Attached files
| file | filename |
|---|---|
| EX-99.3 - EXHIBIT 99.3 - EAGLE PHARMACEUTICALS, INC. | tm218403d1_ex99-3.htm |
| EX-99.1 - EXHIBIT 99.1 - EAGLE PHARMACEUTICALS, INC. | tm218403d1_ex99-1.htm |
| 8-K - FORM 8-K - EAGLE PHARMACEUTICALS, INC. | tm218403d1_8k.htm |
Exhibit 99.2

1 1 INVESTOR Update March 2, 2021

2 Forward - Looking Statements This presentation contains forward - looking information within the meaning of the Private Securities Litigation Reform Act of 199 5, as amended, and other securities laws. Forward - looking statements are statements that are not historical facts. Words and phrases such as “anticipated,” “forward,” “will,” “wo uld,” “may,” “remain,” “potential,” “prepare,” “expected,” “believe,” “plan,” “near future,” “belief,” “guidance,” and similar expressions are intended to identify forward - loo king statements. These statements include, but are not limited to, statements regarding future events such as: the number and timing of potential product launches, development init iat ives or new indications for the Company’s product candidates; the period of market exclusivity for any of the Company’s product candidates; the Company's clinical development pla n for its fulvestrant product candidate, EA - 114, as well as the development efforts for the other product candidates in its portfolio; the potential benefits and efficacy of RYA NODEX, including the potential for RYANODEX as a treatment for nerve agent exposure and additional indications; the timing, scope or likelihood of regulatory filings and appr ova ls from the FDA for the Company’s product candidates; the timing of the Company's PEMFEXY launch, if ever; the success of the Company's collaborations with its strate gic partners and the timing and results of these partners’ preclinical studies and clinical trials, including the Company’s collaboration with its Japanese licensing partner, Sy mBio, with respect to the commercialization of SymBio’s product TREAKISYM, and the timing of the potential product launch of TREAKISYM; the ability of the Company’s fulvest ran t product candidate, EA - 114, to improve clinical outcomes for post - menopausal metastatic breast cancer patients; the Company’s timing and ability to enroll patients in ongoing and upcoming clinical trials; the ability of the Company to obtain and maintain coverage and adequate reimbursement for its products; the implementation of certain health car e reform measures; the Company's timing and ability to repurchase additional shares of the Company's common stock, if any, under its share repurchase program; the Compan y's ability to deliver value in 2021 and over the long term; and the Company's plans and ability to advance the products in its pipeline. All of such statements are subject to ce rtain risks and uncertainties, many of which are difficult to predict and generally beyond the Company's control, that could cause actual results to differ materially from th ose expressed in, or implied or projected by, the forward - looking information and statements. Such risks and uncertainties include, but are not limited to: the impacts of the COVID - 19 pa ndemic, including disruption or impact in the sales of the Company's marketed products, interruptions or other adverse effects to clinical trials, delays in regulatory review, m anu facturing and supply chain interruptions, adverse effects on healthcare systems, disruption in the operations of the Company's third party partners and disruption of the globa l e conomy, and the overall impact of the COVID - 19 pandemic on the Company's business, financial condition and results of operations; risks that the Company's business, financi al condition and results of operations will be impacted by the spread of COVID - 19 in the geographies where the Company's third - party partners operate; whether the Company will incur unforeseen expenses or liabilities or other market factors; risks that results from in vitro laboratory tests of RYANODEX are not necessarily predictive of future cli nical trial and in vivo results; whether the Company will successfully implement its development plan for its fulvestrant product candidate, EA - 114, or other product candidates; del ay in or failure to obtain regulatory approval of the Company's product candidates; whether the Company can successfully market and commercialize its product candidates; the succe ss of the Company's relationships with its partners, including the University of Pennsylvania, Teva, Tyme, NorthShore University HealthSystem and SymBio and the parties' ability to work effectively together; the availability and pricing of third party sourced products and materials; the outcome of litigation involving any of our produc ts or that may have an impact on any of our products; successful compliance with the FDA and other governmental regulations applicable to product approvals, manufacturing faciliti es, products and/or businesses; general economic conditions, including the potential adverse effects of public health issues, including the COVID - 19 pandemic, on economic activi ty and the performance of the financial markets generally; the strength and enforceability of the Company's intellectual property rights or the rights of third parties; comp eti tion from other pharmaceutical and biotechnology companies and the potential for competition from generic entrants into the market; the risks inherent in the early stages of dru g development and in conducting clinical trials; and those risks and uncertainties identified in the “Risk Factors” sections of the Company's Annual Report on Form 10 - K for the year ended December 31, 2020, which the Company expects to file on March 2, 2021, as updated by the company’s subsequent filings with the Securities and Exchange Commission. Re aders are cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof, and the Company does not undertake any oblig ation to revise and disseminate forward - looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non - occurrence of any ev ents.

3 Non - GAAP Financial Performance Measures In addition to financial information prepared in accordance with U.S. GAAP, this press release also contains adjusted non - GAAP n et income and adjusted non - GAAP earnings per share attributable to Eagle. The Company believes these measures provide investors and management with supplemental informati on relating to operating performance and trends that facilitate comparisons between periods and with respect to projected information. Adjusted non - GAAP net income excludes amortization expense, stock - based compensation expense, depreciation expense, expense rela ted to collaboration with Tyme Technologies, Inc, severance, non - cash interest expense, fair value adjustments on equity investment, fair value adjustments on unsettled accelerated share repurchase agreement and the tax effect of these adjustments. The Company believes these non - GAAP financial measures help indicate underlyi ng trends in the Company’s business and are important in comparing current results with prior period results and understanding projected operating performance. Non - GAAP financial measures provide the Company and its investors with an indication of the Company’s baseline performance before items that are considered by the Company not to be reflective of the Company’s ongoing results. These adjusted measures are non - GAAP and should be considered in addition to, but not as a substitute for, the information prepa red in accordance with U.S. GAAP. The Company strongly encourages investors to review its consolidated financial statements and publicly - filed reports in their entire ty and cautions investors that the non - GAAP measures used by the Company may differ from similar measures used by other companies, even when similar terms are used to id ent ify such measures.
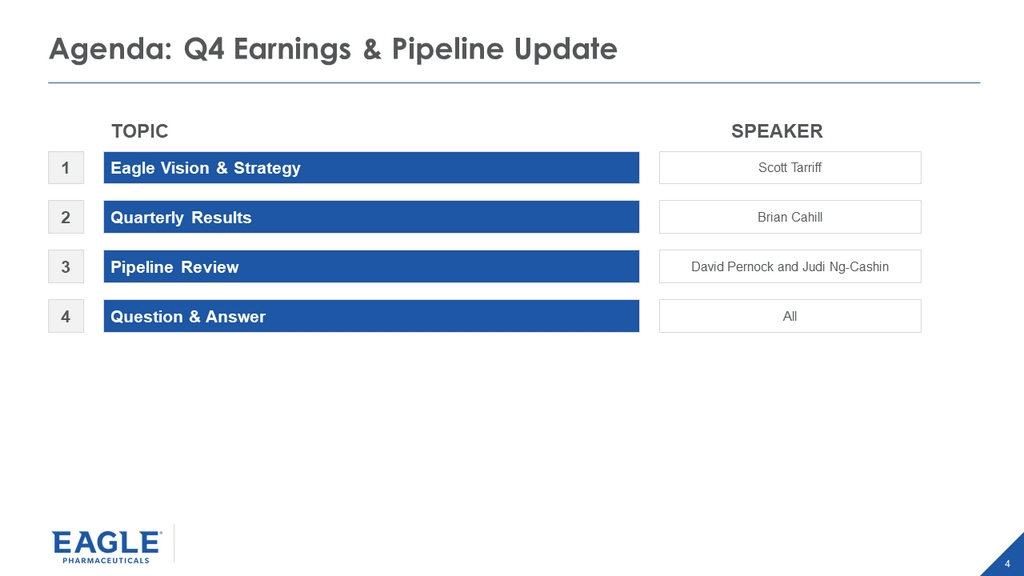
4 Agenda: Q4 Earnings & Pipeline Update Eagle Vision & Strategy Quarterly Results Pipeline Review Question & Answer 1 2 3 4 Scott Tarriff Brian Cahill David Pernock and Judi Ng - Cashin All SPEAKER TOPIC

5 Todays Speakers Scott Tarriff Founder, Chief Executive Officer and Director Brian Cahill Chief Financial Officer Judi Ng - Cashin Chief Medical Officer David Pernock Chief Operating Officer

6 6 Eagle Vision & Strategy Scott Tarriff

7 Strong Foundation for Potential Long - Term Growth Invested $231mm (21%+ of revenue) in R&D (2013 – 2020) $257mm in cash flow from operations (2015 – 2020) $207mm repurchased since August 2016; approx. 13 mm basic shares outstanding as of December 2020 Highly Efficient Business Model Successful Capital Reinvestment Sustainable Profitability Robust Balance Sheet No net debt and flexibility to actively deploy capital for opportunities

8 8 Q4 2020 Results Brian Cahill

9 9 Pipeline Review David Pernock and Judi Ng - Cashin

10 10 External Partnerships David Pernock

11 External Partnerships SM - 88 • In 2020 Eagle and TYME entered into a share purchase agreement and a co - promotion agreement for SM - 88 • SM - 88 is a novel investigational agent in two Phase II/III studies for pancreatic cancer • For SM - 88 Eagle shall earn 15% of U.S. net sales and will be responsible for 25% of the promotional effort • Tyme may buy out Eagle’s rights at any time under the co - promotion agreement for $200mm • SymBio received approval of TREAKISYM Ready - To - Dilute (“RTD”) bendamustine formulation and launched in January 2021 • SymBio is currently conducting a clinical trial for a rapid infusion bendamustine product and pursuing additional indications • Eagle earns tiered royalties on net sales of licensed products and a sales milestone for potential peak revenue of $10mm to $25mm

12 12 PEMFEXY Ρ
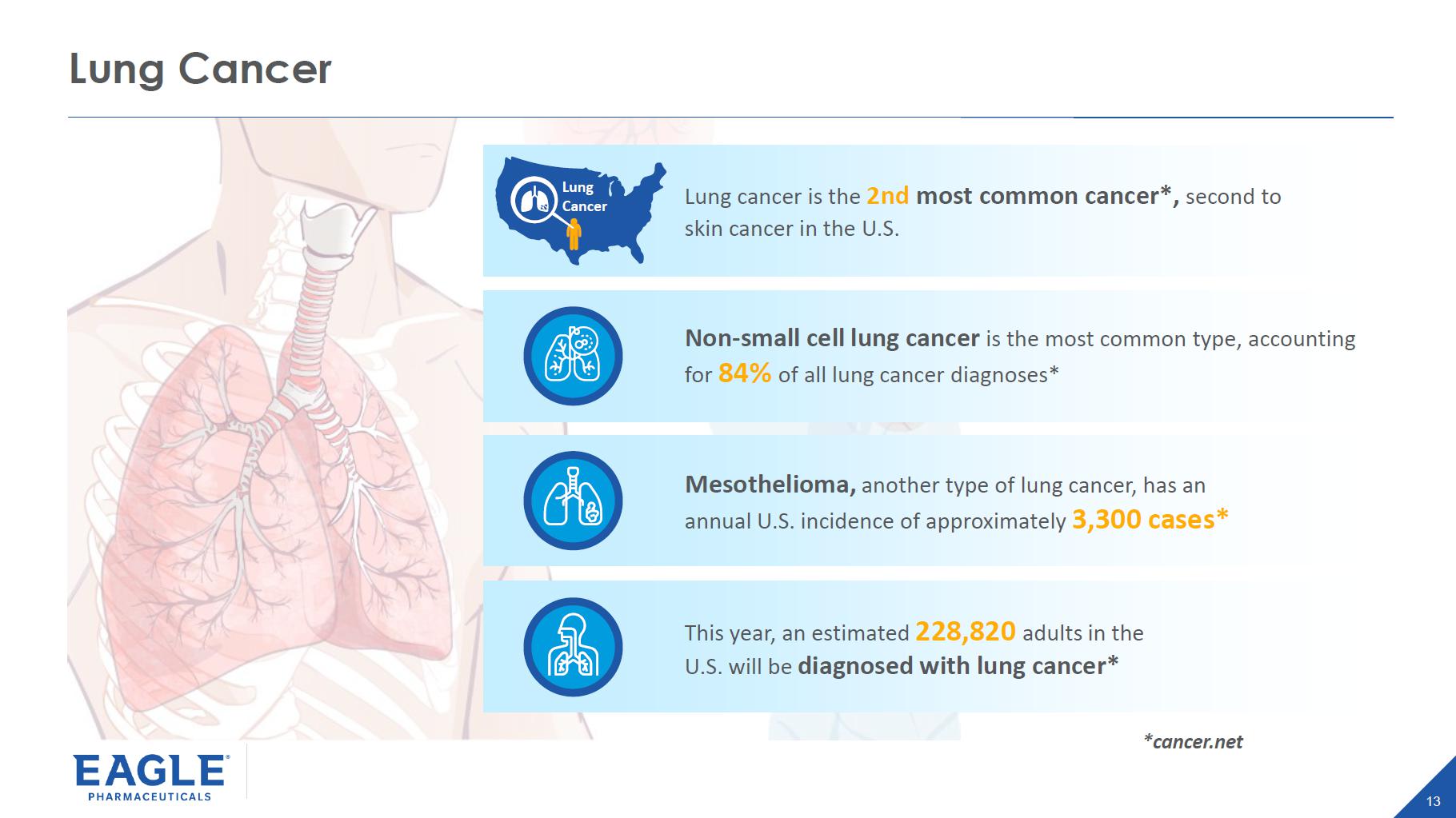
13 Lung Cancer *cancer.net Lung cancer is the 2nd most common cancer*, second to skin cancer in the U.S. Non - small cell lung cancer is the most common type, accounting for 84% of all lung cancer diagnoses* Mesothelioma, another type of lung cancer, has an annual U.S. incidence of approximately 3,300 cases* This year, an estimated 228,820 adults in the U.S. will be diagnosed with lung cancer* Lung Cancer

14 PEMFEXY ΠIs FDA - Approved For: Nonsquamous Non - Small Cell Lung Cancer Nonsquamous Non - Small Cell Lung Cancer in combination with cisplatin for initial treatment or locally in combination for advanced or metastatic disease Nonsquamous Non - Small Cell Lung Cancer maintenance, when disease has not progressed after four cycles of platinum - based first - line chemotherapy Nonsquamous Non - Small Cell Lung Cancer after prior chemotherapy as a single agent for locally advanced or metastatic disease Mesothelioma Mesothelioma in combination with cisplatin for malignant pleural mesothelioma when disease is unresectable.

15 Currently marketed by Lilly as ALIMTA® (pemetrexed) 100mg and 500mg powder single dose vials ALIMTA ® (Eli Lilly) - PEMFEXY Œ (Eagle) – U.S. Sales of $1.265B* 1.2B Eagle first to market 505(b)(2) PEMFEXY Ρ (pemetrexed) 500mg liquid multi - dose vial – Granted unique J - code by CMS – Launch planned February 2022 – Generic entrants blocked until May 24, 2022 *Eli Lilly Q4 2020 Earnings

16 Eagle’s Differentiated PEMFEXY Œ (pemetrexed): Other pemetrexed formulations are single - dose powder, which require reconstitution Eagle’s formulation is available in a 500mg liquid ready - to - dilute multi - dose vial Some patients may need 2 - 3 vials; time - consuming for pharmacist/nurse and wastage occurs frequently because they are not multi - dose vials PEMFEXY Ρ eliminates the reconstitution process wastage and helps prevent medication errors. The vial can be reused under refrigeration for 28 days.
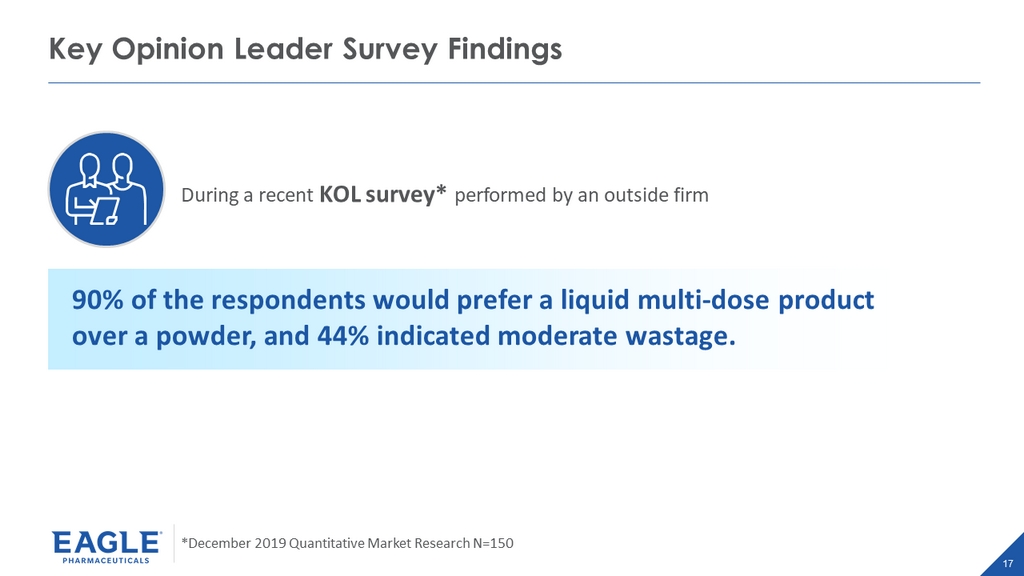
17 Key Opinion Leader Survey Findings During a recent KOL survey* performed by an outside firm 90% of the respondents would prefer a liquid multi - dose product over a powder, and 44% indicated moderate wastage. *December 2019 Quantitative Market Research N=150

18 18 Vasopressin
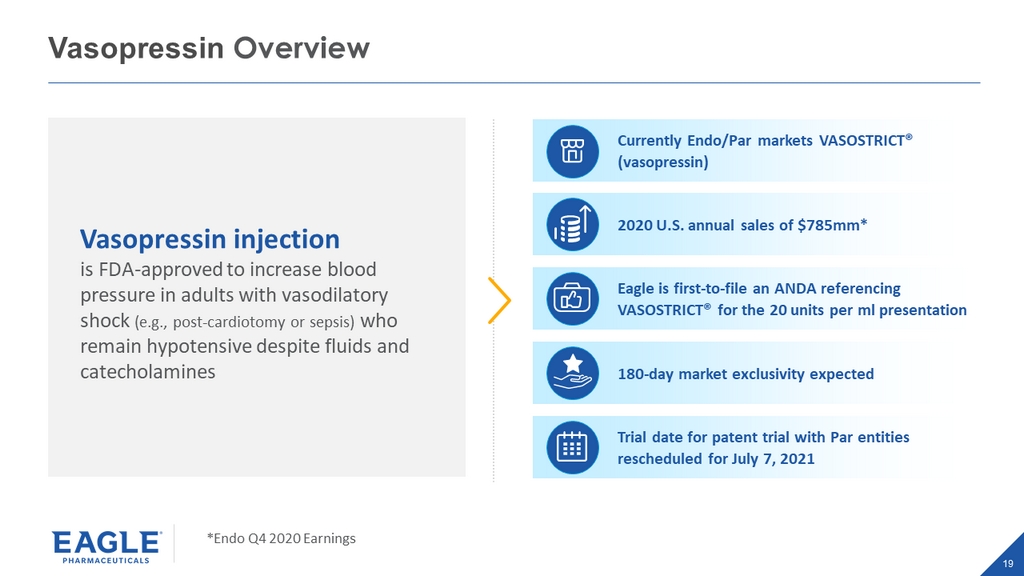
19 Vasopressin Overview Currently Endo/Par markets VASOSTRICT® (vasopressin) 2020 U.S. annual sales of $785mm* Eagle is first - to - file an ANDA referencing VASOSTRICT® for the 20 units per ml presentation 180 - day market exclusivity expected Trial date for patent trial with Par entities rescheduled for July 7, 2021 Vasopressin injection is FDA - approved to increase blood pressure in adults with vasodilatory shock (e.g., post - cardiotomy or sepsis) who remain hypotensive despite fluids and catecholamines *Endo Q4 2020 Earnings

20 20 EA - 114 (Fulvestrant)
21 Current Treatment Landscape in HR+ / HER2 – Breast Cancer Blocking ovarian function permanently with surgery or temporary with drugs called gonadotropin - releasing hormone (GnRH) agonists Blocking estrogen production using drugs called aromatase inhibitors (AI), such as letrozole, anastrozole and exemestane. These agents block the activity of an enzyme called aromatase which the body uses to make estrogen. Selective Estrogen Receptor Modulators (SERM) – Selective Estrogen Receptor Modulators (SERMS) such as tamoxifen and toremifene bind to estrogen receptors, preventing estrogen from binding. Selective Estrogen Receptor Down - Regulators (SERDs) – Selective Estrogen Receptor Down - Regulators (SERDs) work in a somewhat different way to block estrogen’s effects. SERDs are antiestrogens that have the main characteristic of being pure receptor antagonists. Fulvestrant is the only FDA approved SERD that downregulates estrogen receptors. Treatment Strategies for Hormone - Sensitive Breast Cancer

22 EA - 114 (Fulvestrant) Insights and Path Forward ▪ Eagle conducted two clinical trials comparing Eagle’s formulation to Faslodex® ▪ These studies followed 750 subjects for 140 - 280 days ▪ Analyzed thousands of collected data points and discerned the need for a distinctive delivery system ▪ Sought KOL feedback on proposed delivery approach ▪ Based on Eagle’s work and clinical insight, we believe that a significant population of patients may clinically benefit from this new approach to fulvestrant delivery ▪ Have agreement with the FDA on trial design and study endpoints ▪ Following additional formulation work we intend to begin a new clinical trial in patients, which will harness the lessons learned from our in - depth work and clinical insights Our goal is to introduce a new approach to fulvestrant delivery that provides an efficient path to approval

23 23 Nerve Agent Medical Countermeasure

24 RYANODEX Potential for Nerve Agent (NA) Exposure Nerve agents are the most toxic of the known chemical warfare agents • Rapid treatment with available agents decreases risk of mortality but does not ameliorate risk of brain damage. NA survivors may experience permanent neurologic damage and death • Agreement with the United States Army Medical Research Institute of Chemical Defense (USAMRICD) to evaluate the neuroprotective effects of RYANODEX in an accepted NA model • Results of study conducted with USAMRICD demonstrated a statistically significant reduction in brain damage secondary to NA exposure in RYANODEX - treated animals, compared with controls (p value ≤0.04) • Initiating dose ranging studies in another animal model using IV administration of RYANODEX®; will include an arm using an IM formulation of EA - 111. Preliminary results expected to allow the Company to update its SPA (Special Protocol Assessment) with FDA First - of - its - kind neuroprotective medical countermeasure for the amelioration of brain damage due to nerve agent exposure and, if approved, may receive orphan drug exclusivity (ODE) for organophosphate exposure

25 EA - 111 (New Chemical Entity) Developing the next generation of ryanodine receptor antagonists • Significant benefits of an intramuscular (IM) formulation • EA - 111 would allow for easier and more rapid administration in emergency situations (military and civilian) • Enables point - of - care administration to patients in need • Eliminates need for IV - infusion
26 Eagle’s Nerve Agent Medical Countermeasure Program Eagle has put forth a significant amount of effort in developing Ryanodex for the treatment of brain damage secondary to nerve agent toxicity. Proof of Concept study using rat soman model conducted at MRIGlobal ( EGL - DTL - NC - 1704 ) Pivotal Good Laboratory Practices (GLP) study using rat soman model conducted at Research Laboratories of USAMRICD ( USAMRICD - SR - 1 - 19 - U - 1091 ) Results of these studies have demonstrated that Ryanodex - treated animals exhibited lower neuronal necrosis in brain cortical areas compared to animals treated with standard therapy alone.

27 Proof - of - Concept Study: MRIGlobal - EGL - DTL - NC - 1704 The positive outcome of this study provided the basis for the design of an adequately powered GLP study in a well - established rat model of soman exposure. Fronto - Parietal Cortex: Photomicrographs Fronto - Parietal Cortex: Mean Necrosis Scores 1.4 1.4 1 0.7 2.6 10 mg/kg 50 min 30 mg/kg 50 min 10 mg/kg 20 min 30 mg/kg 20 min water 50 min

28 Pivotal GLP Study in Rat Soman Model: USAMRICD - SR - 1 - 19 - U - 1091 Photomicrographs: Fronto - Parietal Cortex Most neurons were necrotic (denoted by black arrows) and only a few neurons (denoted by white arrows) were unaffected

29 Nerve Agent Medical Countermeasure Program Next Steps 29 We are planning further studies in Non - Human Primate soman models The FDA agrees with our model selection and has requested that Eagle submit a SPA for review prior to conducting the pivotal GLP studies Eagle will conduct preliminary PK/PD studies in soman model animals to fully understand the efficacy and dose range of Ryanodex to enable maximum benefit in such indications The pivotal GLP study potentially will be a PK/PD evaluation of Ryanodex in the characterized NHP Soman model to demonstrate efficacy and to help predict human dosing Eagle is also evaluating EA - 111 for an IM route of administration in parallel to the IV dosing approach of Ryanodex

30 30 Question & Answer

31 31 Thank You!
