Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d8k.htm |
| EX-99.2 - POSTER - Paratek Pharmaceuticals, Inc. | dex992.htm |
 Intermezzo
®
Clinical
data
slide
supplement
Intermezzo
®
Clinical
data
slide
supplement
April 8, 2010
Exhibit 99.1 |
 Important
note to readers Important note to readers
The information in this presentation is provided for informational purposes only. The
clinical results and other information included in this presentation should not in
any way be construed as predictive of the outcome of any future studies,
including, without limitation, a repeat of the same or similar studies or any study
conducted to assess the effect of Intermezzo
®
on study subjects’
ability to drive or to be predictive of the sufficiency of current or future data that may
be generated to support FDA approval of
Intermezzo
®
for its intended indication.
To the extent investors may consider such results and the information included on these
slides to be predictive of the outcome
of
any
future
study
or
the
outcome
of
FDA
review
of
a
re-submitted
Intermezzo
®
New
Drug
Application,
investors
should be aware that the actual results of any future studies may differ materially from
those presented herein due to various risks and uncertainties, including, but not
limited to, •
identical study designs evaluating identical endpoints may produce different study
results; •
different study designs intended to measure the same or similar endpoints may produce
different results; •
different studies in different or progressively larger patient populations could reveal more
frequent, more severe or additional side effects that were not seen in earlier
studies; •
the unpredictable nature of clinical trials generally;
•
whether
additional
data
can
be
generated
from
new
clinical
studies
to
demonstrate
sufficiently
to
the
FDA
that
Intermezzo
®
would not present an unacceptable risk of residual effects, including residual effects that
impair next day driving ability; and •
FDA
decisions
on
the
sufficiency
of
other
data
submitted
or
to
be
submitted
in
support
of
a
re-submitted
Intermezzo
®
NDA
to receive approval for its intended indication and any further delays in, and the final
form of, any FDA approval of Intermezzo
®
.
For more information please read the risk factors section in the
Transcept
Form 10-K filed with the Securities and Exchange
Commission.
2 |
 Phase 1
Pharmacokinetic (PK) / Pharmacodynamic
(PD) study (2005)
Phase 1 Pharmacokinetic (PK) /
Pharmacodynamic
(PD) study (2005) |
 Intermezzo
®
: Phase 1 PK/PD study (2005)
Intermezzo
®
: Phase 1 PK/PD study (2005)
Study design:
Double-blind, placebo-controlled, daytime, crossover
study
of
1.0
mg,
1.75
mg
and
3.5
mg
sublingual
zolpidem
tartrate
tablet
in 24 healthy adults (only 1.75 mg and 3.5 mg data shown)
Pharmacokinetic
assessment:
Blood
was
collected
for
up
to
12
hours
post-dose
and
plasma
was
assayed
for
zolpidem
by
LC-MS/MS
Pharmacodynamic
assessment:
–
Digit Symbol Substitution Test (DSST)
–
Choice Reaction Test (CRT)
–
Symbol Copying Test (SCT)
–
Subject rating of sedation (100mm VAS)
–
Bushke
Word Recall Test
4 |
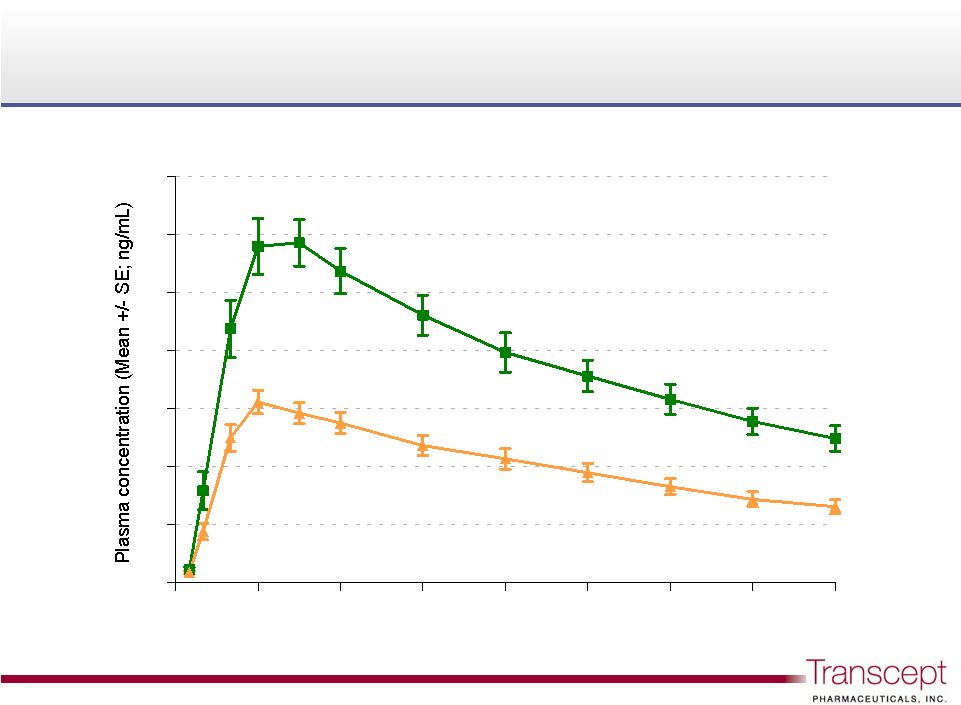 Intermezzo
®
3.5 mg and 1.75 mg plasma
concentration profile
Intermezzo
®
3.5 mg and 1.75 mg plasma
concentration profile
Intermezzo
®
3.5 mg
Intermezzo
®
1.75 mg
Phase 1 PK/PD Study n=24
Tmax 30-40 min
5
0
10
20
30
40
50
60
70
0
30
60
90
120
150
180
210
240
Time (min) |
 Intermezzo
®
: mean DSST scores (
from baseline)
Intermezzo
®
: mean DSST scores (
from baseline)
Placebo
Intermezzo
®
1.75 mg
Intermezzo
®
3.5 mg
Phase 1 PK/PD Study n=24
6
0
20
60
90
120
150
180
240
300
-16
-14
-12
-10
-8
-6
-4
-2
0
2
4
6
Time (min) |
 Phase 1
Pharmacokinetic (PK) comparison study (2007)
Phase 1 Pharmacokinetic (PK)
comparison study (2007) |
 Intermezzo
®
: Phase 1 PK comparison study (2005)
Intermezzo
®
: Phase 1 PK comparison study (2005)
Study
design:
Randomized,
open-label,
comparative,
pharmacokinetic
study
of
Intermezzo
®
3.5mg
and
Ambien
®
10mg
in
33
healthy
adults
Pharmacokinetic
assessment:
Blood
was
collected
prior
to
dosing
(T=0
minutes) and at 5, 10, 15, 20, 25, 30, 40 and 50 minutes and 1, 1.5, 2, 3,
4, 5, 6 and 8 hours post-dose
Key
assessments:
Comparison
between
AUC
0-5min
,
AUC
0-10min
,
AUC
0-15min
,
AUC
0-20min
,
and
C
max
under
fasted
conditions
for
Intermezzo
®
3.5mg
and
Ambien
®
10mg
8 |
 0
20
40
60
80
100
120
140
0
1
2
3
4
5
6
7
8
Time (hours)
3.5
mg
Intermezzo
®
vs.
10
mg
Ambien
®
plasma
profile
3.5
mg
Intermezzo
®
vs.
10
mg
Ambien
®
plasma
profile
Intermezzo
®
3.5mg vs. Ambien
®
10mg PO after an overnight fasting, n=33
9
Intermezzo
®
3.5
mg
Ambien
®
10 mg |
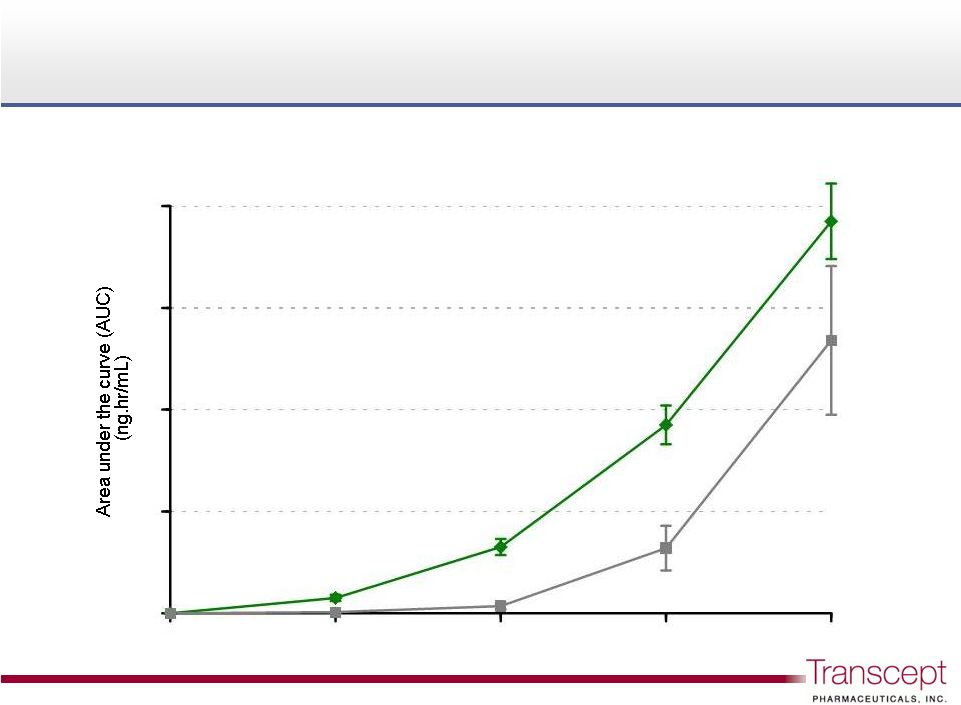 Intermezzo
®
3.5 mg delivered greater early zolpidem
bioavailability
than
a
~3x
higher
Ambien
®
dose
Intermezzo
®
3.5 mg delivered greater early zolpidem
bioavailability
than
a
~3x
higher
Ambien
®
dose
10
Intermezzo
®
3.5mg vs. Ambien
®
10mg PO after an overnight fasting, n=33
9.3x
2.9x
1.4x
Intermezzo
®
3.5 mg
Ambien
®
10 mg
0
1
2
3
4
0
5
10
15
20
Time (min) |
 Intermezzo
®
Phase 3 clinical results
Intermezzo
®
Phase 3 clinical results
Low dose zolpidem
for
middle of the night (MOTN) insomnia |
 Intermezzo
®
: Phase 3 sleep lab study (2006)
Intermezzo
®
: Phase 3 sleep lab study (2006)
Principal investigators:
Thomas Roth, PhD and Martin Scharf, PhD
Study design:
Double-blind, placebo-controlled study for objective
(PSG)
evaluation
of
sleep-onset
safety
and
efficacy
of
1.75
mg
and
3.5
mg
Intermezzo
®
after
scheduled
MOTN
awakening
Study population:
82 non-elderly primary insomnia patients with a
history of difficulty falling back to sleep after MOTN awakening. The
patients should have demonstrated a two night average MOTN
awakening
of
20
min
but
not
less
than
15
min
on
any
one
night
during the sleep lab screening period
Primary
endpoint:
Intermezzo
®
3.5
mg
Latency
to
Persistent
Sleep
(LPS
MOTN
) vs. placebo
12 |
 Intermezzo
®
: Objective Latency to Persistent Sleep
following a middle of the night awakening
Intermezzo
®
: Objective Latency to Persistent Sleep
following a middle of the night awakening
Phase 3 Sleep Lab Study n = 82
13
0
10
20
30
40
50
37.29
23.71
14.16
44.64
30.66
16.97
Placebo
Intermezzo®
1.75 mg
Intermezzo®
3.5 mg
Intermezzo
®
1.75mg
p<0.001
vs.
Placebo
p<0.003
vs.
Placebo
p<0.001
vs.
Placebo
p<0.001
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
>
60 min awakening (37/82)
All Patients (82/82) |
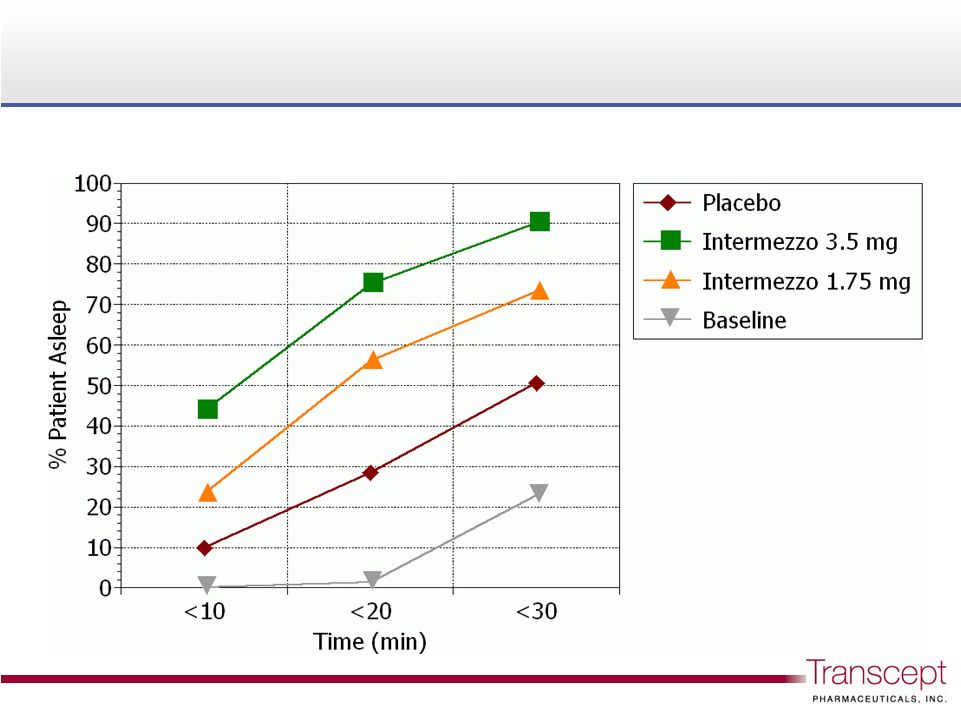 Intermezzo
®
: proportion of patients asleep following a
middle of the night awakening
Intermezzo
®
: proportion of patients asleep following a
middle of the night awakening
75%
56%
28%
Phase 3 Sleep Lab Study n = 82
14 |
 Next-day
residual effects of Intermezzo ®
1.75 mg
and 3.5 mg vs. placebo
Next-day residual effects of Intermezzo
®
1.75 mg
and 3.5 mg vs. placebo
15
Phase 3 Sleep Lab Study n = 82
0
10
20
30
40
50
60
70
Objective (DSST)
61.88
61.59
60.57
Placebo
Intermezzo®
1.75 mg
Intermezzo®
3.5 mg
Intermezzo
®
1.75mg
p<0.62
vs.
Placebo
p<0.44
vs.
Placebo
p<0.15
vs.
Placebo
p<0.91
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
0
10
20
30
40
50
60
70
Subjective (VAS)
62.35
64.25
63.56 |
 Intermezzo
®
: “Good”
or “Excellent”
next day ratings
Intermezzo
®
: “Good”
or “Excellent”
next day ratings
Phase 3 Sleep Lab Study n = 82
16
Sleep Quality
Refreshing Sleep
Ability to Function
0
10
20
30
40
50
23.5
32.9
42.6
23.5
36.5
40.0
39.5
47.6
51.3
Placebo
Intermezzo®
1.75 mg
Intermezzo®
3.5 mg
Intermezzo
®
1.75mg
NS
p<0.017
vs.
Placebo
p<0.001
vs.
Placebo
p<0.001
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
p<0.024
vs.
Placebo
p<0.009
vs.
Placebo |
 Principal
investigators: Andrew Krystal, MD and Thomas Roth, PhD
Study design:
Randomized, double-blind, placebo controlled,
outpatient
study
to
evaluate
the
safety
and
efficacy
of
Intermezzo
®
3.5 mg for the prn
treatment of insomnia after MOTN awakening
Study population:
294 non-elderly primary insomnia patients
demonstrating
at
least
2
prolonged
MOTN
awakenings
of >
60
min,
and
at
least
4
of
>
30
min
during
an
initial
two
week
placebo
run-in
Primary
endpoint:
Post-MOTN
Latency
to
Sleep
Onset
(LSO
MOTN
)
vs.
placebo averaged over the 4-week treatment period
Intermezzo
®
: Phase 3 outpatient study (2008)
Intermezzo
®
: Phase 3 outpatient study (2008)
17 |
 Intermezzo
®
: Latency to Sleep Onset, 4 week avg
Intermezzo
®
: Latency to Sleep Onset, 4 week avg
Phase 3 outpatient study n = 294
18
0
10
20
30
40
50
60
70
80
69.42
56.37
68.13
38.22
Baseline
p<0.0001
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
Baseline
4 week
treatment
Baseline
4 week
treatment
Baseline |
 Intermezzo
®
: Latency to Sleep Onset, weekly
Intermezzo
®
: Latency to Sleep Onset, weekly
Phase 3 outpatient study n = 294
19
Baseline
week 1
week 2
week 3
week 4
0
10
20
30
40
50
60
70
Placebo
Intermezzo® 3.5 mg
Intermezzo
®
3.5mg
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo
p<0.0001
vs.
Placebo |
 Intermezzo
®
: next day sleepiness/alertness scores,
4 week avg
Intermezzo
®
: next day sleepiness/alertness scores,
4 week avg
Phase 3 outpatient study n = 294
20
0
1
2
3
4
5
6
7
8
9
4.71
5.24
4.87
5.59
Baseline
p<0.0041
vs.
Placebo
Intermezzo
®
3.5mg
Placebo
Baseline
4 week
treatment
Baseline
4 week
treatment
Baseline
MORE
ALERT
MORE
SLEEPY |
 Intermezzo
®
: average weekly treatment exposure
Intermezzo
®
: average weekly treatment exposure
21
Placebo
wk 1
Placebo
wk 2
week 1
week 2
week 3
week 4
0
1
2
3
4
5
Placebo
Intermezzo® 3.5 mg
Intermezzo
®
3.5mg
Placebo
Phase 3 outpatient study n = 294 |
 Distribution
of self-reported MOTN awakenings Distribution of self-reported MOTN
awakenings Phase 3 outpatient study n = 294
22
MOTN treatment call to IVRS
0
200
400
600
800
1000
1200
1400
1600
8-9pm
9-10pm
10
-
11pm
11
-
12am
12-1am
1-2am
2-3am
3-4am
4-5am
5-6am
6-7am |
 Intermezzo
®
is a trademark of Transcept
Pharmaceuticals, Inc.
23 |
