Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Shrink Nanotechnologies, Inc. | ex991.htm |
| 8-K - INKN FORM 8-K (5-3-10) - Shrink Nanotechnologies, Inc. | inknform8k050310.htm |
| EX-99.2 - EXHIBIT 99.2 - Shrink Nanotechnologies, Inc. | ex992.htm |
| EX-10.2 - EXHIBIT 10.2 - Shrink Nanotechnologies, Inc. | ex102.htm |
| EX-99.4 - EXHIBIT 99.4 - Shrink Nanotechnologies, Inc. | ex994.htm |
| EX-99.3 - EXHIBIT 99.3 - Shrink Nanotechnologies, Inc. | ex993.htm |
SPONSORED
RESEARCH AGREEMENT
THIS SPONSORED RESEARCH
AGREEMENT (“Agreement”) is entered into on May 1, 2010 (the “Effective
Date”) by and between THE REGENTS OF THE UNIVERSITY OF CALIFORNIA, on behalf of
its Irvine campus ("UCI") and Shrink Technologies, Inc., a California
Corporation, with an office at 2038 Corte Del Nogal, Suite 110, Carlsbad, CA
92011, (“Sponsor”).
RECITALS
UCI,
through the Henry Samueli School of Engineering, has valuable experience, skill,
and ability in “Manufacturable, Shrinkable Plastic Microdevices for Biomedical
Applications.”
Sponsor
desires to have UCI undertake a research project in accordance with the scope of
work described in Exhibit A, “Statement of Work and Tasks” (the
“Research”).
Sponsor
and The Regents of the University of California (“The Regents”) have entered
into an agreement (the “Option”) whereby The Regents will forebear licensing
certain patents (“Regents’ Patent Rights”) that Sponsor may need to practice any
grant of rights under any License Agreement contemplated by this
Agreement.
The research program contemplated by
this Agreement is of mutual interest and benefit to UCI and Sponsor, and will
further UCI’s instructional and research objectives in a manner consistent with
its status as a non-profit, tax exempt educational institution.
NOW, THEREFORE, in
consideration of the promises and mutual covenants herein contained, the parties
hereto hereby agree as follows:
|
1.
|
STATEMENT
OF WORK. UCI agrees to perform the research project described
in Exhibit A (“the Statement of Work and Tasks”), which Exhibit is
incorporated herein. Access to work carried on in UCI’s
laboratories in the course of the Research shall be entirely under the
supervision, direction, and control of UCI
personnel.
|
UCI shall
not contract any consultant or contractor (“Subcontractor”) to perform any
portion of the Research without Sponsor’s prior written consent.
|
2.
|
KEY
PERSONNEL.
|
|
(A)
|
In
addition to appropriate staffing levels necessary to complete the
Research, the following individual is identified as a key personnel for
the performance of the Research at
UCI:
|
Michelle
Khine, Principal Investigator
|
3.
|
PERIOD
OF PERFORMANCE. This Agreement is effective for the period
commencing on the Effective Date and continuing until the later of (a)
three (3) years after the Effective Date, or (b) completion of the
Research and may be extended only by written agreement of the
parties. If, prior to the end of such three (3) year period,
Sponsor agrees in writing to continue to sponsor the Research with a
financial commitment substantially similar to that contained herein, then
this Agreement shall continue for an additional period of up to three (3)
years based on the level of Sponsor’s
commitment.
|
|
4.
|
REIMBURSEMENT
OF COSTS. UCI shall be reimbursed by the Sponsor for all direct
and indirect costs incurred in connection with the Research up to the
amount of $632,051 in accordance with the budget attached as Exhibit
B. While it is estimated that this amount is sufficient to
conduct the Research, UCI may submit to the Sponsor a revised budget
requesting additional funds. UCI shall not be obligated to
expend funds in excess of those provided under this Agreement to conduct
the Research.
|
PAYMENT
TERMS
Sponsor
shall advance the following amount (“Advance Payment”) upon submission of an
invoice with reasonable detail from UCI at the time shown:
Amount
Due
Date Due
|
|
$20,000
|
Within
five (5) days following the Effective
Date
|
During
the project period, beginning on the ninetieth (90th) day
subsequent to the execution of the Agreement, UCI shall invoice the Sponsor each
three (3) months for actual costs incurred in the performance of the Research.
Payment
of such invoices shall be due no later than thirty (30) days after Sponsor
approval of the invoice.
Payment
shall be made by wire transfer to the following account: to be provided under separate
cover.
|
5.
|
TITLE
TO EQUIPMENT.
|
In the
event that UCI purchases equipment, title to such equipment will vest in UCI
upon acquisition.
|
6.
|
INTELLECTUAL
PROPERTY.
|
|
(A)
|
All
rights to inventions or discoveries conceived and reduced to practice in
the performance of Research conducted under this Agreement (the
“Additional IP”) shall belong to The Regents of the University of
California and shall be disposed of in accordance with University of
California Office of the President and UCI’s intellectual property
policies and as set forth in this Agreement. Inventions or discoveries
developed from July 2009 to the Effective Date solely in the UCI
laboratory of Dr. Michelle Khine that name Dr. Khine as the primary
inventor, and which are not under pre-existing obligations to another
third party or sponsor, and which is covered under the Statement of Work
and Tasks (Exhibit A) shall be included under Additional IP subject to the
approval of Dr. Khine and UCI.
|
|
(B)
|
UCI
hereby grants to the Sponsor the right to execute and include the
Additional IP in a license agreement with terms substantially similar to
the terms identified in Appendix B (“License”) provided that Sponsor is
not in default of any payment or funding obligations required under this
Agreement. If Sponsor should exercise this right, Sponsor shall assume all
costs associated with the preparation and filing of any patents (“Patent
Costs”) for the Additional IP and the Additional IP shall be included in
the License.
|
|
(C)
|
Sponsor
will advise UCI within (9) months from disclosure whether or not it wishes
to exercise its right to include under the License said Additional IP.
Notwithstanding the above, if UCI incurs any Patent Costs for the
Additional IP, Sponsor shall advise UCI in writing within three (3) months
of incurring the first Patent Costs. If Sponsor does not advise UCI within
the time stated above, rights to the Additional IP shall be disposed of in
accordance with UCI policies with no further obligation to Sponsor. For
the avoidance of doubt, Sponsor’s exercise of such right prior to the
completion of the Research shall not terminate the Research, and the
Research and Sponsor’s funding obligations shall continue in accordance
with this Agreement.
|
|
(D)
|
UCI
shall promptly disclose to Sponsor any Additional IP made under this
Agreement. Sponsor shall hold such disclosure on a confidential
basis and will not disclose the information to any third party, other than
its actual or prospective investors, on a confidential basis without
consent of UCI. Sponsor shall advise UCI in writing within the
period described in Section 6(B) whether or not it wishes to include
under the License the Additional IP. If Sponsor elects to
include the Additional IP under the License Sponsor shall assume all
Patent Costs whether or not a patent(s)
issues.
|
|
(E)
|
Ownership
of any software first created in the performance of the Research shall
belong to the employer of the author of such software and shall be
determined in accordance with U.S. Copyright law. Upon receipt
of a copy of such software, Sponsor shall have ninety (90) days,
extendible upon the mutual consent of both parties, to negotiate the terms
of a copyright license agreement and UCI agrees to negotiate these license
terms in good faith. During this period, UCI will not offer a
commercial license to any other
party.
|
(F) UCI
agrees to promptly notify Sponsor if it receives any third-party funding that
will affect Sponsor’s intellectual property rights under this
Agreement.
|
7.
|
PUBLICATION. UCI
agrees to provide Sponsor, in confidence, with an advanced copy of any
publication resulting from the Research not less than thirty (30) days
prior to the submission to a journal or any other public
disclosure. At the request of the Sponsor, UCI agrees to delay
the publication for a period of not more than ninety (90) days from the
date the publication was originally provided to the Sponsor for the
purpose of filing relevant patent applications to protect any new
data.
|
|
8.
|
CONFIDENTIALITY.
|
|
(A)
|
Unless
otherwise required by law, Sponsor will safeguard from disclosure
information, oral or written, provided to it by the UCI (“Confidential
Information”) and will only disclose to its actual or prospective
investors in confidence.
|
|
(B)
|
Confidential
Information does not include information
which:
|
|
(i)
|
was
known to Sponsor prior to the disclosure
hereunder;
|
|
(ii)
|
was
received from a third party not under an obligation of confidence to
UCI;
|
|
(iii)
|
is
in the public domain at the time of disclosure hereunder or subsequently
entered the public domain without the fault of the
Sponsor;
|
|
(iv)
|
is
independently known prior to receipt thereof or is discovered
independently by an employee of Sponsor without the use of the information
supplied by UCI under this Agreement;
or
|
|
(v)
|
is
required to be disclosed by law.
|
|
(C)
|
The
obligations of confidentiality under this paragraph shall survive and
continue for five (5) years after the expiration of or early termination
of this Agreement.
|
|
9.
|
REPORTS. UCI
shall hold monthly meetings with Sponsor during the term of this Agreement
summarizing the work conducted. A final report setting forth
the accomplishments and significant research findings shall be prepared by
UCI and submitted to the Sponsor within ninety (90) days of the expiration
or early termination of this
Agreement.
|
|
10.
|
TERMINATION. This
Agreement may be terminated by either party at any time upon the receipt
of ninety (90) days written notice to the other party. The
following Provisions shall survive such early termination: 6,
8, 10, 13, and 14. In addition, if Sponsor fails to make any
payment required hereunder, this agreement shall terminate on the
ninetieth (90th)
day after UCI mails notice of such failure, unless payment is received
before such ninetieth (90th)
day. UCI may also terminate immediately if there is a Force
Majuere event as detailed in Provision 15 below. Upon
notification, UCI shall proceed in an orderly fashion to limit or
terminate any outstanding commitments and/or to conclude the
research. All costs associated with termination shall be
allowable, including non-cancelable commitments incurred prior to receipt
of termination notice and all expenses which have not been reimbursed to
UCI by Sponsor. In the event of termination, UCI shall submit
to Sponsor a final financial report in accordance with Paragraph 4 of
this Agreement. Any costs and commitments incurred in excess of
funds provided will be invoiced to Sponsor and will be payable by Sponsor
within thirty (30) days. Any funds remaining from the advanced
payment under Section 4 shall be returned to Sponsor within thirty
(30) days.
|
|
11.
|
NOTICES. Any
notices given under this Agreement shall be in writing and delivered by
certified or registered return receipt mail, postage prepaid, or by
facsimile addressed to the parties as
follows:
|
For
Sponsor:
For
UCI:
Ben
Holstein, Principal Contract and Grant Officer
Sponsored
Projects
Mark L.
Baum,
Esq. Office
of Research - Research Administration
SHRINK
Nanotechnologies,
Inc.
University of California, Irvine
2038
Corte Del Nogal, Suite
110 300
University Tower
Carlsbad,
CA
92011 Irvine,
CA 92697-7600
(760)
804-8844
(Telephone) (949)
8224-8109 (Telephone)
|
12.
|
PUBLICITY. Neither
party shall use the name, trade names, or trademarks of the other party or
the other party’s employees in connection with any products, promotion, or
advertising without the prior written permission of an authorized
representative of the other party. The foregoing shall not,
however, preclude any legally required disclosure, reports generated in
the normal course of business, or acknowledgement of sponsorship as
required by the guidelines of an academic
organization.
|
|
13.
|
USE
OF RESEARCH RESULTS AND PRODUCT LIABILITY. To the extent it
would otherwise be liable under applicable law, Sponsor agrees
to hold harmless, indemnify and defend UCI from all liabilities, demands,
damages, expenses and losses arising out of use by the Sponsor, or by any
party acting on behalf of or under authorization from the Sponsor, or out
of any use, sale of other disposition by the Sponsor, or by any party
acting on behalf of or under authorization from the Sponsor, of products
made by use of the results of the Research performed
hereunder. The provisions of this paragraph shall survive
termination.
|
|
14.
|
INDEMNIFICATION. To
the extent it would otherwise be liable under applicable law, Sponsor
hereby waives and agrees to indemnify, defend, and hold harmless The
Regents of the University of California, it’s officers, trustees, agents,
employees and students from any loss, claim of damages, or liability of
any kind, including legal fees, court costs and other expenses in
litigation or settlement of any claims, arising out of or in connection
with this Agreement., except to the extent resulting from a breach of this
Agreement by UCI. The provisions of this paragraph shall
survive termination of this
Agreement.
|
|
15.
|
FORCE
MAJEURE. Neither Party shall be liable for any failure to
perform as required by this Agreement, to the extent such failure to
perform is caused by any reason beyond their control, or by reason of any
of the following occurrences: labor disturbances or labor disputes of any
kind, accidents, failure of any governmental approval required for full
performance, civil disorders or commotion’s, acts of aggression, floods,
earthquakes, acts of God, energy or other conservation measures,
explosion, failure of utilities, material shortages, disease, or other
such occurrences.
|
|
16.
|
ASSIGNMENT. This
Agreement is personal to the Sponsor. The Sponsor may not
assign or transfer this Agreement, without The Regents’ prior written
consent; provided, however, that Sponsor may, without such consent, assign
this Agreement and its rights and obligations hereunder in connection with
the transfer or sale of all or substantially all of its business or
assets, or in the event of its merger, consolidation, change in control or
other similar transaction. Any other attempted assignment by
Sponsor without the written consent of The Regents will be null and
void. This Agreement is binding upon and will inure to the
benefit of The Regents, its successors and
assigns.
|
|
17.
|
SEVERABILITY. In
the event a court of competent jurisdiction holds any provision of this
Agreement to be invalid, such holding shall have no effect on the
remaining provisions of this Agreement, and they shall continue in full
force and effect.
|
|
18.
|
INDEPENDENT
CONTRACTOR. Each party shall be deemed to be an independent
contractor of the other party, and neither shall be considered an agent,
employee, joint venture or partner of the other, except to the extent that
Dr. Michelle Khine is an employee of UCI. Neither party shall
have authority to make warranties or representations or enter agreements
on behalf of the other, nor shall either party be bound by the acts,
statements or conduct of the other.
|
|
19.
|
INDEPENDENT
INQUIRY. Nothing in this Agreement shall be construed to limit
the freedom of researchers who are participants in this Agreement, whether
paid under this Agreement, or not, from engaging in similar research
inquiries made independently under other grants, contracts or agreements
with parties other than the
Sponsor.
|
|
20.
|
HEADINGS. The
paragraph headings herein are for convenience only and shall not affect
the construction or interpretation of this
Agreement.
|
|
21.
|
ENTIRE
AGREEMENT CHANGES. This Agreement and its appendices, together
with the Option Agreement entered into between the parties of even date
herewith and any license agreements that result from this Agreement,
contain the entire agreement between the parties, and supersede any prior
agreements between the parties, written or oral regarding the subject
matter thereof. No amendments or changes to this Agreement
shall be effective unless made in writing and signed by authorized
representatives of University of California Office of the President, UCI,
and Sponsor. All correspondence regarding terms of this
Agreement shall be sent as specified in Provision
11.
|
|
22.
|
GOVERNING
LAW. This Agreement will be governed and construed by the laws
of the State of California.
|
IN WITNESS WHEREOF, the
parties hereto have executed this Agreement in duplicate by proper persons
thereunto duly authorized.
Shrink
Technologies,
Inc.
The Regents of the University of California
By: /s/ Mark L. Baum,
Esq.
By: /s/ Ben
Holstein
Name: Mark
L. Baum,
Esq.
Name: Ben
Holstein
Title: Chief
Executive
Officer
Title: Principal Contract and Grant Officer Sponsored
Projects
Date:
Date:
EXHIBIT A:
Statement
of Work and Tasks
Project
Title:
Manufacturable,
Shrinkable Plastic Microdevices for Biomedical Applications
PI: Dr. Michelle Khine,
University of California, Irvine
Email:
mkhine@uci.edu
Phone:
(949) 824-4051
Scope
of Work:
This
Sponsored Research Project focuses on developing shrinkable plastic-based
microfluidic chips for biomedical applications that can be manufactured in
high-volume. Based on the prototype microdevices that Shrink Nanotechnologies
and Dr. Khine have previously engineered, the scope of the current research
project is to develop these technologies into robust products. This effort
incorporates the following major tasks:
|
1.
|
Develop
integrated, manufacturable, nanostructured substrates for
biosensing
|
This task
includes the further development of novel plastic nanostructures, developing and
testing new bioassays using these structures, developing methods to integrate
multiple devices within the plastic substrate, and improving the properties and
performance of the plastic based on the needs of specific biosensing and/or
diagnostic application.
Previous
devices were constructed from various types of polystyrene sheets using several
different fabrication methods (Figure 1). When exposed to heat these sheets
contract, allowing millimeter and micrometer-sized features to be reduced to
micro- and nano-sized features respectively. Several prototype devices were
developed that take advantage of the shrinking properties of polystyrene such as
microfluidic biochips and metal nanostructured surfaces for extremely sensitive
biosensing and cell alignment.
Under
this scope of work further development and optimization of these processes will
take place in order to improve the device performance as well as transition to
high volume manufacturing. Also, new shrinkable materials (such as polyolefin)
will be explored.
|
2.
|
Develop
useful stem cell tools using shrinkable, plastic microfluidic
technologies
|
This task
includes developing and demonstrating the utility of the plastic microfluidic
tools for stem cell culture and differentiation. As shown in Figure 1, patterns
can be printed onto plastic sheets prior to shrinking. While shrinking, the
toner formed raised features that can be used to mold negative replicas. These
replicas will have indented features (such as well arrays). Alternatively,
sheets can be scored and the scored features form channels when the plastic
sheet shrinks. Both of these methods can be used to create complex microscale
well/channel networks that will be useful for stem cell studies.
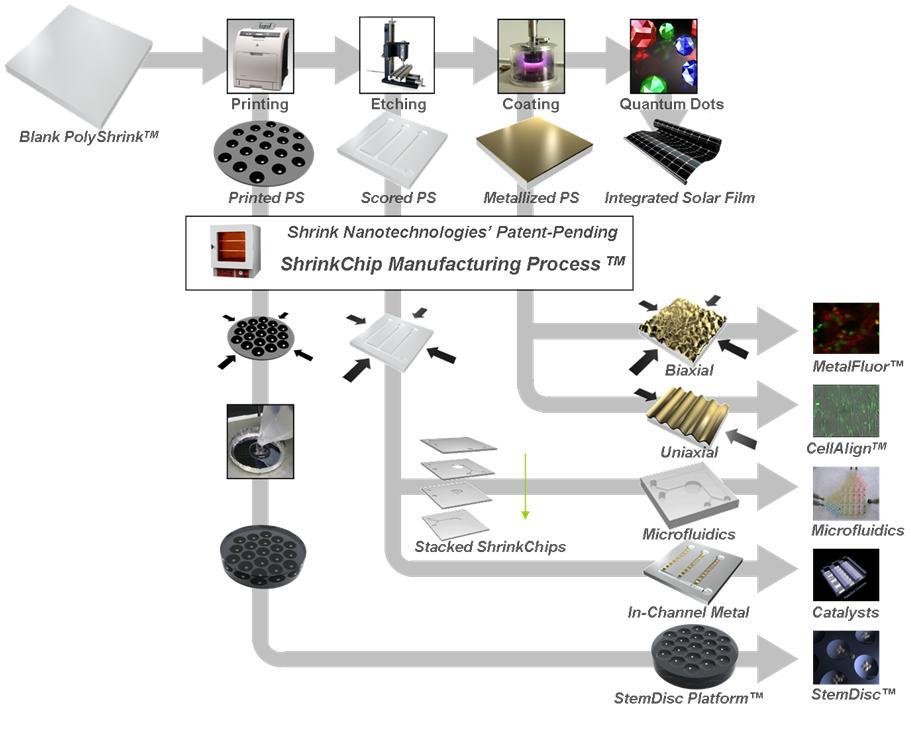
Figure
1. Diagram showing various fabrication procedures and resulting prototype
devices utilizing shrinkable plastic films.
The
period of performance for this project is 3
years. Progress on both tasks will be reported to Shrink
Nanotechnologies, Inc. every month. Milestones per year are as
follows:
Year
1
|
·
|
Design
and test at least one microfluidic stem cell
device.
|
|
·
|
Design
and optimize nanostructured biosensing
surface.
|
|
·
|
Characterize
alternative materials to polystyrene as nanostructured substrate
material.
|
Year
2
|
·
|
Design
and begin prototyping microdevices in manufacturable
process.
|
|
·
|
Develop
methods to integrate multiple
components.
|
|
·
|
Develop
new bioassays for biosensing and/or
diagnostics.
|
Year
3
|
·
|
Develop
a manufacturable process for nanostructured substrates and microfluidic
devices.
|
|
·
|
Demonstrate
shrinkable plastic microdevice for stem cell differentiation
studies.
|
|
·
|
Demonstrate
performance of nanostructured biosensing surface is better than currently
available technology.
|
EXHIBIT
B
Project
Budget
|
PI
NAME
|
Michelle
Khine, UCI
|
|||
|
AGENCY
|
Shrink
Nanotechnologies, Inc.
|
|||
|
Project
Title
|
Manufacturable,
Shrinkable Plastic Microdevices for Biomedical
Applications
|
|||
|
PROJECT
PERIOD
|
May
1, 2010 - April 30, 2013
|
|||
|
5/1/10
- 4/30/11
|
5/1/11
- 4/30/12
|
5/1/12
- 4/30/13
|
||
|
SUMMARY
|
YEAR
1
|
YEAR
2
|
YEAR
3
|
Cummulative
|
|
PERSONNEL
|
||||
|
PI
- Michelle Khine (salary & fringe)
|
$12,254
|
$12,713
|
$13,845
|
$38,811
|
|
Technician
(salary & fringe)
|
$42,319
|
$46,349
|
$50,545
|
$139,212
|
|
Post-doc
(salary & fringe)
|
$43,707
|
$45,982
|
$48,511
|
$138,200
|
|
TRAVEL
|
$2,000
|
$2,000
|
$2,000
|
$6,000
|
|
SUPPLIES
|
$4,000
|
$3,800
|
$3,546
|
$11,346
|
|
OTHER
COST:
|
||||
|
INRF
Clean Rm Fac Access Fees @ $ 500/mo X 2
|
$12,000
|
$12,000
|
$12,000
|
$36,000
|
|
INRF
Clean Room Equip Fees @ $500/mo X 2
|
$12,000
|
$12,000
|
$12,000
|
$36,000
|
|
INRF
One-Time User Training Fee @ $500 X 2
|
$1,000
|
$0
|
$0
|
$1,000
|
|
EQUIPMENT
|
$5,000
|
$5,000
|
$0
|
$10,000
|
|
TOTAL
DIRECT COST
|
$134,279
|
$139,844
|
$142,447
|
$416,569
|
|
MTDC
|
$129,279
|
$134,844
|
$142,447
|
$406,569
|
|
F&A
(53%)
|
$68,518
|
$71,467
|
$75,497
|
$215,482
|
|
|
||||
|
TOTAL
|
$202,796
|
$211,311
|
$217,944
|
$632,051
|
Each
three month period commencing on the Effective Date during the term of this
Agreement, Sponsor shall pay up to 1/4th of the applicable Total Cash
Contributed amount set forth above for the applicable contract
year.
The
overall budget may be amended by either Party at the end of each annual budget
period for this Agreement.
EXCLUSIVE
LICENSE AGREEMENT
for
PROCESSES
FOR MICROFLUIDIC FABRICATION AND OTHER INVENTIONS
This license agreement (“Agreement”) is
made effective this ______ day of ______________________, 2010 (“Effective
Date”), by and between The Regents of the University of California, a California
corporation, having its statewide administrative offices at 1111 Franklin
Street, 12th Floor, Oakland, California 94607-5200 through the University of
California, Irvine, Office of Technology Alliances, having its offices at 4199
Campus Drive, Suite 380, Irvine, CA, 92612 (“The Regents”) and Shrink
Technologies, Inc., a California corporation, having a principal place of
business at 2038 Corte Del Nogal, suite 110, Carlsbad, California 92011
(“Licensee”).
BACKGROUND
A. Certain
inventions, generally characterized as Processes for Microfluidic
Fabrication_______________ (collectively “Invention”), were made in the course
of research at the University of California, Irvine by Dr. Michelle Khine and
others and are claimed in Patent Rights as defined below.
B. [IF
APPLICABLE] To the extent of the actual knowledge of the licensing officer
responsible for the administration of this Agreement, as of the Effective Date,
no federal funds were used in the development of the Invention. In
the event that the development of the Invention was sponsored by the United
States Federal Government, then, as a consequence, this license would be subject
to overriding obligations to the United States Federal Government under 35
U.S.C. §§ 200-212 and applicable regulations including a non-exclusive,
non-transferable, irrevocable, paid-up license to practice or have practiced the
Invention for or on behalf of the United States Government throughout the
world.
C. [IF
APPLICABLE] To the extent of the actual knowledge of the licensing officer
responsible for the administration of this Agreement, as of the Effective Date,
the Invention having UC Case No. __________, characterized as “____________”
were sponsored in part by the California Institute for Regenerative Medicine
(“CIRM”) and are subject to CIRM Grant Nos. _______ and ________
granted by CIRM to The Regents. Under the terms of CIRM’s
Intellectual Property Requirements for Non-Profit Organizations (17 Cal.Code of
Regs. Section 100300), The Regents agrees i) to make its CIRM-funded patented
inventions readily accessible on reasonable terms, either directly or through a
licensee, to other Grantee Organizations for non-commercial purposes upon
request from such Grantee Organization; ii) if The Regents grants an exclusive
license, The Regents is required to document the development and
commercialization capabilities of the intended licensee and to include terms in
the license agreement addressing all relevant therapeutic and diagnostic uses
for which the invention is applicable and the licensee agrees to diligently
develop and to include commercial development milestones and benchmarks so that
development can be assessed and monitored; iii) if The Regents grants an
exclusive license, the licensee must have a plan in place at the time of
commercialization to provide access to resultant therapies and diagnostics for
uninsured California patients and the exclusive licensee must agree to provide
drugs at prices negotiated pursuant to the California Discount Prescription Drug
Program (commencing with California Health and Safety Code section 130500 et
seq.) to eligible Californians under that program and CIRM may make such plan
available for review by the ICOC on an annual basis; iv) The Regents is required
to monitor development activities of the licensee and to make reports of
monitoring activities annually to CIRM; v) The Regents is required to report to
CIRM administrative action to modify or terminate license rights where
necessary; vi) The Regents is required to notify CIRM prior to any press release
that refers to research findings, collaborations, inventions, patents or
licensing activities that arise as a consequence of CIRM funding and CIRM may
decide to participate in a joint press release; and vii) with regard to
CIRM-funded patented inventions, CIRM has certain March-in
Rights. The CIRM Intellectual Property Requirements for Non-profit
Organizations is attached in Appendix A. [MODIFICATIONS TO LICENSE
MAY BE REQUIRED TO COMPLY WITH ANY PREEXISTING OBLIGATIONS THAT THE REGENTS HAS
WITH CIRM AND CIRM INTELLECTRUAL PROPERTY REQUIREMENTS]
D. The Licensee has
evaluated the Invention under a Secrecy Agreement with The Regents (UC Control
No. _______ with an effective date of ______.
E. [IF APPLICABLE] The
Licensee and The Regents have executed an Option Agreement (UC Control No.
______) with an effective date of_______.
F. The Licensee and The
Regents, acting on behalf of the University of California, Irvine, have entered
into a research agreement on _____ (“Research Agreement”).
G. The Licensee wishes
to obtain certain rights from The Regents for the commercial development of the
Invention, in accordance with the terms and conditions set forth herein and The
Regents is willing to grant those rights so that the Invention may be developed
and the benefits enjoyed by the general public.
H. The scope of such
rights granted by The Regents is intended to extend to the scope of the patents
and patent applications in Patent Rights, but only to the extent that The
Regents has proprietary rights in and to the Valid Claims of such Patent
Rights.
I. The Licensee is a
“small business firm” as defined in 15 U.S.C. §632.
J. Both parties
recognize and agree that Earned Royalties are due under this Agreement with
respect to products, services and methods and that such royalties will be paid
with respect to both pending patent applications and issued patents, in
accordance with the terms and conditions set forth herein.
K. Both parties
recognize and agree that Earned Royalties due under this Agreement will be based
on the Licensee's or a Sublicensee's last act of infringement of Patent Rights
within the control of the Licensee or a Sublicensee, regardless of whether the
Licensee or a Sublicensee had control over prior infringing acts; the parties
intend that Earned Royalties due under this Agreement will be calculated based
on the Net Sales of the product or service resulting from the last act of
infringement by the Licensee and its Sublicensees.
- - oo 0 oo - -
The parties agree as
follows:
1. DEFINITIONS
As
used in this Agreement, the following terms, whether used in the singular or
plural, shall have the following meanings:
1.1 “Affiliate”
of the Licensee means any entity which, directly or indirectly, Controls the
Licensee, is Controlled by the Licensee or is under common Control with the
Licensee. “Control” means (i) having the actual, present capacity to
elect a majority of the directors of such affiliate; (ii) having the power to
direct at least forty percent (40%) of the voting rights entitled to elect
directors; or (iii) in any country where the local law will not permit foreign
equity participation of a majority, ownership or control, directly or
indirectly, of the maximum percentage of such outstanding stock or voting rights
permitted by local law.
1.2 “Attributed
Income” means the total gross proceeds (exclusive of Earned Royalties of
Sublicensees but including, without limitation, any license fees, maintenance
fees, or milestone payments), whether consisting of cash or any other forms of
consideration and whether any rights other than Patent Rights are granted, which
gross proceeds are received by or payable to the Licensee, any Affiliate and/or
Joint Venture from any Sublicensee in consideration of the grant of a sublicense
under this Agreement. Notwithstanding the foregoing, Attributed Income shall not
include proceeds attributed in such sublicense or such agreement, arrangement or
other relationship to bona fide (i) debt financing; (ii) equity (and conditional
equity, such as warrants, convertible debt and the like) investments in the
Licensee at market value; (iii) reimbursements of Patent Prosecution Costs
actually incurred by the Licensee; and (iv) reimbursement for the actual cost of
documented research and/or development services undertaken by the Licensee prior
to the effective date of the sublicense for the applicable field of use of such
sublicense and reimbursement for the actual cost of research and/or development
services provided after the effective date of the sublicense by Licensee for the
applicable Sublicensee under such sublicense on the basis of full-time
equivalent (“FTE”) efforts of personnel at or below commercially reasonable and
standard FTE rates. For the avoidance of doubt, any gross proceeds
meeting the definition set forth above in this Article 1.2 shall be “Attributed Income” irrespective of
whether such gross proceeds are received under one or more separate agreements
and irrespective of how such gross proceeds are referred to or characterized by
the Licensee or the Sublicensee. Also for avoidance of doubt, once a
research and development expense is deducted from Attributed Income for any
sublicense it can not be deducted again for that sublicense or any other
sublicense.
1.3 “Combination
Product” means a combined Product that contains or uses a Licensed Product or
Licensed Service and at least one other Product or process (a “Combination
Product Component”), where (i) such Combination Product Component is not a
Licensed Product or Licensed Service, (ii) if such Combination Product Component
were removed from such combined Product or service, the manufacture, use, Sale
or import of the resulting Product or service in or into a particular country
would infringe, but for a license, the same Valid Claim in the country where
such manufacture, use, Sale or import occurs as such combined Product or
service, (iii) such Combination Product Component and such Licensed Product or
Licensed Service are Sold separately from such combined Product or service by
the Licensee or any Affiliate, Joint Venture or Sublicensee, and (iv) the market
price of such combined Product is higher than the market price for such Licensed
Product or Licensed Service as a result of such combined Product or serviced
containing or using such Combination Product Component.
1.4 “Earned
Royalty” means Sublicensee Royalty (as defined in Paragraph 8.2) and Royalty (as defined in Paragraph 9.1)
1.5 “Field of
Use” means all fields unless otherwise amended by the parties to this
Agreement.
1.6 “First
Commercial Sale” means a bona fide good faith Sale of a Licensed Product in
quantities sufficient to meet market demand.
1.7 “Joint
Venture” means any separate entity established pursuant to an agreement between
a third party and the Licensee and/or Sublicensee to constitute a vehicle for a
joint venture, in which the separate entity manufactures, uses, purchases, Sells
or acquires Licensed Products from the Licensee or Sublicensee.
1.8 “Licensed
Method” means any process, art or method the use or practice of which, but for
the license granted in this Agreement, would infringe, or contribute to, or
induce the infringement of, any Patent Rights in any country were they issued at
the time of the infringing activity in that country.
1.9 “Licensed
Product” means any Product, including, without limitation, a Product for use or
used in practicing a Licensed Method and any Product made by practicing a
Licensed Method, the manufacture, use, Sale, offer for Sale or import of which,
but for the license granted in this Agreement, would infringe, or contribute to,
or induce the infringement of, any Patent Rights in any country were they issued
at the time of the infringing activity in that country.
1.10 “Licensed
Service” means any service provided for consideration (whether in cash or any
other form), when such service (i) involves the use of a Licensed Product; or
(ii) involves the practice of a Licensed Method.
1.11 “Net
Invoice Price” means:
|
1.11.1
|
For
Licensed Products and Licensed Services Sold to public companies with a
market cap of seventy-five million or less or private companies with
retained earnings of five million or less: (a) the gross invoice price
charged and the value of any other consideration owed to the Licensee
and/or any Sublicensee for such Licensed Product or Licensed Service, or
(b) for Combination Products, the gross invoice price charged and the
value of any other consideration owed to the Licensee and/or any
Sublicensee for such Licensed Product or Licensed Service used in the
Combination Product when such Licensed Product or Licensed Service is Sold
separately from such Combination Product, less the following items, but
only to the extent that they actually pertain to the disposition of such
Licensed Product or Licensed Service, are included in the gross invoice
price charged or other consideration owed, and are identified separately
on a bill or invoice:
|
|
1.11.1.1
|
Allowances
actually granted to customers for rejections, chargebacks, returns and
prompt payment and volume discounts;
|
|
1.11.1.2
|
uncollectible
debt up to a maximum of two percent (2%) of the gross invoice
price;
|
|
1.11.1.3
|
Freight,
transport packing and insurance charges associated with
transportation;
|
|
1.11.1.4
|
Taxes,
including Deductible Value Added Tax, tariffs or import/export duties
based on Sales when included in the gross invoice price, but excluding
value-added taxes other than Deductible Value Added Tax or taxes assessed
on income derived from Sales. “Deductible Value Added Tax”
means only the portion of the value added tax that is actually incurred
and is not reimbursable, refundable or creditable under the tax authority
of any country; and
|
|
1.11.1.5
|
Rebates
and discounts paid or credited pursuant to applicable law.
|
|
1.11.2
|
For
Licensed Products and Licensed Services Sold to public companies with a
market cap of more than seventy-five million or private companies with
retained earnings of more than five million: (a) the amount of
consideration actually received by the Licensee and/or any Sublicensee for
such Licensed Product or Licensed Service, or (b) for Combination
Products, the amount of consideration actually received by the Licensee
and/or any Sublicensee for such Licensed Product or Licensed Service used
in the Combination Product when such Licensed Product or Licensed Service
is Sold separately from such Combination Product, less the following
items, but only to the extent that they actually pertain to the
disposition of such Licensed Product or Licensed Service, are included in
the gross invoice price charged or other consideration owed, and are
identified separately on a bill or invoice:
|
|
1.11.2.1
|
Allowances
actually granted to customers for rejections, chargebacks, returns and
prompt payment and volume discounts;
|
|
1.11.2.2
|
Freight,
transport packing and insurance charges associated with
transportation;
|
|
1.11.2.3
|
Taxes,
including Deductible Value Added Tax, tariffs or import/export duties
based on Sales when included in the gross invoice price, but excluding
value-added taxes other than Deductible Value Added Tax or taxes assessed
on income derived from Sales. “Deductible Value Added Tax”
means only the portion of the value added tax that is actually incurred
and is not reimbursable, refundable or creditable under the tax authority
of any country; and
|
|
1.11.2.4
|
Rebates
and discounts paid or credited pursuant to applicable law.
|
1.12 “Net
Sale” means:
|
1.12.1
|
except
in the instances described in Paragraphs 1.12.2, 1.12.3 and 1.12.4 of this Paragraph, the Net Invoice
Price;
|
|
1.12.2
|
for
any Relationship-Influenced Sale of a Licensed Product or Licensed
Service, Net Sales shall be based on the Net Invoice Price at which the
Relationship-Influenced Sale Purchaser re-Sells such Licensed Product or
Licensed Service;
|
|
1.12.3
|
in
those instances where Licensed Product or Licensed Service is not Sold,
but is otherwise exploited (except with respect to limited commercially
reasonable quantities of Licensed Product or Licensed Service that are
provided solely for demonstration, evaluation and feasibility purposes for
no consideration) , the Net Sales for such Licensed Product or Licensed
Service shall be the Net Invoice Price of products or services of the same
or similar kind and quality, Sold in similar quantities, currently being
offered for Sale by the Licensee and/or any Sublicensee. Where
such products or services are not currently being offered for Sale by the
Licensee and/or any Sublicensee, the Net Sales for Licensed Product or
Licensed Service otherwise exploited, for the purpose of computing
royalties, shall be the average Net Invoice Price at which products or
services of the same or similar kind and quality, Sold in similar
quantities, are then currently being offered for Sale by other
manufacturers. Where such products or services are not
currently Sold or offered for Sale by the Licensee and/or any Sublicensee
or others, then the Net Sales shall be the Licensee's and/or any
Sublicensee's cost of manufacture of Licensed Product or the cost of
conducting the service, determined according to Generally Accepted
Accounting Principles (“GAAP”), plus 100 percent (100%); and
|
|
1.12.4
|
for
a Reacquisition Sale or Exploitation, Net Sales shall mean the Net Invoice
Price upon the Reacquisition Sale or Exploitation of a Licensed Product or
Licensed Service.
|
1.13 “New
Developments” means inventions, or claims to inventions, which constitute
advancements, developments or improvements, whether or not patentable and
whether or not the subject of any patent application, which are not sufficiently
supported by the specification of a previously-filed patent or patent
application within the Patent Rights to be entitled to the priority date of the
previously-filed patent or patent application.
1.14 “Patent
Prosecution Costs” is defined in Paragraph 22.3.
1.15 “Patent
Rights” means the Valid Claims of, to the extent assigned to or otherwise
obtained by The Regents, the following United States patents and patent
applications:
|
UC
Case Number
|
United
States Application Number or
United
States Patent Number
|
Filing
or Issue Date
|
Patent
Rights shall further include the Valid Claims of, to the extent assigned to or
otherwise obtained by The Regents, the corresponding foreign patents and patent
applications (requested under Paragraph 22.4
herein) and any reissues, extensions, substitutions, continuations, divisions,
and continuation-in-part applications (but only those Valid Claims in the
continuation-in-part applications that are entirely supported in the
specification and entitled to the priority date of the parent application) and
any patent that issues on any application included in the
foregoing. This definition of Patent Rights excludes any rights in
and to New Developments, except to the extent added by amendment pursuant to
Article 11. By mutual written agreement
the parties to this Agreement may include or exclude patent rights from this
definition including patent rights claiming inventions first conceived and
reduced to practice under the Research Agreement. To the extent any
of the claims in Patent Rights has an inventor that is not a student, faculty or
staff member from Dr. Michelle Khine's lab and/or The Regents owe a right to a
third party who sponsored the research of such inventor that resulted in the
claimed invention, then The Regents and Licensee shall negotiate in good faith
the rights that will be available to Licensee with respect to such
claims.
1.16 “Product”
means any kit, article of manufacture, composition of matter, material,
compound, component or product.
1.17 “Reacquisition
Sale or Exploitation” means those instances where the Licensee or a Sublicensee
acquires a Licensed Product or Licensed Service and then subsequently Sells or
otherwise exploits such Licensed Product or Licensed Service.
1.18 “Related
Party” means a corporation, firm or other entity with which, or individual with
whom, the Licensee and/or any Sublicensee (or any of its respective
stockholders, subsidiaries or Affiliates) have any agreement, understanding or
arrangement (for example, but not by way of limitation, an option to purchase
stock or other equity interest, or an arrangement involving a division of
revenue, profits, discounts, rebates or allowances) unrelated to the Sale or
exploitation of the Licensed Products or Licensed Services without which such
other agreement, understanding or arrangement, the amounts, if any, charged by
the Licensee or any Sublicensee to such entity or individual for the
Licensed Product or Licensed Service, would be higher than the Net Invoice Price
actually received, or if such agreement, understanding or arrangement results in
the Licensee or any Sublicensee extending to such entity or individual lower
prices for such Licensed Product or Licensed Service than those charged to
others without such agreement, understanding or arrangement buying similar
products or services in similar quantities.
1.19 “Relationship-Influenced
Sale” means a Sale of a Licensed Product or Licensed Service, or any
exploitation of the Licensed Product or Licensed Method, between the Licensee
and/or any Sublicensee and (i) an Affiliate; (ii) a Joint Venture; (iii) a
Related Party or (iv) the Licensee or a Sublicensee.
1.20 “Relationship-Influenced
Sale Purchaser” means the purchaser of Licensed Product or Licensed Service in a
Relationship-Influenced Sale.
1.21 “Sale”
means the act of selling, leasing or otherwise transferring, providing, or
furnishing for use for any consideration. Correspondingly, “Sell”
means to make or cause to be made a Sale and “Sold” means to have made or caused
to be made a Sale.
1.22 “Sublicensee”
means any person or entity (including any Affiliate or Joint Venture) to which
any of the license rights granted to the Licensee hereunder are
sublicensed.
1.23 “Sublicense
Fee” is defined in Paragraph 8.1.
1.24 “Valid
Claim” means a claim of a patent or patent application in any country that (i)
has not expired; (ii) has not been disclaimed; (iii) has not been cancelled or
superseded, or if cancelled or superseded, has been reinstated; and (iv) has not
been revoked, held invalid, or otherwise declared unenforceable or not allowable
by a tribunal or patent authority of competent jurisdiction over such claim in
such country from which no further appeal has or may be taken.
2. GRANT
2.1 Subject
to the limitations and other terms and conditions set forth in this Agreement
including, if applicable, the license granted to the United States Government
set forth in the Background and in Paragraph 2.3.1,
The Regents grants to the Licensee a license under its rights in and to Patent
Rights to make, use, Sell, offer for Sale and import Licensed
Products and Licensed Services and to practice Licensed Methods, in the United
States and in other countries where The Regents may lawfully grant such
licenses, only in the Field of Use.
2.2 Except as
otherwise provided for in this Agreement, the license granted under Patent
Rights in Paragraph 2.1 is
exclusive.
|
2.3.1
|
If
applicable, the obligations to the United States Government under 35
U.S.C. §§ 200-212 and all applicable governmental implementing
regulations, as amended from time to time, including the obligation to
report on the utilization of the Invention as set forth in 37 CFR. §
401.14(h), and all applicable provisions of any license to the United
States Government executed by The Regents; and
|
|
2.3.2
|
the
National Institutes of Health “Principles and Guidelines for Recipients of
NIH Research Grants and Contracts on Obtaining and Disseminating
Biomedical Research Resources,” 64 F.R. 72090 (Dec. 23, 1999), as amended
from time to time.
|
2.4 The
license granted in Paragraphs 2.1 and 2.2 is limited to methods and products that are within
the Field of Use. In the event that the Field of Use is amended upon
mutual written agreement to exclude certain fields, then the Licensee has no
license under this Agreement for such excluded fields.
2.5 The
Regents reserves and retains the right (and the rights granted to the Licensee
in this Agreement shall be limited accordingly) to make, use and practice the
Invention and any technology relating to the Invention and to make and use any
Products and to practice any process that is the subject of the Patent Rights
(and to grant any of the foregoing rights to other educational and non-profit
institutions) for educational, clinical and research purposes, including without
limitation, any sponsored research performed for or on behalf of commercial
entities and including publication and other communication of any research
results. For the avoidance of doubt, to the extent the Invention and
any technology relating to the Invention are not the subject of the exclusive
license under the Patent Rights granted to the Licensee hereunder, The Regents
shall be free to make, use, Sell, offer to Sell, import, practice and otherwise
commercialize and exploit (including to transfer, license to, or have exercised
by, third parties) for any purpose whatsoever and in its sole discretion, such
Invention and any Products or processes that are the subject of any of the
foregoing.
2.6 In the
event that the Invention was made under funding provided by the United States
Government, then the Licensed Products, the Invention, and any products
embodying the Invention sold in the United States will be substantially
manufactured in the United States.
2.7 This
Agreement will terminate immediately if a claim which includes, in any way, the
assertion that any portion of Patent Rights is invalid or unenforceable is filed
by the Licensee or an Interested Party. For the purposes of this
Paragraph 2.7, an Interested Party means any third
party who files such a claim on behalf of the Licensee or at the written urging
of the Licensee, or is a Sublicensee of Licensee. Notwithstanding the above, if
Licensee terminates a Sublicensee who violates this provision within fifteen
(15) days following receipt of written notice from The Regents of such violation
and the Sublicensee did not violate this provision at Licensee’s urging, then
this Agreement will remain in effect.
3. SUBLICENSES
3.1 The
Regents also grants to the Licensee the right to sublicense to third parties
(including to Affiliates and Joint Ventures) the rights granted to the Licensee
hereunder, with no right to further sublicense except as provided for in
Paragraph 3.2 below, as long as the Licensee has
current exclusive rights thereto under this Agreement. Each
Sublicensee must be subject to a written sublicense agreement. All
sublicenses will include all of the rights of, and will require the performance
of all the obligations due to, The Regents (and, if applicable, the United
States Government and other sponsors), other than those rights and obligations
specified in Article 6 (License Issue Fee), Article
7 (License Maintenance Fee) and Paragraph 9.5 (Minimum Annual Royalty) and Paragraphs 22.3 and 22.5
(reimbursement of Patent Prosecution Costs). For the avoidance of
doubt, Affiliates and Joint Ventures shall have no licenses under this Agreement
unless such Affiliates and Joint Ventures are granted a sublicense. For the
purposes of this Agreement, the operations of all Sublicensees shall be deemed
to be the operations of the Licensee, for which the Licensee shall be
responsible.
3.2 Under the
terms of each sublicense, each such Sublicensee shall have the limited right (as
described below) to grant three (3) further sublicenses (“Further Sublicenses”)
to the Sublicensee’s affiliated companies and/or other third parties (each, a
“Further Sublicensee”). Each Further Sublicensee shall also have the
limited right to grant two (2) additional further sublicenses (“Additional
Further Sublicenses”) to an affiliated company and/or other third party (each an
“Additional Further Sublicensee”). In each case the term “affiliated
company” shall have the same definition as Affiliate in Section 1.1 of this
Agreement, with the appropriate sublicensee substituted for Licensee in the
definition. Such Further Sublicenses and Additional Further
Sublicenses may only be granted to the extent that such Sublicensee or Further
Sublicensee deems that they are reasonably needed for the development and
commercialization of Licensed Products and the maximization of sales in
accordance with this Agreement. Each Sublicensee and each permitted
Further Sublicensee and Additional Further Sublicensee shall be subject to a
written sublicense agreement that shall be consistent with and not in violation
of all of the applicable terms, conditions, obligations, restrictions and other
terms of this Agreement that protect or benefit The Regents’ (and, if
applicable, the U.S. Government’s and other sponsors’) rights and
interests. Licensee shall attach a copy of this Agreement to each
sublicense issued under this Paragraph 3.2 and
shall specify in the sublicense that the sublicensee must comply with the terms
of the Agreement. Licensee may redact the following information from
the Agreement, should it wish to do so: License Issue Fee, License Maintenance
Fee, Earned Royalties and Minimum Royalties, Milestone Payments, Fees for Patent
Rights Added After Effective Date and the Patent Rights not included in the
sublicense. Licensee agrees that it shall require appropriate audited
and auditable reporting from each Sublicensee, its Further Sublicensees and
Additional Further Sublicensees to establish all amounts owed hereunder, and
shall make such reports available to The Regents. Licensee shall
require each Sublicensee to submit to Licensee progress reports and audited
financial reports consistent with the Agreement, and each Sublicensee shall
require each Further Sublicensee and Additional Further Sublicensee to submit
such progress reports and audited financial reports to Sublicensee which it will
deliver to Licensee. Licensee shall make these reports available to
The Regents. Licensee shall require that each Sublicensee agree to
indemnification procedures and insurance coverages consistent with the
obligations imposed on Licensee by Article 25 of
the Agreement. Licensee shall also require each Sublicensee to obtain
comparable indemnification provisions from each Further Sublicensee and each
Additional Further Sublicensee.
3.3 In the
event that The Regents and the Licensee each own an undivided interest in any
Patent Rights licensed hereunder, the Licensee will not separately grant a
license to any third party under its rights without concurrently granting a
license under The Regents' rights on the terms and conditions described in this
Article 3 (Sublicenses).
3.4 The
Licensee will notify The Regents of each sublicense granted hereunder and will
provide The Regents with a complete copy of each sublicense (along with a
summary of the material terms of each such sublicense) and each amendment to
such sublicense within thirty (30) days after issuance of such sublicense or
such amendment. The Licensee will collect from Sublicensees and pay
to The Regents all fees, payments, royalties and the cash equivalent of any
consideration due The Regents. The Licensee will guarantee all monies
due The Regents from Sublicensees. For clarity, if the Licensee grants a
sublicense that contains a provision for payment of royalties by any Sublicensee
in an amount that is less than the Sublicensee Royalty required to be paid under
Paragraph 8.2 below, then the Licensee will pay to
The Regents a total amount equal to the Sublicensee Royalty based on the
Sublicensees’ Net Sales as provided for in Paragraph 8.2. The Licensee will require Sublicensees
to provide it with copies of all progress reports and royalty reports in
accordance with the provisions herein and the Licensee will collect and deliver
all such reports due The Regents from Sublicensees.
3.5 If
Licensee licenses patent rights assigned to or otherwise acquired by it
(“Licensee's Patent Rights”), and it believes, in good faith, that the recipient
of such license will infringe Patent Rights in practicing the Licensee's Patent
Rights, then the Licensee will not separately grant a license to such recipient
under Licensee's Patent Rights without concurrently granting a sublicense under
Patent Rights on the terms required under this Agreement.
3.6 Upon any
expiration or termination of this Agreement for any reason, all sublicensed
rights conveyed to any Sublicensee (but not Further Sublicensees or Additional
Further Sublicensees), granted pursuant to Article 3 of this Agreement will remain in effect and will be
assumed by The Regents as binding obligations provided that (a) such Sublicensee
is not in breach of its sublicense at the time of expiration or termination of
this Agreement; (b) all of the terms of this Agreement are agreed to fully in
writing by such Sublicensee; and (c) such Sublicensee acquires no rights from or
obligations on the part of The Regents other than those that are specifically
granted under this Agreement and such Sublicensee assumes all
liability and obligations to The Regents required of Licensee by this Agreement
with respect to The Regents' sublicensed rights, including past due obligations
existing at the time of assignment of this Agreement by Licensee. Moreover, The
Regents will have the sole right to modify each such assigned sublicense to
include all of the rights of The Regents (and, if applicable, the United States
Government and other sponsors) that are contained in this Agreement, including
the payment of Earned Royalties directly to The Regents by the Sublicensee as if
it were the Licensee at a rate that is no lower than the rate set forth in
Article 9 (Earned Royalties and Minimum Annual
Royalties) in accordance with Article 5 (Payment
Terms). If the Sublicensee fails to meet the above provisions
described in this Paragraph 3.6 (a – c)
then The Regents may terminate its sublicense, in accordance with
Article 16 (Termination by The
Regents). The Regents will not be bound to perform any duties or
obligations set forth in any sublicense that extend beyond the duties and
obligations of The Regents set forth in this Agreement, and the Licensee’s
obligations to The Regents hereunder will be binding upon the
Sublicensee.
3.7 In the
event that the sublicense granted to the Sublicensee under this Agreement
terminates or expires while this Agreement remains in effect, all Further
Sublicenses and Additional Further Sublicenses shall automatically terminate or
expire, as appropriate.
4. MANDATORY
SUBLICENSING
4.1 Commencing
on the date that is eighteen (18) months after the Effective Date, if The
Regents (as represented by the actual knowledge of the licensing professional
responsible for administration of this Agreement) becomes aware of, or if a
third party becomes aware of and notifies such licensing professional of, an
application or use for Products within the licensed Field of Use but for which
Licensed Products have not been developed or are not, at such time, being
developed by Licensee (“New Application”), then The Regents, through the Office
of Technology Transfer, may give written notice to Licensee
thereof.
4.2 Within
ninety (90) days of such notice, Licensee shall give The Regents written notice
stating whether Licensee agrees to develop and commercialize Licensed Products
for such New Application (“New Licensed Products”). Such notice shall
be accompanied by (i) a detailed development schedule, including specific
diligence requirements and development milestones, for the development of New
Licensed Products; and (ii) a detailed business plan for the development,
marketing and commercialization of New Licensed Products (collectively, the
“Development Plan”). If Licensee has not notified The Regents within
such ninety (90) day period, in accordance with the foregoing, that Licensee
agrees to develop and commercialize such New Licensed Products, or if the
Development Plan is not reasonably acceptable to The Regents, then Licensee
shall be deemed to not so agree.
4.3 If
Licensee agrees, as set forth in Paragraph 4.2, to
develop and commercialize such New Licensed Products, then Licensee shall in
accordance with the Development Plan (i) diligently proceed with the
development, manufacture and commercialization of such New Licensed Products and
(ii) after such New Licensed Product has been developed, earnestly and
diligently endeavor to market the same in quantities sufficient to meet market
demand. Licensee shall submit a written progress report setting forth
in detail the status of such development, manufacture and commercialization
every six (6) months to The Regents.
4.4 If
Licensee does not agree, as set forth in Paragraph 4.2 to develop and commercialize such New Licensed
Products, or if Licensee fails to diligently pursue the development and
commercialization thereof in accordance with the Development Plan, then The
Regents shall have the right to seek one or more third parties for the
development and commercialization of such New Licensed Products and refer such
third party to Licensee so that such third party may request a sublicense
allowing for development and commercialization of such New Licensed
Products. If the third party requests a sublicense, then Licensee
shall report such request, together with the terms and conditions thereof, to
The Regents within thirty (30) days from the date of such request.
4.5 If
Licensee does not grant a sublicense to the third party for the New Application
within a reasonable time after such request (and, in any event, within one
hundred (100) days after such request), or refuses to grant such sublicense,
then Licensee shall promptly, or, in the event of such refusal, within thirty
(30) days after such refusal, submit to The Regents a written
report. Such report will include a written justification for the
Licensee's refusal or failure to grant such sublicense and the license terms
proposed by the third party, if any. The Regents, at its sole
discretion, shall have the right to grant to the third party (and the rights
granted to Licensee in this Agreement shall be limited accordingly) a license to
make, have made, use, sell, offer for sale and import Licensed Products, to
provide Licensed Services and to practice the Licensed Methods within the New
Application under terms that The Regents determines to be
reasonable. All amounts received by The Regents pursuant to such
license, after recovery by The Regents of its expenses in obtaining the license,
shall be divided between The Regents and the Licensee as
follows: sixty percent (60%) to The Regents and forty percent (40%)
to the Licensee. The Regents shall deliver to Licensee its portion
thereof.
5. PAYMENT
TERMS
5.1 Paragraphs
1.8, 1.9, 1.10 and 1.15 define Licensed
Method, Licensed Product, Licensed Service and Patent Rights, so that Earned
Royalties are payable on products and methods covered by both pending patent
applications and issued patents. Earned Royalties will accrue in each
country for the duration of Patent Rights in that country and will be payable to
The Regents when Licensed Products or Licensed Services are invoiced, or if not
invoiced, when delivered or otherwise exploited by the Licensee or Sublicensee
in a manner constituting a Net Sale as defined in Paragraph 1.12. Sublicense Fees with respect to any
Attributed Income shall accrue to The Regents within thirty (30) days of the
date that such Attributed Income is due to the Licensee.
5.2 The
Licensee will pay to The Regents all Earned Royalties, Sublicense Fees and other
consideration payable to The Regents quarterly on or before February 28 (for the
calendar quarter ending December 31), May 31 (for the calendar quarter ending
March 31), August 31 (for the calendar quarter ending June 30) and November 30
(for the calendar quarter ending September 30) of each calendar
year. Each payment will be for Earned Royalties, Sublicense Fees and
other consideration which has accrued within the Licensee's most recently
completed calendar quarter.
5.3 All
consideration due The Regents will be payable and will be made in United States
dollars by check payable to “The Regents of the University of California” or by
wire transfer to an account designated by The Regents. The Licensee
is responsible for all bank or other transfer charges. When Licensed
Products or Licensed Services are Sold for monies other than United States
dollars, the Earned Royalties and other consideration will first be determined
in the foreign currency of the country in which such Licensed Products or
Licensed Services were Sold and then converted into equivalent United States
dollars. The exchange rate will be the average exchange rate quoted
in the The Wall Street
Journal during the last thirty (30) days of the reporting
period.
5.4 Sublicense
Fees and Earned Royalties on Net Sales of Licensed Products or Licensed Services
and other consideration accrued in, any country outside the United States may
not be reduced by any taxes, fees or other charges imposed by the government of
such country, except those taxes, fees and charges allowed under the provisions
of Paragraph 1.12 (Net Sales).
5.5 Notwithstanding
the provisions of Article 29 (Force Majeure) if at
any time legal restrictions prevent the prompt remittance of Earned Royalties or
other consideration owed to The Regents by the Licensee with respect to any
country where a sublicense is issued or a Licensed Product or Licensed Service
is Sold or otherwise exploited, then the Licensee shall convert the amount owed
to The Regents into United States dollars and will pay The Regents directly from
another source of funds in order to remit the entire amount owed to The
Regents.
5.6 In the
event that any patent or claim thereof included within the Patent Rights is held
invalid in a final decision by a court of competent jurisdiction and last resort
and from which no appeal has or can be taken, then all obligation to pay
royalties based on that patent or claim or any claim patentably indistinct
therefrom will cease as of the date of final decision. The Licensee
will not, however, be relieved from paying any royalties that accrued before
such final decision and the Licensee shall be obligated to pay the full amount
of royalties due hereunder to the extent that The Regents licenses one or more
Valid Claims within the Patent Rights to the Licensee with respect to Licensed
Products or Licensed Services.
5.7 If
applicable, no Earned Royalties will be collected or paid hereunder to The
Regents on Licensed Products or Licensed Services Sold to, or otherwise
exploited for, the account of the United States Government as provided for in
the license to the United States Government. The Licensee and its
Sublicensees will reduce the amount charged for Licensed Products or Licensed
Services Sold to, or otherwise exploited by, the United States Government by an
amount equal to the Earned Royalty for such Licensed Products or Licensed
Services otherwise due The Regents. Such reduction in Earned Royalties will be
in addition to any other reductions in price required by the United States
Government.
5.8 In the
event that royalties, fees, reimbursements for Patent Prosecution Costs or other
monies owed to The Regents are not received by The Regents when due, the
Licensee will pay to The Regents interest at a rate of ten percent (10%) simple
interest per annum. Such interest will be calculated from the date
payment was due until actually received by The Regents. Such accrual
of interest will be in addition to and not in lieu of, enforcement of any other
rights of The Regents due to such late payment.
6. LICENSE
ISSUE FEE
6.1 The
Licensee will pay a fee of five thousand dollars ($5,000) for each patent
application and patent under Patent Rights as of the Effective Date of the
Agreement, and if not yet filed, for each case identified by UC Case Number
under Patent Rights as of the Effective Date of the Agreement.
7. LICENSE
MAINTENANCE FEE
7.1 The
Licensee will also pay to The Regents a license maintenance fee of
ten thousand dollars ($10,000) beginning on the fourth-year
anniversary of the Effective Date and continuing annually on each anniversary of
the Effective Date. The license maintenance fee is not due on any
anniversary of the Effective Date if on that date, the Licensee is Selling or
otherwise exploiting Licensed Products or Licensed Services and is paying an
Earned Royalty to The Regents on the Net Sales of such Licensed Product or
Licensed Services. The license maintenance fee is non-refundable and
is not an advance or otherwise creditable against any royalties or other
payments required to be paid under the terms of this Agreement.
8. PAYMENTS
ON SUBLICENSES
8.1 The
Licensee will pay to The Regents the following non-refundable and non-creditable
sublicense fees (“Sublicense Fees”):
|
8.1.1
|
thirty
percent (30%) of all Attributed Income.
|
8.2 The
Licensee will also pay to The Regents, with respect to each Sublicensee, an
earned royalty on the
Net Sales of each Licensed Product, Licensed Method or Licensed Service as
provided for in Paragraphs 8.3 and 8.4 below (“Sublicensee Royalty”).
8.3 For each
Licensed Product, Licensed Method or Licensed Service covered by a patent
application or patent included in Patent Rights as of the Effective Date of the
Agreement, the royalty rate for that Licensed Product, Licensed Method or
Licensed Service shall be as follows:
|
8.3.1
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs within three (3) years after the Effective Date,
two and one-half percent (2.5%) of Net Sales;
|
|
8.3.2
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs between three (3) and six (6) years after the
Effective Date, four percent (4%) of Net Sales; or
|
|
8.3.3
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs beyond six (6) years after the Effective Date, five
percent (5%) of Net Sales.
|
8.4 For
Licensed Products, Licensed Method or Licensed Service covered by a patent
application or patent included in Patent Rights by amendment after the Effective
Date of the Agreement, the royalty rate shall be as follows:
|
8.4.1
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs within three (3) years after the effective date of
the amendment under which the patent application or patent is included in
Patent Rights, two and one-half percent (2.5%) of Net Sales;
|
|
8.4.2
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs between three (3) and six (6) years after the
effective date of the amendment under which the patent application or
patent is included in Patent Rights, four percent (4%) of Net Sales;
or
|
|
8.4.3
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs beyond six (6) years after the effective date of
the amendment under which the patent application or patent is included in
Patent Rights, five percent (5%) of Net Sales.
|
8.5 In the
event that the Sublicensee (other than an Affiliate or Joint Venture) uses the
Licensed Products or practices the Licensed Method internally as a research
tool, then the Licensee will also pay to The Regents, with respect to each
Sublicensee (other than an Affiliate or Joint Venture), an Earned Royalty at a
rate to be agreed upon between the parties, but which in no event will be at a
rate lower than the rate charged for similar research tools licensed
from The Regents by others.
9. EARNED
ROYALTIES AND MINIMUM ANNUAL ROYALTIES
9.1 The
Licensee will also pay to The Regents an earned royalty on the Net
Sales of each Licensed Product, Licensed Method or Licensed Service by the
Licensee or any Affiliate or Joint Venture as provided for in Paragraphs 9.2 and 9.3 below
(“Royalty”).
9.2 For each
Licensed Product, Licensed Method or Licensed Service covered by a patent
application or patent included in Patent Rights as of the Effective Date of the
Agreement, the royalty rate for that Licensed Product, Licensed Method or
Licensed Service shall be as follows:
|
9.2.1
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs within three (3) years after the Effective Date,
two and one-half percent (2.5%) of Net Sales;
|
|
9.2.2
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs between three (3) and six (6) years after the
Effective Date, four percent (4%) of Net Sales; or
|
|
9.2.3
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs beyond six (6) years after the Effective Date, five
percent (5%) of Net Sales.
|
9.3 For
Licensed Products, Licensed Method or Licensed Service covered by a patent
application or patent included in Patent Rights by amendment after the Effective
Date of the Agreement, the royalty rate shall be as follows:
|
9.3.1
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs within three (3) years after the effective date of
the amendment under which the patent application or patent is included in
Patent Rights, two and one-half percent (2.5%) of Net Sales;
|
|
9.3.2
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs between three (3) and six (6) years after the
effective date of the amendment under which the patent application or
patent is included in Patent Rights, four percent (4%) of Net Sales;
or
|
|
9.3.3
|
For
a Licensed Product, Licensed Method or Licensed Service where a First
Commercial Sale occurs beyond six (6) years after the effective date of
the amendment under which the patent application or patent is included in
Patent Rights, five percent (5%) of Net Sales.
|
9.4 In the
event that the Licensee, an Affiliate or Joint Venture uses the Licensed
Products or practices the Licensed Method internally as a research tool (but
excluding Licensee’s research and development efforts to develop Licensed
Products, Licensed Methods and Licensed Services), then the Licensee will also
pay to The Regents, with respect to each Sublicensee (other than an Affiliate or
Joint Venture), an Earned Royalty at a rate to be agreed upon between the
parties, but which in no event will be at a rate lower than the rate
charged for similar research tools licensed from The Regents by
others.
9.5 The
Licensee will also pay to The Regents a minimum annual royalty for the
life of Patent Rights. Beginning with the year of the First Commercial Sale of a
Licensed Product or Licensed Service, the Licensee will pay a minimum annual
royalty of fifteen thousand dollars ($15,000). Beginning on the date
of the First Commercial Sale of the fourth Licensed Product, the Licensee will
pay a minimum annual royalty of twenty thousand dollars
($20,000). For each First Commercial Sale of a Licensed Product after
the fourth Licensed Product, the minimum annual royalty due to The Regents will
increase by five thousand dollars ($5,000). For example, upon the
First Commercial Sale of the fifth Licensed Product or Licensed Service, the
Licensee will pay a minimum annual royalty of twenty-five thousand dollars
($25,000). A Licensed Product that is repackaged or has minor
improvements shall not be considered a “new” Licensed Product for the purposes
of this Paragraph. However, for avoidance of doubt, if both the old
product and “new” repackaged or modified product are being Sold, then such
repackaged or modified product shall be considered a “new” Licensed
Product.
9.6 The
minimum annual royalty will be paid to The Regents by February 28 of each year
and will be credited against the Earned Royalty due for the calendar year in
which the minimum payment was made.
10. MILESTONE
PAYMENTS
10.1 The
Licensee will pay to The Regents the following non-refundable, non-creditable
amounts based on total accumulated Net Sales:
|
10.1.1
|
For
total accumulated Net Sales of fifty million dollars ($50,000,000), the
Licensee will pay to The Regents a milestone payment of one hundred
thousand dollars ($100,000);
|
|
10.1.2
|
For
total accumulated Net Sales of one hundred and fifty million dollars
($150,000,000) the Licensee will pay to The Regents a milestone payment
of, five hundred thousand dollars ($500,000); and
|
|
10.1.3
|
For
total accumulated Net Sales of five hundred million dollars
($500,000,000), the Licensee will pay to The Regents a
milestone payment of two million dollars ($2,000,000).
|
10.2 For the
avoidance of doubt, each milestone payment will be payable regardless of whether
the applicable milestone event has been achieved by the Licensee or any
Affiliate, Joint Venture, or Sublicensee.
10.3 All
milestone payments are due to The Regents within thirty (30) days of the
occurrence of the applicable milestone event.
11. FEES FOR
PATENT RIGHTS ADDED AFTER EFFECTIVE DATE
11.1 The
Licensee will pay a fee of five thousand dollars ($5,000) per Base Case added to
the Agreement after the Effective Date. Base Case means rights in the
Valid Claims of any patent or patent application being added to Patent Rights
after the Effective Date, which patent or patent application was not included
within the scope of claims of Patent Rights prior to the date of such addition,
to the extent assigned to or otherwise obtained by The Regents, any
corresponding foreign patents and patent applications, and any reissues,
extensions, substitutions, continuations, divisions, and continuation-in-part
applications (but only those Valid Claims in the continuation-in-part
applications that are entirely supported in the specification and entitled to
the priority date of the parent application added).
11.2 For any
Licensed Product covered by patents or patent applications not included in the
Agreement as of the Effective Date, in the event that the earned royalty for
Licensed Products provided for in Articles 8 and 9 above is not in compliance with legal requirements,
the Licensee and The Regents agree to negotiate in good faith an earned royalty
for such Licensed Products which is legally compliant.
12. DUE
DILIGENCE
12.1 The
Licensee, upon execution of this Agreement, will diligently proceed with the
development and manufacture of Licensed Products and Licensed Services and after
a Licensed Product or Licensed Service has been developed, will
earnestly and diligently Sell and market the same after execution of this
Agreement and in quantities sufficient to meet the market demands
therefor.
12.2 The
Licensee will obtain all necessary governmental approvals in each country where
Licensed Products and Licensed Services are manufactured, used, Sold, offered
for Sale or imported.
12.3 The
Licensee will:
|
12.3.1
|
no
later than three (3) years after the Effective Date, either (a) produce a
good faith working prototype of a first Licensed Product or (b) have a
minimum market capitalization of thirty million dollars ($30,000,00) at
some time within the first three (3) years of the Effective
Date;
|
|
12.3.2
|
within
five (5) years after the Effective Date, have a First Commercial Sale of a
Licensed Product or have executed a sublicense agreement;
|
|
12.3.3
|
within
seven (7) years after the Effective Date, have a First Commercial Sale of
a second Licensed Product or have a second executed sublicense agreement;
and
|
|
12.3.4
|
fill
the market demand for Licensed Products and Licensed Services following
commencement of marketing at any time during the exclusive period of this
Agreement.
|
12.4 If the
Licensee is unable to perform any of the above provisions, then The Regents has
the right and option to either terminate this Agreement or reduce the exclusive
license granted to the Licensee to a nonexclusive license in accordance with
Paragraph 12.7 below. This right, if
exercised by The Regents, supersedes the rights granted in Article 2 (Grant).
12.5 Notwithstanding
the provisions of Paragraph 12.4, The Regents
recognizes that, taking into account the need for further research and
development of the technology before it will be possible for Licensee to
commercialize a Licensed Product, it may be necessary from time to time to amend
the milestones of Paragraphs 12.3.2 and 12.3.3. Accordingly, The Regents hereby
agrees to consider in good faith reasonable proposals from Licensee to amend the
milestones of these two Paragraphs in light of Licensee's
experience in implementing its development plan for Licensed Products under this
Agreement, and The Regents and Licensee agree to discuss and negotiate in good
faith the diligence requirements of Paragraphs 12.3.2 and 12.3.3 for a
period of ninety (90) days if despite diligent effort by Licensee, Licensee is
unable to meet the specified milestone.
12.6 If,
however, notwithstanding good faith negotiation, the parties are unable to agree
upon any modification to Paragraph 12.3.2 and 12.3.3, then the parties will be under no further
obligation to negotiate, and the Agreement's terms shall govern. No
amendment or modification of this Agreement is valid or binding on the parties
unless made in writing and signed on behalf of each party.
12.7 To
exercise either the right to terminate this Agreement or to reduce the exclusive
license granted to the Licensee to a non-exclusive license for lack of diligence
required in this Article 12 (Due Diligence), The
Regents will give the Licensee written notice of the deficiency. The
Licensee thereafter has sixty (60) days to cure the deficiency. If
The Regents has not received written tangible evidence satisfactory to The
Regents that the deficiency has been cured by the end of the sixty (60)-day
period, then The Regents may, at its option, terminate this Agreement
immediately without the obligation to provide sixty (60) days' notice as set
forth in Article 16 (Termination by The Regents) or
reduce the exclusive license granted to the Licensee to a non-exclusive license
by giving written notice to the Licensee.
12.8 Notwithstanding
Paragraphs 12.4 and 12.7, if Licensee is Selling a Licensed Product at the
time of termination, then the Licensee will have the right to a limited
exclusive license under the Patent Rights but only to the extent required to
continue Selling such Licensed Product provided that such Sales are subject to
the terms of this Agreement, including but not limited to the rendering of
reports and payment of earned royalties as required under this
Agreement. If Licensee ceases Selling such existing Licensed Product
then its continuing rights under this Paragraph 12.8 terminate.
13. PROGRESS
AND ROYALTY REPORTS
13.1 Beginning
on June 30, 2010, and semi-annually thereafter, the Licensee will submit to The
Regents a written progress report as described in Paragraph 13.2 below covering the Licensee's (and any
Affiliates', Joint Ventures', or Sublicensee's) activities related to
the development and testing of all Licensed Products and Licensed Services and
related to the obtaining of the governmental approvals necessary for marketing
and the activities required and undertaken in order to meet the diligence
requirements set forth in Article 12 (Due
Diligence). Progress reports are required for each Licensed Product
and Licensed Service until the first Sale or other exploitation of that Licensed
Product or Licensed Service occurs in the United States and shall be again
required if Sales of such Licensed Product or Licensed Service are suspended or
discontinued.
13.2 Progress
reports submitted under Paragraph 13.1 shall
include, but are not limited to, a detailed summary of the following topics so
that The Regents will be able to determine the progress of the development of
Licensed Products and Licensed Services and will also be able to determine
whether or not the Licensee has met its diligence obligations set forth in
Article 12 (Due Diligence) above:
|
13.2.1
|
summary
of work completed as of the submission date of the progress
report;
|
|
13.2.2
|
key
scientific discoveries as of the submission date of the progress
report;
|
|
13.2.3
|
summary
of work in progress as of the submission date of the progress
report;
|
|
13.2.4
|
current
schedule of anticipated events and milestones, including those event and
milestones specified in Article 12 (Due
Diligence);
|
|
13.2.5
|
market
plans for introduction of Licensed Products and Licensed Services
including the anticipated and actual market introduction dates of each
Licensed Product or Licensed Service;
|
|
13.2.6
|
Sublicensees’
activities relating to the above items, if there are any
Sublicensees;
|
|
13.2.7
|
a
summary of resources (dollar value) spent in the reporting period;
and
|
|
13.2.8
|
Licensee's
progress in developing any New Licensed Products elected for commercial
development by Licensee pursuant to Paragraph 4.3 of this Agreement.
|
13.3 If the
Licensee fails to submit a timely progress report to The Regents, then The
Regents will be entitled to terminate this Agreement under the provisions of
Paragraph 16. If either party terminates
this Agreement before any Licensed Products or Licensed Services are Sold or
before this Agreement's expiration, then a final progress report covering the
period prior to termination must be submitted within thirty (30) days of
termination or expiration.
13.4 The
Licensee has a continuing responsibility to keep The Regents informed of the
business entity status (small business entity status or large business entity
status as defined by the United States Patent and Trademark Office) of itself,
any Affiliates, Joint Ventures, or Sublicensees. The Licensee will
notify The Regents of any change of its status or that of any Affiliate, Joint
Venture, or Sublicensee within thirty (30) days of the change in
status.
13.5 The
Licensee will report to The Regents the date of first Sale or other exploitation
of a Licensed Product or Licensed Service in each country in its first progress
and royalty reports following such first Sale of a Licensed Product or Licensed
Service.
13.6 Beginning
with the earlier of (i) the first Sale or other exploitation of a Licensed
Product or Licensed Service or (ii) the first transaction that results in
Sublicense Fees accruing to The Regents, the Licensee will make quarterly
royalty and Sublicensee Fee reports to The Regents on or before each February 28
(for the quarter ending December 31), May 31 (for the quarter ending March 31),
August 31 (for the quarter ending June 30) and November 30 (for the quarter
ending September 30) of each year. Each royalty and Sublicensee Fee
report will cover Licensee's most recently completed calendar quarter and will,
at a minimum, show:
|
13.6.1
|
the
gross invoice prices and Net Sales of Licensed Products or Licensed
Services Sold or otherwise exploited (itemizing the applicable gross
proceeds and any deductions therefrom), any Attributed Income (itemizing
the applicable gross proceeds and any deductions therefrom) due to the
Licensee;
|
|
13.6.2
|
the
quantity of each type of Licensed Product and/or Licensed Service Sold or
otherwise exploited;
|
|
13.6.3
|
the
country in which each Licensed Product and Licensed Service was made, used
or Sold or otherwise exploited;
|
|
13.6.4
|
the
Earned Royalties, in United States dollars, payable with respect to Net
Sales;
|
|
13.6.5
|
the
Sublicense Fees, in United States dollars, payable with respect to
Attributed Income;
|
|
13.6.6
|
the
method used to calculate the Earned Royalty, specifying all deductions
taken and the dollar amount of each such deduction;
|
|
13.6.7
|
the
exchange rates used, if any;
|
|
13.6.8
|
the
amount of the cash and the amount of the cash equivalent of any non-cash
consideration including the method used to calculate the non-cash
consideration;
|
|
13.6.9
|
for
each Licensed Product and each Licensed Service, the specific Patent
Rights identified by UC Case Number exercised by the Licensee or any
Affiliate, Joint Venture, or Sublicensee in the course of making, using,
selling, offering for Sale or importing such Licensed Product and/or
using, selling or offering for Sale such Licensed Service;
and
|
|
13.6.10
|
any
other information reasonably necessary to confirm Licensee's calculation
of its financial obligations hereunder.
|
13.7 If no
Sales of Licensed Products and Licensed Services have been made and no Licensed
Products and Licensed Services have been otherwise exploited and no Attributed
Income is due to the Licensee during any reporting period, then a statement to
this effect must be provided by the Licensee in the immediately subsequent
royalty and Sublicense Fee report.
14. BOOKS AND
RECORDS
14.1 The
Licensee will keep accurate books and records showing all Licensed Product under
development, manufactured, used, offered for Sale, imported, Sold and or
otherwise exploited; all Licensed Service Sold or otherwise provided; all Net
Sales, all Attributed Income, and other amounts payable hereunder; and all
sublicenses granted under the terms of this Agreement. Such books and records
will be preserved for at least five (5) years after the date of the payment to
which they pertain and will be open to examination by representatives or agents
of The Regents at reasonable times to determine their accuracy and assess the
Licensee's compliance with the terms of this Agreement.
14.2 The
Regents shall pay the fees and expenses of such examination. If,
however, an error in royalties of more than five percent (5%) of the total
royalties due for any year is discovered in any examination, then the Licensee
shall bear the fees and expenses of such examination and shall remit such
underpayment to The Regents within thirty (30) days of the examination
results.
15. LIFE OF
THE AGREEMENT
15.1 Unless
otherwise terminated by operation of law, Paragraph 15.2, or by acts of the parties in accordance with the
terms of this Agreement, this Agreement will remain in effect from the Effective
Date until the expiration or abandonment of the last of the Patent Rights
licensed hereunder.
15.2 This
Agreement will automatically terminate without the obligation to provide 60
days' notice as set forth in Article 16 (Termination
By The Regents) upon the filing of a petition for relief under the United States
Bankruptcy Code by or against the Licensee as a debtor or alleged
debtor.
15.3 Any
termination or expiration of this Agreement will not affect the rights and
obligations set forth in the following Articles:
Article 1 Definitions
Paragraph 5.8 Late
Payments
Article 6 License
Issue Fee
Article 8 Payments
on Sublicenses
Article 14 Books
and Records
Article 15 Life
of the Agreement
|
|
Article
18
|
Disposition
of Licensed Products and Licensed Services on Hand Upon Termination or
Expiration
|
|
Article
19
|
Use
of Names and Trademarks
|
|
|
Article
20
|
Limited
Warranty
|
|
|
Article
21
|
Limitation
of Liability
|
|
Article
25
|
Indemnification
|
|
|
Article
26
|
Notices
|
|
|
Article
30
|
Governing
Laws; Venue; Attorneys Fees
|
|
|
Article
33
|
Confidentiality
|
15.4 The
termination or expiration of this Agreement will not relieve the Licensee of its
obligation to pay any fees, royalties or other payments owed to The Regents at
the time of such termination or expiration and will not impair any accrued right
of The Regents, including the right to receive Earned Royalties in accordance
with Articles 8 (Payments on Sublicenses), 9 (Earned Royalties and Minimum Annual Royalties) and
18 (Disposition of Licensed Products and Licensed
Services Upon Termination or Expiration).
16. TERMINATION
BY THE REGENTS
If the
Licensee fails to perform or violates any term of this Agreement, then The
Regents may give written notice of such default (“Notice of Default”) to the
Licensee. If the Licensee fails to repair such default within sixty
(60) days after the effective date of such notice, then The Regents will have
the right to immediately terminate this Agreement and its licenses by providing
a written notice of termination (“Notice of Termination”) to the
Licensee.
17. TERMINATION
BY LICENSEE
The
Licensee has the right at any time to terminate this Agreement by providing a
Notice of Termination to The Regents. Moreover, the Licensee will be
entitled to terminate the rights under Patent Rights on a country-by-country
basis by giving notice in writing to The Regents. Termination of this Agreement
(but not termination of any patents or patent applications under Patent Rights,
which termination is subject to Paragraph 22.5) will
be effective sixty (60) days from the effective date of such
notice.
18. DISPOSITION
OF LICENSED PRODUCT AND LICENSED SERVICES UPON TERMINATION OR
EXPIRATION
18.1 Upon
termination (but not expiration) of this Agreement, within a period of one
hundred and twenty (120) days after the date of termination, the Licensee is
entitled to (i) dispose of all previously made or partially made Licensed
Product, but no more and (ii) provide previously contracted-for Licensed
Services, provided that the Sale or use of such Licensed Product and the
provision of such Licensed Services are subject to the terms of this Agreement,
including, but not limited to, the rendering of reports and payment of Earned
Royalties, Sublicense Fees and any other payments therefor required under this
Agreement. The Licensee will not otherwise make, use, Sell, offer for
Sale or import Licensed Products or Licensed Services, or practice the Licensed
Method after the date of termination.
18.2 If
applicable Patent Rights exist at the time of any making, Sale, offer for Sale,
or import of a Licensed Product or the time of any
Sale, offer for Sale, or rendering of a Licensed Service, then Earned Royalties
shall be paid at the times provided herein and royalty reports shall be rendered
in connection therewith, notwithstanding the absence of applicable Patent Rights
with respect to such Licensed Product or Licensed Service at any later
time. Otherwise, no Earned Royalties shall be paid on the Sales of
such product or service. Any fees or other payments owed to The
Regents at the time of expiration not based on the Sales of a Licensed Product
or Licensed Service will be paid to The Regents at the time such fee or other
payment would have been due had this Agreement not expired.
19. USE OF
NAMES AND TRADEMARKS
Nothing
contained in this Agreement will be construed as conferring any right to either
party to use in advertising, publicity or other promotional activities any name,
trade name, trademark or other designation of the other party (including a
contraction, abbreviation or simulation of any of the
foregoing). Without the Licensee's consent case-by-case, The Regents
may list Licensee's name as a licensee of technology from The Regents without
further identifying the technology. Unless required by law or unless
consented to in writing by Executive Director, Office of Technology Transfer of
The Regents, the use by the Licensee of the name “The Regents of the University
of California” or the name of any campus of the University of California in
advertising, publicity or other promotional activities is expressly
prohibited.
20. LIMITED
WARRANTY
20.1 The
Regents warrants to the Licensee that it has the lawful right to grant this
license.
20.2 Except as
expressly set forth in this Agreement, this license and the associated
Invention, Patent Rights, Licensed Products, Licensed Services, and Licensed
Methods are provided by The Regents WITHOUT WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER WARRANTY OF ANY
KIND, EXPRESS OR IMPLIED. THE REGENTS MAKES NO EXPRESS OR IMPLIED
REPRESENTATION OR WARRANTY THAT THE INVENTION, PATENT RIGHTS, LICENSED PRODUCTS,
LICENSED SERVICES, OR LICENSED METHODS WILL NOT INFRINGE ANY PATENT, COPYRIGHT,
TRADEMARK OR OTHER RIGHTS.
20.3 This
Agreement does not:
|
20.3.1
|
express
or imply a warranty or representation as to the validity, enforceability,
or scope of any Patent Rights; or
|
|
20.3.2
|
express
or imply a warranty or representation that anything made, used, Sold,
offered for Sale or imported or otherwise exploited under any license
granted in this Agreement is or will be free from infringement of patents,
copyrights, or other rights of third parties; or
|
|
20.3.3
|
obligate
The Regents to bring or prosecute actions or suits against third parties
for patent infringement except as provided in Article 24 (Patent Infringement); or
|
|
20.3.4
|
confer
by implication, estoppel or otherwise any license or rights under any
patents or other rights of The Regents other than Patent Rights,
regardless of whether such patents are dominant or subordinate to Patent
Rights; or
|
|
20.3.5
|
obligate
The Regents to furnish any New Developments, know-how, technology or
information not provided in Patent
Rights.
|
21. LIMITATION
OF LIABILITY
EXCEPT
FOR LICENSEE’S INDEMNIFICATION OBLIGATIONS FOR CLAIMS OF THIRD PARTIES UNDER
ARTICLE 25 NEITHER PARTY WILL BE LIABLE FOR ANY
LOST PROFITS, COSTS OF PROCURING SUBSTITUTE GOODS OR SERVICES, LOST BUSINESS,
ENHANCED DAMAGES FOR INTELLECTUAL PROPERTY INFRINGEMENT OR ANY INDIRECT,
INCIDENTAL, CONSEQUENTIAL, PUNITIVE OR OTHER SPECIAL DAMAGES SUFFERED BY THE
REGENTS, LICENSEE, SUBLICENSEES, JOINT VENTURES, OR AFFILIATES ARISING OUT OF OR
RELATED TO THIS AGREEMENT FOR ALL CAUSES OF ACTION OF ANY KIND (INCLUDING TORT,
CONTRACT, NEGLIGENCE, STRICT LIABILITY AND BREACH OF WARRANTY) EVEN IF THE
REGENTS OR THE LICENSEE HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH
DAMAGES.
22. PATENT
PROSECUTION AND MAINTENANCE
22.1 As long
as the Licensee has paid Patent Prosecution Costs as provided for in this
Article 22 (Patent Prosecution and Maintenance), The
Regents will diligently prosecute and maintain the United States and foreign
patents comprising the Patent Rights using counsel of its choice. The
Regents' counsel will take instructions only from The Regents. The
Regents will provide the Licensee with copies of all relevant documentation so
that the Licensee will be informed of the continuing prosecution and may comment
upon such documentation sufficiently in advance of any initial deadline for
filing a response, provided, however, that if the Licensee has not commented
upon such documentation in a reasonable time for The Regents to sufficiently
consider the Licensee’s comments prior to a deadline with the relevant
government patent office, or The Regents must act to preserve the Patent Rights,
The Regents will be free to respond without consideration of the Licensee’s
comments, if any. The Licensee agrees to keep this documentation
confidential as provided for in Article 33
(Confidentiality).
22.2 The
Regents shall use reasonable efforts to amend any patent application to include
claims reasonably requested by the Licensee to protect the products and services
contemplated to be Sold, or the Licensed Method to be practiced, under this
Agreement.
22.3 The
Licensee will bear the costs of preparing, filing, prosecuting and maintaining
all United States and foreign patent applications contemplated by this Agreement
(“Patent Prosecution Costs”). Patent Prosecution Costs billed by The
Regents' counsel will be rebilled to the Licensee and are due within thirty (30)
days of rebilling by The Regents. These Patent Prosecution Costs will
include, without limitation, patent prosecution costs for the Invention incurred
by The Regents prior to the execution of this Agreement and any patent
prosecution costs that may be incurred for patentability opinions,
re-examination, re-issue, interferences, oppositions or inventorship
determinations. Prior Patent Prosecution Costs will be due upon execution of
this Agreement and billing by The Regents and are at least thirty thousand
dollars ($30,000).
22.4 The
Licensee may request that The Regents obtain patent protection on the Invention
in foreign countries, if available and if it so desires. The Licensee
will notify The Regents of its decision to obtain or maintain foreign patents
not less than ninety (90) days prior to the deadline for any payment, filing or
action to be taken in connection therewith. This notice concerning
foreign filing must be in writing, must identify the countries desired and must
reaffirm the Licensee's obligation to pay the Patent Prosecution Costs
thereof. The absence of such a notice from the Licensee to The
Regents will be considered an election not to obtain or maintain foreign Patent
Rights.
22.5 The
Licensee will be obligated to pay any Patent Prosecution Costs incurred during
the three (3)-month period after receipt by either party of a Notice of
Termination, even if the invoices for such Patent Prosecution Costs are received
by the Licensee after the end of the three (3)-month period following receipt of
a Notice of Termination. The Licensee may terminate its obligation to
pay Patent Prosecution Costs with respect to any given patent application or
patent under Patent Rights in any or all designated countries upon three
(3)-months' written notice to The Regents. The Regents may continue prosecution
and/or maintenance of such application(s) or patent(s) at its sole discretion
and expense, provided, however, that the Licensee will have no further right or
licenses thereunder. Non-payment of Patent Prosecution Costs may be
deemed by The Regents as an election by the Licensee not to maintain such
application(s) or patent(s).
22.6 The
Regents may file, prosecute or maintain patent applications or patents at its
own expense in any country in which the Licensee has not elected to file,
prosecute or maintain patent applications or patents in accordance with this
Article 22 (Patent Prosecution and Maintenance) and
those applications, resultant patents and patents will not be subject to this
Agreement.
23. PATENT
MARKING
The
Licensee will mark all Licensed Products made, used or Sold under the terms of
this Agreement or their containers in accordance with the applicable patent
marking laws.
24. PATENT
INFRINGEMENT
24.1 In the
event that The Regents (to the extent of the actual knowledge of the licensing
professional responsible for the administration of this Agreement) or the
Licensee learns of infringement of potential commercial significance of any
patent licensed under this Agreement, the knowledgeable party will provide the
other (i) with written notice of such infringement and (ii) with any evidence of
such infringement available to it (the “Infringement Notice”). During
the period in which, and in the jurisdiction where, the Licensee has exclusive
rights under this Agreement, neither The Regents nor the Licensee will notify a
possible infringer of infringement or put such infringer on notice of the
existence of any Patent Rights without first obtaining consent of the
other. If the Licensee puts such infringer on notice of the existence
of any Patent Rights with respect to such infringement without first obtaining
the written consent of The Regents and if a declaratory judgment action is filed
by such infringer against The Regents, then Licensee’s right to initiate a suit
against such infringer for infringement under Paragraph 24.2 below will terminate immediately without the
obligation of The Regents to provide notice to the
Licensee. Both The Regents and the Licensee will use their
diligent efforts to cooperate with each other to terminate such infringement
without litigation.
24.2 If
infringing activity of potential commercial significance by the infringer has
not been abated within ninety (90) days following the date the Infringement
Notice takes effect, then the Licensee may institute suit for patent
infringement against the infringer. The Regents may voluntarily join
such suit at its own expense, but may not otherwise commence suit against the
infringer for the acts of infringement that are the subject of the Licensee's
suit or any judgment rendered in that suit. The Licensee may not join
The Regents as a party in a suit initiated by the Licensee without The Regents'
prior written consent. If, in a suit initiated by the Licensee, The
Regents is involuntarily joined other than by the Licensee, then the Licensee
will pay any costs incurred by The Regents arising out of such suit, including
but not limited to, any legal fees of counsel that The Regents selects and
retains to represent it in the suit.
24.3 If,
within a hundred and twenty (120) days following the date the Infringement
Notice takes effect, infringing activity of potential commercial significance by
the infringer has not been abated and if the Licensee has not brought suit
against the infringer, then The Regents may institute suit for patent
infringement against the infringer. If The Regents institutes such
suit, then the Licensee may not join such suit without The Regents' consent and
may not thereafter commence suit against the infringer for the acts of
infringement that are the subject of The Regents' suit or any judgment rendered
in that suit.
24.4 Any
recovery or settlement received in connection with any suit will first be shared
by The Regents and the Licensee equally to cover any litigation costs each
incurred and next shall be paid to The Regents or the Licensee to cover any
litigation costs it incurred in excess of the litigation costs of the
other. In any suit initiated by the Licensee, any recovery in excess
of litigation costs will be shared between Licensee and The Regents as
follows: (a) for any recovery other than amounts paid for willful
infringement: (i) The Regents will receive fifteen percent (15%) of the recovery
if The Regents was not a party in the litigation and did not incur any
litigation costs, (ii) The Regents will receive twenty-five percent (25%) of the
recovery if The Regents was a party in the litigation whether joined as a party
under the provisions of Paragraph 24.2 or otherwise,
but The Regents did not incur any litigation costs, and (iii) The Regents will
receive fifty percent (50%) of the recovery if The Regents incurred any
litigation costs in connection with the litigation; and (b) for any recovery for
willful infringement, The Regents will receive fifty percent (50%) of
the recovery. In any suit initiated by The Regents, any recovery in
excess of litigation costs will belong to The Regents. The Regents
and the Licensee agree to be bound by all determinations of patent infringement,
validity and enforceability (but no other issue) resolved by any adjudicated
judgment in a suit brought in compliance with this Article 24 (Patent Infringement).
24.5 Any
agreement made by the Licensee for purposes of settling litigation or other
dispute shall comply with the requirements of Article 3 (Sublicenses) of this Agreement.
24.6 Each
party will cooperate with the other in litigation proceedings instituted
hereunder but at the expense of the party who initiated the suit (unless such
suit is being jointly prosecuted by the parties).
24.7 Any
litigation proceedings will be controlled by the party bringing the suit, except
that The Regents may be represented by counsel of its choice in any suit brought
by the Licensee.
25. INDEMNIFICATION
25.1 The
Licensee will, and will require its Sublicensees to, indemnify, hold harmless
and defend The Regents, the sponsors of the research that led to the
Invention any invention claimed in patents or patent applications
under Patent Rights (including the Licensed Products, Licensed Services and
Licensed Methods contemplated thereunder) and their employers, and the officers,
employees and agents of any of the foregoing, against any and all claims, suits,
losses, damage, costs, fees and expenses resulting from, or arising out of, the
exercise of this license or any sublicense. This indemnification will
include, but not be limited to, any product liability. If The
Regents, in its sole discretion, believes that there will be a conflict of
interest or it will not otherwise be adequately represented by counsel chosen by
the Licensee to defend The Regents in accordance with this Paragraph 25.1, then The Regents may retain counsel of its choice
to represent it and the Licensee will pay all expenses for such
representation.
25.2 The
Licensee, at its sole cost and expense, will insure its activities in connection
with any work performed hereunder and will obtain and maintain the following
insurance:
|
25.2.1
|
Commercial
Form General Liability Insurance (contractual liability included) with
limits as follows:
|
|
Each
Occurrence
|
$ 500,000
|
|
Products/Completed
Operations Aggregate
|
$
|
0
|
|
Personal
and Advertising Injury
|
$ 500,000
|
|
General
Aggregate (commercial form only)
|
$1,000,000
|
If the
above insurance is written on a claims-made form, it shall continue for three
(3) years following termination or expiration of this Agreement. The
insurance shall have a retroactive date of placement prior to or coinciding with
the Effective Date of this Agreement;
|
25.2.2
|
and
Worker's Compensation as legally required in the jurisdiction in which the
Licensee is doing business.
|
25.3 Notwithstanding
the provisions of Paragraph 25.2 above and except
for the provisions of Paragraph 25.4
below, no later than sixty (60) days before the anticipated date of market
introduction of any Licensed Product or Licensed Service under this Agreement
where such Licensed Product or Licensed Service is not used or Sold for human
use, the Licensee, at its sole cost and expense, shall insure its activities in
connection with any work performed under this Agreement and obtain, keep in
force and maintain the following insurance:
|
25.3.1
|
Commercial
Form General Liability Insurance (contractual liability included) with
limits as follows:
|
Each
Occurrence $2,000,000
Products/Completed Operations
Aggregate $5,000,000
Personal and Advertising
Injury $2,000,000
General Aggregate (commercial form
only) $5,000,000
If the
above insurance is written on a claims-made form, it shall continue for three
(3) years following termination or expiration of this Agreement. The
insurance shall have a retroactive date of placement prior to or coinciding with
the Effective Date of this Agreement;
|
25.3.2
|
and
Worker's Compensation as legally required in the jurisdiction in which the
Licensee is doing business.
|
25.4 Notwithstanding
the provisions of Paragraphs 25.2 and 25.3 above, no later than sixty (60) days before the
anticipated date of market introduction of any Licensed Product or Licensed
Service under this Agreement where such Licensed Product or Licensed Service is
used or Sold for human use, the Licensee, at its sole cost and expense, shall
insure its activities in connection with any work performed under this Agreement
and obtain, keep in force and maintain the following insurance:
|
25.4.1
|
Commercial
Form General Liability Insurance (contractual liability included) with
limits as follows:
|
Each
Occurrence $5,000,000
Products/Completed Operations
Aggregate $10,000,000
Personal and Advertising
Injury $5,000,000
General Aggregate (commercial form
only) $10,000,000
If the
above insurance is written on a claims-made form, it shall continue for three
(3) years following termination or expiration of this Agreement. The
insurance shall have a retroactive date of placement prior to or coinciding with
the Effective Date of this Agreement;
|
25.4.2
|
To
the extent Licensee conducts clinical trials under this Agreement,
Licensee, at its sole cost and expense, will obtain, keep in force, and
maintain insurance coverage for each clinical trial in an amount that is
customary for such trial in the industry; and
|
|
25.4.3
|
Worker's
Compensation as legally required in the jurisdiction in which the Licensee
is doing business.
|
25.5 The
coverage and limits referred to in Paragraphs 25.2.1, 25.3.1, 25.4.1, and 25.4.2 above
will not in any way limit the liability of the Licensee under this Article 25 (Indemnification). Upon the execution of
this Agreement, the Licensee will furnish The Regents with certificates of
insurance evidencing compliance with all requirements. Such
certificates will:
|
|
-
|
Provide
for thirty (30) days' (ten (10) days for non-payment of premium) advance
written notice to The Regents of any cancellation of insurance
coverage; the Licensee will promptly notify The Regents of any
material modification of the insurance coverage;
|
|
|
-
|
Indicate
that The Regents has been endorsed as an additional insured under the
coverage described above in Paragraphs 25.2.1, 25.3.1, and
25.4.1; and
|
|
|
-
|
Include
a provision that the coverage will be primary and will not participate
with, nor will be excess over, any valid and collectable insurance or
program of self-insurance maintained by The
Regents.
|
25.6 The
Regents will promptly notify the Licensee in writing of any claim or suit
brought against The Regents for which The Regents intends to invoke the
provisions of this Article 25
(Indemnification). The Licensee will keep The Regents informed of its
defense of any claims pursuant to this Article 25
(Indemnification).
26. NOTICES
26.1 Any
notice or payment required to be given to either party under this Agreement will
be in writing and will be deemed to have been properly given and to be effective
as of the date specified below if delivered to the respective address given
below or to another address as designated by written notice given to the other
party:
|
26.1.1
|
on
the date of delivery if delivered in person;
|
|
26.1.2
|
on
the date of mailing if mailed by first-class certified mail, postage paid;
or
|
|
26.1.3
|
on
the date of mailing if mailed by any global express carrier service that
requires the recipient to sign the documents demonstrating the delivery of
such notice or payment.
|
In the
case of
Licensee: SHRINK
TECHNOLOGIES, INC.
2038 Corte Del Nogal, Suite
110
Carlsbad, CA 92011
Attention: Mark Baum
In the
case of The
Regents: The
Regents of the University
of California
Office of Technology
Transfer
1111 Franklin Street, 5th
Floor
Oakland, CA 94607-5200
Attention: Executive Director
Research
Administration and Technology
Transfer
With copy
to: University
of California, Irvine
Office of Technology
Transfer
Biomedical Engineering
4199 Campus Drive
Suite 380
Irvine, CA 92612
27. ASSIGNABILITY
This
Agreement is personal to the Licensee. The Licensee may not assign or
transfer this Agreement, including by merger, operation of law, or otherwise,
without The Regents' prior written consent, except that such consent will not be
required in the case of assignment or transfer to a party that succeeds to all
or substantially all of Licensee's business or assets relating to this
Agreement, whether by sale, merger, operation of law or otherwise, provided that
such assignee or transferee promptly agrees to be bound by the terms and
conditions of this Agreement and Licensee and such assignee or transferee signs
The Regents' standard substitution of party letter (the form of which is
attached hereto as Appendix B). Any attempted assignment by Licensee
without the written consent of The Regents will be null and
void. This Agreement is binding upon and will inure to the benefit of
The Regents, its successors and assigns.
28. WAIVER
No waiver
by either party of any breach or default of any of the agreements contained
herein will be deemed a waiver as to any subsequent and/or similar breach or
default. No waiver will be valid or binding upon the parties unless
made in writing and signed by a duly authorized officer of each
party.
29. FORCE
MAJEURE
29.1 Except
for the Licensee's obligation to make any payments to The Regents hereunder, the
parties shall not be responsible for any failure to perform due to the
occurrence of any events beyond their reasonable control which render their
performance impossible or onerous, including, but not limited
to: accidents (environmental, toxic spill, etc.); acts of God;
biological or nuclear incidents; casualties; earthquakes; fires; floods;
governmental acts; orders or restrictions; inability to obtain suitable and
sufficient labor, transportation, fuel and materials; local, national or state
emergency; power failure and power outages; acts of terrorism; strike; and
war.
29.2 Either
party to this Agreement, however, will have the right to terminate this
Agreement upon thirty (30) days’ prior written notice if either party is unable
to fulfill its obligations under this Agreement due to any of the causes
specified in Paragraph 29.1 for a period of one (1)
year.
30. GOVERNING
LAWS; VENUE; ATTORNEYS’ FEES
30.1 THIS
AGREEMENT WILL BE INTERPRETED AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE
STATE OF CALIFORNIA, excluding any choice of law rules that would direct the
application of the laws of another jurisdiction and without regard to which
party drafted particular provisions of this Agreement, but the scope and
validity of any patent or patent application will be governed by the applicable
laws of the country of such patent or patent application.
30.2 Any legal
action brought by the parties hereto relating to this Agreement will be
conducted in San Francisco, California.
30.3 The
prevailing party in any suit related to this Agreement will be entitled to
recover its reasonable attorneys' fees in addition to its costs and necessary
disbursements.
31. GOVERNMENT
APPROVAL OR REGISTRATION
If this
Agreement or any associated transaction is required by the law of any nation to
be either approved or registered with any governmental agency, the Licensee will
assume all legal obligations to do so. The Licensee will notify The
Regents if it becomes aware that this Agreement is subject to a United States or
foreign government reporting or approval requirement. The Licensee will make all
necessary filings and pay all costs including fees, penalties and all other
out-of-pocket costs associated with such reporting or approval
process.
32. COMPLIANCE
WITH LAWS
The
Licensee shall comply with all applicable international, national, state,
regional and local laws and regulations in performing its obligations hereunder
and in its use, manufacture, Sale or import of the Licensed Products, Licensed
Services or practice of the Licensed Method. The Licensee will
observe all applicable United States and foreign laws with respect to the
transfer of Licensed Products and related technical data and the provision of
Licensed Services to foreign countries, including, without limitation, the
International Traffic in Arms Regulations (ITAR) and the Export Administration
Regulations. The Licensee shall manufacture Licensed Products and
practice the Licensed Method in compliance with applicable government
importation laws and regulations of a particular country for Licensed Products
made outside the particular country in which such Licensed Products are used,
Sold or otherwise exploited.
33. CONFIDENTIALITY
33.1 The
Licensee and The Regents will treat and maintain the other party’s proprietary
business, patent prosecution, software, engineering drawings, process and
technical information and other proprietary information, including the
negotiated terms of this Agreement and any progress reports and royalty reports
and any sublicense agreement issued pursuant to this Agreement (“Proprietary
Information”) in confidence using at least the same degree of care as the
receiving party uses to protect its own proprietary information of a like nature
from the date of disclosure until five (5) years after the termination or
expiration of this Agreement. This confidentiality obligation will apply to the
information defined as “Data” under the Secrecy Agreement and such Data will be
treated as Proprietary Information hereunder.
33.2 The
Licensee and The Regents may use and disclose Proprietary Information to their
employees, agents, consultants, contractors and, in the case of the Licensee,
its Sublicensees, provided that such parties are bound by a like duty of
confidentiality as that found in this Article 33
(Confidentiality). Notwithstanding anything to the contrary contained
in this Agreement, The Regents may release this Agreement or any sublicense,
including any terms thereof, and information regarding royalty payments or other
income received in connection with this Agreement to the inventors, senior
administrative officials employed by The Regents and individual Regents upon
their request. If such release is made, The Regents will request that
such terms be kept in confidence in accordance with the provisions of this
Article 33 (Confidentiality). In
addition, notwithstanding anything to the contrary in this Agreement, if a third
party inquires whether a license to Patent Rights is available, then The Regents
may disclose the existence of this Agreement and the extent of the grant in
Articles 2 (Grant) and 3
(Sublicenses) and related definitions to such third party, but will not disclose
the name of the Licensee unless Licensee has already made such disclosure
publicly and will not disclose the financial terms contained in this
Agreement.
33.3 All
written Proprietary Information will be labeled or marked confidential or
proprietary. If the Proprietary Information is orally disclosed, it
will be reduced to writing or some other physically tangible form, marked and
labeled as confidential or proprietary by the disclosing party and delivered to
the receiving party within thirty (30) days after the oral
disclosure.
33.4 Nothing
contained herein will restrict or impair, in any way, the right of the Licensee
or The Regents to use or disclose any Proprietary Information:
|
33.4.1
|
that
recipient can demonstrate by written records was previously known to it
prior to its disclosure by the disclosing party;
|
|
33.4.2
|
that
recipient can demonstrate by written records is now, or becomes in the
future, public knowledge other than through acts or omissions of
recipient;
|
|
33.4.3
|
that
recipient can demonstrate by written records was obtained lawfully and
without restrictions on the recipient from sources independent of the
disclosing party; and
|
|
33.4.4
|
that
The Regents is required to disclose pursuant to the California Public
Records Act or other applicable law.
|
The
Licensee or The Regents also may disclose Proprietary Information that is
required to be disclosed (i) to a governmental entity or agency in connection
with seeking any governmental or regulatory approval, governmental audit, or
other governmental contractual requirement or (ii) by law, provided that the
recipient uses reasonable efforts to give the party owning the Proprietary
Information sufficient notice of such required disclosure to allow the party
owning the Proprietary Information reasonable opportunity to object to, and to
take legal action to prevent, such disclosure.
33.5 Upon
termination of this Agreement, the Licensee and The Regents will destroy or
return any of the disclosing party’s Proprietary Information in its possession
within fifteen (15) days following the termination of this
Agreement. The Licensee and The Regents will provide each other,
within thirty (30) days following termination, with written notice that such
Proprietary Information has been returned or destroyed. Each party
may, however, retain one copy of such Proprietary Information for archival
purposes in non-working files.
34. MISCELLANEOUS
34.1 The
headings of the several sections are inserted for convenience of reference only
and are not intended to be a part of or to affect the meaning or interpretation
of this Agreement.
34.2 This
Agreement is not binding on the parties until it has been signed below on behalf
of each party. It is then effective as of the Effective
Date.
34.3 No
amendment or modification of this Agreement is valid or binding on the parties
unless made in writing and signed on behalf of each party.
34.4 This
Agreement embodies the entire understanding of the parties and supersedes all
previous communications, representations or understandings, either oral or
written, between the parties relating to the subject matter
hereof. The Secrecy Agreement (UC Control No. 2009-20-0554) dated
April 9, 2009 is hereby superseded. The Option Agreement remains
in effect.
34.5 In case
any of the provisions contained in this Agreement is held to be invalid, illegal
or unenforceable in any respect, such invalidity, illegality or unenforceability
will not affect any other provisions of this Agreement and this Agreement will
be construed as if such invalid, illegal or unenforceable provisions had never
been contained in it.
34.6 This
Agreement includes the attached Appendix(es) A and B.
34.7 No
provisions of this Agreement are intended or shall be construed to confer upon
or give to any person or entity other than The Regents and the Licensee any
rights, remedies or other benefits under, or by reason of, this
Agreement.
34.8 In
performing their respective duties under this Agreement, each of the parties
will be operating as an independent contractor. Nothing contained
herein will in any way constitute any association, partnership, or joint venture
between the parties hereto, or be construed to evidence the intention of the
parties to establish any such relationship. Neither party will have
the power to bind the other party or incur obligations on the other party's
behalf without the other party's prior written consent.
IN
WITNESS WHEREOF, both The Regents and the Licensee have executed this Agreement,
in duplicate originals, by their respective and duly authorized officers on the
day and year written.
SHRINK
TECHNOLOGIES,
INC. THE
REGENTS OF THE UNIVERSITY
OF CALIFORNIA
By: __________________________ By: ___________________________
(Signature) (Signature)
Name: __________________________ Name: __________________________
(Please Print)
Title: __________________________ Title: __________________________
Date: __________________________ Date: __________________________
APPENDIX
A
CIRM
Intellectual
Property Requirements for Non-Profit Organizations
APPENDIX
B
UC Case
No. XX-XXX
CONSENT
TO SUBSTITUTION OF PARTY
This substitution of parties
(“Agreement”) is effective this day of , 200_, among
The Regents of the University of California (“The Regents), a California
corporation, having its statewide administrative offices at 1111 Franklin
Street, 12th Floor, Oakland, California 94607-5200; [original Licensee name]
[(“XXX”)], a [insert state] corporation, having a principal place of business
at ________________________________; and [new licensee name]
[(“YYY”)] a ______________________ corporation, having a principal place of
business at _________________________________.
BACKGROUND
A. The Regents and [XXX]
entered into a or License Agreement effective ________________ (UC Control No.
__-__-____), entitled _________________ (“[type] Agreement”), wherein
[XXX] was granted certain rights. [include amendments, if
any]
B. [XXX] desires
that [YYY] be substituted as [Licensee] (defined in the [type]
Agreement) in place of [XXX], and The Regents is agreeable to such
substitution.
|
C.
|
[YYY]
has read the [type] Agreement and agrees to abide by its terms and
conditions.
|
The parties agree as
follows:
1. [YYY]
assumes all liability and obligations under the [type] Agreement and is bound by
all its terms in all respects as if it were the original [Licensee] of the
[type] Agreement in place of [XXX].
2. [YYY] is
substituted for [XXX], provided that [YYY] assumes all liability and obligations
under the [type] Agreement as if [YYY] were the original party named as
[Licensee] as of the effective date of the [type] Agreement, for all liability
and obligations under the [type] Agreement arising before or after the effective
date of this Agreement.
3. The
Regents releases [XXX] from all liability and obligations under the [type]
Agreement arising before or after the effective date of this
Agreement.
The parties have executed this
Agreement in triplicate originals by their respective authorized officers on the
following day and year.
[XXX]
COMPANY THE
REGENTS OF THE
UNIVERSITY OF CALIFORNIA
|
|
By:
|
______________________________
|
By:
|
_____________________________
|
(Signature)
Name: ______________________________ Name: [Licensing
Officer Name]
(Please print)
Title: ______________________________ Title: [Licensing
Officer]
Office of Technology
Transfer
Date: ______________________________ Date: _____________________________
[YYY]
COMPANY
By: ___________________________
(Signature)
Name: ___________________________
(Please print)
Title: ___________________________
Date: ___________________________
EXCLUSIVE
LICENSE AGREEMENT
between
THE
REGENTS OF THE UNIVERSITY OF CALIFORNIA
and
SHRINK
TECHNOLOGIES, INC.
for
PROCESSES
FOR MICROFLUIDIC FABRICATIION AND OTHER INVENTIONS
TABLE OF
CONTENTS
Article
No. Title Page
BACKGROUND [INSERT PAGE NUMBER]
1. DEFINITIONS[INSERT PAGE NUMBER]
2. GRANT[INSERT PAGE NUMBER]
3. SUBLICENSES[INSERT PAGE NUMBER]
4. MANDATORY SUBLICENSING[INSERT PAGE NUMBER]
5. PAYMENT TERMS[INSERT PAGE NUMBER]
6. LICENSE ISSUE FEE[INSERT PAGE NUMBER]
7. LICENSE MAINTENANCE FEE[INSERT PAGE NUMBER]
8. PAYMENTS ON SUBLICENSES[INSERT PAGE NUMBER]
9. EARNED ROYALTIES AND MINIMUM ANNUAL
ROYALTIES[INSERT PAGE NUMBER]
10. MILESTONE PAYMENTS[INSERT PAGE NUMBER]
11. FEES FOR PATENT RIGHTS ADDED AFTER
EFFECTIVE DATE[INSERT PAGE
NUMBER]
12. DUE DILIGENCE[INSERT PAGE NUMBER]
13. PROGRESS AND ROYALTY REPORTS[INSERT PAGE NUMBER]
14. BOOKS AND RECORDS[INSERT PAGE NUMBER]
15. LIFE OF THE AGREEMENT[INSERT PAGE NUMBER]
16. TERMINATION BY THE REGENTS[INSERT PAGE NUMBER]
17. TERMINATION BY LICENSEE[INSERT PAGE NUMBER]
18. DISPOSITION OF LICENSED PRODUCT AND
LICENSED SERVICES UPON TERMINATION OR EXPIRATION[INSERT PAGE NUMBER]
19. USE OF NAMES AND TRADEMARKS[INSERT PAGE NUMBER]
20. LIMITED WARRANTY[INSERT PAGE NUMBER]
21. LIMITATION OF LIABILITY[INSERT PAGE NUMBER]
22. PATENT PROSECUTION AND
MAINTENANCE[INSERT PAGE NUMBER]
23. PATENT MARKING[INSERT PAGE NUMBER]
24. PATENT INFRINGEMENT[INSERT PAGE NUMBER]
25. INDEMNIFICATION[INSERT PAGE NUMBER]
26. NOTICES[INSERT PAGE NUMBER]
27. ASSIGNABILITY[INSERT PAGE NUMBER]
28. WAIVER[INSERT PAGE NUMBER]
29. FORCE MAJEURE[INSERT PAGE NUMBER]
30. GOVERNING LAWS; VENUE; ATTORNEYS’
FEES[INSERT PAGE NUMBER]
31. GOVERNMENT APPROVAL OR
REGISTRATION[INSERT PAGE
NUMBER]
32. COMPLIANCE WITH LAWS[INSERT PAGE NUMBER]
33. CONFIDENTIALITY[INSERT PAGE NUMBER]
34. MISCELLANEOUS[INSERT PAGE NUMBER]
APPENDIX
A [INSERT PAGE NUMBER]
APPENDIX
B [INSERT PAGE NUMBER]
APPENDIX
B
