Attached files
| file | filename |
|---|---|
| EX-32.2 - RespireRx Pharmaceuticals Inc. | ex32-2.htm |
| EX-32.1 - RespireRx Pharmaceuticals Inc. | ex32-1.htm |
| EX-31.2 - RespireRx Pharmaceuticals Inc. | ex31-2.htm |
| EX-31.1 - RespireRx Pharmaceuticals Inc. | ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
[X] QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended March 31, 2021
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number: 1-16467
RESPIRERX PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
| Delaware | 33-0303583 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification Number) |
126 Valley Road, Suite C
Glen Rock, New Jersey 07452
(Address of principal executive offices)
(201) 444-4947
(Registrant’s telephone number, including area code)
Not applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| N/A | N/A | N/A |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes [X] No [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [X] | Smaller reporting company [X] |
| Emerging growth company [ ] |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [X]
As of May 19, 2021, the Company had 89,496,596, shares of common stock, $0.001 par value, issued and outstanding.
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
TABLE OF CONTENTS
| 2 |
In this Quarterly Report on Form 10-Q, the terms “RespireRx,” the “Company,” “we,” “us” and “our” refer to RespireRx Pharmaceuticals Inc. a Delaware corporation, and, unless the context indicates otherwise, its consolidated subsidiaries.
INTRODUCTORY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q of RespireRx Pharmaceuticals Inc. (“RespireRx” and together with RespireRx’s wholly owned subsidiary, Pier Pharmaceuticals, Inc. (“Pier”), the “Company, “we,” or “our,” unless the context indicates otherwise) contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Company intends that such forward-looking statements be subject to the safe harbor created thereby. These might include statements regarding the Company’s future plans, targets, estimates, assumptions, financial position, business strategy and other plans and objectives for future operations, and assumptions and predictions about research and development efforts, including, but not limited to, preclinical and clinical research design, execution, timing, costs and results, future product demand, supply, manufacturing, costs, marketing and pricing factors.
In some cases, forward-looking statements may be identified by words including “assumes,” “could,” “ongoing,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” “anticipates,” “believes,” “intends,” “estimates,” “expects,” “plans,” “contemplates,” “targets,” “continues,” “budgets,” “may,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words, and such statements may include, but are not limited to, statements regarding (i) future research plans, expenditures and results, (ii) potential collaborative arrangements, (iii) the potential utility of the Company’s products candidates, (iv) reorganization plans, and (v) the need for, and availability of, additional financing. Forward-looking statements are based on information available at the time the statements are made and involve known and unknown risks, uncertainties and other factors that may cause our results, levels of activity, performance or achievements to be materially different from the information expressed or implied by the forward-looking statements in this report.
These factors include but are not limited to, regulatory policies or changes thereto, available cash, research and development results, issuance of patents, competition from other similar businesses, interest of third parties in collaborations with us, and market and general economic factors, and other risk factors disclosed in “Item 1A. Risk Factors” in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2020, as filed with the SEC on April 15, 2021 (the “2020 Form 10-K”).
You should read these risk factors and the other cautionary statements made in the Company’s filings as being applicable to all related forward-looking statements wherever they appear in this report. We cannot assure you that the forward-looking statements in this report will prove to be accurate and therefore prospective investors are encouraged not to place undue reliance on forward-looking statements. You should read this report completely. Other than as required by law, we undertake no obligation to update or revise these forward-looking statements, even though our situation may change in the future.
We caution investors not to place undue reliance on any forward-looking statement that speaks only as of the date made and to recognize that forward-looking statements are predictions of future results, which may not occur as anticipated. Actual results could differ materially from those anticipated in the forward-looking statements and from historical results, due to the risks and uncertainties described in the 2020 Form 10-K and in this report, as well as others that we may consider immaterial or do not anticipate at this time. These forward-looking statements are based on assumptions regarding the Company’s business and technology, which involve judgments with respect to, among other things, future scientific, economic, regulatory and competitive conditions, collaborations with third parties, and future business decisions, all of which are difficult or impossible to predict accurately and many of which are beyond the Company’s control. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. Our expectations reflected in our forward-looking statements can be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties, including those described in the 2020 Form 10-K and in this report. These risks and uncertainties are not exclusive and further information concerning us and our business, including factors that potentially could materially affect our financial results or condition, may emerge from time to time.
For more information about the risks and uncertainties the Company faces, see “Item 1A. Risk Factors” in our 2020 Form 10-K. Forward-looking statements speak only as of the date they are made. The Company does not undertake and specifically declines any obligation to update any forward-looking statements or to publicly announce the results of any revisions to any statements to reflect new information or future events or developments. We advise investors to consult any further disclosures we may make on related subjects in our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K that we file with or furnish to the SEC.
| 3 |
PART I - FINANCIAL INFORMATION
ITEM 1. CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
CONDENSED CONSOLIDATED BALANCE SHEETS
| March 31, 2021 | December 31, 2020 | |||||||
| (unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 5,347 | $ | 825 | ||||
| Deferred financing costs | - | 52,609 | ||||||
| Prepaid expenses | 100,280 | 31,653 | ||||||
| Total current assets | 105,627 | 85,087 | ||||||
| Total assets | $ | 105,627 | $ | 85,087 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses, including amounts owed to related parties | $ | 5,066,983 | $ | 4,923,947 | ||||
| Accrued compensation and related expenses | 1,934,109 | 1,540,809 | ||||||
| Convertible notes payable, including accrued interest of $82,512 and $85,693 at March 31, 2021 and December 31, 2020, respectively, which includes accrued interest to related parties (Note 4) | 224,043 | 414,860 | ||||||
| Note payable to SY Corporation, including accrued interest of $423,214 and $411,385 at March 31, 2021 and December 31, 2020, respectively (payment obligation currently in default – Note 4) | 846,652 | 864,551 | ||||||
| Notes payable to officer, including accrued interest (Note 4) | 211,101 | 213,067 | ||||||
| Notes payable to former officer, including accrued interest (Note 4) | 191,165 | 185,565 | ||||||
| Other short-term notes payable | 86,192 | 4,608 | ||||||
| Total current liabilities | 8,560,245 | 8,148,407 | ||||||
| Commitments and contingencies (Note 8) | ||||||||
| Stockholders’ deficiency: (Note 6) | ||||||||
| Series B convertible preferred stock, $0.001 par value; $0.6667 per share liquidation preference; aggregate liquidation preference $25,001; shares authorized: 37,500; shares issued and outstanding: 1 common share issuable upon conversion at 0.000030 common shares per Series B share | 21,703 | 21,703 | ||||||
| Common stock, $0.001 par value; shares authorized: 2,000,000,000; shares issued and outstanding: 89,496,596 at March 31, 2021 and 71,271,095 at December 31, 2020, respectively (Note 2 and Note 6) | 89,497 | 71,271 | ||||||
| Additional paid-in capital | 163,094,727 | 162,654,002 | ||||||
| Accumulated deficit | (171,660,545 | ) | (170,810,296 | ) | ||||
| Total stockholders’ deficiency | (8,454,618 | ) | (8,063,320 | ) | ||||
| Total liabilities and stockholders’ deficiency | $ | 105,627 | $ | 85,087 | ||||
See accompanying notes to condensed consolidated financial statements (unaudited).
| 4 |
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
| Three-months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Operating expenses: | ||||||||
| General and administrative, including related parties | $ | 645,376 | $ | 365,280 | ||||
| Research and development, including related parties | 154,764 | 155,290 | ||||||
| Total operating costs and expenses | $ | 800,140 | $ | 520,570 | ||||
| Loss from operations | (800,140 | ) | (520,570 | ) | ||||
| Loss on extinguishment of debt in exchange for equity | - | (323,996 | ) | |||||
| Interest expense, including related parties | (79,470 | ) | (140,710 | ) | ||||
| Foreign currency transaction gain | 29,361 | 38,558 | ||||||
| Net loss attributable to common stockholders | $ | (850,249 | ) | $ | (946,718 | ) | ||
| Net loss per common share - basic and diluted | $ | (0.01 | ) | $ | (1.42 | ) | ||
| Weighted average common shares outstanding - basic and diluted | 78,148,365 | 668,660 | ||||||
See accompanying notes to condensed consolidated financial statements (unaudited).
| 5 |
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIENCY
(Unaudited)
Three-months Ended March 31, 2021
| Series B | ||||||||||||||||||||||||||||
| Convertible | ||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional | Total | |||||||||||||||||||||||||
| Shares | Amount | Shares | Par Value | Paid-in Capital |
Accumulated Deficit |
Stockholders’ Deficiency |
||||||||||||||||||||||
| Balance, December 31, 2020 | 37,500 | $ | 21,703 | 71,271,095 | $ | 71,271 | $ | 162,654,002 | $ | (170,810,296 | ) | $ | (8,063,320 | ) | ||||||||||||||
| Sale of common stock | - | - | 3,600,000 | 3,600 | 113,699 | - | 117,299 | |||||||||||||||||||||
| Costs of stock issuance | - | - | - | (52,609) | - | (52,609 | ) | |||||||||||||||||||||
| Issuance of note commitment shares and beneficial conversion feature | - | - | 2,000,000 | 2,000 | 95,500 | - | 97,500 | |||||||||||||||||||||
| Issuance of common stock upon conversion of convertible notes | - | - | 12,625,557 | 12,626 | 239,885 | - | 252,511 | |||||||||||||||||||||
| Stock -based compensation | - | - | - | - | 44,250 | 44,250 | ||||||||||||||||||||||
| Deferred financing costs | - | - | - | - | (52,609 | ) | - | (52,609 | ) | |||||||||||||||||||
| Adjustment due to reverse stock split | - | - | (56 | ) | - | - | - | - | ||||||||||||||||||||
| Net loss | (850,249 | ) | (850,249 | ) | ||||||||||||||||||||||||
| Balance, March 31, 2021 | 37,500 | $ | 21,703 | 89,496,596 | $ | 89,497 | $ | 163,094,727 | $ | (171,660,545 | ) | $ | (8,454,618 | ) | ||||||||||||||
Three-months Ended March 31, 2020
| Series B | ||||||||||||||||||||||||||||
| Convertible | ||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional | Total | |||||||||||||||||||||||||
| Shares | Amount | Shares | Par Value | Paid-in Capital | Accumulated Deficit | Stockholders’ Deficiency | ||||||||||||||||||||||
| Balance, December 31, 2019 | 37,500 | $ | 21,703 | 417,507 | $ | 418 | $ | 159,042,145 | $ | (166,509,085 | ) | $ | (7,444,819 | ) | ||||||||||||||
| Issuances of common stock | - | - | 2,951,878 | 2,952 | 937,166 | - | 940,118 | |||||||||||||||||||||
| Net loss | (946,718 | ) | (946,718 | ) | ||||||||||||||||||||||||
| Balance, March 31, 2020 | 37,500 | $ | 21,703 | 2,956,053 | $ | 3,370 | $ | 159,979,311 | $ | (167,455,803 | ) | $ | (7,451,419 | ) | ||||||||||||||
See accompanying notes to condensed consolidated financial statements (unaudited).
| 6 |
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
Three-months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (850,249 | ) | $ | (946,718 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Loss on extinguishment of debt | - | 323,996 | ||||||
| Amortization of original issue discount to interest expense | 10,443 | 102,806 | ||||||
| Amortization of capitalized note costs and debt discounts | 39,423 | - | ||||||
| Stock-based compensation included in - | ||||||||
| General and administrative expenses | 28,000 | - | ||||||
| Research and development expenses | 16,250 | - | ||||||
| Foreign currency transaction (gain) loss | (29,361 | ) | (38,558 | ) | ||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) decrease in - | ||||||||
| Prepaid expenses | (68,627 | ) | (71,390 | ) | ||||
| Fees paid with shares of Common Stock | 4,000 | - | ||||||
| Increase (decrease) in - | ||||||||
| Accounts payable and accrued expenses | 143,036 | 313,097 | ||||||
| Accrued compensation and related expenses | 393,300 | 190,784 | ||||||
| Accrued interest payable | 26,924 | 108,124 | ||||||
| Net cash used in operating activities | (286,861 | ) | (17,859 | ) | ||||
| Cash flows from financing activities: | ||||||||
| Proceeds from convertible note financing, net of note costs and original issue discount | 97,500 | 1,250 | ||||||
| Borrowings on short-term notes payable | 81,584 | - | ||||||
| Proceeds from sale of Common Stock | 117,299 | - | ||||||
| Repayment of officer advance | (5,000 | ) | - | |||||
| Net cash provided by financing activities | 291,383 | 1,250 | ||||||
| Cash and cash equivalents: | ||||||||
| Net increase/(decrease) | 4,522 | (16,609 | ) | |||||
| Balance at beginning of period | 825 | 16,690 | ||||||
| Balance at end of period | $ | 5,347 | $ | 81 | ||||
(Continued)
| 7 |
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
(Continued)
Three-months Ended March 31, | ||||||||
| 2021 | 2020 | |||||||
| Supplemental disclosures of cash flow information: | ||||||||
| Cash paid for - | ||||||||
| Interest | $ | 410 | $ | - | ||||
| Income taxes | $ | - | $ | - | ||||
| Non-cash financing activities: | ||||||||
| Amortization of deferred financing costs | $ | 52,609 | $ | - | ||||
| Debt discounts established for convertible debt | $ | 97,500 | $ | - | ||||
| Issuance of common stock in exchange for extinguishment of convertible notes payable | $ | 252,511 | $ | 634,118 | ||||
| Issuance of common stock as commitment for convertible note | $ | 100,000 | $ | 306,000 | ||||
See accompanying notes to condensed consolidated financial statements (unaudited).
| 8 |
RESPIRERX PHARMACEUTICALS INC.
AND SUBSIDIARY
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Organization and Basis of Presentation
Organization
RespireRx Pharmaceuticals Inc. (“RespireRx”) was formed in 1987 under the name Cortex Pharmaceuticals, Inc. to engage in the discovery, development and commercialization of innovative pharmaceuticals for the treatment of neurological and psychiatric disorders. On December 16, 2015, RespireRx filed a Certificate of Amendment to its Second Restated Certificate of Incorporation (as amended, the “Certificate of Incorporation”) with the Secretary of State of the State of Delaware to amend its Second Restated Certificate of Incorporation to change its name from Cortex Pharmaceuticals, Inc. to RespireRx Pharmaceuticals Inc. In August 2012, RespireRx acquired Pier Pharmaceuticals, Inc. (“Pier”), which is now a wholly owned subsidiary. Pier was a clinical stage biopharmaceutical company developing a pharmacologic treatment for obstructive sleep apnea (“OSA”) and had been engaged in research and clinical development activities which activities are now in RespireRx.
Basis of Presentation
The condensed consolidated financial statements are of RespireRx and its wholly-owned subsidiary, Pier (collectively referred to herein as the “Company,” “we” or “our,” unless the context indicates otherwise). The condensed consolidated financial statements of the Company at March 31, 2021 and for the three-months ended March 31, 2021 and 2020, are unaudited. In the opinion of management, all adjustments (including normal recurring adjustments) have been made that are necessary to present fairly the condensed consolidated financial position of the Company as of March 31, 2021, the results of its condensed consolidated operations for the three-months ended March 31, 2021 and 2020, changes in its condensed consolidated statements of stockholders’ deficiency for the three-months ended March 31, 2021 and 2020 and its condensed consolidated cash flows for the three-months ended March 31, 2021 and 2020. Condensed consolidated operating results for the interim periods presented are not necessarily indicative of the results to be expected for a full fiscal year. The consolidated balance sheet at December 31, 2020 has been derived from the Company’s audited consolidated financial statements at such date. For comparative purposes, certain 2020 and 2019 amounts, including, but not limited to, share and per share amounts, par value and additional paid-in capital have been adjusted to a post-reverse stock split basis which occurred on January 5, 2021.
The condensed consolidated financial statements and related notes have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Accordingly, certain information and note disclosures normally included in financial statements prepared in accordance with United States generally accepted accounting principles (“GAAP”) have been omitted pursuant to such rules and regulations. These condensed consolidated financial statements should be read in conjunction with the consolidated financial statements and other information included in the Company’s 2020 Form 10-K.
2. Business
The mission of the Company is to develop innovative and revolutionary treatments to combat disorders caused by disruption of neuronal signaling. We are developing treatment options that address conditions that affect millions of people, but for which there are limited or poor treatment options, including OSA, attention deficit hyperactivity disorder (“ADHD”) epilepsy, chronic pain, including inflammatory and neuropathic pain, recovery from spinal cord injury (“SCI”), as well as other areas of interest based on results of animal studies to date.
RespireRx is developing a pipeline of new drug products based on our broad patent portfolios across two distinct drug platforms:
| (i) | our pharmaceutical cannabinoids platform (which we refer to as ResolutionRx), including dronabinol (a synthetic form of ∆9-tetrahydrocannabinol (“Δ9-THC”)), which acts upon the nervous system’s endogenous cannabinoid receptors, and | |
| (ii) | our neuromodulators platform (which we refer to as EndeavourRx) is made up of two programs: (a) our AMPAkines program, including proprietary compounds that are positive allosteric modulators (“PAMs”) of AMPA-type glutamate receptors to promote neuronal function and (b) our GABAkines program, including proprietary compounds that are PAMs of GABAA receptors, which was recently established pursuant to our entry with the University of Wisconsin-Milwaukee Research Foundation, Inc., an affiliate of the University of Wisconsin-Milwaukee (“UWMRF”), into a patent license agreement (the UWMRF Patent License Agreement”). |
Financing our Platforms
Our major challenge has been to raise substantial equity or equity-linked financing to support research and development plans for our cannabinoid and neuromodulator platforms, while minimizing the dilutive effect to pre-existing stockholders. At present, we believe that we are hindered primarily by our public corporate structure, our OTCQB listing, and low market capitalization as a result of our low stock price.
For this reason, the Company has implemented an internal restructuring plan through which our two drug platforms have been reorganized into separate business units and may in the future be organized into subsidiaries of RespireRx. We believe that by creating one or more subsidiaries to further the aims of ResolutionRx and EndeavourRx, it may be possible, through separate finance channels, to optimize the asset values of each. We are also planning to commence a securities offering by the Company pursuant to Regulation A under the Securities Act by filing a Form 1-A.
| 9 |
Going Concern
The Company’s condensed consolidated financial statements have been presented on the basis that it is a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The Company has incurred net losses of $850,249 for the three-months ended March 31, 2021 and $4,301,211 for the fiscal year ended December 31, 2020, as well as negative operating cash flows of $286,861 for the three-months ended March 31, 2021 and $513,001 for the fiscal year ended December 31, 2020. The Company also had a stockholders’ deficiency of $8,454,618 at March 31, 2021 and expects to continue to incur net losses and negative operating cash flows for at least the next few years. As a result, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern, and the Company’s independent registered public accounting firm, in its report on the Company’s consolidated financial statements for the year ended December 31, 2020, expressed substantial doubt about the Company’s ability to continue as a going concern.
The Company is currently, and has for some time, been in significant financial distress. It has extremely limited cash resources and current assets and has no ongoing source of sustainable revenue. Management is continuing to address various aspects of the Company’s operations and obligations, including, without limitation, debt obligations, financing requirements, intellectual property, licensing agreements, legal and patent matters and regulatory compliance, and has taken steps to continue to raise new debt and equity capital to fund the Company’s business activities from both related and unrelated parties.
The Company is continuing its efforts to raise additional capital in order to be able to pay its liabilities and fund its business activities on a going forward basis, including the pursuit of the Company’s planned research and development activities. The Company regularly evaluates various measures to satisfy the Company’s liquidity needs, including development and other agreements with collaborative partners and, when necessary, seeking to exchange or restructure the Company’s outstanding securities. The Company is evaluating certain changes to its operations and structure to facilitate raising capital from sources that may be interested in financing only discrete aspects of the Company’s development programs. Such changes could include a significant reorganization, which may include the formation of one or more subsidiaries into which one or more programs may be contributed. As a result of the Company’s current financial situation, the Company has limited access to external sources of debt and equity financing. Accordingly, there can be no assurances that the Company will be able to secure additional financing in the amounts necessary to fully fund its operating and debt service requirements. If the Company is unable to access sufficient cash resources, the Company may be forced to discontinue its operations entirely and liquidate.
3. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying condensed consolidated financial statements are prepared in accordance with GAAP and include the financial statements of RespireRx and its wholly-owned subsidiary, Pier. Intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions. These estimates and assumptions affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include, among other things, accounting for potential liabilities, and the assumptions used in valuing stock-based compensation issued for services. Actual amounts may differ from those estimates.
Reverse Stock Split on January 5, 2021
On January 5, 2021, the Company effected a ten to one reverse-stock split of its common stock. Every ten shares of the “old” common stock was exchanged for one “new” share of common stock rounded down to the nearest whole share with any fractional shares of common stock paid to the stockholder in cash. Option and warrant issuances prior to January 5, 2021 have also been proportionately adjusted by dividing the number of shares into which such options and warrants may exercise by ten and multiplying the exercise price by ten. The effect of the reverse-stock split has been reflected retroactively in the Company’s consolidated financial statements as of December 31, 2020 and any interim periods in 2020. Certain amount with respect to 2019 that appear in these condensed consolidated financial statements have also been reflected on a post reverse-stock split basis.
| 10 |
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company limits its exposure to credit risk by investing its cash with high quality financial institutions. The Company’s cash balances may periodically exceed federally insured limits. The Company has not experienced a loss in such accounts to date.
Value of Financial Instruments
The authoritative guidance with respect to value of financial instruments established a value hierarchy that prioritizes the inputs to valuation techniques used to measure value into three levels and requires that assets and liabilities carried at value be classified and disclosed in one of three categories, as presented below. Disclosure as to transfers into and out of Levels 1 and 2, and activity in Level 3 value measurements, is also required.
Level 1. Observable inputs such as quoted prices in active markets for an identical asset or liability that the Company has the ability to access as of the measurement date. Financial assets and liabilities utilizing Level 1 inputs include active-exchange traded securities and exchange-based derivatives.
Level 2. Inputs, other than quoted prices included within Level 1, which are directly observable for the asset or liability or indirectly observable through corroboration with observable market data. Financial assets and liabilities utilizing Level 2 inputs include fixed income securities, non-exchange based derivatives, mutual funds, and fair-value hedges.
Level 3. Unobservable inputs in which there is little or no market data for the asset or liability which requires the reporting entity to develop its own assumptions. Financial assets and liabilities utilizing Level 3 inputs include infrequently-traded, non-exchange-based derivatives and commingled investment funds, and are measured using present value pricing models.
The Company determines the level in the value hierarchy within which each value measurement falls in its entirety, based on the lowest level input that is significant to the value measurement in its entirety. In determining the appropriate levels, the Company performs an analysis of the assets and liabilities at each reporting period end.
The carrying amounts of financial instruments (consisting of cash, cash equivalents, and accounts payable and accrued expenses) are considered by the Company to be representative of the respective values of these instruments due to the short-term nature of those instruments. With respect to the note payable to SY Corporation Co., Ltd. (“SY Corporation”) and the convertible notes payable, management does not believe that the credit markets have materially changed for these types of borrowings since the original borrowing date. The Company considers the carrying amounts of the notes payable to officers, inclusive of accrued interest, to be representative of the respective values of such instruments due to the short-term nature of those instruments and their terms.
Deferred Financing Costs
Costs incurred in connection with ongoing debt and equity financings, including legal fees, are deferred until the related financing is either completed or abandoned.
Costs related to abandoned debt or equity financings are charged to operations in the period of abandonment. Costs related to completed equity financings are netted against the proceeds.
Debt Issuance Costs
The Company presents debt issuance costs related to debt obligations in its consolidated balance sheet as a direct deduction from the carrying amount of that debt obligation, consistent with the presentation for debt discounts.
| 11 |
Convertible Notes Payable
Convertible notes are evaluated to determine if they should be recorded at amortized cost. To the extent that there are associated warrants, commitment shares of Common Stock or a beneficial conversion feature, the convertible notes and equity or equity-linked securities are evaluated to determine if there are embedded derivatives to be identified, bifurcated and valued in connection with and at the time of such financing.
Extinguishment of Debt and Settlement of Liabilities
The Company accounts for the extinguishment of debt and settlement of liabilities by comparing the carrying value of the debt or liability to the value of consideration paid or assets given up and recognizing a loss or gain in the condensed consolidated statement of operations in the amount of the difference in the period in which such transaction occurs. See Note 4. Notes Payable.
Prepaid Insurance
Prepaid insurance represents the premium paid in March 2021 for directors and officers insurance. The amounts of prepaid insurance amortizable in the ensuing twelve-month period are recorded as prepaid insurance in the Company’s consolidated balance sheet at each reporting date and amortized to the Company’s consolidated statement of operations for each reporting period.
Stock-Based Awards
The Company periodically issues common stock and stock options to officers, directors, Scientific Advisory Board members, consultants and vendors for services rendered. Such issuances vest and expire according to terms established at the issuance date of each grant.
The Company accounts for stock-based payments to officers, directors, outside consultants and vendors by measuring the cost of services received in exchange for equity awards based on the grant date fair value of the awards, with the cost recognized as compensation expense on the straight-line basis in the Company’s consolidated financial statements over the vesting period of the awards.
Stock grants and stock options, which are sometimes subject to time-based vesting, are measured at the grant date fair value and charged to operations ratably over the vesting period.
The value of stock options granted as stock-based payments is determined utilizing the Black-Scholes option-pricing model, and is affected by several variables, the most significant of which are the life of the equity award, the exercise price of the stock option as compared to the fair market value of the common stock on the grant date, and the estimated volatility of the common stock over the term of the equity award. Estimated volatility is based on the historical volatility of the Company’s common stock. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The fair market value of common stock is determined by reference to the quoted market price of the Company’s common stock.
Stock and stock option grants and warrants issued to non-employees as compensation for services to be provided to the Company or in settlement of debt are accounted for based upon the fair value of the services provided or the estimated fair value of the stock option or warrant, whichever can be more clearly determined. Management uses the Black-Scholes option-pricing model to determine the fair value of the stock options and warrants issued by the Company. The Company recognizes this expense over the period in which the services are provided.
| 12 |
There were no stock or stock option grants during the three-months ended March 31, 2021.
The Company recognizes the amortized value of stock-based payments in general and administrative costs and in research and development costs, as appropriate, in the Company’s condensed consolidated statements of operations. The Company issues new shares of common stock to satisfy stock option and warrant exercises. There were no stock options exercised during the three-months ended March 31, 2021 and 2020, respectively.
There were no warrants issued as compensation or for services during the three-months ended March 31, 2021 and 2020. Warrants, if issued for services, are typically issued to placement agents or brokers for fund raising services, or to lenders, and are not issued from any of the Company’s stock and option plans, from which options issued to non-employees for services are typically issued.
Income Taxes
The Company accounts for income taxes under an asset and liability approach for financial accounting and reporting for income taxes. Accordingly, the Company recognizes deferred tax assets and liabilities for the expected impact of differences between the financial statements and the tax basis of assets and liabilities.
The Company records a valuation allowance to reduce its deferred tax assets to the amount that is more likely than not to be realized. In the event the Company was to determine that it would be able to realize its deferred tax assets in the future in excess of its recorded amount, an adjustment to the deferred tax assets would be credited to operations in the period such determination was made. Likewise, should the Company determine that it would not be able to realize all or part of its deferred tax assets in the future, an adjustment to the deferred tax assets would be charged to operations in the period such determination was made.
Pursuant to Internal Revenue Code Sections 382 and 383, use of the Company’s net operating loss and credit carryforwards may be limited if a cumulative change in ownership of more than 50% occurs within any three-year period since the last ownership change. The Company may have had a change in control under these Sections. However, the Company does not anticipate performing a complete analysis of the limitation on the annual use of the net operating loss and tax credit carryforwards until the time that it anticipates it will be able to utilize these tax attributes.
As of March 31, 2021, the Company did not have any unrecognized tax benefits related to various federal and state income tax matters and does not anticipate any material amount of unrecognized tax benefits within the next 12 months.
The Company is subject to U.S. federal income taxes and income taxes of various state tax jurisdictions. As the Company’s net operating losses have yet to be utilized, all previous tax years remain open to examination by Federal authorities and other jurisdictions in which the Company currently operates or has operated in the past.
The Company accounts for uncertainties in income tax law under a comprehensive model for the financial statement recognition, measurement, presentation and disclosure of uncertain tax positions taken or expected to be taken in income tax returns as prescribed by GAAP. The tax effects of a position are recognized only if it is “more-likely-than-not” to be sustained by the taxing authority as of the reporting date. If the tax position is not considered “more-likely-than-not” to be sustained, then no benefits of the position are recognized. As of March 31, 2020, the Company had not recorded any liability for uncertain tax positions. In subsequent periods, any interest and penalties related to uncertain tax positions will be recognized as a component of income tax expense.
Foreign Currency Transactions
The note payable to SY Corporation, which is denominated in a foreign currency (the South Korean Won), is translated into the Company’s functional currency (the United States Dollar) at the exchange rate on the balance sheet date. The foreign currency exchange gain or loss resulting from translation is recognized in the related condensed consolidated statements of operations.
| 13 |
Research and Development
Research and development costs include compensation paid to management directing the Company’s research and development activities, including but not limited to compensation paid to our Chief Scientific Officer who is also our Executive Chairman, and fees paid to consultants and outside service providers and organizations (including research institutes at universities), and other expenses relating to the acquisition, design, development and clinical testing of the Company’s treatments and product candidates.
License Agreements
Obligations incurred with respect to mandatory payments provided for in license agreements are recognized ratably over the appropriate term, as specified in the underlying license agreement, and are recorded as liabilities in the Company’s condensed consolidated balance sheet, with a corresponding charge to research and development costs in the Company’s condensed consolidated statement of operations. Obligations incurred with respect to milestone payments provided for in license agreements are recognized when it is probable that such milestone will be reached and are recorded as liabilities in the Company’s condensed consolidated balance sheet, with a corresponding charge to research and development expenses in the Company’s condensed consolidated statement of operations.
Patent Costs
Due to the significant uncertainty associated with the successful development of one or more commercially viable products based on the Company’s research efforts and any related patent applications, all patent costs, including patent-related legal and filing fees, are expensed as incurred and recorded as general and administrative expenses.
Earnings per Share
The Company’s computation of earnings per share (“EPS”) includes basic and diluted EPS. Basic EPS is measured as the income (loss) attributable to common stockholders divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., warrants and options) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
Net loss attributable to common stockholders consists of net loss, as adjusted for actual and deemed preferred stock dividends declared, amortized or accumulated.
Loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the respective periods. Basic and diluted loss per common share is the same for all periods presented because all warrants and stock options outstanding are anti-dilutive.
At March 31, 2021 and 2020 the Company excluded the outstanding securities summarized below, which entitle the holders thereof to acquire shares of common stock, from its calculation of earnings per share, as their effect would have been anti-dilutive.
| March 31, | ||||||||
| 2021 | 2020 | |||||||
| Series B convertible preferred stock | 1 | 1 | ||||||
| Convertible notes payable | 6,674,704 | 12,653,757 | ||||||
| Common stock warrants | 28,800,757 | 219,104 | ||||||
| Common stock options | 7,112,907 | 428,607 | ||||||
| Total | 42,588,369 | 13,301,469 | ||||||
Reclassifications
Certain comparative figures in 2020 have been reclassified to conform to the current quarter’s presentation. These reclassifications were immaterial, both individually and in the aggregate.
| 14 |
Recent Accounting Pronouncements
In August 2020, the FASB issued Accounting Standards Update No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). The subtitle is Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. This Accounting Standard Update (“ASU”) addresses complex financial instruments that have characteristics of both debt and equity. The application of this ASU would reduce the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models would result in fewer embedded conversion features being separately recognized from the host contract as compared with current GAAP. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. The Company has historically issued complex financial instruments and has considered whether embedded conversion features have existed within those contracts or whether derivatives would appropriately be bifurcated. To date, no such bifurcation has been necessary. However, it is possible that this ASU may have a substantial impact on the Company’s financial statements. Management is evaluating the potential impact. This ASU becomes effective for fiscal years beginning after December 15, 2023.
In January 2020, the FASB issued ASU 2020-01, Clarifying the Interactions between Topic 321, Topic 323, Equity Method and Joint Ventures, and Topic 815, Derivatives and Hedging which represents an amendment clarifying the interaction between accounting standards related to equity securities, equity method investments and certain derivatives. The guidance is effective for fiscal years beginning after December 15, 2020. Management is currently evaluating the impact the guidance will have on our consolidated financial statements.
4. Notes Payable
Convertible Notes Payable
The Company periodically issues convertible notes with similar characteristics. As described in the table below, during the three-months ended March 31, 2021, there were four such notes outstanding, two of which were satisfied in full by conversion of both principal and interest and one of which was satisfied in part, principal only, during that period. These notes all have or had a fixed conversion price of $0.02 per share of Common Stock, subject to adjustment in certain circumstances. All notes had an annual interest rate of 10% which was guaranteed in full. The convertible notes had an original issue discount (“OID”), debt issuance costs (“DIC”) that were capitalized by the Company, a warrant (“WT”) or commitment shares (“CS”) and in two cases a beneficial conversion feature (“BCF”), The OID, CN, WTs, CSs and BCF allocated values are amortized over the life of the notes to interest expense. All notes mature or matured nine to fifteen months from their issuance date. All notes were prepayable by the Company during the first six months, subject to prepayment premiums that range from 110% to 115% of the maturity amount plus accrued interest. If not earlier paid, the notes were convertible by the holder into the Company’s Common Stock. Two of the notes were paid before maturity.
| 15 |
The table below summarizes the convertible notes outstanding as of March 31, 2021 and the repayments by conversion during the three-months ended March 31, 2021:
| Inception Date | Maturity date | Original Principal Amount | Interest rate | Original aggregate DIC, OID, Wts, CS and BCF | Cumulative amortization of DIC, OID, Wts, CS and BCF | Accrued coupon interest | Repayment by conversion | Balance sheet carrying amount at March 31, 2021 inclusive of accrued interest | ||||||||||||||||||||||
| July 2, 2020 | April 2, 2021 | $ | 137,500 | 10.00 | % | $ | (44,423 | ) | $ | 44,423 | $ | 6,875 | $ | (144,375 | ) | $ | — | |||||||||||||
| July 28, 2020 | July 28, 2021 | $ | 40,000 | 10.00 | % | $ | — | $ | — | $ | $2,069 | $ | (25,000 | ) | $ | 17,069 | ||||||||||||||
| July 30, 2020 | October 30, 2021 | $ | 75,000 | 10.00 | % | $ | (27,778 | ) | $ | 27,778 | $ | 4,136 | $ | (79,136 | ) | $ | — | |||||||||||||
| February 17, 2021 | November 17, 2021 | $ | 112,000 | 10.00 | % | $ | (112,000 | ) | $ | 16,531 | $ | 1,654 | $ | — | $ | 18,185 | ||||||||||||||
| $ | $ | $ | ||||||||||||||||||||||||||||
| Total | $ | 364,500 | $ | (184,201 | ) | $ | 88,732 | $ | 14,734 | $ | (248,511 | ) | $ | 35,254 | ||||||||||||||||
In addition to what appears in the table above, there is outstanding accrued interest of $2,747 from a prior floating rate convertible note that has not been paid in cash or by conversion as of March 31, 2021.
On December 31, 2018 and January 2, 2019, the Company issued convertible notes to a single investor totaling $35,000 of maturity amount with accrued interest of $8,214 as of March 31, 2021. The number of shares of common stock (or preferred stock) into which these notes may convert is not determinable. The warrants to purchase 19,000 shares of common stock issued in connection with the sale of these notes and other convertible notes issued December 2018 and March 2019 are exercisable at a fixed price of $15.00 per share of common stock, provide no right to receive a cash payment, and included no reset rights or other protections based on subsequent equity transactions, equity-linked transactions or other events and expire on December 30, 2023.
Other convertible notes were also sold to investors in 2014 and 2015 (“Original Convertible Notes), which aggregated a total of $579,500, and had a fixed interest rate of 10% per annum. The Original Convertible Notes have no reset rights or other protections based on subsequent equity transactions, equity-linked transactions or other events. The warrants to purchase shares of common stock issued in connection with the sale of the convertible notes have either been exchanged as part of April and May 2016 note and warrant exchange agreements or expired on September 15, 2016.
| 16 |
The remaining outstanding Original Convertible Notes (including those for which default notices have been received) consist of the following at March 31, 2021 and December 31, 2020:
| March 31, 2021 | December 31, 2020 | |||||||
| Principal amount of notes payable | $ | 75,000 | $ | 75,000 | ||||
| Accrued interest payable | 67,787 | 64,357 | ||||||
| $ | 142,787 | $ | 139,357 | |||||
As of March 31, 2021, principal and accrued interest on the Original Convertible Note that is subject to a default notice accrues annual interest at 12% instead of 10%, totaled $49,899, of which $24,899 was accrued interest. As of December 31, 2020, principal and accrued interest on Original Convertible Notes subject to default notices totaled $48,700 of which $23,700 was accrued interest.
As of March 31, 2021 all of the outstanding Original Convertible Notes, inclusive of accrued interest, were convertible into an aggregate of 1,255 shares of the Company’s common stock. Such Original Convertible Notes will continue to accrue interest until exchanged, paid or otherwise discharged. There can be no assurance that any of the additional holders of the remaining Original Convertible Notes will exchange their Original Convertible Notes.
Note Payable to SY Corporation Co., Ltd.
On June 25, 2012, the Company borrowed 465,000,000 Won (the currency of South Korea, equivalent to approximately $400,000 United States Dollars as of that date) from and executed a secured note payable to SY Corporation Co., Ltd., (“SY Corporation”). The note accrues simple interest at the rate of 12% per annum and had a maturity date of June 25, 2013. The Company has not made any payments on the promissory note. At June 30, 2013 and subsequently, the promissory note was outstanding and in default, although SY Corporation has not issued a notice of default or a demand for repayment. Management believes that SY Corporation is in default of its obligations under its January 2012 license agreement, as amended, with the Company, but the Company has not yet issued a notice of default. The Company has in the past made several efforts towards a comprehensive resolution of the aforementioned matters involving SY Corporation. During the three-months ended March 31, 2021, there were no further communications between the Company and SY Corporation.
The promissory note is secured by collateral that represents a lien on certain patents owned by the Company, dating back to January, August and September 2007, including composition of matter patents for certain of the Company’s high impact ampakine compounds and the low impact ampakine compounds CX2007 and CX2076, and other related compounds that the Company is no longer developing and where patent rights date back to January, August and September 2007. The security interest does not extend to the Company’s patents for its ampakine compounds CX1739 and CX1942 or certain related method of use patents.
The note payable to SY Corporation consists of the following at March 31, 2021 and December 31, 2020:
| March 31, 2021 | December 31, 2020 | |||||||
| Principal amount of note payable | $ | 399,774 | $ | 399,774 | ||||
| Accrued interest payable | 423,214 | 411,384 | ||||||
| Foreign currency transaction adjustment | 23,664 | 53,393 | ||||||
| $ | 846,652 | $ | 864,551 | |||||
Interest expense with respect to this promissory note was $11,829 and $11,960 for the three-months ended March 31, 2021 and 2020, respectively.
Notes Payable to Officers and Former Officers
For the three-months ended March 31, 2021 and 2020, $3,034 and $2,816 was charged to interest expense with respect to Dr. Arnold S. Lippa’s notes, respectively.
For the three-months ended March 31, 2021 and 2020, $4,600 and $4,212 was charged to interest expense with respect to Dr. James S. Manuso’s notes, respectively.
As of September 30, 2018, Dr. James S. Manuso resigned as executive officer in all capacities and as a member of the board of directors of RespireRx (the “Board of Directors”).
| 17 |
Other Short-Term Notes Payable
Other short-term notes payable at March 31, 2021 and December 31, 2020 consisted of premium financing agreements with respect to various insurance policies. At March 31, 2021, a premium financing agreement was payable in the initial amount of $81,672 (after payment of a deposit of $20,347), with interest at 11% per annum, in eight monthly installments of $10,635. In addition, there is $2,317 of short term financing of office and clinical trials insurance premiums. At March 31, 2021 and December 31, 2020, the aggregate amount of the short-term notes payable was $86,192 and $4,608 respectively.
5. Settlement and Payment Agreements
On February 21, 2020, Sharp Clinical Services, Inc. (“Sharp”), a vendor of the Company, filed a complaint against the Company in the Superior Court of New Jersey Law Division, Bergen County related to a December 16, 2019 demand for payment of past due invoices inclusive of late fees totaling $103,890 of which $3,631 related to late fees, seeking $100,259 plus 1.5% interest per month on outstanding unpaid invoices. On May 29, 2020, a default was entered against the Company, and on September 4, 2020, a final judgment by default was entered against the Company in the amount of $104,217. The Company has recorded a liability to Sharp of $103,859 as of March 31, 2021.
By letter dated February 5, 2016, the Company received a demand from a law firm representing Salamandra, LLC (“Salamandra”) alleging an amount due and owing for unpaid services rendered. On January 18, 2017, following an arbitration proceeding, an arbitrator awarded Salamandra the full amount sought in arbitration of $146,082. Additionally, the arbitrator granted Salamandra attorneys’ fees and costs of $47,937. All such amounts have been accrued as of March 31, 2021 and December 31, 2029, including accrued interest at 4.5% annually from February 26, 2018, the date of the judgment, through March 31, 2021, totaling $26,031.
On February 23, 2021, our bank received two New Jersey Superior Court Levies totaling $320,911 related to amounts owed to Sharp and Salamandra which amounts were not in dispute. The bank debited our accounts and restricted access to those accounts pursuant to the liens placed on the accounts. Our accounts were debited for $1,559 on February 23, 2021, which represented all of the cash in our accounts on that date.
On March 3, 2021, we executed a settlement agreement with Sharp (the “Sharp Settlement Agreement”). The Sharp Settlement Agreement calls for a payment schedule of ten $10,000 payments due on April 1, 2021 every other month thereafter and permits early settlement at $75,000 if the Company pays Sharp that lower total by August 1, 2021. The first $10,000 payment which was due on April 1, 2021, was paid on March 23, 2021. On March 9, 2021, Sharp requested of the Bergen (NJ) County Sheriff, the return of the Writ of Execution which resulted in a release of the lien in favor of Sharp.
The Company had previously entered into a settlement agreement with Salamandra that is no longer in effect. RespireRx has approached Salamandra seeking to negotiate a new settlement agreement. The Salamandra lien with respect to that bank is still in effect.
The due date of the $100,000 annual amount payable to the University of Illinois that was originally due on December 31, 2020 pursuant to the 2014 License Agreement was extended to April 19, 2021 and was paid in full on April 1, 2021.
By email dated July 21, 2016, the Company received a demand from an investment banking consulting firm that represented the Company in 2012 in conjunction with the Pier transaction alleging that $225,000 is due and payable for investment banking services rendered. Such amount has been included in accrued expenses at March 31, 2021 and December 31, 2020.
The Company is periodically the subject of various pending and threatened legal actions and claims. In the opinion of management of the Company, adequate provision has been made in the Company’s consolidated financial statements as of March 31, 2021 and December 31, 2020 with respect to such matters, including, specifically, the matters noted above. The Company intends to vigorously defend itself if any of the matters described above results in the filing of a lawsuit or formal claim.
| 18 |
6. Stockholders’ Deficiency
Preferred Stock
RespireRx has authorized a total of 5,000,000 shares of preferred stock, par value $0.001 per share. As of March 31, 2021 and December 31, 2020, 37,500 shares were designated as Series B Convertible Preferred Stock (non-voting, “Series B Preferred Stock”).
Series B Preferred Stock outstanding as of March 31, 2021 and 2020 consisted of 37,500 shares issued in a May 1991 private placement. The shares of Series B Preferred Stock are convertible into 1 share of common stock. RespireRx may redeem the Series B Preferred Stock for $25,001 at any time upon 30 days prior notice.
Although other series of preferred stock have been designated, no other shares of preferred stock are outstanding. As of March 31, 2021 and December 31, 2020, 3,504,424.1552578 shares of preferred stock were undesignated and may be issued with such rights and powers as the Board of Directors may designate.
Common Stock
RespireRx has authorized 2,000,000,000 (2 billion) shares of Common Stock, par value $0.001 (“Common Stock”). There are 89,496,596 shares of the Company’s Common Stock outstanding as of March 31, 2021. After reserving for conversions of convertible debt and convertible preferred stock, as well as exercises of common stock purchase options (granted and available for grant within the 2014 and 2015 stock and stock option plans) and warrants and the issuance of Pier contingent shares and before accounting for incremental contract excess reserves, there were 1,859,151,502 shares of the Company’s Common Stock available for future issuances as of March 31, 2021. After accounting for incremental excess reserves contractually required by the various convertible notes and certain warrants, there were 1,817,007,866, shares of common stock available for future issuances as of March 31, 2021. No warrants or options were exercised after March 31, 2021. See Note 9. Subsequent Events in the notes to our condensed consolidated financial statements as of March 31, 2021.
Common Stock Warrants
Information with respect to the issuance and exercise of common stock purchase warrants in connection with the Convertible Note Payable and Warrant Purchase Agreement, and Notes Payable to Officers, is provided at Note 4.
A summary of warrant activity for the three-months ended March 31, 2021 is presented below.
| Number
of Shares | Weighted Average Exercise Price | Weighted
Average Remaining Contractual Life (in Years) | ||||||||||
| Warrants outstanding at December 31, 2020 | 28,809,352 | $ | 0.1528 | 2.64 | ||||||||
| Issued | - | - | ||||||||||
| Expired | (8,595 | ) | 79.3000 | |||||||||
| Warrants outstanding at March 31, 2021 | 28,800,757 | $ | 0.1292 | 2.39 | ||||||||
| Warrants exercisable at March 31, 2020 | 219,104 | 18.711 | 2.40 | |||||||||
| Warrants exercisable at March 31, 2021 | 28,800,757 | $ | 0.1292 | 2.39 | ||||||||
The exercise prices of common stock warrants outstanding and exercisable are as follows at March 31, 2021:
Exercise Price | Warrants Outstanding (Shares) | Warrants Exercisable (Shares) | Expiration Date | |||||||||
| $ | 0.016 | 2,212,500 | 2,212,500 | May 17, 2022 | ||||||||
| $ | 0.070 | 26,439,926 | 26,439,926 | September 30, 2023 | ||||||||
| $ | 11.00 -27.50 | 148,331 | 148,331 | December 31, 2021-December 30, 2023 | ||||||||
| 28,800,757 | 28,800,757 | |||||||||||
| 19 |
Based on a value of $0.045 per share on March 31, 2021, there were 2,212,500 exercisable in-the-money common stock warrants as of March 31, 2021.
A summary of warrant activity for the three-months ended March 31, 2020 is presented below.
| Number
of Shares | Weighted Average Exercise Price | Weighted
Average Remaining Contractual Life (in Years) | ||||||||||
| Warrants outstanding at December 31, 2019 | 219,104 | $ | 18.7109 | |||||||||
| Issued | - | - | ||||||||||
| Expired | - | - | ||||||||||
| Warrants outstanding at March 31, 2020 | 219,104 | $ | 18.7109 | 2.40 | ||||||||
| Warrants exercisable at March 31, 2020 | 219,104 | $ | 18.7109 | 2.40 | ||||||||
The exercise prices of common stock warrants outstanding and exercisable at March 31, 2020 ranged from $5.00 to $79.30 with respect to warrants exercisable into an aggregate of 219,104 shares which warrants expired or will expire between February 28, 2021 and October 22, 2024.
Based on a value of $0.115 per share on March 31, 2020, there were no exercisable in-the-money common stock warrants as of March 31, 2020.
Stock Options
On March 18, 2014, the stockholders of RespireRx holding a majority of the votes to be cast on the issue approved the adoption of RespireRx’s 2014 Equity, Equity-Linked and Equity Derivative Incentive Plan (the “2014 Plan”), which had been previously adopted by the Board of Directors, subject to stockholder approval. The Plan permits the grant of options and restricted stock in addition to stock appreciation rights and phantom stock, to directors, officers, employees, consultants and other service providers of the Company.
On June 30, 2015, the Board of Directors adopted the 2015 Stock and Stock Option Plan (as amended, the “2015 Plan”). As of March 31, 2021, there are 8,756,559 shares available in the 2015 Plan. The Company has not and does not intend to present the 2015 Plan to stockholders for approval.
Information with respect to the Black-Scholes variables used in connection with the evaluation of the fair value of stock-based compensation costs and fees is provided at Note 3.
A summary of stock option activity for the three-months ended March 31, 2021 is presented below.
Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (in Years) |
||||||||||
| Options outstanding at December 31, 2020 | 7,165,215 | $ | 1.96 | 4.98 | ||||||||
| Expired | (52,308 | ) | 73.78 | - | ||||||||
| Options outstanding at March 31, 2021 | 7,112,907 | $ | 1.43 | 4.38 | ||||||||
| Options exercisable at March 31, 2021 | 6,912,907 | $ | 1.47 | 4.38 | ||||||||
| 20 |
The exercise prices of common stock options outstanding and exercisable were as follows at March 31, 2021:
| Exercise Price | Options Outstanding (Shares) | Options Exercisable (Shares) | Expiration Date | |||||||||
| $ | 0.0540 | 1,700,000 | 1,500,000 | September 30, 2025 | ||||||||
| $ | 0.072 | 5,050,000 | 5,050,000 | July 31, 2025 | ||||||||
| $ | 7.00-$195.00 | 362,907 | 362,907 | September 12, 2021 - December 9, 2027 | ||||||||
| 7,112,907 | 6,912,907 | |||||||||||
There was no deferred compensation expense for the outstanding and unvested stock options at March 31, 2021.
Based on a fair value of $0.045 per share on March 31, 2021, there were no exercisable in-the-money common stock options as of March 31, 2021.
Reserved and Unreserved Shares of Common Stock
As of March 31, 2021, there are 2,000,000,000 shares of Common Stock, par value $0.001 authorized, of which 89,496,596 are issued and outstanding. As of March 31, 2021, there were outstanding options to purchase 7,112,907 share of Common Stock and 6,325 and 8,704,251 shares available for issuance under the 2014 Plan and 2015 Plan respectively. There are 649 Pier contingent shares of Common Stock that may be issued under certain circumstances. As of March 31, 2021, there are 6,674,704 issuable upon conversion of convertible notes. As of March 31, 2021, there are 28,800,757 shares that may be issued upon exercise of outstanding warrants. As of March 31, 2021, the Series B Preferred Stock may convert into 1 share of Common Stock. Therefore, the Company is reserving 51,351,902 shares of Common Stock for future issuances with respect to conversions and exercises as well as for the Pier contingent shares. In addition, certain convertible notes and related warrants impose an additional contractual reserve requirement, above the number of shares into which such convertible notes and related warrants may convert or exercise respectively. Although the Company does not anticipate having to issue such shares, such incremental additional contractual reserves total 42,143,636 shares of Common Stock.
7. Related Party Transactions
Dr. Arnold S. Lippa and Jeff E. Margolis, officers and directors of RespireRx since March 22, 2013, have indirect ownership and managing membership interests in Aurora Capital LLC (“Aurora”) through interests held in its members, and Jeff. E. Margolis is also an officer of Aurora. Aurora is a boutique investment banking firm specializing in the life sciences sector that ceased its securities related activities in April 2021 and on May 5, 2021 filed to withdraw its membership with FINRA and its registration with the SEC. Although Aurora has not provided services to RespireRx during the three-months ended March 31, 2021 or the fiscal year ended December 31, 2020, Aurora had previously provided services to the Company and there remains $96,000 owed to Aurora by RespireRx which amount is included in accounts payable and accrued expenses as of March 31, 2021.
A description of advances and notes payable to officers is provided at Note 4.
8. Commitments and Contingencies
Pending or Threatened Legal Action and Claims
The Company is periodically the subject of various pending and threatened legal actions and claims. In the opinion of management of the Company, adequate provision has been made in the Company’s condensed consolidated financial statements as of March 31, 2021 and 2020 with respect to such matters. See Note 5. Settlement and Payment Agreements to the condensed consolidated financial statements as of March 31, 2021 for additional items and details.
Significant Agreements and Contracts
Consulting Agreements
Richard Purcell, the Company’s Senior Vice President of Research and Development on at-will basis since October 15, 2014, provides his services to the Company on a month-to-month basis through his consulting firm, DNA Healthlink, Inc., through which the Company has contracted for his services for a monthly cash fee of $12,500. Cash compensation expense pursuant to this agreement totaled $0 and $37,500 for the three-months ended March 31, 2021 and 2020, respectively, which is included in research and development expenses in the Company’s consolidated statements of operations for such periods. Mr. Purcell did not provide services to the Company during the three-months ended March 31, 2021 and Mr. Purcell and the Company are in discussions to amend the related contract to change the fee from a monthly fixed rate to a rate of $250 per hour.
| 21 |
The Company entered into a consulting contract with David Dickason effective September 15, 2020 pursuant to which Mr. Dickason was appointed to and serves as the Company’s Senior Vice President of Pre-Clinical Product Development on an at-will basis at the rate of $250 per hour.
Employment Agreements
Timothy L. Jones, Arnold S. Lippa and Jeff E. Margolis have similar employment agreements. Mr. Jones was appointed as RespireRx’s President and Chief Executive Officer on May 6, 2020. Dr. Lippa is RespireRx’s Chief Scientific Officer and Executive Chairman and Mr. Margolis is the Company’s Senior Vice President, Chief Financial Officer, Treasurer and Secretary. Dr. Lippa’s and Mr. Margolis’ employment agreements became effective on August 18, 2015. All three agreements are subject to automatic annual extensions on September 30th of each year beginning with the initial termination date if not earlier terminated, subject to notice in accordance with the terms of the agreements. Mr. Jones’ initial termination date is September 30, 2023 and Dr. Lippa’s and Mr. Margolis’ agreements are in their automatic extension periods.
The table below summarized the current cash commitments to each individual through the next September 30th renewal date and in the case of Mr. Jones, through September 30, 2023.
| Contract year ending | Contract year ending | Contract year ending | ||||||||||||||||||||||||||||||||||||||||||||||
| September 30, 2021 | September 30, 2022 | September 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||
| Six months | Twelve months | Twelve months | ||||||||||||||||||||||||||||||||||||||||||||||
| Base | Guaranteed | Base | Guaranteed | Base | Guaranteed | |||||||||||||||||||||||||||||||||||||||||||
| Salary | Benefits | Bonus | Total | Salary | Benefits | Bonus | Total | Salary | Benefits | Bonus | Total | |||||||||||||||||||||||||||||||||||||
| Timothy L. Jones | $ | 150,000 | $ | 19,800 | $ | 150,000 | $ | 319,800 | $ | 300,000 | $ | 39,600 | $ | 300,000 | $ | 639,600 | $ | 300,000 | $ | 39,600 | $ | 300,000 | $ | 639,600 | ||||||||||||||||||||||||
| Arnold S. Lippa | 150,000 | 19,800 | — | 169,800 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| Jeff E. Margolis | 150,000 | 10,800 | — | 160,800 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||
| $ | 450,000 | $ | 50,400 | $ | 150,000 | $ | 650,400 | $ | 300,000 | $ | 39,600 | $ | 300,000 | $ | 639,600 | $ | 300,000 | $ | 39,600 | $ | 300,000 | $ | 639,600 | |||||||||||||||||||||||||
Under certain circumstances base salaries may be contractually increased or the executives may become eligible for additional benefits and base salaries may be increased at the discretion of the Board of Directors. All executives are eligible for stock and stock option and similar grants at the discretion of the Board or Directors.
The payment of certain amounts reflected in the table above have been voluntarily deferred indefinitely and payments against accrued compensation may be made based upon the Company’s ability to make such payments.
UWMRF Patent License Agreement
On August 1, 2020, the (“Effective Date”), the Company and UWMRF executed the UWMRF Patent License Agreement pursuant to which, the Company has an exclusive license to commercialize GABAkine products based on UWMRF’s rights in certain patents and patent applications, and a non-exclusive license to commercialize products based on UWMRF’s rights in certain technology that is not the subject of the patents or patent applications. UWMRF maintains the right to use, and, upon the approval of the Company, to license, these patent and technology rights for any non-commercial purpose, including research and education. The UWMRF Patent License Agreement expires upon the later of the expiration of the Company’s payment obligations to UWMRF or the expiration of the last remaining licensed patent granted thereunder, subject to early termination upon the occurrence of certain events. The License Agreement also contains a standard indemnification provision in favor of UWMRF and confidentiality provisions obligating both parties.
| 22 |
Under the UWMRF Patent License Agreement, in consideration for the licenses granted, the Company will pay to UWMRF the following: (i) patent filing and prosecution costs incurred by UWMRF prior to the effective date, paid in yearly installments over three years from the Effective Date; (ii) annual maintenance fees, beginning on the second anniversary of the Effective Date, which annual maintenance fees terminate upon the Company’s payment of royalties pursuant to clause (iv) below; (iii) milestone payments, paid upon the occurrence of certain dosing events of patients during clinical trials and certain approvals by the FDA; and (iv) royalties on net sales of products developed with the licenses, subject to minimum annual payments and to royalty rate adjustments based on whether separate royalty payments by the Company yield an aggregate rate beyond a stated threshold. The Company has also granted UWMRF certain stock appreciation rights with respect to the Company’s neuromodulator programs, subject to certain limitations, and will pay to UWMRF certain percentages of revenues generated from sublicenses of the licenses provided under the UWMRF Patent License Agreement by the Company to third parties.
University of Illinois 2014 Exclusive License Agreement
The Company and the University of Illinois entered into the Exclusive License Agreement (the “2014 License Agreement”) effective September 18, 2014, pursuant to which the Company obtained (i) exclusive rights to several issued and pending patents in numerous jurisdictions and (ii) the non-exclusive right to certain technical information that is generated by the University of Illinois in connection with certain clinical trials as specified in the 2014 License Agreement, all of which relate to the use of cannabinoids for the treatment of sleep related breathing disorders. The Company is developing dronabinol (Δ9-tetrahydrocannabinol), a cannabinoid, for the treatment of OSA, the most common form of sleep apnea.
The 2014 License Agreement provides for various commercialization and reporting requirements that commenced on June 30, 2015. In addition, the 2014 License Agreement provides for various royalty payments, including a royalty on net sales of 4%, payment on sub-licensee revenues of 12.5%, and a minimum annual royalty beginning in 2015 of $100,000, which is due and payable on December 31 of each year beginning on December 31, 2015. The minimum annual royalty obligation of $100,000 due on December 31, 2020, was extended to April 19, 2021 and was paid in full on April 1, 2021. One-time milestone payments may become due based upon the achievement of certain development milestones. $350,000 will be due within five days after the dosing of the first patient is a Phase III human clinical trial anywhere in the world. $500,000 will be due within five days after the first NDA filing with FDA or a foreign equivalent. $1,000,000 will be due within twelve months of the first commercial sale. One-time royalty payments may also become due and payable. Annual royalty payments may also become due. In the year after the first application for market approval is submitted to the FDA or a foreign equivalent and until approval is obtained, the minimum annual royalty will increase to $150,000. In the year after the first market approval is obtained from the FDA or a foreign equivalent and until the first sale of a product, the minimum annual royalty will increase to $200,000. In the year after the first commercial sale of a product, the minimum annual royalty will increase to $250,000.
During the three-months ended March 31, 2021 and 2020, the Company recorded charges to operations of $25,000, respectively, with respect to its 2021 and 2020 minimum annual royalty obligation, which is included in research and development expenses in the Company’s condensed consolidated statement of operations for the three-months ended March 31, 2021 and 2020. As discussed above, the Company did not pay the amount due on December 31, 2020 for which the Company was granted an extension until April 19, 2021 and which was paid in full on April 1, 2021.
| 23 |
Noramco Inc. - Dronabinol Development and Supply Agreement
On September 4, 2018, RespireRx entered into a dronabinol Development and Supply Agreement with Noramco Inc., one of the world’s major dronabinol manufacturers, which Noramco subsequently assigned to its subsidiary, Purisys LLC (the “Purisys Agreement”). Under the terms of the Purisys Agreement, Purisys has agreed to (i) provide all of the active pharmaceutical ingredient (“API”) estimated to be needed for the clinical development process for both the first- and second-generation products (each a “Product” and collectively, the “Products”), three validation batches for New Drug Application (“NDA”) filing(s) and adequate supply for the initial inventory stocking for the wholesale and retail channels, subject to certain limitations, (ii) maintain or file valid drug master files (“DMFs”) with the FDA or any other regulatory authority and provide the Company with access or a right of reference letter entitling the Company to make continuing reference to the DMFs during the term of the agreement in connection with any regulatory filings made with the FDA by the Company, (iii) participate on a development committee, and (iv) make available its regulatory consultants, collaborate with any regulatory consulting firms engaged by the Company and participate in all FDA or Drug Enforcement Agency (“DEA”) meetings as appropriate and as related to the API. We now refer to the second-generation product as our proprietary formulation or proprietary product and have de-emphasized the first-generation product.
In consideration for these supplies and services, the Company has agreed to purchase exclusively from Purisys during the commercialization phase all API for its Products (as defined in the Development and Supply Agreement) at a pre-determined price subject to certain producer price index adjustments and agreed to Purisys’ participation in the economic success of the commercialized Product or Products up to the earlier of the achievement of a maximum dollar amount or the expiration of a period of time.
Transactions with Bausch Health Companies Inc. (formerly known as Biovail Laboratories International SRL)
Beginning in March 2010, the Company entered into a series of asset purchase and license agreements with Biovail Laboratories International SRL which later merged with Valeant Pharmaceuticals International, Inc. which was later renamed Bausch Health Companies Inc. (“Bausch”).
In March 2011, the Company entered into a new agreement with Bausch to reacquire the ampakine compounds, patents and rights that Bausch had acquired from the Company in March 2010. The new agreement provided for potential future payments of up to $15,150,000 by the Company based upon the achievement of certain developments, including new drug application submissions and approval milestones pertaining to an intravenous dosage form of the ampakine compounds for respiratory depression, a therapeutic area not currently pursued by the Company. Bausch is also eligible to receive additional payments of up to $15,000,000 from the Company based upon the Company’s net sales of an intravenous dosage form of the compounds for respiratory depression.
At any time following the completion of Phase 1 clinical studies and prior to the end of Phase 2A clinical studies, Bausch retains an option to co-develop and co-market intravenous dosage forms of an ampakine compound as a treatment for respiratory depression and vaso-occlusive crises associated with sickle cell disease. In such an event, the Company would be reimbursed for certain development expenses to date and Bausch would share in all such future development costs with the Company. If Bausch makes the co-marketing election, the Company would owe no further milestone payments to Bausch and the Company would be eligible to receive a royalty on net sales of the compound by Bausch or its affiliates and licensees.
There was no activity during the three-months ended March 31, 2021 or 2020 that affect the Bausch agreement.
Summary of Principal Cash Obligations and Commitments
The following table sets forth the Company’s principal cash obligations and commitments for the next five fiscal years as of March 31, 2021, aggregating $2,380,070. License agreement amounts included in the 2021 column represents amounts contractually due from April 1, 2021 through December 31, 2020 (nine months) and in each of the subsequent years, represents the full year. Employment agreement amounts included in the 2021 column represent amounts contractually due at from April 1, 2021 through September 30, 2021 (six months) when such contracts expire unless extended pursuant to the terms of the contracts.
| Payments Due By Year | ||||||||||||||||||||||||
| Total | 2021 | 2022 | 2023 | 2024 | 2025 | |||||||||||||||||||
| License agreements | $ | 535,370 | $ | 75,000 | $ | 115,092 | $ | 115,093 | $ | 130,185 | $ | 100,000 | ||||||||||||
| Employment agreements (1) | 1,844,700 | 650,400 | 639,600 | 554,700 | - | - | ||||||||||||||||||
| Total | $ | 2,380,070 | $ | 725,400 | $ | 754,692 | $ | 669,793 | $ | 130,185 | $ | 100,000 | ||||||||||||
(1) The payment of certain of such amounts has been deferred indefinitely, as described above in “Employment Agreements”.
| 24 |
9. Subsequent Events
On April 1, 2021, May 3, 2021 and on May 10, 2021, the Company closed on financings pursuant to three convertible notes issued to three separate investors, due in each case, one year from the effective date (which for the first two closings was March 31, 2021 and April 30, 2021 respectively), with maturity amounts of $112,500, $150,000 and $150,000 respectively. In addition, the noteholders received as consideration warrants to purchase 2,400,000, 3,200,000 and 3,200,000 shares of Common Stock, respectively, each exercisable at $0.02 per share for five years. The Company received net proceeds of $96,750, $123,400 and $123,400 respectively, for an aggregate of $343,550. The difference between the maturity amounts and the net proceeds were due to original issue discounts, investor legal fees and in two cases, broker fees. The three notes are convertible at a fixed price of $0.02 per share and bear interest at 10% per year which interest is guaranteed regardless of prepayment. The Company has the right to prepay the notes during the first six months subject to prepayment premiums that range from 0% to 15% (100% to 115% of the maturity amount plus accrued interest and any default interest and similar costs). These notes are similar to the convertible notes described in Note 4 to our condensed consolidated financial statements as of March 31, 2021.
On April 29, 2021, RespireRx agreed to a payment and settlement agreement with the University of California Innovation and Entrepreneurship to a payment schedule with respect to accounts payable in an amount that was not in dispute and is reflected in accounts payable and accrued expenses in the Company’s condensed consolidated financial statements as of March 31, 2021. The total amount due is $234,657. The agreed payment schedule is for the Company to pay $10,000 on each of July 1, 2021, September 1, 2021, November 1, 2021, January 1, 2022 and March 31, 2022. If RespireRx pays an aggregate of $175,000 on or before March 31, 2022, the amounts will be considered paid in full with no further amounts due. If an aggregate of $175,000 has not been paid by March 31, 2022, the remaining unpaid amount up to an aggregate of the original amount of $234,657 would be due and payable.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with the condensed consolidated financial statements (unaudited) and notes related thereto appearing elsewhere in this document, as well as the audited consolidated financial statements, notes related thereto, and Management’s Discussion and Analysis of Financial Condition and Results of Operations in our 2020 Form 10-K.
Overview
The mission of the Company is to develop innovative and revolutionary treatments to combat disorders caused by disruption of neuronal signaling. We are developing treatment options that address conditions that affect millions of people, but for which there are limited or poor treatment options, including obstructive sleep apnea (“OSA”), attention deficit hyperactivity disorder (“ADHD”) epilepsy, chronic pain, including inflammatory and neuropathic pain, recovery from spinal cord injury (“SCI”), as well as other areas of interest based on results of animal studies to date.
RespireRx is developing a pipeline of new drug products based on our broad patent portfolios across two distinct drug platforms:
| (i) | our pharmaceutical cannabinoids platform (which we refer to as ResolutionRx), including dronabinol (a synthetic form of ∆9-tetrahydrocannabinol (“Δ9-THC”)), which acts upon the nervous system’s endogenous cannabinoid receptors, and | |
| (ii) | our neuromodulators platform (which we refer to as EndeavourRx) is made up of two programs: (a) our ampakines program, including proprietary compounds that are positive allosteric modulators (“PAMs”) of AMPA-type glutamate receptors to promote neuronal function and (b) our GABAkines program, including proprietary compounds that are PAMs of GABAA receptors, which was recently established pursuant to our entry with the University of Wisconsin-Milwaukee Research Foundation, Inc., an affiliate of the University of Wisconsin-Milwaukee (“UWMRF”), into a patent license agreement (the UWMRF Patent License Agreement”). |
| 25 |
Financing our Platforms
Our major challenge has been to raise substantial equity or equity-linked financing to support research and development plans for our cannabinoid and neuromodulator platforms, while minimizing the dilutive effect to pre-existing stockholders. At present, we believe that we are hindered primarily by our public corporate structure, our OTCQB listing, and low market capitalization as a result of our low stock price.
For this reason, the Company has affected an internal restructuring plan through which our two drug platforms have been reorganized into separate business units and may in the future, be organized into subsidiaries of RespireRx. We believe that by creating one or more subsidiaries to further the aims of ResolutionRx and EndeavourRx, it may be possible, through separate finance channels, to optimize the asset values of each. We are also planning to commence a securities offering by the Company pursuant to Regulation A by filing a Form 1-A.
Below is a chart that represents our current development status for each of our product candidates for the disorders for which they are being developed. Preclinical testing is pre-human testing and includes in vitro and animal studies. Phase 1 clinical trials are primarily safety, generally conducted in healthy adults. Phase 2 clinical trials are generally somewhat larger than Phase 1 and often include dose finding, additional safety and preliminary efficacy. Phase 3 clinical trials are larger studies designed to test efficacy and safety in a broader population. See “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our 2020 Form 10-K for more information on our proposed regulatory approach and development plans for our product candidates.
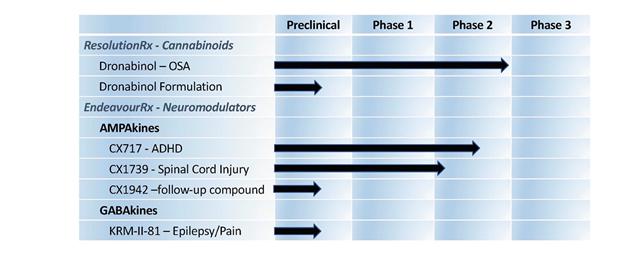
Below is our current business organization with ResolutionRx and EndeavourRx currently operating as divisions and which are planned to become, initially, wholly-owned subsidiaries.
Technology Rights
University of Illinois License Agreement
See Note 8. Commitments and Contingencies – Significant Agreements and Contracts – University of Illinois 2014 Exclusive License Agreement to our condensed consolidated financial statements at March 31, 2021.
As of March 31, 2021, the Company received an extension of time to make a $100,000 payment that would have due on such date until April 19, 2021. The payment was made on April 1, 2021.
UWMRF Patent License Agreement
See Notes 1, 2 and 8 to our condensed consolidated financial statements at March 31, 2021.
Going Concern
See Note 2. Business – Going Concern to our condensed consolidated financial statements at March 31, 2021.
The Company’s regular efforts to raise capital and to evaluate measures to permit sustainability are time-consuming and intensive. Such efforts may not prove successful and may cause distraction, disruption or other adversity that limits the Company’s development program efforts.
Recent Accounting Pronouncements
See Note 2 to the Company’s condensed consolidated financial statements at March 31, 2021.
Management does not believe that any recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
Concentration of Risk
See Note 2. Significant Accounting Policies – Concentration of Credit Risk to the Company’s condensed consolidated financial statements at March 31, 2021.
See Note 8. Commitments and Contingencies – University of Illinois 2014 Exclusive License Agreement to the Company’s condensed consolidated financial statements at March 31, 2021.
See Note 8. Commitments and Contingencies – UWMRF Patent License Agreement to the Company’s condensed consolidated financial statements at March 31, 2021.
| 26 |
Critical Accounting Policies and Estimates
The Company prepared its condensed consolidated financial statements in accordance with GAAP. The preparation of these condensed consolidated financial statements requires the use of estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of revenues and expenses during the reporting period. Management periodically evaluates the estimates and judgments made. Management bases its estimates and judgments on historical experience and on various factors that are believed to be reasonable under the circumstances. Actual results may differ from these estimates as a result of different assumptions or conditions.
Critical accounting policies and estimates are described in the notes to the Company’s condensed consolidated financial statements and include:
| - | Stock-based awards | |
| - | Research and Development Costs | |
| - | License Agreements | |
| - | Patent Costs | |
| - | Convertible Notes |
See Critical Accounting Policies and Estimates in our 2020 Form 10-K for a complete description.
Results of Operations
The Company’s consolidated statements of operations as discussed herein are presented below.
Three-months Ended March 31, (unaudited) | ||||||||
| 2021 | 2020 | |||||||
| Operating expenses: | ||||||||
| General and administrative | $ | 645,376 | $ | 365,280 | ||||
| Research and development | 154,764 | 155,920 | ||||||
| Total operating costs and expenses | 800,140 | 520,570 | ||||||
| Loss from operations | (800,140 | ) | (520,570 | ) | ||||
| Loss on extinguishment of debt in exchange for equity | - | (323,996 | ) | |||||
| Interest expense | (79,470 | ) | (140,710 | ) | ||||
| Foreign currency transaction gain (loss) | 29,361 | 38,558 | ) | |||||
| Net loss attributable to common stockholders | $ | (850,249 | ) | $ | (946,718 | ) | ||
| Net loss per common share - basic and diluted | $ | (0.01 | ) | $ | (1.42 | ) | ||
| Weighted average common shares outstanding – basic and diluted | 78,148,365 | 668,660 | ||||||
Three-months Ended March 31, 2021 and 2020
Revenues. The Company had no revenues during the three-months ended March 31, 2021 and 2020.
General and Administrative. For the three-months ended March 31, 2021 general and administrative expenses were $645,376, an increase of $280,096, as compared to $365,280 for the three-months ended March 31, 2020. The increase in general and administrative expenses for the three-months ended March 31, 2021, as compared to the three-months ended March 31, 2020, is primarily due to a contractual bonus of $200,000 and quarterly base compensation and benefits of $84,900 to the Company’s President and Chief Executive Officer in the current period with no bonus in the prior year period, and increases in accounting fees of $16,650, stock-based expenses of $28,000 and transfer agent fees of $14,625, offset by decreases in general legal fees of $42,195, Board of Directors fees of $10,484 and patent fees of $9,886, and the net effect of increases and decreases other general and administrative expenses.
| 27 |
There was stock-based compensation of $28,000 in general and administrative expenses for the three-months ended March 31, 2021 and none for the three-months ended March 31, 2020.
Research and Development. For the three-months ended March 31, 2021, research and development expenses were $154,764, a decrease of $526, as compared to $155,290 for the three-months ended March 31, 2020. The decrease in research and development expenses for the three-months ended March 31, 2021, as compared to the three-months ended March 31, 2020, is primarily a decrease in utilization of consultants and licensing fees associated with the one-time fee to UWMRF during the three-months ended March 31, 2020, offset by the increase in stock-based compensation.
There was stock-based compensation of $16,250 in research and development expenses for the three-months ended March 31, 2020 and none for the three-months ended March 31, 2020.
Loss on Extinguishment of Convertible Debt. There was no loss on extinguishment of debt during the three-months ended March 31, 2021. The loss on extinguishment of convertible debt during the three-months ended March 31, 2020 was $323,996.
Interest Expense. During the three-months ended March 31, 2021, interest expense was $79,470 as compared to $140,710 for the three-months ended March 31, 2020. The decrease of $61,240 is primarily the result of payments of debt substantially in the early portion of the three-months ended March 31, 2021 reducing the total amount of debt during the quarter as compared to the three-months ended March 31, 2020.
Foreign Currency Transaction (Loss) Gain. Foreign currency transaction gain was $29,361 for the three-months ended March 31, 2021, as compared to a foreign currency transaction gain of $38,558 for the three-months ended March 31, 2020. The foreign currency transaction (loss) gain relates to the $399,774 loan from SY Corporation, made in June 2012, which is denominated in the South Korean Won.
Net Loss Attributable to Common Stockholders. For the three-months ended March 31, 2021, the Company incurred a net loss of $850,249 as compared to a net loss of $946,718 for the three-months ended March 31, 2020. Included in the net loss for the three-months ended March 31, 2020 is a loss on the extinguishment of debt of 323,996.
Liquidity and Capital Resources - March 31, 2021
The Company’s condensed consolidated financial statements have been presented on the basis that it is a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The Company has incurred net losses of $850,249 and net losses from operations of $800,140 for the three-months ended March 31, 2021 and $4,301,210 for the fiscal year ended December 31, 2020, and negative operating cash flows of $286,861 for the three-months ended March 31, 2021 and $513,001 for the fiscal year ended December 31, 2020, had a stockholders’ deficiency of $8,454,618 at March 31, 2021, and expects to continue to incur net losses and negative operating cash flows for at least the next few years. As a result, management has concluded that there is substantial doubt about the Company’s ability to continue as a going concern, and the Company’s independent registered public accounting firm, in its report on the Company’s consolidated financial statements for the year ended December 31, 2020, expressed substantial doubt about the Company’s ability to continue as a going concern.
At March 31, 2021, the Company had a working capital deficit of $8,454,618, as compared to a working capital deficit of $8,063,320 at December 31, 2020 reflecting an increase in the working capital deficit of $391,298 for the three-months ended March 31, 2021. The increase in the working capital deficit is primarily due to a larger increase in our current liabilities, notably accrued compensation and related benefits and accounts payable and accrued expenses which increased as expenses were incurred but not paid within the three-month period ended March 31, 2021, as compared to a more modest increase in current assets which resulted from an increase in cash balances from financings and an increase in prepaid expenses. During the fiscal year ended December 31, 2020, there was forgiveness of certain compensation and related benefits by certain executive officers aggregating $1,684,218 in exchange for equity, having the effect of reducing accrued compensation and related benefits by that amount that did not occur during the three-month period ended March 31, 2021. See also “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations-Forgiveness of Accrued Compensation and Related Costs” in our 2020 Form 10-K. In addition, a portion of accounts payable due to two vendors totaling $241,109 was exchanged for equity during the fiscal year ended December 31, 2020. No similar exchanges occurred during the three-months ended March 31, 2021.
At March 31, 2021, the Company had cash of $5,347, as compared to $825 at December 31, 2020, reflecting an increase in cash of $4,522 for the three-months ended March 31, 2021.
In general, substantially all of the cash raised in financings during the three-months ended March 31, 2021 and during fiscal year ended December 31, 2020 was utilized to pay general and administrative expenses or the related accounts payable, including, but not limited to, payments to our licensors, our independent registered public accounting firm, our patent and intellectual property law firm and for other patent and intellectual property services, our transfer agent, our financial printer and limited cash payments of compensation. Cash was also utilized to pay research and development expenses or the related accounts payable for new formulation work performed by a contractor with respect to the development of a new proprietary formulation of dronabinol. Cash was also utilized, among other purposes, to make payments pursuant to directors and officers insurance and other insurance financings, repay, in part, certain advances made by officers and one vendor and payments of certain outstanding accounts payable.
| 28 |
The Company is currently, and has for some time, been in significant financial distress. It has limited cash resources and current assets and has no ongoing source of sustainable revenue. Management is continuing to address various aspects of the Company’s operations and obligations, including, without limitation, debt obligations, financing requirements, intellectual property, licensing agreements, legal and patent matters and regulatory compliance, and has continued to raise new debt and equity capital to fund the Company’s general and administrative and research and development activities from both related and unrelated parties.
The Company is continuing its efforts to raise additional capital in order to be able to pay its liabilities and fund its business activities on a going forward basis, including the pursuit of the Company’s planned research and development activities. We anticipate filing a Form 1-A with the SEC, which if qualified would enable the Company to engage in a Regulation A Offering. We provide no assurance that we will file a Form 1-A, or if filed that the Regulation A Offering would be qualified, or if qualified, would result in a financing on terms as to price per share or other securities offered, amount of funds raised or other terms acceptable to the Company or at all. The Company regularly evaluates various other measures to satisfy the Company’s liquidity needs, including development and other agreements with collaborative partners and, when necessary, seeking to exchange or restructure the Company’s outstanding securities. The Company is evaluating certain changes to its operations and structure to facilitating raising capital from sources that may be interested in financing only discrete aspects of the Company’s development programs. Such changes could include a significant reorganization, which may include the formation of one or more subsidiaries into which one or more programs may be contributed. As a result of the Company’s current financial situation, the Company has limited access to external sources of debt and equity financing. Accordingly, there can be no assurances that the Company will be able to secure additional financing in the amounts necessary to fully fund its operating and debt service requirements. If the Company is unable to access sufficient cash resources, the Company may be forced to discontinue its operations entirely and liquidate.
Operating Activities. For the three-months ended March 31, 2021, operating activities utilized cash of $286,861, as compared to utilizing cash of $17,859 for the three-months ended March 31, 2020, to support the Company’s ongoing general and administrative expenses as well as its research and development activities.
Financing Activities. For the three-months ended March 31, 2021, financing activities consisted of the $97,500 from a convertible note financing, net of original issue discount and note costs, $117,299 from the sale of Common Stock and $81,564 financing of a new directors and officers insurance policy offset by a $5,000 partial repayment of an officer advance.
Principal Commitments
Employment Agreements
See Note 8. Commitments and Contingencies – Significant Agreements and Contracts – Employment Agreements to our condensed consolidated financial statements at March 31, 2021.
University of Illinois 2014 Exclusive License Agreement
See Note 8. Commitments and Contingencies – Significant Agreements and Contracts – University of Illinois 2014 Exclusive License Agreement to our condensed consolidated financial statements at March 31, 2021.
UWM Research Foundation Patent License Agreement
See Note 8. Commitments and Contingencies – Significant Agreements and Contracts, UWM Research Foundation Patent License Agreement to our condensed consolidated financial statement at March 31, 2021.
A table setting forth the Company’s principal cash obligations and commitments for the next five fiscal years as of March 31, 2021, aggregating $2,380,070, is set forth in Note 8. Commitments and Contingencies – Summary of Principal Cash Obligations and Commitments
Off-Balance Sheet Arrangements
At March 31, 2021, the Company did not have any transactions, obligations or relationships that could be considered off-balance sheet arrangements.
| 29 |
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Not applicable.
ITEM 4. CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are designed to ensure that information required to be disclosed in the reports that the Company files with the Securities and Exchange Commission (the “SEC”) under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to the Company’s management, including its Chief Executive Officer and Chief Financial Officer, to allow for timely decisions regarding required disclosures.
The Company carried out an evaluation, under the supervision and with the participation of its management, consisting of its principal executive officer and principal financial officer, of the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act). Based upon that evaluation, the Company’s principal executive officer and principal financial officer concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures were not effective to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized and reported within the required time periods and is accumulated and communicated to the Company’s management, consisting of the Company’s principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
Management has been focusing on developing replacement controls and procedures that are adequate to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized and reported within the required time periods and is accumulated and communicated to the Company’s management to allow timely decisions regarding required disclosure. Although the senior executive team has been developing more formalized and regular communication and collaboration processes, thereby improving the control environment, these controls and procedures are not yet sufficient to remediate the Company’s control weaknesses. The Company is current in its SEC periodic reporting obligations, but as of the date of the filing of this Quarterly Report on Form 10-Q, the Company had not yet established adequate internal controls over financial reporting.
The Company’s management, consisting of its principal executive officer and principal financial officer, does not expect that its disclosure controls and procedures or its internal controls will prevent all error or fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Furthermore, the design of a control system must reflect the fact that there are resource constraints and the benefits of controls must be considered relative to their costs. Due to the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. In addition, as conditions change over time, so too may the effectiveness of internal controls. However, management believes that the financial information included in this Quarterly Report on Form 10-Q fairly present, in all material respects, the Company’s financial condition, results of operations and cash flows for the periods presented.
(b) Changes in Internal Controls over Financial Reporting
The Company’s management, consisting of its principal executive officer and principal financial officer, has determined that no change in the Company’s internal control over financial reporting (as that term is defined in Rules 13(a)-15(f) and 15(d)-15(f) of the Securities Exchange Act of 1934) occurred during or subsequent to the end of the period covered in this report that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
| 30 |
We are periodically subject to various pending and threatened legal actions and claims. See Note 8. Commitments and Contingencies – Pending or Threatened Legal Actions and Claims to our condensed consolidated financial statements at March 31, 2021 for details regarding these matters.
As of the date of this filing, there have been no material changes to the Risk Factors included in the Company’s 2020 Form 10-K. The Risk Factors set forth in the 2020 Form 10-K should be read carefully in connection with evaluating the Company’s business and in connection with the forward-looking statements contained in this Quarterly Report on Form 10-Q. Any of the risks described in the 2020 Form 10-K could materially adversely affect the Company’s business, financial condition or future results and the actual outcome of matters as to which forward-looking statements are made. These are not the only risks that the Company faces. Additional risks and uncertainties not currently known to the Company or that the Company currently deems to be immaterial also may materially adversely affect the Company’s business, financial condition and/or operating results.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
There were no unregistered sales of equity securities during the three-months ended March 31, 2021 that were not disclosed by the Company on a Current Report on Form 8-K. There were conversions of convertible notes inclusive of accrued interest as disclosed in Note 4. Notes Payable – Convertible Notes Payable of our condensed consolidated financial statements at March 31 2021 and Part I, Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – March 31, 2021.
Additional information with respect to the transactions described above is provided in the Notes to the Condensed Consolidated Financial Statements for the three-months ended March 31, 2021.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
Note Payable to SY Corporation Co., Ltd.
On June 25, 2012, the Company borrowed 465,000,000 Won (the currency of South Korea, equivalent to approximately $400,000 United States Dollars) from and executed a secured note payable to SY Corporation, The note accrues simple interest at the rate of 12% per annum and had a maturity date of June 25, 2013. The Company has not made any payments on the promissory note. At June 30, 2013 and subsequently, the promissory note was outstanding and in technical default, although SY Corporation has not issued a notice of default or a demand for repayment. The Company believes that SY Corporation is in default of its obligations under its January 2012 license agreement, as amended, with the Company, but the Company has not yet issued a notice of default. The Company has in the past made several efforts towards a comprehensive resolution of the aforementioned matters involving SY Corporation. During the three-months ended March 31, 2021, there were no further communications between the Company and SY Corporation.
The note payable to SY Corporation consists of the following at March 31, 2021 and December 31, 2020:
| March 31, 2021 | December 31, 2020 | |||||||
| Principal amount of note payable | $ | 399,774 | $ | 399,774 | ||||
| Accrued interest payable | 423,214 | 411,384 | ||||||
| Foreign currency transaction adjustment | 23,664 | 53,393 | ||||||
| $ | 846,652 | $ | 864,551 | |||||
Interest expense with respect to this promissory note was $11,829 and $11,960 for the three-months ended March 31, 2021 and 2020, respectively.
Default on Convertible Notes Payable
At March 31, 2021, the amount owed on the one remaining Original Convertible Note in default was $49,899, including principal and interest.
| 31 |
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
The information below is reported in lieu of information that would be reported under Items 1.01 and 2.03 under Form 8-K.
On April 29, 2021, RespireRx agreed to a payment and settlement agreement with the University of California Innovation and Entrepreneurship to a payment schedule with respect to accounts payable in an amount that was not in dispute and is reflected in accounts payable and accrued expenses in the Company’s condensed consolidated financial statements as of March 31, 2021. The total amount due is $234,657. The agreed payment schedule is for the Company to pay $10,00 on each of July 1, 2021, September 1, 2021, November 1, 2021, January 1, 2022 and March 31, 2022. If RespireRx pays an aggregate of $175,000 on or before March 31, 2022, the amounts will be considered paid in full with no further amounts due. If an aggregate of $175,000 has not been paid by March 31, 2022, the remaining unpaid amount up to an aggregate of the original amount of $234,657 would be due and payable.
INDEX TO EXHIBITS
The following documents are filed as part of this report:
| Exhibit Number | Description of Document | |
3.1 |
||
10.1 |
||
10.2 |
||
10.3 |
||
10.4 |
||
10.5 |
||
| 31.1* | Officer’s Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2* | Officer’s Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1** | Officer’s Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2** | Officer’s Certification Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101.INS*** | XBRL Instance Document | |
| 101.SCH*** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL*** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.LAB*** | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE*** | XBRL Taxonomy Extension Presentation Linkbase Document | |
| 101.DEF*** | XBRL Taxonomy Extension Definition Linkbase Document |
| * Filed herewith. | |
| ** Furnished herewith. | |
| *** In accordance with Regulation S-T, the XBRL related information on Exhibit No. 101 to this Quarterly Report on Form 10-Q shall be deemed “furnished” herewith not “filed.” |
| 32 |
In accordance with the requirements of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| RESPIRERX PHARMACEUTICALS INC. | ||
| (Registrant) | ||
| Date: May 24, 2021 | By: | /s/ Timothy Jones |
| Timothy Jones | ||
| President and Chief Executive Officer | ||
| Date: May 24, 2021 | By: | /s/ Jeff Eliot Margolis |
| Jeff Eliot Margolis | ||
| Senior Vice President, Chief Financial Officer, Treasurer and Secretary | ||
| 33 |
