Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Apyx Medical Corp | a2019q410-kexhibit322.htm |
| EX-32.1 - EXHIBIT 32.1 - Apyx Medical Corp | a2019q410-kexhibit321.htm |
| EX-31.2 - EXHIBIT 31.2 - Apyx Medical Corp | a2019q410-kexhibit312.htm |
| EX-31.1 - EXHIBIT 31.1 - Apyx Medical Corp | a2019q410-kexhibit311.htm |
| EX-23.2 - EXHIBIT 23.2 - Apyx Medical Corp | exhibit232fdconsent.htm |
| EX-23.1 - EXHIBIT 23.1 - Apyx Medical Corp | exhibit231bdoconsent.htm |
| EX-21.1 - EXHIBIT 21.1 - Apyx Medical Corp | a2019q410-kexhibit211.htm |
| EX-14.1 - EXHIBIT 14.1 - Apyx Medical Corp | apyxmedicalcodeofbusines.htm |
| EX-4.2 - EXHIBIT 4.2 - Apyx Medical Corp | apyxdescriptionofsecurit.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019 |
or |
o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _____ to _____ |
Commission File Number: 0-12183 |
 |
APYX MEDICAL CORPORATION |
(Exact name of registrant as specified in its charter) |
Delaware | 11-2644611 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
5115 Ulmerton Road, Clearwater, FL 33760
(Address of principal executive offices, zip code)
(727) 384-2323
(Issuer’s telephone number)
Securities registered pursuant to Section 12(b) of the Act:
Title of each Class | Trading Symbol | Name of each Exchange on which registered | ||
Common Stock, $.001 Par Value | APYX | NASDAQ Stock Market LLC | ||
Securities registered under Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes: o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes: o No ý
Indicate by check mark whether the registrant (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes: ý No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes: ý No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company” and "emerging growth company" in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer | o | Accelerated filer | ý | |
Non-accelerated filer | o | (Do not check if a smaller reporting company) | Smaller reporting company | ý |
Emerging growth company | o | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. | o | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes: o No ý
The aggregate market value of the common stock held by non-affiliates and non-voting equity held by non-affiliates computed by reference to the price at which the common stock was last sold, or the average bid and asked prices of such common stock as of June 30, 2019, the registrant’s most recently completed second fiscal quarter, was approximately $226.5 million.
As of March 27, 2020, 34,174,314 shares of the registrant’s $.001 par value common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None.
December 31, 2019
Part I | Page | |||
Item 1 | ||||
Item 1A | ||||
Item 1B | ||||
Item 2 | ||||
Item 3 | ||||
Item 4 | ||||
Part II | ||||
Item 5 | ||||
Item 6 | ||||
Item 7 | ||||
Item 7A | ||||
Item 8 | ||||
Item 9 | ||||
Item 9A | ||||
Item 9B | ||||
Part III | ||||
Item 10 | ||||
Item 11 | ||||
Item 12 | ||||
Item 13 | ||||
Item 14 | ||||
Part IV | ||||
Item 15 | ||||
Signatures | ||||
i
APYX MEDICAL CORPORATION
Cautionary Notes Regarding “Forward-Looking” Statements
We have included or incorporated by reference into this report, and from time to time may make in our public filings, press releases or other public statements, certain statements that may constitute forward-looking statements. These include without limitation those under “Business” in Part I, Item 1, “Risk Factors” in Part I, Item 1A, “Legal Proceedings” in Part I, Item 3, “Management's Discussion and Analysis of Financial Condition and Results of Operations” in Part II, Item 7, and “Quantitative and Qualitative Disclosures about Market Risk” in Part II, Item 7A. In addition, our management my make forward-looking statements to analysts, investors, representatives of the media and others. These forward-looking statements are not historical facts and represent only our beliefs regarding future events, many of which, by their nature, are inherently uncertain and beyond our control. We may, in some cases, use words such as “project”, “believe”, “anticipate”, “plan”, “expect”, “estimate”, “intend”, “should”, “would”, “could”, “potentially”, “may” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements.
In connection with the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we are identifying important factors that, individually or in the aggregate, could cause actual results to differ materially from those contained in any forward- looking statements made by us. Any such forward-looking statements are qualified by reference to the following cautionary statements.
Forward-looking statements in this report are subject to a number of risks and uncertainties, some of which are beyond our control, including, among other things:
• | changes in general economic, business or demographic conditions or trends in the U.S. or throughout the world or changes in the political environment, including changes in GDP, interest rates and inflation; |
• | our ability to conclude a sufficient number of attractive growth projects, deploy growth capital in amounts consistent with our objectives in the prosecution of those and achieve targeted risk-adjusted returns on any growth project, including the commercialization of our Helium Plasma technology; |
• | the regulatory environment, including our ability to gain requisite approval from the Food and Drug Administration and other governmental and regulatory bodies, and the ability to estimate compliance costs, comply with any changes thereto, rates implemented by regulators, and our relationships and rights under and contracts with governmental agencies and authorities; |
• | disruptions or other extraordinary or force majeure events and the ability to insure against losses resulting from such events or disruptions, including disruptions caused by the Corona virus; |
• | sudden or extreme volatility in commodity prices and availability; |
• | changes in competitive dynamics affecting our business and the medical device industry as a whole; |
• | technological innovations leading to increased competition in the medical device industry; |
• | changes in healthcare policy; |
• | our ability to make alternate arrangements to account for any disruptions or shutdowns that may affect suppliers’ facilities or the operations upon which our business is dependent; |
• | continued aggressive EPA state regulation of Ethylene oxide sterilization (EtO) commercial plants resulting in additional plant closures, leading to a reduced availability of our handpieces, which are commercially sterilized; |
• | our ability to implement operating and internal growth strategies; |
• | environmental risks, including the impact of climate change and weather conditions; |
• | the impact of weather events, including potentially hurricanes, tornadoes and/or seasonal extremes; |
• | unplanned outages and/or failures of technical and mechanical systems; |
• | cybersecurity breaches impacting critical systems or data; |
• | work interruptions or other labor stoppages; |
Our actual results, performance, prospects or opportunities could differ materially from those expressed in or implied by the forward-looking statements. A description of risks that could cause our actual results to differ appears under the caption “Risk Factors” in Part I, Item 1A and elsewhere in this report. It is not possible to predict or identify all risk factors and you should not consider that description to be a complete discussion of all potential risks or uncertainties that could cause actual results to differ.
In light of these risks, uncertainties and assumptions, you should not place undue reliance on any forward-looking statements. The forward-looking events discussed in this report may not occur. These forward-looking statements are made as of the date of this report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. You should, however, consult further disclosures we may make in future filings with the Securities and Exchange Commission (SEC). Past performance is not an indicator of future results.
ii
APYX MEDICAL CORPORATION
PART I
ITEM 1. Business
General
Apyx Medical Corporation (“Company”, “Apyx Medical”, “we”, “us”, or “our”), formerly known as Bovie Medical Corporation, was incorporated in 1982, under the laws of the State of Delaware and has its principal executive office at 5115 Ulmerton Road, Clearwater, FL 33760.
We are an advanced energy technology company with a passion for elevating people’s lives through innovative products in the cosmetic and surgical markets. Known for our innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients we serve. Our Helium Plasma Technology is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers plastic surgeons, fascial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision and virtually eliminating unintended tissue trauma. We also leverage our deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers.
On August 30, 2018, we closed on a definitive asset purchase agreement with Specialty Surgical Instrumentation Inc., a Tennessee Corporation and wholly-owned subsidiary of Symmetry Surgical Inc. (“Symmetry”), pursuant to which we divested and sold our electrosurgical "Core" business segment and related intellectual property, including the Bovie® brand and trademarks, to Symmetry for gross proceeds of $97 million in cash. The divestiture and sale of our Core business segment to Symmetry allows us to further focus on our strategic objective of commercializing our Helium Plasma Technology, including the expansion of our Renuvion® brand in the cosmetic surgery market.
In connection with the asset purchase agreement, we also entered into an Electrosurgical Disposables and Accessories, Cauteries and Other Products Supply Agreement with Symmetry for up to a four-year term, whereby we will manufacture certain Core products and sell them to Symmetry at agreed upon prices. Any revenue, costs and expenses resulting from this agreement are netted and reported in our Consolidated Statements of Operations as Other gains or losses.
In connection with the asset purchase agreement, we also entered into a Manufacture and Supply Agreement with Symmetry for a ten-year term, whereby we will manufacture certain products and sell them to Symmetry at agreed upon prices. Revenue, costs and expenses resulting from this agreement are reported in our Consolidated Statements as income or loss from operations of our OEM reporting segment.
Our objective is to achieve profitable, sustainable growth by increasing our market share in the advanced energy category, including the commercialization of products that have the potential to be transformational with respect to the results they produce for surgeons and patients. In order to achieve this objective, we plan to leverage our long history in the industry, along with the reputation for quality and reliability that our brand enjoys within the medical community.
Significant Subsidiaries
Apyx Bulgaria, EOOD, formerly known as Bovie Bulgaria, is a wholly-owned limited liability company incorporated under Bulgarian law, located in Sofia, Bulgaria. It is engaged in the business of development and manufacturing of our advanced energy generators, as well as the manufacturing of disposable hand piece subassemblies and OEM generators and accessories. The facility also distributes products directly to customers in certain international markets and provides warranty and repair services.
Industry
The cosmetic surgery market is a special segment of the medical field which is involved in the restoration, reconstruction, or alteration of the human body so as to enhance the body’s appearance. The market for cosmetic surgery includes surgical, minimally invasive and nonsurgical cosmetic procedures. This market is expected to have steady growth year over year and this growth is driven by social and cultural factors such as influence of social media, peer pressure for appearance and beauty, and increasing disposable income.
We believe that Apyx Medical has sustainable, competitive advantages in the cosmetic market for several reasons: our long history of developing unique energy devices to meet the needs of physicians, our unique Helium Plasma Technology, and our outstanding
1
APYX MEDICAL CORPORATION
product quality supported by strong engineering and research and development capabilities. We believe that our equipment and devices have and will continue to improve the lives of doctors and their patients.
Intellectual Property
We rely on our intellectual property that we have developed or acquired over the years including patents, trade secrets, technical innovations and various licensing agreements to provide our future growth and build our competitive position. We have been issued 37 patents in the United States and 26 foreign patents. We have 16 pending patent applications in the United States and 26 pending foreign applications. We have 6 US registered trademarks and 2 pending US trademark applications. As we continue to expand our intellectual property portfolio we believe it is critical for us to continue to invest in filing patent applications to protect our technology, inventions and improvements. However, we can give no assurance that competitors will not infringe on our patent rights or otherwise create similar or non-infringing competing products that are technically patentable in their own right.
Manufacturing and Suppliers
We are committed to producing the most technically advanced and highest quality products of their kind available on the market. We manufacture the majority of our products on our premises in Clearwater, Florida and at our facility located in Sofia, Bulgaria, which are certified under the ISO international quality standards and are subject to continuing regulation and routine inspections by the FDA to ensure compliance with regulations relating to our quality system, medical device complaint reporting and adherence to FDA restrictions on promotion and advertising. In addition, we are subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act and other federal, state and local regulations.
Our wholly-owned subsidiary, Apyx Bulgaria, EOOD operates an approximately 20,000 square foot ISO13485 certified and FDA registered manufacturing facility located in the capital city of Sofia, which houses manufacturing, development and assembly operations.
We maintain collaborative arrangements with three foreign suppliers, including our contract component manufacturer located in Ningbo, China, under which we request the development of certain products which we purchase pursuant to purchase orders. Our purchase order commitments are never more than one year in duration and are supported by our sales forecasts. During late 2019, we entered into a joint venture with our Chinese supplier to establish a foundation for the manufacturing and sale of our products into the Chinese market. The joint venture is in an unfunded startup phase, and accordingly, the activity associated with it is not material.
Backlog
The value of unshipped factory orders is not material.
Employees
At December 31, 2019, we had 266 full-time employees world-wide, of whom 4 were executive officers, 27 supervisory personnel, 34 sales personnel and 201 technical support, administrative and production employees. None of our current employees are covered by a collective bargaining agreement and we have never experienced a work stoppage.
2
APYX MEDICAL CORPORATION
Our Two Business Segments
We currently have two reportable operating segments: Advanced Energy and OEM. The Corporate and Other category includes certain unallocated corporate and administrative costs which are not specifically attributed to any reportable segment. Net assets are shared, therefore, not allocated to the reportable segments.
For the year ended December 31, 2019, our OEM segment contributed 19.7% of our consolidated total revenue and our Advanced Energy segment contributed 80.3% of our consolidated total revenue.
Advanced Energy Segment
Overview
Our product portfolio consists of our Helium Plasma Technology that is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers plastic surgeons, facial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision, virtually eliminating unintended tissue trauma. This technology has US FDA clearance, CE mark, and clearance for sale in multiple other countries and is generally indicated for the cutting, coagulation and ablation of soft tissue. The system consists of an electrosurgical generator unit (ESU), a handpiece and a supply of helium gas. The proprietary radiofrequency (RF) energy is delivered to the handpiece by the ESU and used to energize an electrode. When helium gas passes over the energized electrode, helium plasma is generated which allows for conduction of the RF energy from the electrode to the patient in the form of a precise helium plasma beam. The energy delivered to the patient via the helium plasma beam is very precise and cooler in temperature in comparison to other surgical energy modalities such as standard RF monopolar energy. This technology has been the subject of eight white papers and ten peer-reviewed, published journal articles and book chapters. It also continues to be the subject of numerous presentations at traditional and cosmetic surgery conferences around the world.
This technology initially received FDA clearance in 2012 and a CE mark in December, 2014, which enables us to sell the product in the European Union. In 2014, we created and trained a direct sales force dedicated to sell this technology. In 2015, we continued the commercialization process for our helium plasma technology with a multi-faceted strategy designed to accelerate adoption of the product. This strategy primarily involved deployment of a dedicated sales force, developing product line extensions and expanding the specialties in which this technology can become the “standard of care“ for certain procedures.
During 2019, we continued our full scale commercialization efforts for Renuvion®. As of December 31, 2019 we had a direct sales force of 30 field-based selling professionals and a network of 4 independent sales agencies. We also had 4 sales managers. This selling organization is focused on the use of Renuvion® in the cosmetic surgery market. In addition, we have invested in training programs and marketing-related activities to support accelerated adoption of Renuvion® into physicians' practices.
From 2015 through 2019, we launched numerous new extensions to our helium plasma product lines in an effort to target new surgical procedures, users, and markets. As a result of our sales, marketing and product development initiatives, we have significantly increased the number of physicians using our Helium Plasma Technology by expanding usage to include the cosmetic surgery market in the US, and the cosmetic surgery market as well as the surgical oncology market outside the US.
In order to assist us in leveraging our Helium Plasma Technology's precision and effectiveness in multiple surgical specialties, in 2019 we added 4 additional doctors to our Medical Advisory Board, bringing the total number to 6 members representing the plastic surgery, facial plastic surgery, and cosmetic procedure specialties.
In 2019, our commercial strategy in the U.S. was primarily focused on advancing the usage of Renuvion® in the cosmetic surgery market. In our international markets, we focused on both the cosmetic surgery and on our J-Plasma® technology for the surgical oncology market. Also in 2019, we continued a clinical and regulatory strategy to support our market focus. Once complete, we will launch a corresponding marketing campaign.
We are continuing to make substantial investments in the development and marketing of our Renuvion® technology for the long-term benefit of the Company and its stakeholders and this may adversely affect our short term profitability and cash flow, particularly over the next 12 to 24 months. While we believe that these investments have the potential to generate additional revenues and profits in the future, there can be no assurance that our Helium Plasma Technology will be successful or that such future revenues and profitability will be realized.
3
APYX MEDICAL CORPORATION
Customers
In the US, we primarily sell our Renuvion® products through our direct sales force to physicians, cosmetic surgery offices and surgical centers. Outside of the US, all of our products are sold through our distributor network.
Products
During 2019, Advanced Energy Products consisted of our Helium Plasma Technology lines (Renuvion® and J-Plasma®). These product lines consist of a multifunction generator, a handpiece and a supply of helium gas. Radiofrequency (RF) energy is delivered to the handpiece by the generator and used to energize an electrode. When helium gas passes over the energized electrode, helium plasma is generated which allows for conduction of the RF energy from the electrode to the patient in the form of a precise helium plasma beam. The energy delivered to the patient via the helium plasma beam is very precise and cooler in temperature in comparison to other surgical energy modalities such as standard RF monopolar energy.
Helium Plasma Generator
In June 2017, we launched the newest version of the Apyx Ultimate™ generator. The Apyx Ultimate 2.0 is a high frequency electrosurgical generator that can be used for delivery of RF energy and/or helium plasma to cut, coagulate and ablate soft tissue during open and laparoscopic surgical procedures. The generator offers the users monopolar, bipolar and helium plasma energy in a single unit. It also powers the Cool-Coag technology that has been incorporated into the new Precise Open handpieces that were released in December 2017. These new product releases continue to expand the procedure base for our Helium Plasma Technology by providing surgeons with the tools they need to access additional anatomic locations and perform specific procedures.
Disposable Portfolio
We offer a variety of different hand pieces for open and laparoscopic procedures. The helium-based plasma generated from these devices have been shown to provide increased precision and control and cause less thermal damage to tissue than CO2 laser, argon plasma and RF energy products currently available on the market. The technology has a general indication and can be used for cutting, coagulating and ablating soft tissue. The two primary specialties that are targeted are the cosmetic surgery and surgical oncology markets. The advantages of helium plasma continue to be studied throughout the medical and scientific communities. We believe that surgical applications are just one area of opportunity for this technology.
Competition
Currently, we are the only company with helium-based plasma and retractable blade products. However, there are RF based competitors, argon plasma competitors, and CO2 laser competitors for our target market. We believe our competitive position did not change in 2019.
OEM Segment
Overview
The Company leverages its expertise in the design, development and manufacturing of electrosurgical equipment by producing generators and related accessories for large, well-known medical device manufacturers through original equipment manufacturing (OEM) agreements, as well as start-up companies with the need for our energy based designs. In connection with the Asset Purchase Agreement with Symmetry Surgical we entered into a Manufacturing and Supply Agreement for a ten-year term, whereby we will manufacture certain products and sell to them at agreed upon prices. Revenue, costs and expenses resulting from this agreement are reported in our Consolidated Statements of Operations as a component of income or loss from operations of our OEM reporting segment.
4
APYX MEDICAL CORPORATION
ITEM 1A. Risk factors
In addition to risks and uncertainties in the ordinary course of business, important risk factors that may affect us are discussed below. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impact or impair our business operations.
Risks Related to Our Industry
The energy-based medical device industry in the aesthetics market is highly competitive and we may be unable to compete effectively.
The energy-based medical device industry for the aesthetics market is highly competitive. Many competitors in this industry are well-established, do a substantially greater amount of business and have greater financial resources and facilities than we do.
We have invested and continue to invest, substantial resources to develop and monetize our J-Plasma®/Renuvion® technology. We believe we must continue to develop new applications for our products and obtain new indications for use in order to stay competitive. If we are unable to gain acceptance of our technology in the marketplace, or obtain new indications for use, our business and results of operations and cash flows may be materially and adversely affected.
We also compete by private labeling our products for major distributors under their label. This allows us to increase our position in the marketplace and thereby compete from two different approaches, our Apyx label and our customers’ private label. Our private label customers distribute our products under their name through their internal sales force. We believe our main competitors do not private label their products.
Maintaining strong relationships with plastic surgeons, cosmetic physicians, and other healthcare professionals, is critical to the success of our product commercialization strategy.
Part of our strategy depends on developing strong working relationships with key plastic surgeons, cosmetic physicians and other healthcare professionals. The guidance we get from these relationships is important from both a commercialization strategy and product development standpoint. Without these relationships, the development and commercialization of our products could suffer which could have a material adverse impact on our business.
We rely on our internal sales force and sales partners to market and sell our products. If we are unable to hire, effectively train, and retain these professionals, future sales and profitability may be adversely affected.
The implementation of our growth strategy largely depends on our ability to hire, train, and retain our sales professionals. We train our sales professionals to thoroughly understand our Helium Plasma Technology and the marketplace in which we compete, including how our technologies can increase our customer's revenue and the results they are able to achieve for their patients. It may take time for these sales professionals to become productive and there can be no assurance that recently recruited sales professionals will be adequately trained in a timely manner, that our direct sales productivity will improve, or that we will not experience significant levels of attrition in the future.
If we are unable to successfully introduce new products or fail to keep pace with competitive advances in technology, our business, financial condition and results of operations could be adversely affected. In addition, our research and development efforts rely upon investments and alliances and we cannot guarantee that any previous or future investments or alliances will be successful.
Our research and development activities are an essential component of our efforts to develop new and innovative products for introduction in the marketplace. New and improved products play a critical role in our sales growth. We continue to place emphasis on the development of proprietary products, such as our J-Plasma®/Renuvion® technology, and product improvements to complement and expand our existing product lines. We maintain close working relationships with physicians and medical personnel in hospitals and universities who assist in product research and areas of development.
These activities are primarily conducted internally and are expensed as incurred. Expenses include direct expenses for wages, materials and services associated with the development of our products net of any reimbursements from customers. Research and development expenses do not include any portion of general and administrative expenses. Our research and development activities are conducted at our Clearwater, Florida and Sofia, Bulgaria facilities. We expect to continue making future investments to enable
5
APYX MEDICAL CORPORATION
us to develop and market new technologies and products to further our strategic objectives and strengthen our existing business. However, we cannot guarantee that any of our previous or future investments in both facilities will be successful or that our new products will gain market acceptance, the failure of which would have a material adverse effect on our business and results of operations.
The amount expended by us on research and development of our products during the years 2019, 2018 and 2017, totaled approximately $3.7 million, $2.5 million and $1.9 million, respectively. During the past three years, we invested substantial resources in the development and marketing of our Advanced Energy product technology. We have not incurred any direct costs relating to environmental regulations or requirements. For 2020, we expect to invest approximately 10% to 15% of revenue for research and development activities.
Even if we are successful in developing new, or enhancing our existing products, there are various circumstances that could prevent their successful commercialization.
Our ability to successfully commercialize our products will depend on a number of factors, any of which could delay or prevent commercialization, including:
•our inability to obtain the necessary regulatory clearances or approvals for expanded indications, new products,
or product modifications;
•we are unable to demonstrate, if required, the safety and efficacy of new products with data from preclinical
studies and clinical trials;
•our product is determined to be ineffective or unsafe following approval and is removed from the market or we
are required to perform additional research and development to further prove the safety and effectiveness of the
product before re-entry into the market;
•the regulatory approvals of our new products are delayed (or denied) or we are required to conduct further
research and development of our products prior to receiving regulatory approval;
•we are unable to build and maintain a sales and marketing group to successfully launch and sell our new products;
•we are required to allocate available funds to litigation matters;
•the needs of our physicians or their patients are not sufficiently met;
•we are unable to manufacture the quantity of product needed (in accordance with quality manufacturing
standards) to meet market demand, or at all;
•competition from other products or technologies prevents or reduces market acceptance of our products;
•we do not have and cannot obtain the intellectual property rights needed to manufacture or market our products
without infringing on another company’s patents; or
•we are unsuccessful in defending against patent infringement or other intellectual property rights, claims that
could be brought against us, our products or technologies;
The failure to successfully commercialize products will have a material and adverse effect on the future growth of our business, financial condition and results of operations.
6
APYX MEDICAL CORPORATION
Our industry is highly regulated by the U.S. Food and Drug Administration and international regulatory authorities, as well as other governmental, state and federal agencies which have substantial authority to establish criteria which must be complied with in order for us to continue in operation.
United States
Our products and research and development activities are subject to regulation by the FDA and other regulatory bodies. FDA regulations govern, among other things, the following activities:
•Product development
•Product testing
•Product labeling
•Product storage
•Pre-market clearance or approval
•Advertising and promotion
•Product traceability
•Product indications
•Post Market performance (complaints, adverse events, field actions)
In the United States, medical devices are classified by the FDA on the basis of control deemed necessary to reasonably ensure the safety and effectiveness of the device.
Currently, we only manufacture Class I and Class II devices. Our "Class II" devices require Pre-Market Notification, otherwise known as a "510(k) clearance". A 510(k) is a premarket submission made to FDA to demonstrate that the device to be marketed is at least as safe and effective, that is, substantially equivalent, to a legally marketed device that is not subject to PMA. Submitters must compare their device to one or more similar legally marketed devices and make and support their substantial equivalence claims. Before marketing a device, each submitter must receive an order, in the form of a letter, from FDA which finds the device to be substantially equivalent (SE) and states that the device can be marketed in the U.S. This order "clears" the device for commercial distribution.
International Regulation
To market products in the European Union, our products must bear the “CE” mark. Manufacturers of medical devices bearing the CE mark have gone through a conformity assessment process that assures that products are manufactured in compliance with a recognized quality system in the areas of design, development and manufacturing requirements and that they comply with the European Medical Devices Directive (MDD).
Each device that bears a CE mark has associated technical documentation that includes a description of the following:
•Description of the device and its components,
•A Summary of how the device complies with the essential requirements of the Medical Devices Directive,
•Safety (risk assessment) and performance of the device,
•Clinical evaluations with respect to the device,
•Methods, facilities and quality controls used to manufacture the device and
•Proposed labeling for the device.
Manufacturing and distribution of a device is subject to ongoing surveillance by the appropriate regulatory body to ensure continued compliance with quality system and reporting requirements.
The European Union’s (EU) Medical Device Regulation (MDR), officially passed in April 2017, represents the first major changes to the EU medical device regulatory environment in more than 20 years. The new EU MDR has significantly raised the compliance bar for the medical device industry and will cause significant changes to the regulatory obligations of legal manufacturers, importers and distributors involved in the medical device distribution chain. Enforcement of this new regulation will transition in over the next two years. The EU MDR is far more complex than the existing Medical Devices Directive (MDD) and it presents new challenges for manufacturers. Classification has changed for some product categories and strict new requirements have been imposed on clinical data, risk management, post market surveillance, and supplier management.
7
APYX MEDICAL CORPORATION
Outside of the European Union, regulations vary significantly from country to country and are becoming increasingly stringent and country specific. Territories and countries around the world continue to develop their own unique regulatory requirements and these individual governments are passing laws that enforce these new regulations and also imposing fees to register products in their country. The time and effort required to obtain approval to market products may be longer or shorter than that required in the United States or the European Union. Certain European countries outside of the European Union and other countries in the world do not recognize the CE mark certification or FDA clearance and have their own regulatory requirements to register and sell products in these territories. We are required to meet regulatory requirements and obtain registrations for our products in all countries that have these regulatory requirements prior to selling our products in that country.
Environmental Regulation
The medical device industry continues to be the subject of intense scrutiny and stringent regulation and the demand for green, sustainable products is rapidly increasing. There are increasing requirements for efficient and accurate processes for hazardous substance handling, supplier disclosures and regulatory reporting in order to comply with numerous global health and environmental regulatory requirements and restrictions, including but not limited to:
•Restriction on Hazardous Substances (RoHS) Directive
•Packaging and Packing Waste Directive
•REACH Regulation
•Proposition 65
•Hazardous Air Pollutants: Ethylene Oxide
Compliance with existing and future environmental regulations may have an impact on the manufacturing and sterilization of our medical devices. Environmental regulations in the US and EU limit or prohibit the use of certain chemicals, substances and materials in the manufacture of our medical devices such as Prop 65 in California and others in the EU such as REACH, RoHS, and WEEE Directive. With the current global concerns over climate change and the tangible effects human beings are having on the environment, there is no doubt that the amount of environmental legislation is primed to increase still further, with EU being at the forefront of this movement.
Ethylene oxide is used to sterilize approximately 50% of medical devices in the U.S. While some alternative methods currently exist, potential device incompatibility issues exist with these alternatives. The U.S. Environmental Protection Agency (EPA) classified EtO as a carcinogen after linking it to cases of breast cancer, lymphoma and leukemia. Over the course of 2019, public concerns about the emissions from sterilization facilities using EtO resulted recently in the permanent closure of two facilities (one in Illinois and the other in Michigan) as well as temporary closures for at least two facilities in Georgia and a new facility at risk in Illinois. In addition, the Illinois state legislature is considering a bill that would phase out hospital in-house use of EtO and require EtO commercial sterilization facilities operating within the state to relocate to “scarcely populated areas.” Currently, shortages due to current closures are not expected, but any additional commercial sterilization facility closures could result in shortages for certain devices. Our devices are not currently impacted by these closures however, it is unknown if the current EtO facilities utilized by Apyx Medical could be impacted in the future.
The FDA is closely monitoring the supply chain effects of closures and potential closures of certain facilities that use ethylene oxide to sterilize medical devices prior to their use. The Agency is concerned about the future availability of sterile medical devices and the potential for medical device shortages that might impact patient care. However, the FDA does not have oversight authority over ethylene oxide emissions, that responsibility is within the purview of the Environmental Protection Agency.
Anti-Corruption Regulation
As we grow our international presence and global operations, we will be increasingly exposed to statutes, anti-corruption trade policies, economic sanctions and other restrictions imposed by the United States and other foreign governments and organizations, including the U.S. Foreign Corrupt Practices Act, or the FCPA, and other federal statutes and regulations, including those established by the Office of Foreign Assets Control, or OFAC. In addition, other foreign statutes, such as the U.K. Bribery Act of 2010, or the Bribery Act, prohibits both domestic and international bribery, as well as bribery across both private and public sectors.
8
APYX MEDICAL CORPORATION
We have implemented policies and procedures designed to ensure compliance by our directors, officers, employees, representatives, consultants and agents with the FCPA, OFAC restrictions, the Bribery Act and other export control, anti-corruption, anti-money-laundering and anti-terrorism laws and regulations. However, there can be no assurance that our policies and procedures are or will be sufficient to prevent violations from occurring. Violations of the FCPA, OFAC restrictions, the Bribery Act or other export control, anti-corruption, anti-money laundering and anti-terrorism laws or regulations may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could have a material adverse effect on our reputation, financial condition and results of operations.
Our international operations subject us to foreign currency fluctuations and other risks associated with operating in foreign countries.
We operate internationally and enter into transactions denominated in foreign currencies. To date, we have not hedged our exposure to changes in foreign currency exchange rates and as a result, we are subject to foreign currency transaction and translation gains and losses. We purchase goods and services in U.S. dollars and Euros. Foreign exchange risk is managed primarily by satisfying foreign denominated expenditures with cash flows or assets denominated in the same currency therefore we are subject to some foreign currency fluctuation risk. Our currency value fluctuations were not material for 2019.
There are also other risks inherent with operating on a global scale, not limited to the following:
•Inability to obtain country-specific product registrations and clearances
•Compliance with local regulations
•Challenges with staffing our foreign operating facilities
•Insufficient commercialization efforts in new countries due to lower than expected distributor performance
•Theft or compromise of our intellectual property
•Political changes or economic instability
Changes in U.S. trade policies could significantly increase the cost of imported goods into the United States, which may materially reduce our sales or profitability.
Changes in U.S. trade policy could trigger retaliatory actions by affected countries, resulting in "trade wars," in increased costs for goods imported into the United States, which may reduce customer demand for these products if the parties having to pay those tariffs increase their prices, or in trading partners limiting their trade with the United States. If these consequences are realized, the volume of economic activity in the United States, may be materially reduced. Such a reduction may materially and adversely affect our sales volumes. Further, the realization of these matters may increase our cost of goods and, if those costs cannot be passed on to our customers, our business and profits may be materially and adversely affected.
Our operations may experience higher costs to produce our products as a result of changes in prices for oil, gasoline and other commodities.
We use plastics and other petroleum-based materials along with precious metals contained in electronic components as raw materials in many of our products. Prices of oil and gasoline also significantly affect our costs for freight and utilities. Oil, gasoline and precious metal prices are volatile and may increase, resulting in higher costs to produce and distribute our products. Due to the highly competitive nature of the healthcare industry and the cost-containment efforts of our customers we may be unable to pass along cost increases through higher prices. If we are unable to fully recover these costs through price increases or offset through other cost reductions, our results of operations could be materially and adversely affected.
Risks Relating to Our Business
We manufacture the majority of our products at our Clearwater, Florida and Sofia, Bulgaria facilities. Components, labor-intensive assemblies and sub-assemblies, and sterilization services are out-sourced to third party manufacturers/services and produced to our specifications.
We are also dependent on OEM customers who have no legal obligation to purchase products from us. Should such customers fail to give us purchase orders for the product after development, our future business could be negatively affected. Furthermore, no assurance can be given that such customers will give sufficient high priority to our products. Finally, disagreements or disputes may arise between us and our customers, which could adversely affect production and sales of our products.
9
APYX MEDICAL CORPORATION
The recent Coronavirus outbreak has been declared a pandemic by the World Health Organization and recently has spread to the United States and many other parts of the world and may continue to adversely affect our business operations, employee availability, financial condition, liquidity and cash flow for an extended period of time.
The outbreak of the Coronavirus (“COVID-19”) continues to grow both in the U.S. and globally, and related government and private sector responsive actions may continue to adversely affect our business operations. It is impossible to predict the effect and ultimate impact of the COVID-19 pandemic as the situation is rapidly evolving.
Ongoing significant reductions in business related activities could result in further loss of sales and profits and other material adverse effects. The extent of the impact of COVID-19 on our business, financial results, liquidity and cash flows will depend largely on future developments, including new information that may emerge concerning the severity and action taken to contain or prevent further spread within the U.S. and the related impact on consumer confidence and spending, all of which are highly uncertain and cannot be predicted.
If the COVID-19 outbreak continues and persists for an extended period of time, we expect there will be significant and material disruptions to our supply chain and operations, and delays in the manufacturing and shipment of our products, which may then have a material adverse effect on our business and results of operations.
These and other potential impacts of COVID-19, could therefore materially and adversely affect our business, financial condition and results of operations.
We rely on certain suppliers, subcontractors, and manufacturers for raw materials and other products and are vulnerable to fluctuations in the availability and price of such products and services.
Fluctuations in the price, availability and quality of the raw materials and subcontracting services we use in our manufacturing could have a negative effect on our cost of sales and our ability to meet the demands of our customers. Inability to meet the demands of our customers could result in the loss of future sales.
In addition, the costs to manufacture our products depend in part on the market prices of the raw materials used to produce them. We may not be able to pass along to our customers all or a portion of our higher costs of raw materials due to competitive and marketing pressures, which could decrease our earnings and profitability.
We also have collaborative arrangements with three key foreign suppliers under which we request the development of certain items and components and we purchase them pursuant to purchase orders. Our purchase order commitments are never more than one year in duration and are supported by our sales forecasts. The majority of our raw materials are purchased from sole-source suppliers. While we believe we could ultimately procure other sources for these components, should we experience any significant disruptions in this key supply chain, there are no assurances that we could do so in a timely manner which could render us unable to meet the demands of our customers, resulting in a material and adverse effect on our business and operating results. For example, we source certain materials used in the manufacture of our products from China; the coronavirus outbreak or other similar global disruptions could make access to our existing supply chain difficult and could impact our business.
If we are unable to protect our patents or other proprietary rights, or if we infringe on the patents or other proprietary rights of others, our competitiveness and business prospects may be materially damaged.
We have been issued 37 patents in the United States and 26 foreign patents. We have 16 pending patent applications in the United States and 26 pending foreign applications. Our intellectual property portfolio for our J-Plasma®/Renuvion® products continues to grow on an annual basis. We intend to continue to seek legal protection, primarily through patents, for our proprietary technology. Seeking patent protection is a lengthy and costly process and there can be no assurance that patents will be issued from any pending applications, or that any claims allowed from existing or pending patents will be sufficiently broad or strong to protect our proprietary technology. There is also no guarantee that any patents we hold will not be challenged, invalidated or circumvented, or that the patent rights granted will provide competitive advantages to us. Our competitors have developed and may continue to develop and obtain patents for technologies that are similar or superior to our technologies. In addition, the laws of foreign jurisdictions in which we develop, manufacture or sell our products may not protect our intellectual property rights to the same extent as do the laws of the United States.
10
APYX MEDICAL CORPORATION
Adverse outcomes in current or future legal disputes regarding patent and other intellectual property rights could result in the loss of our intellectual property rights, subject us to significant liabilities to third parties, require us to seek licenses from third parties on terms that may not be reasonable or favorable to us, prevent us from manufacturing, importing or selling our products, or compel us to redesign our products to avoid infringing third parties’ intellectual property. As a result, our product offerings may be delayed and we may be unable to meet customers’ requirements in a timely manner. Regardless of the merit of any related legal proceeding, we have incurred in the past and may be required to incur in the future substantial costs to prosecute, enforce or defend our intellectual property rights. Even in the absence of infringement by our products of third parties’ intellectual property rights, or litigation related to trade secrets, we have elected in the past and may in the future elect to enter into settlements to avoid the costs and risks of protracted litigation and the diversion of resources and management’s attention. However, if the terms of settlements entered into with certain of our competitors are not observed or enforced, we may suffer further costs and risks. Any of these circumstances could have a material adverse effect on our business, financial condition, results of operations or cash flows.
Our ability to develop intellectual property depends in large part on hiring, retaining and motivating highly qualified design and engineering staff with the knowledge and technical competence to advance our technology and productivity goals. To protect our trade secrets and proprietary information, generally we have entered into confidentiality agreements with our employees, as well as with consultants and other parties. If these agreements prove inadequate or are breached, our remedies may not be sufficient to cover our losses.
We have been and may in the future become subject to litigation proceedings that could materially and adversely affect our business.
The medical device industry is characterized by frequent claims and litigation, and we are and may become subject to various claims, lawsuits and proceedings in the ordinary course of our business, including claims by current or former employees, distributors and competitors, and with respect to our products and product liability claims, lawsuits and proceedings.
We are involved in a number of legal actions relating to the use of our technology. The outcomes of these legal actions are not within our complete control and may not be known for prolonged periods of time. In the opinion of management, the Company has meritorious defenses, and such claims are adequately covered by insurance, or are not expected, individually or in the aggregate, to result in a material, adverse effect on our financial condition. However, in the event that damages exceed the aggregate coverage limits of our policy or if our insurance carriers disclaim coverage, we believe it is possible that costs associated with these claims could have a material adverse impact on our consolidated earnings, financial position or cash flows (see below ITEM 3: Legal Proceedings).
Intellectual Property Litigation or Trade Secrets
We have in the past, experienced certain allegations of infringement of intellectual property rights and use of trade secrets and may receive other such claims, with or without merit, in the future. Previously, claims of infringement of intellectual property rights have sometimes evolved into litigation against us and they may continue to do so in the future. It is inherently difficult to assess the outcome of litigation. Although we believe we have had adequate defenses to these claims and that the outcome of the litigation will not have a material adverse impact on our business, financial condition, or results of operations, there can be no assurances that we will prevail. Any such litigation could result in substantial cost to us, significantly reduce our cash resources and create a diversion of the efforts of our technical and management personnel, which could have a material and adverse effect on our business, financial condition and operating results. If we are unable to successfully defend against such claims, we could be prohibited from future sales of the allegedly infringing product or products, which could materially and adversely affect our future growth.
Our business is subject to the potential for recalls or safety alerts, litigation and negative publicity associated with defects or failures of our products, or their misuse\off-label use by physicians.
Manufacturing flaws, component failures, design defects, misuse uses by physicians, or inadequate disclosure of product-related information could result in an unsafe condition or the injury or death of a patient. In addition, there may be increased risk of injury if physicians or others attempt to use our products off-label. The FDA does not restrict or regulate a physician's use of a medical device within the practice of medicine, and we cannot prevent a physician from using our products for an off-label use. The use of our products for indications other than for those for which our products have been approved or cleared by the FDA may not effectively treat the conditions not referenced in our product indications. These problems could lead to litigation, a recall of, or issuance of a safety alert relating to our products, either of which could result in significant costs and negative publicity. Due to the strong name recognition of our brands, an adverse event involving one of our products could result in reduced market acceptance
11
APYX MEDICAL CORPORATION
and demand for all products within that brand and could harm our reputation and our ability to market our products in the future. In some circumstances, adverse events arising from or associated with the design, manufacture or marketing of our products could result in the suspension or delay of our current regulatory reviews of our applications for new product approvals. We also may undertake voluntarily to recall products or temporarily shut down certain production lines based on internal safety and quality monitoring and testing data. Any of the foregoing problems could disrupt our business and have a material adverse effect on our business, results of operations, financial condition and cash flows.
Economic conditions could adversely affect our business and financial condition.
Negative conditions in the national or global economy could adversely affect our business and financial condition. During times of an uncertain economy and tight credit markets our customers may be unwilling or unable to borrow money to properly finance their operations. This may include difficulty in obtaining credit lines to purchase our products, or to maintain their business at current levels. The cosmetic procedure market can be particularly vulnerable to negative trends in the local or global economy since the end-user may have less discretionary income or be unsure about spending their discretionary income. This could result in less demand for procedures where our products are used. Additionally, some end users may have difficulty in obtaining financing at an acceptable interest rate that would be necessary to purchase the procedures in which our products are being used.
These factors could result in reduced revenues from the sale of our product, slower adoption of new technologies, longer sales cycles, increased price competition and increased difficulties in the collection of accounts receivables as, in certainly countries, payments by our customers are dependent on the financial stability of the economy. With the current economic state in many countries outside the United States, we will continue to monitor the ability of our customers to pay their obligations to us as any weaknesses in their end-user market could affect their cashflow and results in delays in their payments to us. This would increase the risk in our credit exposure and delay our ability to recognize revenues of current and future sales to these customers. Any of these events could harm our business and have material adverse effects on our financial condition.
There may be circumstances that arise that may cause us to use some of our existing financial assets ineffectively.
As a result of the proceeds received from the sale of our Core business in 2018, we were able to pay off all significant debt obligations and invest in cash and cash equivalents to support our continued growth initiatives over the coming years. Future decisions made to invest in certain initiatives may not have the expected results, which could materially and adversely affect our business and operations and impact overall stockholder value. In addition, due to fluctuations in interest rates, our investments may not always yield a favorable rate of return.
We may, in the future, identify deficiencies in controls over financial reporting.
As disclosed in Part II, Item 9A, we have concluded that two of the three material weaknesses in our internal controls over financial reporting identified during the year ended December 31, 2018 were not fully remediated due to the need for additional qualified accounting personnel and the lack of sufficient documentation or timeliness in completing certain business processes. In 2019 we also identified a material weakness at our subsidiary in Bulgaria related to the purchasing of goods and services and the processing and payment of vendor invoices. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of a company's annual or interim financial statements will not be prevented or detected on a timely basis.
While we are in the process of identifying and implementing remedial measures to address the control deficiencies that led to these material weaknesses, there can be no assurance that remediation will be fully completed in 2020 or that the remedial measures will prevent future control deficiencies or material weaknesses. If we are unable to remediate these material weaknesses, or we identify additional material weaknesses in our internal controls over financial reporting in the future, our ability to analyze, record and report financial information free of material misstatements, and to prepare our financial statements within the time periods specified by the rules and forms of the SEC will likely be adversely affected.
We are at risk of being the victim of a cyber-attack or a security breach that may expose confidential customer, product and Company data or compromise our internal IT infrastructure. This could lead to liabilities resulting from failure to comply with US and foreign data security and privacy regulations and negative impacts to our business operations.
We store in our computer systems and network various elements of data and information related to our customers, products and company that could be compromised as the result of a cyber-attack or security breach. If an individual or group of individuals, including a Company employee, were to compromise confidential information, or if customer confidential information is
12
APYX MEDICAL CORPORATION
inappropriately disclosed due to a security breach of our computer systems, system failures or otherwise, we may face substantial liabilities or incur penalties in connection with any violation of applicable privacy laws or regulations. We also rely heavily on our internal systems, network and data. Any attacks on our IT infrastructure could have a significant impact on our daily manufacturing and customer service functions which could result in a material adverse impact on our financial results.
Our business is dependent on the security of our IT networks and those of our customers. Internal or external attacks on any of those could disrupt the normal operations of our engagements and impede our ability to provide critical services to our customers, thereby subjecting us to liability under our contracts. Additionally, our business involves the use, storage and transmission of information about our employees, our customers and clients of our customers. While we take measures to protect the security of, and unauthorized access to, our systems, as well as the privacy of personal and proprietary information, it is possible that our security controls over our systems, as well as other security practices we follow or those systems of our customers into which we operate and rely upon, may not prevent the improper access to or disclosure of personally identifiable or proprietary information. Such disclosure could harm our reputation and subject us to liability under our contracts and laws that protect personal data, resulting in increased costs or loss of revenue.
Data privacy is subject to frequently changing rules and regulations, which sometimes conflict among the various jurisdictions and countries in which we provide services and continue to develop in ways which we cannot predict. We are subject to U.S. federal and state laws regarding data privacy and security including Section 5 of the Federal Trade Commission Act, or FTC Act. We are also subject to foreign data privacy and security laws, including the Global Data Protection Regulation, or GDPR, the European Union-wide legal framework to govern data collection, use and sharing and related consumer privacy rights. The GDPR includes significant penalties for non-compliance. Our failure to adhere to or successfully implement processes in response to changing regulatory requirements in this area could result in legal liability or impairment to our reputation in the marketplace, which could have a material adverse effect on our business, financial condition and results of operations.
Our manufacturing facilities are located in Clearwater, Florida and Sophia, Bulgaria and could be affected due to multiple weather risks, including risks to our Florida facility from hurricanes and similar phenomena.
Our manufacturing facilities are located in Clearwater, Florida and Sophia, Bulgaria and could be affected by multiple weather risks. Most notably hurricanes in Clearwater, Florida. Although we carry property and casualty insurance and business interruption insurance, future possible disruptions of operations or damage to property, plant and equipment due to hurricanes or other weather risks could result in impaired production and affect our ability to meet our commitments to our customers and impair important business relationships, the loss of which could adversely affect our operations and profitability. We do however maintain a backup generator at our Clearwater facility and a disaster recovery plan is in place to help mitigate this risk.
Risks Related to Our Stock
The market price of our stock has been and may continue to be highly volatile.
Our common stock is listed on The NASDAQ Stock Market LLC under the ticker symbol “APYX”. The market price of our stock has been and may continue to be highly volatile and announcements by us or by third parties may have a significant impact on our stock price. These announcements may include:
•our listing status on the The NASDAQ Stock Market LLC;
•our operating results falling below the expectations of public market analysts and investors;
•developments in our relationships with or developments affecting our major customers;
•negative regulatory action or regulatory non-approval with respect to our new products;
•government regulation, governmental investigations, or audits related to us or to our products;
•developments related to our patents or other proprietary rights or those of our competitors and
•changes in the position of securities analysts with respect to our stock.
The stock market has from time to time experienced extreme price and volume fluctuations, which have particularly affected the market prices for the medical technology sector companies and which have often been unrelated to their operating performance. These broad market fluctuations may adversely affect the market price of our common stock.
In addition, future sales by our security holders may lower the price of our common stock, which could result in losses to our stockholders. Future sales of substantial amounts of common stock in the public market, or the possibility of such sales occurring, could adversely affect prevailing market prices for our common stock. Substantially all of our common stock is freely tradable in
13
APYX MEDICAL CORPORATION
the public market without restriction under the Securities Act, unless these shares are held by our “affiliates”, as that term is defined in Rule 144 under the Securities Act.
We have no present intention to pay dividends on our common stock and, even if we change that policy, we may be unable to pay dividends on our common stock.
We currently do not anticipate paying any dividends on our common stock in the foreseeable future. We currently intend to retain future earnings, if any, to finance operations and invest in our business. Any declaration and payment of future dividends to holders of our common stock will be at the discretion of our board of directors and will depend on many factors, including our financial condition, earnings, capital requirements, level of indebtedness, statutory and contractual restrictions applying to the payment of dividends and other considerations that our board of directors deems relevant.
If we change that policy and commence paying dividends, we will not be obligated to continue paying those dividends and our stockholders will not be guaranteed, or have contractual or other rights, to receive dividends. If we commence paying dividends in the future, our board of directors may decide, in its discretion, at any time, to decrease the amount of dividends, otherwise modify or repeal the dividend policy or discontinue entirely the payment of dividends. Under the Delaware law, our board of directors may not authorize the payment of a dividend unless it is either paid out of our statutory surplus.
Exercise of options issued by us will dilute the ownership interest of existing stockholders.
As of December 31, 2019, our outstanding stock options to our employees, officers, directors and consultants amounted to 3,966,858 shares of our common stock, representing approximately 11.6% of our outstanding common stock.
The exercise of some or all of our stock options will dilute the ownership interests of existing stockholders. Any sales in the public market of the common stock issuable upon such conversion or exercise could adversely affect prevailing market prices of our common stock.
ITEM 1B. Unresolved Staff Comments
None
ITEM 2. Properties
We currently own and maintain a 60,000 square foot facility which consists of office, warehousing, manufacturing and research space located at 5115 Ulmerton Rd., Clearwater, Florida.
In October, 2015, through our acquisition of Apyx Bulgaria, we acquired a lease for approximately 20,000 square feet of office, warehousing and manufacturing facilities located in Sofia, Bulgaria. The rental cost of the facility is approximately $9,000 per month.
ITEM 3. Legal Proceedings
The medical device industry is characterized by frequent claims and litigation, and we are and may become subject to various claims, lawsuits and proceedings in the ordinary course of our business. Such claims include claims by current or former employees, distributors and competitors, claims concerning the marketing and promotion of our products and product liability claims.
We are involved in a number of legal actions relating to the use of our Helium Plasma technology. The outcomes of these legal actions are not within our complete control and may not be known for prolonged periods of time. We believe that such claims are adequately covered by insurance; however, in the case of one of our carriers, we are in a dispute regarding the total level of coverage available. Notwithstanding the foregoing, in the opinion of management, the Company has meritorious defenses and such claims are not expected, individually or in the aggregate, to result in a material, adverse effect on our financial condition. However, in the event that damages exceed the aggregate coverage limits of our policies or if our insurance carriers disclaim coverage, we believe it is possible that costs associated with these claims could have a material adverse impact on our consolidated results of operations, financial position or cash flows.
In addition, as previously disclosed with the U.S. Securities and Exchange Commission on the Company’s Report on Form 8-K filed April 26, 2019, on April 17, 2019, a complaint (the “Complaint”) was filed in the United States District Court for the Middle District of Florida by plaintiff Kyle Pritchard, individually and on behalf of all others similarly situated against the Company and Charles D. Goodwin (“Goodwin”), the Company’s President and Chief Executive Officer and a member of the Company’s Board of Directors, alleging certain violations of the Securities Exchange Act of 1934, as amended. On July 16, 2019, the Court appointed a lead plaintiff for the putative class and approved the lead plaintiff’s selection of counsel. On or about September 3, 2019, Plaintiff filed an amended complaint (the “Amended Complaint”) with the Court.
The Amended Complaint seeks class action status on behalf of all persons and entities that acquired the Company’s securities between December 21, 2018 and April 1, 2019 and alleges violations by the Company and Goodwin of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended and Rule 10b-5 thereunder, primarily related to certain public statements concerning the Premarket Notification 510(k) submission made to the US Food and Drug Administration for a new indication for the Company’s J-Plasma® technology for use in dermal resurfacing procedures. The Amended Complaint seeks an unspecified amount of compensatory damages, an award of interest, reasonable attorneys’ fees, expert fees and other costs, and equitable relief as the court may deem just and proper. On October 3, 2019, the Company and Goodwin filed a Motion to Dismiss the Amended Complaint. Plaintiff’s opposition to the motion to dismiss was served on November 4, 2019. On March 11, 2020, the Court issued an order denying the Company’s motion to dismiss. The Company intends to vigorously defend its interests against the allegations contained in the complaint.
14
APYX MEDICAL CORPORATION
Although the ultimate outcome of this matter cannot be determined with certainty, the Company believes that the allegations stated in the Amended Complaint are entirely without merit. The Company and Goodwin intend to defend themselves vigorously in the suit. In the opinion of management, such claims are adequately covered by insurance, however, in the event that damages exceed the aggregate coverage limits of our policy or if our insurance carriers disclaim coverage, we believe it is possible that costs associated with this claim could have a material adverse impact on our consolidated earnings, financial position or cash flows. We initially accrued $500,000 for defense costs and upon the denial of the motion to dismiss, we accrued an additional $500,000, which is our insurance deductible related to the matter. $820,000 of the $1,000,000 is still accrued as of December 31, 2019.
We accrue a liability in our consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is recorded. If a loss is reasonably possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded, actual results may differ from these estimates.
ITEM 4. Mine Safety Disclosures
Not Applicable.
15
APYX MEDICAL CORPORATION
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock currently is traded on the NASDAQ Stock Market LLC. As of March 27, 2020, we had approximately 600 stockholders of record. Since many stockholders choose to hold their shares under the name of their brokerage firm, we estimate that the actual number of stockholders was over 3,500 shareholders.
Securities Authorized for Issuance Under Equity Compensation Plans
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) | Weighted Average Exercise Price of Outstanding Options, Warrants and Rights (b) | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) (c) | |||||||
Equity compensation plans approved by security holders | 3,770,715 | $ | 4.59 | 3,546,035 | |||||
Equity compensation plans not approved by security holders (1) | 196,143 | $ | 6.08 | — | |||||
Total | 3,966,858 | $ | 4.67 | 3,546,035 | |||||
Dividend Policy
We have never declared or paid any cash dividends on our common stock and we currently do not anticipate paying cash dividends in the foreseeable future. We currently expect to retain any future earnings to fund the operation and expansion of our business.
16
APYX MEDICAL CORPORATION
Five Year Performance Graph
The following line graph compares the cumulative total return of our common shares with the cumulative total return of the Russell 2000 Stock Index and the Russell 3000 Stock Index. The line graph assumes, in each case, an initial investment of $100 on December 31, 2015, based on the market prices at the end of each fiscal year through and including December 31, 2019, and reinvestment of dividends.
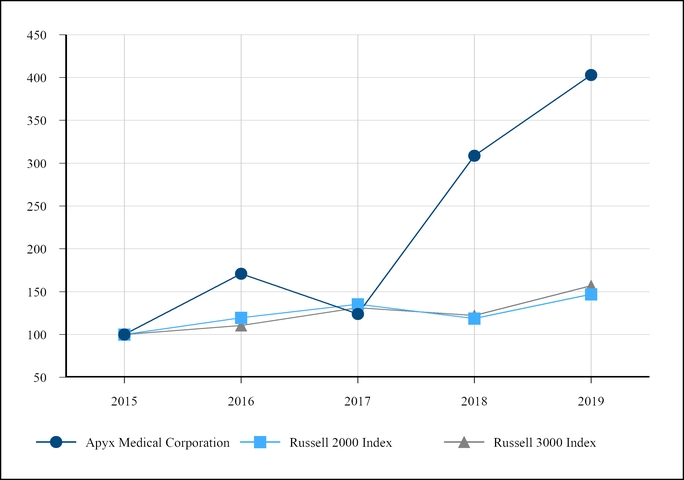
December 31, | ||||||||||||||
2015 | 2016 | 2017 | 2018 | 2019 | ||||||||||
Apyx Medical Corporation | 100.00 | 170.95 | 123.81 | 308.57 | 402.86 | |||||||||
Russell 2000 Index | 100.00 | 119.48 | 135.18 | 118.72 | 146.88 | |||||||||
Russell 3000 Index | 100.00 | 110.42 | 131.24 | 122.07 | 156.91 | |||||||||
17
APYX MEDICAL CORPORATION
ITEM 6. Selected Financial Data
The following selected consolidated financial data (presented in thousands, except per share amounts and employee data) are derived from our consolidated financial statements. On August 30th, 2018, we sold our Core business segment and discontinued those operations. All the information in this table has been restated to reflect this disposition. This data should be read in conjunction with the consolidated financial statements and notes thereto and with Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
2019 | 2018 as Restated | 2017 | |||||||||
Sales | $ | 28,235 | $ | 16,605 | $ | 10,234 | |||||
Cost of sales | 9,141 | 5,779 | 3,276 | ||||||||
Gross profit | 19,094 | 10,826 | 6,958 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 3,731 | 2,549 | 1,941 | ||||||||
Professional services | 8,507 | 3,133 | 1,769 | ||||||||
Salaries and related costs | 14,025 | 9,272 | 6,920 | ||||||||
Selling, general and administrative | 13,700 | 9,407 | 8,689 | ||||||||
Severance and related expense | — | 741 | 1,524 | ||||||||
Total other costs and expenses | 39,963 | 25,102 | 20,843 | ||||||||
Loss from operations | (20,869 | ) | (14,276 | ) | (13,885 | ) | |||||
Interest income | 1,392 | 616 | — | ||||||||
Interest expense | (8 | ) | (104 | ) | (136 | ) | |||||
Other losses, net | (351 | ) | (947 | ) | — | ||||||
Change in fair value of derivative liabilities | — | 20 | 183 | ||||||||
Total other income, net | 1,033 | (415 | ) | 47 | |||||||
Loss before income taxes | (19,836 | ) | (14,691 | ) | (13,838 | ) | |||||
Income tax benefit | (130 | ) | (3,907 | ) | (156 | ) | |||||
Net loss from continuing operations | (19,706 | ) | (10,784 | ) | (13,682 | ) | |||||
Income from discontinued operations, net of tax | — | 5,099 | 8,620 | ||||||||
Gain on sale of the Core Business, net of tax | — | 68,404 | — | ||||||||
Income from discontinued operations, net of tax | — | 73,503 | 8,620 | ||||||||
Net income (loss) | $ | (19,706 | ) | $ | 62,719 | $ | (5,062 | ) | |||
Balance Sheet Information: | |||||||||||
Total current assets | $ | 76,733 | $ | 89,835 | $ | 22,547 | |||||
Short term investments | — | 61,678 | — | ||||||||
Working Capital | 64,422 | 81,224 | 16,574 | ||||||||
Total assets | 84,745 | 95,928 | 30,988 | ||||||||
Long Term Liabilities | 1,175 | 140 | 2,983 | ||||||||
Total Stockholder's Equity | 71,259 | 87,177 | 22,032 | ||||||||
18
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis in conjunction with our financial statements and related notes contained elsewhere in this report. This discussion contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of a variety of factors discussed in this report and those discussed in other documents we file with the SEC. In light of these risks, uncertainties and assumptions, readers are cautioned not to place undue reliance on such forward-looking statements. These forward-looking statements represent beliefs and assumptions as of the date of this report. While we may elect to update forward-looking statements and at some point in the future, we specifically disclaim any obligation to do so, even if our estimates change. Past performance does not guarantee future results.
Executive Level Overview
We are an advanced energy technology company with a passion for elevating people’s lives through innovative products in the cosmetic and surgical markets. Known for our innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients it serves. Our Helium Plasma Technology is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers plastic surgeons, fascial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision and virtually eliminating unintended tissue trauma. We also leverage our deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers.
Total revenue from continuing operations increased by 70.0% or approximately $11.6 million for the year ended December 31, 2019 when compared with 2018. Advanced Energy segment sales increased 74.6% or approximately $9.7 million for the year ended December 31, 2019 when compared with 2018.
International sales represented approximately 30.6% of total revenues in 2019, 22.6% in 2018 and 13.2% in 2017. Management estimates our products have been sold in more than 40 countries through local dealers coordinated by sales, marketing and logistics personnel at our Clearwater, Florida and Sofia, Bulgaria facilities.
Throughout 2019, we continued to drive growth in our Advanced Energy business by increasing the adoption and utilization of our generators and handpieces in the U.S. cosmetic surgery market and fulfilling demand from distributors in our international markets. We also saw contributions from our OEM business, which increased $1.9 million, or 53.6%, as compared to last year. This was driven primarily by contributions from our electrosurgical generator and supply agreement with Symmetry Surgical.
We believe that our investment and focus on the following strategic initiatives in 2019 and beyond will position the Company for long-term growth in the cosmetic surgery market:
•To formalize our regulatory strategy to pursue specific clinical indications that will enable us to sell our
Renuvion® products for targeted procedures
•To secure new clinical evidence demonstrating the safety and efficacy of our Helium Plasma Technology
•To provide enhanced physician and practice support for our cosmetic surgery customers
•To improve our manufacturing capabilities and efficiencies
In regards to our operating segments, our results are aggregated into reportable segments only if they exhibit similar economic characteristics. In addition to similar economic characteristics, we also consider the following factors in determining the reportable segments: the nature of business activities, the management structure directly accountable to our chief operating decision maker for operating and administrative activities, availability of discrete financial information and information presented to the Board of Directors and investors. Asset information is not reviewed by the chief operating decision maker by segment and is not available by segment, and accordingly, we have not presented a measure of assets by segment.
Our reportable segments are disclosed as principally organized and managed as two operating segments: Advanced Energy and OEM. "Corporate & Other" includes certain unallocated corporate and administrative costs which are not specifically attributed to any reportable segment. The OEM segment is primarily development and manufacturing contract and product driven, and all related expenses are recorded as cost of sales, therefore no segment specific operating expenses are incurred.
19
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
We strongly encourage investors to visit our website: www.apyxmedical.com to view the most current news and to review our filings with the Securities and Exchange Commission.
As discussed under "Item 1A. Risk Factors," an outbreak of a novel strain of the coronavirus, COVID-19, was recently identified in China and has subsequently been recognized as a pandemic by the World Health Organization. This coronavirus outbreak has severely restricted the level of economic activity around the world. In response to this coronavirus outbreak the governments of many countries, states, cities and other geographic regions have taken preventative or protective actions, such as imposing restrictions on travel and business operations and advising or requiring individuals to limit or forego their time outside of their homes. Temporary closures of businesses have been ordered and numerous other businesses have temporarily closed voluntarily. These actions have expanded significantly in the past several weeks and are expected to continue to expand. Given the uncertainty regarding the spread of this coronavirus, the related financial impact cannot be reasonably estimated at this time, although the aforementioned actions and related impacts are expected to continue and may also significantly affect the Company's business in other geographic areas in which the coronavirus has spread and may continue to spread. The Company intends to continue to execute on its strategic plans and operational initiatives during the coronavirus outbreak. However, the uncertainties associated with the protective and preventative measures being put in place or recommended by both governmental entities and other businesses, among other uncertainties, may result in delays or modifications to these plans and initiatives.
The following financial statement analysis has been updated for the effects of the restatement to the results of operations and financial position of the Company in 2018 as discussed in Note 4 to the consolidated financial statements.
Results of Operations
Sales
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Sales by Reportable Segment | |||||||||||||||||||||
Advanced Energy | $ | 22,676 | $ | 12,987 | 74.6 | % | $ | 12,987 | $ | 7,636 | 70.1 | % | |||||||||
OEM | 5,559 | 3,618 | 53.6 | % | 3,618 | 2,598 | 39.3 | % | |||||||||||||
Total | $ | 28,235 | $ | 16,605 | 70.0 | % | $ | 16,605 | $ | 10,234 | 62.3 | % | |||||||||
Sales by Domestic and International | |||||||||||||||||||||
Domestic | $ | 19,584 | $ | 12,858 | 52.3 | % | $ | 12,858 | $ | 8,887 | 44.7 | % | |||||||||
International | 8,651 | 3,747 | 130.9 | % | 3,747 | 1,347 | 178.2 | % | |||||||||||||
Total | $ | 28,235 | $ | 16,605 | 70.0 | % | $ | 16,605 | $ | 10,234 | 62.3 | % | |||||||||
Total revenue from continuing operations increased by 70.0% or approximately $11.6 million for the year ended December 31, 2019 when compared with 2018. Advanced Energy segment sales increased 74.6% or approximately $9.7 million for the year ended December 31, 2019 when compared with 2018. The increase is a result of the impact made by the additional sales force in the U.S. and new international distributors. In both the U.S. and internationally, strong sales growth of generators was coupled with utilization based demand for our handpieces. In addition, we entered four new markets in 2019, the largest of which were Mexico and Canada.
The OEM product line consists of proprietary products designed specifically for third party equipment manufacturers; revenue for this product line increased 53.6% or approximately $1.9 million when compared to 2018. The increase from 2018 is primarily attributable to sales to Symmetry under our Manufacture and Supply Agreement, which commenced following the disposition of the Core Business in August 2018.
20
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Overall sales from continuing operations increased by 62.3% or approximately $6.4 million for the year ended December 31, 2018 when compared with 2017. Advanced Energy segment sales increased 70.1% or approximately $5.4 million for the year ended December 31, 2018 when compared with 2017. The increase was primarily driven by a continued focus of our selling into the cosmetic surgery market and sales growth in international markets. The OEM product line consists of proprietary products designed specifically for third party equipment manufacturers; revenue for this product line increased 39.3% or approximately $1.0 million when compared to 2017.
Gross Profit
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Cost of sales | $ | 9,141 | $ | 5,779 | 58.2 | % | $ | 5,779 | $ | 3,276 | 76.4 | % | |||||||||
Percentage of sales | 32.4 | % | 34.8 | % | 34.8 | % | 32.0 | % | |||||||||||||
Gross profit | $ | 19,094 | $ | 10,826 | 76.4 | % | $ | 10,826 | $ | 6,958 | 55.6 | % | |||||||||
Percentage of sales | 67.6 | % | 65.2 | % | 2.4 | % | 65.2 | % | 68.0 | % | (2.8 | )% | |||||||||
Our gross profit margin as a percentage of sales increased by 2.4%, or $8.3 million during the year ended December 31, 2019 compared with 2018. The increase was primarily driven by higher Advanced Energy sales as a percentage of total sales in 2019 as well as efficiencies realized in the manufacturing processes in late 2019. These increases were partially offset by an increase in international sales as a percentage of total sales in 2019 as compared to 2018, which typically carry lower margins that U.S. sales, and OEM sales to Symmetry, which carry lower margins than typical OEM sales.
Our gross profit margin as a percentage of sales decreased by 2.8% but increased by approximately $3.9 million during the year ended December 31, 2018, compared with 2017. The decrease was driven by lower year over year margins in Advanced Energy from increased international sales offset by increased year over year margins in the OEM segment.
In conjunction with the divestment of our Core business segment in 2018, we performed a review of our standard costs, including the composition of our overhead cost pools. As a result, we reclassified certain overhead costs related to quality and regulatory to Salaries and Related Costs, in the amount of approximately $0.1 million in the third quarter and approximately $0.4 million for the last quarter of 2018. This change in estimate was necessary in order to better reflect the change in operations to our Advanced Energy segment.
21
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Other Costs and Expenses
Research and development
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Research and Development expense | $ | 3,731 | $ | 2,549 | 46.4 | % | $ | 2,549 | $ | 1,941 | 31.3 | % | |||||||||
Percentage of sales | 13.2 | % | 15.4 | % | 15.4 | % | 19.0 | % | |||||||||||||
Our expenditures for R&D related activities increased by 46.4% or approximately $1.2 million for the year ended December 31, 2019, compared with 2018. This was mainly driven by continued spending on clinical studies and research projects related to the cosmetic surgery market, including the development of new handpieces which the Company introduced to the market during 2019 as well as the submission of two IDE applications to the FDA in 2019.
Our expenditures for R&D related activities increased by 31.3% or approximately $0.6 million for the year ended December 31, 2018, compared with 2017. This was mainly driven by continued spending on clinical studies and research projects related to the cosmetic surgery market.
Professional services
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Professional services expense | $ | 8,507 | $ | 3,133 | 171.5 | % | $ | 3,133 | $ | 1,769 | 77.1 | % | |||||||||
Percentage of sales | 30.1 | % | 18.9 | % | 18.9 | % | 17.3 | % | |||||||||||||
Professional services expenses increased 171.5% for the year ended December 31, 2019, compared with 2018. The change was primarily attributable to increases in physician consulting expenses, including stock option grants, related to the Advanced Energy segment (increase of $2.0M), increased legal fees primarily associated with our class action lawsuit (increase of $1.0M), our use of third party IT support in 2019 (increase of $0.6M), third party assistance with internal controls in 2019 (increase of $0.5M), and accounting and auditing fees for services provided by our independent accountants ($0.3M).
Professional services costs increased 77.1% for the year ended December 31, 2018, compared with 2017. The change was attributable to increased legal and non-R&D consulting expenses related to the Advanced Energy segment.
Salaries and related costs
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Salaries and related expenses | $ | 14,025 | $ | 9,272 | 51.3 | % | $ | 9,272 | $ | 6,920 | 34.0 | % | |||||||||
Percentage of sales | 49.7 | % | 55.8 | % | 55.8 | % | 67.6 | % | |||||||||||||
During 2019, salaries and related expenses increased approximately 51.3% or approximately $4.8 million compared to 2018. The increase was primarily attributable to additional headcount in 2019 (net increase of 44 employees in 2019), many of whom had a salary in excess of our average salaries in 2018, and employee stock option grants in 2019, which drove an increase in employee stock option expense of $0.6M in 2019.
During 2018, salaries and related expenses increased approximately 34.0% or approximately $2.4 million compared to 2017. The increase was primarily attributable to increased incentive compensation of $1.5 million.
22
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
In conjunction with the divestment of our Core business segment, we performed a review of our standard costs, including the composition of our overhead cost pools. As a result, we reclassified certain overhead costs related to quality and regulatory to Salaries and Related Costs, in the amount of approximately $0.1 million in the third quarter and approximately $0.4 million for the last quarter of 2018. This change in estimate was necessary in order to better reflect the change in operations to our Advanced Energy segment.
Selling, general and administrative expenses
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
SG&A Expense | $ | 13,700 | $ | 9,407 | 45.6 | % | $ | 9,407 | $ | 8,689 | 8.3 | % | |||||||||
Percentage of sales | 48.5 | % | 56.7 | % | 56.7 | % | 84.9 | % | |||||||||||||
Selling, general and administrative expense increased by 45.6% or approximately $4.3 million for the year ended December 31, 2019, compared with 2018. The increase is primarily attributable to higher selling and marketing related expenses, including sales commissions (increase of $0.9M), travel expenses (increase of $0.9M), and advertising including trade shows (increase of $0.7M) to support sales growth in the Advanced Energy segment. Additionally, we incurred additional regulatory expenses (increase of $0.7M) in 2019 associated with obtaining clearance to sell our products, both domestically and internationally.
Selling, general and administrative expense increased by 8.3% or approximately $0.7 million for the year ended December 31, 2018, compared with 2017. The increase is primarily attributable to higher sales and marketing related expenses to support sales growth in the Advanced Energy segment.
Severance
Jay D. Ewers, the Chief Financial Officer, resigned as an officer of the Company effective December 31, 2018, although he continued on as an employee during the first quarter of 2019. In connection with this departure, the Company and Mr. Ewers entered into a separation agreement, dated November 12, 2018. Severance costs incurred included salary, option expense and other benefits of approximately $624,000, approximately $532,000 is included in operational cash outflows during 2019, the remainder will be included in operational cash outflows during 2020.
Jack McCarthy, the Chief Commercialization Officer, was terminated without cause from his position with the Company effective November 6, 2017. Severance costs incurred included salary, option expense and other benefits of approximately $582,000, of which approximately $397,000 was included in operational cash outflows during 2018.
Robert L. Gershon, the Chief Executive Officer and a director, resigned from all of his positions with the Company effective December 15, 2017. In connection with this departure, the Company and Mr. Gershon entered into a separation agreement, dated December 15, 2017. Severance costs incurred included salary, option expense and other benefits of approximately $767,000, of which approximately $670,000 was included in operational cash outflows during 2018.
Other Income (Expense), net
Year Ended December 31, | Year Ended December 31, | ||||||||||||||||||||
(In thousands) | 2019 | 2018 as Restated | Change | 2018 as Restated | 2017 | Change | |||||||||||||||
Interest income | $ | 1,392 | $ | 616 | 126.0 | % | $ | 616 | $ | — | — | % | |||||||||
Interest expense | (8 | ) | (104 | ) | (92.3 | )% | (104 | ) | (136 | ) | (23.5 | )% | |||||||||
Percentage of sales | 4.9 | % | 3.1 | % | 3.1 | % | (1.3 | )% | |||||||||||||
23
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Interest income (expense)
Total net interest income was higher for the year ended December 31, 2019, as compared with 2018. This increase is due to short term investments in U.S. Treasury Securities which we purchased with the proceeds from the sale of the Core business, which were outstanding for all of 2019 as compared to approximately 4 months in 2018. This increase is offset by lower returns due to a lower average yield in 2019 and lower average principal invested.
Total net interest income was higher for the year ended December 31, 2018, as compared with 2017. This increase is primarily related to short term investments in U.S. Treasury Securities which we purchased with the proceeds from the sale of the Core business.
Income Taxes
The income tax benefit was approximately $0.1 million for the year ended December 31, 2019 as compared to an income tax benefit from continuing operations of approximately $3.9 million in 2018. In 2019, our income tax benefit is composed primarily of return to provision adjustments related to the 2018 tax year (benefit of approximately $0.3M), partially offset by the accrual of interest and penalties on our uncertain tax positions (expense of approximately $0.2M).
During 2018, the Company recorded a large gain on the sale of our Core business to Symmetry Surgical. This gain allowed the Company to utilize deferred tax assets (primarily a net operating loss carryforward) that had been fully reserved through a valuation allowance to offset our taxable position in 2018.
Liquidity and Capital Resources
At December 31, 2019, we had approximately $58.8 million in Cash and Cash Equivalents as compared to approximately $78.3 in Cash, Cash Equivalents and Short Term Investments at December 31, 2018. Our working capital at December 31, 2019 was approximately $64.4 million compared with $81.2 million at December 31, 2018. The decrease in working capital at December 31, 2019 from December 31, 2018 was primarily due to the net loss incurred by the Company in 2019.
For the year ended December 31, 2019, net cash used in operating activities is approximately $18.5 million compared with net cash used in operating activities of approximately $20.9 million in 2018. This decrease in cash used is primarily driven by a reduction of taxes paid, and partially offset by a higher net loss from continuing operations.
Net cash from investing activities for the year ended December 31, 2019, is $60.5 million, primarily related to the maturity of short term investments and reinvestment in cash equivalents. Net cash from investing activities for the year ended December 31, 2018 is $29.3 million, primarily related to $91.1 million in net proceeds from the disposition of the Core business, offset by net purchases of marketable securities of $61.4 million.
Cash from financing activities of approximately $0.1 million primarily relates to cash collected for stock options during the year ended December 31, 2019. Cash used in 2018 financing activities was $2.5 million and primarily related to the repayment of the mortgage on our Clearwater, FL, facility.
At December 31, 2019, we had purchase commitments for inventories totaling approximately $2 million, substantially all of which is expected to be purchased by the end of 2020.
Critical Accounting Estimates
In preparing the consolidated financial statements in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP), we have adopted various accounting policies. Our most significant accounting policies are disclosed in Note 2 to the consolidated financial statements.
24
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires us to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Our estimates and assumptions, including those related to inventories, legal proceedings, research and development, warranty obligations, product liability, sales returns and discounts, stock based compensation and income taxes are updated as appropriate, which in most cases is at least quarterly. We base our estimates on historical experience, or various assumptions that are believed to be reasonable under the circumstances and the results form the basis for making judgments about the reported values of assets, liabilities, revenues and expenses. Actual results may materially differ from these estimates.
Estimates are considered to be critical if they meet both of the following criteria: (1) the estimate requires assumptions about material matters that are uncertain at the time the accounting estimates are made and (2) other materially different estimates could have been reasonably made or material changes in the estimates are reasonably likely to occur from period to period. Our critical accounting estimates include the following:
Stock-based Compensation
Under our stock option plans, options to purchase common shares of the Company may be granted to employees, officers and directors of the Company by the Board of Directors. The Company accounts for stock options in accordance with FASB ASC Topic 718-10, Compensation-Stock Compensation, with compensation expense amortized over the vesting period. Options are valued using the Black-Scholes model in 2019 and the trinomial lattice option-pricing model in prior years, both of which includes a number of estimates that affect the amount of our expense. The Company has determined that the most critical of these estimates are the estimates of expected life, forfeiture rate and volatility used in the calculations.
Expected life
For employee stock-based compensation awards, we estimate the expected life of awards utilizing the SEC's simplified method. We utilize this method, as the we have not historically granted stock-based compensation awards to employees in sufficient volumes to determine a reasonable estimate of the life of awards. For awards granted to non-employees, we calculate expected life using a combination of past exercise behavior, the contractual term and expected remaining exercise behavior.
Forfeiture rate
We estimate forfeiture rates at the time stock-based compensation awards are granted. We utilize historical employee turnover by employee class to estimate these rates. Forfeiture estimates are lower for employees in executive and managerial positions than for other employee groups. At a minimum, we record compensation expense on those awards that have vested. Following the disposition of the Core business, we experienced turnover higher than an our average historical turnover, which resulted in actual results differing from these estimates. During the third quarter of 2019, we determined that our estimates at the grant date were not consistent with actual results and that we had not re-evaluated our original forfeiture estimate or recorded compensation cost for the value of awards that had vested. This resulted in a cumulative difference in the compensation cost that had been recognized on these awards of $0.2 million from the estimated amount, which was corrected in a revision included in our 2019 Q3 10-Q.
Volatility
The Company determines the volatility by utilizing the historical volatility of our stock over the period of the awards expected life. The SEC allows us to include periods in excess of the useful life if we determine that they provide a more reasonable basis for the volatility of our stock. Additionally, ASC 718-10 allows us to exclude periods from the volatility if they pertain to events or circumstances that in our judgment are specific to us and if the event or transaction is not reasonably expected to occur again during the expected term of the awards. We have not included any additional periods, nor disregarded any periods, in calculating our volatility.
25
APYX MEDICAL CORPORATION
MANAGEMENT'S DISCUSSION AND ANAYLSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS - Continued
Inventory reserves
We maintain a reserve for excess and obsolete inventory resulting from the potential inability to sell our products at prices in excess of current carrying costs. The markets in which we operate are highly competitive, with new products and surgical procedures introduced on an ongoing basis. Such marketplace changes may cause our products to become obsolete. We make estimates regarding the future recoverability of the costs of these products and record a provision for excess and obsolete inventories based on historical experience and expected future trends. If actual product life cycles, product demand or acceptance of new product introductions are less favorable than projected by management, additional inventory write-downs may be required, which would unfavorably affect future operating results.
Litigation Contingencies
In accordance with authoritative guidance, we record a liability in our consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded; actual results may differ from these estimates.
Income Taxes
The provision for income taxes includes federal, foreign, state and local income taxes currently payable and those deferred because of temporary differences between the financial statement and tax bases of assets and liabilities. Deferred tax assets or liabilities are computed based on the difference between the financial statement and income tax bases of assets and liabilities using enacted marginal tax rates. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. Deferred income tax expenses or credits are based on the changes in the asset or liability from period to period.
As a result of historical losses exclusive of discontinued operations, and our expectation to continue to generate losses in the near future, we recorded a valuation allowance on the net deferred tax asset and do not anticipate recording an income tax benefit related to these deferred tax assets. We will reassess the realization of deferred tax assets each reporting period and will be able to reduce the valuation allowance to the extent the financial results of continuing operations improve and it becomes more likely than not that the deferred tax assets will be realizable. As management expects the Company to continue to generate losses in the foreseeable future after 2019, we will continue to record a valuation allowance on the remaining deferred tax asset balance as of December 31, 2019.
We assess the financial statement impact of an uncertain tax position taken or expected to be taken on an income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized in the financial statements unless it is more likely than not of being sustained.
Inflation
Inflation has not materially impacted the operations of our Company.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements at this time.
Recent Accounting Pronouncements
See Note 3 of the Notes to Consolidated Financial Statements.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
Not required.
26
APYX MEDICAL CORPORATION
ITEM 8. Financial Statements and Supplementary Data
INDEX TO FINANCIAL INFORMATION
Page | |
Reports of Independent Registered Certified Public Accounting Firms | |
27
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Shareholders and Board of Directors
Apyx Medical Corporation
Clearwater, Florida
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Apyx Medical Corporation (the “Company”) as of December 31, 2019, the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for year ended December 31, 2019, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019, and the results of its operations and its cash flows for year ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and our report dated March 31, 2020 expressed an adverse opinion thereon.
Change in Accounting Principle
As discussed in Notes 3 and 9 to the consolidated financial statements, effective January 1, 2019, the Company adopted Accounting Standards Codification Topic 842, Leases.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company's auditor since 2019.
Tampa, Florida
March 31, 2020
28
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Apyx Medical Corporation
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Apyx Medical Corporation (formerly Bovie Medical Corporation) and subsidiaries (the "Company") as of December 31, 2018, and the related consolidated statements of operations, changes in stockholders' equity and cash flows for the years ended December 31, 2018 and 2017, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018, and the results of their operations and cash flows for the years ended December 31, 2018 and 2017, in conformity with accounting principles generally accepted in the United States of America.
Restatement to Correct 2018 Misstatements
As discussed in Note 4 to the consolidated financial statements, the 2018 financial statements have been restated to correct misstatements.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Frazier & Deeter, LLC
Tampa, Florida
March 13, 2019 (March 31, 2020 as to the effects of the restatement discussed in Note 4)
We served as the Company's auditor from 2007 to 2018.
29
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Shareholders and Board of Directors
Apyx Medical Corporation
Clearwater, Florida
Opinion on Internal Control over Financial Reporting
We have audited Apyx Medical Corporation’s (the “Company’s”) internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). In our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
We do not express an opinion or any other form of assurance on management’s statements referring to any corrective actions taken by the Company after the date of management’s assessment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheet of the Company as of December 31, 2019, the related consolidated statements of operations, changes in stockholders’ equity, and cash flows for the year ended December 31, 2019, and the related notes (collectively referred to as “the financial statements”)” and our report dated March 31, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Controls and Procedures. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit of internal control over financial reporting in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and described in management’s assessment: 1) an ineffective control environment requiring additional qualified accounting personnel with an appropriate level of knowledge and experience with generally accepted accounting principles; 2) ineffective control activities due to the lack of documentation and timeliness in executing certain business process controls, specifically related to procure to pay and inventory processes and footnote reporting disclosures related to income tax accounts, primarily related to the Company’s United States Operations; and 3) ineffective control environment and control activities in the Company’s Bulgarian subsidiary relating to the purchasing of goods and services, including the processing and payment of vendor invoices. These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2019 financial statements, and this report does not affect our report dated March 31, 2020 on those financial statements.
30
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ BDO USA, LLP
Tampa, Florida
March 31, 2020
31
(In thousands, except share and per share data)
December 31, 2019 | December 31, 2018 as Restated | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 58,812 | $ | 16,596 | |||
Short term investments | — | 61,678 | |||||
Trade accounts receivable, net of allowance of $273 and $428 | 7,987 | 3,721 | |||||
Other receivables | 1,233 | 1,359 | |||||
Inventories, net of provision for obsolescence of $392 and $439 | 5,068 | 3,146 | |||||
Prepaid expenses and other current assets | 3,633 | 3,335 | |||||
Total current assets | 76,733 | 89,835 | |||||
Property and equipment, net | 6,618 | 5,788 | |||||
Operating lease right-of-use assets | 350 | — | |||||
Finance lease right-of-use assets | 653 | — | |||||
Other assets | 391 | 305 | |||||
Total assets | $ | 84,745 | $ | 95,928 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 2,438 | $ | 1,423 | |||
Accrued expenses and other current liabilities | 9,396 | 7,188 | |||||
Current portion of operating lease liabilities | 108 | — | |||||
Current portion of finance lease liabilities | 229 | — | |||||
Related party note payable | 140 | — | |||||
Total current liabilities | 12,311 | 8,611 | |||||
Related party note payable | — | 140 | |||||
Long-term operating lease liabilities | 235 | — | |||||
Long-term finance lease liabilities | 421 | — | |||||
Other liabilities | 519 | — | |||||
Total liabilities | 13,486 | 8,751 | |||||
COMMITMENTS AND CONTINGENCIES (NOTE 18) | |||||||
STOCKHOLDERS' EQUITY | |||||||
Common stock, $0.001 par value; 75,000,000 shares authorized; 34,312,527 issued and 34,169,952 outstanding as of December 31, 2019, and 33,847,100 issued and 33,704,525 outstanding as of December 31, 2018 | 34 | 34 | |||||
Additional paid-in capital | 56,708 | 52,920 | |||||
Retained earnings | 14,517 | 34,223 | |||||
Total stockholders’ equity | 71,259 | 87,177 | |||||
Total liabilities and stockholders’ equity | $ | 84,745 | $ | 95,928 | |||
The accompanying notes are an integral part of the consolidated financial statements.
32
(In thousands, except per share data)
Year Ended December 31, | |||||||||||
2019 | 2018 as Restated | 2017 | |||||||||
Sales | $ | 28,235 | $ | 16,605 | $ | 10,234 | |||||
Cost of sales | 9,141 | 5,779 | 3,276 | ||||||||
Gross profit | 19,094 | 10,826 | 6,958 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 3,731 | 2,549 | 1,941 | ||||||||
Professional services | 8,507 | 3,133 | 1,769 | ||||||||
Salaries and related costs | 14,025 | 9,272 | 6,920 | ||||||||
Selling, general and administrative | 13,700 | 9,407 | 8,689 | ||||||||
Severance and related expense | — | 741 | 1,524 | ||||||||
Total other costs and expenses | 39,963 | 25,102 | 20,843 | ||||||||
Loss from operations | (20,869 | ) | (14,276 | ) | (13,885 | ) | |||||
Interest income | 1,392 | 616 | — | ||||||||
Interest expense | (8 | ) | (104 | ) | (136 | ) | |||||
Other losses, net | (351 | ) | (947 | ) | — | ||||||
Change in fair value of derivative liabilities | — | 20 | 183 | ||||||||
Total other income, net | 1,033 | (415 | ) | 47 | |||||||
Loss from continuing operations before income taxes | (19,836 | ) | (14,691 | ) | (13,838 | ) | |||||
Income tax benefit | (130 | ) | (3,907 | ) | (156 | ) | |||||
Net loss from continuing operations | (19,706 | ) | (10,784 | ) | (13,682 | ) | |||||
Income from discontinued operations, net of tax | — | 5,099 | 8,620 | ||||||||
Gain on sale of the Core Business, net of tax | — | 68,404 | — | ||||||||
Total income from discontinued operations, net of tax | — | 73,503 | 8,620 | ||||||||
Net income (loss) | $ | (19,706 | ) | $ | 62,719 | $ | (5,062 | ) | |||
Loss per share from continuing operations | |||||||||||
Basic and Diluted | $ | (0.58 | ) | $ | (0.32 | ) | $ | (0.44 | ) | ||
Income per share from discontinued operations | |||||||||||
Basic | — | 2.21 | 0.27 | ||||||||
Diluted | — | 2.14 | 0.27 | ||||||||
Income (loss) per share from all operations | |||||||||||
Basic | (0.58 | ) | 1.89 | (0.16 | ) | ||||||
Diluted | (0.58 | ) | 1.83 | (0.17 | ) | ||||||
Weighted average number of shares outstanding basic | 34,069 | 33,185 | 31,420 | ||||||||
Weighted average number of shares outstanding diluted | 34,069 | 34,366 | 31,427 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
33
(In thousands)
Preferred Stock | Common Stock | ||||||||||||||||||||||||
Shares | Par Value | Shares | Par Value | Additional Paid-In Capital | Retained Earnings (Accumulated Deficit) | Total Stockholders' Equity | |||||||||||||||||||
Balance December 31, 2016 | 976 | $ | 1 | 30,860 | $ | 31 | $ | 49,625 | $ | (23,434 | ) | $ | 26,223 | ||||||||||||
Conversion of Series B convertible preferred to common stock | (976 | ) | (1 | ) | 1,951 | 2 | (1 | ) | — | — | |||||||||||||||
Stock based compensation | — | — | — | — | 871 | — | 871 | ||||||||||||||||||
Shares issued on net settlement of stock options | — | — | 47 | — | — | — | — | ||||||||||||||||||
Shares issued on net settlement of warrants | — | — | 20 | — | — | — | — | ||||||||||||||||||
Net loss | — | — | — | — | — | (5,062 | ) | (5,062 | ) | ||||||||||||||||
Balance December 31, 2017 | — | $ | — | 32,878 | $ | 33 | $ | 50,495 | $ | (28,496 | ) | $ | 22,032 | ||||||||||||
Options exercised for cash | — | — | 88 | — | 202 | — | 202 | ||||||||||||||||||
Stock based compensation - as Restated | — | — | — | — | 2,224 | — | 2,224 | ||||||||||||||||||
Shares issued on net settlement of stock options | — | — | 721 | 1 | (1 | ) | — | — | |||||||||||||||||
Shares issued on net settlement of warrants | — | — | 18 | — | — | — | — | ||||||||||||||||||
Net income - as Restated | — | — | — | — | — | 62,719 | 62,719 | ||||||||||||||||||
Balance December 31, 2018 - as Restated | — | $ | — | 33,705 | $ | 34 | $ | 52,920 | $ | 34,223 | $ | 87,177 | |||||||||||||
Options exercised for cash | — | — | 61 | — | 207 | — | 207 | ||||||||||||||||||
Stock based compensation | — | — | — | — | 3,581 | — | 3,581 | ||||||||||||||||||
Shares issued on net settlement of stock options | — | — | 223 | — | — | — | — | ||||||||||||||||||
Vested restricted stock issued | — | — | 181 | — | — | — | — | ||||||||||||||||||
Net loss | — | — | — | — | — | (19,706 | ) | (19,706 | ) | ||||||||||||||||
Balance December 31, 2019 | — | $ | — | 34,170 | $ | 34 | $ | 56,708 | $ | 14,517 | $ | 71,259 | |||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
34
(In thousands)
Year Ended December 31, | |||||||||||
2019 | 2018 as Restated | 2017 | |||||||||
Cash flows from operating activities | |||||||||||
Net income (loss) | $ | (19,706 | ) | $ | 62,719 | $ | (5,062 | ) | |||
Adjustments to reconcile net income (loss) to net cash used in operating activities: | |||||||||||
Gain on sale of the Core Business, net of tax | — | (68,404 | ) | — | |||||||
Depreciation and amortization | 754 | 669 | 696 | ||||||||
Provision for inventory obsolescence | 132 | — | — | ||||||||
Provision for product warranties | 321 | — | — | ||||||||
Loss on disposal of property and equipment | 89 | — | 5 | ||||||||
Stock based compensation | 3,581 | 2,224 | 871 | ||||||||
Change in fair value of derivative liabilities | — | (20 | ) | (183 | ) | ||||||
Realized and unrealized gains on short term investments | (164 | ) | (247 | ) | — | ||||||
Provision (benefit) for allowance for doubtful accounts | (163 | ) | 224 | 179 | |||||||
Benefit of deferred taxes | — | (368 | ) | (196 | ) | ||||||
Changes in current assets and liabilities, net of effect of disposition: | |||||||||||
Receivables | (3,970 | ) | (447 | ) | (303 | ) | |||||
Prepaid expenses and other assets | (406 | ) | (2,851 | ) | (30 | ) | |||||
Inventories | (2,367 | ) | 1,185 | (368 | ) | ||||||
Accounts payable | 1,054 | (224 | ) | (23 | ) | ||||||
Accrued expenses and other liabilities | 2,370 | (15,341 | ) | 710 | |||||||
Net cash used in operating activities | (18,475 | ) | (20,881 | ) | (3,704 | ) | |||||
Cash flows from investing activities | |||||||||||
Purchases of property and equipment | (1,301 | ) | (363 | ) | (624 | ) | |||||
Proceeds from the disposition of Core business | — | 91,095 | — | ||||||||
Purchases of marketable securities | (18,884 | ) | (87,189 | ) | — | ||||||
Proceeds of marketable securities | 80,726 | 25,758 | — | ||||||||
Net cash provided by (used in) investing activities | 60,541 | 29,301 | (624 | ) | |||||||
Cash flows from financing activities | |||||||||||
Proceeds from stock option exercises | 207 | 202 | — | ||||||||
Repayment of finance lease liabilities | (60 | ) | — | — | |||||||
Repayment of mortgage note payable | — | (2,694 | ) | (239 | ) | ||||||
Net cash provided by (used in) financing activities | 147 | (2,492 | ) | (239 | ) | ||||||
Effect of exchange rates on cash | 3 | — | — | ||||||||
Net change in cash, cash equivalents and restricted cash | 42,216 | 5,928 | (4,567 | ) | |||||||
Cash, cash equivalents and restricted cash, beginning of period | 16,596 | 10,668 | 15,235 | ||||||||
Cash, cash equivalents and restricted cash, end of period | $ | 58,812 | $ | 16,596 | $ | 10,668 | |||||
Cash paid for: | |||||||||||
Interest expense | $ | 8 | $ | 104 | $ | 136 | |||||
Income taxes | 325 | 13,283 | 32 | ||||||||
Non cash operating activities: | |||||||||||
Transfer of other assets to fixed assets | $ | 42 | $ | — | $ | — | |||||
Transfer of inventory to fixed assets | 277 | — | — | ||||||||
Non cash financing activities: | |||||||||||
35
Cashless exercise of stock options/warrants | $ | 612 | $ | 3,237 | $ | 557 | |||||
The accompanying notes are an integral part of the consolidated financial statements.
36
NOTE 1. DESCRIPTION OF BUSINESS
Apyx Medical Corporation (“Company”, “Apyx Medical”, “we”, “us”, or “our”), formerly known as Bovie Medical Corporation, was incorporated in 1982, under the laws of the State of Delaware and has its principal executive office at 5115 Ulmerton Road, Clearwater, FL 33760.
We are an advanced energy technology company with a passion for elevating people’s lives through innovative products in the cosmetic and surgical markets. Known for our innovative Helium Plasma Technology, Apyx is solely focused on bringing transformative solutions to the physicians and patients we serve. Our Helium Plasma Technology is marketed and sold as Renuvion® in the cosmetic surgery market and J-Plasma® in the hospital surgical market. Renuvion® offers plastic surgeons, fascial plastic surgeons and cosmetic physicians a unique ability to provide controlled heat to the tissue to achieve their desired results. The J-Plasma® system allows surgeons to operate with a high level of precision and virtually eliminating unintended tissue trauma. We also leverage our deep expertise and decades of experience in unique waveforms through original equipment manufacturing (OEM) agreements with other medical device manufacturers.
NOTE 2. SIGNIFICANT ACCOUNTING POLICIES
Consolidated Financial Statements
The accompanying consolidated financial statements include the accounts of Apyx and its wholly owned subsidiary, Apyx Bulgaria, EOOD, (collectively, the “Company” or “we”, “our” or “us”). All intercompany transactions and balances have been eliminated in consolidation.
On August 30th, 2018, we sold our Core business and discontinued those operations.
The Company concluded that the divestiture of the Core business on August 30th, 2018 met the criteria for discontinued operations set forth in FASB ASC Topic No. 205, " Presentation of Financial Statements. " The Company reclassified its discontinued operations for all periods presented and has excluded the results of its discontinued operations from continuing operations and from segment results for all periods presented.
Use of Estimates in the Preparation of Financial Statements
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements. The reported amounts of revenues and expenses during the reporting period may be affected by the estimates and assumptions we are required to make.
Cash and Cash Equivalents
Holdings of highly liquid investments with original maturities of three months or less from the date of purchase are considered to be cash equivalents.
Short-term Investments
Our short-term investments principally consisted of US Treasury Bills, which are classified available-for-sale and are carried at their fair value as of the balance sheet date. The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. Short-term investments generally mature between three months and one year from the purchase date. Marketable securities less than or equal to three months are identified as cash equivalents while marketable securities with a maturity duration over three months are considered short term investments. Currently all of our US Treasury Bills have original maturities of three months or less and are included in cash and cash equivalents.
The Treasury Bill investments accrue interest monthly, which is treated as interest income. Realized gains or losses are determined on the specific identification method and are reflected in other income. Net unrealized gains and losses are recorded on a quarterly basis in interest income.
37
Fair Values of Financial Instruments and Concentration of Credit Risk
The carrying amounts of our financial instruments included in current assets and liabilities approximate fair value due to their short term nature.
Financial instruments, which potentially subject us to significant concentrations of credit risk, consists primarily of short term investments and trade accounts receivable. With respect to cash, we frequently maintain cash and cash equivalent balances in excess of federally insured limits. We have not experienced any losses in such accounts.
Accounts Receivable and Allowance for Doubtful Accounts
Our standard credit terms for our billings range from net 10 days to net 90 days, depending on the customer agreement. Accounts receivable are determined to be past due if payments are not made in accordance with such agreements and an allowance is generally recorded for accounts that become three months past due, or sooner if there are other indicators that the receivables may not be recovered. Customary collection efforts are initiated, and receivables are written off when we determine they are not collectible and abandon these collection efforts.
We evaluate the allowance for doubtful accounts on a regular basis for adequacy based upon our periodic review of the collectability of the receivables in light of historical experience, adverse situations that may affect our customers’ ability to pay and prevailing economic conditions. This evaluation is inherently subjective, as it requires estimates that are susceptible to significant revision as more information becomes available. Management believes that the allowances for doubtful accounts of approximately $0.3 million and $0.4 million at December 31, 2019 and 2018, respectively, are, or were, adequate to provide for possible bad debts.
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is determined on a first in, first out basis. Finished goods and work-in-process inventories include material, labor and overhead costs. Factory overhead costs are allocated to inventory manufactured in-house based upon labor hours.
We monitor usage reports to determine if the carrying value of any items should be adjusted due to lack of demand for the item and adjust the inventory for estimated obsolescence or unusable inventory equal to the difference between the cost of inventory and the estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by management, additional inventory write-downs may be required.
Property and Equipment
Property and equipment are recorded at cost. Depreciation and amortization are provided for using the straight-line method over the estimated useful lives of the assets. The amortization of leasehold improvements is based on the shorter of the lease term or the life of the improvement. Betterments and major improvements, which extend the life of the asset, are capitalized, whereas maintenance and repairs and routine improvements are expensed as incurred. The estimated useful lives are: machinery and equipment, 3-10 years; buildings, 39 years; molds, 7-15 years; furniture and fixtures, 5-10 years; and computer equipment and software, 3-5 years.
Goodwill
Goodwill of $0.2 million resulted from our acquisition of Apyx Bulgaria, EOOD and is included in other assets in the Company's consolidated balance sheets.
Valuation of Long-Lived Assets
We review long-lived assets for recoverability if events or changes in circumstances indicate that the assets may have been impaired. This circumstance exists when the carrying amount of the asset exceeds the sum of the undiscounted cash flows expected to result from its use and eventual disposition. In those cases an impairment loss is recognized to the extent that the assets’ carrying amount exceeds its fair value. Any impairment losses are not restored in the future if the fair value increases. At December 31, 2019, we believe the remaining carrying values of our long-lived assets are recoverable.
38
Product Warranties
We provide a four years limited warranty on end-user sales of our Renuvion®/J-Plasma® generators, a two years warranty on mounting fixtures, and a one year warranty on some accessories. We estimate and provide for future costs for product warranties in cost of sales at the time revenue is recognized. We base product warranty costs on related material costs, repair labor costs and shipping costs. We estimate the future cost of product warranties by considering historical material, repair labor, and shipping costs, and applying the experience rates to the outstanding warranty period for products sold. It is reasonably possible that actual results could differ from those estimates.
Revenue Recognition
ASU No. 2014-09 (ASC 606), Revenue from Contracts with Customers became effective for us beginning with the first quarter of 2018, and we adopted the new accounting standard using the modified retrospective transition approach. The modified retrospective transition approach recognized any changes from the beginning of the year of initial application through retained earnings with no restatement of comparative periods. Management performed an evaluation to determine the effects of adopting ASC 606 and we determined that the adoption and the application of the transition requirements of the new standard presented no material impact on our consolidated financial statements, and no entry was recorded to opening retained earnings. We have disaggregated revenue by segment and geography in Note 20 Geographic and Segment Information.
Revenue is recognized when a customer obtains control of promised goods or services in an amount that reflects the consideration that we expect to receive for those goods or services. To recognize revenue, we (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when, or as, we satisfy the performance obligation(s). For sales of our Advanced Energy products (Renuvion®/J-Plasma®), this is at a point in time when title has been transferred to the customer, which is generally at the time of shipment or receipt by customer for FOB destination terms. For sales of products under our OEM agreements, the Company recognizes revenue over time when no alternative use exists for the manufactured goods and the Company has rights to payment. Presently, the Company does not stock any significant completed goods under our OEM agreements, accordingly, the recognition of revenue under these agreements approximates point in time recognition. The following policies apply to our major categories of revenue transactions:
• | The majority of our sales to customers are evidenced by firm purchase orders. Generally, title and the risks and rewards of ownership are transferred to the customer when the product is shipped. Payment by the customer is due under fixed payment terms. |
• | Product returns are only accepted at our discretion and in accordance with our “Returned Goods Policy”. Historically, the level of product returns has not been significant. We accrue for sales returns, rebates and allowances as a reduction of revenue based upon an analysis of historical customer returns and credits, rebates, discounts and current market conditions. |
• | Our terms of sale to customers generally do not include any obligations to perform future services. Limited warranties are generally provided for sales and provisions for warranty are provided at the time of product sale based upon an analysis of historical data. |
• | In connection with the execution of our OEM supply agreements, the Company may enter into an accompanying product development agreement. If the Company enters into a product development agreement, and development of the goods does not represent a performance obligation on a standalone basis, the Company defers the development fees billed to customers and the associated costs. Recognition of the revenues and cost of sales occurs as revenue is recognized on the accompanying supply agreement. At December 31, 2019, the Company had recorded approximately $0.4 million of contract liabilities and $0.1 million of contract assets related to the deferral of revenues and expenses under these agreements. |
Advertising Costs
All advertising costs are expensed as incurred. The amounts of advertising costs, including trade shows, were approximately $1.5 million, $0.8 million and $1.1 million for the years ended December 31, 2019, 2018 and 2017, respectively.
39
Stock-Based Compensation
We account for stock-based compensation in accordance with FASB ASC Topic 718, Compensation-Stock Compensation. FASB ASC 718 requires recognizing compensation costs for all share-based payment awards made to employees, directors and non-employees based upon the awards’ grant date fair value. The Company currently accounts for forfeitures using the estimate method. The standard covers employee stock options, restricted stock and other equity awards. We currently utilize a Black-Scholes model and in prior years utilized a trinomial lattice option-pricing model to estimate the grant date fair value of stock option awards. For employee and director awards compensation cost is recognized on a straight-line basis over the awards’ vesting periods. For non-employee awards, compensation cost is recorded for non-forfeitable fully vested awards at the grant date. For other awards granted to non-employees, compensation cost is recognized as services are provided, which approximates a straight-line basis over the vesting period.
Litigation Contingencies
In accordance with authoritative guidance, we accrue a liability in our consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded; actual results may differ from those estimates.
Income (Loss) Per Share
We compute basic (loss) earnings attributable to common shareholders per share by dividing net (loss) income attributable to common shareholders by the weighted average number of common shares outstanding for the reporting period. Diluted (loss) earnings per share attributable to common shareholders gives effect to all potential dilutive shares outstanding during the period. The number of dilutive shares is calculated using the treasury stock method which reduces the effective number of shares by the amount of shares we could purchase with the proceeds of assumed exercises.
Research and Development Costs
Research and development expenses are charged to operations as incurred. We have expended approximately $3.7 million and $2.5 million and $1.9 million for the years ended 2019, 2018 and 2017 respectively.
Income Taxes
The Company utilizes the liability method of accounting for income taxes as set forth in FASB ASC Topic 740, "Income Taxes". Under the liability method, deferred taxes are determined based on temporary differences between the financial statement and tax bases of assets and liabilities using tax rates expected to be in effect during the years in which the basis difference. The Company accounts for interest and penalties on income taxes as income tax expense. A valuation allowances is recorded when it is more likely than not that a tax benefit will not be realized. In determining the need for valuation allowances the Company considers projected future taxable income, the timing of reversals of temporary differences, and the availability of tax planning strategies. As of December 31, 2019 and 2018, the Company recorded a valuation allowance on the net deferred tax asset.
The Company will reassess the realization of deferred tax assets each reporting period and will be able to reduce the valuation allowance to the extent the financial results of continuing operations improve and it becomes more likely than not that the deferred tax assets will be realizable. As Management expects the Company to continue to generate losses in the foreseeable future after 2019, the Company will continue to record a valuation allowance on the remaining deferred tax assets balance as of December 31, 2019.
We assess the financial statement impact of an uncertain tax position taken or expected to be taken on an income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized in the financial statements unless it is more likely than not of being sustained.
Foreign Currency Transactions
40
The functional currency of Apyx Bulgaria is the U.S. dollar. The monetary assets and liabilities that are denominated in a currency other than U.S. dollar are remeasured into U.S. dollars at the exchange rate on the balance sheet date, while nonmonetary items are remeasured at historical rates. Revenue and expenses are remeasured at weighted average exchange rates during the period. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in selling, general and administrative expenses in the consolidated statements of operations and were not material for the years ended December 31, 2019, 2018 and 2017.
Reclassifications
We have reclassified certain amounts presented in prior years to conform to the current year presentation. These reclassifications had no impact on previously reported net income, retained earnings or operating cash flows for the periods presented.
41
NOTE 3. RECENT ACCOUNTING PRONOUNCEMENTS
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). Topic 842 establishes a new lease model, referred to as the right-of-use model that brings substantially all leases on the balance sheet. This standard requires lessees to recognize leased assets and lease liabilities on the balance sheet and disclose key information about the leasing arrangements in their financial statements. Leases are classified as finance or operating, with classification affecting the pattern and classification of expense recognition in the statement of operations. The Company adopted Topic 842 effective January 1, 2019 using the modified retrospective transition approach that allows a reporting entity to use the effective date as its date of initial application and not restate the comparative periods in the period of adoption when transitioning to the new standard. Consequently, the requisite financial information and disclosures under the new standard are excluded for dates and periods prior to January 1, 2019. In addition, the Company elected to use a number of optional simplification and practical expedients permitted under the transition guidance within the new standard, including allowing the Company to combine fixed lease and non-lease components, apply the short-term lease exception to all leases of one year or less, and utilize the ‘package of practical expedients’, which permits the Company to not reassess prior accounting conclusions with respect to lease identification, lease classification and initial direct costs under Topic 842. Adoption of this new standard resulted in the recognition of approximately $212,000 of operating lease liabilities and right-of-user assets, which represents the present value of the remaining lease payments at the adoption date of approximately $221,000, discounted using the Company’s incremental borrowing rate of 4.00%. Please see Note 9 for a full discussion of the impacts of adoption on the current year consolidated financial statements.
No other new accounting pronouncement issued or effective during the fiscal year had or is expected to have a material impact on our consolidated financial statements or disclosures.
42
NOTE 4. RESTATEMENTS
Throughout 2019, the Company has been making efforts to remediate its material weaknesses in internal control as of December 31, 2018, including investing in new personnel that have expertise in a broad array of accounting topics. As a result of these investments and remediation efforts, the Company reevaluated the accounting for a broad array of items and discovered numerous immaterial errors. On March 12, 2020, our Management and the Audit Committee of the Board of Directors, following discussion with our predecessor independent registered public accounting firm, concluded that the Company's previously filed financial statements for the twelve months ended December, 31 2018 and the quarterly statements for the three and nine months ended September 30, 2018 and three months ended March 31, 2019, were no longer able to be relied upon as the result of the aggregation of errors identified by Management and the Company’s new accounting personnel during 2019 related to the following:
As identified during preparation of the fiscal year 2019 Form 10-K:
• | The Company reevaluated its subsidiary consolidation process and discovered an inaccuracy in its accounting for the elimination of markup on intercompany sales. This resulted in the Company incorrectly including the markup in US inventory purchased from Apyx Bulgaria and resulted in an overstatement of cost of sales and a corresponding understatement of other costs and expenses when the inventory was sold, which did not have any impact on net income (loss) or financial position. |
◦ | For the three months ended March 31, 2019, the total impact included increases to both gross profit and to operating expenses of approximately $113,000. |
• | During the first quarter of 2020, while reconciling the 2019 income tax provision back to the corresponding records, we determined that when employees exercised non-qualified stock options, we did not collect and remit the employee’s income and payroll taxes on the exercises and did not accrue and remit the employer portion of payroll taxes. Due to statutory requirements, we have joint and several liability on the amounts that we did not withhold from employees and remit to the proper taxing authorities. While further investigating the issue, we determined that during 2018 we did not report the correct amount of income to employees on their form W-2 for both non-qualified and incentive stock option exercises and misclassified some non-qualified stock option exercises as incentive stock option exercises. |
◦ | For the three and nine months ended September 30, 2018 and year ended December 31, 2018, the total aggregated impact included an increase of approximately $51,000 to operating expenses, an increase of approximately $713,000 to other losses and an increase to net loss of approximately $764,000. |
◦ | For the three months ended March 31, 2019, the total aggregated impact included an increase to operating expenses of $16,000, an increase of approximately $301,000 to other losses and an increase to net loss of approximately $317,000. |
• | Other minor items primarily related to the appropriate cutoff of transactions at the balance sheet date and the duplicate recording of a State income tax payment. |
◦ | For the three and nine months ended September 30, 2018, the total aggregated impact included an increase of approximately $59,000 to operating expenses, operating loss and net loss. |
◦ | For the year ended December 31, 2018, the total aggregated impact included a decrease to sales of $81,000, an increase to gross profit of $33,000, a decrease to operating income from continuing operations of $74,000 and a decrease to net loss from continuing operations of $56,000. |
◦ | For the three months ended March 31, 2019, the total aggregated impact included a decrease to operating loss of $90,000 and an increase to net loss of $40,000. |
43
As previously disclosed and adjusted in Form 10-Q for the three and nine months ended September 2019 filed on November 11, 2019:
• | The Company reevaluated its accounting for stock-based compensation expense and during the three months ended September 30, 2019, the Company discovered errors in its accounting for certain items included in stock-based compensation expense. These errors related to its accounting for forfeitures, the vesting periods over which the expense was recognized, modifications, fair value measurements, and other minor miscellaneous items, all of which relate to the prior year. Additionally, the Company identified an issue relating to grants in the first quarter of 2019, whereby compensation was not recognized over the correct vesting period. |
◦ | For the year ended December 31, 2018, the total impact included increases to operating expenses, operating loss and net loss of approximately $582,000 each. |
◦ | For the three months ended March 31, 2019, the total impact included increases to operating expenses, operating loss and net loss of approximately $453,000 each. |
• | During the three months ended September 30, 2019, the Company reevaluated its accounting for pre-development activities on certain OEM contracts. In performing the review, the Company determined that the it has not completed its performance obligations on its pre-development activities in these contracts. Accordingly, the Company determined that it had prematurely recognized revenues during the first quarter relating to these activities and did not defer the accompanying costs. |
◦ | For the three months ended March 31, 2019, the total impact included decreases to sales of approximately $194,000, decreases to operating expenses of approximately $77,000 and increases to both operating loss and net loss of approximately $117,000. |
The Company has made all of the restatement adjustments as of and for the year ending December 31, 2018 in the accompanying consolidated financial statements presented here. The Company will file 10-Q/A's for the three and nine months ended September 30, 2018 and the three months ended March 31, 2019 as soon as practicable.
A reconciliation of the originally reported amounts to the restated amounts for the adjustments noted above for each of the affected periods is presented below.
44
Consolidated Balance Sheet as of September 30, 2018:
(In thousands) | As Originally Reported | Adjustments | As Restated | |||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 40,663 | $ | — | $ | 40,663 | ||||||
Short term investments | 55,480 | — | 55,480 | |||||||||
Trade accounts receivable, net | 4,080 | 59 | 4,139 | |||||||||
Inventories, net | 6,037 | — | 6,037 | |||||||||
Prepaid expenses and other current assets | 627 | — | 627 | |||||||||
Total current assets | 106,887 | 59 | 106,946 | |||||||||
Property and equipment, net | 5,842 | — | 5,842 | |||||||||
Other assets* | 385 | — | 385 | |||||||||
Total assets | $ | 113,114 | $ | 59 | $ | 113,173 | ||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 2,348 | $ | — | $ | 2,348 | ||||||
Accrued expenses and other current liabilities | 19,324 | 764 | 20,088 | |||||||||
Total current liabilities | 21,672 | 764 | 22,436 | |||||||||
Related party note payable | 140 | — | 140 | |||||||||
Total liabilities | 21,812 | 764 | 22,576 | |||||||||
STOCKHOLDERS' EQUITY | ||||||||||||
Common stock, $0.001 par value; 75,000,000 shares authorized; 33,763,019 issued and 33,620,444 outstanding | 33 | — | 33 | |||||||||
Additional paid-in capital | 51,798 | — | 51,798 | |||||||||
Retained earnings | 39,471 | (705 | ) | 38,766 | ||||||||
Total stockholders’ equity | 91,302 | (705 | ) | 90,597 | ||||||||
Total liabilities and stockholders’ equity | $ | 113,114 | $ | 59 | $ | 113,173 | ||||||
* The Company has condensed the presentation of amounts presented in the balance sheet to conform to presentation guidelines. | ||||||||||||
45
Consolidated Statement of Operations for the three months ended September 30, 2018:
(In thousands) | As Originally Reported | Adjustments | As Restated | ||||||||
Sales | $ | 3,672 | $ | — | $ | 3,672 | |||||
Cost of sales | 1,151 | — | 1,151 | ||||||||
Gross profit | 2,521 | — | 2,521 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 613 | — | 613 | ||||||||
Professional services | 628 | — | 628 | ||||||||
Salaries and related costs | 2,119 | 51 | 2,170 | ||||||||
Selling, general and administrative | 1,957 | (59 | ) | 1,898 | |||||||
Total other costs and expenses | 5,317 | (8 | ) | 5,309 | |||||||
Loss from operations | (2,796 | ) | 8 | (2,788 | ) | ||||||
Interest income (expense), net | 105 | — | 105 | ||||||||
Other losses | (155 | ) | (713 | ) | (868 | ) | |||||
Total other losses, net | (50 | ) | (713 | ) | (763 | ) | |||||
Loss from continuing operations before income taxes | (2,846 | ) | (705 | ) | (3,551 | ) | |||||
Income tax benefit | (2,408 | ) | — | (2,408 | ) | ||||||
Net loss from continuing operations | (438 | ) | (705 | ) | (1,143 | ) | |||||
Income from discontinued operations, net of tax | 540 | — | 540 | ||||||||
Gain on sale of the Core Business, net of tax | 69,072 | — | 69,072 | ||||||||
Total income from discontinued operations, net of tax | 69,612 | — | 69,612 | ||||||||
Net income (loss) | $ | 69,174 | $ | (705 | ) | $ | 68,469 | ||||
Loss per share from continuing operations | |||||||||||
Basic and Diluted | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.03 | ) | ||
Income per share from discontinued operations | |||||||||||
Basic | 2.09 | — | 2.09 | ||||||||
Diluted | 1.99 | — | 1.99 | ||||||||
Income (loss) per share from all operations | |||||||||||
Basic | 2.08 | (0.02 | ) | 2.06 | |||||||
Diluted | 1.98 | (0.02 | ) | 1.96 | |||||||
Weighted average number of shares outstanding basic | 33,275 | 33,275 | 33,275 | ||||||||
Weighted average number of shares outstanding diluted | 34,934 | 34,934 | 34,934 | ||||||||
46
Consolidated Statement of Operations for the nine months ended September 30, 2018:
(In thousands) | As Originally Reported | Adjustments | As Restated | ||||||||
Sales | $ | 10,760 | $ | — | $ | 10,760 | |||||
Cost of sales | 3,490 | — | 3,490 | ||||||||
Gross profit | 7,270 | — | 7,270 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 1,890 | — | 1,890 | ||||||||
Professional services | 1,815 | — | 1,815 | ||||||||
Salaries and related costs | 5,734 | 51 | 5,785 | ||||||||
Selling, general and administrative | 6,280 | (59 | ) | 6,221 | |||||||
Total other costs and expenses | 15,719 | (8 | ) | 15,711 | |||||||
Loss from operations | (8,449 | ) | 8 | (8,441 | ) | ||||||
Interest income (expense), net | 33 | — | 33 | ||||||||
Other losses | (155 | ) | (713 | ) | (868 | ) | |||||
Change in fair value of derivative liabilities | 20 | — | 20 | ||||||||
Total other losses, net | (102 | ) | (713 | ) | (815 | ) | |||||
Loss from continuing operations before income taxes | (8,551 | ) | (705 | ) | (9,256 | ) | |||||
Income tax benefit | (2,384 | ) | — | (2,384 | ) | ||||||
Net loss from continuing operations | (6,167 | ) | (705 | ) | (6,872 | ) | |||||
Income from discontinued operations, net of tax | 5,062 | — | 5,062 | ||||||||
Gain on sale of the Core Business, net of tax | 69,072 | — | 69,072 | ||||||||
Total income from discontinued operations, net of tax | 74,134 | — | 74,134 | ||||||||
Net income (loss) | $ | 67,967 | $ | (705 | ) | $ | 67,262 | ||||
Loss per share from continuing operations | |||||||||||
Basic and Diluted | $ | (0.19 | ) | $ | (0.02 | ) | $ | (0.21 | ) | ||
Income per share from discontinued operations | |||||||||||
Basic | 2.25 | — | 2.25 | ||||||||
Diluted | 2.19 | — | 2.19 | ||||||||
Income (loss) per share from all operations | |||||||||||
Basic | 2.06 | (0.02 | ) | 2.04 | |||||||
Diluted | 2.00 | (0.02 | ) | 1.98 | |||||||
Weighted average number of shares outstanding basic | 33,014 | 33,014 | 33,014 | ||||||||
Weighted average number of shares outstanding diluted | 33,952 | 33,952 | 33,952 | ||||||||
47
Consolidated Balance Sheet as of December 31, 2018:
(In thousands) | As Originally Reported | Adjustments | As Restated | |||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 16,466 | $ | 130 | $ | 16,596 | ||||||
Short term investments | 61,678 | — | 61,678 | |||||||||
Trade accounts receivable, net** | 3,656 | 65 | 3,721 | |||||||||
Other receivables** | 1,359 | — | 1,359 | |||||||||
Inventories, net** | 3,061 | 85 | 3,146 | |||||||||
Prepaid expenses and other current assets** | 3,297 | 38 | 3,335 | |||||||||
Total current assets | 89,517 | 318 | 89,835 | |||||||||
Property and equipment, net | 5,788 | — | 5,788 | |||||||||
Other assets* | 305 | — | 305 | |||||||||
Total assets | $ | 95,610 | $ | 318 | $ | 95,928 | ||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 1,423 | $ | — | $ | 1,423 | ||||||
Accrued expenses and other current liabilities* | 6,279 | 909 | 7,188 | |||||||||
Total current liabilities | 7,702 | 909 | 8,611 | |||||||||
Related party note payable | 140 | — | 140 | |||||||||
Total liabilities | 7,842 | 909 | 8,751 | |||||||||
STOCKHOLDERS' EQUITY | ||||||||||||
Common stock, $0.001 par value; 75,000,000 shares authorized; 33,847,100 issued and 33,704,525 outstanding | 34 | — | 34 | |||||||||
Additional paid-in capital | 52,221 | 699 | 52,920 | |||||||||
Retained earnings | 35,513 | (1,290 | ) | 34,223 | ||||||||
Total stockholders’ equity | 87,768 | (591 | ) | 87,177 | ||||||||
Total liabilities and stockholders’ equity | $ | 95,610 | $ | 318 | $ | 95,928 | ||||||
* The Company has condensed the presentation of amounts presented in the balance sheet to conform to presentation guidelines. | ||||||||||||
** The Company has reclassified balances to conform to current presentation. | ||||||||||||
48
Consolidated Statement of Operations for the year ended December 31, 2018:
(In thousands) | As Originally Reported | Adjustments | As Restated | ||||||||
Sales | $ | 16,686 | $ | (81 | ) | $ | 16,605 | ||||
Cost of sales | 5,893 | (114 | ) | 5,779 | |||||||
Gross profit | 10,793 | 33 | 10,826 | ||||||||
Other costs and expenses: | |||||||||||
Research and development | 2,469 | 80 | 2,549 | ||||||||
Professional services | 3,072 | 61 | 3,133 | ||||||||
Salaries and related costs | 8,673 | 599 | 9,272 | ||||||||
Selling, general and administrative | 9,438 | (31 | ) | 9,407 | |||||||
Severance and related expense | 741 | — | 741 | ||||||||
Total other costs and expenses | 24,393 | 709 | 25,102 | ||||||||
Loss from operations | (13,600 | ) | (676 | ) | (14,276 | ) | |||||
Interest income | 616 | — | 616 | ||||||||
Interest expense | (104 | ) | — | (104 | ) | ||||||
Other losses | (203 | ) | (744 | ) | (947 | ) | |||||
Change in fair value of derivative liabilities | 20 | — | 20 | ||||||||
Total other income (losses), net | 329 | (744 | ) | (415 | ) | ||||||
Loss from continuing operations before income taxes | (13,271 | ) | (1,420 | ) | (14,691 | ) | |||||
Income tax benefit | (3,777 | ) | (130 | ) | (3,907 | ) | |||||
Net loss from continuing operations | (9,494 | ) | (1,290 | ) | (10,784 | ) | |||||
Income from discontinued operations, net of tax | 5,099 | — | 5,099 | ||||||||
Gain on sale of the Core Business, net of tax | 68,404 | — | 68,404 | ||||||||
Total income from discontinued operations, net of tax | 73,503 | — | 73,503 | ||||||||
Net income (loss) | $ | 64,009 | $ | (1,290 | ) | $ | 62,719 | ||||
Loss per share from continuing operations | |||||||||||
Basic and Diluted | $ | (0.29 | ) | $ | (0.04 | ) | $ | (0.32 | ) | ||
Income per share from discontinued operations | |||||||||||
Basic | 2.21 | — | 2.21 | ||||||||
Diluted | 2.14 | — | 2.14 | ||||||||
Income (loss) per share from all operations | |||||||||||
Basic | 1.93 | (0.04 | ) | 1.89 | |||||||
Diluted | 1.86 | (0.04 | ) | 1.83 | |||||||
Weighted average number of shares outstanding basic | 33,185 | 33,185 | 33,185 | ||||||||
Weighted average number of shares outstanding diluted | 34,366 | 34,366 | 34,366 | ||||||||
49
Consolidated Balance Sheet as of March 31, 2019:
(In thousands) | As Originally Reported | Adjustments | As Restated | |||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 32,415 | $ | — | $ | 32,415 | ||||||
Short term investments | 40,885 | — | 40,885 | |||||||||
Trade accounts receivable, net** | 4,006 | 38 | 4,044 | |||||||||
Other receivables** | 925 | — | 925 | |||||||||
Inventories, net** | 3,013 | — | 3,013 | |||||||||
Prepaid expenses and other current assets** | 3,996 | 38 | 4,034 | |||||||||
Total current assets | 85,240 | 76 | 85,316 | |||||||||
Property and equipment, net** | 6,015 | — | 6,015 | |||||||||
Operating lease right-of-use assets** | 178 | — | 178 | |||||||||
Other assets* | 270 | 77 | 347 | |||||||||
Total assets | $ | 91,703 | $ | 153 | $ | 91,856 | ||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 1,504 | $ | — | $ | 1,504 | ||||||
Accrued expenses and other current liabilities* | 5,868 | 1,141 | 7,009 | |||||||||
Current portion of operating lease liabilities** | 103 | — | 103 | |||||||||
Total current liabilities | 7,475 | 1,141 | 8,616 | |||||||||
Related party note payable | 140 | — | 140 | |||||||||
Long-term portion of operating lease liabilities | 75 | — | 75 | |||||||||
Other long-term liabilities | — | 194 | 194 | |||||||||
Total liabilities | 7,690 | 1,335 | 9,025 | |||||||||
STOCKHOLDERS' EQUITY | ||||||||||||
Common stock, $0.001 par value; 75,000,000 shares authorized; 34,033,255 issued and 33,891,255 outstanding | 34 | — | 34 | |||||||||
Additional paid-in capital | 53,147 | 1,035 | 54,182 | |||||||||
Retained earnings | 30,832 | (2,217 | ) | 28,615 | ||||||||
Total stockholders’ equity | 84,013 | (1,182 | ) | 82,831 | ||||||||
Total liabilities and stockholders’ equity | $ | 91,703 | $ | 153 | $ | 91,856 | ||||||
* The Company has condensed the presentation of amounts presented in the balance sheet to conform to presentation guidelines. | ||||||||||||
** The Company has reclassified balances to conform to current presentation. | ||||||||||||
50
Consolidated Statement of Operations for the three months ended March 31, 2019:
(In thousands) | As Originally Reported | Adjustments | As Restated | ||||||||
Sales | $ | 5,823 | $ | (194 | ) | $ | 5,629 | ||||
Cost of sales | 2,103 | (37 | ) | 2,066 | |||||||
Gross profit | 3,720 | (157 | ) | 3,563 | |||||||
Other costs and expenses: | |||||||||||
Research and development | 810 | (80 | ) | 730 | |||||||
Professional services | 1,791 | 327 | 2,118 | ||||||||
Salaries and related costs | 3,221 | 267 | 3,488 | ||||||||
Selling, general and administrative | 3,101 | (144 | ) | 2,957 | |||||||
Total other costs and expenses | 8,923 | 370 | 9,293 | ||||||||
Loss from operations | (5,203 | ) | (527 | ) | (5,730 | ) | |||||
Interest income | 423 | — | 423 | ||||||||
Other losses | (25 | ) | (270 | ) | (295 | ) | |||||
Total other losses, net | 398 | (270 | ) | 128 | |||||||
Loss from continuing operations before income taxes | (4,805 | ) | (797 | ) | (5,602 | ) | |||||
Income tax (benefit) expense | (124 | ) | 130 | 6 | |||||||
Net loss | $ | (4,681 | ) | $ | (927 | ) | $ | (5,608 | ) | ||
Loss per share | |||||||||||
Basic and Diluted | $ | (0.14 | ) | $ | (0.03 | ) | $ | (0.17 | ) | ||
Weighted average number of shares outstanding basic and diluted | 33,343 | 33,343 | 33,343 | ||||||||
NOTE 5. REVISIONS
As a part of the efforts discussed in Note 4, the Company discovered additional errors in the consolidated financial statements for the three and six months ended June 30, 2019 and the three and nine months ended September 30, 2019. The Company has determined that the aggregated effects of correcting these errors are immaterial to the financial statements for the three and six month periods ending June 30, 2019 and the three and nine months ended September 30, 2019 but would materially misstate the three month period ended December 31, 2019 if recorded then, and has accordingly corrected the errors by revising the previously reported amounts for the three and six month periods ended June 30, 2019 and the three and nine month period ended September 30, 2019 in the following tables. The revised amounts below will be disclosed in the next filing discussing the periods that were subject to the revision.
A summary of the revisions as of each reporting period affected is as follows:
51
For the three months ended June 30, 2019:
(In thousands) | As Reported | Adjustments | As Revised | ||||||||||
Balance Sheet | |||||||||||||
Trade accounts receivable, net | $ | 5,233 | $ | 59 | $ | 5,292 | [3] | ||||||
Accrued and other liabilities | 6,149 | 1,081 | 7,230 | [1] | |||||||||
Retained earnings | 25,342 | (1,022 | ) | 24,320 | [1],[3] | ||||||||
Statement of Operations | |||||||||||||
Sales | $ | 6,568 | $ | 81 | $ | 6,649 | [3] | ||||||
Cost of sales | 2,096 | (121 | ) | 1,975 | [2],[3] | ||||||||
Professional services | 1,633 | 28 | 1,661 | [2] | |||||||||
Salaries and related costs | 3,333 | 177 | 3,510 | [2] | |||||||||
Selling, general and administrative | 3,083 | (46 | ) | 3,037 | [2] | ||||||||
Loss per share - all | $ | (0.13 | ) | $ | — | $ | (0.13 | ) | [2],[3] | ||||
Gross profit | 4,472 | 202 | 4,674 | [2] | |||||||||
Gross profit % | 68.1 | % | 2.2 | % | 70.3 | % | [2] | ||||||
[1] Adjustments relate to payroll tax corrections | |||||||||||||
[2] Adjustments relate to intercompany gross profit | |||||||||||||
[3] Other corrections primarily related to balance sheet cutoff | |||||||||||||
For the six months ended June 30, 2019:
(In thousands) | As Reported | Adjustments | As Revised | ||||||||||
Balance Sheet | |||||||||||||
Trade accounts receivable, net | $ | 5,233 | $ | 59 | $ | 5,292 | [3] | ||||||
Accrued and other liabilities | 6,149 | 1,081 | 7,230 | [1] | |||||||||
Retained earnings | 25,342 | (1,022 | ) | 24,320 | [1],[3] | ||||||||
Statement of Operations | |||||||||||||
Sales | $ | 12,197 | $ | 81 | $ | 12,278 | [3] | ||||||
Cost of sales | 4,199 | (158 | ) | 4,041 | [2],[3] | ||||||||
Research and development | 1,698 | (80 | ) | 1,618 | [2],[3] | ||||||||
Professional services | 3,760 | 19 | 3,779 | [2],[3] | |||||||||
Salaries and related costs | 6,671 | 327 | 6,998 | [1],[2] | |||||||||
Selling, general and administrative | 6,107 | (113 | ) | 5,994 | [2],[3] | ||||||||
Other income (losses), net | (225 | ) | (270 | ) | (495 | ) | [1],[3] | ||||||
Loss per share - all | $ | (0.29 | ) | $ | (0.01 | ) | $ | (0.30 | ) | [1],[3] | |||
Gross profit | 7,998 | 239 | 8,237 | [2],[3] | |||||||||
Gross profit % | 65.6 | % | 1.5 | % | 67.1 | % | [2],[3] | ||||||
[1] Adjustments relate to payroll tax corrections | |||||||||||||
[2] Adjustments relate to intercompany gross profit | |||||||||||||
[3] Other corrections primarily related to balance sheet cutoff | |||||||||||||
52
For the three months ended September 30, 2019:
(In thousands) | As Reported | Adjustments | As Revised | ||||||||||
Balance Sheet | |||||||||||||
Accrued expenses and other liabilities | $ | 7,285 | $ | 1,081 | $ | 8,366 | [1] | ||||||
Retained earnings | 21,031 | (1,081 | ) | 19,950 | [1] | ||||||||
Statement of Operations | |||||||||||||
Cost of sales | $ | 2,558 | $ | (277 | ) | $ | 2,281 | [2] | |||||
Research and development | 936 | 80 | 1,016 | [2] | |||||||||
Professional services | 1,996 | 43 | 2,039 | [2] | |||||||||
Salaries and related costs | 3,020 | 139 | 3,159 | [2] | |||||||||
Selling, general and administrative | 3,762 | 74 | 3,836 | [2],[3] | |||||||||
Loss per share - all | $ | (0.13 | ) | $ | — | $ | (0.13 | ) | [3] | ||||
Gross profit | 5,017 | 277 | 5,294 | [2] | |||||||||
Gross profit % | 66.2 | % | 3.7 | % | 69.9 | % | [2] | ||||||
[1] Adjustments relate to payroll tax corrections | |||||||||||||
[2] Adjustments relate to intercompany gross profit | |||||||||||||
[3] Other corrections primarily related to balance sheet cutoff | |||||||||||||
For the nine months ended September 30, 2019:
(In thousands) | As Reported | Adjustments | As Revised | ||||||||||
Balance Sheet | |||||||||||||
Accrued expenses and other liabilities | $ | 7,285 | $ | 1,081 | $ | 8,366 | [1] | ||||||
Retained earnings | 21,031 | (1,081 | ) | 19,950 | [1] | ||||||||
Statement of Operations | |||||||||||||
Sales | $ | 19,772 | $ | 81 | $ | 19,853 | [3] | ||||||
Cost of sales | 6,757 | (435 | ) | 6,322 | [2],[3] | ||||||||
Research and development | 2,634 | — | 2,634 | [2],[3] | |||||||||
Professional services | 5,756 | 62 | 5,818 | [2],[3] | |||||||||
Salaries and related costs | 9,691 | 466 | 10,157 | [1],[2] | |||||||||
Selling, general and administrative | 9,869 | (39 | ) | 9,830 | [2],[3] | ||||||||
Other income (losses), net | 5 | (270 | ) | (265 | ) | [1],[3] | |||||||
Loss per share - all | $ | (0.41 | ) | $ | (0.01 | ) | $ | (0.42 | ) | [1], [3] | |||
Gross profit | 13,015 | 516 | 13,531 | [2],[3] | |||||||||
Gross profit % | 65.8 | % | 2.4 | % | 68.2 | % | [2],[3] | ||||||
[1] Adjustments relate to payroll tax corrections | |||||||||||||
[2] Adjustments relate to intercompany gross profit | |||||||||||||
[3] Other corrections primarily related to balance sheet cutoff | |||||||||||||
53
NOTE 6. DISPOSITION OF THE CORE BUSINESS
On August 30, 2018, we closed on a definitive asset purchase agreement (the "Asset Purchase Agreement") with Specialty Surgical Instrumentation Inc., a Tennessee Corporation and wholly-owned subsidiary of Symmetry Surgical Inc. (“Symmetry”), pursuant to which the Company divested and sold the Company's electrosurgical "Core" business segment and related intellectual property, including the Bovie® brand and trademarks, to Symmetry for gross proceeds of $97 million in cash.
In connection with the Asset Purchase Agreement, we entered into an Electro Surgical Disposables and Accessories, Cauteries and Other Products Supply Agreement with Symmetry for a four-year term, whereby we will manufacture certain Core products and sell them to Symmetry at agreed upon prices. Any revenue, costs and expenses resulting from this agreement are netted and reported in our Consolidated Statements of Operations as other gains or (losses) in the amount of $0.1 million and $(0.2) million for 2019 and 2018, respectively. Core activity in 2019 amounted to $9.4 million with cost of sales of $8.8 million and related operating expenses of $0.5 million. Core activity in 2018 following the divestiture amounted to $1.5 million with cost of sales of $1.5 million and related operating expenses of $0.2 million.
Additionally, in connection with the Asset Purchase Agreement, we entered into a Manufacture and Supply Agreement with Symmetry for a ten-year term, whereby we will manufacture certain products and sell them to Symmetry at agreed upon prices. Revenue, costs and expenses resulting from this agreement are reported as a component in our Consolidated Statements as income or loss from operations of our OEM reporting segment.
We concluded that the divestiture of the Core business met the criteria for discontinued operations set forth in FASB ASC Topic No. 205, "Presentation of Financial Statements". Gross sales of the Core business prior to the divestiture during 2018 amounted to $19.6 million with a cost of sales of $10.5 million and related operating expenses of $2.8 million. The table below summarizes the cash consideration and the carrying values of disposed assets at the disposition date of August 30, 2018 included as part of discontinued operations:
(In thousands) | |||
Gross consideration from the sale of the Core Business | $ | 97,000 | |
Closing and transaction costs | 5,905 | ||
Net proceeds from sale of the Core Business before taxes | $ | 91,095 | |
Non-cash commitment to provide inventory | $ | 2,305 | |
Book value of the Core Business | |||
Current assets: | |||
Inventories, net | $ | 2,195 | |
Prepaid expenses and other current assets | 57 | ||
Total current assets | 2,252 | ||
Property and equipment, net of depreciation | 375 | ||
Brand name and trademark | 1,510 | ||
Purchased technology and license rights, net of depreciation | 112 | ||
Total non-current assets | 1,997 | ||
Total assets | $ | 4,249 | |
Net gain on sale of the Core Business before taxes | 84,541 | ||
Income tax expense | 16,137 | ||
Net gain on sale of the Core Business after income taxes | $ | 68,404 | |
Cash flows associated with discontinued operations are shown in the table below:
54
(in thousands) | 2019 | 2018 | 2017 | |||
Net Income from discontinued operations | — | 73,503 | 8,620 | |||
Depreciation and amortization | — | 126 | 529 | |||
Change in current assets from discontinued operations | — | (2,378 | ) | 362 | ||
Change in non current assets from discontinued liabilities | — | (1,997 | ) | (632 | ) | |
Change in current liabilities from discontinued operations | — | (1,021 | ) | (1,451 | ) | |
Net cash provided by operating activities | — | 68,233 | 7,428 | |||
55
NOTE 7. INVENTORIES
Inventories consisted of the following:
(In thousands) | December 31, 2019 | December 31, 2018 as Restated | |||||
Raw materials | $ | 2,935 | $ | 2,594 | |||
Work in process | 1,209 | 406 | |||||
Finished goods | 1,316 | 585 | |||||
Gross inventories | 5,460 | 3,585 | |||||
Less: provision for obsolescence | (392 | ) | (439 | ) | |||
Inventories, net | $ | 5,068 | $ | 3,146 | |||
NOTE 8. PROPERTY AND EQUIPMENT
Property and equipment consisted of the following:
(In thousands) | December 31, 2019 | December 31, 2018 | |||||
Land | $ | 1,600 | $ | 1,600 | |||
Building and improvements | 4,423 | 4,338 | |||||
Machinery and equipment | 2,187 | 2,881 | |||||
Furniture and fixtures | 292 | 461 | |||||
Computer equipment and software | 1,409 | 1,741 | |||||
Leasehold improvements | 156 | 108 | |||||
Molds | 805 | 1,017 | |||||
Total property, plant and equipment | 10,872 | 12,146 | |||||
Less: accumulated depreciation and amortization | (4,403 | ) | (6,358 | ) | |||
Property and equipment in service | 6,469 | 5,788 | |||||
Construction in process | 149 | — | |||||
Property and equipment, net | $ | 6,618 | $ | 5,788 | |||
Total depreciation expense from continuing operations was $0.7 million, $0.4 million and $0.6 million for the years ended December 31, 2019, 2018 and 2017, respectively. Depreciation expense is included primarily within cost of goods sold in the consolidated statements of operations.
56
NOTE 9. LEASES
The Company does not recognize leases with terms less than twelve months in duration in our consolidated balance sheet as right-of-use assets and lease liabilities. Leases with an initial term of 12 months or less or that have variable only payments are not recorded on the balance sheet. The Company has adopted the practical expedient which allows for the Company to not separate lease and non-lease components of contracts. Accordingly, non-lease components are included in the measurement of the Company's leases and right-of-use assets. If the Company is aware of the implicit rate in leases, the Company determines the operating lease liability using the implicit rate. For those leases where the Company is not aware of the implicit rate in the lease, the Company utilizes an incremental borrowing rate of 4.00%, which is indicative of our collateralized borrowing rate.
Operating Leases
The Company leases its facility in Sofia, Bulgaria and vehicles in Clearwater, Florida under non-cancelable operating lease agreements. The Company's lease on the Bulgaria facility includes rent escalation over the term of the lease. Rent expense on the lease is accounted for on a straight-line basis over the lease term. During 2019, the Bulgaria facility lease was extended for an additional 2 years. In accordance with operating lease guidance under Topic 842, the extension was accounted for as a lease modification and the right-of-use asset and lease liability were remeasured at the modification date. The Company's operating leases have terms expiring through December 2022.
Finance Leases
During 2019, the Company entered into non-cancelable finance leases for certain computer equipment and a vehicle in Clearwater, Florida. The Company's finance leases have terms expiring through August 2023.
Information about the Company’s lease costs are as follows:
Year Ended December 31, 2019 | |||
Lease costs (in thousands): | |||
Operating lease costs | $ | 115 | |
Finance lease costs: | |||
Amortization of right-of-use assets | 57 | ||
Interest on lease liabilities | 8 | ||
Variable lease costs | 16 | ||
Total lease costs | $ | 196 | |
Cash and non cash information related to our leases are as follows:
Year Ended December 31, 2019 | ||||||
(in thousands) | Operating | Finance | ||||
Non cash information: | ||||||
Right-of-use assets capitalized and lease liabilities recognized upon adoption of Topic 842 | $ | 212 | $ | — | ||
Right-of-use assets capitalized and lease liabilities recognized upon lease remeasurement | $ | 207 | $ | — | ||
Right-of-use assets capitalized and lease liabilities recognized upon execution of lease | $ | 28 | $ | 710 | ||
Cash information: | ||||||
Cash paid for lease liabilities | $ | 106 | $ | 68 | ||
57
Information about the Company’s weighted average remaining lease terms and discount rate assumptions are as follows:
Year Ended December 31, 2019 | ||
Operating | Finance | |
Weighted average remaining lease term (in years) | 3.0 | 2.7 |
Weighted average discount rate | 4.04% | 4.00% |
Maturities of lease liabilities as of December 31, 2019 are as follows:
(In thousands) | Operating | Finance | ||||
2020 | $ | 120 | $ | 251 | ||
2021 | 124 | 236 | ||||
2022 | 121 | 183 | ||||
2023 | — | 18 | ||||
Total lease payments | 365 | 688 | ||||
Less imputed interest | (22 | ) | (38 | ) | ||
Present value of lease liabilities | 343 | 650 | ||||
Less current portion of lease liabilities | (108 | ) | (229 | ) | ||
Long-term portion of lease liabilities | $ | 235 | $ | 421 | ||
NOTE 10. ACCRUED EXPENSES AND OTHER CURRENT LIABILTIES
Accrued expenses and other current liabilities consisted of the following:
(in thousands) | December 31, 2019 | December 31, 2018 as Restated | |||||
Accrued severance and related | $ | 116 | $ | 610 | |||
Accrued payroll | 694 | 418 | |||||
Accrued bonus | 1,306 | 972 | |||||
Accrued commissions | 877 | 379 | |||||
Accrued product warranties | 452 | 348 | |||||
Accrued insurance | 1,170 | 725 | |||||
Accrued professional fees | 1,383 | 331 | |||||
Joint and several payroll liability | 1,045 | 713 | |||||
Uncertain tax positions | 1,491 | 1,325 | |||||
Other accrued expenses and current liabilities | 862 | 1,367 | |||||
Total accrued expenses and other current liabilities | $ | 9,396 | $ | 7,188 | |||
NOTE 11. PRODUCT WARRANTIES
Our product warranty activity consisted of the following for the years ended:
(In thousands) | December 31, 2019 | December 31, 2018 | |||||
Beginning balance | $ | 348 | $ | 233 | |||
Provision for product warranties | 321 | 253 | |||||
Product warranty expenses incurred | (217 | ) | (138 | ) | |||
Accrued product warranties | $ | 452 | $ | 348 | |||
NOTE 12. JOINT AND SEVERAL PAYROLL LIABILITY
As discussed in Restatements (Note 4) and Revisions (Note 5), the Company did not report the correct amount of income to employees, nor did we collect and remit the employees' portion of income and payroll taxes, related to stock option exercises as required by the IRS. Due to IRS statutory requirements, we have joint and several liability for the full amount that was not withheld and remitted to the proper taxing authorities. This amount of the liability was approximately $1.0 million and $0.7 million at December 31, 2019 and 2018 respectively. The Company has recognized these amounts in other income (losses), net in the accompanying Consolidated Statements of Operations. If we can establish that our employees have in fact paid these obligations, either presently or in the future, we will be relieved of our liability.
58
NOTE 13. EARNINGS PER SHARE
We compute basic earnings per share (“basic EPS”) by dividing the net income or loss by the weighted average number of common shares outstanding for the reporting period. Diluted earnings per share (“diluted EPS”) gives effect to all dilutive potential shares outstanding. The following table provides the computation of basic and diluted earnings per share.
Year Ended December 31, | |||||||||||
(in thousands, except per share data) | 2019 | 2018 as Restated | 2017 | ||||||||
Numerators: | |||||||||||
Net (loss) and numerator for dilutive (loss) per share - continuing operations | $ | (19,706 | ) | $ | (10,784 | ) | $ | (13,682 | ) | ||
Numerator for dilutive income per share - discontinued operations | — | 73,503 | 8,620 | ||||||||
Net income (loss) from all operations | (19,706 | ) | 62,719 | (5,062 | ) | ||||||
Derivative liability warrants | — | — | (183 | ) | |||||||
Numerator for full dilutive (loss) income per share - all | (19,706 | ) | 62,719 | (5,245 | ) | ||||||
Denominator for dilutive income (loss) per common share - continuing operations | 34,069 | 33,185 | 31,420 | ||||||||
Denominator - discontinued operations: | |||||||||||
Weighted average shares used to compute basic (loss) | 34,069 | 33,185 | 31,420 | ||||||||
Effect of dilutive securities: | |||||||||||
Stock options | — | 1,181 | — | ||||||||
Denominator for dilutive income (loss) per common share - discontinued operations | 34,069 | 34,366 | 31,420 | ||||||||
Denominator - all operations: | |||||||||||
Weighted average shares used to compute basic income (loss) | 34,069 | 33,185 | 31,420 | ||||||||
Effect of dilutive securities: | |||||||||||
Derivative liability warrants | — | — | 7 | ||||||||
Stock options | — | 1,181 | — | ||||||||
Denominator for dilutive income (loss) per common share | 34,069 | 34,366 | 31,427 | ||||||||
Loss per share from continuing operations | |||||||||||
Basic and diluted | $ | (0.58 | ) | $ | (0.32 | ) | $ | (0.44 | ) | ||
Income per share from discontinued operations | |||||||||||
Basic | $ | — | $ | 2.21 | $ | 0.27 | |||||
Diluted | $ | — | $ | 2.14 | $ | 0.27 | |||||
Income (loss) per share from all operations | |||||||||||
Basic | $ | (0.58 | ) | $ | 1.89 | $ | (0.16 | ) | |||
Diluted | $ | (0.58 | ) | $ | 1.83 | $ | (0.17 | ) | |||
Anti-dilutive instruments excluded from diluted (loss) per common share - continuing operations: | |||||||||||
Warrants | — | — | 7 | ||||||||
Options | 3,967 | 1,181 | 646 | ||||||||
Anti-dilutive instruments excluded from diluted income per common share - discontinued operations: | |||||||||||
Warrants | — | — | 7 | ||||||||
59
Options | 3,967 | — | 646 | ||||||||
Anti-dilutive instruments excluded from diluted income (loss) per common share - all operations: | |||||||||||
Options | 3,967 | — | 646 | ||||||||
60
NOTE 14. FINANCIAL INSTRUMENTS
Cash and Cash Equivalents at December 31, 2019, consists of approximately $2,237,000 in cash and $56,575,000 in US Treasury Securities with maturities of 3 months or less.
Cash, Cash Equivalents and Marketable Securities, as of December 31, 2018 consists of the following:
(In thousands) | Adjusted Cost | Unrealized Gains(3) | Fair Value(3) | Cash and Cash Equivalents (1) | Short-term Marketable Securities | ||||||||||||||
Cash - As Restated | $ | 6,467 | $ | — | $ | 6,467 | $ | 6,467 | $ | — | |||||||||
Level 1 (2) | |||||||||||||||||||
U.S. Treasury Securities, maturities less than three months | 10,129 | — | 10,129 | 10,129 | — | ||||||||||||||
U.S. Treasury Securities, maturities greater than three months | 61,431 | 247 | 61,678 | — | 61,678 | ||||||||||||||
Total | $ | 78,027 | $ | 247 | $ | 78,274 | $ | 16,596 | $ | 61,678 | |||||||||
(1) The company considers all highly liquid instruments with maturities of three months or less at the time of purchase to be cash equivalents.
(2) The fair value of the debt securities consisting of U.S. Treasury bills is based on their quoted market prices. The fair value of these financial instruments are classified as Level 1 in the fair value hierarchy. The original purchase of U.S. Treasury bills occurred in 2018 utilizing the proceeds from the sale of our Core business.
(3) ASC 825-10, Financial Instruments, allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable, unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings within interest income at each subsequent reporting date. At the date of purchase, the Company elected the fair value option for all investments with maturities of three months or greater at the time of purchase.
NOTE 15. INCOME TAXES
61
Components of the provision for income taxes from continuing operations are as follows:
(In thousands) | December 31, 2019 | December 31, 2018 as Restated | December 31, 2017 | ||||||||
Current: | |||||||||||
Federal | $ | (12 | ) | $ | (3,073 | ) | $ | — | |||
State | (205 | ) | (884 | ) | 22 | ||||||
Foreign | 87 | 20 | 17 | ||||||||
(130 | ) | (3,937 | ) | 39 | |||||||
Deferred: | |||||||||||
Federal | (3,989 | ) | (171 | ) | 1,581 | ||||||
State | (741 | ) | 7 | (401 | ) | ||||||
(4,730 | ) | (164 | ) | 1,180 | |||||||
Valuation allowance | 4,730 | 194 | (1,375 | ) | |||||||
Total provision for income tax from continuing operations | $ | (130 | ) | $ | (3,907 | ) | $ | (156 | ) | ||
The Company recognized tax expense of $1.20 million attributable to income from discontinued operations and $16.14 million attributable to the gain on sales of the Core business in the Income Statement in the year ended December 31, 2018.
Below is a reconciliation of the statutory federal income tax rate to our effective tax rate:
Year Ended December 31, | ||||||||
2019 | 2018 | 2017 | ||||||
Federal tax provision | 21.0 | % | 21.0 | % | 34.0 | % | ||
State taxes (net of federal benefit) | 4.3 | % | 5.6 | % | 4.8 | % | ||
Warrant gains | — | % | — | % | 0.4 | % | ||
Valuation allowance | (23.8 | )% | (1.3 | )% | 28.9 | % | ||
Change in federal tax rate | — | % | — | % | (71.2 | )% | ||
Other | (0.8 | )% | 1.3 | % | 6.2 | % | ||
Total | 0.7 | % | 26.6 | % | 3.1 | % | ||
Major components of the Company’s deferred tax assets (liabilities) are as follows:
62
(In thousands) | December 31, 2019 | December 31, 2018 as Restated | December 31, 2017 | ||||||||
Deferred tax assets: | |||||||||||
Loss and credit carry-forwards | $ | 4,779 | $ | — | $ | 7,722 | |||||
Stock-based compensation | 1,004 | 721 | 549 | ||||||||
Inventory Reserve | 99 | 115 | 494 | ||||||||
Intangibles | 34 | 146 | — | ||||||||
Other | 1,000 | 910 | 178 | ||||||||
Total deferred tax assets | 6,916 | 1,892 | 8,943 | ||||||||
Valuation allowance | (6,472 | ) | (1,742 | ) | (8,756 | ) | |||||
Total deferred tax assets, net of valuation allowance | 444 | 150 | 187 | ||||||||
Deferred tax liabilities: | |||||||||||
State taxes (capital) | — | — | (17 | ) | |||||||
Property and equipment | (245 | ) | (150 | ) | (294 | ) | |||||
Intangibles | — | — | (244 | ) | |||||||
Lease right-of-use assets | (199 | ) | — | — | |||||||
Total deferred tax liabilities | (444 | ) | (150 | ) | (555 | ) | |||||
Net deferred tax liabilities | $ | — | $ | — | $ | (368 | ) | ||||
We consider all positive and negative evidence regarding the realization of deferred tax assets, including past operating results and future sources of taxable income.
We consider the earnings of Apyx Bulgaria, EOOD to be indefinitely invested outside the United States on the basis of estimates that future domestic cash generation will be sufficient to meet future domestic cash needs and our specific plans for reinvestment of those subsidiary earnings. We have not recorded a deferred tax liability related to the U.S. Federal and State income taxes and foreign withholding taxes on the undistributed earnings of Apyx Bulgaria, EOOD indefinitely invested outside the United States. If we decide to repatriate the foreign earnings, we would need to adjust our income tax provision in the period we determined that the earnings will no longer be indefinitely invested outside the United States.
We assess the financial statement impact of an uncertain tax position taken or expected to be taken on an income tax return at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized in the financial statements unless it is more likely than not of being sustained. As of December 31, 2019 and 2018, we have reserved approximately $1.3 million of potential tax benefits and accrued approximately $0.2 million of interest and penalties on these positions. It is expected that the amount of unrecognized tax benefit change within the next 12 months will not be significant.
The following is a roll-forward of the Company's total gross unrecognized tax benefits, not including interest and penalties, for the year ended December 31, 2019.
(in thousands) | Gross Unrealized Tax Benefits | |||
Balance at January 1, 2019 | $ | 1,313 | ||
Additions of tax positions related to the current year | — | |||
Additions of tax positions related to the prior year | — | |||
Decreases for tax positions related to prior year | — | |||
Balance at December 31, 2019 | $ | 1,313 | ||
63
The Company is subject to U.S. federal and state income tax examination. The Company’s 2016 through 2018 U.S. federal income tax returns are subject to examination by the Internal Revenue Service. The Company’s state income tax returns are subject to examination for the 2015 through 2018 tax years.
NOTE 16. RETIREMENT PLAN
The Company provides a tax-qualified profit-sharing retirement plan under section 401(k) of the Internal Revenue Code for the benefit of eligible employees with an accumulation of funds for retirement on a tax-deferred basis and provides for annual discretionary contribution to individual trust funds.
All employees are eligible to participate upon completing three months of service. The employees may make voluntary contributions to the plan up to the maximum percentage allowed by the Internal Revenue Code. Vesting in employee matching contributions is graded and depends on the years of service. After three years from their date of hire, the employees are 100% vested. The Company makes matching contributions of 50% of the employee contributions up to a total of 3% of participant payroll. Matching contributions made by the Company totaled $0.3 million for each of the years ended December 31, 2019, 2018 and 2017, respectively.
NOTE 17. RELATED PARTY TRANSACTIONS
Several relatives of Nikolay Shilev, Apyx Bulgaria’s Managing Director, are considered related parties. Teodora Shileva, Mr. Shilev’s spouse, is an employee of the Company working in the accounting department. Antoaneta Dimitrova Shileva-Toromanova, Mr. Shilev’s sister, is the Manager of Production and Human Resources. Svetoslav Shilev, Mr. Shilev’s son, is an engineer in the quality assurance department.
In addition, as part of the purchase of the Bulgaria manufacturing facility, Mr. Shilev was issued a note payable for $0.1 million to be paid 5 years after the original purchase date, which is in October 2020.
NOTE 18. COMMITMENTS AND CONTINGENCIES
Litigation
The medical device industry is characterized by frequent claims and litigation, and we are and may become subject to various claims, lawsuits and proceedings in the ordinary course of our business. Such claims include claims by current or former employees, distributors and competitors, claims concerning the marketing and promotion of our products and product liability claims.
We are involved in a number of legal actions relating to the use of our Helium Plasma technology. The outcomes of these legal actions are not within our complete control and may not be known for prolonged periods of time. We believe that such claims are adequately covered by insurance; however, in the case of one of our carriers, we are in a dispute regarding the total level of coverage available. Notwithstanding the foregoing, in the opinion of management, the Company has meritorious defenses and such claims are not expected, individually or in the aggregate, to result in a material, adverse effect on our financial condition. However, in the event that damages exceed the aggregate coverage limits of our policies or if our insurance carriers disclaim coverage, we believe it is possible that costs associated with these claims could have a material adverse impact on our consolidated results of operations, financial position or cash flows.
In addition, as previously disclosed with the U.S. Securities and Exchange Commission on the Company’s Report on Form 8-K filed April 26, 2019, on April 17, 2019, a complaint (the “Complaint”) was filed in the United States District Court for the Middle District of Florida by plaintiff Kyle Pritchard, individually and on behalf of all others similarly situated against the Company and Charles D. Goodwin (“Goodwin”), the Company’s President and Chief Executive Officer and a member of the Company’s Board of Directors, alleging certain violations of the Securities Exchange Act of 1934, as amended. On July 16, 2019, the Court appointed a lead plaintiff for the putative class and approved the lead plaintiff’s selection of counsel. On or about September 3, 2019, Plaintiff filed an amended complaint (the “Amended Complaint”) with the Court.
The Amended Complaint seeks class action status on behalf of all persons and entities that acquired the Company’s securities between December 21, 2018 and April 1, 2019 and alleges violations by the Company and Goodwin of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended and Rule 10b-5 thereunder, primarily related to certain public statements concerning the Premarket Notification 510(k) submission made to the US Food and Drug Administration for a new indication for
64
the Company’s J-Plasma® technology for use in dermal resurfacing procedures. The Amended Complaint seeks an unspecified amount of compensatory damages, an award of interest, reasonable attorneys’ fees, expert fees and other costs, and equitable relief as the court may deem just and proper. On October 3, 2019, the Company and Goodwin filed a Motion to Dismiss the Amended Complaint. Plaintiff’s opposition to the motion to dismiss was served on November 4, 2019. On March 11, 2020, the Court issued an order denying the Company’s motion to dismiss. The Company intends to vigorously defend its interests against the allegations contained in the complaint.
Although the ultimate outcome of this matter cannot be determined with certainty, the Company believes that the allegations stated in the Amended Complaint are entirely without merit. The Company and Goodwin intend to defend themselves vigorously in the suit. In the opinion of management, such claims are adequately covered by insurance, however, in the event that damages exceed the aggregate coverage limits of our policy or if our insurance carriers disclaim coverage, we believe it is possible that costs associated with this claim could have a material adverse impact on our consolidated earnings, financial position or cash flows. We initially accrued $500,000 for defense costs and upon the denial of the motion to dismiss, we accrued an additional $500,000, which is our insurance deductible related to the matter. $820,000 of the $1,000,000 is still accrued as of December 31, 2019.
We accrue a liability in our consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is recorded. If a loss is reasonably possible, but not known or probable, and can be reasonably estimated, the estimated loss or range of loss is disclosed in the notes to the consolidated financial statements. In most cases, significant judgment is required to estimate the amount and timing of a loss to be recorded, actual results may differ from these estimates.
Purchase Commitments
At December 31, 2019, we had purchase commitments for inventories totaling approximately $2 million, substantially all of which is expected to be purchased by the end of 2020.
China Joint Venture
In late 2019, we executed a joint venture agreement with our Chinese supplier. The agreement requires the Company to make a capital contribution into the newly formed entity of approximately $0.4M. We expect this capital contribution will be made in the first quarter of 2020.
Severance
Jay D. Ewers, the Chief Financial Officer, resigned as an officer of the Company effective December 31, 2018, although he continued on as an employee during the first quarter of 2019. In connection with this departure, the Company and Mr. Ewers entered into a separation agreement, dated November 12, 2018. Severance costs incurred included salary, option expense and other benefits of approximately $624,000, approximately $532,000 is included in operational cash outflows during 2019, the remainder will be included in operational cash outflows during 2020.
Jack McCarthy, the Chief Commercialization Officer, was terminated without cause from his position with the Company effective November 6, 2017. Severance costs incurred included salary, option expense and other benefits of approximately $582,000, of which approximately $397,000 was included in operational cash outflows during 2018.
Robert L. Gershon, the Chief Executive Officer and a director, resigned from all of his positions with the Company effective December 15, 2017. In connection with this departure, the Company and Mr. Gershon entered into a separation agreement, dated December 15, 2017. Severance costs incurred included salary, option expense and other benefits of approximately $767,000, of which approximately $670,000 was included in operational cash outflows during 2018.
Concentrations
Sales to one customer within the OEM segment represented 11% of sales for the year ended December 31, 2019. The Company had no customers who comprised 10% or more of sales for the years ended December 31, 2018 and 2017.
65
66
NOTE 19. STOCK OPTIONS
On October 30, 2007, our stockholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan (the “Plan”) to increase the maximum aggregate number of shares of common stock reserved for issuance under the Plan from 1.2 million shares (already reserved against outstanding options) to 1.7 million shares. Except for the increase in the number of shares covered by the Plan, the Plan remained otherwise unchanged. In 2001, the Board of Directors adopted the 2001 Executive and Employee Stock Option Plan which reserved for issuance 1.2 million stock options. Stock options to employees typically have a ten-year life and currently vest over periods between one and seven years.
In July of 2012, our stockholders approved the 2012 Share Incentive Plan covering a total of 750,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 160,000 are available to be issued in this plan.
In July of 2015, our stockholders approved the 2015 Executive and Employee Stock Option Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 900,000 are available to be issued in this plan.
In August of 2017, our stockholders approved the 2017 Executive and Employee Stock Option Plan covering a total of 3,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 480,000 are available to be issued in this plan.
In August of 2019, our stockholders approved the 2019 Share Incentive Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019, all 2,000,000 are available to be issued in this plan.
On January 15, 2020, the Company granted employees approximately 1,300,000 options to purchase common shares of the Company's stock. All options granted were pursuant to the plans noted above. The options vest over a period of three years.
The status of our stock options and warrants are summarized as follows:
Number of options and warrants | Weighted average exercise price | |||||
Outstanding at December 31, 2016 | 3,526,287 | $ | 3.17 | |||
Granted | 1,728,000 | 3.09 | ||||
Exercised | (176,750 | ) | 2.41 | |||
Canceled and forfeited | (443,302 | ) | 3.95 | |||
Outstanding at December 31, 2017 | 4,634,235 | $ | 3.10 | |||
Granted | 225,000 | 2.40 | ||||
Exercised | (1,378,615 | ) | 2.43 | |||
Canceled and forfeited | (225,841 | ) | 2.10 | |||
Outstanding at December 31, 2018 | 3,254,779 | $ | 3.18 | |||
Granted | 1,379,500 | 7.70 | ||||
Exercised | (410,635 | ) | 2.99 | |||
Canceled and forfeited | (256,785 | ) | 4.76 | |||
Outstanding at December 31, 2019 | 3,966,858 | $ | 4.67 | |||
67
Number of options | Weighted average grant date fair value | |||||
Non-vested at December 31, 2018 | 1,436,429 | $ | 1.68 | |||
Granted | 1,379,500 | 4.57 | ||||
Vested | (1,119,000 | ) | 1.90 | |||
Forfeited | (212,000 | ) | 2.31 | |||
Non-vested at December 31, 2019 | 1,484,929 | $ | 4.11 | |||
Common shares required to be issued upon the exercise of stock options would be issued from our authorized and unissued shares. Prior to 2019, options issued were valued utilizing a trinomial lattice model. During the current year, the Company began granting stock option awards deeper within the Company. The Company does not have the relevant history with grants to these employees to support the inputs required in a trinomial valuation model. Accordingly, the Company adopted the use of the Black-Scholes model in 2019. For employee grants, we calculate expected life via the simplified method as we do not have sufficient history to determine actual expected life. For, non-employee grants, we calculate expected life using a combination of past exercise behavior, the contractual term and expected remaining exercise behavior. Inputs used in the valuation models are as follows:
2019 Grants | 2018 Grants | 2017 Grants | |||||||||
Option value | $7.15 | - | $7.91 | $1.46 | - | $3.04 | $1.73 | - | $2.34 | ||
Risk-free rate | 1.7% | - | 2.6% | 1.9% | - | 2.5% | 1.5% | - | 1.9% | ||
Expected dividend yield | —% | —% | —% | ||||||||
Expected volatility | 64.9% | - | 66.4% | 60.9% | - | 68.8% | 62.1% | - | 68.0% | ||
Expected term (in years) | 4.5 | - | 6 | 6 | 6 | ||||||
We recognized approximately $3,581,000, $2,224,000 and $871,000 in stock-based compensation expense during the years ended December 31, 2019, 2018 and 2017, respectively.
The intrinsic value of each option share is the difference between the fair market value of our common stock and the exercise price of such option share to the extent it is “in-the-money”. Aggregate intrinsic value represents the value that would have been received by the holders of in-the-money options had they exercised their options on the last trading day of the year and sold the underlying shares at the closing stock price on such day. The intrinsic value calculation at December 31, 2019 is based on the $8.46 closing stock price of our common stock on December 31, 2019, the last trading day of 2019.
As of December 31, 2019, there were 3,772,139 stock options outstanding and expected to vest with an aggregate intrinsic value of approximately $14,820,000. These options have a weighted average exercise price of $4.53 and a weighted average remaining contractual term of approximately 7 years.
As of December 31, 2019, there were 2,481,929 stock options outstanding and exercisable with an aggregate intrinsic value of approximately $12,730,000. These options have a weighted average exercise price of $3.33 and a weighted average remaining contractual term of approximately 6 years.
The total intrinsic value of in the money options exercised during the years ended December 31, 2019, 2018 and 2017, was approximately $1,420,000, $4,460,000 and $220,000, respectively. Intrinsic value of exercised shares is the total value of such shares on the date of exercise less the cash received from the option holder to exercise the options or other consideration paid.
The total fair value of options granted during the years ended December 31, 2019, 2018 and 2017, was approximately $6,300,000, $540,000, and $3,140,000, respectively. The weighted average fair value of options granted during the years ended December 31, 2019, 2018 and 2017, was $4.57, $2.40, and $1.82, respectively. The total fair value of option shares vested during the years ended December 31, 2019, 2018, and 2017, was approximately $2,130,000, $1,440,000 and $810,000, respectively.
The Company allows employees to exercise stock-based awards by surrendering stock-based awards with a fair value of the stock-based awards exercised, referred to as net settlements. These surrenders are included in stock options exercised in the options rollforward above. During the years ended December 31, 2019, 2018 and 2017, the Company received 125,948, 570,343, and
68
129,378 options as payment in the exercise of 222,601, 720,772 and 47,372 options, respectively. During the years ended December 31, 2018 and 2017, the Company received 22,711 and 34,687 warrants as payment in the exercise of 17,289 and 19,688 warrants, respectively.
During the year ended December 31, 2018, the Company modified the terms of awards granted to two employees by immediately vesting the remaining unvested options at the modification dates. The modifications resulted in the recognition of approximately an additional $190,000 in compensation cost due to the revaluation of the stock options.
As of December 31, 2019, there was approximately $3,520,000 of total unrecognized stock-based compensation cost, related to unvested stock options granted under the Amended Plan. This cost is expected to be recognized over a weighted-average period of approximately 1 year.
During October 2015, the Company granted 225,922 restricted stock units that vest ratably over a period of 5 years. As of December 31, 2019, 180,740 of the shares had vested, with the remaining shares vesting in October 2020. At December 31, 2019, the Company has approximately $70,000 of stock-based compensation expense to be recognized through October 2020.
69
NOTE 20. GEOGRAPHIC AND SEGMENT INFORMATION
Operating segments are aggregated into reportable segments only if they exhibit similar economic characteristics. In addition to similar economic characteristics, we also consider the following factors in determining the reportable segments: the nature of business activities, the management structure directly accountable to our chief operating decision maker for operating and administrative activities, availability of discrete financial information and information presented to the Board of Directors and investors. Asset information is not reviewed by the chief operating decision maker by segment and is not available by segment, accordingly, we have not presented a measure of assets by segment.
Our reportable segments are disclosed as principally organized and managed as two operating segments: Advanced Energy and OEM. "Corporate & Other" includes certain unallocated corporate and administrative costs which were not specifically attributed to any reportable segment. The OEM segment is primarily development and manufacturing contract and product driven, all related expenses are recorded as cost of sales, therefore no segment specific operating expenses are incurred.
Summarized financial information with respect to reportable segments is as follows:
Year ended December 31, 2019 | ||||||||||||||
(In thousands) | Advanced Energy | OEM | Corporate (Other) | Total | ||||||||||
Sales | $ | 22,676 | $ | 5,559 | $ | — | 28,235 | |||||||
Income (loss) from operations | (8,045 | ) | 2,136 | (14,960 | ) | (20,869 | ) | |||||||
Interest income | — | — | 1,392 | 1,392 | ||||||||||
Interest expense | — | — | (8 | ) | (8 | ) | ||||||||
Other losses, net | — | — | (351 | ) | (351 | ) | ||||||||
Income tax benefit | — | — | 130 | 130 | ||||||||||
Year ended December 31, 2018 as Restated | |||||||||||||||
(In thousands) | Advanced Energy | OEM | Corporate (Other) | Total | |||||||||||
Sales | $ | 12,987 | $ | 3,618 | $ | — | $ | 16,605 | |||||||
Income (loss) from operations | (6,326 | ) | 1,795 | (9,745 | ) | (14,276 | ) | ||||||||
Interest income | — | — | 616 | 616 | |||||||||||
Interest expense | — | — | (104 | ) | (104 | ) | |||||||||
Other losses, net | — | — | (947 | ) | (947 | ) | |||||||||
Change in fair value of derivative liabilities | — | — | 20 | 20 | |||||||||||
Income tax benefit | — | — | 3,907 | 3,907 | |||||||||||
70
Year ended December 31, 2017 | |||||||||||||||
(In thousands) | Advanced Energy | OEM | Corporate (Other) | Total | |||||||||||
Sales | $ | 7,636 | $ | 2,598 | $ | — | $ | 10,234 | |||||||
Income (loss) from operations | (3,957 | ) | 1,353 | (11,281 | ) | (13,885 | ) | ||||||||
Interest expense | — | — | (136 | ) | (136 | ) | |||||||||
Change in fair value of derivative liabilities | — | — | 183 | 183 | |||||||||||
Income tax benefit | — | — | 156 | 156 | |||||||||||
International sales in 2019, 2018 and 2017 were 30.6%, 22.6% and 13.2% of sales, respectively. Substantially all of these sales are denominated in U.S. dollars. Revenue by geographic region, based on the "ship to" location on the invoice are as follows:
Year Ended December 31, | |||||||||||
(In thousands) | 2019 | 2018 | 2017 | ||||||||
Sales by Domestic and International | |||||||||||
Domestic | $ | 19,584 | $ | 12,858 | $ | 8,887 | |||||
International | 8,651 | 3,747 | 1,347 | ||||||||
Total | $ | 28,235 | $ | 16,605 | $ | 10,234 | |||||
71
NOTE 21. SUPPLEMENTAL UNAUDITED QUARTERLY FINANCIAL INFORMATION OF CONTINUING OPERATIONS
The following table sets forth certain unaudited quarterly data of continuing operations for each of the four quarters in the years ended December 31, 2019, and 2018, respectively. The data has been derived from the Company’s unaudited consolidated financial statements that, in management’s opinion, include all adjustments (consisting of normal recurring adjustments) necessary for a fair presentation of such information when read in conjunction with the Consolidated Financial Statements and Notes thereto. The results of operations for any quarter are not necessarily indicative of the results of operations for any future period. The results presented have been updated for restatements and revisions as discussed in Notes 4 and 5.
(In thousands, except per share data) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||
Year ended December 31, 2019 | |||||||||||||||
Sales | $ | 5,629 | $ | 6,649 | $ | 7,575 | $ | 8,382 | |||||||
Gross profit | 3,563 | 4,674 | 5,294 | 5,563 | |||||||||||
Net loss | (5,608 | ) | (4,295 | ) | (4,370 | ) | (5,433 | ) | |||||||
Basic loss per common share | (0.17 | ) | (0.13 | ) | (0.13 | ) | (0.16 | ) | |||||||
Year ended December 31, 2018 | |||||||||||||||
Sales | $ | 3,397 | $ | 3,691 | $ | 3,672 | $ | 5,845 | |||||||
Gross profit | 2,212 | 2,537 | 2,521 | 3,556 | |||||||||||
Net loss | (2,791 | ) | (2,938 | ) | (1,143 | ) | (3,912 | ) | |||||||
Basic loss per common share | (0.08 | ) | (0.09 | ) | (0.03 | ) | (0.12 | ) | |||||||
*Fourth quarter 2018 period includes approximately $0.7 million of non-recurring severance and expenses related to former members of the Company’s executive management team.
72
NOTE 22. SUBSEQUENT EVENTS
In March 2020, the World Health Organization recognized the novel strain of coronavirus, COVID-19, as a pandemic. This coronavirus outbreak has severely restricted the level of economic activity around the world. In response to this coronavirus outbreak, the governments of many countries, states, cities and other geographic regions have taken preventative or protective actions, such as imposing restrictions on travel and business operations and advising or requiring individuals to limit or forego their time outside of their homes. Temporary closures of businesses have been ordered and numerous other businesses have temporarily closed voluntarily. This coronavirus outbreak has started to have a significant decline on the Company's sales results to date in fiscal 2020. Given the uncertainty regarding the spread of this coronavirus, the related financial impact cannot be reasonably estimated at this time.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. Controls and Procedures
Disclosure Controls and Procedures
Our management has established and maintains disclosure controls and procedures that are designed to ensure that the information required to be disclosed by us in reports that we file or submit under the Securities Exchange Act of 1934, as amended (the "Exchange Act"), is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. Management carried out an evaluation, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures as of the end of the period covered by this report. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2019, the Company's disclosure controls and procedures were not effective because of the material weakness in our internal control over financial reporting as discussed below.
Notwithstanding such material weaknesses, which is described below in Management’s Report on Internal Control over Financial Reporting, our management has concluded that the consolidated financial statements included in this Form 10-K present fairly, in all material respects, our financial position, results of operations and cash flows for the periods presented in conformity with GAAP.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). The Company's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, management carried out an evaluation of the effectiveness of the Company's internal control over financial reporting as of December 31, 2019, based on the framework set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013). Based on that evaluation, management concluded that, as of December 31, 2019, the Company's internal control over financial reporting was not effective as a result of the material weaknesses described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that material misstatements of the Company's annual or interim financial statements will not be prevented or detected on a timely basis.
Our management has determined that the following previously reported material weaknesses that existed as of December 31, 2018 have not been remediated and continue to constitute material weaknesses as of December 31, 2019:
73
APYX MEDICAL CORPORATION
• | An ineffective control environment requiring additional qualified accounting personnel with an appropriate level of knowledge and experience with generally accepted accounting principles. |
• | Ineffective control activities due to the lack of documentation and timeliness in executing certain business process controls, specifically related to procure to pay and inventory processes and footnote reporting disclosures related to income tax accounts, primarily related to our United States operations. |
Additionally, our management has determined the following material weakness also exists as of December 31, 2019:
• | Ineffective control environment and control activities over financial reporting in our Bulgarian subsidiary related to the purchasing of goods and services, including the processing and payment of vendor invoices. |
Remediation Efforts to Address Material Weaknesses
Management is committed to maintaining a strong internal control environment. In response to the identified material weaknesses, management, with the oversight of the Audit Committee of the Board of Directors, has taken actions toward the remediation of the respective material weaknesses in internal control over financial reporting as outlined below.
• | We are in the process of remediating the material weakness associated with the lack of sufficient qualified accounting personnel with an appropriate level of knowledge and experience with generally accepted accounting principles by hiring a new Chief Financial Officer in January 2019 and, in September 2019, a new Corporate Controller with experience in internal controls and financial reporting. Both have been actively engaged in remediation efforts to address the material weaknesses to date and will continue throughout fiscal year 2020. We will continue to recruit qualified professionals with appropriate levels of knowledge and experience to assist in resolving accounting issues related to non-routine and complex transactions. We have also enhanced our policies, procedures, and controls for all key business processes. In addition, management will continue to train personnel to ensure consistent application of accounting principles and adherence to the Company’s policies, procedures, and controls. |
• | We are in the process of remediating the material weakness associated with the ineffective control activities due to the lack of documentation and timeliness in executing business process controls by enhancing our processes and review controls associated with the processes noted above. We have reviewed current financial controls to assess if additional management review controls are necessary and will continue to work with all finance personnel to ensure the appropriate documentation criteria for the existing controls, including evidence of review, timeliness and variance thresholds. We will continue to work with the third-party specialists we engaged to review, document, and enhance the design of our controls, with the goal of designing and implementing controls that address the completeness and accuracy of data used in the performance of certain controls as well as the precision of management's review, but also enhance our ability to manage our business. |
• | We have enhanced, or are in the process of enhancing, certain controls over purchasing and disbursements in our Bulgarian subsidiary, including approving and validating vendor invoices received by verifying the related purchase authorization and the receipt of the goods or services. |
Management believes the steps outlined above, along with the implementation of a new financial reporting system, will remediate the material weaknesses described above. The Audit Committee of the Board of Directors and management will continue to monitor the implementation of these remediation measures and the effectiveness of our internal controls over financial reporting on an ongoing basis.
As of December 31, 2019, our remediation of these deficiencies is incomplete.
The effectiveness of our internal control over financial reporting as of December 31, 2019 has been audited by BDO USA LLP, an independent registered public accounting firm, as stated in their reports included in this Annual Report on Form 10-K. This report, which appears in Part II, Item 8 of this Annual Report on Form 10-K, contains an adverse opinion on the effectiveness of our internal control over financial reporting.
Remediation of Prior Material Weaknesses
We have remediated the material weakness previously reported in our Annual Report on Form 10-K for the year ended December 31, 2018 associated with ineffective monitoring controls to ascertain whether the components of internal control were present and
74
APYX MEDICAL CORPORATION
functioning by (i) the hiring of additional resources with an appropriate level of knowledge and expertise, (ii) supplementing the monitoring staff with qualified co-sourcing resources to ensure an adequate level of technical competency, (iii) evaluating deficiencies objectively in accordance with the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013) and (iv) better reporting of results to management, the Audit Committee and the Board of Directors.
Changes in Internal Control Over Financial Reporting
Except as set forth above, there were no changes in our internal control over financial reporting that occurred during the three months ended December 31, 2019 that materially affected, or that are reasonably likely to materially affect our internal control over financial reporting.
ITEM 9B. Other Information
None.
75
APYX MEDICAL CORPORATION
Part III
ITEM 10. Directors, Executive Officers and Corporate Governance
BACKGROUND AND EXPERIENCE OF DIRECTORS
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable the Board of Directors (“Board”) to satisfy its oversight responsibilities effectively in light of the Company’s business and structure, the Governance and Nominating Committee focused primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth immediately below. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business. As more specifically described in such person’s individual biographies set forth below, our directors possess relevant and industry-specific experience and knowledge in the medical, engineering and business fields, as the case may be, which we believe enhances the Board’s ability to oversee, evaluate and direct our overall corporate strategy. The Governance and Nominating Committee annually reviews and makes recommendations to the Board regarding the composition and size of the Board so that the Board consists of members with the proper expertise, skills, attributes and personal and professional backgrounds needed by the Board, consistent with applicable regulatory requirements.
The Governance and Nominating Committee believes that all directors, including nominees, should possess the highest personal and professional ethics, integrity and values and be committed to representing the long-term interests of our stockholders. The Governance and Nominating Committee will consider criteria including the nominee’s current or recent experience as a senior executive officer, whether the nominee is independent, as that term is defined in existing independence requirements of The NASDAQ Stock Market LLC, the business, scientific or engineering experience currently desired on the Board, geography, the nominee’s industry experience and the nominee’s general ability to enhance the overall composition of the Board.
The Governance and Nominating Committee does not have a formal policy on diversity; however, in recommending directors, the Board and the Committee consider the specific background and experience of the Board members and other personal attributes in an effort to provide a diverse mix of capabilities, contributions and viewpoints which the Board believes enables it to function effectively as the Board of Directors of a company with our size and nature of business.
Directors serve for one-year terms and are elected at the annual stockholders’ meeting. Set forth below is information regarding the executive officers, directors and key employees of Apyx Medical Corporation as of March 13, 2020.
Name | Age | Position | Director Since | |||
Charles D. Goodwin | 54 | Chief Executive Officer and Director | December 2017 | |||
Tara Semb | 50 | Chief Financial Officer, Treasurer and Secretary | N/A | |||
Todd Hornsby | 44 | Executive Vice President | N/A | |||
Moshe Citronowicz | 67 | Senior Vice President | N/A | |||
Andrew Makrides | 78 | Chairman of the Board | December 1982 | |||
Lawrence J. Waldman | 73 | Director | March 2011 | |||
Michael Geraghty | 74 | Director | March 2011 | |||
John Andres | 62 | Vice-Chairman of the Board | July 2014 | |||
Craig Swandal | 59 | Director | March 2018 | |||
Minnie Baylor-Henry | 72 | Director | August 2019 | |||
Andrew Makrides, Esq. age 78, Chairman of the Board of Directors since December 1982, received a Bachelor of Arts degree in Psychology from Hofstra University and a Juris Doctor Degree from Brooklyn Law School. He is a member of the Bar of the State of New York and practiced law from 1968 until joining Apyx Medical Corporation as a co-founder and Executive Vice President and director, in 1982. Mr. Makrides became President of the Company in 1985 and the CEO in December 1998 and served as such until March 18, 2011 at which point he relinquished his position as President, but remained CEO until December 2013. Mr. Makrides employment contract expired December 31, 2016. Mr. Makrides has over 30 years of executive experience in the medical industry. The Company believes Mr. Makrides is qualified to serve as Chairman because of his over 30 years of experience in the medical device industry as well as with his previous tenure with the Company.
76
APYX MEDICAL CORPORATION
Charles D. Goodwin, age 54, Chief Executive Officer and a Director of Apyx Medical since December 2017, is an accomplished senior executive with over 25 years of experience in the healthcare industry. Before joining Apyx Medical in December 2017, Mr. Goodwin was the Chief Executive Officer of MIS Implants Technologies, Inc., a privately held company specializing in dental implants. Prior to this position, Mr. Goodwin spent more than 11 years with Olympus/Gyrus ACMI in a variety of commercial and leadership roles of increasing responsibility. Mr. Goodwin began as a regional sales director for Gyrus in 2002 and was later promoted to Vice President of Sales, overseeing the Company’s strong commercial ramp and assisting Gyrus’ executive leadership team in the successful acquisition of American Cytoscope Makers, or “ACMI”, for $500 million in 2005. As President of Gyrus ACMI’s surgical division, Mr. Goodwin developed the company’s global distribution network and achieved average annual sales growth of 35% for three consecutive years, resulting in a promotion to President of Worldwide Sales in 2007. As President of Worldwide Sales for Gyrus ACMI, Mr. Goodwin was responsible for a global business with approximately 700 employees and was a key contributor to the successful sale of Gyrus ACMI to Olympus for $2.2 billion in 2008. Mr. Goodwin served as Group Vice President of Olympus Corporation’s global surgical energy group, where he was responsible for commercial strategy, R&D and operations for a business with more than 500 employees worldwide. Mr. Goodwin held this position for five years before joining MIS Implants Technologies, Inc. in 2014. Mr. Goodwin holds a B.A. Finance and Economics from Eastern Washington University. The Company believes Mr. Goodwin is qualified to serve as a Director given his over 25 years of experience in the medical device industry.
Tara Semb, age 50, Chief Financial Officer, Treasurer and Secretary since January 2019. Prior to joining Apyx Medical, Ms. Semb was the Chief Financial Officer for AVAIL Vapor LLC, a manufacturer and retailer of e-liquid for use in electronic vapor devices, from 2015 until 2018. Ms. Semb previously worked for Amsted Industries, a diversified global manufacturer of industrial components, in multiple positions of increasing responsibility from 2006 until 2015, culminating in her promotion to Director of Finance for the company’s rail bearings division in 2013. Before joining Amsted Industries as Director of Internal Audit in 2006, she held financial and operational roles at Blyth Industries, a manufacturer and seller of candles and home fragrance products, and Anixter International, a global distributor of network & security solutions. She began her career in 1991 as an auditor at Price Waterhouse. Ms. Semb holds a Bachelor of Science degree in Accounting from the University of Illinois, as well as an MBA from Washington University in St. Louis. She is a Certified Public Accountant (CPA).
Todd Hornsby, age 44, Executive Vice President since January 2019, has responsibility for global Commercial operations. He is an accomplished Senior Executive with more than 19 years of success in the medical device and biotech industries. Throughout his career, Todd has held various leadership positions and has extensive experience in sales, sales management, and with building strong teams and launching new technologies. Since joining Apyx™ Medical in August 2014, Todd has focused primarily on the commercialization of Apyx’s Renuvion / J-Plasma advanced energy system. Prior to joining Apyx, Todd held roles of increasing seniority and responsibility at CryoLife, Inc. During his tenure, Todd directed the US Sales team, with a diversified product portfolio of biological heart valves and vascular grafts, surgical adhesives and hemostatic agents, dialysis access and CHF chronic heart failure products. Todd also directed successful integrations of three acquisitions into the US sales channel. Early in his medical device career, Todd held positions with Ethicon - Endo Surgery and Medex Medical. Todd holds a BA in Psychology from Hope College. He is also the recipient of many awards for sales achievement and growth.
Moshe Citronowicz, age 67, Senior Vice President since 2012, came to the United States in 1978 and has worked in a variety of manufacturing and high technology industries. In October 1993, Mr. Citronowicz joined the Company as Vice President of Operations and served as our Chief Operating Officer until November 2011. Currently, he is serving as the Senior Vice President. Mr. Citronowicz’s employment contract extends to December 31, 2020.
Lawrence J. Waldman, CPA, age 73, Director, Audit Committee Chair, and Lead Independent Director since March 2011. Mr. Waldman has over thirty-five years of experience in public accounting. Mr. Waldman currently serves as a senior advisor to First Long Island Investors, LLC, an investment and wealth management firm since May 2016. Prior to that Mr. Waldman served as an advisor to the accounting firm of EisnerAmper LLP, where he was previously the Partner-in-Charge of Commercial Audit Practice Development for Long Island since September 2011. Prior to joining EisnerAmper LLP, Mr. Waldman was the Partner-in-Charge of Commercial Audit Practice Development for Holtz Rubenstein Reminick, LLP from July 2006 to August 2011. Mr. Waldman was the Managing Partner of the Long Island office of KPMG LLP from 1994 through 2006, the accounting firm where he began his career in 1972. Mr. Waldman was elected to the Board of Directors of Comtech Telecommunications Corp. in August of 2015 and since December 2015, serves as Chair of its audit committee. In October 2016, Mr. Waldman was appointed and subsequently in December 2016 elected to the Board of Directors of CVD Equipment Corporation, and serves as the Chair of the audit committee and as Lead Independent Director Mr. Waldman served through October 2018 as a member of the Board of Directors of Northstar/RXR Metro Income Fund, a non-traded Real Estate Investment Trust and has served as a member of its audit committee since
77
APYX MEDICAL CORPORATION
2014. Mr. Waldman is also the Chair of the Supervisory Committee of Bethpage Federal Credit Union. Mr. Waldman also served as a member of the State University of New York’s Board of Trustees and as chair of its audit committee. He previously served as the Chairman of the Board of Trustees of the Long Island Power Authority and as Chair and a member of the finance and audit committee of its Board of Trustees. Mr. Waldman meets the definition of a financial expert as defined by the SEC and The NASDAQ Stock Market LLC. The Company believes Mr. Waldman is qualified to serve as Director, Audit Committee Chair and Lead Independent Director because of his over 35 years experience in public accounting and his positions on various boards.
Michael Geraghty, age 74, has served as a director since March 2011 and was previously employed as the President of Global Sales at Optos, Inc., a developer and manufacturer of retinal imaging devices for screening, detection and diagnosis of eye related conditions. From 2005 through 2008, he was the President of International Sales at Gyrus Acmi where he first started in 2000 as Senior Vice President of Sales for Gyrus Medical. Prior to this, Mr. Geraghty was the Vice President of Sales and Marketing for Everest Medical, Inc. and before that was the Director of Marketing for Advanced Products at Arthrocare Corporation. Mr. Geraghty specializes in building independent direct sales teams in the medical device industry and has extensive domestic and international sales and marketing experience. He received his bachelor’s degree from St. Mary’s University and graduate degree in Executive Sales Management from the University of Minnesota. The Company believes Mr. Geraghty is qualified to serve as Director and Compensation Committee Chair because of his extensive domestic and international sales, marketing, and management experience.
Craig Swandal, age 59, Director since March 2018. Mr. Swandal has over 30 years of experience at public and privately-held medical technology and electronics manufacturing companies. He began his career in 1981 at Unisys Corporation, a manufacturer of main frame computer systems, where he held a variety of manufacturing positions of increasing responsibility. In 1995 he joined Silent Knight, a manufacturer of industrial fire and security systems, as a Manufacturing Manager and was promoted to Vice President of Operations.
In 2001, Mr. Swandal joined Gyrus, a manufacturer of surgical devices, where he was responsible for the company’s manufacturing operations as Director of Operations and later Vice President of Operations. During his tenure, he improved manufacturing efficiencies by leading his manufacturing group through the implementation of lean and Six Sigma techniques. Following Gyrus’s acquisition of ACMI in 2005, Mr. Swandal was promoted to Senior Vice President and was responsible for the global operations of the combined company, which included 12 facilities across 8 countries. He developed and executed Gyrus ACMI’s strategy to consolidate its manufacturing, distribution, customer service and service and repair operations and was a member of the leadership team that successfully sold the company to Olympus Corporation for $2.2 billion in 2008.
Following the acquisition of Gyrus ACMI, Mr. Swandal served on the executive leadership teams of several companies, including ATS Medical, ACELL and Tendyne, where he was focused on operational development and currently holds a position. He is currently the Principal of Lead 2 Change Consulting, where he assists companies in identifying and implementing new manufacturing initiatives. Mr. Swandal serves as a member of the Board of Managers for Tiumed LLC a nontraded Medical Device start up. Mr. Swandal holds a Bachelor’s degree in Organizational Management and Communications from Concordia University, as well as a mini Master of Business Administration in Medical Technology from the University of St Thomas. The Company believes Mr. Swandal is qualified to serve as Director because of his extensive experience in manufacturing operations.
John Andres, age 62, Vice Chairman of the Board of Directors and Nominating Chair since July 2014, has over thirty years of experience in the medical device industry. Since April, 2004, Mr. Andres has been a private consultant, doing business through John C. Andres, LLC, specializing in patent/business strategy development and execution. He also is a partner of Hawk Healthcare, LLC, which provides strategic transaction management to private individuals and companies. Mr. Andres also holds a position with Electrocore.
In 2017, Mr. Andres joined the Longeviti Neuro Solutions, LLC Board of Directors which is developing cranial implant products for cranial reconstruction. In 2004, Mr. Andres helped found K2M, Inc. (KTWO) and from 2004 until 2010 served as a member of the Board of Directors of K2M, Inc. Prior to 2004, Mr. Andres held various legal and strategic business development positions at the Surgical Division of Tyco Healthcare Group, LLP, now Medtronic (NYSE: MDT) and its predecessor, United States Surgical Corporation. Before joining U.S. Surgical, Mr. Andres worked at the New York law firm of Morgan & Finnegan. He received his Associate of Applied Science degree from Rochester Institute of Technology, his Bachelor of Arts degree from Lehigh University and his Juris Doctor from Pace University School of Law. The Company believes Mr. Andres is qualified to serve as Vice Chairman and Nominating Committee Chair because of his extensive experience in patent and business strategy development and execution.
78
APYX MEDICAL CORPORATION
Minnie Baylor-Henry, age 72, Director and Regulator Compliance Committee Chair since August 2019. Ms. Baylor-Henry has over 25 years of regulatory affairs experience. She is the President of B-Henry & Associates, LLC, a consulting firm that she founded to provide regulatory strategic support to life sciences companies. Prior to starting her consulting company, she held various executive level positions over a 15-year period at Johnson & Johnson (J&J). Before retiring from J&J in 2015, she was the Worldwide Vice President of Regulatory Affairs-Medical Devices. During her time at J&J, she also had served as the Vice President-Medical & Regulatory Affairs in the Over-the Counter Group, as well as Senior Director, Regulatory Affairs-Pharmaceuticals. Ms. Baylor-Henry also worked for Deloitte & Touche (2008-2010) as the National Director Regulatory Affairs-Life Sciences. Prior to joining the private sector, she worked for the US Food & Drug Administration (1991-1999) in many roles, including serving as the Director of the Division of Drug, Marketing, Advertising & Communications and the FDA’s National Health Fraud Coordinator.
In 2018, Ms. Baylor-Henry joined the Board of Directors of scPharmaceuticals, a publicly-held company focused on developing technologies that enable subcutaneous administration of therapies and in 2019 the Board of Directors of PolarityTE, a publicly-held regenerative medicine company. Ms. Baylor-Henry received her pharmacy degree from Howard University’s College of Pharmacy and a law degree from Catholic University’s Columbus School of Law. The Company believes Ms. Baylor-Henry is qualified to serve as Director and Regulatory and Compliance Committee Chair because of her extensive experience in global and regulatory management and compliance.
Involvement in Certain Legal Proceedings
None
Independent Board Members
During 2019, the Board had five independent members, John Andres, Michael Geraghty, Craig Swandal, Lawrence J. Waldman, and Minnie Baylor-Henry who meet the existing independence requirements of the NASDAQ Stock Market LLC.
Board Leadership
The independent directors appointed Lawrence J. Waldman as the Lead Independent Director. The Lead Independent Director is appointed by the Board and is responsible for coordinating the activities of the independent directors and coordinating with the Chief Executive Officer of the Company to set agendas for Board meetings and chair executive sessions of the independent directors. The Lead Independent Director is also responsible for meeting, from time to time, with the Company’s Compensation Committee to discuss the Chief Executive Officer’s performance.
Our Corporate Governance Policies also contain several features which the Company believes will ensure that the Board maintains effective and independent oversight of management, including the following:
• | Executive sessions without management and non-independent directors present are a standing Board agenda item. Executive sessions of the independent directors are held at any time requested by an independent director and, in any event, are held in connection with at least 100% of regularly scheduled Board meetings. |
• | The Board regularly meets in executive session with the CEO without other members of management present. |
• | All Board committee members are independent directors. The committee chairs have authority to hold executive sessions without management and non-independent directors present. |
While our Board has no formal policy with respect to separation of the positions of Chairman and CEO or with respect to whether the Chairman should be a member of management or an independent director, our Board leadership structure currently separates the positions of the Chairman and CEO. We believe that these are matters that should be discussed and determined by the Board from time to time. The Chief Executive Officer of the Company, Charlie Goodwin, is tasked with the responsibility of implementing our corporate strategy, we believe he is best suited for leading discussions, at the Board level, regarding performance relative to our corporate strategy and this discussion accounts for a significant portion of the time devoted at our Board meetings.
79
APYX MEDICAL CORPORATION
Board Evaluations
The Board has adopted a policy to evaluate its performance and effectiveness as well as that of the three standing committees on an annual basis. The purpose of the evaluation is to track progress in certain areas targeted for improvement from year to year and to identify ways to enhance the Board’s effectiveness. As part of the evaluation, each Director may complete a written questionnaire developed by the Governance and Nominating Committee to provide feedback on the effectiveness of the Board, the Committees, as well as each individual Director’s own contributions. The collective ratings and comments of the Directors are compiled and then presented to the Governance and Nominating Committee and to the full Board for discussion and action as necessary.
Risk Management
The Board believes that risk management is an important component of the Company’s corporate strategy. While we assess specific risks at our committee levels, the Board, as a whole, oversees our risk management process and discusses and reviews with management major policies with respect to risk assessment and risk management. The Board is regularly informed through its interactions with management and committee reports about risks we face in the course of our business. Our Audit Committee also takes an active role in risk assessment and risk management.
Audit Committee
The Audit Committee assists the Board in its general oversight of our financial reporting, internal controls and audit functions and is directly responsible for the appointment, compensation and oversight of the work of our independent registered public accounting firm. The Audit Committee reviews and discusses with management and our independent accountants the annual audited and quarterly financial statements (including the disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations”), reviews the integrity of the financial reporting processes, both internal and external, reviews the qualifications, performance and independence of our independent accountants and prepares the Audit Committee Report included in this Annual Report on Form 10-K in accordance with rules and regulations of the Securities and Exchange Commission. The Audit Committee has the power to investigate any matter brought to its attention within the scope of its duties. It also has the authority to retain counsel and advisors to fulfill its responsibilities and duties. The Audit Committee also acts as a qualified legal compliance committee.
During 2019, our Audit Committee consisted of four independent members of the Board of Directors, Lawrence J. Waldman, John Andres, Michael Geraghty and Craig Swandal. As a smaller reporting company, we are required to have at least two independent members comprising our Audit Committee in accordance with Rule 10A-3 of the Securities Exchange Act of 1934 and the rules of The NASDAQ Stock Market LLC. During 2019, Mr. Waldman served as the Audit Committee Chairman and financial expert. The Audit Committee meets as often as it determines necessary but not less frequently than once every fiscal quarter. During 2019, the Audit Committee met four times.
Governance and Nominating Committee
The Governance and Nominating Committee is responsible for matters relating to the corporate governance of our company and the nomination of members of the board and committees thereof. During 2019, our Governance and Nominating Committee consisted of three independent members of the Board of Directors, John Andres who serves as Chairman, Lawrence J. Waldman and Michael Geraghty. The Governance and Nominating Committee meets as often as it determines necessary, but not less than once a year. During 2019, the Governance and Nominating Committee met once.
Compensation Committee
The Compensation Committee is responsible for overseeing our compensation and employee benefit plans (including those involving the issuance of our equity securities) and practices, including formulating, evaluating and approving the compensation of our executive officers and reviewing and recommending to the full Board of Directors the compensation of our Chief Executive Officer. During 2019, our Compensation Committee consisted of three independent members of the Board of Directors, Michael Geraghty who served as Chairman, John Andres and Lawrence J. Waldman. The Compensation Committee meets as often as it determines necessary, but not less than once a year. During 2019, the Compensation Committee met three times.
80
APYX MEDICAL CORPORATION
Regulatory Compliance Committee
The Regulatory Compliance Committee, formed in the third quarter of 2019, is responsible for matters relating to the Company’s overall non-financial regulatory and compliance strategies and systems. Specifically, the Committee will provide oversight of management’s efforts to comply with the requirements for a medical device company operating in a regulatory environment, with respect to healthcare compliance, product quality and safety, and other areas as directed by the Board. During 2019, our Regulatory Compliance Committee consisted of three independent members of the Board of Directors, Minnie Baylor-Henry who serves as Chairman, John Andres and Craig Swandal. The Regulatory Compliance Committee meets as often as it determines necessary, but not less than once a year. During 2019, Regulatory and Compliance Committee met once.
Code of Ethics
The Company made revisions to the Code of Ethics in the fourth quarter of 2019.
A copy of the code of ethics, which expressly includes the fiduciary responsibilities of the CEO and CFO, along with a summary of the changes made in 2019, is available on our website at https://apyxmedical.com/code-of-ethics-and-conduct/.
ITEM 11. Executive Compensation Discussion and Analysis
General Compensation Philosophy
The primary objective of our compensation program for employees, including our compensation program for executive officers, is to attract, retain and motivate qualified individuals and reward them in a manner that is fair to all stockholders. We strive to provide incentives for every employee that rewards them for their contribution to the Company.
Our compensation program is designed to be competitive with other employment opportunities and to align the interests of all employees, including executive officers, with the long-term interests of our stockholders. Historically, for our executive officers, we link a much higher percentage of total compensation to incentive compensation such as stock based compensation than we do for other employees.
With these objectives in mind, our Board has built executive and non-executive compensation programs that consist of three principal elements - base salary, performance bonuses and grants of stock options and/or shares of restricted stock.
To understand the competitiveness of compensation arrangements provided to our executive officers, in 2014 the Compensation Committee engaged Pearl Meyer & Partners to perform a competitive assessment of base salaries, bonuses for on-target performance and grants of equity incentives. In 2018 and again in 2019, Pearl Meyer & Partners updated the competitive frame of reference for the study to consist of the following group of pre-selected companies that were of comparable size and operated in our industry category.
Corindus Vascular Robotics, Inc. | IRIDEX Corporation | Restoration Robotics, Inc. |
Cutera, Inc. | Misonix, Inc. | Sensus Healthcare, Inc. |
Ekso Bionics Holdings, Inc. | Neuronetics, Inc. | Utah Medical Products Inc. |
iCAD, Inc. | Nuvectra Corporation | Viveve Medical, Inc. |
IRadimed Corporation | OrthoPediatrics Corp. | |
In addition to the peer group, Pearl Meyer referenced industry-specific, size-adjusted market survey data where appropriate.
The results of the survey confirmed that, consistent with our desired philosophy, our compensation arrangements were competitive with the marketplace, with some variation by individual.
81
APYX MEDICAL CORPORATION
Compensation Program
Base Salary
We pay base salaries to our Executive Officers in order to provide a consistent, minimum level of pay that sustained individual performance warrants. We also believe that a competitive annual base salary is important to attract and retain an appropriate caliber of talent for each position over time.
The annual base salaries of our Executive Officers are determined by our Compensation Committee and approved by the Board of Directors. All salary decisions are based on each Executive Officer’s level of responsibility, experience and recent and past performance, as determined by the Compensation Committee. The Compensation Committee benchmarks base salaries using a major independent consulting firm and using their recommendations and other information the Committee evaluates and establishes the base compensation for our executives.
82
APYX MEDICAL CORPORATION
Performance Bonus
The second component of executive compensation is performance bonuses which are earned when defined metrics are achieved.
For 2019, the Company established a combination of financial, operational and personal objectives as the broad criteria that would determine annual performance bonus amounts for the year.
(In millions) | Target | Achievement | Overall Weight | Achievement | Calculation | ||||||||||
Revenue | 30.1 | 28.2 | 50 | % | 93 | % | 47 | % | |||||||
Operating Income/(Loss) | (19.7 | ) | (20.3 | ) | 25 | % | 97 | % | 25 | % | |||||
Cash Burn | (16.3 | ) | (13.1 | ) | 25 | % | 120 | % | 30 | % | |||||
Total | 100 | % | 102.0 | % | |||||||||||
After careful review and consideration of the measures that comprise the 2019 bonus, the Compensation Committee approved the following performance bonuses:
Name | Bonus | |||
Charles D. Goodwin | $ | 344,250 | ||
Moshe Citronowicz | 82,620 | |||
Todd Hornsby | 168,300 | |||
Tara Semb | 110,265 | |||
Total | $ | 705,435 | ||
Stock Options
The third component of executive compensation is equity grants which have mainly come in the form of stock options. We believe that equity ownership in our Company is important to provide our Executive Officers and key employees with long-term incentives to better align interests of executives with the interests of stockholders and build value for our stockholders. In addition, the equity compensation is designed to attract and retain the executive management team. Stock options have value only if the stock price increases over time and, therefore, provide executives with an incentive to build Apyx’s value. This characteristic ensures that the Executive Officers and key employees have a meaningful portion of their compensation tied to future stock price increases and rewards management for long-term strategic planning through the resulting enhancement of the stock price.
Stock option awards to Executive Officers and key employees are entirely discretionary. The CEO recommends to the Compensation Committee awards for individuals other than himself. The Compensation Committee considers this recommendation along with the prior contribution of these individuals and their expected future contributions to our growth. The Committee formulates and presents its recommended allocation of stock option awards to the Board of Directors for approval. The Compensation Committee then would make an independent determination on CEO stock option awards, again formulating and presenting its recommendation for the allocation of stock option awards to the Board of Directors for approval. The Board of Directors approves, rejects, or, if necessary, modifies the Committee’s recommendations.
83
APYX MEDICAL CORPORATION
Perquisites and Other Benefits
Our Executive Officers are eligible for the same health and welfare programs and benefits as the rest of our employees in their respective locations.
Our Executive Officers are entitled to participate in and receive employer contributions to Apyx's 401(k) Savings Plan. For more information on employer contributions to the 401(k) Savings Plan see the Summary Compensation Table and its footnotes.
Tax and Accounting Considerations
Section 162(m) of the Internal Revenue Code of 1986, as amended (the “Code”), places a limit of $1.0 million on the amount of compensation that we may deduct as a business expense in any year with respect to each of our most highly paid executives unless, among other things, such compensation is performance-based and has been approved by stockholders. The non-performance-based compensation paid to our executive officers for the 2019 fiscal year did not exceed the $1.0 million limit per executive officer. Accounting considerations also play an important role in the design of our executive compensation program. Accounting rules, such as FASB ASC Topic 718-10-10, Share-Based Payment, require us to expense the cost of our stock option grants which reduces the amount of our reported profits. Because of option expensing and the impact of dilution on our stockholders, we pay close attention to the number and value of the shares underlying stock options we grant.
Compensation of Executive Officers
The following table sets forth the compensation paid to each of our Executive Officers for the three years ended December 31, 2019, 2018, and 2017 for services to our Company in all capacities:
Name and Principal Position | Year | Salary | Bonus ($) | Stock Awards ($) | Option Awards ($) (1) | Non-Equity Incentive Plan Compensation Earnings ($) | Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) (6) | Total ($) | ||||||||||||||||||||||||
Charles D. Goodwin | 2019 | $ | 450,000 | $ | 344,250 | $ | — | 1,135,160 | $ | — | $ | — | $ | 15,848 | $ | 1,945,258 | |||||||||||||||||
CEO and Director | 2018 | $ | 400,000 | $ | 1,075,500 | $ | — | — | $ | — | $ | — | $ | 14,233 | $ | 1,785,385 | |||||||||||||||||
2017 | $ | 15,385 | $ | — | $ | — | 1,770,000 | $ | — | $ | — | $ | — | $ | — | ||||||||||||||||||
Moshe Citronowicz | 2019 | $ | 270,000 | $ | 82,620 | $ | — | 346,320 | $ | — | $ | — | $ | 22,415 | $ | 721,355 | |||||||||||||||||
Senior Vice President | 2018 | $ | 226,410 | $ | 342,006 | $ | — | — | $ | — | $ | — | $ | 19,904 | $ | 588,320 | |||||||||||||||||
2017 | $ | 226,410 | $ | 300 | (4) | $ | — | 137,340 | $ | — | $ | — | $ | 18,968 | $ | 383,018 | |||||||||||||||||
Todd Hornsby | 2019 | $ | 330,000 | $ | 168,300 | $ | — | 365,560 | $ | — | $ | — | $ | 28,400 | $ | 892,260 | |||||||||||||||||
Executive Vice President(*) | 2018 | $ | 277,886 | $ | 200,500 | $ | — | — | $ | — | $ | — | $ | 25,713 | $ | 504,099 | |||||||||||||||||
2017 | $ | 504,152 | $ | 300 | $ | — | 206,430 | $ | — | $ | — | $ | 25,076 | $ | 735,958 | ||||||||||||||||||
Tara Semb(**) | 2019 | $ | 271,000 | $ | 110,265 | $ | — | 312,000 | $ | — | $ | — | $ | 5,922 | $ | 699,187 | |||||||||||||||||
Chief Financial Officer, | 2018 | $ | — | $ | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||
Treasurer and Secretary | 2017 | $ | — | $ | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||
*Assumed role as Executive Vice President on January 2, 2019. **Assumed role as CFO, Treasurer and Secretary on January 2nd, 2019.
(1) | These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation). Pursuant to SEC rule changes effective February 28, 2010, we are required to reflect the total grant date fair values of the option grants in the year of grant, rather than the portion of this amount that was recognized for financial |
84
APYX MEDICAL CORPORATION
statement reporting purposes in a given fiscal year which was required under the prior SEC rules, resulting in a change to the amounts reported in prior Annual Reports.
(2) | The amounts for 2019 include compensation under the following plans and programs: |
C.D. Goodwin | M. Citronowicz | T. Hornsby | T. Semb | |||||||||||||
Life insurance premiums | 110 | 72 | 110 | 110 | ||||||||||||
Long-term disability premiums | 392 | 392 | 392 | 392 | ||||||||||||
Health insurance premiums | 6,946 | 14,369 | 20,748 | — | ||||||||||||
Employer 401(k) contribution | 8,400 | 7,582 | 7,150 | 5,420 | ||||||||||||
Total | $ | 15,848 | $ | 22,415 | $ | 28,400 | $ | 5,922 | ||||||||
Amounts in the table above are pro-rated where applicable.
Employment Agreements and Potential Payments Upon Termination or Change in Control
At December 31, 2019, we were obligated under four employment agreements.
Name | Contract Expiration Date | |
Charles D. Goodwin | N/A(1) | |
Tara Semb | N/A(1) | |
Todd Hornsby | N/A(1) | |
Moshe Citronowicz | December 31, 2020 | |
(1) | Employment contracts provide for the Executives to remain employed by the Company until such time as their employment is terminated pursuant to the terms of their Employment Agreement. |
Employment contracts, other than for Messr. Citronowicz, commence on effective date and continue until terminated. The employment agreements provide, among other things, that the executive may be terminated as follows:
(a) | Upon the death of the executive, in which case the executive’s estate shall be paid the basic annual compensation due the employee pro-rated through the date of death. |
(b) | By the resignation of the executive at any time upon at least thirty (30) days prior written notice to Apyx in which case Apyx shall be obligated to pay the employee the basic annual compensation due him pro-rated to the effective date of termination. |
(c) | By Apyx, “for cause” if during the term of the employment agreement the employee violates the non-competition provisions of his employment agreement, or is found guilty in a court of law of any crime of moral turpitude in which case the contract would be terminated and provisions for future compensation forfeited. |
(d) | By Apyx, without cause, with the majority approval of the Board of Directors, for Mr. Goodwin and Mr. Citronowicz at any time upon at least thirty (30) days prior written notice to the executive. In this case Apyx shall be obligated to pay the executive compensation in effect at such time, including all bonuses, accrued or prorated and expenses up to the date of termination. Thereafter for Messr. Citronowicz, Apyx shall pay the executive three times the salary in effect at the time of termination payable in one lump sum. |
85
APYX MEDICAL CORPORATION
(e) | If Apyx fails to meet its obligations to the executive on a timely basis, or if there is a change in the control of Apyx, the executive may elect to terminate his employment agreement. Upon any such termination or breach of any of its obligations under the employment agreement, Apyx shall pay Mr. Citronowicz a lump sum severance equal to three times the annual salary and bonus in effect the month preceding such termination or breach as well as any other sums which may be due under the terms of the employment agreement up to the date of termination. Mr. Goodwin shall be paid two times their annual salary and bonus in effect the month preceding such termination or breach as well as any other sums which may be due under the terms of their respective employment agreement up to the date of termination. Ms. Semb shall be paid her annual salary and bonus in twelve monthly installments in effect the month following such termination. |
There are no other employment contracts that have non-cancelable terms in excess of one year.
Outstanding Equity Awards
The following table presents information with respect to each unexercised stock option held by our Executive Officers as of December 31, 2019:
Name | # of Securities Underlying Unexercised Options (# Exercisable) | # of Securities Underlying Unexercised Options (# Unexercisable) | Weighted Average Option Exercise Price ($/Sh) | Option Expiration Range After Grant Date | ||||||||||
Charles D. Goodwin | 1,078,667 | 157,333 | $ | 3.93 | 12/15/2027 | - | 1/9/2029 | |||||||
Moshe Citronowicz | 143,500 | 65,500 | $ | 4.49 | 7/12/2022 | - | 1/9/2029 | |||||||
Todd Hornsby | 151,583 | 104,417 | $ | 4.45 | 8/27/2024 | - | 1/9/2029 | |||||||
Tara Semb | 21,667 | 43,333 | $ | 7.91 | 1/9/2029 | |||||||||
*** These columns represent the grant date fair value of the awards as calculated in accordance with FASB ASC 718 (Stock Compensation).
In 2019, our Board of Directors consisted of Charles D. Goodwin, Andrew Makrides, John Andres, Lawrence J. Waldman, Michael Geraghty, Craig Swandal, and Minnie Baylor-Henry. Ms. Baylor-Henry became a member of the Board in August 2019.
In 2003, the Board of Directors adopted and our stockholders approved Apyx's 2003 Executive and Employee Stock Option Plan covering a total of 1,200,000 shares of common stock issuable upon exercise of options to be granted under the Plan.
On October 30, 2007, our stockholders approved and the Board of Directors adopted an amendment to the 2003 Executive and Employee Stock Option Plan to increase the maximum aggregate number of shares of common stock reserved for issuance under the 2003 Plan from 1.2 million shares (already reserved against outstanding options) to 1.7 million shares, or an increase of 500,000 shares of common stock for future issuance pursuant to the terms of the plan. Except for the increase in the number of shares covered by the plan, the plan remains otherwise unchanged from its present status. In 2011, the Board of Directors granted 25,000 options to purchase a like number of shares of common stock.
In July of 2012, our stockholders approved the 2012 Share Incentive Plan covering a total of 750,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 160,000 are available to be issued in this plan.
In July of 2015, our stockholders approved the 2015 Executive and Employee Stock Option Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 900,000 are available to be issued in this plan.
In August of 2017, our stockholders approved the 2017 Executive and Employee Stock Option Plan covering a total of 3,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019 approximately 480,000 are available to be issued in this plan.
86
APYX MEDICAL CORPORATION
In August of 2019, our stockholders approved the 2019 Share Incentive Plan covering a total of 2,000,000 shares of common stock issuable upon exercise of options to be granted under the plan. At December 31, 2019, all 2,000,000 are available to be issued in this plan.
There have been no changes in the pricing of any options previously or currently awarded.
Compensation Committee Interlocks and Insider Participation
The Compensation Committee of the Board of Directors is responsible for determining the compensation of executive officers of the Company, as well as compensation awarded pursuant to the Company’s equity incentive plans.
In 2019, our Compensation Committee consisted of three independent members of the Board of Directors, Michael Geraghty (Chairman), John Andres and Lawrence J. Waldman.
No member of the Compensation Committee is or has been an officer or employee of the Company or any of its subsidiaries. In addition, no member of the Compensation Committee had any relationships with the Company or any other entity that require disclosure under the proxy rules and regulations promulgated by the SEC.
COMPENSATION COMMITTEE REPORT
Our Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K with management. Based on our Compensation Committee’s review of and the discussions with management with respect to the Compensation Discussion and Analysis, our Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in our Proxy Statement and in this Annual Report on Form 10-K for the fiscal year ended December 31, 2019 for filing with the SEC. During the majority of 2019, our Compensation Committee consisted of three independent members of the Board of Directors, Michael Geraghty, who served as Chairman, John Andres and Lawrence J. Waldman.
87
APYX MEDICAL CORPORATION
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
See “ITEM 5. Market for Registrant’s Common Equity and Related Stockholder Matters”.
Security Ownership of Certain Beneficial Owners
The following table sets forth certain information as of March 27, 2020, with respect to the beneficial ownership of the Company’s common stock by its executive officers, directors, all persons known by the Company to be the beneficial owners of more than 5% of its outstanding shares and by all officers and directors as a group.
Number of Shares | |||||||||||
Name and Address | Title | Owned (i) | Nature of Ownership | Percentage of Ownership (i) | |||||||
William Weeks Vanderfelt | Common | 3,158,414 | Beneficial | 9.2 | % | ||||||
Coralis 44, Azzuri Village 44 | |||||||||||
Roches Noires, 31201 Mauritius | |||||||||||
Archon Capital Management, LLC | Common | 2,690,026 | Beneficial | 7.9 | % | ||||||
1100 19th Avenue E | |||||||||||
Seattle, WA 98122 | |||||||||||
Andrew Makrides | Common | 661,972 | (ii) | Beneficial | 1.9 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Charles D. Goodwin II | Common | 1,078,667 | (iii) | Beneficial | 3.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Moshe Citronowicz | Common | 570,004 | (iv) | Beneficial | 1.6 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Lawrence Waldman | Common | 149,000 | (v) | Beneficial | 0.4 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Todd Hornsby | Common | 151,583 | (vi) | Beneficial | 0.4 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
88
APYX MEDICAL CORPORATION
Michael E. Geraghty | Common | 98,000 | (vii) | Beneficial | 0.3 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Craig Swandal | Common | 45,916 | (viii) | Beneficial | 0.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
John Andres | Common | 70,500 | (ix) | Beneficial | 0.2 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Tara Semb | Common | 21,667 | (x) | Beneficial | 0.1 | % | |||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Minnie Baylor-Henry | Common | — | Beneficial | — | % | ||||||
5115 Ulmerton Rd. | |||||||||||
Clearwater, FL 33760 | |||||||||||
Officers and Directors as a group (10 people) | 2,827,309 | (xi) | 7.9 | % | |||||||
(i) Based on 34,174,314 outstanding shares of Common Stock and 2,951,762 exercisable outstanding options to acquire a like number of shares of Common Stock as of March 27, 2020, of which officers and directors owned a total of 971,000 vested options and 1,033,476 shares at March 27, 2020 (and exercisable within 60 days thereafter). We have calculated the percentage on the basis of the amount of outstanding securities plus, for each person or group, any securities that person or group has current or future right to acquire pursuant to options, warrants, conversion privileges or other rights based on the 13G and 13D SEC filings.
(ii) Includes 607,972 shares and 54,000 vested options out of a total of 71,000 ten year options owned by Mr. Makrides to purchase shares of Common Stock of the Company at an exercise price between $2.54 and $7.28. Options vest equally over a one year period.
(iii) Includes 0 shares and 1,078,667 vested options out of a total of 1,479,000 ten year options owned by Mr. Goodwin to purchase shares of Common Stock of the Company at an exercise price between $2.99 and $8.18. Options vest equally over a two or three year period.
(iv)Includes 426,504 shares and 143,500 vested options out of a total of 281,000 ten year options owned by Mr. Citronowicz to purchase shares of Common Stock of the Company at an exercise price ranging from $1.80 to $8.18. Options vest equally over a three or four year period.
(v) Includes 0 shares and 149,000 vested options out of a total of 166,000 ten year options owned by Mr. Waldman to purchase shares of Common Stock of the Company at an exercise price ranging from $1.88 to $7.28. Options vest in one year.
(vi) Includes 0 shares and 151,583 vested options out of a total of 356,000 ten year options owned by Mr. Hornsby to purchase shares of Common Stock of the Company at an exercise price ranging from $1.77 to $8.18. Options vest equally over a three to four year period.
(vii) Includes 0 shares and 98,000 vested options out of a total of 115,000 ten year options owned by Mr. Geraghty to purchase shares of Common Stock of the Company at an exercise price ranging from $1.88 to $7.28 Options vest over a one year period.
(viii) Includes 45,916 shares and 0 vested options out of a total of 7,084 ten year options owned by Mr. Swandal to purchase shares of Common Stock of the Company at an exercise price of $7.28. Options vest equally over a one year period.
(ix) Includes 0 shares and 70,500 vested options out of a total of 87,500 ten year options owned by Mr. Andres to purchase shares of Common Stock of the Company at an exercise price ranging from $1.88 to $7.28. Options vest equally over a one year period.
(x) Includes 0 shares and 21,667 vested options out of a total of 161,000 ten year options owned by Ms. Semb to purchase shares of Common Stock of the Company at an exercise price ranging from $7.91 to $8.18. Options vest equally over a three year period.
(xi) Includes 1,766,917 vested options out of a total of 2,733,500 ten year outstanding options and 1,033,476 shares owned by all Executive Officers and directors as a group. The last date the options can be exercised is January 14, 2030.
Section 16(a) Beneficial Ownership Reporting Compliance
89
APYX MEDICAL CORPORATION
Section 16(a) of the Securities Exchange Act of 1934 requires our officers and directors and persons who own more than ten percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the Securities and Exchange Commission. Officers, directors and greater than ten-percent shareholders (the “Reporting Persons”) are required by SEC regulation to furnish us with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on its review of the copies of such reports received or written representations from certain Reporting Persons that no other reports were required, the Company believes that during its fiscal year ended December 31, 2019 all filing requirements applicable to the Reporting Persons were timely met, with the exception of the 4 named Executives, who did not timely file their Form 4s showing 1 transaction, and Craig A. Swandal who did not timely file his Form 4s for 3 separate transactions.
90
APYX MEDICAL CORPORATION
ITEM 13. Certain Relationships and Related Transactions and Director Independence
Certain Relationships and Related Transactions
Several relatives of Nikolay Shilev, Apyx Bulgaria’s Managing Director, are considered related parties. Teodora Shileva, Mr. Shilev’s spouse, is an employee of the Company working in the accounting department. Antoaneta Dimitrova Shileva-Toromanova, Mr. Shilev’s sister, is the manager of production and human resources. Svetoslav Shilev, Mr. Shilev’s son, is an engineer in the quality assurance department. In addition, as part of the purchase of the Bulgaria manufacturing facility, Mr. Shilev was issued a note payable for $0.1 million to be paid 5 years after the original purchase date, which is in October 2020.
Independent Board Members
The Board currently has five independent members, John Andres, Michael Geraghty, Lawrence J. Waldman, Craig Swandal, and Minnie Baylor-Henry, who meet the existing independence requirements of The NASDAQ Stock Market LLC and the Securities and Exchange Commission.
ITEM 14. Principal Accountant Fees and Services
The following table sets forth the aggregate fees billed to us and expected to be billed to us by BDO USA, LLP and Frazier & Deeter, LLC, our principal accountants for 2019 and 2018, respectively:
Year Ended December 31, | |||||||
(In thousands) | 2019 | 2018 | |||||
Audit fees (1) | $ | 515 | $ | 443 | |||
Audit related fees (2) | 14 | 112 | |||||
Tax fees (3) | 81 | — | |||||
All other fees (4) | — | — | |||||
Total fees billed | $ | 610 | $ | 555 | |||
(1) | Audit fees consist of billed and unbilled fees for professional services rendered for the audit of Apyx's annual financial statements and reviews of its interim consolidated financial statements included in quarterly reports and other services related to statutory and regulatory filings or engagements. |
(2) | Audit related fees consist of billed and unbilled fees for assurance and related services that are reasonably related to the performance of the audit or reviews of Apyx's consolidated financial statements and are not reported under “Audit Fees”. |
(3) | Tax fees consist of billed and unbilled fees for professional services rendered for tax compliance and tax advice (domestic and international). These services include assistance regarding federal, state and international tax compliance, acquisitions and international tax planning. |
(4) | All other fees consist of fees for products and services other than the services reported above. |
91
APYX MEDICAL CORPORATION
PART IV
ITEM 15. Exhibits and Financial Statement Schedules
(a)(1) | LISTING OF FINANCIAL STATEMENTS | Page | ||
The following consolidated financial statements of the Company are included in Item 8 of this Report: | ||||
(a)(2) | FINANCIAL STATEMENT SCHEDULES | |||
All financial statement schedules have been omitted, since the required information is not applicable or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the consolidated financial statements and notes thereto included in this Report. | ||||
(a)(3) EXHIBITS
92
APYX MEDICAL CORPORATION
3.1 | ||
3.2 | ||
3.3 | Certificate of Amendment of the Certificate of Incorporation of the Registrant (Incorporated by reference to the Registrant's Quarterly Report on Form 10-Q filed on November 3, 2017) | |
3.4 | Certificate of Elimination of the Series A 6% Convertible Preferred Stock and Series B Convertible Preferred Stock (Incorporated by reference to the Registrant's Current Report on Form 8-K filed on May 3, 2018) | |
3.5 | Certificate of Amendment of the Certificate of Incorporation of the Registrant (Incorporated by reference to the Registrant's Current Report on Form 8-K filed on December 28, 2018) | |
4.1 | Indenture (Incorporated by reference to the Registrant's Registration Statement on Form S-3 filed on May 4, 2018) | |
4.2* | ||
10.1** | Charles D. Goodwin II Employment Agreement, dated December 15, 2017 (Incorporated by the reference to the Registrant's Annual Report on Form 10-K filed on March 13, 2018) | |
10.2** | Separation Agreement and General Release, dated November 12, 2018, by and between the Company and Jay D. Ewers (Incorporated by the reference to the Registrant's Annual Report on Form 10-K filed on March 14, 2019) | |
10.3** | Tara Semb Employment Agreement, dated January 2, 2019 (Incorporated by the reference to the Registrant's Annual Report on Form 10-K filed on March 14, 2019) | |
14.1* | ||
16.1 | Letter from Frazier & Deeter, LLC (Incorporated by the reference to the Registrant's Current Report on Form 8-K filed on June 4, 2019) | |
21.1* | ||
23.1* | ||
23.2* | ||
31.1* | ||
31.2* | ||
32.1* | ||
32.2* | ||
101.INS*** | XBRL Instance Document | |
101.SCH*** | XBRL Taxonomy Extension Schema Document | |
101.CAL*** | XBRL Taxonomy Extension Calculation Linkbase Document | |
101.DEF*** | XBRL Taxonomy Extension Definition Linkbase Document | |
101.LAB*** | XBRL Taxonomy Extension Label Linkbase Document | |
101.PRE*** | XBRL Taxonomy Extension Label Presentation Document | |
* Filed herewith.
** Management contract or compensatory arrangement.
*** XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended and otherwise is not subject to liability under these sections.
93
APYX MEDICAL CORPORATION
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in Clearwater, Florida on March 31, 2020.
Apyx Medical Corporation | |||
By: | /s/ Charles D. Goodwin II | ||
Charles D. Goodwin II | |||
President, Chief Executive Officer and Director | |||
(Principal Executive Officer) | |||
By: | /s/ Tara Semb | ||
Tara Semb | |||
Chief Financial Officer, | |||
Treasurer and Secretary | |||
(Principal Financial Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Name | Title | Date | ||
Directors: | ||||
/s/ ANDREW MAKRIDES | Chairman of the Board | March 31, 2020 | ||
Andrew Makrides | ||||
/s/ CHARLES D. GOODWIN II | Chief Executive Officer and Director | March 31, 2020 | ||
Charles D. Goodwin II | ||||
/s/ TARA SEMB | Chief Financial Officer, Treasurer and Secretary | March 31, 2020 | ||
Tara Semb | ||||
/s/ JOHN ANDRES | Vice Chairman of the Board | March 31, 2020 | ||
John Andres | ||||
/s/ LAWRENCE J. WALDMAN | Director | March 31, 2020 | ||
Lawrence J. Waldman | ||||
/s/ MICHAEL GERAGHTY | Director | March 31, 2020 | ||
Michael Geraghty | ||||
/s/ CRAIG SWANDAL | Director | March 31, 2020 | ||
Craig Swandal | ||||
/s/ MINNIE BAYLOR-HENRY | Director | March 31, 2020 | ||
Minnie Baylor-Henry | ||||
94
