Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Acutus Medical, Inc. | afib-ex322_7.htm |
| EX-32.1 - EX-32.1 - Acutus Medical, Inc. | afib-ex321_6.htm |
| EX-31.2 - EX-31.2 - Acutus Medical, Inc. | afib-ex312_8.htm |
| EX-31.1 - EX-31.1 - Acutus Medical, Inc. | afib-ex311_9.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark one)
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2020
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-39430
ACUTUS MEDICAL, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
45-1306615 |
|
(State or other jurisdiction of |
(I.R.S. Employer |
|
|
|
|
2210 Faraday Ave., Suite 100, Carlsbad, CA |
92008 |
|
(Address of principal executive offices) |
(zip code) |
(Registrant’s telephone number, including area code) (442) 232-6080
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol |
|
Name of each exchange on which registered |
|
Common Stock, par value $0.001 per share |
|
AFIB |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the registrant has submitted electronically, if any, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
|
☐ |
Accelerated filer |
|
☐ |
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
☒ |
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date.
|
Class of Common Stock |
|
Outstanding Shares as of November 9, 2020 |
|
Common Stock, $0.001 par value |
|
27,846,083 |
ACUTUS MEDICAL, INC. AND SUBSIDIARIES
Form 10-Q
For the Quarter Ended September 30, 2020
Table of Contents
|
|
|
|
|
|
|
Page |
|
PART I. FINANCIAL INFORMATION |
|
|
|
|
|
|
|
Item 1. |
1 |
|
|
|
|
|
|
|
Condensed Consolidated Balance Sheets as of September 30, 2020 (unaudited) and December 31, 2019 |
1 |
|
|
|
|
|
|
2 |
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
Notes to Condensed Consolidated Financial Statements (unaudited) |
6 |
|
|
|
|
|
Item 2. |
Management’s Discussion and Analysis of the Results of Operations |
31 |
|
|
|
|
|
Item 3. |
47 |
|
|
|
|
|
|
Item 4. |
47 |
|
|
|
|
|
|
|
||
|
|
|
|
|
Item 1. |
48 |
|
|
|
|
|
|
Item 1A. |
48 |
|
|
|
|
|
|
Item 2. |
48 |
|
|
|
|
|
|
Item 6. |
49 |
|
|
|
|
|
|
50 |
||
Acutus Medical, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
(in thousands, except share and per share amounts)
|
|
|
September 30, 2020 |
|
|
December 31, 2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
ASSETS: |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
58,302 |
|
|
$ |
9,452 |
|
|
Marketable securities, short-term |
|
|
99,742 |
|
|
|
62,351 |
|
|
Restricted cash |
|
|
150 |
|
|
|
150 |
|
|
Accounts receivable |
|
|
1,893 |
|
|
|
263 |
|
|
Inventory |
|
|
10,932 |
|
|
|
8,424 |
|
|
Prepaid expenses and other current assets |
|
|
4,635 |
|
|
|
1,816 |
|
|
Total current assets |
|
|
175,654 |
|
|
|
82,456 |
|
|
|
|
|
|
|
|
|
|
|
|
Marketable securities, long-term |
|
|
8,789 |
|
|
|
— |
|
|
Property and equipment, net |
|
|
9,940 |
|
|
|
4,427 |
|
|
Right-of-use asset, net |
|
|
1,838 |
|
|
|
2,341 |
|
|
Intangible assets, net |
|
|
3,780 |
|
|
|
4,110 |
|
|
Goodwill |
|
|
12,026 |
|
|
|
12,026 |
|
|
Other assets |
|
|
482 |
|
|
|
95 |
|
|
Total assets |
|
$ |
212,509 |
|
|
$ |
105,455 |
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS' EQUITY (DEFICIT) |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
4,723 |
|
|
$ |
3,882 |
|
|
Accrued liabilities |
|
|
6,500 |
|
|
|
10,076 |
|
|
Contingent consideration, short-term |
|
|
4,000 |
|
|
|
8,200 |
|
|
Operating lease liabilities, short-term |
|
|
907 |
|
|
|
833 |
|
|
Common and preferred stock warrant liability |
|
|
— |
|
|
|
8,919 |
|
|
Total current liabilities |
|
|
16,130 |
|
|
|
31,910 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease liabilities, long-term |
|
|
1,365 |
|
|
|
2,054 |
|
|
Long-term debt |
|
|
38,762 |
|
|
|
38,244 |
|
|
Contingent consideration, long-term |
|
|
3,600 |
|
|
|
5,700 |
|
|
Other long-term liabilities |
|
|
8 |
|
|
|
— |
|
|
Total liabilities |
|
|
59,865 |
|
|
|
77,908 |
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies (Note 12) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible preferred stock |
|
|
|
|
|
|
|
|
|
Series A convertible preferred stock, $0.001 par value; no shares authorized, issued and outstanding as of September 30, 2020; 3,848,696 shares authorized and 391,210 shares issued and outstanding as of December 31, 2019; liquidation preference of $3,245 as of December 31, 2019 |
|
|
— |
|
|
|
3,059 |
|
|
Series B convertible preferred stock, $0.001 par value; no shares authorized, issued and outstanding as of September 30, 2020; 30,032,100 shares authorized and 3,088,444 shares issued and outstanding as of December 31, 2019; liquidation preference of $41,294 as of December 31, 2019 |
|
|
— |
|
|
|
40,685 |
|
|
Series C convertible preferred stock, $0.001 par value; no shares authorized, issued and outstanding as of September 30, 2020; 48,184,000 shares authorized and 4,499,921 shares issued and outstanding as of December 31, 2019; liquidation preference of $75,000 as of December 31, 2019 |
|
|
— |
|
|
|
74,575 |
|
|
Series D convertible preferred stock, $0.001 par value; no shares authorized, issued and outstanding as of September 30, 2020; 90,000,000 shares authorized and 8,200,297 issued and outstanding as of December 31, 2019; liquidation preference of $136,675 as of December 31, 2019 |
|
|
— |
|
|
|
135,039 |
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders' equity (deficit) |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 5,000,000 shares authorized as of September 30, 2020 and no shares authorized as of December 31, 2019; no shares issued and outstanding as of each of September 30, 2020 and December 31, 2019, respectively |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.001 par value; 260,000,000 shares authorized as of each of September 30, 2020 and December 31, 2019; 27,826,408 and 695,902 shares issued and outstanding as of September 30, 2020 and December 31, 2019, respectively |
|
|
28 |
|
|
|
1 |
|
|
Additional paid-in capital |
|
|
484,162 |
|
|
|
33,252 |
|
|
Accumulated deficit |
|
|
(331,613 |
) |
|
|
(259,034 |
) |
|
Accumulated other comprehensive income (loss) |
|
|
67 |
|
|
|
(30 |
) |
|
Total stockholders' equity (deficit) |
|
|
152,644 |
|
|
|
(225,811 |
) |
|
Total liabilities, convertible preferred stock and stockholders' equity (deficit) |
|
$ |
212,509 |
|
|
$ |
105,455 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
1
Acutus Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share amounts)
(Unaudited)
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
Revenue |
|
$ |
3,173 |
|
|
$ |
646 |
|
|
$ |
5,890 |
|
|
$ |
2,167 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of products sold |
|
|
5,141 |
|
|
|
2,267 |
|
|
|
10,998 |
|
|
|
6,878 |
|
|
Research and development |
|
|
8,343 |
|
|
|
5,865 |
|
|
|
24,492 |
|
|
|
15,489 |
|
|
Research and development - license acquired |
|
|
— |
|
|
|
15,000 |
|
|
|
— |
|
|
|
15,000 |
|
|
Selling, general and administrative |
|
|
15,833 |
|
|
|
7,978 |
|
|
|
35,193 |
|
|
|
18,998 |
|
|
Change in fair value of contingent consideration |
|
|
118 |
|
|
|
700 |
|
|
|
(1,466 |
) |
|
|
700 |
|
|
Total costs and operating expenses |
|
|
29,435 |
|
|
|
31,810 |
|
|
|
69,217 |
|
|
|
57,065 |
|
|
Loss from operations |
|
|
(26,262 |
) |
|
|
(31,164 |
) |
|
|
(63,327 |
) |
|
|
(54,898 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrant liability and embedded derivative |
|
|
(3,683 |
) |
|
|
(3 |
) |
|
|
(5,555 |
) |
|
|
(608 |
) |
|
Loss on debt extinguishment |
|
|
— |
|
|
|
(49 |
) |
|
|
— |
|
|
|
(1,447 |
) |
|
Interest income |
|
|
23 |
|
|
|
525 |
|
|
|
393 |
|
|
|
733 |
|
|
Interest expense |
|
|
(1,366 |
) |
|
|
(1,394 |
) |
|
|
(4,090 |
) |
|
|
(20,905 |
) |
|
Total other expense, net |
|
|
(5,026 |
) |
|
|
(921 |
) |
|
|
(9,252 |
) |
|
|
(22,227 |
) |
|
Net loss |
|
$ |
(31,288 |
) |
|
$ |
(32,085 |
) |
|
$ |
(72,579 |
) |
|
$ |
(77,125 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized (loss) gain on marketable securities |
|
|
(9 |
) |
|
|
40 |
|
|
|
(50 |
) |
|
|
47 |
|
|
Foreign currency translation adjustment |
|
|
78 |
|
|
|
(45 |
) |
|
|
147 |
|
|
|
(57 |
) |
|
Comprehensive loss |
|
$ |
(31,219 |
) |
|
$ |
(32,090 |
) |
|
$ |
(72,482 |
) |
|
$ |
(77,135 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per common share, basic and diluted |
|
$ |
(1.95 |
) |
|
$ |
(47.21 |
) |
|
$ |
(12.36 |
) |
|
$ |
(115.66 |
) |
|
Weighted average shares outstanding, basic and diluted |
|
|
16,080,467 |
|
|
|
679,591 |
|
|
|
5,870,861 |
|
|
|
666,823 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
2
Acutus Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands, except share amounts)
(Unaudited)
For the Three Months Ended September 30, 2020
|
|
|
Series A |
|
|
Series B |
|
|
Series C |
|
|
Series D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
Total |
|
||||||||||||||||||||||
|
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Other Comprehensive |
|
|
Equity Stockholders' |
|
|||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Income (Loss) |
|
|
(Deficit) |
|
||||||||||||||
|
Balance as of June 30, 2020 (unaudited) |
|
|
391,210 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
8,593,360 |
|
|
$ |
142,236 |
|
|
|
|
775,403 |
|
|
$ |
1 |
|
|
$ |
36,355 |
|
|
$ |
(300,325 |
) |
|
$ |
(2 |
) |
|
$ |
(263,971 |
) |
|
Unrealized loss on marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(9 |
) |
|
|
(9 |
) |
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
78 |
|
|
|
78 |
|
|
Conversion of convertible preferred stock into common stock upon IPO |
|
|
(391,210 |
) |
|
|
(3,059 |
) |
|
|
(3,088,444 |
) |
|
|
(40,685 |
) |
|
|
(4,499,921 |
) |
|
|
(74,575 |
) |
|
|
(8,593,360 |
) |
|
|
(142,236 |
) |
|
|
|
16,572,935 |
|
|
|
17 |
|
|
|
260,538 |
|
|
|
— |
|
|
|
— |
|
|
|
260,555 |
|
|
Issuance of common stock for cash, net of issuance costs of $16,361 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
10,147,058 |
|
|
|
10 |
|
|
|
166,276 |
|
|
|
— |
|
|
|
— |
|
|
|
166,286 |
|
|
Reclassification of warrant liability to stockholders' equity |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
14,474 |
|
|
|
— |
|
|
|
— |
|
|
|
14,474 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
27,661 |
|
|
|
— |
|
|
|
145 |
|
|
|
— |
|
|
|
— |
|
|
|
145 |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
303,351 |
|
|
|
— |
|
|
|
6,374 |
|
|
|
— |
|
|
|
— |
|
|
|
6,374 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(31,288 |
) |
|
|
— |
|
|
|
(31,288 |
) |
|
Balance as of September 30, 2020 (unaudited) |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
27,826,408 |
|
|
$ |
28 |
|
|
$ |
484,162 |
|
|
$ |
(331,613 |
) |
|
$ |
67 |
|
|
$ |
152,644 |
|
For the Three Months Ended September 30, 2019
|
|
|
Series A |
|
|
Series B |
|
|
Series C |
|
|
Series D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|||||||||||||||||||||
|
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Other Comprehensive |
|
|
Total Stockholders' |
|
|||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Income (Loss) |
|
|
Deficit |
|
||||||||||||||
|
Balance as of June 30, 2019 (unaudited) |
|
|
391,210 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
6,418,437 |
|
|
$ |
106,702 |
|
|
|
|
671,960 |
|
|
$ |
1 |
|
|
$ |
31,569 |
|
|
$ |
(207,035 |
) |
|
$ |
15 |
|
|
$ |
(175,450 |
) |
|
Unrealized gain on marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
40 |
|
|
|
40 |
|
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(45 |
) |
|
|
(45 |
) |
|
Issuance of Series D convertible preferred stock for cash, net of issuance costs of $1,358 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,781,860 |
|
|
|
28,341 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
4,612 |
|
|
|
— |
|
|
|
812 |
|
|
|
— |
|
|
|
— |
|
|
|
812 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
7,452 |
|
|
|
— |
|
|
|
19 |
|
|
|
— |
|
|
|
— |
|
|
|
19 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(32,085 |
) |
|
|
— |
|
|
|
(32,085 |
) |
|
Balance as of September 30, 2019 (unaudited) |
|
|
391,210 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
8,200,297 |
|
|
$ |
135,043 |
|
|
|
|
684,024 |
|
|
$ |
1 |
|
|
$ |
32,400 |
|
|
$ |
(239,120 |
) |
|
$ |
10 |
|
|
$ |
(206,709 |
) |
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
Acutus Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands, except share amounts)
(Unaudited)
For the Nine Months Ended September 30, 2020
|
|
|
Series A |
|
|
Series B |
|
|
Series C |
|
|
Series D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional |
|
|
Total |
|
||||||||||||||||||||||
|
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Other Comprehensive |
|
|
Stockholders' Equity |
|
|||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Income (Loss) |
|
|
(Deficit) |
|
||||||||||||||
|
Balance as of December 31, 2019 |
|
|
391,210 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
8,200,297 |
|
|
$ |
135,039 |
|
|
|
|
695,902 |
|
|
$ |
1 |
|
|
$ |
33,252 |
|
|
$ |
(259,034 |
) |
|
$ |
(30 |
) |
|
$ |
(225,811 |
) |
|
Unrealized loss on marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(50 |
) |
|
|
(50 |
) |
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
147 |
|
|
|
147 |
|
|
Issuance of Series D convertible preferred stock for the Biotronik Asset Purchase |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
273,070 |
|
|
|
5,000 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of Series D convertible preferred stock for the contingent consideration related to the Rhythm Xience Acquisition |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
119,993 |
|
|
|
2,197 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Conversion of convertible preferred stock into common stock upon IPO |
|
|
(391,210 |
) |
|
|
(3,059 |
) |
|
|
(3,088,444 |
) |
|
|
(40,685 |
) |
|
|
(4,499,921 |
) |
|
|
(74,575 |
) |
|
|
(8,593,360 |
) |
|
|
(142,236 |
) |
|
|
|
16,572,935 |
|
|
|
17 |
|
|
|
260,538 |
|
|
|
— |
|
|
|
— |
|
|
|
260,555 |
|
|
Issuance of common stock for cash, net of issuance costs of $16,361 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
10,147,058 |
|
|
|
10 |
|
|
|
166,276 |
|
|
|
— |
|
|
|
— |
|
|
|
166,286 |
|
|
Reclassification of warrant liability to stockholders' equity |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
14,474 |
|
|
|
— |
|
|
|
— |
|
|
|
14,474 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
92,223 |
|
|
|
— |
|
|
|
350 |
|
|
|
— |
|
|
|
— |
|
|
|
350 |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
318,290 |
|
|
|
— |
|
|
|
9,272 |
|
|
|
— |
|
|
|
— |
|
|
|
9,272 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(72,579 |
) |
|
|
— |
|
|
|
(72,579 |
) |
|
Balance as of September 30, 2020 (unaudited) |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
27,826,408 |
|
|
$ |
28 |
|
|
$ |
484,162 |
|
|
$ |
(331,613 |
) |
|
$ |
67 |
|
|
$ |
152,644 |
|
For the Nine Months Ended September 30, 2019
|
|
|
Series A |
|
|
Series B |
|
|
Series C |
|
|
Series D |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|||||||||||||||||||||
|
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
Convertible Preferred Stock |
|
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Accumulated |
|
|
Other Comprehensive |
|
|
Total Stockholders' |
|
|||||||||||||||||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Income (Loss) |
|
|
Deficit |
|
||||||||||||||
|
Balance as of December 31, 2018 |
|
|
388,558 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
656,654 |
|
|
$ |
1 |
|
|
$ |
30,150 |
|
|
$ |
(161,995 |
) |
|
$ |
20 |
|
|
$ |
(131,824 |
) |
|
Unrealized gain on marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
47 |
|
|
|
47 |
|
|
Foreign currency translation adjustment |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(57 |
) |
|
|
(57 |
) |
|
Issuance of Series A preferred stock for cashless warrant exercise |
|
|
2,652 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of Series D convertible preferred stock for cash, net of issuance costs of $1,632 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,091,819 |
|
|
|
66,567 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of Series D convertible preferred stock for 2018 Convertible Notes and 2019 Convertible Notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,108,478 |
|
|
|
68,476 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
11,449 |
|
|
|
— |
|
|
|
2,174 |
|
|
|
— |
|
|
|
— |
|
|
|
2,174 |
|
|
Stock option exercises |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
15,921 |
|
|
|
— |
|
|
|
76 |
|
|
|
— |
|
|
|
— |
|
|
|
76 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(77,125 |
) |
|
|
— |
|
|
|
(77,125 |
) |
|
Balance as of September 30, 2019 (unaudited) |
|
|
391,210 |
|
|
$ |
3,059 |
|
|
|
3,088,444 |
|
|
$ |
40,685 |
|
|
|
4,499,921 |
|
|
$ |
74,575 |
|
|
|
8,200,297 |
|
|
$ |
135,043 |
|
|
|
|
684,024 |
|
|
$ |
1 |
|
|
$ |
32,400 |
|
|
$ |
(239,120 |
) |
|
$ |
10 |
|
|
$ |
(206,709 |
) |
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
Acutus Medical, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(in thousands)
(Unaudited)
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(72,579 |
) |
|
$ |
(77,125 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
Depreciation expense |
|
|
1,754 |
|
|
|
1,676 |
|
|
Amortization of intangible assets |
|
|
330 |
|
|
|
125 |
|
|
Stock-based compensation expense |
|
|
9,272 |
|
|
|
2,174 |
|
|
Amortization of premiums/(accretion of discounts) on marketable securities, net |
|
|
113 |
|
|
|
(100 |
) |
|
Amortization of debt issuance costs |
|
|
518 |
|
|
|
17,438 |
|
|
Amortization of right-of-use assets |
|
|
507 |
|
|
|
470 |
|
|
Research and development - license acquired |
|
|
— |
|
|
|
15,000 |
|
|
Gain on disposal of property and equipment |
|
|
— |
|
|
|
(1 |
) |
|
Loss on debt extinguishment |
|
|
— |
|
|
|
1,447 |
|
|
Change in fair value of warrant liability and embedded derivative |
|
|
5,555 |
|
|
|
608 |
|
|
Change in fair value of contingent consideration |
|
|
(1,466 |
) |
|
|
700 |
|
|
Changes in operating assets and liabilities, net of effect from business combination: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(1,630 |
) |
|
|
(697 |
) |
|
Inventory |
|
|
(1,865 |
) |
|
|
(3,829 |
) |
|
Prepaid expenses and other current assets |
|
|
(2,729 |
) |
|
|
(306 |
) |
|
Other assets |
|
|
(387 |
) |
|
|
(8 |
) |
|
Accounts payable |
|
|
753 |
|
|
|
2,873 |
|
|
Accrued liabilities |
|
|
1,423 |
|
|
|
9,268 |
|
|
Operating lease liabilities |
|
|
(615 |
) |
|
|
(536 |
) |
|
Other long-term liabilities |
|
|
8 |
|
|
|
— |
|
|
Net cash used in operating activities |
|
|
(61,038 |
) |
|
|
(30,823 |
) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
|
Purchases of available-for-sale marketable securities |
|
|
(108,528 |
) |
|
|
(68,735 |
) |
|
Sales of available-for-sale marketable securities |
|
|
17,095 |
|
|
|
— |
|
|
Maturities of available-for-sale marketable securities |
|
|
45,000 |
|
|
|
11,550 |
|
|
Purchases of property and equipment |
|
|
(7,822 |
) |
|
|
(683 |
) |
|
Purchase of research and development license |
|
|
— |
|
|
|
(10,000 |
) |
|
Cash paid, net of cash acquired for the Rhythm Xience Acquisition |
|
|
— |
|
|
|
(3,000 |
) |
|
Net cash used in investing activities |
|
|
(54,255 |
) |
|
|
(70,868 |
) |
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
|
Proceeds from issuance of debt and warrants |
|
|
— |
|
|
|
77,000 |
|
|
Repayment of debt |
|
|
— |
|
|
|
(15,000 |
) |
|
Payment of issuance and extinguishment costs related to debt |
|
|
— |
|
|
|
(2,332 |
) |
|
Payment of contingent consideration |
|
|
(2,636 |
) |
|
|
— |
|
|
Proceeds from issuance of convertible preferred stock, net of issuance costs |
|
|
— |
|
|
|
66,567 |
|
|
Proceeds from issuance of common stock upon IPO, net of issuance costs |
|
|
166,286 |
|
|
|
— |
|
|
Proceeds from stock options exercises |
|
|
350 |
|
|
|
76 |
|
|
Net cash provided by financing activities |
|
|
164,000 |
|
|
|
126,311 |
|
|
|
|
|
|
|
|
|
|
|
|
Effect of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
143 |
|
|
|
(50 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net change in cash, cash equivalents and restricted cash |
|
|
48,850 |
|
|
|
24,570 |
|
|
Cash, cash equivalents and restricted cash, at the beginning of the period |
|
|
9,602 |
|
|
|
9,775 |
|
|
Cash, cash equivalents and restricted cash, at the end of the period |
|
$ |
58,452 |
|
|
$ |
34,345 |
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
Cash paid for income taxes |
|
$ |
— |
|
|
$ |
— |
|
|
Cash paid for interest |
|
$ |
3,526 |
|
|
$ |
4,647 |
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of noncash investing and financing activities: |
|
|
|
|
|
|
|
|
|
Issuance of Series D convertible preferred stock for 2018 Convertible Notes and 2019 Convertible Notes |
|
$ |
— |
|
|
$ |
68,476 |
|
|
Issuance of Series D convertible preferred stock for Biotronik asset purchase |
|
$ |
5,000 |
|
|
$ |
— |
|
|
Issuance of Series D convertible preferred stock for Rhythm Xience Acquisition |
|
$ |
2,197 |
|
|
$ |
— |
|
|
Change in unrealized (gain) loss on marketable securities |
|
$ |
50 |
|
|
$ |
(47 |
) |
|
Warrants issued in conjunction with OrbiMed debt |
|
$ |
— |
|
|
$ |
872 |
|
|
Right-of-use assets exchanged for operating lease liabilities |
|
$ |
— |
|
|
$ |
2,978 |
|
|
Accrued purchase of research and development-license |
|
$ |
— |
|
|
$ |
5,000 |
|
|
Unpaid purchases of property and equipment |
|
$ |
88 |
|
|
$ |
35 |
|
|
Conversion of convertible preferred stock into common stock upon IPO |
|
$ |
260,555 |
|
|
$ |
— |
|
|
Reclassification of warrant liability to stockholders' equity |
|
$ |
14,474 |
|
|
$ |
— |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
5
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 1—Organization and Description of Business
Acutus Medical, Inc. (the “Company”) is an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated. The Company designs, manufactures and markets a range of tools for catheter-based ablation procedures to treat various arrhythmias. The Company’s product portfolio includes novel access sheaths, transseptal crossing tools, diagnostic and mapping catheters, ablation catheters, mapping and imaging consoles and accessories, as well as supporting algorithms and software programs. The Company was incorporated in the state of Delaware on March 25, 2011, and is located in Carlsbad, California.
Reverse Stock Split
The Company’s board of directors approved a reverse split of shares of the Company’s common stock and convertible preferred stock on a 9.724-for-one basis (the “Reverse Stock Split”), which was effected on July 28, 2020. The par value and the number of authorized shares of the convertible preferred stock and common stock were not adjusted in connection with the Reverse Stock Split. All references to common stock, convertible preferred stock, warrants to purchase common stock, warrants to purchase convertible preferred stock, options to purchase common stock, restricted stock units, restricted stock awards, share data, per share data and related information contained in the condensed consolidated financial statements have been retrospectively adjusted to reflect the effect of the Reverse Stock Split for all periods presented. No fractional shares of the Company’s common stock were issued in connection with the Reverse Stock Split. Any fractional share resulting from the Reverse Stock Split was rounded down to the nearest whole share, and any stockholder entitled to a fractional share as a result of the Reverse Stock Split received a cash payment in lieu of receiving fractional shares.
Initial Public Offering
On August 10, 2020, the Company issued 10,147,058 shares of common stock in an initial public offering (“IPO”), which included 1,323,529 shares of common stock issued upon the underwriters’ exercise in full of an option to purchase, at the public offering price less underwriting discounts and commissions, up to an additional 1,323,529 shares. The price to the public for each share was $18.00. The Company received proceeds of $166.3 million from its IPO, net of underwriting discounts and commissions and other offering expenses.
On August 10, 2020, in connection with the closing of the IPO, 391,210 shares of Series A, 3,088,444 shares of Series B, 4,499,921 shares of Series C and 8,593,360 shares of Series D convertible preferred stock, respectively, automatically converted into an equal number of shares of common stock and the warrants to purchase 446,990 shares of Series D convertible preferred stock were automatically converted to an equal number of warrants to purchase common stock at an exercise price of $16.67 per share.
As a result of the IPO, the underwriters’ exercise of their option, and the conversions of the Series A, B, C and D convertible preferred stock, the Company’s total number of outstanding shares increased by 26,719,993 immediately following the closing of the IPO.
Going Concern, Liquidity and Capital Resources
The Company has limited revenue, has incurred operating losses since inception and expects to continue to incur significant operating losses for at least the next several years and may never become profitable. As of September 30, 2020 and December 31, 2019, the Company had an accumulated deficit of $331.6 million and $259.0 million, respectively, and working capital of $159.5 million and $50.5 million, respectively. The Company has historically funded its operations primarily through the sale of debt and equity securities, as well as other indebtedness. With the closing of the Company’s IPO, the Company’s current cash, cash equivalents and marketable securities are sufficient to fund operations for at least the next 12 months. However, the Company may need to raise additional funds through one or more of the following: issuance of additional debt, equity or both. Until such time, if ever, the Company can generate revenue sufficient to achieve profitability, the Company expects to finance its operations through equity or debt financings, which may not be available to the Company on the timing needed or on terms that the Company deems to be favorable. To the extent that the Company raises additional capital through the sale of equity or convertible debt securities, the ownership interest of its stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting the Company’s ability to take specific actions, such as incurring additional
6
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
debt, making acquisitions or capital expenditures or declaring dividends. If the Company is unable to maintain sufficient financial resources, its business, financial condition and results of operations will be materially and adversely affected. The Company may be required to delay, limit, reduce or terminate its product discovery and development activities or future commercialization efforts. There can be no assurance that the Company will be able to obtain the needed financing on acceptable terms or at all.
Beginning in early March 2020, the COVID-19 pandemic and the measures imposed to contain this pandemic disrupted the Company’s business. The effects of the pandemic began to decrease in late April 2020 as electrophysiology labs began reopening and procedure volumes began increasing as compared to COVID-19 related low points in March 2020. The Company could experience similar, or even more sustained, access restrictions or decreases in procedural activities as hospitals continue to deal with the COVID-19 pandemic. If cases of COVID-19 increase and hospitals again prioritize those patients, additional restrictions may be implemented which would adversely impact our business and financial results.
Note 2—Summary of Significant Accounting Policies
Basis of Presentation
The condensed consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial information and the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, they do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, the condensed consolidated financial statements reflect all adjustments, which include only normal recurring adjustments necessary for the fair statement of the balances and results for the periods presented. Certain information and note disclosures normally included in the Company’s annual financial statements prepared in accordance with U.S. GAAP have been condensed or omitted. These condensed consolidated financial statement results are not necessarily indicative of results to be expected for the full fiscal year or any future period.
Principles of Consolidation
The condensed consolidated financial statements include the accounts of Acutus Medical, Inc. and its wholly-owned subsidiary Acutus Medical NV (“Acutus NV”), which was incorporated under the laws of Belgium in August 2013. All intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates and Assumptions
The preparation of the condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and disclosures of contingent assets and liabilities. The most significant estimates and assumptions in the Company’s condensed consolidated financial statements include, but are not limited to, revenue recognition, useful lives of intangible assets, assessment of impairment of goodwill, provisions for income taxes, measurement of operating lease liabilities, and the fair value of common stock, stock options, warrants, intangible assets, contingent consideration and goodwill. These estimates and assumptions are based on current facts, historical experience and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of expenses that are not readily apparent from other sources. Actual results could differ from those estimates.
Segments
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business in one operating segment.
7
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Cash and Cash Equivalents and Restricted Cash
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. All of the Company’s cash equivalents have liquid markets and high credit ratings. The Company maintains its cash in bank deposits and other accounts, the balances of which, at times and as of September 30, 2020 and December 31, 2019, exceeded federally insured limits.
Restricted cash serves as collateral for the Company’s corporate credit card program. The following table reconciles cash, cash equivalents and restricted cash in the condensed consolidated balance sheets to the totals shown on the condensed consolidated statements of cash flows (in thousands):
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
58,302 |
|
|
$ |
9,452 |
|
|
Restricted cash |
|
|
150 |
|
|
|
150 |
|
|
Total cash, cash equivalents and restricted cash |
|
$ |
58,452 |
|
|
$ |
9,602 |
|
Marketable Securities
The Company considers its debt securities to be available-for-sale securities. Available-for-sale securities are classified as cash equivalents or short-term or long-term marketable securities based on the maturity date at time of purchase and their availability to meet current operating requirements. Marketable securities that mature in three months or less from the date of purchase are classified as cash equivalents. Marketable securities, excluding cash equivalents, that mature in one year or less are classified as short-term available-for-sale securities and are reported as a component of current assets.
Securities that are classified as available-for-sale are measured at fair value with temporary unrealized gains and losses reported in other comprehensive loss, and as a component of stockholders’ equity (deficit) until their disposition or maturity. See “Fair Value Measurements” below. The Company reviews all available-for-sale securities at each period end to determine if they remain available-for-sale based on the Company’s current intent and ability to sell the security if it is required to do so. Realized gains and losses from the sale of marketable securities, if any, are calculated using the specific-identification method.
Marketable securities are subject to a periodic impairment review. The Company may recognize an impairment charge when a decline in the fair value of investments below the cost basis is determined to be other-than-temporary. In determining whether a decline in market value is other-than-temporary, various factors are considered, including the cause, duration of time and severity of the impairment, any adverse changes in the investees’ financial condition and the Company’s intent and ability to hold the security for a period of time sufficient to allow for an anticipated recovery in market value. Declines in value judged to be other-than-temporary are included in the Company’s condensed consolidated statements of operations and comprehensive loss. The Company did not record any other-than-temporary impairments related to marketable securities in the Company’s condensed consolidated statements of operations and comprehensive loss for the nine-month periods ended September 30, 2020 and 2019.
Concentrations of Credit Risk and Off-Balance Sheet Risk
Financial instruments that potentially subject the Company to credit risk consist principally of cash, cash equivalents, restricted cash, accounts receivable and marketable securities. Cash and restricted cash are maintained in accounts with financial institutions, which, at times may exceed the Federal depository insurance coverage of $0.25 million. The Company has not experienced losses on these accounts and management believes, based upon the quality of the financial institutions, that the credit risk with regard to these deposits is not significant. The Company’s marketable securities portfolio consists primarily of investments in money market funds, commercial paper and short-term high credit quality corporate debt securities.
8
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Revenue from Contracts with Customers
The Company accounts for revenue earned from contracts with customers under Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (“ASC 606”). The core principle of the revenue standard is that a company should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. The following five steps are applied to achieve that core principle:
|
|
• |
Step 1: Identify the contract with the customer. |
|
|
• |
Step 2: Identify the performance obligations in the contract. |
|
|
• |
Step 3: Determine the transaction price. |
|
|
• |
Step 4: Allocate the transaction price to the performance obligations in the contract. |
|
|
• |
Step 5: Recognize revenue when, or as, the company satisfies a performance obligation. |
The Company usually places its medical diagnostic equipment, AcQMap System, at customer sites under loan agreements and generates revenue from disposable products used with the AcQMap System. Disposable products include AcQMap Catheters and AcQGuide Steerable Sheaths. The Company provides the disposable products in exchange for consideration, which occurs when a customer submits a purchase order and the Company provides disposables at the agreed upon prices in the invoice. Generally, customers purchase disposable products using separate purchase orders after the equipment has been provided to the customer for free with no binding agreement or requirement to purchase any disposable products. The Company also sells the AcQMap System to customers along with software updates on a when-and-if-available basis and equipment service. The Company has elected the practical expedient and accounting policy election to account for the shipping and handling as activities to fulfill the promise to transfer the disposable products and not as a separate performance obligation.
During the three months ended September 30, 2020, the Company entered into deferred equipment agreements that are generally structured such that the Company agrees to provide an AcQMap System at no up-front charge, with title of the device transferring to the customer at the end of the contract term, in exchange for the customer’s commitment to purchase disposables at a specified price over the term of the agreement, which generally ranges from two to four years. The Company determined that the deferred equipment agreements include embedded sales-type leases. The Company allocates contract consideration under deferred equipment agreements containing fixed annual disposable purchase commitments to the underlying lease and non-lease components at contract inception. The Company expenses the cost of the device at the inception of the agreement and records a financial lease asset equal to the gross consideration allocated to the lease. The lease asset will be reduced by payments for minimum disposable purchases that are allocated to the lease.
The following table sets forth the Company’s revenue for disposables and systems/service for the three and nine months ended September 30, 2020 and 2019 (in thousands):
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||||||||||
|
Disposables |
|
$ |
2,179 |
|
|
$ |
643 |
|
|
$ |
4,358 |
|
|
$ |
2,155 |
|
|
Systems |
|
|
965 |
|
|
|
— |
|
|
|
1,485 |
|
|
|
— |
|
|
Service/Other |
|
|
29 |
|
|
|
3 |
|
|
|
47 |
|
|
|
12 |
|
|
Total |
|
$ |
3,173 |
|
|
$ |
646 |
|
|
$ |
5,890 |
|
|
$ |
2,167 |
|
The Company’s contracts only include fixed consideration. There are no discounts, rebates, returns or other forms of variable consideration. Customers are generally required to pay within 30 to 60 days.
The delivery of disposable products are performance obligations satisfied at a point in time. The disposable products are shipped Free on Board (“FOB”) shipping point or FOB destination. For disposable products that are shipped FOB shipping point, the customer has the significant risks and rewards of ownership and legal title to the assets when the disposable products leave the Company’s shipping facilities, thus the customer obtains control and revenue is recognized at that point in time. Revenue is recognized on delivery for disposable products shipped via FOB destination.
9
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The installation and delivery of the AcQMap System is satisfied at a point in time when the installation is complete, which is when the customer can benefit and has control of the system. The Company’s software updates and equipment service performance obligations are satisfied evenly over time as the customer simultaneously receives and consumes the benefits of the Company’s performance for these services throughout the service period.
The Company allocates the transaction price to each performance obligation identified in the contract based on the relative standalone selling price (“SSP”). The Company determines SSP for the purposes of allocating the transaction price to each performance obligation based on the adjusted market assessment approach that maximizes the use of observable inputs, which includes, but is not limited to, transactions where the specific performance obligations are sold separately, list prices, and offers to customers.
The Company’s contracts with customers generally have an expected duration of one year or less, and therefore the Company has elected the practical expedient in ASC 606 to not disclose information about its remaining performance obligations. Any incremental costs to obtain contracts are recorded as selling, general and administrative expense as incurred due to the short duration of the Company’s contracts. The Company’s contract balances consisted solely of accounts receivable as of September 30, 2020 and December 31, 2019.
In May 2020, the Company entered into bi-lateral distribution agreements with Biotronik SE & Co. KG (“Biotronik”) (the “Bi-Lateral Distribution Agreements”). Pursuant to the Bi-Lateral Distribution Agreements, the Company obtained a non- exclusive license to distribute a range of Biotronik’s products and accessories in the United States, Canada, China, Hong Kong and multiple Western European countries under the Company’s private label. Moreover, if an investigational device exemption (“IDE”) clinical trial is required for these products to obtain regulatory approval in the United States, or a clinical trial is required for these products to obtain regulatory approval in China, the Company will obtain an exclusive distribution right in such territories for a term of up to five years commencing on the date of regulatory approval if the Company covers the cost of the IDE or other clinical trial and the Company conducts such study within a specified period. Biotronik also agreed to distribute the Company’s products and accessories in Germany, Japan, Mexico, Switzerland and multiple countries in Asia-Pacific, Eastern Europe, the Middle East and South America. The Company also granted Biotronik a coexclusive right to distribute these products in Hong Kong. Each party will pay to the other party specified transfer prices on the sale of the other party’s products and, accordingly, will earn a distribution margin on the sale of the other party’s products.
The following table provides revenue by geographic location for the three and nine months ended September 30, 2020 and 2019 (in thousands):
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||||||||||
|
United States |
|
$ |
1,867 |
|
|
$ |
147 |
|
|
$ |
3,195 |
|
|
$ |
603 |
|
|
Europe |
|
|
1,306 |
|
|
|
499 |
|
|
|
2,695 |
|
|
|
1,564 |
|
|
Total Revenue |
|
$ |
3,173 |
|
|
$ |
646 |
|
|
$ |
5,890 |
|
|
$ |
2,167 |
|
Inventory
Inventory is comprised of raw materials, direct labor and manufacturing overhead and is stated at the lower of cost (first-in, first-out basis) or net realizable value. The Company recorded write-downs for excess and obsolete inventory based on management’s review of inventories on hand, compared to estimated future usage and sales, shelf-life and assumptions about the likelihood of obsolescence of $37,000 and $0.3 million, for the three months ended September 30, 2020 and 2019, respectively, and $0.1 million and $0.5 million, for the nine months ended September 30, 2020 and 2019, respectively.
Accounts Receivable
Trade accounts receivable are recorded net of allowances for uncollectible accounts. The Company evaluates the collectability of its accounts receivable based on various factors including historical experience, the length of time the receivables are past due and the financial health of the customer. The Company reserves specific receivables if collectability is no longer reasonably assured. Based upon the assessment of these factors, the Company did not record an allowance for uncollectible accounts as of September 30, 2020 and December 31, 2019.
10
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Property and equipment are recorded at cost. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets, generally three to five years, or, in the case of leasehold improvements, over the lesser of the useful life of the related asset or the lease term.
Intangible Assets
Intangible assets consist of acquired developed technology, acquired in-process technology, trademarks and trade names and a customer-related intangible which were acquired as part of the acquisition of Rhythm Xience, Inc. (“Rhythm Xience”) in June 2019. The Company determines the appropriate useful life of its finite-lived intangible assets by performing an analysis of expected cash flows of the acquired assets. Finite-lived intangible assets are amortized over their estimated useful lives using the straight-line method, which approximates the pattern in which the economic benefits are consumed. Acquired in-process technology was classified as an indefinite-lived intangible asset, until the receipt of FDA approval for the technology in January 2020. Once the FDA approval was received, the in-process technology was classified as a finite-lived intangible and amortization for in-process technology began. Indefinite-lived intangible assets are tested for impairment at least annually and are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Indefinite-lived intangible assets are impaired if their estimated fair values are less than their carrying value.
Goodwill
Goodwill represents the excess of the purchase price of an entity over the estimated fair value of the assets acquired and liabilities assumed, and it is presented as goodwill in the accompanying condensed consolidated balance sheets. Under ASC 350, Intangibles – Goodwill and Other (“ASC 350”), goodwill is not amortized but is subject to periodic impairment testing. ASC 350 requires that an entity assign its goodwill to reporting units and test each reporting unit’s goodwill for impairment at least on an annual basis and between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. In the evaluation of goodwill for impairment, which is performed annually during the fourth quarter, the Company first assesses qualitative factors to determine whether the existence of events or circumstances led to a determination that it was more likely than not that the fair value of a reporting unit is less than its carrying amount. If, after assessing the totality of events or circumstances, it is determined that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the Company is required to perform the quantitative goodwill impairment test. The Company has one reporting unit. For the nine months ended September 30, 2020, the qualitative testing did not indicate any impairment for the carrying amount of goodwill.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and equipment and finite-lived intangible assets, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss is recognized when the asset’s carrying value exceeds the total undiscounted cash flows expected from its use and eventual disposition. The amount of the impairment loss is determined as the excess of the carrying value of the asset over its fair value. For the three and nine months ended September 30, 2020 and 2019, the Company determined that there was no impairment of property and equipment or intangible assets.
Foreign Currency Translation and Transactions
The assets, liabilities and results of operations of Acutus NV are measured using their functional currency, the Euro, which is the currency of the primary foreign economic environment in which this subsidiary operates. Upon consolidating this entity with the Company, its assets and liabilities are translated to U.S. dollars at currency exchange rates as of the balance sheet date and its revenues and expenses are translated at the weighted average currency exchange rates during the applicable reporting periods. Translation adjustments resulting from the process of translating this entity’s financial statements are reported in accumulated other comprehensive income (loss) in the condensed consolidated balance sheets and foreign currency translation adjustment in the condensed consolidated statements of operations and comprehensive loss.
Lessee Leases
Effective January 1, 2019, the Company accounts for its lessee leases under ASC 842, Leases (“ASC 842”). Under this guidance, arrangements meeting the definition of a lease are classified as operating or financing leases, and are recorded on the
11
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
condensed consolidated balance sheet as both a right-of-use asset and a lease liability, calculated by discounting fixed lease payments over the lease term at the rate implicit in the lease or the Company’s incremental borrowing rate. Lease liabilities are increased by interest and reduced by payments each period, and the right-of-use asset is amortized over the lease term. For operating leases, interest on the lease liability and the amortization of the right-of-use asset results in straight-line rent expense over the lease term. Variable lease expenses are recorded when incurred.
In calculating the right-of-use asset and lease liability, the Company elects to combine lease and non-lease components. The Company excludes short-term leases having initial terms of 12 months or less from the new guidance as an accounting policy election.
Cost of Products Sold
Cost of products sold includes raw materials, direct labor, manufacturing overhead, shipping and receiving costs and other less significant indirect costs related to the production of the Company’s products.
Research and Development
The Company is actively engaged in new product research and development efforts. Research and development expenses consist primarily of salaries and employee-related costs (including stock-based compensation) for personnel directly engaged in research and development activities, clinical trial expenses, equipment costs, material costs, allocated rent and facilities costs and depreciation. Research and development expenses also include payments for the asset acquisition from Biotronik and VascoMed GmbH (collectively, the “Biotronik Parties”) for certain licenses of patents, technology, know-how rights and equipment (the “Biotronik Asset Acquisition”).
Research and development expenses relating to possible future products are expensed as incurred. The Company also accrues and expenses costs for activities associated with clinical trials performed by third parties as incurred. All other costs relative to setting up clinical trial sites are expensed as incurred. Clinical trial site costs related to patient enrollment are accrued as patients are entered into the trials.
Selling, General and Administrative
Selling, general and administrative (“SG&A”) expenses consist primarily of salaries and employee-related costs (including stock-based compensation) for personnel in sales, executive, finance and other administrative functions, allocated rent and facilities costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting and consulting services, marketing costs and insurance costs. The Company expenses all SG&A costs as incurred.
Fair Value Measurements
Fair value measurements are based on the premise that fair value is an exit price representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the following three-tier fair value hierarchy has been used in determining the inputs used in measuring fair value:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Observable inputs other than Level 1 prices for similar assets or liabilities that are directly or indirectly observable in the marketplace.
Level 3—Unobservable inputs which are supported by little or no market activity and that are financial instruments whose values are determined using pricing models, discounted cash flow methodologies or similar techniques, as well as instruments for which the determination of fair value requires significant judgment or estimation.
Financial instruments measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. Management’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability. The use of different assumptions and/or estimation methodologies may have a material effect on estimated fair values. Accordingly, the fair value estimates disclosed or initial amounts recorded may not be indicative of the amount that the Company or holders of the instruments could realize in a current market
12
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
exchange. There were no transfers made among the three levels in the fair value hierarchy for the three and nine months ended September 30, 2020 and 2019.
As of September 30, 2020 and December 31, 2019, the Company’s cash (excluding cash equivalents which are recorded at fair value on a recurring basis), restricted cash, accounts receivable, accounts payable and accrued expenses were carried at cost, which approximates the fair values due to the short-term nature of the instruments.
The carrying amount of the Company’s long-term debt approximates fair value due to its variable market interest rate and management’s opinion that current rates and terms that would be available to the Company with the same maturity and security structure would be essentially equivalent to that of the Company’s long-term debt.
The following tables classify the Company’s financial assets and liabilities measured at fair value on a recurring basis into the fair value hierarchy as of September 30, 2020 and December 31, 2019 (in thousands):
|
|
|
Fair Value Measured at September 30, 2020 (unaudited) |
|
|
|
|
|
|||||||||
|
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Fair Value at September 30, 2020 |
|
||||
|
Assets included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market securities |
|
$ |
53,063 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
53,063 |
|
|
Marketable securities at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities |
|
|
— |
|
|
|
31,455 |
|
|
|
— |
|
|
|
31,455 |
|
|
U.S. treasury securities |
|
|
— |
|
|
|
14,361 |
|
|
|
— |
|
|
|
14,361 |
|
|
Commercial paper |
|
|
— |
|
|
|
53,926 |
|
|
|
— |
|
|
|
53,926 |
|
|
Asset-backed securities |
|
|
— |
|
|
|
8,789 |
|
|
|
— |
|
|
|
8,789 |
|
|
Total fair value |
|
$ |
53,063 |
|
|
$ |
108,531 |
|
|
$ |
— |
|
|
$ |
161,594 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,600 |
|
|
$ |
7,600 |
|
|
Total fair value |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
7,600 |
|
|
$ |
7,600 |
|
13
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
|
|
|
Fair Value Measured at December 31, 2019 |
|
|
|
|
|
|||||||||
|
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
|
Fair Value at December 31, 2019 |
|
||||
|
Assets included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market securities |
|
$ |
8,901 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
8,901 |
|
|
Marketable securities at fair value |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities |
|
|
— |
|
|
|
28,224 |
|
|
|
— |
|
|
|
28,224 |
|
|
Asset-backed securities |
|
|
— |
|
|
|
17,121 |
|
|
|
— |
|
|
|
17,121 |
|
|
U.S. treasury securities |
|
|
— |
|
|
|
5,032 |
|
|
|
— |
|
|
|
5,032 |
|
|
Commercial paper |
|
|
— |
|
|
|
11,974 |
|
|
|
— |
|
|
|
11,974 |
|
|
Total fair value |
|
$ |
8,901 |
|
|
$ |
62,351 |
|
|
$ |
— |
|
|
$ |
71,252 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities included in: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
13,900 |
|
|
$ |
13,900 |
|
|
Common and preferred stock warrant liability |
|
|
— |
|
|
|
— |
|
|
|
8,919 |
|
|
|
8,919 |
|
|
Total fair value |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
22,819 |
|
|
$ |
22,819 |
|
The fair value of the Company’s money market funds is determined using quoted market prices in active markets for identical assets.
The Company’s portfolio of marketable securities is comprised of commercial paper, asset-backed securities, U.S. treasury securities and short-term highly liquid, high credit quality corporate debt securities. The fair value for the available-for-sale marketable securities is determined based on valuation models using inputs that are observable either directly or indirectly (Level 2 inputs), such as quoted prices for similar assets or liabilities, yield curve, volatility factors, credit spreads, default rates, loss severity, current market and contractual prices for the underlying instruments or debt, broker and dealer quotes, as well as other relevant economic measures.
The following table presents changes in Level 3 liabilities measured at fair value for the nine months ended September 30, 2020 (in thousands):
|
|
|
Common and Preferred Stock Warrant Liability |
|
|
Contingent Consideration |
|
|
Total |
|
|||
|
Balance, December 31, 2019 |
|
$ |
8,919 |
|
|
$ |
13,900 |
|
|
$ |
22,819 |
|
|
Payment of contingent consideration |
|
|
— |
|
|
|
(2,636 |
) |
|
|
(2,636 |
) |
|
Issuance of preferred stock for contingent consideration |
|
|
— |
|
|
|
(2,197 |
) |
|
|
(2,197 |
) |
|
Reclassification of warrant liability to stockholders' equity |
|
|
(14,474 |
) |
|
|
— |
|
|
|
(14,474 |
) |
|
Change in fair value |
|
|
5,555 |
|
|
|
(1,466 |
) |
|
|
4,089 |
|
|
Balance, September 30, 2020 (unaudited) |
|
$ |
— |
|
|
$ |
7,600 |
|
|
$ |
7,600 |
|
Unrealized gains and losses associated with liabilities within the Level 3 category include changes in fair value that were attributable to both observable (e.g., changes in market interest rates) and unobservable (e.g., changes in unobservable long-dated volatilities) inputs.
The fair value of the common and preferred stock warrant liability has been estimated using a Monte Carlo simulation in the first quarter of 2019 and as an output of the Hybrid Method for the second and third quarter of 2019 and the first and second quarter of 2020. The underlying equity included in the Monte Carlo simulation and the Hybrid Method was determined based on the equity
14
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
value implied from the preferred stock transactions and from examination of income and market approaches for measurement dates in which a preferred transaction was not applicable. Additionally, the expected IPO value was considered in the determination of the equity value. The fair value of the warrants was impacted by the model selected as well as assumptions surrounding unobservable inputs including the underlying equity value, risk-free interest rate, expected dividend yield, contractual term and expected volatility. On August 10, 2020 when the warrants were converted and became equity classified warrants, the fair value of the common and preferred stock warrant liability was estimated using a Black-Scholes model. The underlying equity included in the Black-Scholes model was based on the IPO share price of $18.00. The weighted average (in aggregate) significant unobservable inputs (Level 3 inputs) used in measuring the common and preferred stock warrant liability as of August 10, 2020 (date of conversion to equity classified warrants) and December 31, 2019 were as follows:
|
|
|
August 10, |
|
|
December 31, |
|
|
|
2020 |
|
|
2019 |
|
|
|
(unaudited) |
|
|
|
|
Risk-free interest rate |
|
0.39% |
|
|
1.59% - 1.60% |
|
Expected dividend yield |
|
— |
|
|
— |
|
Contractual term in years |
|
7.8 - 8.8 |
|
|
0.7 - 1.0 |
|
Expected volatility |
|
65.0% |
|
|
60.0% - 110.5% |
The fair value of the contingent consideration from the acquisition of Rhythm Xience represents the estimated fair value of future payments due to the sellers of Rhythm Xience based on the achievement of certain milestones and revenue-based targets in certain years. The initial fair value of the revenue-based contingent consideration was calculated through the use of a Monte Carlo simulation using revenue projections for the respective earn-out period, corresponding targets and approximate timing of payments as outlined in the purchase agreement. The analyses used the following assumptions: (i) expected term; (ii) risk-adjusted net sales or earnings; (iii) risk-free interest rate and (iv) expected volatility of earnings. Estimated payments, as determined through the respective model, were further discounted by a credit spread assumption to account for credit risk. The fair value of the milestones-based contingent consideration was determined by probability weighting and discounting to the respective valuation date at the Company’s cost of debt. The Company’s cost of debt was determined by performing a synthetic credit rating for the Company and selecting yields based on companies with a similar credit rating. The contingent consideration is revalued to fair value each period, and any increase or decrease is recorded in operating loss. The fair value of the contingent consideration may be impacted by certain unobservable inputs, most significantly with regard to discount rates, expected volatility and historical and projected performance. Significant changes to these inputs in isolation could result in a significantly different fair value measurement. The weighted average (in aggregate) significant unobservable inputs (Level 3 inputs) used in measuring the contingent consideration from the acquisition of Rhythm Xience as of September 30, 2020 and December 31, 2019 were as follows:
|
|
|
September 30, 2020 |
|
|
December 31, 2019 |
|
|
|
|
|
(unaudited) |
|
|
|
|
|
|
Risk-free interest rate |
|
0.20% |
|
|
1.60% |
|
|
|
Expected term in years |
|
1.0 - 2.0 |
|
|
1.0 - 2.0 |
|
|
|
Expected volatility |
|
20.4% |
|
|
11.8% |
|
|
Stock-Based Compensation
The Company accounts for all stock-based payments to employees and non-employees, including grants of stock options, restricted stock awards (“RSAs”), restricted stock units (“RSUs”) and restricted stock units with non-market performance and service conditions (“PSUs”) to be recognized in the condensed consolidated financial statements, based on their respective grant date fair values. The Company estimates the fair value of stock option grants using the Black-Scholes option pricing model. The RSAs, RSUs and PSUs are valued based on the fair value of the Company’s common stock on the date of grant. The assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. The Company expenses stock-based compensation related to stock options, RSAs and RSUs over the requisite service period. As the PSUs have a performance condition, compensation expense was recognized for each vesting tranche over the respective requisite service period of each tranche upon the IPO closing on August 10, 2020, when the Company’s management deemed it probable that the performance conditions were satisfied. The Company recognized a cumulative true-up adjustment related to PSUs once the conditions became probable of being satisfied as the related service period had been completed in a prior period. All stock-based compensation costs are recorded in cost of products sold, research and development expense or SG&A
15
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
expense in the condensed consolidated statements of operations and comprehensive loss based upon the respective employee’s or non-employee’s roles within the Company. Forfeitures are recorded as they occur. See “Note 16—Stock-Based Compensation” below.
Income Taxes
Income taxes are recorded in accordance with ASC 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the condensed consolidated financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse, and net operating loss (“NOL”) carryforwards and research and development (“R&D”) tax credit carryforwards. Valuation allowances are provided if, based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with the provisions of ASC 740. When uncertain tax positions exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized assuming examination by the taxing authority. The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position as well as consideration of the available facts and circumstances. To date, there have been no interest or penalties charged in relation to the unrecognized tax benefits.
Warrant Liability
The Company accounted for certain common stock warrants and convertible preferred stock warrants outstanding as a liability, in accordance with ASC 815, Derivatives and Hedging (“ASC 815”), at fair value. This liability was subject to re-measurement at each reporting period and upon conversion to equity-classified warrants, and any change in fair value is recognized in the condensed consolidated statements of operations and comprehensive loss. In connection with the IPO on August 10, 2020, the common stock warrants and convertible preferred stock warrants that had been liability-classified, were automatically converted into an equal number of warrants to purchase common stock and became equity-classified. The fair value of the outstanding liability on the conversion date was reclassified to additional paid-in capital in the Company’s condensed consolidated balance sheet.
Asset Acquisitions (Research and Development—License Acquired)
The Company accounts for asset acquisitions, where substantially all of the fair value of the assets acquired is concentrated in a group of similar assets (i.e., intellectual property) and therefore the acquisitions do not constitute a business, in accordance with ASC 805, Business Combinations (“ASC 805”), under the asset acquisition method. Under the asset acquisition method of accounting, the Company is required to fair value the assets transferred. The cost of the assets acquired, including transaction costs, is allocated to the individual assets acquired based on their relative fair values and does not give rise to goodwill.
Business Combinations
The Company accounts for business acquisitions using the acquisition method of accounting based on ASC 805, which requires recognition and measurement of all identifiable assets acquired and liabilities assumed at their fair value as of the date control is obtained. The Company determines the fair value of assets acquired and liabilities assumed based upon its best estimates of the acquisition-date fair value of assets acquired and liabilities assumed in the acquisition. Goodwill represents the excess of the purchase price over the fair value of the net tangible and identifiable intangible assets acquired. Subsequent adjustments to fair value of any contingent consideration are recorded to the Company’s condensed consolidated statements of operations and comprehensive loss.
16
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Accounting Pronouncements to Be Adopted
In June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-13, Financial Instruments – Credit Losses (Topic 326). The ASU sets forth a “current expected credit loss” model which requires the Company to measure all expected credit losses for financial instruments held at the reporting date based on historical experience, current conditions and reasonable supportable forecasts. This replaces the existing incurred loss model and is applicable to the measurement of credit losses on financial assets measured at amortized cost, available-for-sale debt securities and applies to certain off-balance sheet credit exposures. This ASU is effective for smaller reporting companies in 2023. The Company is currently assessing the impact of the adoption of this ASU on its condensed consolidated financial statements.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU No. 2019-12 removes certain exceptions to the general principles in ASC 740 and also clarifies and amends existing guidance to improve consistent application. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company is currently evaluating the impact of this standard on its condensed consolidated financial statements.
In March 2020, the FASB issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting, which provides temporary optional guidance to ease the potential burden in accounting for reference rate reform. The new guidance provides optional expedients and exceptions for applying U.S. GAAP to transactions affected by reference rate reform if certain criteria are met. These transactions include: contract modifications, hedging relationships and sale or transfer of debt securities classified as held-to-maturity. Entities may apply the provisions of the new standard as of the beginning of the reporting period when the election is made (i.e., as early as the first quarter 2020). Unlike other topics, the provisions of this update are only available until December 31, 2022, when the reference rate replacement activity is expected to have been completed. The Company is currently evaluating the impact of this standard on its condensed consolidated financial statements and has yet to elect an adoption date.
Note 3—Asset Acquisition and Business Combination
Biotronik Asset Acquisition
In July 2019, the Company entered into a License and Distribution Agreement with the Biotronik Parties to obtain certain licenses to the Biotronik Parties’ patents, whereby the Company acquired certain manufacturing equipment and obtained from the Biotronik Parties a license under certain patents and technology to develop, commercialize, distribute and manufacture the AcQBlate Force ablation catheters and Qubic Force device. In exchange for the rights granted to the Company, the Company made cash payments totaling $10.0 million during the year ended December 31, 2019, and issued 273,070 shares of Series D convertible preferred stock with an implied value of $5.0 million during the nine months ended September 30, 2020. The implied value of $5.0 million was recorded as an accrued liability as of December 31, 2019. In accordance with ASC 805, the Biotronik Asset Acquisition was accounted for as an asset acquisition as substantially all of the $15.0 million value transferred to Biotronik was allocated to intellectual property. On the acquisition date, the products licensed had not yet received regulatory approval and the intellectual property did not have an alternative use. Accordingly, the $15.0 million paid to Biotronik was immediately charged to research and development expense—licensed acquired in the condensed consolidated statement of operations and comprehensive loss in July 2019.
Additional contingent milestone payments of up to $10.0 million are to be made to the Biotronik Parties contingent upon certain regulatory approvals and first commercial sale. In further consideration of the rights granted, beginning with the Company’s first commercial sale of the first force sensing ablation catheter within the licensed product line, the Company will also make per unit royalty payments. The Company has determined that as of the acquisition date and as of September 30, 2020 and December 31, 2019, the contingent milestone and royalty payments are not probable and estimable and therefore have not been recorded as a liability. Upon regulatory approval of the Company’s force sensing ablation catheter in Europe, the milestone payments will be capitalized and amortized, and the royalty payments will be recorded as cost of products sold as sales of catheters are recognized.
Rhythm Xience Business Combination
On June 18, 2019 (the “Acquisition Date”), the Company acquired an integrated family of transseptal crossing and steerable introducer systems through its acquisition of Rhythm Xience for $3.0 million in cash in exchange for all of the stock of Rhythm
17
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Xience (the “Rhythm Xience Acquisition”). The cash payment did not include the potential $17.0 million in earn out consideration, of which $2.2 million was paid with the issuance of Series D convertible preferred stock in February 2020 and the remainder is to be paid based on the achievement of certain regulatory milestones and revenue milestones. In accordance with ASC 805, the Rhythm Xience Acquisition is accounted for as a business combination.
Purchase Price Allocation
The following table summarizes the allocation of the purchase price to the assets acquired and liabilities assumed for the Rhythm Xience Acquisition (in thousands):
|
Accounts receivable, net |
|
$ |
3 |
|
|
Prepaid expenses and other current assets |
|
|
8 |
|
|
Property and equipment, net |
|
|
3 |
|
|
Intangible assets |
|
|
4,360 |
|
|
Goodwill |
|
|
12,026 |
|
|
Contingent consideration |
|
|
(13,400 |
) |
|
Cash consideration |
|
$ |
3,000 |
|
The Company recorded $12.0 million of goodwill that arose out of synergies from the Rhythm Xience Acquisition. The Company does not expect goodwill to be deductible for tax purposes.
As part of Rhythm Xience Acquisition, the Company recorded a contingent consideration liability for potential additional payments due to the sellers of Rhythm Xience if certain regulatory approval milestones and revenue milestones are achieved. The initial contingent consideration liability of $13.4 million was based on the fair value of the contingent consideration liability at the acquisition date. During the nine months ended September 30, 2020, the Company issued 119,993 shares of Series D convertible preferred stock and paid $2.6 million of the contingent consideration for the achievement of certain regulatory milestones and revenue milestones. Additionally, the Company recorded a $0.1 million and $0.7 million increase for the three months ended September 30, 2020 and 2019, respectively, and a $1.5 million decrease and a $0.7 million increase for the nine months ended September 30, 2020 and 2019, respectively, to the fair value of the contingent consideration liability which is included in change in fair value of contingent consideration in the condensed consolidated statement of operations and comprehensive loss. As of September 30, 2020, the contingent consideration liability of $7.6 million is the fair value of the remaining payments due to the sellers of Rhythm Xience if certain additional regulatory approval milestones and revenue milestones are achieved.
Note 4—Marketable Securities
Marketable securities consisted of the following as of September 30, 2020 and December 31, 2019 (in thousands):
|
|
|
September 30, 2020 (unaudited) |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
|
Available-for-sale securities - short-term: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities |
|
$ |
31,459 |
|
|
$ |
— |
|
|
$ |
(4 |
) |
|
$ |
31,455 |
|
|
U.S. treasury securities |
|
|
14,362 |
|
|
|
— |
|
|
|
(1 |
) |
|
|
14,361 |
|
|
Commercial paper |
|
|
53,926 |
|
|
|
— |
|
|
|
— |
|
|
|
53,926 |
|
|
Total available-for-sale securities - short-term |
|
|
99,747 |
|
|
|
— |
|
|
|
(5 |
) |
|
|
99,742 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Asset-backed securities, long-term |
|
|
8,789 |
|
|
|
|
|
|
|
— |
|
|
|
8,789 |
|
|
Total available-for-sale securities |
|
$ |
108,536 |
|
|
$ |
— |
|
|
$ |
(5 |
) |
|
$ |
108,531 |
|
18
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
|
|
|
December 31, 2019 |
|
|||||||||||||
|
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
|
Available-for-sale securities - short-term: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Corporate debt securities |
|
$ |
28,204 |
|
|
$ |
20 |
|
|
$ |
— |
|
|
$ |
28,224 |
|
|
Asset-backed securities |
|
|
17,108 |
|
|
|
13 |
|
|
|
— |
|
|
|
17,121 |
|
|
U.S. treasury securities |
|
|
5,020 |
|
|
|
12 |
|
|
|
— |
|
|
|
5,032 |
|
|
Commercial paper |
|
|
11,974 |
|
|
|
— |
|
|
|
— |
|
|
|
11,974 |
|
|
Total available-for-sale securities |
|
$ |
62,306 |
|
|
$ |
45 |
|
|
$ |
— |
|
|
$ |
62,351 |
|
As of December 31, 2019, all of the Company’s available-for-sale securities mature in one year or less. As of September 30, 2020, the Company’s available-for-sale securities classified as short-term of $99.7 million mature in one year or less and the available-for-sale securities classified as long-term of $8.8 million mature within two years.
Note 5—Inventory
Inventory as of September 30, 2020 and December 31, 2019 consisted of the following (in thousands):
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Raw materials |
|
$ |
5,980 |
|
|
$ |
5,492 |
|
|
Work in process |
|
|
864 |
|
|
|
1,605 |
|
|
Finished goods |
|
|
4,088 |
|
|
|
1,327 |
|
|
Total inventory |
|
$ |
10,932 |
|
|
$ |
8,424 |
|
Note 6—Lessor Sales-Type Leases
The Company recognizes revenue and costs, as well as a lease receivable, at the time embedded sales-type leases within its deferred equipment agreements commence. Lease revenue related to sales-type leases for each of the three and nine months ended September 30, 2020 was $0.7 million and is included within revenue in the accompanying condensed consolidated statements of operations and comprehensive loss. There was no lease revenue for the three and nine months ended September 30, 2019. Costs related to embedded leases within the Company’s deferred equipment agreements are included in cost of products sold in the accompanying condensed consolidated statements of operations and comprehensive loss.
The Company has a short-term lease receivable of $0.3 million and a long-term lease receivable of $0.4 million included in prepaid expenses and other current assets and other assets, respectively, as of September 30, 2020. There was no lease receivable as of December 31, 2019.
As of September 30, 2020, estimated future maturities of customer sales-type lease receivables for each of the following years are as follows (in thousands):
|
Three months ending December 31, 2020 |
|
$ |
68 |
|
|
Year ending December 31, 2021 |
|
|
273 |
|
|
Year ending December 31, 2022 |
|
|
254 |
|
|
Year ending December 31, 2023 |
|
|
46 |
|
|
Year ending December 31, 2024 |
|
|
27 |
|
|
Lease receivable |
|
$ |
668 |
|
19
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 7—Property and Equipment, Net
The Company’s property and equipment, net, consisted of the following as of September 30, 2020 and December 31, 2019 (in thousands):
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Medical diagnostic equipment |
|
$ |
10,155 |
|
|
$ |
5,492 |
|
|
Furniture and fixtures |
|
|
388 |
|
|
|
159 |
|
|
Office equipment |
|
|
1,391 |
|
|
|
1,321 |
|
|
Laboratory equipment and software |
|
|
3,409 |
|
|
|
2,807 |
|
|
Leasehold improvements |
|
|
600 |
|
|
|
507 |
|
|
Construction in process |
|
|
141 |
|
|
|
306 |
|
|
Total property and equipment |
|
|
16,084 |
|
|
|
10,592 |
|
|
Less: accumulated depreciation |
|
|
(6,144 |
) |
|
|
(6,165 |
) |
|
Property and equipment, net |
|
$ |
9,940 |
|
|
$ |
4,427 |
|
Property and equipment includes certain medical diagnostic equipment, AcQMap Systems, located at customer premises. The Company retains the ownership of the equipment and has the right to remove the equipment if it is not being used according to expectations.
Depreciation expense was $0.8 million and $0.5 million for the three months ended September 30, 2020 and 2019, respectively, and $1.8 million and $1.7 million for the nine months ended September 30, 2020 and 2019, respectively.
Note 8—Goodwill and Intangible Assets
The table below summarizes goodwill and intangible assets activities as of September 30, 2020 and December 31, 2019 (in thousands):
|
|
|
Goodwill |
|
|
Intangible Assets |
|
||
|
Balance at December 31, 2019 |
|
$ |
12,026 |
|
|
$ |
4,110 |
|
|
Amortization expense |
|
|
— |
|
|
|
(330 |
) |
|
Balance at September 30, 2020 (unaudited) |
|
$ |
12,026 |
|
|
$ |
3,780 |
|
|
|
|
Estimated Useful |
|
Weighted Average Remaining |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Life (in years) |
|
Life (in years) |
|
|
Intangible Assets |
|
|
Accumulated Amortization |
|
|
September 30, 2020 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|
Developed technology |
|
10 |
|
|
8.8 |
|
|
$ |
4,200 |
|
|
$ |
(495 |
) |
|
$ |
3,705 |
|
|
Trademarks and trade names |
|
0.5 |
|
— |
|
|
|
60 |
|
|
|
(60 |
) |
|
|
— |
|
|
|
Customer-related intangible |
|
5 |
|
3.75 |
|
|
|
100 |
|
|
|
(25 |
) |
|
|
75 |
|
|
|
Total |
|
|
|
|
|
|
|
$ |
4,360 |
|
|
$ |
(580 |
) |
|
$ |
3,780 |
|
20
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
|
|
|
Estimated Useful |
|
Weighted Average Remaining |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Life (in years) |
|
Life (in years) |
|
Intangible Assets |
|
|
Accumulated Amortization |
|
|
December 31, 2019 |
|
|||
|
Developed technology |
|
10 |
|
9.5 |
|
$ |
3,600 |
|
|
$ |
(180 |
) |
|
$ |
3,420 |
|
|
In-process technology |
|
indefinite |
|
|
|
|
600 |
|
|
|
— |
|
|
|
600 |
|
|
Trademarks and trade names |
|
0.5 |
|
— |
|
|
60 |
|
|
|
(60 |
) |
|
|
— |
|
|
Customer-related intangible |
|
5 |
|
4.5 |
|
|
100 |
|
|
|
(10 |
) |
|
|
90 |
|
|
Total |
|
|
|
|
|
$ |
4,360 |
|
|
$ |
(250 |
) |
|
$ |
4,110 |
|
Acquired in-process technology was classified as an indefinite-lived intangible asset until the receipt of FDA approval for the technology in January 2020. Once the FDA approval was received, the in-process technology was reclassified as developed technology and amortization began. The Company recorded amortization expense related to the above intangible assets of $0.1 million and $0.1 million for the three months ended September 30, 2020 and 2019, respectively, and $0.3 million and $0.1 million for the nine months ended September 30, 2020 and 2019, respectively.
The following table shows the remaining amortization expense associated with amortizable intangible assets as of September 30, 2020 (in thousands):
|
|
|
Developed Technology |
|
|
Customer- Related Intangible |
|
|
Total Amortization |
|
|||
|
Three months ending December 31, 2020 |
|
$ |
105 |
|
|
$ |
5 |
|
|
$ |
110 |
|
|
Year ending December 31, 2021 |
|
|
420 |
|
|
|
20 |
|
|
|
440 |
|
|
Year ending December 31, 2022 |
|
|
420 |
|
|
|
20 |
|
|
|
440 |
|
|
Year ending December 31, 2023 |
|
|
420 |
|
|
|
20 |
|
|
|
440 |
|
|
Year ending December 31, 2024 |
|
|
420 |
|
|
|
10 |
|
|
|
430 |
|
|
Thereafter |
|
|
1,920 |
|
|
|
— |
|
|
|
1,920 |
|
|
Total |
|
$ |
3,705 |
|
|
$ |
75 |
|
|
$ |
3,780 |
|
Note 9—Accrued Liabilities
Accrued liabilities consisted of the following as of September 30, 2020 and December 31, 2019 (in thousands):
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Payroll and related expenses |
|
$ |
5,083 |
|
|
$ |
3,785 |
|
|
Professional fees |
|
|
290 |
|
|
|
188 |
|
|
Deferred revenue |
|
|
290 |
|
|
|
311 |
|
|
Sales and use tax |
|
|
140 |
|
|
|
151 |
|
|
Biotronik Asset Acquisition - accrued purchase price |
|
|
— |
|
|
|
5,000 |
|
|
Other |
|
|
697 |
|
|
|
641 |
|
|
Total accrued liabilities |
|
$ |
6,500 |
|
|
$ |
10,076 |
|
21
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Outstanding debt as of September 30, 2020 and December 31, 2019 consisted of the following (in thousands):
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|
|
|
|
|
|
2019 Credit Agreement (1) |
|
$ |
44,550 |
|
|
$ |
44,550 |
|
|
Total debt, gross |
|
|
44,550 |
|
|
|
44,550 |
|
|
Less: Unamortized debt discount and fees |
|
|
(5,788 |
) |
|
|
(6,306 |
) |
|
Total long-term debt |
|
$ |
38,762 |
|
|
$ |
38,244 |
|
|
(1) |
The 2019 Credit Agreement includes final payment fees of $4.6 million. |
2019 Credit Agreement
On May 20, 2019, the Company entered into a Credit Agreement (the “2019 Credit Agreement”). The 2019 Credit Agreement provided the Company with a senior term loan facility in aggregate principal amount of $70.0 million, of which the Company borrowed $40.0 million upon closing. Of the remaining amount of the facility, $10.0 million was available for borrowing by the Company on or prior to June 30, 2020 and $20.0 million is available for borrowing by the Company on or prior to December 31, 2020, in each case subject to the achievement of specified trailing revenue levels. The Company did not achieve the trailing revenue levels to draw the $10.0 million by June 30, 2020 but the $20.0 million remains available for borrowing on or prior to December 31, 2020 if the specified trailing revenue levels are met. The 2019 Credit Agreement bears interest per annum at 7.75% plus LIBOR for such interest period and the principal amount of term loans outstanding under the 2019 Credit Agreement is due on May 20, 2024. The 2019 Credit Agreement provides for final payment fees of an additional $4.6 million that are due upon prepayment or on the maturity date or upon acceleration.
Upon the occurrence and during an event of default, which includes but is not limited to payment default, covenant default or the occurrence of a material adverse change, the lenders may declare all outstanding principal and accrued and unpaid interest immediately due and payable, all unfunded commitments would be terminated, there would be an increase in the applicable interest rate by 10.0% per annum, and the lenders would be entitled to exercise their other rights and remedies provided for under the 2019 Credit Agreement. Additionally, the lenders may request repayment of a portion of obligations outstanding under the 2019 Credit Agreement to the extent of the Company’s receipt of any (i) net casualty proceeds or (ii) net asset sales proceeds, as defined. These acceleration and early payment features are an embedded derivative that is separately measured from the loan host instrument and classified with the loan host instrument.
In connection with the issuance of the 2019 Credit Agreement, the Company issued liability-classified warrants with a fair value of $0.9 million to purchase 419,992 shares of Series C convertible preferred stock at $16.67 per share. These warrants were subsequently automatically converted into warrants to purchase an equal number of shares of the Company’s Series D convertible preferred stock at a price of $16.67 per share.
The initial recognition of the warrant liability and direct fees of $1.2 million and final payment fees of $4.6 million for the 2019 Credit Agreement resulted in a discount of $6.7 million, which is being amortized to interest expense over the term of the 2019 Credit Agreement using the effective interest method.
The Company’s obligations under the 2019 Credit Agreement are secured by substantially all of its assets, including its intellectual property, and is guaranteed by Acutus NV. The 2019 Credit Agreement contains customary affirmative and negative covenants, including with respect to the Company’s ability to enter into fundamental transactions, incur additional indebtedness, grant liens, pay any dividend or make any distributions to its holders, make investments and merge or consolidate with any other person or engage in transactions with its affiliates, but does not include any financial covenants, other than a minimum liquidity requirement. As of and for the nine months ended September 30, 2020 and 2019, the Company was in compliance with all such covenants.
2019 Convertible Notes
On May 20, 2019, the Company sold and issued $37.0 million in aggregate principal amount of convertible promissory notes (the “2019 Convertible Notes”). The 2019 Convertible Notes bore interest at 13% per annum and were due on December 31, 2019.
22
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The 2019 Convertible Notes, including accrued interest, automatically converted into preferred stock at the lowest price per share paid by a cash investor in a qualified equity financing of at least $23.0 million (excluding the principal of any debt that is cancelled or converted into preferred stock in the equity financing), which occurred on June 12, 2019. In accordance with ASC 480, Distinguishing Liabilities from Equity, the 2019 Convertible Notes were recorded as share-settled debt at fair value. As the 2019 Convertible Notes converted to Series D convertible preferred stock on June 12, 2019, the initial proceeds of $37.0 million is the fair value and the settlement value of the 2019 Convertible Notes.
Note 11—Operating Leases
The Company leases approximately 50,800 square feet of office space for its corporate headquarters and manufacturing facility in Carlsbad, California under a noncancelable operating lease that expires on December 31, 2022. The lease is subject to variable charges for common area maintenance and other costs that are determined annually based on actual costs. The base rent is subject to an annual increase each year. The Company has a renewal option for an additional five-year term upon the expiration date of the lease, which has been excluded from the calculation of the right-of-use asset as it is not reasonably certain to be exercised.
The Company also leases approximately 3,900 square feet of office space in Zaventem, Belgium under a noncancelable operating lease that expires on December 31. 2021. The lease is subject to variable charges that are determined annually for common area maintenance and other costs based on actual costs, and base rent is subject to an annual increase each year based on an index rate. The Company has a renewal option for an additional three-year term upon the expiration date of the lease, which has been included in the calculation of the right-of-use asset as it is reasonably certain to be exercised.
The following table summarizes quantitative information about the Company’s operating leases for the nine months ended September 30, 2020 and 2019 (dollars in thousands):
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Operating cash flows from operating leases |
|
$ |
760 |
|
|
$ |
725 |
|
|
Right-of-use assets exchanged for operating lease liabilities |
|
$ |
— |
|
|
$ |
2,978 |
|
|
Weighted average remaining lease term – operating leases (in years) |
|
|
1.7 |
|
|
|
2.2 |
|
|
Weighted average discount rate – operating leases |
|
|
7.0 |
% |
|
|
7.0 |
% |
The following table provides the components of the Company’s lease cost (in thousands):
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||||||||||
|
Operating leases |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating lease cost |
|
$ |
216 |
|
|
$ |
216 |
|
|
$ |
648 |
|
|
$ |
648 |
|
|
Variable lease cost |
|
|
73 |
|
|
|
65 |
|
|
|
238 |
|
|
|
169 |
|
|
Total rent expense |
|
$ |
289 |
|
|
$ |
281 |
|
|
$ |
886 |
|
|
$ |
817 |
|
23
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
As of September 30, 2020, future minimum payments under the non-cancelable operating leases under ASC 842 were as follows (in thousands):
|
Three months ending December 31, 2020 |
|
$ |
253 |
|
|
Year ending December 31, 2021 |
|
|
1,044 |
|
|
Year ending December 31, 2022 |
|
|
1,074 |
|
|
Year ending December 31, 2023 |
|
|
51 |
|
|
Year ending December 31, 2024 |
|
|
51 |
|
|
Total |
|
|
2,473 |
|
|
Less: present value discount |
|
|
(201 |
) |
|
Operating lease liabilities |
|
$ |
2,272 |
|
Note 12—Commitments and Contingencies
The Company is not a party to any material legal proceedings and is not aware of any pending or threatened claims. From time to time however, the Company may be subject to various legal proceedings and claims that arise in the ordinary course of its business activities.
Note 13—Warrants
As of September 30, 2020 and December 31, 2019, the outstanding warrants to purchase the Company’s common stock were comprised of the following:
|
|
|
Equity Upon |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise |
|
|
|
|
|
|
|
September 30, |
|
|
December 31, |
|
||
|
|
|
(After Conversion) |
|
Exercise Price |
|
|
Expiration Date |
|
2020 |
|
|
2019 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
(unaudited) |
|
|
|
|
|
|
|
Warrants issued in 2015 |
|
Common Stock |
|
$ |
5.25 |
|
|
1/30/25 |
|
|
7,616 |
|
|
|
7,616 |
|
|
Warrants issued with 2018 Convertible Notes |
|
Common Stock |
|
$ |
0.10 |
|
|
6/7/28 |
|
|
501,946 |
|
|
|
501,946 |
|
|
Warrants issued with 2018 Term Loan |
|
Common Stock |
|
$ |
16.67 |
|
|
7/31/28 |
|
|
26,998 |
|
|
|
26,998 |
|
|
Warrants issued with 2019 Credit Agreement |
|
Common Stock |
|
$ |
16.67 |
|
|
5/20/29 |
|
|
419,992 |
|
|
|
419,992 |
|
|
Total Warrants |
|
|
|
|
|
|
|
|
|
|
956,552 |
|
|
|
956,552 |
|
The Company has no warrant activity for the nine months ended September 30, 2020. The remaining weighted average contractual life is 7.7 years as of September 30, 2020.
Warrants Classified as Liabilities
During 2019, in connection with the Company’s entry into the 2019 Credit Agreement, the Company issued warrants to purchase 419,992 shares of its Series C convertible preferred stock with an exercise price of $16.67 per share. These warrants were subsequently automatically converted into warrants to purchase an equal number of shares of the Company’s Series D convertible preferred stock at a price of $16.67 per share. During 2018, in connection with the issuance of the 2018 Convertible Notes and the 2018 Term Loan, the Company issued ten-year warrants to purchase 501,946 shares of common stock with an exercise price of $0.10 per share and 26,998 shares of Series C convertible preferred stock with an exercise price of $16.67 per share, respectively. The warrants for Class C convertible preferred stock were subsequently automatically converted into warrants to purchase an equal number of shares of the Company’s Series D convertible preferred stock at a price of $16.67 per share. On August 10, 2020, in connection with the closing of the IPO, the warrants to purchase 446,990 shares of Series D convertible preferred stock were automatically converted to an equal number of warrants to purchase common stock at an exercise price of $16.67 per share.
24
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The Company’s warrants provide the holder the option to purchase a specified number of shares for a specified price. The holder may exercise the warrant in cash or exercise pursuant to a cashless exercise whereby a calculated number of shares are withheld upon exercise to satisfy the exercise price. The warrants do not provide the holder any voting rights until the warrants are exercised.
Prior to the IPO, in accordance with ASC 815, other than the warrants issued in 2015, the warrants were recorded as liabilities at fair value at the issuance date. Changes in the fair value were recognized in change in fair value of warrant liability and embedded derivative in the condensed consolidated statements of operations and comprehensive loss at the end of each reporting period. On August 10, 2020, in connection with the closing of the IPO, the warrants recorded as liabilities no longer met the definition of a derivative. Accordingly, the fair value of the common and preferred stock warrant liability of $14.5 million was reclassified to stockholders’ equity (deficit) in the condensed consolidated balance sheet.
Warrants Classified as Equity
In accordance with ASC 815, the warrants issued in 2015 do not meet the definition of a derivative and are classified in stockholders’ equity (deficit) in the condensed consolidated balance sheets.
Note 14—Convertible Preferred Stock
In February 2020, the Company issued 119,993 shares of its Series D convertible preferred stock with an implied value of $2.2 million in connection with a contingent consideration payment related to the Rhythm Xience Acquisition.
In February 2020, the Company issued 273,070 shares of its Series D convertible preferred stock with an implied value of $5.0 million for the stock issuance portion of the purchase consideration of the Biotronik Asset Acquisition.
On August 10, 2020, in connection with the closing of the IPO, all of the 391,210 shares of Series A, 3,088,444 shares of Series B, 4,499,921 shares of Series C and 8,593,360 shares of Series D convertible preferred stock, respectively, automatically converted into an equal number of shares of common stock.
Redemption
The convertible preferred stock was not unconditionally redeemable at the option of the holder thereof. However, the convertible preferred stock was contingently redeemable upon certain liquidation events. As redemption by the holders was not solely within the control of the Company, all of the outstanding convertible preferred stock was classified as temporary equity in the condensed consolidated balance sheets, prior to the conversion to common stock on August 10, 2020.
Dividends
The holders of shares of convertible preferred stock were entitled to receive dividends, out of any assets legally available therefore, prior and in preference to any declaration or payment of any dividend on the common stock of the Company, at the applicable dividend rate, payable on a pro rata, pari passu basis when, as and if declared by the Company’s board of directors. The dividend rate was $0.68 per annum for each share of Series A convertible preferred stock, $1.07 per annum for each share of Series B convertible preferred stock and $1.36 per annum for each share of Series C convertible preferred stock and Series D convertible preferred stock, as adjusted. The dividend rights were not cumulative.
Liquidation
The holders of the Series D convertible preferred stock were entitled to receive a liquidation preference prior to any distribution to the holders of Series A convertible preferred stock, Series B convertible preferred stock and Series C convertible preferred stock (collectively the “Junior Preferred Stock”) and the holders of common stock, in the amount of the original issue price plus declared but unpaid dividends on such shares (the “Series D Liquidation Preference”). The holders of the Junior Preferred Stock were entitled to receive a liquidation preference prior to any distribution to the holders of common stock, after payment of the Series D Liquidation Preference, in the amount of the applicable original issue price plus declared but unpaid dividends on such shares.
25
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Each share of preferred stock was convertible, at the option of the holder thereof, at any time after the date of issuance of such share, into such number of fully paid and nonassessable shares of the Company’s common stock as was determined by dividing the original issue price, as adjusted, for such series by the applicable conversion price for such series in effect on the date the certificate is surrendered for conversion. The initial conversion price per share for each series of convertible preferred stock was the original issue price applicable to such series as follows:
|
Series |
|
Conversion Price |
|
|
|
Series A convertible preferred stock |
|
$ |
8.295 |
|
|
Series B convertible preferred stock |
|
$ |
13.370 |
|
|
Series C convertible preferred stock |
|
$ |
16.667 |
|
|
Series D convertible preferred stock |
|
$ |
16.667 |
|
Each share of convertible preferred stock was automatically convertible into fully-paid, non-assessable shares of common stock at the conversion rate at the time in effect for such series of preferred stock immediately upon: (i) the date, or the occurrence of an event, specified by vote or written consent or agreement of the requisite investors; or (ii) the closing of the sale of shares of common stock to the public, at a price of at least $50.00 per share, as adjusted, in a firm-commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933, as amended, resulting in at least $50.0 million of net proceeds to the Company. As noted above, on August 10, 2020, all shares of Series A, Series B, Series C and Series D convertible preferred stock were converted into common stock.
Note 15—Stockholders’ Equity (Deficit)
On August 10, 2020, the Company issued 10,147,058 shares of common stock in its IPO, which included 1,323,529 shares of common stock issued upon the underwriters’ exercise in full of an option to purchase, at the public offering price less underwriting discounts and commissions, up to an additional 1,323,529 shares. The price to the public for each share was $18.00. The Company received gross proceeds of $182.6 million from the IPO. Net of underwriting discounts and commission and other offering expenses, the Company received net proceeds of $166.3 million from the IPO.
On August 10, 2020, in connection with the closing of the IPO, the Company filed an amended and restated certificate of incorporation (the “A&R Certificate”) with the Secretary of State of the State of Delaware. The A&R Certificate amended and restated the Company’s authorized shares of common stock to 260,000,000 and authorized shares of undesignated preferred stock to 5,000,000.
As of December 31, 2019, the Company’s Amended and Restated Certificate of Incorporation authorized the issuance of 220,000,000 shares of common stock, $0.001 par value per share. Each share of common stock is entitled to one voting right. Common stock owners are entitled to dividends when funds are legally available and declared by the Board.
During the nine months ended September 30, 2020 and 2019, stock options to acquire 92,223 and 15,921 shares, respectively, were exercised for shares of common stock. The Company received approximately $0.4 million and $0.1 million for the exercise price of the stock options for the nine months ended September 30, 2020 and 2019, respectively.
Note 16—Stock-Based Compensation
2020 Equity Incentive Plan
The 2020 Equity Incentive Plan (“2020 Plan”) which permits the granting of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units, performance shares and other equity-based awards to employees, directors and consultants became effective on August 5, 2020. As of September 30, 2020, 2,191,600 shares of common stock were authorized for issuance under the 2020 Plan and 1,101,996 shares remain available for issuance under the 2020 Plan.
2011 Equity Incentive Plan
The Company’s 2011 Equity Incentive Plan (the “2011 Plan”) permits the granting of incentive stock options, non-statutory stock options, restricted stock, restricted stock units and other stock-based awards to employees, directors, officers and consultants. As
26
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
of September 30, 2020, 2,823,333 shares of common stock were authorized for issuance under the 2011 Plan and no shares remain available for issuance under the 2011 Plan. No additional awards will be granted under the 2011 Plan. Shares that become available for issuance from the outstanding awards under the 2011 Plan due to forfeiture, or otherwise, will become available for issuance of future awards under the 2020 Plan.
Stock Options
The stock options generally vest over four years and have a ten-year contractual term. The fair value of each employee and non-employee stock option grant is estimated on the date of grant using the Black-Scholes option pricing model. The Company became publicly traded in August 2020 and lacks company-specific historical and implied volatility information. Therefore, it estimates its expected stock volatility based on the historical volatility of a set of publicly traded peer companies. Due to the lack of historical exercise history, the expected term of the Company’s stock options has been determined using the “simplified” method for awards. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is zero based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
The following assumptions were used to estimate the fair value of stock option for the nine months ended September 30, 2020 and 2019:
|
|
|
Nine Months Ended September 30, |
|
||||
|
|
|
2020 |
|
|
2019 |
|
|
|
|
|
(unaudited) |
|
||||
|
Risk-free interest rate |
|
0.4% - 0.9% |
|
|
1.6% - 2.1% |
|
|
|
Expected dividend yield |
|
— |
|
|
— |
|
|
|
Expected term in years |
|
|
7.0 |
|
|
6.4 - 10.0 |
|
|
Expected volatility |
|
70% - 80% |
|
|
80% |
|
|
The following table summarizes stock option activity during the nine months ended September 30, 2020:
|
|
|
Stock Options |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Life (in years) |
|
|
Aggregate Intrinsic Value (in thousands) |
|
||||
|
Outstanding as of December 31, 2019 |
|
|
2,041,205 |
|
|
$ |
9.52 |
|
|
|
7.7 |
|
|
$ |
7,857 |
|
|
Options granted |
|
|
1,746,415 |
|
|
|
16.69 |
|
|
|
|
|
|
|
|
|
|
Option exercised |
|
|
(139,597 |
) |
|
|
7.24 |
|
|
|
|
|
|
|
|
|
|
Options forfeited |
|
|
(231,678 |
) |
|
|
11.91 |
|
|
|
|
|
|
|
|
|
|
Outstanding as of September 30, 2020 (unaudited) |
|
|
3,416,345 |
|
|
$ |
13.12 |
|
|
|
8.2 |
|
|
$ |
57,110 |
|
|
Options vested and exercisable as of September 30, 2020 (unaudited) |
|
|
1,310,459 |
|
|
$ |
9.22 |
|
|
|
6.5 |
|
|
$ |
26,974 |
|
The aggregate intrinsic value in the above table is calculated as the difference between the fair value of the Company’s common stock as of September 30, 2020 and the exercise price of the stock options. The weighted average grant date fair value per share for the stock option awards granted during the nine months ended September 30, 2020 was $11.49. As of September 30, 2020, the total unrecognized compensation related to unvested stock option awards granted was $29.1 million, which the Company expects to recognize over a weighted average period of approximately 3.0 years.
Performance-Based Restricted Stock Units and Restricted Stock Units
In June 2019, the Company granted 567,509 PSUs, with a grant date fair value of $13.37. Vesting of the PSUs was dependent upon the satisfaction of both a service condition and a performance condition, which is an IPO or a change of control. The Company began recording compensation expense related to the PSUs upon the declaration of the Company’s IPO becoming effective on August 5, 2020, as the performance conditions were satisfied. The compensation expense was determined using the original grant date fair value and is being recognized over the remaining service period.
27
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The Company’s PSU and RSU activity for the nine months ended September 30, 2020 was as follows:
|
|
|
Number of Shares |
|
|
Weighted Average Grant Price |
|
||
|
Unvested as of December 31, 2019 |
|
|
567,509 |
|
|
$ |
13.37 |
|
|
Granted |
|
|
215,846 |
|
|
|
18.35 |
|
|
Forfeited |
|
|
(1,388 |
) |
|
|
18.00 |
|
|
Vested |
|
|
(285,375 |
) |
|
|
13.37 |
|
|
Unvested as of September 30, 2020 (unaudited) |
|
|
496,592 |
|
|
$ |
15.52 |
|
Restricted Stock
The Company’s RSA activity for the nine months ended September 30, 2020 was as follows:
|
|
|
Number of Shares |
|
|
Weighted Average Grant Price |
|
||
|
Unvested as of December 31, 2019 |
|
|
— |
|
|
$ |
— |
|
|
Granted |
|
|
32,921 |
|
|
|
16.55 |
|
|
Vested |
|
|
(32,921 |
) |
|
|
16.55 |
|
|
Unvested as of September 30, 2020 (unaudited) |
|
|
— |
|
|
$ |
— |
|
The following table summarizes the total stock-based compensation expense for the stock options, PSUs, RSUs and RSAs recorded in the condensed consolidated statements of operations and comprehensive loss for the three and nine months ended September 30, 2020 and 2019 (in thousands):
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||||||||||
|
Cost of products sold |
|
$ |
126 |
|
|
$ |
56 |
|
|
$ |
292 |
|
|
$ |
162 |
|
|
Research and development |
|
|
319 |
|
|
|
179 |
|
|
|
697 |
|
|
|
471 |
|
|
Selling, general and administrative |
|
|
5,929 |
|
|
|
577 |
|
|
|
8,283 |
|
|
|
1,541 |
|
|
Total stock-based compensation |
|
$ |
6,374 |
|
|
$ |
812 |
|
|
$ |
9,272 |
|
|
$ |
2,174 |
|
Employee Stock Purchase Plan
The 2020 Employee Stock Purchase Plan (“2020 ESPP”), which permits employees to purchase shares of the Company’s common stock, became effective on August 10, 2020 and 387,063 shares of common stock were authorized for sale under the 2020 ESPP. As of September 30, 2020, there were no purchase of shares under the 2020 ESPP.
28
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
Note 17—Net Loss Per Common Share
Basic net loss per common share is computed by dividing net loss attributable to common stockholders by the weighted average number of shares of common stock outstanding for the period. Diluted net loss per common share excludes the potential impact of the Company’s convertible preferred stock, common stock options and warrants because their effect would be anti-dilutive due to the Company’s net loss. Since the Company had a net loss in the periods presented, basic and diluted net loss per common share are the same.
The table below provides potentially dilutive securities not included in the calculation of the diluted net loss per common share because to do so would be anti-dilutive:
|
|
|
Nine Months Ended September 30, |
|
|||||
|
Shares issuable upon: |
|
2020 |
|
|
2019 |
|
||
|
Conversion of Series A Preferred Stock |
|
|
— |
|
|
|
391,210 |
|
|
Conversion of Series B Preferred Stock |
|
|
— |
|
|
|
3,088,444 |
|
|
Conversion of Series C Preferred Stock |
|
|
— |
|
|
|
4,499,921 |
|
|
Conversion of Series D Preferred Stock |
|
|
— |
|
|
|
6,418,437 |
|
|
Exercise of stock options |
|
|
3,416,345 |
|
|
|
1,751,616 |
|
|
Exercise of common stock warrants |
|
|
956,552 |
|
|
|
509,562 |
|
|
Exercise of preferred stock warrants |
|
|
— |
|
|
|
446,990 |
|
|
Vesting of PSUs and RSUs |
|
|
496,592 |
|
|
|
— |
|
|
Total |
|
|
4,869,489 |
|
|
|
17,106,180 |
|
For the nine months ended September 30, 2019, the PSUs are not included in the above table as awards with performance conditions are not included in the calculation of diluted earnings per share until the performance conditions for the PSU are considered probable.
Note 18—401(k) Retirement Plan
The Company has a 401(k) retirement savings plan that provides retirement benefits to substantially all full-time U.S. employees. Eligible employees may contribute a percentage of their annual compensation, subject to Internal Revenue Service limitations. The Company did not provide any contributions to the 401(k) retirement savings plan for the nine months ended September 30, 2020 and 2019.
Note 19—Related Party Transactions
The Company licenses certain patent rights from a former director and shareholder. The license agreement provides for royalty payments to the shareholder of 3% of net product sales, as defined in the agreement. Royalties earned prior to the director’s resignation were $22,000 and $10,000 for the three months ended September 30, 2020 and 2019, respectively, and $61,000 and $32,000 for the nine months ended September 30, 2020 and 2019, respectively. Additionally, the former director and shareholder also works for one of the Company’s customers and can significantly influence the customer to purchase the Company’s product. Prior to the director’s resignation, the Company recorded sales to this customer of $8,000 and $50,000 for the three months ended September 30, 2020 and 2019, respectively, and $0.5 million and $0.2 million for the nine months ended September 30, 2020 and 2019, respectively.
The Company has a consulting agreement with a director and chairman of the Company’s board of directors. The Company recorded $55,000 and $81,000 for the three months ended September 30, 2020 and 2019, respectively, and $0.1 million and $0.2 million for the nine months ended September 30, 2020 and 2019, respectively, in SG&A expense in the condensed consolidated statements of operations and comprehensive loss, for the consulting services. With the completion of the IPO, the performance condition was satisfied for certain performance stock units granted to the director and chairman of the Company’s board of directors. The Company recorded $4.0 million of stock-based compensation expense for these awards for the three and nine months ended September 30, 2020 in SG&A expense in the condensed consolidated statements of operations and comprehensive loss.
29
Acutus Medical, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(Unaudited)
The Company had a consulting agreement with an officer of the Company in the prior year. The Company recorded $93,000 for the three months ended September 30, 2019, and $0.3 million for the nine months ended September 30, 2019 in SG&A expense in the condensed consolidated statements of operations and comprehensive loss, for the consulting services.
The Company had a consulting arrangement with a current director and officer of the Company, prior to his full-time employment. The Company recorded $0.2 million and $0.4 million for the three and nine months ended September 30, 2019, respectively, in SG&A expense in the condensed consolidated statements of operations and comprehensive loss, for the consulting services.
Multiple preferred stock shareholders entered into the 2018 and 2019 Convertible Notes that also contained detached warrants. Additionally, Orbimed Royalty Opportunities II, LP and Deerfield Private Design Fund II, L.P. entered into the 2019 Credit Agreement with the Company in 2019 for a total of $70.0 million, with $40.0 million being drawn as of September 30, 2020. The Company recorded $1.4 million and $1.4 million for the three months ended September 30, 2020 and 2019, respectively, and $4.1 million and $2.1 million for the nine months ended September 30, 2020 and 2019, respectively, in interest expense related to these debt agreements.
30
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results of operations together with our condensed consolidated financial statements and related notes and other financial information included elsewhere in this Form 10-Q. Some of the information contained in this discussion and analysis or set forth elsewhere in this Form 10-Q, including information with respect to our plans and strategy for our business, includes “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). In some cases, you can identify these statements by forward-looking words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “should,” “estimate,” or “continue,” and similar expressions or variations. Such forward-looking statements are subject to risks, uncertainties and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included in this Form 10-Q. The forward-looking statements in this Form 10-Q represent our views as of the date of this Form 10-Q. Except as may be required by law, we assume no obligation to update these forward-looking statements or the reasons that results could differ from these forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Form 10-Q.
Overview
We are an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated. Despite several decades of effort by the incumbents in this field, the clinical and economic challenges associated with arrhythmia treatment continue to be a huge burden for patients, providers and payors. We are committed to advancing the field of electrophysiology with a unique array of products and technologies which will enable more physicians to treat more patients more effectively and efficiently. Through internal product development, acquisitions and global partnerships, we have established a global sales presence delivering a broad portfolio of highly differentiated electrophysiology products. Our goal is to provide our customers with a complete solution for catheter-based treatment of cardiac arrhythmias in each of our geographic markets.
Our product portfolio includes novel access catheters, diagnostic and mapping catheters, ablation catheters, mapping and imaging consoles and accessories, as well as supporting algorithms and software programs. Our foundational and most highly differentiated product is our AcQMap imaging and mapping system. Our paradigm-shifting AcQMap System offers a novel approach to mapping the drivers and maintainers of arrhythmias with unmatched speed and precision. With the ability to rapidly and accurately identify ablation targets and to confirm both ablation success and procedural completion, we believe our AcQMap System addresses the primary unmet need in electrophysiology procedures today.
We were incorporated in the State of Delaware on March 25, 2011 and are headquartered in Carlsbad, California. Early versions of our AcQMap System and certain related accessory products have been used in the United States since May 2018 and Western Europe since July 2016 in a limited, pilot launch capacity, where our focus was on optimizing workflow and validating our value proposition. We fully commenced the launch of our commercial-grade console and software products in the first quarter of 2020. Critical to our launch were a series of recent strategic transactions and regulatory approvals, including: FDA 510(k) clearance and CE Mark of our second-generation AcQMap console and SuperMap software suite; the addition of an integrated family of transseptal crossing and steerable introducer systems to our product portfolio through our acquisition of Rhythm Xience, Inc., or Rhythm Xience; and the acquisition of our AcQBlate Force sensing product line from Biotronik SE & Co. KG, or Biotronik. Since our full launch, we have continued to enhance our product portfolio and global presence by entering into bi-lateral distribution agreements with Biotronik in May 2020, which added a full suite of diagnostic and ablation catheters to our product portfolio and significantly expanded our international distribution and market development capabilities.
31
The diagram below depicts a chronology of these and other key events since our inception:
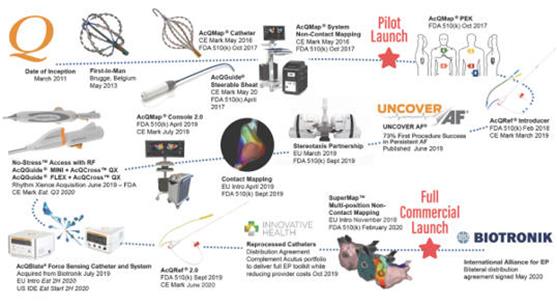
We market our electrophysiology products worldwide to hospitals and electrophysiologists that treat patients with arrhythmias. We have strategically developed a direct selling presence in the United States and select markets in Western Europe where cardiac ablation is a standard of care and third-party reimbursement is well-established. In these markets, we install our AcQMap console and workstation with customer accounts and then sell our disposable products to those accounts for use with our system. In other international markets, we leverage our partnership with Biotronik to install our AcQMap console and workstation with customer accounts and then to sell our disposable products to those accounts. Once an AcQMap console and workstation is established in a customer account, our revenue from that account becomes predominantly recurring in nature and derived from the sale of our portfolio of disposable products used with our system. Our currently marketed disposable products include access sheaths, transseptal crossing tools, diagnostic and mapping catheters, ablation catheters and accessories. We plan to leverage the geographically concentrated nature of procedure volumes and the recurring nature of our sales to drive an increasingly efficient commercial model.
As of September 30, 2020, our commercial organization consisted of 62 individuals with substantial applicable medical device, sales and clinical experience, including sales managers, sales representatives and mappers. Over time, we plan to selectively add highly qualified personnel to our commercial organization with a strategic mix of sales representatives and mappers to cover the concentrated group of hospitals that we believe perform the majority of the cardiac ablation procedures in our direct markets.
Our revenue has historically consisted predominantly of sales of our disposable products (principally our mapping catheters and related access sheaths, and to a lesser extent our transseptal crossing tools, ablation catheters and other accessories), as we generally loaned our first-generation AcQMap console and workstation to our customers without charge to facilitate the use of our disposable products. Beginning in late 2019, we began to install our second-generation AcQMap console and workstation with customers under evaluation contracts. Under these evaluation contracts, we place our AcQMap console and workstation with customers for no upfront fee to the customer during the applicable evaluation period and seek to reach agreement with the customer for purchase of the console and workstation in the form of a contractual commitment to purchase a minimum amount of our disposable products or a cash purchase. In addition, we have also generated a small portion of our revenue from service agreements with our customers.
We currently manufacture our novel access sheaths, transseptal crossing tools, diagnostic and mapping catheters, ablation catheters, mapping and imaging consoles and accessories at our approximately 50,800 square foot facility in Carlsbad, California. This facility provides approximately 15,750 square feet of space for our production and distribution operations, including manufacturing, quality control and storage. In addition, we stock inventory of raw materials, components and finished goods at our facility in Carlsbad and, to a limited extent, with our sales representatives, who travel to our customers’ locations as part of their sales efforts. We rely on a single or limited number of suppliers for certain raw materials and components, and we generally have no long-term supply arrangements with our suppliers, as we generally order on a purchase order basis. Furthermore, we rely on third parties to manufacture certain products we offer our customers as part of our product portfolio, including Biotronik for diagnostic and ablation catheters, radiofrequency, or RF, generators and irrigation pumps, Innovative Health for reprocessed diagnostic catheters and MedFact for robotic navigation enabled ablation catheters.
32
As of September 30, 2020, we have completed three clinical trials that collectively evaluated 223 subjects across 16 centers in multiple countries. We are currently conducting two post-market trials to provide physicians with additional safety and effectiveness data on the use of our AcQMap System. Global data has been collected on over 100 participants. We are planning two investigational device exemption (IDE) studies to support U.S. regulatory approval of our AcQBlate Force ablation catheters. Our ongoing and planned trials are anticipated to involve an aggregate of over 700 subjects in at least 35 centers in the United States and internationally. We expect to provide data readouts from these ongoing and planned trials at various points in time through early 2024.
For the nine months ended September 30, 2020, and 2019, we generated revenue of $5.9 million and $2.2 million, respectively, of which 46% and 72%, respectively, was from customers located outside of the United States. Since our inception, we have generated significant losses. Our net loss was $72.6 million and $77.1 million for the nine months ended September 30, 2020 and 2019, respectively. As of September 30, 2020 and December 31, 2019, we had an accumulated deficit of $331.6 million and $259.0 million, respectively, and working capital of $159.5 million and $50.5 million, respectively. Prior to our initial public offering (“IPO”) on August 10, 2020, our operations have been financed primarily by aggregate net proceeds from the sale of our convertible preferred stock and principal of our converted debt of $253.9 million, as well as other indebtedness.
We intend to continue to make significant investments in our sales and marketing organization. We believe increasing the number of sales representatives and expanding our international marketing programs will help facilitate further adoption of our products among existing customer accounts as well as broaden awareness of our products to new accounts. We also expect to continue to make substantial investments in our ongoing clinical trials and in additional clinical trials that are designed to provide clinical evidence of the safety and effectiveness of our existing and future generations of products. We expect to continue to make investments in research and development and regulatory affairs to develop future generations of products based on our technology, supported with appropriate regulatory submissions. We may in the future seek to acquire or invest in additional businesses, products or technologies that we believe could complement or expand our portfolio, enhance our technical capabilities or otherwise offer growth opportunities. We will also incur costs as a public company that we have not previously incurred or have previously incurred at lower rates, including increased costs for employee-related expenses, director and officer insurance premiums, audit and legal fees, investor relations fees, fees to members of our board of directors and expenses for compliance with public-company reporting requirements under the Exchange Act and rules implemented by the SEC, as well as Nasdaq rules. Because of these and other factors, we expect to continue to incur substantial net losses and negative cash flows from operations for at least the next several years.
Biotronik Agreements
Biotronik License Agreement
In July 2019, we entered into the Biotronik License Agreement with Biotronik and VascoMed GmbH, or VascoMed (who we refer to together as the Biotronik Parties), whereby we acquired certain manufacturing equipment and obtained from the Biotronik Parties a license under certain patents and technology to develop, commercialize, distribute and manufacture our AcQBlate Force ablation catheters and Qubic Force device. We refer to this transaction as the Biotronik Asset Acquisition. Pursuant to the Biotronik License Agreement, we paid Biotronik a $3.0 million upfront fee at the time the agreement was signed, as well as a technology transfer fee consisting of $7.0 million in cash in December 2019 and $5.0 million in shares of our Series D convertible preferred stock in February 2020.
The Biotronik License Agreement also requires that we pay the Biotronik Parties certain milestone payments as follows: (i) $2.0 million upon receipt of marketing approval for the sale of our AcQBlate Force ablation catheters in Europe; (ii) $5.0 million upon the receipt of marketing approval for the sale of our AcQBlate Force ablation catheters in the United States; and (iii) $3.0 million upon the first commercial sale of our AcQBlate Force ablation catheters in the United States. We are also required to pay the Biotronik Parties unit-based royalties on any sales we make of our AcQBlate Force ablation catheters following commercialization.
Bi-Lateral Distribution Agreements
In May 2020, we entered into more expansive bi-lateral distribution agreements with Biotronik. We refer to these agreements as the Bi-Lateral Distribution Agreements and our relationship with Biotronik as the Acutus/Biotronik Global Alliance for Electrophysiology. Pursuant to our Bi-Lateral Distribution Agreements, we obtained a non-exclusive license to distribute a range of Biotronik’s therapeutic electrophysiology products and accessories (including the AlCath family of RF ablation catheters) in the United States, Canada, China, Hong Kong and multiple Western European countries under our own private label. Moreover, if an IDE clinical trial is required for these products to obtain regulatory approval in the United States, or a clinical trial is required for these products to obtain regulatory approval in China, we will obtain an exclusive distribution right in such territories for a term of up to five years commencing on the date of regulatory approval if we cover the cost of the IDE or other clinical trial and we conduct such study within a specified period. We also obtained a non-exclusive license to distribute a range of Biotronik’s diagnostic electrophysiology products and accessories in each of the foregoing territories under our own private label.
33
Pursuant to the Bi-Lateral Distribution Agreements, Biotronik has also agreed to distribute our products, including our AcQMap System, our Qubic Force device and our disposable products (including our AcQBlate Force catheters) and accessories in Germany, Japan, Mexico, Switzerland and multiple countries in Asia-Pacific, Eastern Europe, the Middle East and South America. We also granted Biotronik a co-exclusive right to distribute these products in Hong Kong. Biotronik is required to use our branding with respect to the AcQMap console and workstation, but retains the right to distribute our disposable products and accessories under its private label. Each party will pay to the other party specified transfer prices on the sale of the other party’s products under the Bi-Lateral Distribution Agreements and, accordingly, will earn a distribution margin on the sale of the other party’s products.
Key Business Metric
We regularly review a number of operating and financial metrics, including the following key business metric, to evaluate our business, measure our performance, identify trends affecting our business, formulate financial projections and make strategic decisions. We believe that the following metric is representative of our current business. However, we anticipate this metric may change or may be substituted for additional or different metrics as our business grows and as we introduce new products.
Installed Base
Once an AcQMap console and workstation is established in a customer account, our revenue from that account becomes predominantly recurring in nature and derived from the sale of our portfolio of disposable products used with our system. We believe our installed base is one of the key indicators of our ability to drive customer adoption of our products. We define our installed base as the cumulative number of AcQMap consoles and workstations placed into service at customer sites. Beginning in late 2019, we began to install our second-generation AcQMap console and workstation with customers under evaluation contracts. Under these evaluation contracts, we place our AcQMap console and workstation with customers for no upfront fee to the customer during the applicable evaluation period and seek to reach agreement with the customer for the purchase of the console and workstation in the form of a contractual commitment to purchase a minimum amount of our disposable products or a cash purchase. Our total installed base as of September 30, 2020 and 2019 is set forth in the table below:
|
|
|
As of September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
US |
|
|
29 |
|
|
|
6 |
|
|
Europe |
|
|
15 |
|
|
|
17 |
|
|
Total Acutus Direct |
|
|
44 |
|
|
|
23 |
|
|
Biotronik |
|
|
5 |
|
|
|
— |
|
|
Total installed base |
|
|
49 |
|
|
|
23 |
|
Our net increase in installed base for the three and nine months ended September 30, 2020 and 2019, exclusive of transfers between Acutus and Biotronik, is set forth in the table below:
|
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
2020 |
|
|
2019 |
|
||||
|
|
|
(unaudited) |
|
|
(unaudited) |
|
||||||||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US |
|
|
9 |
|
|
|
(2 |
) |
|
|
19 |
|
|
|
1 |
|
|
Europe |
|
|
2 |
|
|
|
(1 |
) |
|
|
3 |
|
|
|
1 |
|
|
Total Acutus Direct |
|
|
11 |
|
|
|
(3 |
) |
|
|
22 |
|
|
|
2 |
|
|
Net systems to Biotronik |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total net system placements |
|
|
11 |
|
|
|
(3 |
) |
|
|
22 |
|
|
|
2 |
|
Growth in our quarterly installed base can fluctuate due to a number of factors, including the commercial effectiveness of our sales representatives and strategic partners such as Biotronik, and the procurement and budgeting cycles of many of our customers, especially those where unused funds may be forfeited or future budgets may be reduced if purchases are not made by their fiscal year end. We also believe the timing of installations has been impacted and will continue to be impacted by the timing of product introductions and transitions. In addition, the growth of our market in certain geographic regions and our continued efforts to service these regions impact unit volumes quarter to quarter.
34
Factors Affecting Our Performance
There are a number of factors that have impacted, and we believe will continue to impact, or that we expect to impact, our results of operations and growth. These factors include:
|
|
• |
Market Acceptance. The growth of our business will depend substantially on our ability to increase our installed base. Once an AcQMap console and workstation is established in a customer account, our revenue from that account becomes predominantly recurring in nature and derived from the sale of our portfolio of disposable products used with our system. Our ability to increase our installed base will depend on our ability to gain broader acceptance of our AcQMap System by continuing to make physicians and other hospital staff aware of the benefits of the AcQMap System, thereby generating increased demand for system installations and the frequency of use of our disposable products. Although we are attempting to increase our installed base through our established relationships and focused sales efforts, we cannot provide assurance that our efforts will be successful. |
|
|
• |
Commercial Organization Size and Effectiveness. As of September 30, 2020, our commercial organization consisted of 62 individuals with substantial applicable medical device, sales and clinical experience, including sales managers, sales representatives and mappers. We intend to continue to make significant investments in our commercial organization by increasing the number of our sales representatives, sales managers and mappers, as well as by expanding our global marketing and training programs, to help facilitate further adoption of our products among existing and new customer accounts. The rate at which we grow our commercial organization and the speed at which newly hired personnel become effective can impact our revenue growth or our costs incurred in anticipation of such growth. |
|
|
• |
Strategic Partnerships and Acquisitions. We have in the past, and may in the future, enter into strategic partnerships and acquire complementary businesses, products or technologies. For example, we have entered into strategic partnerships with Innovative Health and Stereotaxis and, most recently, we entered into our Global Alliance for Electrophysiology with Biotronik in May 2020. In addition, we added an integrated family of transseptal crossing and steerable introducer systems to our product portfolio through our acquisition of Rhythm Xience in June 2019 and acquired our AcQBlate Force sensing product line from Biotronik in July 2019. Our strategic partnerships and acquisitions have helped us establish a global sales presence delivering a broad portfolio of highly differentiated electrophysiology products. Our ability to grow our revenue will depend substantially on our ability to leverage our strategic partnerships and acquisitions to achieve distribution at a global scale, broaden our product portfolio and enable and accelerate global connectivity. |
|
|
• |
Continued Investment in Innovation. Our business strategy relies significantly on innovation to develop and introduce new products and to differentiate our products from our competitors. For example, in 2019, our research and development team released five new disposable products, two hardware products, including a major generational update to our AcQMap System, and 15 software updates. We expect our research and development expenditures to increase as we make additional investments to support our growth strategies. We plan to increase our research and development expenditures with internal initiatives, as well as potentially licensing or acquiring technology from third parties. We also expect expenditures associated with our manufacturing organization to grow over time as production volume increases and we bring new products to market. Our internal and external investments will be focused on initiatives that we believe will offer the greatest opportunity for growth and profitability. With a significant investment in research and development, a strong focus on innovation and a well-managed innovation process, we believe we can continue to innovate and grow. Introducing additional, innovative products is also expected to help support our existing installed base and help drive demand for additional installations of our system. If, however, our future innovations are not successful in meeting customers’ needs or prove to be too costly relative to their perceived benefit, we may not be successful. Moreover, as cost of products sold, operating expenses and capital expenditures fluctuate over time, we may experience short-term, negative impacts to our results of operations and cash flows, but we are undertaking such investments in the belief that they will contribute to long-term growth. |
|
|
• |
Product and Geographic Mix; Timing. Our financial results, including our gross margins, may fluctuate from period to period due to a variety of factors, including: average selling prices; production volumes; the cost of direct materials; the timing of customer orders or medical procedures and the timing and number of system installations; the number of available selling days in a particular period, which can be impacted by a number of factors such as holidays or days of severe inclement weather in a particular geography; the mix of products sold and the geographic mix of where products are sold; the level of reimbursement available for our products; discounting practices; manufacturing costs; product yields; headcount; and cost-reduction strategies. For example, gross margins on the sale of our products by our direct selling organization in the United States and Western Europe are higher than gross margins on the sale of our products by Biotronik in other parts of the world. Moreover, gross margins on the sale of our proprietary products are generally higher than gross margins on the sale of products we source through our strategic partnerships with third parties. Future selling prices and gross margins for our products may fluctuate due to a variety of other factors, including the introduction by others of competing products or the attempted integration by third parties of capabilities similar to ours into their existing products. We aim to mitigate downward pressure on our selling prices by increasing the value proposition offered by our products through innovation. While we have not yet experienced significant seasonality in our results, it is not uncommon in our industry to experience seasonally weaker revenue during the summer months and end-of-year holiday season. |
35
|
|
• |
Competition. Our industry is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. Our most significant competitors are large, well-capitalized companies. We must continue to successfully compete considering our competitors’ existing and future products and related pricing and their resources to successfully market to the physicians who could use our products. Publications of clinical results by us, our competitors and other third parties can also have a significant influence on whether, and the degree to which, we are able to gain market share and increase utilization of our products. |
|
|
• |
COVID-19 Pandemic. Beginning in early March 2020, the COVID-19 pandemic and the measures imposed to contain this pandemic disrupted and are expected to continue to impact our business. For example, on March 19, 2020, the Executive Department of the State of California issued Executive Order N-33-20, ordering all individuals in the State of California to stay home or at their place of residence except as needed to maintain continuity of operations of the federal critical infrastructure sectors. Our primary operations are located in Carlsbad, California. As a result of such order, the majority of our employees have telecommuted, which may impact certain of our operations over the near term and long term. Moreover, beginning in March 2020, access to hospitals and other customer sites was restricted to essential personnel, which negatively impacted our ability to install our AcQMap consoles and workstations in new accounts and for our sales representatives and mappers to promote the use of our products with physicians. Moreover, hospitals and other therapeutic centers suspended many elective procedures, resulting in a significantly reduced volume of procedures using our products. In addition, all clinical trials in Europe were suspended with follow-ups for clinical trials done via telecom, and we believe enrollment timing in our planned clinical trials will be slowed due to COVID-19 driven delayed access to enrollment sites. As a result of the interruptions to our business due to COVID-19, we enacted a cash conservation program, which included delaying certain non-critical capital expenditures and other projects and implementing a hiring freeze, headcount reductions and temporary compensation reductions (through August 2020). Although the effects of the pandemic began to decrease in late April 2020 as electrophysiology labs began reopening and procedure volumes began increasing as compared to COVID-19 related low points in March 2020, the magnitude of the impact of the COVID-19 pandemic on our productivity, results of operations and financial position, and its disruption to our business and our clinical programs and timelines, will depend, in part, on the length and severity of these restrictions and on our ability to conduct business in the ordinary course. Quarantines, shelter-in-place and similar government orders have also impacted, and may continue to impact, our third-party manufacturers and suppliers, and could in turn adversely impact the availability or cost of materials, which could disrupt our supply chain. |
In addition, we may experience meaningful variability in our quarterly revenue and gross profit/loss as a result of a number of factors, including, but not limited to: inventory write-offs and write-downs; costs, benefits and timing of new product introductions; the availability and cost of components and raw materials; and fluctuations in foreign currency exchange rates. Additionally, we may experience quarters in which our costs and operating expenses, in particular our research and development expenses, fluctuate depending on the stage and timing of product development.
While certain of these factors may present significant opportunities for us, they all pose significant risks and challenges that we must address. See the section titled “Risk Factors” for more information.
Components of Results of Operations
Revenue
Our revenue consists of: (i) revenue from the sale of our disposable products; and (ii) systems and service revenue. In the United States and select markets in Western Europe where we have developed a direct selling presence, we install our AcQMap console and workstation with our customer accounts and then generate revenue from the sale of our disposable products to these accounts for use with our system. In other international markets, we leverage our partnership with Biotronik to install our AcQMap console and workstation with customer accounts and then generate revenue from Biotronik’s sale of our disposable products to these accounts for use with our system. Our currently marketed disposable products include access sheaths, transseptal crossing tools, diagnostic and mapping catheters, ablation catheters and accessories.
36
Our revenue has historically consisted predominantly of sales of our disposable products (principally our mapping catheters and related access sheaths, and to a lesser extent our transseptal crossing tools, ablation catheters and other accessories), as we generally loaned our first-generation AcQMap console and workstation to our customers without charge to facilitate the use of our disposable products. Beginning in late 2019, we began to install our second-generation AcQMap console and workstation with customers under evaluation contracts. Under these evaluation contracts, we place our second-generation AcQMap console and workstation with customers for no upfront fee to the customer during the applicable evaluation period and seek to reach agreement with the customer for the purchase of the console and workstation in the form of a contractual commitment to purchase a minimum amount of our disposable products or a cash purchase. When a sale of a second-generation AcQMap system is made, the sale includes installation of the equipment, software updates and maintenance, and equipment service. Evaluation contracts are not accounted for as sales under Accounting Standards Codification, or ASC, 606, Revenue from Contracts with Customers. In addition, we also generate a small portion of our revenue from service agreements. Revenue is recognized when the customer obtains control of the promised goods or services, generally at a point in time, and is recognized in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. For the nine months ended September 30, 2020 and 2019, approximately 46% and 72%, respectively, of our sales were denominated in currencies other than U.S. dollars, primarily in Euros and the British Pound Sterling, or GBP. Our revenue is subject to fluctuation based on the foreign currency in which our products are sold.
Costs and Operating Expenses
Cost of Products Sold
Cost of products sold consist primarily of raw materials, direct labor, manufacturing overhead associated with the production and sale of our disposable products and, to a more limited extent, production and depreciation of our AcQMap console and workstation that we install with our customer accounts. We depreciate equipment over a three-year period. Cost of products sold also includes expenditures for warranty, field service, freight, royalties and inventory reserve provisions. We expect cost of products sold to increase in absolute dollars in future periods as our revenue increases.
Gross profit is calculated as revenue less cost of products sold. Gross margin is gross profit expressed as a percentage of revenue. Our gross margins may fluctuate from period to period due to a variety of factors, including: average selling prices; production volumes; the cost of direct materials; the timing of customer orders or medical procedures and the timing and number of system installations; the number of available selling days in a particular period, which can be impacted by a number of factors such as holidays or days of severe inclement weather in a particular geography; the mix of products sold and the geographic mix of where products are sold; the level of reimbursement available for our products; discounting practices; manufacturing costs; product yields; headcount; and cost-reduction strategies. For example, gross margins on the sale of our products by our direct selling organization in the United States and Western Europe are higher than gross margins on the sale of our products by Biotronik in other parts of the world. Moreover, gross margins on the sale of our proprietary products are generally higher than gross margins on the sale of products we source through our strategic partnerships with third parties. Future selling prices and gross margins for our products may fluctuate due to a variety of other factors, including the introduction by others of competing products or the attempted integration by third parties of capabilities similar to ours into their existing products. We aim to mitigate downward pressure on our selling prices by increasing the value proposition offered by our products through innovation.
In addition, we have experienced negative gross margins in recent periods as a result of significant investments in our infrastructure to support our commercial launch and to enable our production volumes to scale as our business grows. We expect our gross margins to increase over the long term to the extent we are successful in increasing our sales volume and are therefore able to leverage our fixed costs. We intend to use our design, engineering and manufacturing capabilities to further advance and improve the efficiency of our manufacturing processes, which, if successful, we believe will reduce costs and enable us to increase our gross margins. Such manufacturing cost improvement efforts may involve moving production of key subassemblies in house, volume driven supplier cost reductions and process redesigns. While we expect gross margins to increase over the long term, they will likely fluctuate from quarter to quarter as we continue to introduce new products and adopt new manufacturing processes and technologies.
Research and Development Expenses
Research and development expenses consist primarily of salaries and employee-related costs (including stock-based compensation) for personnel directly engaged in research and development activities, clinical trial expenses, equipment costs, materials costs, allocated rent and facilities costs and depreciation.
Research and development expenses related to possible future products are expensed as incurred. We also accrue and expense costs for activities associated with clinical trials performed by third parties as incurred. All other costs relative to setting up clinical trial sites are expensed as incurred. Clinical trial site costs related to patient enrollment are accrued as patients are entered into the trials.
37
We expect our research and development expenses to increase in absolute dollars for the foreseeable future, though they may vary from period to period as a percentage of revenue, as we hire additional research and development personnel, as well as continue to develop new products, enhance existing products and technologies and perform activities related to obtaining additional regulatory approvals or clearances.
Research and Development Expenses—License Acquired
In July 2019, we entered into the Biotronik License Agreement with the Biotronik Parties in connection with the Biotronik Asset Acquisition. In accordance with ASC 805, Business Combinations, the Biotronik Asset Acquisition was accounted for as an asset acquisition as substantially all of the $15.0 million in value transferred to Biotronik was allocated to intellectual property. On the acquisition date, the products licensed had not yet received regulatory approval and the intellectual property did not have an alternative use. Accordingly, the $15.0 million paid to Biotronik was immediately charged to research and development expenses—license acquired in our consolidated statement of operations and comprehensive loss in July 2019. Additional contingent milestone payments of up to $10.0 million are to be made to the Biotronik Parties upon certain regulatory approvals and first commercial sale, as described above. In further consideration of the rights granted, beginning with our first commercial sale of the first force sensing ablation catheter within the licensed product line, we will also make per unit royalty payments. We have determined that as of the acquisition date and as of September 30, 2020 and December 31, 2019, the contingent milestone and royalty payments are not probable and estimable and therefore have not been recorded as a liability. Upon regulatory approval of our force sensing ablation catheter in Europe, the milestone payments will be capitalized and amortized, and the royalty payments will be recorded as cost of products sold as sales of catheters are recognized.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of salaries and employee-related costs (including stock-based compensation) for personnel in sales, executive, finance and other administrative functions, allocated rent and facilities costs, legal fees relating to intellectual property and corporate matters, professional fees for accounting and consulting services, marketing costs and insurance costs.
We expect our selling, general and administrative expenses to increase in absolute dollars for the foreseeable future, though they may vary from period to period as a percentage of revenue, as we expand our sale force and increase the number of our mappers, increase our professional education and physician training, as well as to support our expanded infrastructure and incur increased costs associated with operating as a public company. These increases are expected to include increased costs for fees to members of our board of directors, increased employee-related expenses, and increased director and officer insurance premiums, audit and legal fees, investor relations fees and expenses for compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC, as well as stock exchange rules.
In connection with our IPO, certain performance-based restricted stock awards vested as the completion of our IPO constituted the relevant performance condition. As a result, we recorded an incremental stock-based compensation charge of $3.8 million for the awards that vested upon the IPO as part of selling, general and administrative expenses for the quarter ended September 30, 2020 in our condensed consolidated financial statements.
Other Income (Expense)
Change in Fair Value of Warrant Liability
We accounted for certain of our freestanding warrants to purchase shares of our common stock and preferred stock as liabilities at fair value. We accounted for certain features of our convertible notes issued in 2018, or the 2018 Convertible Notes (which were converted into shares of our Series D convertible preferred stock in 2019), that were determined to be an embedded derivative requiring bifurcation and separate accounting at fair value. The warrants and embedded derivative were subject to re-measurement at each balance sheet date and upon reclassification to stockholders’ equity with gains and losses reported in our condensed consolidated statements of operations and comprehensive loss. On August 5, 2020, in connection with the IPO being declared effective, the warrants recorded as liabilities no longer meet the definition of a derivative. Accordingly, the fair value of the common and preferred stock warrant liability was reclassified to stockholders’ equity (deficit) in the condensed consolidated balance sheet.
Loss on Debt Extinguishment
During 2019, we repaid the entire principal amount of our loan under our loan and security agreement with Oxford Finance LLC, or the 2018 Term Loan. We recorded a loss on debt extinguishment for the write off of deferred financing fees, the prepayment penalty and related fees upon our prepayment of this loan.
38
Interest income consists primarily of interest earned on our cash, cash equivalents and marketable securities.
Interest Expense
Interest expense relates to our: (i) Credit Agreement with Orbimed Royalty Opportunities II, LP and Deerfield Private Design Fund II, L.P., or the 2019 Credit Agreement; (ii) 2018 Term Loan, which was repaid during 2019; (iii) 2018 Convertible Notes; and (iv) convertible notes issued in 2019, or the 2019 Convertible Notes. Our 2018 Convertible Notes and our 2019 Convertible Notes were converted into shares of our Series D convertible preferred stock during 2019.
Results of Operations for the Three Months Ended September 30, 2020 and 2019
The results of operations presented below should be reviewed in conjunction with our condensed consolidated financial statements and related notes included elsewhere in this Form 10-Q. The following table sets forth our results of operations for the three months ended September 30, 2020 and 2019:
|
|
|
Three Months Ended September 30, |
|
|
Change |
|
||||||||||
|
(dollars in thousands) |
|
2020 |
|
|
2019 |
|
|
$ |
|
|
% |
|
||||
|
|
|
(Unaudited) |
|
|
|
|
|
|
|
|
|
|||||
|
Revenue(2) |
|
$ |
3,173 |
|
|
$ |
646 |
|
|
$ |
2,527 |
|
|
|
391 |
% |
|
Costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Costs of products sold(1) |
|
|
5,141 |
|
|
|
2,267 |
|
|
|
2,874 |
|
|
|
127 |
% |
|
Research and development(1) |
|
|
8,343 |
|
|
|
5,865 |
|
|
|
2,478 |
|
|
|
42 |
% |
|
Research and development - license acquired |
|
|
— |
|
|
|
15,000 |
|
|
|
(15,000 |
) |
|
NM |
|
|
|
Selling, general and administrative(1) |
|
|
15,833 |
|
|
|
7,978 |
|
|
|
7,855 |
|
|
|
98 |
% |
|
Change in fair value of contingent consideration |
|
|
118 |
|
|
|
700 |
|
|
|
(582 |
) |
|
|
(83 |
%) |
|
Total costs and operating expenses |
|
|
29,435 |
|
|
|
31,810 |
|
|
|
(2,375 |
) |
|
|
(7 |
%) |
|
Loss from operations |
|
|
(26,262 |
) |
|
|
(31,164 |
) |
|
|
4,902 |
|
|
|
(16 |
%) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrant liability |
|
|
(3,683 |
) |
|
|
(3 |
) |
|
|
(3,680 |
) |
|
NM |
|
|
|
Loss on debt extinguishment |
|
|
— |
|
|
|
(49 |
) |
|
|
49 |
|
|
NM |
|
|
|
Interest income |
|
|
23 |
|
|
|
525 |
|
|
|
(502 |
) |
|
|
(96 |
%) |
|
Interest expense |
|
|
(1,366 |
) |
|
|
(1,394 |
) |
|
|
28 |
|
|
|
(2 |
%) |
|
Total other expense, net |
|
|
(5,026 |
) |
|
|
(921 |
) |
|
|
(4,105 |
) |
|
NM |
|
|
|
Net loss |
|
$ |
(31,288 |
) |
|
$ |
(32,085 |
) |
|
$ |
797 |
|
|
|
(2 |
%) |
|
Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on marketable securities |
|
|
(9 |
) |
|
|
40 |
|
|
|
(49 |
) |
|
NM |
|
|
|
Foreign currency translation adjustment |
|
|
78 |
|
|
|
(45 |
) |
|
|
123 |
|
|
NM |
|
|
|
Comprehensive loss |
|
$ |
(31,219 |
) |
|
$ |
(32,090 |
) |
|
$ |
871 |
|
|
|
(3 |
%) |
NM - Not meaningful
|
(1) |
The following table sets forth the stock-based compensation expense included in our results of operations for the three months ended September 30, 2020 and 2019: |
|
|
|
Three Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Cost of products sold |
|
$ |
126 |
|
|
$ |
56 |
|
|
Research and development |
|
|
319 |
|
|
|
179 |
|
|
Selling, general and administrative |
|
|
5,929 |
|
|
|
577 |
|
|
Total stock-based compensation |
|
$ |
6,374 |
|
|
$ |
812 |
|
|
(2) |
The following tables sets forth our revenue for disposables and systems/service for the three months ended September 30, 2020 and 2019: |
39
|
|
|
Three Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
Disposables |
|
$ |
1,680 |
|
|
$ |
635 |
|
|
Systems |
|
|
965 |
|
|
|
— |
|
|
Service/Other |
|
|
29 |
|
|
|
3 |
|
|
Total Acutus direct revenue |
|
|
2,674 |
|
|
|
638 |
|
|
Distribution agreements |
|
|
499 |
|
|
|
8 |
|
|
Total revenue |
|
$ |
3,173 |
|
|
$ |
646 |
|
|
|
|
Three Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
United States |
|
$ |
1,790 |
|
|
$ |
147 |
|
|
Europe |
|
|
884 |
|
|
|
491 |
|
|
Total Acutus direct revenue |
|
|
2,674 |
|
|
|
638 |
|
|
Distribution Agreements |
|
|
|
|
|
|
|
|
|
United States |
|
|
77 |
|
|
|
— |
|
|
Europe |
|
|
422 |
|
|
|
8 |
|
|
Total revenue through distribution |
|
|
499 |
|
|
|
8 |
|
|
Total revenue |
|
$ |
3,173 |
|
|
$ |
646 |
|
Revenue
Revenue was $3.2 million for the three months ended September 30, 2020, compared to $0.6 million for the three months ended September 30, 2019. This increase of $2.5 million, or 391%, was primarily attributable to a $1.6 million increase in purchase volume of our disposable products used in electrophysiology procedures as a result of a higher installed base and a $1.0 million increase in AcQMap System sales.
Revenue, classified by the major geographic areas, was $1.9 million for the United States and $1.3 million for all other countries in the three months ended September 30, 2020, compared to $0.1 million for the United States and $0.5 million for all other countries for the comparative period in 2019.
Costs and Operating Expenses
Cost of Products Sold
Cost of products sold was $5.1 million for the three months ended September 30, 2020, compared to $2.3 million for the three months ended September 30, 2019. This increase of $2.9 million, or 127%, was driven by an increase of $2.2 million of costs from growth in our sales volume, $0.4 million of depreciation and freight expense to support the higher installed base, and $0.3 million increase in warranty and field service. Gross margin was negative 62% for the three months ended September 30, 2020 compared to negative 251% for the three months ended September 30, 2019. This improvement in gross margin was primarily attributable to increased sales volume and manufacturing efficiencies.
Research and Development Expenses
Research and development expenses were $8.3 million for the three months ended September 30, 2020, compared to $5.9 million for the three months ended September 30, 2019. This increase of $2.5 million, or 42%, was primarily attributable to $1.7 million in increased compensation and related costs from higher headcount, and $0.8 million in increased materials and supplies costs related to higher engineering project spending.
40
Research and Development Expenses – License Acquired
Research and development expenses – license acquired were $15.0 million for the three months ended September 30, 2019, related to the Biotronik Asset Acquisition, where we acquired intellectual property. Since the acquired intellectual property had no alternative use and was for products that have not yet received regulatory approval, the intellectual property was immediately charged to research and development expenses—license acquired.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $15.8 million for the three months ended September 30, 2020, compared to $8.0 million for the three months ended September 30, 2019. This increase of $7.9 million, or 98%, was primarily attributable to $3.8 million of stock-based compensation for certain performance-based awards as the applicable performance conditions were achieved upon the completion of our IPO and $3.5 million in increased compensation and related costs due to our investment in our commercial organization in support of our full commercial launch in the United States which started in the first quarter of 2020.
Change in Fair Value of Contingent Consideration
For the three months ended September 30, 2020 and 2019, we recorded a change in fair value of contingent consideration of $0.1 million and $0.7 million, respectively, for the increase in the fair value of the contingent consideration for the acquisition of Rhythm Xience.
Other Income (Expense)
Other expense, net was $5.0 million for the three months ended September 30, 2020, compared to $0.9 million for the three months ended September 30, 2019. This increase of $4.1 million was primarily attributable to an increase of $3.7 million in the fair value of the warrant liability.
Results of Operations for the Nine Months Ended September 30, 2020 and 2019
The results of operations presented below should be reviewed in conjunction with our condensed consolidated financial statements and related notes included elsewhere in this Form 10-Q. The following table sets forth our results of operations for the nine months ended September 30, 2020 and 2019:
|
|
|
Nine Months Ended September 30, |
|
|
Change |
|
||||||||||
|
|
|
2020 |
|
|
2019 |
|
|
$ |
|
|
% |
|
||||
|
|
|
(Unaudited) |
|
|
|
|
|
|
|
|
|
|||||
|
Revenue(2) |
|
$ |
5,890 |
|
|
$ |
2,167 |
|
|
$ |
3,723 |
|
|
|
172 |
% |
|
Costs and operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Costs of products sold(1) |
|
|
10,998 |
|
|
|
6,878 |
|
|
|
4,120 |
|
|
|
60 |
% |
|
Research and development(1) |
|
|
24,492 |
|
|
|
15,489 |
|
|
|
9,003 |
|
|
|
58 |
% |
|
Research and development - license acquired |
|
|
— |
|
|
|
15,000 |
|
|
|
(15,000 |
) |
|
NM |
|
|
|
Selling, general and administrative(1) |
|
|
35,193 |
|
|
|
18,998 |
|
|
|
16,195 |
|
|
|
85 |
% |
|
Change in fair value of contingent consideration |
|
|
(1,466 |
) |
|
|
700 |
|
|
|
(2,166 |
) |
|
NM |
|
|
|
Total costs and operating expenses |
|
|
69,217 |
|
|
|
57,065 |
|
|
|
12,152 |
|
|
|
21 |
% |
|
Loss from operations |
|
|
(63,327 |
) |
|
|
(54,898 |
) |
|
|
(8,429 |
) |
|
|
15 |
% |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrant liability and embedded derivative |
|
|
(5,555 |
) |
|
|
(608 |
) |
|
|
(4,947 |
) |
|
NM |
|
|
|
Loss on debt extinguishment |
|
|
— |
|
|
|
(1,447 |
) |
|
|
1,447 |
|
|
NM |
|
|
|
Interest income |
|
|
393 |
|
|
|
733 |
|
|
|
(340 |
) |
|
|
(46 |
%) |
|
Interest expense |
|
|
(4,090 |
) |
|
|
(20,905 |
) |
|
|
16,815 |
|
|
|
(80 |
%) |
|
Total other expense, net |
|
|
(9,252 |
) |
|
|
(22,227 |
) |
|
|
12,975 |
|
|
|
(58 |
%) |
|
Net loss |
|
$ |
(72,579 |
) |
|
$ |
(77,125 |
) |
|
$ |
4,546 |
|
|
|
(6 |
%) |
|
Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on marketable securities |
|
|
(50 |
) |
|
|
47 |
|
|
|
(97 |
) |
|
NM |
|
|
|
Foreign currency translation adjustment |
|
|
147 |
|
|
|
(57 |
) |
|
|
204 |
|
|
NM |
|
|
|
Comprehensive loss |
|
$ |
(72,482 |
) |
|
$ |
(77,135 |
) |
|
$ |
4,653 |
|
|
|
(6 |
%) |
NM - Not meaningful
41
|
(1) |
The following table sets forth the stock-based compensation expense included in our results of operations for the nine months ended September 30, 2020 and 2019: |
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Cost of products sold |
|
$ |
292 |
|
|
$ |
162 |
|
|
Research and development |
|
|
697 |
|
|
|
471 |
|
|
Selling, general and administrative |
|
|
8,283 |
|
|
|
1,541 |
|
|
Total stock-based compensation |
|
$ |
9,272 |
|
|
$ |
2,174 |
|
|
(2) |
The following tables sets forth our revenue for disposables and systems/service for the nine months ended September 30, 2020 and 2019: |
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
Disposables |
|
$ |
3,599 |
|
|
$ |
2,123 |
|
|
Systems |
|
|
1,485 |
|
|
|
— |
|
|
Service/Other |
|
|
47 |
|
|
|
12 |
|
|
Total Acutus direct revenue |
|
|
5,131 |
|
|
|
2,135 |
|
|
Distribution agreements |
|
|
759 |
|
|
|
32 |
|
|
Total revenue |
|
$ |
5,890 |
|
|
$ |
2,167 |
|
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(unaudited) |
|
|||||
|
Acutus Direct |
|
|
|
|
|
|
|
|
|
United States |
|
$ |
3,103 |
|
|
$ |
603 |
|
|
Europe |
|
|
2,028 |
|
|
|
1,532 |
|
|
Total Acutus direct revenue |
|
|
5,131 |
|
|
|
2,135 |
|
|
Distribution Agreements |
|
|
|
|
|
|
|
|
|
United States |
|
|
92 |
|
|
|
— |
|
|
Europe |
|
|
667 |
|
|
|
32 |
|
|
Total revenue through distribution |
|
|
759 |
|
|
|
32 |
|
|
Total revenue |
|
$ |
5,890 |
|
|
$ |
2,167 |
|
Revenue
Revenue was $5.9 million for the nine months ended September 30, 2020, compared to $2.2 million for the nine months ended September 30, 2019. This increase of $3.7 million, or 172%, was primarily attributable to a $2.2 million increase in purchase volume of our disposable products used in electrophysiology procedures as a result of a higher installed base and $1.5 million of AcQMap systems sales.
Revenue, classified by the major geographic areas, was $3.2 million for the United States and $2.7 million for all other countries in the nine months ended September 30, 2020, compared to $0.6 million for the United States and $1.6 million for all other countries for the comparative period in 2019.
Costs and Operating Expenses
Cost of Products Sold
Cost of products sold was $11.0 million for the nine months ended September 30, 2020, compared to $6.9 million for the nine months ended September 30, 2019. This increase of $4.1 million, or 60%, was primarily driven by an increase of $2.9 million of costs
42
from growth in our sales volume, $0.7 million increase in warranty and field service expense to support the higher installed base, $0.2 million increase in royalties, and $0.2 million increase in freight. Gross margin was negative 87% for the nine months ended September 30, 2020 compared to negative 217% for the nine months ended September 30, 2019. This improvement in gross margin was primarily attributable to increased sales volume of our disposable products.
Research and Development Expenses
Research and development expenses were $24.5 million for the nine months ended September 30, 2020, compared to $15.5 million for the nine months ended September 30, 2019. This increase of $9.0 million, or 58%, was primarily attributable to $5.8 million in increased materials and supplies costs related to higher engineering project spending and $3.2 million in increased compensation and related costs from higher headcount.
Research and Development Expenses – License Acquired
Research and development expenses – license acquired were $15.0 million for the nine months ended September 30, 2019, related to the Biotronik Asset Acquisition, where we acquired intellectual property. Since the acquired intellectual property had no alternative use and was for products that have not yet received regulatory approval, the intellectual property was immediately charged to research and development expenses—license acquired.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $35.2 million for the nine months ended September 30, 2020, compared to $19.0 million for the nine months ended September 30, 2019. This increase of $16.2 million, or 85%, was primarily attributable to $10.2 million in increased compensation and related costs due to our investment in our commercial organization in support of our full commercial launch in the United States, $3.8 million of stock-based compensation for certain performance-based awards as the applicable performance conditions were achieved upon the completion of our IPO and $0.7 million in increased consulting expenses.
Change in Fair Value of Contingent Consideration
For the nine months ended September 30, 2020 and 2019, we recorded a change in fair value of contingent consideration of $1.5 million for the decrease and $0.7 million for the increase, respectively, of the contingent consideration for the acquisition of Rhythm Xience.
Other Income (Expense)
Other expense, net was $9.3 million for the nine months ended September 30, 2020, compared to $22.2 million for the nine months ended September 30, 2019. This decrease of $13.0 million, or 58%, was primarily attributable to a decrease of $16.8 million in interest expense primarily related to the 2019 Credit Agreement and 2018 Convertible Notes, partially offset by an increase of $4.9 million in the fair value of the warrant liability.
Liquidity and Capital Resources
We have incurred significant operating losses and negative cash flows from operations since our inception, and we anticipate that we will incur significant losses for at least the next several years. As of September 30, 2020 and December 31, 2019, we had cash and cash equivalents and marketable securities of $166.8 million and $71.8 million, respectively. For the nine months ended September 30, 2020 and the years ended December 31, 2019 and 2018, our net losses were $72.6 million, $97.0 million and $47.9 million, respectively, and our net cash used in operating activities was $61.0 million, $56.0 million and $33.8 million, respectively. We had an accumulated deficit of $331.6 million and $259.0 million as of September 30, 2020 and December 31, 2019, respectively.
43
Prior to our IPO in August 2020, our operations had been financed primarily by aggregate net proceeds from the sale of our convertible preferred stock and principal of our converted debt of $253.9 million, as well as other indebtedness. In June and July 2019, we completed an equity financing pursuant to which we issued 8,200,297 shares of Series D convertible preferred stock in a private placement. The Series D convertible preferred stock issuance was comprised of: (i) 4,091,819 shares at $16.67 per share for cash proceeds of $66.6 million, net of fees of $1.6 million; and (ii) 1,884,565 shares at $13.33 per share (including a 20% discount) for the conversion of our 2018 Convertible Notes (and related accrued interest) and 2,223,913 shares at $16.67 per share for the conversion of our 2019 Convertible Notes (and related accrued interest), in an aggregate amount of $68.5 million, including the fair value of the embedded derivative of $6.3 million relating to the 20% discount for the conversion of the 2018 Convertible Notes. On August 10, 2020, we issued 10,147,058 shares of common stock in our IPO, which included 1,323,529 shares of common stock issued upon the exercise in full by the underwriters of an option to purchase, at the public offering price less underwriting discounts and commissions, up to an additional 1,323,529 shares. The price to the public for each share was $18.00.
Our future liquidity and capital funding requirements will depend on numerous factors, including:
|
|
• |
our revenue growth; |
|
|
• |
our research and development efforts; |
|
|
• |
our sales and marketing activities; |
|
|
• |
our success in leveraging our strategic partnerships, including with Biotronik, as well as entrance into any other strategic partnerships or strategic transactions in the future; |
|
|
• |
our ability to raise additional funds to finance our operations; |
|
|
• |
the outcome, costs and timing of any clinical trial results for our current or future products; |
|
|
• |
the emergence and effect of competing or complementary products; |
|
|
• |
the availability and amount of reimbursement for procedures using our products; |
|
|
• |
our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
|
|
• |
our ability to retain our current employees and the need and ability to hire additional management and sales, scientific and medical personnel; |
|
|
• |
the terms and timing of any collaborative, licensing or other arrangements that we have or may establish; |
|
|
• |
debt service requirements; |
|
|
• |
the extent to which we acquire or invest in businesses, products or technologies; and |
|
|
• |
the impact of the COVID-19 pandemic. |
Our primary uses of capital are, and we expect will continue to be, investment in our commercial organization and related expenses, clinical research and development services, laboratory and related supplies, legal and other regulatory expenses, general administrative costs and working capital. In addition, we have acquired, and may in the future seek to acquire or invest in, additional businesses, products or technologies that we believe could complement or expand our portfolio, enhance our technical capabilities or otherwise offer growth opportunities. For example, in June 2019, we acquired Rhythm Xience, a medical device company specializing in the design and manufacture of transseptal crossing and steerable introducer systems, for $3.0 million in cash. The cash payment did not include the potential $17.0 million in earn out consideration to be paid based on the achievement of certain regulatory milestones and revenue milestones. In February 2020, we issued to the former owners of Rhythm Xience 119,993 shares of our Series D convertible preferred stock and paid them $2.6 million in connection with the regulatory and revenue milestones earned to date. In addition, pursuant to the Biotronik License Agreement, we paid Biotronik a $3.0 million upfront fee at the time the agreement was signed, as well as a technology transfer fee consisting of $7.0 million in cash in December 2019 and $5.0 million in shares of our Series D convertible preferred stock in February 2020. We are required to pay the Biotronik Parties up to $10.0 million upon the achievement of various regulatory and sales-related milestones, as well as unit-based royalties on any sales of force sensing catheters. We will also incur costs as a public company that we have not previously incurred or have previously incurred at lower rates.
44
With the closing of our IPO, our current cash, cash equivalents and marketable securities are sufficient to fund operations for at least the next 12 months. However, we will need to raise additional funds through one or more of the following: issuance of additional debt, equity or both. Until such time, if ever, that we can generate revenue sufficient to achieve profitability, we expect to finance our operations through equity or debt financings, which may not be available to us on the timing needed or on terms that we deem to be favorable. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures or declaring dividends. If we are unable to maintain sufficient financial resources, our business, financial condition and results of operations will be materially and adversely affected. We may be required to delay, limit, reduce or terminate our product discovery and development activities or future commercialization efforts. There can be no assurance that we will be able to obtain the needed financing on acceptable terms or at all.
Debt Obligations
During 2019, we repaid our 2018 Term Loan and our 2018 Convertible Notes and our 2019 Convertible Notes were converted into shares of our Series D convertible preferred stock.
On May 20, 2019, we entered into the 2019 Credit Agreement. The 2019 Credit Agreement provided us with a senior term loan facility in aggregate principal amount of $70.0 million, of which we borrowed $40.0 million upon closing. Of the remaining $30.0 million, $10.0 million is no longer available for borrowing and $20.0 million is available for borrowing by us on or prior to December 31, 2020, subject to our achievement of specified trailing revenue levels. The 2019 Credit Agreement bears interest per annum at 7.75% plus LIBOR for such interest period, and the principal amount of term loans outstanding under the 2019 Credit Agreement is due on May 20, 2024. The 2019 Credit Agreement can be prepaid but is subject to prepayment penalties. The 2019 Credit Agreement provides for final payment fees of an additional $4.6 million that are due upon prepayment, on the maturity date or upon acceleration.
Our obligations under the 2019 Credit Agreement are secured by substantially all of our assets, including our intellectual property, and is guaranteed by our subsidiary. The 2019 Credit Agreement contains customary affirmative and restrictive covenants, including with respect to our ability to enter into fundamental transactions, incur additional indebtedness, grant liens, pay any dividend or make any distributions to our holders, make investments and merge or consolidate with any other person or engage in transactions with our affiliates, but does not include any financial covenants, other than a minimum liquidity requirement.
In connection with our entry into the 2019 Credit Agreement, we issued liability-classified warrants with a fair value of $0.9 million to purchase 419,992 shares of our Series C convertible preferred stock at $16.67 per share. These warrants were subsequently automatically converted into warrants to purchase an equal number of shares of our Series D convertible preferred stock at a price of $16.67 per share. Upon closing of our IPO, these warrants were automatically converted into warrants to purchase an equal number of shares of our common stock at a price of $16.67 per share.
Cash Flows
The following table shows a summary of our cash flows for the nine months ended September 30, 2020 and 2019 (in thousands):
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2020 |
|
|
2019 |
|
||
|
|
|
(Unaudited) |
|
|||||
|
Net cash used in operating activities |
|
$ |
(61,038 |
) |
|
$ |
(30,823 |
) |
|
Net cash used in investing activities |
|
|
(54,255 |
) |
|
|
(70,868 |
) |
|
Net cash provided by financing activities |
|
|
164,000 |
|
|
|
126,311 |
|
|
Effect of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
143 |
|
|
|
(50 |
) |
|
Net change in cash, cash equivalents and restricted cash |
|
$ |
48,850 |
|
|
$ |
24,570 |
|
45
During the nine months ended September 30, 2020, operating activities used $61.0 million of cash, an increase of $30.2 million from the nine months ended September 30, 2019. This increase was primarily attributable to a $23.0 million decrease of non-cash items, primarily driven by an decrease of $16.9 million in amortization of debt issuance costs in the nine months ended September 30, 2020 and a $15.0 million write-off of intellectual property acquired in the Biotronik Asset Acquisition in the nine months ended September 30, 2019, partially offset by an increase in stock-based compensation expense of $7.1 million and an increase in the change in fair value of warrant liability and embedded derivative of $4.9 million. This increase was also due to a $11.8 million increase in use of cash from operating assets and liabilities, primarily resulting from (i) a $2.7 million increase in the prepaid expenses and other current assets balance from December 31, 2019 to September 30, 2020, as compared to a relatively consistent prepaid expenses and other current assets balance from December 31, 2018 to September 30, 2019, and (ii) a $1.4 million increase in the accrued liabilities balance from December 31, 2019 to September 30, 2020, excluding non-cash items, as compared to a $9.3 million increase in the accrued liabilities balance from December 31, 2018 to September 30, 2019, excluding non-cash items. The decrease in non-cash items and the increase in use of cash from operating assets and liabilities was partially offset by a $4.5 million decrease in net loss.
Investing Activities
During the nine months ended September 30, 2020, investing activities used $54.3 million of cash, a decrease of $16.6 million from the nine months ended September 30, 2019. This decrease was attributable to (a) an increase of maturities of marketable securities of $33.5 million and sales of marketable securities of $17.1 million, and (b) cash paid of $10.0 million for the Biotronik research and development license and $3.0 million for the Rhythm Xience acquisition for the nine months ended September 30, 2019. This decrease is partially offset by an additional $39.8 million of purchases of marketable securities and an additional $7.1 million purchases of property and equipment.
Financing Activities
During the nine months ended September 30, 2020, financing activities provided $164.0 million of cash, an increase of $37.7 million from the nine months ended September 30, 2019. The primary financing activities for the nine months ended September 30, 2020 was the closing of the Company’s IPO which resulted in net proceeds of $166.3 million and payment of contingent consideration of $2.6 million related to the Rhythm Xience acquisition for the achievement of certain regulatory milestones and revenue targets. The primary financing activities for the nine months ended September 30, 2019 included $40.0 million resulting from the closing of the 2019 Credit Agreement, $66.6 million from the issuance of shares of our Series D convertible preferred stock in June 2019 and $37.0 million from the issuance of the 2019 Convertible Notes in May 2019, partially offset by $17.3 million in debt repayments related to the repayment of the 2018 Term Loan and payments of issuance and extinguishment costs.
Contractual Obligations and Commitments
During the nine months ended September 30, 2020, there have been no material changes outside the ordinary course of business to our contractual obligations from those disclosed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in the prospectus dated August 5, 2020 (the “Prospectus”) that forms a part of the Company’s registration statement on Form S-1 (File No. 333-239873), as filed with the SEC pursuant to Rule 424(b)(4) promulgated under the Securities Act of 1933, as amended, (the “Securities Act”).
Off-Balance Sheet Arrangements
As of September 30, 2020 and December 31, 2019, we did not have, and we do not currently have, any off-balance sheet arrangements, as defined in the SEC rules and regulations.
Critical Accounting Policies and Estimates
Management’s discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or U.S. GAAP. The preparation of these condensed consolidated financial statements requires us to make estimates and assumptions for the reported amounts of assets, liabilities, revenue and expenses. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material.
46
During the nine months ended September 30, 2020, there have been no material changes to our critical accounting policies and estimates from those disclosed in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in the Prospectus that forms a part of the Company’s registration statement on Form S-1 (File No. 333-239873), as filed with the SEC pursuant to Rule 424(b)(4) promulgated under the Securities Act.
Our significant accounting policies are described in the Note 2 to our condensed consolidated financial statements.
Recent Accounting Pronouncements
See Note 2 to our condensed consolidated financial statements for a description of recent accounting pronouncements applicable to our condensed consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, the Company is not required to provide the information required by this item.
Item 4. Controls and Procedures
Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in Securities and Exchange Commission rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and our Chief Financial Officer, to allow timely decisions regarding required disclosure.
The design of any disclosure controls and procedures also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
With respect to the quarter ended September 30, 2020, under the supervision and with the participation of our management, we conducted an evaluation of the effectiveness of the design and operations of our disclosure controls and procedures. Based upon this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective. Management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of internal control over financial reporting can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been or will be detected.
Changes in Internal Control over Financial Reporting:
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended September 30, 2020 which have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
47
From time to time, we are involved in various legal proceedings arising from the normal course of our business activities. We are not presently a party to any litigation the outcome of which, we believe, if determined adversely to us, would individually or taken together have a material adverse effect on our business, operating results, cash flows or financial condition. We have received, and may from time to time receive, letters from third parties alleging patent infringement, violation of employment practices or trademark infringement, and we may in the future participate in litigation to defend ourselves. The results of any current or future litigation cannot be predicted with certainty, and regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
As of the date of this Quarterly Report on Form 10-Q, there have been no material changes from the risk factors disclosed in our Prospectus as filed by us with the SEC pursuant to Rule 424(b)(4) under the Securities Act, relating to our registration statement on Form S-1 (File No. 333-239873). Any of these factors could result in a significant or material adverse effect on our result of operations or financial conditions. Additional risk factors not presently known to us or that we currently deem immaterial may also impair our business or results of operations. We may disclose changes to such factors or disclose additional factors from time to time in our future filings with the SEC.
Item 2. Recent Sales of Unregistered Securities.
(a) Sales of Unregistered Securities
Between July 1, 2020 and September 30, 2020:
|
|
• |
we issued and sold to our current and former officers and employees an aggregate of 18,631 shares of common stock upon the exercise of options under our equity compensation plans at exercise prices ranging from $1.85 to $13.38 per share, for an aggregate amount of $0.1 million. |
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. Unless otherwise stated, the sales of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act (or Regulation D promulgated thereunder) or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions.
48
|
|
|
|
|
Incorporated by Reference |
||||||||
|
Exhibit No. |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation of Acutus Medical, Inc. |
|
8-K |
|
001-39430 |
|
3.1 |
|
August 10, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Amended and Restated Bylaws of the Registrant Acutus Medical, Inc. |
|
8-K |
|
001-39430 |
|
3.2 |
|
August 10, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 |
|
|
S-1 |
|
333-239873 |
|
10.12 |
|
July 15, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2 |
|
2020 Equity Incentive Plan and forms of agreements thereunder. |
|
S-1/A |
|
333-239873 |
|
10.14 |
|
July 30, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3 |
|
|
S-1/A |
|
333-239873 |
|
10.15 |
|
July 30, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4 |
|
|
S-1 |
|
333-239873 |
|
10.16 |
|
July 15, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.1* |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
32.2* |
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
101 |
|
The following financial information from the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2020, formatted in Inline Extensible Business Reporting Language (iXBRL): (i) the Condensed Consolidated Balance Sheets, (ii) the Condensed Consolidated Statements of Operations and Comprehensive Loss, (iii) the Condensed Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit), (iv) the Condensed Consolidated Statements of Cash Flows, and (v) Notes to the Condensed Consolidated Financial Statements (filed herewith). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
|
|
|
|
|
* |
The certifications furnished in Exhibit 32 hereto are deemed to accompany this Quarterly Report on Form 10-Q and will not be deemed “filed” for purposes of Section 18 of the Securities Act, except to the extent that the registrant specifically incorporates them by reference. |
49
Pursuant to the requirements of the Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
Acutus Medical, Inc. |
|
|
|
(Registrant) |
|
|
|
|
|
|
Date: November 12, 2020 |
By: |
/s/ Vince Burgess |
|
|
Vince Burgess |
|
|
|
President and Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
|
|
|
|
|
|
Date: November 12, 2020 |
By: |
/s/ Gary W. Doherty |
|
|
Gary W. Doherty |
|
|
|
Chief Financial Officer |
|
|
|
(Principal Financial Officer) |
|
50
