Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - EAGLE PHARMACEUTICALS, INC. | tm218403d1_ex99-2.htm |
| EX-99.1 - EXHIBIT 99.1 - EAGLE PHARMACEUTICALS, INC. | tm218403d1_ex99-1.htm |
| 8-K - FORM 8-K - EAGLE PHARMACEUTICALS, INC. | tm218403d1_8k.htm |
Exhibit 99.3

1 1 INVESTOR Update March 2, 2021

2 Forward - Looking Statements This presentation contains forward - looking information within the meaning of the Private Securities Litigation Reform Act of 199 5, as amended, and other securities laws. Forward - looking statements are statements that are not historical facts. Words and phrases such as “anticipated,” “forward,” “will,” “wo uld,” “may,” “remain,” “potential,” “prepare,” “expected,” “believe,” “plan,” “near future,” “belief,” “guidance,” and similar expressions are intended to identify forward - loo king statements. These statements include, but are not limited to, statements regarding future events such as: the number and timing of potential product launches, development init iat ives or new indications for the Company’s product candidates; the period of market exclusivity for any of the Company’s product candidates; the Company's clinical development pla n for its fulvestrant product candidate, EA - 114, as well as the development efforts for the other product candidates in its portfolio; the potential benefits and efficacy of RYA NODEX, including the potential for RYANODEX as a treatment for nerve agent exposure and additional indications; the timing, scope or likelihood of regulatory filings and appr ova ls from the FDA for the Company’s product candidates; the timing of the Company's PEMFEXY launch, if ever; the success of the Company's collaborations with its strate gic partners and the timing and results of these partners’ preclinical studies and clinical trials, including the Company’s collaboration with its Japanese licensing partner, Sy mBio, with respect to the commercialization of SymBio’s product TREAKISYM, and the timing of the potential product launch of TREAKISYM; the ability of the Company’s fulvest ran t product candidate, EA - 114, to improve clinical outcomes for post - menopausal metastatic breast cancer patients; the Company’s timing and ability to enroll patients in ongoing and upcoming clinical trials; the ability of the Company to obtain and maintain coverage and adequate reimbursement for its products; the implementation of certain health car e reform measures; the Company's timing and ability to repurchase additional shares of the Company's common stock, if any, under its share repurchase program; the Compan y's ability to deliver value in 2021 and over the long term; and the Company's plans and ability to advance the products in its pipeline. All of such statements are subject to ce rtain risks and uncertainties, many of which are difficult to predict and generally beyond the Company's control, that could cause actual results to differ materially from th ose expressed in, or implied or projected by, the forward - looking information and statements. Such risks and uncertainties include, but are not limited to: the impacts of the COVID - 19 pa ndemic, including disruption or impact in the sales of the Company's marketed products, interruptions or other adverse effects to clinical trials, delays in regulatory review, m anu facturing and supply chain interruptions, adverse effects on healthcare systems, disruption in the operations of the Company's third party partners and disruption of the globa l e conomy, and the overall impact of the COVID - 19 pandemic on the Company's business, financial condition and results of operations; risks that the Company's business, financi al condition and results of operations will be impacted by the spread of COVID - 19 in the geographies where the Company's third - party partners operate; whether the Company will incur unforeseen expenses or liabilities or other market factors; risks that results from in vitro laboratory tests of RYANODEX are not necessarily predictive of future cli nical trial and in vivo results; whether the Company will successfully implement its development plan for its fulvestrant product candidate, EA - 114, or other product candidates; del ay in or failure to obtain regulatory approval of the Company's product candidates; whether the Company can successfully market and commercialize its product candidates; the succe ss of the Company's relationships with its partners, including the University of Pennsylvania, Teva, Tyme, NorthShore University HealthSystem and SymBio and the parties' ability to work effectively together; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of our produc ts or that may have an impact on any of our products; successful compliance with the FDA and other governmental regulations applicable to product approvals, manufacturing faciliti es, products and/or businesses; general economic conditions, including the potential adverse effects of public health issues, including the COVID - 19 pandemic, on economic activi ty and the performance of the financial markets generally; the strength and enforceability of the Company's intellectual property rights or the rights of third parties; comp eti tion from other pharmaceutical and biotechnology companies and the potential for competition from generic entrants into the market; the risks inherent in the early stages of dru g development and in conducting clinical trials; and those risks and uncertainties identified in the “Risk Factors” sections of the Company's Annual Report on Form 10 - K for the year ended December 31, 2020, which the Company expects to file on March 2, 2021, as updated by the company’s subsequent filings with the Securities and Exchange Commission. Re aders are cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof, and the Company does not undertake any oblig ation to revise and disseminate forward - looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non - occurrence of any ev ents.

3 3 EA - 114 (Fulvestrant)

4 EA - 114 (Fulvestrant) Insights and Path Forward ▪ Eagle conducted two clinical trials comparing Eagle’s formulation to Faslodex® ▪ These studies followed 750 subjects for 140 - 280 days ▪ Analyzed thousands of collected data points and discerned the need for a distinctive delivery system ▪ Sought KOL feedback on proposed delivery approach ▪ Based on Eagle’s work and clinical insight, we believe that a significant population of patients may clinically benefit from this new approach to fulvestrant delivery ▪ Have agreement with the FDA on trial design and study endpoints ▪ Following additional formulation work we intend to begin a new clinical trial in patients, which will harness the lessons learned from our in - depth work and clinical insights Our goal is to introduce a new approach to fulvestrant delivery that provides an efficient path to approval

5 5 Nerve Agent Medical Countermeasure
6 Eagle’s Nerve Agent Medical Countermeasure Program Eagle has put forth a significant amount of effort in developing Ryanodex for the treatment of brain damage secondary to nerve agent toxicity. Proof of Concept study using rat soman model conducted at MRIGlobal ( EGL - DTL - NC - 1704 ) Pivotal Good Laboratory Practices (GLP) study using rat soman model conducted at Research Laboratories of USAMRICD ( USAMRICD - SR - 1 - 19 - U - 1091 ) Results of these studies have demonstrated that Ryanodex - treated animals exhibited lower neuronal necrosis in brain cortical areas compared to animals treated with standard therapy alone.
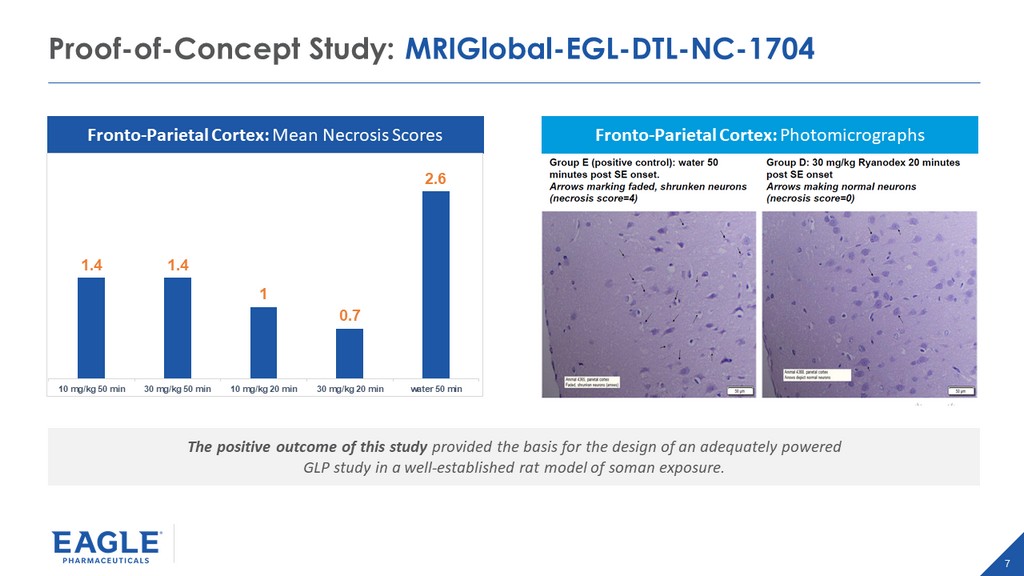
7 Proof - of - Concept Study: MRIGlobal - EGL - DTL - NC - 1704 The positive outcome of this study provided the basis for the design of an adequately powered GLP study in a well - established rat model of soman exposure. Fronto - Parietal Cortex: Photomicrographs Fronto - Parietal Cortex: Mean Necrosis Scores 1.4 1.4 1 0.7 2.6 10 mg/kg 50 min 30 mg/kg 50 min 10 mg/kg 20 min 30 mg/kg 20 min water 50 min
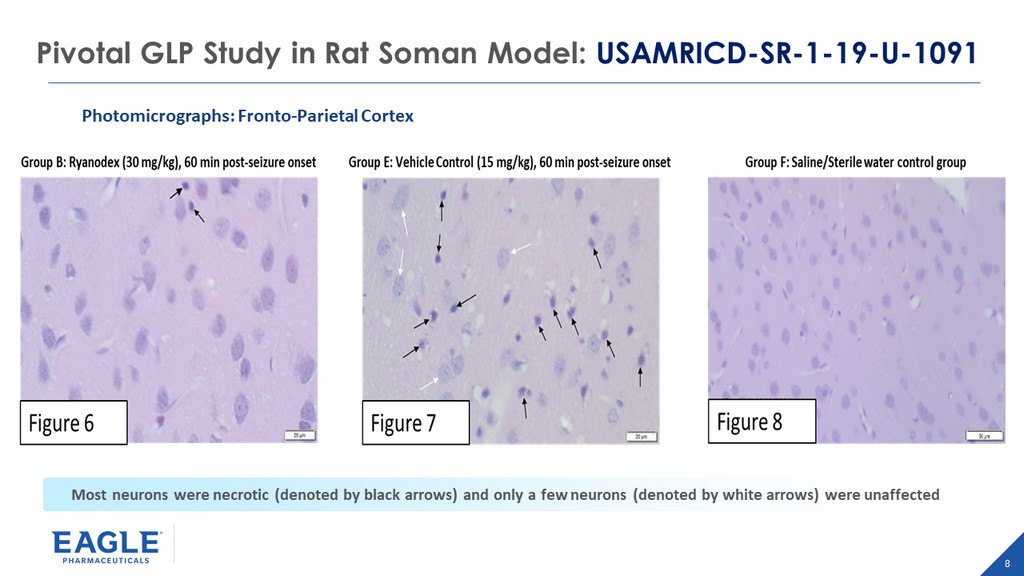
8 Pivotal GLP Study in Rat Soman Model: USAMRICD - SR - 1 - 19 - U - 1091 Photomicrographs: Fronto - Parietal Cortex Most neurons were necrotic (denoted by black arrows) and only a few neurons (denoted by white arrows) were unaffected
9 Nerve Agent Medical Countermeasure Program Next Steps 9 We are planning further studies in Non - Human Primate soman models The FDA agrees with our model selection and has requested that Eagle submit a SPA for review prior to conducting the pivotal GLP studies Eagle will conduct preliminary PK/PD studies in soman model animals to fully understand the efficacy and dose range of Ryanodex to enable maximum benefit in such indications The pivotal GLP study potentially will be a PK/PD evaluation of Ryanodex in the characterized NHP Soman model to demonstrate efficacy and to help predict human dosing Eagle is also evaluating EA - 111 for an IM route of administration in parallel to the IV dosing approach of Ryanodex
