Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - CRISPR Therapeutics AG | crsp-ex993_179.htm |
| EX-99.1 - EX-99.1 - CRISPR Therapeutics AG | crsp-ex991_11.htm |
| 8-K - 8-K - CRISPR Therapeutics AG | crsp-8k_20210611.htm |
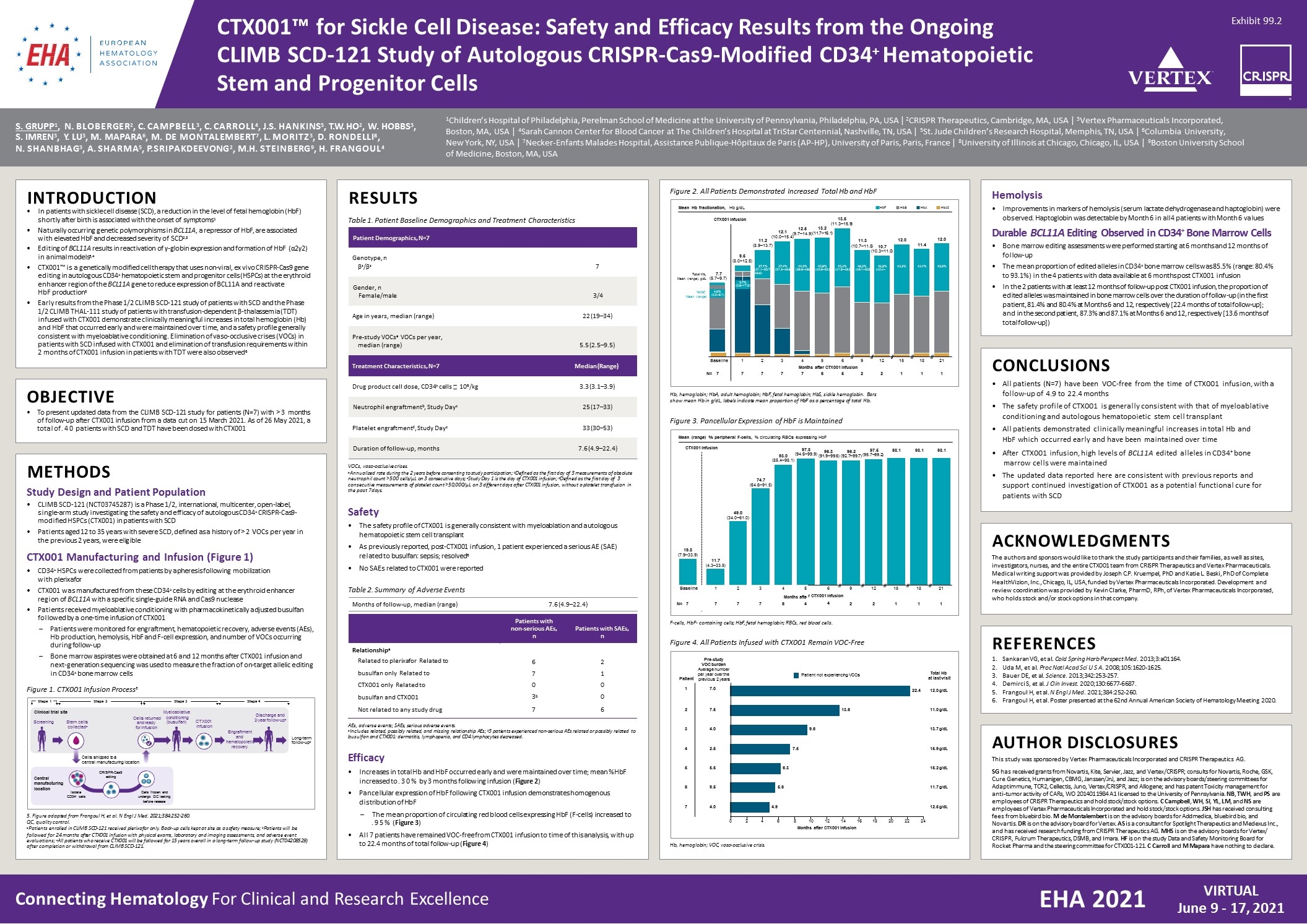
Connecting Hematology For Clinical and Research Excellence VIRTUAL June 9 - 17, 2021 EHA 2021 CTX001™ for Sickle Cell Disease: Safety and Efficacy Results from the Ongoing CLIMB SCD-121 Study of Autologous CRISPR-Cas9-Modified CD34+ Hematopoietic Stem and Progenitor Cells S. GRUPP1, N. BLOBERGER2, C. CAMPBELL3, C. CARROLL4, J.S. HANKINS5, T.W. HO2, W. HOBBS3, S. IMREN3, Y. LU3, M. MAPARA6, M. DE MONTALEMBERT7, L. MORITZ3, D. RONDELLI8, N. SHANBHAG3, A. SHARMA5, P. SRIPAKDEEVONG2, M.H. STEINBERG9, H. FRANGOUL4 1Children’s Hospital of Philadelphia, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA | 2CRISPR Therapeutics, Cambridge, MA, USA | 3Vertex Pharmaceuticals Incorporated, Boston, MA, USA | 4Sarah Cannon Center for Blood Cancer at The Children’s Hospital at TriStar Centennial, Nashville, TN, USA | 5St. Jude Children’s Research Hospital, Memphis, TN, USA | 6Columbia University, New York, NY, USA | 7Necker-Enfants Malades Hospital, Assistance Publique-Hôpitaux de Paris (AP-HP), University of Paris, Paris, France | 8University of Illinois at Chicago, Chicago, IL, USA | 9Boston University School of Medicine, Boston, MA, USA INTRODUCTION In patients with sickle cell disease (SCD), a reduction in the level of fetal hemoglobin (HbF) shortly after birth is associated with the onset of symptoms1 Naturally occurring genetic polymorphisms in BCL11A, a repressor of HbF, are associated with elevated HbF and decreased severity of SCD2,3 Editing of BCL11A results in reactivation of γ-globin expression and formation of HbF (α2γ2) in animal models3,4 CTX001™ is a genetically modified cell therapy that uses non-viral, ex vivo CRISPR-Cas9 gene editing in autologous CD34+ hematopoietic stem and progenitor cells (HSPCs) at the erythroid enhancer region of the BCL11A gene to reduce expression of BCL11A and reactivate HbF production5 Early results from the Phase 1/2 CLIMB SCD-121 study of patients with SCD and the Phase 1/2 CLIMB THAL-111 study of patients with transfusion-dependent β-thalassemia (TDT) infused with CTX001 demonstrate clinically meaningful increases in total hemoglobin (Hb) and HbF that occurred early and were maintained over time, and a safety profile generally consistent with myeloablative conditioning. Elimination of vaso-occlusive crises (VOCs) in patients with SCD infused with CTX001 and elimination of transfusion requirements within 2 months of CTX001 infusion in patients with TDT were also observed6 OBJECTIVE To present updated data from the CLIMB SCD-121 study for patients (N=7) with >3 months of follow-up after CTX001 infusion from a data cut on 15 March 2021. As of 26 May 2021, a total of .40 patients with SCD and TDT have been dosed with CTX001 METHODS Study Design and Patient Population CLIMB SCD-121 (NCT03745287) is a Phase 1/2, international, multicenter, open-label, single-arm study investigating the safety and efficacy of autologous CD34+ CRISPR-Cas9- modified HSPCs (CTX001) in patients with SCD Patients aged 12 to 35 years with severe SCD, defined as a history of >2 VOCs per year in the previous 2 years, were eligible CTX001 Manufacturing and Infusion (Figure 1) CD34+ HSPCs were collected from patients by apheresis following mobilization with plerixafor CTX001 was manufactured from these CD34+ cells by editing at the erythroid enhancer region of BCL11A with a specific single-guide RNA and Cas9 nuclease Patients received myeloablative conditioning with pharmacokinetically adjusted busulfan followed by a one-time infusion of CTX001 Patients were monitored for engraftment, hematopoietic recovery, adverse events (AEs), Hb production, hemolysis, HbF and F-cell expression, and number of VOCs occurring during follow-up Bone marrow aspirates were obtained at 6 and 12 months after CTX001 infusion and next-generation sequencing was used to measure the fraction of on-target allelic editing in CD34+ bone marrow cells Safety The safety profile of CTX001 is generally consistent with myeloablation and autologous hematopoietic stem cell transplant As previously reported, post-CTX001 infusion, 1 patient experienced a serious AE (SAE) related to busulfan: sepsis; resolved6 No SAEs related to CTX001 were reported Efficacy Increases in total Hb and HbF occurred early and were maintained over time; mean %HbF increased to .30% by 3 months following infusion (Figure 2) Pancellular expression of HbF following CTX001 infusion demonstrates homogenous distribution of HbF – The mean proportion of circulating red blood cells expressing HbF (F-cells) increased to .95% (Figure 3) All 7 patients have remained VOC-free from CTX001 infusion to time of this analysis, with up to 22.4 months of total follow-up (Figure 4) CONCLUSIONS All patients (N=7) have been VOC-free from the time of CTX001 infusion, with a follow-up of 4.9 to 22.4 months The safety profile of CTX001 is generally consistent with that of myeloablative conditioning and autologous hematopoietic stem cell transplant All patients demonstrated clinically meaningful increases in total Hb and HbF which occurred early and have been maintained over time After CTX001 infusion, high levels of BCL11A edited alleles in CD34+ bone marrow cells were maintained The updated data reported here are consistent with previous reports and support continued investigation of CTX001 as a potential functional cure for patients with SCD Hemolysis Improvements in markers of hemolysis (serum lactate dehydrogenase and haptoglobin) were observed. Haptoglobin was detectable by Month 6 in all 4 patients with Month 6 values Durable BCL11A Editing Observed in CD34+ Bone Marrow Cells Bone marrow editing assessments were performed starting at 6 months and 12 months of follow-up The mean proportion of edited alleles in CD34+ bone marrow cells was 85.5% (range: 80.4% to 93.1%) in the 4 patients with data available at 6 months post CTX001 infusion In the 2 patients with at least 12 months of follow-up post CTX001 infusion, the proportion of edited alleles was maintained in bone marrow cells over the duration of follow-up (in the first patient, 81.4% and 80.4% at Months 6 and 12, respectively [22.4 months of total follow-up]; and in the second patient, 87.3% and 87.1% at Months 6 and 12, respectively [13.6 months of total follow-up]) ACKNOWLEDGMENTS The authors and sponsors would like to thank the study participants and their families, as well as sites, investigators, nurses, and the entire CTX001 team from CRISPR Therapeutics and Vertex Pharmaceuticals. Medical writing support was provided by Joseph C.P. Kruempel, PhD and Katie L. Beski, PhD of Complete HealthVizion, Inc., Chicago, IL, USA, funded by Vertex Pharmaceuticals Incorporated. Development and review coordination was provided by Kevin Clarke, PharmD, RPh, of Vertex Pharmaceuticals Incorporated, who holds stock and/or stock options in that company. REFERENCES Sankaran VG, et al. Cold Spring Harb Perspect Med. 2013;3:a01164. Uda M, et al. Proc Natl Acad Sci U S A. 2008;105:1620-1625. 3. Bauer DE, et al. Science. 2013;342:253-257. Demirci S, et al. J Clin Invest. 2020;130:6677-6687. Frangoul H, et al. N Engl J Med. 2021;384:252-260. Frangoul H, et al. Poster presented at the 62nd Annual American Society of Hematology Meeting 2020. AUTHOR DISCLOSURES This study was sponsored by Vertex Pharmaceuticals Incorporated and CRISPR Therapeutics AG. SG has received grants from Novartis, Kite, Servier, Jazz, and Vertex/CRISPR; consults for Novartis, Roche, GSK, Cure Genetics, Humanigen, CBMG, Janssen/JnJ, and Jazz; is on the advisory boards/steering committees for Adaptimmune, TCR2, Cellectis, Juno, Vertex/CRISPR, and Allogene; and has patent Toxicity management for anti-tumor activity of CARs, WO 2014011984 A1 licensed to the University of Pennsylvania. NB, TWH, and PS are employees of CRISPR Therapeutics and hold stock/stock options. C Campbell, WH, SI, YL, LM, and NS are employees of Vertex Pharmaceuticals Incorporated and hold stock/stock options. JSH has received consulting fees from bluebird bio. M de Montalembert is on the advisory boards for Addmedica, bluebird bio, and Novartis. DR is on the advisory board for Vertex. AS is a consultant for Spotlight Therapeutics and Medexus Inc., and has received research funding from CRISPR Therapeutics AG. MHS is on the advisory boards for Vertex/ CRISPR, Fulcrum Therapeutics, DSMB, and Imara. HF is on the study Data and Safety Monitoring Board for Rocket Pharma and the steering committee for CTX001-121. C Carroll and M Mapara have nothing to declare. RESULTS Table 1. Patient Baseline Demographics and Treatment Characteristics Patient Demographics, N=7 Genotype, n βs/βs 7 Gender, n Female/male 3/4 Age in years, median (range) 22 (19–34) Pre-study VOCsa VOCs per year, median (range) 5.5 (2.5–9.5) Treatment Characteristics, N=7 Median (Range) Drug product cell dose, CD34+ cells 106/kg3.3 (3.1–3.9) Neutrophil engraftmentb, Study Dayc25 (17–33) Platelet engraftmentd, Study Dayc33 (30–53) Duration of follow-up, months7.6 (4.9–22.4) VOCs, vaso-occlusive crises. aAnnualized rate during the 2 years before consenting to study participation; bDefined as the first day of 3 measurements of absolute neutrophil count >500 cells/µL on 3 consecutive days; cStudy Day 1 is the day of CTX001 infusion; dDefined as the first day of 3 consecutive measurements of platelet count >50,000/µL on 3 different days after CTX001 infusion, without a platelet transfusion in the past 7 days. Table 2. Summary of Adverse Events Months of follow-up, median (range) 7.6 (4.9–22.4) Patients with non-serious AEs, n Patients with SAEs, n Relationshipa Related to plerixafor Related to busulfan only Related to CTX001 only Related to busulfan and CTX001 6 7 0 3b 2 1 0 0 Total Hb, 7.7 Mean (range), g/dL (5.7–9.7) 9.5 (8.0–12.5) 11.2 (8.9–13.7) 12.1 (10.0–15.4) 12.5 13.2 (9.7–14.9)(11.7–16.1) 13.5 (11.3–15.9) 11.3 (10.7–11.8) 10.7 (10.3–11.0) 12.0 11.4 12.0 %HbF, Mean (range) Baseline 1 2 3 456 Months after CTX001 Infusion 9 12 15 18 21 N= 7 CTX001 Infusion Mean Hb fractionation, Hb g/dL 7 7 7 765 2 2 1 1 1 HbF HbS HbA HbA2 27.1%37.4%44.2%45.9%45.3%48.2%46.0% (21.1–33.7) (31.3–46.8) (39.6–49.5) (40.6–50.9) (41.9–48.0) (46.1–50.2) (42.4–49.6) 43.2% 43.1% 42.0% 5.7% (0.8–17.3) 4.0% (0.0–9.1) Hb, hemoglobin; HbA, adult hemoglobin; HbF, fetal hemoglobin; HbS, sickle hemoglobin. Bars show mean Hb in g/dL, labels indicate mean proportion of HbF as a percentage of total Hb. Figure 2. All Patients Demonstrated Increased Total Hb and HbF 13.6 9.6 7.5 6.3 5.6 4.9 17.0 Total Hb at last visit Pre-study VOC burden Average number per year over the Patient previous 2 years 4.0 024681012141618202224 Months after CTX001 Infusion 7 9.5 6 5.5 5 2.5 4 4.0 3 7.5 22.412.0 g/dL 12.5 g/dL 11.7 g/dL 15.2 g/dL 15.9 g/dL 13.7 g/dL 11.0 g/dL 2 Patient not experiencing VOCs Hb, hemoglobin; VOC, vaso-occlusive crisis. Figure 4. All Patients Infused with CTX001 Remain VOC-Free 19.8 (7.9–33.9) 11.7 (4.3–33.5) 49.0 (34.0–61.0) 74.7 (64.6–91.5) (85.4–98.1) 96.396.2 97.897.5 93.0 (94.6–99.9) (91.9–99.6) (92.7–99.7) (95.7–99.2) 98.1 98.1 98.1 CTX001 Infusion Baseline 1 2 345 N= 7 7 7 Months afte 764 6 r CTX001 Infusion 4 9 12 15 18 21 2 2 1 1 1 Mean (range) % peripheral F-cells, % circulating RBCs expressing HbF F-cells, HbF- containing cells; HbF, fetal hemoglobin; RBCs, red blood cells. Figure 3. Pancellular Expression of HbF is Maintained 5. Figure adapted from Frangoul H, et al. N Engl J Med. 2021;384:252-260. QC, quality control. aPatients enrolled in CLIMB SCD-121 received plerixafor only. Back-up cells kept at site as a safety measure; bPatients will be followed for 24 months after CTX001 infusion with physical exams, laboratory and imaging assessments, and adverse event evaluations; cAll patients who receive CTX001 will be followed for 15 years overall in a long-term follow-up study (NCT04208529) after completion or withdrawal from CLIMB SCD-121. Figure 1. CTX001 Infusion Process5 Stage 1Stage 2Stage 3 Stage 4 ScreeningStem cellsand ready collectedafor infusion Clinical trial siteMyeloablative Cells returned conditioning (busulfan)CTX001 infusion Not related to any study drug76 AEs, adverse events; SAEs, serious adverse events. aIncludes related, possibly related, and missing relationship AEs; b3 patients experienced non-serious AEs related or possibly related to busulfan and CTX001: dermatitis, lymphopenia, and CD4 lymphocytes decreased. Discharge and 2-year follow-upb Engraftment andLong-term hematopoieticfollow-upc recovery Cells frozen and undergo QC testing before release Central manufacturing location Cells shipped to a central manufacturing location CRISPR-Cas9 editing Isolate CD34+ cells Exhibit 99.2
