Attached files
| file | filename |
|---|---|
| EX-31.1 - EX-31.1 - MARINUS PHARMACEUTICALS INC | mrms-20200331ex3110e4ab1.htm |
| EX-32.1 - EX-32.1 - MARINUS PHARMACEUTICALS INC | mrms-20200331ex321bc1d99.htm |
| EX-31.2 - EX-31.2 - MARINUS PHARMACEUTICALS INC | mrms-20200331ex31213f47b.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
☒QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE QUARTERLY PERIOD ENDED MARCH 31, 2020
OR
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
COMMISSION FILE NUMBER 001-36576

MARINUS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
20-0198082 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
5 Radnor Corporate Center, Suite 500
100 Matsonford Rd
Radnor, PA 19087
(Address of registrant’s principal executive offices)
Registrant’s telephone number, including area code: (484) 801-4670
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.001 per share |
|
MRNS |
|
Nasdaq Global Market |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes☐ No.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐ |
|
Accelerated filer |
☒ |
|
|
|
|
|
|
Non-accelerated filer ☐ |
|
Smaller reporting company |
☒ |
|
|
|
|
|
|
Emerging growth company ☐ |
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No.
The number of outstanding shares of the registrant’s common stock, par value $0.001 per share, as of May 1, 2020 was: 86,732,035.
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
FOR THE QUARTER ENDED MARCH 31, 2020
2
FINANCIAL INFORMATION
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
(in thousands, except share and per share amounts)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
December 31, |
|
||
|
|
|
2020 |
|
2019 |
|
||
|
|
|
|
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
70,326 |
|
$ |
90,943 |
|
|
Short-term investments |
|
|
7,460 |
|
|
739 |
|
|
Prepaid expenses and other current assets |
|
|
2,212 |
|
|
2,452 |
|
|
Total current assets |
|
|
79,998 |
|
|
94,134 |
|
|
Property and equipment, net |
|
|
2,186 |
|
|
2,265 |
|
|
Other assets |
|
|
2,374 |
|
|
2,443 |
|
|
Total assets |
|
$ |
84,558 |
|
$ |
98,842 |
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
2,845 |
|
$ |
2,763 |
|
|
Accrued expenses |
|
|
7,479 |
|
|
5,268 |
|
|
Total current liabilities |
|
|
10,325 |
|
|
8,031 |
|
|
Other long-term liabilities |
|
|
2,921 |
|
|
3,042 |
|
|
Total liabilities |
|
|
13,245 |
|
|
11,073 |
|
|
Series A convertible preferred stock, $0.001 par value; 25,000,000 shares authorized, 30,000 shares issued and outstanding at March 31, 2020 and December 31, 2019 |
|
|
28,200 |
|
|
28,200 |
|
|
Commitments and contingencies (Note 9) |
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
Common stock, $0.001 par value; 150,000,000 shares authorized, 86,761,266 issued and 86,732,035 outstanding at March 31, 2020 and 86,500,353 issued and 86,471,122 outstanding at December 31, 2019 |
|
|
87 |
|
|
87 |
|
|
Additional paid-in capital |
|
|
306,124 |
|
|
295,056 |
|
|
Treasury stock at cost, 29,231 shares at March 31, 2020 and December 31, 2019 |
|
|
— |
|
|
— |
|
|
Accumulated other comprehensive income |
|
|
28 |
|
|
— |
|
|
Accumulated deficit |
|
|
(263,126) |
|
|
(235,574) |
|
|
Total stockholders’ equity |
|
|
43,113 |
|
|
59,569 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
84,558 |
|
$ |
98,842 |
|
See accompanying notes to consolidated financial statements.
3
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share amounts)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
|
||||
|
|
|
2020 |
|
2019 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
Expenses: |
|
|
|
|
|
|
|
|
|
Research and development |
|
$ |
15,004 |
|
$ |
8,872 |
|
|
|
General and administrative |
|
|
3,850 |
|
|
3,667 |
|
|
|
Loss from operations |
|
|
(18,854) |
|
|
(12,539) |
|
|
|
Interest income |
|
|
222 |
|
|
96 |
|
|
|
Other expense |
|
|
(40) |
|
|
(40) |
|
|
|
Net loss |
|
|
(18,672) |
|
|
(12,483) |
|
|
|
Deemed dividends on convertible preferred stock |
|
|
(8,880) |
|
|
— |
|
|
|
Net loss applicable to common shareholders |
|
$ |
(27,552) |
|
$ |
(12,483) |
|
|
|
Per share information: |
|
|
|
|
|
|
|
|
|
Net loss per share of common stock—basic and diluted |
|
$ |
(0.32) |
|
$ |
(0.24) |
|
|
|
Basic and diluted weighted average shares outstanding |
|
|
86,661,845 |
|
|
52,465,207 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(18,672) |
|
$ |
(12,483) |
|
|
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
Unrealized gain on available-for-sale securities |
|
|
28 |
|
|
2 |
|
|
|
Total comprehensive loss |
|
$ |
(18,644) |
|
$ |
(12,481) |
|
|
See accompanying notes to consolidated financial statements.
4
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
|
|
|
Three Months Ended March 31, |
|
||||
|
|
|
2020 |
|
2019 |
|
||
|
|
|
|
|
|
|
|
|
|
Cash flows from operating activities |
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(18,672) |
|
$ |
(12,483) |
|
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
84 |
|
|
36 |
|
|
Stock-based compensation expense |
|
|
1,876 |
|
|
1,836 |
|
|
Loss on disposal of fixed assets |
|
|
— |
|
|
42 |
|
|
Noncash lease expense |
|
|
62 |
|
|
46 |
|
|
Noncash lease liability |
|
|
95 |
|
|
(55) |
|
|
Amortization of premium on investments |
|
|
1 |
|
|
(1) |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets |
|
|
258 |
|
|
(847) |
|
|
Accounts payable and accrued expenses |
|
|
2,259 |
|
|
(238) |
|
|
Net cash used in operating activities |
|
|
(14,037) |
|
|
(11,664) |
|
|
Cash flows from investing activities |
|
|
|
|
|
|
|
|
Maturities of short-term investments |
|
|
1,737 |
|
|
5,000 |
|
|
Purchases of short-term investments |
|
|
(8,433) |
|
|
— |
|
|
Deposit on property and equipment |
|
|
— |
|
|
(80) |
|
|
Purchases of property and equipment |
|
|
(1) |
|
|
(52) |
|
|
Net cash (used in) provided by investing activities |
|
|
(6,697) |
|
|
4,868 |
|
|
Cash flows from financing activities |
|
|
|
|
|
|
|
|
Proceeds from exercise of stock options |
|
|
312 |
|
|
65 |
|
|
Financing costs |
|
|
(195) |
|
|
(148) |
|
|
Net cash provided by (used in) financing activities |
|
|
117 |
|
|
(83) |
|
|
Net decrease in cash and cash equivalents |
|
|
(20,617) |
|
|
(6,879) |
|
|
Cash and cash equivalents—beginning of year |
|
|
90,943 |
|
|
67,727 |
|
|
Cash and cash equivalents—end of year |
|
$ |
70,326 |
|
$ |
60,848 |
|
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
Property and equipment in accounts payable and accrued expenses |
|
$ |
— |
|
$ |
48 |
|
|
Operating lease liability |
|
$ |
— |
|
$ |
3,357 |
|
|
Operating right-of-use asset |
|
$ |
— |
|
$ |
2,458 |
|
See accompanying notes to consolidated financial statements.
5
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
(unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
Additional |
|
|
|
|
|
|
Other |
|
|
|
Total |
|
||||
|
|
|
Common Stock |
|
Paid-in |
|
Treasury Stock |
|
Comprehensive |
|
Accumulated |
|
Stockholders’ |
|
||||||||||
|
|
|
Shares |
|
Amount |
|
Capital |
|
Shares |
|
Amount |
|
Income |
|
Deficit |
|
Equity |
|
||||||
|
Balance, December 31, 2018 |
|
52,548,244 |
|
$ |
53 |
|
$ |
249,727 |
|
29,231 |
|
|
— |
|
$ |
(2) |
|
$ |
(181,453) |
|
$ |
68,325 |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
1,836 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
1,836 |
|
|
Exercise of stock options |
|
55,812 |
|
|
— |
|
|
65 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
65 |
|
|
Forfeiture of restricted stock |
|
(20,200) |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Unrealized gain on investments |
|
— |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
2 |
|
|
— |
|
|
2 |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(12,483) |
|
|
(12,483) |
|
|
Balance, March 31, 2019 |
|
52,583,856 |
|
$ |
53 |
|
$ |
251,628 |
|
29,231 |
|
|
— |
|
$ |
— |
|
$ |
(193,936) |
|
$ |
57,745 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2019 |
|
86,500,353 |
|
$ |
87 |
|
$ |
295,056 |
|
29,231 |
|
|
— |
|
$ |
— |
|
$ |
(235,574) |
|
$ |
59,569 |
|
|
Stock-based compensation expense |
|
— |
|
|
— |
|
|
1,876 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
1,876 |
|
|
Exercise of stock options |
|
239,913 |
|
|
— |
|
|
312 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
312 |
|
|
Issuance of restricted stock |
|
21,000 |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Unrealized gain on investments |
|
— |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
28 |
|
|
— |
|
|
28 |
|
|
Deemed dividend on beneficial conversion feature - Series A convertible preferred stock |
|
— |
|
|
— |
|
|
8,880 |
|
— |
|
|
— |
|
|
— |
|
|
(8,880) |
|
|
— |
|
|
Net loss |
|
— |
|
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
(18,672) |
|
|
(18,672) |
|
|
Balance, March 31, 2020 |
|
86,761,266 |
|
$ |
87 |
|
$ |
306,124 |
|
29,231 |
|
|
— |
|
$ |
28 |
|
$ |
(263,126) |
|
$ |
43,113 |
|
See accompanying notes to consolidated financial statements.
6
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of the Business and Liquidity
We are a clinical stage pharmaceutical company focused on developing and commercializing innovative therapeutics to treat patients suffering from rare seizure disorders. Our clinical stage product candidate, ganaxolone, is a positive allosteric modulator of GABAA that is being developed in formulations for two different routes of administration: intravenous (IV) and oral. Ganaxolone is a synthetic analog of allopregnanolone, an endogenous neurosteroid. The different formulations are intended to maximize potential therapeutic applications of ganaxolone for adult and pediatric patient populations, in both acute and chronic care, and for both in‑patient and self‑administered settings. Ganaxolone acts at both synaptic and extrasynaptic GABAA receptors, a target known for its anti‑seizure, antidepressant and anxiolytic potential.
In December 2019, an outbreak of a novel strain of coronavirus (COVID-19) was identified in Wuhan, China. This virus has been declared a pandemic by the World Health Organization and has spread to other countries, including the United States. Efforts to contain the spread of COVID-19 have intensified and many countries, including the United States, have implemented severe travel restrictions, business shutdowns and social distancing measures that have impacted clinical development through supply chain shortages and clinical trial enrollment difficulties as hospitals reduce and divert staffing, divert resources to patients suffering from the infectious disease and limit hospital access for non-patients. The outbreak of COVID-19 poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting normal business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities.
The continued spread of COVID-19 globally has impacted our operations but did not have a material impact on our business, operating results, financial condition or cash flows as of and for the three months ended March 31, 2020. For example, our Phase 1 supportive clinical trials of oral ganaxolone in CDD have experienced delays in enrollment due to COVID-19. Further, in response to COVID-19, for our ongoing clinical trials, we have implemented multiple measures consistent with the U.S. Food and Drug Administration’s guidance on the conduct of clinical trials of medical products during the COVID-19 pandemic, including implementing remote site monitoring and remote visits using telemedicine where needed. Although operations have not been materially affected by the COVID-19 pandemic as of and for the three months ended March 31, 2020, we are unable to predict the impact that COVID-19 will have in the future on our business, financial position, operating results and cash flows due to numerous uncertainties. The duration and severity of the pandemic and its long-term impact on our business are uncertain at this time, and our ability to raise sufficient additional financing depends on many factors beyond our control, including the current volatility in the capital markets as a result of the COVID-19 pandemic
Liquidity
We have not generated any product revenues and have incurred operating losses since inception, including losses of $18.7 million for the three months ended March 31, 2020. There is no assurance that profitable operations will ever be achieved, and if achieved, could be sustained on a continuing basis. In addition, development activities, clinical and preclinical testing, and commercialization of our product candidates will require significant additional financing. Our accumulated deficit as of March 31, 2020 was $263.1 million and we expect to incur substantial losses in future periods. We plan to finance our future operations with a combination of proceeds from the issuance of equity securities, the issuance of debt, collaborations, licensing transactions and other commercial transactions and revenues from future product sales, if any. We have not generated positive cash flows from operations, and there are no assurances that we will be successful in obtaining an adequate level of financing for the development and commercialization of our product candidates.
In connection with the closing of concurrent equity financings during the fourth quarter of 2019, we issued a total of 32,200,000 shares of common stock in an underwritten public offering and 30,000 shares of Series A convertible preferred stock in a private placement resulting in aggregate net proceeds, after underwriting discounts and commissions
7
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
in the public offering and other estimated offering expenses, of $65.7 million. We also raised, during the fourth quarter of 2019, net proceeds of $2.1 million in connection with the sale of 1,692,289 shares of common stock under our equity distribution agreement.
2. Summary of Significant Accounting Policies
Basis of Presentation
The unaudited interim consolidated financial statements included herein have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (SEC). Accordingly, they do not include all information and disclosures necessary for a presentation of our financial position, results of operations and cash flows in conformity with generally accepted accounting principles in the United States of America (GAAP) for annual financial statements. In the opinion of management, these unaudited interim consolidated financial statements reflect the elimination of all intercompany accounts and transactions and all adjustments, consisting primarily of normal recurring accruals, necessary for a fair presentation of our financial position and results of operations and cash flows for the periods presented. The results of operations for interim periods are not necessarily indicative of the results for the full year. These unaudited interim consolidated financial statements should be read in conjunction with the audited consolidated financial statements for the year ended December 31, 2019 and accompanying notes thereto included in our annual report on Form 10-K filed with the SEC on March 16, 2020.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of income and expenses during the reporting period. Actual results could differ from such estimates.
New Accounting Pronouncements
In June 2016, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The ASU replaces the incurred loss impairment methodology in current GAAP with a methodology that reflects expected credit losses and requires consideration of a broader range of reasonable and supportable information to inform credit loss estimates. We adopted the ASU effective as of January 1, 2020.
Our cash equivalents and short-term investments are accounted for as available-for-sale debt instruments and certificates of deposit, recorded at fair value. Interest income on these instruments is recorded as “Other income” on the interim consolidated statements of operations and comprehensive loss. We have never experienced a credit loss on the principal or interest receivable of our cash equivalents or short-term investments. Our available-for-sale debt securities represent United States (U.S.) treasury securities, and our certificates of deposit are each individually and fully insured by the Federal Deposit Insurance Corporation (FDIC). Accordingly, we did not measure an allowance for credit losses on these securities and we did not record a cumulative-effect adjustment to accumulated deficit during the three months ended March 31, 2020 upon adoption of ASU No. 2016-13.
3. Fair Value Measurements
FASB accounting guidance defines fair value as the price that would be received to sell an asset or paid to transfer a liability (the exit price) in an orderly transaction between market participants at the measurement date. The accounting guidance outlines a valuation framework and creates a fair value hierarchy in order to increase the consistency and comparability of fair value measurements and the related disclosures. In determining fair value, we use
8
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
quoted prices and observable inputs. Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from independent sources.
The fair value hierarchy is broken down into three levels based on the source of inputs as follows:
|
· |
Level 1 — Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities. |
|
· |
Level 2 — Valuations based on observable inputs and quoted prices in active markets for similar assets and liabilities. |
|
· |
Level 3 — Valuations based on inputs that are unobservable and models that are significant to the overall fair value measurement. |
If the inputs used to measure fair value fall within different levels of the hierarchy, the category level is based on the lowest priority level input that is significant to the fair value measurement of the instrument.
Valuation Techniques - Level 2 Inputs
We estimate the fair values of our financial instruments categorized as level 2 in the fair value hierarchy, including U.S. Treasury securities, by taking into consideration valuations obtained from third-party pricing services. The pricing services use industry standard valuation models, including both income- and market-based approaches, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include reported trades of and broker/dealer quotes on the same or similar securities, benchmark yields, issuer credit spreads, benchmark securities, and other observable inputs. We obtain a single price for each financial instrument and do not adjust the prices obtained from the pricing service. We validate the prices provided by our third-party pricing services by reviewing their pricing methods, obtaining market values from other pricing sources and comparing them to the share prices presented by the third-party pricing services. After completing our validation procedures, we did not adjust or override any fair value measurements provided by our third-party pricing services as of March 31, 2020.
The following fair value hierarchy table presents information about each major category of our financial assets and liabilities measured at fair value on a recurring basis (in thousands):
|
|
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|
||||
|
March 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds (cash equivalents) |
|
$ |
60,095 |
|
$ |
— |
|
$ |
— |
|
$ |
60,095 |
|
|
Certificates of deposit (cash equivalents) |
|
|
498 |
|
|
— |
|
|
— |
|
|
498 |
|
|
Certificates of deposit |
|
|
5,446 |
|
|
— |
|
|
— |
|
|
5,446 |
|
|
U.S. Treasury securities |
|
|
— |
|
|
2,015 |
|
|
— |
|
|
2,015 |
|
|
Total assets |
|
$ |
66,039 |
|
$ |
2,015 |
|
$ |
— |
|
$ |
68,054 |
|
|
December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market funds (cash equivalents) |
|
$ |
85,395 |
|
$ |
— |
|
$ |
— |
|
$ |
85,395 |
|
|
Certificates of deposit |
|
|
739 |
|
|
— |
|
|
— |
|
|
739 |
|
|
Total assets |
|
$ |
86,134 |
|
$ |
— |
|
$ |
— |
|
$ |
86,134 |
|
9
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Accrued Expenses
Accrued expenses consisted of the following (in thousands):
|
|
|
March 31, |
|
December 31, |
|
||
|
|
|
2020 |
|
2019 |
|
||
|
Payroll and related costs |
|
$ |
1,349 |
|
$ |
2,514 |
|
|
Clinical trials and drug development |
|
|
5,047 |
|
|
1,849 |
|
|
Professional fees |
|
|
574 |
|
|
396 |
|
|
Short-term lease liabilities |
|
|
462 |
|
|
446 |
|
|
Other |
|
|
47 |
|
|
63 |
|
|
Total accrued expenses |
|
$ |
7,479 |
|
$ |
5,268 |
|
5. Investments
As of March 31, 2020, our short-term investments consisted of U.S. Treasury securities, maturing at various dates through November 2020, and certificates of deposit with various financial institutions maturing at various dates through February 2021. U.S. Treasury securities are classified as short-term investments on our interim consolidated balance sheets and certificates of deposit are classified as short-term investments or cash equivalents on our interim consolidated balance sheets. U.S Treasury securities and certificates of deposit are classified as available-for-sale and are recorded at fair value. The total fair value of our available-for-sale securities as of March 31, 2020 was $7.5 million.
6. Loss Per Share of Common Stock
Basic loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as convertible preferred stock, convertible notes payable, warrants, stock options, and unvested restricted stock, which would result in the issuance of incremental shares of common stock. In computing the basic and diluted net loss per share applicable to common stockholders, the weighted average number of shares remains the same for both calculations due to the fact that when a net loss exists, dilutive shares are not included in the calculation. These potentially dilutive securities are more fully described in Note 7, and summarized in the table below:
|
|
|
March 31, |
|||
|
|
|
2020 |
|
2019 |
|
|
Convertible preferred stock |
|
24,000,000 |
|
— |
|
|
Restricted stock |
|
21,000 |
|
32,400 |
|
|
Stock options |
|
13,056,165 |
|
6,492,571 |
|
|
|
|
37,077,165 |
|
6,524,971 |
|
7. Stockholders’ Equity
In 2005, we adopted the 2005 Stock Option and Incentive Plan (2005 Plan) that authorizes us to grant options, restricted stock and other equity-based awards. As of March 31, 2020, 327,421 options to purchase shares of common stock were outstanding pursuant to grants in connection with the 2005 Plan. No additional shares are available for issuance under the 2005 Plan.
In August 2014, we adopted our 2014 Equity Incentive Plan, amended in May 2017 (2014 Plan), that authorizes us to grant options, restricted stock, and other equity-based awards, subject to adjustment in accordance with the 2014 Plan. The amount, terms of grants, and exercisability provisions are determined and set by our board of directors. As of
10
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
March 31, 2020, 11,384,244 options to purchase shares of common stock and 21,000 shares of restricted stock were outstanding pursuant to grants in connection with the 2014 Plan, and 615,008 shares of common stock were available for future issuance. The amount, terms of grants, and exercisability provisions are determined and set by our board of directors.
Stock Options
There were 13,056,165 stock options outstanding as of March 31, 2020 at a weighted-average exercise price of $3.25 per share. During the three months ended March 31, 2020, 5,038,200 options were granted to employees and directors at a weighted-average exercise price of $2.04 per share. Of the options granted, 4,749,700 options were granted pursuant to the 2014 Plan and 288,500 were granted outside of the 2014 Plan as inducements for new employees.
Total compensation cost recognized for all stock option awards in the statements of operations is as follows (in thousands):
|
|
|
Three Months Ended |
|
|
||||
|
|
|
March 31, |
|
|
||||
|
|
|
2020 |
|
2019 |
|
|
||
|
Research and development |
|
$ |
729 |
|
$ |
649 |
|
|
|
General and administrative |
|
|
1,146 |
|
|
1,177 |
|
|
|
Total |
|
$ |
1,875 |
|
$ |
1,826 |
|
|
Restricted Stock
All issued and outstanding restricted shares of common stock are time-based, and become vested between one and three years after the grant date. Compensation expense is recorded ratably over the requisite service period. Compensation expense related to restricted stock is measured based on the fair value using the closing market price of our common stock on the date of the grant.
We issued 21,000 and 0 restricted shares of common stock during the three months ended March 31, 2020 and 2019, respectively. As of March 31, 2020 there were 21,000 restricted shares of common stock outstanding, and 32,400 shares vested during the three months ended March 31, 2020.
Total compensation cost recognized for all restricted stock awards in the statements of operations is as follows (in thousands):
|
|
|
|
Three Months Ended |
|
||||
|
|
|
|
March 31, |
|
||||
|
|
|
|
2020 |
|
2019 |
|
||
|
Research and development |
|
|
$ |
— |
|
$ |
5 |
|
|
General and administrative |
|
|
|
1 |
|
|
5 |
|
|
Total |
|
|
$ |
1 |
|
$ |
10 |
|
Deemed Dividends
On March 31, 2020, our stockholders approved an amendment to our company charter with the Secretary of State of the State of Delaware to increase the the number of authorized shares of common stock, which resulted in the recognition of a beneficial conversion feature on the Series A convertible preferred stock. Accordingly, we recorded $8.9 million in deemed dividends in the three months ended March 31, 2020, which was calculated as the difference
11
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
between the conversion price and the fair value of our common stock on the commitment date (transaction date) in connection with the closing of concurrent equity financings during the fourth quarter of 2019.
8. Leases
We have entered into operating leases for real estate. These leases have terms which range from 36 to 78 months, and include renewal terms which can extend the lease terms by 24 to 60 months, which are included in the lease term when it is reasonably certain that we will exercise the option. As of March 30, 2020, our operating leases had a weighted average remaining lease term of 65 months. These right-of-use (ROU) assets are included in "Other assets" on our interim consolidated balance sheets as of March 31, 2020 and December 31, 2019, and represent our right to use the underlying asset for the lease term. Our obligations to make lease payments are included in "Accrued expenses" and "Other long-term liabilities" on our interim consolidated balance sheets as of March 31, 2020 and December 31, 2019. The ROU assets were initially measured at cost, which comprises the initial amount of the lease liability adjusted for lease payments made at or before the lease commencement date, plus any initial direct costs incurred, less any lease incentives received. The ROU assets are subsequently measured throughout the lease term at the carrying amount of the lease liability, plus initial direct costs, plus (minus) any prepaid (accrued) lease payments, less the unamortized balance of lease incentives received. Our ROU assets as of March 31, 2020 and December 31, 2019 have been adjusted for $0.9 million in lease incentives.
As of March 31, 2020 and December 31, 2019, ROU assets were $2.2 million at each period, and operating lease liabilities were $3.4 million and $3.5 million, respectively. We have entered into various short-term operating leases, primarily for clinical trial equipment, with an initial term of twelve months or less. These leases are not recorded on our consolidated balance sheets. All operating lease expense is recognized on a straight-line basis over the lease term. During the three months ended March 31, 2020 and December 31, 2019, we recognized $0.2 million and $0.7 million, respectively, in total lease costs, which included less than $0.1 million in short-term lease costs related to short-term operating leases.
Because the rate implicit in each lease is not readily determinable, we use our incremental borrowing rate to determine the present value of the lease payments. The weighted average incremental borrowing rate used to determine the initial value of ROU assets and lease liabilities was 11.0%, derived from a corporate yield curve based on a synthetic credit rating model using a market signal analysis. We have certain contracts for real estate which may contain lease and non-lease components which we have elected to treat as a single lease component.
ROU assets for operating leases are periodically reduced by impairment losses. We use the long-lived assets impairment guidance in Accounting Standards Codification (ASC) Subtopic 360-10, Property, Plant, and Equipment – Overall, to determine whether an ROU asset is impaired, and if so, the amount of the impairment loss to recognize. As of March 31, 2020 and December 31, 2019, we have not recognized any impairment losses for our ROU assets.
We monitor for events or changes in circumstances that require a reassessment of one of our leases. When a reassessment results in the remeasurement of a lease liability, a corresponding adjustment is made to the carrying amount of the corresponding ROU asset unless doing so would reduce the carrying amount of the ROU asset to an amount less than zero. In that case, the amount of the adjustment that would result in a negative ROU asset balance is recorded in our interim consolidated statements of operations and comprehensive loss.
12
MARINUS PHARMACEUTICALS, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Maturities of operating lease liabilities as of March 31, 2020 were as follows (in thousands):
|
|
|
|
|
|
|
|
|
|
|
|
|
Remaining nine months of 2020 |
|
$ |
608 |
|
|
2021 |
|
|
818 |
|
|
2022 |
|
|
807 |
|
|
2023 |
|
|
823 |
|
|
2024 |
|
|
840 |
|
|
Thereafter |
|
|
641 |
|
|
|
|
|
4,537 |
|
|
Less: imputed interest |
|
|
(1,154) |
|
|
Total lease liabilities |
|
$ |
3,383 |
|
|
|
|
|
|
|
|
Current operating lease liabilities |
|
$ |
462 |
|
|
Non-current operating lease liabilities |
|
|
2,921 |
|
|
Total lease liabilities |
|
$ |
3,383 |
|
9. Commitments
Severance Arrangements
In March 2019, we entered into a Severance Agreement and General Release (Severance Agreement) with Christopher M. Cashman (Cashman), our former Chief Executive Officer. In connection with this Severance Agreement, we agreed to pay certain severance benefits for one year to Cashman, consisting of base salary and benefits continuation and a prorated bonus totaling $0.6 million. As of March 31, 2020, all severance benefits have been paid to Mr. Cashman.
13
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Cautionary Note Regarding Forward-Looking Statements
This Quarterly Report on Form 10-Q contains forward-looking statements, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, that involve substantial risks and uncertainties. In some cases, you can identify forward-looking statements by the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” “will,” or “would,” and or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this Quarterly Report on Form 10-Q, we caution you that these statements are based on a combination of facts and factors currently known by us and our expectations of the future, about which we cannot be certain.
The forward-looking statements in this Quarterly Report on Form 10-Q include, among other things, statements about:
|
· |
our ability to develop and commercialize ganaxolone; |
|
· |
status, timing and results of preclinical studies and clinical trials; |
|
· |
design of and enrollment in clinical trials, availability of data from ongoing clinical trials, expectations for regulatory approvals, or the attainment of clinical trial results that will be supportive of regulatory approvals; |
|
· |
the potential benefits of ganaxolone; |
|
· |
the timing of seeking marketing approval of ganaxolone; |
|
· |
our ability to obtain and maintain marketing approval; |
|
· |
our estimates of expenses and future revenue and profitability; |
|
· |
our estimates regarding our capital requirements and our needs for additional financing; |
|
· |
our plans to develop and market ganaxolone and the timing of our development programs; |
|
· |
our estimates of the size of the potential markets for ganaxolone; |
|
· |
our selection and licensing of ganaxolone; |
|
· |
our ability to attract collaborators with acceptable development, regulatory and commercial expertise; |
|
· |
the benefits to be derived from corporate collaborations, license agreements, and other collaborative or acquisition efforts, including those relating to the development and commercialization of ganaxolone; |
|
· |
sources of revenue, including contributions from corporate collaborations, license agreements, and other collaborative efforts for the development and commercialization of ganaxolone and our product candidates; |
|
· |
our ability to create an effective sales and marketing infrastructure if we elect to market and sell ganaxolone directly; |
14
|
· |
the rate and degree of market acceptance of ganaxolone; |
|
· |
the timing and amount of reimbursement for ganaxolone; |
|
· |
the success of other competing therapies that may become available; |
|
· |
the manufacturing capacity for ganaxolone; |
|
· |
our intellectual property position; |
|
· |
our ability to maintain and protect our intellectual property rights; |
|
· |
our results of operations, financial condition, liquidity, prospects, and growth strategies; |
|
· |
the industry in which we operate; |
|
· |
the extent to which our business may be adversely impacted by the effects of the COVID-19 coronavirus pandemic or by other pandemics, epidemics or outbreaks; and |
|
· |
the trends that may affect the industry or us. |
You should refer to Part II Item 1A. “Risk Factors” of this Quarterly Report on this Form 10-Q for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Quarterly Report on Form 10-Q will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this Quarterly Report on Form 10-Q and the documents that we reference in this Quarterly Report on Form 10-Q and have filed as exhibits to this Quarterly Report on Form 10-Q completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with: (i) the interim consolidated financial statements and related notes thereto which are included in this Quarterly Report on Form 10-Q; and (ii) our annual consolidated financial statements for the year ended December 31, 2019 which are included in our Annual Report on Form 10-K filed with the SEC on March 16, 2020.
Overview
We are a clinical stage pharmaceutical company focused on developing and commercializing innovative therapeutics to treat patients suffering from rare seizure disorders. Our clinical stage product candidate, ganaxolone, is a positive allosteric modulator of GABAA that is being developed in formulations for two different routes of administration: intravenous (IV) and oral. Ganaxolone is a synthetic analog of allopregnanolone, an endogenous neurosteroid. The different formulations are intended to maximize potential therapeutic applications of ganaxolone for adult and pediatric patient populations, in both acute and chronic care, and for both in‑patient and self‑administered settings. Ganaxolone acts at both synaptic and extrasynaptic GABAA receptors, a target known for its anti‑seizure, antidepressant and anxiolytic potential.
15
Our Pipeline
We are developing ganaxolone in indications where there is a mechanistic rationale for ganaxolone to provide a benefit, including the following indications:
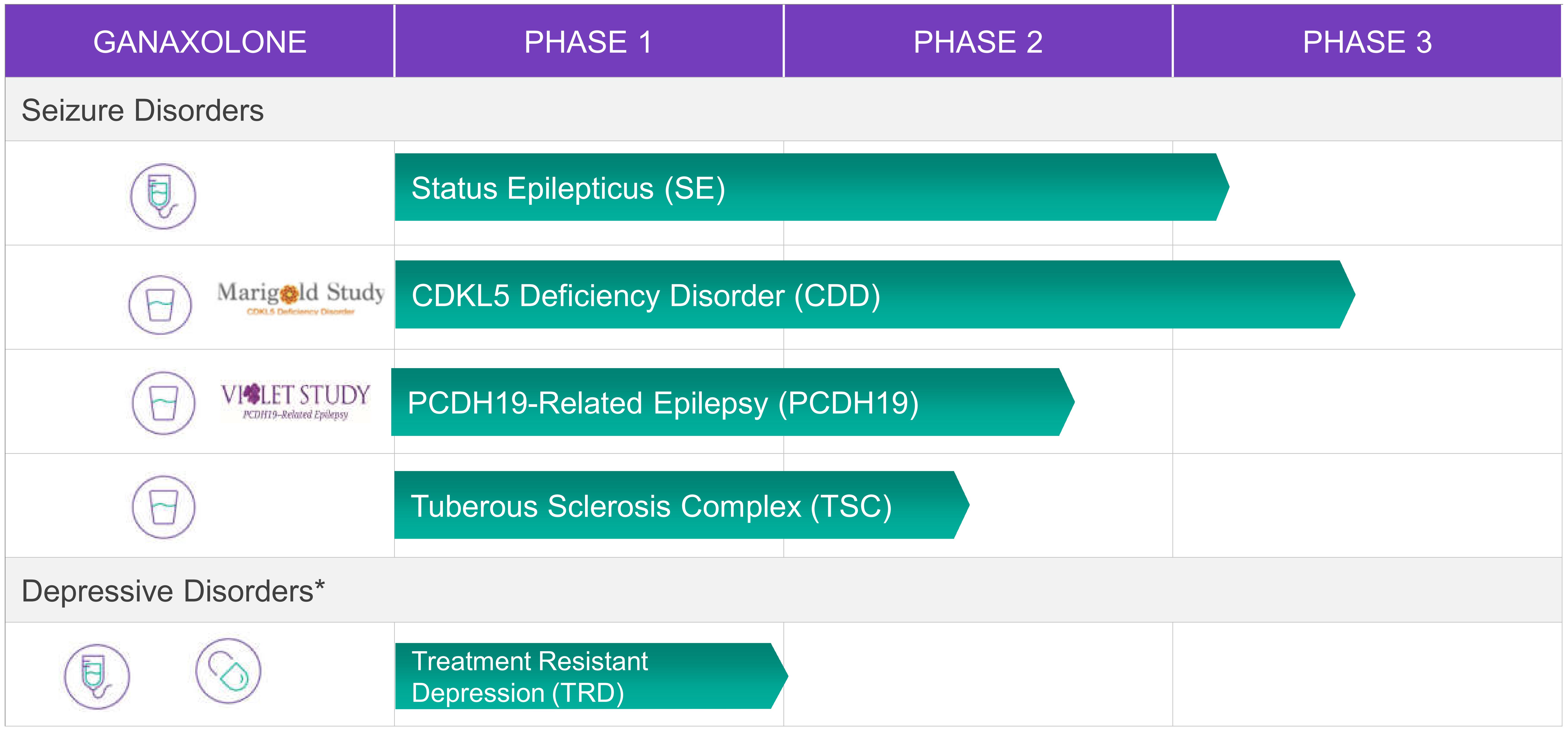
*Programs on hold pending decision to conduct additional trials.
Status Epilepticus (SE)
Status epilepticus (SE) is a life‑threatening occurrence of continuous or intermittent seizures lasting more than five minutes in duration without recovery of consciousness. If SE is not treated immediately, permanent neuronal damage may occur, which contributes to high rates of morbidity and mortality. SE patients who do not respond to first-line treatment and one second-line antiepileptic drug (AED) are classified as having refractory SE. In refractory SE, certain synaptic GABAA receptors are internalized into the neuron, and therefore are unavailable to drugs that target them, such as benzodiazepines. Refractory SE patients who fail to respond to at least two AEDs may be given an IV anesthetic to stop seizures and avoid neuronal injury. Patients who remain in SE after an attempt to wean IV anesthesia are referred to as having super refractory status epilepticus (SRSE).
During the first quarter of 2020, we completed an end-of-phase 2 meeting with the Food and Drug Administration (FDA), and are making preparations to initiate a Phase 3 pivotal clinical trial in SE in the third quarter of 2020. We plan to examine co-primary endpoints that focus on status cessation within 30-minutes and suppression of status for at least 24 hours. Ganaxolone will be administered intravenously for 48 hours, the first 12 hours of which is expected to target a 500 ng/ml serum concentration. We anticipate the trial will enroll approximately 125 patients to provide greater than 90 percent power to detect a 30 percent efficacy difference between ganaxolone and placebo. We expect to enroll patients who have previously failed a benzodiazepine and at least two second-line antiepileptic drugs. Topline data are expected in the first half of 2022.
CDKL5 Deficiency Disorder (CDD)
CDD is a serious and rare genetic disorder that is caused by a mutation of the cyclin‑dependent kinase‑like 5 (CDKL5) gene, located on the X chromosome. It predominantly affects females and is characterized by early‑onset, difficult‑to‑control seizures and severe neuro‑developmental impairment. The CDKL5 gene encodes proteins essential for normal brain function. Most children affected by CDD cannot walk normally, talk, or care for themselves. Many also suffer from scoliosis, visual impairment, gastrointestinal difficulties and sleep disorders. There are no approved therapies
16
or treatments for CDD. Genetic testing is available to determine if a patient has a mutation in the CDKL5 gene. To our knowledge, no previous late-stage clinical trials have been conducted in this patient population.
During the first quarter of 2020, we completed enrollment in a pivotal Phase 3 clinical trial (Marigold Study), which is evaluating the use of oral ganaxolone in children and young adults with CDD. The Marigold Study is a global, double‑blind, placebo‑controlled, clinical trial that has enrolled 101 patients between the ages of 2 and 21 with a confirmed disease‑related CDKL5 gene variant. Patients randomized to ganaxolone receive up to 600 mg of oral liquid suspension three times a day. The primary endpoint of the trial is percent change in 28‑day seizure frequency. The discontinuation rate in this trial is in-line with expectations (less than 10 percent), and enrollment in the open label extension part of the trial continues to be high. We expect to report top‑line data from the clinical trial in the the third quarter of 2020, with no material delays presently expected due to COVID-19. We have also begun preparations for a potential New Drug Application (NDA) filing and development of a commercial strategy. Phase 1 supportive clinical trials have experienced delays in enrollment due to COVID-19, which are not expected to impact timing for a potential NDA filing.
Tuberous Sclerosis Complex (TSC)
TSC is a rare genetic disorder that affects many organs and causes non‑malignant tumors in the brain, skin, kidney, heart, eyes, and lungs. The condition is caused by inherited mutations in either the TSC1 gene or the TSC2 gene. TSC occurs with a frequency of 1:6,000 and a mutation is found in 85% of patients. While the disease phenotype can be extremely variable, neurologic manifestations such as epilepsy can be seen in up to 90% of TSC patients. TSC is a leading cause of genetic epilepsy, often occurring in the first year of life as either focal seizures or infantile spasms. There are currently limited disease-specific treatments approved for seizure types that occur in TSC patients.
We are conducting a Phase 2 open‑label trial to evaluate the safety and tolerability of adjunctive ganaxolone treatment in patients with TSC. The trial is expected to enroll approximately 30 patients ages 2 to 65. Patients will undergo a four‑week baseline period followed by a 12‑week treatment period where they will receive up to 600 mg of ganaxolone (oral liquid suspension) three times a day. Patients who meet eligibility criteria may continue ganaxolone treatment in a 24‑week extension to the trial. The primary endpoint for the trial is percent change in 28‑day primary seizure frequency for the treatment period relative to baseline. We also plan to analyze allopregnanolone sulfate levels as part of the trial efficacy analysis. We do not currently anticipate any material impact on enrollment or trial progress due to COVID-19, and expect to report top line data in the first quarter of 2021.
PCDH19-Related Epilepsy (PCDH19-RE)
PCDH19‑RE is a rare and serious epileptic syndrome characterized by early‑onset seizures, cognitive and sensory impairment of varying degrees, and psychiatric and behavioral disturbances. Seizures occur in clusters lasting from several hours to days. It is caused by a mutation in the PCDH19 gene on the X-chromosome. Unlike other X-linked disorders, it selectively affects females, with very few cases reported in males. The gene encodes a protein involved in cell adhesion and is widely expressed in the central nervous system. Genetic testing is available to determine if a patient has the PCDH19 mutation. Currently, there are no drugs approved specifically for the general treatment of various seizures associated with PCDH19-RE.
In April 2020, we decided to transition the ongoing Phase 3 clinical trial (Violet Study) to a Phase 2 proof-of-concept (POC) clinical trial evaluating allopregnanolone sulfate as a biomarker in the patients with a confirmed PCDH19-RE mutation. We have decided to limit trial enrollment due to several factors, including the challenges to enroll a global clinical trial (including COVID-19 impact), the episodic nature of seizures in PCDH-19 patients, and potential commercial challenges. We now expect to complete the double-blind portion of the clinical trial with approximately 15-20 patients, and we plan to continue to evaluate if the allopregnanolone sulfate biomarker hypothesis could have broader utility in other targeted indications. The POC biomarker clinical trial will stratify patients into one of two biomarker groups based on baseline allopregnanolone sulfate levels and randomized (ganaxolone or placebo) within each stratum. The trial will consist of a 12‑week prospective baseline period to collect seizure data, followed by a 17‑week double‑blind treatment phase. Patients randomized to ganaxolone will titrate over four weeks to a dose of up to 600 mg of ganaxolone (oral liquid suspension) three times a day and maintain that dose for the following 13‑weeks.
17
We will continue to develop a program to ensure access to ganaxolone for PCDH19-RE patients who saw benefits in the Violet Study and expect to announce results of this POC clinical trial in the first half of 2021.
COVID-19
In December 2019, an outbreak of a novel strain of coronavirus (COVID-19) was identified in Wuhan, China. This virus has been declared a pandemic by the World Health Organization and has spread to other countries, including the United States. Efforts to contain the spread of COVID-19 have intensified and many countries, including the United States, have implemented severe travel restrictions, business shutdowns and social distancing measures that have impacted clinical development through supply chain shortages and clinical trial enrollment difficulties as hospitals reduce and divert staffing, divert resources to patients suffering from the infectious disease and limit hospital access for non-patients. The outbreak of COVID-19 poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting normal business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities.
The continued spread of COVID-19 globally has impacted our operations but did not have a material impact on our business, operating results, financial condition or cash flows as of and for the three months ended March 31, 2020. For example, our Phase 1 supportive clinical trials of oral ganaxolone in CDD have experienced delays in enrollment due to COVID-19. Further, in response to COVID-19, for our ongoing clinical trials, we have implemented multiple measures consistent with the U.S. Food and Drug Administration’s guidance on the conduct of clinical trials of medical products during the COVID-19 pandemic, including implementing remote site monitoring and remote visits using telemedicine where needed. Although operations have not been materially affected by the COVID-19 pandemic as of and for the three months ended March 31, 2020, we are unable to predict the impact that COVID-19 will have in the future on our business, financial position, operating results and cash flows due to numerous uncertainties. The duration and severity of the pandemic and its long-term impact on our business are uncertain at this time, and our ability to raise sufficient additional financing depends on many factors beyond our control, including the current volatility in the capital markets as a result of the COVID-19 pandemic.
Operations
Our operations to date have consisted primarily of organizing and staffing our company and developing ganaxolone, including conducting preclinical studies, clinical trials and raising capital. We have funded our operations primarily through sales of equity and debt securities. At March 31, 2020, we had cash, cash equivalents and investment balances of $77.8 million. We have no products currently available for sale, have incurred operating losses since inception, have not generated any product sales revenue and have not achieved profitable operations. We incurred a net loss of $18.7 million and $12.5 million for the three months ended March 31, 2020 and 2019, respectively. Our accumulated deficit as of March 31, 2020 was $263.1 million, and we expect to continue to incur substantial losses in future periods. We anticipate that our operating expenses will increase substantially as we continue to advance our clinical-stage product candidate, ganaxolone.
We anticipate that our expenses will increase substantially as we:
conduct later stage clinical trials in targeted indications, which could include SE, CDD, TSC, PCDH19-RE and possibly other indications;
|
· |
continue the research, development and scale-up manufacturing capabilities to optimize ganaxolone and dose forms for which we may obtain regulatory approval; |
|
· |
conduct other preclinical studies and clinical trials to support the filing of New Drug Applications (NDAs) with the Food and Drug Administration (FDA) and other regulatory agencies in other countries; |
|
· |
acquire the rights to other product candidates and fund their development; |
|
· |
maintain, expand and protect our global intellectual property portfolio; |
18
|
· |
hire additional clinical, manufacturing and scientific personnel; and |
|
· |
add operational, financial and management information systems and personnel, including personnel to support our drug development and potential future commercialization efforts. |
We believe that our cash, cash equivalents and investment balances as of March 31, 2020, will enable us to fund our operating expenses and capital expenditure requirements into the third quarter of 2021. However, we will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, licensing transactions, other commercial transactions or other sources, in order to carry out all of our planned research and development activities with respect to ganaxolone.
Financial Overview
Research and Development Expenses
Our research and development expenses consist primarily of costs incurred for the development of ganaxolone, which include:
employee-related expenses, including salaries, benefits, travel and stock-based compensation expense;
expenses incurred under agreements with clinical research organizations (CROs) and investigative sites that conduct our clinical trials and preclinical studies;
the cost of acquiring, developing and manufacturing clinical trial materials;
facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other supplies; and
costs associated with preclinical activities and regulatory operations.
We expense research and development costs when we incur them. We record costs for some development activities, such as clinical trials, based on an evaluation of the progress to completion of specific tasks using data such as patient enrollment, clinical site activations and information our vendors provide to us.
We will incur substantial costs beyond our present and planned clinical trials in order to file an NDA and Supplemental New Drug Applications (sNDAs), or equivalent Marketing Authorization Applications (MAA) outside the US, for ganaxolone for various clinical indications, and in each case, the nature, design, size and cost of further clinical trials and other studies will depend in large part on the outcome of preceding studies and trials and discussions with regulators. It is difficult to determine with certainty the costs and duration of our current or future clinical trials and preclinical studies, or if, when or to what extent we will generate revenue from the commercialization and sale of ganaxolone if we obtain regulatory approval. We may never succeed in achieving regulatory approval for ganaxolone. The duration, costs and timing of clinical trials and development of ganaxolone will depend on a variety of factors, including the uncertainties of future clinical trials and preclinical studies, uncertainties in clinical trial enrollment rate and significant and changing government regulation.
In addition, the probability of success for our clinical programs will depend on numerous factors, including competition, manufacturing capability and commercial viability. See “Risk Factors.” Our commercial success depends upon attaining significant market acceptance, if approved, among physicians, patients, healthcare payers and the medical community. We will determine which programs to pursue and how much to fund each program in response to the scientific and clinical success, as well as an assessment of commercial potential.
19
General and Administrative Expenses
General and administrative expenses consist principally of salaries and related costs for executive and other administrative personnel and consultants, including stock-based compensation and travel expenses. Other general and administrative expenses include professional fees for legal, patent review, consulting and accounting services. General and administrative expenses are expensed when incurred. We expect that our general and administrative expenses will increase in the future as a result of employee hiring and our scaling-up of operations commensurate with supporting more advanced clinical trials and in preparation for commercial infrastructure. These increases will likely include increased costs for insurance, hiring of additional personnel, outside consultants, legal counsel and accountants, among other expenses.
Interest Income
Interest income consists principally of interest income earned on cash and cash equivalents and investment balances.
Results of Operations
Research and Development Expenses
We allocate direct research and development expenses, consisting principally of external costs, such as fees paid to investigators, consultants, central laboratories and CROs in connection with our clinical trials, and costs related to manufacturing or purchasing clinical trial materials, to specific product programs. We do not allocate employee and contractor-related costs, costs associated with our facility expenses, including depreciation or other indirect costs, to specific product programs because these costs are deployed across multiple product programs under research and development and, as such, are separately classified. The table below shows our research and development expenses incurred with respect to each active program, in thousands. The primary drivers of our research and development expenditures are currently in our programs in SE, CDD, TSC and PCDH19-RE. We expect our research and development expenses for ganaxolone will continue to increase during subsequent periods. We did not allocate research and development expenses to any other specific product programs during the periods presented (in thousands):
|
|
|
Three Months Ended |
|
|
||||
|
|
|
March 31, |
|
|
||||
|
|
|
2020 |
|
2019 |
|
|
||
|
CDKL5 deficiency disorder (1) |
|
$ |
4,010 |
|
$ |
1,314 |
|
|
|
Postpartum depression (2) |
|
|
— |
|
|
2,461 |
|
|
|
Status epilepticus (3) |
|
|
4,792 |
|
|
507 |
|
|
|
PCDH19-related epilepsy (4) |
|
|
1,562 |
|
|
1,331 |
|
|
|
Tuberous Sclerosis (5) |
|
|
306 |
|
|
— |
|
|
|
Indirect research and development (6) |
|
|
4,334 |
|
|
3,259 |
|
|
|
Total |
|
$ |
15,004 |
|
$ |
8,872 |
|
|
Note: Certain prior year expenses have been reclassified to conform to current year presentation.
|
(1) |
The increase is due to enhanced drug development activity, including clinical trials and preclinical studies and manufacturing activities in preparation for commercialization. |
|
(2) |
We completed our clinical trials in PPD in 2019, and have placed further development on hold pending a decision to conduct additional trials based upon regulatory interactions and funding. |
|
(3) |
The increase is due primarily to enhanced drug development activity, including preclinical studies and manufacturing activities in preparation for a Phase 3 clinical trial expected to commence in the third quarter of 2020. |
20
|
(4) |
Expenses for this indication were consistent with the prior year as the clinical trial was continuing to enroll until we decided to revise the nature of the trial in April 2020. |
|
(5) |
We began making preparations for a Phase 2 clinical trial in TSC during the first quarter of 2020. |
|
(6) |
Indirect research and development expenses in support of all our programs have increased due to the overall increase in preclinical, clinical, and manufacturing activities. |
General and Administrative Expenses
General and administrative expenses were $3.9 million and $3.7 million for the three months ended March 31, 2020 and 2019, respectively. The primary drivers of the decrease was $1.0 million in severance expenses related to the departure of Christopher M. Cashman, our former Chief Executive Officer, recorded in 2019, partially offset by an increase of approximately $0.6 million in noncash stock-based compensation expense.
Liquidity and Capital Resources
Since inception, we have incurred net losses and negative cash flows from our operations. We incurred net losses of $18.7 million and $12.5 million for the three months ended March 31, 2020 and 2019, respectively. Our cash used in operating activities was $14.0 million for the three months ended March 31, 2020 compared to $11.7 million for the same period a year ago. Historically, we have financed our operations principally through the sale of common stock, preferred stock and convertible debt, and the use of term loans. At March 31, 2020, we had cash, cash equivalents and investment balances of $77.8 million.
In October 2017, we entered into an Equity Distribution Agreement (EDA) with JMP Securities LLC (JMP), under which JMP, as our exclusive agent, at our discretion and at such times that we may determine from time to time, may sell over a three-year period from the execution of the agreement up to a maximum of $50 million of shares of our common stock. During the year ended December 31, 2019, we issued 1,692,289 shares of our common stock pursuant to the EDA for aggregate net proceeds to us of $2.2 million. We did not issue any shares of our common stock pursuant to the EDA during the three months ended March 31, 2020.
Cash Flows
Operating Activities. Cash used in operating activities increased to $14.0 million for the three months ended March 31, 2020 compared to $11.7 million for the same period a year ago. The increase was driven primarily by a $6.2 million increase in net loss, offset by an increase of $3.6 million in the change in our operating accounts payable and accrued expenses, which was due primarily to increased research and development activities as described above.
Investing Activities. Cash used in investing activities for the three months ended March 31, 2020 represents the maturity of short-term investments of $1.7 million offset by $8.4 million in purchases of investments. Cash provided by investing activities for the three months ended March 31, 2019 represents $5.0 million in maturities of short-term investments offset by $0.1 million in cash paid for property, plant and equipment.
Financing Activities. Cash provided by financing activities for the three months ended March 31, 2020 represents $0.3 million in proceeds from the exercise of stock options, offset by payment of $0.2 million for financing costs. Cash used in financing activities for the three months ended March 31, 2019 represents $0.1 million in proceeds from the sale of stock options, offset by payment of $0.1 million for financing costs.
Funding Requirements
We have not achieved profitability since our inception, and we expect to continue to incur net losses for the foreseeable future. We expect our cash expenditures to increase in the near term as we fund our continuing and planned clinical trials for ganaxolone.
21
We believe that our cash, cash equivalents and investments as of March 31, 2020, will enable us to fund our operating expenses and capital expenditure requirements into the third quarter of 2021. However, we will need to raise substantial additional financing in the future to fund our operations. In order to meet these additional cash requirements, we may seek to sell additional equity or convertible debt securities that may result in dilution to our stockholders. If we raise additional funds through the issuance of convertible debt securities, these securities could have rights senior to those of our common stock and could contain covenants that restrict our operations. There can be no assurance that we will be able to obtain additional equity or debt financing on terms acceptable to us, if at all. Further, the continued spread of COVID-19 has also led to severe disruption and volatility in the global capital markets, which could increase our cost of capital and adversely affect our ability to access the capital markets in the future. Our failure to obtain sufficient funds on acceptable terms when needed could have a negative impact on our business, results of operations, and financial condition.
Our future capital requirements will depend on many factors, including:
|
· |
the effects of the COVID-19 pandemic on our business, the medical community and the global economy; |
|
· |
the results of our preclinical studies and clinical trials; |
|
· |
the development, formulation and commercialization activities related to ganaxolone; |
|
· |
the scope, progress, results and costs of researching and developing ganaxolone or any other future product candidates, and conducting preclinical studies and clinical trials; |
|
· |
the timing of, and the costs involved in, obtaining regulatory approvals for ganaxolone or any other future product candidates; |
|
· |
the cost of commercialization activities if ganaxolone or any other future product candidates are approved for sale, including marketing, sales and distribution costs; |
|
· |
the cost of manufacturing and formulating ganaxolone, or any other future product candidates, to internal and regulatory standards for use in preclinical studies, clinical trials and, if approved, commercial sale; |
|
· |
our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of such agreements; |
|
· |
any product liability, infringement or other lawsuits related to our product candidates and, if approved, products; |
|
· |
capital needed to attract and retain skilled personnel; |
|
· |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; and |
|
· |
the timing, receipt and amount of sales of, or royalties on, future approved products, if any. |
Please see “Risk Factors” for additional risks associated with our substantial capital requirements.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
22
Discussion of Critical Accounting Policies and Significant Judgments and Estimates
The preparation of financial statements in conformity with GAAP requires us to use judgment in making certain estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities and the reported amounts of revenues and expenses in our financial statements and accompanying notes. Critical accounting policies are those that are most important to the portrayal of our financial condition and results of operations and require difficult, subjective and complex judgments by management in order to make estimates about the effect of matters that are inherently uncertain. During the three months ended March 31, 2020, there were no significant changes to our critical accounting policies from those described in our annual financial statements for the year ended December 31, 2019, which we included in our Annual Report on Form 10-K and was filed with the SEC on March 16, 2020.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended (Exchange Act) and are not required to provide the information under this item.
Item 4. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Quarterly Report on Form 10-Q to ensure that the information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosures. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost benefit relationship of possible controls and procedures. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were not effective at the reasonable assurance level as of March 31, 2020 due to the material weakness described in Item 9A of the Annual Report on Form 10-K filed with the SEC on March 16, 2020 not having been fully remediated by the first quarter of 2020.
Notwithstanding the identified material weakness and management’s assessment that our disclosure controls and procedures were not effective at the reasonable assurance level as of March 31, 2020, management believes that the interim consolidated financial statements included in this Quarterly Report on Form 10-Q fairly present, in all material respects, our financial condition, results of operations and cash flows as of and for the periods presented in accordance with generally accepted accounting principles.
(b) Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act that occurred during the quarter ended March 31, 2020 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
23
OTHER INFORMATION
From time to time, we may become subject to litigation and claims arising in the ordinary course of business. We are not currently a party to any material legal proceedings, and we are not aware of any pending or threatened legal proceedings against us that we believe could have a material adverse effect on our business, operating results or financial condition.
This section augments and updates certain risk factors disclosed in Item 1A of Part I of our Annual Report on Form 10-K for the year ended December 31, 2019 (the “Annual Report”). The following risk factor should be read together with the other risk factors disclosed in the Annual Report. In addition to the other information in this Quarterly Report on Form 10-Q, all of the risk factors should be carefully considered in evaluating us and our common stock. Any of these risks, many of which are beyond our control, could materially and adversely affect our financial condition, results of operations or cash flows, or cause our actual results to differ materially from those projected in any forward-looking statements. We may also face other risks and uncertainties that are not presently known, are not currently believed to be material, or are not identified below because they are common to all businesses. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. For more information, see “Cautionary Note Regarding Forward-Looking Statements” in this Quarterly Report on Form 10-Q.
The COVID-19 coronavirus pandemic could adversely affect our business.
In December 2019, an outbreak of a novel strain of coronavirus (COVID-19) was identified in Wuhan, China. This virus has been declared a pandemic by the World Health Organization and has spread to other countries, including the United States. Efforts to contain the spread of COVID-19 have intensified and many countries, including the United States, have implemented severe travel restrictions, business shutdowns and social distancing measures that have impacted clinical development through supply chain shortages and clinical trial enrollment difficulties as hospitals reduce and divert staffing, divert resources to patients suffering from the infectious disease and limit hospital access for non-patients. The outbreak of COVID-19 poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting normal business activities for an indefinite period of time, including due to shutdowns that may be requested or mandated by governmental authorities.
The continued spread of COVID-19 globally has impacted our operations and such impacts may continue and become material to our operations. For example, our Phase 1 supportive clinical trials of oral ganaxolone in CDD have experienced delays in enrollment due to COVID-19. Further, in response to COVID-19, for our ongoing clinical trials, we have implemented multiple measures consistent with the FDA’s guidance on the conduct of clinical trials of medical products during the COVID-19 pandemic, including implementing remote site monitoring and remote visits using telemedicine where needed.
There is also a risk that clinical supplies of our product candidates may be significantly delayed or may become unavailable as a result of COVID-19 and the resulting impact on our suppliers’ labor forces and operations, including as a result of governmental restrictions on business operations and the movement of people and goods in an effort to curtail the spread of the virus. There can be no assurance that we would be able to timely implement any mitigation plans. Disruptions in our supply chain, whether as a result of restricted travel, quarantine requirements or otherwise, could negatively impact clinical supplies of our product candidates, which could materially adversely impact our clinical trial and development timelines.
The continued spread of COVID-19 has also led to severe disruption and volatility in the global capital markets, which could increase our cost of capital and adversely affect our ability to access the capital markets in the future. It is
24
likely that the continued spread of COVID-19 will cause an economic slowdown or recession or cause other unpredictable events, each of which could adversely affect our business, results of operations or financial condition.
The extent to which COVID-19 impacts our business will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the COVID-19 outbreak and the actions to contain the outbreak or treat its impact, among others. Moreover, the COVID-19 outbreak has begun to have indeterminable adverse effects on general commercial activity and the world economy, and our business and results of operations could be adversely affected to the extent that COVID-19 or any other pandemic harms the global economy generally.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
None.
|
Exhibit |
|
Exhibit Description |
|
|
|
|
|
3.1 |
|
|
|
|
|
|
|
3.2 |
|
|
|
|
|
|
|
3.3 |
|
|
|
|
|
|
|
3.4 |
|
|
|
4.1 |
|
|
|
|
|
|
|
4.2 |
|
|
|
31.1 |
|
25
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
101.LAB |
|
XBRL Taxonomy Extension Labels Linkbase Document |
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
26
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ SCOTT BRAUNSTEIN |
|
President and Chief Executive Officer (principal executive officer) and Director |
|
May 4, 2020 |
|
Scott Braunstein |
|
|||
|
|
|
|
|
|
|
/s/ EDWARD F. SMITH |
|
Vice President, Chief Financial Officer, Treasurer and Secretary (principal financial and accounting officer) |
|
May 4, 2020 |
|
Edward F. Smith |
|
27
