Attached files
| file | filename |
|---|---|
| EX-10.1 - EXHIBIT 10.1 - GENEREX BIOTECHNOLOGY CORP | ex10_1.htm |
| 8-K - FORM 8-K - GENEREX BIOTECHNOLOGY CORP | gnbt060520form8k.htm |
APPENDIX A- Statement of Work
This Statement of Work #1 (“SOW”), dated and effective May 18, 2020, submitted in connection with the Laboratory Services Agreement by and between Cellular Technology Limited (“CTL”) and Generex Biotechnology Corporation, a public company organized under the laws of Delaware , with its principal place of business located at 10102 USA Today Way, Miramar, FL 33025 (“Company”) (“Company”) dated May 15, 2020 (“Agreement”), is hereby agreed to by the Parties.
Pursuant to Article 2 of the Agreement, this SOW (including any attachments hereto) shall be governed by the terms and conditions of the Agreement and, if applicable, any modifications to the Agreement agreed to by the Parties and set forth in this SOW under the section below, entitled “Modifications to Agreement.” Any such modifications shall apply only to this SOW, and not to any previous or subsequent SOWs, unless expressly stated otherwise in such other SOW.
| CTL | Company | |
| /s/ Madalena Tarey-Lehmann | By: | /s/ Joe Moscato |
| Magdalena Tary-Lehmann, M.D., Ph.D. | Print Name: | Joe Moscato |
| Chief Scientific Officer | Title: | President & Chief Executive Officer |
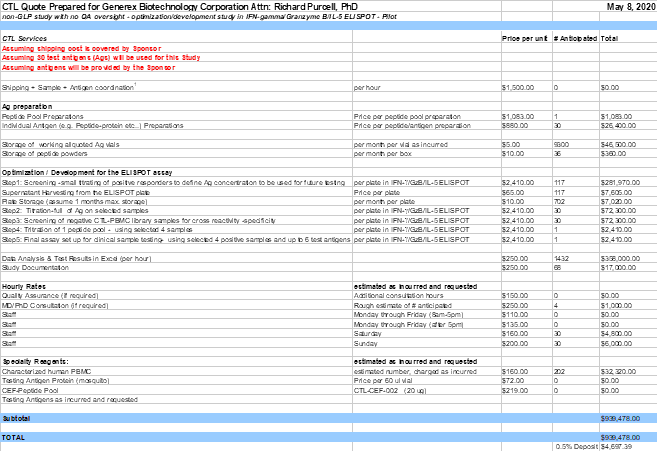
- non-GLP study with no QA oversight - Pilot T cell ELISPOT project
Company will:
| • | Use its own preferred carrier for shipments of materials to CTL. |
| • | Provide thirty (30) peptides- test antigens (Ags). |
| • | Provide up to thirty-nine (39) cryopreserved PBMC samples from different subjects with up to one time points. |
CTL will:
- Antigen preparation: CTL will dissolve, pool, prepare and store working aliquots of the Company’s antigens for a fee. These synthesized antigens are provided by the Company. Currently up to thirty (30) individual antigen preparations and one (1) peptide pool preparation is assumed. Specifics on the antigens will be provided by the Company (e.g. sequence, purity, which Ags to be pooled etc.). In the case that antigens are requested to be ordered by CTL, then CTL will be reimbursed by the Company (cost not included in the work order).
- Optimization phase for ELISPOT: CTL will optimize the antigen-specific triple color fluorescent IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay for the testing of human samples using the Company’s antigens of interest.
- Step 1: Screening-small titration of the up to thirty-nine (39) cryopreserved positive responders PBMC in three Ag concentrations (e.g. 100 ug/ml, 25 ug/ml and 2.5 ug/ml) per provided antigen.
- The supernatant (SN) from IFN-gamma/Granzyme B/IL-5 ELISPOT plate will be collected, frozen and stored until further instructions have been received by the Company.
- Step 2: Titration of the Ags in the IFN-gamma/Granzyme B/IL-5 ELISPOT assay on up to four (4) antigen positive identified samples to define correct Ag concentration to be used for each Ag.
- Step 3: Screening of thirty (30) purchased sample(s) from CTL ePBMC® library with the thirty (30) antigens at one concentration, in the recall antigen specific IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay using one (1) sample per plate.
- Step 4: Titration of the one (1) peptide pool in the IFN-gamma/Granzyme B/IL-5 ELISPOT assay on up to four (4) selected samples to define Ag concentration to be used.
- Step 5: Final assay set up for clinical sample testing- using selected four (4) positive samples and up to six (6) test antigens in the recall antigen specific IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay, whereby the test antigens are comprised of one (1) peptide pool and up to five (5) individual peptides at one (1) concentration
| • | Human samples from CTL ePBMC® library used will contains approximately 1 x 107 cells per vial. |
- A CTL Development Report will be generated once the Pilot study has been finalized.
| • | Additional optimization experiments (with input from Company before each experiment would be initiated) might be performed, however than an Amendment to this Work Order will need to be prepared. |
| • | Results from each experiment will be forwarded to the Company. Results will be presented in the form of an Excel workbook, including among others, viability and recovery of cells, and mean and standard deviation of the spot counts, with a brief (1-2 pages) Internal Experimental Report that will summarize the results of the experiment performed. |
- This phase of the study is a non GLP study; however, if QA oversight would be requested by the Company, an additional 10% fee on all included activities (but not supplies) would be incurred. If this option would be chosen, then CTL, Quality Assurance will inspect this non-regulated study, which will include inspection of the raw data and final draft report. No QA Statement will be included in the final development report, but records of inspection will be maintained with CTL QA.
| • | Overtime and weekend work will be billed as incurred for the ELISPOT assays. |
| • | Short Term Storage: For the month in which the sample or antigen vial is received, storage is free. Thereafter a storage fee per vial will apply as incurred. |
| • | Store the cryopreserved samples under liquid nitrogen vapor. CTL staff will enter specimen information into its sample management database in accordance with CTL standard operating procedures (SOPs). Long term storage of cryopreserved samples will incur a placement and a pulling fee per vial and a storage fee per vial per month as outlined in the attachment. |
| • | Provide M.D./Ph.D. consultation at a rate of $250/hour. |
| B. | General Provisions |
| • | If Company later desires CTL to accelerate (rush) the schedule for any of the services to be rendered by CTL under the agreement or applicable work order, CTL will attempt to find a mutually agreeable modification of the schedule to accommodate Company’s request. However, CTL’s ability to meet such a request may depend upon the complexity of services involved, the volume of work requested, the availability of materials and/or personnel needed to meet the request, and the time frame involved. An acceleration of the specified dates outlined in the agreement or work order may result in accelerated services surcharges based upon the aforementioned factors. |
| • | CTL Consumables: CTL has available multiple consumables for standardization of the assay performed at CTL. These include: vials of isolated and cryopreserved PBMC from a large batch (single time point), serum-free media (wash, test, and freezing media), peptides, control antigens, and protocols and training for the processing/cryopreservation of PBMC. All are available upon request and will be charged separately if incurred. |
- CTL could offer the consultation services of its staff for the support of the study and interpretation and management of the data. This includes biostatisticians, PhD’s, technicians, etc.
| • | All records generated by CTL will be scanned. The Company will be given the option to request all materials to be shipped to a defined location or the records generated by CTL will be retained at CTL for two (2) years from the study completion date and signed final report. After two (2) years have elapsed, the Company will be contacted for further instructions and, if additional storage of records is requested, additional charges will be incurred. All records generated by CTL could be retained at CTL for two (2) years from the study completion date for no additional fee. Additional storage could be accommodated for a fee of $43.00 per inch binder per month. |
| • | ELISPOT plates will be discarded after experimental data has been approved by the CTL Study Director/Responsible Scientist/ PI. |
| • | Sample vials stored at CTL incur the storage fee per vial per month starting 1 month after their arrival. After completion of the study and the final report has been signed, samples, antigens and aliquots will be stored at CTL for up to four (4) weeks. CTL will send written instructions to the Company regarding onward shipping or disposal of the samples and aliquots stored at CTL. If onward shipping is requested or further storage by the Company, additional charges might occur. |
- After two (2) years have elapsed, CTL will make three (3) contacting attempts by mail or e-mail. If the Company will not respond to CTL within 30 days of the third attempt, then this will automatically authorize CTL to discard the remaining study records, materials and samples pertaining to this study.
| C. | Payment Terms |
In consideration of the Services performed by CTL, Company hereby agrees to pay CTL in accordance with the payment terms and schedules set forth below as outlined and in budget attachment:
Part A: non-GLP study with no QA oversight - Pilot T cell ELISPOT project
0.5% of the costings ($4,697.39 USD) are due once work order has been signed. Cost for this part is $939,478.00USD.
Note: All deposits are non-refundable, and will be used against work performed.
The balance of the costings will be invoiced on a monthly basis for activities completed the prior month.
CTL will invoice Company only for actual services performed and materials provided; (see Attachment), incorporated herein by reference, for itemized pricing. Company will pay CTL’s invoices within 30 (thirty) days of receipt. Upon the expiration of thirty (30) days from the date of the invoices, unpaid balances shall bear interest at the rate of one and one-half percent (1 ½%) per month. Invoices shall be calculated and payments due in U.S. Dollars. If Company has specific invoicing instructions or requirements, e.g. the issue of a purchase order or billing to a separate address, please advise. In the event costs exceed the estimated total amount for each project by ten (10) percent or more. CTL shall seek written approval from Company. Additional storage will be accommodated for a fee of $43.00 per inch binder per month. Company will be contacted for further instructions and, if additional storage of records is requested, additional charges will be incurred.
All original invoices shall be sent to: ________________________________________
Invoices must reference: __________________________________________________
Payments to CTL should be made to: If wiring payment, the wiring Instructions are:
Cellular Technology Limited (CTL), Beneficiary: Cellular Technology Limited.
Attn: Accounting Department Bank: PNC Bank
20521 Chagrin Boulevard, Account Number: 4228503614
Shaker Heights, Routing Number: 041000124
OH 44122, USA (Domestic Wires: Centralized Columbus, Ohio)
Email: Swift Code: PNCCUS33
CROBilling@immunospot.com (International Wire Transfers only)
Tax ID number: 34-1870041
This Work Order will remain in effect until the Services have been performed, unless earlier terminated as provided in the Agreement.
Attachment 1
non-GLP study with no QA oversight - Pilot T cell ELISPOT project