Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Alkermes plc. | alks-ex991_7.htm |
| 8-K - 8-K - Alkermes plc. | alks-8k_20201029.htm |

Third Quarter 2020 Financial Results & Business Update October 29, 2020 Exhibit 99.2

Forward-Looking Statements and Non-GAAP Financial Information Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s expectations with respect to its future financial and operating performance, business plans or prospects; the potential therapeutic and commercial value of the company’s marketed and development products; the company’s expectations and assumptions regarding the future impacts of COVID-19 on its business; the company’s expectations concerning future regulatory activities and interactions, including expected timing of the U.S. Food and Drug Administration’s (“FDA”) target Prescription Drug User Fee Act (“PDUFA”) action date for the new drug application (“NDA”) for ALKS 3831 and, if approved, Drug Enforcement Administration de-scheduling of ALKS 3831; the company’s expectations concerning future development activities, including with respect to the ongoing ARTISTRY clinical development program and plans to present data from the program at a medical meeting; the company’s expectations concerning its commercial activities and capabilities, including launch planning strategies and activities for the potential launch of ALKS 3831. The company cautions that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: the impacts of the ongoing COVID-19 pandemic and continued efforts to mitigate its spread on the company’s business, results of operations or financial condition, including impacts on the vendors or distribution channels in its supply chain and the company’s ability to continue to manufacture its products, impacts on its ability to continue its discovery activities, impacts on the conduct of its clinical trials, impacts on healthcare systems that serve people living with opioid dependence, alcohol dependence and schizophrenia and on patient and healthcare provider access to the company’s medicines, impacts on the regulatory agencies with which the company interacts in the development, review, approval and commercialization of its medicines, impacts on reimbursement for its products, including its Medicaid rebate liability, and for services related to the use of its products, and impacts on the U.S., Irish and/or global economies more broadly; the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of the company’s products, which may lead to competition from generic drug manufacturers; clinical development activities may not be completed on time or at all; the results of the company’s clinical development activities may not be positive, or predictive of real-world results or of results in subsequent clinical trials; regulatory submissions may not occur or be submitted in a timely manner; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products, including decisions not to approve the company’s NDAs, including the NDA for ALKS 3831; data from clinical trials may be interpreted by the FDA in different ways than the company or an advisory committee interprets it; the FDA may not agree with the company’s regulatory approval strategies or components of the NDA for ALKS 3831 or the Company’s other regulatory filings, including clinical trial designs, conduct and methodologies, manufacturing processes and facilities, and the adequacy of the data and other information included in the company’s filings to meet the FDA’s requirements for approval, including the risk/benefit profile of the company’s product candidates; the company and its licensees may not be able to continue to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov, and on the company’s website at www.alkermes.com in the ‘Investors – SEC filings’ section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Non-GAAP Financial Measures: This presentation includes information about certain financial measures that are not prepared in accordance with generally accepted accounting principles in the U.S. (GAAP), including non-GAAP net income. These non-GAAP measures are not based on any standardized methodology prescribed by GAAP and are not necessarily comparable to similar measures presented by other companies. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the Alkermes plc Current Report on Form 8-K filed with the SEC on Oct. 29, 2020. Note Regarding Trademarks: The company is the owner of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, ARISTADA INITIO® and VIVITROL®. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto.

Introduction Sandy Coombs, VP, Investor Relations Q3 2020 Financial Results; 2020 Financial Expectations Jim Frates, Chief Financial Officer Q3 2020 Commercial Review Todd Nichols, Chief Commercial Officer R&D Pipeline and Business Update Richard Pops, Chief Executive Officer Agenda
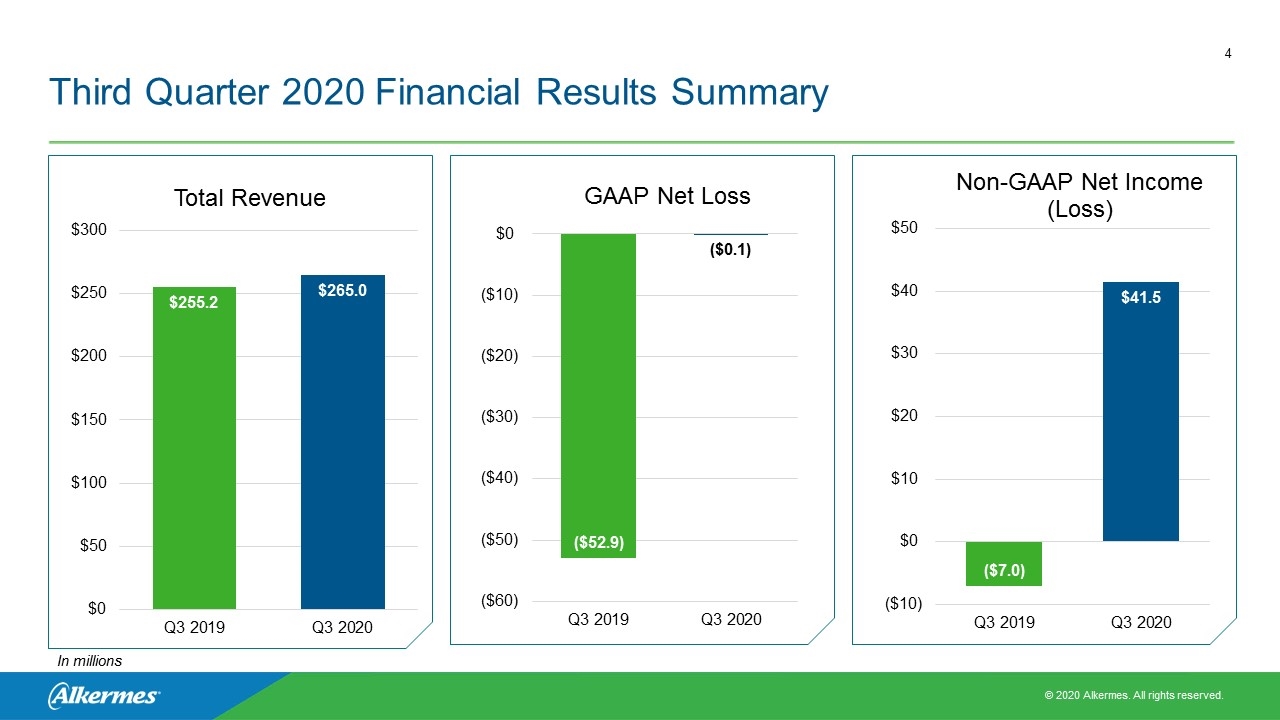
Third Quarter 2020 Financial Results Summary In millions
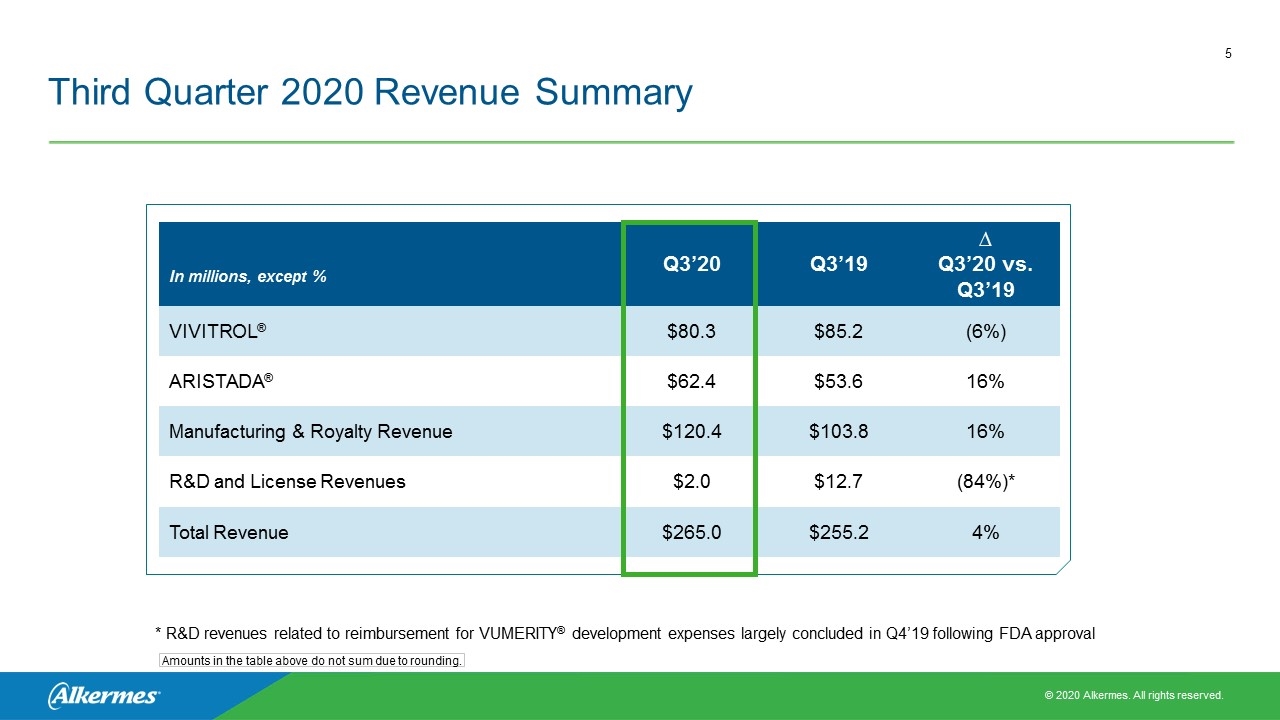
Third Quarter 2020 Revenue Summary In millions, except % Q3’20 Q3’19 ∆ Q3’20 vs. Q3’19 VIVITROL® $80.3 $85.2 (6%) ARISTADA® $62.4 $53.6 16% Manufacturing & Royalty Revenue $120.4 $103.8 16% R&D and License Revenues $2.0 $12.7 (84%)* Total Revenue $265.0 $255.2 4% Amounts in the table above do not sum due to rounding. * R&D revenues related to reimbursement for VUMERITY® development expenses largely concluded in Q4’19 following FDA approval
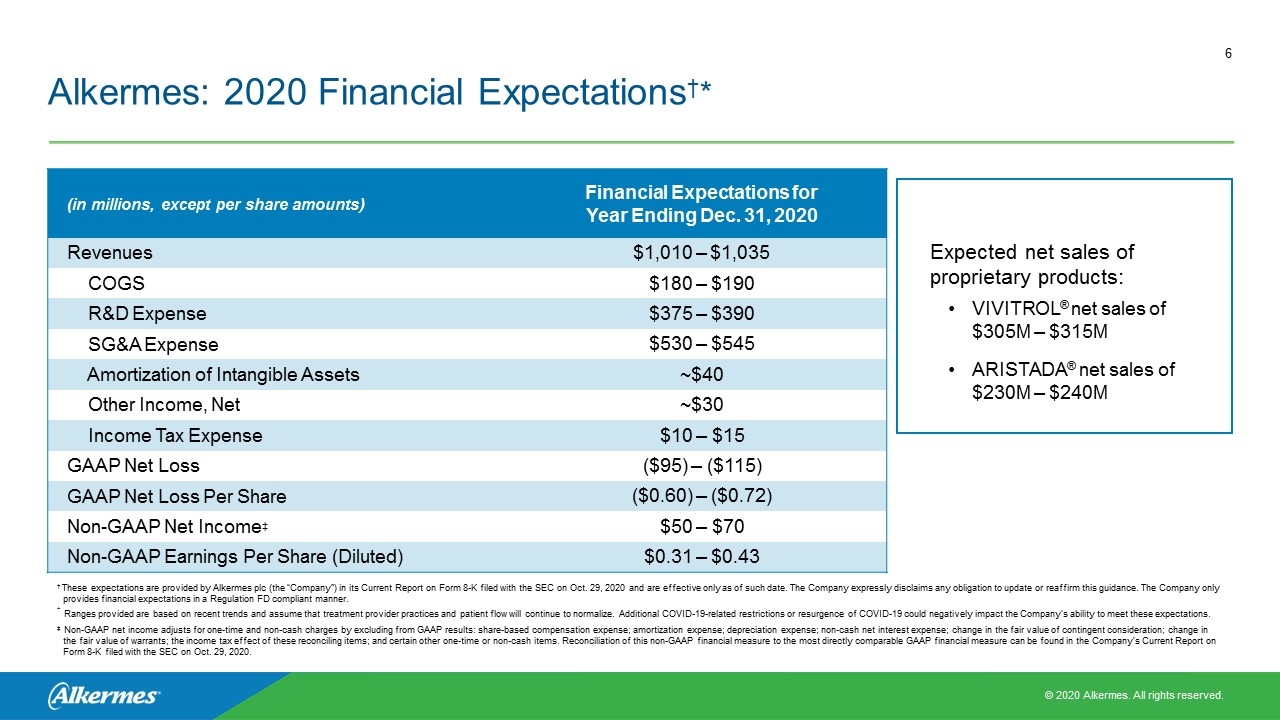
(in millions, except per share amounts) Financial Expectations for Year Ending Dec. 31, 2020 Revenues $1,010 – $1,035 COGS $180 – $190 R&D Expense $375 – $390 SG&A Expense $530 – $545 Amortization of Intangible Assets ~$40 Other Income, Net ~$30 Income Tax Expense $10 – $15 GAAP Net Loss ($95) – ($115) GAAP Net Loss Per Share ($0.60) – ($0.72) Non-GAAP Net Income‡ $50 – $70 Non-GAAP Earnings Per Share (Diluted) $0.31 – $0.43 † These expectations are provided by Alkermes plc (the “Company”) in its Current Report on Form 8-K filed with the SEC on Oct. 29, 2020 and are effective only as of such date. The Company expressly disclaims any obligation to update or reaffirm this guidance. The Company only provides financial expectations in a Regulation FD compliant manner. * Ranges provided are based on recent trends and assume that treatment provider practices and patient flow will continue to normalize. Additional COVID-19-related restrictions or resurgence of COVID-19 could negatively impact the Company’s ability to meet these expectations. ‡ Non-GAAP net income adjusts for one-time and non-cash charges by excluding from GAAP results: share-based compensation expense; amortization expense; depreciation expense; non-cash net interest expense; change in the fair value of contingent consideration; change in the fair value of warrants; the income tax effect of these reconciling items; and certain other one-time or non-cash items. Reconciliation of this non-GAAP financial measure to the most directly comparable GAAP financial measure can be found in the Company’s Current Report on Form 8-K filed with the SEC on Oct. 29, 2020. Expected net sales of proprietary products: VIVITROL® net sales of $305M – $315M† ARISTADA® net sales of $230M – $240M Alkermes: 2020 Financial Expectations†*
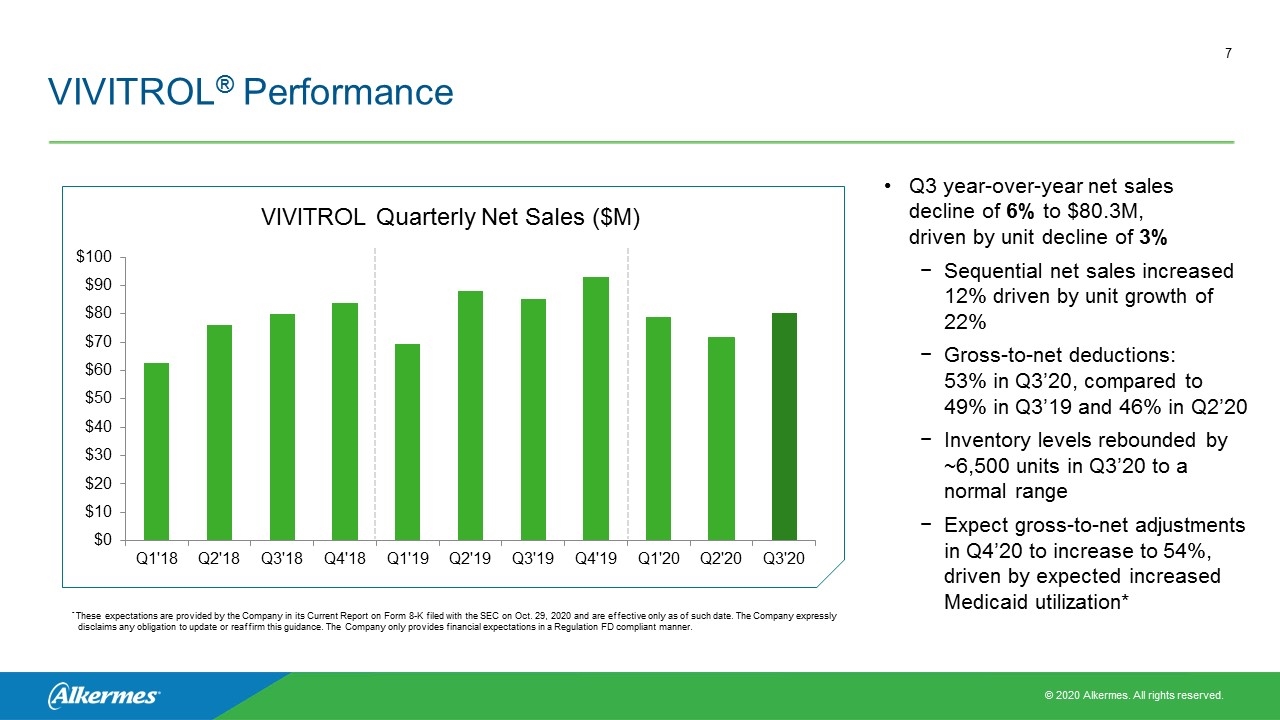
VIVITROL® Performance Q3 year-over-year net sales decline of 6% to $80.3M, driven by unit decline of 3% Sequential net sales increased 12% driven by unit growth of 22% Gross-to-net deductions: 53% in Q3’20, compared to 49% in Q3’19 and 46% in Q2’20 Inventory levels rebounded by ~6,500 units in Q3’20 to a normal range Expect gross-to-net adjustments in Q4’20 to increase to 54%, driven by expected increased Medicaid utilization* VIVITROL Quarterly Net Sales ($M) * These expectations are provided by the Company in its Current Report on Form 8-K filed with the SEC on Oct. 29, 2020 and are effective only as of such date. The Company expressly disclaims any obligation to update or reaffirm this guidance. The Company only provides financial expectations in a Regulation FD compliant manner.
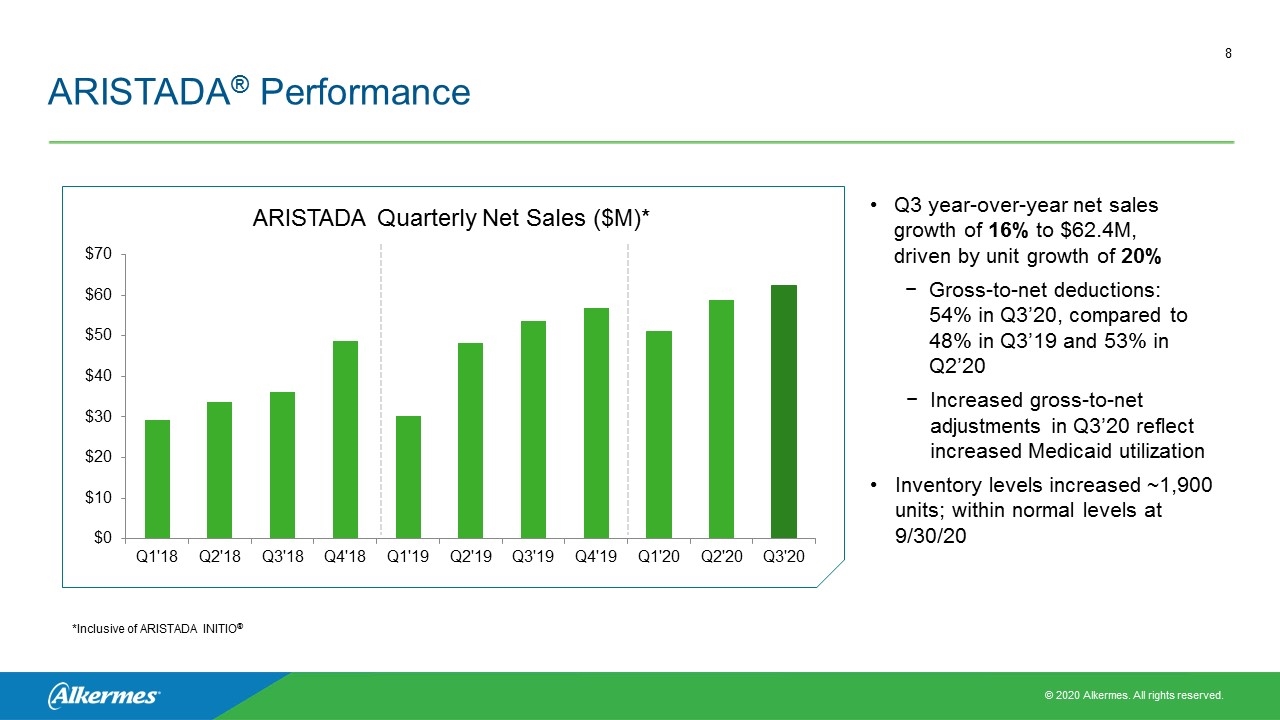
ARISTADA® Performance Q3 year-over-year net sales growth of 16% to $62.4M, driven by unit growth of 20% Gross-to-net deductions: 54% in Q3’20, compared to 48% in Q3’19 and 53% in Q2’20 Increased gross-to-net adjustments in Q3’20 reflect increased Medicaid utilization Inventory levels increased ~1,900 units; within normal levels at 9/30/20 ARISTADA Quarterly Net Sales ($M)* *Inclusive of ARISTADA INITIO®
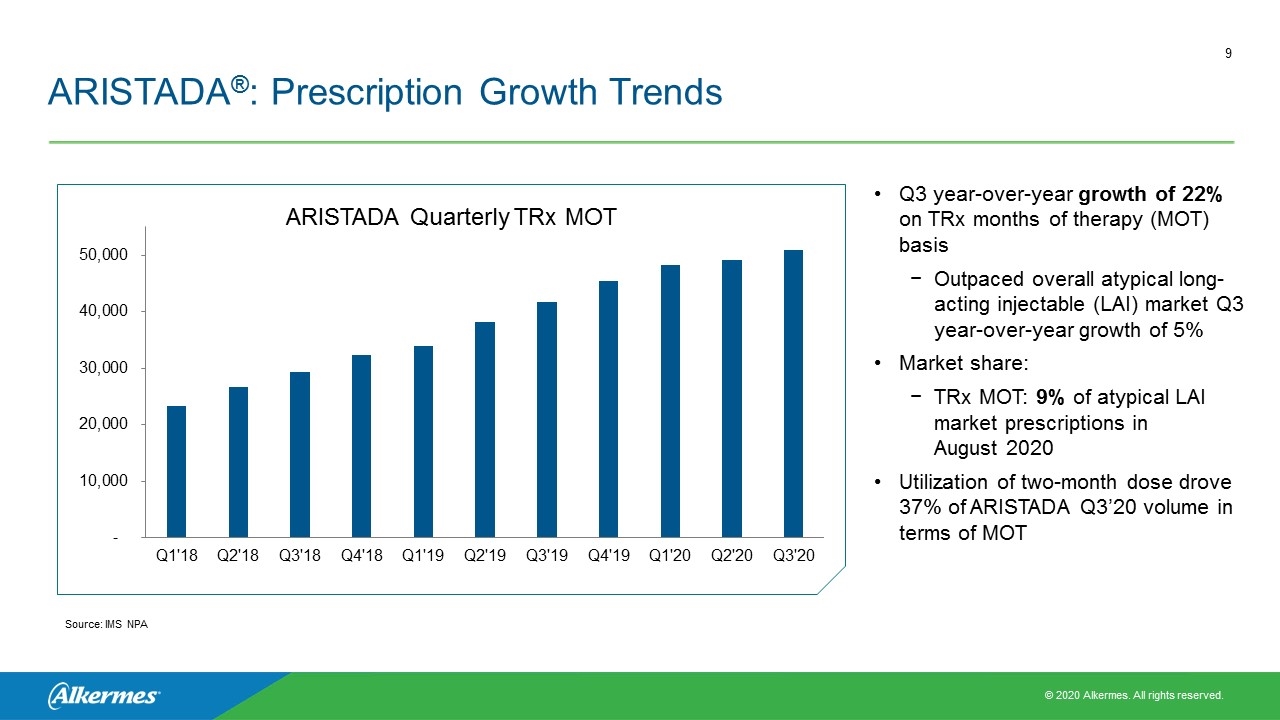
ARISTADA®: Prescription Growth Trends Q3 year-over-year growth of 22% on TRx months of therapy (MOT) basis Outpaced overall atypical long-acting injectable (LAI) market Q3 year-over-year growth of 5% Market share: TRx MOT: 9% of atypical LAI market prescriptions in August 2020 Utilization of two-month dose drove 37% of ARISTADA Q3’20 volume in terms of MOT ARISTADA Quarterly TRx MOT Source: IMS NPA

ALKS 3831 Update Payer engagement strategy Expect that access will improve throughout first year of launch as formulary decisions are made Plan to implement patient access programs designed to mitigate payer restrictions early in launch Sales force planning Focus on leveraging existing commercial organization Hybrid promotional model permanently incorporates both in-person and virtual engagements to efficiently target a broader footprint of prescribers for oral antipsychotics Well-defined healthcare provider call universe Plan to target healthcare providers that represent ~ 70% of the oral antipsychotic market, and ~ 80% of the branded oral antipsychotic market Commercial launch pending FDA approval and DEA de-scheduling Launch Planning Activities Regulatory Review Positive outcome of Advisory Committee meeting Prescription Drug User Fee Act (PDUFA) target action date of Nov. 15, 2020

ALKS 4230: Advancing the Clinical Development Program ARTISTRY-1 data presented at the European Society for Medical Oncology (ESMO) Virtual Congress included evidence of ALKS 4230 antitumor activity, both as monotherapy and in combination with pembrolizumab, with durable and deepening responses observed in a diverse set of difficult-to-treat tumor types ARTISTRY-2 dose escalation is ongoing for both once-weekly and once-every-three-week subcutaneous dosing regimens Data to be presented at the Society for Immunotherapy of Cancer (SITC) Annual Meeting in November will include pharmacokinetic, pharmacodynamic, safety and tolerability data from the initial dose escalation cohorts

www.alkermes.com
