Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Turning Point Brands, Inc. | hc10009574x1_ex32-1.htm |
| EX-31.3 - EXHIBIT 31.3 - Turning Point Brands, Inc. | hc10009574x1_ex31-3.htm |
| EX-31.2 - EXHIBIT 31.2 - Turning Point Brands, Inc. | hc10009574x1_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - Turning Point Brands, Inc. | hc10009574x1_ex31-1.htm |
| EX-23 - EXHIBIT 23 - Turning Point Brands, Inc. | hc10009574x1_ex23.htm |
| EX-21 - EXHIBIT 21 - Turning Point Brands, Inc. | hc10009574x1_ex21.htm |
| EX-10.48 - EXHIBIT 10.48 - Turning Point Brands, Inc. | hc10009574x1_ex10-48.htm |
| EX-4.2 - EXHIBIT 4.2 - Turning Point Brands, Inc. | hc10009574x1_ex4-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
(Mark One)
| ☑ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
OR
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from_______________ to ________________
Commission file number: 001-37763
TURNING POINT BRANDS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
20-0709285
|
|
(State or other jurisdiction of
incorporation or organization) |
(I.R.S. Employer
Identification No.) |
|
|
|
|
5201 Interchange Way, Louisville, KY
|
40229
|
|
(Address of principal executive offices)
|
(Zip Code)
|
(502) 778-4421
(Registrant’s telephone number, including area code)
Former name, former address and former fiscal year, if changed since last report: not applicable
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
|
Common Stock, $0.01 par value
|
TPB
|
New York Stock Exchange
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. o Yes ☑ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. o Yes ☑ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☑ Yes o No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☑ Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
o
|
Accelerated filer
|
☑
|
|
Non-accelerated filer
|
o
|
Smaller reporting company
|
o
|
|
Emerging growth company
|
☑
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☑
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes ☑ No
As of June 30, 2019, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $410 million based on the closing sale price of the common stock as reported on the New York Stock Exchange.
At February 28, 2020, there were 19,723,080 shares outstanding of the registrant’s voting common stock, par value $0.01 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for use in connection with its annual meeting of stockholders to be held on April 28, 2020, expected to be filed with the Securities and Exchange Commission on or about March 19, 2020, are incorporated by reference into Part III hereof.
Cautionary Note Regarding Forward-Looking Statements
This annual report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. Forward-looking statements may generally be identified using words such as “anticipate,” “believe,” “expect,” “intend,” “plan” and “will” or, in each case, their negative, or other variations or comparable terminology. These forward-looking statements include all matters that are not historical facts. By their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. As a result, actual events may differ materially from those expressed in or suggested by the forward-looking statements. Any forward-looking statement made by TPB in this annual report on Form 10-K speaks only as of the date hereof. New risks and uncertainties come up from time to time, and it is impossible for TPB to predict these events or how they may affect it. TPB has no obligation, and does not intend, to update any forward-looking statements after the date hereof, except as required by federal securities laws. Factors that could cause these differences include, but are not limited to:
| • | declining sales of tobacco products, and expected continuing decline of sales, in the tobacco industry overall; |
| • | our dependence on a small number of third-party suppliers and producers; |
| • | the possibility that we will be unable to identify or contract with new suppliers or producers in the event of a supply or product disruption; |
| • | our business may be damaged by events outside of our suppliers’ control, such as the impact of epidemics (e.g., coronavirus), political upheavals, or natural disasters; |
| • | the possibility that our licenses to use certain brands or trademarks will be terminated, challenged or restricted; |
| • | failure to maintain consumer brand recognition and loyalty of our customers; |
| • | substantial and increasing U.S. regulation; |
| • | regulation of our products by the FDA, which has broad regulatory powers; |
| • | our products are subject to developing and unpredictable regulation, for example, current court action moving forward certain substantial Pre Market Tobacco Application obligations; |
| • | some of our products contain nicotine, which is considered to be a highly addictive substance; |
| • | uncertainty related to the regulation and taxation of our NewGen products; |
| • | possible significant increases in federal, state and local municipal tobacco- and vapor-related taxes; |
| • | possible increasing international control and regulation; |
| • | our reliance on relationships with several large retailers and national chains for distribution of our products; |
| • | our amount of indebtedness; |
| • | the terms of our credit facilities, which may restrict our current and future operations; |
| • | intense competition and our ability to compete effectively; |
| • | uncertainty and continued evolution of markets containing our NewGen products; |
| • | significant product liability litigation; |
| • | the scientific community’s lack of information regarding the long-term health effects of certain substances contained in some of our products; |
| • | requirement to maintain compliance with master settlement agreement escrow account; |
| • | competition from illicit sources; |
| • | our reliance on information technology; |
| • | security and privacy breaches; |
| • | contamination of our tobacco supply or products; |
2
| • | infringement on our intellectual property; |
| • | third-party claims that we infringe on their intellectual property; |
| • | failure to manage our growth; |
| • | failure to successfully integrate our acquisitions or otherwise be unable to benefit from pursuing acquisitions; |
| • | fluctuations in our results; |
| • | exchange rate fluctuations; |
| • | adverse U.S. and global economic conditions; |
| • | sensitivity of end-customers to increased sales taxes and economic conditions; |
| • | failure to comply with certain regulations; |
| • | departure of key management personnel or our inability to attract and retain talent; |
| • | imposition of significant tariffs on imports into the U.S.; |
| • | reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors, potentially decreasing our stock price; |
| • | failure to maintain our status as an emerging growth company before the five-year maximum time period a company may retain such status; |
| • | our principal stockholders will be able to exert significant influence over matters submitted to our stockholders and may take certain actions to prevent takeovers; |
| • | our certificate of incorporation and bylaws, as well as Delaware law and certain regulations, could discourage or prohibit acquisition bids or merger proposals, which may adversely affect the market price of our common stock; |
| • | our certificate of incorporation limits the ownership of our common stock by individuals and entities that are Restricted Investors. These restrictions may affect the liquidity of our common stock and may result in Restricted Investors being required to sell or redeem their shares at a loss or relinquish their voting, dividend and distribution rights; |
| • | future sales of our common stock in the public market could reduce our stock price, and any additional capital raised by us through the sale of equity or convertible securities may dilute your ownership in us; |
| • | we may issue preferred stock whose terms could adversely affect the voting power or value of our common stock; and |
| • | our status as a “controlled company” could make our common stock less attractive to some investors or otherwise harm our stock price. |
3
PART I
| Item 1. | Business |
Turning Point Brands, Inc., Overview
Turning Point Brands, Inc. (the “Company,” “we,” “our,” or “us”) is a leading, independent provider of Other Tobacco Products (“OTP”) in the U.S. We estimate the OTP industry generated approximately $11.5 billion of manufacturer revenue in 2019. In contrast to manufactured cigarettes, which have been experiencing declining volumes for decades based on data published by the Alcohol and Tobacco Tax and Trade Bureau (“TTB”), the OTP industry is demonstrating increased consumer appeal with low to mid-single digit consumer unit growth as reported by Management Science Associates, Inc. (“MSAi”), a third-party analytics and information company. We were the 6th largest competitor in terms of total OTP consumer units sold during 2019. We sell a wide range of products across the OTP spectrum; however, we do not sell cigarettes. Our portfolio of brands includes some of the most widely recognized names in the OTP industry, such as Zig-Zag®, Beech-Nut®, Stoker’s®, Trophy®, VaporBeast®, Solace®, and VaporFi®. We currently ship to approximately 800 distributors with an additional 100 secondary, indirect wholesalers in the U.S. that carry and sell our products. We operate in three segments: (i) Smokeless products, (ii) Smoking products, and (iii) NewGen products.
We have identified additional growth opportunities in the emerging alternatives market. In January 2019, we established our subsidiary, Nu-X Ventures (“Nu-X”), a new company and wholly owned subsidiary dedicated to the development, production and sale of alternative products and acquisitions in related spaces. The creation of Nu-X allows us to leverage our expertise in traditional OTP management to alternative products. The TPB management team has over 100 years of experience navigating federal, state and local regulations that are directly applicable to the growing alternatives market. In July 2019, we acquired the assets of Solace Technology (“Solace”). Solace is an innovative product development company which established one of the top e-liquid brands and has since grown into a leader in alternative products. Solace’s legacy and innovation will enhance Nu-X’s strong and nimble development engine. In July 2019, we acquired a 30% stake in ReCreation Marketing (“ReCreation”). ReCreation is a specialty marketing and distribution firm focused on building brands in the Canadian smoking and alternative products categories. The investment will leverage ReCreation’s significant expertise in marketing and distributing tobacco and cannabis products throughout Canada. We plan to make additional investments, partnerships and acquisitions to drive the business of Nu-X. These endeavors will enable us to continue to identify unmet customer needs and provide quality products that we believe will result in genuine customer satisfaction and foster the growth of revenue.
We believe there are meaningful opportunities to grow through acquisitions and joint ventures across all product categories. As of December 31, 2019, our products are available in approximately 185,000 U.S. retail locations which, with the addition of retail stores in Canada, brings our total North American retail presence to an estimated 210,000 points of distribution. Our sales team targets widespread distribution to all traditional retail channels, including convenience stores.
Smokeless Segment
Our Smokeless segment includes both loose leaf chewing tobacco and moist snuff tobacco (“MST”). Our Smokeless focus brand is Stoker’s in both chewing tobacco and MST. Stoker’s® chewing tobacco has grown considerable share over the last several years and is presently the #1 discount brand and the second largest brand in the industry, with approximately a 20% market share. Our status in the chew market is further strengthened by Beech-Nut®, the #3 premium brand and #7 overall, as well as Trophy®, Durango®, and the five Wind River Brands we acquired in 2016. Collectively, the company is the #2 marketer of chewing tobacco with approximately 29% market share. Our chewing tobacco operations are facilitated through our long-standing relationship with Swedish Match, the manufacturer of our loose-leaf chewing tobaccos.1
In MST, Stoker’s remains among the fastest growing brands and holds an 8.1% share in the stores with distribution and a 4.5% share of the total U.S. MST market. Stoker’s pioneered the large 12 oz. tub packaging format and is manufactured using a proprietary process that we think results in a superior product. In late 2015, we extended the Stoker’s® MST franchise to include traditional 1.2 oz. cans to broaden retail availability. Our proprietary manufacturing process is conducted at our Dresden, Tennessee, plant and packaged in both our Dresden, Tennessee, and Louisville, Kentucky facilities.1
| 1 | Brand rankings and market share percentages obtained from MSAi as of December 31, 2019. |
4
Smoking Segment
Our Smoking segment principally includes cigarette papers and Make-Your-Own (“MYO”) cigar wraps. The iconic strength of the Zig-Zag® brand drives our leadership position in both the cigarette papers and MYO cigar wrap markets. In cigarette papers, Zig-Zag® is the #1 premium cigarette paper in the U.S. with approximately 35% total market share. Management estimates also indicate that Zig-Zag® is the #1 brand in the promising Canadian market. Cigarette paper operations are aided by our sourcing relationships with Bolloré.1
In MYO cigar wraps, the Zig-Zag® brand commands about three-quarters of the market and continues to innovate in novel ways, including our recent introduction of Zig-Zag® ‘Rillo sized wraps which are similar in size to cigarillos, the most popular and fastest growing type of machine-made cigars. MYO cigar wraps operations are facilitated by our long-standing commercial relationship with the patent holder, Durfort.1
NewGen Segment
Our NewGen segment includes our Nu-X subsidiary dedicated to the development, production and sale of alternative products as well as our various acquisitions in the vape space. Nu-X is dedicated to the development, production and sale of alternative products, which was enhanced by the acquisition of Solace in July 2019. VaporBeast is a leading distributor of vapor products servicing the non-traditional retail channel. International Vapor Group and its subsidiaries (collectively, “IVG”), operate a strong B2C eCommerce business with direct sales to consumers nationwide and abroad through the Direct-Vapor and VaporFi brands. Refer to Note 3 of our Notes to Consolidated Financial Statements for further details regarding these acquisitions. In late summer 2019, the vapor market experienced a significant disruption due to consumer illnesses and, thereafter, the FDA flavor regulation announcement. As a result, on November 1, 2019, we announced our intention to evaluate strategic alternatives as they relate to the vapor business and implemented a restructuring effort to right-size the business including a company-wide workforce reduction of ten percent and the consolidation of warehouses, elimination of unprofitable platforms and store closures. Coinciding with our restructuring announcement, we communicated our intention to pivot from the third-party vaping business and to focus sales and marketing resources on our proprietary brands.
Competitive Strengths
We believe our competitive strengths include the following:
Large, Leading Brands with Significant Scale
We have built a portfolio of leading brands with significant scale that are well recognized by consumers, retailers, and wholesalers. Our Stoker’s® and Zig-Zag® brands are each well established and date back 80 and 120 years, respectively. The NewGen segment has been built primarily through the acquisitions of Solace, VaporBeast, and IVG, leading sellers of e-liquids, devices, and accessories.
| • | Stoker’s® is the #2 loose leaf chewing tobacco brand and among the fastest growing MST brands in the industry. We manufacture Stoker’s® MST using only 100% American Leaf, utilizing a proprietary process to produce what we believe is a superior product. |
| • | Zig-Zag® is the #1 premium cigarette paper brand in the U.S., with significant distribution in Canada. Zig-Zag® is also the #1 MYO cigar wrap brand in the U.S., as measured by MSAi. |
We believe the Stoker’s® brand is seen as an innovator in both the loose-leaf chewing tobacco and moist snuff markets. Zig-Zag® is an iconic brand and has strong, enduring brand recognition among a wide audience of consumers. The Solace acquisition provides us with a proven line of e-liquid and a strong new product development platform from which we intend to launch additional novel products, including a variety of actives. VaporBeast is a powerful distribution engine that allows us to further penetrate non-traditional retail outlets. IVG provides us direct access to the highly attractive, high margin B2C segment via the flagship VaporFi® brand.
5
Successful Track Record of New Product Launches and Category Expansions
We have successfully launched new products and entered new product categories by leveraging the strength of our brands. We methodically target markets which we believe have significant growth potential. We have been successful in entering new product categories by extending existing products and brands in addition to introducing new products:
| • | We leveraged the proud legacy and value of the Stoker’s® brand to introduce a 12 oz. MST tub, a product whose size was not offered by any other market participant at the time of introduction. Stoker’s® MST has been among the fastest growing moist snuff brands in the industry in terms of pounds sold. While competitors have introduced larger format tub packaging, the early entry and differentiation of the Stoker’s® product have firmly established us as the market leader with over 50% of the Tub market. In third quarter 2015, we introduced Stoker’s® MST in 1.2 oz. cans to further expand retail penetration, particularly in convenience stores. |
| • | In 2009, we extended the Zig-Zag® tobacco brand into the MYO cigar wraps market and captured a 50% market share within the first two years. We are now the market share leader for MYO cigar wraps with approximately a 75% share. We believe our success was driven by the Zig-Zag® tobacco branding, which we feel is widely understood by consumers to represent a favorable, customizable experience ideally suited to MYO products. |
| • | In 2019 we launched the Nu-X brand focused on product development in the alternative market including cannabidiol isolate (“CBD”). |
| • | VaporBeast quickly established itself as a leading marketer and distributor of liquid vapor products to the non-traditional retail universe. With its national footprint, VaporBeast is leveraging its regional consumer preference insights to further accelerate sales advances. |
| • | The IVG acquisition, and specifically the VaporFi B2C marketing engine, offers us the opportunity to leverage the marketing competencies and processes to sell novel proprietary products across multiple channels and platforms. |
| • | The Solace acquisition in 2019 provided us with a leading line of liquids and a powerful new product development platform. |
We strategically target product categories that we believe demonstrate significant growth potential and for which the value of our brands is likely to have a meaningful impact. We believe that our track record and existing portfolio of brands provide growth advantages as we continue to evaluate opportunities to extend our product lines and expand into new categories.
Extensive Distribution Network and Data Driven Sales Organization
We have taken important steps to enhance our selling and distribution network and consumer marketing capabilities while keeping our capital expense requirements relatively low. We service our traditional tobacco and vapor customer bases with an experienced sales and marketing organization of approximately 178 professionals who possess in-depth knowledge of the OTP market. We extensively use data supported by leading technology to enable our salesforce to analyze changing trends and effectively identify evolving consumer preferences at the store level. We subscribe to a sales tracking system provided by MSAi that measures all OTP product shipments by all market participants, on a weekly basis, from approximately 900 wholesalers to over 250,000 traditional retail stores in the U.S. This system enables us to understand share and volume trends across multiple categories at the individual store level, allowing us to allocate field salesforce coverage to the highest opportunity stores, thereby enhancing the value of new store placements and sales activity. Within our Stoker’s product categories, we have seen a positive correlation between the frequency of store calls by our salesforce and our retail market share. As the initial sales effort is critical to the success of a product launch, we believe our experienced salesforce, expansive distribution network, and leading market analytics put us in a strong position to swiftly execute new product launches in response to evolving consumer and market preferences.
6
Long-standing, Strong Relationships with an Established Set of Producers
As part of our asset-light operating model we built long-standing and extensive relationships with leading, high-quality producers. In 2019, our three most important suppliers were:
| • | Swedish Match, which manufactures our loose-leaf chewing tobacco; |
| • | Bolloré, which provides us with exclusive access to the Zig-Zag® cigarette paper and accessories brand for the U.S. and Canada; and |
| • | Durfort, from which we source our MYO cigar wraps. |
By outsourcing the production of products that represent more than 80% of our net sales to a select group of suppliers with whom we have strong relationships, we are able to maintain low overhead costs and minimal capital expenditures, which together drive our margins.
Experienced Management Team
With an average of approximately 26 years of consumer products experience, including an average of 24 years in the tobacco industry, our senior management team has enabled us to grow and diversify our business while improving operational efficiency. Members of management have previous experience at other leading tobacco companies, including Altria Group, Inc. (formerly Philip Morris); Liggett & Myers Tobacco Company (now Liggett Group, a subsidiary of Vector Group ltd); Swedish Match; and American Brands, Inc. Given the professional experience of the senior management team we, are able to analyze risks and opportunities from a variety of perspectives. Our senior leadership has embraced a collaborative culture in which the combined experience, analytical rigor, and creativity are leveraged to assess opportunities and deliver products that satisfy consumers’ demands.
Growth Strategies
We are focused on building sustainable margin streams, expanding the availability of our products, developing new products through innovation, and enhancing overall operating efficiencies with the goal of improving margins and cash flow. We adopted the following strategies to drive growth in our business and build stockholder value:
Grow Share of Existing Product Lines, Domestically and Internationally
We intend to remain a consumer centric organization with an innovative view and understanding of the OTP market. We believe there are meaningful opportunities for growth within the OTP market and in the emerging alternatives market which includes CBD. We expect to continue to identify unmet consumer needs and provide quality products that we believe will result in genuine consumer satisfaction and foster the growth of revenue. We maintain a robust product pipeline and plan to strategically introduce new products in attractive, growing OTP segments, both domestically and internationally. For example, in addition to our successful launch of Stoker’s® smaller 1.2 oz. MST cans, we believe there are opportunities for new products in the MST pouch and MYO cigar wrap markets. CBD products in the NewGen products segment are currently in our pipeline. We believe we have successfully built strong, powerful brands possessing significant potential.
In 2019, less than 5% of our revenues were generated outside of the U.S. Having established a strong infrastructure and negotiated relationships across multiple segments and products, we are pursuing an international growth strategy to broaden sales and strengthen margins. We believe international sales represent a meaningful growth opportunity. Our goals include expanding our presence in the worldwide OTP industry on a targeted basis. For example, we are selling our Stoker’s® MST products in South America and expanding Zig-Zag’s retail penetration and product assortment in Canada.
Expand into Adjacent Categories through Innovation and New Partnerships
We continually evaluate opportunities to expand into adjacent product categories by leveraging our current portfolio or through new partnerships. In 2009, we leveraged the Zig-Zag® tobacco brand and introduced Zig-Zag® MYO cigar wraps with favorable results. We now command the #1 market share position for that segment. We are currently expanding our MYO cigar wraps business through the expansion of hemp cigar wraps which are similar to traditional cigar wraps, but are made of fine quality hemp, lack any tobacco or nicotine and, therefore, are not subject to federal excise tax. Additionally, we leveraged the big value equity in Stoker’s to launch a highly
7
differentiated and proprietary MST product that remains among the fastest growing brands in the category. We have identified a number of new adjacencies and we intend to leverage our existing brands and partnerships to continue the process of commercializing winning products that satisfy consumer needs.
Continue to Grow a Strong NewGen Platform
The OTP category is continually evolving as consumers actively seek out new products and product forms. Given this market demand, we have developed a NewGen product platform which we believe will serve new and evolving consumer demands across multiple product categories.
Moving forward, we have identified additional opportunities in both CBD and other actives which we intend to take to market under Nu-X. Through our partnership with Canadian American Standard Hemp Inc. (“CASH”) and the keen insights we have attained in the alternative channel space over the last several years, we intend to fully leverage the total TPB infrastructure to place novel Nu-X products at retail and online via our B2C expertise.
We believe the categories within our NewGen segment are poised to be the key industry growth drivers in the future, and we are well-positioned to capitalize on this growth. We intend to continue to pursue growth of our NewGen product platform by offering unique and innovative products to address evolving consumer demands.
Strategically Pursue Acquisitions
We believe there are meaningful acquisition opportunities in the fragmented OTP space. We regularly evaluate acquisition opportunities across the OTP landscape. In evaluating acquisition opportunities, our focus is on identifying acquisitions that strengthen our current distribution platform and product offerings or enable category expansion in areas with high growth potential.
Substantially all of our 2019 U.S. gross profit was derived from sales of products currently regulated by the U.S. Food and Drug Administration (“FDA”) Center for Tobacco Products. We have significant experience in complying with the FDA regulatory regime with a compliance infrastructure composed of legal and scientific professionals. We believe many smaller OTP manufacturers currently lack this infrastructure, which we believe is necessary to comply with the broad scope of FDA regulations. We believe our regulatory compliance infrastructure, combined with our skilled management and strong distribution platform, position us to act as a consolidator within the OTP industry.
We have a strong track record of enhancing our OTP business with strategic and accretive acquisitions. For example, our acquisition of the North American Zig-Zag® cigarette papers distribution rights in 1997 has made us the #1 premium cigarette paper brand in the U.S., as measured by MSAi. Perhaps more importantly, we own the Zig-Zag® tobacco trademark in the U.S. and have leveraged this asset effectively with approximately 52% of our total 2019 Zig-Zag branded net sales under our own Zig-Zag® marks rather than those we license from Bolloré. In 2003, we acquired the Stoker’s® brand. We have since built the brand to a strong #2 position in the chewing tobacco industry while successfully leveraging the brand’s value through our MST expansion where it remains among the fastest growing MST brands in the industry. More recently, we have completed a series of acquisitions since our IPO in 2016 including (i) smokeless tobacco brands from Wind River, (ii) VaporBeast, (iii) IVG, and (iv) Solace. Our strategic minority interest in CASH gives us access to a pipeline of novel CBD products that we believe will be a dynamic force in the industry. Additionally, our investment in ReCreation Marketing in Canada is expected to accelerate Zig-Zag’s growth through alternative channel penetration.
Maintain Lean, Low-Cost Operating Model
We have a lean, asset-light manufacturing and sourcing model which requires low capital expenditures and utilizes outsourced supplier relationships. We believe our asset-light model provides marketplace flexibility and allows us to achieve favorable margins. Our market analytics allow us to efficiently and effectively address evolving consumer and market demands. Our supplier relationships allow us to increase the breadth of our product offerings and quickly enter new OTP markets as management is able to focus on brand building and innovation. We intend to continue to optimize our asset-light operating model as we grow in order to maintain a low cost of operations and healthy margins. In 2019, over 80% of our net sales were derived from outsourced production operations. Our capital expenditures have ranged between $1.6 million and $4.8 million per year over the previous 5 years. We do not intend to outsource our MST production as a result of our proprietary manufacturing processes which are substantively different than those of our competitors.
8
Raw Materials, Product Supply, and Inventory Management
We source our products through a series of longstanding, highly valued relationships which allow us to conduct our business on an asset-light, distribution-focused basis.
The components of inventories were as follows (in thousands):
December 31, 2019 |
December 31, 2018 |
|||||
Raw materials and work in process |
$ | 7,050 | $ | 2,722 | ||
Leaf tobacco |
32,763 | 34,977 | ||||
Finished goods - Smokeless products |
5,680 | 6,321 | ||||
Finished goods - Smoking products |
13,138 | 14,666 | ||||
Finished goods - NewGen products |
17,111 | 37,194 | ||||
Other |
989 | 738 | ||||
Gross Inventory |
76,731 | 96,618 | ||||
LIFO reserve |
(5,752 | ) |
(5,381 | ) |
||
Net Inventory |
$ | 70,979 | $ | 91,237 | ||
Smokeless Products
Our loose-leaf chewing and moist snuff tobaccos are produced from air-cured and fire-cured leaf tobacco, respectively. We utilize recognized suppliers that generally maintain 12- to 24-month supplies of our various types of tobacco at their facilities. We do not believe we are dependent on any single country or supplier source for tobacco. We generally maintain up to a two-month supply of finished, loose leaf chewing tobacco and moist snuff. This supply is maintained at our Louisville, Kentucky, facility and in two regional public warehouses to facilitate distribution.
We also utilize a variety of suppliers for the sourcing of additives used in our smokeless products and for the supply of our packaging materials. Thus, we believe we are not dependent on a single supplier for these products. There are no current U.S. federal regulations that restrict tobacco flavor additives in smokeless products. The additives that we use are food-grade, generally accepted ingredients.
All of our loose-leaf chewing tobacco production is fulfilled through our agreement with Swedish Match. See the “Distribution and Supply Agreements” section for our discussion of the Swedish Match Manufacturing Agreement. All of our moist snuff products are manufactured at our facility in Dresden, Tennessee. Packaging occurs at the Dresden, Tennessee, location in addition to the facility in Louisville, Kentucky.
Smoking Products
Pursuant to our distribution agreements with Bolloré (discussed in more detail, below, under the heading “Distribution and Supply Agreements”), we are required to purchase from Bolloré all cigarette papers, cigarette tubes, and cigarette injecting machines that we sell, subject to Bolloré fulfilling its obligations under these distribution agreements. If Bolloré is unable or unwilling to perform its obligations or ceases its cigarette paper manufacturing operations, in each case, as set forth in the Distribution Agreements, we may seek third-party suppliers and continue the use of the Zig-Zag® trademark to market these products. To ensure we have a steady supply of premium cigarette paper products, as well as cigarette tubes and injectors, Bolloré is required to maintain, at its expense, a two-month supply of inventory in a bonded, public warehouse in the U.S.
We obtain our MYO cigar wraps from the patent holder under our agreement with Durfort in the Dominican Republic. We also obtain our Zig-Zag branded cigar products from the Dominican Republic.
NewGen Products
We have sourcing relationships that are capable of providing liquid vapor products for other companies’ brands and for producing our own branded product lines in the category. Our acquisitions of VaporBeast, IVG and Solace have (i) accelerated our entry into the non-traditional retail channel, where we believe a significant portion of CBD and liquid vapor products are sold; (ii) provided enhanced distribution of products; and (iii) established best-in-class distribution and B2C platforms combining eCommerce selling skills with a national, retail salesforce. We believe the VaporBeast B2B competency coupled with the IVG B2C selling strengths and our national retail salesforce is a
9
genuine competitive advantage and one that we intend to leverage on behalf of Nu-X CBD and other actives products. Furthermore, we have established a sourcing group in Asia to ensure timely and cost-effective access to marketplace winners and new product launches, while also maximizing margin through thoughtful logistics strategies.
Distribution and Supply Agreements
Bolloré Distribution and License Agreements
We are party to two long-term distribution and license agreements with Bolloré with respect to sales of cigarette papers, cigarette tubes, and cigarette injector machines—one with respect to distribution in the U.S. and one with respect to distribution in Canada (collectively, the “Distribution Agreements”). Under the Distribution Agreements, Bolloré granted us the exclusive right to purchase products bearing the Zig-Zag® brand name from Bolloré for resale in the U.S. and Canada. We have the sole right to determine pricing and other terms upon which we may resell any products purchased from Bolloré, including the right to determine the ultimate distributors of such products within these countries. Furthermore, on March 19, 2013, we entered into an additional License and Distribution Agreement with Bolloré (the “Bolloré License Agreement”), which permits us the exclusive use of the Zig-Zag® brand name in the U.S. for e-cigarettes and any related accessories, including vaporizers and e-liquids. The Bolloré License Agreement terminates upon termination of the Distribution Agreements. We also entered into a License and Distribution Agreement with Bolloré permitting us the exclusive use of the Zig-Zag brand in the U.S. and Canada for paper cone products. This agreement also terminates upon termination of the Distribution Agreements.
Each of the Distribution Agreements were entered into on November 30, 1992, by a predecessor in interest for an initial twenty-year term. The Distribution Agreements automatically renewed in November 2012 for a second twenty-year term and will automatically renew for successive twenty-year terms unless terminated in accordance with the provisions of such agreement. The Distribution Agreements provide that, in order to assure each of the parties receives commercially reasonable profits in light of inflationary trends and currency fluctuation factors, 120 days prior to December 31, 2004, and each fifth-year anniversary from such date thereafter, the parties are required to enter into good faith negotiations to agree on an index and currency adjustment formula to replace the index and formula currently in effect. If the parties are unable to agree, the dispute is to be submitted to binding arbitration. Pursuant to the Distribution Agreements, if at any time the price received by Bolloré fails to cover its costs, Bolloré may give us notice of this deficiency, and the parties must promptly negotiate in good faith to adjust prices. If the parties cannot agree on new prices, we may purchase products from an alternative supplier reasonably acceptable to Bolloré until the next price adjustment period (subject to certain price-matching rights available to Bolloré and other terms and conditions). Further, Bolloré sources its needs for our orders from an affiliate of one of our competitors. For further details, see “Risk Factors—We depend on a small number of key third-party suppliers and producers for our products”.
Pursuant to the Distribution Agreements, export duties, insurance, and shipping costs are the responsibility of Bolloré. Import duties and taxes in the U.S. and Canada are our responsibility. Under the Distribution Agreements, we must purchase cigarette papers, cigarette tubes, and cigarette injector machines from Bolloré, subject to Bolloré fulfilling its obligations under these agreements. Bolloré is required to provide us with the quantities of the products that we order consistent with specific order-to-delivery timelines detailed in the agreement. The Distribution Agreements provide us with certain safeguards to ensure that we will be able to secure a steady supply of product, including (i) granting us the right to seek third-party suppliers with continued use of the Zig-Zag® trademark if Bolloré is unable to perform its obligations or ceases its cigarette paper manufacturing operation, in each case as set forth in the Distribution Agreements, and (ii) maintaining a two-month supply of safety stock inventory of the premium papers, tubes, and injector machines in the U.S. at Bolloré’s expense.
Under the Distribution Agreements, we have agreed that for a period of five years after the termination of the agreements we will not engage, directly or indirectly, in the manufacturing, selling, distributing, marketing, or otherwise promoting, in the U.S. and Canada, of cigarette paper or cigarette paper booklets of a competitor without Bolloré’s consent, except for certain de minimis acquisitions of debt or equity securities of such a competitor and certain activities with respect to an alternative supplier used by us as permitted under the Distribution Agreements.
Each of the Distribution Agreements permits Bolloré to terminate such agreement (i) if certain minimum purchases (which, in the case of both Distribution Agreements, have been significantly exceeded in recent years) of cigarette paper booklets have not been made by us for resale in the jurisdiction covered by such agreement within a calendar year, (ii) if we assign such agreement without the consent of Bolloré, (iii) upon a change of control without the consent of Bolloré, (iv) upon certain acquisitions of our equity securities by one of our competitors or certain
10
investments by our significant stockholders in one of our competitors, (v) upon certain material breaches, including our agreement not to promote, directly or indirectly, cigarette paper or cigarette paper booklets of a competitor, or (vi) upon our bankruptcy, insolvency, liquidation, or other similar event. Additionally, the Canada Distribution Agreement is terminable by either us or Bolloré upon the termination of the U.S. Distribution Agreement.
Swedish Match Manufacturing Agreement
On September 4, 2008, we entered into a manufacturing and distribution agreement with Swedish Match whereby Swedish Match became the exclusive manufacturer of our loose-leaf chewing tobacco. Under the agreement, production of our loose-leaf chewing tobacco products was completely transitioned to Swedish Match’s plant located in Owensboro, Kentucky, on September 18, 2009. We source all of the tobacco Swedish Match uses to manufacture our products along with certain proprietary flavorings and retain all marketing, design, formula, and trademark rights over our loose-leaf products. We also have the right to approve all product modifications and are solely responsible for decisions related to package design and branding of the loose-leaf tobacco produced for us. Responsibilities related to process control, manufacturing activities, and inventory management with respect to our loose-leaf products are allocated between us and Swedish Match as specified in the agreement. We also have rights to monitor production and quality control processes on an ongoing basis.
The agreement had an initial ten-year term and will automatically be renewed for five successive ten-year terms unless either party provides at least 180 days’ notice prior to a renewal term of its intent to terminate the agreement, or unless otherwise terminated by mutual agreement of the parties in accordance with the provisions of the agreement. If a notice of non-renewal is delivered, the contract will expire two years after the date on which the agreement would have otherwise been renewed. The terms allow the agreement to be assumed by a buyer, terminated for uncured material breach, or terminated by us subject to a buyout. We also hold a right of first refusal to acquire the manufacturing plant as well as Swedish Match’s chewing tobacco unit. The agreement was automatically renewed for the first of five 10-year renewal periods on September 4, 2018.
Production and Quality Control
We primarily outsource our manufacturing and production processes and focus on packaging, marketing, and distribution. We currently manufacture less than 20% of our products as measured by net sales. Our in-house manufacturing operations are principally limited to (i) the manufacturing of our moist snuff products, which occurs at our facility in Dresden, Tennessee; (ii) the packaging of our moist snuff products at our facilities in Dresden, Tennessee, and Louisville, Kentucky; and (iii) the manufacturing of e-liquids at our Louisville, Kentucky, facility. Our MST products are processed in-house, rather than outsourced, as a result of our proprietary manufacturing processes which are substantively different than those of our competitors.
We use proprietary production processes and techniques, including strict quality controls. Our quality control group routinely tests the quality of the tobacco, flavorings, application of flavorings, premium cigarette papers, tubes and injectors, cigars, MYO cigar wraps, liquid vapor products, and packaging materials. We utilize sophisticated quality controls to test and closely monitor the quality of our products. The high quality of our tobacco products is largely the result of using high-grade tobacco leaf and food-grade flavorings and, on an ongoing basis, analyzing the tobacco cut, flavorings, and moisture content together with strict specifications for sourced products.
Given the importance of contract manufacturing to our business, our quality control group ensures that established, written procedures and standards are adhered to by each of our contract manufacturers. Responsibilities related to process control, manufacturing activities, quality control, and inventory management with respect to our loose leaf are allocated between us and Swedish Match under the manufacturing agreement.
Sales and Marketing
We have grown the size and capacity of our salesforce and intend to continue strengthening the organization to advance our ability to deepen and broaden the retail availability of our products and brands.
As of December 31, 2019, we had a nationwide sales and marketing organization of approximately 178 professionals. Our sales and marketing group focuses on priority markets and sales channels and seeks to operate with a high level of efficiency. In 2019, our tobacco-related sales and marketing efforts enabled our products to reach an estimated 210,000 retail doors in North America and over 800 direct wholesale customers with an additional 100 secondary, indirect wholesalers in the U.S.
11
Our tobacco sales efforts are focused on wholesale distributors and retail merchants in the independent and chain convenience store, tobacco outlet, food store, mass merchandising, drug store, and non-traditional retail channels. Our NewGen sales efforts are focused on alternative channels and winning new stores, increasing store share of requirements and growing the B2C engine to capture a greater share of online sales direct to the consumer. We have expanded, and intend to continue to expand, the sales of our products into previously underdeveloped geographic markets and retail channels. In 2019, we derived more than 95% of our net sales from sales in the U.S., with the remainder primarily from sales in Canada.
We subscribe to a sales tracking system from MSAi that records all traditional OTP product shipments (ours as well as those of our competitors) from approximately 900 wholesalers to over 250,000 traditional retail stores in the U.S. This system enables us to understand individual product share and volume trends across multiple categories down to the individual retail store level, allowing us to allocate field salesforce coverage to the highest opportunity stores. Additionally, the ability to select from a range of parameters and to achieve this level of granularity means we can analyze marketplace trends in a timely manner and swiftly evolve our business planning to meet market opportunities.
We employ marketing activities to grow awareness, trial, and sales including selective trade advertising to expand wholesale availability, point-of-sale advertising and merchandising and permanent and temporary displays to improve consumer visibility, and social media. We comply with all regulations relating to the marketing of tobacco products, such as directing marketing efforts to adult consumers, and are committed to full legal compliance in the sales and marketing of our products. To date, we have neither relied upon, nor conducted, any substantial advertising in the consumer media for our tobacco products.
In the years ended December 31, 2019, 2018, and 2017, we did not have any customer that accounted for 10% or more of our net sales. Our customers use an open purchase order system to buy our products and are not obligated to do so pursuant to ongoing contractual obligations. We perform periodic credit evaluations of our customers and generally do not require collateral on trade receivables. Historically, we have not experienced material credit losses. Sales to customers within our NewGen segment are generally prepaid.
Competition
Many of our competitors are better capitalized than we are and have greater resources, financial and otherwise. We believe our ability to effectively compete and strong market positions in our principal product lines are due to the high recognition of our brand names, the perceived quality of each of our products, and the efforts of our sales, marketing, and distribution teams. We compete against “big tobacco,” including Altria Group, Inc. (formerly Philip Morris); British American Tobacco p.l.c. (formerly Reynolds); Swedish Match; Swisher International; and manufacturers including U.K. based Imperial Brands, PLC, across our segments. “Big tobacco” has substantial resources and a customer base that has historically demonstrated loyalty to their brands.
Competition in the OTP market is based upon not only brand quality and positioning but also on price, packaging, promotion, and retail availability and visibility. Given the decreasing prevalence of cigarette consumption, the “big tobacco” companies continue to demonstrate an increased interest and participation in a number of OTP markets.
Smokeless Products
Our three principal competitors in the loose-leaf chewing tobacco market are Swedish Match, the American Snuff Company, LLC (a unit of British American Tobacco p.l.c.), and Swisher International Group, Inc. We believe moist snuff products are used interchangeably with loose leaf products by many consumers. In the moist snuff category, we face the same competitors with the addition of U.S. Smokeless Tobacco Company (a division of Altria Group, Inc.).
Smoking Products
Our principle competitors for premium cigarette paper sales are Republic Tobacco, L.P. and HBI International. Our two major competitors for MYO cigar wraps are New Image Global, Inc., and Blunt Wrap USA. We believe MYO cigar wrap products are used interchangeably with both rolling papers and finished cigar products by many consumers.
12
NewGen Products
In the NewGen products segment, aside from the established operations of Juul Labs, our competitors are varied as the market is relatively new and highly fragmented. Our direct competitors sell products that are substantially similar to our products through the same channels in which we sell our liquid vapor products. We compete with these direct competitors for sales through wholesalers and retailers including, but not limited to, vapor stores, national chain stores, tobacco shops, and convenience stores and in the online direct to consumer environment. Through our acquisitions we now also compete directly with other non-traditional distributors and retailers.
Patents, Trademarks, and Trade Secrets
We have numerous registered trademarks relating to our products, including: Beech-Nut®, Trophy®, Havana Blossom®, Durango®, Stoker’s®, Tequila Sunrise®, Fred’s Choice®, Old Hillside®, Our Pride®, Red Cap®, Tennessee Chew®, Big Mountain®, Springfield Standard®, Snake River®, VaporBeast®, Vapor Shark®, DirectVapor®, VaporFi®, SouthBeachSmoke®, and Nu-X Ventures®. The registered trademarks, which are significant to our business, expire periodically and are renewable for additional 10-year terms upon expiration. Flavor and blend formula trade secrets relating to our tobacco products, which are key assets of our businesses, are maintained under strict secrecy.
The Zig-Zag® trade dress trademark for premium cigarette papers and related products are owned by Bolloré and have been exclusively licensed to us in the U.S. and Canada. The Zig-Zag® trademark for e-cigarettes is also owned by Bolloré and has been exclusively licensed to us in the U.S. We own the Zig-Zag® trademark with respect to its use in connection with products made with tobacco including, without limitation, cigarettes, cigars, and MYO cigar wraps in the U.S.
Research and Development and Quality Assurance
We have a research and development and quality assurance function that tests raw materials and finished products in order to maintain a high level of product quality and consistency. Research and development largely bases its new product development efforts on our high-tech data systems. We spent approximately $2.5 million, $2.5 million, and $2.3 million dollars on research and development and quality control efforts for the years ended December 31, 2019, 2018, and 2017, respectively.
Employees
As of February 28, 2020, we employed 466 full-time and part-time employees. None of our employees are represented by unions. We believe we have a positive relationship with our employees.
Internet Address and Company SEC Filings
Our primary Internet address is www.turningpointbrands.com. On the investor relations portion of our website, www.turningpointbrands.com/investor-relations, we provide a link to our electronic filings with the U.S. Securities and Exchange Commission (the “SEC”), including our annual report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K, and any amendments to these reports. We make all such filings available free of charge as soon as reasonably practicable after filing. The information found on our website is not part of this or any other report we file with or furnish to the SEC.
13
| Item 1A. | Risk Factors |
Risks related to our business and industry include the following:
Sales of tobacco products are generally expected to continue to decline.
As a result of restrictions on advertising and promotions, increases in regulation and excise taxes, health concerns, a decline in the social acceptability of tobacco and tobacco-related products, increased pressure from anti-tobacco groups, and other factors, the overall U.S. market for tobacco products has generally been declining in terms of volume of sales and is expected to continue to decline. The general climate of declining sales of tobacco products is principally driven by the long-standing declines in cigarettes. OTP, on the other hand, as measured by MSAi, have been generating modest consumer unit volume gains. For instance, while loose-leaf chewing tobacco products have declined for over a decade, the MST segment pouch products and snus have been growing in the low single digits over the same period. Additionally, cigarillo cigars and MYO cigar wraps have each demonstrated MSAi volume gains in recent years. Our tobacco products comprised approximately 58% of our total 2019 net sales and, while some of our sales volume declines have been offset by higher prices or by increased sales in other product categories, there can be no assurance that these price increases or increased sales can be sustained, especially in an environment of increased regulation, product characteristic restrictions, and taxation and changes in consumer spending habits.
We depend on a small number of key third-party suppliers and producers for our products.
Our operations are largely dependent on a small number of key suppliers and producers to supply or manufacture our products pursuant to long-term contracts. In 2019, our three most important suppliers and producers were: (i) Swedish Match, which produces all of our loose leaf chewing tobacco in the U.S., (ii) Bolloré, which provides us with exclusive access to the Zig-Zag® cigarette paper and related accessories in the U.S. and Canada, and (iii) Durfort, from which we source our MYO cigar wraps.
All of our loose-leaf tobacco products are manufactured for us by Swedish Match pursuant to a ten-year renewable agreement, which we entered into in 2008. The agreement will automatically be renewed for five successive ten-year terms unless either party provides at least 180 days’ notice prior to a renewal term of its intent to terminate the agreement or unless otherwise terminated in accordance with the provisions of the agreement. If a notice of non-renewal is delivered, the contract will expire two years after the date on which the agreement would have otherwise been renewed. Under this agreement, we retain the rights to all marketing, distribution and trademarks over the loose-leaf brands that we own or license. The agreement renewed for an additional ten-year term in 2018. We share responsibilities with Swedish Match related to process control, manufacturing activities, quality control, and inventory management with respect to our loose-leaf products. We rely on the performance by Swedish Match of its obligations under the agreement for the production of our loose-leaf tobacco products. Any significant disruption in Swedish Match’s manufacturing capabilities or our relationship with Swedish Match, a deterioration in Swedish Match’s financial condition, or an industry-wide change in business practices with respect to loose leaf tobacco products could have a material adverse effect on our business, results of operations, and financial condition.
All of our Zig-Zag® premium cigarette papers, cigarette tubes, and injectors are sourced from Bolloré, pursuant to a renewable 20-year exclusive agreement. This agreement was most recently renewed in 2012. In addition, under the terms of the agreement with Bolloré, we renegotiate pricing terms every five years. Further, Bolloré sources its needs for certain of our orders from an affiliate of one of our competitors.
We source our MYO cigar wraps through the patent holder, Durfort, pursuant to an agreement entered into in October 2008. The agreement extends until expiration of the patents or cancellation of the agreement by either party. We rely on Durfort to produce and package our MYO cigar wraps to our specifications. Any significant disruption in our relationship with Durfort, a deterioration in Durfort’s financial condition, an industry-wide change in business practices relating to MYO cigar wraps, Durfort’s ability to comply with regulatory requirements, or our ability to source the MYO cigar wraps from them could have a material adverse effect on our business, results of operations, and financial condition.
Pursuant to agreements with certain suppliers, we have agreed to store tobacco inventory purchased on our behalf and generally maintain a 12- to 24-month supply of our various tobacco products at their facilities. We cannot guarantee our supply of these products will be adequate to meet the demands of our customers. Further, a major fire, violent weather conditions, or other disasters that affect us or any of our key suppliers or producers, including Bolloré, Swedish Match, or Durfort, as well as those of our other suppliers and vendors, could have a material adverse
14
effect on our operations. Although we have insurance coverage for some of these events, a prolonged interruption in our operations, as well as those of our producers, suppliers, or vendors, could have a material adverse effect on our business, results of operations, and financial condition. In addition, we do not know whether we will be able to renew any or all of our agreements on a timely basis, on terms satisfactory to us, or at all.
Any disruptions in our relationships with Bolloré, Swedish Match, or Durfort, a failure to renew any of our agreements, an inability or unwillingness by any supplier to produce sufficient quantities of our products in a timely manner or finding a new supplier would have a significant impact on our ability to continue distributing the same volume and quality of products and maintain our market share, even during a temporary disruption, which could have a material adverse effect on our business, results of operations and financial condition.
We may be unable to identify or contract with new suppliers or producers in the event of a disruption to our supply.
In order to continue selling our products in the event of a disruption to our supply, we would have to identify new suppliers or producers that would be required to satisfy significant regulatory requirements. Only a limited number of suppliers or producers may have the ability to produce our products at the volumes we need, and it could be costly or time-consuming to locate and approve such alternative sources. Moreover, it may be difficult or costly to find suppliers to produce small volumes of our new products in the event we are looking only to supplement current supply as suppliers may impose minimum order requirements. In addition, we may be unable to negotiate pricing or other terms with our existing or new suppliers as favorable as those we currently enjoy. Even if we were able to successfully identify new suppliers and contract with them on favorable terms, these new suppliers would also be subject to stringent regulatory approval procedures that could result in prolonged disruptions to our sourcing and distribution processes.
Furthermore, there is no guarantee that a new third-party supplier could accurately replicate the production process and taste profile of our existing products. We cannot guarantee that a failure to adequately replace our existing suppliers would not have a material adverse effect on our business, results of operations, and financial condition.
Our business may be damaged by events outside of our suppliers’ control, such as the impact of epidemics (e.g., coronavirus), political upheavals, or natural disasters.
We have critical suppliers of raw materials and finished products in other countries where events may prevent them from performing their obligations to us, through no fault of any party. Examples of such events could include the effect of potential epidemics, such as coronavirus; political upheavals including violent changes in government, widespread labor unrest, or breakdowns in civil order; and natural disasters, such as hurricanes, earthquakes or floods. If such events were to occur and disrupt our supply arrangements, there can be no assurance that we could quickly replace the supply and there could be a material adverse impact on our business, results of operations, and financial condition.
Our licenses to use certain brands and trademarks may be terminated or not renewed.
We are reliant upon brand recognition in the OTP markets in which we compete as the OTP industry is characterized by a high degree of brand loyalty and a reluctance to switch to new or unrecognizable brands on the part of consumers. Some of the brands and trademarks under which our products are sold are licensed to us for a fixed period of time in respect of specified markets, such as our distribution and license agreement with Bolloré for use of the Zig-Zag® name and associated trademarks in connection with certain of our cigarette papers and related products.
We have three licensing agreements with Bolloré, the first of which governs licensing and the use of the Zig-Zag® name with respect to cigarette papers, cigarette tubes, and cigarette injector machines, the second of which governs licensing and the use of the Zig-Zag® name with respect to e-cigarettes, vaporizers, and e-liquids, and the third of which governs the licensing, sourcing and use of the Zig-Zag trademark on paper cones. In 2019, we generated approximately $108 million in net sales of Zig-Zag® products, of which approximately $52 million was generated from products sold through our license agreement with Bolloré. In the event the licensing agreements with Bolloré are not renewed, the terms of the agreements bind us under a five-year non-compete clause, under which we cannot engage in direct or indirect manufacturing, selling, distributing, marketing, or otherwise promoting of
15
cigarette papers of a competitor without Bolloré’s consent, except in limited instances. We do not know whether we will renew these agreements on a timely basis, on terms satisfactory to us, or at all. As a result of these restrictions, if our agreements with Bolloré are terminated, we may not be able to access the markets with recognizable brands that would be positioned to compete in these segments.
In the event that the licenses to use the brands and trademarks in our portfolio are terminated or are not renewed after the end of the term, there is no guarantee we will be able to find a suitable replacement, or if a replacement is found, that it will be on favorable terms. Any loss in our brand-name appeal to our existing customers as a result of the lapse or termination of our licenses could have a material adverse effect on our business, results of operations, and financial condition.
We may not be successful in maintaining the consumer brand recognition and loyalty of our products.
We compete in a market that relies on innovation and the ability to react to evolving consumer preferences. The tobacco industry in general, and the OTP industry, in particular, are subject to changing consumer trends, demands, and preferences. Therefore, products once favored may over time become disfavored by consumers or no longer perceived as the best option. Consumers in the OTP market have demonstrated a high degree of brand loyalty, but producers must continue to adapt their products in order to maintain their status among these customers as the market evolves. The Zig-Zag® brand has strong brand recognition among smokers, and our continued success depends in part on our ability to continue to differentiate the brand names that we own or license and maintain similarly high levels of recognition with target consumers. Trends within the OTP industry change often. Our failure to anticipate, identify, or react to changes in these trends could, among other things, lead to reduced demand for our products. Factors that may affect consumer perception of our products include health trends and attention to health concerns associated with tobacco, price-sensitivity in the presence of competitors’ products or substitute products, and trends in favor of new NewGen products that are currently being researched and produced by participants in our industry. For example, in recent years, we have witnessed a shift in consumer purchases from chewing tobacco to moist snuff due to its increased affordability. Along with our biggest competitors in the chewing tobacco market, which also produce moist snuff, we have been able to shift priorities and adapt to this change. A failure to react to similar trends in the future could enable our competitors to grow or establish their brands’ market shares in these categories before we have a chance to respond.
Consumer perceptions of the overall health of tobacco-based products is likely to continue to shift, and our success depends, in part, on our ability to anticipate these shifting tastes and the rapidity with which the markets in which we compete will evolve in response to these changes on a timely and affordable basis. If we are unable to respond effectively and efficiently to changing consumer preferences, the demand for our products may decline, which could have a material adverse effect on our business, results of operations, and financial condition.
Regulations may be enacted in the future, particularly in light of increasing restrictions on the form and content of marketing of tobacco products, that would make it more difficult to appeal to our consumers or to leverage existing recognition of the brands that we own or license. Furthermore, even if we are able to continue to distinguish our products, there can be no assurance that the sales, marketing, and distribution efforts of our competitors will not be successful in persuading consumers of our products to switch to their products. Many of our competitors have greater access to resources than we do, which better positions them to conduct market research in relation to branding strategies or costly marketing campaigns. Any loss of consumer brand loyalty to our products or reduction of our ability to effectively brand our products in a recognizable way will have a material effect on our ability to continue to sell our products and maintain our market share, which could have a material adverse effect on our business, results of operations, and financial condition.
We are subject to substantial and increasing regulation.
The tobacco industry has been under public scrutiny for over 50 years. Industry critics include special interest groups, the U.S. Surgeon General, and many legislators and regulators at the local, state and federal levels. A wide variety of federal, state, and local laws limit the advertising, sale, and use of tobacco, and these laws have proliferated in recent years. Together with changing public attitudes towards tobacco consumption, the constant expansion of regulations has been a major cause of the overall decline in the consumption of tobacco products since the early 1970s. These regulations relate to, among other things, the importation of tobacco products and shipping throughout the U.S. market, increases in the minimum age to purchase tobacco products, imposition of taxes, sampling and advertising bans or restrictions, flavor bans or restrictions, ingredient and constituent disclosure requirements, and
16
media campaigns and restrictions on where smokers can smoke. Additional restrictions may be legislatively imposed or agreed to in the future. These limitations may make it difficult for us to maintain the value of any brand.
Moreover, the current trend is toward increasing regulation of the tobacco industry, which is likely to differ between the various U.S. states and Canadian provinces in which we currently conduct the majority of our business. Extensive and inconsistent regulation by multiple states and at different governmental levels could prove to be particularly disruptive to our business as we may be unable to accommodate such regulations in a cost-effective manner that allows us to continue to compete in an economically viable way. Regulations are often introduced without the tobacco industry’s input and have been a significant reason behind reduced industry sales volumes and increased illicit trade.
In 1986, federal legislation was enacted regulating smokeless tobacco products (including dry and moist snuff and chewing tobacco) by, among other things, requiring health warnings on smokeless tobacco packages and prohibiting the advertising of smokeless tobacco products on media subject to the jurisdiction of the Federal Communications Commission (“FCC”). Since 1986, other proposals have been made at the federal, state, and local levels for additional regulation of tobacco products. It is likely that additional proposals will be made in the coming years. For example, the Prevent All Cigarette Trafficking Act prohibits the use of the U.S. Postal Service to mail most tobacco products and amends the Jenkins Act, which established cigarette sales reporting requirements for state excise tax collection, to require individuals and businesses that make interstate sales of cigarettes or smokeless tobacco comply with state tax laws. See “—There is uncertainty related to the federal regulation of NewGen products, cigars and pipe tobacco products” for further details. Additional federal or state regulation relating to the manufacture, sale, distribution, advertising, labeling, mandatory ingredients disclosure and nicotine yield information disclosure of tobacco products could reduce sales, increase costs, and have a material adverse effect on our business, results of operations, and financial condition.
On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act (the “Tobacco Control Act”) authorized the FDA for regulatory authority over tobacco products. The Act also amended the Federal Cigarette Labeling and Advertising Act, which governs how cigarettes can be advertised and marketed, as well as the Comprehensive Smokeless Tobacco Health Education Act (“CSTHEA”), which governs how smokeless tobacco can be advertised and marketed. In addition to the FDA and FCC, we are subject to regulation by numerous other federal agencies, including the Federal Trade Commission (“FTC”), the Department of Justice (“DOJ”), the Alcohol and Tobacco Tax and Trade Bureau (“TTB”), the U.S. Environmental Protection Agency (“EPA”), the U.S. Department of Agriculture (“USDA”), the Consumer Product Safety Commission (“CPSC”), the U.S. Customs and Border Protection (“CBP”) and the U.S. Center for Disease Control and Prevention’s (“CDC”) Office on Smoking and Health. There have also been adverse legislative and political decisions and other unfavorable developments concerning cigarette smoking and the tobacco industry, which have received widespread public attention. FDA has, and other governmental entities have, expressed concerns about the use of flavors in tobacco products and an interest in significant regulation of such use, up to and including de facto bans in certain products. There can be no assurance as to the ultimate content, timing or effect of any regulation of tobacco products by governmental bodies, nor can there be any assurance that potential corresponding declines in demand resulting from negative media attention would not have a material adverse effect on our business, results of operations and financial condition.
Our products are regulated by the FDA, which has broad regulatory powers.
Substantially all of our 2019 U.S. net sales are derived from the sale of products that are currently regulated by the FDA. The Tobacco Control Act grants the FDA broad regulatory authority over the design, manufacture, sale, marketing and packaging of tobacco products. Among the regulatory powers conferred to the FDA under the Tobacco Control Act is the authority to impose tobacco product standards that are appropriate for the protection of the public health, require manufacturers to obtain FDA review and authorization for the marketing of certain new or modified tobacco products and impose various additional restrictions. Such restrictions may include requiring reduction or elimination of the use of particular constituents or components, requiring product testing, or addressing other aspects of tobacco product construction, constituents, properties or labeling.
Specifically, the Tobacco Control Act (i) increases the number of health warnings required on cigarette and smokeless tobacco products, increases the size of warnings on packaging and in advertising, requires the FDA to develop graphic warnings for cigarette packages, and grants the FDA authority to require new warnings, (ii) imposes restrictions on the sale and distribution of tobacco products, including significant restrictions on tobacco product advertising and promotion as well as the use of brand and trade names, (iii) bans the use of “light,” “mild,” “low”
17
or similar descriptors on tobacco products, (iv) bans the use of “characterizing flavors” in cigarettes other than tobacco or menthol, (v) requires manufacturers to report ingredients and harmful constituents and requires the FDA to disclose certain constituent information to the public, (vi) authorizes the FDA to require the reduction of nicotine and the potential reduction or elimination of other constituents or additives, including menthol, (vii) establishes potentially expensive and time-consuming pre-market and “substantial equivalence” review pathways for tobacco products that are considered new, (viii) gives FDA broad authority to deny product applications thereby preventing the sale or distribution of the product subject to the application (and requiring such product to be removed from the market, if applicable), and (ix) requires tobacco product manufacturers (and certain other entities) to register with the FDA.
The FDA charges user fees based on the USDA unit calculations pro-rated to the annualized FDA congressionally allocated budget. These fees only apply to certain products currently regulated by the FDA, which include our smokeless and smoking products (other than cigarette paper products), but we may in the future be required to pay such fees on more of our products, and we cannot accurately predict which additional products may be subject to such fees or the magnitude of such fees, which could become significant.
Although the FDA is prohibited from issuing regulations banning all cigarettes, all smokeless tobacco products, all little cigars, all cigars other than little cigars, all pipe tobacco, or all roll-your-own tobacco, or requiring the reduction of nicotine yields of a tobacco product to zero, it is likely that its regulations in accordance with the Tobacco Control Act could result in a decrease in sales of these products in the U.S. We believe that such regulation could adversely affect our ability to compete against our larger competitors, who may be able to more quickly and cost-effectively comply with these new rules and regulations. Our ability to gain efficient market clearance for new tobacco products, or even to keep existing products on the market, could also be affected by FDA rules and regulations. Some of our currently marketed products that are subject to FDA regulation will require marketing authorizations from the FDA for us to continue marketing them (e.g., pre-market or substantial equivalence marketing authorizations, as applicable to the product), which we cannot guarantee we will be able to obtain. In addition, failure to comply with new or existing tobacco laws under which the FDA imposes regulatory requirements could result in significant financial penalties and government investigations of us. To the extent we are unable to respond to, or comply with, new FDA regulations it could have a material adverse effect on our business, results of operations and financial condition.
Some of our products are subject to developing and unpredictable regulation.
Some of our NewGen products marketed through our Nu-X subsidiary and similar third-party products sold through our NewGen distribution vehicles may be subject to uncertain federal, state and local regulations concerning hemp, CBD and other non-tobacco consumable products. Enforcement initiatives by those authorities are therefore unpredictable and impossible to anticipate. We anticipate that all levels of government are likely to seek in some way to regulate these products, but the type, timing, and impact of such regulations remains uncertain. Accordingly, we cannot give any assurance that such actions would not have a material adverse effect on this emerging business.
Many of our products contain nicotine, which is considered to be a highly addictive substance.
Many of our products contain nicotine, a chemical that is considered to be highly addictive. The Tobacco Control Act empowers the FDA to regulate the amount of nicotine found in tobacco products, but not to require the reduction of nicotine yields of a tobacco product to zero. Any FDA regulation, whether of nicotine levels or other product attributes, may require us to reformulate, recall and/or discontinue certain of the products we may sell from time to time, which may have a material adverse effect on our ability to market our products and have a material adverse effect on our business, results of operations and financial condition.
There is uncertainty related to the federal regulation of NewGen products, cigars and pipe tobacco products. Increased regulatory compliance burdens could have a material adverse impact on our NewGen business development efforts.
Since their introduction, there has been significant uncertainty regarding whether, how and when tobacco regulations would apply to NewGen products, such as electronic cigarettes or other vaporizer products. Based on a decision in December 2010 by the U.S. Court of Appeals for the D.C. Circuit (the “Sottera decision”), the FDA is permitted to regulate electronic cigarettes containing tobacco-derived nicotine as “tobacco products” under the Tobacco Control Act.
18
Effective August 8, 2016, FDA’s regulatory authority under the Tobacco Control Act was extended to all remaining tobacco products, including: (i) certain NewGen products (such as electronic cigarettes, vaporizers and e-liquids) and their components or parts (such as tanks, coils and batteries); (ii) cigars and their components or parts (such as cigar tobacco); (iii) pipe tobacco; (iv) hookah products; or (v) any other tobacco product “newly deemed” by FDA. These deeming regulations apply to all products made or derived from tobacco intended for human consumption, but excluding accessories of tobacco products (such as lighters).
The deeming regulations require us to (i) register with the FDA and report product and ingredient listings; (ii) market newly deemed products only after FDA review and approval; (iii) only make direct and implied claims of reduced risk if the FDA approves after finding that scientific evidence supports the claim and that marketing the product will benefit public health as a whole; (iv) refrain from distributing free samples; (v) implement minimum age and identification restrictions to prevent sales to individuals under age 18; (vi) develop an approved warning plan and include prescribed health warnings on packaging and advertisements; and (vii) refrain from selling the products in vending machines, unless the machine is located in a facility that never admits youth. Newly deemed tobacco products are also subject to the other requirements of the Tobacco Control Act, such as that they not be adulterated or misbranded. The FDA could in the future promulgate good manufacturing practice regulations for these and our other products, which could have a material adverse impact on our ability and the cost to manufacture our products.
Marketing authorizations will be necessary in order for us to continue our distribution of NewGen and cigar and pipe tobacco products. As a result of recent litigation and subsequent FDA Guidance, newly-deemed products will require marketing applications no later than May 12, 2020, with the exception of our “grandfathered” products (products in commerce as of February 15, 2007) which are already authorized, unless FDA grants extensions to these compliance periods. We intend to timely file for the appropriate authorizations to allow us to sell our products in the U.S. We have no assurances that the outcome of such processes will result in our products receiving marketing authorizations from the FDA. We also have certain previously regulated tobacco products which FDA removed from review but remain subject to “provisional” substantial equivalence filings made on March 22, 2011; however, FDA has the discretion to reinitiate review of these products. If the FDA establishes regulatory processes that we are unable or unwilling to comply with, our business, results of operations, financial condition and prospects could be adversely affected.
The anticipated costs of complying with future FDA regulations will be dependent on the rules issued by the FDA, the timing and clarity of any new rules or guidance documents accompanying these rules, the reliability and simplicity (or complexity) of the electronic systems utilized by FDA for information and reports to be submitted, and the details required by FDA for such information and reports with respect to each regulated product (which have yet to be issued by FDA). Failure to comply with existing or new FDA regulatory requirements could result in significant financial penalties and could have a material adverse effect on our business, results of operations, financial condition and ability to market and sell our products. Compliance and related costs could be substantial and could significantly increase the costs of operating in our NewGen and cigar and pipe tobacco product markets.
In addition, failure to comply with the Tobacco Control Act and with FDA regulatory requirements could result in litigation, criminal convictions or significant financial penalties and could impair our ability to market and sell our electronic and vaporizer products. At present, we are not able to predict whether the Tobacco Control Act will impact our products to a greater degree than competitors in the industry, thus affecting our competitive position.
Furthermore, neither the Prevent All Cigarette Trafficking (“PACT”) Act nor the Federal Cigarette Labeling and Advertising Act currently apply to NewGen products; however, there is pending federal legislation that seeks to include certain NewGen products under the requirements of the PACT Act. There may, in the future, also be increased regulation of additives in tobacco products and internet sales of NewGen products. The application of either or both of these federal laws, and of any new laws or regulations which may be adopted in the future, to NewGen products or such additives could result in additional expenses and require us to change our advertising and labeling, and methods of marketing and distribution of our products, any of which could have a material adverse effect on our business, results of operations and financial condition.
Significant increases in state and local regulation of our NewGen products have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions.
There has been increasing activity on the state and local levels with respect to scrutiny of NewGen products. State and local governmental bodies across the U.S. have indicated NewGen products may become subject to new laws and regulations at the state and local levels. Further, some states and cities, have enacted regulations that require
19
obtaining a tobacco retail license in order to sell electronic cigarettes and vaporizer products. If one or more states from which we generate or anticipate generating significant sales of NewGen products bring actions to prevent us from selling our NewGen products unless we obtain certain licenses, approvals or permits, and if we are not able to obtain the necessary licenses, approvals or permits for financial reasons or otherwise and/or any such license, approval or permit is determined to be overly burdensome to us, then we may be required to cease sales and distribution of our products to those states, which could have a material adverse effect on our business, results of operations and financial condition.
Certain states and cities have already restricted the use of electronic cigarettes and vaporizer products in smoke-free venues, imposed excise taxes, or limited sales of flavored NewGen products. Additional city, state or federal regulators, municipalities, local governments and private industry may enact additional rules and regulations restricting electronic cigarettes and vaporizer products. Because of these restrictions, our customers may reduce or otherwise cease using our NewGen products, which could have a material adverse effect on our business, results of operations and financial condition.
Increases in tobacco-related taxes have been proposed or enacted and are likely to continue to be proposed or enacted in numerous jurisdictions.
Tobacco products, premium cigarette papers and tubes have long been subject to substantial federal, state and local excise taxes. Such taxes have frequently been increased or proposed to be increased, in some cases significantly, to fund various legislative initiatives or further disincentivize tobacco usage. Since 1986, smokeless products have been subject to federal excise tax. Smokeless products are taxed by weight (in pounds or fractional parts thereof) manufactured or imported.
Since the State Children’s Health Insurance Program (“S-CHIP”) reauthorization in early 2009, which utilizes, among other things, taxes on tobacco products to fund health insurance coverage for children, the federal excise tax increases adopted have been substantial and have materially reduced sales in the “roll your own” (“RYO”) /MYO cigarette smoking products market, and also caused volume declines in other markets. Although the RYO/MYO cigarette smoking tobacco and related products market had been one of the fastest growing markets in the tobacco industry in the five years prior to 2009, the reauthorization of S-CHIP increased the federal excise tax on RYO tobacco from $1.10 to $24.78 per pound, and materially reduced the MYO cigarette smoking tobacco market in the U.S. There have not been any increases announced since 2009, but we cannot guarantee that we will not be subject to further increases, nor whether any such increases will affect prices in a way that further deters consumers from purchasing our products and/or affects our net revenues in a way that renders us unable to compete effectively.
In addition to federal excise taxes, every state and certain city and county governments have imposed substantial excise taxes on sales of tobacco products, and many have raised or proposed to raise excise taxes in recent years. Approximately one-half of the states tax MST on a weight-based versus ad valorem system of taxation. Additional states may consider adopting such revised tax structures as well. Tax increases, depending on their parameters, may result in consumers switching between tobacco products or depress overall tobacco consumption, which is likely to result in declines in overall sales volumes.
Any future enactment of increases in federal or state excise taxes on our tobacco products or rulings that certain of our products should be categorized differently for excise tax purposes could adversely affect demand for our products and may result in consumers switching between tobacco products or a depression in overall tobacco consumption, which would have a material adverse effect on our business, results of operations and financial condition.
If our NewGen products become subject to increased taxes it could adversely affect our business.
Presently the federal government and many states do not tax the sale of NewGen products like the sale of conventional cigarettes or other tobacco products, all of which generally have high tax rates and have faced significant increases in the amount of taxes collected on their sales. In recent years, however, state and local governments have taken actions to move towards imposing excise taxes on NewGen products. As of December 31, 2019, nearly half of the states and certain localities impose excise taxes on electronic cigarettes and/or liquid vapor. These tax structures may benefit one type of NewGen product over another, which may result in consumers switching between NewGen products, other traditional tobacco products, or depress overall consumption in general. Should federal, state and local governments and or other taxing authorities begin or continue to impose excise taxes similar to those levied against conventional cigarettes and tobacco products on NewGen products, it may have a
20
material adverse effect on the demand for these products, as consumers may be unwilling to pay the increased costs, which in turn could have a material adverse effect on our business, results of operations and financial condition.
We may be subject to increasing international control and regulation.
The World Health Organization’s Framework Convention on Tobacco Control (“FCTC”) is the first international public health treaty that establishes a global agenda to reduce initiation of tobacco use and regulate tobacco in an effort to encourage tobacco cessation. Over 170 governments worldwide have ratified the FCTC. The FCTC has led to increased efforts to reduce the supply and demand of tobacco products and to encourage governments to further regulate the tobacco industry. The tobacco industry expects significant regulatory developments to take place over the next few years, driven principally by the FCTC. Regulatory initiatives that have been proposed, introduced or enacted include:
| • | the levying of substantial and increasing tax and duty charges; |
| • | restrictions or bans on advertising, marketing and sponsorship; |
| • | the display of larger health warnings, graphic health warnings and other labeling requirements; |
| • | restrictions on packaging design, including the use of colors and generic packaging; |
| • | restrictions or bans on the display of tobacco product packaging at the point of sale, and restrictions or bans on cigarette vending machines; |
| • | requirements regarding testing, disclosure and performance standards for tar, nicotine, carbon monoxide and other smoke constituents levels; |
| • | requirements regarding testing, disclosure and use of tobacco product ingredients; |
| • | increased restrictions on smoking in public and work places and, in some instances, in private places and outdoors; |
| • | elimination of duty-free allowances for travelers; and |
| • | encouraging litigation against tobacco companies. |
If the U.S. becomes a signatory to the FCTC and/or national laws are enacted in the U.S. that reflect the major elements of the FCTC, our business, results of operations and financial condition could be materially and adversely affected. If NewGen products become subject to one or more of the significant regulatory initiatives proposed under the FCTC, our NewGen products segment may also be materially adversely affected.
As part of our strategy, we have begun strategic international expansions, such as introducing our moist snuff tobacco products in South America. This and other future expansions may subject us to additional or increasing international regulation, either by the countries that are the object of the strategic expansion or through international regulatory regimes, such as the FCTC, to which those countries may be signatories.
Canada and some Canadian provinces have restricted or are contemplating restrictions on the sales and marketing of electronic cigarettes. Furthermore, some Canadian provinces have limited the use of electronic cigarettes and vaporizer products in public places. These measures, and any future measures taken to limit the marketing, sale and use of NewGen products may have a material adverse effect on our business, results of operations and financial condition.
To the extent our existing or future products become subject to international regulatory regimes that we are unable to comply with or fail to comply with, they may have a material adverse effect on our business, results of operations and financial condition.
Our distribution efforts rely in part on our ability to leverage relationships with large retailers and national chains.
Our distribution efforts rely in part on our ability to leverage relationships with large retailers and national chains to sell and promote our products, which is dependent upon the strength of the brand names that we own or license and our salesforce effectiveness. In order to maintain these relationships, we must continue to supply products that will bring steady business to these retailers and national chains. We may not be able to sustain these relationships or establish other relationships with such entities, which could have a material adverse effect on our ability to execute
21
our branding strategies, our ability to access the end-user markets with our products or our ability to maintain our relationships with the producers of our products. For example, if we are unable to meet benchmarking provisions in contracts or if we are unable to maintain and leverage our retail relationships on a scale sufficient to make us an attractive distributor, it would have a material adverse effect on our ability to source products, and on our business, results of operations and financial condition.In addition, there are factors beyond our control that may prevent us from leveraging existing relationships, such as industry consolidation. If we are unable to develop and sustain relationships with large retailers and national chains, or are unable to leverage those relationships due to factors such as a decline in the role of brick-and-mortar retailers in the North American economy, our capacity to maintain and grow brand and product recognition and increase sales volume will be significantly undermined. In such an event, we may ultimately be forced to pursue and rely on local and more fragmented sales channels, which will have a material adverse effect on our business, results of operations and financial condition.
We have a substantial amount of indebtedness that could affect our financial condition.
As of February 28, 2020, we had $146.0 million outstanding under our credit facility with the ability to borrow an additional $46.3 million under our revolving credit facility. In addition, we had $172.5 million outstanding under our Convertible Senior Notes. If we cannot generate sufficient cash flow from operations to service our debt, we may need to further refinance our debt, dispose of assets or issue equity to obtain necessary funds. We do not know whether we will be able to do any of this on a timely basis or on terms satisfactory to us or at all.
Our substantial amount of indebtedness could limit our ability to:
| • | obtain necessary additional financing for working capital, capital expenditures or other purposes in the future; |
| • | plan for, or react to, changes in our business and the industries in which we operate; |
| • | make future acquisitions or pursue other business opportunities; |
| • | react in an extended economic downturn; and |
| • | pay dividends. |
The terms of the agreement governing our indebtedness may restrict our current and future operations, which would adversely affect our ability to respond to changes in our business and to manage our operations.
Our 2018 Credit Facility contains, and any future indebtedness of ours would likely contain, a number of restrictive covenants that impose significant operating and financial restrictions on us, including restrictions on our ability to, among other things:
| • | incur additional debt; |
| • | pay dividends and make other restricted payments; |
| • | create liens; |
| • | make investments and acquisitions; |
| • | engage in sales of assets and subsidiary stock; |
| • | enter into sale-leaseback transactions; |
| • | enter into transactions with affiliates; |
| • | transfer all or substantially all of our assets or enter into merger or consolidation transactions; and |
| • | enter into certain hedging agreements. |
Our 2018 Credit Facility requires, us to maintain certain financial ratios. As of December 31, 2019, we were in compliance with the financial and restrictive covenants of the 2018 Credit Facility. However, a failure by us to comply with the covenants or financial ratios in our debt instruments could result in an event of default under the applicable facility, which could adversely affect our ability to respond to changes in our business and manage our operations. In the event of any default under our 2018 Credit Facility, the lenders under our debt instruments could elect to declare all amounts outstanding under such instruments to be due and payable and require us to apply all of our available cash to repay these amounts. If the indebtedness under our 2018 Credit Facility were to be accelerated,
22
which would cause an event of default and a cross-acceleration of our obligations under our other debt instruments, there can be no assurance that our assets would be sufficient to repay this indebtedness in full, which could have a material adverse effect on our business, results of operations, and financial condition.
We face intense competition and may fail to compete effectively.
We are subject to significant competition across our segments and compete against companies in all segments that have access to significant resources in terms of technology, relationships with suppliers and distributors and access to cash flow and financial markets. The OTP industry is characterized by brand recognition and loyalty, with product quality, price, marketing and packaging constituting the primary methods of competition. Substantial marketing support, merchandising display, competitive pricing and other financial incentives generally are required to introduce a new brand or to improve or maintain a brand’s market position. Our principal competitors are “big tobacco,” Altria Group, Inc. (formerly Phillip Morris) and British American Tobacco p.l.c. (formerly Reynolds) as well as Swedish Match, Swisher International and manufacturers of electronic cigarettes, including U.K.-based Imperial Brands PLC. These competitors are significantly larger than us and aggressively seek to limit the distribution or sale of other companies’ products, both at the wholesale and retail levels. For example, certain competitors have entered into agreements limiting retail-merchandising displays of other companies’ products or imposing minimum prices for OTP products, thereby limiting their competitors’ ability to offer discounted products. In addition, the tobacco industry is experiencing a trend toward industry consolidation, most recently evidenced by the December 2018 investment in Juul Labs by Altria, the July 2017 acquisition of Reynolds American, Inc., by British American Tobacco p.l.c., and the June 2015 acquisition of Lorillard, Inc., by Reynolds American, Inc. Industry consolidation could result in a more competitive environment if our competitors are able to increase their combined resources, enhance their access to national distribution networks, or become acquired by established companies with greater resources than ours. Any inability to compete due to our smaller scale as the industry continues to consolidate and be dominated by “big tobacco” could have a material adverse effect on our business, results of operations and financial condition.
The competitive environment and our competitive position are also significantly influenced by economic conditions, the state of consumer confidence, competitors’ introduction of low-priced products or innovative products, higher taxes, higher absolute prices and larger gaps between price categories and product regulation that diminishes the consumer’s ability to differentiate tobacco products. Due to the impact of these factors, as well as higher state and local excise taxes and the market share of deep discount brands, the tobacco industry has become increasingly price competitive. As we seek to adapt to the price competitive environment, our competitors that are better capitalized may be able to sustain price discounts for long periods of time by spreading the loss across their expansive portfolios, with which we are not positioned to compete.
“Big tobacco” has also established its presence in the NewGen products market. There can be no assurance that our products will be able to compete successfully against these companies or any of our other competitors, some of which have far greater resources, capital, experience, market penetration, sales and distribution channels than us. In addition, there are currently no U.S. restrictions on advertising electronic cigarettes and vaporizer products and competitors, including “big tobacco,” may have more resources than us for advertising expenses, which could have a material adverse effect on our ability to build and maintain market share, and thus have a material adverse effect on our business, results of operations and financial condition.
The market for NewGen products is subject to a great deal of uncertainty and is still evolving.
Vaporizer products and electronic cigarettes, having recently been introduced to market, are at an early stage of development, and represent core components of a market that is evolving rapidly and is characterized by a number of market participants. Rapid growth in the use of, and interest in, vaporizer products and electronic cigarettes is recent, and may not continue on a lasting basis. The demand and market acceptance for these products is subject to a high level of uncertainty. Therefore, we are subject to all of the business risks associated with a new enterprise in an evolving market. Continued evolution, uncertainty and the resulting increased risk of failure of our new and existing product offerings in this market could have a material adverse effect on our ability to build and maintain market share and on our business, results of operations and financial condition. Further, there can be no assurance that we will be able to continue to effectively compete in the NewGen products marketplace.
We are subject to significant product liability litigation.
The tobacco industry has experienced, and continues to experience, significant product liability litigation. Most tobacco liability lawsuits have been brought against manufacturers and sellers of cigarettes by individual
23
plaintiffs, often participating on a class-action basis, for injuries allegedly caused by cigarette smoking or by exposure to cigarette smoke. However, several lawsuits have also been brought against us and other manufacturers and sellers of smokeless products for injuries to health allegedly caused by use of smokeless products. There are several such suits pending against us with limited activity. In addition to the risks to our business, results of operations and financial condition resulting from adverse results in any such action, ongoing litigation may divert management’s attention and resources, which could have an impact on our business and operations. We cannot predict with certainty the outcome of these claims and there can be no assurance that we will not sustain losses in connection with such lawsuits and that such losses will not have a material adverse effect on our business, results of operations and financial condition.
In addition to current and potential future claims related to our smoking and smokeless products, we are subject to several lawsuits alleging personal injuries resulting from malfunctioning vaporizer devices or consumption of e-liquids and may be subject to claims in the future relating to our other NewGen products. We are still evaluating these claims and the potential defenses to them. As a result of their relative novelty, electronic cigarette and vaporizer product manufacturers and sellers have only recently become subject to litigation. We may see increasing litigation over NewGen products or the regulation of our products, as the regulatory regimes surrounding these products develop. For a description of current material litigation to which we or our subsidiaries are a party, see “Item 3. Legal Proceedings”.
As a result, we may face substantial costs due to increased product liability litigation relating to new regulations or other potential defects associated with NewGen products we ship, which could have a material adverse effect on our business, results of operations and financial condition.
The scientific community has not yet studied extensively the long-term health effects of certain substances contained in some of our products.
Electronic cigarettes, vaporizers and many of our NewGen products were recently developed and therefore the scientific community has not had a sufficient period of time to study the long-term health effects of their use. Currently, there is no way of knowing whether these products are safe for their intended use. If the scientific community were to determine conclusively that use of any or all of these products poses long-term health risks, market demand for these products and their use could materially decline. Such a determination could also lead to litigation and significant regulation. Loss of demand for our product, product liability claims and increased regulation stemming from unfavorable scientific studies on these products could have a material adverse effect on our business, results of operations and financial condition.
We are required to maintain cash amounts within an escrow account in order to be compliant with a settlement agreement between us and certain U.S. states and territories.
In November 1998, the major U.S. cigarette manufacturers entered into the Master Settlement Agreement (“MSA”) and the Smokeless Tobacco Master Settlement Agreement (“STMSA”) with 46 U.S. states and certain U.S. territories and possessions. Pursuant to the MSA and subsequent states’ statutes, a “cigarette manufacturer” (which is defined to also include a manufacturer of RYO/MYO cigarette tobacco) has the option of either becoming a signatory to the MSA, or, as we have elected, operating as a non-participating manufacturer (“NPM”) by funding and maintaining an escrow account, with sub-accounts on behalf of each settling state. These NPM escrow accounts are governed by states’ escrow and complementary statutes that are generally monitored by the Office of the State Attorney General. The statutes require NPM companies to deposit, on an annual basis, into qualified banks’ escrow funds based on the number of cigarettes or cigarette equivalents, which is measured by pounds of RYO/MYO tobacco sold. NPM companies are, within specified limits, entitled to direct the investment of the escrowed funds and withdraw any interest or appreciation, but cannot withdraw the principal for twenty-five years from the year of each annual deposit, except to withdraw funds deposited pursuant to an individual state’s escrow statute to pay a final judgment to that state’s plaintiffs in the event of such a final judgment. The investment vehicles available to us are specified in the state escrow agreements and are limited to low-risk government securities.
Various states have enacted or proposed complementary legislation intended to curb the activity of certain manufacturers and importers of cigarettes or MYO tobacco that are selling into MSA states without signing the MSA or who have failed to properly establish and fund a qualifying escrow account. We believe we have been fully compliant with all applicable laws, regulations, and statutes, although compliance-related issues may, from time to time, be disruptive to our business, any of which could have a material adverse effect on our business, results of operations, and financial condition.
24
Pursuant to the NPM escrow account statutes, in order to be compliant with the NPM escrow requirements, we are required to deposit such funds for each calendar year into a qualifying escrow account by April 15 of the following year with each year’s deposit being released from escrow after 25 years. We discontinued our MYO tobacco line in the third quarter of 2017. During 2019 no monies were deposited into this qualifying escrow account. As of December 31, 2019, we had made deposits of approximately $32.1 million. Thus, pending a change in MSA legislation, we have no remaining product lines covered by the MSA and will not be required to make future escrow deposits.
Although no such legislation has been proposed or enacted, future changes to the MSA, such as legislation that extends the MSA to products to which it does not currently apply or legislation that limits the ability of companies to receive unused escrow funds after 25 years, may have a material adverse effect on our business, results of operations and financial condition. Despite the amounts maintained and funded to the escrow account, compliance with the funding requirements for the escrow account does not necessarily prevent future federal and/or state regulations with respect to the OTP industry from having a material adverse effect on our business, results of operations and financial condition.
Competition from illicit sources may have an adverse effect on our overall sales volume, restricting the ability to increase selling prices and damaging brand equity.
Illicit trade and tobacco trafficking in the form of counterfeit products, smuggled genuine products and locally manufactured products on which applicable taxes or regulatory requirements are evaded, represent a significant and growing threat to the legitimate tobacco industry. Factors such as increasing tax regimes, regulatory restrictions, and compliance requirements are encouraging more consumers to switch to illegal, cheaper tobacco products and providing greater rewards for smugglers. Illicit trade can have an adverse effect on our overall sales volume, restrict the ability to increase selling prices, damage brand equity and may lead to commoditization of our products.
Although we combat counterfeiting of our products by engaging in certain tactics, such as requiring all sales force personnel to randomly collect our products from retailers in order to be tested by our quality control team, maintaining a quality control group that is responsible for identifying counterfeit products and using a private investigation firm to help perform surveillance of retailers we suspect are selling counterfeit products, no assurance can be given that we will be able to detect or stop sales of all counterfeit products. In addition, we have in the past and will continue to bring suits against retailers and distributors that sell certain counterfeit products. While we have been successful in securing financial recoveries from and helping to obtain criminal convictions of counterfeiters in the past, no assurance can be given that we will be successful in any such suits or that such suits will be successful in stopping other retailers or distributors from selling counterfeit products. Even if we are successful, such suits could consume a significant amount of management’s time and could also result in significant expenses to the company. Any failure to track and prevent counterfeiting of our products could have a material adverse on our ability to maintain or effectively compete for the products we distribute under our brand names, which would have a material adverse effect on our business, results of operations and financial condition.
Reliance on information technology means a significant disruption could affect our communications and operations.
We increasingly rely on information technology systems for our internal communications, controls, reporting and relations with customers and suppliers and information technology is becoming a significantly important tool for our sales staff. Our marketing and distribution strategy are dependent upon our ability to closely monitor consumer and market trends on a highly specified level, for which we are reliant on our highly sophisticated data tracking systems, which are susceptible to disruption or failure. In addition, our reliance on information technology exposes us to cyber-security risks, which could have a material adverse effect on our ability to compete. Security and privacy breaches may expose us to liability and cause us to lose customers or may disrupt our relationships and ongoing transactions with other entities with whom we contract throughout our supply chain. The failure of our information systems to function as intended, or the penetration by outside parties’ intent on disrupting business processes, could result in significant costs, loss of revenue, assets or personal or other sensitive data and reputational harm.
Security and privacy breaches may expose us to liability and cause us to lose customers.
Federal and state laws require us to safeguard our wholesalers’ and retailers’ financial information, including credit information. Although we have established security procedures to protect against identity theft and the theft of our customers’ and distributors’ financial information, our security and testing measures may not prevent security
25
breaches and breaches of privacy may occur and could harm our business. Typically, we rely on encryption and authentication technology licensed from third parties to enhance transmission security of confidential information in relation to financial and other sensitive information that we have on file. Advances in computer capabilities, new discoveries in the field of cryptography, inadequate facility security or other developments may result in a compromise or breach of the technology used by us to protect customer data. Any compromise of our security could harm our reputation or financial condition and, therefore, our business. In addition, a party who is able to circumvent our security measures or exploit inadequacies in our security measures, could, among other effects, misappropriate proprietary information, cause interruptions in our operations or expose customers and other entities with which we interact to computer viruses or other disruptions. Actual or perceived vulnerabilities may lead to claims against us. To the extent the measures we have taken prove to be insufficient or inadequate, we may become subject to litigation or administrative sanctions, which could result in significant fines, penalties or damages and harm to our reputation.
Contamination of, or damage to, our products could adversely impact sales volume, market share and profitability.
Our market position may be affected through the contamination of our tobacco supply or products during the manufacturing process or at different points in the entire supply chain. We keep significant amounts of inventory of our products in warehouses and it is possible that this inventory could become contaminated prior to arrival at our premises or during the storage period. If contamination of our inventory or packaged products occurs, whether as a result of a failure in quality control by us or by one of our suppliers, we may incur significant costs in replacing the inventory and recalling products. We may be unable to meet customer demand and may lose customers who purchase alternative brands or products. In addition, consumers may lose confidence in the affected product.
Under the terms of our contracts, we impose requirements on our suppliers to maintain quality and comply with product specifications and requirements, and on our third-party co-manufacturer to comply with all federal, state and local laws. These third-party suppliers, however, may not continue to produce products that are consistent with our standards or that are in compliance with applicable laws, and we cannot guarantee that we will be able to identify instances in which our third-party suppliers fail to comply with our standards or applicable laws. A loss of sales volume from a contamination event may occur, and such a loss may affect our ability to supply our current customers and to recapture their business in the event they are forced to switch products or brands, even if on a temporary basis. We may also be subject to legal action as a result of a contamination, which could result in negative publicity and affect our sales. During this time, our competitors may benefit from an increased market share that could be difficult and costly to regain. Such a contamination event could have a material adverse effect on our business, results of operations and financial condition.
Our intellectual property may be infringed.
We currently rely on trademark and other intellectual property rights to establish and protect the brand names and logos we own or license. Third parties have in the past infringed, and may in the future infringe, on these trademarks and our other intellectual property rights. Our ability to maintain and further build brand recognition is dependent on the continued and exclusive use of these trademarks, service marks and other proprietary intellectual property, including the names and logos we own or license. Despite our attempts to ensure these intellectual property rights are protected, third parties may take actions that could materially and adversely affect our rights or the value of this intellectual property. Any litigation concerning our intellectual property rights, whether successful or unsuccessful, could result in substantial costs to us and diversions of our resources. Expenses related to protecting our intellectual property rights, the loss or compromise of any of these rights or the loss of revenues as a result of infringement could have a material adverse effect on our business, results of operations and financial condition, and may prevent the brands we own or license from growing or maintaining market share.
Third parties may claim that we infringe their intellectual property and trademark rights.
Competitors in the tobacco products and NewGen markets may claim that we infringe their proprietary rights. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources, injunctions against us or the payment of damages. Further, our vapor distribution businesses distribute third party product brands with those suppliers’ branding and imagery. If that branding or imagery is alleged by other parties to infringe or otherwise violate intellectual property rights, we could be drawn into such litigation.
26
We may fail to manage our growth.
We have expanded over our history and intend to grow in the future. We acquired the Stoker’s® brand in 2003 and have continued to develop it through the introduction of new products, such as moist snuff. Our acquisition of the VaporBeast® brand in 2016 accelerated our entry into non-traditional retail channels while the 2018 acquisition of IVG added a top B2C platform which enhances our marketing and selling of proprietary and third-party vapor products to adult consumers. More recently, the acquisition of Solace provided us with a leading line of liquids and a powerful new product development platform. We have also focused on growing our relationships with our key suppliers through expansion into new product lines such as MYO cigar wraps, which are sourced from Durfort. However, any future growth will place additional demands on our resources, and we cannot be sure we will be able to manage our growth effectively. If we are unable to manage our growth while maintaining the quality of our products and profit margins, or if new systems that we implement to assist in managing our growth do not produce the expected benefits, our business, financial position, results of operations and cash flows could be adversely affected. We may not be able to support, financially or otherwise, future growth, or hire, train, motivate and manage the required personnel. Our failure to manage growth effectively could also limit our ability to achieve our goals as they relate to streamlined sales, marketing and distribution operations and the ability to achieve certain financial metrics.
We may fail to successfully integrate our acquisitions or otherwise be unable to benefit from pursuing acquisitions.
We believe there are meaningful opportunities to grow through acquisitions and joint ventures across all OTP product categories and we expect to continue a strategy of selectively identifying and acquiring businesses with complementary products. We may be unable to identify, negotiate, and complete suitable acquisition opportunities on reasonable terms. There can be no assurance that any business acquired by us will be successfully integrated with our operations or prove to be profitable to us. We may incur future liabilities related to acquisitions. Should any of the following problems, or others, occur as a result of our acquisition strategy, the impact could be material:
| • | difficulties integrating personnel from acquired entities and other corporate cultures into our business; |
| • | difficulties integrating information systems; |
| • | the potential loss of key employees of acquired companies; |
| • | the assumption of liabilities and exposure to undisclosed or unknown liabilities of acquired companies; or |
| • | the diversion of management attention from existing operations. |
We are subject to fluctuations in our results that make it difficult to track trends and develop strategies in the short-term.
In response to competitor actions and pricing pressures, we have engaged in significant use of promotional and sales incentives. We regularly review the results of our promotional spending activities and adjust our promotional spending programs in an effort to maintain our competitive position. Accordingly, unit sales volume and sales promotion costs in any period are not necessarily indicative of sales and costs that may be realized in subsequent periods. Additionally, promotional activity significantly increases net sales in the month in which it is initiated, and net sales are adversely impacted in the month after a promotion. Accordingly, based upon the timing of our marketing and promotional initiatives, we have and may continue to experience significant variability in our results, which could affect our ability to formulate strategies that allow us to maintain our market presence across volatile periods. If our fluctuations obscure our ability to track important trends in our key markets, it may have a material adverse effect on our business, results of operations and financial condition.
We are subject to the risks of exchange rate fluctuations.
Currency movements and suppliers’ price increases relating to premium cigarette papers and cigarette tubes are the primary factors affecting our cost of sales. These products are purchased from Bolloré and we make payments in euros. Thus, we bear certain foreign exchange rate risk for certain of our inventory purchases. In addition, as part of our strategy, we have begun strategic international expansions. As a result, we may be more sensitive to the risks of exchange rate fluctuations. To manage this risk, we sometimes utilize short-term forward currency contracts to purchase euros for our inventory purchases. We have a foreign exchange currency policy which governs our hedging
27
of risk. While we engage in hedging transactions from time to time, no assurance can be made that we will be successful in eliminating currency exchange risks or that changes in currency rates will not have a material adverse effect on our business, results of operations and financial condition.
Adverse U.S. and global economic conditions could negatively impact our business, prospects, results of operations, financial condition or cash flows.
Our business and operations are sensitive to global economic conditions. These conditions include interest rates, energy costs, inflation, recession, fluctuations in debt and equity capital markets and the general condition of the U.S. and world economy. A material decline in the economic conditions affecting consumers, which cause a reduction in disposable income for the average consumer, may change consumption patterns, and may result in a reduction in spending on OTP or a switch to cheaper products or products obtained through illicit channels. Electronic cigarettes, vaporizer and e-liquid products are relatively new to market and may be regarded by users as a novelty item and expendable. As such, demand for our NewGen products may be particularly sensitive to economic conditions such as inflation, recession, high energy costs, unemployment, changes in interest rates and money supply, changes in the political environment and other factors beyond our control, any combination of which could result in a material adverse effect on our business, results of operations and financial condition.
Our supply to our wholesalers and retailers is dependent on the demands of their customers who are sensitive to increased sales taxes and economic conditions affecting their disposable income.
Consumer purchases of tobacco products are historically affected by economic conditions, such as changes in employment, salary and wage levels, the availability of consumer credit, inflation, interest rates, fuel prices, sales taxes, and the level of consumer confidence in prevailing and future economic conditions. Discretionary consumer purchases, such as of OTP, may decline during recessionary periods or at other times when disposable income is lower, and taxes may be higher.
In addition, states such as New York, Hawaii, Rhode Island, Georgia and North Carolina have begun collecting taxes on internet sales where companies have used independent contractors in those states to solicit sales from residents of those states. These taxes apply to our online sales of NewGen products into those states and may result in reduced demand from the independent wholesalers who may not be able to absorb the increased taxes or successfully pass them onto the end-user without experiencing reduced demand. Further, as a result of South Dakota v. Wayfair, states are now able to impose sales tax on internet purchases made from out-of-state sellers, even if the seller does not have a physical presence in the taxing state. Consequently, additional states are likely to seek or have begun to impose sales tax on our online sales. The requirement to collect, track and remit taxes may require us to increase our prices, which may affect demand for our products or conversely reduce our net profit margin, which could have a material adverse effect on our business, results of operations and financial condition.
Our failure to comply with certain environmental, health and safety regulations could adversely affect our business.
The storage, distribution and transportation of some of the products that we sell are subject to a variety of federal and state environmental regulations. In addition, our manufacturing facilities are similarly subject to federal, state and local environmental laws. We are also subject to operational, health and safety laws and regulations. Our failure to comply with these laws and regulations could cause a disruption in our business, an inability to maintain our manufacturing resources, and additional and potentially significant remedial costs and damages, fines, sanctions or other legal consequences that could have a material adverse effect on our business, results of operations and financial condition.
The departure of key management personnel and the failure to attract and retain talent could adversely affect our operations.
Our success depends upon the continued contributions of our senior management. Our ability to implement our strategy of attracting and retaining the best talent may be impaired by the decreasing social acceptance of tobacco usage. The tobacco industry competes for talent with the consumer products industry and other companies that enjoy greater societal acceptance. As a result, we may be unable to attract and retain the best talent, which could have a material adverse effect on our business, results of operations and financial condition.
28
Imposition of significant tariffs on imports into the U.S., could have a material and adverse effect on our business.
We are required to purchase all our cigarette papers, cigarette tubes and cigarette injector machines from Bolloré in France. Additionally, a substantial portion of our NewGen products are sourced from China. In 2018, President Trump and his administration imposed significant additional tariffs on certain goods imported from outside the U.S. and could impose additional tariffs in the future. These additional tariffs apply to a significant portion of our NewGen products and may result in increased prices for our customers. These increased prices may reduce demand where customers are unable to absorb the increased prices or successfully pass them onto the end-user. If the U.S. were to impose additional tariffs on goods we import, it is likely to make it more costly for us to import goods from other countries. As a result, our business, financial condition and results of operations could be materially adversely affected.
The reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors, potentially decreasing our stock price.
We are an “emerging growth company” as defined under the federal securities laws. For as long as we continue to be an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth Companies. Investors may find our common stock less attractive because we may rely on these exemptions, which include but are not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (“Section 404”), reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, Section 107 of the JOBS Act (“Section 107”) provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. We have elected to opt out of the extended transition period for complying with the revised accounting standards.
If investors find our common stock less attractive as a result of exemptions and reduced disclosure requirements, there may be a less active trading market for our common stock and our stock price may be more volatile or decrease.
We may lose our status as an emerging growth company before the five-year maximum time period a company may retain such status.
We have elected to rely on certain exemptions and reduced disclosure requirements applicable to emerging growth companies and expect to continue to do so. However, we may choose to “opt out” of such reduced disclosure requirements and provide disclosure required for companies that do not qualify as emerging growth companies. In addition, we chose to opt out of the provision of the JOBS Act that permits us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. Section 107 provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards would be irrevocable.
Furthermore, although we are able to remain an emerging growth company for up to five years, we may lose such status at an earlier time if (i) our annual gross revenues exceed $1 billion, (ii) we become a “large accelerated filer” as defined in Rule 12b-2 under the Exchange Act, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) we issued more than $1 billion in non-convertible debt during the preceding three-year period.
When we lose our emerging growth company status, whether due to an election, the end of the five-year period, or one of the circumstances listed in the preceding paragraph, the emerging growth company exemptions will cease to apply and we expect we will incur additional expenses and devote increased management effort toward ensuring compliance with the non-emerging growth company requirements. We cannot predict or estimate the amount of additional costs we may incur as a result of the change in our status or the timing of such costs, though such costs may be substantial.
Our principal stockholders are able to exert significant influence over matters submitted to our stockholders and may take certain actions to prevent takeovers.
Standard Diversified Inc. (“SDI”), which is controlled by funds managed by Standard General L.P. (together with the funds it manages, “Standard General”), is a significant stockholder. SDI owns approximately 50.0% of our stock and Standard General directly owns approximately 3.4% of our common stock. The existence of these and other
29
significant stockholders may have the effect of deterring hostile takeovers, delaying or preventing changes in control or changes in management, or limiting the ability of our other stockholders to approve transactions that they may deem to be in the best interests of our company. In addition, our significant stockholders will be able to exert significant influence over the decision, if any, to authorize additional capital stock, which, if issued, could have a significant dilutive effect on holders of common stock.
Our certificate of incorporation provides that the doctrine of “corporate opportunity” will not apply against SDI and Standard General in a manner that would prohibit them from investing in competing businesses or doing business with our customers. To the extent they invest in such other businesses, SDI and Standard General may have differing interests than our other stockholders. In addition, SDI and Standard General are permitted to engage in business activities or invest in or acquire businesses which may compete with or do business with any competitors of ours.
Furthermore, Standard General is in the business of managing investment funds and therefore may pursue acquisition opportunities that may be complementary to our business and, as a result, such acquisition opportunities may not be available to us.
Our certificate of incorporation and bylaws, as well as Delaware law and certain regulations, could discourage or prohibit acquisition bids or merger proposals, which may adversely affect the market price of our common stock.
Our certificate of incorporation authorizes our board of directors to issue preferred stock without stockholder approval. If our board of directors elects to issue preferred stock, it could be more difficult for a third party to acquire us. In addition, some provisions of our certificate of incorporation, bylaws and applicable law could make it more difficult for a third party to acquire control of us, even if the change of control would be beneficial to our stockholders, including:
| • | limitations on the removal of directors; |
| • | limitations on the ability of our stockholders to call special meetings; |
| • | limitations on stockholder action by written consent; |
| • | establishing advance notice provisions for stockholder proposals and nominations for elections to the board of directors to be acted upon at meetings of stockholders; and |
| • | limitations on the ability of our stockholders to fill vacant directorships or amend the number of directors constituting our board of directors. |
Our certificate of incorporation limits the ownership of our common stock by individuals and entities that are Restricted Investors. These restrictions may affect the liquidity of our common stock and may result in Restricted Investors being required to sell or redeem their shares at a loss or relinquish their voting, dividend and distribution rights.
For so long as we or one of our subsidiaries is party to any of the Bolloré distribution agreements, our certificate of incorporation will limit the ownership of our common stock by any “Restricted Investor” to 14.9% of our outstanding common stock and shares convertible or exchangeable therefor (including our non-voting common stock) (the “Permitted Percentage”). A “Restricted Investor” is defined as: (i) any entity that directly or indirectly manufactures, sells, markets, distributes or otherwise promotes cigarette paper booklets, filter tubes, injector machines or filter tips in the United States, the District of Columbia, the territories, possessions and military bases of the United States and the Dominion of Canada (a “Bolloré Competitor”), (ii) any entity that owns more than a 20% equity interest in any Bolloré Competitor, or (iii) any person who serves as a director or officer of, or any entity that has the right to appoint an officer or director of, any Bolloré Competitor or of any entity that owns more than a 20% equity interest in any Bolloré Competitor (each, a “Restricted Investor”). Our certificate of incorporation further provides that any issuance or transfer of shares to a Restricted Investor in excess of the Permitted Percentage will be ineffective as against us and that neither we nor our transfer agent will register the issuance or transfer of shares or be required to recognize the transferee or owner as a holder of our common stock for any purpose except to exercise our remedies described below. Any shares in excess of the Permitted Percentage in the hands of a Restricted Investor will not have any voting or dividend rights and are subject to redemption by us in our discretion. The liquidity or market value of the shares of our common stock may be adversely impacted by such transfer restrictions.
30
As a result of the above provisions, a proposed transferee of our common stock that is a Restricted Investor may not receive any return on its investment in shares it purchases or owns, as the case may be, and it may sustain a loss. We are entitled to redeem all or any portion of such shares acquired by a Restricted Investor in excess of the Permitted Percentage (“Excess Shares”) at a redemption price based on a fair market value formula that is set forth in our certificate of incorporation, which may be paid in any form, including cash or promissory notes, at our discretion. Excess Shares not yet redeemed will not be accorded any voting, dividend or distribution rights while they constitute Excess Shares. As a result of these provisions, a stockholder who is a Restricted Investor may be required to sell its shares of our common stock at an undesirable time or price and may not receive any return on its investment in such shares. However, we may not be able to redeem Excess Shares for cash because our operations may not have generated sufficient excess cash flow to fund the redemption and we may incur additional indebtedness to fund all or a portion of such redemption, in which case our financial condition may be materially weakened.
Our certificate of incorporation permits us to require that owners of any shares of our common stock provide certification of their status as a Restricted Investor. In the event that a person does not submit such documentation, our certificate of incorporation provides us with certain remedies, including the suspension of the payment of dividends and distributions with respect to shares held by such person and deposit of any such dividends and distributions into an escrow account. As a result of non-compliance with these provisions, an owner of the shares of our common stock may lose significant rights associated with those shares.
Although our certificate of incorporation contains the above provisions intended to assure compliance with the restrictions on ownership of our common stock by Restricted Investors, we may not be successful in monitoring or enforcing the provisions. A failure to enforce or otherwise maintain compliance could lead Bolloré to exercise its termination rights under the agreements, which would have a material and adverse effect on the Company’s financial position and its results of operations.
In addition to the risks described above, the foregoing restrictions could delay, defer or prevent a transaction or change in control that might involve a premium price for our common stock or that might otherwise be in the best interest of our stockholders.
Future sales of our common stock in the public market could reduce our stock price, and any additional capital raised by us through the sale of equity or convertible securities may dilute our stockholders.
We may sell additional shares of common stock in subsequent public offerings. We may also issue additional shares of common stock or convertible securities.
We cannot predict the size of future issuances of our common stock or securities convertible into common stock or the effect, if any, that future issuances and sales of shares of our common stock will have on the market price of our common stock. Sales of substantial amounts of our common stock (including shares issued in connection with an acquisition), or the perception that such sales could occur, may adversely affect prevailing market prices of our common stock.
We may issue preferred stock whose terms could adversely affect the voting power or value of our common stock.
Our certificate of incorporation authorizes us to issue, without the approval of our stockholders, one or more classes or series of preferred stock having such designations, preferences, limitations and relative rights, including preferences over our common stock respecting dividends and distributions, as our board of directors may determine. The terms of one or more classes or series of preferred stock could adversely impact the voting power or value of our common stock. For example, we might grant holders of preferred stock the right to elect some number of our directors in all events or on the happening of specified events or the right to veto specified transactions. Similarly, the repurchase or redemption rights or liquidation preferences we might assign to holders of preferred stock could affect the residual value of the common stock.
Our status as a “controlled company” could make our common stock less attractive to some investors or otherwise harm our stock price.
Because we qualify as a “controlled company” under the corporate governance rules for NYSE-listed companies we are not required to have, and could elect in the future not to have, a majority of our board of directors be independent, a compensation committee, or an independent nominating function. Accordingly, should the interests
31
of our controlling stockholder differ from those of other stockholders, the other stockholders may not have the same protections afforded to stockholders of companies subject to all of the corporate governance rules for NYSE-listed companies. Our status as a controlled company could make our common stock less attractive to some investors or otherwise harm our stock price.
| Item 1B. | Unresolved Staff Comments |
None
| Item 2. | Properties |
As of December 31, 2019, we operated manufacturing, distribution, retail, office, and warehouse space in the U.S. with a total floor area of approximately 398,000 square feet, all of which is leased with the exception of our Dresden, Tennessee, manufacturing facility which we purchased in 2016. To provide a cost-efficient supply of products to our customers, we maintain centralized management of internal manufacturing and nationwide distribution facilities. Our two manufacturing and distribution facilities are located in Louisville, Kentucky and Dresden, Tennessee. We believe our facilities are generally adequate for our current and anticipated future use.
The following table describes our principal properties as of December 31, 2019:
|
Location
|
Principal Use
|
Segments that use
the Property(ies) |
Square
Feet |
Owned or
Leased |
|
Darien, CT
|
Administrative office
|
All segments
|
1,950
|
Leased
|
|
|
|
|
|
|
|
Louisville, KY
|
Corporate offices, manufacturing,
R&D, warehousing, and distribution |
All segments
|
248,800
|
Leased
|
|
|
|
|
|
|
|
Carlsbad, CA
|
Administrative office
|
NewGen
|
10,491
|
Leased
|
|
|
|
|
|
|
|
Dresden, TN
|
Manufacturing and administration
|
Smokeless
|
76,600
|
Owned
|
|
|
|
|
|
|
|
Miami, FL
|
Administrative offices
|
NewGen
|
22,522
|
Leased
|
|
|
|
|
|
|
|
Simi Valley, CA
|
Administrative office
|
NewGen
|
10,340
|
Leased
|
|
|
|
|
|
|
|
Various cities in southern Florida
|
Nine retail stores
|
NewGen
|
13,184
|
Leased
|
|
|
|
|
|
|
|
Various cities in Oklahoma City
|
Seven retail stores
|
NewGen
|
14,235
|
Leased
|
| Item 3. | Legal Proceedings |
We are a party from time to time to various proceedings in the ordinary course of business. For a description of the Master Settlement Agreement, to which we are a party, refer to Note 2 in our Notes to Consolidated Financial Statements, “Summary of Significant Accounting Policies: Risk and Uncertainties”. Other than the proceedings mentioned below, there is no material litigation, arbitration or governmental proceeding currently pending against us or any of our officers or directors in their capacity as such, and we and our officers and directors have not been subject to any such proceeding.
Other major tobacco companies are defendants in product liability claims. In a number of these cases, the amounts of punitive and compensatory damages sought are significant and could have a material adverse effect on our business and results of operations. The Company is subject to several lawsuits alleging personal injuries resulting from malfunctioning vaporizer devices or consumption of e-liquids and may be subject to claims in the future relating to our other NewGen products. The Company is still evaluating these claims and the potential defenses to them. For example, the Company did not design or manufacture the products at issue; rather, we were merely the distributor. Nonetheless, there can be no assurance that we will prevail in these cases, and they could have a material adverse effect on our business and results of operations. Because of their relative novelty, electronic cigarette and vaporizer product manufacturers and sellers have only recently become subject to litigation.
32
We engaged in discussions and mediation with VMR, which was acquired in 2018. Pursuant to a Distribution and Supply agreement (“VMR Agreement”), VMR was providing us with V2 e-cigarettes for the exclusive distribution in bricks-and-mortar stores in the United States. Under the terms of the VMR Agreement, in the event of termination following a change in control, the acquirer was required to make a payment to us under a formula designed to provide us with a fair share of the value created by our performance under the VMR Agreement. The discussions have been completed and we received $6.7 million in the second quarter 2019 to settle the issue. Net of legal costs and reserves for anticipated future returns associated with the discontinuance, we recorded a $5.5 million gain in the second quarter, which is recorded as a reduction to selling, general, and administrative expenses.
We have several subsidiaries engaged in making, distributing and retailing (online and in bricks-and-mortar) vapor products. As a result of the overall publicity and controversy surrounding the vapor industry generally, many companies have received informational subpoenas from various regulatory bodies and in some jurisdictions regulatory lawsuits have been filed regarding marketing practices and possible underage sales. We expect that our subsidiaries will be subject to some such cases and information requests. In the acquisition of the vapor businesses, we negotiated financial “hold-backs”, which we expect to be able to use to defray expenses associated with the information production and the cost of defending any such lawsuits. To the extent that litigation becomes necessary, we believe that the subsidiaries have strong factual and legal defenses against claims that they unfairly marketed vapor products.
On October 8, 2019, the City of New York filed a complaint against twenty-three companies, including IVG and VaporFi, making various allegations including selling to consumers over the age of 18 but under 21. In response, those subsidiaries have ceased all sales into New York City, which was an immaterial market for those businesses. This proceeding was settled for monetary terms which were not material and certain structural remedies that the subsidiaries deemed acceptable.
See “Risk Factors—We are subject to significant product liability litigation” for additional details.
| Item 4. | Mine Safety Disclosures |
Not applicable.
Information about our Executive Officers
Listed below are the executive officers of the Company. Our executive officers are appointed by, and serve at the discretion of, our board of directors. There are no family relationships between any of the executive officers, and there is no arrangement or understanding between any executive officer and any other person pursuant to which the executive officer was selected.
Lawrence S. Wexler, age 67, has served as our President and CEO since June 2009 and as President and Chief Operating Officer of NATC, our primary operating subsidiary since June 2006. Prior to June 2006, Mr. Wexler had been the Chief Operating Officer of NATC since June 2005, and prior to that, the President and Chief Operating Officer of one of our other subsidiaries since December 2003. Mr. Wexler was a consultant to a number of emerging marketing, communication, and financial companies, advising them on financial, marketing and strategic matters, at times in an operating role, from 1998 to 2003. From 1977 to 1998, he was employed by Philip Morris, USA in various positions in the Sales, Marketing, and Finance Departments. As Group Director, Discount Brands, his group introduced the Basic and Alpine brands. He served as Senior Vice President of Marketing from 1992 to 1993 and Senior Vice President Finance, Planning, and Information Services from 1993 until his departure in 1998. Mr. Wexler holds a Bachelor of Science in administrative science from Yale and a Master of Business Administration from Stanford.
Graham Purdy, age 48, was appointed as Chief Operating Officer in November 2019 after serving as President of our New Ventures Division since December 2017. Mr. Purdy joined us in 2004 and has held various leadership positions since that time. Prior to joining us, Mr. Purdy spent 7 years at Philip Morris, USA where he served in senior sales and sales management positions. Mr. Purdy holds a Bachelor of Arts from California State University, Chico.
Robert Lavan, age 37, joined us as Chief Financial Officer in March 2018 and served as a consultant for us since January 2018. Prior to joining the company, Mr. Lavan was the Chief Financial Officer of General Wireless Operations from January 2017 to January 2018, where he was responsible for revamping the company’s financial reporting systems and building a robust distribution platform that linked multiple eCommerce sites and Amazon.
33
From 2014 until Mr. Lavan’s appointment as Chief Financial Officer of General Wireless Operations, Mr. Lavan served as an analyst for Standard General LP, a New York-based investment firm that is the majority shareholder of Standard Diversified Inc. (SDI), TPB’s majority shareholder. Before that, Mr. Lavan worked at SAC Capital and J. Goldman & Co. LP in various analyst and portfolio manager roles covering a wide range of industries. He began his career at The Blackstone Group. Mr. Lavan holds a Bachelor of Science in engineering from the University of Pennsylvania.
James W. Dobbins, age 60, has been our Senior Vice President, General Counsel, and Secretary since June 1999 and has served in various roles in our legal department since joining us in June 1999. Prior to joining us, Mr. Dobbins was in private practice in North Carolina and held various positions in the legal department of Liggett Group, Inc., a major cigarette manufacturer, including, at the time he left that company, Vice President, General Counsel, and Secretary. Mr. Dobbins has also practiced as an outside litigation attorney with Webster & Sheffield, a New York law firm, representing a variety of clients including Liggett Group, Inc. Prior to joining Webster & Sheffield, he served as a law clerk to the Honorable J. Daniel Mahoney, U.S. Circuit Judge for the Second Circuit Court of Appeals. Mr. Dobbins holds a Bachelor of Arts in mathematics and political science from Drew University and a J.D. from Fordham University School of Law.
34
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
The principal stock exchange on which Turning Point Brands, Inc.’s common stock (par value $0.01 per share) is listed is the New York Stock Exchange under the symbol “TPB.” At February 28, 2020, there were 65 holders of record of Turning Point Brands, Inc.’s common stock.
Dividends. On November 9, 2017, our Board of Directors approved the initiation of a cash dividend to shareholders. The initial quarterly dividend of $0.04 per common share was paid on December 15, 2017 to shareholders of record at the close of business on November 27, 2017. The most recent dividend of $0.05 per common share, an increase of approximately 11%, will be paid on April 10, 2020, to shareholders of record at the close of business on March 20, 2020.
Performance graph. The graph below compares the cumulative total shareholder return of Turning Point Brands, Inc.’s common stock since our initial public offering on May 11, 2016, with the Russell 3000 Index and the S&P Small Cap 600 Consumer Staples Index. The information presented assumes an initial investment of $100 on May 11, 2016, and that all dividends were reinvested. The cumulative returns shown represent the value that these investments would have had on December 31, 2019.
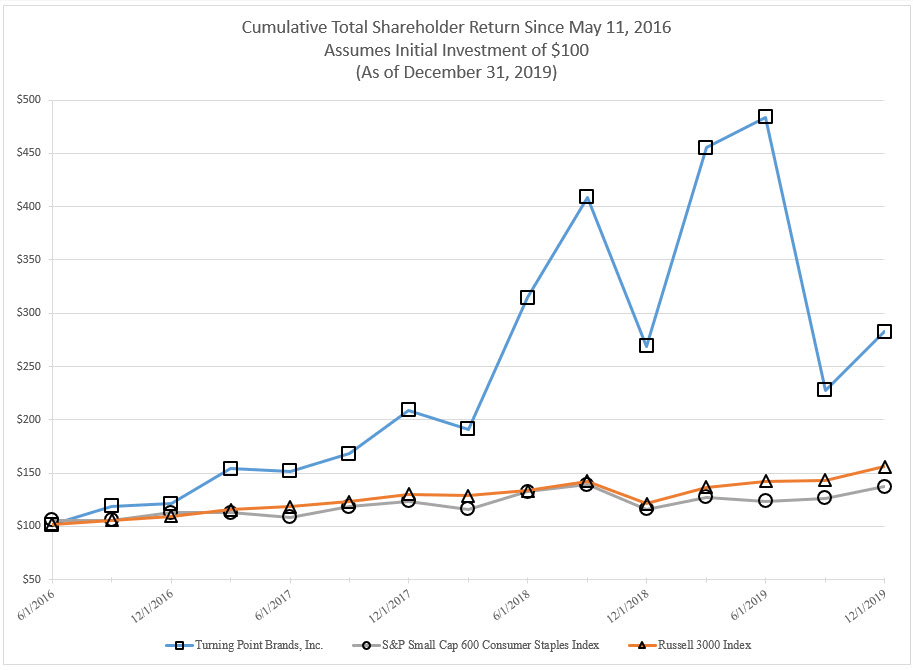
Issuer purchases of equity securities. No shares of common stock were purchased during 2019.
35
| Item 6. | Selected Financial Data |
The following selected financial data should be read in conjunction with “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and consolidated financial statements and notes thereto contained in “Item 8. Financial Statements and Supplementary Data” of this report. A reconciliation of non-GAAP measures to the most directly comparable GAAP financial measure is presented following the Selected Financial Data.
(dollars in thousands) |
Year Ended December 31, |
||||||||||||||
2019 |
2018 |
2017 |
2016 |
2015 |
|||||||||||
Consolidated Statement of Operations Data: |
|||||||||||||||
Net sales |
$ | 361,989 | $ | 332,683 | $ | 285,777 | $ | 206,228 | $ | 197,256 | |||||
Cost of sales |
225,243 | 190,124 | 160,807 | 105,683 | 100,775 | ||||||||||
Gross profit |
136,746 | 142,559 | 124,970 | 100,545 | 96,481 | ||||||||||
Selling, general and administrative expenses |
109,887 | 94,075 | 75,290 | 56,626 | 51,758 | ||||||||||
Operating income |
26,859 | 48,484 | 49,680 | 43,919 | 44,723 | ||||||||||
Interest expense, net |
17,342 | 14,819 | 16,889 | 26,621 | 34,284 | ||||||||||
Investment income |
(2,648 | ) |
(424 | ) |
(438 | ) |
(768 | ) |
— | ||||||
Loss on extinguishment of debt |
1,308 | 2,384 | 6,116 | 2,824 | — | ||||||||||
Net periodic benefit (income) cost, excluding service cost |
(4,961 | ) |
131 | 180 | 334 | 212 | |||||||||
Income before income taxes |
15,818 | 31,574 | 26,933 | 14,908 | 10,227 | ||||||||||
Income tax expense (benefit) |
2,044 | 6,285 | 7,280 | (12,005 | ) |
1,078 | |||||||||
Consolidated net income |
13,774 | 25,289 | 19,653 | 26,913 | 9,149 | ||||||||||
Net loss attributable to non-controlling interest |
— | — | (556 | ) |
— | $ | — | ||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | $ | 20,209 | $ | 26,913 | $ | 9,149 | |||||
Basic income per common share: |
|||||||||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 0.70 | $ | 1.31 | $ | 1.06 | $ | 1.63 | $ | 1.27 | |||||
Diluted income per common share: |
|||||||||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 0.69 | $ | 1.28 | $ | 1.04 | $ | 1.49 | $ | 1.10 | |||||
Weighted average common shares outstanding: |
|||||||||||||||
Basic |
19,627,093 | 19,355,607 | 18,989,177 | 16,470,352 | 7,198,081 | ||||||||||
Diluted |
20,037,540 | 19,827,562 | 19,513,008 | 18,015,545 | 8,354,387 | ||||||||||
Other Financial Information: |
|||||||||||||||
Net cash provided by operating activities |
$ | 37,795 | $ | 13,090 | $ | 29,690 | $ | 9,128 | $ | 24,430 | |||||
Net cash provided by (used in) investing activities |
15,901 | (24,669 | ) |
(1,116 | ) |
(55,888 | ) |
(2,030 | ) |
||||||
Net cash provided by (used in) financing activities |
67,966 | 9,930 | (28,016 | ) |
15,734 | (26,032 | ) |
||||||||
Capital expenditures |
(4,815 | ) |
(2,267 | ) |
(2,021 | ) |
(3,207 | ) |
(1,602 | ) |
|||||
Depreciation and amortization |
4,089 | 3,111 | 2,328 | 1,285 | 1,059 | ||||||||||
EBITDA(1) |
38,557 | 51,888 | 52,822 | 45,638 | 45,570 | ||||||||||
Adjusted EBITDA(1) |
67,337 | 64,610 | 60,024 | 52,449 | 50,604 | ||||||||||
Leverage Ratio(2) |
2.8 | x |
3.4 | x |
3.3 | x |
4.1 | x |
5.7 | x |
|||||
Balance Sheet Data: |
|||||||||||||||
Cash |
$ | 95,250 | $ | 3,306 | $ | 2,607 | $ | 2,865 | $ | 4,835 | |||||
Working capital |
133,364 | 48,088 | 41,263 | 37,289 | 42,815 | ||||||||||
Total assets |
446,584 | 339,377 | 282,277 | 285,020 | 242,463 | ||||||||||
Notes payable and long-term debt |
284,191 | 220,715 | 202,040 | 218,225 | 292,440 | ||||||||||
Total liabilities |
339,999 | 256,754 | 228,953 | 250,962 | 324,075 | ||||||||||
Total stockholders’ equity (deficit) |
106,585 | 82,623 | 53,324 | 34,058 | (81,612 | ) |
|||||||||
| (1) | To supplement our financial information presented in accordance with generally accepted accounting principles in the United States, or U.S. GAAP, we use non-U.S. GAAP financial measures including EBITDA and Adjusted EBITDA. We define “EBITDA” as net income before interest expense, loss on extinguishment of debt, income taxes, depreciation, and amortization. We define “Adjusted EBITDA” as net income before interest expense, loss on extinguishment of debt, income taxes, depreciation, amortization, other non-cash items, and other |
36
items that we do not consider ordinary course in our evaluation of ongoing, operating performance. We present EBITDA and Adjusted EBITDA in this Form 10-K because they are key metrics used by management and our board of directors to assess our financial performance and are also used by management to assess performance for the purposes of our executive compensation programs. EBITDA and Adjusted EBITDA are also frequently used by analysts, investors and other interested parties to evaluate companies in our industry. We believe that EBITDA and Adjusted EBITDA are appropriate measures of operating performance because they eliminate the impact of expenses that do not relate to business performance. EBITDA and Adjusted EBITDA have limitations as analytical tools, and you should not consider them in isolation, or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
| (i) | They do not reflect our cash expenditures, or future requirements for capital expenditures or contractual commitments; |
| (ii) | They do not reflect changes in, or cash requirements for, our working capital needs; |
| (iii) | They do not reflect our significant interest expense, or the cash requirements necessary to service interest or principal payments on our debt; and |
| (iv) | Although depreciation and amortization are non-cash charges, the assets being depreciated and amortized often will have to be replaced in the future, and EBITDA and Adjusted EBITDA do not reflect any cash requirements for such replacements. |
| (2) | Leverage Ratio - We calculate our Leverage Ratio by dividing Notes payable and long-term debt, less Cash, by Adjusted EBITDA. |
(in thousands) |
Years ended December 31, |
||||||||||||||
2019 |
2018 |
2017 |
2016 |
2015 |
|||||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | $ | 20,209 | $ | 26,913 | $ | 9,149 | |||||
Add: |
|||||||||||||||
Interest expense, net |
17,342 | 14,819 | 16,889 | 26,621 | 34,284 | ||||||||||
Loss on extinguishment of debt |
1,308 | 2,384 | 6,116 | 2,824 | — | ||||||||||
Income tax expense |
2,044 | 6,285 | 7,280 | (12,005 | ) |
1,078 | |||||||||
Depreciation expense |
2,638 | 2,105 | 1,626 | 1,227 | 1,059 | ||||||||||
Amortization expense |
1,451 | 1,006 | 702 | 58 | — | ||||||||||
EBITDA |
$ | 38,557 | $ | 51,888 | $ | 52,822 | $ | 45,638 | $ | 45,570 | |||||
Components of Adjusted EBITDA |
|||||||||||||||
Other(a) |
360 | 366 | 1,317 | 1,451 | 250 | ||||||||||
Stock options, restricted stock, and incentives expense(b) |
4,626 | 1,410 | 668 | 180 | 234 | ||||||||||
Transactional expenses and strategic initiatives(c) |
1,764 | 4,482 | 2,133 | 1,587 | 2,259 | ||||||||||
New product launch costs(d) |
6,185 | 1,835 | 2,414 | 2,678 | 1,915 | ||||||||||
FDA PMTA(e) |
2,153 | — | — | — | — | ||||||||||
Corporate and vapor restructuring(f) |
19,214 | 4,629 | 563 | — | 376 | ||||||||||
Vendor settlement(g) |
(5,522 | ) |
— | — | — | — | |||||||||
Bonus(h) |
— | — | 107 | 915 | — | ||||||||||
Adjusted EBITDA |
$ | 67,337 | $ | 64,610 | $ | 60,024 | $ | 52,449 | $ | 50,604 | |||||
| (a) | Represents LIFO adjustment, non-cash pension expense (income) and foreign exchange hedging. |
| (b) | Represents non-cash stock options, restricted stock, incentives expense and Solace PRSUs. |
| (c) | Represents the fees incurred for transaction expenses and strategic initiatives. |
| (d) | Represents product launch costs for our new product lines. |
| (e) | Represents costs associated with applications related to FDA PMTA. |
| (f) | Represents costs associated with corporate and vapor restructuring including severance and inventory reserves. |
| (g) | Represents net gain associated with the settlement of a vendor contract. |
| (h) | Represents bonuses associated with the December 2017 Tax Cuts and Jobs Act and non-recurring compensation expenses incurred coinciding with the May 2016 IPO. |
37
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion of the historical financial condition and results of operations in conjunction with our historical consolidated financial statements and accompanying notes, which are included elsewhere in this Annual Report on Form 10-K. In addition, this discussion includes forward-looking statements subject to risks and uncertainties that may result in actual results differing from statements we make. See “Cautionary Note Regarding Forward-Looking Statements.” Factors that could cause actual results to differ include those risks and uncertainties discussed in “Risk Factors.”
The following discussion relates to the audited financial statements of Turning Point Brands, Inc., included elsewhere in this Annual Report on Form 10-K. In this discussion, unless the context requires otherwise, references to “our Company” “we,” “our,” or “us” refer to Turning Point Brands, Inc., and its consolidated subsidiaries. References to “TPB” refer to Turning Point Brands, Inc., without any of its subsidiaries. We were incorporated in 2004 under the name North Atlantic Holding Company, Inc. On November 4, 2015, we changed our name to Turning Point Brands, Inc. Many of the amounts and percentages in this discussion have been rounded for convenience of presentation.
Organizational Structure
We, Turning Point Brands, Inc., are a holding company which owns North Atlantic Trading Company, Inc. (“NATC”), and its subsidiaries, Turning Point Brands, LLC (“TPLLC”), and its subsidiaries, and Turning Point Brands (Canada) Inc. (“TPBC”). NATC includes subsidiaries National Tobacco Company, L.P. (“NTC”), National Tobacco Finance, LLC (“NTFLLC”), North Atlantic Operating Company, Inc. (“NAOC”), North Atlantic Cigarette Company, Inc. (“NACC”), and RBJ Sales, Inc. (“RBJ”). TPLLC includes subsidiaries Intrepid Brands, LLC (“Intrepid”), TPB Beast, LLC (“VaporBeast”), TPB Shark, LLC, and its subsidiaries (collectively, “Vapor Shark”), TPB International, LLC and its subsidiaries (collectively, “IVG”), and Nu-X Ventures, LLC (“Nu-X”).
Overview
We are a leading independent provider of Other Tobacco Products (“OTP”) in the U.S. We sell a wide range of products across the OTP spectrum including moist snuff tobacco (“MST”), loose leaf chewing tobacco, premium cigarette papers, make-your-own (“MYO”) cigar wraps, cigars, and liquid vapor products; but, we do not sell cigarettes. We estimate the OTP industry generated approximately $11.5 billion in manufacturer revenue in 2019. In contrast to manufactured cigarettes, which have been experiencing declining volumes for decades based on data published by the Alcohol and Tobacco Tax and Trade Bureau (“TTB”), the OTP industry is demonstrating increased consumer appeal with low to mid-single digit consumer unit growth as reported by Management Science Associates, Inc. (“MSAi”), a third-party analytics and informatics company. Under the leadership of a senior management team with an average of 24 years of experience in the tobacco industry, we have grown and diversified our business through new product launches, category expansions, and acquisitions while concurrently improving operational efficiency.
Products
We operate in three segments: Smokeless products, Smoking products and NewGen products. In our Smokeless products segment, we (i) manufacture and market moist snuff and (ii) contract for and market loose leaf chewing tobacco products. In our Smoking products segment, we principally (i) market and distribute cigarette papers, tubes, and related products; and (ii) market and distribute finished cigars and MYO cigar wraps. In our NewGen products segment, we (i) market and distribute CBD, liquid vapor products and certain other products without tobacco and/or nicotine; (ii) distribute a wide assortment of products to non-traditional retail via VaporBeast; and (iii) market and distribute a wide assortment of products to individual consumers via the VaporFi B2C online platforms. Refer to the ‘Recent Developments’ section below for details regarding the Solace acquisition and ReCreation investment.
38
Our portfolio of brands includes some of the most widely recognized names in the OTP industry, such as Stoker’s® in the Smokeless segment, Zig-Zag® in the Smoking segment, and VaporBeast® and VaporFi® in the NewGen segment. The following table sets forth the market share and category rank of our core products and demonstrates their industry positions:
|
Brand
|
Product
|
TPB Segment
|
Market
Share(1) |
Category Rank(1)
|
|
Stoker’s®
|
Chewing Tobacco
|
Smokeless Products
|
20.0%
|
#1 discount, #2 overall
|
|
Stoker’s®
|
Moist Snuff
|
Smokeless Products
|
4.5%
|
#4 discount, #6 overall
|
|
Zig-Zag®
|
Cigarette Papers
|
Smoking Products
|
35.0%
|
#1 premium
|
|
Zig-Zag®
|
MYO Cigar Wraps
|
Smoking Products
|
75.0%
|
#1 overall
|
| (1) | Market share and category rank data for all products are derived from MSAi data as of 12/31/19. |
Operations
As of December 31, 2019, our products are available in approximately 185,000 U.S. retail locations which, with the addition of retail stores in Canada, brings our total North American retail presence to an estimated 210,000 points of distribution. We subscribe to a sales tracking system from MSAi that records all OTP product shipments (ours as well as those of our competitors) from approximately 900 wholesalers to over 250,000 traditional retail stores in the U.S. This system enables us to understand individual product share and volume trends across multiple categories down to the individual retail store level, allowing us to allocate field salesforce coverage to the highest opportunity stores. Our sales and marketing group of approximately 178 professionals utilizes the MSAi system to efficiently target markets and sales channels with the highest sales potential.
Our core tobacco business (Smokeless and Smoking segments) primarily generates revenues from the sale of our products to wholesale distributors who, in turn, resell the products to retail operations. Our acquisition of VaporBeast in 2016 expanded our revenue streams as we began selling directly to non-traditional retail outlets. Our acquisition of IVG in 2018 enhanced our business-to-consumer revenue stream with the addition of the Vapor-Fi online platform. The acquisition of Solace provided us with a line of leading liquids and a powerful new product development platform. Our net sales, which include federal excise taxes, consist of gross sales net of cash discounts, returns, and selling and marketing allowances.
We rely on long-standing relationships with high-quality, established manufacturers to provide the majority of our produced products. More than 80% of our production, as measured by net sales, is outsourced to suppliers. The remaining production consists primarily of our moist snuff tobacco operations located in Dresden, Tennessee, and Louisville, Kentucky and the proprietary e-liquids operations located in Louisville, Kentucky. Our principal operating expenses include the cost of raw materials used to manufacture the limited number of our products which we produce in-house; the cost of finished products, which are generally purchased goods; federal excise taxes; legal expenses; and compensation expenses, including benefits and costs of salaried personnel. Our other principal expenses include interest expense and other expenses.
Key Factors Affecting Our Results of Operations
We consider the following to be the key factors affecting our results of operations:
| • | Our ability to further penetrate markets with our existing products; |
| • | Our ability to introduce new products and product lines that complement our core business; |
| • | Decreasing interest in tobacco products among consumers; |
| • | Price sensitivity in our end-markets; |
| • | Marketing and promotional initiatives, which cause variability in our results; |
| • | General economic conditions, including consumer access to disposable income; |
| • | Cost and increasing regulation of promotional and advertising activities; |
| • | Cost of complying with regulation, including newly passed “deeming regulations”; |
39
| • | Counterfeit and other illegal products in our end-markets; |
| • | Currency fluctuations; |
| • | Our ability to identify attractive acquisition opportunities in OTP; and |
| • | Our ability to integrate acquisitions. |
Recent Developments
Vaping Business Review
The Board of Directors is reviewing strategic alternatives for our third-party vaping distribution business. We are committed to capitalizing on our core competencies in branding, distribution, product development, and regulatory affairs to create market-leading adult actives products. This includes investing in the FDA premarket tobacco product application (“PMTA”) process for our proprietary brands. However, the expected future returns from third-party vaping distribution may not justify the required investment of human and financial resources going forward. There can be no assurance that this process will result in the approval or completion of any particular strategic alternative or transaction in the future. See “Item 1. Business” for further details.
British American Tobacco (“BAT”) Partnership
In December 2019, we announced we had executed a binding letter of intent with BAT’s Canadian subsidiary, its Canadian partner and distributor of Zig-Zag rolling papers (“BAT Canada”). The newly executed agreement provides the foundation for accelerated success in the dynamic Canadian marketplace with stronger TPB Zig-Zag rolling paper margins and the ability to complement the traditional Direct-Store-Delivery network of BAT Canada with supplemental distribution in the alternative channels space, including dispensaries, through our recently established partnership with ReCreation Marketing. Our first Zig-Zag paper purchase order from ReCreation Marketing was received in February 2020.
Share Repurchase Authorization
On February 25, 2020, the TPB board of directors approved a $50 million share repurchase authorization, which is intended for opportunistic execution based upon a variety of factors including marketing dynamics. The program will be subject to the ongoing discretion of the board.
Standard Diversified Inc. (“SDI”) Reorganization
On November 18, 2019, our parent company, SDI, announced plans to pursue a corporate reorganization with us. SDI has indicated that the reorganization is expected to consist of a statutory merger implemented via Delaware law pursuant to which SDI would be merged with a wholly-owned subsidiary of us with us as the survivor of the merger. Pursuant to the merger, which would be designed to constitute a tax-free “downstream reorganization” for U.S. federal income tax purposes, holders of SDI common stock would receive, in turn, for their SDI common stock, shares of our common stock. We have formed a committee to engage in discussions with SDI, but no decisions have been made. There can be no assurance that any definitive agreement will be executed or that any transaction will be approved or consummated.
Solace Technologies Acquisition
In July 2019, we purchased the assets of E-Vape 12, Inc and Solace Technologies LLC (“Solace”) for $9.4 million in total consideration, comprised of $7.7 million in cash and $1.1 million earn-out fair value, and $0.5 million holdback for 18 months, which was adjusted by $0.2 million for a working capital deficiency. The earn-out consists of 44,295 shares of TPB to be issued to the former owners upon the achievement of certain annual milestones. Immediately following the acquisition, 88,582 performance based restricted stock with a fair value of $4.62 million were issued to former owners who became employees. Refer to Note 17 of our Notes to Consolidated Financial Statements for further information. Solace is an innovative product development company that has grown from the creator of one of the leading vape juice brands in the industry into a leader of alternative ingredients product development. We intend to incorporate Solace’s innovative products as well as the legacy vapor products into our Nu-X Ventures development engine.
40
ReCreation Marketing Investment
In July 2019 we obtained a 30% stake in Canadian distribution entity, ReCreation Marketing (“ReCreation”). For $1.0 million paid at closing through our newly created subsidiary, Turning Point Brands (Canada) Inc. We may invest an additional $2.0 million, if certain performance metrics are achieved, with options to acquire up to a 50% ownership position. We received board seats aligned with our ownership position.
ReCreation Marketing is a specialty marketing and distribution firm focused on building brands in the Canadian smoking, vaping and alternative products categories. ReCreation’s management has significant expertise in marketing and distributing tobacco and cannabis products throughout Canada. ReCreation’s management and advisory team has over 50 years combined experience building and managing a portfolio of premium brands, all supported by an expert team of sales associates working across Canada to provide service to over 30,000 traditional retail outlets and newly constructed cannabis dispensaries.
Critical Accounting Policies and Uses of Estimates
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States. When more than one accounting principle, or the method of its application, is generally accepted, we select the principle or method that is appropriate in the specific circumstances. Application of these accounting principles requires us to make estimates about the future resolution of existing uncertainties. Actual results could differ from these estimates. We evaluate our estimates, including those related to revenue recognition, collectability of accounts receivable, inventory valuation and obsolescence, goodwill, intangibles, pension and post-retirement obligations, income taxes, litigation, and contingencies on an ongoing basis. We base these estimates on our historical experience and other assumptions we believe are appropriate under the circumstances. In preparing these consolidated financial statements, we have made our best estimates and judgments of the amounts and disclosures included in the consolidated financial statements.
Revenue Recognition
We adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes nearly all existing revenue recognition guidance under U.S. GAAP, on January 1, 2018. We recognize revenues, which include excise taxes and shipping and handling charges billed to customers, net of cash discounts for prompt payment, sales returns and sales incentives, upon delivery of goods to the customer—at which time our performance obligation is satisfied—at an amount that we expect to be entitled to in exchange for those goods in accordance with the five-step analysis outlined in Topic 606: (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) performance obligations are satisfied. We exclude from the transaction price, sales taxes and value-added taxes imposed at the time of sale (which do not include excise taxes on smokeless tobacco, cigars or vaping products billed to customers).
We record an allowance for sales returns, based principally on historical volume and return rates, which is included in accrued liabilities on the consolidated balance sheets. We record sales incentives, which consist of consumer incentives and trade promotion activities, as a reduction in revenues (a portion of which is based on amounts estimated as being due to wholesalers, retailers and consumers at the end of the period) based principally on historical volume and utilization rates. Expected payments for sales incentives are included in accrued liabilities on the consolidated balance sheets.
A further requirement of ASU 2014-09 is for entities to disaggregate revenue recognized from contracts with customers into categories that depict how the nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factors. Our management views business performance through segments that closely resemble the performance of major product lines. Thus, the primary, and most useful, disaggregation of our contract revenue for decision making purposes is the disaggregation by segment which can be found in Note 21 of our Notes to Consolidated Financial Statements. An additional disaggregation of contract revenue by sales channel can be found within Note 21 as well.
Derivative Instruments
We use foreign currency forward contracts to hedge a portion of our exposure to changes in foreign currency exchange rates from time to time. We account for our forward contracts under the provisions of ASC 815, Derivatives and Hedging. Under our policy, as amended, we may hedge up to 100% of our anticipated purchases of inventory
41
in the denominated invoice currency over a forward period not to exceed twelve months. We may also, from time to time, hedge up to ninety percent of our non-inventory purchases in the denominated invoice currency. Forward contracts that qualify as hedges are adjusted to their fair value through other comprehensive income as determined by market prices on the measurement date except any hedge ineffectiveness which is recognized currently in income. Gains and losses on these contracts are transferred from other comprehensive income into net income as the related inventories are received. Changes in fair value of any contracts that do not qualify for hedge accounting or are not designated as hedges are recognized in income currently.
Interest Rate Swaps
We enter into interest rate swap contracts to manage interest rate risk and reduce the volatility of future cash flows. We account for interest rate swap contracts under the provisions of ASC 815, Derivatives and Hedging. Swap contracts that qualify as hedges are adjusted to their fair value through other comprehensive income as determined by market prices on the measurement date, except any hedge ineffectiveness which is recognized currently in income. Gains and losses on these swap contracts are transferred from other comprehensive income into net income upon settlement of the derivative position or at maturity of the interest rate swap contract. Changes in fair value of any contracts that do not qualify for hedge accounting or are not designated as hedges are recognized currently in income.
Goodwill and Other Intangible Assets
We follow the provisions of ASC 350, Intangibles – Goodwill and Other in accounting for our goodwill and other intangible assets. Goodwill and indefinite-lived intangible assets are reviewed for impairment annually on December 31, or more frequently if certain indicators are present, in accordance with ASC 350-20-35 and ASC 350-30-35, respectively. If the carrying value of the goodwill or indefinite-life intangible asset exceeds its fair value, determined using the discounted cash flows method and the relief-from-royalty method, respectively, the goodwill or intangible asset is considered impaired. The carrying value of the goodwill or indefinite-life intangible asset would then be reduced to fair value. For goodwill, the determination of a reporting unit’s fair value involves, among other things, our market capitalization and application of the income approach, which includes developing forecasts of future cash flows and determining an appropriate discount rate.
Based on our annual goodwill impairment testing, the estimated fair values of each of our reporting units were in excess of the respective carrying values at December 31, 2019. We had no such impairment of goodwill or other intangible assets during the year ended December 31, 2019. However, there could be an impairment of the goodwill of the NewGen reporting unit if future revenues do not achieve our expected future cash flows or if macroeconomic conditions result in future increases in the weighted average cost of capital used to estimate fair value. Refer to Note 10 of Notes to Consolidated Financial Statements for further details regarding our goodwill and other intangible assets as of December 31, 2019.
Fair Value
GAAP establishes a framework for measuring fair value. That framework provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1) and the lowest priority to unobservable inputs (level 3). The three levels of the fair value hierarchy under GAAP are described below:
| • | Level 1 – Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets at the measurement date. |
| • | Level 2 – Inputs to the valuation methodology include: quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in inactive markets; inputs other than quoted prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
| • | Level 3 – Unobservable inputs that reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. |
Under GAAP, certain convertible debt instruments that may be settled in cash on conversion are required to be separately accounted for as liability and equity components of the instrument in a manner that reflects the issue’s non-convertible debt borrowing rate. Accordingly, in accounting for the issuance of the Convertible Senior Notes, we
42
separated the Convertible Senior Notes into liability and equity components. The carrying amount of the liability component was calculated by measuring the fair value of a similar liability that does not have an associated convertible feature. This evaluation can be complex and requires management to make assumptions to determine the fair value.
Retirement Plans
We follow the provisions of ASC 715, Compensation – Retirement Benefits in accounting for our retirement plans, which requires an employer to (i) recognize in its statement of financial position the funded status of a benefit plan, measured as the difference between the fair value of plan assets and benefit obligations; (ii) recognize, net of tax, the gains or losses and prior service costs or credits that arise during the period but are not recognized as components of net periodic benefit cost; and (iii) measure defined benefit plan assets and obligations as of the date of the employer’s statement of financial position.
Income Taxes
We account for income taxes under ASC 740. We record the effects of income taxes under the liability method in which deferred income tax assets and liabilities are recognized based on the difference between the financial and tax basis of assets and liabilities using the enacted tax rates in effect for the years in which the differences are expected to reverse. We assess our ability to realize future benefits of deferred tax assets by determining if they meet the “more likely than not” criteria in ASC 740, Income Taxes. If we determine that future benefits do not meet the “more likely than not” criteria, a valuation allowance is recorded.
Stock-Based Compensation
We measure stock compensation costs related to our stock options on the fair value-based method under the provisions of ASC 718, Compensation – Stock Compensation, which requires compensation cost for stock options to be recognized based on the fair value of stock options granted. We determined the fair value of these awards using the Black-Scholes option pricing model.
We grant performance-based restricted stock units (“PRSU”) subject to both performance-based and service-based vesting conditions. The fair value of each PRSU is our stock price on the date of grant. For purposes of recognizing compensation expense as services are rendered in accordance with ASC 718, we assume all employees involved in the PRSU grant will provide service through the end of the performance period. Stock compensation expense is recorded based on the probability of achievement of the performance conditions specified in the PRSU grant.
Accounts Receivable
Accounts receivable are recognized at their net realizable value. All accounts receivable are trade-related and are recorded at the invoiced amount and do not bear interest. We maintain allowances for doubtful accounts receivable for estimated uncollectible invoices resulting from the customer’s inability to pay, which may result in write-offs. We recorded an allowance for doubtful accounts of $0.3 million and less than $0.1 million at December 31, 2019 and 2018, respectively.
Inventories
Inventories are stated at the lower of cost or market. Cost was determined using the LIFO method for approximately 49.4% of the inventories as of December 31, 2019. Leaf tobacco is presented in current assets in accordance with standard industry practice, notwithstanding the fact that such tobaccos are carried longer than one year for the purpose of curing. We recorded an inventory valuation allowance of $21.5 million and $2.5 million at December 31, 2019 and 2018, respectively.
Jumpstart Our Business Startups Act of 2012
We chose to “opt out” of the provision of the JOBS Act that permits us, as an “emerging growth company,” to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. As a result, we will comply with new or revised accounting standards as required for public companies. Our decision to opt out of the extended transition period provided in the JOBS Act is irrevocable.
43
Results of Operations
Summary
The table and discussion set forth below relates to our consolidated results of operations for the years ended December 31 (in thousands):
For the year ended December 31, |
|||||||||||||||
2019 |
2018 |
% Change |
2017 |
% Change |
|||||||||||
Consolidated Results of Operations Data: |
|||||||||||||||
Net sales |
|||||||||||||||
Smokeless products |
$ | 99,894 | $ | 90,031 | 11.0 | % |
$ | 84,560 | 6.5 | % |
|||||
Smoking products |
108,733 | 111,507 | -2.5 | % |
109,956 | 1.4 | % |
||||||||
NewGen products |
153,362 | 131,145 | 16.9 | % |
91,261 | 43.7 | % |
||||||||
Total net sales |
361,989 | 332,683 | 8.8 | % |
285,777 | 16.4 | % |
||||||||
Cost of sales |
225,243 | 190,124 | 18.5 | % |
160,807 | 18.2 | % |
||||||||
Gross profit |
|||||||||||||||
Smokeless products |
52,277 | 46,490 | 12.4 | % |
42,703 | 8.9 | % |
||||||||
Smoking products |
59,386 | 57,043 | 4.1 | % |
57,146 | -0.2 | % |
||||||||
NewGen products |
25,083 | 39,026 | -35.7 | % |
25,121 | 55.4 | % |
||||||||
Total gross profit |
136,746 | 142,559 | -4.1 | % |
124,970 | 14.1 | % |
||||||||
Selling, general, and administrative expenses |
109,887 | 94,075 | 16.8 | % |
75,290 | 25.0 | % |
||||||||
Operating income |
26,859 | 48,484 | -44.6 | % |
49,680 | -2.4 | % |
||||||||
Interest expense, net |
17,342 | 14,819 | 17.0 | % |
16,889 | -12.3 | % |
||||||||
Investment income |
(2,648 | ) |
(424 | ) |
524.5 | % |
(438 | ) |
-3.2 | % |
|||||
Loss on extinguishment of debt |
1,308 | 2,384 | -45.1 | % |
6,116 | -61.0 | % |
||||||||
Net periodic benefit (income) cost, excluding service cost |
(4,961 | ) |
131 | -3887.0 | % |
180 | -27.2 | % |
|||||||
Income before income taxes |
15,818 | 31,574 | -49.9 | % |
26,933 | 17.2 | % |
||||||||
Income tax expense |
2,044 | 6,285 | -67.5 | % |
7,280 | -13.7 | % |
||||||||
Consolidated net income |
13,774 | 25,289 | -45.5 | % |
19,653 | 28.7 | % |
||||||||
Net loss attributable to non-controlling interest |
— | — | NM | (556 | ) |
-100.0 | % |
||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | -45.5 | % |
$ | 20,209 | 25.1 | % |
|||||
Comparison of Year Ended December 31, 2019, to Year Ended December 31, 2018
Net Sales. For the year ended December 31, 2019, overall net sales increased to $362.0 million from $332.7 million for the year ended December 31, 2018, an increase of $29.3 million or 8.8%. The increase in net sales was primarily driven by Stoker’s MST, Zig-Zag cigar wraps, and Nu-X including the acquisition of Solace in 2019.
For the year ended December 31, 2019, net sales in the Smokeless products segment increased to $99.9 million from $90.0 million for the year ended December 31, 2018, an increase of $9.9 million or 11.0%. For the year ended December 31, 2019, Smokeless products volume increased 7.3% and price/mix increased 3.7%. The increase in net sales was primarily driven by the continuing growth of Stoker’s® MST partially offset by declines in chewing tobacco attributable to increased competition, our promotional timing, and a continuing segment shift to lower price products. MST represented 54% of Smokeless revenue in 2019, up from 47% a year earlier.
For the year ended December 31, 2019, net sales in the Smoking products segment decreased to $108.7 million from $111.5 million for the year ended December 31, 2018, a decrease of $2.8 million or 2.5%. For the year ended December 31, 2019, Smoking products volumes decreased 4.9%, while price/mix increased 2.4%. The decrease in net sales is primarily due to the delay of Canadian paper orders in the first half of the year as a result of the new packaging regulations in Canada as well as our strategic decision to de-emphasize the low margin cigar and MYO / pipe products businesses. Cigar and MYO / pipe product sales declined by $2.4 million to $7.2 million in the year ended December 31, 2019.
44
For the year ended December 31, 2019, net sales in the NewGen products segment increased to $153.4 million from $131.1 million for the year ended December 31, 2018, an increase of $22.2 million or 16.9%. The increase in net sales was primarily driven by higher Nu-X alternative products sales in 2019 (includes the Solace acquisition) and an additional eight months of IVG net sales in 2019. Net sales were negatively impacted by the vape disruption in the fourth quarter of 2019.
Gross Profit. For the year ended December 31, 2019, overall gross profit decreased to $136.7 million from $142.6 million for the year ended December 31, 2018, a decrease of $5.8 million or 4.1%, primarily as a result of certain restructuring activities in the fourth quarter 2019. Consolidated gross profit for the year ended December 31, 2019, included $0.4 million of unfavorable LIFO adjustments, $1.2 million of introductory launch costs, and $23.0 million of restructuring costs, primarily inventory reserves, compared to $0.1 million, $1.0 million, and $2.9 million, respectively, in the year ended December 31, 2018. Gross profit as a percentage of net sales weakened to 37.8% for the year ended December 31, 2019, from 42.9% for the year ended December 31, 2018, primarily due to the aforementioned restructuring expenses, including the inventory reserves and write-off associated with our pivot from third-party vaping products.
For the year ended December 31, 2019, gross profit in the Smokeless products segment increased to $52.3 million from $46.5 million for the year ended December 31, 2018, an increase of $5.8 million or 12.4%. Smokeless gross profit for the year ended December 31, 2019, included $0.3 million of unfavorable LIFO adjustments and $0.0 million of introductory launch costs compared to $0.1 million and $0.2 million, respectively, for the year ended December 31, 2018. Gross profit as a percentage of net sales increased to 52.3% of net sales for the year ended December 31, 2019, from 51.6% of net sales for the year ended December 31, 2018 driven by Stoker MST gains.
For the year ended December 31, 2019, gross profit in the Smoking products segment increased to $59.4 million from $57.0 million for the year ended December 31, 2018, an increase of $2.3 million or 4.1%. Smoking gross profit for the year ended December 31, 2018 included $0.6 million of introductory launch costs and $1.3 million of line rationalization expenses. Gross profit as a percentage of net sales increased to 54.6% of net sales for the year ended December 31, 2019, from 51.2% of net sales for the year ended December 31, 2018. The increase in gross profit as a percentage of net sales is primarily due to declining sales of lower margin, low priority products.
For the year ended December 31, 2019, gross profit in the NewGen products segment decreased to $25.1 million from $39.0 million for the year ended December 31, 2018, a decrease of $13.9 million or 35.7%. NewGen gross profit for the year ended December 31, 2019, included $1.2 million of introductory launch costs and $23.2 million of restructuring expenses compared to $0.3 million and $1.5 million, respectively, for the year ended December 31, 2018. Additionally, gross profit includes $9.3 million of tariff expenses in 2019 compared to $1.1 million in 2018. Gross profit as a percentage of net sales decreased to 16.4% of net sales for the year ended December 31, 2019, from 29.8% of net sales for the year ended December 31, 2018, primarily due to the aforementioned restructuring expenses associated with our pivot from third-party vaping products.
Selling, General and Administrative Expenses. For the year ended December 31, 2019, selling, general and administrative expenses increased to $109.9 million from $94.1 million for the year ended December 31, 2018, an increase of $15.8 million or 16.8%. Selling, general, and administrative expenses for the year ended December 31, 2019, include $1.7 million of expenses relating to the inclusion of our 2019 investment in Solace, $1.8 million of transaction costs (primarily relating to Solace and ReCreation as well as earnout expense for IVG), $5.0 million of introductory launch costs, $3.2 million of restructuring expenses, and $2.2 million in PMTA expenses. Selling, general, and administrative expenses for the year ended December 31, 2018, include $4.5 million of transaction and strategic initiative costs (primarily relating to IVG and Vapor Supply transaction costs), $0.9 million of company-wide introductory launch costs, and $1.8 million of restructuring costs.
Interest Expense, net. For the year ended December 31, 2019, interest expense, on a net basis, increased to $17.3 million from $14.8 million for the year ended December 31, 2018, primarily as a result of the amortization of the discount on the Convertible Senior Notes in 2019 of $2.9 million.
Investment Income. For the year ended December 31, 2019, investment income increased to $2.6 million from $0.4 million for the year ended December 31, 2018, primarily due to the $2.0 million gain on the CASH investment as a result of marking the investment to fair value.
45
Loss on Extinguishment of Debt. For the year ended December 31, 2019, loss on extinguishment of debt was $1.3 million as the result of paying off the 2018 Second Lien Credit Facility. For the year ended December 31, 2018, loss on extinguishment of debt was $2.4 million as the result of refinancing our credit facility in the first quarter of 2018.
Net periodic benefit (income) cost, excluding service cost. For the year ended December 31, 2019, net periodic income was $5.0 million primarily due to the gain on the termination of the postretirement plan. For the year ended December 31, 2018, net periodic benefit cost was $0.1 million.
Income Tax Expense. The Company’s income tax expense of $2.0 million, or 12.9% of income before income taxes, for the year ended December 31, 2019, is lower than the expected annual effective tax rate as a result of discrete tax benefits of $4.6 million from the exercise of stock options during the year. The Company’s income tax expense of $6.3 million, or 19.9% of income before income taxes, for the year ended December 31, 2018, is lower than the expected annual effective tax rate as a result of discrete tax benefits of $5.4 million from the exercise of stock options during the year.
Consolidated Net Income. Due to the factors described above, net income for the year ended December 31, 2019 and 2018, was $13.7 million and $25.3 million, respectively.
Comparison of Year Ended December 31, 2018, to Year Ended December 31, 2017
Net Sales. For the year ended December 31, 2018, overall net sales increased to $332.7 million from $285.8 million for the year ended December 31, 2017, an increase of $46.9 million or 16.4%. The increase in net sales was primarily driven by continued VaporBeast momentum and the acquisitions of Vapor Supply and IVG.
For the year ended December 31, 2018, net sales in the Smokeless products segment increased to $90.0 million from $84.6 million for the year ended December 31, 2017, an increase of $5.5 million or 6.5%. For the year ended December 31, 2018, Smokeless products volume increased 2.6% and price/mix increased 3.9%. The increase in net sales was primarily driven by the continuing growth of Stoker’s® MST partially offset by declines in chewing tobacco attributable to increased competition, our promotional timing, and a continuing segment shift to lower price products.
For the year ended December 31, 2018, net sales in the Smoking products segment increased to $111.5 million from $110.0 million for the year ended December 31, 2017, an increase of $1.6 million or 1.4%. For the year ended December 31, 2018, Smoking products volumes decreased 0.7%, while price/mix decreased 0.7%. The increase in net sales is primarily due to volume growth for our Zig-Zag® branded papers and cigar wraps offset by our strategic decision to de-emphasize the low margin cigar products business and line rationalization of our MYO tobacco products. Cigar product sales declined by $3.0 million to $5.5 million in the year ended December 31, 2018.
For the year ended December 31, 2018, net sales in the NewGen products segment increased to $131.1million from $91.3 million for the year ended December 31, 2017, an increase of $39.9 million or 43.7%. The increase in net sales was primarily driven by continued VaporBeast momentum along with the acquisitions of Vapor Supply and IVG.
Gross Profit. For the year ended December 31, 2018, overall gross profit increased to $142.6 million from $125.0 million for the year ended December 31, 2017, an increase of $17.6 million or 14.1%, primarily due to growth in the NewGen segment. Consolidated gross profit for the year ended December 31, 2018, included $0.1 million of unfavorable LIFO adjustments, $1.0 million of introductory launch costs and $2.9 million of product line rationalizations compared to $1.1 million, $0.7 million and $0.5 million, respectively, in the year ended December 31, 2017. Gross profit as a percentage of net sales weakened to 42.9% for the year ended December 31, 2018, from 43.7% for the year ended December 31, 2017, primarily due to the majority of the sales growth coming from the NewGen segment, which has lower margins.
For the year ended December 31, 2018, gross profit in the Smokeless products segment increased to $46.5 million from $42.7 million for the year ended December 31, 2017, an increase of $3.8 million or 8.9%. Smokeless gross profit for the year ended December 31, 2018, included $0.1 million of unfavorable LIFO adjustments, $0.2 million of introductory launch costs, and $0.1 million of restructuring expenses compared to $0.7 million, $0.7 million, and less than $0.1 million, respectively, for the year ended December 31, 2017. Gross profit as a percentage of net sales increased to 51.6% of net sales for the year ended December 31, 2018, from 50.5% of net sales for the year ended December 31, 2017.
46
For the year ended December 31, 2018, gross profit in the Smoking products segment decreased to $57.0 million from $57.1 million for the year ended December 31, 2017, a decrease of $0.1 million or 0.2%. Smoking gross profit for the year ended December 31, 2018, included less than $0.1 million of unfavorable LIFO adjustments, $0.6 million of introductory launch costs, and $1.3 million of restructuring expenses compared to $0.4 million, $0.0, and $0.2 million, respectively, for the year ended December 31, 2017. Gross profit as a percentage of net sales decreased to 51.2% of net sales for the year ended December 31, 2018, from 52.0% of net sales for the year ended December 31, 2017. The decrease in gross profit as a percentage of net sales is primarily due to introductory launch costs and restructuring expenses on discontinued products.
For the year ended December 31, 2018, gross profit in the NewGen products segment increased to $39.0 million from $25.1 million for the year ended December 31, 2017, an increase of $13.9 million or 55.4%. NewGen gross profit for the year ended December 31, 2018, included $0.3 million of introductory launch costs and $1.5 million of restructuring expenses compared to less than $0.1 million, $0.2 million, and $0, respectively, for the year ended December 31, 2017. Additionally, the Company paid $2.8 million for newly imposed tariffs on goods from outside the United States in 2018, $1.1 million of which was included in cost of goods sold for the year ended December 31, 2018. Gross profit as a percentage of net sales increased to 29.8% of net sales for the year ended December 31, 2018, from 27.5% of net sales for the year ended December 31, 2017, primarily due to acquisition activity which has resulted in business-to-consumer sales, which generally have higher margins, becoming a larger share of the NewGen segment.
Selling, General and Administrative Expenses. For the year ended December 31, 2018, selling, general and administrative expenses increased to $94.1 million from $75.3 million for the year ended December 31, 2017, an increase of $18.8 million or 25.0%. Selling, general, and administrative expenses for the year ended December 31, 2018, include $10.5 million of expenses relating to the inclusion of our 2018 acquisitions IVG and Vapor Supply, $4.5 million of transaction costs (primarily relating to IVG and Vapor Supply) and strategic initiatives, $0.9 million of introductory launch costs, $1.8 million of restructuring costs, and a $2.0 million net reduction to selling, general and administrative expenses related to the VMR Loan. Selling, general, and administrative expenses for the year ended December 31, 2017, included $2.1 million of transaction and strategic initiative costs, $1.7 million of launch costs, and $0.1 million of restructuring expenses. Other items leading to the increase in selling, general, and administrative expenses in the year ended December 31, 2018, when compared to the year ended December 31, 2017, include higher legal and litigation expenses associated with our anti-counterfeiting initiative and variable logistics costs associated with increased sales at VaporBeast partially offset by a receivable reserve reversal and prepayment penalty, both of which are associated with the loan issued to a supplier in the second quarter of 2018 that was repaid during the third quarter of 2018.
Interest Expense, net. For the year ended December 31, 2018, interest expense, on a net basis, decreased to $14.8 million from $16.9 million for the year ended December 31, 2017, primarily as a result of lower interest rates from our March 2018 refinancing of our credit facility.
Investment Income. For the year ended December 31, 2018 and 2017, investment income relating to investments of the MSA escrow deposits was $0.4 million and $0.4 million, respectively.
Loss on Extinguishment of Debt. For the year ended December 31, 2018, loss on extinguishment of debt was $2.4 million as the result of refinancing our credit facility in the first quarter of 2018. For the year ended December 31, 2017, loss on extinguishment of debt was $6.1 million as the result of refinancing our credit facility in the first quarter of 2017.
Net Periodic Benefit Cost, excluding service cost. For the year ended December 31, 2018, net periodic benefit cost, excluding service cost was $0.1 million. For the year ended December 31, 2017, net periodic benefit cost was $0.2 million.
Income Tax Expense. The Company’s income tax expense of $6.3 million, or 19.9% of income before income taxes, for the year ended December 31, 2018, is lower than the expected annual effective tax rate as a result of discrete tax benefits of $5.4 million from the exercise of stock options during the year. The Company’s income tax expense of $7.3 million, or 27% of income before income taxes, for the year ended December 31, 2017, is lower than the expected annual effective tax rate as a result of discrete tax benefits of $4.2 million from the exercise of stock options during the year.
47
Consolidated Net Income. Due to the factors described above, net income for the year ended December 31, 2018 and 2017, was $25.3 million and $19.7 million, respectively.
Net Loss Attributable to Non-Controlling Interest. Net loss attributable to non-controlling interest of $0.6 million for the year ended December 31, 2017, is related to Vapor Shark, which was accounted for as a VIE during the second quarter of 2017.
Net Income Attributable to Turning Point Brands, Inc. Due to the factors described above, net income for the year ended December 31, 2018 and 2017, was $25.3 million and $20.2 million, respectively.
Liquidity and Capital Reserves
Our principal uses for cash are working capital, debt service, and capital expenditures. We believe our cash flows from operations and borrowing availability under our 2018 Revolving Credit Facility (as defined herein) are adequate to satisfy our operating cash requirements for the foreseeable future.
Our working capital, which we define as current assets less current liabilities, increased $85.3 million to $133.4 million at December 31, 2019, compared with $48.1 million at December 31, 2018. The increase in working capital is primarily due to the cash proceeds from the issuance of the Convertible Senior Notes.
As of |
||||||
(in thousands) |
December 31, 2019 |
December 31, 2018 |
||||
Current assets |
$ | 189,250 | $ | 111,854 | ||
Current liabilities |
55,886 | 63,766 | ||||
Working capital |
$ | 133,364 | $ | 48,088 | ||
During the year ended December 31, 2019, we invested $4.8 million in capital expenditures. We had unrestricted cash on hand of $95.3 million and $3.3 million as of December 31, 2019 and 2018, respectively. We had restricted assets of $32.1 million and $30.6 million as of December 31, 2019 and 2018, respectively. Restricted assets consist of escrow deposits under the MSA. On the 25th anniversary of each annual deposit, we are entitled to receive reimbursement of the principal amount of escrow remaining for that year. See “Master Settlement Agreement” below for details.
Cash Flows from Operating Activities
For the year ended December 31, 2019, net cash provided by operating activities increased to $37.8 million from $13.1 million for the year ended December 31, 2018, an increase of $24.7 million or 189%. Primarily due to inventory buys in 2018 that reduced cash flow.
For the year ended December 31, 2018, net cash provided by operating activities decreased to $13.1 million from $29.7 million for the year ended December 31, 2017, a decrease of $16.6 million or 56%, primarily due to inventory increases from pre-tariff inventory buys within our existing operations.
Cash Flows from Investing Activities
For the year ended December 31, 2019, net cash provided by investing activities was $15.9 million compared to cash used in investing activities of $24.7 million for the year ended December 31, 2018, an increase of $40.6 million or 164%, primarily due to the change in MSA escrow deposits from investments to cash holdings as well as lower cash paid for acquisitions.
For the year ended December 31, 2018, net cash used in investing activities increased to $24.7 million from $1.9 million for the year ended December 31, 2017, an increase of $23.6 million or 2110%, primarily due to the Vapor Supply and IVG acquisitions.
Cash Flows from Financing Activities
For the year ended December 31, 2019, net cash provided by financing activities increased to $68.0 million from $9.9 million for the year ended December 31, 2018, an increase of $58.0 million or 584%, primarily due to the proceeds from the issuance of the Convertible Senior Notes offset by payments on the 2018 Revolving Credit Facility, the 2018 Second Lien Credit Facility and payment for the call options.
48
For the year ended December 31, 2018, net cash provided by financing activities was $9.9 million compared to net cash used in financing activities of $28.0 million for the year ended December 31, 2017, an increase of $37.9 million, primarily due to borrowings against our 2018 Revolving Credit Facility to fund our investing activities.
Long-Term Debt
Notes payable and long-term debt consisted of the following at December 31, 2019 and 2018, in order of preference:
December 31, 2019 |
December 31, 2018 |
|||||
2018 Revolving Credit Facility |
$ | — | $ | 26,000 | ||
2018 First Lien Term Loan |
146,000 | 154,000 | ||||
2018 Second Lien Term Loan |
— | 40,000 | ||||
Convertible Senior Notes |
172,500 | — | ||||
Note payable - IVG |
4,240 | 4,000 | ||||
Gross notes payable and long-term debt |
322,740 | 224,000 | ||||
Less deferred finance charges |
(6,466 | ) |
(3,285 | ) |
||
Less debt discount |
(32,083 | ) |
— | |||
Less revolving credit facility |
— | (26,000 | ) |
|||
Less current maturities |
(15,240 | ) |
(8,000 | ) |
||
Net notes payable and long-term debt |
$ | 268,951 | $ | 186,715 | ||
2018 Credit Facility
On March 7, 2018, the Company entered into a $250 million credit facility consisting of a $160 million 2018 First Lien Term Loan with Fifth Third Bank, as administrative agent, and other lenders, and a $50 million 2018 Revolving Credit Facility (collectively, the “2018 First Lien Credit Facility”) in addition to a $40 million 2018 Second Lien Term Loan (together with the 2018 First Lien Credit Facility, the “2018 Credit Facility”) with Prospect Capital Corporation, as administrative agent, and other lenders. The 2018 Credit Facility retained the $40 million accordion feature of the 2017 Credit Facility. Proceeds from the 2018 Credit Facility were used to repay, in full, the 2017 Credit Facility. The Company incurred a loss on extinguishment of debt of $2.4 million in the first quarter of 2018 as a result of the refinancing.
The 2018 Credit Facility contains customary events of default including payment defaults, breaches of representations and warranties, covenant defaults, cross-defaults to certain other material indebtedness in excess of specified amounts, certain events of bankruptcy and insolvency, certain ERISA events, judgments in excess of specified amounts, and change in control defaults. The 2018 Credit Facility also contains certain negative covenants customary for facilities of these types including covenants that, subject to exceptions described in the 2018 Credit Facility, restrict the ability of the Company and its subsidiary guarantors: (i) to pledge assets, (ii) to incur additional indebtedness, (iii) to pay dividends, (iv) to make distributions, (v) to sell assets, and (vi) to make investments. Refer to Note 23 of Notes to Consolidated Financial Statements for further information regarding dividend restrictions.
2018 First Lien Credit Facility: The 2018 First Lien Term Loan and the 2018 Revolving Credit Facility bear interest at LIBOR plus a spread of 2.75% to 3.50% based on our senior leverage ratio. The 2018 First Lien Term Loan has quarterly required payments of $2.0 million beginning June 30, 2018, increasing to $3.0 million on June 30, 2020, and increasing to $4.0 million on June 30, 2022. The 2018 First Lien Credit Facility has a maturity date of March 7, 2023. The 2018 First Lien Term Loan is secured by a first priority lien on substantially all of the assets of the borrowers and the guarantors thereunder, including a pledge of our capital stock, other than certain excluded assets (the “Collateral”). In connection with the Senior Notes offering, we entered into a First Amendment (“the Amendment”) to the First Lien Credit Agreement, with Fifth Third Bank, as administrative agent, and other lenders and certain other lending other lending parties thereto. The Amendment was entered into primarily to permit us to issue up to $200 million of convertible senior notes, enter into certain capped call transactions in connection with the issuance of such notes and to use the proceeds from the issuance of the notes to repay amounts outstanding under our Second Lien Credit Agreement and use the remaining proceeds for acquisitions and investments. In connection with the Amendment, fees of $0.7 million were incurred. The 2018 First Lien Credit Facility contains
49
certain financial covenants, which were amended in connection with the Convertible Senior Notes offering in the third quarter 2019. The covenants include maximum senior leverage ratio of 3.00x with step-downs to 2.50x, a maximum total leverage ratio of 5.50x with step-downs to 5.00x, and a minimum fixed charge coverage ratio of 1.20x. In the first quarter of 2020, the financial covenants were amended to permit certain add-backs related to PMTA in the definition of Consolidated EBITDA for the period of October 1, 2019 until September 30, 2020. Based on an excess cash covenant for the facility, a principal payment of $4.5 million was due in the second quarter 2019. All parties agreed to waive the payment, resulting in consent fees of $0.1 million. The weighted average interest rate of the 2018 First Lien Term Loan was 4.55% at December 31, 2019. At December 31, 2019, we had no borrowings outstanding under the 2018 Revolving Credit Facility. The $50.0 million unused portion of the 2018 Revolving Credit Facility is reduced by letters of credit from Fifth Third Bank totaling $3.7 million, resulting in $46.3 million of availability under the 2018 Revolving Credit Facility at December 31, 2019.
2018 Second Lien Credit Facility: The 2018 Second Lien Credit Facility bore interest at a rate of LIBOR plus 7.00% and had a maturity date of March 7, 2024. The 2018 Second Lien Term Loan was secured by a second priority interest in the Collateral and was guaranteed by the same entities as the 2018 First Lien Term Loan. The 2018 Second Lien Credit Facility contained certain financial covenants including a maximum senior leverage ratio of 3.75x with step-downs to 3.50x, a maximum total leverage ratio of 4.75x with step-downs to 4.50x, and a minimum fixed charge coverage ratio of 1.10x. Based on an excess cash covenant for the facility, a $4.5 million principal payment was made in the second quarter 2019, resulting in $0.2 million loss on extinguishment of debt. We used a portion of the proceeds from the issuance of the Convertible Senior Notes to prepay all outstanding amounts related to the 2018 Second Lien Credit Facility in the third quarter 2019. The principal paid in the third quarter 2019 amounted to $35.5 million, and the transaction resulted in a $1.2 million loss on extinguishment of debt.
Convertible Senior Notes
In July 2019 we closed an offering of $172.5 million in aggregate principal amount of our 2.50% Convertible Senior Notes due July 15, 2024 (the “Convertible Senior Notes”). The Convertible Senior Notes bear interest at a rate of 2.50% per year, payable semiannually in arrears on January 15 and July 15 of each year, beginning on January 15, 2020. The Convertible Senior Notes will mature on July 15, 2024, unless earlier repurchased, redeemed or converted. The Convertible Senior Notes are senior unsecured obligations.
The Convertible Senior Notes are convertible into approximately 3,202,808 shares of our voting common stock under certain circumstances prior to maturity at a conversion rate of 18.567 shares per $1,000 principal amount of the Convertible Senior Notes, which represents a conversion price of approximately $53.86 per share, subject to adjustment under certain conditions, but will not be adjusted for any accrued and unpaid interest. Upon conversion, we may pay cash, shares of our common stock or a combination of cash and stock, as determined by us at our discretion. The conditions required to allow the holders to convert their Convertible Senior Notes were not met as of December 31, 2019.
Under GAAP, certain convertible debt instruments that may be settled in cash on conversion are required to be separately accounted for as liability and equity components of the instrument in a manner that reflects the issuer’s non-convertible debt borrowing rate. Accordingly, in accounting for the issuance of the Convertible Senior Notes, we separated the Convertible Senior Notes into liability and equity components. The carrying amount of the liability component was calculated by measuring the fair value of a similar liability that does not have an associated convertible feature. The carrying amount of the equity component, which is recognized as a debt discount, represents the difference between the proceeds from the issuance of the Convertible Senior Notes and the fair value of the liability component of the Convertible Senior Notes. The excess of the principal amount of the liability component over its carrying amount (“debt discount”), $35.0 million, will be amortized to interest expense using an effective interest rate of 7.5% over the expected life of the Convertible Senior Notes. The equity component is not remeasured as long as it continues to meet the criteria for equity classification. Interest expense includes $2.9 million of amortization for the year ended December 31, 2019.
In accounting for the debt issuance costs related to the issuance of the Convertible Senior Notes, we allocated the total amount incurred to the liability and equity components based on their relative values. Debt issuance costs attributable to the liability component are amortized to the interest expense using the effective interest method over the expected life of the Convertible Senior Notes, $4.7 million, and the debt issuance costs attributable to the equity component, $1.2 million, are netted with the equity component of stockholders’ equity (deficit).
50
In connection with the Convertible Senior Notes offering, we entered into privately negotiated capped call transactions with certain financial institutions. The capped call transactions have a strike price of $53.86 per and a cap price of $82.86 per, and are exercisable when, and if, the Convertible Senior Notes are converted. We paid $20.53 million for these capped calls and charged that amount to additional paid-in capital.
Note Payable – IVG
In September 2018, the Company issued a note payable to IVG’s former shareholders (“IVG Note”). The IVG Note is $4.0 million principal with 6.0% interest compounding annually and matures on March 5, 2020. The IVG Note is subject to customary defaults including defaults for nonpayment, nonperformance, any material breach under the purchase agreement, and bankruptcy or insolvency. The carrying amount of the IVG Note is $4.2 million as of December 31, 2019.
Distribution Agreements
For a description of our material distribution agreements, see “Business—Distribution and Supply Agreements.”
Master Settlement Agreement
On November 23, 1998, the major U.S. cigarette manufacturers, Philip Morris USA, Inc., Brown & Williamson Tobacco Corporation, Lorillard Tobacco Company and R.J. Reynolds Tobacco Company, entered into the MSA with attorneys general representing states that agreed to settle certain recovery actions (the “Settling States”). In order to be in compliance with the MSA and subsequent states’ statutes, we were required to fund an escrow account with each of the Settling States based on the number of cigarettes or cigarette equivalents (which is measured by pounds of MYO cigarette smoking tobacco) sold in such state. Funding of the escrow deposit by us in 2018 was less than $0.1 million in respect of sales of smoking products in 2017. We estimate the total deposits relating to 2018 sales will be less than $0.1 million. Under current MSA legislation, we will not be required to make escrow deposits after making deposits for 2017 sales as our last remaining product line subject to MSA legislation, MYO cigarette smoking tobacco, was discontinued in the third quarter of 2017. Each year’s deposit will be released from escrow after 25 years. We are scheduled to begin receiving payments as our escrow deposits are released from escrow beginning in 2024.
The following table summarizes our escrow deposit balances (in thousands) by sales year as of:
Sales Year |
Deposits as of December 31, |
|||||
2019 |
2018 |
|||||
1999 |
$ | 211 | $ | 211 | ||
2000 |
1,017 | 1,017 | ||||
2001 |
1,673 | 1,673 | ||||
2002 |
2,271 | 2,271 | ||||
2003 |
4,249 | 4,249 | ||||
2004 |
3,714 | 3,714 | ||||
2005 |
4,553 | 4,552 | ||||
2006 |
3,847 | 3,847 | ||||
2007 |
4,167 | 4,167 | ||||
2008 |
3,364 | 3,364 | ||||
2009 |
1,619 | 1,619 | ||||
2010 |
406 | 406 | ||||
2011 |
193 | 193 | ||||
2012 |
199 | 199 | ||||
2013 |
173 | 173 | ||||
2014 |
143 | 143 | ||||
2015 |
101 | 101 | ||||
2016 |
91 | 91 | ||||
2017 |
83 | 83 | ||||
Total |
$ | 32,074 | $ | 32,073 | ||
51
Off-balance Sheet Arrangements
During 2019 we did not execute any forward contracts. During 2018 we executed various forward contracts, none of which met hedge accounting requirements, for the purchase of €14.5 million with maturity dates ranging from March 2018 to January 2019. During 2017, we executed no forward contracts. At December 31, 2019 and 2018, we had forward contracts for the purchase of €0.0 million and €1.5 million, respectively. The Company had swap contracts for a total notional amount of $70 million at December 31,2019 and 2018. The fair values of the swap contracts are based upon quoted market prices and resulted in a liability of $2.5 million and $0.9 million, respectively, as of December 31, 2019 and 2018, included in other long-term liabilities.
Contractual Obligations
The following table summarizes our contractual obligations at December 31, 2019 (in thousands):
Payments due by period |
|||||||||||||||
Total |
Less than 1 year |
1-3 years |
4-5 years |
More than 5 years |
|||||||||||
Long-term debt obligations, including interest |
$ | 362,850 | $ | 25,907 | $ | 51,230 | $ | 285,713 | $ | — | |||||
Operating lease obligations |
16,926 | 2,924 | 4,895 | 2,810 | 6,297 | ||||||||||
Purchase obligations |
23,930 | 23,930 | — | — | — | ||||||||||
$ | 403,706 | $ | 52,761 | $ | 56,125 | $ | 288,523 | $ | 6,297 | ||||||
The total lease expense included in the consolidated statements of income for the years ended December 31, 2019, 2018, and 2017, was $4.3 million, $3.6 million, and $2.6 million, respectively.
Inflation
We believe that any effect of inflation at current levels will be minimal. Historically, we have been able to increase prices at a rate equal to or greater than that of inflation and believe that we will continue to be able to do so for the foreseeable future. In addition, we have been able to maintain a relatively stable variable cost structure for our products due, in part, to our successful procurement with regard to our tobacco products and, in part, to our existing contractual agreement for the purchase of our premium cigarette papers.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Foreign Currency Sensitivity
Our inventory purchases from Bolloré are denominated in euros. Accordingly, we have exposure to potentially adverse movements in the euro exchange rate. In addition, Bolloré provides a contractual hedge against catastrophic currency fluctuation in our agreement. We do not use derivative financial instruments for speculative trading purposes, nor do we hedge our foreign currency exposure in a manner that offsets the effects of changes in foreign exchange rates.
We regularly review our foreign currency risk and hedging programs and may as part of that review determine at any time to change our hedging policy. During 2019 we did not execute any forward contracts. At December 31, 2019, we had no forward contracts. A 10% change in the euro to U.S. dollars exchange rate would change pre-tax income by approximately $1.3 million per year.
Credit Risk
At December 31, 2019 and 2018, we had bank deposits, including MSA escrows, in excess of federally insured limits of approximately $126.0 million and $4.4 million, respectively. The Company has chosen to invest a portion of the MSA escrows, from time to time, in U.S. Government securities including Treasury Notes and Treasury Bonds.
We sell our products to distributors, retail establishments, and individual consumers throughout the U.S. and also have sales of Zig-Zag® premium cigarette papers in Canada. In 2019, 2018, and 2017, we had no customers that accounted for more than 10% of our net sales. We perform periodic credit evaluations of our customers and generally do not require collateral on trade receivables. Historically, we have not experienced significant losses due to customer credit issues.
52
Interest Rate Sensitivity
We have exposure to interest rate volatility principally relating to interest rate changes applicable to loans under our 2018 Credit Facility. As of December 31, 2019, all of our debt with the exception of the IVG Note Payable and Convertible Senior Notes bears interest at variable rates. However, the Company had swap contracts for a total notional amount of $70 million at December 31, 2019. The fair values of the swap contracts are based upon quoted market prices and resulted in a liability of $2.5 million as of December 31, 2019. We believe that the effect, if any, of reasonably possible near-term changes in interest rates on our consolidated financial position, results of operations or cash flows would not be significant. A 1% change in the interest rate would change pre-tax income by approximately $0.8 million per year.
53
| Item 8. | Financial Statements and Supplementary Data |
TURNING POINT BRANDS, INC.
CONTENTS
Page |
|||
Financial Statements: |
|||
54
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Turning Point Brands, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Turning Point Brands, Inc. and its subsidiaries (the Company) as of December 31, 2019 and 2018, the related consolidated statements of income, comprehensive income, changes in stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2019, and the related notes to the consolidated financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for leases in 2019.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ RSM US LLP
We have served as the Company’s auditor since 2006.
Greensboro, North Carolina
March 12, 2020
55
Turning Point Brands, Inc. and Subsidiaries
Consolidated Balance Sheets
December 31, 2019 and 2018
(dollars in thousands except share data)
December 31, 2019 |
December 31, 2018 |
|||||
ASSETS |
||||||
Current assets: |
||||||
Cash |
$ | 95,250 | $ | 3,306 | ||
Accounts receivable, net of allowances of $280 in 2019 and $42 in 2018 |
6,906 | 2,617 | ||||
Inventories |
70,979 | 91,237 | ||||
Other current assets |
16,115 | 14,694 | ||||
Total current assets |
189,250 | 111,854 | ||||
Property, plant, and equipment, net |
13,816 | 10,589 | ||||
Right of use assets |
12,130 | — | ||||
Deferred financing costs, net |
890 | 870 | ||||
Goodwill |
154,282 | 145,939 | ||||
Other intangible assets, net |
33,469 | 35,339 | ||||
Master Settlement Agreement (MSA) escrow deposits |
32,074 | 30,550 | ||||
Other assets |
10,673 | 4,236 | ||||
Total assets |
$ | 446,584 | $ | 339,377 | ||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
||||||
Current liabilities: |
||||||
Accounts payable |
$ | 14,126 | $ | 6,841 | ||
Accrued liabilities |
26,520 | 22,925 | ||||
Current portion of long-term debt |
15,240 | 8,000 | ||||
Revolving credit facility |
— | 26,000 | ||||
Total current liabilities |
55,886 | 63,766 | ||||
Notes payable and long-term debt |
268,951 | 186,715 | ||||
Deferred income taxes |
1,572 | 2,291 | ||||
Postretirement benefits |
— | 3,096 | ||||
Lease liabilities |
11,067 | — | ||||
Other long-term liabilities |
2,523 | 886 | ||||
Total liabilities |
339,999 | 256,754 | ||||
Commitments and contingencies |
||||||
Stockholders’ equity: |
||||||
Preferred stock; $0.01 par value; authorized shares 40,000,000; issued and outstanding shares -0- |
— | — | ||||
Common stock, voting, $0.01 par value; authorized shares, 190,000,000; issued and outstanding shares - 19,680,673 at December 31, 2019, and 19,553,857 at December 31, 2018 |
197 | 196 | ||||
Common stock, nonvoting, $0.01 par value; authorized shares, 10,000,000; issued and outstanding shares -0- |
— | — | ||||
Additional paid-in capital |
125,469 | 110,466 | ||||
Accumulated other comprehensive loss |
(3,773 | ) |
(2,536 | ) |
||
Accumulated deficit |
(15,308 | ) |
(25,503 | ) |
||
Total stockholders’ equity |
106,585 | 82,623 | ||||
Total liabilities and stockholders’ equity |
$ | 446,584 | $ | 339,377 | ||
The accompanying notes are an integral part of the consolidated financial statements.
56
Turning Point Brands, Inc. and Subsidiaries
Consolidated Statements of Income
for the years ended December 31, 2019, 2018, and 2017
(dollars in thousands except share data)
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Net sales |
$ | 361,989 | $ | 332,683 | $ | 285,777 | |||
Cost of sales |
225,243 | 190,124 | 160,807 | ||||||
Gross profit |
136,746 | 142,559 | 124,970 | ||||||
Selling, general, and administrative expenses |
109,887 | 94,075 | 75,290 | ||||||
Operating income |
26,859 | 48,484 | 49,680 | ||||||
Interest expense, net |
17,342 | 14,819 | 16,889 | ||||||
Investment income |
(2,648 | ) |
(424 | ) |
(438 | ) |
|||
Loss on extinguishment of debt |
1,308 | 2,384 | 6,116 | ||||||
Net periodic benefit (income) cost, excluding service cost |
(4,961 | ) |
131 | 180 | |||||
Income before income taxes |
15,818 | 31,574 | 26,933 | ||||||
Income tax expense |
2,044 | 6,285 | 7,280 | ||||||
Consolidated net income |
13,774 | 25,289 | 19,653 | ||||||
Net loss attributable to non-controlling interest |
— | — | (556 | ) |
|||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | $ | 20,209 | |||
Basic income per common share: |
|||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 0.70 | $ | 1.31 | $ | 1.06 | |||
Diluted income per common share: |
|||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 0.69 | $ | 1.28 | $ | 1.04 | |||
Weighted average common shares outstanding: |
|||||||||
Basic |
19,627,093 | 19,355,607 | 18,989,177 | ||||||
Diluted |
20,037,540 | 19,827,562 | 19,513,008 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
57
Turning Point Brands, Inc. and Subsidiaries
Consolidated Statements of Comprehensive Income
for the years ended December 31, 2019, 2018, and 2017
(dollars in thousands)
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | $ | 20,209 | |||
Other comprehensive income (loss), net of tax |
|||||||||
Amortization of unrealized pension and postretirement gain (loss), net of tax of $136 in 2019, $435 in 2018, and $543 in 2017 |
(1,150 | ) |
1,361 | 889 | |||||
Unrealized gain (loss) on investments, net of tax of $351 in 2019, $31 in 2018, and $114 in 2017 |
1,174 | (266 | ) |
187 | |||||
Unrealized loss on interest rate swaps, net of tax of $377 in 2019 and $204 in 2018 |
(1,261 | ) |
(682 | ) |
— | ||||
(1,237 | ) |
413 | 1,076 | ||||||
Comprehensive income |
$ | 12,537 | $ | 25,702 | $ | 21,285 | |||
The accompanying notes are an integral part of the consolidated financial statements.
58
Turning Point Brands, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
for the years ended December 31, 2019, 2018, and 2017
(dollars in thousands)
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Cash flows from operating activities: |
|||||||||
Consolidated net income |
$ | 13,774 | $ | 25,289 | $ | 19,653 | |||
Adjustments to reconcile net income to net cash provided by operating activities: |
|||||||||
Loss on extinguishment of debt |
1,308 | 2,384 | 6,116 | ||||||
Loss on sale of property, plant, and equipment |
7 | — | 150 | ||||||
Impairment loss |
301 | — | — | ||||||
Gain on postretirement plan termination |
(4,915 | ) |
— | — | |||||
Gain on investments |
(2,000 | ) |
— | — | |||||
Depreciation expense |
2,638 | 2,105 | 1,626 | ||||||
Amortization of other intangible assets |
1,451 | 1,005 | 702 | ||||||
Amortization of debt discount and deferred financing costs |
4,365 | 951 | 1,071 | ||||||
Deferred income taxes |
(4,219 | ) |
2,565 | 5,181 | |||||
Stock compensation expense |
3,629 | 1,411 | 720 | ||||||
Noncash lease expense |
357 | — | — | ||||||
Changes in operating assets and liabilities: |
|||||||||
Accounts receivable |
(3,464 | ) |
824 | (1,067 | ) |
||||
Inventories |
21,036 | (20,650 | ) |
495 | |||||
Other current assets |
(1,196 | ) |
(5,097 | ) |
1,495 | ||||
Other assets |
(2,864 | ) |
75 | (334 | ) |
||||
Accounts payable |
6,608 | 2,523 | (5,702 | ) |
|||||
Accrued postretirement liabilities |
(168 | ) |
(97 | ) |
(24 | ) |
|||
Accrued liabilities and other |
1,147 | (198 | ) |
(392 | ) |
||||
Net cash provided by operating activities |
$ | 37,795 | $ | 13,090 | $ | 29,690 | |||
Cash flows from investing activities: |
|||||||||
Capital expenditures |
$ | (4,815 | ) |
$ | (2,267 | ) |
$ | (2,021 | ) |
Restricted cash, MSA escrow deposits |
29,718 | (1,241 | ) |
816 | |||||
Acquisitions, net of cash acquired |
(7,704 | ) |
(19,161 | ) |
268 | ||||
Proceeds on sale of property, plant and equipment |
123 | — | — | ||||||
Payments for investments |
(1,421 | ) |
(2,000 | ) |
(179 | ) |
|||
Issuance of note receivable |
— | (6,500 | ) |
— | |||||
Repayment of note receivable |
— | 6,500 | — | ||||||
Net cash provided by (used in) investing activities |
$ | 15,901 | $ | (24,669 | ) |
$ | (1,116 | ) |
|
The accompanying notes are an integral part of the consolidated financial statements.
59
Turning Point Brands, Inc. and Subsidiaries
Consolidated Statements of Cash Flows (cont.)
for the years ended December 31, 2019, 2018, and 2017
(dollars in thousands)
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Cash flows from financing activities: |
|||||||||
Proceeds from 2018 first lien term loan |
$ | — | $ | 160,000 | $ | — | |||
Payments of 2018 first lien term loan |
(8,000 | ) |
(6,000 | ) |
— | ||||
Proceeds from 2018 second lien term loan |
— | 40,000 | — | ||||||
Payments of 2018 second lien term loan |
(40,000 | ) |
— | — | |||||
Proceeds from 2018 revolving credit facility |
— | 26,000 | — | ||||||
Payments of 2018 revolving credit facility |
(26,000 | ) |
— | — | |||||
Proceeds from Convertible Senior Notes |
172,500 | — | — | ||||||
Payments for call options |
(20,528 | ) |
— | — | |||||
Payment of dividends |
(3,531 | ) |
(2,318 | ) |
(768 | ) |
|||
Proceeds from 2017 first lien term loan |
— | — | 145,000 | ||||||
Payments of 2017 first lien term loan |
— | (140,613 | ) |
(4,387 | ) |
||||
Proceeds from 2017 second lien term loan |
— | — | 55,000 | ||||||
Payments of 2017 second lien term loan |
— | (55,000 | ) |
— | |||||
Proceeds from (payments of) 2017 revolving credit facility, net |
— | (8,000 | ) |
8,000 | |||||
Payments of VaporBeast Note Payable |
— | (2,000 | ) |
— | |||||
Proceeds from release of restricted funds |
— | 1,107 | — | ||||||
Payments of financing costs |
(7,117 | ) |
(3,286 | ) |
(4,783 | ) |
|||
Exercise of options |
738 | 833 | 1,431 | ||||||
Redemption of options |
(12 | ) |
(623 | ) |
(1,740 | ) |
|||
Surrender of restricted stock |
(84 | ) |
— | (1,000 | ) |
||||
Payment to terminate acquired capital lease |
— | (170 | ) |
— | |||||
Payments of first lien term loan |
— | — | (147,362 | ) |
|||||
Payments of second lien term loan |
— | — | (60,000 | ) |
|||||
Proceeds from (payments of) revolving credit facility |
— | — | (15,083 | ) |
|||||
Payments of Vapor Shark loans |
— | — | (1,867 | ) |
|||||
Prepaid equity issuance costs |
— | — | (453 | ) |
|||||
Distribution to non-controlling interest |
— | — | (4 | ) |
|||||
Net cash provided by (used in) financing activities |
$ | 67,966 | $ | 9,930 | $ | (28,016 | ) |
||
Net increase (decrease) in cash |
$ | 121,662 | $ | (1,649 | ) |
$ | 558 | ||
Cash, beginning of period: |
|||||||||
Unrestricted |
3,306 | 2,607 | 2,865 | ||||||
Restricted |
2,356 | 4,704 | 3,888 | ||||||
Total cash at beginning of period |
5,662 | 7,311 | 6,753 | ||||||
Cash, end of period: |
|||||||||
Unrestricted |
95,250 | 3,306 | 2,607 | ||||||
Restricted |
32,074 | 2,356 | 4,704 | ||||||
Total cash at end of period |
$ | 127,324 | $ | 5,662 | $ | 7,311 | |||
Supplemental disclosures of cash flow information: |
|||||||||
Cash paid during the period for interest |
$ | 11,828 | $ | 14,238 | $ | 15,828 | |||
Cash paid during the period for income taxes, net |
$ | 11,332 | $ | 3,215 | $ | 1,811 | |||
Supplemental schedule of noncash investing activities: |
|||||||||
Investment in General Wireless |
$ | — | $ | 421 | $ | — | |||
Supplemental schedule of noncash financing activities: |
|||||||||
Issuance of shares for acquisition |
$ | — | $ | 5,292 | $ | — | |||
Issuance of note payable for acquisition |
$ | — | $ | 4,000 | $ | — | |||
Dividends declared not paid |
$ | 962 | $ | 915 | $ | — | |||
The accompanying notes are an integral part of the consolidated financial statements.
60
Turning Point Brands, Inc. and Subsidiaries
Consolidated Statements of Changes in Stockholders’ Equity (Deficit)
for the years ended December 31, 2019, 2018, and 2017
(dollars in thousands)
Voting Shares |
Common Stock, Voting |
Additional Paid-In Capital |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Non- Controlling Interest |
Total |
|||||||||||||||
Beginning balance January 1, 2017 |
18,402,022 | $ | 184 | $ | 104,895 | $ | (4,049 | ) |
$ | (66,972 | ) |
$ | — | $ | 34,058 | ||||||
Unrecognized pension and postretirement cost adjustment, net of tax of $543 |
— | $ | — | $ | — | $ | 889 | $ | — | $ | — | $ | 889 | ||||||||
Unrealized gain on MSA investments, net of tax of $114 |
— | — | — | 185 | — | — | 185 | ||||||||||||||
Unrealized gain on other investments, net of tax of $1 |
— | — | — | 2 | — | — | 2 | ||||||||||||||
Stock compensation expense |
— | — | 648 | — | — | — | 648 | ||||||||||||||
Restricted stock forfeitures |
(4,831 | ) |
— | (63 | ) |
— | — | — | (63 | ) |
|||||||||||
Acquisition of non-controlling interest |
— | — | (560 | ) |
— | — | 560 | — | |||||||||||||
Distribution to non-controlling interest |
— | — | — | — | — | (4 | ) |
(4 | ) |
||||||||||||
Exercise of options |
813,442 | 9 | 1,422 | — | — | — | 1,431 | ||||||||||||||
Surrender of options |
— | — | (1,000 | ) |
— | — | — | (1,000 | ) |
||||||||||||
Redemption of options |
— | (1 | ) |
(1,702 | ) |
— | — | — | (1,703 | ) |
|||||||||||
Dividends |
— | — | — | — | (772 | ) |
— | (772 | ) |
||||||||||||
Net income |
— | — | — | — | 20,209 | (556 | ) |
19,653 | |||||||||||||
Ending balance December 31, 2017 |
19,210,633 | $ | 192 | $ | 103,640 | $ | (2,973 | ) |
$ | (47,535 | ) |
$ | — | $ | 53,324 | ||||||
Unrecognized pension and postretirement cost adjustment, net of tax of $435 |
— | $ | — | $ | — | $ | 1,361 | $ | — | $ | — | $ | 1,361 | ||||||||
Unrealized loss on MSA investments, net of tax of $31 |
— | — | — | (263 | ) |
— | — | (263 | ) |
||||||||||||
Unrealized loss on other investments, net of tax of $1 |
— | — | — | (3 | ) |
— | — | (3 | ) |
||||||||||||
Unrealized loss on interest rate swaps, net of tax of $204 |
— | — | — | (682 | ) |
— | — | (682 | ) |
||||||||||||
Stock compensation expense |
— | — | 1,336 | — | — | — | 1,336 | ||||||||||||||
Restricted stock forfeitures |
(3,128 | ) |
— | (8 | ) |
— | — | — | (8 | ) |
|||||||||||
Exercise of options |
193,273 | 2 | 831 | — | — | — | 833 | ||||||||||||||
Redemption of options |
— | — | (623 | ) |
— | — | — | (623 | ) |
||||||||||||
Dividends |
— | — | — | — | (3,233 | ) |
— | (3,233 | ) |
||||||||||||
Reclassification of tax effects from accumulated other comprehensive income |
— | — | — | 24 | (24 | ) |
— | — | |||||||||||||
IVG issuance of stock |
153,079 | 2 | 5,290 | — | — | — | 5,292 | ||||||||||||||
Net income |
— | — | — | — | 25,289 | — | 25,289 | ||||||||||||||
Ending balance December 31, 2018 |
19,553,857 | $ | 196 | $ | 110,466 | $ | (2,536 | ) |
$ | (25,503 | ) |
$ | — | $ | 82,623 | ||||||
Unrecognized pension and postretirement cost adjustment, net of tax of $136 |
— | $ | — | $ | — | $ | (1,150 | ) |
$ | — | $ | — | $ | (1,150 | ) |
||||||
Unrealized gain on MSA investments, net of tax of $351 |
— | — | — | 1,174 | — | — | 1,174 | ||||||||||||||
Unrealized loss on interest rate swaps, net of tax of $377 |
— | — | — | (1,261 | ) |
— | — | (1,261 | ) |
||||||||||||
Stock compensation expense |
— | — | 3,600 | — | — | — | 3,600 | ||||||||||||||
Restricted stock forfeitures |
(1,947 | ) |
— | (84 | ) |
— | — | — | (84 | ) |
|||||||||||
Exercise of options |
128,763 | 1 | 738 | — | — | — | 739 | ||||||||||||||
Redemption of options |
— | — | (12 | ) |
— | — | — | (12 | ) |
||||||||||||
Dividends |
— | — | — | — | (3,579 | ) |
— | (3,579 | ) |
||||||||||||
Purchase of call options, net of tax of $5,195 |
— | — | (15,332 | ) |
— | — | — | (15,332 | ) |
||||||||||||
Issuance of Convertible Senior Notes, net of tax of $8,857 |
— | — | 24,938 | — | — | — | 24,938 | ||||||||||||||
Fair value of earn-out |
— | — | 1,155 | — | — | — | 1,155 | ||||||||||||||
Net income |
— | — | — | — | 13,774 | — | 13,774 | ||||||||||||||
Ending balance December 31, 2019 |
19,680,673 | $ | 197 | $ | 125,469 | $ | (3,773 | ) |
$ | (15,308 | ) |
$ | — | $ | 106,585 | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
61
Turning Point Brands, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(dollars in thousands, except where designated and per share data)
Note 1. Organizations and Basis of Presentation
Organizations
Turning Point Brands, Inc. (the “Company”), is a holding company which owns North Atlantic Trading Company, Inc. (“NATC”), and its subsidiaries, Turning Point Brands, LLC (“TPLLC”), and its subsidiaries, and Turning Point Brands (Canada), Inc. (“TPBC”). Except where the context indicates otherwise, references to the Company include the Company; NATC and its subsidiaries National Tobacco Company, L.P. (“NTC”), National Tobacco Finance, LLC (“NTFLLC”), North Atlantic Operating Company, Inc. (“NAOC”), North Atlantic Cigarette Company, Inc. (“NACC”), and RBJ Sales, Inc. (“RBJ”); and TPLLC and its subsidiaries Intrepid Brands, LLC (“Intrepid”), TPB Beast, LLC (“VaporBeast,”), TPB Shark, LLC, and its subsidiaries (collectively, “Vapor Shark,”), TPB International, LLC and its subsidiaries (collectively, “IVG”), and Nu-X Ventures LLC (“Nu-X”).
Basis of Presentation
The consolidated financial statements include the Company, as well as its wholly owned subsidiaries. All intercompany transactions have been eliminated. The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities, disclosure of contingent assets and liabilities as of the dates of the financial statements, and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. The Company’s significant estimates include those affecting the valuation of goodwill and other intangible assets, assumptions used in determining pension and postretirement benefit obligations, deferred income tax valuation allowances and the valuation of inventory, including reserves.
Certain prior year amounts have been reclassified to conform to the current year’s presentation. The changes did not have an impact on the Company’s consolidated financial position, results of operations, or cash flows in any of the periods presented.
Note 2. Summary of Significant Accounting Policies
Consolidation
The consolidated financial statements include the accounts of the Company, its subsidiaries, all of which are wholly owned, and the results of Vapor Shark from April 1, 2017, through June 30, 2017. All significant intercompany transactions have been eliminated. From April 1 through June 30, 2017, Vapor Shark was a variable interest entity (“VIE”) for which the Company was considered the primary beneficiary due to an April 2017 management agreement in which the Company was granted the right to purchase 100% of the equity interest of Vapor Shark. The Company did not own Vapor Shark during the second quarter of 2017; however, Vapor Shark’s financial results are included in the Company’s consolidated results as a VIE. On June 30, 2017, the Company exercised a warrant to purchase all of the issued and outstanding equity of Vapor Shark. Beginning June 30, 2017, Vapor Shark became a wholly owned subsidiary of the Company.
Revenue Recognition
The Company adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes nearly all existing revenue recognition guidance under U.S. GAAP, on January 1, 2018. The Company recognizes revenues, which includes excise taxes and shipping and handling charges billed to customers, net of cash discounts for prompt payment, sales returns and sales incentives, upon delivery of goods to the customer—at which time the Company’s performance obligation is satisfied—at an amount that the Company expects to be entitled to in exchange for those goods in accordance with the five-step analysis outlined in Topic 606: (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations, and (v) recognize revenue when (or as) performance obligations are satisfied. The Company excludes from the transaction price, sales taxes and value-added taxes imposed at the time of sale (which do not include excise taxes on smokeless tobacco, cigars, or vaping products billed to customers).
62
The Company records an allowance for sales returns, based principally on historical volume and return rates, which is included in accrued liabilities on the consolidated balance sheets. The Company records sales incentives, which consist of consumer incentives and trade promotion activities, as a reduction in revenues (a portion of which is based on amounts estimated as being due to wholesalers, retailers and consumers at the end of the period) based principally on historical volume and utilization rates. Expected payments for sales incentives are included in accrued liabilities on the consolidated balance sheets.
A further requirement of ASU 2014-09 is for entities to disaggregate revenue recognized from contracts with customers into categories that depict how the nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factors. Company management views business performance through segments that closely resemble the performance of major product lines. Thus, the primary and most useful disaggregation of the Company’s contract revenue for decision making purposes is the disaggregation by segment which can be found in Note 21, “Segment Information”. An additional disaggregation of contract revenue by sales channel can be found within Note 21 as well.
Derivative Instruments
Foreign Currency Forward Contracts: The Company enters into foreign currency forward contracts to hedge a portion of its exposure to changes in foreign currency exchange rates on inventory purchase commitments. The Company accounts for its forward contracts under the provisions of ASC 815, Derivatives and Hedging. Under the Company’s policy, the Company may hedge up to 100% of its anticipated purchases of inventory in the denominated invoice currency over a forward period not to exceed twelve months. The Company may also, from time to time, hedge up to ninety percent of its non-inventory purchases in the denominated invoice currency. Forward contracts that qualify as hedges are adjusted to their fair value through other comprehensive income as determined by market prices on the measurement date, except any hedge ineffectiveness which is recognized currently in income. Gains and losses on these forward contracts are transferred from other comprehensive income into net income as the related inventories are received. Changes in fair value of any contracts that do not qualify for hedge accounting or are not designated as hedges are recognized currently in income.
Interest Rate Swap Agreements: The Company enters into interest rate swap contracts to manage interest rate risk and reduce the volatility of future cash flows. The Company accounts for its interest rate swap contracts under the provisions of ASC 815, Derivatives and Hedging. Swap contracts that qualify as hedges are adjusted to their fair value through other comprehensive income as determined by market prices on the measurement date, except any hedge ineffectiveness which is recognized currently in income. Gains and losses on these swap contracts are transferred from other comprehensive income into net income upon settlement of the derivative position or at maturity of the interest rate swap contract. Changes in fair value of any contracts that do not qualify for hedge accounting or are not designated as hedges are recognized currently in income.
Shipping Costs
The Company records shipping costs incurred as a component of selling, general and administrative expenses. Shipping costs incurred were approximately $18.1 million, $15.1 million, and $10.4 million in 2019, 2018, and 2017, respectively.
Research and Development and Quality Assurance Costs
Research and development and quality assurance costs are expensed as incurred. These expenses, classified as selling, general and administrative expenses, were approximately $2.5 million, $2.5 million, and $2.3 million in 2019, 2018, and 2017, respectively.
Cash and Cash Equivalents
The Company considers any highly liquid investments with a maturity of three months or less from the date of purchase to be cash equivalents.
Inventories
Cost is determined using the last-in, first-out (“LIFO”) method for approximately 49.4% of the inventories and first-in, first-out (“FIFO”) for the remaining inventories as of December 31, 2019. Inventories that are measured
63
using the LIFO method are stated at the lower of cost or market. Inventories that are measured using the FIFO method are stated at the lower of cost or net realizable value. Leaf tobacco is presented in current assets in accordance with standard industry practice, notwithstanding the fact that such tobaccos are carried longer than one year for the purpose of curing.
Property, Plant and Equipment
Property, Plant and Equipment are stated at cost less accumulated depreciation and impairment. Depreciation is provided using the straight-line method over the lesser of the estimated useful lives of the assets or the life of the leases for leasehold improvements (4 to 7 years for machinery, equipment and furniture, 10 to 15 years for leasehold improvements, and up to 15 years for buildings and building improvements). Expenditures for repairs and maintenance are charged to expense as incurred. The costs of major renewals and improvements are capitalized and depreciated over their estimated useful lives. Upon disposition of fixed assets, the costs and related accumulated depreciation amounts are relieved. Any resulting gain or loss is reflected in operations during the period of disposition. Long-lived assets are reviewed for impairment when changes in circumstances indicate that the carrying amount of an asset may not be recoverable.
Goodwill and Other Intangible Assets
The Company follows the provisions of ASC 350, Intangibles – Goodwill and Other in accounting for goodwill and other intangible assets. Goodwill and indefinite-lived intangible assets are reviewed for impairment annually on December 31, or more frequently if certain indicators are present, in accordance with ASC 350-20-35 and ASC 350-30-35, respectively. If the carrying value of a reporting unit including goodwill exceeds its fair value, which is determined using the discounted cash flows, goodwill is considered impaired. The amount of impairment loss is measured as the difference between the carrying value and the fair value of the reporting unit, but is limited to the total goodwill allocated to the reporting unit. If the carrying value of an indefinite-life intangible asset exceeds its fair value, which is determined using discontinued cash flows or relief-from-royalty, the intangible asset is considered impaired and is reduced to fair value. For goodwill, the determination of a reporting unit’s fair value involves, among other things, the Company’s market capitalization and application of the income approach, which includes developing forecasts of future cash flows and determining an appropriate discount rate.
Based on the Company’s annual goodwill impairment testing, the estimated fair values of each of our reporting units were in excess of the respective carrying values at December 31, 2019. The Company had no such impairment of goodwill or other intangible assets during the year ended December 31, 2019. However, there could be an impairment of the goodwill of the NewGen reporting unit if future revenues do not achieve our expected future cash flows or if macroeconomic conditions result in a future increase in the weighted average cost of capital used to estimate fair value. See Note 10, “Goodwill and Other Intangible Assets”, for further details regarding the Company’s goodwill and other intangible assets as of December 31, 2019.
Fair Value
GAAP establishes a framework for measuring fair value. That framework provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (level 1) and the lowest priority to unobservable inputs (level 3).
The three levels of the fair value hierarchy under GAAP are described below:
| • | Level 1 – Inputs to the valuation methodology are unadjusted quoted prices for identical assets or liabilities in active markets at the measurement date. |
| • | Level 2 – Inputs to the valuation methodology include: quoted prices for similar assets or liabilities in active markets; quoted prices for identical or similar assets or liabilities in inactive markets; inputs other than quoted prices that are observable for the asset or liability; and inputs that are derived principally from or corroborated by observable market data by correlation or other means. |
| • | Level 3 – Unobservable inputs that reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. |
64
Retirement Plans
The Company follows the provisions of ASC 715, Compensation – Retirement Benefits. ASC 715-30, Defined Benefit Plans – Pensions, which requires an employer to (a) recognize in its statement of financial position the funded status of a benefit plan, measured as the difference between the fair value of plan assets and benefit obligations, (b) recognize net of tax, the gains or losses and prior service costs or credits that arise during the period but are not recognized as components of net periodic benefit cost, and (c) measure defined benefit plan assets and obligations as of the date of the employer’s statement of financial position.
Deferred Financing Costs
Deferred financing costs are amortized over the terms of the related debt obligations using the effective interest method. Unamortized amounts are expensed upon extinguishment of the related borrowings. Deferred financing costs are presented as a direct deduction from the carrying amount of that debt liability except for deferred financing costs relating to our revolving credit facility, which are presented as an asset.
Income Taxes
The Company records the effects of income taxes under the liability method in which deferred income tax assets and liabilities are recognized based on the difference between the financial and tax basis of assets and liabilities using the enacted tax rates in effect for the years in which the differences are expected to reverse. The Company assesses its ability to realize future benefits of deferred tax assets by determining if they meet the “more likely than not” criteria in ASC 740, Income Taxes. If the Company determines that future benefits do not meet the “more likely than not” criteria, a valuation allowance is recorded.
Advertising and Promotion
Advertising and promotion costs, including point of sale materials, are expensed as incurred and amounted to $12.0 million, $5.6 million, and $3.4 million for the years ending December 31, 2019, 2018, and 2017, respectively.
Stock-Based Compensation
The Company measures stock-based compensation costs related to its stock options on the fair value-based method under the provisions of ASC 718, Compensation – Stock Compensation. The fair value-based method requires compensation cost for stock options to be recognized over the requisite service period based on the fair value of stock options granted. The Company determined the fair value of these awards using the Black-Scholes option pricing model.
The Company grants performance-based restricted stock units (“PRSU”) subject to both performance-based and service-based vesting conditions. The fair value of each PRSU is the Company’s stock price on the date of grant. For purposes of recognizing compensation expense as services are rendered in accordance with ASC 718, the Company assumes all employees involved in the PRSU grant will provide service through the end of the performance period. Stock compensation expense is recorded based on the probability of achievement of the performance conditions specified in the PRSU grant.
Risks and Uncertainties
Manufacturers and sellers of tobacco products are subject to regulation at the federal, state, and local levels. Such regulations include, among others, labeling requirements, limitations on advertising, and prohibition of sales to minors. The tobacco industry is likely to continue to be heavily regulated. There can be no assurance as to the ultimate content, timing, or effect of any regulation of tobacco products by any federal, state, or local legislative or regulatory body, nor can there be any assurance that any such legislation or regulation would not have a material adverse effect on the Company’s financial position, results of operations, or cash flows. Recently, several state governors have reacted to perceived issues around nicotine vapor products by unilaterally, without regard to the legislative process, proclaiming bans on vapor products, particularly those that are flavored. Many of these executive actions have been challenged and temporarily restrained, but no assurance can be given that such state or local flavor bans will not be enacted or ultimately upheld. Depending on the number and location of such bans, such executive actions and legislation could have a material adverse effect on the Company’s financial position, results of operations or cash flows. Food Drug and Administration (“FDA”) continues to consider various restrictive regulations around our products, including targeted flavor bans; however, the details, timing, and ultimate implementation of such measures remain unclear.
65
The tobacco industry has experienced and is experiencing significant product liability litigation. Most tobacco liability lawsuits have been brought against manufacturers and sellers of cigarettes for injuries allegedly caused by smoking or exposure to smoke. However, several lawsuits have been brought against manufacturers and sellers of smokeless products for injuries to health allegedly caused by use of smokeless products. Typically, such claims assert that use of smokeless products is addictive and causes oral cancer. Additionally, several lawsuits have been brought against manufacturers and distributors of NewGen products due to malfunctioning devices. There can be no assurance the Company will not sustain losses in connection with such lawsuits and that such losses will not have a material adverse effect on the Company’s financial position, results of operations, or cash flows.
Master Settlement Agreement (MSA): Forty-six states, certain U.S. territories, and the District of Columbia are parties to the Master Settlement Agreement (“MSA”) and the Smokeless Tobacco Master Settlement Agreement (“STMSA”). To the Company’s knowledge, signatories to the MSA include 49 cigarette manufacturers and/or distributors. The only signatory to the STMSA is US Smokeless Tobacco Company. In the Company’s opinion, the fundamental basis for each agreement is the states’ consents to withdraw all claims for monetary, equitable, and injunctive relief against certain tobacco products manufacturers and others and, in return, the signatories have agreed to certain marketing restrictions and regulations as well as certain payment obligations.
Pursuant to the MSA and subsequent states’ statutes, a “cigarette manufacturer” (which is defined to also include MYO cigarette tobacco) has the option of either becoming a signatory to the MSA or opening, funding, and maintaining an escrow account, with sub-accounts on behalf of each settling state. The STMSA has no similar provisions. The MSA escrow accounts are governed by states’ statutes that expressly give the manufacturers the option of opening, funding, and maintaining an escrow account in lieu of becoming a signatory to the MSA. The statutes require companies who are not signatories to the MSA to deposit, on an annual basis, into qualified banks, escrow funds based on the number of cigarettes or cigarette equivalents, i.e., the pounds of MYO tobacco, sold. The purpose of these statutes is expressly stated to be to eliminate the cost disadvantage the settling manufacturers have as a result of entering into the MSA. Such companies are entitled to direct the investment of the escrowed funds and withdraw any appreciation, but cannot withdraw the principal for twenty-five years from the year of each annual deposit, except to withdraw funds deposited pursuant to an individual state’s escrow statute to pay a final judgment to that state’s plaintiffs in the event of such a final judgment against the company. Either option – becoming an MSA signatory or establishing an escrow account – is permissible.
The Company chose to open and fund an MSA escrow account as its means of compliance. It is management’s opinion, due to the possibility of future federal or state regulations, though none have to date been enacted, that entering into one or both of the settlement agreements or establishing and maintaining an escrow account would not necessarily prevent future regulations from having a material adverse effect on the results of operations, financial position, and cash flows of the Company.
Various states have enacted or proposed complementary legislation intended to curb the activity of certain manufacturers and importers of cigarettes that are selling into MSA states without signing the MSA or who have failed to properly establish and fund a qualifying escrow account. To the best of the Company’s knowledge, no such statute has been enacted which could inadvertently and negatively impact the Company, which has been, and is currently, fully compliant with all applicable laws, regulations, and statutes. However, there can be no assurance that the enactment of any such complementary legislation in the future will not have a material adverse effect on the results of operations, financial position, or cash flows of the Company.
Pursuant to the MSA escrow account statutes, in order to be compliant with the MSA escrow requirements, companies selling products covered by the MSA are required to deposit such funds for each calendar year into a qualifying escrow account by April 15 of the following year. At December 31, 2019, the Company had on deposit approximately $32.1 million, the fair value of which was approximately $32.1 million. Inputs to the valuation methodology of the MSA escrow deposits when funds are invested include unadjusted quoted prices for identical assets or liabilities in active markets at the measurement date. During 2019 no monies were deposited into this qualifying escrow account. The investment vehicles available to the Company are specified in the state escrow agreements and are limited to low-risk government securities.
Effective April 1, 2009, the federal excise tax on MYO products was increased from $1.0969 per pound to $24.78 per pound of tobacco. With this significant increase in the federal excise tax, the Company discontinued its
66
generic category of MYO. The Company’s Zig-Zag branded MYO cigarette smoking tobacco line was discontinued in the third quarter of 2017. Thus, pending a change in MSA legislation, the Company has no remaining product lines covered by the MSA and will not be required to make future escrow deposits.
The Company has chosen to invest a portion of the MSA escrow, from time to time, in U.S. Government securities including TIPS, Treasury Notes, and Treasury Bonds. These investments are classified as available-for-sale and carried at fair value. Realized losses are prohibited under the MSA; thus, any investment in an unrealized loss position will be held until the value is recovered, or until maturity. The following shows the fair value of the MSA escrow account:
As of December 31, 2019 |
As of December 31, 2018 |
||||||||||||||
Cost and Estimated Fair Value |
Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||||
Cash and cash equivalents |
$ | 32,074 | $ | 2,361 | $ | — | $ | — | $ | 2,361 | |||||
U.S. Governmental agency obligations (unrealized gain position < 12 months) |
— | 1,193 | 9 | — | 1,202 | ||||||||||
U.S. Governmental agency obligations (unrealized loss position < 12 months) |
— | 1,000 | — | (3 | ) |
997 | |||||||||
U.S. Governmental agency obligations (unrealized loss position > 12 months) |
— | 27,519 | — | (1,529 | ) |
25,990 | |||||||||
Total |
$ | 32,074 | $ | 32,073 | $ | 9 | $ | (1,532 | ) |
$ | 30,550 | ||||
The following shows the maturities of the U.S. Governmental agency obligations:
As of |
|||
December 31, 2018 |
|||
Less than one year |
$ | 1,499 | |
One to five years |
13,591 | ||
Five to ten years |
11,152 | ||
Greater than ten years |
3,470 | ||
Total |
$ | 29,712 | |
The following shows the amount of deposits by sales year for the MSA escrow account:
Sales Year |
Deposits as of December 31, |
|||||
2019 |
2018 |
|||||
1999 |
$ | 211 | $ | 211 | ||
2000 |
1,017 | 1,017 | ||||
2001 |
1,673 | 1,673 | ||||
2002 |
2,271 | 2,271 | ||||
2003 |
4,249 | 4,249 | ||||
2004 |
3,714 | 3,714 | ||||
2005 |
4,553 | 4,552 | ||||
2006 |
3,847 | 3,847 | ||||
2007 |
4,167 | 4,167 | ||||
2008 |
3,364 | 3,364 | ||||
2009 |
1,619 | 1,619 | ||||
2010 |
406 | 406 | ||||
2011 |
193 | 193 | ||||
2012 |
199 | 199 | ||||
2013 |
173 | 173 | ||||
2014 |
143 | 143 | ||||
2015 |
101 | 101 | ||||
2016 |
91 | 91 | ||||
2017 |
83 | 83 | ||||
Total |
$ | 32,074 | $ | 32,073 | ||
67
Federal Excise Taxes: Tobacco products, cigarette papers, and cigarette tubes are subject to federal excise taxes. The following table outlines the federal excise tax rate by product category effective as of April 1, 2009:
|
Product Category
|
Cigarette and Tobacco Rates effective April 1, 2009
|
|
Cigarettes
|
$1.0066 per pack
|
|
Large Cigars
|
52.75% of manufacturer’s price; cap of $0.4026 per cigar
|
|
Little Cigars
|
$1.0066 per pack
|
|
Pip Tobacco (including Shisha)
|
$2.8311 per pound
|
|
Chewing Tobacco
|
$0.5033 per pound
|
|
Snuff
|
$1.51 per pound
|
|
RYO/MYO and Cigar Wrappers
|
$24.78 per pound
|
|
Cigarette Papers
|
$0.0315 per 50 papers
|
|
Cigarette Tubes
|
$0.063 per 50 tubes
|
Any future enactment of increases in federal excise taxes on the Company’s products could have a material adverse effect on the results of operations or financial condition of the Company. The Company is unable to predict the likelihood of passage of future increases in federal excise taxes. As of December 31, 2019, federal excise taxes are not assessed on e-cigarettes and related products.
As of December 31, 2019, nearly half of the states and certain localities impose excise taxes on electronic cigarettes and/or liquid vapor. In addition, there are several local taxing jurisdictions with an excise tax on e-cigarettes. Several states have also implemented additional measures on e-cigarettes, such as licensing requirements.
Food and Drug Administration (“FDA”): On June 22, 2009, the Family Smoking Prevention and Tobacco Control Act (“FSPTCA”) authorized the Food and Drug Administration to immediately regulate the manufacture, sale, and marketing of four categories of tobacco products – cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco. On August 8, 2016, the FDA deeming regulation became effective. The deeming regulation gave the FDA the authority to additionally regulate cigars, pipe tobacco, e-cigarettes, vaporizers, and e-liquids as “deemed” tobacco products under the FSPTCA.
The FDA assesses tobacco product user fees on six classes of regulated tobacco products and computes user fees using a methodology similar to the methodology used by the U.S Department of Agriculture to compute the Tobacco Transition Payment Program (“TTPP,” also known as the “Tobacco Buyout”) assessment. First, the total, annual, congressionally established user fee assessment is allocated among the various classes of tobacco products using the federal excise tax weighted market share of tobacco products subject to regulation. Then, the assessment for each class of tobacco products is divided among individual manufacturers and importers.
In August 2016, the FDA’s regulatory authority under the Tobacco Control Act (the “TCA”) was extended to all tobacco products not previously covered, including: (i) certain NewGen products (such as electronic cigarettes, vaporizers and e-liquids) and their components or parts (such as tanks, coils and batteries); (ii) cigars and their components or parts (such as cigar tobacco); (iii) pipe tobacco; (iv) hookah products; and (v) any other tobacco product “newly deemed” by the FDA. These “deeming regulations” apply to all products made or derived from tobacco intended for human consumption, but excluding accessories of tobacco products (such as lighters). Accordingly, the FDA has since regulated our pipe tobacco, cigar, and cigar wrap products as well as our vapor products containing tobacco-derived nicotine and products intended or reasonably expected to be used to consume such e-liquids.
Under the deeming regulations, the FDA has responsibility for conducting premarket review of “new tobacco products”—defined as those products not commercially marketed in the United States as of February 15, 2007. There are three pathways for obtaining premarket authorization, including submission of a premarket tobacco product application (“PMTA”).
When the FDA initially issued the deeming regulations, it recognized that many products in the deemed categories that were already on the market qualified as “new tobacco products” and lacked a marketing order. In August 2017, the FDA issued a compliance policy (the “August 2017 Guidance”) that allowed new tobacco products to remain on the market without an FDA authorization until specified deadlines had passed. Under the August 2017 Guidance, compliance dates vary depending upon the type of application submitted, but all
68
newly-deemed products require an application no later than August 8, 2021, for “combustible” products (e.g. cigar and pipe), and August 8, 2022, for “non-combustible” products (e.g. vapor products) with the exception of “grandfathered” products (products in commerce as of February 15, 2007) which are already authorized.
On March 27, 2018, several public health organizations filed a lawsuit (the “Maryland Lawsuit”) challenging the August 2017 Guidance. The plaintiffs asserted, among other arguments, that the modification to the deeming regulations included in the August 2017 Guidance conflicts with the TCA and exceeds FDA’s statutory authority. The plaintiffs also expressed concern that the August 2017 Guidance allows vapor products to remain marketed for a significant period of time without required premarket review.
The court found in favor of the plaintiffs in May 2019 and vacated the August 2017 Guidance. On July 12, 2019, the court issued its remedy order (the “Remedy Order”). Specifically, the court ordered that: (1) for all deemed new tobacco products, marketers must file applications within 10 months of the Remedy Order to continue marketing such products; (2) such a product may remain on the market pending FDA review of a timely filed application for a period not to exceed one year from the date of the application’s submission; (3) in its discretion, the FDA may enforce the premarket review requirements against such products for which marketers do not file applications within 10 months; and (4) the FDA will have the ability to exempt deemed new tobacco products from these application submission requirements for good cause, on a case-by-case basis. On October 24, 2019, FDA filed a Notice of Appeal from the Remedy Order and other actions adverse to FDA. The court-ordered modification to the compliance policy remains subject to change as a result of potential appeals or litigation brought or pending in other venues.
Currently, the deadline to submit an application and to continue marketing a deemed new product remains May 12, 2020. In January, the FDA indicated it intended to maintain this deadline irrespective of the outcome of the pending appeal in the Maryland Lawsuit.
On September 11, 2019, President Donald Trump and the Department of Health and Human Services Secretary, Alex Azar, indicated FDA would adopt a regulatory policy restricting all flavors in vapor products. In January 2020, FDA issued a Guidance document (the “January 2020 Guidance”) that stated it would be prioritizing enforcement of several categories of electronic nicotine delivery system (“ENDS”) products: (1) flavored, cartridge-based ENDS products (other than tobacco- or menthol-flavored ENDS products; (2) ENDS products for which the manufacturer has failed to take (or is failing to take) adequate measures to prevent minors’ access; (3) ENDS products targeted to minors or whose marketing is likely to promote the use of ENDS by minors; and (4) ENDS products offered for sale after May 12, 2020, for which the manufacturer has not submitted a premarket application. The policy outlined several factors the agency would consider in its enforcement of flavored cigars going forward but did not restrict those products as it had considered in the March 2019 Guidance proposal. The agency’s policy on these and other regulated products may change or expand over time in ways not yet known; however, such a policy could significantly impact our products and our plans for PMTA filings.
As a result of the Remedy Order and subsequent January 2020 Guidance, we would not be permitted to continue marketing our existing line of vapor products that the FDA regulates as tobacco products past May 12, 2020, unless we file an application for each such product by that date. We expect to be able to make appropriate PMTA applications by the deadlines and to supplement and complete the applications within FDA’s discretionary timeline. A successful PMTA must demonstrate that the subject product is “appropriate for the protection of public health,” taking into account the effect of the marketing of the product on all sub-populations. On September 25, 2019, FDA published a proposed rule outlining certain required elements of PMTA filings. This rule is not yet final, and its requirements may shift before being finalized. We believe we have products that meet the requisite standard and that we will be able to efficiently produce satisfactory PMTA filings. However, there is no assurance that the FDA’s guidance or ultimate regulation will not change, the Remedy Order will not be altered or that unforeseen circumstances will not arise that prevent us from filing applications or otherwise increase the amount of time and money we are required to spend to successfully file all necessary PMTAs. Even if we successfully file all of our PMTAs in a timely manner, no assurance can be given that the applications will ultimately be successful. Given the shorter time frame mandated by the Remedy Order, which if not amended or successfully appealed, may result in the prioritization of meeting requisite deadlines by selecting high priority SKUs in our inventory position, and future revenues may be adversely impacted.
In addition, we currently distribute many third-party manufactured vapor products for which we will be completely dependent on the manufacturer complying with the premarket filing requirements. There can be no assurances that some products that we currently distribute will be able to be sold to end consumers after May 2020.
69
While we will take measures to pursue regulatory compliance for our own privately-branded or proprietary vape products that compete with these third-party products, there is no assurance that such proprietary products would be as successful in the marketplace or can fully displace third-party products that are currently being distributed by us, which could adversely affect our results of operations and liquidity.
Consumer Product Safety Commission (“CPSC”): On July 26, 2016, the CPSC began requiring that e-liquid containers be packaged in child-resistant packaging, as outlined in the Poison Prevention Packaging Act. We are not able to predict whether additional packaging requirements will be necessary for our e-liquid products in the future.
Concentration of Credit Risk: At December 31, 2019 and 2018, the Company had bank deposits, including MSA escrow accounts, in excess of federally insured limits of approximately $126.0 million and $4.4 million, respectively. During 2019 and 2018, the Company invested a portion of the MSA escrow accounts in U.S. Government securities including TIPS, Treasury Notes, and Treasury Bonds.
The Company sells its products to distributors, retail establishments, and consumers throughout the United States and also sells Zig-Zag® premium cigarette papers in Canada and some smaller quantities in other countries. The Company had no customers that accounted for more than 10% of net sales for 2019, 2018, or 2017. The Company performs periodic credit evaluations of its customers and generally does not require collateral on trade receivables. Historically, the Company has not experienced significant credit losses.
Accounts Receivable
Accounts receivable are recognized at their net realizable value. All accounts receivable are trade related, recorded at the invoiced amount, and do not bear interest. The Company maintains allowances for doubtful accounts receivable for estimated uncollectible invoices resulting from a customer’s inability to pay (bankruptcy, out of business, etc., i.e. “bad debt” which results in write-offs). The activity of allowance for doubtful accounts during 2019 and 2018 is as follows:
December 31, 2019 |
December 31, 2018 |
|||||
Balance at beginning of period |
$ | 42 | $ | 17 | ||
Additions to allowance account during period |
238 | 25 | ||||
Deductions of allowance account during period |
— | — | ||||
Balance at end of period |
$ | 280 | $ | 42 | ||
Recent Accounting Pronouncements Adopted
Effective January 1, 2019, the Company adopted Accounting Standards Update (“ASU”) No. 2016-02, “Leases.” This ASU requires substantially all leases be recorded on the balance sheet as right of use assets and lease obligations. The Company adopted the ASU using a modified retrospective adoption method at January 1, 2019, as outlined in ASU No. 2018-11, “Leases - Targeted Improvements.” Under this method of adoption, there is no impact to the comparative consolidated statement of income and consolidated balance sheet. The Company determined that there was no cumulative-effect adjustment to beginning retained earnings on the consolidated balance sheet. The Company will continue to report periods prior to January 1, 2019 in its financial statements under prior guidance as outlined in Accounting Standards Codification Topic 840, “Leases”. In addition, the Company elected the package of practical expedients permitted under the transition guidance within the new standard, which among other things, allowed carry forward of historical lease classifications. Adoption of this standard did not materially impact the Company’s income before income taxes or the statement of cash flows. See Note 16, “Lease Commitments”, for further details.
Recent Accounting Pronouncements Not Yet Adopted
In June 2016, the FASB issued ASU 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 is intended to improve financial reporting by requiring timelier recording of credit losses on loans and other financial instruments held by financial institutions and other organizations. This ASU applies to financial assets measured at amortized cost, including loans, held-to-maturity debt securities, net investments in leases, and trade accounts receivable as well as certain off-balance sheet credit exposures, such as loan commitments. The ASU replaces the current incurred loss impairment methodology with a
70
methodology to reflect current expected credit losses (“CECL”) and requires consideration of a broader range of reasonable and supportable information to explain credit loss estimates. The guidance must be adopted using a modified retrospective transition method through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The ASU is effective for the Company beginning in the first quarter of 2020. The Company does not expect the ASU to have a significant impact to the Company’s financial statements and related disclosures.
In December 2019, the FASB issued ASU 2019-12 to simplify the accounting in ASC 740, Income Taxes. This guidance removes certain exceptions related to the approach for intra-period tax allocation, the methodology for calculating income taxes in an interim period, and the recognition of deferred tax liabilities for outside basis differences. This guidance also clarifies and simplifies other areas of ASC 740. This ASU will be effective beginning in the first quarter of the Company’s fiscal year 2021. Early adoption is permitted. Certain amendments in this update must be applied on a prospective basis, certain amendments must be applied on a retrospective basis, and certain amendments must be applied on a modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The Company is currently evaluating the impact this ASU will have on the financial statements and related disclosures, as well as the timing of adoption.
Note 3. Acquisitions
Solace Technologies
In July 2019, the Company purchased the assets of E-Vape 12, Inc and Solace Technologies LLC (“Solace”) for $9.4 million in total consideration, comprised of $7.7 million in cash, $1.1 million earn-out fair value, and $0.5 million holdback for 18 months, which was adjusted by $0.2 million for a working capital deficiency. The earn-out consists of 44,295 shares of the Company’s common stock to be issued to the former owners upon the achievement of certain annual milestones. Immediately following the acquisition, 88,582 performance based restricted stock units with a fair value of $4.62 million were issued to former owners who became employees. See Note 17, “Share Incentive Plans”, for further details. Solace is an innovative product development company that has grown from the creator of one of the leading vape juice brands in the industry into a leader of alternative ingredients product development. The Company intends to incorporate Solace’s innovative products as well as the legacy vapor products into our Nu-X development engine. As of December 31, 2019, the Company had not completed the accounting for the acquisition. The following purchase price and goodwill and other intangibles are based on the excess of the acquisition price over the estimated fair value of the tangible assets acquired and are based on management’s preliminary estimates:
Total consideration transferred |
$ | 9,405 | |
Adjustments to consideration transferred: |
|||
Cash acquired |
(45 | ) |
|
Working capital |
(235 | ) |
|
Adjusted consideration transferred |
9,125 | ||
Assets acquired: |
|||
Working capital (primarily AR and inventory) |
1,132 | ||
Fixed assets and Other long term assets |
414 | ||
Intangible assets |
1,352 | ||
Other liabilities |
(209 | ) |
|
Net assets acquired |
$ | 2,689 | |
Goodwill |
$ | 6,436 |
The goodwill of $6.4 million consists of the synergies and scale expected from combining the operations and is currently deductible for tax purposes.
IVG
In September 2018, the Company acquired 100% of the equity interest of IVG for total consideration of $23.8 million satisfied through $14.5 million paid in cash, 153,079 shares of the Company’s common stock with a fair value of $5.3 million, and a $4.0 million note payable to IVG’s shareholders (“IVG Note”) which matures 18 months from the acquisition date, on March 5, 2020. All principal and accrued and unpaid interest under the IVG Note is subject to
71
indemnification obligations of the sellers pursuant to the International Vapor Group Stock Purchase Agreement dated as of September 5, 2018. The Company has tracked liabilities subject to indemnification obligations and believes that such obligations exceed $4 million. The Purchase Agreement provides a mechanism under which the parties either agree on the indemnity amount or litigate disputed amounts. The Purchase Agreement provides that the amount of the indemnity is to initially be determined as of March 5, 2020. Some of the liabilities are identified but not yet fixed, such as product liability expenses. The Purchase Agreement and related agreements include an additional $4.5 million of earnouts with both performance-based and service-based conditions payable to former IVG owners who became employees of the Company as a result of the acquisition. Such amounts will be considered compensation and are not a component of the IVG purchase price. The portion of earnout payments a recipient will receive will be calculated by reference to certain performance metrics not to exceed a two-year period as specified within the acquisition agreement. The Company recorded earnout expense of approximately $0.9 million and $1.5 million, respectively, within selling, general, and administrative expenses in the consolidated statements of income for the years ended December 31, 2019 and 2018, based on the probability of achieving the performance conditions.
IVG markets and sells a broad array of proprietary and third-party vapor products directly to adult consumers through an online platform under brand names such as VaporFi, South Beach Smoke, and Direct-Vapor. IVG operates company-owned stores under the VaporFi brand and also operates as a franchisor to franchisee-owned stores. The acquisition of IVG adds a significant business-to-consumer distribution platform to the Company’s NewGen portfolio. The Company completed the accounting for the acquisition during the third quarter 2019. The following purchase price and goodwill are based on the excess of the acquisition price over the fair value of the tangible and intangible assets acquired:
Total consideration transferred |
$ | 24,292 | |
Adjustments to consideration transferred: |
|||
Cash acquired, net of debt assumed |
(221 | ) |
|
Working capital |
(245 | ) |
|
Adjusted consideration transferred |
23,826 | ||
Assets acquired: |
|||
Working capital (primarily inventory) |
3,218 | ||
Fixed assets |
1,274 | ||
Intangible assets |
7,880 | ||
Net assets acquired |
$ | 12,372 | |
Goodwill |
$ | 11,454 |
The goodwill of $11.5 million consists of the synergies and scale expected from combining the operations and is currently deductible for tax purposes.
Vapor Supply
On April 30, 2018, the Company purchased the assets of Vapor Supply LLC, vaporsupply.com, and some of its affiliates including the Ecig.com domain through its subsidiary Vapor Acquisitions Company, LLC, for total consideration of $4.8 million paid in cash to strengthen its presence within the NewGen segment. Vapor Supply is a business-to-business e-commerce distribution platform servicing independent retail vape shops. Additionally, Vapor Supply manufactures and markets proprietary e-liquids under the DripCo brand and operates company-owned stores. The accounting for the acquisition of these assets was finalized during the second quarter 2019. The following purchase price and goodwill are based on the excess of the acquisition price over the estimated fair value of the tangible and intangible assets acquired:
Total consideration transferred |
$ | 4,800 | |
Assets acquired: |
|||
Working capital (primarily inventory) |
2,500 | ||
Fixed assets |
272 | ||
Intangible assets |
256 | ||
Net assets acquired |
3,028 | ||
Goodwill |
$ | 1,772 |
72
Upon finalization of the acquisition accounting during the second quarter 2019, $1.8 million was transferred between intangible assets and goodwill. The goodwill of $1.8 million is related to the expected increased retail presence in geographic regions not previously served by the Company and is currently deductible for tax purposes.
Note 4. Derivative Instruments
Foreign Currency
The Company’s policy is to manage the risks associated with foreign exchange rate movements. The policy allows hedging up to 100% of its anticipated purchases of inventory over a forward period that will not exceed 12 rolling and consecutive months. The Company may, from time to time, hedge currency for non-inventory purchases, e.g., production equipment, not to exceed 90% of the purchase price. The Company did not execute any forward contracts during 2019. During 2018 the Company executed various forward contracts, none of which met hedge accounting requirements, for the purchase of €14.5 million with maturity dates ranging from March 2018 to January 2019. At December 31, 2019 and 2018, the Company had forward contracts for the purchase of €0.0 million and €1.5 million, respectively.
Interest Rate Swaps
The Company’s policy is to manage interest rate risk by reducing the volatility of future cash flows associated with debt instruments bearing interest at variable rates. In March 2018, the Company executed various interest rate swap agreements for a notional amount of $70 million with an expiration of December 2022. The swap agreements fix LIBOR at 2.755%. The swap agreements met the hedge accounting requirements; thus, any change in fair value is recorded to other comprehensive income. The Company uses the Shortcut Method to account for the swap agreements. The Shortcut Method assumes the hedge to be perfectly effective; thus, there is no ineffectiveness to be recorded in earnings.
Note 5. Fair Value of Financial Instruments
The estimated fair value amounts have been determined by the Company using the methods and assumptions described below. However, considerable judgment is required to interpret market data to develop estimates of fair value. Accordingly, the estimates presented herein are not necessarily indicative of the amounts the Company could realize in a current market exchange. The use of different market assumptions and/or estimation methodologies may have a material effect on the estimated fair value amounts.
Cash and Cash Equivalents
Cash and cash equivalents are, by definition, short-term. Thus, the carrying amount is a reasonable estimate of fair value.
Accounts Receivable
The fair value of accounts receivable approximates their carrying value due to their short-term nature.
Revolving Credit Facility
The fair value of the revolving credit facility approximates its carrying value as the interest rate fluctuates with changes in market rates.
Note Payable – IVG
The fair value of the IVG Note approximates its carrying value of $4.2 million due to the recency of the note’s issuance, relative to the year ended December 31, 2019.
Long-Term Debt
The Company’s 2018 Credit Facility bears interest at variable rates that fluctuate with market rates. The carrying values of the long-term debt instruments approximate their respective fair values. As of December 31, 2019, the fair value of the 2018 First Lien Term Loan approximated $146.0 million. As of December 31, 2018, the fair values of the 2018 First Lien Term Loans and the 2018 Second Lien Term Loan approximated $154.0 million and $40.0 million, respectively.
73
The Convertible Senior Notes bear interest at a rate of 2.50% per year and the fair value approximated $140.1 million, with a carrying value of $172.5 million as of December 31, 2019.
See Note 13, “Notes Payable and Long-Term Debt”, for further information regarding the Company’s long-term debt.
Foreign Exchange
At December 31, 2019 and 2018, we had forward contracts for the purchase of €0.0 million and €1.5 million, respectively. The fair value of the foreign exchange contracts was based upon the quoted market price that resulted in no gain or loss for the year ended December 31, 2019 and a loss of approximately $0.1 million for the year ended December 31, 2018. As there were no open contracts as of December 31, 2019, there is no resulting balance sheet position related to the fair value. The fair value of the foreign exchange contracts resulted in a liability of approximately $0.1 million as of December 31, 2018.
Interest Rate Swaps
The Company had swap contracts for a total notional amount of $70 million at December 31, 2019 and 2018. The fair values of the swap contracts are based upon quoted market prices for similar instruments, thus leading to a level 2 distinction within the fair value hierarchy, and resulted in a liability of $2.5 million and $0.9 million, respectively, as of December 31, 2019 and 2018.
Note 6. Inventories
The components of inventories are as follows:
December 31, 2019 |
December 31, 2018 |
|||||
Raw materials and work in process |
$ | 7,050 | $ | 2,722 | ||
Leaf tobacco |
32,763 | 34,977 | ||||
Finished goods - Smokeless products |
5,680 | 6,321 | ||||
Finished goods - Smoking products |
13,138 | 14,666 | ||||
Finished goods - NewGen products |
17,111 | 37,194 | ||||
Other |
989 | 738 | ||||
Gross Inventory |
76,731 | 96,618 | ||||
LIFO reserve |
(5,752 | ) |
(5,381 | ) |
||
Net Inventory |
$ | 70,979 | $ | 91,237 | ||
The following represents the inventory valuation allowance roll-forward, for the years ended December 31:
2019 |
2018 |
|||||
Balance at beginning of period |
$ | (2,504 | ) |
$ | (459 | ) |
Charged to cost and expense |
(20,001 | ) |
(2,132 | ) |
||
Deductions for inventory disposed |
1,003 | 263 | ||||
Other |
— | (176 | ) |
|||
Balance at end of period |
$ | (21,502 | ) |
$ | (2,504 | ) |
Inventory reserves increased as a result of additional reserves necessary for products in our NewGen segment primarily from increased regulation.
74
Note 7. Other Current Assets
Other current assets consists of:
December 31, 2019 |
December 31, 2018 |
|||||
Inventory deposits |
$ | 4,012 | $ | 9,739 | ||
Prepaid taxes |
3,673 | 580 | ||||
Other |
8,430 | 4,375 | ||||
Total |
$ | 16,115 | $ | 14,694 | ||
Note 8. Property, Plant and Equipment
Property, plant and equipment consists of:
December 31, 2019 |
December 31, 2018 |
|||||
Land |
$ | 22 | $ | 22 | ||
Buildings and improvements |
2,655 | 2,320 | ||||
Leasehold improvements |
2,567 | 2,101 | ||||
Machinery and equipment |
14,516 | 13,292 | ||||
Furniture and fixtures |
8,502 | 5,045 | ||||
Gross property, plant and equipment |
28,262 | 22,780 | ||||
Accumulated depreciation |
(14,446 | ) |
(12,191 | ) |
||
Net property, plant and equipment |
$ | 13,816 | $ | 10,589 | ||
Note 9. Deferred Financing Costs
Deferred financing costs relating to the revolving credit facility consist of:
December 31, 2019 |
December 31, 2018 |
|||||
Deferred financing costs, net of accumulated amortization of $410 and $174, respectively |
$ | 890 | $ | 870 | ||
Note 10. Goodwill and Other Intangible Assets
The following table summarizes goodwill by segment:
Smokeless |
Smoking |
NewGen |
Total |
|||||||||
Balance as of December 31, 2017 |
$ | 32,590 | $ | 96,107 | $ | 5,923 | $ | 134,620 | ||||
Acquisitions |
— | — | 11,319 | 11,319 | ||||||||
Balance as of December 31, 2018 |
$ | 32,590 | $ | 96,107 | $ | 17,242 | $ | 145,939 | ||||
Adjustments |
— | — | 1,907 | 1,907 | ||||||||
Acquisitions |
— | — | 6,436 | 6,436 | ||||||||
Balance as of December 31, 2019 |
$ | 32,590 | $ | 96,107 | $ | 25,585 | $ | 154,282 | ||||
The following tables summarize information about the Company’s allocation of other intangible assets. Gross carrying amounts of unamortized, indefinite life intangible assets are shown below:
December 31, 2019 |
December 31, 2018 |
|||||||||||||||||
Smokeless |
NewGen |
Total |
Smokeless |
NewGen |
Total |
|||||||||||||
Unamortized, indefinite life intangible assets: |
||||||||||||||||||
Trade names |
$ | 10,871 | $ | 10,786 | $ | 21,657 | $ | 10,871 | $ | 10,786 | $ | 21,657 | ||||||
Formulas |
53 | — | 53 | 53 | — | 53 | ||||||||||||
Total |
$ | 10,924 | $ | 10,786 | $ | 21,710 | $ | 10,924 | $ | 10,786 | $ | 21,710 | ||||||
75
Amortized intangible assets included within the NewGen segment consists of:
December 31, 2019 |
December 31, 2018 |
|||||||||||
Gross Carrying |
Accumulated Amortization |
Gross Carrying |
Accumulated Amortization |
|||||||||
Amortized intangible assets: |
||||||||||||
Customer relationships (useful life of 8-10 years) |
$ | 6,936 | $ | 2,283 | $ | 6,936 | $ | 1,453 | ||||
Trade names (useful life of 15 years) |
7,158 | 714 | 7,578 | 208 | ||||||||
Franchise agreements (useful life of 8 years) |
780 | 130 | 780 | 44 | ||||||||
Non-compete agreements (useful life of 3.5 years) |
100 | 88 | 100 | 60 | ||||||||
Total |
$ | 14,974 | $ | 3,215 | $ | 15,394 | $ | 1,765 | ||||
Annual amortization expense for each of the next five years is estimated to be approximately $1.4 million, assuming no additional transactions occur that require the amortization of intangible assets.
Note 11. Other Assets
Other assets consists of:
December 31, 2019 |
December 31, 2018 |
|||||
Equity investments |
$ | 5,421 | $ | 2,421 | ||
Pension assets |
1,686 | 1,223 | ||||
Other |
3,566 | 592 | ||||
Total |
$ | 10,673 | $ | 4,236 | ||
In July 2019 we obtained a 30% stake in Canadian distribution entity, ReCreation Marketing (“ReCreation”) for $1 million paid at closing. We may invest an additional $2 million, if certain performance metrics are achieved, with options to acquire up to a 50% ownership position. We received board seats aligned with our ownership position. 2019 sales to ReCreation of RipTide products was $0.2 million, which was included in accounts receivable at December 31, 2019.
In November 2018, the Company paid $2.0 million to acquire a minority ownership position (19.99%) in Canadian American Standard Hemp (“CASH”). CASH is headquartered in Warwick, Rhode Island, and manufactures cannabidiol isolate (“CBD”) developed through highly efficient and proprietary processes. The investment in CASH positions the Company to participate in the market for hemp-derived products. In the fourth quarter 2019 CASH completed a fundraising round, resulting in the fair value of our investment increasing to $4.0 million. This resulted in a gain of $2 million which is recorded in investment income for 2019. 2019 purchases of inventory from CASH was $0.6 million. There were no amounts outstanding at December 31, 2019.
In December 2018, the Company acquired a minority ownership position in General Wireless Operations, Inc. (d/b/a RadioShack; “RadioShack”) from 5G gaming LLC, which is owned by Standard General LP, for $0.4 million. Standard General LP has a controlling interest in the Company and qualifies as a related party. The Company will work together with RadioShack on product development and sourcing teams in China. Furthermore, the Company paid $0.2 million in consulting fees in 2019 and purchased $1.1 million of finished goods inventory from Radio Shack during 2018. There were no amounts outstanding at December 31, 2019.
Note 12. Accrued Liabilities
Accrued liabilities at consists of:
December 31, 2019 |
December 31, 2018 |
|||||
Accrued payroll and related items |
$ | 5,267 | $ | 6,063 | ||
Customer returns and allowances |
6,160 | 3,634 | ||||
Taxes payable |
705 | 2,138 | ||||
Lease liabilities |
2,218 | — | ||||
Accrued interest |
1,909 | 363 | ||||
Other |
10,261 | 10,727 | ||||
Total |
$ | 26,520 | $ | 22,925 | ||
76
Note 13. Notes Payable and Long-Term Debt
Notes payable and long-term debt consists of the following in order of preference:
December 31, 2019 |
December 31, 2018 |
|||||
2018 First Lien Term Loan |
$ | 146,000 | $ | 154,000 | ||
2018 Second Lien Term Loan |
— | 40,000 | ||||
Convertible Senior Notes |
172,500 | — | ||||
Note payable - IVG |
4,240 | 4,000 | ||||
Gross notes payable and long-term debt |
322,740 | 198,000 | ||||
Less deferred finance charges |
(6,466 | ) |
(3,285 | ) |
||
Less debt discount |
(32,083 | ) |
— | |||
Less current maturities |
(15,240 | ) |
(8,000 | ) |
||
Net notes payable and long-term debt |
$ | 268,951 | $ | 186,715 | ||
2018 Credit Facility
On March 7, 2018, the Company entered into a $250 million credit facility consisting of a $160 million 2018 First Lien Term Loan with Fifth Third Bank, as administrative agent, and other lenders, and a $50 million 2018 Revolving Credit Facility (collectively, the “2018 First Lien Credit Facility”) in addition to a $40 million 2018 Second Lien Term Loan (together with the 2018 First Lien Credit Facility, the “2018 Credit Facility”) with Prospect Capital Corporation, as administrative agent, and other lenders. The 2018 Credit Facility retained the $40 million accordion feature of the 2017 Credit Facility. Proceeds from the 2018 Credit Facility were used to repay, in full, the 2017 Credit Facility. The Company incurred a loss on extinguishment of debt of $2.4 million in the first quarter of 2018 as a result of the refinancing.
The 2018 Credit Facility contains customary events of default including payment defaults, breaches of representations and warranties, covenant defaults, cross-defaults to certain other material indebtedness in excess of specified amounts, certain events of bankruptcy and insolvency, certain ERISA events, judgments in excess of specified amounts, and change in control defaults. The 2018 Credit Facility also contains certain negative covenants customary for facilities of these types including covenants that, subject to exceptions described in the 2018 Credit Facility, restrict the ability of the Company and its subsidiary guarantors: (i) to pledge assets, (ii) to incur additional indebtedness, (iii) to pay dividends, (iv) to make distributions, (v) to sell assets, and (vi) to make investments. See Note 23, “ Dividends”, for further information regarding dividend restrictions.
2018 First Lien Credit Facility: The 2018 First Lien Term Loan and the 2018 Revolving Credit Facility bear interest at LIBOR plus a spread of 2.75% to 3.50% based on the Company’s senior leverage ratio. The 2018 First Lien Term Loan has quarterly required payments of $2.0 million beginning June 30, 2018, increasing to $3.0 million on June 30, 2020, and increasing to $4.0 million on June 30, 2022. The 2018 First Lien Credit Facility has a maturity date of March 7, 2023. The 2018 First Lien Term Loan is secured by a first priority lien on substantially all of the assets of the borrowers and the guarantors thereunder, including a pledge of the Company’s capital stock, other than certain excluded assets (the “Collateral”). In connection with the Convertible Senior Notes offering, the Company entered into a First Amendment (“the Amendment”) to the First Lien Credit Agreement, with Fifth Third Bank, as administrative agent, and other lenders and certain other lender parties thereto. The Amendment was entered into primarily to permit the Company to issue up to $200 million of convertible senior notes, enter into certain capped call transactions in connection with the issuance of such notes and to use the proceeds from the issuance of the notes to repay amounts outstanding under the Company’s Second Lien Credit Agreement and use the remaining proceeds for acquisitions and investments. In connection with the Amendment, fees of $0.7 million were incurred. The 2018 First Lien Credit Facility contains certain financial covenants, which were amended in connection with the Convertible Senior Notes offering in the third quarter 2019. The covenants include maximum senior leverage ratio of 3.00x with step-downs to 2.50x, a maximum total leverage ratio of 5.50x with step-downs to 5.00x, and a minimum fixed charge coverage ratio of 1.20x. In the first quarter of 2020, the financial covenants were amended to permit certain add-backs related to PMTA in the definition of Consolidated EBITDA for the period of October 1, 2019 until September 30, 2020. Based on an excess cash covenant for the facility, a principal payment of $4.5 million was due in the second quarter 2019. All parties agreed to waive the payment, resulting in consent fees of $0.1 million. The weighted average interest rate of the 2018 First Lien Term Loan was 4.55% at December 31, 2019.
77
At December 31, 2019, the Company had no borrowings outstanding under the 2018 Revolving Credit Facility. The $50.0 million unused portion of the 2018 Revolving Credit Facility is reduced by letters of credit from Fifth Third Bank totaling $3.7 million, resulting in $46.3 million of availability under the 2018 Revolving Credit Facility at December 31, 2019.
2018 Second Lien Credit Facility: The 2018 Second Lien Credit Facility bore interest at a rate of LIBOR plus 7.00% and had a maturity date of March 7, 2024. The 2018 Second Lien Term Loan was secured by a second priority interest in the Collateral and was guaranteed by the same entities as the 2018 First Lien Term Loan. The 2018 Second Lien Credit Facility contained certain financial covenants including a maximum senior leverage ratio of 3.75x with step-downs to 3.50x, a maximum total leverage ratio of 4.75x with step-downs to 4.50x, and a minimum fixed charge coverage ratio of 1.10x. Based on an excess cash covenant for the facility, a $4.5 million principal payment was made in the second quarter 2019, resulting in a $0.2 million loss on extinguishment of debt. The Company used a portion of the proceeds from the issuance of the Convertible Senior Notes to prepay all outstanding amounts related to the 2018 Second Lien Credit Facility in the third quarter 2019. The principal paid in the third quarter amounted to $35.5 million, and the transaction resulted in a $1.1 million loss on extinguishment of debt.
Convertible Senior Notes
In July 2019 the Company closed an offering of $172.5 million in aggregate principal amount of our 2.50% Convertible Senior Notes due July 15, 2024 (the “Convertible Senior Notes”). The Convertible Senior Notes bear interest at a rate of 2.50% per year, payable semiannually in arrears on January 15 and July 15 of each year, beginning on January 15, 2020. The Convertible Senior Notes will mature on July 15, 2024, unless earlier repurchased, redeemed or converted. The Convertible Senior Notes are senior unsecured obligations of the Company.
The Convertible Senior Notes are convertible into approximately 3,202,808 shares of our voting common stock under certain circumstances prior to maturity at a conversion rate of 18.567 shares per $1,000 principal amount of the Convertible Senior Notes, which represents a conversion price of approximately $53.86 per share, subject to adjustment under certain conditions, but will not be adjusted for any accrued and unpaid interest. Upon conversion, the Company may pay cash, shares of common stock or a combination of cash and stock, as determined by the Company at its discretion. The conditions required to allow the holders to convert their Convertible Senior Notes were not met as of December 31, 2019.
Under GAAP, certain convertible debt instruments that may be settled in cash on conversion are required to be separately accounted for as liability and equity components of the instrument in a manner that reflects the issuer’s non-convertible debt borrowing rate. Accordingly, in accounting for the issuance of the Convertible Senior Notes, the Company separated the Convertible Senior Notes into liability and equity components. The carrying amount of the liability component was calculated by measuring the fair value of a similar liability that does not have an associated convertible feature. The carrying amount of the equity component, which is recognized as a debt discount, represents the difference between the proceeds from the issuance of the Convertible Senior Notes and the fair value of the liability component of the Convertible Senior Notes. The excess of the principal amount of the liability component over its carrying amount (“debt discount”), $35.0 million, will be amortized to interest expense using an effective interest rate of 7.5% over the expected life of the Convertible Senior Notes. The equity component is not remeasured as long as it continues to meet the criteria for equity classification. Interest expense includes $2.9 million of amortization for the year ended December 31, 2019.
In accounting for the issuance costs related to the issuance of the Convertible Senior Notes, the Company allocated the total amount incurred to the liability and equity components based on their relative values. Debt issuance costs attributable to the liability component are amortized to interest expense using the effective interest method over the expected life of the Convertible Senior Notes, $4.7 million, and the debt issuance costs attributable to the equity component, $1.2 million, are netted with the equity component of stockholders’ equity (deficit).
In connection with the Convertible Senior Notes offering, the Company entered into privately negotiated capped call transactions with certain financial institutions. The capped call transactions have a strike price of $53.86 per and a cap price of $82.86 per, and are exercisable when, and if, the Convertible Senior Notes are converted. The Company paid $20.53 million for these capped calls and charged that amount to additional paid-in capital.
78
Note Payable – IVG
In September 2018, the Company issued a note payable to IVG’s former shareholders (“IVG Note”). The IVG Note is $4.0 million principal with 6.0% interest compounding annually and matures on March 5, 2020. The IVG Note is subject to customary defaults including defaults for nonpayment, nonperformance, any material breach under the purchase agreement, and bankruptcy or insolvency.
The carrying amount of the IVG Note is $4.2 million as of December 31, 2019.
Note 14. Income Taxes
Income tax expense (benefit) for the years ended December 31 consists of the following components:
2019 |
2018 |
2017 |
|||||||||||||||||||||||||
Current |
Deferred |
Total |
Current |
Deferred |
Total |
Current |
Deferred |
Total |
|||||||||||||||||||
Federal |
$ | 5,281 | $ | (3,282 | ) |
$ | 1,999 | $ | 2,326 | $ | 3,165 | $ | 5,491 | $ | 329 | $ | 4,772 | $ | 5,101 | ||||||||
State and Local |
982 | (937 | ) |
45 | 1,394 | (600 | ) |
794 | 1,770 | 409 | 2,179 | ||||||||||||||||
Total |
$ | 6,263 | $ | (4,219 | ) |
$ | 2,044 | $ | 3,720 | $ | 2,565 | $ | 6,285 | $ | 2,099 | $ | 5,181 | $ | 7,280 | ||||||||
Deferred tax assets and liabilities consists of:
December 31, 2019 |
December 31, 2018 |
|||||||||||
Assets |
Liabilities |
Assets |
Liabilities |
|||||||||
Inventory |
$ | 7,705 | $ | — | $ | 3,004 | $ | — | ||||
Property, plant, and equipment |
— | 2,076 | — | 1,445 | ||||||||
Goodwill and other intangible assets |
— | 7,672 | — | 7,386 | ||||||||
Accrued pension and post-retirement costs |
— | 943 | 202 | — | ||||||||
State NOL carryforward |
3,225 | — | 2,842 | — | ||||||||
Unrealized loss on investments |
580 | — | 351 | — | ||||||||
Leases |
3,393 | 3,099 | — | — | ||||||||
Original issue discount |
4,806 | 8,118 | — | — | ||||||||
Other |
4,407 | 555 | 3,424 | 440 | ||||||||
Gross deferred income taxes |
24,116 | 22,463 | 9,823 | 9,271 | ||||||||
Valuation allowance |
(3,225 | ) |
— | (2,842 | ) |
— | ||||||
Net deferred income taxes |
$ | 20,891 | $ | 22,463 | $ | 6,981 | $ | 9,271 | ||||
At December 31, 2019, the Company had state NOL carryforwards for income tax purposes of approximately $65.2 million, which expire between 2020 and 2039, $13.0 million of which has an indefinite carryforward period. The Company has determined that, at December 31, 2019 and 2018, its ability to realize future benefits of its state NOL carryforwards does not meet the “more likely than not” criteria in ASC 740, Income Taxes. Therefore, a valuation allowance of $3.2 million and $2.8 million has been recorded at December 2019 and 2018, respectively.
ASC 740-10-25 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not to be sustained upon examination by taxing authorities. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. The Company has determined that they did not have any uncertain tax positions requiring recognition as a result of the provisions of ASC 740-10-25. The Company’s policy is to recognize interest and penalties accrued on uncertain tax positions as part of interest expense. For the years ended December 31, 2019, 2018, and 2017, no estimated interest or penalties were recognized for the uncertainty of tax positions taken. The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions. In general, the Company is no longer subject to U.S. federal and state tax examinations for years prior to 2016.
79
Reconciliation of the federal statutory rate and the effective income tax rate for the years ended December 31 is as follows:
2019 |
2018 |
2017 |
|||||||
Federal statutory rate |
21.0 | % |
21.0 | % |
35.0 | % |
|||
State taxes |
0.0 | % |
3.3 | % |
8.1 | % |
|||
Permanent differences |
-6.7 | % |
-2.9 | % |
-16.1 | % |
|||
Other |
-3.8 | % |
-0.8 | % |
0.0 | % |
|||
Valuation allowance |
2.4 | % |
-0.7 | % |
0.0 | % |
|||
Effective income tax rate |
12.9 | % |
19.9 | % |
27.0 | % |
|||
The permanent differences for the year ended December 31, 2019, 2018, and 2017 are primarily related to income tax benefits of $4.6 million ($1.0 million tax effected), $5.4 million ($1.1 million tax effected), and $4.2 million ($1.1 million tax effected), respectively, as a result of stock option exercises.
Note 15. Pension and Postretirement Benefit Plans
The Company has a defined benefit pension plan. Benefits for hourly employees were based on a stated benefit per year of service, reduced by amounts earned in a previous plan. Benefits for salaried employees were based on years of service and the employees’ final compensation. The defined benefit pension plan is frozen. The Company’s policy is to make the minimum amount of contributions that can be deducted for federal income taxes. The Company expects to make no contributions to the pension plan in 2020. In the second quarter of 2018, the Company made mutually agreed upon lump-sum payments to certain individuals covered by the defined benefit pension plan which resulted in a curtailment loss of approximately $0.3 million during the second quarter of 2018, which is reported within “Net periodic benefit (income), excluding service cost” within the Consolidated Statements of Income. In the fourth quarter 2019, the Company elected to terminate the defined benefit pension plan, effective December 31, 2019 with final distributions to be made in 2020.
The Company sponsored a defined benefit postretirement plan that covered hourly employees. This plan provides medical and dental benefits. This plan is contributory with retiree contributions adjusted annually. The Company’s policy is to make contributions equal to benefits paid during the year. In the fourth quarter 2019, the Company amended the plan to cease benefits effective June 30, 2020. The plan amendment eliminated a significant amount of the benefits under the plan, resulting in a curtailment of $3.1 million. The curtailment resulted in $1.8 million being reclassified from other comprehensive income to income. The total gain on the curtailment was $4.9 million and is recorded in net periodic (benefit) expense, excluding service cost in the income statement. The Company expects to contribute approximately $0.1 million to its postretirement plan in 2020 for payment of benefits.
The following tables provide a reconciliation of the changes in the plans’ benefit obligations and fair value of assets for the years ended December 31, 2019 and 2018, and a statement of the funded status:
Pension Benefits |
Postretirement Benefits |
|||||||||||
2019 |
2018 |
2019 |
2018 |
|||||||||
Reconciliation of benefit obligations: |
||||||||||||
Benefit obligation at January 1 |
$ | 13,700 | $ | 17,121 | $ | 3,305 | $ | 4,217 | ||||
Service cost |
104 | 104 | — | — | ||||||||
Interest cost |
520 | 553 | 101 | 117 | ||||||||
Actuarial loss (gain) |
916 | (1,157 | ) |
— | (527 | ) |
||||||
Assumptions |
— | — | — | (323 | ) |
|||||||
Settlement/curtailment |
— | (1,866 | ) |
(3,207 | ) |
— | ||||||
Benefits paid |
(1,023 | ) |
(1,055 | ) |
(84 | ) |
(179 | ) |
||||
Benefit obligation at December 31 |
$ | 14,217 | $ | 13,700 | $ | 115 | $ | 3,305 | ||||
80
Pension Benefits |
Postretirement Benefits |
|||||||||||
2019 |
2018 |
2019 |
2018 |
|||||||||
Reconciliation of fair value of plan assets: |
||||||||||||
Fair value of plan assets at January 1 |
$ | 14,923 | $ | 17,517 | $ | — | $ | — | ||||
Actual return on plan assets |
2,003 | 327 | — | — | ||||||||
Employer contributions |
— | — | 84 | 179 | ||||||||
Settlement/curtailment |
— | (1,866 | ) |
— | — | |||||||
Benefits paid |
(1,023 | ) |
(1,055 | ) |
(84 | ) |
(179 | ) |
||||
Fair value of plan assets at December 31 |
$ | 15,903 | $ | 14,923 | $ | — | $ | — | ||||
Funded status: |
||||||||||||
Funded status at December 31 |
$ | 1,686 | $ | 1,223 | $ | (115 | ) |
$ | (3,305 | ) |
||
Unrecognized net actuarial loss (gain) |
1,827 | 2,416 | (54 | ) |
(1,929 | ) |
||||||
Net amount recognized |
$ | 3,513 | $ | 3,639 | $ | (169 | ) |
$ | (5,234 | ) |
||
Accumulated benefit obligations did not exceed plan assets at December 31, 2019 or 2018, for the Company’s pension plan.
The asset allocation for the Company’s defined benefit plan, by asset category, follows:
Target Allocation |
Percentage of Plan Assets at December 31, |
||||||||
2020 |
2019 |
2018 |
|||||||
Asset category: |
|||||||||
Debt securities |
100.0 | % |
88.5 | % |
84.8 | % |
|||
Cash |
0.0 | % |
11.5 | % |
15.2 | % |
|||
Total |
100.0 | % |
100.0 | % |
100.0 | % |
|||
The asset’s or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
Following is the description of the valuation methodologies used for assets measured at fair value subsequent to initial recognition. These methods may produce a fair value calculation that may not be indicative of net realizable value or reflective of future fair values. Furthermore, while the Company believes its valuation methods are appropriate and consistent with those of other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different fair value measurement at the reporting date. There have been no changes in the methodologies used at December 31, 2019 and 2018.
| • | Pooled Separate Accounts. Valued at the net asset value (NAV) of shares held by the plan at year end. |
| • | Guaranteed Deposit Account. Valued at contract value, which approximates fair value. |
| • | Assets measured at fair value on a recurring basis. The table below presents the balances of the plan’s assets measured at fair value on a recurring basis by level within the fair value hierarchy: |
Total |
Level 1 |
Level 2 |
Level 3 |
|||||||||
Pooled separate accounts |
$ | 14,079 | $ | — | $ | 14,079 | $ | — | ||||
Guaranteed deposit account |
1,824 | — | — | 1,824 | ||||||||
Total assets at fair value as of December 31, 2019 |
$ | 15,903 | $ | — | $ | 14,079 | $ | 1,824 | ||||
Pooled separate accounts |
$ | 12,658 | $ | — | $ | 12,658 | $ | — | ||||
Guaranteed deposit account |
2,265 | — | — | 2,265 | ||||||||
Total assets at fair value as of December 31, 2018 |
$ | 14,923 | $ | — | $ | 12,658 | $ | 2,265 | ||||
81
The table below sets forth a summary of the changes in the fair value of the Guaranteed Deposit Account:
Guaranteed Deposit Account |
|||
Balance at January 1, 2018 |
$ | 4,721 | |
Total gains (losses), realized/unrealized |
|||
Return on plan assets |
81 | ||
Purchases, sales, and settlements, net |
(2,537 | ) |
|
Balance at December 31, 2018 |
$ | 2,265 | |
Total gains (losses), realized/unrealized |
|||
Return on plan assets |
$ | 45 | |
Purchases, sales, and settlements, net |
(486 | ) |
|
Balance at December 31, 2019 |
$ | 1,824 | |
The Company’s investment philosophy is to earn a reasonable return without subjecting plan assets to undue risk. The Company uses one management firm to manage plan assets, which are invested in equity and debt securities. The Company’s investment objective is to match the duration of the debt securities with the expected payments.
The following table provides the amounts recognized in the consolidated balance sheets as of December 31:
Pension Benefits |
Postretirement Benefits |
|||||||||||
2019 |
2018 |
2019 |
2018 |
|||||||||
Prepaid asset |
$ | 1,686 | $ | 1,223 | $ | — | $ | — | ||||
Accrued benefit cost |
— | — | (115 | ) |
(3,305 | ) |
||||||
Accumulated other comprehensive loss, unrecognized net gain (loss) |
1,827 | 2,416 | (54 | ) |
(1,929 | ) |
||||||
Total |
$ | 3,513 | $ | 3,639 | $ | (169 | ) |
$ | (5,234 | ) |
||
The amounts in accumulated other comprehensive income that are expected to be recognized in net periodic benefit costs in 2020 is a loss of $1.8 million for pension.
The following table provides the components of net periodic pension and postretirement benefit costs and total costs for the plans for the years ended December 31:
Pension Benefits |
Postretirement Benefits |
|||||||||||||||||
2019 |
2018 |
2017 |
2019 |
2018 |
2017 |
|||||||||||||
Service cost |
$ | 104 | $ | 104 | $ | 104 | $ | — | $ | — | $ | — | ||||||
Interest cost |
520 | 553 | 649 | 101 | 117 | 144 | ||||||||||||
Expected return on plan assets |
(645 | ) |
(949 | ) |
(1,024 | ) |
— | — | — | |||||||||
Amortization of (gains) losses |
147 | 186 | 463 | (169 | ) |
(81 | ) |
(52 | ) |
|||||||||
Curtailment loss (gain) |
— | 306 | — | (4,915 | ) |
— | — | |||||||||||
Net periodic benefit cost |
$ | 126 | $ | 200 | $ | 192 | $ | (4,983 | ) |
$ | 36 | $ | 92 | |||||
The Company is required to make assumptions regarding such variables as the expected long-term rate of return on plan assets and the discount rate applied to determine service cost and interest cost. The rate of return on assets used is determined based upon analysis of the plans’ historical performance relative to the overall markets and mix of assets. The assumptions listed below represent management’s review of relevant market conditions and have been adjusted as appropriate. A discount rate was not used for postretirement benefits in 2019 as all benefits will be paid in less than one year. The weighted average assumptions used in the measurement of the Company’s benefit obligation are as follows:
Pension Benefits |
Postretirement Benefits |
||||||||
2019 |
2018 |
2018 |
|||||||
Discount rate |
3.00 | % |
4.00 | % |
4.25 | % |
|||
82
The weighted average assumptions used to determine net periodic pension and postretirement costs are as follows:
Pension Benefits |
Postretirement Benefits |
||||||||
2019 |
2018 |
2018 |
|||||||
Discount rate |
4.0 | % |
3.8 | % |
3.3 | % |
|||
Expected return on plan assets |
4.5 | % |
6.0 | % |
0.0 | % |
|||
The following benefit payments, which reflect expected future service, as appropriate, are expected to be paid:
Period |
Pension Benefits |
||
2020 |
$ | 1,036 | |
2021 |
1,028 | ||
2022 |
1,003 | ||
2023 |
994 | ||
2024 |
964 | ||
2025 - 2029 |
$ | 4,489 | |
The Company also sponsors a voluntary 401(k) retirement savings plan. Eligible employees may elect to contribute up to 15% of their annual earnings subject to certain limitations. For the 2019 and 2018 Plan Years, the Company contributed 4% to those employees contributing 4% or greater. For those employees contributing less than 4%, the Company matched the contribution by 100%. Additionally, for all years presented, the Company made discretionary contributions of 1% to all employees, regardless of an employee’s contribution level. Company contributions to this plan were approximately $1.5 million for 2019, $1.2 million for 2018, and $0.9 million for 2017.
Note 16. Lease Commitments
As of January 1, 2019, the Company adopted ASU 2016-02, Leases (Topic 842). The main impact to the financial statements is the recognition of lease liabilities and right of use assets. The Company’s leases consist primarily of leased property for manufacturing warehouse, head offices and retail space as well as vehicle leases. In general, the Company does not recognize any renewal periods within the lease terms as there are not significant barriers to ending the lease at the initial term. Lease and non-lease components are accounted for as a single lease component.
Leases with an initial term of 12 months or less are not recorded on the balance sheet. Lease expense for these leases is recognized on a straight-line basis over the lease term.
The components of lease expense consists of the following:
For the year ended December 31, 2019 |
|||
Operating lease cost |
|||
Cost of sales |
$ | 874 | |
Selling, general and administrative |
2,973 | ||
Variable lease cost(1) |
463 | ||
Short-term lease cost |
147 | ||
Sublease income |
(110 | ) |
|
Total |
$ | 4,347 | |
| (1) | Variable lease cost includes elements of a contract that do not represent a good or service but for which the lessee is responsible for paying. |
83
December 31, 2019 |
|||
Assets: |
|||
Right of use assets |
$ | 12,130 | |
Total lease assets |
$ | 12,130 | |
Liabilities: |
|||
Current lease liabilities(2) |
$ | 2,218 | |
Long-term lease liabilities |
11,067 | ||
Total lease liabilities |
$ | 13,285 | |
| (2) | Reported within accrued liabilities on the balance sheet |
|
|
As of
December 31, 2019 |
|
Weighted-average remaining lease term - operating leases
|
8.1 years
|
|
Weighted-average discount rate - operating leases
|
6.07%
|
Nearly all the lease contracts for the Company do not provide a readily determinable implicit rate. For these contracts, the Company estimated the incremental borrowing rate based on information available upon adoption of ASU 2016-02. The Company applied a consistent method in periods after the adoption of ASU 2016-02 to estimate the incremental borrowing rate.
Maturities of lease liabilities consisted of the following:
December 31, 2019 |
|||
2020 |
$ | 2,924 | |
2021 |
2,730 | ||
2022 |
2,165 | ||
2023 |
1,782 | ||
2024 |
1,028 | ||
Years thereafter |
6,297 | ||
Total lease payments |
$ | 16,926 | |
Less: Imputed interest |
3,641 | ||
Present value of lease liabilities |
$ | 13,285 | |
Minimum lease payments for operating leases that had initial or remaining non-cancelable lease terms in excess of one year consisted of the following:
Year |
Payments |
||
2019 |
$ | 1,938 | |
2020 |
1,613 | ||
2021 |
727 | ||
2022 |
276 | ||
2023 |
114 | ||
2024 |
21 | ||
Total |
$ | 4,689 | |
At December 31, 2019, the Company had operating leases with lease liabilities of $1.5 million which had not yet commenced. The leases are primarily related to vehicles for business use. The Company recognized $0.3 million impairment of right of use assets in the fourth quarter 2019 related to planned store closures.
84
Note 17. Share Incentive Plans
On April 28, 2016, the Board of Directors of the Company adopted the Turning Point Brands, Inc., 2015 Equity Incentive Plan (the “2015 Plan”), pursuant to which awards may be granted to employees, non-employee directors, and consultants. In addition, the 2015 Plan provides for the granting of nonqualified stock options to employees of the Company or any subsidiary of the Company. Pursuant to the 2015 Plan, 1,400,000 shares of the Company’s voting common stock are reserved for issuance as awards to employees, non-employee directors, and consultants as compensation for past or future services or the attainment of certain performance goals. The 2015 Plan is scheduled to terminate on April 27, 2026. The 2015 Plan is administrated by a committee (the “Committee”) of the Company’s Board of Directors. The Committee determines the vesting criteria for the awards, with such criteria to be specified in the award agreement. As of December 31, 2019, 16,159 shares of restricted stock, 355,258 performance-based restricted stock units, and 459,070 options have been granted to employees of the Company under the 2015 Plan, net of forfeitures. There are 569,513 shares available for grant under the 2015 Plan.
On February 8, 2006, the Board of Directors of the Company adopted the 2006 Equity Incentive Plan (the “2006 Plan”) of North Atlantic Holding Company, Inc., pursuant to which awards may be granted to employees. The 2006 Plan provides for the granting of nonqualified stock options and restricted stock awards to employees. Upon the adoption of the Company’s 2015 Equity Incentive Plan in connection with its IPO, the Company determined no additional grants would be made under the 2006 Plan. However, all awards issued under the 2006 Plan that have not been previously terminated or forfeited remain outstanding and continue unaffected.
There are no shares available for grant under the 2006 Plan. Stock option activity for the 2006 and 2015 Plans is summarized below:
Stock Option Shares |
Weighted Average Exercise Price |
Weighted Average Grant Date Fair Value |
|||||||
Outstanding, December 31, 2017 |
763,672 | $ | 5.73 | $ | 2.36 | ||||
Granted |
124,100 | 21.27 | 6.33 | ||||||
Exercised |
(209,943 | ) |
3.97 | 1.47 | |||||
Forfeited |
(18,255 | ) |
13.46 | 3.90 | |||||
Outstanding, December 31, 2018 |
659,574 | 9.00 | 3.34 | ||||||
Granted |
180,780 | 43.89 | 14.34 | ||||||
Exercised |
(129,067 | ) |
5.72 | 2.58 | |||||
Forfeited |
(14,571 | ) |
34.55 | 11.10 | |||||
Outstanding, December 31, 2019 |
696,716 | $ | 18.13 | $ | 6.17 | ||||
Under the 2006 Plan, the total intrinsic value of options exercised during the years ended December 31, 2019, 2018, and 2017, was $5.0 million, $5.7 million, and $11.9 million, respectively.
At December 31, 2019, under the 2006 Plan, the outstanding stock options’ exercise price for 310,319 options is $3.83 per share, all of which are exercisable. The weighted average of the remaining lives of the outstanding stock options is approximately 3.85 years for the options with the $3.83 exercise price. The Company estimates the expected life of these stock options is ten years from the date of grant. For the $3.83 per share options, the weighted average fair value of options was determined using the Black-Scholes model assuming a ten-year life from grant date, a current share price and exercise price of $3.83, a risk-free interest rate of 3.57%, a volatility of 40%, and no assumed dividend yield. Based on these assumptions, the fair value of these options is approximately $2.17 per share option granted.
At December 31, 2019, under the 2015 Plan, the risk-free interest rate is based on the U.S. Treasury rate for the expected life at the time of grant. The expected volatility is based on the average long-term historical volatilities of peer companies. We intend to continue to consistently use the same group of publicly traded peer companies to determine expected volatility until sufficient information regarding volatility of our share price becomes available or the selected companies are no longer suitable for this purpose. Due to our limited trading history, we are using the “simplified method” to calculate expected holding periods, which represent the periods of time for which options
85
granted are expected to be outstanding. We will continue to use this method until we have sufficient historical exercise experience to give us confidence in the reliability of our calculations. The fair values of these options were determined using the Black-Scholes option pricing model.
The following table outlines the assumptions based on the number of options granted under the 2015 Plan.
February 10, 2017 |
May 17, 2017 |
March 7, 2018 |
March 13, 2018 |
March 20, 2019 |
October 24, 2019 |
|||||||||||||
Number of options granted |
40,000 | 93,819 | 98,100 | 26,000 | 155,780 | 25,000 | ||||||||||||
Options outstanding at December 31, 2019 |
28,700 | 71,514 | 87,353 | 26,000 | 147,830 | 25,000 | ||||||||||||
Number exercisable at December 31, 2019 |
17,150 | 47,529 | 30,362 | 17,420 | — | — | ||||||||||||
Exercise price |
$ | 13.00 | $ | 15.41 | $ | 21.21 | $ | 21.49 | $ | 47.58 | $ | 20.89 | ||||||
Remaining lives |
7.12 | 7.38 | 8.19 | 8.21 | 9.22 | 9.82 | ||||||||||||
Risk free interest rate |
1.89 | % |
1.76 | % |
2.65 | % |
2.62 | % |
2.34 | % |
1.58 | % |
||||||
Expected volatility |
27.44 | % |
26.92 | % |
28.76 | % |
28.76 | % |
30.95 | % |
31.93 | % |
||||||
Expected life |
6.000 | 6.000 | 6.000 | 5.495 | 6.000 | 6.000 | ||||||||||||
Dividend yield |
— | — | 0.83 | % |
0.82 | % |
0.42 | % |
0.95 | % |
||||||||
Fair value at grant date |
$ | 3.98 | $ | 4.60 | $ | 6.37 | $ | 6.18 | $ | 15.63 | $ | 6.27 | ||||||
The Company has recorded compensation expense related to the options based on the provisions of ASC 718 under which the fixed portion of such expense is determined as the fair value of the options on the date of grant and amortized over the vesting period. The Company recorded compensation expense related to the options of approximately $1.7 million and $0.7 million for the years ended December 31, 2019 and 2018, respectively. Total unrecognized compensation expense related to options at December 31, 2019, is $1.1 million, which will be expensed over 1.94 years.
Performance-based restricted stock units (“PRSUs”) are restricted stock units subject to both performance-based and service-based vesting conditions. The number of shares of common stock a recipient will receive upon vesting of a PRSU will be calculated by reference to certain performance metrics related to the Company’s performance over a five-year period. PRSUs will vest on the measurement date, which is no more than 65 days after the performance period, provided the applicable service and performance conditions are satisfied. At December 31, 2019, there are 355,258 PRSUs outstanding, all of which are unvested.
March 31, 2017 |
March 7, 2018 |
March 20, 2019 |
March 20, 2019 |
July 19, 2019 |
|||||||||||
Number of PRSUs granted |
94,000 | 96,000 | 92,500 | 4,901 | 88,582 | ||||||||||
PRSUs outstanding at December 31, 2019 |
83,000 | 93,000 | 85,800 | 4,876 | 88,582 | ||||||||||
Fair value as of grant date |
$ | 15.60 | $ | 21.21 | $ | 47.58 | $ | 47.58 | $ | 52.15 | |||||
Remaining lives |
2.00 | 3.00 | 4.00 | — | 3.00 | ||||||||||
The Company recorded compensation expense related to the PRSUs of approximately $1.9 million and $0.6 million in the consolidated statements of income for the years ended December 31, 2019 and 2018, respectively, based on the probability of achieving the performance condition. Total unrecognized compensation expense related to these awards at December 31, 2019, is $9.4 million, which will be expensed over the service period based on the probability of achieving the performance condition.
Note 18. Contingencies
Other major tobacco companies are defendants in product liability claims. In a number of these cases, the amounts of punitive and compensatory damages sought are significant and could have a material adverse effect on our business and results of operations.
The Company is subject to several lawsuits alleging personal injuries resulting from malfunctioning vaporizer devices or consumption of e-liquids and may be subject to claims in the future relating to other NewGen products. The Company is still evaluating these claims and the potential defenses to them. For example, the Company did not design or manufacture the products at issue; rather, the Company was merely the distributor. Nonetheless, there can be no assurance that the Company will prevail in these cases, and they could have a material adverse effect on the financial position, results of operations, or cash flows of the Company.
86
The Company has several subsidiaries engaged in making, distributing and retailing (online and in bricks-and-mortar) vapor products. As a result of the overall publicity and controversy surrounding the vapor industry generally, many companies have received informational subpoenas from various regulatory bodies and in some jurisdictions regulatory lawsuits have been filed regarding marketing practices and possible underage sales. The Company expects that its subsidiaries will be subject to some such cases and information requests. In the acquisition of the vapor businesses, the Company negotiated financial “hold-backs”, which it expects to be able to use to defray expenses associated with the information production and the cost of defending any such lawsuits. To the extent that litigation becomes necessary, the Company believes that the subsidiaries have strong factual and legal defenses against claims that it unfairly marketed vapor products.
On October 8, 2019, the City of New York filed a complaint against twenty-three companies, including IVG and VaporFi, making various allegations including selling to consumers over the age of 18 but under 21. In response, those subsidiaries have ceased all sales into New York City, which was an immaterial market for those businesses. This proceeding was settled for monetary terms which were not material and certain structural remedies that the subsidiaries deemed acceptable.
Note 19. Legal Settlement
The company engaged in discussions and mediation with VMR Products LLC (“VMR”), which was acquired in 2018. Pursuant to a Distribution and Supply agreement (“VMR Agreement”), VMR was providing the Company with V2 e-cigarettes for the exclusive distribution in bricks-and-mortar stores in the United States. Under the terms of the VMR Agreement, in the event of termination following a change in control, the acquirer was required to make a payment to the Company under a formula designed to provide the Company with a fair share of the value created by the Company’s performance under the VMR Agreement. The discussions have been completed and the Company received $6.7 million in the second quarter 2019 to settle the issue. Net of legal costs and reserves for anticipated future returns associated with the discontinuance, the Company recorded a $5.5 million gain in the second quarter, which is recorded as a reduction to selling, general, and administrative expenses.
Note 20. Income Per Share
The following is a reconciliation of the numerators and denominators of the basic and diluted EPS computations of net income:
December 31, 2019 |
December 31, 2018 |
December 31, 2017 |
|||||||||||||||||||||||||
Income |
Shares |
Per Share |
Income |
Shares |
Per Share |
Income |
Shares |
Per Share |
|||||||||||||||||||
Net income attributable to Turning Point Brands, Inc. |
$ | 13,774 | $ | 25,289 | $ | 20,209 | |||||||||||||||||||||
Basic EPS: |
|||||||||||||||||||||||||||
Weighted average |
19,627,093 | $ | 0.70 | 19,355,607 | $ | 1.31 | 18,989,177 | $ | 1.06 | ||||||||||||||||||
Diluted EPS: |
|||||||||||||||||||||||||||
Effect of dilutive securities: |
|||||||||||||||||||||||||||
Stock options |
410,447 | 471,955 | 523,831 | ||||||||||||||||||||||||
20,037,540 | $ | 0.69 | 19,827,562 | $ | 1.28 | 19,513,008 | $ | 1.04 | |||||||||||||||||||
For the year ended December 31, 2019, the effect of the 3,202,808 shares issuable upon conversion of the Convertible Senior Notes were excluded from the diluted net income per share calculation because the Company’s average stock price did not exceed $53.86 during the period.
Note 21. Segment Information
In accordance with ASC 280, Segment Reporting, the Company has three reportable segments, (1) Smokeless products; (2) Smoking products; and (3) NewGen products. The Smokeless products segment (a) manufactures and markets moist snuff and (b) contracts for and markets chewing tobacco products. The Smoking products segment (a) markets cigarette papers, tubes, and related products; (b) markets and distributes finished cigars and MYO cigar wraps; and (c) processes, packages, markets, and distributes traditional pipe tobaccos. The NewGen products segment
87
(a) markets and distributes e-cigarettes, e-liquids, vaporizers, and certain other products without tobacco and/or nicotine; (b) markets and distributes a wide assortment of vaping and CBD related products to non-traditional retail outlets via VaporBeast, Vapor Shark, Vapor Supply, IVG and Solace; and (c) markets and distributes a wide assortment of vapor and CBD related products to individual consumers via Vapor Shark and VaporFi branded retail outlets in addition to online platforms. Smokeless and Smoking products are distributed primarily through wholesale distributors in the United States while NewGen products are distributed primarily through e-commerce to non-traditional retail outlets in the United States. The Other segment includes the costs and assets of the Company not assigned to one of the three reportable segments such as intercompany transfers, deferred taxes, deferred financing fees, and investments in subsidiaries. The Company had no customer that accounted for more than 10% of net sales in 2019, 2018, or 2017.
The accounting policies of these segments are the same as those of the Company. Corporate costs are not directly charged to the three reportable segments in the ordinary course of operations. The Company evaluates the performance of its segments and allocates resources to them based on operating income.
The tables below present financial information about reported segments:
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Net sales |
|||||||||
Smokeless products |
$ | 99,894 | $ | 90,031 | $ | 84,560 | |||
Smoking products |
108,733 | 111,507 | 109,956 | ||||||
NewGen products |
153,362 | 131,145 | 91,261 | ||||||
Total |
$ | 361,989 | $ | 332,683 | $ | 285,777 | |||
Gross profit |
|||||||||
Smokeless products |
$ | 52,277 | $ | 46,490 | $ | 42,703 | |||
Smoking products |
59,386 | 57,043 | 57,146 | ||||||
NewGen products |
25,083 | 39,026 | 25,121 | ||||||
Total |
$ | 136,746 | $ | 142,559 | $ | 124,970 | |||
Operating income (loss) |
|||||||||
Smokeless products |
$ | 35,978 | $ | 28,920 | $ | 28,005 | |||
Smoking products |
45,058 | 42,650 | 43,816 | ||||||
NewGen products |
(20,629 | ) |
6,752 | 3,178 | |||||
Corporate unallocated(1) |
(33,548 | ) |
(29,838 | ) |
(25,320 | ) |
|||
Total |
$ | 26,859 | $ | 48,484 | $ | 49,680 | |||
Interest expense, net |
17,342 | 14,819 | 16,889 | ||||||
Investment income |
(2,648 | ) |
(424 | ) |
(438 | ) |
|||
Loss on extinguishment of debt |
1,308 | 2,384 | 6,116 | ||||||
Net periodic benefit (income) cost, excluding service cost |
(4,961 | ) |
131 | 180 | |||||
Income before income taxes |
$ | 15,818 | $ | 31,574 | $ | 26,933 | |||
Capital expenditures |
|||||||||
Smokeless products |
$ | 2,823 | $ | 1,559 | $ | 1,928 | |||
Smoking products |
— | — | — | ||||||
NewGen products |
1,992 | 708 | 93 | ||||||
Total |
$ | 4,815 | $ | 2,267 | $ | 2,021 | |||
Depreciation and amortization |
|||||||||
Smokeless products |
$ | 1,608 | $ | 1,360 | $ | 1,400 | |||
Smoking products |
— | — | — | ||||||
NewGen products |
2,481 | 1,750 | 928 | ||||||
Total |
$ | 4,089 | $ | 3,110 | $ | 2,328 | |||
| (1) | Includes corporate costs that are not allocated to any of the three reportable segments. |
88
December 31, 2019 |
December 31, 2018 |
|||||
Assets |
||||||
Smokeless products |
$ | 120,723 | $ | 99,441 | ||
Smoking products |
145,831 | 142,520 | ||||
NewGen products |
90,899 | 95,397 | ||||
Corporate unallocated (1) |
89,131 | 2,019 | ||||
Total |
$ | 446,584 | $ | 339,377 | ||
| (1) | Includes assets not assigned to the three reportable segments. All goodwill has been allocated to the reportable segments. |
Revenue Disaggregation—Sales Channel
Revenues of the Smokeless and Smoking segments are primarily comprised of sales made to wholesalers while NewGen sales are made business to business and business to consumer, both online and through our corporate retail stores. NewGen net sales are broken out by sales channel below.
NewGen Segment |
|||||||||
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Business to Business |
$ | 112,580 | $ | 105,736 | $ | 82,596 | |||
Business to Consumer - Online |
31,348 | 15,624 | 5,021 | ||||||
Business to Consumer - Corporate store |
9,273 | 9,631 | 3,607 | ||||||
Other |
161 | 154 | 37 | ||||||
Total |
$ | 153,362 | $ | 131,145 | $ | 91,261 | |||
Net Sales: Domestic and Foreign
The following table shows a breakdown of consolidated net sales between domestic and foreign.
For the year ended December 31, |
|||||||||
2019 |
2018 |
2017 |
|||||||
Domestic |
$ | 347,616 | $ | 317,046 | $ | 272,927 | |||
Foreign |
14,373 | 15,637 | 12,850 | ||||||
Total |
$ | 361,989 | $ | 332,683 | $ | 285,777 | |||
Note 22. Selected Quarterly Financial Information (Unaudited)
The following table presents the quarterly operating results:
1st |
2nd |
3rd |
4th |
|||||||||
2019 |
||||||||||||
Net sales |
$ | 91,628 | $ | 93,339 | $ | 96,800 | $ | 80,222 | ||||
Gross profit |
40,464 | 41,183 | 42,816 | 12,283 | ||||||||
Consolidated net income |
6,560 | 13,205 | 6,274 | (12,265 | )(2)(3) |
|||||||
Basic net income (loss) per share |
0.34 | 0.67 | 0.32 | (0.62 | ) |
|||||||
Diluted net income (loss) per share |
$ | 0.33 | $ | 0.66 | $ | 0.31 | $ | (0.62 | ) |
|||
2018 |
||||||||||||
Net sales |
$ | 73,942 | $ | 81,101 | $ | 83,349 | $ | 94,291 | ||||
Gross profit |
31,809 | 35,795 | 36,211 | 38,744 | ||||||||
Consolidated net income |
3,032 | (1) |
9,319 | 7,954 | 4,984 | |||||||
Basic net income per share |
0.16 | 0.48 | 0.41 | 0.25 | ||||||||
Diluted net income per share |
$ | 0.15 | $ | 0.47 | $ | 0.40 | $ | 0.25 | ||||
| (1) | Includes $1,883 of loss on extinguishment of debt, net of tax of $501 |
| (2) | Includes corporate and vapor restructuring costs of $12.7 million net of tax of $5.1 million |
| (3) | Includes out of period non-cash interest expense adjustment related to the convertible debt of $0.8 million, net of tax of $0.3 million related to the prior quarters of 2019 |
89
The amounts presented in the table above are computed independently for each quarter. As a result, their sum may not equal the total year amounts.
Note 23. Dividends
On November 9, 2017, the Company’s Board of Directors approved the initiation of a cash dividend to shareholders. The initial quarterly dividend of $0.04 per common share was paid on December 15, 2017 to shareholders of record at the close of business on November 27, 2017. The most recent dividend of $0.05 per common share, an increase of approximately 11%, will be paid on April 10, 2020, to shareholders of record at the close of business on March 20, 2020.
Dividends, among other disbursements assets, are classified as restricted payments within the 2018 Credit Facility. The Company is generally permitted to make restricted payments provided that, at the time of payment, or as a result of payment, the Company is not in default. Additional restrictions limit the aggregate amount of restricted, quarterly dividends during a fiscal year to the aggregate amount of mandatory and voluntary principal payments made on the priority term loans during the fiscal year.
Note 24. Subsequent Events
On February 25, 2020 the Company’s board of directors approved a $50.0 million share repurchase authorization, which is intended for opportunistic execution based upon a variety of factors including market dynamics. The authorization will be subject to the ongoing discretion of the board.
90
| Item 9. | Changes In and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
As of December 31, 2019, the Company’s management, with participation of the Company’s President and Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), evaluated the effectiveness of the Company’s disclosure controls and procedures as defined in Exchange Act Rules 13a-15(e) and 15d-15(e). Based on that evaluation, the CEO and CFO concluded that the Company’s disclosure controls and procedures were not effective as of December 31, 2019 solely due to the material weakness in our internal control over financial reporting related to the third-party valuation of our convertible debt as described below.
Internal Control
Pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, we have included a report that provides management’s assessment of our internal control over financial reporting as part of this Annual Report on Form 10-K for the year ended December 31, 2019. Management’s report is included below under the caption entitled “Management’s Report on Internal Control Over Financial Reporting,” and is incorporated herein by reference. Our independent registered public accounting firm is not yet required to formally attest to the effectiveness of our internal controls over financial reporting and will not be required to do so for as long as we are an “emerging growth company” pursuant to the provisions of the Jumpstart Our Business Startups Act of 2012.
Management’s Report on Internal Control over Financial Reporting
The consolidated financial statements appearing in this Annual Report have been prepared by the management that is responsible for their preparation, integrity, and fair presentation. The statements have been prepared in accordance with U.S. generally accepted accounting principles, which requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Securities Exchange Act of 1934, as amended). Our internal control system was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Further, because of changes in conditions, the effectiveness of an internal control system may vary over time.
Under the supervision and with the participation of our management, including our CEO, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2019, based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (“COSO ICIF”).
A material weakness is defined as a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Based on our evaluation under the framework in COSO ICF, our management concluded that our internal control over financial reporting was not effective as of December 31, 2019 solely due to a material weakness in our internal control over financial reporting related to the valuation of our convertible debt as described below. In conducting management's evaluation as described above, Solace was excluded. The operations of Solace excluded from management's assessment of internal control over financial reporting, represent approximately 0.8% of the Company's consolidated revenues and approximately 2.5% of total assets as of December 31, 2019.
During the preparation of our annual financial statements and the conduct of the annual financial statements audit, management identified a material weakness in our internal control over financial reporting relating to oversight
91
and review of the work of the third-party valuation specialists retained to conduct the valuation of our convertible debt issued in the third quarter of 2019 which contains an equity classified embedded derivative. This material weakness resulted in errors in our financial statements and related disclosures related to the valuation of our convertible debt as of and for the quarter ended September 30, 2019. As a result, the debt discount on our convertible debt was increased by $32 million as of and for the quarter ended December 31, 2019 with an offset to additional paid-in-capital and deferred income taxes, which debt discount will be amortized to interest expense over the life of the loan.
To remediate the material weakness, we have subsequently enhanced the design and expanded our management review controls around the use of third-party valuation specialists. Specifically, management has implemented procedures to review the qualifications of third-party valuation specialists and to perform additional steps to evaluate and accept the work product of such specialists. The remediation was complete and deemed effective as of March 6, 2020.
Our independent registered public accounting firm is not yet required to formally attest to the effectiveness of our internal controls over financial reporting and will not be required to do so for as long as we are an “emerging growth company” pursuant to the provisions of the Jumpstart Our Business Startups Act of 2012.
Changes in Internal Controls over Financial Reporting
Other than the steps taken to remediate the material described above under “—Management’s Report on Internal Control over Financial Reporting” , management has determined that there were no changes in the Company’s internal controls over financial reporting during the fiscal quarter ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
|
/s/ Lawrence S. Wexler
|
/s/ Robert Lavan
|
/s/ Brian Wigginton
|
|
Lawrence S. Wexler
|
Robert Lavan
|
Brian Wigginton
|
|
President and Chief Executive Officer
|
Chief Financial Officer
|
Chief Accounting Officer
|
|
|
|
|
|
Date: March 12, 2020
|
Date: March 12, 2020
|
Date: March 12, 2020
|
| Item 9B. | Other Information |
None.
92
PART III
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required for this Item is incorporated by reference from our Proxy Statement to be filed in connection with our 2020 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2019.
| Item 11. | Executive Compensation |
The information required for this Item is incorporated by reference from our Proxy Statement to be filed in connection with our 2020 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2019.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required for this Item is incorporated by reference from our Proxy Statement to be filed in connection with our 2020 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2019.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required for this Item is incorporated by reference from our Proxy Statement to be filed in connection with our 2020 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2019.
| Item 14. | Principal Accountant Fees and Services |
The information required for this Item is incorporated by reference from our Proxy Statement to be filed in connection with our 2020 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2019.
93
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
| a) | Financial Information |
| (1) | Financial Statements: See “Index to Consolidated Financial Statements” in Part II, Item 8 of this Annual Report on Form 10-K. |
| (2) | Financial Statement Schedule: Information required by this item is included within the consolidated financial statements or notes in Item 8 of this Annual Report on Form 10-K. |
| (3) | Exhibits – See (b) below |
| b) | Exhibits Index to Exhibits |
94
Index to Exhibits
|
Exhibit
No. |
Description
|
|
International Vapor Group Stock Purchase Agreement dated as of September 5, 2018, between Turning Point Brands, Inc. and International Vapor Group, LLC (incorporated by reference to Exhibit 2.1 to the Registrant’s Quarterly Report on Form 10-Q filed on November 7, 2018).
|
|
|
|
|
|
Second Amended and Restated Certificate of Incorporation of Turning Point Brands, Inc. (incorporated by reference to Exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on May 16, 2016).
|
|
|
|
|
|
Amended and Restated By-laws (incorporated by reference to Exhibit 3.3 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 24, 2015).
|
|
|
|
|
|
Registration Rights Agreement of Turning Point Brands, Inc. dated May 10, 2016, between Turning Point Brands, Inc. and the Stockholders named therein (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on May 16, 2016).
|
|
|
|
|
|
Description of Securities*
|
|
|
|
|
|
Indenture dated as of July 30, 2019, between Turning Point Brands, Inc. and GLAS Trust Company LLC, (including the form of Note as Exhibit A thereto) (incorporated by reference to Exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on July 30, 2019).
|
|
|
|
|
|
Turning Point Brands, Inc. 2015 Equity Incentive Plan (the “2015 Plan”) (incorporated by reference to Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1/A
(File No. 333-207816) filed on November 5, 2015). † |
|
|
|
|
|
Form of Stock Option Award Agreement under the 2015 Plan (incorporated by reference to Exhibit 10.2 to the Registrant’s Annual Report on Form 10-K filed on March 13, 2017). †
|
|
|
|
|
|
Form of Performance-Based Restricted Stock Unit Award Agreement under the Turning Point Brands, Inc. 2015 Equity Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on May 11, 2017).†
|
|
|
|
|
|
2006 Equity Incentive Plan of Turning Point Brands, Inc. (incorporated by reference to Exhibit 10.3 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 5, 2015). †
|
|
|
|
|
|
Amendment No. 1 to the 2006 Equity Incentive Plan of North Atlantic Holding Company, Inc. (incorporated by reference to Exhibit 10.4 to the Registrant’s Annual Report on Form 10-K filed on March 13, 2017). †
|
|
|
|
|
|
Amendment No. 2 to the 2006 Equity Incentive Plan of North Atlantic Holding Company, Inc. (incorporated by reference to Exhibit 10.5 to the Registrant’s Annual Report on Form 10-K filed on March 13, 2017). †
|
|
|
|
|
|
Amendment No. 3 to the 2006 Equity Incentive Plan of North Atlantic Holding Company, Inc. (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on February 7, 2017). †
|
|
|
|
|
|
Amendment No. 4 to the 2006 Equity Incentive Plan of North Atlantic Holding Company, Inc. (incorporated by reference to Exhibit 10.54 to the Registrant’s Annual Report on Form 10-K filed on March 13, 2017). †
|
|
|
|
|
95
|
Exhibit
No. |
Description
|
|
Form of Award Agreement under the 2006 Plan (incorporated by reference to Exhibit 10.4 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 5, 2015). †
|
|
|
|
|
|
Form of Cash-Out Agreement under the 2006 Plan (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on February 7, 2017). †
|
|
|
|
|
|
Form of Indemnification Agreement between Turning Point Brands, Inc. and certain directors and officers (incorporated by reference to Exhibit 10.10 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 24, 2015).
|
|
|
|
|
|
Form of Indemnification Agreement between Turning Point Brands, Inc. and Standard General Master Fund, L.P. (incorporated by reference to Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 24, 2015).
|
|
|
|
|
|
Employment Agreement between Turning Point Brands, Inc. and Lawrence Wexler dated November 23, 2015 (incorporated by reference to Exhibit 10.9 to the Registrant’s Current Report on Form 8-K filed on May 16, 2016). †
|
|
|
|
|
|
Employment Agreement between Turning Point Brands, Inc. and James Dobbins dated November 23, 2015 (incorporated by reference to Exhibit 10.10 to the Registrant’s Current Report on Form 8-K filed on May 16, 2016). †
|
|
|
|
|
|
Employment Agreement between Turning Point Brands, Inc. and Mr. Robert M. Lavan dated March 13, 2018 (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on March 19, 2018).†
|
|
|
|
|
|
Contract Manufacturing, Packaging and Distribution Agreement dated as of September 4, 2008, between National Tobacco Company, L.P. and Swedish Match North America, Inc. (incorporated by reference to Exhibit 10.17 to the Registrant’s Registration Statement on Form S-1/A
(File No. 333-207816) filed on November 24, 2015). |
|
|
|
|
|
Amended and Restated Distribution and License Agreement dated as of November 30, 1992, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc., as predecessor to North Atlantic Operating Company, Inc. (U.S.) (incorporated by reference to Exhibit 10.2 to Amendment No. 2 to the Registrant’s Registration Statement (Reg. No. 333-31931) on Form S-4/A filed with the Commission on September 17, 1997).
|
|
|
|
|
|
Amended and Restated Distribution and License Agreement dated as of November 30, 1992, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc., as predecessor to North Atlantic Operating Company, Inc. (Canada) (incorporated by reference to Exhibit 10.4 to Amendment No. 2 to the Registrant’s Registration Statement (Reg. No. 333-31931) on Form S-4/A filed with the Commission on September 17, 1997).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated March 31, 1993 between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.22 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreements dated June 10, 1996, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.23 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
96
|
Exhibit
No. |
Description
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated September 1996, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.24 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Restated Amendment to the Amended and Restated Distribution and License Agreement between Bolloré Technologies, S.A. and North Atlantic Operating Company, Inc. dated June 25, 1997 (U.S. & Canada) (incorporated by reference to Exhibit 10.5 to Amendment No. 2 to the Registrant’s Registration Statement (Reg. No. 333-31931) on Form S-4/A filed with the Commission on September 17, 1997).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated October 22, 1997, between Bolloré Technologies, S.A. and North Atlantic Operating Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.31 to the Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 1997).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated June 19, 2002, between Bolloré S.A. and North Atlantic Operating Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.31 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Trademark Consent Agreement, dated March 26, 1997, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc. (incorporated by reference to Exhibit 10.25 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated February 28, 2005, between Bolloré S.A. and North Atlantic Operating Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.33 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated April 20, 2006, between Bolloré S.A. and North Atlantic Operating Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2006).
|
|
|
|
|
|
Amendment to the Amended and Restated Distribution and License Agreement dated March 10, 2010, between Bolloré S.A. and North Atlantic Operating Company, Inc. (U.S. & Canada) (incorporated by reference to Exhibit 10.35 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Consent Agreement dated as of April 4, 1997, between Bolloré Technologies, S.A. and North Atlantic Trading Company, Inc. (incorporated by reference to Exhibit 10.26 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Amendment No. 1 to Consent Agreement dated as of April 9, 1997, between Bolloré Technologies, S.A. and North Atlantic Operating Company, Inc. (incorporated by reference to Exhibit 10.27 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Amendment No. 2 to Consent Agreement dated as of June 25, 1997, between Bolloré Technologies, S.A. and North Atlantic Operating Company, Inc. (incorporated by reference to Exhibit 10.28 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
97
|
Exhibit
No. |
Description
|
|
Trademark Consent Agreement dated July 31, 2003, among Bolloré Technologies, S.A., North Atlantic Trading Company, Inc. and North Atlantic Operating Company, Inc. (incorporated by reference to Exhibit 10.32 to the Registrant’s Registration Statement on Form S-1
(File No. 333-207816) filed on November 5, 2015). |
|
|
|
|
|
Amendment No. 2 to Trademark Consent Agreement dated December 17, 2012, between Bolloré S.A. and North Atlantic Operating Company, Inc. (incorporated by reference to Exhibit 10.36 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
License and Distribution Agreement dated March 19, 2013 between Bolloré S.A. and North Atlantic Operating Company, Inc. (incorporated by reference to Exhibit 10.37 to the Registrant’s Registration Statement on Form S-1 (File No. 333-207816) filed on November 5, 2015).
|
|
|
|
|
|
Distributors Supply Agreement dated as of April 1, 2013, between National Tobacco Company, L.P. and JJA Distributors, LLC (incorporated by reference to Exhibit 10.38 to the Registrant’s Registration Statement on Form S-1/A (File No. 333-207816) filed on November 24, 2015).
|
|
|
|
|
|
First Lien Credit Agreement dated as of February 17, 2017, by and among Turning Point Brands, Inc., Fifth Third Bank, and the lenders party thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on February 17, 2017).
|
|
|
|
|
|
Second Lien Credit Agreement dated as of February 17, 2017, by and among Turning Point Brands, Inc., as the Borrower, Prospect Capital Corporation, as administrative agent, and the lenders party thereto (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on February 17, 2017).
|
|
|
|
|
|
First Lien Guaranty and Security Agreement dated as of February 17, 2017, by and among Turning Point Brands, Inc., Fifth Third Bank, and the lenders party thereto (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on February 17, 2017).
|
|
|
|
|
|
Second Lien Guaranty and Security Agreement dated as of February 17, 2017, by and among Turning Point Brands, Inc., Prospect Capital Corporation, and the lenders party thereto (incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed on February 17, 2017).
|
|
|
|
|
|
Intercreditor Agreement dated as of February 17, 2017, by and among Turning Point Brands, Inc., the other grantors party thereto, Fifth Third Bank, as first lien collateral agent, and Prospect Capital Corporation, as second lien collateral agent (incorporated by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K filed on February 17, 2017).
|
|
|
|
|
|
Amended and Restated First Lien Credit Agreement, dated as of March 7, 2018, by and among Turning Point Brands, Inc. and its subsidiaries, as the obligors, Fifth Third Bank, as administrative agent, and the lenders party thereto (incorporated by reference to Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on March 8, 2018).
|
|
|
|
|
|
Amended and Restated Second Lien Credit Agreement, dated as of March 7, 2018, by and among Turning Point Brands, Inc. and its subsidiaries, as obligors, Prospect Capital Corporation, as administrative agent, and the lenders party thereto (incorporated by reference to Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on March 8, 2018).
|
|
|
|
|
98
|
Exhibit
No. |
Description
|
|
Omnibus Amendment, Reaffirmation Agreement and Joinder, dated as of March 7, 2018, by and among Turning Point Brands, Inc. and its subsidiaries, as the Grantors, Fifth Third Bank, as administrative agent, and the lenders party thereto (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on March 8, 2018).
|
|
|
|
|
|
Second Lien Omnibus Amendment, Reaffirmation Agreement and Joinder, dated as of March 7, 2018, by and among Turning Point Brands, Inc. and its subsidiaries, as the Grantors, Fifth Third Bank, as administrative agent, and the lenders party thereto (incorporated by reference to Exhibit 10.4 to the Registrant’s Current Report on Form 8-K filed on March 8, 2018).
|
|
|
|
|
|
First Amendment to Second Lien Intercreditor Agreement, dated as of March 7, 2018, by and among Turning Point Brands, Inc., and the other grantors party thereto, Fifth Third Bank, as first lien collateral agent, and Prospect Capital Corporation, as second lien collateral agent (incorporated by reference to Exhibit 10.5 to the Registrant’s Current Report on Form 8-K filed on March 8, 2018).
|
|
|
|
|
|
First Amendment to the First Lien Credit Agreement (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 10-Q for the period ended June 30, 2019).
|
|
|
|
|
|
Form of Capped Call Agreement (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on July 30, 2019).
|
|
|
|
|
|
Second Amendment to the First Lien Credit Agreement. *
|
|
|
|
|
|
Subsidiaries of Turning Point Brands, Inc.*
|
|
|
|
|
|
Consent of RSM US, LLP.*
|
|
|
|
|
|
Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.*
|
|
|
|
|
|
Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.*
|
|
|
|
|
|
Certification of Principal Accounting Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.*
|
|
|
|
|
|
Certifications of Principal Executive Officer and Principal Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.*
|
|
|
|
|
|
101
|
XBRL (eXtensible Business Reporting Language). The following materials from Turning Point Brands, Inc.’s Annual Report on Form 10-K for the years ended December 31, 2019, 2018, and 2017, formatted in XBRL: (i) consolidated balance sheets, (ii) consolidated statements of income, (iii) consolidated statements of comprehensive income, (iv) consolidated statements of changes in stockholder’s equity (deficit), (v) consolidated statements of cash flows, and (vi) notes to the consolidated financial statements.*
|
| * | Filed herewith |
| † | Compensatory plan or arrangement |
| Item 16. | Form 10-K Summary |
Not applicable.
99
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized, on March 12, 2020.
|
|
TURNING POINT BRANDS, INC.
|
|
|
|
|
|
|
|
By:
|
/s/ Lawrence S. Wexler
|
|
|
Name:
|
Lawrence S. Wexler
|
|
|
Title:
|
Chief Executive Officer
|
|
|
|
|
|
|
By:
|
/s/ Robert Lavan
|
|
|
Name:
|
Robert Lavan
|
|
|
Title:
|
Chief Financial Officer
|
|
|
|
|
|
|
By:
|
/s/ Brian Wigginton
|
|
|
Name:
|
Brian Wigginton
|
|
|
Title:
|
Chief Accounting Officer
|
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
|
|
|
|
|
|
|
By:
|
/s/ Lawrence S. Wexler
|
Director, Chief Executive Officer
|
March 12, 2020
|
|
|
Lawrence S. Wexler
|
|
|
|
|
|
|
|
|
By:
|
/s/ Robert Lavan
|
Chief Financial Officer
|
March 12, 2020
|
|
|
Robert Lavan
|
|
|
|
|
|
|
|
|
By:
|
/s/ Brian Wigginton
|
Chief Accounting Officer
|
March 12, 2020
|
|
|
Brian Wigginton
|
|
|
|
|
|
|
|
|
By:
|
/s/ David Glazek
|
Chairman of the Board of Directors
|
March 12, 2020
|
|
|
David Glazek
|
|
|
|
|
|
|
|
|
By:
|
/s/ Gregory H. A. Baxter
|
Director
|
March 12, 2020
|
|
|
Gregory H. A. Baxter
|
|
|
|
|
|
|
|
|
By:
|
/s/ H. C. Charles Diao
|
Director
|
March 12, 2020
|
|
|
H. C. Charles Diao
|
|
|
|
|
|
|
|
|
By:
|
/s/ Peggy Hebard
|
Director
|
March 12, 2020
|
|
|
Peggy Hebard
|
|
|
|
|
|
|
|
|
By:
|
/s/ Arnold Zimmerman
|
Director
|
March 12, 2020
|
|
|
Arnold Zimmerman
|
|
|
|
|
|
|
|
|
By:
|
/s/ Ashley Davis Frushone
|
Director
|
March 12, 2020
|
|
|
Ashley Davis Frushone
|
|
|
100
