Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | c96348e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - Unilife Corp | c96348exv99w1.htm |
Exhibit 99.2

| An emerging global leader for innovative safety medical devices February 2010 |

| Cautionary Note Regarding Forward-Looking Statements This presentation contains forward-looking statements. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements. These forward-looking statements are based on management's beliefs and assumptions and on information currently available to our management. Our management believes that these forward-looking statements are reasonable as and when made. However, you should not place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. We do not undertake any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results, events and developments to differ materially from our historical experience and our present expectations or projections. These risks and uncertainties include, but are not limited to, those described in "Item 1A. Risk Factors" and elsewhere in our registration statement on Form 10 and those described from time to time in our future reports which we will file with the Securities and Exchange Commission. |

| Well positioned to succeed in fast-growing healthcare & pharmaceutical markets being driven to mandatory use First range of prefilled & clinical syringes with a technology platform of automatic, fully integrated safety features Sanofi-aventis (largest prefilled syringe purchaser) paying $40m for Unifill industrialization and right to purchase^ Discussions with additional pharmaceutical companies Building a world-class team and industrial capabilities Business Overview ^ €10m exclusivity fee (conversion on receipt $14m) and scheduled milestone payments under €17m Unifill industrialization program |

| The Global Transition to Safety Syringes 1.3m^ people die annually from unsafe injection practices Needlestick injuries a serious threat to healthcare workers US mandates and enforces safety syringes within facilities Europe and other international markets now following Pharmaceutical companies must also comply with laws Passive, integrated safety preferred but rarely available Healthcare workers still awaiting products of choice ^ World Health Organization (WHO) Injection Safety Fact Sheet. October 2006 |
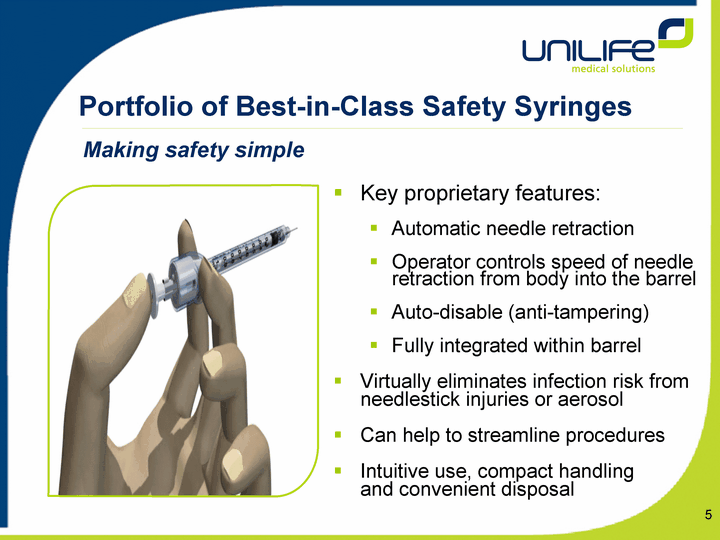
| Portfolio of Best-in-Class Safety Syringes Key proprietary features: Automatic needle retraction Operator controls speed of needle retraction from body into the barrel Auto-disable (anti-tampering) Fully integrated within barrel Virtually eliminates infection risk from needlestick injuries or aerosol Can help to streamline procedures Intuitive use, compact handling and convenient disposal Making safety simple |

| Unilife Brand Architecture Ready-to-Fill (Prefilled) syringes (glass barrel) Supplied to pharmaceutical companies Clinical syringes (plastic barrel for use with vials) Supplied for use by healthcare workers and self-injecting patients (i.e. insulin) |

| Key Target Markets Targeting fast-growing and non-crowded markets that are pre-conditioned to demand premium safety devices Unilife Target Market Volumes^ (billions) Annual Growth Rate Capacity For Premium Devices Market Transition to Safety Level of Comp. Pharmaceutical High 2.7 High High Mature Low Mature healthcare* Moderate 12 Medium High Mature Moderate Patient self-injection Moderate 6 Medium Moderate Emerging High Harm reduction Moderate 0.4 Medium Moderate Emerging Low Emerging healthcare Low 12 Low Low Poor High Vaccinations Low 2 Medium Low Emerging Moderate ^ Internal Unilife estimates based upon 2004 IASIT estimate of 35 billion syringes used globally * Includes healthcare facilities for North America, European Union, Japan and Australia |

| Pharmaceutical Market for Prefilled Syringes More than 2.7* billion prefilled syringes used globally $1.5** billion market growing at more than 15% annually 50+ injectable drugs and vaccines are now prefilled Market now restricted to attaching ancillary safety devices to standard prefilled syringes Expensive to purchase and attach, with high packaging, transport and storage costs * Greystone Associates. Prefilled Syringes. 2009 ** OnDrug Delivery. Prefilled Syringe Edition. 2009 |

| Agreements with sanofi-aventis Long-term relationship with 'top 5' pharmaceutical leader Exclusive Agreement signed in July 2008 €10m fee for exclusive right to negotiate Unifill(tm) purchase Agreed to start and fund the industrialization program Industrialization Agreement signed in June 2009 Committed to fund the €17m industrialization program* Negotiating for desired areas therapeutic exclusivity Retained right to negotiate with other companies World's largest purchaser of prefilled syringes * Subject to continued completion of industrialization program milestone payments |

| The world's first true prefilled safety syringe Insertion into standard prefilled fill-finish systems Use of proven materials for components in fluid path All safety features fully integrated inside glass barrel Streamlines filling and packaging processes Compact in size for easy handling & convenient disposal Strong product differentiation in competitive markets Expansion into new markets (self-injection at home) The Unifill(tm) Syringe |

| Unifill(tm) is the only primary syringe container with safety mechanism inside the glass barrel Competitive Position Unifill (tm) syringes Glass Prefilled Syringe Fully Integrated External Sheath Controlled Retraction into Barrel Active Activation Needle Guard Passive Activation Stand - Alone Needles External Sheath The Unifill(tm) Syringe |

| Ancillary Safety Products Attached onto Standard Prefilled Syringes Unifill syringe Ancillary Safety Products Attached onto Standard Prefilled Syringes The Unifill(tm) Syringe Competitive Comparison |

| Unifill(tm) Industrialization program Commenced in July 2008 Target completion late 2010 Mikron engaged for assembly Assembly process validated 60m / year assembly line ready late 2010 Further assembly lines to have 150m per year capacity |
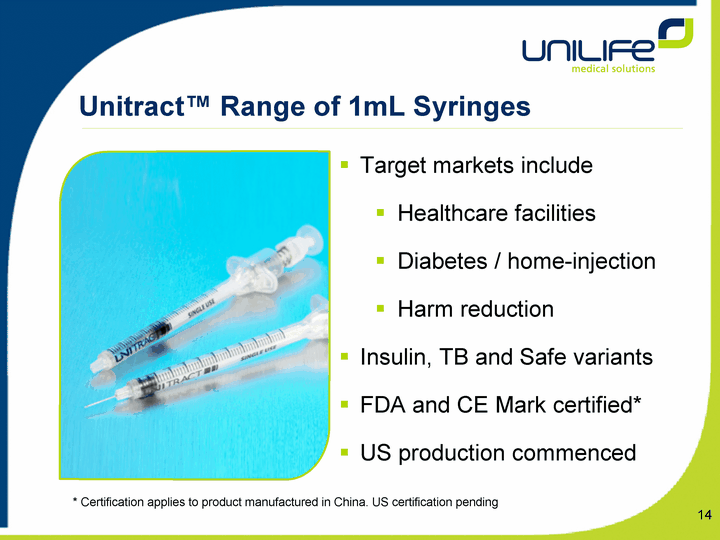
| Unitract(tm) Range of 1mL Syringes Target markets include Healthcare facilities Diabetes / home-injection Harm reduction Insulin, TB and Safe variants FDA and CE Mark certified* US production commenced * Certification applies to product manufactured in China. US certification pending |

| ISO-13485 certified with FDA-registered facilities in PA Team of more than 120 world-class staff Strong patent position for our proprietary products Strong cash position, multiple funding / revenue streams Successfully redomiciled from Australia to the US NASDAQ listing supports ongoing expansion activities Positioning Unilife for Success Building value for shareholders |

| Positioning Unilife for Success New Global Headquarters and Production Center State-of-the-art facility to meet highest industry standards Stage one capacity supports 400 million syringes a year 2nd stage approval supports production of 1 billion units Ready for operations in late 2010 |

| Positioning Unilife for Success Additional pharmaceutical relationships |

| Positioning Unilife for Success Glass ready-to-fill Unifill(tm) Select Attachable needles Plastic for vial Unitract Clinical Range 3 & 5mL with Luer needle Development of Pipeline Products |

| Strong Product Pipeline Current 2010-2011 2012 + Unifill(tm) Select attachable needles^ Unitract(tm) 3 & 5mL with luer needle Unifill(tm) syringe with fixed needle Unitract(tm) 1mL Insulin, TB and Safe ^ subject to securing agreement with an interested pharmaceutical party |

| Background Financial Data Selected Data Strong cash position $41.4m (as of 12/31/09) $14m from sanofi-aventis in 2008, regular milestone payments Raised approx $47.5m in US / Australian markets Nov. 2009 Secured $5.2m package from PA Government in Oct. 2009 Cap. Structure Approx 50m shares of common stock tradable on Nasdaq* CHESS Depositary Interests (CDI) traded on Australian Stock Exchange (ASX ticker code UNS) * Upon commencement of NASDAQ trading |

| Upcoming Milestones Commercial release of US-made Unitract(tm) 1mL syringes Completion of negotiations for Unifill(tm) exclusivity list Listing on NASDAQ Open new global headquarters and production facility in PA Completion of Unifill(tm) industrialization program Agreements with additional pharmaceutical companies |

| Summary Unifill(tm) syringe represents 'disruptive technology' World's major prefilled buyer committed $40m^ (pre-sales) Industry certified with FDA-registered production facilities Strong cash position, multiple funding & revenue streams Well-positioned to attain strong market share in the pharmaceutical market for prefilled safety syringes World-class team with strong industry credentials ^ €10m exclusivity fee (conversion on receipt $14m) and scheduled milestone payments under €17m Unifill industrialization program |
