc.
Zoloft
®
is a registered trademark of Pfizer Inc. |
