Attached files
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
10-K
x ANNUAL
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
fiscal year ended: December 31,
2009
¨ TRANSITION REPORT
PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
For the
transition period from ____________ to _____________
Commission
File Number: 000-52860

Shrink
Nanotechnologies, Inc.
(Exact
name of registrant as specified in its charter)
|
Delaware
|
20-2197964
|
|
(State
or other jurisdiction of
incorporation
or organization)
|
(I.R.S.
Employer
Identification
No.)
|
|
2038
Corte del Nogal, Suite 110
Carlsbad,
California 92011
|
(760)
804-8844
(Registrant’s
telephone number, including area code)
|
|
(Address
of principal executive offices)
|
Securities
registered pursuant to Section 12(b) of the
Act: None
Securities
registered pursuant to Section 12(g) of the Exchange
Act: Common Stock, par value $0.001 per
share
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in
Rule 405 of the Securities Act. Yes ¨ No x
Indicate
by check mark if the registrant is not required to file reports pursuant to
Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate
by check mark whether the registrant (1) has filed all reports required to be
filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the
preceding 12 months (or for such shorter period that the registrant was required
to file such reports), and (2) has been subject to such filing requirements for
the past 90 days. Yes x No
¨
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Website, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files). Yes No
x
Indicate
by check mark if disclosure of delinquent filers pursuant to Item 405 of
Regulation S-K is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or information statements
incorporated by reference in Part III of this Form 10-K or any amendment to this
Form 10-K.
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer, or a smaller reporting
company. (See the definitions of “large accelerated
filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.)
Large
Accelerated
Filer
Accelerated Filer
Non-Accelerated
Filer (Do
not check if a smaller reporting
company)
Smaller reporting company
Indicate
by check mark whether registrant is a shell company (as defined in Rule 12b-2 of
the Act). Yes No x
As of
June 30, 2009 (the last business day of the registrant’s most recently completed
second fiscal quarter), the aggregate market value of the shares of the
registrant’s common stock held by non-affiliates (based upon the closing price
of such shares as reported on the Over-the-Counter Bulletin Board) was
approximately $1,700,000.
Shares of
the registrant’s common stock held by each executive officer and director and
each by each person who owns 10% or more of the outstanding common stock have
been excluded from the calculation in that such persons may be deemed to be
affiliates of the registrant. This determination of affiliate status is not
necessarily a conclusive determination for other purposes.
There
were a total of 191,303,985 shares of the registrant’s common stock outstanding
as of April 14, 2010 (post stock split).

SHRINK
NANOTECHNOLOGIES, INC.
Annual
Report on FORM 10-K
For
the Fiscal Year Ended December 31, 2009
|
TABLE
OF CONTENTS
|
Page
|
|
|
PART
I
|
1
|
|
|
ITEM
1.
|
BUSINESS
|
1
|
|
ITEM
1A.
|
RISK FACTORS
|
13
|
|
ITEM
1B.
|
UNRESOLVED
STAFF COMMENTS
|
25
|
|
ITEM
2.
|
PROPERTIES
|
25
|
|
ITEM
3.
|
LEGAL
PROCEEDINGS
|
25
|
|
PART
II
|
26
|
|
|
ITEM
4.
|
MARKET
FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER
PURCHASES OF EQUITY SECURITIES
|
28
|
|
ITEM
5.
|
SELECTED
FINANCIAL DATA
|
28
|
|
ITEM
6.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
29
|
|
ITEM
6A.
|
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
29
|
|
ITEM
7.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA
|
F-1
- F-39
|
|
ITEM
8.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE
|
40
|
|
ITEM
9A.
|
CONTROLS
AND PROCEDURES
|
41
|
|
ITEM
9B.
|
OTHER
INFORMATION
|
41
|
|
PART
III
|
42
|
|
|
ITEM
10.
|
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
42
|
|
ITEM
11.
|
EXECUTIVE
COMPENSATION
|
45
|
|
ITEM
12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS
|
47
|
|
ITEM
13.
|
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE
|
49
|
|
ITEM
14.
|
PRINCIPAL
ACCOUNTANT FEES AND SERVICES
|
50
|
|
PART
IV
|
51
|
|
|
ITEM
15.
|
EXHIBITS,
FINANCIAL STATEMENT SCHEDULES
|
51
|
|
SIGNATURES
|
52
|
PART
I
|
ITEM
1.
|
BUSINESS
|
FORWARD
LOOKING STATEMENTS
Certain
information contained in this Annual Report on Form 10-K (this “Report” or
“Annual Report”) includes forward-looking statements within the meaning of the
Securities Act, and Section 21E of the Securities Exchange Act of 1934, as
amended, (thee “Exchange Act”). These statements are expressed in
good faith and based upon what we believe are reasonable assumptions, but there
can be no assurance that these expectations will be achieved or
accomplished. These forward looking statements can be identified by
the use of terms and phrases such as “believe,” “plan,” “intend,” “anticipate,”
“target,” “estimate,” “expect,” and the like, and/or future-tense or conditional
constructions (“will,” “may,” “could,” “should,” etc.).
The
statements herein which are not historical reflect our current expectations and
projections about the Company’s future results, performance, liquidity,
financial condition, prospects and opportunities and are based upon information
currently available to the Company and management and their interpretation of
what are believed to be significant factors affecting the businesses, including
many assumptions regarding future events. Such forward-looking
statements include statements regarding, without limitation:
|
·
|
the
development, commercialization and market acceptance of our recently
acquired microfluidic, “shrinkable plastic” and solar concentrator
technologies, and other related technologies, and the related costs to us
for the foregoing,
|
|
·
|
the
market acceptance of our technologies to develop lower cost microfluidic
chips and related devices and
systems,
|
|
·
|
our
immediate and growing need to raise the capital needed or obtain
government or educational grants to implement our business
plan,
|
|
·
|
items
contemplating or making assumptions about the progress of our research and
development activities,
|
|
·
|
our
ability to further acquire, hold and defend our intellectual
property,
|
|
·
|
the
projected growth in stem cell
research,
|
|
·
|
alternative
energy demands (specifically, products ultimately derived from our solar
concentrator technology) and our ability to fund our solar chip
products,
|
|
·
|
the
presumed size and growth of the market for lab-on-a-chip devices,
and
|
|
·
|
liquidity
and sufficiency of existing cash.
|
These
forward looking statements should be considered in addition to our risk factors
(contained in this Annual Report under the heading “Risk
Factors”). Additional risks not described above, or unknown to us,
may also adversely affect the Company or its results.
Additional
risks not described above, or unknown to us, may also adversely affect the
Company or its results. Readers are urged not to place undue reliance on these
forward-looking statements, which speak only as of the date of this Annual
Report. We assume no obligation to update any forward-looking
statements in order to reflect any event or circumstance that may arise after
the filing date of this Annual Report, other than as may be required by
applicable law or regulation. Readers are urged to carefully review
and consider the various disclosures made by us in our reports filed with the
SEC (which shall also include by reference herein and incorporate the same as if
fully included in there entirety, all Form 10-Ks, Form 10-Qs, Form 8-Ks and
other periodic reports filed by us in the SEC’s EDGAR filing system
(www.sec.gov)) which attempt to update interested parties of the risks and
factors and other disclosures that may affect our business, financial condition,
results of operation and cash flows.
Our
actual results could differ materially from those anticipated in these
forward-looking statements as a result of various factors, including those set
forth in the Risk Factors section in this Annual Report.
1
Use
of Terms
Except as
otherwise indicated by the context, references in this report to (i) the “Shrink
Parent” or “Parent” or similar terms refer to the publicly traded parent holding
company and registrant, Shrink Nanotechnologies, Inc., a Delaware
corporation, (ii) the “Company”, “we”, “us”, or “our”,
refer to the combined business of Shrink Parent, together with its wholly owned
subsidiary, Shrink, a California corporation, unless the context requires
otherwise, (iii) “Shrink” refers to Shrink Technologies, Inc., a California
corporation acquired by us in May 2010, inclusive of its R&D activities
through universities or third parties, (iv) “Forward Split” refers to the 5 for
1 forward split of the Parent’s stock effective as of April 8, 2010, (v) “SEC”
are to the United States Securities and Exchange Commission, (vi) “Securities
Act” are to Securities Act of 1933, as amended, and (vii) “Exchange Act” are to
the Securities Exchange Act of 1934, as amended.
Business
Overview
General. We are,
through our subsidiary, a research, design and development company dedicated to
the commercialization of a nanotechnology platform called the ShrinkChip
Manufacturing Solution™, which we believe represents a new low cost alternative
for fabrication of otherwise costly diagnostic chips and other nano-size
devices. Our technology can be used to manufacture chips and other
devices used as measuring tools and energy and content transfer devices for a
wide range of applications from the life sciences, drug and chemical analysis
industries to the optoelectronics components and solar/renewable energy
businesses.
Platform
Focus. Our core shrink-film technology is a “platform
technology” which is to say that it has a variety of applications in numerous
fields that relate to the use of and functionalization of nano-sized structures,
including channels, tubes, holes, wrinkles as well as “cracked”
surfaces. We are focusing on commercializing and developing our
technologies in three specific business segments which are discussed in greater
depth in this Annual Report: (1) our NanoShrink business, (2) our StemDisc
business and (3) our Solar Concentrator and related solar
business. All of these business segments are relatively immature and
require additional development before products may ultimately reach a commercial
customer.
Core Ability. Out
technologies allow for the flexible and rapid design, prototyping and
manufacture of certain complex chips, microfluidic diagnostic tools and
measuring devices. This is made possible by our ability to design at
a larger scale and then use the inherent properties of our NanoShrink™ plastic
material to functionalize a device by controllably shrinking the same by a
factor of up to 95%, through a proprietary process. While the markets
for what we can do is meaningful, we believe that our “shrinkable technology”
offers, if successfully accepted, a low cost and flexible replacement for
existing chips and related devices manufactured through very expensive and
relatively inflexible manufacturing processes.
Technology and Intellectual
Property. We own, or license through various agreements with
the University of California, Merced (“UC Merced”) and the University of UC
Regents (the “UC Regents”), various patent applications and rights relating to
the ShrinkChip Manufacturing Solution™. A description of these
agreements and related technologies, applications and cost estimates is provided
below along with a list of patents and other intellectual property owned by, or
licensed to us. Additionally, we have filed applications with the US
Patent and Trademark Office (“USPTO”) and we possess significant know-how as it
relates to using shrinkable films in the manner in which we seek to design and
build products for our focused markets.
Corporate
History and Structure
General.
We were
incorporated in the state of Delaware on January 15, 2002 as Jupiter Processing,
Inc. On January 13, 2005, the Company changed its name to
Audiostocks, Inc. On May 14, 2009, the Parent company changed its
name to Shrink Nanotechnologies, Inc. in anticipation of its acquisition of
Shrink Technologies, Inc. On May 29, 2009, the Company entered into
and completed a share exchange agreement with the former principals of Shrink
Technologies, Inc., for the acquisition of Shrink which held and continues to
hold, most of our related business assets and has agreements with both UC Merced
and the UC Regents granting the Company the exclusive rights to the Shrink
related technologies discussed herein.
The
exchange of shares with Shrink’s owner has been accounted for as a reverse
acquisition under the purchase method of accounting with the business of Shrink
Technologies, Inc. as the surviving company for accounting and financial
reporting purposes.
2
We have
limited liquidity and our funds are allocated towards research, development and
commercialization of our technologies and products, identifying and retaining
executive management that will be able to clear the Company’s path into the life
sciences industry.
We have
been continually disposing of our Audiostocks related operations and
assets. The Company intends to liquidate any remaining assets
relating to its AudioStocks business. Nonetheless, certain
information relating to our previous AudioStocks business is contained towards
the end of this “Business” section, below but should not be relied upon as
meaningful in assessing our new Shrink operations.
Our
common stock was quoted on the Over-the-Counter Bulletin Board under the symbol
“INKN” through Tuesday, April 13, 2010. On April 14, 2010, the
Company was notified by FINRA that its 5 for 1 forward stock split was accepted
and that on April 15, 2010, the Company’s common stock would trade, for 20
business days, under the stock symbol “INKND” and that immediately subsequent to
the 20th business day following April 15, 2010, our stock symbol would revert
back to INKN (see “Recent Developments” at the end of this “Business” section,
below.) All references to share amounts, exercise, sale or conversion
prices in this Annual Report are as adjusted to reflect post Forward Split
amounts and prices.
Our
principal offices are located at 2038 Corte Del Nogal, Suite 110, Carlsbad,
California, 92011, Phone No. 760-804-8844, Contact person Mark L. Baum,
Esq. Our website is www.shrinknano.com.
Our
License Agreements
Research
Agreement
When we
acquired our Shrink subsidiary in May 2009, Shrink was and continues to be, a
party to a research agreement (the “Research Agreement”) with UC
Merced. Pursuant to the Research Agreement, UC Merced agreed to
undertake a research project and Shrink agreed to reimburse it for all direct
and indirect costs incurred in connection with the research, up to the amount of
$640,935 and in accordance with an agreed-upon budget. The Research
Agreement provides Shrink with the right to elect to receive an exclusive
royalty-bearing license to make, use, sell, offer for sale and import any
products and practice any methods in the inventions or discoveries conceived and
reduced to practice in the performance of the research conducted under the
Research Agreement. We have exercised our rights under the Research
Agreement and entered into a license agreement with the UC
Regents. As of the date of this filing, the Research Agreement
remains in tact and valid.
Our
Exclusive License Agreement with the UC Regents; Exclusive Patent
Licenses
The
underlying intellectual property and patent rights to Shrink’s proprietary
technologies are owned by the UC Regents. On April 29, 2009 and
pursuant to the terms of the Research Agreement, Shrink and the UC Regents
entered into an Exclusive License Agreement for Processes for Microfluidic
Fabrication and Other Inventions (the “License Agreement”). The
License Agreement licenses a broad array of intellectual property and inventions
vital to our planned operations. As of the date of this filing, the
License Agreement remains in tact and valid.
The
following description of the License Agreement is a summary only, and is
qualified in its entirety by the actual agreement which has been filed in our
public filings and which is incorporated herein.
In
accordance with the License Agreement, we will be required to pay royalties
based on net sales of each licensed product, licensed method or licensed service
by the Shrink, any of its affiliates or joint-ventures. Specific
details relating to the economic terms of our License Agreement with the UC
Regents is provided below, in this Report, under the section titled “Item 7.
Management’s Discussion and Analysis of Financial Condition and Results of
Operations” below.
The term
of the License Agreement commenced April 29, 2009, and will continue in effect
until the expiration or abandonment of the last patent covered by the agreement,
unless we terminate it on 60 days’ notice or the UC Regents terminated it on 60
days’ notice if we default on our obligations under the License Agreement and
fail to cure such default during the 60 day period.
3
Pursuant
to the License Agreement, Shrink acquired, subject to certain limitations, an
exclusive license to the UC Regents’ rights in and to specified patents, patent
applications and intellectual property for which patent applications have not
yet been filed relating to various inventions (the “Licensed
Rights”). Under the License Agreement, Shrink is permitted to make,
use, sell, offer for sale the licensed intellectual property and import licensed
products and services and to practice licensed methods in all fields and in all
locations in which the UC Regents may lawfully grant the Licensed Rights. The
patents, patent applications and other intellectual property licensed were
originally developed by Dr. Michelle Khine and others in the course of research
which took place primarily at the UC Merced and at University of California,
Berkeley. A list of these patent applications follows:
|
Shrink
Reference Number
|
Filing Date
|
USPTO
Application Number
|
Title
|
|
1
|
11/13/2007
|
61/003,113
|
Processes
for Microfluidic Fabrication
|
|
2
|
1/3/2008
|
61/018,881
|
Processes
for Microfluidic Fabrication
(Petition
to Amend Inventorship under 37 CFR 1.48(d))--adding Luke Lee as
inventor
|
|
3
|
11/12/2008
|
PCT/US2008/083283
|
Processes
for Rapid Microfabrication Using Thermoplastics and Devices
Thereof
|
|
4
|
3/18/2009
|
61/161,388
|
Honeycomb
Shrink Wells For Stem Cell Culture
|
|
5
|
3/19/2009
|
61/161,738
|
Aligning
Cells on Wrinkled Surface
|
|
6
|
3/25/2009
|
61/163,343
|
Quantum
Dot Solar Concentrator
|
|
7
|
5/12/2009
|
61/177,402
|
Aligning
Cells on Wrinkled Surface
|
|
8
|
5/13/2009
|
61/177,871
|
Honeycomb
Shrink Wells For Stem Cell Culture
|
|
9
|
5/13/2009
|
61/177,916
|
Textured
Metal Nanopetals
|
|
10
|
5/13/2009
|
61/177,952
|
Thermoplastic
Substrates with Wrinkled Metallic Surfaces for Chemical and Biological
Sensing
|
|
11
|
5/13/2009
|
61/177,990
|
High
Resolution Light Emitting Devices
|
|
12
|
5/13/2009
|
61/178,005
|
Metal-Coated
Shrinkable Polystyrene and Methods for Using
Same
|
There is
a difference between a patent application and a full patent
filing. The above items (save Shrink Reference Number 3) relates only
to patent applications. These patent applications are designed to be
protective of ideas and intellectual property and expire after one
year. At or prior to the expiration of the patent applications, to
the extent the Company believes there is merit to incur the additional expense
in making a full patent application filing, we will agree to incur the costs of
doing so, and request that the UC Regents, file full applications. To
the extent we decide not to make these requests, we may lose our rights to the
same under our License Agreement (because we agreed to pay for all costs of
filing patent applications and related costs). To date, the UC
Regents have filed full patent applications on Shrink Reference Numbers
1-8. Items 9, 10, 11 and 12, as well as applications we have made
(which are referenced below) remain subject to the same deadlines and costs,
except that we would be the owner, in whole or in part, in some cases, of the
patent rights. To date, we have not made any full patent application
filings.
Inventions
or intellectual property rights which constitute advancements, developments or
improvements in which the UC Regents acquires rights, whether or not patentable
or subject to a patent application, are not automatically covered by the
license. The License Agreement must be amended to add such
intellectual property and we are required to pay a $5,000 fee for the addition
to the license. However, we have a one year option to license any
such intellectual property if it was developed under the terms of the Research
Agreement.
As is
common with license agreements with educational institutions, the License
Agreement permits the UC Regents to use or exploit any Licensed Rights for
educational, clinical or research purposes, including, without limitation, any
sponsored research performed for or on behalf of commercial entities and for
publication or other communication of any research results. In
addition, to the extent that an invention or technology discovered by them is
not part of the Licensed Rights, the UC Regents may exploit (or sublicense) such
technology and rights to third parties.
While we
may sublicense our rights, we may only do so in writing. Among other
conditions, any sublicense must include all of the rights of, and require the
performance of all the obligations due to the UC Regents, except for certain
payment obligations. To the extent we own any undivided interest in
any Licensed Right with the UC Regents, we cannot grant any third-party a
license to such Licensed Right unless we also grant a license to the UC
Regents. The license to the UC Regents would have to be on the same
terms and conditions set forth in the License Agreement on which we are entitled
to grant licenses in the Licensed Rights which we do not have an ownership
interest.
4
Intellectual
Property We Own (in Whole or in Part)
In
addition to the patents acquired through our License Agreement with UC Merced
and the UC Regents, in August through September of 2009, we filed and obtained
four (4) additional provisional patents as originally more fully disclosed in
our Current Report on Form 8-K dated August 31, 2009, the provisions of which
are incorporated by reference herein and described below:
|
Shrink
Reference Number
|
Filing Date
|
Application Number
|
Title
|
|
13
|
8/26/2009
|
61/237,224
|
Quantum
Dot Solar Concentrator
|
|
14
|
8/26/2009
|
61/237,245
|
Aligning
Cells On Wrinkled Surface
|
|
15
|
8/27/2009
|
61/237,623
|
High
Resolution Light Emitting Devices
|
|
16
|
9/4/2009
|
61/240,129
|
High
Surface Area Micro and Nano Beads
|
|
17
|
10/13/2009
|
61/251,242
|
Process
for Fabrication of Nanoholes, Related Structures, and Devices
Thereof
|
Trademarks
We hold a
number of trade or service marks and have been using most since late 2008 to
early 2009. These marks have also been filed with the USPTO and
include the following:
|
Trademark
|
Shrink
Trademarks:
|
Date
of Filing
|
|
T06435US0
|
StemDisc™
|
9/4/2009
|
|
T06438US0
|
CellAlign™
|
9/4/2009
|
|
T06439US0
|
PolyShrink™
|
9/4/2009
|
|
T0644OUS0
|
MetalFluor™
|
9/4/2009
|
|
T06437US0
|
OptiSol™
|
9/4/2009
|
|
T06441US0
|
ShrinkPatch™
|
9/4/2009
|
|
T06436US0
|
Quantumsol™
|
9/4/2009
|
|
T06434US0
|
ShrinkChip™
|
9/4/2009
|
|
T06433US0
|
Shrink™
|
9/4/2009
|
|
T06433US0
|
ShrinkNano™
|
9/4/2009
|
Our
Patent Filing Policy
We are
required by the License Agreement to pay the costs of obtaining patents and the
Company’s board of directors along with the Shrink Scientific Advisory Board,
intend to regularly reassess the technologies and applications we have, develop,
or acquire an interest in to determine, in good faith, whether the benefits of
obtaining a patent outweigh the costs of obtaining it and the resulting public
disclosure of our processes, technologies or proprietary
technologies.
No
assurance can be made that the Company will be able to assess the
patent-worthiness of all its products, or, if a patent is desirable, that the
Company will have sufficient funds or wherewithal to secure or prosecute its
intellectual property rights.
Technology
Description Overview
The heart
of our technology is our use of polystyrene and other pliant and pre-stressed
materials used in numerous industrial applications. Our material
which we use is branded as NanoShrink™. The use of NanoShrink within
our systems allows for the ultra-rapid direct patterning of complex or even
three dimensional, stacked polystyrene micro and nanostructures. The
benefits of using a shrinkable medium, such as NanoShrink™, result in part from
its ability, through our patent-pending processes, to uniformly shrink during
heating. Our patent-pending shrinkable chip technology is a “platform
technology” with potential applicability to a number of different business and
scientific areas.
5
Our
technologies, primarily owned or licensed by Shrink, involves the creation and
manufacture of certain complex chips or microfluidic diagnostic tools and
measuring devices on a larger micro-scale on shrinkable, pre-stressed
polystyrene sheets (or other plastic-derived materials such as NanoShrink™) and
then shrinking them down significantly in size through a patent-pending
process. Our research indicates, to date, that the technology and
materials under development by Shrink result in a relatively “uniform” shrinking
process, resulting in a “true” and relatively undisturbed end product that
retains its vital properties even at the micro or
nano-scale. We sometimes refer to this platform, system and
technology as the Shrink Chip Manufacturing Solution™.
Shrink
has successfully created a number of highly complex prototype chips including
microfluidic chips and components for integrated circuitry systems, solar cell
concentrators, metal-enhanced fluorescent diagnostic and biological testing
chips, “lab-on-a-chip” systems, as well as three dimensional multi-level chip
designs.
Our
Shrink Chip Manufacturing Solution™ technology, if further developed,
commercialized and applied properly, will not only allow for the inexpensive
development of innovative and complex chips that can be used in a broad range of
applications, from (i) chemical, biological and environmental measuring tools
for the life and environmental science industries to (ii) use in electronic
components, but also allows for the manufacture of related devices and systems
to be completed without the high capital costs and laborious processing steps
traditionally associated with micro fabrication, such as “clean rooms” and
complex robotics.
Our
Proprietary Material
NanoShrink™
and other shrinkable plastic substrates that we use are used in countless
industrial applications because of there flexible material properties and
relative material stability. By taking advantage of these inherent
characteristics, NanoShrink allows for the ultra-rapid direct patterning of
complex, even three-dimensional, stacked polystyrene micro- and nanostructures
as well as advanced optoelectronic devices. Management believes that
NanoShrink™ and similar materials readily available at economic rates both in
the US and abroad.
Advantages
of Our Manufacturing Process and Technologies
What
follows are some examples of technological and/or cost benefits of our
shrinkable polystyrene and plastic-based technologies over other competing
technologies.
Quicker
Manufacturing. Developing intricate
micro patterns into substrates of silicon, glass, or quartz requires large
investments in capital equipment, manufacturing “clean rooms”, and costly
consumables. The soft lithography processes which we use, we believe,
can accelerates chip fabrication from months (using standard silicon technology)
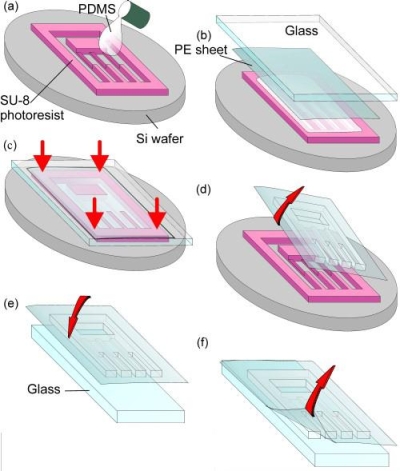
to typically less than 2 days. This process still requires patterning
of a silicon wafer (see Figure A. below). This wafer is used as the
mold, with expensive photo-sensitive polymers that define the features by
photolithography.
Figure A:
6
Reduced Capital
Costs. We believe that Shrink’s technology obviates the
requirement for high cost manufacturing equipment, the need for multiple and
complex micro fabrication designs, and the use of high stringent environments
and clean rooms. Due to ease of use, the Company will also be able to
rapidly integrate into most pre-existing manufacturing lines. With
the relative ease of prototype design and creation, Shrink is able to accelerate
research and development time without the added cost of resources or
manpower.
Better Material
Characteristics. We believe that our shrinkable
technology using
NanoShrink is
advantageous over other substrates such as silicon because of its desirable flow
characteristics in microfluidic applications. Microfluidic devices
typically require dimensions in the 100 μm range, whereas we are able to achieve
resolutions near 70 μm in width and operate well within the requirements and
capabilities of current lithography techniques. So far,
testing indicates that NanoShrink™ is not as easily impacted by solvents or
environmental factors such as extreme heat, cold or physical
impact. For this reason as well as the other reasons relating to ease
and economics in manufacture, we believe that shrinkable polystyrene materials
(such as NanoShrink™) are an ideal material for products such as cell-culture
dishes and plates used in drug discovery. Many hydrophobic molecules
absorb easily into the porous PDMS matrix of computing technologies, potentially
affecting results which have intolerable in many analytical
applications. We believe that this is one property that links
industry adoption of PDMS for applications in drug discovery and other sensitive
assays.
Design and Manufacturing
Efficiencies; Three Dimensional Structures. Traditional photolithography creates
planar or 2-dimensional structures. Rounded and/or multi-height
features are therefore difficult to create and require additional processing
steps. To achieve multi-heights, several masks and high precision
alignment are required. Our processes, we believe, creates inherently
rounded features, and multi-heights are easily achievable. Additionally, because
of the unique characteristics of our shrinkable polystyrene technologies, design
and fabrication can occur sequentially, with immediate results allowing for
quick turn-around prototype
testing.
Rapid Deployment. Shrink’s Shrink technologies
allow for the quick and easily delivery of custom microfluidic chips for low
volume prototype or manufacturing needs. Through the integrated
website we are developing, www.ShrinkChip.com,
users will be able to establish a secure account and in a few steps upload,
store and manage their computer assisted drawing, or CAD, of micro-chip
patterns, select the size, quantity and other characteristics, receive a price
quote, place the order, and have the chips shipped directly to their
lab. Shrink intends to continue to make use of industry-standard
xerography printing machines to provide prospective customers the highest levels
of quality control.
Business
Strategy
Our
business strategy, based on the FIGA™ model (www.figa.org), is to use outside
experts from various industries in order to meet commercial ends. We
execute on our strategy by funding research in outside (typically academic)
research labs, all of which we have established technology licensing
arrangements with. Around our “hub” of mainly academic research
relationships, we connect teams consisting of people with financial, related
industry (physical or life sciences) and government (typically regulatory)
backgrounds. We use these teams to vet technology that is produced
and patentable to first determine the commercial viability of the technology,
and then to create specific commercialization plans for the same
technology.
To date,
we have used our business model to create three businesses, which we continue to
develop:
NanoShrink™
Our
NanoShrink™ business (“NanoShrink™”) seeks to exploit a proprietary polymer
material which we seek to sell to academic labs and research organizations
around the world. NanoShrink™ can be used as a flexible platform to
create a myriad of life science and physical science
applications. NanoShrink™ will eventually be operated as a separate
subsidiary entity with a dedicated management team.
StemDisc™
Our
StemDisc™ business (“StemDisc™”) is based on a biomedical research tools
platform designed to grow and differentiate various biological samples,
including embryoid bodies (EBs), human and animal single cell studies, human
embryonic stem cells (HESCs) and the new and growing field of induced
pluri-potent stem cells (IPSCs). Our StemDisc business will
eventually be operated as a separate subsidiary entity with a dedicated
management team.
Shrink
Solar™
Our
Shrink Solar business (“Shrink Solar™”) seeks to design and market optical solar
concentrators based on a version of NanoShrink™ material which is “doped” with a
proprietary blend of photovoltaic nanoparticles called quantum
dots. The balance of the Shrink Solar system, which in total
comprises the finished products we seek to sell, will be used to solarize
surfaces, including turning windows into solar collection devices and as a low
cost replacement to and even accessory item for traditional flat panel
crystalline silicon solar cells. Our technology does not require
mirrors, lens or tracking devices. We believe the final commercial
products we will seek to sell will be extremely low in cost, relatively equal to
existing technologies in terms of efficiency and will offer advantages that will
allow for system upgrades as technology improves. Our Shrink Solar
business will eventually be operated as a separate subsidiary entity with a
dedicated management team.
7
Product
Commercialization and Marketing
We will
require substantial additional capital to further develop and commercialize our
manufacturing processes. In each of our businesses, consisting of
NanoShrink™, StemDisc™ and Shrink Solar™, we have established relationships with
contract suppliers of the products we seek to market. In each
business, and presuming adequate funding and that no unusual impediments are
incurred, we do not foresee any limitation on our ability to continue to design
and eventually market the related products for the businesses.
Strategic
Marketing Agreement with Inabata
In
September of 2009, we entered into a Strategic Marketing and Development
Agreement (the “Marketing Agreement”) with Inabata America Corporation
(“Inabata”), a subsidiary of Inabata & Co. Ltd., which provides for the
appointment of Inabata as the Company’s non-exclusive representative
for the purposes of marketing and promoting the Company’s solar concentrator
technology and ShrinkChip™ RPS solar products to third parties (the “Solar
Products”). Pursuant to the Marketing Agreement, Inabata is required
to use best efforts in, among other things, introducing the Solar Products to
potential purchasers, licensees, customers, development partners or possible
funding sources, and to provide certain technical assistance and
training. In exchange therefore, Inabata was granted a good faith
right of first negotiation to act as non-exclusive distributor of the Solar
Products. In addition, the Marketing Agreement provides for fees to
be paid to Inabata if it introduces a funding, grant or development funding
source which completes a capital transaction with the Company up to 50% of which
may be paid in stock, at the sole discretion of the Company.
Research
and Development Priorities and Expenditures
We
estimated that Shrink has invested approximately $122,416 into its technologies
in 2009, primarily from government and university grants, and $79,352
into its technologies in 2008, most of which has stemmed from payments from
Shrink through UC Merced or, to a limited extent, CIRM. (See “Item
7. Management’s Discussion and Analysis of Financial Condition and
Results of Operations” below).
In
determining our allocation of resources, we assess which fields we believe are
closer to commercial development and which we believe hold greater financial and
strategic potential for us. Our goal is to penetrate those areas that
we are most prepared to commercialize and that have the fewest relative barriers
to market acceptance. In order to best describe the fields and the
relative priority of each to us at this time, what follows is a summary of
applications currently being pursued in order of currently intended
priority.
Specifically,
Shrink has focused its early development towards metal-enhanced fluorescence
diagnostic systems, stem cell research, cellular
growth substrates, Lab-on-a-Chip devices and our solar concentrator
technologies. Our StemDiscTM
product is about to enter “second generation” testing, and comprehensive R&D
trials will be performed and analyzed by Shrink’s scientific
partners.
Employees
We
currently do not have any full time employees for Shrink Nanotechnologies, Inc.,
including its subsidiaries. We obtain administrative services from
BCGU, LLC (“BCGU”), an entity indirectly controlled by James B. Panther II, and
Mark L. Baum, Esq., who are two of our directors, for a fee of $30,000 per month
pursuant to an operating agreement. In the past, because we have not
had the cash on hand to pay BCGU, we have accrued these amounts
owed. The foregoing is a summary only of our operating agreement with
BCGU, LLC, a copy of which is attached as an Exhibit to our Current Report on
Form 8-K, Filed June 5, 2009.
We hire
independent contractor labor for executive management, legal, accounting and
other administrative functions, on an as needed basis and have entered into
consulting arrangements with certain directors and advisory board members in
exchange for stock or derivative securities. We have not entered into a
collective bargaining agreement with any union.
8
Prospective
Business Goals
The goals
we will need to attain for our various businesses are currently, as follows
(although management constantly monitors market trends, as well as available
capital and has in the past, and continues to, refine and re-prioritize the
Company’s goals, so as to attain commercialization as quickly as
possible):
NanoShrink™
Business
|
·
|
Finish
development on the first iteration of the NanoShrink™ product
line.
|
|
·
|
Complete
the protection of our NanoShrink™ intellectual property
and know how as well as optimize the propriety of the ancillary devices we
wish to make available in order to enhance the value of using
NanoShrink™ and
the balance of the systems we intend to commercially
offer.
|
|
·
|
Enter
into a joint development agreement to develop additional NanoShrink™ films (plain), as
well as single, multi-layered metalized films and structured
NanoShrink™ films
for diagnostic assays and biomedical research
applications.
|
|
·
|
Build
a web platform to allow researchers and users of the products to provide
feedback, order products and collaborate with other user researchers
around the world.
|
|
·
|
Seed
ideas to our NanoShrink™ user community, post
research results and publish in academic journals in order to spread the
word about the flexibility and usefulness of the NanoShrink™ platform for
diagnostic and microfluidic assay
prototyping.
|
|
·
|
Continued
academic publishing related to the uses of NanoShrink™.
|
StemDisc™
Business
|
·
|
Develop
the StemDisc “family of products” for biological materials as small as the
single cell – all the way up to products that foster research on full
scale patches of tissue (cardiac patches,
etc.).
|
|
·
|
Create
a product development advisory committee (the “PDAC”) to distribute our
products to leading researchers and receive feedback regarding the use of
the materials and ways to be design the
product.
|
|
·
|
Develop
scalable commercial quality manufacturing processes for StemDisc
devices.
|
|
·
|
Commercially
manufacture StemDisc devices.
|
|
·
|
OEM
StemDisc products through one of several large multi-national
distributors.
|
Solar
Concentrator
|
·
|
Unveil
our initial working prototype devices and discuss efficiency of the device
as well as potential scaled cost per watt
calculations,
|
|
·
|
Improve
and optimize our solar concentrator
technology,
|
|
·
|
Commence
to develop a branding strategy for the
technology,
|
|
·
|
Accessing
adequate supply arrangements for
components,
|
|
·
|
Develop
reliable commercial scale “solarizable” film and plastic sheet
relationships,
|
|
·
|
Secure
supply arrangements for system connector
components,
|
|
·
|
Establish
relationships with market leading product design and manufacturing
entities – in the window covering, residential and commercial window
manufacturing, siding, roof tile and conventional solar panel businesses
to integrate our products and systems into their designs to in effect
“solarize” previously non-energy producing
surfaces.
|
9
Competition
There are
numerous competitors in every space/market we seek to exploit, with the
strongest competitors in the life sciences industry boasting large market
capitalizations and liquidity, as well as scientific and marketing resources and
recognition.
While
competing technologies and companies exist for all of our products, we believe
that we have certain opportunities that we can exploit which may increase our
opportunity to be successful in the markets we seek to
exploit. However, even if our technologies are successful, no
assurance can be made that we will be able to compete effectively and yield
significant or sustained value for our shareholders.
|
NanoShrink
|
The
business itself is what may reasonably be referred to as an attempt to
shift the paradigm in the manner in which prototype devices are made in
the physical and life sciences. The idea of designing and
printing at the macro scale and then shrinking (a polymer), resulting in
feature diminution, leaving a functional device with micro or nano scale
features, is a relatively new concept and there are not any direct
competitors in this field other than those companies which offer more
traditional methods of device prototyping.
The
pool of potential dollars flowing into this “market” is relatively finite
– although it does increase and decrease as research and academic,
governmental and commercial research and development budgets ebb and
flow.
Here
is a partial list of entities competing for these dollars:
· TransLume
(http://www.translume.com)
· Micralyne
(http://www.micralyne.com)
· Siloam Biosciences
(http://www.siloambio.com)
· Stanford
Rapid Prototyping Laboratory, Microfluidics Foundry etc.
· Dr.
George M. Whitesides, a legend in the microfluidics area, has proposed a
similar shrink based technology in 1997, but has not initiated
commercialization.
|
|
StemDisc
|
The
stem cell research tools business is a relatively new, dynamic and growing
field. As such, we are aware of several companies who directly
compete in the market we seek to enter. A few of these
companies are listed below. Additionally, we are aware of many
academic laboratories around the United States (and we are sure there are
many more around the world) who are dedicating resources to building newer
technologies that may compete with and potentially displace any interest
in our devices.
· Stem Cell Technologies
(http://www.stemcell.com)
· PicoVitro
(http://www.picovitro.com)
· Nunc (http://www.nunc.com)
|
|
Shrink
Solar
|
The
solar field is extremely crowded with numerous different technologies
competing for a growing market for renewable energy investment, including
power generation, power conservation and in general, “green(er)
technologies.”
Below
is a list of a few companies in the solar space that offer products in the
market we seek to enter:
· Covalent Solar
(http://www.covalentsolar.com/)
· Morgan Solar
(http://www.morgansolar.com/)
· SolarWorld
(http://www.solarworld-usa.com/
· Kyocera
(http://www.kyocera.com/)
· SunPower
(http://www.sunpower.com/)
· Dow Chemical (http://www.dowsolar.com/)
|
10
Government
Regulation
Shrink is
a research and development business and to date, has not commercialized or sold
any products using its technologies. Therefore, the Company cannot be
certain exactly which governmental regulations will ultimately be applicable to
the final products. However, certain of our products and manufacturing
processes will be subject to regulation under various portions of the
U.S. Federal Food, Drug and Cosmetic Act, to the extent they are used in or
as part of medical devices or in vitro diagnostic devices. The effects of
this are that, among other things, our manufacturing facilities, when and if
created, may be subject to periodic inspection by the U.S. Food and Drug
Administration, or FDA, other product-oriented federal agencies and various
state and local authorities in the U.S. for compliance with the requirements of
the FDA’s Quality System Regulation (formerly known as Good Manufacturing
Practices), other federal, state and local regulations and other quality
standards such as ISO 9001 or ISO 13485.
To the
extent our products are used in medical devices or other regulated applications,
our products may be subject to extensive medical, FDA or other government
regulation, testing and pre-market approval or clearance processes. Prior
to application for approval or clearance, we may need to conduct clinical trials
to test the safety and efficacy of our products or we may be required to
identify predicate devices to which we can establish substantial
equivalence. In addition, we may be required to satisfy testing criterion
or submit product applications in other countries where we intend to market our
goods. These processes may take an extensive period of time and would
necessarily require significant capital outlays, which we have no commitments
for at this time.
We do not
use and we anticipate that we will not utilize, manufacture or operate with
substances deemed controlled under the Controlled Substances Act, administered
by the Drug Enforcement Agency, or DEA, and therefore, no special procedures
need be in place.
Because
we do not actually do any material amount of testing, but rather, the
manufacturing of devices that will be used as “tools”, we do not employ Centers
for Disease Control/National Institutes of Health, Guidelines for Research
Involving Recombinant DNA Molecules, Biosafety in Microbiological and Biomedical
Laboratories, but, if and as our operations expand, may be required to do so in
the future.
We are
subject to federal, state, and local laws and regulations regulating the
discharge of materials into the environment, or otherwise relating to the
protection of the environment, in those jurisdictions where we operate or
maintain facilities. Our operations to date have only been R&D and
within universities in the State of California. Therefore, we do not
believe that any liability arising under, or compliance with, these laws and
regulations will have a material effect on our business, and no material capital
expenditures are expected for environmental control.
Certain
Regulation Relating To Our License Agreement
Because
certain inventions licensed to us under the License Agreement were funded by
CIRM grants, as discussed above and in “Item 7. Management’s
Discussion and Analysis of Financial Condition and Results of Operations.”
Below, we are subject to the terms of the State of California’s CIRM’s
Intellectual Property requirements for Non-Profit Organizations (17 Cal. Code of
Regs. Section 1003000, et.
seq.
The
license granted by the License Agreement is also subject to the obligations to
the U.S. government, under 35 U.S.C. Sections 200-212, including the obligations
to report on utilization of inventions under 37 CFR 401.14(h), and is subject to
the National Institute Of Health’s “Principals and Guidelines for Recipients of
NIH Research Grants and Contracts on Obtaining and Disseminating Biomedical
Research Resources” (64 F.R. 72090 Dec. 1999, as amended).
We do not
believe, based on our existing Research Agreement and License Agreement, that
any of our funding grants to date involved the use of federal
funds. U.S. Federal rules relating to ownership of intellectual
property may be applicable to our intellectual property. In the
event that we accept federally funded grants or projects in the future, which we
hope to do, our use of technologies developed will be subject to strict
licensing guidelines and rules.
AudioStocks
and Previous Business Revenues
In 2008
and 2009, we continued to wind down our AudioStocks business. We do
not anticipate any material revenues from our AudioStocks business in 2010 as we
completed the sale of our AudioStocks business and related assets in
2009. Because we no longer operate in this segment, management
believes that extensive disclosure on this business is not material to an
understanding of our new Shrink business.
11
Recent
Developments
Forward
Split and Increase in Capitalization
Effective
as of April 8, 2010, we filed a Certificate of Amendment to our Certificate of
Incorporation (the “Certificate of Amendment”) which both:
|
·
|
increased
our authorized capital from 100,000,000 shares of which 95,000,000 shares
are common stock, par value $0.001 per share and 5,000,000 are preferred
stock, par value $0.001per share, to 500,000,000 shares, of which
475,000,000 are shares of Common Stock, par value $0.001 per share and
25,000,000 are preferred stock (the “Capitalization Increase”);
and
|
|
·
|
effectuated
a forward split of our outstanding common stock on a 5 for 1 basis,
increasing our issued and outstanding shares of common stock from
38,260,797 to 191,303,985 shares, par value $0.001 (the “Forward
Split”).
|
The
Forward split had a pari
pasu effect on our 4,000,000 shares of outstanding Series A Preferred
Stock, which is now increased to 20,000,000 shares of Series A Preferred
Stock.
As a
result of the Forward Split and Capitalization Increase, we now have remaining
283,696,015 shares of Common Stock authorized, but unissued and available for
issuance, and an additional 5,000,000 shares of “blank check” preferred stock
authorized, which have not been designated or reserved for issuance by the
board.
All
references in this Annual Report to share amounts, exercise or conversion prices
reflect post split numbers and amounts, unless expressly indicated
otherwise.
Convertible
Note Financing
During
the months of January and February of 2010, the Company issued, on a private
basis, $335,000 in 12% convertible notes initially convertible at $.10 per
share, and 1,675,000 Series A Common Stock Purchase Warrants, exercisable at
$0.20 per share as part of a confidential private financing in exchange for
$335,000. The notes and warrants are identical to $100,000 of 12%
notes and warrants issued in November of 2009 (i.e. an aggregate of $435,000
principal amount of convertible notes and warrants). The notes are
repayable one year from issuance and the warrants are exercisable commencing 6
months from issuance and expire 36 months from issuance. In the event
of a default, or in certain other events, the notes become exercisable at 80% of
the then effective conversion price (initially $.08 per share), and the Company
may force conversion at the discounted rate at such time.
When
combined with notes and warrants sold during fiscal 2009 (and not including any
prior 14% convertible notes issued to Noctua Fund, L.P.) the following is a
brief summary of the notes and warrants outstanding:
|
·
|
$435,000
principal amount 12% convertible notes, currently convertible at $.10 per
share into an aggregate of 4,350,000 shares of common stock (plus shares
issuable as interest), payable one year from their respective issuance
dates, and
|
|
·
|
2,175,000
Series A Common Stock Purchase Warrants, exercisable at $.20 per share,
and expiring three years from their respective issuance
dates.
|
The
foregoing is a summary only of the note and warrant
financing. Details of the same may be found below in this Annual
Report in the section titled “Item 5. Market for Registrant’s Common Equity,
Related Stockholder Matters and Issuer Purchases of Equity
Securities.”
Issuances
of Shares to Consultants
As part
of the Company’s business plan and when feasible, it regularly elects to pay
consulting fees in stock, as opposed to shares, to professionals so as to
conserve capital. In particular, during March of 2010, the
Company issued an aggregate of 18,382,500 shares of restricted common stock to
seven consultants and professional service providers for services
rendered. Additional information relating to these issuances and the
underlying agreements and details can be found below in this Annual Report in
the section titled “Item 5. Market for Registrant’s Common Equity, Related
Stockholder Matters and Issuer Purchases of Equity Securities” and “Item
7. Management Discussion and Analysis of Financial Condition and
Results of Operations” below.
12
|
ITEM
1A.
|
RISK
FACTORS
|
We
have not yet commercialized our ShrinkChip™ related technologies and have not
attained revenues from them. The nature of our business activities
subjects us to certain hazards and risks and uncertainties. We are a
research and development stage company that will need substantial additional
capital and development to be able to commercialize and gain market acceptance
for our innovative products. You should carefully consider the risk
factors and all of the other information included in, or incorporated by
reference into, this Annual Report or any supplement, including those included
in our most recent Reports or documents provided as exhibits to this Report or
other Reports as may be incorporated from time to time.
Our
independent accounting firm and our board of directors, given the present
resources and condition of the company agree that our internal controls are
ineffective and that we may not be able to continue as a going
concern. We are taking steps to correct our internal controls and are
also moving to improve the financial condition of the company.
General
Risks:
You
will suffer immediate and subsequent dilution and the costs of the securities
you may purchase at exceed the price paid by current principal
shareholders.
Many of
the present owners of our issued and outstanding securities acquired such
securities at a cost substantially less than that of the open
market. In addition, the Company currently is indebted to Noctua
Fund, LP for loans made to the Company or Shrink since 2008, as reflected on two
14% convertible promissory notes in the aggregate initial principal amounts of
$118,121.28 and $100,000, respectively, plus interest, which are convertible at
$.04 per share based on conversion prices at the times of the loans
(approximately 5,848,725 shares, in aggregate as of April 10, 2010), both of
which are currently in default. Therefore, the shareholders will bear
a substantial portion of the risk of dilution and losses.
Some
of our debt is in default and could result in us losing some or all of our
assets.
The
“default” status with respect to the notes owned by Noctua Fund L.P., an
affiliate of certain management, provides them with the right to take
potentially aggressive actions, including levying on some or all of our assets,
in order to recover monies owed.
We
may not be able to continue operations as a going concern and we need
substantial additional capital to continue our research and development
activities and begin commercialization plans. Research and
Development costs of Shrink have been completely paid for to date by the UC
Regents, and to a lesser extent, CIRM and, we do not have any capital
commitments at this time.
Our
independent auditors have expressed doubt about ability to continue as a going
concern. Shrink is currently a research and development company
only. We do not have grant or capital commitments at this time and
previous Shrink research has been funded by the UC Regents and
CIRM. We do not believe that the sale of our remaining AudioStocks
business related assets or from recent capital raises will be sufficient to fund
our operations or research to any material degree. Nonetheless, we
have no further commitments for grants and grant funds cannot, generally, be
used towards commercialization and manufacturing
efforts. Accordingly, the corporation will be dependent on obtaining
additional external sources of capital in order to fund its operations or even
continue as a going concern.
A future
capital raise could involve a private or public sales of equity securities or
the incurrence of additional indebtedness. Additional funding may not be
available on favorable terms, or at all. If we borrow additional
funds, we likely will be obligated to make periodic interest or other debt
service payments and may be subject to additional restrictive covenants. If we
fail to obtain sufficient additional capital in the future, we could be forced
to curtail our growth strategy by reducing or delaying capital expenditures,
selling assets or downsizing or restructuring our operations. If we
raise additional funds through public or private sales of equity securities, the
sales may be at prices below the market price of our stock, and our shareholders
may suffer significant dilution as a result of such sale.
13
Management
owns a controlling interest of our stock, including, without limitation, our
Series A Preferred Stock; the existence of these derivative securities, may
adversely affect your stock price and our ability to raise capital or into
business ventures and transactions.
Currently,
twenty million shares (20,000,000) of Series A Preferred Stock are issued
and outstanding and held by certain principal shareholders or their
affiliates. These shares are all convertible and contain strict anti
dilution provisions and other restrictive covenants. As a result,
over 90% of our voting control is vested in three beneficial
owners. Moreover, as shares of preferred stock are converted and
sold, such sales are likely to have a highly dilutive effect on our stock
price.
Additionally,
the existence of our outstanding preferred stock may hinder our ability to raise
capital at favorable prices if and as needed, or to make
acquisitions.
As a result, these members of
management will be able to:
|
·
|
control
the composition of our board of directors; control our management and
policies;
|
|
·
|
determine
the outcome of significant corporate transactions, including changes in
control that may be beneficial to stockholders;
and
|
|
·
|
act
in each of their own interests, which may conflict with, or be different
from, the interests of each other or the interests of the other
stockholders.
|
Our
principal stockholders have the ability to exert significant control in matters
requiring stockholder vote and could delay, deter or prevent a change in control
of our company.
Since our
stock ownership is concentrated among a limited number of holders, those holders
have significant influence over all actions requiring stockholder approval,
including the election of our board of directors. Specifically, these
members of management are Marshall Khine, Esq., Mark L. Baum and James B.
Panther, II (director only). Through their concentration of voting
power, they could delay, deter or prevent a change in control of our company or
other business combinations that might otherwise be beneficial to our other
stockholders. In deciding how to vote on such matters, they may be
influenced by interests that conflict with other stockholders. Accordingly,
investors should not invest in the Company’s securities without being willing to
entrust the Company’s business decisions to such persons.
Conflicts
of interest between the stockholders and our company or our directors could
arise because we do not comply with the listing standards of any exchange with
regard to director independence.
There are
a variety of conflicts of interests and related party transactions with members
of management, which control the vast majority of our shares and our
board. We are not listed on a stock exchange and our Board of
Directors does not comply with the independence and committee requirements which
would be imposed upon us if we were listed on an exchange. In the absence
of a majority of independent directors, our directors could establish policies
and enter into transactions without independent review and approval. This could
present the potential for a conflict of interest between the stockholders and
our company or our directors.
14
We
have a significant amount of preferred stock, convertible debt and warrants
outstanding, all of which have anti dilution provisions. If we issue
additional shares or derivative securities as we raise capital, the notes are
converted or the warrants are exercised, or if the conversion prices are
adjusted downward as a result of a stock issuance at our current market rates,
you will suffer immediate and substantial dilution to your common
stock.
The
bylaws allow the board to issue common shares without stockholder
approval. Currently, the board is authorized to issue a total of
475,000,000 common shares, of which less than 45% have been issued or reserved
for issuance as of April 2010. In addition, the board is authorized
to issue up to 25,000,000 preferred shares of which 20,000,000 are already
designated and issue and an addition 5,000,000 “blank check” preferred may be
issued. Currently, 191,303,985 shares of common stock are issued and
outstanding and, the following securities with anti-dilution provisions are
outstanding:
|
·
|
20,000,000
shares of Series A Preferred Stock, owned primarily by Messers Baum and
Panther, II, convertible on a one-for-one basis into common
stock,
|
|
·
|
$435,000
principal amount of unsecured 12% convertible notes, initially convertible
at $.10 per share, into an aggregate of 4,350,000, in addition to shares
that may be issued in respect of interest payments,
and
|
|
·
|
2,175,000
shares issuable upon exercise of Series A Common Stock Purchase Warrants
issued between November 2009 and April of 2010, at $.20 per share,
and
|
|
·
|
$118,121.28
principal amount of 14% convertible promissory note, issued to Noctua
Fund, L.P. in May 2009, an affiliate of Messers Baum and Panther, II,
convertible at $.04 per share into an aggregate of 2,953,032 shares (or
3,223,725 inclusive of 270,693 shares underlying $10,827.72 or accrued
interest, through April 10, 2010), which note is currently in
default,
|
|
·
|
$100,000
principal amount of 14% convertible promissory Note issued to Noctua Fund,
L.P., an affiliate of Messers Baum and Panther, II, reflecting loans made
by it in 2008 and 2009, convertible at $0.04 per share into an aggregate
of 2,500,000 shares (or 2,625,000 shares inclusive of 125,000 shares
underlying $5,000 of accrued interest, through April 10, 2010), which note
is also currently in default,
|
Sales of
a substantial number of shares of our common stock in the public market could
depress the market price of our common stock and impair our ability to raise
capital through the sale of additional equity securities.
In
addition, it is not likely that we will be able to raise convertible debt or
equity financing without issuing shares at or below market rates, which, in
addition to obvious dilution to existing shareholders, would reduce the
conversion and exercise prices of the above securities causing further dilution
to shareholders. Our board of directors has the authority, without
the consent of any of the stockholders, to cause us to issue more shares or our
common stock and shares of our preferred stock at such prices and on such terms
and conditions as are determined by the Board in our sole
discretion.
Moreover,
anti-dilution provisions in warrants have an additional adverse effect on the
Company in that we would be required to reflect the value of such warrants as a
potential liability to the Company. No assurance can be made,
therefore, that we will be able to raise capital, or, if we do, that the same
will not have a material adverse effect on our capitalization or to our balance
sheet.
If
additional funds are raised through the issuance of equity securities, the
percentage of equity ownership of the existing stockholders will be reduced. The
issuance of additional shares of capital stock by us would materially dilute the
stockholders’ ownership in us.
Presently,
we have under-qualified management operating the company.
The
present management team is not experienced enough and sufficient in number to
realize the potential of Shrink. It is very likely that more
qualified additional managers, with significantly more specific experience in
the businesses we seek to engage in, will need to be hired. The
sooner we can hire these people the better. These persons will likely
require substantial salaries and compensation packages that the company cannot
presently afford. Additionally, there may be substantial fees
associated with recruiting new additional or replacement managers. To
the extent that we are unable to ultimately bring managers into the company who
are more qualified than our present team, the company and it’s shareholders will
be negatively impacted.
15
Risks
Relating to an Investment in Our Securities:
Anti-takeover
provisions in our organizational documents and Delaware law may limit the
ability of our stockholders to control our policies and effect a change of
control of our company and may prevent attempts by our stockholders to replace
or remove our current management, which may not be in your best
interests.
There are
provisions in our certificate of incorporation and bylaws that may discourage a
third party from making a proposal to acquire us, even if some of our
stockholders might consider the proposal to be in their best interests, and may
prevent attempts by our stockholders to replace or remove our current
management. These provisions include the following:
|
·
|
Currently,
we have 20 million shares of Series A Preferred Stock outstanding (post
Forward Split) and owned primarily by management, all of which contain
significant anti-takeover and change of control
deterrents. Additionally, our certificate of incorporation
authorizes our board of directors to designate any special priority or
preference or other rights of, and issue up to 5,000,000 additional shares
of “blank check” preferred stock without stockholder approval and to
establish the preferences and rights of any preferred stock issued, which
would allow the board to issue one or more classes or series of preferred
stock that could discourage or delay a tender offer or change in
control;
|
|
·
|
our
certificate of incorporation prohibits our stockholders from filling board
vacancies, calling special stockholder meetings or taking action by
written consent;
|
|
·
|
our
certificate of incorporation provides for the removal of a director only
with cause and by the affirmative vote of the holders of 75% or more of
the shares then entitled to vote at an election of
directors; and
|
|
·
|
our
bylaws require advance written notice of stockholder proposals and
director nominations. Additionally, we are subject to Section 203 of
the Delaware General Corporation Law, which, in general, imposes
restrictions upon acquirers of 15% or more of our stock. Finally, the
board of directors may in the future adopt other protective measures, such
as a stockholder rights plan, which could delay, deter or prevent a change
of control.
|
Compliance
with Sarbanes-Oxley could be time consuming and costly, which could cause our
independent registered public accounting firm to conclude that our internal
control over financial reporting is not effective.
As a
public company, we are required to document and test our internal control
procedures in order to satisfy the requirements of Section 404 of the
Sarbanes-Oxley Act, which will require annual management assessments of the
effectiveness of our internal control over financial reporting and a report by
our independent registered public accounting firm that both addresses
management’s assessment of the effectiveness of internal control over financial
reporting and the effectiveness of internal control over financial
reporting.
During
the course of our testing, we may identify deficiencies which we may not be able
to remediate in time to meet our deadline for compliance with Section 404.
Testing and maintaining internal controls can divert our management’s attention
from other matters that are important to our business. We also expect the new
regulations to increase our legal and financial compliance cost, make it more
difficult to attract and retain qualified officers and members of our Board of
Directors (particularly to serve on an audit committee) and make some activities
more difficult, time consuming and costly. We may not be able to
conclude on an ongoing basis that we have effective internal control over
financial reporting in accordance with Section 404. Our independent
registered public accounting firm may not be able or willing to issue an
unqualified report on the effectiveness of our internal control over financial
reporting.
If we
conclude that our internal control over financial reporting is not effective, we
cannot be certain as to the timing of completion of our evaluation, testing and
remediation actions or their effect on our operations since there is presently
no precedent available by which to measure compliance adequacy.
If we are
unable to conclude that we have effective internal control over financial
reporting or our independent auditors are unable to provide us with an
unqualified report as required by Section 404, then we may be unable to continue
to have our common stock traded on the Over-the-Counter Bulletin Board and
investors could lose confidence in our reported financial information, which
could have a negative effect on the trading price of our stock.
16
Lack
of economic review may interfere with investors’ ability to fully assess merits
and risks associated with purchase of our stock.
The
procurement by prospective investors of an independent review of the investments
merits of a proposed subscription for our stock would be costly and can normally
be conducted only by those prospective investors whose subscriptions will be of
sufficient magnitude that they, either alone or by a pooling of their resources
with those of other prospective investors, could afford the expense of such
independent analysis, including the retaining of independent consultants. Such a
review might or might not prove favorable. Accordingly, subscription
to the stock is suitable only for such proposed investors willing and able to
accept the risks created by a failure to conduct an independent analysis of this
investment.
We
do not anticipate paying cash dividends, which could reduce the value of your
stock.
We have
never paid cash dividends on our common stock and do not anticipate paying cash
dividends in the foreseeable future. Even if we do attain revenues
and become profitable, we intend on reinvesting profits, if any. The
payment of dividends on our common stock will depend on earnings, financial
condition and other business and economic factors affecting it at such time as
our board of directors may consider relevant. Our common stock may be
less valuable because a return on your investment will only occur if our stock
price appreciates.
There
is limited historical information available for investors to evaluate our
performance or a potential investment in our shares.
We are a
development stage company with little historical information available to help
prospective investors evaluate our performance or an investment in our shares,
and our historical financial statements are not necessarily a meaningful guide
for evaluating our future performance because we have not begun to manufacture
and market our products.
Our
common stock does not trade in a mature market and therefore has limited
liquidity.
Our
common stock trades on the over-the-counter market. The average daily
trading volume of our common stock on the over-the-counter market has not been
consistent. Our daily volume remains limited and there is no assurance that
increased volume, if any occurs, will continue. Holders of the our common stock
may not be able to liquidate their investments in a short time period or at the
market prices that currently exist at the time a holder decides to
sell. Because of this limited liquidity, it is unlikely that shares
of our common stock will be accepted by lenders as collateral for
loans.
The
Company’s shareholders may face significant regulatory restrictions relating to
the sale of their stock, as a result of penny stock and similar
rules.
The
Company’s stock differs from many stocks in that it is a “penny stock.” The
Commission has adopted a number of rules to regulate “penny stocks” including,
but not limited to, those rules from the Securities Act as follows:
|
·
|
3a51-1 which
defines penny stock as, generally speaking, those securities which are not
listed on either NASDAQ or a national securities exchange and are priced
under $5, excluding securities of issuers that have net tangible assets
greater than $2 million if they have been in operation at least three
years, greater than $5 million if in operation less than three years, or
average revenue of at least $6 million for the last three
years;
|
|
·
|
15g-1
which outlines transactions by broker/dealers which are exempt from 15g-2
through 15g-6 as those whose commissions from traders are lower than 5%
total commissions;
|
|
·
|
15g-2
which details that brokers must disclose risks of penny stock on Schedule
15G;
|
|
·
|
15g-3
which details that broker/dealers must disclose quotes and other
information relating to the penny stock
market;
|
|
·
|
15g-4
which explains that compensation of broker/dealers must be
disclosed;
|
|
·
|
15g-5
which explains that compensation of persons associated in connection with
penny stock sales must be
disclosed;
|
|
·
|
15g-6
which outlines that broker/dealers must send out monthly account
statements; and
|
|
·
|
15g-9
which defines sales practice
requirements.
|
17
Since the
Company’s securities constitute a “penny stock” within the meaning of the rules,
the rules would apply to us and our securities. Because these rules provide
regulatory burdens upon broker-dealers, they may affect the ability of
shareholders to sell their securities in any market that may develop; the rules
themselves may limit the market for penny stocks. Additionally, the market among
dealers may not be active. Investors in penny stock often are unable to sell
stock back to the dealer that sold them the stock. The mark-ups or commissions
charged by the broker-dealers may be greater than any profit a seller may make.
Because of large dealer spreads, investors may be unable to sell the stock
immediately back to the dealer at the same price the dealer sold the stock to
the investor. In some cases, the stock may fall quickly in value. Investors may
be unable to reap any profit from any sale of the stock, if they can sell it at
all. Shareholders should be aware that, according to Commission Release No.
34-29093 dated April 17, 1991, the market for penny stocks has suffered
from patterns of fraud and abuse. These patterns include:
|
·
|
control
of the market for the security by one or a few broker-dealers that are
often related to the promoter or
issuer;
|
|
·
|
manipulation
of prices through prearranged matching of purchases and sales and false
and misleading press releases;
|
|
·
|
“boiler
room” practices involving high pressure sales tactics and unrealistic
price projections by inexperienced sales
persons;
|
|
·
|
excessive
and undisclosed bid-ask differentials and markups by selling
broker-dealers; and
|
|
·
|
the
wholesale dumping of the same securities by promoters and broker-dealers
after prices have been manipulated to a desired level, along with the
inevitable collapse of those prices with consequent investor
losses.
|
Our
previous Audiostocks related businesses have dramatically diminished and we do
not intend to pursue them in any significant manner. Our Shrink subsidiary is a
research and development startup company and has not generated any operating
revenues and may never achieve profitability, and we have yet to commercialize
its inventions.
We sold
the assets relating to our AudioStocks internet based investor relations
business in late 2008, with the exception of certain proprietary software which
we intend to sell in the future, if possible. Therefore, we do not
have any revenues or significant assets from our prior businesses.
Our
recently acquired subsidiary, Shrink, is a risky and unproven
development stage company and, to date, has not generated any revenues from
sales. We cannot assure you that we will generate revenues or that we
can achieve or sustain profitability in the future. Our operations
are subject to the risks and competition inherent in the establishment of a
business enterprise. There can be no assurance that future operations will be
profitable. Revenues and profits, if any, will depend upon various
factors, including whether our product development can be completed, and if we
can achieve market acceptance. We may not achieve our business
objectives and the failure to achieve such goals would have an adverse impact on
us.
Risks
Related To Development And Commercialization Of Our Products:
Our
technologies are unproven and new. No assurance can be made that our
technologies will work as anticipated, or, even if they do work, will be
commercially accepted.
We are a
primarily R&D company with a newly developing and novel manufacturing
technology. We do not have agreements with purchasers yet and no
assurance can be made that we will be able to make our technology
viable. Even if we are able to continue to fund our research and
development and make our technologies viable, no assurance can be made that our
chips will attain market acceptance in light of the larger, more established
competitors in the life sciences and solar industries. If we are
unable to raise sufficient capital or commercialize our unproven products, our
stock price will decline and you will be adversely affected.
If
we are unable to obtain required clearances or approvals for the
commercialization of our products in the United States, we may not be able to
sell products and our sales could be adversely affected.
Our
future performance depends on, among other matters, our estimates as to when and
at what cost we will receive regulatory approval for our products. Regulatory
approval can be a lengthy, expensive and uncertain process, making the timing,
cost and ability to obtain approvals difficult to predict.
In the
United States, clearance or approval to commercially distribute new medical
devices is received from the FDA through clearance of a Premarket Notification,
or 510(k), or through approval of a Premarket Approval, or PMA. To receive
510(k) clearance, a new product must be substantially equivalent to a medical
device first marketed in interstate commerce prior to May 1976.
The FDA
may determine that a new product is not substantially equivalent to a device
first marketed in interstate commerce prior to May 1976 or that additional
information is needed before a substantial equivalence determination can be
made.
A “not
substantially equivalent” determination, or a request for additional
information, could prevent or delay the market introduction of new products that
fall into this category. The 510(k) clearance and PMA review processes can be
expensive, uncertain and lengthy. It generally takes from three to five months
from submission to obtain 510(k) clearance, and from six to eighteen months from
submission to obtain a PMA approval; however, it may take longer, and 510(k)
clearance or PMA approval may never be obtained.
Modifications
or enhancements that could significantly affect safety or effectiveness, or
constitute a major change in the intended use of the device, require new 510(k)
or PMA submissions.
18
We
may suffer the loss of key personnel or may be unable to attract and retain
qualified personnel to maintain and expand our business.
Our
success is highly dependent on the continued services of a limited number of
skilled scientists, especially, Dr. Michelle Khine and Dr. Heiner
Dreismann. The company does not have an agreement for employment or
consulting with Dr. Khine. We have a consulting agreement with Dr.
Dreismann. In addition, our success will depend upon, among other
factors, our ability to attract, retain and motivate qualified research and
development, engineering and operating personnel, generally and during periods
of rapid growth, especially in those areas of our businesses focused on new
products and advanced manufacturing processes. There can be no assurance that we
will be able to retain existing employees or to attract and retain additional
personnel on acceptable terms. The competition for qualified
personnel in industrial, academic and nonprofit research sectors is
significant.
The loss
of the services of any of our key research and development, engineering or
operational personnel or senior management without adequate replacement, or the
inability to attract new qualified personnel, would have a material adverse
effect on our operations.
Significant existing or additional
governmental regulation could subject us to unanticipated delays and costs,
which would adversely affect our revenues.
Our
products are still in the development stage. However certain products
which we may produce may be subject to government regulation depending upon
their ultimate use. The successful implementation of our business strategy
depends in part on our ability to get our products into the market as quickly as
possible. Additional laws and regulations, or changes to existing laws and
regulations, applicable to our business may be enacted or promulgated and the
interpretation, application or enforcement of existing laws and regulations may
change. We cannot predict the nature of any future laws, regulations,
interpretations, applications or enforcements, or the specific effects any of
these might have on our business. Any future laws, regulations, interpretations,
applications, or enforcements could delay or prevent regulatory approval or
clearance of our products and our ability to market our products. Moreover, if
we do not comply with existing or future laws or regulations, we could be
subject to the following types of enforcement actions by the FDA and other
agencies:
|
·
|
Fines;
|
|
·
|
Injunctions;
|
|
·
|
Civil
penalties;
|
|
·
|
Recalls
or seizures of our products;
|
|
·
|
Total
or partial suspension of the production of our
products;
|
|
·
|
Withdrawal
of existing approvals or premarket clearances of our
products;
|
|
·
|
Refusal
to approve or clear new applications or notices relating to our
products;
|
|
·
|
Recommendations
by the FDA that we not be allowed to enter into government contracts;
and
|
|
·
|
Criminal
prosecution.
|
19
We
rely in part, on UC Merced and the UC Regents to file and secure our
patents.
In
addition to the option agreement with the UC Regents, Shrink has entered into a
research and development agreement with UC Merced in order to fund certain
research based on the intellectual property we have licensed and have the right
to license from the UC Regents. The agreement with UC Merced provides
Shrink with an exclusive opportunity to license any technology that flows
(derivative IP) from the research Shrink funds at UC Merced. Should
any intellectual property flow from this research and development agreement and
Shrink elects to exercise such rights, it will be required to make annual
payments to the UC Regents as well as royalty payments as commercialization
occurs.
Under
our research and development agreements we rely on third party laboratories that
can experience manufacturing problems or delays, which could result in decreased
revenue or increased costs.
All of
our research and development agreements require processes that are complex and
require specialized and expensive equipment. Replacement parts for this
specialized equipment can be expensive and, in some cases, can require lead
times of up to a year to acquire. There can be no assurance that our funding
commitment under the UC Merced research and development agreements will be
adequate to fund the equipment requirements. We also rely on numerous
third parties to supply production materials and in some cases there may not be
alternative sources immediately available, or they may not be available at a
reasonable cost.
We
will be subject to applicable regulatory approval requirements of the foreign
countries in which we could sell products, which are costly and may prevent or
delay us from marketing our products in those countries.
In
addition to regulatory requirements in the United States, we will be subject to
the regulatory approval requirements for each foreign country to which could
export our products. In the European Union, regulatory compliance requires
affixing the “CE” mark to product labeling. Although our new products could be
made eligible for CE marking through self-certification, this process can be
lengthy and expensive. In Canada, as another example, our new products could
require approval by Health Canada prior to commercialization along with
International Standards Organization, or ISO, 13485/CMDCAS certification. It
generally takes from three to six months from submission to obtain a Canadian
Device License. Any changes in foreign approval requirements and processes may
cause us to incur additional costs or lengthen review times of our new products,
if any. We may not be able to obtain foreign regulatory approvals on a timely
basis, if at all, and any failure to do so may cause us to incur additional
costs or prevent us from marketing our products in foreign countries, which may
have a material adverse effect on our business, financial condition and results
of operations.
We
may experience difficulties that may delay or prevent our development,
introduction or marketing of new or enhanced products.
We intend
to continue to invest in product and technology development. The development of
new or enhanced products is a complex and uncertain process. We may experience
research and development, manufacturing, marketing and other difficulties that
could delay or prevent our development, introduction or marketing of new
products or enhancements. We cannot be certain that:
· any of
the products under development will prove to be effective in generating sales
demand;
|
·
|
we
will be able to obtain, in a timely manner or at all, regulatory approval,
if required, to market any of our products that are in development or
contemplated; and
|
|
·
|
the
products we develop can be manufactured at acceptable cost and with
appropriate quality; or these products, if and when approved, can be
successfully marketed.
|
The
factors listed above, as well as manufacturing or distribution problems, or
other factors beyond our control, could delay new product launches. In addition,
we cannot assure you that the market will accept these
products. Accordingly, there is no assurance that our overall revenue
will increase if and when new products are launched.
20
Intense
competition could limit our ability to secure market share which could impair
our ability to sell our products and harm our financial
performance.
The
microfluidics, biochip and renewable energy industries, along with nearly every
market we seek to penetrate, are new and underdeveloped markets and industries
are rapidly evolving, and developments are expected to continue at a rapid pace.
Competition in this industry, which includes competition from professional
diagnostic, consumer diagnostic and renewable energy businesses, among others,
is intense and expected to increase as new products and technologies become
available and new competitors enter the market. Our competitors in the United
States and abroad are numerous and include, among others, diagnostic testing,
medical products and renewable energy companies, universities and other research
institutions.
Our
future success depends upon maintaining a competitive position in the
development of products and technologies in our areas of focus. Our competitors
may:
|
·
|
develop
technologies and products that are more effective, less expensive, safer
or more readily available than our products or that render our
technologies or products obsolete or
noncompetitive;
|
|
·
|
obtain
patent protection or other intellectual property rights that would prevent
us from developing potential
products; or
|
|
·
|
obtain
regulatory approval for the commercialization of our products more rapidly
or effectively than we do.
|
As a
result, our products or processes may not compete successfully, and research and
development by others may render our products or processes obsolete or
uneconomical.
Also, the
possibility of patent disputes with competitors holding domestic and/or foreign
patent rights may limit or delay expansion possibilities. In addition, many of
our existing or potential competitors have or may have substantially greater
research and development capabilities, clinical, manufacturing, regulatory and
marketing experience and financial and managerial resources, giving them a
competitive advantage in the markets in which we hope to operate.
The
market for the sale of our products is also highly competitive. This competition
is based principally upon price, quality of products, customer service and
marketing support. We believe that a number of our competitors are substantially
larger than we are and have greater financial resources, and have a more
established sales and marketing force already operating in the market, which
would give them a competitive advantage over us.
We must be able to manufacture new
and improved products to meet customer demand on a timely and cost-effective
basis.
We do not
have any manufacturing facilities. We are negotiating with third parties for the
development and manufacture of our products initially. However, even
if we are able to develop our products, and penetrate the drug and life science
industries, we may have to construct or acquire a manufacturing facility or
utilize third parties, both of which would require us to train appropriate
personnel. To build a manufacturing facility, we will need
substantial additional capital and we do not have any capital commitments to
fund such construction. In addition, the recent financial crisis has
made it extremely difficult to obtain commercial property or industrial
financing. In addition, we must be able to resolve in a timely manner
manufacturing issues that may arise from time to time as we commence production
of our complex products. With a start up manufacturing facility,
unexpected problems may be more frequent as new equipment is put on line and
operators are inexperienced in its operation. These and other
unanticipated difficulties or delays in manufacturing our products during
commercialization, in sufficient quantities to meet customer demand could
diminish future demand for our products, cause us to incur greater debt and
materially harm our business.
A significant portion of our sales
will be dependent upon our customers’ capital spending policies and research and
development budgets, and government funding of research and development programs
at universities and other organizations, which are subject to significant and
unexpected fluctuations.
Our
target markets include pharmaceutical and biotechnology companies, academic
institutions, government laboratories, and private foundations. Fluctuations in
the research and development budgets at these organizations could have a
significant effect on the demand for our products and services. Research and
development budgets fluctuate due to changes in available resources, mergers of
pharmaceutical and biotechnology companies, spending priorities, general
economic conditions, and institutional and governmental budgetary policies. Our
business could be seriously damaged by any significant decrease in capital
equipment purchases or life sciences research and development expenditures by
pharmaceutical and biotechnology companies, academic institutions, government
laboratories, or private foundations.
The
timing and amount of revenues from customers that rely on government funding of
research may vary significantly due to factors that can be difficult to
forecast. Research funding for life science research has increased
more slowly during the past several years compared to the previous years and has
declined in some countries, and some grants have been frozen for extended
periods of time or otherwise become unavailable to various institutions,
sometimes without advance notice. Although the level of research funding
increased significantly during the years 1999 through 2003, increases for fiscal
2004 through 2008 were significantly lower. Government funding of research and
development is subject to the political process, which is inherently fluid and
unpredictable. Other programs, such as homeland security or defense, or general
efforts to reduce the federal budget deficit could be viewed by the
U.S. government as a higher priority. These budgetary pressures may result
in reduced allocations to government agencies that fund research and development
activities. Past proposals to reduce budget deficits have included reduced NIH
and other research and development allocations. Any shift away from
the funding of life sciences research and development or delays surrounding the
approval of government budget proposals may seriously damage our
business.
21
Many
of our current and potential competitors have longer operating histories, larger
customer or user bases, greater brand recognition, greater access to brand name
suppliers, and significantly greater financial, marketing and other resources
than we do.
Many of
these current and potential competitors can devote substantially more resources
to the development of their business operations than we can at
present. Currently, we do not have a marketing or manufacturing
infrastructure in place. In addition, larger, well-established
and well-financed entities may acquire, invest in or form joint ventures with
other established competitors or with specific product manufacturers, which will
allow them pricing advantages due to economies of scale or pursuant to
distribution agreements with suppliers. Some large product distributors may also
have exclusive distribution agreements or protected territories in which they
can sell specific brand name products at a significant discount, or territories
in which they may seek to exclude us from selling a specific brand of product.
These types of arrangements between our competitors and manufacturers and
suppliers may limit our ability to distribute certain products and could
adversely affect our revenues.
There
can be no assurance that new products we introduce will achieve significant
market acceptance or will generate significant revenue.
The
market for products in micro-fluidics, bio-sensing and renewable energy
industries is characterized by rapid technological advances, evolving standards
in technology and frequent new product and service introductions and
enhancements. Possible short life cycles for products we sell may necessitate
high levels of expenditures for continually selecting new products and
discontinuing the sale of obsolete product lines. To obtain a competitive
position, we must continue to introduce new products and new versions of
existing products that will satisfy increasingly sophisticated customer
requirements and achieve market acceptance. Our inability or failure
to position and/or price our new or existing products competitively, in response
to changes in evolving standards in technology, could have a material adverse
effect on our business, results of operations or financial
position.
If we deliver products with defects,
our credibility may be harmed, market acceptance of our products may decrease
and we may be exposed to liability.
The
manufacturing and marketing of our products will involve an inherent risk of
product liability claims. In addition, our product development is and production
will be extremely complex and could expose our products to defects. Any defects
could harm our credibility and decrease market acceptance of our products. In
the event that we are held liable for a claim for which we are not indemnified
or insured that claim could materially damage our business and financial
condition. Even if we are indemnified, we may not be able to obtain
reimbursement of our damages from the indemnifying party, because for example
they do not have sufficient funds or insurance coverage to pay us. If
a claim is made for which we have insurance coverage, we can not be certain that
the amount of such coverage will be sufficient, or that we will be able to
collect on a claim when we make it.
Because
we have not yet begun to sell our products, we have not obtained insurance
covering product liability claims or product recall claims against
us. When we begin to market our products, such insurance may not be
available to us, or not available on terms which we find
acceptable. We may determine that the cost of such insurance is
too high when compared to the risks of not having coverage and we may determine
not to obtain policies insuring such risks, or we may obtain policies with a
high deductible.
22
If we are not able to adequately
protect our intellectual property, we may not be able to compete
effectively.
Our
success depends, to a significant degree, upon the protection of our proprietary
technologies. While we currently license certain U.S. patents and patent
applications from UC Merced, we may need to pursue additional protections for
our intellectual property as we develop new products and enhance existing
products and technologies.
We may
not be able to obtain appropriate protections for our intellectual property in a
timely manner, or at all. Our inability to obtain appropriate protections for
our intellectual property may allow competitors to enter our markets and produce
or sell the same or similar products.
The risks
and uncertainties that we face with respect to our patents and other proprietary
rights include the following:
|
·
|
the
pending patent applications we have filed or to which we have exclusive
rights may not result in issued patents or may take longer than we expect
to result in issued patents;
|
|
·
|
the
claims of any patents which are issued may not provide meaningful
protection;
|
|
·
|
we
may not be able to develop additional proprietary technologies that are
patentable; the patents licensed or issued to us or our customers may not
provide a competitive advantage;
|
|
·
|
other
parties may challenge patents or patent applications licensed or issued to
us or our customers;
|
|
·
|
patents
issued to other companies may harm our ability to do
business; and
|
|
·
|
other
companies may design around technologies we have patented, licensed or
developed.
|
We may
need to obtain licenses to patents or other proprietary rights from third
parties. We may not be able to obtain the licenses required under any patents or
proprietary rights, or they may not be available on acceptable terms. If we do
not obtain required licenses, we may encounter delays in product development or
find that the development, manufacture or sale of products requiring licenses
could be foreclosed.
We may,
from time to time, support and collaborate in research conducted by universities
and governmental research organizations, such as UC Merced, UC Irvine and MF3 at
UC Irvine. We may not be able to acquire exclusive rights to the inventions or
technical information derived from these collaborations, and disputes may arise
over rights in derivative or related research programs conducted by us or our
collaborators.
In
addition to patents, we rely on a combination of trade secrets, nondisclosure
agreements and other contractual provisions and technical measures to protect
our intellectual property rights. Nevertheless, these measures may
not be adequate to safeguard the technology underlying our products. If these
measures do not protect our rights, third parties could use our technology and
our ability to compete in the market would be reduced. In addition, employees,
consultants and others who participate in the development of our products may
breach their agreements with us regarding our intellectual property, and we may
not have adequate remedies for the breach.
We also
may not be able to effectively protect our intellectual property rights in some
foreign countries.
In order
to protect or enforce our patent rights, we may initiate patent litigation
against third parties, such as infringement suits or interference
proceedings. Litigation may be necessary to:
|
·
|
assert
claims of infringement;
|
|
·
|
enforce
our patents;
|
|
·
|
protect
our trade secrets or
know-how; or
|
|
·
|
determine
the enforceability, scope and validity of the proprietary rights of
others.
|
Any
lawsuits that we initiate could be expensive, take significant time and divert
management’s attention from other business concerns. Litigation also puts our
patents, if any, at risk of being invalidated or interpreted narrowly and our
patent applications at risk of not issuing. Additionally, we may provoke third
parties to assert claims against us.
Litigation
may be necessary to enforce intellectual property rights. Litigation is
inherently uncertain and the outcome is often unpredictable. Patent law relating
to the scope of claims in the technology fields in which we operate is still
evolving and, consequently, patent positions in our industry are generally
uncertain. We may not prevail in any and the damages or other remedies awarded,
if any, may not be commercially valuable.
For a
variety of reasons, for example, because we do not have adequate funds, we may
decide not to file for patent, copyright or trademark protection or prosecute
potential infringements of our patents. Our trade secrets may also become known
through other means not currently foreseen by us. Despite our efforts to protect
our intellectual property, our competitors or customers may independently
develop similar or alternative technologies or products that are equal or
superior to our technology and products without infringing on any of our
intellectual property rights or design around our proprietary technologies.
Changes in laws concerning intellectual property would affect our ability to
protect our intellectual property, if any.
23
We could suffer monetary damages,
incur substantial costs or be prevented from using technologies important to our
products as a result of a legal proceedings being commenced against
us.
Because
of the nature of our business, we may be subject at any particular time to
commercial disputes, consumer product claims, negligence or various other
lawsuits arising in the ordinary course of our business, including employment
matters, and we expect that this will continue to be the case in the future.
Such lawsuits generally seek damages, sometimes in substantial amounts, for
commercial or personal injuries allegedly suffered and can include claims for
punitive or other special damages. An adverse ruling or rulings in one or more
such lawsuits could, individually or in the aggregate, have a material adverse
effect on our sales, operations or financial performance. We cannot
assure you that any future lawsuits relating to our businesses will not have a
material adverse effect on us.
Risks
Relating to Our Operations:
We are currently in a growth stage
and may experience setbacks in both business and product
development.
We are
subject to all of the risks inherent in both the creation of a new business and
the development of new and existing products. Our cash flows may be
insufficient to meet expenses relating to our operations and the growth of our
business, and may be insufficient to allow us to develop new and existing
products. We currently so not manufacture or market any product and we cannot be
certain that we will ever be able to develop, manufacture, market or sell any
product.
We
currently do not have adequate insurance coverage for claims against
us.
We face
the risk of loss resulting from product liability, securities, fiduciary
liability, intellectual property, antitrust, contractual, warranty,
environmental, fraud and other lawsuits, whether or not such claims are
valid. In addition, we do not have adequate or in some cases, any
product liability, fiduciary, directors and officers, property, natural
catastrophe and comprehensive general liability insurance. To the
extent we secure adequate insurance it may not be adequate to cover such claims
or may not be available to the extent we expect. If we are able to secure
adequate insurance our costs could be volatile and, at any time, can increase
given changes in market supply and demand. We may not be able to obtain adequate
insurance coverage in the future at acceptable costs. A successful
claim that exceeds or is not covered by our policies could require us to pay
substantial sums. Even to the extent we are able to acquire adequate
insurance, we may not be able to afford to continue coverage through a policy
period or in multiple and successive policy periods.
Although
we have implemented safeguards to prevent unauthorized access to our ecommerce
sites, there always exists certain security risks, which may cause
interruptions, delays or cessation in service.
Despite
the implementation of security measures, our network infrastructure may be
vulnerable to computer viruses or problems caused by third parties, which could
lead to interruptions, delays or cessation in service to our clients.
Inappropriate use of the Internet by third parties could also potentially
jeopardize the security or deter certain persons from using our services. Such
inappropriate use of the Internet would include attempting to gain unauthorized
access to information or systems - commonly known as "cracking" or "hacking."
Although we intend to continue to implement security measures, such measures
have been circumvented in the past, and there can be no assurance that measures
implemented will not be circumvented in the future. Alleviating problems caused
by computer viruses or other inappropriate uses or security breaches may require
interruptions, delays or cessation in service to our operations. There can be no
assurance that customers or others will not assert claims of liability against
us as a result of failures. Further, until more comprehensive security
technologies are developed, the security and privacy concerns of existing and
potential customers may inhibit the growth of the Internet service industry in
general and our customer base and revenues in particular.
24
|
ITEM
1B.
|
UNRESOLVED
STAFF COMMENTS
|
Not
Applicable.
|
ITEM
2.
|
PROPERTIES
|
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010.
We
believe that all our properties have been adequately maintained, are generally
in good condition, and are suitable and adequate for our business.
|
ITEM
3.
|
LEGAL
PROCEDINGS
|
From time
to time, we may become involved in various lawsuits and legal proceedings which
arise in the ordinary course of business, in particular, that may relate to
defense of our intellectual property rights. However, litigation is
subject to inherent uncertainties, and an adverse result in these or other
matters may arise from time to time that may harm our business. We
are currently not aware of any such legal proceedings or claims that we believe
will have a material adverse affect on our business, financial condition or
operating results.
|
|
[Remainder
of Page Intentionally Left Blank]
25
PART
II
|
ITEM 4.
|
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER
PURCHASES OF EQUITY SECURITIES.
|
Market
Information
As a
result of our 5 for 1 Forward Split, since April 12, 2010, our common stock has
been quoted under the symbol INKN on the OTC Bulletin Board
system. Our shares trade on a limited and sporadic basis and the
trading price of our shares is not necessarily of the existence of a trading
market for our securities or indicative of our value. The following
table sets forth, for the periods indicated, the high and low closing prices of
our common stock. These prices reflect inter-dealer prices, without
retail mark-up, mark-down or commission as adjusted to reflect to Forward Split,
and may not represent actual transactions. As of April 12, 2010,
there was not a bid and ask price of our common stock available, the most recent
trading price of $0.17 on March 22, 2010.
|
Closing Bid Prices(1)
|
||||
|
High
|
Low
|
|||
|
Quarter
Ended December 31, 2010
|
$
|
0.254
|
0.102
|
|
|
Year Ended
December 31, 2009
|
||||
|
4th
Quarter
|
$
|
0.256
|
$
|
0.190
|
|
3rd
Quarter
|
0.256
|
0.202
|
||
|
2nd
Quarter
|
0.250
|
0.102
|
||
|
1st
Quarter
|
0.204
|
0.01
|
||
|
Year
Ended December 31, 2008
|
||||
|
4th
Quarter
|
$
|
0.200
|
$
|
0.200
|
|
3rd
Quarter
|
0.200
|
0.050
|
||
|
2nd
Quarter
|
0.350
|
0.050
|
||
|
1st Quarter
|
0.350
|
0.350
|
||
(1) The
above table sets forth the range of high and low closing bid prices per share of
our common stock as reported by www.quotemedia.com for the periods indicated, as
adjusted for the 5 for 1 Forward Split in April of 2010.
Approximate
Number of Holders of Our Common Stock
As of
April 12, 2010, there were 222 holders of record of our common
stock. This number excludes the shares of our common stock owned by
stockholders holding stock under nominee security position listings. In
aggregate, we had 191,303,985 shares of common stock outstanding as of April 12,
2010.
Dividends
We have
never declared or paid a cash dividend and do not foresee paying one in the near
future. Any future decisions regarding dividends will be made by our board
of directors. We currently intend to retain and use any future
earnings for the development and expansion of our business and do not anticipate
paying any cash dividends in the foreseeable future. Our board of
directors has complete discretion on whether to pay dividends, subject to the
approval of our stockholders. Even if our board of directors decides
to pay dividends, the form, frequency and amount will depend upon our future
operations and earnings, capital requirements and surplus, general financial
condition, contractual restrictions and other factors that the board of
directors may deem relevant.
Securities
Authorized for Issuance Under Equity Compensation Plans
The
company does not full time employees and does not have any stock option plan,
whether approved by shareholders or otherwise, however, the Company does, on
occasion issued equity or options as compensation to consultants. Additional
detailed information relating to stock and option grants during 2009 can be
found below in “Item 12, Security Ownership of Certain Beneficial Owners and
Management and Related Stockholder Matters — Securities Authorized for Issuance
Under Equity Compensation Plans.”, the provisions of which are incorporated
herein.
26
Recent
Sales of Unregistered Securities
Private
Placement of 12% Convertible Notes and Series A Warrants
Between
November 2009 and March of 2010, we sold in a private placement to 7 investors,
$435,000 in principal amount of 12% convertible notes (the “12% Notes”),
exercisable initially into 4,350,000 shares of common stock (subject to
adjustment) at $.10 per share and due one year from issuance, and Series A
Common Stock Purchase Warrants to purchase 2,175,000 shares, exercisable at $.20
per share, exercisable 6 months after issuance and expiring three years from
issuance (the “Series A Warrants”). The Notes and Series A Warrants
were sold at face value for each dollar amount of Notes sold. These
12% Notes and Series A Warrants contain various anti dilution and similar
protective provisions which could be dilutive to investors. No placement agent
compensation has been paid in this financing.
The terms
of the 12% convertible notes issued provide that they are initially convertible
at $.10 (as adjusted for the Forward Split) and that:
|
·
|
Investors
in notes have a right to invest outstanding principal and interest in the
notes for the purchase price of new securities in any new private equity
linked financing while the notes are
outstanding,
|
|
·
|
The
Company may, at any time up to 30 days prior to maturity, offer
noteholders right to early repayment if the company enters into and closes
an equity based financing with net proceeds of not less than $6 million of
net proceeds to the Company,
|
|
·
|
In
the event of default the conversion price is reduced to 80% of the then
existing conversion price in effect and, after such default, the Company
may mandate conversion as said discounted rate, as an alternative to
paying cash.
|
The
foregoing is a summary only of the 12% Note and Series A Warrant, a copy of the
forms of which, along with the form of subscription agreement entered into with
investors in this offering, are each annexed as exhibits to this Annual Report,
the provisions of which are incorporated herein.
All of
the issuances of securities described above were restricted share issuances and
deemed to be exempt from registration in reliance on Rule 506 of Regulation D
and/or Section 4(2) of the Securities Act as transactions by an issuer not
involving a public offering. Each investor represented that they were
accredited investors, as defined in Rule 501 of Regulation D and, there was no
general solicitation or general advertising used to market the securities.
We made available to each investor with disclosure of all aspects of our
business, including providing the investor with press releases, access to our
auditors, and other financial, business, and corporate
information. All securities issued were restricted with an
appropriate restrictive legend on certificates for notes and warrants issued
stating that the securities (and underlying shares) have not been registered
under the Securities Act and cannot be sold or otherwise transferred without an
effective registration or an exemption therefrom.
Issuances
to Consultants for Services Rendered
As part
of the Company’s business plan, it regularly elects to pay consulting fees in
stock, as opposed to shares, so as to conserve capital. In
particular, during March of 2010, the Company made the following 18,382,500
issuances of restricted stock to consultants and professional service
providers:
|
·
|
7,250,000
shares were issued to a consultant in satisfaction of a consulting
agreement entered into in January, 2010, for public relations, marketing
and structuring services;
|
|
·
|
8,700,000
shares were issued to a consultant in satisfaction of a consulting
agreement entered into in January of 2010, for public relations,
assistance with press releases and branding, research and web/statistical
content and investor relations;
|
|
·
|
1,500,000
shares were issued to a consultant in satisfaction of a consulting
agreement entered into in February of 2010, for services relating to
marketing of our medical device products, and marketing to researches,
academia and medical professionals;
|
|
·
|
600,000
shares were issued to an accounting professional for professional
services;
|
|
·
|
40,000
shares (in addition to 60,000 shares issued in November 2010) were issued
as payment for professional services
rendered;
|
|
·
|
180,000
shares were issued to a consultant in satisfaction of a consulting
agreement entered into in March 2010, for services rendered;
and
|
|
·
|
112,500
shares were issued to a member of the Scientific Advisory board, pursuant
to an agreement dated as of March 1,
2010.
|
27
The
Company reasonably believes that all of the issuances of securities described
above were deemed to be exempt from registration in reliance on Section 4(2) of
the Securities Act as transactions by an issuer not involving a public offering.
Each investor had a pre-existing relationship with the Company,
represented that it had the financial wherewithal, knowledge and sophistication
to invest in the securities of the Company and only legended restricted
securities were issued. In addition, each recipient represented that
they are acquiring the securities as an investment only and not with a view
towards distribution of the same to the public. We made available to
each investor with disclosure of all aspects of our business, including
providing the investor with press releases, access to our auditors, and other
financial, business, and corporate information. All securities issued
were restricted with an appropriate restrictive legend on certificates for notes
and warrants issued stating that the securities (and underlying shares) have not
been registered under the Securities Act and cannot be sold or otherwise
transferred without an effective registration or an exemption
therefrom.
Purchases
of Equity Securities
No
repurchases of our common stock were made during the fourth quarter of
2009.
ITEM
5. SELECTED FINANCIAL DATA.
Not
Applicable.
|
|
[Remainder
of Page Intentionally Left Blank]
28
|
ITEM 6.
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
The
following discussion and analysis should be read in conjunction with our
unaudited financial statements and related notes included in this report and the
“Forward Looking Statements” section in the forepart of this Report (see above)
and the “Risk Factors” set forth in Part II of this Report, as may be amended or
updated from time to time. The statements contained in this Report
that are not historic in nature, particularly those that utilize terminology
such as “may,” “will,” “should,” “expects,” “anticipates,” “estimates,”
“believes,” or “plans” or comparable terminology are forward-looking statements
based on current expectations and assumptions. These statements are
based on current information available to management. No assurance
can be made that any of our forward looking statements will materialize as
planned.
Various
risks and uncertainties could cause actual results to differ materially from
those expressed in forward-looking statements. Factors that could
cause actual results to differ from expectations include, but are not limited
to, those set forth under the sections “Forward Looking Statements” above, and
“Risk Factors” and other business risks and information, set forth herein, as
well as the “Business” section above.
Background
The
Company, originally named Jupiter Processing Inc. incorporated under the laws of
the State of Delaware on January 22, 2002. We changed our name to
Audiostocks, Inc. on January 13, 2005. Effective May 14, 2009, we
changed our name to Shrink Nanotechnologies, Inc., in contemplation of our
acquisition of Shrink Technologies, Inc., a California corporation, or Shrink,
which was completed on May 29, 2009 and is referred to herein as the “Shrink
Acquisition”.
Prior to
the Shrink Acquisition, the Company was operating solely as an Internet-based
publishing platform designed to compile, catalogue, distribute and make
functional, financial content and data related to that content. Due
to certain regulatory changes in the securities and financial markets following
the Sarbanes-Oxley Act of 2002, as well as the most recent global financial
crisis and resulting limits on smaller public company budgets dedicated towards
public relations, our ability to find paying customers for our internet
publishing services has been severely challenged. While the Company
has remaining operations in this sector, including the vertical search business
through StockVert.com, it intends to wind it down and, if possible, liquidate or
sell its previous internet publishing business and remaining assets used in this
sector. The Company intends on liquidating any remaining assets relating to its
AudioStocks business.
Shrink
As Accounting Acquirer
As a
result of our Shrink Acquisition from its former shareholder Mr. Marshall Khine,
we have succeeded to the business and research and development operations of
Shrink, which now constitute our primary asset and
operations. Accordingly, Shrink is deemed the financial acquirer for
accounting related purposes and, the financial statements presented in Part I of
this Report, and in this Management Discussion and Analysis of Financial
Condition and Results of Operations, are those of Shrink, unless the context
requires otherwise.
Summary
of Shrink Acquisition Terms
We
acquired 100% ownership of Shrink from its sole shareholder, Marshall
Khine. Shrink’s (and therefore our) primary asset is a license
agreement with the Regents of the UC Regents granting Shrink intellectual
property rights in patent rights held by the UC Regents. In addition
Shrink owns certain trademarks and other intellectual property.
As
consideration for the acquisition of Shrink, the Company issued an aggregate of
44,444,440 shares of its common stock and 50 shares of its Series C Preferred
Stock, which has since been cancelled. In addition, the Company (i)
assumed pursuant to the terms of a note exchange agreement, the outstanding
notes due to Noctua Fund LP, which had an aggregate currently outstanding
balance (at the time) of approximately $118,121, (ii) agreed to issue 495,500
shares of common stock to the UC Regents as the initial consideration under
Shrink’s license agreement with it (iii) provided Seller with one seat on the
Company’s Board of Directors which went to Mr. Marshall Khine, Esq., and (iv)
agreed to comply with all outstanding commitments made by Shrink and its
consultants, including members of Shrink’s Scientific Advisory Board and its
Business Advisory Board. Additional information relating the Shrink
Acquisition and Shrink itself is contained in our Current Report on Form 8-K,
Filed on June 5, 2009, and exhibits thereto the provisions of which are
incorporated herein.
Plans
for Preexisting Operations
Prior to
and since the Shrink Acquisition we were operating and looking to liquidate, our
investor relations and other internet businesses. We intend on
selling or licensing our previous investor relations and related internet
business (i.e. the AudioStocks business) software and do not intend to continue
operations in that operating segment any longer. No assurance can be
made that we will be able to do so, or, if we do so, that it will be at
favorable terms.
Because
we were operational and have revenues from non Shrink related operations in
previous periods prior to the Shrink Acquisition, certain portions of this
Report and specifically, this Management Discussion and Analysis of Financial
Condition and Results of Operations, also contains information that may relate
to our previous, non-Shrink related operations, which are no longer material to
our business. As our operations switched to those of Shrink as of May
29, 2009 with Shrink as the surviving accounting acquirer, and since Shrink is a
development stage company, a comparison for our pre-Shrink Acquisition business
revenues and operations with those of Shrink’s would is necessarily be
appropriate or meaningful in this Report. Moreover, as Shrink on its
own has limited operations or history, there is little historic information upon
which shareholders may assess our business prospects.
29
Recent
Events
Forward
Split and Increase in Capitalization
Effective
as of April 8, 2010, we filed a Certificate of Amendment to our Certificate of
Incorporation (the “Certificate of Amendment”) which both:
|
·
|
increased
our authorized capital from 100,000,000 shares of which 95,000,000 shares
are common stock, par value $0.001 per share and 5,000,000 are preferred
stock, par value $0.001per share, to 500,000,000 shares, of which
475,000,000 are shares of Common Stock, par value $0.001 per share and
25,000,000 are preferred stock;
and
|
|
·
|
effectuated
a forward split of our outstanding common stock on a 5 for 1 basis,
increasing our issued and outstanding shares of common stock from
38,260,797 to 191,303,987 shares, par value
$0.001.
|
The
Forward split had a pari
pasu effect on our 4,000,000 shares of outstanding Series A Preferred
Stock, which is now increased to 20,000,000 shares of Series A Preferred
Stock.
As a
result of the Forward Split and capitalization increase, we now have remaining
283,696,013 shares of Common Stock authorized, but unissued and available for
issuance, and an additional 5,000,000 shares of “blank check” preferred stock
authorized, which have not been designated or reserved for issuance by the
board.
The above
forward stock split was accepted and made effective by FINRA (www.finra.org) on
April 15, 2010, and the Company’s common stock, following 20 days from April 15,
2010 will remain INKN.
Convertible
Note Financing
Between
November of 2009 and March of 2010, the Company sold, in a private placement,
$435,000 in 12% convertible notes initially convertible at $.10 per share, and
1,675,000 Series A Common Stock Purchase Warrants, exercisable at $0.20 per
share, with aggregate proceeds to the Company of $435,000. The notes
are due 1 year from issuance and the warrants are exercisable commencing 6
months from issuance and expire 36 months from issuance. In the event
of default, or in certain other events, the notes become exercisable at 80% of
the then effective conversion price (initially $.08 per share) and the Company
may force conversion at the discounted rate at such time.
The
foregoing is a summary only of the note and warrant financing, details of which
can be found above in this Annual Report in the section titled “Item 5. Market
for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases
of Equity Securities.”
Strategic
Marketing and Development Agreement Relating to Solar Products
Effective
as of September 1, 2009 and as previously reported, the Company entered into a
Strategic Marketing and Development Agreement with Inabata America Corporation,
a subsidiary of Inabata & Co. Ltd. The Marketing Agreement
provides for the appointment of Inabata by the Company, as the Company’s
non-exclusive representative for the purposes of marketing and promoting the
Company’s solar concentrator technology and SHRINKCHIP™ RPS solar products to
third parties (the “Solar Products”). Pursuant to the Marketing
Agreement, Inabata is required to use best efforts in, among other things,
introducing the Solar Products to potential purchasers, licensees, customers,
development partners or possible funding sources, and to provide certain
technical assistance and training. In exchange therefore, Inabata was
granted a good faith right of first negotiation to act as non-exclusive
distributor of the Solar Products. In addition, the Marketing
Agreement provides for fees to be paid to Inabata if it introduces a funding,
grant or development funding source which completes a capital transaction with
the Company up to 50% of which may be paid in stock, at the sole discretion of
the Company.
30
Year Ended December 31,
2009Compared to the
Year ended December 31, 2008
Results
of Operations; Material Changes in Financial Condition; Development Stage
Company
The most
significant event and change to our Company during the year ended December 31,
2009, was the acquisition of Shrink on May 29, 2009, which is currently our only
material business, and completion of our recent equity financing. The
Shrink Acquisition resulted in Shrink being the surviving entity for financial
and accounting reporting purposes. Our discussion herein, therefore,
primarily relates to this business (except as indicated otherwise), as Shrink is
our surviving entity for accounting purposes. Our acquisition of
Shrink, along with our gradual liquidation of AudioStocks assets has resulted in
a material change to our business that occurred during 2009.
Shrink
was formed on January 15, 2008. Accordingly, any comparison data
herein relating to periods of Shrink in 2008, includes such period, from the
date of inception of Shrink.
As Shrink
is a development stage company, we expect to incur substantial additional
investment expenses in commercializing our Shrink related
technologies.
Since our
acquisition of Shrink we have also made various trademark applications relating
to our ShrinkChip technologies and entered into additional service or research
contracts to develop our manufacturing process.
The
Company, including its subsidiary, has not had any revenues in either 2009 or
2008 as it is a research and development stage company.
Operating
Expenses
Our
Shrink related business had total operating expenses of $1,468,818 for the year
ended December 31, 2009 and $88,493, as compared to the same period for the
Shrink related business in 2008. We expect operating expenses to
continue as we look to expand our current business operations and invest further
in research and development activities.
General and
Administrative Expenses. Our general and administration
expenses increased to $1,164,804, in the fiscal year ended December 31, 2009
from $9,141 in 2008. This increase was mainly due to a significant increase in
operating activities and company development.
Professional
Fees. Professional fees are generally related to public
company reporting and governance expenses as well as costs related to our
acquisitions. Our costs for professional fees increased to $151,116
in the fiscal year ended December 31, 2009 from $0 in 2008. This increase
was mainly due to the reverse acquisition of Shrink Nanotechnologies, Inc. which
led to an increase in public company reporting requirements, a significant
increase in operating activities and company development. We expect
our costs for professional services for public company reporting and corporate
governance expenses to remain significant.
Depreciation and
Amortization. Our depreciation expenses increased to $27,146 in the
fiscal year ended December 31, 2009 from $0 in 2008, and our amortization
expenses increased to $3,336 in the fiscal year ended December 31, 2009 from $0
in 2008. The increase in depreciation expenses was mainly due to the acquisition
of assets during reverse acquisition of Shrink Nanotechnologies, Inc., and the
increase in amortization expenses was mainly due to an increase of patent
pending inventions acquired during the year.
Interest
Expense.
Interest expense increased to $34,588 in the fiscal year ended December 31,
2009 from $0 in 2008, primarily due to an increase of financing through
convertible note issuances.
Net
Loss From Operations
For the
year ended December 31, 2009, we had a net loss of $1,621,527 from continuing
operations as compared to a net loss of $88,493 from continuing operations for
the period ended December 31, 2008. Management attributes the
increase in net loss to an increase in operational and research activities
and no revenues during 2009.
We
anticipate, continued losses relating to investment into our research and
development activities relating to Shrink, and to our capital raising and
investor relations activities. We intend to fund our R&D
activities, as we have in the past, through government grants, partnerships and
arrangements with universities (such as those that are in effect with the
Regents and Merced) and equity and debt financings.
31
Net
Gain from Discontinued Operations – Audiostocks.com Business
Division
In 2008
and 2009, we continued to operate our AudioStocks business while selling off its
assets and winding it down through November of 2009.
We
recorded a gain of $143,825 from our discontinued operations in 2009, as
compared to $0 for fiscal 2008.
We
anticipate that further gains and losses relating to our previous non-Shrink
related business will diminish as we have wound down or sold these related
operations in late 2008 and 2009. We do not currently incur material
expenses related to these businesses.
Because
we no longer operate in this segment, management believes that extensive
disclosure on this business is not material to an understanding of our new
Shrink business. In addition, a comparison of our new research and
development activities to our previous internet and investor relations business
would not be meaningful to investors. Accordingly, the focus of this Report is
on our Shrink related research and development business and business plans and
the previous Shrink business, which is deemed the accounting acquirer after the
Shrink Acquisition in May 2009.
Net
Loss
In the
fiscal year ended December 31, 2009, we generated a net loss of $1,477,702
compared to a net loss of $88,493 in 2008. The increase in net loss is due to an
overall increase in our business operations.
Liquidity
and Capital Resources
Our cash
on hand at December 31, 2009 was $58,539 as compared to $37,940 on hand December
31, 2008. The recent increase is primarily attributable to our recent
equity and debt financings.
Given our
current commitments and working capital, we cannot support our operations for
the next 12 months without additional capital (See “Need for Additional Capital”
below).
The
following table provides detailed information about our net cash flow for all
financial statement periods presented in this report.
|
Year
Ended December 31,
|
||||
|
2009
|
2008
|
|||
|
Net
cash provided by used in operating activities
|
$
|
(390,794)
|
$
|
(53,155)
|
|
Net
cash provided by (used in) investing activities
|
(76,107)
|
-
|
||
|
Net
cash provided by (used in) financing activities
|
487,500
|
91,095
|
||
|
Net
Increase in Cash and Cash Equivalents
|
20,599
|
37,940
|
||
|
Cash
and Cash Equivalent at Beginning of the Year
|
37,940
|
-
|
||
|
Cash
and Cash Equivalent at End of the Year
|
58,539
|
37,940
|
||
Operating
activities
Net cash
used in operating activities was $390,794 for the year ended December 31, 2009,
as compared to $53,155 used in operating activities during 2008. The increase in
net cash used in operating activities was mainly due to an increase in operating
activities and a lack of revenue.
Investing
activities
Net cash
used in investing activities for the year ended December 31, 2009 was $76,107,
as compared to $0 net cash used in investing activities in 2008. The increase in
net cash used in investing activities was mainly attributable to an increase in
patent pending and other intangible asset acquisitions.
32
Net cash
provided by financing activities for the year ended December 31, 2009 was
$487,500, as compared to $91,095 net cash provided by financing activities in
2008. The increase of net cash provided by financing activities was mainly
attributable to financing efforts made by management in order to obtain certain
assets, maintain current business operations and research and development
activities.
To date,
a material portion of our operations, and of the operations of our Shrink
subsidiary, have been funded by certain members of management and Noctua Fund,
L.P., which is an affiliate of Messrs. Baum and Panther, II, our current
directors. Specifically, and without limitation, Noctua Fund, L.P.
loaned the Company over $100,000 between 2008 and April 2009, as represented by
the 14% convertible promissory note, convertible at $.04 per share; and the
identical $118,000 14% convertible promissory note, convertible at $.04 per
share, reflecting loans to our Shrink subsidiary prior to our acquisition of
them, to fund their operations. Interest to date on the notes exceeds
$15,000 and both of the foregoing notes to Noctua are in default.
The
Company raised $100,000 through a Convertible Note and Series A Warrant
financing during the year ended December 31, 2009, and an additional $335,000
from January 2010 through March 2010, with gross proceeds to the Company of
$435,000.
Our
expectations are based on certain assumptions concerning the anticipated costs
associated with any new projects. These assumptions concern future
events and circumstances that our officers believe to be significant to our
operations and upon which our working capital requirements will
depend. Some assumptions will invariably not materialize and some
unanticipated events and circumstances occurring subsequent to the date of this
annual report. A portion of our research is being conducted by the
University of California, through grants. We will continue to seek to
fund our capital requirements over the next 12 months from the additional sale
of our securities; however, it is possible that we will be unable to obtain
sufficient additional capital through the sale of our securities as
needed.
The
amount and timing of our future capital requirements will depend upon many
factors, including the level of funding received by us anticipated private
placements of our common stock and the level of funding obtained through other
financing sources, and the timing of such funding.
We intend
to retain any future earnings to retire any existing debt, finance the expansion
of our business and any necessary capital expenditures, and for general
corporate purposes.
The
Company estimates that it will cost approximately $9,000,000 in deficit cash
flows until sustained potential profitability, and that substantial additional
costs will be incurred in order to commercialize its Shrink related
technologies.
Trends
To a
great extent, we expect government grants and university/academic funding to be
cyclical based on public policy (e.g. brain stem research limitations), the
amount allocated by governments and universities generally to research, and
general economic trends. We expect that we will have to compete diligently in
order to secure grants and research funding from universities. We
also anticipate that, for the foreseeable future, our ability to attain
conventional bank or secured financing for our products will be difficult as a
result of our limited fungible assets and banking constraints that limit
commercial loans.
Research
Agreement with UC Merced
Shrink is
a party to a research agreement with UC Merced, which agreement is referred to
in this Report as the Research Agreement. Pursuant to the Research
Agreement, UC Merced agreed to undertake a research project and Shrink agreed to
reimburse it for all direct and indirect costs incurred in connection with the
research, up to the amount of $640,935 and in accordance with an agreed-upon
budget. The Research Agreement provides Shrink with the right to
elect to receive an exclusive royalty-bearing license to make, use, sell, offer
for sale and import any products and practice any methods in the inventions or
discoveries conceived and reduced to practice in the performance of the research
conducted under the Research Agreement. We have exercised our rights
under this agreement and entered into a license agreement with the California
Regent.
33
Our
Exclusive License Agreement
The
underlying intellectual property and patent rights to Shrink’s proprietary
technologies are owned by the UC Regents. On April 29, 2009, and
pursuant to the terms of the Research Agreement, Shrink and the UC Regents
entered into an Exclusive License Agreement for Processes for Microfluidic
Fabrication and Other Inventions, which is referred to in this Report as the
License Agreement. The License Agreement licenses a broad array of
intellectual property and inventions vital to our planned
operations. The following description of the License Agreement is a
summary only, and is qualified in its entirety by the actual agreement which is
filed as an Exhibit to our Current Report on Form 8-K, filed June 5,
2009.
The term
of the License Agreement commenced April 29, 2009 and will continue in effect
until the expiration or abandonment of the last patent covered by the agreement,
unless we terminate it on 60 days’ notice or the UC Regents terminated it on 60
days’ notice if we default on our obligations under the agreement and fail to
cure such default during the 60 day period.
In
accordance with the License Agreement, we will be required to pay royalties
based on net sales of each licensed product, licensed method or licensed service
by the Shrink, any of its affiliates or joint-ventures. The License
Agreement requires that we pay royalties from all income, which includes gross
proceeds of all income (other than certain royalties required to be paid by our
sub licensees, if any), whether in cash or other considerations and, whether
paid to Shrink or its affiliates or assigns, but explicitly excluding, among
other things, revenues or receipts from debt or equity financings, or
reimbursements for our R&D activities or actual patent prosecution
costs.
Our fees
to the Regents under the License Agreement include:
|
·
|
A $100,000
contract initiation fee paid to the Regents in the form of 495,500 shares
of common stock of the Company; however, we have the option to reacquire
the shares for $100,000 at any time through April
2010;
|
|
·
|
A $25,000
annual maintenance fee, unless, (i) by the time that such annual
maintenance fee is due, Shrink has already commercialized its principal
product and begun paying earned royalties; or (ii) the Company maintains
the Research Agreement or another research agreement at the University of
California, Merced.
|
|
·
|
A
fee of 30% of all income attributed to revenues from a sublicensed product
or technology;
|
|
·
|
Milestone
payments of $100,000 for total accumulated nets sales of $50,000,000;
$500,000 for accumulated net sales of $150,000,000; and
$2,000,000 for net sales of $500,000,000;
and
|
|
·
|
Earned
royalty payments based on net sales of licensed
products.
|
A minimum
$15,000 earned royalty payment will be due each year following the first
commercial sale of a licensed product. The minimum payment will
increase to $20,000 on the first commercial sale of the fourth licensed product,
and by an additional $5,000 after a commercial sale of each additional licensed
product. The minimum payments are payable by February 28 of each year
in which they are due. Earned royalties in excess of the minimums are
payable quarterly.
The
License Agreement encourages early commercialization of the Licensed Rights, by
providing that our earned royalty payments based on revenues shall be set at
between 2.5% of net sales in the event that the first commercial sale commences
prior to April of 2012; 4% of net sales in the event that the first commercial
sale commences after April 2012 but before the April 2015; and 5% of net sales
if the first commercial sale commences thereafter. First commercial
sale is defined as bona fide good faith sale of products that are part of our
Licensed Rights, in quantities sufficient to meet market demands, and includes
sales for any consideration.
If we use
the licensed products or methods as research tools, we are required to pay
royalties also, at a rate to be agreed upon, but no less than the rate charged
by the UC Regents for similar research tools licensed to others.
If we
sublicense the licensed intellectual property, we are also required to pay
licensing fee of 30% of the gross income from the license, as well as earned
royalty payments on sublicensed sold.
Other
Limitations
The
license granted by the License Agreement is further subject to the obligations
to the U.S. government, under 35 U.S.C. Sections 200-212, including the
obligations to report on utilization of inventions under 37 CFR 401.14(h), and
is subject to the National Institute Of Health’s “Principals and Guidelines for
Recipients of NIH Research Grants and Contracts on Obtaining and Disseminating
Biomedical Research Resources” (64 F.R. 72090 Dec. 1999, as
amended).
No
assurance can be made that we will be able to continue developing or, if
developed successfully, commercialize our technologies licensed through UC
Merced. The foregoing is a summary only of the License Agreement with
UC Merced, a copy of which is filed with our current report on Form 8-K filed
June 6, 2009, the provisions of which are incorporated by reference
herein.
34
Off-Balance
Sheet Arrangements
We do not
have any off balance sheet arrangements that are reasonably likely to have a
current or future effect on our financial condition, revenues, and results of
operations, liquidity or capital expenditures.
Contractual
Obligations
The
Company and its subsidiary is a party to agreements with the Regents, numerous
consultants on its science and business Advisory Boards, other non-science
consultants (specifically our Operating Agreement with BCGU), and other
agreements mentioned above and which have been disclosed in our Reports and
which are incorporated herein.
We
currently do not have any full time employees. We have obtained
certain administrative services from BCGU, LLC (BCGU), an entity which is owned
and controlled by Mr. James B. Panther II and Mark L. Baum, Esq., both directors
of the Company. BCGU is to be paid a fee of $30,000 per month,
although BCGU has never actually received a cash fee equal to the contracted
amount as a result of our limited access to cash. Monies under this
agreement accrue on our financial records
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010. The foregoing is a
summary only of our sublease with Business Consulting Group Unlimited, Inc., a
copy of which is attached as an Exhibit to this Report, the provisions of which
are incorporated by reference herein.
The
Company previously shared office space with the Noctua Fund Manager, LLC.
On January 1, 2009 we began accruing a $2,500 rental expense each month for the
use of the space. There was no written agreement; it is a verbal agreement
between the two companies. The verbal agreement ceased on May 29, 2009
pursuant the Company’s execution of a sublease.
Recent
Accounting Pronouncements
We are
not aware of any additional pronouncements that materially affect our financial
position or results of operations.
Research
and Development
The
Company estimates that at least $122,416 has been spent by Shrink during fiscal
2009 and $79,352 during fiscal 2008 on research and development activities, most
of which has stemmed from payments from Shrink through UC Merced or, to a
limited extent, CIRM.
Employees
We
currently do not have any full time employees. We obtain certain
administrative services from BCGU, LLC, or BCGU, an entity indirectly controlled
by James B. Panther II, and Mark L. Baum, Esq., who are two of our directors,
for a fee of $30,000 per month pursuant to an operating
agreement. The foregoing is a summary only of our operating agreement
with BCGU, LLC, a copy of which is attached as an Exhibit to our Current Report
on Form 8-K, Filed June 5, 2009. (See also “Item
13. Certain Relationships and Related Party Transactions”
below).
We hire
independent contractor labor on an as needed basis and have entered into
consulting arrangements with certain directors and advisory board members in
exchange for stock or derivative securities. We have not entered into a
collective bargaining agreement with any union.
Properties
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010. The foregoing is a
summary only of our sublease with Business Consulting Group Unlimited, Inc., a
copy of which was attached as an Exhibit to our Current Report on Form 8-K, as
filed June 5, 2010, the provisions of which agreement are incorporated by
reference herein. (See also “Iem 13. Certain Relationships
and Related Party Transactions” below).
35
Recent
Developments
On
February 10, 2010 the Company resolved all its claims with American Scientific
Resources, Inc. As a result of the settlement all claims were dismissed,
including all action against the Company. The settlement has no
adverse impact on the Company’s financial results.
During
the month of March 2010, pursuant to agreements and commitments the Company
issued 18,382,500 shares of common stock with a value of $3,127,025 as payment
for various bona fide services.
Taxation
Income
taxes are accounted for under ASC740 "Accounting for Income
Taxes.” ASC740 requires the asset and liability method of accounting
for deferred income taxes. Deferred tax assets and liabilities are
determined based on the difference between the financial statement and tax bases
of assets and liabilities. Deferred tax assets or liabilities at the
end of each period are determined using the tax rate expected to be in effect
when taxes are actually paid or recovered. Even if we were to become
profitable, we may be unable to utilize our deferred tax asset.
ASC740
also requires that a valuation allowance be established when it is more likely
than not that all or a portion of a deferred tax asset will not be
realized. A review of all available positive and negative evidence
needs to be considered, including a company’s current and past performance, the
market environment in which the company operates, length of carryback and
carryforward periods and existing contracts that will result in future
profits.
Forming a
conclusion that a valuation allowance is not needed is difficult when there is
negative objective evidence such as cumulative losses in recent
years. Cumulative losses weigh heavily in the overall
assessment. As a result, we determined that it was appropriate to
establish a valuation allowance for the full amount of our deferred tax
assets.
ASC740
also prescribes a comprehensive model for recognizing, measuring, presenting and
disclosing in the consolidated financial statements tax positions taken or
expected to be taken on a tax return, including a decision whether to file or
not to file in a particular jurisdiction.
The
calculation of our tax liabilities involves the inherent uncertainty associated
with the application of complex tax laws. We are subject to examination by
various taxing authorities. We believe that as a result of our losses sustained
to date, any examination would result in a reduction of our net operating losses
rather than a tax liability. As such, we have not provided for
additional taxes estimated under ASC740, Accounting for Uncertainty in Income
Taxes.
The above
listing is not intended to be a comprehensive list of all of our accounting
policies. In many cases, the accounting treatment of a particular
transaction is specifically dictated by accounting principles, generally
accepted in the United States of America, with no need for management’s judgment
in their application. There are also areas in which management’s
judgment in selecting any viable alternative would not produce a materially
different result. See our audited financial statements and notes
thereto which contain accounting policies and other disclosures required by
accounting principles generally accepted in the United States of
America.
Obligations
Under Material Contracts
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010, and will continue as a month to month
tenancy thereafter. At December 31, 2009 $42,000 had been paid
to Business Consulting Group Unlimited, Inc and nothing was owed to Business
Consulting Group Unlimited, Inc.
On May
29, 2009, the Company signed an Operating Agreement with BCGU, LLC, an entity
indirectly controlled by James B. Panther II, and Mark L. Baum, Esq., who are
two of our directors, for a fee of $6,000 per month. During October
2009, the Company amended the Operating Agreement with BCGU, LLC. The
Amended Operating Agreement allows us to retain certain day-to-day
administrative services and management in consideration of a monthly fee of
$30,000 per month. The Amended Operating Agreement also included a
$270,000 signature bonus. The Amended Operating Agreement expires October
1, 2012. At December 31, 2009, $42,000 had been paid to BCGU, LLC and
there was $342,000 owed to BCGU, LLC. There is an option within the
Amended Operating Agreement that allows BCGU, LLC to convert outstanding
payables related to the Operating Agreement into a 10% convertible
note. The Company believes, based on review of its independent
directors, that the foregoing transactions and agreements are no less favorable
(or even more favorable, given the flexibility) to the Company than would
otherwise be available from independent third parties.
36
The
Company has also incurred debt in order to fund its operations, as discussed
above and elsewhere in this report in respect of $435,000 of 12% convertible
notes issued between November and March 2010, and $218,121.28 or debt funded by
Noctua Fund L.P. to the Company or its Shink subsidiary since 2008, as evidenced
by the two 14% Convertible notes, in the aggregate principal amount of
$118,121.28 and $100,000, respectively, specifically, some of our notes and
related obligations include:
|
·
|
$435,000
principal amount of unsecured 12% convertible notes, initially convertible
at $.10 per share, into an aggregate of 4,350,000, in addition to shares
that may be issued in respect of interest payments,
and
|
|
·
|
2,175,000
shares issuable upon exercise of Series A Common Stock Purchase Warrants
issued between November 2009 and April of 2010, at $.20 per share,
and
|
|
·
|
$118,121.28
principal amount of 14% convertible promissory note, issued to Noctua
Fund, L.P. in May 2009, an affiliate of Messrs. Baum and Panther, II,
convertible at $.04 per share into an aggregate of 2,953,032 shares (or
3,223,725 inclusive of 270,693 shares underlying $10,827.72 or accrued
interest, through April 10, 2010).
|
|
·
|
$100,000
principal amount of 14% convertible promissory Note issued to Noctua Fund,
L.P., an affiliate of Messrs. Baum and Panther II, reflecting loans made
by it in 2008 and 2009, convertible at $0.04 per share into an aggregate
of 2,500,000 shares (or 2,625,000 shares inclusive of 125,000 shares
underlying $5,000 of accrued interest, through April 10,
2010).
|
The notes
to Noctua Fund L.P. are currently in default.
Agreements
with Universities
As
indicated above, Shrink is a party to a Research Agreement with UC Merced and to
an Exclusive License Agreement with the UC Regents. A more detailed
description of these agreements is contained above, in this section, and
elsewhere in this Report.
37
Agreements
for Consulting Services
The
Company does not pay cash or issue securities to promote its stock or to make a
market or assist in making a market for its securities. Our sole
executive officer has instructed all company representatives of this
policy.
The
Company strives to save liquid capital by paying for services or satisfying
liabilities, whenever able, by issuance of stock to consultants, services
providers and consultants. The issuance of restricted stock in lieu
of cash payment sometimes requires that the company pay a premium for such
services. Management believes, nonetheless, that given credit crisis,
and difficulty in raising capital for R&D companies such as our own, that
the benefits of issuing restricted stock for services outweigh the downside in
that it leaves cash available to satisfy debts with vendors where
able. Below is a list material agreements entered into with
consultants for services, all of which consultants are independent
parties.
In March
of 2010, we issued 7,250,000 shares valued at $1,232,500 to a consultant in
satisfaction of a consulting agreement entered into in January, 2010, for public
relations, marketing and structuring services.
In March
of 2010, we issued 8,700,000 shares valued at $1,479,000 to a consultant in
satisfaction of a consulting agreement entered into in January of 2010, for
public relations, assistance with press releases and branding, research and
web/statistical content and investor relations.
In March
of 2010, we issued 1,500,000 shares valued at $255,000 to a consultant in
satisfaction of a consulting agreement entered into in February of 2010, for
services relating to marketing of our medical device products, and marketing to
researches, academia and medical professionals.
In March
of 2010, we issued 600,000 shares valued at $102,000 to an accounting
professional for previous and ongoing accounting, bookkeeping and financial
statement preparation related services.
In March
of 2010, we issued 180,000 shares valued at $30,600 to a consultant in
satisfaction of a consulting agreement entered into in March 2010, for services
rendered.
In March
of 2010, we issued 112,500 shares valued at $19,125 to a member of the
Scientific Advisory Board, pursuant to an agreement dated as of March 1,
2010.
In March
of 2010, we issued 40,000 shares valued at $6,800 (in addition to 60,000 shares
issued in November 2010) as payment for professional services
rendered.
Some of
the agreements under which these shares were issued have not been committed to a
writing and were entered into based on an oral agreement between the independent
contractor(s) and a company representative. These shares are cancellable based
on performance of said independent contractor.
The
foregoing agreements do not require us to make further issuances (except for the
issuances for professional services which continue insofar as such persons
continue to provide services and agree to accept shares in lieu of cash
payment. The foregoing is a summary only of the foregoing agreements,
copies of which, to the extent material, are annexed as exhibits to this Annual
Report.
All stock
issuances are subject to Board approval.
Need
for Additional Capital
As
indicated above, management does not believe that the Company has sufficient
capital to sustain its operations beyond 12 months or commercializing its
technologies without raising additional capital. We presently do not
have any available credit, bank financing or other external sources of
liquidity. Accordingly, we expect that we will require additional
funding through additional equity and/or debt financings. However,
there can be no assurance that any additional financing will become
available to us, and if available, on terms acceptable to us.
The
conversion of our outstanding notes and exercise of our outstanding warrants
into shares of common stock would have a dilutive effect on our common stock,
which would in turn reduce our ability to raise additional funds on favorable
terms. In addition, the subsequent sale on the open market of any
shares of common stock issued upon conversion of our outstanding notes and
exercise of our outstanding warrants could impact our stock price which would in
turn reduce our ability to raise additional funds on favorable
terms.
Any
financing, if available, may involve restrictive covenants that may impact our
ability to conduct our business or raise additional funds on acceptable
terms. If we are unable to raise additional capital when required or
on acceptable terms, we may have to delay, scale back or discontinue our
expansion plans. In the event we are unable to raise additional
capital we will not be able to sustain any growth or continue to
operate.
Effects
of Inflation
Inflation
and changing prices have not had a material effect on our business and we do not
expect that inflation or changing prices will materially affect our business in
the foreseeable future. However, our management will closely monitor
the price change and continually maintain effective cost control in
operations.
Off
Balance Sheet Arrangements
We do not
have any off balance sheet arrangements that have or are reasonably likely to
have a current or future effect on our financial condition, changes in financial
condition, revenues or expenses, results of operations, liquidity or capital
expenditures or capital resources that is material to an investor in our
securities.
38
Seasonality
Our
operating results and operating cash flows historically have not been subject to
seasonal variations. This pattern may change, however, as a result of new market
opportunities or new product introductions.
Critical
Accounting Policies and Estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States requires our management to make
assumptions, estimates and judgments that affect the amounts reported in the
financial statements, including the notes thereto, and related disclosures of
commitments and contingencies, if any. We consider our critical accounting
policies to be those that require the more significant judgments and estimates
in the preparation of financial statements, including the
following:
|
·
|
Management
believes the critical accounting policies discussed below affect its more
significant estimates and assumptions used in the preparation of its
consolidated financial statements. For a complete discussion of all of the
Company’s significant accounting policies, see Note 2. to the Notes to
Consolidated Financial Statements in this Form
10-K.
|
|
ITEM 6A.
|
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISK.
|
Not
Applicable.
|
ITEM 7.
|
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA.
|
The full
text of our audited consolidated financial statements as of December 31, 2009
and 2008 begins on page F-1 of this Annual Report.
[Remainder
of Page Intentionally Left Blank]
39
REPORT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the
Board of Directors
Shrink
Technologies, Inc
Carlsbad,
California
We have
audited the accompanying balance sheets of Shrink Technologies,
Inc (a development stage company) as of December 31, 2009 and
2008, and the related statements of operations, stockholders' equity
(deficit) and cash flows for the years then ended and from January 15, 2008
(inception), through December 31, 2009. These financial statements are the
responsibility of the Company's management. Our responsibility is to express an
opinion on these financial statements based on our audits.
We
conducted our audits in accordance with the standards of the Public Company
Accounting Oversight Board (United States). Those standards require that we plan
and perform the audits to obtain reasonable assurance about whether the
financial statements are free of material misstatement. The Company is not
required to have, nor were we engaged to perform, an audit of its internal
controls over financial reporting. Our audits included consideration of internal
control over financial reporting as a basis for designing audit procedures that
are appropriate in the circumstances, but not for the purpose of expressing an
opinion on the effectiveness of the Company’s internal control over financial
reporting. Accordingly, we express no such opinion. An audit includes examining,
on a test basis, evidence supporting the amounts and disclosures in the
financial statements. An audit also includes assessing the accounting principles
used and significant estimates made by management, as well as evaluating the
overall financial statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our
opinion, the financial statements referred to above present fairly, in all
material respects, the financial position of Shrink Technologies, Inc. as of
December 31, 2009 and 2008, and the results of its operations and its
cash flows for the years then ended and from January 15, 2008 (inception)
through December 31, 2009 in conformity with accounting principles generally
accepted in the United States of America.
The
accompanying financial statements have been prepared assuming that the Company
will continue as a going concern. The Company has suffered losses from
operations and is dependent on financing to continue operations, which raises
substantial doubt about its ability to continue as a going concern.
Management’s plans in regard to these matters are described in Note 3.
The financial statements do not include any adjustments that might result
from the outcome of this uncertainty.
/s/ Chisholm, Bierwolf,
Nilson & Morrill
Chisholm,
Bierwolf, Nilson & Morrill, LLC
Bountiful,
Utah
April 14,
2010
F-1
|
(A
Development Stage Company)
|
||||
|
CONSOLIDATED
- BALANCE SHEETS
|
||||
|
December
31,
|
December
31,
|
|||
|
2009
|
2008
|
|||
|
ASSETS
|
||||
|
Current
assets
|
||||
|
Cash
|
$
58,539
|
$
37,940
|
||
|
Litigation
receivable
|
108,011
|
-
|
||
|
Prepaid
expenses
|
120,333
|
-
|
||
|
Total
current assets
|
286,883
|
37,940
|
||
|
Prepaid
expenses, non-current
|
257,408
|
-
|
||
|
Property,
plant and equipment, net
|
140,054
|
-
|
||
|
Intangible
assets, net
|
219,062
|
-
|
||
|
TOTAL
ASSETS
|
$
903,407
|
$
37,940
|
||
|
LIABILITIES
AND STOCKHOLDERS' DEFICIT
|
||||
|
Current
liabilities
|
||||
|
Accounts
payable and accrued expenses
|
$
973,840
|
$ 35,338
|
||
|
Due
to former preferred stock shareholders
|
143,825
|
-
|
||
|
Due
to a related party
|
12,500
|
-
|
||
|
Convertible
debentures, net of discount
|
13,611
|
-
|
||
|
Convertible
debentures, net of discount, default - related party
|
218,121
|
45,547
|
||
|
Total
current liabilities
|
1,361,897
|
80,885
|
||
|
Long
term liabilities
|
||||
|
Convertible
debentures, net of discount - related party
|
-
|
45,547
|
||
|
TOTAL
LIABILITIES
|
1,361,897
|
126,432
|
||
|
COMMITMENTS
|
||||
|
STOCKHOLDERS'
DEFICIT
|
||||
|
Preferred
stock, 25,000,000 shares authorized, $0.001 par value
|
||||
|
issued
and outstanding 20,000,000 and 50
|
||||
|
at
December 31, 2009 and December 31, 2008, respectively
|
4,000
|
-
|
||
|
Common
stock, 475,000,000 shares authorized, $0.001 par value
|
||||
|
issued
and outstanding 172,921,485 and 44,444,440
|
||||
|
at
December 31, 2009 and December 31, 2008, respectively
|
34,584
|
8,889
|
||
|
Additional
paid in capital
|
1,069,121
|
(8,888)
|
||
|
Accumulated
deficit
|
(1,566,195)
|
(88,493)
|
||
|
TOTAL
STOCKHOLDERS' DEFICIT
|
(458,490)
|
(88,492)
|
||
|
TOTAL
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
$
903,407
|
$
37,940
|
||
|
The
accompanying notes are an integral part of these financial
statements
|
||||
F-2
|
(A
Development Stage Company)
|
||||
|
CONSOLIDATED
- STATEMENTS OF OPERATIONS
|
||||
|
For
the year
|
From
Inception
|
From
Inception
|
||
|
ended
|
January
15, 2008
|
January
15, 2008
|
||
|
December
31,
|
through
December 31,
|
through
December 31,
|
||
|
2009
|
2008
|
2009
|
||
|
Net
revenues
|
$
-
|
$ -
|
$
-
|
|
|
Expenses
|
||||
|
Research
and development
|
122,416
|
79,352
|
201,768
|
|
|
Professional
fees
|
151,116
|
-
|
151,116
|
|
|
General
and administrative
|
1,164,804
|
9,141
|
1,173,945
|
|
|
Depreciation,
depletion, and amortization
|
30,482
|
-
|
30,482
|
|
|
Total
operating expenses
|
1,468,818
|
88,493
|
1,557,311
|
|
|
Loss
from operations
|
(1,468,818)
|
(88,493)
|
(1,557,311)
|
|
|
Other
Income (Expense)
|
||||
|
Interest
expense
|
(34,588)
|
-
|
(34,588)
|
|
|
Loss
from extinguishment of debt
|
(118,121)
|
-
|
(118,121)
|
|
|
Total
Other Income (Expense)
|
(152,709)
|
-
|
(152,709)
|
|
|
Net
loss from continued operations
|
(1,621,527)
|
(88,493)
|
(1,710,020)
|
|
|
Discontinued
Operations
|
||||
|
Gain
from discontinued operations of Audiostocks business
|
143,825
|
-
|
143,825
|
|
|
Income
from discontinued operations
|
143,825
|
-
|
143,825
|
|
|
(Loss)
Before Income Taxes
|
(1,477,702)
|
(88,493)
|
(1,566,195)
|
|
|
Income
Taxes
|
-
|
-
|
-
|
|
|
NET
(LOSS)
|
$
(1,477,702)
|
$
(88,493)
|
$
(1,566,195)
|
|
|
Net
income (loss) per common share, basic and diluted:
|
||||
|
Net
(loss) from continued operations
|
(0.01)
|
(0.00)
|
-
|
|
|
Net
income from discontinued operations
|
0.00
|
-
|
-
|
|
|
Net
(loss) per common share:
|
$
(0.01)
|
$
(0.00)
|
-
|
|
|
Weighted
average common and common equivalent shares outstanding
|
||||
|
Basic
and diluted
|
117,799,762
|
44,444,440
|
81,839,262
|
|
|
The
accompanying notes are an integral part of these financial
statements
|
||||
F-3
|
SHRINK
NANOTECHNOLOGIES, INC.
|
||||
|
(A
Development Stage Company)
|
||||
|
CONSOLIDATED
- STATEMENTS OF CASH FLOWS
|
||||
|
For
the year
|
From
Inception
|
From
Inception
|
||
|
ended
|
January
15, 2008
|
January
15, 2008
|
||
|
December
31,
|
through
December 31,
|
through
December 31,
|
||
|
2009
|
2008
|
2009
|
||
|
CASH
FLOWS FROM OPERATING ACTIVITIES
|
||||
|
Net
loss
|
$
(1,477,702)
|
$
(88,493)
|
$
(1,566,195)
|
|
|
Adjustments
to reconcile net earnings to net cash used
|
||||
|
by
operating activities:
|
||||
|
Depreciation,
amortization
|
30,482
|
-
|
30,482
|
|
|
Debt
discount amortization
|
13,611
|
-
|
13,611
|
|
|
Non-cash
share-based payments
|
539,799
|
-
|
539,799
|
|
|
Loss
from extinguishment of debt
|
118,121
|
-
|
118,121
|
|
|
Changes
in assets and liabilities, net of effects from
acquisitions
|
||||
|
Prepaid
expenses
|
(377,741)
|
-
|
(377,741)
|
|
|
Accounts
receivable
|
(106,339)
|
-
|
(106,339)
|
|
|
Accounts
payable and accrued liabilities
|
868,975
|
35,338
|
904,313
|
|
|
NET
CASH USED IN OPERATING ACTIVITIES
|
(390,794)
|
(53,155)
|
(443,949)
|
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES
|
||||
|
Cash
purchased at acquisition
|
62,404
|
-
|
62,404
|
|
|
Additions
to fixed assets
|
(16,113)
|
-
|
(16,113)
|
|
|
Additions
to intangible assets
|
(122,398)
|
-
|
(122,398)
|
|
|
NET
CASH FROM INVESTING ACTIVITIES
|
(76,107)
|
-
|
(76,107)
|
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES
|
||||
|
Proceeds
from subsidiary prior to merger
|
12,500
|
-
|
12,500
|
|
|
Proceeds
from issuance of common stock
|
355,000
|
1
|
355,001
|
|
|
Proceeds
from convertible debentures
|
120,000
|
91,094
|
211,094
|
|
|
NET
CASH FROM FINANCING ACTIVITIES
|
487,500
|
91,095
|
578,595
|
|
|
NET
CHANGE IN CASH
|
20,599
|
37,940
|
58,539
|
|
|
CASH
BALANCES
|
||||
|
Beginning
of period
|
37,940
|
-
|
-
|
|
|
End
of period
|
$
58,539
|
$
37,940
|
$
58,539
|
|
|
SUPPLEMENTAL
DISCLOSURE:
|
||||
|
Interest
paid
|
$
312
|
$
-
|
$
-
|
|
|
Income
taxes paid
|
$
-
|
$
-
|
$ -
|
|
|
NON-CASH
INVESTING AND FINANCING TRANSACTIONS :
|
||||
|
Non-cash
assets acquired through reverse acquisition
|
$
152,759
|
$
-
|
$
-
|
|
|
Liabilities
assumed through reverse acquisition
|
$
186,297
|
$ -
|
$
-
|
|
|
License
acquired through issuance of stock
|
$
100,000
|
$
-
|
$
-
|
|
|
Stock
issued to satisfy convertible debt obligation
|
$
57,500
|
$
-
|
$
-
|
|
|
The
accompanying notes are an integral part of these financial
statements
|
||||
F-4
|
SHRINK
NANOTECHNOLOGIES, INC.
|
|||||||
|
(Development
Stage Company)
|
|||||||
|
STATEMENTS
OF STOCKHOLDERS'' EQUITY (DEFICIT)
|
|||||||
|
From
January 15, 2008 date of inception through December 31,
2009
|
|||||||
|
Preferred
Stock
|
Common
Stock
|
Additional
|
Retained
|
Total
|
|||
|
Par
|
Par
|
Paid-in
|
Earnings
|
||||
|
Shares
|
Value
|
Shares
|
Value
|
Capital
|
and
Deficit
|
||
|
Issuance
founder shares
|
50
|
$
-
|
44,444,440
|
$8,889
|
$(8,888)
|
$ -
|
$ 1
|
|
Net
Loss
|
-
|
-
|
-
|
-
|
-
|
(88,493)
|
(88,493)
|
|
Balance
at December 31, 2008
|
50
|
-
|
44,444,440
|
8,889
|
(8,888)
|
(88,493)
|
(88,492)
|
|
Reverse
merger
|
20,000,100
|
4,000
|
115,004,875
|
23,001
|
(45,892)
|
-
|
(18,891)
|
|
Beneficial
conversion feature convertible notes
|
-
|
-
|
-
|
-
|
69,900
|
-
|
69,900
|
|
Value
associated with warrants detached
|
|||||||
|
from
notes
|
-
|
-
|
-
|
-
|
30,100
|
-
|
30,100
|
|
Conversion
of convertible debt
|
-
|
-
|
1,916,670
|
383
|
57,117
|
-
|
57,500
|
|
Issuance
of shares for license issuance fee
|
-
|
-
|
495,500
|
99
|
99,901
|
-
|
100,000
|
|
Shares
issued for cash at $.20 per share
|
-
|
-
|
8,875,000
|
1,775
|
353,225
|
-
|
355,000
|
|
Issuance
of shares for services valued at
|
|||||||
|
$1.08
per share
|
-
|
-
|
2,185,000
|
437
|
469,461
|
-
|
469,898
|
|
Value
of stock options granted
|
-
|
-
|
-
|
-
|
69,901
|
-
|
69,901
|
|
Cancellation
of series c preferred stock
|
(150)
|
-
|
-
|
-
|
(143,825)
|
-
|
(143,825)
|
|
Loss
from extinguished debt
|
-
|
-
|
-
|
-
|
118,121
|
-
|
118,121
|
|
Net
Loss
|
-
|
-
|
-
|
-
|
-
|
(1,477,702)
|
(1,477,702)
|
|
Balance
at December 31, 2009
|
20,000,000
|
$4,000
|
172,921,485
|
$34,584
|
$
1,069,121
|
$(1,566,195)
|
$
(458,490)
|
|
The
accompanying notes are an integral part of these financial
statements
|
|||||||
F-5
SHRINK
NANOTECHNOLOGIES, INC.
Notes
to the Consolidated Financial Statements
For
the year ended December 31, 2009 and 2008
NOTE
1. ORGANIZATION
Shrink
Nanotechnologies, Inc. (the “Company or the “Successor Entity” or “us” or “we”)
was incorporated in the state of Delaware on January 15, 2002, as Jupiter
Processing, Inc. On January 13, 2005, the Company changed its name to
Audiostocks, Inc. On May 14, 2009, the Company changed its name to
Shrink Nanotechnologies, Inc. Following a shareholder
action described in a Form 14C filed on with the Securities and Exchange
Commission on February 15, 2010, the Board of Directors effected a 5 for 1
forward split. All per share amounts and calculations in our
presentation reflect this change.
On May 29, 2009, the
Company entered into and completed a share exchange agreement with Shrink
Technologies, Inc., (“Shrink”) a privately-owned California corporation which
held and continues to hold, most of our Shrink related business and intellectual
property assets. The exchange of shares with Shrink
Technologies, Inc. has been accounted for as a reverse acquisition with the
business of Shrink Technologies, Inc. as the surviving Company for accounting
and financial reporting purposes. Accordingly, the acquisition has
been recorded as a recapitalization of the Company. Therefore, the
historical financial statements presented prior to the acquisition date, are
those of Shrink Technologies, Inc., the operating entity and consolidated with
the Company post acquisition.
The
Company is a research, design and development company dedicated to the
commercial adoption of a nanotechnology platform called the ShrinkChip
Manufacturing Solution™, which we believe represents a new paradigm in the rapid
design and low-cost fabrication of diagnostic chips and other nano-size devices
that we intend to commercialize as measuring tools and energy and content
transfer devices for a wide range of applications from the life sciences, drug
and chemical analysis industries to the optoelectronics components and renewable
energy businesses. ( Collectively, an incomplete but abbreviated description of
Shrink’s operational business, which shall sometimes be referred to as the
“Shrink Business”).
NOTE
2. SUMMARY OF ACCOUNTING POLICIES
Principles
of Consolidation
The
consolidated balance sheets include the accounts of Shrink Nanotechnologies,
Inc. and its wholly owned subsidiary Shrink Technologies, Inc., thereby
reflecting the transactions related to the May 29, 2009 effective date of the
Exchange Agreement. The consolidated statements of operations include the
operations (which consisted mostly of research and development) of the
predecessor entity, Shrink Technologies, Inc. from inception on January 15,
2008 and the Company from May 29, 2009, the effective date of the
acquisition of the Shrink business. All significant intercompany
accounts and transactions have been eliminated in consolidation.
Shrink
Technologies, Inc. operated its research and development operations prior to the
Share Exchange and is continuing to do so. The accompanying financial statements
include the Balance Sheet of Shrink Technologies, Inc. as of December 31, 2008,
and the Statement of Operations, Statement of Cash Flows, and Statement of
Changes in Equity (Deficit) for the year then ended.
Accounting
Method
The
financial statements are prepared using the accrual method of accounting.
The Company has elected a December 31 year-end.
Use
of Estimates
The
preparation of financial statements in conformity with accounting principles
generally accepted in the United States of America requires management to make
estimates and assumptions that affect the reported amounts of assets and
liabilities and disclosure of contingent assets and liabilities at the date of
the financial statements and the reported amounts of revenues and expenses
during the reporting period. Actual results could differ from those
estimates. Estimates
and assumptions principally relate to the fair value and forfeiture rates of
stock based transactions, and asset depreciation and amortization.
F-6
Cash
and Cash Equivalents
Cash
equivalents include short-term, highly liquid investments with maturities of
three months or less at the time of acquisition.
Property,
Plant and Equipment
Property,
plant and equipment are stated at cost less accumulated depreciation.
Depreciation is computed using the straight-line method over estimated useful
lives of the asset. During the year ended December 31, 2009 and 2008
the Company recorded $27,146 and $0 in depreciation expenses,
respectively.
|
December
31,
|
December
31,
|
|
|
2009
|
2008
|
|
|
Property
Plant and Equipment, net:
|
||
|
Computer
Software and Hardware
|
$
138,166
|
$ -
|
|
Furniture
and Equipment
|
28,181
|
-
|
|
Building
and Improvements
|
853
|
-
|
|
Accumulated
Depreciation
|
(27,146)
|
-
|
|
Total
|
$
140,054
|
$
-
|
Provision
for Income Taxes
The
Financial Accounting Standards Board (FASB) has issued FASB ASC
740-10. FASB ASC 740-10 clarifies the accounting for uncertainty in
income taxes recognized in an enterprise's financial statements in accordance
with prior literature FASB Statement No. 109, Accounting for Income Taxes.
This standard requires a company to determine whether it is more likely
than not that a tax position will be sustained upon examination based upon the
technical merits of the position. If the more-likely-than- not threshold
is met, a company must measure the tax position to determine the amount to
recognize in the financial statements. As a result of the implementation
of this standard, the Company performed a review of its material tax positions
in accordance with recognition and measurement standards established by FASB ASC
740-10.
Deferred
taxes are provided on a liability method whereby deferred tax assets are
recognized for deductible temporary differences and operating loss and tax
credit carryforwards and deferred tax liabilities are recognized for taxable
temporary differences. Temporary differences are the differences between
the reported amounts of assets and liabilities and their tax basis.
Deferred tax assets are reduced by a valuation allowance when, in the
opinion of management, it is more likely than not that some portion or all of
the deferred tax assets will not be realized. Deferred tax assets and
liabilities are adjusted for the effects of changes in tax laws and rates on the
date of enactment.
The
components of current income tax expense as of December 31, 2009 and 2008
consist of the following:
|
December 31,
|
December 31,
|
|
|
2009
|
2008
|
|
|
Current
federal tax expense
|
$ -
|
$ -
|
|
Current
state tax expense
|
-
|
-
|
|
Change
in NOL benefits
|
(497,791)
|
(30,088)
|
|
Change
in depreciation differences
|
-
|
-
|
|
Change
in contribution benefits
|
-
|
-
|
|
Change
in valuation allowance
|
497,791
|
30,088
|
|
$ -
|
$ -
|
F-7
The net
deferred income taxes in the accompanying balance sheet include the following
amounts of deferred income tax assets:
|
December 31,
|
December 31,
|
|
|
2009
|
2008
|
|
|
Net
income tax assets:
|
||
|
Net
operating loss carryforward
|
$
527,879
|
$
30,088
|
|
Contribution
carryforward
|
-
|
-
|
|
Less:
Deferred income tax liabilities
|
||
|
Depreciation
differences
|
-
|
-
|
|
Less:
Valuation allowance
|
(527,879)
|
(30,088)
|
|
Net
deferred income tax asset
|
$
-
|
$
-
|
The
following is a reconciliation of the provision for income taxes at the United
States of America federal income tax rate to the income taxes reflected in the
statements of operations:
Tax
expense (credit) at statutory rate – federal -35%
State tax
expense net of federal tax -9%
Change in
valuation allowance -44%
Tax
expense at actual rate 0%
As of
December 31, 2009, the Company’s net deferred tax assets are offset by a
valuation allowance of $527,879. In assessing the realizability of deferred tax
assets, management considers whether it is more likely than not that some
portion or all of the deferred tax assets will not be realized. The ultimate
realization of deferred tax assets is dependent upon the generation of future
taxable income during the year in which those temporary differences become
deductible. Management considers projected future taxable income and tax
planning strategies in making this assessment.
As of
December 31, 2009 and 2008, the Company has approximately $1,552,000 and
$88,000, respectively, of net operating loss carryforwards available to reduce
future taxable income. These carryforwards will begin to expire in
2028.
A
reconciliation of the beginning and ending amount of unrecognized tax benefits
is as follows:
|
Year
ended December 31,
|
||||
|
2009
|
2008
|
|||
|
Beginning
balance
|
$
|
-
|
$
|
-
|
|
Additions
based on tax positions related to current year
|
-
|
-
|
||
|
Additions
for tax positions of prior years
|
-
|
-
|
||
|
Reductions
for tax positions of prior years
|
-
|
-
|
||
|
Reductions
in benefit due to income tax expense
|
-
|
-
|
||
|
Ending
balance
|
$
|
-
|
$
|
-
|
At
December 31, 2009, the Company had no unrecognized tax benefits that, if
recognized, would affect the effective tax rate.
The
Company did not have any tax positions for which it is reasonably possible that
the total amount of unrecognized tax benefits will significantly increase or
decrease within the next 12 months.
The
Company includes interest and penalties arising from the underpayment of income
taxes in the consolidated statements of operations in the provision for income
taxes. As of December 31, 2009 and 2008, the Company had no accrued
interest or penalties related to uncertain tax positions.
The tax
years that remain subject to examination by major taxing jurisdictions are those
for the years ended December 31, 2009 and 2008.
F-8
Basic
Net Loss per Share of Common Stock
In
accordance with ASC 260-10, basic net loss per common share is based on the
weighted average number of shares outstanding during the periods presented.
Diluted earnings per share are computed using weighted average number of
common shares plus dilutive common share equivalents outstanding during the
period. There is $318,121 in convertible debt currently outstanding,
20,000,000 preferred shares and stock options that total 28,571,570 in common
stock equivalents. Common stock equivalents resulting from the
issuance of these stock options have been considered, but have not been included
in the per share calculations because such inclusion would be
anti-dilutive.
|
From
inception
|
||
|
For
the year ended
|
January
15, 2008
|
|
|
December
31, 2009
|
through
December 31, 2008
|
|
|
Numerator
– (loss)
|
$
(1,621,527)
|
$
(88,493)
|
|
Denominator
– weighted average
|
||
|
number
of shares outstanding
|
117,799,762
|
44,444,440
|
|
Loss
per share – continuing operations
|
$
(0.01)
|
$
(0.00)
|
Research
and Development
In
accordance with ASC 730-10, the Company expenses all costs related to research
and development as they are incurred. During the year ended December
31, 2009 and 2008, the Company expensed $122,416 and $79,352 and in research and
development costs, respectively.
Recent
Accounting Pronouncements
In March
2008, the FASB issued FASB ASC 815-10 (Prior authoritative literature: SFAS No.
161, “Disclosures about
Derivative Instruments and Hedging Activities”), which is effective
January 1, 2009. FASB ASC 815-10 requires enhanced disclosures about derivative
instruments and hedging activities to allow for a better understanding of their
effects on an entity’s financial position, financial performance, and cash
flows. Among other things, this standard requires disclosures of the fair values
of derivative instruments and associated gains and losses in a tabular formant.
This standard is not currently applicable to the Company since we do not have
derivative instruments or engage in hedging activity.
In May
2008, the FASB issued FASB ASC 944 (Prior authoritative literature: SFAS No.
163, "Accounting for Financial
Guarantee Insurance Contracts - an interpretation of FASB Statement No. 60").
FASB ASC 944 interprets Statement 60 and amends existing accounting
pronouncements to clarify their application to the financial guarantee insurance
contracts included within the scope of that Statement. This standard is
effective for financial statements issued for fiscal years beginning after
December 15, 2008, and all interim periods within those fiscal
years. This standard did not have a material effect on our financial
statements.
In April,
2009, the FASB issued FASB ASC 810-10-65 (Prior authoritative literature: SFAS
No. 164, “Not-for-Profit
Entities: Mergers and Acquisitions”) which governs the information that a
not-for-profit entity should provide in its financial reports about a
combination with one or more other not-for-profit entities, businesses or
nonprofit activities and sets out the principles and requirements for how a
not-for-profit entity should determine whether a combination is in fact a merger
or an acquisition. This standard is effective for mergers occurring on or after
Dec. 15, 2009 and for acquisitions where the acquisition date is on or after the
beginning of the first annual reporting period beginning on or after December
15, 2009. This standard does not apply to the Company since the Company is
considered a for-profit entity.
In May
2009, FASB issued FASB ASC 855-10 (Prior authoritative
literature: SFAS No. 165, "Subsequent Events"). FASB
ASC 855-10 establishes principles and requirements for the reporting of events
or transactions that occur after the balance sheet date, but before financial
statements are issued or are available to be issued. FASB ASC 855-10 is
effective for financial statements issued for fiscal years and interim periods
ending after June 15, 2009. Adoption of FASB ASC 855-10 did not have a material
effect on our financial statements.
In
June 2009, the FASB ASC 860-10 (Prior authoritative literature: issued SFAS
No. 166, “Accounting for
Transfers of Financial Assets, an Amendment of FASB Statement
No. 140”), which eliminates the concept of a qualifying
special-purpose entity (“QSPE”), clarifies and amends the de-recognition
criteria for a transfer to be accounted for as a sale, amends and clarifies the
unit of account eligible for sale accounting and requires that a transferor
initially measure at fair value and recognize all assets obtained and
liabilities incurred as a result of a transfer of an entire financial asset or
group of financial assets accounted for as a sale. This standard is effective
for fiscal years beginning after November 15, 2009. The Company is
currently evaluating the potential impact of this standard on its financial
statements, but does not expect it to have a material effect.
In
June 2009, the FASB issued FASB ASC 810-10-65 (Prior authoritative
literature: SFAS No. 167, “Amendments to FASB Interpretation
No. 46(R)”) which amends the consolidation guidance applicable to a
variable interest entity (“VIE”). This standard also amends the guidance
governing the determination of whether an enterprise is the primary beneficiary
of a VIE, and is therefore required to consolidate an entity, by requiring a
qualitative analysis rather than a quantitative analysis. Previously, the
standard required reconsideration of whether an enterprise was the primary
beneficiary of a VIE only when specific events had occurred. This standard is
effective for fiscal years beginning after November 15, 2009, and for
interim periods within those fiscal years. Early adoption is prohibited. The
Company is currently evaluating the potential impact of the adoption of this
standard on its financial statements, but does not expect it to have a material
effect.
F-9
In June
2009, FASB issued ASC 105-10 (Prior authoritative literature: SFAS
No. 168, "The FASB Accounting
Standards Codification TM and the Hierarchy of Generally Accepted Accounting
Principles - a replacement of FASB Statement No. 162").FASB ASC 105-10
establishes the FASB Accounting Standards Codification TM (Codification) as the
source of authoritative accounting principles recognized by the FASB to be
applied by nongovernmental entities in the preparation of financial statements
in conformity with GAAP. FASB ASC 105-10 is effective for financial statements
issued for fiscal years and interim periods ending after September 15, 2009.
Adoption of FASB ASC 105-10 did not have a material effect on the Company’s
financial statements.
Financial
Instruments
In
determining fair value, the Company uses various valuation approaches within the
ASC 820-10 fair value measurement framework. Fair value measurements are
determined based on the assumptions that market participants would use in
pricing an asset or liability. ASC 820-10 establishes a hierarchy for
inputs used in measuring fair value that maximizes the use of observable inputs
and minimizes the use of unobservable inputs by requiring that the most
observable inputs be used when available. ASC 820-10 defines levels within the
hierarchy based on the reliability of inputs as follows:
|
·
|
Level
1 - Quoted prices in active markets for identical assets or
liabilities.
|
|
·
|
Level
2 - Inputs, other than the quoted prices in active markets, that are
observable either directly or
indirectly.
|
|
·
|
Level
3 - Unobservable inputs based on the Company’s
assumptions.
|
ASC
820-10 requires the use of observable market data if such data is available
without undue cost and effort. The Company’s adoption of ASC 820-10
did not result in any changes to the accounting for its financial assets and
liabilities.
The
recorded amounts of financial instruments, including cash equivalents accounts
payable and accrued expenses, and long-term debt approximate their market values
as of December 31, 2009 and December 31, 2008.
F-10
Convertible
Notes
In
accordance with ASC 470-20 we calculated the value of the beneficial conversion
feature embedded in the Convertible Notes. If the note is
contingently convertible, the intrinsic value of the beneficial conversion
feature is not recorded until the note becomes convertible.
Convertible
notes are split into two components: a debt component and a component
representing the embedded derivatives in the debt. The debt component represents
the Company’s liability for future interest coupon payments and the redemption
amount. The embedded derivatives represent the value of the option that
debtholders have to convert into ordinary shares of the Company. If
the number of shares that may be required to be issued upon conversion of the
Convertible Notes is indeterminate, the embedded conversion option of the
Convertible Notes are accounted for as derivative instrument liabilities rather
than equity as in accordance with ASC 815-40.
The debt
component of the convertible note is measured at amortized cost and therefore
increases as the present value of the interest coupon payments and redemption
amount increases, with a corresponding charge to finance cost – other than
interest. The debt component decreases by the cash interest coupon payments
made. The embedded derivatives are measured at fair value at each balance sheet
date, and the change in the fair value is recognized in the income
statement.
Concentration
of Risk
A
financial instrument which potentially subjects the Company to concentrations of
credit risk is cash. The Company places its cash with financial
institutions deemed by management to be of high credit quality. The amount on
deposit in any one institution that exceeds federally insured limits of $250,000
is subject to credit risk.
Intangible
Assets
Intangible
assets consist of intellectual property rights of an exclusive license to
several patent pending inventions surrounding our core
technologies. The Company accounts for goodwill and other
intangible assets in accordance with ASC 350-10. Goodwill and other
intangible assets are required to be tested at least on an annual basis for
impairment or on an interim basis if an event occurs or circumstances change
that would reduce the fair value of a reporting unit below its carrying value.
The fair value of definite lived intangible assets is determined by using a
“relief from royalty” approach. Intangible assets with estimable
useful lives and those assets with defined lives due to the legal nature of the
asset are amortized over their estimated useful lives, 15 years, using the
straight-line method. During the year ended December 31, 2009 and
2008 the Company recorded $3,336 and $0 in amortization expenses,
respectively.
Intangible
assets consisted of the following at December 31, 2009 and December 31,
2008:
|
2009
|
2008
|
|
|
Intangible
Assets, net:
|
||
|
Patents
|
$
98,559
|
$
-
|
|
License
|
112,902
|
-
|
|
Trademarks
|
10,938
|
-
|
|
222,399
|
-
|
|
|
Less:
Amortization
|
(3,336)
|
-
|
|
$
219,062
|
$
-
|
Accounts
Receivable
Accounts
receivable are stated at the amount management expects to collect from
outstanding balances. The Company provides for probable uncollectible
amounts through a charge to earnings and a credit to a valuation allowance based
on its assessment of the current status of individual
accounts. Balances that are still outstanding after management has
used reasonable collection efforts are written off through a charge to the
valuation allowance and a credit to accounts receivable. There was no provision
for bad debt at December 31, 2009 and 2008.
NOTE
3. GOING
CONCERN
The
accompanying financial statements have been prepared assuming the Company will
continue as a going concern. Because of the recurring operating losses, no
revenues and the excess of current liabilities over current assets, there is
substantial doubt about the Company’s ability to continue as a going concern.
As a
result of the aforementioned conditions the Company may be unable to meet
certain obligations to fund future research and development
activities. The Company’s continuation as a going concern is
dependent on obtaining additional outside financing, as it is not anticipated
that the Company will have profitable operations from its research and
development activities during the near term. The Company has funded losses
from its research and development and other operations primarily from the
issuance of debt and, equity. The Company believes that the issuance
of equity and debt will continue to fund operating losses in the short-term
until the Company can generate revenues sufficient to fund its
operations. If management can’t achieve its plans there is a
possibility that operations will discontinue.
NOTE
4. REVERSE ACQUISITION-RELATED PARTY
On May
29, 2009, the Company entered into and completed a reverse acquisition
pursuant to the Share Exchange Agreement pursuant to which Shrink
Nanotechnologies, Inc. acquired 100% ownership of Shrink Technologies, Inc., and
settled and restructured certain debts in exchange for the issuance of
44,444,440 shares of the of common stock and 50 shares of preferred stock of
Shrink Nanotechnologies, Inc. The assets and business that were acquired
by us as a result of the Share Exchange comprised primarily of an exclusive
licensing agreement (the “License Agreement”) with the Regents of the University
of California (the “Regents”). The License Agreement licenses an
array of intellectual property and inventions vital to our planned
operations. The Statement of Operations includes the full year
activity of Shrink Technologies (the accounting acquirer) and seven months
beginning May 29 of Shrink Nanotechnologies, Inc. (the public
parent).
F-11
This
transaction was accounted for as a reverse acquisition. As a result, all
financial information prior to May 29, 2009 is that of Shrink Technologies,
Inc. Following the merger, a reverse merger adjustment was made to
reflect Shrink Nanotechnologies, Inc. capital structure. All of the
assets and liabilities acquired in the reverse acquisition were recorded at
cost.
The
following is a condensed balance sheet disclosing the fair values of the Shrink
Business assets and liabilities acquired.
Assets
|
Cash
|
$
687
|
|
|
Website
Development
|
4,200
|
|
|
Patents
and other IP
|
26,928
|
|
|
Total
Assets
|
$
31,815
|
|
|
Liabilities
and Stockholders’ Deficit
|
||
|
Accounts
Payable
|
$
94,218
|
|
|
Notes
Payable
|
118,121
|
|
|
Total
Liabilities
|
212,339
|
|
|
Stockholders’
Deficit
|
(180,524)
|
|
|
Total
Liabilities and Stockholders’ Deficit
|
$
31,815
|
|
The
following represents the approximate pro-forma effect assuming the acquisition
with the companies had occurred on January 1, 2009, the beginning of the
Company’s current fiscal year, and on January 15, 2008 the date of inception,
including proforma adjustments for depreciation and interest
expense.
|
From
Inception
|
||
|
For
the year ended
|
January
15, 2008 to
|
|
|
December
31, 2009
|
December
31, 2008
|
|
|
Net
Loss
|
$
(1,141,554)
|
$
(4,512,119)
|
|
Earning
per share
|
$
(0.01)
|
$
(0.01)
|
Shrink
Technologies, Inc.’s president is and was Mark L. Baum, Esq., a director and
shareholder of the Company prior to the acquisition. Mr. Baum is now
president and CEO of Shrink Nanotechnologies, Inc.
NOTE
5. RESEARCH AND LICENSE AGREEEMENT
The
intellectual property and patent rights to our proprietary technology are owned
by the Regents. On June 16, 2008 (before we acquired them)
Shrink Technologies, Inc (“Shrink”), entered into an agreement (the “Option
Agreement”) with the Regents. Parallel to the Option Agreement,
Shrink entered into another agreement (the “Research Agreement”) with the
Regents, based on work which was to be performed at the University of
California, Merced (“UCM”).
The
Option Agreement provided Shrink with the exclusive right to license two patent
pending inventions (the “Regents Inventions”), and required Shrink to make
annual payments to the Regents as well as royalty payments on any commercialized
products based on the Regents Inventions. Shrink’s rights under
the Option Agreement required customary measures of performance on the part of
Shrink in terms of patent cost maintenance and other payments of costs
associated with the Regents Inventions. With respect to the Option
Agreement, Shrink’s rights were broad in terms of the potential access Shrink
had to use the Regents Inventions in products, and services and many of the key
economic terms of a future license, should Shrink exercise it’s rights under the
Option Agreement, were agreed to in the Option Agreement.
F-12
We
exercised our rights under the Option Agreement and as a result, entered into
the License Agreement for processes for microfluidic fabrication and other
inventions with the Regent which is currently our flagship asset.
The
License Agreement licenses an array of intellectual property and inventions
vital to our planned operations.
The term
of the License Agreement commenced April 29, 2009, and will continue in effect
until the expiration or abandonment of the last patent covered by the Agreement,
unless we terminate it on 60 days notice or the Regents terminated it on 60 days
notice if we default on our obligations under the agreement and fail to cure
such default during the 60 day period. Under the License Agreement
Shrink is permitted to make, use, sell and offer for sale the licensed
intellectual property and import licensed products and services and to practice
licensed methods in all fields and in all locations in which the Regents may
lawfully grant the licensed rights.
Our fees
to the Regents under the License Agreement include:
|
·
|
A $100,000
contract initiation fee paid to the Regents in the form of 495,500 shares
of common stock of the Company; however, we have the option to reacquire
the shares for $100,000 at any time through April
2010;
|
|
·
|
A $25,000
annual maintenance fee, unless, (i) by the time that such
annual maintenance fee is due, Shrink has already commercialized its
principal product and begun paying earned royalties; or (ii) the Company
maintains the Research Agreement or another research agreement at the
University of California, Merced.
|
|
·
|
A
fee of 30% of all income attributed to revenues from a sublicensed product
or technology;
|
|
·
|
Milestone
payments of $100,000 for total accumulated nets sales of $50,000,000;
$500,000 for accumulated net sales of $150,000,000; and
$2,000,000 for net sales of $500,000,000;
and
|
|
·
|
Earned
royalty payments based on net sales of licensed
products.
|
A minimum
$15,000 earned royalty payment will be due each year following the first
commercial sale of a licensed product. The minimum payment will
increase to $20,000 on the first commercial sale of the fourth licensed product,
and by an additional $5,000 after a commercial sale of each additional licensed
product. The minimum payments are payable by February 28 of each year
in which they are due. Earned royalties in excess of the minimums are
payable quarterly.
The
License Agreement encourages early commercialization of the licensed rights, by
providing that our earned royalty payments based on revenues shall be set at
between 2.5% of net sales in the event that the first commercial sale commences
prior to April of 2012; 4% of net sales in the event that the first commercial
sale commences after April 2012 but before the April 2015; and 5% of net sales
if the first commercial sale commences thereafter. First
commercial sale is defined as bona fide good faith sale of products that are
part of our licensed rights, in quantities sufficient to meet market demands,
and includes sales for any consideration.
If
we use the licensed products or methods as research tools, we are required to
pay royalties also, at a rate to be agreed upon, but no less than the rate
charged by the Regents for similar research tools licensed to
others.
The
Research Agreement commits Shrink to fund research based on the Regents
Inventions at UCM up to the amount of $640,935 in accordance with a planned
budget. The Research Agreement provides Shrink with an exclusive
right to license all technology that is discovered from the monies funded to UCM
through the Research Agreement (the “Derivative IP”). To the extent
that Shrink exercises its rights under the Research Agreement, Shrink will be
required to make customary annual payments to the Regents, who shall be the
owners of any Derivative IP, as well as royalty payments as any
commercialization of such Derivative IP occurs. Subsequent to the
execution of the License Agreement, all Derivative IP flows into the same
License Agreement, and the use of said Derivative IP and the relative economic
terms regarding its use, are contained within the License
Agreement.
Shrink
shall reimburse UCM on a quarterly basis for all direct and indirect cost
incurred in connection with the research. These funds will be used to
fund researchers’ salaries, equipment, materials, supplies, and other
miscellaneous expenses incurred by UCM. In accordance with the
planned budget, Shrink has been billed through December 31, 2009 and expects
future expenses to be as follows:
|
Amount
|
|
|
2008
|
79,958
|
|
2009
|
75,644
|
|
2010
|
192,595
|
|
2011
|
192,799
|
|
2012
|
99,939
|
|
Total
|
640,935
|
F-13
NOTE
6. COMMITMENTS AND LEASES – RELATED PARTY
The
Company previously shared office space with the Noctua Fund Manager, LLC, which
is affiliated with two of our directors. On January 1, 2009 we began
accruing a $2,500 rental expense each month for the use of the space. From
January 1, 2009 to May 28, 2009 there was not a written agreement; there was
simply a verbal agreement between the two companies. The verbal agreement
ceased on May 29, 2009, pursuant the Company’s execution of a
sublease.
Mark L.
Baum, Esq. (“Baum”) our CEO and president, is a managing member of the Noctua
Fund Manager, LLC. James B. Panther, II (“Panther”), one of Shrink’s
directors, is also a managing member of the Noctua Fund Manager,
LLC. As of December 31, 2009, there was $12,500 owed to the Noctua
Fund Manager, LLC and no payments have been made to the Noctua Fund Manager,
LLC.
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010, and will continue as a month to month
tenancy thereafter. At December 31, 2009 $42,000 had been paid
to Business Consulting Group Unlimited, Inc and nothing was owed to Business
Consulting Group Unlimited, Inc.
On May
29, 2009, the Company signed an operating agreement (the “Operating Agreement”)
with BCGU, LLC, an entity indirectly controlled by James B. Panther II, and Mark
L. Baum, Esq., who are two of our directors, for a fee of $6,000 per
month. During October 2009, the Company amended the Operating
Agreement with BCGU, LLC. The amended Operating Agreement (“the “Amended
Operating Agreement”) allows us to retain certain day-to-day administrative
services and management in consideration of a monthly fee of $30,000 per
month. The Amended Operating Agreement also included a $270,000 signature
bonus. The Amended Operating Agreement expires October 1,
2012. At December 31, 2009 $42,000 had been paid to BCGU, LLC and
there was $342,000 owed to BCGU, LLC. There is an option within the
Amended Operating Agreement that allows BCGU, LLC to convert outstanding
payables related to the Operating Agreement into a 10% convertible
note. The Company believes, based on review of its independent
directors, that the foregoing transactions and agreements are no less favorable
(or even more favorable, given the flexibility) to the Company than would
otherwise be available from independent third parties.
The
Company’s rent expense for the years ended December 31, 2009 and 2008 was
$54,500 and $0, respectively.
The
following represents future minimum lease payments for the next 5
years:
|
Amount
|
|
|
2010
|
$ 24,000
|
|
2011
|
-
|
|
2012
|
-
|
|
2013
|
-
|
|
2014
|
-
|
|
Total
|
$ 24,000
|
NOTE
7. CONVERTIBLE
DEBENTURES, WARRANTS
In
accordance with ASC 470-20, we recognize the advantageous value of conversion
rights attached to convertible debt. Such rights give the debt holder the
ability to convert his debt into common stock at a price per share that is less
than the trading price to the public on the day the loan is made to the Company.
The beneficial value is calculated as the intrinsic value (the market price of
the stock at the commitment date in excess of the conversion rate) of the
beneficial conversion feature of debentures. This feature is recorded
as a discount to the related debt and an addition to additional paid in capital.
The discount is amortized over the remaining outstanding period of related debt
using the interest method.
F-14
|
A.
|
Convertible
Debentures – Third Party
|
On July
1, 2007, a $25,000 zero coupon convertible debenture was issued in exchange for
cash, which was used to purchase and retire shares from a
shareholder. This debenture was amended on July 1, 2008, to reflect a
10% interest rate and maturity date of December 31, 2008. The
debenture was due and payable on December 31, 2008. It is convertible
into common stock at the price of $0.03 per share. The conversion
price is subject to proportional adjustment for reclassification, stock splits,
combinations and dividends. At the time of the agreement date, there
was no determinable stock price, therefore there is no beneficial conversion
feature that applies to this debenture. On September 1, 2009, the
Company received a notice of conversion for the principal balance of this
debenture and accrued interest. On October 6, 2009, the Company
converted this debenture into 958,335 shares of common stock to satisfy the
conversion demand in full.
|
B.
|
Convertible
Debentures – Related Party
|
On July
1, 2008, we consolidated all obligations due and owing to The Baum Law Firm, PC
and Mark L. Baum, Esq., a related party and one of our executive officers, for
legal services rendered to the Company, and issued to Meaux Street Partners LP,
a Baum related party, a 10% promissory note for $25,000. The note was
due and payable on December 31, 2008. It is convertible into common
stock at the price of $0.03 per share. The conversion price is
subject to proportional adjustment for reclassification, stock splits,
combinations and dividends. At the time of the note agreement date,
the Company recorded a $16,667 discount, which represents the intrinsic value of
the beneficial conversion feature. The discount of $16,667 is being
amortized over the life of the note. On September 1, 2009, the
Company received a notice of conversion for the principal balance of this note
and accrued interest. On October 6, 2009, the Company converted this
note into 958,335 shares of common stock to satisfy the conversion demand in
full.
On May 7,
2009, Shrink Nanotechnologies, Inc. entered into a debt consolidation agreement
(the “Debt Consolidation Agreement”) with Noctua Fund LP to consolidate certain
secured and unsecured liabilities (“Dao Liabilities”) which were originally
assigned to Dao Information Systems, Inc. (“Dao”). While the Company
did make an assignment of the Dao Liabilities to Dao, the Company was legally
responsible to Noctua Fund LP for the principal and interest related to the Dao
Liabilities. The principal amount of the secured part of the Dao
Liabilities was $76,500 and the principal amount of the unsecured part of the
Dao Liabilities was approximately $5,000. All of the promissory notes
underlying the Dao Liabilities have matured and were in default.
The Debt
Consolidation Agreement consolidates all monies presently owed to Noctua Fund LP
which are consolidated into one new secured convertible promissory note with a
principal amount of $100,000. The note’s maturity date is October 1,
2012. The new note accrues interest at fourteen percent (14%) but
does not begin to accrue interest until October 1, 2009. Interest
payments on the note are due monthly. The note has a conversion price
of $.04 per share. In accordance with ASC 470-50, the
Company determined this to be a substantial modification to the debt instruments
and prior to the merger applied debt extinguishment accounting to record a loss
on extinguishment of debt of $100,000.
At
December 31, 2009 the Company had not made any of the required interest payments
and as a result this note was in default and accruing interest at its default
rate of 18%.
On May
29, 2009, we assumed convertible notes and accrued interest of $118,121 owed to
Noctua Fund LP, as part of the share exchange agreement with the shareholders of
Shrink Technologies, Inc. The terms of these notes were then renegotiated into a
new note with a principal amount of $118,121. The note’s maturity
date is October 1, 2012. The note accrues interest at fourteen
percent (14%) but did not begin to accrue interest until October 1,
2009. Interest payments on the note are due monthly. The
note is convertible into shares of our common stock at an original conversion
price of $.04 per share. In accordance with ASC 470-50, the Company
has determined this to be a substantial modification to the debt instruments and
has applied debt extinguishment accounting to record a loss on extinguishment of
debt of $118,121 for the year ended December 31, 2009.
At
December 31, 2009 the Company had not made any of the required interest payments
and as a result this note was in default and accruing interest at its default
rate of 18%.
Baum,
our president and CEO, and Panther, a director, are equal indirect beneficial
owners of Noctua Fund Manager, LLC, the general partner of Noctua Fund, L.P.,
and are non-voting minority investors in the Noctua Fund,
L.P.
F-15
|
C.
|
Convertible
Debentures, Warrants - Private Placement
Offering
|
During
November of 2009, a confidential financing offering (“the Offering”) was made by
the Company to various private accredited investors. The Offering was
set to close on March 31, 2010. The principal amount of the Offering
is set at $1,000,000 with excess of $1,000,000 accepted at the option of the
Company and consists of convertible notes and stock purchase
warrants. The notes will mature at the one year anniversary of their
effective date, be sold at their face value, accrue interest at 12% and have a
conversion feature that allows the investor to convert the note and accrued
interest into common stock at a price of $0.10 per share. The Class A
common stock purchase warrants are exercisable via cashless exercise commencing
six months after each respective closing, at $0.20 per share, expiring 3 years
from the first closing of this Offering. The investors shall be
issued warrants to purchase such number of common stock as equals fifty percent
(50%) of the number of common shares underlying the convertible note based on
the fixed conversion price. The warrants shall contain a standard cashless
provision, which permits the holder to exercise the warrants if the stock price
is above the exercise price, by turning in warrants and not paying
cash.
On
November 12, 2009, a $100,000 12% convertible note and detachable
series A warrants to purchase up 500,000 shares of common stock were issued in
exchange of cash as part of the Offering. The note’s maturity date is
November 12, 2010. At the time of the issuance date, the Company
recorded a $69,900 discount, which represents the intrinsic value of the
beneficial conversion feature. The discount is being accreted over 12
month life of the note.
In
accordance with FASB ASC 470-20, the Company used the effective conversion price
based on the proceeds received to compute the intrinsic value of the embedded
conversion option. The Company allocated the proceeds received from
the offering to the convertible instrument and the detachable warrants included
in the exchange on a relative fair value basis. The Company then
calculated an effective conversion price and used that price to measure the
intrinsic value of the embedded conversion option. The conversion
price of the convertible notes is equal to $0.50 per share of the Company’s
common stock. The number of shares issuable upon conversion of the
notes shall be determined by dividing the outstanding principal amount, together
with accrued but unpaid interest, to be converted by the conversion price in
effect on the conversion date.
Warrants
associated with the Offering were issued to purchase up to 500,000 shares at an
exercise price of $.20 per share. These warrants are valued at
$30,100 and were combined with the
beneficial conversion feature of $69,900, to record a 100,000 discount on the
convertible note. The warrants are exercisable at 6 months following
their effective date and begin to expire 11/12/2012. The warrants
were valued using a Black-Scholes valuation model. The variables used
in this option-pricing model included: (1) discount rate of 1.36% (2) expected
warrant life is the actual remaining life of the warrant as of each period end,
(3) expected volatility of 286% and (4) zero expected dividends.
There are
no warrants immediately exercisable at December 31, 2009 and 2008.
Notes
Payable consists of the following at December 31, 2009 and 2008:
|
2009
|
2008
|
|
|
14%
convertible note due October 1, 2012
|
$
218,121
|
$
91,094
|
|
12%
convertible note due November 12, 2011
|
100,000
|
-
|
|
Total
convertible notes payable
|
$
318,121
|
$
91,094
|
|
Less:
Discount on notes
|
(86,389)
|
-
|
|
Less:
Current portion
|
(231,732)
|
(45,547)
|
|
Long-term
portion
|
$
-
|
$
45,547
|
The
following represents minimum payments due for notes payable:
|
Amount
|
|
|
2010
|
318,121
|
|
2011
|
-
|
|
2012
|
-
|
|
Total
|
$ 318,121
|
The
Company has notes payable in the amount of $218,121 with a maturity date of
October 1, 2012. These notes are currently in default due to
non-payment of monthly interest accruals and are classified as current
liabilities on the balance sheet. Interest payments are due
monthly on these notes.
F-16
NOTE
8. COMMON
STOCK
All share
amounts have been restated to reflect a 5 for 1 forward stock
split.
The
Company has made various private issuances of securities to fund its operations
and satisfy obligations from time to time.
Pursuant
to the reverse merger the Company issued 44,444,440 shares of common stock to
the shareholders of shrink Technologies. Reverse merger accounting requires
these shares to be shown retroactively as though a stock split had
occurred. Therefore this issuance adjusted the initial issuance to
the founders at inception.
Pursuant
to this same reverse acquisition, the statement of stockholders equity is then
adjusted to reflect the shareholders of the public entity (115,004,875) while
establishing the equity of the parent Company. The retained deficit of the
parent Company is removed and reorganized to reflect only the history of the
subsidiary.
During
June 2009, the Company issued 3,625,000 shares of common stock in exchange for
cash of $145,000.
During
June 2009, the Company issued 100,000 shares of common stock to consultants with
a value of $20,400 as payment for services.
During
June 2009, the Company issued 495,500 shares of common stock with a value of
$100,000 as payment for a contract initiation fee related to our license
agreement.
During
July 2009, the Company issued 150,000 shares of common stock to consultants with
a value of $30,600 as payment for services rendered.
During
July 2009, the Company issued 250,000 shares of common stock in exchange for
cash of $10,000.
During
August 2009, the Company issued 1,250,000 shares of common stock in exchange for
cash of $50,000.
During
August 2009, the Company issued 1,741,670 shares of common stock to consultants
with a value of $374,800 as payment for services
During
September 2009, the Company issued 3,500,000 shares of common stock in exchange
for cash of $140,000.
During
September 2009, the Company issued 25,000 shares of common stock to an employee
with a value of $5,100 as payment for services.
During
October 2009, the Company issued 250,000 shares of common stock in exchange for
cash of $10,000
During
October 2009, the Company issued 66,670 shares of common to consultants with a
value of $15,600 as payment for services.
During
November 2009, the Company issued 1,916,670 shares of common stock for
conversion of convertible notes and accrued interest totaling
$57,500.
During
December 2009, the Company issued 101,660 shares of common stock to consultants
with a value of $23,398 as payment for services.
F-17
NOTE
9. PREFERRED STOCK
The
Preferred Series A shares (“Series A”) provide the holder of the same with
certain voting rights, among other rights, that may equal, when cast, a majority
of the votes which could be cast at a meeting of the Company’s
shareholders. The Series A holders have such number of votes as is
determined by multiplying (a) the number of shares of Series A held by such
holder, (b) the number of issued and outstanding shares of the Company’s Series
A and common stock on a fully diluted basis as at the record date for the vote
and (c) .0000002. Series A have no express dividend right, and are
convertible into common shares at a ratio of 1:1.
Pursuant
to the reverse acquisition, the statement of stockholders equity was adjusted to
reflect the Preferred Series A shareholders of the public entity (20,000,000)
while establishing the equity of the parent Company.
The
Preferred Series B shares provide the holder with a significant dividend right
(59.5% of gross revenues), among other rights. These shares carry no
voting rights and are not convertible into common shares. The Company
currently has no shares of Preferred Series B Stock issued.
The
Preferred Series C shares provide the holder the right to receive a cash
dividend of 15% of the Company’s gross revenues. This dividend amount
will be payable in the form of cash, but the holders may elect to accept stock
as payment. The value of any stock payable to the holders will be
determined by the closing price of stock on the date the dividend is
declared. The holders of the series C preferred shares have no voting
rights.
Pursuant
to the reverse merger 50 Preferred Series C shares were issued to our
founder as part of a founder issuance. The statement of
stockholders equity was then adjusted to reflect the Preferred Series C
shareholders of the public entity (100) while establishing the equity of the
parent Company. During October 2009, the Company cancelled all of its
Preferred Series C stock. The holders of the Series C Stock in return
received certain legacy assets of the Company. Pursuant to the
settlement of a legal matter, described in Note 11, the Company recorded a
reduction of stockholders equity for $143,825, which is due to the former
Preferred Series C shareholders as part of their cancellation
agreement. The Company currently has no shares of
Preferred Series C stock issued.
NOTE
10. STOCK BASED COMPENSATION
The
Company has issued stock options to key employees, consultants, and
non-employee's advisors and directors of the Company. These issuances shall be
accounted for based on the fair value of the consideration received or the fair
value of the equity instruments issued, or whichever is more readily
determinable. The Company has elected to account for the stock option plan in
accordance with ASC 718-10 where the compensation to employees should be
recognized over the period(s) in which the related services are rendered. In
accordance with ASC 718-10 the fair value of a stock option granted is estimated
using an option-pricing model. As of December 31, 2009, the following stock
options were outstanding:
275,000
options were issued to the members of our advisory board and employees. These
options are valued at $141,713 the options are exercisable for 1-2 years
following their effective dates that begin to expire 9/1/2010. The
options were valued using a binomial valuation model. The variables
used in this option-pricing model included: (1) discount rates of 0.67-1.04%,
(2) expected option life is the actual remaining life of the options as of each
period end, (3) expected volatility of 200%-286% and (4) zero expected
dividends.
The
following summarizes options as of December 31, 2009 and 2008:
|
Amount
|
Weighted
Average Exercise Price
|
|
|
Outstanding
January 15, 2008 (date of inception)
|
-
|
$
-
|
|
Expired/Retired
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Issued
|
-
|
-
|
|
Outstanding
December 31, 2008
|
-
|
$
-
|
|
Expired/Retired
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Issued
|
275,000
|
.216
|
|
Outstanding
December 31, 2009
|
275,000
|
$
.216
|
F-18
The
following summarizes the changes in options outstanding for the year ended
December 31, 2009:
|
Options
Outstanding
|
Options
Exercisable
|
|||||||||||
|
Number
|
Weighted
Average
|
Remaining
Exercise
|
Number
|
Weighted
Average
|
||||||||
|
Date
of Grant
|
of
Shares
|
Exercise
Price
|
Life
in Years
|
Exercisable
|
Exercise
Price
|
|||||||
|
Second
Quarter Fiscal 2009
|
75,000
|
$ .204
|
1.41
|
375,000
|
$
.204
|
|||||||
|
Third
Quarter Fiscal 2009
|
75,000
|
.22
|
1.66
|
375,000
|
.22
|
|||||||
|
Fourth
quarter fiscal 2009
|
25,000
|
.22
|
1.87
|
125,000
|
.22
|
|||||||
|
Fourth
quarter fiscal 2009
|
75,000
|
.22
|
1.91
|
375,000
|
.22
|
|||||||
|
Fourth
quarter fiscal 2009
|
25,000
|
.22
|
1.92
|
125,000
|
.22
|
|||||||
|
Total
at December 31, 2009
|
275,000
|
$
.216
|
275,000
|
$ .216
|
||||||||
The
following summarizes stock purchase warrants (Note 7) as of December 31, 2009
and 2008:
|
Amount
|
Weighted
Average Exercise Price
|
|
|
Outstanding
January 15, 2008 (date of inception)
|
-
|
$
-
|
|
Expired/Retired
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Issued
|
-
|
-
|
|
Outstanding
December 31, 2008
|
-
|
$
-
|
|
Expired/Retired
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Issued
|
500,000
|
.20
|
|
Outstanding
December 31, 2009
|
500,000
|
$
.20
|
The
following summarizes the changes in warrants (Note 7) outstanding for the year
ended December 31, 2009:
|
Warrants
Outstanding
|
Warrants
Exercisable
|
|||||
|
Number
|
Weighted
Average
|
Remaining
Exercise
|
Number
|
Weighted
Average
|
||
|
Date
of Grant
|
of
Shares
|
Exercise
Price
|
Life
in Years
|
Exercisable
|
Exercise
Price
|
|
|
Fourth
quarter fiscal 2009
|
500,000
|
$
.20
|
2.87
|
-
|
$
-
|
|
|
Total
at December 31, 2009
|
500,000
|
$
.20
|
-
|
$
-
|
||
NOTE
11. LEGAL MATTERS – DISCONTINUED
OPERATIONS
On
October 28, 2009, the Company filed a complaint in the Superior Court for the
County of San Diego against American Scientific Resources, Inc. (“ASFX”) for,
among other causes of action, breach of contract. The Company believes ASFX
intentionally misled the Company by issuing untradeable restricted stock as
compensation for services rendered pursuant to the Company’s services agreements
with ASFX. ASFX cross-claimed against the Company for breach of
contract and other claims related to the services provided by the
Company. This agreement is related to a discontinued business
division, Audiostocks.com. Proceeds from this litigation were
recorded as part of our discontinued operations.
In
December 2009, the Company settled part of the litigation, received proceeds and
recorded a gain of $35,814. In February 2010, the Company reached a
final settlement agreement with ASFX and all claims were dismissed, including
all action against the Company. As a result of the final settlement agreement,
the Company recorded another gain of $108,011, during the year ended December
31, 2009 The net gain recorded from the litigation was $143,825. Pursuant to the
cancellation agreement with the former Preferred Series C shareholders (Note 9),
the Company also recorded a liability for the $143,825 due to the former
shareholders and reduced stockholders equity by the same
amount. Management believes at this time that the final resolution of
the matter will not have a material adverse effect upon the Company’s
consolidated annual results of operations, cash flows or financial
position.
F-19
NOTE
12. SUBSEQUENT EVENTS
The
Company has performed an evaluation of events occurring subsequent to year end
through the issuance date of this report. Based on our evaluation, nothing other
than the below events need to be disclosed.
During
the months of January and February of 2010, the Company issued $335,000 in
convertible notes and 335,000 warrants as part of the confidential financing
offering (Note 7) in exchange of $335,000.
On
February 10, 2010 the Company resolved all its claims with American Scientific
Resources, Inc. (Note 11). As a result of the settlement all claims
were dismissed, including all action against the Company. The
settlement has no adverse affect effect on the Company’s financial
results.
During
the month of March 2010, pursuant to agreements and commitments the Company
issued 18,382,500 shares of common stock with a value of $3,127,025 as payment
for various bona fide services.
Following
a shareholder action described in a Form 14C filed on with the Securities and
Exchange Commission on February 15, 2010, the Board of Directors effected a 5
for 1 forward split. All per share amounts and calculations in our
presentation reflect this change.
F-20
|
ITEM
8.
|
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE.
|
None.
|
ITEM
9A
|
CONTROLS
AND PROCEDURES.
|
Evaluation
of Disclosure Controls and Procedures
We
maintain disclosure controls and procedures (as defined in Rule 13a-15(e) under
the Exchange Act) that are designed to ensure that information that would be
required to be disclosed in Exchange Act reports is recorded, processed,
summarized and reported within the time period specified in the SEC’s rules and
forms, and that such information is accumulated and communicated to our
management, including to our Chief Executive Officer and Chief Financial
Officer, as appropriate, to allow timely decisions regarding required
disclosure.
As
required by Rule 13a-15 under the Exchange Act, our management,
including our Chief Executive Officer and Chief Financial Officer,
evaluated the effectiveness of the design and operation of our disclosure
controls and procedures as of December 31, 2009. Based on that
evaluation, our Chief Executive Officer and Chief Financial
Officer concluded that as of December 31, 2009, and as of the date that the
evaluation of the effectiveness of our disclosure controls and procedures was
completed, our disclosure controls and procedures were not effective to satisfy
the objectives for which they are intended.
Management’s
Annual Report on Internal Control Over Financial Reporting
Our
management is responsible for establishing and maintaining adequate internal
control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f)
under the Exchange Act. The Exchange Act defines internal control over financial
reporting as a process designed by, or under the supervision of, our principal
executive and principal financial officers and effected by our board of
directors, management and other personnel, to provide reasonable assurance
regarding the reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with accounting
principles generally accepted in the United States of America and includes those
policies and procedures that:
|
·
|
Pertain
to the maintenance of records that in reasonable detail accurately and
fairly reflect the transactions and dispositions of the assets of the
Company;
|
|
·
|
Provide
reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with accounting
principles generally accepted in the United States of America, and that
our receipts and expenditures are being made only in accordance with
authorizations of our management and directors;
and
|
|
·
|
Provide
reasonable assurance regarding prevention or timely detection of
unauthorized acquisition, use or disposition of our assets that could have
a material effect on our financial
statements.
|
All
internal control systems, no matter how well designed, have inherent
limitations. Therefore, even those systems determined to be effective can
provide only reasonable assurance with respect to financial statement
preparation and presentation. Also, projections of any evaluation of
effectiveness to future periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree of compliance
with the policies or procedures may deteriorate.
Management
assessed the effectiveness of our internal control over financial reporting as
of December 31, 2009. In making this assessment, management used the framework
set forth in the report entitled Internal Control - Integrated Framework issued
by the Committee of Sponsoring Organizations of the Treadway Commission, or
COSO. The COSO framework summarizes each of the components of a company’s
internal control system, including (i) the control environment, (ii) risk
assessment, (iii) control activities, (iv) information and communication, and
(v) monitoring. Based on our assessment we determined that, as of December 31,
2009, our internal control over financial reporting is not effective at the
reasonable assurance level based on those criteria, due to the following
weakness described below.
Insufficient segregation of duties
in our finance and accounting functions due to limited personnel.
During the year ended December 31, 2009, the company internally performed
all aspects of our financial reporting process, including, but not limited to,
access to the underlying accounting records and systems, the ability to post and
record journal entries and responsibility for the preparation of the financial
statements. Due to the fact these duties were performed oftentimes by the
same people, a lack of review was created over the financial reporting process
that might result in a failure to detect errors in spreadsheets, calculations,
or assumptions used to compile the financial statements and related disclosures
as filed with the SEC. These control deficiencies could result in a
material misstatement to our interim or annual financial statements that would
not be prevented or detected.
40
Insufficient corporate governance
policies. Although we have a code of ethics which provides broad
guidelines for corporate governance, our corporate governance activities and
processes are not always formally documented. Specifically, decisions made
by the board to be carried out by management should be documented and
communicated on a timely basis to reduce the likelihood of any misunderstandings
regarding key decisions affecting our operations and management.
We intend
to take appropriate and reasonable steps to make the necessary improvements to
remediate these deficiencies and we intend to consider the results of our
remediation efforts and related testing as part of our next year-end assessment
of the effectiveness of our internal control over financial
reporting.
This
annual report does not include an attestation report of our registered public
accounting firm regarding internal control over financial reporting.
Management’s report was not subject to attestation by our registered public
accounting firm pursuant to temporary rules of the SEC that permit the Company
to provide only management’s report in this annual report.
Changes
in Internal Control Over Financial Reporting
There
have been no changes in our internal control over financial reporting during the
fourth quarter of fiscal year 2009 that have materially affected, or are
reasonably likely to materially affect, our internal control over financial
reporting.
|
ITEM 9B
|
OTHER
INFORMATION.
|
During
the fourth quarter of 2010, the holders of our 150 shares of Series C Preferred
Stock returned all shares of Series C Preferred Stock to the Company for
cancellation, in exchange for $143,825 in consideration. No
distributions or rights have ever been issued in respect of such Series C
Preferred Stock. We have no information to disclose that was required
to be disclosed in a report on Form 8-K during fourth quarter of fiscal year
2009, but was not reported.
41
PART
III
|
ITEM
10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE.
|
Directors
and Executive Officers
The
following table lists the officers and directors of Shrink
Nanotechnologies:
|
Name
|
Age
|
Position
|
|
Mark
L. Baum, Esq.
|
37
|
CEO
and Director
|
|
Marshall
Khine, Esq.
|
37
|
Director,
Secretary
|
|
James
B. Panther, II
|
37
|
Chairman
of the Board of Directors
|
|
Heiner
Dreismann, Ph.D.
|
56
|
Director
|
|
Luis
J. Leung
|
37
|
President,
CEO, CFO, Director (Resigned as of May 29,
2009)
|
Mark L. Baum, Esq. is an
executive with more than 15 years experience financing, operating and advising
smallcap and microcap publicly traded enterprises. As a manager of
capital, including that of Black Forest International LLC and Noctua Fund LP,
two funds he founded and co-manages, he has completed more than 125 rounds of
financing for more than 40 publicly traded companies. Mr. Baum has participated
in numerous public company spin-offs, restructurings and recapitalizations,
venture fundings, private-to-public mergers, and multi-million dollar asset
acquisitions and divestitures. In additional to the operation of Black Forest
and Noctua, Mr. Baum has operational experience in areas as diverse as
diagnostic testing businesses, closed door pharmacies, cleaner and renewable
energy companies and retail furniture. Mr. Baum co-founded Shrink
Nanotechnologies, Inc., a nanotechnology company based on international award
winning science, and FIGA.org, an expert network service that brings together
professionals from the worlds of finance, industry, government and
academia. As a restructuring officer and advisor for financially
troubled companies, Mr. Baum has a history of Boards of Directors and Boards of
Advisors service for numerous domestic and international private and public
companies, including Chembio Diagnostics, Inc., AudioStocks, Inc., Co-Connect,
Inc., VisiTrade, Inc., ProtoKinetix, Inc., AlphaNutra, Inc., Metaphor
Corporation and PNG Ventures, Inc., a company which in 2009 filed under Chapter
11 of the US Bankruptcy Code. Mr. Baum founded and capitalized
the Mark L. Baum Scholarship which has funded grants to students at the
University of Texas at Arlington and other educational and religious
institutions. He is a trustee of the Collier de Bleu Trust, which is
dedicated to funding educational opportunities for non-English speaking children
in Mexico. Mr. Baum is a published inventor and a licensed attorney
in the State of California.
Marshall Khine, Esq. is an
Assistant District Attorney for the San Francisco District Attorney’s
Office. For the past four years, Mr. Khine has served on the Homicide
Trial Team prosecuting murder cases. He has been a trial lawyer for over
ten years with extensive experience in many areas of complex litigation
including sexual assault crimes, hate crimes, theft and fraud related crimes,
grand jury work, forensic evidence litigation, psychiatric evidence litigation
and wiretap operations. He graduated from Cornell University in
Ithaca, New York in 1994. He attended California Western School of Law in
San Diego, California on a full academic scholarship and graduated early with
honors in 1997. While in law school and after law school, he worked for
the In-house Legal Department of Cubic Corporation in San Diego. Since
1998, Mr. Khine has resided in the San Francisco Bay Area.
James B. Panther, II is a
co-founder, along with Mr. Mark L. Baum, Esq. and Dr. Michelle Khine of
Shrink. Mr. Panther II has served as one of our
directors since May 5, 2009. He currently is responsible for the day to day
investment decision for Noctua Fund, LP. He brings significant experience in
managing fund raising, financing, M & A, and advisory services in a merchant
banking environment. Mr. Panther II has raised, funded and participated in more
than five hundred million dollars of debt and equity financing for private and
publicly traded companies. As Managing Partner of Bristol Partners he was
responsible for portfolio investments, leading over 20 transactions ranging from
$15 million to $300 million in valuation and building a team of professionals
that closed over 40 transactions. As a restructuring officer and advisor for
financially troubled companies, Mr. Panther II served on the Board of Directors
for PNG Ventures, Inc., a company which in 2009 filed under Chapter 11 of the US
Bankruptcy Code. Mr. Panther II holds B.A. in Finance from
Boston College and a Degree in Economics from the University of Granada,
Granada, Spain. He is fluent in English and Spanish.
42
Heiner Dreismann, Ph.D., joined the board on June 22,
2009 and, is an international executive with more than 24 years experience in
the biotech and healthcare industries. Dr. Dreismann has a proven track record
in general management with P&L responsibility as well as management of
corporate functions such as research and development, manufacturing, marketing,
business development, licensing, mergers and acquisitions and several board
memberships of public and private companies. Dr. Dreismann served as
the President and CEO of Roche Molecular Systems, Inc., from 2000-2006, leading
a staff of more than 1,600 and growing the RMS molecular IVD business from $640M
to $1.2B in annual sales. Prior to serving as the senior executive at
Roche Molecular Systems, Inc., Dr. Dreismann served as the Head of Global
Business Development at Roche Diagnostics, responsible for business development
for the Roche Diagnostics $7B IVD business. From 1997 to 1999, Dr.
Dreismann served as the Head of Integration for Roche Diagnostics, managing the
merger of the Roche Diagnostics’ IVD business with Boehringer
Manheim. Dr. Dreismann served in a number of other leadership
capacities at Roche Diagnostics and Roehm GmbH, dating back to
1983. Dr. Dreismann’s post-doctoral fellowship was earned at the
French Center for Nuclear Research in Saclay (near Paris). He earned
his Masters Degree Diploma in Biology from Westfaelische Wilhelms University,
Muenster, a Ph.D. in Microbiology/Molecular Biology from Westfaelische Wilhelms
University, Muenster (summa cum laude). Dr. Dreismann currently
serves on the Boards of GeneNews, Sirius Genomics, Smart Holograms, OxFord
BioDynamics, Magellan Diagnostics, Singulex, MedBioGene, BioHelix, Celula,Inc.,
AlliedPath and Stratos Genomics.
Luis J. Leung, Mr. Leung was
our Director and served in various executive officer capacities through May 29,
2010, at which time he resigned as director and from all other
positions. Mr. Leung was affiliated with various entities and
ventures with Messers. Baum and Panther, II, including other privately held or
public companies. Mr. Leung began his career in São Paulo, Brazil in
1988 with Smar Equipamentos Industrias Ltd as a Research and Development
Software Engineer. In 1991 he was helped establish the company’s operations in
the United States and was promoted to CIO and Comptroller. From 1994 to
1997 Mr. Leung helped structure operations and systems for the company’s
headquarters as well as for its subsidiaries in Germany, France and
Singapore. In 2004, Mr. Leung co-founded Scitus Corporation, a
Microsoft VAR and ISV, and the Alba Spectrum Group a consulting company. The
Alba Spectrum Group has operations in Chicago, Houston and São Paulo, Brazil.
Its line of products includes Microsoft, SAP and Oracle. In 2001, Mr. Leung
co-founded Advent Corporation, a software development company and Microsoft
Partner. The company merged with Enterlogix Corporation in 1992. Enterlogix
Corporation, after the merger, was the largest Microsoft Great Plains
implementation practice in the state of Texas. In 1997, Mr. Leung started
his consulting career at Hein + Associates LLP in Houston, TX as a Project
Manager. In 1999 he was hired by Grant Thornton, LLP as a Consulting Manager and
participated in several multi-country systems implementations. In 2000, he
worked at eParners Inc as a CRM Practice Leader. Mr. Leung holds a
Bachelors of Science from Instituto Tecnologico Aerospacial,
Brazil.
Scientific Advisory
Board
The
Company has created a scientific advisory board and appointed members who
previously served on the Scientific Advisory Board of Shrink. Members
of the Scientific Advisory Board are not employees of the Company or members of
our Board of Directors.
Sayantani Ghosh, PhD is
Assistant Professor at the School of Natural Sciences at the University of
California Merced. She has performed significant research and is published in
the fields of spintronics, liquid crystal quantum dot ensembles, plasmonics, and
photovoltaics.
Fabian Pease, PhD is Professor
of Electrical Engineering at Stanford University where his group's areas of
research include micro- and nano-fabrication and their application to electronic
and magnetic devices and structures. Dr. Pease conducted research on the
synthesis of DNA microarrays at Affymetrix Corporation and initiated programs in
Advanced Microelectronics and Molecular-Level Printing the Defense Advanced
Research Projects Agency. He has served as a consultant to IBM, Xerox, Etec
Systems, and Lawrence Livermore Labs and is on the Technical Advisory Boards of
Ultratech Stepper, San Jose, CA and Affymetrix, Santa Clara, CA. He has
published over 200 articles and authored several book chapters. Dr. Pease holds
BA, MA, and PhD degrees from Cambridge University, United Kingdom.
Professor David D. Awschalom
received his B.Sc. in physics from the University of Illinois at
Urbana-Champaign, and his Ph.D. in experimental physics from Cornell University.
He was a Research Staff member and Manager of the Nonequilibrium Physics
Department at the IBM Watson Research Center in Yorktown Heights, New York. In
1991 he joined the University of California-Santa Barbara as a Professor of
Physics, and in 2001 was additionally appointed as a Professor of Electrical and
Computer Engineering. He is presently the Peter J. Clarke Professor and Director
of the California NanoSystems Institute, and Director of the Center for
Spintronics and Quantum Computation.Professor Awschalom received an IBM
Outstanding Innovation Award (1987), the Outstanding Investigator Prize from the
Materials Research Society (1992), the International Magnetism Prize and Néel
Medal from the International Union of Pure and Applied Physics (2003), the
Oliver E. Buckley Prize from the American Physical Society (2005), the Agilent
Europhysics Prize from the European Physical Society (2005), the Newcomb
Cleveland Prize from the American Association for the Advancement of Science
(2006), and the UC Faculty Research Lecturer Award (2008). Dr. Awschalom is a
Fellow of the American Physical Society, the American Association for the
Advancement of Science, the American Academy of Arts and Sciences, and a member
of the National Academy of Sciences.
43
Andrew Isaacs is Adjunct
Professor of Entrepreneurship at the Haas School of Business, University of
California, Berkeley. Mr. Isaacs is involved in numerous directorships and is
the President of California Technology Intl, Inc. He has served as Vice
President of Kevex Instruments, and Senior Scientist at NASA Johnson Space
Center. As an educator, he has guided many start up companies to success. Mr.
Isaacs holds B.S. and M.S. degrees the University of Michigan.
Kara McCloskey, PhD is
Professor at School of Engineering, Universiy of California, Merced. She has
conducted significant research in the field of tissue engineering with a
specific focus on the cardiovascular system and stem cells. Professor McCloskey
received a BS and an MS from Ohio State University and a PhD from Cleveland
Clinic Foundation, conferred by Ohio State University.
Bruce R. Conklin, MD, is a
Senior Investigator at the Gladstone Institute of Cardiovascular Disease and a
Professor at the University of California, San Francisco, with appointments in
the Departments of Medicine and Cellular and Molecular Pharmacology. His
research combines genetic techniques and bioinformatics to study G-Protein
Couple Receptor hormone signaling involved in murine embryonic stem cells (mESC)
and pluripotent embryonic stem (ES) cells. The cell lines can be used in to
analyze toxic side effects of pharmaceuticals and study the differentiation of
embryonic stem cells into cardiac tissues. Dr. Conklin served as the founding
director of the Gladstone Genomics Core and is currently the founding director
of the Gladstone Stem Cell Core. From 1995 to 2001, Dr. Conklin was the
Associate Director of the General Clinical Research Center at San Francisco
General Hospital. He is a member of several honorary societies including the
American Society for Clinical Investigation. Dr. Conklin was the co-chair of the
UCSF public science project in 1994, and is co-chair of the UCSF-California
Academy of Sciences Affiliation Task Force. Dr. Conklin is the Associate
Director of the Gladstone CIRM Scholars Training Program, and the recipient of a
CIRM New Stem Cell Lines grant. Dr. Conklin is a key advisor to iPierian, Inc.,
a Kleiner Perkins-backed biopharmaceutical company located in San
Francisco.
Tianhong Cui, PhD, is a Nelson
Associate Professor in the Department of Mechanical Engineering at the
University of Minnesota. He has performed significant research and is
published in fields of micro/nano electro mechanical systems,
micro/nanomanufacturing and flexible electronics. He holds a B.S. in
Mechanical Engineering from the Chinese Academy of Sciences and a PhD in
Mechanical Engineering from Nanjing University of Aeronautics & Stronautics
in China.
A
detailed description of the Company’s management, their compensation, and all
beneficial ownership reporting is incorporated by reference from the Reports,
the provisions of which are incorporated by reference herein.
There are
no agreements or understandings for any of our executive officers or director to
resign at the request of another person and no officer or director is acting on
behalf of nor will any of them act at the direction of any other
person.
Directors
are elected until their successors are duly elected and qualified.
Family
Relationships
There is
no family relationship among any of our officers or directors.
Involvement
in Certain Legal Proceedings
To the
best of our knowledge, none of our directors or executive officers has been
convicted in a criminal proceeding, excluding traffic violations or similar
misdemeanors, or has been a party to any judicial or administrative proceeding
during the past ten years that resulted in a judgment, decree or final order
enjoining the person from future violations of, or prohibiting activities
subject to, federal or state securities laws, or a finding of any violation of
federal or state securities laws, except for matters that were dismissed without
sanction or settlement. Except as set forth in our discussion below
in Item 13, “Certain Relationships and Related Transactions, and Director
Independence,” none of our directors, director nominees or executive officers
has been involved in any transactions with us or any of our directors, executive
officers, affiliates or associates which are required to be disclosed pursuant
to the rules and regulations of the SEC.
44
Board
Composition and Committees
Our board
of directors is currently composed of 4 members: Messrs. Baum, Panther, II,
Khine, and Dreismann. Our board of directors has determined that
only Mr. Dreismann is an independent director at this time, under the rules
of the American Stock Exchange Company Guide, or the AMEX Company Guide, because
he does not currently own a significant percentage our shares, are not currently
employed by the Company, has not been actively involved in the management of the
Company and do not fall into any of the enumerated categories of people who
cannot be considered independent directors under the AMEX Company
Guide.
We do not
have an audit or compensation committee at this time, however, the Company has
adopted an Audit Committee Charter.
Section
16(A) Beneficial Ownership Reporting Compliance
Under
U.S. securities laws, directors, certain executive officers and persons holding
more than 10% of our common stock must report their initial ownership of the
common stock, and any changes in that ownership, to the SEC. The SEC has
designated specific due dates for these reports. Based solely on our review of
copies of such reports filed with the SEC by and written representations of our
directors and executive offers, we believe that our directors and executive
offers filed the required reports on time in 2009 fiscal year, except that Mr.
Khine was late with an initial report and a Form 4, and Messrs. Baum and
Panther, II, each filed a Form 4 late. No short term profits were
reported.
Code of Ethics
On
February 10, 2006 we adopted a Code of Ethics. The same was filed as
Exhibit 11.1.8 on a Form 10 Annual Report filed on April 14, 2009.
|
ITEM
11.
|
EXECUTIVE
COMPENSATION.
|
As a
result of the reverse merger and acquisition by Shrink Technologies, Inc., a
California corporation, of Shrink Nanotechnologies, Inc., a Delaware
corporation, we did not experience any cash flow event as a result of any
payment to an executive. Additionally, there were no stock awards or
options granted to executives during this same 2008 and 2009
period. We have not provided retirement benefits or severance or
change of control benefits to our named executive officer, Mark L.
Baum. No unexercised options or warrants were held by any of our
executive officers at year ended 2009. No equity awards were made during the
year ended December 31, 2009.
|
Annual
Compensation
|
Long-Term
Compensation
|
||||||||||||||
|
Common
|
|||||||||||||||
|
Shares
|
|||||||||||||||
|
Underlying
|
All
|
||||||||||||||
|
Restricted
|
Options
|
Other
|
|||||||||||||
|
Other
Annual
|
Stock
|
Granted
|
Compensation
|
||||||||||||
|
Name
and Position
|
Year
|
Salary
|
Bonus
|
Compensation
|
Awards
|
(#
Shares)
|
|||||||||
|
Mark
L. Baum, Esq.(1)
|
2009
|
$
-
|
-
|
-
|
-
|
-
|
-
|
||||||||
|
President
and Chief
|
|||||||||||||||
|
Executive
Officer and
Principal
Executive Officer
|
2008
|
$
-
|
-
|
-
|
-
|
-
|
-
|
||||||||
|
Luis
Leung (2)
|
2009
|
$
-
|
-
|
-
|
-
|
-
|
-
|
||||||||
|
President,
Chief
|
|||||||||||||||
|
Executive
Officer, Chief
|
2008
|
$
103,511
|
-
|
-
|
1,218,722
|
-
|
-
|
||||||||
|
Financial
Officer and
|
|||||||||||||||
|
Secretary
|
|||||||||||||||
|
(1)
|
Mr.
Baum, although he has not received any cash compensation for employment
services, he is entitled to receive compensation through an agreement the
Company has with BCGU, LLC. Mr. Baum co-owns BCGU, LLC with Mr.
Panther, II, who also receives 50% of the fees generated from the services
rendered pursuant to this
agreement.
|
|
(2)
|
Luis
Leung was formally our senior and sole executive at the legal parent
level. He received no compensation other than what has been
previously disclosed in our SEC
filings.
|
45
Compensation
of Directors
The table
below sets forth the compensation of our directors for the fiscal year ended
December 31, 2009.
|
Name
|
Fees
earned or paid in cash ($)
|
Stock
awards ($)
|
Option
awards
($)
|
Non-equity
incentive plan compensation ($)
|
Nonqualified
deferred
compensation
earnings
($)
|
All
other compensation ($)
|
Total
($)
|
|
|
Mark
L. Baum, Esq.
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
James
B. Panther, II
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
Marshall
Khine, Esq.
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
|
Heiner
Dreismann
|
36,666
|
-
|
-
|
-
|
-
|
-
|
36,666
(1)(2)
|
|
|
(1)
|
We
did not compensate our directors in 2008 or 2009. These cash
fees remain unpaid.
|
(2) Effective
as of June 22, 2009, and simultaneously with the appointment of Dr. Dreismann as
a director of the Company, the Company entered into a Consulting Agreement with
Dr. Dreismann. The material terms of the Consulting Agreement
provide, among other terms, that Dr. Dreismann shall dedicate not
less than two days per month, as reasonably requested by the Company and as the
Consultant’s schedule permits, towards providing life sciences related advisory
services and assist in procuring strategic partners and
relationships. The term of the Consulting Agreement is one year,
provided that it may be extended at the end of each year.
Our compensation to Dr. Dreismann under
the agreement is as follows:
Quarterly Cash Fee in the form of a
base fee and a success fee. The base fee (the “Quarterly Cash Fee”) to be
paid is $20,000 each quarter, paid, at Dr. Dreismann’s consultants
discretion, in cash or common stock of the Company, provided, that a limitation
on payments in cash is to the extent of multiplying: (i) fifteen percent (15%)
and (ii) the aggregate of: (A) the (net of fees) amount, exclusive of a pass
through investment, of any new debt or equity investment into the Company by a
non-affiliated third party during the term of the Agreement; and (B) all
Strategic Party Income, as defined below,
Additional Success
Fees. The Consulting Agreement also provides for a “success
based” fee to be paid within 90 days of the end of the Company’s fiscal year
from cash received from either (i) sublicensing agreements entered into with
certain third parties introduced by the Mr. Dreismann or (ii) revenues generated
from the sale of goods or services to parties introduced by Mr. Dreismann
(“Strategic Party
Income”). Specifically, the consultant will be paid 5% of
Strategic Party Income, net of all royalties and dividends payments, or
3% of the revenues received by the Company from the sale of goods and
services to or through a strategic party introduced by Mr. Dreismann, net of
costs of goods or services and a pro-rata allocation of sales, general and
administrative costs.
Notwithstanding
the foregoing, the Company shall receive a credit towards any Success Fee
otherwise payable during a year, of an amount equal to the amount of Quarterly
Cash Fees paid to the consultant at the time a Success Fee is due, and in any
event, such an amount shall not exceed $80,000; with an upper limit on Success
Fees of five million dollars ($5,000,000).
The
foregoing is a brief summary only of the Consulting Agreement, a copy of which
is filed as an exhibit to our Current Report on Form 8-K, filed June 25, 2009,
the provisions of which are incorporated by reference herein.
Compensation
Discussion and Analysis
The
Company does not have any full time employees and has not entered into long term
executive or non-executive employment agreements, so as to limit the Company’s
exposure and liability. As indicated elsewhere in this Report, the Company
regularly engages outside consultants, accountants and other professional
service providers for purposes of providing services to the Company or its Shink
subsidiary. The Company endeavors, where able, to issue shares in
lieu of cash compensation, so as to preserve capital where needed and limit cash
risk exposure.
Historically,
funding for the Company, or for Shrink prior to our acquisition of it, were
funded by management and its affiliates, namely, Noctua Fund, L.P., which has
loaned over $218,000 (on a consolidated basis) as reflected in a note to them,
which is now in default and forbearance. In addition, the Company,
utilizes services, bookkeeping, administrative staff, equipment and
extensive management and day to day operational services from BCGU, LLC, an
entity co-owned by Messrs. Baum and Panther, II, which have provided such
services under the Amended Operating Agreement since 2009 and, prior to such
time, free of charge. The Corporation believes that, given the
extensive securities law experience of Mr. Baum, and the extensive small cap and
micro cap M&A and financing experience of Messrs. Baum and Panther, II, the
years of experience for each of them, and the current opportunity cost factor
for each of them, as combined with the fact that BCGU, LLC has continued to
provide services and funding despite non-payment of over $234,000 since mid
2009, that the amount of compensation provided under the Amended Operating
Agreement is fair and reasonable for the Corporation.
Additionally,
the Corporation has, in the past, sought to retain management, which would
require that the Corporation enter into long term, inflexible employment
arrangements with persons that may have a limited stake in the Corporation’s
success as compared to our principals.
46
The
Corporation also limits cash compensation to outside or internal directors and
does not have a cash compensation policy. The Company endeavors to
enter into performance based compensation consulting packages with members of
the Company’s SAB or board of directors. Performance based
compensation tends to be conditioned on achieving milestones such as achieving
certain minimum net revenue streams from sources introduced by the director or
SAB member, securing government grants or similar financing for our research and
development, or other specific activities or items for which a particular board
or SAB member may have skills.
|
ITEM
12.
|
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS.
|
The
following table sets forth certain information regarding the beneficial
ownership of common stock of the Company by (i) each person who, to the
Company’s knowledge, owns more than 5% of its Common Stock, (ii) each of the
Company’s named executive officers and directors, and (iii) all of the Company’s
named executive officers and directors as a group. Shares of the
Company’s Common Stock subject to options, warrants, or other rights currently
exercisable, or exercisable within 60 days of the date hereof, are deemed to be
beneficially owned and outstanding for computing the share ownership and
percentage of the person holding such options, warrants or other rights, but are
not deemed outstanding for computing the percentage of any other person. As of
the date hereof, are reflecting the 5 for 1 Forward Split implemented April 8,
2010, the Company has 191,303,985 shares of Common Stock issued and
outstanding. All of the below share amounts are adjusted to reflect
the Forward Split.
|
Name and Address
|
Amount
and Nature of Beneficial Ownership
(Post Forward Split)
|
Percentage
of Voting Securities
|
|
Mark
L. Baum, Esq. (1)(2)
|
109,029,010(3)
|
63.05%
|
|
James
B. Panther, II (2)(4)
|
106,085,695(5)
|
61.34%
|
|
Marshall
Khine(6)
|
43,194,440
|
24.98%
|
|
Heiner
Dreismann (6)
|
0
|
0
|
|
Luis
Leung
|
10,853,085
|
6.27
|
|
All
Officers and Directors as a group (4 persons) (7)
|
259,939,645
|
98.83
|
(1) The
address for Mr. Baum is 1302 Waugh Drive, Suite 618, Houston Texas
77019.
(2) The
address for Noctua Fund, L.P., is Noctua Fund Manager, LLC c/o Mr. Baum,
2038 Corte Del Nogal, Suite 110, Carlsbad, CA 92011. Noctua Fund
Manager, LLC, is an entity equally beneficially owned and controlled by Mr. Baum
and Mr. Panther II through entities owned or controlled by them. Such
persons are non-voting minority limited partners in the Noctua Fund, L.P.
itself. Accordingly, Messers. Baum and Panther II disclaims the
economic ownership of securities held by Noctua Fund L.P.
(3) Includes
10,000,000 shares underlying shares of Series A Preferred Stock, and 11,733,530
shares owned by entities principally owned and controlled by Mr.
Baum. Also includes: 76,422,395 shares held by BCGU, LLC, of which
Mr. Baum and Panther II are equal indirect beneficial owners and control
persons; and 20,000 shares and 10,853,085 shares underlying an immediately
exercisable option to acquire shares from a former director, owned by Noctua
Fund, L.P. Mr. Baum and Mr. Panther II, are equal indirect beneficial
owners of BCGU, LLC and of Noctua Fund Manager, LLC, the general partner of
Noctua Fund, L.P., but are only non-voting minority investors in the Noctua
Fund, L.P. Accordingly, Mr. Baum disclaims economic ownership of half
(38,211,195 shares) of all shares owned by BCGU, LLC and of any securities held
by Noctua Fund L.P. Does not include up to 5,578,032 shares, in
aggregate, issuable upon conversion of $118,121.28 and $100,000 principal amount
of 14% convertible notes plus interest issued Noctua Fund, L.P., convertible at
$0.04 per share, subject to certain limitations.
47
(4) The
address for Mr. James B. Panther, II, is 381 Casa Linda Plaza, Suite 408,
Dallas, Texas 75218.
(5) Includes
9,800,000 shares underlying shares of Series A Preferred Stock, and 8,880,975
shares of Common Stock owned by an entity indirectly beneficially owned and
controlled by Mr. Panther II. Also includes: 76,422,395 shares
held by BCGU, LLC, of which Mr. Baum and Panther II are equal indirect
beneficial owners and control persons; and 20,000 shares and 10,853,085shares
underlying an immediately exercisable option to acquire shares from a former
director, owned by Noctua Fund, L.P. Mr. Baum and Mr. Panther II, are
equal indirect beneficial owners of BCGU, LLC and of Noctua Fund Manager, LLC,
the general partner of Noctua Fund, L.P., but are only non-voting minority
investors in the Noctua Fund, L.P. Accordingly, Mr. Panther, II
disclaims economic ownership of half (38,211,195 shares) of all shares owned by
BCGU, LLC and of any securities held by Noctua Fund L.P. Does not include up to
5,578,032 shares, in aggregate, issuable upon conversion of $118,121.28 and
$100,000 principal amount of 14% convertible notes plus interest issued Noctua
Fund, L.P., convertible at $0.04 per share, subject to certain
limitations.
(6) The
address for Mr. Marshall Khine and Mr. Dreismann, is c/o the Company, 2038 Corte
Del Nogal, Suite 110, Carlsbad, CA 92011.
(7) The
address for Mr. Luis J. Leung is c/o 15818 Sweetrose Place, Houston, Texas
77095. Includes 11,253,085 shares subject to sale under the four year
option to the Noctua Fund, L.P. as disclosed in notes 3 and 5
above.
Changes
in Control
There are
no arrangements known to us, including any pledge by any person of our
securities, the operation of which may at a subsequent date result in a change
in control of the Company.
Securities
Authorized for Issuance Under Equity Compensation Plans
The
following table includes the information as of the end of fiscal year 2009 for
each category of our equity compensation plan:
|
Plan
category
|
Number
of securities to be issued upon exercise of outstanding options,
restricted stock, warrants and rights
(a)
|
Weighted-average
exercise price of outstanding options, restricted stock, warrants and
rights
(b)
|
Number
of securities remaining available for future issuance under equity
compensation plans (excluding securities reflected in column
(a))
(c)
|
|
Equity
compensation plans approved by security holders
|
-
|
-
|
-
|
|
Equity
compensation plans not approved by security holders (1)
|
275,000
|
$.216
|
-
|
|
Total
|
275,000
|
$.216
|
-
|
|
(1)
|
While
the Company does not have a stock option plan approved by the
shareholders, the Company does issue share or option based compensation on
occasion.
|
[Remainder
of Page Intentionally Left Blank]
48
|
ITEM
13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS,
AND DIRECTOR INDEPENDENCE.
|
A variety
of conflicts of interest exist, and may continue to exist, from time to time,
primarily as a result of our principal owners maintaining control of the
Company, and our continued sublease and operating agreements with a principal
shareholder and director, Mark. L. Baum, and Mr. James B. Panther,
II. They also own and control, the Series A Preferred Stock which
gives its holders voting control over the Company.
Sublease
With Affiliates
The
Company previously shared office space with the Noctua Fund Manager, LLC.
On January 1, 2009 we began accruing a $2,500 rental expense each month for the
use of the space. There is currently no written agreement; it is a verbal
agreement between the two companies. The verbal agreement ceased on May
29, 2009 pursuant the Company’s execution of a sublease, as described
below.
The
Company subleases space from Business Consulting Group Unlimited, Inc., an
entity owned by two of our directors, James B. Panther II, and Mark L. Baum,
Esq. pursuant to which the Company leases approximately 3,000 square feet of
office and administrative space, as well as use of, among other things,
internet, postage, copy machines, electricity, furniture, fixtures etc. at a
rate of $6,000 per month. The lease with Business Consulting Group
Unlimited, Inc. expires April 30, 2010. An aggregate of $42,000 was
paid during the year ended December 31, 2009 under this agreement and no amounts
are due and owing.
On May
29, 2009, the Company signed an Operating Agreement with BCGU, LLC, an entity
indirectly controlled by James B. Panther II, and Mark L. Baum, Esq., who are
two of our directors, for a fee of $6,000 per month. During October
2009, the Company amended the Operating Agreement with BCGU, LLC. The
Amended Operating Agreement allows us to retain certain day-to-day
administrative services and management in consideration of a monthly fee of
$30,000 per month. The Amended Operating Agreement also included a
$270,000 signature bonus. The Amended Operating Agreement expires October
1, 2012. At December 31, 2009 $42,000 had been paid to BCGU, LLC and
there was $342,000 owed to BCGU, LLC. There is an option within the
Amended Operating Agreement that allows BCGU, LLC to convert outstanding
payables related to the Operating Agreement into a 10% convertible
note.
The
Company believes, that the terms of the foregoing arrangements are no less
favorable to the Company than as would otherwise be available from independent
commercial third parties. At the time of both the above
transactions, Mr. Baum and Panther, II were in control of the Company with
limited independent directors or management.
Familial
and other Relationships Relating to Shrink
Mr. Baum
and Mr. Panther, II, through Noctua Fund L.P. and other entities, regularly
invest in and manage, companies or other investments together.
Mr. Baum
and Mr. Panther, II, are also principals of Business Consulting Group Unlimited,
Inc. and BCGU, LLC, both of which are parties to material compensation
agreements as provided above.
Mr. Baum
and Mr. Panther, II, have been business partners for more than 8
years. Mr. Khine and Dr. Michelle Khine are brother and sister,
respectively. Mr. Baum and Mr. Khine are long time friends and
attended law school together from 1995 to 1998.
Mr.
Khine, who is now one of our new directors appointed at the time of the Shrink
acquisition, is the person from whom we acquired Shrink and was issued
44,444,440 shares of our common stock and 50 shares of our Series C Preferred
Stock (which have since been cancelled).
Series
C Preferred Stock
Noctua
Fund L.P. and Khine collectively owned 150 shares of Series Preferred Stock at
the time of the Shrink Acquisition, issued for consideration valued at $15,000
and contained certain onerous protective provisions. In late October
of 2010, each of these persons agreed to return all of the Series C Preferred
Stock in exchange for cash consideration and certain
releases. Neither the issuance nor cancellation of the Series C
Preferred Stock were valued by independent valuation agents.
49
Other
Contracts
In
addition, we may enter into contracts, agreements of arrangements from time to
time with such persons, or with Mr. Marshall Khine, a member of our board of
director and significant shareholder, or other persons on our scientific
advisory board. The Board endeavors to require that all interested
party transactions be approved by a majority of the disinterested
directors. In addition, the Board, in good faith, negotiates or
intends to negotiate transactions with affiliates at terms that are no less
favorable to the Company than would otherwise be available from bona fide third
party sources.
As a
result of control by these persons, and their ability to appoint board members
and officers, and, as a result of the outstanding Series A Preferred Stock, such
persons has and will continue to have and be able to exert, substantial control
over the Company’s and Shrink’s day to day operations, contracts, and long term
prospects.
Mark L.
Baum and James B. Panther II are both limited partners in Noctua, and through
entities which Mr. Baum and Mr. Panther II own, equally own and control Noctua
Fund Manager LLC, the general partner of Noctua. As part of the
consideration for the acquisition of Shrink, the Company assumed, pursuant to
the terms of a note exchange agreement, the outstanding notes due to Noctua Fund
LP, which had an aggregate currently outstanding balance of approximately
$118,121, in consideration of Noctua consenting to the
transaction. Noctua also loaned the company $10,000 on March 31,
2009, at 15% interest, with a maturity date of March 31, 2010. This
loan was reflected as an amended promissory note.
Promoters
and Certain Control Persons
We did
not have any promoters at any time during the past five fiscal
years.
Director
Independence
We
currently do not have any independent directors other than Dr. Heiner Dreismann,
as the term “independent” is defined by the rules of the Nasdaq Stock
Market.
|
ITEM
14.
|
PRINCIPAL
ACCOUNTING FEES AND SERVICES.
|
Independent
Auditors’ Fees
The
following is a summary of the fees billed to the Company by its principal
accountants for professional services rendered for the fiscal years ended
December 31, 2009 and 2008:
|
Fiscal
Year Ended December 31,
|
||||
|
2009
|
2008
|
|||
|
Audit
Fees
|
$
|
21,801
|
$
|
20,500
|
|
Audit-Related
Fees
|
-
|
-
|
||
|
All
Other Fees
|
-
|
-
|
||
|
TOTAL
|
$
|
21,801
|
$
|
20,500
|
“Audit
Fees” consisted of the aggregate fees billed for professional services rendered
for the audit of our annual financial statements and the reviews of the
financial statements included in our Forms 10-Q and for any other services that
were normally provided in connection with our statutory and regulatory filings
or engagements. Audit fees were paid to Chisholm, Bierwolf, Nilson &
Morrill, LLC, as our auditors for the years ended December 31, 2008 and
2009.
Our
present account balance owed to Chisholm Bierwolf, Nilson & Morrill, LLC is
approximately $5,000, all of which are for audit services.
Chisholm,
Bierwolf, Nilson & Morrill, LLC billed us $18,000 in fees for review of our
quarterly financial statements and annual audit for the year ended December 31,
2008 and $28,343 for the year ended 2009.
“Audit
Related Fees” consisted of the aggregate fees billed for professional services
rendered for assurance and related services that were reasonably related to the
performance of the audit or review of our financial statements and were not
otherwise included in Audit Fees. We were not invoiced by our auditors for any
such Audit Related Fees” or “Tax Fees” during 2008 and 2009 and our auditors did
not provide such services.
“Tax
Fees” consisted of the aggregate fees billed for professional services rendered
for tax compliance, tax advice and tax planning. Included in such Tax Fees were
fees for preparation of our tax returns and consultancy and advice on other tax
planning matters. We did not pay any fees to Chisholm, Bierwolf,
Nilson & Morrill, LLC for tax compliance, tax advice, tax planning or other
work during our fiscal years ending December 31, 2009 or December 31,
2008. During 2009, we paid $6,027 in fees to our tax preparation and
filing firm (which is not Chisholm, Bierwolf, Nilson & Morrill,
LLC).
“All
Other Fees” consisted of the aggregate fees billed for products and services
provided and not otherwise included in Audit Fees, Audit Related Fees or Tax
Fees.
Pre-Approval
Policies and Procedures
Under the
Sarbanes-Oxley Act of 2002, all audit and non-audit services performed by our
auditors must be approved in advance by our board of directors to assure that
such services do not impair the auditors’ independence from us. In
accordance with its policies and procedures, our board of directors pre-approved
the audit and non-audit service performed by our auditors for our consolidated
financial statements as of and for the year ended December 31,
2009.
50
PART
IV
|
ITEM
15.
|
EXHIBITS,
FINANCIAL STATEMENT SCHEDULES.
|
Financial
Statements and Schedules
The
financial statements are set forth under Item 8 of this Annual Report on Form
10-K. Financial statement schedules have been omitted since they are either not
required, not applicable, or the information is otherwise included.
Exhibit
List
The
following Exhibits have been previously filed in the below referenced filings or
have been attached hereto, and in any case, as is stated on the cover of this
Report, all of the below Exhibits are incorporated herein by
reference.
|
Form
10-SB
|
10/19/2006
|
|
3.1.1
|
Certificate
of Incorporation
|
|
3.1.2
|
Amended
and Restated Certificate of Incorporation
|
|
3.2
|
Bylaws
|
|
Form
8-K
|
8/24/2007
|
|
10.1
|
Lock-Up
and Leak Out Agreement with BCGU LLC
|
|
10.2
|
Lock-Up
and Leak Out Agreement with Luis Leung
|
|
Amended
Form 8-K
|
9/5/2007
|
|
10.1
|
Lock-Up
and Leak Out Agreement with BCGU LLC
|
|
10.2
|
Lock-Up
and Leak Out Agreement with Luis Leung
|
|
Form
10-QSB
|
11/14/2007
|
|
3.1.3
|
Certificate
of Designation (Series A and B Preferred Stock)
|
|
Form
10-Q
|
11/19/2008
|
|
10.1
|
Asset
Purchase Agreement
|
|
10.2
|
Meaux
Street Partners LP 10% Convertible Promissory Note
|
|
10.3
|
The
Sonkei Trust Amended 10% Convertible Promissory Note
|
|
10.4
|
September
30, 2008 Settlement Agreements with Peer and SixTech
|
|
10.5
|
September
30, 2008 Settlement Agreements with David F. Rubin and John
Burkett
|
|
10.6
|
September
30, 2008 Settlement Agreement with Luis Leung
|
|
Form
8-K
|
1/21/2009
|
|
10.1
|
License
Consent Agreement
|
|
10.2
|
First
Amended License Consent Agreement
|
|
Form
10-K Report
|
4/14/2009
|
|
11.1.1
|
September
30, 2008 Settlement Agreements with For Goodness
|
|
11.1.2
|
September
30, 2008 Settlement Agreements with Mathew Luchak
|
|
11.1.3
|
October
1, 2008 Omnibus Settlement Agreement with Luis Leung
|
|
11.1.4
|
November
30, 2008 Second Amended License Consent Agreement with
BCGU
|
|
11.1.5
|
March
19, 2009 Amendment to the Articles of Incorporation
|
|
11.1.6
|
March
19, 2008 Designation of Series C Preferred Stock
|
|
11.1.7
|
Code
of Ethics adopted on February 10, 2006
|
|
11.1.8
|
Share
Exchange Agreement with BCGU
|
|
Form
8-K
|
5/8/2009
|
|
10.1
|
Debt
Consolidation Agreement with Noctua Fund L.P.
|
|
10.2
|
Convertible
Secured Promissory Note
|
|
Form
8-K
|
5/12/2009
|
|
10.1
|
Board
of Directors appointment of Mark L. Baum, Esq. as
Director
|
|
Form
8-K
|
5/13/2009
|
|
3.1.3
|
Binding
Letter of Intent to acquire Shrink Technologies, Inc.
|
|
Form
8-K
|
5/15/2009
|
|
3.1
|
Certificate
of ownership and merger, name change to Shrink Nanotechnologies,
Inc.
|
|
Form
8-K
|
6/5/2009
|
|
10.1
|
Share
Exchange Agreement
|
|
10.2
|
Exclusive
License Agreement
|
|
10.3
|
Research
Agreement
|
|
10.4
|
Office
space sublease
|
|
10.5
|
Operating
Agreement
|
|
10.6
|
Debt
Consolidation Agreement
|
|
10.7
|
Note
Exchange Agreement
|
|
10.8
|
Consulting
Agreement with Dr. Michelle Khine
|
|
21.1
|
List
of Subsidiaries of Registrant
|
|
99.1
|
Unaudited
Pro Forma Financial Statements: Shrink Nanotechnologies,
Inc.
|
|
99.2
|
Audited
Financial Statements of Shrink Technologies, Inc.
|
|
Form
8-K
|
6/19/2009
|
|
3.2
|
Amended
and restated Bylaws of Shrink Nanotechnologies, Inc.
|
|
Form
8-K
|
6/25/2009
|
|
10.1
|
Consulting
agreement Heiner Dreismann
|
|
Form
10-Q
|
11/25/2009
|
|
10.1
|
First
Amended Operating Agreement
|
|
Form
8-K
|
12/29/2009
|
|
10.1
|
First
Amended Asset Purchase Agreement with Dao Information Systems,
Inc.
|
|
Form
8-K
|
2/3/2010
and Amended on 4/8/2010
|
|
99.1
|
Overview
of Shrink Nanotechnologies, Inc.
|
|
This
Form 10-K Report
|
|
|
3.1
|
Certificate
of Amendment to Articles of Incorporation
|
|
4.1
|
Form
of Convertible Note 12%
|
|
4.2
|
Form
of Series A Warrant
|
|
10.1
|
Subscription
Agreement
|
|
21.1
|
Subsidiaries
|
|
23.1
|
Consent
of Independent Registered Public Accounting Firm Chisholm Bierwolf, Nilson
& Morrill, LLC
|
|
31.1
|
Certification
of Mark L. Baum, Esq., President and Chief Executive Officer, pursuant to
Rule 13a-14(a) or 15d-14(a) of the Securities and Exchange Act of
1934, as adopted pursuant to Section 302 of the Sarbanes- Oxley Act
of 2002.
|
|
31.2
|
Certification
of Mark L. Baum, Esq., Chief Financial Officer, pursuant to
Rule 13a-14(a) or 15d-14(a) of the Securities and Exchange Act of
1934, as adopted pursuant to Section 302 of the Sarbanes- Oxley Act
of 2002
|
|
32.1
|
Certification
pursuant to 18 U.S.C Section 1350 as adopted pursuant to section 906 The
Sarbanes-Oxley Act of 2002, executed by Mark L. Baum, Esq. Chief Executive
Officer and President
|
|
32.2
|
Certification
pursuant to 18 U.S.C Section 1350 as adopted pursuant to section 906 The
Sarbanes-Oxley Act of 2002, executed by Mark L. Baum, Esq. Chief Financial
and Accounting Officer
|
51
SIGNATURES
In
accordance with section 13 or 15(d) of the Securities Exchange Act of 1934, the
Registrant caused this Report on Form 10-K to be signed on its behalf by the
undersigned, thereto duly authorized individual.
Date:
April 14, 2010
In
accordance with the Securities Exchange Act of 1934, this report has been signed
below by the following persons on behalf of the Registrant and in the capacities
and on the dates indicated.
Each
person whose signature appears below hereby authorizes Mark L. Baum, Esq. as
attorney-in-fact to sign on his or her behalf, individually, and in each
capacity stated below, and to file all amendments and/or supplements to this
Annual Report on Form 10-K.
|
Signature
|
Title
|
Date
|
|
|
/s/
Mark L. Baum, Esq.
|
CEO
and President
|
April
14, 2010
|
|
|
Mark
L. Baum, Esq.
|
(Principal
Executive Officer, Principal Financial Officer and Principal Accounting
Officer)
|
||
|
/s/
James B. Panther, II
|
|
April
14, 2010
|
|
|
James
B. Panther, II
|
Chairman
of the Board of Directors
|
||
|
/s/
Marshall Khine, Esq.
|
April
14, 2010
|
||
|
Marshall
Khine, Esq.
|
Director
|
||
|
/s/
Heiner Dreismann, Ph.D
|
Director
|
April
14, 2010
|
|
|
Heiner
Dreismann, Ph.D
|
|||
52
