Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Ovid Therapeutics Inc. | ovid-ex321_7.htm |
| EX-31.2 - EX-31.2 - Ovid Therapeutics Inc. | ovid-ex312_6.htm |
| EX-31.1 - EX-31.1 - Ovid Therapeutics Inc. | ovid-ex311_8.htm |
| EX-23.1 - EX-23.1 - Ovid Therapeutics Inc. | ovid-ex231_9.htm |
| EX-10.19 - EX-10.19 - Ovid Therapeutics Inc. | ovid-ex1019_672.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2017
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from to
Commission File Number: 001-38085
Ovid Therapeutics Inc.
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
|
|
Delaware |
|
2834 |
|
46-5270895 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(Primary Standard Industrial Classification Code Number) |
|
(I.R.S. Employer Identification Number) |
1460 Broadway, Suite 15044
New York, New York 10036
(646) 661-7661
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.001 per share |
|
Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to the filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large Accelerated Filer |
|
☐ |
|
Accelerated Filer |
|
☐ |
|
Non-accelerated Filer |
|
☒ (Do not check if a smaller reporting company) |
|
Smaller Reporting Company |
|
☐ |
|
Emerging growth company |
|
☒ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act.) Yes ☐ No ☒
As of June 30, 2017, the last day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the Common Stock held by non-affiliates of the registrant was approximately $155.6 million based on the closing price of the registrant’s common stock on June 30, 2017. The calculation excludes shares of the registrant’s common stock held by current executive officers, directors and stockholders that the registrant has concluded are affiliates of the registrant. This determination of affiliate status is not a determination for other purposes.
As of March 22, 2018, there were 24,617,979 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2018 Annual Meeting of Stockholders, which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission not later than 120 days after the registrant’s fiscal year ended December 31, 2017, are incorporated by reference into Part III of this Annual Report on Form 10-K.
|
|
|
|
|
Page |
|
|
ii |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 1. |
|
|
1 |
|
|
Item 1A. |
|
|
32 |
|
|
Item 1B. |
|
|
60 |
|
|
Item 2. |
|
|
60 |
|
|
Item 3. |
|
|
60 |
|
|
Item 4. |
|
|
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 5. |
|
|
61 |
|
|
Item 6. |
|
|
62 |
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
63 |
|
Item 7A. |
|
|
71 |
|
|
Item 8. |
|
|
71 |
|
|
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
71 |
|
Item 9A. |
|
|
71 |
|
|
Item 9B. |
|
|
71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 10. |
|
|
72 |
|
|
Item 11. |
|
|
72 |
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
72 |
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
72 |
|
Item 14. |
|
|
72 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 15. |
|
|
73 |
|
|
Item 16. |
|
|
74 |
|
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are “forward-looking statements” for purposes of this Annual Report on Form 10-K. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “could,” “estimate,” “expects,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative or plural of those terms, and similar expressions.
Forward-looking statements include, but are not limited to, statements about:
|
|
• |
the initiation, timing, progress and results of our current and future preclinical studies and clinical trials and our research and development programs; |
|
|
• |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
|
• |
our ability to identify additional novel compounds with significant commercial potential to acquire or in-license; |
|
|
• |
our ability to successfully acquire or in-license additional drug candidates on reasonable terms; |
|
|
• |
our ability to obtain regulatory approval of our current and future drug candidates; |
|
|
• |
our expectations regarding the potential market size and the rate and degree of market acceptance of such drug candidates; |
|
|
• |
our ability to fund our working capital requirements; |
|
|
• |
the implementation of our business model and strategic plans for our business and drug candidates; |
|
|
• |
developments or disputes concerning our intellectual property or other proprietary rights; |
|
|
• |
our ability to maintain and establish collaborations or obtain additional funding; |
|
|
• |
our expectations regarding government and third-party payor coverage and reimbursement; |
|
|
• |
our ability to compete in the markets we serve; |
|
|
• |
the impact of government laws and regulations; |
|
|
• |
developments relating to our competitors and our industry; and |
|
|
• |
the factors that may impact our financial results. |
Factors that may cause actual results to differ materially from current expectations include, among other things, those set forth in Part I, Item 1A, “Risk Factors,” herein and for the reasons described elsewhere in this Annual Report on Form 10-K. Any forward-looking statement in this Annual Report on Form 10-K reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our operations, results of operations, industry and future growth. Given these uncertainties, you should not rely on these forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Annual Report on Form 10-K also contains estimates, projections and other information concerning our industry, our business and the markets for certain drugs and consumer products, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources and we have not independently verified the data from third party sources. In some cases, we do not expressly refer to the sources from which these data are derived.
In this Annual Report on Form 10-K, unless otherwise stated or as the context otherwise requires, references to “Ovid,” “the Company,” “we,” “us,” “our” and similar references refer to Ovid Therapeutics Inc. and its wholly owned subsidiaries. This Annual Report on Form 10-K also contains references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
ii
Overview
We are a biopharmaceutical company focused exclusively on developing impactful medicines for patients and families living with rare neurological disorders. We believe these disorders represent an attractive area for drug development as the understanding of the underlying biology has grown meaningfully over the last few years; yet has remained underappreciated by the industry. Our experienced team began with a vision to integrate the biology and symptomology of rare neurological conditions to employ innovative research and clinical strategies for the development of our drug candidates. Based on recent scientific advances in genetics and the biological pathways of the brain, we created a proprietary map of disease-relevant pathways and used it to identify and acquire novel compounds for the treatment of rare neurological disorders. We are executing on our strategy by in-licensing and collaborating with leading biopharmaceutical companies and academic institutions. We are developing a robust pipeline of clinical assets with an initial focus on neurodevelopmental disorders and rare epileptic encephalopathies. OV101, has commenced and completed randomization of a Phase 2 trial, which is primarily a safety trial that is designed to provide proof-of-concept on efficacy parameters, in adults and adolescents with Angelman syndrome. We completed a Phase 1 trial in adolescents with Angelman syndrome or Fragile X syndrome in which, OV101 was found to be generally well tolerated and its pharmacokinetic, or PK profile in adolescents was similar to previous data generated in young adults. Along with our collaborator, Takeda Pharmaceutical Company Limited (“Takeda”), in June 2017 we initiated patient recruitment in our Phase 1b/2a trial of OV935 in adults with rare epileptic encephalopathies. We expect data from both programs in 2018.
Our most advanced drug candidate is OV101, which we acquired from H. Lundbeck A/S, or Lundbeck, in 2015. We received orphan drug designation for OV101 for the treatment of Angelman syndrome from the U.S. Food and Drug Administration, or FDA, in September 2016. In October 2017 the FDA granted orphan drug designation for OV101 for the treatment of Fragile X syndrome, in December 2017 the FDA granted fast track designation for the treatment of Angelman syndrome and in March 2018 the FDA granted fast track designation for the treatment of Fragile X syndrome. We believe our development plan for OV101 highlights our ability to translate new scientific insights into drug candidates that target an unexplored disease-relevant pathway. OV101 targets disorders characterized by diminished tonic inhibition, a neurological signaling abnormality that has been identified as a potential central cause of the symptoms seen in a number of disorders of the brain. This can lead to certain symptoms including, but not limited to motor deficiencies, sleep abnormalities, behavioral manifestations and seizures. We believe modulating tonic inhibition may have a meaningful clinical impact in patients with Angelman syndrome and Fragile X syndrome.
In January 2017, we entered into a collaboration with Takeda for its compound TAK-935, which we refer to as OV935. The collaboration enables us to share equally in building upon Takeda’s discoveries, bringing together the capabilities of both companies in development, regulatory and commercialization activities. We believe that OV935’s inhibition of the cholesterol metabolism pathway may down regulate the excitatory signals involved in epilepsy, which may suppress seizures and also lead to a long-term disease-modifying effect. We are initially targeting OV935 for rare epileptic encephalopathies with high unmet medical need, including Dravet syndrome, Lennox-Gastaut syndrome and Tuberous Sclerosis Complex. OV935 has completed four Phase 1 trials in certain epileptic encephalopathies, preliminarily demonstrating favorable tolerability at doses that we believe may be therapeutically relevant. In December 2017, the FDA granted orphan drug designation for OV935 for the treatment of Dravet syndrome and Lennox-Gastaut syndrome.
Our management team is a critical component to the execution of our overall strategy and our business model. We have assembled a team with significant experience in translational science, drug evaluation, clinical development, regulatory affairs and business development. We believe these capabilities will drive our ability to identify, acquire, develop and commercialize novel compounds that have the potential to modify the devastating course of rare neurological disorders. We believe our expertise will make us the partner of choice for leading biopharmaceutical companies or academic institutions that wish to maximize the value of their neurology drug candidates. The members of our team have been collectively involved in the development and approval of over 20 marketed drugs. Further, we believe that we are particularly well positioned to execute on our business development strategy given the extensive network and breadth of expertise of our Chairman and Chief Executive Officer Dr. Jeremy Levin and the other members of our management team. Our management team is supported by our board of directors, which has extensive experience in the biopharmaceutical industry.
1
Our Focus: Rare Neurological Disorders
Rare neurological disorders are among the most devastating in their impact on patients and their families. Patients suffering from these disorders typically require full-time care, and yet are among the most underserved. We believe that there are at least 100 neurodevelopmental disorders, epileptic encephalopathies and other related rare neurological disorders that we may be able to target. These disorders are characterized by impairments and pathologies in the growth and development of the brain. Due to a historical overwhelming preference in the drug industry to develop drugs for broader neurological indications, many of these disorders have no approved therapies. As a result, recent scientific advancements have been overlooked, which we believe presents us with an opportunity to pursue these indications. These reasons include:
|
|
• |
High penetrance linking genetic defect to disorder pathology. Rare neurological disorders that are genetic in origin typically have a strong correlation, or penetrance, between the presence of a gene and the manifestation of the corresponding disease pathology. As a result, we believe we can develop drug candidates that will be efficacious in patients with a given genetic profile. |
|
|
• |
Predictive genetic and other models. Recent advances in genetics enable us to employ predictive in vivo genetic models of certain of these disorders. These models allow us to evaluate and observe a drug candidate’s potential activity prior to initiation of clinical trials. Through these models, we believe we will be able to select the most relevant clinical endpoints for our trials and increase the potential for clinical success. |
|
|
• |
Overlapping pathophysiology and symptoms. Neurological disorders are often characterized by a number of overlapping symptoms, such as seizures, sleep disturbances, movement deficiencies and behavioral manifestations. We believe these commonalities will enable us to employ clinical endpoints that may be translatable from one disorder to another, and to develop drugs that may provide a clinical benefit across multiple indications. |
|
|
• |
Early observation of proof-of-concept. By employing clinical endpoints that are highly relevant and are designed to detect meaningful clinical benefits, we anticipate that many of our studies may provide early proof-of-concept in clinical development. |
|
|
• |
Potential ability to affect disease progression. We are focusing on disorders that are typically diagnosed in early childhood when the brain is still developing. We believe that we may be able to meaningfully address symptoms and potentially alter the progression of disease, especially if the drug can be administered early in life. |
|
|
• |
Motivated and accessible patient populations. We are targeting our programs for disorders with motivated and accessible patient populations. We believe that the patients and caregivers affected by these disorders are avid users of social media, in order to learn about and share relevant information and experiences. We use digital platforms to efficiently identify new patients for our clinical trials, raise disease awareness and help connect the patient and caregiver communities. |
The Ovid Approach
The Ovid approach to drug development for rare neurological disorders is scientifically driven, patient focused and business development oriented.
Scientifically Driven
We are taking a scientifically driven approach to identifying promising drug candidates for our pipeline. We are building our portfolio based on the existence of clear biological rationales, including a focus on disorders that have, where possible, a direct genetic linkage. We used our proprietary map to identify initial drug candidates across all stages of development for potential acquisition or in-licensing. As we advance our drug candidates into the clinic, we are building on the emerging body of scientific and clinical insights developed by us and others in the biopharmaceutical industry to target these new disease pathways of the brain. As we evaluate data from previous and ongoing preclinical studies and clinical trials, we intend to refine our scientific approach and apply these insights to continue to build our pipeline.
In particular, the Ovid approach is driven by the following scientific principles:
|
|
• |
map biological pathways that are relevant for rare neurological disorders with significant unmet need; |
|
|
• |
target biological pathways for which proof-of-concept has been established via in vitro or animal models; |
|
|
• |
focus on biological pathways that cause the pathology of the disorder and that have common symptoms that we can target; and |
|
|
• |
identify and utilize biomarkers that can provide evidence of the activity of our drug candidates. |
2
We are highly focused on the patient communities affected by the rare neurological disorders we are addressing. We believe this aspect of our approach is critical, given that these disorders affect a small population of patients, but carry serious morbidities and require extensive involvement from the patients’ families, caregivers, physicians and patient advocacy groups.
The Ovid approach is driven by the following patient-focused principles:
|
|
• |
develop close relationships with patients, caregivers, families, disease foundations and key opinion leaders, to better understand the history of these disorders, raise awareness, identify patients and facilitate enrollment of clinical trials; |
|
|
• |
identify clinically meaningful endpoints based on input from patients and their physicians and caregivers; and |
|
|
• |
develop digital capabilities to engage, foster and maintain close relationships with patient communities. |
Business Development Oriented
We are building a broad pipeline of potential drug candidates to treat rare neurological disorders through the in-licensing or partnering of drug candidates. Central to the success of this process is a highly focused and disciplined business development effort aimed at securing relevant assets in each of our selected rare neurological disorders.
We are developing a specialized, scalable and robust infrastructure that we believe will make us a leader in rare neurological disorders and the partner of choice for leading biopharmaceutical companies or academic institutions that wish to maximize the value of their neurology drug candidates in these areas. This infrastructure spans from research, translational science, clinical development and regulatory affairs to business development, market access and relationships with patient advocacy groups. If and when our drug candidates are approved, we also plan to establish a highly focused commercial and distribution network dedicated to rare neurological disorders in the United States and Europe, where we believe the patient populations and medical specialists are sufficiently concentrated to effectively market our drug candidates.
We believe that we are particularly well positioned to execute on our business development strategy because of the extensive network of our Chairman and Chief Executive Officer Dr. Jeremy Levin and the other members of our management team, who collectively have a track record of success in orphan drug development and evaluation.
Our Pipeline
The following table sets forth the status and mechanism of action of our drug candidates:

|
* |
Also known as TAK-935 under a co-development program with Takeda Pharmaceutical Company Limited pursuant to a license and collaboration agreement. |
3
We are developing OV101 for the treatment of Angelman syndrome and Fragile X syndrome, two neurodevelopmental disorders that are characterized by similar symptoms due to decreased tonic inhibition, an important mechanism whereby it is believed that the brain distinguishes signal from noise. Angelman syndrome and Fragile X syndrome have overlapping symptoms, including sleep disorder, aberrant behavior, anxiety and cognitive or intellectual disabilities. Both of these disorders are typically diagnosable in early childhood and require full-time care for the patients affected. Although the FDA has not yet made any determination regarding the safety and efficacy of OV101, in previously conducted clinical trials in primary insomnia enrolling over 4,000 adults, OV101 was observed to have favorable safety and oral bioavailability profiles. Success in these previous trials does not ensure that our clinical trials of OV101 will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of OV101. We commenced and completed randomization in our Phase 2 trial of OV101, which is primarily a safety trial that is designed to provide proof-of-concept on efficacy parameters in adults and adolescents with Angelman syndrome. We completed a Phase 1 trial in adolescents with Angelman syndrome or Fragile X syndrome, in which OV101 was found to be generally well tolerated and its PK profile in adolescents was similar to previous data generated in young adults. In September 2016, the FDA granted orphan drug designation for OV101 for the treatment of Angelman syndrome, in October 2017 the FDA granted orphan drug designation for OV101 for the treatment of Fragile X syndrome, and in December 2017 the FDA granted fast track designation for the treatment of Angelman syndrome and in March 2018, the FDA granted fast track designation for the treatment of Fragile X syndrome. We plan to initiate a Phase 2 clinical trial investigating OV101 for the treatment of adolescents and young adults with Fragile X syndrome in 2018. The Phase 2 multi-dose, three-arm clinical trial is expected to enroll up to 30 male patients aged 13 to 22 diagnosed with Fragile X syndrome. The primary endpoint of the study will be the safety and tolerability of OV101. Secondary endpoints include an evaluation of changes in behavior during 12 weeks of treatment with OV101.
OV101 and Tonic Inhibition
Tonic inhibition is a critical regulatory mechanism that allows a healthy human brain to decipher excitatory and inhibitory neurological signals correctly without being overloaded. Defects of this system are thought to play a role in multiple disease states, including Angelman syndrome and Fragile X syndrome. OV101 represents a promising compound targeting this mechanism.
Decreased tonic inhibition results in an imbalance in the ratio of excitation to inhibition. If tonic inhibition is reduced, the brain becomes inundated with signals and loses the ability to separate background noise from critical information. This imbalance disrupts normal brain functioning, including sensory processing and integration. This can lead to symptoms characteristic of neurodevelopmental disorders, including those related to behavior, learning, cognitive development, motor function, sleep disturbances and seizures. By modulating tonic inhibition, OV101 may have the potential to alleviate important symptoms and provide a meaningful clinical benefit to patients across several neurodevelopmental disorders including Angelman syndrome and Fragile X syndrome.
4
Tonic Inhibition and Neurodevelopmental Disorders
Neurotransmission
The brain is composed of a vast network of interconnected neurons that facilitate the communication between cells. These communications are governed by the release of chemical signals, or neurotransmitters, from one neuron to another. The neuron that releases the neurotransmitter is called the presynaptic neuron. The neuron that receives the neurotransmitter is called the postsynaptic neuron. The presynaptic neuron releases a neurotransmitter into a physical gap separating the two neurons, which is called the synaptic gap. The neurotransmitter then diffuses across the synaptic gap to bind to a receptor on the postsynaptic neuron. This binding then triggers a signal to stimulate, inhibit or otherwise modulate the activity of the postsynaptic neuron. For example, gamma-aminobutyric acid, or GABA, is an inhibitory neurotransmitter that plays a role in anxiety, sleep, seizure, motor functions and certain other brain functions. The neurotransmitter GABA binds to receptors in the synaptic gap, called synaptic receptors, as well as receptors outside the synaptic gap, called extrasynaptic GABA receptors. The following figure depicts the synaptic gap and extrasynaptic GABA receptors outside the synaptic gap:

Figure 1: The neurotransmitter GABA binds to synaptic receptors in the synaptic gap and extrasynaptic GABA
receptors outside the synaptic gap.
5
The Role of GABA in Phasic and Tonic Inhibition
There is a fine balance between an appropriate and an inappropriate response of the postsynaptic neuron to an incoming stimulus. Neurons are typically responsive to incoming stimulus at two levels through processes known as phasic and tonic inhibition.
Phasic Inhibition. Phasic inhibition is short-acting in nature and takes place in the synaptic gap. The brain processes signals passed between neurons in milliseconds. To keep the system in balance, the body sets up a feedback loop so that signaling is interpreted appropriately. When the body determines that it should cease responding to a specific signal, it triggers this feedback loop, which inhibits the receiving neuron from responding too strongly. This is done when the neurotransmitter GABA binds to receptors in the synaptic gap, or synaptic GABAA receptors. Once the receiving postsynaptic neuron has been inhibited, balance can be restored and the receiving neuron can prepare to receive a new stimulus.
Tonic Inhibition. Tonic inhibition is longer-acting in nature and takes place in the extrasynaptic region. Extrasynaptic GABAA receptors contain specific subunits that make them different from synaptic GABA A receptors in structure and activity. In particular, many extrasynaptic GABA A receptors contain a specific domain called the δ (delta) subunit, which is not expressed in synaptic GABA A receptors. These extrasynaptic GABA A receptors that contain the δ subunit receptors act with slower kinetics, hence the longer-acting inhibitory response of tonic inhibition. The following figure depicts the location and domains of the extrasynaptic GABA A receptor:

Figure 2: Structure and localization of the δ (delta) subunit contained in the extrasynaptic GABAA receptor.
6
The following figure depicts physiologic levels of phasic inhibition and tonic inhibition, the two levels of signaling between neurons:

Figure 3: Phasic inhibition is rapid and short-lived. Tonic inhibition is more persistent.
The same neurotransmitter, GABA, which stimulates the short-acting receptors in the synapse in the process of phasic inhibition, also stimulates the longer-acting receptors outside the synapse in the process of tonic inhibition.
Under normal conditions there is sufficient GABA present in and around the synaptic region and both sets of receptors are stimulated. However, in certain neurodevelopmental disorders, the overall levels of GABA are reduced. This reduction can lead to a situation in which there is enough GABA in the synapse to maintain normal short-term signaling, but GABA outside the synapse is insufficient to occupy the extrasynaptic receptors and maintain longer-term signaling. The decline in tonic inhibition triggered by the shortage of extrasynaptic GABA leads to the chronic activation of the receiving postsynaptic neurons and disruption of normal brain network activity.
Tonic inhibition is a key mechanism of neural regulation that has not yet, to our knowledge, been specifically addressed by any approved drug. Tonic inhibition has been shown to be important in helping to discriminate important signals from the “noise” generated by the multitude of sensory signals entering the brain. In patients with decreased tonic inhibition, the flood of incoming signals overwhelms the ability of the brain to process them.
The clinical manifestations of decreased tonic inhibition are seen across several neurodevelopmental disorders, including Angelman syndrome and Fragile X syndrome. When tonic inhibition is decreased, the body has trouble functioning normally in the presence of this chronic overstimulation of neurons. The result is profound pathology that is manifested by seizures, anxiety and disturbances in motor function, behavior, sleep, cognition, learning, memory and ability to communicate effectively.
In addition, learning and memory are closely linked to the ability of the brain to establish and maintain connections among nerve cells. Many brain processes, including tonic inhibition, play a role in the creation and maintenance of these connections. One of the long-term consequences of decreased tonic inhibition is the disruption of memory. The restoration of tonic inhibition has been observed to lead to improvements in memory in adult animal models.
OV101 and Tonic Inhibition in Angelman Syndrome and Fragile X Syndrome
OV101 is a delta selective extrasynaptic GABAA receptor agonist. An agonist is a chemical that binds to a receptor and activates the receptor to produce a biological response. OV101 specifically exerts its biological activity through the δ subunit of extrasynaptic GABAA receptors. Based on the biological pathway and existing preclinical data, we anticipate developing OV101 as an orally active selective extrasynaptic GABA A agonist to compensate for the deficit in GABA concentrations observed in patients with certain rare neurological disorders. We believe OV101 is the only drug candidate in development that exerts its biological activity preferentially through the δ subunit of extrasynaptic receptors. We are initially developing OV101 for Angelman syndrome and Fragile X syndrome, and we believe it has the potential to address multiple neurodevelopmental disorders characterized by decreased tonic inhibition.
7
The following figure depicts how OV101 addresses tonic inhibition by restoring activity of the extrasynaptic GABAA receptors:

Figure 4. How OV101 is designed to address tonic inhibition by restoring activity of the extrasynaptic GABAA receptors. The figures represent the synapse between a GABAergic neuron and a postsynaptic cell. The top panel shows normal release and uptake of GABA with activation of both synaptic and extrasynaptic receptors resulting in normal phasic and tonic inhibition. In the middle panel, an increase in GABA uptake (Angelman syndrome) or a reduction in GABA release (Fragile X syndrome) leads to a reduction in extracellular concentrations of GABA, preferentially impacting extrasynaptic GABA receptor signaling and resulting in reduced tonic inhibition. In the bottom panel, OV101 acts selectively on the extrasynaptic GABA A receptors restoring tonic inhibition.
8
Overview. Angelman syndrome is a rare genetic disorder that is typically diagnosed in the United States after one year of age when parents notice severe developmental delays or the child suffers seizures. Characteristic features of this disorder include delayed development, intellectual disability, severe speech impairment, problems with movement and balance, seizures, sleep disorders and anxiety. Individual patients with Angelman syndrome can have varied symptoms, including the inability to walk or control motor movement, which can limit their ability to handle daily functions such as feeding, dressing or bathing. These patients are also often hyperactive, leading to various behavioral problems. Angelman syndrome symptoms, such as poor sleeping patterns, can lead to serious consequences, including increased frequency of seizures and exacerbation of behavioral manifestations. Most Angelman syndrome patients require full-time care, which can represent a substantial emotional and financial burden on their families. In addition, Angelman syndrome has been associated with poor parental sleep and high parental stress.
According to the National Organization for Rare Disorders, the approximate prevalence of Angelman syndrome is between 1 in 12,000 and 1 in 20,000 people. There are no FDA-approved therapies for the treatment of Angelman syndrome. The current standard of care for seizures associated with Angelman syndrome is traditional anticonvulsants, which are not designed to trigger tonic inhibition. There is no widely accepted standard of care for other symptoms of Angelman syndrome, including sleep disruptions, motor dysfunction and behavioral abnormalities.
Tonic Inhibition and Angelman Syndrome. In 1997, scientists traced the genetic causes of Angelman syndrome to mutations and other disruptions in the UBE3A gene. The UBE3A gene encodes the UBE3A protein, which plays a central role in protein degradation. Protein degradation is the breakdown of damaged or unnecessary proteins within the cell, which is an important aspect of maintaining normal cellular function. The UBE3A protein triggers the attachment of a protein called ubiquitin to other cellular proteins. These ubiquitin attachments serve as tags that mark the tagged cellular proteins for degradation. Alterations in the UBE3A gene, and therefore the UBE3A protein, result in deficiencies in the tagging of proteins for degradation, leading to inappropriate protein accumulation within the cell.
One of the proteins that the UBE3A protein normally tags for degradation is GABAA Transporter 1, or GAT1, a protein that is responsible for the uptake of GABA by neurons. The disruption in the UBE3A gene results in an overabundance of GAT1, leading to an exaggerated uptake of GABA. This results in low levels of GABA in both the synaptic and extrasynaptic regions. The deficiency of GABA in the extrasynaptic region culminates in a decrease of tonic inhibition, triggering a chronic activation of downstream neurons. The following figure depicts the biological pathway by which the UBE3A gene alteration present in Angelman syndrome patients leads to decreased tonic inhibition:

Figure 5. Linkage between UBE3A gene alteration and tonic inhibition.
9
By adding OV101, a GABA agonist, we believe it is possible to address this decreased tonic inhibition by compensating for the low GABA levels. Once OV101 is added, the extrasynaptic receptors are activated to transport chloride, or Cl - ions, from outside the cell into the postsynaptic neuron. Negatively charged CI - ions inside the postsynaptic neuron increase tonic inhibition, reducing the excessive activation of downstream neurons.
Preclinical Data in Angelman Syndrome. In preclinical studies conducted by an independent academic group in Angelman syndrome mouse models, OV101 was observed to increase tonic inhibition and alleviate the key motor symptoms that are also observed in Angelman syndrome patients.
In 2012, researchers published a study in the journal Science Translational Medicine, reporting that they had created mice that lacked a functional copy of the UBE3A gene. In these mouse models, the researchers observed several features of Angelman syndrome, including a loss of controlled bodily movements and motor deficits. The researchers further observed that mice lacking a functional copy of the UBE3A gene had deficiencies in tonic inhibition and that increased GABA could partially restore this deficiency.
In this study, analysis of the activity of individual nerve cells in mice with a defective UBE3A gene demonstrated the effects of decreased tonic inhibition. Decreased tonic inhibition resulted in excessive neuronal activity, which caused disruption of the normal, tightly coordinated and regulated signaling within the brain. The direct addition of OV101 to these nerve cells largely restored their activity and regional brain network activity. Based on these results, we believe that OV101 may have a similar effect in increasing tonic inhibition in Angelman syndrome patients.
The utility of these Angelman syndrome mouse models is also demonstrated by the effects of OV101 on walking gait. Angelman syndrome patients often have an altered walking gait where the legs are wide-spaced and feet are turned out. The Angelman syndrome mouse models were observed to have an altered gait, involving rotation of the hind paws outward. In this study, Angelman syndrome mouse models and normal mice were each administered OV101 and a placebo. Administration of OV101 was observed to result in statistically significant reductions in hind paw rotation in the Angelman syndrome mice, but had no effect in the normal mice. The following figure depicts the decrease in hind-paw rotation in the Angelman syndrome mice administered OV101 versus those that were administered a placebo:

Figure 6: Administration of OV101 led to a reduction in the degree of hind paw rotation.
|
|
** |
A p-value of 0.05 or less represents statistical significance, meaning that there is a less than 1-in-20 likelihood that the observed results occurred by chance. A p-value of 0.01 or less means that there is a less than 1-in-100 likelihood that the observed results occurred by chance. |
|
OV101 administration in Angelman syndrome mouse models was also observed to result in improved overall motor and clasping reflex function as measured by a rotarod test and tail suspension. In the rotarod test, mice are required to perch atop a rotating cylinder and use their legs and body in a coordinated fashion in order to avoid falling off. Angelman syndrome mice that were administered OV101 were observed to have an increased time on the rotarod versus those that were administered a placebo. Administration of OV101 was not observed to have an effect in normal mice. The experiment showed that the increased amount of time shown on the rotarod after treatment was statistically significant (approximately 20%, p<0.05) relative to the time spent on the rotarod before injection. In the tail suspension test, Angelman syndrome mice showed a mild to moderate clasping reflex, represented by forelimb clasping and flexion to the body and smaller forelimb flexion. Angelman syndrome mice treated with OV101 showed improved clasping reflexes and significantly increased forelimb angles (p<0.01).
10
Overview. Fragile X syndrome is a genetic condition that results in intellectual disability, anxiety disorders, behavioral and learning challenges and various physical disabilities. Patients with Fragile X syndrome exhibit autism-like symptoms, including cognitive impairment, anxiety, mood swings, hyperactivity, attention deficit and heightened sensitivity to various stimuli, such as sound. The severity of an individual patient’s impairment can range from mild learning disabilities to more severe cognitive or intellectual disabilities. Fragile X syndrome is one of the most commonly inherited intellectual disability disorders. Children with Fragile X syndrome also often have unusual sleep patterns and may have difficulty with routine activities such as feeding and dressing. The challenges presented by Fragile X syndrome often extend beyond the patient and can lead to significant hardships on the emotional and financial health of their families.
Fragile X syndrome is caused by mutations in the fragile X mental retardation gene, or FMR1 gene. FMR1 is a gene that leads to the synthesis of the fragile X mental retardation protein, FMRP, which is needed for normal brain development. The FMR1 gene normally contains in its sequence between 5 and 44 copies of a short, repeated motif, or recurring pattern in DNA. In Fragile X syndrome, there are more than 200 copies of this motif in the FMR1 gene, a genetic change that prevents the synthesis of FMRP. Patients with intermediate numbers of repeats are able to make some FMRP and have milder symptoms.
According to the National Fragile X Foundation, Fragile X syndrome affects approximately 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. The average age of diagnosis of Fragile X syndrome is approximately three years. Currently, there are no approved therapies for the treatment of Fragile X syndrome. The current standard of care for the psychiatric challenges of Fragile X syndrome is tailored to each patient and may include antipsychotics, antidepressants and drugs to treat attention deficit and sleep disorders. Special education and symptomatic treatments for anxiety and irritability are often employed to lessen the burden of illness. Fragile X syndrome patients also may experience seizures, which are treated with traditional anticonvulsants.
Tonic Inhibition and Fragile X Syndrome. Due to mutations in the FMR1 gene, patients with Fragile X syndrome have deficiencies in the levels of FMRP, an RNA binding protein that regulates the synthesis of proteins such as the two forms of glutamic acid decarboxylase, or GAD65 and GAD67, which we refer to together as GAD65/67. GAD65/67 are the key enzymes required for synthesis of GABA. Knocking out the FMR1 gene results in reduced expression of GAD65/67 in mouse models. The following figure depicts the reduced expression of GAD65/67 in mice containing a knockout of the FMR1 gene:

Figure 7: Knockout of the FMR1 gene leads to reduced expression of
GAD65/67 in mice.
11
The reduced expression of GAD65/67 in this model results in decreased GABA production and subsequently lower extrasynaptic levels of GABA and decreased tonic inhibition required for normal tonic inhibition. Decreased tonic inhibition is believed to be responsible for a number of Fragile X syndrome-related symptoms, such as behavioral and cognitive problems. The following figure depicts the biological pathway by which an alteration in the FMR1 gene leads to decreased tonic inhibition:

Figure 8: Linkage between FMR1 gene alteration and tonic inhibition.
Preclinical Data in Fragile X Syndrome. The association between the FMR1 gene and physiological changes observed in Fragile X syndrome patients has been evaluated in preclinical studies conducted by an independent academic group. Mice containing a knockout of the FMR1 gene exhibit behaviors similar to those observed in Fragile X syndrome patients, such as hyperactivity, anxiety and increased sensitivity to sounds.
In these studies, hyperactivity in FMR1-deficient mice was assessed by the distance traveled and average speed in an open field test. FMR1 -deficient mice were significantly more active than normal mice. It was observed that treatment of these mice with OV101 led to a statistically significant decrease in the distance traveled and average speed. These results were considered to be indicative of a reduction in hyperactivity, which we believe resulted from increased tonic inhibition. The following figure depicts the normalization of hyperactivity in mice containing a knockout of the FMR1 gene when administered OV101:
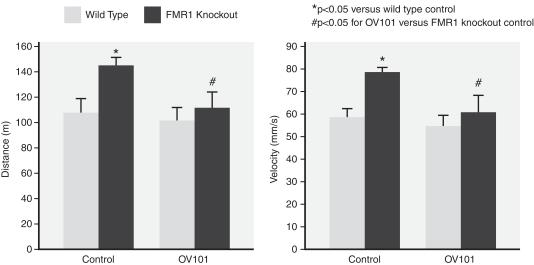
Figure 9: OV101 was observed to reduce signs of hyperactivity in mice containing a knockout of the FMR1 gene.
12
In these studies, OV101 partially normalized the mice’s response to startling sounds in the pre-pulse inhibition test in which a pre-stimulus is given to suppress the startle response and improve the signal to noise ratio. Additionally, mice with a knockout of the FMR1 gene were also observed to have higher neuronal activity as compared to normal mice and a lower threshold for action potential, or AP, generation leading to increased AP bursts, which reverted to normal levels with the administration of OV101, as shown in the following figure:

Figure 10. OV101 was observed to reduce levels of neuronal activity in mice with a knockout of
the FMR1 gene.
OV101 and its Potential Impact on Neurogenesis and on Learning and Memory
Based on preclinical data, we believe OV101 may facilitate neurogenesis, or the creation and maturation of new neurons, which may lead to cognitive benefits. In a preclinical study in mice conducted by an independent academic group, OV101 was observed to promote the performance of certain memory behaviors and facilitate neurogenesis.
Extrasynaptic GABAA receptors contain a unique δ subunit, which is a specific domain on extrasynaptic GABAA receptors that is not expressed in synaptic GABA A receptors. OV101 mediates its pharmacological properties via the activity of this δ subunit and we believe that is a key differentiating aspect of our drug. It is believed that GABA A receptor-dependent signaling regulates memory and also facilitates the growth of neurons after birth in the brain.
Long-term administration of OV101 in normal mice, but not in mice deficient in the GABAA receptor delta subunit, was observed to result in significant increases in neurogenesis. These treated mice also performed better on challenges that were dependent on long-term memory. We believe that these results in mice suggest that long-term treatment with OV101 in patients with Angelman syndrome and Fragile X syndrome may lead to improvement in cognitive abilities.
Previous Clinical Development of OV101
We acquired worldwide rights to OV101 from Lundbeck in March 2015. Prior to the acquisition, Lundbeck filed an investigational new drug application, or IND, with the FDA for the treatment of insomnia. Pursuant to this IND, Lundbeck and Merck & Co., Inc., or Merck, partnered to conduct several Phase 3 trials for primary insomnia between 2004 and 2007. Over the course of the development of OV101, over 4,000 adults were administered OV101, resulting in an OV101 exposure of approximately 950 patient years. These trials were primarily randomized, placebo-controlled short-term and long-term safety and efficacy clinical studies, using classic sleep parameters such as total sleep time, time to sleep onset, wakefulness after sleep onset, and number of nocturnal awakenings as clinical endpoints.
The Phase 3 program consisted of three trials: two 3-month placebo controlled trials conducted in the United States and one 2-week trial conducted in Europe and Canada, each evaluating OV101 against placebo and the active comparator, zolpidem (Ambien). The primary endpoints of the trials included total sleep time and time-to-sleep onset and the secondary endpoints included number of nocturnal awakenings, wakefulness after sleep onset and daytime function. In Phase 3 trials, which were conducted for durations of up to 12 months, OV101 was observed to have efficacy that was largely comparable to zolpidem (Ambien) on several sleep metrics. In the first 3-month trial, a dose of 15mg of OV101 met both of the primary endpoints as well as the secondary endpoints at week one and month three compared to placebo. In the second 3-month trial, the same dose met only total sleep time and number of awakenings at week one, but significance was lost after adjusting for multiplicity at month three. The 2-week trial met all primary endpoints and wakefulness after sleep onset, for 15mg of OV101 at weeks one and two. Additionally, subjects who were administered OV101 showed no evidence of withdrawal symptoms or rebound insomnia after discontinuation of short-term treatment, whereas transient rebound insomnia was observed in subjects receiving zolpidem. In addition, clear differences were observed between OV101 and zolpidem from a sleep architecture perspective. OV101 has shown consistent increases in slow wave sleep compared to zolpidem with no significant effect on stage 2 or REM sleep in healthy adult, elderly subjects. It is believed that slow wave sleep is important for encoding long-term, fact-based memories. Slow wave sleep has been associated with physical changes in neuronal connections.
13
Overall, OV101 was observed to be well-tolerated in adult patients aged 18-64 years in the Phase 2 and Phase 3 trials at doses of 5mg to 15mg given as evening doses. The FDA has not, however, made any determination regarding the safety and efficacy of OV101. Success in these previous trials does not ensure that our clinical trials in OV101 will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of OV101. The most common reported adverse events were headache, nausea, vomiting, somnolence and dizziness. In general, the adverse events appeared to be dose-related. The majority of the serious adverse events, or SAEs, observed were considered to not be related to treatment with OV101. One SAE, fatigue, was considered by Lundbeck and Merck as probably related to OV101 treatment. Among the 9 SAEs considered by Lundbeck and Merck to be possibly related to OV101 treatment, there were three cases of fainting and one case each of: radius fracture, abnormal QRS axis, transient ischemic attack, non-cardiac chest pain, unresponsive to stimuli and atrial fibrillation. Across trials there were no apparent clinical trends regarding SAEs with respect to frequency, distribution across system organ classes or preferred terms. Also, there were no apparent clinical differences versus placebo. In the Phase 3 trials at the 15mg dose, at least one SAE was observed in 1.3% of subjects at two weeks and 3 months and 3.0% of subjects at 12 months versus 0.0% to 1.0% on placebo over the same timeframe.
Consistent with the clinical development of other insomnia drugs, the FDA requested that Lundbeck and Merck conduct a series of preclinical and clinical abuse studies as part of their development program. In preclinical studies, OV101 demonstrated low abuse potential. In one clinical trial, the abuse potential of OV101 was investigated in doses up to 45mg in male and female subjects with a history of hypnotic/sedative abuse and other drug abuse. Safety results showed that OV101 administered at doses of 30mg and 45mg in women and 45mg in men was not tolerated in this population of drug abusers, contrary to previous experience with the same doses in healthy volunteers. This indicated that a history of drug abuse decreased tolerability to OV101 in these subjects. Adverse events that were associated with a dose-dependent lack of tolerability in this trial included psychiatric, nervous system, musculoskeletal and gastrointestinal disorders.
In 2007, following the completion of all clinical trials for OV101 in insomnia, Lundbeck and Merck discontinued the development program for insomnia, and announced that the overall clinical profile did not support further development of OV101 for insomnia.
OV101 Clinical Development Plan
We have commenced and completed randomization in our Phase 2 trial of OV101 in adults and adolescents with Angelman syndrome, which we refer to as the STARS trial, and have completed a Phase 1 pharmacokinetic, or PK, trial in adolescents with Angelman syndrome or Fragile X syndrome. We anticipate topline data for the STARS trial in the second half of 2018. The Phase 1 PK data in adolescents with Angelman syndrome and Fragile X syndrome showed OV101 to be generally safe and well tolerated and its PK profile in adolescents was similar to previous data generated in young adults. With the data from the adolescent PK trial, we amended the STARS protocol to allow for enrollment of adolescent subjects aged 13 years and older and the STARS trial is now fully enrolled. With the initial trials being conducted in adults and adolescents, our goal is to initiate subsequent pediatric clinical trials given the high unmet medical need in the pediatric population.
14
We have designed and initiated the STARS trial to assess safety and tolerability in the target patient populations and to explore multiple endpoints that have the potential to inform the design of our future clinical trials. The STARS trial is fully enrolled. The trial is a randomized, double-blind, placebo-controlled trial. The primary endpoint will evaluate the safety and tolerability of OV101 from baseline to week 12 in two dosing schedules either once a day or QD or twice daily, or BID. The QD dose is an evening dose up to 15mg. The BID doses involve one morning dose of up to 10mg and one evening dose of up to 15mg. The exploratory endpoints will evaluate measures of clinical global impression, maladaptive behavior, sleep, gross and fine motor skills and health-related quality of life questionnaires. While we are also evaluating indications of efficacy as exploratory endpoints, this is primarily a safety trial that is designed to provide a proof-of-concept on efficacy parameters. The following figure depicts the design of the STARS trial:

Figure 11: The STARS trial is a randomized, double-blind placebo-controlled Phase 2 trial to assess the safety and tolerability of two dose schedules in adults and adolescents with a confirmed diagnosis of Angelman syndrome.
In parallel to the STARS trial, we completed a Phase 1 single-dose, single-arm, open-label, PK trial of OV101 in adolescents with Angelman syndrome or Fragile X syndrome aged 13 to 17 years. The trial enrolled seven male and five female adolescent patients who had been diagnosed with either Angelman syndrome or Fragile X syndrome. Participants received a single 5mg oral dose of OV101. Overall results of the PK study met the objectives and showed that PK parameters in adolescents with Angelman and Fragile X syndrome were not significantly different from previous data generated in young adults. The dosage of 5mg of OV101 in the PK trial is consistent with the dose that was used in the trials conducted by Lundbeck and Merck for insomnia and lower than those used in the clinical abuse trial.
OV935
OV935 for Epileptic Encephalopathies
We are developing OV935 in collaboration with Takeda for the treatment of rare epileptic encephalopathies. OV935 is a potent, highly selective inhibitor of the enzyme cholesterol 24-hydroxylase, or CH24H. We believe, if approved, OV935 has the potential to become a first-in-class inhibitor of CH24H. CH24H is predominantly expressed in the brain, where it plays a central role in cholesterol homeostasis. Recent literature suggests that modulation of CH24H may have an impact on over-activation of neurotransmitter pathways that have been implicated in a number of neurological disorders, such as epilepsy. OV935 has completed four Phase 1 trials demonstrating favorable tolerability at doses that are believed to be therapeutically relevant. We and Takeda commenced a Phase 1b/2a proof-of-concept trial in patients with Dravet syndrome, Lennox-Gastaut syndrome and Tuberous Sclerosis Complex in June 2017, each of which are rare epileptic encephalopathies that we believe, based on their biology, may be treated by OV935. In December 2017, the FDA granted orphan drug designation for OV935 for the treatment of Dravet syndrome and Lennox-Gastaut syndrome. We believe that OV935 offers the possibility not only to suppress seizures, as was observed in preclinical studies, but also to modulate the underlying biological pathways that lead to the development of seizures. This may offer the possibility of a long-term, disease-modifying therapy.
15
Dravet syndrome is a severe form of childhood epilepsy largely genetically driven by the mutation of the SCN1A gene that typically presents during the first year of life. Eighty percent of patients have a mutation of the SCN1A-gene. Children experience frequent seizures, loss of muscle control, cognitive deficits and, in approximately 10% of cases, death before the age of 12 years. Children continue to suffer from seizures and severe cognitive and developmental impairment throughout their lifetime. While some patients may survive into adulthood, their long-term intellectual development and seizure outcomes are typically extremely poor. The incidence of Dravet syndrome in the United States ranges from 1 in 15,700 to 1 in 20,900 births. Patients are frequently treated with combinations of classic anti-epileptic drugs, none of which are particularly effective. However, no drug has been approved specifically for the treatment of Dravet syndrome in the United States and only one drug, the anticonvulsant stiripentol, has been approved in Europe.
Lennox-Gastaut Syndrome
Lennox-Gastaut syndrome is a rare disorder that is often diagnosed between three and five years of age. Patients diagnosed with Lennox-Gastaut syndrome experience a multitude of seizure types that are difficult to manage and have many of the same symptomologies as other rare pediatric epilepsies. Lennox -Gastaut syndrome affects over 30,000 people in the United States with approximately half being children under the age of 18. Some patients have de novo genetic mutations, including a mutation of the SCN2A gene. The annual incidence of Lennox - Gastaut syndrome in childhood is estimated to be 2 per 100,000 children. It is also estimated that between 1% and 4% of childhood epilepsies are a result of Lennox - Gastaut syndrome. Only 10% of these patients have seizures that are fully controlled by existing therapies.
Tuberous Sclerosis Complex
Tuberous Sclerosis Complex is a genetic disorder that causes non-malignant tumors to form in many different organs, and primarily in the brain, eyes, heart, kidney, skin and lungs and is often diagnosed in childhood. The brain and skin are the most affected organs. Tuberous Sclerosis Complex results from a mutation in tumor suppression genes TSC1 or TSC2. Most cases of Tuberous Sclerosis Complex are caused by de novo mutations of the TSC1 or TSC2 genes. According to the Tuberous Sclerosis Alliance, Tuberous Sclerosis Complex is estimated to affect approximately 50,000 patients in the United States and occurs in 1 of 6,000 live births. The most common symptom of Tuberous Sclerosis Complex is epilepsy, which occurs in 60% to 90% of patients, of which 70% experience seizure onset in their first year of life. Despite available therapies, a significant number of Tuberous Sclerosis Complex patients have treatment-resistant seizures. There are significant co-morbidities associated with Tuberous Sclerosis Complex, including cognitive impairment in 50%, autism spectrum disorders in up to 40% and neurobehavioral disorders in over 60% of individuals with Tuberous Sclerosis Complex.
The Role of Cholesterol Metabolism in Epileptic Encephalopathies
The brain is a cholesterol-rich organ, containing about 25% of the total cholesterol in the body. Cholesterol is an essential component of cellular membranes, including the synaptic membranes that aid in the transmission of signals between cells. Cholesterol is also a key component of myelin, the protective layer of lipids and proteins that serves as an insulating sheath and facilitates electrical conduction in nerve cells.
Cholesterol in the brain is entirely synthesized and metabolized locally to maintain physiologic levels. When cholesterol is metabolized in the brain, it is broken down to 24-hydroxycholesterol, or 24HC, by CH24H, an enzyme predominantly expressed in the brain. Converting cholesterol to 24HC enables it to pass through the blood-brain barrier and enter into the circulatory system, allowing it to be eliminated from the body. Since CH24H is primarily present in the brain, there is a strong correlation between circulating blood levels and brain levels of 24HC. The levels of 24HC can therefore serve as a biomarker of CH24H activity in the brain.
16
24HC levels can profoundly impact key signaling pathways in the brain including glutamatergic signaling, or signaling by the neurotransmitter glutamate. In one subtype of glutamate receptors called N-Methyl-D-Aspartate, or NMDA, receptors, elevated levels of 24HC have been shown in various cellular and tissue models to lead to increased activation of the glutamate signaling pathway. Activation of NMDA receptors has been implicated in a number of neurological disorders, including Alzheimer’s disease and epilepsy. As a result, modulation of cholesterol metabolism has been proposed as a potential therapeutic approach for several neurological disorders. We believe that decreasing 24HC levels, and thereby modulating NMDA receptor activity, represents a sound rationale for addressing the underlying biology of epileptic encephalopathies. The following figure depicts inhibition of CH24H by OV935 and its impact on excitatory signaling in the brain.

Figure 12: Through inhibition of CH24H, OV935 is believed to reduce brain levels of 24HC, thereby reducing excitatory signaling.
The glutamatergic pathway and NMDA receptors have been the targets of a number of approved drugs. Many of these approved drugs, including anesthetics such as ketamine, were developed as antagonists of NMDA receptors, thus blocking the receptor. These drugs have not been used in disorders such as epilepsy, where the goal is not to entirely block the NMDA receptor, but rather to modulate its activity. The complete blockade of the NMDA receptor with long-term use is frequently associated with poor tolerability. In some cases, low doses of NMDA receptor antagonists have demonstrated clinical benefit outside their prescribed use, including for treatment of neurological disorders. For example, memantine, a low affinity NMDA receptor antagonist, has been used to treat moderate to severe Alzheimer’s disease patients because it does not completely block the NMDA receptor. Modulation of the NMDA receptor, rather than NMDA receptor antagonism, could provide a more effective method for treatment of neurological disorders.
A number of publications by independent academic groups have shown that 24HC is a potent modulator of the NMDA receptor. These publications describe 24HC as a positive allosteric modulator, a molecule that induces a conformational change within the protein structure of the receptor and increases its activity. Mice lacking CH24H expression have reduced brain levels of 24HC and decreased NMDA receptor signaling. Therefore reducing 24HC levels in the brain offers an alternate mechanism for modulating NMDA receptor activity without blocking normal receptor function. We believe that reducing 24HC levels represents an innovative approach to impacting the glutamatergic pathway to treat epileptic encephalopathies. The novelty of this approach, along with the strength of the data supporting it, are what attracted us to OV935 as a potential treatment for these disorders.
OV935 Preclinical Data
OV935 has been evaluated in multiple preclinical epilepsy and seizure models. In these studies, OV935 was observed to have anti-convulsive activity in genetic, pharmacologic and inflammation-induced seizure models. Based on the preclinical data available to us, OV935 may have diverse effects on overall brain function, affecting both seizure intensity and frequency, and potentially modifying the underlying disease biology. We believe the data provide a rationale to test OV935 in multiple epileptic encephalopathies. The models are summarized as follows:
|
Model |
|
Observed OV935 Activity |
|
SCN1A Knock-In Model of DS |
|
Increased temperature threshold for hyperthermia-induced seizures |
|
Pentylenetetrazol (PTZ) Kindling Model |
|
Reduced PTZ-induced seizure progression |
|
Fring’s Audiogenic Seizure Model |
|
Reduced audiogenic seizures |
|
APP/PS1 Transgenic Mouse Model |
|
Prolonged overall survival |
|
TMEV Mouse Model |
|
Decreased seizure activity and duration |
17
SCN1A Knock-In Model of Dravet Syndrome
OV935 was tested in a knock-in model of Dravet syndrome constructed by inactivation of the SCN1A gene. This gene encodes a voltage-gated sodium channel that plays a critical role in the normal functioning of inhibitory pathways in the brain. Deficiencies in the functioning of this channel allow brain excitatory pathways to function unchecked, resulting in severe seizures. The majority of Dravet syndrome cases are caused by mutations in this gene. Mice containing the SCN1A gene mutation have hyperthermia-induced or high-temperature-induced seizures. Mice treated with OV935 were observed to have a significantly raised threshold temperature for developing these seizures after seven days of dosing, relative to untreated mice, as depicted below.

Source: RIKEN Brain Science Institute, Neurogenetics laboratory (Lab head; Kazuhiro Yamakawa).
Figure 13: OV935 increases the threshold for temperature-induced seizures in mice containing
the SCN1A gene mutation.
PTZ Kindling Model
OV935 was tested in a preclinical kindling seizure model. Scientists use kindling models to study the effects of repeat seizures in the brain. One such kindling model described here is the pentylenetetrazol, or PTZ, model. As depicted in the figure below, it has been observed that repeat stimulation can increase the likelihood of seizures, presumably because there is a threshold for seizures to occur and the repeat stimulation lowers this threshold. In this model, PTZ is used to chemically stimulate mice at sub-convulsive levels. PTZ also increases the density and sensitivity of glutamate receptors in specific regions of the brain. Mice that were dosed daily with OV935 demonstrated a significant delay in seizure development in this model.

Figure 14: OV935 delayed seizure development in the PTZ-induced kindling mouse model.
18
Fring’s Audiogenic Seizure Model
OV935 was tested in a preclinical audiogenic seizure model. The Fring’s audiogenic seizure model is widely used by scientists to investigate the effects of investigational drugs in a model sensitive to sound-induced seizures. In the experiment, OV935 was dosed once a day for either one or three days. The effect of OV935 on the duration of seizures was determined after the induction of a seizure either one hour or 24 hours following administration of the last dose. As depicted in the figure below, it was observed that OV935 treatment resulted in a significant dose-dependent reduction in sound-induced seizures following single and 3-day repeat dosing.

Figure 15: OV935 dose-dependently reduced seizure in the Fring’s audiogenic seizure model.
90APP/PS1 Transgenic Mouse Model
19
OV935 was tested in a preclinical Alzheimer’s disease model where an amyloid precursor protein, or APP, overexpressing mouse was crossed with a presenilin-1, or PS1, mutant mouse that is highly prone to developing spontaneous seizures. We believe that results obtained in this model yield some valuable insights into neurological disorders, and particularly into the biochemistry of the brain. Mice in this model typically have a high incidence of sudden death with only 50% of them surviving after three months. As depicted in the figure below, mice treated with OV935 in this model were observed to have a significant increase in overall survival. While it is unclear if the increase in survival is directly related to reduction in seizures, increase in survival has previously been observed in APP/PS1 mice when one copy of the gene for CH24H is inactivated, suggesting that the survival benefit observed after treatment with OV935 may be due to inhibition of its intended target, CH24H.

Figure 16: OV935 increased survival in APP/PS1 transgenic mouse model.
TMEV Mouse Model
OV935 was tested in a Theiler’s murine encephalomyelitis virus, or TMEV, model to determine the role of OV935 in reducing inflammation-induced seizures. Infection with TMEV in this model leads to acute seizures and significant elevations in inflammatory signaling molecules, known as cytokines. A large fraction of the mice develop spontaneous, recurrent seizures and various behavioral co-morbidities weeks later. As depicted below, treatment with OV935 demonstrated reduction in the overall number and average severity of seizures in the mice in the acute phase. In addition, long-term benefits were observed, based on significant improvements in anxiety assays such as the open field test.

Figure 17: OV935 reduced the number of seizures and the number of severe (Stage 4/5) seizures in the
TMEV model.
OV935 Clinical Data
OV935 has been tested in 86 healthy volunteers across four Phase 1 trials. Single oral doses of up to 1,350mg of OV935 were well-tolerated. The most frequently reported adverse events were headache, ECG electrode application site dermatitis and nausea. All reported events were mild with no apparent dose-response. In a 14-day repeat dosing trial, doses of 100mg once a day, or QD, 300mg QD and 400mg QD were well-tolerated. One volunteer at the 300mg BID experienced an event of confusional state and another volunteer at the 600mg QD dose experienced acute psychosis. Both volunteers discontinued the trial at day 11. One volunteer receiving placebo reported events of nightmares, spatial disorientation, insomnia and dizziness. All treatment emergent adverse events, or TEAEs, resolved with continued dosing through day 15. No serious adverse events were reported. Overall, no safety issues of concern were identified in the Phase 1 trials based on assessments of physical examinations, vital sign measurements, clinical laboratory values or 12-lead electrocardiogram findings.
20
The following table summarizes each trial:
|
Trial |
|
Purpose |
|
Design |
|
Number of Volunteers |
|
Dosage |
|
1 |
|
Safety and tolerability |
|
Phase 1, randomized, double-blind, placebo-controlled, single ascending dose trial |
|
48 |
|
15-1,350mg, oral |
|
|
|
|
|
|
|
|
|
|
|
2 |
|
Safety and tolerability |
|
Phase 1, randomized, double-blind, placebo-controlled, multiple ascending dose trial |
|
40 |
|
100-600mg QD, and 300mg BID, 14 days, oral |
|
|
|
|
|
|
|
|
|
|
|
3 |
|
Brain CH24H enzyme occupancy using positron emission tomography, or PET |
|
Open-label, non-randomized |
|
11 |
|
50-600mg, oral |
|
|
|
|
|
|
|
|
|
|
|
4 |
|
Relative bioavailability of tablet versus solution formulation; effect of food |
|
Phase 1, randomized, open-label, single dose trial |
|
9 |
|
300mg (tablet), oral; 300mg (solution), oral |
In the Phase 1 PET imaging trial, following administration of OV935, levels of plasma 24HC decreased as the dose increased, reaching an apparent plateau of a 60% reduction at a dose of 300mg, as depicted in the figure below.

Figure 18: Dose-dependent reduction in plasma 24HC by OV935 in a Phase 1 multiple ascending-dose trial.
OV935 Clinical Development Plan
In June 2017, along with our development partner, Takeda, we commenced a Phase 1b/2a proof-of-concept trial in adult patients with rare epileptic encephalopathies, including Dravet syndrome, Lennox-Gastaut syndrome and Tuberous Sclerosis Complex. We are conducting this trial pursuant to an IND that Takeda submitted to the FDA. This trial is currently ongoing and we expect data from this trial the second half of 2018.
Other Programs
Epilepsy
We are developing additional preclinical-stage compounds for rare epilepsy disorders that may provide the opportunity to exploit novel pathways or offer differentiated profiles over existing therapies.
OV102
We are exploring opportunities to develop OV102, an intravenous formulation of OV101, for indications in several clinical settings. We may choose to develop OV102 internally, or to collaborate externally.
21
License and Collaboration Agreements
License Agreement with H. Lundbeck A/S
In March 2015, we entered into a license agreement with Lundbeck, or the Lundbeck agreement, pursuant to which we obtained from Lundbeck an exclusive (subject to certain reserved non-commercial rights), worldwide license to develop, manufacture, and commercialize OV101, also known as gaboxadol, for the treatment of human disease. Under the Lundbeck agreement, we are responsible for and will use commercially reasonable efforts to carry out all future development and commercialization of OV101. Initially, we will purchase OV101 compound from Lundbeck’s existing inventory at a specified price. Following the depletion of the existing inventory, we may purchase the compound from a third party or, if the parties agree, Lundbeck may continue to supply the compound to us. We are also obligated to make certain manufacturing-related payments to Lundbeck, including for its preparation of a drug master file for OV101. We granted Lundbeck a right of first negotiation if we decide at any time to seek a partner to develop or commercialize OV101 in one or more specified countries.
In connection with the Lundbeck agreement, we issued 489,756 shares of our common stock to Lundbeck. We also agreed to pay to Lundbeck milestone payments up to an aggregate of $181.0 million upon the achievement of certain global development, regulatory and sales milestone events. In addition, if we successfully develop and commercialize OV101, we will be obligated to pay to Lundbeck tiered royalties in the range of low to middle teens based on the net sales of OV101, subject to certain reductions for generic product sales and for royalties paid for licenses to third party intellectual property. In the event that we commercialize OV101 with a partner in China, Japan or South Korea, each, an Asian Partner, we will instead share with Lundbeck specified percentages of the payments we receive from the Asian Partner, including any upfront payment, milestone payments and royalties, provided that we may deduct certain OV101 development expenses from the amounts owed to Lundbeck. Our obligation to make royalty payments, and to share amounts received from Asian Partners, will expire on a country-by-country basis upon the later of the expiration of the last solely owned licensed patent or 10 years after the first commercial sale. If Lundbeck manufactures OV101 compound for us after the expiration of the royalty term, we will pay to Lundbeck, in addition to the fully burdened cost of such manufacture, a low, single digit manufacturing royalty on the net sales of OV101 manufactured by Lundbeck.
The Lundbeck agreement will continue until the expiration of all relevant royalty terms, and may be earlier terminated by either party for the other party’s uncured material breach or insolvency. In addition, we can terminate the Lundbeck agreement upon advance notice for convenience at any time prior to first regulatory approval of OV101. If the Lundbeck agreement is terminated by us for convenience or by Lundbeck for our breach or insolvency, the OV101 compound will revert to Lundbeck and we will grant Lundbeck an exclusive license to develop and commercialize OV101; such license will be royalty-bearing if we filed an application for regulatory approval prior to termination. If we terminate the Lundbeck agreement for Lundbeck’s breach or insolvency, our license will continue and our obligations to make royalty payments to Lundbeck and to share Asian Partner payments with Lundbeck will continue but we will not be obligated to make further milestone payments to Lundbeck or to purchase any additional quantities of OV101 compound from Lundbeck’s existing inventory.
License and Collaboration Agreement with Takeda
In January 2017, we entered into a license and collaboration agreement with Takeda, or the Takeda license agreement. All activities of the collaboration regarding OV935 will be guided by the Takeda/Ovid “One Team” concept, an integrated and interdisciplinary team from both companies devoted to the successful advancement of OV935 across rare epilepsy syndromes. Pursuant to the Takeda license agreement, we will take the lead in clinical development activities and commercialization of the compound OV935 and products containing this compound (as well as certain other similar compounds, including any prodrug where TAK-935 is the primary pharmacologically active metabolite) for the treatment of certain rare neurological diseases in the United States, Canada, the European Union and Israel. Takeda will take the lead in commercialization of OV935 in Japan and has the option to lead in Asia and the rest of the world, or the Takeda Territory. While we and Takeda have agreed to initially focus on certain rare neurological disorders, the scope of the collaboration may in the future include other mutually agreed upon rare neurological disorders.
Under the Takeda license agreement, Takeda granted to us an exclusive license in our territory under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. Takeda also granted to us a worldwide, co-exclusive license to develop, manufacture and otherwise exploit (but not commercialize) OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
Under the Takeda license agreement, we granted to Takeda an exclusive license in the Takeda Territory under certain patents and other intellectual property controlled by us to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. We also granted to Takeda a worldwide, co-exclusive license to develop, manufacture and otherwise exploit (but not commercialize) OV935 and products containing OV935 for the treatment of certain rare neurological disorders and a co-exclusive license in certain countries to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders that are subsequently included in the collaboration.
22
We and Takeda will collaborate in the development of OV935. Pursuant to the terms of the Takeda license agreement, each party is required to use commercially reasonable efforts to develop OV935 for the treatment of certain rare neurological disorders in accordance with a mutually agreed upon development plan. We are primarily responsible for activities related to the development of OV935, and as such Takeda will transition certain development activities to us. Takeda is initially responsible for regulatory activities in all countries (excluding Israel). We are initially responsible for regulatory activities in Israel, and, upon regulatory approval in the United States, Canada, and the European Union, we will assume responsibility for further regulatory activities in such jurisdictions.
We and Takeda will collaborate in the commercialization of OV935. Pursuant to the terms of the Takeda license agreement, each party is required to use commercially reasonable efforts to commercialize OV935 for the treatment of certain rare neurological disorders in its territory. We are responsible for commercialization of OV935 in the United States, Canada, the European Union and Israel, and Takeda is responsible for commercialization of OV935 in Japan, and has the first right to elect to commercialize the products in the Takeda Territory. Additionally, Takeda has the right to jointly commercialize the products with us in the United States and/or the European Union for any additional mutually agreed upon rare neurological indication.
Under the Takeda license agreement, we and Takeda will initially share equally all development and commercialization costs and expenses prior to launch of a product and all revenues and commercialization costs and expenses after launch. In the event that we and Takeda agree to expand the scope of the collaboration to include additional rare neurological disorders, either party may elect not to fund all or a portion of the development of such indication, in which case such party’s overall share of revenues and commercialization costs and expenses after launch of a product may be reduced under certain circumstances.
During the period commencing on the effective date of the Takeda license agreement, we and Takeda have both agreed that we will not, directly or indirectly, and will cause all of our respective affiliates, not to, alone or with others, commercialize any competing product in the field of rare neurological disorders. For these purposes, a competing product is any product or compound directed against CH24H as its primary, intended mode of action. If, during such period, we or any of our affiliates is acquired by a third party that is commercializing a competing product, then we must divest our interest or terminate the commercialization of the competing product or cause our affiliate to do so.
The Takeda license agreement will expire upon the cessation of commercialization of the products by both us and Takeda. Either party may terminate the Takeda license agreement as a result of the other party’s uncured material breach or insolvency, for safety reasons, or, after completion of the first proof of mechanism clinical trial, for convenience. Takeda may terminate the Takeda license agreement for our (or our sublicensee’s) challenge to the patents licensed under the Takeda license agreement. If the agreement is terminated by Takeda for our material breach, bankruptcy or patent challenge or by us for convenience or safety reasons, our rights to the products will cease, we will transition all activities related to the products to Takeda, and we will grant Takeda an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by us to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. If the agreement is terminated by us for Takeda’s material breach or bankruptcy or by Takeda for convenience or safety reasons, Takeda’s rights to the products will cease, Takeda will transition all activities related to the products to us, and Takeda will grant us an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
Under the Takeda license agreement, in the event of an acquisition of us by certain types of acquirers prior to the final dosing of a patient in the first Phase 3 trial, Takeda would have the right to elect to take over all development and commercialization activities with respect to the products, so long as Takeda at such time has (or will have) sufficient commercial infrastructure to commercialize the products. Even if Takeda exercises such right to take over all development and commercialization activities with respect to the products, we and Takeda will continue to share equally all development and commercialization costs and expenses prior to launch of a product and all revenues and commercialization costs and expenses after launch, unless otherwise set forth in the agreement.
In connection with the Takeda license agreement and in consideration of certain license rights granted to us by Takeda, we issued 1,781,996 shares of our Series B-1 convertible preferred stock to Takeda. Under the Takeda license agreement, we are obligated to pay Takeda future payments if and when certain milestones are achieved. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial disorders we and Takeda are focusing on, we are obligated to issue to Takeda the number of unregistered shares of our common stock equal to the lesser of (a) 8% of our outstanding capital stock on the issuance date or (b) $50.0 million divided by the applicable share price, unless certain events occur. In the event such payment would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of our common stock at our election.
23
On December 15th, 2016, we entered into a license agreement with Northwestern University, or Northwestern, pursuant to which Northwestern granted us an exclusive, worldwide license to patent rights in certain inventions, or the Northwestern Patent Rights, which relate to a specific compound and related methods of use for such compound, along with certain Know-How related to the practice of the inventions claimed in the Northwestern Patents.
Under the Northwestern agreement, we were granted exclusive rights to research, develop, manufacture and commercialize products utilizing the Northwestern Patent Rights for all uses. We have agreed that we will not use the Northwestern Patent Rights to develop any products for the treatment of cancer, but Northwestern may not grant rights in the technology to others for use in cancer. We also have an option, exercisable during the term of the agreement to an exclusive license under certain intellectual property rights covering novel compounds with the same or similar mechanism of action as the primary compound that is the subject of the license agreement. Northwestern has retained the right, on behalf of itself and other non-profit institutions, to use the Northwestern Patent Rights and practice the inventions claimed therein for educational and research purposes and to publish information about the inventions covered by the Northwestern Patent Rights.
Upon entry into the Northwestern agreement, the Company paid an upfront non-creditable one-time license issuance fee of $75,000, and is required to pay an annual license maintenance fee of $20,000, which will be creditable against any royalties payable to Northwestern following first commercial sale of licensed products under the agreement. The Company is responsible for all ongoing costs of filing, prosecuting and maintaining the Northwestern Patents, but also has the right to control such activities using its own patent counsel. In consideration for the rights granted to the Company under the Northwestern agreement, the Company is required to pay to Northwestern up to an aggregate of $5.3 million upon the achievement of certain development and regulatory milestones for the first product covered by the Northwestern Patents, and, upon commercialization of any such products, will be required to pay to Northwestern a tiered royalty on net sales of such products by the Company, its affiliates or sublicensees, at percentages in the low to mid single-digits, subject to standard reductions and offsets. The Company’s royalty obligations continue on a product-by-product and country-by-country basis until the later of the expiration of the last-to-expire valid claim in a licensed patent covering the applicable product in such country and 10 years following the first commercial sale of such product in such country. If the Company sublicenses a Northwestern Patent Right, it will be obligated to pay to Northwestern a specified percentage of sublicense revenue received by the Company, ranging from the high single digits to the low-teens.
The Northwestern agreement requires that the Company use commercially reasonable efforts to develop and commercialize at least one product that is covered by the Northwestern Patent Rights.
Unless earlier terminated, the Northwestern agreement will remain in force until the expiration of the Company’s payment obligations thereunder. The Company has the right to terminate the agreement for any reason upon prior written notice or for an uncured material breach by Northwestern. Northwestern may terminate the agreement for the Company’s uncured material breach or insolvency.
Sales and Marketing
Given our stage of development, we have not yet established a commercial organization or distribution capabilities. We plan to build focused capabilities in the United States and European Union to commercialize our development programs focused on orphan disorders of the brain, where we believe the patient populations and medical specialists for the indications we are targeting are sufficiently concentrated to allow us to effectively promote our product, if approved for commercial sale, with a targeted sales team. In other markets for which commercialization may be less capital efficient for us, we may selectively pursue strategic collaborations with third parties in order to maximize the commercial potential of our drug candidates.
Manufacturing and Supply
We currently have no manufacturing facilities and we intend to use our collaborators and contract manufacturers for the foreseeable future. However, certain members of our management have broad experience in manufacturing, which we believe may provide a competitive advantage.
We currently rely on Lundbeck to provide the drug substance supply for our planned clinical trials in OV101. Pursuant to the Lundbeck agreement, we agreed to purchase from Lundbeck, and Lundbeck agreed to sell to us, the entirety of their existing inventory of the OV101 compound. We have purchased and imported a portion of this inventory, which was requalified by Lundbeck, and expect that this supply will be sufficient to meet our needs through the completion of our planned Phase 2 trials in Angelman syndrome and Fragile X syndrome. We further expect that Lundbeck’s remaining existing inventory will be sufficient to meet our needs through the completion of all of our planned clinical trials in OV101 and potentially into commercialization, if approved. Following the depletion of Lundbeck’s existing inventory, we may purchase the OV101 compound from a third party or, if the parties agree, Lundbeck may continue to supply the compound to us at the fully burdened cost of manufacture. We have contracted with a third-party contract development and manufacturing organization to manufacture the drug product for our planned and ongoing clinical trials in OV101, and we expect to engage another third party to package, label and distribute the drug. We plan to continue to rely upon Lundbeck and/or one or more alternative contract manufacturers to supply us with commercial quantities of drug substance and drug product supply, including for OV101, if approved.
24
We will continue to rely on Takeda to provide the drug product supply for our planned clinical trials in OV935 and, if approved, drug substance supply for commercial use of OV935. We will be required to contract with a third-party development and manufacturing organization to manufacture the drug product for our commercial use, and we expect to engage another third party to package, label and distribute the drug.
Competition
Currently, there are no therapies approved for the treatment of Angelman syndrome or Fragile X syndrome. However, certain symptomatic treatments, including traditional anticonvulsants, sedatives and antianxiety drugs, are employed to lessen the burden of these disorders. We believe SAGE Therapeutics, Inc., Marinus Pharmaceuticals, Inc. and Zynerba Pharmaceuticals, Inc. are our most direct competitors with respect to OV101. We believe Zogenix, Inc., GW Pharmaceuticals plc, Sage Therapeutics, Inc., Marinus Pharmaceuticals, Inc., Zynerba Pharmaceuticals, Inc. and PTC Therapeutics, Inc. are our most direct competitors with respect to OV935.
Drug development is highly competitive and subject to rapid and significant technological advancements. Our ability to compete will significantly depend upon our ability to complete necessary clinical trials and regulatory approval processes, and effectively market any drug that we may successfully develop. Our current and potential future competitors include pharmaceutical and biotechnology companies, academic institutions and government agencies. The primary competitive factors that will affect the commercial success of any drug candidate for which we may receive marketing approval include efficacy, safety and tolerability profile, dosing convenience, price, coverage and reimbursement. Many of our existing or potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of drug candidates, as well as in obtaining regulatory approvals of those drug candidates in the United States and in foreign countries.
Our current and potential future competitors also have significantly more experience commercializing drugs that have been approved for marketing. Mergers and acquisitions in the pharmaceutical and biotechnology industries could result in even more resources being concentrated among a small number of our competitors.
Accordingly, our competitors may be more successful than us in obtaining regulatory approval for therapies and in achieving widespread market acceptance of their drugs. It is also possible that the development of a cure or more effective treatment method for the disorders we are targeting by a competitor could render our current or future drug candidates non- competitive or obsolete or reduce the demand for our drug candidates before we can recover our development and commercialization expenses.
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our current and future drug candidates, novel discoveries, product development technologies and know-how, to operate without infringing on the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing or in-licensing U.S. and foreign patents and patent applications related to technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trademarks, trade secrets, copyright protection, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position. For example, the proprietary map of disease-relevant biological pathways underlying orphan disorders of the brain that we developed would not be appropriate for patent protection and, as a result, we rely on trade secrets to protect this aspect of our business.
While we seek broad coverage under our existing patent applications, there is always a risk that an alteration to the product or process may provide sufficient basis for a competitor to avoid infringement claims. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued and courts can reinterpret patent scope after issuance. Moreover, many jurisdictions including the United States permit third parties to challenge issued patents in administrative proceedings, which may result in further narrowing or even cancellation of patent claims. Moreover, we cannot provide any assurance that any patents will be issued from our pending or any future applications or that any potentially issued patents will adequately protect our intellectual property.
As of December 31, 2017, we have exclusively licensed a portfolio of issued U.S. and international patents from Lundbeck directed to polymorphic forms of OV101 and their preparation, and these patents expire on dates ranging from 2025 to 2028. In addition, we have exclusively licensed from Lundbeck a pending application directed to an OV101 manufacturing processes that, if issued, would have a statutory expiration in 2036. We have also filed, and own, multiple patent families directed to methods of treatment and formulations with OV101. In particular, we currently own two issued U.S. patents directed to treatment of Angelman syndrome with OV101 that expire in 2035, excluding any regulatory extensions. Additional applications are pending that are directed to methods of treating neurodegenerative diseases and developmental disorders, including Fragile X syndrome. We are, or will, seek patent protection for these inventions in numerous countries and regions including, among others, Europe, Australia, Canada, Mexico, Israel, Japan, China, and Korea.
25
On January 6, 2017, we licensed from Takeda a portfolio of U.S. and international patents and applications directed to the OV935 composition of matter, and these patents and applications expire in 2032, excluding any regulatory extensions.
Individual patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, utility patents issued for applications filed in the United States are granted a term of 20 years from the earliest effective filing date of a non-provisional patent application. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office, or the USPTO, delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. The actual protection afforded by a patent may vary on a product by product basis, from country to country and can depend upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
Furthermore, we rely upon trade secrets and know-how and continuing technological innovation to develop and maintain our competitive position. We seek to protect our proprietary information, in part, using confidentiality agreements with our employees and consultants and any potential commercial partners and collaborators and invention assignment agreements with our employees. We also have or intend to implement confidentiality agreements or invention assignment agreements with our selected consultants and any potential commercial partners. These agreements are designed to protect our proprietary information and, in the case of the invention assignment agreements, to grant us ownership of technologies that are developed through a relationship with a third party. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our commercial partners, collaborators, employees and consultants use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our drugs or processes, obtain licenses or cease certain activities. Our breach of any license agreements or failure to obtain a license to proprietary rights that we may require to develop or commercialize our future drugs may have an adverse impact on us. Since patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months or potentially longer, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in Interference, Derivation, Reexam, Post-Grant Review, Inter Partes Review, or Opposition proceedings brought by third parties or declared by the USPTO.
Government Regulation
The FDA and comparable regulatory authorities in state and local jurisdictions and in other countries impose substantial and burdensome requirements upon companies involved in the clinical development, manufacture, marketing and distribution of drugs, such as those we are developing. These agencies and other federal, state and local entities regulate, among other things, the research and development, testing, manufacture, quality control, safety, effectiveness, labeling, storage, record keeping, approval, advertising and promotion, distribution, post-approval monitoring and reporting, sampling and export and import of our drug candidates.
U.S. Government Regulation
In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statutes and regulations requires the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to a variety of administrative or judicial sanctions, such as the FDA’s refusal to approve pending New Drug Applications, or NDAs, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
|
|
• |
completion of preclinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
|
|
• |
submission to the FDA of an IND which must become effective before human clinical trials may begin; |
|
|
• |
approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated; |
26
|
|
• |
submission to the FDA of an NDA; |
|
|
• |
satisfactory completion of an FDA advisory committee review, if applicable; |
|
|
• |
satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current good manufacturing practice, or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
|
|
• |
FDA review and approval of the NDA. |
Preclinical Studies
Preclinical studies include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies to assess potential safety and efficacy. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data and any available clinical data or literature, among other things, to the FDA as part of an IND. Some preclinical testing may continue even after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to human patients under the supervision of qualified investigators in accordance with GCP requirements, which include the requirement that all research patients provide their informed consent in writing for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. In addition, an IRB at each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution. Information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on their www.clinicaltrials.gov website.
Human clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
|
|
• |
Phase 1 clinical trial: The drug is initially introduced into healthy human volunteers or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness. |
|
|
• |
Phase 2 clinical trial: The drug is administered to a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
|
|
• |
Phase 3 clinical trial: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product. |
Progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently if serious adverse events occur. Each of Phase 1, Phase 2 and Phase 3 clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
A drug being studied in clinical trials may be made available to individual patients in certain circumstances. Pursuant to the 21st Century Cures Act, or Cures Act, which was signed into law in December 2016, the manufacturer of an investigational drug for a serious disease or condition is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for individual patient access to such investigational drug. This requirement applies on the later of 60 calendar days after the date of enactment of the Cures Act or the first initiation of a Phase 2 or Phase 3 trial of the investigational drug.
27
Assuming successful completion of the required clinical testing, the results of the preclinical studies and clinical trials, together with detailed information relating to the product’s chemistry, manufacture, controls and proposed labeling, among other things, are submitted to the FDA as part of an NDA requesting approval to market the product for one or more indications. In most cases, the submission of an NDA is subject to a substantial application user fee. Under the Prescription Drug User Fee Act, or PDUFA, guidelines that are currently in effect, the FDA has a goal of ten months from the date of “filing” of a standard NDA for a new molecular entity to review and act on the submission. This review typically takes twelve months from the date the NDA is submitted to FDA because the FDA has approximately two months to make a “filing” decision.
In addition, under the Pediatric Research Equity Act of 2003, or PREA, as amended and reauthorized, certain NDAs or supplements to an NDA must contain data that are adequate to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements.
The FDA also may require submission of a risk evaluation and mitigation strategy, or REMS, plan to ensure that the benefits of the drug outweigh its risks. The REMS plan could include medication guides, physician communication plans, assessment plans, or elements to assure safe use, such as restricted distribution methods, patient registries, or other risk minimization tools. The FDA conducts a preliminary review of all NDAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. The FDA reviews an NDA to determine, among other things, whether the drug is safe and effective and whether the facility in which it is manufactured, processed, packaged or held meets standards designed to assure the product’s continued safety, quality and purity.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA may inspect one or more clinical trial sites to assure compliance with GCP requirements.
After evaluating the NDA and all related information, including the advisory committee recommendation, if any, and inspection reports regarding the manufacturing facilities and clinical trial sites, the FDA may issue an approval letter, or, in some cases, a complete response letter. A complete response letter generally contains a statement of specific conditions that must be met in order to secure final approval of the NDA and may require additional clinical or preclinical testing in order for FDA to reconsider the application. Even with submission of this additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval. If and when those conditions have been met to the FDA’s satisfaction, the FDA will typically issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
Even if the FDA approves a product, it may limit the approved indications for use of the product, require that contraindications, warnings or precautions be included in the product labeling, require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess a drug’s safety after approval, require testing and surveillance programs to monitor the product after commercialization, or impose other conditions, including distribution and use restrictions or other risk management mechanisms under a REMS, which can materially affect the potential market and profitability of the product. The FDA may prevent or limit further marketing of a product based on the results of post-marketing studies or surveillance programs. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes, and additional labeling claims, are subject to further testing requirements and FDA review and approval.
Orphan Drug Act
Under the Orphan Drug Act of 1983, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the name of the sponsor, identity of the drug or biologic and its potential orphan use are disclosed publicly by the FDA. The orphan drug designation does not shorten the duration of the regulatory review or approval process, but does provide certain advantages, such as a waiver of PDUFA fees, enhanced access to FDA staff and potential waiver of pediatric research requirements.
28
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same drug or biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the application user fee. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
The FDA granted orphan drug designation for OV101 for the treatment of Angelman syndrome and for the treatment of Fragile X syndrome in September 2016 and October 2017, respectively. The FDA granted orphan drug designation for OV935 for the treatment of Dravet syndrome and Lennox-Gastaut syndrome both in December 2017. We intend to pursue orphan drug designation for OV101 in additional indications, as well as for OV935 and potential other future drug candidates as we deem it appropriate. Even if we were to obtain orphan drug designation for a drug candidate, we may not obtain orphan exclusivity and that exclusivity may not effectively protect the drug from the competition of different drugs for the same condition, which could be approved during the exclusivity period.
Post-Approval Requirements
Drugs manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, product sampling and distribution, advertising and promotion and reporting of adverse experiences with the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims are subject to prior FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA may impose a number of post-approval requirements as a condition of approval of an NDA. For example, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization.
In addition, drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and state agencies, and are subject to periodic unannounced inspections by the FDA and these state agencies for compliance with cGMP requirements. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain cGMP compliance.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
|
|
• |
restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
|
|
• |
fines, warning letters or holds on post-approval clinical trials; |
|
|
• |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals; |
|
|
• |
product seizure or detention, or refusal to permit the import or export of products; or |
|
|
• |
injunctions or the imposition of civil or criminal penalties. |
The FDA strictly regulates marketing, labeling, advertising and promotion of products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability.
29
Sales of our drug candidates, if approved, will depend, in part, on the extent to which such products will be covered by third-party payors, such as government health care programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly limiting coverage or reducing reimbursements for medical products and services. In addition, the U.S. government, state legislatures and foreign governments have continued implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any drug candidates that we develop will be made on a payor-by-payor basis. Each payor determines whether or not it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and on what tier of its formulary it will be placed. The position on a payor’s list of covered drugs, or formulary, generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our drug candidates or a decision by a third-party payor to not cover our drug candidates could reduce physician usage of our drug candidates, once approved, and have a material adverse effect on our sales, results of operations and financial condition.
Other Healthcare Laws
Because of our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors, we will also be subject to healthcare regulation and enforcement by the federal government and the states and foreign governments in which we will conduct our business, including our clinical research, proposed sales, marketing and educational programs. Failure to comply with these laws, where applicable, can result in the imposition of significant civil, criminal, and administrative penalties.
The U.S. laws that may affect our ability to operate, among others, include: the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, which governs the conduct of certain electronic healthcare transactions and protects the security and privacy of protected health information; certain state laws governing the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; the federal Anti-Kickback Statute, which prohibits, among other things, individuals or entities from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as the Medicare and Medicaid programs; federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent; federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; the Physician Payments Sunshine Act, which requires manufacturers of drugs, devices, biologics, and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and ownership and investment interests held by physicians and their immediate family members.
In addition, many states have similar laws and regulations, such as anti-kickback and false claims laws that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, to the extent that our product is sold in a foreign country, we may be subject to similar foreign laws.
Healthcare Reform
Current and future legislative proposals to further reform healthcare or reduce healthcare costs may result in lower reimbursement for our products. The cost containment measures that payors and providers are instituting and the effect of any healthcare reform initiative implemented in the future could significantly reduce our revenues from the sale of our products.
For example, implementation of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively the Affordable Care Act, or the PPACA, has substantially changed healthcare financing and delivery by both governmental and private insurers, and significantly impacted the pharmaceutical industry. The PPACA, among other things, established an annual, nondeductible fee on any entity that manufactures or imports certain specified branded prescription drugs and biologic agents, revised the methodology by which rebates owed by manufacturers to the state and federal government for covered outpatient drugs under the Medicaid Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, provided incentives to programs that increase the federal government’s comparative effectiveness research and created a licensure frame work for follow-on biologic products. Since its enactment there have been judicial and Congressional challenges to certain aspects of the PPACA. Since January 2017, President
30
Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provisions of the PPACA or otherwise circumvent some of the requirements for health insurance mandated by the PPACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the PPACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the PPACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain PPACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the PPACA, effective January 1, 2019, to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. Congress could consider additional legislation to repeal or repeal and replace other elements of the PPACA. Thus, the full impact of the PPACA on our business remains unclear.
In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. In August 2011, then President Obama signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals for spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments, including the BBA, will remain in effect through 2027 unless additional Congressional action is taken. Additionally, in January 2013, then President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. More recently, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products. For example, there have been several recent Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products.
We expect that additional federal and state, as well as foreign, healthcare reform measures will be adopted in the future, any of which could result in reduced demand for our products or additional pricing pressure.
Employees
As of December 31, 2017, we had 43 full-time employees, 17 of whom were primarily engaged in research and development activities and 21 of whom had an MD or PhD degree. None of our employees is represented by a labor union and we consider our employee relations to be good.
Facilities
We lease the space for our principal executive offices, which are located at 1460 Broadway, New York, New York, on a monthly basis. We believe that our facilities are adequate to meet our current needs.
Corporate and Other Information
We were incorporated in Delaware in April 2014. Our principal executive offices are located at 1460 Broadway, Suite 15044, New York, New York 10036 and our telephone number is (646) 661-7661. Our corporate website address is www.ovidrx.com. Information contained on or accessible through our website is not a part of this Annual Report, and the inclusion of our website address in this Annual Report is an inactive textual reference only.
We file electronically with the Securities and Exchange Commission, or the SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make available on our website at www.ovidrx.com under “Investors,” free of charge, copies of these reports as soon as reasonably practicable after filing or furnishing these reports with the SEC.
31
This Annual Report on Form 10-K contains forward-looking information based on our current expectations. Because our business is subject to many risks and our actual results may differ materially from any forward-looking statements made by or on behalf of us, this section includes a discussion of important factors that could affect our business, operating results, financial condition and the trading price of our common stock. You should carefully consider these risk factors, together with all of the other information included in this Annual Report on Form 10-K as well as our other publicly available filings with the Securities and Exchange Commission.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant operating losses since inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future and may never achieve or maintain profitability.
Since inception in April 2014, we have incurred significant operating losses. Our net loss was $64.8 million for the year ended December 31, 2017. As of December 31, 2017, we had an accumulated deficit of $100.7 million. We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. Since inception, we have devoted substantially all of our efforts to research and preclinical and clinical development of our drug candidates, as well as to hiring employees and building our infrastructure. It could be several years, if ever, before we have a commercialized drug. The net losses we incur may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially if, and as, we:
|
|
• |
continue the ongoing and planned preclinical and clinical development of our drug candidates; |
|
|
• |
continue to build a portfolio of drug candidates through the acquisition or in-license of drugs, drug candidates or technologies; |
|
|
• |
initiate preclinical studies and clinical trials for any additional drug candidates that we may pursue in the future; |
|
|
• |
seek marketing approvals for our current and future drug candidates that successfully complete clinical trials; |
|
|
• |
establish a sales, marketing and distribution infrastructure to commercialize any drug candidate for which we may obtain marketing approval; |
|
|
• |
develop, maintain, expand and protect our intellectual property portfolio; |
|
|
• |
implement operational, financial and management systems; and |
|
|
• |
attract, hire and retain additional administrative, clinical, regulatory and scientific personnel. |
In addition, because of the numerous risks and uncertainties associated with pharmaceutical products and development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Our expenses could increase and profitability could be further delayed if we decide to or are required by the U.S. Food and Drug Administration, or FDA, or other regulatory authorities such as the European Medicines Agency, or EMA, to perform studies or trials in addition to those currently expected, or if there are any delays in the development, or in the completion of any planned or future preclinical studies or clinical trials of our current and future drug candidates. Even if we complete the development and regulatory processes described above, we anticipate incurring significant costs associated with launching and commercializing our current and future drug candidates.
Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, maintain our research and development efforts, expand our business or continue our operations.
We have a limited operating history and have never generated any revenue from drug sales. Our limited operating history may make it difficult to evaluate the success of our business to date and to assess our future viability.
We are a clinical-stage company founded in April 2014. Our operations have consumed substantial amounts of cash since our inception, primarily due to organizing and staffing our company, business planning, raising capital, acquiring assets and undertaking the development of OV101. We have not yet demonstrated the ability to obtain marketing approvals, manufacture a commercial-scale drug or conduct sales and marketing activities necessary for successful commercialization. Consequently, any predictions about our future success or viability may not be as accurate as they could be if we had more experience developing drug candidates.
32
Our ability to generate revenue from drug sales and achieve profitability depends on our ability, alone or with any current or future collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, our current and future drug candidates. We do not anticipate generating revenue from drug sales for the next several years, if ever. Our ability to generate revenue from drug sales depends heavily on our, or any current or future collaborators’, success in:
|
|
• |
timely and successfully completing preclinical and clinical development of our current and future drug candidates; |
|
|
• |
obtaining regulatory approvals for our current and future drug candidates for which we successfully complete clinical trials; |
|
|
• |
launching and commercializing any drug candidates for which we obtain regulatory approval by establishing a sales force, marketing and distribution infrastructure or, alternatively, collaborating with a commercialization partner; |
|
|
• |
qualifying for coverage and adequate reimbursement by government and third-party payors for any drug candidates for which we obtain regulatory approval, both in the United States and internationally; |
|
|
• |
developing, validating and maintaining a commercially viable, sustainable, scalable, reproducible and transferable manufacturing process for our current and future drug candidates that is compliant with current good manufacturing practices, or cGMP; |
|
|
• |
establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate amount and quality of drugs and services to support clinical development, as well as the market demand for our current and future drug candidates, if approved; |
|
|
• |
obtaining market acceptance, if and when approved, of our current or any future drug candidate as a viable treatment option by physicians, patients, third-party payors and others in the medical community; |
|
|
• |
effectively addressing any competing technological and market developments; |
|
|
• |
implementing additional internal systems and infrastructure, as needed; |
|
|
• |
negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations pursuant to such arrangements; |
|
|
• |
our ability to obtain and maintain orphan drug exclusivity for any of our current and future drug candidates for which we obtain regulatory approval; |
|
|
• |
maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how; |
|
|
• |
avoiding and defending against third-party interference or infringement claims; and |
|
|
• |
securing appropriate pricing in the United States, the European Union and other countries. |
We expect our financial condition and operating results to continue to fluctuate from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. We will need to eventually transition from a company with a research and development focus to a company capable of undertaking commercial activities. We may encounter unforeseen expenses, difficulties, complications and delays and may not be successful in such a transition.
We will require additional capital to finance our operations, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate certain of our drug development efforts or other operations.
Our operations have consumed substantial amounts of cash since our inception. We expect our expenses to increase in connection with our ongoing and planned activities, particularly as we continue to develop and commercialize our drug candidates, in addition to costs associated with the acquisition or in-licensing of any additional drug candidates we may pursue. Our expenses could increase beyond expectations if the FDA or other regulatory authorities require us to perform clinical and other studies in addition to those that we currently anticipate. In addition, if we obtain marketing approval for our drug candidates, we expect to incur significant expenses related to manufacturing, marketing, sales and distribution. Furthermore, we expect to incur additional costs associated with operating as a public company.
As of December 31, 2017, our cash and cash equivalents was $87.1 million. We believe that our existing cash and cash equivalents will fund our current operating plans through at least the next 12 months from the filing of this annual report on Form 10-K. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, third-party funding, marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements, or any combination of these approaches.
33
In any event, we will require more capital to pursue additional preclinical and clinical activities, regulatory approval and the commercialization of our current or future drug candidates. Even if we believe we have sufficient capital for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. Any additional capital raising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our current and future drug candidates.
If we do not raise additional capital in sufficient amounts, or on terms acceptable to us, we may be prevented from pursuing development and commercialization efforts, which will harm our business, operating results and prospects.
Raising additional capital or acquiring or licensing assets by issuing equity or debt securities may cause dilution to our stockholders, and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
Until such time as we can generate substantial revenue from drug sales, if ever, we expect to finance our cash needs through a combination of equity and debt financings, strategic alliances, and license and development agreements in connection with any collaborations. We do not have any committed external source of funds. To the extent that we issue additional equity securities, our stockholders may experience substantial dilution, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a stockholder. In addition, we may issue equity or debt securities as consideration for obtaining rights to additional compounds. For example, in our arrangement with Takeda Pharmaceutical Company Limited, or Takeda, upon the achievement of a certain development milestone, we will be obligated to issue to Takeda additional securities equal to up to 8% of our outstanding capital stock in certain situations which will dilute our stockholders. In addition, further dilution may occur if we elect to issue shares of common stock to Takeda as payment for the remaining potential global commercial and regulatory milestone payments, which aggregate to approximately $35.0 million. For more information, see “Business—License and Collaboration Agreements—License and Collaboration Agreement with Takeda.”
Debt and equity financings, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as redeeming our shares, making investments, incurring additional debt, making capital expenditures, declaring dividends or placing limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could negatively impact our ability to conduct our business. If we raise additional capital through future collaborations, strategic alliances or third-party licensing arrangements, we may have to relinquish valuable rights to our intellectual property, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us.
If we are unable to raise additional capital when needed, we may be required to delay, limit, reduce or terminate our drug development or future commercialization efforts, or grant rights to develop and market drug candidates that we would otherwise develop and market ourselves.
We may be required to make significant payments in connection with our licenses of OV101 from Lundbeck and OV935 from Takeda.
We acquired rights to OV101 pursuant to a license agreement with H. Lundbeck A/S, or Lundbeck, in March 2015, or the Lundbeck agreement. Under the Lundbeck agreement, we are subject to significant obligations, including payment obligations upon achievement of specified milestones and royalties on drug sales, as well as other material obligations. We are obligated to pay Lundbeck milestone payments up to an aggregate of $181.0 million upon the achievement of certain development, regulatory and sales milestone events. In addition, we are obligated to pay Lundbeck tiered royalties based on net sales of OV101. If these payments become due under the terms of the Lundbeck agreement, we may not have sufficient funds available to meet our obligations and our development efforts may be harmed.
We also acquired rights to OV935 pursuant to a license and collaboration agreement with Takeda, or the Takeda license agreement, in January 2017. Under the Takeda license agreement, we are obligated to pay Takeda future payments upon achievement of specified milestones. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial indications we and Takeda are focusing on pursuant to the Takeda license agreement, we are obligated to issue to Takeda the number of unregistered shares of our common stock equal to the lesser of (i) 8% of our outstanding capital stock on the issuance date or (ii) $50.0 million divided by the applicable share price, unless certain events occur. In the event such payment would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of our common stock at our election, unless certain events occur in which Takeda can require us to pay such payments in cash. If these payments become due under the terms of the Takeda license agreement and we can only pay, or choose to pay, these payments in cash, we may not have sufficient funds available to meet our obligations and our development efforts may be harmed.
34
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses since inception and do not expect to become profitable in the near future, if ever. To the extent that we continue to generate taxable losses, unused losses will carry forward to offset future taxable income, if any, until such unused losses expire. Under Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in its equity ownership by certain stockholders over a three-year period, the corporation’s ability to use its pre-change net operating loss carryforwards, or NOLs, and other pre-change tax attributes (such as research tax credits) to offset its post-change income or taxes may be limited. We may have experienced ownership changes in the past and may experience ownership changes in the future as a result of shifts in our stock ownership (some of which shifts are outside our control). As a result, if we earn net taxable income, our ability to use our pre-change NOLs to offset such taxable income will be subject to limitations. Similar provisions of state tax law may also apply to limit our use of accumulated state tax attributes. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed. As a result, even if we attain profitability, we may be unable to use a material portion of our NOLs and other tax attributes, which could negatively impact our future cash flows.
Comprehensive tax reform legislation could adversely affect our business and financial condition.
On December 22, 2017, the Tax Cuts and Jobs Act of 2017, or the Tax Act, was signed into law. The Tax Act, among other things, contains significant changes to corporate taxation, including (i) reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, (ii) limitation of the tax deduction for interest expense to 30% of adjusted earnings (except for certain small businesses), (iii) limitation of the deduction for net operating losses to 80% of current year taxable income in respect of net operating losses generated during or after 2018 and elimination of net operating loss carrybacks, (iv) one-time taxation of offshore earnings at reduced rates regardless of whether they are repatriated, (v) immediate deductions for certain new investments instead of deductions for depreciation expense over time, and (vi) modifying or repealing many business deductions and credits. Any federal net operating loss incurred in 2018 and in future years may now be carried forward indefinitely pursuant to the Tax Act. It is uncertain if and to what extent various states will conform to the newly enacted federal tax law. We will continue to examine the impact the Tax Act may have on our business.
Risks Related to the Development and Commercialization of Our Drug Candidates
Our future success is dependent on the successful clinical development, regulatory approval and commercialization of our current and future drug candidates. If we are not able to obtain required regulatory approvals, we will not be able to commercialize our drug candidates, and our ability to generate revenue will be adversely affected.
We do not have any drugs that have received regulatory approval. Our business is dependent on our ability to successfully complete preclinical and clinical development of, obtain regulatory approval for, and, if approved, successfully commercialize our current and future drug candidates in a timely manner. Activities associated with the development and commercialization of our current and future drug candidates are subject to comprehensive regulation by the FDA and other regulatory agencies in the United States and similar regulatory authorities outside the United States. Failure to obtain regulatory approval in the United States or other jurisdictions would prevent us from commercializing and marketing our current and future drug candidates.
Even if we obtain approval from the FDA and comparable foreign regulatory authorities for our current and future drug candidates, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval study or risk management requirements. If we are unable to obtain regulatory approval, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of that drug candidate or any other drug candidate that we may in-license, develop or acquire in the future.
Furthermore, even if we obtain regulatory approval for our current and future drug candidates, we will still need to develop a commercial organization, establish a commercially viable pricing structure and obtain approval for adequate reimbursement from third-party and government payors. If we are unable to successfully commercialize our current and future drug candidates, we may not be able to generate sufficient revenue to continue our business.
Because the results of preclinical studies or earlier clinical trials are not necessarily predictive of future results, our drug candidates may not have favorable results in planned or future preclinical studies or clinical trials, or may not receive regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a drug candidate. Frequently, drug candidates that have shown promising results in early clinical trials have subsequently suffered significant setbacks in later clinical trials. For instance, although OV101 was observed to have a favorable safety and oral bioavailability profile in previously conducted clinical trials in primary insomnia and in a Phase 1 study in adolescents diagnosed with Angelman syndrome or Fragile X syndrome, OV101 has not been previously tested for efficacy in patients with Angelman syndrome and Fragile X syndrome and OV935 has not been tested for efficacy in patients with rare epileptic encephalopathies and the FDA has not yet made any determination regarding safety and
35
efficacy of either OV101 or OV935 in these indications. The results from preclinical studies of OV101 and OV935 in animal models and the results from the OV101 clinical trials in primary insomnia may not be predictive of the effects of these compounds in patients with the targeted disease. Our approach of targeting the extrasynaptic GABA A receptor with OV101 and cholesterol 24-hydroxylase with OV935 are both novel and unproven and as such, the cost and time needed to develop OV101 and OV935 is difficult to predict and our efforts may not be successful. If we do not observe favorable results in clinical trials of our drug candidates, we may decide to delay or abandon clinical development of such drug candidate. Any such delay or abandonment could harm our business, financial condition, results of operations and prospects.
Risks associated with the in-licensing or acquisition of drug candidates could cause substantial delays in the preclinical and clinical development of our drug candidates.
Prior to March 2015, we had no involvement with or control over the preclinical and clinical research and development of OV101. We have relied on Lundbeck or its prior licensee to have conducted such research and development in accordance with the applicable protocol, legal, regulatory and scientific standards, having accurately reported the results of all clinical trials conducted prior to our acquisition of OV101 and having correctly collected and interpreted the data from these trials. If the research and development processes or the results of the development programs prior to our acquisition of OV101 prove to be unreliable, this could result in increased costs and delays in the development of OV101, which could adversely affect any future revenue from this drug candidate.
Similarly, we acquired rights to OV935 from Takeda in January 2017. Because we were not involved in the development of OV935 prior to January 2017, we may experience difficulties in the transition of certain development activities from Takeda and its affiliates to us, which may result in delays in clinical trials, as well as problems in our development efforts, particularly if we do not receive all of the necessary products, information, reports and data from Takeda and its affiliates in a timely manner. Further, we have had no involvement with or control over the preclinical and clinical development of OV935 to date. We have relied on Takeda having conducted such research and development in accordance with the applicable protocol, legal, regulatory and scientific standards, having accurately reported the results of all clinical trials conducted prior to our agreement with Takeda and having correctly collected and interpreted the data from these trials. To the extent any of these has not occurred, expected development time and costs may be increased which could adversely affect any future revenue from this drug candidate.
We may also acquire or in-license additional drug candidates for preclinical or clinical development in the future as we continue to build our pipeline. The risks associated with acquiring or in-licensing current or future drug candidates could result in delays in the commencement or completion of our preclinical studies and clinical trials, if ever, and our ability to generate revenues from our drug candidates may be delayed.
We may encounter substantial delays in our clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
Before obtaining marketing approval from regulatory authorities for the sale of our drug candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the drug candidate for its intended indications. Clinical trials are expensive, time-consuming and uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
|
|
• |
delays in reaching a consensus with regulatory authorities on trial design; |
|
|
• |
delays in reaching agreement on acceptable terms with prospective clinical research organizations, or CROs, and clinical trial sites; |
|
|
• |
delays in opening sites and recruiting suitable patients to participate in our clinical trials; |
|
|
• |
imposition of a clinical hold by regulatory authorities as a result of a serious adverse event, concerns with a class of drug candidates or after an inspection of our clinical trial operations or trial sites; |
|
|
• |
delays in having patients complete participation in a trial or return for post-treatment follow-up; |
|
|
• |
occurrence of serious adverse events associated with the drug candidate that are viewed to outweigh its potential benefits; or |
|
|
• |
changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
Further, clinical endpoints for certain diseases we are targeting, such as Angelman syndrome and Fragile X syndrome, have not been established, and accordingly we may have to develop new modalities or modify existing endpoints to measure efficacy, which may increase the time it takes for us to commence or complete clinical trials. In addition, we believe investigators in this area may be inexperienced in conducting trials in this area due to the current lack of drugs to treat these disorders, which may result in increased time and expense to train investigators and open clinical sites.
36
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue from future drug sales and regulatory and commercialization milestones. In addition, if we make manufacturing or formulation changes to our drug candidates, we may need to conduct additional testing to bridge our modified drug candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our drug candidates, if approved, or allow our competitors to bring comparable drugs to market before we do, which could impair our ability to successfully commercialize our drug candidates and may harm our business, financial condition, results of operations and prospects.
Additionally, if the results of our clinical trials are inconclusive or if there are safety concerns or serious adverse events associated with our drug candidates, we may:
|
|
• |
be delayed in obtaining marketing approval, if at all; |
|
|
• |
obtain approval for indications or patient populations that are not as broad as intended or desired; |
|
|
• |
obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
|
|
• |
be subject to additional post-marketing testing requirements; |
|
|
• |
be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements; |
|
|
• |
have regulatory authorities withdraw, or suspend, their approval of the drug or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy, or REMS; |
|
|
• |
be subject to the addition of labeling statements, such as warnings or contraindications; |
|
|
• |
be sued; or |
|
|
• |
experience damage to our reputation. |
Our drug development costs will also increase if we experience delays in testing or obtaining marketing approvals. We do not know whether any of our preclinical studies or clinical trials will begin as planned, need to be restructured or be completed on schedule, if at all.
Further, we, the FDA or an institutional review board, or IRB, may suspend our clinical trials at any time if it appears that we or our collaborators are failing to conduct a trial in accordance with regulatory requirements, including the FDA’s current Good Clinical Practice, or GCP, regulations, that we are exposing participants to unacceptable health risks, or if the FDA finds deficiencies in our investigational new drug, or IND, applications or the conduct of these trials. Therefore, we cannot predict with any certainty the schedule for commencement and completion of future clinical trials. If we experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our drug candidates could be negatively impacted, and our ability to generate revenues from our drug candidates may be delayed.
Angelman syndrome has no FDA-approved treatments, and the clinical endpoints to obtain approval are not well defined.
We intend to seek a broad indication for OV101 to treat Angelman syndrome. However, Angelman syndrome is characterized by a variety of signs and symptoms, such as delayed development, intellectual disability, severe speech impairment, problems with movement and balance, seizures, sleep disorders and anxiety. In order to obtain a broad indication for treatment of Angelman syndrome from the FDA, we would need to demonstrate efficacy on several of the key symptoms of Angelman syndrome. If we fail to do so, our clinical development may be delayed or our label may be limited. In addition, the FDA has not endorsed any primary efficacy endpoints with respect to development of drugs to treat Angelman syndrome. As a result, we must develop acceptable endpoints and seek the FDA’s agreement before seeking approval of OV101. If we fail to reach such an agreement with the FDA as to how to measure efficacy in Angelman syndrome patients in our trials, our clinical development plan will be delayed.
Our primary endpoint in the Phase 2 trial of OV101 in adults and adolescents with Angelman syndrome is safety and tolerability. While we are also evaluating indications of efficacy as exploratory endpoints, this is primarily a safety trial that is designed to provide a proof-of-concept on the efficacy parameters. Hence, we do not know whether we will be able to obtain a statistically significant result in any of these exploratory endpoints.
While our OV101 Phase 2 trial remains on track to generate data in 2018, we may have difficulty demonstrating efficacy in this patient population.
The FDA has requested that we obtain certain pharmacokinetic and tolerability data in adolescents prior to enrolling them in our clinical trials. Therefore, we are currently conducting a Phase 2 trial of OV101 initially in adults over the age of 18 with Angelman syndrome and in parallel conducted a Phase 1 trial evaluating pharmacokinetic, or PK and tolerability of OV101 in adolescents with Angelman syndrome or Fragile X syndrome.
37
After the completion of the Phase 1 trial in adolescents, which showed that PK parameters in adolescents with Angelman and Fragile X syndrome were not significantly different from previous data generated in young adults, we subsequently amended the Phase 2 adult trial to include both adults and adolescents. We expect to maintain our previously disclosed plan to release data from this trial in 2018. Genetic testing for Angelman syndrome is fairly new, and most patients who have been conclusively tested for Angelman syndrome are young. Because older patients often do not undergo genetic testing since there are currently no approved therapies for this disorder, we believe that many adult Angelman syndrome patients have not received a confirmed diagnosis of Angelman syndrome.
In addition, certain aspects of Angelman syndrome, such as sleep disturbances, may change with age. As a result, demonstrating a statistically significant and clinical meaningful effect in adults with respect to these symptoms may be more difficult or may require more patients than demonstrating an effect in adolescents or pediatric patients.
We must develop a new liquid pediatric formulation of OV101 for use in young children initially, and eventually for infants and toddlers, and we may be unable to successfully develop an appropriate formulation.
Our existing formulation of OV101 is an oral capsule. We have recently developed lower strength capsules that can be opened and sprinkled on applesauce or similar semi-solid foods. However, for use in very young pediatric patients, we will need to develop an oral liquid formulation of OV101. While we have begun developing this formulation, we do not know if our efforts will be successful or if the FDA will agree that the new formulation is comparable to our current formulation. We may experience manufacturing problems such as with solubility or stability or we may discover that the new formulation is less effective than an oral capsule. In addition, we will need to conduct bridging studies to demonstrate that the new formulation is equivalent to our oral capsule, which could result in delays in development and additional costs.
We may not be able to obtain or maintain orphan drug designations or exclusivity for our drug candidates, which could limit the potential profitability of our drug candidates.
Regulatory authorities in some jurisdictions, including the United States, may designate drugs for relatively small patient populations as orphan drugs. Under the Orphan Drug Act of 1983, the FDA may designate a drug as an orphan drug if it is a drug intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals in the United States. Generally, if a drug with an orphan drug designation subsequently receives the first marketing approval for an indication for which it receives the designation, then the drug is entitled to a period of marketing exclusivity that precludes the applicable regulatory authority from approving another marketing application for the same drug for the same indication for the exclusivity period except in limited situations. For purposes of small molecule drugs, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the drug in question. A designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation.
For OV101, the FDA granted orphan drug designation for OV101 for the treatment of Angelman syndrome and for the treatment of Fragile X syndrome in September 2016 and October 2017, respectively, and fast track designation for the treatment of Angelman Syndrome and Fragile X syndrome in December 2017 and March 2018, respectively. The FDA granted orphan drug designation for OV935 for the treatment of Dravet syndrome and Lennox-Gastaut syndrome both in December 2017. We intend to pursue orphan drug designation for OV101 in additional indications, as well as for OV935 and potential other future drug candidates. Obtaining orphan drug designations is important to our business strategy; however, obtaining an orphan drug designation can be difficult and we may not be successful in doing so. Even if we were to obtain orphan drug designation for a drug candidate, we may not obtain orphan exclusivity and that exclusivity may not effectively protect the drug from the competition of different drugs for the same condition, which could be approved during the exclusivity period. Additionally, after an orphan drug is approved, the FDA could subsequently approve another application for the same drug for the same indication if the FDA concludes that the later drug is shown to be safer, more effective or makes a major contribution to patient care. Orphan drug exclusive marketing rights in the United States also may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition. The failure to obtain an orphan drug designation for any drug candidates we may develop, the inability to maintain that designation for the duration of the applicable period, or the inability to obtain or maintain orphan drug exclusivity could reduce our ability to make sufficient sales of the applicable drug candidate to balance our expenses incurred to develop it, which would have a negative impact on our operational results and financial condition.
38
If we are not successful in discovering, developing and commercializing additional drug candidates, our ability to expand our business and achieve our strategic objectives would be impaired.
A key element of our strategy is to develop and potentially commercialize a portfolio of drug candidates to treat rare neurological disorders. We intend to do so by in-licensing and entering into collaborations with leading biopharmaceutical companies or academic institutions for new drug candidates. Identifying new drug candidates requires substantial technical, financial and human resources, whether or not any drug candidates are ultimately identified. Our approach to business development, including our efforts to map the biological pathways related to orphan disorders of the brain and our relationships among the pharmaceutical industry, may not result in viable drug candidates for clinical development. Even if we identify drug candidates that initially show promise, we may fail to in-license or acquire these assets and may also fail to successfully develop and commercialize such drug candidates for many reasons, including the following:
|
|
• |
the research methodology used may not be successful in identifying potential drug candidates; |
|
|
• |
competitors may develop alternatives that render any drug candidate we develop obsolete; |
|
|
• |
any drug candidate we develop may nevertheless be covered by third parties’ patents or other exclusive rights; |
|
|
• |
a drug candidate may, on further study, be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise does not meet applicable regulatory criteria; |
|
|
• |
a drug candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
|
|
• |
a drug candidate may not be accepted as safe and effective by physicians, patients, the medical community or third-party payors. |
We have limited financial and management resources and, as a result, we may forego or delay pursuit of opportunities with other drug candidates or for other indications that later prove to have greater market potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial drugs or profitable market opportunities. If we do not accurately evaluate the commercial potential or target market for a particular drug candidate, we may relinquish valuable rights to that drug candidate through collaboration, licensing or other royalty arrangements in circumstances under which it would have been more advantageous for us to retain sole development and commercialization rights to such drug candidate.
If we are unsuccessful in identifying and developing additional drug candidates or are unable to do so, our key growth strategy and business will be harmed.
We are heavily dependent on our relationship with Takeda for the development and commercialization of OV935. Any disruption in our relationship with Takeda could lead to delays in, or the termination of, the development of OV935, which would materially harm our business.
We are jointly developing OV935 with Takeda pursuant to the Takeda license agreement, which also granted us intellectual property rights to OV935. The development and commercialization of OV935 is highly dependent upon our relationship with Takeda, including Takeda’s submission of the IND to the FDA. If for any reason the Takeda license agreement is terminated, or we otherwise lose the intellectual property rights to OV935, our business would be adversely affected. The Takeda license agreement imposes on us rights and obligations, including but not limited to exclusivity, territorial rights, development, commercialization, funding, payment, diligence, sublicensing, insurance and intellectual property protection. After a negotiated time period, each party has the right to terminate the license for convenience upon six to twelve months’ notice to the other party, which would result in us being unable to co-develop and sell OV935. Further, if we breach any material obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages to Takeda, and Takeda may have the right to terminate the license. Takeda could also breach its obligations under the agreement, or may not commit a sufficient amount of resources to satisfy its obligations, which would result in the development of OV935 being materially delayed or terminated.
We may explore additional strategic collaborations that may never materialize or may fail.
Our business strategy is based on acquiring or in-licensing compounds directed at rare neurological disorders. As a result, we intend to periodically explore a variety of possible additional strategic collaborations in an effort to gain access to additional drug candidates or resources. At the current time, we cannot predict what form such a strategic collaboration might take. We are likely to face significant competition in seeking appropriate strategic collaborators, and strategic collaborations can be complicated and time consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations because of the numerous risks and uncertainties associated with establishing them.
39
Clinical trials are very expensive, time-consuming and difficult to design and implement.
Our drug candidates will require clinical testing before we are prepared to submit a new drug application, or NDA, for regulatory approval. We cannot predict with any certainty if or when we might submit an NDA for regulatory approval for any of our drug candidates or whether any such NDA will be approved by the FDA. Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. For instance, the FDA may not agree with our proposed endpoints for any future clinical trial of our drug candidates, which may delay the commencement of our clinical trials. In addition, we may not succeed in developing and validating disease-relevant clinical endpoints based on insights regarding biological pathways for the disorders we are studying. The clinical trial process is also time-consuming. We estimate that the successful completion of clinical trials of our drug candidates will take at least several years to complete, if not longer. Furthermore, failure can occur at any stage and we could encounter problems that cause us to abandon or repeat clinical trials.
Enrollment and retention of patients in clinical trials is an expensive and time-consuming process and could be made more difficult or rendered impossible by multiple factors outside our control.
Identifying and qualifying patients to participate in our clinical trials is critical to our success. The number of patients suffering from Angelman syndrome, Fragile X syndrome and rare epileptic encephalopathies, such as Dravet syndrome, Lennox-Gastaut syndrome and Tuberous Sclerosis Complex, is small and has not been established with precision. If the actual number of patients with these disorders is smaller than we anticipate, we may encounter difficulties in enrolling patients in our clinical trials, thereby delaying or preventing development and approval of our drug candidates. Even once enrolled we may be unable to retain a sufficient number of patients to complete any of our trials. Patient enrollment and retention in clinical trials depends on many factors, including the size of the patient population, the nature of the trial protocol, the existing body of safety and efficacy data, the number and nature of competing treatments and ongoing clinical trials of competing therapies for the same indication, the proximity of patients to clinical sites and the eligibility criteria for the trial. Because we are focused on addressing rare neurological disorders, there are limited patient pools from which to draw in order to complete our clinical trials in a timely and cost-effective manner. Furthermore, our efforts to build relationships with patient communities may not succeed, which could result in delays in patient enrollment in our clinical trials. In addition, any negative results we may report in clinical trials of our drug candidate may make it difficult or impossible to recruit and retain patients in other clinical trials of that same drug candidate. Delays or failures in planned patient enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our drug candidates, or could render further development impossible. In addition, we may rely on CROs and clinical trial sites to ensure proper and timely conduct of our future clinical trials and, while we intend to enter into agreements governing their services, we will be limited in our ability to compel their actual performance.
Our drug candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial potential or result in significant negative consequences following any potential marketing approval.
During the conduct of clinical trials, patients report changes in their health, including illnesses, injuries and discomforts, to their doctor. Often, it is not possible to determine whether or not the drug candidate being studied caused these conditions. Regulatory authorities may draw different conclusions or require additional testing to confirm these determinations, if they occur. In addition, it is possible that as we test our drug candidates in larger, longer and more extensive clinical programs, or as use of these drug candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts and other adverse events that were observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by subjects. Many times, side effects are only detectable after investigational drugs are tested in large-scale, Phase 3 trials or, in some cases, after they are made available to patients on a commercial scale after approval. For example, in one of the trials conducted by Lundbeck, there were reports of hallucinations in drug abusers at 30mg and 45mg doses of OV101, which are higher than the 10mg and 15mg doses that were effective for insomnia. In addition, some patients treated with OV101 in the Lundbeck Phase 3 trials experienced headaches, nausea and dizziness. Patients in our ongoing or planned clinical trials may experience similar or other side effects after treatment with OV101. If additional clinical experience indicates that any of our current drug candidates, including OV101 and OV935, and any future drug candidates has side effects or causes serious or life-threatening side effects, the development of the drug candidate may fail or be delayed, or, if the drug candidate has received regulatory approval, such approval may be revoked, which would harm our business, prospects, operating results and financial condition.
Moreover, if we elect, or are required, to delay, suspend or terminate any clinical trial of our drug candidates, the commercial prospects of our drug candidates may be harmed and our ability to generate revenue through their sale may be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly. Additionally, if any of our drug candidates receive marketing approval, the FDA could require us to include a black box warning in our label or adopt REMS to ensure that the benefits outweigh its risks, which may include, among other things, a medication guide outlining the risks of the drug for distribution to patients and a communication plan to health care practitioners. Furthermore, if we or others later identify undesirable side effects caused by our drug candidates, several potentially significant negative consequences could result, including:
|
|
• |
regulatory authorities may suspend or withdraw approvals of such drug candidate; |
|
|
• |
regulatory authorities may require additional warnings on the label; |
|
|
• |
we may be required to change the way a drug candidate is administered or conduct additional clinical trials; |
40
|
|
• |
we may need to conduct a recall; and |
|
|
• |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our drug candidates and could significantly harm our business, prospects, financial condition and results of operations.
We may be required to relinquish important rights to and control over the development and commercialization of our drug candidates to any future collaborators.
Our current and future collaborations could subject us to a number of risks, including:
|
|
• |
we may be required to undertake the expenditure of substantial operational, financial and management resources; |
|
|
• |
we may be required to issue equity securities that would dilute our stockholders’ percentage of ownership; |
|
|
• |
we may be required to assume substantial actual or contingent liabilities; |
|
|
• |
we may not be able to control the amount and timing of resources that our strategic collaborators devote to the development or commercialization of our drug candidates; |
|
|
• |
strategic collaborators may delay clinical trials, provide insufficient funding, terminate a clinical trial or abandon a drug candidate, repeat or conduct new clinical trials or require a new version of a drug candidate for clinical testing; |
|
|
• |
strategic collaborators may not pursue further development and commercialization of products resulting from the strategic collaboration arrangement or may elect to discontinue research and development programs; |
|
|
• |
strategic collaborators may not commit adequate resources to the marketing and distribution of our drug candidates, limiting our potential revenues from these products; |
|
|
• |
we rely on our current collaborators to manufacture drug substance and drug product and may do so with respect to future collaborators, which could result in disputes or delays; |
|
|
• |
disputes may arise between us and our strategic collaborators that result in the delay or termination of the research, development or commercialization of our drug candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources; |
|
|
• |
strategic collaborators may experience financial difficulties; |
|
|
• |
strategic collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
|
|
• |
business combinations or significant changes in a strategic collaborator’s business strategy may also adversely affect a strategic collaborator’s willingness or ability to complete its obligations under any arrangement; |
|
|
• |
strategic collaborators could decide to move forward with a competing drug candidate developed either independently or in collaboration with others, including our competitors; and |
|
|
• |
strategic collaborators could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing our drug candidates. |
If the market opportunities for our drug candidates are smaller than we believe they are, even assuming approval of a drug candidate, our business may suffer. Because the patient populations in the market for our drug candidates may be small, we must be able to successfully identify patients and acquire a significant market share to achieve profitability and growth.
We focus our research and drug development on treatments for rare neurological disorders. Given the small number of patients who have the disorders that we are targeting, our eligible patient population and pricing estimates may differ significantly from the actual market addressable by our drug candidates. Our projections of both the number of people who have these disorders, as well as the subset of people with these disorders who have the potential to benefit from treatment with our drug candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including the scientific literature, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these disorders. The number of patients may turn out to be lower than expected. Likewise, the potentially addressable patient population for each of our drug candidates may be limited or may not be amenable to treatment with our drug candidates, and new patients may become increasingly difficult to identify or gain access to, which would adversely affect our results of operations and our business.
41
We face substantial competition, which may result in others developing or commercializing drugs before or more successfully than us.
The development and commercialization of new drugs is highly competitive. We face competition with respect to our current drug candidates and will face competition with respect to any other drug candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are a number of large pharmaceutical and biotechnology companies that currently market and sell drugs or are pursuing the development of drug candidates for the treatment of the indications that we are pursuing. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
More established companies may have a competitive advantage over us due to their greater size, resources and institutional experience. In particular, these companies have greater experience and expertise in securing reimbursement, government contracts, relationships with key opinion leaders, conducting testing and clinical trials, obtaining and maintaining regulatory approvals and distribution relationships to market products, and marketing approved drugs. These companies also have significantly greater research and marketing capabilities than we do. If we are not able to compete effectively against existing and potential competitors, our business and financial condition may be harmed.
As a result of these factors, our competitors may obtain regulatory approval of their drugs before we are able to, which may limit our ability to develop or commercialize our drug candidates. Our competitors may also develop therapies that are safer, more effective, more widely accepted and cheaper than ours, and may also be more successful than us in manufacturing and marketing their drugs. These appreciable advantages could render our drug candidates obsolete or non-competitive before we can recover the expenses of such drug candidates’ development and commercialization.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Even if our current or future drug candidates receive marketing approval, they may fail to achieve market acceptance by physicians, patients, third-party payors or others in the medical community necessary for commercial success.
Even if our current or future drug candidates receive marketing approval, they may fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. If they do not achieve an adequate level of acceptance, we may not generate significant drug revenue and may not become profitable. The degree of market acceptance of our current or future drug candidates, if approved for commercial sale, will depend on a number of factors, including but not limited to:
|
|
• |
the efficacy and potential advantages compared to alternative treatments and therapies; |
|
|
• |
effectiveness of sales and marketing efforts; |
|
|
• |
the strength of our relationships with patient communities; |
|
|
• |
the cost of treatment in relation to alternative treatments and therapies, including any similar generic treatments; |
|
|
• |
our ability to offer such drug for sale at competitive prices; |
|
|
• |
the convenience and ease of administration compared to alternative treatments and therapies; |
|
|
• |
the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
|
|
• |
the strength of marketing and distribution support; |
|
|
• |
the availability of third-party coverage and adequate reimbursement; |
|
|
• |
the prevalence and severity of any side effects; and |
|
|
• |
any restrictions on the use of the drug together with other medications. |
Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of our drug candidates may require significant resources and may never be successful. Such efforts may require more resources than are typically required due to the complexity and uniqueness of our drug candidates. Because we expect sales of our drug candidates, if approved, to generate substantially all of our drug revenues for the foreseeable future, the failure of our drugs to find market acceptance would harm our business and could require us to seek additional financing.
42
Even if we obtain regulatory approval for our current or future drug candidates, they will remain subject to ongoing regulatory oversight.
Even if we obtain any regulatory approval for our current or future drug candidates, such approvals will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record-keeping and submission of safety and other post-market information. Any regulatory approvals that we receive for our current or future drug candidates may also be subject to a REMS, limitations on the approved indicated uses for which the drug may be marketed or to the conditions of approval, or contain requirements for potentially costly post-marketing testing, including Phase 4 trials, and surveillance to monitor the quality, safety and efficacy of the drug.
In addition, drug manufacturers and their facilities are subject to payment of user fees and continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP requirements and adherence to commitments made in the NDA or foreign marketing application. If we, or a regulatory authority, discover previously unknown problems with a drug, such as adverse events of unanticipated severity or frequency, or problems with the facility where the drug is manufactured or if a regulatory authority disagrees with the promotion, marketing or labeling of that drug, a regulatory authority may impose restrictions relative to that drug, the manufacturing facility or us, including requesting a recall or requiring withdrawal of the drug from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of our current or future drug candidates, a regulatory authority may:
|
|
• |
issue an untitled letter or warning letter asserting that we are in violation of the law; |
|
|
• |
seek an injunction or impose administrative, civil or criminal penalties or monetary fines; |
|
|
• |
suspend or withdraw regulatory approval; |
|
|
• |
suspend any ongoing clinical trials; |
|
|
• |
refuse to approve a pending NDA or comparable foreign marketing application (or any supplements thereto) submitted by us or our strategic partners; |
|
|
• |
restrict the marketing or manufacturing of the drug; |
|
|
• |
seize or detain the drug or otherwise require the withdrawal of the drug from the market; |
|
|
• |
refuse to permit the import or export of drug candidates; or |
|
|
• |
refuse to allow us to enter into supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our current or future drug candidates and harm our business, financial condition, results of operations and prospects.
In addition, the FDA’s policies, and those of equivalent foreign regulatory agencies, may change and additional government regulations may be enacted that could cause changes to or delays in the drug review process, or suspend or restrict regulatory approval of our drug candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability, which would harm our business, financial condition, results of operations and prospects.
If we are unable to establish sales and marketing capabilities, or enter into agreements with third parties to market and sell our current or any future drug candidates, we may be unable to generate any revenue from drug sales.
To successfully commercialize any drug candidate that may result from our development programs, we will need to build out our sales and marketing capabilities, either on our own or with others. The establishment and development of our own commercial team or the establishment of a contract sales force to market any drug candidate we may develop will be expensive and time-consuming and could delay any drug launch. Moreover, we cannot be certain that we will be able to successfully develop this capability. We may seek to enter into additional collaborations with other entities to utilize their established marketing and distribution capabilities, but we may be unable to enter into such agreements on favorable terms, if at all. If any current or future collaborators do not commit sufficient resources to commercialize our drug candidates, or we are unable to develop the necessary capabilities on our own, we will be unable to generate sufficient revenue to sustain our business. We compete with many companies that currently have extensive, experienced and well-funded marketing and sales operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our current and future drug candidates. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
43
Even if we obtain and maintain approval for our current or future drug candidates from the FDA, we may never obtain approval for our current or future drug candidates outside of the United States, which would limit our market opportunities and could harm our business.
Approval of a drug candidate in the United States by the FDA does not ensure approval of such drug candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our current and future drug candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a drug candidate, comparable regulatory authorities of foreign countries also must approve the manufacturing and marketing of the drug candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and more onerous than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a drug candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for any drug candidates, if approved, is also subject to approval. Obtaining approval for our current and future drug candidates in the European Union from the European Commission following the opinion of the EMA, if we choose to submit a marketing authorization application there, would be a lengthy and expensive process. Even if a drug candidate is approved, the FDA or the European Commission, as the case may be, may limit the indications for which the drug may be marketed, require extensive warnings on the drug labeling or require expensive and time-consuming additional clinical trials or reporting as conditions of approval. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our current and future drug candidates in certain countries.
Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Also, regulatory approval for our drug candidates may be withdrawn. If we fail to comply with the regulatory requirements, our target market will be reduced and our ability to realize the full market potential of our current and future drug candidates will be harmed and our business, financial condition, results of operations and prospects could be harmed.
If we seek approval to commercialize our current or future drug candidates outside of the United States, in particular in the European Union and Israel, a variety of risks associated with international operations could harm our business.
If we seek approval of our current or future drug candidates outside of the United States, we expect that we will be subject to additional risks in commercialization including:
|
|
• |
different regulatory requirements for approval of therapies in foreign countries; |
|
|
• |
reduced protection for intellectual property rights; |
|
|
• |
unexpected changes in tariffs, trade barriers and regulatory requirements; |
|
|
• |
economic weakness, including inflation, or political instability in particular foreign economies and markets; |
|
|
• |
compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
|
|
• |
foreign currency fluctuations, which could result in increased operating expenses and reduced revenues, and other obligations incident to doing business in another country; |
|
|
• |
foreign reimbursement, pricing and insurance regimes; |
|
|
• |
workforce uncertainty in countries where labor unrest is more common than in the United States; |
|
|
• |
production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
|
|
• |
business interruptions resulting from geopolitical actions, including war and terrorism or natural disasters including earthquakes, typhoons, floods and fires. |
We have no prior experience in these areas. In addition, there are complex regulatory, tax, labor and other legal requirements imposed by both the European Union, Israel and many of the individual countries in and outside of Europe with which we will need to comply. Many biopharmaceutical companies have found the process of marketing their own products in foreign countries to be very challenging.
44
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of any drug candidate that we may develop.
We face an inherent risk of product liability exposure related to the testing of our current and any future drug candidates in clinical trials and may face an even greater risk if we commercialize any drug candidate that we may develop. If we cannot successfully defend ourselves against claims that any such drug candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
|
|
• |
decreased demand for any drug candidate that we may develop; |
|
|
• |
loss of revenue; |
|
|
• |
substantial monetary awards to trial participants or patients; |
|
|
• |
significant time and costs to defend the related litigation; |
|
|
• |
withdrawal of clinical trial participants; |
|
|
• |
the inability to commercialize any drug candidate that we may develop; and |
|
|
• |
injury to our reputation and significant negative media attention. |
Although we maintain product liability insurance coverage, such insurance may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage each time we commence a clinical trial and if we successfully commercialize any drug candidate. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Regulatory Compliance
Our relationships with customers, physicians, and third-party payors will be subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any drug candidates for which we obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors may subject us to various federal and state fraud and abuse laws and other health care laws, including, without limitation, the federal Anti-Kickback Statute, the federal civil and criminal false claims laws and the law commonly referred to as the Physician Payments Sunshine Act and regulations. These laws will impact, among other things, our clinical research, proposed sales, marketing and educational programs. In addition, we may be subject to patient privacy laws by both the federal government and the states in which we conduct or may conduct our business. The laws that will affect our operations include, but are not limited to:
|
|
• |
the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, in return for the purchase, recommendation, leasing or furnishing of an item or service reimbursable under a federal healthcare program, such as the Medicare and Medicaid programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers and formulary managers on the other. The Patient Protection and Affordable Care Act, as amended, or the PPACA, amended the intent requirement of the federal Anti-Kickback Statute. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it; |
|
|
• |
federal civil and criminal false claims laws, including, without limitation, the False Claims Act, and civil monetary penalty laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid or other government payors that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. The PPACA provides, and recent government cases against pharmaceutical and medical device manufacturers support, the view that federal Anti-Kickback Statute violations and certain marketing practices, including off-label promotion, may implicate the False Claims Act; |
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit a person from knowingly and willfully executing a scheme or making false or fraudulent statements to defraud any healthcare benefit program, regardless of the payor (e.g., public or private); |
|
|
• |
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization on health plans, health care clearinghouses and certain health care providers, and their respective business associates that perform certain services involving the use or disclosure of individually identifiable health information; |
45
|
|
• |
state and foreign law equivalents of each of the above federal laws, state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, and state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or to adopt compliance programs as prescribed by state laws and regulations, or that otherwise restrict payments that may be made to healthcare providers; and |
|
|
• |
state and foreign laws that govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Because of the breadth of these laws and the narrowness of the statutory exceptions and regulatory safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws.
It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws and the curtailment or restructuring of our operations.
The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
Coverage and adequate reimbursement may not be available for our current or any future drug candidates, which could make it difficult for us to sell profitably, if approved.
Market acceptance and sales of any drug candidates that we commercialize, if approved, will depend in part on the extent to which reimbursement for these drugs and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations and other private health insurers. Third-party payors decide which therapies they will pay for and establish reimbursement levels. Third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any drug candidates that we develop will be made on a payor-by-payor basis. One payor’s determination to provide coverage for a drug does not assure that other payors will also provide coverage, and adequate reimbursement, for the drug. Additionally, a third-party payor’s decision to provide coverage for a therapy does not imply that an adequate reimbursement rate will be approved. Each payor determines whether or not it will provide coverage for a therapy, what amount it will pay the manufacturer for the therapy, and on what tier of its formulary it will be placed. The position on a payor’s list of covered drugs, or formulary, generally determines the co-payment that a patient will need to make to obtain the therapy and can strongly influence the adoption of such therapy by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our drugs unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our drugs.
A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any drug that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any drug for which we obtain marketing approval. If coverage and adequate reimbursement are not available, or are available only to limited levels, we may not be able to successfully commercialize our current and any future drug candidates that we develop.
46
Healthcare legislative reform measures may have a negative impact on our business and results of operations.
In the United States and some foreign jurisdictions, there have been, and continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of drug candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any drug candidates for which we obtain marketing approval.
Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the PPACA was passed, which substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The PPACA, among other things: (i) addresses a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; (ii) increases the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extends the rebate program to individuals enrolled in Medicaid managed care organizations; (iii) establishes annual fees and taxes on manufacturers of certain branded prescription drugs; (iv) expands the availability of lower pricing under the 340B drug pricing program by adding new entities to the program; and (v) establishes a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% (and 70% commencing January 1, 2019) point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D. Additionally, in the United States, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated approval pathway for biologic drugs that are demonstrated to be “biosimilar or interchangeable” with an FDA-approved biologic drug. This new pathway could allow competitors to reference data from biologic drugs already approved after 12 years from the time of approval. This could expose us to potential competition by lower-cost biosimilars even if we commercialize a biologic drug candidate faster than our competitors. Some of the provisions of the PPACA have yet to be fully implemented, while certain provisions have been subject to judicial and Congressional challenges, as well as recent efforts by the Trump administration to repeal or replace certain aspects of the PPACA. Since January 2017, President Trump has signed two Executive Orders and other directives designed to delay the implementation of certain provisions of the PPACA or otherwise circumvent some of the requirements for health insurance mandated by the PPACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the PPACA. While Congress has not passed comprehensive repeal legislation, two bills affecting the implementation of certain taxes under the PPACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision repealing, effective January 1, 2019, the tax-based shared responsibility payment imposed by the PPACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain PPACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, or the BBA, among other things, amends the PPACA, effective January 1, 2019, to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole”. Congress may consider additional legislation to repeal or repeal and replace other elements of the PPACA. We continue to evaluate the effect that the PPACA and its possible repeal and replacement has on our business.
Other legislative changes have been proposed and adopted since the PPACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013, and due to subsequent legislative amendments to the statute, including the BBA, will remain in effect through 2027 unless additional Congressional action is taken. The American Taxpayer Relief Act of 2012, among other things, further reduced Medicare payments to several providers, including hospitals and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could have an adverse effect on customers for our drug candidates, if approved, and, accordingly, our financial operations.
Additional changes that may affect our business include the expansion of new programs such as Medicare payment for performance initiatives for physicians under the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, which will be fully implemented in 2019. At this time, it is unclear how the introduction of the Medicare quality payment program will impact overall physician reimbursement. Also, there has been heightened governmental scrutiny recently over the manner in which drug manufacturers set prices for their marketed products, which have resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the federal level, the Trump administration’s budget proposal for fiscal year 2019 contains further drug price control measures that could be enacted during the 2019 budget process or in other future legislation, including, for example, measures to permit Medicare Part D plans to negotiate the price of certain drugs under Medicare Part B, to allow some states to negotiate drug prices under Medicaid, and to eliminate cost sharing for generic drugs for low-income patients. While any proposed measures will require authorization through additional legislation to become effective, Congress and the Trump administration indicated that they will continue to seek new legislative and/or administrative measures to control drug costs. At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, legislation designed to encourage importation from other countries and bulk purchasing.
47
We expect that these and other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved drug. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our drugs.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our current or any future drug candidates, or if the scope of the patent protection obtained is not sufficiently broad, we may not be able to compete effectively in our markets.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our development programs and drug candidates. Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our current and any future drug candidates. We seek to protect our proprietary position by filing patent applications in the United States and abroad related to our current and future development programs and drug candidates. The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
Pursuant to the Lundbeck Agreement, we obtained an exclusive, worldwide license to develop, manufacture and commercialize OV101 for the treatment of human disease. However, the Lundbeck Agreement permits Lundbeck and certain other entities to manufacture and research OV101 and, in certain situations, to perform additional non-commercial activities involving OV101, all of which could result in new patentable inventions concerning the manufacture or use of OV101. While the Lundbeck Agreement prohibits Lundbeck from filing certain patent applications regarding OV101 and obligates Lundbeck to include certain newly filed patents in the license granted to us, if new patents issue that cover valuable methods for making or using OV101, we would be prohibited from employing such methods to manufacture or use OV101 unless we obtain a license to such patents.
It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our current or any future drug candidates in the United States or in other foreign countries. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our current or any future drug candidates, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, invalidated, or held unenforceable. Any successful opposition to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any drug candidates or companion diagnostic that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a drug candidate and companion diagnostic under patent protection could be reduced.
If the patent applications we hold or have in-licensed with respect to our development programs and drug candidates fail to issue, if their breadth or strength of protection is threatened, or if they fail to provide meaningful exclusivity for our current or any future drug candidates, it could dissuade companies from collaborating with us to develop drug candidates and threaten our ability to commercialize, future drugs. Any such outcome could have a negative effect on our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than United States law does. Publications of discoveries in scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our owned or licensed patents or pending patent applications, or that we were the first to file for patent protection of such inventions. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or drugs, in whole or in part, or which effectively prevent others from commercializing competitive technologies and drugs. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On December 16, 2011, the Leahy-Smith America Invents Act, (the “Leahy-Smith Act”), was signed into law. The Leahy-Smith Act includes a number of significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The United States Patent Office recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could harm our business and financial condition.
48
Moreover, we may be subject to a third-party pre-issuance submission of prior art to the U.S. Patent and Trademark Office, (the “USPTO”), or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or drugs and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize drugs without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future drug candidates.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and drugs, or limit the duration of the patent protection of our technology and drugs. Moreover, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years from the earliest filing date of a non-provisional patent application. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Without patent protection for our current or future drug candidates, we may be open to competition from generic versions of such drugs. Given the amount of time required for the development, testing and regulatory review of new drug candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing drugs similar or identical to ours.
We may be unable to prevent third parties from selling, making, promoting, manufacturing, or distributing alternative polymorphic forms of OV101.
We currently have issued patents directed to polymorphic forms of OV101. These patents would not prevent a third-party from creating, making and marketing alternative polymorphic forms that fall outside the scope of these patent claims. There can be no assurance that any such alternative polymorphic forms will not be therapeutically equivalent and/or commercially feasible. In the event an alternative polymorphic form of OV101 is developed and approved for use in indications that we may seek approval for, the marketability and commercial success of OV101, if approved, could be materially harmed.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or applications will be due to be paid to the USPTO and various government patent agencies outside of the United States over the lifetime of our owned and licensed patents and/or applications and any patent rights we may own or license in the future. We rely on our outside counsel or our licensing partners to pay these fees due to non-U.S. patent agencies. The USPTO and various non-U.S. government patent agencies require compliance with several procedural, documentary, fee payment and other similar provisions during the patent application process. We employ reputable law firms and other professionals to help us comply and we are also dependent on our licensors to take the necessary action to comply with these requirements with respect to our licensed intellectual property. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could harm our business.
Patent terms may be inadequate to protect our competitive position on our drug candidates for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new drug candidates such as OV101, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we are prosecuting patents. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the USPTO in the United States, and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their drug earlier than might otherwise be the case.
49
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business. The following examples are illustrative:
|
|
• |
others may be able to make compounds or formulations that are similar to our drug candidates but that are not covered by the claims of any patents, should they issue, that we own or control; |
|
|
• |
we or any strategic partners might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own or control; |
|
|
• |
we might not have been the first to file patent applications covering certain of our inventions; |
|
|
• |
others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; |
|
|
• |
it is possible that our pending patent applications will not lead to issued patents; |
|
|
• |
issued patents that we own or control may not provide us with any competitive advantages, or may be held invalid or unenforceable as a result of legal challenges; |
|
|
• |
our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive drugs for sale in our major commercial markets; |
|
|
• |
we may not develop additional proprietary technologies that are patentable; and |
|
|
• |
the patents of others may have an adverse effect on our business. |
The proprietary map of disease-relevant biological pathways underlying orphan disorders of the brain that we developed would not be appropriate for patent protection and, as a result, we rely on trade secrets to protect this aspect of our business.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a negative impact on the success of our business.
Our commercial success depends, in part, upon our ability and the ability of our current or future collaborators to develop, manufacture, market and sell our current and any future drug candidates and use our proprietary technologies without infringing the proprietary rights and intellectual property of third parties. The biotechnology and pharmaceutical industries are characterized by extensive and complex litigation regarding patents and other intellectual property rights. We may in the future become party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our current and any future drug candidates and technology, including interference proceedings, post grant review and inter partes review before the USPTO. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. There is a risk that third parties may choose to engage in litigation with us to enforce or to otherwise assert their patent rights against us. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could have a negative impact on our ability to commercialize our current and any future drug candidates. In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. If we are found to infringe a third party’s valid and enforceable intellectual property rights, we could be required to obtain a license from such third party to continue developing, manufacturing and marketing our drug candidate(s) and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease developing, manufacturing and commercializing the infringing technology or drug candidate. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right. A finding of infringement could prevent us from manufacturing and commercializing our current or any future drug candidates or force us to cease some or all of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business, financial condition, results of operations and prospects. See the section herein titled “Legal Proceedings” for additional information.
50
We may be subject to claims asserting that our employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants or advisors are currently, or were previously, employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, while it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
We may be involved in lawsuits to protect or enforce our patents, the patents of our licensors or our other intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors may infringe or otherwise violate our patents, the patents of our licensors or our other intellectual property rights. To counter infringement or unauthorized use, we may be required to file legal claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing. The initiation of a claim against a third party may also cause the third party to bring counter claims against us such as claims asserting that our patents are invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement or lack of statutory subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant material information from the USPTO, or made a materially misleading statement, during prosecution. Third parties may also raise similar validity claims before the USPTO in post-grant proceedings such as ex parte reexaminations, inter partes review, or post-grant review, or oppositions or similar proceedings outside the United States, in parallel with litigation or even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. For the patents and patent applications that we have licensed, we may have limited or no right to participate in the defense of any licensed patents against challenge by a third party. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any future patent protection on our current or future drug candidates. Such a loss of patent protection could harm our business.
We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Our business could be harmed if in litigation the prevailing party does not offer us a license on commercially reasonable terms. Any litigation or other proceedings to enforce our intellectual property rights may fail, and even if successful, may result in substantial costs and distract our management and other employees.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common stock.
Changes in U.S. patent law or the patent law of other countries or jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our current and any future drug candidates.
The United States has recently enacted and implemented wide-ranging patent reform legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce patents that we have licensed or that we might obtain in the future. Similarly, changes in patent law and regulations in other countries or jurisdictions or changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces patent laws or regulations may weaken our ability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future.
51
We may not be able to protect our intellectual property rights throughout the world, which could negatively impact our business.
Filing, prosecuting and defending patents covering our current and any future drug candidates throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own drugs and, further, may export otherwise infringing drugs to territories where we may obtain patent protection, but where patent enforcement is not as strong as that in the United States. These drugs may compete with our drugs in jurisdictions where we do not have any issued or licensed patents and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
If we rely on third parties to manufacture or commercialize our current or any future drug candidates, or if we collaborate with additional third parties for the development of our current or any future drug candidates, we must, at times, share trade secrets with them. We may also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with our advisors, employees, third-party contractors and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure could have an adverse effect on our business and results of operations.
In addition, these agreements typically restrict the ability of our advisors, employees, third-party contractors and consultants to publish data potentially relating to our trade secrets. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any third-party collaborators. A competitor’s discovery of our trade secrets would harm our business.
Risks Related to Our Dependence on Third Parties
We do not have our own manufacturing capabilities and will rely on third parties to produce clinical and commercial supplies of our current and any future drug candidates.
We do not own or operate, and we do not expect to own or operate, facilities for drug manufacturing, storage and distribution, or testing. We will be dependent on third parties to manufacture the clinical supplies of our drug candidates. The drug substance for OV101 was manufactured by Lundbeck. We believe that the drug substance transferred from Lundbeck under the Lundbeck agreement will be sufficient for us to complete our ongoing and future clinical trials. We will also continue to rely on Takeda to provide the drug product supply for our planned clinical trials of OV935.
Further, we also will rely on third-party manufacturers to supply us with sufficient quantities of our drug candidates, including OV101 and OV935, to be used, if approved, for commercialization. Any significant delay in the supply of a drug candidate, or the raw material components thereof, for an ongoing clinical trial due to the need to replace a third-party manufacturer could considerably delay completion of our clinical trials, product testing and potential regulatory approval of our drug candidates.
Further, our reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured drug candidates ourselves, including:
|
|
• |
inability to meet our drug specifications and quality requirements consistently; |
|
|
• |
delay or inability to procure or expand sufficient manufacturing capacity; |
|
|
• |
issues related to scale-up of manufacturing; |
|
|
• |
costs and validation of new equipment and facilities required for scale-up; |
|
|
• |
failure to comply with cGMP and similar foreign standards; |
|
|
• |
inability to negotiate manufacturing agreements with third parties under commercially reasonable terms, if at all; |
|
|
• |
termination or nonrenewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; |
|
|
• |
reliance on single sources for drug components; |
|
|
• |
lack of qualified backup suppliers for those components that are currently purchased from a sole or single source supplier; |
52
|
|
• |
operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier; and |
|
|
• |
carrier disruptions or increased costs that are beyond our control. |
Any of these events could lead to clinical trial delays, failure to obtain regulatory approval or impact our ability to successfully commercialize our current or any future drug candidates once approved. Some of these events could be the basis for FDA action, including injunction, request for recall, seizure, or total or partial suspension of production.
We intend to rely on third parties to conduct, supervise and monitor our preclinical studies and clinical trials, and if those third parties perform in an unsatisfactory manner, it may harm our business.
We do not currently have the ability to independently conduct any clinical trials. We intend to rely on CROs and clinical trial sites to ensure the proper and timely conduct of our preclinical studies and clinical trials, and we expect to have limited influence over their actual performance. We intend to rely upon CROs to monitor and manage data for our clinical programs, as well as the execution of future nonclinical studies. We expect to control only certain aspects of our CROs’ activities. Nevertheless, we will be responsible for ensuring that each of our preclinical studies or clinical trials are conducted in accordance with the applicable protocol, legal, regulatory and scientific standards and our reliance on the CROs does not relieve us of our regulatory responsibilities.
We and our CROs will be required to comply with good laboratory practices, (“GLPs”), and good clinical practices, (“GCPs”), which are regulations and guidelines enforced by the FDA and are also required by the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities in the form of International Conference on Harmonization guidelines for any of our drug candidates that are in preclinical and clinical development. The regulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and clinical trial sites. Although we will rely on CROs to conduct GCP-compliant clinical trials, we remain responsible for ensuring that each of our GLP preclinical studies and clinical trials is conducted in accordance with its investigational plan and protocol and applicable laws and regulations, and our reliance on the CROs does not relieve us of our regulatory responsibilities. If we or our CROs fail to comply with GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. Accordingly, if our CROs fail to comply with these regulations or fail to recruit a sufficient number of subjects, we may be required to repeat clinical trials, which would delay the regulatory approval process.
While we will have agreements governing their activities, our CROs will not be our employees, and we will not control whether or not they devote sufficient time and resources to our future clinical and nonclinical programs. These CROs may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials, or other drug development activities which could harm our business. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by CROs, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for any other reasons, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize any drug candidate that we develop. As a result, our financial results and the commercial prospects for any drug candidate that we develop would be harmed, our costs could increase, and our ability to generate revenue could be delayed.
If our relationship with these CROs terminates, we may not be able to enter into arrangements with alternative CROs or do so on commercially reasonable terms. Switching or adding additional CROs involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays occur, which can negatively impact our ability to meet our desired clinical development timelines. Though we intend to carefully manage our relationships with our CROs, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not have a negative impact on our business, financial condition and prospects.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, we may be required to report some of these relationships to the FDA. The FDA may conclude that a financial relationship between us and a principal investigator has created a conflict of interest or otherwise affected interpretation of the trial. The FDA may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of marketing approval of our current and future drug candidates.
53
Risks Related to Our Business Operations, Employee Matters and Managing Growth
We are highly dependent on the services of our senior management team, including our Chairman and Chief Executive Officer, Dr. Jeremy Levin, and if we are not able to retain these members of our management team or recruit and retain additional management, clinical and scientific personnel, our business will be harmed.
We are highly dependent on our senior management team, including our Chairman and Chief Executive Officer, Dr. Levin. The employment agreements we have with these officers do not prevent such persons from terminating their employment with us at any time. The loss of the services of any of these persons could impede the achievement of our research, development and commercialization objectives.
In addition, we are dependent on our continued ability to attract, retain and motivate highly qualified additional management, clinical and scientific personnel. If we are not able to retain our management and to attract, on acceptable terms, additional qualified personnel necessary for the continued development of our business, we may not be able to sustain our operations or grow. This risk may be further amplified given the particularly competitive hiring market in New York City, the location of our corporate headquarters.
We may not be able to attract or retain qualified personnel in the future due to the intense competition for qualified personnel among biotechnology, pharmaceutical and other businesses. Many of the other pharmaceutical companies that we compete against for qualified personnel and consultants have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates and consultants than what we have to offer. If we are unable to continue to attract, retain and motivate high-quality personnel and consultants to accomplish our business objectives, the rate and success at which we can discover and develop drug candidates and our business will be limited and we may experience constraints on our development objectives.
Our future performance will also depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our drug candidates, harming future regulatory approvals, sales of our drug candidates and our results of operations. Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or any of our employees.
We will need to expand our organization, and we may experience difficulties in managing this growth, which could disrupt our operations.
As of December 31, 2017, we had 43 employees. As our development and commercialization plans and strategies develop, we expect to need additional managerial, operational, sales, marketing, financial, legal and other resources. Our management may need to divert a disproportionate amount of its attention away from our day-to-day operations and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational inefficiencies, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of our current and potential future drug candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance, our ability to commercialize drug, develop a scalable infrastructure and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk that our employees, consultants, distributors, and collaborators may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates the regulations of the FDA and non-U.S. regulators, including those laws requiring the reporting of true, complete and accurate information to such regulators, manufacturing standards, healthcare fraud and abuse laws and regulations in the United States and abroad or laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry, including the sale of pharmaceuticals, are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and
54
administrative penalties, damages, monetary fines, imprisonment, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
Risks Related to Ownership of Our Common Stock
The market price of our common stock may be volatile and fluctuate substantially, which could result in substantial losses for our common stock.
The market price of our common stock is likely to be volatile. The stock market in general and the market for biopharmaceutical or pharmaceutical companies in particular, has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may lose all or part of your investment in our common stock since you might be unable to sell your shares at or above the price you paid for the shares.
The market price for our common stock may be influenced by many factors, including:
|
|
• |
results of clinical trials of our current and any future drug candidates or those of our competitors; |
|
|
• |
the success of competitive drugs or therapies; |
|
|
• |
regulatory or legal developments in the United States and other countries; |
|
|
• |
developments or disputes concerning patent applications, issued patents or other proprietary rights; |
|
|
• |
the recruitment or departure of key personnel; |
|
|
• |
the level of expenses related to our current and any future drug candidates or clinical development programs; |
|
|
• |
the results of our efforts to discover, develop, acquire or in-license additional drug candidates; |
|
|
• |
actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
|
|
• |
our inability to obtain or delays in obtaining adequate drug supply for any approved drug or inability to do so at acceptable prices; |
|
|
• |
disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
|
|
• |
significant lawsuits, including patent or stockholder litigation; |
|
|
• |
variations in our financial results or those of companies that are perceived to be similar to us; |
|
|
• |
changes in the structure of healthcare payment systems; |
|
|
• |
market conditions in the pharmaceutical and biotechnology sectors; |
|
|
• |
general economic, industry and market conditions; and |
|
|
• |
the other factors described in this “Risk Factors” section. |
In addition, in the past, stockholders have initiated class action lawsuits against companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources.
Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Based upon our shares of our common stock outstanding as of March 22, 2018, our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock will, in the aggregate, beneficially own shares representing approximately 56.2% of our outstanding common stock.
55
Takeda, a greater than 5% holder, may receive additional securities upon the achievement of certain development, commercial and regulatory milestones pursuant to the Takeda license agreement. Specifically, we will be obligated to issue additional securities to Takeda equal to the lesser of 8% of our outstanding capital stock or $50.0 million unless certain events occur, and may issue, at our discretion, additional securities to Takeda upon the achievement of other milestones. Further, pursuant to the Series B-1 preferred stock purchase agreement entered into with Takeda in January 2017, or the Takeda stock purchase agreement, Takeda has agreed to, among other things, (i) a standstill provision, (ii) restrictions on its ability to sell or otherwise transfer it shares of our stock, (iii) vote its shares on certain matters in accordance with the holders of a majority of shares of our common stock and (iv) restrictions on the percentage of our outstanding common stock it may own. See “Business—License and Collaboration Agreements—License and Collaboration Agreement with Takeda” for additional information.
If our executive officers, directors and stockholders who owned more than 5% of our outstanding common stock acted together, they may be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. The concentration of voting power, Takeda standstill provisions, voting obligations and transfer restrictions could delay or prevent an acquisition of our company on terms that other stockholders may desire or result in the management of our company in ways with which other stockholders disagree.
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our stock, the price of our stock could decline.
The trading market for our common stock relies, in part, on the research and reports that industry or financial analysts publish about us or our business. We do currently have research coverage offered by several industry or financial analysts. If one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our stock, which in turn could cause our stock price to decline.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
If we engage in future acquisitions or strategic partnerships, this may increase our capital requirements, dilute our stockholders, cause us to incur debt or assume contingent liabilities and subject us to other risks.
Our business plan is to continue to evaluate various acquisitions and strategic partnerships, including licensing or acquiring complementary drugs, intellectual property rights, technologies, or businesses. Any potential acquisition or strategic partnership may entail numerous risks, including:
|
|
• |
increased operating expenses and cash requirements; |
|
|
• |
the assumption of additional indebtedness or contingent liabilities; |
|
|
• |
assimilation of operations, intellectual property and drugs of an acquired company, including difficulties associated with integrating new personnel; |
|
|
• |
the diversion of our management’s attention from our existing drug programs and initiatives in pursuing such a strategic partnership, merger or acquisition; |
|
|
• |
retention of key employees, the loss of key personnel, and uncertainties in our ability to maintain key business relationships; |
|
|
• |
risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing drugs or drug candidates and regulatory approvals; and |
|
|
• |
our inability to generate revenue from acquired technology and/or drugs sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs. |
In addition, if we engage in future acquisitions or strategic partnerships, we may issue dilutive securities, assume or incur debt obligations, incur large one-time expenses and acquire intangible assets that could result in significant future amortization expense. Moreover, we may not be able to locate suitable acquisition opportunities and this inability could impair our ability to grow or obtain access to technology or drugs that may be important to the development of our business.
56
A significant portion of our total outstanding shares are restricted from immediate resale but may be sold into the market in the near future, which could cause the market price of our common stock to drop significantly, even if our business is performing well.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. All of the stockholders who held shares of our capital stock prior to our initial public offering, or IPO, are subject to lock-up agreements with the underwriters of our IPO that restrict such stockholders’ ability to transfer shares of our common stock that they held prior to the consummation of our IPO. Moreover, holders of an aggregate of approximately 19,601,936 shares of our common stock will have rights, subject to some conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We recently registered all shares of common stock that we may issue under our equity compensation plans. They can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” or EGC, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an EGC until the earlier of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of our IPO in May 2017; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or SEC. For so long as we remain an EGC, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
|
|
• |
not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404; |
|
|
• |
not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; |
|
|
• |
being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced ‘‘Management’s Discussion and Analysis of Financial Condition and Results of Operations’’ disclosure; |
|
|
• |
reduced disclosure obligations regarding executive compensation; and |
|
|
• |
exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
We currently intend to take advantage of some, but not all, of the reduced regulatory and reporting requirements that will be available to us so long as we qualify as an EGC. For example, our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an EGC, which may increase the risk that material weaknesses or significant deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an EGC, we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the SEC, which may make it more difficult for investors and securities analysts to evaluate our company. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile and may decline.
In addition, the JOBS Act provides that an EGC may take advantage of an extended transition period for complying with new or revised accounting standards. This allows an EGC to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not an EGC.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, and particularly after we are no longer an EGC, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC and The Nasdaq Stock Market LLC have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance.
57
Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we remain an EGC, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed time frame that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Provisions in our corporate charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our corporate charter and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. In addition, because our board of directors is responsible for appointing the members of our management team, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors. Among other things, these provisions:
|
|
• |
establish a classified board of directors such that not all members of the board are elected at one time; |
|
|
• |
allow the authorized number of our directors to be changed only by resolution of our board of directors; |
|
|
• |
limit the manner in which stockholders can remove directors from the board; |
|
|
• |
establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
|
|
• |
require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
|
|
• |
limit who may call stockholder meetings; |
|
|
• |
authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a stockholder rights plan, or so-called “poison pill,” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
|
|
• |
require the approval of the holders of at least 66 2/3% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our charter or bylaws. |
Moreover, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Additionally, the Takeda standstill provisions and transfer restrictions in the Takeda Stock Purchase Agreement may delay or prevent a merger, acquisition or other change in control of us that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares.
We may be subject to securities litigation, which is expensive and could divert management attention.
The market price of our common stock may be volatile, and in the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
58
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. Commencing after the filing of our initial annual report on Form 10-K, we will be required, under Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, for as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of the exemption permitting us not to comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 will require that we incur substantial expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting once that firm begins its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by the Nasdaq Global Select Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would benefit our stockholders and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These provisions include:
|
|
• |
authorizing the issuance of “blank check” preferred stock, the terms of which we may establish and shares of which we may issue without stockholder approval; |
|
|
• |
prohibiting cumulative voting in the election of directors, which would otherwise allow for less than a majority of stockholders to elect director candidates; |
|
|
• |
prohibiting stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; |
|
|
• |
eliminating the ability of stockholders to call a special meeting of stockholders; and |
|
|
• |
establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings. |
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or the DGCL, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under the DGCL, a corporation may not, in general, engage in a business combination with any holder of 15% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our amended and restated certificate of incorporation or amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change of control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
59
Item 1B. Unresolved Staff Comments
None.
We lease the space for our principal executive offices, which are located at 1460 Broadway, New York, New York, on a monthly basis. We believe that our facilities are adequate to meet our current needs.
On July 25, 2017, a notice of opposition was filed at the Trademark Trial and Appeal Board of the U.S. Patent and Trademark Office (the “Appeal Board”) in which Ovid Technologies, Inc. objected to our pending trademark applications for the marks OVID THERAPEUTICS and OVID THERAPEUTICS BOLDMEDICINE (and Design) for pharmaceutical product development and related services based on Ovid Technologies’ registrations for the marks OVID and OVIDMD for computer software, computer information services, medical information services, and other services. On July 25, 2017, a petition for cancellation was filed at the Appeal Board in which Ovid Technologies, Inc. seeks cancellation of our trademark registrations for the mark OVID for pharmaceutical product research, development, evaluation, and related services based on Ovid Technologies’ registrations for the OVID and OVIDMD marks. The only issue decided in these proceedings is the right to register a trademark and the only remedy is to allow, deny or cancel registration. No form of monetary or injunctive relief is available in these proceedings.
Item 4. Mine Safety Disclosures
Not applicable.
60
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock began trading on The Nasdaq Global Select Market on May 5, 2017, under the symbol “OVID” Prior to that time, there was no public market for our common stock. The following table sets forth the high and low closing sales prices per share of our common stock as reported on The Nasdaq Global Select Market for the periods indicated.
|
Year Ended December 31, 2017 |
|
High |
|
|
Low |
|
||
|
Second Quarter (Beginning May 5, 2017) |
|
$ |
15.00 |
|
|
$ |
10.49 |
|
|
Third Quarter |
|
$ |
10.23 |
|
|
$ |
5.32 |
|
|
Fourth Quarter |
|
$ |
12.31 |
|
|
$ |
6.12 |
|
Comparative Stock Performance Graph
The following performance graph and related information shall not be deemed “soliciting material” or to be “filed” with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act or Exchange Act. The following graph shows a comparison from May 5, 2017 (the date our common stock commenced trading on the Nasdaq Global Select Market) through December 31, 2017, of the cumulative total return for our common stock, the Nasdaq Composite Index, and the Nasdaq Biotechnology.

The graph assumes an initial investment of $100 on May 5, 2017. The comparisons in the graph are not intended to forecast or be indicative of possible future performance of our common stock.
Holders of Record
As of March 22, 2018, we had approximately 26 holders of record of our common stock. Certain shares are held in “street” name and accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings to fund the development and growth of our business. We do not expect to pay any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on then-existing conditions, including our financial conditions, operating results, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
Use of Proceeds
On May 10, 2017, we completed our IPO and sold 5,000,000 shares of common stock at the initial public offering price of $15.00 per share, for gross proceeds of $75.0 million and $66.7 million in net proceeds after deducting underwriting discounts and commissions and other offering expenses payable by us. None of these expenses consisted of payments made by us to directors, officers or persons owning 10% or more of our common stock or to their associates, or to our affiliates.
61
The offer and sale of the shares in our IPO were registered pursuant to our Registration Statement on Form S-1 (File No. 333-217245), which was declared effective by the SEC on May 4, 2017. Citigroup Global Markets Inc. and Cowen and Company, LLC acted as joint book-running managers for the offering, and William Blair & Company, L.L.C. and JMP Securities LLC acted as co-managers for the offering.
There has been no material change in the planned use of proceeds from our IPO as described in our Prospectus that forms a part of the Company’s Registration Statement on Form S-1 (File No. 333-217245), which was filed with the SEC pursuant to Rule 424 on May 5, 2017. As of December 31, 2017, we consumed approximately $18.3 million of net proceeds from the IPO, primarily to advance OV101 and OV935 through clinical trials, and for working capital and general corporate purposes. We invested the remaining funds received in cash equivalents and other short-term investments in accordance with our investment policy.
Item 6. Selected Financial Data
The following table sets forth our selected financial data. We derived the statement of operations data for the years ended December 31, 2017, 2016, and 2015 and the balance sheet data as of December 31, 2017 and 2016, from our audited financial statements, included elsewhere in this Annual Report on Form 10-K. We have derived the balance sheet data as of December 31, 2015 from our audited financial statements not included in this report. Our historical results are not necessarily indicative of results to be expected for any period in the future. The selected financial data presented below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes thereto, included elsewhere in this Annual Report on Form 10-K. The selected financial data in this section are not intended to replace our financial statements and the related notes thereto.
Statement of Operations Data:
|
|
|
For the Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
(in thousands, except share and per share data) |
|
|||||||||
|
Selected Statements of Operations and Comprehensive Loss Data: |
|
|
|
|||||||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
49,972 |
|
|
$ |
9,585 |
|
|
$ |
6,612 |
|
|
General and administrative |
|
|
15,035 |
|
|
|
12,950 |
|
|
|
6,578 |
|
|
Total operating expenses |
|
|
65,007 |
|
|
|
22,535 |
|
|
|
13,190 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations |
|
|
(65,007 |
) |
|
|
(22,535 |
) |
|
|
(13,190 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
202 |
|
|
|
121 |
|
|
|
30 |
|
|
Net loss and comprehensive loss |
|
$ |
(64,805 |
) |
|
$ |
(22,414 |
) |
|
$ |
(13,160 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(64,805 |
) |
|
$ |
(22,414 |
) |
|
$ |
(13,160 |
) |
|
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(3.35 |
) |
|
$ |
(2.28 |
) |
|
$ |
(1.36 |
) |
|
Weighted -average common shares outstanding basic and diluted |
|
|
19,344,355 |
|
|
|
9,838,590 |
|
|
|
9,699,247 |
|
Balance Sheet Data:
|
|
|
As of December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Selected Balance Sheet Data: |
|
|
|
|||||||||
|
Cash and cash equivalents |
|
|
87,126 |
|
|
|
51,940 |
|
|
|
69,944 |
|
|
Working capital |
|
|
82,567 |
|
|
|
48,678 |
|
|
|
67,974 |
|
|
Total assets |
|
|
89,458 |
|
|
|
53,028 |
|
|
|
70,377 |
|
|
Total liabilities |
|
|
6,021 |
|
|
|
3,733 |
|
|
|
2,309 |
|
|
Accumulated deficit |
|
|
(100,716 |
) |
|
|
(35,910 |
) |
|
|
(13,495 |
) |
|
Total stockholders’ equity |
|
|
83,437 |
|
|
|
49,294 |
|
|
|
68,067 |
|
62
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with “Selected Financial Data” and our financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion and analysis and other parts of this Annual Report on Form 10-K contain forward-looking statements based upon current beliefs, plans and expectations that involve risks, uncertainties and assumptions, such as statements regarding our plans, objectives, expectations, intentions and projections. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this Annual Report on Form 10-K. You should carefully read the “Risk Factors” section of this Annual Report on Form 10-K to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section entitled “Special Note Regarding Forward-Looking Statements.”
Overview
We are a biopharmaceutical company focused exclusively on developing impactful medicines for patients and families living with rare neurological disorders. We believe these disorders represent an attractive area for drug development as the understanding of the underlying biology has grown meaningfully over the last few years; yet has remained underappreciated by the industry. Our experienced team began with a vision to integrate the biology and symptomology of rare neurological conditions to employ innovative research and clinical strategies for the development of our drug candidates. Based on recent scientific advances in genetics and the biological pathways of the brain, we created a proprietary map of disease-relevant pathways and used it to identify and acquire novel compounds for the treatment of rare neurological disorders. We are executing on our strategy by in-licensing and collaborating with leading biopharmaceutical companies and academic institutions. We are developing a robust pipeline of clinical assets with an initial focus on neurodevelopmental disorders and rare epileptic encephalopathies. Our most advanced candidate, OV101, has completed randomization of a Phase 2 trial, which is primarily a safety trial that is designed to provide proof-of-concept on efficacy parameters, in adults and adolescents with Angelman syndrome. We completed a Phase 1 trial in adolescents with Angelman syndrome or Fragile X syndrome in which, OV101 was found to be generally well tolerated and its pharmacokinetic, or PK, profile in adolescents was similar to previous data generated in young adults. Along with our collaborator, Takeda Pharmaceutical Company Limited, or Takeda, we initiated patient recruitment in our Phase 1b/2a trial of OV935 in adults with rare epileptic encephalopathies in June 2017. We expect data from both programs in 2018.
Since our inception in April 2014, we have devoted substantially all of our efforts to organizing and planning our business, building our management and technical team, acquiring operating assets and raising capital.
On May 1, 2017, we effected a 1-for-2.15 reverse stock split of our outstanding common stock and convertible preferred stock. Stockholders entitled to fractional shares because of the reverse stock split received a cash payment in lieu of receiving fractional shares. All of our historical share and per share information shown in the accompanying audited financial statements and related notes for all periods presented have been adjusted to give effect to this reverse stock split.
On May 10, 2017, we completed our initial public offering, or IPO, of 5,000,000 shares of our common stock at a public offering price of $15.00 per share. The gross proceeds from the IPO were $75.0 million and the net proceeds were $66.7 million, after deducting underwriting discounts and commissions and other offering expenses payable by us.
Since our inception, we have not generated any revenue and have funded our business primarily through the sale of our capital stock. Through December 31, 2017, we have raised net proceeds of $142.3 million from the sale of convertible preferred stock and our IPO. As of December 31, 2017, we had $87.1 million in cash and cash equivalents. We recorded net losses of $64.8 million, which includes a non-cash charge of $25.9 million related to our Takeda collaboration (as defined below) and $22.4 million for the years ended December 31, 2017 and 2016, respectively. As of December 31, 2017, we had an accumulated deficit of approximately $100.7 million.
We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. Our net losses may fluctuate significantly from period to period, depending on the timing of our planned clinical trials and expenditures on our other research and development and commercial development activities. We expect our expenses will increase substantially over time as we:
|
|
• |
continue the ongoing and planned preclinical and clinical development of our drug candidates; |
|
|
• |
build a portfolio of drug candidates through the acquisition or in-license of drugs, drug candidates or technologies; |
|
|
• |
initiate preclinical studies and clinical trials for any additional drug candidates that we may pursue in the future; |
|
|
• |
seek marketing approvals for our current and future drug candidates that successfully complete clinical trials; |
|
|
• |
establish a sales, marketing and distribution infrastructure to commercialize any drug candidate for which we may obtain marketing approval; |
63
|
|
• |
implement operational, financial and management systems; and |
|
|
• |
attract, hire and retain additional administrative, clinical, regulatory and scientific personnel. |
License and Collaboration Agreement with Takeda Pharmaceutical Company Limited
In January 2017, we entered into a license and collaboration agreement with Takeda or the Takeda Collaboration. All activities of the collaboration regarding OV935 will be guided by the Takeda/Ovid “One Team” concept, an integrated and interdisciplinary team from both companies devoted to the successful advancement of OV935 across rare epilepsy syndromes. Under the agreement, we will take the lead in clinical development activities and commercialization of the compound OV935 and products containing this compound for the treatment of certain rare neurological disorders in the United States, Canada, the European Union and Israel. Takeda will take the lead in commercialization of OV935 in Japan and has the option to lead in Asia and other selected geographies. We and Takeda will initially share equally all development and commercialization costs and expenses prior to launch of a product and all revenues and commercialization costs and expenses after launch.
Financial Operations Overview
Revenue
We have not generated any revenue from commercial drug sales and do not expect to generate any revenue unless or until we obtain regulatory approval of and commercialize one or more of our current or future drug candidates. In the future, we may also seek to generate revenue from a combination of research and development payments, license fees and other upfront or milestone payments.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our product discovery efforts and the development of our product candidates, which include, among other things:
|
|
• |
fees related to the acquisition of the rights to OV101 and OV935; |
|
|
• |
employee-related expenses, including salaries, benefits and stock-based compensation expense; |
|
|
• |
fees paid to consultants for services directly related to our drug development and regulatory effort; |
|
|
• |
expenses incurred under agreements with contract research organizations, as well as contract manufacturing organizations and consultants that conduct preclinical studies and clinical trials; |
|
|
• |
costs associated with preclinical activities and development activities; |
|
|
• |
costs associated with technology and intellectual property licenses; |
|
|
• |
milestone payments and other costs under licensing agreements; and |
|
|
• |
depreciation expense for assets used in research and development activities. |
Costs incurred in connection with research and development activities are expensed as incurred. Costs for certain development activities, such as clinical trials, are recognized based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations or other information provided to us by our vendors.
Research and development activities are and will continue to be central to our business model. We expect our research and development expenses to increase for the foreseeable future as we advance our current and future drug candidates through preclinical studies and clinical trials. The process of conducting preclinical studies and clinical trials necessary to obtain regulatory approval is costly and time-consuming. It is difficult to determine with certainty the duration and costs of any preclinical study or clinical trial that we may conduct. The duration, costs and timing of clinical trial programs and development of our current and future drug candidates will depend on a variety of factors that include, but are not limited to, the following:
|
|
• |
number of clinical trials required for approval and any requirement for extension trials; |
|
|
• |
per patient trial costs; |
|
|
• |
number of patients who participate in the clinical trials; |
|
|
• |
number of sites included in the clinical trials; |
|
|
• |
countries in which the clinical trial is conducted; |
|
|
• |
length of time required to enroll eligible patients; |
64
|
|
• |
drop-out or discontinuation rates of patients; |
|
|
• |
potential additional safety monitoring or other studies requested by regulatory agencies; |
|
|
• |
duration of patient follow-up; and |
|
|
• |
efficacy and safety profile of the drug candidate. |
In addition, the probability of success for any of our current or future drug candidates will depend on numerous factors, including competition, manufacturing capability and commercial viability. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success of each drug candidate, as well as an assessment of each drug candidate’s commercial potential.
General and Administrative Expenses
General and administrative expenses consist primarily of employee-related expenses, including salaries, benefits and stock-based compensation expense, related to our executive, finance, business development and support functions. Other general and administrative expenses include costs associated with operating as a public company described below, travel expenses, conferences, professional fees for auditing, tax and legal services and facility-related costs.
We expect that general and administrative expenses will increase in the future as we expand our operating activities and incur additional costs associated with being a publicly traded company. These increases will include legal and accounting fees, costs associated with maintaining compliance with The Nasdaq Global Select Market LLC and the Securities and Exchange Commission, or the SEC, directors’ and officers’ liability insurance premiums and fees associated with investor relations. In addition, if our current or future drug candidates are approved for sale, we expect that we would incur expenses associated with building our commercial and distribution infrastructure.
Interest Income
Interest income consists of interest income earned on our cash and cash equivalents maintained in money market funds.
Results of Operations
Comparison of the Years Ended December 31, 2017 and 2016
The following table summarizes the results of our operations for the periods indicated:
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2017 |
|
|
2016 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Research and development |
|
$ |
49,972 |
|
|
$ |
9,585 |
|
|
$ |
40,387 |
|
|
General and administrative |
|
|
15,035 |
|
|
|
12,950 |
|
|
|
2,085 |
|
|
Total operating expenses |
|
|
65,007 |
|
|
|
22,535 |
|
|
|
42,472 |
|
|
Loss from operations |
|
|
(65,007 |
) |
|
|
(22,535 |
) |
|
|
(42,472 |
) |
|
Interest income |
|
|
202 |
|
|
|
121 |
|
|
|
81 |
|
|
Loss |
|
|
(64,805 |
) |
|
|
(22,414 |
) |
|
|
(42,391 |
) |
|
Net loss and comprehensive loss |
|
$ |
(64,805 |
) |
|
$ |
(22,414 |
) |
|
$ |
(42,391 |
) |
Research and Development Expenses
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2017 |
|
|
2016 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Preclinical and development expense |
|
$ |
41,181 |
|
|
$ |
4,819 |
|
|
$ |
36,362 |
|
|
Payroll and payroll-related expenses |
|
|
7,668 |
|
|
|
4,212 |
|
|
|
3,456 |
|
|
Other expenses |
|
|
1,123 |
|
|
|
554 |
|
|
|
569 |
|
|
Total research and development |
|
$ |
49,972 |
|
|
$ |
9,585 |
|
|
$ |
40,387 |
|
65
Research and development expenses were $50.0 million for the year ended December 31, 2017 compared to $9.6 million for the year ended December 31, 2016. The increase of $40.4 million was primarily due to an increase in preclinical and development expenses for the clinical studies of OV101 and OV935, the issuance of Series B-1 Preferred Stock to Takeda as an upfront payment upon signing the collaboration agreement, and an increase in headcount to support our expanded operations. During the year ended December 31, 2017, total research and development expenses consisted of $41.2 million in preclinical and development expenses, of which $25.9 million related to the issuance of Series B-1 Preferred Stock associated with the collaboration rights to OV935 and $4.3 million represents amounts reimbursed to Takeda in respect of the Takeda Collaboration, $7.7 million in payroll and payroll-related expenses, of which $2.4 million related to stock-based compensation, and $1.1 million in other expenses. During the year ended December 31, 2016, total research and development expenses consisted of $4.8 million in preclinical and development expenses, which included $0.5 million in consulting expenses, $4.2 million in compensation expenses, of which $1.5 million related to stock-based compensation, and $0.6 million in other expenses.
General and Administrative Expenses
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2017 |
|
|
2016 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Payroll and payroll-related expenses |
|
$ |
9,087 |
|
|
$ |
6,753 |
|
|
$ |
2,334 |
|
|
Legal and professional fees |
|
|
3,806 |
|
|
|
4,330 |
|
|
|
(524 |
) |
|
General office expenses |
|
|
2,142 |
|
|
|
1,867 |
|
|
|
275 |
|
|
Total general and administrative |
|
$ |
15,035 |
|
|
$ |
12,950 |
|
|
$ |
2,085 |
|
General and administrative expenses were $15.0 million for the year ended December 31, 2017 compared to $13.0 million for the year ended December 31, 2016. The increase of $2.0 million was primarily due to the increase in payroll and payroll-related expenses of $2.3 million resulting from an increase in headcount. During the year ended December 31, 2017, total payroll and payroll-related expenses consisted of $4.0 million related to stock-based compensation compared to $2.1 million for the year ended December 31, 2016.
Interest Income
Interest income increased to $0.2 million for the year ended December 31, 2017 from $0.1 million for the year ended December 31, 2016. The increase is attributable to increased interest on our cash and cash equivalents due to the proceeds received from our IPO.
Income Taxes
There was no provision for income taxes for the years ended December 31, 2017 and 2016 because we have historically incurred operating losses and we maintain a full valuation allowance against our net deferred tax assets. The valuation allowance was approximately $35.3 million and $16.3 million at December 31, 2017 and 2016, respectively.
Comparison of the Years Ended December 31, 2016 and 2015
The following table summarizes the results of our operations for the periods indicated:
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2016 |
|
|
2015 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Research and development |
|
$ |
9,585 |
|
|
$ |
6,612 |
|
|
$ |
2,973 |
|
|
General and administrative |
|
|
12,950 |
|
|
|
6,578 |
|
|
|
6,372 |
|
|
Total operating expenses |
|
|
22,535 |
|
|
|
13,190 |
|
|
|
9,345 |
|
|
Loss from operations |
|
|
(22,535 |
) |
|
|
(13,190 |
) |
|
|
(9,345 |
) |
|
Interest income |
|
|
121 |
|
|
|
30 |
|
|
|
91 |
|
|
Loss |
|
|
(22,414 |
) |
|
|
(13,160 |
) |
|
|
(9,254 |
) |
|
Net loss and comprehensive loss |
|
$ |
(22,414 |
) |
|
$ |
(13,160 |
) |
|
$ |
(9,254 |
) |
66
Research and Development Expenses
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2016 |
|
|
2015 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Preclinical and development expense |
|
$ |
4,819 |
|
|
$ |
4,325 |
|
|
$ |
494 |
|
|
Payroll and payroll-related expenses |
|
|
4,212 |
|
|
|
1,604 |
|
|
|
2,608 |
|
|
Other expenses |
|
|
554 |
|
|
|
683 |
|
|
|
(129 |
) |
|
Total research and development |
|
$ |
9,585 |
|
|
$ |
6,612 |
|
|
$ |
2,973 |
|
Research and development expenses were $9.6 million for the year ended December 31, 2016 compared to $6.6 million for the year ended December 31, 2015. The increase of $3.0 million was primarily due to an increase in preclinical and development expenses for the clinical studies of OV101 and an increase in headcount. During the year ended December 31, 2016, total research and development expenses consisted of $4.8 million in preclinical and development expenses, which included $0.5 million in consulting expenses, $4.2 million in payroll and payroll-related expenses, of which $1.5 million related to stock-based compensation, and $0.6 million in other expenses. During the year ended December 31, 2015, total research and development expenses consisted of $4.3 million of costs associated with the acquisition of the rights to OV101, $1.1 million in compensation expenses, and $0.7 million in other expenses which included $0.6 million in consulting expenses.
General and Administrative Expenses
|
|
|
Year Ended |
|
|
Year Ended |
|
|
|
|
|
||
|
|
|
December 31, |
|
|
December 31, |
|
|
|
|
|
||
|
|
|
2016 |
|
|
2015 |
|
|
Change $ |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Payroll and payroll-related expenses |
|
$ |
6,753 |
|
|
$ |
3,579 |
|
|
$ |
3,174 |
|
|
Legal and professional fees |
|
|
4,330 |
|
|
|
2,258 |
|
|
|
2,072 |
|
|
General office expenses |
|
|
1,867 |
|
|
|
741 |
|
|
|
1,126 |
|
|
Total general and administrative |
|
$ |
12,950 |
|
|
$ |
6,578 |
|
|
$ |
6,372 |
|
Selling, general and administrative expenses were $13.0 million for the year ended December 31, 2016 compared to $6.6 million for the year ended December 31, 2015. The increase of $6.4 million was due to the increases in (a) payroll and payroll-related expenses of $3.2 million as a result of increased headcount, of which $1.2 million and $0.8 million related to stock-based compensation and employee severance, respectively, (b) legal and professional fees of $2.1 million related to our efforts to build our management and operational team, develop potential pipelines and expand our operations, and (c) general office expenses of $1.1 million related to our continued growth of operations.
Interest Income
Interest income increased to $0.1 million for the year ended December 31, 2016 from $30 thousand for the year ended December 31, 2015. The increase was primarily due to interest earned on higher cash and cash equivalents due to the receipt of aggregate net proceeds of $70.6 million from the sale of our Series B convertible preferred stock in August 2015.
Income Taxes
There was no provision for income taxes for the years ended December 31, 2016 and 2015 because we have historically incurred operating losses and we maintain a full valuation allowance against our net deferred tax assets.
Liquidity and Capital Resources
Overview
As of December 31, 2017, we had total cash and cash equivalents of $87.1 million as compared to $51.9 million as of December 31, 2016. The $35.2 million increase in total cash was due primarily to the receipt of $66.7 million of net proceeds from the completion of our IPO after deducting underwriting discounts and commissions and other offering expenses payable by us, offset by cash-based operating expenses.
We have incurred losses and experienced negative operating cash flows since our inception and anticipate that we will continue to incur losses for at least the next several years. We incurred net losses of approximately $64.8 million, which includes a non-cash charge of $25.9 million related to our Takeda Collaboration, $22.4 million, and $13.2 million for the years ended December 31, 2017, 2016, and 2015, respectively. As of December 31, 2017, we had an accumulated deficit of approximately $100.7 million, working capital of $82.6 million and cash and cash equivalents of $87.1 million.
67
We believe that our existing cash and cash equivalents as of December 31, 2017 will be sufficient to fund our current operating plans through at least the next 12 months from the filing of this annual report on Form 10-K.
Until such time, if ever, as we can generate revenue from drug sales, we expect to finance our cash needs through a combination of equity offerings, debt financings and potential collaborations and license and development agreements. To the extent that we raise additional capital through future equity offerings or debt financings, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt and equity financings, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. There can be no assurance that such financings will be obtained on terms acceptable to us, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us we may have to significantly delay, scale back or discontinue our research and development programs or future commercialization efforts. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties for one or more of our current or future drug candidates, we may be required to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates or to grant licenses on terms that may not be favorable to us. Our failure to raise capital as and when needed would have a material adverse effect on our financial condition and our ability to pursue our business strategy.
Cash Flows
The following table summarizes our cash flows for the periods indicated:
|
|
|
Year Ended |
|
|
Year Ended |
|
|
Year Ended |
|
|||
|
|
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(31,470 |
) |
|
$ |
(17,802 |
) |
|
$ |
(5,485 |
) |
|
Investing activities |
|
|
(47 |
) |
|
|
(189 |
) |
|
|
(56 |
) |
|
Financing activities |
|
|
66,703 |
|
|
|
(14 |
) |
|
|
70,639 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
35,186 |
|
|
$ |
(18,005 |
) |
|
$ |
65,098 |
|
Net Cash Used in Operating Activities
Net cash used in operating activities was $31.5 million for the year ended December 31, 2017, which consisted of net losses of $64.8 million offset by $32.3 million of non-cash charges, of which $25.9 million related to the issuance of Series B-1 Preferred Stock associated with the collaboration rights to OV935. Net cash used in operating activities was $17.8 million for the year ended December 31, 2016, which consisted of net losses of $22.4 million offset by $3.6 million of non-cash charges. The increase of $13.7 million in net cash used in operating activities was primarily due to an increase in our costs related to our research and development programs and an increase in our payroll and payroll-related expenses as the result of increased headcount as we continue to build our management team and expand our operations.
Net cash used in operating activities was $17.8 million for the year ended December 31, 2016 compared to $5.5 million for the year ended December 31, 2015. The increase of $12.3 million in net cash used in operating activities was primarily due to an increase in our research and development programs and in our payroll and payroll-related expenses as the result of increased headcount as we continue to build our management team and expand our operations and the payment of the 2015 accrued bonus of $1.6 million.
Net Cash Used in Investing Activities
Net cash used in investing activities was de minimis for each of the periods presented. The Company used $47 thousand for the year ended December 31, 2017 compared to $0.2 million for the year months ended December 31, 2016. The decrease in cash used was primarily due to a decrease in external software development costs.
Net Cash Provided by Financing Activities
Net cash provided by financing activities of $66.7 million for the year ended December 31, 2017 was primarily due to the receipt of $66.7 million of net proceeds from the completion of our IPO, after deducting underwriting discounts and commissions and other offering expenses payable by us.
Net cash provided by financing activities of $70.6 million for the year ended December 31, 2015 was due to $70.6 million of net proceeds received from the sales of our Series B convertible preferred stock.
68
Contractual Obligations and Commitments
As of December 31, 2017, we had no contractual obligations or commitments. We had no long-term debt or capital leases and no material non-cancelable purchase commitments with service providers, as we have generally contracted on a cancelable, purchase order basis. We excluded any potential contingent payments upon the achievement by us of clinical, regulatory and commercial events, as applicable, or royalty payments that we may be required to make under license agreements we have entered into with various entities pursuant to which we have in-licensed certain intellectual property as contractual obligations or commitments, including our license agreement with H. Lundbeck A/S, Northwestern, and our Takeda license agreement. Pursuant to these license agreements, we have agreed to make milestone payments up to an aggregate of $271.3 million upon the achievement of certain development, regulatory and sales milestones. We excluded these contingent payments given that the timing and amount, if any, of such payments cannot be reasonably estimated at this time. See the section titled “Business—License and Collaboration Agreements—License Agreement with H. Lundbeck A/S”, Business—License and Collaboration Agreements—Northwestern License,” and “Business—License and Collaboration Agreements—License and Collaboration Agreement with Takeda” for additional information.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
|
|
• |
reduced disclosure about our executive compensation arrangements; |
|
|
• |
no non-binding stockholder advisory votes on executive compensation or golden parachute arrangements; and |
|
|
• |
exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We have taken advantage of reduced reporting requirements in this Annual Report on Form 10-K. In particular, in this Annual Report on Form 10-K, we have not included all of the executive compensation related information that would be required if we were not an emerging growth company. We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenues as of the end of any fiscal year, if we are deemed to be a large accelerated filer under the rules of the SEC or if we issue more than $1.0 billion of non- convertible debt over a three-year period. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the revenue and expenses incurred during the reported periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in more detail in Note 2 to our audited financial statements appearing elsewhere in this Form 10-K. We believe the following critical accounting policies are most important to understanding and evaluating our reported financial results.
Accrued Clinical Expenses
When preparing our financial statements, we are required to estimate our accrued clinical expenses. This process involves reviewing open contracts and communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or
69
otherwise notified of actual cost. Payments under some of the contracts we have with third parties depend on factors, such as successful enrollment of certain numbers of patients, site initiation and the completion of clinical trial milestones.
When accruing clinical expenses, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from our service providers. However, we may be required to estimate the cost of these services based only on information available to us. If we underestimate or overestimate the cost associated with a trial or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Historically, our estimated accrued clinical expenses have approximated actual expense incurred.
Stock-Based Compensation
We account for stock-based compensation awards in accordance with the Financial Accounting Standards Board Accounting Standards Codification, or ASC, Topic 718, Compensation—Stock Compensation, or ASC 718. ASC 718 requires all stock-based compensation awards to employees to be recognized as expense based on their grant date fair values. We recognized expenses over the requisite service period, which is generally the vesting period of the award under the straight-line method. We account for forfeitures as they occur. We record the expense for stock-based compensation awards subject to performance-based milestone vesting over the remaining service period when management determines that achievement of the milestone is probable. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions at each reporting date.
We recognize stock-based compensation expense related to stock options granted to non-employees issued in exchange for services based on the estimated fair value of the awards on the date of grant. We estimate the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model. Option awards granted to non-employees are accounted for in accordance with ASC Topic 505, Equity. Compensation expense is recognized over the vesting period in which the services are rendered. At the end of each financial reporting period prior to the time at which the award is fully vested, the fair value of each non-employee award is remeasured based on the current fair value of our common stock at that time with the updated assumption inputs in the Black-Scholes option-pricing model. The resulting increase or decrease in value, if any, is recognized as expense or income, respectively, during the period the related services are rendered.
The Black-Scholes option-pricing model uses highly subjective assumptions. These assumptions include:
|
|
• |
Expected Volatility. Due to the lack of a public market for the trading of our common stock and a lack of company-specific historical and implied volatility data, the expected volatility was estimated using weighted average measures of implied volatility and the historical volatility of a representative group of small, publicly traded drug development companies at a similar stage of development as ourselves. |
|
|
• |
Expected Term. The expected term of the options outstanding is determined using the “simplified” method for “plain vanilla” options based on the mid-point between the vesting date and the end of the contractual term as prescribed by Staff Accounting Bulletin No. 107, Share-Based Payment. |
|
|
• |
Risk-Free Interest Rate. The risk-free interest rate is based on U.S. Treasury notes with remaining terms similar to the expected term of the option. |
|
|
• |
Expected Dividends. The dividend yield assumption is zero since we have never paid cash dividends and do not plan to pay cash dividends in the foreseeable future. |
We expect the impact of our stock-based compensation expense for stock options granted to employees and non-employees to grow in future periods due to the potential increases in the value of our common stock and in headcount.
70
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
The primary objectives of our investment activities are to ensure liquidity and to preserve capital. As of December 31, 2017, we had cash equivalents of $87.1 million that were held in an interest-bearing money market account. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. Due to the short-term maturities of our cash equivalents and the low risk profile of our investments, an immediate 100 basis point change in interest rates would not have a material effect on the fair market value of our cash equivalents. To minimize the risk in the future, we intend to maintain our portfolio of cash equivalents in institutional market funds that are comprised of U.S. Treasury and U.S. Treasury-backed repurchase agreements.
Item 8. Financial Statements and Supplementary Data
Our financial statements, together with the report of our independent registered public accounting firm, appear in this Annual Report on Form 10-K beginning on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures.
Management’s Evaluation of our Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is (1) recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms and (2) accumulated and communicated to our management, including our principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
As of December 31, 2017 our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Our management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives, and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Our principal executive officer and principal financial officer have concluded based upon the evaluation described above that, as of December 31, 2017 our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Due to a transition period established by SEC rules applicable to newly public companies, our management is not required to evaluate the effectiveness of our internal control over financial reporting until after the filing of our Annual Report on Form 10-K for the year ended December 31, 2017.
Attestation Report of the Registered Public Accounting Firm
This Annual Report on Form 10-K does not include an attestation report of our registered public accounting firm due to an exemption established by the JOBS Act for “emerging growth companies.”
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting that occurred during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
71
Item 10. Directors, Executive Officers, and Corporate Governance
The information required by this item is incorporated by reference to the information set forth in the sections titled “Proposal 1 – Election of Directors,” “Executive Officers,” and “Information Regarding the Board and Corporate Governance” and “Section 16(a) Beneficial Ownership Reporting Compliance” in our 2018 Proxy Statement.
Item 11. Executive Compensation
The information required by this item is incorporated by reference to the information set forth in the section titled “Executive Officer and Director Compensation” in our 2018 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated by reference to the information set forth in the section titled “Security Ownership of Certain Beneficial Owners and Management and “Equity Compensation Plan Information” in our 2018 Proxy Statement.
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required by this item is incorporated by reference to the information set forth in the section titled “Transactions with Related Persons” and “Information regarding the Board and Corporate Governance – Board Independence” in our 2018 Proxy Statement.
Item 14. Principal Accountant Fees and Services
The information required by this item is incorporated by reference to the information set forth in the section titled “Independent Registered Public Accounting Firm Fees” contained in Proposal 2 in our 2018 Proxy Statement.
72
Item 15. Exhibits, Financial Statements and Schedules
(a)(1) Financial Statements.
The response to this portion of Item 15 is set forth under Item 8 hereof.
(a)(2) Financial Statement Schedules.
All schedules have been omitted because they are not required or because the required information is given in the Financial Statements or Notes thereto.
(a)(3) Exhibits.
The exhibits listed below are filed as part of this Form 10-K other than Exhibit 32.1, which shall be deemed furnished
|
Number |
|
Description |
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
4.1 |
|
|
|
|
|
|
|
4.2 |
|
|
|
10.1+ |
|
|
|
10.2+ |
|
|
|
10.3+ |
|
|
|
10.4+ |
|
|
|
10.5+ |
|
|
|
10.6+ |
|
|
|
10.7+ |
|
|
|
10.8+ |
|
|
|
10.9+ |
|
|
|
10.10+ |
|
|
|
10.11+ |
|
73
|
|
||
|
10.13+ |
|
|
|
10.14+ |
|
|
|
10.15+ |
|
|
|
10.16† |
|
|
|
10.17† |
|
|
|
10.18† |
|
|
|
10.19†† |
|
|
|
23.1 |
|
|
|
24.1 |
|
Power of Attorney (included on the signature page to this report). |
|
31.1 |
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
32.1* |
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
* |
Furnished herewith and not deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and shall not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
|
+ |
Indicates a management contract or compensatory plan. |
|
† |
Confidential treatment has been granted for certain portions of this exhibit. These portions have been omitted and filed separately with the SEC. |
|
†† |
Portions of this exhibit have been omitted pursuant to a request for confidential treatment that will be separately filed with the Securities and Exchange Commission |
Not applicable.
74
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
|
OVID THERAPEUTICS INC. |
||
|
|
|
|
|
|
|
Date: March 29, 2018 |
|
By: |
|
/s/ Jeremy M. Levin |
|
|
|
|
|
Jeremy M. Levin |
|
|
|
|
|
Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
Date: March 29, 2018 |
|
By: |
|
/s/ Timothy Daly |
|
|
|
|
|
Timothy Daly |
|
Each person whose individual signature appears below hereby authorizes and appoints Jeremy M. Levin, DPhil, MB BChir and Timothy Daly, and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his or her substitute or substitutes may lawfully do or cause to be done by virtue thereof. Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated. |
||||
|
|
|
|
|
|
|
Signature |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ Jeremy M. Levin, DPhil, MB BChir Jeremy M. Levin, DPhil, MB BChir |
|
Chief Executive Officer and Director (Principal Executive Officer) |
|
March 29, 2018 |
|
|
|
|
||
|
/s/ Timothy Daly Timothy Daly |
|
Senior Vice President, Finance and Corporate Controller (Principal Financial and Accounting Officer) |
|
March 29, 2018 |
|
|
|
|
||
|
/s/ Matthew During, MD, DSc Matthew During, MD, DSc |
|
President, Chief Scientific Officer and Director |
|
March 29, 2018 |
|
|
|
|
||
|
/s/ Karen Bernstein, PhD Karen Bernstein, PhD |
|
Director |
|
March 29, 2018 |
|
|
|
|
|
|
|
/s/ Barbara Duncan |
|
Director |
|
March 29, 2018 |
|
Barbara Duncan |
|
|
||
|
|
|
|
||
|
/s/ Bart Friedman Bart Friedman |
|
Director |
|
March 29, 2018 |
|
|
|
|
||
|
/s/ Douglas Williams, PhD Douglas Williams, PhD |
|
Director |
|
March 29, 2018 |
75
Index to Financial Statements
|
|
F-2 |
|
|
|
|
F-3 |
|
|
|
|
F-4 |
|
|
|
|
F-5 |
|
|
|
|
F-6 |
|
|
|
|
F-7–F-19 |
|
F-1
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Ovid Therapeutics Inc.:
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Ovid Therapeutics Inc. (the Company) as of December 31, 2017 and 2016, the related statements of operations and comprehensive loss, changes in stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2017, and the related notes (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2015.
New York, New York
March 29, 2018
F-2
OVID THERAPEUTICS INC.
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2017 |
|
|
2016 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
87,125,600 |
|
|
$ |
51,939,661 |
|
|
Prepaid and other current assets |
|
|
1,462,448 |
|
|
|
221,507 |
|
|
Due from related parties |
|
|
- |
|
|
|
7,369 |
|
|
Deferred transaction costs |
|
|
- |
|
|
|
242,673 |
|
|
Total current assets |
|
|
88,588,048 |
|
|
|
52,411,210 |
|
|
|
|
|
|
|
|
|
|
|
|
Security deposit |
|
|
88,940 |
|
|
|
407,785 |
|
|
Property, plant and equipment, net |
|
|
51,775 |
|
|
|
43,591 |
|
|
Other assets |
|
|
728,840 |
|
|
|
165,301 |
|
|
Total assets |
|
$ |
89,457,603 |
|
|
$ |
53,027,887 |
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders' Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
2,025,766 |
|
|
$ |
857,169 |
|
|
Accrued expenses |
|
|
3,995,334 |
|
|
|
2,876,243 |
|
|
Total current liabilities |
|
|
6,021,100 |
|
|
|
3,733,412 |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity: |
|
|
|
|
|
|
|
|
|
Preferred Series A - zero and 5,121,453 shares authorized at December 31, 2017 and 2016, respectively zero and 2,382,069 issued and outstanding at December 31, 2017 and 2016, respectively |
|
|
- |
|
|
|
2,382 |
|
|
Preferred Series B - zero and 12,038,506 shares authorized at December 31, 2017 and 2016, respectively zero and 5,599,282 issued and outstanding at December 31, 2017 and 2016, respectively |
|
|
- |
|
|
|
5,599 |
|
|
Common stock, $0.001 par value; 125,000,000 and 58,000,000 shares authorized at December 31, 2017 and 2016, respectively, 24,606,256 and 9,838,590 shares issued and outstanding at December 31, 2017 and 2016, respectively |
|
|
24,606 |
|
|
|
9,839 |
|
|
Additional paid-in-capital |
|
|
184,127,565 |
|
|
|
85,186,269 |
|
|
Accumulated deficit |
|
|
(100,715,668 |
) |
|
|
(35,909,614 |
) |
|
Total stockholders' equity |
|
|
83,436,503 |
|
|
|
49,294,475 |
|
|
Total liabilities and stockholders' equity |
|
$ |
89,457,603 |
|
|
$ |
53,027,887 |
|
See accompanying notes to these financial statements
F-3
Statements of Operations and Comprehensive Loss
|
|
|
For the Year Ended December 31, |
|
|
For the Year Ended December 31, |
|
|
For the Year Ended December 31, |
|
|||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
49,972,102 |
|
|
$ |
9,585,649 |
|
|
$ |
6,611,948 |
|
|
General and administrative |
|
|
15,035,461 |
|
|
|
12,949,525 |
|
|
|
6,578,426 |
|
|
Total operating expenses |
|
|
65,007,563 |
|
|
|
22,535,174 |
|
|
|
13,190,374 |
|
|
Loss from operations |
|
|
(65,007,563 |
) |
|
|
(22,535,174 |
) |
|
|
(13,190,374 |
) |
|
Interest income |
|
|
201,509 |
|
|
|
120,822 |
|
|
|
30,281 |
|
|
Net loss and comprehensive loss |
|
$ |
(64,806,054 |
) |
|
$ |
(22,414,352 |
) |
|
$ |
(13,160,093 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(64,806,054 |
) |
|
$ |
(22,414,352 |
) |
|
$ |
(13,160,093 |
) |
|
Net loss per share attributable to common stockholders, basic and diluted |
|
$ |
(3.35 |
) |
|
$ |
(2.28 |
) |
|
$ |
(1.36 |
) |
|
Weighted-average common shares outstanding basic and diluted |
|
|
19,344,355 |
|
|
|
9,838,590 |
|
|
|
9,699,247 |
|
See accompanying notes to these financial statements
F-4
Statement of Changes in Stockholders’ Equity
|
|
|
Series A Preferred Stock |
|
|
Series B Preferred Stock |
|
|
Series B-1 Preferred Stock |
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated |
|
|
|
|
|
||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Total |
|
|||||||||||
|
Balance, December 31, 2014 |
|
|
2,382,069 |
|
|
$ |
2,382 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
- |
|
|
|
9,302,323 |
|
|
$ |
9,302 |
|
|
$ |
5,061,622 |
|
|
$ |
(335,169 |
) |
|
$ |
4,738,137 |
|
|
Issuance of common stock for research and development activities |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
489,756 |
|
|
|
490 |
|
|
|
4,011,352 |
|
|
|
- |
|
|
|
4,011,842 |
|
|
Issuance of Series B Preferred Stock, net of issuance cost of $4,360,721 |
|
|
- |
|
|
|
- |
|
|
|
5,599,282 |
|
|
|
5,599 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
70,633,563 |
|
|
|
- |
|
|
|
70,639,162 |
|
|
Issuance of common stock for separation agreement |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
46,511 |
|
|
|
47 |
|
|
|
380,953 |
|
|
|
- |
|
|
|
381,000 |
|
|
Stock-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,457,313 |
|
|
|
- |
|
|
|
1,457,313 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(13,160,093 |
) |
|
|
(13,160,093 |
) |
|
Balance, December 31, 2015 |
|
|
2,382,069 |
|
|
|
2,382 |
|
|
|
5,599,282 |
|
|
|
5,599 |
|
|
|
- |
|
|
|
- |
|
|
|
9,838,590 |
|
|
|
9,839 |
|
|
|
81,544,803 |
|
|
|
(13,495,262 |
) |
|
|
68,067,361 |
|
|
Stock-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
3,641,466 |
|
|
|
- |
|
|
|
3,641,466 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(22,414,352 |
) |
|
|
(22,414,352 |
) |
|
Balance, December 31, 2016 |
|
|
2,382,069 |
|
|
$ |
2,382 |
|
|
|
5,599,282 |
|
|
$ |
5,599 |
|
|
|
- |
|
|
$ |
- |
|
|
|
9,838,590 |
|
|
$ |
9,839 |
|
|
$ |
85,186,269 |
|
|
$ |
(35,909,614 |
) |
|
$ |
49,294,475 |
|
|
Issuance of Series B-1 Preferred Stock |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,781,996 |
|
|
|
1,782 |
|
|
|
- |
|
|
|
- |
|
|
|
25,859,446 |
|
|
|
- |
|
|
|
25,861,228 |
|
|
Proceeds from Initial Public Offering, net of underwriting costs and commissions |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
5,000,000 |
|
|
|
5,000 |
|
|
|
69,745,000 |
|
|
|
- |
|
|
|
69,750,000 |
|
|
Deferred offering costs reclassified to additional paid-in capital |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(3,087,481 |
) |
|
|
- |
|
|
|
(3,087,481 |
) |
|
Conversion of preferred stock into common stock |
|
|
(2,382,069 |
) |
|
|
(2,382 |
) |
|
|
(5,599,282 |
) |
|
|
(5,599 |
) |
|
|
(1,781,996 |
) |
|
|
(1,782 |
) |
|
|
9,763,346 |
|
|
|
9,763 |
|
|
|
(464 |
) |
|
|
- |
|
|
|
(464 |
) |
|
Issuance of common stock from exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,320 |
|
|
|
4 |
|
|
|
27,039 |
|
|
|
|
|
|
|
27,043 |
|
|
Stock-based compensation expense |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
6,397,756 |
|
|
|
- |
|
|
|
6,397,756 |
|
|
Net loss |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(64,806,054 |
) |
|
|
(64,806,054 |
) |
|
Balance, December 31, 2017 |
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
|
- |
|
|
$ |
- |
|
|
|
24,606,256 |
|
|
$ |
24,606 |
|
|
$ |
184,127,565 |
|
|
$ |
(100,715,668 |
) |
|
$ |
83,436,503 |
|
See accompanying notes to these financial statements
F-5
|
|
|
Year Ended December 31, |
|
|
Year Ended December 31, |
|
|
Year Ended December 31, |
|
|||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(64,806,054 |
) |
|
$ |
(22,414,352 |
) |
|
$ |
(13,160,093 |
) |
|
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash research and development expense |
|
|
25,861,228 |
|
|
|
- |
|
|
|
4,011,842 |
|
|
Common stock issuance for separation agreement |
|
|
- |
|
|
|
- |
|
|
|
381,000 |
|
|
Stock-based compensation expense |
|
|
6,397,756 |
|
|
|
3,641,466 |
|
|
|
1,457,313 |
|
|
Depreciation and amortization |
|
|
80,310 |
|
|
|
56,512 |
|
|
|
11,778 |
|
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
(793,258 |
) |
|
|
58,825 |
|
|
|
(279,563 |
) |
|
Deferred transaction costs |
|
|
229,000 |
|
|
|
(229,000 |
) |
|
|
- |
|
|
Security deposit |
|
|
(36,590 |
) |
|
|
(362,935 |
) |
|
|
(38,325 |
) |
|
Other assets |
|
|
(604,646 |
) |
|
|
(28,011 |
) |
|
|
- |
|
|
Accounts payable |
|
|
1,076,349 |
|
|
|
186,624 |
|
|
|
591,406 |
|
|
Accrued expenses |
|
|
1,118,627 |
|
|
|
1,238,643 |
|
|
|
1,608,384 |
|
|
Due from/ to related parties |
|
|
7,369 |
|
|
|
50,257 |
|
|
|
(69,222 |
) |
|
Net cash used in operating activities |
|
|
(31,469,909 |
) |
|
|
(17,801,971 |
) |
|
|
(5,485,480 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment |
|
|
(38,907 |
) |
|
|
(30,364 |
) |
|
|
(32,082 |
) |
|
Software development and other assets |
|
|
(8,480 |
) |
|
|
(158,623 |
) |
|
|
(24,250 |
) |
|
Net cash used in investing activities |
|
|
(47,387 |
) |
|
|
(188,987 |
) |
|
|
(56,332 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from initial public offering |
|
|
69,750,000 |
|
|
|
- |
|
|
|
- |
|
|
Proceeds from issuance of preferred stock, net of issuance costs |
|
|
- |
|
|
|
- |
|
|
|
70,639,162 |
|
|
Payments for transaction costs |
|
|
(3,073,808 |
) |
|
|
(13,673 |
) |
|
|
- |
|
|
Proceeds from exercise of options |
|
|
27,043 |
|
|
|
- |
|
|
|
- |
|
|
Net cash provided by (used in) financing activities |
|
|
66,703,235 |
|
|
|
(13,673 |
) |
|
|
70,639,162 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
35,185,939 |
|
|
|
(18,004,631 |
) |
|
|
65,097,350 |
|
|
Cash and cash equivalents, at beginning of period |
|
|
51,939,661 |
|
|
|
69,944,292 |
|
|
|
4,846,942 |
|
|
Cash and cash equivalents, at end of period |
|
$ |
87,125,600 |
|
|
$ |
51,939,661 |
|
|
$ |
69,944,292 |
|
See accompanying notes to these financial statements
F-6
NOTES TO FINANCIAL STATEMENTS
Ovid Therapeutics Inc. (the “Company”) was incorporated under the laws of the state of Delaware on April 1, 2014 and maintains its principal executive office in New York, New York. The Company commenced operations on April 1, 2014 (date of inception). The Company is a biopharmaceutical company focused exclusively on developing impactful medicines for patients and families living with rare neurological disorders.
Since its inception, the Company has devoted substantially all of its efforts to business development, research and development, recruiting management and technical staff, and raising capital, and has financed its operations through issuance of convertible preferred stock (“Preferred Stock”), common stock and other equity instruments. The Company has not generated any revenue. The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development and regulatory success, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations, and ability to secure additional capital to fund operations.
On May 10, 2017, the Company completed its initial public offering (“IPO”) of 5,000,000 shares of the Company's common stock at a public offering price of $15.00 per share. The gross proceeds from the IPO were $75.0 million and the net proceeds were $66.7 million, after deducting underwriting discounts and commissions and other offering expenses payable by us. At the time of the IPO the Series A Preferred Stock, the Series B Preferred Stock, and the Series B-1 Preferred Stock were automatically converted into common stock (see Note 6).
The Company has incurred operating losses since inception and had an accumulated deficit of $100.7 million as of December 31, 2017. The Company expects to continue to incur net losses for at least the next several years and is highly dependent on its ability to find additional sources of funding in the form of debt or equity financing to fund its operations. Management believes that the Company’s existing cash and cash equivalents as of December 31, 2017, will be sufficient to fund its current operating plans through at least the next 12 months from the date of filing of this 10-K. Management expects that future sources of funding may include new or expanded partnering arrangements and sales of equity or debt securities. Adequate additional funding may not be available to the Company on acceptable terms or at all. The failure to raise capital as and when needed could have a negative impact on the Company’s financial condition and ability to pursue business strategies. The Company may be required to delay, reduce the scope of or eliminate research and development programs, or obtain funds through arrangements with collaborators or others that may require the Company to relinquish rights to certain drug candidates that the Company might otherwise seek to develop or commercialize independently.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(A) Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Actual results could differ materially from those estimates.
(B) Reverse Stock Split
In connection with the IPO, the Board of Directors and the stockholders of the Company approved a one-for-2.15 reverse stock split of the Company’s issued and outstanding common stock and preferred stock. The reverse stock split became effective on May 1, 2017. All share and per share amounts in the financial statements have been adjusted for all periods presented to give effect to the reverse stock split.
(C) Risks and Uncertainties
The Company is subject to risks common to companies in the development stage including, but not limited to, dependency on the clinical and commercial success of its drug candidates, ability to obtain regulatory approval of its drug candidates, the need for substantial additional financing to achieve its goals, uncertainty of broad adoption of its approved products, if any, by physicians and consumers, and significant competition and untested manufacturing capabilities.
F-7
(D) Deferred Transaction Costs
Deferred transaction costs, primarily consisted of direct incremental legal, accounting, and other fees related to the Company’s contemplated initial public offering (“IPO”), and were capitalized as incurred. The deferred transaction costs have been offset against IPO proceeds upon the consummation of the offering.
(E) Comprehensive Loss
Comprehensive loss includes net loss as well as other changes in stockholders’ equity that result from transactions and economic events other than those with stockholders. For all periods presented, there was no difference between net loss and comprehensive loss.
(F) Collaboration Arrangement
License and Collaboration Agreement with Takeda Pharmaceutical Company Limited
The Company accounts for the license and collaboration agreement with Takeda Pharmaceutical Company Limited (“Takeda”) in accordance with Accounting Standard Codification (“ASC”) 808 – “Collaborative Arrangements.” As Ovid and Takeda are sharing 50/50 in the drug development and throughout the life of this compound, the Company records 50% of the development costs in research and development. When Ovid incurs the majority of the costs and Takeda transfers a payment to Ovid to equalize the costs, Ovid records the participation by Takeda as a reduction of its research and development expenses, as the parties under the collaboration are sharing in the costs and the payment represents reimbursement of costs by Takeda. When Takeda incurs the majority of the costs and Ovid transfers a payment to Takeda (to equalize the costs), Ovid records the participation in Takeda’s expenses as research and development costs in its statement of operations, as Ovid and Takeda are sharing in the research and development activities and this participation represents Ovid’s share of the research and development costs in the specific period.
(G) Cash and Cash Equivalents
The Company’s cash and cash equivalents consist of cash held in checking accounts and money market funds. The Company considers all highly liquid investments with an original maturity date of three months or less to be cash and cash equivalents. Accounts held at U.S. financial institutions are insured by the FDIC up to $250,000. Cash balances could exceed insured amounts at any given time.
(H) Property and Equipment
Property and equipment are stated at cost and depreciated over their estimated useful lives of three years using the straight-line method. Repairs and maintenance costs are expensed. The Company reviews the recoverability of all long-lived assets, including the related useful life, whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset might not be recoverable.
(I) Research and Development Expenses
The Company expenses the cost of research and development as incurred. Research and development expenses comprise costs incurred in performing research and development activities, including clinical trial costs, manufacturing costs for both clinical and pre-clinical materials as well as other contracted services, license fees, and other external costs. Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity is performed or when the goods have been received, rather than when the cost is incurred, in accordance with Accounting Standards Codification (“ASC”) 730, Research and Development.
(J) Stock-Based Compensation
The Company accounts for its stock-based compensation in accordance with ASC 718, Compensation—Stock Compensation, which establishes accounting for stock-based awards granted to employees for services and requires companies to expense the estimated fair value of these awards over the requisite service period. The Company estimates the fair value of all awards granted using the Black-Scholes valuation model. Key inputs and assumptions include the expected term of the option, stock price volatility, risk-free interest rate, dividend yield, stock price and exercise price. Many of the assumptions require significant judgment and any changes could have a material impact in the determination of stock-based compensation expense. The Company elected an accounting policy to record forfeitures as they occur. The Company recognizes employee stock-based compensation expense based on the fair value of the award on the date of the grant. The compensation expense is recognized over the vesting period under the straight-line method.
The Company accounts for options awards granted to nonemployee consultants and directors under ASC 505 Equity. The fair value of the option issued or committed to be issued is used to measure the transaction, as this is more reliable than the fair value of the services received. The fair value is measured at the value of the Company’s common stock at the earlier of the date that the commitment for performance by the counterparty has been reached or the counterparty’s performance is complete. Awards granted to
F-8
nonemployees are remeasured to fair value at each period end date until vested and expensed on a straight-line basis over the vesting period.
(K) Fair Value of Financial Instruments
Financial Accounting Standards Board (“FASB”) guidance specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
|
|
• |
Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. The Company’s Level 1 assets consisted of money market funds totaling $86.6 million and $51.6 million as of December 31, 2017 and 2016, respectively. |
|
|
• |
Level 2—Inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly or indirectly (e.g., quoted prices of similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in markets that are not active). Level 2 includes financial instruments that are valued using models or other valuation methodologies. The Company had no Level 2 assets or liabilities as of December 31, 2017 and 2016. |
|
|
• |
Level 3—Unobservable inputs for the asset or liability. Financial instruments are considered Level 3 when their fair values are determined using pricing models, discounted cash flows or similar techniques and at least one significant model assumption or input is unobservable. The Company had no Level 3 assets or liabilities as of December 31, 2017 and 2016. |
The carrying amounts reported in the balance sheets for cash and cash equivalents, other current assets, accounts payable, and accrued expenses approximate their fair value based on the short-term maturity of these instruments.
(L) Income Taxes
The Company accounts for income taxes under the asset and liability method, which requires deferred tax assets and liabilities to be recognized for the estimated future tax consequences attributable to differences between financial statement carrying amounts and respective tax bases of existing assets and liabilities, as well as net operating loss carryforwards and research and development credit. Valuation allowances are provided if it is more likely than not that some portion or all of the deferred tax assets will not be realized. The impact of a change in the tax laws is recorded in the period in which the law is enacted.
(M) Net Loss Per Common Share
Basic and diluted net loss per common share is determined by dividing net loss attributable to common stockholders by the weighted-average common shares outstanding during the period. For all periods presented, the common shares underlying the Preferred Stock and options have been excluded from the calculation because their effect would be anti-dilutive. Therefore, the weighted-average shares outstanding used to calculate both basic and diluted loss per common share are the same.
The following potentially dilutive securities have been excluded from the computations of diluted weighted-average shares outstanding as they would be anti-dilutive:
|
|
|
For the Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Stock options to purchase common stock |
|
|
4,298,802 |
|
|
|
2,987,729 |
|
|
|
1,890,690 |
|
|
Preferred stock convertible into common stock |
|
|
- |
|
|
|
7,981,351 |
|
|
|
7,981,351 |
|
|
Total |
|
|
4,298,802 |
|
|
|
10,969,080 |
|
|
|
9,872,041 |
|
(N) Segment Data
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions.
(O) Retirement Plan
The Company maintains a 401(k)-retirement plan for its employees that is intended to qualify under Sections 401(a) and 501(a) of the U.S. Internal Revenue Code of 1986, as amended (“Code”), in 2016. The Company provides all active employees with a 100% matching contribution equal to 3% of an employee’s eligible compensation deferred and 50% matching contributions on employee
F-9
contributions that are between 3% and 5% of an employee’s eligible compensation deferred. These safe harbor contributions vest immediately. For the years ended December 31, 2017 and 2016 the Company contributed $163,942 and $36,606, respectively.
(P) Recent Accounting Pronouncements
Recent accounting standards which have been adopted
In March 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-09, "Compensation - Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting." ASU 2016-09 simplifies various aspects of the accounting for share-based payments. The simplifications include: (a) recording all tax effects associated with stock-based compensation through the income statement, as opposed to recording certain amounts in other paid-in capital, which eliminates the complications of tracking a “windfall pool,” but will increase the volatility of income tax expense; (b) allowing entities to withhold shares to satisfy the employer’s statutory tax withholding requirement up to the highest marginal tax rate applicable to employees rather than the employer’s minimum statutory rate, without requiring liability classification for the award; (c) modifying the requirement to estimate the number of awards that will ultimately vest by providing an accounting policy election to either estimate the number of forfeitures or recognize forfeitures as they occur; and (d) changing certain presentation requirements in the statement of cash flows, including removing the requirement to present excess tax benefits as an inflow from financing activities and an outflow from operating activities, and requiring the cash paid to taxing authorities arising from withheld shares to be classified as a financing activity. ASU 2016-09 is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Early adoption is permitted for any entity in any interim or annual period.
The Company early adopted ASU 2016-09 as of September 30, 2016 on a retroactive basis to the beginning of the period. In connection with the early adoption, the Company elected an accounting policy to record forfeitures as they occur. There was no financial statement impact upon adoption for the above accounting policy election. In addition, there was no financial statement impact of adopting ASU 2016-09 provisions regarding recognition of tax effects associated with stock-based compensation, as the Company is in a net operating loss (“NOL”) position with a full valuation allowance. Also, for the period from inception through December 31, 2016, the Company did not record an income statement benefit for excess tax benefits as there were no exercises of options during the period.
New accounting standards which have not yet been adopted
In May 2017, the FASB issued ASU No. 2017-09, Compensation - Stock Compensation (Topic 718): Scope of Modification Accounting. This new standard clarifies when to account for a change to the terms or conditions of share-based payment award as a modification. Under the new guidance, modification accounting is required unless the fair value, the vesting conditions, and the classification of the award remain the same as the original award. ASU 2017-09 is effective for public companies for fiscal years beginning on or after December 15, 2017. The adoption of this standard is not expected to have a material impact on the Company’s financial statements.
In January 2017, the FASB issued ASU No. 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business. This new standard clarifies the definition of a business and provides a screen to determine when an integrated set of assets and activities is not a business. The screen requires, among others, that when substantially all of the fair value of the gross assets acquired (or disposed of) is concentrated in a single identifiable asset or a group of similar identifiable assets, the set is not a business. ASU 2017-01 is effective for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. The adoption of this standard is not expected to have a material impact on the Company’s financial statements.
F-10
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments. The new standard clarifies certain aspects of the statement of cash flows, including the classification of debt prepayment or debt extinguishment costs, settlement of zero-coupon debt instruments or other debt instruments with coupon interest rates that are insignificant in relation to the effective interest rate of the borrowing, contingent consideration payments made after a business combination, proceeds from the settlement of insurance claims, proceeds from the settlement of corporate-owned life insurance policies, distributions received from equity method investees and beneficial interests in securitization transactions. The new standard also clarifies that an entity should determine each separately identifiable source or use within the cash receipts and cash payments on the basis of the nature of the underlying cash flows. In situations in which cash receipts and payments have aspects of more than one class of cash flows and cannot be separated by source or use, the appropriate classification should depend on the activity that is likely to be the predominant source or use of cash flows for the item. ASU 2016-15 is effective for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2017. The adoption of this standard is not expected to have a material impact on the Company’s statements of cash flows upon adoption.
In February 2016, the FASB issued ASU 2016-02, “Leases (Topic 842).” ASU 2016-02 was issued to increase transparency and comparability among entities by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about lease arrangements. ASU 2016-02 is effective for public companies for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The Company does not expect ASU 2016-02 to have a material impact on its results of operations and financial position.
NOTE 3 – PRECLINICAL AND CLINICAL AGREEMENTS
On August 26, 2016, the Company contracted with a clinical research organization for the study entitled “Safety and Efficacy of Gaboxadol in Angelman Syndrome: A Phase 2 Study of OV101 in adolescents and adults.”. In connection with the execution of this contract, the Company provided an upfront retainer of $355,435. This retainer is reflected within current assets on the balance sheet. During the year ended December 31, 2017, the Company has expensed approximately $4,286,256 related to this contract.
NOTE 4 – PROPERTY AND EQUIPMENT AND INTANGIBLE ASSETS
Property and equipment is summarized as follows:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2017 |
|
|
2016 |
|
||
|
Furniture and equipment |
|
$ |
102,690 |
|
|
$ |
63,783 |
|
|
Less accumulated depreciation |
|
|
(50,915 |
) |
|
|
(20,192 |
) |
|
Total property, plant and equipment, net |
|
$ |
51,775 |
|
|
$ |
43,591 |
|
Depreciation expense was $26,917, $16,289, $3,903 for the years ended December 31, 2017, 2016, and 2015, respectively.
Intangible assets, net of accumulated amortization, were $124,194 and $110,074 as of December 31, 2017 and December 31, 2016, respectively, and are included in other assets. Amortization expense was $53,393, $40,223, and $7,875 for the years ended December 31, 2017, 2016, and 2015 respectively.
NOTE 5 – ACCRUED EXPENSES
Accrued expenses consist of the following:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2017 |
|
|
2016 |
|
||
|
Collaboration agreement accrual |
|
$ |
754,841 |
|
|
$ |
- |
|
|
Payroll and bonus accrual |
|
|
1,919,120 |
|
|
|
1,324,649 |
|
|
Professional fees accrual |
|
|
321,852 |
|
|
|
874,525 |
|
|
Clinical trials accrual |
|
|
753,018 |
|
|
|
409,804 |
|
|
Other |
|
|
246,503 |
|
|
|
267,265 |
|
|
Total |
|
$ |
3,995,334 |
|
|
$ |
2,876,243 |
|
NOTE 6 – STOCKHOLDERS’ EQUITY AND PREFERRED STOCK
The Company’s capital structure consists of common stock and Preferred Stock with certain rights and privileges summarized below.
The Company was initially authorized to issue 1,000 shares of common stock at $0.001 par value per share. The Company’s certificate of incorporation was amended on January 6, 2017 to increase the authorized shares of common stock available for issuance to 62,000,000 at $0.001 par value, and shares of Preferred Stock to 20,991,252.
F-11
The holders of common stock are entitled to one vote for each share held. The holders of common stock have no preemptive or other subscription rights, and there are no redemption or sinking fund provisions with respect to such shares. The common stock is subordinate to all series of Preferred Stock with respect to dividend rights and rights upon liquidation, winding up and dissolution of the Company. The holders of common stock are entitled to liquidation proceeds after all liquidation preferences for the Preferred Stock are satisfied.
On May 10, 2017, the Company filed an amended and restated certificate of incorporation with the Secretary of the State of Delaware, which was approved by the Company’s Board of Directors and stockholders on April 12, 2017 and April 24, 2017, respectively, and which went effective immediately after the closing of the Company’s IPO on May 10, 2017. Pursuant to the amended and restated certificate of incorporation, the Company is authorized to issue 125,000,000 shares of common stock and 10,000,000 shares of preferred stock. Upon completion of its IPO, on May 10, 2017, the Company issued 5,000,000 shares of its common stock, and 2,382,069 shares of Series A Preferred Stock, 5,599,282 shares of Series B Preferred Stock and 1,781,996 shares Series B-1 Preferred Stock were converted into 9,763,346 shares of common stock.
The holders of the Preferred Stock had the following rights and preferences:
Voting Rights
The holders of Preferred Stock are entitled to vote, together with the holders of common stock, on all matters submitted to stockholders for a vote, except the election of common stock directors and except as required by law. Each preferred stockholder is entitled to the number of votes equal to the number of shares of common stock into which each share of Preferred Stock is convertible as of the record date for determining stockholders entitled to vote on such matter.
Liquidation Preferences
In the event that the Company liquidates, dissolves or winds up, whether voluntarily or involuntarily, or sells all or substantially all of its assets, or sells the Company or a controlling interest in the Company or if certain events deemed to be a liquidation occur, then first, the holders of Series B Preferred Stock and the holders of Series B-1 Preferred Stock shall be entitled to receive, in each case on a pari passu basis, in preference to holders of Series A Preferred Stock and common stock, an amount per share equal to the greater of the (i) the original purchase price for the Series B Preferred Stock and Series B-1 Preferred Stock, as applicable, plus any dividends, if declared but unpaid thereon, or (ii) amount per share as would have been payable had all shares of Series B Preferred Stock or Series B-1 Preferred Stock, as applicable, been converted into common stock immediately prior to the liquidation event. After payment of required amounts to the holder of Series B Preferred Stock and Series B-1 Preferred Stock, the holders of shares of Series A Preferred Stock shall be entitled to receive in preference to holders of common stock, an amount per share equal to the greater of the (i) the original purchase price for the Series A Preferred Stock, plus any dividends, if declared but unpaid thereon, or (ii) amount per share as would have been payable had all shares of Series A Preferred Stock been converted into common stock immediately prior to the liquidation event. Following all preferential payments to holders of Preferred Stock as required, any remaining undistributed assets shall be shared ratably with all common stockholders.
Dividends
The holders of the Preferred Stock are entitled to receive, if declared by the Board, non-cumulative dividends at the rate of 8% of the original purchase price per annum. Such dividends shall only be payable when, and if declared and are not cumulative. If dividends are declared, then preference is given in order to the Series B Preferred Stock and Series B-1 Preferred Stock, the Series A Preferred Stock and then the common stock.
The holders of Series B Preferred Stock and the holders of Series B-1 Preferred Stock have liquidation and dividend rights in preference to holders of Series A Preferred and common stock. The holders of Series A Preferred Stock have liquidation and dividend rights in preference to holders of common stock. No dividends on the common stock shall be declared and paid unless dividends on the Preferred Stock have been declared and paid. Through December 31, 2017, the Company has not declared any dividends.
Redemption Rights
The Preferred Stock is not redeemable at the option of the holder.
Conversion Rights
Each share of Preferred Stock is convertible at any time at the option of the stockholder into fully paid and nonassessable shares of common stock determined by dividing the original purchase price by the conversion price in effect at the time of conversion. The original purchase price for Series A Preferred Stock, Series B Preferred Stock and Series B-1 Preferred Stock is $2.125, $13.395 and $14.513 per share, respectively. In the event that the Company issues additional shares of stock, stock splits and combination, dividends and distributions, the conversion price may be adjusted, with certain exceptions. In the event of a liquidation, dissolution, winding up or deemed liquidation event, the conversion rights will be terminated at the close of business on the last day preceding the date fixed for payment of liquidation amounts to the holders of Preferred Stock.
F-12
Mandatory Conversion
All outstanding shares of Preferred Stock will be automatically converted into shares of common stock upon a trigger event. A trigger event is defined as either (a) the closing of the sale of shares of common stock to the public in a firm-commitment underwritten public offering on the New York Stock Exchange, The Nasdaq Stock Market or other internationally recognized stock exchange, pursuant to an effective registration statement under the Securities Act of 1933, as amended, resulting in at least fifty million dollars ($50,000,000) of gross proceeds or (b) the date and time, or the occurrence of an event, specified by vote or written consent of the holders of at least sixty percent (60%) of the then outstanding shares of Preferred Stock and the holders of a majority of the Series B Preferred Stock and Series B-1 Preferred Stock (voting together as a single class).
The Preferred Stock is classified as permanent equity because the shares contain redemption features that are within the control of the Company. The Company believes the shares are not currently redeemable and it is not probable that a deemed liquidation event (including merger, acquisition or sale of all or substantially all of the Company’s assets) will occur to trigger redemption. There was no accretion of Preferred Stock to redemption value recorded as of December 31, 2017.
Conversion of Preferred Stock Upon IPO
Prior to the Company’s IPO, the holders of the Company’s Series A Preferred Stock, Series B Preferred Stock and Series B-1 Preferred Stock had certain voting rights, dividend rights, liquidation preferences and conversion privileges. Upon completion of the Company’s IPO, all shares of outstanding convertible preferred stock were automatically converted into an aggregate of 9,763,346 shares of common stock. All rights, preferences and privileges associated with the outstanding convertible preferred stock were terminated upon this conversion.
As of December 31, 2017, no shares of preferred stock were issued or outstanding.
NOTE 7 – STOCK-BASED COMPENSATION
On August 29, 2014, the Company’s Board of Directors adopted and approved the 2014 Equity Incentive Plan (the “2014 Plan”), which authorized the Company to grant shares of common stock in the form of incentive stock options, nonstatutory stock options, stock appreciation rights, restricted stock and restricted stock units. The types of stock-based awards, including share purchase rights amount, terms, and exercisability provisions of grants are determined by the Company’s Board of Directors. The purpose of the 2014 Plan is to provide the Company with the flexibility to issue stock-based awards as part of an overall compensation package to attract and retain qualified personnel.
The Company's Board of Directors adopted and the Company's stockholders approved the 2017 equity incentive plan (“2017 Plan”), which became effective immediately prior to the execution of the underwriting agreement related to the IPO on May 4, 2017. The 2017 Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance-based stock awards, and other forms of stock-based awards. Additionally, the 2017 Plan provides for the grant of performance cash awards. The Company's employees, officers, directors and consultants and advisors are eligible to receive awards under the 2017 Plan. With the adoption of the 2017 Plan, no further awards will be granted under the 2014 Plan. The Company may issue up to 3,052,059 shares of common stock under the 2017 Plan. The 2017 Plan allows for each January 1, pursuant to the terms of the 2017 Equity Incentive Plan, the Plan Limit shall be increased by the lesser of (x) 5% of the number of shares of Common Stock outstanding as of the immediately preceding December 31 and (y) such lesser number as the Board of Directors may determine in its discretion.
The Company's Board of Directors adopted and the Company's stockholders approved the 2017 employee stock purchase plan (the “2017 ESPP”), which became effective immediately prior to the execution of the underwriting agreement related to the IPO on May 4, 2017. The initial reserve of shares of common stock that may be issued under the 2017 ESPP is 279,069 shares. On September 20, 2017, the Company’s Compensation Committee approved an offering period under the 2017 ESPP, which began on October 20, 2017. The 2017 ESPP allows employees to purchase common stock of the Company at a 15% discount to the market price on designated exercise dates. During the year ended December 31, 2017, no shares were purchased under the 2017 ESPP and the Company recorded expense of $12,100.
Unless specified otherwise in an individual option agreement, stock options granted under the 2014 Plan and 2017 Plan generally have a ten-year term and a four-year graded vesting period. The vesting requirement is conditioned upon the grantee’s continued service with the Company during the vesting period. Once vested, all awards are exercisable from the date of grant until they expire. The option grants are non-transferable. Vested options generally remain exercisable for 90 days subsequent to the termination of the option holder’s service with the Company. In the event of option holder’s death or disability while employed by or providing service to the Company, the exercisable period extends to twelve months.
F-13
Performance-based option awards generally have similar vesting terms, with vesting commencing on the date the performance condition is achieved and expire in accordance to the specific terms of the agreement. At December 31, 2017, there were 50,000 performance-based options outstanding and unvested. These awards immediately vest upon meeting certain business development conditions. The Company recognizes stock-based compensation expense based on the grant date fair value of the award over the vesting period when the performance condition becomes probable of being achieved.
The fair value of options granted during the years ended December 31, 2017, 2016 and 2015 was estimated using the Black-Scholes option valuation model. The inputs for the Black-Scholes option valuation model require management’s significant assumptions and are detailed in the table below. Prior to the IPO, the common stock price was determined by the Board of Directors. In the absence of market data for the Company’s common stock, the Board of Directors considered various factors in estimating the fair value of the common stock at the time of each option grant which included but was not limited to the common stock valuation performed by a third party independent valuation firm, the Company’s performance and future economic outlook, the potential financing available to the Company, and the valuation of common stock of similar companies in the industry. The risk-free interest rates were based on the rate for U.S. Treasury securities at the date of grant with maturity dates approximately equal to the expected life at the grant date. The expected life was based on the simplified method in accordance with the SEC Staff Accounting Bulletin Nos. 107 and 110 as the Company’s shares just recently became publicly traded. The expected volatility was estimated based on historical volatility information of peer companies that are publicly available.
All assumptions used to calculate the grant date fair value of nonemployee options are generally consistent with the assumptions used for options granted to employees. In the event the Company terminates any of its consulting agreements, the unvested options underlying the agreements would also be cancelled. Unvested nonemployee options are marked-to-market at each reporting period.
The Company granted 27,906, 34,882, and 11,627 stock options to nonemployee consultants for services rendered during the years ended December 31, 2017, 2016, and 2015, respectively. There were 37,066, 73,398, and 59,591 unvested nonemployee options outstanding as of December 31, 2017, 2016, and 2015, respectively. Total expense recognized related to the nonemployee stock options for the years ended December 31, 2017, 2016, and 2015 was $416,457, $351,384 and $449,010, respectively. Total unrecognized compensation expenses related to the nonemployee stock options was $271,294 as of December 31, 2017. During the year ended December 31, 2017, the Company recognized $162,700 in expenses for non-employee performance-based option awards.
The Company granted 1,510,436, 1,336,573, and 1,872,087 stock options to employees during the years ended December 31, 2017, 2016, and 2015, respectively. There were 2,528,063, 2,231,275, and 1,872,087 unvested employee options outstanding as of December 31, 2017, 2016, and 2015, respectively. Total expense recognized related to the employee stock options for the years ended December 31, 2017, 2016, and 2015 was $5,969,204, $2,485,323, and $1,293,388 (of which $381,000 was issued in connection with a separation agreement) respectively. Total unrecognized compensation expense related to employee stock options was $12,237,394 as of December 31, 2017. During the year ended December 31, 2017, the Company recognized $830,997 in expenses for employee performance-based option awards.
The Company’s stock-based compensation expense was recognized in operating expense as follows:
|
|
|
For the Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Research and development |
|
$ |
2,417,727 |
|
|
$ |
1,506,036 |
|
|
$ |
513,669 |
|
|
General and administrative |
|
|
3,980,029 |
|
|
|
2,135,430 |
|
|
|
943,644 |
|
|
Total |
|
$ |
6,397,756 |
|
|
$ |
3,641,466 |
|
|
$ |
1,457,313 |
|
The fair value of employee options granted during the years ended December 31, 2017, 2016, and 2015, respectively, was estimated by utilizing the following assumptions:
|
|
|
For the Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
Weighted Average |
|
|
Weighted Average |
|
|
Weighted Average |
|
|||
|
Volatility |
|
|
80.39 |
% |
|
|
82.78 |
% |
|
|
76.38 |
% |
|
Expected term in years |
|
|
6.08 |
|
|
|
6.07 |
|
|
|
6.03 |
|
|
Dividend rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
Risk-free interest rate |
|
|
2.06 |
% |
|
|
1.45 |
% |
|
|
1.82 |
% |
|
Fair value of option on grant date |
|
$ |
6.30 |
|
|
$ |
4.95 |
|
|
$ |
5.53 |
|
F-14
The fair value of nonemployee options granted and remeasured during the years ended December 31, 2017, 2016, and 2015, respectively, was estimated by utilizing the following assumptions:
|
|
|
For the Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
|
|
Weighted Average |
|
|
Weighted Average |
|
|
Weighted Average |
|
|||
|
Volatility |
|
|
81.44 |
% |
|
|
82.62 |
% |
|
|
79.90 |
% |
|
Expected term in years |
|
|
4.39 |
|
|
|
4.89 |
|
|
|
5.13 |
|
|
Dividend rate |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
|
0.00 |
% |
|
Risk-free interest rate |
|
|
2.08 |
% |
|
|
1.37 |
% |
|
|
1.63 |
% |
|
Fair value of option on grant date |
|
$ |
6.80 |
|
|
$ |
4.76 |
|
|
$ |
7.59 |
|
The following table summarizes the number of options outstanding and the weighted average exercise price:
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted |
|
|
Average |
|
|
|
|
|
||
|
|
|
|
|
|
|
Average |
|
|
Remaining |
|
|
Aggregate |
|
|||
|
|
|
Number of |
|
|
Exercise |
|
|
Contractual |
|
|
Intrinsic |
|
||||
|
|
|
Shares |
|
|
Price |
|
|
Life in Years |
|
|
Value |
|
||||
|
Options outstanding at December 31, 2014 |
|
|
69,766 |
|
|
$ |
0.22 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
1,883,714 |
|
|
|
8.24 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
Options Outstanding December 31, 2015 |
|
|
1,953,480 |
|
|
$ |
7.95 |
|
|
|
8.18 |
|
|
$ |
780,500 |
|
|
Vested and exercisable at December 31, 2015 |
|
|
23,255 |
|
|
$ |
0.22 |
|
|
|
8.71 |
|
|
$ |
567,000 |
|
|
Granted |
|
|
1,294,711 |
|
|
$ |
6.87 |
|
|
|
|
|
|
|
|
|
|
Exercised |
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(260,462 |
) |
|
|
8.18 |
|
|
|
|
|
|
|
|
|
|
Options Outstanding December 31, 2016 |
|
|
2,987,729 |
|
|
$ |
7.46 |
|
|
|
8.82 |
|
|
$ |
837,036 |
|
|
Vested and exercisable at December 31, 2016 |
|
|
683,070 |
|
|
$ |
7.71 |
|
|
|
8.47 |
|
|
$ |
253,969 |
|
|
Granted |
|
|
1,538,342 |
|
|
|
9.03 |
|
|
|
9.19 |
|
|
|
|
|
|
Exercised |
|
|
(4,320 |
) |
|
|
6.26 |
|
|
|
|
|
|
|
|
|
|
Forfeited |
|
|
(222,949 |
) |
|
|
6.61 |
|
|
|
|
|
|
|
|
|
|
Options Outstanding December 31, 2017 |
|
|
4,298,802 |
|
|
$ |
8.07 |
|
|
|
8.32 |
|
|
$ |
8,174,686 |
|
|
Vested and exercisable at December 31, 2017 |
|
|
1,733,673 |
|
|
$ |
7.65 |
|
|
|
7.92 |
|
|
$ |
3,865,036 |
|
At December 31, 2017 there was approximately $12,508,689 of unamortized share–based compensation expense, which is expected to be recognized over a remaining average vesting period of 2.57 years.
NOTE 8 – INCOME TAXES
At December 31, 2017, the Company has available approximately $59,943,000 and $62,478,000 of unused NOL carryforwards for federal and state tax purposes, respectively, that may be applied against future taxable income. The Company also has approximately $59,701,000 of unused NOL carryforwards for New York City purposes. The NOL carryforwards will begin to expire in the year 2035 if not utilized prior to that date. There is no provision for income taxes because the Company has historically incurred operating losses and maintains a full valuation allowance against its net deferred tax assets. The valuation allowance increased by approximately $18,971,000, $10,174,000, and $5,992,200 during the years 2017, 2016, and 2015, respectively, and was approximately $35,285,000, $16,314,000, and $6,140,000 at December 31, 2017, 2016, and 2015, respectively.
The Tax Cuts and Jobs Act (“Tax Act”) was enacted on December 22, 2017. The Act reduces the U.S. corporate rate from 34% to 21% beginning in 2018. The Company re-measured its deferred tax assets based upon the new 21% tax rate. As a result, the Company decreased its deferred tax assets by $10,605,000, with a corresponding adjustment to its valuation allowance for the year ended December 31, 2017
The Company may be subject to the NOL utilization provisions of Section 382 of the Code. The effect of an ownership change would be the imposition of an annual limitation on the use of NOL carryforwards attributable to periods before the change. The amount of the annual limitation depends upon the value of the Company immediately before the change, changes to the Company’s capital during a specified period prior to the change, and the federal published interest rate. The Company has not completed a Section 382 analysis to determine if a change in ownership has occurred. Until an analysis is completed, there can be no assurance that the existing net operating loss carry-forwards or credits are not subject to significant limitation.
F-15
The Company’s reserves related to taxes are based on a determination of whether and how much of a tax benefit taken by the Company in its tax filings or positions is more likely than not to be realized following resolution of any potential contingencies related to the tax benefit. For the years ended December 31, 2017, 2016, and 2015, the Company had no unrecognized tax benefits or related interest and penalties accrued. The Company has not, yet, conducted a study of research and development credit carryforwards. This study may result in an adjustment to the Company’s research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company’s research and development credits and, if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. Thus, there would be no impact to the balance sheet or statement of operations if an adjustment were required. The Company would recognize both accrued interest and penalties related to unrecognized benefits in income tax expense. The Company’s uncertain tax positions are related to years that remain subject to examination by relevant tax authorities. Since the Company is in a loss carryforward position, the Company is generally subject to examination by the U.S. federal, state and local income tax authorities for all tax years in which a loss carryforward is available.
The tax effects of temporary differences that gave rise to significant portions of the deferred tax assets were as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
Deferred tax assets/liabilities: |
|
|
|
|
|
|
|
|
|
Net operating loss carryovers |
|
$ |
20,880,120 |
|
|
$ |
11,711,643 |
|
|
Research and development tax credits |
|
|
714,087 |
|
|
|
348,816 |
|
|
Share-based compensation |
|
|
3,392,111 |
|
|
|
2,035,042 |
|
|
Accrued compensation |
|
|
633,745 |
|
|
|
600,663 |
|
|
Depreciation |
|
|
(10,778 |
) |
|
|
(10,318 |
) |
|
Charitable contributions |
|
|
53,038 |
|
|
|
- |
|
|
Intangible assets |
|
|
9,622,535 |
|
|
|
1,628,471 |
|
|
Total gross deferred tax assets/liabilities |
|
|
35,284,858 |
|
|
|
16,314,317 |
|
|
Valuation allowance |
|
|
(35,284,858 |
) |
|
|
(16,314,317 |
) |
|
Net deferred tax assets (liabilities) |
|
$ |
- |
|
|
$ |
- |
|
A reconciliation of the statutory U.S. Federal rate to the company’s effective tax rate is as follows:
|
|
|
December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Federal income tax benefit at statutory rate |
|
|
(34.00 |
) |
|
|
(34.00 |
) |
|
|
(34.00 |
) |
|
State income tax, net of federal benefit |
|
|
(11.77 |
) |
|
|
(11.35 |
) |
|
|
(11.33 |
) |
|
Permanent items |
|
|
0.89 |
|
|
|
1.16 |
|
|
|
0.31 |
|
|
Change in valuation allowance |
|
|
29.18 |
|
|
|
45.64 |
|
|
|
45.45 |
|
|
Research and development tax credits |
|
|
(0.56 |
) |
|
|
(1.26 |
) |
|
|
(0.51 |
) |
|
Deferred re-measurement |
|
|
16.31 |
|
|
|
- |
|
|
|
- |
|
|
Other |
|
|
(0.05 |
) |
|
|
(0.19 |
) |
|
|
0.08 |
|
|
Effective income tax (benefit) expense rate |
|
|
0 |
% |
|
|
0 |
% |
|
|
0 |
% |
NOTE 9 – COMMITMENTS AND CONTINGENCIES
License Agreements
On March 26, 2015, the Company entered into an exclusive agreement with H. Lundbeck A/S (“Lundbeck”) for a worldwide perpetual licensing right related the research, development and commercialization of OV101.
Pursuant to the Lundbeck license agreement, the Company agreed to make milestone payments totaling up to $181 million upon the achievement of certain development, regulatory and sales milestones. The first payment of $10 million is due upon the successful completion of the first Phase 3 trial for a product in which OV101 is an active ingredient. In addition, the agreement calls for the Company to pay royalties for an initial term based on a low double-digit percentage of sales and provides for the reduction of royalties in certain limited circumstances.
In connection with the Lundbeck license agreement, the Company issued 489,756 shares of common stock valued at $4,011,842. The value of the common stock was based on the fair value of the Company’s common stock of $8.20 per share as determined by the Company’s Board of Directors in the same period for the purpose of option grants.
F-16
In connection with the Lundbeck license agreement, the Company paid $250,000 to another entity as a milestone payment for securing the license granted under the Lundbeck license agreement.
Since the intangibles acquired in the Lundbeck license agreement do not have alternative future use, all costs incurred were treated as research and development expense. The Company recorded a total of $4,270,024 as research and development expenses related to this agreement in 2015.
On December 15th, 2016, we entered into a license agreement with Northwestern University, or Northwestern, pursuant to which Northwestern granted us an exclusive, worldwide license to patent rights in certain inventions, or the Northwestern Patent Rights, which relate to a specific compound and related methods of use for such compound, along with certain Know-How related to the practice of the inventions claimed in the Northwestern Patents.
Under the Northwestern agreement, the Company was granted exclusive rights to research, develop, manufacture and commercialize products utilizing the Northwestern Patent Rights for all uses. The Company has agreed that we will not use the Northwestern Patent Rights to develop any products for the treatment of cancer, but Northwestern may not grant rights in the technology to others for use in cancer. The Company also has an option, exercisable during the term of the agreement to an exclusive license under certain intellectual property rights covering novel compounds with the same or similar mechanism of action as the primary compound that is the subject of the license agreement. Northwestern has retained the right, on behalf of itself and other non-profit institutions, to use the Northwestern Patent Rights and practice the inventions claimed therein for educational and research purposes and to publish information about the inventions covered by the Northwestern Patent Rights.
Upon entry into the Northwestern agreement, the Company paid an upfront non-creditable one-time license issuance fee of $75,000, and is required to pay an annual license maintenance fee of $20,000, which will be creditable against any royalties payable to Northwestern following first commercial sale of licensed products under the agreement. The Company is responsible for all ongoing costs of filing, prosecuting and maintaining the Northwestern Patents, but also has the right to control such activities using its own patent counsel. In consideration for the rights granted to the Company under the Northwestern agreement, the Company is required to pay to Northwestern up to an aggregate of $5.3 million upon the achievement of certain development and regulatory milestones for the first product covered by the Northwestern Patents, and, upon commercialization of any such products, will be required to pay to Northwestern a tiered royalty on net sales of such products by the Company, its affiliates or sublicensees, at percentages in the low to mid single-digits, subject to standard reductions and offsets. The Company’s royalty obligations continue on a product-by-product and country-by-country basis until the later of the expiration of the last-to-expire valid claim in a licensed patent covering the applicable product in such country and 10 years following the first commercial sale of such product in such country. If the Company sublicenses a Northwestern Patent Right, it will be obligated to pay to Northwestern a specified percentage of sublicense revenue received by the Company, ranging from the high single digits to the low-teens.
The Northwestern agreement requires that the Company use commercially reasonable efforts to develop and commercialize at least one product that is covered by the Northwestern Patent Rights.
Unless earlier terminated, the Northwestern agreement will remain in force until the expiration of the Company’s payment obligations thereunder. The Company has the right to terminate the agreement for any reason upon prior written notice or for an uncured material breach by Northwestern. Northwestern may terminate the agreement for the Company’s uncured material breach or insolvency.
Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, and penalties and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred. The Company is not currently involved in any legal matters arising in the normal course of business.
Under the terms of their respective employment agreements, each of our named executive officers is eligible to receive severance payments and benefits upon a termination without “cause” or due to “permanent disability,” or upon “resignation for good reason,” contingent upon the named executive officer’s delivery to us of a satisfactory release of claims, and subject to the named executive officer’s compliance with non-competition and non-solicitation restrictive covenants for two years following the termination date.
NOTE 10 – COLLABORATION AGREEMENT
Takeda Collaboration
On January 6, 2017, the Company entered into a license and collaboration agreement with Takeda, pursuant to which Takeda granted the Company an exclusive license to commercialize the compound TAK-935, which the Company now refers to as OV935, in certain territories, and a co-exclusive worldwide license, together with Takeda, to develop OV935. In consideration of certain license rights granted to the Company pursuant to the Takeda collaboration, the Company issued 1,781,996 shares of its Series B-1 Preferred Stock (Note 6), pursuant to a Series B-1 preferred stock purchase agreement entered into on January 6, 2017, at an ascribed price per share
F-17
of $14.513 on January 6, 2017 for an aggregate fair value of $25,861,228, which was recorded as research and development expense at the date of the transaction. Under the Takeda collaboration, the Company is obligated to pay Takeda future payments if and when certain milestones are achieved. Upon the first patient enrollment in the first Phase 3 trial for the first of the initial indications the Company and Takeda are focusing on in the Takeda collaboration, the Company is obligated to issue to Takeda the number of unregistered shares of the Company’s common stock equal to the lesser of (a) 8% of the Company outstanding capital stock on the issuance date or (b) $50.0 million divided by the applicable share price, unless certain events occur. In the event such payment would cause Takeda to own over 19.99% of our outstanding capital stock or other events occur, such payment must be paid in cash. The remaining potential global commercial and regulatory milestone payments equal approximately $35.0 million and can be satisfied in cash or unregistered shares of the Company’s common stock at its election, unless certain events occur. During the year ended December 31, 2017, the Company recognized $4,321,951 in research and development expenses representing research and development expenses reimbursed to Takeda in respect of this collaboration agreement. The 1,781,996 shares of Series B-1 Preferred Stock held by Takeda automatically converted into 1,781,996 shares of the Company’s common stock upon the completion of its IPO.
The Takeda collaboration will expire upon the cessation of commercialization of the products by both the Company and Takeda. Either party may terminate the Takeda collaboration because of the other party’s uncured material breach or insolvency, for safety reasons, or, after completion of the first proof of mechanism clinical trial, for convenience. Takeda may terminate the Takeda collaboration for the Company’s (or the Company’s sublicensee’s) challenge to the patents licensed under the Takeda collaboration. If the collaboration is terminated by Takeda for material breach by the Company, bankruptcy or patent challenge or by the Company for convenience or safety reasons, the Company’s rights to the products will cease, the Company will transition all activities related to the products to Takeda, and the Company will grant Takeda an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by the Company to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders. If the collaboration is terminated by the Company for Takeda’s material breach or bankruptcy or by Takeda for convenience or safety reasons, Takeda’s rights to the products will cease, Takeda will transition all activities related to the products to us, and Takeda will grant us an exclusive, royalty-bearing license under certain patents and other intellectual property controlled by Takeda to commercialize OV935 and products containing OV935 for the treatment of certain rare neurological disorders.
NOTE 11 – RELATED PARTY TRANSACTIONS
As of December 31, 2016, amounts due from related parties represented travel related expenses.
NOTE 12 – SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
The following tables contain selected quarterly financial information from 2017 and 2016. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair statement of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
|
|
Three Months Ended |
|
|||||||||||||
|
(in thousands, except per share data) |
March 31, |
|
|
June 30, |
|
|
September 30, |
|
|
December 31, |
|
||||
|
|
2017 |
|
|
2017 |
|
|
2017 |
|
|
2017 |
|
||||
|
Total operating expenses |
$ |
34,262 |
|
|
$ |
10,288 |
|
|
$ |
9,409 |
|
|
$ |
11,048 |
|
|
Total other income (expense), net |
|
23 |
|
|
|
40 |
|
|
|
51 |
|
|
|
88 |
|
|
Net loss |
$ |
(34,239 |
) |
|
$ |
(10,248 |
) |
|
$ |
(9,358 |
) |
|
$ |
(10,960 |
) |
|
Net loss applicable to common stockholders |
$ |
(34,239 |
) |
|
$ |
(10,248 |
) |
|
$ |
(9,358 |
) |
|
$ |
(10,960 |
) |
|
Net loss per share applicable to common stockholders - basic and diluted |
|
(3.48 |
) |
|
|
(0.57 |
) |
|
|
(0.38 |
) |
|
|
(0.45 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|||||||||||||
|
(in thousands, except per share data) |
March 31, |
|
|
June 30, |
|
|
September 30, |
|
|
December 31, |
|
||||
|
|
2016 |
|
|
2016 |
|
|
2016 |
|
|
2016 |
|
||||
|
Total operating expenses |
$ |
3,714 |
|
|
$ |
5,417 |
|
|
$ |
5,815 |
|
|
$ |
7,589 |
|
|
Total other income (expense), net |
|
32 |
|
|
|
31 |
|
|
|
30 |
|
|
|
27 |
|
|
Net loss |
$ |
(3,682 |
) |
|
$ |
(5,386 |
) |
|
$ |
(5,785 |
) |
|
$ |
(7,562 |
) |
|
Net loss applicable to common stockholders |
$ |
(3,682 |
) |
|
$ |
(5,386 |
) |
|
$ |
(5,785 |
) |
|
$ |
(7,562 |
) |
|
Net loss per share applicable to common stockholders - basic and diluted |
|
(0.37 |
) |
|
|
(0.55 |
) |
|
|
(0.59 |
) |
|
|
(0.77 |
) |
F-18
Equity Awards
From January 1, 2018 through the date of the filing of this Form 10-K, the Company has granted option awards for an aggregate of 976,476 shares to employees with a weighted average exercise price of $9.02.
Income Taxes
On February 15, 2018, the Company was approved for a $186,218 refundable credit towards future New York City tax expense. The credit is for qualified emerging technology companies (“QETCS”) focused on biotechnology located in New York City.
F-19
