Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - MARINUS PHARMACEUTICALS INC | tm2018461d1_ex99-1.htm |
| 8-K - FORM 8-K - MARINUS PHARMACEUTICALS INC | tm2018461-1_8k.htm |
Exhibit 99.2

NASDAQ: MRNS @ MarinusPharma Corporate Overview 2020

©2020 Marinus Pharmaceuticals. All Rights Reserved I Safe Harbor Statement To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they ar e forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Secu rit ies Litigation Reform Act of 1995. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify forward - looking statements. Examples of forwar d - looking statements contained in this presentation include, among others, statements regarding our clinical development plans for ganaxolone; exp ect ed dosing in our clinical trials; the clinical development schedule and milestones; our expected timing to begin and complete enrollment in our clinica l t rials, including our expectation to dose the first patient in our Phase 3 clinical trial for SE in September 2020; the expected trial design, targ et patient population and endpoints for our clinical trials; interpretation of scientific basis for ganaxolone use; timing for availability and release of data, inc luding the expected release of data from the Marigold Study in the third quarter of 2020 and from the proof of concept study in PCDH19 in the first half of 2021; th e potential safety and efficacy of ganaxolone; the therapeutic potential of ganaxolone; and our expectations regarding the effect of the COVID - 19 pandemic on ou r business and clinical development plans. Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward - loo king statements. Such risks and uncertainties include, among others, uncertainties and delays relating to the design, enrollment, completion, and r esu lts of clinical trials; unanticipated costs and expenses; clinical trial results may not support further development in a specified indication or at all ; actions or advice of the U.S. Food and Drug Administration may affect the design, initiation, timing, continuation and/or progress of clinical trials or re sul t in the need for additional clinical trials; our ability to obtain and maintain regulatory approval for our product candidate; delays, interruptions or f ail ures in the manufacture and supply of our product candidate; our ability to raise additional capital; the effect of the COVID - 19 pandemic on our business, the medi cal community and the global economy; and the availability or potential availability of alternative products or treatments for conditions targeted by us t hat could affect the availability or commercial potential of our product candidate. Marinus undertakes no obligation to update or revise any forward - looking statemen ts. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward - looki ng statements, as well as risks relating to the business of the Company in general, see filings Marinus has made with the Securities and Exchange Commission. Y ou may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov . 2
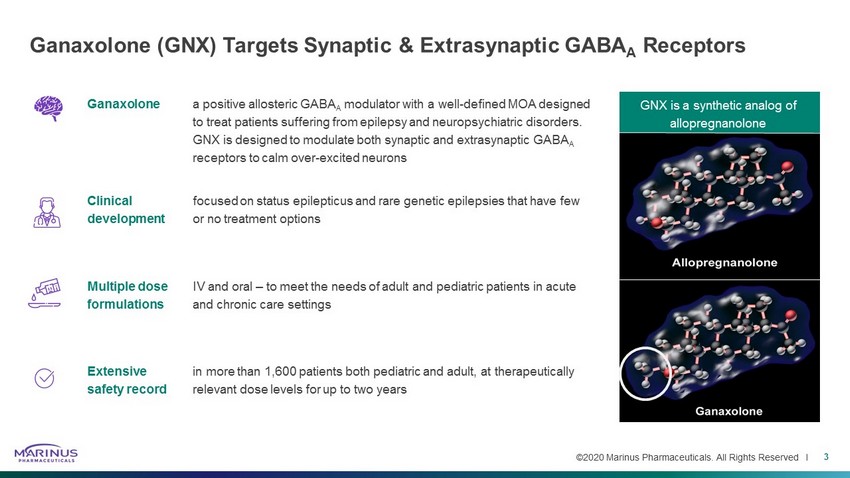
©2020 Marinus Pharmaceuticals. All Rights Reserved I Ganaxolone (GNX) Targets Synaptic & Extrasynaptic GABA A Receptors 3 Ganaxolone a positive allosteric GABA A modulator with a well - defined MOA designed to treat patients suffering from epilepsy and neuropsychiatric disorders. GNX is designed to modulate both synaptic and extrasynaptic GABA A receptors to calm over - excited neurons Clinical development focused on status epilepticus and rare genetic epilepsies that have few or no treatment options Multiple dose formulations IV and oral – to meet the needs of adult and pediatric patients in acute and chronic care settings Extensive safety record in more than 1,600 patients both pediatric and adult, at therapeutically relevant dose levels for up to two years GNX is a synthetic analog of allopregnanolone
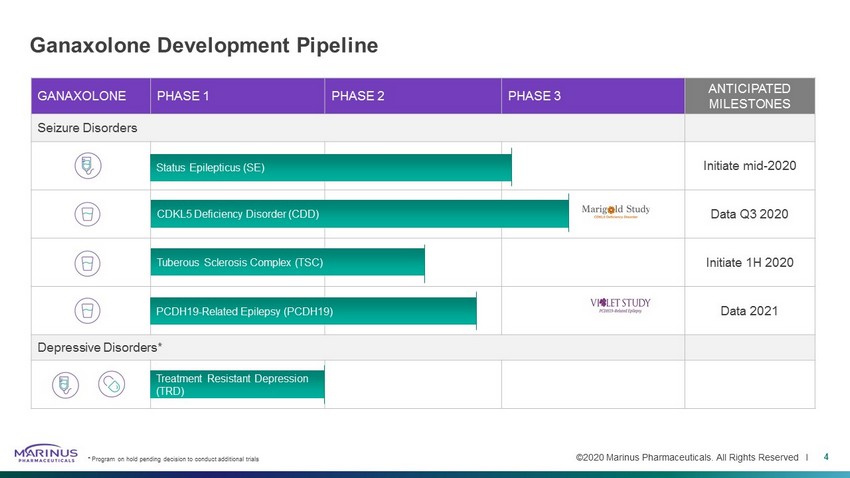
©2020 Marinus Pharmaceuticals. All Rights Reserved I GANAXOLONE PHASE 1 PHASE 2 PHASE 3 ANTICIPATED MILESTONES Seizure Disorders Initiate mid - 2020 Data Q3 2020 Initiate 1H 2020 Data 2021 Depressive Disorders* Ganaxolone Development Pipeline Treatment Resistant Depression (TRD) Status Epilepticus (SE) 4 * Program on hold pending decision to conduct additional trials PCDH19 - Related Epilepsy (PCDH19) CDKL5 Deficiency Disorder (CDD) Tuberous Sclerosis Complex (TSC)

Status Epilepticus Indication
©2020 Marinus Pharmaceuticals. All Rights Reserved I Status Epilepticus (SE): Definition and Epidemiology Background • Continuous seizures lasting >5 min • Heterogenous patient population with various etiologies, including glioblastoma, vascular disease, encephalitis, drug or alcohol withdrawal or overdose • Pre - existing epilepsy in less than half of SE cases • Prolonged seizure activity can result in permanent neuronal damage and contribute to the high morbidity and mortality rates • SE becomes more difficult to control as its duration increases and is associated with increased mortality 6 SE is the second most common neurologic emergency in the U.S.
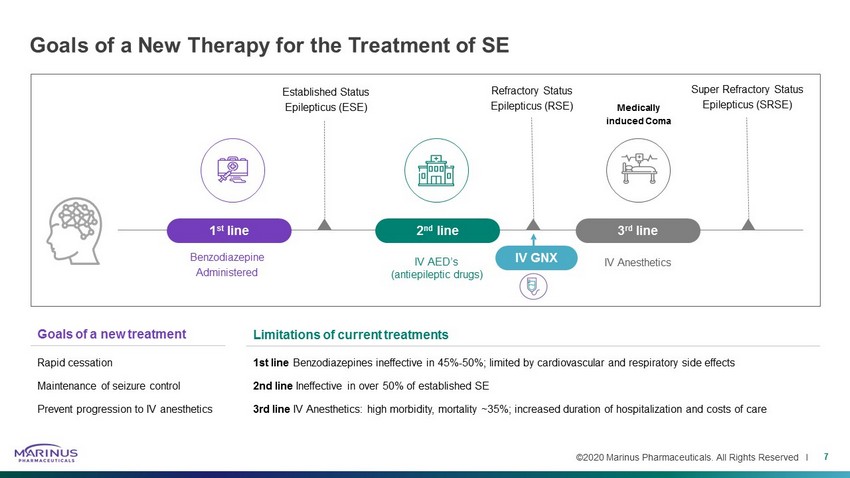
©2020 Marinus Pharmaceuticals. All Rights Reserved I Goals of a new treatment Rapid cessation Maintenance of seizure control Prevent progression to IV anesthetics Goals of a New Therapy for the Treatment of SE 7 Benzodiazepine Administered Medically induced Coma Established Status Epilepticus (ESE) 1 st line 2 nd line IV AED’s (antiepileptic drugs) 3 rd line IV Anesthetics Super Refractory Status Epilepticus (SRSE) Refractory Status Epilepticus (RSE) IV GNX Limitations of current treatments 1st line Benzodiazepines ineffective in 45% - 50%; limited by cardiovascular and respiratory side effects 2nd line Ineffective in over 50% of established SE 3rd line IV Anesthetics: high morbidity, mortality ~35%; increased duration of hospitalization and costs of care
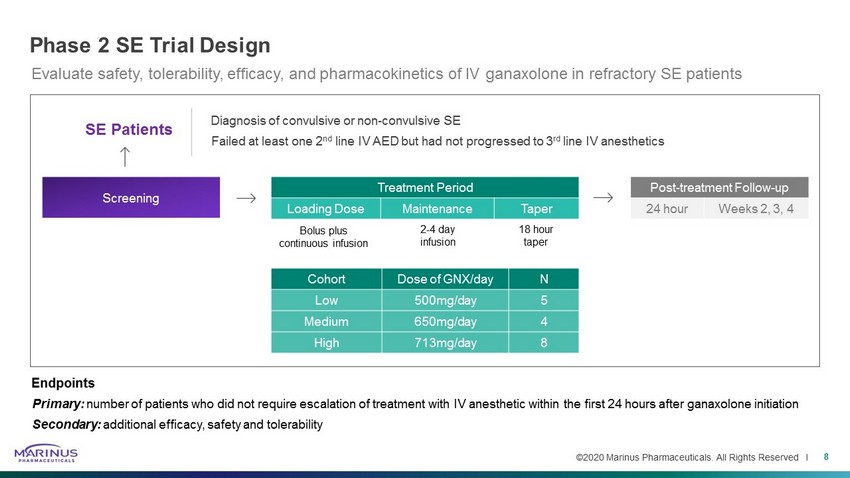
©2020 Marinus Pharmaceuticals. All Rights Reserved I Treatment Period Loading Dose Maintenance Taper Phase 2 SE Trial Design 8 Diagnosis of convulsive or non - convulsive SE Failed at least one 2 nd line IV AED but had not progressed to 3 rd line IV anesthetics Bolus plus continuous infusion 2 - 4 day infusion 18 hour taper Screening Post - treatment Follow - up 24 hour Weeks 2, 3, 4 SE Patients Cohort Dose of GNX/day N Low 500mg/day 5 Medium 650mg/day 4 High 713mg/day 8 Evaluate safety, tolerability, efficacy, and pharmacokinetics of IV ganaxolone in refractory SE patients Endpoints Primary: number of patients who did not require escalation of treatment with IV anesthetic within the first 24 hours after ganaxolone ini tiation Secondary: additional efficacy, safety and tolerability
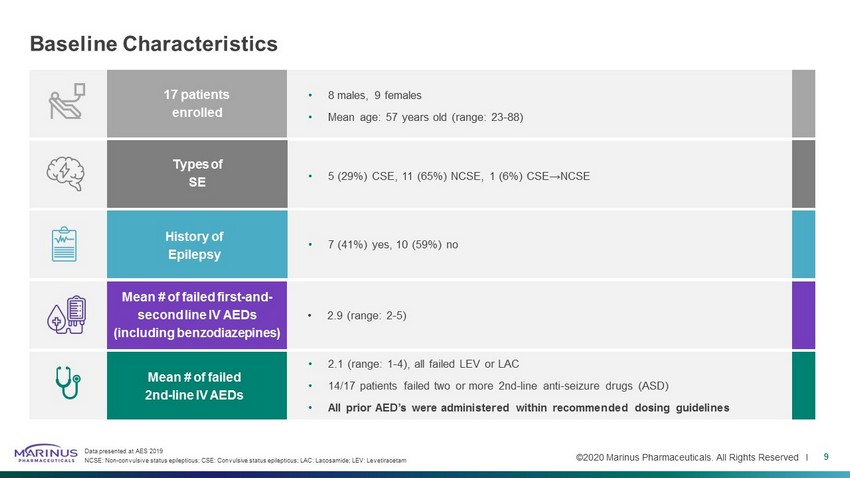
©2020 Marinus Pharmaceuticals. All Rights Reserved I Baseline Characteristics 9 Data presented at AES 2019 NCSE: Non - convulsive status epilepticus; CSE: Convulsive status epilepticus; LAC: Lacosamide ; LEV: Levetiracetam • 8 males, 9 females • Mean age: 57 years old (range: 23 - 88) • 5 (29%) CSE, 11 (65%) NCSE, 1 (6%) CSE→NCSE • 7 (41%) yes, 10 (59%) no 17 patients enrolled Types of SE History of Epilepsy Mean # of failed first - and - second line IV AEDs (including benzodiazepines) Mean # of failed 2nd - line IV AEDs • 2.9 (range: 2 - 5) • 2.1 (range: 1 - 4), all failed LEV or LAC • 14/17 patients failed two or more 2nd - line anti - seizure drugs (ASD) • All prior AED’s were administered within recommended dosing guidelines
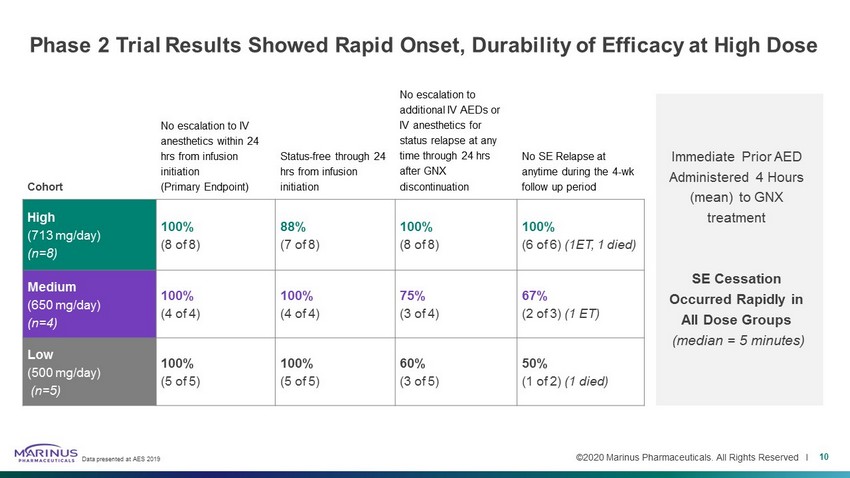
©2020 Marinus Pharmaceuticals. All Rights Reserved I Phase 2 Trial Results Showed Rapid Onset, Durability of Efficacy at High Dose Cohort No escalation to IV anesthetics within 24 hrs from infusion initiation (Primary Endpoint) Status - free through 24 hrs from infusion initiation No escalation to additional IV AEDs or IV anesthetics for status relapse at any time through 24 hrs after GNX discontinuation No SE Relapse at anytime during the 4 - wk follow up period High (713 mg/day) (n=8) 100% (8 of 8) 88% (7 of 8) 100% (8 of 8) 100% (6 of 6) (1ET, 1 died) Medium (650 mg/day) (n=4) 100% (4 of 4) 100% (4 of 4) 75% (3 of 4) 67% (2 of 3) (1 ET) Low (500 mg/day) (n=5) 100% (5 of 5) 100% (5 of 5) 60% (3 of 5) 50% (1 of 2) (1 died) Immediate Prior AED Administered 4 Hours (mean) to GNX treatment SE Cessation Occurred Rapidly in All Dose Groups (median = 5 minutes) Data presented at AES 2019 10
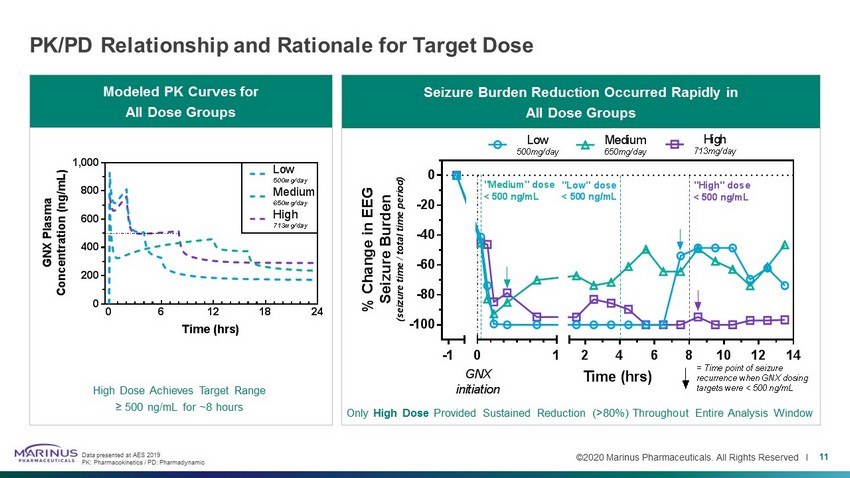
©2020 Marinus Pharmaceuticals. All Rights Reserved I PK/PD Relationship and Rationale for Target Dose 11 Seizure Burden Reduction Occurred Rapidly in All Dose Groups Modeled PK Curves for All Dose Groups High Dose Achieves Target Range ≥ 500 ng/mL for ~8 hours Only High Dose Provided Sustained Reduction (>80%) Throughout Entire Analysis Window Data presented at AES 2019 PK: Pharmacokinetics / PD: Pharmadynamic 0 6 12 18 24 0 200 400 600 800 1,000 Time (hrs) G N X P l a s m a C o n c e n t r a t i o n ( n g / m L ) Low 500mg/day Medium 650mg/day High 713mg/day -1 -100 -80 -60 -40 -20 0 0 1 2 4 6 8 10 12 14 Time (hrs) % C h a n g e i n E E G S e i z u r e B u r d e n ( s e i z u r e t i m e / t o t a l t i m e p e r i o d ) GNX initiation High 713mg/day Medium 650mg/day Low 500mg/day "High" dose < 500 ng/mL = Time point of seizure recurrence when GNX dosing targets were < 500 ng/mL "Low" dose < 500 ng/mL "Medium" dose < 500 ng/mL
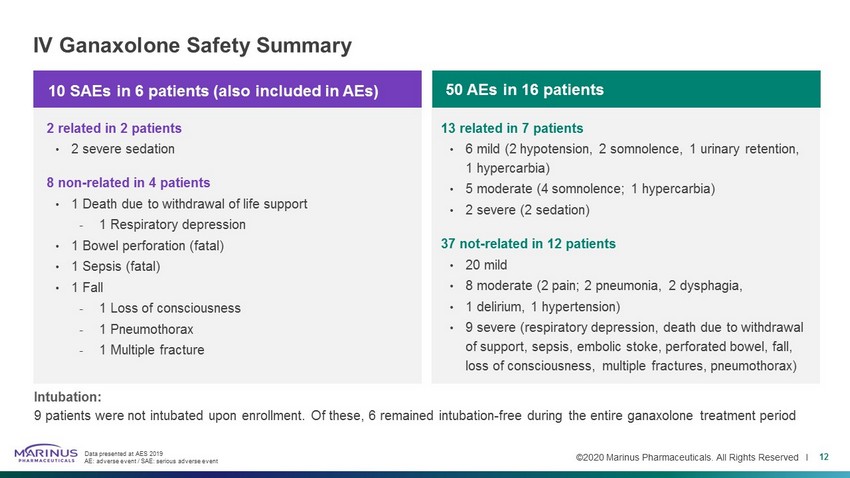
©2020 Marinus Pharmaceuticals. All Rights Reserved I IV Ganaxolone Safety Summary 12 Intubation: 9 patients were not intubated upon enrollment. Of these, 6 remained intubation - free during the entire ganaxolone treatment perio d Data presented at AES 2019 AE: adverse event / SAE: serious adverse event 10 SAEs in 6 patients (also included in AEs) 2 related in 2 patients • 2 severe sedation 8 non - related in 4 patients • 1 Death due to withdrawal of life support - 1 Respiratory depression • 1 Bowel perforation (fatal) • 1 Sepsis (fatal) • 1 Fall - 1 Loss of consciousness - 1 Pneumothorax - 1 Multiple fracture 13 related in 7 patients • 6 mild (2 hypotension, 2 somnolence, 1 urinary retention, 1 hypercarbia) • 5 moderate (4 somnolence; 1 hypercarbia) • 2 severe (2 sedation) 37 not - related in 12 patients • 20 mild • 8 moderate (2 pain; 2 pneumonia, 2 dysphagia, • 1 delirium, 1 hypertension) • 9 severe (respiratory depression, death due to withdrawal of support, sepsis, embolic stoke, perforated bowel, fall, loss of consciousness, multiple fractures, pneumothorax) 50 AEs in 16 patients
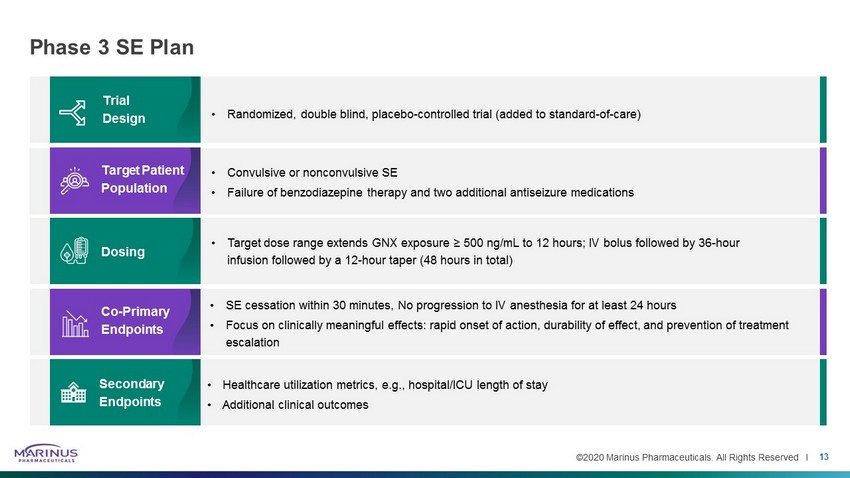
©2020 Marinus Pharmaceuticals. All Rights Reserved I Phase 3 SE Plan 13 • Target dose range extends GNX exposure ≥ 500 ng/mL to 12 hours; IV bolus followed by 36 - hour infusion followed by a 12 - hour taper (48 hours in total) • SE cessation within 30 minutes, No progression to IV anesthesia for at least 24 hours • Focus on clinically meaningful effects: rapid onset of action, durability of effect, and prevention of treatment escalation • Randomized, double blind, placebo - controlled trial (added to standard - of - care) • Convulsive or nonconvulsive SE • Failure of benzodiazepine therapy and two additional antiseizure medications • Healthcare utilization metrics, e.g., hospital/ICU length of stay • Additional clinical outcomes Trial Design Target Patient Population Dosing Co - Primary Endpoints Secondary Endpoints
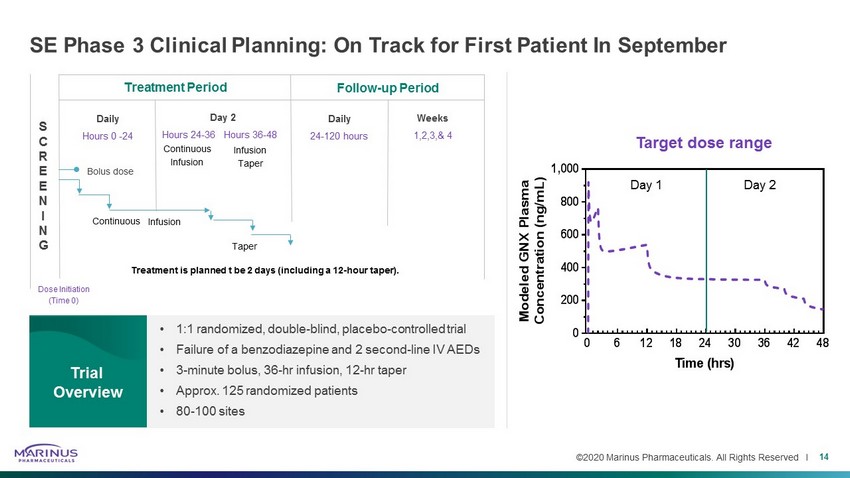
©2020 Marinus Pharmaceuticals. All Rights Reserved I SE Phase 3 Clinical Planning: On Track for First Patient In September 14 • 1:1 randomized, double - blind, placebo - controlled trial • Failure of a benzodiazepine and 2 second - line IV AEDs • 3 - minute bolus, 36 - hr infusion, 12 - hr taper • Approx. 125 randomized patients • 80 - 100 sites Trial Overview S C R E E N I N G Dose Initiation (Time 0) Treatment Period Follow - up Period Weeks 1,2,3,& 4 Daily 24 - 120 hours Day 2 Hours 24 - 36 Hours 36 - 48 Daily Hours 0 - 24 Bolus dose Continuous Infusion Taper Treatment is planned t be 2 days (including a 12 - hour taper). 0 6 12 18 24 30 36 42 48 0 200 400 600 800 1,000 Time (hrs) M o d e l e d G N X P l a s m a C o n c e n t r a t i o n ( n g / m L ) Target dose range Day 1 Day 2 Continuous Infusion Infusion Taper

CDKL5 Deficiency Disorder
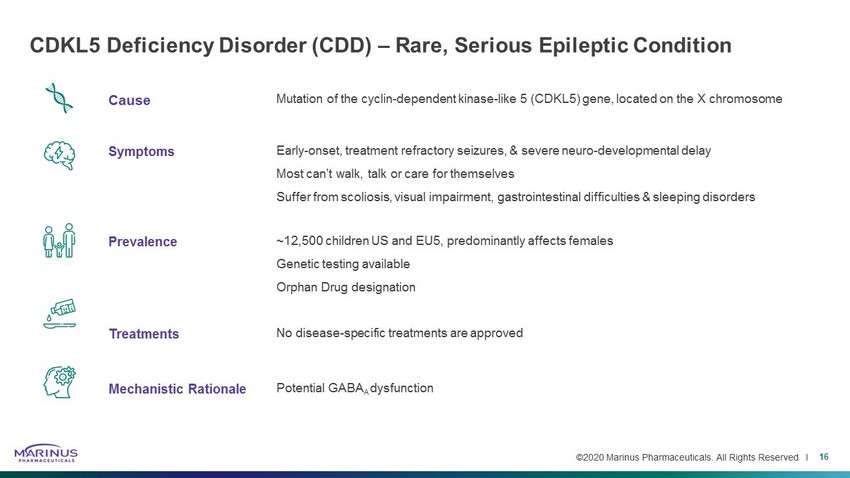
©2020 Marinus Pharmaceuticals. All Rights Reserved I Cause Mutation of the cyclin - dependent kinase - like 5 (CDKL5) gene, located on the X chromosome Symptoms Early - onset, treatment refractory seizures, & severe neuro - developmental delay Most can’t walk, talk or care for themselves Suffer from scoliosis, visual impairment, gastrointestinal difficulties & sleeping disorders Prevalence ~12,500 children US and EU5, predominantly affects females Genetic testing available Orphan Drug designation Treatments No disease - specific treatments are approved Mechanistic Rationale Potential GABA A dysfunction CDKL5 Deficiency Disorder (CDD) – Rare, Serious Epileptic Condition 16 Cause
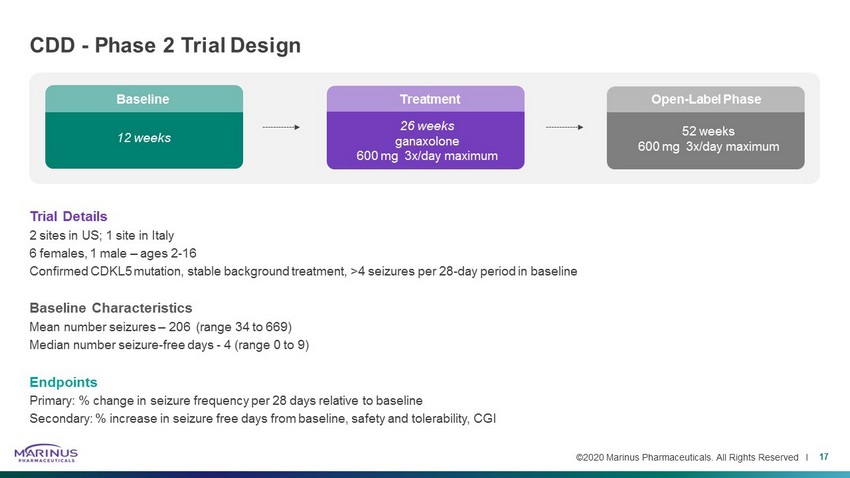
©2020 Marinus Pharmaceuticals. All Rights Reserved I 26 weeks ganaxolone 600 mg 3x/day maximum 12 weeks Trial Details 2 sites in US; 1 site in Italy 6 females, 1 male – ages 2 - 16 Confirmed CDKL5 mutation, stable background treatment, >4 seizures per 28 - day period in baseline Baseline Characteristics Mean number seizures – 206 (range 34 to 669) Median number seizure - free days - 4 (range 0 to 9) Endpoints Primary: % change in seizure frequency per 28 days relative to baseline Secondary: % increase in seizure free days from baseline, safety and tolerability, CGI CDD - Phase 2 Trial Design 17 Open - Label Phase 52 weeks 600 mg 3x/day maximum Treatment Baseline
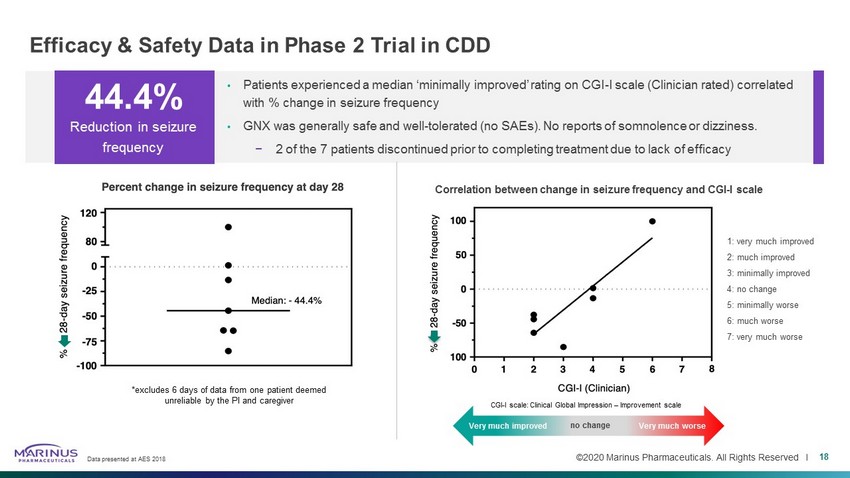
©2020 Marinus Pharmaceuticals. All Rights Reserved I Efficacy & Safety Data in Phase 2 Trial in CDD Data presented at AES 2018 18 • Patients experienced a median ‘minimally improved’ rating on CGI - I scale (Clinician rated) correlated with % change in seizure frequency • GNX was generally safe and well - tolerated (no SAEs). No reports of somnolence or dizziness. − 2 of the 7 patients discontinued prior to completing treatment due to lack of efficacy 44.4% Reduction in seizure frequency *excludes 6 days of data from one patient deemed unreliable by the PI and caregiver Very much improved Very much worse 1: very much improved 2: much improved 3: minimally improved 4: no change 5: minimally worse 6: much worse 7: very much worse Correlation between change in seizure frequency and CGI - I scale CGI - I scale: Clinical Global Impression – Improvement scale Very much improved no change Very much worse

©2020 Marinus Pharmaceuticals. All Rights Reserved I Durable Responses Seen out to 18 Months in CDD Phase 2 Extension • 4 of 7 patients entered the extension period • Ganaxolone demonstrated preliminary evidence of sustained, long - term (out to 18 months) efficacy in a small cohort of CDD patients • Efficacy of existing AEDs and the ketogenic diet in patients with the CDKL5 mutation is low and the durability of effect is s hor t 1 19 Clinical data presented at AES 2018 1 Müller, A. et al., European Journal of Paediatric Neurology, Volume 20 (2016), 147 - 151. OLE: open - label extension 54% Median change in seizure frequency at 6 months 66% Median change frequency improved to 66% in extension period (as of 12/31) Patients Entering OLE Patient 1 Patient 2 Patient 3 Patient 4 %
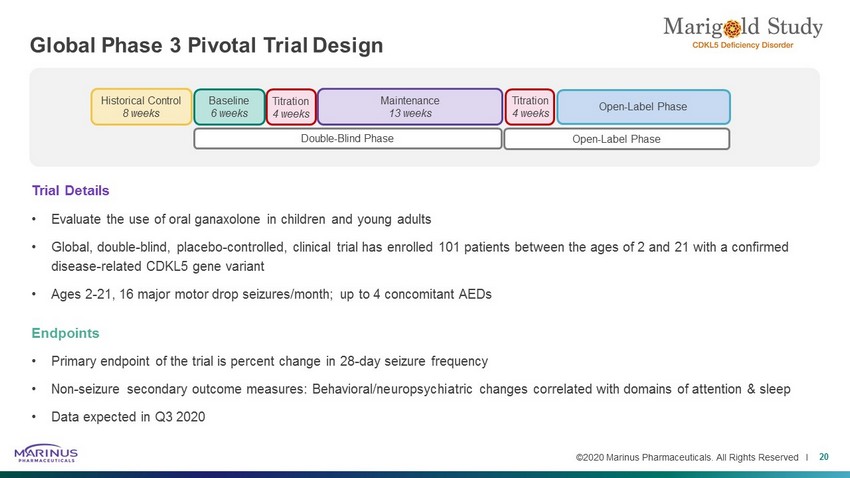
©2020 Marinus Pharmaceuticals. All Rights Reserved I Global Phase 3 Pivotal Trial Design 20 Baseline 6 weeks Historical Control 8 weeks Double - Blind Phase Open - Label Phase Maintenance 13 weeks Titration 4 weeks Titration 4 weeks Open - Label Phase Trial Details • Evaluate the use of oral ganaxolone in children and young adults • Global, double - blind, placebo - controlled, clinical trial has enrolled 101 patients between the ages of 2 and 21 with a confirmed disease - related CDKL5 gene variant • Ages 2 - 21, 16 major motor drop seizures/month; up to 4 concomitant AEDs Endpoints • Primary endpoint of the trial is percent change in 28 - day seizure frequency • Non - seizure secondary outcome measures: Behavioral/neuropsychiatric changes correlated with domains of attention & sleep • Data expected in Q3 2020

Tuberous Sclerosis Complex
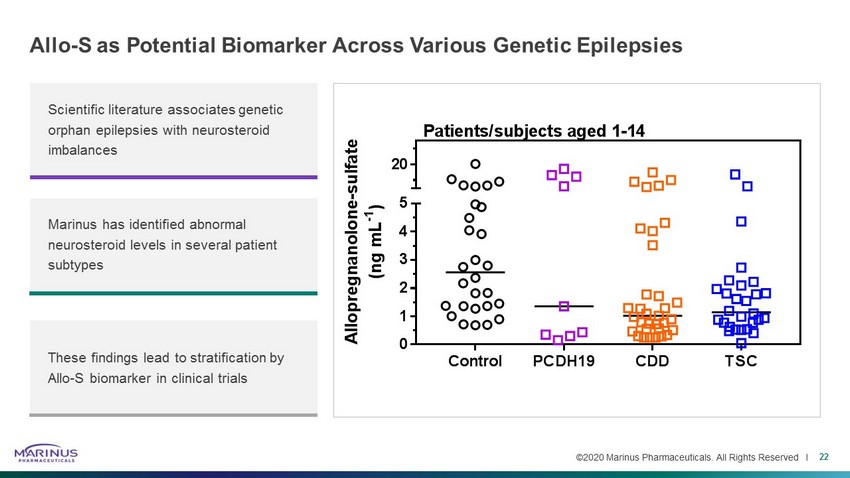
©2020 Marinus Pharmaceuticals. All Rights Reserved I Allo - S as Potential Biomarker Across Various Genetic Epilepsies 22 Scientific literature associates genetic orphan epilepsies with neurosteroid imbalances Marinus has identified abnormal neurosteroid levels in several patient subtypes These findings lead to stratification by Allo - S biomarker in clinical trials Control PCDH19 CDD TSC 0 1 2 3 4 5 20 A l l o p r e g n a n o l o n e - s u l f a t e ( n g m L - 1 ) Patients/subjects aged 1-14
©2020 Marinus Pharmaceuticals. All Rights Reserved I Cause Defect or mutation of TSC1 and/or TSC2 genes Symptoms Benign tumors, seizures, cognitive impairment, behavioral problems, skin abnormalities Prevalence ~25K-40K refractory TSC patients in the U.S.* Treatments Limited approved treatments Mechanistic Rationale Potential neurosteroid deficiency 1 Tuberous Sclerosis Complex – Rare, Serious Genetic Disorder 23 1 diMichele , et al, J. Neuro Neurosurg Psychiatry , 2003 *Failure of two prior antiseizure medications with ongoing, frequent seizures.
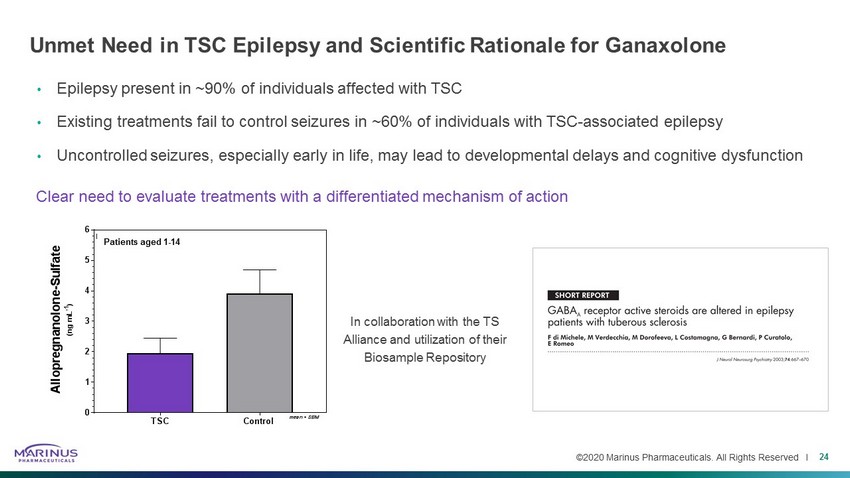
©2020 Marinus Pharmaceuticals. All Rights Reserved I Unmet Need in TSC Epilepsy and Scientific Rationale for Ganaxolone • Epilepsy present in ~90% of individuals affected with TSC • Existing treatments fail to control seizures in ~60% of individuals with TSC - associated epilepsy • Uncontrolled seizures, especially early in life, may lead to developmental delays and cognitive dysfunction 24 Clear need to evaluate treatments with a differentiated mechanism of action In collaboration with the TS Alliance and utilization of their Biosample Repository TSC Control 0 1 2 3 4 5 6 A l l o p r e g n a n o l o n e - S u l f a t e ( n g m L - 1 ) Patients/subjects aged 1-14 mean + SEM Patients aged 1 - 14
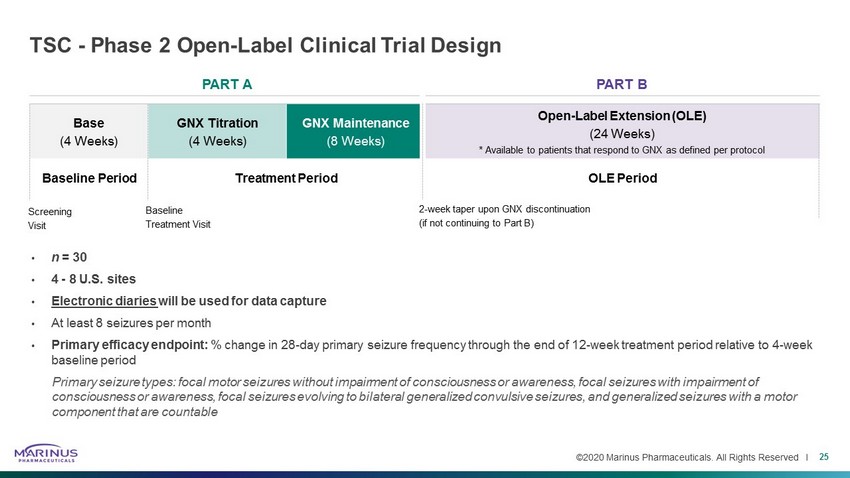
©2020 Marinus Pharmaceuticals. All Rights Reserved I PART A PART B Base (4 Weeks) GNX Titration (4 Weeks) GNX Maintenance (8 Weeks) Open - Label Extension (OLE) (24 Weeks) * Available to patients that respond to GNX as defined per protocol TSC - Phase 2 Open - Label Clinical Trial Design • n = 30 • 4 - 8 U.S. sites • Electronic diaries will be used for data capture • At least 8 seizures per month • Primary efficacy endpoint: % change in 28 - day primary seizure frequency through the end of 12 - week treatment period relative to 4 - week baseline period Primary seizure types: focal motor seizures without impairment of consciousness or awareness, focal seizures with impairment of consciousness or awareness, focal seizures evolving to bilateral generalized convulsive seizures, and generalized seizures wi th a motor component that are countable 25 Baseline Period Treatment Period OLE Period Screening Visit Baseline Treatment Visit 2 - week taper upon GNX discontinuation (if not continuing to Part B)
PCDH19 Related Epilepsy
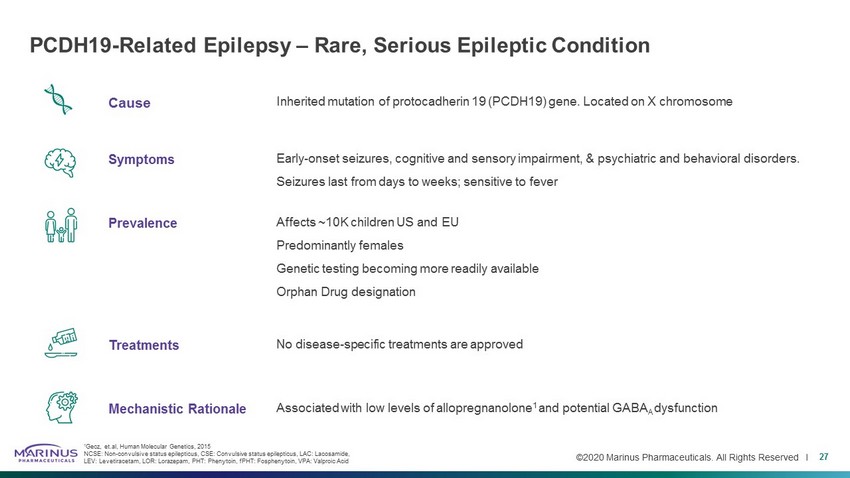
©2020 Marinus Pharmaceuticals. All Rights Reserved I Cause Inherited mutation of protocadherin 19 (PCDH19) gene. Located on X chromosome Symptoms Early - onset seizures, cognitive and sensory impairment, & psychiatric and behavioral disorders. Seizures last from days to weeks; sensitive to fever Prevalence Affects ~10K children US and EU Predominantly females Genetic testing becoming more readily available Orphan Drug designation Treatments No disease - specific treatments are approved Mechanistic Rationale Associated with low levels of allopregnanolone 1 and potential GABA A dysfunction PCDH19 - Related Epilepsy – Rare, Serious Epileptic Condition 27 1 Gecz, et.al , Human Molecular Genetics, 2015 NCSE: Non - convulsive status epilepticus, CSE: Convulsive status epilepticus, LAC: Lacosamide , LEV: Levetiracetam, LOR: Lorazepam, PHT: Phenytoin, fPHT : Fosphenytoin , VPA: Valproic Acid
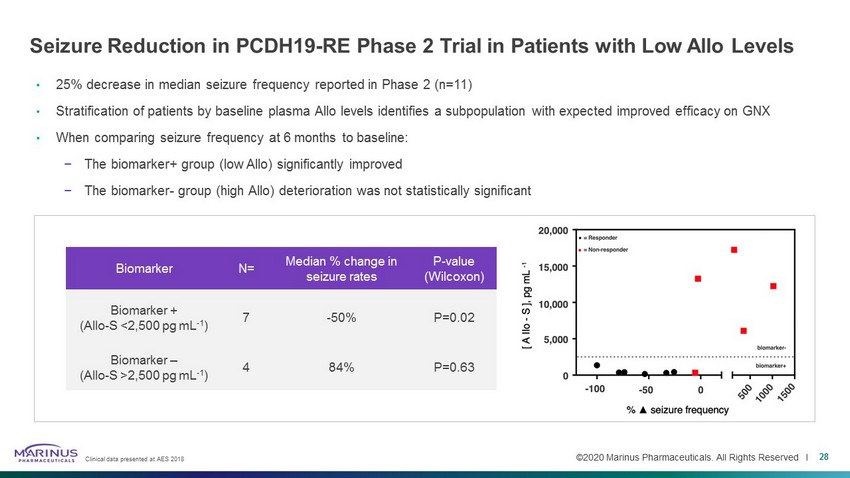
©2020 Marinus Pharmaceuticals. All Rights Reserved I Seizure Reduction in PCDH19 - RE Phase 2 Trial in Patients with Low Allo Levels • 25% decrease in median seizure frequency reported in Phase 2 (n=11) • Stratification of patients by baseline plasma Allo levels identifies a subpopulation with expected improved efficacy on GNX • When comparing seizure frequency at 6 months to baseline: − The biomarker+ group (low Allo ) significantly improved − The biomarker - group (high Allo ) deterioration was not statistically significant Biomarker N= Median % change in seizure rates P - value (Wilcoxon) Biomarker + ( Allo - S <2,500 pg mL - 1 ) 7 - 50% P=0.02 Biomarker – ( Allo - S >2,500 pg mL - 1 ) 4 84% P=0.63 28 Clinical data presented at AES 2018
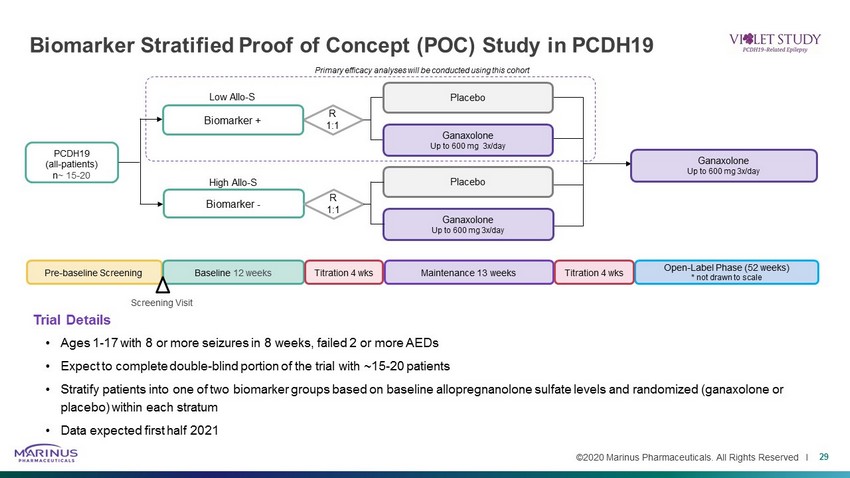
©2020 Marinus Pharmaceuticals. All Rights Reserved I Biomarker Stratified Proof of Concept (POC) Study in PCDH19 29 Trial Details • Ages 1 - 17 with 8 or more seizures in 8 weeks, failed 2 or more AEDs • Expect to complete double - blind portion of the trial with ~15 - 20 patients • Stratify patients into one of two biomarker groups based on baseline allopregnanolone sulfate levels and randomized ( ganaxolone or placebo) within each stratum • Data expected first half 2021 R 1:1 PCDH19 (all - patients) n ~ 15 - 20 Biomarker + Low Allo - S Primary efficacy analyses will be conducted using this cohort Biomarker - High Allo - S R 1:1 Ganaxolone Up to 600 mg 3x/day Placebo Ganaxolone Up to 600 mg 3x/day Placebo Ganaxolone Up to 600 mg 3x/day Pre - baseline Screening Maintenance 13 weeks Baseline 12 weeks Titration 4 wks Open - Label Phase (52 weeks) * not drawn to scale Titration 4 wks Screening Visit

Intellectual Property
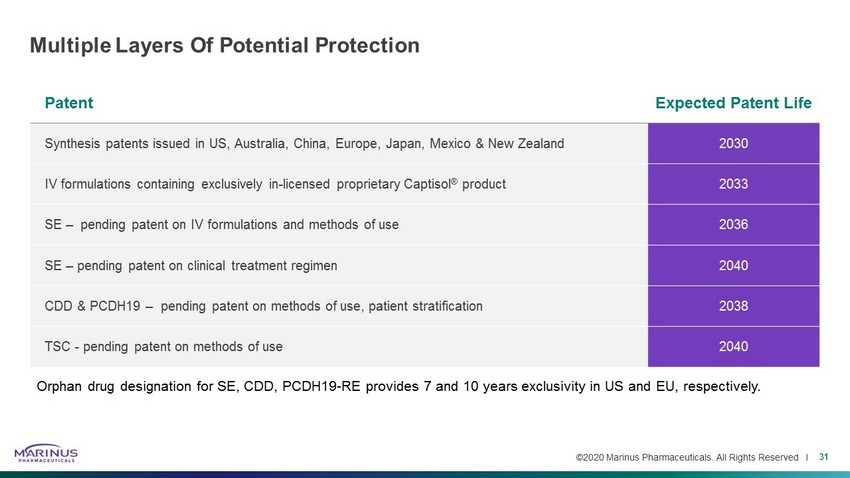
©2020 Marinus Pharmaceuticals. All Rights Reserved I Patent Expected Patent Life Synthesis patents issued in US, Australia, China, Europe, Japan, Mexico & New Zealand 2030 IV formulations containing exclusively in - licensed proprietary Captisol ® product 2033 SE – pending patent on IV formulations and methods of use 2036 SE – pending patent on clinical treatment regimen 2040 CDD & PCDH19 – pending patent on methods of use, patient stratification 2038 TSC - pending patent on methods of use 2040 Multiple Layers Of Potential Protection 31 Orphan drug designation for SE, CDD, PCDH19 - RE provides 7 and 10 years exclusivity in US and EU, respectively.

Thank You
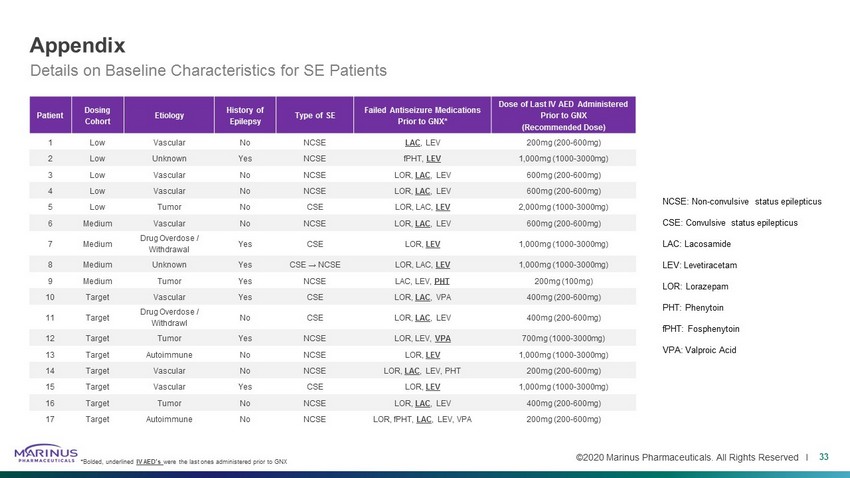
©2020 Marinus Pharmaceuticals. All Rights Reserved I Appendix 33 NCSE: Non - convulsive status epilepticus CSE: Convulsive status epilepticus LAC: Lacosamide LEV: Levetiracetam LOR: Lorazepam PHT: Phenytoin fPHT : Fosphenytoin VPA: Valproic Acid Details on Baseline Characteristics for SE Patients *Bolded, underlined IV AED’s were the last ones administered prior to GNX Patient Dosing Cohort Etiology History of Epilepsy Type of SE Failed Antiseizure Medications Prior to GNX* Dose of Last IV AED Administered Prior to GNX (Recommended Dose) 1 Low Vascular No NCSE LAC , LEV 200mg (200 - 600mg) 2 Low Unknown Yes NCSE fPHT , LEV 1,000mg (1000 - 3000mg) 3 Low Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 4 Low Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 5 Low Tumor No CSE LOR, LAC, LEV 2,000mg (1000 - 3000mg) 6 Medium Vascular No NCSE LOR, LAC , LEV 600mg (200 - 600mg) 7 Medium Drug Overdose / Withdrawal Yes CSE LOR, LEV 1,000mg (1000 - 3000mg) 8 Medium Unknown Yes CSE → NCSE LOR, LAC, LEV 1,000mg (1000 - 3000mg) 9 Medium Tumor Yes NCSE LAC, LEV, PHT 200mg (100mg) 10 Target Vascular Yes CSE LOR, LAC , VPA 400mg (200 - 600mg) 11 Target Drug Overdose / Withdrawl No CSE LOR, LAC , LEV 400mg (200 - 600mg) 12 Target Tumor Yes NCSE LOR, LEV, VPA 700mg (1000 - 3000mg) 13 Target Autoimmune No NCSE LOR, LEV 1,000mg (1000 - 3000mg) 14 Target Vascular No NCSE LOR, LAC , LEV, PHT 200mg (200 - 600mg) 15 Target Vascular Yes CSE LOR, LEV 1,000mg (1000 - 3000mg) 16 Target Tumor No NCSE LOR, LAC , LEV 400mg (200 - 600mg) 17 Target Autoimmune No NCSE LOR, fPHT , LAC , LEV, VPA 200mg (200 - 600mg)
