Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - 9 METERS BIOPHARMA, INC. | ex-991pressrelease71921.htm |
| 8-K - 8-K - 9 METERS BIOPHARMA, INC. | nmtr-20210719.htm |

9 Meters – Lobesity Asset Purchase Acquisition July 19, 2021

Forward Looking Statements This presentation includes forward-looking statements based upon the Company's current expectations. Forward- looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: risks associated with making and integrating acquisitions; uncertainties associated with the clinical development and regulatory approval of product candidates; uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; risks related to the failure to realize any value from product candidates and preclinical programs being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; the impact of COVID-19 on our operations, enrollment in and timing of clinical trials; and risks related to the inability of the Company to obtain sufficient additional capital to continue to advance these product candidates and its preclinical programs. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements because of these risks and uncertainties. These and other risks and uncertainties are more fully described in periodic filings with the SEC, including the factors described in the section entitled "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended December 31, 2020, Form 10-Q for the quarter ended March 31, 2021, and in other filings that the Company has made and future filings the Company will make with the SEC. You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof or as of the dates indicated in the forward-looking statements. The Company expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. 2

Lobesity Company Overview • Privately-held company based in Cleveland, OH (Lead investor is Metro Health System) • Founded in 2015 by researchers Michael Wolfe, MD (inventor of Pepcid Complete® and former Chair of the Advisory Board for GI Drugs at FDA) and Michael Boylan, PhD (recognized expert in GI hormones and receptors) • Company is based on the founders’ years-long work on glucose-dependent insulinotropic peptide (GIP) – an incretin hormone secreted by the intestinal tract post-prandially which controls blood glucose and energy • A proprietary anti-GIP mAb, herein known as NM-136, targets circulating GIP ligand preventing it from binding its’ receptor which could potentially have profound effects in numerous metabolic disorders, including an orphan designated metabolic disease known as Prader-Willi Syndrome (PWS) 3
Transacted Key Deal Terms 4 9 Meters and Lobesity have agreed to an asset purchase agreement, pursuant to which 9 Meters will acquire NM-136 (formerly LOB-0136), related analogues, and all related intellectual property and other related assets of Lobesity for: • A combination of cash and equity consideration in the form of a $5 million upfront payment, as 40% cash and 60% equity* • Plus the right to contingent payments including certain worldwide regulatory and clinical milestone payments totaling up to $45.5 million • Global sales-related milestone payments totaling up to $50 million • And subject to certain adjustments, a mid-single digit royalty on worldwide net sales. *priced at 9 Meters’ 30-day volume weighted average price immediately prior to the closing of the transaction and issued in the form of unregistered common stock

▪ Glucose-dependent insulinotropic peptide (GIP) is a 42-amino acid hormone made in K-cells localized to the proximal gut, and it is secreted after eating food containing glucose and fat ▪ Although originally named asan acid blocker, the principal property of GIPis the stimulation of insulin release from pancreatic islet β-cells in the presence of glucose Accordingly, GIP is also known as “glucose-dependent insulinotropic polypeptide” ▪ GIPenhances gut nutrient absorption and increases glucose uptake into fat cells. - GIP also increases insulin & further increases nutrient uptake and storage NM-136 is a specific humanized anti-GIP monoclonal antibody: ▪ Lobesity has developed a highly specific monoclonal antibody which binds circulating GIP and removes it – unable to agonize the GIP receptor – behaving as a GIP antagonist Lobesity and GIP Monoclonal Antibody Obesity and related disorders of increased nutrient uptake and storage are facilitated byGIP. 5 Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007 May;132(6):2131-57.

NM-136 Complements and Adds Potential Strategic Optionality to 9 Meters Pipeline NM-136 Mechanism of Action: • GIP mAb decreases nutrient uptake & fat storage and potentially effective in metabolic and obesity-related disorders, where this is a key problem • GIP mAb also potentially decreases intestinal nutrient absorption 6 Boylan MO, Glazebrook PA, Tatalovic M, Wolfe MM. Am J Physiol Endocrinol Metab. 2015 Dec 15;309(12):E1008-18 NM-136 (proprietary anti-GIP mAb)

NM-136 Pre-Clinical Data Showed Key Improvements • In this study done in mice, NM-136 (30 mg/kg) attenuated the insulin response to oral glucose by 2/3 and eliminated the insulin response to co-administered glucose. • The effect at 60 mg/kg weekly on weight gain decreased by 46.5% and was equivalent to the low-fat diet control mice. • There were NOdifferences in the amount of food consumed among the treatmentgroups. Source: Boylan MO et al. Am JPhysiol 2015; 309:E1008-E1018: https://doi.org/10.1152/ajpendo.00345.2015 7

Prader-Willi Syndrome Overview Prader-Willi syndrome is characterized by: • Weight gain caused by hyperphagia: Chronic feeling of insatiable hunger causing morbid obesity • Hyperphagia starts at very young age and usually lasts until death • Intellectual/emotional disabilities and psychiatric disorders • Multiple hormonal deficiencies resulting in short stature and incomplete development • Metabolic dysfunction and early death Up to 20,000 13,000 –18,000 ~1:20,000INCIDENCE 30-40 YEARSMEDIAN AGE / MORTALITY PREVALENT CASES U.S. EUROPE 8 Bohonowych J, Miller J, McCandless SE, Strong TV. The Global Prader–Willi Syndrome Registry: Development, Launch, and Early Demographics. Genes. 2019; 10(9):713.
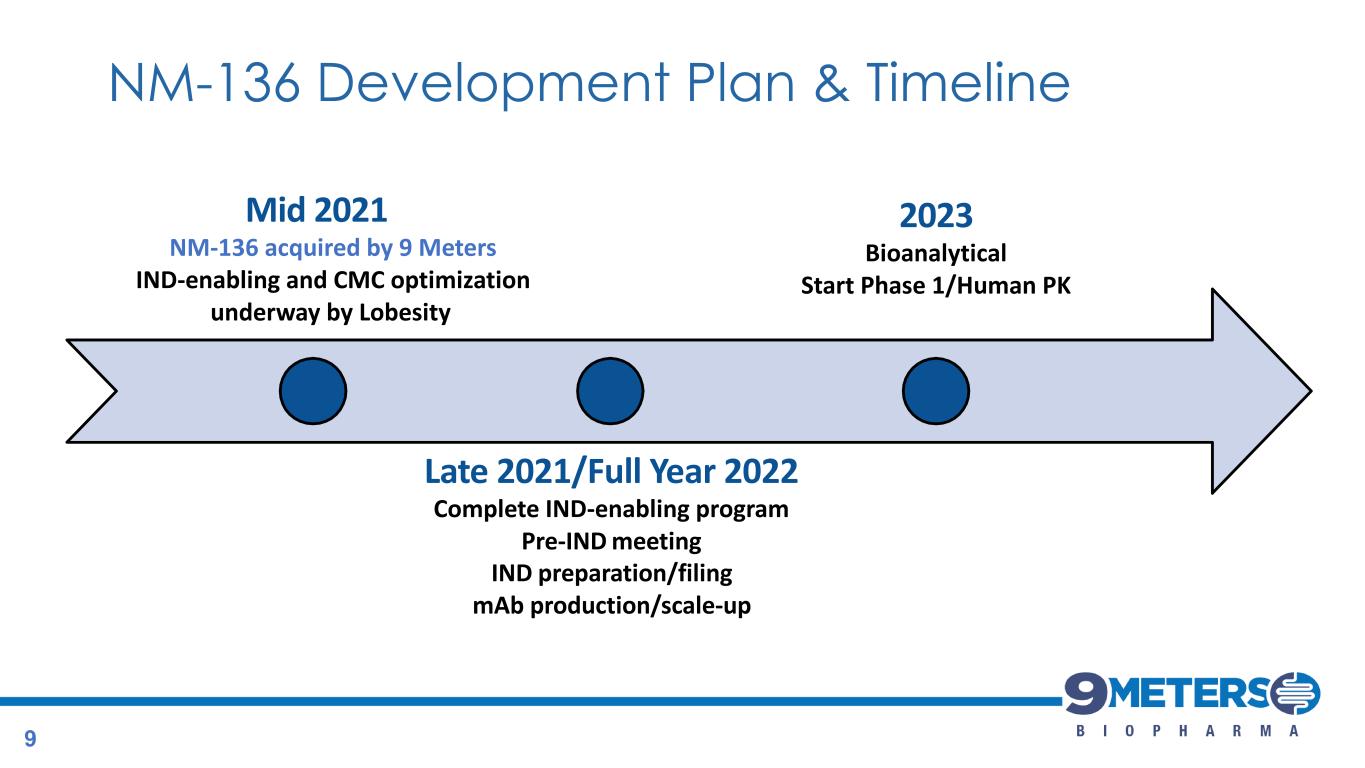
Mid 2021 NM-136 acquired by 9 Meters IND-enabling and CMC optimization underway by Lobesity Late 2021/Full Year 2022 Complete IND-enabling program Pre-IND meeting IND preparation/filing mAb production/scale-up 2023 Bioanalytical Start Phase 1/Human PK NM-136 Development Plan & Timeline 9

Thank You John Temperato President & CEO jtemperato@9meters.com Edward Sitar Chief Financial Officer esitar@9meters.com 8480 Honeycutt Road, Suite 120 Raleigh, NC 27615 Telephone: 1-919-275-1933 info@9meters.com www.9meters.com Twitter LinkedIn
