Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - IOVANCE BIOTHERAPEUTICS, INC. | tm2120950d1_ex99-1.htm |
| 8-K - FORM 8-K - IOVANCE BIOTHERAPEUTICS, INC. | tm2120950d1_8k.htm |
Exhibit 99.2

IOV - COM - 202 Cohort 3B : Clinical Data for LN - 145 in Patients with Metastatic Non - Small Cell Lung Cancer June 29, 2021 © 2021, Iovance Biotherapeutics, Inc 1

21.4% ORR for LN - 145 in Previously Treated Patients with mNSCLC © 2021, Iovance Biotherapeutics, Inc 2 IOV - COM - 202 Cohort 3B (NCT03645928) • After a median study follow - up of 8.2 months, median DOR was not reached • 24/28 patients (85.7%), including all responders, had received ≥2 prior lines of systemic therapy − All patients received prior anti − PD - 1/ anti − PD - L1 therapy and all responders also received prior chemotherapy • TEAEs were consistent with the underlying disease and known adverse event profiles of non - myeloablative lymphodepletion and IL - 2 (1) As assessed by investigator using RECIST 1.1. (2) One patient is reported as a CR based on a negative FDG - PET scan by investigator. DOR, duration of response; ICI, immune checkpoint inhibitors; IL - 2, interleukin - 2; mNSCLC, metastatic non - small cell lung cancer ; PD - 1, programmed cell death protein - 1; PD - L1, programmed death ligand - 1; TEAEs, treatment - emergent adverse events; TIL, tumor - infiltrating lymphocytes. Response, n (%) (1 ) Cohort 3B n=28 Objective Response Rate 6 (21.4) Complete Response (2 ) 1 (3.6) Partial Response 5 (17.9) Stable Disease 12 (42.9) Progressive Disease 6 (21.4) Non - Evaluable 4 (14.3) Disease Control Rate 18 (64.3) Median Duration of Response Not Reached Min, Max (months) 1.2+, 20.7+
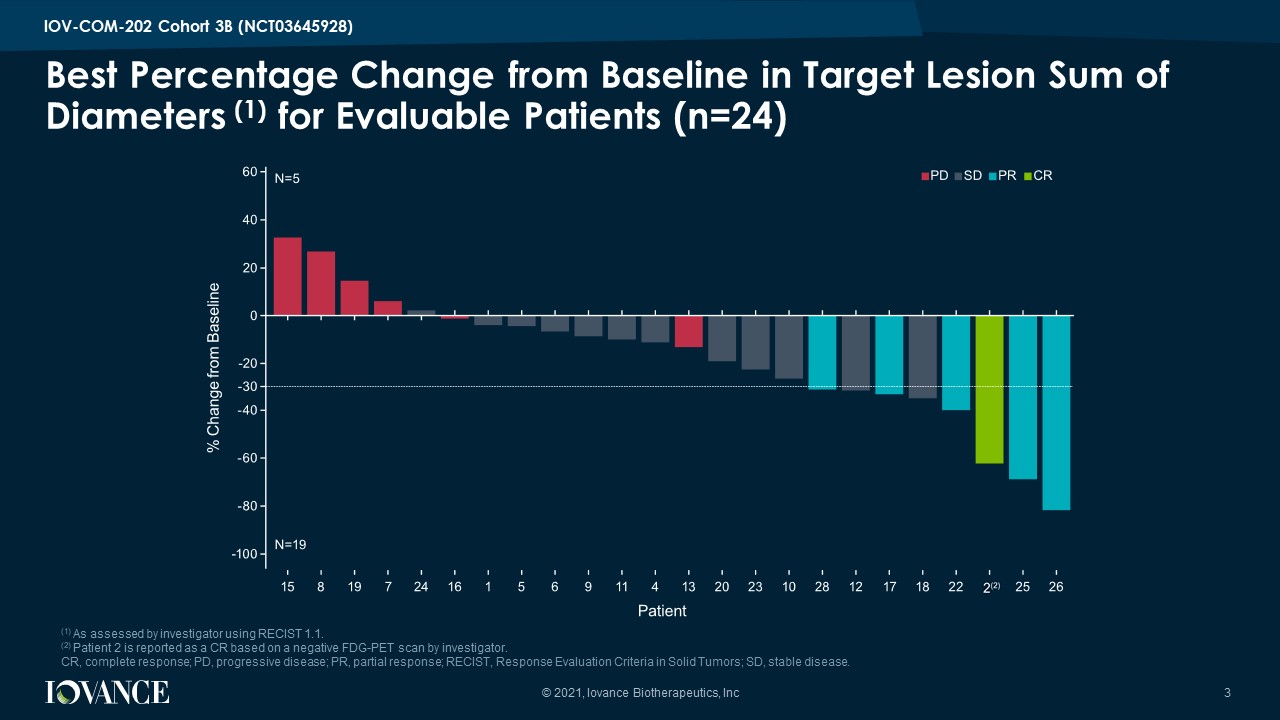
Best Percentage Change from Baseline in Target Lesion Sum of Diameters (1) for Evaluable Patients (n=24) © 2021, Iovance Biotherapeutics, Inc 3 IOV - COM - 202 Cohort 3B (NCT03645928) (1) As assessed by investigator using RECIST 1.1. (2) Patient 2 is reported as a CR based on a negative FDG - PET scan by investigator. CR, complete response; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; S D, stable disease.

(1) As assessed by investigator using RECIST 1.1. (2) Patient 2 is reported as a CR based on a negative FDG - PET scan by investigator. CR, complete response; PD, progressive disease; PD - L1, programmed death ligand - 1; PR, partial response; RECIST, Response Evaluat ion Criteria in Solid Tumors; TIL, tumor - infiltrating lymphocytes. Time to Response (1) for Confirmed Responders (PR or Better; n=6) © 2021, Iovance Biotherapeutics, Inc 4 IOV - COM - 202 Cohort 3B (NCT03645928)

• Treatment options are limited for patients with metastatic NSCLC who progress or relapse after standard - of - care front - line therapies, including platinum - doublet chemotherapy and ICI or TKI • In 28 patients with advanced or metastatic NSCLC who progressed after systemic therapy (including ICI or targeted therapy for those with actionable oncogene - driven tumors), LN - 145 resulted in: − 21.4% ORR (1 CR and 5 PRs) − Median DOR not reached at 8.2 months of median study follow up • The safety profile was consistent with the underlying disease and known adverse event profiles of non - myeloablative lymphodepletion and IL - 2 • These results are encouraging and warrant continued investigation of LN - 145 TIL cell therapy in patients with advanced or metastatic NSCLC in ongoing trials − IOV - COM - 202 (NCT03645928) Cohorts 3A and 3C − IOV - LUN - 202 (NCT04614103) • Updated data will be presented at a future meeting © 2021, Iovance Biotherapeutics, Inc 5 IOV - COM - 202 Cohort 3B (NCT03645928) IOV - COM - 202 Cohort 3B: Summary of Data in NSCLC CR, complete response; DOR, duration of response; ICI, immune checkpoint inhibitors; IL - 2, interleukin - 2; NSCLC, non - small cell lung cancer; ORR, objective response rate; PR, partial response; TIL, tumor - infiltrating lymphocytes; TKI, tyrosine kinase inhibitors.

• Nearly 70% of patients with NSCLC present with locally advanced or metastatic disease at the time of diagnosis (1) • LN - 145, an autologous TIL cell therapy, is being investigated (IOV - COM - 202, NCT03645928) for the treatment of patients with locally advanced or metastatic NSCLC − Patients must have previously received treatment with ICI as part of 1 – 3 prior lines of systemic therapy, except for patients with actionable oncogene - driven tumors, who must have progressed on ≥1 line of targeted therapy © 2021, Iovance Biotherapeutics, Inc 6 IOV - COM - 202 Cohort 3B (NCT03645928) IOV - COM - 202 Cohort 3B: Background (1) Molina JR, et al. Mayo Clin Proc . 2008;83(5):584 - 94. ICI, immune checkpoint inhibitors; NSCLC, non - small cell lung cancer; TIL, tumor - infiltrating lymphocytes.
