Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | c14915e8vk.htm |
Exhibit
99.1

| ReZolve(tm) Bioresorbable Coronary Stent Challenges Dr. Alexandre Abizaid, MD, PhD, FACC Instituto Dante Pazzanese, Sao Paulo, Brazil Columbia University, New York, USA |

| Disclosure Statement of Financial Interest Consultant for REVA Medical, Inc. 1 4/5/11 |

| To develop a stent that provides the benefits of metal without the permanency Strength Maintain acute lumen gain Maintain support during the healing process Performance Expansion range to accommodate "real world" clinical use Visibility Visualize entire stent during and after deployment Goals of the ReZolve(tm) Stent Program 2 4/5/11 |

| Unique Stent Geometry Unique Material The REVA Approach 3 4/5/11 |

| Steel-like performance in a polymer stent Exceptional radial strength Negligible recoil Extremely flexible design Slide & Lock Design 4 4/5/11 Deploys (expands) in artery with sliding, locking parts rather than material deformation Improved performance with no change to clinical practice |

| Tyrosine-derived polycarbonate Developed for stent performance Inherently radiopaque, x-ray visible Benign resorption Biocompatible MRI/CT compatible Proprietary Bioresorbable Material 5 4/5/11 Radiopacity of the ReZolve Bioresorbable Coronary Stent shortly after implant in a porcine animal model Desired features for optimal polymer stent performance |

| Initiated June 2007 Clinical sites in Brazil and Germany 25 patients received a REVA bioresorbable stent Stent successfully dilated lesions at implant Evidenced by acute gain and reduction in %DS Vessel diameter remained stable over time Higher than anticipated TLR rate Patients approaching 4 year time point RESORB First-in-Man with Gen. 1 Stent 4/5/11 6 |

| Leveraging the Gen. 1 Clinical Experience Key Learnings: Slight modification of the polymer was necessary Design modifications would further improve performance More predictive bench and preclinical tests required for polymer stenting 4/5/11 7 |

| ReZolve(tm) Sirolimus-Eluting Bioresorbable Coronary Stent 8 Drug-eluting (Sirolimus) Stronger and More Resilient Polymer Optimized Uniform Design 4/5/11 Restore. Remodel. Resorb. |

| Maintains Radiopacity Clinically-relevant expansion range Enhancements More uniform scaffolding In-board locking mechanism Improved deliverability Drug eluting Design Comparison Gen 1 to ReZolve(tm) 9 4/5/11 Modular Gen 1 Design Spiral ReZolve Design |

| 1-Year Animal Results Serial Imaging 3 months 6 months 12 months 1 0 4/5/11 |
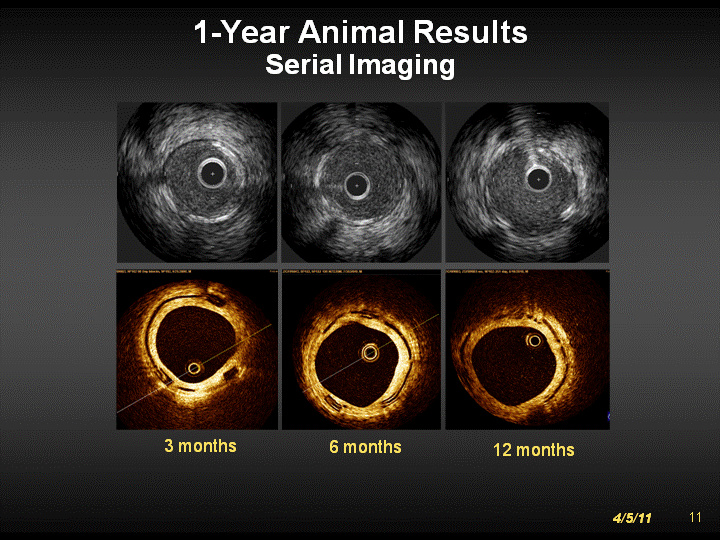
| 1-Year Animal Results Serial Imaging 3 months 6 months 12 months 1 1 4/5/11 |

| ReZolve(tm) Stent Histology 3 month time point 12 4/5/11 Proximal (Left), Mid (Center), Distal (Right) Struts captured and excellent healing in the presence of drug |

| Enhanced Test Methods Rigorous destructive challenge of structural robustness 13 4/5/11 Stent Type Cycles Cycles Time Time Stent Type Range Average Range Average ReZolve 127,202 - 507,854 246,230 29 - 120 Hrs 62 Hrs Metal Control 61,986 - 108,248 80,956 14.5 - 25 Hrs 18.9 Hrs |

| Initiating Q2 2011 Pilot Safety Study 50 Patient enrollment Sites in Brazil & Europe PI: Dr. Alexandre Abizaid Primary Endpoint: freedom from ischemic-driven TLR at 6 months QCA/IVUS parameters at 12 months 14 RESTORE Clinical Trial ReZolve Sirolimus-Eluting Bioresorbable Coronary Stent 4/5/11 |

| End 15 4/5/11 |
