Attached files
| file | filename |
|---|---|
| EX-10.2 - EXHIBIT 10.2 - GENEREX BIOTECHNOLOGY CORP | ex10_2.htm |
| EX-10.1 - EXHIBIT 10.1 - GENEREX BIOTECHNOLOGY CORP | ex10_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549 FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 2, 2020
GENEREX BIOTECHNOLOGY CORPORATION
(Exact of registrant as specified in its charter)
| DELAWARE | 000-29169 | 98-0178636 |
| State or other jurisdiction of incorporation | Commission File Number | IRS Employer Identification No. |
10102 USA Today Way, Miramar, Florida 33025 (Address of principal executive offices) (Zip Code)
(416) 364-2551
(Registrant’s telephone number, including area code) (Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13 (a) of the Exchange Act. ☐
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| N/A | N/A | N/A |
| 1 |
Item 1.01 Entry into a Material Definitive Agreement.
On June 2, 2020, Generex Biotechnology Corporation (“Generex”) entered into a Laboratory Services Agreement and Statement of Work Agreement (together, the “Agreement”) with Cellular Technology Limited (“CTL”). The Agreement calls for CTL to provide certain laboratory testing and analysis of approximately thirty (30) peptides-test antigens and thirty-nine (39) cryopreserved PBMC samples from different subjects with up to one time points. These services provided by CTL to Generex are part of the development of a potential vaccine for COVID-19 based upon NuGenerex Immuno-Oncology Inc’s (“NGIO”) Ii-Key vaccine technology. NGIO is a majority owned subsidiary of Generex. Generex/NGIO will own the intellectual property generated by CTL’s work.
Pursuant to the Agreement, Generex will pay to CTL as follows: Fee For Work Plan Completion: $939,478
This Current Report contains summaries of the material terms of the Agreement. The summary of the Agreement contained in this Current Report is subject to, and is qualified in its entirety by, reference to the Agreement, which is filed as an exhibit hereto and incorporated herein by reference.
Forward-Looking Statements
Statements in this report may contain certain forward-looking statements. All statements included concerning activities, events or developments that the Generex expects, believes or anticipates will or may occur in the future are forward-looking statements. Actual results could differ materially from the results discussed in the forward-looking statements. Forward-looking statements are based on current expectations and projections about future events and involve known and unknown risks, uncertainties and other factors that may cause actual results and performance to be materially different from any future results or performance expressed or implied by forward-looking statements. Known risks and uncertainties also include those identified from time to time in the reports filed by Generex with the Securities and Exchange Commission, which should be considered together with any forward-looking statement. No forward-looking statement is a guarantee of future results or events, and one should avoid placing undue reliance on such statements. Generex undertakes no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Generex cannot be sure when or if it will be permitted by regulatory agencies to undertake additional clinical trials or to commence any particular phase of clinical trials. Because of this, statements regarding the expected timing of clinical trials or ultimate regulatory approval cannot be regarded as actual predictions of when Generex will obtain regulatory approval for any “phase” of clinical trials or when it will obtain ultimate regulatory approval by a particular regulatory agency. Generex claims the protection of the safe harbor for forward-looking statements that is contained in the Private Securities Litigation Reform Act. Additional information on these and other risks, uncertainties and factors is included in the Company’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8- K and other documents filed with the SEC.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
The list of exhibits called for by this Item are incorporated by reference to the Exhibit Index filed with this report.
| 2 |
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: June 5, 2020
GENEREX BIOTECHNOLOGY CORPORATION
/s/Joseph Moscato
By: Joseph Moscato, CEO, President
| 3 |
Exhibit Index
| Exhibit No. | Description |
| 10.1 | Laboratory Services Agreement by and between Generex Biotechnology Corporation and Cellular Technology Limited, June 2, 2020 |
| 10.2 | APPENDIX A STATEMENT OF WORK No. 1 |
| 4 |
LABORATORY SERVICES AGREEMENT
This Laboratory Services Agreement (“Agreement”) is made and entered into as of May 15, 2020 (“Effective Date”) by and between Cellular Technology Limited, an Ohio limited liability company organized under the laws of Ohio, with its principal place of business located at 20521 Chagrin Boulevard, Shaker Heights, Ohio 44122 (“CTL”), and Generex Biotechnology Corporation, a public company organized under the laws of Delaware , with its principal place of business located at 10102 USA Today Way, Miramar, FL 33025 (“Company”). CTL and the Company are each referred to herein individually as a “Party” and collectively as the “Parties.”
Recitals
WHEREAS, Company desires to retain the services of CTL from time to time to perform certain high throughput immune monitoring laboratory services and Laboratory desires to provide such services.; and
WHEREAS, CTL desires to provide such services under the terms and conditions of this Agreement and any attached exhibits.
NOW, THEREFORE, in consideration of the promises and mutual covenants herein contained, and other valuable consideration, the Parties agree as follows:
1. DEFINITIONS
1.1 “Confidential Information” is defined in Section 8.1.
1.2 “Good Laboratory Practices” means the practices and procedures set forth in Title 21, United States Code of Federal Regulations, Part 58, and any other regulations, guidelines or guidance documents relating to good laboratory practices.
1.3 “Inventions” is defined in Section 5.1.
1.4 “Materials” means any tangible materials provided by Company to CTL under this Agreement or any SOW, including ______________________________________________________________________.
1.5 “Person” means any person or entity, including any individual, trustee, corporation, partnership, trust, unincorporated organization, limited liability company, business association, firm, joint venture or governmental agency or authority.
1.6 “Records” is defined in Section 4.1.1.
1.7 “Reports” means any reports or documentation prepared by CTL pursuant to Section 2.3.
1.8 “Services” means the services to be performed by CTL under this Agreement, including the testing and analysis of Materials and the generation and delivery of any Reports, as further defined from time to time in a SOW.
1.9 “SOW” is defined in Section 2.1.1.
1.10 “Third Party” means any Person other than a Party to this Agreement.
2. Statements of Work; Performance of Services; RePorts
2.1 Statements of Work.
a). All Services to be performed under this Agreement are set forth in a Statement of Work (“SOW”), attached hereto as Appendix A. This Agreement, together with each individual SOW (including any attachments or schedules thereto), but separate and apart from any other SOW, shall constitute the entire agreement between the Parties for the performance of any Services defined in the applicable SOW.
b). The performance of all Services shall be controlled by the terms and conditions of this Agreement (including any applicable SOW). The terms and conditions of any business forms used by CTL for purposes of invoicing, delivering Reports, or otherwise shall not form part of this Agreement unless otherwise stated in an applicable SOW. In the event of a conflict between the terms and conditions of this Agreement and any SOW, the terms and conditions of the Applicable SOW shall control.
2.2 Performance of Services.
a). CTL shall perform the Services in accordance with the terms and conditions of this Agreement and the applicable SOW.
b). The Services shall, unless otherwise specified in the applicable SOW, be conducted in compliance with Good Laboratory Practices and shall use such personnel, methods, procedures and resources specified in the applicable SOW. All Services shall be conducted in a professional and competent manner, in compliance with all applicable laws, ordinances, rules, regulations and guidelines.
c). In the event of a material error by CTL in the performance of the Services, upon Company’s request to do so, CTL will conduct again, at its own cost and within a reasonable period of time, that part of the work affected by such error.
2.3 Reports. CTL shall prepare and deliver to Company in a timely fashion, all reports and other documentation required under a SOW.
3. Payments
3.1 Costs. In compensation for the Service, Company shall pay CTL the fees and costs in the amounts and in accordance with the terms, conditions and payment schedules as set forth in the applicable SOW.
3.2 Invoicing. Once each calendar month, or as Services are rendered, CTL shall submit to Company a written invoice detailing the amounts due to CTL for the performance of the Services detailed in a SOW. Unless otherwise specified in the applicable SOW, invoices shall be sent to Company via e-mail at the following addresses:
jterrell@generex.com
3.3 Payment. Within thirty (30) days of receipt of an invoice issued pursuant to Section 3.2, Company shall pay to CTL the invoiced amount. Payment shall be made in United States currency by check or wire transfer sent as directed by CTL.
3.4 Change in Scope of Services. Any change in the scope of Services which impacts the cost for Services rendered under this Agreement and applicable SOW shall require a written change order or similar document signed by both CTL and the Company. Unless otherwise agreed, any increase or reduction in total cost associated with a change in scope shall be established based upon the fee structure set forth in the applicable SOW.
3.5 Acceleration of Schedule. If the Company or the sponsor later desire CTL to accelerate the schedule for any of the Services, CTL will attempt to find a mutually agreeable modification of the schedule to accommodate the Company’s and/or the sponsor’s request. However, CTL’s ability to meet such a request may depend upon the complexity of Services involved, the volume of work requested, the availability of materials and/or personnel needed to meet the request, and the time frame involved. An acceleration of the specified dates outlined in the Agreement or applicable SOW may result in accelerated services surcharges based upon the aforementioned factors.
4. Record Keeping; Inspections
4.1 Records.
a). CTL shall maintain complete and accurate records, in appropriate detail for patent and regulatory purposes, fully and properly reflecting all Services performed by it and the results thereof, including, without limitation, such data and materials as are required to be maintained pursuant to any applicable law, ordinance, rule, regulation or guideline, Good Laboratory Practices, and any applicable SOW (“Records”).
b). To the extent practical, all Records shall be kept separately from documentation and materials associated with other CTL laboratory activities.
c). CTL shall maintain the Records for two (2) years after CTL’s completion of Services under the applicable SOW or such other longer amount of time as is required by applicable law or the applicable SOW. Prior to destroying any Records, Laboratory shall give Company at least thirty (30) days prior written notice of CTL’s intention to destroy such. Upon Company’s written request, and at Company’s expense, Laboratory shall deliver to Company all original Records.
d). All Records shall be the sole property of Company and shall not be used by CTL for any purpose other than performing the Services or as otherwise required by this Agreement or applicable Work Order.
4.2 Inspections and Right to Audit.
a). CTL agrees to promptly notify Company of any government or regulatory inspection or audit of CTL that may impact or relate to the Services, Materials or Records. CTL shall inform Company of the results such governmental or regulatory agency inspection or audit and shall promptly take any reasonable steps required to cure any deficiencies shown as a result of such audit or inspection.
b). During the period of time in which CTL is providing Services and until the end of the period time in which CTL is maintaining the Records, Company may upon reasonable written notice to CTL, visit during normal business hours CTL’s facilities where Services have been or are being performed to: (i) inspect and audit the facilities; and (ii) review, copy and inspect all Records pertaining to the Services and any CTL standard operating services pertinent to the providing of the Services. All representatives of or on behalf of Company who visit, inspect or audit CTL’s facilities shall be subject to and bound by the terms of any confidentiality agreements between the Parties.
5. Ownership; Intellectual Property
5.1 Ownership Generally. The Parties acknowledge and agree that the existing inventions and technologies of each of CTL and Company are their separate property, respectively, and are not affected by this Agreement and neither Party shall have any claims to or rights in such existing inventions and technologies of the other party.
5.2. Company’s Property; Inventions. Subject to subsection 5.3, Company shall solely own (and CTL hereby assigns to Company) all right, title and interest in and to all intellectual property created or arising in connection with, or related to, the Materials, the performance of any Services or the preparation of any Reports or Records regardless of whether such intellectual property was conceived, reduced to practice or otherwise created or authored solely by CTL or jointly by CTL and Company or a Third Party (“Inventions”). CTL shall promptly disclose to Company all Inventions. If any part of the Services or Inventions cannot be fully exploited without violating intellectual property rights owned or licensed by CTL and not assigned hereunder, CTL hereby grants Company a perpetual, irrevocable, worldwide, royalty-free, non-exclusive, license to fully exploit and exercise all such intellectual property rights.
5.3. CTL’s Property. Notwithstanding the provisions of subsection 5.3 above, CTL’s Property shall remain the sole and exclusive property of CTL, and such CTL Property shall not be deemed to constitute Inventions. “CTL’s Property” means any intellectual property which has been created by or in the possession of CTL prior to the date of this Agreement and all subsequent (i) inventions (whether or not patentable), know-how, works of authorship, technology, software, techniques, developments, ideas, concepts, discoveries, designs, algorithms, models, formulations, improvements, protocols, data and proprietary information; and (ii) patents, copyrights, trademarks, service marks, trade secrets, or other intellectual property rights associated with the foregoing, which (A) is an improvement, modification or development of CTL’s equipment, assays, materials, laboratory testing, data collection or data management processes, procedures, or methods (whether or not created, developed or produced pursuant to this Agreement), and (B) does not contain any, or is not developed using any Company Property.
5.4 Retained Rights. No rights are granted to CTL under any intellectual property owned or controlled by Company, except to the extent strictly necessary to allow CTL to perform the Services and fulfill its obligations under this Agreement. Upon completion of such Services or fulfillment of such obligations, or expiration or termination of this Agreement, the limited rights granted to Laboratory under this Section 5.4 shall immediately terminate.
6. REPRESENTATIONS; Disclaimers
6.1 CTL Representations. CTL hereby represents and warrants to Company that:
a). CTL has the full right, power and authority, and has obtained all approvals, permits or consents necessary, to enter into this Agreement and to perform all of its obligations hereunder.
b). CTL has not, prior to the Effective Date, entered into and shall not, following the Effective Date, enter into any agreement and has not granted any now existing, or agreed to grant any future, license, right or privilege which agreement, license, right or privilege conflicts in any way with this Agreement or CTL’s obligations hereunder.
6.2 Company’s Representations. Company hereby represents and warrants to CTL that:
a). Company has the full right, power and authority, and has obtained all approvals, permits or consents necessary, to enter into this Agreement and to perform all of its obligations hereunder.
b). Company has not, prior to the Effective Date, entered into and shall not, following the Effective Date, enter into any agreement and has not granted any now existing, or agreed to grant any future, license, right or privilege which agreement, license, right or privilege conflicts in any way with this Agreement or Company’s obligations hereunder.
c). Company represents that it owns or has the right to use all copyright, trademark, patents, and other intellectual property rights to the Confidential Information (including any Materials) supplied by Company to CTL and that such do not infringe upon any third party’s patents, trade secrets, copyrights or other intellectual property rights.
6.3 Disclaimer. EXCEPT AS OTHERWISE PROVIDED IN SECTION 6.2 ABOVE, THE CONFIDENTIAL INFORMATION (INCLUDING ANY MATERIALS) SUPPLIED BY COMPANY TO CTL IS BEING SUPPLIED “AS IS” AND COMPANY MAKES NO WARRANTIES WITH REGARD THERETO, AND DISCLAIMS ALL IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY, AND FITNESS FOR A PARTICULAR PURPOSE.
7. INDEMNIFICATION
7.1 General Indemnification. CTL and Company (each, respectively, being the “Indemnifying Party”) shall protect, defend, indemnify, and save harmless each other , and their respective Affiliates and their respective officers, directors, employees, and agents (the “Indemnified Party”) from and against any and all claims, losses, demands, causes of action, and any and all related costs and expenses of every kind (including, but not limited to, reasonable attorneys’ fees, costs, and expenses suffered by the parties hereto and/or their employees, and/or suffered by any other person or entity) on account of (i) personal injury, death, or damage to property occurring, growing out of, incident to, or resulting directly or indirectly from, but only to the extent of, the negligence or willful misconduct of the Indemnifying Party, its agents, representatives, employees, and/or Subcontractors, and (ii) a breach by the Indemnifying Party or any subcontractor of any of its representations, warranties, covenants, agreements or obligations under this Agreement or any SOW.
7.2 Defense; Selection of Counsel. The Indemnifying Party shall have the right to select defense counsel and to direct the defense or settlement of any such Claim. The Indemnifying Party shall promptly provide the Indemnified Party with reasonable written notice of any Claim. The Indemnified party shall reasonably cooperate with the Indemnifying party and its legal representatives in the investigation and defense of any claim. The Indemnified Party may obtain representation by separate legal counsel, at its own expense.
7.3 Survival of Obligations. The terms of this Section 7 and the Parties’ obligations hereunder, shall survive the ttermination or expiration of this Agreement.
8. Confidential Information
8.1 Confidentiality of Information. During the course of performing Services, CTL may receive, or have access to confidential information of Company and Company might receive or have access to confidential information of Laboratory. Such confidential Information includes, but is not limited to, the following: Materials; Records; Reports; Inventions; CTL’s equipment, assays, and materials; CTL’s laboratory testing processes, procedures, or methods; and CTL’s data collection or data management processes, procedures, or methods. (“Confidential Information”) Each Party receiving (the “Receiving Party”) Confidential Information of the other party (the “Disclosing Party”) agrees to hold in strict confidence the Disclosing Party’s Confidential Information.
8.2 Use of Confidential Information. The Receiving Party will use the Disclosing Party’s Confidential Information only for the purpose of fulfilling its obligations and exercising its rights under this Agreement and will not disclose it, without the prior written consent of the Disclosing Party, to any third party except to the Receiving Party’s employees or agents who have a need to know such information and are bound in writing by obligations of confidentiality and restrict use that protect Confidential Information and that are at least as stringent as those provided herein. The obligations of confidentiality hereunder shall continue for a period of ten (10) years from the later of the (i) expiration or early termination of the Agreement, or (ii) date of completion or early termination of the Services. Such obligations shall not apply to information that the Receiving Party can establish:
a) was in the public domain at the time of disclosure;
b) is in the public domain, by publication or otherwise, after disclosure or development through no fault of the Receiving Party, its agents or employees;
c) was already in the possession of the Receiving Party at the time of disclosure or development, as established by its contemporaneous written records;
d) was independently developed by the Receiving Party without use of or reliance on any Confidential Information; or
e) is required to be disclosed by any law, rule, regulation, court order or other legal obligation provided that the Receiving Party promptly provides written notice to the Disclosing Party of its notice of any such disclosure requirement to provide the Disclosing Party the opportunity to seek a protective order or other appropriate remedy.
8.3 Publicity. CTL shall not issue any press release or other publicity materials, or make any public presentation or representation with respect to the existence of, or any of the terms or conditions of, this Agreement without the prior written consent of Company.
8.4 Availability of Injunctive Relief. Each Party acknowledges that the disclosure of Confidential Information without the other Party’s express written permission may cause irreparable harm, and that the breach or threatened breach of the non-disclosure and/or non-use provisions of this Agreement will entitle the other party to injunctive relief, in addition to any other legal or equitable remedies that may be available to it.
8.5 Data Protection. All Confidential Information containing personal data and information shall be handled by CTL in accordance with all applicable laws and regulation, including if applicable any patient privacy requirements.
9. TERM AND TERMINATION
9.1 Term. The term of this Agreement (the “Term”) shall commence on the Effective Date and, unless sooner terminated by mutual agreement or pursuant to any other provision of this Agreement, shall terminate five (5) years from the Effective Date.
9.2 Default. Either Party may terminate this Agreement for any material breach by the other Party, provided that the terminating Party gives the breaching Party written notice of such breach and the breach remains uncured after the expiration of thirty (30) days after such written notice was given.
9.3 Effect of Termination or Expiration. Termination or expiration of this Agreement through any means and for any reason shall not relieve the Parties of any obligation accruing prior thereto and shall be without prejudice to the rights and remedies of either Party with respect to any antecedent breach of any of the provisions of this Agreement.
9.4 Survival. Sections 3, 4, 5, 6, 7, and 8 shall survive expiration or termination of this Agreement for any reason.
10. LIMITATION OF DAMGAGES
UNDER NO CIRCUMSTANCES SHALL EITHER PARTY BE ENTITLED TO, NOR SHALL EITHER PARTY BE RESPONSIBLE FOR, ANY INCIDENTAL, INDIRECT, CONSEQUENTIAL OR SPECIAL DAMAGES (INCLUDING WITHOUT LIMITATION, LOSS OF PROFITS) ARISING IN CONNECTION WITH ANY DEFAULT OR BREACH OF OBLIGATIONS UNDER THIS AGREEMENT OR ANY DOCUMENTS RELATED HERETO.
11. NOTICES
The term “notice” as used throughout this Agreement shall mean written notice, except where specifically provided to the contrary. Notice shall be delivered to the parties addressed below by (i) certified mail, return receipt requested (or the equivalent), (ii) hand delivery with receipt acknowledged, or (iii) overnight courier service that provides a delivery receipt to the following addresses or to such other address or person as a party may specify by notice given in accordance with this Section.
If to Company: Generex Biotechnology Corporation
10102 USA Today Way
Miramar, FL 33025 Attention: Jason Terrell, MD, Chief Medical Officer
jterrell@generex.com
with copies to:
rpurcell@nugenerex.com
mwernicke@nugenerex.com
If to CTL:
Cellular Technology Limited:
Magdalena Tary-Lehmann, M.D., Ph.D.
Chief Scientific Officer
Cellular Technology Limited
20521 Chagrin Boulevard
Shaker Heights, Ohio 44122
Telephone: (216) 791-5084 ext. 124
FAX: (216) 791-8814
E mail: magda.tary-lehmann@immunospot.com
Notice given in accordance with this Section shall be deemed delivered (i) when received, or (ii) upon refusal of receipt.
12 MISCELLANEOUS
12.1 Independent Contractors. CTL and Company are independent contractors and are not, and shall not represent themselves as, principal and agent or as a joint venture. Nothing herein shall create any association, partnership, joint venture, fiduciary duty or the relation of principal and agent between the Parties hereto, and neither Party shall have the authority to assume or create any obligations or responsibilities, expressed or implied, of the other or the other’s representatives in any way. The employees and agents of each Party are not the employees or agents of the other Party.
12.2 Force Majeure. Neither Party will be liable hereunder by reason of any failure or delay in the performance of its obligations hereunder on account of strikes, shortages, riots, insurrection, fires, flood, storm, explosions, acts of God, war, governmental action, labor conditions, earthquakes, or any other similar cause which is beyond the reasonable control of such party; provided, however, that the Party affected shall promptly notify the other of the force majeure condition and to resume performance of its obligations as soon as reasonably possible.
| 12.3 | Waiver. The failure of either Party to require performance by the other Party of any provision of this Agreement will not affect the full right to require such performance at any time thereafter nor will the waiver by either Party of a breach of any provision of this Agreement be taken or held to be a waiver of the provision itself. |
12.4 Severability. If any provision of this Agreement is found to be unenforceable, illegal or invalid under any applicable law by a court of competent jurisdiction, such unenforceability, illegality or invalidity will not render this Agreement unenforceable, illegal or invalid as a whole, and shall not affect the validity or enforceability of any other provision of this Agreement. The Parties shall make a good faith effort to substitute a valid, legal and enforceable provision that reflects the intent of such invalid, illegal or unenforceable provision and implements the purpose of the provision.
| 12.5 | Governing Law. This Agreement shall be governed and construed in accordance with the laws of the State of Delaware, U.S.A., without regard to conflicts of law. |
| 12.6 | Headings. The section headings appearing in this Agreement are inserted only as a matter of convenience and in no way define, limit, construe or describe the scope or extent of such section and in no manner affect this Agreement. |
12.7 Authority. Each Party warrants that it has full power and authority to enter into and perform its obligations under this Agreement, (ii) this Agreement has been duly authorized by and is binding and enforceable upon such Party and (iii) the person signing this Agreement on that Party's behalf has been duly authorized and empowered to enter into this Agreement. Each Party further acknowledges that it has read this Agreement, understands it, and agrees to be bound by it.
12.8 Counterparts. This Agreement may be signed in counterparts each of which shall be deemed to be original, but which together will form a single agreement as if both parties had executed the same document.
12.9 Assignment. Neither Party may not assign any of its rights or delegate any of its obligations under this Agreement without the express written consent of the other Party, which consent may be unreasonably withheld. No permitted assignment will relieve either Party of its liability for obligations under this Agreement.
12.10. Entire Agreement. This Agreement sets forth the entire understanding and agreement of the parties as to the matters covered hereby. This Agreement supersedes any prior or collateral agreements with respect to the matters covered by this Agreement.
12.11 Binding Effect. This Agreement shall be binding upon, inure to the benefit of, and be enforceable by CTL and Company, and their respective affiliates, successors, heirs, executors administrators, and permitted assigns
| 12.12 | Amendments. This Agreement may only be amended in a writing signed by duly authorized representatives of CTL and Company. |
INTENDING TO BE LEGALLY BOUND, the Parties have executed this Agreement by their duly authorized representative.
| CTL | Company | |
| /s/ Madalena Tarey-Lehmann | By: | /s/ Joe Moscato |
| Magdalena Tary-Lehmann, M.D., Ph.D. | Print Name: | Joe Moscato |
| Chief Scientific Officer | Title: | President & Chief Executive Officer |
APPENDIX A- Statement of Work
This Statement of Work (“SOW”), dated and effective ___________, 20____, submitted in connection with the Laboratory Services Agreement by and between Cellular Technology Limited (“CTL”) and __________________________________________ (“Company”) dated _____________, 20___ (“Agreement”), is hereby agreed to by the Parties.
Pursuant to Article 2 of the Agreement, this SOW (including any attachments hereto) shall be governed by the terms and conditions of the Agreement and, if applicable, any modifications to the Agreement agreed to by the Parties and set forth in this SOW under the section below, entitled “Modifications to Agreement.” Any such modifications shall apply only to this SOW, and not to any previous or subsequent SOWs, unless expressly stated otherwise in such other SOW.
| CTL | Company | |
| /s/ Madalena Tarey-Lehmann | By: | /s/ Joe Moscato |
| Magdalena Tary-Lehmann, M.D., Ph.D. | Print Name: | Joe Moscato |
| Chief Scientific Officer | Title: | President & Chief Executive Officer |
| 5 |
APPENDIX A
STATEMENT OF WORK No. 1
APPENDIX A- Statement of Work
This Statement of Work #1 (“SOW”), dated and effective May 18, 2020, submitted in connection with the Laboratory Services Agreement by and between Cellular Technology Limited (“CTL”) and Generex Biotechnology Corporation, a public company organized under the laws of Delaware , with its principal place of business located at 10102 USA Today Way, Miramar, FL 33025 (“Company”) (“Company”) dated May 15, 2020 (“Agreement”), is hereby agreed to by the Parties.
Pursuant to Article 2 of the Agreement, this SOW (including any attachments hereto) shall be governed by the terms and conditions of the Agreement and, if applicable, any modifications to the Agreement agreed to by the Parties and set forth in this SOW under the section below, entitled “Modifications to Agreement.” Any such modifications shall apply only to this SOW, and not to any previous or subsequent SOWs, unless expressly stated otherwise in such other SOW.
| CTL | Company | |
| /s/ Madalena Tarey-Lehmann | By: | /s/ Joe Moscato |
| Magdalena Tary-Lehmann, M.D., Ph.D. | Print Name: | Joe Moscato |
| Chief Scientific Officer | Title: | President & Chief Executive Officer |
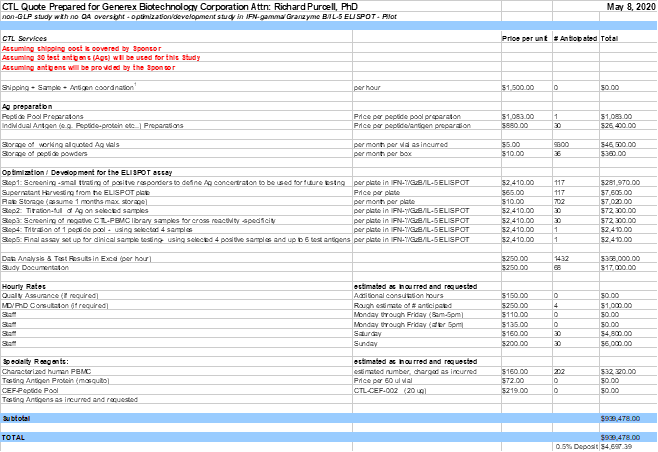
- non-GLP study with no QA oversight - Pilot T cell ELISPOT project
Company will:
| • | Use its own preferred carrier for shipments of materials to CTL. |
| • | Provide thirty (30) peptides- test antigens (Ags). |
| • | Provide up to thirty-nine (39) cryopreserved PBMC samples from different subjects with up to one time points. |
CTL will:
- Antigen preparation: CTL will dissolve, pool, prepare and store working aliquots of the Company’s antigens for a fee. These synthesized antigens are provided by the Company. Currently up to thirty (30) individual antigen preparations and one (1) peptide pool preparation is assumed. Specifics on the antigens will be provided by the Company (e.g. sequence, purity, which Ags to be pooled etc.). In the case that antigens are requested to be ordered by CTL, then CTL will be reimbursed by the Company (cost not included in the work order).
- Optimization phase for ELISPOT: CTL will optimize the antigen-specific triple color fluorescent IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay for the testing of human samples using the Company’s antigens of interest.
- Step 1: Screening-small titration of the up to thirty-nine (39) cryopreserved positive responders PBMC in three Ag concentrations (e.g. 100 ug/ml, 25 ug/ml and 2.5 ug/ml) per provided antigen.
- The supernatant (SN) from IFN-gamma/Granzyme B/IL-5 ELISPOT plate will be collected, frozen and stored until further instructions have been received by the Company.
- Step 2: Titration of the Ags in the IFN-gamma/Granzyme B/IL-5 ELISPOT assay on up to four (4) antigen positive identified samples to define correct Ag concentration to be used for each Ag.
- Step 3: Screening of thirty (30) purchased sample(s) from CTL ePBMC® library with the thirty (30) antigens at one concentration, in the recall antigen specific IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay using one (1) sample per plate.
- Step 4: Titration of the one (1) peptide pool in the IFN-gamma/Granzyme B/IL-5 ELISPOT assay on up to four (4) selected samples to define Ag concentration to be used.
- Step 5: Final assay set up for clinical sample testing- using selected four (4) positive samples and up to six (6) test antigens in the recall antigen specific IFN-gamma/ Granzyme B/ IL-5 ELISPOT assay, whereby the test antigens are comprised of one (1) peptide pool and up to five (5) individual peptides at one (1) concentration
| • | Human samples from CTL ePBMC® library used will contains approximately 1 x 107 cells per vial. |
- A CTL Development Report will be generated once the Pilot study has been finalized.
| • | Additional optimization experiments (with input from Company before each experiment would be initiated) might be performed, however than an Amendment to this Work Order will need to be prepared. |
| • | Results from each experiment will be forwarded to the Company. Results will be presented in the form of an Excel workbook, including among others, viability and recovery of cells, and mean and standard deviation of the spot counts, with a brief (1-2 pages) Internal Experimental Report that will summarize the results of the experiment performed. |
- This phase of the study is a non GLP study; however, if QA oversight would be requested by the Company, an additional 10% fee on all included activities (but not supplies) would be incurred. If this option would be chosen, then CTL, Quality Assurance will inspect this non-regulated study, which will include inspection of the raw data and final draft report. No QA Statement will be included in the final development report, but records of inspection will be maintained with CTL QA.
| • | Overtime and weekend work will be billed as incurred for the ELISPOT assays. |
| • | Short Term Storage: For the month in which the sample or antigen vial is received, storage is free. Thereafter a storage fee per vial will apply as incurred. |
| • | Store the cryopreserved samples under liquid nitrogen vapor. CTL staff will enter specimen information into its sample management database in accordance with CTL standard operating procedures (SOPs). Long term storage of cryopreserved samples will incur a placement and a pulling fee per vial and a storage fee per vial per month as outlined in the attachment. |
| • | Provide M.D./Ph.D. consultation at a rate of $250/hour. |
| B. | General Provisions |
| • | If Company later desires CTL to accelerate (rush) the schedule for any of the services to be rendered by CTL under the agreement or applicable work order, CTL will attempt to find a mutually agreeable modification of the schedule to accommodate Company’s request. However, CTL’s ability to meet such a request may depend upon the complexity of services involved, the volume of work requested, the availability of materials and/or personnel needed to meet the request, and the time frame involved. An acceleration of the specified dates outlined in the agreement or work order may result in accelerated services surcharges based upon the aforementioned factors. |
| • | CTL Consumables: CTL has available multiple consumables for standardization of the assay performed at CTL. These include: vials of isolated and cryopreserved PBMC from a large batch (single time point), serum-free media (wash, test, and freezing media), peptides, control antigens, and protocols and training for the processing/cryopreservation of PBMC. All are available upon request and will be charged separately if incurred. |
- CTL could offer the consultation services of its staff for the support of the study and interpretation and management of the data. This includes biostatisticians, PhD’s, technicians, etc.
| • | All records generated by CTL will be scanned. The Company will be given the option to request all materials to be shipped to a defined location or the records generated by CTL will be retained at CTL for two (2) years from the study completion date and signed final report. After two (2) years have elapsed, the Company will be contacted for further instructions and, if additional storage of records is requested, additional charges will be incurred. All records generated by CTL could be retained at CTL for two (2) years from the study completion date for no additional fee. Additional storage could be accommodated for a fee of $43.00 per inch binder per month. |
| • | ELISPOT plates will be discarded after experimental data has been approved by the CTL Study Director/Responsible Scientist/ PI. |
| • | Sample vials stored at CTL incur the storage fee per vial per month starting 1 month after their arrival. After completion of the study and the final report has been signed, samples, antigens and aliquots will be stored at CTL for up to four (4) weeks. CTL will send written instructions to the Company regarding onward shipping or disposal of the samples and aliquots stored at CTL. If onward shipping is requested or further storage by the Company, additional charges might occur. |
- After two (2) years have elapsed, CTL will make three (3) contacting attempts by mail or e-mail. If the Company will not respond to CTL within 30 days of the third attempt, then this will automatically authorize CTL to discard the remaining study records, materials and samples pertaining to this study.
| C. | Payment Terms |
In consideration of the Services performed by CTL, Company hereby agrees to pay CTL in accordance with the payment terms and schedules set forth below as outlined and in budget attachment:
Part A: non-GLP study with no QA oversight - Pilot T cell ELISPOT project
0.5% of the costings ($4,697.39 USD) are due once work order has been signed. Cost for this part is $939,478.00USD.
Note: All deposits are non-refundable, and will be used against work performed.
The balance of the costings will be invoiced on a monthly basis for activities completed the prior month.
CTL will invoice Company only for actual services performed and materials provided; (see Attachment), incorporated herein by reference, for itemized pricing. Company will pay CTL’s invoices within 30 (thirty) days of receipt. Upon the expiration of thirty (30) days from the date of the invoices, unpaid balances shall bear interest at the rate of one and one-half percent (1 ½%) per month. Invoices shall be calculated and payments due in U.S. Dollars. If Company has specific invoicing instructions or requirements, e.g. the issue of a purchase order or billing to a separate address, please advise. In the event costs exceed the estimated total amount for each project by ten (10) percent or more. CTL shall seek written approval from Company. Additional storage will be accommodated for a fee of $43.00 per inch binder per month. Company will be contacted for further instructions and, if additional storage of records is requested, additional charges will be incurred.
All original invoices shall be sent to: ________________________________________
Invoices must reference: __________________________________________________
Payments to CTL should be made to: If wiring payment, the wiring Instructions are:
Cellular Technology Limited (CTL), Beneficiary: Cellular Technology Limited.
Attn: Accounting Department Bank: PNC Bank
20521 Chagrin Boulevard, Account Number: 4228503614
Shaker Heights, Routing Number: 041000124
OH 44122, USA (Domestic Wires: Centralized Columbus, Ohio)
Email: Swift Code: PNCCUS33
CROBilling@immunospot.com (International Wire Transfers only)
Tax ID number: 34-1870041
This Work Order will remain in effect until the Services have been performed, unless earlier terminated as provided in the Agreement.
Attachment 1
non-GLP study with no QA oversight - Pilot T cell ELISPOT project
| 6 |
