Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Theravance Biopharma, Inc. | tm2125603d1_ex99-1.htm |
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | tm2125603d1_8k.htm |
Exhibit 99.2

Izencitinib (TD - 1473/JNJ - 8398) Oral gut - selective pan - JAK inhibitor to treat inflammatory bowel diseases August 23, 2021 THERAVANCE BIOPHARMA ® , THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries). All third party trademarks used herein are the property of their respective owners. © 2021 Theravance Biopharma. All rights reserved.

Forward - looking statements 2 Under the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , the company cautions investors that any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward - looking statements or projections . Examples of forward - looking statements in this presentation may include the Company’s goals, designs, strategies, plans and objectives, the Company’s regulatory strategies and timing of clinical studies (including the data therefrom), the potential characteristics, benefits and mechanisms of action of the Company’s product and product candidates, the potential that the Company’s research programs will progress product candidates into the clinic, the Company’s expectations for product candidates through development, the Company's expectations regarding its allocation of resources, potential regulatory approval and commercialization (including their differentiation from other products or potential products), product sales or profit share revenue and the Company’s expectations for its expenses, excluding share - based compensation and other financial results . The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to the impacts on the COVID - 19 global pandemic on our business, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s compounds or product candidates are unsafe, ineffective or not differentiated, risks that product candidates do not obtain approval from regulatory authorities, the feasibility of undertaking future clinical trials for our product candidates based on policies and feedback from regulatory authorities, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure, disagreements with Innoviva, Inc . and TRC LLC, the uncertainty of arbitration and litigation and the possibility that an arbitration award or litigation result could be adverse to the Company . Other risks affecting Theravance Biopharma are in the company's Form 10 - Q filed with the SEC on August 5 , 2021 , and other periodic reports filed with the SEC .
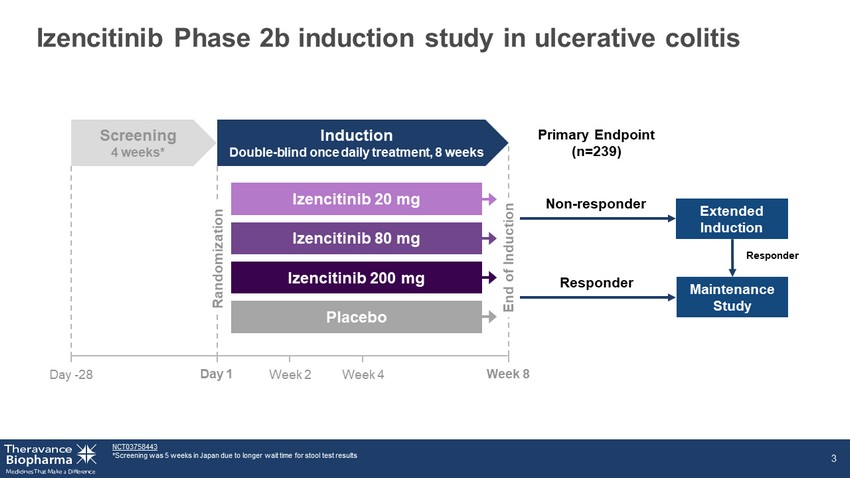
Izencitinib Phase 2b induction study in ulcerative colitis NCT03758443 *Screening was 5 weeks in Japan due to longer wait time for stool test results 3 Week 8 Non - responder Extended Induction Day 1 Week 2 Week 4 Primary Endpoint (n=239) Responder Maintenance Study Day - 28 Screening 4 weeks* Induction Double - blind once daily treatment, 8 weeks Placebo Izencitinib 20 mg Izencitinib 80 mg Izencitinib 200 mg Randomization End of Induction Responder
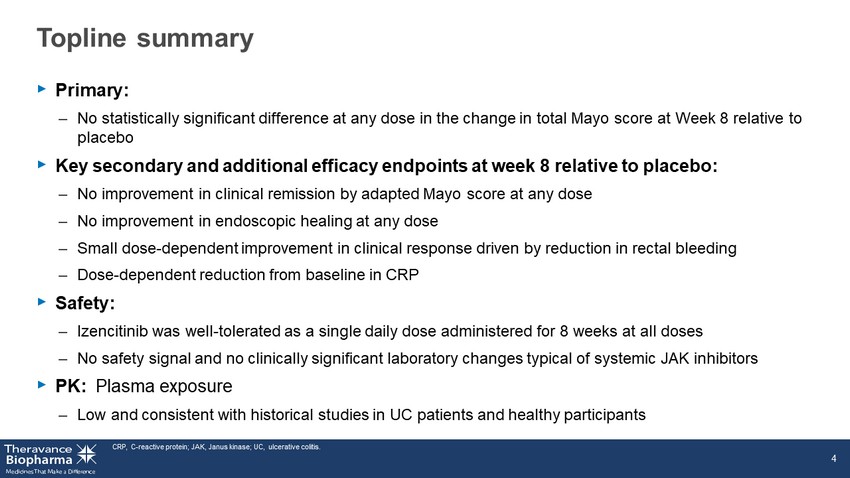
Topline summary ‣ Primary: – No statistically significant difference at any dose in the change in total Mayo score at Week 8 relative to placebo ‣ Key secondary and additional efficacy endpoints at week 8 relative to placebo: – No improvement in clinical remission by adapted Mayo score at any dose – No improvement in endoscopic healing at any dose – Small dose - dependent improvement in clinical response driven by reduction in rectal bleeding – Dose - dependent reduction from baseline in CRP ‣ Safety: – Izencitinib was well - tolerated as a single daily dose administered for 8 weeks at all doses – No safety signal and no clinically significant laboratory changes typical of systemic JAK inhibitors ‣ PK: Plasma exposure – Low and consistent with historical studies in UC patients and healthy participants CRP, C - reactive protein; JAK, Janus kinase; UC, ulcerative colitis. 4
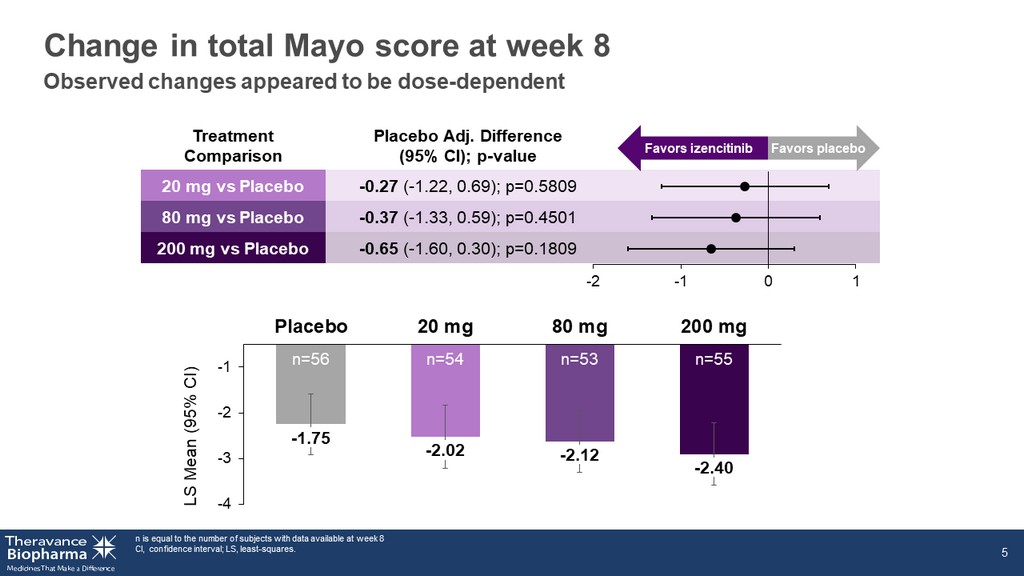
- 1.75 - 2.02 - 2.12 - 2.40 -4 -3 -2 -1 Placebo 20 mg 80 mg 200 mg Treatment Comparison Placebo Adj. Difference (95% CI); p - value 20 mg vs Placebo - 0.27 ( - 1.22, 0.69); p=0.5809 80 mg vs Placebo - 0.37 ( - 1.33, 0.59); p=0.4501 200 mg vs Placebo - 0.65 ( - 1.60, 0.30); p=0.1809 Change in total Mayo score at week 8 n is equal to the number of subjects with data available at week 8 CI, confidence interval; LS, least - squares. 5 Favors izencitinib Favors placebo LS Mean (95% CI) n=56 n=54 n=53 n=55 -2 -1 0 1 Observed changes appeared to be dose - dependent

Rates of key secondary endpoints at 8 weeks Clinical Remission: by adapted Mayo score components: a stool frequency score of 0 or 1, a rectal bleeding subscore of 0, and a Mayo endoscopy subscore of 0 or 1. Clinical Response: reduction in total Mayo score ≥ 3 and ≥ 30%, with a reduction in the rectal bleeding subscore ≥ 1 or an ab sol ute subscore ≤ 1. Endoscopic Remission: Mayo endoscopic subscore = 0; Endoscopic Healing: Mayo endoscopic subscore ≤ 1. Percentages are calculated based on the number of subjects randomized in that treatment group. 6 Placebo 20 mg 80 mg 200 mg Izencitinib 9.8 24.6 0 11.5 9.8 31.1 4.9 13.1 6.8 32.2 3.4 11.9 6.9 39.7 3.4 8.6 0 20 40 60 80 100 Clinical Remission Clinical Response Endoscopic Remission Endoscopic Healing %
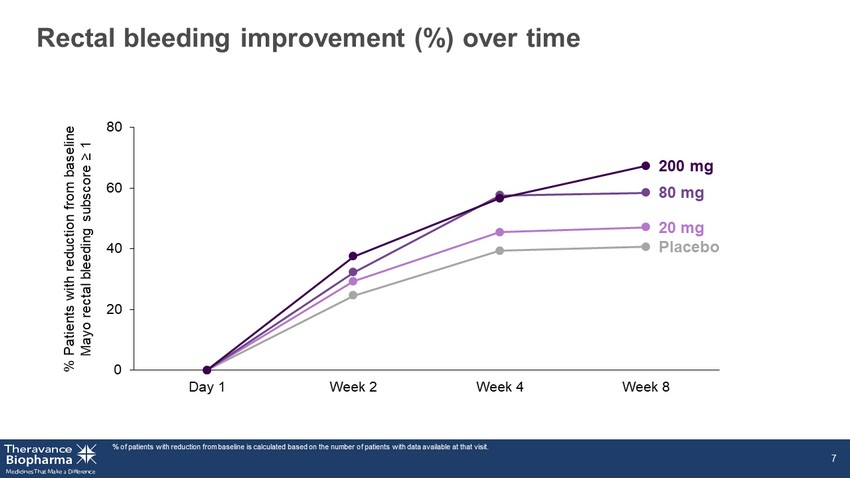
0 20 40 60 80 Day 1 Week 2 Week 4 Week 8 Rectal bleeding improvement (%) over time % of patients with reduction from baseline is calculated based on the number of patients with data available at that visit. 7 % Patients with reduction from baseline Mayo rectal bleeding subscore ≥ 1 Placebo 20 mg 80 mg 200 mg

Dose - dependent reduction in CRP (median change over time) CRP, C - reactive protein. 8 -1 -0.8 -0.6 -0.4 -0.2 0 0.2 0.4 0.6 0.8 Day 1 Week 2 Week 4 Week 8 mg/L Placebo 20 mg 80 mg 200 mg

Summary of safety results ‣ Izencitinib up to 200 mg daily for 8 weeks was well - tolerated ‣ Serious adverse events (n=13) were well balanced amongst the treatment groups and not considered related to study treatment; no deaths ‣ Most TEAEs were mild or moderate – UC exacerbation was slightly more common in izencitinib groups, but rates were consistent with other studies – No safety signal (including adverse events of special interest*) ‣ Unremarkable laboratory values, ECG parameters, and vital signs – Liver or kidney function – Creatine phosphokinase – Lipid parameters – Blood cell counts (leukocytes, lymphocytes, neutrophils, platelets, reticulocytes, hemoglobin, hematocrit) *Includes malignancy, thromboembolic events, major cardiovascular events, opportunistic infections, serious infections, compl ica ted zoster, and laboratory abnormalities of interest. ECG, electrocardiogram; TEAE, treatment - emergent adverse event. 9

Topline summary ‣ Primary: – No statistically significant difference at any dose in the change in total Mayo score at Week 8 relative to placebo ‣ Key secondary and additional efficacy endpoints at week 8 relative to placebo: – No improvement in clinical remission by adapted Mayo score at any dose – No improvement in endoscopic healing at any dose – Small dose - dependent improvement in clinical response driven by reduction in rectal bleeding – Dose - dependent reduction from baseline in CRP ‣ Safety: – Izencitinib was well - tolerated as a single daily dose administered for 8 weeks at all doses – No safety signal and no clinically significant laboratory changes typical of systemic JAK inhibitors ‣ PK: Plasma exposure – Low and consistent with historical studies in UC patients and healthy participants CRP, C - reactive protein; JAK, Janus kinase; UC, ulcerative colitis. 10

Analyze data 16 - week extended induction 44 - week maintenance 12 - week induction Based on the ulcerative colitis results, the Company will seek to minimize future expenses associated with the izencitinib program. Izencitinib program current plan 11 Crohn's Disease Phase 2 study Ulcerative Colitis Internal review upcoming Continue to monitor Top - line results late Q4'21 or early Q1'22

Q&A Session Rick E Winningham Chairman and Chief Executive Officer Andrew A. Hindman Senior Vice President, Chief Financial Officer Richard A. Graham Senior Vice President, Development

