Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aquestive Therapeutics, Inc. | brhc10018765_8k.htm |
Exhibit 99.1

January 2021 Aquestive TherapeuticsCorporate Presentation

Forward Looking Statement 2 This presentation includes forward-looking statements within
the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believe,” “anticipate,” “plan,” “expect,” “estimate,” “intend,” “may,” “will,” or the negative of those terms, and similar expressions, are intended to identify
forward-looking statements. These forward-looking statements include, but are not limited to, statements regarding therapeutic benefits and plans and objectives for regulatory approvals of Libervant™ and AQST-108; ability to address the
concerns identified in the FDA’s Complete Response Letter dated September 25, 2020 regarding the New Drug Application for Libervant and obtain FDA approval of Libervant for U.S. market access; ability to obtain FDA approval and advance
AQST-108, Libervant and our other product candidates to the market; about our growth and future financial and operating results and financial position; regulatory approval and pathway; clinical trial timing and plans; our and our competitors’
orphan drug approval and resulting drug exclusivity for our products or products of our competitors; short-term and long-term liquidity and cash requirements, cash funding and cash burn; business strategies, market opportunities, and other
statements that are not historical facts. These forward-looking statements are also subject to the uncertain impact of the COVID-19 global pandemic on our business including with respect to our clinical trials including site initiation, patient
enrollment and timing and adequacy of clinical trials; on regulatory submissions and regulatory reviews and approvals of our product candidates; pharmaceutical ingredient and other raw materials supply chain, manufacture, and distribution; sale
of and demand for our products; our liquidity and availability of capital resources; customer demand for our products and services; customers’ ability to pay for goods and services; and ongoing availability of an appropriate labor force and
skilled professionals. Given these uncertainties, the Company is unable to provide assurance that operations can be maintained as planned prior to the COVID-19 pandemic. These forward-looking statements are based on our current expectations and
beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Such risks and uncertainties include, but are not limited to, risks
associated with the Company's development work, including any delays or changes to the timing, cost and success of our product development activities and clinical trials and plans; risk of delays in FDA approval of Libervant and our other drug
candidates or failure to receive approval; risk of our ability to demonstrate to the FDA “clinical superiority” within the meaning of the FDA regulations of our drug candidate Libervant relative to FDA-approved diazepam rectal gel and nasal
spray products including by establishing a major contribution to patient care within the meaning of FDA regulations relative to the approved products as well as risks related to other potential pathways or positions which are or may in the
future be advanced to the FDA to overcome the seven year orphan drug exclusivity granted by the FDA for the approved nasal spray product of a competitor in the U.S. and there can be no assurance that we will be successful; risk that a
competitor obtains FDA orphan drug exclusivity for a product with the same active moiety as any of our other drug products for which we are seeking FDA approval and that such earlier approved competitor orphan drug blocks such other product
candidates in the U.S. for seven years for the same indication; risk inherent in commercializing a new product (including technology risks, financial risks, market risks and implementation risks and regulatory limitations); risks and
uncertainties concerning the royalty and other revenue stream of the KYNMOBI® monetization, achievement of royalty targets worldwide or in any jurisdiction and certain other commercial targets required for contingent payments under the
monetization transaction, and of sufficiency of net proceeds of the monetization transaction after satisfaction of and compliance with 12.5% Senior Notes obligations, as applicable, and for funding the Company’s operations; risk of development
of our sales and marketing capabilities; risk of legal costs associated with and the outcome of our patent litigation challenging third party at risk generic sale of our proprietary products; risk of sufficient capital and cash resources,
including access to available debt and equity financing and revenues from operations, to satisfy all of our short-term and longer term cash requirements and other cash needs, at the times and in the amounts needed; risk of failure to satisfy
all financial and other debt covenants and of any default; risk related to government claims against Indivior for which we license, manufacture and sell Suboxone® and which accounts for the substantial part of our current operating revenues;
risk associated with Indivior’s cessation of production of its authorized generic buprenorphine naloxone film product, including the impact from loss of orders for the authorized generic product and risk of eroding market share for Suboxone and
risk of sunsetting product; risks related to the outsourcing of certain marketing and other operational and staff functions to third parties; risk of the rate and degree of market acceptance of our product and product candidates; the success of
any competing products, including generics; risk of the size and growth of our product markets; risks of compliance with all FDA and other governmental and customer requirements for our manufacturing facilities; risks associated with
intellectual property rights and infringement claims relating to the Company's products; risk of unexpected patent developments; the impact of existing and future legislation and regulatory provisions on product exclusivity; legislation or
regulatory actions affecting pharmaceutical product pricing, reimbursement or access; claims and risks that may arise regarding the safety or efficacy of the Company's products and product candidates; risk of loss of significant customers;
risks related to legal proceedings, including patent infringement, investigative and antitrust litigation matters; changes in government laws and regulations; risk of product recalls and withdrawals; uncertainties related to general economic,
political, business, industry, regulatory and market conditions and other unusual items; and other uncertainties affecting the Company described in the “Risk Factors” section and in other sections included in our Annual Report on Form 10 K, in
our Quarterly Reports on Form 10-Q, and in our Current Reports on Form 8-K filed with the Securities Exchange Commission (SEC). Given those uncertainties, you should not place undue reliance on these forward-looking statements, which speak only
as of the date made. All subsequent forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by this cautionary statement. The Company assumes no obligation to update
forward-looking statements or outlook or guidance after the date of this press release whether as a result of new information, future events or otherwise, except as may be required by applicable law.PharmFilm® and the Aquestive logo are
registered trademarks of Aquestive Therapeutics, Inc. All other registered trademarks referenced herein are the property of their respective owners.

Advancing Medicines to Improve Therapeutics Proven track record of success in developing,
obtaining FDA approval, and manufacturing differentiated therapeutics with PharmFilm® technologies5 FDA approved products, both proprietary and out-licensed Capital, including current cash, and revenue from licensed & proprietary
therapeutics extends cash into 3Q21 and potentially beyondExecuted KYNMOBITM monetization agreement providing up to $125 million for royalty rightsVarious capital sources may extend runway further Advancing late-stage pipeline of
differentiated therapeutics for complex conditionsReceived CRL from FDA for LIBERVANTTM in Sept. 2020, Type A meeting held on Nov. 12th and revised modeling submitted to FDA in Dec. 2020Received FDA Fast Track designation for AQST-108
(epinephrine) in August 2020 and outlined topline results from second pilot PK study in Jan. 2021 3 Libervant™ (diazepam) buccal film is an investigational drug being evaluated for use in children and adults with refractory seizures, who
remain on stable regimens of antiepileptic drugs, to control bouts of increased seizure activity. The product profile, data from our trials, and related statements have not been approved by the FDA. Aquestive has received conditional acceptance
of the use of this trade name, which is subject to final FDA review and acceptance.

PharmFilm®: Usable Medication for Undertreated Patients 4 VS Can deliver rapid onset of
action with entry into systemic circulation Ease of administration Demonstrated bioequivalence, safety and tolerability Non-invasive DiastatRectal Gel EpinephrineInjection Customizable taste masking profile Uniform
distribution & reproducible delivery of API's AlternativeNasal Sprays

Track Record of Success Innovative formulation capabilities Novel and transformative
products Expertise in oral transmucosal permeationScalable technology for consistent performance Clinical and regulatory success5 FDA approved products, 2 under FDA review, several in developmentMultiple clinical studies planned200+ worldwide
patents Commercial success in collaboration Commercial success on our own Suboxone® Sublingual Film Buprenorphine/Naloxone (Opioid Dependence)Global licenseOver 2 billion doses manufactured Exservan™ Oral Film Riluzole (ALS)*Zuplenz®
Ondansetron (CINV/PINV) SYMPAZAN® Oral Film Clobazam (Lennox-Gastaut syndrome)FDA approved in October 2018 Prescriber base exceeds 750 HCPsPrecursor for LIBERVANT™ launch with significant overlap in prescribersFor information and Boxed
WARNING: https://www.sympazan.com/pdfs/pi.pdf 5 *Exservan is in EMA approval process

Diversified Portfolio and Pipeline AQST-108 (Epinephrine)(Anaphylaxis)FDA completed safety
review of IND; completed dosing in Phase 1 PK trial in Oct. 2020 Pre-Clinical Clinical Filed Marketed Exservan™ Oral Film (Riluzole)(ALS)Zambon EU license / Seeking US licensee LIBERVANT™ (Diazepam) Buccal Film(Refractory Seizures)CRL
received on Sept. 25, 2020; Type A meeting held Nov. 12; agreed to a follow-up FDA meeting prior to resubmission Suboxone® Sublingual Film (Buprenorphine/Naloxone) (Opioid Dependence)Indivior license FDA Approved 2010 Zuplenz® (Ondansetron)
(CINV/PINV)Fortivia US license & Hypera Brazil License FDA Approved 2010 SYMPAZAN® Oral Film (Clobazam)(Lennox-Gastaut syndrome)Launched in December 2018 FDA Approved 2018 *Aquestive holds rights for worldwide
commercialization 6 LICENSED COMMERCIAL PRODUCTS AND PIPELINE CANDIDATES PROPRIETARY GROWTH DRIVERS* AQST-305* (Octreotide)(Acromegaly/Carcinoid Syndrome)Reformulation after 1st round Human POC studies KynmobiTM Sublingual Film
(Apomorphine HCI)(Parkinson’s)Sunovion License FDA Approved 2020 FDA Approved 2019

15 Solving problems in EPILEPSY: LIBERVANTTM (diazepam) Buccal Film In development for
management of selected, refractory, patients with epilepsy, on stable regimens of AEDs, who require intermittent use of diazepam to control bouts of increased seizure activityPotential to become the preferred rescue medication by patients and
providers looking for clinically differentiated treatment in an oral dosage form ≈92%of patients with refractory seizures will not interact with the historically available treatment2 ≈1M suffer from
uncontrolled, Epilepsypatients1 refractory seizures 7 1M EMERGENCY DEPARTMENTvisits annually3 Seizuresaccount for

Historic Rescue Market – Highly Fragmented2 8
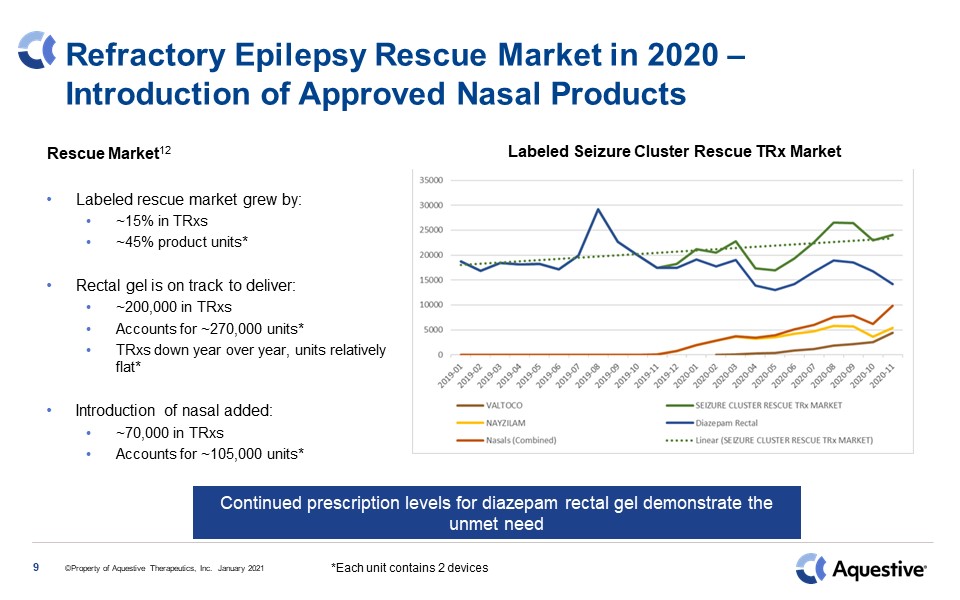
Refractory Epilepsy Rescue Market in 2020 – Introduction of Approved Nasal Products Rescue
Market12Labeled rescue market grew by:~15% in TRxs~45% product units*Rectal gel is on track to deliver:~200,000 in TRxsAccounts for ~270,000 units*TRxs down year over year, units relatively flat*Introduction of nasal added:~70,000 in
TRxsAccounts for ~105,000 units* 9 Continued prescription levels for diazepam rectal gel demonstrate the unmet need Labeled Seizure Cluster Rescue TRx Market *Each unit contains 2 devices
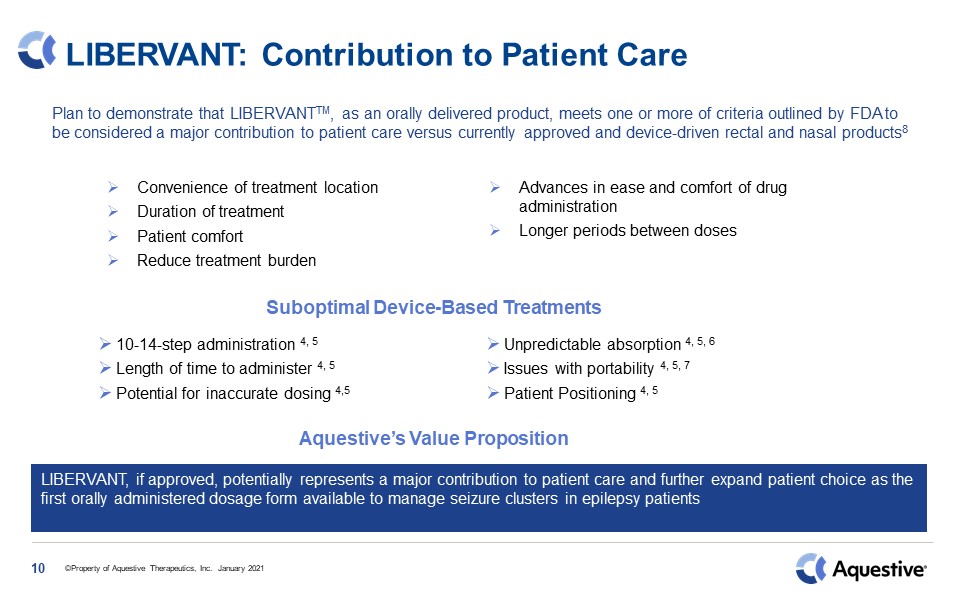
LIBERVANT: Contribution to Patient Care Plan to demonstrate that LIBERVANTTM, as an orally
delivered product, meets one or more of criteria outlined by FDA to be considered a major contribution to patient care versus currently approved and device-driven rectal and nasal products8 10 Convenience of treatment locationDuration of
treatmentPatient comfortReduce treatment burdenAdvances in ease and comfort of drug administrationLonger periods between doses LIBERVANT, if approved, potentially represents a major contribution to patient care and further expand patient
choice as the first orally administered dosage form available to manage seizure clusters in epilepsy patients Suboptimal Device-Based Treatments Aquestive’s Value Proposition 10-14-step administration 4, 5Length of time to administer 4,
5Potential for inaccurate dosing 4,5Unpredictable absorption 4, 5, 6Issues with portability 4, 5, 7Patient Positioning 4, 5

LIBERVANT Commentary 11 “…what was found in the usability studies9 was that a caregiver had
no trouble administering this into the buccal space.…They are at school and at work, but they always have a concern that a seizure could occur…We don’t have an FDA-approved therapy that they can carry with them.” – Michael Rogawski, MD, PhD,
Professor of Neurology and Pharmacology, School of Medicine, University of California, Davis “I will say from a pediatric standpoint, getting a child to put something in their nose and hold it still is very difficult.”– Syndi Seinfeld, DO, MS,
Director of Epilepsy, Joe DiMaggio Children’s Hospital , Miami, FL Portability Convenience of treatment location Advances in ease and comfort of administration, patient comfort

LIBERVANT: Received CRL from FDA, Plan to Resubmit 1H21 12 In PK Crossover Study 180323,
FDA acknowledged overall Cmax geometric mean ratio (GMR) of LibervantTM versus Diastat was comparableFDA cited that two weight groups showed lower Cmax levels when compared to Diastat:51-62 kg (n=6) (12.5 mg Libervant/ 15.0 mg Diastat)76-87 kg
(n=7) (15.0 mg Libervant / 17.5 mg Diastat)FDA noted that 4 subjects in the weight groups identified above and 1 additional subject in the 63-75 kg weight group had a median Cmax level that was approximately half of the median Diastat level
(180 ng/mL vs. 375 ng/mL respectively)No other safety, clinical pharmacology / biopharmaceutics, CMC or other non-clinical issues identified in CRL*At a Type A meeting held November 12, 2020, FDA confirmed issues identified in the CRL related
to the NDA for drug candidate LIBERVANT™ (diazepam) Buccal Film may be addressed by utilizing modeling and simulations based upon the information provided by Aquestive in its FDA meeting package submitted in October 2020Aquestive resubmitted a
revised weight-based dosing regimen along with modeling and simulations in December 2020Based on correspondence from the FDA, Aquestive expects to receive feedback and guidance from the FDA in late January, which may require the need for
additional meetings with the FDAAquestive intends to resubmit the NDA as quickly as possible, based on further FDA feedback, during the first half of 2021 *The FDA cited a small number of protocol deviations in blood draws in one of the studies
in the NDA which the Company believes have been addressed in its FDA submitted meeting package.

Difficult administrationPainful intramuscular injectionsInconvenient portability Suboptimal
TreatmentEpiPen® ≈$1.5B market with ~3M total prescriptions13 AQST-108: Solving Problems in ANAPHYLAXIS Oral sublingual film formulation of epinephrine for the treatment of allergic reactions (Type 1), including anaphylaxisPhase 1 dose
escalation proof-of-concept study in healthy subjects demonstrated ability to deliver systemic epinephrine using proprietary PharmFilm® technologiesAt constructive pre-IND meeting held on February 4, 2020, FDA confirmed clinical development
will be reviewed under the regulatory 505(b)(2) pathway as proposed by AquestiveFDA completed safety review of IND in July 2020Received FDA Fast Track designation in August 2020 13 Hospital Admissions by 500-700% in last 10-15
years11 Increased Approximately 186 225 deaths per year12 to Episodes 50to112 Per100,000 people per year11

AQST-108: Solving Problems in ANAPHYLAXIS Encouraging results from two pilot
pharmacokinetic (PK) studiesClinical dosing as a sublingual filmGenerated median Tmax similar to subcutaneous and intramuscular injectionsGeometric mean Cmax ranges between approximately 200 pg/mL and 400 pg/mL depending on dosing strengthAs
comparators, the 0.3mg subcutaneous and IM injections administered in the most recent Phase 1 study resulted in geometric mean Cmax levels of approximately 385 ng/mL and 475 ng/mL respectivelyEpiPen studies indicate a range of potential Cmax
outcomesStudies conducted by Aquestive indicate a Cmax range for EpiPen between 350 pg/mL and 400 pg/mL (n=18, n=9)Recent public presentations indicate a Cmax for EpiPen of approximately 310 pg/mL (n=55)*14Intellect Summary Basis of Approval
(SBA) indicates a Cmax for EpiPen of 520 pg/mL (n=135)Anticipate conducting a virtual R&D event in Q1 2021Outline the market need for new innovationProvide an in-depth understanding of our novel delivery systemOutline clinical results and
upcoming milestones 14

Continue to Expand in Our Epilepsy Franchise Cont’d 15 SYMPAZAN®: Continued Growth
National trend for total prescriptions were down 7% below prior year trends driven by fewer new prescriptionsIn spite of Q2 and Q4 challenges from COVID SYMPAZAN grew ~110% in TRx and ~80% in prescriber base
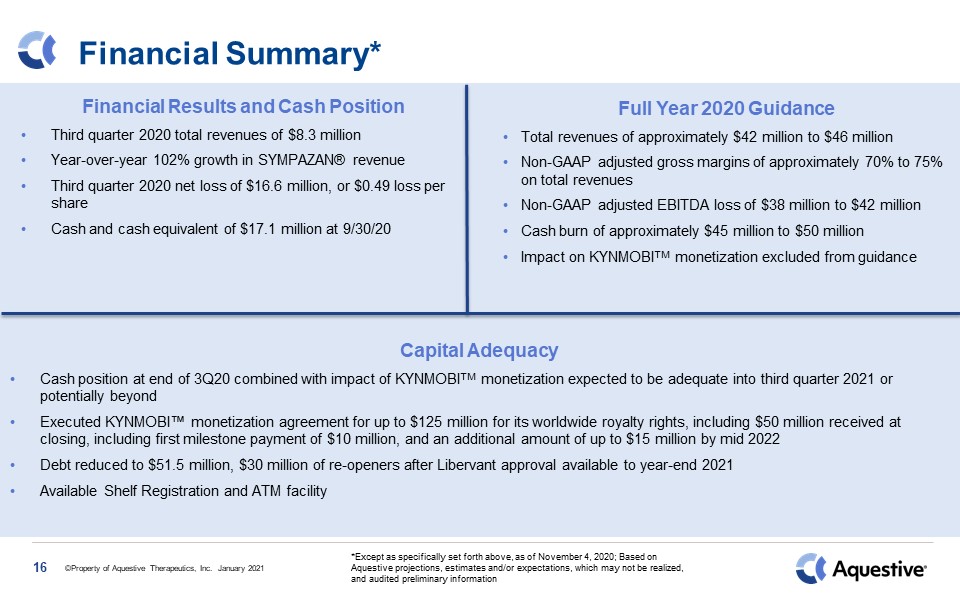
Financial Summary* 16 Financial Results and Cash PositionThird quarter 2020 total
revenues of $8.3 million Year-over-year 102% growth in SYMPAZAN® revenueThird quarter 2020 net loss of $16.6 million, or $0.49 loss per shareCash and cash equivalent of $17.1 million at 9/30/20 Full Year 2020 Guidance Total revenues of
approximately $42 million to $46 millionNon-GAAP adjusted gross margins of approximately 70% to 75% on total revenuesNon-GAAP adjusted EBITDA loss of $38 million to $42 million Cash burn of approximately $45 million to $50 millionImpact on
KYNMOBITM monetization excluded from guidance *Except as specifically set forth above, as of November 4, 2020; Based on Aquestive projections, estimates and/or expectations, which may not be realized, and audited preliminary
information Capital AdequacyCash position at end of 3Q20 combined with impact of KYNMOBITM monetization expected to be adequate into third quarter 2021 or potentially beyondExecuted KYNMOBI™ monetization agreement for up to $125 million for
its worldwide royalty rights, including $50 million received at closing, including first milestone payment of $10 million, and an additional amount of up to $15 million by mid 2022Debt reduced to $51.5 million, $30 million of re-openers after
Libervant approval available to year-end 2021Available Shelf Registration and ATM facility

Our Focus in 2021 Continue to expand in our epilepsy franchiseFocus on the FDA approval
and launch of LIBERVANT™Generate continued growth in SYMPAZAN® prescriptions Continue to strengthen the capital position of the companyEvaluate various paths to capital Advance our novel epinephrine delivery platform Continue to pursue
clinical developmentProgress towards regulatory interactions and filings 17

18 CORPORATE INFORMATION , PHARMFILM® TECHNOLOGY, SYMPAZAN®, LIBERVANT™ AND EPINEPHRINE
DATAData on fileLIBERVANT™ REFRACTORY SEIZURESLaxer, K. et al, The consequences of Refractory Epilepsy and its treatment; Epilepsy & Behavior; Vol 37, Aug 2014, Pgs 59-70; https://doi.org/10.1016/j.yebeh.2014.05.031Triangle Insights Group
(2017). Synthesis of Epilepsy (ARS) Primary Research. Internal Aquestive report: unpublished.Seizure visits to ED: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657249/Diastat administration and disposal instructions www.diastat.comValtoco®
instructions for use www.valtoco.com Cereghino JJ. Identification and treatment of acute repetitive seizures in children and adults. Curr Treat Options Neurol 2007;9(4):249-255.Penovich PE, Buelow J, Steinberg K, Sirven J, Wheless J. Burden of
seizure clusters on patients with epilepsy and caregivers: survey of patient, caregiver, and clinician perspectives. Neurologist 2017;22(6):207-214.See “Orphan Drug Regulations”, Final Rule, Federal Register/Vol.78, No. 113/June 12, 2013.
Usability study conducted by Aquestive Therapeutics. Data on file. (2019).HCP preference study conducted by Aquestive Therapeutics. Data on file. (2019). ANAPHYLAXIS11. Epidemiology of anaphylaxis. Tejedor Alonso MA, Moro M, Mugica Garcia MV,
Clin Exp Allergy. 45(6):1027-39, Jun 201512. Wood, Camargo, et al Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J ALLERGY CLIN IMMUNOL VOLUME 133, Ma L, Danoff TM, Borish L. Case fatality and
population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2013;133(4):1075-83. doi: 10.1016/j.jaci.2013.10.02913. Source: Symphony Health 2020 data on file.14. Pharmacokinetic and Pharmacodynamic Effects of
Intranasal Epinephrine Versus Intramuscular Epinephrine in Adults, David Dworaczyk, PhD and Allen Hunt, MD; Presented at the American Academy of Allergy, Asthma & Immunology (AAAAI) National Conference, March 16, 2020, Philadelphia, PA,
USA References

Thank You
