Attached files
| file | filename |
|---|---|
| EX-99.1 - RespireRx Pharmaceuticals Inc. | ex99-1.htm |
| 8-K - RespireRx Pharmaceuticals Inc. | form8k.htm |
Exhibit 99.2

| RespireRx Pharmaceuticals Inc. | Company at a Glance |
|
The primary mission of RespireRx Pharmaceuticals is to develop innovative and revolutionary treatments to combat diseases caused by disruption of neuronal signaling. We are developing treatment options that address conditions affecting millions of people, but for which there are few or poor treatment options, including obstructive sleep apnea (“OSA”), attention deficit hyperactivity disorder (“ADHD”), epilepsy, chronic pain and recovery from spinal cord injury (“SCI”). Based on our broad patent portfolios, RespireRx is developing a pipeline of new drug products within its two distinct business units:
(i) ResolutionRx is developing pharmaceutical cannabinoids, particularly dronabinol, a synthetic form of ∆9-tetrahydrocannabinol (“Δ9-THC”) for the treatment of OSA
(ii) EndeavourRx is developing neuromodulators, which include AMPAkines and GABAkines, proprietary compounds that positively modulate (positive allosteric modulators or “PAMs”) AMPA-type glutamate receptors and GABAA receptors, respectively.
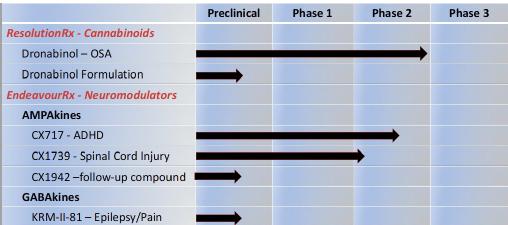
Status of Lead Products Under Development
Summary
● Highly desirable assets with 3 clinical stage drugs and confirmed efficacy ● Expanding portfolio of novel preclinical compounds across multiple therapeutic categories and indications ● World Class Management Team and Board of Directors ● Broad flexibility in modeling mechanisms of investment ● Funding commitments secured through 2021 ● Strategic Partners afforded the opportunity to share in the financial growth from early clinical to commercialization ● Exemplary regulatory and financial compliance history with government agencies ● Key clinical supply chains for dronabinol established
|
ResolutionRx – Two successful Phase 2 clinical trials have been completed demonstrating the ability of dronabinol to reduce the symptoms of OSA. The Company has designed and is developing proprietary, patent-pending dronabinol formulations that, if successful, could prove to be superior to existing formulations and not only extend market exclusivity for the treatment of OSA, but other indications as well.
EndeavourRx - CX717 and CX1739, our lead clinical AMPAkines, have successfully completed multiple Phase 1 safety trials with no drug-associated serious adverse events. Both compounds have also completed Phase 2 efficacy trials demonstrating target engagement, by antagonizing the process of opioid-induced respiratory depression (“OIRD”). CX717 has successfully completed a Phase 2 trial demonstrating the ability to significantly reduce the symptoms of adult ADHD.
KRM-II-81, our lead GABAkine, has demonstrated highly desirable properties in animal models of drug resistant epilepsy and chronic pain, with greatly reduced liability to produce side effects associated with current treatments. The anticonvulsant action of KRM-II-81 was confirmed by microelectrode recordings from slices obtained from freshly excised cortex from epileptic patients.
Executive Management Team Tim Jones, CEO and President Arnold Lippa, CSO Jeff Margolis, CFO
OFFICE LOCATION 126 Valley Rd. Glen Rock, NJ 07452
Website www.RespireRx.com
Market OTC: RSPI
Contact: Jeff Margolis jmargolis@respirerx.com (917) 834-7206
|
For additional information, the reader is directed to SEC filings that can be found on the Company website - www.RespireRx.com
Special Note Regarding Forward-Looking Statements.
In some cases, you can identify forward-looking statements by the following words: “anticipate,” “assume,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words, and such statements may include, but are not limited to, statements regarding (i) future research plans, expenditures and results, (ii) potential collaborative arrangements, (iii) the potential utility of the Company’s product candidates, (iv) reorganization plans, and (v) the need for, and availability of, additional financing. Forward-looking statements are not a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements contained herein.
These factors include but are not limited to, regulatory policies or changes thereto, available cash, research and development results, issuance of patents, competition from other similar businesses, interest of third parties in collaborations with us, and market and general economic factors.
We cannot assure you that the forward-looking statements contained herein will prove to be accurate and therefore prospective investors are encouraged not to place undue reliance on forward-looking statements. We caution investors to recognize that forward-looking statements are predictions of future results, which may not occur as anticipated. Actual results could differ materially from those anticipated in the forward-looking statements and from historical results. These forward-looking statements are based on assumptions regarding the Company’s business and technology, which involve judgments with respect to, among other things, future scientific, economic, regulatory and competitive conditions, collaborations with third parties, and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the Company’s control. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. Our expectations reflected in our forward-looking statements can be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties. We advise investors and all other interested parties to consult any further disclosures we may make on related subjects in our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K that we file with or furnish to the SEC.