Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Green Thumb Industries Inc. | d853417dex322.htm |
| EX-32.1 - EX-32.1 - Green Thumb Industries Inc. | d853417dex321.htm |
| EX-31.2 - EX-31.2 - Green Thumb Industries Inc. | d853417dex312.htm |
| EX-31.1 - EX-31.1 - Green Thumb Industries Inc. | d853417dex311.htm |
| EX-23.2 - EX-23.2 - Green Thumb Industries Inc. | d853417dex232.htm |
| EX-23.1 - EX-23.1 - Green Thumb Industries Inc. | d853417dex231.htm |
| EX-16.1 - EX-16.1 - Green Thumb Industries Inc. | d853417dex161.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 000-56132
GREEN THUMB INDUSTRIES INC.
(Exact name of registrant as specified in its charter)
| British Columbia | 98-1437430 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. employer identification no.) | |
| 325 West Huron Street, Suite 412 Chicago, Illinois |
60654 | |
| (Address of principal executive offices) | (zip code) | |
Registrant’s telephone number, including area code - (312) 471-6720
Securities registered pursuant to Section 12(g) of the Act:
Subordinate Voting Shares
Multiple Voting Shares
Super Voting Shares
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
As of March 31, 2020, there were 144,810,322 shares of the registrant’s Subordinate Voting Shares, 23,850,400 shares of the registrant’s Multiple Voting Shares (on an as converted basis) and 39,081,400 of the registrant’s Super Voting Shares (on an as converted basis). Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the Subordinate Voting Shares, and Multiple Voting Shares and Super Voting Shares (on an as converted basis, based on the closing price of these shares on the Canadian Stock Exchange) on June 30, 2019, the last business day of the registrant’s most recently completed second fiscal quarter, held by nonaffiliates was $1,455,373,948
DOCUMENTS INCORPORATED BY REFERENCE
Part III incorporates certain information by reference from the definitive proxy statement to be filed by the registrant in connection with the 2020 Annual Meeting of Stockholders (the “2020 Proxy Statement”). The 2020 Proxy Statement will be filed by the registrant with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the year ended December 31, 2019.
Table of Contents
Table of Contents
Use of Names
In this Annual Report on Form 10-K, unless the context otherwise requires, the terms “we,” “us,” “our,” “Company,” “Corporation” or “GTI” refer to Green Thumb Industries Inc. together with its wholly-owned subsidiaries. References to “Bayswater” refer to the Company prior to completion of the Transaction (as hereinafter defined).
Currency
Unless otherwise indicated, all references to “$” or “US$” in this document refer to United States dollars, and all references to “C$” refer to Canadian dollars.
Disclosure Regarding Forward-Looking Statements
This Annual Report on Form 10-K contains statements that we believe are, or may be considered to be, “forward-looking statements.” All statements other than statements of historical fact included in this document regarding the prospects of our industry or our prospects, plans, financial position or business strategy may constitute forward-looking statements. In addition, forward-looking statements generally can be identified by the use of forward-looking words such as “may,” “will,” “expect,” “intend,” “estimate,” “foresee,” “project,” “anticipate,” “believe,” “plan,” “forecast,” “continue” or “could” or the negative of these terms or variations of them or similar terms. Furthermore, forward-looking statements may be included in various filings that we make with the Securities and Exchange Commission (the “SEC”) or press releases or oral statements made by or with the approval of one of our authorized executive officers. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that these expectations will prove to be correct. These forward-looking statements are subject to certain known and unknown risks and uncertainties, as well as assumptions that could cause actual results to differ materially from those reflected in these forward-looking statements. Readers are cautioned not to place undue reliance on any forward-looking statements contained in this document, which reflect management’s opinions only as of the date hereof. Except as required by law, we undertake no obligation to revise or publicly release the results of any revision to any forward-looking statements. You are advised, however, to consult any additional disclosures we make in our reports to the SEC. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained in this document.
1
Table of Contents
PART I
Background
Green Thumb Industries Inc. is a reporting issuer in Canada listed for trading on the Canadian Securities Exchange (“CSE”) under the symbol “GTII.” The Company’s Subordinate Voting Shares (as hereinafter defined) are also traded in the United States on the OTCQX Best Market (the “OTCQX”) under the symbol “GTBIF.”
Originally founded in 2014, GTI began operations in 2015 upon the award of a medical marijuana license for cultivation/processing and retail in Illinois. The Company has since expanded its operational footprint to 11 additional U.S. markets, including California, Colorado, Connecticut, Florida, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania. Currently, GTI owns, manufactures, and distributes a portfolio of cannabis consumer packaged goods brands (which we refer to as our consumer packaged goods business), including Beboe, Dogwalkers, Dr. Solomon’s, incredibles, Rythm and The Feel Collection, primarily to third-party licensed retail cannabis stores across the United States as well as to GTI-owned retail stores. The Company also owns and operates a rapidly growing national chain of retail cannabis stores called Rise and, in the Las Vegas, Nevada area, a chain of stores called Essence, which both sell GTI and third-party products (which we refer to as our retail business).
The Company, through its subsidiaries, owns interests in several state-licensed medical and/or adult use marijuana businesses in Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania. The Company also licenses its intellectual property and certain brands to licensees in California and Colorado. The following organizational chart describes the organizational structure of the Company as of December 31, 2019. See Exhibit 21.1 to this document for a list of subsidiaries of the Company. All lines represent 100% ownership of outstanding securities of the applicable subsidiary unless otherwise noted in Exhibit 21.1. In part, the complexity of our organization structure is due to state licensing requirements that mandate that we maintain the corporate identity of our operating license holders.
2
Table of Contents
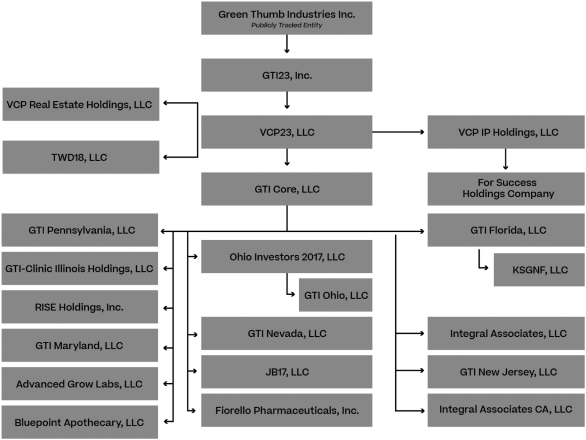
The registered office of GTI is located at 250 Howe Street, 20th Floor, Vancouver, British Columbia V6C 3R8. The head office is located at 325 W. Huron Street, Suite 412, Chicago, Illinois 60654.
History of the Company
The Company was incorporated under the Company Act (British Columbia) on June 26, 1979 under the name “Dalmatian Resources Ltd.” On February 18, 2002, the Company changed its name to “Enwest Ventures Corp.” Further, on February 25, 2003, the Company changed its name to “Bayswater Ventures Corp.” In August 2006, the Company changed its name from Bayswater Ventures Corp. to “Bayswater Uranium Corporation” following a Canadian amalgamation transaction with Pathfinder Resources Ltd.
On July 18, 2007, under a plan of arrangement, the Company amalgamated with Kilgore Minerals Ltd., a company incorporated under the Canada Business Corporations Act (the “CBCA”) on June 21, 2002 and continued into the Province of British Columbia under the Business Corporations Act (British Columbia) (the “BCBCA”) on December 7, 2006. Following the plan of arrangement, Kilgore Minerals Ltd. changed its name to “Bayswater Uranium Corporation” on July 24, 2007.
Subsequent to the Company’s financial year ended February 28, 2018, the Company completed the Transaction (as hereinafter defined), filed Articles of Amendment in the Province of British Columbia to effect the name change from “Bayswater Uranium Corporation” to “Green Thumb Industries Inc.” and continued the business of VCP23, LLC (“VCP”). VCP was formed in the State of Delaware on November 27, 2017. The entity had no activity or financials in 2017.
3
Table of Contents
General Development of the Business
The Transaction
On January 1, 2018, the Corporation completed a restructuring to consolidate its organizational structure. RCP23, LLC, which had operations in Maryland, Massachusetts, Nevada and Pennsylvania, and GTI-Clinic Illinois Holdings, LLC, which had operations in Illinois, restructured and each entity contributed certain assets and real estate to VCP or its subsidiaries. Simultaneously, GTI-Clinic Illinois Holdings, LLC transferred its membership interests in the Illinois licensed medical businesses to GTI Core, LLC. Prior to the closing of the Transaction, VCP was acquired by GTI23, Inc. (“GTI23”) and the members of VCP exchanged their membership interests in VCP in exchange for shares of GTI23. The transaction did not include outside parties that were not previously members of RCP23, LLC or GTI clinic Illinois Holdings, LLC. The transaction did involve certain current directors and officers of the Corporation that, at the time, were members of RCP23, LLC and/or GTI Clinic Illinois Holdings, LLC.
On June 12, 2018, the Company, 1165318 B.C. Ltd. a wholly-owned subsidiary of Bayswater (“Subco”), VCP, GTI23 and GTI Finco Inc. (“GTI Finco”) entered into a Business Combination Agreement whereby the Company, Subco, VCP, GTI23 and GTI Finco combined their respective businesses (the “Transaction”). The Transaction was structured as a series of transactions, including a Canadian three-cornered amalgamation transaction and a series of U.S. reorganization steps for the purpose of raising capital from third-party investors simultaneously with the closing of the Transaction. The Company (formerly Bayswater) had no active business operations leading up to completion of the Transaction.
In connection with the Transaction, the Company disposed of its uranium-based assets, changed its name from “Bayswater Uranium Corporation” to “Green Thumb Industries Inc.” and consolidated its existing common shares on the basis of one Subordinate Voting Share for each 368 existing common shares of the Company.
At a meeting of the Company’s shareholders on June 11, 2018, the shareholders approved a resolution to restructure the Company’s share capital to, among other things, re-designate its existing common shares as subordinate voting shares (“Subordinate Voting Shares”) and create a class of multiple voting shares (“Multiple Voting Shares”) and super voting shares (“Super Voting Shares”).
The Company, Subco and GTI Finco were parties to a Canadian three-cornered amalgamation (the “Amalgamation”) whereby:
| (i) | GTI Finco shareholders received Subordinate Voting Shares of the Company on a one-for-one basis; |
| (ii) | members of VCP contributed their membership interests to GTI23 for shares of GTI23; and |
| (iii) | members of VCP then contributed their shares of GTI23 to GTI in exchange for Super Voting Shares and Multiple Voting Shares of GTI. |
The SR Offering
Prior to the Transaction, GTI Finco (a special purpose corporation wholly-owned by VCP), completed a brokered and a non-brokered subscription receipt financing at a price of C$7.75 per subscription receipt for aggregate gross proceeds of approximately C$87 million (the “SR Offering”). As part of closing the Transaction, the investors in the SR Offering received Subordinate Voting Shares of GTI on an economically equivalent basis. The brokered portion of the SR Offering was co-led by GMP Securities L.P. and Canaccord Genuity Corp., with a syndicate that included Beacon Securities Limited, Echelon Wealth Partners Inc. and Eight Capital Corp. In connection with the Transaction and pursuant to the SR Offering, a total of 11,245,434 Subordinate Voting
4
Table of Contents
Shares were issued and outstanding after completion of the Transaction, including Subordinate Voting Shares issued to former holders of GTI Finco subscription receipts issued in the SR Offering.
The Subordinate Voting Shares began trading on the CSE on June 13, 2018 under the symbol “GTII.”
Financing Activities
On March 6, 2020, the Company closed on a sale and lease back transaction to sell its Oglesby, Illinois cultivation and processing facility to an unrelated third party, Innovative Industrial Properties (“IIP”). Under a long-term agreement, the Company will lease back the facility and continue to operate and manage it. The purchase price for the property was $9.0 million, excluding transaction costs. The Company is also expected to make certain improvements to the property that will significantly enhance production capacity, for which IIP has agreed to provide reimbursement of up to $41 million. Assuming full reimbursement for such improvements, IIPs total investment in the property will be $50 million.
On January 31, 2020, the Corporation closed on a sale and leaseback transaction to sell its Toledo, Ohio processing facility to IIP. Under a long-term agreement, the Corporation will lease back the facility and continue to operate the space and manage it. The purchase price for the property was $2.9 million, excluding transaction costs. The Corporation is also expected to make certain improvements to the property that will significantly enhance production capacity, for which IIP has agreed to provide reimbursement of up to $4.3 million. Assuming full reimbursement for such improvements, IIP’s total investment in the property will be $7.2 million.
On November 12, 2019, the Company closed on a sale and lease back transaction to sell its Danville, Pennsylvania cultivation and processing facility to IIP. Under a long-term agreement, the Company will lease back the facility and continue to operate and manage it. The purchase price for the property was $20.3 million, excluding transaction costs. The Company is also expected to make certain improvements to the property that will significantly enhance production capacity, for which IIP has agreed to provide reimbursement of up to $19.3 million. Assuming full reimbursement for such improvements, IIP’s total investment in the property will be $39.6 million.
On May 22, 2019, the Company closed a $105 million senior secured non-brokered private placement financing through the issuance of three-year senior secured notes (the “Notes”) pursuant to the Note Purchase Agreement (the “Note Purchase Agreement”). The financing generated funds for general working capital purposes and various growth initiatives and to retire the Company’s existing debt, including the Bridge Notes (as hereinafter defined). The Notes have a maturity date of May 22, 2022 and will bear interest from the date of issue at 12% per annum, payable quarterly, with an option, at the discretion of the Company, to extend an additional 12 months. Upon the execution of the Note Purchase Agreement, the Purchasers of the Notes received warrants to purchase 1,822,771 Subordinate Voting Shares at an exercise price of C$19.39 per share, which can be exercised for 60 months from the date of issuance. The Company entered into the First Amendment to the Note Purchase Agreement (the “Note Purchase Agreement Amendment”) on November 9, 2019. The Note Purchase Agreement Amendment reduced the borrowing capacity from $150 million to $130 million, which allows the Company to borrow an additional $24.5 million over a period of 12 months from the closing date of the Note Purchase Agreement. Upon the execution of the Note Purchase Agreement Amendment, the Purchasers of the Notes received warrants to purchase 365,076 Subordinate Voting Shares at an exercise price of C$12.04 per share, which can be exercised for 60 months from the date of issuance.
On April 12, 2019, the Company closed on a private placement of $12.5 million in six-month senior secured promissory notes (the “Bridge Notes”). The Bridge Notes accrue interest at an annual rate of 10.5% payable on a monthly basis, commencing June 1, 2019. The Bridge Notes included warrants to purchase 218,964 Subordinate Voting Shares at an exercise price of C$22.90 per share, which can be exercised for 42 months from the closing date of the transaction. On May 22, 2019, the Company repaid the full principal amount and accrued interest for the Bridge Notes with the proceeds from the private placement financing discussed above.
5
Table of Contents
On October 17, 2018, the Company closed a $78.6 million (C$101.7 million) bought deal financing, which included proceeds from the sale of Subordinate Voting Shares following the full exercise by the underwriters, namely GMP Securities L.P. (as lead underwriter and sole bookrunner), Beacon Securities Limited, Cormark Securities Inc., Echelon Wealth Partners Inc. and Eight Capital Corp., of an over-allotment option. The financing generated funds for the Company’s continued growth, including wholesale capacity, strategic initiatives and general corporate purposes.
On August 2, 2018, the Company closed a $61.7 million (C$80.3 million) bought deal financing, co-led by Canaccord Genuity Corp. and GMP Securities L.P., and including Beacon Securities Limited, Echelon Wealth Partners Inc. and Eight Capital Corp., to fund the Company’s continued growth, including the acquisition of one of ten licenses in the regulated New York cannabis market and the buildout of five dispensaries in Ohio pursuant to licenses awarded by the Ohio State Board of Pharmacy in June 2018, and for working capital purposes.
On June 12, 2018, GTI Finco (a special purpose corporation wholly-owned by VCP), completed the SR Offering, a brokered and a non-brokered subscription receipt financing at a price of C$7.75 per subscription receipt for aggregate gross proceeds of approximately $64.1 million (C$87 million). The investors received 11,245,434 Subordinate Voting Shares on an economically equivalent basis. The brokered portion of the financing was co-led by GMP Securities L.P. and Canaccord Genuity Corp., with a syndicate that included Beacon Securities Limited, Echelon Wealth Partners Inc. and Eight Capital Corp.
On April 30, 2018, the Company closed a private placement offering to sell $45 million in a convertible promissory note (“Convertible Promissory Note”) to VCP Convert, LLC, a Delaware limited liability company owned by accredited investors. The Convertible Promissory Note was converted into common units of VCP immediately prior to the Transaction.
On December 31, 2017, RCP23, LLC, closed a $67 million private placement offering to sell investor member units (“RCP Investor Member Units”) in RCP23, LLC to fund growth opportunities and working capital of the Company.
Certain Recent Developments
On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic and recommended containment and mitigation measures worldwide. The Company is monitoring this closely, and although operations have not been materially affected by the COVID-19 outbreak to date, the ultimate severity of the outbreak and its impact on the economic environment is uncertain. Operations of the Company are currently ongoing as the cultivation, processing and sale of cannabis products is currently considered an essential business by the states in which we operate with respect to all customers (or medical patients only in Massachusetts). The uncertain nature of the spread of COVID-19 globally may impact our business operations for reasons including the potential quarantine of our employees, customers, and third-party service providers. At this time, the Company is unable to estimate the impact of this event on its operations.
On August 23, 2019, the Company closed on its acquisition of Fiorello Pharmaceuticals, Inc. (“Fiorello”). Fiorello is one of only ten companies in New York licensed to grow, process and dispense medical cannabis. Fiorello has one cultivation/processing facility and four dispensing locations, with three of such dispensing locations currently operating.
On June 5, 2019, the Company closed on its acquisition of Integral Associates, LLC (“Integral Nevada”), a leading cannabis operator in Nevada, and Integral Associates CA, LLC (“Integral California,” together with Integral Nevada, “Integral Associates”). In addition to the initial consideration paid at closing, the membership interest purchase agreement (the “Membership Interest Purchase Agreement”) provides for the payment by the Company of additional consideration upon the achievement of certain performance targets (including a potential EBITDA earn-out payment) and regulatory license awards. Milestone payments for each
6
Table of Contents
license won are 50% payable at the initial license award, and the remaining 50% will be paid once the final licenses are issued. The transaction consideration included $52.8 million paid in cash and approximately 20.8 million in Subordinate Voting Shares which were valued at $235.4 million, and an additional 3.3 million milestone shares with a fair value of $37.7 million, for a total value of $273.1 million in share issuances. The acquisition includes: (i) Integral Associates’ three Essence retail stores located across the Las Vegas, Nevada area; (ii) eight additional adult use retail licenses in Nevada, five in the Las Vegas area and three in Northern Nevada; (iii) West Hollywood, California retail license, one of only five with a consumption lounge and delivery service; (iv) Pasadena, California and Culver City, California retail licenses; (v) Desert Grown Farms, a 54,000 square foot state-of-the-art cultivation and processing facility with an award-winning genetics library of 100+ strains; and (vi) Cannabiotix NV, a 41,000 square foot cultivation and processing facility which has been a recognized High Times Cannabis Cup award winner.
On February 21, 2019, the Company closed on its acquisition of For Success Holding Company, the Los Angeles-based creator of the lifestyle suite of Beboe branded cannabis products. Beboe is best known for, among other things, the thoughtfully designed aesthetic of its iconic rose gold vaporizing pens and edible pastilles, with each product curated using a unique blend of socially dosed THC (as hereinafter defined) and CBD (as hereinafter defined). The acquisition was an all-stock transaction, and consideration was satisfied through the issuance of Subordinate Voting Shares. The purchase agreement also includes additional consideration based on future performance targets.
On February 12, 2019, the Company closed on its acquisition of Connecticut-based Advanced Grow Labs LLC (“AGL”). AGL is one of four companies in Connecticut licensed to grow and process cannabis. AGL operates a 41,000 square foot manufacturing facility in West Haven, Connecticut, with expansion potential. AGL also has an ownership stake in a dispensary that is located in Westport, Connecticut. AGL produces and distributes a wide range of cannabis products to the operating stores in the state. The transaction consideration included $15.5 million in cash and approximately 7.3 million Subordinate Voting Shares. The purchase agreement also includes additional consideration based on future performance targets.
On November 8, 2018, the Company acquired KSGNF, LLC, the holder of a license to operate a vertically-integrated medical marijuana treatment center in Florida, in exchange for approximately $48.6 million in cash and Subordinate Voting shares valued at approximately $49.6 million. KSGNF, LLC operates a cultivation/processing facility in Homestead, Florida with six open and operating dispensaries across the state.
Description of the Business
Overview of the Company
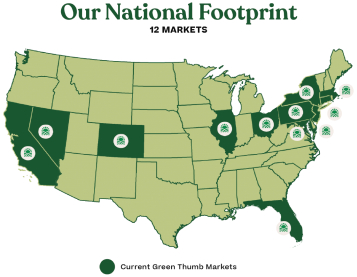
Established in 2014 and headquartered in Chicago, Illinois, GTI is promoting well-being through the power of cannabis, while being committed to community and sustainable profitable growth. As of March 31, 2020, GTI has operations across 12 U.S. markets, employs approximately 1,700 people and serves hundreds of thousands of patients and customers annually.
GTI’s core business is manufacturing, distributing and marketing a portfolio of owned cannabis consumer packaged goods brands (which we refer to as our consumer packaged goods business), including Beboe, Dogwalkers, Dr. Solomon’s, incredibles, Rythm and The Feel Collection, primarily to third-party licensed retail cannabis stores across the United States as well as to GTI-owned retail stores.
The Company’s consumer packaged goods portfolio is produced in 13 owned and operated manufacturing facilities and consists of stock keeping units (“SKUs”) across a range of product categories, including flower, pre-rolls, concentrates, vape, capsules, tinctures, edibles, topicals and other cannabis-related products.
GTI also owns and operates a national cannabis retail chain called Rise, and in the Las Vegas, Nevada area, a chain of stores called Essence, which are relationship-centric retail experiences aimed to deliver a superior level of customer service through high-engagement consumer interaction, a consultative, transparent and education-forward selling approach and a consistently available assortment of cannabis products. In addition, we own stores under other names, primarily where we co-own the stores or naming is subject to licensing or similar restrictions. (We refer to the operation of these retail stores as our retail business.) The income from our retail
7
Table of Contents
stores is primarily from the sale of cannabis-related products, which includes the sale of GTI produced products as well as those produced by third parties, with an immaterial (under 10%) portion of this income resulting from the sale of other merchandise (such as t-shirts and accessories for cannabis use). Our Rise stores currently are located in eight of the states in which we operate (including Nevada). Our Essence stores were acquired in connection with the 2019 acquisition of Integral Associates and are located in Nevada. The Essence stores differ from the Rise stores mainly in geographic location. As of December 31, 2019, the Company had 39 open and operating retail locations, with state licensed permission to open a total of 96 stores. Our new store opening plans will remain fluid depending on market conditions, obtaining local licensing, construction and other permissions and subject to our capital allocation plans and the evolving situation with respect to the Coronavirus.
Financial Highlights and Revenue Streams
The Company has consolidated financial statements across its operating businesses with revenue from the manufacture, sale and distribution of branded cannabis products to third-party licensed retail customers as well as the sale of finished products to consumers in its retail stores.
The percentage of total revenue contributed by operations of consumer package goods was, 36%, 33% and 40% for the years ended December 31, 2019, 2018 and 2017 respectively. The percentage of total revenue contributed by retail operations was, 64%, 67% and 60% for the years ended December 31, 2019, 2018 and 2017, respectively. See Item 7—“Management Discussion and Analysis” for details on key financial highlights.
As of the year ended December 31, 2019, GTI has operating revenue in 12 markets (California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New York, New Jersey, Ohio and Pennsylvania).
Geographic Information
GTI operates in 12 U.S. states: California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania.
Product Research, Design and Development
The Company’s branded products portfolio includes stock keeping units (“SKUs”) across a range of product categories, including flower, pre-rolls, concentrates, vape, capsules, tinctures, edibles, topicals and other cannabis-related products.
8
Table of Contents
GTI engages in research and development activities focused on developing new extracted or infused cannabis consumer packaged products.
Manufacturing
Our branded products are produced in manufacturing facilities across 12 U.S. states in which the primary activity is the cultivation, processing and manufacture of cannabis consumer packaged goods.
The majority of our finished goods production is manufactured by our owned production facilities. However, we also have entered into manufacturing agreements with third parties, primarily for our cannabidiol (“CBD”) business lines, none of which account for more than 1% of finished goods production.
We aim to maintain strict brand and quality assurance standards and have implemented standard operating procedures across all production facilities to ensure continuity of product and consistent consumer experience across all operating markets.
Sources and Availability of Materials
Almost all of the raw material input, except packaging materials, used by the Company to produce finished cannabis consumer packaged goods are cultivated or processed internally for further use in the manufacturing process.
Significant Customers
Customers of our consumer packaged goods business include legal state-licensed cannabis dispensaries within each U.S. state in which we operate, as well as national retail channels, including department stores and specialty boutiques. The majority of our branded consumer packaged goods are distributed to unrelated third-party licensed retail cannabis stores. GTI is not dependent upon a single customer, or a few customers, the loss of any one or more of which would have a material adverse effect on the business. No customer accounted for 10% or more of our consolidated net revenue during fiscal 2019, 2018 or 2017.
Merchandise
To meet the array of unique customer needs, we offer a variety of cannabis products at each of our Rise and Essence stores, totaling thousands of SKUs in managed inventory, comprehensive of product categories including flower, concentrates, topicals (bath and beauty products) and edibles (confection, beverages, snacks).
We leverage our owned retail channel, Rise, Essence and our other stores to distribute our branded product portfolio, such as Beboe, Dogwalkers, Dr. Solomon’s, incredibles, Rythm and The Feel Collection, among others.
All products sold have passed state-mandated third-party testing as required by applicable law to help assure that they do not contain impermissible levels of toxins, microbials and other harmful substances, are inventoried in comprehensive seed-to-sale tracking software to minimize product slippage and deviated inventory and meet the Company’s vendor requirements for quality assurance and reliability.
Omnichannel Distribution
Products sold at Rise and Essence are delivered directly to our stores primarily by our manufacturing and distribution vendor partners.
Our primary retail presence is traditional brick and mortar. However, as regulations allow, we will continue to expand our e-commerce, in-store guest pick-up and direct to consumer delivery capabilities as part of our commitment to providing a consistent retail brand experience no matter where the consumer might be.
9
Table of Contents
Intellectual Property – Patents and Trademarks
We believe that brand protection is critical to our business strategy. We regularly seek to protect our intellectual property rights in connection with our operating names (e.g., Green Thumb and Rise), our consumer packaged goods (e.g., Dogwalkers and Rythm) and certain patentable goods and services. The U.S. trademark statute, The Lanham Act, allows for the protection of trademarks and service marks on products and services used, or intended for use, lawfully. Because cannabis-related products and services remain illegal at the federal level under the Controlled Substances Act (21 U.S.C. § 811) (the “CSA”), we are not able to fully protect our intellectual property at the federal level; therefore, we currently seek trademark protections at the state level where commercially feasible. Nonetheless, our success depends upon other areas of our business such as product development and design, production and marketing and not exclusively upon trademarks, patents and trade secrets.
From the time the Company became licensed to cultivate marijuana, we have developed proprietary cultivation techniques. The Company has also developed certain proprietary intellectual property for operating butane extraction, carbon dioxide extraction and ethanol extraction machinery, including production best practices, procedures and methods. This requires specialized skills in cultivation, extraction and refining.
The Company relies on non-disclosure/confidentiality agreements to protect its intellectual property rights. To the extent the Company describes or discloses its proprietary cultivation or extraction techniques in its applications for cultivation or processing licenses, the Company redacts or requests redaction of such information prior to public disclosure.
GTI has sought U.S. patent protection for certain of its Dr. Solomon’s products, namely a utility patent for compositions and methods for treating skin and neuropathic conditions and disorders. Where commercially reasonable, we will seek further U.S. patent protection on other eligible products and services. The Company owns several website domains, including www.gtigrows.com, numerous social media accounts across all major platforms and various phone and web application platforms.
GTI has successfully registered over 20 trademarks across three countries and nine states for brands offered within those jurisdictions and has additional trademark applications pending. GTI is in the process of registering several brands for state trademark protection at the Canadian federal level, U.S. federal level and/or in the states in which the brands are offered, including Beboe, Dogwalkers, Dr. Solomon’s, incredibles, Rythm and The Feel Collection. GTI anticipates feedback on outstanding submitted applications on a rolling basis. As such, GTI will continue to rely on common law protection for these brands during the trademark registration process. Moreover, GTI will proactively seek intellectual property protection for brand expansions in current markets as well as any new market expansion. For additional details on the risks associated with the lack of trademark protection, see Item 1A—“Risk Factors” with respect to intellectual property.
For incredibles and Beboe branded cannabis products, the Company has entered into licensing and distribution contracts with third parties that hold licenses to engage in the sale of cannabis in the states of California and Colorado, where the Company does not currently have permission to operate cultivation and processing facilities. Such third parties directly engage in or arrange for the sourcing, manufacturing, laboratory testing, quality assurance, storage, marketing, sales, distribution and delivery of products containing cannabis and remit licensing fees to the Company.
Joint Ventures
We utilize joint ventures when necessary to comply with state regulatory requirements in certain states. Partnering with one or more non-affiliated third parties provides the Company with the opportunity to mitigate certain operational and financial risks while ensuring continued compliance with the applicable regulatory guidelines. Currently, the Company has joint ventures for a vertically integrated license in Ohio and for the
10
Table of Contents
operation of (i) a medical marijuana dispensary in Effingham, Illinois (Illinois Disp, LLC) of which the Company holds a 50% interest, (ii) a medical marijuana dispensary in Westport, Connecticut (Bluepoint Wellness of Westport, LLC) of which the Company holds a 46% interest, and (iii) a registered marijuana dispensary and a cultivation and processing facility in Chicopee, Massachusetts (Cal Funding, LLC) of which the Company holds less than a 10% interest. See additional discussion of the Company’s investments in Note 13—Investment in Associates, Note 19—Variable Interest Entities and Note 14—Share Capital.
Working Capital
Effective inventory management is critical to the Company’s ongoing success and the Company uses a variety of demand and supply forecasting, planning and replenishment techniques. The Company strives to maintain sufficient levels of inventory of core product categories, maintain positive vendor and customer relationships and carefully plan to minimize markdowns and inventory write-offs.
For additional details on liquidity and Capital Resources, see Item 7—“Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Number of Employees
As of March 31, 2020 GTI employs approximately 1,700 team members nationwide including corporate, retail, manufacturing and part-time employees, including but not limited to: finance and accounting, legal and compliance, supply chain and operations, sales and marketing, commercial and cannabis agriculture, chemists, customer service, construction and project management, real estate and human resources. We offer a comprehensive package of company-sponsored benefits to our team. Eligibility depends on the full-time or part-time status, location and other factors, and benefits include 401(k), medical and dental plans, disability insurance, employee assistance programs and life insurance. Additionally, we believe in aligned incentives and utilize employee stock and incentive plans for a competitive total rewards program.
The Company began executing furloughs mainly in the Nevada market where its Retail operations have been impacted by COVID-19. The total furloughed workforce represents less than 8% of the Company’s total employee base with approximately 74% of those impacted being part time employees. The Company continues to closely monitor and assess each and every market accordingly.
Environmental Compliance
Expenditures for compliance with federal, state and local environmental laws and regulations are consistent from year to year and are not material to the Company’s financials. The Company is compliant with all applicable regulations and does not use materials that would pose any known risk under normal conditions.
Competitive Conditions and the Company’s Position in the Industry
Competition
The markets in which the Company’s products are distributed and its retail stores are operated are highly competitive markets. The Company’s operations exist in markets with relatively high barriers to entry given the licensed nature of the cannabis industry. The Company competes directly with cannabis producers and retailers within single-state operating markets, as well as those that operate across several U.S. markets. More broadly, GTI views manufacturers of other consumer products, such as those in the pharmaceuticals, alcohol, tobacco, health and beauty and functional wellness industries, as potential competitors. Product quality, performance, new product innovation and development, packaging, customer experience and consumer price/value are important differentiating factors.
The Company faces intense competition from other companies that may have a longer operating history, a higher capitalization, additional financial resources, more manufacturing and marketing experience, greater access to public equity and debt markets and more experienced management than the Company. Increased competition by larger and better financed competitors could materially affect the business, financial condition and results of operations of the Company. The vast majority of both manufacturing and retail competitors in our markets consist of localized businesses (i.e. doing business in only a single state market).
11
Table of Contents
There are a few multistate operators with whom the Company competes directly in several of the Company’s operating markets. Aside from this direct competition, out-of-state operators that are capitalized well enough to enter those markets through acquisitions are also part of the competitive landscape. Similarly, as the Company executes its national U.S. growth strategy, operators in our future state markets will inevitably become direct competitors.
Because of the early stage of the industry in which the Company operates, the Company faces additional competition from new entrants. If the number of consumers of medical and adult use cannabis in the states in which the Company operates its business increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company will require a continued high level of investment in research and development, marketing, sales and client support. The Company may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis, which could materially and adversely affect the business, financial condition and results of its operations.
See Item 1A—“Risk Factors” with respect to competition.
Medical-Only Markets
All of the medical-only markets that the Company does business in (Illinois until January 1, 2020, Connecticut, Florida, Maryland, New Jersey, New York, Ohio and Pennsylvania) have written regulations that impose limitations on the number of cannabis business licenses that can be awarded. In each of these markets, the Company has a proven track record of: (i) entering the market through state-granted awards based on the merit of its application and business plans; and/or (ii) expanding market reach through accretive mergers, acquisitions and partnership ventures.
Adult Use Markets
The adult use markets in which the Company operates (Illinois as of January 1, 2020, California, Colorado, Massachusetts and Nevada) have fewer barriers to entry and more closely reflect free market dynamics typically seen in mature retail and manufacturing industries. The growth of these markets poses a risk of increased competition. However, given the Company’s additional growth opportunities as an original operator in these states, which have historically been limited supply markets, management views the Company’s market share as less at risk than operators without a current operating footprint.
Overview of Government Regulation
Below is a discussion of the federal and state-level regulatory regimes in those jurisdictions where the Company is currently directly involved through its subsidiaries. The Company’s subsidiaries are directly engaged in the manufacture, possession, sale or distribution of cannabis in the adult use and/or medicinal cannabis marketplace in the states of California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania.
Federal Regulation of Cannabis
In 2005, the U.S. Supreme Court ruled that Congress has the power to regulate cannabis.
The U.S. federal government regulates drugs through the Controlled Substances Act (21 U.S.C. § 811) (the “CSA”), which places controlled substances, including cannabis, in a schedule. Cannabis is classified as a Schedule I controlled substance. A Schedule I controlled substance is defined as a substance that has no currently accepted medical use in the United States, a lack of safety for use under medical supervision and a high potential
12
Table of Contents
for abuse. The Department of Justice (the “DOJ”) defines Schedule I drugs, substances or chemicals as “drugs with no currently accepted medical use and a high potential for abuse.” However, the Food and Drug Administration (the “FDA”) has approved Epidiolex, which contains a purified form of the drug CBD, a non-psychoactive ingredient in the cannabis plant, for the treatment of seizures associated with two epilepsy conditions. The FDA has not approved cannabis or cannabis compounds as a safe and effective drug for any other condition. Moreover, under the 2018 Farm Bill or Agriculture Improvement Act of 2018 (the “Farm Bill”), CBD remains a Schedule I controlled substance under the CSA, with a narrow exception for CBD derived from hemp with a tetrahydrocannabinol (“THC”) concentration of less than 0.3%.
Marijuana is largely regulated at the state level.
State laws that permit and regulate the production, distribution and use of cannabis for adult use or medical purposes are in direct conflict with the CSA, which makes cannabis use and possession federally illegal. Although certain states and territories of the U.S. authorize medical and/or adult use cannabis production and distribution by licensed or registered entities, under U.S. federal law, the possession, use, cultivation and transfer of cannabis and any related drug paraphernalia is illegal and any such acts are criminal acts under federal law under any and all circumstances under the CSA. Although the Company’s activities are believed to be compliant with applicable state and local laws, strict compliance with state and local laws with respect to cannabis may neither absolve the Company of liability under U.S. federal law, nor may it provide a defense to any federal proceeding which may be brought against the Company.
As of December 31, 2019, 33 states, plus the District of Columbia (and the territories of Guam, Puerto Rico, the U.S. Virgin Islands and the Northern Mariana Islands), have legalized the cultivation and sale of cannabis for medical purposes. In 11 states, the sale and possession of cannabis is legal for both medical and adult use, and the District of Columbia has legalized adult use but not commercial sale.
The risk of federal enforcement and other risks associated with the Company’s business are described in Item 1A—“Risk Factors.”
Regulation of the Cannabis Market at State and Local Level
Following the thesis that distributing brands at scale will win, the Company enters markets where it believes that it can profitably and sustainably operate and command significant market share, and thus maximize consumer and brand awareness. The regulatory frameworks installed by the states, which are similar to the limited and controlled issuance of gaming or alcohol distributorship licenses, provide macro-level indication of whether certain state markets will be sustainable and profitable.
Below is a summary overview of the regulatory and competitive frameworks in each of the Company’s operating markets. See Appendix A to this Annual Report on Form 10-K for a state-by-state list of the licenses and permits held by the Company.
California
In 1996, California was the first state to legalize medical marijuana through Proposition 215, the Compassionate Use Act of 1996. This legalized the use, possession and cultivation of medical marijuana by patients with a physician recommendation for treatment of cancer, anorexia, AIDS, chronic pain, spasticity, glaucoma, arthritis, migraine or any other illness for which marijuana provides relief. In 2003, Senate Bill 420 was signed into law establishing an optional identification card system for medical marijuana patients.
In September 2015, the California legislature passed three bills collectively known as the Medical Cannabis Regulation and Safety Act (“MCRSA”). The MCRSA established a licensing and regulatory framework for medical marijuana businesses in California. In November 2016, voters in California overwhelmingly passed
13
Table of Contents
Proposition 64, the Adult Use of Marijuana Act (“AUMA”) creating an adult use marijuana program for adults 21 years of age or older. Some provisions of AUMA conflicted with MCRSA, so the California State Legislature passed Senate Bill No. 94, known as Medicinal and Adult Use Cannabis Regulation and Safety Act (“MAUCRSA”) in June 2017, amalgamating MCRSA and AUMA to provide a single set of regulations to govern a medical and adult use licensing regime for cannabis businesses in the State of California. MAUCRSA went into effect on January 1, 2018.
The three agencies that regulate cannabis at the state level are: (a) the California Department of Food and Agriculture, via CalCannabis, which issues licenses to cannabis cultivators; (b) the California Department of Public Health, via the Manufactured Cannabis Safety Branch, which issues licenses to cannabis manufacturers; and (c) the California Department of Consumer Affairs, via the Bureau of Cannabis Control, which issues licenses to cannabis distributors, testing laboratories, retailers and micro-businesses.
On June 6, 2018, a proposal by the California Department of Consumer Affairs, the California Department of Public Health and the California Department of Food and Agriculture to re-adopt their emergency cannabis regulations went into effect. Among the changes, applicants may now complete one license application, allowing for both medical and adult use cannabis activity. On January 16, 2019, California’s three state cannabis licensing authorities announced that the Office of Administrative Law officially approved state regulations for cannabis businesses. The final cannabis regulations took effect immediately and superseded the previous emergency regulations.
In order to legally operate a medical or adult use cannabis business in California, the operator must have both a local and state license. This requires license holders to operate in cities with cannabis licensing programs. Municipalities in California are allowed to determine the number of licenses they will issue to cannabis operators or can choose to ban cannabis businesses outright.
California License and Regulations
There are three principal license categories in California: (1) cultivation, (2) processing and (3) retailer. A license holder that does not submit a completed license renewal application to the state within 30 calendar days after the expiration of a current license forfeits their eligibility to apply for a license renewal and, instead, would be required to submit a new license application.
Currently, the Company does not have any operational licenses in the State of California. GTI has been granted conditional licenses, permitting the Company to retail medical and adult use cannabis and cannabis related products.
Cultivation licenses permit commercial cannabis cultivation activity involving the planting, growing, harvesting, drying, curing, grading or trimming of cannabis. Such licenses further permit the production, labeling and packaging of a limited number of non-manufactured cannabis products and permit the licensee to sell cannabis to certain licensed entities (both medical and adult use licensees) within the State of California for resale or manufacturing purposes.
Processing licenses authorize manufacturers to process marijuana biomass into certain value-added products with the use of volatile or non-volatile solvents, depending on the license type.
Retailer licenses permit the sale of cannabis and cannabis products to both medical patients and adult use customers. Only certified physicians may provide medicinal marijuana recommendations. An adult use retailer license permits the sale of cannabis and cannabis products to any adult 21 years of age or older. It does not require the individual to possess a physician’s recommendation. Under the terms of such licenses, the holder is permitted to sell adult use cannabis and cannabis products to any person, provided the local jurisdiction permits the sale of adult use cannabis and the person presents a valid government-issued photo identification demonstrating that they are 21 years of age or older.
14
Table of Contents
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in California.
California Reporting Requirements
The California Cannabis Track-and-Trace (“CCTT”) system is the T&T system used statewide to record the inventory and movement of cannabis and cannabis products through the commercial cannabis supply chain from seed to sale. The CCTT system must be used by all annual and provisional cannabis licensees, including those with licenses for cannabis cultivation, manufacturing, retail, distribution, testing labs and microbusinesses. The state’s contracted service provider for the CCTT system is METRC. Licensees are required to maintain records for at least seven years from the date a record is created.
Colorado
On November 7, 2000, Colorado voters approved Amendment 20, which amended the state constitution to allow the use of marijuana in the state by approved patients with written medical consent. Conditions recognized for medical marijuana in Colorado include: cancer, chronic pain, epilepsy, HIV/AIDS, multiple sclerosis and nausea.
Amendment 64 passed on November 6, 2012, which amended the state constitution to establish a cannabis program in Colorado and permit the commercial cultivation, manufacture and sale of marijuana to adults 21 years of age or older. The commercial sale of marijuana for adult use to the general public began on January 1, 2014 at cannabis businesses licensed under the regulatory framework.
In Colorado, cannabis businesses must comply with local licensing requirements in addition to state licensing requirements in order to operate. Colorado localities are allowed to limit or prohibit the operation of marijuana cultivation facilities, product manufacturing facilities or retail dispensary facilities.
Colorado License and Regulations
There are three principal license categories in Colorado: (1) cultivation, (2) product manufacturer and (3) medical center/retail store. Each facility is authorized to engage only in the type of activity for which it is licensed. A licensee must apply for renewal before the expiration date of a license.
The Company does not have any licenses in the State of Colorado. The Company has entered into licensing and distribution contracts with third parties that hold licenses to engage in the sale of cannabis in Colorado for incredibles and Beboe branded cannabis products. Such third parties directly engage in or arrange for the sourcing, manufacturing, laboratory testing, quality assurance, storage, marketing, sales, distribution and delivery of products containing cannabis and remit licensing fees to GTI. See Item 1—“Intellectual Property – Patents and Trademarks” for details on licenses with respect to operations in Colorado.
Regulations for the production and sale of marijuana in Colorado are published through the Marijuana Enforcement Division of the Department of Revenue (the “MED”).
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Colorado.
Colorado Reporting Requirements
Colorado uses METRC as the MED’s marijuana inventory tracking system for all medical and adult use licensees. Marijuana is required to be tracked and reported with specific data points from seed to sale through METRC for compliance purposes under Colorado marijuana laws and regulations.
15
Table of Contents
Connecticut
The State of Connecticut has authorized cultivation, possession and distribution of marijuana for medical purposes by certain licensed Connecticut marijuana businesses. The Medical Marijuana Program in Connecticut (the “CT Program”) registers qualifying patients, primary caregivers, Dispensary Facilities (“DFs”) and Dispensary Facility Employees (“DFEs”). The CT Program was established by Connecticut General Statutes §§ 21a-408–21a-429. DFs and production facilities are separately licensed.
Connecticut’s medical cannabis program was introduced in May 2012 when the General Assembly passed legislation PA 12-55 “An Act Concerning the Palliative Use of Marijuana.”
The program launched with six dispensary licensees and four producer licensees. The first dispensaries sold to patients in September 2014.
In January 2016, the Connecticut Department of Consumer Protection (“CTDCP”), the agency that oversees and administers the program, approved three additional dispensary licenses. In December 2018, the CTDCP issued nine additional dispensary licenses, bringing the total to 18 licensed dispensaries in the state. As of December 31, 2019, 15 of these dispensaries were operational.
Connecticut Licenses and Regulations
There are two principal license categories in Connecticut: (1) cultivation/processing and (2) dispensary. The Company is licensed to operate one medical marijuana cultivation/processing facility and two medical marijuana dispensaries. All licenses are, as of the date hereof, active with the State of Connecticut. The licenses are independently issued for each approved activity for use at the Company’s facilities in Connecticut.
The CTDCP has issued regulations regarding the CT Program. Patients with certain debilitating medical conditions qualify to participate in the CT Program. A physician or advanced practice registered nurse must issue a written certification for a CT Program patient, and the qualifying patient or caregiver must choose one designated DF where the patient’s marijuana will be obtained. Under the CT Program, dispensary licenses are renewed annually. Renewal applications must be submitted 45 days prior to license expiration and any renewal submitted more than 30 days after expiration will not be renewed.
Medical marijuana cultivation/processing licenses permit the Company to operate a secure, indoor facility to cultivate and process medical marijuana and wholesale to dispensaries.
Medical marijuana dispensary facility licenses qualify a dispensary to purchase medical cannabis from licensed medical cannabis producers and to dispense cannabis to qualifying patients or primary caregivers that are registered under the CT Program. Dispensaries must have a pharmacist on staff.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Connecticut.
Connecticut Reporting Requirements
Connecticut does not mandate the use of a particular unified T&T system by which all dispensary license holders submit data directly to the state. However, the CT Program does provide strict guidelines for reporting via the license holder’s T&T program. Every cannabis sale must be documented at the point of sale, including recording the date. At least once per day, all sales must be uploaded via the T&T system to the Connecticut Prescription Monitoring Program which accumulates and tracks medical cannabis purchases across all Connecticut dispensaries.
16
Table of Contents
Florida
In 2014, the Florida Legislature passed the Compassionate Use Act, which was the first legal medical cannabis program in the state’s history. The original Compassionate Use Act only allowed for low-THC cannabis to be dispensed and purchased by patients suffering from cancer and epilepsy. In 2016, the Legislature passed the Right To Try Act which allowed for full potency cannabis to be dispensed to patients suffering from a diagnosed terminal condition. Also, in 2016, the Florida Medical Marijuana Legalization Initiative was introduced by citizen referendum and passed on November 8. This language, known as “Amendment 2,” amended the state constitution and mandated an expansion of the state’s medical cannabis program.
Amendment 2, and the resulting expansion of qualifying medical conditions, became effective on January 3, 2017. The Florida Department of Health, physicians, dispensing organizations and patients are bound by Article X Section 29 of the Florida Constitution and Florida Statutes Section 381.986. On June 9, 2017, the Florida House of Representatives and Florida Senate passed respective legislation to implement the expanded program by replacing large portions of the existing Compassionate Use Act, which officially became law on June 23, 2017.
The State of Florida Statutes Section 381.986(8)(a) provides a regulatory framework that requires licensed producers, which are statutorily defined as “Medical Marijuana Treatment Centers” (the “MMTC”), to cultivate, process and dispense medical cannabis in a vertically-integrated marketplace.
Florida Licenses and Regulations
There is one principal license category in Florida: vertically-integrated MMTC license. The Company is licensed to operate one medical cannabis cultivation/processing facility and up to 35 medical dispensaries. All licenses are, as of the date hereof, active with the State of Florida. The licenses are independently issued for each approved activity for use at the Company’s facilities in Florida.
Licenses are issued by the Florida Department of Health and must be renewed biennially, provided the license meets the requirements under Florida law and the license holder pays a renewal fee. License holders can only own one license. Currently, the dispensaries can be in any geographic location within the state, provided that the local municipality’s zoning regulations authorize such a use, the proposed site is zoned for a pharmacy and the site is not within 500 feet of a church or school.
The MMTC license permits the Company to sell medical cannabis to qualified patients to treat certain medical conditions in Florida, which are delineated in Florida Statutes Section 386.981. As the Company’s operations in Florida are vertically-integrated, the Company is able to cultivate, harvest, process and sell/dispense/deliver its own medical cannabis products. Under the terms of its Florida license, the Company is permitted to sell medical cannabis only to qualified medical patients that are registered with the State. Only certified physicians who have successfully completed a medical cannabis educational program can register patients on the Florida Office of Compassionate Use Registry.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Florida.
Florida Reporting Requirements
The Florida Department of Health requires that any licensee establish, maintain and control a computer software tracking system that traces cannabis from seed to sale and allows real-time, 24-hour access by the Florida Department of Health to this data. The tracking system must allow for integration of other seed-to-sale systems and, at minimum, include notification of when marijuana seeds are planted, when marijuana plants are harvested and destroyed and when cannabis is transported, sold, stolen, diverted or lost. Additionally, the Florida Department of Health maintains a patient and physician registry, and the Company must comply with all requirements and regulations related to providing required data or proof of key events to the tracking system.
17
Table of Contents
Illinois
The Compassionate Use of Medical Cannabis Program Act, which allows individuals diagnosed with a debilitating medical condition access to medical marijuana, became effective January 1, 2014 and is now permanent. There are more than 40 qualifying conditions as part of the medical program, including chronic pain, migraines, epilepsy, traumatic brain injury and post-traumatic stress disorder (“PTSD”). Licenses were awarded based on merit in a highly competitive application process to applicants who demonstrated strong operational expertise and financial backing.
On May 31, 2019, Illinois lawmakers passed a bill legalizing adult use marijuana. The bill permits adult use sales to adults 21 years of age or older which began in the state of Illinois on January 1, 2020. Governor J.B. Pritzker signed the bill into law, making Illinois the 11th state to legalize the adult use and commercial sale of cannabis to adults.
Illinois Licenses and Regulations
There are two principal license categories in Illinois: (1) cultivation/processing and (2) dispensary. The Company is licensed to grow cannabis for medical and adult use sales at the Company’s two cultivation/processing facilities. The Company has applied for and received state approval for its five existing medical dispensary retail locations in Illinois to also make sales to adult use customers, subject to local zoning approval. The Company applied for and received five “secondary” adult use dispensary licenses, subject to local zoning approval, which by law will serve only adult use customers, not medical patients. That will bring the Company to a total of ten dispensary licenses in Illinois, which is the statutory cap. All licenses are, as of the date hereof, active with the State of Illinois. The licenses are independently issued for each approved activity for use at the Company’s facilities in Illinois.
All cultivation/processing establishments must register with Illinois Department of Agriculture. All dispensaries must register with the Illinois Department of Financial and Professional Regulation. If applications contain all required information, establishments are issued a marijuana establishment registration certificate. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. Pursuant to Illinois law, registration renewal applications must be received 45 days prior to expiration and may be denied if the license has a history of non-compliance and penalties.
The cultivation/processing licenses permit the Company to acquire, possess, cultivate, manufacture/process into edible marijuana products and/or marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to marijuana dispensaries.
The retail dispensary licenses permit the Company to purchase marijuana and marijuana products from cultivation/processing facilities, as well as allow the sale of marijuana and marijuana products.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Illinois.
Illinois Reporting Requirements
The state of Illinois uses BioTrack as the state’s computerized track-and-trace (“T&T”) system for seed-to-sale reporting. Individual licensees, whether directly or through third-party integration systems, are required to push data to the state to meet all reporting requirements. The Company uses an in-house computerized seed-to-sale software, which integrates with the state’s BioTrack program and captures the necessary data points as required in the Illinois Compassionate Use of Medical Cannabis Program Act.
18
Table of Contents
Maryland
In 2012, a state law was enacted in Maryland to establish a state-regulated medical marijuana program. Legislation was signed in May 2013 and the program became operational on December 1, 2017. The Maryland Medical Cannabis Commission (the “MMCC”) regulates the state program and awarded operational licenses in a highly competitive application process. 102 dispensary licenses were awarded out of a pool of over 800 applicants, while an original 15 cultivation licenses were awarded out of a pool of over 150 applicants. In April 2018, Maryland lawmakers agreed to expand the state’s medical marijuana industry by authorizing an additional 20 licenses, seven for cultivation and 13 for processing. The state program was written to allow access to medical marijuana for patients with any condition that is considered “severe” for which other medical treatments have proven ineffective, including: chronic pain, nausea, seizures, glaucoma and PTSD.
Maryland Licenses and Regulations
There are three principal license categories in Maryland: (1) cultivation, (2) processing and (3) dispensary. The Company has control and/or ownership over one cultivation license, one processing license and three retail dispensaries. All licenses are, as of the date hereof, active with the State of Maryland. The licenses are independently issued for each approved activity for use at the Company facilities in Maryland.
All cultivation, processing and dispensary establishments must register with the MMCC under the provisions of the Maryland Medical Cannabis Law, Section 13-3301 et seq. If applications contain all required information, establishments are issued a medical marijuana establishment registration certificate. Registration certificates are valid for a period of six years and are subject to annual renewals after required fees are paid and the business remains in good standing. After the first expiration of the approved license, the dispensary, cultivation and processing licensee is required to renew every two years. Licensees are required to submit a renewal application per the guidelines published by the MMCC. 90 days prior to the expiration of a license, the MMCC notifies the licensee of the date on which the license expires and provides the instructions and fee required to renew the license along with the consequences of failure to renew. At least 30 business days before a license expires, the licensee must submit the renewal application as provided by the MMCC.
The medical cultivation licenses permit the Company to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries, facilities for the production of medical marijuana products and/or medical marijuana-infused products or other medical marijuana cultivation facilities.
The medical processing license permits the Company to acquire, possess, manufacture, deliver, transfer, transport, supply, or sell marijuana products or marijuana infused products to other medical marijuana production facilities or medical marijuana dispensaries.
The retail dispensary licenses permit the Company to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities and marijuana from other medical marijuana dispensaries, as well as allow the sale of marijuana and marijuana products.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Maryland.
Maryland Reporting Requirements
The state of Maryland uses METRC as the state’s computerized T&T system for seed-to-sale reporting. Individual licensees, whether directly or through third-party integration systems, are required to push data to the state to meet all reporting requirements. The Company uses an in-house computerized seed-to-sale software, which integrates with the state’s METRC program and captures the required data points for retail as required in the Maryland Medical Cannabis Law. The Company uses METRC directly for cultivation and manufacturing.
19
Table of Contents
Massachusetts
Massachusetts legalized medical marijuana when voters passed a ballot initiative in 2012. The Massachusetts Medical Use of Marijuana Program was formed pursuant to the Act for the Humanitarian Medical Use of Marijuana. Adult use marijuana became legal in Massachusetts as of December 15, 2016, following a ballot initiative in November 2016. Dispensaries for the adult use of cannabis in Massachusetts began operating in July 2018.
In Massachusetts, Registered Marijuana Dispensaries (“RMDs”) are “vertically-integrated,” which means RMDs grow, process and dispense their own marijuana. An RMD must have a retail facility, as well as cultivation and processing operations. Some RMDs elect to conduct cultivation, processing and retail operations all in one location, which is commonly referred to as a “co-located” operation. An RMD may also choose to have a retail dispensary in one location and grow marijuana at a remote cultivation location. An RMD may process marijuana at either a retail dispensary location or a remote cultivation location. The remote cultivation location need not be in the same municipality, or the same county, as the retail dispensary.
Massachusetts Licenses and Regulations
There is one principal license category in Massachusetts: vertically-integrated RMD license. The Company is licensed to operate one medical and adult use cultivation/processing facility and up to two medical and adult use retail dispensaries. All licenses are, as of the date hereof, active with the State of Massachusetts. The licenses are independently issued for each approved activity for use at the Company’s facilities in Massachusetts.
The Massachusetts Department of Public Health was the regulatory body that oversaw the original Massachusetts medical program, including all cultivation, processing and dispensary facilities. The Cannabis Control Commission (the “CCC”), a regulatory body created in 2018, now oversees the medical and adult use programs, including licensing of cultivation, processing and dispensary facilities. Licensed medical dispensaries are given priority status in adult use licensing.
Each Massachusetts dispensary, cultivator and processor license is valid for one year and must be renewed no later than 60 calendar days prior to expiration. The CCC can deny or revoke licenses and renewals for multiple reasons, including (a) submission of materially inaccurate, incomplete or fraudulent information, (b) failure to comply with any applicable law or regulation, including laws relating to taxes, child support, workers compensation and insurance coverage, (c) failure to submit or implement a plan of correction, (d) attempting to assign registration to another entity, (e) insufficient financial resources, (f) committing, permitting, aiding or abetting of any illegal practices in the operation of the RMD, (g) failure to cooperate or give information to relevant law enforcement related to any matter arising out of conduct at an RMD and (h) lack of responsible RMD operations, as evidenced by negligence, disorderly or unsanitary facilities or permitting a person to use a registration card belonging to another person.
The RMD license permits the Company to cultivate, process and dispense medical and adult use cannabis.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Massachusetts.
Massachusetts Reporting Requirements
The Commonwealth of Massachusetts uses the MMJ Online system through the Virtual Gateway portal as the state’s computerized T&T system for seed-to-sale reporting. Individual licensees, whether directly or through third-party integration systems, are required to push data to the state to meet all reporting requirements.
20
Table of Contents
The Company uses an in-house computerized seed-to-sale software, which integrates with the state’s program and captures the required data points for cultivation, manufacturing and retail as required in the Massachusetts marijuana laws and regulations.
Nevada
Nevada became a medical marijuana state in 2001. In 2013, the Nevada legislature passed SB374, providing for state licensing of medical marijuana establishments. On November 8, 2016, Nevada voters passed NRS 435D by ballot initiative allowing for the sale of marijuana for adult use starting on July 1, 2017. In 2018, the Nevada Department of Taxation (the “DOT”) opened up applications for additional adult use marijuana dispensary licenses. Only those companies that held medical marijuana licenses in the state could apply. In December 2018, 61 additional marijuana dispensary licenses were issued by the DOT.
Nevada Licenses and Regulations
There are three principal license categories in Nevada: (1) cultivation, (2) processing and (3) dispensary. The Company is licensed to operate two medical and adult use cultivation facilities, three medical and adult use processing facilities, five medical dispensary licenses and up to 13 adult use retail locations. All licenses are, as of the date hereof, active with the State of Nevada. The licenses are independently issued for each approved activity for use at the Company’s facilities in Nevada.
Under applicable laws, the licenses permit the Company to cultivate, manufacture, process, package, sell and purchase marijuana pursuant to the terms of the licenses, which are issued by the DOT under the provisions of Nevada Revised Statutes section 453A. If applications contain all required information, establishments are issued a marijuana establishment registration certificate. In a local governmental jurisdiction that issues business licenses, the issuance by DOT of a marijuana establishment registration certificate is considered provisional until the local government has issued a business license for operation and an establishment is in compliance with all applicable local governmental ordinances. Final registration certificates are valid for a period of one year and the Nevada DOT shall issue a renewal license within ten days after the receipt of a renewal application and applicable fee if the license is not then under suspension or has not been revoked.
The cultivation licenses permit the Company to acquire, possess, cultivate, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to marijuana dispensaries, facilities for the production of edible marijuana products and/or marijuana-infused products or other marijuana cultivation facilities.
The processing license permits the Company to acquire, possess, manufacture, deliver, transfer, transport, supply or sell edible marijuana products or marijuana infused products to other marijuana production facilities or marijuana dispensaries.
The retail dispensary licenses permit the Company to purchase marijuana from cultivation facilities, marijuana and marijuana products from product manufacturing facilities and marijuana from other retail stores, as well as allow the sale of marijuana and marijuana products.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Nevada.
Nevada Reporting Requirements
The state of Nevada uses Marijuana Enforcement Tracking Regulation and Compliance (“METRC”) as the state’s computerized T&T system for seed-to-sale reporting. Individual licensees, whether directly or
21
Table of Contents
through third-party integration systems, are required to push data to the state to meet all reporting requirements. The Company has designated an in-house computerized seed-to-sale software that integrates with METRC via API, which captures the required data points for cultivation, manufacturing and retail as required in Nevada Revised Statutes section 453A.
New Jersey
On January 18, 2010, the governor of New Jersey signed into law S.119, the Compassionate Use Medical Marijuana Act (the “NJ Act”), permitting the use of medical cannabis for persons with certain debilitating conditions. The law permits the New Jersey Department of Health (“NJDOH”) to create rules to add other illnesses to the permitted conditions. The NJ Act mandates that cannabis must be acquired through Alternative Treatment Centers (each an “ATC”) licensed by the State.
A single ATC license allows for the cultivation, processing and dispensing of medical marijuana products. Originally, each ATC was permitted to open one dispensary. With the Executive Order 6 Report, each ATC can now open two additional satellite dispensaries within their NJDOH-designated region for a total of three dispensaries each, as well as satellite production facilities, subject to regulatory approval.
On March 27, 2018 through executive order No. 6 (2018), Governor Phil Murphy expanded the medical marijuana program, announcing the 20-plus recommendations presented by the NJDOH on March 23, 2018. The NJDOH’s recommendations and next steps included certain measures that took effect immediately (e.g. the addition of debilitating conditions and the reduction of registration fees) and other recommendations (e.g. the home delivery model) that require further regulatory or statutory enactment.
On July 2, 2019, Governor Phil Murphy signed the Jake Honig Compassionate Use Medical Cannabis Act into law (“CUMCA”), which amended the NJ Act. Previously, New Jersey law only permitted applicants to apply for vertically-integrated licenses. Under CUMCA, the permit process includes three different permit types. The new permit types are medical cannabis cultivator, dispensary and manufacturer permits, which are to be applied for individually. The vertically-integrated ATC will continue to be able to cultivate, manufacture and dispense medical cannabis. These new permit types are still in the application stage and have not yet been awarded.
New Jersey Licenses and Regulations
There is currently one principal license category in New Jersey: vertically-integrated ATC license. The Company is licensed to operate one medical cultivation and processing facility and up to three retail medical cannabis dispensaries in the state of New Jersey. All licenses are, as of the date hereof, active with the State of New Jersey. The licenses are independently issued for each approved activity for use at the Company facilities in New Jersey.
The NJDOH is responsible for issuing permits and administering the NJ Act to ensure qualifying patients’ access to safe cannabis for medical use in New Jersey.
ATC permits expire annually on December 31. A permit renewal application must be submitted at least 60 days prior to the expiration date. An ATC that seeks to renew its permit shall submit to the permitting authority an application for renewal with all required documentation and the required fees. Prior to the issuance of any permit, the Company must certify that it submits to the jurisdiction of the courts of the State of New Jersey and agrees to comply with all the requirements of the laws of New Jersey pertaining to New Jersey’s Medicinal Marijuana Program.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in New Jersey.
22
Table of Contents
New Jersey Reporting Requirements
New Jersey does not have a unified T&T system. All information is forwarded to the medical marijuana program through email. The ATC collects and submits to the NJDOH for each calendar year statistical data on (a) the number of registered qualified patients and registered primary caregivers, (b) the debilitating medical conditions of the qualified patients, (c) patient demographic data, (d) summary of the patient surveys and evaluation of services and (e) other information as the NJDOH may require. The ATC must retain records for at least two years. The Company also uses an in-house computerized seed-to-sale software, which captures the required data points for cultivation, manufacturing and retail as required in the New Jersey marijuana laws and regulations.
New York
In July 2014, the New York Legislature and Governor enacted the Compassionate Care Act (the “CCA”) to provide a comprehensive, safe and effective medical marijuana program. The CCA provides access to the program for those who suffer from qualifying serious conditions including: cancer, HIV/AIDS, ALS and PTSD. The program allows ten “Registered Organizations” to hold vertically-integrated licenses and service qualified patients and caregivers. Each Registered Organization has one cultivation/processing license and four dispensary licenses.
Under the terms of licenses in the state of New York, licensees are permitted to sell NYSDOH-approved medical marijuana manufactured products to any qualified patient who possesses a physician’s recommendation, provided that the patient presents a valid government-issued photo identification and NYSDOH-issued Registry Identification Card proving that the patient or designated caregiver meets the statutory conditions to be a qualified patient or designated caregiver. The card contains the recommendation from the physician and the limitation on form or dosage of medical marijuana.
In order for a patient or registered caregiver to receive dispensed marijuana, they must be logged into the Prescription Monitoring Program (“PMP”) registry. The PMP registry is monitored by the NYSDOH and contains controlled substance prescription dispensing history and medical marijuana dispensing history to ensure that patients only receive a maximum of 30 days’ worth of dispensed product from one Registered Organization. Only registered pharmacists can dispense medical marijuana to approved patients and caregivers.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in New York.
New York Licenses and Regulations
There is one principal license category in New York: vertically-integrated license. The Company is licensed to operate one medical marijuana cultivation/manufacturing facility and up to four medical marijuana dispensaries. All licenses are, as of the date hereof, active with the State of New York. The licenses are independently issued for each approved activity for use at the Company’s facilities in New York.
The New York State Department of Health (“NYSDOH”) is the regulatory agency that oversees the medical marijuana program in New York. New York is a vertically-integrated system; however, the state does allow Registered Organizations to wholesale manufactured products to one another. As such, the Company has the ability to be vertically-integrated and cultivate, harvest, process, transport, sell and dispense cannabis products. Delivery is allowed from dispensaries to patients, however the delivery plan must be pre-approved by the NYSDOH.
New York Reporting Requirements
The State of New York has selected BioTrackTHC’s system as the state’s T&T system used to track commercial cannabis activity and seed-to-sale. The BioTrackTHC system must serve as all Registered
23
Table of Contents
Organizations’ patient verification system but is optional as Registered Organizations’ tracking system. The Company currently uses BioTrackTHC as its seed-to-sale tracking system, but GTI also integrates its in-house seed-to-sale tracking system with BioTrackTHC.
Every month the NYSDOH requests a dispensing report in Excel format, via email, showing all products dispensed for the month. This is the only report that the Company is required to submit to the NYSDOH. All other data is pulled by the NYSDOH directly from the Company’s seed-to-sale tracking system.
Ohio
House Bill 523, effective on September 8, 2016, legalized medical marijuana in Ohio. The Ohio Medical Marijuana Control Program allows people with certain medical conditions, upon the recommendation of an Ohio-licensed physician certified by the State Medical Board, to purchase and use medical marijuana. In November 2018, the state issued 12 ‘Level I’ cultivation licenses, which permit up to 25,000 square feet of canopy, and 12 ‘Level II’ cultivation licenses, which permit up to 3,000 square feet of canopy. In June 2018, the state issued 56 dispensary licenses. In August 2018, the state issued seven processing licenses and, over the next few months, issued seven additional processing licenses. In January 2019, the state issued an additional 26 processing licenses for a total of 40 across Ohio. On December 3, 2019, the State of Ohio Board of Pharmacy awarded one additional medical marijuana provisional dispensary license. The licenses were awarded after an extensive review of 376 submitted dispensary applications.
By rule, the State of Ohio Board of Pharmacy is currently limited to issuing up to 60 dispensary licenses across the state. Under the program rules, the Board will consider, on at least a biennial basis, whether enough medical marijuana dispensaries exist, considering the state population, the number of patients seeking to use medical marijuana and the geographic distribution of dispensary sites.
Ohio License and Regulations
There are three principal license categories in Ohio: (1) cultivation, (2) processing and (3) dispensary. The Company is licensed to operate one medical marijuana cultivation facility, one medical marijuana processing facility and up to five retail medical marijuana dispensaries in the state of Ohio. All licenses are, as of the date hereof, active with the State of Ohio. The licenses are independently issued for each approved activity for use at the Company facilities in Ohio.
The three following state government agencies are responsible for the operation of Ohio’s Medical Marijuana Control Program: (1) the Ohio Department of Commerce oversees medical marijuana cultivators, processors and testing laboratories; (2) the State of Ohio Board of Pharmacy oversees medical marijuana retail dispensaries, the registration of medical marijuana patients and caregivers, the approval of new forms of medical marijuana and coordinating the Medical Marijuana Advisory Committee; and (3) the State Medical Board of Ohio certifies physicians to recommend medical marijuana and may add to the list of qualifying conditions for which medical marijuana can be recommended.
Certificates of operation for dispensaries carry two year terms, while certificates of operation for cultivators and processors must be renewed annually. A certificate of operation will expire on the date identified on the certificate. A dispensary licensee will receive written or electronic notice 90 days before the expiration of its certificate of operation. The dispensary licensee must submit the renewal information at least 45 days prior to the date the existing certificate expires. A processor or cultivator licensee must submit the renewal application at least 30 days prior to the expiration date of the certificate of operation. If a licensee’s renewal application is not filed prior to the expiration date of the certificate of operation, the certificate of operation will be suspended for a maximum of 30 days. After 30 days, if the licensee has not successfully renewed the certificate of operation, including the payment of all applicable fees, the certificate of operations will be deemed expired.
24
Table of Contents
The medical cultivation licenses permit the Company to acquire, possess, cultivate, manufacture/process into medical marijuana products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
The medical processor license permits the Company to manufacture and produce medical marijuana products.
The dispensary licenses will permit the Company to purchase marijuana and marijuana products from cultivation and/or processing facilities, as well as allow the sale of marijuana and marijuana products to registered patients.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Ohio.
Ohio Reporting Requirements
Ohio uses METRC as its seed-to-sale tracking system. Licensees are required to use METRC in Ohio to push data to the state to meet all of the reporting requirements. The Company integrates its in-house seed-to-sale tracking system with METRC to capture the required data points as required in the Ohio medical marijuana laws and regulations.
Pennsylvania
The Pennsylvania medical marijuana program was signed into law on April 17, 2016 under Act 16 and provided access to state residents with one or more of 17 qualifying conditions, including: epilepsy, chronic pain and PTSD. The state originally awarded only 12 licenses to cultivate/process and 27 licenses to operate retail dispensaries (which entitled holders to up to three medical dispensary locations per retail license).
On March 22, 2018, it was announced that the final phase of the Pennsylvania medical marijuana program would initiate its rollout, which included 13 additional cultivation/processing licenses and 23 additional dispensary licenses. Additionally, the list of qualifying conditions was expanded from 17 to 21.
Pennsylvania Licenses and Regulations
There are two principal license categories in Pennsylvania: (1) cultivation/processing and (2) dispensary. The Company’s subsidiary GTI Pennsylvania, LLC is licensed to operate a medical cultivation/processing facility and is also licensed to operate medical retail locations. The Company’s subsidiary KW Ventures Holdings, LLC is also licensed to operate medical retail locations. All licenses are, as of the date hereof, active with the Commonwealth of Pennsylvania. The licenses are independently issued for each approved activity for use at the Company facilities in Pennsylvania.
All cultivation/processing establishments and dispensaries must register with Pennsylvania Department of Health. Registration certificates are valid for a period of one year and are subject to annual renewals after required fees are paid and the business remains in good standing. The Pennsylvania Department of Health must renew a permit unless it determines the applicant is unlikely to maintain effective control against diversion of medical cannabis and the applicant is unlikely to comply with all laws as prescribed under the Pennsylvania medical marijuana program. Under applicable laws, the licenses permit the license holder to cultivate, manufacture, process, package, sell and purchase medical marijuana pursuant to the terms of the licenses, which are issued by the Pennsylvania Department of Health under the provisions of Medical Marijuana Act and Pennsylvania regulations.
The medical cultivation/processing licenses permit the Company to acquire, possess, cultivate, manufacture/process into medical marijuana products and/or medical marijuana-infused products, deliver, transfer, have tested, transport, supply or sell marijuana and related supplies to medical marijuana dispensaries.
25
Table of Contents
The retail dispensary licenses permit the Company to purchase marijuana and marijuana products from cultivation/processing facilities, as well as allow the sale of marijuana and marijuana products.
See Appendix A to this Annual Report for a list of the licenses issued to the Company with respect to its operations in Pennsylvania.
Pennsylvania Reporting Requirements
Pennsylvania uses MJ Freeway as the state’s computerized T&T system for seed-to-sale reporting. Individual licensees are required to use MJ Freeway to push data to the state to meet all reporting requirements. The Company uses MJ Freeway as its in-house computerized seed-to-sale software, which integrates with the state’s MJ Freeway program and captures the required data points for cultivation, manufacturing and retail as required in the Pennsylvania medical marijuana laws and regulations.
Compliance with Applicable Federal Law
The Company is in compliance with applicable cannabis licensing requirements and the regulatory framework enacted by each state in which it operates. The Company is not subject to any material citations or notices of violation of applicable licensing requirements or the regulatory framework enacted by each applicable state which may have an adverse impact on its licenses, business activities or operations.
The Company has in place a detailed compliance program and an internal legal and compliance department and is building out its operational compliance team across all states in which it operates. The Company also has external state and local regulatory/compliance counsel engaged in every jurisdiction in which it operates.
The Company provides training for all employees, including on the following topics:
| • | Compliance with state and local laws |
| • | Safe cannabis use |
| • | Dispensing procedures |
| • | Security and safety policies and procedures |
| • | Inventory control |
| • | T&T training session |
| • | Quality control |
| • | Transportation procedures |
The Company emphasizes security and inventory control to ensure strict monitoring of cannabis and inventory, from delivery by a licensed distributor to sale or disposal. Only authorized, properly trained employees are allowed to access the Company’s computerized seed-to-sale system.
The Company monitors all compliance notifications from the regulators and inspectors in each market and timely resolves any issues identified. The Company keeps records of all compliance notifications received from the state regulators or inspectors, as well as how and when an issue was resolved. Moreover, the Company monitors news sources for information regarding developments at the state and federal level relating to the regulation and criminalization of cannabis.
Further, the Company has created comprehensive standard operating procedures that include detailed descriptions and instructions for receiving shipments of inventory, inventory tracking, recordkeeping and record
26
Table of Contents
retention practices related to inventory. The Company also has comprehensive standard operating procedures in place for performing inventory reconciliation, and ensuring the accuracy of inventory tracking and recordkeeping. The Company maintains accurate records of its inventory at all licensed facilities. Adherence to the Company’s standard operating procedures is mandatory and ensures that the Company’s operations are compliant with the rules set forth by the applicable state and local laws, regulations, ordinances, licenses and other requirements. The Company ensures adherence to standard operating procedures by regularly conducting internal inspections and ensures that any issues identified are resolved quickly and thoroughly.
Federal Law
The inconsistencies between federal and state regulation of cannabis were addressed in a memorandum which then-Deputy Attorney General James Cole sent to all U.S. District Attorneys in August 2013 (the “Cole Memorandum”) outlining certain priorities for the DOJ relating to the prosecution of cannabis offenses. The Cole Memorandum acknowledged that, notwithstanding the designation of cannabis as a Schedule I controlled substance at the federal level, several states had enacted laws authorizing the use of cannabis for medical purposes. The Cole Memorandum noted that jurisdictions that have enacted laws legalizing cannabis in some form have also implemented strong and effective regulatory and enforcement systems to control the cultivation, processing, distribution, sale and possession of cannabis. As such, conduct in compliance with those laws and regulations is less likely to implicate the Cole Memorandum’s enforcement priorities. The DOJ did not provide (and has not provided since) specific guidelines for what regulatory and enforcement systems would be deemed sufficient under the Cole Memorandum. In light of limited investigative and prosecutorial resources, the Cole Memorandum concluded that the DOJ should be focused on addressing only the most significant threats related to cannabis, such as distribution of cannabis from states where cannabis is legal to those where cannabis is illegal, the diversion of cannabis revenues to illicit drug cartels and sales of cannabis to minors.
On January 4, 2018, former U.S. Attorney General Jeff Sessions issued a new memorandum (the “Sessions Memorandum”), which rescinded the Cole Memorandum. The Sessions Memorandum stated, in part, that current law reflects “Congress’ determination that cannabis is a dangerous drug and cannabis activity is a serious crime,” and Mr. Sessions directed all U.S. Attorneys to enforce the laws enacted by Congress by following well-established principles when pursuing prosecutions related to cannabis activities. The Company is not aware of any prosecutions of investment companies doing routine business with licensed marijuana related businesses in light of the new DOJ position. However, there can be no assurance that the federal government will not enforce federal laws relating to cannabis in the future. As a result of the Sessions Memorandum, federal prosecutors are now free to utilize their prosecutorial discretion to decide whether to prosecute cannabis activities, despite the existence of state-level laws that may be inconsistent with federal prohibitions. No direction was given to federal prosecutors in the Sessions Memorandum as to the priority they should ascribe to such cannabis activities, and thus it is uncertain how active U.S. federal prosecutors will be in relation to such activities.
While federal prosecutors appear to continue to use the Cole Memorandum’s priorities as an enforcement guide, the Company believes it is too soon to determine what prosecutorial effects will be created by the rescission of the Cole Memorandum and the implementation of the Sessions Memorandum. The sheer size of the cannabis industry, in addition to participation by state and local governments and investors, suggests that a large-scale federal enforcement operation would more than likely create unwanted political backlash for the DOJ and the current administration. It is also possible that the revocation of the Cole Memorandum could motivate Congress to reconcile federal and state laws. Indeed, the U.S. House Judiciary Committee approved a bill on November 20, 2019 that removes cannabis from Schedule I of the CSA. This legislation will next be voted upon by the U.S. House of Representatives. If the bill passes the House of Representatives, it will then advance to the U.S. Senate. While Congress is considering legislation that may address these issues, there can be no assurance that such legislation passes. Regardless, at this time, cannabis remains a Schedule I controlled substance at the federal level. The U.S. federal government has always reserved the right to enforce federal law in regard to the sale and disbursement of medical or adult use cannabis, even if state law authorizes such sale and disbursement. It is unclear whether the risk of enforcement has been altered.
27
Table of Contents
On June 7, 2018, the Strengthening the Tenth Amendment Through Entrusting States Act (the “STATES Act”) was introduced in the Senate by Republican Senator Cory Gardner of Colorado and Democratic Senator Elizabeth Warren of Massachusetts. A companion bill was introduced in the House by Democratic representative Jared Polis of Colorado. The bill provides in relevant part that the provisions of the CSA, as applied to marijuana, “shall not apply to any person acting in compliance with state law relating to the manufacture, production, possession, distribution, dispensation, administration, or delivery of marihuana.” Even though marijuana will remain within Schedule I of the CSA under the STATES Act, the bill makes the CSA unenforceable to the extent it conflicts with state law. In essence, the bill extends the limitations afforded by the protection within the federal budget – which prevents the DOJ and the DEA from using funds to enforce federal law against state-legal medical cannabis commercial activity – to both medical and adult use cannabis activity in all states where it has been legalized. By allowing continued prohibition to be a choice by the individual states, the STATES Act does not fully legalize cannabis on a national level. In that respect, the bill emphasizes states’ rights under the Tenth Amendment, which provides that “the powers not delegated to the United States by the Constitution, nor prohibited by it to the States, are reserved to the States respectively, or to the people.” Under the STATES Act companies operating legal cannabis businesses would no longer be considered “trafficking” under the CSA, and this would likely assist financial institutions in transacting with individuals and businesses in the cannabis industry without the threat of money laundering prosecution, civil forfeiture and other criminal violations that could lead to a charter revocation. The STATES Act was reintroduced on April 4, 2019 in both the House and the Senate. Since the STATES Act is currently draft legislation, there is no guarantee that the STATES Act will become law in its current form.
One legislative safeguard for the medical cannabis industry, appended to the federal budget bill, remains in place following the rescission of the Cole Memorandum. For fiscal years 2015, 2016, 2017 and 2018, Congress adopted a so-called “rider” provision to the Consolidated Appropriations Acts (formerly referred to as the Rohrabacher-Farr Amendment and currently referred to as the “Rohrabacher-Blumenauer Amendment”) to prevent the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state and local law. The Rohrabacher-Blumenauer Amendment was included in the fiscal year 2018 budget passed on March 23, 2018. The Rohrabacher-Blumenauer Amendment was included in the consolidated appropriations bill signed into legislation by President Trump in February 2019. In signing the Rohrabacher-Blumenauer Amendment, President Trump issued a signing statement noting that the Rohrabacher-Blumenauer Amendment “provides that the Department of Justice may not use any funds to prevent implementation of medical marijuana laws by various States and territories,” and further stating “I will treat this provision consistent with the President’s constitutional responsibility to faithfully execute the laws of the United States.” On June 20, 2019, the House approved a broader amendment that, in addition to protecting state medical cannabis programs, would also protect state adult use programs. On September 26, 2019, the Senate Appropriations Committee declined to take up the broader amendment but did approve the Rohrabacher-Blumenauer Amendment for the fiscal year 2020 spending bill. On September 27, 2019, the Rohrabacher-Blumenauer Amendment was reviewed as part of a stopgap spending bill, in effect through November 21, 2019. The Rohrabacher-Blumenauer Amendment may or may not be renewed as part of a subsequent stopgap spending bill or omnibus appropriations package.
Despite the rescission of the Cole Memorandum, the DOJ appears to continue to adhere to the enforcement priorities set forth in the Core Memorandum. Accordingly, as an industry best practice, the Company continues to employ the following policies to ensure compliance with the guidance provided by the Cole Memorandum:
| • | the operations of the Company and its subsidiaries are compliant with all licensing requirements as established by the applicable state, county, municipality, town, township, borough and other political/administrative divisions; to this end, the Company retains appropriately experienced legal counsel to conduct the necessary due diligence to ensure compliance of such operations with all applicable state and local laws; |
28
Table of Contents
| • | the cannabis-related activities adhere to the scope of the licensing obtained – for example, in states where only medical cannabis is permitted, the products are only sold to patients who hold the necessary documentation to permit the possession of the cannabis; in states where cannabis is permitted for adult use, the products are only sold to individuals who meet the requisite age requirements; |
| • | the Company only works through licensed operators, which must pass a range of requirements, adhere to strict business practice standards and be subject to strict regulatory oversight to ensure that no revenue is distributed to criminal enterprises, gangs or cartels; |
| • | the Company has implemented an inventory tracking system and necessary procedures to ensure that such compliance system is effective in tracking inventory and preventing diversion of cannabis or cannabis products into those states where cannabis is not permitted by state law or cross any state lines in general; |
| • | the Company’s state-authorized cannabis business activity is not used as a cover or pretense for trafficking of other illegal drugs, and the Company is not engaged in any other illegal activity or any activities that are contrary to any applicable anti-money laundering statutes; and |
| • | the Company conducts reviews of products and product packaging to ensure that the products comply with applicable regulations and contain necessary disclaimers about the contents of the products to prevent adverse public health consequences from cannabis use and prevent impaired driving. |
The Cole Memorandum and the Rohrabacher-Blumenauer Amendment gave licensed cannabis operators (particularly medical cannabis operators) and investors in states with legal regimes greater certainty regarding the DOJ’s enforcement priorities and the risk of operating cannabis businesses. While the Sessions Memorandum has introduced some uncertainty regarding federal enforcement, the cannabis industry continues to experience growth in legal medical and adult use markets across the United States. U.S. Attorney General Jeff Sessions resigned on November 7, 2018. On February 14, 2019, William Barr was confirmed as U.S. Attorney General. It is unclear what impact, if any, this development will have on U.S. federal government enforcement policy. However, in a written response to questions from U.S. Senator Cory Booker made as a nominee, U.S. Attorney General Barr stated: “I do not intend to go after parties who have complied with state law in reliance on the Cole Memo.” With respect to the STATES Act, Mr. Barr stated: “Personally, I would still favor one uniform federal rule against marijuana but, if there is not sufficient consensus to obtain that, then I think the way to go is to permit a more federal approach so states can make their own decisions within the framework of the federal law and so we’re not just ignoring the enforcement of federal law.” Mr. Barr has also stated the need for more legal growers of marijuana for research and acknowledged that the Farm Bill has broad implications for the sale of cannabis products. Nonetheless, there is no guarantee that state laws legalizing and regulating the sale and use of cannabis will remain in place or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. Unless and until the United States Congress amends the CSA with respect to cannabis (and as to the timing or scope of any such potential amendments there can be no assurance), there is a risk that federal authorities may enforce current U.S. federal law criminalizing cannabis.
The Company will continue to monitor compliance on an ongoing basis in accordance with its compliance program and standard operating procedures. While the Company’s operations are in full compliance with all applicable state laws, regulations and licensing requirements, such activities remain illegal under federal law. For the reasons described above and the risks further described in Item 1A—“Risk Factors,” there are significant risks associated with the business of the Company. Readers of this Form are strongly encouraged to carefully read all of the risk factors contained in Item 1A—“Risk Factors.”
29
Table of Contents
Ability to Access Public and Private Capital
Due to the present state of the laws and regulations governing financial institutions in the U.S., banks often refuse to provide banking services to businesses involved in the marijuana industry. Consequently, it may be difficult for the Company to obtain financing from large U.S. financial institutions.
The Company has historically, and continues to have, access to equity and debt financing from non-public (i.e., private placement) markets. The Company’s executive team and Board of Directors (the “Board”) have extensive relationships with sources of capital (such as funds and high net worth individuals).
In addition to the Company’s working capital, the Company continues to generate adequate cash to fund its operations from capital raising transactions, including:
| • | the Convertible Promissory Note; |
| • | the SR Offering; |
| • | the C$80.3 million bought deal financing in August 2018; |
| • | the C$101.7 million bought deal financing in October 2018; |
| • | the Bridge Notes; |
| • | the Notes; and |
| • | the $39.6 million sale and lease back transaction with IIP for the Danville, Pennsylvania facility in November 2019. |
| • | the $7.2 million sale and leaseback transaction with IIP for the Toledo, Ohio facility in January 2020. |
| • | The $50.0 million sale and lease back transaction with IIP for the Oglesby, Illinois facility in March 2020. |
The Company’s business plan continues to include aggressive growth, both in the form of additional acquisitions and through facility expansion and improvements. Accordingly, the Company expects to raise additional capital, both in the form of debt and new equity offerings during the next few years.
However, there can be no assurance that additional financing will be available to the Company when needed or on terms which are acceptable.
Restricted Access to Banking and Other Financial Services
The U.S. Department of the Treasury’s Financial Crimes Enforcement Network (“FinCEN”) issued a memorandum on February 14, 2014 (the “FinCEN Memorandum”) with respect to financial institutions providing banking services to cannabis businesses. These include burdensome due diligence expectations and reporting requirements. The FinCEN Memorandum outlines the pathways for financial institutions to bank state-sanctioned cannabis businesses in compliance with federal enforcement priorities. The FinCEN Memorandum echoed the enforcement priorities of the Cole Memorandum and states that, in some circumstances, it is permissible for banks to provide services to cannabis-related businesses without risking prosecution for violation of federal money laundering laws. Under these guidelines, financial institutions must submit a Suspicious Activity Report (“SAR”) in connection with all cannabis-related banking activities by any client of such financial institution, in accordance with federal money laundering laws. These cannabis-related SARs are divided into three categories – cannabis limited, cannabis priority, and cannabis terminated – based on the financial institution’s belief that the business in question follows state law, is operating outside of compliance with state law, or where the banking relationship has been terminated, respectively.
30
Table of Contents
Former U.S. Attorney General Sessions’ revocation of the Cole Memorandum has not affected the status of the FinCEN Memorandum, nor has the Department of the Treasury given any indication that it intends to rescind the FinCEN Memorandum itself. Shortly after the Sessions Memorandum was issued, FinCEN did state that it would review the FinCEN Memorandum, but FinCEN has not yet issued further guidance. The FinCEN Memorandum is a standalone document which explicitly lists the eight enforcement priorities originally cited in the Cole Memorandum. As such, the FinCEN Memorandum remains intact, indicating that the Department of the Treasury and FinCEN intend to continue abiding by its guidance.
However, the FinCEN Memorandum does not provide any safe harbors or legal defenses from examination or regulatory or criminal enforcement actions by the DOJ, FinCEN or other federal regulators. Thus, most banks and other financial institutions in the United States do not appear comfortable providing banking services to cannabis-related businesses or relying on this guidance, given that it has the potential to be amended or revoked by the current administration. In addition to the foregoing, banks may refuse to process debit card payments and credit card companies generally refuse to process credit card payments for cannabis-related businesses. As a result, the Company may have limited or no access to banking or other financial services in the United States. In addition, federal money laundering statutes and regulations under the U.S. Currency and Foreign Transactions Reporting Act of 1970 (commonly known as the “Bank Secrecy Act”), as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (the “USA Patriot Act”), discourage financial institutions from working with any organization that sells a controlled substance, regardless of whether the state it operates in permits cannabis sales. The inability or limitation of the Company’s ability to open or maintain bank accounts, obtain other banking services and/or accept credit card and debit card payments may make it difficult for the Company to operate and conduct its business as planned or to operate efficiently.
Banks and other depository institutions are currently hindered by federal law from providing financial services to marijuana businesses, even in states where those businesses are regulated. On March 7, 2019, Democratic representative Ed Perlmutter of Colorado introduced house bill H.R. 1595, known as the Secure and Fair Enforcement (SAFE) Banking Act of 2019 (the “SAFE Banking Act”), which would protect banks and their employees from punishment for providing services to cannabis businesses that are legal on a state level. The bill was advanced by the House Financial Services Committee on March 28, 2019 and passed with strong bipartisan support in the House of Representatives on September 25, 2019.
Newly Established Legal Regime
The Company’s business activities rely on newly established and/or developing laws and regulations in the states in which it operates. These laws and regulations are rapidly evolving and subject to change with minimal notice. Regulatory changes may adversely affect the Company’s profitability or cause it to cease operations entirely. The cannabis industry may come under further scrutiny by the FDA, the SEC, the DOJ, the Financial Industry Regulatory Advisory and other regulatory authorities that supervise or regulate the production, distribution, sale and use of cannabis for medical and nonmedical purposes in the United States. It is impossible to determine the extent of the impact of new laws, regulations or initiatives that may be proposed. The regulatory uncertainty surrounding the industry may adversely affect the business and operations of the Company, including without limitation, the costs to remain compliant with applicable laws and the impairment of its business or the ability to raise additional capital.
Available Information
Our website address is www.gtigrows.com. Through this website, our filings with the SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and all amendments to those reports, will be accessible (free of charge) as soon as reasonably practicable after materials are electronically filed with or furnished to the SEC. The information provided on our website is not part of this document.
31
Table of Contents
You also may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
Certain factors may have a material adverse effect on our business, financial condition, and results of operations. You should carefully consider the following risks, together with all of the other information contained in this Annual Report on Form 10-K, including the sections titled “Disclosure Regarding Forward-Looking Statements” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes included elsewhere in this Annual Report on Form 10-K. Any of the following risks could have an adverse effect on our business, financial condition, operating results, or prospects and could cause the trading price of our common stock to decline, which would cause you to lose all or part of your investment. Our business, financial condition, operating results, or prospects could also be harmed by risks and uncertainties not currently known to us or that we currently do not believe are material.
Risks Related to Our Business
Marijuana remains illegal under federal law, and enforcement of cannabis laws could change.
Cannabis is illegal under U.S. federal law. In those states in which the use of cannabis has been legalized, its use remains a violation of federal law pursuant to the CSA. The CSA classifies cannabis as a Schedule I controlled substance, and as such, medical and adult use cannabis use is illegal under U.S. federal law. Unless and until Congress amends the CSA with respect to cannabis (and the President approves such amendment), there is a risk that federal authorities may enforce current federal law. If that occurs, the Company may be deemed to be producing, cultivating or dispensing cannabis and drug paraphernalia in violation of federal law. Since federal law criminalizing the use of cannabis pre-empts state laws that legalize its use, enforcement of federal law regarding cannabis is a significant risk and would greatly harm the Company’s business, prospects, revenue, results of operation and financial condition.
The activities of the Company are, and will continue to be, subject to evolving regulation by governmental authorities. The Company is directly or indirectly engaged in the medical and adult use cannabis industry in the United States where local state law permits such activities. The legality of the production, cultivation, extraction, distribution, retail sales, transportation and use of cannabis differs among states in the United States. Due to the current regulatory environment in the United States, new risks may emerge, and management may not be able to predict all such risks.
As of December 31, 2019, there are 33 states, plus the District of Columbia (and the territories of Guam, Puerto Rico, the U.S. Virgin Islands and the Northern Mariana Islands), that have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and consumer use of cannabis in connection with medical treatment. In addition, Alaska, California, Colorado, Illinois (beginning on January 1, 2020), Maine, Massachusetts, Michigan, Nevada, Oregon, Vermont, Washington and the District of Columbia have legalized cannabis for adult use.
The Company’s activities in the medical and adult use cannabis industry may be illegal under the applicable federal laws of the United States. There can be no assurances that the federal government of the United States will not seek to enforce the applicable laws against the Company. The consequences of such enforcement would be materially adverse to the Company and the Company’s business, including its reputation, profitability and the market price of its publicly traded shares, and could result in the forfeiture or seizure of all or substantially all of the Company’s assets.
32
Table of Contents
Due to the conflicting views between state legislatures and the federal government regarding cannabis, cannabis businesses are subject to inconsistent laws and regulations. The prior U.S. administration attempted to address the inconsistent treatment of cannabis under state and federal law in the Cole Memorandum that Deputy Attorney General James Cole sent to all U.S. Attorneys in August 2013, which outlined certain priorities for the DOJ relating to the prosecution of cannabis offenses. The Cole Memorandum noted that, in jurisdictions that have enacted laws legalizing cannabis in some form and that have also implemented strong and effective regulatory and enforcement systems to control the cultivation, processing, distribution, sale and possession of cannabis, conduct in compliance with such laws and regulations was not a priority for the DOJ. However, the DOJ did not provide (and has not provided since) specific guidelines for what regulatory and enforcement systems would be deemed sufficient under the Cole Memorandum.
On January 4, 2018, former U.S. Attorney General Jeff Sessions formally issued the Sessions Memorandum, which rescinded the Cole Memorandum effective upon its issuance. The Sessions Memorandum stated, in part, that current law reflects “Congress’ determination that cannabis is a dangerous drug and cannabis activity is a serious crime,” and Mr. Sessions directed all U.S. Attorneys to enforce the laws enacted by Congress and to follow well-established principles when pursuing prosecutions related to cannabis activities.
As a result of the Sessions Memorandum, federal prosecutors are now free to utilize their prosecutorial discretion to decide whether to prosecute cannabis activities, despite the existence of state-level laws that may be inconsistent with federal prohibitions. No direction was given to federal prosecutors in the Sessions Memorandum as to the priority they should ascribe to such cannabis activities, and thus it is uncertain how active U.S. federal prosecutors will be in relation to such activities.
There can be no assurance that the federal government will not enforce federal laws relating to cannabis and seek to prosecute cases involving cannabis businesses that are otherwise compliant with state laws in the future. Jeff Sessions resigned as U.S. Attorney General on November 7, 2018. On February 14, 2019, William Barr was confirmed as U.S. Attorney General. It is unclear what impact this development will have on U.S. federal government enforcement policy.
Additionally, the Rohrabacher-Blumenauer Amendment may or may not be renewed as part of a subsequent stopgap spending bill or omnibus appropriations package in order to prevent the federal government from using congressionally appropriated funds to enforce federal cannabis laws against regulated medical cannabis actors operating in compliance with state and local law. If the Rohrabacher-Blumenauer Amendment is not renewed, potential proceedings could involve significant restrictions being imposed upon the Company or third parties and divert the attention of key executives. Such proceedings could also have a material adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition, as well as the Company’s reputation, even if such proceedings were concluded successfully in favor of the Company. Further, there is no guarantee that draft legislation such as the STATES Act will become law in its current form.
The uncertainty of U.S. federal enforcement practices going forward and the inconsistency between U.S. federal and state laws and regulations present major risks for the Company.
The Company may be subject to action by the U.S. federal government.
Since the cultivation, processing, production, distribution and sale of cannabis for any purpose, medical, adult use or otherwise, remain illegal under U.S. federal law, it is possible that the Company may be forced to cease activities. The U.S. federal government, through, among others, the DOJ, its sub-agency the Drug Enforcement Administration (“DEA”) and the U.S. Internal Revenue Service (the “IRS”), has the right to actively investigate, audit and shut down cannabis growing facilities, processors and retailers. The U.S. federal government may also attempt to seize the property of the Company. Any action taken by the DOJ, the DEA and/or the IRS to interfere with, seize or shut down the operations of the Company will have an adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition.
33
Table of Contents
Since federal law criminalizing the use of cannabis pre-empts state laws that legalize its use, the federal government can assert criminal violations of federal law despite state laws permitting the use of cannabis. While it does not appear that federal law enforcement and regulatory agencies are focusing resources on licensed marijuana related businesses that are operating in compliance with state law, the stated position of the current administration is hostile to legal cannabis. As the recession of the Cole Memorandum and the implementation of the Sessions Memorandum demonstrate, the DOJ may at any time issue additional guidance that directs federal prosecutors to devote more resources to prosecuting marijuana related businesses. In the event that the DOJ under U.S. Attorney General Barr aggressively pursues financiers or equity owners of cannabis-related businesses, and U.S. Attorneys follow the DOJ policies through pursuing prosecutions, then the Company could face:
| (i) | seizure of its cash and other assets used to support or derived from its cannabis subsidiaries; |
| (ii) | the arrest of its employees, directors, officers, managers and investors; and |
| (iii) | ancillary criminal violations of the CSA for aiding and abetting, and conspiracy to violate the CSA by providing financial support to cannabis companies that service or provide goods to state-licensed or permitted cultivators, processors, distributors and/or retailers of cannabis. |
Because the Cole Memorandum was rescinded, the DOJ under the current administration or an aggressive federal prosecutor could allege that the Company, its Board and it’s executive officers and, potentially, its shareholders, “aided and abetted” violations of federal law by providing finances and services to its portfolio cannabis companies. Under these circumstances, federal prosecutors could seek to seize the assets of the Company, and to recover the “illicit profits” previously distributed to shareholders resulting from any of the Company’s financing or services. In these circumstances, the Company’s operations would cease, shareholders may lose their entire investments and directors, officers and/or shareholders may be left to defend any criminal charges against them at their own expense and, if convicted, be sent to federal prison.
Additionally, there can be no assurance as to the position any new administration may take on marijuana, and a new administration could decide to enforce the federal laws strongly. Any enforcement of current federal marijuana laws could cause significant financial damage to the Company and its shareholders. Further, future presidential administrations may choose to treat marijuana differently and potentially enforce the federal laws more aggressively.
Violations of any federal laws and regulations could result in significant fines, penalties, administrative sanctions, convictions or settlements arising from civil proceedings conducted by either the federal government or private citizens, or criminal charges, including, but not limited to, disgorgement of profits, cessation of business activities or divestiture. These results could have a material adverse effect on the Company, including its reputation and ability to conduct business, its holding (directly or indirectly) of cannabis licenses in the United States, the listing of its securities on various stock exchanges, its financial position, operating results, profitability or liquidity or the market price of its Subordinate Voting Shares. In addition, it is difficult to estimate the time or resources that would be needed for the investigation or final resolution of any such matters because: (i) the time and resources that may be needed depend on the nature and extent of any information requested by the authorities involved, and (ii) such time or resources could be substantial.
State regulation of cannabis is uncertain.
There is no assurance that state laws legalizing and regulating the sale and use of cannabis will not be repealed or overturned, or that local governmental authorities will not limit the applicability of state laws within their respective jurisdictions. If the U.S. federal government begins to enforce U.S. federal laws relating to cannabis in states where the sale and use of cannabis is currently legal, or if existing state laws are repealed or curtailed, the Company’s business or operations in those states or under those laws would be materially and adversely affected. Federal actions against any individual or entity engaged in the cannabis industry or a substantial repeal of cannabis related legislation could adversely affect the Company, its business and its assets or investments.
34
Table of Contents
The rulemaking process at the state level that applies to cannabis operators in any state will be ongoing and result in frequent changes. As a result, a compliance program is essential to manage regulatory risk. All operating policies and procedures implemented by the Company are compliance-based and are derived from the state regulatory structure governing ancillary cannabis businesses and their relationships to state-licensed or permitted cannabis operators, if any. Notwithstanding the Company’s efforts and diligence, regulatory compliance and the process of obtaining regulatory approvals can be costly and time-consuming. No assurance can be given that the Company will receive the requisite licenses, permits or cards to continue operating its businesses.
In addition, local laws and ordinances could restrict the Company’s business activity. Although the Company’s operations are legal under the laws of the states in which the Company’s business operate, local governments have the ability to limit, restrict and ban cannabis businesses from operating within their jurisdiction. Land use, zoning, local ordinances and similar laws could be adopted or changed and have a material adverse effect on the Company’s business.
Multiple states where medical and/or adult use cannabis is legal have or are considering special taxes or fees on businesses in the marijuana industry. It is uncertain at this time whether other states are in the process of reviewing such additional taxes and fees. The implementation of special taxes or fees could have a material adverse effect upon the Company’s business, prospects, revenue, results of operation and financial condition.
The Company currently operates in California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania and intends to operate in other states as deemed appropriate by management.
State regulatory agencies may require the Company to post bonds or significant fees.
There is a risk that a greater number of state regulatory agencies will begin requiring entities engaged in certain aspects of the business or industry of legal marijuana to post a bond or significant fees when applying, for example, for a dispensary license or renewal as a guarantee of payment of sales and franchise taxes. The Company is not able to quantify at this time the potential scope of such bonds or fees in the states in which it currently operates or may in the future operate. Any bonds or fees of material amounts could have a negative impact on the ultimate success of the Company’s business.
The Company may be subject to heightened scrutiny by Canadian regulatory authorities.
Currently, the Company is traded on the CSE and on over-the-counter markets in the United States. The business, operations and investments of the Company in the United States, and any future business, operations or investments, may become the subject of heightened scrutiny by regulators, stock exchanges and other authorities in Canada and the United States. As a result, the Company may be subject to significant direct and indirect interaction with public officials. There can be no assurance that this heightened scrutiny will not in turn lead to the imposition of certain restrictions on the Company’s ability to operate or invest in the United States or any other jurisdiction, in addition to those described herein.
In 2017, there were concerns that the Canadian Depository for Securities Limited, through its subsidiary CDS Clearing and Depository Services Inc. (“CDS”), Canada’s central securities depository (clearing and settling trades in the Canadian equity, fixed income and money markets), would refuse to settle trades for cannabis issuers that have investments in the United States. However, CDS has not implemented this policy.
On February 8, 2018, the Canadian Securities Administrators published Staff Notice 51-352 describing the Canadian Securities Administrators’ disclosure expectations for specific risks facing issuers with cannabis-related activities in the U.S. Staff Notice 51-352 confirms that a disclosure-based approach remains appropriate for issuers with U.S. cannabis-related activities. Staff Notice 51-352 includes additional disclosure expectations
35
Table of Contents
that apply to all issuers with U.S. cannabis-related activities, including those with direct and indirect involvement in the cultivation and distribution of cannabis, as well as issuers that provide goods and services to third parties involved in the U.S. cannabis industry.
On February 8, 2018, following discussions with the Canadian Securities Administrators and recognized Canadian securities exchanges, the TMX Group, which is the owner and operator of CDS, announced the signing of a Memorandum of Understanding (“MOU”) with Aequitas NEO Exchange Inc., the CSE, the Toronto Stock Exchange and the TSX Venture Exchange (“TSXV”). The MOU outlines the parties’ understanding of Canada’s regulatory framework applicable to the rules, procedures and regulatory oversight of the exchanges and CDS as it relates to issuers with cannabis-related activities in the United States. The MOU confirms, with respect to the clearing of listed securities, that CDS relies on the Canadian securities exchanges to review the conduct of listed issuers. The MOU notes that securities regulation requires that the rules of each of the exchanges must not be contrary to the public interest and that the rules of each of the exchanges have been approved by the securities regulators. Pursuant to the MOU, CDS will not ban accepting deposits of or transactions for clearing and settlement of securities of issuers with cannabis-related activities in the United States. Even though the MOU indicated that there are no plans to ban the settlement of securities through CDS, there can be no guarantee that this approach to regulation will continue in the future. If such a ban were implemented at a time when Subordinate Voting Shares are listed on a Canadian stock exchange, it would have a material adverse effect on the ability of holders of Subordinate Voting Shares to make and settle trades. In particular, the Subordinate Voting Shares would become highly illiquid until an alternative (if available) was implemented, and investors would have no ability to effect a trade of Subordinate Voting Shares through the facilities of the applicable Canadian stock exchange.
The Company may face limitations on ownership of cannabis licenses.
In certain states, the cannabis laws and regulations limit not only the number of cannabis licenses issued, but also the number of cannabis licenses that one person or entity may own. Such limitations on the ownership of additional licenses within certain states may limit the Company’s ability to grow in such states. The Company employs joint ventures from time to time to ensure continued compliance with the applicable regulatory guidelines. Currently, the Company has joint ventures with third parties in Connecticut, Illinois, Massachusetts and Ohio. The Company structures its joint ventures on a case-by-case basis but generally maintains operational control over the joint venture business and a variable economic interest through the applicable governing documents.
The Company may become subject to FDA or ATF regulation.
Cannabis remains a Schedule I controlled substance under U.S. federal law. If the federal government reclassifies cannabis to a Schedule II controlled substance, it is possible that the FDA would seek to regulate cannabis under the Food, Drug and Cosmetics Act of 1938. Additionally, the FDA may issue rules and regulations, including good manufacturing practices, related to the growth, cultivation, harvesting and processing of medical cannabis. Clinical trials may be needed to verify the efficacy and safety of cannabis. It is also possible that the FDA would require facilities where medical use cannabis is grown to register with the FDA and comply with certain federally prescribed regulations. In the event that some or all of these regulations are imposed, the impact they would have on the cannabis industry is unknown, including the costs, requirements and possible prohibitions that may be enforced. If the Company is unable to comply with the potential regulations or registration requirements prescribed by the FDA, it may have an adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition.
It is also possible that the federal government could seek to regulate cannabis under the U.S. Bureau of Alcohol, Tobacco, Firearms and Explosives (“ATF”). The ATF may issue rules and regulations related to the use, transporting, sale and advertising of cannabis or cannabis products, including smokeless cannabis products.
36
Table of Contents
Cannabis businesses are subject to applicable anti-money laundering laws and regulations and have restricted access to banking and other financial services.
The Company is subject to a variety of laws and regulations in the United States that involve money laundering, financial record-keeping and proceeds of crime, including the Bank Secrecy Act, as amended by Title III of the USA Patriot Act, and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the United States. Accordingly, pursuant to the Bank Secrecy Act, banks or other financial institutions that provide a cannabis business with a checking account, debit or credit card, small business loan or any other service could be found guilty of money laundering, aiding and abetting, or conspiracy.
FinCEN issued the FinCEN Memorandum on February 14, 2014, outlining the pathways for financial institutions to bank cannabis businesses in compliance with federal enforcement priorities. The FinCEN Memorandum states that in some circumstances, it is permissible for banks to provide services to cannabis-related businesses without risking prosecution for violation of federal money laundering laws. The FinCEN Memorandum refers to the Cole Memorandum’s enforcement priorities.
The revocation of the Cole Memorandum has not yet affected the status of the FinCEN Memorandum, nor has FinCEN given any indication that it intends to rescind the FinCEN Memorandum itself. Shortly after the Sessions Memorandum was issued, FinCEN did state that it would review the FinCEN Memorandum, but FinCEN has not yet issued further guidance.
Although the FinCEN Memorandum remains intact, it is unclear whether the current administration will continue to follow its guidelines. The DOJ continues to have the right and power to prosecute crimes committed by banks and financial institutions, such as money laundering and violations of the Bank Secrecy Act, that occur in any state including states that have in some form legalized the sale of cannabis. Further, the conduct of the DOJ’s enforcement priorities could change for any number of reasons. A change in the DOJ’s priorities could result in the prosecution of banks and financial institutions for crimes that were not previously prosecuted.
If the Company’s operations, or proceeds thereof, dividend distributions or profits or revenues derived from the Company’s operations were found to be in violation of money laundering legislation or otherwise, such transactions may be viewed as proceeds from a crime (the sale of a Schedule I drug) under the Bank Secrecy Act’s money laundering provisions. This may restrict the ability of the Company to declare or pay dividends or effect other distributions.
The FinCEN Memorandum does not provide any safe harbors or legal defenses from examination or regulatory or criminal enforcement actions by the DOJ, FinCEN or other federal regulators. Thus, most banks and other financial institutions in the United States do not appear comfortable providing banking services to cannabis-related businesses or relying on this guidance given that it has the potential to be amended or revoked by the current administration. In addition to the foregoing, banks may refuse to process debit card payments and credit card companies generally refuse to process credit card payments for cannabis-related businesses. As a result, the Company may have limited or no access to banking or other financial services in the United States. In addition, federal money laundering statutes and Bank Secrecy Act regulations discourage financial institutions from working with any organization that sells a controlled substance, regardless of whether the state it operates in permits cannabis sales. The inability or limitation of the Company’s ability to open or maintain bank accounts, obtain other banking services and/or accept credit card and debit card payments may make it difficult for the Company to operate and conduct its business as planned or to operate efficiently.
Banks and other depository institutions are currently hindered by federal law from providing financial services to marijuana businesses, even in states where those businesses are regulated. On March 7, 2019, Democratic representative Ed Perlmutter of Colorado introduced house bill H.R. 1595, known as the SAFE Banking Act, which would protect banks and their employees from punishment for providing services to cannabis businesses that are legal on a state level. The bill was advanced by the House Financial Services Committee on March 28, 2019 and passed with strong bipartisan support in the House of Representatives on September 25, 2019.
37
Table of Contents
The Company may face difficulties acquiring additional financing.
The Company may require equity and/or debt financing to support on-going operations, to undertake capital expenditures or to undertake acquisitions and/or other business combination transactions. There can be no assurance that additional financing will be available to the Company when needed or on terms which are acceptable. The Company’s inability to raise financing through traditional banking to fund on-going operations, capital expenditures or acquisitions could limit its growth and may have a material adverse effect upon the Company’s business, prospects, revenue, results of operation and financial condition.
The Company lacks access to U.S. bankruptcy protections.
Many courts have denied cannabis businesses bankruptcy protections because the use of cannabis is illegal under federal law. In the event of a bankruptcy, it would be very difficult for lenders to recoup their investments in the cannabis industry. If the Company were to experience a bankruptcy, there is no guarantee that U.S. federal bankruptcy protections would be available to the Company, which would have a material adverse effect on the Company.
We operate in a highly regulated sector and may not always succeed in complying fully with applicable regulatory requirements in all jurisdictions where we carry on business.
Our business and activities are heavily regulated in all jurisdictions where we carry on business. Our operations are subject to various laws, regulations and guidelines by state and local governmental authorities relating to the manufacture, marketing, management, transportation, storage, sale, pricing and disposal of cannabis and cannabis oil, and also including laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over our activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services. Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by these governmental authorities and obtaining all necessary regulatory approvals for the manufacture, production, storage, transportation, sale, import and export, as applicable, of our products. The commercial cannabis industry is still a new industry at the state and local level. The effect of relevant governmental authorities’ administration, application and enforcement of their respective regulatory regimes and delays in obtaining, or failure to obtain, applicable regulatory approvals which may be required may significantly delay or impact the development of markets, products and sales initiatives and could have a material adverse effect on our business, prospects, revenue, results of operation and financial condition.
While we endeavor to comply with all relevant laws, regulations and guidelines and, to our knowledge, we are in compliance or are in the process of being assessed for compliance with all such laws, regulations and guidelines, any failure to comply with the regulatory requirements applicable to our operations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate our business; the suspension or expulsion from a particular market or jurisdiction or of our key personnel; the imposition of additional or more stringent inspection, testing and reporting requirements; and the imposition of fines and censures. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increase compliance costs or give rise to material liabilities and/or revocation of our licenses and other permits, which could have a material adverse effect on our business, results of operations and financial condition. Furthermore, governmental authorities may change their administration, application or enforcement procedures at any time, which may adversely impact our ongoing costs relating to regulatory compliance.
The Company may face difficulties in enforcing its contracts.
Because the Company’s contracts involve cannabis and other activities that are not legal under federal law and in some state jurisdictions, the Company may face difficulties in enforcing its contracts in federal courts
38
Table of Contents
and certain state courts. The Company cannot be assured that it will have a remedy for breach of contract, which could have a material adverse effect on the Company.
The Company has limited trademark protection.
The Company will not be able to register any federal trademarks for its cannabis products. Because producing, manufacturing, processing, possessing, distributing, selling and using cannabis is a crime under the CSA, the Patent and Trademark Office will not permit the registration of any trademark that identifies cannabis products. As a result, the Company likely will be unable to protect its cannabis product trademarks beyond the geographic areas in which it conducts business. The use of the Company’s trademarks outside the states in which it operates by one or more other persons could have a material adverse effect on the value of such trademarks.
The Company may be subject to constraints on marketing its products.
There may be restrictions on sales and marketing activities imposed by government regulatory bodies that can hinder the development of the Company’s business and operating results. Restrictions may include regulations that specify what, where and to whom product information and descriptions may appear and/or be advertised. Marketing, advertising, packaging and labeling regulations also vary from state to state, potentially limiting the consistency and scale of consumer branding communication and product education efforts. The regulatory environment in the U.S. limits the Company’s ability to compete for market share in a manner similar to other industries. If the Company is unable to effectively market its products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for its products, the Company’s sales and operating results could be adversely affected.
The Company faces risks related to the results of future clinical research.
Research regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis or isolated cannabinoids (such as CBD and THC) remains in early stages. There have been relatively few clinical trials on the benefits of cannabis or isolated cannabinoids (such as CBD and THC). Although the Company believes that various articles, reports and studies support its beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Given these risks, uncertainties and assumptions, holders and prospective purchasers of Subordinate Voting Shares should not place undue reliance on such articles and reports. Future research studies and clinical trials may draw opposing conclusions to those stated in this Annual Report on Form 10-K or reach negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing, social acceptance or other facts and perceptions related to cannabis, which could have a material adverse effect on the demand for the Company’s products, with the potential to have a material adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition.
The Company is subject to taxation in Canada and the United States.
The Company is and will continue to be a Canadian corporation as of the date of this Annual Report on Form 10-K. The Company is treated as a Canadian resident company (as defined in the Income Tax Act (Canada) (the “ITA”)) subject to Canadian income taxes. The Company is also treated as a U.S. corporation subject to U.S. federal income tax pursuant to Section 7874 of the Internal Revenue Code (“IRC”) and is subject to U.S. federal income tax on its worldwide income. As a result, the Company is subject to taxation both in Canada and the United States, which could have a material adverse effect on its financial condition and results of operations.
It is unlikely that the Company will pay any dividends on the Subordinate Voting Shares in the foreseeable future. However, dividends received by shareholders who are residents of Canada for purposes of the ITA will be subject to U.S. withholding tax. Any such dividends may not qualify for a reduced rate of withholding tax under the Canada-United States tax treaty. In addition, a foreign tax credit or a deduction in respect of foreign taxes may not be available.
39
Table of Contents
Dividends received by U.S. shareholders will not be subject to U.S. withholding tax but will be subject to Canadian withholding tax. Dividends paid by the Company will be characterized as U.S. source income for purposes of the foreign tax credit rules under the IRC. Accordingly, U.S. shareholders generally will not be able to claim a credit for any Canadian tax withheld unless, depending on the circumstances, they have an excess foreign tax credit limitation due to other foreign source income that is subject to a low or zero rate of foreign tax.
Dividends received by shareholders that are neither Canadian nor U.S. shareholders will be subject to U.S. withholding tax and will also be subject to Canadian withholding tax. These dividends may not qualify for a reduced rate of U.S. withholding tax under any income tax treaty otherwise applicable to a shareholder of the Company, subject to examination of the relevant treaty.
Because the Subordinate Voting Shares are treated as shares of a U.S. domestic corporation, the U.S. gift, estate and generation-skipping transfer tax rules generally apply to a non-U.S. shareholder of Subordinate Voting Shares.
Each shareholder should seek tax advice, based on such shareholder’s particular circumstances, from an independent tax advisor.
Cannabis businesses are subject to unfavorable tax treatment.
Under Section 280E of the IRC, no deduction or credit is allowed for any amount paid or incurred during the taxable year in carrying on business if the business (or the activities which comprise the trade or business) consists of trafficking in controlled substances (within the meaning of Schedules I and II of the CSA). The IRS has applied this provision to cannabis operations, prohibiting them from deducting expenses associated with cannabis businesses. Section 280E may have a lesser impact on cannabis cultivation and manufacturing operations. Accordingly, Section 280E has a significant impact on the operations of cannabis companies and an otherwise profitable business may operate at a loss, after taking into account its U.S. income tax expenses.
Cannabis businesses may be subject to civil asset forfeiture.
Any property owned by participants in the cannabis industry used in the course of conducting such business, or that is the proceeds of such business, could be subject to seizure by law enforcement and subsequent civil asset forfeiture because of the illegality of the cannabis industry under federal law. Even if the owner of the property is never charged with a crime, the property in question could still be seized and subject to an administrative proceeding by which, with minimal due process, it could be subject to forfeiture.
The Company is subject to proceeds of crime statutes.
The Company will be subject to a variety of laws that concern money laundering, financial recordkeeping and proceeds of crime. These include: the Bank Secrecy Act, as amended by Title III of the USA Patriot Act, the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), the rules and regulations under the Criminal Code of Canada and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities in the United States and Canada.
In the event that any of the Company’s license agreements, or any proceeds thereof, in the United States were found to be in violation of money laundering legislation or otherwise, such transactions may be viewed as proceeds of crime under one or more of the statutes noted above, or any other applicable legislation. This could have a material adverse effect on the Company and, among other things, could restrict or otherwise jeopardize the ability of the Company to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada.
40
Table of Contents
The Company faces security risks.
The business premises of the Company’s operating locations are targets for theft. While the Company has implemented security measures at each location and continues to monitor and improve such security measures, its cultivation, processing and dispensary facilities could be subject to break-ins, robberies and other breaches in security. If there was a breach in security and the Company fell victim to a robbery or theft, the loss of cannabis plants, cannabis oils, cannabis flowers and cultivation and processing equipment could have a material adverse impact on the business, prospects, revenue, results of operation and financial condition of the Company.
As the Company’s business involves the movement and transfer of cash which is collected from dispensaries or patients/customers and deposited into its bank, there is a risk of theft or robbery during the transport of cash. The Company has engaged a security firm to provide security in the transport and movement of large amounts of cash. Employees sometimes transport cash and/or products and, if requested, may be escorted by armed guards. While the Company has taken robust steps to prevent theft or robbery of cash during transport, there can be no assurance that there will not be a security breach during the transport and the movement of cash involving the theft of product or cash.
The Company faces exposure to fraudulent or illegal activity.
The Company faces exposure to the risk that employees, independent contractors or consultants may engage in fraudulent or other illegal activities. Misconduct by these parties could be intentional, reckless and/or negligent conduct. There may be disclosure of unauthorized activities that violate government regulations, manufacturing standards, healthcare laws, abuse laws and other financial reporting laws. Further, it may not always be possible for the Company to identify and deter misconduct by its employees and other third parties, and the precautions taken by the Company to detect and prevent these activities may not always be effective. As a result, the Company could face potential penalties and litigation.
The Company is a holding company.
The Company is a holding company and essentially all of its assets are the capital stock of its subsidiaries in its 12 markets, including California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania. As a result, investors in the Company are subject to the risks attributable to its subsidiaries. As a holding company, the Company conducts substantially all of its business through its subsidiaries, which generate substantially all of its revenues. Consequently, the Company’s cash flows and ability to complete current or desirable future enhancement opportunities are dependent on the earnings of its subsidiaries and the distribution of those earnings to the Company. The ability of these entities to pay dividends and other distributions depends on their operating results and is subject to applicable laws and regulations, which require that solvency and capital standards be maintained by the subsidiaries and contractual restrictions are contained in the instruments governing their debt. In the event of a bankruptcy, liquidation or reorganization of any of the Company’s material subsidiaries, holders of indebtedness and trade creditors may be entitled to payment of their claims from the assets of those subsidiaries before the Company.
Our internal controls over financial reporting may not be effective, and our independent auditors may not be able to certify as to their effectiveness, which could have a significant and adverse effect on our business.
We are subject to various SEC reporting and other regulatory requirements. We have incurred and will continue to incur expenses and, to a lesser extent, diversion of our management’s time in our efforts to comply with Section 404 of the Sarbanes-Oxley Act regarding internal controls over financial reporting. Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement
41
Table of Contents
required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm when required, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retrospective changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our Subordinate Voting Shares.
Material acquisitions, dispositions and other strategic transactions involve a number of risks for the Company.
Material acquisitions, dispositions and other strategic transactions involve a number of risks for the Company, including:
| (i) | potential disruption of its ongoing business; |
| (ii) | distraction of management; |
| (iii) | increased financial leverage; |
| (iv) | the anticipated benefits and cost savings of those transactions may not be realized or may take longer to realize than anticipated; |
| (v) | increased scope and complexity of the Company’s operations; and |
| (vi) | loss or reduction of control over certain of the Company’s assets. |
Additionally, the Company may issue additional Subordinate Voting Shares in connection with such transactions, which would dilute a shareholder’s holdings in the Company.
The presence of one or more material liabilities of an acquired company that are known, but believed to be immaterial, or unknown to the Company at the time of acquisition could have a material adverse effect on the business, prospects, revenue, results of operation and financial condition of the Company. A strategic transaction may result in a significant change in the nature of the Company’s business, operations and strategy. In addition, the Company may encounter unforeseen obstacles or costs in implementing a strategic transaction or integrating any acquired business into the Company’s operations.
The Company may invest in pre-revenue companies which may not be able to meet anticipated revenue targets in the future.
The Company may make investments in companies with no significant sources of operating cash flow and no revenue from operations. The Company’s investments in such companies will be subject to risks and uncertainties that new companies with no operating history may face. In particular, there is a risk that the Company’s investment in these pre-revenue companies will not be able to meet anticipated revenue targets or will generate no revenue at all. The risk is that underperforming pre-revenue companies may lead to these businesses failing, which could have a material adverse effect on the business, prospects, revenue, results of operation and financial condition of the Company.
Our use of joint ventures may expose us to risks associated with jointly owned investments.
We currently operate parts of our business through joint ventures with other companies, and we may enter into additional joint ventures and strategic alliances in the future. Joint venture investments may involve risks not otherwise present in investments made solely by us, including: (i) we may not control the joint ventures; (ii) our joint venture partners may not agree to distributions that we believe are appropriate; (iii) where we do not
42
Table of Contents
have substantial decision-making authority, we may experience impasses or disputes with our joint venture partners on certain decisions, which could require us to expend additional resources to resolve such impasses or disputes, including litigation or arbitration; (iv) our joint venture partners may become insolvent or bankrupt, fail to fund their share of required capital contributions or fail to fulfil their obligations as a joint venture partner; (v) the arrangements governing our joint ventures may contain certain conditions or milestone events that may never be satisfied or achieved; (vi) our joint venture partners may have business or economic interests that are inconsistent with ours and may take actions contrary to our interests; (vii) we may suffer losses as a result of actions taken by our joint venture partners with respect to our joint venture investments; and (viii) it may be difficult for us to exit a joint venture if an impasse arises or if we desire to sell our interest for any reason. Any of the foregoing risks could have a material adverse effect on our business, financial condition and results of operations. In addition, we may, in certain circumstances, be liable for the actions of our joint venture partners.
There can be no assurance that our current and future strategic alliances or expansions of scope of existing relationships will have a beneficial impact on our business, financial condition and results of operations.
We currently have, and may in the future enter into, additional strategic alliances with third parties that we believe will complement or augment our existing business. Our ability to complete strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance our business and may involve risks that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will continue to achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, if at all. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operations.
Competition for the acquisition and leasing of properties suitable for the cultivation, production and sale of medical and adult use cannabis may impede our ability to make acquisitions or increase the cost of these acquisitions, which could adversely affect our operating results and financial condition.
We compete for the acquisition of properties suitable for the cultivation, production and sale of medical and adult use cannabis with entities engaged in agriculture and real estate investment activities, including corporate agriculture companies, cultivators, producers and sellers of cannabis. These competitors may prevent us from acquiring and leasing desirable properties, may cause an increase in the price we must pay for properties or may result in us having to lease our properties on less favorable terms than we expect. Our competitors may have greater financial and operational resources than we do and may be willing to pay more for certain assets or may be willing to accept more risk than we believe can be prudently managed. In particular, larger companies may enjoy significant competitive advantages that result from, among other things, a lower cost of capital and enhanced operating efficiencies. Our competitors may also adopt transaction structures similar to ours, which would decrease our competitive advantage in offering flexible transaction terms. In addition, due to a number of factors, including but not limited to potential greater clarity of the laws and regulations governing medical use cannabis by state and federal governments, the number of entities and the amount of funds competing for suitable investment properties may increase, resulting in increased demand and increased prices paid for these properties. If we pay higher prices for properties or enter into leases for such properties on less favorable terms than we expect, our profitability and ability to generate cash flow and make distributions to our stockholders may decrease. Increased competition for properties may also preclude us from acquiring those properties that would generate attractive returns to us.
43
Table of Contents
Our reputation and ability to do business may be negatively impacted by the improper conduct by our business partners, employees or agents.
We depend on third-party suppliers to produce and timely ship our orders. Products purchased from our suppliers are resold to our customers. These suppliers could fail to produce products to our specifications or quality standards and may not deliver units on a timely basis. Any changes in our suppliers to resolve production issues could impact our ability to fulfill orders and could also disrupt our business due to delays in finding new suppliers.
Furthermore, we cannot provide assurance that our internal controls and compliance systems will protect us from acts committed by our employees, agents or business partners in violation of U.S. federal or state or local laws. Any improper acts or allegations could damage our reputation and subject us to civil or criminal investigations and related shareholder lawsuits, could lead to substantial civic and criminal monetary and non-monetary penalties and could cause us to incur significant legal and investigatory fees.
The Company faces risks related to its products.
As a relatively new industry, there are not many established players in the adult use cannabis industry whose business model the Company can follow or build on the success of. Similarly, there is no information about comparable companies available for potential investors to review in making a decision about whether to invest in the Company.
Shareholders and investors should consider, among other factors, the Company’s prospects for success in light of the risks and uncertainties encountered by companies, like the Company, that are in their early stages. For example, unanticipated expenses and problems or technical difficulties may occur, which may result in material delays in the operation of the Company’s business. The Company may fail to successfully address these risks and uncertainties or successfully implement its operating strategies. If the Company fails to do so, it could materially harm the Company’s business to the point of having to cease operations and could impair the value of the Subordinate Voting Shares to the point where investors may lose their entire investments.
The Company has committed and expects to continue committing significant resources and capital to develop and market existing products and new products and services. These products are relatively untested in the marketplace, and the Company cannot assure shareholders and investors that it will achieve market acceptance for these products, or other new products and services that the Company may offer in the future. Moreover, these and other new products and services may be subject to significant competition with offerings by new and existing competitors in the business. In addition, new products and services may pose a variety of challenges and require the Company to attract additional qualified employees. The failure to successfully develop and market these new products and services could seriously harm the Company’s business, prospects, revenue, results of operation and financial condition.
The Company is dependent on the popularity of consumer acceptance of the Company’s brand portfolio.
The Company’s ability to generate revenue and be successful in the implementation of the Company’s business plan is dependent on consumer acceptance of and demand for the Company’s products. Acceptance of the Company’s products depends on several factors, including availability, cost, ease of use, familiarity of use, convenience, effectiveness, safety and reliability. If these customers do not accept the Company’s products, or if such products fail to adequately meet customers’ needs and expectations, the Company’s ability to continue generating revenues could be reduced.
The Company’s business is subject to the risks inherent in agricultural operations.
The Company’s business involves the growing of cannabis, an agricultural product. The Company’s business is subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar
44
Table of Contents
agricultural risks. Although the Company’s cultivation is substantially completed indoors under climate control, some cultivation is completed outdoors, and there can be no assurance that natural elements will not have a material adverse effect on any future production.
The Company may be adversely impacted by rising or volatile energy costs.
The Company’s cannabis growing operations consume considerable energy, which makes it vulnerable to rising energy costs. Accordingly, rising or volatile energy costs may adversely affect the business of the Company and its ability to operate profitably.
The Company may encounter unknown environmental risks.
There can be no assurance that the Company will not encounter hazardous conditions, such as asbestos or lead, at the sites of the real estate used to operate its businesses, which may delay the development of its businesses. Upon encountering a hazardous condition, work at the facilities of the Company may be suspended. If the Company receives notice of a hazardous condition, it may be required to correct the condition prior to continuing construction. If additional hazardous conditions were present, it would likely delay construction and may require significant expenditure of the Company’s resources to correct the conditions. Such conditions could have a material impact on the investment returns of the Company.
The Company faces risks related to its information technology systems, and potential cyber-attacks and security breaches.
The Company’s operations depend, in part, on how well the Company and its suppliers protect networks, equipment, information technology (“IT”) systems and software against damage and threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism and theft. The Company’s operations also depend on the timely maintenance and replacement of network equipment, IT systems and software, as well as pre-emptive expenses to mitigate associated risks. Given the nature of the Company’s products and the lack of legal availability outside of channels approved by the federal government, as well as the concentration of inventory in its facilities, there remains a risk of shrinkages, as well as theft. If there was a breach in security and the Company fell victim to theft or robbery, the loss of cannabis plants, cannabis oils, cannabis flowers and cultivations and processing equipment, or if there was a failure in information systems, it could adversely affect the Company’s reputation and business continuity.
Additionally, the Company may store and collect personal information about customers and is responsible for protecting that information from privacy breaches that may occur through procedural or process failure, IT malfunction or deliberate unauthorized intrusions. Any such theft or privacy breach would have a material adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition.
We are subject to laws, rules and regulations in the United States (such as the California Consumer Privacy Act (“CCPA”), which will become effective on January 1, 2020) and other jurisdictions relating to the collection, processing, storage, transfer and use of personal data. Our ability to execute transactions and to possess and use personal information and data in conducting our business subjects us to legislative and regulatory burdens that may require us to notify regulators and customers, employees and other individuals of a data security breach. Evolving compliance and operational requirements under the CCPA and the privacy laws, rules and regulations of other jurisdictions in which we operate impose significant costs that are likely to increase over time. In addition, non-compliance could result in proceedings against us by governmental entities and/or significant fines, could negatively impact our reputation and may otherwise adversely impact our business, financial condition and operating results.
45
Table of Contents
The Company faces risks related to its insurance coverage and uninsurable risks.
The Company’s business is subject to a number of risks and hazards generally, including adverse environmental conditions, accidents, labor disputes and changes in the regulatory environment. Such occurrences could result in damage to assets, personal injury or death, environmental damage, delays in operations, monetary losses and possible legal liability.
Although the Company intends to continue to maintain insurance to protect against certain risks in such amounts as it considers to be reasonable, its insurance will not cover all the potential risks associated with its operations. The Company may also be unable to maintain insurance to cover these risks at economically feasible premiums. Insurance coverage may not continue to be available or may not be adequate to cover any resulting liability. Moreover, insurance against risks such as environmental pollution or other hazards encountered in the operations of the Company is not generally available on acceptable terms. The Company might also become subject to liability for pollution or other hazards which it may not be insured against or which the Company may elect not to insure against because of premium costs or other reasons. Losses from these events may cause the Company to incur significant costs that could have a material adverse effect upon its financial performance and results of operations.
The Company is dependent on key inputs, suppliers and skilled labor.
The marijuana business is dependent on a number of key inputs and their related costs, including raw materials and supplies related to growing operations, as well as electricity, water and other local utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs, such as the raw material cost of cannabis, could materially impact the business, financial condition, results of operations or prospects of the Company. Some of these inputs may only be available from a single supplier or a limited group of suppliers. If a sole source supplier was to go out of business, the Company might be unable to find a replacement for such source in a timely manner, or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Company in the future. Any inability to secure required supplies and services, or to do so on appropriate terms, could have a materially adverse impact on the business, prospects, revenue, results of operation and financial condition of the Company. The Company provides its vendor base with annual projections so that its vendors can aim to ensure a steady supply of raw materials and packaging. It checks in with its vendors at least once quarterly to update them to relevant real time changes in its annual plan. For most important raw materials and packaging, the Company has both a primary vendor supplier and a secondary vendor supplier to ensure redundancy.
The ability of the Company to compete and grow will be dependent on it having access, at a reasonable cost and in a timely manner, to skilled labor, equipment, parts and components. No assurances can be given that the Company will be successful in maintaining its required supply of skilled labor, equipment, parts and components. This could have an adverse effect on the financial results of the Company.
The Company must attract and maintain key personnel or our business will fail.
Success of the Company is dependent upon the ability, expertise, judgment, discretion and good faith of its senior management and key personnel. We compete with other companies both within and outside the cannabis industry to recruit and retain competent employees. If we cannot maintain qualified employees to meet the needs of our anticipated growth, our business and financial condition could be materially adversely effected.
The Company’s sales are difficult to forecast.
As a result of recent and ongoing regulatory and policy changes in the medical and adult use cannabis industries, the market data available is limited and unreliable. The Company must rely largely on its own market research to forecast sales, as detailed forecasts are not generally obtainable from other sources in the states in
46
Table of Contents
which the Company’s business operates. Additionally, any market research and projections by the Company of estimated total retail sales, demographics, demand and similar consumer research, are based on assumptions from limited and unreliable market data. A failure in the demand for its products to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations and financial condition of the Company.
The Company may be subject to growth-related risks.
The Company may be subject to growth-related risks, including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. The inability of the Company to deal with this growth may have a material adverse effect on the Company’s business, prospects, revenue, results of operation and financial condition.
The Company may be subject to litigation.
The Company may become party to litigation from time to time in the ordinary course of business, which could adversely affect its business. Should any litigation in which the Company becomes involved be determined against the Company, such a decision could adversely affect the Company’s ability to continue operating and the market price for the Subordinate Voting Shares and could potentially use significant resources of the Company. Even if the Company is involved in litigation and wins, litigation can redirect significant resources of the Company and/or its subsidiaries.
The Company faces an inherent risk of product liability claims.
As a distributor of products designed to be ingested by humans, the Company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the sale of the Company’s products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Company’s products alone or in combination with other medications or substances could occur. The Company may be subject to various product liability claims, including, among others, that the Company’s products caused injury, illness or death, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect the Company’s reputation with its clients and consumers generally and could have a material adverse effect on the business, results of operations and financial condition of the Company. There can be no assurances that the Company will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of the Company’s potential products.
The Company may be exposed to infringement or misappropriation claims by third parties, which, if determined adversely to the Company, could subject the Company to significant liabilities and other costs.
The Company’s success may depend on its ability to use and develop new extraction technologies, recipes, know-how and new strains of marijuana without infringing the intellectual property rights of third parties. The Company cannot assure that third parties will not assert intellectual property claims against it. The Company is subject to additional risks if entities licensing intellectual property to the Company do not have adequate rights to the licensed materials. If third parties assert copyright or patent infringement or violation of other intellectual property rights against the Company, the Company will be required to defend itself in litigation or administrative proceedings, which can be both costly and time consuming and may significantly divert the
47
Table of Contents
efforts and resources of management personnel. An adverse determination in any such litigation or proceedings to which the Company may become a party could subject the Company to significant liability to third parties, require the Company to seek licenses from third parties, require the Company to pay ongoing royalties or subject the Company to injunctions that may prohibit the development and operation of its applications.
The Company’s products may be subject to product recalls.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. If any of the Company’s products are recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Company may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin, if at all. In addition, a product recall may require significant management attention. Although the Company has detailed procedures in place for testing its products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the Company’s significant brands were subject to recall, the image of that brand and the Company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Company’s products and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the Company’s operations by the FDA, or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
The Company may face unfavorable publicity or consumer perception.
Management believes the adult use cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the adult use cannabis produced. Consumer perception of the Company’s products may be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of adult use cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the adult use cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that is perceived as less favorable than, or questions earlier research reports, findings or publicity could have a material adverse effect on the demand for the Company’s products and the business, results of operations, financial condition and cash flows of the Company. The Company’s dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the demand for the Company’s products and the business, results of operations, financial condition and cash flows of the Company. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of adult use cannabis in general, or the Company’s products specifically, or associating the consumption of adult use cannabis with illness or other negative effects or events, could have such a material adverse effect. Adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed.
Certain of our products are e-vapor or “vape” products. The use of vape products and vaping may pose health risks. According to the Centers for Disease Control, vape products may contain ingredients that are known to be toxic to humans and may contain other ingredients that may not be safe. Because clinical studies about the safety and efficacy of vape products have not been submitted to the FDA, consumers currently have no way of knowing whether they are safe for their intended use or what types or concentrations of potentially harmful chemicals or by-products are found in these products. It is also uncertain what implications the use of vape or other inhaled products, such as flower that is smoked, may have on respiratory illnesses such as that caused by
48
Table of Contents
the Coronavirus Disease 2019, or COVID 19. Adverse findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of vape or other inhaled products, including adverse publicity regarding underage use of vape or other inhaled products, may adversely affect the Company.
The Company faces intense competition.
The Company faces intense competition from other companies, some of which have longer operating histories and more financial resources and manufacturing and marketing experience than the Company. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and results of operations of the Company.
Because of the early stage of the industry in which the Company operates, the Company faces additional competition from new entrants. If the number of consumers of cannabis in the states in which the Company operates its business increases, the demand for products will increase and the Company expects that competition will become more intense, as current and future competitors begin to offer an increasing number of diversified products. To remain competitive, the Company will require a continued high level of investment in research and development, marketing, sales and client support. The Company may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis, which could materially and adversely affect the business, financial condition and results of its operations.
A decline in the price of the Subordinate Voting Shares could affect the Company’s ability to raise further working capital and adversely impact its ability to continue operations.
A prolonged decline in the price of the Subordinate Voting Shares could result in a reduction in the liquidity of the Subordinate Voting Shares and a reduction in its ability to raise capital. Because a significant portion of the Company’s operations have been and will be financed through the sale of equity securities, a decline in the price of its common stock could be especially detrimental to the Company’s liquidity and its operations. Such reductions may force the Company to reallocate funds from other planned uses and may have a significant negative effect on the Company’s business plan and operations, including its ability to develop new products and continue its current operations. If the Company’s stock price declines, there can be no assurance that the Company will be able to raise additional capital or generate funds from operations sufficient to meet its obligations. If the Company is unable to raise sufficient capital in the future, the Company may not be able to have the resources to continue its normal operations.
The Company may have increased labor costs based on union activity.
Labor unions are working to organize workforces in the cannabis industry in general. Currently, there is no labor organization that has been recognized as a representative of GTI employees. However, it is possible that certain retail and/or manufacturing locations will be organized in the future, which could lead to work stoppages or increased labor costs and adversely affect our business. We cannot predict how stable our relationships with U.S. labor organizations would be or whether we would be able to meet any unions’ requirements without impacting our financial condition. Labor unions may also limit our flexibility in dealing with our workforce. Work stoppages and instability in our union relationships could delay the production and sale of our products, which could strain relationships with customers and cause a loss of revenues which would adversely affect our operations.
The Company is subject to general economic risks.
The Company’s operations could be affected by the economic context should the unemployment level, interest rates or inflation reach levels that influence consumer trends and spending and, consequently, impact the Company’s sales and profitability.
49
Table of Contents
The Company may be negatively impacted by challenging global economic conditions.
The Company’s business, financial condition, results of operations and cash flow may be negatively impacted by challenging global economic conditions.
A global economic slowdown would cause disruptions and extreme volatility in global financial markets, increased rates of default and bankruptcy and declining consumer and business confidence, which can lead to decreased levels of consumer spending. These macroeconomic developments could negatively impact the Company’s business, which depends on the general economic environment and levels of consumer spending. As a result, the Company may not be able to maintain its existing customers or attract new customers, or it may be forced to reduce the price of its products. The Company is unable to predict the likelihood of the occurrence, duration or severity of such disruptions in the credit and financial markets or adverse global economic conditions. Any general or market-specific economic downturn could have a material adverse effect on the business, financial condition, results of operations and cash flow of the Company.
Additionally, the U.S. has imposed and may impose additional quotas, duties, tariffs, retaliatory or trade protection measures or other restrictions or regulations and may adversely adjust prevailing quota, duty or tariff levels, which can affect both the materials that we use to package our products and the sale of finished products. For example, the tariffs imposed by the U.S. on materials from China are impacting materials that we import for use in packaging in the U.S. Measures to reduce the impact of tariff increases or trade restrictions, including geographical diversification of our sources of supply, adjustments in packaging design and fabrication or increased prices, could increase our costs, delay our time to market and/or decrease sales. Other governmental action related to tariffs or international trade agreements has the potential to adversely impact demand for our products and our costs, customers, suppliers and global economic conditions and cause higher volatility in financial markets. While we actively review existing and proposed measures to seek to assess the impact of them on our business, changes in tariff rates, import duties and other new or augmented trade restrictions could have a number of negative impacts on our business, including higher consumer prices and reduced demand for our products and higher input costs.
The Company is subject to risks arising from epidemic diseases, such as the recent outbreak of the COVID-19 illness.
The recent outbreak of COVID-19 which has been declared by the World Health Organization to be a “pandemic” has spread across the globe and is impacting worldwide economic activity. A public health epidemic, including COVID-19, or the fear of a potential pandemic, poses the risk that we or our employees, contractors, suppliers, and other partners may be prevented from conducting business activities for an indefinite period of time, including due to shutdowns or other preventative measures taken to limit the potential impact from a public health epidemic that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the impact that COVID-19 (or any other actual or potential pandemic) could have on our business, the continued spread of COVID-19 (or any other actual or potential pandemic) and the measures taken by the governments of countries affected could disrupt the supply chain and the manufacture or shipment or sale of our products and adversely impact our business, financial condition or results of operations. It could also affect the health and availability of our workforce at our facilities, as well as those of our suppliers, particularly those in China and India. The COVID-19 outbreak and mitigation measures may also have an adverse impact on global economic conditions which could have an adverse effect on our business and financial condition. The extent to which the COVID-19 outbreak impacts our results will depend on future developments that are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of the virus and the actions to contain its impact. Because cannabis remains federally illegal, it is possible that the Company would not be eligible to participate in any government relief programs (such as federal loans or access to capital) resulting from COVID-19 or any other actual or potential pandemic.
50
Table of Contents
Risks Related to Our Securities
A return on the Company’s securities is not guaranteed.
There is no guarantee that the Company’s Subordinate Voting Shares will earn any positive return in the short term or long term. A holding of Subordinate Voting Shares is speculative and involves a high degree of risk and should be undertaken only by holders whose financial resources are sufficient to enable them to assume such risks and who have no need for immediate liquidity in their investment. A holding of Subordinate Voting Shares is appropriate only for holders who have the capacity to absorb a loss of some or all of their holdings.
The Company may be affected by currency fluctuations.
The Company faces exposure to significant currency fluctuations because of its present operations in the U.S. Recent events in the global financial markets have been coupled with increased volatility in the currency markets. All or substantially all of the Company’s revenue is earned in U.S. dollars, but a portion of its operating expenses are incurred in Canadian dollars. The Company does not have currency hedging arrangements in place and there is no expectation that the Company will put any currency hedging arrangements in place in the future. Fluctuations in the exchange rate between the U.S. dollar and the Canadian dollar may have a material adverse effect on the Company’s business, financial position or results of operations.
The Company’s voting control is concentrated.
The Company’s senior executives exercise a significant majority of the voting power with respect to the Company’s outstanding shares because of the Super Voting Shares that they hold. These officials include Benjamin Kovler, the Company’s Founder, Chairman and Chief Executive Officer, Andrew Grossman, the Company’s Head of Capital Markets, and Anthony Georgiadis, the Company’s Chief Financial Officer. Subordinate Voting Shares are entitled to one vote per share, Multiple Voting Shares are entitled to 100 votes per share, and Super Voting Shares are entitled to up to 1,000 votes per share. As a result, Mr. Kovler, Mr. Grossman and Mr. Georgiadis potentially have the ability to control the outcome of matters submitted to the Company’s shareholders for approval, including the election and removal of directors and any arrangement or sale of all or substantially all of the assets of the Company.
This concentrated control could delay, defer or prevent a change of control of the Company, arrangement or merger involving the Company or sale of all or substantially all of the assets of the Company that its other shareholders may support. Conversely, this concentrated control could allow the holders of the Super Voting Shares to consummate such a transaction that the Company’s other shareholders do not support. In addition, the holders of the Super Voting Shares may make long-term strategic investment decisions and take risks that may not be successful and/or may seriously harm the Company’s business.
The Company’s capital structure and voting control may cause unpredictability.
Although other Canadian-based companies have dual class or multiple voting share structures, given the unique capital structure in respect of the Company and the concentration of voting control that is held by the holders of the Super Voting Shares, this structure and control could result in a lower trading price for or greater fluctuations in the trading price of the Company’s Subordinate Voting Shares, adverse publicity to the Company or other adverse consequences.
Additional issuances of Super Voting Shares, Multiple Voting Shares or Subordinate Voting Shares may result in dilution.
The Corporation may issue additional equity or convertible debt securities in the future, which may dilute an existing shareholder’s holdings in the Corporation. The Corporation’s articles permit the issuance of an unlimited number of Super Voting Shares, Multiple Voting Shares and Subordinate Voting Shares, and existing
51
Table of Contents
shareholders will have no pre-emptive rights in connection with such further issuances. The Corporation’s Board has discretion to determine the price and the terms of further issuances, and such terms could include rights, preferences and privileges superior to those existing holders of Subordinate Voting Shares. Moreover, additional Subordinate Voting Shares will be issued by the Corporation on the conversion of the Multiple Voting Shares and Super Voting Shares in accordance with their terms. To the extent holders of the Corporation’s options or other convertible securities convert or exercise their securities and sell Subordinate Voting Shares they receive, the trading price of the Subordinate Voting Shares may decrease due to the additional amount of Subordinate Voting Shares available in the market. The Corporation cannot predict the size or nature of future issuances or the effect that future issuances and sales of Subordinate Voting Shares will have on the market price of the Subordinate Voting Shares. Issuances of a substantial number of additional Subordinate Voting Shares, or the perception that such issuances could occur, may adversely affect prevailing market prices for the Subordinate Voting Shares. With any additional issuance of Subordinate Voting Shares, investors will suffer dilution to their voting power and economic interest in the Corporation.
Sales of substantial amounts of Subordinate Voting Shares may have an adverse effect on the market price of the Subordinate Voting Shares.
Sales of substantial amounts of Subordinate Voting Shares, or the availability of such securities for sale, could adversely affect the prevailing market prices for the Subordinate Voting Shares. A decline in the market prices of the Subordinate Voting Shares could impair the Company’s ability to raise additional capital through the sale of securities should it desire to do so.
The market price for the Subordinate Voting Shares may be volatile.
The market price for securities of cannabis companies generally are likely to be volatile. In addition, the market price for the Subordinate Voting Shares has been and may be subject to wide fluctuations in response to numerous factors beyond the Company’s control, including, but not limited to:
| • | actual or anticipated fluctuations in the Company’s quarterly results of operations; |
| • | recommendations by securities research analysts; |
| • | changes in the economic performance or market valuations of companies in the industry in which the Company operates; |
| • | addition or departure of the Company’s executive officers and other key personnel; |
| • | release or expiration of transfer restrictions on outstanding Subordinate Voting Shares; |
| • | sales or perceived sales of additional Subordinate Voting Shares; |
| • | operating and financial performance that varies from the expectations of management, securities analysts and investors; |
| • | regulatory changes affecting the Company’s industry generally and its business and operations both domestically and abroad; |
| • | announcements of developments and other material events by the Company or its competitors; |
| • | fluctuations in the costs of vital production materials and services; |
| • | changes in global financial markets, global economies and general market conditions, such as interest rates and pharmaceutical product price volatility; |
| • | significant acquisitions or business combinations, strategic partnerships, joint ventures or capital commitments by or involving the Company or its competitors; |
52
Table of Contents
| • | operating and share price performance of other companies that investors deem comparable to the Company or from a lack of market comparable companies; and |
| • | news reports relating to trends, concerns, technological or competitive developments, regulatory changes and other related issues in the Company’s industry or target markets. |
Financial markets have at times historically experienced significant price and volume fluctuations that: (i) have particularly affected the market prices of equity securities of companies and (ii) have often been unrelated to the operating performance, underlying asset values or prospects of such companies. Accordingly, the market price of the Subordinate Voting Shares from time to time may decline even if the Company’s operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that may result in impairment losses to the Company. There can be no assurance that further fluctuations in price and volume of equity securities will not occur. If increased levels of volatility and market turmoil continue, the Company’s operations could be adversely impacted, and the trading price of the Subordinate Voting Shares may be materially adversely affected.
If securities or industry analysts do not publish or cease publishing research or reports or publish misleading, inaccurate or unfavorable research about us, our business or our market, our stock price and trading volume could decline.
The trading market for our Subordinate Voting Shares will be influenced by the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. If no or few securities or industry analysts cover our Corporation, the trading price and volume of our shares would likely be negatively impacted. If one or more of the analysts who covers us downgrades our shares or publishes inaccurate or unfavorable research about our business, or provides more favorable relative recommendations about our competitors, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our shares could decrease, which could cause our stock price or trading volume to decline.
The Company faces liquidity risks.
The Company’s Subordinate Voting Shares currently trade on the CSE and on over-the-counter markets in the U.S. The Company cannot predict at what prices the Subordinate Voting Shares will continue to trade, and there is no assurance that an active trading market will be sustained.
The Company’s Subordinate Voting Shares do not currently trade on any U.S. securities exchange. In the event the Company’s Subordinate Voting Shares do trade on any U.S. securities exchange, the Company cannot predict at what prices the Subordinate Voting Shares will trade and there is no assurance that an active trading market will develop or be sustained. There is a significant liquidity risk associated with an investment in the Company.
The Company is subject to increased costs as a result of being a public company in Canada and the United States.
As a public company in Canada and the United States, the Company is subject to the reporting requirements, rules and regulations under the applicable Canadian and American securities laws and rules of stock exchanges on which the Company’s securities may be listed. The requirements of existing and potential future rules and regulations will increase the Company’s legal, accounting and financial compliance costs, make some activities more difficult, time-consuming or costly and may place undue strain on its personnel, systems and resources, which could adversely affect its business, financial condition and results of operations.
The Company faces costs of maintaining a public listing.
As a public company, there are costs associated with legal, accounting and other expenses related to regulatory compliance. Securities legislation and the rules and policies of the CSE require listed companies to,
53
Table of Contents
among other things, adopt corporate governance and related practices, and to continuously prepare and disclose material information, all of which add to a company’s legal and financial compliance costs. The Company may also elect to devote greater resources than it otherwise would have on communication and other activities typically considered important by publicly traded companies.
The Company does not intend to pay dividends on our common shares and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common shares.
We have never declared or paid any cash dividend on our Subordinate Voting Shares and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings, if materialized, for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Therefore, the success of an investment in our common shares will depend upon any future appreciation in their value. There is no guarantee that our common shares will appreciate in value or even maintain the price at which you purchased them.
The Company is eligible to be treated as an “emerging growth company” as defined in the JOBS Act, and the Company cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make the Subordinate Voting Shares less attractive to investors.
The Company is an “emerging growth company,” as defined in the JOBS Act. For as long as the Company continues to be an emerging growth company, it may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, (2) reduced disclosure obligations regarding executive compensation in this document and periodic reports and proxy statements, and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. The Company could be an emerging growth company for up to five years, although circumstances could cause the Company to lose that status earlier, including if the market value of the Subordinate Voting Shares held by non-affiliates exceeds $700 million as of June 30, 2020, or if the Company has total annual gross revenue of $1.07 billion or more during any fiscal year before that time, in which case the Company would no longer be an emerging growth company as of the following December 31. Additionally, if the Company issues more than $1.0 billion in non-convertible debt during any three-year period before June 30, 2020, it would cease to be an emerging growth company immediately. The Company cannot predict if investors will find the Subordinate Voting Shares less attractive because the Company may rely on these exemptions. If some investors find the Subordinate Voting Shares less attractive as a result, there may be a less active trading market for the Subordinate Voting Shares, and the stock price may be more volatile.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
| ITEM 2. | PROPERTIES |
The following tables set forth the Company’s principal physical properties.
| Corporate Properties | ||||
| Type |
Location |
Leased / Owned | ||
| Headquarters | Chicago, IL | Leased | ||
54
Table of Contents
| Production Properties | ||||
| Type |
Location |
Leased / Owned | ||
| West Haven Facility | West Haven, CT | Leased | ||
| Homestead Facility | Homestead, FL | Owned | ||
| Oglesby Facility | Oglesby, IL | Owned* | ||
| Rock Island Facility | Rock Island, IL | Owned | ||
| Centreville Facility | Centreville, MD | Leased | ||
| Holyoke Facility | Holyoke, MA | Leased | ||
| Carson City Facility | Carson City, NV | Leased | ||
| Las Vegas Facility 1 | Las Vegas, NV | Leased | ||
| Las Vegas Facility 2 | Las Vegas, NV | Leased | ||
| Paterson Facility | Paterson, NJ | Leased | ||
| Schenectady Facility | Glenville, NY | Leased | ||
| Toledo Facility | Toledo, OH | Owned** | ||
| Danville Facility | Danville, PA | Leased | ||
| Retail Properties | ||||
| Type |
Location |
Leased / Owned | ||
| Bluepoint Wellness Branford | Branford, CT | Leased | ||
| Bluepoint Wellness Westport | Westport, CT | Leased | ||
| Rise Bonita Springs | Bonita Springs, FL | Leased | ||
| Rise Deerfield Beach | Deerfield Beach, FL | Leased | ||
| Rise Hallandale Beach | Hallandale Beach, FL | Leased | ||
| Rise Oviedo | Oviedo, FL | Leased | ||
| Rise Pinellas Park | Pinellas Park, FL | Leased | ||
| Rise West Palm Beach | West Palm Beach, FL | Leased | ||
| Rise Canton | Canton, IL | Leased | ||
| The Clinic Effingham | Effingham, IL | Leased | ||
| 3C Joliet | Joliet, IL | Leased | ||
| Rise Joliet | Joliet, IL | Leased | ||
| Rise Mundelein | Mundelein, IL | Owned | ||
| 3C Naperville | Naperville, IL | Leased | ||
| Rise Quincy | Quincy, IL | Leased | ||
| Rise Bethesda | Bethesda, MD | Leased | ||
| Rise Joppa | Joppa, MD | Owned | ||
| Rise Silver Spring | Silver Spring, MD | Leased | ||
| Rise Amherst | Amherst, MA | Leased | ||
| Rise Paterson | Paterson, NJ | Leased | ||
| Rise Carson City | Carson City, NV | Leased | ||
| Essence Henderson | Henderson, NV | Leased | ||
| Essence The Strip | Las Vegas, NV | Leased | ||
| Essence Tropicana West | Las Vegas, NV | Leased | ||
| Rise Spanish Springs | Spanish Springs, NV | Leased | ||
| Fp WELLNESS Halfmoon | Clifton Park, NY | Leased | ||
| Fp WELLNESS Manhattan | New York, NY | Leased | ||
| Fp WELLNESS Rochester | Rochester, NY | Leased | ||
| Rise Cleveland | Cleveland, OH | Leased | ||
| Rise Lakewood | Lakewood, OH | Leased | ||
| Rise Lorain | Lorain, OH | Owned | ||
| Rise Toledo | Toledo, OH | Leased | ||
| Rise Carlisle | Carlisle, PA | Leased | ||
| Rise Erie | Erie, PA | Owned | ||
55
Table of Contents
| Retail Properties | ||||
| Type |
Location |
Leased / Owned | ||
| Rise Hermitage | Hermitage, PA | Leased | ||
| Rise King of Prussia | King of Prussia, PA | Leased | ||
| Rise Latrobe | Latrobe, PA | Leased | ||
| Rise Mechanicsburg | Mechanicsburg, PA | Leased | ||
| Rise New Castle | New Castle, PA | Leased | ||
| Rise Steelton | Steelton, PA | Leased | ||
| Rise York | York, PA | Leased | ||
* - As of December 31, 2019, the Oglesby Facility was owned by the Company. On March 6, 2020, the Company closed on a sale and lease back transaction to sell the facility to an unrelated third party. GTI will lease back the facility via a long-term agreement and continue to operate and manage it. See additional discussion in Note 21.
** As of December 31, 2019, the Toledo Processing Facility was owned by the Company. On January 31, 2020, the Company closed on a sale and lease back transaction to sell the facility to an unrelated third party. GTI leased back the facility via a long-term agreement and continues to operate and manage it. See additional discussion in Note 21.
Properties Subject to an Encumbrance. Pursuant to the Note Purchase Agreement, the Company collateralized the facilities in (i) Rock Island, Illinois, (ii) and Homestead, Florida.
| ITEM 3. | LEGAL PROCEEDINGS |
Legal Proceedings
The Company has no legal proceedings, pending or threatened, which would have a material impact on the operations or financial condition of the Company.
| ITEM 4. | MINE SAFETY DISCLOSURE |
Not applicable.
56
Table of Contents
PART II
ITEM 5. MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANTS COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Trading Price and Volume
The Subordinate Voting Shares of the Company are traded on the CSE under the symbol “GTII.” During fiscal 2018, the common shares of Bayswater traded on the TSXV under the symbol “BYU.H.”
The following table sets forth trading information for the Subordinate Voting Shares for the periods indicated (from June 13, 2018, the date of their initial trading on the CSE), as quoted on the CSE.(1)
| Period |
Low Trading Price (C$) |
High Trading Price (C$) |
Volume | |||||||||
| First Quarter (March 31, 2019) |
$ | 11.60 | $ | 20.90 | 22,190,482 | |||||||
| Second Quarter (June 30, 2019) |
$ | 13.10 | $ | 21.71 | 20,904,219 | |||||||
| Third Quarter (September 30, 2019) |
$ | 10.55 | $ | 14.67 | 25,325,632 | |||||||
| Fourth Quarter (December 31, 2019) |
$ | 10.26 | $ | 13.26 | 18,498,123 | |||||||
| Year Ended December 31, 2018 |
||||||||||||
| Fourth Quarter (December 31, 2018) |
$ | 10.22 | $ | 24.68 | 12,829,887 | |||||||
| Third Quarter (September 30, 2018) |
$ | 8.58 | $ | 30.01 | 13,462,742 | |||||||
| June 13, 2018—June 30, 2018 |
$ | 8.95 | $ | 16.04 | 2,882,771 | |||||||
Notes:
| (1) | Source: Bloomberg. |
The following table sets forth trading information for the pre-Transaction common shares of Bayswater for the periods indicated (until June 12, 2018, the date of their delisting on the TSXV), as quoted on the TSXV.(1)
| Period |
Low Trading Price (C$) |
High Trading Price (C$) |
Volume | |||||||||
| Year Ended December 31, 2018 |
||||||||||||
| April 1, 2018—June 12, 2018(2) |
$ | 9.20 | $ | 12.88 | 881 | |||||||
| First Quarter (March 31, 2018) |
$ | 9.20 | $ | 14.72 | 4,402 | |||||||
| Year Ended December 31, 2017 |
||||||||||||
| Fourth Quarter (December 31, 2017) |
$ | 11.04 | $ | 14.72 | 5,022 | |||||||
| Third Quarter (September 30, 2017) |
$ | 7.36 | $ | 18.40 | 2,170 | |||||||
| Second Quarter (June 30, 2017) |
$ | 12.88 | $ | 22.08 | 4,788 | |||||||
| First Quarter (March 31, 2017) |
$ | 12.88 | $ | 31.28 | 19,845 | |||||||
Notes:
| (1) | Source: Bloomberg. |
| (2) | Bayswater common shares were halted from trading in connection with the Transaction and subsequently delisted from the TSXV on June 12, 2018. |
The Subordinate Voting Shares of the Company are also traded on the OTCQX under the symbol “GTBIF.”
57
Table of Contents
The following table sets forth trading information for the Subordinate Voting Shares for the periods indicated (from July 9, 2018, the date of their initial trading on the OTCQX), as quoted on the OTCQX.(1)
| Period |
Low Trading Price ($) |
High Trading Price ($) |
Volume | |||||||||
| First Quarter (March 31, 2019) |
$ | 8.54 | $ | 15.60 | 15,697,374 | |||||||
| Second Quarter (June 30, 2019) |
$ | 9.78 | $ | 16.20 | 15,817,344 | |||||||
| Third Quarter (September 30, 2019) |
$ | 7.99 | $ | 11.47 | 17,225,728 | |||||||
| Fourth Quarter (December 31, 2019) |
$ | 7.86 | $ | 9.98 | 15,167,168 | |||||||
| Period |
Low Trading Price ($) |
High Trading Price ($) |
Volume | |||||||||
| Year Ended December 31, 2018 |
||||||||||||
| Fourth Quarter (December 31, 2018) |
$ | 7.55 | $ | 18.96 | 13,570,767 | |||||||
| July 9, 2018—September 30, 2018 |
$ | 6.57 | $ | 23.16 | 16,519,410 | |||||||
Notes:
| (1) | Source: Bloomberg. |
Shareholders
As of March 19, 2020, there are 47,513 holders of record of our Subordinate Voting Shares.
Dividends
The Company has not declared distributions on Subordinate Voting Shares in the past. The Company currently intends to reinvest all future earnings to finance the development and growth of its business. As a result, the Company does not intend to pay dividends on Subordinate Voting Shares in the foreseeable future. Any future determination to pay distributions will be at the discretion of the Board and will depend on the financial condition, business environment, operating results, capital requirements, any contractual restrictions on the payment of distributions and any other factors that the Board deems relevant. The Company is not bound or limited in any way to pay dividends in the event that the Board determines that a dividend is in the best interest of its shareholders.
Equity Compensation Plans
For more information on equity compensation plans, see Item 12 of Part III of the Annual Report.
58
Table of Contents
Peer Performance Table
The following graph compares the cumulative total shareholder return on Green Thumb Industries Inc. Subordinate Voting Shares from June 12, 2018, when Green Thumb Industries Inc. began trading on the Canadian Securities Exchange (CSE), through December 31, 2019, with the comparable cumulative return of the Russell 2000 Index and a selected peer group of companies. The comparison assumes all dividends have been reinvested (if any) and an initial investment of $100 on June 12, 2018. The returns of each company in the peer group have been weighted to reflect their market capitalizations. All amounts below are disclosed in US Dollars. The stock price performance on the following graph is not necessarily indicative of future stock price performance.
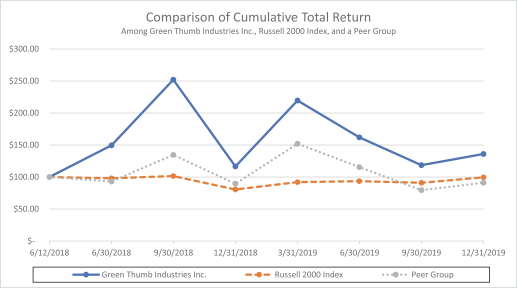
| Base Period 6/12/2018 |
6/30/2018 | 9/30/2018 | 12/31/2018 | 3/31/2019 | 6/30/2019 | 9/30/2019 | 12/31/2019 | |||||||||||||||||||||||||
| Green Thumb Industries Inc. |
$ | 100.00 | $ | 149.27 | $ | 251.94 | $ | 116.50 | $ | 219.50 | $ | 161.59 | $ | 118.46 | $ | 135.81 | ||||||||||||||||
| Russell 2000 |
$ | 100.00 | $ | 98.00 | $ | 101.19 | $ | 80.44 | $ | 91.84 | $ | 93.44 | $ | 90.86 | $ | 99.26 | ||||||||||||||||
| Peer Group |
$ | 100.00 | $ | 92.59 | $ | 134.51 | $ | 89.30 | $ | 151.90 | $ | 115.20 | $ | 79.21 | $ | 90.79 | ||||||||||||||||
Below are the specific companies included in the peer group.
Peer Group Companies
| - Acreage Holdings, Inc. | - Harvest Health and Recreation, Inc. | - Trulieve Cannabis Corp. | ||
| - Cresco Labs Inc. | - iAnthus Capital Holdings, Inc. | |||
| - Curaleaf Holdings, Inc | - MedMen Enterprises Inc. |
This performance graph and other information furnished under this Part II Item 5 of this Form 10-K shall not be deemed to be “soliciting material” or to be “filed” with the SEC or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Exchange Act.
59
Table of Contents
Recent Sales of Unregistered Securities
The following information represents securities sold by the Company for the period covered by this Annual Report on Form 10-K which were not registered under the Securities Act. Included are new issues, securities issued in exchange for property, services or other securities, securities issued upon conversion from other share classes and new securities resulting from the modification of outstanding securities. The Company sold all of the securities listed below pursuant to the exemption from registration provided by Section 4(a)(2) of the Securities Act, or Regulation D or Regulation S promulgated thereunder.
Subordinate Voting Shares
On February 11, 2019, the Company issued 6,539,746 Subordinate Voting Shares for 100% of the membership interest of Advanced Grow Labs, LLC.
On May 22, 2019 and May 24, 2019, the Company issued 19,875 Subordinate Voting Shares to the lead lender pursuant to a Note Purchase Agreement.
On June 5, 2019, June 21, 2019 and August 12, 2019, the Company issued, in total, 24,665,193 Subordinate Voting Shares to the owners of Integral Associates, LLC and Integral Associates CA, LLC for 100% of the membership interest of both entities, as well as for certain achieved milestone payments earned pursuant to a Membership Interest Purchase Agreement.
Between July 2, 2019 and November 19, 2019, the Company issued, in total, 1,165,630 Subordinate Voting Shares to holders of the Company’s restricted stock units, which vested over the same period.
Multiple Voting Shares
Beginning on December 3, 2018 and through December 31, 2018, the shareholders of the Company converted 8,896 Super Voting Shares into 8,896 Multiple Voting Shares and, continuing through November 30, 2019, the shareholders of the Company converted an additional 22,224 Super Voting Shares into 22,224 Multiple Voting Shares.
On January 8, 2019, the Company issued 31,000 Multiple Voting Shares to buyout the membership interest of a joint venture partner pursuant to an agreement between the parties.
Super Voting Shares
Beginning on December 3, 2018 and through December 31, 2018, the shareholders of the Company converted 8,896 Super Voting Shares into 8,896 Multiple Voting Shares and, continuing through November 30, 2019, the shareholders of the Company converted an additional 22,224 Super Voting Shares into 22,224 Multiple Voting Shares.
60
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA
The data set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and accompanying notes presented in Item 15 of this Annual Report. The Company’s Consolidated Financial Statements have been prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) and on a going-concern basis that contemplates continuity of operations and realization of assets and liquidation of liabilities in the ordinary course of business.
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (In thousands) | ||||||||||||
| Total Revenues, net of discounts |
$ | 216,433 | $ | 62,494 | $ | 16,529 | ||||||
| Cost of Goods Sold |
$ | 109,402 | $ | 34,177 | $ | 9,808 | ||||||
| Gross Profit |
$ | 107,031 | $ | 28,317 | $ | 6,721 | ||||||
| Total Expenses |
$ | 134,721 | $ | 54,657 | $ | 11,491 | ||||||
| Other Income (Expense) |
$ | (22,512 | ) | $ | 56,091 | $ | 112 | |||||
| Net Income (Loss) Attributable to GTI |
$ | (59,116 | ) | $ | (5,244 | ) | $ | (4,250 | ) | |||
| Total Assets |
$ | 1,167,537 | $ | 418,349 | $ | 86,213 | ||||||
| Long-Term Liabilities |
$ | 212,961 | $ | 28,310 | $ | 7,508 | ||||||
ITEM 7. MANAGEMENT DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following information should be read in conjunction with the consolidated financial statements and related notes thereto included in this Annual Report on Form 10-K.
In addition to historical information, this report contains forward-looking statements that involve risks and uncertainties which may cause our actual results to differ materially from plans and results discussed in forward-looking statements. We encourage you to review the risks and uncertainties discussed in the sections entitled Item 1A. “Risk Factors” and “Disclosure Regarding Forward-Looking Statements” included at the beginning of this Annual Report on Form 10-K. The risks and uncertainties can cause actual results to differ significantly from those forecast in forward-looking statements or implied in historical results and trends.
We caution readers not to place undue reliance on any forward-looking statements made by us, which speak only as of the date they are made. We disclaim any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to reflect any change in our expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements.
This management discussion and analysis (“MD&A”) of the financial condition and results of operations of Green Thumb Industries Inc. (the “Company” or “GTI”) is for the years ended December 31, 2019, 2018 and 2017. It is supplemental to, and should be read in conjunction with, the Company’s consolidated financial statements for the years ended December 31, 2019 and 2018 and the combined financial statements for the year ended December 31, 2017 and the accompanying notes for each respective period. The Company’s financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). Financial information presented in this MD&A is presented in United States dollars (“$” or “US$”), unless otherwise indicated.
This MD&A contains certain “forward-looking statements” and certain “forward-looking information” as defined under applicable United States securities laws. Please refer to the discussion of forward-looking statements and information set out under the heading “Cautionary Note Regarding Forward-Looking Information,” identified in the ‘‘Risks and Uncertainties’’ section of this MD&A. As a result of many factors, the
61
Table of Contents
Company’s actual results may differ materially from those anticipated in these forward-looking statements and information.
OVERVIEW OF THE COMPANY
Established in 2014 and headquartered in Chicago, Illinois, GTI is promoting well-being through the power of cannabis, while being committed to community and sustainable profitable growth. As of March 31, 2020, GTI has operations across 12 U.S. markets, employs approximately 1,700 people and serves hundreds of thousands of patients and customers annually.
GTI’s core business is manufacturing, distributing and marketing a portfolio of owned cannabis consumer packaged goods brands (which we refer to as our consumer packaged goods business), including Beboe, Dogwalkers, Dr. Solomon’s, incredibles, Rythm and The Feel Collection. We distribute and market these products primarily to third-party licensed retail cannabis stores across the United States as well as to GTI-owned retail stores (which we refer to as our retail business).
The Company’s consumer packaged goods portfolio is primarily generated from plant material that GTI grows and processes itself, which we use to produce our consumer packaged goods in 13 manufacturing facilities. This portfolio consists of stock keeping units (“SKUs”) across a range of cannabis product categories, including flower, pre-rolls, concentrates, vape, capsules, tinctures, edibles, topicals and other cannabis-related products (none of which product categories are individually material to the Company).
GTI also owns and operates a national cannabis retail chain called Rise, and in the Las Vegas, Nevada area, a chain of stores called Essence, which are relationship-centric retail experiences aimed to deliver a superior level of customer service through high-engagement consumer interaction, a consultative, transparent and education-forward selling approach and a consistently available assortment of cannabis products. In addition, we own stores under other names, primarily where we co-own the stores or naming is subject to licensing or similar restrictions. The income from our retail stores is primarily from the sale of cannabis-related products, which includes the sale of GTI produced products as well as those produced by third parties, with an immaterial (under 10%) portion of this income resulting from the sale of other merchandise (such as t-shirts and accessories for cannabis use). Our Rise stores currently are located in eight of the states in which we operate (including Nevada). Our Essence stores were acquired in connection with the 2019 acquisition of Integral Associates and are located in Nevada. The Essence stores differ from the Rise stores mainly in geographic location. As of December 31, 2019, the Company had 39 open and operating retail locations, with state licensed permission to open a total of 96 stores. Our new store opening plans will remain fluid depending on market conditions, obtaining local licensing, construction and other permissions and subject to our capital allocation plans and the evolving situation with respect to the Coronavirus.
Results of Operations - Consolidated
Revenue Streams
The Company has consolidated financial statements across its operating businesses with revenue from the manufacture, sale and distribution of branded cannabis products to third-party retail customers as well as the sale of finished products to consumers in its retail stores.
As of the year ended December 31, 2019, GTI has operating revenue in all of its 12 markets (California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania).
Year Ended December 31, 2019
Revenue
Revenue for the year ended December 31, 2019 was $216,433 thousand, up 246% from $62,494 thousand for the year ended December 31, 2018 driven by contribution from both consumer packaged goods and retail
62
Table of Contents
sales across all 12 markets (California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania). Key performance drivers are: distribution expansion of GTI’s branded product portfolio primarily in Illinois, Massachusetts and Pennsylvania; new store openings and increased store traffic to GTI’s 39 open and operating retail stores, particularly in Florida, Illinois, Massachusetts and Pennsylvania; and the addition of revenue from the acquisition of Connecticut-based AGL and Nevada-based Integral Associates.
Cost of Goods Sold
Cost of goods sold are derived from cost related to the internal cultivation and production of cannabis and from retail purchases made from other licensed producers operating within our state markets.
Cost of goods sold for the year ended December 31, 2019 was $109,402 thousand, up 220% from $34,177 thousand for the year ended December 31, 2018, driven by growth from both Consumer Packaged Goods and Retail sales across all 12 markets (California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania).
Gross Profit
Gross profit for the year ended December 31, 2019 was $107,031 thousand, representing a gross margin on the sale of finished cannabis consumer packaged goods of 49%. This is compared to gross profit for the year ended December 31, 2018 of $28,317 thousand or a 45% gross margin driven by increased harvested cannabis and consumer packaged goods shipments, along with incremental contribution from increased volume from Retail sales.
Total Expenses
Total expenses for the year ended December 31, 2019 were $134,721 thousand or 62% of Total Revenues, net of discounts, an increase of $80,064 thousand. Total expenses for the year ended December 31, 2018 were $54,657 thousand or 87% of Total Revenues, net of discounts.
Increase in total expenses was attributable to an increase in general and administrative expenses, mainly due to non-cash charges related to equity incentive compensation of $18,285 thousand, an increase of $6,137 thousand compared to the year ended December 31, 2018. Salaries and benefits also contributed to the increase as a result of new headcount from the Company’s Retail facilities in Florida, Illinois, Nevada, Maryland, Massachusetts and Pennsylvania, along with corporate staff development.
Additionally, the Company had professional fees of $17,714 thousand which represented an increase of $7,025 thousand over the 2018 amount of $10,689 thousand, primarily driven by acquisition related support, and other regulatory and growth-related activities.
Total Other Income (Expense)
Total other expense for the year ended December 31, 2019 was ($22,512) thousand, compared to income of $56,091 thousand for the year ended December 31, 2018, mainly due to a favorable adjustment to the fair values of the Company’s investments recorded in 2018, as further described in the Liquidity, Financing Activities During the Period, and Capital Resources section of this MD&A.
Provision for Income Taxes
Income tax expense is recognized based on the expected tax payable on the taxable income for the year, using tax rates enacted at year-end. For the year ended December 31, 2019, federal and state income tax
63
Table of Contents
expense totaled $9,344 thousand compared to $7,184 thousand for the year ended December 31, 2018. The net expense of $9,344 thousand for the year ended December 31, 2019 includes current tax expense of $22,761 thousand and deferred tax benefit of $13,417 thousand in the current period. The deferred tax benefit is mainly driven by changes in the fair value of investments and amortization of intangibles.
Income (Loss) From Operations
Net operating loss before provision for income taxes and non-controlling interest for the year ended December 31, 2019 was ($50,203) thousand compared to income of $29,751 thousand for the year ended December 31, 2018.
As presented in the Non-GAAP section, after adjusting for non-cash equity incentive compensation of $18,285 thousand as described above, as well as other non-operating items, adjusted operating EBITDA was $27,762 thousand and ($9,007) thousand for the year ended December 31, 2019 and 2018, respectively.
Year Ended December 31, 2018
Revenue
Revenue for the year ended December 31, 2018 was $62,494 thousand, up 278% from $16,529 thousand for the year ended December 31, 2017 due to revenue contribution from Consumer Packaged Goods and Retail sales across Illinois, Maryland, Massachusetts, Nevada and Pennsylvania. Year over year consumer packaged goods growth is driven by expanded distribution to third-party retail customers of GTI’s branded product portfolio, including Rythm, The Feel Collection and Dogwalkers, primarily across Illinois, Maryland and Pennsylvania. Retail sales growth is driven by increased foot traffic in Illinois retail stores, incremental revenue from two Illinois stores which were acquired in October 2017, new store openings of Rise (three in Maryland and four in Pennsylvania) and the commencement of adult use sales for both Nevada Rise stores as of January 1, 2018, all incremental compared to the year ending December 31, 2017.
Cost of Goods Sold
Cost of goods sold are derived from cost related to the internal cultivation and production of cannabis and from retail purchases made from other licensed producers operating within our state markets.
Year ended December 31, 2018 cost of goods sold of $34,177 thousand was up $24,369 thousand or 249% compared to year ended December 31, 2017, driven by expanded production of consumer packaged goods in new markets Maryland and Pennsylvania, as well as material increases from retail sales driven by new store openings and increases in daily transactions across Illinois, Maryland, Massachusetts, Nevada and Pennsylvania.
Gross Profit
Gross profit for the year ended December 31, 2018 was $28,317 thousand, representing a gross margin on the sale of branded cannabis flower and processed and packaged products including concentrates, edibles, topicals and other cannabis products, of 45%. This is compared to gross profit for the year ended December 31, 2017 of $6,721 thousand or a 41% gross margin.
Total Expenses
Total expenses for year ended December 31, 2018 were $54,657 thousand, an increase of $43,166 thousand, compared to year ended December 31, 2017.
Increase in total expenses was attributable to an increase in general and administrative expenses, mainly due to non-cash charges related to equity incentive compensation of $12,148 thousand, which is all
64
Table of Contents
incremental compared to the prior year. Salaries and benefits also contributed to the increase as a result of new headcount from the Company’s Retail facilities in Illinois, Nevada, Maryland and Pennsylvania along with corporate staff development.
Additionally, the Company had professional fees of $10,689 thousand which represented an increase of $7,171 thousand over the 2017 amount of $3,518 thousand due to the reverse takeover transaction, acquisition related support, and other regulatory and growth related activities.
Total Other Income
Total other income for year ended December 31, 2018 was $56,091 thousand, an increase of $55,979 thousand compared to 2017, due to the iAnthus Warrants and other investments recorded at fair value, as further described in the Liquidity, Financing Activities During the Period, and Capital Resources section of this MD&A.
Provision for Income
Income tax expense is recognized based on the expected tax payable on the taxable income for the year, using tax rates enacted at year-end. For year ended December 31, 2018, Federal and State income tax expense totaled $7,184 thousand compared to $214 thousand provision for income taxes for the year ended December 31, 2017. Deferred tax expense of $4,061 thousand is included in the $7,184 thousand for the current period. This expense is driven by the fair value of Warrants and investments, partially offset by deferred tax benefit related to net operating losses and stock-based compensation.
Income (Loss) From Operations
Net operating income before provision for income taxes and non-controlling interest for year ended December 31, 2018 was $29,751 thousand, compared to a loss of ($4,658) for the year ended December 31, 2017. The increase in net operating income was driven by the fair value of the iAnthus Warrants and other investments recorded at fair value, partially offset by equity incentive compensation as described above, in addition to start-up costs for new markets this year.
As presented in the Non-GAAP section, after adjusting for non-cash equity incentive compensation of $12,148 thousand as described above adjusted operating EBITDA was ($9,007) thousand and ($4,080) thousand for the year ended December 31, 2018 and 2017, respectively.
Year Ended December 31, 2017
Revenue
Revenue for the year ended December 31, 2017 was $16,529 thousand, up 129% from $7,214 thousand for the year ended December 31, 2016 due to revenue contribution from Consumer Packaged Goods in Illinois and Retail sales across Illinois and Nevada. Year over year consumer packaged goods growth is driven by expanded distribution to third-party retail customers of GTI’s branded product portfolio including Rythm, The Feel Collection and Dogwalkers. Retail sales growth is driven by increased foot traffic in Illinois and Nevada retail stores and incremental revenue from two Illinois stores which were acquired in October 2017.
Cost of Goods Sold
Cost of goods sold are derived from cost related to the internal cultivation and production of cannabis and from retail purchases made from other licensed producers operating within our state markets.
65
Table of Contents
Year ended December 31, 2017 cost of goods sold of $9,808 thousand was up $4,259 thousand or 77% compared to year ended December 31, 2016, driven by expanded production of consumer packaged goods in Illinois, as well as increases from retail sales driven by increases in daily transactions in Illinois and Nevada.
Gross Profit
Gross profit for the year ended December 31, 2017 was $6,721 thousand, representing a gross margin on the sale of branded cannabis flower and processed and packaged products including concentrates, edibles, topicals and other cannabis products, of 41%. This is compared to gross profit for the year ended December 31, 2016 of $1,664 thousand or a 23% gross margin.
Total Expenses
Total expenses for year ended December 31, 2017 were $11,491 thousand, an increase of $6,827 thousand, compared to year ended December 31, 2016.
Increase in total expenses was attributable to an increase in general and administrative expenses driven by salaries and benefits as a result of new headcount from the Company’s Retail facilities in Illinois and Nevada, along with corporate staff development and professional fees related to growth related activities.
Provision for Income Taxes
Income tax expense is recognized based on the expected tax payable on the taxable income for the year, using tax rates enacted at year-end. For year ended December 31, 2017, Federal and State income tax expense totaled $214 thousand compared to zero provision for income taxes for the year ended December 31, 2016.
Loss From Operations
Net operating loss before provision for income taxes and non-controlling interest for year ended December 31, 2017 was $4,658 thousand, an increase of $1,125 thousand compared to the year ended December 31, 2016. The increase in net operating income was driven by start-up costs for upcoming new markets along with growth related professional fees.
As presented in the Non-GAAP section, after adjusting for non-operating items, adjusted operating EBITDA was ($4,080) thousand and ($2,506) thousand for the year ended December 31, 2017 and 2016, respectively.
Results of Operations - by Segment
The following tables summarize revenues net of sales discounts by segment for the years ended December 31, 2019, 2018 and 2017:
| Year Ended December 31, | 2019 vs. 2018 | 2018 vs. 2017 | ||||||||||||||||||||||||||
| 2019 | 2018 | 2017 | $ Change | % Change | $ Change | % Change | ||||||||||||||||||||||
| Consumer Packaged Goods |
$ | 109,930,160 | $ | 25,706,134 | $ | 8,375,953 | 84,224,026 | 328 | % | 17,330,181 | 207 | % | ||||||||||||||||
| Retail |
137,809,904 | 41,994,791 | 9,924,970 | 95,815,113 | 228 | % | 32,069,821 | 323 | % | |||||||||||||||||||
| Intersegment Eliminations |
(31,307,459 | ) | (5,207,245 | ) | (1,772,144 | ) | (26,100,214 | ) | n.m. | (3,435,101 | ) | n.m. | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total Revenues, Net of Discounts |
$ | 216,432,605 | $ | 62,493,676 | $ | 16,528,779 | 153,938,929 | 246 | % | 45,964,897 | 278 | % | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
66
Table of Contents
Year Ended December 31, 2019
Revenues, net of discounts for the Consumer Packaged Goods Segment were $109,930,160, an increase of $84,224,026 or 328%, compared to the year ended December 31, 2018. The increase in Consumer Packaged Goods revenues, net of discounts, was primarily driven by increased sales volume in established markets such as Illinois, Massachusetts, Maryland and Pennsylvania as well as the acquisition of Advanced Grow Labs, LLC’s cultivation and processing facility and Integral Associates, LLC’s Desert Grown Farms cultivation and processing facility.
Revenues, net of discounts for the Retail Segment were $137,809,904, an increase of $95,815,113 or 228%, compared to the year ended December 31, 2018. The increase in Retail revenues, net of discounts, was primarily driven by new store openings, the acquisition of Integral Associates, LLC’s Essence branded dispensaries and increased sales volume at existing stores.
Due to the vertically integrated nature of the business, the Company reviews its revenue at the Retail and CPG level while reviewing its operating results on a consolidated basis.
Year Ended December 31, 2018
Revenues, net of discounts for the Consumer Packaged Goods Segment were $25,706,134, an increase of $17,330,181 or 207%, compared to the year ended December 31, 2017. The increase in Consumer Packaged Goods revenues, net of discounts, was primarily driven by new markets in Pennsylvania and Massachusetts along with increased sales volume in the established markets of Illinois and Maryland.
Revenues, net of discounts for the Retail Segment were $41,994,791, an increase of $32,069,821 or 323%, compared to the year ended December 31, 2017. The increase in Retail revenues, net of discounts, was primarily driven by new store openings in Pennsylvania and Maryland, increased sales volume at existing stores in the established markets of Illinois and Nevada as well as the revenue from the acquisition of two Illinois stores in October 2017.
Due to the vertically integrated nature of the business, the Company reviews its revenue at the Retail and CPG level while reviewing its operating results on a consolidated basis.
Drivers of Results of Operations
Revenue
The Company derives its revenue from two revenue streams: a Consumer Packaged Good business in which it manufactures, sells and distributes its portfolio of finished consumer packaged goods, including brands Rythm, Dogwalkers, The Feel Collection and Beboe, among others, primarily to third-party retail customers; and a Retail business in which it sells finished goods sourced primarily from third-party cannabis manufacturers direct to the end consumer in its brick and mortar retail stores, as well as direct-to-consumer delivery where applicable by state law.
For the year ended December 31, 2019, revenue was contributed from consumer packaged goods and retail sales across California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania.
Gross Profit
Gross profit is revenue less cost of goods sold. Cost of goods sold includes the costs directly attributable to product sales and includes amounts paid for finished goods, such as flower, edibles, and concentrates, as well as packaging and other supplies, fees for services and processing, and allocated overhead which includes allocations of rent, utilities and related costs. Cannabis costs are affected by various state
67
Table of Contents
regulations that limit the sourcing and procurement of cannabis product, which may create fluctuations in gross profit over comparative periods as the regulatory environment changes. Gross margin measures our gross profit as a percentage of revenue.
During the year ended December 31, 2019, the Company continued to be focused on executing sustainable, profitable growth of the Company’s base business while pursuing national expansion. GTI expects to continue its growth strategy for the foreseeable future as the Company expands its consumer packaged goods and retail footprint within its current markets with acquisitions and partnerships, and scales resources into new markets.
Total Expenses
Total expenses other than the cost of goods sold consist of selling costs to support customer relationships and marketing and branding activities. It also includes a significant investment in the corporate infrastructure required to support ongoing business.
Selling costs generally correlate to revenue. As a percentage of sales, the Company expects selling costs to remain relatively flat in the more established operational markets (Connecticut, Illinois, Nevada, Maryland, Massachusetts and Pennsylvania) and increase in the up and coming markets as business continues to grow (Florida, New Jersey, New York and Ohio). The increase is expected to be driven primarily by the growth of our Retail channels and the ramp up from pre-revenue to sustainable market share.
General and administrative expenses also include costs incurred at the corporate offices, primarily related to personnel costs, including salaries, incentive compensation, benefits, stock-based compensation and other professional service costs. The Company expects to continue to invest considerably in this area to support aggressive expansion plans and to support the business by attracting and retaining top-tier talent. Furthermore, the Company expects to continue to incur acquisition and transaction costs related to these expansion plans and anticipates an increase in stock compensation expenses related to recruiting and hiring talent, along with legal and professional fees associated with being a publicly traded company.
Provision for Income Taxes
The Company is subject to income taxes in the jurisdictions in which it operates and, consequently, income tax expense is a function of the allocation of taxable income by jurisdiction and the various activities that impact the timing of taxable events. As the Company operates in the legal cannabis industry, it is subject to the limitations of IRC Section 280E under which taxpayers are only allowed to deduct expenses directly related to sales of product. This results in permanent differences between ordinary and necessary business expenses deemed non-allowable under IRC Section 280E and a higher effective tax rate than most industries. Therefore, the effective tax rate can be highly variable and may not necessarily correlate to pre-tax income or loss.
Non-GAAP Measures
EBITDA, Adjusted Operating EBITDA, and Adjusted EBITDA are non-GAAP measures and do not have standardized definitions under GAAP. The following information provides reconciliations of the supplemental non-GAAP financial measures, presented herein to the most directly comparable financial measures calculated and presented in accordance with GAAP. The Company has provided the non-GAAP financial measures, which are not calculated or presented in accordance with GAAP, as supplemental information and in addition to the financial measures that are calculated and presented in accordance with GAAP. These supplemental non-GAAP financial measures are presented because management has evaluated the financial results both including and excluding the adjusted items and believe that the supplemental non-GAAP financial measures presented provide additional perspective and insights when analyzing the core operating performance of the business. These supplemental non-GAAP financial measures should not be considered
68
Table of Contents
superior to, as a substitute for or as an alternative to, and should be considered in conjunction with, the GAAP financial measures presented.
| December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (in thousands) | ||||||||||||
| Net Income (Loss) Before Non-Controlling Interest |
$ | (59,547 | ) | $ | 22,568 | $ | (4,872 | ) | ||||
| Interest Income |
(1,466 | ) | (1,953 | ) | — | |||||||
| Interest Expense |
13,659 | 2,279 | 432 | |||||||||
| Provision For Income Taxes |
9,344 | 7,184 | 214 | |||||||||
| Other Income |
10,319 | (56,417 | ) | (544 | ) | |||||||
| Depreciation and amortization |
31,153 | 5,184 | 690 | |||||||||
|
|
|
|
|
|
|
|||||||
| Earnings before interest, taxes, depreciation and amortization (EBITDA) (non-GAAP measure) |
$ | 3,462 | $ | (21,155 | ) | $ | (4,080 | ) | ||||
|
|
|
|
|
|
|
|||||||
| Stock-based compensation, non-cash |
18,285 | 12,148 | — | |||||||||
| Acquisition, transaction and other non-operating costs |
6,015 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Adjusted Operating EBITDA (non-GAAP measure) |
$ | 27,762 | $ | (9,007 | ) | $ | (4,080 | ) | ||||
|
|
|
|
|
|
|
|||||||
Liquidity, Financing Activities During the Period, and Capital Resources
As of December 31, 2019, and 2018, the Company had total current liabilities of $111,367 thousand and $47,852 thousand, respectively, and cash and cash equivalents of $46,667 thousand and $145,986 thousand, respectively to meet its current obligations. As of December 31, 2019, the Company had working capital of ($2,305) thousand, down $123,515 thousand as compared to December 31, 2018, driven primarily by liabilities arising from the completion of business acquisitions during the year ended December 31, 2019. Contingent consideration payable of $50,391 thousand and liability for acquisition of noncontrolling interest of $5,500 thousand are two such current liabilities, both of which the Company has the ability to settle in cash or Subordinated Voting Shares. In each instance, the Company anticipates settling these liabilities in full with Subordinated Voting Shares, thereby increasing working capital (adjusted for these non-cash liabilities) to $53,587 thousand for the year ended December 31, 2019.
The Company is an early-stage growth company. It is generating cash from sales and is deploying its capital reserves to acquire and develop assets capable of producing additional revenues and earnings over both the immediate and near term. Capital reserves are being utilized for acquisitions in the medical and adult use cannabis markets, for capital expenditures and improvements in existing facilities, product development and marketing, as well as customer, supplier and investor and industry relations.
Cash Flows
Cash Used in Operating Activities
Net cash used in operating activities for the years ended December 31, 2019, 2018 and 2017, were as follows:
| Years Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| Net Cash Used in Operating Activities |
($ | 18,014 | ) | ($ | 17,683 | ) | ($ | 4,289 | ) | |||
Cash Flow from Investing Activities
Net cash used in investing activities for the years ended December 31, 2019, 2018 and 2017, were as follows:
| Years Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| Net Cash Used in Investing Activities |
($ | 174,671 | ) | ($ | 111,421 | ) | ($ | 26,025 | ) | |||
69
Table of Contents
Cash Flow from Financing Activities
Net cash provided by financing activities for the years ended December 31, 2019, 2018 and 2017, were as follows:
| Years Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| Net Cash Provided by Financing Activities |
$ | 93,366 | $ | 245,525 | $ | 46,924 | ||||||
Contractual Cash Obligations and Other Commitments and Contingencies
The following table quantifies the Company’s future contractual obligations as of December 31, 2019:
| Total | 2020 | 2021 | 2022 | 2023 | 2024 | Thereafter | ||||||||||||||||||||||
| Notes Payable(a) |
$ | 105,466,429 | $ | — | $ | — | $ | 105,466,429 | $ | — | $ | — | $ | — | ||||||||||||||
| Charitable Contributions |
970,957 | 206,675 | 184,913 | 188,958 | 193,092 | 197,319 | — | |||||||||||||||||||||
| Interest Due on Notes Payable |
30,304,983 | 12,655,971 | 12,655,971 | 4,993,041 | — | — | — | |||||||||||||||||||||
| Operating Leases - Third Party |
104,768,501 | 10,041,583 | 10,862,998 | 10,261,431 | 10,039,912 | 8,750,062 | 54,812,515 | |||||||||||||||||||||
| Operating Leases - Related Parties |
18,676,425 | 1,392,233 | 1,424,852 | 1,458,247 | 1,492,438 | 1,384,036 | 11,524,619 | |||||||||||||||||||||
| Contingent Consideration(b) |
58,936,739 | 50,391,181 | 8,545,558 | — | — | — | — | |||||||||||||||||||||
| Liability for Acquisition of Noncontrolling Interest(b) |
5,500,000 | 5,500,000 | — | — | — | — | — | |||||||||||||||||||||
| Construction Commitments |
10,877,000 | 10,877,000 | — | — | — | — | — | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total as of December 31, 2019 |
$ | 335,501,034 | $ | 91,064,643 | $ | 33,674,292 | $ | 122,368,106 | $ | 11,725,442 | $ | 10,331,417 | $ | 66,337,134 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
(a) - The amount excludes $15,090,517 of unamortized debt discount as of December 31, 2019. See Note 10 - Notes Payable for details.
(b) - The Company has the ability to settle these liabilities in cash or Subordinated Voting Shares. In each instance, the Company anticipates settling these liabilities in full through the issuance of Subordinate Voting Shares.
Off-Balance Sheet Arrangements
As of December 31, 2019, the Company does not have any off-balance-sheet arrangements that have, or are reasonably likely to have, a current or future effect on the results of operations or financial condition of the Company, including, and without limitation, such considerations as liquidity and capital resources.
Pending and Subsequent Transactions
On January 31, 2020, the Company closed on a sale and lease back transaction to sell its Toledo, Ohio processing facility to an unrelated third party. GTI will lease back the facility via a long-term agreement and continue to operate and manage it. The purchase price for the property was $2.9 million, excluding transaction costs. GTI is also expected to make certain improvements to the property that will significantly enhance production capacity, for which GTI will be reimbursed up to $4.3 million. Assuming full reimbursement for such improvements, the total investment in the property will be $7.2 million.
On March 6, 2020, the Company closed on a sale and lease back transaction to sell its Oglesby, Illinois cultivation and processing facility to an unrelated third party. GTI will lease back the facility via a long-term agreement and continue to operate and manage it. The purchase price for the property was $9.0 million, excluding transaction costs. GTI is also expected to make certain improvements to the property that will significantly enhance production capacity, for which GTI will be reimbursed up to $41.0 million. Assuming full reimbursement for such improvements, the total investment in the property will be $50.0 million.
70
Table of Contents
Changes in or Adoption of Accounting Practices
The following GAAP standards have been recently issued by the accounting standards board. The Company is assessing the impact of these new standards on future consolidated financial statements.
| (i) | In February 2016, the FASB issued Accounting Standards Update No. 2016-02 “Leases (Topic 842)” (“ASU 2016-02”), which requires lessees to record most leases on the balance sheet but recognize expense on the income statement in a manner similar to current accounting. For lessors, ASU 2016-02 also modifies the classification criteria and the accounting for sales-type and direct financing leases. The standard requires a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements and is effective in the first quarter of 2019. |
Upon adoption of ASU 2016-02, the Company recorded right-of-use assets of $11,197,339 and corresponding lease liabilities of $11,695,585 with the difference of $498,246 recorded in opening retained earnings.
| (ii) | In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires the measurement of current expected credit losses for financial assets held at the reporting date based on historical experience, current conditions and reasonable and supportable forecasts. Adoption of ASU 2016-13 will require financial institutions and other organizations to use forward-looking information to better formulate their credit loss estimates. In addition, the ASU amends the accounting for credit losses on available for sale debt securities and purchased financial assets with credit deterioration. This update will be effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company expects to implement the provisions of ASU 2016-13 as of January 1, 2020. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (iii) | In January 2017, the FASB issued Accounting Standards Update No. 2017-04 “Intangibles— Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment” (“ASU 2017-04”), which simplifies the accounting for goodwill impairment. ASU 2017-04 requires entities to record an impairment charge based on the excess of a reporting unit’s carrying amount over its fair value (Step 1 under the current impairment test). The standard eliminates Step 2 from the current goodwill impairment test, which included determining the implied fair value of goodwill and comparing it with the carrying amount of that goodwill. ASU 2017-04 must be applied prospectively and is effective in the first quarter of 2020. Early adoption is permitted. The Company intends to adopt the new standard in the first quarter of 2020. |
| (iv) | In August 2018, the FASB issued ASU 2018-13, Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement (Topic 820). ASU 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements. ASU 2018-13 is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (v) | In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740)—Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (vi) | In January 2020, the FASB issued ASU 2020-01, Investments—Equity Securities (Topic 321), Investments—Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) (“ASU 2020-01”), which is intended to clarify the interaction of the accounting for |
71
Table of Contents
| equity securities under Topic 321 and investments accounted for under the equity method of accounting in Topic 323 and the accounting for certain forward contracts and purchased options accounted for under Topic 815. ASU 2020-01 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
CRITICAL ACCOUNTING ESTIMATES
The preparation of the Company’s consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
Significant judgments, estimates and assumptions that have the most significant effect on the amounts recognized in the consolidated financial statements are described below.
Estimated Useful Lives and Amortization of Intangible Assets
Amortization of intangible assets is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any. Intangible assets that have indefinite useful lives are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they may be impaired.
Business Combinations
Classification of an acquisition as a business combination or an asset acquisition depends on whether the assets acquired constitute a business, which can be a complex judgment. Whether an acquisition is classified as a business combination or asset acquisition can have a significant impact on the entries made on and after acquisition.
In determining the fair value of all identifiable assets, liabilities and contingent liabilities acquired, the most significant estimates relate to contingent consideration and intangible assets. Management exercises judgement in estimating the probability and timing of when earn-outs are expected to be achieved, which is used as the basis for estimating fair value. For any intangible asset identified, depending on the type of intangible asset and the complexity of determining its fair value, an independent valuation expert or management may develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows.
Cannabis licenses are the primary intangible asset acquired in business combinations as they provide the Corporation the ability to operate in each market. However, some cannabis licenses are subject to renewal and therefore there is some risk of non-renewal for several reasons, including operational, regulatory, legal or economic. To appropriately consider the risk of non-renewal, the Corporation applies probability weighting to the expected future net cash flows in calculating the fair value of these intangible assets. The key assumptions used in these cash flow projections include discount rates and terminal growth rates. Of the key assumptions used, the impact of the estimated fair value of the intangible assets have the greatest sensitivity to the estimated discount rate used in the valuation. Management selected discount rates ranging from 12% to 18% primarily dependent upon the markets in which each of the acquisitions operates. The terminal growth rate represents the rate at which these businesses will continue to grow into perpetuity. Management selected terminal growth rates between 2% and 3%. Other significant assumptions include revenue, gross profit, operating expenses and anticipated capital expenditures which are based upon the Corporation’s historical operations along with management projections.
72
Table of Contents
The evaluations are linked closely to the assumptions made by management regarding the future performance of these assets and any changes in the discount rate applied.
Inventories
The net realizable value of inventories represents the estimated selling price for inventories in the ordinary course of business, less all estimated costs of completion and costs necessary to make the sale. The determination of net realizable value requires significant judgment, including consideration of factors such as shrinkage, the aging of and future demand for inventory, expected future selling price the Company expects to realize by selling the inventory and the contractual arrangements with customers. Reserves for excess and obsolete inventory are based upon quantities on hand, projected volumes from demand forecasts and net realizable value. The estimates are judgmental in nature and are made at a point in time, using available information, expected business plans and expected market conditions. As a result, the actual amount received on sale could differ from the estimated value of inventory. Periodic reviews are performed on the inventory balance. The impact of changes in inventory reserves is reflected in cost of goods sold.
Investments in Private Holdings
Investments include private company investments which are carried at fair value based on the value of the Company’s interests in the private companies determined from financial information provided by management of the companies, which may include operating results, subsequent rounds of financing and other appropriate information. Any change in fair value is recognized on the consolidated statement of operations.
Goodwill Impairment
Goodwill is tested for impairment annually and whenever events or changes in circumstances indicate that the carrying amount of goodwill has been impaired. In order to determine if the value of goodwill has been impaired, the reporting unit to which goodwill has been assigned or allocated must be valued using present value techniques. When applying this valuation technique, the Company relies on a number of factors, including historical results, business plans, forecasts and market data. Changes in the conditions for these judgments and estimates can significantly affect the assessed value of goodwill.
Determination of Reporting Units
The Company’s assets are aggregated into two reportable segments (Retail and Consumer Packaged Goods). For the purposes of testing goodwill, GTI has identified 22 reporting units. The Company analyzed its reporting units by first reviewing the operating segments based on the geographic areas in which GTI conducts business (or each market). The markets were then further divided into reporting units based on the market operations (Retail and Consumer Packaged Goods) which were primarily determined based on the licenses each market holds. See Note 20 - Segment Reporting for additional details.
Consolidation
Judgment is applied in assessing whether the Company exercises control and has significant influence over entities in which the Company directly or indirectly owns an interest. The Company has control when it has the power over the subsidiary, has exposure or rights to variable returns and has the ability to use its power to affect the returns. Significant influence is defined as the power to participate in the financial and operating decisions of the subsidiaries. Where the Company is determined to have control, these entities are consolidated. Additionally, judgment is applied in determining the effective date on which control was obtained. See Note 14 – Variable Interest Entities for further details.
73
Table of Contents
Allowance for Uncollectible Accounts
Management determines the allowance for uncollectible accounts by evaluating individual receivable balances and considering accounts and other receivable financial condition and current economic conditions. Accounts receivable and financial assets recorded in other receivables are written off when deemed uncollectible. Recoveries of accounts receivable previously written off are recorded as income when received. All receivables are expected to be collected within one year of the balance sheet date.
Stock-Based Payments
Valuation of stock-based compensation and warrants requires management to make estimates regarding the inputs for option pricing models, such as the expected life of the option, the volatility of the Company’s stock price, the vesting period of the option and the risk-free interest rate are used. Actual results could differ from those estimates. The estimates are considered for each new grant of stock options or warrants.
Fair Value of Financial Instruments
The individual fair values attributed to the different components of a financing transaction, including derivative financial instruments, are determined using valuation techniques. The Company uses judgment to select the methods used to make certain assumptions and in performing the fair value calculations in order to determine (a) the values attributed to each component of a transaction at the time of their issuance; (b) the fair value measurements for certain instruments that require subsequent measurement at fair value on a recurring basis; and (c) for disclosing the fair value of financial instruments. These valuation estimates could be significantly different because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market.
Financial Instruments and Financial Risk Management
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, member contribution receivable, notes receivable, due from related parties, investments, accounts payable and accrued liabilities, notes payable, derivative liability, liability for acquisition of noncontrolling interest and contingent consideration payable.
Financial instruments recorded at fair value are classified using a fair value hierarchy that reflects the significance of the inputs to fair value measurements. The three levels of hierarchy are:
Level 1 – Unadjusted quoted prices in active markets for identical assets or liabilities;
Level 2 – Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and
Level 3 – Inputs for the asset or liability that are not based on observable market data.
ITEM 7A. QUANTITAVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board mitigates these risks by assessing, monitoring and approving the Company’s risk management processes.
Credit Risk
Credit risk is the risk of a potential loss to the Company if a customer or third party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure at December 31, 2019 is the carrying amount of cash and cash equivalents. The Company does not have significant credit risk with respect to its customers. All cash and cash equivalents are placed with major U.S. financial institutions.
74
Table of Contents
The Company provides credit to its customers in the normal course of business. The Company has established credit evaluation and monitoring processes to mitigate credit risk but has limited risk as the majority of its sales are transacted with cash.
Liquidity Risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company manages liquidity risk through the effective management of its capital structure. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity at all times to settle obligations and liabilities when due.
Market Risk
Market risk is the risk of loss arising from adverse changes in market rates and prices, such as interest rates, foreign exchange, raw material and other commodity prices.
Currency Risk. The operating results and financial position of the Company are reported in U.S. dollars. Some of the Company’s financial transactions are denominated in currencies other than the U.S. dollar. The results of the Company’s operations are subject to currency transaction risks. The Company has no hedging agreements in place with respect to foreign exchange rates. The Company has not entered into any agreements or purchased any instruments to hedge possible currency risks at this time.
Interest Rate Risk. Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. Cash and cash equivalents bear interest at market rates. The Company’s financial debts have fixed rates of interest and therefore expose the Company to a limited interest rate fair value risk.
Commodities Price Risk. Price risk is the risk of variability in fair value due to movements in equity or market prices. The primary raw materials used by the Company aside from those cultivated internally are labels and packaging. Management believes a hypothetical 10% change in the price of these materials would not have a significant effect on the Company’s consolidated annual results of operations or cash flows, as these costs are generally passed through to its customers. However, such an increase could have an impact on our customers’ demand for our products, and we are not able to quantify the impact of such potential change in demand on our combined annual results of operations or cash flows.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial information required by Item 8 is located beginning on page F-1 of this report.
ITEM 9. CHANGES AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Prior Independent Registered Accounting Firm
Following the Transaction, the independent registered public accounting firm of the Corporation was MNP LLP (“MNP”) located at 111 Richmond Street W, Suite 300, Toronto, Ontario M5H 2G4, Canada. The Corporation engaged MNP on February 21, 2019, and MNP completed an audit of the Corporation for the year ended December 31, 2018. MNP resigned as the Corporation’s auditor effective October 15, 2019. The resignation of MNP was approved by the Audit Committee and the Board.
During the year ended December 31, 2018 and the subsequent period through October 15, 2019, the date of MNP’s resignation, there were no (1) disagreements with MNP on any matter of accounting principles or
75
Table of Contents
practices, financial statement disclosure or auditing scope or procedure, which, if not resolved to MNP’s satisfaction, would have caused MNP to make reference thereto in its report on the consolidated financial statements of the Corporation (as described in Item 304(a)(1)(iv) of Regulation S-K) or (2) reportable events (as described in Item 304(a)(1)(v) of Regulation S-K).
MNP’s report on the consolidated financial statements of the Corporation for the year ended December 31, 2018 did not contain an adverse opinion or a disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope or accounting principles.
We have provided MNP with a copy of the foregoing disclosures and requested that MNP furnish us with a letter addressed to the SEC stating whether MNP agrees with the statements made herein and, if not, stating the respects in which it does not agree. A copy of the letter is filed as Exhibit 16.1 to this document.
Engagement of Macias Gini & O’Connell LLP
The Corporation appointed Macias Gini & O’Connell LLP (“MGO”) located at 2029 Century Park East, Suite 1500, Los Angeles, California 90067 as its independent registered public accounting firm effective October 17, 2019. The engagement of MGO was approved by the Audit Committee and the Board. MGO will complete an audit of the Corporation for the year ended December 31, 2019. Additionally, in connection with this Annual Report on Form 10-K, MGO provided audits of the Corporation for the years ended December 31, 2016 and 2017.
During the period from October 17, 2019 through November 30, 2019, the Company did not consult with MGO regarding any of the following:
| • | the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Corporation’s financial statements, and neither a written report nor oral advice was provided to the Corporation that MGO concluded was an important factor considered by the Corporation in reaching a decision as to an accounting, auditing or financial reporting issue; or |
| • | any matter that was either the subject of a disagreement (as described in Item 304(a)(1)(iv) of Regulation S-K) or a reportable event (as described in Item 304(a)(1)(v) of Regulation S-K). |
MGO’s reports on the combined financial statements of the Corporation for the years ended December 31, 2016 and 2017 did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principle.
ITEM 9A. CONTROLS AND PROCEDURES
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report of the company’s registered public accounting firm due to a transition period established by rules of the Securities and Exchange Commission for newly public companies.
None.
76
Table of Contents
PART III
ITEM 10. EXECUTIVE OFFICERS OF GREEN THUMB INDUSTRIES INC.
Information regarding directors and executive officers is incorporated herein by reference to the 2020 proxy statement.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item will be set forth under Executive Compensation in the Green Thumb Industries Inc. Proxy Statement for the 2020 Annual Meeting of Shareholders, which are incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Information regarding security ownership of certain beneficial owners and management is incorporated herein by reference to the 2020 Proxy Statement.
Equity Compensation Plans
The shareholders and the Board approved the Stock and Incentive Plan on June 11, 2018, and amended it effective August 30, 2019. The granting of awards under the Stock and Incentive Plan is intended to promote the interests of the Company and its shareholders by aiding the Company in attracting and retaining persons capable of assuring the future success of the Company, to offer such persons incentives to put forth maximum efforts for the success of the Company’s business and to compensate such persons through various stock and cash-based arrangements and provide them with opportunities for stock ownership in the Company, thereby aligning the interests of such persons with the Company’s shareholders. Eligible participants under the Stock and Incentive Plan include non-employee directors, officers (including the named executive officers), employees, consultants and advisors of the Company and its subsidiaries. The Stock and Incentive Plan will be administered by the Board or a committee thereof appointed by the Board (the “Stock and Incentive Plan Committee”).
Pursuant to the Stock and Incentive Plan, the Company may issue equity-based compensation (denominated in Subordinate Voting Shares) in the form of stock options, stock appreciation rights, restricted stock awards, restricted stock units, performance shares, performance units and dividend equivalent awards to eligible participants. The Stock and Incentive Plan Committee or its permitted delegates has the power and discretionary authority to determine the amount, terms and conditions of the Stock and Incentive Plan awards, including, without limitation, (i) the exercise price of any stock options or stock appreciation rights, (ii) the method of payment for shares purchased pursuant to any award, (iii) the method for satisfying any tax withholding obligation arising in connection with any award, including by net exercise or the withholding or delivery of shares, (iv) the timing, terms and conditions of the exercisability, vesting or payout of any award or any shares acquired pursuant thereto, (v) the performance criteria, if any, applicable to any award and the extent to which such performance criteria have been attained, (vi) the time of the expiration of any award, (vii) the effect of the participant’s termination of service on any of the foregoing, and (viii) all other terms, conditions and restrictions applicable to any award or shares acquired pursuant thereto as the Board shall consider to be appropriate and not inconsistent with the terms of the Stock and Incentive Plan. A non-employee director may not be granted Stock and Incentive Plan awards that exceed in the aggregate $1,500,000 in any calendar year. The foregoing limit does not apply to any award made pursuant to any election by the director to receive an award in lieu of all or a portion of annual and committee retainers and meeting fees.
77
Table of Contents
The following table sets forth securities authorized for issuance under the Stock and Incentive Plan as of fiscal 2019-year end.
| Plan Category |
(a) Number of securities to be issued upon exercise of outstanding Options, warrants and rights |
(b) Weighted-average exercise price of outstanding Options, warrants and rights |
(c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by shareholders(1) |
5,221,667 | C$ | 14.18 | 15,434,719 | ||||||||
| Equity compensation plans not approved by shareholders |
Nil | Nil | Nil | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL |
5,221,667 | C$14.18 | 15,434,719 | |||||||||
|
|
|
|
|
|
|
|||||||
Note:
| (1) | The maximum number of Subordinate Voting Shares issuable under the Stock and Incentive Plan of the Corporation as of December 31, 2019 was 20,656,386, representing 10% of the number of the issued and outstanding Subordinate Voting Shares (including, for these purposes, the number of Subordinate Voting Shares underlying the Multiple Voting Shares and the Super Voting Shares on an “as if converted” basis) (the “Outstanding Share Number”). |
As at December 31, 2019, the following Awards were outstanding under the Stock and Incentive Plan: (i) a total of 3,821,905 Options, representing approximately 2% of the then Outstanding Share Number; and (ii) a total of 1,399,762 RSUs, representing approximately 1% of the then Outstanding Share Number. As at December 31, 2019, an aggregate of 15,434,719 Subordinate Voting Shares remained available for issuance under the Stock and Incentive Plan, representing approximately 7% of the then Outstanding Share Number.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Information regarding certain relationships and related transactions and director independence is incorporated herein by reference to the 2020 Proxy Statement.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Information regarding principal accounting fees and services is incorporated herein by reference to the 2020 Proxy Statement.
78
Table of Contents
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| (a) | Financial Statements |
The financial statements listed in the accompanying index (page F-1) to the financial statements are filed as part of this Annual Report on Form 10-K.
| (b) | Exhibits |
The exhibits listed on the accompanying index on page E-1 are filed as part of this Annual Report on Form 10-K.
| (c) | Financial Statements Schedules omitted |
Certain schedules have been omitted because the required information is included in the consolidated financial statements and notes thereto or because they are not applicable or not required.
ITEM 15 . EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a). INDEX TO FINANCIAL STATEMENTS
| Page | ||||
| Consolidated Balance Sheets as of December 31, 2019 and 2018 |
F-2 | |||
| F-4 | ||||
| F-5 | ||||
| F-7 | ||||
| F-9 | ||||
| F-56 | ||||
(b). EXHIBITS
A list of exhibits filed with this Annual Report on Form 10-K is included in the Exhibit Index immediately preceding the signature page to this registration statement and is incorporated herein by reference.
Not applicable.
79
Table of Contents
| * | Certain confidential information has been excluded from this exhibit because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed. |
| # | Previously filed as an exhibit to our registration statement on Form 10 filed on December 20, 2019. |
E-1
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| GREEN THUMB INDUSTRIES INC. |
| /s/ Benjamin Kovler |
| By: Benjamin Kovler |
| Title: Chief Executive Officer |
Date: April 15, 2020
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacity and on the dates indicated.
| Name and Signature |
Title |
Date | ||
| /s/ Benjamin Kovler Benjamin Kovler |
Chairman of the Board and Chief Executive Officer (Principal Executive Officer) |
April 15, 2020 | ||
| /s/ Anthony Georgiadis Anthony Georgiadis |
Director and Chief Financial Officer (Principal Financial Officer) |
April 15, 2020 | ||
| /s/ Wendy Berger Wendy Berger |
Director | April 15, 2020 | ||
| /s/ William Gruver William Gruver |
Director | April 15, 2020 | ||
| /s/ Wes Moore Wes Moore |
Director | April 15, 2020 | ||
| /s/ Glen Senk Glen Senk |
Director | April 15, 2020 | ||
| /s/ Alex Yemenidjian Alex Yemenidjian |
Director | April 15, 2020 | ||
S-1
Table of Contents
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| Consolidated Balance Sheets as of December 31, 2019 and 2018 |
F-2 | |||
| F-4 | ||||
| F-5 | ||||
| F-7 | ||||
| F-9 | ||||
| F-56 | ||||
F-1
Table of Contents
Green Thumb Industries Inc.
December 31, 2019 and 2018
(Amounts Expressed in United States Dollars)
| December 31, 2019 |
December 31, 2018 |
|||||||
| ASSETS |
|
|||||||
| Current Assets: |
||||||||
| Cash and Cash Equivalents |
$ | 46,667,334 | $ | 145,986,072 | ||||
| Accounts Receivable |
7,530,253 | 4,574,404 | ||||||
| Inventories |
46,034,481 | 12,359,064 | ||||||
| Notes Receivable |
— | 3,500,000 | ||||||
| Prepaid Expenses |
6,780,657 | 1,501,555 | ||||||
| Other Current Assets |
2,049,886 | 1,140,926 | ||||||
|
|
|
|
|
|||||
| Total Current Assets |
109,062,611 | 169,062,021 | ||||||
| Property and Equipment, Net |
155,596,675 | 65,324,080 | ||||||
| Right of Use Assets, Net |
63,647,812 | — | ||||||
| Investments |
14,068,821 | 40,933,283 | ||||||
| Investment in Associate |
10,350,000 | 5,850,000 | ||||||
| Notes Receivable |
815,937 | 7,424,727 | ||||||
| Intangible Assets, Net |
435,246,898 | 88,365,678 | ||||||
| Goodwill |
375,084,991 | 39,204,360 | ||||||
| Deposits and Other Assets |
3,662,879 | 2,184,417 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 1,167,536,624 | $ | 418,348,566 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|||||||
| LIABILITIES |
||||||||
| Current Liabilities: |
||||||||
| Accounts Payable |
$ | 9,990,377 | $ | 8,928,528 | ||||
| Accrued Liabilities |
35,939,850 | 7,326,156 | ||||||
| Current Portion of Notes Payable |
206,675 | 1,480,660 | ||||||
| Current Portion of Lease Liabilities |
3,833,268 | — | ||||||
| Liability for Acquisition of Noncontrolling Interest |
5,500,000 | 25,420,009 | ||||||
| Contingent Consideration Payable |
50,391,181 | — | ||||||
| Derivative Liability |
— | 4,238,701 | ||||||
| Income Tax Payable |
5,505,904 | 457,585 | ||||||
|
|
|
|
|
|||||
| Total Current Liabilities |
111,367,255 | 47,851,639 | ||||||
| Long-Term Liabilities: |
||||||||
| Lease Liabilities |
61,115,737 | — | ||||||
| Notes Payable, Net of Current Portion |
91,140,194 | 5,733,797 | ||||||
| Contingent Consideration Payable |
8,545,558 | 9,035,250 | ||||||
| Warrant Liability |
15,879,843 | — | ||||||
| Deferred Income Taxes |
36,279,361 | 13,541,000 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
324,327,948 | 76,161,686 | ||||||
F-2
Table of Contents
| December 31, 2019 |
December 31, 2018 |
|||||||
| COMMITMENTS AND CONTINGENCIES |
||||||||
| SHAREHOLDERS’ EQUITY |
||||||||
| Subordinate Voting Shares (Shares Authorized, Issued and Outstanding at December 31, 2019: Unlimited, 128,999,964 and 128,999,964, respectively at December 31, 2018: Unlimited, 43,920,131, and 43,920,131, respectively) |
— | — | ||||||
| Multiple Voting Shares (Shares Authorized, Issued and Outstanding at December 31, 2019: Unlimited, 373,350 and 373,350, respectively at December 31, 2018: Unlimited, 677,230 and 677,230, respectively) |
— | — | ||||||
| Super Voting Shares (Shares Authorized, Issued and Outstanding at December 31, 2019: Unlimited, 402,289 and 402,289, respectively at December 31, 2018: Unlimited, 424,513 and 424,513, respectively) |
— | — | ||||||
| Share Capital |
980,638,701 | 397,590,465 | ||||||
| Shares to be Issued |
— | 27,773,234 | ||||||
| Contributed Surplus |
3,960,854 | 14,202,659 | ||||||
| Deferred Share Issuances |
16,587,798 | — | ||||||
| Accumulated Deficit |
(160,491,590 | ) | (100,876,937 | ) | ||||
|
|
|
|
|
|||||
| Equity of Green Thumb Industries Inc. |
840,695,763 | 338,689,421 | ||||||
| Noncontrolling interests |
2,512,913 | 3,497,459 | ||||||
|
|
|
|
|
|||||
| TOTAL SHAREHOLDERS’ EQUITY |
843,208,676 | 342,186,880 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY |
$ | 1,167,536,624 | $ | 418,348,566 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Table of Contents
Green Thumb Industries Inc.
Consolidated Statements of Operations
Years Ended December 31, 2019, 2018 and 2017
(Amounts Expressed in United States Dollars)
| December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Revenues, Net of Discounts |
$ | 216,432,605 | $ | 62,493,680 | $ | 16,528,779 | ||||||
| Cost of Goods Sold, Net |
(109,401,914 | ) | (34,177,259 | ) | (9,807,775 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Gross Profit |
107,030,691 | 28,316,421 | 6,721,004 | |||||||||
|
|
|
|
|
|
|
|||||||
| Expenses: |
||||||||||||
| Selling, General, and Administrative |
134,721,393 | 54,656,579 | 11,490,772 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Expenses |
134,721,393 | 54,656,579 | 11,490,772 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss From Operations |
(27,690,702 | ) | (26,340,158 | ) | (4,769,768 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other Income (Expense): |
||||||||||||
| Other Income (Expense), Net |
(10,318,936 | ) | 56,417,421 | 544,399 | ||||||||
| Interest Income |
1,465,705 | 1,952,945 | — | |||||||||
| Interest Expense |
(13,658,904 | ) | (2,278,834 | ) | (432,448 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total Other Income (Expense) |
(22,512,135 | ) | 56,091,532 | 111,951 | ||||||||
|
|
|
|
|
|
|
|||||||
| Income (Loss) Before Provision for Income Taxes And Non-Controlling Interest |
(50,202,837 | ) | 29,751,374 | (4,657,817 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Provision For Income Taxes |
9,344,033 | 7,183,595 | 214,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net Income (Loss) Before Non-Controlling Interest |
(59,546,870 | ) | 22,567,779 | (4,871,817 | ) | |||||||
| Net (Loss) Income Attributable to Non-Controlling Interest |
(430,463 | ) | 27,811,696 | (622,042 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net Loss Attributable to Green Thumb Industries Inc. |
$ | (59,116,407 | ) | $ | (5,243,917 | ) | $ | (4,249,775 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net Loss Per Share Attributable to Green Thumb Industries Inc. - Basic and Diluted |
$ | (0.31 | ) | $ | (0.04 | ) | $ | (0.07 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted Average Number of Shares Outstanding - Basic and Diluted |
190,602,400 | 130,102,523 | 63,123,183 | |||||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Table of Contents
F-5
Table of Contents
| Green Thumb Industries Inc. Consolidated Statements of Changes in Shareholders’ Equity Years Ended December 31, 2019, 2018 and 2017 (Amounts Expressed in United States Dollars)
|
||||||||||||||||||||||||||||
| Share Capital |
Shares to Be Issued |
Contributed Surplus |
Deferred Share Issuances |
Accumulated Earnings (Deficit) |
Non-Controlling Interest |
Total | ||||||||||||||||||||||
| Balance, January 1, 2019 |
$ | 397,590,465 | $ | 27,773,234 | $ | 14,202,659 | $ | — | $ | (100,876,937 | ) | $ | 3,497,459 | $ | 342,186,880 | |||||||||||||
| Adoption of ASC 842, Leases |
— | — | — | — | (498,246 | ) | — | (498,246 | ) | |||||||||||||||||||
| Noncontrolling interests adjustment for change in ownership |
22,461,256 | (27,773,234 | ) | (1,128,776 | ) | — | — | 5,311,978 | (1,128,776 | ) | ||||||||||||||||||
| Contributions from limited liability company unit holders |
— | — | — | — | — | 1,650,000 | 1,650,000 | |||||||||||||||||||||
| Issuance of shares under business combinations and investments |
530,697,606 | — | (23,697,894 | ) | — | — | — | 506,999,712 | ||||||||||||||||||||
| Deferred share issuances |
— | — | — | 16,587,798 | — | — | 16,587,798 | |||||||||||||||||||||
| Issuance of shares for redemption of noncontrolling interests |
29,889,374 | — | (4,469,365 | ) | — | — | — | 25,420,009 | ||||||||||||||||||||
| Stock based compensation |
— | — | 18,285,377 | — | — | — | 18,285,377 | |||||||||||||||||||||
| Shares issued in consideration of professional fees |
— | — | 228,761 | — | — | — | 228,761 | |||||||||||||||||||||
| Issuance of shares upon Exercise of broker options |
— | — | 665,152 | — | — | — | 665,152 | |||||||||||||||||||||
| Shares withheld in lieu of cash |
— | — | (125,060 | ) | — | — | — | (125,060 | ) | |||||||||||||||||||
| Distributions to limited liability company unit holders |
— | — | — | — | — | (7,516,061 | ) | (7,516,061 | ) | |||||||||||||||||||
| Net loss |
— | — | — | — | (59,116,407 | ) | (430,463 | ) | (59,546,870 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, December 31, 2019 |
$ | 980,638,701 | $ | — | $ | 3,960,854 | $ | 16,587,798 | $ | (160,491,590 | ) | $ | 2,512,913 | $ | 843,208,676 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Table of Contents
Green Thumb Industries Inc.
Consolidated Statements of Cash Flows
Years Ended December 31, 2019, 2018 and 2017
(Amounts Expressed in United States Dollars)
| 2019 | 2018 | 2017 | ||||||||||
| CASH FLOW FROM OPERATING ACTIVITIES |
||||||||||||
| Net loss attributable to Green Thumb Industries Inc. |
$ | (59,116,407 | ) | $ | (5,243,917 | ) | $ | (4,249,775 | ) | |||
| Net income attributable to non-controlling interest |
(430,463 | ) | 27,811,696 | (622,042 | ) | |||||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
31,482,340 | 5,183,980 | 689,988 | |||||||||
| Amortization of operating lease assets |
7,291,154 | — | — | |||||||||
| Loss on disposal of property and equipment |
— | 667,837 | — | |||||||||
| Loss from investment in associate |
56,423 | 55,750 | — | |||||||||
| Deferred rent |
— | (20,978 | ) | 301,105 | ||||||||
| Deferred income taxes |
(13,680,913 | ) | 4,061,000 | — | ||||||||
| Stock based compensation |
18,285,377 | 12,148,251 | — | |||||||||
| Decrease (increase) in fair value of investments |
5,586,480 | (51,942,861 | ) | — | ||||||||
| Decrease in fair value conversion feature |
— | (1,293,474 | ) | — | ||||||||
| Changes in value of liabilities related to put option and purchase of noncontrolling interests |
132,523 | (2,518,180 | ) | — | ||||||||
| Interest on convertible note payable |
— | 434,000 | — | |||||||||
| Interest on contingent consideration payable and acquisition liabilities |
3,908,529 | 178,030 | — | |||||||||
| Decrease in fair value of warrants |
(4,159,687 | ) | — | — | ||||||||
| Decrease in fair value of convertible note receivable |
6,608,790 | — | — | |||||||||
| Amortization of debt discount |
5,177,775 | — | — | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(791,709 | ) | (3,682,031 | ) | (592,026 | ) | ||||||
| Inventory |
(19,928,761 | ) | (7,441,790 | ) | (1,804,844 | ) | ||||||
| Prepaid expenses and other current assets |
(5,656,786 | ) | (2,092,697 | ) | (394,922 | ) | ||||||
| Deposits and other assets |
(306,795 | ) | (679,072 | ) | (901,768 | ) | ||||||
| Accounts payable |
(153,713 | ) | 4,725,096 | 3,098,293 | ||||||||
| Accrued liabilities |
9,122,121 | 1,722,772 | (26,717 | ) | ||||||||
| Operating lease liabilities |
(6,488,207 | ) | — | — | ||||||||
| Income tax payable |
5,048,319 | 243,585 | 214,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| NET CASH USED IN OPERATING ACTIVITIES |
(18,013,610 | ) | (17,683,003 | ) | (4,288,708 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOW FROM INVESTING ACTIVITIES |
||||||||||||
| Investments in debentures |
— | (42,550,000 | ) | — | ||||||||
| Repayment of debenture investments |
— | 20,000,000 | — | |||||||||
| Investment in associates |
— | (4,387,500 | ) | — | ||||||||
| Purchases of property and equipment |
(88,557,016 | ) | (27,432,847 | ) | (14,244,340 | ) | ||||||
| Proceeds from disposal of assets |
20,325,557 | — | — | |||||||||
| Advances to related parties |
— | (2,750,000 | ) | (1,188,686 | ) | |||||||
| Repayments from related parties |
— | 583,686 | — | |||||||||
| Repayment of note receivable |
3,000,000 | — | — | |||||||||
| Issuance of notes receivable |
— | (3,500,000 | ) | — | ||||||||
| Consolidation of variable interest entities |
— | 154,776 | — | |||||||||
| Purchases of licenses |
— | (49,999 | ) | (220,000 | ) | |||||||
| Purchase of businesses, net of cash acquired |
(109,439,921 | ) | (51,489,384 | ) | (10,372,385 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET CASH USED IN INVESTING ACTIVITIES |
(174,671,380 | ) | (111,421,268 | ) | (26,025,411 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOW FROM FINANCING ACTIVITIES |
||||||||||||
| Contributions from limited liability company unit holders |
1,650,000 | 21,748,211 | 66,160,076 | |||||||||
| Distributions to limited liability company unit holders |
(7,516,061 | ) | (17,368,034 | ) | (34,007,599 | ) | ||||||
| Payment for change in ownership interests of subsidiary |
— | (700,000 | ) | — | ||||||||
| Proceeds from shares issued pursuant to private placement |
— | 66,805,295 | — | |||||||||
| Proceeds from exchangeable notes payable |
— | 45,000,000 | — | |||||||||
| Proceeds from fundraise transactions |
— | 140,289,093 | — | |||||||||
| Proceeds from exercise of options and RSUs |
540,089 | 906,296 | — | |||||||||
| Reverse takeover, private placement, and fundraise transaction financing costs |
— | (10,627,423 | ) | — | ||||||||
| Proceeds from issuance of notes payable |
117,435,724 | 825,000 | 15,000,000 | |||||||||
| Principal repayment of notes payable |
(18,743,500 | ) | (1,353,592 | ) | (228,379 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES |
93,366,252 | 245,524,846 | 46,924,098 | |||||||||
|
|
|
|
|
|
|
|||||||
| NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS |
(99,318,738 | ) | 116,420,575 | 16,609,979 | ||||||||
| CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD |
145,986,072 | 29,565,497 | 12,955,518 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH AND CASH EQUIVALENTS, END OF PERIOD |
$ | 46,667,334 | $ | 145,986,072 | $ | 29,565,497 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Table of Contents
Green Thumb Industries Inc.
Consolidated Statements of Cash Flows
Years Ended December 31, 2019, 2018 and 2017
(Amounts Expressed in United States Dollars)
| 2019 | 2018 | 2017 | ||||||||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION |
||||||||||||
| Interest paid |
$ | 5,019,465 | $ | 874,298 | $ | 149,081 | ||||||
|
|
|
|
|
|
|
|||||||
| SUPPLEMENTAL DISCLOSURE OF NONCASH INVESTING AND FINANCING ACTIVITIES |
||||||||||||
| Purchase of property and equipment with cancellation of note receivable |
$ | — | $ | 605,000 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Distributions payable to member |
$ | — | $ | — | $ | 279,750 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of notes payable into equity |
$ | — | $ | 8,613,740 | $ | 2,279,452 | ||||||
|
|
|
|
|
|
|
|||||||
| Compensation options issued for reverse takeover services |
$ | — | $ | 906,366 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Initial consolidation of variable interest entities, net of cash |
$ | — | $ | (319,411 | ) | $ | 934,472 | |||||
|
|
|
|
|
|
|
|||||||
| Due from investors for equity contributions |
$ | — | $ | — | $ | 2,785,998 | ||||||
|
|
|
|
|
|
|
|||||||
| Accrued capital expenditures |
$ | 14,949,980 | $ | 2,710,085 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Distributions of investments |
$ | — | $ | 26,134,851 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Liability related to put option of convertible note payable |
$ | — | $ | 7,108,043 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Increase in Right of Use Assets |
$ | (63,477,013 | ) | $ | — | $ | — | |||||
|
|
|
|
|
|
|
|||||||
| Increase in Lease Liabilities |
$ | 63,975,259 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Net liability upon adoption of ASC 842, Leases |
$ | 498,246 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Exercise of put options |
$ | (1,128,776 | ) | $ | — | $ | — | |||||
|
|
|
|
|
|
|
|||||||
| Warrant liability attributable to debt issuance |
$ | 20,039,530 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Liability for purchase of noncontrolling interest |
$ | (25,420,009 | ) | $ | 25,068,847 | $ | — | |||||
|
|
|
|
|
|
|
|||||||
| Issuance of shares under acquisition agreement for contingent consideration |
$ | 10,999,040 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Deferred share issuances |
$ | 16,587,798 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Issuance of shares under business combinations |
$ | 495,737,729 | $ | 51,151,649 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Acquisitions |
||||||||||||
| Inventory |
$ | 13,746,656 | $ | 975,329 | $ | 483,058 | ||||||
| Accounts receivable |
2,164,140 | — | — | |||||||||
| Prepaid assets |
531,276 | 26,635 | — | |||||||||
| Property and equipment |
16,628,952 | 3,938,703 | 397,015 | |||||||||
| Investments |
9,900,000 | — | — | |||||||||
| Right of use assets |
7,461,953 | — | — | |||||||||
| Identifiable intangible assets |
377,163,592 | 76,650,639 | 10,600,480 | |||||||||
| Goodwill |
331,208,108 | 39,016,100 | 188,260 | |||||||||
| Deposits and other assets |
1,171,667 | 239,808 | — | |||||||||
| Liabilities assumed |
(9,729,371 | ) | (2,088,369 | ) | (1,296,428 | ) | ||||||
| Lease liabilities |
(7,461,953 | ) | — | — | ||||||||
| Contingent liabilities |
(56,992,000 | ) | (8,857,220 | ) | — | |||||||
| Equity interests issued |
(495,806,400 | ) | (49,689,149 | ) | — | |||||||
| Conversion of notes receivable previously issued |
(27,121,559 | ) | — | — | ||||||||
| Acquisition liability |
(17,378,866 | ) | — | — | ||||||||
| Deferred income taxes |
(36,046,274 | ) | (6,680,000 | ) | — | |||||||
| Noncontrolling interests |
— | (2,043,092 | ) | — | ||||||||
|
|
|
|
|
|
|
|||||||
| $ | 109,439,921 | $ | 51,489,384 | $ | 10,372,385 | |||||||
|
|
|
|
|
|
|
|||||||
| Initial consolidation of controlled entities, net of cash |
||||||||||||
| Inventory |
$ | — | $ | 79,083 | $ | — | ||||||
| Prepaid expenses and other current assets |
— | — | 1,921 | |||||||||
| Property and equipment |
— | 2,433,950 | 1,287,356 | |||||||||
| Deposits and other assets |
— | 9,000 | 160,000 | |||||||||
| Liabilities assumed |
— | (2,841,444 | ) | (514,805 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| $ | — | $ | (319,411 | ) | $ | 934,472 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Table of Contents
Green Thumb Industries Inc.
Notes to Consolidated Financial Statements
| 1. | NATURE OF OPERATIONS |
GTI is empowering the right to wellness by progressing responsible adult use of cannabis through branded consumer packaged goods and people-first retail experiences, while being committed to community and sustainable profitable growth. GTI owns, manufactures, and distributes a portfolio of cannabis consumer packaged goods brands including Rythm, Dogwalkers, The Feel Collection, incredibles, Dr. Solomon’s and Beboe, primarily to third-party retail stores across the United States as well as to GTI owned retail stores. The Company also owns and operates a rapidly growing national chain of retail cannabis stores called Risetm and Essence. As of the year ended December 31, 2019, GTI has operating revenue in twelve markets (California, Colorado, Connecticut, Florida, Illinois, Nevada, Maryland, Massachusetts, New York, New Jersey, Ohio, and Pennsylvania).
In addition to the States listed above, the Company also conducts pre-licensing activities in other markets. In these markets, the Company has either applied for licenses, or plans on applying for licenses, but does not currently own any cultivation, production or retail licenses. The Company also provides management services and solutions to state licensed cannabis cultivators and dispensaries.
On June 12, 2018, the Company completed a reverse takeover transaction (“RTO”) further described in Note 3. Following the RTO, the Company is listed on the Canadian Securities Exchange (the “CSE”) under ticker symbol “GTII” and on the OTCQX, part of the OTC Markets Group, under the ticker “GTBIF”.
The Company’s registered office is located at 885 West Georgia Street, Suite 2200, Vancouver, British Columbia, V6C 3E8, Canada. The Company’s U.S. headquarters are at 325 W. Huron St., Chicago, IL 60654.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
| (a) | Basis of Preparation and Statement of Compliance |
The consolidated financial statements as of December 31, 2019 and 2018 (the “financial statements”), have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
| (b) | Basis of Measurement |
These consolidated financial statements have been prepared on the going concern basis, under the historical cost convention, except for certain financial instruments that are measured at fair value as described herein.
| (c) | Functional and Presentation Currency |
The Company’s functional currency, as determined by management, is the United States (“U.S.”) dollar. These consolidated and combined financial statements are presented in U.S. dollars.
| (d) | Basis of Consolidation |
The consolidated financial statements for the years ended December 31, 2019 and 2018 include the accounts of the Company, its wholly-owned subsidiaries, its partially-owned subsidiaries, and those controlled by the Company by virtue of agreements, on a consolidated basis after elimination of intercompany transactions and balances.
F-9
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (d) | Basis of Consolidation (Continued) |
Control exists when the Company has power over an investee, when the Company is exposed, or has rights, to variable returns from the investee, and when the Company has the ability to affect those returns through its power over the investee. The financial statements of entities controlled by the Company by virtue of agreements are fully consolidated from the date that control commences and deconsolidated from the date control ceases.
On January 1, 2018, the members of GTI-Clinic Illinois Holdings, LLC (representing GTI’s Illinois operations and ownership) and RCP23, LLC (representing GTI’s non-Illinois operations that included Nevada, Pennsylvania, Massachusetts, and Maryland ownership) closed on a restructuring, which combined all of GTI’s operational and ownership structure within VCP23, LLC. Prior to January 1, 2018, these businesses were managed and controlled by GTI senior management. Subsequent to January 1, 2018, VCP23, LLC was controlled by the members of GTI-Clinic Illinois Holdings, LLC and RCP23, LLC.
On June 12, 2018, the Company completed a reverse takeover transaction with Bayswater Uranium Corporation (Bayswater). The Transaction was structured as a series of transactions, including a Canadian three-cornered amalgamation transaction and a series of U.S. reorganization steps as explained further in Note 3.
The following are the Company’s wholly owned subsidiaries that are included in these consolidated financial statements as of and for the years ended December 31, 2019 and 2018:
| Subsidiaries |
Jurisdiction | Interest | ||||||
| GTI23, Inc. |
Delaware | 100 | % | |||||
| VCP23, LLC |
Delaware | 100 | % | |||||
| GTI Core, LLC |
Delaware | 100 | % | |||||
F-10
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (d) | Basis of Consolidation (Continued) |
The following are VCP23, LLC’s and GTI Core, LLC’s wholly owned subsidiaries and entities over which the Company has control, that are included in these consolidated financial statements for the year ended December 31, 2019:
| Subsidiaries |
Ownership | Jurisdiction | Purpose | |||
| JB17, LLC |
100% | Maryland | Management company | |||
| GTI-Clinic Illinois Holdings, LLC |
100% | Illinois | License holder | |||
| Illinois Disp, LLC |
50% | Illinois | License holder | |||
| RISE Holdings, Inc. |
100% | Massachusetts | License holder | |||
| GTI Maryland, LLC |
100% | Maryland | License holder | |||
| Ohio Investors 2017, LLC |
99% | Ohio | Holding Company | |||
| GTI Ohio, LLC |
99% | Ohio | License holder | |||
| GTI Nevada, LLC |
100% | Nevada | License holder | |||
| GTI Pennsylvania, LLC |
100% | Pennsylvania | License holder | |||
| GTI Florida, LLC |
100% | Florida | Holding company | |||
| KSGNF, LLC |
100% | Florida | License holder | |||
| GTI New Jersey, LLC |
100% | New Jersey | License holder | |||
| KW Ventures Holdings, LLC |
100% | Pennsylvania | License holder | |||
| Chesapeake Alternatives, LLC |
0% | Maryland | License holder | |||
| Meshow, LLC |
0% | Maryland | License holder | |||
| Advanced Grow Labs, LLC |
100% | Connecticut | License holder | |||
| Bluepoint Wellness of Westport, LLC |
46% | Connecticut | License holder | |||
| Bluepoint Apothecary, LLC |
100% | Connecticut | License holder | |||
| Integral Associates, LLC |
100% | Nevada | License holder | |||
| Integral Associates CA, LLC |
100% | California | License holder | |||
| Fiorello Pharmaceuticals, Inc. |
100% | New York | License holder | |||
| MC Brands, LLC |
100% | Colorado | Intellectual property | |||
| For Success Holding Company |
100% | California | Intellectual property | |||
| VCP IP Holdings, LLC |
100% | Delaware | Intellectual property | |||
| Vision Management Services, LLC |
100% | Delaware | Management company | |||
| TWD18, LLC |
100% | Delaware | Investment company | |||
| VCP Real Estate Holdings, LLC |
100% | Delaware | Real Estate holding company |
| (e) | Investment in Associates |
Associates are all entities over which the Company has significant influence but not control, generally accompanying a shareholding of between 20% and 50% of the voting rights. Investments in associates are accounted for using the equity method and are initially recognized at cost. Unrealized gains on transactions between the Company and its associates are eliminated to the extent of the Company’s interest in the associates. Accounting policies of associates have been adjusted where necessary to ensure consistency with the policies adopted by the Company. Dilution gains and losses arising in investments in associates are recognized in the consolidated statements of operations. See Note 13 - Investment in Associates for additional details.
F-11
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (e) | Investment in Associates (Continued) |
The Company assesses annually whether there is any objective evidence that its interest in associates is impaired. If impaired, the carrying value of the Company’s share of the underlying assets of associates is written down to its estimated recoverable amount (being the higher of fair value less costs of disposal or value in use) and charged to the consolidated statement of operations. If the financial statements of an associate are prepared on a date different from that used by the Company, adjustments are made for the effects of significant transactions or events that occur between that date and the date of these consolidated financial statements.
| (f) | Non-controlling Interests |
Non-controlling interests (“NCI”) represent equity interests owned by outside parties. NCI may be initially measured at fair value or at the NCI’s proportionate share of the recognized amounts of the acquiree’s identifiable net assets. The choice of measurement is made on a transaction by transaction basis. GTI elected to measure each NCI at its proportionate share of the recognized amounts of the acquiree’s identifiable net assets. The share of net assets attributable to NCI are presented as a component of equity. Their share of net income or loss and comprehensive income or loss is recognized directly in equity. Total comprehensive income or loss of subsidiaries is attributed to the shareholders of the Company and to the NCI, even if this results in the NCI having a deficit balance.
| (g) | Cash and Cash Equivalents |
Cash and cash equivalents include cash deposits in financial institutions, other deposits that are readily convertible into cash, with original maturities of three months or less, and cash held at retail locations.
| (h) | Accounts Receivable |
Accounts receivable are recorded net of an allowance for doubtful accounts. The Company estimates the allowance for doubtful accounts based on existing contractual payment terms, actual payment patterns of its customers and individual customer circumstances. For the year ended December 31, 2019 the Company recorded approximately $139,000 in allowance for doubtful accounts. For the year ended December 31, 2018, the Company determined that an allowance for doubtful accounts was not required. The Company wrote off approximately $161,700 during the year ended December 31, 2019 and no accounts were written off during the year ended December 31, 2018.
| (i) | Inventories |
Inventories of purchased finished goods and packing materials are initially valued at cost and subsequently at the lower of cost and net realizable value.
Costs incurred during the growing and production process are capitalized as incurred to the extent that cost is less than net realizable value. These costs include materials, labor and manufacturing overhead used in the growing and production processes.
Net realizable value is determined as the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale. Cost is determined using the weighted average cost basis. Products for resale and supplies and consumables are valued at lower of cost and net realizable value. The Company reviews inventory for obsolete, redundant and slow-moving goods and any such inventories are written down to net realizable value.
F-12
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (j) | Property and Equipment |
Property and equipment are stated at cost, including capitalized borrowing costs, net of accumulated depreciation and impairment losses, if any. Expenditures that materially increase the life of the assets are capitalized. Ordinary repairs and maintenance are expensed as incurred. Depreciation is calculated on a straight-line basis over the estimated useful life of the asset using the following terms and methods:
| Land |
Not Depreciated | |
| Buildings and Improvements |
39 Years | |
| Furniture and Fixtures |
5 – 7 Years | |
| Computer Equipment and Software |
5 Years | |
| Leasehold Improvements |
Remaining Life of Lease | |
| Production and Processing Equipment |
5 – 7 Years | |
| Assets Under Construction |
Not Depreciated |
The assets’ residual values, useful lives and methods of depreciation are reviewed at each financial year-end and adjusted prospectively if appropriate. An item of equipment is derecognized upon disposal or when no future economic benefits are expected from its use. Any gain or loss arising on de-recognition of the asset (calculated as the difference between the net disposal proceeds and the carrying value of the asset) is included in operations in the year the asset is derecognized.
The Company evaluates the recoverability of other long-lived assets, including property, plant and equipment, and certain identifiable intangible assets, whenever events or changes in circumstances indicate that the carrying value of an asset or asset group may not be recoverable. The Company performs impairment tests of indefinite-lived intangible assets on an annual basis or more frequently in certain circumstances. Factors which could trigger an impairment review include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of the assets or the strategy for the overall business, a significant decrease in the market value of the assets or significant negative industry or economic trends. When the Company determines that the carrying value of long-lived assets may not be recoverable based upon the existence of one or more of the indicators, the assets are assessed for impairment based on the estimated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. If the carrying value of an asset exceeds its estimated future undiscounted cash flows, an impairment loss is recorded for the excess of the asset’s carrying value over its fair value. There were no impairment charge or disposals related to intangible assets or property, plant and equipment for the years ended December 31, 2019 and 2018, respectively.
| (k) | Convertible Notes Receivable and Investments in Equity |
Convertible notes investments and investments in equity of private companies are classified as financial assets at fair value through profit or loss. Upon initial recognition, the investment is recognized at fair value with directly attributable transaction costs expensed as incurred. Subsequent changes in fair value are recognized in profit or loss.
F-13
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (l) | Intangible Assets |
Intangible assets are recorded at cost less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization periods of assets with finite lives are based on management’s estimates at the date of acquisition and were as follows for each class of intangible asset as of December 31, 2019:
| Licenses and Permits |
15 years | |
| Tradenames |
3-15 years | |
| Customer Relationships |
3-7 years | |
| Non-competition Agreement |
2-5 years |
Intangible assets with finite lives are amortized over their estimated useful lives. The estimated useful lives, residual values, and amortization methods are reviewed at each year end, and any changes in estimates are accounted for prospectively.
| (m) | Goodwill |
Goodwill represents the excess of the purchase price paid for the acquisition of an entity over the fair value of the net tangible and intangible assets acquired. Goodwill is either assigned to a specific reporting unit or allocated between reporting units based on the relative fair value of each reporting unit.
Goodwill is not subject to amortization and is tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. The Company reviews indefinite-lived intangible assets, which includes goodwill, annually at fiscal year-end for impairment or more frequently if events or circumstances indicate that the carrying value may not be recoverable. An impaired asset is written down to its estimated fair value based on the most recent information available. The Company assesses the fair values of its intangible assets, and its reporting unit for goodwill testing purposes, using an income-based approach. Under the income approach, fair value is based on the present value of estimated future cash flows. The income approach is dependent on a number of factors, including forecasted revenues and expenses, appropriate discount rates and other variables. The annual impairment review utilizes the estimated fair value of the intangible assets and the overall reporting unit and compares the estimated fair values to the carrying values as of the testing date. If the carrying value of these intangible assets or the reporting unit exceeds the fair values, the Company would then use the fair values to measure the amount of any required impairment charge. No impairment charge was recognized for intangible assets for any of the fiscal periods presented.
| (n) | Income Taxes |
Deferred taxes are provided using an asset and liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax basis. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
Deferred tax assets and liabilities are measured using the enacted taxes rates. The effect on deferred tax assets and liabilities of a change in tax law or tax rates is recognized in income in the period that enactment occurs.
As discussed further in Note 11, the Company is subject to the limitations of IRC Section 280E.
F-14
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (o) | Revenue Recognition |
Revenue is recognized by the Company in accordance with ASU 2014-09, Revenue from Contracts with Customers (Topic 606). Through application of the standard, the Company recognizes revenue to depict the transfer of promised goods or services to the customer in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services.
In order to recognize revenue under ASU 2014-09, the Company applies the following five (5) steps:
| • | Identify a customer along with a corresponding contract; |
| • | Identify the performance obligation(s) in the contract to transfer goods or provide distinct services to a customer; |
| • | Determine the transaction price the Company expects to be entitled to in exchange for transferring promised goods or services to a customer; |
| • | Allocate the transaction price to the performance obligation(s) in the contract; |
| • | Recognize revenue when or as the Company satisfies the performance obligation(s). |
Revenues consist of consumer packaged goods and retail sales of cannabis, which are generally recognized at a point in time when control over the goods have been transferred to the customer and is recorded net of sales discounts. Payment is typically due upon transferring the goods to the customer or within a specified time period permitted under the Company’s credit policy. Sales discounts were not material during the years ended December 31, 2019, 2018 and 2017.
Revenue is recognized upon the satisfaction of the performance obligation. The Company satisfies its performance obligation and transfers control upon delivery and acceptance by the customer.
For some of its locations, the Company offers a loyalty reward program to its dispensary customers. A portion of the revenue generated in a sale must be allocated to the loyalty points earned. The amount allocated to the points earned is deferred until the loyalty points are redeemed or expire. As of December 31, 2019, the loyalty liability totaled $1,000,010 and is included in accounts payable on the consolidated balance sheet. There were no loyalty liabilities as of December 31, 2018.
Based on the Company’s assessment, the adoption of this new standard had no impact on the amounts recognized in its consolidated financial statements.
| (p) | Stock-Based Payments |
The Company operates equity settled stock-based remuneration plans for its eligible directors, officers, employees and consultants. All goods and services received in exchange for the grant of any stock-based payments are measured at their fair value unless the fair value cannot be estimated reliably. If the Company cannot estimate reliably the fair value of the goods and services received, the Company shall measure their value indirectly by reference to the fair value of the equity instruments granted. For transactions with employees and others providing similar services, the Company measures the fair value of the services by reference to the fair value of the equity instruments granted.
Equity settled stock-based payments under stock-based payments plans are ultimately recognized as an expense in profit or loss with a corresponding credit to reserve for stock-based payments, in equity.
The Company recognizes compensation expense for Restricted Stock Units (“RSUs”) and options on a straight-line basis over the requisite service period of the award. Non-market vesting conditions are included in the assumptions about the number of options that are expected to become exercisable. Estimates are subsequently revised if there is any indication that the number of share options expected to vest differs from the previous estimate. Any cumulative adjustment prior to vesting is recognized in the current period. No adjustment is made to any expense recognized in prior period if share options ultimately exercised are different to that estimated on vesting.
F-15
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (q) | Fair Value of Financial Instruments (See also Note 18) |
The Company applies fair value accounting for all financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded at fair value, the Company considers all related factors of the asset by market participants in which the Company would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as inherent risk, transfer restrictions, and credit risk.
The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels, and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
| Level 1 – | Unadjusted quoted prices in active markets for identical assets or liabilities; | |
| Level 2 – | Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and | |
| Level 3 – | Inputs for the asset or liability that are not based on observable market data. |
| (r) | Commitments and Contingencies |
The Company is subject to lawsuits, investigations and other claims related to employment, commercial and other matters that arise out of operations in the normal course of business. Periodically, the Company reviews the status of each significant matter and assesses the potential financial exposure. If the potential loss from any claim or legal proceeding is considered probable, and the amount can be reliably estimated, such amount is recognized in other liabilities.
Contingent liabilities are measured at management’s best estimate of the expenditure required to settle the obligation at the end of the reporting period and are discounted to present value where the effect is material. The Company performs evaluations to identify onerous contracts and, where applicable, records contingent liabilities for such contracts.
Contingent consideration is measured upon acquisition and is estimated using probability weighting of potential payouts. Subsequent changes in the estimated contingent consideration from the final purchase price allocation are recognized in the Company’s consolidated statement of operations.
| (s) | Share Capital |
Common shares are classified as equity. Incremental costs directly attributable to the issuance of shares are recognized as a deduction from equity. The proceeds from the exercise of stock options or warrants together with amounts previously recorded in reserves over the vesting periods are recorded as share capital. Income tax relating to transaction costs of an equity transaction is accounted for in accordance with Accounting Standards Codification (ASC) 740, Income Taxes.
| (t) | Loss per Share |
Basic loss per share is calculated using the treasury stock method, by dividing the net loss attributable to shareholders by the weighted average number of common shares outstanding during each of the years presented. Contingently issuable shares (including shares held in escrow) are not considered outstanding common shares and consequently are not included in the loss per share calculations. Diluted income per share is calculated by adjusting the weighted average number of common shares outstanding to assume conversion of all dilutive potential common shares. The Company has three categories of potentially dilutive common share equivalents: restricted stock units, stock options and warrants. The Company had 3,839,017 and 1,677,192 options outstanding, 1,399,762 and 1,589,000
F-16
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (t) | Loss per Share (Continued) |
restricted stock units outstanding and 2,387,470 and no warrants outstanding as of December 31, 2019 and 2018, respectively. The Company did not have any options, restricted stock units or warrants outstanding as of December 31, 2017.
In order to determine diluted income per share, it is assumed that any proceeds from the exercise of dilutive stock options would be used to repurchase common shares at the average market price during the period. The diluted income per share calculation excludes any potential conversion of stock options and convertible debt that would decrease loss per share. As a result, the Company’s calculation of basic earnings per share and diluted earnings per share include the same number of share equivalents for the years ended December 31, 2019, 2018 and 2017.
| (u) | Business Combinations |
Business combinations are accounted for using the acquisition method. The consideration transferred in a business combination is measured at fair value at the date of acquisition. Acquisition related transaction costs are expensed as incurred. Identifiable assets and liabilities, including intangible assets, of acquired businesses are recorded at their fair value at the date of acquisition. When the Company acquires control of a business, any previously held equity interest also is remeasured to fair value. The excess of the purchase consideration and any previously held equity interest over the fair value of identifiable net assets acquired is goodwill. If the fair value of identifiable net assets acquired exceeds the purchase consideration and any previously held equity interest, the difference is recognized in the Consolidated Statements of Operations immediately as a gain or loss on acquisition.
Contingent consideration is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Contingent consideration that is classified as equity is not remeasured at subsequent reporting dates and its subsequent settlement is accounted for within equity. Contingent consideration that is classified as an asset or a liability is remeasured at subsequent reporting dates in accordance with Accounting Standards Codification (ASC) 450, Contingencies, as appropriate, with the corresponding gain or loss being recognized in profit or loss.
| (v) | Foreign Currency |
Assets and liabilities denominated in currencies other than GTI’s functional currency are initially measured in the functional currencies at the transaction date exchange rate. Monetary assets are re-measured at the rate of exchange in effect as of the balance sheet date. Revenues and expenses are translated at the transaction date exchange rate. Foreign currency gains and losses resulting from translation are reflected in net comprehensive loss for the period.
| (w) | Impairment of Other Long-Lived Assets |
The Company evaluates the recoverability of other long-lived assets, including property, plant and equipment, and certain identifiable intangible assets, whenever events or changes in circumstances indicate that the carrying value of an asset or asset group may not be recoverable. The Company performs impairment tests of indefinite-lived intangible assets on an annual basis or more frequently in certain circumstances. Factors which could trigger an impairment review include significant underperformance relative to historical or projected future operating results, significant changes in the manner of use of the assets or the strategy for the overall business, a significant decrease in the market value of the assets or significant negative industry or economic trends.
When the Company determines that the carrying value of long-lived assets may not be recoverable based upon the existence of one or more of the indicators, the assets are assessed for impairment based on the estimated future undiscounted cash flows expected to result from the use of the asset and its eventual disposition. If the carrying value of an asset exceeds its estimated future undiscounted cash
F-17
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (w) | Impairment of Other Long-Lived Assets (Continued) |
flows, an impairment loss is recorded for the excess of the asset’s carrying value over its fair value. There was no impairment charge related to intangible assets or property, plant and equipment for the years ended December 31, 2019 and 2018.
| (x) | Significant Accounting Judgments, Estimates and Assumptions |
The preparation of the Company’s consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
Significant judgments, estimates and assumptions that have the most significant effect on the amounts recognized in the consolidated financial statements are described below.
| (i) | Estimated Useful Lives and Amortization of Intangible Assets (Also see Note 8) |
Amortization of intangible assets is recorded on a straight-line basis over their estimated useful lives, which do not exceed the contractual period, if any.
| (ii) | Business Combinations |
Classification of an acquisition as a business combination or an asset acquisition depends on whether the assets acquired constitute a business, which can be a complex judgment. Whether an acquisition is classified as a business combination or asset acquisition can have a significant impact on the entries made on and after acquisition.
In determining the fair value of all identifiable assets, liabilities and contingent liabilities acquired, the most significant estimates relate to contingent consideration and intangible assets. Management exercises judgement in estimating the probability and timing of when earn-outs are expected to be achieved which is used as the basis for estimating fair value. For any intangible asset identified, depending on the type of intangible asset and the complexity of determining its fair value, an independent valuation expert or management may develop the fair value, using appropriate valuation techniques, which are generally based on a forecast of the total expected future net cash flows. The evaluations are linked closely to the assumptions made by management regarding the future performance of these assets and any changes in the discount rate applied. See Note 7 - Acquisitions for additional details.
| (iii) | Inventories |
The net realizable value of inventories represents the estimated selling price for inventories in the ordinary course of business, less all estimated costs of completion and costs necessary to make the sale. The determination of net realizable value requires significant judgment, including consideration of factors such as shrinkage, the aging of and future demand for inventory, expected future selling price the Company expects to realize by selling the inventory, and the contractual arrangements with customers. Reserves for excess and obsolete inventory are based upon quantities on hand, projected volumes from demand forecasts and net realizable value. The estimates are judgmental in nature and are made at a point in time, using available information, expected business plans, and expected market conditions. As a result, the actual amount received on sale could differ from the estimated value of inventory. Periodic reviews are performed on the inventory balance. The impact of changes in inventory reserves is reflected in cost of goods sold.
F-18
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (x) | Significant Accounting Judgments, Estimates and Assumptions (Continued) |
| (iv) | Investments in Private Holdings |
Investments include private company investments which are carried at fair value based on the value of the Company’s interests in the private companies determined from financial information provided by management of the companies, which may include operating results, subsequent rounds of financing and other appropriate information. Any change in fair value is recognized on the consolidated statement of operations.
| (v) | Goodwill Impairment |
Goodwill is tested for impairment annually and whenever events or changes in circumstances indicate that the carrying amount of goodwill has been impaired. In order to determine if the value of goodwill has been impaired, the reporting unit to which goodwill has been assigned or allocated must be valued using present value techniques. When applying this valuation technique, the Company relies on a number of factors, including historical results, business plans, forecasts and market data. Changes in the conditions for these judgments and estimates can significantly affect the assessed value of goodwill.
| (vi) | Determination of Reporting Units |
The Company’s assets are aggregated into two reportable segments (retail and consumer packaged goods). For the purposes of testing goodwill, GTI has identified 22 reporting units. The Company analyzed it’s reporting units by first reviewing the operating segments based on the geographic areas in which GTI conducts business (or each market). The markets were then further divided into reporting units based on the market operations (retail and consumer packaged goods) which were primarily determined based on the licenses each market holds. The following represent the markets in which GTI’s operates as of December 31, 2019: California, Colorado, Connecticut, Florida, Illinois, Maryland, Massachusetts, Nevada, New Jersey, New York, Ohio and Pennsylvania.
| (vii) | Consolidation |
Judgment is applied in assessing whether the Company exercises control and has significant influence over entities in which the Company directly or indirectly owns an interest. The Company has control when it has the power over the subsidiary, has exposure or rights to variable returns, and has the ability to use its power to affect the returns. Significant influence is defined as the power to participate in the financial and operating decisions of the subsidiaries. Where the Company is determined to have control, these entities are consolidated. Additionally, judgment is applied in determining the effective date on which control was obtained.
| (viii) | Allowance for Uncollectible Accounts |
Management determines the allowance for uncollectible accounts by evaluating individual receivable balances and considering accounts and other receivable financial condition and current economic conditions. Accounts receivable and financial assets recorded in other receivables are written off when deemed uncollectible. Recoveries of accounts receivable previously written off are recorded as income when received. All receivables are expected to be collected within one year of the balance sheet date.
F-19
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (x) | Significant Accounting Judgments, Estimates and Assumptions (Continued) |
| (ix) | Stock-Based Payments |
Valuation of stock-based compensation and warrants requires management to make estimates regarding the inputs for option pricing models, such as the expected life of the option, the volatility of the Company’s stock price, the vesting period of the option and the risk-free interest rate are used. Actual results could differ from those estimates. The estimates are considered for each new grant of stock options or warrants.
| (x) | Fair Value of Financial Instruments |
The individual fair values attributed to the different components of a financing transaction, including derivative financial instruments, are determined using valuation techniques. The Company uses judgment to select the methods used to make certain assumptions and in performing the fair value calculations in order to determine (a) the values attributed to each component of a transaction at the time of their issuance; (b) the fair value measurements for certain instruments that require subsequent measurement at fair value on a recurring basis; and (c) for disclosing the fair value of financial instruments. These valuation estimates could be significantly different because of the use of judgment and the inherent uncertainty in estimating the fair value of these instruments that are not quoted in an active market.
| (y) | New and Revised Standards |
| (i) | In February 2016, the FASB issued Accounting Standards Update No. 2016-02 “Leases (Topic 842)” (“ASU 2016-02”), which requires lessees to record most leases on the balance sheet but recognize expense on the income statement in a manner similar to current accounting. For lessors, ASU 2016-02 also modifies the classification criteria and the accounting for sales-type and direct financing leases. The standard requires a modified retrospective approach for leases that exist or are entered into after the beginning of the earliest comparative period in the financial statements and is effective in the first quarter of 2019. |
Upon adoption of ASU 2016-02, the Company recorded right-of-use assets of $11,197,339 and corresponding lease liabilities of $11,695,585 with the difference of $498,246 recorded in opening retained earnings.
| (ii) | In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires the measurement of current expected credit losses for financial assets held at the reporting date based on historical experience, current conditions and reasonable and supportable forecasts. Adoption of ASU 2016-13 will require financial institutions and other organizations to use forward-looking information to better formulate their credit loss estimates. In addition, the ASU amends the accounting for credit losses on available for sale debt securities and purchased financial assets with credit deterioration. This update will be effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. The Company expects to implement the provisions of ASU 2016-13 as of January 1, 2020. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (iii) | In January 2017, the FASB issued Accounting Standards Update No. 2017-04 “Intangibles— Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment” (“ASU 2017-04”), which simplifies the accounting for goodwill impairment. ASU 2017-04 requires entities to record an impairment charge based on the excess of a reporting unit’s carrying amount over its fair value (Step 1 under the current impairment test). The standard eliminates Step 2 from the current goodwill impairment test, which included determining the implied fair value of |
F-20
Table of Contents
| 2. | SIGNIFICANT ACCOUNTING POLICIES (Continued) |
| (y) | New and Revised Standards (Continued) |
| goodwill and comparing it with the carrying amount of that goodwill. ASU 2017-04 must be applied prospectively and is effective in the first quarter of 2020. Early adoption is permitted. The Company intends to adopt the new standard in the first quarter of 2020. |
| (iv) | In August 2018, the FASB issued ASU 2018-13, Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement (Topic 820). ASU 2018-13 adds, modifies, and removes certain fair value measurement disclosure requirements. ASU 2018-13 is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (v) | In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740) - Simplifying the Accounting for Income Taxes, which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. ASU 2019-12 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| (vi) | In January 2020, the FASB issued ASU 2020-01, Investments - Equity Securities (Topic 321), Investments - Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815) (“ASU 2020-01”), which is intended to clarify the interaction of the accounting for equity securities under Topic 321 and investments accounted for under the equity method of accounting in Topic 323 and the accounting for certain forward contracts and purchased options accounted for under Topic 815. ASU 2020-01 is effective for the Company beginning January 1, 2021. The Company is currently evaluating the effect of adopting this ASU on the Company’s financial statements. |
| 3. | REVERSE TAKEOVER TRANSACTION |
In April 2018, the Company raised approximately $65.1 million in subscription receipts, gross of approximately $4.0 million in transaction costs. The subscription receipts were for the potential purchase of shares in GTI Finco Inc. (“GTI Finco”) and were held in an escrow account until the reverse takeover transaction. Additionally, the Company issued 285,000 options to consultants with a strike prices of C$7.75 per option. The value of the options was approximately $900,000 under the Black-Scholes option pricing model.
At a meeting of shareholders on June 11, 2018, the Company’s shareholders approved a resolution to restructure the Company’s share capital to, among other things, re-designate its existing common shares as subordinate voting shares (“Subordinate Voting Shares”) and create a class of multiple voting shares (“Multiple Voting Shares”) and super voting shares (“Super Voting Shares”).
On June 12, 2018, Green Thumb Industries Inc., 1165318 B.C. Ltd. (a wholly-owned subsidiary of Bayswater) (“Subco”), VCP23, LLC (“VCP”), GTI23, Inc. (“GTI23”) and GTI Finco entered into a Business Combination Agreement whereby the Company, Subco, VCP, GTI23 and GTI Finco combined their respective businesses (the “Transaction”). The Transaction was structured as a series of transactions, including a Canadian three-cornered amalgamation transaction and a series of U.S. reorganization steps. The subscription receipts of GTI Finco were then released from escrow.
In connection with the Transaction completed on June 12, 2018, the Company changed its name from “Bayswater Uranium Corporation” to “Green Thumb Industries Inc.” and consolidated its existing common shares on the basis of one Subordinate Voting Share for each 368 existing common shares of the Company. Such share consolidation has been reflected retrospectively in these consolidated financial statements.
F-21
Table of Contents
| 3. | REVERSE TAKEOVER TRANSACTION (Continued) |
The Company, Subco and GTI Finco were parties to a three-cornered amalgamation (“Amalgamation”) whereby GTI Finco shareholders received Subordinate Voting Shares of the Company on a one-for-one basis and members of VCP contributed their membership interests to GTI23 for shares of GTI23 and then contributed their shares of GTI23 to GTI in exchange for Super Voting Shares and Multiple Voting Shares of GTI.
GTI was the acquirer for accounting purposes and the net assets of Bayswater acquired were nil.
Pursuant to the reverse merger, the historical financial statements of Green Thumb Industries Inc. (the accounting acquirer) become the historical financial statements of Bayswater Uranium Corporation (legal acquirer) on a go forward basis. As a result, Green Thumb Industries Inc. has retroactively restated its share capital on a per share basis pursuant to Accounting Standards Codification (ASC) 805, Business Combinations to reflect that of the legal acquirer.
| 4. | INVENTORIES |
The Company’s inventories include the following at December 31, 2019 and December 31, 2018:
| December 31, 2019 |
December 31, 2018 |
|||||||
| Raw Material |
$ | 6,375,032 | $ | 527,456 | ||||
| Packaging and Miscellaneous |
4,887,970 | 2,511,769 | ||||||
| Work in Process |
10,394,590 | 5,231,630 | ||||||
| Finished Goods |
26,408,762 | 4,088,209 | ||||||
| Reserve for Obsolete Inventory |
(2,031,873 | ) | — | |||||
|
|
|
|
|
|||||
| Total Inventories |
$ | 46,034,481 | $ | 12,359,064 | ||||
|
|
|
|
|
|||||
The reserve for obsolete inventory primarily relates to packaging (raw materials) for certain products that the Company has re-branded during 2019 of otherwise determined to be unsuitable.
| 5. | NOTES RECEIVABLE |
On October 16, 2018, the Company executed a promissory note to an unrelated third party. The value of the note is variable in nature as the note is secured by warrants which expire in January 2021. The maturity date of the note is tied directly to the expiration date of the warrants, both being January 2021. The initial fair value upon execution of the note was $11,630,867. The fair value as of December 31, 2019 and December 31, 2018 was $815,937 and $7,424,727, respectively, resulting in an adjustment to fair value of ($6,608,790) and ($4,206,141) during those same periods, respectively, which is recorded in other income (expense) on the audited consolidated statements of operations. Repayment of the note is due within ten days of exercise of the underlying security, at which time it will bear interest at the lowest applicable federal rate. The principal amount due is based on the actual value of the underlying security at the time of exercise. The Company used the Black Scholes option pricing model to estimate the fair value of the note receivable as of December 31, 2019 and 2018 using the assumptions below.
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Risk-free Rate |
1.90% | 1.86% | ||||||
| Exercise Price of Underlying Securities |
$ | 1.998 | $ | 1.998 | ||||
| Share Price of Underlying Security |
1.90 | $ | 4.03 - $5.50 | |||||
| Volatility |
71.50% | 71.50% | ||||||
| Remaining Life (in years) |
1.0 | 2.0 | ||||||
F-22
Table of Contents
| 5. | NOTES RECEIVABLE (Continued) |
An increase or decrease to the underlying share price and volatility rate of 5% would result in a nominal change to the fair value.
On October 22, 2018, the Company issued a line of credit to an entity, allowing for maximum borrowings of $1,000,000, of which $500,000 was drawn as of December 31, 2018. The note had a term of one year and bears interest at a rate of 8%. The $500,000 note receivable was included as part of the acquisition consideration of Beboe. See Note 7 for details.
On October 31, 2018, the Company issued a $3,000,000 promissory note to an unrelated third party. The note had a term of one year and bears interest at a rate of 8% which was repaid in 2019. The Company accounted for this investment as held-to-maturity.
At each reporting date, the Company applies its judgment to evaluate the collectability of the notes receivable and makes a provision based on the assessed amount of expected credit loss. This judgment is based on parameters such as interest rates, specific country risk factors, and creditworthiness of the creditor. The Company has not experienced an increase in credit risk since the initial recognition of the notes receivable.
| 6. | PROPERTY AND EQUIPMENT |
At December 31, 2019, property and equipment consisted of the following:
| Cost |
Land | Buildings and Improvements |
Furniture and Fixtures |
Computer Equipment and Software |
Leasehold Improvements |
Production and Processing Equipment |
Assets Under Construction |
Total | ||||||||||||||||||||||||
| As at January 1, 2019 |
$ | 2,243,085 | $ | 20,861,988 | $ | 2,328,847 | $ | 2,093,205 | $ | 18,435,893 | $ | 6,579,446 | $ | 16,664,958 | $ | 69,207,422 | ||||||||||||||||
| Transfers |
892,056 | 9,400,171 | 40,167 | 143,882 | 13,981,780 | 4,075,642 | (28,533,698 | ) | — | |||||||||||||||||||||||
| Additions |
3,500,974 | 18,817,329 | 34,669 | 4,667,127 | 24,020,898 | 14,147,722 | 35,711,782 | 100,900,501 | ||||||||||||||||||||||||
| Additions from acquisitions |
— | — | 106,235 | 169,593 | 12,242,926 | 3,977,534 | 29,074 | 16,525,362 | ||||||||||||||||||||||||
| Disposals |
(3,363,676 | ) | (15,702,017 | ) | — | — | — | (2,854,198 | ) | — | (21,919,891 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| As at December 31, 2019 |
$ | 3,272,439 | $ | 33,377,471 | $ | 2,509,918 | $ | 7,073,807 | $ | 68,681,497 | $ | 25,926,146 | $ | 23,872,116 | $ | 164,713,394 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Accumulated Depreciation |
||||||||||||||||||||||||||||||||
| As at January 1, 2019 |
$ | — | $ | 1,351,230 | $ | 489,956 | $ | 249,423 | $ | 1,007,998 | $ | 784,735 | $ | — | $ | 3,883,342 | ||||||||||||||||
| Depreciation |
— | 1,866,400 | 127,060 | 1,308,757 | 1,990,289 | 1,535,205 | — | 6,827,711 | ||||||||||||||||||||||||
| Disposals |
— | (981,376 | ) | — | — | — | (612,958 | ) | — | (1,594,334 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| As at December 31, 2019 |
$ | — | $ | 2,236,254 | $ | 617,016 | $ | 1,558,180 | $ | 2,998,287 | $ | 1,706,982 | $ | — | $ | 9,116,719 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net book value |
||||||||||||||||||||||||||||||||
| As at January 1, 2019 |
$ | 2,243,085 | $ | 19,510,758 | $ | 1,838,891 | $ | 1,843,782 | $ | 17,427,895 | $ | 5,794,711 | $ | 16,664,958 | $ | 65,324,080 | ||||||||||||||||
| As at December 31, 2019 |
$ | 3,272,439 | $ | 31,141,217 | $ | 1,892,902 | $ | 5,515,627 | $ | 65,683,210 | $ | 24,219,164 | $ | 23,872,116 | $ | 155,596,675 | ||||||||||||||||
F-23
Table of Contents
| 6. | PROPERTY AND EQUIPMENT (Continued) |
At December 31, 2018, property and equipment consisted of the following:
| Cost |
Land | Buildings and Improvements |
Furniture and Fixtures |
Computer Equipment and Software |
Leasehold Improvements |
Production and Processing Equipment |
Assets Under Construction |
Total | ||||||||||||||||||||||||
| As at January 1, 2018 |
$ | 1,626,989 | $ | 13,999,703 | $ | 505,268 | $ | 381,029 | $ | 2,350,287 | $ | 1,128,835 | $ | 12,762,563 | $ | 32,754,674 | ||||||||||||||||
| Transfers |
— | 83,609 | 796,512 | 213,667 | 7,820,415 | 408,733 | (9,322,936 | ) | — | |||||||||||||||||||||||
| Additions |
60,500 | 3,278,221 | 874,487 | 1,310,292 | 8,009,792 | 3,321,472 | 13,893,168 | 30,747,932 | ||||||||||||||||||||||||
| Additions from acquisitions |
555,596 | 3,500,455 | 152,580 | 188,217 | 255,399 | 1,720,406 | — | 6,372,653 | ||||||||||||||||||||||||
| Disposals |
— | — | — | — | — | — | (667,837 | ) | (667,837 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| As at December 31, 2018 |
$ | 2,243,085 | $ | 20,861,988 | $ | 2,328,847 | $ | 2,093,205 | $ | 18,435,893 | $ | 6,579,446 | $ | 16,664,958 | $ | 69,207,422 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Accumulated Depreciation |
||||||||||||||||||||||||||||||||
| As at January 1, 2018 |
$ | — | $ | 752,962 | $ | 195,832 | $ | 28,517 | $ | 82,809 | $ | 136,197 | $ | — | $ | 1,196,317 | ||||||||||||||||
| Depreciation |
— | 598,268 | 294,124 | 220,906 | 925,189 | 648,538 | — | 2,687,025 | ||||||||||||||||||||||||
| Disposals |
— | — | — | — | — | — | — | — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| As at December 31, 2018 |
$ | — | $ | 1,351,230 | $ | 489,956 | $ | 249,423 | $ | 1,007,998 | $ | 784,735 | $ | — | $ | 3,883,342 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Net book value |
||||||||||||||||||||||||||||||||
| As at January 1, 2018 |
$ | 1,626,989 | $ | 13,246,741 | $ | 309,436 | $ | 352,512 | $ | 2,267,478 | $ | 992,638 | $ | 12,762,563 | $ | 31,558,357 | ||||||||||||||||
| As at December 31, 2018 |
$ | 2,243,085 | $ | 19,510,758 | $ | 1,838,891 | $ | 1,843,782 | $ | 17,427,895 | $ | 5,794,711 | $ | 16,664,958 | $ | 65,324,080 | ||||||||||||||||
Assets under construction represent construction in progress related to both cultivation and dispensary facilities not yet completed or otherwise not ready for use. Included within assets under construction was $2,500,000 and $0 of capitalized interest additions as of December 31, 2019 and 2018 respectively.
On November 12, 2019, the Company closed on a sale and lease back transaction to sell its Danville Pennsylvania cultivation and processing facility to IIP. Under the long-term agreement, the Company will lease back the facility and continue to operate and manage it. As a result of the sale, the Company disposed of $3,363,676 of land, $15,702,017 of buildings and improvements, and $2,854,198 in production and processing equipment. There was no gain or loss on the sale. For further information regarding the transaction, see Note 9 - Leases.
Depreciation expense for the years ended December 31, 2019, 2018 and 2017 totaled $6,827,711, $2,687,025 and $646,928, respectively of which $4,246,524, $1,346,632 and $428,724, respectively, is included in cost of goods sold.
| 7. | ACQUISITIONS |
The Company has determined that the below acquisitions are business combinations under Accounting Standards Codification (ASC) 805, Business Combinations. Those acquisitions that are determined to be the acquisition of a business are accounted for by applying the acquisition method, whereby the assets acquired and the liabilities assumed are recorded at their fair values with any excess of the aggregate consideration over the fair values of the identifiable net assets allocated to goodwill. Operating results have been included in these consolidated financial statements from the date of the acquisition. Any goodwill recognized is attributed based on reporting units.
The Company completed preliminary allocations of the purchase prices of the assets acquired and liabilities assumed as follows with the assistance of an independent valuation firm. The Company is still in the process of
F-24
Table of Contents
| 7. | ACQUISITIONS (Continued) |
completing the valuations. The preliminary allocations of the purchase prices were based upon preliminary valuations and our estimates and assumptions are subject to change within the purchase price allocation period (generally one year from the acquisition dates). The primary areas of the purchase price allocations that are not yet finalized relate to the valuation of the tangible and intangible assets acquired and the residual goodwill.
| Advanced Grow Labs, LLC |
Integral Associates, LLC |
Other Acquisitions |
||||||||||
| Cash Paid |
$ | 15,481,967 | $ | 52,807,500 | $ | 44,147,694 | ||||||
| Share Issuances |
79,709,170 | 273,146,014 | 142,882,549 | |||||||||
| Deferred Share Issuances |
5,380,000 | — | 11,207,798 | |||||||||
| Conversion of Previous Notes Receivable |
— | — | 27,121,559 | |||||||||
| Acquisition Liability |
— | 791,068 | — | |||||||||
| Contingent Consideration |
8,081,000 | 39,985,000 | 8,926,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Consideration |
$ | 108,652,137 | $ | 366,729,582 | $ | 234,285,600 | ||||||
|
|
|
|
|
|
|
|||||||
The following table summarizes the initial accounting estimates:
| Advanced Grow Labs, LLC |
Integral Associates, LLC |
Other Acquisitions |
||||||||||
| Cash |
$ | 1,406,377 | $ | 744,825 | $ | 777,371 | ||||||
| Inventory |
1,906,828 | 10,107,303 | 1,732,525 | |||||||||
| Accounts Receivable |
420,649 | 1,477,535 | 265,956 | |||||||||
| Prepaid Expenses |
— | 492,571 | 38,705 | |||||||||
| Property and Equipment |
5,934,295 | 8,107,836 | 2,586,821 | |||||||||
| Right-of-Use Asset |
565,336 | 4,840,609 | 2,056,008 | |||||||||
| Investment in CAL Funding |
9,900,000 | — | — | |||||||||
| Deposits and Other Assets |
246,843 | 122,826 | 801,998 | |||||||||
| Intangible Assets: |
||||||||||||
| Licenses and Permits |
28,920,000 | 175,845,000 | 48,300,000 | |||||||||
| Tradename |
930,000 | 57,425,000 | 38,740,592 | |||||||||
| Customer Relationships |
12,462,000 | 2,678,000 | 9,298,000 | |||||||||
| Non-competition Agreements |
100,000 | — | 2,465,000 | |||||||||
| Liabilities Assumed |
(1,230,441 | ) | (11,091,246 | ) | (4,869,639 | ) | ||||||
| Deferred Tax Liabilities |
(14,238,349 | ) | — | (21,807,925 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total Identifiable Net Assets |
47,323,538 | 250,750,259 | 80,385,412 | |||||||||
| Goodwill |
61,328,599 | 115,979,323 | 153,900,188 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net Assets |
$ | 108,652,137 | $ | 366,729,582 | $ | 234,285,600 | ||||||
|
|
|
|
|
|
|
|||||||
Other acquisitions consists of For Success Holdings Company, Fiorello Pharmaceuticals, Inc., MC Brands, LLC as well as two dispensaries. The details of the significant transactions are discussed below. The Company also incurred approximately $812,000 of acquisition related costs which were expensed in the current period.
F-25
Table of Contents
| 7. | ACQUISITIONS (Continued) |
Pro Forma Financial Information
The following table summarizes the revenue and net income (loss) of Advanced Grow Labs, LLC and Integral Associates, LLC from the transaction date (the date of acquisition for Advanced Grow Labs, LLC of February 12, 2019 and for Integral Associates, LLC of June 5, 2019) through December 31, 2019 and Revenues, net of discounts and Net income (loss) for Advanced Grow Labs, LLC and Integral Associates, LLC for the years ended December 31, 2018 and 2017:
| Transaction Date Through December 31, 2019 |
For the Year Ended December 31, 2018 |
For the Year Ended December 31, 2017 |
||||||||||||||||||||||
| Advanced Grow Labs, LLC |
Integral Associates, LLC |
Advanced Grow Labs, LLC |
Integral Associates, LLC |
Advanced Grow Labs, LLC |
Integral Associates, LLC |
|||||||||||||||||||
| Revenues, net of discounts |
$ | 16,377,199 | $ | 39,246,745 | $ | 17,016,743 | $ | 60,261,432 | $ | 13,979,514 | $ | 30,420,101 | ||||||||||||
| Net income (loss) |
3,612,455 | (6,375,800 | ) | 7,497,696 | 13,218,159 | 7,721,627 | 6,983,028 | |||||||||||||||||
The following table summarizes the pro forma combined revenue and net income (loss) of Green Thumb Industries Inc., Advanced Grow Labs, LLC and Integral Associates, LLC for the period from January 1, 2019 through December 31, 2019 (presented as if the acquisitions had occurred at January 1, 2019):
| For the Year Ended December 31, 2019 | ||||||||||||||||||||||||
| Green Thumb Industries Inc. |
Advanced Grow Labs, LLC |
Integral Associates, LLC |
Pro Forma Adjustments |
Notes | Pro Forma Combined |
|||||||||||||||||||
| Unaudited | Unaudited | |||||||||||||||||||||||
| Revenues, net of discounts |
$ | 160,808,662 | $ | 18,516,074 | $ | 79,146,937 | $ | — | $ | 258,471,673 | ||||||||||||||
| Net income (loss) attributable to Green Thumb Industries Inc. |
(56,353,062 | ) | 4,586,122 | (1,110,610 | ) | (8,394,854 | ) | (a | ), (b) | (61,272,404 | ) | |||||||||||||
| (a) | Represents estimated amortization expense on intangible assets acquired as part of the acquisition of Advance Grow Labs, LLC and Integral Associates, LLC of $327,302 representing one month of amortization expense and $6,639,127 representing five months of amortization expense, respectively. |
| (b) | Represents estimated income tax expense of Advanced Grow Labs, LLC of $164,780 and Integral Associates, LLC of $1,263,645 based on a 24% effective tax rate. |
The following table summarizes the pro forma combined revenue and net income (loss) of Green Thumb Industries Inc., Advanced Grow Labs, LLC and Integral Associates, LLC for the period from January 1, 2018 through December 31, 2018 (presented as if the acquisitions had occurred at January 1, 2018):
| For the Year Ended December 31, 2018 | ||||||||||||||||||||||||
| Green Thumb Industries Inc. |
Advanced Grow Labs, LLC |
Integral Associates, LLC |
Pro Forma Adjustments |
Notes | Pro Forma Combined |
|||||||||||||||||||
| Unaudited | Unaudited | |||||||||||||||||||||||
| Revenues, net of discounts |
$ | 62,493,680 | $ | 17,016,743 | $ | 60,261,432 | $ | — | $ | 139,771,855 | ||||||||||||||
| Net income (loss) attributable to Green Thumb Industries Inc. |
(5,243,917 | ) | 7,497,696 | 13,218,159 | (24,006,529 | ) | (a | ), (b) | (8,534,591 | ) | ||||||||||||||
| (a) | Represents estimated amortization expense on intangible assets acquired as part of the acquisition of Advance Grow Labs, LLC and Integral Associates, LLC of $3,927,619 and $15,933,905 each representing twelve months of amortization expense, respectively. |
F-26
Table of Contents
| 7. | ACQUISITIONS (Continued) |
| (b) | Represents estimated income tax expense of Advanced Grow Labs, LLC of $972,647 and Integral Associates, LLC of $3,172,358 based on a 24% effective tax rate. |
The following table summarizes the pro forma combined revenue and net income (loss) of Green Thumb Industries Inc., Advanced Grow Labs, LLC and Integral Associates, LLC for the period from January 1, 2017 through December 31, 2017 (presented as if the acquisitions had occurred at January 1, 2017):
| For the Year ended December 31, 2017 | ||||||||||||||||||||||||
| Green Thumb Industries |
Advanced Grow Labs, LLC |
Integral Associates, LLC |
Pro Forma Adjustments |
Notes | Pro Forma Combined |
|||||||||||||||||||
| Unaudited | Unaudited | |||||||||||||||||||||||
| Revenues, net of discounts |
$ | 16,528,779 | $ | 13,979,514 | $ | 30,420,101 | $ | 60,928,394 | ||||||||||||||||
| Net income (loss) attributable to Green Thumb Industries Inc. |
(4,249,775 | ) | 7,721,627 | 6,983,028 | (22,563,841 | ) | (a),(b) | $ | (12,108,961 | ) | ||||||||||||||
| (a) | Represents estimated amortization expense on intangible assets acquired as part of the acquisition of Advance Grow Labs, LLC and Integral Associates, LLC of $3,927,619 and $15,933,905 each representing twelve months of amortization expense, respectively. |
| (b) | Represents estimated income tax expense of Advanced Grow Labs, LLC of $1,026,390 and Integral Associates, LLC of $1,675,927 based on a 24% effective tax rate. |
Business Acquisitions:
| (a) | Acquisition of Advanced Grow Labs, LLC |
On February 12, 2019, the Company acquired 100% of the ownership interests of Connecticut-based Advanced Grow Labs, LLC (“AGL”). AGL is licensed in Connecticut to grow and process cannabis. The acquisition includes a manufacturing license and an ownership stake in a recently awarded Connecticut-based dispensary. The transaction consideration included $15.5 million of cash, approximately 7.3 million Subordinate Voting Shares of GTI which were valued at approximately $85.1 million, based on the fair value of the securities on their date of issuance, which was the closing price of GTI’s Subordinate Voting Shares as traded on the CSE on the date of the transaction. The purchase agreement also included additional consideration ranging from $0 to $15 million in shares of GTI depending upon the EBITDA results of AGL over the twelve-month period following the close of the transaction. As of December 31, 2019, the Company estimated the fair value of contingent consideration (which had an initial value of $8.1 million as of February 12, 2019) to be $8.6 million using a probability weighting of the potential payouts.
| (b) | Acquisition of Integral Associates, LLC |
On June 5, 2019, the Company acquired 100% of the ownership interests of Integral Associates, LLC. The acquisition included Integral Associate’s retail brand Essence, three retail locations, as well as two cultivation and processing facilities. The transaction consideration included $52.8 million paid in cash and approximately 20.8 million in Subordinate Voting Shares which were valued at $235.4 million, and an additional 3.3 million milestone shares with a fair value of $37.7 million, for a total value of $273.1 million in share issuances. The fair value of the securities was based upon the closing price of GTI’s Subordinate Voting Shares as traded on the CSE on the date of the transaction. The purchase agreement includes additional consideration based upon future milestone targets of which an additional 0.9 million Subordinate Voting Shares have been issued to-date. The former owners of Integral Associates may receive additional contingent consideration of up to $57 million in shares of GTI depending upon the EBITDA results of Integral Associates over the twelve-month period following the close of the transaction along with
F-27
Table of Contents
| 7. | ACQUISITIONS (Continued) |
conditional and/or final dispensary operating licenses. As of December 31, 2019, the Company estimated the fair value of contingent consideration (which had an initial value of $40 million as of June 5, 2019) to be $39.6 million using a probability weighting of the potential payouts.
| (c) | Acquisition of For Success Holding Company |
On February 21, 2019, the Company acquired 100% of the ownership interests of For Success Holding Company, the Los Angeles-based creator of the lifestyle suite of Beboe branded products. Beboe is currently available in certain retail locations in California and Colorado and via home delivery across California. The acquisition was an all stock transaction whereby consideration was satisfied through the issuance 6,463,553 of GTI Subordinate Voting Shares (including 793,448 deferred shares) which were valued at $94.5 million, based on the fair value of the securities on their date of issuance, which was the closing price of GTI’s Subordinate Voting Shares as traded on the CSE on the date of the transaction. The purchase agreement also includes additional consideration ranging from $0 to $15 million in cash or shares of GTI subject to Beboe achieving the placement of its products in specified retailers during the twelve months post acquisition of which $6.9 million was earned and paid during 2019 in the form of 808,614 Subordinated Voting Shares. As of December 31, 2019, the Company estimated the fair value of contingent consideration to be $2.4 million using a probability weighting of the potential payouts.
| (d) | Acquisition of Fiorello Pharmaceuticals, Inc |
On August 23, 2019, the Company acquired 100% of the ownership interests of New York-based Fiorello Pharmaceuticals, Inc. The acquisition consideration was paid using $42.6 million of cash and 1.7 million of the company’s Subordinate Voting Shares which were valued at $14.1 million, based on the fair value of the securities on their date of issuance, which was the closing price of GTI’s Subordinate Voting Shares as traded on the CSE on the date of the transaction. The acquisition includes the license and assets for one cultivation, one processing, and four retail facilities in the state of New York.
| (e) | MC Brands, LLC |
On June 27, 2019, the Company acquired the remaining 75% interest in MC Brands, LLC which is based in Colorado through the issuance of 1.7 million Subordinated Voting Shares valued at $19.4 million. The transaction was accounted for as an asset acquisition.
| 8. | INTANGIBLE ASSETS AND GOODWILL |
Intangible assets are recorded at cost less accumulated amortization and impairment losses, if any. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Amortization of definite life intangibles is provided on a straight-line basis over their estimated useful lives. The estimated useful lives, residual values, and amortization methods are reviewed at each year end, and any changes in estimates are accounted for prospectively.
F-28
Table of Contents
| 8. | INTANGIBLE ASSETS AND GOODWILL (Continued) |
At December 31, 2019, intangible assets consisted of the following:
| Licenses and Permits |
Tradenames | Customer Relationships |
Non-Competition Agreements |
Total | ||||||||||||||||
| Cost |
||||||||||||||||||||
| As at January 1, 2019 |
$ | 89,705,213 | $ | 360,000 | $ | 820,000 | $ | 20,480 | $ | 90,905,693 | ||||||||||
| Additions from acquisitions |
247,249,000 | 97,095,590 | 24,438,000 | 2,565,000 | 371,347,590 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As at December 31, 2019 |
$ | 336,954,213 | $ | 97,455,590 | $ | 25,258,000 | $ | 2,585,480 | $ | 462,253,283 | ||||||||||
| Accumulated Amortization |
||||||||||||||||||||
| As at January 1, 2019 |
$ | 2,322,715 | $ | — | $ | 204,500 | $ | 12,800 | $ | 2,540,015 | ||||||||||
| Amortization |
16,154,785 | 4,121,800 | 3,727,916 | 461,869 | 24,466,370 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As at December 31, 2019 |
$ | 18,477,500 | $ | 4,121,800 | $ | 3,932,416 | $ | 474,669 | $ | 27,006,385 | ||||||||||
| Net book value |
||||||||||||||||||||
| As at January 1, 2019 |
$ | 87,382,498 | $ | 360,000 | $ | 615,500 | $ | 7,680 | $ | 88,365,678 | ||||||||||
| As at December 31, 2019 |
$ | 318,476,713 | $ | 93,333,790 | $ | 21,325,584 | $ | 2,110,811 | $ | 435,246,898 | ||||||||||
At December 31, 2018, intangible assets consisted of the following:
| Licenses and Permits |
Tradenames | Customer Relationships |
Non-Competition Agreements |
Total | ||||||||||||||||
| Cost |
||||||||||||||||||||
| As at January 1, 2018 |
$ | 13,004,575 | $ | 360,000 | $ | 820,000 | $ | 20,480 | $ | 14,205,055 | ||||||||||
| Additions |
49,999 | — | — | — | 49,999 | |||||||||||||||
| Additions from acquisitions |
76,650,637 | — | — | — | 76,650,639 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As at December 31, 2018 |
$ | 89,705,213 | $ | 360,000 | $ | 820,000 | $ | 20,480 | $ | 90,905,693 | ||||||||||
| Accumulated Amortization |
||||||||||||||||||||
| As at January 1, 2018 |
$ | — | $ | — | $ | 40,500 | $ | 2,560 | $ | 43,060 | ||||||||||
| Amortization |
2,322,715 | — | 164,000 | 10,240 | 2,496,955 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As at December 31, 2018 |
$ | 2,322,715 | $ | — | $ | 204,500 | $ | 12,800 | $ | 2,540,015 | ||||||||||
| Net book value |
||||||||||||||||||||
| As at January 1, 2018 |
$ | 13,004,575 | $ | 360,000 | $ | 779,500 | $ | 17,920 | $ | 14,161,995 | ||||||||||
| As at December 31, 2018 |
$ | 87,382,498 | $ | 360,000 | $ | 615,500 | $ | 7,680 | $ | 88,365,678 | ||||||||||
The Company recorded amortization expense for the years ended December 31, 2019, 2018 and 2017 of $24,466,370, $2,496,955 and $43,060, respectively.
The following table outlines the estimated annual amortization expense related to intangible assets as of December 31, 2019:
| Year Ending December 31 |
Estimated Amortization |
|||
| 2020 |
$ | 33,427,493 | ||
| 2021 |
33,198,048 | |||
| 2022 |
32,767,307 | |||
| 2023 |
32,684,492 | |||
| 2024 |
32,103,159 | |||
| Thereafter |
271,066,399 | |||
|
|
|
|||
| $ | 435,246,898 | |||
|
|
|
|||
F-29
Table of Contents
| 8. | INTANGIBLE ASSETS AND GOODWILL (Continued) |
Goodwill
At December 31, 2019, Goodwill consisted of the following:
| Retail | Consumer Packaged Goods |
Total | ||||||||||
| As at January 1, 2019 |
$ | 15,286,360 | $ | 23,918,000 | $ | 39,204,360 | ||||||
| Acquisition of Advanced Grow Labs, LLC |
16,756,250 | 44,572,349 | 61,328,599 | |||||||||
| Acquisition of Integral Associates, LLC |
46,655,753 | 69,323,570 | 115,979,323 | |||||||||
| Other Acquisitions |
32,936,590 | 120,963,598 | 153,900,188 | |||||||||
| Adjustments to Purchase Price Allocations |
8,238,808 | (3,566,285 | ) | 4,672,523 | ||||||||
|
|
|
|
|
|
|
|||||||
| As at December 31, 2019 |
$ | 119,873,759 | $ | 255,211,232 | $ | 375,084,991 | ||||||
|
|
|
|
|
|
|
|||||||
At December 31, 2018, Goodwill consisted of the following:
| Retail | Consumer Packaged Goods |
Total | ||||||||||
| As at January 1, 2018 |
$ | 188,260 | $ | — | $ | 188,260 | ||||||
| Acquisition of KSGNF, LLC |
10,250,880 | 23,918,000 | 34,168,880 | |||||||||
| Acquisition of Compassionate Organics, LLC |
4,847,220 | — | 4,847,220 | |||||||||
|
|
|
|
|
|
|
|||||||
| As at December 31, 2018 |
$ | 15,286,360 | $ | 23,918,000 | $ | 39,204,360 | ||||||
|
|
|
|
|
|
|
|||||||
As described in Notes 2(l) and 2(m), a two-step method was used for determining goodwill impairment. In the first step (“Step One”), the Company compared the estimated fair value of each reporting unit to its carrying value, including goodwill. If the carrying value of a reporting unit exceeded the estimated fair value, the second step (“Step Two”) is completed to determine the amount of the impairment charge. Step Two requires the allocation of the estimated fair value of the reporting unit to the assets, including any unrecognized intangible assets, and liabilities in a hypothetical purchase price allocation. Any remaining unallocated fair value represents the implied fair value of goodwill, which is compared to the corresponding carrying value of goodwill to compute the goodwill impairment charge. The results of Step One of the goodwill impairment test indicated that the estimated fair values for all reporting units exceed their respective carrying values. The Company’s reporting unit’s to which goodwill has been assigned include California Consumer Packaged Goods, Connecticut Retail and Consumer Packaged Goods, Florida Retail and Consumer Packaged Goods, Illinois Retail, Massachusetts Retail, Nevada Retail and Consumer Packaged Goods and New York Retail and Consumer Packaged Goods.
To estimate the fair value of each reporting unit, management utilized an income approach. The key assumptions used in the calculation of the fair value of each reporting unit include management’s projections of future cash flows for a five-year period and after projections end, a growth rate of 3.0%, a discount rate of 13.5 %—14.8% with variability within the range based on the risk associated with the reporting unit. If the growth rate and discount rate were to be increased or decreased by 5%, the recoverable amount of goodwill would still be higher than the carrying amounts.
F-30
Table of Contents
| 9. | LEASES |
In February 2016, the FASB issued Accounting Standards Update No. 2016-02 “Leases (Topic 842)” (“ASU 2016-02”), which requires lessees to put most leases on the balance sheet but recognize expense on the income statement in a manner similar to current accounting. On January 1, 2019, the Company adopted the standard and all related amendments, using the optional transition method (modified retrospective approach) applied to leases at the adoption date. Under the modified retrospective approach, comparative periods have not been restated and continue to be reported under the accounting standards in effect for those periods. Additionally, an adjustment was recorded to retrained earnings to account for the initial adoption of the standard.
The Company elected the optional package of practical expedients to not reassess prior conclusions related to contracts containing leases, lease classification and initial direct costs. The Company also elected the practical expedient to not separate lease components from non-lease components for real estate leases. As a result of the adoption of ASU 2016-02, the Company recorded right-of-use assets of $11,197,339 and corresponding lease liabilities of $11,695,585 with the difference of $498,246 recorded in opening retained earnings.
The Company has operating leases for certain Rise and Essence retail dispensaries located throughout the US and processing and cultivation facilities in Connecticut, Florida, Massachusetts, Maryland, Nevada, New York and Pennsylvania as well as corporate office space in Illinois and Nevada. Operating lease ROU assets and operating lease liabilities are recognized based on the present value of future minimum lease payments over the lease term at commencement date.
Upon adoption of ASU 2016-02, ROU assets were adjusted for deferred rent and prepaids as of January 1, 2019. Lease expense is recognized on a straight-line basis over the expected lease term. The Company’s incremental borrowing rate is used in determining the present value of future payments at the commencement date of the lease, or for the adoption of ASU 2016-02, at January 1, 2019. Balances related to operating leases are included in ROU assets and noncurrent lease liabilities on the consolidated balance sheet.
All real estate leases are recorded on the balance sheet. Equipment and other non-real estate leases with an initial term of twelve months or less are not recorded on the balance sheet. Lease agreements for some locations provide for rent escalations and renewal options. Many leases include one or more options to renew the lease at the end of the initial term. The Company considered renewals in its ROU assets and operating lease liabilities. Certain real estate leases require payment for taxes, insurance and maintenance which are considered non-lease components. The Company accounts for real estate leases and the related fixed non-lease components together as a single component
The Company determines if an arrangement is a lease at inception. The Company must consider whether the contract conveys the right to control the use of an identified asset. Certain arrangements require significant judgment to determine if an asset is specified in the contract and if the Company directs how and for what purpose the asset is used during the term of the contract.
For the year ended December 31, 2019, the Company recorded $7,291,154 in operating lease expense.
Other information related to operating leases as of and for the year ended December 31, 2019 were as follows:
| Year Ended December 31, 2019 |
||||
| Weighted average remaining lease term (in years) |
7.42 | |||
| Weighted average discount rate |
12.0 | % | ||
F-31
Table of Contents
| 9. | LEASES (Continued) |
Maturities of lease liabilities for operating leases as of December 31, 2019 were as follows:
| Maturities of Lease Liability | ||||||||||||
| Year Ending December 31 |
Third Party | Related Party | Total | |||||||||
| 2020 |
$ | 10,041,583 | $ | 1,392,233 | $ | 11,433,816 | ||||||
| 2021 |
10,862,998 | 1,424,852 | 12,287,850 | |||||||||
| 2022 |
10,261,431 | 1,458,247 | 11,719,678 | |||||||||
| 2023 |
10,039,912 | 1,492,438 | 11,532,350 | |||||||||
| 2024 |
8,750,062 | 1,384,036 | 10,134,098 | |||||||||
| 2025 and Thereafter |
54,812,515 | 11,524,619 | 66,337,134 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Lease Payments |
104,768,501 | 18,676,425 | 123,444,926 | |||||||||
|
|
|
|
|
|
|
|||||||
| Less: Interest |
(49,018,620 | ) | (9,477,301 | ) | (58,495,921 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Present Value of Lease Liability |
$ | 55,749,881 | $ | 9,199,124 | $ | 64,949,005 | ||||||
|
|
|
|
|
|
|
|||||||
Related Party Operating Leases
During 2019, GTI entered into three additional related party transactions with respect to its leasing arrangements for three GTI dispensaries operating in Florida, Illinois and Nevada.
With respect to leasing arrangements in Florida and Illinois, Wendy Berger, a director of the Company, is a principal of WBS Equities, LLC, which is the Manager of Mosaic Real Estate, LLC. Additionally, Mosaic Real Estate, LLC is owned in part by Ms. Berger (through the Wendy Berger 1998 Revocable Trust), Benjamin Kovler, the Chief Executive Officer and a director of the Company (through KP Capital, LLC), and Mr. Georgiadis, the Chief Financial Officer and a director of the Company (through Three One Four Holdings, LLC). The terms of these leases range from 7 years to 15 years. For the year ended December 31, 2019, the Company recorded lease expense of $710,525 associated with these lease arrangements.
In addition to the leases entered into in 2019, the Company had pre-existing operating leases with the same related parties for three dispensaries located in Maryland, Nevada and Massachusetts. These leases commenced in 2017 and have terms of 7 to 15 years. For the years ended December 31, 2019, 2018 and 2017, rent expense associated with these leases was $519,825, $515,064 and $87,705, respectively.
In regards to the leasing arrangement in Nevada, and as a result of the acquisition of Integral Associates, LLC, Armen Yemenidjian, the President of the Integral Associates, LLC, and Alejandro Yemenidjian, a director of Integral Associates, LLC, each own 50% of Armenco Capital LLC, which owns 50% of Durango Teco Partners, LLC. Durango Teco Partners, LLC owns the building in which an Essence dispensary leases. The term of the lease is 10 years. For the year ended December 31, 2019, the Company recorded lease expense of $42,396 associated with this lease.
Sales Lease Back Transaction
On November 12, 2019, the Company closed on a sale and lease back transaction to sell its Danville, Pennsylvania cultivation and processing facility to IIP. Under a long-term agreement, the Company will lease back the facility and continue to operate and manage it. The purchase price for the property was $20,300,000, excluding transaction costs. The Company is also expected to make certain improvements to the property that will significantly enhance production capacity, for which IIP has agreed to provide reimbursement of up to $19,300,000. Assuming full reimbursement for such improvements, IIP’s total investment in the property will be $39.6 million. The lease was recorded as an operating lease and resulted in a right of use asset and related lease liability of $25,165,505 each and was recorded net of the improvements allowance of $19,300,000.
F-32
Table of Contents
| 9. | LEASES (Continued) |
Disclosures related to period prior to adoption of ASU 2016-02
Future minimum rental commitments under non-cancelable operating leases as of December 31, 2018 were expected to be as follows:
| Year Ending December 31 |
Third Party |
Related Party |
Total | |||||||||
| 2019 |
$ | 1,188,865 | $ | 574,477 | $ | 1,763,342 | ||||||
| 2020 |
1,127,754 | 585,966 | 1,713,720 | |||||||||
| 2021 |
1,123,769 | 597,686 | 1,721,455 | |||||||||
| 2022 |
1,040,481 | 504,255 | 1,544,736 | |||||||||
| 2023 |
1,067,783 | 366,802 | 1,434,585 | |||||||||
| 2024 and Thereafter |
3,119,021 | 695,291 | 3,814,312 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Future Minimum Lease Payments |
$ | 8,667,673 | $ | 3,324,477 | $ | 11,992,150 | ||||||
|
|
|
|
|
|
|
|||||||
The Company leases certain business facilities from third parties under operating lease agreements that specify minimum rentals. The Company’s net rent expense for the years ended December 31, 2018 and 2017 totaled approximately $1,901,000 and $927,000 for these third-party leases.
| 10. | NOTES PAYABLE |
At December 31, 2019 and December 31, 2018, notes payable consisted of the following:
| December 31, 2019 |
December 31, 2018 |
|||||||
| Promissory note dated October 2, 2017, in the original amount of $2,250,000 issued to accredited investors, which matures October 1, 2022; monthly payments of $55,611 including interest at 12.0% per annum |
$ | — | $ | 2,007,256 | ||||
| Promissory note dated October 2, 2017, in the original amount of $5,000,000 issued to accredited investors, which matures October 1, 2022; monthly payments of $112,490 including interest at 12.5% per annum |
— | 4,084,885 | ||||||
| In connection with an acquisition completed in 2017, the Company is required to make quarterly charitable contributions of $50,000 through October 2024. The net present value of these required payments has been recorded as a liability with an interest rate of 2.17% |
970,957 | 1,122,316 | ||||||
| Private placement debt dated May 22, 2019, in the original amount of $105,466,429, which matures on May 22, 2022. The debt was issued at a discount, the carrying value of which is $15,090,517 as of December 31, 2019, and bears interest of 12.00% per annum. |
90,375,912 | — | ||||||
|
|
|
|
|
|||||
| Total notes payable |
91,346,869 | 7,214,457 | ||||||
| Less: current portion of notes payable |
(206,675 | ) | (1,480,660 | ) | ||||
|
|
|
|
|
|||||
| Notes payable, net of current portion |
$ | 91,140,194 | $ | 5,733,797 | ||||
|
|
|
|
|
|||||
F-33
Table of Contents
| 10. | NOTES PAYABLE (Continued) |
(a) Bridge Financing
On April 12, 2019, the Company completed a private placement of $12,500,000 in six-month senior secured promissory notes (the “Bridge Notes”). These Notes included Warrants to purchase 218,964 Subordinate Voting Shares at an exercise price of C$22.90, which can be exercised 42 months after the closing.
The exercise price of the warrants is denominated in Canadian dollars whereas the Company’s functional currency is USD. As such, upon issuance, the Company recorded a warrant liability and debt discount of $2,291,189 which was measured at fair value using a Monte Carlo simulation model. On May 22, 2019, the Company repaid the full principal amount and accrued interest of $12,645,833 for the Bridge Notes. The Company recognized $2,291,189 in interest expense for accretion of the debt discount upon the repayment of the April 12, 2019 Bridge Notes.
The warrant liability associated with the April 12, 2019 warrants is revalued as of each reporting date and requires various inputs, including management’s estimate of volatility, remaining term and risk-free rate. The following table summarizes the significant assumptions utilized as part of estimate of the fair value of the warrant liability at April 12, 2019 and December 31, 2019:
| Date of Measurement – Warrant Liability |
Volatility | Remaining Term |
Risk-Free Rate |
Estimated Fair Value |
||||||||||||
| April 12, 2019 |
123.64 | % | 3.50 Years | 1.64 | % | $ | 2,291,189 | |||||||||
| December 31, 2019 |
100.90 | % | 2.78 Years | 1.69 | % | $ | 1,385,000 | |||||||||
As of December 31, 2019, the Company recorded $805,788 as gain on fair value of warrants which is included in other income (expense) on the consolidated statement of operations.
| (b) | Private Placement Financing |
On May 22, 2019, the Company completed a private placement of $105,466,429 in three-year senior secured promissory notes and extinguished the bridge notes issued on April 12, 2019 and the promissory notes dated October 2, 2017 in the original amounts of $2,500,000 and $5,000,000. The notes accrue interest at an annual rate of 12.16%, payable on a quarterly basis commencing June 30, 2019 and all principal is due on the maturity date. The Company also has the sole discretion to extend the financing an additional twelve months.
The notes contain certain covenants which require the Company to maintain (on a daily basis) unrestricted cash and cash equivalents in an amount greater than or equal to the amount of interest that is scheduled to become due in the next 365-days and to not permit the ratio of net debt to stockholders’ equity to exceed a certain maximum as of the last day of any fiscal quarter. The Company was in compliance with such covenants as of December 31, 2019. Additionally, there are certain covenants which will require the Company to maintain a specific debt to EBITDA ratio and an interest coverage ratio which will be measured beginning June 30, 2020 as well as a fixed coverage ratio beginning December 31, 2020. Such covenants were not in effect as of December 31, 2019.
For the year ended December 31, 2019, the Company recognized $7,732,279 in interest expense associated with the promissory notes and $3,317,291 in interest expense for accretion of the debt discount.
As part of the transactions, the purchasers of the promissory notes also received warrants to purchase 1,822,771 Subordinate Voting Shares at an exercise price of C$19.39, which can be exercised at any time until 60 months after the closing of the transaction. The exercise price of the warrants is denominated in Canadian dollars whereas the Company’s functional currency is USD. As such, upon issuance, the Company recorded a warrant liability and debt discount of $15,543,468 which was measured at fair value using a Monte Carlo simulation model. In addition to the value of warrants, the debt discount included $228,761 of professional fees, and transaction related fees of $430,704 and is being accreted to interest expense over the term of the debt which approximates the effective interest method. As of December 31, 2019, the carrying value of the debt discount, net of amortization was $12,885,643.
F-34
Table of Contents
| 10. | NOTES PAYABLE (Continued) |
The warrant liability associated with the May 22, 2019 warrants is revalued as of each reporting date and requires various inputs, including management’s estimate of volatility, remaining term and risk-free rate. The following table summarizes the significant assumptions utilized as part of estimate of the fair value of the warrant liability at May 22, 2019 and December 31, 2019:
| Date of Measurement – Warrant Liability |
Volatility | Remaining Term |
Risk-Free Rate |
Estimated Fair Value |
||||||||||||
| May 22, 2019 |
121.14 | % | 5.00 Years | 1.62 | % | $ | 15,543,468 | |||||||||
| December 31, 2019 |
114.65 | % | 4.39 Years | 1.68 | % | $ | 12,189,169 | |||||||||
As of December 31, 2019, the Company recorded $3,354,299 to gain on fair value of warrants which is included in other income (expense) on the consolidated statement of operations.
| (c) | Modification of Private Placement Financing |
On November 9, 2019, the Company amended the May 22, 2019 Private Placement Financing to allow for additional financing through sales lease back arrangements and to clarify certain aspects of the financing agreement with the lenders. Specifically, the calculation of the effective interest rate on the note was clarified to refer to a 365-day calendar year rather than LIBOR-based 360-day year. The result of this change was a reduction in the effective interest rate by approximately 16 basis points (from 12.16% to 12.0%). The Amendment also reduced the borrowing capacity from $150,000,000 to $130,000,000, which allows the Company to borrow an additional $24,533,571 over a period of 12 months from the closing date of the Note Purchase Agreement. The Company evaluated the terms of the November 9, 2019 amendment and concluded that the transaction resulted in a debt modification, which resulted in the value of the warrants being recorded as additional debt discount and is being accreted to interest expense over the remaining term of the debt which approximates the effective interest method. As part of this transaction, the Company issued 365,076 warrants allowing the lenders to purchase Subordinate Voting Shares at an exercise price of C$12.04, which can be exercised 60 months following the close of the transaction. The exercise price of the warrants is denominated in Canadian dollars whereas the Company’s functional currency is USD. As such, upon issuance, the Company recorded an additional amount to debt discount and warrant liability of $2,304,874 which was measured at fair value using a Monte Carlo simulation model. The Company did not incur any other fees related to the amendment. As of December 31, 2019, the carrying value of the debt discount, net of amortization was $2,204,874.
The warrant liability is revalued as of each reporting date and requires various inputs including management’s estimate of volatility, remaining term and risk-free rate. The following table summarizes the significant assumptions utilized as part of estimate of the fair value of the warrant liability at November 9, 2019 and December 31, 2019:
| Date of Measurement – Warrant Liability |
Volatility | Remaining Term |
Risk-Free Rate |
Estimated Fair Value |
||||||||||||
| November 9, 2019 |
117.43 | % | 5.00 Years | 1.55 | % | $ | 2,304,874 | |||||||||
| December 31, 2019 |
116.58 | % | 4.86 Years | 1.68 | % | $ | 2,304,874 | |||||||||
As the fair value of the November 9, 2019 warrants approximated the fair value recorded upon issuance, no fair value adjustment was required as of December 31, 2019.
F-35
Table of Contents
| 10. | NOTES PAYABLE (Continued) |
(d) Related Parties
The private placement debt and related warrant liability are held by related parties of the Company as well as un-related third parties. Benjamin Kovler, the Chief Executive Officer and a director of the Corporation (through KP Capital, LLC); Andrew Grossman, the Executive Vice President of Capital Markets (through AG Funding Group, LLC) and Anthony Georgiadis, the Chief Financial Officer and a director of the Corporation (through Three One Four Holdings, LLC and ABG, LLC) all participated in the private placement financing. The following table summarizes the ownership interests in the private placement debt and warrant liability held as of December 31, 2019:
| Related Parties – Private Placement Debt |
Third Parties |
Related Parties |
Total Notes Payable |
|||||||||
| Private Placement Debt dated May 22, 2019 |
$ | 89,576,809 | $ | 799,103 | $ | 90,375,912 | ||||||
| Related Parties – Warrant Liability |
Third Parties |
Related Parties |
Total Warrant Liability |
|||||||||
| April 12, 2019 |
$ | 1,385,400 | $ | — | $ | 1,385,400 | ||||||
| May 22, 2019 |
12,081,392 | 107,777 | 12,189,169 | |||||||||
| April 12, 2019 |
2,284,920 | 20,354 | 2,305,274 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Warrant Liability |
$ | 15,751,712 | $ | 128,131 | $ | 15,879,843 | ||||||
|
|
|
|
|
|
|
|||||||
| (e) | Notes Payable Maturities |
Maturities of the Company’s Notes Payable as of December 31, 2019 were as follows:
| Maturities of Notes Payable | ||||||||||||
| Private Placement Debt |
Charitable Contributions |
Total | ||||||||||
| 2020 |
$ | — | $ | 206,675 | $ | 206,675 | ||||||
| 2021 |
— | 184,913 | 184,913 | |||||||||
| 2022 |
105,466,429 | (a) | 188,958 | 105,655,387 | ||||||||
| 2023 |
— | 193,092 | 193,092 | |||||||||
| 2024 |
— | 197,319 | 197,319 | |||||||||
| 2025 and Thereafter |
$ | — | $ | — | $ | — | ||||||
| (a) | The amount excludes $15,090,517 of unamortized debt discount as of December 31, 2019. |
| 11. | INCOME TAXES |
On January 1, 2018, the Company, through a tax-free transfer under IRC Section 351, transferred ownership in GTI-Clinic Illinois Holdings, LLC (taxed as a partnership) to GTI Core, LLC (taxed as a “C” corporation). As a result of the transaction, the Company now accounts for income taxes in accordance with ASC 740—Income Taxes, under which deferred tax assets and liabilities are recognized based upon anticipated future tax consequences attributable to differences between financial statement carrying values of assets and liabilities and the respective tax bases.
Taxable income is computed for GTI Core, LLC and its respective LLC ownership interests up through the RTO date of June 12, 2018 and for all GTI companies and subsidiaries from this date forward. Effective with the Company’s reverse takeover transaction on June 12, 2018, all GTI companies and subsidiaries have elected to be taxed as “C” corporations.
F-36
Table of Contents
| 11. | INCOME TAXES (Continued) |
Green Thumb Industries Inc. is based in Canada but maintains all of its operations in the United States. Due to this inverted entity structure, the Company is subject to both US and Canadian taxation, however there was no Canadian tax liability and accordingly the Company filed a nil return with Canadian tax authorities.
For the years ended December 31, 2019 and 2018, income taxes expense consisted of:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | 18,095,946 | $ | 2,842,696 | $ | 214,000 | ||||||
| State |
4,665,000 | 279,899 | — | |||||||||
| Foreign |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current |
22,760,946 | 3,122,595 | 214,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred: |
||||||||||||
| Federal |
(12,535,000 | ) | 3,330,000 | — | ||||||||
| State |
(881,913 | ) | 731,000 | — | ||||||||
| Foreign |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Deferred |
(13,416,913 | ) | 4,061,000 | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 9,344,033 | $ | 7,183,595 | $ | 214,000 | ||||||
|
|
|
|
|
|
|
|||||||
The difference between the income tax expense for the years ended December 31, 2019, 2018 and 2017 and the expected income taxes based on the statutory tax rate applied to pre-tax earnings (loss) arises as follows:
| 2019 | 2018 | 2017 | ||||||||||
| Income/(Loss) before Income Taxes |
$ | (50,202,837 | ) | $ | 29,751,374 | $ | (4,150,756 | ) | ||||
| Statutory Tax Rates |
21 | % | 21 | % | 34 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Expense/(Recovery) based on Statutory Rates |
(10,542,596 | ) | 6,247,788 | (1,411,257 | ) | |||||||
| Pass-throughs and Non-controlling Interests |
49,203 | (1,062,111 | ) | (211,494 | ) | |||||||
| State Taxes |
(1,536,694 | ) | (279,899 | ) | — | |||||||
| Provision to Return Adjustment |
(1,209,592 | ) | 53,304 | — | ||||||||
| Adjustments for Stock Compensation |
(1,952,083 | ) | — | — | ||||||||
| Non-deductible Expenses |
14,166,223 | 2,263,978 | 1,842,735 | |||||||||
| Change in State Rate Reconciliation |
513,338 | — | — | |||||||||
| Change in Valuation Allowance |
7,604,098 | — | — | |||||||||
| Change in Uncertain Tax Position |
2,113,263 | — | — | |||||||||
| Other Differences |
138,873 | (39,465 | ) | (5,984 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Income Tax Expense |
$ | 9,344,033 | $ | 7,183,595 | $ | 214,000 | ||||||
|
|
|
|
|
|
|
|||||||
As the Company operates in the cannabis industry, it is subject to the limitations of IRC Section 280E under which the Company is only allowed to deduct expenses directly related to sales of product. This results in permanent differences between ordinary and necessary business expenses deemed non-allowable under IRC Section 280E. Therefore, the effective tax rate can be highly variable and may not necessarily correlate with pre-tax income or loss.
Deferred taxes are provided using an asset and liability method whereby deferred tax assets are recognized based on the rates at which they are expected to reverse in the future. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax basis. The effect on deferred tax assets and liabilities of a change in tax law or tax rates is recognized in income in the period that enactment occurs.
F-37
Table of Contents
| 11. | INCOME TAXES (Continued) |
At December 31, 2019 and December 31, 2018, the components of deferred tax assets and liabilities were as follows:
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Deferred Tax Assets |
||||||||
| Net Operating Losses |
$ | 12,997,000 | $ | 1,046,000 | ||||
| Stock-based Compensation |
4,592,000 | — | ||||||
| Other |
1,596,000 | 804,000 | ||||||
| Valuation Allowance |
(7,604,098 | ) | — | |||||
|
|
|
|
|
|||||
| Total Deferred Tax Assets |
11,580,902 | 1,850,000 | ||||||
|
|
|
|
|
|||||
| Deferred Tax Liabilities |
||||||||
| Fair Value Investments |
$ | (1,852,000 | ) | $ | (5,911,000 | ) | ||
| Intangibles |
(43,895,000 | ) | (9,480,000 | ) | ||||
|
|
|
|
|
|||||
| Total Deferred Tax Liabilities |
(45,747,000 | ) | (15,391,000 | ) | ||||
|
|
|
|
|
|||||
| Net Deferred Tax Liabilities |
$ | (34,166,098 | ) | $ | (13,541,000 | ) | ||
|
|
|
|
|
|||||
As of December 31, 2019, the Net Deferred Tax Liability of $36,279,361, as presented on the consolidated balance sheet, includes unrecognized tax benefits of $2,113,263 as further described below.
Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. We assess the positive and negative evidence to determine if sufficient future taxable income will be generated to use the existing deferred tax assets.
As of December 31, 2019, we had federal net operating loss carryforwards with no expiration of $45 million and $45 million of state net operating loss carryforwards that begin to expire in 2031. Our evaluation of evidence resulted in management concluding that the majority of our net operating losses will not be realized. A valuation allowance in the amount of $7.6 million is maintained as of December 31, 2019.
Pursuant to Section 382 and 383 of the Internal Revenue Code of 1986, as amended, utilization of our net operating losses and credits may be subject to annual limitations in the event of any significant future changes in its ownership structure. These annual limitations may result in the expiration of net operating losses and credits prior to utilization.
The Company operates in a number of tax jurisdictions and are subject to examination of its income tax returns by tax authorities in those jurisdictions who may challenge any item on these returns. Because the tax matters challenged by tax authorities are typically complex, the ultimate outcome of these challenges is uncertain. In accordance with ASC 740 (Topic 740, “Income Taxes”), the Company recognizes the benefits of uncertain tax positions in our consolidated financial statements only after determining that it is more likely than not that the uncertain tax positions will be sustained.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Balance as of 12/31/2018 |
$ | — | ||
| Additions for current year |
1,720,865 | |||
| Additions for prior year |
392,398 | |||
| Subtractions for current year |
— | |||
|
|
|
|||
| Balance as of 12/31/2019 |
$ | 2,113,263 | ||
|
|
|
F-38
Table of Contents
| 11. | INCOME TAXES (Continued) |
The Company recognizes accrued interest and penalties related to unrecognized tax benefits in the provision for income taxes. As of December 31, 2019 and 2018, we recognized an immaterial amount of interest and penalties. There are no positions for which it is reasonably possible that the uncertain tax benefit will significantly increase or decrease within twelve months. We file income tax returns in the United States, various state jurisdictions, and Canada with varying statutes of limitations. The federal statute of limitation remains open for the 2016 tax year to the present. The state income tax returns generally remain open for the 2016 tax year through the present. Net operating loss arising prior to these years are also open to examination if and when utilized.
| 12. | INVESTMENTS |
The Company applies fair value accounting for all financial assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded at fair value, the Company considers all related factors of the asset by market participants in which the Company would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as inherent risk, transfer restrictions, and credit risk.
The Company applies the following fair value hierarchy, which prioritizes the inputs used to measure fair value into three levels, and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement:
Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities;
Level 2 — Inputs other than quoted prices that are observable for the asset or liability, either directly or indirectly; and
Level 3 — Inputs for the asset or liability that are not based on observable market data.
The Company participated in various fundraises of other cannabis companies throughout 2018. The investments include convertible notes with terms to maturity ranging from 1 to 2 years that carry simple interest of 6.00% per annum and convert into common shares at pre-defined numbers of units. Management estimated that market interest rates on similar borrowings without the conversion feature was approximately 18% and has used an implied volatility of 58% in measuring the fair value. At December 31, 2019 and 2018, the fair value of these investments is $7,533,000 and $30,336,000, respectively.
The Company also made direct equity investments. Management estimated that market yields were approximately 15% and used an implied volatility of 106% in measuring the fair value. At December 31, 2019 and 2018, the fair value of these investments was $6,535,821 and $10,597,283, respectively.
In addition to the investments discussed above, the Company also held an equity interest in a publicly traded company (which is considered a Level 1 investment and was accounted for as a trading investment at the date of acquisition) in the amount of $0 and $966,541 as of December 31, 2019 and 2018, respectively. All of these investments are measured at fair value for financial reporting purposes. As these convertible notes (as described above) and equity investments are not traded in an active market; their fair values are estimated by using market data. Any resulting change in fair value is reflected on the consolidated statement of operations under the classification other income (expense).
F-39
Table of Contents
| 12. | INVESTMENTS (Continued) |
The following table summarizes the change in the Company’s investments as of December 31, 2019:
| Convertible Notes Receivable |
Equity | Total | ||||||||||
| Balance at December 31, 2018 |
$ | 30,336,000 | $ | 10,597,283 | $ | 40,933,283 | ||||||
| Fair value adjustment |
(1,398,000 | ) | (4,061,462 | ) | (5,459,462 | ) | ||||||
| Applied to consideration in business combination |
(21,405,000 | ) | — | (21,405,000 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31, 2019 |
$ | 7,533,000 | $ | 6,535,821 | $ | 14,068,821 | ||||||
|
|
|
|
|
|
|
|||||||
The following table summarizes the change in the Company’s investments as of December 31, 2018:
| Warrants | Convertible Notes Receivable |
Equity | Total | |||||||||||||
| Balance at December 31, 2017 |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Additions |
22,153,692 | 16,750,000 | 5,800,000 | 44,703,692 | ||||||||||||
| Fair value adjustment |
15,612,026 | 13,586,000 | 4,797,283 | 33,995,309 | ||||||||||||
| Exchange for note receivable (see Note 5) |
(11,630,867 | ) | — | — | (11,630,867 | ) | ||||||||||
| Distributions |
(26,134,851 | ) | — | — | (26,134,851 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance at December 31, 2018 |
$ | — | $ | 30,336,000 | $ | 10,597,283 | $ | 40,933,283 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The calculated fair values are recorded as a Level 3 fair value investment as of December 31, 2019 and 2018 (see Note 18 Financial Instrument and Financial Risk Management for additional details). The convertible notes receivable were valued using the Binomial Lattice Model, which is based on a generalized binomial option pricing formula, using the following assumptions:
| Year Ended December 31, | ||||
| 2019 | 2018 | |||
| Risk free rate |
1.58% - 2.46% | 2.54% - 2.63% | ||
| Equity Volatility |
58% - 106% | 100% | ||
| Market Yield |
15% - 18% | 15% | ||
| Probability of Qualified Financing |
0% | 0% | ||
| Probability of Sale |
30% | 50%-80% | ||
| Probability of No Event |
70% | 20%-50% | ||
| 13. | INVESTMENT IN ASSOCIATE |
The Company’s investments in associates are as follows:
| Ownership Interest | ||||||||||
| Investment in associates |
Jurisdiction | 2019 | 2018 | |||||||
| MC Brands, LLC |
Colorado | 100 | % | 25 | % | |||||
| Cal Funding, LLC |
Massachusetts | 9.9 | % | — | ||||||
During 2019, the Company acquired a 9.9% interest in Cal Funding, LLC, an investment entity that owns a Massachusetts cannabis operator as part of the acquisition of Advanced Grow Labs, LLC. In addition to the 9.9% interest acquired, the Company also indirectly controls a portion of Cal Funding, LLC through operating agreements with the former owners of the entity. This investment is being accounted for as an equity method investment as the Company exerts significant influence over Cal Funding, LLC. See Note 7—Acquisitions for additional details.
F-40
Table of Contents
| 13. | INVESTMENT IN ASSOCIATE (Continued) |
Presented below is the summarized unaudited balance sheet of Cal Funding, LLC as of December 31, 2019:
| 2019 | ||||
| Cash and Cash Equivalents |
$ | 2,095,631 | ||
| Other Current Assets |
1,061,707 | |||
|
|
|
|||
| Total Current Assets |
3,157,338 | |||
| Non-Current Assets |
6,955,118 | |||
| Current Liabilities |
584,557 | |||
| Long-Term Liabilities |
1,000,000 | |||
| Net Assets |
$ | 8,527,899 | ||
For the year ended December 31, 2019, Cal Funding, LLC had revenues of $7,053,953 and net income of $2,003,887 from which, the Company recorded a nominal amount in income from the investee within Other Income (Expense) in the consolidated statement of operations. The balance of GTI’s Investment was $10,350,000 as of December 31, 2019.
During 2018, the Company acquired a 25% interest in MC Brands, LLC, a Colorado based intellectual property business that licenses its edibles and extracts brand and product formulation to various cannabis operators. Presented below is the summarized unaudited balance sheet of MC Brands, LLC as of December 31, 2018.
| 2018 | ||||
| Cash and Cash Equivalents |
$ | 9,313 | ||
| Other Current Assets |
80,719 | |||
|
|
|
|||
| Total Current Assets |
90,032 | |||
| Non-Current Assets |
515,282 | |||
| Current Liabilities |
235,236 | |||
| Net Assets |
370,078 | |||
During 2018, the investee had nominal profit and loss activity from the date of the Company’s investment through December 31, 2018, and thus the Company did not record an adjustment to the carrying balance of the investment in associate. In June 2019, the Company acquired the remaining ownership interests of MC Brands, LLC. The value of the investment of $6.5 million as of the transaction date was included as part of the consideration. As of December 31, 2019, MC Brands, LLC was included within the consolidated financial statements. See Note 7 for details.
F-41
Table of Contents
| 14. | SHARE CAPITAL |
Common shares are classified as equity. Incremental costs directly attributable to the issuance of shares are recognized as a deduction from equity. The proceeds from the exercise of stock options or warrants together with amounts previously recorded in reserves over the vesting periods are recorded as share capital. Income tax relating to transaction costs of an equity transaction is accounted for in accordance with Accounting Standards Codification (ASC) 740, Income Taxes.
| (a) | Authorized |
The Company has the following classes of Share Capital, with each class having no par value:
| (i) | Subordinate Voting Shares |
The holders of the Subordinate Voting shares are entitled to receive dividends which may be declared from time to time, and are entitled to one vote per share at shareholder meetings of the Company. All Subordinate Voting shares are ranked equally with regard to the Company’s residual assets. The Company is authorized to issue an unlimited number of no par value Subordinate Voting shares. During the year ended December 31, 2019, the shareholders of the corporation converted 357,104 Multiple Voting Shares into 35,710,400 Subordinate Voting Shares.
| (ii) | Multiple Voting Shares |
Each Multiple Voting share is exchangeable for 100 Subordinate Voting shares and is entitled to one hundred votes per share at shareholder meetings of the Company. At December 31, 2019, the Company has 373,350 issued and outstanding multiple voting shares, which convert into 37,335,000 subordinate voting shares. The Company is authorized to issue an unlimited number of Multiple Voting shares. During the year ended December 31, 2019, the shareholders of the Corporation converted 22,224 Super Voting Shares into 22,224 Multiple Voting Shares.
| (iii) | Super Voting Shares |
Each Super Voting share is exchangeable for 100 Subordinate Voting shares and is entitled to one thousand votes per share at shareholder meetings of the Company. At December 31, 2019, the Company has 402,289 issued and outstanding Super Voting shares which convert into 40,228,900 subordinate voting shares. The Company is authorized to issue an unlimited number of super voting shares. During the year ended December 31, 2019, the shareholders of the Corporation converted 22,224 Super Voting Shares into 22,224 Multiple Voting Shares.
| (b) | Issued and Outstanding |
A reconciliation of the beginning and ending amounts of the issued and outstanding shares by class is as follows:
| Issued and Outstanding | ||||||||||||
| Subordinate Voting Shares |
Multiple Voting Shares |
Super Voting Shares |
||||||||||
| As of December 31, 2018 |
43,920,131 | 677,230 | 424,513 | |||||||||
| Issuance of shares under business combinations and investments |
45,571,444 | — | — | |||||||||
| Noncontrolling interests adjustment for change in ownership |
— | 31,000 | — | |||||||||
| Issuance of shares for redemption of noncontrolling interests |
2,498,404 | — | — | |||||||||
| Issuance of shares upon vesting of RSUs |
1,165,630 | — | — | |||||||||
| Issuance of shares upon exercise of broker options |
114,080 | — | — | |||||||||
| Issuance of shares for professional fees |
19,875 | — | — | |||||||||
| Exchange of shares |
35,710,400 | (334,880 | ) | (22,224 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| As at December 31, 2019 |
128,999,964 | 373,350 | 402,289 | |||||||||
|
|
|
|
|
|
|
|||||||
F-42
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
| (c) | Deferred Share Issuances |
In relation to certain acquisitions as described in Note 7 - Acquisitions, certain transactions involved consideration in the form of deferred shares which are to be issued to the previous owners of each respective entity at a date post acquisition. The following is a summary of the shares to be issued as of December 31, 2019:
| December 31, 2019 | ||||||||||||
| Transaction |
Date of Transaction |
Units | Value | |||||||||
| Advanced Grow Labs, LLC |
February 12, 2019 | 472,500 | $ | 5,380,000 | ||||||||
| For Success Holdings Company |
February 21, 2019 | 793,448 | 9,900,000 | |||||||||
| Rise Canton |
May 15, 2019 | 101,695 | 1,307,798 | |||||||||
|
|
|
|
|
|||||||||
| 1,367,643 | $ | 16,587,798 | ||||||||||
|
|
|
|
|
|||||||||
The deferred shares for each acquisition are to be issued upon the passage of 12-24 months from the close of each respective transaction as defined within each agreement. The value associated with the deferred shares is recorded within the Company’s consolidated statement of shareholders’ equity as of December 31, 2019.
| (d) | Private Placement of Shares in Connection with Reverse Takeover |
In contemplation of its reverse takeover (RTO) transaction, the Company issued $45,000,000 in convertible notes payable to various investors. The original maturity of the convertible notes payable was three years from the funding date of April 30, 2018, and the notes bore simple interest at a rate of 8% per year. At June 12, 2018, the carrying value of the convertible notes payable, including accrued interest, was $15,245,960 and the fair value assigned to the conversion feature of the notes was $28,894,566. The Black Scholes options pricing model assumptions used in calculating the fair value include a risk free rate of 2.04%, volatility of 100%, an expected term of 60 days, and a share price of $6.00. The fair value adjustment related to the conversion feature was $1,981,358, and is included in other income on the consolidated statement of operations.
An increase in the share price and volatility assumptions of 5% would result in an increase in the fair value estimate of approximately $3,700,000, and a decrease in the share price and volatility assumptions of 5% would result in a decrease in the fair value estimate of approximately $3,694,000. Upon the RTO transaction, the convertible notes payable were converted into 122,442 Multiple Voting shares and 2,211 Super Voting shares, carrying a total value of $44,140,526.
On April 25, 2018, Subscription Receipts were sold at a price of C$7.75 per Subscription Receipt, for gross proceeds of $64,075,295 less issuance costs of $4,014,585. The Subscription Receipts were for the potential purchase of shares in GTI FinCo Inc. and were to be held in an escrow account until the reverse takeover transaction were to occur. Upon the RTO transaction, simultaneously with the issuance of shares of the Company to the holders of the Subscription Receipts, the funds held in the escrow account were released to the Company, and the shares converted into 10,744,995 Subordinate Voting shares of the Company. Also upon the RTO transaction, 4,550 Multiple Voting shares, which are convertible into 455,000 Subordinate Voting shares, were issued for gross proceeds of $2,730,000. Last, in connection with the private placement, the Company issued 285,000 options to consultants as compensation for the services provided. The options provided the recipients the right to purchase Subordinate Voting shares at an exercise price of C$7.75 per share. The options vested immediately and had a contractual life of two years. The value of the options was $906,366 under the Black-Scholes option pricing model. The total of the gross Subscription Receipts and Multiple Voting shares issued, less the direct costs of the Subscription Receipts and the value assigned to the options, resulted in an increase of $61,884,344 to share capital.
As discussed in Note 3, the RTO transaction was executed on June 12, 2018. Pursuant to the RTO transaction, Bayswater Uranium Corporation’s existing 185,186,988 common shares were converted into 500,439 Subordinate Voting shares of the Company. The value assigned to these shares was $3,002,634,
F-43
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
which was based on a per-share price of $6.00 (US Dollars) on the RTO date. Also pursuant to the RTO transaction, 130,435,783 Common Units and 119,266,258 Preferred Units of VCP23, LLC were converted into 431,198 Super Voting shares and 644,083 Multiple Voting shares, respectively, of the Company.
| (e) | Fundraise Transactions |
On August 2, 2018, the Company closed on a brokered fundraise transaction (the “First Offering”) for 7,300,000 Subordinate Voting shares, at a price of C$11.00 per share, for gross proceeds of $61,726,497. Financing costs related to the First Offering totaled $3,133,722.
On October 17, 2018, the Company closed on a brokered fundraise transaction (the “Second Offering”) for 5,083,000 Subordinate Voting shares, at a price of C$20.00 per share, for gross proceeds of $78,562,596. Financing costs related to the Second Offering totaled $3,479,116.
| (f) | Changes in Ownership and Noncontrolling Interests |
| (i) | Acquisition of Noncontrolling Interest in KW Ventures Holdings, LLC (Firefly) |
On January 1, 2019, the Company closed on its acquisition of KW Ventures Holdings, LLC (Firefly). The acquisition was an all stock transaction whereby consideration was satisfied through the issuance of 542,416 Subordinate Voting Shares at a fair value of $5,364,048. In addition to the shares issued on January 1, 2019 an additional 48,450 Subordinate Voting Shares with a fair value of $434,069 were held back as part of the closing agreement. The value of those shares was recorded as a current liability and included within Accrued Liabilities on the consolidated balance sheet as of December 31, 2019. Such shares were distributed to the noncontrolling interest members in February 2020.
Prior to January 1, 2019, 100% of Firefly was owned by the noncontrolling interest members. However, GTI considered Firefly to be a variable interest entity and accordingly, consolidated Firefly within GTI’s consolidated financial statements as GTI was determined to be the primary beneficiary of the operations of Firefly and GTI possessed the power to direct the activities of Firefly through a management services agreement. Consequently, when GTI acquired the noncontrolling interest, there was no change in control, and as a result, no gain or loss was recognized nor was there any excess purchase price recorded as a result of the transaction. The transaction resulted in an increase to share capital and a reduction to noncontrolling interest of $552,472. During 2019, GTI also made contributions to Firefly of $444,046 to cover the noncontrolling interest member’s 2018 tax liability.
| (ii) | Acquisition of Noncontrolling Interest in GTI Ohio, LLC |
On April 19, 2019, GTI Core, LLC, a wholly owned subsidiary of Green Thumb Industries Inc. entered into a membership interest purchase agreement with George Management Ltd. (George Management) to acquire 59% of the 60% interest that George Management held in the Retail and Processing License. On June 7, 2019, GTI consummated the acquisitions through the issuance of 1,233,014 Subordinate Voting shares with a fair value of $13,980,441 as well as $5,150,000 in cash of which $1,650,000 was contributed by George Management during 2019 as part of a capital call.
As part of the purchase agreement, and in consideration of the cultivation license for which GTI held a 40% interest as of the date of the purchase agreement, the Company and George Management entered into a reciprocal purchase agreement that would allow GTI to purchase the remaining 59% interest through a call option or, alternatively, allow George Management to put the 59% interest to GTI. The option may only be exercised in the event the Ohio Department of Commerce approves GTI’s cultivation license application.
GTI evaluated the reciprocal purchase option and determined that it represented a derivative liability that required remeasurement on a periodic basis with changes in fair value recorded through the statement of operations. As of the transaction date, the Company recorded a derivative liability of $4,526,401 using a Black Scholes option pricing model. During 2019, the Company recognized nominal gains as a result of changes in the fair value of the liability.
F-44
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
| (f) | Changes in Ownership and Noncontrolling Interests (Continued) |
| (ii) | Acquisition of Noncontrolling Interest in GTI Ohio, LLC (Continued) |
On December 29, 2019, subsequent to the Ohio Department of Commerce’s approval of GTI’s license application, George Management exercised their put option allowing GTI to purchase 59% of the remaining 60% interest in the cultivation license. As of December 31, 2019, the Company and George Management have been unable to agree upon the value of the Ohio cultivation license. The Company derecognized the derivative liability and recorded a current liability of $5,500,000 representing management’s estimate of the expected value to be paid to George Management as of December 31, 2019. The transactions resulted in an increase to share capital and a reduction to noncontrolling interest of $4,198,173.
| (iii) | Acquisition of Noncontrolling Interest in GTI New Jersey, LLC |
On April 23, 2019, the Company closed on its acquisition of GTI New Jersey, LLC to acquire the remaining 16% interest held by unrelated third parties. The acquisition was an all stock transaction whereby consideration was satisfied through the issuance of 671,317 Subordinate Voting Shares at a fair value of $5,766,613. Prior to April 23, 2019, 16% of GTI-NJ was owned by the noncontrolling interest members. However, GTI considered GTI-NJ to be a variable interest entity and accordingly, consolidated GTI-NJ within GTI’s consolidated financial statements as GTI was determined to be the primary beneficiary of the operations of GTI-NJ and GTI possessed the power to direct the activities of GTI-NJ through a management services agreement. Consequently, when GTI acquired the noncontrolling interest, there was no change in control, and as a result, no gain or loss was recognized nor was there any excess purchase price recorded as a result of the transaction. The transaction resulted in an increase to share capital and a reduction to noncontrolling interest of $570,078. As part of the acquisition of GTI New Jersey, LLC, the Company also agreed to award the previous owners of the entity $1,000,000 in Subordinate Voting Shares for each additional dispensary successfully opened, up to a $3,000,000 cap. However, the ambiguity in cannabis regulations in the state of New Jersey make it unknown whether the Company would be allowed to open and operate additional dispensaries. Given the uncertainty surrounding the Company’s ability to open and operate additional dispensaries, the Company has considered the possibility of successful openings to be unlikely, and as such no amount has been recorded on the consolidated balance sheet as an obligation as of December 31, 2019.
| (iv) | Acquisition of Noncontrolling Interest in JB17, LLC |
On June 12, 2018, the Company acquired all of the noncontrolling interests in JB17, LLC. The consideration paid was $700,000 and the issuance of 59,900 Multiple Voting shares, which were convertible into 5,990,000 Subordinate Voting shares, at a value of $6.00 per Subordinate Voting Share-equivalent. This resulted in an increase to share capital of $35,940,000, and a decrease of $33,662,548, in accumulated deficit, which has been presented as a reduction to accumulated deficit, after the reclassification of the noncontrolling interest carrying balance.
| (v) | Acquisition of Noncontrolling Interest in GTI Pennsylvania, LLC |
In December 2018, the Company issued the reciprocal put and call options discussed in Note 15 to the noncontrolling interest holders of GTI Pennsylvania, LLC. The noncontrolling interests were acquired by the Company subsequent to year-end, in January 2019. As it was determined that the Company had effective control over GTI Pennsylvania, LLC as of the put and call option issuance date, an increase to shares to be issued was reflected in the statement of equity of $27,773,234, representing the fair value of the 31,000 Multiple Voting Shares to be issued upon the subsequent acquisition date, along with a corresponding decrease of $30,663,670, which has been presented as a reduction to accumulated deficit, and the removal of the existing noncontrolling interest carrying balance as of December 31, 2018.
F-45
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
| (f) | Changes in Ownership and Noncontrolling Interests (Continued) |
| (vi) | Acquisition of Noncontrolling Interest in GTI Nevada, LLC |
In December 2018, the Company acquired the noncontrolling interests of GTI Nevada, LLC, in exchange for 2,987,751 Subordinate Voting Shares of the Company. The shares are to be issued in seven tranches, the first of which was delivered in December 2018. The removal of the noncontrolling interests carrying balance, as well as the recording of the liability to issue the shares, resulted in a decrease of $25,917,883 to accumulated deficit as of December 31, 2018. The balance of the remaining liability at December 31, 2018 is $25,420,009 and is recorded in liability for acquisition of noncontrolling interest on the consolidated balance sheet. The remaining shares were delivered in 2019.
| (g) | Stock-Based Compensation |
The Company operates equity settled stock-based remuneration plans for its eligible directors, officers, employees and consultants. All goods and services received in exchange for the grant of any stock-based payments are measured at their fair value unless the fair value cannot be estimated reliably. If the Company cannot estimate reliably the fair value of the goods and services received, the Company shall measure their value indirectly by reference to the fair value of the equity instruments granted. For transactions with employees and others providing similar services, the Company measures the fair value of the services by reference to the fair value of the equity instruments granted.
Equity settled stock-based payments under stock-based payments plans are ultimately recognized as an expense in profit or loss with a corresponding credit to reserve for stock-based payments, in equity.
The Company recognizes compensation expense for Restricted Stock Units (“RSUs”) and options on a straight-line basis over the requisite service period of the award. Non-market vesting conditions are included in the assumptions about the number of options that are expected to become exercisable. Estimates are subsequently revised if there is any indication that the number of share options expected to vest differs from the previous estimate. Any cumulative adjustment prior to vesting is recognized in the current period. No adjustment is made to any expense recognized in prior period if share options ultimately exercised are different to that estimated on vesting.
In June 2018, the Company established the GTII Stock and Incentive Plan (the “Plan”). The maximum number of shares issued under the Plan shall not exceed 10% of the issued and outstanding shares. Option and RSU grants generally vest over one to three years, and Options typically have a life of ten years. Option grants are determined by the Compensation Committee of the Board with the option price set at no less than 100% of the fair market value of a share on the date of grant. Stock option activity is summarized as follows:
| Number of Shares |
Weighted Average Exercise Price (CAD) |
Weighted Average Contractual Life |
Aggregate Intrinsic Value |
|||||||||||||
| Balance as at December 31, 2018 |
1,677,192 | 13.23 | 8.72 | $ | 27,698 | |||||||||||
| Granted |
3,040,906 | 13.35 | 5.00 | |||||||||||||
| Exercised |
(114,080 | ) | 11.79 | 3.19 | ||||||||||||
| Forfeited |
(765,001 | ) | 14.01 | 8.35 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as at December 31, 2019 |
3,839,017 | 13.21 | 5.81 | |||||||||||||
| Vested |
492,833 | 13.63 | 7.84 | |||||||||||||
| Exercisable at December 31, 2019 |
116,320 | 6.67 | 3.19 | $ | 218,234 | |||||||||||
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between the Company’s closing stock price on December 31, 2019 and 2018, respectively, and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option
F-46
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
| (g) | Stock-Based Compensation (Continued) |
holders had all option holders exercised their in-the-money options on December 31, 2019 and 2018. This amount will change in future periods based on the fair market value of the Company’s stock and the number of options outstanding.
The Company used the Black-Scholes option pricing model to estimate the fair value of the options at the grant dates during the year ended December 31, 2019 using the following ranges of assumptions:
| Risk-free interest rate |
1.18% - 2.33% | |||
| Expected dividend yield |
0% | |||
| Expected volatility |
61% - 100% | |||
| Expected option life |
3 – 10 years |
As the Company became publicly traded in June 2018, sufficient historical trading information was not available to determine an expected volatility rate. The volatility rate was based on comparable companies within the same industry. As permitted under ASC 718, the Company has made an accounting policy choice to account for forfeitures when they occur.
The following table summarizes the number of nonvested restricted stock unit awards as of December 31, 2019 and 2018 and the changes during the year ended December 31, 2019:
| Number of Shares |
Weighted Average Grant Date Fair Value (CAD) |
|||||||
| Nonvested Shares at December 31, 2018 |
1,589,000 | 10.28 | ||||||
| Granted |
1,192,678 | 11.70 | ||||||
| Forfeited |
(201,873 | ) | 14.77 | |||||
| Vested |
(1,180,043 | ) | 12.37 | |||||
|
|
|
|
|
|||||
| Nonvested Shares at December 31, 2019 |
1,399,762 | 9.30 | ||||||
The stock-based compensation expense for the years ended December 31, 2019, 2018 and 2017 was as follows:
| For the Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Stock Options Expense |
$ | 6,393,277 | $ | 1,876,627 | $ | — | ||||||
| Restricted Stock Units |
11,892,100 | 4,748,444 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Stock-Based Compensation Expense |
$ | 18,285,377 | $ | 6,625,071 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| (h) | Warrants |
As part of the April 12, 2019 bridge loan financing, the May 22, 2019 private placement financing and the November 9, 2019 modification, the Company issued warrants to related parties as well as un-related third-party lenders. The related parties consisted of Benjamin Kovler, the Chief Executive Officer and a director of the Corporation (through KP Capital, LLC); Andrew Grossman, the Executive Vice President of Capital Markets (through AG Funding Group, LLC) and Anthony Georgiadis, the Chief Financial Officer and a director of the Corporation (through Three One Four Holdings, LLC and ABG, LLC). The related parties and un-related third parties held warrants that were valued at $128,131 and $15,751,712, respectively as of December 31, 2019. The warrant exercise prices are denominated in Canadian dollars whereas the Company’s functional currency is USD. As such, the Company classified the warrants as a liability and valued them using a Monte Carlo simulation model. See Note 10 – Notes Payable for additional details regarding the valuation methodology and significant assumptions used within the valuation. Changes in the fair value of the warrants since the transaction dates were recorded in the consolidated statement of
F-47
Table of Contents
| 14. | SHARE CAPITAL (Continued) |
| (h) | Warrants (Continued) |
operations within other income (expense). The following table summarizes the number warrants issued to related parties and third parties as of December 31, 2019 and 2018 and the changes during the year ended December 31, 2019:
| Third Party Number of Shares |
Related Party Number of Shares |
Weighted Average Exercise Price (CAD) |
Weighted Average Contractual Life |
|||||||||||||
| Balance as at December 31, 2018 |
— | — | 0.00 | 0.00 | ||||||||||||
| Granted |
2,387,470 | 19,341 | 18.59 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as at December 31, 2019 |
2,387,470 | 19,341 | 18.59 | 4.86 | ||||||||||||
| (i) | Profits Interests Units |
During the year ended December 31, 2018, the Company granted 7,657,700 membership units to certain employees and consultants as compensation for services. These membership units qualify as profits interests for U.S. federal income tax purposes and were accounted for in accordance with Accounting Standards Codification (ASC) 718, Stock Compensation. The Company amortizes awards over the service period until awards are fully vested.
The following table summarizes the status of the unvested profits interests at December 31, 2018:
| Number of Units |
Weighted Average Grant Date Fair Value |
|||||||
| Unvested, Beginning of Period |
— | $ | — | |||||
| Granted |
7,657,700 | 0.44 | ||||||
| Forfeited |
— | — | ||||||
| Vested |
(7,657,700 | ) | 0.44 | |||||
|
|
|
|
|
|||||
| Unvested, End of Period |
— | n/a | ||||||
The Company recorded $5,523,180 as compensation expense in connection with these awards during the year ended December 31, 2018. At December 31, 2018, there was no unamortized expense related to unvested profits interests.
| 15. | NONCONTROLLING INTERESTS PUT AND CALL OPTIONS |
The Company has entered into agreements during 2019 and 2018 with certain of its noncontrolling interests whereby the agreements contain a put option, which provides the holder with the right to require the Company to purchase their retained interest for deemed fair market value at the time the put is exercised. The Company has also negotiated reciprocal call options, which would require the same non-controlling interests to sell their retained interest to the Company for deemed fair market value at the time the call is exercised.
For the year ended December 31, 2019, the Company recorded a liability of $5,500,000 in connection with a 2019 agreement with George Management where the noncontrolling party exercised its put option during the year for the Company to purchase the noncontrolling interest. The transaction is expected to close in early 2020. See further discussion in Note 14—Share Capital.
For the year ended December 31, 2018, the Company recorded a derivative liability in connection with the 2018 agreement for the put and call options using the Black Scholes options pricing model which approximates the value that would be obtained using a lattice model. The assumptions used in the calculating the fair value include a risk free rate of 2.44%, volatility of 100%, an expected term of 30 days, and a share price of $8.07. Upon initial recognition, the Company recorded a derivative liability of $7,078,792. The Company recorded a gain of $2,869,342 on revaluation of the derivative liability, which is included other income on the consolidated
F-48
Table of Contents
| 15. | NONCONTROLLING INTERESTS PUT AND CALL OPTIONS (Continued) |
statement of operations for the year ended December 31, 2018. The value of the derivative at December 31, 2018 is $4,238,701 and is recorded as a derivative liability on the consolidated balance sheet. The options were exercised and shares were issued on January 8, 2019.
| 16. | OTHER INCOME (EXPENSE) |
For the years ended December 31, 2019, 2018 and 2017 other income (expense) was comprised of the following:
| For the Years Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Fair value adjustments on equity investments |
$ | (3,530,969 | ) | $ | 4,797,283 | $ | — | |||||
| Fair value adjustments on convertible notes receivable |
(1,771,420 | ) | 13,586,000 | — | ||||||||
| Fair value adjustment on put and call options |
(132,523 | ) | 2,869,342 | — | ||||||||
| Fair value adjustments on warrants received |
— | 37,765,718 | — | |||||||||
| Fair value adjustments on variable note receivable |
(6,608,790 | ) | (4,206,141 | ) | — | |||||||
| Fair value adjustment on convertible note payable in connection with RTO |
— | 1,981,358 | — | |||||||||
| Fair value adjustments on warrants issued |
4,159,687 | — | ||||||||||
| Fair value adjustments on contingent consideration |
(3,686,921 | ) | — | — | ||||||||
| Other |
1,252,000 | (376,139 | ) | 544,399 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total Other Income (Expense) |
$ | (10,318,936 | ) | $ | 56,417,421 | $ | 544,399 | |||||
|
|
|
|
|
|
|
|||||||
| 17. | COMMITMENTS AND CONTINGENCIES |
The Company is subject to lawsuits, investigations and other claims related to employment, commercial and other matters that arise out of operations in the normal course of business. Periodically, the Company reviews the status of each significant matter and assesses the potential financial exposure. If the potential loss from any claim or legal proceeding is considered probable, and the amount can be reliably estimated, such amount is recognized in other liabilities.
Contingent liabilities are measured at management’s best estimate of the expenditure required to settle the obligation at the end of the reporting period and are discounted to present value where the effect is material. The Company performs evaluations to identify onerous contracts and, where applicable, records contingent liabilities for such contracts.
Contingent consideration is measured upon acquisition and is estimated using probability weighting of potential payouts. Subsequent changes in the estimated contingent consideration from the final purchase price allocation are recognized in the Company’s consolidated statement of operations.
| (a) | Contingencies |
The Company’s operations are subject to a variety of local and state regulation. Failure to comply with one or more of those regulations could result in fines, restrictions on its operations, or losses of permits that could result in the Company ceasing operations in that specific state or local jurisdiction. While management of the Company believes that the Company is in compliance with applicable local and state regulations at December 31, 2019, medical cannabis regulations continue to evolve and are subject to differing interpretations. As a result, the Company may be subject to regulatory fines, penalties, or restrictions in the future.
| (b) | Claims and Litigation |
From time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. At December 31, 2018, there were no pending or
F-49
Table of Contents
| 17. | COMMITMENTS AND CONTINGENCIES (Continued) |
| (b) | Claims and Litigation (Continued) |
threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s consolidated operations. There are also no proceedings in which any of the Company’s directors, officers or affiliates is an adverse party or has a material interest adverse to the Company’s interest.
| (c) | Construction Commitments |
As of December 31, 2019, the Company held approximately $10,877,000 of open commitments to contractors on work being performed.
| 18. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT |
Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, accounts receivable, member contribution receivable, notes receivable, due from related parties, investments, accounts payable and accrued liabilities, notes payable, derivative liability, liability for acquisition of noncontrolling interest and contingent consideration payable. The Company’s financial instruments and their classifications at December 31, 2019 are as follows:
| As of December 31, 2019 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Cash and Cash Equivalents |
$ | 46,667,334 | $ | — | $ | — | $ | 46,667,334 | ||||||||
| Notes Receivable |
— | — | 815,937 | 815,937 | ||||||||||||
| Investments |
— | — | 14,068,821 | 14,068,821 | ||||||||||||
| Liability of Redemption of Noncontrolling Interest |
— | — | (5,500,000 | ) | (5,500,000 | ) | ||||||||||
| Warrant Liability |
— | — | (15,879,843 | ) | (15,879,843 | ) | ||||||||||
| Contingent Consideration Payable |
— | — | (58,936,739 | ) | (58,936,739 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 46,667,334 | $ | — | $ | (65,431,824 | ) | $ | (18,764,490 | ) | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company’s financial instruments and their classification at December 31, 2018 is as follows:
| As of December 31, 2018 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Cash and Cash Equivalents |
$ | 145,986,072 | $ | — | $ | — | $ | 145,986,072 | ||||||||
| Notes Receivable |
— | — | 10,924,727 | 10,924,727 | ||||||||||||
| Investments |
966,541 | — | 39,966,742 | 40,933,283 | ||||||||||||
| Liability for Redemption of Noncontrolling Interest |
— | — | (25,420,009 | ) | (25,420,009 | ) | ||||||||||
| Derivative Liability |
— | — | (4,238,701 | ) | (4,238,701 | ) | ||||||||||
| Contingent Consideration Payable |
— | — | (9,035,250 | ) | (9,035,250 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 146,952,613 | $ | — | $ | 12,197,509 | $ | 159,150,122 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
There have been no transfers between fair value levels during the periods ended December 31, 2019 and 2018.
F-50
Table of Contents
| 18. | FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT (Continued) |
Financial Risk Management
The Company is exposed in varying degrees to a variety of financial instrument related risks. The Board mitigates these risks by assessing, monitoring and approving the Company’s risk management processes:
| (a) | Credit Risk |
Credit risk is the risk of a potential loss to the Company if a customer or third party to a financial instrument fails to meet its contractual obligations. The maximum credit exposure at December 31, 2018 is the carrying amount of cash and cash equivalents. The Company does not have significant credit risk with respect to its customers. All cash and cash equivalents are placed with major U.S. financial institutions.
The Company provides credit to its customers in the normal course of business and has established credit evaluation and monitoring processes to mitigate credit risk but has limited risk as the majority of its sales are transacted with cash. As of December 31, 2018, the Company had approximately $1,013,000 of accounts receivable that were past due. Given management’s expectation that any credit losses will be nominal, no provision is provided.
The Company has also issued notes receivable to certain counterparties, as described in Note 5. These notes are issued to creditworthy entities with which the Company is familiar, and thus the Company expects that any credit losses will be nominal. This note was fully repaid in June 2019.
| (b) | Liquidity Risk |
Liquidity risk is the risk that the Company will not be able to meet its financial obligations associated with financial liabilities. The Company manages liquidity risk through the effective management of its capital structure. The Company’s approach to managing liquidity is to ensure that it will have sufficient liquidity at all times to settle obligations and liabilities when due.
In addition to the commitments outlined in Note 17, the Company has the following contractual obligations:
| <1 Year | 1 to 3 Years | 3 to 5 Years | >5 Years | Total | ||||||||||||||||
| Accounts Payable and Accrued Liabilities |
$ | 45,930,227 | $ | — | $ | — | $ | — | $ | 45,930,227 | ||||||||||
| Notes Payable |
206,675 | 90,942,875 | 197,319 | — | 91,346,869 | |||||||||||||||
| Construction Commitments |
10,877,000 | — | — | — | 10,877,000 | |||||||||||||||
| Contingent Consideration Payable |
50,391,181 | 8,545,558 | — | — | 58,936,739 | |||||||||||||||
| (c) | Market Risk |
Interest Rate Risk
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. Cash and cash equivalents bear interest at market rates. The Company’s financial debts have fixed rates of interest and therefore expose the Company to a limited interest rate fair value risk.
F-51
Table of Contents
| 19. | VARIABLE INTEREST ENTITIES |
The following table represents the summarized financial information about the Company’s consolidated variable interest entities (VIEs) which are included in the consolidated balance sheets and statements of operation as of December 31, 2019 and 2018. All of these entities were determined to be VIEs as the Company possesses the power to direct activities through management services agreements (MSAs).
| December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||
| Chesapeake Alternatives, LLC |
Illinois Disp, LLC |
Other Non- material VIEs |
Chesapeake Alternatives, LLC |
Illinois Disp, LLC |
Other Non- material VIEs |
|||||||||||||||||||
| Current assets |
$ | 19,455,533 | $ | 1,381,716 | $ | 1,352,935 | $ | 6,508,304 | 791,134 | $ | 1,279,049 | |||||||||||||
| Non-current assets |
22,384,663 | 3,083,659 | 2,534,297 | 1,728,594 | 2,958,845 | 78,910 | ||||||||||||||||||
| Current liabilities |
14,219,204 | 149,498 | 783,682 | 3,014,764 | 138,549 | 1,392,559 | ||||||||||||||||||
| Non-current liabilities |
1,169,989 | 137,736 | 855,440 | 10,892 | — | 3,000,000 | ||||||||||||||||||
| Noncontrolling interests |
350,206 | 2,089,071 | (22,488 | ) | 1,486,062 | 1,957,147 | (105,402 | ) | ||||||||||||||||
| Equity attributable to Green Thumb Industries Inc. |
6,645,263 | 2,089,070 | 2,270,598 | — | 1,957,147 | 1,529,037 | ||||||||||||||||||
| Revenues |
16,056,521 | 5,857,946 | 3,516,164 | 13,783,876 | 643,898 | 4,555,543 | ||||||||||||||||||
| Net income (loss) attributable to noncontrolling interests |
— | 699,624 | (112,245 | ) | 1,407,266 | 60,601 | (582,280 | ) | ||||||||||||||||
| Net income (loss) attributable to Green Thumb Industries Inc. |
1,807,229 | 699,625 | (79,402 | ) | — | 60,600 | (102,037 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net income (loss) |
$ | 1,807,229 | $ | 1,399,249 | $ | (191,647 | ) | $ | 1,407,266 | $ | 121,201 | $ | (684,317 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
VIEs included in the Other Non-material VIEs are Bluepoint Wellness of Westport LLC and Meshow, LLC as of December 31, 2019. VIEs included in the Other Non-material VIEs are KW Ventures Holdings, LLC; Meshow, LLC; and Ohio Investors 2017, LLC as of December 31, 2018.
The net change in the consolidated VIEs and Other Noncontrolling Interest are as follows for the years ended December 31, 2019 and 2018:
| Variable Interest Entities | ||||||||||||||||||||
| Chesapeake Alternatives, LLC |
Illinois Disp, LLC |
Other Non- material VIEs |
Other Noncontrolling Interests |
Total | ||||||||||||||||
| Balance as at December 31, 2017 |
$ | 170,049 | $ | — | $ | — | $ | 3,196,301 | $ | 3,366,350 | ||||||||||
| Acquisitions |
— | 1,896,546 | (164,635 | ) | — | 1,731,911 | ||||||||||||||
| Contributions |
193,628 | — | 683,513 | 21,106,610 | 21,983,751 | |||||||||||||||
| Distributions |
(95,000 | ) | — | (42,000 | ) | (40,819,508 | ) | (40,956,508 | ) | |||||||||||
| Net income (loss) |
1,217,385 | 60,601 | (582,280 | ) | 27,115,990 | 27,811,696 | ||||||||||||||
| Changes in ownership |
— | — | — | (10,439,741 | ) | (10,439,741 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as at December 31, 2018 |
1,486,062 | 1,957,147 | (105,402 | ) | 159,652 | 3,497,459 | ||||||||||||||
| Contributions |
— | — | — | 1,650,000 | 1,650,000 | |||||||||||||||
| Distributions |
(1,135,856 | ) | (567,700 | ) | — | (5,812,505 | ) | (7,516,061 | ) | |||||||||||
| Net income (loss) |
— | 699,624 | (112,245 | ) | (1,017,842 | ) | (430,463 | ) | ||||||||||||
| Changes in ownership |
— | — | — | 5,311,978 | 5,311,978 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as at December 31, 2019 |
$ | 350,206 | $ | 2,089,071 | $ | (217,647 | ) | $ | 291,283 | $ | 2,512,913 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
F-52
Table of Contents
| 19. | VARIABLE INTEREST ENTITIES (Continued) |
During 2019, the Company acquired the noncontrolling interests for Ohio Investors 2017, LLC; KW Ventures Holdings, LLC (Firefly); and GTI New Jersey, LLC. The activity for these entities is included within the Other Noncontrolling Interests column in the above table. See Note 14—Share Capital for additional discussion.
| 20. | SEGMENT REPORTING |
The Company operates in two segments: the cultivation, production and sale of cannabis products to retail stores (consumer packaged goods), and retailing of cannabis to patients and consumers (retail). The below table presents revenues by type for the years ended December 31, 2019, 2018 and 2017:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Revenues, Net of Discounts |
||||||||||||
| Consumer Packaged Goods |
$ | 109,930,160 | $ | 25,706,134 | $ | 8,375,953 | ||||||
| Retail |
137,809,904 | 41,994,791 | 9,924,970 | |||||||||
| Intersegment Eliminations |
(31,307,459 | ) | (5,207,245 | ) | (1,772,144 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total Revenues, Net of Discounts |
$ | 216,432,605 | $ | 62,493,680 | $ | 16,528,779 | ||||||
|
|
|
|
|
|
|
|||||||
| Depreciation and Amortization |
||||||||||||
| Consumer Packaged Goods |
$ | 28,670,519 | $ | 2,666,603 | $ | 526,404 | ||||||
| Retail |
2,811,821 | 2,461,117 | 111,880 | |||||||||
| Intersegment Eliminations |
— | 56,260 | 8,644 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Depreciation and Amortization |
$ | 31,482,340 | $ | 5,183,980 | $ | 646,928 | ||||||
|
|
|
|
|
|
|
|||||||
| Income Taxes |
||||||||||||
| Consumer Packaged Goods |
$ | 3,727,292 | $ | 2,245,450 | $ | — | ||||||
| Retail |
9,905,876 | 4,938,145 | 214,000 | |||||||||
| Intersegment Eliminations |
(4,289,135 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total Income Taxes |
$ | 9,344,033 | $ | 7,183,595 | $ | 214,000 | ||||||
|
|
|
|
|
|
|
|||||||
The following table presents total assets by type as of December 31, 2019 and 2018:
| Year Ended December 31, | ||||||||
| 2019 | 2018 | |||||||
| Consumer Packaged Goods |
$ | 648,080,341 | $ | 104,514,162 | ||||
| Retail |
164,308,187 | 105,293,320 | ||||||
| Corporate |
1,961,227,868 | 209,879,995 | ||||||
| Intersegment Eliminations |
(1,606,079,772 | ) | (1,338,911 | ) | ||||
|
|
|
|
|
|||||
| Total Assets |
$ | 1,167,536,624 | $ | 418,348,566 | ||||
|
|
|
|
|
|||||
Goodwill assigned to the Consumer Packaged Goods segment as of December 31, 2019 and 2018 was $255,211,232 and $23,918,000, respectively. Intangible assets, net assigned to the Consumer Packaged Goods segment as of December 31, 2019 and 2018 was $228,795,692 and $46,540,000, respectively.
Goodwill assigned to the Retail segment as of December 31, 2019 and 2018 was $119,873,759 and $15,286,360, respectively. Intangible assets, net assigned to the Retail segment as of December 31, 2019 and 2018 was $207,002,644 and $41,825,678, respectively.
F-53
Table of Contents
| 20. | SEGMENT REPORTING (Continued) |
The Company’s assets are aggregated into two reportable segments (Retail and Consumer Packaged Goods). For the purposes of testing goodwill, GTI has identified 22 reporting units. The Company analyzed its reporting units by first reviewing the operating segments based on the geographic areas in which GTI conducts business (or each market). The markets were then further divided into reporting units based on the market operations (Retail and Consumer Packaged Goods) which were primarily determined based on the licenses each market holds. All revenues are derived from customers domiciled in the United States and all assets are located in the United States.
| 21. | SUBSEQUENT EVENTS |
| (a) | Sale and Leaseback Transaction |
On January 31, 2020, the Company closed on a sale and lease back transaction to sell its Toledo, Ohio processing facility to an unrelated third party. GTI will lease back the facility via a long-term agreement and continue to operate and manage it. The purchase price for the property was $2.9 million, excluding transaction costs. GTI is also expected to make certain improvements to the property that will significantly enhance production capacity, for which GTI will be reimbursed up to $4.3 million. Assuming full reimbursement for such improvements, the total investment in the property will be $7.2 million.
On March 6, 2020, the Company closed on a sale and lease back transaction to sell its Oglesby, Illinois cultivation and processing facility to an unrelated third party. GTI will lease back the facility via a long-term agreement and continue to operate and manage it. The purchase price for the property was $9.0 million, excluding transaction costs. GTI is also expected to make certain improvements to the property that will significantly enhance production capacity, for which GTI will be reimbursed up to $41.0 million. Assuming full reimbursement for such improvements, the total investment in the property will be $50.0 million.
| (b) | COVID-19 Global Pandemic |
On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic and recommended containment and mitigation measures worldwide. The Company is monitoring this closely, and although operations have not been materially affected by the COVID-19 outbreak to date, the ultimate severity of the outbreak and its impact on the economic environment is uncertain. Operations of the Company are currently ongoing as the cultivation, processing and sale of cannabis products is currently considered an essential business by the states in which we operate with respect to all customers (or medical patients only in Massachusetts). The uncertain nature of the spread of COVID-19 globally may impact our business operations for reasons including the potential quarantine of our employees, customers, and third-party service providers. At this time, the Company is unable to estimate the impact of this event on its operations.
F-54
Table of Contents
| 22. | QUARTERLY FINANCIAL DATA (UNAUDITED) |
The following table contains selected quarterly data for 2019 and 2018. The information should be read in conjunction with the Company’s financial statements and related notes included elsewhere in this report. The Company believes that the following information reflects all normal recurring adjustments necessary for a fair presentation of the information for the periods presented. The operating results for any quarter are not necessarily indicative of results for any future period.
| First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
Full Year |
||||||||||||||||
| 2019 |
||||||||||||||||||||
| Net Sales |
$ | 27,913,163 | $ | 44,726,777 | $ | 67,990,907 | $ | 75,801,758 | $ | 216,432,605 | ||||||||||
| (Loss) Income from operations |
(13,629,660 | ) | (9,326,730 | ) | 1,376,718 | (6,111,030 | ) | (27,690,702 | ) | |||||||||||
| Net loss attributable to Green Thumb Industries Inc. |
(9,563,056 | ) | (20,892,049 | ) | (14,590,793 | ) | (14,070,509 | ) | (59,116,407 | ) | ||||||||||
| Net loss per share |
(0.06 | ) | (0.11 | ) | (0.07 | ) | (.07 | ) | (0.31 | ) | ||||||||||
| Weighted average number of common shares outstanding |
167,171,886 | 182,261,947 | 204,709,085 | 207,666,666 | 190,602,400 | |||||||||||||||
| 2018 |
||||||||||||||||||||
| Net Sales |
$ | 10,925,898 | $ | 13,624,658 | $ | 17,171,710 | $ | 20,771,414 | $ | 62,493,680 | ||||||||||
| Income from operations |
(622,698 | ) | (3,325,725 | ) | (5,598,127 | ) | (16,793,608 | ) | (26,340,158 | ) | ||||||||||
| Net (loss) earnings attributable to Green Thumb Industries Inc. |
(1,012,009 | ) | 2,595,022 | (3,523,222 | ) | (3,303,708 | ) | (5,243,917 | ) | |||||||||||
| Net (loss) earnings per share |
(0.01 | ) | 0.02 | (0.03 | ) | (0.02 | ) | (0.04 | ) | |||||||||||
| Weighted average number of common shares outstanding |
107,528,100 | 113,492,970 | 142,365,356 | 151,274,619 | 130,102,523 | |||||||||||||||
F-55
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Green Thumb Industries Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Green Thumb Industries Inc. (the “Company”) as of December 31, 2019, and the related consolidated statement of operations, changes in shareholders’ equity, and cash flows for the year then ended December 31 2019, and the related notes (collectively referred to as the “consolidated financial statements”).
In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2019, and the consolidated results of its operations and its cash flows for the year then ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Other Matter
The consolidated financial statements of the Company for the year ended December 31, 2018 were audited by another auditor.
Changes in Accounting Principles
As discussed in Note 1 to the consolidated financial statements, the Company has changed its accounting method of accounting for leases on January 1, 2019, due to the adoption of Financial Accounting Standard Board’s Accounting Standards Codification 842, Leases, and related amendments.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
F-56
Table of Contents
We have served as the Company’s auditor since 2019.
/s/ Macias Gini & O’Connell LLP
Los Angeles, California
April 15, 2020
F-57
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Green Thumb Industries Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Green Thumb Industries Inc. (the “Company”) as of December 31, 2018, and the related consolidated statement of operations, changes in shareholders’ equity, and cash flows for the year then ended, and the related notes (collectively referred to as the consolidated financial statements).
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Other Matter
The combined financial statements of the Company for the year ended December 31, 2017 were audited by another auditor.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
| ||
| December 16, 2019 |
Chartered Professional Accountants | |
| Toronto, Ontario |
Licensed Public Accountants |
We have served as the Company’s auditor since 2018.

F-58
Table of Contents

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Members and Board of Directors of
Green Thumb Industries (GTI) Group of Companies
Opinion on the Combined Financial Statements
We have audited the accompanying combined statements of operations, changes in members’ equity, and cash flows of Green Thumb Industries (GTI) Group of Companies (the “Company”) for the year ended December 31, 2017, and the related notes (collectively referred to as the “combined financial statements”).
In our opinion, the combined financial statements present fairly, in all material respects, the combined results of its operations of the Company and its cash flows for the year ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These combined financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s combined financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the combined financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the combined financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the combined financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the combined financial statements. We believe that our audit provides a reasonable basis for our opinion.
We have served as the Company’s auditor since 2019.
| /s/ Macias Gini & O’Connell LLP |
| Los Angeles, California |
| December 20, 2019 |
F-59
