Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ovid Therapeutics Inc. | ovid-8k_20210818.htm |

Ovid Therapeutics Corporate Overview August 2021 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “believe,” “expect,” “plan,” “anticipate” and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. Forward-looking statements contained in this presentation may include statements regarding the progress, timing, development of the Company’s product candidates and pipeline programs; scope of clinical trials; the potential clinical benefit of the Company’s product candidates and pipeline programs; regulatory development; the success of any licensing or partnering opportunities; the potential commercialization of product candidates and pipeline programs; the potential value of the 2021 royalty, license and termination agreement with Takeda; the success of Takeda’s trials in soticlestat and the potential commercialization of soticlestat; and the Company’s expectations regarding its operating expenses, and use of its cash, cash equivalents and short-term investments to the development the Company’s pipeline and pursue business development opportunities. Each of these forward-looking statements involves risks and uncertainties. These statements are based on the Company’s current expectations and projections made by management and are not guarantees of future performance. Therefore, actual events, outcomes and results may differ materially from what is expressed or forecast in such forward-looking statements. Factors that may cause actual results to differ materially from these forward-looking statements include the fact that initial data from clinical trials may not be indicative, and are not guarantees, of the final results of the clinical trials and are subject to the risk that one or more clinical outcomes may materially change as patient enrollment continues and or more patient data becomes available; Takeda’s ability to successfully complete clinical development of, obtain regulatory approval for and, if approved successfully commercialize soticlestat; and uncertainties in the development and regulatory approval process. Additional risks that could cause actual results to differ materially from those in the forward-looking statements are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including the "Risk Factors" sections contained therein. Such risks may be amplified by the COVID-19 pandemic and its potential impact on the Company’s business and the global economy. Except as otherwise required under federal securities laws, we do not have any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events, changes in assumptions or otherwise. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. 2 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
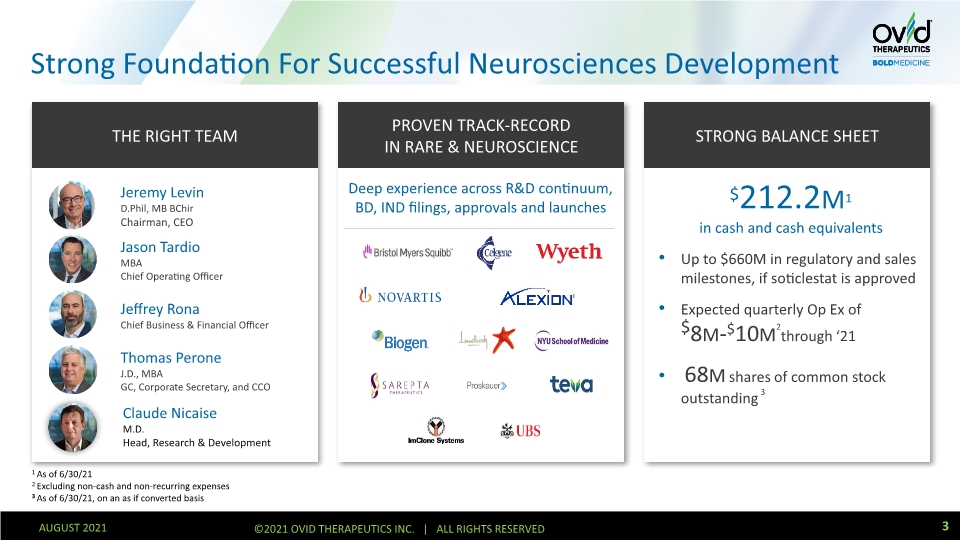
Strong Foundation For Successful Neurosciences Development Build a unique company that delivers first-in-class therapeutics for rare disorders of the CNS 1 As of 6/30/21 2 Excluding non-cash and non-recurring expenses 3 As of 6/30/21, on an as if converted basis THE RIGHT TEAM PROVEN TRACK-RECORD IN RARE & NEUROSCIENCE STRONG BALANCE SHEET Deep experience across R&D continuum, BD, IND filings, approvals and launches $212.2M1 in cash and cash equivalents Up to $660M in regulatory and sales milestones, if soticlestat is approved Expected quarterly Op Ex of $8M-$10M2through ‘21 68M shares of common stock outstanding 3 3 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED Jeremy Levin D.Phil, MB BChir Chairman, CEO Jason Tardio MBA Chief Operating Officer Jeffrey Rona Chief Business & Financial Officer Thomas Perone J.D., MBA GC, Corporate Secretary, and CCO Claude Nicaise M.D. Head, Research & Development

Ovid Therapeutics Focus & Approach Developer of novel first-in-class / best-in-class therapeutics that seek to make a BOLD impact in rare neurological and related CNS disorders. 4 FOCUS Translation engine Target identification, pathology & translation with academic partners APPROACH Modality & delivery Pairing optimal modalities (small molecule & next generation) to deliver therapeutics across the blood-brain barrier Enabling technologies & screening tools De-risking potential candidates earlier with enabling tools and screening technologies Clinical stage pipeline acquisitions that complement existing pipeline and areas of focus Accelerated development by collaboratively engaging patient communities in every stage of development Harness successful development capabilities in small molecule CNS medicines and expand to multiple modalities AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
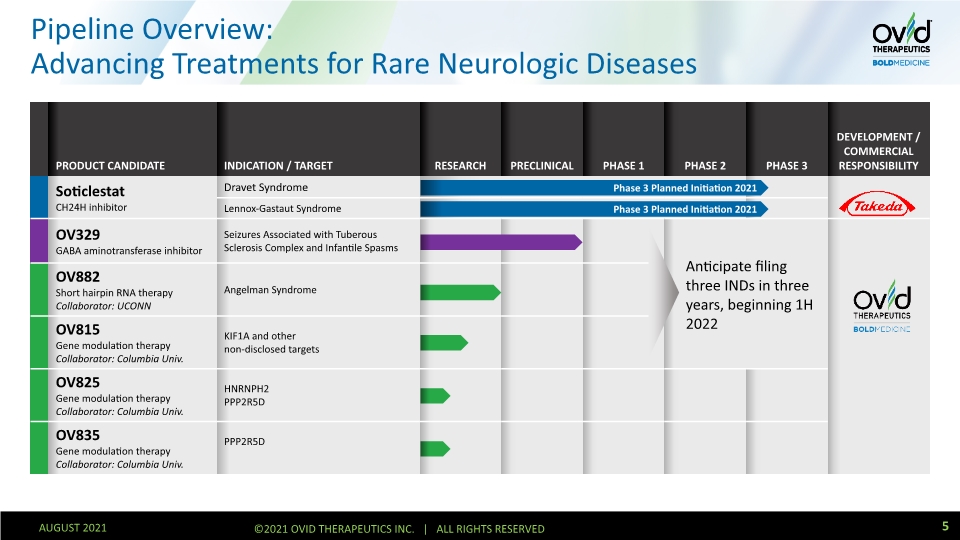
Pipeline Overview: Advancing Treatments for Rare Neurologic Diseases 5 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED Phase 3 Planned Initiation 2021 Phase 3 Planned Initiation 2021 Anticipate filing three INDs in three years, beginning 1H 2022
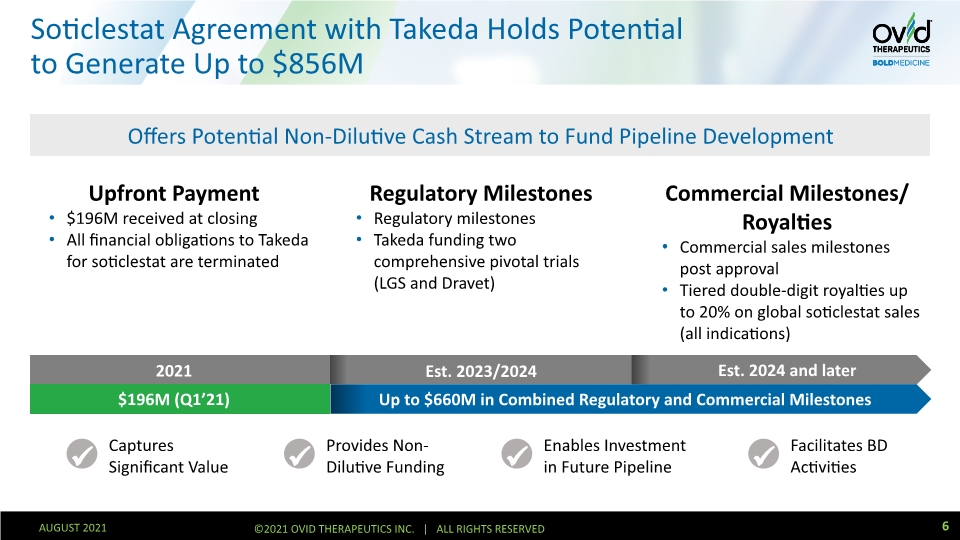
Soticlestat Agreement with Takeda Holds Potential to Generate Up to $856M Upfront Payment $196M received at closing All financial obligations to Takeda for soticlestat are terminated Regulatory Milestones Regulatory milestones Takeda funding two comprehensive pivotal trials (LGS and Dravet) Commercial Milestones/ Royalties Commercial sales milestones post approval Tiered double-digit royalties up to 20% on global soticlestat sales (all indications) Offers Potential Non-Dilutive Cash Stream to Fund Pipeline Development 2021 Est. 2023/2024 Est. 2024 and later Up to $660M in Combined Regulatory and Commercial Milestones $196M (Q1’21) Captures Significant Value Provides Non-Dilutive Funding Enables Investment in Future Pipeline Facilitates BD Activities 6 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
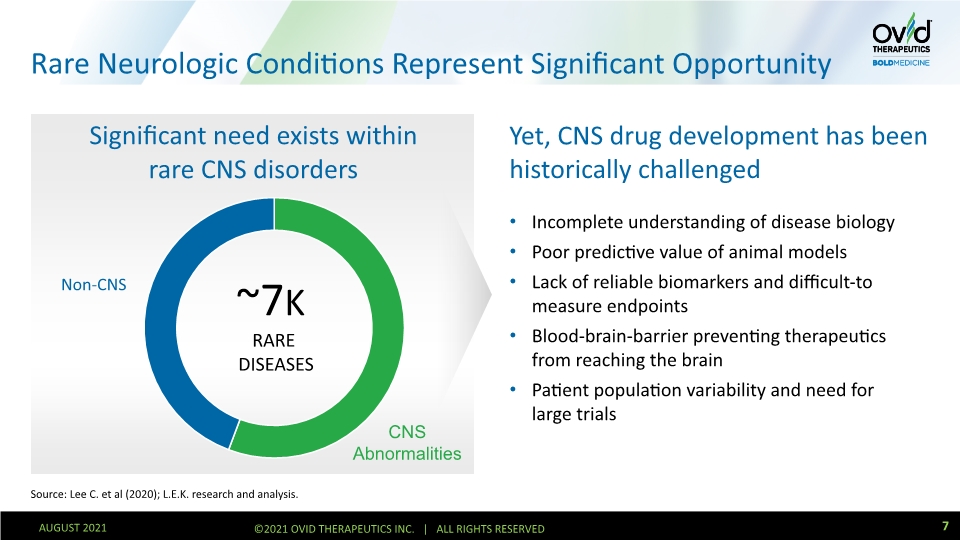
Rare Neurologic Conditions Represent Significant Opportunity Yet, CNS drug development has been historically challenged Incomplete understanding of disease biology Poor predictive value of animal models Lack of reliable biomarkers and difficult-to measure endpoints Blood-brain-barrier preventing therapeutics from reaching the brain Patient population variability and need for large trials Source: Lee C. et al (2020); L.E.K. research and analysis. ~7K RARE DISEASES 7 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
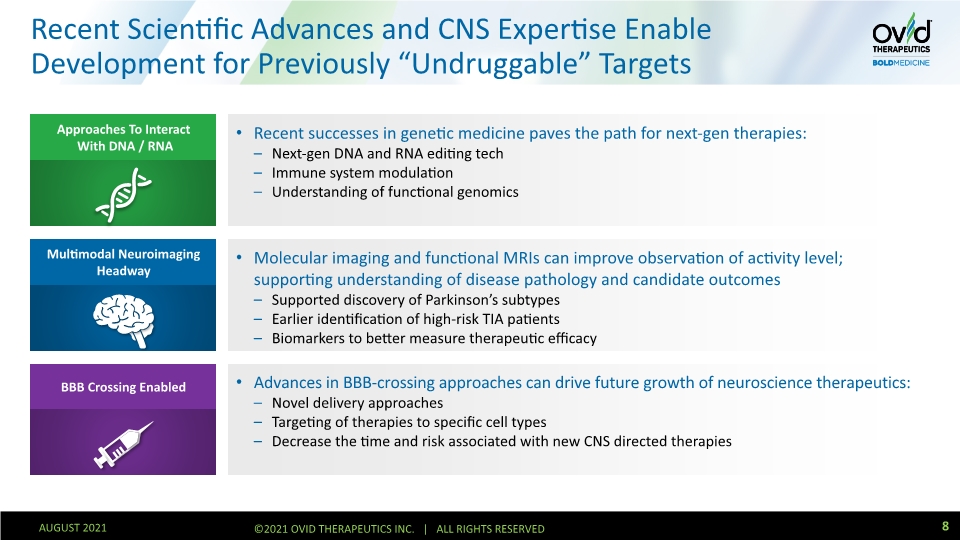
Recent Scientific Advances and CNS Expertise Enable Development for Previously “Undruggable” Targets Recent successes in genetic medicine paves the path for next-gen therapies: Next-gen DNA and RNA editing tech Immune system modulation Understanding of functional genomics Molecular imaging and functional MRIs can improve observation of activity level; supporting understanding of disease pathology and candidate outcomes Supported discovery of Parkinson’s subtypes Earlier identification of high-risk TIA patients Biomarkers to better measure therapeutic efficacy Advances in BBB-crossing approaches can drive future growth of neuroscience therapeutics: Novel delivery approaches Targeting of therapies to specific cell types Decrease the time and risk associated with new CNS directed therapies Approaches To Interact With DNA / RNA Multimodal Neuroimaging Headway BBB Crossing Enabled 8 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED

Proprietary and differentiated enabling technologies and delivery systems to support development of next-generation CNS therapeutics Tools that can be applied and offer utility for current pre-clinical and future CNS assets Assays that support penetration across the blood brain barrier, and allow in vitro testing Delivery platforms that minimize immunogenicity, enable precision targeting, and address manufacturing issues Strong IP position Complement existing pre-clinical pipeline with actionable assets near IND or later Complementary to existing pipeline and strategy Leverage core capabilities in rare CNS diseases Potential for first, or best-in-class medicines that are disease modifying or that establish a new standard of care Business Development Activities Support Our Approach 9 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED

Pipeline Candidates A closer look AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
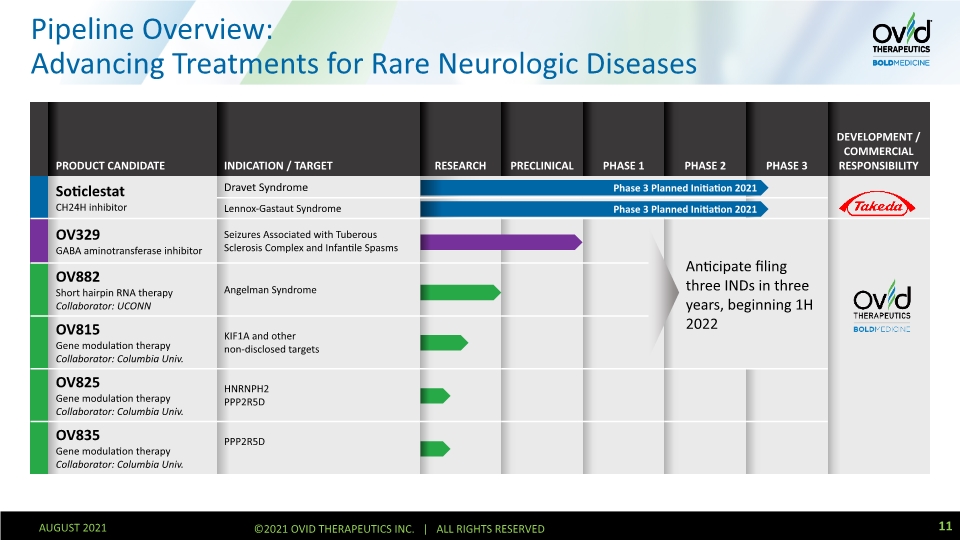
Pipeline Overview: Advancing Treatments for Rare Neurologic Diseases 11 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED Phase 3 Planned Initiation 2021 Phase 3 Planned Initiation 2021 Anticipate filing three INDs in three years, beginning 1H 2022
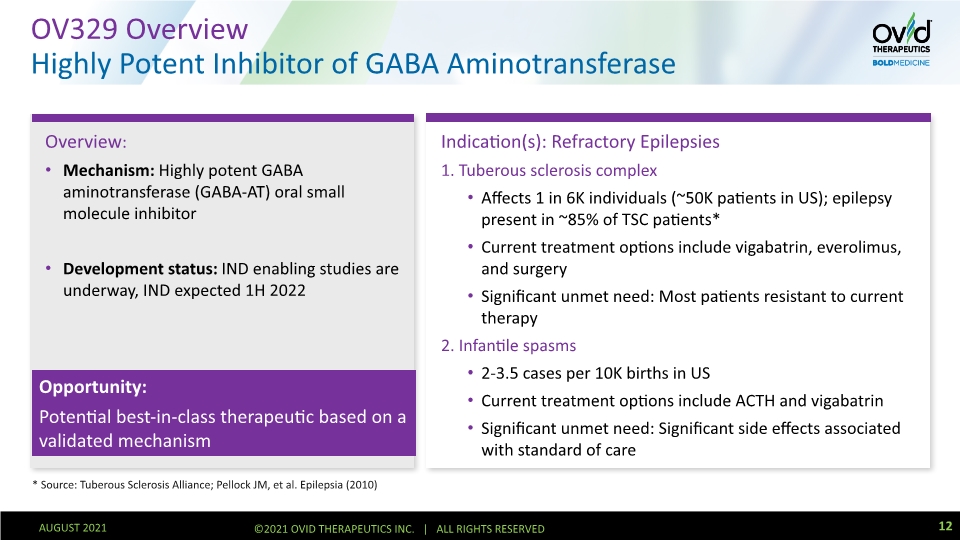
Indication(s): Refractory Epilepsies 1. Tuberous sclerosis complex Affects 1 in 6K individuals (~50K patients in US); epilepsy present in ~85% of TSC patients* Current treatment options include vigabatrin, everolimus, and surgery Significant unmet need: Most patients resistant to current therapy 2. Infantile spasms 2-3.5 cases per 10K births in US Current treatment options include ACTH and vigabatrin Significant unmet need: Significant side effects associated with standard of care Overview: Mechanism: Highly potent GABA aminotransferase (GABA-AT) oral small molecule inhibitor Development status: IND enabling studies are underway, IND expected 1H 2022 OV329 Overview Highly Potent Inhibitor of GABA Aminotransferase * Source: Tuberous Sclerosis Alliance; Pellock JM, et al. Epilepsia (2010) 12 Opportunity: Potential best-in-class therapeutic based on a validated mechanism AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
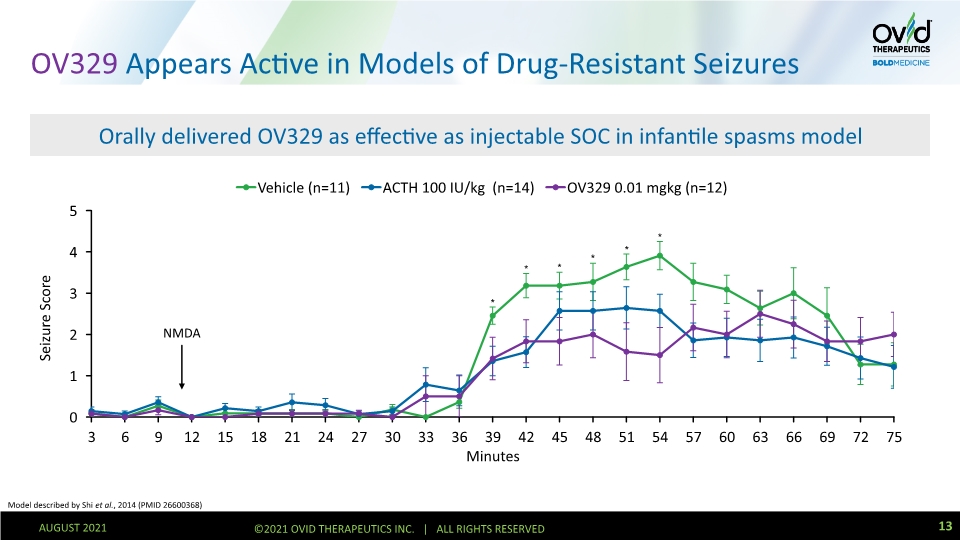
OV329 Appears Active in Models of Drug-Resistant Seizures Orally delivered OV329 as effective as injectable SOC in infantile spasms model NMDA Model described by Shi et al., 2014 (PMID 26600368) 13 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
Indication: Angelman Syndrome Affects 1 in 15K individuals Characterized by developmental delay, ataxia, sleep disorder, seizures, and speech impairments Current treatment options are symptomatic (e.g., anti-seizure) Significant unmet need: No specific treatments available which target the neuropathophysiology of Angelman syndrome ASOs** are being investigated by others; approach may have challenges Overview Mechanism: Short hairpin RNA that interacts with non-coding RNA to inhibit the silencing of paternal UBE3A gene Development status: POC* activity confirmed in vitro; currently undergoing pre-clinical validation Collaboration with Connecticut Autism Language Lab under Associate Professor Stormy Chamberlain OV882 Overview Potential Disease-Modifying Genetic Therapy for Angelman Syndrome Source: Foundation for Angelman Syndrome Therapeutics (FAST) * Proof of Concept (POC), ** Antisense oligonucleotides 14 Opportunity: Potential disease modifying treatment Targets the mechanism of silencing without affecting the gene, minimizing off-target effects, and potentially increases treatment duration compared to ASOs AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
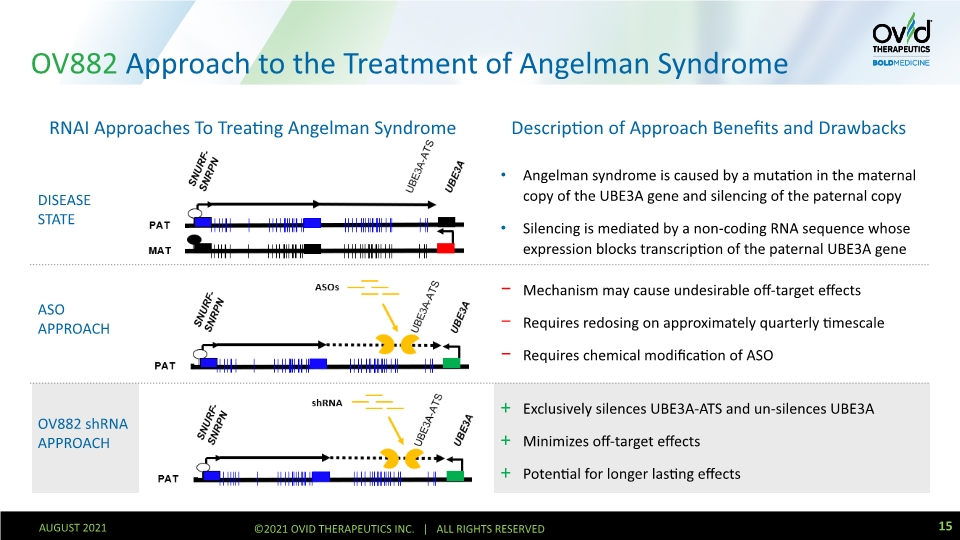
OV882 Approach to the Treatment of Angelman Syndrome DISEASE STATE ASO APPROACH OV882 shRNA APPROACH Mechanism may cause undesirable off-target effects Requires redosing on approximately quarterly timescale Requires chemical modification of ASO Angelman syndrome is caused by a mutation in the maternal copy of the UBE3A gene and silencing of the paternal copy Silencing is mediated by a non-coding RNA sequence whose expression blocks transcription of the paternal UBE3A gene Exclusively silences UBE3A-ATS and un-silences UBE3A Minimizes off-target effects Potential for longer lasting effects Description of Approach Benefits and Drawbacks RNAI Approaches To Treating Angelman Syndrome 15 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
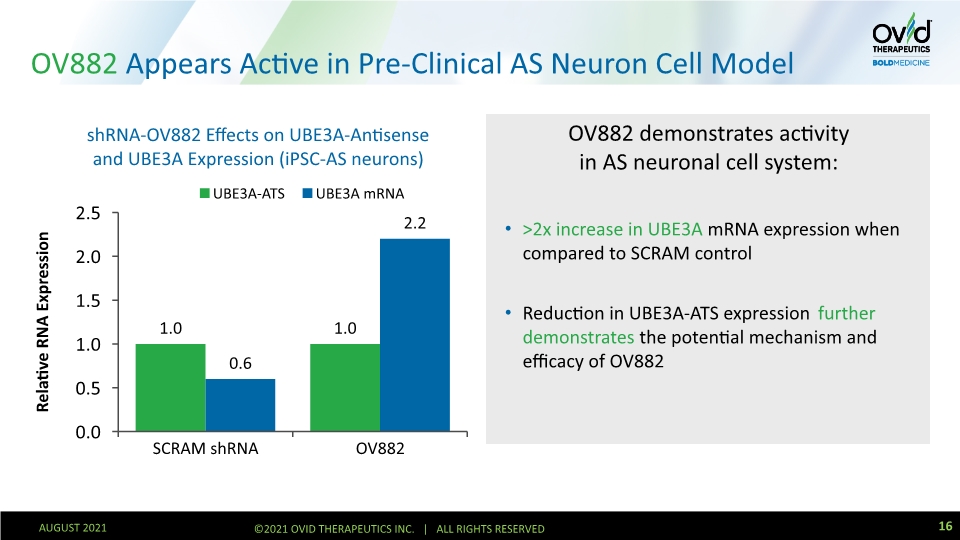
OV882 Appears Active in Pre-Clinical AS Neuron Cell Model OV882 demonstrates activity in AS neuronal cell system: >2x increase in UBE3A mRNA expression when compared to SCRAM control Reduction in UBE3A-ATS expression further demonstrates the potential mechanism and efficacy of OV882 16 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
Indication: KAND* ~200** patients worldwide with documented diagnoses; total number of affected patients likely in the thousands Broader kinesin superfamily opportunity Symptoms associated with KAND include hereditary spastic paraplegia, ataxia, epilepsy, hypotonia, autism, and ADHD Current treatment options are symptomatic Significant unmet need: No specific treatments available Overview Mechanism: Genetic / molecular approach targeting KIF1A Development status: Currently in screening stage for aptamer and gene silencing technologies In collaboration with OV815 Overview Potential Advanced Genetic Therapy for KAND and KIF-associated Diseases Notes: * KIF1A-Associated Neurological Disorder Source: **KIF1A.org 17 Opportunity: Leverage knowledge gained from KIF1A to access the broader kinesin superfamily associated diseases AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
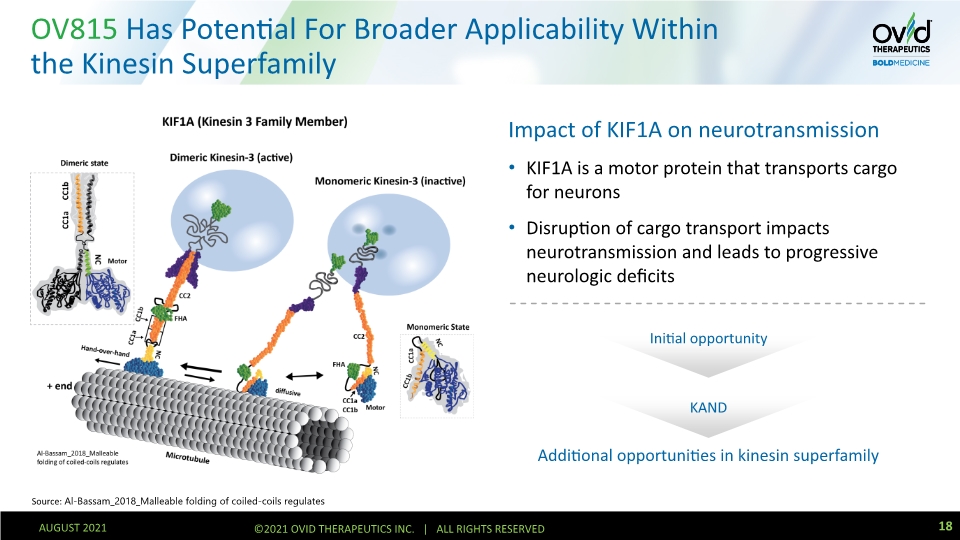
OV815 Has Potential For Broader Applicability Within the Kinesin Superfamily Impact of KIF1A on neurotransmission KIF1A is a motor protein that transports cargo for neurons Disruption of cargo transport impacts neurotransmission and leads to progressive neurologic deficits Additional opportunities in kinesin superfamily Source: Al-Bassam_2018_Malleable folding of coiled-coils regulates KAND Initial opportunity 18 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
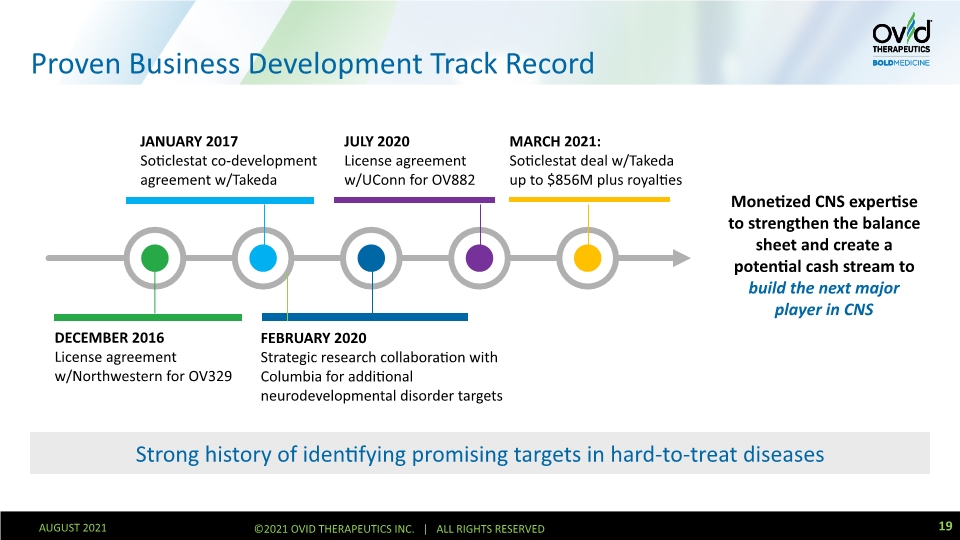
Strong history of identifying promising targets in hard-to-treat diseases Proven Business Development Track Record Monetized CNS expertise to strengthen the balance sheet and create a potential cash stream to build the next major player in CNS DECEMBER 2016 License agreement w/Northwestern for OV329 January 2017 Soticlestat co-development agreement w/Takeda FEBRUARY 2020 Strategic research collaboration with Columbia for additional neurodevelopmental disorder targets JULY 2020 License agreement w/UConn for OV882 March 2021: Soticlestat deal w/Takeda up to $856M plus royalties 19 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
The Ingredients to Be a Major Player in CNS 20 Modality & delivery mechanisms to cross the BBB Enabling technologies & screening tools increase efficiency Effective prosecution of R&D; coupled with pipeline acquisitions THE OVID APPROACH Apply insights from small molecule neurologic therapeutics to expand to multiple modalities that treat challenging, unaddressed diseases of the brain >$200M to Invest Deep CNS Expertise Proven BD Track Record Strong academic relationships for discovery Further Potential Soticlestat Monetization ACCELERATED DEVELOPMENT AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED

Thank you August 2021 AUGUST 2021 ©2021 OVID THERAPEUTICS INC. | ALL RIGHTS RESERVED
