Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Arbutus Biopharma Corp | ex991-abx729updatepressrel.htm |
| 8-K - 8-K - Arbutus Biopharma Corp | a2020-03x8xkxabx729update.htm |

AB-729-001 Preliminary Results March 26, 2020 NASDAQ: ABUS www.arbutusbio.com

Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, and forward- looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). Forward-looking statements in this presentation include statements about our expectations regarding the timing and clinical development of our product candidates, including the evaluation of multiple dose and additional single-dose cohorts in our Phase 1a/1b clinical trial for AB-729 during 2020; and the potential for our drug candidates to improve upon the standard of care and contribute to a curative combination regimen for chronic HBV. With respect to the forward-looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things: the effectiveness and timeliness of preclinical studies and clinical trials, and the usefulness of the data; the timeliness of regulatory approvals; the continued demand for Arbutus’ assets; and the stability of economic and market conditions. While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties and contingencies, including uncertainties and contingencies related to the ongoing COVID-19 pandemic. Additionally, there are known and unknown risk factors which could cause Arbutus’ actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements contained herein. Known risk factors include, among others: anticipated pre-clinical studies and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate; changes in Arbutus’ strategy regarding its product candidates and clinical development activities; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus’ products; economic and market conditions may worsen; market shifts may require a change in strategic focus; and the ongoing COVID-19 pandemic could significantly disrupt our clinical development programs. A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus’ Annual Report on Form 10-K, Arbutus’ Quarterly Reports on Form 10-Q and Arbutus’ continuous and periodic disclosure filings, which are available at www.sedar.com and at www.sec.gov. All forward-looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward-looking statements or to publicly announce the result of any revisions to any of the forward-looking statements contained herein to reflect future results, events or developments, except as required by law. COVID-19. In December 2019 an outbreak of a novel strain of coronavirus (COVID-19) was identified in Wuhan, China. This virus continues to spread globally, has been declared a pandemic by the World Health Organization and has spread to nearly every country in the world. The impact of this pandemic has been, and will likely continue to be, extensive in many aspects of society. The pandemic has resulted in and will likely continue to result in significant disruptions to businesses. A number of countries and other jurisdictions around the world have implemented extreme measures to try and slow the spread of the virus. These measures include the closing of businesses and requiring people to stay in their homes, the latter of which raises uncertainty regarding the ability to travel to hospitals in order to participate in clinical trials. Additional measures that have had, and will likely continue to have, a major impact on clinical development, at least in the near- term, include shortages and delays in the supply chain, and prohibitions in certain countries on enrolling subjects in new clinical trials (e.g. in Australia). It is not possible to predict if the COVID-19 pandemic will negatively impact our plans and timelines. NASDAQ: ABUS www.arbutusbio.com
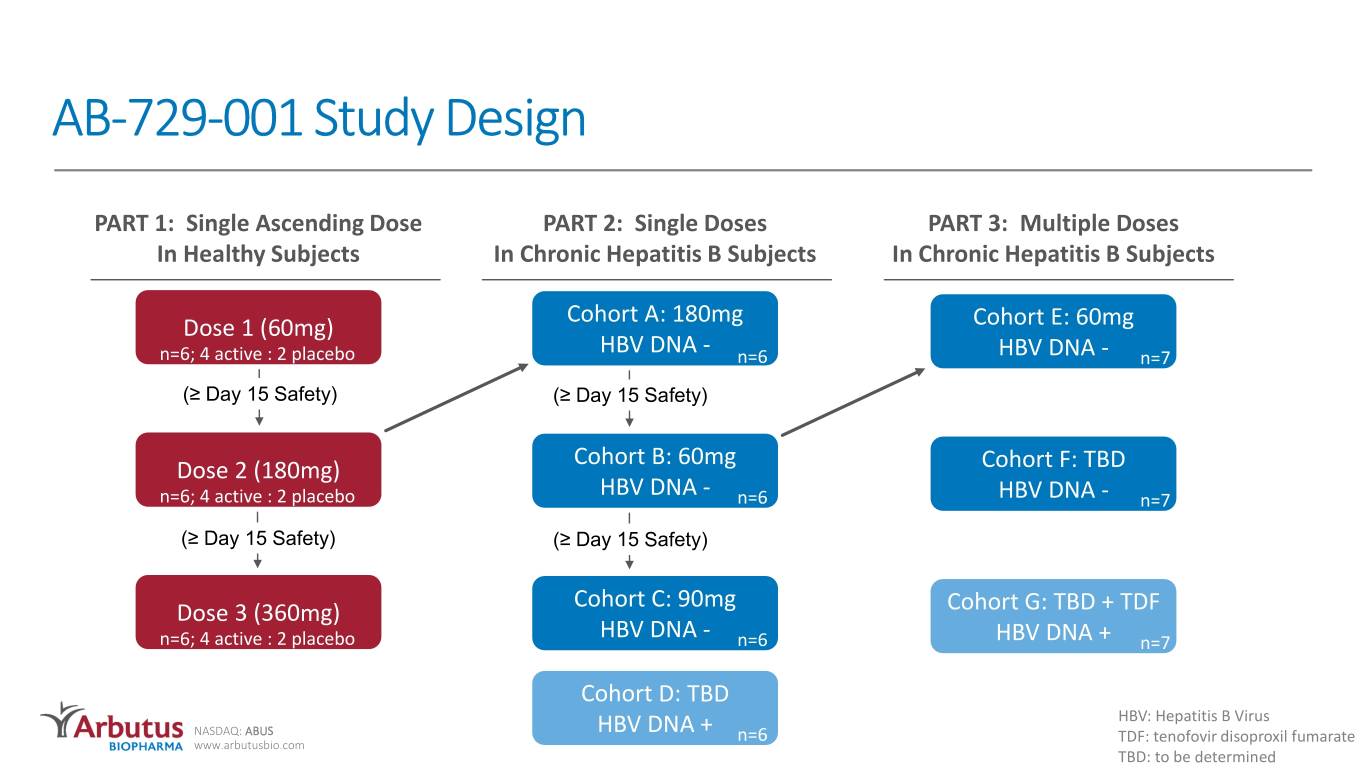
AB-729-001 Study Design PART 1: Single Ascending Dose PART 2: Single Doses PART 3: Multiple Doses In Healthy Subjects In Chronic Hepatitis B Subjects In Chronic Hepatitis B Subjects Cohort A: 180mg Dose 1 (60mg) Cohort E: 60mg HBV DNA - n=6; 4 active : 2 placebo n=6 HBV DNA - n=7 (≥ Day 15 Safety) (≥ Day 15 Safety) Cohort B: 60mg Dose 2 (180mg) Cohort F: TBD HBV DNA - n=6; 4 active : 2 placebo n=6 HBV DNA - n=7 (≥ Day 15 Safety) (≥ Day 15 Safety) Cohort C: 90mg Dose 3 (360mg) Cohort G: TBD + TDF HBV DNA - n=6; 4 active : 2 placebo n=6 HBV DNA + n=7 Cohort D: TBD HBV: Hepatitis B Virus NASDAQ: ABUS HBV DNA + n=6 www.arbutusbio.com TDF: tenofovir disoproxil fumarate TBD: to be determined

AB-729-001 Key Inclusion/Exclusion Criteria 1. Documented chronic hepatitis B infection; confirmed HBeAg positive or negative 2. HBV-DNA at screening: a) For HBV-DNA negative subjects (on a NA for at least 6 months): HBV-DNA <LLOQ b) For HBV-DNA positive subjects: HBV-DNA ≥1,000 IU/mL 3. HBsAg ≥250 IU/mL at screening 4. Non-cirrhotic with mild/moderate fibrosis defined by: a) Liver biopsy Metavir Fibrosis Score of F0-2 (or equivalent) within 12 months OR Fibroscan® result of ≤10 kPa within 6 months 5. ALT/AST <5x ULN for Part 2 and <2x ULN for Part 3; Tbili <1.5x ULN for all Parts NASDAQ: ABUS www.arbutusbio.com
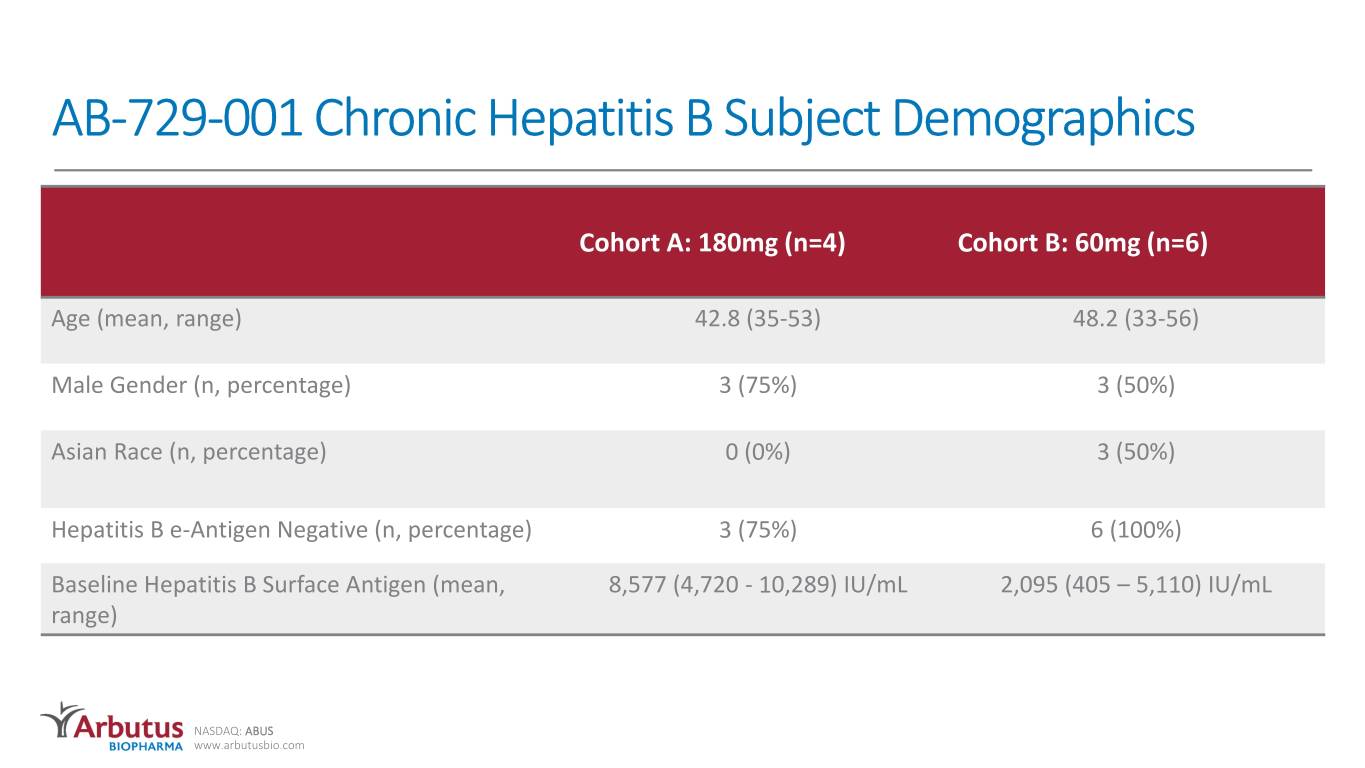
AB-729-001 Chronic Hepatitis B Subject Demographics Cohort A: 180mg (n=4) Cohort B: 60mg (n=6) Age (mean, range) 42.8 (35-53) 48.2 (33-56) Male Gender (n, percentage) 3 (75%) 3 (50%) Asian Race (n, percentage) 0 (0%) 3 (50%) Hepatitis B e-Antigen Negative (n, percentage) 3 (75%) 6 (100%) Baseline Hepatitis B Surface Antigen (mean, 8,577 (4,720 - 10,289) IU/mL 2,095 (405 – 5,110) IU/mL range) NASDAQ: ABUS www.arbutusbio.com

AB-729-001: Healthy Volunteer Safety •No SAEs across all doses •15 AEs in total; 4 study drug-related AEs •Dose 1 (60 mg): • No clinically significant changes in vital signs, lab parameters or ECG abnormalities •Dose 2 (180 mg): • 1 Grade 1 drug-related AE (erythema at injection site) • 1 Lab abnormality (not drug-related): 1 Grade 2 ALT elevation (resolved) • No clinically significant changes in vital signs, other lab parameters or ECG abnormalities •Dose 3 (360 mg): • 3 Lab abnormalities: 2 subjects with Grade 3 and 1 subject with Grade 1 asymptomatic ALT elevations (resolved). These 3 subjects had Grade 2 and Grade 1 AST elevations. • No clinically significant changes in vital signs, other lab parameters or ECG abnormalities NASDAQ: ABUS www.arbutusbio.com
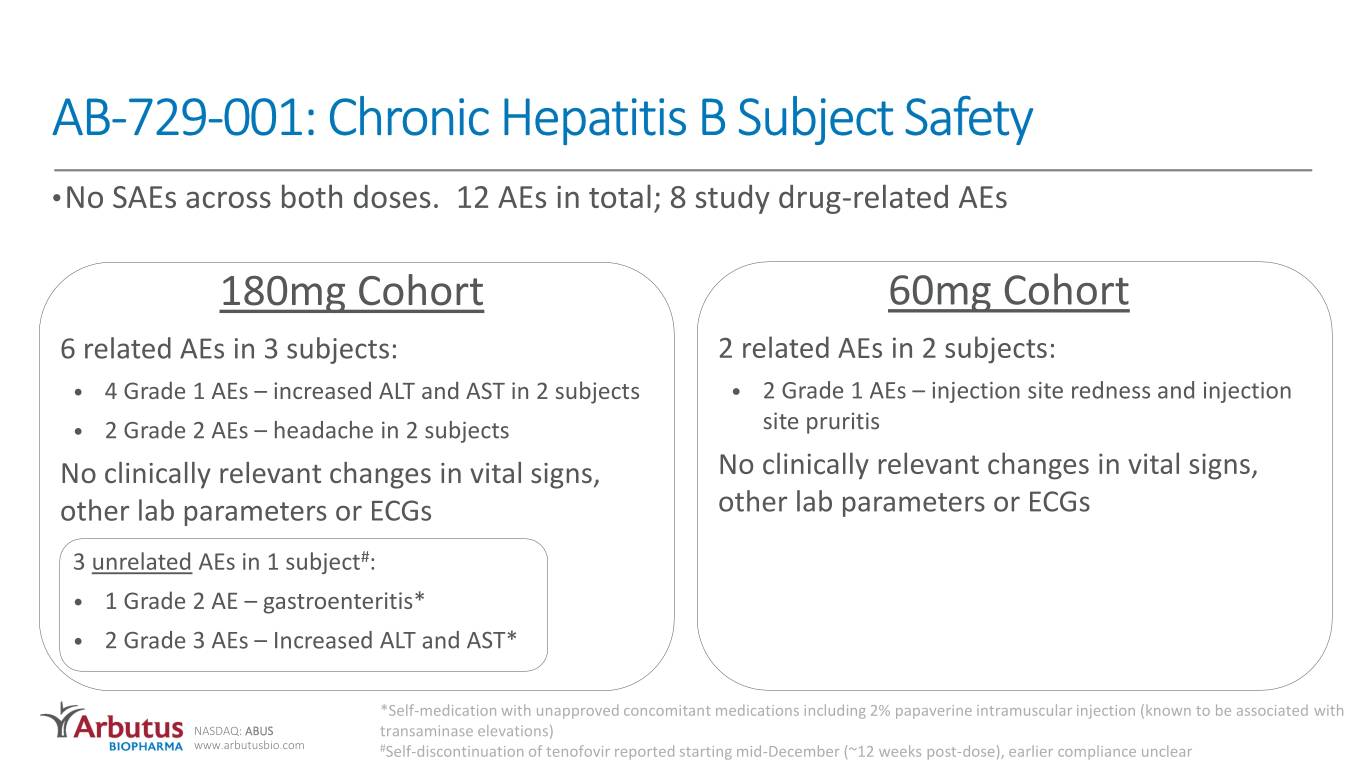
AB-729-001: Chronic Hepatitis B Subject Safety •No SAEs across both doses. 12 AEs in total; 8 study drug-related AEs 180mg Cohort 60mg Cohort 6 related AEs in 3 subjects: 2 related AEs in 2 subjects: • 4 Grade 1 AEs – increased ALT and AST in 2 subjects • 2 Grade 1 AEs – injection site redness and injection • 2 Grade 2 AEs – headache in 2 subjects site pruritis No clinically relevant changes in vital signs, No clinically relevant changes in vital signs, other lab parameters or ECGs other lab parameters or ECGs 3 unrelated AEs in 1 subject#: • 1 Grade 2 AE – gastroenteritis* • 2 Grade 3 AEs – Increased ALT and AST* *Self-medication with unapproved concomitant medications including 2% papaverine intramuscular injection (known to be associated with NASDAQ: ABUS transaminase elevations) www.arbutusbio.com #Self-discontinuation of tenofovir reported starting mid-December (~12 weeks post-dose), earlier compliance unclear

Individual HBsAg Change From Baseline in Chronic Hepatitis B Subjects 29 Days After a Single Dose of AB-729 60 mg Cohort 180 mg Cohort Mean Day 29: -0.44 log10 IU/mL Mean Day 29: (n=3) -0.24 log10 IU/mL (n=6) Mean Day 29: Mean Day 29: -0.81 log10 IU/mL -0.51 log10 IU/mL (n=3) (n=4) -1.94 log10 IU/mL NASDAQ: ABUS www.arbutusbio.com ATAFD: actual time after first dose
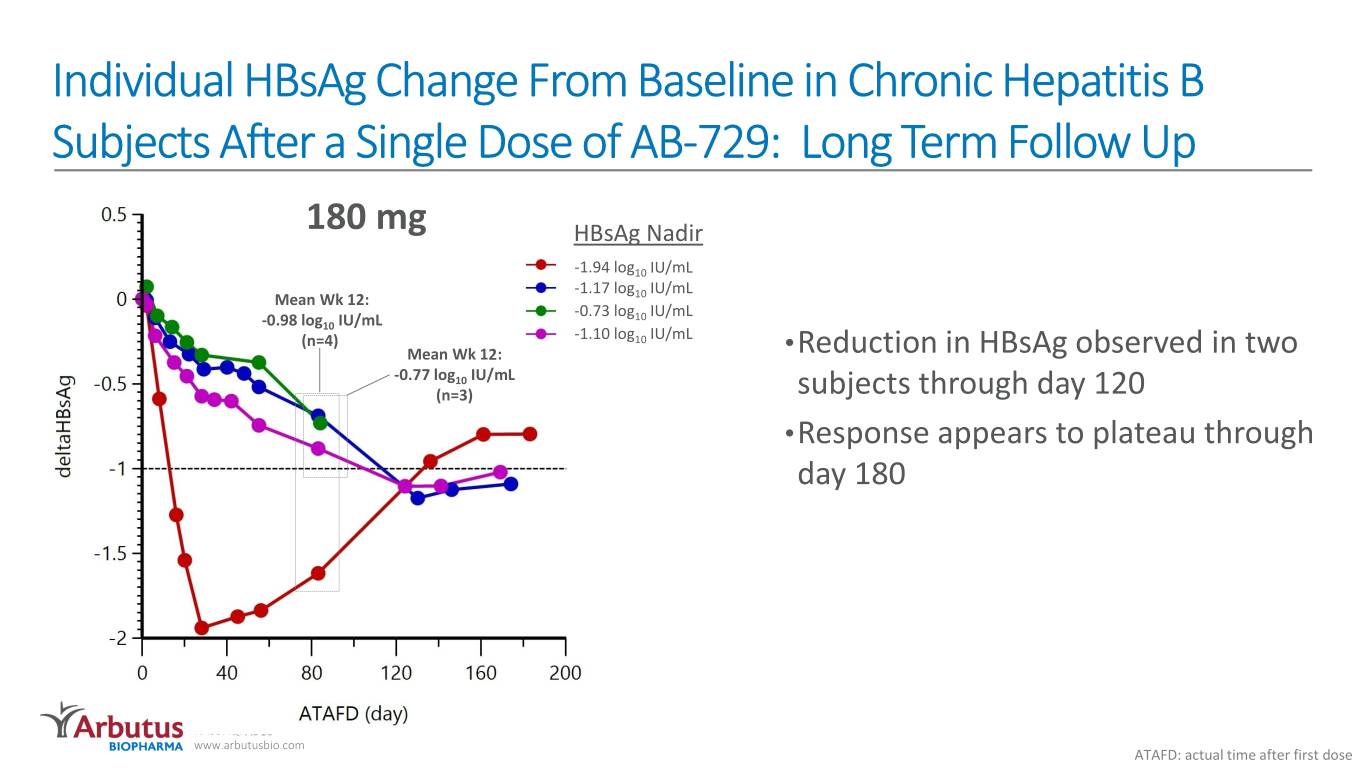
Individual HBsAg Change From Baseline in Chronic Hepatitis B Subjects After a Single Dose of AB-729: Long Term Follow Up 180 mg HBsAg Nadir -1.94 log10 IU/mL -1.17 log IU/mL Mean Wk 12: 10 -0.73 log10 IU/mL -0.98 log10 IU/mL (n=4) -1.10 log10 IU/mL Mean Wk 12: •Reduction in HBsAg observed in two -0.77 log10 IU/mL (n=3) subjects through day 120 •Response appears to plateau through day 180 NASDAQ: ABUS www.arbutusbio.com ATAFD: actual time after first dose
Q&A
