Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Arbutus Biopharma Corp | f8k_050521.htm |
| EX-99.1 - PRESS RELEASE - Arbutus Biopharma Corp | exh_991.htm |
EXHIBIT 99.2

Corporate P r ese n t a ti o n May 2021 NASDAQ: ABUS www.arbutusbio.com

NASDAQ: ABUS www.arbutusbio.com F o r w a r d - Loo k i n g S t a t eme n t s 2 This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to : the potential market opportunity for HBV ; Arbutus’ ability to meet a significant unmet medical need ; the sufficiency of Arbutus’ cash and cash equivalents to extend through the third quarter of 2022 ; the potential for AB - 729 to be a well - tolerated low dose treatment for HBV with a minimum of injections ; the potential for AB - 836 to be low dose with a greater therapeutic window and to address known capsid resistant variants T 33 N and I 105 T ; the potential for AB - 836 to be once daily dosing ; Arbutus’ expectations regarding the timing and clinical development of Arbutus’ product candidates including its 2021 key clinical objectives with respect to AB - 729 and AB - 836 and its clinical collaboration with Assembly Biosciences ; the timeline to a combination cure for HBV ; Arbutus’ coronavirus strategy ; Arbutus’ expectations regarding its technology licensed to Genevant ; and other statements relating to our future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies including uncertainties and contingencies related to the ongoing COVID - 19 pandemic . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; and market shifts may require a change in strategic focus ; and the ongoing COVID - 19 pandemic could significantly disrupt our clinical development programs . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law .

NASDAQ: ABUS www.arbutusbio.com I n v e s t me n t Hi g h li g h t s Therapeutic focus – curing chronic Hepatitis B Virus (HBV) Infection 3 HCV: Hepatitis C Virus | HIV Human Immunodeficiency Virus Significant Unmet Medical N ee d i n H B V Undetectable HBV DNA and HBsAg delivered through finite duration treatment with a combination of drugs with different modes of action G o a l o f H B V Functional Cure Broad HBV P o r t f o li o HBV assets include: RNAi C a ps i d I nh i b i t o r s PD - L1 HBV RNA Destabilizers C o r o n a vi r u s Research Initiative Focused on direct acting antivirals targeting the viral polymerase and protease T e a m w it h Antiviral Ex p e r ti s e & P r ov e n T r ac k Record Applying knowledge gained from HIV and HCV success to HBV and C o r on a v i r uses Global HBV prevalence double that of HCV, potential for larger market opportunity 1 6 % Ow n e r s hi p i n Genevant Rights to potential future royalties and sublicense revenues for LNP Technology

NASDAQ: ABUS www.arbutusbio.com Proven Lea d e r s h i p Team 4 Successful track records in the discovery, development, and commercialization of multiple antivirals including sofosbuvir, etravirine, rilpivirine, telaprevir and simeprevir William H. Collier President and CEO David C. Hastings Chief Financial Officer Gaston Picchio, PhD Chief Development Officer Michael J. Sofia, PhD Chief Scientific Officer Michael J. McElhaugh Chief Business Officer Elizabeth Howard, PhD, JD EVP, General Counsel and Chief Compliance Officer

NASDAQ: ABUS www.arbutusbio.com Sources : Global Hepatitis Report and Hepatitis B Fact Sheet, WHO (2017) http://www.who.int/mediacentre/factsheets/fs204/en/ Kowdley et al. Hepatology (2012) Prevalence of Chronic Hepatitis B Among Foreign - Born Persons Living in the US by Country of Origin H B V P r e s e n t s a S i gn i f i c a n t U n m e t Med i c a l N ee d 5 ~ 900k people die every y ear as a consequence despite the availability of effective vaccines and antivirals. >257M people are chronically infected with HBV, globally. 90M China 15M Europe 2M United States

NASDAQ: ABUS www.arbutusbio.com N e w H B V T he r a p i e s rate of HBsAg Loss rate of Undetectable HBV DNA + SOC: Standard Of Care | HBsAg : HBV Surface Antigen | PegIFN: Pegylated Interferon Source: EASL HBV Clinical Practice Guidelines, 2017 - Pegasys, PEG - Intron, Baraclude and Viread Package Inserts Significant Opportunity t o I m p r o v e H B V C u r e R a t e s PegIFN Entecavir Tenofovir Dosing Duration 48 - weeks Chronic Chronic HBV DNA Undetectable (<60 - 80 IU/ml) 7 - 19% 67 - 90% 76 - 93% HBsAg Loss ~3 - 7% ~1 - 2% ~1 - 3% Achievable HBV Cure Rates with Current SOC = HIGHER CURES RATES HBV cures are achievable with today’s SOC in <5% of patients . Sustained HBsAg and HBV DNA loss after end - of - treatment* is rare. *undetectable HBsAg and HBV DNA 6 months after end - of - treatment accepted as a functional cure. 6 STANDARD OF CARE THERAPIES FOR CHRONIC HBV

NASDAQ: ABUS www.arbutusbio.com An HBV curative regimen would substantially increase diagnosis Compelling Growth Opportunity in the HBV Market 7 SOC: Standard Of Care Source: Global Hepatitis Report and Hepatitis B Fact Sheet, WHO (2017) http://www.who.int/mediacentre/factsheets/fs204/en/ 10.5% Diagnosed 1.8% Treated Low due to sub - optimal SOC cure rate and asymptomatic nature of disease. and treatment rates to unlock significant market growth opportunities. 27M 257M c h r on i c H B V 4 . 5 M

NASDAQ: ABUS www . a r bu t usb i o . c o m 3 2 1 2 H B V L i f ec y c l e I ll u s t r a t e s K e y P o i n t s f o r I nt e r v e n t i o n A combination of agents with complementary MOA is needed to cure HBV 1. Nucleoside Analogue 2. C a p s i d Inhibi t o r 3. R N A i & R N A Destabilizer NASDAQ: ABUS www.arbutusbio.com MOA: Mechanism of Action

NASDAQ: ABUS www.arbutusbio.com 9 Block Replication ▪ NA ▪ Ca p s i d In h i b i t or ▪ RNAi ▪ RNA Destabilizer Reduce cccDNA Pool ▪ Capsid Inhibitor Block HBsAg ▪ RNAi ▪ RNA Destabilizer Immuno - modulation ▪ PD - L1 Inhibitor ▪ Interferon Leading to an HBV CURE K e y s t o T h e r a p e ut i c Success MOA: Mechanism of Action | NA: Nucleoside Analogue | HBsAg : HBV Surface Antigen Suppress HBV DNA and viral antigens Reawaken host immune response Therapeutic success will require a combination of agents with complementary MOAs R e duc e / Supp r ess Viral DNA 1 Reawaken / Boost Host Immune Response 3 Block HBsAg ▪ RNAi ▪ RNA Destabilizer Reduce/Suppress Viral Antigens 2

NASDAQ: ABUS www.arbutusbio.com Ar butu s P i p e li n e 10 Phase I HBV Lead Optimization CTA/I ND Enabling Healthy Subjects HBV Subjects Phase II HBsAg Reduction RNAi AB - 729 HBV RNA Destabilizers Next Gen HBV DNA Suppression Capsid Inhibitor AB - 836 Immune Reawakening PD - L1 1st gen COVID - 19/Coronaviruses Lead Optimization CTA/I ND Enabling Phase I Phase II Pan - Coronavirus Agents

NASDAQ: ABUS www.arbutusbio.com AB - 729 RNAi Therapeutic 11 Proprietary GalNAc - conjugate delivery technology provides liver targeting and enables subcutaneous dosing Single trigger RNAi agent targeting all HBV transcripts Inhibits HBV replication and lowers all HBV antigens Pan - genotypic activity across HBV genotypes Demonstrated complementarity with capsid inhibitors Actively targets the liver Active against cccDNA derived and integrated HBsAg transcripts Clean profile in long term preclinical safety studies Linker Gal N A c n sAg Polymerase, Core Ag, eAg, pgRNA s A g H B x

NASDAQ: ABUS www.arbutusbio.com Cohort J: 90 mg Q12W HBV DNA - AB - 729 - 00 1 St ud y Part 1: Single Ascending Dose In Healthy Subjects Dose 1 (60 mg) n=6; 4 active : 2 placebo Dose 2 (180 mg) n=6; 4 active : 2 placebo Dose 3 (360 mg) n=6; 4 active : 2 placebo (≥ Day 15 Safety) Cohort A: 180 mg HBV DNA - Cohort B: 60 mg HBV DNA - Cohort C: 90 mg HBV DNA - (≥ Day 15 Safety) (≥ Day 15 Safety) (≥ Day 15 Safety) Cohort D: 90 mg HBV DNA + n =6 n =6 n =6 n =6 Cohort E: 60 mg Q4W HBV DNA - Cohort F: 60 mg Q8W HBV DNA - n =7 n =7 n =7 Part 2: Single Doses In Chronic Hepatitis B Subjects Part 3: Multiple Doses In Chronic Hepatitis B Subjects HBV: Hepatitis B Virus | TDF: tenofovir disoproxil fumarate | TBD: to be determined Cohort I: 90 mg Q8W HBV DNA - n =7 n =7 12 Cohort G: 90 mg Q8W + TDF HBV DNA +

NASDAQ: ABUS www.arbutusbio.com S i n gl e D os e s o f A B - 72 9 R e s u l t i n C o mp a r a b l e M e a n H B s A g Declines at Week 12 Followed by a Sustained Plateau Phase AB - 729 60 mg single dose (N=6) * AB - 729 90 mg single dose (N=6) ** AB - 729 180 mg single dose (N=4) # Week 12 mean (SE): - 0.99 (0.24) Week 12 mean (SE): - 1.23 (0.18) Week 12 mean (SE): - 0.98 (0.43) 3/6 HBsAg <100 IU/mL 1/6 HBsAg <10 IU/mL 5/6 HBsAg <100 IU/mL 1/6 HBsAg <10 IU/mL 0/4 HBsAg <100 IU/mL mean in d ivid ua l *N=5 at Week 10, 14, 18, 22, 28, and 32 **N=4 at Week 14 and 16; N=3 at Weeks 18 – 24 # N = 3 a f te r W ee k 12; nom i n a l v isi t s “ 7 d a ys 13

NASDAQ: ABUS www.arbutusbio.com R e p e a t D osi n g o f A B - 72 9 6 0 m g E v e r y 4 W ee k s R e s u l t s i n Continuous Mean HBsAg Declines Beyond Week 12 Mean ( “ SE) HBsAg declines across single and repeat dose Cohorts *N=5 at Week 10, 14, 18 and 22 **N=4 at Week 14 - 20; N=3 at Weeks 22 – 24 # N=6 at Week 6 SD: single dose; Q4W: every 4 weeks 14 #

Mean (SE) Week 20 : - 1.71 (0.18) Mean (SE) Week 24 : - 1.84 (0.16) R e p e a t D osi n g o f A B - 72 9 6 0 m g E v e r y 8 W ee k s R e s u l t s i n C o mp a r a b l e Mean HBsAg Declines to 60 mg Every 4 Weeks at Week 16 Mean (SE) Week 12: - 1.10 (0.15) Mean (SE) Week 16: - 1.44 (0.18) at Week 24: 5/7 HBsAg <100 IU/mL 2/7 HBsAg <10 IU/mL Mean Individual NASDAQ: ABUS www.arbutusbio.com AB - 729 60 mg Q4W AB - 729 60 mg Q8W 15 Me a n I ndivid ua l Mean (SE) Week 12: - 1.02 (0.11) Mean (SE) Week 16: - 1.39 (0.07)

NASDAQ: ABUS www.arbutusbio.com 16 R e p e a t D osi n g o f AB - 72 9 6 0 m g E v e r y 8 W ee k s R e s u l t s i n C o mp a r a b l e M e a n H B s A g D e c li n e s t o 6 0 m g E v e r y 4 W ee k s a t W ee k 1 6 Mean ( “ SE) HBsAg declines across repeat dose Cohorts *N=6 at Week 6 Q4W: every 4 weeks; Q8W: every 8 weeks

NASDAQ: ABUS www.arbutusbio.com AB - 729 90 mg Single Dose Reduces HBsAg and HBV DNA in H B V DN A P osi t i v e C H B s ub j e c t s These data continue to support dosing intervals of up to 12 weeks 17 - 2.5 - 2.0 - 1.5 - 1.0 - 0.5 0.0 0.5 1.0 0 4 8 12 Week 16 2 0 24 individual mean (N=5) ∆log 10 HBsAg (IU/mL) - 2.5 - 2.0 - 1.5 - 1.0 - 0.5 0.0 0.5 1.0 0 4 8 12 Week 16 ∆log 10 HBV DNA (IU/mL) 2 0 24 individual mean (N=5) Week 12 mean (SE): - 1.02 (0.13) Week 12 mean (SE): - 1.53 (0.24)

NASDAQ: ABUS www.arbutusbio.com AB - 72 9 W a s S a f e a n d W e l l T o l e r a t e d A f t e r S i n g l e a n d R e p e a t D o s e s ▪ No SAEs or discontinuations due to AEs ▪ No treatment - related Grade 3 or 4 AEs* ▪ No Grade 3 or 4 laboratory abnormalities* ▪ Grade 1 and Grade 2 ALT elevations have decreased with continued treatment ▪ Injection site TEAEs were mild (erythema, pain, pruritis, bruising) or moderate (pain) and transient ▪ No clinically meaningful changes in ECGs or vital signs ▪ All subjects in cohort E consented to an additional 6 months of dosing * 1 subject (Cohort A) with rapid decline in HBsAg of ~2.0 log10 IU/mL had an unrelated Gr 2 AE of food poisoning resulting in unrelated transient Grade 3 AEs of ALT/AST elevation (without bilirubin changes) 18

NASDAQ: ABUS www.arbutusbio.com 19 AB - 72 9 C li n i c a l S u mma r y Repeat 60 mg Q4W dosing with AB - 729 resulted in a continuous and robust mean HBsAg decline at week 24 ( - 1.84 log10 IU/mL, N=7) Repeat dosing of AB - 729 60 mg every 8 weeks results in comparable mean HBsAg declines relative to 60 mg every 4 weeks at week 16 ( - 1.44 log10 IU/mL vs - 1.37 log10 IU/mL, p <0.7) In HBV DNA positive CHB subjects, a single 90 mg AB - 729 dose resulted in robust mean HBsAg ( - 1.02 log10 IU/mL) and HBV DNA ( - 1.53 log10 IU/mL) declines at week 12, as well as decreases in HBV RNA and core - related antigen ▪ Similar mean HBsAg reductions were observed in HBV DNA positive and negative CHB subjects ▪ These findings support complete target engagement by AB - 729 AB - 729 remains generally safe and well tolerated These results support advancing AB - 729 to Phase 2 combination studies with AB - 729 dosing as infrequently as every 8 or 12 weeks

NASDAQ: ABUS www.arbutusbio.com NA: Nucleoside Analogue | HBeAg : HBV e Antigen AB - 72 9 C li n i c a l Collaboration w i t h A ss e m b l y Biosciences Provides accelerated AB - 729 combination proof of concept (POC) with a capsid inhibitor and NA with the potential for functional cure 20 F o l l ow U p AB - 729 + vebicorvir + NA AB - 729 + NA vebicorvir + NA Initiated Phase 2 Clinical Trial Feb 2021 Baseline Wk 72 Wk 48 Wk 24 ~60 virologically - suppressed subjects with chronic HBV infection Equal sharing of expertise and costs for this POC open - label trial No financial requirements or restrictions and no business requirements or restrictions

NASDAQ: ABUS www.arbutusbio.com Novel chemical series differentiated from AB - 506 and other competitor compounds in the Class II capsid inhibitor space Leverages a novel binding site within the core protein dimer - dimer interface Improved intrinsic potency with EC50 < 10 nM Active against NA resistant variants Potential to address known capsid resistant variants T33N and I105T Provides the potential for low dose and wide therapeutic window Projected to be once daily dosing Pangenotypic Combinable with other MOA agents AB - 836 Capsid Inh i b i t o r In March 2021, received regulatory approval to initiate Phase 1a/1b clinical trial Potential for increased efficacy and enhanced resistance profile relative to earlier generation capsid inhibitors 21
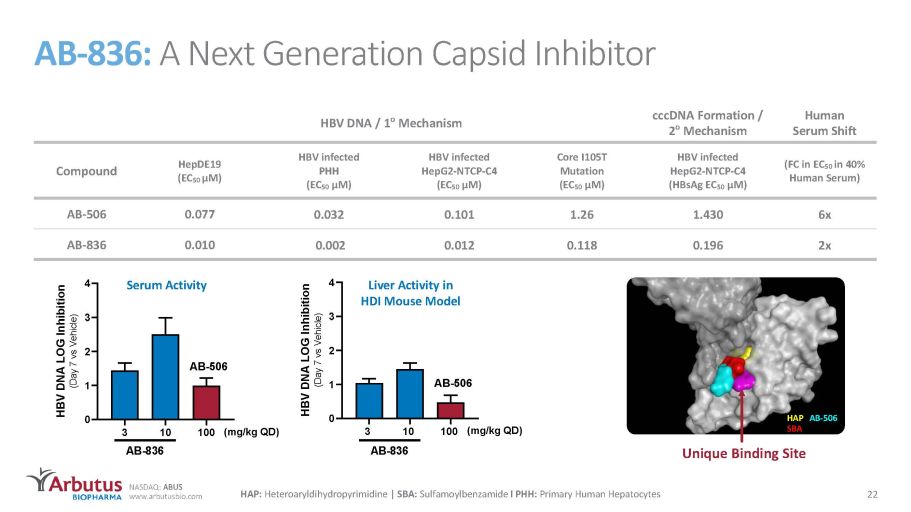
NASDAQ: ABUS www.arbutusbio.com AB - 836 : A N e x t G ene r a t i o n Ca p s i d Inh i b i t o r HBV DNA / 1 o Mechanism cccDNA Formation / 2 o Mechanism Human Serum Shift Compound HepDE19 ( E C 5 0 μ M ) HBV infected PHH (EC 50 μM) HBV infected He pG 2 - N T CP - C4 (EC 50 μM) Core I 105 T Mutation (EC 50 μM) HBV infected HepG2 - NTCP - C4 (HBsAg EC 50 μM) (FC in EC 50 in 40% Human Serum) AB - 506 0.077 0.032 0.101 1.26 1.430 6x AB - 836 0.010 0.002 0.012 0.118 0.196 2x 3 1 0 100 0 1 2 3 4 HBV DNA LOG Inhibition (Day 7 vs Vehicle) (mg/kg QD) A B - 8 3 6 A B - 5 0 6 Serum Activity 3 10 100 0 1 2 3 4 HBV DNA LOG Inhibition (Day 7 vs Vehicle) (mg/kg QD) A B - 8 3 6 A B - 5 0 6 Liver Activity in HDI Mouse Model Unique Binding Site HAP: Heteroaryldihydropyrimidine | SBA: Sulfamoylbenzamide I PHH: Primary Human Hepatocytes HAP AB - 506 SBA 22

NASDAQ: ABUS www.arbutusbio.com AB - 836 - 00 1 St ud y 23 Dose 6 ≤ 6 00 mg Dose 4 ≤ 2 00 mg Dose 3 ≤ 100 mg Dose 1 10mg Dose 5 ≤ 4 00 mg C o ho r t B Dose 2 ≤35mg C o ho r t A Dose 5 FE ≤400mg Dose 7 ≤ 8 00 mg Part 1: Single Ascending Dose In Healthy Subjects Alternating Cohorts A and B n=8/cohort; 6 active: 2 placebo Part 2: Multiple Ascending Dose in Healthy Subjects Cohort C (≤ 75mg QD) x 10 days N = 10; 8 active: 2 placebo Cohort D (≤200mg QD) x 10 days N = 10; 8 active: 2 placebo Cohort E (≤ 400mg QD) x 10 days N = 10; 8 active: 2 placebo Part 3: Multiple Doses In Chronic Hepatitis B Subjects Cohort F (≤ Cohort C) x 28 days DNA + N = 12; 10 active: 2 placebo Cohort G (≤ Cohort D) x 28 days DNA+ N = 12; 10 active: 2 placebo Cohort H (Dose TBD) x 28 days DNA+ N = 12; 10 active: 2 placebo Cohort I (Dose TBD) + NA x 28 days DNA - N = 12; 10 active: 2 placebo Cohort J (Dose TBD) + TDF x 28 days DNA+ N = 12; 10 active: 2 placebo

NASDAQ: ABUS www.arbutusbio.com N e x t G e n R N A D e s t a b ili z e r P r o g r a m W e beli e v e t hi s a pp r o ac h o f f e r s p o t e n t i a l f o r a n o r a l H B s A g r edu c in g a g e n t an d al l o r a l c o m bin a ti o n therapy C o n t inuin g ac t i v e r e s e a r c h an d d e v el o p m e n t o f a n e x t g ene r a ti o n s m al l m o le c ul e O f f e r s a n o v e l m e c h a ni s m o f ac t i o n t o r edu c e H B s A g an d o the r v i r a l p r o t ein s an d v i r a l R N A 24

NASDAQ: ABUS www.arbutusbio.com Leveraging our proven expertise and capabilities in antiviral drug discovery and development Long term commitment Pan - coronavirus focused Small Molecule Direct - Acting Antivirals Directed Effort ▪ nsp12 Viral Polymerase - nucleos(t)ides ▪ nsp5 Main Viral Protease - de novo design Screening Effort ▪ Proprietary library screening through COVID R&D consortium C o r o n a v ir u s St r a t eg y COVID - 19 Virus nsp5 / 3CLpro Viral Polymerase Viral Protease +RNA Virus 31 kb Genome nsp 5 protease & nsp 12 polymerase essential enzymes for replication 25

NASDAQ: ABUS www.arbutusbio.com 202 1 K e y Ob j ec t i v e s Cash balance of ~ $132M as of March 31, 2021, cash runway through 3Q 2022 Objective Anticipated Timing 2021 Additional data from AB - 729 90 mg single - dose in HBV DNA positive subjects 1H x Initiate a Phase 2 combination clinical trial to evaluate AB - 729 in combination with Assembly Biosciences’ lead core/capsid inhibitor candidate vebicorvir (VBR) and an NrtI 1H x Initiate a Phase 1a/1b clinical trial of AB - 836, our next - generation oral capsid inhibitor 1H x Additional data from AB - 729 60 mg multi - dose (4 wk / 8 wk dosing intervals) 1H / 1H Initial data from AB - 729 90 mg multi - dose (8 wk / 12 wk dosing intervals) 1H / 2H Initial data from AB - 729 90 mg multi - dose (8 wk dosing interval) in HBV DNA positive subjects 2H Initiate two Phase 2 combination clinical trials in HBV subjects; both including AB - 729, with one or more approved or investigational agents 2H 26
