Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CLINICAL DATA INC | b81084e8vk.htm |
| EX-99.3 - EX-99.3 - CLINICAL DATA INC | b81084exv99w3.htm |
| EX-99.1 - EX-99.1 - CLINICAL DATA INC | b81084exv99w1.htm |
| EX-99.6 - EX-99.6 - CLINICAL DATA INC | b81084exv99w6.htm |
| EX-99.4 - EX-99.4 - CLINICAL DATA INC | b81084exv99w4.htm |
| EX-99.5 - EX-99.5 - CLINICAL DATA INC | b81084exv99w5.htm |
| EX-99.8 - EX-99.8 - CLINICAL DATA INC | b81084exv99w8.htm |
| EX-99.7 - EX-99.7 - CLINICAL DATA INC | b81084exv99w7.htm |
Exhibit 99.2
Poster NR4-2
Efficacy and Tolerability of Vilazodone, a Dual-Acting Serotonergic Antidepressant, in the Treatment of Patients With Major Depressive Disorder (MDD)
Arif Khan, MD,1,2 Andrew J. Cutler, MD,3,4
Daniel K. Kajdasz,
PhD,5 Maria Athanasiou, PhD,5
Donald S. Robinson, MD,6 Heidi
Whalen, MHS,5 Carol R. Reed, MD5
1Northwest Clinical Research Center, Bellevue, Washington; 2Department of
Psychiatry and Behavioral Sciences, Duke University, Durham, North Carolina; 3University
of Florida, Gainesville, Florida; 4Florida Clinical Research Center, LLC, Bradenton,
Florida; 5PGxHealth, LLC, New Haven, Connecticut; 6Worldwide Drug
Development, Burlington, Vermont
Abstract
Objective
To assess the efficacy, safety, and tolerability of vilazodone HCl (VLZ), a dual-acting selective
serotonin reuptake inhibitor and 5-HT1A receptor partial agonist, in the treatment of
major depressive disorder (MDD).
Method
This phase 3, randomized, double-blind, placebo (PBO)-controlled study enrolled patients (pts)
18-70 years of age with MDD and a baseline HAM-D-17 score ³22. Pts received VLZ or PBO QD titrated
to 40 mg over 2 weeks and continued for up to 8 weeks (ITT population, n = 463; 388 completers).
The primary objective was to assess VLZ efficacy measured by change in MADRS score. Secondary
efficacy measures included MADRS response, HAM-D, HAM-A, CGI-S, and CGI-I. Safety and tolerability
assessments included treatment-emergent adverse events (AEs) and labs, ECGs, and the Changes in
Sexual Function Questionnaire (CSFQ).
Results
VLZ-treated pts had significantly greater improvement from baseline in MADRS total score at end
point (P = 0.009) than PBO (ITT, LS mean changes, -13.3 and -10.8, respectively). Significantly
more VLZ-treated than PBO pts (44% vs 30%, P = 0.002) were MADRS responders (decrease in MADRS
total score ³50%). VLZ-treated pts also had significant improvements from baseline in HAM-D-17 (P =
0.026), HAM-A (P = 0.037), CGI-S (P = 0.004), and CGI-I (P = 0.004) at end point vs PBO.
Discontinuations due to AEs were VLZ 5.1% and PBO 1.7%. The most frequent AEs in VLZ vs PBO pts
were headache (13% vs 10%), nausea (26% vs 6%), and diarrhea (31% vs 11%) (>93% of these judged
to be mild or moderate in intensity). There were no clinically significant changes in weight, vital
signs, ECGs, or labs. No clinically significant treatment-related effects on sexual function were
seen on the CSFQ in males or females. Libido decrease was the most common AE related to sexual
function (11 [4.7%] VLZ pts and 0 PBO pts).
Conclusions
Vilazodone treatment was associated with significant improvement in depression and anxiety symptoms
and was safe and well tolerated in this study of adult patients with MDD.
Introduction
| • | Despite a variety of available antidepressants, most patients with major depressive disorder (MDD) do not experience an adequate response to their first course of treatment | |
| • | In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study |
| — | 47% of patients responded to SSRI treatment1 | ||
| — | Switching to different regimens was associated with increased intolerability2,3 |
| • | Alternative agents offering good efficacy and tolerability are needed | |
| • | Vilazodone HCl is a dual-acting potent and selective serotonin reuptake inhibitor and 5-HT1A receptor partial 1A agonist that exerts its effects at the serotonin transporter and at pre- and post-synaptic 5-HT1A receptors4,5 |
| — | The unique dual modulation of serotonin neurotransmission by vilazodone is hypothesized to decrease endogenous serotonin negative feedback and enhance post-synaptic 5-HT effects |
| • | A previous placebo-controlled, phase 3 study found vilazodone effective for the treatment of patients with MDD, with good tolerability at a dose of 40 mg/day6 |
Objectives
| • | Primary |
| — | To compare efficacy between vilazodone-treated and placebo groups using change from baseline to end of treatment (EOT) on the Montgomery-Åsberg Depression Rating Scale (MADRS) total score up to 8 weeks of treatment |
| • | Secondary |
| — | To assess the safety and tolerability profile of vilazodone | ||
| — | To evaluate the efficacy of vilazodone compared with placebo on the following secondary measures: 17-item Hamilton Depression Scale (HAM-D-17), Hamilton Anxiety Scale (HAM-A), and Clinical Global Impression-Severity (CGI-S) and CGI-Improvement (CGI-I) scales |
Presented at the 163rd Annual Meeting of the American Psychiatric Association, May 22-26, 2010, New Orleans, Louisiana |
Supported by PGxHealth, LLC |
Methods
| • | All patients provided written informed consent before study procedures | |
| • | The protocol was approved by the institutional review board of each center |
Study Population
| • | Selected inclusion criteria |
| — | Male and female patients; 18 to 70 years of age | ||
| — | Diagnosis of MDD, single episode or recurrent, according to DSM-IV-TR criteria | ||
| — | Current episode ³4 weeks’ and <2 years’ duration | ||
| — | HAM-D-17 score ³22 and HAM-D item 1 (depressed mood) score ³2 at screening and baseline visits |
| • | Selected exclusion criteria |
| — | Axis I disorder of posttraumatic stress disorder, obsessive-compulsive disorder, or eating disorder within 6 months | ||
| — | History of schizophrenia, schizoaffective disorder, or bipolar I or II disorder | ||
| — | Psychotherapy within 12 weeks of screening | ||
| — | Failure to respond to 2 consecutive antidepressants of different classes during the present episode |
Study Design
| • | Placebo-controlled, randomized, double-blind, multicenter, parallel-group study of 8 weeks’ duration (Figure 1) | |
| • | Patients randomized to vilazodone were titrated to the target dose of 40 mg once daily (QD) over a 2-week period according to a fixed-titration schedule | |
| • | A sample of 470 patients with 1:1 randomization to vilazodone or placebo provided 90% power to detect a 3-point difference in mean change from baseline between treatments (2-sided testing at 0.05 significance level) |
Figure 1. Study design and treatment.
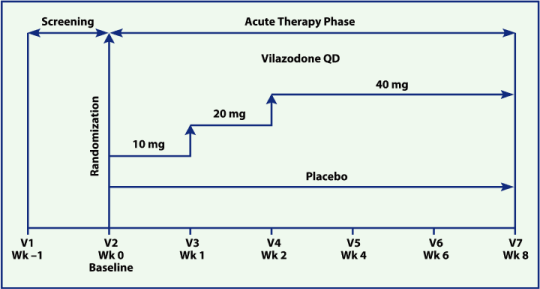
V, study visit.
• Efficacy Assessments
| — |
MADRS, HAM-D-17, HAM-A, and CGI-S at baseline and at weeks 1, 2, 4, 6, and 8 (or EOT) |
| • | Safety Assessments |
| — | Adverse events (AEs), clinical laboratory evaluations, electrocardiography (ECG), physical examinations, and vital signs | ||
| — | Patient-rated Changes in Sexual Function Questionnaire (CSFQ) for change in sexual function during treatment | ||
| — | Columbia-Suicide Severity Rating Scale to assess risk for suicidal ideation and/or behavior |
Statistical Analysis
| • | Efficacy evaluations were based on the intent-to-treat (ITT) population, defined as all randomized patients dispensed study drug with ³1 postbaseline efficacy assessment | |
| • | Safety evaluations were based on the safety population, defined as all randomized patients dispensed study drug with ³1 postbaseline safety measure | |
| • | Treatment group comparisons of continuous measures were based on differences in least-squares mean (LSM) changes from baseline to EOT from an analysis of covariance model containing terms for treatment and center, with baseline included as a covariate | |
| • | A mixed-effects model repeated measures (MMRM) analysis was conducted with terms for treatment, center, visit, and treatment-by-visit interaction, with baseline and baseline-by-visit interaction included as covariates | |
| • | Statistical comparisons were 2-sided and considered significant at the 0.05 level | |
| • | No statistical testing for safety measures was performed |
Results
| • | 481 patients were randomized, and 388 (80.7%) completed the study (Figure 2) |
Figure 2. Patient disposition.
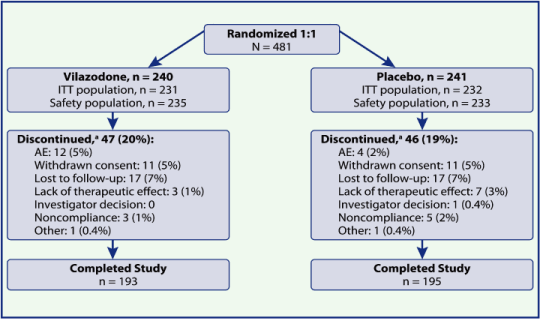
a n (%) of randomized patients in each group.
| • | Demographic characteristics were similar between vilazodone-treated and placebo groups (Table 1) |
Table 1. Baseline Demographics and Disease Characteristics (Safety Population)
Characteristic |
Vilazodone n = 235 |
Placebo n = 233 |
||||||
Sex, n (%) |
||||||||
Male |
96 (40.9 | ) | 109 (46.8 | ) | ||||
Female |
139 (59.1 | ) | 124 (53.2 | ) | ||||
Race, n (%) |
||||||||
White |
182 (77.4 | ) | 191 (82.0 | ) | ||||
Black/African American |
35 (14.9 | ) | 31 (13.3 | ) | ||||
Other |
18 (7.7 | ) | 11 (4.7 | ) | ||||
Mean age, y (SD) |
41.1 (12.2 | ) | 42.4 (12.5 | ) | ||||
Range, y |
18-69 | 19-70 | ||||||
Duration of current MDD episode, n (%) |
||||||||
1-6 mo |
110 (46.8 | ) | 120 (51.5 | ) | ||||
>6-12 mo |
61 (26.0 | ) | 59 (25.3 | ) | ||||
>12 mo |
63 (26.8 | ) | 54 (23.2 | ) | ||||
Severity of current episode, n (%) |
||||||||
Moderate |
175 (74.5 | ) | 165 (70.8 | ) | ||||
Severe |
60 (25.5 | ) | 68 (29.2 | ) | ||||
• Treatment compliance was >90% for vilazodone-treated and placebo patients at each visit
Efficacy
| • | Vilazodone-treated patients showed significantly greater improvement at EOT in MADRS scores than did placebo patients (Table 2) | |
| • | Vilazodone-treated patients also experienced significantly greater improvement at EOT in HAM-D-17, HAM-A, and CGI-S and CGI-I scores than did placebo patients (Table 2) |
Table 2. Mean Change in Efficacy Assessments From Baseline to EOT
(ITT Population, Last-Observation-Carried-Forward Analysis)
(ITT Population, Last-Observation-Carried-Forward Analysis)
| Baseline | LSM Change at EOT | |||||||||||||||||||
| Vilazodone | Placebo | Vilazodone | Placebo | |||||||||||||||||
| Assessment | n = 231 | n = 232 | n = 231 | n = 232 | P | |||||||||||||||
MADRS |
31.9 ± 3.5 | 32.0 ± 3.6 | -13.3 ± 0.9 | -10.8 ± 0.9 | 0.009 | |||||||||||||||
HAM-D-17 |
25.0 ± 2.4 | 25.3 ± 2.6 | -10.7 ± 0.7 | -9.1 ± 0.7 | 0.026 | |||||||||||||||
HAM-A |
18.0 ± 5.3 | 18.1 ± 5.8 | -7.0 ± 0.55 | -5.7 ± 0.55 | 0.037 | |||||||||||||||
CGI-S |
4.5 ± 0.5 | 4.5 ± 0.5 | -1.4 ± 0.1 | -1.1 ± 0.1 | 0.004 | |||||||||||||||
CGI-I |
— | — | 2.5 ± 0.1 | 2.8 ± 0.1 | 0.004 | |||||||||||||||
| • | MMRM analysis showed greater improvement in MADRS total score for vilazodone-treated patients than for placebo patients at week 6 (P = 0.019) and week 8 (P = 0.007) (Figure 3) | |
| • | MADRS response rate (³50% decrease from baseline at EOT) was significantly greater (P = 0.002) for vilazodone-treated patients (44%) than for placebo patients (30%); remission (EOT score <10) was not significantly greater for vilazodone-treated patients (27%) than for placebo patients (20%) |
Figure 3. Mean change from baseline in MADRS total score by week (ITT Population, MMRM Analysis)
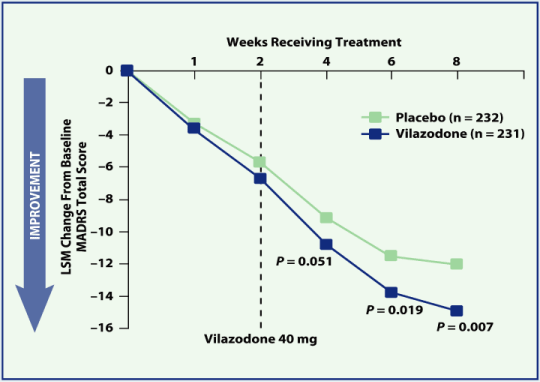
Safety and Tolerability
| • | Most treatment-emergent adverse events (TEAEs) were mild or moderate in intensity (>93% in both groups) | |
| • | The most frequent TEAEs in the vilazodone group (compared with the placebo group) were diarrhea (31% vs 11%), nausea (26% vs 6%), and headache (13% vs 10%) (Table 3) |
Table 3. TEAEs Occurring in ³5% of Vilazodone Patients (Safety Population)
| Vilazodone | Placebo | |||||||
| n = 235 | n = 233 | |||||||
| MedDRA Preferred Term | Patients, n (%) | |||||||
Diarrhea |
72 (30.6 | ) | 25 (10.7 | ) | ||||
Nausea |
61 (26.0 | ) | 13 (5.6 | ) | ||||
Headache |
30 (12.8 | ) | 24 (10.3 | ) | ||||
Dry mouth |
21 (8.9 | ) | 9 (3.9 | ) | ||||
Dizziness |
21 (8.9 | ) | 9 (3.9 | ) | ||||
Insomnia |
17 (7.2 | ) | 7 (3.0 | ) | ||||
Abnormal dreams |
14 (6.0 | ) | 4 (1.7 | ) | ||||
Vomiting |
12 (5.1 | ) | 1 (0.4 | ) | ||||
MedDRA, Medical Dictionary for Regulatory Activities.
| • | 12 of 235 (5.1%) vilazodone patients and 4 of 233 (1.7%) placebo patients discontinued due to TEAEs | |
| • | Overall, mean changes in laboratory values from screening (hematology, chemistry, and urinalysis) were similar and clinically unimportant between vilazodone and placebo groups | |
| • | No clinically significant treatment-related ECG abnormalities were reported; vital sign and weight changes were not clinically remarkable (Table 4) |
Table 4. Blood Pressure (Sitting), Pulse, and Weight at Baseline and Change From Baseline (Safety Population)
| Vilazodone | Placebo | |||||||||||||||
| n = 235 | n = 233 | |||||||||||||||
| Mean Change From | Mean Change From | |||||||||||||||
| Parameter | Mean | Baseline | Mean | Baseline | ||||||||||||
SBP, mm Hg |
||||||||||||||||
Baseline |
120.3 | — | 119.9 | — | ||||||||||||
Week 8 |
119.3 | -1.3 | 119.8 | -0.1 | ||||||||||||
Early termination |
122.8 | -0.1 | 120.9 | 1.9 | ||||||||||||
DBP, mm Hg |
||||||||||||||||
Baseline |
76.2 | — | 77.1 | — | ||||||||||||
Week 8 |
76.5 | 0.2 | 76.4 | -0.5 | ||||||||||||
Early termination |
79.2 | 2.2 | 77.8 | 0.3 | ||||||||||||
Pulse, bpm |
||||||||||||||||
Baseline |
73.4 | — | 72.9 | — | ||||||||||||
Week 8 |
74.4 | 1.2 | 74.3 | 1.6 | ||||||||||||
Early termination |
74.2 | -0.4 | 76.6 | 3.7 | ||||||||||||
Weight, kg |
||||||||||||||||
Baseline |
86.36 | — | 88.86 | — | ||||||||||||
Week 8 |
87.80 | 0.21 | 90.26 | 0.36 | ||||||||||||
Early termination |
83.64 | 0.19 | 87.46 | -0.25 | ||||||||||||
SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per minute.
| • | Overall sexual function for men and women (as measured by CSFQ) was similar for vilazodone and placebo groups | |
| • | The TEAE decreased libido was more common among patients treated with vilazodone (11 [4.7%]) than among those given placebo (0%) |
Conclusions
| • | Vilazodone produced statistically significant and clinically meaningful improvement of symptoms in patients with MDD based on disease-specific and global improvement measures (MADRS, HAM-D, HAM-A, and CGI) during 8 weeks of treatment in this study | |
| • | Vilazodone treatment at a dose of 40 mg/day was safe and well-tolerated | |
| • | This study replicates efficacy, safety, and tolerability findings demonstrated in a previous 8-week, phase 3, placebo-controlled study of vilazodone in adults with MDD6 |
References
| 1. | Trivedi MH et al. Am J Psychiatry. 2006;163:28-40. | |
| 2. | Rush AJ. Am J Psychiatry. 2007;164:201-204. | |
| 3. | Rush AJ et al. Am J Psychiatry. 2006;163:1905-1917. | |
| 4. | Hughes A et al. Eur J Pharmacol. 2005;510:49-57. | |
| 5. | Dawson L et al. CNS Neurosci Ther. 2009;15:107-117. | |
| 6. | Rickels K et al. J Clin Psychiatry. 2009;70:326-333. |
