Attached files
Exhibit 99.3
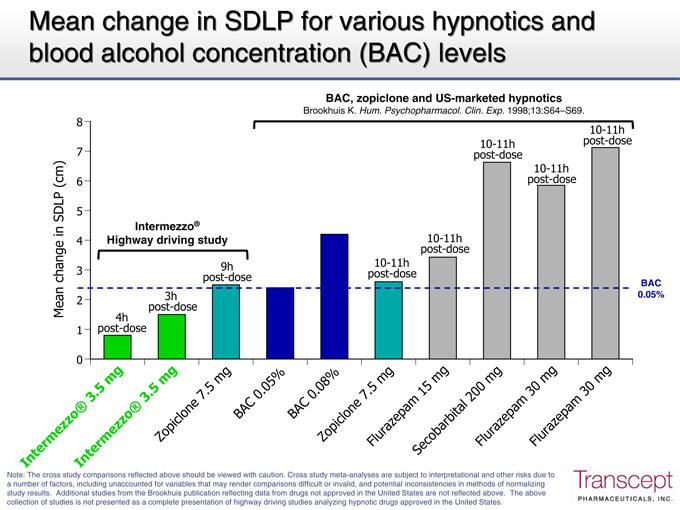
Mean change in SDLP for various hypnotics and blood alcohol concentration (BAC) levels
BAC, zopiclone and US-marketed hypnotics
Brookhuis K. Hum. Psychopharmacol. Clin. Exp. 1998;13:S64–S69.
8 10-11h
10-11h post-dose
7 post-dose
10-11h
(cm) 6 post-dose
SDLP 5
Intermezzo®
in 4 Highway driving study 10-11h
post-dose
3 9h post-dose 10-11h
change post-dose BAC
2 3h 0.05%
Mean post-dose
4h
1 post-dose
0
Inter me In zzo® t e3 . 5 rmezmg zo ® 3.
5
Zopiclone mg 7 . 5 m g BAC
0 . 05% BAC
0. Zo 08 piclone % 7 . 5 m Flurazepam g Se1 c 5 obarbital mg Fl200 uramg z ep am 30 Flurmg azepam 30 m g
Note: The cross study comparisons reflected above should be viewed with caution. Cross study meta-analyses are subject to interpretational and other risks due to a number of factors, including unaccounted for variables that may render comparisons difficult or invalid, and potential inconsistencies in methods of normalizing study results. Additional studies from the Brookhuis publication reflecting data from drugs not approved in the United States are not reflected above. The above collection of studies is not presented as a complete presentation of highway driving studies analyzing hypnotic drugs approved in the United States.
