Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - Arbutus Biopharma Corp | exh_991.htm |
| 8-K - FORM 8-K - Arbutus Biopharma Corp | f8k_062621.htm |
EXHIBIT 99.2

NASDAQ: ABUS www.arbutusbio.com AB - 729 Development Update EASL 2021 ILC TM Dr. Gaston Picchio Chief Development Officer June 28, 2021

F o r w a r d - L oo kin g S ta t eme n t s This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to Arbutus’ expectations regarding the timing, clinical development and potential of Arbutus’ product candidates and other statements relating to Arbutus’ future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies including uncertainties and contingencies related to the ongoing COVID - 19 pandemic . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; and market shifts may require a change in strategic focus ; and the ongoing COVID - 19 pandemic could significantly disrupt our clinical development programs . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law .

AB - 729 - 00 1 Stud y O v e r vi e w Part 1: Single Ascending Dose in Healthy Subjects Dose 3 (360 mg) n=6; 4 active : 2 placebo (≥ Day 15 Safety) Dose 1 (60 mg) n=6; 4 active : 2 placebo Dose 2 (180 mg) n=6; 4 active : 2 placebo HBV DNA - Cohort B: 60 mg HBV DNA - Cohort C: 90 mg HBV DNA - (≥ Day 15 Safety) (≥ Day 15 Safety) (≥ Day 15 Safety) n=4 n=6 n=6 Cohort D: 90 mg HBV DNA + n=6 Cohort E: 60 mg Q4W HBV DNA - n=7 Part 2: Single Doses in Chronic Hepatitis B Subjects (open - label) Cohort A: 180 mg Part 3: Repeat Doses in Chronic Hepatitis B Subjects (open - label) Cohort I: 90 mg Q8W HBV DNA - Cohort J: 90 mg Q12W HBV DNA - n=7 Cohort F: 60 mg Q8W HBV DNA - n=7 Cohort G: 90 mg Q8W + TDF: HBV DNA + n=7 Cohort K: 90 mg Q8W HBV DNA - , HBeAg+ n=7 HBV: Hepatitis B Virus | Q4W: every 4 weeks | Q8W: every 8 weeks | Q12W: every 12 weeks | TDF: tenofovir disoproxil fumarate Initially, AB - 729 was dosed for 6 months . An optional 6 month treatment extension was amended to the protocol, with 48 n=6 weeks of follow - up.

Ba s elin e Cha r ac t er i s t ic s Baseline Measure Cohort E AB - 729 60 mg Q4W* (N=7) Cohort F AB - 729 60 mg Q8W (N=7) Cohort I AB - 729 90 mg Q8W (N=6) Age in years, mean (range) 45.1 (33 – 63) 44.0 (31 – 59) 45.7 (38 – 54) Male gender, n (%) 4 (57%) 4 (57%) 4 (67%) BMI, mean (SD) 27.7 (5.01) 23.7 (2.17) 25.5 (3.11) Race, n (%) Asian 1 (14%) 5 (71%) 5 (83%) Black 0 1 (14%) 0 White 6 (86%) 1 (14%) 1 (14%) ALT (U/L), mean (SD) 22.4 (10.52) 23.4 (15.22) 26.0 (10.20) HBV eAg negative, n (%) 7 (100%) 6 (71%)** 5 (83%) HBsAg (IU/mL), mean (range) 5,372 (584 – 11,761) 5,354 (667 – 18,605) 4,691 (338 – 19,017) *subjects switched to AB - 729 60 mg Q12W after the Week 20 dose ** 1 subject counted as HBeAg negative was identified as “HBeAg borderline” (baseline HBeAg = 0.18 IU/mL, LLOQ = 0.11 IU/mL) ▪ All subjects were virologically suppressed on an NA (ETV, TDF or TAF) with HBV DNA < LLOQ (20 IU/mL) ▪ HBV genotype was not determined

Repeat dosing of AB - 729 60 mg and 90 mg results in comparable HBsAg decline profiles with 75 percent of subjects reaching <100 IU/mL Plateau in response observed around Week 20, regardless of dose or dosing interval AB - 729 60 mg Q4W [N=7] * AB - 729 60 mg Q8W [N=7] AB - 729 90 mg Q8W [N=6] *Due to the prolonged pharmacodynamic activity observed after a single dose of AB - 729 (Yuen, AASLD 2020), subjects switched to AB - 729 60 mg Q12W after Week 20 5/7 HBsAg < 100 IU/mL 5/7 HBsAg < 100 IU/mL 5/6 HBsAg < 100 IU/mL
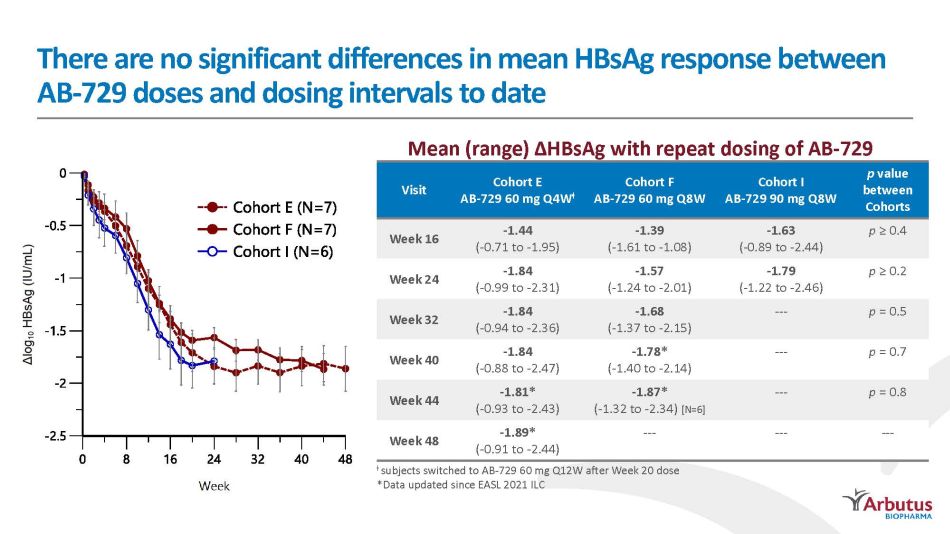
There are no significant differences in mean HBsAg response between AB - 72 9 d os e s an d d os in g i n t e r v al s t o d a t e Visit Cohort E AB - 729 60 mg Q4W ܶ Cohort F AB - 729 60 mg Q8W Cohort I AB - 729 90 mg Q8W p value b e t w een Cohorts Week 16 - 1.44 ( - 0.71 to - 1.95) - 1.39 ( - 1.61 to - 1.08) - 1.63 ( - 0.89 to - 2.44) p ≥ 0.4 Week 24 - 1.84 ( - 0.99 to - 2.31) - 1.57 ( - 1.24 to - 2.01) - 1.79 ( - 1.22 to - 2.46) p ≥ 0.2 Week 32 - 1.84 ( - 0.94 to - 2.36) - 1.68 ( - 1.37 to - 2.15) --- p = 0.5 Week 40 - 1.84 ( - 0.88 to - 2.47) - 1.78* ( - 1.40 to - 2.14) --- p = 0.7 Week 44 - 1.81* ( - 0.93 to - 2.43) - 1.87* ( - 1.32 to - 2.34) [N=6] --- p = 0.8 Week 48 - 1.89* ( - 0.91 to - 2.44) --- --- --- Mean (range) ∆HBsAg with repeat dosing of AB - 729 ܶ subjects switched to AB - 729 60 mg Q12W after Week 20 dose *Data updated since EASL 2021 ILC
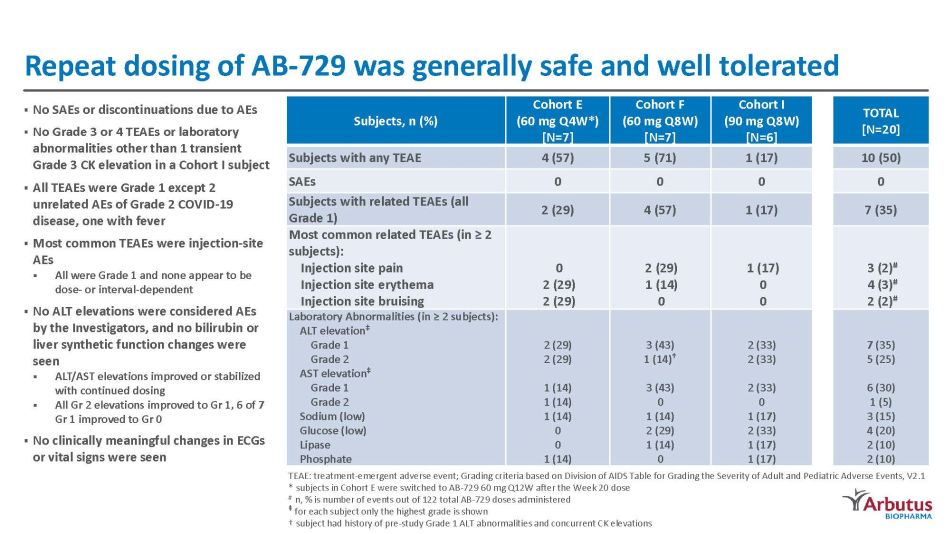
Repeat dosing of AB - 729 was generally safe and well tolerated Subjects, n (%) Cohort E (60 mg Q4W*) [N=7] Cohort F (60 mg Q8W) [N=7] Cohort I (90 mg Q8W) [N=6] TOTAL [N=20] Subjects with any TEAE 4 (57) 5 (71) 1 (17) 10 (50) SAEs 0 0 0 0 Subjects with related TEAEs (all Grade 1) 2 (29) 4 (57) 1 (17) 7 (35) Most common related TEAEs (in ≥ 2 subjects): Injection site pain Injection site erythema Injection site bruising 0 2 (29) 2 (29) 2 (29) 1 (14) 0 1 (17) 0 0 3 (2) # 4 (3) # 2 (2) # Laboratory Abnormalities (in ≥ 2 subjects): A L T ele v a t ion ‡ Grade 1 G r ade 2 A S T ele v a t i on ‡ Grade 1 Grade 2 Sodium (low) Glucose (low) Lipase Phosphate 2 ( 2 9) 2 ( 2 9) 1 ( 1 4) 1 ( 1 4) 1 ( 1 4) 0 0 1 ( 1 4) 3 (43) 1 ( 1 4 ) † 3 ( 4 3) 0 1 ( 1 4) 2 ( 2 9) 1 ( 1 4) 0 2 ( 3 3) 2 ( 3 3) 2 ( 3 3) 0 1 ( 1 7) 2 ( 3 3) 1 ( 1 7) 1 ( 1 7) 7 ( 3 5) 5 ( 2 5) 6 ( 3 0) 1 (5) 3 ( 1 5) 4 ( 2 0) 2 ( 1 0) 2 ( 1 0) ▪ No SAEs or discontinuations due to AEs ▪ No Grade 3 or 4 TEAEs or laboratory abnormalities other than 1 transient Grade 3 CK elevation in a Cohort I subject ▪ All TEAEs were Grade 1 except 2 unrelated AEs of Grade 2 COVID - 19 disease, one with fever ▪ Most common TEAEs were injection - site AEs ▪ All were Grade 1 and none appear to be dose - or interval - dependent ▪ No ALT elevations were considered AEs by the Investigators, and no bilirubin or liver synthetic function changes were seen ▪ ALT/AST elevations improved or stabilized with continued dosing ▪ All Gr 2 elevations improved to Gr 1, 6 of 7 Gr 1 improved to Gr 0 ▪ No clinically meaningful changes in ECGs or vital signs were seen TEAE: treatment - emergent adverse event; Grading criteria based on Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, V2.1 * subjects in Cohort E were switched to AB - 729 60 mg Q12W after the Week 20 dose # n, % is number of events out of 122 total AB - 729 doses administered ‡ for each subject only the highest grade is shown † subject had history of pre - study Grade 1 ALT abnormalities and concurrent CK elevations

AB - 729 mediated HBsAg reduction is associated with increased HBV - specific immune responses in 3/5* evaluable subjects #2823

AB - 72 9 i s acti v e a g ain s t al l i so f o rm s * o f H B s A g From Thi, et al. Poster #2822, EASL 2021 *As measured with the RUO Abbott isoform assay MHB = medium HBsAg LHB = large HBsAg

AB - 72 9 r educe s H B V RN A i n r api d an d sl o w r esponde r s demon s t r a tin g broad target engagement From Thi, et al. Poster #282 3 , EASL 2021 #2822

Takeaways • Clinical data supports our view that AB - 729 60 mg every 8 weeks is an appropriate and convenient dose to explore in Phase 2a combination trials • Long - term dosing with AB - 729 results in 75 percent of subjects reaching <100 IU/mL of HBsAg , a clinically relevant threshold informing when to stop all therapies • Preliminary data suggest that long - term suppression of HBsAg with AB - 729 results in increased HBV - specific immune response • Based on these findings, we expect to initiate several proof - of - concept Phase 2a combination trials using AB - 729 as the cornerstone agent in 2H/2021
