Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Neoleukin Therapeutics, Inc. | nltxer8-kform1.htm |
| EX-99.1 - EX-99.1 - Neoleukin Therapeutics, Inc. | ex-991xpressreleaseofneole.htm |


Forward Looking Statements

Leader in Therapeutic Protein Design First Program: Cancer Immunotherapy 2018 FOUNDED 2019 PUBLIC NL-201 program: Platform technology: de novo NASDAQ: NLTX 2020 IND SUBMISSION SEATTLE WA NL-201 2021 CLINICAL TRIALS Systemic Local

Functional De Novo Proteins Better Immunotherapies by Design de novo de novo 2019 2020

Neoleukin Progress in 2021

Carl Walkey, Ph.D. Senior VP, Corporate Development Previous: Postdoctoral Fellow, UW-IPD Priti Patel, M.D., M.S. Chief Medical Officer Previous: AstraZeneca, Acerta Pharma Umut Ulge, M.D., Ph.D. VP, Clinical Development Previous: Postdoctoral Fellow, UW-IPD Jonathan Drachman, M.D. Chief Executive Officer Previous: CMO, EVP R&D, Seattle Genetics Robert Ho Chief Financial Officer Previous: Morgan Stanley & Co., DaVita Samantha Willing VP, People Previous: Seattle Genetics, Microsoft Holly Vance, J.D., Pharm.D. General Counsel Previous: Bill & Melinda Gates Foundation Leadership Team

NL-201: De Novo IL-2/IL-15 Agonist Designed to retain benefits of IL-2 without drawbacks Nature α β hIL-2Neoleukin-2/15 and and

IL-2 Binds Strongly to Non-Target Cells, Causing Toxicity and Limiting Efficacy α β β IL-2 Off-Target Cells Effector Cells IL-2IL-2

Building a Neoleukin Cytokine Mimetic in 4 Steps 1 2 3 4

Crystal Structure Shows Neo-2/15 Binding Beta/Gamma as Predicted ALPHA BETA GAMMA IL-2 Neo-2/15 BETA GAMMA 14% Nature

NL-201 Stimulates CD8 Effector T and NK Cells More Selectively Than IL-2 0. 00 01 0. 00 1 0. 01 0. 1 1 10 10 0 1, 00 0 1,500 2,000 2,500 3,000 Treatment (nM) M e a n p S T A T 5 CD8 Signaling 11X 0. 00 01 0. 00 1 0. 01 0. 1 1 10 10 0 1, 00 0 1,500 1,750 2,000 2,250 2,500 2,750 Treatment (nM) NK Signaling 3.1X 0. 00 01 0. 00 1 0. 01 0. 1 1 10 10 0 1, 00 0 2,000 3,000 4,000 5,000 Treatment (nM) Treg Signaling 31X Walkey et. al, AACR Virtual Annual Meeting II, Abstract #4518, June 2020

NL-201 Stimulates Dose-Dependent CD8:Treg and NK:Treg Proliferation More Potently Than IL-2 0 0.3 1.0 3.0 10 30 0 1 2 3 4 5 6 7 Treatment (ng/ml) % K i6 7 + C D 8 :T re g CD8:Treg Proliferation 0 0.3 1.0 3.0 10 30 0 5 10 15 20 25 30 Treatment (ng/ml) % K i6 7 + N K :T re g NK:Treg Proliferation Walkey et. al, AACR Virtual Annual Meeting II, Abstract #4518, June 2020

NL-201 is Well Tolerated and Promotes Durable Anti-tumor Activity 0 7 14 21 28 35 0 1000 2000 3000 Study Day T u m o r V o lu m e ( m m 3 ) Re-Challenge 9 16 23 30 -5 0 5 10 15 20 25 30 Study Day % B o d y w e ig h t C h a n g e Tolerability ** 9 16 23 30 37 44 51 58 65 72 79 0 25 50 75 100 Study Day % T u m o rs < 1 0 0 0 m m 3 Tumor Growth Inhibition 6/15 (40%) tumor-freeNL-201 aPD-1 aPD-L1 Walkey et. al, AACR Virtual Annual Meeting II, Abstract #4518, June 2020

NL-201 Demonstrates Robust Single-Agent Activity in Multiple Tumor Models PBS NL-201 0 1000 2000 3000 4000 T u m o r V o lu m e ( m m 3 ) Hepa1-6 (Liver) Day 24 90% TGI PBS NL-201 0 1000 2000 3000 4000 T u m o r V o lu m e ( m m 3 ) LL/2 (Lung) Day 35 64% TGI PBS NL-201 0 1000 2000 3000 4000 MC38 (Colon) Day 21 88% TGI PBS NL-201 0 1000 2000 3000 4000 EMT-6 (Breast) Day 28 62% TGI PBS NL-201 0 1000 2000 3000 4000 CT26 (Colon) Day 21 78% TGI PBS NL-201 0 1000 2000 3000 Pan02 (Pancreatic) Day 52 60% TGI PBS NL-201 0 1000 2000 3000 4000 H22 (Liver) Day 18 77% TGI PBS NL-201 0 1000 2000 3000 4000 Renca (Kidney) Day 23 53% TGI PBS NL-201 0 1000 2000 3000 4000 5000 A20 (Lymphoma) Day 26 77% TGI PBS NL-201 0 1000 2000 3000 4000 B16F10 (Melanoma) Day 18 34% TGI PBS NL-201 0 1000 2000 3000 4000 5000 RM-1 (Prostate) Day 20 71% TGI PBS NL-201 1000 2000 3000 4000 B16BL6 (Melanoma) Day 22 28% TGI Walkey et. al, AACR Virtual Annual Meeting II, Abstract #4518, June 2020

NL-201 Shows Minimal Immunogenicity in NHPs 1 2 3 4 5 6 7 8 9 10 1 2 3 4 5 6 1 2 3 4 5 6 7 8 9 10 100 1000 10000 100000 Animal # A s s a y S ig n a l (A U ) HPC MPC LPC NC 5ug/kg 15ug/kg 50ug/kg Abstract #4518, Walkey et. al, AACR Virtual Annual Meeting II, June 2020

Similar Pharmacodynamics and Tolerability Observed in ADA+ vs ADA- NHPs Adapted from Abstract #4518, Walkey et. al, AACR Virtual Annual Meeting II, June 2020 Pr e 24 h 3d 5d 7d Pr e 24 h 3d 0 20 40 60 80 100 % K i6 7 + C D 8 + T C e ll s (% o f to ta l C D 8 + ) CD8+ Proliferation (5mg/kg) 1st Dose 5th Dose Pr e 24 h 3d 5d 7d Pr e 24 h 3d 0 20 40 60 80 100 CD8+ Proliferation (50mg/kg) 1st Dose 5th Dose Pr e 24 h 3d 5d 7d Pr e 24 h 3d 0 20 40 60 80 100 CD8+ Proliferation (15mg/kg) 1st Dose 5th Dose

NL-201 Phase 1 Clinical Trials Systemic administration: Local administration:

NL-201 Upregulates PD-1 Expression by CD8+ T Cells Walkey et. Al, SITC 2020, Abstract #576, November 2020

NL-201 Enhances Activity of Checkpoint Inhibitors in Preclinical Models NL-201 enhances activity of CPIs in breast and kidney cancer models Combination with NL-201 beneficial in CPI-resistant syngeneic tumors 0 7 14 21 28 35 42 0 20 40 60 80 100 Study Day % S u rv iv in g EMT-6 (Breast) Vehicle aPD-1 NL-201 NL-201 + aPD-1 7/9 tumor-free 0 7 14 21 28 35 42 0 20 40 60 80 100 Study Day % S u rv iv in g Renca (Kidney) Vehicle aPD-1 + aCTLA-4 NL-201 NL-201 + aPD-1 + aCTLA-4 5/9 tumor-free p=0.0001: ⍺ ⍺ ⍺ ⍺ p=0.0006: ⍺ ⍺ p=0.0029: ⍺ ⍺ p<0.0001: ⍺ NL-201: ⍺PD-1: ⍺CTLA-4: Treatment began when tumors reached ~90mm3

NL-201 Potently Expands CAR-T Cells and Promotes Antitumor Activity Subcurative doses of CAR-T cells combined with NL-201 induce deep tumor control and achieve 100% survival. NL-201 greatly enhances intratumoral CD8: Treg ratios (approximately 1000x compared to 50x for IL-2). Leung et. al, AACR Virtual Annual Meeting II, Abstract #2222, June 2020

NL-201 Enhances Activity of Tumor-Targeting Antibodies in Multiple Preclinical Models Walkey et. Al, SITC 2020, Abstract #576, November 2020

Neoleukin Cytokine Mimetics are Hyperstable and Easily Modified Nature

De Novo Split Technology - Conditionally Active IL-2 Mimetic Cell signaling (murine CTLL2 cells) Part A + B Quijano-Rubio et. Al., AACR Virtual Annual Meeting II, Abstract #1075, Jun/2020 Part-A Part-B Reconstituted Neo-2/15

Targeted Split Neo-2/15 Increases Therapeutic Window Weight change D12 Percent Survival ⍺ ⍺PDL1-Part A + ⍺PDL1-Part B (8 nmol) Quijano-Rubio et. Al., AACR Virtual Annual Meeting II, Abstract #1075, Jun/2020

De Novo Platform Potential – COVID-19 ACE2 NL-CVX1 - de novo ACE2 decoy Binds to SARS-CoV2 spike protein Inhibits viral infection in vitro Designed, tested, optimized in ~10 weeks Cell Virus Spike protein Cell Virus ACE2 Spike protein SARS-CoV-2 ACE2 NL-CVX1

NL-CVX1 – De Novo Protein Decoy De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2 T. W. Linsky et. al. Science. 10.1126/science.abe0075 (2020)
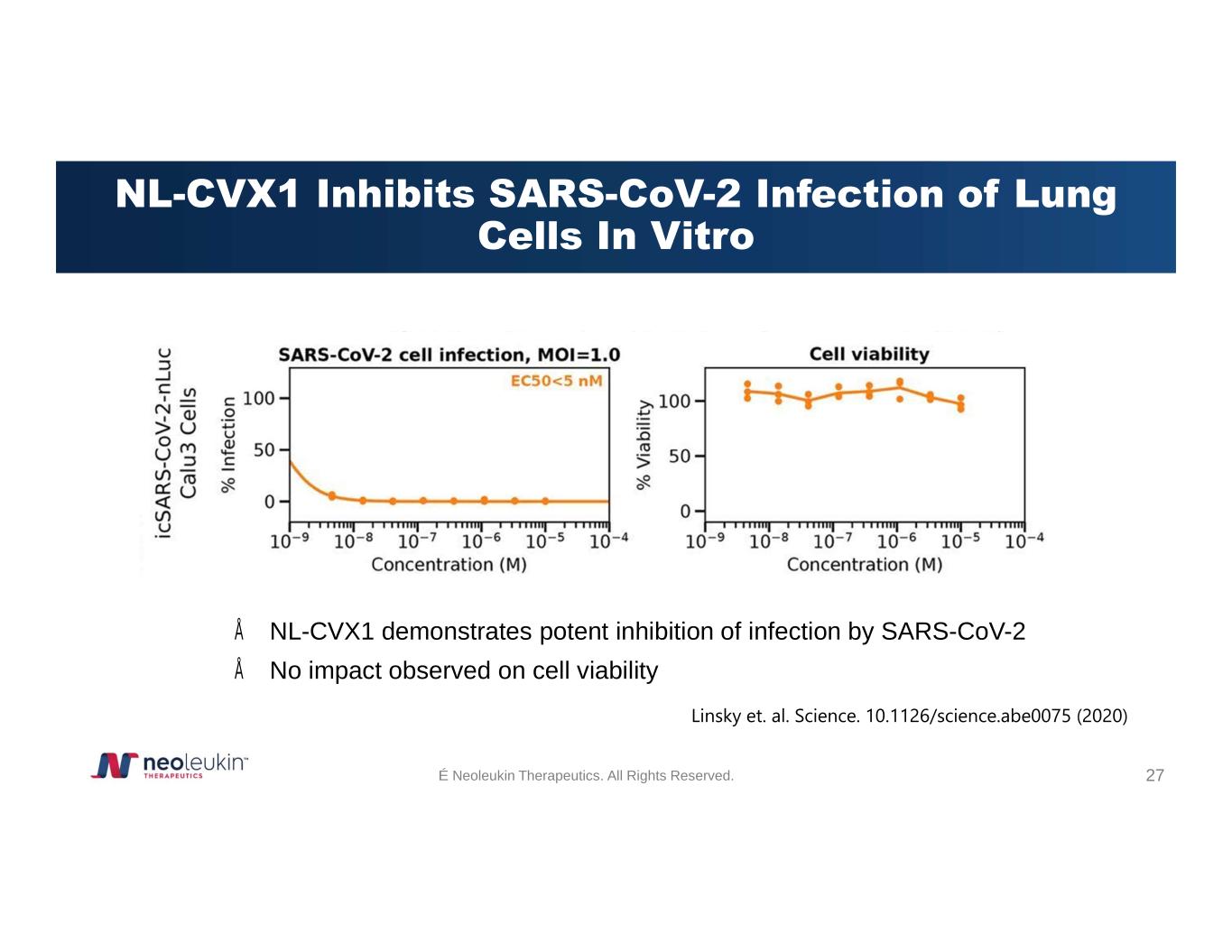
NL-CVX1 Inhibits SARS-CoV-2 Infection of Lung Cells In Vitro Linsky et. al. Science. 10.1126/science.abe0075 (2020)

Single Dose of NL-CVX1 Rescues Animals from Lethal SARS-CoV-2 Challenge 0 1 2 3 4 5 6 7 8 9 10 11 0 25 50 75 100 Days Post-Challenge S u rv iv a l 12h pre-challenge 0 1 2 3 4 5 6 7 8 9 10 11 80 85 90 95 100 105 Days Post-Challenge B o d y w e ig h t (% ) 12h pre-challenge Mock CTC445.2d Vehicle Linsky et. al. Science. 10.1126/science.abe0075 (2020)

Anticipated Milestones de novo

Financial Highlights

Improving on nature. Designing for life.
