Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Assertio Therapeutics, Inc | a10-7670_18k.htm |
Exhibit 99.1
|
|
Investor Newsletter A message from our CEO, Carl A. Pelzel April 6, 2010 Dear Shareholders of Depomed, 2010 is off to a productive start at Depomed. I am pleased with the significant progress made on our two lead product candidates at just the midpoint of the first quarter. First, we announced that our partner Abbott Products (formerly Solvay Pharmaceuticals) has submitted the New Drug Application for DM-1796 for post-herpetic neuralgia. The FDA’s acceptance of the NDA expected in the second quarter of 2010 will trigger a $10 million milestone payment from Abbott. Approval of the NDA, which could occur in the first quarter of 2011, triggers a $35 million milestone for the approval, and a milestone of up to $25 million depending on the product label. We look forward to the approval of DM-1796 and its commercial potential in the market. DM-1796 will address a large and dynamic market with >$6 billion in sales at branded prices. Lyrica initially had a strong launch but has only grown by $50 million in sales over the past 2 years despite Pfizer’s sales force and DTC promotion efforts. Meanwhile, generic gabapentin has grown by $800 million in sales at branded prices, underlining Lyrica’s lack of product differentiation and the resulting limits it faces with managed care. With the efficacy and side effect profile that emerged from the Phase 3 trial completed in the third quarter of last year, we see DM-1796 competing favorably in the market against Lyrica and other PHN treatments (see page 2). Based on our physician market research, DM-1796 addresses the No. 1 unmet need of low tolerability causing less than optimal results in almost 50% of patients. Therefore, physicians actually anticipate DM-1796 as the most frequently prescribed first-line treatment (see page 3). We believe the clinical differentiation will be compelling to physicians and managed care. We have also advanced SeradaTM, our extended release gabapentin product candidate formulated with our Acuform® drug delivery technology. We expect input from the FDA later this quarter on a Special Protocol Assessment for Breeze 3, our upcoming Phase 3 trial evaluating SeradaTM for the treatment of menopausal hot flashes. We anticipate enrolling patients in the randomized, double-blind, placebo-controlled trial of up to 600 patients by the end of April. Patients will receive either placebo or a total dose of 1800mg of Serada dosed 600mg in the morning and 1200mg in the evening. In Breeze 3, we believe we have a trial that will accurately measure Serada’s drug effect without overstating the placebo effect. First, we have mitigated the impact individual outliers can have on achievement of Breeze 3’s endpoints by using a non-parametric statistical analysis (based on median, rather than mean, frequency of hot flashes). Breeze 3’s single active treatment arm also means that patients will have a 50% chance of receiving placebo, rather than a 33% chance, and hence are less likely to believe they are on active. Also, with a single active arm, the required statistical p-value is .05 rather than .025, as was the case in Breeze 1 and 2. Finally, we have addressed the tendency patients in clinical trials have to regress by increasing the run-in period to two weeks in Breeze 3 (compared to one week in Breeze 1 and 2). The longer run-in period may result in a more stable baseline, and thereby reduce the placebo effect as observed in the Pristiq studies with a ~55% placebo response. Our commercialized product Glumetza® (extended release metformin hydrochloride tablets) continues to perform well. Glumetza is the number one branded metformin product in the United States, reaching a $54m gross revenue run rate in February. In 2010, we will continue to work hard with our partners at Santarus to increase revenues and prescriptions. A $3 million sales milestones is payable to Depomed by Santarus if annual net product sales of Glumetza exceed $50 million this year. I anticipate 2010 will be a productive year on the business development front as well. Our team will focus on out licensing our early-stage non-core assets such as our GERD product candidate and our Parkinson’s Disease product candidate. Technology deals such as our Covidien and Merck collaborations are also a priority. Finally, we believe an ex-US transaction for DM-1796 could meaningfully impact our near-term balance sheet and our medium and long-term royalty revenue. Thank you again for your continued support and interest in Depomed. Sincerely, Carl A. Pelzel President and CEO Please see note regarding forward-looking statements and risk factors on last page |
|
|
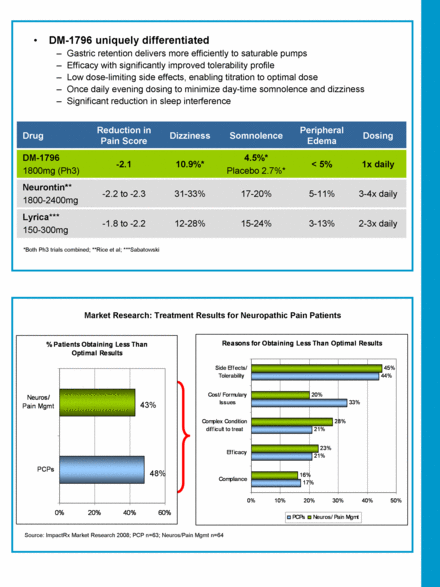
-1.8 to -2.2 -2.2 to -2.3 -2.1 Reduction in Pain Score 1x daily < 5% 4.5%* Placebo 2.7%* 10.9%* DM-1796 1800mg (Ph3) 3-13% 5-11% Peripheral Edema 2-3x daily 3-4x daily Dosing 15-24% 12-28% Lyrica*** 150-300mg 17-20% 31-33% Neurontin** 1800-2400mg Somnolence Dizziness Drug DM-1796 uniquely differentiated Gastric retention delivers more efficiently to saturable pumps Efficacy with significantly improved tolerability profile Low dose-limiting side effects, enabling titration to optimal dose Once daily evening dosing to minimize day-time somnolence and dizziness Significant reduction in sleep interference *Both Ph3 trials combined; **Rice et al; ***Sabatowski Source: ImpactRx Market Research 2008; PCP n=63; Neuros/Pain Mgmt n=64 Market Research: Treatment Results for Neuropathic Pain Patients 43% 48% Reasons for Obtaining Less Than Optimal Results 17% 21% 21% 33% 44% 16% 23% 28% 20% 45% 0% 10% 20% 30% 40% 50% Compliance Efficacy Complex Condition difficult to treat Cost/ Formulary Issues Side Effects/ Tolerability PCPs Neuros/ Pain Mgmt % Patients Obtaining Less Than Optimal Results 0% 20% 40% 60% |
|
|
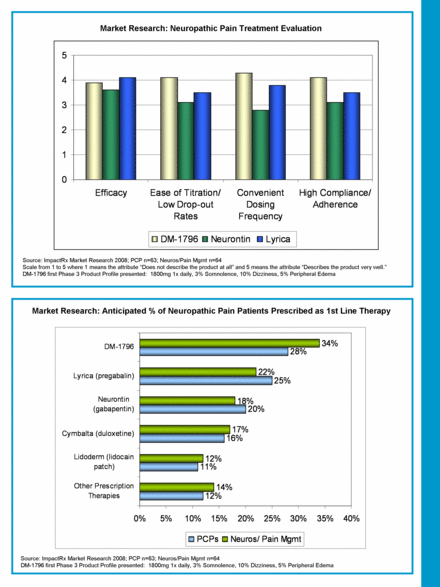
Source: ImpactRx Market Research 2008; PCP n=63; Neuros/Pain Mgmt n=64 Scale from 1 to 5 where 1 means the attribute “Does not describe the product at all” and 5 means the attribute “Describes the product very well.” DM-1796 first Phase 3 Product Profile presented: 1800mg 1x daily, 3% Somnolence, 10% Dizziness, 5% Peripheral Edema Market Research: Neuropathic Pain Treatment Evaluation Source: ImpactRx Market Research 2008; PCP n=63; Neuros/Pain Mgmt n=64 DM-1796 first Phase 3 Product Profile presented: 1800mg 1x daily, 3% Somnolence, 10% Dizziness, 5% Peripheral Edema Market Research: Anticipated % of Neuropathic Pain Patients Prescribed as 1st Line Therapy 0 1 2 3 4 5 Efficacy Ease of Titration/ Low Drop-out Rates Convenient Dosing Frequency High Compliance/ Adherence DM-1796 Neurontin Lyrica 12% 11% 16% 20% 25% 28% 14% 12% 17% 18% 22% 34% 0% 5% 10% 15% 20% 25% 30% 35% 40% Other Prescription Therapies Lidoderm (lidocain patch) Cymbalta (duloxetine) Neurontin (gabapentin) Lyrica (pregabalin) DM-1796 PCPs Neuros/ Pain Mgmt |
|
|
NOTE REGARDING FORWARD-LOOKING STATEMENTS AND RISK FACTORS. Statements in this newsletter that are not historical facts are forward-looking statements that involve risks and uncertainties. The inclusion of forward-looking statements, including those related to expectations regarding the commercialization of SeradaTM potential business transactions, financial matters, clinical development programs, and potential benefits of our products and product candidates, should not be regarded as a representation that any of our plans will be achieved. Actual results may differ materially from those set forth in this newsletter due to the risks and uncertainties inherent in our business, including, without limitation, risks and uncertainties related to: our research and development efforts, including pre-clinical and clinical testing; regulation by the FDA and other government agencies; the timing of regulatory applications and product launches; our ability to successfully commercialize our products; the success of our collaborative arrangements with development and commercialization partners; and other risks detailed in our filings with the Securities and Exchange Commission filings, including our most recent Annual Report on Form 10-K and our most recent Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We undertake no obligation to revise or update this newsletter to reflect events or circumstances that occur after the date of this newsletter. Investment in our common stock involves a high degree of risk. This newsletter is not intended to be, and is not, a prospectus. You should review our most recent Annual Report on Form 10-K and any subsequently filed Quarterly Report on Form 10-Q or Current Report on Form 8-K before making any investment decision related to our securities. You should pay particular attention to the “Risk Factors” sections in the Form 10-K and Form 10-Q. Each of the risks described in those documents could adversely affect our business, financial condition, results of operations and prospects, and could result in a complete loss of any investment you may have in our securities. Requires statistical p-value of .05 rather than .025 to achieve statistical significance Two active arms (1200mg QD, 1800mg BID) Single active arm (1800mg BID) Reduces regression to the mean observed in Breeze 1 and 2, resulting in a more stable baseline thereby potentially reducing the placebo effect One-week run in period prior to randomization Two-week run in period prior to randomization Increased statistical power Maximum 180 patients per active treatment arm Up to 300 patients in the active treatment arm Designed to reduce influence that significant outliers can have on the achievement of efficacy endpoints Standard parametric analysis Non-parametric analysis – alternative statistical analysis Reason for Modification Breeze 1 and 2 Breeze 3 Why we believe Breeze 3 will be successful Breeze 3 assessed under SPA – Statistical modifications improve likelihood of success Higher number of patients per one treatment arm, p<0.05, and non-parametric analysis reducing the effects of outliers increase statistical power Placebo response reduction measures compared to Breeze 1 Two groups rather than three. Patient now has 50% chance of receiving placebo (vs. 33%) and hence is less likely to think they are on active. Two week run in rather than one week run in to establish a more stable baseline and reduce regression to the mean (observed in Pristiq studies with ~55% placebo response) Reduction of patient interaction with clinical center. One visit will be removed and two visits will be replaced by telephone calls to reduce the clinical care impact on the placebo response Diary collection on only 4 weeks of the final 12 to reduce boredom and mechanical diary completion Breeze 3 Modifications |