Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ANI PHARMACEUTICALS INC | a11-3023_18k.htm |
| EX-99.1 - EX-99.1 - ANI PHARMACEUTICALS INC | a11-3023_1ex99d1.htm |
Exhibit 99.2
|
|
BioSante and LibiGel® Market Overview January 2011 |
|
|
To the extent any statements made in this presentation deal with information that is not historical, these are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about BioSante’s plans, objectives, expectations and intentions with respect to future operations and products, future market acceptance, size and potential of LibiGel and other statements identified by words such as “would,” “will,” “potential,” “could,” “can,” “believe,” “intends,” “continue,” “plans,” “expects,” “anticipates,” “estimates,” “may,” other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause BioSante’s actual results to be materially different than those expressed in or implied by BioSante’s forward-looking statements. For BioSante, particular uncertainties and risks include, among others, the difficulty of developing pharmaceutical products, obtaining regulatory and other approvals and achieving market acceptance; the marketing success of BioSante’s licensees or sublicensees; the success of clinical testing; and BioSante’s need for and ability to obtain additional financing. More detailed information on these and additional factors that could affect BioSante’s actual results are described in BioSante’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10-K and subsequent quarterly reports on Form 10-Q. All forward-looking statements in this presentation speak only as of the date of this presentation. BioSante undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. Disclaimer—Forward-looking Statements |
|
|
BioSante Overview Company Information |
|
|
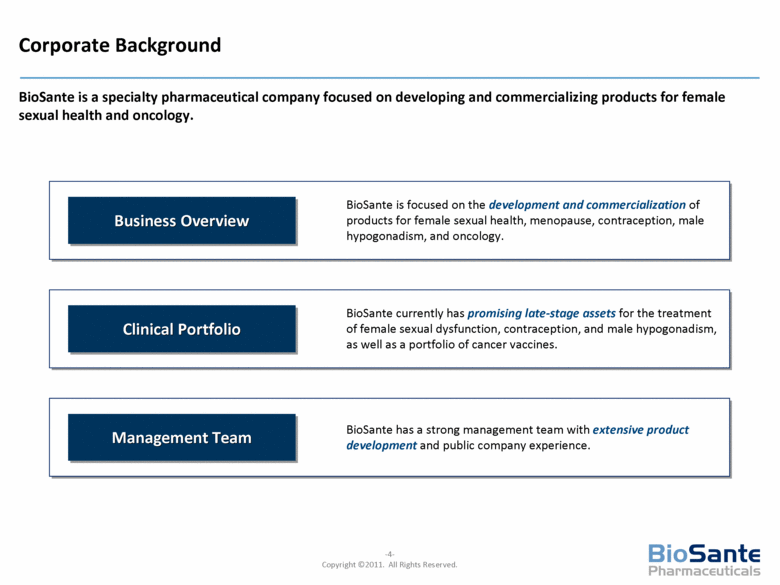
BioSante is focused on the development and commercialization of products for female sexual health, menopause, contraception, male hypogonadism, and oncology. Business Overview BioSante has a strong management team with extensive product development and public company experience. Management Team BioSante currently has promising late-stage assets for the treatment of female sexual dysfunction, contraception, and male hypogonadism, as well as a portfolio of cancer vaccines. Clinical Portfolio Corporate Background BioSante is a specialty pharmaceutical company focused on developing and commercializing products for female sexual health and oncology. |
|
|
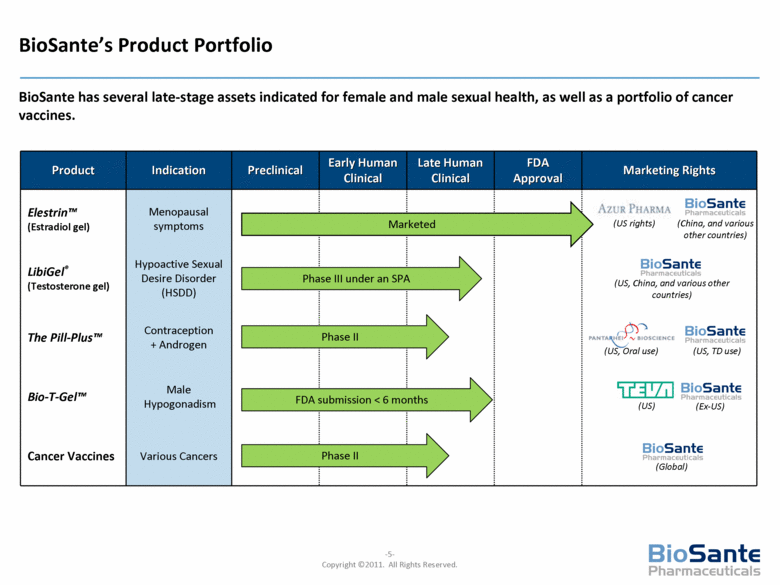
Product Indication Preclinical Early Human Clinical Late Human Clinical FDA Approval Marketing Rights Elestrin™ (Estradiol gel) Menopausal symptoms LibiGel® (Testosterone gel) Hypoactive Sexual Desire Disorder (HSDD) The Pill-Plus™ Contraception + Androgen Bio-T-Gel™ Male Hypogonadism Cancer Vaccines Various Cancers Marketed BioSante’s Product Portfolio BioSante has several late-stage assets indicated for female and male sexual health, as well as a portfolio of cancer vaccines. Phase III under an SPA Phase II FDA submission < 6 months (US, China, and various other countries) (US, Oral use) (Ex-US) (US) (US, TD use) Phase II (Global) (China, and various other countries) (US rights) |
|
|
LibiGel® Overview |
|
|
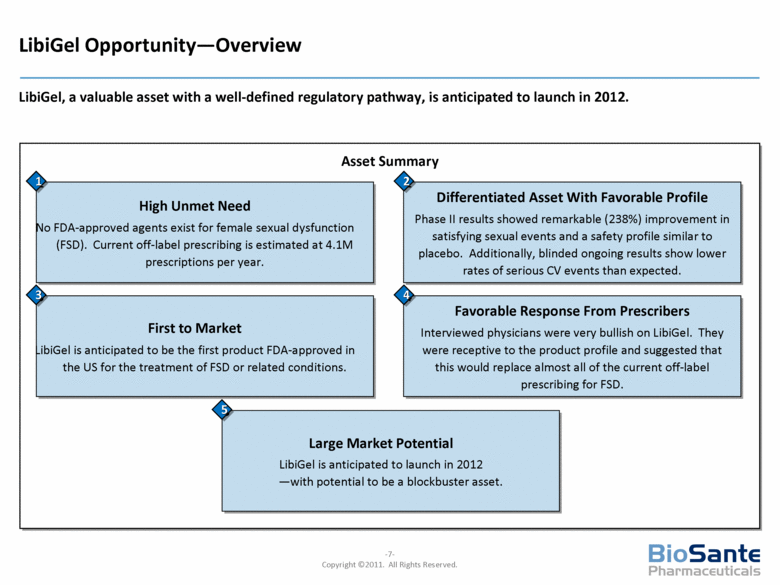
LibiGel Opportunity—Overview LibiGel, a valuable asset with a well-defined regulatory pathway, is anticipated to launch in 2012. Asset Summary Differentiated Asset With Favorable Profile Phase II results showed remarkable (238%) improvement in satisfying sexual events and a safety profile similar to placebo. Additionally, blinded ongoing results show lower rates of serious CV events than expected. High Unmet Need No FDA-approved agents exist for female sexual dysfunction (FSD). Current off-label prescribing is estimated at 4.1M prescriptions per year. 1 2 Favorable Response From Prescribers Interviewed physicians were very bullish on LibiGel. They were receptive to the product profile and suggested that this would replace almost all of the current off-label prescribing for FSD. First to Market LibiGel is anticipated to be the first product FDA-approved in the US for the treatment of FSD or related conditions. 3 4 Large Market Potential LibiGel is anticipated to launch in 2012 —with potential to be a blockbuster asset. 5 |
|
|
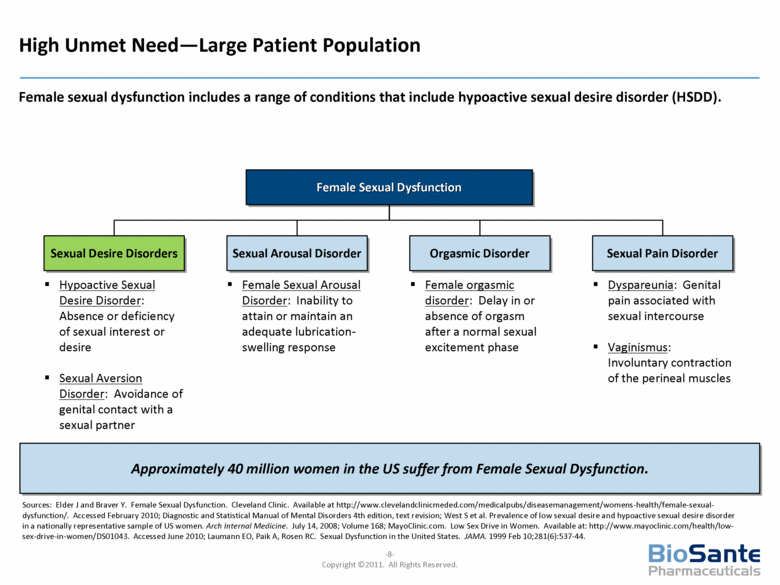
Female Sexual Dysfunction Sexual Desire Disorders Orgasmic Disorder Sexual Pain Disorder Sexual Arousal Disorder Sources: Elder J and Braver Y. Female Sexual Dysfunction. Cleveland Clinic. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/female-sexual-dysfunction/. Accessed February 2010; Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision; West S et al. Prevalence of low sexual desire and hypoactive sexual desire disorder in a nationally representative sample of US women. Arch Internal Medicine. July 14, 2008; Volume 168; MayoClinic.com. Low Sex Drive in Women. Available at: http://www.mayoclinic.com/health/low-sex-drive-in-women/DS01043. Accessed June 2010; Laumann EO, Paik A, Rosen RC. Sexual Dysfunction in the United States. JAMA. 1999 Feb 10;281(6):537-44. Hypoactive Sexual Desire Disorder: Absence or deficiency of sexual interest or desire Sexual Aversion Disorder: Avoidance of genital contact with a sexual partner Female Sexual Arousal Disorder: Inability to attain or maintain an adequate lubrication-swelling response Female orgasmic disorder: Delay in or absence of orgasm after a normal sexual excitement phase Dyspareunia: Genital pain associated with sexual intercourse Vaginismus: Involuntary contraction of the perineal muscles Female sexual dysfunction includes a range of conditions that include hypoactive sexual desire disorder (HSDD). High Unmet Need—Large Patient Population Approximately 40 million women in the US suffer from Female Sexual Dysfunction. |
|
|
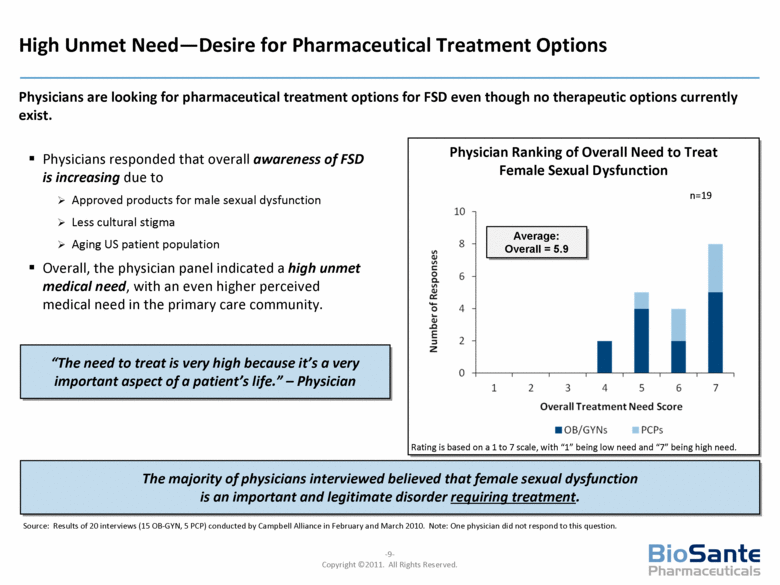
Physician Ranking of Overall Need to Treat Female Sexual Dysfunction Physicians responded that overall awareness of FSD is increasing due to Approved products for male sexual dysfunction Less cultural stigma Aging US patient population Overall, the physician panel indicated a high unmet medical need, with an even higher perceived medical need in the primary care community. Rating is based on a 1 to 7 scale, with “1” being low need and “7” being high need. n=19 Average: Overall = 5.9 The majority of physicians interviewed believed that female sexual dysfunction is an important and legitimate disorder requiring treatment. Source: Results of 20 interviews (15 OB-GYN, 5 PCP) conducted by Campbell Alliance in February and March 2010. Note: One physician did not respond to this question. High Unmet Need—Desire for Pharmaceutical Treatment Options Physicians are looking for pharmaceutical treatment options for FSD even though no therapeutic options currently exist. “The need to treat is very high because it’s a very important aspect of a patient’s life.” – Physician |
|
|
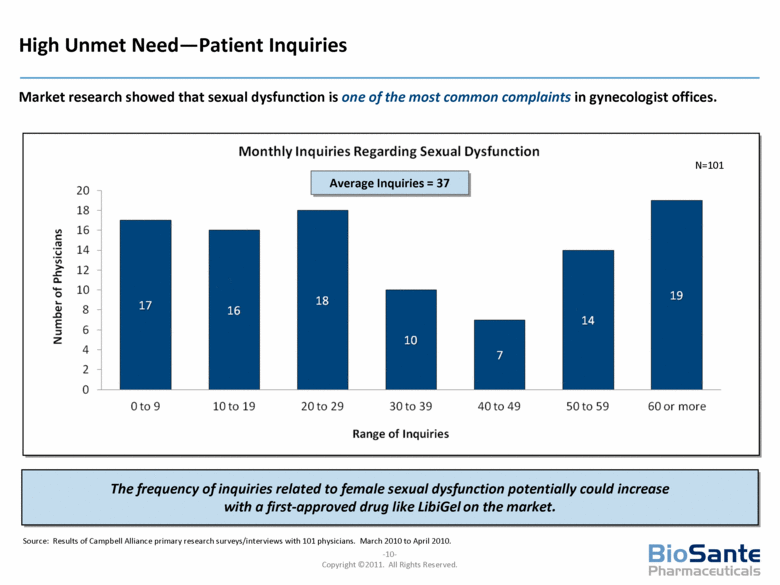
High Unmet Need—Patient Inquiries Market research showed that sexual dysfunction is one of the most common complaints in gynecologist offices. Average Inquiries = 37 The frequency of inquiries related to female sexual dysfunction potentially could increase with a first-approved drug like LibiGel on the market. N=101 Source: Results of Campbell Alliance primary research surveys/interviews with 101 physicians. March 2010 to April 2010. |
|
|
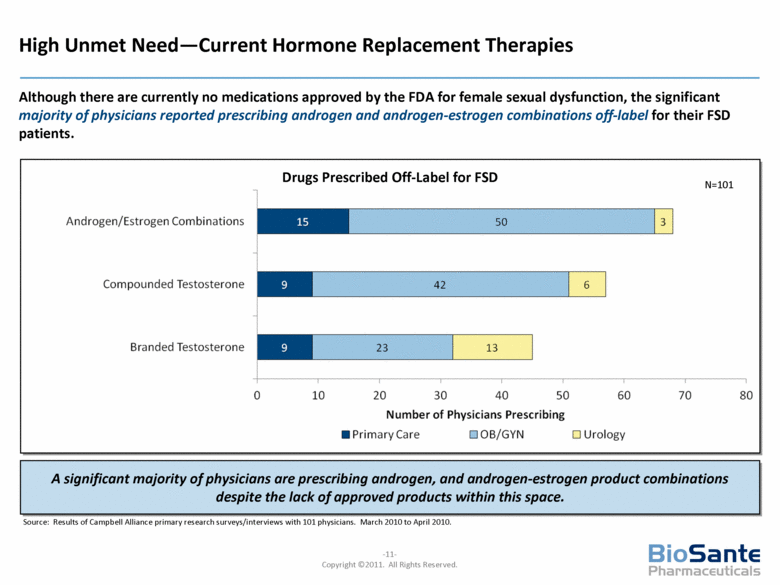
Drugs Prescribed Off-Label for FSD High Unmet Need—Current Hormone Replacement Therapies N=101 Source: Results of Campbell Alliance primary research surveys/interviews with 101 physicians. March 2010 to April 2010. Although there are currently no medications approved by the FDA for female sexual dysfunction, the significant majority of physicians reported prescribing androgen and androgen-estrogen combinations off-label for their FSD patients. A significant majority of physicians are prescribing androgen, and androgen-estrogen product combinations despite the lack of approved products within this space. |
|
|
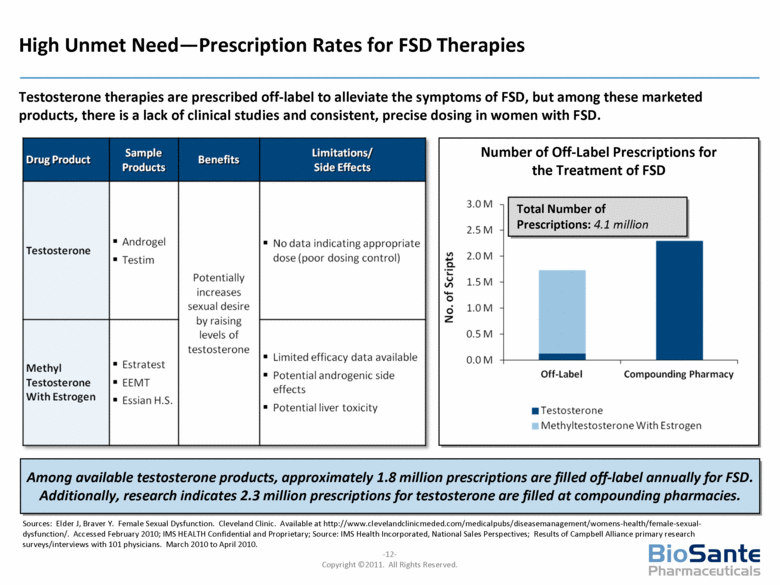
Sources: Elder J, Braver Y. Female Sexual Dysfunction. Cleveland Clinic. Available at http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/womens-health/female-sexual-dysfunction/. Accessed February 2010; IMS HEALTH Confidential and Proprietary; Source: IMS Health Incorporated, National Sales Perspectives; Results of Campbell Alliance primary research surveys/interviews with 101 physicians. March 2010 to April 2010. High Unmet Need—Prescription Rates for FSD Therapies Testosterone therapies are prescribed off-label to alleviate the symptoms of FSD, but among these marketed products, there is a lack of clinical studies and consistent, precise dosing in women with FSD. Among available testosterone products, approximately 1.8 million prescriptions are filled off-label annually for FSD. Additionally, research indicates 2.3 million prescriptions for testosterone are filled at compounding pharmacies. Number of Off-Label Prescriptions for the Treatment of FSD Total Number of Prescriptions: 4.1 million |
|
|
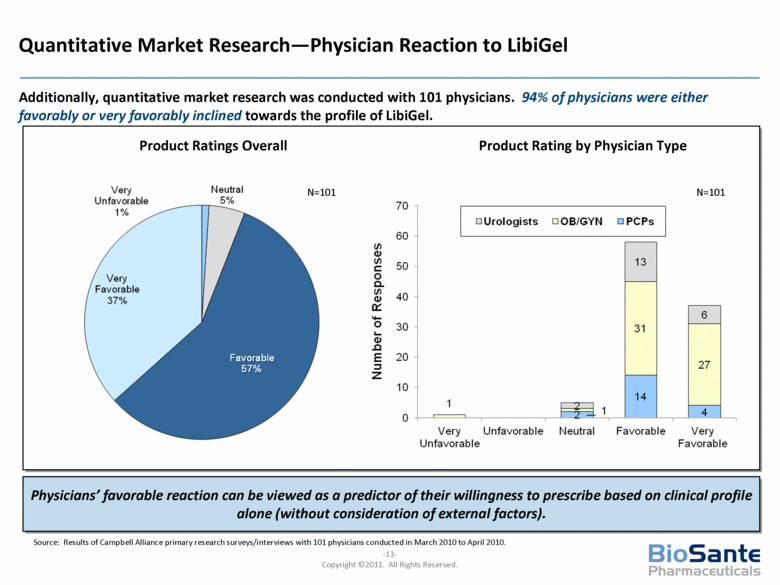
Quantitative Market Research—Physician Reaction to LibiGel Additionally, quantitative market research was conducted with 101 physicians. 94% of physicians were either favorably or very favorably inclined towards the profile of LibiGel. Product Rating by Physician Type Physicians’ favorable reaction can be viewed as a predictor of their willingness to prescribe based on clinical profile alone (without consideration of external factors). N=101 N=101 Product Ratings Overall Source: Results of Campbell Alliance primary research surveys/interviews with 101 physicians conducted in March 2010 to April 2010. |
|
|
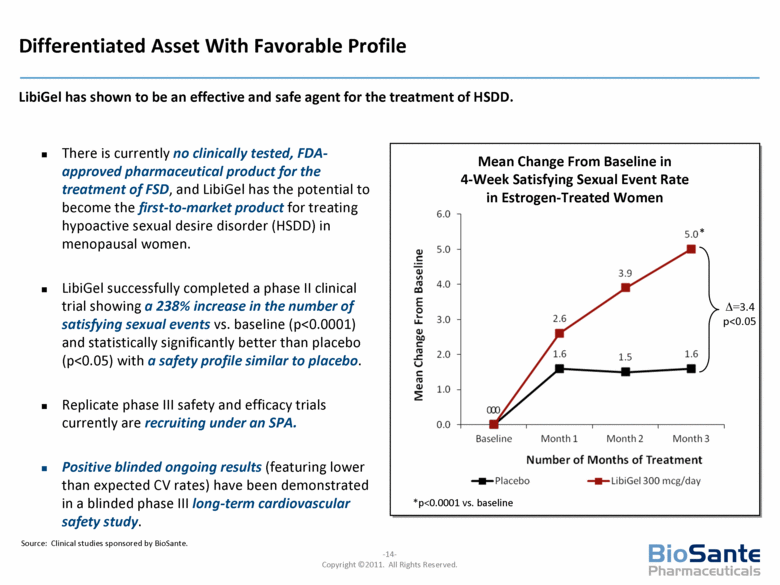
Differentiated Asset With Favorable Profile There is currently no clinically tested, FDA-approved pharmaceutical product for the treatment of FSD, and LibiGel has the potential to become the first-to-market product for treating hypoactive sexual desire disorder (HSDD) in menopausal women. LibiGel successfully completed a phase II clinical trial showing a 238% increase in the number of satisfying sexual events vs. baseline (p<0.0001) and statistically significantly better than placebo (p<0.05) with a safety profile similar to placebo. Replicate phase III safety and efficacy trials currently are recruiting under an SPA. Positive blinded ongoing results (featuring lower than expected CV rates) have been demonstrated in a blinded phase III long-term cardiovascular safety study. LibiGel has shown to be an effective and safe agent for the treatment of HSDD. Mean Change From Baseline in 4-Week Satisfying Sexual Event Rate in Estrogen-Treated Women =3.4 p<0.05 *p<0.0001 vs. baseline * Source: Clinical studies sponsored by BioSante. |
|
|
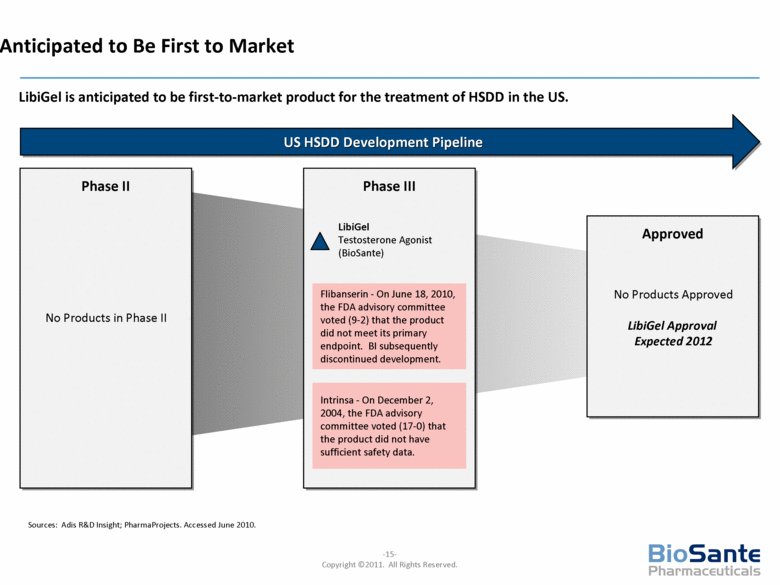
Anticipated to Be First to Market LibiGel is anticipated to be first-to-market product for the treatment of HSDD in the US. Sources: Adis R&D Insight; PharmaProjects. Accessed June 2010. US HSDD Development Pipeline Phase II Approved No Products Approved LibiGel Approval Expected 2012 No Products in Phase II Phase III LibiGel Testosterone Agonist (BioSante) Flibanserin - On June 18, 2010, the FDA advisory committee voted (9-2) that the product did not meet its primary endpoint. BI subsequently discontinued development. Intrinsa - On December 2, 2004, the FDA advisory committee voted (17-0) that the product did not have sufficient safety data. |
|
|
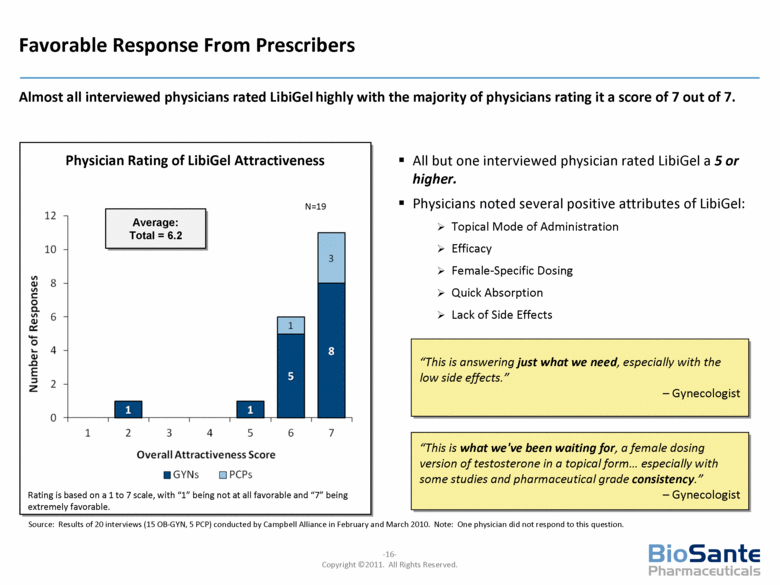
Physician Rating of LibiGel Attractiveness Favorable Response From Prescribers Almost all interviewed physicians rated LibiGel highly with the majority of physicians rating it a score of 7 out of 7. All but one interviewed physician rated LibiGel a 5 or higher. Physicians noted several positive attributes of LibiGel: Topical Mode of Administration Efficacy Female-Specific Dosing Quick Absorption Lack of Side Effects “This is answering just what we need, especially with the low side effects.” – Gynecologist N=19 Average: Total = 6.2 Rating is based on a 1 to 7 scale, with “1” being not at all favorable and “7” being extremely favorable. “This is what we've been waiting for, a female dosing version of testosterone in a topical form... especially with some studies and pharmaceutical grade consistency.” – Gynecologist Source: Results of 20 interviews (15 OB-GYN, 5 PCP) conducted by Campbell Alliance in February and March 2010. Note: One physician did not respond to this question. |
|
|
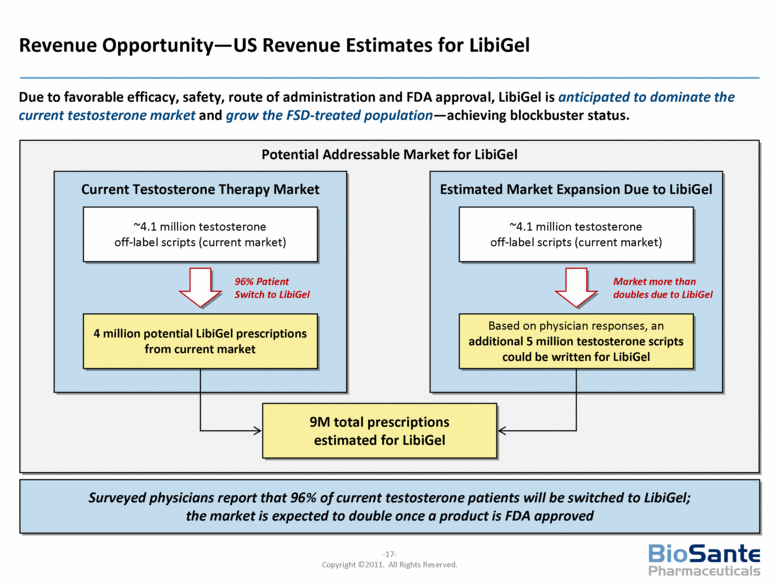
Revenue Opportunity—US Revenue Estimates for LibiGel Due to favorable efficacy, safety, route of administration and FDA approval, LibiGel is anticipated to dominate the current testosterone market and grow the FSD-treated population—achieving blockbuster status. Potential Addressable Market for LibiGel Current Testosterone Therapy Market Estimated Market Expansion Due to LibiGel ~4.1 million testosterone off-label scripts (current market) 4 million potential LibiGel prescriptions from current market ~4.1 million testosterone off-label scripts (current market) Based on physician responses, an additional 5 million testosterone scripts could be written for LibiGel 96% Patient Switch to LibiGel Market more than doubles due to LibiGel 9M total prescriptions estimated for LibiGel Surveyed physicians report that 96% of current testosterone patients will be switched to LibiGel; the market is expected to double once a product is FDA approved |
|
|
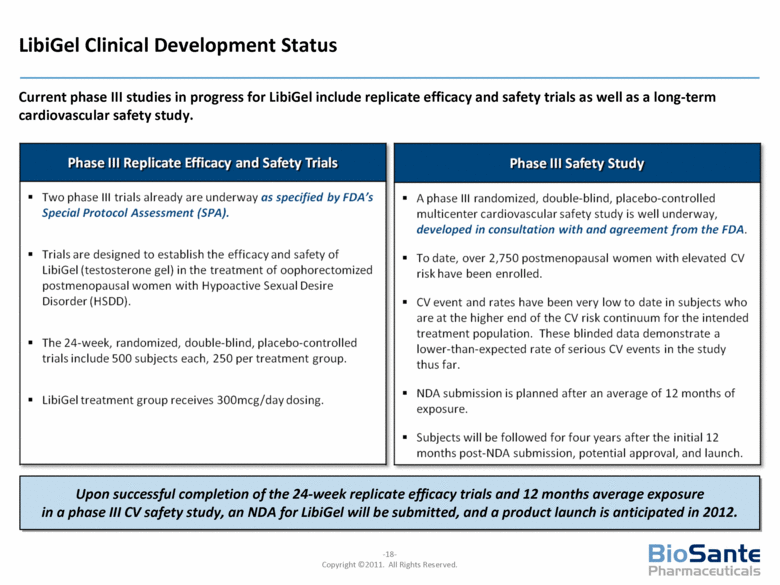
LibiGel Clinical Development Status Current phase III studies in progress for LibiGel include replicate efficacy and safety trials as well as a long-term cardiovascular safety study. Upon successful completion of the 24-week replicate efficacy trials and 12 months average exposure in a phase III CV safety study, an NDA for LibiGel will be submitted, and a product launch is anticipated in 2012. Phase III Replicate Efficacy and Safety Trials Two phase III trials already are underway as specified by FDA's Special Protocol Assessment (SPA). Trials are designed to establish the efficacy and safety of LibiGel (testosterone gel) in the treatment of oophorectomized postmenopausal women with Hypoactive Sexual Desire Disorder (HSDD). The 24-week, randomized, double-blind, placebo-controlled trials include 500 subjects each, 250 per treatment group. LibiGel treatment group receives 300mcg/day dosing. Phase III Safety Study A phase III randomized, double-blind, placebo-controlled multicenter cardiovascular safety study is well underway, developed in consultation with and agreement from the FDA. To date, over 2,750 postmenopausal women with elevated CV risk have been enrolled. CV event and rates have been very low to date in subjects who are at the higher end of the CV risk continuum for the intended treatment population. These blinded data demonstrate a lower-than-expected rate of serious CV events in the study thus far. NDA submission is planned after an average of 12 months of exposure. Subjects will be followed for four years after the initial 12 months post-NDA submission, potential approval, and launch. |