Attached files
| file | filename |
|---|---|
| EX-32.2 - CERTIFICATION - GI DYNAMICS, INC. | f10k2020ex32-2_gidynamics.htm |
| EX-32.1 - CERTIFICATION - GI DYNAMICS, INC. | f10k2020ex32-1_gidynamics.htm |
| EX-31.2 - CERTIFICATION - GI DYNAMICS, INC. | f10k2020ex31-2_gidynamics.htm |
| EX-31.1 - CERTIFICATION - GI DYNAMICS, INC. | f10k2020ex31-1_gidynamics.htm |
| EX-23.1 - CONSENT OF WOLF & COMPANY, P.C - GI DYNAMICS, INC. | f10k2020ex23-1_gidynamics.htm |
| EX-4.7 - DESCRIPTION OF REGISTRANT'S SECURITIES - GI DYNAMICS, INC. | f10k2020ex4-7_gidynamics.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2020
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 000-55195
GI DYNAMICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 84-1621425 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) | |
| 320 Congress Street, 3rd Floor | ||
| Boston, Massachusetts | 02210 | |
| (Address of Principal Executive Offices) | (Zip Code) | |
(781) 357-3300
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Exchange Act: None
Securities registered pursuant to Section 12(g) of the Exchange Act: Common Stock, $0.01 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days: Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files): Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Accelerated filer ☐ | |
| Large accelerated filer ☐ | Smaller reporting company ☒ |
| Non-accelerated filer ☒ | Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): ☐ Yes ☒ No
The registrant’s common stock, in the form of CHESS Depositary Interests (“CDI” in singular, “CDIs” in plural), each CDI represented 1/50th of one share of common stock computed by reference to the price at which the CDIs were last sold on June 30, 2020, the last business day of the registrant’s most recently completed second quarter, as reported on the Australian Securities Exchange (“ASX”), was $3,767,612 (A$5,489,744).
On July 22, 2020, the registrant’s common stock was removed from the Official List of the Australian Securities Exchange and the CHESS Depository Trust was dissolved and all CDIs were automatically converted to shares of common stock, with fractional shares being redeemed for cash payment. The total number of shares of the registrant’s common stock outstanding on March 5, 2021 was 88,095,659.
DOCUMENTS INCORPORATED BY REFERENCE
The registrant intends to file with the Securities and Exchange Commission a proxy statement pursuant to Regulation 14A or an amendment to this report filed under cover of Form 10-K/A containing the information required to be disclosed under Part III of Form 10-K within 120 days of the end of its fiscal year ended December 31, 2020. Portions of such proxy statement or Form 10-K/A are incorporated by reference into Part III of this Annual Report on Form 10-K.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements as defined in Section 27A of the United States Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended. Throughout this Annual Report on Form 10-K, all references to the “Company” or “GI Dynamics” unless where the context requires otherwise, refers to the consolidated entity of GI Dynamics, Inc. These forward-looking statements concern the Company’s business, operations, financial performance and condition as well as plans, objectives and expectations for the Company’s business, operations and financial performance and condition. Any statements contained in this Annual Report on Form 10-K that are not of historical facts may be deemed to be forward-looking statements. The forward-looking statements are contained principally in the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Forward-looking statements include, but are not limited to, statements about the Company’s:
| ● | expectations with respect to the Company’s intellectual property position; |
| ● | expectations with respect to clinical trials for EndoBarrier®; |
| ● | expectations with respect to regulatory submissions and receipt and maintenance of regulatory approvals; |
| ● | the impact of the ongoing COVID-19 pandemic on our clinical trials, business plan and the global economy; | |
| ● | ability to commercialize products; |
| ● | ability to develop and commercialize new products; |
| ● | expectation with regard to product manufacture and inventory; and |
| ● | estimates regarding capital requirements and need for additional financing. |
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “aims,” “assumes,” “goal,” “intends,” “objective,” “potential,” “positioned,” “target,” “continue,” “seek,” “vision,” or the negative thereof and similar expressions intended to identify forward-looking statements.
These forward-looking statements are based on current expectations, estimates, forecasts and projections about the Company’s business and the industry in which it operates and management’s beliefs and assumptions. These forward-looking statements are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond the Company’s control. As a result, any or all forward-looking statements in this Annual Report on Form 10-K may later become inaccurate. The Company may not actually achieve the plans, intentions or expectations disclosed in any forward-looking statements, and actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements made by the Company. The Company has included important factors in the cautionary statements included in this Annual Report on Form 10-K, particularly in the “Risk Factors” section, that could cause actual results or events to differ materially from the forward-looking statements made.
You are urged to consider these factors carefully in evaluating the forward-looking statements and are cautioned not to place undue reliance on the forward-looking statements. You should read this Annual Report on Form 10-K and the documents that the Company has filed as exhibits to the GI Dynamics Form 10-K completely and with the understanding that actual future results may be materially different from what the Company expects. These forward-looking statements speak only as at the date of this Annual Report on Form 10-K. Unless required by law, the Company does not intend to publicly update or revise any forward-looking statements to reflect new information or future events or otherwise. You should, however, review the factors and risks described in the reports the Company will file from time to time with the SEC after the date of this Annual Report on Form 10-K.
GI DYNAMICS, INC.
ANNUAL REPORT ON FORM 10-K
FOR THE YEAR ENDED DECEMBER 31, 2020
TABLE OF CONTENTS
i
Overview
GI Dynamics, Inc. (“GI Dynamics” or “the Company”) is a clinical stage medical device company focused on the development and commercialization of EndoBarrier, a medical device system intended for treatment of patients with type 2 diabetes and reduction of obesity. EndoBarrier is a medical implant designated for the treatment of type 2 diabetes and the reduction of obesity with nearly 4,000 implants since inception and is the subject of an approved FDA pivotal trial in the United States under which the Company initiated patient implant procedures in January 2020. The Company believes that EndoBarrier represents a paradigm-breaking approach to traditional management of type 2 diabetes, which has focused historically solely on lifestyle intervention and diabetes medications. According to the article “Challenges in Diabetes Management: Glycemic Control, Medication Adherence, and Healthcare Costs,” published in the American Journal of Managed Care in 2017, these historical treatments for type 2 diabetes are limited in efficacy, with approximately 40% of patients achieving glycemic control.
EndoBarrier represents the first material departure from this historical approach. The Company believes that EndoBarrier offers an adjunct to non-insulin diabetes pharmacotherapy, while providing patients a chance to significantly reduce or eliminate insulin and increase the likelihood of avoiding invasive and permanent bariatric or metabolic surgery. EndoBarrier is designed to mimic the mechanism of action of the duodenal-jejunal exclusion currently created by gastric bypass surgery (which is referred to as Roux-en-Y Gastric Bypass, or RYGB), as explained in the article “EndoBarrier: A Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes?”, published in Obesity Surgery in 2018 by Nisha Patel. After implanting EndoBarrier, the patient’s food that exits the stomach will flow through the inside of the EndoBarrier Liner, and therefore will not contact the wall of the upper intestine (duodenum and jejunum). The food will also not mix with fluids from the pancreas and bile duct until these fluids and the food reach the far end of the EndoBarrier Liner. It is believed that, in a similar fashion to RYGB, shunting the food to a more distant location in the upper intestines triggers hormonal responses that regulate key factors that help control hunger, satiety, and insulin sensitivity, which is also explained in the article by Nisha Patel.
EndoBarrier has been shown in multiple independent clinical studies to lower blood sugar levels (as measured by hemoglobin A1c or HbA1c), reduce excess body weight, increase insulin sensitivity, and positively affect other health metrics and comorbidities. Because it has been utilized in approximately 4,000 procedures, EndoBarrier has a thoroughly characterized benefit-risk profile. A recent independent meta-analysis published in 2018 by Pichamol Jirapinyo in American Diabetes Association (ADA) Diabetes Care, “Effects of the Duodenal-Jejunal Bypass Liner on Glycemic Control in Patients with Type 2 Diabetes with Obesity: A Meta-Analysis with Secondary Analysis on Weight Loss and Hormonal Changes,” provides the most comprehensive review and meta-analysis of EndoBarrier clinical data to date. In addition, two ongoing EndoBarrier registries in the UK and Germany have captured and reported clinical results on more than 800 patients between the two databases. These registries as well as other investigator-initiated trials continue to release clinical data on an annual basis, largely centered around the annual meetings hosted by DDW (Digestive Disease Week) in May, ADA (American Diabetes Association) in June, EASD (European Association for the Study of Diabetes) in October, and Obesity Week in November.
Business Strategy
The Company’s goal is to become the leading provider of alternative options for treating type 2 diabetes and concurrently reducing obesity. The Company’s corporate priorities include:
| 1) | US Clinical Operations: Conduct Stage 1 of the STEP-1 EndoBarrier pivotal trial in the United States. |
The Company is focused on the successful execution of the STEP-1 pivotal trial in the United States. In August 2018, GI Dynamics received notification from the FDA that it had approved the Investigational Device Exemption (“IDE”) application for EndoBarrier, and received required Institutional Review Board (IRB) approval, in February 2019. The approved EndoBarrier IDE represents the pivotal clinical trial for EndoBarrier.
1
The STEP-1 clinical trial is a randomized, controlled, double blinded trial with 3:1 randomization between patients who receive the EndoBarrier implant (3) and the control group made up of patients who will receive a sham procedure and not receive the EndoBarrier implant (1). Both arms will maintain pharmacotherapy within ADA guidelines and will receive lifestyle and nutritional counseling consistent with ADA guidelines. The primary endpoint of the trial is reduction of HbA1c from baseline to removal of the EndoBarrier at twelve months as compared to the control group. The study includes numerous secondary endpoints, including changes in weight, cardiovascular risk metrics, as well as health metrics related to NAFLD (non-alcoholic fatty liver disease) and NASH (non-alcoholic steatohepatitis), and CKD (chronic kidney disease).
The STEP-1 clinical trial consists of two stages. The first stage consists of 50 EndoBarrier patients and approximately 17 control patients. At the end of stage 1, the Company will submit four DMC (Data Monitoring Committee) reports to the FDA for review. Upon a successful completion of the stage 1 review, the Company will submit a request to the FDA to complete the study by conducting stage 2, which the Company anticipates will be comprised of 130 EndoBarrier patients and approximately 43 control patients for a study total of 240 patients (180 EndoBarrier and 60 control patients). There is no guarantee that this will be the final composition of the study, as the number of remaining stages or remaining patients may vary based on the stage 1 clinical data and the outcome of the stage 1 review with FDA. In addition to the multiple staged study, the FDA has mandated certain stopping rules which, if triggered, could result in the STEP-1 study having enrollment or treatment delayed or stopped.
On January 27, 2020, the first patient was randomized into the STEP-1Trial Protocol. On March 11, 2020, the World Health Organization declared the outbreak of a coronavirus (“COVID-19”) pandemic. From the time of that declaration, the public safety restrictions adopted at the federal, state, and individual clinic policy level have impacted the ability to enroll patients after March 11, 2020. The EndoBarrier implantation procedure is classified as an elective procedure and was thus impacted by policies that disallowed elective procedures. In the fourth quarter of 2020, select clinical trial sites have been engaged in pre-screening activities to identify potential enrollees, although an increase in COVID-19 positivity rates and resulting policies on elective procedures, patient caution, and individual infections have prevented randomization of any additional enrollees prior to March 11, 2021. The Company continues to focus on potential patient identification and will resume enrollment and randomization as early as possible, presumably in the first half of 2021 due to the availability of the SARS-Cov2 vaccines and the assumption that the vaccines will continue to provide protection against emergent viral variants. Pending timely release of the COVID-19 restrictions, the Company expects to complete the enrollment of Stage 1 by the end of 2021.
| 2) | India Partnership: Conduct the I-STEP EndoBarrier pivotal trial in India as part of the Apollo Sugar partnership. |
GI Dynamics is focused on the successful execution of the EndoBarrier trial in India as part of its partnership with Apollo Sugar, which was announced on November 27, 2018.
The I-STEP study is also a randomized, controlled, double blinded trial with 3:1 randomization between patients who receive the EndoBarrier implant (3) and the control group made up of patients who will not receive the EndoBarrier implant (1). The primary endpoint of the trial is reduction of HbA1c from baseline to removal of the EndoBarrier at twelve months as compared to the control group. The study includes numerous secondary endpoints, including changes in weight and reduction in cardiovascular risk metrics. The study includes 100 patients in total, which consists of 75 patients who will receive the EndoBarrier implant and 25 control patients. Up to five clinical sites in India will participate in the study.
Apollo Sugar is a collaboration between Apollo Health & Lifestyle Limited and Sanofi. Apollo Sugar is a division of Apollo Hospitals Group (Apollo) focused on the treatment of metabolic disorders and operates an integrated network of centers of excellence for diabetes, obesity and endocrinology. Apollo is the largest private hospital system in India and has emerged as Asia’s foremost integrated healthcare services provider, maintaining a robust presence of hospitals, pharmacies, primary care and diagnostic clinics across the healthcare ecosystem. Since its inception, Apollo has treated over 65 million patients from 141 countries.
2
In April 2020, the Company and Apollo Sugar filed a pre-trial application with the Central Drugs Standards Control Organization (CDSCO) in India to obtain approval to initiate the I-STEP trial. As of March 11, 2021, the Company has not received communication from CDSCO regarding the timing of a response to the application, with the delay likely related to COVID-19 disruption in India. The Company expects to receive approval and initiate the trial in the first half of 2021, pending the removal of COVID-19 disruption and restrictions. Once initiated, the partners anticipate trial enrollment will take less than 12 months.
GI Dynamics and Apollo Sugar leadership are working to finalize the terms of the GI Dynamics-Apollo Sugar partnership that will focus on the marketing, distribution and clinical support of EndoBarrier to appropriate patients in India, Southeast Asia and the Middle East. Final terms of the proposed collaboration are subject to negotiation and will be disclosed upon completion.
| 3) | Gain CE Mark: Work with new Notified Body to gain EndoBarrier CE mark. |
CE Marking of a product is a manufacturer’s declaration that a product meets the applicable health, safety, and environmental requirements outlined in the appropriate European product legislation and has undergone the relevant conformity assessment procedure. A CE Mark is required before the Company can market EndoBarrier in the EU and certain Middle Eastern countries. In 2020, the standards for a medical device CE mark were scheduled to change from the Medical Device Directive (“MDD”) to the more stringent Medical Device Regulation (“MDR”), but largely due to the impacts of COVID-19, the deadline for conversion to MDR has been postponed. The Company has initiated the review process under MDD regulation with a new Notified Body, the independent third party commissioned to evaluate the conformity of products and the associated quality systems for manufacturers that seek to sell products in Europe.
Summary
In summary, GI Dynamics believes that EndoBarrier represents the most effective new treatment option in a market dominated by pharmaceutical companies generating annual revenue in excess $40 billion. The Company believes that EndoBarrier is poised to have a transformative and disruptive effect on the type 2 diabetes market. EndoBarrier is one of the few treatment options that treats type 2 diabetes, concurrently reduces obesity, and continues to show lasting treatment effects post treatment in many patients. As the duration of the EndoBarrier treatment effect continues to increase in published literature and because, to date, there has been minimal observed clinical risk after EndoBarrier removal, the EndoBarrier risk-benefit balance continues to evolve in an increasingly positive manner.
Background of the Disease
Diabetes mellitus type 2 (also known as type 2 diabetes) is a long-term progressive metabolic disorder characterized by high blood sugar, insulin resistance, and reduced insulin production. People with type 2 diabetes represent 90-95% of the worldwide diabetes population; only 5-10% of this population is diagnosed with type 1 diabetes (a form of diabetes mellitus wherein little to no insulin is produced).
Being overweight is a condition where the patient’s body mass index (BMI) is greater than 25 (kg/m2); obesity is a condition where the patient’s BMI is greater than 30, with some countries applying a lower metric to both definitions. Obesity and its comorbidities contribute to the progression of type 2 diabetes. Many experts believe obesity contributes to higher levels of insulin resistance, which creates a feedback loop that increases the severity of type 2 diabetes for many patients.
When considering treatment for type 2 diabetes, it is optimal to address obesity concurrently with diabetes. According to the article “Mechanisms of Insulin Resistance in Obesity,” published in Frontiers of Medicine in 2013 by Jianping Ye, absent the concurrent reduction of obesity with the treatment of type 2 diabetes, obesity will likely continue to contribute to the progressive nature of type 2 diabetes. It is the Company’s belief that the inability of the current pharmacologic options to fully treat type 2 diabetes is due to the fact that diabetes medications generally treat blood sugar levels only and do not contribute substantially to weight loss.
3
Current Treatment Options
According to the American Diabetes Association 2018 Standards of Medical Care in Diabetes, the current treatment paradigm for type 2 diabetes is lifestyle therapy combined with pharmacological treatment, whereby treating clinicians prescribe a treatment regimen of one to four concurrent medications that could include insulin. Insulin usage carries a significant risk of increased mortality and may contribute to weight gain, which in turn may lead to higher levels of insulin resistance and increased levels of blood sugar, as explained in the article “Insulin-associated weight gain in diabetes--causes, effects and coping strategies,” by Russel-Jones, D. Only 40% of patients treated pharmacologically for type 2 diabetes are adequately managed, meaning that medication does not lower blood sugar adequately and does not halt the progressive nature of diabetes for these patients, as explained in the article “Adherence to Therapies in Patients with Type 2 Diabetes,” by Luis-Emilio Garcia-Perez.
The current pharmacological treatment algorithms for type 2 diabetes fall short of ideal, creating a large and unfilled efficacy gap. GI Dynamics believes that EndoBarrier, which is designed to mimic the mechanism of action of duodenal-jejunal exclusion currently created by RYGB gastric bypass surgery and operates in a relatively similar manner by reducing both weight and long-term blood sugar levels, can fill this gap by treating type 2 diabetes and reducing obesity in a unique minimally invasive and reversible manner.
Market Opportunity
Unmet Clinical Needs in the Treatment of Type 2 Diabetes and Obesity.
In 2019, the International Diabetes Federation estimated there were approximately 463 million people with type 2 diabetes worldwide. Diabetes is the leading cause of cardiovascular disease, kidney failure, blindness, and lower-limb amputation in almost all countries.
Three years after initial diagnosis, over half of patients with type 2 diabetes require multiple drug therapies. Studies have shown that only 40% of the type 2 diabetes population is adequately managed pharmacologically, as explained in the article “Challenges in Diabetes Management: Glycemic Control, Medication Adherence, and Healthcare Costs,” published in the American Journal of Managed Care in 2017. At ten years post diagnosis, most patients, despite insulin use in many, struggle to reach their hemoglobin A1c (HbA1c) treatment goals. HbA1c is a glycosylated hemoglobin molecule found in the bloodstream that is formed when red blood cells are exposed to blood glucose. HbA1c has become the generally accepted gold standard biomarker for measuring levels of diabetes control in clinical practice and in human trials.
Many patients and health care systems struggle to meet the financial burden imposed by the numerous concurrent medications required to attempt to control the progressive nature of type 2 diabetes.
According to the World Health Organization, in 2016 more than 1.9 billion adults 18 and older were overweight. Of these, 650 million people worldwide are diagnosed with obesity (BMI ³ > 30 kg/m 2), a condition often leading to serious health consequences such as cardiovascular disease, diabetes, musculoskeletal disorders, and some cancers.
Those suffering from both type 2 diabetes and obesity, also referred to as diabesity, total more than 169 million worldwide, represent a significant public health problem not only in the United States (US) but also globally, as explained in the article “Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications,” published in the Circulation Research in 2016 by Shilpa Bhupathiraju. GI Dynamics believes that the unchecked worldwide rate of growth of the type 2 diabetes and obesity patient population represents one of the greatest unmanaged health risks in all of health care.
GI Dynamics believes EndoBarrier can treat patients with type 2 diabetes and reduce obesity in a safe, effective, nonpharmacological, and nonpermanent manner.
The Efficacy Gap
The Company’s intent in developing and seeking regulatory approval for EndoBarrier is to help clinicians deliver a unique treatment option to those patients suffering from type 2 diabetes, a disease state that sorely lacks innovative and broadly effective new treatment options.
4
GI Dynamics and its scientific advisors feel there must be a change in how the medical establishment currently treats obese patients suffering from type 2 diabetes because current treatment options are not effective. According to the International Diabetes Federation, the number of obese patients progressing to later stages of type 2 diabetes continues to grow at an alarming rate. Yet less than half of all type 2 diabetes patients are adequately managed by pharmacotherapy, and insulin carries serious risks and may in many cases contribute to the further progression of obesity. At the extreme end of the treatment spectrum, the treatment options are limited to different types of bariatric or metabolic surgery, which are highly invasive and irreversible procedures. Less than 1% of patients who are eligible for bariatric or metabolic surgery opt to undergo the procedure, as explained in the article “Recent advances in clinical practice challenges and opportunities in the management of obesity,” published by Andres Acosta in 2015 in the Gut .
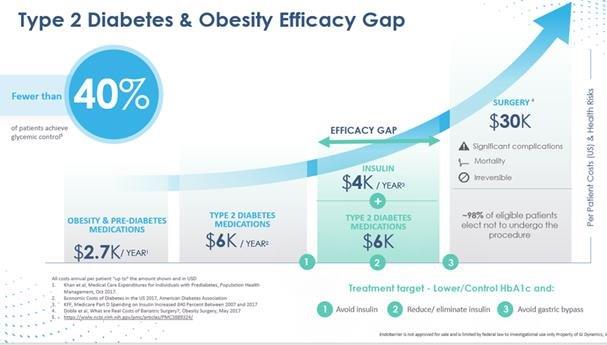
The graphic above illustrates the multiple treatment options during the course of progression of type 2 diabetes:
| ● | Risk associated with treatment increases from left to right. |
| ● | Progression of diabetes and obesity health risk and cost increase vertically. |
| ● | Lifestyle therapy (lifestyle counseling including nutrition and exercise) is the first line of defense against the progression of type 2 diabetes and obesity. |
| ● | Pharmacotherapy |
| o | Oral monotherapy follows lifestyle therapy, often with metformin as the first line of treatment. |
| o | Multiple combinations may then be administered, as recommended by the American Diabetes Association and other diabetes associations around the world. |
| o | Ultimately, as disease progression continues, injected insulin may be prescribed. |
| ● | Bariatric or metabolic gastric bypass surgery may represent a final option. |
5
A significant and rapidly growing patient population falls into the efficacy gap, wherein the patient is inadequately managed by medication yet unwilling to undergo gastric bypass surgery. For patients who elect to undergo bariatric or metabolic surgery, the clinical gains may not be permanent, while the risk associated with the procedure is permanent. These risks include, but are not limited to, excessive bleeding, blood clots, infection and in some cases death. GI Dynamics believes that because EndoBarrier is minimally invasive, can mimic the effects of gastric bypass, and has minimal side-effects, EndoBarrier can uniquely fill this efficacy gap, as explained in the article “EndoBarrier: A Safe and Effective Novel Treatment for Obesity and Type 2 Diabetes?” published in Obesity Surgery in 2018 by Nisha Patel.
The EndoBarrier Solution
EndoBarrier is intended for the treatment of type 2 diabetes and the reduction of obesity in a minimally invasive and reversible manner. EndoBarrier is designed to mimic the mechanism of action of duodenal-jejunal exclusion created by gastric bypass surgery. The EndoBarrier System is provided sterile and consists of an EndoBarrier Liner preloaded, packaged and sterilized within the EndoBarrier Delivery System. The EndoBarrier Delivery System is utilized to deliver the EndoBarrier Liner to the proximal small intestine. The EndoBarrier Liner is removed using the EndoBarrier Retrieval System.
The EndoBarrier System consists of three primary components:

| ● | EndoBarrier — The EndoBarrier or EndoBarrier Liner is a 60-cm-long implant consisting of a thin, flexible, impermeable fluoropolymer liner coupled to a proprietary nitinol Anchor Mechanism. A gastroenterologist implants the EndoBarrier Liner into the patient’s duodenum in a minimally invasive manner using the EndoBarrier Delivery System and standard gastroscope. The EndoBarrier Liner is placed endoscopically (through the mouth and esophagus and into the stomach without cutting tissue). Once the EndoBarrier Liner is properly positioned in the patient’s duodenal bulb, the area within the duodenum just below the stomach, the EndoBarrier Delivery System is removed and the EndoBarrier Liner remains, held in place by a proprietary Anchor Mechanism. The EndoBarrier Liner remains in the body for a maximum intended duration of twelve months until removal, again via a minimally invasive endoscopic procedure using the EndoBarrier Retrieval System. |
6
| ● | EndoBarrier Delivery System — The EndoBarrier Liner is delivered using the Company’s proprietary sterile, single-use EndoBarrier Delivery System. This includes a custom-made Outer Catheter that is sufficiently flexible to allow for insertion through the patient’s mouth, through the stomach, and into the intestine without kinking. The EndoBarrier Liner is pre-assembled during manufacturing into a collapsed position inside the Capsule, which is located at the end of the Outer Catheter. The clinician uses the EndoBarrier Delivery System to advance the Capsule into the duodenum and deploy the EndoBarrier Liner from the Capsule. The delivery procedure is typically an outpatient procedure, during which the patient is under anesthesia or semi-sedation. |
| ● | EndoBarrier Removal System — The EndoBarrier Liner is removed at the end of the treatment period via a minimally invasive endoscopic procedure using the Company’s proprietary Retrieval System which is passed by the clinician through a standard gastroscope. The Hook of the EndoBarrier Removal System is used to pull either of two drawstrings located in the Anchor Mechanism. As the drawstring is pulled, the Anchor Mechanism is collapsed inward and disengages from the wall of the duodenum. The Hood, pre-placed on the end of a gastroscope, allows the EndoBarrier anchor assembly to be pulled and collapsed into the Hood with the Hood positioned to cover portions of the anchor. The EndoBarrier Liner is then safely removed through the patient’s stomach, esophagus, and mouth. The retrieval procedure is typically an outpatient procedure, during which the patient is under anesthesia or semi-sedation. |
EndoBarrier has been shown in multiple company-sponsored and independent clinical studies to lower blood sugar levels (hemoglobin A1c or HbA1c), reduce excess body weight, and positively affect other health metrics and comorbidities.
EndoBarrier is not currently approved or commercially available in any jurisdiction. In August 2018, GI Dynamics received notification from the FDA that it had approved its IDE application, pending IRB approval which was received in February 2019. To gain regulatory approval to commercialize EndoBarrier in the United States, the Company must submit a pre-market authorization (PMA) application for review and approval by the FDA. In January 2020, GI Dynamics initiated treatment of patients in the STEP-1 pivotal clinical trial, which is the registration trial for the PMA application.
The EndoBarrier Effect
Obesity has been shown to exacerbate insulin resistance and contribute to the progression of type 2 diabetes. In situations where lifestyle modification and pharmacotherapy have failed and surgery is not an option or is considered a therapy of last resort, EndoBarrier is intended to reduce blood sugar and weight. In clinical trials in both the United States and outside of the United States, EndoBarrier has been shown to:
| ● | significantly lower glucose levels; |
| ● | significantly lower body weight; |
| ● | lower cardiovascular-related risk factors. |
EndoBarrier utilization accomplishes this in many patients by affecting key hormones involved in insulin sensitivity, glucose metabolism, satiety, and food intake. (See the section titled “How EndoBarrier Works: EndoBarrier Mechanism of Action.”)
The Company’s intent in positioning EndoBarrier to fill the type 2 diabetes efficacy gap with concurrent reduction of obesity is to help patients and clinicians avoid the initiation of insulin therapy by helping many patients maintain lower HbA1c levels and slow or halt the progression of type 2 diabetes. Furthermore, for those patients whose type 2 diabetes has progressed to the point where insulin therapy is necessary, EndoBarrier has been shown in many cases to lower HbA1c levels to the point where insulin dosage can be reduced, or insulin is no longer needed.
7
Finally, if the progression of type 2 diabetes is severe enough to warrant gastric bypass surgery, the Company expects that EndoBarrier either may serve as an opportunity to control type 2 diabetes so that surgery may not be needed, or at least better prepare the patient for bariatric surgery by lowering weight and helping control other comorbidities prior to surgery.
How EndoBarrier Works: EndoBarrier Mechanism of Action
The EndoBarrier mechanism of action is thought to be based on its functional similarities in many ways to RYGB. Once the EndoBarrier is implanted into the duodenum and proximal jejunum, ingested food passing through the EndoBarrier during the normal digestive process is prevented from interacting with the epithelium, microbiota, mucosal layer, or biliopancreatic secretions within the duodenum and proximal jejunum. In addition, EndoBarrier acts as a physical barrier that prevents the interaction of food with pancreatic enzymes and bile until reaching the end of the EndoBarrier Liner. Pancreatic enzymes and bile pass outside EndoBarrier and mix with the food at the distal end of the liner, where absorption ultimately takes place in the intestine. Thus, EndoBarrier creates a functional and reversible bypass of the upper intestine. Unlike RYGB surgery, EndoBarrier does not require an invasive and permanent surgical procedure or permanent physical modification of the stomach and exclusion of the distal stomach from the alimentary flow.
The Company’s scientific team and advisors have postulated the following EndoBarrier mechanisms of action, based on scientific evidence from such articles as “Effects of the Duodenal-Jejunal Bypass Liner on Glycemic Control in Patients with Type 2 Diabetes with Obesity: A Meta-Analysis with Secondary Analysis on Weight Loss and Hormonal Changes,” published in Diabetes Care in 2018 by Pichamol Jirapinyo and “The EndoBarrier: Duodenal-Jejunal Bypass Liner for Diabetes and Weight Loss,” published in Hindawi in 2018 by Aruchuna Ruban.:
Exclusion of the duodenum — This may offset an abnormality of gastrointestinal physiology responsible for insulin resistance and type 2 diabetes.
| ● | Increased nutrient delivery to the distal small bowel — Additional findings suggest that the exclusion of the proximal intestine (foregut theory) and increase in nutrient delivery to the distal small bowel (hindgut theory) created by EndoBarrier likely induce neuro-hormonal changes and nutrient sensing that affect energy balance and glucose homeostasis. |
| ● | Secretion of GLP-1 — Partially digested nutrients reach the distal ileum, which stimulates the secretion of GLP-1 by L-cells located in this area. GLP-1 is known to regulate insulin secretion and action. |
| ● | Increase in gut hormones — This contributes to the restoration of energy and glucose homeostasis. |
| ● | Elevated GLP-1 and PYY levels — Both levels are elevated as quickly as one-week post-implantation. Both hormones may play a role in satiety and body weight control. |
There is no known evidence of occurrence of clinically significant caloric malabsorption with EndoBarrier. EndoBarrier covers only 60 cm of duodenal and proximal jejunal mucosa, which most likely represents less than 10% of the length of the small intestine and leaves almost the entire jejunum and ileum for digestion and absorption.
Operations and Financing
GI Dynamics began selling EndoBarrier in Europe and South America in 2010 and in Australia in 2011. Since inception, the Company has distributed almost 4,000 units of EndoBarrier and generated a total of $7.8 million in revenue. The Company has incurred net losses in each year since its inception.
The Company has five subsidiaries: GI Dynamics Securities Corporation, a Massachusetts-incorporated nontrading entity; GID Europe Holding B.V., a Netherlands-incorporated nontrading holding company; GID Europe B.V., a Netherlands-incorporated company that conducts certain European business operations; GID Germany GmbH, a German-incorporated company that conducts certain European business operations; and GI Dynamics Australia Pty Ltd, an Australia-incorporated company that conducts Australian business operations.
8
GI Dynamics has raised net proceeds of approximately $283 million through sales of the Company’s equity and placement of debt, of which $9.5 million was received in 2020 that included the debt portion of the 2019 financing and the Initial Close of the September 2020 Series A Preferred Stock Financing. Prior to going public on the Australian Securities Exchange (“ASX”), the Company generated $75.7 million in proceeds, net of expenses, through the sale of convertible preferred stock to a number of US venture capital firms, two global medical device manufacturers, and qualified individual investors. In June 2011, the Company issued convertible term promissory notes to several of its stockholders totaling $6 million. In September 2011, the Company conducted an Initial Public Offering (“IPO”) in Australia and simultaneously conducted a private placement of CDIs to accredited investors in the United States. Net proceeds from these financings approximated $72.5 million, net of expenses and repayment of the June 2011 notes. In connection with the IPO, all existing shares of preferred stock were converted into common stock.
Between July 2013 and December 2016, GI Dynamics raised approximately $84.3 million, net of expenses, in offerings of the Company’s CDIs to qualified investors. In January 2017, GI Dynamics raised approximately $0.2 million, net of expenses, in an offering of the Company’s CDIs under a Security Purchase Plan (“SPP”) and in June 2017, GI Dynamics placed a Convertible Secured Term Promissory Note (the “2017 Note”, see Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In February and March 2018, GI Dynamics raised approximately $1.6 million in an offering of the Company’s CDIs to qualified investors and in May 2018, GI Dynamics placed a Convertible Term Promissory Note and Warrant (the “2018 Note and Warrant”, see Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing). In September and November 2018, GI Dynamics raised approximately $5 million in an offering of the Company’s CDIs to qualified investors.
In October 2018, GI Dynamics announced that it signed an agreement with its new notified body Intertek to pursue CE marking of EndoBarrier in Europe. The Company has since suspended its relationship with Intertek and is working with a different notified body. In November 2018, the Company signed a clinical trial agreement and a memorandum of understanding with its clinical and potential commercial partner Apollo Sugar, a division of Apollo Hospitals Group located in India. In December 2018, GI Dynamics announced the appointment of Charles Carter, a consultant, as the Company’s Chief Financial Officer and Secretary. Mr. Carter became an employee of the Company on September 1, 2019.
In March 2019, the Company completed a Note Purchase Agreement (“March 2019 NPA”) detailing a convertible term promissory note (the “March 2019 Note”) and warrant (the “March 2019 Warrant”) financing with Crystal Amber for a gross amount of $1 million (see Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing). In May 2019, the Company completed a convertible term promissory note (the “May 2019 Note”) and warrant (the “May 2019 Warrant”) financing with Crystal Amber for a gross amount of $3 million. (see Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing). On June 30, 2019, Crystal Amber elected to convert the 2018 Note, the March 2019 Note and the May 2019 Note into an aggregate of 453,609,963 CDIs (representing approximately 9,072,197 shares of common stock).
On August 21, 2019, the Company and Crystal Amber entered into a securities purchase agreement for a total funding of up to approximately $10 million (the “August 2019 SPA”, see Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing). In a series of scheduled transactions between August 2019 and November 2019, Crystal Amber exercised the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant per the terms of the August 2019 SPA. In December 2019, the Company and Crystal Amber agreed to defer the funding of the August 2019 Note to January 2020.
On January 13, 2020, the $4.6 million August 2019 Note was funded by Crystal Amber and the August 2019 Warrant was issued to Crystal Amber, providing for the purchase of up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) at $0.02 per CDI.
On June 18, 2020, the Company entered into a Note Purchase Agreement (“June 2020 NPA”) by and between the Company and Crystal Amber. Pursuant to the June 2020 NPA, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $750 thousand, with terms including mandatory conversion in the event of the close of a Series A Preferred Stock offering.
9
On July 13, 2020, Crystal Amber provided the Company with a notice of optional conversion of the 2017 Note. On the conversion date, the principal of $5 million and the accrued and unpaid interest of $390,240 totaling $5,390,240 was converted into 2,574,873,400 CDIs, which was equivalent to 51,497,468 shares of Common Stock. The conversion price equaled $0.002093 per CDI or $0.10467 per share of Common Stock. On receipt of the notice issued the available 38,401,704 shares and the Company and Crystal Amber executed a Right to Shares and Waiver Agreement in which the Company agreed to issue the remaining 13,095,764 shares of Common Stock owed under the conversion when the Company had received shareholder approval and had increased the authorized number of shares of common stock to allow for issuance of the remaining shares. The additional shares were issued September 3, 2020.
On July 22, 2020, GI Dynamics was removed from the Official List of the ASX and all CDIs were converted to shares of Common Stock, with cash redemption of fractional shares. The CHESS Depository Nominee was subsequently dissolved. Although the Company’s securities are not traded on any exchange, the Company remains subject to SEC reporting requirements.
On August 4, 2020, the Company entered into a Note Purchase Agreement (“August 2020 NPA”) by and between the Company and Crystal Amber. Pursuant to the August 2020 NPA, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $500 thousand (the “August 2020 Note”) with terms including mandatory conversion in the event of the close of a Series A Preferred Stock offering. The Company received $250 thousand on August 3, 2020 and the remaining $250 thousand on August 6, 2020.
On September 4, 2020, the Company executed a series of financing-related agreements (“the September 2020 Financing”) which included the cancellation of the August 2019 Warrant, the restructuring of the August 2019 Note into the September 2020 Note (see below for a detailed description of the September 2020 Note and the accounting classification of the August 2019 Note restructuring into the September 2020 Note), the conversion of the June and August 2020 Convertible Notes into shares of Series A Preferred Stock (see below for a detailed description of the June and August 2020 Notes and Note 10 to the Consolidated Financial Statements for a description of the Series A Preferred Stock), and the sale of $8.75 million of Series A Preferred Stock to Crystal Amber under the Series A Preferred Stock Purchase Agreement (the “Series A SPA”, see Note 11 to the Consolidated Financial Statements for a description of the Series A SPA terms). The Initial Close of the September 2020 Financing occurred on September 4, 2020, and included all components of the September 2020 Financing, including the Crystal Amber purchase of 42,310,730 shares of Series A Preferred Stock for gross proceeds of $3.75. Pursuant to the Series A SPA and subsequent amendments, the second and final close (the “Second Close”) was originally to occur on October 31, 2020 but through a series of extensions, was extended until February 24, 2021. On February 24, 2021, an individual investor closed the purchase of 600,000 shares of Series A Preferred Stock for approximate proceeds of $53 thousand. Effective February 24, 2021, Crystal Amber executed a contract amendment restructuring the Second Close to include the sale of 16,924,292 shares of Series A Preferred Stock for proceeds of $1.5 million to close on March 4, 2021, the sale of 11,282,861 shares of Series A Preferred Stock for proceeds of $1.5 million to close no later than March 25, 2021, and the remaining 27,607,153 shares of Series A Preferred Stock for proceeds of $2.45 million to close no later than May 28, 2021.
On October 8, 2020, Mr. Joseph Virgilio was appointed Chief Operating Officer of the Company.
On October 20, 2020, Ginger Glaser was appointed a Director of the Company.
On November 2, 2020, Mr. Scott Schorer resigned as President and Chief Executive Officer of the Company and Mr. Joseph Virgilio was appointed Chief Executive Officer and a director of the Company.
On December 31, 2020, Charles Carter, the Company’s Chief Financial Officer, Secretary and Treasurer, ceased to be an employee of the Company and executed a retention bonus agreement and a consulting agreement. Mr. Carter remains the Company’s Chief Financial Officer, Secretary and Treasurer.
The rights of the Company’s stockholders are governed by Delaware general corporation law.
10
GI Dynamics is headquartered in Boston, Massachusetts, where the majority of the Company’s employees work. The Company has subsidiaries in the Netherlands, Germany, and Australia, with employees in Germany and Australia.
Strategic Focus in 2021: The Path Forward
The global COVID-19 pandemic and associated public health restrictions caused major disruption to the Company’s operations, specifically in the clinical hold on elective procedures pausing the Step-1 clinical trial enrollment in the United States, the COVID-19 related delay in receiving approval to initiate the I-Step trial in India, the COVID-19 related changes in the schedule and priorities of critical vendors in the application and review for CE Mark. With the majority of 2020 being disrupted due to the ongoing COVID-19 pandemic, our strategic goals for 2021 involve the resumption of the pursuit of our corporate strategy with an aggressive focus on time compression of key activities to make up for COVID-19 related delays. The complete resumption of activity will be dependent on both a release of the restrictions that impacted operations in 2020, but also a processing of prioritized backlogs by critical agencies and vendors.
The Company’s goal is to become the leading provider of alternative options for treating type 2 diabetes and concurrently reducing obesity. GI Dynamics intends to do this by conducting the following activities:
| 1) | U.S. clinical operations and pivotal trial: | Complete enrollment in Stage 1 of the STEP-1 pivotal trial in the US. | |
| 2) | India partnership and pivotal trial: | Initiate enrollment in the I-STEP pivotal trial in India. | |
| 3) | Gain CE Mark: | Work with the Company’s notified body to gain the EndoBarrier CE mark. |
The Company will need to raise additional capital in 2021.
As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. GI Dynamics is currently focused on obtaining an EndoBarrier CE mark and enrolling the Company’s clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2020 and had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
The Company will need to arrange additional financing before August 1, 2021 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources, however, the Company has no guaranteed source of capital that will sustain operations past August 1, 2021. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these Consolidated Financial Statements are issued.
11
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s Consolidated Financial Statements include a going-concern disclosure. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources” for further information regarding the Company’s funding requirements.
Implications of Being a Smaller Reporting Company
We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares of common stock held by non-affiliates does not equal or exceed $250.0 million as of the prior June 30th, or (2) our annual revenues did not equal or exceed $100.0 million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any reduced disclosure obligations, it may make comparison of our financial statements with other public companies difficult or impossible.
Employees
At March 11, 2021, GI Dynamics has 12 full-time employees, 9 of whom are in the United States. None of the Company employees are represented by labor unions or covered by collective bargaining agreements.
Available Information
Financial and other information about GI Dynamics is available on the Company website, whose address is www.gidynamics.com. The Company makes available on its website, free of charge, copies of its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after the Company electronically files such material with, or furnishes it to, the U.S. Securities and Exchange Commission (SEC).The information contained in the Company website is not intended to be part of this filing.
12
The Company’s business faces many risks. The Company believes the risks described below are the material risks that it faces. However, the risks described below may not be the only risks that it faces. Additional unknown risks or risks that are currently considered immaterial, may also impair the Company’s business operations. If any of the events or circumstances described below actually occur, the Company’s business, financial condition or results of operations could suffer. You should consider the specific risk factors discussed below together with the cautionary statements under the caption “Forward-Looking Statements” and the other information and documents that the Company files from time to time with the Securities and Exchange Commission (SEC).
Risks Related to the Company’s Business
GI Dynamics will need substantial additional funding and may be unable to raise capital when needed, which could force the Company to delay, reduce, or eliminate planned activities or result in its inability to operate as a going concern.
As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. GI Dynamics is currently focused on obtaining an EndoBarrier CE mark and enrolling the Company’s clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2020 and had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
The Company will need to arrange additional financing before August 1, 2021 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources, however, the Company has no guaranteed source of capital that will sustain operations past August 1, 2021. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these Consolidated Financial Statements are issued.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s Consolidated Financial Statements include a going-concern disclosure. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources” for further information regarding the Company’s funding requirements.
Failure to achieve a positive outcome in the U.S. clinical trial of EndoBarrier could negatively impact the Company’s ability to raise additional capital and obtain regulatory approval in other countries.
GI Dynamics is conducting the U.S. pivotal trial of EndoBarrier under the FDA’s IDE. The Company refers to this trial as the GI Dynamics STEP-1 clinical trial in its statements and filings. Failure to achieve a positive outcome in this clinical trial could result in the failure of EndoBarrier to gain regulatory approval in the U. S. This outcome could negatively impact the Company’s ability to raise additional capital and obtain regulatory approval in other countries.
13
In order to complete the STEP-1 clinical trial, GI Dynamics will need to enroll patients that ultimately complete the trial protocol. If the Company is unable to enroll patients, if the enrollment pace is slower than anticipated, or if a number of enrolled patients do not complete the study protocol, it could have a negative effect on the Company’s ability to complete the clinical trial, which could adversely affect the Company’s business, operating results and prospects.
Conducting successful clinical studies will require the enrollment of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the sufficient resolution of COVID-19 related public health restrictions to allow enrollment and implantation of patients, the sufficient decline in the perception of risk of infection with SARS-CoV2, or emerging or new variants, related to physical presence in a clinical setting, the size of the target patient population, the nature of the trial protocol, the desirability of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators and support staff, the proximity of patients to clinical sites, the ability of patients to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in the Company’s clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of the Company’s product or if they determine that the treatments received under the trial protocols are not desirable or involve unacceptable risk or discomfort.
Development of sufficient and appropriate clinical protocols to demonstrate safety and efficacy are required and GI Dynamics may not adequately develop such protocols to support clearance or approval. Further, the FDA may require the Company to submit data on a greater number of patients than originally anticipated and/or for a longer follow-up period or change the data collection requirements or data analysis applicable to the Company’s clinical trials. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may cause an increase in costs and delays in the approval or clearance and attempted commercialization of the Company’s product or result in the failure of the clinical trial. In addition, despite considerable time and expense invested in clinical trials, the FDA may not consider the Company’s data adequate to demonstrate safety and efficacy. Such increased costs and delays or failures could adversely affect the Company’s business, operating results and prospects.
GI Dynamics or third parties upon whom it depends may be adversely affected by natural disasters and/or health epidemics (specifically, COVID-19), and the Company’s business, financial condition and results of operations could continue to be adversely affected.
In December 2019, a novel strain of coronavirus was reported to have surfaced in Wuhan, China. In January 2020, this coronavirus spread to other parts of the world, including the United States and Europe, and efforts to contain the spread of this coronavirus intensified. In March 2020, the World Health Organization declared the Coronavirus outbreak a “pandemic.” The Company operations experienced significant restrictions and delays in 2020, including the following operational impacts:
| ● | The pause of enrollment of the Step-1 trial due to categorization of the EndoBarrier implantation procedure as an elective procedure. In the face of the epidemic and as a measure to reduce the spread of SARS-CoV-2, many states and/or clinics ceased all elective procedures. All of the Step-1 clinical sites ceased enrollment for a period in 2020 and as of March 11, 2021, all sites were able to enroll, pending procedural backlog clearance in individual clinics. |
| ● | The private and public regulatory agencies required to approve the initiation of clinical trials, review and audit quality systems, and review applications for regulatory approvals all had to adapt to COVID-19 restricted work protocols that may have impacted productivity, priorities and schedules. As such, many regulatory applications the Company submitted were delayed in submission and follow-up response from the agencies were dramatically delayed. In one instance, the Company was able to incur substantial cost in enabling a timely in-person audit under COVID-19 safety precautions. In other instances, the Company cannot accelerate the timing of agency response. |
| ● | The economic impact of the pandemic and resulting restrictions may have affected investor perception of available funds for investment and risk tolerance. |
| ● | Business travel was largely restricted or eliminated in 2020, potentially limiting the effectiveness of fundraising and other important business meetings. |
As of March 2021, various vaccines are being administered at differential rates in global communities and while the spread of SARS-CoV-2 is being impacted positively, a state of global economic and public health safety uncertainty remains as SARS-CoV-2 variants have emerged. Currently, the existing vaccines provide protection against the known variants, but the emergence of a variant for which the current vaccines are not effective may occur. If such a variant does emerge, the same effects experienced in 2020 may be repeated as restrictions are again required to minimize the impact of the re-emergent pandemic.
Should the coronavirus continue to spread in the United States and Europe or should a novel strain develop for which there is no vaccine or effective treatments, GI Dynamics business operations will likely continue to be delayed or interrupted. For instance, the Company’s clinical trials may be impacted by containment policies enacted by the clinical practice, the respective state or the Federal government. Further, as a result of 2020 restrictions, there is a growing backlog of patient procedures which may be prioritized higher than EndoBarrier implantation. These, and other COVID-19 related effects could result in lower than anticipated patient registration or enrollment and GI Dynamics may be forced to temporarily delay or pause ongoing trials. Further, if the spread of the coronavirus continues and the Company’s operations are impacted, GI Dynamics risks a delay, default and/or nonperformance under existing agreements arising from force majeure. If any of the foregoing were to occur, it could materially adversely affect the Company’s financial condition.
14
The FDA has mandated certain stopping rules in the STEP-1 clinical trial. If the stopping rules are triggered or other unanticipated adverse issues occur during the STEP-1 clinical trial, the FDA may not permit the trial to continue. If that were to happen, the Company’s business, operating results and prospects would be materially and adversely affected.
Clinical trials involve the administration of the biological product candidate to patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, and the parameters to be used to monitor subject safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur. In the context of the GI Dynamics STEP-1 clinical trial, hepatic bleeding is an example of an adverse event that would be investigated and could trigger such stopping rules.
If, during the STEP-1 clinical trial, the stopping rules are triggered or other unanticipated adverse issues occur, the FDA may prevent the Company from enrolling additional patients in the clinical trial or may not permit the clinical trial to be completed. If that were to happen, the Company’s business, operating results and prospects would be materially and adversely affected.
In order to commercialize the Company’s product in the U.S. and certain other countries, GI Dynamics will need to obtain regulatory and other approvals. The Company’s inability to achieve, or a delay in achieving, such approvals could lead to the denial of marketing approval for EndoBarrier® or any of its other products.
At present, GI Dynamics has no regulatory approvals for the marketing and sale of EndoBarrier. In November 2017, the Company received notification from SGS, the Company’s now former notified body in Europe, that they were withdrawing the Certificate of Conformity for EndoBarrier. The Certificate of Conformity is required for the sale of any product under CE marking. As a result, GI Dynamics is not permitted to supply the EndoBarrier, EndoBarrier Delivery System and EndoBarrier Retrieval System in Europe.
There is no guarantee that GI Dynamics will obtain additional approvals from regulatory bodies in the future, including the FDA in the U.S. In the U.S., the Company stopped its pivotal trial of EndoBarrier in 2015. The Company will not be able to obtain FDA approval to commercialize EndoBarrier in the U.S. until the STEP-1 clinical trial is successfully completed and regulatory approval is obtained from the FDA through the PMA review process. The STEP-1 clinical trial will be conducted in two stages. At the end of the first stage, the FDA will review data collected to date from the STEP-1 clinical trial and must approve the continuation of the STEP-1 clinical trial to the second stage. There can be no assurance that the FDA will approve continuation of the trial at the end of the first stage.
Regulatory authorities in other countries may also require additional clinical trials. Any such clinical trials may be delayed, suspended or prematurely terminated because costs are greater than anticipated or for a variety of other reasons, including: COVID-19 related delays, delay or failure in reaching agreement with the foreign regulatory authority(ies) on a trial design that the Company is able to execute; delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical trial; or regulators may require that the Company suspend or terminate its clinical trials for various reasons, including noncompliance with regulatory requirements, unforeseen safety issues or adverse side effects, or a finding that the participants are being exposed to unacceptable health risks. Any of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of marketing approval for EndoBarrier or any of the Company’s other products. Necessary regulatory approvals could also be delayed, which could significantly impact the Company’s ability to commercialize the Company’s technology in the U.S. and other countries.
15
GI Dynamics has a history of net losses and may never achieve or maintain profitability.
GI Dynamics is a medical device company with a limited history of operations and has limited commercial experience with its product. As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. The Company continues to evaluate which markets are appropriate to continue pursuing reimbursement, market awareness and general market development and selling efforts, and continues to restructure its business and costs, establish new priorities, and evaluate strategic options. The Company expects to continue to incur significant operating losses for the foreseeable future as it incurs costs, including those associated with commercializing the Company’s products, conducting clinical trials to test its products, attempting to secure regulatory approvals for the Company’s products (in the U.S. and other countries) and ongoing costs associated with being an SEC reporting company in the U.S. As a result, if the Company remains in business, it expects to incur significant operating losses for the next several years.
The Company has incurred operating losses since inception and at December 31, 2020, had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
GI Dynamics cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
GI Dynamics depends heavily on the success of the Company’s product, EndoBarrier. The Company cannot give any assurance that EndoBarrier will receive regulatory approval, which is necessary before it can be commercialized.
The Company’s ability to generate product revenues will depend heavily on the successful commercialization of its product, EndoBarrier, if approved by regulatory agencies in the U.S. or abroad. GI Dynamics cannot be certain that EndoBarrier will be successful in clinical trials or receive regulatory approval. Further, EndoBarrier may not receive regulatory approval even if it is successful in clinical trials. If the Company does not receive regulatory approvals for EndoBarrier, it may not be able to continue operations.
If GI Dynamics can obtain the required regulatory approvals in the U.S. and certain other countries, it expects to derive substantially all of its revenue from sales of EndoBarrier. Accordingly, the Company’s ability to generate revenues in the future relies on its ability to market and sell this product.
The degree of market acceptance for EndoBarrier will depend on a number of factors, including:
| ● | the efficacy, ease of use and perceived advantages and disadvantages of EndoBarrier over other available treatments and technologies for managing type 2 diabetes; |
| ● | the prevalence and severity of any adverse events or side effects of EndoBarrier; |
| ● | the extent to which physicians adopt EndoBarrier (which may be influenced by the Company’s ability to provide additional clinical data regarding the potential long-term benefits provided by EndoBarrier and the strength of the Company’s sales and marketing initiatives); |
| ● | the price of EndoBarrier and the third-party coverage and reimbursement for procedures using EndoBarrier; |
| ● | the extent to which reimbursement may be secured for each country in which EndoBarrier is commercialized; and |
| ● | the Company’s ability to attract and retain professional sales personnel to drive EndoBarrier revenue. |
16
GI Dynamics cannot predict the outcome and timing of its current and future human clinical trials of EndoBarrier products. If the trials do not produce positive results, the commercial prospects for EndoBarrier will be impaired.
The results of current and future human clinical trials, whether investigator initiated, or Company sponsored, cannot be predicted. If EndoBarrier or new products that the Company develops and tests in the future cause serious adverse events in future human clinical trials, these trials may need to be delayed or stopped. For example, GI Dynamics stopped its original U.S. pivotal trial, the ENDO trial, in 2015 because of adverse events associated with EndoBarrier in that trial. In addition, these clinical trials may not produce positive safety or efficacy results or may produce results that are not as favorable as those seen in previous clinical trials.
Negative safety or efficacy results of any future human clinical trials could require that the Company attempt to modify the EndoBarrier device or the treatment guidelines to address these issues and there is no guarantee that any potential modifications would be successfully developed.
If future human clinical trials of EndoBarrier products do not meet the required clinical specifications or cause serious adverse or unexpected events, such as those experienced in the ENDO trial, then these results could affect regulatory approvals and adoption and materially impact potential product sales and reimbursement. If the Company is not able to adequately address any adverse or unexpected events through training, education, changes in product design or product claims, this may significantly impair the commercial prospects for EndoBarrier.
Doctors may not accept EndoBarrier as a treatment option, which would harm the Company’s business and future revenues, if any.
The commercial success of EndoBarrier will require acceptance by physicians, who may be slow to adopt the Company’s product for the following reasons (among others):
| ● | lack of long-term clinical data supporting patient benefits or cost savings over existing alternative treatments; |
| ● | lack of experience with EndoBarrier and training time required before it can be used, which may drive preferences for other products or procedures; |
| ● | lack of adequate payment to the physician for implanting the device or caring for the patient (driven by availability of adequate coverage and reimbursement for hospitals and implanting physicians); |
| ● | perceived strength of products, procedures or pharmacotherapies as alternatives to EndoBarrier; and |
| ● | perceived liability risks associated generally with the use of new products and procedures. |
Although the Company has developed relationships with physicians who are key opinion leaders in certain countries, GI Dynamics cannot assure that these existing relationships and arrangements can be maintained or that new relationships will be established in support of its products. If physicians do not consider the Company’s products to be adequate for the treatment of type 2 diabetes or if a sufficient number of physicians recommend and use competing products or pharmacotherapies, it could harm the Company’s business and future revenues, if any.
The Company has limited sales, marketing and distribution experience; therefore, it may be unable to successfully commercialize its products.
There can be no guarantee that the Company will be able to effectively commercialize its products. Developing direct sales, distribution and marketing capabilities will require the devotion of significant resources and require GI Dynamics to ensure compliance with all legal and regulatory requirements for sales, marketing and distribution. Failure to develop these capabilities and meet these requirements could jeopardize the Company’s ability to market its products or could subject it to substantial liability. In addition, for those countries where the Company commercializes its products through distributors or other third parties, GI Dynamics will rely heavily on the ability of its partners to effectively market and sell its products to physicians and other end users in those countries. The Company cannot guarantee that distributors or other third parties will be effective in commercializing GI Dynamics products.
17
GI Dynamics may compete against companies that have longer operating histories, more established or approved products, and greater resources, which may prevent the Company from achieving market penetration with its products.
Competition in the medical device industry is intense and EndoBarrier will likely compete in part against more established procedures and products for the treatment of type 2 diabetes and obesity. Bariatric surgery, including gastric bypass surgery and the gastric band, have been used for many years with extensive publication histories on clinical effectiveness. Large multinational medical device companies sell supplies for these procedures and are formidable competitors to new entrants. In addition, certain drugs have been approved, and are used, for the treatment of type 2 diabetes and obesity. Pharmaceutical companies with significantly greater resources than the Company market these drugs, and GI Dynamics may be unable to compete effectively against these companies.
Many of the Company’s competitors have significantly greater sales, marketing, financial and manufacturing capabilities than it has and have established reputations and/or significantly greater name recognition. Accordingly, there is no assurance that GI Dynamics will be able to win market share from these competitors or that these competitors will not succeed in developing products that are more effective or economic.
Additionally, GI Dynamics is likely to compete with companies offering new technologies in the future. The Company may also face competition from other medical therapies, which may focus on the Company’s target market as well as competition from manufacturers of pharmaceutical and other devices that have not yet been developed. Competition from these companies could adversely affect the Company’s business.
GI Dynamics does not have data regarding the long-term benefits of EndoBarrier, which may affect market acceptance. Furthermore, if the long-term data, once obtained, does not indicate that EndoBarrier is as safe or effective as other treatment options, the Company’s business would be harmed.
An important factor that may be relevant to market acceptance of EndoBarrier is whether it improves or maintains glycemic control and maintains weight loss over extended periods of time after removal of the device. While the Company has tested and evaluated its technology in several clinical trials with hundreds of patients which, in the aggregate, have shown that EndoBarrier is an effective treatment for type 2 diabetes and also reduces obesity, GI Dynamics does not yet have sufficient data to demonstrate and claim any longer-term benefits of its product in the treatment of type 2 diabetes and the reduction of obesity following removal of the device from the patient.
GI Dynamics is continuing to monitor some patients who participated in the Company’s clinical trials after device removal to determine the ongoing effects and longevity of results, however, the Company does not currently have long-term data that supports the safety and efficacy of EndoBarrier. Accordingly, the Company cannot provide assurance that the long-term data, once obtained, will prove lower HbA1c levels compared to alternative treatment options for type 2 diabetes. If the results obtained from the Company clinical trials indicate that EndoBarrier is not as safe or effective as other treatment options or as effective as the Company’s current short-term data would suggest, EndoBarrier may not be approved, or its adoption may suffer, and the Company’s business would be harmed.
If GI Dynamics fails to obtain and maintain adequate levels of reimbursement for its products by health insurers and other third-party payers, there may be no commercially viable markets for its products, or the markets may be much smaller than expected.
If GI Dynamics products are approved for sale, health care providers, including hospitals and physicians that may purchase the Company’s products, generally rely on third-party payers, particularly government-sponsored health care and private health insurance providers, to pay for all or a portion of the costs of the procedures, including the cost of the products used in such procedures. Reimbursement and health care payment systems vary significantly by country. Third-party payers may attempt to limit coverage and the level of reimbursement of new therapeutic products.
If GI Dynamics fails to obtain and maintain adequate levels of reimbursement for its products by health insurers and other third-party payers, there may be few commercially viable markets for its products, or the markets may be much smaller than expected. Third-party payers may demand additional clinical data requiring new clinical trials or economic models showing the cost savings of using the Company’s product, each of which would consume resources and may delay the decision on reimbursement. If the results of such studies are not satisfactory to third-party payers, then reimbursement may not be received in an acceptable amount or at all. In addition, the efficacy, safety, performance and cost-effectiveness of the Company’s products in comparison to any competing products or therapies may determine the availability and level of reimbursement.
18
The Company believes that future reimbursement may be subject to increased restrictions both in the U.S. and in international markets. Future legislation, regulation or reimbursement policies of health insurers or third-party payers may adversely affect the demand for the Company’s current and future products or limit the Company’s ability to sell these products on a profitable basis.
The Company’s products may be subject to extensive, dynamic and ongoing regulation in countries where it plans to sell EndoBarrier, which may impede or hinder the approval or sale of its products and, in some cases, may ultimately result in the inability to obtain approval of certain products or may result in the recall or seizure of previously approved products.
The medical device industry is regulated extensively by governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies and authorities, such as the E.U., legislative bodies and the European Economic Area, or EEA, Member State Competent Authorities. Additionally, the exit of Great Britain from the E.U. may create a separate and distinct regulatory agency with different requirements for regulatory approval in Great Britain.
Before GI Dynamics can market its products in the E.U., and in many other parts of the world, it must obtain and maintain CE Mark certification, which indicates that a product meets the essential requirements of applicable E.U. Directives and has been subject to the appropriate conformity assessment route. This conformity assessment procedure is often done through a self-certification, but depending on the type of product, may also require verification by an independent certification body, called a “notified body.” Notified bodies will also periodically conduct audits to ensure continued compliance with the applicable requirements. The CE Mark allows free movement of products in the E.U., the EEA and Switzerland although any of the member countries may require medical devices to be registered and impose requirements relating to the language of the device information. Many non-European countries also recognize and accept the CE Mark. Failure to support product performance claims and demonstrate continued compliance with the applicable E.U. requirements, can result in the loss of the right to affix the CE Mark to the product, which prevents the product from being sold within the territory and in other countries that recognize the CE Mark.
Before GI Dynamics can market its products in other parts of the world, which are not regulated via CE mark or FDA approvals, it must obtain and maintain regulatory approvals in those countries. These approvals are dependent on clinical data and may require specific clinical trial data to be collected before applications for approval can be made. The Company does not currently have any assurance in any jurisdiction that such regulatory approvals will be granted or that more extensive clinical trials will not be required to secure such approvals. In India, GI Dynamics is partnering with Apollo Sugar to initiate and conduct clinical trials to generate data required by the Indian regulatory authorities, but as with any market, the Company does not have any assurance that these trials will generate the complete data set required to support a marketing approval.
In addition, even if the Company receives regulatory approval of its products in existing markets, the Company will be subject to ongoing regulatory requirements relating to its existing products in those markets. These include the requirement to timely file various reports with regulatory authorities in the countries in which the Company markets products, including reports of adverse events such as those experienced in the ENDO trial, events that may have caused or contributed to a death or serious injury and malfunctions that would likely cause or contribute to a death or serious injury if the malfunction were to recur. If these reports are not timely filed, regulators may impose sanctions, including temporarily suspending existing market authorizations, and sales of EndoBarrier may suffer. In that case, the Company may be subject to product liability or regulatory enforcement actions, all of which could harm the Company’s business. The Company’s failure to comply with EEA or other foreign regulations applicable in the countries where it operates could lead to the issuance of warning letters or untitled letters, the imposition of injunctions, suspensions or loss of regulatory clearance or approvals, product recalls, termination of distribution, product seizures or civil penalties. In the most extreme cases, criminal sanctions or closure of the Company’s manufacturing facilities are possible.
19
In addition, numerous new regulatory changes in the E.U. came into effect in 2016. The Company may not be able to comply with the new regulations and standards, despite compliance with prior regulations and standards.
In some countries, GI Dynamics may rely on its foreign distributors and agents to assist in complying with foreign regulatory requirements, and the Company cannot be sure that they will always do so. If the Company or any of its suppliers, third-party manufacturers, distributors, agents or customers fail to comply with applicable requirements, the Company may face:
| ● | adverse publicity; | |
| ● | investigations by governmental authorities; | |
| ● | fines and prosecutions; | |
| ● | inability to raise capital; | |
| ● | inability to attract and retain sales professionals; | |
| ● | inability to attract and agree to terms with business partners; | |
| ● | increased difficulty in obtaining required approvals; | |
| ● | loss of approvals already granted; | |
| ● | delays in purchasing decisions by customers or cancellation of existing orders; and | |
| ● | the inability to sell or import the Company’s products into such countries. |
Regulatory requirements affecting the development, manufacture and sale of medical devices are evolving and subject to future change. GI Dynamics cannot predict what impact, if any, those changes might have on its business. Failure to comply with regulatory requirements could have a material adverse effect on the Company’s business, financial condition and results of operations. Later discovery of previously unknown problems with a product or manufacturer could result in fines, delays or suspensions of regulatory approvals. The failure to receive product approval on a timely basis, or the withdrawal of product approval by regulatory agencies, could have a material adverse effect on the Company’s business, financial condition or results of operations.
GI Dynamics has had historical challenges with regulatory compliance, which led to a 2014 shipping hold in the E.U., multiple observations by the Therapeutic Goods Administration (“TGA”) in Australia regarding the Company’s failure to comply with Essential Principals of the TGA, and compliance issues that led to the TGA’s cancellation of the EndoBarrier listing on the Australian Register of Therapeutic Goods (“ARTG”) in 2016. In addition, in May 2017, GI Dynamics received notification from its notified body, SGS, that the CE Mark for EndoBarrier had been suspended pending closure of non-conformances related to the Company’s quality management system required under International Organization for Standardization (“ISO”) regulations. On November 10, 2017, the Company received notification from SGS that SGS was withdrawing the Certificate of Conformance for EndoBarrier, ending the CE Marking of EndoBarrier in Europe and select Middle East countries.
Given the past challenges the Company has had in meeting all the requirements of a comprehensive global quality system, a risk exists that the Company will have compliance issues in the future.
Claims that the Company’s current or future products infringe or misappropriate the proprietary rights of others could adversely affect the Company’s ability to sell those products and cause it to incur additional costs.
If any third-party intellectual property claim against the Company is successful, the Company could be prevented from commercializing EndoBarrier or other Company products.
20
There are numerous issued patents and pending patent applications in the U.S. and internationally that are owned by third parties and that contain patent claims in areas that are the focus of the Company’s product development efforts. GI Dynamics is aware of patents owned by third parties, to which it does not have licenses, that relate to, among other things, liner materials and anchoring. The Company has also employed individuals who were previously employed at other medical device companies, including competitors or potential competitors, which may result in claims from third parties that the Company has inadvertently or otherwise used or disclosed alleged trade secrets or other proprietary information of former employers of its employees.
In addition, because patent applications can take many years to issue, there may be currently pending applications, unknown to the Company, which may later result in issued patents that pose a material risk to the Company.
GI Dynamics expects that it could be subject to third-party infringement claims if the number of competitors grows and the functionality of products and technology in different industry segments overlap. Third parties may currently have, or may eventually be issued, patents on which its current or future technologies may infringe.
If GI Dynamics is unable to obtain, maintain and enforce intellectual property protection covering its products, others may be able to make, use, or sell products substantially the same as the Company’s, which could adversely affect the Company’s ability to compete in the market.
The Company’s commercial success is dependent in part on obtaining, maintaining and enforcing intellectual property rights, including patents, covering EndoBarrier and the Company’s future products. If GI Dynamics is unable to obtain, maintain and enforce intellectual property protection covering its products, others may be able to make, use or sell products that are substantially the same as the Company’s without incurring the sizeable development costs that the Company has incurred, which would adversely affect the Company’s ability to compete in the market. Certain patents covering EndoBarrier will begin to expire in 2023.
Even if GI Dynamics patents are determined by a court to be valid and enforceable, they may not be sufficiently broad or enforceable to prevent others from marketing products similar to the Company’s or designing around the Company’s patents, despite its patent rights. GI Dynamics products may also not have freedom to operate unimpeded by the patent rights of others.
In addition to patented technology, the Company relies on a combination of non-patented proprietary technology, trade secrets, processes, procedures, technical knowledge and know-how accumulated or acquired since inception. Any of the Company’s intellectual property rights could be challenged, invalidated, circumvented or misappropriated. In order to preserve and enforce the Company’s patent and other intellectual property rights, GI Dynamics may need to make claims or file lawsuits against third parties. This can entail significant costs and divert management’s attention from developing and commercializing EndoBarrier.
GI Dynamics relies on suppliers for certain key components in the manufacture of the EndoBarrier system. If these suppliers were to become unavailable to the Company, there could be delays in the commercialization of the Company’s products.
GI Dynamics relies on suppliers for several key components of EndoBarrier, in particular the material used to manufacture the sleeve used in EndoBarrier. Except as discussed below, the Company or its manufacturing partner, PPMD, have supply agreements in place with suppliers of all components and services required to manufacture the EndoBarrier System. While GI Dynamics currently has adequate material required to make the sleeve to support the Company well into commercialization efforts, the Company does not presently have an active supply agreement with a manufacturer for additional material. The reliance on third-party suppliers subjects the Company to risks that could harm its business, particularly with respect to the supply of key components or processes. Although the Company believes that alternative suppliers are available, the process of identifying and qualifying new suppliers who can produce the components to the Company’s specifications could cause delays in the commercialization of the Company products.
21
The use, misuse or off-label use of the Company’s products by physicians may harm the Company’s image in the marketplace or result in injuries that lead to product liability.
GI Dynamics cannot prevent a physician from using EndoBarrier for purposes outside of its eventual approved and intended use, which is known as off-label use. If physicians attempt to use the Company’s products off-label, there may be an increased risk of adverse events. Further, the use of the Company’s products for uses other than those uses for which the products have been approved may not effectively treat such conditions, which could harm the Company’s reputation in the marketplace among physicians and patients.
Physicians may also misuse the Company’s products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability for the Company. Physicians may also treat patients from other countries where the product is not approved, and the physician is then unable to properly monitor the patient’s progress. If GI Dynamics is deemed to have engaged in the promotion of any of its products for off-label use, the Company could be subject to action by regulatory authorities and the imposition of sanctions, which could also affect the Company’s reputation and position within the industry.
Patients may not follow instructions from their physicians. Patients who ignore training and ignore their clinician’s request to comply with dietary instructions or pharmacotherapy label instructions, or to remove EndoBarrier at the end of the 12-month indication for treatment may be exposed to greater physical and clinical risk.
Product liability claims could damage the Company’s reputation or adversely affect its business or financial position.
GI Dynamics may be exposed to the risk of product liability claims, which are inherent in the design, manufacturing, marketing and use of medical devices and, in particular, implantable medical devices. The Company holds product liability insurance; however, adequate product liability insurance may not continue to be available on commercially acceptable terms. Product liability claims may damage the Company’s reputation and, if the Company’s insurance coverage proves inadequate, may harm or destroy the Company’s business. Defending a suit, regardless of its merits, could be costly and could divert Company management’s attention from the Company’s core business activities.
GI Dynamics will rely on a third-party manufacturer for its products and this manufacturer is required to comply with regulatory requirements. Failure of the Company’s manufacturer or suppliers to comply with regulatory requirements could disrupt the manufacturing process, which would, in turn, have a material adverse effect on the Company’s business and results of operations.
Future clinical trials and commercialization of GI Dynamics products requires access to manufacturing facilities that meet applicable regulatory standards to manufacture a sufficient supply of the Company’s products. GI Dynamics will rely on a third-party manufacturer to meet the applicable regulatory requirements. Suppliers of components and products used to manufacture the Company’s products and the Company’s third-party manufacturer must also comply with applicable regulatory requirements. These often require significant time, resources, record-keeping and quality assurance efforts and subject the Company, its suppliers and third-party manufacturer to potential regulatory inspections and stoppages. Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored by regulatory authorities. Failure by GI Dynamics, its third-party manufacturer or suppliers to comply with regulatory requirements or failure to take satisfactory corrective action in response to an adverse inspection could result in enforcement actions that could have a material adverse effect on the Company’s business and results of operations.
In order to manufacture EndoBarrier in the quantities that GI Dynamics anticipates will be required to meet its clinical trial needs and potential future market demand, the Company will need to increase production capacity and efficiency over current levels, and the Company’s third-party manufacturer must therefore be able to provide the Company with sufficient quantities of its product in compliance with regulatory requirements, in accordance with agreed upon specifications, at acceptable cost and on a timely basis. The Company’s potential future growth could strain the ability of its third-party manufacturer to deliver the Company’s product and obtain materials and components in sufficient quantities. Third-party manufacturers often experience difficulties in scaling up production, including problems with production yields and quality control and assurance. If the Company is unable to obtain a sufficient or consistent supply of EndoBarrier or any other product it is developing, or if it cannot do so efficiently, the Company’s revenue, business and financial prospects would be adversely affected.
22
The Company announced on July 26, 2017 that it executed an agreement with PPMD to manufacture the Company’s product. GI Dynamics does not have extensive experience with this manufacturer, and the Company cannot guarantee that PPMD will manufacture its product in a validated manner or to acceptable levels of quality. The Company is seeking other suppliers who will be able to manufacture the Company’s product should the relationship with PPMD not prove successful.
GI Dynamics has been focused on efficiently running the Company’s operations in order to conserve resources which may impact its ability to retain its remaining employees and therefore the Company may not be able to sustain its business.
At March 11, 2021, 2020, GI Dynamics has 12 full time employees. Since the Company’s initial restructuring efforts in 2015, the Company has been heavily dependent upon its ability to retain its remaining employees. The loss of service of any of the Company’s remaining employees, particularly members of the management team, may have an adverse effect on the Company’s business. Given the long period of reduced staff, the morale of the Company’s remaining employees may be lower, employees may be distracted and any one of the remaining employees could terminate his or her employment with the Company at any time. A departing employee’s expertise would be difficult to replace and the failure to do so on a timely basis could have a material adverse effect on the Company’s ability to achieve its business goals. There can be no assurance that the Company will have the financial resources to, or otherwise be successful in, retaining the Company’s remaining personnel and failure to do so could have a material adverse effect on the Company’s business, financial condition and results of operations.
If GI Dynamics is unable to retain or hire key personnel, it may not be able to sustain or grow its business.
The Company’s ability to operate successfully and manage potential future growth depends significantly upon the ability to attract, retain and motivate highly skilled and qualified research, technical, clinical, regulatory, sales, marketing, managerial and financial personnel. The competition for qualified employees in the medical device industry is intense and there are a limited number of persons with the necessary skills and experience.
The Company’s performance is substantially dependent on senior management and key technical staff to continue to develop and manage the Company operations. The loss or the inability to recruit and retain high-caliber staff could have a material adverse effect on the Company. GI Dynamics also relies on the technical and management abilities of certain key directors, key members of the executive team and employees, consultants and scientific advisers. The loss of any of these directors, members of the executive team, employees, consultants, or scientific advisers could have an adverse effect on the Company’s business.
GI Dynamics may be unable to effectively manage its anticipated growth.
To manage the Company’s anticipated growth and to commercialize its products, GI Dynamics will need to expand operations (research and development, product development, quality, regulatory, manufacturing, sales, marketing and administrative). This expansion will place a significant strain on management, infrastructure, and operational and financial resources, particularly in light of the uncertainties related to operational execution and methods after the COVID-19 pandemic, for example, international business travel versus virtual presence. Specifically, the Company will need to manage relationships with various persons and entities participating in the Company’s clinical trials and quality systems, manufacturers, suppliers and other organizations, including various regulatory bodies in the U.S. and other jurisdictions. GI Dynamics may not be able to implement the required improvements in an efficient and timely manner and may discover deficiencies in existing systems and controls. The failure to accomplish any of these tasks could materially harm the Company’s business and its ability to commercialize EndoBarrier. As a result, the Company’s revenue, business and financial prospects would be adversely affected.
23
If the Company fails to maintain proper and effective internal controls over financial reporting, its ability to produce accurate and timely financial statements could be impaired, which could harm the Company’s operating results, and investors could lose confidence in the Company’s financial reports, which could adversely affect the value of the Company.
As an SEC reporting company in the U.S., GI Dynamics is required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by management on, among other things, the effectiveness of its internal control over financial reporting as of the end of the fiscal year. The controls and other procedures are designed to ensure that information required to be disclosed by the Company in the reports that the Company files with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. The Company’s first report on compliance with Section 404 was furnished in connection with the GI Dynamics financial statements for the year ended December 31, 2014. As a former emerging growth company and a current smaller reporting company, the Company has been and currently is exempted from the requirement to include an external auditor opinion on the Company’s internal controls in its filings. GI Dynamics continues to update, document and evaluate its internal control over financial reporting to the requirements of Section 404.
In Item 9A of this report, the Company discloses that with respect to the standards of Section 404, the Company concluded that its internal control over financial reporting was effective as of December 31, 2020. For additional information on this item, please see Item 9A. Controls and Procedures.
Although the Company concluded that its internal control over financial reporting was effective as of December 31, 2020, the Company has identified and reported material weaknesses in prior periods, and the Company cannot be certain that its internal control practices will ensure that the Company will have or maintain adequate internal control over financial reporting in future periods. Any failure to have or to maintain such internal control could harm the Company’s operating results, and investors could lose confidence in the Company’s financial reports, which could adversely affect the value of the Company.
Fluctuations in foreign currency exchange rates could adversely affect the Company’s financial results.
In the past, when GI Dynamics sold EndoBarrier outside of the U.S., the Company’s activities produced revenues and GI Dynamics incurred expenses in a variety of different currencies, which exposed the Company to significant exchange rate risk. If GI Dynamics is successful in once again obtaining CE Mark designation or other foreign regulatory approvals that will allow it to sell EndoBarrier outside of the U.S., the Company will again be exposed to significant exchange rate risk. Additionally, expenses may be incurred in foreign currencies, subjecting the Company to exchange rate risk at the time of cash disbursement. Exchange rate risk may affect the Company’s financial performance and position. Furthermore, some of the Company’s funds may be held in euros, Australian dollars or other currencies, while the Company’s functional currency is U.S. dollars. GI Dynamics is not currently hedging against exchange rate fluctuations, and consequently will be at the risk of any adverse movement in the U.S. dollar exchange rate if the Company exchanges funds held in one currency into another currency.
Risks Related to the Medical Device Industry
The medical device industry is subject to rapid technology change, which may result in obsolescence of the Company’s products.
The medical device industry is subject to rapid technology change. For the Company to remain competitive and to retain and build market share, it must continually develop new products as well as improve its existing ones. Accordingly, The Company must devote substantial resources to research, development and commercialization activities.
There can be no assurance that GI Dynamics will be successful in developing competitive new products and/or improving existing products so that such products remain competitive and avoid obsolescence. There can also be no assurance that any or all Company research and development projects for new products will demonstrate safety and efficacy and result in commercial products, or that if such products are successfully designed and launched, they will be profitable.
Health care reform legislation could adversely affect the Company’s future revenue and financial condition.
In recent years, there have been numerous initiatives by governments throughout the world for comprehensive reforms affecting the delivery of and payment for health care. GI Dynamics cannot predict the changes that will be made and the effect such changes will have on the use of EndoBarrier. Decisions to increase or decrease reimbursement or coverage for treatments for type 2 diabetes and/or obesity could have a material impact on the Company’s business and results of operations.
24
CE Mark designation requires ongoing compliance, reporting and testing requirements established by regulatory agencies. While the Company has adopted comprehensive compliance programs to attempt to comply with these regulations, it is possible that some of its business activities could be subject to challenge. Furthermore, the regulations may become more stringent in the future and historical data may or may not suffice in meeting the new regulations. If the Company’s past or present operations, or those of its former independent sales agents and distributors, are found to be in violation of any of such applicable governmental regulations, GI Dynamics may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from health care reimbursement, product recall, or retraction of approval to market the Company’s product.
New legislation in the U.S. has also been enacted that imposes additional reporting requirements and penalties on the medical device industry. While GI Dynamics has adopted comprehensive compliance programs to attempt to comply with these regulations, it is possible that some of the Company’s business activities could be subject to challenge under one or more of such laws. If the Company’s past or present operations, or those of its former independent sales agents and distributors, are found to be in violation of any of such laws or any other applicable governmental regulations, GI Dynamics may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from health care reimbursement, product recall, or retraction of approval to market the Company’s product.
Subsequent to future regulatory approvals for EndoBarrier, pricing pressures in the health care industry could lead to demands for price concessions, which could have an adverse effect on the Company’s business, financial condition or results of operations.
Due to the significant rise in health care costs over the past decade, numerous initiatives and reforms initiated by governments and third-party payers to curb these costs have resulted in difficulties in maintaining or raising the number and price of procedures. The increase in pricing pressure is driven by the competitive environment in the medical device industry as many larger companies cut prices as they struggle to retain market share.
The type 2 diabetes and obesity market has seen increasing resistance from payers regarding local and national reimbursement coverage. GI Dynamics expects that market demand, government regulation, third-party reimbursement policies and societal pressures could exert downward pressure on the prices of the Company’s products in the future after regulatory approval is granted and may adversely impact the Company’s business, financial condition or results of operations on a country by country basis.
Manufacturing facilities for medical devices must comply with applicable regulatory requirements.
Completion of the Company’s current and future clinical trials and commercialization of its products requires access to manufacturing facilities that meet applicable regulatory standards to manufacture an adequate supply of the Company’s products. The third-party manufacturer and suppliers of components and parts that the Company intends to use to manufacture its products must also comply with applicable regulatory requirements, which often require significant time, money, resources and record-keeping and quality assurance efforts and subject the Company, its third-party manufacturer and its suppliers to potential regulatory inspections and stoppages.
Compliance with applicable regulatory requirements is subject to continual review and is rigorously monitored through periodic inspections by regulatory authorities. Failure by the Company, its third-party manufacturer or its suppliers to comply with regulatory requirements or failure to take satisfactory corrective action in response to an adverse inspection could result in enforcement actions, including a public warning letter, a shutdown of, or restrictions on, the Company’s ability to obtain sufficient quantities of its products, delays in approving or clearing a product, refusal to permit the import or export of its products or other enforcement action.
25
Critical elements of the manufacturing process may be found to be hazardous and alternative technologies may cost more and/or may be less effective.
Numerous materials are used in the process of manufacturing a patient-ready device. As chemical testing and empirical data collection continues, certain chemicals or processes may be declared hazardous and while suitable alternatives will be sought, there may be a period of short or no availability before a replacement chemical or process is adopted. For example, a widespread process used in medical device manufacture is the use of ethylene oxide (“EtO”) for low temperature gas sterilization. In early 2019, a large EtO sterilization plant was closed by state-level authorities over health concerns. The medical device industry, the contract manufacturing industry, state authorities, federal authorities, and scientists are seeking a suitable alternative. Until such a suitable replacement is found, there is a risk that there will be a shortage of sterilization options, which may require design changes, process changes or which may cause a delay in device production.
Inadequate funding or pandemic-related limitations for the FDA, the SEC and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent EndoBarrier from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of the Company’s business rely, which could negatively impact the Company’s business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which the Company’s operations may rely, including those that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs and medical devices to be reviewed and/or approved by necessary government agencies, which would adversely affect the Company’s business. For example, over the last several years, including beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly affect the ability of the FDA to timely review and process the Company’s regulatory submissions, which could have a material adverse effect on the Company’s business. Further, in the Company’s operations as a public company, future government shutdowns could impact the ability to access the public markets and obtain necessary capital in order to properly capitalize and continue operations.
Risks Related to GI Dynamics common stock
There is no current trading market for GI Dynamics common stock and no such market may develop.
Subsequent to the Company’s removal from the official list of the Australian Securities Exchange on July 22, 2020, there is no current trading market for the Company’s securities. Should at some stage in the future, GI Dynamics submit a listing application with Nasdaq (or any other exchange) to list its common stock on the Nasdaq Capital Market (or any other exchange), Nasdaq may not approve the listing application. Even if Nasdaq (or such other exchange) approves the Company’s listing application, an active trading market for the Company’s common stock may never develop in the U.S. (or other relevant country) and stockholders may not be able to transfer or resell their common stock at its fair value, or at all.
Changes in economic conditions may adversely affect the Company’s business.
Changes in the general economic climate in which the Company operates may adversely affect its financial performance and the value of its assets. Factors that contribute to that general economic climate include:
| ● | contractions in the world economy or increases in the rate of inflation; | |
| ● | international currency fluctuations;
| |
| ● | Economic impact of any world health and public safety concerns and resulting policies; |
26
| ● | changes in interest rates; | |
| ● | new or increased government taxes or duties or changes in taxation laws; or | |
| ● | changes in government regulatory policy. |
Some current stockholders can exert control over the Company and may not make decisions that are in the best interests of all stockholders.
At December 31, 2020, Crystal Amber owned approximately 93% of the Company’s outstanding shares of common stock and would be able to exert a significant degree of influence over the Company’s management of its affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. The second largest stockholder, on matters on which Crystal Amber was ineligible to vote, would have approximately 46% of the voting power, and would be able to exert a significant degree of influence over the Company’s management of its affairs and over matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may harm the value of the Company’s common stock by delaying or preventing a change in control, even if a change is in the best interests of other stockholders. This concentration of ownership may also delay, deter, or prevent acts that may be favored by other stockholders, as the interests of these controlling stockholders may not always coincide with the interests of other stockholders.
Provisions of the Company’s certificate of incorporation, the Company’s bylaws and Delaware law could make an acquisition of the Company more difficult.
Certain provisions of the Company’s certificate of incorporation and bylaws could discourage, delay or prevent a merger, acquisition or other change of control that the Company’s stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their common stock. These provisions could also limit the price that investors might be willing to pay in the future for the common stock, thereby depressing the market price of these securities. Stockholders who wish to participate in these transactions may not have the opportunity to do so. In addition, GI Dynamics is incorporated in the State of Delaware and, as such, is governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on GI Dynamics common stock, from merging or combining with the Company for a prescribed period of time.
GI Dynamics does not intend to pay cash dividends on its common stock in the foreseeable future.
GI Dynamics has never declared or paid any cash dividends on its shares of common stock, and it currently does not anticipate paying any cash dividends in the foreseeable future. The Company intends to retain any earnings to finance the development and expansion of the Company’s products and business. Accordingly, the Company’s stockholders will not realize a return on their investment unless the trading price of GI Dynamics common stock appreciates.
The Company’s ability to use its net operating loss carryforwards and certain other tax attributes may be limited.
The Company’s ability to utilize its federal net operating losses and federal tax credits may be limited under Sections 382 and 383 of the Internal Revenue Code. The limitations apply if an ownership change, as defined by Section 382, occurs. Generally, an ownership change occurs when certain stockholders increase their aggregated ownership by more than 50 percentage points over their lowest ownership percentage in a testing period (typically three years). The Company may already be subject to Section 382 limitations due to previous ownership changes. In addition, future changes in stock ownership may also trigger an ownership change and, consequently, a Section 382 limitation. Due to the significant complexity and cost associated with a change in control study, and the expectation of continuing to incur losses whereby the net operating losses and federal tax credits are not anticipated to be used in the foreseeable future, the Company has not assessed whether there have been changes in control since its formation. If GI Dynamics has experienced changes in control at any time since its formation, utilization of its net operating losses and research and development credit carryforwards would be subject to annual limitations under Sections 382 and 383, respectively. Any limitation may result in expiration of a portion of the net operating loss or research and development credit carryforwards before utilization, which would reduce the Company’s gross deferred tax assets and corresponding valuation allowance. As a result, if GI Dynamics earns net taxable income, its ability to use the pre-change net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to the Company.
27
Item 1B. UNRESOLVED STAFF COMMENTS
None.
On April 22, 2019, GI Dynamics entered into a lease for 3,520 square feet of office space in Boston, Massachusetts. The lease period commenced on May 1, 2019 and expires in May 2022.
GI Dynamics is currently not involved in any litigation that it believes could have a materially adverse effect on its financial condition or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of the executive officers of the Company or any of its subsidiaries, threatened against or affecting the Company, its common stock, any of its subsidiaries or of the Company’s or its subsidiaries’ officers or directors in their capacities as such, in which an adverse decision could have a material adverse effect. From time to time, the Company may become involved legal proceedings, lawsuits, claims and regulations in the ordinary course of its business.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable.
28
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
GI Dynamics CDIs, each representing one-fiftieth of one share of GI Dynamics common stock, were listed on the ASX under the trading symbol “GID” from September 7, 2011 to July 22, 2020. No public market for the Company’s securities existed prior to September 7, 2011 or after July 22, 2020.
On July 22, 2020, the last reported sale price of GI Dynamics CDIs was A$0.002 per CDI, or $0.07 per share of common stock.
As of December 31, 2020, 491,636 shares of common stock were subject to outstanding options and warrants to purchase shares of common stock.
Holders
At March 5, 2021, the Company had 88,095,659 shares of common stock issued and outstanding with 716 registered holders of record.
The Company’s transfer agent and registrar for its common stock is American Stock Transfer & Trust Company, LLC, 6201 15th Avenue, Brooklyn, New York 11219.
Equity Compensation Plan Information
The information required to be disclosed by Item 201(d) of Regulation S-K, “Securities Authorized for Issuance Under Equity Compensation Plans” is referenced under Item 12 of Part III of this Annual Report on Form 10-K.
ITEM 6. SELECTED CONSOLIDATED FINANCIAL DATA
Not applicable.
29
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the Company’s financial condition and results of operations should be read in conjunction with the Company’s Consolidated Financial Statements and related notes to those financial statements appearing elsewhere in this Annual Report on Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. You should review the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements described in the following discussion and analysis.
Overview
GI Dynamics is a clinical stage medical device company located in Boston, Massachusetts. The Company has developed EndoBarrier, a medical device intended to treat patients with type 2 diabetes and to reduce obesity. GI Dynamics is taking the steps necessary to obtain the regulatory approvals required to market this product. In order to market EndoBarrier in the U.S., GI Dynamics must obtain approval from the FDA. In order to market EndoBarrier outside of the U.S., GI Dynamics is required to comply with various regulations imposed by the countries in which it seeks to sell the product.
In 2010, EndoBarrier received CE Marking for sale in the European Union and in 2011, EndoBarrier was listed on the Australian Register of Therapeutic Goods. As a result, during 2013 and 2014, the Company received approximately $2.8 million and $2.3 million, respectively, in revenue from the sale of EndoBarrier in Europe, South America and the Asia Pacific region. In the U.S. in 2013, the Company began enrollment of patients in the initial pivotal trial of EndoBarrier, which is referred to as the ENDO trial.
In the third quarter of 2015, the Company announced its decision to discontinue the ENDO trial because patients were experiencing a higher than previously observed level of hepatic (liver) abscesses. In the fourth quarter of 2016, GI Dynamics received formal notification from the TGA of the Australian government of the cancellation of EndoBarrier’s inclusion on the ARTG. In the fourth quarter of 2017 the Company received formal notification of CE mark withdrawal from its notified body in Europe, preventing the sale of EndoBarrier in Europe and select Middle Eastern countries. The Company undertook comprehensive cost-cutting measures throughout 2015 and 2016, including significantly reducing the number of its employees.
Following the decision to discontinue the ENDO trial, GI Dynamics undertook significant investigational and scientific analyses with the goal of reducing the incidence rate and severity of hepatic abscess concurrent with the EndoBarrier treatment. This investigational work focused on understanding the root cause of hepatic abscess and how to reduce the rate of occurrence. This included: DNA analysis of normal EndoBarrier removals as well as hepatic abscess EndoBarrier removals, numerous meta-analyses and responder cohort analyses, investigation into the contributing factors represented by proton pump inhibitors (PPIs), leaky gut syndrome and microbiome analyses, among other research. This allowed the Company to modify the medications utilized with EndoBarrier, most notably discontinuation of chronic double-dose PPI usage during EndoBarrier implant.
As a result of the efforts described above, in August 2018, GI Dynamics received an IDE from the FDA to begin enrollment in a pivotal trial evaluating the safety and efficacy of EndoBarrier in the United States pending IRB approval, which was received on February 13, 2019. In this report, the Company refers to this pivotal trial as the GI Dynamics STEP-1 clinical trial. On January 27, 2020, the first patient was randomized into the STEP-1 Trial Protocol.
For financial reporting purposes, the Company have one reportable segment, which designs, manufactures and plans to market EndoBarrier.
To date, GI Dynamics has devoted substantially all of its efforts to research and development, business planning, clinical research, clinical study management, reimbursement development, product commercialization, acquiring operating assets and raising capital. GI Dynamics has incurred significant operating losses since its inception in 2003. As of December 31, 2020, the Company had an accumulated deficit of approximately $296 million. The Company expects to incur net losses for the next several years while it continues to evaluate which markets are appropriate to continue pursuing reimbursement, market awareness and general market development efforts, and continues to restructure its business and costs, establish new priorities, continue limited research, and evaluate strategic options.
30
GI Dynamics has raised net proceeds of approximately $283 million through sales of the Company’s equity and placement of debt, of which $9.5 million was received in 2020 that included the debt portion of the 2019 financing and the Initial Close of the September 2020 Financing.
In March 2019, the Company completed a convertible term promissory note (the “March 2019 Note”) and warrant (the “March 2019 Warrant”) financing with Crystal Amber for a gross amount of $1 million. The March 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in March 2024. Per the March 2019 NPA, the Company agreed to issue a warrant (the “March 2019 Warrant”) to Crystal Amber, pending Stockholder Approval, to purchase 78,984,823 CDIs (representing 1,579,696 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issuance of the March 2019 Warrant required the approval of stockholders and was not exercisable until it was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In March 2019, the maturity date of the 2017 Note was extended to May 1, 2019. In April 2019, the maturity date of the 2017 Note was extended to July 1, 2019. Further extensions of the maturity date of the 2017 Note are described below.
In May 2019, the Company completed a convertible term promissory note (the “May 2019 Note”) and warrant (the “May 2019 Warrant”) financing with Crystal Amber for a gross amount of $3 million. The May 2019 Note compounded interest annually at 10% and, subject to Stockholder Approval required under ASX Listing Rules, Crystal Amber was conferred the right to optionally convert all unpaid principal and interest to CDIs at $0.0127 per CDI before the Note Maturity date in May 2024. Per the May 2019 NPA, the Company agreed to issue a warrant (the “May 2019 Warrant”) to Crystal Amber, pending Stockholder Approval, to purchase 236,220,472 CDIs (representing 4,724,409 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issuance of the May 2019 Warrant required the approval of stockholders and was not exercisable until it was approved on June 30, 2019. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
In June 2019, the maturity date of the 2017 Note was extended to October 1, 2019.
On June 30, 2019, Crystal Amber elected to convert the 2018 Note, the March 2019 Note and the May 2019 Note into CDIs. Under the terms of the respective notes, an aggregate of 453,609,963 CDIs (representing approximately 9,072,197 shares of common stock) were subscribed but unissued on conversion and concurrent cancellation of the 2018 Note, the March 2019 Note and the May 2019 Note. The CDIs were issued on July 3, 2019.
On August 21, 2019, the Company and Crystal Amber entered into a securities purchase agreement for a total funding of up to approximately $10 million (the “August 2019 SPA”). The initial $5.4 million was comprised of existing warrant exercises scheduled between August 25, 2019 and November 15, 2019. Exercises included the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant issued to Crystal Amber, as further detailed above. The remaining amount of the August 2019 funding was represented by a Convertible Term Promissory Note (“August 2019 Note”) of up to approximately $4.6 million and a related Warrant (“August 2019 Warrant”) in substantially the same form as the March 2019 and May 2019 convertible term promissory note. Under the terms of the August 2019 SPA and August 2019 Note, the Company, at its sole discretion, could elect to request any of the $4.6 million to be funded at any date on or before December 6, 2019. The conversion feature allows the conversion of the August 2019 Note’s unpaid principal and interest at $0.02 per CDI and the August 2019 Warrant, upon its issue, allowed the purchase for $0.02 per CDI a number of CDIs represented by the August 2019 Note principal divided by $0.02 per CDI. (See Note 9 of the Consolidated Financial Statements for a more complete description of the terms and conditions of the financing).
On August 21, 2019, the maturity date of the 2017 Note was extended to March 31, 2020.
31
On August 25, 2019, Crystal Amber exercised the 2018 Warrant and a portion of the March 2019 Warrant in accordance with the terms of the August 2019 SPA. For an aggregate cash payment of $2 million, 97,222,200 CDIs (representing approximately 1,944,444 shares of common stock) were issued at $0.0144 per CDI under the 2018 Warrant and 47,244,119 CDIs (representing approximately 944,882 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant.
On September 30, 2019, Crystal Amber exercised the remaining portion of the March 2019 Warrant and a portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $2 million, 31,740,704 CDIs (representing approximately 634,814 shares of common stock) were issued at $0.0127 per CDI under the March 2019 Warrant and 125,739,610 CDIs (representing approximately 2,514,792 shares of common stock) were issued on October 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. As of September 30, 2019, this was recorded as a subscription receivable from a related party and the cash was received on October 1, 2019.
On October 31, 2019, Crystal Amber exercised another portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of $1 million, 78,740,157 CDIs (representing approximately 1,574,803 shares of common stock) were issued on November 4, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on October 31, 2019.
On November 15, 2019, Crystal Amber exercised the final portion of the May 2019 Warrant in accordance with the August 2019 SPA. For an aggregate cash payment of approximately $0.4 million, 31,740,748 CDIs (representing approximately 634,814 shares of common stock) were issued on November 18, 2019 at $0.0127 per CDI under the May 2019 Warrant. Cash was received on November 15, 2019.
On December 2, 2019, GI Dynamics provided notice to Crystal Amber that the Company elected to place the August 2019 Note at the full amount, on or before December 6, 2019. In December, the Company and Crystal Amber agreed to confer the right to tranche the funding of the August 2019 Note in amounts and per timing chosen solely by Crystal Amber, provided the August 2019 Note total was funded on or before January 15, 2020.
On December 16, 2019, GI Dynamics’ stockholders approved the August 2019 Note conversion feature and the issuance of the August 2019 Warrant, pending funding of the August 2019 Note by Crystal Amber.
On January 13, 2020, GI Dynamics received approximately $4.6 million in cash representing the full funding of the August 2019 Note. As stockholder approval had been obtained in December 2019, the August 2019 Note became convertible at Crystal Amber’s sole discretion until maturity in January 2025. Additionally, the August 2019 Warrant was issued to Crystal Amber, providing for the purchase of up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) at $0.02 per CDI.
On March 31, 2020, the maturity date of the 2017 Note was extended to May 1, 2020. On April 30, 2020, the maturity date of the 2017 Note was extended to May 15, 2020. On May 15, 2020, the maturity date of the 2017 Note was extended to June 15, 2020. On June 15, 2020, the maturity date of the 2017 Note was extended to June 29, 2020. On June 29, 2020, the maturity date of the 2017 Note was extended to July 31, 2020.
On May 11, 2020, the Company received approximately $200 thousand from a lender under the Paycheck Protection Program (“PPP”) Loan program of the 2020 CARES Act. The PPP loan accrues interest at 1% with payments deferred for six months and a maturity date two years from loan approval. The Company may apply for loan forgiveness subject to completion of an application and appropriate use of the loan proceeds as defined in the PPP loan structure. The Company must use a defined portion of the proceeds for payroll and the remainder for qualified facilities expenses during a defined measurement period. The Company has completed the measurement period and believes all conditions for forgiveness were met. The lender through which the Company received the PPP Loan has been resolving implications of ongoing legislative updates, the most recently being in December 2020, and as such, the Company has not been able to submit an application for forgiveness. The ability to submit forgiveness applications has been frozen as of February 16, 2021, but the Company will apply for forgiveness when the lender accepts forgiveness applications.
32
On June 18, 2020, the Company entered into a Note Purchase Agreement (“June 2020 NPA”) with Crystal Amber, pursuant to which, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $750 thousand (the “June 2020 Note”). The note and accrued interest mandatorily converted at the September 4, 2020 Initial Close of the September 2020 Financing into approximately 10.6 million shares of Series A Preferred Stock.
On July 13, 2020, Crystal Amber provided the Company with a notice of optional conversion of the 2017 Note, thereby converting the principal of $5 million and the accrued and unpaid interest of $390 thousand into 2,574,873,400 CDIs, which is equal to 51,497,468 shares of Common Stock. On receipt of the notice of conversion, the Company did not have sufficient authorized shares to allow full conversion. The available 38,401,704 shares were issued to CDN, allowing the allotment of 1,920,085,200 CDIs to Crystal Amber and a Right to Shares and Waiver Agreement in which the Company agreed to issue the remaining 13,095,764 shares of Common Stock owed under the conversion when the Company has filed an amended and restated certification of incorporation with the Delaware Secretary of State after the Company was delisted from the ASX and in connection with the consummation of the September 2020 Financing.
On July 22, 2020, GI Dynamics was removed from the Official List of the ASX and all CDIs were converted to shares of Common Stock. Although the Company’s securities are not traded on any exchange, the Company remains subject to SEC reporting requirements.
On August 4, 2020, the Company entered into a Note Purchase Agreement (“August 2020 NPA”) with Crystal Amber, pursuant to which, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $500 thousand (the “August 2020 Note”). The note and accrued interest mandatorily converted at the September 4, 2020, Initial Close of the September 2020 Financing into approximately 7.1 million shares of Series A Preferred Stock.
On September 3, 2020, Shareholders approved an increase in shares of authorized Common Stock to 280 million and authorized 118 million shares of a new Series A Preferred class of capital stock. The approval for the increase in the authorized shares of Common Stock allowed for the immediate issuance of the remaining 13,095,764 shares of Common Stock owed to Crystal Amber under the Right to Shares and Waiver Agreement.
On September 4, 2020, the Company executed a series of financing-related agreements (“the September 2020 Financing”) which included the cancellation of the August 2019 Warrant, the restructuring of the August 2019 Note into the September 2020 Note (see below for a detailed description of the September 2020 Note and the accounting classification of the August 2019 Note restructuring into the September 2020 Note), the conversion of the June and August 2020 Convertible Notes into shares of Series A Preferred Stock (see below for a detailed description of the June and August 2020 Notes and Note 10 to the Consolidated Financial Statements for a description of the Series A Preferred Stock), and the sale of $8.75 million of Series A Preferred Stock to Crystal Amber under the Series A Preferred Stock Purchase Agreement (the “Series A SPA”, see Note 11 to the Consolidated Financial Statements for a description of the Series A SPA terms). The Initial Close of the September 2020 Financing occurred on September 4, 2020, and included all components of the September 2020 Financing, including the Crystal Amber purchase of 42,310,730 shares of Series A Preferred Stock for gross proceeds of $3.75. Pursuant to the Series A SPA and subsequent amendments, the Second Close was originally to occur on October 31, 2020 but through a series of extensions, was extended until February 24, 2021. On February 24, 2021, an individual investor closed the purchase of 600,000 shares of Series A Preferred Stock for approximate proceeds of $53 thousand. Effective February 24, 2021, Crystal Amber executed a contract amendment restructuring the Second Close to include the sale of 16,924,292 shares of Series A Preferred Stock for proceeds of $1.5 million to close on March 4, 2021, the sale of 11,282,861 shares of Series A Preferred Stock for proceeds of $1.0 million to close no later than March 25, 2021, and the remaining 27,607,153 shares of Series A Preferred Stock for proceeds of $2.45 million to close no later than May 28, 2021.
The Company’s costs include employee salaries and benefits, compensation paid to consultants, materials and supplies for research, costs associated with development activities including materials, sub-contractors, travel and administration, legal expenses, sales and marketing costs, general and administrative expenses, and other costs associated with a clinical and emerging commercial stage medical technology company. At March 11, 2021, GI Dynamics has 12 full-time employees. The number of employees required to support the Company’s activities as it moves the EndoBarrier through the GI Dynamics STEP-1 and the I-step clinical trials, as well as in the areas of research and development, sales and marketing, and general and administrative functions, may increase. GI Dynamics expects to continue to incur consulting expenses related to technology development that will increase as it enters into the recruitment phase of the STEP-1 and I -Step clinical trials, and the Company expects to continue to incur expenses to protect its intellectual property.
33
The amount that GI Dynamics spends for any specific purpose may vary significantly from quarter to quarter or year to year, and could depend on a number of factors including, but not limited to, the pace of progress of the GI Dynamics STEP-1 and I-Step clinical trials, commercialization and development efforts and actual needs with respect to development and research.
Research, development, and commercial acceptance of new medical technologies are, by their nature, unpredictable. Although the Company will undertake development and commercialization efforts with reasonable diligence, there can be no assurance that the net proceeds from the Company’s securities offerings will be sufficient to enable the Company to develop the Company’s technology to the extent needed to create future sales to sustain operations. If the net proceeds from these offerings are insufficient for this purpose, GI Dynamics will consider other options to continue its path to commercialization, including, but not limited to, additional financing through follow-on equity offerings, debt financing, co-development agreements, sale or licensing of developed intellectual or other property, or other alternatives.
GI Dynamics cannot assure that its technology will be accepted, that it will ever earn revenues sufficient to support its operations, or that it will ever be profitable. Furthermore, the Company has no committed source of financing and cannot assure that it will be able to raise money as and when it needs it to continue operations. If the Company cannot raise funds as and when it needs them, it may be required to scale back development plans by reducing expenditures for employees, consultants, business development and marketing efforts or to otherwise severely curtail, or even to cease, business operations.
The Company’s corporate headquarters are located in Boston, Massachusetts. The Company leases 3,520 square feet of office space with a lease expiration in May 2022. This space is adequate for current operations.
Critical Accounting Policies and Estimates
The Company’s discussion and analysis of its financial condition and results of operations is based upon the Company’s Consolidated Financial Statements prepared in accordance with generally accepted accounting principles in the United States. GI Dynamics believes that its application of the following accounting policies, each of which require significant judgments and estimates on the part of management, are the most critical to aid in fully understanding and evaluating the Company’s reported financial results. The Company’s significant accounting policies are more fully described in Note 2, “Summary of Significant Accounting Policies and Basis of Presentation”, to the Company’s Consolidated Financial Statements appearing elsewhere in this Annual Report on Form 10-K.
Use of Estimates
The preparation of Consolidated Financial Statements in accordance with generally accepted accounting principles in the U.S. requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. On an ongoing basis, the Company’s management evaluates its estimates, including those related to impairment of long-lived assets, income taxes including the valuation allowance for deferred tax assets, research and development, contingencies, valuation of any derivative liabilities, estimates used to assess its ability to continue as a going concern and stock-based compensation. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known.
34
Going Concern Evaluation and Presentation
GI Dynamics analyzes its ability to continue as a going concern in each reporting period, assessing the likelihood that the company will be able to fund operations for the twelve months after the filing date of the financial statements. For further information regarding the Company’s funding requirements, see Note 1, “Nature of Business – Going Concern Evaluation”, to the Company’s consolidated financial statements. The Company has concluded and disclosed that there is substantial doubt in its ability to continue a going concern for a twelve-month period after the filing of this Annual Report on Form 10-K.
While there remains substantial doubt of the Company continuing as a going concern, the Company is seeking alternative financing sources and evaluating contingency plans that, while not guaranteed to yield the ability to fund operations at current levels, provide a reasonable probability that the Company will be able to maintain at least a subset of business operations. Therefore, the accompanying Consolidated Financial Statements have been prepared assuming GI Dynamics will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The Consolidated Financial Statements for the year ended December 31, 2020 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern within one year after the date that the Consolidated Financial Statements are issued.
Fair Value of Derivative Instruments and Debt Instruments
GI Dynamics examines all financial instruments to determine if the financial instrument or any component feature that is a derivative requires liability treatment, predominantly due to a cash settlement feature. Such derivative liabilities are initially recorded at fair value with subsequent changes in value recorded in other income (expense) in the statements of operations. If the derivative instruments subsequently meet the requirements for classification as equity, the Company reclassifies the then fair value of the instrument to equity. The Company values free-standing warrants and any other derivative liabilities identified using the Black-Scholes option pricing model. If multiple outcomes are probable, management assigns probability adjustments to determine the most likely probability adjusted fair value.
GI Dynamics examines all debt to determine if any component feature of the debt requires fair value measurement or requires fair value measurement to allocate debt proceeds to the various components and features. When assumptions are reasonably known, the net present value of the cash flows of the debt are used to estimate the fair value of the note component, while a Black-Scholes option pricing model is used to value warrants and beneficial conversion features are measured at the intrinsic value of the effective conversion price.
Research and Development Costs
Research and development costs are expensed when incurred. Research and development costs include costs of all basic research activities as well as other research, engineering, and technical effort required to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include preapproval regulatory and clinical trial expenses.
Stock-Based Compensation
GI Dynamics accounts for stock-based compensation in accordance with the Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification (“ASC”) 718, Stock Compensation (“ASC 718”), which requires that stock-based compensation be measured at the grant date fair value and recognized as an expense in the consolidated financial statements.
For awards that vest based on service conditions, the Company uses the straight-line method to allocate compensation expense to reporting periods. The grant date fair value of options granted is calculated using the Black-Scholes option pricing model, which requires the use of subjective assumptions including volatility, expected term and the fair value of the underlying common stock, among others.
35
The assumptions used in determining the fair value of stock-based awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change, and the Company uses different assumptions, its stock-based compensation could be materially different in the future. The risk-free interest rate used for each grant is based on a zero-coupon U.S. Treasury instrument with a remaining term similar to the expected term of the stock-based award. Because the Company does not have a sufficient stable history to estimate the expected term, the simplified method for estimating the expected term is used. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. The Company uses the Company’s stock equivalents or that of comparable companies to estimate historical price volatility. GI Dynamics has not paid and does not anticipate paying cash dividends on shares of common stock; therefore, the expected dividend yield is assumed to be zero. The Company accounts for forfeitures as they occur, rather than applying an estimated forfeiture rate to the value of the grant on the grant date.
GI Dynamics periodically issues performance-based awards. For these awards, vesting will occur upon the achievement of certain milestones. When achievement of the milestone is deemed probable, the Company expenses the compensation of the respective stock award over the implicit service period.
Stock awards to non-employees are accounted for in accordance with ASC 718, as the Company adopted FASB Accounting Standards Update (“ASU”) No. 2018-07, Compensation – Stock Compensation (Topic 718: Improvements to nonemployee share-based payment accounting) (“ASU 2018-07”) on January 1, 2019. This ASU provides measurement provisions and clarifications for the accounting for Non-employee Share-Based Payments (“NESBP”). Most of the guidance within ASC 718 associated with employee share-based payments, including most of its requirements related to classification and measurement, now applies to NESBP. The Company elects to use the contractual term of each award as the expected term for NESBP awards. At December 31, 2020, the Company had options to purchase 15,000 shares of common stock granted to non-employees.
Impairment of Long-Lived Assets
GI Dynamics regularly reviews the carrying amount of its long-lived assets to determine whether indicators of impairment may exist that warrant adjustments to carrying values or estimated useful lives. If indications of impairment exist, projected future undiscounted cash flows associated with the asset are compared to the carrying amount to determine whether the asset’s value is recoverable. If the carrying value of the asset exceeds such projected undiscounted cash flows, the asset will be written down to its estimated fair value.
Smaller Reporting Company Status
The Securities and Exchange Commission amended regulation S-K, section 210 in September 2018 to reduce the scale of Smaller Reporting Companies. Smaller Reporting Companies are defined based on public capital and revenue limits and GI Dynamics qualifies as a Smaller Reporting Company for the year ended December 31, 2020. As a result, the Company’s reporting is compliant with the Smaller Reporting Company requirements.
36
Results of Operations
The following is a description of significant components of the Company’s operations, including significant trends and uncertainties that are believed to be important to an understanding of the Company’s business and results of operations.
Year Ended December 31, 2020 Compared to Year Ended December 31, 2019
GI Dynamics did not record any revenue in the years ended December 31, 2020 or 2019.
Operating Expenses
| Years Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2020 | 2019 | $ | % | |||||||||||||
| (dollars in thousands) | ||||||||||||||||
| Research and development expense | $ | 3,484 | $ | 4,225 | $ | (741 | ) | (17.5 | )% | |||||||
| Sales and marketing expense | - | 22 | (22 | ) | (100 | )% | ||||||||||
| General and administrative expense | 5,621 | 5,295 | 326 | 6.2 | % | |||||||||||
| Total operating expenses | $ | 9,105 | $ | 9,542 | $ | (437 | ) | (4.6 | )% | |||||||
Research and Development Expense. The decrease in research and development expense of approximately $741 thousand for the year ended December 31, 2020 compared to the year ended December 31, 2019 was primarily due to a decrease in spending on internal and external resources as the COVID-19 pandemic caused clinical pauses on trial enrollment and slowed international regulatory reviews to start the I-Step Trial and perform CE mark certification audits. In 2019 the Company incurred expenses to prepared for and initiate the STEP-1 clinical trial in the United States, the I-STEP trial in India (under joint venture with Apollo Sugar) and prepare for audits related to filing for its CE Mark designation.
Sales and Marketing Expense. The decrease in sales and marketing expense of approximately $22 thousand for the year ended December 31, 2020 compared to the year ended December 31, 2019 was primarily due to limited spending on sales and marketing given COVID-19 pandemic delays and the focus of resources on a marketing project that remains a prepaid expense until fully implemented.
General and Administrative Expense. The increase in general and administrative expense of approximately $326 thousand for the year ended December 31, 2020 compared to the year ended December 31, 2019 was primarily related to increased compensation expenses offset by reductions in travel-related expenses due to COVID-19, board of directors related expenses and costs associates with public listing on the ASX.
GI Dynamics continues to look for ways to streamline the Company’s expenses, especially in General and Administrative departments.
37
Other Income (Expense), Net
| Years Ended | ||||||||||||||||
| December 31, | Change | |||||||||||||||
| 2020 | 2019 | $ | % | |||||||||||||
| (dollars in thousands) | ||||||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | $ | - | $ | 3 | $ | (3 | ) | N/A | ||||||||
| Interest expense | (1,547 | ) | (6,178 | ) | (4,631 | ) | (75 | )% | ||||||||
| Foreign exchange gain (loss) | (18 | ) | 11 | (29 | ) | (254 | )% | |||||||||
| Loss on extinguishment of debt | (678 | ) | - | (678 | ) | N/A | ||||||||||
| Re-measurement of derivative liabilities | - | (1,683 | ) | 1,683 | N/A | |||||||||||
| Gain on write-off of accounts payable | - | 101 | (101 | ) | N/A | |||||||||||
| Other income | 236 | - | 236 | N/A | ||||||||||||
| Total other expense, net | $ | (2,007 | ) | $ | (7,746 | ) | $ | 5,739 | (36 | )% | ||||||
Interest income. The decrease in interest income of approximately $3 thousand for the year ended December 31, 2020 compared to the year ended December 31, 2019 was due to lower average cash balances in 2020.
Interest expense. Interest expense decreased by approximately $4.6 million as the debt discount amortization in the year ended December 31, 2020 was less than the aggregate debt discount amortization in the year ended December 31, 2019 and non-cash interest expenses related to the June 2019 conversion of the 2018, March 2019 and May 2019 Notes payable to Crystal Amber.
Foreign exchange gain (loss). The $29 thousand difference in the foreign exchange gain (loss) reflects foreign exchange rate fluctuations on immaterially changed balances.
Loss on extinguishment of debt. The $678 thousand loss related to the restructuring of the August 2019 Note and cancellation of the August 2019 Warrant into the September 2020 Note which occurred in the year ended December 31, 2020 with no similar transaction in the year ended December 31, 2019.
Re-measurement of derivative liabilities. There were no re-measurements of derivative liabilities in 2020, while re-measurement of derivative liabilities totaled approximately $1.7 million for the year ended December 31, 2019, which was primarily due to the revaluation of the March and May 2019 warrants prior to stockholder approval. During the intervening period between Note issuance and derivative remeasurement, the market price per CDI increased significantly which resulted in a higher value driven by the widened spread between the exercise price and the market price and less so in the increased volatility resulting from the change in the price of the CDIs.
Gain on write-off of accounts payable. The change in the gain on write off of accounts payable reflects approximately $101 thousand of settled accounts payable in 2019 versus none in 2020.
Other expenses (income). The $236 thousand of Other Income recorded in the year ended December 31, 2020 is predominantly the $165 reversal of reserves for product returns given the expiration of all product previously eligible for return, $42 thousand in foreign COVID-19 related payroll assistance programs, and $10 thousand redemption of credit card cash back rewards. There were no similar transactions in the year ended December 31, 2019.
38
Liquidity and Capital Resources
As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. GI Dynamics is currently focused on obtaining an EndoBarrier CE mark and enrolling the Company’s clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2020 and had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
The Company will need to arrange additional financing before August 1, 2021 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources, however, the Company has no guaranteed source of capital that will sustain operations past August 1, 2021. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these Consolidated Financial Statements are issued.
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s Consolidated Financial Statements include a going-concern disclosure.
During the year ended December 31, 2020, the Company’s cash and restricted cash balance decreased by approximately $1.3 million primarily due to cash payments related to, among other things, research and development and general and administrative expenses as GI Dynamics continued to focus on clinical and regulatory strategies that exceeded its various financings.
The following table sets forth the major sources and uses of cash for each of the periods set forth below:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| (in thousands) | ||||||||
| Net cash (used in) provided by: | ||||||||
| Operating activities | $ | (10,846 | ) | $ | (10,612 | ) | ||
| Investing activities | - | (11 | ) | |||||
| Financing activities | 9,506 | 9,316 | ||||||
| Net decrease in cash and restricted cash | $ | (1,340 | ) | $ | (1,307 | ) | ||
39
Cash Flows used in Operating Activities
Net cash used in operating activities totaled approximately $10.8 million for the year ended December 31, 2020. The primary uses of cash were:
| ● | to fund the Company’s net loss of approximately $11.1 million; |
| ● | a net negative adjustment to cash flow from changes in working capital of approximately $2.6 million resulting primarily from increases in prepaid expenses related to a directors and officers liability insurance run-off policy offset by a reduction of the prepaid balance on other liability insurance policies, the funding of a severance escrow account, and material decreases in accounts payable and accrued expenses ; and |
| ● | a net positive adjustment to cash flow from non-cash items of approximately $2.8 million, primarily from non-cash interest expense, loss on extinguishment of debt, and stock-based compensation. |
Net cash used in operating activities totaled approximately $10.6 million for the year ended December 31, 2019. The primary uses of cash were:
| ● | to fund the Company’s net loss of approximately $17.3 million; |
| ● | a net negative adjustment to cash flow from changes in working capital of approximately $1.6 million resulting primarily from increases in prepaid expenses and decreases in accounts payable and accrued expenses; and |
| ● | a net positive adjustment to cash flow from non-cash items of approximately $8.3 million, primarily from non-cash interest expense, change in derivative valuation and stock-based compensation. |
Cash Flows used in Investing Activities
No cash was used in investing activities for the year ended December 31, 2020.
Cash used in investing activities for the year ended December 31, 2019 totaled approximately $11 thousand from the purchase of office equipment.
Cash Flows provided by Financing Activities
Cash provided by financing activities for the year ended December 31, 2020 totaled approximately $9.5 million and primarily resulted from the proceeds from short and long-term debt from a related party and the net proceeds from the sale of Series A Preferred Stock.
Cash provided by financing activities for the year ended December 31, 2019 totaled approximately $9.3 million and primarily resulted from the exercise of related party warrants and proceeds from short and long-term debt from a related party.
Funding Requirements
As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. GI Dynamics is currently focused on obtaining an EndoBarrier CE mark and enrolling the Company’s clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market at this time. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2020 and had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
40
Due to the numerous risks and uncertainties associated with securing regulatory approval for EndoBarrier, at this time GI Dynamics is unable to estimate precisely the amounts of capital outlays and operating expenditures necessary to complete the task of obtaining regulatory approval for EndoBarrier. Funding requirements will depend on many factors, including, but not limited to, the following:
| ● | the timing, cost, and resulting success of research and development efforts, including the impact of COVID-19 related restrictions and complications in conducting clinical trials and regulatory reviews; | |
| ● | the rate of progress and cost of commercialization activities after regulatory approval; | |
| ● | the expenses the Company may incur in marketing and selling EndoBarrier subject to future regulatory approvals; | |
| ● | the timing and decisions of payer organizations related to reimbursement; |
| ● | the revenue generated by sales of EndoBarrier; | |
| ● | the safety and efficacy of the Company’s product in treating diabetes and reducing obesity; | |
| ● | the success of the Company’s investment in its manufacturing and supply chain infrastructure; | |
| ● | the time and costs involved in obtaining regulatory approvals for EndoBarrier in new markets; | |
| ● | the costs associated with any additional clinical trial(s) required in the U.S. and other countries on a case by case basis; | |
| ● | the ability to ship CE marked products; | |
| ● | the emergence of competing or complementary developments; and | |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights. |
GI Dynamics will continue to manage its capital structure and to consider all financing opportunities, whenever they may occur, that could strengthen its long-term liquidity profile. Any such capital transactions may or may not be similar to transactions in which the Company has engaged in the past and the ownership interests of existing stockholders may be materially diluted.
Off–Balance Sheet Arrangements
GI Dynamics does not have any relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities, that would have been established for the purpose of facilitating off-balance sheet arrangements (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) or other contractually narrow or limited purposes. As such, the Company is not exposed to any financing, liquidity, market or credit risk that could arise if it had engaged in those types of relationships. GI Dynamics enters into guarantees in the ordinary course of business related to the guarantee of its own performance and the performance of its subsidiaries.
Contractual Obligations and Commitments
The Company’s commitments for operating leases relate to the lease of office space in Boston, Massachusetts which expires in May 2022.
Recent Accounting Pronouncements
For a discussion of recent accounting pronouncements please refer to Note 2, “Summary of Significant Accounting Policies and Basis of Presentation”, to the Company’s Consolidated Financial Statements included in this Annual Report on Form 10-K.
41
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
GI Dynamics is focused on developing clinical data and regulatory approvals for EndoBarrier and the Company earnings and cash flows are exposed to market risk from changes in currency exchange rates and interest rates.
Interest Rate Sensitivity
The Company’s cash and restricted cash balance of $1.2 million at December 31, 2020 consisted of cash and restricted cash, all of which will be used for working capital purposes. Because of the short-term nature of the Company’s cash and restricted cash, GI Dynamics does not believe that it has any material exposure to changes in their fair values as a result of changes in interest rates.
Foreign Currency Risk
GI Dynamics conducts business in foreign countries. For U.S. reporting purposes, all assets and liabilities of non-U.S. entities are remeasured at the period-end exchange rate and income and expenses at the average exchange rates in effect during the periods. The net effect of these remeasurement adjustments is shown in the accompanying Consolidated Financial Statements as a component of net loss. Continued fluctuation of these exchange rates could result in financial results that are not comparable from quarter to quarter. The Company does not currently utilize foreign currency contracts to mitigate the gains and losses generated by the remeasurement of non-functional currency assets and liabilities and does not currently hold material cash reserves in foreign currencies in which certain expenditures are denominated.
Effects of Inflation
GI Dynamics does not believe that inflation and changing prices over the years ended December 31, 2020 and 2019 had a significant impact on its results of operations.
ITEM 8. CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Company’s consolidated financial statements, together with the independent registered public accounting firm report thereon, appear on pages F-1 through F-33 of this Annual Report on Form 10-K.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
In accordance with Exchange Act Rules 13a-15 and 15d-15(f), GI Dynamics carried out an evaluation, under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer (the “Certifying Officers”), of the effectiveness of the Company’s disclosure controls and procedures as of the end of the period covered by this report. Based on that evaluation, the Company’s Certifying Officers concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2020 in enabling GI Dynamics to record, process, summarize and report information required to be included in its periodic SEC filings within the required time period.
Management’s Annual Report on Internal Control over Financial Reporting
As a company registered with the SEC in the U.S., GI Dynamics is required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by management on, among other things, the effectiveness of its internal control over financial reporting as of the end of the fiscal year. The controls and other procedures are designed to ensure that information required to be disclosed by the Company in the reports that the Company files with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms. The Company’s first report on compliance with Section 404 was furnished in connection with the GI Dynamics financial statements for the year ended December 31, 2014. As a former emerging growth company and a current smaller reporting company, the Company has been and currently is exempted from the requirement to include an external auditor opinion on the Company’s internal controls in its filings. GI Dynamics continues to update, document and evaluate its internal control over financial reporting to the requirements of Section 404.
42
The Company’s Certifying Officers are responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act.
Internal control over financial reporting is a process designed by, or under the supervision of, the Certifying Officers and effected by the Company’s Board of Directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the Company’s assets; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that the Company’s receipts and expenditures are being made only in accordance with authorizations of the Company’s management and directors; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition or disposition of the Company’s assets that could have a material effect on the financial statements. |
Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives because of its inherent limitations. It is a process that involves human diligence and compliance and is subject to lapses in judgment or breakdowns resulting from human failures. Internal control over financial reporting also can be circumvented by collusion or improper management override. While process safeguards can reduce risks, because of inherent limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company, under the supervision and with the participation of the Certifying Officers, has evaluated the effectiveness of the Company’s internal control over financial reporting as of December 31, 2020 based upon the framework in Internal Control Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on such evaluations, the Certifying Officers have concluded that the Company’s internal control over financial reporting was effective as of December 31, 2020.
Changes in Internal Control Over Financial Reporting
As required by Rule 13a-15(d) of the Exchange Act, the Certifying Officers conducted an evaluation of the internal control over financial reporting to determine whether any changes occurred during the fourth fiscal quarter that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting. The evaluation performed by the Certifying Officers resulted in a conclusion that such changes occurred. In the third quarter, a material weakness was identified related to reconciliation of accrued interest which has been address by changing the mandatory review and approval processes related to period closure and journal entries, including those related to accrued interest. Also in the third quarter, a material weakness was identified related to stock based compensation charges relating to an inaccurate interpretation of a contractual vesting clause which has been addressed by implementing a formal legal review of contracts whose terms may impact financial reporting, including stock award vesting and cancellation clauses.
None.
43
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Certain information required by this Item 10 relating to the Company’s directors and executive officers is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2021 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2020.
Code of Business Conduct and Ethics
GI Dynamics has adopted a code of business conduct and ethics applicable to directors, executive officers and all other employees. A copy of that code is available on the Company’s corporate website at http://www.gidynamics.com. Any amendments to the code of business conduct and ethics, and any waivers thereto involving the Company executive officers, also will be available on the corporate website. A printed copy of these documents will be made available upon request. The content on the Company website is not incorporated by reference into this Annual Report on Form 10-K.
Directors of the Registrant
The following table sets forth the name, age and position of each of the Company’s directors at March 11, 2021:
| Name | Age | Position | ||
| Mark Lerdal | 62 | Non-executive Chairman of the Board | ||
| Ginger Glaser | 53 | Non-executive Director | ||
| Joseph Virgilio | 47 | Non-executive Director |
All three directors are members of the audit committee, the compensation committee, and the nominating and corporate governance committee. At March 11, 2021, none of the committees have elected a specific chairperson.
Mark Lerdal has served as the Chairman of the Board of Directors of GI Dynamics since August 2020. Mr. Lerdal’s extensive knowledge and experience as a board director in financial and business development, as well as a proven track record leading multiple mergers and acquisitions, makes him qualified to serve on the Company’s board of directors.
Mr. Lerdal founded KC Holdings, Inc., a company formed to take KENETECH Corporation private through a management buyout. Mr. Lerdal previously held the position as President and CEO of KENETECH Corporation, one of the world’s largest developer, constructor and operator of wind energy plants.
Mr. Lerdal currently serves as Chairman of the Board for Leaf Clean Energy, Element Markets, LLC and Empower Energies, Inc., and sits on the Board of Directors for Southern Current, Allied Minds, PLC (LSE:ALM), and acts as a consultant to Northleaf Capital Partners. Mr. Lerdal previously served on the Board of Directors to Onsite Energy Corporation, Medley Capital Corporation, Trading Emissions, PLC, TerraForm Power, Inc. and TerraForm Global, Inc.
Mr. Lerdal graduated Cum Laude from Northwestern University School of Law and earned his Bachelor of Arts in Economics from Stanford University.
44
Ginger Glaser has been a director of GI Dynamics since October 2020. Ms. Glaser’s 25 years of quality and regulatory experience, proven leadership of successful medical device organizations, as well as her breadth of knowledge in clinical affairs and engineering makes her qualified to serve on the Company’s board of directors.
Ms. Glaser currently is the Principal for G2 Consulting Services and the Chief Technology Officer for Monteris Medical, where she previously held vice president positions. Ms. Glaser held vice president roles with Boston Scientific and American Medical Systems, prior to its acquisition by Boston Scientific.
Ms. Glaser has a strong understanding of FDA processes and culture. She has led industry-wide task forces and helped numerous organizations obtain global regulatory approvals, including “first in the world” achievements.
Ms. Glaser graduated Cum Laude from Texas A&M University, where she earned her B.S. in Bioengineering and M.S. in Biomedical Engineering.
Joseph Virgilio has been a director and the Company’s chief executive officer since November 2020. Mr. Virgilio’s more than 20 years of sales, marketing, and general management experience in the medical device industry makes him qualified to serve on the Company’s board of directors. Mr. Virgilio possesses a proven track record of aggressive revenue growth through the development and execution of strategic sales and marketing plans with demonstrated abilities to recruit, train, and motivate a highly effective and specialized cross-functional team. His areas of expertise also include GPO/IDN contracting, organizational development and restructuring, corporate partnerships, and distribution strategies. Additionally, Mr. Virgilio possesses broad experience leading organizational turn-around and integration activities.
Prior to joining GI Dynamics, Mr. Virgilio served as President and General Manager for Amann Girrbach, a global leader in dental CAD/CAM technology Mr. Virgilio has also held the position of Vice President of Sales, The Americas at Surgical Specialties Corporation, as well as Vice President of Sales and Global Marketing at Aptus Endosystems (acquired by Medtronic). Mr. Virgilio spent the early part of his medical device career at Boston Scientific followed by almost a decade at Medtronic in roles of increasing responsibility.
Mr. Virgilio holds a Bachelor of Arts degree from Colgate University and earned continued education credits from the University of Pennsylvania, Wharton School of Business and from the University of North Carolina, Chapel Hill, Kenan-Flagler School of Business.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2021 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2020.
45
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information relating to security ownership of certain beneficial owners of the Company’s common stock and information relating to the security ownership of management required by this Item 12 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2021 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2020.
The table below sets forth information with regard to shares authorized for issuance under the Company’s equity compensation plans as of December 31, 2020. As of December 31, 2020, GI Dynamics had three active equity compensation plans, each of which was approved by GI Dynamics stockholders:
| ● | The GI Dynamics 2003 Omnibus Stock Plan; and |
| ● |
The GI Dynamics 2011 Employee, Director and Consultant Equity Incentive Plan; and |
| ● | The GI Dynamics 2020 Employee, Director and Consultant Equity Incentive Plan. |
| Plan Category | Number of shares to be issued upon exercise of outstanding options or vesting of restricted stock units |
Weighted- average exercise price of outstanding options |
Number of shares remaining available for future issuance under equity compensation plans |
|||||||||
| Equity compensation plans approved by security holders | 463,104 | $ | 2.04 | 41,710,968 | ||||||||
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2021 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2020.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item 13 is incorporated by reference herein to the Company’s Proxy Statement to be filed with the SEC in connection with its 2021 Annual Meeting of Stockholders or an amendment to this report filed under cover of Form 10-K/A not later than 120 days after the end of the fiscal year ended December 31, 2020.
46
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a) | List of documents filed as part of this Annual Report on Form 10-K |
| (1) | Consolidated Financial Statements listed under Part II, Item 8 and included herein by reference. |
| (2) | Consolidated Financial Statement Schedules |
No schedules are submitted because they are not applicable, not required or because the information is included in the Consolidated Financial Statements or Notes to Consolidated Financial Statements.
| (3) | Exhibits |
The exhibits listed in the accompanying Exhibit Index are filed or incorporated by reference as part of this Annual Report on Form 10-K.
Not applicable.
47
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, hereunto duly authorized.
| GI Dynamics, Inc. | ||
| Date: March 11, 2021 | By: | /s/ Joseph Virgilio |
| Name: | Joseph Virgilio | |
| Title: | President, Chief Executive Officer, and Director | |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ JOSEPH VIRGILIO | President, Chief Executive Officer | March 11, 2021 | ||
| Joseph Virgilio | (Principal Executive Officer) and Director | |||
| /s/ CHARLES R. CARTER | Chief Financial Officer, Treasurer, Secretary | March 11, 2021 | ||
| Charles R. Carter | (Principal Financial and Accounting Officer) | |||
| /s/ MARK LERDAL | Chairman and Director | March 11, 2021 | ||
| Mark Lerdal | ||||
| /s/ GINGER GLASER | Director | March 11, 2021 | ||
| Ginger Glaser | ||||
48
EXHIBIT INDEX
49
50
| * | Filed herewith. |
| ‡ | Furnished herewith. |
| † | Management contract or compensatory plan or arrangement. |
51
GI Dynamics, Inc. and Subsidiaries
Index to Consolidated Financial Statements
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
GI Dynamics, Inc.
Boston, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of GI Dynamics, Inc. and Subsidiaries (the Company) as of December 31, 2020 and 2019, the related consolidated statements of operations, stockholders’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America (U.S. GAAP).
Emphasis of Matter Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered losses from operations since inception and has an accumulated deficit that raises substantial doubt about the Company’s ability to continue as a going concern. Management's evaluation of the events and conditions and management's plans regarding these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
F-2
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Description of the Matter
Elements of the Company’s financing activities represent critical audit matters due to management judgments in the application of accounting guidance under U.S. GAAP and assumptions applied in the valuation of instruments issued. The valuation methodologies and the assumptions applied impact the fair values of the convertible promissory notes, related conversion features, warrants for the purchase of common stock and the fair values determined in the extinguishment of convertible promissory notes and warrants.
How We Addressed the Matter in Our Audit
Our audit procedures related to the Company’s application of accounting guidance and valuation estimates included the following, among others:
| ● | We reviewed the Company’s technical accounting memorandums for each financing transaction, which describe the terms of financing and management’s evaluation and conclusion on the accounting treatment. Our review also incorporated a review of executed agreements and cited accounting guidance. |
| ● | We tested management’s valuation of the convertible promissory notes, warrants for the purchase of common stock and beneficial conversion features, as applicable. We tested the accuracy and reasonableness of inputs and assumptions applied to agreements and other relevant evidence. Assumptions were also reviewed for management bias. We also tested the mathematical accuracy of management’s calculations. |
| ● | For the exchange of convertible promissory notes, we tested management’s evaluation of the significance of the changes including the present value of expected future cash flows under the newly issued convertible promissory note upon exchange. We also tested the mathematical accuracy of management’s calculations. |
/s/ Wolf and Company, P.C.
We have served as the Company's auditor since 2019.
Boston, Massachusetts
March 11, 2021
F-3
GI Dynamics, Inc. and Subsidiaries
Consolidated Balance Sheets
(In thousands, except share and per share amounts)
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 1,159 | $ | 2,499 | ||||
| Restricted cash | 30 | 30 | ||||||
| Prepaid expenses and other current assets | 3,029 | 1,230 | ||||||
| Total current assets | 4,218 | 3,759 | ||||||
| Property and equipment, net | 14 | 42 | ||||||
| Right-of-use operating lease, net of amortization | 244 | 386 | ||||||
| Total assets | $ | 4,476 | $ | 4,187 | ||||
| Liabilities and stockholders’ deficit | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 430 | $ | 636 | ||||
| Accrued expenses | 583 | 1,353 | ||||||
| Short term debt | 263 | — | ||||||
| Short term debt to related party, net of discount | — | 5,000 | ||||||
| Derivative liabilities | — | 10 | ||||||
| Operating lease liability | 172 | 169 | ||||||
| Total current liabilities | 1,448 | 7,168 | ||||||
| Long term debt to related party, net of discount | 3,496 | — | ||||||
| Operating lease liability, non-current | 67 | 217 | ||||||
| Total liabilities | 5,011 | 7,385 | ||||||
| Commitments (Note 10) | ||||||||
| Stockholders’ deficit: | ||||||||
| Preferred stock, $0.01 par value – 118,000,000 and 0 shares authorized at December 31, 2020 and 2019, respectively; 60,085,583 and 0 shares issued and outstanding at December 31, 2020 and 2019, respectively | 601 | - | ||||||
| Common stock, $0.01 par value – 280,000,000 and 75,000,000 shares authorized at December 31, 2020 and 2019, respectively; 88,095,659 and 36,598,291 shares issued and outstanding at December 31, 2020 and 2019, respectively | 881 | 366 | ||||||
| Additional paid-in capital | 293,611 | 280,928 | ||||||
| Accumulated deficit | (295,628 | ) | (284,492 | ) | ||||
| Total stockholders’ deficit | (535 | ) | (3,198 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 4,476 | $ | 4,187 | ||||
See report of independent registered public accounting firm and the accompanying notes to these consolidated financial statements.
F-4
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Operations
(In thousands, except share and per share amounts)
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 3,484 | $ | 4,225 | ||||
| Sales and marketing | — | 22 | ||||||
| General and administrative | 5,621 | 5,295 | ||||||
| Total operating expenses | 9,105 | 9,542 | ||||||
| Loss from operations | (9,105 | ) | (9,542 | ) | ||||
| Other income (expense): | ||||||||
| Interest income | — | 3 | ||||||
| Interest expense | (1,547 | ) | (6,178 | ) | ||||
| Foreign exchange gain (loss) | (18 | ) | 11 | |||||
| Loss on extinguishment of debt | (678 | ) | — | |||||
| Re-measurement of derivative liabilities | — | (1,683 | ) | |||||
| Gain on write-off of accounts payable | — | 101 | ||||||
| Other income | 236 | - | ||||||
| Other expense, net | (2,007 | ) | (7,746 | ) | ||||
| Loss before income tax expense | (11,112 | ) | (17,288 | ) | ||||
| Income tax expense | 24 | 45 | ||||||
| Net loss | $ | (11,136 | ) | $ | (17,333 | ) | ||
| Basic and diluted net loss per common share | $ | (0.19 | ) | $ | (0.67 | ) | ||
| Weighted-average number of common shares used in basic and diluted net loss per common share | 58,858,758 | 25,886,615 | ||||||
See report of independent registered public accounting firm and the accompanying notes to these consolidated financial statements.
F-5
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit
(In thousands, except share amounts)
| Additional | Total | |||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Paid-in | Accumulated | Stockholders’ | ||||||||||||||||||||||||
| Shares | Par Value | Shares | Par Value | Capital | Deficit | Deficit | ||||||||||||||||||||||
| Balance at December 31, 2018 | — | — | 19,277,545 | 193 | 263,521 | (267,159 | ) | (3,445 | ) | |||||||||||||||||||
| Reclassification of derivative liabilities to additional paid-in capital upon stockholder approval | — | — | — | — | 5,785 | — | 5,785 | |||||||||||||||||||||
| Issuance of common stock upon conversion of notes payable to related party | — | _ | 9,072,197 | 91 | 5,913 | — | 6,004 | |||||||||||||||||||||
| Issuance of common stock upon exercise of related party warrants | — | — | 8,248,549 | 82 | 5,321 | — | 5,403 | |||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | 388 | — | 388 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (17,333 | ) | (17,333 | ) | |||||||||||||||||||
| Balance at December 31, 2019 | _ | — | $ | 36,598,291 | $ | 366 | $ | 280,928 | $ | (284,492 | ) | $ | (3,198 | ) | ||||||||||||||
| Relative fair value of warrants and beneficial conversion feature in connection with August 2019 Note | — | — | — | — | 2,765 | — | 2,765 | |||||||||||||||||||||
| Conversion of 2017 Note | — | — | 51,497,468 | 515 | 4,875 | — | 5,390 | |||||||||||||||||||||
| Redemption of fractional shares on conversion from CDIs to shares of common stock | — | — | (100 | ) | — | — | — | — | ||||||||||||||||||||
| Conversion of June 2020 and August 2020 Convertible | 17,774,853 | 178 | — | — | 1,397 | — | 1,575 | |||||||||||||||||||||
| Sale of Series A Preferred Stock, net of issuance costs of $286 | 42,310,730 | 423 | — | — | 3,041 | — | 3,464 | |||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | 605 | — | 605 | |||||||||||||||||||||
| Net loss | — | — | — | — | — | (11,136 | ) | (11,136 | ) | |||||||||||||||||||
| Balance at December 31, 2020 | 60,085,583 | $ | 601 | 88,095,659 | $ | 881 | $ | 293,611 | $ | (295,628 | ) | $ | (535 | ) | ||||||||||||||
See report of independent registered public accounting firm and the accompanying notes to these consolidated financial statements.
F-6
GI Dynamics, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | (11,136 | ) | $ | (17,333 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 28 | 32 | ||||||
| Stock-based compensation expense | 605 | 388 | ||||||
| Non-cash interest expense | 1,542 | 6,173 | ||||||
| Reclassification of warrant from derivative liabilities to other income | (10 | ) | ||||||
| Re-measurement of derivative liabilities | — | 1,683 | ||||||
| Loss on extinguishment of debt | 678 | — | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (1,741 | ) | (700 | ) | ||||
| Accounts payable | (206 | ) | (414 | ) | ||||
| Accrued expenses | (606 | ) | (441 | ) | ||||
| Net cash used in operating activities | (10,846 | ) | (10,612 | ) | ||||
| Investing activities: | ||||||||
| Purchases of property and equipment | — | (11 | ) | |||||
| Net cash used in investing activities | — | (11 | ) | |||||
| Financing activities: | ||||||||
| Debt issuance costs | — | (87 | ) | |||||
| Proceeds from exercise of related party warrants | — | 5,403 | ||||||
| Proceeds from Paycheck Protection Program loan | 195 | — | ||||||
| Proceeds from short term and long term debt, related party | 5,847 | 4,000 | ||||||
| Proceeds from sale of preferred stock | 3,750 | — | ||||||
| Issuance costs related to sale of preferred stock | (286 | ) | — | |||||
| Net cash provided by financing activities | 9,506 | 9,316 | ||||||
| Net decrease in cash and cash equivalents | (1,340 | ) | (1,307 | ) | ||||
| Cash and cash equivalents and restricted cash at beginning of year | 2,529 | 3,836 | ||||||
| Cash and cash equivalents and restricted cash at end of year | $ | 1,189 | $ | 2,529 | ||||
| Supplemental cash flow disclosures | ||||||||
| Income taxes paid | 15 | 45 | ||||||
| Interest paid | 5 | 394 | ||||||
| Conversion of notes payable to related party into common stock | 5,390 | 6,004 | ||||||
| Fair value of warrants issued with notes to related party | 2,330 | 4,061 | ||||||
| Beneficial conversion feature in connection with August 2019 Note to related party | 435 | — | ||||||
| Insurance premium financing plan | 150 | — | ||||||
| Capitalization of accrued interest into September 2020 Note | 297 | — | ||||||
| Conversion of notes and accrued interest payable to related party to preferred stock | 1,260 | — | ||||||
| Right-of-use asset obtained in exchange for lease liability | — | 463 | ||||||
| Reclassification of warrant from derivative liabilities to additional paid-in capital | — | 5,785 | ||||||
See report of independent registered public accounting firm and the accompanying notes to these consolidated financial statements.
F-7
GI Dynamics, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Nature of Business
GI Dynamics® is a clinical stage medical device company focused on the development and commercialization of EndoBarrier, a medical device intended to treat patients with type 2 diabetes and to reduce obesity.
Diabetes mellitus type 2 (also known as type 2 diabetes) is a long-term progressive metabolic disorder characterized by high blood sugar, insulin resistance, and reduced insulin production. People with type 2 diabetes represent 90-95% of the worldwide diabetes population; only 5-10% of this population is diagnosed with type 1 diabetes (a form of diabetes mellitus wherein little to no insulin is produced).
Being overweight is a condition where the patient’s BMI is greater than 25 (kg/m2); obesity is a condition where the patient’s BMI is greater than 30. Obesity and its comorbidities contribute to the progression of type 2 diabetes. Many experts believe obesity contributes to higher levels of insulin resistance, which creates a feedback loop that increases the severity of type 2 diabetes.
When considering treatment for type 2 diabetes, it is optimal to address obesity concurrently with diabetes.
EndoBarrier® is intended for the treatment of type 2 diabetes and to reduce obesity in a minimally invasive and reversible manner.
The current treatment paradigm for type 2 diabetes is lifestyle therapy combined with pharmacological treatment, whereby treating clinicians prescribe a treatment regimen of one to four concurrent medications that could include insulin for patients with higher levels of blood sugar. Insulin carries a significant risk of increased mortality and may contribute to weight gain, which in turn may lead to higher levels of insulin resistance and increased levels of blood sugar. Fewer than 50% of patients treated pharmacologically for type 2 diabetes are adequately managed, meaning that medication does not lower blood sugar adequately and does not halt the progressive nature of diabetes of these patients.
The current pharmacological treatment algorithms for type 2 diabetes fall short of ideal, creating a large and unfilled efficacy gap.
The GI Dynamics vision is to make EndoBarrier the essential nonpharmacological and non-anatomy-altering treatment for patients with type 2 diabetes. The Company intends to achieve this vision by providing a safe and effective device, focusing on optimal patient care, supporting treating clinicians, adding to the extensive body of clinical evidence around EndoBarrier, gaining appropriate regulatory approvals, continuing to improve its products and systems, operating the Company in a lean fashion, and maximizing stockholder value.
EndoBarrier® is intended for the treatment of type 2 diabetes and to reduce obesity in a minimally invasive and reversible manner and is designed to mimic the mechanism of action of duodenal-jejunal exclusion created by gastric bypass surgery.
Since incorporation, the Company has devoted substantially all of its efforts to product commercialization, research and development, business planning, recruiting management and technical staff, acquiring operating assets, and raising capital. The Company currently operates in one reportable business segment.
F-8
COVID-19 Pandemic and Impacts to Business Operations
In December 2019, a novel strain of coronavirus was reported to have surfaced in Wuhan, China. In January 2020, this coronavirus spread to other parts of the world, including the United States and Europe, and efforts to contain the spread of this coronavirus intensified. In March 2020, the World Health Organization declared the Coronavirus (“SARS-CoV-2”) outbreak a “pandemic.” The Company operations experienced significant restrictions and delays in 2020, including the following operational impacts:
| ● | The pause of enrollment of the Step-1 trial due to categorization of the EndoBarrier implantation procedure as an elective procedure. In the face of the epidemic and as a measure to reduce the spread of SARS-CoV-2, many states and/or clinics ceased all elective procedures. All of the Step-1 clinical sites ceased enrollment for a period in 2020 and as of March 11, 2021, all sites were able to enroll, pending procedural backlog clearance in individual clinics. | |
| ● | The private and public regulatory agencies required to approve the initiation of clinical trials, review and audit quality systems, and review applications for regulatory approvals all had to adapt to COVID-19 restricted work protocols that may have impacted productivity, priorities and schedules. As such, many regulatory applications the Company submitted were delayed in submission and follow-up response from the agencies were dramatically delayed. In one instance, the Company was able to incur substantial cost in enabling a timely in-person audit under COVID-19 safety precautions. In other instances, the Company cannot accelerate the timing of agency responses. | |
| ● | The economic impact of the pandemic and resulting restrictions may have affected investor perception of available funds for investment and risk tolerance. | |
| ● | Business travel was largely restricted or eliminated in 2020, potentially limiting the effectiveness of fundraising and other important business meetings. |
As of March 2021, various vaccines are being administered at differential rates in global communities and while the spread of SARS-CoV-2 is being impacted positively, a state of global economic and public health safety uncertainty remains as SARS-CoV-2 variants have emerged. Currently, the existing vaccines provide protection against the known variants, but the emergence of a variant for which the current vaccines are not effective may occur. If such a variant does emerge, the same effects experienced in 2020 may be repeated as restrictions are again required to minimize the impact of the re-emergent pandemic.
Going Concern Evaluation
As of December 31, 2020, the Company’s primary source of liquidity is its cash and restricted cash balances. GI Dynamics is currently focused on obtaining an EndoBarrier CE mark and enrolling the Company’s clinical trials, which will support future regulatory submissions and potential commercialization activities. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market. Without revenues, GI Dynamics is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.
The Company has incurred operating losses since inception and at December 31, 2020 had an accumulated deficit of approximately $296 million. The Company expects to incur significant operating losses for the next several years. At December 31, 2020, the Company had approximately $1.2 million in cash and restricted cash.
The Company will need to arrange additional financing before August 1, 2021 in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources, however, the Company has no guaranteed source of capital that will sustain operations past August 1, 2021. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. If the Company is unable to raise sufficient capital on the Company’s required timelines and on acceptable terms to existing stockholders and the Board of Directors, it could be forced to cease operations, including activities essential to support regulatory applications to commercialize EndoBarrier. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these Consolidated Financial Statements are issued.
F-9
In addition, if the Company does not meet its payment obligations to third parties as they become due, the Company may be subject to litigation claims and its credit worthiness would be adversely affected. Even if the Company is successful in defending these claims, litigation could result in substantial costs and would be a distraction to management and may have other unfavorable results that could further adversely impact the Company’s financial condition.
As a result of the factors described above, the Company’s Consolidated Financial Statements include a going-concern disclosure. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations — Liquidity and Capital Resources” for further information regarding the Company’s funding requirements.
The accompanying Consolidated Financial Statements have been prepared assuming GI Dynamics will continue as a going concern, which contemplates the realization of assets and liabilities and commitments in the normal course of business. The Consolidated Financial Statements for the year ended December 31, 2020 do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern within one year after the date that these Consolidated Financial Statements are issued.
2. Summary of Significant Accounting Policies and Basis of Presentation
Principles of Consolidation
The accompanying Consolidated Financial Statements include the accounts of GI Dynamics, Inc. and its wholly owned subsidiaries. All intercompany transactions and balances are eliminated in consolidation.
The functional currency of GID Europe Holding B.V., GID Europe B.V., GID Germany GmbH and GI Dynamics Australia Pty Ltd, each wholly-owned subsidiaries of the Company, is the U.S. dollar. Consolidated balance sheet accounts of the Company’s subsidiaries are remeasured into U.S. dollars using the exchange rate in effect at the consolidated balance sheet date while expenses are remeasured using the average exchange rate in effect during the period. Gains and losses arising from remeasurement of the wholly owned subsidiaries’ financial statements are included in the determination of net loss.
Segment Reporting
The Company has one reportable segment which designs, develops, manufactures and markets medical devices for non-surgical approaches to treating type 2 diabetes.
GI Dynamics does not report geographic segments as there were no product sales in 2020 or 2019 and at December 31, 2020 and 2019, all long-lived assets comprised of property and equipment were held in the U.S.
F-10
Use of Estimates
The preparation of Consolidated Financial Statements in accordance with generally accepted accounting principles in the U.S. requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates, including those related to the impairment of long-lived assets, income taxes including the valuation allowance for deferred tax assets, research and development, contingencies, valuation of warrant and other derivative liabilities, estimates used to assess its ability to continue as a going concern and stock-based compensation. GI Dynamics bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known.
Cash and Cash Equivalents and Restricted Cash
At December 31, 2020 and 2019, GI Dynamics had approximately $0.03 million and $0.01 million, respectively, of cash and cash equivalents denominated in Australian dollars that is subject to foreign currency gain and loss.
GI Dynamics has $30 thousand in restricted cash used to secure a corporate credit card account.
Property and Equipment
Property and equipment, are recorded at cost and are depreciated when placed in service using the straight-line method based on their estimated useful lives as follows:
| Asset Description |
Estimated Useful Life (In Years) | |
| Laboratory and manufacturing equipment | 5 | |
| Computer equipment and software | 3 | |
| Office furniture and equipment | 5 |
Maintenance and repair costs for fixed assets are expensed as incurred.
F-11
Derivative Liabilities
GI Dynamics examines all financial instruments to determine if the financial instrument or any component feature is a derivative under Financial Accounting Standards Board, (“FASB”) Accounting Standards Codification (“ASC”) 815, Derivatives and Hedging (“ASC 815”) and therefore requires liability classification. Certain warrants to purchase common stock did not meet the requirements for equity classification and were considered derivative instruments due to their cash settlement features. The derivative warrants were initially recorded at fair value with subsequent changes in fair value recorded in other income (expense) in the statements of operations. The Company estimates fair value using the Black-Scholes option pricing model. See Note 5 for inputs and assumptions used in the determination of the fair value. If the derivative instruments subsequently meet the requirements for classification as equity, the Company reclassifies the then fair value of the instrument to equity. If multiple outcomes are probable, management assigns probability adjustments to determine the most likely probability adjusted fair value.
Research and Development Costs
Research and development costs are expensed when incurred. Research and development costs include costs of all basic research activities as well as other research, engineering, and technical effort required to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include preapproval regulatory and clinical trial expenses.
Patent Costs
GI Dynamics expenses as incurred all costs, including legal expenses, associated with obtaining patents until the patented technology becomes feasible. All costs incurred after the patented technology is feasible will be capitalized as an intangible asset. As of December 31, 2020, no such costs had been capitalized since inception of the Company. GI Dynamics has expensed approximately $200 thousand of patent costs within general and administrative expenses in the consolidated statements of operations in each of the years ended December 31, 2020 and 2019.
Stock-Based Compensation
GI Dynamics accounts for stock-based compensation in accordance with ASC 718, Stock Compensation (“ASC 718”), which requires that stock-based compensation be measured at the grant date fair value and recognized as an expense in the financial statements. .
For awards that vest based on service conditions, GI Dynamics uses the straight-line method to allocate compensation expense to reporting periods. The grant date fair value of options granted is calculated using the Black-Scholes option pricing model, which requires the use of subjective assumptions including volatility, expected term and the fair value of the underlying common stock, among others.
The assumptions used in determining the fair value of stock-based awards represent management’s best estimates, but these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change, and management uses different assumptions, the Company’s stock-based compensation could be materially different in the future. The risk-free interest rate used for each grant is based on a zero-coupon U.S. Treasury instrument with a remaining term similar to the expected term of the stock-based award. Because GI Dynamics does not have a sufficient stable history to estimate the expected term, it uses the simplified method for estimating the expected term. The simplified method is based on the average of the vesting tranches and the contractual life of each grant. Prior to delisting from the ASX in July 2020, the Company estimated the expected stock volatility at the grant date based on the appropriate historical ASX price volatility.
GI Dynamics has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
F-12
GI Dynamics recognizes the impact of share-based award forfeitures only as they occur rather than by applying an estimated forfeiture rate.
GI Dynamics periodically issues performance-based awards. For these awards, vesting will occur upon the achievement of certain milestones. When achievement of the milestone is deemed probable, the Company records as compensation expense, the value of the respective stock award over the implicit remaining service period.
Stock awards to non-employees are also accounted for in accordance with ASC 718.. The Company elects to use the contractual term of each award as the expected term for NESBP awards.
Impairment of Long-Lived Assets
GI Dynamics regularly reviews the carrying amount of its long-lived assets to determine whether indicators of impairment may exist that merit adjustments to carrying values or estimated useful lives. If indications of impairment exist, projected future undiscounted cash flows associated with the asset are compared to the carrying amount to determine whether the asset’s value is recoverable. If the carrying value of the asset exceeds such projected undiscounted cash flows, the asset will be written down to its estimated fair value. No such impairments were recorded in 2020 or 2019.
Income Taxes
GI Dynamics uses the asset and liability method of accounting for income taxes. The Company records deferred tax assets and liabilities for the expected future tax consequences of temporary differences between its financial reporting and the tax bases of assets and liabilities measured using the enacted tax rates in effect in the years in which the differences are expected to reverse. The Company regularly assesses the need for a valuation allowance against its deferred tax assets. Future realization of the Company’s deferred tax assets ultimately depends on the existence of sufficient taxable income within the available carryback or carryforward periods. Sources of taxable income include taxable income in prior carryback years, future reversals of existing taxable temporary differences, tax planning strategies, and future taxable income. The Company records a valuation allowance to reduce its deferred tax assets to an amount it believes is more-likely-than-not to be realized. Deferred tax assets are reduced by a valuation allowance to reflect the uncertainty associated with their ultimate realization.
The Company assesses its income tax positions and records tax benefits based upon management’s evaluation of the facts, circumstances, and information available at the reporting date. For those tax positions where it is more-likely-than-not that a tax benefit will be sustained, the Company records the largest amount of tax benefit with a greater than 50 percent likelihood of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. For those income tax positions where it is not more-likely-than-not that a tax benefit will be sustained, no tax benefit is recognized in the financial statements. The Company classifies interest and penalties on uncertain tax positions as income tax expense.
Concentrations of Credit Risk
Financial instruments that subject GI Dynamics to credit risk primarily consist of cash and restricted cash. Cash balances are all maintained with high quality financial institutions, and consequently, the Company believes that such funds are subject to minimal credit risk.
F-13
Guarantees
GI Dynamics has identified the guarantees described below as disclosable, in accordance with ASC 460, Guarantees.
As permitted under Delaware law, GI Dynamics indemnifies its officers and directors for certain events or occurrences while the officer or director is, or was, serving at the Company’s request in such capacity. The maximum potential amount of future payments the Company could be required to make is unlimited; however, the Company maintains directors’ and officers’ insurance coverage that should limit its exposure and enable it to recover a portion of any future amounts paid.
GI Dynamics is a party to a number of agreements entered into in the ordinary course of business that contain typical provisions that obligate it to indemnify the other parties to such agreements upon the occurrence of certain events. Such indemnification obligations are usually in effect from the date of execution of the applicable agreement for a period equal to the applicable statute of limitations. The aggregate maximum potential future liability of the Company under such indemnification provisions is uncertain.
As of December 31, 2020, and 2019, GI Dynamics had not experienced any material losses related to these indemnification obligations, and no material claims with respect thereto were outstanding. The Company does not expect significant claims related to these indemnification obligations and, consequently, concluded that the fair value of these obligations is negligible. As a result, no related reserves have been established.
Leases
The Company applies ASC 842, Leases, which requires that most operating leases be recorded on the balance sheet unless the practical expedient is elected for short-term operating leases. The Company elected to apply the practical expedient as it relates to short-term leases. For other leases subject to this guidance, the Company will record a lease liability, which is the Company’s obligation to make lease payments arising from its leases, measured on a discounted basis, and a right-of-use asset, which is an asset representing the Company’s right to use the underlying asset for the lease term.
Recently Adopted Accounting Pronouncements
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820), Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement, or ASU 2018-13, which provides guidance focused on the disclosure requirements for disclosing fair value estimates, assumptions, and methodology. Requirements removed include the requirement to disclose details around amount and reasoning for level 1 to level 2 transfers, timing policies for transfer between levels and the valuation processes for level 3 fair value measurements. Modified requirements include details regarding net asset redemption restrictions and timing related to uncertainty disclosures. Requirements added include disclosures of changes in unrealized gains and losses for recurring level 3 measurements held as of the reporting date and disclosures around the range and weighted average of significant inputs used to develop level 3 fair value measurements. These amendments are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019. The amendments on changes in unrealized gains and losses, the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements and the narrative description of measurement uncertainty should be applied prospectively for only the most recent interim or annual period presented in the initial fiscal year of adoption. All other amendments should be applied retrospectively to all periods presented upon their effective date. Early adoption was permitted, however the Company declined early adoption and adopted this ASU effective January 1, 2020. The adoption had no impact to its consolidated financial statements.
Recently Issued Accounting Pronouncements
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes, or ASU 2019-12, which changes the treatment for a number of specific situations, with the most relevant topic being the tax effects of items not included in continuing operations when reporting a loss from continuing operations for the period. The guidance is effective for public business entities for fiscal years beginning after December 15, 2020, and for interim periods within those fiscal years. The Company has elected not to adopt ASU 2019-12 early and is evaluating the potential impact of adoption to its consolidated financial statements.
F-14
In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity, or ASU 2020-06, which simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts in an entity’s own equity. The changes include the accounting for beneficial conversion features and will result in less debt discount interest expense amortization. There are also reduced requirements for equity classification of contracts in an entity’s own equity. Additionally, expanded disclosures will be required for convertible debt instruments. These changes may have impact on earnings per share calculations. A full or modified retrospective approach can be adopted and ASU 2020-06 must be adopted by smaller reporting companies for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. The Company has elected not to adopt ASU 2020-06 early and is evaluating the potential impact of adoption to its consolidated financial statements.
3. Net Loss per Common Share
Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Potential common stock equivalents are determined using the treasury stock method. For diluted net loss per share purposes, stock options and other share-based awards are excluded as inclusion would have an anti-dilutive effect in periods in which a net loss is reported. Likewise, for diluted net loss per share purposes, warrants conferring the right to purchase common shares and because CDIs and common shares are interchangeable at the election of the stockholder, warrants conferring the right to purchase CDIs are excluded from diluted net loss per share calculations as inclusion would have an anti-dilutive effect in periods in which a net loss is reported. Shares of preferred stock that are convertible into shares of common stock are excluded from diluted net loss per share calculations as inclusion would also have an anti-dilutive effect. During each of the years ended December 31, 2020 and 2019, common stock equivalents were excluded from the calculation of diluted net loss per common share, as their effect was anti-dilutive due to the net loss incurred. Therefore, basic and diluted net loss per share was the same in all years presented.
The following potentially dilutive securities have been excluded from the computation of diluted weighted- average shares outstanding as of December 31, 2020 and 2019, as they would be anti-dilutive:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Warrants to purchase common stock | 28,532 | 28,532 | ||||||
| Options to purchase common stock and other stock-based awards | 463,104 | 3,331,154 | ||||||
| Total | 491,636 | 3,359,686 | ||||||
4. Warrants to Purchase Common Stock or CDIs
There was one warrant outstanding and exercisable at December 31, 2020 and 2019. On May 4, 2016, the Company entered into a consulting agreement pursuant to which a consulting firm provides strategic advisory, finance, accounting, human resources and administrative functions, including chief financial officer services, to the Company. In connection with the consulting agreement, the Company granted the consulting firm a warrant (“Consultant Warrant”) to purchase up to 28,532 shares of the Company’s common stock at an exercise price per share equal to $0.64. The Consultant Warrant is fully vested and expires on May 4, 2021. As of December 31, 2020, the Consultant Warrants had not been exercised.
The following table rolls forward the warrant activity during the year ended December 31, 2020:
| Shares underlying warrants | Exercise price per share | |||||||
| Outstanding and Exercisable at December 31, 2019 | 28,532 | $ | 0.64 | |||||
| Issuance of August 2019 Warrant | 4,596,893 | 1.00 | ||||||
| Cancellation of August 2019 Warrant | (4,596,893 | ) | 1.00 | |||||
| Outstanding and Exercisable at December 31, 2020 | 28,532 | $ | 0.64 | |||||
F-15
On August 21, 2019, GI Dynamics and Crystal Amber entered into a securities purchase agreement (“SPA”) for a total funding of up to approximately $10 million (the “August 2019 SPA”) comprised of the scheduled exercise of the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant as detailed above and the sale of an Unsecured Convertible Note for up to approximately $4.6 million (the “August 2019 Note”), which included an agreement to issue a warrant (the “August 2019 Warrant”) to purchase up to 229,844,650 CDIs (representing 4,596,893 shares of common stock) for an exercise price of $0.02 per CDI. On December 16, 2019, stockholder approval for the warrant to be issued was obtained and it was issued on January 13, 2020. The August 2019 Warrant was issued on January 13, 2020 and estimated fair value was determined to allocate relative fair values of the August 2019 Note and the August 2019 Warrant. The assumptions used to estimate the fair value of $2.3 million included an expected volatility of approximately 141%, an expected term of 5 years, a risk-free interest rate of 1.65%, and no dividend yield.
On September 4, 2020, the Company and Crystal Amber executed financing documents (the “September 2020 Financing”) that included the sale of up to $10 million of shares of Series A Preferred Stock, the cancellation of the August 2019 Warrants, and the restructuring of the August 2019 Note (described more fully in Notes 8 and 11 of the consolidated financial statements).
5. Fair Value Measurements
Information about the Company’s assets and liabilities that are measured at fair value initially or on a recurring basis as of December 31, 2020 and 2019 is provided below and includes the fair value hierarchy of the valuation techniques the Company used to determine such fair value. In general, fair values determined by Level 1 inputs utilize observable inputs such as quoted prices in active markets for identical assets or liabilities. Fair values determined by Level 2 inputs utilize data points that are either directly or indirectly observable, such as quoted prices, interest rates and yield curves. Fair values determined by Level 3 inputs utilize unobservable data points in which there is little or no market data, requiring the Company to develop its own assumptions for the asset or liability.
On March 31, 2020, the derivative liability classification of the Consultant Warrant was re-evaluated, and the Company re-classified it as an equity instrument. On March 31, 2020, this reclassification resulted in an immaterial credit to Other Income.
On June 18, 2020 and on August 4, 2020, convertible notes were issued to Crystal Amber, the terms of which included a mandatory conversion of outstanding principal and interest into shares issued in the next Qualified Financing, which was the September 2020 Financing, at a conversion price equal to 80% of the price per share of Series A Preferred Stock. The fair value of each the June 2020 Note and the August 2020 Note at issuance was determined using directly observable Level 2 inputs including the time period to the Proposed Offering, accrued interest and the fixed conversion premium. The Company elected the interest method of accretion to approximate fair value, so the June 2020 Note was only remeasured at August 1, 2020 when the timing and conversion discount methodology was clarified to be different from the original assumptions. The August 2020 Note applied the same methodology with known values for valuation variables. The June 2020 and the August 2020 Notes were converted into shares of Series A Preferred Stock on September 4, 2020 as part of the September 2020 financing.
Cash, restricted cash, prepaid expenses and other current assets, accounts payable, accrued expenses, short-term debt and other current liabilities at September 30, 2020 and December 31, 2019 are carried at amounts that approximate fair value due to their short-term maturities and the highly liquid nature of these instruments.
At December 31, 2020, there are no assets or liabilities that require remeasurement of fair value on a recurring basis.
F-16
6. Prepaid Expenses and Other Current Assets
Prepaid expenses consisted of the following (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Prepaid insurance | $ | 1,426 | $ | 293 | ||||
| Escrowed severance reserves | 544 | - | ||||||
| Prepaid clinical trial expenses | 489 | 488 | ||||||
| Prepaid corporate identity project | 165 | 134 | ||||||
| Other | 405 | 315 | ||||||
| Total | $ | 3,029 | $ | 1,230 | ||||
7. Property and Equipment
Property and equipment consisted of the following (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Laboratory and manufacturing equipment | $ | 591 | $ | 591 | ||||
| Computer equipment and software | 1,193 | 1,193 | ||||||
| Office furniture and equipment | 183 | 183 | ||||||
| 1,967 | 1,967 | |||||||
| Less accumulated depreciation and amortization | (1,953 | ) | (1,925 | ) | ||||
| Total | $ | 14 | $ | 42 | ||||
At December 31, 2020 and 2019, the Company had no property and equipment assets financed in any capital lease arrangement.
8. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Payroll and related liabilities | $ | 203 | $ | 531 | ||||
| Professional fees | 177 | 335 | ||||||
| Credit refunds | - | 164 | ||||||
| Interest payable | 80 | 250 | ||||||
| Other | 123 | 73 | ||||||
| Total | $ | 583 | $ | 1,353 | ||||
In 2017, following the notification by the United Kingdom’s Medicines and Healthcare products Regulatory Agency, the Company notified its customers to return their inventory on hand. The Company calculated an estimate for returns, reversed its revenue and recorded an accrued expense estimate of $202 thousand of product return related costs in addition to $77 thousand of credit memos granted to customers. Through December 31, 2019, accumulated returns had reduced the reserve total to approximately $164 thousand. Given the Company’s expectation that there will not be additional valid claims now that all product has fully expired, the reserves were reversed to income in 2020.
F-17
9. Notes Payable
2017 Convertible Note Financing
On June 15, 2017, the Company entered into a Note Purchase Agreement (“2017 NPA”) by and between the Company and Crystal Amber, a Related Party. Pursuant to the 2017 NPA, the Company issued and sold to Crystal Amber, a Senior Secured Convertible Promissory Note in an aggregate original principal amount of $5.0 million (the “2017 Note”).
The 2017 Note accrued interest at an annually compounded rate of 5% per annum, other than during the continuance of an event of default, when the 2017 Note would accrue interest at a rate of 8% per annum. The entire outstanding principal balance and all unpaid accrued interest thereon was initially due on the original maturity date of December 31, 2018, and, as announced on July 1, 2020, was most recently amended to extend the maturity date to July 31, 2020.
The 2017 Note was secured by a first priority security interest in substantially all tangible and intangible assets of the Company, including intellectual property (the “Collateral”). In the event of an uncured default, Crystal Amber, was authorized to sell, transfer, assign or otherwise deal in or with the Collateral or the proceeds thereof or any related goods securing the Collateral, as fully and effectually as if Crystal Amber were the absolute owner thereof. The ASX provided the Company with a waiver to allow all asset liens (the “Security”) to be granted to Crystal Amber, without the customary requirement of having to obtain stockholder approval for the grant of a security to a Related Party of the Company.
The entire outstanding principal balance under the 2017 Note and all unpaid accrued interest thereon was convertible into CDIs (i) prior to the maturity date, at the option of Crystal Amber at a conversion price calculated based on the five-day volume weighted average price (“VWAP”) of the Company’s CDIs traded on the ASX (“Optional Conversion Price”), or (ii) automatically upon the occurrence of an equity financing in which the Company raises at least $10 million (a “Qualified Financing”) at the price per CDI of the CDIs issued and sold in such financing.
On July 13, 2020, Crystal Amber provided the Company with a notice of optional conversion of the 2017 Note. On the conversion date, the principal of $5 million and the accrued and unpaid interest of $390 thousand totaling $5,390,240 was converted into 2,574,873,400 CDIs, which was equivalent to 51,497,468 shares of Common Stock. The conversion price equaled the 5-day VWAP per CDI for the 5 trading days immediately preceding the date of notice, which equaled $0.002093 per CDI or $0.10467 per share of Common Stock.
On receipt of the notice of conversion, the Company did not have sufficient authorized shares to issue to CHESS Depository Nominees, Ltd. (“CDN”) to enable the required number of CDIs to be allotted to Crystal Amber. The available 38,401,704 shares were issued to CDN, allowing the allotment of 1,920,085,200 CDIs to Crystal Amber. The Company and Crystal Amber executed a Right to Shares and Waiver Agreement in which the Company agreed to issue the remaining 13,095,764 shares of Common Stock owed under the conversion when the Company filed an amended and restated certification of incorporation with the Delaware Secretary of State after the Company was delisted from the ASX and in connection with the consummation of the September 2020 Financing. On July 22, 2020, the Company was removed from the Official List of the ASX (“Delisted”) and the CDN trust was subsequently dissolved, causing all CDIs to automatically convert to shares of Common Stock. The remaining 13,095,764 shares of Common Stock owed under the conversion were issued as shares of Common Stock immediately after Shareholders approved the increase in the authorized shares of common stock to 280,000,000 shares at the Special Meeting of Shareholders held on September 3, 2020.
The original debt discount associated with the 2017 Note had been fully amortized prior to December 31, 2018. The Company recorded a debt discount related to the initial maturity extension to March 31, 2019 and amortized over that period. Subsequent maturity date extensions were evaluated under ASC 470 to determine whether debt extinguishment or debt modification accounting applied and the Company concluded that the maturity extensions did not meet the characteristics of debt extinguishments under ASC 470 and no accounting recognition was required. For the years ended December 31, 2020 and 2019, the Company recognized interest expense related to the 2017 Note of $206 and $248 thousand and amortization of debt issuance costs of $0 and $43 thousand, respectively.
F-18
2018 Convertible Note and Warrant Financing
On May 30, 2018, the Company entered into a Note Purchase Agreement (“2018 NPA”) by and between the Company and Crystal Amber, a Related Party. Pursuant to the 2018 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of approximately $1.8 million (the “2018 Note”) with a maturity date of May 30, 2023. Interest accrued at an annually compounded rate of 10% per annum.
Due to a subsequent equity financing in September 2018, the conversion price adjusted to $0.0144 per CDI. During 2019, Crystal Amber submitted notice to convert the 2018 Note consisting of approximately $1.8 million in principal and $192 thousand of accrued interest into 134,852,549 CDIs (representing 2,697,050 shares of common stock).
In connection with the 2018 NPA, GI Dynamics issued to Crystal Amber a warrant (the “2018 Warrant”) to purchase 97,222,200 CDIs (representing 1,944,444 shares of common stock) at an initial exercise price of $0.018 per CDI. As per the 2018 Note conversion price, the warrant exercise price was subsequently adjusted to $0.0144 per CDI. The 2018 Warrant was exercised in full on August 25, 2019 for $1.4 million.
The Company evaluated the guidance ASC 480-10 Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the 2018 Note and 2018 Warrant. On issuance, having already obtained the required stockholder approval to reserve the CDIs underlying the conversion feature and the warrant, the 2018 Warrant was determined to be a freestanding instrument meeting the requirements for equity classification. Accordingly, the fair value estimated for the 2018 Warrant, totaling approximately $743 thousand, was recorded as a discount to the debt with the offset to additional paid-in capital. The 2018 Note was also evaluated for a BCF subsequent to the allocation of proceeds among the 2018 Note and 2018 Warrant. Based upon the effective conversion price of the 2018 Note after considering the stock price at the date of issuance and the allocation of estimated fair value to the 2018 Warrant, it was determined that the 2018 Note contained a BCF. The value of the BCF was computed to be approximately $1.2 million but was capped at approximately $1 million so as to not exceed the total proceeds from the 2018 Note after deducting the value allocated to the 2018 Note and 2018 Warrant. The effective interest rate on the note after the discounts was 26.4%.
The Company recorded the 2018 Note at issuance, net of the total debt discount of $1.8 million and amortized the debt discount over the life of the 2018 Note. For the year ended December 31, 2019, the Company recorded accrued interest expense of $91 thousand and debt discount amortization to interest expense of $146 thousand prior to conversion.
March 2019 Convertible Note and Warrant Financing
On March 15, 2019, the Company entered into a Note Purchase Agreement (“March 2019 NPA”) by and between the Company and Crystal Amber. Pursuant to the March 2019 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of $1 million (the “March 2019 Note”) with a maturity date of March 15, 2024. Interest accrued at an annually compounded rate of 10% per annum and issuance costs related to the March 2019 NPA were $50 thousand.
After the Company obtained stockholder approval to enable Crystal Amber’s conversion right under the March 2019 Note on June 30, 2019, the entire outstanding principal balance under the March 2019 Note and all unpaid accrued interest thereon was convertible into CDIs, at the option of Crystal Amber at a conversion price of $0.0127 per CDI. On June 30, 2019, Crystal Amber elected to convert the March 2019 Note consisting of $1 million of principal and accrued interest of $30 thousand into 81,070,003 CDIs (representing 1,621,400 shares of common stock).
F-19
Per the March 2019 NPA, the Company agreed to issue a warrant (the “March 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 78,984,823 CDIs (representing 1,579,696 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the March 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. On August 25, 2019, a portion of the March 2019 Warrant totaling 47,244,119 CDIs (equivalent to approximately 944,882 shares of common stock) was exercised for $600 thousand. The remaining portion of the March 2019 Warrant, totaling 31,740,704 CDIs (equivalent to 634,814 shares of common stock) was exercised for approximately $400 thousand on September 30, 2019.
The Company evaluated the guidance ASC 480-10, Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the March 2019 Note and March 2019 Warrant. On the date of the issuance of the March 2019 Note, the March 2019 Warrant was determined to be a freestanding instrument meeting the requirements for liability classification due to a cash settlement recourse available on the condition of not obtaining stockholder approval. Accordingly, the fair value estimated for the March 2019 Warrant, totaling approximately $850 thousand, was recorded as a discount to the March 2019 Note with the offset to derivative liabilities. The Company then evaluated the March 2019 Note for a BCF. Based upon the effective conversion price of the March 2019 Note after considering the stock price at the commitment date and the allocation of estimated fair value to the March 2019 Warrant, it was determined that the March 2019 Note contained a contingent BCF. The value of the BCF was computed to be approximately $623 thousand but was capped at approximately $129 thousand so as to not exceed the total proceeds from the March 2019 Note after deducting the value allocated to the March 2019 Warrant.
In addition, upon a change of control of the Company (other than a change of control resulting from a Qualified Financing) in which the Company’s stockholders receive cash consideration, the Company was obligated under the March 2019 Note to pay all accrued and unpaid interest then due plus 110% of the remaining outstanding unconverted principal balance. The Company considered the change in control premium as a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premium, the fair value was considered immaterial upon issuance and at all subsequent reporting period ends until converted.
Upon approval of the conversion features of the March 2019 Note and issuance of the March 2019 Warrants on June 30, 2019, the Company remeasured the warrant liability and recorded a $576 thousand remeasurement loss to the consolidated statement of operations and then reclassified $1.4 million of fair value of March 2019 Warrant from derivative liability to equity as the warrant became immediately exercisable. The total debt discount on the March 2019 Note upon stockholder approval of its conversion feature was $1 million. The effective interest rate on the Note after the discounts was 29.4%. Upon conversion of the 2019 March Note on June 30, 2019, the Company recorded the contingent beneficial conversion feature of $129 thousand as non-cash interest expense and additional paid-in capital.
F-20
For the year ended December 31, 2019, the Company accrued interest expense of $30 thousand and recorded debt discount amortization to interest expense of $1 million.
May 2019 Convertible Note and Warrant Financing
On May 8, 2019, the Company entered into a Note Purchase Agreement (“May 2019 NPA”) by and between the Company and Crystal Amber. Pursuant to the May 2019 NPA, the Company issued and sold to Crystal Amber a Senior Unsecured Convertible Promissory Note in an aggregate original principal amount of $3.0 million (the “May 2019 Note”) with a maturity date of May 8, 2024. Interest accrued at an annually compounded rate of 10% per annum and issuance costs related to the May 2019 NPA were $34 thousand.
After the Company obtained stockholder approval to enable Crystal Amber’s, a Related Party, conversion rights under the May 2019 Note on June 30, 2019, the entire outstanding principal balance under the May 2019 Note and all unpaid accrued interest thereon was convertible into CDIs, at the option of Crystal Amber at a conversion price of $0.0127 per CDI. On June 30, 2019, Crystal Amber elected to convert the May 2019 Note consisting of approximately $3.0 million of principal and accrued interest of $19 thousand into 237,687,411 CDIs (representing 4,753,748 shares of common stock).
Per the May 2019 NPA, the Company agreed to issue a warrant (the “May 2019 Warrant”) to Crystal Amber, pending stockholder approval, to purchase 236,220,472 CDIs (representing 4,724,409 shares of common stock) at an initial exercise price of $0.0127 per CDI. The issue of the May 2019 Warrant required the approval of stockholders and was not exercisable until its issue was approved on June 30, 2019. A portion of the May 2019 Warrant totaling 125,739,610 CDIs (equivalent to 2,514,792 shares of common stock) was exercised by Crystal Amber for approximately $1.6 million on September 30, 2019. Another portion of the May 2019 Warrant totaling 78,740,157 CDIs (equivalent to 1,574,803 shares of common stock) was exercised by Crystal Amber for approximately $1 million on October 31, 2019.
The Company evaluated the guidance ASC 480-10, Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options to determine the appropriate classification of the May 2019 Note and May 2019 Warrant. On the date of the issuance of the May 2019 Note, the May 2019 Warrant was determined to be a freestanding instrument meeting the requirements for liability classification due to a cash settlement recourse available on the condition of not obtaining stockholder approval. Accordingly, the fair value estimated for the May 2019 Warrant, totaling approximately $3.2 million, was recorded to derivative liabilities with offsets of $3 million to a discount on the May 2019 Note and $200 thousand to derivative loss on the consolidated statements of operations. The Company then evaluated the May 2019 Note for a BCF. Based upon the effective conversion price of the May 2019 Note after considering the stock price at commitment date and the allocation of estimated fair value to the May 2019 Warrant, it was determined that the May 2019 Note contained a BCF. The value of the BCF was computed to be approximately $2 million but was not recorded as doing so would exceed the total proceeds from the May 2019 Note after recording the fair value of the May 2019 Warrant.
In addition, upon a change of control of the Company (other than a change of control resulting from a Qualified Financing) in which the Company’s stockholders receive cash consideration, the Company was obligated under the May 2019 Note to pay all accrued and unpaid interest then due plus 110% of the remaining outstanding unconverted principal balance. The Company considered the change in control premium as a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premium, the fair value was considered immaterial upon issuance and at all subsequent reporting period ends.
Upon approval of the conversion features of the May 2019 Note and issuance of the May 2019 Warrant on June 30, 2019, the Company revalued the warrant liability and recorded a $1.1 million remeasurement loss to the consolidated statements of operations and then reclassified approximately $4.3 million of fair value of May 2019 Warrant from derivative liabilities to equity as the May 2019 Warrant became immediately exercisable. The total debt discount on the May 2019 Note upon stockholder approval was $3 million. The effective interest rate on the May 2019 Note after the discounts is 29.4%.
F-21
For the year ended December 31, 2019, the Company accrued interest expense of $19 thousand and recorded debt discount amortization to interest expense of $3 million.
August 2019 Securities Purchase Agreement (“SPA”)
On August 21, 2019, the Company entered into the August 2019 SPA by and between the Company and Crystal Amber. The August 2019 SPA detailed a timeline wherein Crystal Amber would exercise the 2018 Warrant, the March 2019 Warrant, and the May 2019 Warrant. Additionally, pursuant to the August 2019 SPA, the Company issued and sold to Crystal Amber the August 2019 Note in an aggregate principal amount of up to approximately $4.6 million to be funded on December 6, 2019, or such earlier or later date as may be requested by the Company (the “Funding Date”). In conjunction with the August 2019 Note, the Company agreed to issue to Crystal Amber the August 2019 Warrant (see Notes 4 and 9 of the consolidated financial statements) conferring the right to purchase 229,844,650 CDIs (representing 4,596,893 shares of common stock), with warrant issuance subject to the funding of the August 2019 Note and the receipt of required stockholder approval to issue the August 2019 Warrant.
The August 2019 Note accrued interest at a rate equal to 10% per annum from the August 2019 Note Funding Date, compounded annually, other than during the continuance of an event of default, when the August 2019 Note would accrue interest at a rate of 16% per annum. The entire outstanding principal balance and all unpaid accrued interest thereon would become due on the fifth anniversary of the Funding Date. The entire outstanding principal balance under the August 2019 Note and all unpaid accrued interest thereon was immediately convertible into CDIs at the option of Crystal Amber at a conversion price equal to $0.02 per CDI (representing $1.00 per share of Common Stock). In the event that the Company issued additional CDIs to a stockholder other than Crystal Amber in a subsequent equity financing at a price per CDI that was less than the conversion price under the August 2019 Note, the conversion price was to be reduced to the lowest such price per CDI. In addition, upon a change of control of the Company resulting in cash proceeds, Crystal Amber could, at its option, demand that the Company prepay all accrued and unpaid interest plus 110% of the remaining outstanding unconverted principal balance. The Company was unable to prepay the August 2019 Note without the consent of Crystal Amber, a Related Party. If the stockholder approvals required to issue the August 2019 Warrant or to approve the conversion rights under the August 2019 Note were not obtained, the Company was obligated to prepay all accrued and unpaid interest plus 110% of the remaining outstanding unconverted principal balance on the earlier of the Funding Date or the date that was six months following the date of the stockholder meeting at which the requisite approvals were not obtained. The Company considered the change in control premium and the stockholder approval premium to each represent a cash settleable feature, thereby requiring derivative liability classification. On applying a probability adjusted present value of the premiums, the fair values of these features were considered immaterial upon issuance.
The August 2019 SPA contained customary events of default. If a default occurs and is not cured within the applicable cure period or is not waived, any outstanding obligations under the August 2019 Note may be accelerated. The August 2019 SPA and related August 2019 Note and August 2019 Warrant documents also contained additional representations and warranties, covenants and conditions, in each case customary for transactions of this type.
Prior to December 6, 2019, the Company notified Crystal Amber that it had elected to receive the full amount of approximately $4.6 million under the August 2019 Note, but agreed to timing extensions.
On December 16, 2019, stockholder approval was obtained pursuant to ASX Listing Rule 10.11, for the August 2019 Note conversion feature and the issuance of the August 2019 Warrant, contingent on receipt of the August 2019 Note proceeds.
On January 13, 2020, the full amount of approximately $4.6 million was received as proceeds from the August 2019 Note. On receipt of funds, the August 2019 Note was immediately convertible. On January 13, 2020, the Company issued to Crystal Amber an immediately exercisable August 2019 Warrant to purchase 229,844,650 CDIs (representing 4,596,893 shares of common stock) for an exercise price of $0.02 per CDI (see Note 4 of the consolidated financial statements).
F-22
On issuance, having already obtained the required stockholder approval to reserve the CDIs underlying the conversion feature and the August 2019 Warrant, the August 2019 Warrant was determined to be a freestanding instrument meeting the requirements for equity classification in accordance with ASC 480-10 Distinguishing Liabilities from Equity, ASC 815-40 Contracts in an Entity’s Own Equity and ASC 470-20 Debt with Conversion and Other Options. Accordingly, proceeds from the August 2019 SPA were allocated to the August 2019 Note and Warrant based on their relative fair values. The relative fair value of the August 2019 Warrant of approximately $2.3 million was recorded as a debt discount with the offset to additional paid-in capital. Additionally, the Company analyzed the conversion features of the August 2019 Note to determine whether a beneficial conversion feature (BCF) existed. The Company determined a BCF with a value of $435 thousand existed and was recorded as a debt discount with the offset to additional paid-in capital. The total debt discount was to be amortized to interest expense through the January 2025 maturity of the August 2019 Note.
On September 4, 2020, the Company executed a series of financing-related agreements (“the September 2020 Financing”) which included the cancellation of the August 2019 Warrant, the restructuring of the August 2019 Note into the September 2020 Note (see below for a detailed description of the September 2020 Note and the accounting classification of the August 2019 Note restructuring into the September 2020 Note), the conversion of the June and August 2020 Convertible Notes into shares of Series A Preferred Stock (see below for a detailed description of the June and August 2020 Notes and Note 10 for a description of the Series A Preferred Stock), and the sale of $8.75 million of Series A Preferred Stock to Crystal Amber under the Series A Preferred Stock Purchase Agreement (the “Series A SPA”, see Note 11 of the Consolidated Financial Statements for a description of the Series A SPA terms). The Initial Close of the September 2020 Financing occurred on September 4, 2020, and included all components of the September 2020 Financing, except that $5 million of the total Series A Preferred Stock sale was originally deferred to October 31, 2020 (the “Second Close” of the September 2020 Financing, see Note 11 of the Consolidated Financial Statements for a description of the Series A SPA terms).
For the year ended December 31, 2020, the Company recognized interest expense of approximately $300 thousand and amortization of debt issuance costs of $395 thousand related to the August 2019 Note, and interest expense of approximately $80 thousand and amortization of debt issuance costs of $305 thousand related to the September 2020 Note.
Paycheck Protection Program (“PPP”) Loan
On March 27, 2020, the CARES Act was signed into law in the United States providing economic assistance for American workers and families, small businesses, and preserves jobs for American industries. On April 4, 2020, GI Dynamics submitted an application to a lending institution for a loan of approximately $200 thousand under the Paycheck Protection Program. In accordance with the provisions of the PPP, the loan accrues interest at a rate of 1% and all or a portion of the loan may be forgiven if it is used to pay for qualifying costs such as payroll, rent and utilities. Amounts that are not forgiven will be repaid 2 years from the date of the loan. The loan was granted by the lending institution on May 8, 2020 and funds were received into the Company’s bank account on May 11, 2020. The Company believes expenditures of the loan proceeds are fully compliant with the terms for loan forgiveness and while legislation passed in late December 2020 caused a hold on forgiveness applications at most lending institutions, the Company anticipates applying for loan forgiveness after resumption of lender acceptance of forgiveness applications.
June 2020 Convertible Note and August 2020 Convertible Note
On June 18, 2020, the Company entered into a Note Purchase Agreement (“June 2020 NPA”) by and between the Company and Crystal Amber. Pursuant to the June 2020 NPA, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $750 thousand (the “June 2020 Note”).
On August 4, 2020, the Company entered into Note Purchase Agreement (“August 2020 NPA”) by and between the Company and Crystal Amber that was identical to the June 2020 Note in all terms except the principal amount. Pursuant to the August 2020 NPA, the Company issued and sold to Crystal Amber, a Convertible Promissory Note in an aggregate original principal amount of $500 thousand (the “August 2020 Note”). The Company received $250 thousand on August 3, 2020 and the remaining $250 thousand on August 6, 2020.
F-23
The June and August 2020 Notes accrued interest at an annually compounded rate of 5% per annum, other than during the continuance of an event of default, when the June and August 2020 Notes would accrue interest at a rate of 8% per annum. The entire outstanding principal balance and all unpaid accrued interest thereon could become immediately due and payable at the sole discretion of Crystal Amber any time after December 18, 2020 and February 4, 2021, respectively. The entire outstanding principal balance and all unpaid accrued interest under the June and August 2020 Notes mandatorily converted at the Initial Close of the September 2020 Financing into shares of Series A Preferred Stock at a conversion price of $0.0709 per share, which was equal to 80% of the price per share of Series A Preferred Stock sold in the September 2020 Financing.
At issuance, the Company analyzed the June and August 2020 Notes and their settlement features under ASC 480-10 Distinguishing Liabilities from Equity. Having determined that the predominant settlement feature for both notes was the mandatory conversion into shares of Series A Preferred Stock at the close of the September 2020 Financing, the June and August 2020 Notes and settlement features were recorded at fair value as a liability. The Company initially recorded the fair value of the June and August 2020 Notes at the principal value and subsequently used the effective interest rate method to accrete the value of the conversion premium, recorded as a debt discount, and nominal interest over the period to expected conversion.
While the June 2020 and August 2020 Notes were still outstanding, if a Company change of control event had generated cash proceeds for the Company, Crystal Amber could, at its option, demand that the Company pay all accrued and unpaid interest plus 110% of the remaining outstanding unconverted principal balance. The Company could not prepay the June and August 2020 Notes without the consent of Crystal Amber. The Company considered the change in control premium to represent a cash settleable feature, thereby requiring derivative liability classification. On applying a probability-adjusted present value of the premiums, the fair value of the cash settleable feature was considered immaterial.
On August 1, 2020, the assumed values used in determining the fair value of the June 2020 Note became known and the debt was revalued as of August 1. The change in terms used to value the debt were analyzed under ASC 470-60 Troubled Debt Restructurings by Debtors and the lack of a concession by the Creditor led the Company to conclude the changes did not constitute a Troubled Debt Restructuring. Further analysis under ASC 470-50 Debt Modifications and Extinguishments showed insufficient changes to result in extinguishment accounting, so the debt was treated as a modification and recorded as an approximately $40 thousand increase to the note and debt discount.
On September 4, 2020, as part of the September 2020 Financing, the June 2020 Note and August 2020 Note with an aggregate principal of $1.25 million and $10 thousand of accrued interest were converted into approximately 17.7 million shares of Series A Preferred Stock.
For the year ended December 31, 2020, the Company accrued interest expense of $10 thousand and $315 thousand for amortization of the debt discount related to the conversion premium.
September 2020 Note and the Debt Restructuring Transaction
On September 4, 2020, as part of the September 2020 Financing, the August 2019 Warrant was cancelled, the August 2019 Note was extinguished and a new convertible note (the “September 2020 Note”) was issued with a principal amount of approximately $4.9 million, which was the sum of the outstanding principal and accrued interest from the August 2019 Note as of September 4, 2020. The September 2020 Note accrues annually compounded interest at 5% per annum and matures on June 30, 2022. On the continuation of an event of default, the outstanding principal and any accrued and unpaid interest shall be immediately payable in cash. The September 2020 Note can be optionally converted at any time prior to maturity and at the sole discretion of Crystal Amber. On election to convert, the entirety of the outstanding principal and all accrued but unpaid interest (the “Outstanding Amount’) is convertible into the number of shares of common stock equal to the quotient obtained by dividing the Outstanding Amount by $0.17726 (the “Conversion Price”). The conversion price represents 200% of the initial purchase price per share in the September 2020 Financing with gross proceeds of not less than $10 million in the aggregate, pursuant to the terms and subject to the conditions of the September 2020 Preferred Stock Purchase Agreement.
F-24
The Company analyzed the combined August 2019 Warrant cancellation and the August 2019 Note restructuring for the classification of a Troubled Debt Restructuring under ASC 470-60 Troubled Debt Restructurings by Debtors. The Company concluded that the restructuring was not a Troubled Debt Restructuring as the effective interest rate of the new debt exceeded the effective interest rate of the old debt, so the Creditor did not provide a concession. The Company then analyzed the old and new debt to determine whether the restructuring should be treated as a modification or an extinguishment under ASC 470-50 Debt Modifications and Extinguishments. Any restructuring representing a greater than 10% change in the present value of the new debt cash flows relative to the present value of the old debt cash flows requires the old debt to be extinguished and the new debt to be issued in the period of restructuring by adjusting the carrying amount of the old debt to the fair value of the new debt with the adjustment being reflected as a gain or loss. The difference in the present value of the cash flows of the September 2020 Note relative to the present value of the remaining cash flows for the August 2019 Note was more than 10%, therefore, the restructuring requires extinguishment accounting. The September 2020 Note has an interest rate of 5% and no inducements, therefore, the Company considered the equivalent arms-length placement to a third-party investor highly unlikely given the Company’s risk position. The present value of the September 2020 Note cash flows was therefore calculated using a discount rate equal to the effective interest rate of the August 2019 Note as it contained a higher nominal interest rate and 100% warrant coverage as inducements. Based on this analysis, the Company estimated that the fair value of the September 2020 Note was approximately $3.2 million upon issuance.
The September 4, 2020 extinguishment of the August 2019 Note and issuance of the September 2020 Note resulted in a decrease in accrued interest and an increase to long term debt of approximately $0.3 million, a decrease in the related debt discount of approximately $0.7 million for a change in the carrying value of the debt of approximately $1.0 million. A loss on debt extinguishment of approximately $0.7 million was recorded on the consolidated statements of operations for the year ended December 31, 2020.
For the year ended December 31, 2020, the Company accrued interest expense of $10 thousand and recorded $316 thousand for amortization of the debt discount related to the September 2020 Note.
10. Commitments
Lease Commitment
From December 2018 to May 2019, the Company operated under a 6-month membership agreement with WeWork for office space located in Boston, Massachusetts.
On April 22, 2019, the Company entered into a right-of-use lease for 3,520 square feet of office space in Boston, Massachusetts. The lease period commenced May 1, 2019 and expires on May 31, 2022. The lease had a one-month rent-free period, has escalating rent payments and contains no extension or expansion rights. On lease execution, the Company recorded the approximately $463 thousand present value of the lease liability in short-term and long-term liabilities and recorded a related right-of-use asset. The right-of-use asset is amortizing to lease expense and the liability is be reduced by the rent payments over the term of the lease.
The Company’s leases generally do not provide an implicit interest rate and therefore the Company uses 10% as an estimate of its incremental borrowing rate as the discount rate when measuring operating lease liabilities. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease in a similar economic environment.
The Company’s operating lease is reflected in the balance sheets. Lease expense totaled $175 thousand and $102 thousand for the years ended December 31, 2020 and 2019, respectively. Other information related to leases was as follows:
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| (in thousands) | (in thousands) | |||||||
| Operating cash flows from operating leases in lease liability measurement | $ | 178 | $ | 148 | ||||
| Operating cash flows from short term leases | - | 103 | ||||||
| Remaining long-term lease term in years | 1.4 | 2.4 | ||||||
F-25
The maturity of the Company’s operating lease liability as of December 31, 2020 is as follows:
| December 31, | ||||
| 2020 | ||||
| (in thousands) | ||||
| 2021 | 182 | |||
| 2022 | 76 | |||
| Total future minimum lease payments | 258 | |||
| Less: imputed interest | (19 | ) | ||
| Total lease liabilities | $ | 239 | ||
Rent expense on non-cancelable operating leases was approximately $175 and $102 thousand for the years ended December 31, 2020 and 2019, respectively.
11. Stockholders’ Deficit
On December 19, 2019, GI Dynamics stockholders approved an increase of its authorized shares of common stock from 50 million to 75 million.
On May 26, 2020, the Company filed a definitive proxy statement and Notice of Stockholder Meeting with the Securities and Exchange Commission in the United States (the “SEC”) and with ASX in Australia. The proposal to be voted by shareholders was to formally apply for removal from the Official List of the ASX (the “Delisting”). On June 20, 2020, stockholders approved the Delisting and notice was given to the market that a formal Delisting application had been submitted to ASX. The Company was not offering any share purchase facilities under the Delisting plan. After a 30-day trading period ending 4:00 p.m. July 22, 2020 Australian Eastern Standard Time, the Company was delisted. All CDIs were converted to shares of Common Stock before the CDN trust was dissolved. Registered holders converting CDIs such that a fractional common share was generated received cash payment for such fractional share. The Company’s SEC reporting requirements are still in effect, even though the Company’s securities are not listed on any exchange.
As described in Note 8, Crystal Amber provided the Company with a notice of optional conversion of the 2017 Note in July 2019 and converted the principal and accrued interest into Common Stock.
On September 3, 2020, GI Dynamics stockholders approved an increase of its authorized shares of common stock from 75 million to 280 million shares and approved the authorization of 118 million shares of Series A Convertible Preferred Stock (“Series A Preferred”), a newly created class of capital stock with the following rights and privileges.
Voting and Director Nomination Rights
Holders of shares of Series A Preferred will generally be entitled to vote with the holders of shares of common stock, at any stockholder meeting or by written consent in lieu of such meeting. Except as required by applicable law or provided in the Restated Certificate, holders of Series A Preferred will vote together with the holders of common stock as a single class and on an as-converted to common stock basis.
Holders of Series A Preferred will have the right, exclusively and as a separate class, to (a) designate 2 members of the Board, (b) remove such designees from the Board without cause and (c) fill any vacancies with respect to those directorships.
F-26
Protective Provisions
In addition to the foregoing director nomination rights, at any time when at least 5 million shares of Series A Preferred are outstanding, holders of at least a majority of the outstanding shares of Series A Preferred, voting separately as a single class, must approve certain significant actions of the Company, including, among others: (a) the liquidation, dissolution or winding up of the affairs of the Company; (b) any Deemed Liquidation 122 Event (as defined in the Restated Certificate); (c) amendments to the Restated Certificate or the Company’s bylaws, which would adversely affected the rights and privileges of the Series A Preferred; (d) any issuance or authorization of an additional class or series of capital stock of the Company that does not rank junior to the Series A Preferred with respect certain rights and privileges; (e) any reclassification of an existing security of the Company that renders such security senior to the Series A Preferred with respect to certain rights and privileges; and (f) any increase or decrease in the authorized number of members of the Board.
Conversion Rights and Anti-Dilution Adjustments
The Holders of shares of Series A Preferred will have the right to convert such shares into shares of common stock on a 1-for-1 basis, at a conversion price equal to the per share issue price of the Series A Preferred, which initially under the Series A SPA, will be $0.08863 per share. Shares of Series A Preferred will be convertible both at the option of the holder and mandatorily upon either (a) the closing of a Qualified IPO or (b) the date and time, or the occurrence of an event, specified by vote or written consent of the holders of a majority of the then outstanding shares of Series A Preferred.
The Company will be required, at all times, to reserve and keep available out of its authorized and unissued shares of common stock the number of shares that would be issuable upon conversion of all outstanding shares of Series A Preferred. If this reserve is not sufficient at any point to allow for full conversion, the Company must act to sufficiently increase its pool of authorized but unissued shares of common stock.
The conversion price of the Series A Preferred and the number of shares of common stock to be delivered upon conversion of the Series A Preferred will be subject to certain customary anti-dilution protections for certain events, such as (a) stock splits, subdivisions, or combinations of common stock, (b) certain dividends or distributions on shares of common stock and (c) certain mergers, reorganizations, reclassifications, recapitalizations and consolidations of the Company.
Preferential Payments
In the event of a voluntary or involuntary liquidation, dissolution or winding up of the Company, holders of the Series A Preferred then outstanding will be entitled to receive, before any distribution of the assets of the Company to the holders of common stock, an amount per share equal to 1.2 times the original issue price per share in the Series A Preferred financing, plus any declared but unpaid dividends. Holders of Series A Preferred will also be entitled to the same preferential rights upon a Deemed Liquidation Event (as defined in the Restated Certificate), except that in such case, the distributions to holders of Series A Preferred shall be in the form of payments from the proceeds of the transaction constituting such Deemed Liquidation Event.
Dividends
Holders of Series A Preferred will entitled to receive dividends, when and if declared by the Company on any shares of its capital stock (other than on dividends on shares of common stock payable in shares of common stock), prior to or at the same time of the payment of such declared dividends. In such case, the minimum dividend amount payable on shares of Series A Preferred will be determined either on an as-converted to common stock basis or on the basis of the issue price of the capital stock, and will be calculated based on the formulas set forth in the Restated Certificate.
On September 3, 2020, after stockholders authorized the increase in shares of Common Stock, the Company issued the remaining 13,095,764 shares of Common Stock due to Crystal Amber in full satisfaction of the Right to Shares and Waiver Agreement (see Note 9 of the consolidated financial statements)).
F-27
On September 4, 2020, as part of the September 2020 Financing, Crystal Amber converted the amounts owed related the June and August 2020 Notes, which totaled an outstanding principal amount of $1.25 million and a total of approximately $10 thousand of unpaid accrued interest, into 17,774,853 shares of Series A Preferred Stock.
On September 4, 2020, the Company executed a series of financing-related agreements (“the September 2020 Financing”) which included the cancellation of the August 2019 Warrant, the restructuring of the August 2019 Note into the September 2020 Note (see below for a detailed description of the September 2020 Note and the accounting classification of the August 2019 Note restructuring into the September 2020 Note), the conversion of the June and August 2020 Convertible Notes into shares of Series A Preferred Stock (see below for a detailed description of the June and August 2020 Notes and Note 11 for a description of the Series A Preferred Stock), and the sale of $8.75 million of Series A Preferred Stock to Crystal Amber under the Series A Preferred Stock Purchase Agreement (the “Series A SPA”, see Note 11 of the Consolidated Financial Statements for a description of the Series A SPA terms). The Initial Close of the September 2020 Financing occurred on September 4, 2020, and included all components of the September 2020 Financing, including the Crystal Amber purchase of 42,310,730 shares of Series A Preferred Stock for gross proceeds of $3.75. Pursuant to the Series A SPA and subsequent amendments, the Second Close was originally to occur on October 31, 2020 but through a series of extensions, was extended until February 24, 2021. On February 24, 2021, an individual investor closed the purchase of 600,000 shares of Series A Preferred Stock for approximate proceeds of $53 thousand. Effective February 24, 2021, Crystal Amber executed a contract amendment restructuring the Second Close to include the sale of 16,924,292 shares of Series A Preferred Stock for proceeds of $1.5 million to close on March 4, 2021, the sale of 11,282,861 shares of Series A Preferred Stock for proceeds of $1.0 million to close no later than March 25, 2021, and the remaining 27,607,153 shares of Series A Preferred Stock for proceeds of $2.45 million to close no later than May 28, 2021.
12. Share-Based Compensation
The Company has three stock-based compensation plans. The Board of Directors adopted the 2003 Omnibus Stock Plan (the “2003 Plan”), which provided for the grant of qualified incentive stock options and nonqualified stock options or other awards to the Company’s employees, officers, directors, advisors, and outside consultants to purchase up to an aggregate of 922,086 shares of the Company’s common stock. At December 31, 2020, one fully vested grant remained outstanding conferring the right to purchase 5,000 shares for $8.20 per share with an expiry of April 2021.
In August 2011, the Board of Directors adopted the 2011 Employee, Director and Consultant Equity Incentive Plan (the “2011 Plan”) as the successor to the 2003 Plan. Under the 2011 Plan, the Company may grant incentive stock options, nonqualified stock options, restricted and unrestricted stock awards and other stock-based awards. As of August 20, 2020, the date of the adoption of the 2020 Plan detailed below, 863,307 shares of common stock were available for grant under the Company’s 2011 Plan. Existing, outstanding grants issued under the 2011 Plan will remain active unless they are exercised or cancelled due to forfeiture or expiry. The Board of Directors have disallowed the granting of any new awards using shares available under the 2011 Plan.
In August 2020, the Board of Directors adopted the 2020 Employee, Director and Consultant Equity Incentive Plan (the “2020 Plan”) as the successor to the 2011 and 2003 Plans. On September 3, 2020, shareholders approved the adoption of the 2020 Plan. Under the 2020 Plan, the Company may grant incentive stock options, nonqualified stock options, restricted and unrestricted stock awards and other stock-based awards up to a total of 41,710,968 shares. The 2003 Plan, 2011 Plan and 2020 Plan are collectively referred to herein as the “Plans”. As of December 31, 2020, 41,710,968 shares of common stock were available for grant under the Company’s 2020 Plan.
On January 28, 2021, the Board of Directors granted 15 option awards to employees, board members, and a consultant, representing the right to purchase a total of 24,484,059 shares of common stock for a strike price of $0.06, which is the fair value determined by the Board of Directors.
F-28
Stock-Based Compensation
Stock-based compensation is reflected in the consolidated statements of operations as follows for the years ended December 31, 2020 and 2019 (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Research and development | $ | 63 | $ | 77 | ||||
| Sales and marketing | — | — | ||||||
| General and administrative | 542 | 311 | ||||||
| $ | 605 | $ | 388 | |||||
The stock options granted under the Plans generally vest over a four-year period and expire ten years from the date of grant.
The weighted-average assumptions used to estimate the fair value of employee stock options using the Black-Scholes option-pricing model were as follows for the years ended December 31, 2020 and 2019:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Expected volatility | 164.9 | % | 271.0 | % | ||||
| Expected term (in years) | 5.84 | 6.05 | ||||||
| Risk-free interest rate | 0.9 | % | 1.8 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
Stock Options
The following table summarizes share-based activity under the Plans:
| Shares of Common Stock Attributable to Options | Weighted- Average Exercise Price | Weighted- Average Contractual Life | Aggregate Intrinsic Value | |||||||||||||
| (in years) | (in thousands) | |||||||||||||||
| Outstanding at December 31, 2019 | 3,096,154 | $ | 1.47 | 8.30 | $ | — | ||||||||||
| Granted | 90,000 | $ | 0.23 | |||||||||||||
| Cancelled | (2,723,050 | ) | $ | 1.33 | ||||||||||||
| Outstanding at December 31, 2020 | 463,104 | $ | 2.04 | 7.60 | $ | — | ||||||||||
| Exercisable at December 31, 2020 | 254,965 | $ | 3.27 | 6.97 | $ | — | ||||||||||
The majority of the Company’s option grants vest 25% on the first anniversary of the grant date, and in a quarterly straight-line rate thereafter until fully vested on the fourth anniversary of the grant date. The weighted average grant date fair value for options granted in 2020 and 2019, was $0.18 and $1.04, respectively. The unrecognized stock compensation expense at December 31, 2020 was $123 thousand and was expected to be recognized over a weighted average period of 2.08 years from December 31, 2020.
F-29
Performance Stock Units
Each performance stock unit (“PSU”) represents a contingent right to receive one share of the Company’s common stock. There is no consideration payable on the vesting of PSUs issued under the Plans. Upon vesting, the PSUs are exercised automatically and settled in shares of the Company’s common stock. During the years ended December 31, 2020 and 2019, the Company awarded no PSUs to employees and directors of the Company. Due to the resignation of an employee, all PSUs were cancelled in November 2020.
The following table summarizes information related to PSU activity during the year ended December 31, 2020:
| Number of Units | Weighted- Average Contractual Life | Aggregate Intrinsic Value | ||||||||||
| (in years) | (in thousands) | |||||||||||
| Outstanding at December 31, 2019 | 250,000 | 6.23 | $ | 149 | ||||||||
| Cancelled | 250,000 | 5.23 | $ | 15 | ||||||||
| Outstanding at December 31, 2020 | - | - | $ | - | ||||||||
The aggregate intrinsic value at December 31, 2019 noted in the table above represents the closing price of the Company’s common stock multiplied by the number of PSUs outstanding.
During the years ended December 31, 2020 and 2019, the Company recognized no stock-based compensation related to performance-based vesting of PSUs.
13. Retirement Plans
The Company has a 401(k) retirement and savings plan (“401(k) Plan”) covering all qualified U.S. employees. The 401(k) Plan is a defined contribution plan and allows each participant to contribute up to 100% of the participant’s base wages up to an amount not to exceed an annual statutory maximum. The Company has made discretionary contributions to the 401(k) Plan and recorded expenses of approximately $68 and $48 thousand for the years ended December 31, 2020 and 2019, respectively.
The Company maintains a defined contribution plan for certain international employees. The Company contributes 100% of the cost of the defined contribution. The Company recorded expenses of approximately $3 and $9 thousand for the years ended December 31, 2020 and 2019, respectively, under this plan.
14. Income Taxes
Income (loss) before provision for income taxes consisted of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Domestic | $ | (11,291 | ) | $ | (17,376 | ) | ||
| Foreign | 179 | 88 | ||||||
| Total | $ | (11,112 | ) | $ | (17,288 | ) | ||
F-30
The provision for income taxes in the accompanying consolidated statements of operations consisted of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Current Provision: | ||||||||
| Federal | $ | — | $ | — | ||||
| State | 1 | 1 | ||||||
| Foreign | 20 | 42 | ||||||
| Total | 21 | 43 | ||||||
| Deferred Provision: | ||||||||
| Federal | — | — | ||||||
| State | — | — | ||||||
| Foreign | 3 | 2 | ||||||
| Total | 3 | 2 | ||||||
| Total provision | $ | 24 | $ | 45 | ||||
A reconciliation of income taxes from operations computed using the U.S. federal statutory rate of 21% to that reflected in operations follows (in thousands):
Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Income tax benefit using U.S. federal statutory rate | $ | (2,334 | ) | $ | (3,624 | ) | ||
| State rate, net of federal benefit | (644 | ) | (631 | ) | ||||
| Permanent differences | 159 | 1,422 | ||||||
| Tax credits generated | (131 | ) | (147 | ) | ||||
| Change in valuation allowance | 2,595 | 2,888 | ||||||
| Foreign rate differential | (11 | ) | 3 | |||||
| Other items | 122 | 96 | ||||||
| Stock compensation | 268 | 38 | ||||||
| Total income tax expense | $ | 24 | $ | 45 | ||||
F-31
Components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 69,942 | $ | 66,859 | ||||
| Research and development credit carryforwards | 4,150 | 4,083 | ||||||
| Capitalized start-up expenses | 1,778 | 2,133 | ||||||
| Depreciation and other | 431 | 635 | ||||||
| Total deferred tax assets | 76,302 | 73,710 | ||||||
| Valuation allowance | (76,298 | ) | (73,703 | ) | ||||
| Net deferred tax asset | $ | 4 | $ | 7 | ||||
Management of the Company has evaluated the positive and negative evidence bearing upon the realizability of the Company’s deferred tax assets and determined that it is more likely than not that the Company will not recognize the benefits of the deferred tax assets related to the U.S. As a result, a valuation allowance of approximately $76.3 million and $73.7 million was established at December 31, 2020 and 2019, respectively. The valuation allowance increased by approximately $2.6 million during the year ended December 31, 2020, primarily due to the current year change in temporary tax items. As of December 31, 2020, there was a net deferred tax asset in Australia related to future tax benefits which will offset future taxable income.
At December 31, 2020, the Company had U.S. federal and state net operating loss carryforwards of approximately $260 million and $242.5 million, respectively. These operating loss carryforwards will expire at various times beginning in 2024 through 2037 for federal purposes except for $37 million that were generated between 2018 and 2020 that can be carried forward indefinitely. For state purposes the net operating losses begin to expire in 2030 and will continue to expire through 2040.
In addition, at December 31, 2020, the Company also has U.S. federal and state research and development tax credit carryforwards (excluding ASC 740, Income Taxes (“ASC 740”), reserve) of approximately $4 million and $2.0 million, respectively, to offset future income taxes. These tax credit carryforwards will expire at various times beginning in 2023 through 2040 for federal purposes and will expire at various times beginning in 2019 through 2035 for state purposes.
Utilization of net operating loss carryforwards and research and development credit carryforwards may be subject to a substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future in accordance with Section 382 of the Internal Revenue Code of 1986 (“IRC Section 382”) and with Section 383 of the Internal Revenue Code of 1986, as well as similar state provisions. These ownership changes may limit the amount of net operating loss carryforwards and research and development credit carryforwards that can be utilized annually to offset future taxable income and taxes, respectively. In general, an ownership change, as defined by IRC Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. The Company has completed several financings since its inception, which may have resulted in a change in control as defined by IRC Section 382 or could result in a change in control in the future. As of December 31, 2020, the Company has not, as yet, conducted an IRC Section 382 study, which could impact its ability to utilize net operating loss and tax credit carryforwards annually in the future to offset the Company’s taxable income, if any.
F-32
The Company applies ASC 740-10, which provides guidance on the accounting for uncertainty in income taxes recognized in financial statements and requires the impact of a tax position to be recognized in the financial statements if that position is more likely than not of being sustained by the taxing authority. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. At December 31, 2020 and 2019, the Company had unrecognized tax liabilities of approximately $1.5 million and $1.5 million, respectively.
The following is a roll forward of the Company’s unrecognized tax benefits (in thousands):
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| Unrecognized tax benefit – as of the beginning of the year | $ | 1,480 | $ | 1,462 | ||||
| Gross decreases – provision to return tax positions of the prior periods | (13 | ) | (21 | ) | ||||
| Gross increases – current period tax positions | 35 | 39 | ||||||
| Unrecognized tax benefits – as of the end of the year | $ | 1,502 | $ | 1,480 | ||||
The Company will recognize interest and penalties related to uncertain tax positions, should they be assessed, in income tax expense. As of December 31, 2020, and 2019, the Company had no accrued interest or penalties related to uncertain tax positions, and no amounts have been recognized in the Company’s consolidated statements of operations.
The statute of limitations for assessment by the Internal Revenue Service (“IRS”) and state tax authorities is open for tax years ended December 31, 2017 through December 31, 2020, although carryforward attributes that were generated prior to tax year 2017 may still be adjusted upon examination by the IRS or state tax authorities if they either have been or will be used in a future period. The statute of limitations for assessment by foreign tax authorities is open for tax years ended December 31, 2016 through December 31, 2020. There are currently no federal or state audits in progress.
The Company has not yet completed a study of its research and development credit carryforwards. Once completed, this study may result in an adjustment to the Company’s research and development credit carryforwards. A full valuation allowance has been provided against the Company’s research and development credits, and if an adjustment is required at the time the study is completed, this adjustment would be offset by an adjustment to the deferred tax asset established for the research and development credit carryforward and the valuation allowance.
15. Subsequent Events
As discussed in Note 11 to the Consolidated Financial Statements, pursuant to the Series A SPA and subsequent amendments, the Second Close of the Series A Preferred Stock offering was originally to occur on October 31, 2020 but through a series of extensions, was extended until February 24, 2021. On February 24, 2021, an individual investor closed the purchase of 600,000 shares of Series A Preferred Stock for approximate proceeds of $53 thousand. Effective February 24, 2021, Crystal Amber executed a contract amendment restructuring the Second Close to include the sale of 16,924,292 shares of Series A Preferred Stock for proceeds of $1.5 million to close on March 4, 2021, the sale of 11,282,861 shares of Series A Preferred Stock for proceeds of $1.0 million to close no later than March 25, 2021, and the remaining 27,607,153 shares of Series A Preferred Stock for proceeds of $2.45 million to close no later than May 28, 2021.
As discussed in Notes 2 and 12 to the Consolidated Financial Statements, On January 28, 2021, the Board of Directors granted 15 option awards to employees, board members, and a consultant, representing the right to purchase a total of 24,484,059 shares of common stock for a strike price of $0.06 per share, which is the fair value determined by the Board of Directors.
F-33
