Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CLINICAL DATA INC | b81084e8vk.htm |
| EX-99.1 - EX-99.1 - CLINICAL DATA INC | b81084exv99w1.htm |
| EX-99.2 - EX-99.2 - CLINICAL DATA INC | b81084exv99w2.htm |
| EX-99.6 - EX-99.6 - CLINICAL DATA INC | b81084exv99w6.htm |
| EX-99.4 - EX-99.4 - CLINICAL DATA INC | b81084exv99w4.htm |
| EX-99.5 - EX-99.5 - CLINICAL DATA INC | b81084exv99w5.htm |
| EX-99.8 - EX-99.8 - CLINICAL DATA INC | b81084exv99w8.htm |
| EX-99.7 - EX-99.7 - CLINICAL DATA INC | b81084exv99w7.htm |
Exhibit 99.3
Poster NR4-22
A 1-Year Open-Label Study Assessing the Safety and Tolerability of Vilazodone in Patients
With Major Depressive Disorder
With Major Depressive Disorder
Donald S. Robinson, MD,1 Daniel K. Kajdasz, PhD,2 Susan Gallipoli, RN,2
Heidi Whalen, MHS,2 Art Wamil, MD, PhD,2
Carol R. Reed, MD2
Carol R. Reed, MD2
1Worldwide Drug Development, Burlington, Vermont; 2PGxHealth, LLC, New Haven, Connecticut
Abstract
Objective
Vilazodone HCl is a dual-acting selective serotonin reuptake inhibitor and 5-HT1A
receptor partial agonist that has been studied in two 8-week, phase 3 studies in adults with major
depressive disorder (MDD). The objective of this study was to assess the long-term safety profile
of vilazodone (VLZ).
Method
This 52-week, open-label, multicenter study enrolled MDD patients 18-70 years of age with
scores ≥18 on HAM-D17 at screening and baseline. The VLZ dose was titrated to 40 mg QD by day 14
and then continued for up to 52 weeks (safety population, n = 599; completers, n = 254). Drug
safety was assessed by measurement of treatment-emergent adverse events (AEs), lab tests, physical
exams, vital signs, weight, ECGs, discontinuations due to AEs, and the Changes in Sexual Function
Questionnaire (CSFQ). Secondary outcome measures included MADRS, CGI-S, and CGI-I.
Results
Discontinuations due to AEs in ≥1% of patients were nausea (1.3%), diarrhea (1.2%), and
anxiety (1.0%). Most AEs (95.9%) were of mild or moderate intensity; severe AEs occurred in 14.9%
of patients (severe AEs ≥1% incidence: psychiatric, 4.5%; gastrointestinal, 3.5%; and nervous
system, 2.7%). The most frequent AEs were diarrhea (35.7%), nausea (31.6%), and headache (20.0%),
with most (96.3%, 95.8%, 94.2%, respectively) rated mild or moderate in intensity. Most serious AEs
(33 in 23 patients) were judged by investigators as not or unlikely to be related to VLZ. Lab, ECG,
and vital sign
abnormalities were considered to be not clinically relevant. Decreased libido was the most frequent
AE related to sexual function (4.2% of patients). CSFQ scores improved in males and females over
the 52 weeks. MADRS mean total scores (OC) were: baseline, 29.9 (n = 596); week 8, 11.4 (n = 447);
week 24, 8.2 (n = 313), and week 52, 7.1 (n = 254). CGI-I and CGI-S scores also improved.
Conclusions
In this study, vilazodone 40 mg QD treatment of adults with MDD for up to 1 year appeared to
be well tolerated.
Introduction
| • | Major depressive disorder (MDD) is a common mental disorder; an estimated 16.5 million adults in the United States experience major depressive episodes each year1 | |
| • | MDD is a chronic disorder that warrants continuing treatment beyond the acute phase of 4 to 6 months; continuing treatment reduces depressive relapse by approximately two-thirds2 | |
| • | Assessing the safety of the long-term use of antidepressant drugs is important3 | |
| • | Vilazodone HCl is a dual-acting potent and selective serotonin reuptake inhibitor and 5-HT1A receptor partial agonist that exerts its effects at the serotonin transporter and at pre- and post-synaptic 5-HT1A receptors4,5 |
| — | The unique dual modulation of serotonin neurotransmission by vilazodone is hypothesized to decrease endogenous serotonin negative feedback and enhance post-synaptic 5-HT effects |
| • | The safety, tolerability, and efficacy of vilazodone for the short-term treatment of MDD have been shown in two 8-week, randomized, placebo-controlled studies6 (see APA poster NR4-2) |
Objectives
| • | Primary |
| — | To assess the long-term safety and tolerability of vilazodone treatment in adults with MDD |
| • | Secondary |
| — | To assess the effectiveness of vilazodone during long-term, open-label treatment in adults with MDD |
Methods
| • | All patients provided written informed consent | |
| • | The protocol was approved by the institutional review board of each center |
| Presented at the 163rd Annual Meeting of the American Psychiatric Association, May 22-26, 2010, New Orleans, Louisiana | Supported by PGxHealth, LLC |
Study Population
| • | Selected inclusion criteria |
| — | Male and female patients; 18 to 70 years of age | ||
| — | Diagnosis of MDD according to the DSM-IV-TR, as confirmed by Mini-International Neuropsychiatric Interview | ||
| — | 17-item Hamilton Depression Scale (HAM-D-17) score of ≥18 at screening and baseline visits |
| • | Selected exclusion criteria |
| — | History of schizophrenia, schizoaffective disorder, or bipolar I or II disorder | ||
| — | Substance abuse or dependence according to DSM-IV-TR criteria within 1 year of baseline (nicotine and caffeine excepted) | ||
| — | Medical or neurologic condition that made it unlikely the patient could complete 1 year of treatment or that precluded administration of vilazodone |
Study Design
| • | 52-week, open-label, multicenter study (Figure 1) | |
| • | Patients were titrated to the target dose of vilazodone 40 mg once daily (QD) over a 2-week period according to a fixed-titration schedule | |
| • | 600 patients were enrolled to obtain 300 patients with at least 6 months and 100 patients with at least 52 weeks of exposure |
Figure 1. Study design and treatment.
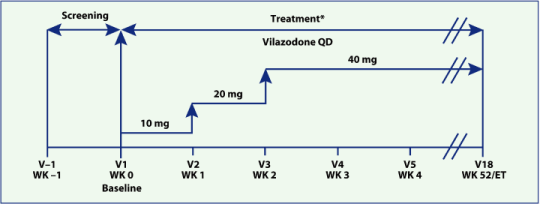
| * | Visits 5 to 7 were 2 weeks apart and visits 7
to 18 were 4 weeks apart. |
| • | Safety and Tolerability Assessments |
| — | Safety assessments |
| § | Adverse events (AEs), clinical laboratory evaluations, electrocardiography (ECG), physical examination, and vital signs |
| — | Patient-rated Changes in Sexual Functioning Questionnaire (CSFQ) for change in sexual function during treatment | ||
| — | Columbia-Suicide Severity Rating Scale to assess risk of suicidal ideation and/or behavior |
| • | Efficacy Assessments |
| — | Montgomery-Åsberg Depression Rating Scale (MADRS) | ||
| — | Clinical Global Impression-Severity (CGI-S) score | ||
| — | Clinical Global Impression-Improvement (CGI-I) score |
Statistical Analysis
Safety and Tolerability
| • | Safety evaluations were based on the safety population, defined as all enrolled patients dispensed study drug who had ≥1 postbaseline safety assessment |
| • | Summary statistics were presented for each assessment visit (observed cases) and change from baseline, where applicable |
| • | AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA, V 11.1), and were summarized by System Organ Class and Preferred Term |
| • | AEs were summarized by number and percentage of patients experiencing each AE |
Effectiveness
| • | Effectiveness evaluations were based on the effectiveness population, defined as all enrolled patients dispensed study drug with ≥1 postbaseline effectiveness measure |
| • | Mean changes from baseline for MADRS total score and CGI-S score were summarized at each postbaseline visit; CGI-I scores were summarized at each postbaseline visit |
| • | No statistical analyses were performed on safety or efficacy measures |
Results
| • | 616 patients were enrolled in the study, and 254 patients (41.2%) completed the study; 313 (51%) completed at least 6 months of the study (Figure 1) |
Figure 1. Subject disposition.
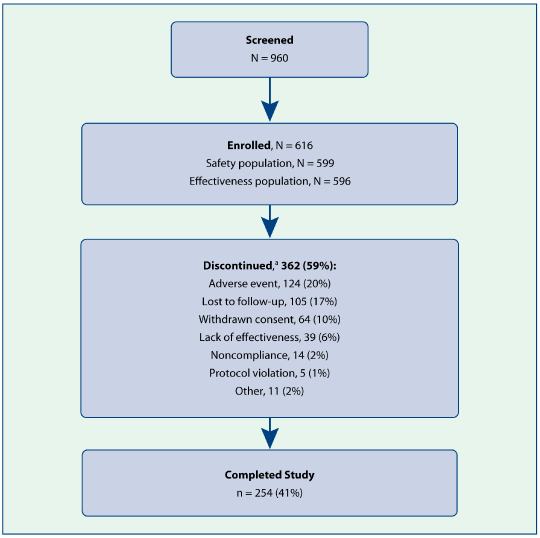
| a | n (%) of enrolled population. |
| • | Baseline demographic and disease characteristics are presented in Table 1 | |
| • | 89.1% of patients were compliant with overall treatment |
Table 1. Baseline Demographic and Disease Characteristics (Safety Population)
| Vilazodone | ||||
| Characteristic | N = 599 | |||
Sex, n (%) |
||||
Male |
192 (32.1 | ) | ||
Female |
407 (67.9 | ) | ||
Race, n (%) |
||||
White |
479 (80.0 | ) | ||
Black/African American |
103 (17.2 | ) | ||
Asian |
10 (1.7 | ) | ||
Other |
7 (1.1 | ) | ||
Mean age, y (SD) |
||||
Median (range) |
42.8 (12.5 | ) | ||
| 44 (18-70 | ) | |||
Duration of current MDD episode, n (%) |
||||
1-6 mo |
215 (35.9 | ) | ||
>6-12 mo |
172 (28.7 | ) | ||
>12 mo |
212 (35.4 | ) | ||
Severity of current episode,a n (%) |
||||
Mild |
11 (1.8 | ) | ||
Moderate |
469 (78.3 | ) | ||
Severe |
119 (19.9 | ) | ||
| a | DSM-IV-TR criteria of MDD severity: mild = 5 or 6 depressive symptoms; severe without psychotic features = presence of most criteria symptoms and observable disability; moderate = intermediate between mild and severe. |
Safety and Tolerability Results
| • | Treatment-emergent AEs (TEAEs) led to discontinuation in 124 of 599 patients (20.7%); specific TEAEs leading to discontinuation in ≥1% of patients were nausea (1.3%), diarrhea (1.2%), and anxiety (1.0%) |
| • | Most TEAEs were rated mild or moderate in intensity (95.9%); 89 patients (14.9%) reported 1 or more severe TEAEs |
| • | TEAEs occurring with the highest incidence were diarrhea (35.7%) and nausea (31.6%) (Table 2), with 96.3% and 95.8% of affected patients, respectively, reporting maximum severity of mild or moderate |
| • | 33 serious AEs (SAEs) occurred in 23 patients (3.8%); most were judged as not or unlikely related to vilazodone; pneumonia was the only SAE reported in more than 1 patient (n = 2) |
| Table 2. TEAEs Occurring in ≥5% of Patients (Safety Population) |
| Vilazodone | ||||
| N = 599 | ||||
| MedDRA Preferred Term | Patients, n (%) | |||
Diarrhea |
214 (35.7 | ) | ||
Nausea |
189 (31.6 | ) | ||
Headache |
120 (20.0 | ) | ||
Upper respiratory tract infection |
82 (13.7 | ) | ||
Insomnia |
78 (13.0 | ) | ||
Dry mouth |
66 (11.0 | ) | ||
Dizziness |
64 (10.7 | ) | ||
Somnolence |
64 (10.7 | ) | ||
Abnormal dreams |
62 (10.4 | ) | ||
Weight increased |
57 (9.5 | ) | ||
Increased appetite |
54 (9.0 | ) | ||
Fatigue |
46 (7.7 | ) | ||
Nasopharyngitis |
45 (7.5 | ) | ||
Vomiting |
44 (7.3 | ) | ||
Anxiety |
36 (6.0 | ) | ||
Urinary tract infection |
35 (5.8 | ) | ||
Back pain |
33 (5.5 | ) | ||
MedDRA, Medical Dictionary for Regulatory Activities.
| • | Mean changes from baseline in systolic and diastolic blood pressure and pulse were considered clinically unimportant (Table 3) |
| • | Mean change in weight was insignificant (1.71 kg) for subjects completing the 52 weeks of the study (Table 3) |
| Table 3. Change From Baseline in Blood Pressure (Sitting), Pulse, and Weight (Safety Population) |
| Vilazodone | ||||||||
| N = 599 | ||||||||
| Parameter | n | Mean Change From Baseline (SD) | ||||||
SBP, mm Hg |
||||||||
Week 8 |
448 | 1.2 (11.96 | ) | |||||
Week 12 |
389 | 0.3 (11.49 | ) | |||||
Week 24 |
313 | 0.4 (12.16 | ) | |||||
Week 52 |
254 | 0.8 (12.57 | ) | |||||
DBP, mm Hg |
||||||||
Week 8 |
448 | 0.3 (7.67 | ) | |||||
Week 12 |
389 | 0.6 (8.19 | ) | |||||
Week 24 |
313 | -0.2 (8.71 | ) | |||||
Week 52 |
254 | 0.5 (8.56 | ) | |||||
Pulse, bpm |
||||||||
Week 8 |
448 | -0.1 (9.51 | ) | |||||
Week 12 |
389 | 1.3 (10.25 | ) | |||||
Week 24 |
313 | -0.1 (10.47 | ) | |||||
Week 52 |
254 | 1.9 (11.15 | ) | |||||
Weight, kg |
||||||||
Week 8 |
448 | 0.29 (2.55 | ) | |||||
Week 12 |
389 | 0.63 (2.93 | ) | |||||
Week 24 |
313 | 0.81 (4.43 | ) | |||||
Week 52 |
254 | 1.71 (5.90 | ) | |||||
SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per minute.
| • | Mean changes from baseline in hematology, blood chemistry, and urinalysis values showed no clinically important trends |
| • | Treatment-emergent elevations in LFTs to ≥3.0 × the upper limit of the normal range at end point were observed in a few patients: ALKPHOS and TBILI, 0 patients; ALT and AST, 2 patients (0.4%); GGTP, 9 patients (1.7%) | |
| • | No clinically significant changes in ECGs occurred |
| • | Overall, CSFQ total scores improved for men and women during treatment (1.5 [n = 192] and 2.7 [n = 407], respectively) |
| • | Decreased libido, which occurred in 25 (4.2%) patients, was the most frequent TEAE related to sexual function |
| • | When mapped to the Columbia-Classification Algorithm for Suicide Assessment, 7 patients (1.2%) met criteria for exhibiting treatment-emergent suicidal behavior |
Effectiveness Results
| • | Effectiveness measures showed improvement over 52 weeks of treatment |
| — | Mean MADRS score was 29.9 at baseline and 7.1 at week 52 (Figure 2) | ||
| — | Mean CGI-S score was 4.3 at baseline and 1.7 at week 52, representing an overall change of -2.6 | ||
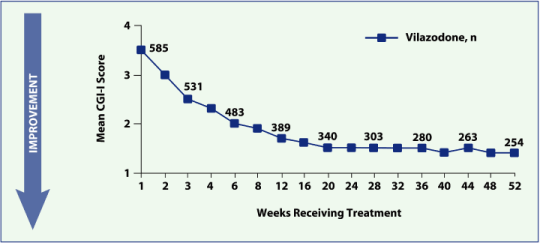
| — | Mean CGI-I score was 3.5 at week 1 and 1.4 at week 52 (Figure 3) |
Figure 2. Mean change from baseline in MADRS total score by week, observed
cases (Effectiveness Population, N = 596).
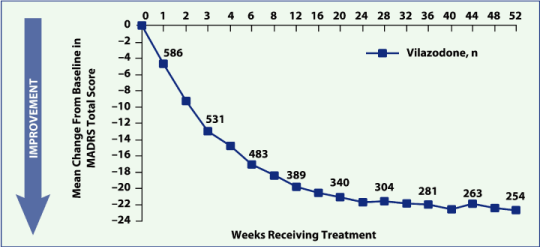
Figure 3. CGI-I score by visit, observed cases (Effectiveness Population, N = 596).
Conclusions
| • | Vilazodone 40 mg QD was safe and well tolerated in this 52-week, open-label treatment study of adults with MDD |
| • | Most TEAEs associated with vilazodone treatment were mild or moderate in intensity and had a pattern similar to that observed in the 8-week, placebo-controlled, short-term treatment studies of vilazodone6 (see APA poster NR4-2) |
| • | Diarrhea and nausea were the most frequent TEAEs, with the majority of occurrences being mild or moderate in severity | |
| • | Changes in vital signs, weight, laboratory values, and ECGs were not of clinical concern | |
| • | Based on changes in CSFQ, sexual functioning improved in men and women | |
| • | Effectiveness findings for MDD symptoms were similar to those observed with vilazodone in two placebo-controlled studies of 8 weeks’ duration6 (see APA poster NR4-2) |
REFERENCES
| 1. | Substance Abuse and Mental Health Services Administration. The NSDUH Report: Major Depressive Episode and Treatment Among Adults. 2009. | |
| 2. | Geddes JR et al. Lancet. 2003;361:653-661. | |
| 3. | US Food and Drug Administration. Guidance for Industry ICH-E1A. 1995. | |
| 4. | Hughes A et al. Eur J Pharmacol. 2005;510:49-57. | |
| 5. | Dawson L et al. CNS Neurosci Ther. 2009;15:107-117. | |
| 6. | Rickels K et al. J Clin Psychiatry. 2009;70:326-333. |
