Attached files
| file | filename |
|---|---|
| EX-21 - EXHIBIT 21 - AFFYMETRIX INC | ex21.htm |
| EX-23 - EXHIBIT 23 - AFFYMETRIX INC | ex23.htm |
| EX-32 - EXHIBIT 32 - AFFYMETRIX INC | ex32.htm |
| EX-31.1 - EXHIBIT 31.1 - AFFYMETRIX INC | ex31-1.htm |
| EX-31.2 - EXHIBIT 31.2 - AFFYMETRIX INC | ex31-2.htm |
| EX-10.23 - EXHIBIT 10.23 - AFFYMETRIX INC | ex10-23.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
(Mark One)
|
|
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2010
OR
|
|
|
o
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
FOR THE TRANSITION PERIOD FROM TO
COMMISSION FILE NUMBER 0-28218
|
|
AFFYMETRIX, INC.
(Exact name of registrant as specified in its charter)
|
DELAWARE
(State or other jurisdiction of
incorporation or organization)
|
77-0319159
(IRS Employer Identification Number)
|
|
3420 CENTRAL EXPRESSWAY
SANTA CLARA, CALIFORNIA
(Address of principal executive offices)
|
95051
(Zip Code)
|
|
(408) 731-5000
(Registrant’s telephone number, including area code)
|
|
|
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of Each Class
Common Stock, $0.01 par value
Preferred Stock Purchase Rights
|
Name of Each Exchange on Which Registered
The Nasdaq Global Select Market
The Nasdaq Global Select Market
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer o
|
Accelerated filer x
|
|
Non-accelerated filer o (Do not check if a smaller reporting company)
|
Smaller reporting company o
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant at June 30, 2010, based on the closing price of such stock on The Nasdaq Global Select Market on such date, was approximately $414 million. The number of shares of the registrant’s Common Stock outstanding on February 22, 2011 was 70,539,515.
DOCUMENTS INCORPORATED BY REFERENCE
Certain sections of the Proxy Statement to be filed in connection with the 2011 Annual Meeting of Stockholders are incorporated by reference into Part III of this Form 10-K where indicated.
AFFYMETRIX, INC.
FORM 10-K
DECEMBER 31, 2010
|
Item No.
|
Page
|
|
ITEM 1. BUSINESS
Forward-Looking Statements
All statements in this Annual Report on Form 10-K that are not historical are "forward-looking statements" within the meaning of the federal securities laws. These include statements regarding our "expectations," "beliefs," "hopes," "intentions," "strategies" or the like. Such statements are based on our current expectations and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. We cannot assure you that actual results or business conditions will not differ materially from those projected or suggested in such forward-looking statements as a result of various factors, including, but not limited to, those discussed in "Risk Factors" contained in Item 1A of this Annual Report on Form 10-K. Unless required by law, we do not undertake to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations with regard thereto or any change in events, conditions, or circumstances on which any such statements are based.
Overview
We develop, manufacture and sell products and services for genetic analysis to the life science research and clinical healthcare markets. Researchers around the world use our technology to better understand the role that genes play in disease, the effectiveness and safety of therapies and many other biological factors that affect human well-being. We sell our products to some of the world’s largest pharmaceutical, diagnostic and biotechnology companies, as well as leading academic, government and not-for profit research institutions. Approximately 23,000 peer-reviewed papers have been published based on work using our products. We have more than 900 employees worldwide and maintain sales and distribution operations across the United States, Europe and Asia.
We were incorporated in California in 1992 and reincorporated in Delaware in 1998. Our principal executive offices are located at 3420 Central Expressway, Santa Clara, CA 95051. Our telephone number is (408) 731-5000.
Our Strategy
Our objective is to be the leading provider of genetic analysis tools that service the needs of a growing base of research and clinical markets. The key elements of our strategy are:
|
·
|
Revenue growth. Our goal is to expand our revenue base by entering new markets, growing our customer base and successfully commercializing our established and acquired technologies. We launched several important new products in 2010, including our Axiom Genotyping Platform that enables the design of catalog and custom array designs for the study of genetic variation associated with disease, health and the environment in humans and other model organisms. We also introduced a new service level offering, OncoScan FFPE Express, that target the analysis of challenging formalin-fixed, paraffin-embedded tissue. Using OncoScan, molecular pathologists and oncologists are able to assess relevant cancer measurements in one assay, ultimately accelerating our understanding of, and approach to, treating cancer.
|
|
·
|
Profitability. Throughout 2010, we remained focused on improving our operating leverage and were successful in achieving operating income in the second half of 2010. Additionally, we have reduced our future interest payments by repurchasing a portion of our 3.50% senior convertible notes due 2038 and significantly lowered our operating expenses in 2010 as compared to 2009. Our new array plate consumables that run on the GeneTitan® and GeneAtlasTM Systems also have lower manufacturing costs than the cartridge-based consumables.
|
|
·
|
Development of new technologies and platforms. We seek to expand our product line with new products that combine automated instrumentation, powerful new biological assays, and new array designs and content. For example, the GeneTitan® System, our mid- to high-throughput instrumentation platform, enables increased efficiency and throughput for researchers conducting array-based experiments. In 2010, we commercially released our GeneAtlasTM System, our low-to-medium throughput instrumentation platform, which targets new users of microarray technology. With the GeneTitan® and GeneAtlasTM instruments, we also introduced a menu of gene expression and genotyping consumables in the array plate format.
|
Our Markets
The market for genetic analysis tools is large, growing and consists of several important end users segments. Traditionally, end users of our technologies and products have been scientific researchers located in major academic university research laboratories and within major pharmaceutical and biotechnology companies. Our products enable a research workflow that consists of several phases:
|
·
|
discovery – describing the differences between individuals,
|
|
·
|
exploration – seeking to further understand the biological relevance of a disease or process, and
|
|
·
|
validation and testing – identifying disease mechanisms and pathways.
|
Discovery and Exploration Markets
The main source of discovery funding comes from a variety of public and private sources, with the National Institute of Health being the largest. A primary end-point of these end users is peer-reviewed scientific publication and our technologies have been referenced in approximately 23,000 scientific publications. The required technologies generally must enable large-scale and highly complex analysis of genetic variation (genotyping) and biological function (gene expression). Our products for large scale genotyping and gene expression applications serve customers in the exploration markets which include academic research centers, government agencies, private research foundations, clinical and commercial reference laboratories and the research departments of pharmaceutical companies.
Validation and Testing Markets
As the adoption of genetic analysis has increased, new end users entered the markets that are downstream of the basic research markets described above. In particular, clinical researchers, molecular pathologists, oncologists and cytogeneticists have become increasingly engaged in applying genetic analysis technologies for the development of new clinical methods to be used in the diagnosis, monitoring and treatment of a wide variety of molecular based diseases. These end users are often located in diagnostic companies, commercial reference laboratories, clinical research departments within academic medical centers, major pharmaceutical, biotechnology companies and agricultural based companies focused on plant and animal breed testing. The required technology is used in a repetitive testing environment and must enable cost effective, flexible analysis of significantly fewer genetic and biological markers. We believe these new users are more likely to generate recurring revenue and the downstream markets are growing at a higher compound annual growth rate than the scientific research markets.
Our genotyping products with diagnostic or copy number applications, our targeted genotyping products and our low- to mid-plex gene expression products target the needs of the new users.
We expect that the following factors, among others, will influence the size and development of the markets served by our technologies:
|
·
|
the availability of genomic sequence and sequence variation data for the human population and for other organisms;
|
|
·
|
technological innovation that increases throughput and lowers the cost of genomic and genetic analysis;
|
|
·
|
the development of new computational techniques to handle and analyze large amounts of genomic data;
|
|
·
|
the availability of government funding for basic and disease-related research;
|
|
·
|
the amount of capital and ongoing expenditures allocated to research and development and outsourced spending by biotechnology, pharmaceutical and diagnostic companies for products and services;
|
|
·
|
the application of genomics to new areas including molecular diagnostics, agriculture, human identity and consumer goods; and
|
|
·
|
the availability of genetic markers and signatures of diagnostic value.
|
Scientific Background
Introduction to the Genome and its Opportunity
In the years following the completion of the Human Genome Project in 2003, an explosion of research in genome structure, function and variation has led to an understanding that human genetic variation is common and takes on many structural forms. Among individual humans, genetic variation ranges from single nucleotide changes to gross alterations of entire chromosomes. Subsequent efforts to identify and catalog human genetic variation, including the HapMap Project and the 1000 Genomes Project, continue to generate tremendous amounts of information that is made freely and quickly available to the public. Genetic variation accounts for many of the differences between individuals, such as eye color and blood group, and also affects a person’s susceptibility to certain diseases including cancer, diabetes, stroke and Alzheimer’s disease. Genetic variation can also determine a person’s response to drug therapies. Further, many cancers are caused by genetic variations in individual cells. Understanding the genome helps us understand the inheritance of biological characteristics. We believe that this will lead to a new healthcare paradigm where disease is understood at the molecular level, allowing patients to be diagnosed according to genetic information and then treated with drugs designed to work on specific molecular targets.
All known genomes are composed of either deoxyribonucleic acid (“DNA”) or ribonucleic acid (“RNA”). The human genome is composed of DNA and RNA. The instructions required for every living cell to develop its characteristic form and function are believed to be represented within discrete regions of the genome known as genes. DNA molecules consist of two long complementary strands held together by base pairs. Four nucleotide bases—adenine-A, cytosine-C, guanine-G and thymine-T form the chemical building blocks of DNA. The two DNA strands are held together by hydrogen bonds between nucleotide bases on one strand to complementary nucleotide bases on the other strand. Only certain pairs of the bases can form these complementary bonds: C pairs with G, and A pairs with T. Therefore, a single DNA strand containing bases in the sequence CGTACGGAT can form a bond with a DNA strand containing bases in the sequence GCATGCCTA. Such paired DNA strands are said to be "complementary" and can form a double helix structure in a process called "hybridization." Our technology uses the principle of hybridization to recognize the presence of specific gene sequences and to analyze genetic information.
Through the process of transcription RNA, copies of the DNA are made from the regions containing genes. Many copies of RNA can be made from each DNA region. The amount of RNA made from any given gene is a measure of the expression level of that gene. Many copies of RNA can be made from the same region of DNA. In the cell, RNA is typically single-stranded, while DNA is double-stranded. One type of RNA, the messenger RNA (mRNA) is central to protein synthesis. There are RNAs with other roles, such as regulating which genes are expressed and carrying genetic information of viruses.
Genotyping
Genotyping is the process of determining the genetic constitution of a cell, organism or individual in order to determine how it is specialized or differs from a group. Typically, each cell in an individual contains a complete copy of its genome. In a population, individuals vary from one another because of differences in gene sequences which are inherited from each parent and sometimes through the introduction of sequence changes due to environmental damage or biological errors in processes like gene replication. Common forms of genetic variation include single-nucleotide polymorphisms, or SNPs, and copy number variations, or CNVs. A SNP is a variation in a single position in a DNA sequence and a CNV is a variation in the number of copies of a segment of the DNA.
Genotyping is a valuable tool for studying genetic contributions to diseases and the efficacy of drug therapies in specified patient populations. While, in some cases, genetic variations, or polymorphisms, have little detectable effect on the biology of the organism, in other cases they may result in a predisposition to disease or an altered biological response to the environment. By screening for these polymorphisms, researchers seek to identify those that might be implicated in specific diseases. Sometimes it is not a single SNP or CNV, but the combination of certain variations that lead to a diseased state. For this reason, researchers look at the patterns of these polymorphisms in a large number of healthy and diseased individuals in order to correlate specific variants with specific diseases or phenotypes. Large scale genotyping can be used, for example, in studies designed to elucidate the genetic contributions to disease and, in the case of clinical trials, to drug response.
Gene Expression Monitoring
Gene expression monitoring is the process of determining which genes are active in a specific cell or group of cells. Timing and level of gene expression is an important mechanism by which the fate and function of cells are regulated. Although most cells contain an organism's full set of genes, each cell expresses only a subset of genes at any given time and the level of expression also varies with the state of that cell. The expression pattern or profile of genes can be correlated with many human diseases such as cancer, as well as with the effectiveness of treatment in specific patient populations. By identifying genes that are differentially expressed in particular diseases or patient populations, novel molecular targets and treatments may be identified and validated. In addition, gene expression signatures may be identified that provide early identification of a predisposition to disease or allow the selection of treatments optimized for an individual.
Gene expression monitoring is a valuable tool for identifying correlations between genes, determining their biological functions and identifying patterns that might be useful in classifying diseases. To monitor gene expression, we design and manufacture arrays with single-stranded DNA molecules that are complementary to sequences within genes or exons of interest. By synthesizing specific probes for multiple genes or exons on a single probe array, we enable researchers to quickly, quantitatively and simultaneously monitor the expression of a large number of genes or exons of interest. By monitoring the expression of such genes under different conditions and at different times, researchers can use the arrays to understand the dynamic relationship between gene expression and biological activity. We believe such information will be an important tool in understanding gene function and for the development of new drugs and diagnostic tools. Increasingly, clinical research is showing that gene expression patterns in tissue samples, particularly those from cancerous tissues, can be used to characterize disease sub-types and hopefully to predict therapeutic responses and likely outcomes.
In order to understand the impact of genomics on health, disease and other aspects of the human condition, scientists must compare both the sequence variation and the gene expression patterns of healthy and diseased individuals, tissues and cells. The use of arrays to identify correlations of gene expression patterns and sequence variation with specific diseases is expected to become increasingly important for gene marker validation, exploration and routine testing for diagnosis of disease.
Our Technologies
Array Technology
Our array technology leverages semiconductor-based photolithographic fabrication techniques, which enables us to synthesize a large variety of predetermined DNA sequences simultaneously in predetermined locations on a small glass chip called an "array."
Photolithography is a technique which uses light to create exposure patterns on the glass chip and to direct chemical reactions. The process begins by coating the chip with light-sensitive chemical compounds that prevent chemical coupling. These light-sensitive compounds are called "protecting groups." Lithographic masks, which consist of predetermined transparent patterns etched into a glass plate that block or transmit light, are used to selectively illuminate the glass surface of the chip. Only those areas exposed to light are deprotected, and thus activated for chemical coupling through removal of the light-sensitive protecting groups. The entire surface is then flooded with a solution containing the first in a series of DNA building blocks (A, C, G or T). Coupling only occurs in those regions that have been deprotected through illumination. The new DNA building block also bears a light-sensitive protecting group so that the cycle can be repeated.
This process of exposure to light and subsequent chemical coupling can be repeated many times on the same chip in order to generate a complex array of DNA sequences of defined length. The intricate illumination patterns allow us to build high-density arrays of many diverse DNA sequences in a small area. Unlike conventional synthesis techniques, which generally use a linear process to create compounds, our synthesis technique is combinatorial, in that the number of different compounds synthesized grows exponentially with the number of cycles in the synthesis. Currently, our commercial arrays contain over six million unique sequences. Each unique sequence is 25 to 50 nucleotides in length and is represented millions of times within a specified area of the array. Just as in the semiconductor industry, we manufacture arrays in a wafer format. Each wafer is approximately five inches square and can contain over 300 million unique probe sequences based on current technology. For our commercial array products, we can manufacture a large number of identical or different DNA probe arrays on a glass wafer, which is then diced into individual chips. The number of chips manufactured per wafer can be varied depending on the desired amount of information on each chip. The chips can be packaged individually, in our cartridge format, in our strip format or in our peg format. A strip format can have four arrays packaged together on a strip and a peg format can have up to 96 arrays packaged together for automated and parallel processing. Given the large amount of unique sequences represented in our arrays, our technology enables the efficient analysis of a multitude of DNA probes to analyze DNA or RNA sequences in a test sample.
The function of each single-stranded sequence on our array is to bind to its complementary single strand of DNA or RNA from a biological sample. Each unique feature on the array contains multiple copies of the same single strand of DNA. The nucleic acid (DNA or RNA) to be tested is isolated from a sample, such as blood, saliva or biopsy tissue, amplified and prepared for hybridization to the array. The test sample is then washed over the array, where the individual nucleic acid sequences that represent the genetic content or expressed genes of the sample hybridize to their complementary sequences bound on the array. The molecules in the test sample may be labeled with fluorescent dye either before or after hybridization. When scanned by a laser in the scanner instrument, the test sample generates a fluorescent signal. The locations where a fluorescent signal is detected by an optical detection system on the scanner instrument correspond to sequences complementary to the test sample. Sequence variation, or the quantification of specific sequences of nucleic acids in the sample, can be determined by detecting the relative strength of these signals since the sequence and position of each complementary DNA probe on the probe array is known. The combination of a particular array, together with an optimized set of reagents and a user protocol describing how to carry out the procedure, is referred to as an "assay."
bDNA Technology
We offer customers a suite of assay products for a wide variety of low- to mid-plex genetic, protein and cellular analysis applications using branched DNA, or bDNA, technology. These assays measure RNA levels directly from samples using a novel signal amplification method without the need for RNA purification, providing customers with improved accuracy, scale and workflow relative to traditional methods based on polymerase chain reaction, or PCR.
Our Products
Overview
We offer a comprehensive line of products for two principal applications: genotyping and gene expression. The majority of our product sales consist of sales of instruments and related consumables. We have three families of instrument systems, GeneChip®, GeneTitan® and GeneAtlasTM that include instruments, consumables and software. Our GeneChip® instruments run arrays packaged in cartridges and our GeneTitan® and GeneAtlasTM instruments run arrays packaged in a peg format for automated high throughput processing.
We also offer a variety of assays for gene expression targeting low- to mid-plex markets that are downstream of our whole genome arrays and a range of reagent kits that are compatible with our platforms as well as the products of other vendors.
GeneChip® Family of Products
Our GeneChip® system provides an integrated solution for gene expression and genotyping analysis. It consists of instruments and consumables that provide for the robust preparation and analysis of samples using our GeneChip® cartridge arrays. The components of the GeneChip® system include (1) disposable probe arrays containing genetic information on a chip, (2) reagents for extracting, amplifying and labeling target nucleic acids, (3) a fluidics station for introducing the test sample to the probe arrays, (4) a hybridization oven for optimizing the binding of samples to the probe arrays, (5) a scanner to read the fluorescent image from the probe arrays, and (6) software to analyze and manage the resulting genetic information.
Our major GeneChip® instrument products include:
|
Product
|
Product Description
|
|
GeneChip® Scanner 3000
|
Instrument for scanning higher-density arrays, including SNP arrays with up to 900,000 SNPs, tiling arrays for transcription and all-exon arrays for whole-genome analysis.
|
|
GeneChip® Scanner 3000Dx
|
This instrument is a version of the GeneChip® Scanner 3000 that is cleared by the United States Food and Drug Administration as an in vitro diagnostic device (“IVD”).
|
|
GeneChip® Fluidics Station
|
Instrument for the wash and stain of GeneChip® arrays.
|
|
GeneChip® Hybridization Oven
|
This instrument provides temperature and rotation control to ensure the successful hybridization of cartridge arrays before scanning.
|
Our major GeneChip® array and reagent products include:
|
Genotyping Catalog Cartridge Arrays
|
· SNP 6.0 Array – This single chip array is a robust tool for studying variation. It enables genotyping of approximately 906,600 SNPs
and assaying of approximately 945,800 non-polymorphic probes for detection of copy number.
· DMET™ Plus – This array features drug markers in FDA-validated genes and enables discovery and measurement of genetic variation
associated with drug response.
· Universal Tag Arrays – These arrays enable targeted genotyping for the analysis of between 1,500 to 20,000 SNPs per sample.
|
|
Gene Expression Catalog Cartridge Arrays
|
· U133 – This array analyzes the expression level of over 47,000 transcripts and variants of the human genome.
· Other Arrays – We also offer a range of catalog expression arrays for the study of rat, mouse and other mammalian and model
organisms.
|
|
Custom Arrays
|
· MyGeneChip™ and CustomSeq™ products are custom expression and sequence arrays designed by our customers to study
organisms of interests to them.
|
GeneTitan® Family of Products
Our GeneTitan® family of products consists of the GeneTitan® instrument system that runs genotyping and gene expression array plates. The GeneTitan® family of products provides a hands-free, automated solution for monitoring gene expression and genome-wide SNP genotyping.
Our GeneTitan® products include:
|
Product
|
Product Description
|
|
GeneTitan®
|
The GeneTitan® instrument automates array processing from target hybridization to data generation by combining a hybridization oven, fluidics processing and imaging device into a single bench-top instrument. It runs array plates and supports both gene expression and genotyping studies.
|
|
Axiom™ Genotyping Solution
|
The Axiom™ Genotyping Solution includes array plates with validated genomic content, complete reagent kits, data analysis tools and a fully automated workflow utilizing the GeneTitan®.
· Axiom™ Human Array Plates – these arrays are designed to maximize genomic coverage of common and novel SNPs and insertions
and deletions in Caucasian, Asian and African populations. We are currently offering the Caucasian and Asian array plates and expect
to offer the African array plates in 2011.
· Axiom™ Custom Arrays – Customers can make custom arrays utilizing a proprietary database of validated genomic markers.
|
|
Gene Expression Array Plates
|
We offer a catalog of gene expression array plates similar to our catalog gene expression cartridge arrays to be used on the GeneTitan® instrument. These arrays are available for the study of human, rat, mouse and a broad range of other mammalian and model organisms.
|
Our GeneAtlasTM products include:
|
Product
|
Product Description
|
|
GeneAtlasTM Personal Microarray System
|
The GeneAtlasTM is a lower-priced instrument for low-to-medium throughput. The GeneAtlasTM utilizes the array strip format, with four arrays per strip, and provides simplified hybridization and simple array processing with common microwell-based labware.
|
|
Gene Expression Array Plates
|
We offer a catalog of gene expression array plates for the study of human, rat and mouse to be used on our GeneAtlasTM system.
|
Low- to Mid-plex Products
We also offer an extensive line of multiplex assays to serve both the discovery and the validation markets. Multiplex assays measure many different targets from the same sample. These products enable drug target identification through analysis of gene silencing, cell signaling and biomarker validation. Our QuantiGene line of products is based on bDNA technology and delivers quantitative gene expression analysis. These products are compatible with a wide variety of samples and tissues.
We offer researchers with an extensive line of reagent kits, enzymes and biochemicals. Our reagents are complementary to our array portfolio, thus enabling us to provide our customers with whole product solutions. In addition, they can be applied to a broad variety of emerging technologies. Our reagents include:
|
·
|
ExoSAP-IT® For PCR Product Clean-Up, a reagent for the rapid clean-up of PCR products used in downstream applications, such as DNA sequencing or SNP analysis.
|
|
·
|
HotStart-IT® line of PCR reagents, reagents that utilize a novel primer binding protein to inhibit primer dimer formation, and results in sensitive and consistent amplification for real-time PCR.
|
Our Services
Our Affymetrix Research Service Laboratory offers high-throughput genotyping services for customers using our genotyping products. Our projects range in size from a few hundred samples to over 10,000 samples. We serve customers requiring quick turnaround times and suitably priced solutions to their large-scale academic and consortia genotyping studies.
Our Collaborative Partners
We collaborate with our partners to expand the applications of our technology and to acquire access to complementary technologies and resources. We collaborate with a number of instrumentation and reagent companies to develop and supply certain components of the user work flow. These companies include Beckman Coulter, Inc., CapitalBio Corporation, Life Technologies Corporation, Genisphere LLC, Takara Bio Inc., New England Biolabs, Inc., Luminex Corporation and Qiagen GmbH.
Through our Powered by Affymetrix™, or PbA Program, we permit commercial entities to license our technologies to develop custom product solutions based upon our arrays, instrumentation and software. Our PbA partners include F. Hoffman-La Roche Ltd., bioMerieux, Inc., Veridex, LLC, a Johnson & Johnson company, Signature Diagnostics and TessArae. We provide our PbA partners custom arrays. Our partners subsequently package these arrays into kits, seek regulatory approval and reimbursement for their diagnostic use, and sell them into the diagnostic markets using their sales channels. An example is the PathChip, a gene expression array used by our PbA partner Pathwork Diagnostics, Inc., in its Pathwork Tissue of Origin test. In July 2008, and again in January 2010, the U.S. Food and Drug Administration (“FDA”) cleared the version of the Pathwork Tissue of Origin test for marketing.
We also collaborate with certain academic, government, and commercial research groups to develop and validate new applications of our technologies. These include the Broad Institute of Harvard, the Massachusetts Institute of Technology and the National Genome Research Institute.
Sales and Distribution
We market and distribute our products directly to customers in North America, Japan and major European markets. In these markets, we have our own sales, service and application support personnel responsible for expanding and managing their respective customer bases. In other markets, such as Mexico, India, the Middle East and Asia Pacific, including the People’s Republic of China, we sell our products principally through third party distributors that specialize in life science supply. For molecular diagnostic and industrial applications market opportunities, we supply our partners with arrays and instruments, which they incorporate into diagnostic products and assume the primary commercialization responsibilities.
Manufacturing and Raw Materials
We manufacture our consumables, including our arrays and reagents, and contract with third parties to manufacture our instruments. We manufacture our reagents in our Cleveland, Ohio facility and our arrays in our Singapore facility. We maintain a pilot manufacturing and process engineering/development facility in Santa Clara, California.
Our array manufacturing process involves wafer preparation, probe synthesis, dicing of synthesized wafers into chips, assembly of chips and quality control. We have developed software programs that extensively automate the design of photolithographic masks used in array manufacturing and that control the array manufacturing lines. Glass wafers are prepared for synthesis through the application of chemical coatings. Arrays are synthesized on the wafers using our proprietary, combinatorial photolithographic process. The completed wafers can then be diced into chips. The chips can be packaged individually, in our cartridge format, in our strip format or in our peg format.
We offer a variety of reagents to our customers, including those that are manufactured in-house, those that are supplied by qualified third-party suppliers and a combination of the two.
Our Singapore facility is fully operational and has been certified to ISO 13485 standards. The Singapore facility operates under the strict standards of our corporate quality plan. In 2009, we certified a portion of our Cleveland, Ohio operations to these standards. We intend to certify the remaining portions of the Cleveland, Ohio operations to these standards in 2011. Third parties who manufacture our instruments will have to meet our quality standards as part of the qualification process.
Key parts of our product lines, such as our GeneTitan® instrument and hybridization ovens, are available from single sources. Likewise, certain raw materials or components used in the synthesis of arrays or the assembly of instrumentation are currently available only from a single source or limited sources. Alternative sources of supply may be time consuming and expensive to qualify. In addition, we are dependent on our vendors to provide components of appropriate quality and reliability, and to meet applicable regulatory requirements. We take what we believe are appropriate measures to prevent the delay or interruption of supplies from these vendors and to ensure the appropriate quality for our customers, since any delay or interruption could delay our ability to deliver our products to our customers.
Research and Development
We believe that a substantial investment in research and development is essential to a long-term sustainable competitive advantage and critical to expansion into the validation and routine testing markets. Our research and development effort is divided into the major areas of basic research, product research and development, and manufacturing technology development. Research efforts are carried out through our Affymetrix Research Laboratories to further advance our platform and develop new concepts and applications that can be commercialized to grow our business. Our product development efforts are focused primarily on the development of new array, assay and reagent products, improving the overall performance of our assays, increasing the information capacity per probe array and simplifying highly complex assays. We are also actively engaged in research aimed at enhancing the manufacturing process currently employed in the production of our arrays.
Our research and development expenses for the years ended December 31, 2010, 2009 and 2008 were $67.9 million, $77.4 million and $84.5 million, respectively.
Intellectual Property
We rely on a combination of patent, copyright, and trade secret laws, know-how and licensing opportunities to establish and protect our proprietary technologies and products. Our success depends in part on our ability to obtain patent protection for our products and processes, to preserve our copyrights and trade secrets, to operate without infringing the proprietary rights of third parties and to acquire licenses related to enabling technology or products used with our technology.
We are pursuing a patent strategy designed to facilitate our research and development program and the commercialization of our current and future products. While no one patent is considered essential to our success, we aggressively seek to protect our patent rights as our patent portfolio as a whole is material to the success of the business.
There are a significant number of United States and foreign patents and patent applications in our areas of interest, and we believe that there will continue to be significant litigation in the industry regarding patent and other intellectual property rights. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. It may be necessary for us to enter into litigation to defend against or assert claims of infringement, to enforce patents issued to us, to protect trade secrets or know-how owned by us or to determine the scope and validity of the proprietary rights of others. From time to time, to determine the priority of inventions, it may be necessary for us to participate in interference proceedings declared by the United States Patent and Trademark Office. Litigation or patent administrative proceedings could result in substantial costs to and distraction from our core business and our efforts in respect to such proceedings may not be successful. For further information regarding intellectual property litigation involving us, see “Item 8. Financial Statements and Supplementary Data—Note 10. Commitments and Contingencies” in this Annual Report on Form 10-K.
We also rely upon copyright and trade secrets to protect our confidential and proprietary information. We seek to protect our proprietary technology and processes by confidentiality agreements with our employees and certain consultants and contractors. These agreements may be breached, we may not have adequate remedies for any breach and our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees or our consultants or contractors use intellectual property owned by others in their work for us, disputes may also arise as to the rights in related or resulting know-how and inventions.
We are party to various option, supply and license agreements with third parties which grant us rights to use certain aspects of our technologies. We take such measures as we believe are appropriate to maintain rights to such technology under these agreements. In addition, our academic collaborators have certain rights to publish data and information in which we have rights. There is considerable pressure on academic institutions to publish discoveries in the genetics and genomics fields. We take such steps as we believe are appropriate to ensure that such publication will not adversely affect our ability to obtain patent protection for information in which we may have a commercial interest.
Competition
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition, new product introductions and strong price competition. We face significant competition as existing companies develop new or improved products, and as companies enter the market with new technologies, such as next-generation sequencing.
In the highly multiplexed genotyping and gene expression markets, existing competitive technologies include DNA sequencing, which we do not offer and is offered by companies such as Illumina, Inc., Life Technologies Corporation and Pacific Biosciences of California, Inc. Other companies developing or marketing competitive DNA array technology include Illumina, Inc., Agilent Technologies, Inc., BD Biosciences, CombiMatrix Corporation, MDS Analytic Technologies/Danaher, Nimblegen/Roche Diagnostics, NuGEN Technologies, Inc. and Sequenom, Inc., some of which offer products directly competitive with our microarrays or reagents. In the low to midplex genotyping and gene expression markets, much of the existing low-plex competition comes from the supplier of realtime PCR products, including Life Technologies Corporation, who has a dominant position, Roche Diagnostics, Agilent Technologies, Inc. and BioRad Laboratories, Inc. In addition, there are new midplex technologies being offered by Fluidigm Corporation, Sequenom, Inc., High Throughput Genomics, Inc., Beckman Coulter, NanoString Technologies and Life Technologies Corporation (BioTrove). In order to compete against existing and emerging technologies, we will need to demonstrate that our products have superior throughput, cost and accuracy advantages over competing products.
In the molecular diagnostic field, competition may likely come from established diagnostic companies, companies developing and marketing DNA probe tests for genetic and other diseases, and other companies conducting research on new technologies to ascertain and analyze genetic information. The market for molecular diagnostic products derived from gene discovery is highly competitive and has high barriers of entry, with several large corporations already having significant market share. Established diagnostic companies such as Beckman Coulter, Becton, Dickinson and Company, bioMérieux, Johnson & Johnson, Gen-Probe Incorporated and Roche Diagnostics have the strategic commitment to diagnostics, the financial and other resources to invest in new technologies, substantial intellectual property portfolios, substantial experience in new product development, regulatory expertise, manufacturing capabilities and the distribution channels to deliver products to customers. Established diagnostic companies also have an installed base of instruments in several markets, including clinical and reference laboratories, which are not compatible with our system and could slow acceptance of our products. In addition, these companies have formed alliances with genomics companies which provide them access to genetic information that may be incorporated into their diagnostic tests.
We will face increased competition in existing and potential markets as the cost of new technologies such as sequencing and other technologies improves. We expect new competitors and technologies to emerge. In addition, pharmaceutical and biotechnology companies have significant needs for genomic information and may choose to develop or acquire competing technologies to meet these needs themselves. We have significantly expanded our network of approved service providers in America, Japan, Europe, and China. While these companies expand the reach of Affymetrix technology and make its analytical power available to a wider base of users they may act as a substitute for outright purchase of instruments and arrays by those end users. In addition, we have several other third-party licensees that could offer products that compete with our product offerings.
Government Regulation
Many of our products are labeled for research use only. Products intended for research use only are not subject to clearance or approval by the FDA. However, research use only products may fall under the FDA’s jurisdiction if these are used for clinical rather than research purposes. Even where a product is not otherwise subject to clearance or approval by the FDA, the FDA may impose restrictions as to the manner in which we can market and sell our products and/or the types of customers to which we can market and sell our products in order to limit sales to those who use the products for research only.
Our GeneChip® Scanner 3000Dx is cleared by the FDA to be used in conjunction with cleared medical devices such as the Roche Diagnostics AmpliChip CYP450 Test. We will continue to develop diagnostic products ourselves or with our collaborative partners that may require regulatory clearance or approval by governmental agencies. Commercially available in vitro diagnostic test kits and the reagents and instrumentation used with in vitro diagnostic tests are regulated as medical devices and are generally subject to rigorous testing and other pre-market review procedures by the FDA in the U.S. and by other regulatory agencies in other countries. The FDA's Quality System Regulations also apply in connection with our manufacture of arrays and systems as components for use in diagnostic products distributed outside of the research environment. Obtaining these clearances or approvals and the compliance with these regulations require the expenditure of substantial resources over a significant period of time, and we cannot assure you that any clearances or approvals will be granted on a timely basis, if at all. Once granted, a clearance or approval may place substantial restrictions on how the device is marketed or labeled or to whom it may be sold. In addition, various federal and state statutes and regulations govern or influence the manufacturing, safety, and storage of our products and components of our products as well as our record keeping.
The FDA, the U.S. Department of Health and Human Services, the States of California and New York, and foreign government regulators are increasingly focused on genetic analysis tools, including the use of microarrays, which are labeled for research use only, by clinical laboratories in laboratory-developed tests offered by these laboratories, including labs certified under the Clinical Laboratory Improvement Amendments, or CLIA, or licensed under California or New York State laboratory regulations or licensed by other states. We cannot predict the nature of future regulatory or policy initiatives with respect to the sale and use of arrays for the development of assays by CLIA-certified, state licensed laboratories, or the extent to which such initiatives will impact our business. If new regulations restrict our customers’ development of laboratory-developed tests using products labeled for research use only, or if we otherwise are required to obtain FDA premarket clearance or approval prior to commercializing products labeled as research use only, our ability to generate revenue from the sale of our products may be delayed or otherwise adversely affected. Moreover, our failure to comply with governmental rules and regulations related to our products could cause us to incur significant adverse publicity, or subject us to investigations and notices of non-compliance or lead to fines or restrictions upon our ability to sell our products. We also may be at risk for liability related to government reimbursement of tests involving the use of our products if it were determined that these tests require FDA-clearance or approval and no such clearance or approval has been obtained.
Medical device laws and regulations are also in effect in many countries, including countries in the European Union, ranging from comprehensive device approval requirements to requests for product data or certifications. The number and scope of these requirements are increasing. We may not be able to obtain regulatory approvals in such countries or may incur significant costs in obtaining or maintaining our foreign regulatory approvals. In addition, the export by us of certain of our products which have not yet been cleared for domestic commercial distribution may be subject to FDA or other export restrictions.
We have agreements relating to the sale of our products to government entities and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government. A failure to comply with these regulations might result in suspension of these contracts or administrative or other penalties, and could have a material adverse effect on our ability to compete for future government grants, contracts and programs.
Reimbursement
The design of our products and the potential market for their use may be directly or indirectly affected by U.S. and other government regulations governing reimbursement for clinical testing services. The availability of third-party reimbursement for our products and services may be limited or uncertain, particularly with respect to genetic tests and other clinical applications products.
Third-party payers may deny reimbursement if they determine that an ordered health care product or service has not received appropriate FDA or other governmental regulatory clearances, is not used in accordance with cost-effective treatment methods as determined by the payer, or is deemed by the third-party payer to be experimental, unnecessary or inappropriate. Under Medicare rules, diagnostic tests must be ordered by a physician who is treating the beneficiary and who uses the test results in patient management. Under this rule, some Medicare contractors may deny coverage for a test, even if the test has been cleared or approved by the FDA, without proof, as determined sufficient by the contractor, that the test is useful in patient management. Furthermore, third-party payers are increasingly challenging the prices charged for health care products and services.
We are currently developing diagnostic and therapeutic products, including those with our collaborative partners which may be subject to reimbursement issues. The commercialization of such products may depend, in part, on the extent to which reimbursement for these products will be available under U.S. and foreign regulations governing reimbursement for clinical testing services by government authorities, private health insurers and other organizations.
In the United States, third-party payer price resistance, the trend towards managed health care, implementation of the Patient Protection and Affordable Care Act of 2010 and other legislative proposals to reform health care or reduce government insurance programs could reduce payment rates for health care products and services, adversely affect the profits of our customers and collaborative partners and thus reduce our future royalties and product sales.
Environmental Matters
We are dedicated to compliance and protection of the environment and individuals. Our operations require the use of hazardous materials (including biological materials) which subject us to a variety of federal, state and local environmental and safety laws and regulations. Some of the regulations under the current regulatory structure allow for "strict liability," holding a party potentially liable without regard to fault or negligence. We could be held liable for damages and fines as a result of our, or others', business operations should contamination of the environment or individual exposure to hazardous substances occur. We cannot predict how changes in these laws or development of new regulations will affect our business operations or the cost of compliance.
Employees
As of February 22, 2011, we had 918 full-time employees. The employee group includes chemists, engineers, computer scientists, mathematicians and molecular biologists with experience in the diagnostic products, medical products, semiconductor, computer software and electronics industries. None of our employees are represented by a collective bargaining agreement, nor have we experienced work stoppages. Our success depends in large part on our ability to attract and retain skilled and experienced employees.
Seasonality
Customer demand for probe arrays and instrumentation systems is typically highest in the fourth quarter of the calendar year as customers spend unused budget allocations before the end of the year.
Backlog
Because most customer orders are shipped in the quarter in which they are received, we believe that backlog at quarter end is typically not a material indicator of future sales. In addition, backlog may not result in sales because of cancellation of orders or other factors. On a few occasions we have experienced, and made public announcements about, short-term increases in backlog as a result of factors such as new product introductions or supply constraints.
Financial Information About Industry Segments
We operate in one business segment, for the development, manufacture, and commercialization of systems for genetic analysis in the life sciences and diagnostic industry. Our operations are treated as one segment as we only report operating information on a total enterprise level to our chief operating decision-maker. Further, resource allocations are also made at the enterprise level by our chief operating decision-maker.
Financial Information About Geographic Areas
Our total revenue from customers outside of the United States for the years ended December 31, 2010, 2009 and 2008 was $132.7, $136.8 million and $141.1 million, or approximately 43%, 42% and 34%, respectively, of our total revenue. A summary of revenues from external customers attributed to each of our geographic areas for the years ended December 31, 2010, 2009 and 2008, is included in “Item 8. Financial Statements and Supplementary Data—Note 14. Segment and Geographic Information”.
Available Information
Our internet address is www.affymetrix.com. Information included on our website is not part of this Form 10-K. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. In addition, copies of our annual reports are available free of charge upon written request. The SEC also maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is www.sec.gov.
ITEM 1A. RISK FACTORS
Risks Related to the Growth of Our Business
If we do not continually develop and commercialize new or enhanced products and services, our business may not grow.
Our success depends in large part on our continual, timely development and commercialization of new or enhanced products and services that address evolving market requirements and are attractive to customers. The genetic analysis tools market, including the RNA/DNA probe array field, is characterized by rapid and significant technological changes, frequent new product introductions and enhancements, evolving industry standards and changing customer needs. Standardization of tools and systems for genetic research is still ongoing and we cannot assure you that our products will emerge as the standard for genetic research. Other companies may introduce new technologies, techniques, products or services that render our products or services obsolete or uneconomical. If we do not appropriately innovate and invest in new technologies, then our technologies will become dated and our customers could move to new technologies offered by our competitors.
As a result, we are continually looking to develop, license or acquire new or enhanced technologies, products and services to further broaden and deepen our offerings. Some of the factors affecting market acceptance of our products and services include:
|
·
|
availability, quality and price as compared to competitive technologies, products and services;
|
|
·
|
the functionality of new and existing products and services, and whether they address market requirements;
|
|
·
|
the timing of introduction of our technologies, products and services as compared to competitive technologies, products and services;
|
|
·
|
the existence of product defects;
|
|
·
|
scientists’ and customers’ opinions of the utility of our products and services and our ability to incorporate their feedback into future products and services;
|
|
·
|
citation of our products in published research; and
|
|
·
|
general trends in life science research and life science informatics software development.
|
Our new or enhanced technologies, products or services may not be accepted by customers in our target markets. For example, once we have developed or obtained a new technology, we may fail to successfully commercialize new products and services based on that technology, particularly to the extent that our new products and services compete with established technologies or the products and services of more established competitors. Risks relating to product adoptions include the inability to accurately forecast demand and difficulties in managing different sales and support requirements due to the type or complexity of the new products.
Our growth depends in part on our ability to acquire new technologies and successfully integrate past acquisitions, which may absorb significant resources and may not be successful.
As part of our strategy to develop and identify new technologies, products and services, we have made and may continue to acquire new technologies. Our integration of the operations of acquired businesses requires significant efforts, including the coordination of information technologies, research and development, sales and marketing, operations, manufacturing and finance. These efforts result in additional expenses and divert significant amounts of management’s time from other projects. Our failure to manage successfully and coordinate the growth of the combined company could also have an adverse impact on our business. In addition, there is no guarantee that some of the businesses we acquire will become profitable or remain so. If our acquisitions do not meet our initial expectations, we may record impairment charges, such as those recorded in 2008.
Factors that will affect the success of our acquisitions include:
|
·
|
our ability to retain key employees of the acquired company;
|
|
·
|
the performance of the acquired business, technology, product or service;
|
|
·
|
our ability to integrate operations, financial and other systems;
|
|
·
|
the ability of the combined company to achieve synergies among its constituent companies, such as increasing sales of the combined company’s products and services, achieving expected cost savings and effectively combining technologies to develop new products and services;
|
|
·
|
any disruption in order fulfillment or loss of sales due to integration processes;
|
|
·
|
the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies;
|
|
·
|
any decrease in customer and distributor loyalty and product orders caused by dissatisfaction with the combined companies’ product lines and sales and marketing practices, including price increases; and
|
|
·
|
our assumption of known contingent liabilities that are realized, known liabilities that prove greater than anticipated, or unknown liabilities that come to light, to the extent that the realization of any of these liabilities increases our expenses or adversely affects our business or financial position.
|
Emerging market opportunities in molecular diagnostics may not develop as quickly as we expect and we depend on the efforts of our partners to be successful.
The clinical applications of our technologies for diagnosing and enabling informed disease management options in the treatment of disease is an emerging market opportunity in molecular diagnostics. At this time, we cannot be certain that molecular diagnostic markets will develop as quickly as we expect. Although we believe that there will be clinical applications of our technologies that will be utilized for diagnosing and enabling informed disease management options in the treatment of disease, there can be no certainty of the technical or commercial success our technologies will achieve in such markets.
Our success in the molecular diagnostics market depends to a large extent on our collaborative relationships and the ability of our collaborative partners to achieve regulatory approval for such products in the United States and in overseas markets, and successfully market and sell products using our technologies.
Risks Related to Our Sales
We face significant competition, and our failure to compete effectively could adversely affect our sales and results of operations.
We compete with companies that develop, manufacture and market genetic analysis tools for the life science and clinical healthcare markets. We face significant competition as our competitors and new companies develop new, improved or more economical products, services and technologies.
The market for molecular diagnostics products and services is highly competitive, has high barriers to entry and has several other large companies with significant market share. For example, companies such as Illumina, Agilent Technologies and Life Technologies have products for genetic analysis that are directly competitive with certain of our products. In addition, Illumina, Inc., Life Technologies Corporation and Pacific Biosciences of California, Inc. also offer DNA sequencing technology which we do not offer. As the costs of DNA sequencing fall, we will face increased competition in our existing and potential markets. We also face competition from established diagnostic companies such as Beckman Coulter, Becton, Dickinson and Company, bioMérieux, Celera Diagnostics, Johnson & Johnson, Gen-Probe Incorporated and Roche Diagnostics, which have made strategic commitments to diagnostics, have financial and other resources to invest in new technologies, and have substantial intellectual property portfolios, substantial experience in new product development and regulatory expertise. In addition, our collaborative partners may compete with us.
Many of our current and potential competitors have significantly greater financial, technical, marketing and other resources than we do. In addition, many current and potential competitors have greater name recognition, more extensive customer bases and access to proprietary genetic content.
Reduction or delay in research and development budgets and government funding may adversely impact our sales.
We expect that our revenue in the foreseeable future will be derived primarily from products and services provided to pharmaceutical and biotechnology companies, as well as a relatively small number of academic, governmental and other research institutions. Our operating results may fluctuate substantially due to reductions and delays in research and development expenditures by these customers.
Factors that could affect the spending levels of our customers include:
|
·
|
weakness in the global economy and changing market conditions that affect our customers;
|
|
·
|
changes in the extent to which the pharmaceutical industry may use genetic information and genetic testing as a methodology for drug discovery and development;
|
|
·
|
changes in government programs that provide funding to companies and research institutions;
|
|
·
|
changes in the regulatory environment affecting life science companies and life science research;
|
|
·
|
impact of consolidation within the pharmaceutical industry; and
|
|
·
|
cost reduction initiatives of customers.
|
As we implement our strategy to expand into new markets, the size and structure of our current sales, marketing and technical support organizations may limit our ability to sell our products and services.
As we implement our strategy to expand into new markets, we may not be able to establish a sales, marketing and technical support organization sufficient to sell, market and support all of our new products, or to cover all of the regions that we target globally. To assist our sales and support activities, we have entered into distribution agreements through certain distributors, principally in markets outside of North America and Europe. In addition, we may enter into distribution arrangements with respect to some of our products that we believe will be better served in such arrangements than our current sales and marketing organizations. We have less control over other third parties on whom we rely for sales, marketing and technical support. In addition, these third parties may decide to develop and sell competitive products or otherwise become our competitors, which could harm our business.
Consolidation trends in both our market and many of our customers’ markets have increased competition.
There has been a trend toward industry consolidation in our markets for the past several years. We expect this trend toward industry consolidation to continue as companies attempt to strengthen or hold their market positions in an evolving industry and as companies are acquired or are unable to continue operations. We believe that industry consolidation may result in stronger competitors that are better able to compete as sole-source vendors for customers. This could lead to more variability in operating results and could harm our business.
Additionally, there has been a trend toward consolidation in many of the customer markets we sell to, in particular the pharmaceutical industry. Consolidation in our customer markets results in increased competition for important market segments and fewer available accounts, and larger consolidated customers may be able to exert increased pricing pressure on companies in our market.
If we are unable to maintain our relationships with collaborative partners, we may have difficulty developing and selling our products and services.
Our commercial success depends in part on our ability to develop and maintain collaborative relationships with key companies as well as with key academic researchers. In particular, we depend on our collaborators for in-licensed technology and components for a variety of our product lines. We collaborate with a number of instrumentation and reagent companies, including Beckman Coulter, CapitalBio Corporation, Genisphere LLC, Life Technologies Corporation, Luminex Corporation, Siemens Medical Solutions Diagnostics, Takara Bio Inc., New England Biolabs, Inc. and Qiagen GmbH. Some of these collaborators, like Life Technologies Corporation, Takara Bio Inc. and New England Biolabs, Inc. and Luminex Corporation, are currently sole suppliers of components of some of our reagent kits but they are also our competitors. Relying on our collaborative relationships is risky to our future success because:
|
·
|
our partners may develop technologies or components competitive with our products and services;
|
|
·
|
our existing collaborations may preclude us from entering into additional future arrangements;
|
|
·
|
our partners may not obtain regulatory approvals necessary to continue the collaborations in a timely manner;
|
|
·
|
some of our agreements may terminate prematurely due to disagreements between us and our partners;
|
|
·
|
our partners may not devote sufficient resources to the development and sale of our products and services;
|
|
·
|
our partners may be unable to provide the resources required for us to progress in the collaboration on a timely basis;
|
|
·
|
our collaborations may be unsuccessful; or
|
|
·
|
some of our agreements have expired and we may not be able to negotiate future collaborative arrangements on acceptable terms.
|
Risks Related to the Manufacturing of Our Products
We depend on a limited number of suppliers. We will be unable to launch or commercialize our products in a timely manner if our suppliers are unable to meet our requirements or if shipments from these suppliers are delayed or interrupted.
We outsource the manufacturing of our instruments to a limited number of suppliers. Some of our instruments and other key parts of our product lines, including components of our manufacturing equipment and certain raw materials used in the manufacture of our products are currently only available from a single supplier. Therefore, we depend on our suppliers to supply our instruments, or components of our products, in required volumes, at appropriate quality and reliability levels, and in compliance with regulatory requirements. If supplies from these vendors do not meet our requirements, or were delayed or interrupted for any reason, we would not be able to commercialize our products successfully or in a timely fashion, and our business could be adversely impacted.
Our business is dependent on our ability to forecast our needs for components and products in our product lines and our suppliers’ ability to deliver such components and products in time to meet critical manufacturing and product release schedules. Our business could be adversely affected, for example, if suppliers fail to meet product release schedules, if we experience supply constraints, if we fail to negotiate favorable pricing or if we experience any other interruption or delay in the supply chain which interferes with our ability to manufacture our products or manage our inventory levels.
We may lose customers or sales if we are unable to meet customer demand for our products on a timely and cost-effective basis, or if we are unable to ensure the proper performance and quality of our products.
We produce our products in an innovative and complicated manufacturing process which has the potential for significant variability in manufacturing yields. We have encountered, and may in the future encounter, difficulties in manufacturing our products and, due to the complexity of our products and our manufacturing process, we may experience delays in the manufacture of our products or fail to ensure their proper performance or quality. As we develop new and enhanced products, we must be able to resolve in a timely, cost-effective manner manufacturing issues that may arise from time to time.
We base our manufacturing capabilities on our forecasted product mix for the quarter. If the actual product mix varies significantly from our forecast, we may not be able to fill some orders during that quarter, which could adversely impact our financial results. Difficulties in meeting customer, collaborator and internal demand could also cause us to lose customers or require us to delay new product introductions, which could in turn result in reduced demand for our products.
We rely on internal quality control procedures to verify our manufacturing processes. Due to the complexity of our products and manufacturing process, however, it is possible that products that do not meet all of our performance specifications may not be identified before they are shipped. If our products do not consistently meet our customers’ performance expectations, demand for our products will decline. In addition, we do not maintain any backup manufacturing capabilities for the production of our products. Any interruption in our ability to continue operations at our existing manufacturing facilities could delay our ability to develop or sell our products, which could result in lost revenue and seriously harm our business, financial condition and results of operations.
We may need to adjust our manufacturing capacity based on business requirements or improvements made to our technological capabilities and there are risks associated with such adjustment.
If demand for our products is reduced or if we implement technologies that increase the density or yields of our wafers, our manufacturing capacity could be under-utilized and some of our long-lived assets, including facilities and equipment, may be impaired, which would increase our expenses. In addition, factory planning decisions may shorten the useful lives of long-lived assets including facilities and equipment, and cause us to accelerate depreciation. These changes in demand for our products, and changes in our customers’ product needs, could have a variety of negative effects on our competitive position and our financial results, and, in certain cases, may reduce our revenue, increase our costs, lower our gross margin percentage or require us to recognize impairments of our assets. In addition, if demand for our products is reduced or we fail to accurately forecast demand, we could be required to write down inventory since certain of our products have a limited shelf life, which would have a negative impact on our gross margin.
We have in the past, and may in the future, adjust our manufacturing capacity based on business requirements, which may include the rationalization of our facilities, including the abandonment of long-lived manufacturing assets and additional charges related to a reduction in capacity. In 2008, we implemented a restructuring plan that included the closure of our West Sacramento, California facility and the consolidation of our manufacturing. This restructuring was completed in the second quarter of 2009. Manufacturing and product quality issues may arise as we launch new products in our Singapore and Ohio facilities and rely increasingly upon manufacturing by third parties. We may lose customers if we are unable to manufacture products or if we experience delays in the manufacture of our products as a result of this transition.
We may not be able to deliver acceptable products to our customers due to the rapidly evolving nature of genetic sequence information upon which our products are based.
The genetic sequence information upon which we rely to develop and manufacture our products is contained in a variety of databases throughout the world. These databases are rapidly expanding and evolving. In addition, the accuracy of these databases and resulting genetic research is dependent on various scientific interpretations and it is not expected that global genetic research efforts will result in standardized genetic sequence databases for particular genomes in the near future.
Although we have implemented ongoing internal quality control efforts to help ensure the quality and accuracy of our products, the fundamental nature of our products requires us to rely on genetic sequence databases and scientific interpretations which are continuously evolving. As a result, these variables may cause us to develop and manufacture products that incorporate sequence errors or ambiguities. The magnitude and importance of these errors will depend upon multiple and complex factors that would be considered in determining the appropriate actions required to remedy any inaccuracies. Our inability to timely deliver acceptable products as a result of these factors would likely adversely affect our relationship with customers, and could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Operations
We may not achieve sustained profitability.
Prior to 2002, we incurred losses each year since our inception, and we reported losses in 2006 and 2008 through 2010. As a result, we had an accumulated deficit of approximately $450.5 million as of December 31, 2010. Our ability to achieve sustained profitability will depend, in part, on the rate of growth, if any, of our revenue and on the level of our expenses. We expect to continue incurring significant expenses related to research and development, sales and marketing efforts to commercialize our products, litigation and non-cash stock based compensation, and we expect to continue to experience fluctuations in our operating results. If our revenue grows more slowly than we anticipate, or if our operating expenses increase more than we expect or cannot be reduced in the event of lower revenue, we may not become profitable on a sustained basis, or at all.
If we do not attract and retain key employees, our business could be impaired.
To be successful, we must attract and retain qualified scientific, engineering, manufacturing, sales, marketing and management personnel. To expand our research, product development and sales efforts we need additional people skilled in areas such as bioinformatics, organic chemistry, information services, regulatory affairs, manufacturing, sales, marketing and technical support. Competition for these people is intense, and our compensation arrangements, such as our equity award programs, may not always be successful in attracting new employees and retaining and motivating our existing employees. For example, our stock price has been volatile in the past three years, resulting in a significant number of stock options granted to our employees having a strike price that is higher than the current trading price of our common stock. If we are unable to hire, train and retain a sufficient number of qualified employees, we will not be able to expand our business or our business could be adversely affected.
We also rely on our scientific advisors and consultants to assist us in formulating our research, development and commercialization strategy. All of these individuals are engaged by other employers and have commitments to other entities that may limit their availability to us.
Due to the international nature of our business, political or economic changes or other factors could harm our business.
A significant amount of our revenue is currently generated from sales outside the United States. Although such transactions are primarily denominated in both U.S. dollars and foreign currencies, our future revenue, gross margin, expenses and financial condition are still affected by such factors as changes in foreign currency exchange rates; unexpected changes in, or impositions of, legislative or regulatory requirements, including export and trade barriers and taxes; longer payment cycles and greater difficulty in accounts receivable collection.
We also are subject to general geopolitical risks in connection with international operations, such as political, social and economic instability, potential hostilities, epidemics and changes in diplomatic and trade relationships. We cannot assure investors that one or more of the foregoing factors will not have a material adverse effect on our business, financial condition and operating results or require us to modify our current business practices.
Our effective tax rate may vary significantly.
Our operations are subject to income and transaction taxes in the United States and in multiple foreign jurisdictions. Estimates and judgments are required in determining our worldwide provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. The ultimate amount of tax liability may be uncertain as a result.
Changes in overall levels and the geographic mix of pretax earnings may adversely impact our effective tax rate. Certain jurisdictions have lower tax rates, and the amount of earnings in these jurisdictions may fluctuate. If we do not have profitable operations in these jurisdictions, our effective tax rate could be adversely impacted. Changes in tax laws, applicability of tax holidays and regulatory requirements in the countries in which we operate could have a material impact on our tax provision. To the extent that we are unable to continue to reinvest a substantial portion of our profits in our foreign operations, we may be subject to effective income tax rate increases in the future. Tax authorities may challenge the allocation of profits between our subsidiaries and we may not prevail in any such challenge. If we were not to prevail, we could be subject to higher tax rates or double tax.
Estimates are required in determining any valuation allowance to be recorded against our net deferred tax assets. Changes in the amount of valuation allowance required may significantly impact our financial results of operations.
Risks Related to Our Investments
Our strategic equity investments may result in losses.
We periodically make strategic equity investments in various public and private companies with businesses or technologies that may complement our business. The market values of these strategic equity investments may fluctuate due to market conditions and other conditions over which we have no control. Other-than-temporary declines in the market price and valuations of the securities that we hold in other companies would require us to record losses relative to our ownership interest. This could result in future charges to our earnings. It is uncertain whether or not we will realize any long-term benefits associated with these strategic investments.
Global credit and financial market conditions could negatively impact the value of our current portfolio of cash equivalents or short-term investments and our ability to meet our financing objectives.
Our cash and cash equivalents are maintained in highly liquid investments with remaining maturities of 90 days or less at the time of purchase. Our short-term investments consist primarily of readily marketable debt securities with remaining maturities of more than 90 days at the time of purchase. While as of the date of this filing, we are not aware of any downgrades, material losses, or other significant deterioration in the fair value of our cash equivalents or short-term investments since December 31, 2010, any significant deterioration in conditions of the global credit and financial markets may negatively impact our current portfolio of cash equivalents or short-term investments or our ability to meet our financing objectives. Other-than-temporary declines in the market price and valuation of any of our short-term investments would require us to adjust the carrying value of the investment through an impairment charge.
Risks Related to Government Regulation and Litigation
We and our customers are subject to various government regulations, and we may incur significant expenses to comply with, and experience delays in our product commercialization as a result of, these regulations.
The FDA has jurisdiction over the commercialization of medical devices, including in vitro diagnostic test kits and the reagents and instrumentation used in these tests. In vitro diagnostic tests, reagents, and instruments may be subject to pre-market review and post-market controls by the FDA. Certain in-vitro diagnostic products must also be approved by the regulatory agencies of foreign governments or jurisdictions before the product can be sold outside the United States. Commercialization of our and our collaborative partners’ in-vitro diagnostic products outside of the research environment may depend upon successful completion of clinical trials. Clinical development is a long, expensive and uncertain process and we do not know whether we, or any of our collaborative partners, will be permitted to undertake clinical trials of any potential in-vitro diagnostic products. It may take us or our collaborative partners many years to complete any such testing, and failure can occur at any stage. Delays or rejections of potential products may be encountered based on changes in regulatory policy during the period of product development and regulatory agency review. Moreover, if and when our projects reach clinical trials, we, or our collaborative partners, may decide to discontinue development of any or all of these projects at any time for commercial, scientific or other reasons. Any of the foregoing matters could have a material adverse effect on our business, financial condition and results of operations.
Many of our products are labeled for “research use only”. Products intended for research use only are not subject to clearance or approval by the Food and Drug Administration. However, research use only products may fall under the FDA’s jurisdiction if these are used for clinical rather than research purposes. Even when a product is exempted from FDA clearance or approval, the FDA may impose restrictions as to the types of customers to which we can market and sell our products. Such restrictions may materially and adversely affect our business, financial condition and results of operations.
The FDA, the U.S. Department of Health and Human Services and foreign government regulators are increasingly focused on genetic analysis tools, including the use of arrays, which are labeled for research use only, by clinical laboratories in laboratory-developed tests (“LDTs”) offered by these laboratories, including labs certified under the Clinical Laboratory Improvement Amendments (“CLIA”). We cannot predict the extent of the FDA’s future efforts in regulation and enforcement policies with respect to the sale and use of arrays for the development of LDTs by CLIA-certified laboratories. If regulations or enforcement policies restrict our customers’ development of LDTs using our products labeled for research use only, or if we otherwise are required to obtain FDA premarket clearance or approval prior to commercializing these products, our ability to generate revenue from the sale of our products may be delayed or otherwise adversely affected. Moreover, our failure to comply with governmental rules and regulations related to our products could cause us to incur significant adverse publicity, subject us to investigations and notices of non-compliance or lead to fines or restrictions upon our ability to sell our products. We also may be at risk for liability related to government reimbursement of tests involving the use of our products if it is determined that these tests require FDA-clearance or approval and no such clearance or approval has been obtained.
Medical device laws and regulations are also in effect in many countries, ranging from comprehensive device approval requirements to requests for product data or certifications. The number and scope of these requirements are increasing. We may not be able to obtain regulatory approvals in such countries or may incur significant costs in obtaining or maintaining our foreign regulatory approvals. In addition, the export by us of certain of our products which have not yet been cleared for domestic commercial distribution may be subject to FDA or other export restrictions.
We have agreements relating to the sale of our products to government entities and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government. A failure to comply with these regulations might result in suspension of these contracts or administrative or other penalties, and could have a material adverse effect on our ability to compete for future government grants, contracts and programs.
Healthcare reform and restrictions on reimbursements may limit our returns on molecular diagnostic products that we may develop with our collaborators.
We are currently collaborating with our partners to develop diagnostic and therapeutic products. The ability of our collaborators to commercialize such products may depend, in part, on the extent to which reimbursement for these products will be available under U.S. and foreign regulations that govern reimbursement for clinical testing services by government authorities, private health insurers and other organizations. In the United States, third-party payer price resistance, the trend towards managed health care and the implementation of the Patient Protection and Affordable Care Act of 2010 could reduce payment rates for health care products and services, adversely affecting the profits of our customers and collaborative partners and reducing our future royalties. Under Medicare rules, diagnostic tests must be ordered by a physician who is treating the beneficiary and who uses the test results in patient management. Under this rule, some Medicare contractors may deny coverage for a test, even if the test has been cleared or approved by the FDA, without proof, as determined sufficient by the contractor, that the test is useful in patient management.
We face risks related to handling of hazardous materials and other regulations governing environmental safety.
Our operations are subject to complex and stringent environmental, health, safety and other governmental laws and regulations that both public officials and private individuals may seek to enforce. Our activities that are subject to these regulations include, among other things, our use of hazardous and radioactive materials and the generation, transportation and storage of waste. We could discover that we or an acquired business is not in material compliance. Existing laws and regulations may also be revised or reinterpreted, or new laws and regulations may become applicable to us, whether retroactively or prospectively, that may have a negative effect on our business and results of operations. It is also impossible to eliminate completely the risk of accidental environmental contamination or injury to individuals. In such an event, we could be liable for any damages that result, which could adversely affect our business.
We may be exposed to liability due to product defects.
The risk of product liability claims is inherent in the testing, manufacturing, marketing and sale of human diagnostic and therapeutic products and we may be subjected to such claims. We may seek to acquire additional insurance for clinical or product liability risks. We may not be able to obtain such insurance or general product liability insurance on acceptable terms or in sufficient amounts. A product liability claim or recall could have a serious adverse effect on our business, financial condition and results of operations.
Ethical, legal and social concerns surrounding the use of genetic information could reduce demand for our products.
Genetic testing has raised ethical issues regarding privacy and the appropriate uses of the resulting information. For these reasons, governmental authorities may call for limits on or regulation of the use of genetic testing or prohibit testing for genetic predisposition to certain conditions, particularly for those that have no known cure. Similarly, such concerns may lead individuals to refuse to use genetics tests even if permissible. Any of these scenarios could reduce the potential markets for our molecular diagnostic products, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Intellectual Property
We may be unable to effectively protect or enforce our intellectual property, which could harm our competitive position.
Maintaining a strong patent position is critical to our business. Patent law relating to the scope of claims in the technology fields in which we operate is uncertain, so we cannot be assured the patent rights we have or may obtain will be valuable. Others have filed, and in the future are likely to file, patent applications that are similar or identical to ours or those of our licensors. To determine the priority of inventions, we may have to participate in interference proceedings declared by the United States Patent and Trademark Office that could result in substantial costs in legal fees and could substantially affect the scope of our patent protection. We cannot be assured our patent applications will have priority over those filed by others. Also, our intellectual property may be subject to significant administrative and litigation proceedings such as opposition proceedings against our patents in Europe, Japan and other jurisdictions.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or interferences against those that have infringed on our patents, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
In addition to patent protection, we also rely upon copyright and trade secret protection, as well as non-disclosure agreements with our employees, consultants and third-parties, to protect our confidential and proprietary information. Such measures may not provide adequate protection for our proprietary information.
Litigation or other proceedings or third party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling our products or services or impact our stock price.
Third parties have asserted and may in the future assert that we are employing their proprietary technology without authorization. As we launch new products and enter new markets, we expect that competitors will claim that our products infringe their intellectual property rights as part of business strategies designed to impede our successful commercialization and entry into new markets. We are currently engaged in litigation with third parties who allege that we have infringed their intellectual property rights, including a lawsuit in which our competitor Illumina alleges that the manufacture and sale of certain of our new peg-based arrays and related instruments infringe two of their patents. See Note 10. “Commitments and Contingencies” to our Condensed Consolidated Financial Statements elsewhere in this Annual Report on Form 10-K for further information. In addition, we are aware of third-party patents that may relate to our technology. We routinely receive notices claiming infringement from third parties as well as invitations to take licenses under third party patents. Third parties may have obtained, and may in the future obtain, patents allowing them to claim that the use of our technologies infringes these patents.
We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a material adverse impact on our stock price, which may be disproportionate to the actual import of the ruling itself. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and obtain one or more licenses from third parties, or be prohibited from selling certain products. In addition, we may be unable to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our ability to grow and maintain profitability.
Risks Related to Our Common Stock
The market price of our common stock has been volatile.
The market price of our common stock is volatile. Our common stock traded as low as $3.75 and as high as $8.38 per share during the twelve-month period ending December 31, 2010. Moreover, the daily volume of our common stock fluctuated from 182,300 to 15,386,500 shares during that period. Our stock price may be affected by a number of factors, including those listed in these “Risk Factors” and other, unknown factors. Our stock price also may be affected by: comments by securities analysts regarding our business or prospects; our inability to meet analysts’ expectations; general fluctuations in the stock market or in the stock prices of our industry peers or our customers; and, general conditions and publicity regarding the genomics, biotechnology, pharmaceutical or life science industries.
Volatility in the stock price of other companies often has led to securities class action litigation against those companies. Any future securities litigation against us could result in substantial costs and divert management’s attention and resources, which could seriously harm our business, financial condition and results of operations.
Our quarterly results have historically fluctuated significantly and may continue to do so. Failure to meet financial expectations may disappoint securities analysts or investors and result in a decline in our stock price.
Our revenue and operating results may fluctuate significantly due in part to factors that are beyond our control and which we cannot predict. The timing of our customers’ orders may fluctuate from quarter to quarter. Historically, we have experienced customer ordering patterns for instrumentation and consumables in which the majority of the shipments occur in the last month of the quarter. These ordering patterns may limit management’s ability to accurately forecast our future revenue or product mix. Additionally, license revenue may also be unpredictable and may fluctuate due to the timing of payments of non-recurring licensing fees. Because our expenses are largely fixed in the short to medium term, any material shortfall in revenue may cause us to experience material losses.
Because of this difficulty in predicting future performance, our operating results may fall below our own expectations and the expectations of securities analysts or investors in some future quarter or quarters. Our failure in the past to meet these expectations has adversely affected the market price of our common stock and may continue to do so.
In addition to factors that affect the spending levels of our customers described above, additional factors could cause our operating results to fluctuate, including:
|
·
|
competition;
|
|
·
|
our inability to produce products in sufficient quantities and with appropriate quality;
|
|
·
|
the frequency of experiments conducted by our customers;
|
|
·
|
our customers’ inventory of products;
|
|
·
|
the receipt of relatively large orders with short lead times; and
|
|
·
|
our customers’ expectations as to how long it takes us to fill future orders.
|
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our corporate headquarters is located in Santa Clara, California, where we lease approximately 200,000 square feet and we have manufacturing facilities located in Singapore and Cleveland, Ohio, where we lease approximately 150,000 square feet and 15,000 square feet, respectively. We lease approximately 140,000 square feet of administrative and research and development space in California (Emeryville and Sunnyvale), Ohio (Cleveland and Maumee), China (Shanghai), Germany (Freiburg), Japan (Osaka and Tokyo) and the United Kingdom (Wooburn Green). We have also entered into agreements to sublease to third parties approximately 90,000 square feet of administrative and research and development space in California (Sacramento) and Massachusetts (Bedford). Additionally, we own a 170,000 square foot facility in West Sacramento, California that we have leased to a charter school. We believe that our existing properties are in good condition and are suitable for the conduct of our business.
ITEM 3. LEGAL PROCEEDINGS
Information pertaining to legal proceedings can be found in “Item 8. Financial Statements and Supplementary Data—Note 10. Commitments and Contingencies” of this Annual Report on Form 10-K, and is incorporated by reference herein.
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
No matters were submitted during the fourth quarter of the year ended December 31, 2010.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER REPURCHASES OF EQUITY SECURITIES
Our common stock is traded on The Nasdaq Global Select Market under the symbol of AFFX. The following table sets forth on a per share basis, for the periods indicated, the low and high closing prices of our common stock as reported by The Nasdaq Global Select Market.
|
Low
|
High
|
|||||||
|
2010
|
||||||||
|
First Quarter
|
$ | 5.28 | $ | 7.98 | ||||
|
Second Quarter
|
$ | 5.81 | $ | 8.33 | ||||
|
Third Quarter
|
$ | 3.80 | $ | 5.86 | ||||
|
Fourth Quarter
|
$ | 4.16 | $ | 5.28 | ||||
|
2009
|
||||||||
|
First Quarter
|
$ | 1.78 | $ | 4.06 | ||||
|
Second Quarter
|
$ | 3.23 | $ | 6.82 | ||||
|
Third Quarter
|
$ | 5.04 | $ | 9.50 | ||||
|
Fourth Quarter
|
$ | 4.69 | $ | 9.83 | ||||
As of February 22, 2010, there were approximately 318 holders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company (“DTC”). All of the shares of common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC and therefore are considered to be held of record by Cede & Co. as one shareholder.
No cash dividends have been paid on our common stock. We currently intend to retain all future earnings, if any, for use in our business and do not anticipate paying any cash dividends on our common stock in the foreseeable future.
No equity securities were sold during 2010 that were not registered under the Securities Act of 1933, as amended (the “Securities Act”). We did not repurchase any shares of our common stock during the fourth quarter of 2010.
For information regarding compensation plans under which equity securities were authorized for issuance, see the section of the Proxy Statement to be filed in connection with our 2011 Annual Meeting of Shareholders entitled “Equity Compensation Plan Information,” incorporated by reference into Item 12 of this Annual Report on Form 10-K.
Performance Graph
The graph below compares the cumulative total return* on our common stock for the period commencing on December 31, 2005 and ending December 31, 2010 compared to the CRSP Total Return Index for the Nasdaq National Market (U.S. companies) and the CRSP Total Return Index for the Nasdaq Pharmaceutical Stocks (SIC 283). The stock price performance shown on the graph below is not necessarily indicative of future price performance.
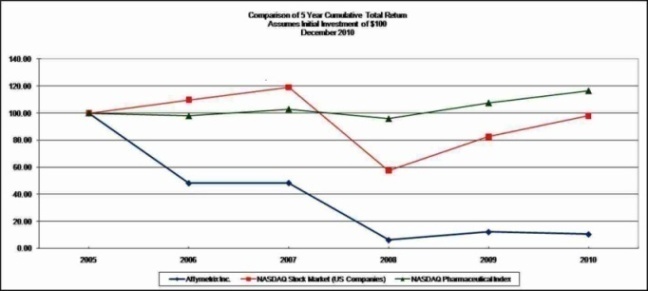
|
|
*
|
Assumes $100 invested on December 31, 2005 in our common stock and in each index listed above. The total return for our common stock and the indices used assumes the reinvestment of dividends, even though dividends have never been declared on our common stock.
|
The information under the caption “Performance Graph” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of Affymetrix under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (the “Exchange Act”), whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation language in such filings.
ITEM 6. SELECTED FINANCIAL DATA
The following selected historical consolidated financial information has been derived from our audited consolidated financial statements. The information below is not necessarily indicative of our future results of operations and should be read in conjunction with Item 1A, “Risk Factors,” Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and Item 8, “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K in order to fully understand the factors that may affect the comparability of the information presented below:
|
Year Ended December 31,
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
Consolidated Statement of Operations Data:
|
(in thousands, except per share amounts)
|
|||||||||||||||||||
|
Total revenue
|
$ | 310,746 | $ | 327,094 | $ | 410,249 | $ | 371,320 | $ | 355,317 | ||||||||||
|
(Loss) income from operations
|
(5,167 | ) | (33,158 | ) | (242,539 | ) | 6,080 | (18,545 | ) | |||||||||||
|
Net (loss) income (1)
|
$ | (10,233 | ) | $ | (23,909 | ) | $ | (307,919 | ) | $ | 12,593 | $ | (13,704 | ) | ||||||
|
Basic net (loss) income per common share
|
$ | (0.15 | ) | $ | (0.35 | ) | $ | (4.49 | ) | $ | 0.18 | $ | (0.20 | ) | ||||||
|
Diluted net (loss) income per common share
|
$ | (0.15 | ) | $ | (0.35 | ) | $ | (4.49 | ) | $ | 0.17 | $ | (0.20 | ) | ||||||
|
Consolidated Balance Sheet Data:
|
||||||||||||||||||||
|
Cash, cash equivalents, and available-for-sale securities
|
$ | 237,184 | $ | 346,574 | $ | 397,739 | $ | 584,274 | $ | 247,752 | ||||||||||
|
Working capital
|
159,932 | 345,486 | 420,768 | 583,067 | 290,302 | |||||||||||||||
|
Total assets (2)
|
460,785 | 630,950 | 713,310 | 1,133,591 | 781,215 | |||||||||||||||
|
Long-term obligations (3)(4)
|
111,821 | 261,394 | 330,896 | 451,143 | 134,662 | |||||||||||||||
|
(1)
|
During 2010 and 2009, we repurchased $151.7 million and $69.1 million, respectively, of our 3.50% senior convertible notes and the resulting gain is labeled as “Gain from repurchase of convertible notes” in a single line item in our Consolidated Statements of Operations (See (4) for further details):
|
|
·
|
In 2010, we recognized a net gain of $6.3 million on repurchases totaling $151.7 million in aggregate principal amount; and
|
|
·
|
In 2009, we recognized a net gain of $17.4 million on the repurchase of $69.1 million in aggregate principal amount.
|
|
|
In 2008, we recognized a goodwill impairment charge of $239.1 million that is presented in a single line item labeled “Goodwill impairment (credit) charges” in our Consolidated Statements of Operations as well as an income tax provision of $65.9 million primarily resulting from a full valuation allowance recorded against all U.S. deferred tax assets.
|
|
|
Additionally, we recognized $2.2 million, $43.7 million and $15.3 million in 2009, 2008 and 2007, respectively, of expense related to our restructuring plans that was presented in a single line item labeled “Restructuring charges” in our Consolidated Statements of Operations.
|
|
(2)
|
In 2008, we completed the acquisitions of USB Corporation (“USB”), True Materials, Inc. (“TMI”), and Panomics, Inc. for aggregate cash consideration of $163.0 million.
|
|
(3)
|
In 2007, we issued $316.3 million aggregate principal amount of 3.50% senior convertible notes.
|
|
(4)
|
In 2010 and 2009, we partially repurchased our 3.50% convertible notes:
|
|
·
|
In 2010, a total of $151.7 million aggregate principal amount was purchased for cash consideration of $143.6 million, including accrued interest and transaction costs; and
|
|
·
|
In 2009, $69.1 million aggregate principal amount was purchased for cash consideration of $50.6 million, including accrued interest and transactions costs.
|
|
|
In 2008, a total of $119.9 million aggregate principal amount of our 0.75% senior convertible notes was redeemed for cash as investors exercised their put right. We repurchased an additional $0.1 million aggregate principal amount of such notes in 2009.
|
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with the consolidated financial statements and the related notes that appear elsewhere in this document.
The purpose of the following discussion and analysis is to provide an overview of the business to help facilitate an understanding of significant factors influencing our historical operating results, financial condition and cash flows and also to convey our expectations of the potential impact of known trends, events, or uncertainties that may impact our future results. The discussion and analysis in this Annual Report on Form 10-K contains forward-looking statements that involve risks and uncertainties, such as statements of our plans, strategies, objectives, expectations, intentions and adequacy of resources. Forward-looking statements are subject to known and unknown risks and uncertainties and are based on potentially inaccurate assumptions that could cause actual results to differ materially from those expected or implied by the forward looking statements. Words such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project” or similar words or phrases, or the negatives of these words, may identify forward-looking statements, but the absence of these words does not necessarily mean that a statement is not forward looking. Examples of forward-looking statements include, among others, statements regarding the integration of our acquired technologies with our existing technology, the commercial launch of new products and the duration which our existing cash and other resources is expected to fund our operating activities. This discussion should be read in conjunction with the other sections of this Annual Report on Form 10-K, including “Item 1: Business”; “Item 1A: Risk Factors”; “Item 6: Selected Financial Data”; and “Item 8: Financial Statements and Supplementary Data.”
Overview
We develop, manufacture and sell products and services for genetic analysis to the life science research and clinical healthcare markets. Researchers around the world use our technology to better understand the role that genes play in disease, the effectiveness and safety of therapies and many other biological factors that affect human well-being. We sell our products to some of the world’s largest pharmaceutical, diagnostic and biotechnology companies, as well as leading academic, government and not-for-profit research institutions. Approximately 23,000 peer-reviewed papers have been published based on work using our products. We have more than 900 employees worldwide and maintain sales and distribution operations across the United States, Europe and Asia.
We offer a comprehensive line of products for two principal applications: gene expression and genotyping. Our product sales consist primarily of sales of instruments and related consumables. We have three instrument systems, GeneTitan®, GeneChip® and GeneAtlas™, that include instruments, consumables and software. Our GeneChip® instruments run arrays packaged in cartridges and our GeneTitan® and GeneAtlasTM instruments run arrays packaged in a peg format.
We also offer a variety of assays for gene expression targeting low- to mid-plex markets that are downstream of our whole genome arrays and a range of reagent kits that are compatible with our platforms as well as the products of other vendors.
Overview of Fiscal Year 2010 and Strategic Initiatives
In 2010, we continued to focus our efforts on meeting the three strategic initiatives that we laid out in 2009. We achieved our goal of improved operating leverage, earning a net profit during the second half of the year ended December 31, 2010, including a profit from operations in the fourth quarter of $5.2 million, and generated positive operating cash flows in each quarter of 2010. In addition, we significantly reduced total operating expenses in 2010 as compared to 2009 and further decreased our future interest payments by repurchasing a portion of our 3.50% convertible notes due 2038. We released several new products and began entering into important new markets that we believe have potential for success in the future.
Below is a summary of our progress on the three strategic initiatives which we remain focused on executing in 2011:
Revenue growth. Our goal is to expand our revenue base by entering new markets, growing our customer base and successfully commercializing our established and acquired technologies. We launched several important new products in 2010, including our Axiom Genotyping Platform that enables the design of catalog and custom array designs for the study of genetic variation associated with disease, health and the environment in humans and other model organisms. We also introduced a new service level offering, OncoScan FFPE Express, that target the analysis of challenging formalin-fixed, paraffin-embedded tissue. Using OncoScan, molecular pathologists and ocologists are able to assess relevant cancer measurements in one assay, ultimately accelerating our understanding of, and approach to, treating cancer.
In 2011, we are looking to further shift our focus to the validation and routine testing markets which we believe are currently expanding at a higher compound annual growth rate than the discovery and exploration markets and will provide opportunities for more recurring revenue growth in the future.
Profitability. Throughout 2010, we remained focused on improving our operating leverage and were successful in achieving operating income in the second half of 2010. We have significantly reduced our future interest payments by repurchasing a portion of our 3.50% senior convertible notes due 2038 and significantly lowered our operating expenses in 2010 as compared to 2009. Our new array plate consumables that run on the GeneTitan® and GeneAtlasTM Systems also have lower manufacturing costs than the cartridge-based consumables.
Development of new technologies and platforms. We seek to expand our product line with new products that combine automated instrumentation, powerful new biological assays, and new array designs and content. For example, the GeneTitan® System, our mid- to high-throughput instrumentation platform, enables increased efficiency and throughput for researchers conducting array-based experiments. In 2010, we commercially released our GeneAtlasTM System, our low-to-medium throughput instrumentation platform, which targets new users of microarray technology. With the GeneTitan® and GeneAtlasTM instruments, we also introduced a menu of gene expression and genotyping consumables in the array plate format.
CRITICAL ACCOUNTING POLICIES & ESTIMATES
General
The following section of Management’s Discussion and Analysis of Financial Condition and Results of Operations is based upon our consolidated financial statements, which have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“US GAAP”). The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are fully described in “Item 8. Financial Statements and Supplementary Data—Note 2. Summary of Significant Accounting Policies.” However, certain accounting policies are particularly important to the reporting of our financial position and results of operations and require the application of significant judgment by our management. An accounting policy is deemed to be critical if it requires an accounting estimate to be made based on assumptions about matters that are highly uncertain at the time the estimate is made, and if different estimates that reasonably could have been used, or changes in the accounting estimates that are reasonably likely to occur periodically, could materially impact the financial statements. Management believes the following critical accounting policies reflect its more significant estimates and assumptions used in the preparation of the consolidated financial statements.
REVENUE RECOGNITION
We enter into contracts to sell our products and, while the majority of our sales agreements contain standard terms and conditions, there are agreements that contain multiple elements or non-standard terms and conditions. As a result, significant contract interpretation is sometimes required to determine the appropriate accounting, including whether the deliverables specified in a multiple element arrangement should be treated as separate units of accounting for revenue recognition purposes, and if so, how the value of the arrangement should be allocated among the deliverable elements, when and how to recognize revenue for each element, and the period over which revenue should be recognized.
Effective January 1, 2010, we early adopted the recently revised accounting guidance on revenue recognition for multiple element arrangements on a prospective basis, which requires us to allocate consideration to all deliverables at the inception of the arrangement using the relative selling price method. The relative selling price method establishes the relative selling price of a deliverable using a hierarchy, first through vendor-specific objective evidence (“VSOE”), second through third-party evidence if VSOE is not available and finally, through estimated selling prices if neither VSOE nor third-party evidence is available. Additionally, the revised accounting guidance also refined the criteria for determining when a deliverable should be accounted for as a separate unit of accounting. Based on this guidance, we identify separate units of accounting for a multiple element arrangement if the delivered item has value to the customer on a standalone basis and, when a general right of return exists, the delivery or performance of an undelivered item is considered probable and under our control. Changes in the allocation of the sales price from delivered to undelivered elements might impact the timing of revenue recognition, but would not change the total revenue recognized on any arrangement.
We generally have been able to determine the selling price of each deliverable in a multiple element arrangement based on the price for such deliverable when it is sold separately. If VSOE is not determinable and when third-party evidence is not available, we would use the estimated selling price of a deliverable which is determined based on several factors, including, but not limited to, the cost to produce the deliverable, the expected margin on that deliverable, our ongoing pricing strategy and policies, the selling price and profit margin of similar deliverables and value-added components of differentiated deliverables, if determinable.
The change in accounting principle for revenue recognition on multiple element arrangements did not have a material impact on our financial results for the year ended December 31, 2010. We anticipate that the effect on the change in accounting principle on subsequent periods will be primarily dependent on the arrangements entered into, the ability to estimate selling prices when VSOE cannot be established and the timing of the delivery of the products and services. Additionally, had the new accounting guidance been applied for the year ended December 31, 2009, there would have been no material impact on the revenue recognized.
INVENTORIES
We enter into inventory purchases and commitments so that we can meet future shipment schedules based on forecasted demand for our products. The business environment in which we operate is subject to rapid changes in technology and customer demand. We perform a detailed assessment of inventory each period, which includes a review of, among other factors, demand requirements, product life cycle and development plans, component cost trends, product pricing, product expiration and quality issues. Based on this analysis, we record adjustments to inventory for potentially excess, obsolete or impaired goods, when appropriate, in order to report inventory at net realizable value. Revisions to our inventory adjustments may be required if actual demand, component costs, supplier arrangements, or product life cycles differ from our estimates. Any such adjustments or revised adjustments could result in a charge to our results of operations.
NON-MARKETABLE EQUITY SECURITIES
As part of our strategic efforts to gain access to potential new products and technologies, we invest in equity securities of certain private biotechnology companies. These investments are included in other assets in our Consolidated Balance Sheets and are carried at cost. We also invest in a limited partnership investment fund that is accounted for under the equity method. We periodically review our investments for impairment; however, the impairment analysis requires significant judgment in identifying events or circumstances that would likely have significant adverse effect on the fair value of the investment. The analysis may include assessment of the investee’s (i) revenue and earnings trend, (ii) business outlook for its products and technologies, (iii) liquidity position and the rate at which it is using its cash, and (iv) likelihood of obtaining subsequent rounds of financing. If an investee obtains additional funding at a valuation lower than our carrying value, we presume that the investment is other than temporarily impaired. We have experienced impairments in our portfolio due to the decline in the value of certain of our non-marketable investments over the past few years.
INCOME TAXES
Income tax expense is based on pretax financial accounting income. Under the asset and liability method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. We must assess the likelihood that the resulting deferred tax assets will be realized. To the extent we believe that realization is not more likely than not, we establish a valuation allowance. Significant estimates are required in determining our provision for income taxes, our deferred tax assets and liabilities, and any valuation allowance to be recorded against our deferred tax assets. Some of these estimates are based on interpretations of existing tax laws or regulations. We believe that our estimates are reasonable and that our reserves for income tax related uncertainties are adequate. Various internal and external factors may have favorable or unfavorable effects on our future effective tax rate. These factors include, but are not limited to, changes in overall levels, character, or geographical mix of pretax earnings, changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, changes in the valuation of our deferred tax assets or liabilities, future levels of research and development spending, nondeductible expenses, applicability of tax holidays, and ultimate outcomes of income tax audits.
The total amount of unrecognized tax benefits as of December 31, 2010 was approximately $20.8 million. If recognized, the amount of unrecognized tax benefits that would impact income tax expense is $2.2 million. As of December 31, 2010, we do not anticipate any material changes to the amount of unrecognized tax benefit during the next twelve months.
We classify interest and penalties related to tax positions as components of income tax expense. For the year ended December 31, 2010, the amount of accrued interest and penalties related to tax uncertainties was approximately $0.1 million for a total cumulative amount of $0.5 million of non-current income taxes payable as of December 31, 2010.
We file U.S. federal, state, and foreign income tax returns in jurisdictions with varying statutes of limitations. The 1992 through 2010 tax years generally remain subject to examination by federal and state tax authorities. In significant foreign jurisdictions, the 2006 through 2010 tax years generally remain subject to examination by their respective tax authorities.
CONTINGENCIES
We are subject to legal proceedings principally related to intellectual property matters. Based on the information available at the balance sheet dates, we assess the likelihood of any adverse judgments or outcomes to these matters, as well as potential ranges of probable losses. If losses are probable and reasonably estimable, we will record a reserve which may change in the future due to new developments in each matter.
ACCOUNTING FOR STOCK-BASED COMPENSATION
We account for employee stock-based compensation by estimating the fair value of our employee stock awards at the date of grant using the Black-Scholes option-pricing model, which requires the use of certain subjective assumptions. The most significant of these assumptions are our estimates of the expected term, volatility and forfeiture rates of the awards. The expected stock price volatility assumption was determined using a combination of historical and implied volatility of our common stock. We determined that blended volatility is more reflective of market conditions and a better indicator of expected volatility than historical volatility. The estimate of these key assumptions is based on historical information and judgment regarding market factors and trends. As required under the accounting rules, we review our valuation assumptions at each grant date and, as a result, we are likely to change our valuation assumptions used to value employee stock-based awards granted in future periods.
US GAAP requires that employee stock-based compensation costs be recognized over the requisite service period, or the vesting period, in a manner similar to all other forms of compensation paid to employees. Accordingly, in 2010, we recognized employee stock-based compensation of $9.9 million, which consisted of $1.0 million in cost of product sales, $2.1 million in research and development expense and $6.8 million in selling, general and administrative expenses. As of December 31, 2010, $15.5 million of total unrecognized compensation cost related to non-vested employee stock awards is expected to be recognized as future cost of sales and operating expenses over a weighted-average period of 2.7 years.
RESULTS OF OPERATIONS
The following discussion compares the historical results of operations for the years ended December 31, 2010, 2009 and 2008.
PRODUCT SALES
The components of product sales are as follows:
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Consumables
|
$ | 252,165 | $ | 255,660 | $ | 248,903 | $ | (3,495 | ) | $ | 6,757 | (1 | )% | 3 | % | |||||||||||||
|
Instruments
|
25,578 | 23,526 | 21,489 | 2,052 | 2,037 | 9 | 9 | |||||||||||||||||||||
|
Total product sales
|
$ | 277,743 | $ | 279,186 | $ | 270,392 | $ | (1,443 | ) | $ | 8,794 | (1 | ) | 3 | ||||||||||||||
Total product sales decreased $1.4 million or 1% in 2010 as compared to 2009. Consumables sales decreased primarily due to a decrease in the sales of our RNA reagents partially offset by an increase in sales of our DNA reagents. In both areas, sales of our lower price products have increased as a percentage of the total mix. Instrument sales grew in 2010 as compared to 2009, primarily due to the increased adoption of GeneTitan® and introduction of GeneAtlasTM families of instruments, which were introduced in late 2009 and early 2010, respectively, partially offset by declines in the volume of sales of older instruments.
Total product sales increased $8.8 million or 3% in 2009 as compared to 2008. Consumables sales increased as a result of a higher volume of sales of our RNA analysis chips and additional revenues from our acquisition of Panomics in December 2008. This was partially offset by a decline in volume of our DNA analysis chips and lower overall average selling prices, partially due to a shift in product mix to lower-priced products. Additionally, instruments revenue increased due to the introduction of the GeneTitan® family of products in 2009.
SERVICES
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Services
|
$ | 20,565 | $ | 39,563 | $ | 32,096 | $ | (18,998 | ) | $ | 7,467 | (48 | )% | 23 | % | |||||||||||||
Total services revenue decreased in 2010 as compared to 2009, primarily due to the completion in late 2009 of several genotyping projects that began in the fourth quarter of 2008, including the Wellcome Trust Case Consortium and the National Institutes of Health projects. We currently have not entered into any new significant contracts for other genotyping projects. We anticipate this trend to remain consistent in 2011.
Total services revenue increased in 2009 as compared to 2008 primarily due to the growth in our scientific services business associated with several genotyping projects as discussed above.
ROYALTY AND OTHER REVENUE
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Royalties and other revenue
|
$ | 12,438 | $ | 8,345 | $ | 107,761 | $ | 4,093 | $ | (99,416 | ) | 49 | % | (92 | )% | |||||||||||||
Royalties and other revenue increased in 2010 as compared to 2009 primarily due to the receipt of a non-recurring $4.8 million license payment in the fourth quarter of 2010.
Royalties and other revenue decreased in 2009 as compared to 2008 primarily due to a non-recurring $90 million intellectual property payment received in January 2008. Additionally, research revenue decreased by $4.1 million in 2009 as compared to 2008 as National Institutes of Health grants expired in 2009.
PRODUCT AND SERVICES GROSS MARGINS
|
Dollars in thousands
|
Dollar/Point
|
|||||||||||||||||||
|
Year ended December 31,
|
change from
|
|||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||
|
Total gross margin on product sales
|
$ | 160,359 | $ | 152,809 | $ | 143,483 | $ | 7,550 | $ | 9,326 | ||||||||||
|
Total gross margin on services
|
4,743 | 15,683 | 6,975 | (10,940 | ) | 8,708 | ||||||||||||||
|
Product gross margin as a percentage of products sales
|
58 | % | 55 | % | 53 | % | 3 | 2 | ||||||||||||
|
Service gross margin as a percentage of services
|
23 | % | 40 | % | 22 | % | (17 | ) | 18 | |||||||||||
Product gross margin increased in 2010 as compared to 2009 primarily due to a reduction in plant consolidation costs from $9.4 million in 2009 to $1.6 million in 2010, as the majority of the West Sacramento manufacturing facility closure took place in 2009. Additionally, favorable pricing and absorption in chips contributed to the increased product margin. These increases were partially offset by higher excess and obsolescence costs for products with finite lives. Additional excess and obsolescence costs may be incurred in the near term as we continue to assess our product portfolio.
The increase in product gross margin in 2009 as compared to 2008 is primarily due to favorable factory utilization from higher production volumes in arrays as a result of the plant consolidation to Singapore in 2009. These increases were partially offset by an unfavorable shift from higher margin array products to lower margin reagent products.
Service gross margin was impacted in 2009 by the Wellcome Trust Case Consortium and National Institutes of Health projects which had higher margins. The margin percentages normalized in 2010 and 2008 when we did not have revenue from the projects.
RESEARCH AND DEVELOPMENT EXPENSES
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Research and development
|
$ | 67,934 | $ | 77,358 | $ | 84,482 | $ | (9,424 | ) | $ | (7,124 | ) | (12 | )% | (8 | )% | ||||||||||||
Research and development expenses decreased in 2010 as compared to 2009 primarily due to savings in head count-related expenses and variable compensation of $5.7 million and decreased spending on supplies of $2.2 million as a result of cost-control measures we implemented.
The decrease in research and development expenses in 2009 of $7.1 million as compared to 2008 was primarily due to decreased spending for consulting and purchased services as grant research projects were completed during the year. Masks, chips and supplies also decreased as certain products were commercialized in 2009. Additionally, we recognized an intangible asset impairment charge of $3.2 million in 2008. The decrease during 2009 was partially offset by an increase in headcount-related expenses and variable compensation.
SELLING, GENERAL AND ADMINISTRATIVE EXPENSES
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Selling, general and administrative
|
$ | 114,773 | $ | 130,838 | $ | 127,161 | $ | (16,065 | ) | $ | 3,677 | (12 | )% | 3 | % | |||||||||||||
Selling, general and administrative expenses decreased in 2010 as compared to 2009 primarily due to lower compensation and benefits expenses of $11.0 million as a result of lower headcount and decreased variable compensation. Additionally, legal expenses decreased by $2.1 million primarily due to timing of legal proceedings with third-parties, consulting and purchased services decreased by $1.8 million as a result of various general and administrative cost reductions, and rent expense decreased by $1.4 million due primarily to the relocation of our Japan office and other site consolidation efforts.
The increase in selling, general and administrative expenses in 2009 of $3.7 million as compared to 2008 was primarily due to increases of $1.6 million in compensation and benefits expense, including commissions and variable compensation expenses, $3.4 million in legal expenses primarily resulting from increased litigation during the year, and $1.5 million in depreciation and amortization expense from intangible assets related to the Panomics acquisition. These increases were partially offset by decreases of $1.3 million from consulting and purchased services, due to lower advertising and travel expenses, and $1.4 million from headcount-related expenses in 2009 compared to 2008.
ACQUIRED IN-PROCESS TECHNOLOGY
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Acquired in-process technology
|
$ | - | $ | - | $ | 6,200 | $ | - | $ | (6,200 | ) | - | % | 100 | % | |||||||||||||
In 2008, for each of the three acquisitions we made, USB, TMI and Panomics, we recognized the estimated fair value of certain research and development programs in-process that had not yet reached technological feasibility and had no alternative future use, totaling $0.8 million, $5.1 million and $0.3 million, respectively. The fair values of these projects were determined using the income approach whereby we estimated each project’s related future net cash flows. The largest research and development program in-process at the acquisition date primarily was the microRNA profiling project, which we completed in 2009.
RESTRUCTURING
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Restructuring
|
$ | - | $ | 2,180 | $ | 43,707 | $ | (2,180 | ) | $ | (41,527 | ) | (100 | )% | (95 | )% | ||||||||||||
In 2008, we undertook a restructuring plan designed to improve our operating leverage, where we closed our West Sacramento manufacturing facility, moving the probe array manufacturing to our Singapore facility, consolidating reagent manufacturing to our Cleveland facility and outsourcing instrument manufacturing operations to third parties. We completed the closure of the West Sacramento facility during the second quarter of 2009 and no expenses were recognized during the year ended December 31, 2010, compared to $2.2 million of expense pertaining to employee severance and relocation benefits recognized during the year ended December 31, 2009. For the year ended December 31, 2008, we recognized $8.2 million of expenses related to employee termination benefits and $37.1 million of expenses related to the abandonment and impairment of certain manufacturing assets.
INTEREST INCOME AND OTHER, NET
The components of interest income and other, net, are as follows:
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Interest income
|
$ | 2,845 | $ | 4,455 | $ | 14,579 | $ | (1,610 | ) | $ | (10,124 | ) | (36 | )% | (69 | )% | ||||||||||||
|
Realized (loss) gain on equity investments, net
|
(4,342 | ) | (916 | ) | 1,201 | (3,426 | ) | (2,117 | ) | 374 | (176 | ) | ||||||||||||||||
|
Currency loss, net
|
(48 | ) | (2,800 | ) | (2,188 | ) | 2,752 | (612 | ) | (98 | ) | 28 | ||||||||||||||||
|
Other
|
58 | 1,850 | 1,037 | (1,792 | ) | 813 | (97 | ) | 78 | |||||||||||||||||||
|
Total interest income and other, net
|
$ | (1,487 | ) | $ | 2,589 | $ | 14,629 | $ | (4,076 | ) | $ | (12,040 | ) | (157 | ) | (82 | ) | |||||||||||
Interest income and other, net decreased in 2010 as compared to 2009 due to the following:
|
·
|
Interest income decreased by $1.6 million in 2010 as compared to 2009 due to lower average cash and investment balances as a result of the repurchase of a portion of our 3.50% convertible notes during 2010, partially offset by higher effective interest rates in 2010;
|
|
·
|
Realized loss in equity investments increased by $3.4 million in 2010 as we recognized $5.6 million of other-than-temporary impairment expense, largely as a result of our recognition of impairment charges on two non-marketable investments. This was partially offset by a net realized gain from our investment in a limited partnership investment fund of $1.3 million. In 2009, we recognized a net realized loss of $0.9 million as described below;
|
|
·
|
Currency loss decreased in 2010 by $2.8 million due to improved hedging activities in 2010; and
|
|
·
|
Other income decreased primarily due to a nonrecurring sale of equipment in 2009 totaling $0.9 million.
|
Interest income and other, net decreased in 2009 as compared to 2008 due to the following:
|
·
|
Interest income decreased by $10.1 million as a result of lower average cash and investment balances from the repurchase of our 3.50% convertible notes totaling $69.1 million, combined with lower effective interest rates in 2009 compared to 2008.
|
|
·
|
Net realized (loss) gain on investments decreased by $2.1 million in 2009 primarily due to other-than-temporary impairment expense of $1.1 million that we recognized on our nonmarketable securities portfolio, partially offset by net realized gains of $0.2 million in our investment in a limited partnership investment fund in 2009. This is compared to net realized gains of $1.2 million in our investment in a limited partnership investment fund in 2008.
|
INTEREST EXPENSE
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Interest expense
|
$ | 7,706 | $ | 10,945 | $ | 14,091 | $ | (3,239 | ) | $ | (3,146 | ) | (30 | )% | (22 | )% | ||||||||||||
Interest expense decreased in 2010 as compared to 2009 primarily due to a lower aggregate principal balance of our 3.50 % convertible notes as a result of our repurchases of totaling $151.7 million in aggregate principal amount during 2010.
Interest expense decreased in 2009 as compared to 2008 primarily due to lower aggregate principal balance of the senior convertible notes resulting from our repurchases of $69.1 million in aggregate principal amount of our 3.50% convertible notes in the second quarter of 2009 and $119.9 million in aggregate principal amount of our 0.75% convertible notes in the fourth quarter of 2008.
INCOME TAX PROVISION (BENEFIT)
|
Dollars in thousands
|
Dollar
|
Percentage
|
||||||||||||||||||||||||||
|
Year ended December 31,
|
change from
|
change from
|
||||||||||||||||||||||||||
|
2010
|
2009
|
2008
|
2009
|
2008
|
2009
|
2008
|
||||||||||||||||||||||
|
Income tax provision (benefit)
|
$ | 2,170 | $ | (158 | ) | $ | 65,918 | $ | 2,328 | $ | (66,076 | ) | (1,473 | )% | (100 | )% | ||||||||||||
The income tax provision in 2010 was approximately $2.2 million, which consisted primarily of foreign taxes. The income tax benefit in 2009 of $0.2 million consisted of foreign taxes similar to 2010 offset by refundable U.S. credits. The provision for income taxes in 2008 was approximately $65.9 million and consisted of foreign taxes, state taxes and the establishment of a full valuation allowance against our U.S. deferred tax assets, net of reserves for uncertain tax positions.
Deferred tax assets are recognized if realization of such assets is more likely than not. As of December 31, 2010, we provided for a valuation allowance of $142.6 million against our net deferred tax assets. As a result of negative evidence based on our cumulative net loss position, we have placed a full valuation allowance on U.S. and certain foreign deferred tax assets, net of reserves for uncertain tax positions. We intend to maintain the valuation allowance until sufficient positive evidence exists to assure realization of these tax benefits through future taxable income.
As of December 31, 2010, we had total net operating loss carryforwards of $259.8 million, comprised of $146.7 million for U.S. federal purposes, which expire in the years 2020 through 2030 if not utilized, and $113.1 million for state purposes, the majority of which expire in the years 2012 through 2030 if not utilized. Utilization of net operating loss carryforwards may be subject to substantial annual limitations due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation may result in the expiration of the net operating loss before utilization.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity
Historically, we have financed our operations primarily through product sales; sales of equity and debt securities such as our 3.50% convertible notes in 2007, collaborative agreements; interest income; and licensing of our technology.
Our cash outflows have generally been as follows: cash used in operating activities such as research and development programs, sales and marketing activity, procurement and growth of inventory, compensation and benefits of our employees and other working capital needs; cash paid for acquisitions; cash paid for litigation activity and settlements; and cash used for our debt repurchases and repurchases of our convertible notes as well as interest payments on our convertible notes obligations.
As of December 31, 2010, we had cash, cash equivalents, and available-for-sale securities of approximately $237.2 million. We anticipate that our existing capital resources along with the cash to be generated from operations will enable us to maintain currently planned operations, acquisitions, debt repayments or repurchases, and capital expenditures, for the foreseeable future.
However, this expectation is based on our current operating and financing plans, which are subject to change, and therefore we could require additional funding. Factors that may cause us to require additional funding may include, but are not limited to, future acquisitions; our ability to maintain existing collaborative and customer arrangements and establish and maintain new collaboration and customer arrangements; the progress of our research and development programs; initiation or expansion of research programs and collaborations; the costs involved in preparing, filing, prosecuting, defending and enforcing intellectual property rights; the effectiveness of product commercialization activities and arrangements; the purchase of patent licenses; and other factors.
As of December 31, 2010, we have no credit facility or other committed sources of capital. To the extent capital resources are insufficient to meet future capital requirements; we will have to raise additional funds to continue the development of our technologies. There can be no assurance that such funds will be available on favorable terms, or at all. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of such securities could result in dilution to our stockholders. If adequate funds are not available, we may be required to curtail operations significantly or to obtain funds by entering into collaboration agreements on unattractive terms. Our inability to raise capital would have a material adverse effect on our business, financial condition and results of operations.
From time to time, we may seek to retire, repurchase, or exchange our convertible securities or common stock in open market purchases, privately negotiated transactions dependent on market conditions, liquidity, and contractual obligations and other factors. For the year ended December 31, 2010, we repurchased an aggregate of $151.7 million of the outstanding principal balance of our 3.50% convertible notes due 2038 for cash payments totaling $143.6 million and recognized a gain of $6.3 million on the repurchase.
Cashflow (in thousands)
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net cash provided by operating activities
|
$ | 47,975 | $ | 9,329 | $ | 83,511 | ||||||
|
Net cash provided by (used in) investing activities
|
66,211 | (7,190 | ) | (151,062 | ) | |||||||
|
Net cash used in financing activities
|
(144,759 | ) | (50,073 | ) | (108,185 | ) | ||||||
|
Effect of foreign currency translation on cash and cash equivalents
|
415 | 284 | 384 | |||||||||
|
Net decrease in cash and cash equivalents
|
$ | (30,158 | ) | $ | (47,650 | ) | $ | (175,352 | ) | |||
Operating Activities
Net cash provided by operating activities for the year ended December 31, 2010 was comprised of a net loss of $10.2 million, net of non-cash charges of $45.2 million and a $13.0 million increase in working capital. Adjustments for non-cash expenses include depreciation and amortization expense of $35.5 million, a net realized loss on investments of $4.6 million, which included other-than-temporary impairment charges totaling $5.6 million, primarily due to two non-marketable equity investments, stock-based compensation expense of $9.9 million and a gain from the repurchase of convertible notes of $6.3 million.
Investing Activities
Our investing activities, other than purchases, sales and maturities of available-for-sale securities, primarily consist of capital expenditures and purchased technology rights.
Cash used for capital expenditures was $7.7 million for the year ended December 31, 2010. Our capital expenditures were primarily related to the purchase of manufacturing equipment and computer hardware.
Financing Activities
Our financing activities generally consist of stock option exercise activity under our employee stock plan. Cash used in the issuance of stock under our employee stock plan, net of treasury shares withheld for taxes, was $1.2 million for the year ended December 31, 2010. Additionally, in 2010, we bought back a total of $151.7 million in aggregate principal balance of our 3.50% convertible notes for $143.6 million in cash consideration, excluding transaction costs of $0.4 million.
Off-Balance Sheet Arrangements and Aggregate Contractual Obligations
As of December 31, 2010, we had no off-balance sheet arrangements. The impact that our contractual obligations, as of December 31, 2010, is expected to have on our liquidity and cash flow in future periods is as follows (in thousands):
|
Total
|
2011
|
2012-2013 | 2014-2015 |
After 2015
|
||||||||||||||||
|
Senior convertible notes
|
$ | 95,472 | $ | - | $ | 95,472 | $ | - | $ | - | ||||||||||
|
Interest payments (1)
|
6,821 | 3,341 | 3,480 | - | - | |||||||||||||||
|
Operating leases
|
28,095 | 8,878 | 15,088 | 4,056 | 73 | |||||||||||||||
|
Purchase commitments (2)
|
3,071 | 3,071 | - | - | - | |||||||||||||||
|
Total contractual obligations
|
$ | 133,459 | $ | 15,290 | $ | 114,040 | $ | 4,056 | $ | 73 | ||||||||||
|
(1)
|
Our 3.50% senior convertible notes are due in 2038. However, holders may require us to repurchase all or a portion of their notes on January 15, 2013, 2018 and 2028. In 2010, we purchased a total of approximately $151.7 million of aggregate principal amount of our 3.50% senior convertible notes for $143.6 million in total cash consideration.
|
|
|
In June 2009, we repurchased approximately $69.1 million of aggregate principal amount for $49.7 million in total cash consideration.
|
|
(2)
|
Purchase commitments include agreements to purchase goods or services that are enforceable and legally binding on Affymetrix and that specify all significant terms, including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Purchase obligations exclude agreements that are cancelable without penalty.
|
The above table does not reflect unrecognized tax benefits of approximately $20.8 million, the timing of which is uncertain. Refer to “Item 8. Financial Statements and Supplementary Data—Note 13. Income Taxes” for additional discussion on unrecognized tax benefits.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Risk
Our exposure to interest rate risk relates primarily to our investment portfolio. Fixed rate securities may have their fair market value adversely impacted due to fluctuations in interest rates, while floating rate securities may produce less income than expected if interest rates fall. Due in part to these factors, our future investment income may fall short of expectations due to changes in interest rates or we may suffer losses in principal if forced to sell securities which have declined in market value due to changes in interest rates.
The primary objective of our investment activities is to preserve principal while at the same time maximize yields without significantly increasing risk. To achieve this objective, we invest our excess cash in debt instruments of the U.S. Government and its agencies and high-quality corporate issuers, and, by policy, restrict our exposure to any single corporate issuer by imposing concentration limits. To minimize the exposure due to adverse shifts in interest rates, we maintain investments at an average maturity of less than three years.
|
Fair Value at
|
||||||||||||||||||||||||
|
Periods of Maturity
|
December 31,
|
|||||||||||||||||||||||
|
2011
|
2012
|
2013 (1)
|
Thereafter
|
Total
|
2010 (1)
|
|||||||||||||||||||
|
ASSETS:
|
||||||||||||||||||||||||
|
Available-for-sale securities
|
$ | 73,490 | $ | 45,556 | $ | 60,549 | $ | 28,573 | $ | 208,168 | $ | 207,517 | ||||||||||||
|
Average interest rate
|
2.2 | % | 2.2 | % | 2.6 | % | 3.8 | % | ||||||||||||||||
|
LIABILITIES:
|
||||||||||||||||||||||||
|
3.50% senior convertible notes due 2038
|
$ | - | $ | - | $ | 95,469 | $ | - | $ | 95,469 | $ | 95,469 | ||||||||||||
|
Average interest rate
|
3.50 | % | ||||||||||||||||||||||
|
Fair Value at
|
||||||||||||||||||||||||
|
Periods of Maturity
|
December 31,
|
|||||||||||||||||||||||
|
2010
|
2011
|
2012
|
Thereafter (1)
|
Total
|
2009 (1)
|
|||||||||||||||||||
|
ASSETS:
|
||||||||||||||||||||||||
|
Available-for-sale securities
|
$ | 237,060 | $ | 59,639 | $ | 4,643 | $ | 1,220 | $ | 302,562 | $ | 303,917 | ||||||||||||
|
Average interest rate
|
1.5 | % | 0.6 | % | 0.1 | % | 1 | % | ||||||||||||||||
|
LIABILITIES:
|
||||||||||||||||||||||||
|
3.50% senior convertible notes due 2038
|
$ | - | $ | - | $ | - | $ | 247,198 | $ | 247,198 | $ | 217,537 | ||||||||||||
|
Average interest rate
|
3.50 | % | ||||||||||||||||||||||
(1) Remaining balance on our 0.75% senior convertible notes is less than $0.1 million.
Foreign Currency Exchange Rate Risk
We derive a portion of our revenues in foreign currencies, predominantly in Europe and Japan. In addition, a portion of our assets are held in nonfunctional currencies of our subsidiaries. We may conduct hedging activities by using foreign currency forward and option contracts with financial institutions to manage a portion of the currency exposures created by our foreign activities. Our net currency loss recognized on business transactions was less than $0.1 million for the year ended December 31, 2010 and is included in interest income and other, net. Management will continue to reevaluate foreign currency exchange rate risk on an ongoing basis. As of December 31, 2010, we had no open hedging contracts.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
AFFYMETRIX, INC.
|
Page No.
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Affymetrix, Inc.
We have audited the accompanying consolidated balance sheets of Affymetrix, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. Our audits also included the financial statement schedule listed in the Index at Item 15(a). These financial statements and schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Affymetrix, Inc. at December 31, 2010 and 2009, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Affymetrix, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 28, 2011 expressed an unqualified opinion thereon.
|
/s/ Ernst & Young LLP
|
|
|
San Jose, California
February 28, 2011
|
AFFYMETRIX, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share amounts)
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS:
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 35,484 | $ | 65,642 | ||||
|
Restricted cash—short-term portion
|
287 | 1,686 | ||||||
|
Available-for-sale securities—short-term portion
|
67,223 | 213,377 | ||||||
|
Accounts receivable, net
|
52,281 | 64,933 | ||||||
|
Inventories
|
49,373 | 54,490 | ||||||
|
Deferred tax assets—short-term portion
|
1,071 | 1,172 | ||||||
|
Prepaid expenses and other current assets
|
9,422 | 15,903 | ||||||
|
Total current assets
|
215,141 | 417,203 | ||||||
|
Available-for-sale securities—long-term portion
|
134,190 | 64,760 | ||||||
|
Property and equipment, net
|
54,177 | 68,182 | ||||||
|
Acquired technology rights, net
|
38,858 | 49,855 | ||||||
|
Deferred tax assets—long-term portion
|
4,894 | 4,720 | ||||||
|
Restricted cash—long-term portion
|
- | 1,109 | ||||||
|
Other long-term assets
|
13,525 | 25,121 | ||||||
|
Total assets
|
$ | 460,785 | $ | 630,950 | ||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY:
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued liabilities
|
$ | 44,259 | $ | 57,183 | ||||
|
Deferred revenue—short-term portion
|
10,950 | 14,534 | ||||||
|
Total current liabilities
|
55,209 | 71,717 | ||||||
|
Deferred revenue—long-term portion
|
4,601 | 3,898 | ||||||
|
Other long-term liabilities
|
11,748 | 10,295 | ||||||
|
Convertible notes
|
95,472 | 247,201 | ||||||
|
Stockholders’ equity:
|
||||||||
|
Convertible redeemable preferred stock, $0.01 par value; 5,000 shares authorized; no shares issued and outstanding at December 31, 2010 and 2009
|
- | - | ||||||
|
Common stock, $0.01 par value; 200,000 shares authorized; 70,578 and 71,000 shares issued and outstanding at December 31, 2010 and 2009, respectively
|
706 | 710 | ||||||
|
Additional paid-in capital
|
742,206 | 733,378 | ||||||
|
Accumulated other comprehensive income (loss)
|
1,376 | 4,051 | ||||||
|
Accumulated deficit
|
(450,533 | ) | (440,300 | ) | ||||
|
Total stockholders’ equity
|
293,755 | 297,839 | ||||||
|
Total liabilities and stockholders’ equity
|
$ | 460,785 | $ | 630,950 | ||||
See Accompanying Notes
AFFYMETRIX, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
REVENUE:
|
||||||||||||
|
Product sales
|
$ | 277,743 | $ | 279,186 | $ | 270,392 | ||||||
|
Services
|
20,565 | 39,563 | 32,096 | |||||||||
|
Royalties and other revenue
|
12,438 | 8,345 | 107,761 | |||||||||
|
Total revenue
|
310,746 | 327,094 | 410,249 | |||||||||
|
COSTS AND EXPENSES:
|
||||||||||||
|
Cost of product sales
|
117,384 | 126,377 | 126,909 | |||||||||
|
Cost of services and other
|
15,822 | 23,949 | 25,231 | |||||||||
|
Research and development
|
67,934 | 77,358 | 84,482 | |||||||||
|
Selling, general and administrative
|
114,773 | 130,838 | 127,161 | |||||||||
|
Acquired in-process technology
|
- | - | 6,200 | |||||||||
|
Restructuring charges
|
- | 2,180 | 43,707 | |||||||||
|
Goodwill impairment (credit) charges
|
- | (450 | ) | 239,098 | ||||||||
|
Total costs and expenses
|
315,913 | 360,252 | 652,788 | |||||||||
|
Loss from operations
|
(5,167 | ) | (33,158 | ) | (242,539 | ) | ||||||
|
Interest income and other, net
|
(1,487 | ) | 2,589 | 14,629 | ||||||||
|
Interest expense
|
7,706 | 10,945 | 14,091 | |||||||||
|
Gain from repurchase of convertible notes
|
6,297 | 17,447 | - | |||||||||
|
Loss before income taxes
|
(8,063 | ) | (24,067 | ) | (242,001 | ) | ||||||
|
Income tax provision (benefit)
|
2,170 | (158 | ) | 65,918 | ||||||||
|
Net loss
|
$ | (10,233 | ) | $ | (23,909 | ) | $ | (307,919 | ) | |||
|
Basic and diluted net loss per common share
|
$ | (0.15 | ) | $ | (0.35 | ) | $ | (4.49 | ) | |||
|
Shares used in computing basic and diluted net loss per common share
|
68,856 | 68,722 | 68,556 | |||||||||
See Accompanying Notes
AFFYMETRIX, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net loss
|
$ | (10,233 | ) | $ | (23,909 | ) | $ | (307,919 | ) | |||
|
Other comprehensive (loss) income:
|
||||||||||||
|
Foreign currency translation adjustment, net of tax
|
415 | 284 | 384 | |||||||||
|
Unrealized gains (losses) on available-for-sale and non-marketable securities, net of tax
|
1,252 | 6,979 | (3,492 | ) | ||||||||
|
Reclassification adjustment for realized gains (losses) recognized in net loss
|
(4,342 | ) | (916 | ) | (1,201 | ) | ||||||
|
Unrealized gains on cash flow hedges, net of tax
|
- | - | 15 | |||||||||
|
Net change in other comprehensive (loss) income
|
(2,675 | ) | 6,347 | (4,294 | ) | |||||||
|
Comprehensive loss
|
$ | (12,908 | ) | $ | (17,562 | ) | $ | (312,213 | ) | |||
See Accompanying Notes
AFFYMETRIX, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Convertible redeemable preferred stock:
|
||||||||||||
|
Balance, beginning of year
|
$ | - | $ | - | $ | - | ||||||
|
Balance, end of year
|
- | - | - | |||||||||
|
Common stock:
|
||||||||||||
|
Balance, beginning of year
|
710 | 703 | 692 | |||||||||
|
Common stock (cancelled) issued
|
(4 | ) | 7 | 11 | ||||||||
|
Balance, end of year
|
706 | 710 | 703 | |||||||||
|
Additional paid-in capital:
|
||||||||||||
|
Balance, beginning of year
|
733,378 | 721,641 | 704,189 | |||||||||
|
Issuance of common stock upon exercise of stock options and warrants
|
||||||||||||
|
and issuances of restricted stock, net
|
(1,178 | ) | (820 | ) | (80 | ) | ||||||
|
Stock-based compensation expense from stock options and restricted stock
|
9,910 | 11,148 | 7,611 | |||||||||
|
Income tax benefit from stock-based compensation
|
96 | 1,409 | 9,921 | |||||||||
|
Balance, end of year
|
742,206 | 733,378 | 721,641 | |||||||||
|
Accumulated other comprehensive gain (loss):
|
||||||||||||
|
Balance, beginning of year
|
4,051 | (2,296 | ) | 1,998 | ||||||||
|
Unrealized (loss) gain on investments, net of tax
|
(3,090 | ) | 6,063 | (4,693 | ) | |||||||
|
Unrealized gain on hedging contracts, net of tax
|
- | - | 15 | |||||||||
|
Foreign currency translation adjustment, net of tax
|
415 | 284 | 384 | |||||||||
|
Balance, end of year
|
1,376 | 4,051 | (2,296 | ) | ||||||||
|
Accumulated deficit:
|
||||||||||||
|
Balance, beginning of year
|
(440,300 | ) | (416,391 | ) | (108,472 | ) | ||||||
|
Net loss
|
(10,233 | ) | (23,909 | ) | (307,919 | ) | ||||||
|
Balance, end of year
|
(450,533 | ) | (440,300 | ) | (416,391 | ) | ||||||
|
Total stockholders' equity
|
$ | 293,755 | $ | 297,839 | $ | 303,657 | ||||||
|
Number of shares of common stock
|
||||||||||||
|
Balance, beginning of year
|
71,000 | 70,267 | 69,217 | |||||||||
|
Issuance of common stock for cash or services
|
(422 | ) | 733 | 1,050 | ||||||||
|
Balance, end of year
|
70,578 | 71,000 | 70,267 | |||||||||
See Accompanying Notes
AFFYMETRIX, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
(10,233 | ) | $ | (23,909 | ) | $ | (307,919 | ) | ||||
|
Adjustments to reconcile net loss to net cash provided by operating activities:
|
||||||||||||
|
Depreciation and amortization
|
22,156 | 29,888 | 37,058 | |||||||||
|
Goodwill impairment
|
- | - | 239,098 | |||||||||
|
Amortization of intangible assets
|
13,304 | 15,237 | 13,453 | |||||||||
|
Charge for acquired in-process technology
|
- | - | 6,200 | |||||||||
|
Amortization of investment premiums, net
|
- | 1,219 | 389 | |||||||||
|
Excess tax benefits for stock-based compensation
|
(416 | ) | (1,409 | ) | (11,794 | ) | ||||||
|
Stock-based compensation
|
9,910 | 11,148 | 7,611 | |||||||||
|
Realized loss on equity investments
|
4,647 | 917 | 421 | |||||||||
|
Deferred tax assets
|
(73 | ) | 1,358 | 60,140 | ||||||||
|
Amortization of debt offering costs
|
841 | 1,207 | 2,080 | |||||||||
|
Gain from repurchase of convertible notes
|
(6,297 | ) | (17,447 | ) | - | |||||||
|
Impairment and loss (gain) on disposal of property and equipment
|
1,132 | (231 | ) | 36,989 | ||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable, net
|
12,599 | (2,207 | ) | 23,803 | ||||||||
|
Inventories
|
5,117 | (3,157 | ) | 495 | ||||||||
|
Prepaid expenses and other assets
|
10,802 | 4,115 | 2,009 | |||||||||
|
Accounts payable and accrued liabilities
|
(12,801 | ) | (5,374 | ) | (18,042 | ) | ||||||
|
Deferred revenue
|
(2,881 | ) | (1,349 | ) | (6,639 | ) | ||||||
|
Other long-term liabilities
|
168 | (677 | ) | (1,841 | ) | |||||||
|
Net cash provided by operating activities
|
47,975 | 9,329 | 83,511 | |||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Capital expenditures
|
(7,726 | ) | (10,249 | ) | (13,846 | ) | ||||||
|
Purchases of available-for-sale securities
|
(453,138 | ) | (381,560 | ) | (449,398 | ) | ||||||
|
Proceeds from sales of available-for-sale securities
|
417,981 | 261,029 | 330,655 | |||||||||
|
Proceeds from maturities of available-for-sale securities
|
110,477 | 124,090 | 138,016 | |||||||||
|
Acquisition of businesses, net of cash acquired
|
- | - | (156,178 | ) | ||||||||
|
Purchase of non-marketable equity investments
|
- | - | (311 | ) | ||||||||
|
Purchase of technology rights
|
(1,383 | ) | (500 | ) | - | |||||||
|
Net cash provided by (used in) investing activities
|
66,211 | (7,190 | ) | (151,062 | ) | |||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||
|
Issuance of common stock, net
|
(1,182 | ) | (813 | ) | (70 | ) | ||||||
|
Repurchase of convertible notes
|
(143,993 | ) | (50,669 | ) | (119,909 | ) | ||||||
|
Excess tax benefits for stock-based compensation
|
416 | 1,409 | 11,794 | |||||||||
|
Net cash used in financing activities
|
(144,759 | ) | (50,073 | ) | (108,185 | ) | ||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
415 | 284 | 384 | |||||||||
|
Net decrease in cash and cash equivalents
|
(30,158 | ) | (47,650 | ) | (175,352 | ) | ||||||
|
Cash and cash equivalents at beginning of year
|
65,642 | 113,292 | 288,644 | |||||||||
|
Cash and cash equivalents at end of year
|
$ | 35,484 | $ | 65,642 | $ | 113,292 | ||||||
|
SUPPLEMENTAL DISCLOSURE OF NONCASH ACTIVITIES:
|
||||||||||||
|
Recognition of deferred tax assets relating to tax benefits from employee stock plans
|
$ | - | $ | - | $ | 5,320 | ||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||||||
|
Cash paid for interest
|
$ | 9,284 | $ | 9,963 | $ | 8,358 | ||||||
|
Cash paid for income taxes
|
$ | 1,450 | $ | 144 | $ | 6,804 | ||||||
See Accompanying Notes
AFFYMETRIX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2010
NOTE 1—NATURE OF OPERATIONS
Affymetrix, Inc. (“Affymetrix” or the “Company”) is engaged in the development, manufacture, sale and service of consumables and systems for genetic analysis in the life sciences and clinical healthcare markets. Affymetrix has developed its GeneChip® system and related microarray technology as the platform of choice for acquiring, analyzing and managing complex genetic information. The Company’s integrated GeneChip® microarray platform includes: disposable DNA probe arrays (chips) consisting of nucleic acid sequences set out in an ordered, high density pattern, certain reagents for use with the probe arrays, a scanner and other instruments used to process the probe arrays, and software to analyze and manage genomic or genetic information obtained from the probe arrays. Related microarray technology also offered by Affymetrix includes licenses for fabricating, scanning, collecting and analyzing results from complementary technologies. The Company currently sells its products directly to pharmaceutical, biotechnology, agrichemical, diagnostics and consumer products companies as well as academic research centers, government research laboratories, private foundation laboratories and clinical reference laboratories in North America and Europe. The Company also sells some of its products through life science supply specialists acting as authorized distributors in Latin America, India, the Middle East and Asia Pacific regions, including China.
NOTE 2—SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
BASIS OF PRESENTATION
The consolidated financial statements include the accounts of Affymetrix and its wholly-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Certain prior year revenue and cost of sales amounts in the Company’s consolidated statements of operations have been reclassified to conform to the current period presentation. Product sales include consumables and instruments while services includes scientific services and associated consumables, field service, and professional service. Royalties and other includes software, subscription fees, licensing and royalties and research revenue.
USE OF ESTIMATES
The preparation of the consolidated financial statements is in conformity with U.S. generally accepted accounting principles (“US GAAP”) which require management to make estimates and assumptions that affect amounts reported in the financial statements and accompanying notes. Actual results could differ materially from those estimates.
FOREIGN CURRENCY
Assets and liabilities of non-U.S. subsidiaries that use the local currency as their functional currency are translated to U.S. dollars at exchange rates in effect at the balance sheet date, with the resulting translation adjustments directly recorded to a separate component of accumulated other comprehensive income (loss) within stockholders’ equity. Income and expense accounts are translated at average exchange rates during the year. Foreign currency transaction gains and losses are recognized, net of hedging activity, in interest income and other, net and were comprised of net losses of less than $0.1 million, $2.8 million and $2.2 million for the years ended December 31, 2010, 2009 and 2008, respectively.
CASH EQUIVALENTS, AVAILABLE-FOR-SALE SECURITIES AND INVESTMENTS
|
|
Marketable Securities
|
The Company’s investments consist of marketable equity and debt securities including U.S. government notes and bonds; corporate notes, bonds and asset-backed securities; mortgage-backed securities, municipal notes and bonds; and publicly traded equity securities. The Company reports all securities with maturities at the date of purchase of 90 days or less that are readily convertible into cash and have insignificant interest rate risk as cash equivalents. The Company’s investments are carried at fair value with unrealized gains and losses reported in accumulated other comprehensive income (loss) in stockholders’ equity. The cost of its marketable securities is adjusted for the amortization of premiums and discounts to maturity. This amortization is included in interest income and other, net. Realized gains and losses, as well as interest income, on available-for-sale securities are also included in interest income and other, net. The cost of securities sold is based on the specific identification method. The fair values of securities are based on quoted market prices. The Company includes its available-for-sale securities that have an effective maturity of less than twelve months as of the balance sheet date in current assets and those with an effective maturity greater than twelve months as of the balance sheet date in non-current assets.
The Company conducts a review of investment securities on a quarterly basis for the presence of impairment that is deemed to be other-than-temporary (“OTTI”). As part of its review, the Company is required to take into consideration current market conditions, fair value in relationship to cost, extent and nature of change in fair value, issuer rating changes and trends, volatility of earnings, current analysts’ evaluations, all available information relevant to the collectability of debt securities, its ability to hold until, and whether it will more likely than not be required to sell prior to, a recovery of fair value, which may be maturity, and other factors when evaluating for the existence of OTTI in its securities portfolio. Under these circumstances, OTTI is considered to have occurred if (1) the Company intends to sell the security; (2) it is “more likely than not” that the Company will be required to sell the security before recovery of its amortized cost basis or (3) the present value of expected cash flows is not expected to recover the entire amortized cost basis. Any credit-related OTTI is to be recognized in earnings while noncredit-related OTTI on securities not expected to be sold is to be recognized in other comprehensive income (“OCI”). Noncredit-related OTTI is based on other factors, including illiquidity. Presentation of OTTI is made in the Condensed Consolidated Statements of Operations on a gross basis with an offset for the amount of OTTI recognized in OCI.
Non-marketable Securities
As part of the Company’s strategic efforts to gain access to potential new products and technologies, it invests in equity securities of certain private biotechnology companies. These investments are included in other assets in the Condensed Consolidated Balance Sheets and are carried at cost. The Company also invests in a limited partnership investment fund that is accounted for under the equity method. The Company periodically monitors the liquidity and financing activities of the respective investments to determine if any impairment exists and accordingly writes down to the extent necessary, the carrying value of the non-marketable equity securities to their estimated fair values. In order to determine whether a decline in value is other-than-temporary, the Company evaluates, among other factors: the duration and extent to which the fair value has been less than the carrying value; the financial condition of and business outlook of the issuer for the company, including key operational and cash flow metrics, current market conditions; and the Company’s intent and ability to retain the investment for a period of time sufficient to allow for any anticipated recovery in estimated fair value. The Company recognized $5.6 million of OTTI expense, primarily related to two investments in private biotechnology companies, and recorded it as part of interest income and other, net in the Condensed Consolidated Statements of Operations for the year ended December 31, 2010. Refer to Note 5. “Fair Value of Financial Instruments” for further information.
ACCOUNTS RECEIVABLE
Trade accounts receivable are recorded at net invoice value. The Company considers amounts past due based on the related terms of the invoice. The Company reviews its exposure to amounts receivable and provides an allowance for specific amounts if collectability is no longer reasonably assured. The Company also provides an allowance for a percentage of the gross trade receivable balance (excluding any specifically reserved amounts) based on its collection history.
DERIVATIVE INSTRUMENTS
The Company has international operations and during the normal course of business is exposed to foreign currency exchange risks as a result of transactions that are denominated in currencies other than the United States dollar. The Company enters into foreign currency forward contracts to manage a portion of the volatility related to transactions that are denominated in foreign currencies. The Company’s foreign currency forward contracts are entered into for periods consistent with the related underlying exposures and do not constitute positions that are independent of those exposures. In addition, the Company does not enter into foreign currency forward contracts for trading or speculative purposes, is not party to any leveraged derivative instrument, and may only enter into derivative agreements with highly rated counterparties.
The foreign currency forward contracts used by the Company are generally short-term in nature, maturing within one quarter. The Company has elected not to designate its derivatives for balance sheet purposes as fair value hedges and have appropriately recorded any changes in fair value to interest income and other, net.
INVENTORIES
Inventory cost is computed on an adjusted standard basis (which approximates actual cost on a first-in, first-out basis). Provisions for slow moving, potentially excess and obsolete inventories are provided based on estimated demand requirements, product life cycle and development plans, component cost trends, product pricing, product expiration and quality issues.
PROPERTY AND EQUIPMENT
Property and equipment are recorded at cost and are depreciated using the straight-line method over the estimated useful lives of the assets or the lease term, whichever is shorter. Equipment and furniture is depreciated over useful lives generally ranging from 3 to 7 years and leasehold improvements are depreciated over the shorter of the expected life of the asset or lease terms generally ranging from 3 to 15 years. Maintenance and repair costs are expensed as incurred. The Company reassesses the useful life on its property and equipment on a periodic basis and may adjust the lives accordingly. For example, the Company extended the useful life of its enterprise resource system as a result of the extended support period committed to by the service provider.
ACQUIRED TECHNOLOGY RIGHTS
Acquired technology rights are carried at cost less accumulated amortization and are comprised of licenses to technology covered by patents held by third parties or acquired by the Company. Purchased intangible assets other than goodwill are required to be amortized over their useful lives unless these lives are determined to be indefinite. Amortization is recorded over the estimated useful life of the underlying patents, which has historically ranged from one to thirteen years. In 2010 and 2009, the Company did not identify any indicators of impairment during its annual test on the acquired technology rights and did not recognize any impairment losses. Refer to Note 8, “Acquired Technology Rights”, for further information.
IMPAIRMENT OF LONG-LIVED ASSETS
Long-lived assets and certain identifiable intangible assets are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their estimated fair values. For the year ended December 31, 2010, no impairment was recorded. For the year ended December 31, 2009, the Company determined that the carrying value of its capitalized software exceeded its net realizable value by $1.0 million, respectively, and recorded the impairment charges as a component of “Cost of product sales” in the Consolidated Statements of Operations.
INCOME TAXES
Income tax expense is based on pre-tax financial accounting income. Under the liability method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. The Company must then assess the likelihood that the resulting deferred tax assets will be realized. To the extent the Company believes that realization of the deferred tax assets is not more likely than not, the Company establishes a valuation allowance. Significant estimates are required in determining the Company’s provision for income taxes, our deferred tax assets and liabilities, and any valuation allowance to be recorded against our net deferred tax assets. Some of these estimates are based on interpretations of existing tax laws or regulations. The Company believes that its estimates are reasonable and that its reserves for income tax related uncertainties are adequate. Various internal and external factors may have favorable or unfavorable effects on the Company’s future effective tax rate. These factors include, but are not limited to, changes in overall levels of characterization and geographical mix of pretax earnings (losses), changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, changes in the valuation of our deferred tax assets or liabilities, future levels of research and development spending, nondeductible expenses, applicability of tax holidays and ultimate outcomes of income tax audits. Relative to uncertain tax positions, the Company only recognizes the tax benefit if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such positions are then measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
CONTINGENCIES
The Company is subject to various legal proceedings principally related to intellectual property matters. Based on the information available at the most recent balance sheet date, the Company assesses the likelihood of any material adverse judgments or outcomes that may result from these matters, as well as the range of possible or probable loss, if any. If losses are probable and reasonably estimable, the Company will record a reserve. Any reserves recorded may change in the future due to new developments in each matter.
REVENUE RECOGNITION
|
|
Overview
|
The Company recognizes revenue when persuasive evidence of an arrangement exists, delivery has occurred, the fee is fixed or determinable, and collectability is reasonably assured. In instances where final acceptance of the product or system is required or performance obligations remain, revenue is deferred until all the acceptance criteria or performance obligations have been met.
The Company derives the majority of its revenue from product sales of probe arrays, reagents, and related instrumentation that may be sold individually or combined with any of the products, services or other sources of revenue. When a sale combines multiple elements upon delivery or performance of multiple products, services and/or rights to use assets, the Company allocates revenue for transactions or collaborations that include multiple elements to each unit of accounting based on its relative fair value, and recognizes revenue for each unit of accounting when the revenue recognition criteria have been met. The price charged when the element is sold separately generally determines fair value.
Effective January 1, 2010, the Company early adopted the recently revised accounting guidance related to revenue recognition for multiple element arrangements on a prospective basis, which establishes the relative selling price method whereby the Company is required to allocate consideration to all deliverables at the inception of the arrangement based on their relative selling prices. In order to determine the selling price of a deliverable, the Company applies the following hierarchy: 1) vendor-specific objective evidence (“VSOE”); 2) third-party evidence if VSOE is not available; and 3) the Company’s best estimate of selling price for the deliverable if neither VSOE nor third-party evidence is available.
The Company primarily expects to utilize VSOE in determining the selling price of each deliverable in a multiple element arrangement as the Company historically has been able to establish fair value of a deliverable based on the price charged when it is sold separately. In the event that VSOE is not determinable, and where no third-party evidence is available, the Company will use estimated selling price in its allocation of arrangement consideration. Several factors are considered when determining the estimated selling price of a deliverable, including, but not limited to, the cost to produce the deliverable, the expected margin on that deliverable, the Company’s ongoing pricing strategy and policies and the value-added components of differentiated deliverables, if determinable.
The revised accounting guidance also refined the criteria for determining when a deliverable should be accounted for as a separate unit of accounting. Both of the following criteria must be met in order to be considered a separate unit of accounting: 1) the delivered item or items have value to the customer on a standalone basis; and 2) when a general right of return exists, the delivery or performance of an undelivered item is considered probable and under the control of the Company. The Company has determined that a deliverable has standalone value when the item is sold separately by the Company or another vendor or can be resold by the customer. The Company’s revenue arrangements generally do not have a general right of return. When a deliverable does not meet the criteria to be considered a separate unit of accounting, the Company groups it with other deliverables that, when combined, meet the criteria, and the appropriate allocation of arrangement consideration and revenue recognition is determined.
|
|
Product Sales
|
Product sales include sales of probe arrays, reagents and related instrumentation. Probe array, reagent and instrumentation revenue is recognized when earned, which is generally upon shipment and transfer of title to the customer and fulfillment of any significant post-delivery obligations. Accruals are provided for anticipated warranty expenses at the time the associated revenue is recognized.
|
|
Services
|
Services revenue includes equipment service revenue; scientific services revenue, which includes associated consumables; and revenue from custom probe array design fees.
Revenue related to extended warranty arrangements is deferred and recognized ratably over the applicable periods. Revenue from custom probe array design fees associated with the Company’s GeneChip® CustomExpress™ and CustomSeq™ products are recognized when the associated products are shipped.
Revenue from scientific and DNA analysis services are recognized upon shipment of the required data to the customer.
|
|
Royalties and Other Revenue
|
Royalties and other revenue include license revenue; royalties earned from third party license agreements; milestones and royalties earned from collaborative product development and supply agreements; subscription fees earned under GeneChip® array access programs; research revenue, which mainly consists of amounts earned under government grants; and non-recurring intellectual property payments.
License revenue is generally recognized upon the execution of the agreement unless the Company has continuing performance obligations, in which case the license revenue is recognized ratably over the period of expected performance. Included in the Consolidated Statements of Operations for the year ended December 31, 2008 is a one-time perpetual license to specified patents for $90 million. The one-time perpetual license was due to the settlement of intellectual property litigation.
Royalty revenue is earned from the sale of products by third parties who have been licensed under the Company’s intellectual property portfolio. Revenue from minimum royalties is amortized over the term of the creditable royalty period. Any royalties received in excess of minimum royalty payments are recognized under the terms of the related agreement, generally upon notification of manufacture or shipment of a product by a licensee.
The Company enters into collaborative arrangements which generally include a research and product development phase and a manufacturing and product supply phase. These arrangements may include up-front nonrefundable license fees, milestones, the rights to royalties based on the sale of final product by the partner, product supply agreements and distribution arrangements.
Any up-front, nonrefundable payments from collaborative product development agreements are recognized ratably over the research and product development period, and at-risk substantive based milestones are recognized when earned. Any payments received which are not yet earned are included in deferred revenue.
|
|
Transactions with Distributors
|
The Company recognizes revenue from transactions with distributors when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the seller’s price is fixed or determinable, and collectability is reasonably assured. The Company’s agreements with distributors do not include rights of return.
RESEARCH AND DEVELOPMENT EXPENSES
Research and development expenses consist of costs incurred for internal, collaborative and grant-sponsored research and development. Research and development expenses include salaries, contractor fees, building costs, utilities and allocations of shared corporate services. In addition, the Company funds research and development at other companies and research institutions under agreements which are generally cancelable. All such costs are charged to research and development expense as incurred.
SOFTWARE DEVELOPMENT COSTS
Development Costs of Software to Be Sold, Leased or Marketed
Certain software development costs subsequent to the establishment of technological feasibility are capitalized. The Company’s software is deemed to have achieved technological feasibility at the point a working model of the software product is developed. For the years ended December 31, 2010 and 2009, the Company did not capitalize any software development costs. Amortization costs for the years ended December 31, 2010 and 2009 on software development costs previously capitalized were $0.7 million and $1.1 million, respectively. The costs of developing routine software enhancements are expensed as research and development as incurred because of the short time between the determination of technological feasibility and the date of general release of the related products.
Long-lived assets and certain identifiable intangible assets are reviewed for impairment when events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Refer to Note 2, “Summary of Significant Accounting Policies – Impairment of Long-Lived Assets” for further information.
Internal-Use Software
For the years ended December 31, 2010 and 2009, the Company capitalized approximately $1.1 million and $0.7 million, respectively, related to costs associated with internal-use software. All costs associated with software developed for internal use will be amortized at the time in which the software is ready for its intended use. As of December 31, 2010, no amortization costs have been recognized.
ADVERTISING COSTS
The Company expenses advertising costs as incurred. Advertising costs recorded for the years ended December 31, 2010, 2009 and 2008 were $1.2 million, $0.9 million and $1.6 million, respectively.
STOCK-BASED COMPENSATION
The Company recognizes the fair value of its stock option awards as compensation expense on a straight-line basis over the requisite service period of each award, generally four years. The fair value of options is estimated at the date of grant using a Black-Scholes option pricing model.
COMPREHENSIVE INCOME (LOSS)
Comprehensive income (loss) is comprised of net loss and other comprehensive income (loss). Other comprehensive income (loss) includes unrealized gains and losses on the Company’s available-for-sale securities that are excluded from net loss, and foreign currency translation adjustments. Total comprehensive income (loss) has been disclosed in the consolidated statement of comprehensive loss.
The components of accumulated other comprehensive income is as follows (in thousands):
|
Year Ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Foreign currency translation adjustment, net of tax
|
$ | 742 | $ | 327 | ||||
|
Unrealized gains on available-for-sale and non-marketable securities, net of tax
|
634 | 3,724 | ||||||
|
Accumulated other comprehensive income
|
$ | 1,376 | $ | 4,051 | ||||
NET LOSS PER COMMON SHARE
Basic net loss per common share is calculated using the weighted-average number of common shares outstanding during the period less the weighted-average shares subject to repurchase. Diluted net loss per common share gives effect to dilutive common stock subject to repurchase, stock options and warrants (calculated based on the treasury stock method), and convertible debt (calculated using an as-if-converted method).
The following table sets forth a reconciliation of basic and diluted net loss per common share (in thousands, except per share amounts):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Numerator:
|
||||||||||||
|
Net loss - basic and diluted
|
$ | (10,233 | ) | $ | (23,909 | ) | $ | (307,919 | ) | |||
|
Denominator:
|
||||||||||||
|
Weighted-average shares outstanding
|
70,783 | 70,758 | 69,818 | |||||||||
|
Less: weighted-average shares of common stock subject to repurchase
|
(1,927 | ) | (2,036 | ) | (1,262 | ) | ||||||
|
Shares used in computing basic and diluted net loss per common share
|
68,856 | 68,722 | 68,556 | |||||||||
|
Basic and diluted net loss per common share
|
$ | (0.15 | ) | $ | (0.35 | ) | $ | (4.49 | ) | |||
Diluted earnings per share, if any, include certain common share equivalents from outstanding stock options (on the treasury stock method), common stock subject to repurchase, outstanding warrants to purchase common stock and convertible notes (on the as-if-converted basis).
The potentially dilutive securities excluded from diluted earnings per common share because their effect would have been anti-dilutive, on an actual outstanding basis, were as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Employee stock options
|
6,636 | 6,216 | 6,071 | |||||||||
|
Restricted stock subject to repurchase
|
1,953 | 2,154 | 1,671 | |||||||||
|
Convertible notes
|
3,170 | 8,207 | 10,502 | |||||||||
|
Total
|
11,759 | 16,577 | 18,244 | |||||||||
RESTRUCTURING
The Company has in recent years engaged in restructuring actions which require management to utilize significant estimates related to expenses for severance and other employee separation costs, lease cancellation, realizable values of assets that may become duplicative or obsolete, and other exit costs. If the actual amounts differ from the Company’s estimates, the amount of the restructuring charges could be materially impacted. Refer to Note 3, “Restructuring”, for further information.
NOTE 3—RESTRUCTURING
In 2008, the Company closed its West Sacramento manufacturing facility, moving its probe array manufacturing to its Singapore facility, consolidating its reagent manufacturing to its Cleveland facility and outsourcing its instrument manufacturing operations to third parties (the “2008 Plan”). The Company made total cash payments of $8.2 million related to employee termination benefits and incurred noncash charges of $36.9 million related to the abandonment and impairment of certain manufacturing assets which were presented as a component of “Restructuring charges” in the Company’s Condensed Consolidated Statements of Operations. The Company completed the closure of the West Sacramento facility during the second quarter of 2009 and no expense was recognized during the year ended December 31, 2010. In 2009 and 2008, the Company recognized $2.4 million and $5.8 million, respectively, of employee termination benefits expense, and $(0.2) million and $37.1 million, respectively, of non-cash credits and charges related to the abandonment and impairment of certain manufacturing assets. The Company does not expect to incur additional expense in connection with the 2008 Plan.
NOTE 4—CONCENTRATIONS OF RISK
Cash equivalents and investments are financial instruments that potentially subject Affymetrix to concentrations of risk to the extent of amounts recorded in the consolidated balance sheets. Company policy restricts the amount of credit exposure to any one issuer and to any one type of investment, other than securities issued by the United States Government.
The Company has not experienced significant credit losses from its accounts receivable. Affymetrix performs a regular review of its customer activity and associated credit risks and does not require collateral from its customers. The Company maintains an allowance for doubtful accounts receivable based upon the expected collectability of accounts receivable.
Certain raw materials or components used in the synthesis of probe arrays or the assembly of instrumentation are currently available only from a single source or limited sources. No assurance can be given that these raw materials or other components of the GeneChip® system will be available in commercial quantities at acceptable costs from other vendors should the need arise. If the Company is required to seek alternative sources of supply, it could be time consuming and expensive.
In addition, the Company is dependent on its vendors to provide components of appropriate quality and reliability and to meet applicable regulatory requirements. Consequently, in the event that supplies from these vendors are delayed or interrupted for any reason, the Company’s ability to develop and supply its products could be impaired, which could have a material adverse effect on the Company’s business, financial condition and results of operations.
For the years ended December 31, 2010, 2009 and 2008, approximately 43%, 42% and 34%, respectively, of the Company’s total revenue was generated from sales outside the United States. The Company’s results of operations are affected by such factors as changes in foreign currency exchange rates, trade protection measures, longer accounts receivable collection patterns and changes in regional or worldwide economic or political conditions. The risks of the Company’s international operations are mitigated in part by the extent to which its sales are geographically distributed.
NOTE 5—FAIR VALUE OF FINANCIAL INSTRUMENTS
Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and consider assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions and risk of nonperformance.
A fair value hierarchy was established which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The three levels of inputs that may be used to measure fair value are as follows:
|
·
|
Level 1: quoted prices in active markets for identical assets or liabilities;
|
|
·
|
Level 2: inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; or
|
|
·
|
Level 3: unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
|
Financial Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table represents the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2010 and 2009 (in thousands):
|
Significant
|
||||||||||||
|
Quoted Prices
|
Other
|
|||||||||||
|
In Active
|
Observable
|
|||||||||||
|
Markets
|
Inputs
|
|||||||||||
|
(Level 1)
|
(Level 2)
|
Total
|
||||||||||
|
December 31, 2010:
|
||||||||||||
|
Assets:
|
||||||||||||
|
U.S. government obligations and agencies*
|
$ | - | $ | 52,056 | $ | 52,056 | ||||||
|
U.S. corporate debt*
|
- | 103,192 | 103,192 | |||||||||
|
U.S. money markets
|
- | 3,634 | 3,634 | |||||||||
|
Non-U.S. government obligations and agencies
|
- | 8,106 | 8,106 | |||||||||
|
Non-U.S. corporate debt
|
804 | 39,725 | 40,529 | |||||||||
|
Total financial assets
|
$ | 804 | $ | 206,713 | $ | 207,517 | ||||||
|
December 31, 2009:
|
||||||||||||
|
Assets:
|
||||||||||||
|
U.S. government obligations and agencies*
|
$ | - | $ | 127,808 | $ | 127,808 | ||||||
|
U.S. corporate debt*
|
- | 100,229 | 100,229 | |||||||||
|
U.S. and Non-U.S. money markets
|
- | 39,094 | 39,094 | |||||||||
|
Non-U.S. government obligations and agencies
|
- | 14,225 | 14,225 | |||||||||
|
Non-U.S. corporate debt
|
1,512 | 18,524 | 20,036 | |||||||||
|
Non-U.S. money markets
|
- | 2,525 | 2,525 | |||||||||
|
Total financial assets
|
$ | 1,512 | $ | 302,405 | $ | 303,917 | ||||||
* Included in these security types were the Company’s mortgage-backed investments which were approximately 4% and 1% of the total portfolio for the year ended December 31, 2010 and 2009, respectively.
The fair value of the convertible notes is estimated based on the quoted market prices for the same or similar issues or on the current rates offered to the Company for the convertible notes of the same remaining maturities. As of December 31, 2010 and 2009, the estimated fair value of the convertible notes was $95.5 million and $217.5 million, respectively. Refer to Note 11, “Senior Convertible Notes”, for further information.
As of December 31, 2010 and 2009, the Company had no financial assets or liabilities requiring Level 3 classification, including those that have unobservable inputs that are supported by little or no market activity and are significant to the fair value of the assets and liabilities.
Investments in Debt and Equity Securities
The fair values of all available-for-sale securities are based on quoted market prices and are included in cash and cash equivalents, available-for-sale securities—short-term and available-for-sale securities—long-term on the Company’s Consolidated Balance Sheets based on the securities maturity. The following is a summary of available-for-sale securities as of December 31, 2010 (in thousands):
|
Gross
|
Gross
|
|||||||||||||||
|
Amortized
|
Unrealized
|
Unrealized
|
||||||||||||||
|
Cost
|
Gains
|
Losses
|
Fair Value
|
|||||||||||||
|
U.S. government obligations and agency securities*
|
$ | 52,047 | $ | 85 | $ | (76 | ) | $ | 52,056 | |||||||
|
U.S. corporate debt*
|
103,601 | 275 | (684 | ) | 103,192 | |||||||||||
|
U.S. money markets
|
3,642 | - | (8 | ) | 3,634 | |||||||||||
|
Non-U.S. government obligations and agency securities
|
8,155 | 24 | (73 | ) | 8,106 | |||||||||||
|
Non-U.S. corporate debt securities
|
40,724 | 48 | (243 | ) | 40,529 | |||||||||||
|
Total securities
|
$ | 208,169 | $ | 432 | $ | (1,084 | ) | $ | 207,517 | |||||||
|
Amounts included in:
|
||||||||||||||||
|
Cash equivalents
|
$ | 6,125 | $ | - | $ | (22 | ) | $ | 6,103 | |||||||
|
Available-for-sale securities
|
202,044 | 432 | (1,062 | ) | 201,414 | |||||||||||
|
Total securities
|
$ | 208,169 | $ | 432 | $ | (1,084 | ) | $ | 207,517 | |||||||
|
Amounts mature in:
|
||||||||||||||||
|
Less than one year
|
$ | 73,490 | $ | 228 | $ | (392 | ) | $ | 73,326 | |||||||
|
One to two years
|
45,556 | 158 | (24 | ) | 45,690 | |||||||||||
|
More than two years
|
89,123 | 46 | (668 | ) | 88,501 | |||||||||||
|
Total securities
|
$ | 208,169 | $ | 432 | $ | (1,084 | ) | $ | 207,517 | |||||||
The following is a summary of available-for-sale securities as of December 31, 2009 (in thousands):
|
Gross
|
Gross
|
|||||||||||||||
|
Amortized
|
Unrealized
|
Unrealized
|
||||||||||||||
|
Cost
|
Gains
|
Losses
|
Fair Value
|
|||||||||||||
|
U.S. government obligations and agency securities*
|
$ | 127,274 | $ | 560 | $ | (26 | ) | $ | 127,808 | |||||||
|
U.S. corporate debt*
|
100,160 | 459 | (390 | ) | 100,229 | |||||||||||
|
U.S. money markets
|
39,079 | 17 | (2 | ) | 39,094 | |||||||||||
|
Non-U.S. government obligations and agency securities
|
14,150 | 76 | (1 | ) | 14,225 | |||||||||||
|
Non-U.S. corporate debt securities
|
19,387 | 677 | (28 | ) | 20,036 | |||||||||||
|
Non-U.S. money markets
|
2,512 | 13 | - | 2,525 | ||||||||||||
|
Total securities
|
$ | 302,562 | $ | 1,802 | $ | (447 | ) | $ | 303,917 | |||||||
|
Amounts included in:
|
||||||||||||||||
|
Cash equivalents
|
$ | 25,791 | $ | 6 | $ | (17 | ) | $ | 25,780 | |||||||
|
Available-for-sale securities
|
276,771 | 1,796 | (430 | ) | 278,137 | |||||||||||
|
Total securities
|
$ | 302,562 | $ | 1,802 | $ | (447 | ) | $ | 303,917 | |||||||
|
Amounts mature in:
|
||||||||||||||||
|
Less than one year
|
$ | 237,061 | $ | 1,315 | $ | (438 | ) | $ | 237,938 | |||||||
|
One to two years
|
59,638 | 442 | (9 | ) | 60,071 | |||||||||||
|
More than two years
|
5,863 | 45 | - | 5,908 | ||||||||||||
|
Total securities
|
$ | 302,562 | $ | 1,802 | $ | (447 | ) | $ | 303,917 | |||||||
* Included in these security types were the Company’s mortgage-backed investments which were approximately 4% and 1% of the total portfolio for the year ended December 31, 2010 and 2009, respectively.
Realized gains for the years ended December 31, 2010 and 2009 were $0.8 million and $0.3 million, respectively. For the years ended December 31, 2010 and 2009, realized losses were $0.3 million and $0.1 million, respectively. Realized gains and losses are included in interest income and other, net in the accompanying Consolidated Statements of Operations. The gross unrealized losses as of December 31, 2010 and 2009 were primarily related to a mortgage-backed security that was impacted by the weakening of the global economy caused by a lack of liquidity in the credit markets which has not fully recovered. No significant facts or circumstances have arisen to indicate that there has been any deterioration in the creditworthiness of the issuers of the Company’s other securities. Based on its review of these securities, including the assessment of the severity of the related unrealized losses, the Company has not recorded any other-than-temporary impairments on these securities for the years ended December 31, 2010 and 2009.
Additionally, the Company did not have any noncredit-related OTTI that was recognized in OCI during the years ended December 31, 2010 and 2009 on securities not expected to be sold and is not more likely than not required to sell before recovery of their amortized cost basis. The Company did not record any cumulative effect adjustments for noncredit-related portion of OTTI losses previously recognized in earnings.
Derivative Financial Instruments
The Company derives a portion of its revenues in foreign currencies, predominantly in Europe and Japan. In addition, a portion of its assets are held in nonfunctional currencies of its subsidiaries. As of December 31, 2010 and 2009, the Company had no open hedging contracts.
Non-Marketable Securities
As of December 31, 2010 and 2009, the carrying amounts of the Company’s non-marketable securities, totaling $6.8 million and $13.1 million, respectively, equaled their estimated fair values. Their estimated fair values were based on liquidation and net realizable values. During the year ended December 31, 2010, the Company recorded impairment charges on its non-marketable securities totaling $5.6 million, primarily due to declines in the estimated fair values of two of its investments in private biotechnology companies that were determined to be other-than-temporary as a result of the respective price per share paid by investors in the most recent round of financing for each company which, in each case, was significantly lower than the carrying value of the Company’s investment. During the year ended December 31, 2009, the Company also determined that declines in estimated fair values of other investments in its non-marketable equity securities portfolio were other-than-temporary and recognized an additional net impairment loss of approximately $1.1 million. Net investment losses are included in interest income and other, net in the Consolidated Statements of Operations. Depending on market conditions, the Company may incur additional charges on this investment portfolio in the future.
NOTE 6—INVENTORIES
Inventories consist of the following at December 31, 2010 and 2009 (in thousands):
|
2010
|
2009
|
|||||||
|
Raw materials
|
$ | 18,321 | $ | 20,012 | ||||
|
Work-in-process
|
13,516 | 9,456 | ||||||
|
Finished goods
|
17,536 | 25,022 | ||||||
|
Total
|
$ | 49,373 | $ | 54,490 | ||||
NOTE 7—PROPERTY AND EQUIPMENT
Property and equipment consists of the following as of December 31, 2010 and 2009 (in thousands):
|
2010
|
2009
|
|||||||
|
Property and equipment:
|
||||||||
|
Construction-in-progress
|
$ | 1,013 | $ | 3,561 | ||||
|
Equipment and furniture
|
147,493 | 146,449 | ||||||
|
Building and leasehold improvements
|
99,206 | 95,535 | ||||||
|
Land
|
1,310 | 1,310 | ||||||
| 249,022 | 246,855 | |||||||
|
Less: accumulated depreciation and amortization
|
(194,845 | ) | (178,673 | ) | ||||
|
Net property and equipment
|
$ | 54,177 | $ | 68,182 | ||||
For the years ended December 31, 2010, 2009 and 2008, the Company recorded depreciation expense of $22.2 million, $29.6 million and $33.1 million, respectively. Additionally, in 2008, the Company wrote down property and equipment in the amount of approximately $37.1 million in connection with its 2008 Plan. Refer to Note 3, “Restructuring”, for further information.
NOTE 8—ACQUIRED TECHNOLOGY RIGHTS
Acquired Technology Rights
Acquired technology rights are comprised of licenses to technology covered by patents owned by third parties or patents acquired by the Company and are amortized over the expected useful lives of these assets, which range from one to fifteen years. Accumulated amortization of these rights amounted to $71.6 million and $59.2 million at December 31, 2010 and 2009, respectively.
During the years ended December 31, 2010 and 2009, the Company concluded that here were no indicators of impairment during its annual impairment test of its acquired technology rights and the $38.9 million balance at December 31, 2010 is expected to be recoverable.
The expected future annual amortization expense of the Company’s acquired technology rights is as follows (in thousands):
|
Amortization
|
||||
|
For the Year Ending December 31,
|
Expense
|
|||
|
2011
|
$ | 12,474 | ||
|
2012
|
10,701 | |||
|
2013
|
8,192 | |||
|
2014
|
6,312 | |||
|
2015
|
979 | |||
|
Thereafter
|
200 | |||
|
Total
|
$ | 38,858 | ||
NOTE 9—ACCOUNTS PAYABLE AND ACCRUED LIABILITIES
Accounts payable and accrued liabilities as of December 31, 2010 and 2009 consist of the following (in thousands):
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Accounts payable
|
$ | 12,897 | $ | 15,243 | ||||
|
Accrued compensation and related liabilities
|
14,840 | 22,266 | ||||||
|
Accrued interest
|
1,531 | 3,965 | ||||||
|
Accrued taxes
|
6,860 | 7,814 | ||||||
|
Accrued legal
|
518 | 1,022 | ||||||
|
Accrued warranties
|
1,493 | 1,685 | ||||||
|
Other
|
6,120 | 5,188 | ||||||
|
Total
|
$ | 44,259 | $ | 57,183 | ||||
NOTE 10—COMMITMENTS AND CONTINGENCIES
Operating Leases
Affymetrix leases laboratory, office and manufacturing facilities under non-cancelable operating leases that expire at various times through 2016. Some of these leases contain renewal options ranging from two to five years and escalation clauses. Rent expense related to operating leases for the years ended December 31, 2010, 2009 and 2008 was approximately $9.7 million, $10.2 million and $11.0 million, respectively. In connection with some of these facility leases, the Company has made security deposits totaling $1.8 million, which are included in long-term other assets in the Consolidated Balance Sheets.
Future minimum lease obligations, net of sublease income, at December 31, 2010 under all non-cancelable operating leases are as follows (in thousands):
|
For the Year Ending December 31,
|
Amount
|
|||
|
2011
|
$ | 8,878 | ||
|
2012
|
8,536 | |||
|
2013
|
6,552 | |||
|
2014
|
2,125 | |||
|
2015
|
1,931 | |||
|
Thereafter
|
73 | |||
|
Total
|
$ | 28,095 | ||
Sublease income is expected to be approximately $0.8 million, $0.9 million and $0.5 million for the years ended December 31, 2011, 2012 and 2013, respectively, and $0 thereafter.
Product Warranty Commitment
The Company provides for anticipated warranty costs at the time the associated revenue is recognized. Product warranty costs are estimated based upon the Company’s historical experience and the warranty period. The Company periodically reviews the adequacy of its warranty reserve and adjusts, if necessary, the warranty percentage and accrual based on actual experience and estimated costs to be incurred. In 2010, we experienced more warranty claims for instruments than expected. Information regarding the changes in the Company’s product warranty liability for the years ended December 31, 2009 and 2008 is as follows (in thousands):
|
Amount
|
||||
|
Balance at December 31, 2008
|
$ | 1,604 | ||
|
Additions charged to cost of product sales
|
3,016 | |||
|
Repairs and replacements
|
(3,526 | ) | ||
|
Adjustments
|
591 | |||
|
Balance at December 31, 2009
|
$ | 1,685 | ||
|
Additions charged to cost of product sales
|
2,673 | |||
|
Repairs and replacements
|
(3,509 | ) | ||
|
Adjustments
|
644 | |||
|
Balance at December 31, 2010
|
$ | 1,493 | ||
Non-Cancelable Supply Agreements
As of December 31, 2010, the Company had approximately $1.7 million of non-cancelable inventory supply agreements that are in effect through 2011.
Indemnifications
From time to time the Company has entered into indemnification provisions under certain of its agreements with other companies in the ordinary course of business, typically with business partners, customers, and suppliers. Pursuant to these agreements, the Company generally indemnifies, holds harmless, and agrees to reimburse the indemnified parties on a case by case basis for losses suffered or incurred by the indemnified parties in connection with any U.S. patent or other intellectual property infringement claim by any third party with respect to its products. The term of these indemnification provisions is generally perpetual from the time of the execution of the agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification provisions is unlimited. In addition, the Company has entered into indemnification agreements with its officers and directors. The Company has not incurred material costs to defend lawsuits or settle claims related to these indemnification provisions. As of December 31, 2010, the Company had not accrued a liability for this guarantee, because the likelihood of incurring a payment obligation in connection with this guarantee is remote.
Legal Proceedings
The Company has been in the past, and continues to be, a party to litigation which has consumed, and may continue to consume, substantial financial and managerial resources. While the results of any litigation or any other legal proceedings are uncertain, the Company does not believe the ultimate resolution of any pending legal matters is likely to have a material adverse effect on our financial position or results of operations.
|
Illumina Lawsuit
|
On May 4, 2009 and November 3, 2009, the Company was named as a defendant in complaints filed by plaintiff, Illumina, Inc., in the United States District Court for the Western District of Wisconsin (the “Court”). In the complaints, plaintiff alleged that the Company is infringing Patent Nos. 7,510,841 and 7,612,020 by making and selling certain of the GeneChip® products. Plaintiff seeks a permanent injunction enjoining the Company from further infringement and unspecified monetary damages. In December 2010, the Court granted the Company’s motion for summary judgment that it does not infringe the patents held by Illumina and directed that the patent lawsuits brought by Illumina be dismissed and the cases closed. Illumina is appealing the Court’s decision.
|
E8 Pharmaceuticals LLC
|
On July 1, 2008, the Company was named as a defendant in a complaint filed by plaintiffs E8 Pharmaceuticals LLC and Massachusetts Institute of Technology ("MIT") in the United States District Court of Massachusetts. In the complaint, the plaintiffs allege that the Company is infringing one patent owned by MIT and licensed to E8 Pharmaceuticals by making and selling the Company’s GeneChip® products to customers and teaching its customers how to use the products. The plaintiffs seek a permanent injunction enjoining the Company from further infringement, unspecified monetary damages, enhanced damages pursuant to 35 U.S.C. § 284, costs, attorneys’ fees and other relief as the court deems just and proper. The Company will vigorously defend against plaintiffs’ claims. There is no trial date set in this matter.
|
Enzo Litigation
|
On October 28, 2003, Enzo Life Sciences, Inc., a wholly-owned subsidiary of Enzo Biochem, Inc. (collectively “Enzo”), filed a complaint against the Company that is pending in the United States District Court for the Southern District of New York for breach of contract, injunctive relief and declaratory judgment. The Enzo complaint relates to a 1998 distributorship agreement with Enzo under which the Company served as a non-exclusive distributor of certain reagent labeling kits supplied by Enzo. In its complaint, Enzo seeks monetary damages and an injunction against the Company from using, manufacturing or selling Enzo products and from inducing collaborators and customers to use Enzo products in violation of the 1998 agreement. Enzo also seeks the transfer of certain Affymetrix patents to Enzo. In connection with its complaint, Enzo provided the Company with a notice of termination of the 1998 agreement effective on November 12, 2003.
On November 10, 2003, the Company filed a complaint against Enzo in the United States District Court for the Southern District of New York for declaratory judgment, breach of contract and injunctive relief relating to the 1998 agreement. In its complaint, the Company alleges that Enzo has engaged in a pattern of wrongful conduct against it and other Enzo labeling reagent customers by, among other things, asserting improperly broad rights in its patent portfolio, improperly using the 1998 agreement and distributorship agreements with others in order to corner the market for non-radioactive labeling reagents, and improperly using the 1998 agreement to claim ownership rights to the Company’s proprietary technology. The Company seeks declarations that it has not breached the 1998 agreement and that nine Enzo patents that are identified in the 1998 agreement are invalid and/or not infringed by it. The Company also seeks damages and injunctive relief to redress Enzo’s alleged breaches of the 1998 agreement, its alleged tortuous interference with the Company’s business relationships and prospective economic advantage, and Enzo’s alleged unfair competition. The Company filed a notice of related case stating that its complaint against Enzo is related to the complaints already pending in the Southern District of New York against eight other former Enzo distributors. The Company’s case has been related to complaints previously pending in the Southern District of New York against eight other former Enzo distributors. There is no trial date in the actions between Enzo and the Company.
|
Administrative Proceedings
|
The Company’s intellectual property is subject to a number of significant administrative actions. These proceedings could result in the Company’s patent protection being significantly modified or reduced, and the incurrence of significant costs and the consumption of substantial managerial resources. As of the year ended December 31, 2010, the Company did not incur significant costs in connection with administrative proceedings.
NOTE 11—SENIOR CONVERTIBLE NOTES
On November 13, 2007, the Company issued $316.3 million principal amount of 3.50% Senior Convertible Notes (the “Notes”) due January 15, 2038. The net proceeds after issuance costs from the Notes offering were approximately $309.4 million. The Notes bear interest of 3.50% per year on the principal amount payable semi-annually in arrears on January 15 and July 15 of each year. The Company incurred underwriter discount and issuance costs of approximately $6.9 million, which are being amortized over the effective life of the Notes which is five years, the period up to the first date that the holders of the Notes (the “Holders”) can require the Company to repurchase the Notes.
The Notes are convertible into 33.1991 shares of Affymetrix common stock per $1,000 principal amount of Notes which equates to 10,499,215 shares of common stock, or a conversion price equivalent of $30.12 per share of common stock. The conversion rate is subject to adjustment upon the occurrence of the following specified events:
|
|
•
|
issuing shares of the Company’s common stock as a dividend or distribution of the Company’s common stock;
|
|
|
•
|
effecting a stock split or stock combination;
|
|
|
•
|
issuing to all or substantially all Holders of the Company’s common stock any rights or warrants under certain circumstances and with certain entitlements;
|
|
|
•
|
distributing shares of the Company’s common stock, evidences of indebtedness or other assets or property, to all or substantially all Holders of the Company’s common stock, with certain exceptions;
|
|
|
•
|
making cash distributions to all or substantially all Holders of the Company’s common stock; or
|
|
|
•
|
should the Company or any of its subsidiaries purchase shares of its common stock pursuant to a tender offer at a premium to market.
|
Holders may convert their Notes into shares of Affymetrix stock at any time at their option at the initial conversion rate, subject to adjustment, prior to the close of business on the business day prior to the maturity date.
On January 15, 2013, 2018 and 2028, the security holders have the option to require the Company to repurchase the Notes at a price equal to 100% of the principal amount of the Notes plus accrued interest. Additionally, on or after January 15, 2013, Affymetrix has the option of redeeming for cash at 100% of the principal amount all or part of the then outstanding Notes plus accrued interest.
The Notes are unsecured and rank equally with the Company’s other existing and future senior indebtedness. The Notes are structurally subordinated to any current or future indebtedness and other liabilities of the Company’s subsidiaries.
In 2010, the Company repurchased a total of $151.7 million of aggregate principal amount of the Notes for total cash consideration of $143.6 million, including accrued interest of $1.5 million. The recognized gain on debt repurchase of $6.3 million is net of transaction costs of $0.4 million and accelerated amortization of deferred financing costs of $1.4 million.
In 2009, the Company repurchased approximately $69.1 million of aggregate principal amount of the Notes for total cash consideration of $50.6 million, including accrued interest of $0.9 million. The recognized gain on debt repurchase of $17.4 million is net of transaction costs of $0.9 million and accelerated amortization of deferred financing costs of $1.0 million.
As of December 31, 2010, the balance remaining on the Notes was $95.5 million.
NOTE 12—STOCKHOLDERS’ EQUITY
Stockholder Rights Plan
In February 2010, the Company’s existing stockholder rights plan expired and no further plan has been adopted.
Stock-Based Compensation Plans
The Company has a stock-based compensation program that provides the Board of Directors broad discretion in creating equity incentives for employees, officers, directors and consultants. This program includes incentive and non-qualified stock options and non-vested stock awards (also known as restricted stock) granted under various stock plans. Stock options and restricted stock awards are generally time-based, vesting 25% on each annual anniversary of the grant date over four years. Stock options expire 7 to 10 years from the grant date. As of December 31, 2010, the Company had approximately 5.8 million shares of common stock reserved for future issuance under its stock-based compensation plans. New shares are issued as a result of stock option exercises, restricted stock units vesting and restricted stock award grants. A more detailed description of the Company’s current stock option plans follows below.
In 1998, the Board of Directors adopted the Affymetrix 1998 Stock Incentive Plan (the “1998 Stock Plan”) under which nonqualified stock options and restricted stock may be granted to employees and outside consultants, except that members of the Board of Directors and individuals who are considered officers of the Company under the rules of the National Association of Securities Dealers shall not be eligible. Options granted under the 1998 Stock Plan expire no later than ten years from the date of grant. The option price shall be at least 100% of the fair value of the Company’s common stock on the date of grant (110% in certain circumstances), as determined by the Board of Directors. Options may be granted with different vesting terms from time to time as determined by the Board of Directors. A total of 3.6 million shares of common stock are authorized for issuance under the 1998 Stock Plan.
In 2000, the Board of Directors adopted the Amended and Restated 2000 Equity Incentive Plan (the “2000 Stock Plan”), which was amended and restated in 2001, under which restricted shares, stock units, stock options and stock appreciation rights may be granted to employees, outside directors and consultants. Options granted under the 2000 Stock Plan expire no later than ten years from the date of grant. The option price shall be at least 100% of the fair value of the Company’s common stock on the date of grant (110% in certain circumstances), as determined by the Board of Directors. Options may be granted with different vesting terms from time to time as determined by the Board of Directors. In the second quarter of 2010, 4.5 million shares of common stock were added under the 2000 Stock Plan bringing the total shares of common stock authorized for issuance under the 2000 Stock Plan to 16.2 million.
The following table sets forth the total stock-based compensation expense resulting from stock options and non-vested stock awards included in the Company’s Condensed Consolidated Statements of Operations (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Costs of sales
|
$ | 994 | $ | 1,677 | $ | 1,305 | ||||||
|
Research and development
|
2,136 | 2,207 | 1,665 | |||||||||
|
Selling, general and administrative
|
6,780 | 7,264 | 4,641 | |||||||||
|
Total stock-based compensation expense
|
$ | 9,910 | $ | 11,148 | $ | 7,611 | ||||||
As of December 31, 2010, $15.5 million of total unrecognized stock-based compensation expense related to non-vested awards is expected to be recognized over the respective vesting terms of each award through 2014. The weighted-average term of the unrecognized stock-based compensation expense is 2.7 years.
Stock Options
The fair value of options was estimated at the date of grant using a Black-Scholes option pricing model with the following weighted-average assumptions:
|
Year Ended December 31,
|
||||||
|
2010
|
2009
|
2008
|
||||
|
Risk free interest rate
|
1.1%
|
1.7%
|
3.0%
|
|||
|
Expected dividend yield
|
0.0%
|
0.0%
|
0.0%
|
|||
|
Expected volatility
|
76%
|
70%
|
44%
|
|||
|
Expected option term (in years)
|
4.1
|
4.1
|
4.5
|
|||
The risk free interest rate for periods within the contractual life of the Company’s stock options is based on the U.S. Treasury yield curve in effect at the time of grant. The expected term is derived from an analysis of the Company’s historical exercise trends over ten years. The expected volatility for the years ended December 31, 2010 and 2009 is based on a blend of historical and market-based implied volatility. Using the assumptions above, the weighted-average grant date fair value of options granted during the years ended December 31, 2010, 2009 and 2008 was $2.66, $3.03 and $4.32, respectively.
Activity under the Company’s stock plans for the year ended December 31, 2010 is as follows (in thousands, except per share amounts):
|
Weighted-Average
|
Weighted-Average
|
Aggregate
|
||||||||||||||
|
Exercise Price
|
Remaining
|
Intrinsic
|
||||||||||||||
|
Shares
|
Per Share
|
Contractual Terms
|
Value
|
|||||||||||||
|
(in years)
|
||||||||||||||||
|
Outstanding at December 31, 2009
|
6,216 | $ | 15.72 | |||||||||||||
|
Grants
|
2,002 | 4.68 | ||||||||||||||
|
Exercises
|
(62 | ) | 3.02 | |||||||||||||
|
Forfeitures or expirations
|
(1,520 | ) | 15.98 | |||||||||||||
|
Outstanding at December 31, 2010
|
6,636 | $ | 12.44 | 4.54 | $ | 2,730 | ||||||||||
|
Exercisable at December 31, 2010
|
2,899 | $ | 19.97 | 4.36 | $ | 430 | ||||||||||
|
Vested and expected to vest at December 31, 2010
|
5,869 | $ | 13.31 | 3.08 | $ | 2,245 | ||||||||||
The following table summarizes information concerning currently outstanding and exercisable options at December 31, 2010:
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||||
|
Weighted-Average
|
Weighted-Average
|
Weighted-Average
|
||||||||||||||||||||
|
Remaining
|
Exercise Price
|
Exercise Price
|
||||||||||||||||||||
|
Range of Exercise Prices
|
Number
|
Contractual Life
|
Per Share
|
Number
|
Per Share
|
|||||||||||||||||
|
(in thousands)
|
(in years)
|
(in thousands)
|
||||||||||||||||||||
| $ | 1.32 - $3.16 | 703 | 4.73 | $ | 2.99 | 197 | $ | 2.96 | ||||||||||||||
| $ | 3.50 - $4.22 | 1,482 | 6.47 | $ | 4.20 | 15 | $ | 3.91 | ||||||||||||||
| $ | 4.26 - $6.51 | 584 | 6.04 | $ | 5.39 | 197 | $ | 5.54 | ||||||||||||||
| $ | 6.57 - $8.29 | 850 | 5.47 | $ | 8.15 | 194 | $ | 8.25 | ||||||||||||||
| $ | 8.71 - $10.29 | 1,052 | 4.04 | $ | 10.19 | 564 | $ | 10.19 | ||||||||||||||
| $ | 10.52 - $25.71 | 950 | 3.34 | $ | 20.28 | 728 | $ | 20.48 | ||||||||||||||
| $ | 25.75 - $34.51 | 699 | 1.50 | $ | 29.73 | 689 | $ | 29.76 | ||||||||||||||
| $ | 34.85 - $61.25 | 316 | 1.89 | $ | 42.49 | 315 | $ | 42.49 | ||||||||||||||
|
Total
|
6,636 | 4.54 | $ | 12.44 | 2,899 | $ | 19.97 | |||||||||||||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (i.e., the difference between the Company’s closing stock price on the last trading day of its fourth quarter of 2010 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2010. The amount changes based on the fair market value of the Company’s common stock. Total intrinsic value of options exercised is $0.2 million, less than $0.1 million and $0.3 million for the years ended December 31, 2010, 2009 and 2008, respectively.
Reserved Shares
At December 31, 2010, the Company has shares reserved for future issuance as follows (in thousands):
|
Options outstanding
|
6,636 | |||
|
Options available for future grants
|
5,806 | |||
|
Convertible subordinated notes
|
6,476 | |||
| 18,918 |
Restricted Stock
The following table summarizes the Company’s non-vested restricted stock activity for the year ended December 31, 2010 (in thousands, except per share amounts):
|
|
Weighted-Average
|
|||||||
|
Number of Shares
|
Grant Date Fair Value
|
|||||||
|
Non-vested stock outstanding at December 31, 2009
|
2,101 | $ | 9.19 | |||||
|
Granted
|
124 | $ | 6.35 | |||||
|
Vested
|
(570 | ) | $ | 10.59 | ||||
|
Forfeited
|
(407 | ) | $ | 9.60 | ||||
|
Non-vested stock outstanding at December 31, 2010
|
1,248 | $ | 8.14 | |||||
Total fair value of shares vested is $10.2 million and $19.3 million for the years ended December 31, 2010 and 2009, respectively.
NOTE 13—INCOME TAXES
The following table presents the U.S. and foreign components of consolidated loss before income taxes and the provision (benefit) for income taxes (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
(LOSS) INCOME BEFORE INCOME TAXES:
|
||||||||||||
|
U.S.
|
$ | (15,722 | ) | $ | (25,150 | ) | $ | (244,479 | ) | |||
|
Foreign
|
7,659 | 1,083 | 2,478 | |||||||||
|
Loss before income taxes
|
$ | (8,063 | ) | $ | (24,067 | ) | $ | (242,001 | ) | |||
|
PROVISION (BENEFIT) FOR INCOME TAXES:
|
||||||||||||
|
Current:
|
||||||||||||
|
Federal
|
$ | - | $ | (2,248 | ) | $ | 12,042 | |||||
|
State
|
37 | 25 | 2,780 | |||||||||
|
Foreign
|
2,222 | 2,123 | 2,598 | |||||||||
|
Subtotal
|
2,259 | (100 | ) | 17,420 | ||||||||
|
Deferred:
|
||||||||||||
|
Federal
|
- | - | 43,012 | |||||||||
|
State
|
- | - | 5,317 | |||||||||
|
Foreign
|
(89 | ) | (58 | ) | 169 | |||||||
|
Subtotal
|
(89 | ) | (58 | ) | 48,498 | |||||||
|
Provision (benefit) for income taxes
|
$ | 2,170 | $ | (158 | ) | $ | 65,918 | |||||
The difference between the provision (benefit) for income taxes and the amount computed by applying the federal statutory income tax rate (35%) to loss before taxes is explained as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Tax at federal statutory rate
|
$ | (2,822 | ) | $ | (8,423 | ) | $ | (84,700 | ) | |||
|
State taxes, net
|
(1,646 | ) | (2,315 | ) | (3,344 | ) | ||||||
|
Non-deductible stock compensation
|
626 | 659 | 294 | |||||||||
|
Foreign rate differential
|
(547 | ) | 1,686 | 1,543 | ||||||||
|
Research credits
|
(991 | ) | (1,772 | ) | (1,142 | ) | ||||||
|
Goodwill impairment
|
- | - | 64,986 | |||||||||
|
Change in valuation allowance
|
7,026 | 8,968 | 85,603 | |||||||||
|
Other
|
524 | 1,039 | 2,678 | |||||||||
|
Provision (benefit) for income taxes
|
$ | 2,170 | $ | (158 | ) | $ | 65,918 | |||||
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s assets are as follows (in thousands):
|
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Deferred tax assets:
|
||||||||
|
Net operating loss carryforwards
|
$ | 52,327 | $ | 48,209 | ||||
|
Tax credit carryforwards
|
40,047 | 38,710 | ||||||
|
Deferred revenue
|
1,717 | 1,802 | ||||||
|
Capitalized research and development costs
|
596 | 799 | ||||||
|
Intangibles
|
21,562 | 22,536 | ||||||
|
Stock-based compensation
|
5,422 | 5,629 | ||||||
|
Accrued compensation
|
2,125 | 2,432 | ||||||
|
Accrued warranty
|
576 | 676 | ||||||
|
Inventory reserve
|
5,013 | 3,101 | ||||||
|
Reserves and other
|
11,236 | 12,323 | ||||||
|
Depreciation and amortization
|
21,569 | 21,184 | ||||||
|
Other, net
|
2,844 | 2,626 | ||||||
|
Total deferred tax assets
|
165,034 | 160,027 | ||||||
|
Valuation allowance for deferred tax assets
|
(142,565 | ) | (139,343 | ) | ||||
|
Net deferred tax assets
|
22,469 | 20,684 | ||||||
|
Net deferred tax liabilities:
|
||||||||
|
Acquired intangibles
|
(3,670 | ) | (4,900 | ) | ||||
|
Cancellation of debt
|
(9,653 | ) | (6,767 | ) | ||||
|
Other, net
|
(3,181 | ) | (3,125 | ) | ||||
|
Total deferred tax liabilities
|
(16,504 | ) | (14,792 | ) | ||||
|
Net deferred tax assets
|
$ | 5,965 | $ | 5,892 | ||||
As of December 31, 2010, the Company had total net operating loss carryforwards of $259.3 million, comprised of $146.7 million for U.S. federal purposes, which expire in the years 2020 through 2030 if not utilized, and $112.6 million for state purposes, the majority of which expire in the years 2012 through 2030 if not utilized. Additionally, the Company has federal research and development tax credit carryforwards of approximately $22.8 million, which expire in the years 2017 through 2030 if not utilized. The Company also has state research and development tax credit carryforwards and other various tax credit carryforwards of approximately $37.1 million. Substantially all of the state tax credits can be carried forward indefinitely. Utilization of net operating loss and tax credit carryforwards may be subject to substantial annual limitations due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such an annual limitation may result in the expiration of the net operating loss before utilization.
As of December 31, 2010, the Company has recorded a full valuation allowance against all U.S. and certain foreign deferred tax assets, net of reserves for uncertain tax positions. The valuation allowance increased by $3.2 million and decreased by $0.4 million for the years ended December 31, 2010 and 2009, respectively. The increase during year ended December 31, 2010 is attributable to U.S. losses. Approximately $31.3 million of the valuation allowance as of December 31, 2010 is attributable to the income tax benefits of stock-based compensation, the benefit of which will be credited to stockholders’ equity when, and if, realized.
Not included in the deferred tax assets as of December 31, 2010 is approximately $4.6 million of tax benefits related to stock-based compensation. When realized, the tax benefit of these assets will be accounted for as a credit to stockholders’ equity, rather than a reduction of the income tax provision.
Of the total tax benefits realized from the stock-based compensation the amounts recorded to stockholders’ equity were approximately $0.1 million and $1.4 million for the years ended December 31, 2010 and 2009, respectively.
As of December 31, 2010, cumulative un-remitted foreign earnings that are considered to be permanently invested outside the United States and on which no U.S. taxes have been provided were approximately $16.6 million. The residual U.S. tax liability, if such amounts were remitted, would be nominal.
A portion of the Company’s operations in Singapore operate under various tax holidays and tax incentive programs, which expire in whole or in part at various dates through 2017. There was a minimal net impact of these tax holidays and tax incentive programs for the year ended December 31, 2010.
The following table presents the total amount of gross unrecognized tax benefits (in thousands):
|
2010
|
2009
|
|||||||
|
Unrecognized tax benefits, beginning of year
|
$ | 19,866 | $ | 18,545 | ||||
|
Gross increases - tax positions in prior period
|
167 | 1,004 | ||||||
|
Gross decreases - tax positions in prior period
|
(361 | ) | (731 | ) | ||||
|
Gross increases - current period tax positions
|
1,086 | 1,048 | ||||||
|
Settlements
|
- | - | ||||||
|
Unrecognized tax benefits, end of year
|
$ | 20,758 | $ | 19,866 | ||||
If recognized, the amount of unrecognized tax benefits that would impact income tax expense is $2.2 million. As of December 31, 2010, the Company does not anticipate any material changes to the amount of unrecognized tax benefits during the next 12 months. The Company classifies interest and penalties related to tax positions as components of income tax expense. For the year ended December 31, 2010, the amount of accrued interest and penalties related to tax uncertainties was approximately $0.1 million for a total cumulative amount included in non-current income taxes payable of $0.5 million as of December 31, 2010.
The Company files U.S. federal, state, and foreign income tax returns in jurisdictions with varying statutes of limitations. Our major tax jurisdictions are the U.S., California, Singapore, and the U.K. The federal and California statute of limitations on assessment remain open for the tax years 1992 through 2010. The major foreign jurisdictions remain open for examination in general for tax years 2006 through 2010.
NOTE 14—SEGMENT AND GEOGRAPHIC INFORMATION
The Company has determined that it operates in one segment as it only reports operating results on an aggregate basis to the chief operating decision maker of the Company.
The Company reported total revenue by region as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Customer location:
|
||||||||||||
|
United States
|
$ | 178,029 | $ | 190,257 | $ | 269,159 | ||||||
|
Europe
|
80,914 | 87,061 | 92,487 | |||||||||
|
Japan
|
22,248 | 22,588 | 23,861 | |||||||||
|
Other
|
29,555 | 27,188 | 24,742 | |||||||||
|
Total
|
$ | 310,746 | $ | 327,094 | $ | 410,249 | ||||||
Excluding a $90 million one-time, non-refundable intellectual property payment received in January 2008, there were no customers representing 10% or more of total revenue in 2010, 2009 and 2008.
The Company’s long-lived assets other than purchased intangible assets, which the Company does not allocate to specific geographic locations as it is impracticable to do so, are composed principally of net property, plant and equipment.
Net property, plant and equipment, classified by major geographic areas in which the Company operates was as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Net property, plant and equipment:
|
||||||||||||
|
United States
|
$ | 42,434 | $ | 51,262 | $ | 67,680 | ||||||
|
Singapore
|
10,206 | 15,510 | 20,164 | |||||||||
|
Other countries
|
1,537 | 1,410 | 1,501 | |||||||||
|
Total
|
$ | 54,177 | $ | 68,182 | $ | 89,345 | ||||||
NOTE 15—DEFINED-CONTRIBUTION SAVINGS PLANS
401(k) Plan
The Company maintains a defined-contribution savings plan which is qualified under Section 401(k) of the Internal Revenue Code. The plan covers substantially all full-time U.S. employees. Participating employees may defer a portion of their pretax earnings, up to the Internal Revenue Service annual contribution limit. The Company’s expense associated with matching employee contributions for the years ended December 31, 2010, 2009 and 2008 totaled $2.8 million, $3.2 million and $3.3 million, respectively. Company contributions to employees vest ratably over four years.
Director and Executive Deferred Compensation Plan
In December 2004, the Board of Directors approved the creation of the Affymetrix, Inc. Deferred Compensation Plan (the “Plan”). The Plan provides directors, executive officers and other eligible employees with the opportunity to enter into agreements to defer specified percentages of their cash compensation derived from base salary, bonus awards and other specified compensation (including director fees). Distributions occur upon termination of service (or the 6-month anniversary after termination), death, or upon such other dates that may be elected by the participant in accordance with the terms of the Plan. Generally, participants may elect for distributions of deferred amounts upon termination or death to be paid in the form of either a lump sum or in annual installments. Distributions would be made in the event of a change of control of Affymetrix. Deferrals are adjusted for gain or loss based on the performance of one or more investment options selected by the participant from among investment funds chosen by the Compensation Committee of the Board. The Company in its sole discretion may suspend or terminate the Plan or revise or amend it in any respect, except that no such action may reduce vested amounts credited to deferral accounts, and such accounts will continue to be owed to the participants or beneficiaries and will continue to be a liability of the Company until paid. For the years ended December 31, 2010 and 2009, the Company incurred no significant expenses in connection with the Plan. The Company terminated the Plan effective December 15, 2010.
NOTE 16—UNAUDITED QUARTERLY FINANCIAL INFORMATION
|
2010
|
2009
|
|||||||||||||||||||||||||||||||
|
Fourth
|
Third
|
Second
|
First
|
Fourth
|
Third
|
Second
|
First
|
|||||||||||||||||||||||||
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
Quarter
|
|||||||||||||||||||||||||
|
(in thousands, except per share amounts)
|
||||||||||||||||||||||||||||||||
|
Total revenue
|
$ | 84,909 | $ | 73,972 | $ | 71,678 | $ | 80,187 | $ | 88,788 | $ | 78,191 | $ | 81,554 | $ | 78,561 | ||||||||||||||||
|
Total cost of goods sold
|
$ | 35,570 | $ | 33,499 | $ | 31,089 | $ | 33,048 | $ | 34,980 | $ | 35,870 | $ | 37,452 | $ | 42,024 | ||||||||||||||||
|
Net income (loss)
|
$ | 3,960 | $ | 968 | $ | (5,541 | ) | $ | (9,620 | ) | $ | 2,795 | $ | (8,819 | ) | $ | 7,320 | $ | (25,205 | ) | ||||||||||||
|
Basic net income (loss) per common share
|
$ | 0.06 | $ | 0.01 | $ | (0.08 | ) | $ | (0.14 | ) | $ | 0.04 | $ | (0.13 | ) | $ | 0.11 | $ | (0.37 | ) | ||||||||||||
|
Diluted net income (loss) per common share
|
$ | 0.06 | $ | 0.01 | $ | (0.08 | ) | $ | (0.14 | ) | $ | 0.04 | $ | (0.13 | ) | $ | 0.11 | $ | (0.37 | ) | ||||||||||||
In 2010 and 2009, the Company recognized total gains of $6.3 million and $17.4 million, respectively, as part of its repurchases of its Notes.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As required by paragraph (b) of Exchange Act Rules 13a-15 or 15d-15, Affymetrix’ management, including our Chief Executive Officer and Chief Financial Officer, conducted an evaluation as of the end of the period covered by this report, of the effectiveness of Affymetrix’ disclosure controls and procedures as defined in Exchange Act Rule 13a-15(e) and 15d-15(e). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that Affymetrix’ disclosure controls and procedures were effective as of the end of the period covered by this report.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f) and 15d-15(f). Under the supervision of our Chief Executive Officer and Chief Financial Officer and with the participation of our management, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2010 based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on that evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2010.
The effectiveness of our internal control over financial reporting as of December 31, 2010, has been audited by Ernst &Young LLP, our independent registered public accounting firm, as stated in their report which is included as follows.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Affymetrix, Inc.
We have audited Affymetrix, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). Affymetrix, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Affymetrix, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Affymetrix, Inc. as of December 31, 2010 and 2009, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for each of the three fiscal years in the period ended December 31, 2010 of Affymetrix, Inc. and our report dated February 28, 2011 expressed an unqualified opinion thereon.
|
/s/ Ernst & Young LLP
|
|
|
San Jose, California
February 28, 2011
|
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during our last fiscal quarter that have materially affected or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Information regarding our directors and executive officers is incorporated by reference to the sections of the Company’s proxy statement for the 2011 Annual Meeting of Stockholders (the “Proxy Statement”) entitled “Election of Directors” and “Management.”
The information concerning our corporate governance, including our audit committee, required by this Item is incorporated by reference to the sections of the Proxy Statement entitled “Governance of the Company” and “Report of the Audit Committee.”
The information concerning compliance with Section 16(a) of the Exchange Act required by this Item is incorporated by reference to the section of the Proxy Statement entitled “Section 16(a) Beneficial Ownership Reporting Compliance.”
CODE OF ETHICS
Affymetrix has adopted a code of business conduct and ethics for directors, officers (including Affymetrix’ Chief Executive Officer, Chief Financial Officer and Corporate Controller) and employees, known as the Code of Business Conduct and Ethics. The Code of Business Conduct and Ethics is available on Affymetrix’ website at www.affymetrix.com in the Corporate Governance section under the “Investors” link. Stockholders may request a free copy of the Code of Business Conduct and Ethics by sending an email request to investor@affymetrix.com.
ITEM 11. EXECUTIVE COMPENSATION
Incorporated by reference to the sections of the Proxy Statement entitled “Executive Compensation,” “Compensation Discussion and Analysis,” “Compensation Committee Report,” “Certain Transactions” and “Compensation of Directors.”
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Incorporated by reference to the section of the Proxy Statement entitled “Stock Ownership of Principal Stockholders and Management.”
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Incorporated by reference to the sections of the Proxy Statement entitled “Certain Transactions” and “Governance of the Company.”
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Information about principal accountant fees and services as well as related pre-approval policies appears under “Fees Paid to Ernst & Young LLP” and “Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Registered Public Accounting Firm” in the Proxy Statement. Those portions of the Proxy Statement are incorporated by reference into this report.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements. The financial statements as set forth under Item 8 of this report on Form 10-K are incorporated herein by reference.
(a)(2) Financial Statement Schedule—Schedule II—Valuation and Qualifying Accounts. All other schedules have been omitted as they are not required, not applicable or the information is otherwise included.
(a)(3) Exhibits:
|
EXHIBIT
NUMBER
|
DESCRIPTION OF DOCUMENT
|
|
2.1(1)
|
Agreement and Plan of Merger by and among Panomics, Inc., the Company, Panda Acquisition Corporation and the Equityholders’ Representative dated as of November 11, 2008.
|
|
3.1(2)
|
Restated Certificate of Incorporation.
|
|
3.2(3)
|
Amended and Restated Bylaws.
|
|
4.1(4)
|
Indenture dated as of December 15, 2003, between the Company and The Bank of New York, as Trustee.
|
|
4.2(5)
|
Indenture dated as of November 16, 2007, between the Company and the Bank of New York Trust Company, N.A. as Trustee.
|
|
10.1(6)‡
|
1993 Stock Plan, as amended.
|
|
10.2(6)‡
|
1996 Nonemployee Directors Stock Option Plan.
|
|
10.3(7)
|
Lease between Sobrato Interests and the Company dated June 12, 1996 (3380 Central Expressway, Santa Clara, CA).
|
|
10.4(7)
|
Lease between Sobrato Interests and the Company dated May 31, 1996 (3450 Central Expressway, Santa Clara, CA).
|
|
10.5(8)‡
|
1998 Stock Incentive Plan.
|
|
10.6(8)‡
|
Form of Officer and Director Indemnification Agreement.
|
|
10.7(9)‡
|
Amendment No. 1 to the 1996 Nonemployee Directors Stock Option Plan of the Company
|
|
10.8(10)‡
|
Amended and Restated 1996 Non-Employee Directors Stock Plan.
|
|
10.9(11)*
|
Common Terms Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.10(11)*
|
License Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.11(11)*
|
Affymetrix Instrument and Chip Supply Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.12(11)*
|
Research & Development Collaboration Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.13(11)*
|
Diagnostic Product and Instrument Agency Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.14(11)*
|
Affymetrix Instrument Agency Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.15(12)
|
Lease between Sobrato Interests and the Company dated July 3, 2002 (3420 Central Expressway, Santa Clara, CA).
|
|
10.16(12)
|
First Amendment to Lease between Sobrato Interests and the Company dated September 30, 2003 (3420 Central Expressway, Santa Clara, CA).
|
|
10.17(13)‡
|
Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan, as adopted effective March 9, 2000 and amended through May 14, 2010.
|
|
10.18(14)‡
|
Form of Non-Qualified Stock Option Agreement under the Affymetrix, Inc. Amended and Restated 1996 Non-Employee Directors Stock Plan.
|
|
10.19(1)‡
|
Form of Stock Option Agreement under the Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan.
|
|
10.20(15)
|
Fifth Amendment to Lease between Sobrato Interests and the Company dated July 3, 2002 (3380 Central Expressway, Santa Clara, CA).
|
|
10.21(15)
|
First Amendment to Lease between Sobrato Interests and the Company dated July 3, 2002 (3450 Central Expressway, Santa Clara, CA).
|
|
10.22(16)
|
Lease between Keppel Logistics Pte Ltd. and Affymetrix Pte Ltd. dated as of January 1, 2006 (7 Gul Circle, Singapore 629363).
|
|
10.23 (27)
|
Addendum to Lease Agreement between Keppel Logistics Pte Ltd. and Affymetrix Pte Ltd. dated June 1, 2010 (7 Gul Circle, Singapore 629363)
|
|
10.24(1)‡
|
Form of Restricted Stock Agreement under the Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan.
|
|
10.25(17)‡
|
Offer Letter from the Company to Kevin M. King dated December 18, 2006.
|
|
10.26(18)**
|
Amendment Agreement dated December 22, 2006 by and among F. Hoffmann-La Roche Ltd., Roche Molecular Systems, Inc. and the Company
|
|
10.27(19)‡
|
Offer Letter from the Company to John C. Batty dated May 16, 2007.
|
|
10.28(20)
|
Settlement and Release Agreement dated January 9, 2008 between the Company and Illumina, Inc.
|
|
10.29(21)
|
Affymetrix, Inc. Change of Control Plan, as amended through May 14, 2010.
|
|
10.30(22)‡
|
Offer Letter from the Company to John F. (Rick) Runkel dated October 6, 2008.
|
|
10.31(23)
|
First Amendment to Affymetrix, Inc. 1998 Stock Incentive Plan.
|
|
10.32(24)
|
Stipulation of Settlement regarding the Affymetrix Derivative Litigation in the United States District Court, Northern District of California.
|
|
10.33(25)‡
|
Offer Letter from the Company to Andrew J. Last, Ph.D. dated November 2, 2009.
|
|
10.34(25)
|
Lease Agreement between SBP Limited Partnership and the Company dated August 10, 2008 (26309 Miles Road, Warrensville Heights, OH).
|
|
10.35(25)
|
First Amendment and Lease Expansion Agreement between SBP Limited Partnership and the Company dated May 20, 2009 (26309 Miles Road, Warrensville Heights, OH).
|
|
10.36(25)
|
Lease Agreement between OTR, acting as the duly authorized nominee of The State Teacher Retirement System of Ohio and Anatrace, Inc. dated February 14, 2001 (434 Dussel Drive, Maumee, OH).
|
|
10.37(25)
|
Assignment and Assumption of Lease between Anatrace, Inc. and USB Acquisition dated April 30, 2005 (434 Dussel Drive, Maumee, OH).
|
|
10.38(26)‡
|
Offer Letter from the Company to Timothy C. Barabe dated March 9, 2010.
|
|
10.39(26)‡
|
Separation Agreement between the Company and John C. Batty dated March 17. 2010.
|
|
10.40(26)
|
Lease Agreement between the Company and Miles/Commerce Ltd. dated April 1, 2010 (26101 Miles Road, Warrensville Heights, OH).
|
|
10.41(26)
|
Lease Agreement between the Company and 26111 Miles Road Ltd. dated April 1, 2010 (26111 Miles Road, Warrensville Heights, OH).
|
|
10.42(21)‡
|
Executive Severance Policy dated May 14, 2010.
|
|
21
|
List of Subsidiaries.
|
|
23
|
Consent of Independent Registered Public Accounting Firm.
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.
|
|
32
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted Pursuant to Section 906 of Sarbanes-Oxley Act of 2002.
|
|
(1)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 2, 2009 (File No. 000-28218).
|
|
(2)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on June 13, 2000 (File No. 000-28218).
|
|
(3)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on August 7, 2009 (File No. 000-28218).
|
|
(4)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-3 as filed on January 29, 2004 (File No. 333-112311).
|
|
(5)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on November 19, 2007 (File No. 000-28218).
|
|
(6)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-1 (File No. 333-3648), as amended.
|
|
(7)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on August 14, 1996 (File No. 000-28218).
|
|
(8)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 31, 1999 (File No. 000-28218).
|
|
(9)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-3 as filed on July 12, 1999 (File No. 333-82685), as amended.
|
|
(10)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 15, 2001 (File No. 000-28218).
|
|
(11)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 15, 2003 (File No. 000-28218).
|
|
(12)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 15, 2004 (File No. 000-28218).
|
|
(13)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-8 as filed on May 17, 2010 (File No. 333-166894).
|
|
(14)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on November 9, 2004 (File No. 000-28218).
|
|
(15)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 16, 2005 (File No. 000-28218).
|
|
(16)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 9, 2006 (File No. 000-28218).
|
|
(17)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on December 19, 2006 (File No. 000-28218).
|
|
(18)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on December 22, 2006 (File No. 000-28218).
|
|
(19)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on June 6, 2007 (File No. 000-28218).
|
|
(20)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on February 29, 2008 (File No. 000-28218).
|
|
(21)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on May 18, 2010 (File No. 000-28218).
|
|
(22)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on November 7, 2008 (File No. 000-28218).
|
|
(23)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-8 as filed on April 18, 2001 (File No. 333-59158).
|
|
(24)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on May 20, 2009 (File No. 000-28218).
|
|
(25)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on February 28, 2010 (File No. 000-28218).
|
|
(26)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 6, 2010 (File No. 000-28218).
|
|
(27)
|
Filed herewith.
|
|
‡
|
Management contract, compensatory plan, contract or arrangement
|
|
*
|
Confidential treatment granted
|
** Confidential treatment requested
AFFYMETRIX, INC.
Schedule II—Valuation and Qualifying Accounts
(in thousands)
|
Additions
|
||||||||||||||||
|
Balance at
|
Charged to
|
|||||||||||||||
|
Beginning of
|
Operations or
|
Write-offs, net
|
Balance at
|
|||||||||||||
|
Period
|
Other Accounts (1)
|
of recoveries
|
End of Period
|
|||||||||||||
|
Allowance for Doubtful Accounts:
|
||||||||||||||||
|
Year Ended December 31, 2010
|
$ | 1,853 | $ | (685 | ) | $ | (219 | ) | $ | 949 | ||||||
|
Year Ended December 31, 2009
|
$ | 2,213 | $ | (87 | ) | $ | (273 | ) | $ | 1,853 | ||||||
|
Year Ended December 31, 2008
|
$ | 2,372 | $ | 683 | $ | (842 | ) | $ | 2,213 | |||||||
|
(1)
|
2008 includes $101K for USB Opening Balance Sheet
|
Pursuant to the requirements of Section 13 of 15(d) of the Securities Exchange Act of 1934, the registrant has caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Affymetrix, Inc.
(Registrant)
|
||
|
February 28, 2011
|
By:
|
/s/ Kevin M. King
|
|
Kevin M. King
DIRECTOR, PRESIDENT AND
CHIEF EXECUTIVE OFFICER
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Each individual whose signature appears below constitutes and appoints John F. Runkel, Jr. and Timothy C. Barabe, and each of them singly, his or her true and lawful attorneys-in-fact, each with the power of substitution, for him or her in any and all capacities, to sign any amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the U.S. Securities and Exchange Commission, hereby ratifies and confirms all that said attorneys-in-fact, or his or her substitute or substitutes, may do or cause to be done by virtue thereof.
|
Name
|
Title
|
Date
|
|||||||
|
By:
|
/s/ Kevin M. King
|
Director, President and Chief Executive Officer
|
February 28, 2011
|
||||||
|
Kevin M. King
|
(Principal Executive Officer)
|
||||||||
|
By:
|
/s/ Timothy C. Barabe
|
Executive Vice President and Chief Financial
|
February 28, 2011
|
||||||
|
Timothy C. Barabe
|
Officer (Principal Financial and Accounting Officer)
|
||||||||
|
By:
|
/s/ Stephen P.A. Fodor, Ph.D.
|
Founder and Executive
Chairman of the Board
|
February 28, 2011
|
||||||
|
Stephen P.A. Fodor, Ph.D.
|
|||||||||
|
By:
|
/s/ Paul Berg, Ph.D.
|
Director
|
February 28, 2011
|
||||||
|
Paul Berg, Ph.D.
|
|||||||||
|
By:
|
/s/ Nelson C. Chan
|
Director
|
February 28, 2011
|
||||||
|
Nelson C. Chan
|
|||||||||
|
By:
|
/s/ John D. Diekman, Ph.D.
|
Director
|
February 28, 2011
|
||||||
|
John D. Diekman, Ph.D.
|
|||||||||
|
By:
|
/s/ Gary S. Guthart, Ph.D.
|
Director
|
February 28, 2011
|
||||||
|
Gary S. Guthart, Ph.D.
|
|||||||||
|
By:
|
/s/ Jami Dover Nachtsheim
|
Director
|
February 28, 2011
|
||||||
|
Jami Dover Nachtsheim
|
|||||||||
|
By:
|
/s/ Robert H. Trice, Ph.D.
|
Director
|
February 28, 2011
|
||||||
|
Robert H. Trice, Ph.D.
|
|||||||||
|
By:
|
/s/ Robert P. Wayman
|
Director
|
February 28, 2011
|
||||||
|
Robert P. Wayman
|
|||||||||
INDEX TO EXHIBITS
|
EXHIBIT
NUMBER
|
DESCRIPTION OF DOCUMENT
|
|
2.1(1)
|
Agreement and Plan of Merger by and among Panomics, Inc., the Company, Panda Acquisition Corporation and the Equityholders’ Representative dated as of November 11, 2008.
|
|
3.1(2)
|
Restated Certificate of Incorporation.
|
|
3.2(3)
|
Amended and Restated Bylaws.
|
|
4.1(4)
|
Indenture dated as of December 15, 2003, between the Company and The Bank of New York, as Trustee.
|
|
4.2(5)
|
Indenture dated as of November 16, 2007, between the Company and the Bank of New York Trust Company, N.A. as Trustee.
|
|
10.1(6)‡
|
1993 Stock Plan, as amended.
|
|
10.2(6)‡
|
1996 Nonemployee Directors Stock Option Plan.
|
|
10.3(7)
|
Lease between Sobrato Interests and the Company dated June 12, 1996 (3380 Central Expressway, Santa Clara, CA).
|
|
10.4(7)
|
Lease between Sobrato Interests and the Company dated May 31, 1996 (3450 Central Expressway, Santa Clara, CA).
|
|
10.5(8)‡
|
1998 Stock Incentive Plan.
|
|
10.6(8)‡
|
Form of Officer and Director Indemnification Agreement.
|
|
10.7(9)‡
|
Amendment No. 1 to the 1996 Nonemployee Directors Stock Option Plan of the Company
|
|
10.8(10)‡
|
Amended and Restated 1996 Non-Employee Directors Stock Plan.
|
|
10.9(11)*
|
Common Terms Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.10(11)*
|
License Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.11(11)*
|
Affymetrix Instrument and Chip Supply Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.12(11)*
|
Research & Development Collaboration Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.13(11)*
|
Diagnostic Product and Instrument Agency Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.14(11)*
|
Affymetrix Instrument Agency Agreement between F. Hoffmann-La Roche Ltd. and the Company dated January 29, 2003.
|
|
10.15(12)
|
Lease between Sobrato Interests and the Company dated July 3, 2002 (3420 Central Expressway, Santa Clara, CA).
|
|
10.16(12)
|
First Amendment to Lease between Sobrato Interests and the Company dated September 30, 2003 (3420 Central Expressway, Santa Clara, CA).
|
|
10.17(13)‡
|
Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan, as adopted effective March 9, 2000 and amended through May 14, 2010.
|
|
10.18(14)‡
|
Form of Non-Qualified Stock Option Agreement under the Affymetrix, Inc. Amended and Restated 1996 Non-Employee Directors Stock Plan.
|
|
10.19(1)‡
|
Form of Stock Option Agreement under the Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan.
|
|
10.20(15)
|
Fifth Amendment to Lease between Sobrato Interests and the Company dated July 3, 2002 (3380 Central Expressway, Santa Clara, CA).
|
|
10.21(15)
|
First Amendment to Lease between Sobrato Interests and the Company dated July 3, 2002 (3450 Central Expressway, Santa Clara, CA).
|
|
10.22(16)
|
Lease between Keppel Logistics Pte Ltd. and Affymetrix Pte Ltd. dated as of January 1, 2006 (7 Gul Circle, Singapore 629363).
|
|
10.23(27)
|
Addendum to Lease Agreement between Keppel Logistics Pte Ltd. and Affymetrix Pte Ltd. dated June 1, 2010 (7 Gul Circle, Singapore 629363)
|
|
10.24(1)‡
|
Form of Restricted Stock Agreement under the Affymetrix, Inc. Amended and Restated 2000 Equity Incentive Plan.
|
|
10.25(17)‡
|
Offer Letter from the Company to Kevin M. King dated December 18, 2006.
|
|
10.26(18)**
|
Amendment Agreement dated December 22, 2006 by and among F. Hoffmann-La Roche Ltd., Roche Molecular Systems, Inc. and the Company
|
|
10.27(19)‡
|
Offer Letter from the Company to John C. Batty dated May 16, 2007.
|
|
10.28(20)
|
Settlement and Release Agreement dated January 9, 2008 between the Company and Illumina, Inc.
|
|
10.29(21)
|
Affymetrix, Inc. Change of Control Plan, as amended through May 14, 2010.
|
|
10.30(22)‡
|
Offer Letter from the Company to John F. (Rick) Runkel dated October 6, 2008.
|
|
10.31(23)
|
First Amendment to Affymetrix, Inc. 1998 Stock Incentive Plan.
|
|
10.32(24)
|
Stipulation of Settlement regarding the Affymetrix Derivative Litigation in the United States District Court, Northern District of California.
|
|
10.33(25)‡
|
Offer Letter from the Company to Andrew J. Last, Ph.D. dated November 2, 2009.
|
|
10.34(25)
|
Lease Agreement between SBP Limited Partnership and the Company dated August 10, 2008 (26309 Miles Road, Warrensville Heights, OH).
|
|
10.35(25)
|
First Amendment and Lease Expansion Agreement between SBP Limited Partnership and the Company dated May 20, 2009 (26309 Miles Road, Warrensville Heights, OH).
|
|
10.36(25)
|
Lease Agreement between OTR, acting as the duly authorized nominee of The State Teacher Retirement System of Ohio and Anatrace, Inc. dated February 14, 2001 (434 Dussel Drive, Maumee, OH).
|
|
10.37(25)
|
Assignment and Assumption of Lease between Anatrace, Inc. and USB Acquisition dated April 30, 2005 (434 Dussel Drive, Maumee, OH).
|
|
10.38(26)‡
|
Offer Letter from the Company to Timothy C. Barabe dated March 9, 2010.
|
|
10.39(26)‡
|
Separation Agreement between the Company and John C. Batty dated March 17. 2010.
|
|
10.40(26)
|
Lease Agreement between the Company and Miles/Commerce Ltd. dated April 1, 2010 (26101 Miles Road, Warrensville Heights, OH).
|
|
10.41(26)
|
Lease Agreement between the Company and 26111 Miles Road Ltd. dated April 1, 2010 (26111 Miles Road, Warrensville Heights, OH).
|
|
10.42(21)‡
|
Executive Severance Policy dated May 14, 2010.
|
|
21
|
List of Subsidiaries.
|
|
23
|
Consent of Independent Registered Public Accounting Firm.
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Section 302 of Sarbanes-Oxley Act of 2002.
|
|
32
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted Pursuant to Section 906 of Sarbanes-Oxley Act of 2002.
|
|
(1)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 2, 2009 (File No. 000-28218).
|
|
(2)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on June 13, 2000 (File No. 000-28218).
|
|
(3)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on August 7, 2009 (File No. 000-28218).
|
|
(4)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-3 as filed on January 29, 2004 (File No. 333-112311).
|
|
(5)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on November 19, 2007 (File No. 000-28218).
|
|
(6)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-1 (File No. 333-3648), as amended.
|
|
(7)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on August 14, 1996 (File No. 000-28218).
|
|
(8)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 31, 1999 (File No. 000-28218).
|
|
(9)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-3 as filed on July 12, 1999 (File No. 333-82685), as amended.
|
|
(10)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 15, 2001 (File No. 000-28218).
|
|
(11)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 15, 2003 (File No. 000-28218).
|
|
(12)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 15, 2004 (File No. 000-28218).
|
|
(13)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-8 as filed on May 17, 2010 (File No. 333-166894).
|
|
(14)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on November 9, 2004 (File No. 000-28218).
|
|
(15)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 16, 2005 (File No. 000-28218).
|
|
(16)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 9, 2006 (File No. 000-28218).
|
|
(17)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on December 19, 2006 (File No. 000-28218).
|
|
(18)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on December 22, 2006 (File No. 000-28218).
|
|
(19)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on June 6, 2007 (File No. 000-28218).
|
|
(20)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on February 29, 2008 (File No. 000-28218).
|
|
(21)
|
Incorporated by reference to Registrant’s Annual Report on Form 8-K as filed on May 18, 2010 (File No. 000-28218).
|
|
(22)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on November 7, 2008 (File No. 000-28218).
|
|
(23)
|
Incorporated by reference to Registrant’s Registration Statement on Form S-8 as filed on April 18, 2001 (File No. 333-59158).
|
|
(24)
|
Incorporated by reference to Registrant’s Current Report on Form 8-K as filed on May 20, 2009 (File No. 000-28218).
|
|
(25)
|
Incorporated by reference to Registrant’s Annual Report on Form 10-K as filed on March 1, 2010 (File No. 000-28218).
|
|
(26)
|
Incorporated by reference to Registrant’s Quarterly Report on Form 10-Q as filed on May 6, 2010 (File No. 000-28218).
|
|
(27)
|
Filed herewith.
|
|
‡
|
Management contract, compensatory plan, contract or arrangement
|
|
*
|
Confidential treatment granted
|
** Confidential treatment requested
