Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | a59584e8vk.htm |
Exhibit 99.1


| Not an Offer for Securities This presentation has been prepared by REVA Medical, Inc. (REVA or the Company) solely for use at the investor presentations to be made by the Company. This presentation does not constitute an offer, invitation, solicitation or recommendation with respect to the purchase or sale of any security in the Company nor does it constitute financial product advice nor take into account your investment objectives, taxation situation, financial situation or needs. An investor must not act on the basis of any matter contained in this presentation but must make its own assessment of the Company and conduct its own investigations and analysis. Information is a synopsis only This presentation only contains a synopsis of information on the Company and accordingly no reliance may be placed for any purpose whatsoever on the sufficiency or completeness of such information The information presented in this presentation is subject to change without notice and neither the Company nor Inteq have any responsibility or obligation to inform you of any matter arising or coming to their notice, after the date of this presentation, which may affect any matter referred to in this presentation. Currency references Financial amounts in this presentation are expressed in Australian dollars or A$. USD or US$ shall mean the lawful currency of the United States of America. Forward looking Statements This announcement contains or may contain forward-looking statements that are based on management's beliefs, assumptions and expectations and on information currently available to management. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements, including without limitation our expectations with respect to regulatory submissions and approvals such as our expectations with respect to our clinical trials, including enrolment in or completion of our clinical trials; our expectations with respect to the integrity or capabilities of our intellectual property position; our ability to commercialize our existing products; our ability to develop and commercialize new products; and our estimates regarding our capital requirements and financial performance, including profitability. Management believes that these forward-looking statements are reasonable as and when made. You should not place undue reliance on forward-looking statements because they speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. REVA may not actually achieve the plans, projections or expectations disclosed in forward-looking statements, and actual results, developments or events could differ materially from those disclosed in the forward-looking statements. Forward-looking statements are subject to a number of risks and uncertainties, described in "Risk Factors" in our Annual Report on Form 10-K or other periodic reports or current reports filed with the U.S. Securities and Exchange Commission (the "SEC"). Disclaimer This presentation has been prepared by the Company based on information which is available to it. The information contained in this presentation is an overview and does not contain all information necessary to make an investment decision. Although reasonable care has been taken to ensure that the facts stated in this presentation are accurate and that the opinions expressed are fair and reasonable, no representation or warranty, express or implied, is made as to the fairness, accuracy, completeness or correctness of the information and opinions contained in this presentation and no reliance should be placed on such information or opinions. To the maximum extent permitted by law, none of the Company, or any of its members, directors, officers, employees or agents, advisers nor any other person accepts any liability whatsoever for any loss, however arising, from the use of the presentation or its contents or otherwise arising in connection with it, including, without limitation, any liability arising from fault or negligence on the part of the Company or any of its directors, officers, employees or agents. REVA 2011 Annual General Meeting |

| "We are devoted to developing and manufacturing novel polymer devices for the treatment of vascular disease that improve health and quality of life." REVA Mission Statement REVA 2011 Annual General Meeting |

| REVA is developing its proprietary bioresorbable stent technology ReZolveTM stent potentially offers significant advantages for patients The market opportunity is significant Globally in 2009 coronary stent sales ~ US$5.3 billion REVA is well positioned to be successful Extensive development work over ten years largely complete Pilot human clinical trial to validate technology commencing 3Q 2011 Funding until end of 2013 Near term milestones and path to market CE Mark approval targeted by the end of 2013 then sales in EU Boston Scientific Corporation has option to distribute - Medtronic are existing shareholders Highly experienced team to deliver Board and management have had significant industry success and investment track record Scientific Advisory Board of recognised leaders in cardiology REVA 2011 Annual General Meeting |
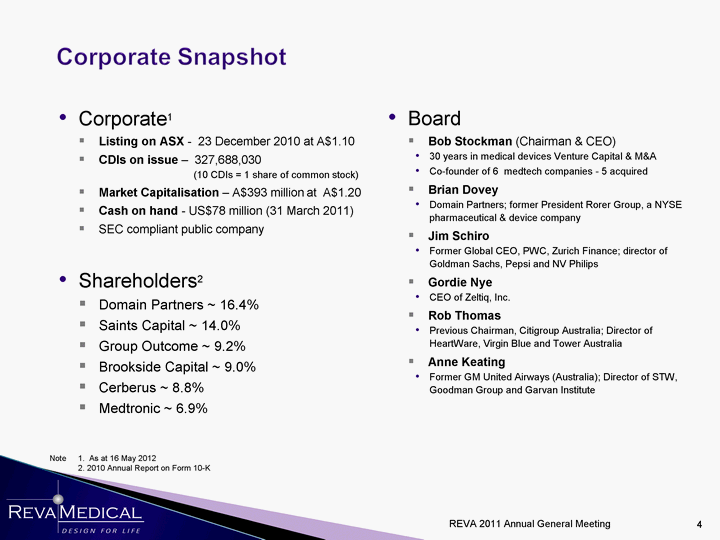
| Corporate1 Listing on ASX - 23 December 2010 at A$1.10 CDIs on issue - 327,688,030 (10 CDIs = 1 share of common stock) Market Capitalisation - A$393 million at A$1.20 Cash on hand - US$78 million (31 March 2011) SEC compliant public company Shareholders2 Domain Partners ~ 16.4% Saints Capital ~ 14.0% Group Outcome ~ 9.2% Brookside Capital ~ 9.0% Cerberus ~ 8.8% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Medtronic ~ 6.9% Note 1. As at 16 May 2012 2. 2010 Annual Report on Form 10-K Board Bob Stockman (Chairman & CEO) 30 years in medical devices Venture Capital & M&A Co-founder of 6 medtech companies - 5 acquired Brian Dovey Domain Partners; former President Rorer Group, a NYSE pharmaceutical & device company Jim Schiro Former Global CEO, PWC, Zurich Finance; director of Goldman Sachs, Pepsi and NV Philips Gordie Nye CEO of Zeltiq, Inc. Rob Thomas Previous Chairman, Citigroup Australia; Director of HeartWare, Virgin Blue and Tower Australia Anne Keating Former GM United Airways (Australia); Director of STW, Goodman Group and Garvan Institute REVA 2011 Annual General Meeting |

| Leading cause of death in many developed countries Treatment evolution Open heart surgery - CABG Late 1970's - balloon angioplasty Early 1990's - bare metal stents Today - drug-eluting stents Drug-eluting stents have reduced retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% retreatment rate to less than 10% REVA 2011 Annual General Meeting |

| Allows artery to heal, then goes away Restores natural movement of the artery May reduce or eliminate blood clots associated with permanent stents May reduce the need for anti-clotting drugs Huge clinical and consumer appeal REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting |

| Unique Stent Geometry Unique Material REVA 2011 Annual General Meeting |

| Patented 'Slide & Lock' design Ratcheting design Patented Strong Unique Patented polymer composition Natural Tunable Visible Safe Safe Safe Safe Safe Safe Safe Safe Safe Safe Safe Safe Top: Backbone and "U" piece of ReZolveTM stent Bottom: X-ray of REVA animal model REVA and metal stent REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting |

| More than 1,000 stents tested Resorbs and clears from body with no reaction All components thoroughly tested (stent, catheter, drug) ReZolveTM lumen area increases Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted Metal lumen area restricted ReZolve lumen area increases REVA 2011 Annual General Meeting |

| 36 Months 6 Months REVA 2011 Annual General Meeting |

| Baseline REVA 2011 Annual General Meeting |

| Post Stent 6 months 34 months 12-months ~30% stenosis REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting |

| Four large multinational device companies > 95% of stent sales Abbott Laboratories Boston Scientific Corporation Medtronic Johnson & Johnson No coronary bioresorbable stent available for sale...yet Bioresorbable coronary stents in or approaching clinical trials Abbott' Absorb(tm) stent- CE mark January 2011, commercialization pending Biotronik's 'Dreams' stent - magnesium based - clinical trial initiated August 2010 REVA's ReZolve (tm) stent to begin its clinical trial in Q3 2011 REVA 2011 Annual General Meeting |

| ~ 250 patents and patent filings - expiring up to 2030 Patents protecting 'Slide & Lock' design are owned by REVA Patents protecting polymer technology under exclusive license from Rutgers University REVA owns polymer patents for non-core applications REVA actively monitors its intellectual property position Core technology protected as follows Patent Families US Patents Non-US Patents Stent design Ten Six granted (four pending) 34 granted (20 pending) Polymer Eight Four granted (four pending) 14 granted (15 pending) REVA 2011 Annual General Meeting |

| IPO on ASX, 23 December 2010, raised A$85 million Impressive cross-section of private and institutional investors Australia, Hong Kong, United Kingdom, United States Key positions staffed Jeff Anderson, VP of Clinical and Regulatory Affairs Don Brandom, VP of Product Development Key hires in finance and operations Production Capacity Increased Stent builds at scale to support pilot study Clean room staff 3X since IPO Additional patent protection secured Spiral geometry utilized in ReZolve(tm) stent Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology Advanced side-chain crystalizable polymer technology REVA 2011 Annual General Meeting |

| ReZolveTM Sirolimus-Eluting Bioresorbable Coronary Stent Pilot trial to begin Q3 2011 Clinical approval obtained in Brazil Import approval pending Submission under review in Germany Additional sites being qualified in Europe and Australia Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial Will support wider CE mark trial REVA 2011 Annual General Meeting |

| Complete clinical readiness of ReZolve(tm) stent Obtain final clinical approvals in Brazil and Europe Demonstrate ReZolve(tm) stent safety in Pilot Study Begin Pivotal Study in 2012 Drive ReZolveTM stent adoption CE Mark 2013 Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries Followed by sales in EU, Australia / NZ and other Non US countries REVA 2011 Annual General Meeting |

| REVA 2011 Annual General Meeting Note: The above Balance Sheets have been prepared in USD under US GAAP. The Balance Sheet as at December 31, 2010 has been audited by Ernst & Young, LLP and included in the Company's 10-k filing. The Balance Sheet as at March 31, 2011 has not been audited and is included in the Company's 10-Q filing. |

| REVA is developing its bioresorbable, stent technology - potentially offers significant benefits for patients REVA believes bioresorbable stents will be the next major advance in coronary stents - US$5.3 billion market opportunity REVA is completing preparation to commence a pilot clinical study in 3Q 2011 with CE Mark targeted by the end of 2013 REVA 2011 Annual General Meeting |
