Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - Recro Pharma, Inc. | reph-ex321_14.htm |
| EX-31.2 - EX-31.2 - Recro Pharma, Inc. | reph-ex312_10.htm |
| EX-31.1 - EX-31.1 - Recro Pharma, Inc. | reph-ex311_8.htm |
| EX-23.1 - EX-23.1 - Recro Pharma, Inc. | reph-ex231_297.htm |
| EX-21.1 - EX-21.1 - Recro Pharma, Inc. | reph-ex211_7.htm |
| EX-10.30 - EX-10.30 - Recro Pharma, Inc. | reph-ex1030_296.htm |
| EX-10.29 - EX-10.29 - Recro Pharma, Inc. | reph-ex1029_299.htm |
| EX-10.5 - EX-10.5 - Recro Pharma, Inc. | reph-ex105_298.htm |
| EX-4.7 - EX-4.7 - Recro Pharma, Inc. | reph-ex47_232.htm |
.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-36329
Recro Pharma, Inc.
(Exact name of registrant as specified in its charter)
|
Pennsylvania |
26-1523233 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
|
490 Lapp Road, Malvern, Pennsylvania |
19355 |
|
(Address of principal executive offices) |
(Zip Code) |
(484) 395-2470
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Trading Symbol |
Name of Exchange on Which Registered |
|
Common Stock, par value $0.01 |
REPH |
Nasdaq Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☒ |
|
Non-accelerated filer |
☐ |
|
Smaller reporting company |
☒ |
|
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
On the last business day of the most recently completed second fiscal quarter, the aggregate market value (based on the closing sale price of its common stock on that date) of the voting stock held by non-affiliates of the registrant was $195.7 million.
As of February 28, 2020, there were 23,401,633 shares of common stock outstanding, par value $0.01 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Part III of this Annual Report on Form 10-K incorporates certain information by reference from the registrant’s proxy statement for the 2020 annual meeting of shareholders to be filed no later than 120 days after the end of the registrant’s fiscal year ended December 31, 2019.
Index
|
|
|
|
|
|
Page |
|
|
|
|
|||
|
|
4 |
||||
|
|
|
|
|
|
|
|
|
Item 1. |
|
|
4 |
|
|
|
|
|
|
|
|
|
|
Item 1A. |
|
|
10 |
|
|
|
|
|
|
|
|
|
|
Item 1B. |
|
|
28 |
|
|
|
|
|
|
|
|
|
|
Item 2. |
|
|
28 |
|
|
|
|
|
|
|
|
|
|
Item 3. |
|
|
28 |
|
|
|
|
|
|
|
|
|
|
Item 4. |
|
|
28 |
|
|
|
|
|
|
|
|
|
|
29 |
||||
|
|
|
|
|
|
|
|
|
Item 5. |
|
|
29 |
|
|
|
|
|
|
|
|
|
|
Item 6. |
|
|
29 |
|
|
|
|
|
|
|
|
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
31 |
|
|
|
|
|
|
|
|
|
Item 7A. |
|
|
38 |
|
|
|
|
|
|
|
|
|
|
Item 8. |
|
|
39 |
|
|
|
|
|
|
|
|
|
|
Item 9. |
|
Changes in Disagreements with Accountants on Accounting and Financial Disclosures |
|
39 |
|
|
|
|
|
|
|
|
|
Item 9A. |
|
|
39 |
|
|
|
|
|
|
|
|
|
|
Item 9B. |
|
|
40 |
|
|
|
|
|
|
|
|
|
|
41 |
||||
|
|
|
|
|
|
|
|
|
Item 10. |
|
|
41 |
|
|
|
|
|
|
|
|
|
|
Item 11. |
|
|
41 |
|
|
|
|
|
|
|
|
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
41 |
|
|
|
|
|
|
|
|
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
41 |
|
|
|
|
|
|
|
|
|
Item 14. |
|
|
41 |
|
|
|
|
|
|
|
|
|
|
42 |
||||
|
|
|
|
|
|
|
|
|
Item 15. |
|
|
42 |
|
This Annual Report on Form 10-K and the documents incorporated by reference herein contain forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this Annual Report on Form 10-K or the documents incorporated by reference herein regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “will,” “would” “could,” “should,” “potential,” “seek,” “evaluate,” “pursue,” “continue,” “design,” “impact,” “affect,” “forecast,” “target,” “outlook,” “initiative,” “objective,” “designed,” “priorities,” “goal,” or the negative of such terms and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such statements are based on assumptions and expectations that may not be realized and are inherently subject to risks, uncertainties and other factors, many of which cannot be predicted with accuracy and some of which might not even be anticipated.
The forward-looking statements in this Annual Report on Form 10-K and the documents incorporated herein by reference include, among other things, statements about:
|
|
▪ |
our estimates regarding expenses, future revenue, cash flow, capital requirements and timing and availability of and the need for additional financing; |
|
|
▪ |
our ability to maintain our relationships, profitability and contracts with our key commercial partners; |
|
|
▪ |
our ability to grow and diversify our business with new customers, including our ability to meet desired project outcomes with development customers; |
|
|
▪ |
our ability to comply with the regulatory schemes applicable to our business and other regulatory developments in the United States and foreign countries; |
|
|
▪ |
our ability to operate under increased leverage and associated lending covenants; to pay existing required interest and principal amortization payments when due; and/or to obtain acceptable refinancing alternatives; |
|
|
▪ |
the performance of third-party suppliers upon which we depend for Active Pharmaceutical Ingredients, or APIs, excipients, capsules, reagents, etc., and other third-parties involved with maintenance of our facilities and equipment; |
|
|
▪ |
our ability to obtain and maintain patent protection for applicable products and defend our intellectual property rights against third-parties; |
|
|
▪ |
pharmaceutical market forces that may impact our commercial customers success for products we produce; |
|
|
▪ |
our ability to defend the securities class action lawsuit filed against us, or any future material litigation filed against us; |
|
|
▪ |
our ability to recruit or retain key scientific, technical, business development, and management personnel and to retain our executive officers; |
|
|
▪ |
our ability to comply with stringent U.S. and foreign government regulation in the manufacture of pharmaceutical products, including Good Manufacturing Practice, or cGMP, compliance and U.S. Drug Enforcement Agency, or DEA, compliance and other relevant regulatory authorities; and |
We may not achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this Annual Report on Form 10-K, particularly under “Risk Factors,” that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures, collaborations or investments we may make. You should read this Annual Report on Form 10-K and the documents that we incorporate by reference herein completely and with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements.
Solely for convenience, tradenames referred to in this Annual Report on Form 10-K appear without the ® symbol, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert its rights, to these tradenames. All trademarks, service marks and tradenames included or incorporated by reference in this Annual Report on Form 10-K are the property of their respective owners.
3
Overview
We are a leading contract development and manufacturing organization, or CDMO, with integrated solutions for the development, formulation, regulatory support, manufacturing, and packaging of oral solid dose drug products. We leverage our formulation and development expertise to develop and manufacture pharmaceutical products using proprietary delivery technologies and know-how for commercial partners who commercialize or plan to commercialize these products. These collaborations result in revenue streams including manufacturing, royalties, profit sharing, and research and development, which support our continued operations. We operate a 97,000 square foot, DEA-licensed manufacturing facility in Gainesville, Georgia, as well as a 24,000 square foot development and high potency product facility in Gainesville, Georgia that we opened in October 2018. We currently develop and/or manufacture the following key products with our key commercial partners: Ritalin LA®, Focalin XR®, Verelan PM®, Verelan SR®, Verapamil PM, Verapamil SR and Zohydro ER®, as well as supporting development stage products.
Our manufacturing and development capabilities include formulation, product development from formulation through commercial manufacturing, and specialized capabilities for solid oral dosage forms, extended release and controlled substance manufacturing, as well as high potency development and manufacturing. In a typical collaboration, we work with our commercial partners to develop product candidates, or new formulations of existing product candidates, and may license certain intellectual property to such commercial partners. We also typically exclusively manufacture and supply clinical and commercial supplies of these proprietary products and product candidates.
Acute Care Spin-Off
In November 2019, our former Acute Care business was spun-out from us through our former wholly-owned subsidiary, Baudax Bio, Inc., or Baudax Bio, when we completed a special dividend distribution of all the outstanding shares of common stock of Baudax Bio to our shareholders. On November 21, 2019, the distribution date, each of our shareholders received one share of Baudax Bio’s common stock, or the Distribution, for every two and one-half shares of our common stock held of record at the close of business on November 15, 2019, the record date for the Distribution. Additionally, we contributed $19 million of cash to Baudax Bio in connection with the separation. As a result of the Distribution, Baudax Bio is now an independent public company whose shares of common stock are trading under the symbol “BXRX” on The Nasdaq Capital Market, or Nasdaq.
Our consolidated results of operations and financial position included in this Annual Report on Form 10-K reflect the financial results of Baudax Bio as a discontinued operation for all periods presented. For additional information on the spin-off of Baudax Bio please read Note 4, Discontinued Operations, to our consolidated financial statements included in this Annual Report on Form 10-K.
Our Strategy
The CDMO market is large and growing and is expected to continue to expand as outsourced penetration is seen due to biotechnology and pharmaceutical companies outsourcing more of their operations. These companies, which include our customers and prospective customers, generally prefer fewer, higher quality suppliers with specialized expertise in addressing their formulation and manufacturing challenges early in the development cycle. Our strategy for growth in this market includes:
|
|
• |
Expand Existing Customer Relationships. We maintain strong customer relationships with large pharmaceutical and biotechnology companies with established and stable pharmaceutical products. We plan to leverage these relationships for new business opportunities. |
|
|
• |
Diversify Our Customer Base. We have taken, and continue to take, steps to diversify and expand our customer base. Beginning in 2018, we increased our focus on business development, hired subject matter experts and set up additional systems and processes in order to expand our offering of development-stage services to attract new customers. In 2019, we added 6 new development customers and expect to further expand our business with new customers in 2020. |
|
|
• |
Invest in our Manufacturing Capabilities. We intend to continue to invest in our facilities and infrastructure to maximize our utilization and support our customers’ development and commercial manufacturing requirements. |
|
|
• |
Explore Acquisitions and Licensing. We may drive growth through the acquisition of businesses, products, product lines, technologies and capabilities. |
4
We believe that the strong relationships we have with our commercial partners result from of our competitive strengths. In particular:
|
|
• |
Our Operational Excellence. We maintain a commitment to continually improve productivity and customer service levels and maintain excellent quality and regulatory compliance systems. |
|
|
• |
Focus on Specialized Markets. We participate in specialized markets where significant technical expertise provides a competitive advantage. This includes differentiated drug delivery, controlled substance and complex formulation. Our core expertise is modified release oral solid dosage form development and manufacturing and custom release profile development, including for DEA controlled substance products. We developed extended, controlled and sustained release mechanisms and other intellectual property for several current commercial products. |
|
|
• |
Our Integrated Development and Manufacturing Facilities. Our early-stage development and high-potency business feeds clinical and commercial manufacturing opportunities to our manufacturing business. |
|
|
• |
Our Customer-Centric, Consultative Approach. We are highly collaborative throughout the product lifecycle, guiding our commercial partners through the development process towards commercialization, including support and guidance on regulatory matters and CMC regulatory document preparation. In particular, we provide differentiated capabilities across a broad array of services that support the ability to serve our commercial partners through the entire development spectrum. |
Services
Manufacturing
Our 97,000 square foot manufacturing facility is located in Gainesville, Georgia. We have a full range of manufacturing capabilities, from scale-up services to commercial manufacture. Our manufacturing platform includes:
|
|
• |
process development and scale-up; |
|
|
• |
prototype, pilot and commercial manufacturing; |
|
|
• |
primary, secondary and tertiary packaging; and |
|
|
• |
analytical method development, validation and quality control with physical testing and analytical method capabilities. |
Development and High Potency
Our 24,000 square foot development and high potency facility is also located in Gainesville, Georgia. Our development team focuses on:
|
|
• |
formulation, system design and engineering; |
|
|
• |
analytical method development and validations; |
|
|
• |
stability programs; |
|
|
• |
prototyping and pilot manufacturing; |
|
|
• |
early stage quality assurance and quality control; |
|
|
• |
nonclinical and early stage clinical development; and |
|
|
• |
pre-commercial manufacturing. |
5
Early-stage coordination with customers at our development and high-potency site helps assure streamlined technology transfer for final scale up and manufacturing at our commercial manufacturing site.
Our Commercial Partners
We are party to agreements with each of our commercial partners governing the development, formulation and/or supply services we provide, as well as any applicable intellectual property licenses. Each commercial partner generally remains responsible for distributing, marketing and promoting their respective products. These collaborations result in revenue streams including royalties and profit sharing. We are dependent on a small number of commercial partners, with our four largest customers (Novartis Pharma AG, or Novartis, Teva Pharmaceutical Industries, Inc., or Teva, Currax Pharmaceutical LLC or Currax and Lannett Company, Inc., or Lannett) having generated 96% of our revenues for the twelve months ended December 31, 2019, of which, Teva, Lannett and Novartis generated 42%, 25% and 24% of our of our revenue, respectively.
The table below details the key products developed and/or manufactured with our key commercial partners:
|
Product |
Indication |
Territory |
Revenue |
Commercial |
Agreement term |
|
|
|
Source |
Partner |
|
|
|
Ritalin LA® |
Attention Deficit Hyperactivity Disorder |
Worldwide |
Manufacturing |
Novartis Pharma AG |
Through December 31, 2023 |
|
Focalin XR® |
Attention Deficit Hyperactivity Disorder |
Worldwide, except Canada |
Manufacturing |
Novartis Pharma AG |
Through December 31, 2023 |
|
Verelan PM®, SR & Verapamil PM |
Hypertension |
United States |
Profit Sharing / Manufacturing |
Lannett Company, Inc. |
Through December 31, 2021 |
|
Verapamil SR |
Hypertension |
United States |
Profit Sharing / Manufacturing |
Teva Pharmaceutical Industries Ltd. |
Through December 31, 2024 |
|
Zohydro ER® |
Severe Pain |
United States |
Royalty / Manufacturing |
Currax Pharmaceutical LLC |
Through March 2029 |
Lannett
We are party to a License and Supply Agreement with Kremers Urban Pharmaceutical, Inc., a subsidiary of Lannett, or the Lannett Agreement, pursuant to which we supply Verelan PM and SR and Verapamil PM to Lannett. We own the NDA related to Verelan and license commercialization rights to Lannett under the Lannett Agreement. The Lannett Agreement expires on December 31, 2021 and will renew thereafter for successive two-year periods. Under the Lannett Agreement, Lannett pays us a share of profits on sales of Verelan PM and SR and Verapamil PM.
Teva
We are party to a License and Supply Agreement with Watson Laboratories, Inc., a subsidiary of Teva, or the Teva Agreement, pursuant to which we are the exclusive supplier of Verapamil SR to Teva. We own the NDA for Verapamil SR and, pursuant to the Teva Agreement, have granted Teva an exclusive license to commercialize and sell Verapamil SR in the United States. The Teva Agreement expires on December 31, 2024, after which it will renew for additional one-year periods unless terminated by either party. Under the Teva Agreement, Teva pays us a share of profits on sales of Verapamil SR.
Novartis
In February 2019 we entered into a Manufacturing and Supply Agreement with Novartis, or the Novartis Agreement, pursuant to which we continued our long-standing relationship with Novartis as the exclusive global supplier to Novartis of Ritalin LA and Focalin XR capsules until December 31, 2023. The Novartis Agreement will renew automatically thereafter for successive one-year periods unless terminated by either party at least 24 months prior to December 31, 2023, or any subsequent one-year term
6
thereafter. Novartis may terminate the Agreement immediately if (i) any governmental regulatory authority prevents Novartis from supplying the active pharmaceutical ingredients in the products and/or exporting, purchasing or selling the products; (ii) any product cannot be reasonably commercialized for medical, scientific or legal reasons; or (iii) we fail to comply with certain health, safety and environmental protection requirements. After the December 31, 2023, Novartis may terminate the Novartis Agreement upon 12 months’ written notice in the event of any sale or divestment by us of our business or assets relating to the products.
Permits and Regulatory Approvals
We hold various licenses and registrations for our manufacturing activities. The primary licenses and registrations held are FDA Registrations of Drug Establishments and DEA Controlled Substance Registration. Due to certain U.S. state law requirements, we also hold certain state licenses for distribution activities throughout certain states. We also hold cGMP certifications for EU importation of products made in Gainesville for sale in the EU and an ANVISA certification for sale in Brazil.
In certain of our commercial partnerships, our commercial partner is the product authorization holder for products that have been developed on behalf of the commercial partner. In other commercial partnerships, we are the authorization holder. When our commercial partner holds the relevant authorization from the FDA or other national regulator, we support this authorization by furnishing a letter of reference to the Drug Master File, or the chemistry, manufacturing and related data to the relevant regulator or sponsor to provide adequate manufacturing support in respect of the product. We generally update this information annually with the relevant regulator.
We hold the approved NDAs for Verelan SR and Veralan PM, which we license to Lannett Company, Inc. and Teva Pharmaceutical Industries, Inc., respectively. Verapamil SR and Verapamil PM are authorized generics.
Environmental and Safety Matters
Certain products manufactured by us involve the use, storage and transportation of toxic or hazardous material. Our operation are subject to extensive laws and regulations relating to the storage, handling, emissions, transportation and discharge of materials into the environment and the maintenance of safe working conditions. We maintain environmental and industrial safety and health compliance programs and training at our facilities.
Prevailing legislation tend to hold companies primarily responsible for the proper disposal of their waste even after transfer to third party waste disposal facilities. Other future developments, such as increasingly strict environmental, health and safety laws and regulations, and enforcement policies, could result in substantial costs and liabilities to us and could subject the handling, manufacture, use, reuse or disposal of substances or pollutants at our facilities to more rigorous scrutiny than at present.
Intellectual Property
We own several issued patents in the United States and several foreign patent applications for abuse resistant pharmaceutical compositions and methods of use related to Zohydro ER®, which provide patent protection through 2034, subject to any extensions or disclaimers. Although certain patents may have expired or may expire in the future, we believe there are other barriers to entry for our commercial partners and competition, including ownership of regulatory filings, NDAs, abbreviated new drug applications or ANDAs, and drug master files or DMF’s, manufacturing trade secrets, proprietary dosage strengths, pricing limitations in various geographies, costs to revalidate with another supplier, maturity and life-cycle stage of products. We have acquired and developed and continue to acquire and develop knowledge and expertise and trade secrets in the provision of formulation, process development and manufacturing services. We intend to rely on a combination of patents and trade secrets, as well as confidentiality agreements and license agreements, to protect our proprietary know-how.
Competition
The pharmaceutical and biotechnology industries are intensely competitive and subject to rapid and significant technological change. Our current and future competitors include pharmaceutical, biotechnology and specialty pharmaceutical companies. Many of our competitors have greater financial and other resources than we have, such as more commercial resources, larger research and development staffs and more extensive marketing and manufacturing organizations.
We compete with contract pharmaceutical formulation and manufacturing companies such as Alcami Corporation, Cambrex Corporation, Mylan N.V., Catalent, Inc., Patheon, a part of Thermo Fisher Scientific, Mikart, LLC, Quotient Sciences, and other formulation, development and manufacture-related service providers.
7
Governmental authorities in the United States at the federal, state and local level, and the equivalent regulatory authorities in other countries, extensively regulate the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, distribution, marketing, export and import of prescription drugs, such as those we are developing and manufacturing. Any drug products developed or manufactured by us are subject to pervasive and continuing regulation by the FDA, including compliance with Good Manufacturing Practices, or GMP, which impose procedural and documentation requirements. The FDA or other regulatory agencies can delay approval of a drug if our manufacturing facilities are not able to demonstrate compliance with GMPs, pass other aspects of pre-approval inspections (i.e., compliance with filed submissions) or properly scale up to produce commercial supplies. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and state agencies, and are subject to periodic announced and unannounced inspections by the FDA and state agencies for compliance with GMP and other regulations. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from GMP and impose reporting and documentation requirements. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with GMPs and other aspects of regulatory compliance. Failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal of product approval, recall or seizure of the product or other voluntary, FDA‑initiated or judicial action that could delay or prohibit further operations.
The Drug Supply Chain Security Act, or DSCSA, added new sections to the Federal Food, Drug & Cosmetic Act, or FD&C, that require manufacturers, repackagers, wholesale distributors, dispensers, and third-party logistics providers to take steps to identify and trace certain prescription drugs to protect against the threats of counterfeit, stolen, contaminated, or otherwise harmful drugs in the supply chain. Among other mandates, the DSCSA requires manufacturers and repackagers to affix or imprint a unique product identifier (comprised of a standardized numerical identifier, lot number, and expiration date of the product) on certain prescription drug packages in both a human-readable and on a machine-readable data carrier. The standardized numerical identifier is comprised of the product’s corresponding National Drug Code combined with a unique alphanumeric serial number. A drug product is misbranded if it does not bear the product identifier as required by Section 582 of the FD&C. Section 582 also established several requirements relating to the verification of product identifiers.
Certain products that we manufacture are regulated as “controlled substances” as defined in the Controlled Substances Act of 1970, or CSA, which establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered and enforced by the United States Drug Enforcement Agency, or DEA. The DEA is concerned with the control and handling of controlled substances, and with the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce. Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, activity and controlled substance schedule.
The DEA regulates controlled substances by controlling them in five schedules. Schedule I and II controlled substances have a high potential for abuse, whereas Schedule III-V controlled substances have relatively decreasing potential for abuse. Therefore, the DEA imposes more stringent controls on Schedule I and II substances than Schedule III-V substances, including stricter security controls, quotas, and increased recordkeeping and reporting requirements. Certain of the products we manufacture and/or develop are regulated as Schedule II controlled substances. The DEA establishes annually an aggregate quota for how much certain controlled substances that we manufacture may be produced in total in the United States, based on the DEA’s estimate of the quantity needed to meet legitimate scientific and medicinal needs. This limited aggregate amount that the DEA allows to be produced in the United States each year is allocated among individual companies, who must submit applications annually to the DEA for individual production and procurement quotas. We must receive an annual quota from the DEA in order to produce any Schedule II substance. The DEA may adjust aggregate production quotas and individual production and procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments. In April 2018, the DEA proposed new guidelines aimed at strengthening the process for setting controls over diversion of controlled substances and making other improvements in the quota managements regulatory system for the production, manufacturing and procurement of controlled substances. Following a public comment period, the DEA published the final guidelines, which were substantially similar to the proposed guidelines, in July 2018. For 2019, the DEA proposed decreased manufacturing quotas for the six most frequently misused opioids, including hydrocodone, which we use in the manufacture of certain products, by an average of 10% as compared to the 2018 quotas. The DEA has proposed further decreasing manufacturing quotas in 2020 for five of the six opioids, including hydrocodone, by an average of 28%. Together with reductions in morphine, this is a 53% decrease since 2016. In October 2019, the DEA proposed additional regulations to amend the manner in which the agency grants quotas to manufacturers. The proposed regulations will establish use-specific quotas, including commercial sales, product development, transfer, replacement, and packaging. To decrease the risk of diversion and increase accountability, inventory allowances will be reduced, and procurement quota certifications will be required.
8
The DEA requires facilities that manufacture controlled substances to adhere to certain security requirements. Security requirements vary by controlled substance schedule, with the most stringent requirements applying to Schedule I and Schedule II substances. Required security measures include background checks on employees and physical control of inventory through measures such as cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances and periodic reports must be made to the DEA, for example, distribution, acquisition, and inventory reports for Schedule I and II controlled substances, Schedule III substances that are narcotics and other designated substances. Reports must also be made for thefts or losses of any controlled substance and suspicious orders. In addition, special authorization and notification requirements apply to imports and exports.
The DEA requires drug manufacturers to design and implement a system that identifies suspicious orders of controlled substances, such as those of unusual size, those that deviate substantially from a normal pattern and those of unusual frequency, prior to completion of the sale. A compliant suspicious order monitoring, or SOM, system includes well-defined due diligence, “know your customer” efforts and order monitoring.
To enforce these requirements, the DEA conducts periodic inspections of registered establishments that handle controlled substances. Individual states also independently regulate controlled substances. We are subject to state regulation of distribution for these products. Failure to maintain compliance with applicable requirements, particularly where noncompliance results in loss or diversion, can result in enforcement action that could have a material adverse effect on our business, results of operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to revoke those registrations, or take other enforcement action. In certain circumstances, violations could result in criminal prosecution.
In addition to DEA regulations, the U.S. government and state legislatures have enacted legislation and regulations intended to fight the opioid epidemic. In February 2016, the FDA released an action plan to address the opioid epidemic, which is part of a broader initiative led by the Department of Health and Human Services, which includes the release of a new Guideline for Prescribing Opioids for Chronic Pain, FDA’s requirement of enhanced warnings and safety labeling, and institution of a class-wide REMs as a condition of approval. Further, the Comprehensive Addiction and Recovery Act, or CARA, was passed in 2016. CARA provides resources to improve state monitoring of controlled substances, including opioids. A Senate bill introduced in February 2018, known as CARA 2.0, would further limit initial prescriptions for opioids to three days, while exempting initial prescriptions for chronic care, cancer care, hospice or end of life care, and palliative care. CARA 2.0 would also increase civil and criminal penalties for opioid manufacturers that fail to report suspicious orders for opioids or fail to maintain effective controls against diversion of opioids. More recently, the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, or Support Act, has been enacted. It provides for further regulation as well as funding for research and development of non-addictive painkillers. State legislatures have followed in the footsteps of the federal government in passing similar laws intended to limit prescription sales and quantities as well as increase the ability to monitor and regulate the manufacture and sale of opioids.
Corporate Information
We were incorporated under the laws of the Commonwealth of Pennsylvania in November 2007. Our principal executive offices are located at 490 Lapp Road, Malvern, PA 19355 and our telephone number is (484) 395-2470.
Employees
As of December 31, 2019 we had 215 employees including 2 part-time employees. None of our employees are covered by collective bargaining agreements, and we consider relations with our employees to be good.
Available Information
Our website address is www.recrogainesville.com. Our Annual Report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, any amendments to those reports, proxy and registration statements filed or furnished with the Securities and Exchange Commission, or SEC, are available free of charge through our website. We make these materials available through our website as soon as reasonably practicable after we electronically file such materials with, or furnish such materials to, the SEC. The reports filed with the SEC by our executive officers and directors pursuant to Section 16 under the Exchange Act are also made available, free of charge on our website, as soon as reasonably practicable after copies of those filings are provided to us by those persons. These materials can be accessed through the “Investor Relations” section of our website. The information contained in, or that can be accessed through, our website is not part of this Report.
9
The following risk factors and other information included in this Annual Report on Form 10-K should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations. Please see page 3 of this Annual Report on Form 10-K for a discussion of some of the forward-looking statements that are qualified by these risk factors. If any of the following risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. All references and risks related to the launch, commercialization or sale of any of our product candidates are predicated on such product candidates receiving the requisite marketing and regulatory approval in the United States and applicable foreign jurisdictions.
Risks Related to Our Business and Industry
Our revenues are dependent on a small number of commercial partners, and the loss of any one of these partners, or a decline in their orders, may adversely affect our business.
We are dependent on a small number of commercial partners, with our four largest customers (Novartis Pharma AG, or Novartis, Teva Pharmaceutical Industries, Inc., or Teva, Currax, and Lannett Company, Inc.) having generated 96% of our revenues for the twelve months ended December 31, 2019, of which Teva generated 42% of our revenue, Lannett generated 25% of our revenue and Novartis generated 24% of our revenue. Our agreement with Teva expires on December 31, 2024, and our agreement with Novartis expires on December 31, 2023. Our other customer contracts range from three to five years. If any one or more of these commercial partners fails to renew their contract, faces increasing or new competition in their market, adjusts pricing, significantly reduces their purchasing volume or experiences financial difficulties such as bankruptcy, our revenues could be adversely affected. Furthermore, the acquisition of or change in strategy by one of our customers could impact projects we are currently working or planning to work in the future. We are actively seeking to develop new customer relationships; but there can be no guarantee that we will be able to expand our customer base. New business may not be secured at the levels we anticipate, or at all.
Our royalty, profit sharing and manufacturing revenues also depend on the ability of our commercial partners to effectively market and sell their products to their customers. A commercial partner may choose to devote its efforts to its other products or reduce or fail to devote the necessary resources to provide effective sales and marketing support for the products we manufacture and supply. Our commercial partners face competition from other pharmaceutical companies for sales of products to end users. Competition from sellers of generic drugs is a major challenge for our commercial partners, and the loss or expiration of intellectual property rights for the products we manufacture can have a significant adverse effect on their sales volume and price. Two of our partners, Currax with respect to Zohydro and Novartis with respect to Ritalin, expect to compete in the near future with generic entrants with respect to the products we manufacture for them, which could impact the sales volume or pricing of those products and our revenues. In addition, as pharmaceutical product pricing faces scrutiny by governments, legislative bodies and enforcement agencies, our commercial partners may lower their prices or adopt cost-savings measures which could be passed on to us or otherwise impact our profit-sharing revenues. These pricing changes and any significant reduction, delay or cancellation of orders from our commercial partners could adversely affect our revenues.
Our and our customers’ failure to receive or maintain regulatory approval for product candidates or products could negatively impact our revenue and profitability.
Our business materially depends upon the regulatory approval of the products we manufacture. As such, if we or our customers experience a delay in, or failure to receive, approval for any of their product candidates or fail to maintain regulatory approval of products, our revenue and profitability could be adversely affected. For example, a customer preparing for commercial launch scale-up received a complete response letter from the FDA and, as a result, cancelled their anticipated commercial launch orders for 2020, which impacted our anticipated revenue. Additionally, if the FDA or a comparable foreign regulatory authority does not approve of our facilities for the manufacture of a customer product or if it withdraws such approval in the future, our customers may choose to identify alternative manufacturing facilities and/or relationships, which could significantly impact our ability to expand our capacity and capabilities.
10
We depend on spending and demand from our customers for our contract manufacturing and development services and any reduction in spending or demand could have a material adverse effect on our business.
The amount that our customers spend on the development and manufacture of their products or product candidates, particularly the amount our customers choose to spend on outsourcing these services to us, substantially impacts our revenue and profitability. The outcomes of our customers’ research, development and marketing also significantly influence the amount that our customers choose to spend on our services and offerings. Our customers determine the amounts that they will spend on our services based upon, among other things, the clinical and market success of their products, available resources, access to capital and their need to develop new products, which, in turn, depend upon a number of other factors, including their competitors’ research, development and product initiatives and the anticipated market for any new products, as well as clinical and reimbursement scenarios for specific products and therapeutic areas. Further, increasing consolidation in the pharmaceutical industry may impact such spending, particularly in the event that any of our customers choose to develop or acquire integrated manufacturing operations. Any reduction in customer spending on development and related services as a result of these and other factors could have a material adverse effect on our business, results of operations and financial condition.
Failure to obtain manufacturing components, supplies and related materials from third-party manufacturers could affect our ability to manufacture and deliver our products.
We rely on third-party manufacturers to supply many of our manufacturing components, supplies and related materials, which in some instances are supplied from a single source. Prolonged disruptions in the supply of any of our key manufacturing components, supplies and related materials, difficulty implementing replacement materials or new sources of supply, or a significant increase in the prices of manufacturing components, supplies and related materials could have a material adverse effect on our operating results, financial condition or cash flows. In particular, manufacturing problems may occur with these suppliers, and if a supplier provides us with manufacturing components, supplies and related materials that are deficient or defective or if a supplier fails to provide us with such materials or supplies in a timely manner, we may have limited ability to find appropriate substitutes or otherwise meet required specifications and deadlines. Moreover, we could experience inventory shortages if we are required to use an alternative supplier on short notice, which also could lead to manufacturing components, supplies and related materials being purchased on less favorable terms than we have with our regular suppliers. If such problems occur, we may not be able to manufacture our products profitably or on time, which could harm our reputation and have a material adverse effect on our business.
Our manufacturing services are highly complex, and if we are unable to provide quality and timely services to our customers, our business could suffer.
The manufacturing services we offer are highly complex, due in part to strict regulatory requirements. A failure of our quality control systems in our facilities could cause problems to arise in connection with facility operations for a variety of reasons, including equipment malfunction, viral contamination, failure to follow specific manufacturing instructions, protocols and standard operating procedures, problems with raw materials or environmental factors. Such problems could affect production of a single manufacturing run or a series of runs, requiring the destruction of products, or could halt manufacturing operations altogether. In addition, our failure to meet required quality standards may result in our failure to timely deliver products to our customers, which in turn could damage our reputation for quality and service. Any such incident could, among other things, lead to increased costs, lost revenue, reimbursement to customers for lost drug substance, damage to and possibly termination of existing customer relationships, time and expense spent investigating the cause and, depending on the cause, similar losses with respect to other manufacturing runs. With respect to our commercial manufacturing, if problems are not discovered before the product is released to the market, we may be subject to regulatory actions, including product recalls, product seizures, injunctions to halt manufacture and distribution, restrictions on our operations, civil sanctions, including monetary sanctions, and criminal actions. In addition, such issues could subject us to litigation, the cost of which could be significant.
The consumers of the products we manufacture for our customers may significantly influence our business, results of operations and financial condition.
We depend on, and have no control over, consumer demand for the products we manufacture for our customers. Consumer demand for our customers’ products could be adversely affected by, among other things, delays in health regulatory approval, the inability of our customers to demonstrate the efficacy and safety of their products, the loss of patent and other intellectual property rights protection, the emergence of competing or alternative products, including generic drugs, the degree to which private and government payment subsidies for a particular product offset the cost to consumers and changes in the marketing strategies for such products. If
11
the products we manufacture for our customers do not gain market acceptance, our revenues and profitability may be adversely affected.
We believe that continued changes to the healthcare industry, including ongoing healthcare reform, adverse changes in government or private funding of healthcare products and services, legislation or regulations governing the privacy of patient information or patient access to care, or the delivery, pricing or reimbursement of pharmaceuticals and healthcare services or mandated benefits, may cause healthcare industry participants to purchase fewer services from us or influence the price that others are willing to pay for our services. Changes in the healthcare industry’s pricing, selling, inventory, distribution or supply policies or practices could also significantly reduce our revenue and profitability.
Our operating results may fluctuate significantly.
Our operating results may be subject to quarterly and annual fluctuations. Our operating results will be affected by numerous factors, including:
|
|
• |
fluctuations in the revenues, including the loss of a major customer or product; |
|
|
• |
the timing of purchasing order patterns, safety stock methodology and habits of our commercial partners; |
|
|
• |
unsuccessful execution, postponement or cancellation of anticipated formulation, development and manufacturing services related to customer projects, |
|
|
• |
variations in the level of expenses related to our production volumes and development programs; |
|
|
• |
any intellectual property infringement lawsuit in which we may become involved; |
|
|
• |
CDMO or pharmaceutical competitors that introduce new products or take increased positions that may emerge and reduce market share for our existing customer/partner products; |
|
|
• |
our execution of any additional collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements; |
|
|
• |
our acquisition, divestiture, spin-off or in-licensing of new technologies or assets. |
Due to the various factors mentioned above, and others, the results of any prior quarterly period should not be relied upon as an indication of our future operating performance. If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
We have a history of operating losses. If we cannot maintain profitability and secure additional business, we may have to raise additional capital.
Prior to the spin-out of our Acute care business in November 2019, we had focused primarily on developing proprietary product candidates through our Acute care business, and incurred significant losses of approximately $18.6 million and $79.7 million for the years ended December 31, 2019 and 2018, respectively. As of December 31, 2019, we had an accumulated deficit of $206.9. We have financed our operations through the sale of debt and equity securities, term loans made under our previous and existing credit facilities, including our current $125.0 million credit facility with Athyrium Opportunities III Acquisition LP, or Athyrium, and operating revenue. We achieved operating profitability for the first time in the third quarter of 2019 and we generated operating profitability from continuing operations for each of the years in the three year period ended December 31, 2019, subsequent to our spin-off of our acute care business, but we cannot provide assurance that we will remain profitable in the future. Although it is difficult to forecast all of our future liquidity requirements, we believe that our cash and cash equivalents on hand combined with our projected cash receipts from services generated under our customer contracts will be sufficient to fund our operations beyond one year after the date our financial statements are issued. In addition, in the event a customer timely cancels its commitments prior to our initiation of manufacturing services, we may be required to refund some or all of the advance payments made to us under those canceled commitments, which would have a negative impact on our liquidity and future revenue.
12
In the event we are unable to maintain sufficient business to support our current operations, we may need to raise additional capital in the future. There can be no assurance that equity financing will be available on acceptable terms or at all. Our ability to raise additional capital in the equity markets to fund our future operations is dependent on a number of factors, including, but not limited to, the market demand for our common stock. The market demand or liquidity of our common stock is subject to a number of risks and uncertainties, including but not limited to, our financial results and economic and market conditions. In addition, even if we are able to raise additional capital, it may not be at a price or on terms that are favorable to us.
We have incurred significant indebtedness, which could adversely affect our business.
As of December 31, 2019, we had an outstanding balance under our credit agreement with Athyrium of $125 million. Our indebtedness could have important consequences to our shareholders. For example, it:
|
|
• |
increases our vulnerability to adverse general economic or industry conditions; |
|
|
• |
limits our flexibility in planning for, or reacting to, changes in our business or the industries in which we operate; |
|
|
• |
reduces proceeds we may receive as a result of any sale; |
|
|
• |
makes us more vulnerable to increases in interest rates, as borrowings under our credit agreement with Athyrium are at variable rates; |
|
|
• |
limits our ability to obtain additional financing or refinancing in the future for working capital or other purposes; and |
|
|
• |
places us at a competitive disadvantage compared to our competitors that have less indebtedness. |
Any of the above-listed factors could materially adversely affect our business, financial condition, results of operations and cash flows. Our credit agreement with Athyrium also contains certain financial and other covenants, including a minimum liquidity requirement and maximum leverage ratios and includes limitations on, among other things, additional indebtedness, paying dividends in certain circumstances, acquisitions and certain investments. The credit agreement provides for certain mandatory prepayment events, including with respect to the proceeds of asset sales, extraordinary receipts, debt issuances and other specified events, based on the terms of the credit agreement with Athyrium. Any failure to comply with the terms, covenants and conditions of the credit agreement may limit our ability to draw upon additional tranches of term loans and may result in an event of default under such agreement, which could have a material adverse effect on our business, financial condition and results of operation.
We operate in a highly competitive market and competition may adversely affect our business.
We operate in a market that is highly competitive. Our competition in the contract manufacturing market includes full-service contract manufacturers and large pharmaceutical companies offering third-party manufacturing services to fill their excess capacity. One of our competitors in the production of verapamil has faced shortage and supply issues in recent years and our sales of verapamil have increased as a result; however, that competitor could return to the manufacturing market for verapamil at any time. If they do we may lose business or face price pressure as a result. We may also compete with the internal operations of those pharmaceutical companies that choose to source their product offerings internally. In addition, most of our competitors may have substantially greater financial, marketing, technical or other resources than we do. Moreover, additional competition may emerge, particularly in lower-cost jurisdictions such as India and China, which could, among other things, result in a decrease in the fees paid for our services, which may adversely affect our results of operations and financial condition.
Our business, financial condition, and results of operations are subject to risks arising from the international scope of our manufacturing and supply relationships.
Some of our customers source raw materials outside the United States. As such, we are subject to risks associated with such international manufacturing relationships, including:
|
|
• |
unexpected changes in regulatory requirements; |
|
|
• |
problems related to markets with different cultural biases or political systems; |
13
|
|
• |
increased risk relating to the transport of products internationally, including damage to our customers’ API, shipment delays relating to the import or export of our products or the delivery of products by means of additional third-party vendors; |
|
|
• |
difficulties importing or exporting supplies or products; |
|
|
• |
unforeseen global instability, including political instability or instability from an outbreak of pandemic or contagious disease (including, for example, the recent coronavirus outbreak); |
|
|
• |
compliance with the U.S. Foreign Corrupt Practices Act and other laws and regulations governing international trade; |
|
|
• |
changes to U.S. and foreign trade policies, including the enactment of tariffs on goods imported into the United States; and |
|
|
• |
imposition of domestic and international customs and tariffs, withholding or other taxes, including any value added taxes. |
Additionally, we are subject to periodic reviews and audits by governmental authorities responsible for administering import/export regulations. To the extent that we are unable to successfully defend against an audit or review, we may be required to pay assessments, penalties, and increased duties on products imported into the United States.
Issues with product quality could have a material adverse effect upon our business, subject us to regulatory actions and cause a loss of customer confidence in us or our products.
Our success depends upon the quality of our products. Quality management plays an essential role in meeting customer requirements, preventing defects, improving our customers product candidates and services and assuring the safety and efficacy of their product candidates. Our future success depends on our ability to maintain and continuously improve our quality management program. A quality or safety issue may result in adverse inspection reports, warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution of products, civil or criminal sanctions, costly litigation, refusal of a government to grant approvals and licenses, restrictions on operations or withdrawal of existing approvals and licenses. An inability to address a quality or safety issue in an effective and timely manner may also cause negative publicity, a loss of customer confidence in us or our future products, which may result in difficulty in successfully launching product candidates and the loss of sales, which could have a material adverse effect on our business, financial condition, and results of operations.
Our development and formulation services projects are typically for a shorter term than our manufacturing projects, and any failure by us to maintain an adequate volume of development and formulation services projects, including due to lower than expected success rates of the products for which we provide services, could have a material adverse effect on our business, results of operations and financial condition.
Our pharmaceutical development services business contracts are generally shorter in term than our manufacturing contracts and typically require us to provide development services within a designated scope. Since our development and formulation services focus on products that are still in developmental stages, their viability depends on the ability of such products to reach their respective subsequent development phases. In many cases, such products do not reach subsequent development phases and, as a result, the profitability of the related pharmaceutical development service project may be limited. Even if a customer wishes to proceed with a project, the product we are developing on such customer’s behalf may fail to receive necessary regulatory approval or may have its development hindered by other factors, such as the development of a competing product.
If we are unable to continue to or timely obtain new projects from existing and new customers, our development and formulation services business could be adversely affected. Furthermore, although our development and formulation services business may act as a pipeline for our manufacturing services business, we cannot predict the conversion rate of our development and formulation services projects to commercial manufacturing services projects, or how successful we will be in winning new projects that lead to a viable
14
product. As such, an increase in the turnover rate of our development and formulation services projects may not benefit our manufacturing services business at a later time.
In addition, our backlog is subject to a number of risks and uncertainties, including risk that a customer timely cancels its commitments, the risk that a customer may experience delays in its program(s) or otherwise, which could result in the postponement or cancellation of anticipated formulation, development and manufacturing services revenue. There is risk that our business development efforts may not materialize as quickly as we have projected, that we may not successfully execute on all customer projects, any of which could have a negative impact on our liquidity, reported backlog and future revenue. Further, the discontinuation of a project as a result of our failure to satisfy a customer’s requirements may also affect our ability to obtain future projects from such customer, as well as from new customers. Any failure by us to maintain a high volume of development and formulation services projects could have a material adverse effect on our business, results of operations and financial condition.
If we fail to meet the stringent requirements of governmental regulation in the manufacture of pharmaceutical products, we could incur substantial costs and a reduction in revenues.
We are required to maintain compliance with cGMP, and our manufacturing facilities are subject to inspections by the FDA and other global regulators to confirm such compliance. Changes of suppliers or modifications of methods of manufacturing may require amending our application(s) to the FDA and acceptance of the change by the FDA prior to release of our manufactured products. Because we produce multiple products at our manufacturing facilities, there are increased risks associated with cGMP compliance. On August 12, 2019 following a six-day pre-approval inspection of our primary manufacturing facility , the FDA issued a Form 483 containing two observations relating to a documentation issue and incomplete investigation. We have promptly responded to these observations as a part of our ongoing obligations under the FDA’s quality system regulation and have implemented corrective and preventative actions to ensure these type of observations do not occur in the future. While we remain committed to continuous improvement and strengthening our quality system and ensuring that all aspects of the system are in full compliance, we can provide no assurance that we will not encounter future inspections resulting in observations not acceptable by the FDA.
Our inability to demonstrate ongoing cGMP compliance could require us to engage in additional lengthy and expensive remediation efforts, withdraw or recall products and/or interrupt commercial supply of any products. Any delay, interruption or other issue that arises in the manufacture, fill/finish, packaging, or storage of any drug product as a result of a failure of our facilities to pass any regulatory agency inspection or maintain cGMP compliance could significantly impair our relationships with our commercial partners, which would substantially harm our business, prospects, operating results and financial condition. Any ongoing or additional findings of non-compliance could also increase our costs and cause us to lose revenue from manufactured products, which could be seriously detrimental to our business, prospects, operating results and financial condition.
Additionally, our manufacturing activities are subject to the Controlled Substances Act and the regulations of the DEA. Accordingly, we must adhere to a number of requirements with respect to controlled substances, including registration, recordkeeping and reporting requirements; labeling and packaging requirements; security controls, procurement and manufacturing quotas; and certain restrictions on refills. Failure to maintain compliance with applicable requirements can result in an enforcement action that could have a material adverse effect on our business, financial condition, operating results and cash flows. The DEA may seek civil penalties, refuse to renew necessary registrations or initiate proceedings to revoke those registrations. In certain circumstances, violations could result in criminal proceedings.
Manufacturers of drug products and their facilities are subject to payment of substantial user fees and continual review and periodic inspections by the FDA and other regulatory authorities, including equivalent regulatory authorities in other countries, for compliance with cGMP regulations and adherence to commitments made in the NDA or the application for marketing authorization. If we, or a regulatory authority, discover previously unknown problems with a product, such as AEs of unanticipated severity or frequency, or problems with a facility where the product is manufactured, a regulatory authority may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market, suspension of manufacturing, or other FDA action or other action by the equivalent regulatory authorities in other countries.
We manufacture opioid products, which are subject to additional regulation by state and federal law enforcement and other regulatory agencies.
We manufacture opioid products, including Zohydro ER, an extended-release opioid treatment, containing hydrocodone. The U.S. government and state legislatures have prioritized combatting the growing misuse and addiction to opioids such as hydrocodone and
15
have enacted legislation and regulations as well as other measures intended to fight the opioid epidemic. Addressing prescription drug abuse is a priority for the current U.S. administration and the FDA and is part of a broader initiative led by the Department of Health and Human Services. Overall, there is greater scrutiny of entities involved in the manufacture, sale and distribution of opioids. These initiatives, existing regulations, and any negative publicity related to opioids may have a material impact on our business and our ability to manufacture opioid products.
Opioids are controlled substance regulated by the DEA. The amount of Schedule II substances that can be obtained is limited by the CSA and DEA regulations. In November 2017, the DEA reduced the amount of almost every Schedule II opiate and opioid medication that may be manufactured in the U.S. in calendar year 2018 by 20%. For 2019, the DEA proposed decreased manufacturing quotas for the six most frequently misused opioids, including oxycodone, by an average of 10% as compared to the 2018 quotas; and DEA has proposed further decreasing manufacturing quotas in 2020 for five of the six opioids (fentanyl, hydrocodone, hydromorphone, oxycodone, oxymorphone), by an average of 28%. Together with reductions in morphine, this is a 53% decrease since 2016. In October 2019, the DEA proposed additional regulations to amend the manner in which the agency grants quotas to manufacturers. The proposed regulations will establish use-specific quotas, including commercial sales, product development, transfer, replacement, and packaging. To decrease the risk of diversion and increase accountability, inventory allowances will be reduced, and procurement quota certifications will be required. If limited supply of opioids impacts demand for products of our partners, our revenues may be adversely impacted. In addition to DEA regulations, the U.S. government and states have enacted other laws that seek to promote improved monitoring of opioids and to increase funding for research and development of non-addictive painkillers. Legislation has also been proposed that would further limit the ability to sell and prescribe opioids. These efforts may result in an additional reduction of demand for opioid products or government action against us if we fail to comply with these laws and could have a material adverse effect on our business
If we use hazardous and biological materials in a manner that causes injury or violates applicable law, we may be liable for damages.
Our operations involve the controlled use of hazardous materials and chemicals. We are subject to federal, state and local laws and regulations in the U.S. governing the use, manufacture, storage, handling and disposal of hazardous materials and chemicals. Although we believe that our procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we may incur significant additional costs to comply with applicable laws in the future. Also, even if we are in compliance with applicable laws, we cannot completely eliminate the risk of contamination or injury resulting from hazardous materials or chemicals. As a result of any such contamination or injury, we may incur liability or local, city, state or federal authorities may curtail the use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our contract manufacturing operations, which could materially harm our business, financial condition and results of operations.
We may not be able to successfully offer new services.
In order to successfully compete, we will need to offer and develop new services. Without the timely introduction of enhanced or new services, our services and capabilities may become obsolete over time, in which case, our revenues and operating results would suffer. The related development costs may require a substantial investment before we can determine their commercial viability, and we may not have the financial resources to fund such initiatives.
In addition, the success of enhanced or new services will depend on several factors, including but not limited to our ability to:
|
|
• |
properly anticipate and satisfy customer needs, including increasing demand for lower cost services; |
|
|
• |
enhance, innovate, develop and manufacture new offerings in an economical and timely manner; |
|
|
• |
differentiate our deliverables from competitors’ offerings; |
|
|
• |
meet quality requirements, authorization requirements, and other regulatory requirements of government agencies; |
|
|
• |
obtain valid and enforceable intellectual property rights; and |
|
|
• |
avoid infringing the proprietary rights of third parties. |
16
Even if we were to succeed in creating enhanced or new services, those services may not result in commercially successful offerings or may not produce revenues in excess of the costs of development and capital investment and may be quickly rendered obsolete by changing customer preferences or by technologies or features offered by our competitors. In addition, innovations may not be accepted quickly in the marketplace due to, among other things, entrenched patterns of clinical practice, the need for regulatory clearance and uncertainty over market access or government or third-party reimbursement. If we are not able to offer new services and effectively compete, our business, financial condition, and results of operations could be negatively impacted.
Technological change may cause our offerings to become obsolete over time. A decrease in our customers’ purchases of our offerings could have a material adverse effect on our business, results of operations and financial condition.
The healthcare industry is characterized by rapid technological change. Demand for our services may change in ways that we may not anticipate because of evolving industry standards or as a result of evolving customer needs that are increasingly sophisticated and varied or because of the introduction by competitors of new services and technologies. In addition, we require capital and resources to support the maintenance and improvement of our facilities, including replacing or repairing aging production equipment and updating overall facility master plans. If we are unable to maintain and improve our facilities, we may experience unscheduled equipment downtime and unpredicted machinery failure and become unable to supply our customers with products or services which may affect business continuity. Any such incident or disruption in business continuity could have a material adverse effect on our business, results of operations and financial condition.
We may be adversely affected by natural disasters or other events that disrupt our business operations, and our business continuity and disaster recovery plans may not adequately protect us from a serious disaster.
Our manufacturing facilities are located in Gainesville, Georgia, where natural disasters or similar events, like hurricanes, blizzards, tornadoes, fires, floods, earthquakes or explosions or large-scale accidents or power outages, could severely disrupt our operations and have a material adverse effect on our business, prospects, results of operations and financial condition. If a disaster, power outage or other event occurred that prevented us from using all or a significant portion of our Gainesville facilities, damaged critical infrastructures, such as manufacturing resource planning and enterprise quality systems, or otherwise disrupted operations at that location, it may be difficult or, in certain cases, impossible for us to continue our development, formulation and manufacturing business for a substantial period of time, which could have a material adverse effect on our business, financial condition, and results of operations.
Currently, we maintain insurance coverage against damage to our property and equipment, and to cover business interruption expenses, in an amount we believe is sufficient for our development, formulation and manufacturing operations. However, there can be no assurance that such insurance will continue to be available on acceptable terms or that such insurance will provide adequate protection against actual losses. Even if we maintain adequate insurance coverage, claims could have a material adverse effect on our financial condition, liquidity and results of operations and on our ability to obtain suitable, adequate or cost-effective insurance in the future.
We must comply with environmental and health and safety laws and regulations, which can be expensive and restrict how we do business.
We are subject to federal, state and local laws, rules, regulations and policies concerning the environment and the health and safety of our employees. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions, which could have a material adverse effect on our business, financial condition, and results of operations.
In addition, our business involves the use, generation and disposal of hazardous materials, including chemicals, solvents, agents and biohazardous materials. As a result, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens and wastes. Although we believe that our safety procedures for storing, handling and disposing of such materials comply with the standards prescribed by those regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. We currently contract with third parties to dispose of these substances that we generate, and we rely on these third parties to properly dispose of these substances in compliance with applicable laws and regulations. If these third parties do not properly dispose of these substances in compliance with applicable laws and regulations, we may be subject to legal action by governmental
17
agencies or private parties for improper disposal of these substances. The costs of defending such actions and the potential liability resulting from such actions are often very large. In the event we are subject to such legal action or we otherwise fail to comply with applicable laws and regulations governing the use, generation and disposal of hazardous materials and chemicals, we could be held liable for any damages that result, and any such liability could exceed our resources. In addition, although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees, including those resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. If we become subject to any of the foregoing liabilities, our business, financial condition, and results of operations could be materially adversely impacted.
We may be subject to litigation or government investigations for a variety of claims, which could adversely affect our operating results, harm our reputation or otherwise negatively impact our business.
We may be subject to litigation or government investigations. These may include claims, lawsuits, and proceedings involving product liability, labor and employment, wage and hour, commercial and other matters. The outcome of any litigation or government investigation, regardless of its merits, is inherently uncertain. Any lawsuits or government investigations, and the disposition of such lawsuits and government investigations, could be time-consuming and expensive to resolve and divert management attention and resources. Any adverse determination related to litigation or government investigations could adversely affect our operating results, harm our reputation or otherwise negatively impact our business. In addition, depending on the nature and timing of any such dispute, a resolution of a legal matter or government investigation could materially affect our future operating results, our cash flows or both.
Our future success depends on our ability to retain and have the full attention of our key executives as well as to attract, retain and motivate other qualified personnel.
We are highly dependent on the principal members of our executive team and, in particular, the services of Gerri A. Henwood, our President and Chief Executive Officer, the loss of whose services would adversely impact the achievement of our objectives. We have entered into employment agreements with each of our executive officers. Recruiting and retaining qualified employees for our business, including business development, scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous pharmaceutical companies for individuals with similar skill sets. In addition, failure to succeed in clinical studies may make it more challenging to recruit and retain qualified personnel. The inability to recruit or loss of the services of any executive or key employee could impede the progress of our business development, research, development, manufacturing, quality and commercialization, growth and diversification objectives.
We may acquire other assets or businesses, or form collaborations or make investments in other companies or technologies, that could have a material adverse effect on our operating results, dilute our shareholders’ ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of assets, including, businesses or strategic alliances and collaborations, to expand our existing technologies and operations. We may not identify or complete these transactions in a timely manner, on a cost‑effective basis, or at all, and we may not realize the anticipated benefits of any such transaction, any of which could have a material adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company or assets may also disrupt ongoing operations, require the hiring of additional personnel and the implementation of additional internal systems and infrastructure, especially the acquisition of commercial assets, and require management resources that would otherwise focus on developing our existing business.
To finance any acquisitions or collaborations, we may choose to issue debt or shares of our common or preferred stock as consideration. Any such issuance of shares would dilute the ownership of our shareholders. If the price of our common stock is low or volatile, we may not be able to acquire other assets or companies or fund a transaction using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
Our employees, partners, independent contractors, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
18
We are exposed to the risk that our employees, partners, independent contractors, consultants and vendors may engage in fraudulent or other illegal activity with respect to our business. Misconduct by these employees could include intentional, reckless and/or negligent conduct or unauthorized activity that violates: (1) FDA or DEA regulations, including those laws requiring the reporting of true, complete and accurate information to the FDA; (2) manufacturing standards; (3) federal and state healthcare fraud and abuse laws and regulations; or (4) laws that require the true, complete and accurate reporting of financial information or data. Activities subject to these laws also involve the improper use of information obtained in the course of clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and serious harm to our reputation. Any incidents or any other conduct that leads to an employee receiving an FDA debarment could result in a loss of business from our partners and severe reputational harm. We have adopted a Code of Business Conduct and Ethics, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business, operating results and financial condition.
We face potential product liability claims, and, if successful claims are brought against us, we may incur substantial liability.
The use of our products exposes us to the risk of product liability claims as well as potential toxic tort and other types of product liability claims that are inherent in the manufacture of pharmaceutical products. Product liability claims might be brought against us by consumers, health care providers, pharmaceutical companies or others selling or otherwise coming into contact with our products. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
|
|
• |
impairment of our business reputation and negative media attention; |
|
|
• |
withdrawal of our customers clinical study participants or adverse effects occurring during such clinical trials; |
|
|
• |
costs due to related litigation; |
|
|
• |
distraction of management’s attention from our primary business; |
|
|
• |
decreased demand for our manufacturing services or loss of any of our commercial partners; |
|
|
• |
substantial monetary awards to patients or other claimants; |
|
|
• |
the inability of our customers to commercialize their product candidates; |
|
|
• |
increased scrutiny and potential investigation by, among others, the FDA, the Department of Justice, the Office of Inspector General of the U.S. Department of Health and Human Services, State Attorneys General, members of Congress and the public. |
Our current product liability insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability.
Changes in tax laws and unanticipated tax liabilities could adversely affect our effective income tax rate and ability to achieve profitability.
We are subject to income taxes in the United States. Our effective income tax rate in the future could be adversely affected by a number of factors including changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, reversal of established valuation allowances, changes to our operations including the discontinuance, licensing, spin-off or sale of any asset, changes to tax strategy, changes in transfer pricing and changes in tax laws.
19
We regularly assess these matters to determine the adequacy of our tax provision, which is subject to discretion. If our assessments are incorrect, it could have an adverse effect on our business and financial condition. There can be no assurance that income tax laws and administrative policies with respect to the income tax consequences generally applicable to our subsidiaries or to us will not be changed in a manner which adversely affects our shareholders. Changes in tax laws and unanticipated tax liabilities could adversely affect our effective income tax rate and ability to achieve profitability, which could have a material adverse effect on our business, financial condition and results of operation.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As of December 31, 2019, we had federal and state net operating loss carry forwards, or NOLs, of approximately $121.6 million and $128.1 million, respectively. The federal carry forwards for 2008 through 2017 will expire in 2028 through 2038. Federal net operating losses incurred in 2018 and onward have an indefinite expiration under the 2017 Tax Cut & Jobs Act. The state carry forwards including those generated in 2019 will expire in 2028 through 2029.
Our NOLs may be subject to audit and future adjustment by the Internal Revenue Service in the U.S. or other state taxing authorities, which could result in a reversal of none, part, or all of the income tax benefit or could result in a benefit higher than the net amount recorded. If the relevant taxing authorities reject or reduce the amount of the income tax benefit related to our NOLs, we may have to pay additional cash income taxes, which could adversely affect our results of operations, financial condition, and cash flows. We cannot guarantee what the ultimate outcome or amount of the benefit we may receive from the NOLs, if any, will be.
Furthermore, utilization of NOLs may be subject to a substantial limitation pursuant to Section 382 of the Code as well as similar state statutes in the event of an ownership change. Such ownership changes have occurred in the past, and could occur again in the future Under Section 382 of the Internal Revenue Code of 1986, as amended, or Section 382, if a corporation undergoes an "ownership change," generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation's ability to use its pre-change NOLs and other pre-change tax attributes (such as research and development tax credits) to offset its post-change income may be limited. We may experience ownership changes in the future as a result of shifts in our stock ownership some of which are outside our control. We completed a detailed study of our NOLs and determined that there was not an ownership change in excess of 50%. Ownership changes in future periods may place additional limits on our ability to utilize net operating loss and tax credit carry forwards. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
We incur increased costs and demands upon our management as a result of complying with the laws and regulations affecting public companies, which could harm our operating results.
We are a public company and, as such, we incur significant legal, accounting, directors & officers insurance and other expenses, including costs associated with public company reporting requirements. We incur costs associated with current corporate governance requirements, including certain of the requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, as well as rules implemented by the SEC and the Nasdaq Capital Market, the stock exchange on which our common stock is listed. If we fail to comply with current corporate governance requirements, our business may be negatively affected, including by having our common stock delisted from the Nasdaq Capital Market.
The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically in recent years. We expect these rules and regulations to continue to significantly impact our legal, insurance and financial compliance costs and to make some activities more time-consuming and costly. We are unable to currently estimate these costs with any degree of certainty. We also expect that these rules and regulations may make it difficult and expensive for us to continue to maintain director and officer liability insurance, and if we are able to maintain such insurance, we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage available to privately-held companies. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors, or the board, or as our executive officers, which could have a material adverse effect on our business.
The security of our information technology systems may be compromised in the event of system failures, unauthorized access, cyberattacks or a deficiency in our cybersecurity, and confidential information, including non-public personal information that we maintain, could be improperly disclosed.
20
We rely extensively on information technology and systems including internet sites, data hosting, physical security, and software applications and platforms. Despite our security measures, our information technology systems, some of which are managed by third parties, may be susceptible to damage, disruptions or shutdowns due to computer viruses, attacks by computer hackers, failures during the process of upgrading or replacing software, power outages, user errors or catastrophic events. A significant breakdown, invasion, corruption, destruction or interruption of critical information technology systems, by our employees, others with authorized access to our systems or unauthorized persons could negatively impact or interrupt operations. For example, the loss of data from completed or ongoing clinical trials for product candidates could result in delays in regulatory approval efforts and significantly increase our costs to recover or reproduce the data. The use of technology, including cloud-based computing, creates opportunities for the unintentional dissemination or intentional destruction of confidential information stored in our systems or our third-party systems. We could also experience a business interruption, theft of confidential information or reputational damage from malware or other cyberattacks, which may compromise our systems or lead to data leakage, either internally or at our third-party providers.
As part of our business, we maintain large amounts of confidential information, including non-public personal information on patients and our employees. The maintenance of such information is governed by various rules and regulations in the jurisdictions in which we conduct our business, including by the General Data Privacy Regulation, or GDPR, in the European Union. Breaches in security, either internally or at our third-party providers, could result in the loss or misuse of this information, which could, in turn, result in potential regulatory actions or litigation, including material claims for damages, interruption to our operations, damage to our reputation or otherwise have a material adverse effect on our business, financial condition and operating results. Although we believe we have appropriate information security policies and systems in place in order to prevent unauthorized use or disclosure of confidential information, including non-public personal information, there can be no assurance that such use or disclosure will not occur.
Any such business interruption, theft of confidential information or reputational damage from malware or other cyberattacks, or violation of personal information laws, could have a material adverse effect on our business, financial condition, and results of operations.
If we fail to comply with data protection laws and regulations, we could be subject to government enforcement actions (which could include civil or criminal penalties), private litigation and/or adverse publicity, which could negatively affect our operating results and business.
We are subject to laws and regulations that address privacy and data security of patients who use our product candidates in the United States and in states in which we conduct our business. In the United States, numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act) govern the collection, use, disclosure, and protection of health-related and other personal information. For instance, HIPAA imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information and imposes notification obligations in the event of a breach of the privacy or security of individually identifiable health information on entities subject to HIPAA and their business associates that perform certain activities that involve the use or disclosure of protected health information on their behalf. Failure to comply with applicable data protection laws and regulations could result in government enforcement actions and create liability for us, which could include civil and/or criminal penalties, as well as private litigation and/or adverse publicity that could negatively affect our operating results and business.
Risks Related to Our Intellectual Property
We own numerous pending patent applications and issued patents in the United States. If our pending patent applications fail to issue or if our issued patents expire or are successfully opposed, invalidated, or rendered unenforceable, our business will be adversely affected.
To protect our proprietary technology, we rely on patents and other intellectual property protections, including trade secrets, nondisclosure agreements and confidentiality provisions.
As of December 31, 2019, we own five issued U.S. patents, and pending applications in the U.S. and several foreign countries relating to Zohydro-ER®, all of which expire on September 12, 2034. We license the Canadian patent application relating to this technology to our commercial partner, Paladin Labs Inc., in Canada. The patent applications that we have filed and have not yet been granted may fail to result in issued patents in the United States or foreign countries. Even if the patents do successfully issue, third parties
21
may challenge the patents or the inventorship thereof, which can lead to an issued patent being found invalid, unenforceable or can otherwise alter the ownership of the patents.
The issuance of any patent is not a certainty. Unless and until our pending applications issue, their protective scope is impossible to determine. It is impossible to predict whether or how many of these applications will result in issued patents and patents that issue may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of patent exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which may limit our ability to prevent others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. In addition, upon expiration of a patent, we may be limited in our ability to prevent others from using or commercializing subject matter covered by the expired patents. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
Patent reform legislation could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. The Leahy Smith America Invents Act, or the Leahy Smith Act, enacted in September 2011, brought significant changes to the U.S. patent system. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The United States Patent Office continues to develop and implement new regulations and procedures to govern administration of the Leahy Smith Act, and many of the substantive changes to patent law associated with the Leahy Smith Act became effective on March 16, 2013. The Leahy Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patent, all of which could have a material adverse effect on our business and financial condition.
Litigation involving patents, patent applications and other proprietary rights is expensive and time-consuming. If we are involved in such litigation, it could interfere with our business.
Our success depends in part on not infringing patents and proprietary rights of third parties. Although we are not currently aware of litigation or other proceedings or third-party claims of intellectual property infringement related to our technologies or business activities, the pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights.
In a patent infringement claim against us, we may assert, as a defense, that we do not infringe the relevant patent claims, that the patent is invalid or both. The strength of our defenses will depend on the patents asserted, the interpretation of these patents and/or our ability to invalidate the asserted patents. However, we could be unsuccessful in advancing non-infringement and/or invalidity arguments in our defense. In the United States, issued patents enjoy a presumption of validity, and the party challenging the validity of a patent claim must present clear and convincing evidence of invalidity, which is a high burden of proof. Conversely, the patent owner need only prove infringement by a preponderance of the evidence, which is a low burden of proof.
If we were found by a court to have infringed a valid patent claim, we could be prevented from using the patented technology or be required to pay the owner of the patent for the right to license the patented technology. If we decide to pursue a license to one or more of these patents, we may not be able to obtain a license on commercially reasonable terms, if at all, or the license we obtain may require us to pay substantial royalties or grant cross licenses to our patent rights. For example, if the relevant patent is owned by a competitor, that competitor may choose not to license patent rights to us. If we decide to develop alternative technology, we may not be able to do so in a timely or cost-effective manner, if at all.
In addition, because patent applications can take years to issue and are often afforded confidentiality for some period of time, there may currently be pending applications, unknown to us, that later result in issued patents that could cover one or more of our products.
It is possible that we may in the future receive, particularly as a public company, communications from competitors and other companies alleging that we may be infringing their patents, trade secrets or other intellectual property rights, offering licenses to such intellectual property or threatening litigation. In addition to patent infringement claims, third parties may assert copyright, trademark or other proprietary rights against us. We may need to expend considerable resources to counter such claims and may not be able to be successful in our defense. Our business may suffer if a finding of infringement is established.
Generic competitors can challenge the U.S. patents protecting our commercial partners’ product candidates by filing an ANDA or an NDA for a generic or a modified version of our commercial partners’ product candidates.
22
Separate and apart from the protection provided under the U.S. patent laws, drug candidates may be subject to the provisions of the Hatch- Waxman Act, which may provide drug candidates with either a three- or five-year period of marketing exclusivity following receipt of FDA approval. The Hatch-Waxman Act prohibits the FDA from accepting the filing of an ANDA application (for a generic product) or a 505(b)(2) NDA (for a modified version of the product) for three years for active drug ingredients previously approved by the FDA or for five years for active drug ingredients not previously approved by the FDA.
There is an exception, however, for newly approved molecules that allows competitors to challenge a patent beginning four years into the five-year exclusivity period by alleging that one or more of the patents listed in the FDA’s list of approved drug products are invalid, unenforceable and/or not infringed and submitting an ANDA for a generic version of a drug candidate. This patent challenge is commonly known as a Paragraph IV certification. Within the past several years, the generic industry has aggressively pursued approvals of generic versions of innovator drugs at the earliest possible point in time.
If a generic company is able to successfully challenge the patents covering drug candidates by obtaining FDA approval for an ANDA, the generic company may choose to launch a generic version of a drug candidate. Any launch of a generic version of our drug candidates prior to the expiration of patent protection will have a material adverse effect on our revenues and our results of operations.
We and our commercial partners have been involved in Paragraph IV litigation in the United States involving some of our patents in respect of Zohydro ER®. These litigations have been, and any other Paragraph IV litigation may be, expensive, distracting to management and protracted. Although we and our commercial partners have successfully settled our Paragraph IV litigation, any future Paragraph IV litigation could result in new or additional generic competition to Zohydro ER®. We have confirmed a generic for the (hydrocodone bitartrate) SR capsules 10, 15, 20, 30 40 and 50 was approved by FDA / OGD on Jan 21, 2020 for Alvogen which could have a material adverse effect on our business, results of operations, financial condition and prospects. In addition, we were previously involved in an interference in front of the United States Patent and Trademark Office with another party, which involved a patent application relating to Zohydro ER®, for which we ultimately were successful on appeal. However, any future interference claims could arise, and if successful, result in the issuance of a patent that could limit our freedom to operate in respect to Zohydro ER®, which could also cause a reduction in revenue and have a material adverse effect on our business, prospects, results of operations and financial condition.
It is difficult and costly to protect our proprietary rights, and we may not be able to ensure their protection.
The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in pharmaceutical patents has emerged in the United States to date. The pharmaceutical patent situation outside of the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in the patents that may be issued from the applications we currently or may in the future own or license from third parties. Further, if any patent license we obtain is deemed invalid and/or unenforceable, it could impact our ability to commercialize or partner our technology.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
|
|
• |
we were the first to make the inventions covered by each of our pending patent applications; |
|
|
• |
we were the first to file patent applications for these inventions; |
|
|
• |
others will not independently develop similar or alternative technologies or duplicate any of our technologies; |
|
|
• |
an individual or party will not challenge inventorship, that if successful, could have an adverse effect on our business; |
|
|
• |
any patents issued to us or our collaborators will provide a basis for commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; or |
|
|
• |
the patents of others will not have an adverse effect on our business. |
23
If we do not adequately protect our proprietary rights, competitors may be able to use our technologies and erode or negate any competitive advantage we may possess, which could materially harm our business, negatively affect our position in the marketplace, limit our ability to commercialize our product candidates and delay or render impossible our achievement of profitability.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
In the future, we may rely on trade secrets to protect our proprietary know-how and technological advances, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. Failure to obtain or maintain trade secret protection could enable competitors to use our proprietary information to develop products that compete with our products or cause additional, material adverse effects on our competitive business position.
Our ability to manufacture products for our commercial partners may be impaired if any of our manufacturing activities, or the activities of third parties involved in our manufacture and supply chain, are found to infringe patents of others.
Our ability to continue to manufacture products for our commercial partners, to utilize third parties to supply raw materials or other products, or to perform fill/finish services or other steps in our manufacture and supply chain, depends on our and their ability to operate without infringing the patents and other intellectual property rights of others. Other parties may allege that our manufacturing activities, or the activities of third parties involved in our manufacturing and supply chain, infringe patents or other intellectual property rights. A judicial decision in favor of one or more parties making such allegations could preclude the manufacture of the products to which those intellectual property rights apply, which could materially harm our business, operating results and financial condition.
Risks Relating to Our Securities
We may incur operational difficulties or be exposed to claims and liabilities as a result of the separation and distribution of Baudax Bio.
On November 21, 2019, we distributed all of the then outstanding shares of Baudax Bio common stock to our shareholders in connection with the separation of our Acute Care business. In connection with the distribution, we entered into a separation and distribution agreement and various other agreements (including a transition services agreement, a tax matters agreement, a manufacturing and supply agreement, an employee matters agreement, an intellectual property matters agreement and certain other commercial agreements). These agreements govern the separation and distribution and the relationship between the two companies going forward, including with respect to potential tax-related losses associated with the separation and distribution. They also provide for the performance of services by each company for the benefit of the other for a period of time.
The separation and distribution agreement provides for indemnification obligations designed to make Baudax Bio financially responsible for any liabilities that may exist relating to its business activities, whether incurred prior to or after the distribution, including any pending or future litigation. It is possible that a court would disregard the allocation agreed to between us and Baudax Bio and require us to assume responsibility for obligations allocated to Baudax Bio. Third parties could also seek to hold us responsible for any of these liabilities or obligations, and the indemnity rights we have under the separation and distribution agreement may not be sufficient to fully cover all of these liabilities and obligations. Even if we are successful in obtaining indemnification, we may have to bear costs temporarily. In addition, our indemnity obligations to Baudax Bio may be significant. These risks could negatively affect our business, financial condition or results of operations.
The separation of Baudax Bio continues to involve a number of risks, including, among other things, the indemnification risks described above and the potential that management’s and our employees’ attention will be significantly diverted by the provision of transitional services. Certain of the agreements described above provide for the performance of services by each company for the benefit of the other for a period of time. If Baudax Bio is unable to satisfy its obligations under these agreements, including its
24
indemnification obligations, we could incur losses. Our inability to effectively manage the separation activities and related events could adversely affect our business, financial condition or results of operations.
The market price and trading volume of our common stock have been and may continue to be volatile, which could result in rapid and substantial losses for our shareholders.
The market price for our common stock has been volatile and may continue to fluctuate or may decline significantly in the future. An active, liquid and orderly market for our common stock may not be sustained, which could depress the trading price of our common stock or cause it to continue to be highly volatile or subject to wide fluctuations. Some of the factors that could negatively affect our share price or result in fluctuations in the price or trading volume of our common stock include, among other things:
|
|
• |
FDA, state or international regulatory actions, including actions on regulatory applications for any of our commercial partners’ product candidates; |
|
|
• |
legislative or regulatory changes; |
|
|
• |
judicial pronouncements interpreting laws and regulations; |
|
|
• |
changes in government programs; |
|
|
• |
announcements of new products, services or technologies, commercial relationships, acquisitions or other events by us or our competitors; |
|
|
• |
changes in demand for or pricing of our customers products; |
|
|
• |
the sales ramp and trajectory for our formulation, development and manufacturing services; |
|
|
• |
market conditions in the pharmaceutical and biotechnology sectors; |
|
|
• |
fluctuations in stock market prices and trading volumes of similar companies; |
|
|
• |
changes in accounting principles; |
|
|
• |
litigation or public concern about the safety of our products or similar products; |
|
|
• |
sales of large blocks of our common stock, including sales by our executive officers, directors and significant shareholders |
|
|
• |
our announcement of financing transactions, including debt, convertible notes, etc.; and |
|
|
• |
actions by institutional or activist shareholders. |
These broad market and industry factors may decrease the market price of our common stock, regardless of our actual operating performance. In addition, in the past, following periods of volatility in the overall market and decreases in the market price of a company’s securities, securities class action litigation has often been instituted against these companies. Following the decrease in our trading price in May 2018, a securities class action lawsuit was filed against us and certain of our officers and directors for alleged violations of Section 10(b) and 20(a) of the Exchange Act and Rule 10(b)(5) promulgated thereunder. The complaint seeks unspecified damages, interest, attorneys’ fees and other costs. On December 10, 2018, lead plaintiff filed an amended complaint that asserted the same claims and sought the same relief but included new allegations and named additional officers and directors as defendants. On February 8, 2019, we filed a motion to dismiss the amended complaint in its entirety which the lead plaintiff opposed on April 9, 2019. On May 9, 2019, we filed our response and briefing was completed on the motion to dismiss. In response to questions from the Judge, the parties submitted supplemental briefs with regard to the motion to dismiss the amended complaint during the fall of 2019. On February 18, 2020, the motion to dismiss was granted without prejudice; however, the plaintiffs have indicated that they intend to file a new complaint. In connection with the separation from Baudax Bio, Baudax Bio accepted
25
assignment from us of all of our obligations in connection with the litigation and agreed to indemnify us for all liabilities related to the litigation. This litigation, and any other securities class actions that may be brought against us, could result in substantial costs and a diversion of our management’s attention and resources.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
Certain holders of our securities are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act. Any sales of shares by these shareholders could have a material adverse effect on the trading price of our common stock.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, our ability to operate our business and investors’ views of us.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be frequently evaluated. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and attestations of the effectiveness of internal controls by independent auditors (the latter requirement does not apply to smaller reporting companies-we qualify as a smaller reporting company). Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock.
As of December 31, 2019, we are no longer an “emerging growth company” and, as a result, are required to comply with increased disclosure and governance requirements.
As of December 31, 2019, we ceased to be an “emerging growth company” as defined in the JOBS Act as of December 31, 2019. We are now subject to certain requirements that apply to other public companies but did not previously apply to us. These requirements include:
|
|
• |
the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; and the “say on pay” provisions (requiring a non-binding stockholder vote to approve compensation of certain executive officers) and |
|
|
• |
the “say on golden parachute” provisions (requiring a non-binding stockholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Act and some of the disclosure requirements of the Dodd-Frank Act relating to compensation of our chief executive office |
Therefore, this Annual Report is subject to Section 404(b) of the Sarbanes-Oxley Act, which requires that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting. Compliance with Section 404 is expensive and time consuming for management and could result in the detection of internal control deficiencies of which we are currently unaware. The loss of “emerging growth company” status and compliance with the additional requirements significantly impacts our legal and financial compliance costs and make some activities more time consuming and costly.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
26
Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision‑making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
If securities or industry analysts do not continue to publish research or reports, or if they publish unfavorable research or reports, about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We currently have limited research coverage by securities and industry analysts. If additional securities or industry analysts do not commence coverage of our company, the trading price for our stock could be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
We have never paid cash dividends on our common stock and do not intend to do so for the foreseeable future.
We have never paid cash dividends on our common stock and we do not anticipate that we will pay any cash dividends on our common stock for the foreseeable future. Accordingly, any return on an investment in our common stock will be realized, if at all, only when shareholders sell their shares. In addition, our failure to pay cash dividends may make our stock less attractive to investors, adversely impacting trading volume and price.
The concentration of our capital stock ownership with our directors and their affiliated entities and our executive officers will limit shareholders’ abilities to influence certain corporate matters.
Our directors and their affiliated entities, and our executive officers, beneficially own, in the aggregate, approximately 18% of our outstanding common stock as of December 31, 2019. As a result, these shareholders are collectively able to influence matters requiring approval of our shareholders, including the election of directors and approval of significant corporate transactions, such as mergers, consolidations or the sale of all or substantially all of our assets. Such influence may delay, prevent or deter a change in control of our company, even when such a change may be in the best interests of some shareholders, impede a merger, consolidation, takeover or other business combination involving us, or could deprive our shareholders of an opportunity to receive a premium for their common stock as part of a sale of our company or our assets and might adversely affect the prevailing market price of our common stock.
Some provisions of our charter documents and Pennsylvania law may have anti‑takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders, and may prevent attempts by our shareholders to replace or remove our current management.
Provisions in our articles of incorporation and amended and restated bylaws could make it more difficult for a third-party to acquire us or increase the cost of acquiring us, even if doing so would benefit our shareholders, or remove our current management. These include provisions that:
|
|
• |
divide our board of directors into three classes with staggered three-year terms; |
|
|
• |
provide that a special meeting of shareholders may be called only by a majority of our board of directors; |
27
|
|
• |
provide that shareholders may only act at a duly organized meeting; and |
|
|
• |
provide that members of our board of directors may be removed from office by our shareholders only for cause by the affirmative vote of 75% of the total voting power of all shares entitled to vote generally in the election of directors. |
These provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Pennsylvania, we are governed by the provisions of the Pennsylvania Business Corporation Law of 1988, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our shareholders. Under Pennsylvania law, a corporation may not, in general, engage in a business combination with any holder of 20% or more of its capital stock unless the holder has held the stock for five years or, among other things, the board of directors has approved the transaction. Any provision of our articles of incorporation or bylaws or Pennsylvania law that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Item 1B.Unresolved Staff Comments
None.
Our principal executive offices are located at 490 Lapp Road, Malvern, PA 19355. We currently operate our owned 97,000 square foot, DEA-licensed facility in Gainesville, Georgia and leased 24,000 square foot development and high potency product services facility, also in Gainesville, GA, which expires on June 30, 2025.
On May 31, 2018, a securities class action lawsuit, or the Securities Litigation, was filed against us and certain of our officers and directors in the U.S. District Court for the Eastern District of Pennsylvania (Case No. 2:18-cv-02279-MMB) that purported to state a claim for alleged violations of Section 10(b) and 20(a) of the Exchange Act and Rule 10(b)(5) promulgated thereunder, based on statements made by us concerning the NDA for IV meloxicam. The complaint seeks unspecified damages, interest, attorneys’ fees and other costs. On December 10, 2018, lead plaintiff filed an amended complaint that asserted the same claims and sought the same relief but included new allegations and named additional officers as defendants. On February 8, 2019, we filed a motion to dismiss the amended complaint in its entirety, which the lead plaintiff opposed on April 9, 2019. On May 9, 2019, we filed our response and briefing was completed on the motion to dismiss. In response to questions from the Judge, the parties submitted supplemental briefs with regard to the motion to dismiss the amended complaint during the fall of 2019. On February 18, 2020, the motion to dismiss was granted without prejudice; however, the plaintiffs have indicated that they intend to file a second amended complaint. In connection with the separation of Baudax Bio, Baudax Bio accepted assignment by us of all of our obligations in connection with the Securities Litigation and agreed to indemnify us for all liabilities related to the Securities Litigation. We believe that the lawsuit is without merit and intend to vigorously defend against it if the plaintiffs file a new complaint. The lawsuit is in the early stages and, at this time, no assessment can be made as to its likely outcome or whether the outcome will be material to us.
Item 4. Mine Safety Disclosures
Not applicable.
28
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Our common stock is traded on the Nasdaq Capital Market under the symbol “REPH.”
Holders of Common Stock
As of February 28, 2020, there were 7 holders of record of our common stock. We believe that the number of beneficial owners of our common stock at that date was substantially greater.
Dividend Policy
We have never declared or paid any cash dividends on our common stock and our ability to pay cash dividends is currently prohibited by the terms of our credit facility with Athyrium. We do not anticipate paying cash dividends on our common stock in the foreseeable future. Payment of future dividends, if any, on our common stock will be at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, anticipated cash needs and plans for expansion.
Issuer Repurchases of Equity Securities
None.
Securities Authorized for Issuance Under Equity Compensation Plans
Other information about our equity compensation plans is incorporated herein by reference to Part III, Item 12 of this Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
None.
Item 6.Selected Financial Data
None.
29
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any of our filings under the Securities Act or the Exchange Act.
The following graph illustrates a comparison of the total cumulative stockholder return for our common stock since December 31, 2014, to two indices: the NASDAQ Composite Index and the NASDAQ Biotechnology Index. The graph assumes an initial investment of $100 on December 31, 2014, in our common stock, the stocks comprising the NASDAQ Composite Index, and the stocks comprising the NASDAQ Biotechnology Index. Historical stockholder return is not necessarily indicative of the performance to be expected for any future periods.
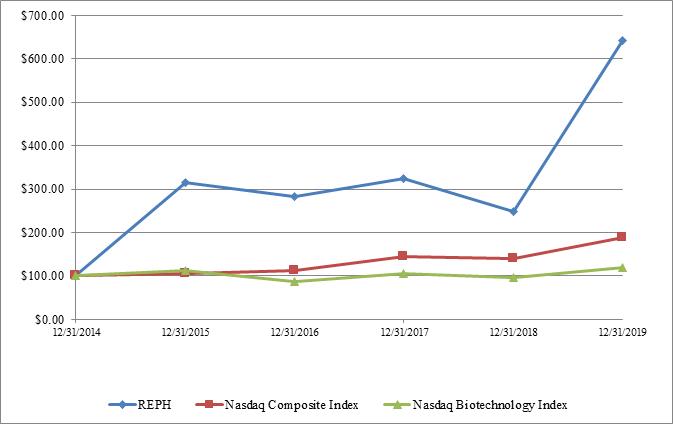
30
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes appearing elsewhere. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions and other factors that could cause actual results to differ materially from those made, projected or implied in the forward-looking statements. Our actual results may differ materially from those discussed below. Please see “Forward-Looking Statements” and “Risk Factors” included in Part I, Item 1A of this Annual Report on Form 10-K for factors that could cause or contribute to such differences.
Overview
We are a leading contract development and manufacturing organization, or CDMO, with integrated solutions for the development, formulation, regulatory support, manufacturing, and packaging of oral solid dose drug products. We operate through a single CDMO business upon completion of the spin-off of our historical Acute Care business, which occurred on November 21, 2019.
We leverage our formulation and development expertise to develop and manufacture pharmaceutical products using proprietary delivery technologies and know-how for commercial partners who commercialize or plan to commercialize these products. These collaborations result in revenue streams including manufacturing, royalties, profit sharing, and research and development, which support our continued operations. We operate a 97,000 square foot, DEA-licensed manufacturing facility in Gainesville, Georgia, as well as a 24,000 square foot development and high potency product facility in Gainesville, Georgia that we opened in October 2018. We currently develop and/or manufacture the following key products with our key commercial partners: Ritalin LA®, Focalin XR®, Verelan PM®, Verelan SR®, Verapamil PM, Verapamil SR and Zohydro ER®, as well as supporting development stage products.
We have used cash flow generated by our business primarily to fund operations at our Gainesville, Georgia manufacturing facilities, fund our historical Acute Care business and to make payments under our credit facility. We believe our business will continue to contribute cash for these and other general corporate purposes.
In November 2019, our former Acute Care business was spun-out from us through our former wholly-owned subsidiary, Baudax Bio, Inc., or Baudax Bio, when we completed a special dividend distribution of all the outstanding shares of common stock of Baudax Bio to our shareholders. On November 21, 2019, the distribution date, each of our shareholders received one share of Baudax Bio’s common stock, or the Distribution, for every two and one-half shares of our common stock held of record at the close of business on November 15, 2019, the record date for the Distribution. Additionally, we contributed $19 million of cash to Baudax Bio in connection with the separation. As a result of the Distribution, Baudax Bio is now an independent public company whose shares of common stock are trading under the symbol “BXRX” on The Nasdaq Capital Market, or Nasdaq.
Our consolidated results of operations and financial position included in this Annual Report on Form 10-K reflect the financial results of Baudax Bio as a discontinued operation for all periods presented. For additional information on the spin-off of Baudax Bio please read Note 4, Discontinued Operations, to our consolidated financial statements included in this Annual Report on Form 10-K.
Financial Overview
Revenues
During the twelve months ended December 31, 2019, 2018 and 2017, we recognized revenues from three revenue streams: manufacturing revenue, royalty revenue and research and development revenue.
Manufacturing revenue
We recognize manufacturing revenue from the sale of products we manufacture for our commercial partners. Manufacturing revenues are recognized upon transfer of control of a product to a customer, generally upon shipment, based on a transaction price that reflects the consideration we expect to be entitled to as specified in the agreement with the commercial partner, which could include pricing and volume-based adjustments.
Royalty revenue
We recognize royalty or profit sharing revenue, collectively referred to as royalty revenue, related to the sale of products by our commercial partners that incorporate our technologies. Royalty revenues are generally recognized under the terms of the applicable license, development and/or supply agreement. For arrangements that include sales-based royalties and the license is deemed to be the predominant item to which the royalties relate, we recognize revenue when the related sales occur by the commercial partner. For arrangements that include sales-based royalties and the license is not deemed to be the predominant item to which the royalties relate, we recognize revenue when the performance obligation to which the royalty has been allocated has been satisfied, which is upon transfer of control of a product to a customer. In this case, significant judgment is used in the estimation of these royalties based on
31
historical customer pricing and deductions and is partially constrained due to items that are outside of our control including the uncertainty of the timing of future commercial partner sales, mix of volume, customer stocking and ordering patterns, as well as unforeseen price adjustments made by our commercial partners.
Research and Development revenue
Research and development revenue consists of revenue that compensates us for services performed, such as formulation, process development, and preparation of pre-clinical and clinical drug product materials under research and development arrangements with partners. Revenues related to research and development are generally recognized as the related services or activities are performed using the output method and in accordance with the contract terms. To the extent that the agreements specify services are to be performed on a fixed basis, revenues are recognized consistent with the pattern of the work performed. In agreements that specify milestones, we evaluate whether the milestones are considered probable of being achieved and estimate the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur, the value of the associated milestone is recognized at a point in time. Non-refundable milestone payments related to arrangements under which we have continuing performance obligations would be deferred and recognized over the period of performance. Milestone payments that are not within our control, such as submission for approval to regulators by a partner or approvals from regulators, are not considered probable of being achieved until those submissions are submitted by the customer or approvals are received.
Research and Development Expenses
Research and development expenses consist of costs incurred for our product and formulation development activities, including regulatory support. We expense research and development costs as incurred. Advanced payments for good and services that will be used in future research and development activities are initially recorded as prepaid expenses and expensed as the activity is performed or when the goods have been received. In 2018 and 2017, these costs included salaries and related costs for personnel in research and development and regulatory functions. In the fourth quarter of 2018, we shifted the focus of these personnel to revenue-generating activities and, as such, these costs are included as a cost of sales beginning in the fourth quarter of 2018.
Selling, General and Administrative Expenses
Selling, General and Administrative expenses consists of salaries and related costs for corporate administrative, public company costs, business development personnel as well as legal, patent-related expenses and consulting fees. Public company costs include compliance, auditing services, tax services, insurance and investor relations. As a significant portion of these corporate public company costs related to a more complex organization with multiple segments, these costs going forward are expected to be in the range of mid to upper single digits, excluding non-cash expenses and new initiatives as they relate to our operations as a stand-alone public company.
We expect our business development expenses to increase in 2020 as we continue to expand our sales team in various geographies, in anticipation of business growth from new formulation and development capabilities.
Amortization of Intangible Assets
We recognize amortization expense related to the intangible asset for our contract manufacturing relationships on a straight-line basis over an estimated useful life of six years.
Change in Fair Value of Warrants
We have classified as liabilities certain warrants outstanding that contain a contingent net cash settlement feature, upon a change in control. The fair value of these warrants are remeasured through settlement or expiration with changes in fair value recognized as a period charge within the Consolidated Statements of Operations and Comprehensive Loss. All remaining liability classified warrants were exercised in November 2019. A fair value determination at the time of the exercise occurred and is included in the change in warrant valuation for the year ended December 31, 2019.
Interest Expense, net
Interest expense, net for the twelve months ended December 31, 2019 and 2018 was a result of interest expense incurred on our Athyrium senior secured term loan and the amortization of the related financing costs. Interest expense for the twelve months ended December 31, 2017 was a result of interest expense incurred on our OrbiMed and Athyrium senior secured term loans and the amortization of the related financing costs. In addition, due to the November 2017 refinancing of our debt, in 2017 we incurred one-
32
time charges for fees related to early extinguishment of the OrbiMed debt and the non-cash write-off of OrbiMed deferred financing costs.
Net Operating Losses and Tax Carryforwards
As of December 31, 2019, we had approximately $121.6 million of federal net operating loss carryforwards. We also had federal and state research and development tax credit carryforwards of $4.4 million available to offset future taxable income. U.S. tax laws limit the time during which these carryforwards may be utilized against future taxes. With the exception of the 2019 and 2018 federal net operating losses, which have an indefinite carry forward period, these federal and state net operating loss and federal and state tax credit carryforwards will begin to expire at various dates beginning in 2028, if not utilized. We believe that it is more likely than not that the deferred income tax assets associated with our U.S. operations will not be realized, and as such, there is a full valuation allowance against our U.S. deferred tax assets.
Results of Operations
Comparison of the Twelve Months Ended December 31, 2019 and 2018:
|
|
|
Year ended December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
|
|
(amounts in thousands) |
|
|||||
|
Revenue |
|
$ |
99,219 |
|
|
$ |
77,347 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of intangible assets) |
|
|
50,981 |
|
|
|
43,160 |
|
|
Research and development |
|
|
— |
|
|
|
4,402 |
|
|
Selling, general and administrative |
|
|
19,909 |
|
|
|
14,437 |
|
|
Amortization of intangible assets |
|
|
2,583 |
|
|
|
2,583 |
|
|
Change in warrant valuation |
|
|
2,116 |
|
|
|
284 |
|
|
Total operating expenses |
|
|
75,589 |
|
|
|
64,866 |
|
|
Operating income from continuing operations |
|
|
23,630 |
|
|
|
12,481 |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(19,005 |
) |
|
|
(8,113 |
) |
|
Income from continuing operations before income taxes |
|
|
4,625 |
|
|
|
4,368 |
|
|
Income tax expense |
|
|
— |
|
|
|
(17,436 |
) |
|
Net income (loss) from continuing operations |
|
|
4,625 |
|
|
|
(13,068 |
) |
|
Loss on discontinued operations (see Note 4) |
|
|
(23,255 |
) |
|
|
(66,655 |
) |
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
Revenue and Cost of sales. Our revenues were $99.2 million and $77.3 million and cost of sales were $51.0 million and $43.2 million for the twelve months ended December 31, 2019 and 2018, respectively. The increase of $21.9 million in revenue was primarily due to increased profit sharing royalties recognized from one of our commercial partners and an increase in product sales to various commercial partners. The increase in cost of sales of $7.8 million was primarily due to product mix and expanded service and development capabilities as well as growth in manufacturing demand.
Research and Development. There were no research and development expenses for the twelve months ended December 31, 2019. Our research and development expenses were $4.4 million for the twelve months ended December 31, 2018. In the fourth quarter of 2018, we shifted the focus of our development activities to support revenue generating activities and therefore such costs are now included in cost of sales above.
Selling, General and Administrative. Our selling, general and administrative expenses were $19.9 million and $14.4 million for the twelve months ended December 31, 2019 and 2018, respectively. The increase of $5.5 million was primarily due to higher public company costs (including corporate initiatives), which increased by $4.1 million to $16.3 million for the year ended December 31, 2019 compared to $12.2 million for the year ended December 31, 2018. The remaining $1.4 million increase was driven by higher business development costs, as we expanded our sales team in various geographies in anticipation of business growth from new formulation and development capabilities.
Amortization of Intangible Assets. Amortization expense was $2.6 million for each of the twelve months ended December 31, 2019 and 2018, respectively, which was exclusively related to the amortization of our royalties and contract manufacturing relationships intangible asset over its estimated useful life.
33
Interest Expense, net. Interest expense, net was $19.0 million and $8.1 million during the twelve months ended December 31, 2019 and 2018, respectively. The increase in interest expense, net, was due to a higher principal balance on our Athyrium senior secured term loan and amortization of the related financing costs.
Income Tax Expense. As a result of recording a full valuation allowance, there was no income tax benefit for the twelve months ended December 31, 2019. For the twelve months ended December 31, 2018, the income tax expense was $17.4 million, which reflects the recording of a full valuation allowance in the fourth quarter of 2018. As discussed in Note 17 to the Consolidated Financial Statements included in this Form 10-K, we believe that it is more likely than not that the deferred income tax assets associated with our U.S. operations will not be realized, and as such, there is a full valuation allowance against our U.S. deferred tax assets.
Comparison of the Years Ended December 31, 2018 and 2017:
|
|
|
Year ended December 31, |
|
|||||
|
|
2018 |
|
|
2017 |
|
|||
|
|
|
(amounts in thousands) |
|
|||||
|
Revenue |
|
$ |
77,347 |
|
|
$ |
71,834 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of intangible assets) |
|
|
43,160 |
|
|
|
38,193 |
|
|
Research and development |
|
|
4,402 |
|
|
|
4,460 |
|
|
Selling, general and administrative |
|
|
14,437 |
|
|
|
14,324 |
|
|
Amortization of intangible assets |
|
|
2,583 |
|
|
|
2,583 |
|
|
Change in warrant valuation |
|
|
284 |
|
|
|
9 |
|
|
Total operating expenses |
|
|
64,866 |
|
|
|
59,569 |
|
|
Operating income from continuing operations |
|
|
12,481 |
|
|
|
12,265 |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(8,113 |
) |
|
|
(11,665 |
) |
|
Income from continuing operations before income taxes |
|
|
4,368 |
|
|
|
600 |
|
|
Income tax expense |
|
|
(17,436 |
) |
|
|
(7,317 |
) |
|
Net loss from continuing operations |
|
|
(13,068 |
) |
|
|
(6,717 |
) |
|
Loss on discontinued operations (see Note 4) |
|
|
(66,655 |
) |
|
|
(43,365 |
) |
|
Net loss |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
Revenue and Cost of sales. Our revenues were $77.3 million and $71.8 million and cost of sales were $43.2 million a $38.2 million for the twelve months ended December 31, 2018 and 2017, respectively. The $5.5 million increase in 2018 revenue versus 2017 was primarily due to higher profit sharing royalties as a result of stronger sales volumes and pricing of one of our products as well as increased manufacturing revenue. These increases were partially offset by decreased royalty revenue due to a change in the mix of generic and brand sales by another of our commercial partners. The increase in cost of sales of $5.0 million was primarily due to changes in the product mix and expanded service and development capabilities as well as growth in manufacturing demand.
Research and Development. Our research and development expenses were $4.4 million and $4.5 million for the twelve months ended December 31, 2018 and 2017, respectively. The decrease of $0.1 million in 2018 was primarily due to lower research and development costs to support commercial operations.
Selling, General and Administrative. Our selling, general and administrative expenses were $14.4 million and $14.3 million for the twelve months ended December 31, 2018 and 2017, respectively. The increase of $0.1 million was primarily due to a $1.0 million increase in administrative expenses and costs to build the business development team to support our new business growth and diversification efforts, which was partially offset by lower public company costs (including legal fees) of $0.9 million.
Amortization of Intangible Assets. Amortization expense was $2.6 million for the twelve months ended December 31, 2018 and 2017, which was exclusively related to the amortization of our royalties and contract manufacturing relationships intangible asset over its estimated useful life.
Interest Expense, net. Interest expense, net was $8.1 million and $11.7 million during the twelve months ended December 31, 2018 and 2017, respectively. The decrease in interest expense, net, was due to the refinancing of our prior credit agreement with OrbiMed in 2017, which resulted in a one-time charge totaling approximately $6.8 million for fees related to early extinguishment of debt and the non-cash write-off of related deferred financing costs. This was partially offset by the higher principal balance on our
34
Athyrium senior secured term loan and amortization of the related financing costs in 2018 contributing to an increase in interest expense, net.
Income Tax Expense. Income tax expense from continuing operations was $17.4 million and $7.3 million for the twelve months ended December 31, 2018 and 2017, respectively. Income tax expense from continuing operations for the twelve months ended December 31, 2018 was attributable to the valuation allowance recorded during such period. Income tax expense from continuing operations for the twelve months ended December 31, 2017 primarily relates to the impact of the change in the U.S. tax rate due to the Tax Cuts and Jobs Act of 2017, which resulted in a non-cash adjustment of $7.9 million for the remeasurement of the net deferred tax items using the recently enacted 21% statutory tax rate.
Liquidity and Capital Resources
As of December 31, 2019, we had $19.1 million in cash and cash equivalents.
Since inception through December 31, 2019, we have financed our product development, operations and capital expenditures primarily from sales of equity and debt securities, including sales of our common stock with net proceeds of $133.5 million, and term loans made under our previous and existing credit facilities, including our credit facility with Athyrium with an outstanding balance of $125 million and contributions of excess cash flow. During the twelve months ended December 31, 2019, our capital expenditures were $8.3 million, which increased primarily related to expansion of the capabilities to support anticipated new business activities.
We may require additional financing and may raise such additional funds through debt refinancing, bank or other loans, through strategic research and development, licensing, including out-licensing activities, sale of assets and/or marketing arrangements or through public or private sales of equity or debt securities from time to time. Financing may not be available on acceptable terms, or at all, and our failure to raise capital when needed could materially adversely impact our growth plans and our financial condition or results of operations. Additional debt or equity financing, if available, may be dilutive to the holders of our common stock and may involve significant cash payment obligations and covenants that restrict our ability to operate our business or access to capital.
On November 17, 2017, we entered into our credit agreement with Athyrium, pursuant to which we drew upon an initial $60.0 million term loan. We used the proceeds from the initial term loan to (i) repay in full all outstanding indebtedness under our credit facility with OrbiMed of approximately $31.7 million, which included the remaining debt principal balance of $27.3 million and early termination charges of $4.4 million and (ii) pay transaction fees associated with the credit facility with Athyrium of approximately $4.2 million. In December 2018 we amended the credit agreement with Athyrium and drew upon a $10.0 million term B-1 loan. In February 2019, we entered into a second amendment to the credit agreement with Athyrium pursuant to which the credit facility was (i) expanded from $100.0 million to $125.0 million and (ii) the two additional $15.0 million tranches were restructured into a $55.0 million term B-2 loan, which was funded on the date of execution of the Second Amendment, net of the original issue discount of $11.4 million. Beginning on March 31, 2021, we must repay the outstanding principal amount in quarterly installments of $3.0 million with the outstanding principal balance due on March 31, 2023. As of December 31, 2019, we had $125.0 million outstanding principal under our credit agreement with Athyrium.
Sources and Uses of Cash
Cash provided by operations from continuing operations was $16.2 million, $11.0 million and $19.1 million for the twelve months ended December 31, 2019, 2018 and 2017, respectively, which represents our operating income from continuing operations plus stock-based compensation, depreciation, non-cash interest expense, loss on early extinguishment of debt, changes in fair value of warrants and amortization of intangibles, as well as changes in operating assets and liabilities.
Cash used in investing activities from continuing operations was $8.3 million, $3.7 million and $9.0 million for the twelve months ended December 31, 2019, 2018 and 2017, respectively, and reflected cash used for the purchase of short-term investments offset by maturities/redemption of investments and for the purchase of property and equipment.
There was $26.0 million of cash provided by financing activities from continuing operations in the twelve months ended December 31, 2019 from net proceeds from issuance of long-term debt from Athyrium of $43.6 million, and net proceeds of $6.0 million from the exercise of options, which was partially offset by the contribution of $19.0 million to Baudax Bio in connection with the Separation, deferred financing costs of $2.9 million from the Athyrium transaction and $1.7 million of payments of withholdings on shares withheld for income taxes. Cash provided by financing activities was $27.7 million for the twelve months ended December 31, 2018 from continuing operations, from proceeds from issuance of long-term debt from Athyrium of $10.0 million, net proceeds of $17.0 million from the sale of shares of common stock through our Common Stock Purchase Agreement with Aspire Capital and proceeds of $1.8 million from the exercise of options, which was partially offset by deferred financing costs of $1.0 million. Cash provided by financing activities from continuing operations for the twelve months ended December 31, 2017 was $23.9
35
million from proceeds from issuance of long-term debt from Athyrium of $60 million, offset by repayment of long term debt for the payoff of the OrbiMed debt of $27.3 million, fees related to early extinguishment of debt paid to OrbiMed of $4.4 million and deferred financing costs from the Athyrium transaction of $4.2 million.
Our future use of operating cash and capital requirements will depend on many forward-looking factors, including the following:
|
|
|
|
|
• |
the extent to which we in-license, acquire or invest in products, businesses and technologies; |
|
|
• |
the timing and extent of our manufacturing and capital expenditures; |
|
|
• |
our ability to maintain our relationships and contracts with our commercial partners; |
|
|
• |
our ability to grow and diversify our business with new customers, including our ability to meet desired project outcomes with development customers; |
|
|
• |
our ability to continue profitability; |
|
|
• |
our ability to comply with stringent U.S. & foreign government regulation in the manufacture of pharmaceutical products, including cGMP and U.S. DEA requirements; |
|
|
• |
the extent to which we choose to establish collaboration, co-promotion, distribution or other similar agreements for product candidates; |
|
|
• |
our ability to raise additional funds through equity or debt financings or sale of certain assets; |
|
|
• |
the costs of preparing, submitting and prosecuting patent applications and maintaining, enforcing and defending intellectual property claims; and |
|
|
• |
the effect of any changes in our effective tax rate due to changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities and changes in tax laws. |
We might use existing cash and cash equivalents on hand, additional debt, equity financing, sale of assets or out-licensing revenue or a combination thereof to fund our operations or product acquisitions. If we increase our debt levels, we might be restricted in our ability to raise additional capital and might be subject to financial and restrictive covenants. Our shareholders may experience dilution as a result of the issuance of additional equity or debt securities. This dilution may be significant depending upon the amount of equity or debt securities that we issue and the prices at which we issue any securities.
Contractual Commitments
The table below reflects our contractual commitments as of December 31, 2019:
|
|
|
Payments Due by Period (in 000s) |
|
|||||||||||||
|
Contractual Obligations |
|
Total |
|
Less than 1 year |
|
1-3 years |
|
3-5 years |
|
More than 5 years |
|
|||||
|
Long-Term Debt Obligations (1): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Athyrium Debt |
|
$ |
126,250 |
|
$ |
— |
|
$ |
126,250 |
|
$ |
— |
|
$ |
— |
|
|
Interest on Debt |
|
$ |
40,463 |
|
|
14,853 |
|
|
25,610 |
|
|
— |
|
|
— |
|
|
Purchase Obligations (2): |
|
$ |
5,593 |
|
|
4,196 |
|
|
1,397 |
|
|
— |
|
|
— |
|
|
Operating Leases (3) |
|
$ |
927 |
|
|
203 |
|
|
321 |
|
|
312 |
|
|
91 |
|
|
Other Long-Term Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreements (4) |
|
$ |
1,018 |
|
|
1,018 |
|
|
— |
|
|
— |
|
|
— |
|
|
Total Contractual Obligations |
|
$ |
174,251 |
|
$ |
20,270 |
|
$ |
153,578 |
|
$ |
312 |
|
$ |
91 |
|
36
|
|
recorded on our Consolidated Balance Sheet. See Note 11 to the Consolidated Financial Statements included in this Annual Report on Form 10-K. |
|
|
(2) |
These obligations consist of cancelable and non-cancelable purchase commitments related to inventory, capital expenditures, transition services agreement costs, and other goods or services. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. See Note 12 to the Consolidated Financial Statements included in this Annual Report on Form 10-K. |
|
|
(3) |
We have become party to certain operating leases for the leased space in Gainesville, Georgia, as well as for office equipment, for which the minimum lease payments are presented. See Note 12(b) to the Consolidated Financial Statements included in this Annual Report on Form 10-K. |
|
|
(4) |
We have entered into employment agreements with certain of our named executive officers. As of December 31, 2019, these employment agreements provided for, among other things, annual base salaries in an aggregate amount of not less than this amount, from that date through calendar year 2020. In accordance with U.S. GAAP, these obligations are not recorded on our Consolidated Balance Sheets. See Note 12(d) to the Consolidated Financial Statements included in this Annual Report on Form 10-K. |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
Critical Accounting Policies and Estimates
This management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses, revenue recognition and stock-based compensation. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Impairment of Goodwill– We are required to review, on an annual basis, the carrying value of goodwill to determine whether impairment may exist. For goodwill, the impairment model prescribes a one-step method for determining impairment. The one-step quantitative test calculates the amount of goodwill impairment as the excess of a reporting unit’s carrying amount over its fair value, not to exceed the total amount of goodwill allocated to the reporting unit.
Impairment of Long-lived Assets—We are required to review the carrying value of long-lived fixed and amortizing intangible assets for recoverability whenever events occur or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. The impairment test is a two-step test. Under step one we assess the recoverability of an asset (or asset group). The carrying amount of an asset (or asset group) is not recoverable if it exceeds the sum of the undiscounted cash flows expected from the use and eventual disposition of the asset (or asset group). The impairment loss is measured in step two as the difference between the carrying value of the asset (or asset group) and its fair value. Assumptions and estimates used in the evaluation of impairment are subjective and changes in these assumptions may negatively impact projected undiscounted cash flows, which could result in impairment charges in future periods. On an ongoing periodic basis, we evaluate the useful life of our long-lived assets and determine if any economic, governmental or regulatory event has modified their estimated useful lives.
Revenue Recognition— We generate revenues from manufacturing, packaging, research and development, and related services for multiple pharmaceutical companies. Our agreements with our commercial partners provide for manufacturing revenues, sales-based royalties and/or profit sharing components. Our revenue policies listed below are reflective of ASU 2014-09, which we adopted effective January 1, 2018. See Note 16 to the Consolidated Financial Statements included in this Form 10-K for additional information regarding our adoption of ASU 2014-09 and its impact on our financial statements.
37
Manufacturing and other related services revenue is recognized upon transfer of control of a product to a customer, generally upon shipment, based on a transaction price that reflects the consideration we expect to be entitled to as specified in the agreement with the commercial partner.
In addition to manufacturing and packaging revenue, certain customer agreements may have intellectual property sales-based royalties and/or profit sharing consideration, collectively referred to as royalties, computed on the net product sales of the commercial partner. Royalty revenues are generally recognized under the terms of the applicable license, development and/or supply agreement. For arrangements that include sales-based royalties where the license for intellectual property is deemed to be the predominant item to which the royalties relate, we recognize revenue when the related sales occur by the commercial partner. For arrangements that include sales-based royalties where the license for intellectual property is not deemed to be the predominant item to which the royalties relate, we recognize revenue upon transfer of control of the manufactured product. In these cases, significant judgment is required to calculate this estimated variable consideration using the most-likely amount method based on historical customer pricing and deductions and is partially constrained due to items that are outside of our control including the uncertainty of the timing of future commercial partner sales, mix of volume, customer stocking and ordering patterns, as well as unforeseen price adjustments made by our commercial partners.
Revenues related to research and development are generally recognized over-time as the related services or activities are performed using the output method and in accordance with the contract terms. In agreements which specify milestones, we evaluate whether the milestones are considered probable of being achieved and estimate the amount to be included in the transaction price using the most likely amount method. Milestone payments related to arrangements under which we have continuing performance obligations would be deferred and recognized over the period of performance. Milestone payments that are not within our control, such as submission for approval to regulators by a commercial partner or approvals from regulators, are not considered probable of being achieved until those submissions are submitted by the customer or approvals are received.
Income taxes - We use the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between the financial statement carrying amount and the tax basis of assets and liabilities and are measured using enacted tax rates and laws that will be in effect when the differences are expected to reverse. We provide a valuation allowance when it is more-likely-than-not that deferred tax assets will not be realized.
On a periodic basis, we evaluate the realizability of our deferred tax assets and adjust such amounts in light of changing facts and circumstances, including but not limited to projections of future taxable income, the reversal of deferred tax liabilities, tax legislation, rulings by relevant tax authorities, tax planning strategies and the progress of ongoing tax examinations. As part of this evaluation, we consider whether it is more likely than not that all or some portion of the deferred tax asset will not be realized. The ultimate realization of a deferred tax asset is dependent upon the generation of future taxable income during the period in which the related temporary difference becomes deductible or the net operating loss, or NOL, and credit carryforwards can be utilized.
We maintain a full valuation allowance against our deferred tax assets where realizability is not certain. We periodically evaluate the likelihood of the realization of deferred tax assets and adjust the carrying amount of these deferred tax assets by a valuation allowance based on the anticipated realizability. The valuation allowance can be reversed if objective negative evidence in the form of cumulative losses is no longer present and additional weight is given to subjective evidence, such as our projection of future growth. This determination depends on a variety of factors, some of which are subjective, including our current year taxable income in the United States, expectations of future taxable income, impact of tax reform, achievement of milestones, carryforward periods available to us for tax reporting purposes, various income tax strategies and other relevant factors. If we determine that the deferred tax assets realizability is impacted, we would record material changes to income tax expense in that period.
We are exposed to market risks in the ordinary course of our business. These market risks are principally limited to interest rate fluctuations. At December 31, 2019, we had approximately $11.6 million invested in money market instruments. We believe our policy of investing in highly-rated securities, whose liquidities are, at December 31, 2019, all less than two months, minimizes such risks. Due to the short-term duration of our investment portfolio and the low-risk profile of our investments, an immediate 10.0% change in interest rates would not have a material effect on the fair market value of our portfolio. Accordingly, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates on our investment portfolio. We do not enter into investments for trading or speculative purposes. Our Athyrium secured term loan interest expense is based on the current committed rate of three-month LIBOR plus 9.75% with a 1.0% LIBOR floor. A fluctuation in LIBOR of 0.25% would result in a charge of $0.3 million of interest expense over a twelve-month period.
38
Our consolidated financial statements and the report of our independent registered public accounting firm are included in this Annual Report on Form 10-K on the pages indicated in Part IV, Item 15.
None.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act) as of December 31, 2019. We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow for timely decisions regarding required disclosure.
A control system, no matter how well conceived and operated, can provide only reasonable, and not absolute, assurance that the objectives of the control system will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the company have been detected. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. However, our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives. Based on the evaluation of our disclosure controls and procedures as of December 31, 2019, our principal executive officer and principal financial officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance of the reliability of financial reporting and of the preparation of financial statements for external reporting purposes, in accordance with U.S. generally accepted accounting principles.
Internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and disposition of assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with the authorization of its management and directors; and (3) provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on its financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of the effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies and procedures included in such controls may deteriorate.
Our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making this assessment, management used the criteria established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control – Integrated Framework (2013). These criteria are in the areas of control environment, risk assessment, control activities, information and communication, and monitoring. Management’s assessment included extensive documentation, evaluating and testing the design and operating effectiveness of its internal controls over financial reporting.
Based on management’s processes and assessment, as described above, management has concluded that, as of December 31, 2019, our internal control over financial reporting was effective.
KPMG LLP, our independent registered public accounting firm, issued an attestation report on our internal control over financial reporting, which is included starting on page F-3 of the financial statements included in this Annual Report on Form 10-K.
39
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. Subsequent to the Separation on November 21, 2019, we rely on certain financial information and resources of Baudax Bio to manage specific aspects of our business and report results in accordance with a transition services agreement. These include investor relations, corporate communications, accounting, tax, legal, human resources, benefit plan administration, benefit plan reporting, general management, real estate, treasury, insurance and risk management. We continue to review our internal controls over financial reporting, and may from time to time make changes aimed at enhancing their effectiveness. These efforts may lead to changes in our internal controls over financial reporting.
None.
40
Information with respect to this item will be set forth in the Proxy Statement for the 2018 Annual Meeting of Shareholders, or the Proxy Statement, under the headings “Board of Directors,” “Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” and “Corporate Governance and Risk Management” and is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
Information with respect to this item will be set forth in the Proxy Statement under the headings “Director Compensation,” “Executive Compensation,” and “Corporate Governance and Risk Management” is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Information with respect to this item will be set forth in the Proxy Statement under the headings “Security Ownership of Directors, Certain Beneficial Owners and Management,” “Executive Compensation,” and “Director Compensation,” and is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
Information with respect to this item will be set forth in the Proxy Statement under the headings “Certain Relationships and Related Party Transactions” and “Corporate Governance and Risk Management” and is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
Information with respect to this item will be set forth in the Proxy Statement under the heading “Independent Registered Public Accounting Firm,” and is incorporated herein by reference. The Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report.
41
(a)(1) Consolidated Financial Statements.
The following consolidated financial statements are filed as a part of this Annual Report on Form 10-K:
Consolidated Financial Statements
Reports of Independent Registered Public Accounting Firm
Consolidated Balance Sheets as of December 31, 2019 and 2018
Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2019, 2018 and 2017
Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2019, 2018 and 2017
Consolidated Statements of Cash Flows for the years ended December 31, 2019, 2018 and 2017
(a)(2) Consolidated Financial Statement Schedules.
Not applicable.
(a)(3); (b) Exhibits:
|
No. |
|
Description |
|
Method of Filing |
|||
|
|
|
|
|
|
|||
|
2.1 |
|
|
Incorporated herein by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed on November 26, 2019 (File No. 001-36329). |
||||
|
|
|
|
|
||||
|
3.1 |
|
Second Amended and Restated Articles of Incorporation of Recro Pharma, Inc. |
|
Incorporated herein by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on March 13, 2014 (File No. 001-36329). |
|||
|
|
|
|
|||||
|
3.2 |
|
|
Incorporated herein by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed on March 13, 2014 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
4.1 |
|
|
Incorporated herein by reference to Exhibit 4.1 to the Company’s Registration Statement on Form S-1/A filed on December 20, 2013 (File No. 333-191879). |
||||
|
|
|
|
|||||
|
4.2 |
|
|
Incorporated herein by reference to Exhibit A of Exhibit 1.1 to the Company’s Registration Statement on Form S-1/A filed on February 11, 2014 (File No. 333-191879). |
||||
|
|
|
|
|||||
|
4.3† |
|
Incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on November 20, 2017 (File No. 001-36329). |
|||||
|
|
|
|
|||||
|
4.4† |
|
|
Incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed on November 20, 2017 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
|
4.5 |
|
|
Incorporated herein by reference to Exhibit 4.8 to the Company’s Annual Report on Form 10-K filed on March 2, 2018 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
42
|
No. |
|
Description |
|
Method of Filing |
|||
|
|
|
Incorporated herein by reference to Exhibit 4.8 to the Company’s Annual Report on Form 10-K filed on February 19, 2019 (File No. 001-36329). |
|||||
|
4.7 |
|
|
Filed herewith. |
||||
|
|
|
|
|||||
|
10.1† |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 26, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.2† |
|
|
Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 26, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.3† |
|
|
Incorporated herein by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on November 26, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.4• |
|
|
Incorporated herein by reference to Exhibit 10.4 to the Company's Current Report on Form 8-K filed on November 26, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.5• |
|
|
Filed herewith. |
||||
|
|
|
|
|||||
|
10.6• |
|
|
Incorporated herein by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q filed on May 12, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.7• |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on December 23, 2015 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
|
10.8• |
|
|
Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on December 28, 2018 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.9• |
Recro Pharma, Inc. 2018 Amended and Restated Equity Incentive Plan. |
Incorporated herein by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed on May 9, 2018 (File No. 001-36329). |
|||||
|
|
|
|
|||||
|
10.10• |
|
|
Incorporated herein by reference to Exhibit 10.10 to the Company’s Registration Statement on Form S-1/A filed on November 29, 2013 (File No. 333-191879). |
||||
|
|
|
|
|||||
|
10.11• |
|
|
Incorporated herein by reference to Exhibit 10.11 to the Company’s Registration Statement on Form S-1/A filed on November 29, 2013 (File No. 333-191879). |
||||
|
|
|
|
|||||
|
10.12• |
|
|
Incorporated herein by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-K filed on March 25, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.13• |
|
|
Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K on December 22, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.14• |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Registration Statement on Form S-8 filed on December 23, 2015 (File No. 333-208750). |
||||
|
|
|
|
|||||
|
10.15• |
|
Form of Award Agreement for Restricted Stock Unit Inducement Awards. |
|
Incorporated herein by reference to Exhibit 10.20 to the Company’s Annual Report on Form 10-K filed on March 2, 2018 (File No. 001-36329). |
|||
43
|
No. |
|
Description |
|
Method of Filing |
|||
|
|
|
|
|||||
|
10.16† |
|
|
Incorporated herein by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q filed on August 14, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.17 |
|
|
Incorporated herein by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q filed on August 14, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.18 |
|
|
Incorporated herein by reference to Exhibit 10.8 to the Company’s Quarterly Report on Form 10-Q filed on August 14, 2015 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.19† |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on April 18, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.20† |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on November 20, 2017 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
|
10.21 |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on January 4, 2019 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.22 |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on March 4, 2019 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
|
10.23 |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed on November 8, 2019 (File No. 001-36329). |
||||
|
|
|
|
|
||||
|
10.24 |
|
|
Incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on November 20, 2017 (File No. 001-36329). |
||||
|
|
|
|
|
|
|||
|
10.25 |
|
|
Incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on December 29, 2017 (File No. 001-36329). |
||||
|
|
|
|
|||||
|
10.26 |
|
|
Incorporated herein by reference to Exhibit 10.38 to the Company’s Annual Report on Form 10-K filed on March 2, 2018 (File No. 001-36329). |
||||
|
10.27 |
|
|
Incorporated herein by reference to Exhibit 10.34 to the Company’s Annual Report on Form 10-K filed on February 19, 2019 (File No. 001-36329). |
||||
|
10.28† |
|
|
Incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed on March 6, 2019 (File No. 001-3632).
|
||||
|
10.29 |
|
|
Filed herewith.
|
||||
|
|
|
|
|
|
|||
44
|
No. |
|
Description |
|
Method of Filing |
|||
|
10.30 |
|
Amendment No. 1 to License and Supply Agreement, dated as of September 6, 2018, by and between Recro Gainesville LLC and Kremers Urban Pharmaceuticals, Inc. |
|
Filed herewith |
|||
|
|
|
|
|||||
|
|
|
|
|||||
|
21.1 |
|
|
Filed herewith. |
||||
|
|
|
|
|||||
|
23.1 |
|
Consent of KPMG LLP, Independent Registered Public Accounting Firm. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
31.1 |
|
Rule 13a-14(a)/15d-14(a) certification of Principal Executive Officer. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
31.2 |
|
Rule 13a-14(a)/15d-14(a) certification of Principal Financial Officer. |
|
Filed herewith. |
|||
|
|
|
|
|
|
|||
|
32.1 |
|
Section 1350 certification, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
101 INS |
|
XBRL Instance Document. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
101 SCH |
|
XBRL Taxonomy Extension Schema. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
101 CAL |
|
XBRL Taxonomy Extension Calculation Linkbase. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
101 DEF |
|
XBRL Taxonomy Extension Definition Linkbase. |
|
Filed herewith. |
|||
|
|
|
|
|||||
|
101 LAB |
|
XBRL Taxonomy Extension Label Linkbase. |
|
Filed herewith. |
|||
|
101 PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
Filed herewith. |
|||
|
• |
Management contract or compensatory plan or arrangement. |
|
† |
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment pursuant to Rule 406 under the Securities Act of 1933. |
(c) Not applicable
Item 16. Form 10-K Summary
None.
45
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, Annual Report on Form 10-K has been signed by the following persons in the capacities held on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Gerri A. Henwood |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 4, 2020 |
|
Gerri A. Henwood |
|
|
||
|
|
|
|
|
|
|
/s/ Ryan D. Lake |
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
|
March 4, 2020
|
|
Ryan D. Lake |
|
|
||
|
|
|
|
|
|
|
/s/ Arnaud Ajdler |
|
Director |
|
March 4, 2020 |
|
Arnaud Ajdler |
|
|
|
|
|
|
|
|
|
|
|
/s/ Alfred Altomari |
|
Director |
|
March 4, 2020 |
|
Alfred Altomari |
|
|
||
|
|
|
|
|
|
|
/s/ William L. Ashton |
|
Director |
|
March 4, 2020 |
|
William L. Ashton |
|
|
||
|
|
|
|
|
|
|
/s/ Michael Berelowitz |
|
Director |
|
March 4, 2020 |
|
Michael Berelowitz |
|
|
||
|
|
|
|
|
|
|
/s/ Winston J. Churchill |
|
Director |
|
March 4, 2020 |
|
Winston J. Churchill |
|
|
||
|
|
|
|
|
|
|
/s/ Bryan M. Reasons |
|
Director |
|
March 4, 2020 |
|
Bryan M. Reasons |
|
|
||
|
|
|
|
|
|
|
/s/ Wayne B. Weisman |
|
Director |
|
March 4, 2020 |
|
Wayne B. Weisman |
|
|
46
RECRO PHARMA, INC. AND SUBSIDIARIES
Index to Consolidated Financial Statements
|
|
|
Page |
|
|
F-2 |
|
|
|
F-4 |
|
|
Consolidated Statements of Operations and Comprehensive Loss |
|
F-5 |
|
|
F-6 |
|
|
|
F-7 |
|
|
|
F-9 |
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Recro Pharma, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Recro Pharma, Inc. and subsidiaries (the Company) as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for each of the years in the three year period ended December 31, 2019, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the three year period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated March 4, 2020 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Changes in Accounting Principle
As discussed in Note 2 to the consolidated financial statements, the Company has changed its method of accounting for leases as of January 1, 2019 due to the adoption of Accounting Standards Update (ASU) No. 2016-02, Leases (Topic 842) and ASU No. 2018-11, Leases (Topic 842), Targeted Improvements.
As discussed in Note 2 to the consolidated financial statements, the Company has changed its method of accounting for revenue as of January 1, 2018 due to the adoption of Accounting Standards Update (ASU) No. 2014-09, Revenue from Contracts with Customers (Topic 606).
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ KPMG LLP
We have served as the Company’s auditor since 2009.
Philadelphia, Pennsylvania
March 4, 2020
F-2
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Recro Pharma, Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Recro Pharma, Inc. and subsidiaries’ (the Company) internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2019 and 2018, the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2019, and the related notes (collectively, the consolidated financial statements), and our report dated March 4, 2020 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 4, 2020
F-3
RECRO PHARMA, INC. AND SUBSIDIARIES
|
(amounts in thousands, except share and per share data) |
|
December 31, 2019 |
|
|
December 31, 2018 |
|
||
|
Assets |
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
19,148 |
|
|
$ |
38,514 |
|
|
Accounts receivable |
|
|
14,389 |
|
|
|
12,866 |
|
|
Contract asset |
|
|
8,851 |
|
|
|
5,201 |
|
|
Inventory |
|
|
15,072 |
|
|
|
10,699 |
|
|
Prepaid expenses and other current assets |
|
|
2,700 |
|
|
|
1,795 |
|
|
Current assets of discontinued operation |
|
|
— |
|
|
|
2,066 |
|
|
Total current assets |
|
|
60,160 |
|
|
|
71,141 |
|
|
Property, plant and equipment, net |
|
|
42,212 |
|
|
|
41,700 |
|
|
Right of use asset |
|
|
485 |
|
|
|
— |
|
|
Intangible assets, net |
|
|
3,283 |
|
|
|
5,866 |
|
|
Goodwill |
|
|
4,319 |
|
|
|
4,319 |
|
|
Non-current assets of discontinued operation |
|
|
— |
|
|
|
32,467 |
|
|
Total assets |
|
$ |
110,459 |
|
|
$ |
155,493 |
|
|
Liabilities and Shareholders’ Equity |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
989 |
|
|
$ |
2,160 |
|
|
Accrued expenses and other current liabilities |
|
|
4,176 |
|
|
|
5,597 |
|
|
Current portion of operating lease liability |
|
|
148 |
|
|
|
— |
|
|
Current liabilities of discontinued operation |
|
|
1,172 |
|
|
|
21,273 |
|
|
Total current liabilities |
|
|
6,485 |
|
|
|
29,030 |
|
|
Long-term debt, net |
|
|
110,319 |
|
|
|
64,243 |
|
|
Warrants and other long-term liabilities |
|
|
— |
|
|
|
1,131 |
|
|
Long-term portion of operating lease liability |
|
|
367 |
|
|
|
— |
|
|
Non-current liabilities of discontinued operation |
|
|
— |
|
|
|
80,589 |
|
|
Total liabilities |
|
|
117,171 |
|
|
|
174,993 |
|
|
Commitments and contingencies (Note 12) |
|
|
|
|
|
|
|
|
|
Shareholders’ equity: |
|
|
|
|
|
|
|
|
|
Preferred stock, $0.01 par value. Authorized, 10,000,000 shares; none issued and outstanding |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.01 par value. Authorized, 50,000,000 shares; issued and outstanding, 23,312,928 shares at December 31, 2019 and 21,799,961 shares at December 31, 2018 |
|
|
233 |
|
|
|
218 |
|
|
Additional paid-in capital |
|
|
199,938 |
|
|
|
168,535 |
|
|
Accumulated deficit |
|
|
(206,883 |
) |
|
|
(188,253 |
) |
|
Total shareholders’ equity (deficit) |
|
|
(6,712 |
) |
|
|
(19,500 |
) |
|
Total liabilities and shareholders’ equity |
|
$ |
110,459 |
|
|
$ |
155,493 |
|
See accompanying notes to consolidated financial statements.
F-4
RECRO PHARMA, INC. AND SUBSIDIARIES
Consolidated Statements of Operations and Comprehensive Loss
|
|
|
For the Year ended December 31, |
|
|||||||||
|
(amounts in thousands, except share and per share data) |
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Revenue |
|
$ |
99,219 |
|
|
$ |
77,347 |
|
|
$ |
71,834 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of sales (excluding amortization of intangible assets) |
|
|
50,981 |
|
|
|
43,160 |
|
|
|
38,193 |
|
|
Research and development |
|
|
— |
|
|
|
4,402 |
|
|
|
4,460 |
|
|
Selling, general and administrative |
|
|
19,909 |
|
|
|
14,437 |
|
|
|
14,324 |
|
|
Amortization of intangible assets |
|
|
2,583 |
|
|
|
2,583 |
|
|
|
2,583 |
|
|
Change in warrant valuation |
|
|
2,116 |
|
|
|
284 |
|
|
|
9 |
|
|
Total operating expenses |
|
|
75,589 |
|
|
|
64,866 |
|
|
|
59,569 |
|
|
Operating income from continuing operations |
|
|
23,630 |
|
|
|
12,481 |
|
|
|
12,265 |
|
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
|
801 |
|
|
|
643 |
|
|
|
369 |
|
|
Interest expense |
|
|
(19,806 |
) |
|
|
(8,756 |
) |
|
|
(12,034 |
) |
|
Income from continuing operations before income taxes |
|
|
4,625 |
|
|
|
4,368 |
|
|
|
600 |
|
|
Income tax expense |
|
|
— |
|
|
|
(17,436 |
) |
|
|
(7,317 |
) |
|
Net income (loss) from continuing operations |
|
|
4,625 |
|
|
|
(13,068 |
) |
|
|
(6,717 |
) |
|
Loss on discontinued operation, net of income taxes |
|
|
(23,255 |
) |
|
|
(66,655 |
) |
|
|
(43,365 |
) |
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Per share information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share from continuing operations, basic |
|
$ |
0.21 |
|
|
$ |
(0.64 |
) |
|
$ |
(0.35 |
) |
|
Net loss per share from discontinued operations, basic |
|
$ |
(1.04 |
) |
|
$ |
(3.26 |
) |
|
$ |
(2.28 |
) |
|
Net loss per share, basic |
|
$ |
(0.83 |
) |
|
$ |
(3.90 |
) |
|
$ |
(2.63 |
) |
|
Weighted average common shares outstanding, basic |
|
|
22,414,194 |
|
|
|
20,465,106 |
|
|
|
19,070,983 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share from continuing operations, diluted |
|
$ |
0.20 |
|
|
$ |
(0.64 |
) |
|
$ |
(0.35 |
) |
|
Net loss per share from discontinued operations, diluted |
|
$ |
(0.99 |
) |
|
$ |
(3.26 |
) |
|
$ |
(2.28 |
) |
|
Net loss per share, diluted |
|
$ |
(0.79 |
) |
|
$ |
(3.90 |
) |
|
$ |
(2.63 |
) |
|
Weighted average common shares outstanding, diluted |
|
|
23,608,862 |
|
|
|
20,465,106 |
|
|
|
19,070,983 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
|
Other comprehensive loss: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain (loss) on available-for-sale securities |
|
|
— |
|
|
|
1 |
|
|
|
(1 |
) |
|
Comprehensive loss |
|
$ |
(18,630 |
) |
|
$ |
(79,722 |
) |
|
$ |
(50,083 |
) |
See accompanying notes to consolidated financial statements.
F-5
RECRO PHARMA, INC. AND SUBSIDIARIES
Consolidated Statements of Shareholders’ Equity
For the Years Ended December 31, 2019, 2018 and 2017
|
|
|
Common Stock |
|
|
Additional |
|
|
|
|
|
|
Accumulated other |
|
|
|
|
|
|||||||
|
(amounts in thousands, except share data) |
|
Shares |
|
|
Amount |
|
|
paid-in capital |
|
|
Accumulated Deficit |
|
|
comprehensive loss |
|
|
Total |
|
||||||
|
Balance, December 31, 2016 |
|
|
19,043,216 |
|
|
$ |
190 |
|
|
$ |
132,691 |
|
|
$ |
(61,266 |
) |
|
$ |
— |
|
|
$ |
71,615 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
5,546 |
|
|
|
— |
|
|
|
— |
|
|
|
5,546 |
|
|
Stock option exercise |
|
|
7,756 |
|
|
|
— |
|
|
|
53 |
|
|
|
— |
|
|
|
— |
|
|
|
53 |
|
|
Issuance of restricted stock units, net of shares withheld for income taxes |
|
|
76,463 |
|
|
|
1 |
|
|
|
(250 |
) |
|
|
— |
|
|
|
— |
|
|
|
(249 |
) |
|
Warrants issued in financing facility, net of related tax effect |
|
|
— |
|
|
|
— |
|
|
|
1,966 |
|
|
|
— |
|
|
|
— |
|
|
|
1,966 |
|
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1 |
) |
|
|
(1 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(50,082 |
) |
|
|
— |
|
|
|
(50,082 |
) |
|
Balance, December 31, 2017 |
|
|
19,127,435 |
|
|
|
191 |
|
|
|
140,006 |
|
|
|
(111,348 |
) |
|
|
(1 |
) |
|
|
28,848 |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
7,129 |
|
|
|
— |
|
|
|
— |
|
|
|
7,129 |
|
|
Stock option exercise |
|
|
352,025 |
|
|
|
4 |
|
|
|
1,811 |
|
|
|
— |
|
|
|
— |
|
|
|
1,815 |
|
|
Issuance of restricted stock units, net of shares withheld for income taxes |
|
|
122,746 |
|
|
|
1 |
|
|
|
(92 |
) |
|
|
— |
|
|
|
— |
|
|
|
(91 |
) |
|
Sale of common stock under equity facility, net of transaction costs |
|
|
1,983,040 |
|
|
|
20 |
|
|
|
17,005 |
|
|
|
— |
|
|
|
— |
|
|
|
17,025 |
|
|
Cashless exercise of warrants |
|
|
214,715 |
|
|
|
2 |
|
|
|
2,587 |
|
|
|
— |
|
|
|
— |
|
|
|
2,589 |
|
|
Revaluation of equity classified warrants |
|
|
— |
|
|
|
— |
|
|
|
89 |
|
|
|
— |
|
|
|
— |
|
|
|
89 |
|
|
Change in other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1 |
|
|
|
1 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(79,723 |
) |
|
|
— |
|
|
|
(79,723 |
) |
|
Cumulative effect of adoption of new accounting standards, net of tax |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
2,818 |
|
|
|
— |
|
|
|
2,818 |
|
|
Balance, December 31, 2018 |
|
|
21,799,961 |
|
|
|
218 |
|
|
|
168,535 |
|
|
|
(188,253 |
) |
|
|
— |
|
|
|
(19,500 |
) |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
9,094 |
|
|
|
— |
|
|
|
— |
|
|
|
9,094 |
|
|
Stock option exercise |
|
|
863,952 |
|
|
|
9 |
|
|
|
5,994 |
|
|
|
— |
|
|
|
— |
|
|
|
6,003 |
|
|
Issuance of restricted stock units, net of shares withheld for income taxes |
|
|
429,926 |
|
|
|
4 |
|
|
|
(1,681 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,677 |
) |
|
Issuance of common stock for equity facility |
|
|
34,762 |
|
|
|
— |
|
|
|
301 |
|
|
|
— |
|
|
|
— |
|
|
|
301 |
|
|
Separation of Baudax Bio, Inc. |
|
|
— |
|
|
|
— |
|
|
|
14,480 |
|
|
|
— |
|
|
|
— |
|
|
|
14,480 |
|
|
Cashless exercise of warrants |
|
|
184,327 |
|
|
|
2 |
|
|
|
3,215 |
|
|
|
— |
|
|
|
— |
|
|
|
3,217 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(18,630 |
) |
|
|
— |
|
|
|
(18,630 |
) |
|
Balance, December 31, 2019 |
|
|
23,312,928 |
|
|
$ |
233 |
|
|
$ |
199,938 |
|
|
$ |
(206,883 |
) |
|
$ |
— |
|
|
$ |
(6,712 |
) |
See accompanying notes to consolidated financial statements.
F-6
RECRO PHARMA, INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
|
|
|
For the Year ended December 31, |
|
|||||||||
|
(amounts in thousands) |
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Cash flows from operating activities, continuing operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
|
Loss on discontinued operations, net of income taxes |
|
|
23,255 |
|
|
|
66,655 |
|
|
|
43,365 |
|
|
Adjustments to reconcile net income (loss) from continuing operations to net cash provided by operating activities from continuing operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock-based compensation |
|
|
6,191 |
|
|
|
4,279 |
|
|
|
4,178 |
|
|
Non-cash interest expense |
|
|
5,412 |
|
|
|
1,287 |
|
|
|
912 |
|
|
Depreciation expense |
|
|
5,817 |
|
|
|
4,872 |
|
|
|
4,793 |
|
|
Loss on early extinguishment of debt |
|
|
— |
|
|
|
— |
|
|
|
6,772 |
|
|
Amortization of intangible assets |
|
|
2,583 |
|
|
|
2,583 |
|
|
|
2,583 |
|
|
Change in warrant valuation |
|
|
2,116 |
|
|
|
284 |
|
|
|
9 |
|
|
Deferred income taxes |
|
|
— |
|
|
|
17,637 |
|
|
|
7,507 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventory |
|
|
(4,373 |
) |
|
|
(860 |
) |
|
|
(1,093 |
) |
|
Contract asset |
|
|
(3,650 |
) |
|
|
(1,446 |
) |
|
|
— |
|
|
Prepaid expenses and other current assets |
|
|
(604 |
) |
|
|
(508 |
) |
|
|
(243 |
) |
|
Right of use asset |
|
|
207 |
|
|
|
— |
|
|
|
— |
|
|
Accounts receivable |
|
|
(1,523 |
) |
|
|
(3,180 |
) |
|
|
725 |
|
|
Accounts payable, accrued expenses and other liabilities |
|
|
(364 |
) |
|
|
(858 |
) |
|
|
(352 |
) |
|
Operating lease liability |
|
|
(213 |
) |
|
|
— |
|
|
|
— |
|
|
Net cash provided by operating activities, continuing operations |
|
|
16,224 |
|
|
|
11,022 |
|
|
|
19,074 |
|
|
Cash flows from investing activities, continuing operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(8,342 |
) |
|
|
(7,198 |
) |
|
|
(5,403 |
) |
|
Purchases of short-term investments |
|
|
(12,100 |
) |
|
|
(6,225 |
) |
|
|
(57,124 |
) |
|
Proceeds from maturity of investments |
|
|
12,100 |
|
|
|
9,750 |
|
|
|
53,500 |
|
|
Net cash used in investing activities, continuing operations |
|
|
(8,342 |
) |
|
|
(3,673 |
) |
|
|
(9,027 |
) |
|
Cash flows from financing activities, continuing operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash contribution to Baudax Bio, Inc. |
|
|
(19,000 |
) |
|
|
— |
|
|
|
— |
|
|
Proceeds from issuance of long-term debt, net of original issue discount of $11,400 |
|
|
43,600 |
|
|
|
10,000 |
|
|
|
60,000 |
|
|
Payments on long-term debt |
|
|
— |
|
|
|
— |
|
|
|
(27,347 |
) |
|
Fees related to early extinguishment of debt |
|
|
— |
|
|
|
— |
|
|
|
(4,420 |
) |
|
Payment of deferred financing costs |
|
|
(2,936 |
) |
|
|
(961 |
) |
|
|
(4,178 |
) |
|
Proceeds from sale of common stock, net of transaction costs |
|
|
— |
|
|
|
16,965 |
|
|
|
— |
|
|
Payments of withholdings on shares withheld for income taxes |
|
|
(1,676 |
) |
|
|
(91 |
) |
|
|
(250 |
) |
|
Proceeds from option exercises |
|
|
6,003 |
|
|
|
1,815 |
|
|
|
53 |
|
|
Net cash provided by financing activities, continuing operations |
|
|
25,991 |
|
|
|
27,728 |
|
|
|
23,858 |
|
|
Net increase in cash and cash equivalents from continuing operations |
|
|
33,873 |
|
|
|
35,077 |
|
|
|
33,905 |
|
|
Discontinued operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows used in operating activities |
|
|
(41,721 |
) |
|
|
(54,137 |
) |
|
|
(36,117 |
) |
|
Cash flows used in investing activities |
|
|
(1,518 |
) |
|
|
(3,410 |
) |
|
|
(1,287 |
) |
|
Cash flows used in financing activities |
|
|
(10,000 |
) |
|
|
— |
|
|
|
— |
|
|
Net decrease in cash and cash equivalents from discontinued operations |
|
|
(53,239 |
) |
|
|
(57,547 |
) |
|
|
(37,404 |
) |
|
Cash and cash equivalents, beginning of year |
|
|
38,514 |
|
|
|
60,984 |
|
|
|
64,483 |
|
|
Cash and cash equivalents, end of year |
|
$ |
19,148 |
|
|
$ |
38,514 |
|
|
$ |
60,984 |
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
F-7
|
|
$ |
14,395 |
|
|
$ |
8,134 |
|
|
$ |
5,341 |
|
|
|
Cash paid for taxes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
467 |
|
|
Purchase of property, plant and equipment included in accrued expenses and accounts payable |
|
$ |
288 |
|
|
$ |
2,301 |
|
|
$ |
235 |
|
|
Common stock issued in connection with equity facility |
|
$ |
301 |
|
|
$ |
357 |
|
|
$ |
— |
|
|
Amortization of deferred equity costs |
|
$ |
— |
|
|
$ |
332 |
|
|
$ |
— |
|
|
Fair value recognized for warrants |
|
$ |
— |
|
|
$ |
89 |
|
|
$ |
2,143 |
|
See accompanying notes to consolidated financial statements.
F-8
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
|
(1) |
Background |
Recro Pharma, Inc., or the Company, was incorporated in Pennsylvania on November 15, 2007. The Company is a leading contract development and manufacturing organization, or CDMO, with integrated solutions for the development, formulation, regulatory support, manufacturing, and packaging of oral solid dose drug products. It leverages its formulation and development expertise to develop and manufacture pharmaceutical products using proprietary delivery technologies and know-how for commercial partners who commercialize or plan to commercialize these products.
In November 2019, the Company’s former Acute Care business was spun-out through its former wholly-owned subsidiary, Baudax Bio, Inc., or Baudax Bio, when the Company completed a special dividend distribution of all the outstanding shares of common stock of Baudax Bio to its shareholders. See Note 4 to the consolidated financial statements for additional information on the spin-off of Baudax Bio.
The Company has incurred losses from operations since inception and has an accumulated deficit of $206,883 as of December 31, 2019, which is mostly related to activities that are presented as discontinued operations upon completion of the spin-off as Baudax Bio. The Company’s future operations are highly dependent on the continued profitability of its manufacturing operations. Management believes that it is probable that the Company will be able to meet its obligations as they become due within one year after the date the financial statements are issued.
|
(2) |
Summary of Significant Accounting Principles |
|
|
(a) |
Basis of Presentation and Principles of Consolidation |
The accompanying consolidated financial statements of the Company and its subsidiaries have been prepared in accordance with U.S. generally accepted accounting principles, or U.S. GAAP. The Company’s consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated.
|
|
(b) |
Use of Estimates |
The preparation of financial statements and the notes to the financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from such estimates.
|
|
(c) |
Cash and Cash Equivalents |
Cash and cash equivalents represents cash in banks and highly liquid short-term investments that have maturities of three months or less when acquired. These highly liquid, short-term investments are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of the changes in interest rates.
|
|
(d) |
Property and Equipment |
Property and equipment are recorded at cost less accumulated depreciation and amortization. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets, which are as follows: three to ten years for furniture and office equipment; six to ten years for manufacturing equipment; two to five years for vehicles; 35 to 40 years for buildings; and the shorter of the lease term or useful life for leasehold improvements. Repairs and maintenance cost are expensed as incurred.
|
|
(e) |
Business Combinations |
In accordance with Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or ASC, Topic 805, “Business Combinations,” or ASC 805, the Company allocates the purchase price of acquired companies to the tangible and intangible assets acquired and liabilities assumed based on their estimated fair values. Valuations are performed to assist in determining the fair values of assets acquired and liabilities assumed, which requires management
F-9
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
to make significant estimates and assumptions, in particular with respect to intangible assets. Management makes estimates of fair value based upon assumptions believed to be reasonable. These estimates are based in part on historical experience and information obtained from management of the acquired companies and expectations of future cash flows. Transaction costs and restructuring costs associated with the transaction are expensed as incurred.
|
|
(f) |
Goodwill and Intangible Assets |
Goodwill represents the excess of purchase price over the fair value of net assets acquired by the Company. Goodwill is not amortized, but assessed for impairment on an annual basis or more frequently if impairment indicators exist. The impairment model prescribes a one-step method for determining impairment.
The one-step quantitative test calculates the amount of goodwill impairment as the excess of a reporting unit’s carrying amount over its fair value, not to exceed the total amount of goodwill allocated to the reporting unit.
Intangible assets include the Company’s royalties and contract manufacturing relationships assets. The royalties and contract manufacturing relationships intangible asset is considered a definite-lived intangible asset and is amortized on a straight-line basis over a useful life of six years. The Company is required to review the carrying value of amortizing intangible assets for recoverability whenever events occur or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. There were no triggering events as of December 31, 2019.
The Company performs its annual goodwill impairment test as of November 30th, or whenever an event or change in circumstances occurs that would require reassessment of the recoverability of goodwill. In performing the evaluation, the Company assesses qualitative factors such as overall financial performance, anticipated changes in industry and market conditions, including recent tax reform, and competitive environments. The Company performed its impairment test as of November 30, 2019 and noted there have been no triggering events or indicators of impairment as of December 31, 2019. As a result of the impairment test, the Company determined that there was no impairment to goodwill for the year ended December 31, 2019.
|
|
(g) |
Revenue Recognition |
The Company generates revenues from manufacturing, packaging, research and development, and related services for multiple pharmaceutical companies. The agreements that the Company has with its commercial partners provide for manufacturing revenues, sales-based royalties and/or profit sharing components. The Company’s revenue policies listed below are reflective of Accounting Standards Update, or ASU, No. 2014-09, “Revenue from Contracts with Customers,” or ASU 2014-09, which the Company adopted effective January 1, 2018. See Note 16 for additional information regarding the Company’s adoption of ASU 2014-09 and its impact on the Company’s financial statements.
Manufacturing and other related services revenue is recognized upon transfer of control of a product to a customer, generally upon shipment, based on a transaction price that reflects the consideration the Company expects to be entitled to as specified in the agreement with the commercial partner, which could include pricing and volume-based adjustments.
In addition to manufacturing and packaging revenue, certain customer agreements may have intellectual property sales-based royalties and/or profit sharing consideration, collectively referred to as royalties, computed on the net product sales of the commercial partner. Royalty revenues are generally recognized under the terms of the applicable license, development and/or supply agreement. For arrangements that include sales-based royalties where the license for intellectual property is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue when the related sales occur by the commercial partner. For arrangements that include sales-based royalties where the license for intellectual property is not deemed to be the predominant item to which the royalties relate, the Company recognizes revenue upon transfer of control of the manufactured product. In these cases, significant judgment is required to calculate this estimated variable consideration using the most-likely amount method based on historical customer pricing and deductions and is partially constrained due to items that are outside of the Company’s control including the uncertainty of the timing of future commercial partner sales, mix of volume, customer stocking and ordering patterns, as well as unforeseen price adjustments made by the Company’s commercial partners.
Revenues related to research and development are generally recognized over-time as the related services or activities are performed using the output method and in accordance with the contract terms. In agreements which specify milestones, the Company evaluates whether the milestones are considered probable of being achieved and estimates the amount to be
F-10
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
included in the transaction price using the most likely amount method. Milestone payments related to arrangements under which the Company has continuing performance obligations would be deferred and recognized over the period of performance. Milestone payments that are not within the control of the Company, such as submission for approval to regulators by a commercial partner or approvals from regulators, are not considered probable of being achieved until those submissions are submitted by the customer or approvals are received.
|
|
(h) |
Concentration of Credit Risk |
Financial instruments that potentially subject the Company to significant concentration of credit risk consist primarily of cash, cash equivalents and accounts receivable. The Company manages its cash and cash equivalents based on established guidelines relative to diversification and maturities to maintain safety and liquidity.
The Company’s accounts receivable balances are concentrated amongst approximately four customers and if any of these customers’ receivable balances should be deemed uncollectible, it could have a material adverse effect on the Company’s results of operations and financial condition.
The Company is dependent on its relationships with a small number of commercial partners, with its four largest customers having generated 96% of its revenues for the year ending December 31, 2019. A portion of the Company’s revenues are dependent on U.S. based customers selling to end-users outside the U.S.
|
|
(i) |
Research and Development |
Research and development expenses consist of costs incurred for product and formulation development activities, including regulatory support. The Company expenses research and development costs as incurred. Advanced payments for goods and services that will be used in future research and development activities are initially recorded as prepaid expenses and expensed as the activity is performed or when the goods have been received. In 2018 and 2017, these costs included salaries and related costs for personnel in research and development and regulatory functions. In the fourth quarter of 2018, the Company shifted the focus of these personnel to revenue-generating activities and, as such, these costs are included as a cost of sales beginning in the fourth quarter of 2018.
|
|
(j) |
Stock-Based Awards |
The Company measures employee stock-based awards at grant-date fair value and recognizes employee compensation expense on a straight-line basis over the vesting period of the award. The Company accounts for forfeitures as they occur.
Determining the appropriate fair value of stock options requires the input of subjective assumptions, including the expected life of the option and expected stock price volatility. The Company uses the Black-Scholes option pricing model to value its stock option awards. The assumptions used in calculating the fair value of stock-based awards represent management’s best estimates and involve inherent uncertainties and the application of management’s judgment. As a result, if factors change and/or management uses different assumptions, stock-based compensation expense could be materially different for future awards.
The expected life of stock options was estimated using the “simplified method,” which is based on the average of the vesting tranches and the contractual life of each grant. For stock price volatility, the Company uses the historical volatility of its publicly traded stock in order to estimate future stock price trends. The risk-free interest rate is based on U.S. Treasury notes with a term approximating the expected life of the option.
For non-employee stock-based awards, the Company recognizes compensation expense on a straight-line basis over the vesting period of each separated vesting tranche of the award, which is known as the accelerated attribution method. The estimation of the number of stock awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from the Company’s current estimates, such amounts are recognized as an adjustment in the period in which estimates are revised.
|
|
(k) |
Income Taxes |
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis, operating losses and tax credit carryforwards. Deferred tax assets and
F-11
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. A valuation allowance is recorded to the extent it is more likely than not that some portion or all of the deferred tax assets will not be realized.
Unrecognized income tax benefits represent income tax positions taken on income tax returns that have not been recognized in the consolidated financial statements. The Company recognizes the benefit of an income tax position only if it is more likely than not (greater than 50%) that the tax position will be sustained upon tax examination, based solely on the technical merits of the tax position. Otherwise, no benefit is recognized. The tax benefits recognized are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement. The Company does not anticipate significant changes in the amount of unrecognized income tax benefits over the next year.
|
|
(l) |
Net Income (Loss) Per Common Share |
Basic net income (loss) per common share is determined by dividing net income (loss) applicable to common shareholders by the weighted average common shares outstanding during the period.
For purposes of calculating diluted net income (loss) per common share, the denominator includes both the weighted average common shares outstanding and the dilutive effect of outstanding common stock options, warrants and unvested restricted stock units, using the treasury stock method, if the inclusion of such instruments would be dilutive.
The following table sets forth the computation of basic and diluted loss per share:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Basic Loss Per Share |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) from continuing operations |
|
$ |
4,625 |
|
|
$ |
(13,068 |
) |
|
$ |
(6,717 |
) |
|
Net income loss from discontinued operations |
|
|
(23,255 |
) |
|
|
(66,655 |
) |
|
|
(43,365 |
) |
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share from continuing operations |
|
$ |
0.21 |
|
|
$ |
(0.64 |
) |
|
$ |
(0.35 |
) |
|
Net loss per share from discontinued operations |
|
$ |
(1.04 |
) |
|
$ |
(3.26 |
) |
|
$ |
(2.28 |
) |
|
Net loss per share of common stock, basic |
|
$ |
(0.83 |
) |
|
$ |
(3.90 |
) |
|
$ |
(2.63 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding, basic |
|
|
22,414,194 |
|
|
|
20,465,106 |
|
|
|
19,070,983 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted Loss Per Share |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) from continuing operations |
|
$ |
4,625 |
|
|
$ |
(13,068 |
) |
|
$ |
(6,717 |
) |
|
Net loss from discontinued operations |
|
|
(23,255 |
) |
|
|
(66,655 |
) |
|
|
(43,365 |
) |
|
Net loss |
|
$ |
(18,630 |
) |
|
$ |
(79,723 |
) |
|
$ |
(50,082 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) per share from continuing operations |
|
$ |
0.20 |
|
|
$ |
(0.64 |
) |
|
$ |
(0.35 |
) |
|
Net loss per share from discontinued operations |
|
$ |
(0.99 |
) |
|
$ |
(3.26 |
) |
|
$ |
(2.28 |
) |
|
Net loss per share of common stock, diluted |
|
$ |
(0.79 |
) |
|
$ |
(3.90 |
) |
|
$ |
(2.63 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding, diluted |
|
|
23,608,862 |
|
|
|
20,465,106 |
|
|
|
19,070,983 |
|
F-12
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The following potentially dilutive securities have been excluded from the computations of diluted weighted average shares outstanding as of December 31, 2019, 2018 and 2017 as they would be anti-dilutive:
|
|
|
December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Options and restricted stock units outstanding |
|
|
298,565 |
|
|
|
4,878,461 |
|
|
|
3,865,468 |
|
|
Warrants |
|
|
— |
|
|
|
838,664 |
|
|
|
1,133,592 |
|
Amounts in the table above reflect the common stock equivalents of the noted instruments.
|
|
(m) |
Segment Information |
The Company determined that it operates in a single segment.
|
|
(n) |
Recent Accounting Pronouncements |
Recently Adopted Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842),” or ASU 2016-02. ASU 2016-02 establishes a wholesale change to lease accounting and introduces a lease model that brings most leases on the balance sheet. It also eliminates the required use of bright-line tests in current U.S. GAAP for determining lease classification. In July 2018, the FASB issued ASU No. 2018-11, Leases (Topic 842), Targeted Improvements, which provides an alternative transition method permitting the recognition of a cumulative-effect adjustment on the date of adoption rather than restating comparative periods in transition as originally prescribed by Topic 842. The new guidance is effective for annual and interim periods beginning after December 15, 2018, with early adoption permitted. The Company adopted this guidance as of January 1, 2019. The Company elected the optional transition method to account for the impact of the adoption with a cumulative-effect adjustment in the period of adoption and did not restate prior periods. The Company opted to elect the package of practical expedients to not reassess prior conclusions related to contracts containing leases, lease classification and initial direct costs, and certain other practical expedients, including the use of hindsight to determine the lease term for existing leases and in assessing impairment of the right-of-use asset, and the exception for short-term leases. For its current classes of underlying assets, the Company did not elect the practical expedient under which the lease components would not be separated from the nonlease components. At January 1, 2019, the Company recorded a right-of-use asset of $692 and an operating lease liability of $728. For additional information regarding how the Company is accounting for leases under the new guidance, refer to Note 12(b).
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606).” ASU 2014-09 represents a comprehensive new revenue recognition model that requires a company to recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which a company expects to be entitled to receive in exchange for those goods or services. This ASU sets forth a new five-step revenue recognition model that replaces the prior revenue recognition guidance in its entirety and is intended to eliminate numerous industry-specific pieces of revenue recognition guidance that have historically existed. In January 2018, the Company adopted the standard using the modified retrospective method. See Note 16 for additional information on the impact of the transition on the Company’s financial statements.
Accounting Pronouncements Not Yet Adopted
In August 2018, the FASB issued ASU 2018-13, “Fair Value Measurement (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement,” or ASU 2018-13. ASU 2018-13 removes, modifies and adds certain disclosure requirements in Topic 820 “Fair Value Measurement”. ASU 2018-13 eliminates certain disclosures related to transfers and the valuations process, clarifies the measurement uncertainty disclosure, and requires additional disclosures for Level 3 fair value measurements, including the range and weighted average of significant unobservable inputs used to develop Level 3 fair value measurements. ASU 2018-13 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2019, with early adoption permitted. The Company is currently evaluating the potential impact on its disclosures.
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments,” or ASU 2016-13. ASU 2016-13 requires companies to measure credit losses utilizing a methodology that reflects expected credit losses and requires consideration of a range of reasonable information
F-13
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
to estimate credit losses on certain types of financial instruments, including trade receivables and available-for-sale debt securities. ASU 2016-13 is effective for fiscal years beginning after December 15, 2022, including those interim periods within those fiscal years. The Company is currently assessing the impact of adopting this standard, but based on a preliminary assessment, does not expect the adoption of this guidance to have a material impact on its consolidated financial statements.
|
(3) |
Acquisition of Gainesville Facility |
On April 10, 2015, the Company completed the Gainesville Transaction. The consideration paid in connection with the Gainesville Transaction consisted of $50,000 cash at closing, a $4,000 working capital adjustment and a seven-year warrant to purchase 350,000 shares of the Company’s common stock at an exercise price of $19.46 per share, according to the original agreement. Under the acquisition method of accounting, the consideration paid was allocated to the fair value of the assets acquired and liabilities assumed.
In December 2018, the Company entered in to an Amendment to the Purchase and Sale Agreement that amended the warrant agreement with Alkermes, which decreased the exercise price of the warrant to $8.26 per share. The warrant was settled in November 2019.
|
(4) |
Discontinued Operations |
On November 21, 2019 (the “Distribution Date”), the Company completed the separation (the “Separation”) of its former Acute Care business by distributing to the Company’s shareholders on a pro rata basis all of the issued and outstanding common stock of Baudax Bio, the entity the Company incorporated to hold such businesses. To effect the Separation, the Company distributed to its shareholders 1 share of Baudax Bio common stock for every 2.5 shares of the Company’s common stock outstanding as of November 15, 2019, the record date for the distribution. Fractional shares of Baudax Bio common stock that otherwise would have been distributed were aggregated and sold into the public market and the proceeds distributed to the Company’s shareholders. Additionally, in connection with the Separation, the Company contributed $19,000 of cash to Baudax Bio.
The accounting requirements for reporting the Separation of Baudax Bio as a discontinued operation were met when the Separation was completed. Accordingly, the accompanying consolidated financial statements for all periods presented reflect this business as a discontinued operation.
In connection with the Separation, the Company and Baudax Bio entered into various agreements to effect the Separation and provide a framework for their relationship after the Separation, including a transition services agreement, an employee matters agreement, a tax matters agreement and an intellectual property matters agreement. These agreements provide for the allocation between the Company and Baudax Bio of assets, employees, liabilities and obligations (including investments, property and employee benefits and tax-related assets and liabilities) attributable to periods prior to, at, and after Baudax Bio’s separation from the Company and govern certain relationships between the Company and Baudax Bio after the Separation.
The historical consolidated balance sheet and statements of operations of the Company and the related notes to the consolidated financial statements have been presented as discontinued operations in the consolidated financial statements and prior periods have been recast. Discontinued operations include results of the Company’s Acute Care business except for certain corporate overhead costs and certain costs associated with transition services provided by Baudax Bio to the Company, following the Separation, which are included in continuing operations.
The Separation and Distribution Agreement with Baudax Bio sets forth, among other things, the assets that were transferred, the liabilities assumed, and the contracts that were assigned to each of Baudax Bio and the Company as part of the Separation of the Company into two companies, and provided for when and how these transfers, assumptions and assignments were to occur.
The tax matters agreement governs the respective rights, responsibilities and obligations of Baudax Bio and the Company with respect to taxes (including taxes arising in the ordinary course of business and taxes, if any, incurred as a result of any failure of the Distribution and certain related transactions to qualify as tax-free for U.S. federal income tax purposes), tax attributes, uncertain tax positions, tax returns, tax proceedings and certain other tax matters.
The employee matters agreement governs certain compensation and employee benefit obligations and allocates liabilities and responsibilities relating to employment matters, employee compensation and benefit plans and programs and other related matters, including the transfer or assignment of employees from the Company to Baudax Bio.
F-14
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
As of December 31, 2019, certain current liabilities of discontinued operations remain on the Company’s consolidated balance sheet due to timing of payment, which consists of $22 of accounts payable and $1,150 of accrued expenses.
The following table shows amounts included in assets and liabilities of discontinued operations, respectively, on the Company’s Consolidated Balance Sheets at December 31, 2018.
|
|
|
December 31, 2018 |
|
|
|
Current assets: |
|
|
|
|
|
Prepaid expenses and other current assets |
|
$ |
2,066 |
|
|
Current assets of discontinued operation |
|
|
2,066 |
|
|
Property, plant and equipment, net |
|
|
3,940 |
|
|
Intangible assets, net |
|
|
26,400 |
|
|
Goodwill |
|
|
2,127 |
|
|
Non-current assets of discontinued operation |
|
|
32,467 |
|
|
Total assets of discontinued operation |
|
$ |
34,533 |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
Accounts payable |
|
$ |
2,351 |
|
|
Accrued expenses and other current liabilities |
|
|
8,568 |
|
|
Current portion of contingent consideration |
|
|
10,354 |
|
|
Current liabilities of discontinued operation |
|
|
21,273 |
|
|
Other long-term liabilities |
|
|
31 |
|
|
Long-term portion of contingent consideration |
|
|
80,558 |
|
|
Non-current liabilities of discontinued operation |
|
|
80,589 |
|
|
Total liabilities of discontinued operation |
|
$ |
101,862 |
|
The following table represents the carrying value of assets and liabilities of discontinued operations distributed as part of the Separation on November 21, 2019, excluding corporate overhead previously included in the Acute Care business:
|
|
|
November 21, 2019 |
|
|
|
Current assets: |
|
|
|
|
|
Cash and cash equivalents |
|
$ |
19,000 |
|
|
Prepaid expenses and other current assets |
|
|
605 |
|
|
Current assets |
|
|
19,605 |
|
|
Right of use asset |
|
|
832 |
|
|
Property, plant and equipment, net |
|
|
4,846 |
|
|
Intangible assets, net |
|
|
26,400 |
|
|
Goodwill |
|
|
2,127 |
|
|
Non-current assets |
|
|
34,205 |
|
|
Total assets |
|
$ |
53,810 |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
Accounts payable |
|
$ |
22 |
|
|
Accrued expenses and other current liabilities |
|
|
1,263 |
|
|
Current portion of operating lease liability |
|
|
356 |
|
|
Current liabilities |
|
|
1,641 |
|
|
Non-current portion of operating lease liability |
|
|
520 |
|
|
Long-term portion of contingent consideration |
|
|
66,129 |
|
|
Non-current liabilities |
|
|
66,649 |
|
|
Total liabilities |
|
$ |
68,290 |
|
F-15
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The following is a summary of the Acute Care business expenses for the years ended December 31, 2019, 2018 and 2017.
|
|
|
Year ended December 31, |
|
|||||||||
|
Operating expenses: |
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Research and development |
|
$ |
19,471 |
|
|
$ |
35,583 |
|
|
$ |
28,635 |
|
|
Selling, general and administrative |
|
|
18,441 |
|
|
|
22,441 |
|
|
|
11,104 |
|
|
Change in contingent consideration valuation |
|
|
(14,783 |
) |
|
|
8,499 |
|
|
|
12,839 |
|
|
Total operating expenses |
|
|
23,129 |
|
|
|
66,523 |
|
|
|
52,578 |
|
|
Other income (expense), net |
|
|
(126 |
) |
|
|
(132 |
) |
|
|
16 |
|
|
Loss on discontinued operations before income taxes |
|
|
(23,255 |
) |
|
|
(66,655 |
) |
|
|
(52,562 |
) |
|
Income tax benefit on discontinued operations |
|
|
— |
|
|
|
— |
|
|
|
9,197 |
|
|
Loss on discontinued operations, net of income taxes |
|
$ |
(23,255 |
) |
|
$ |
(66,655 |
) |
|
$ |
(43,365 |
) |
|
(5) |
Fair Value of Financial Instruments |
The Company follows the provisions of FASB ASC Topic 820, “Fair Value Measurements and Disclosures,” for fair value measurement recognition and disclosure purposes for its financial assets and financial liabilities that are remeasured and reported at fair value each reporting period. The Company measures certain financial assets and liabilities at fair value on a recurring basis, including cash equivalents and warrants. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the valuation of financial assets and financial liabilities and their placement within the fair value hierarchy. Categorization is based on a three-tier valuation hierarchy, which prioritizes the inputs used in measuring fair value, as follows:
|
|
• |
Level 1: Observable inputs such as quoted prices (unadjusted) in active markets for identical assets or liabilities |
|
|
• |
Level 2: Inputs that are other than quoted prices in active markets for identical assets and liabilities, inputs that are quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are either directly or indirectly observable; and |
|
|
• |
Level 3: Unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. |
F-16
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The Company has classified assets and liabilities measured at fair value on a recurring basis as follows:
|
|
|
Fair value measurements at reporting date using |
|
|||||||||
|
|
|
Quoted prices in active markets for identical assets (Level 1) |
|
|
Significant other observable inputs (Level 2) |
|
|
Significant unobservable inputs (Level 3) |
|
|||
|
At December 31, 2018: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market mutual funds (See Note 6) |
|
$ |
24,720 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Commercial paper |
|
|
— |
|
|
|
2,247 |
|
|
|
— |
|
|
U.S. Treasury obligations |
|
|
2,748 |
|
|
|
— |
|
|
|
— |
|
|
Total cash equivalents |
|
$ |
27,468 |
|
|
$ |
2,247 |
|
|
$ |
— |
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrants (See Note 13(d)) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,101 |
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,101 |
|
|
At December 31, 2019: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash equivalents |
|
|
|
|
|
|
|
|
|
|
|
|
|
Money market mutual funds (See Note 6) |
|
$ |
11,609 |
|
|
$ |
— |
|
|
$ |
— |
|
|
Total cash equivalents |
|
$ |
11,609 |
|
|
$ |
— |
|
|
$ |
— |
|
The Company developed its own assumptions to determine the value of the warrants that do not have observable inputs or available market data to support the fair value. This method of valuation involves using inputs such as the fair value of the Company’s common stock, stock price volatility, the contractual term of the warrants, risk free interest rates and dividend yield. Due to the nature of these inputs, the valuation of the warrants is considered a Level 3 measurement.
The reconciliation of warrants measured at fair value on a recurring basis using unobservable inputs (Level 3) is as follows:
|
|
|
Warrants |
|
|
|
Balance at December 31, 2017 |
|
$ |
3,406 |
|
|
Exercise of warrants |
|
|
(2,589 |
) |
|
Remeasurement |
|
|
284 |
|
|
Balance at December 31, 2018 |
|
$ |
1,101 |
|
|
Exercise of warrants |
|
|
(3,217 |
) |
|
Remeasurement |
|
|
2,116 |
|
|
Total at December 31, 2019 |
|
$ |
— |
|
|
|
|
|
|
|
The Company follows the disclosure provisions of FASB ASC Topic 825, “Financial Instruments” (ASC 825), for disclosure purposes for financial assets and financial liabilities that are not measured at fair value. As of December 31, 2019, the financial assets and liabilities recorded on the Consolidated Balance Sheets that are not measured at fair value on a recurring basis include accounts receivable, accounts payable and accrued expenses and approximate fair value due to the short-term nature of these instruments. The fair value of long-term debt, where a quoted market price is not available, is evaluated based on, among other factors, interest rates currently available to the Company for debt with similar terms, remaining payments and considerations of the Company’s creditworthiness. The Company determined that the recorded book value of long-term debt approximated fair value at December 31, 2019 due to the comparison of the terms of the debt, including borrowing rates available to the Company through its recently completed debt refinancing process, availability of additional term loan tranches, and maturity.
F-17
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The following is a summary of cash equivalents:
|
|
|
December 31, 2019 |
|
|||||||||||||
|
|
|
Amortized |
|
|
Gross Unrealized |
|
|
Estimated |
|
|||||||
|
Description |
|
Cost |
|
|
Gain |
|
|
Loss |
|
|
Fair Value |
|
||||
|
Money market mutual funds |
|
$ |
11,609 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,609 |
|
|
Total cash equivalents |
|
$ |
11,609 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,609 |
|
|
|
|
December 31, 2018 |
|
|||||||||||||
|
|
|
Amortized |
|
|
Gross Unrealized |
|
|
Estimated |
|
|||||||
|
Description |
|
Cost |
|
|
Gain |
|
|
Loss |
|
|
Fair Value |
|
||||
|
Money market mutual funds |
|
$ |
24,720 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
24,720 |
|
|
Commercial paper |
|
|
2,247 |
|
|
|
— |
|
|
|
— |
|
|
|
2,247 |
|
|
U.S. Treasury obligations |
|
|
2,747 |
|
|
|
1 |
|
|
|
— |
|
|
|
2,748 |
|
|
Total cash equivalents |
|
$ |
29,714 |
|
|
$ |
1 |
|
|
$ |
— |
|
|
$ |
29,715 |
|
As of December 31, 2019 and 2018, the Company’s cash equivalents had maturities ranging from one to two months. The fair value of the Company’s U.S. Treasury obligations is determined by taking into consideration valuations obtained from third-party pricing services. The third-party pricing services utilize industry standard valuation models, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include reported trades of and broker/dealer quotes on the same or similar securities, issuer credit spreads, benchmark securities, and other observable inputs. To derive the fair value of its commercial paper, the Company uses benchmark inputs and industry standard analytical models.
|
(7) |
Inventory |
Inventory is stated at the lower of cost and net realizable value. Included in inventory are raw materials and work-in-process used in the production of commercial products. Cost is determined using the first-in, first-out method.
Inventory was as follows as of December 31, 2019 and 2018:
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
||
|
Raw materials |
|
$ |
3,240 |
|
|
$ |
2,611 |
|
|
Work in process |
|
|
6,430 |
|
|
|
4,935 |
|
|
Finished goods |
|
|
5,892 |
|
|
|
3,440 |
|
|
|
|
|
15,562 |
|
|
|
10,986 |
|
|
Provision for inventory obsolescence |
|
|
(490 |
) |
|
|
(287 |
) |
|
|
|
$ |
15,072 |
|
|
$ |
10,699 |
|
Adjustments to inventory are determined at the raw materials, work-in-process, and finished good levels to reflect obsolescence or impaired balances. Inventory is primarily ordered to meet specific customer orders and largely reflects demand. Factors influencing inventory obsolescence include changes in demand, product life cycle, product pricing, physical deterioration and quality concerns.
F-18
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
Property, plant and equipment consists of the following:
|
|
|
December 31, 2019 |
|
|
December 31, 2018 |
|
||
|
Land |
|
$ |
3,263 |
|
|
$ |
3,263 |
|
|
Building and improvements |
|
|
20,900 |
|
|
|
17,683 |
|
|
Furniture, office and computer equipment |
|
|
5,847 |
|
|
|
5,604 |
|
|
Manufacturing equipment |
|
|
35,699 |
|
|
|
30,097 |
|
|
Construction in progress |
|
|
729 |
|
|
|
3,610 |
|
|
Property, plant and equipment |
|
|
66,438 |
|
|
|
60,257 |
|
|
Less: accumulated depreciation and amortization |
|
|
24,226 |
|
|
|
18,557 |
|
|
Property, plant and equipment, net |
|
$ |
42,212 |
|
|
$ |
41,700 |
|
Depreciation expense for the years ended December 31, 2019, 2018 and 2017 was $5,817, $4,872 and $4,793, respectively.
|
(9) |
Intangible Assets |
The following represents the balance of the intangible assets at December 31, 2019:
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Net Intangible Assets |
|
|||
|
Royalties and contract manufacturing relationships |
|
$ |
15,500 |
|
|
$ |
12,217 |
|
|
$ |
3,283 |
|
|
Total |
|
$ |
15,500 |
|
|
$ |
12,217 |
|
|
$ |
3,283 |
|
The following represents the balance of intangible assets at December 31, 2018:
|
|
|
Cost |
|
|
Accumulated Amortization |
|
|
Net Intangible Assets |
|
|||
|
Royalties and contract manufacturing relationships |
|
$ |
15,500 |
|
|
$ |
9,634 |
|
|
$ |
5,866 |
|
|
Total |
|
$ |
15,500 |
|
|
$ |
9,634 |
|
|
$ |
5,866 |
|
Amortization expense each year for the years ended December 31, 2019, 2018 and 2017 was $2,583. The amortization expense for the next two years will be $3,283 consisting of $2,583 in 2020 and $700 in the final year.
|
(10) |
Accrued Expenses and Other Current Liabilities |
Accrued expenses and other current liabilities consist of the following:
|
|
|
December 31, |
|
|
December 31, |
|
||
|
|
|
2019 |
|
|
2018 |
|
||
|
Payroll and related costs |
|
|
2,593 |
|
|
|
3,219 |
|
|
Professional and consulting fees |
|
|
370 |
|
|
|
555 |
|
|
Accrued restructuring |
|
|
365 |
|
|
|
— |
|
|
Deferred revenue |
|
|
337 |
|
|
|
66 |
|
|
Property plant and equipment |
|
|
88 |
|
|
|
1,459 |
|
|
Other |
|
|
423 |
|
|
|
298 |
|
|
|
|
$ |
4,176 |
|
|
$ |
5,597 |
|
|
(11) |
Long-Term Debt |
On November 17, 2017, the Company entered into a $100,000 Credit Agreement, or the Credit Agreement, with Athyrium Opportunities III Acquisition LP, or Athyrium. The Credit Agreement provided for a term loan in the original principal amount of $60,000 funded at closing. In December 2018, the Company amended the Credit Agreement, (as amended, the “Amended
F-19
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
Credit Agreement”). Pursuant to the Amended Credit Agreement, the $20,000 term B loan and $20,000 term C loan provided for under the Credit Agreement, were restructured into (i) a $10,000 term B-1 loan, funded on December 28, 2018; (ii) a $15,000 term B-2 loan; and (iii) a $15,000 term C loan.
On February 28, 2019, the Company entered into a Second Amendment to Credit Agreement (the “Second Amendment”) with Athyrium. Pursuant to the Second Amendment, (i) the total commitments of the term loan credit facility governed by the Amended Credit Agreement was increased from $100,000 to $125,000, (ii) the $15,000 term B-2 loan and $15,000 term C loan provided for under the Amended Credit Agreement were restructured into a $55,000 term B-2 loan, which was funded on the date of execution of the Second Amendment and (iii) the maturity date was extended to March 31, 2023 (the “Maturity Date”). Beginning on March 31, 2021, the Company must repay the outstanding principal amount in quarterly installments of $3,000 with the outstanding principal balance due on the Maturity Date.
On October 22, 2019, the Company entered into a Third Amendment to Credit Agreement (the “Third Amendment”) with Athyrium. The Third Amendment authorizes the release of two of the Company’s subsidiaries, Baudax Bio and Baudax Bio N.A. LLC (formerly known as Recro N.A. LLC) (“Baudax Bio N.A.”), from their respective obligations as guarantors and the release of any liens granted to or held by Athyrium on collateral provided by or equity interests in Baudax Bio and Baudax Bio N.A., including the security interest in Baudax Bio Limited (formerly Recro Ireland Limited) (the “Release”) under the Credit Agreement, as amended.
The Release is subject to certain conditions, including consummation of the Distribution. The Release is applicable only to Baudax Bio and Baudax Bio N.A. and will not affect or modify any obligations of the Company or the Guarantors (other than Baudax Bio and Baudax Bio N.A.) under the Existing Credit Agreement.
The term loans bear interest at a rate equal to the three-month LIBOR rate, with a 1% floor plus 9.75% per annum. In addition, in accordance with the Credit Agreement the Company will have to pay a 1% exit fee, which is $1,250 at the current outstanding loan balance and is being accreted to the carrying amount of the debt using the effective interest method over the term of the loan. In addition, if there is an early repayment, there is a sliding scale of prepayment penalties beginning with a 10% penalty and including a make-whole interest payment. No prepayment penalties are assessed for payments made after March 31, 2022.
The Amended Credit Agreement contains certain usual and customary affirmative and negative covenants, as well as financial covenants that the Company will need to satisfy on a monthly and quarterly basis. As of December 31, 2019, the Company was in compliance with the covenants.
As of December 31, 2019, the remaining payments due under the Amended Credit Agreement include a principal payment of $125,000 and an exit fee of $1,250 due at the Maturity Date.
In connection with the Credit Agreement, the Company issued warrants to each of Athyrium and its affiliate, Athyrium Opportunities II Acquisition LP, or Athyrium II, to purchase an aggregate of 348,664 shares of the Company’s common stock with an exercise price of $8.6043 per share. In connection with the Amended Credit Agreement, the warrants were amended to decrease the exercise price to $6.84 per share. See Note 13(d) for additional information. The warrants are exercisable through November 17, 2024. The initial fair value of the warrant and revaluation adjustment from the repricing of the warrants of $2,232 was recorded as a debt issuance cost.
In addition, the Company recorded debt issuance costs for the Amended Credit Agreement of $4,439 at original signing, an amendment fee of $500 as well as certain other fees and expenses in December 2018, and recorded debt issuance costs for the Second Amendment consisting of a $2,500 amendment fee, $436 closing fee and $11,400 original issue discount which, along with the fair value of warrants, are being amortized using the effective interest method over the term of the Second Amendment. Debt issuance cost amortization is included in interest expense within the Consolidated Statements of Operations and Comprehensive Loss. As of December 31, 2019, the effective interest rate was 16.11%, which takes into consideration the non-cash accretion of the exit fee, the amortization of the debt issuance cost and the original issue discount.
The components of the carrying value of the debt as of December 31, 2019, are detailed below:
|
Principal balance outstanding |
|
$ |
125,000 |
|
|
Unamortized deferred issuance costs |
|
|
(15,100 |
) |
|
Exit fee accretion |
|
|
419 |
|
|
Total |
|
$ |
110,319 |
|
F-20
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The Company used proceeds from the Credit Agreement to (i) repay in full all outstanding indebtedness under its previous credit facility, dated April 10, 2015, between the Company’s subsidiary, Recro Gainesville LLC and OrbiMed Royalty Opportunities II, LP, or the OrbiMed Credit Agreement of $31,767, which included the remaining debt principal balance of $27,347 and early termination charges of $4,420 and (ii) pay transaction fees associated with the Credit Agreement of $4,178.
Associated with the refinancing of the OrbiMed Credit Agreement and in accordance with ASC 405-20 “Extinguishments of Liabilities”, in the twelve months ended December 31, 2017, the Company recorded a loss on extinguishment of $6,772, which is reflected in the interest expense line within the Consolidated Statement of Operations and Comprehensive Loss.
The Company recorded debt issuance cost amortization related to the credit agreements of $5,129, $1,313 and $771, for the years ended of December 31, 2019, 2018 and 2017, respectively.
|
(12) |
Commitments and Contingencies |
|
|
(a) |
Litigation |
The Company is involved, from time to time, in various claims and legal proceedings arising in the ordinary course of its business. Except as disclosed below, the Company is not currently a party to any such claims or proceedings that, if decided adversely to it, would either individually or in the aggregate have a material adverse effect on its business, financial condition or results of operations.
On May 31, 2018, a securities class action lawsuit, or the Securities Litigation, was filed against the Company and certain of its officers and directors in the U.S. District Court for the Eastern District of Pennsylvania (Case No. 2:18-cv-02279-MMB) that purported to state a claim for alleged violations of Section 10(b) and 20(a) of the Exchange Act and Rule 10(b)(5) promulgated thereunder, based on statements made by the Company concerning the NDA for IV meloxicam. The complaint seeks unspecified damages, interest, attorneys’ fees and other costs. On December 10, 2018, lead plaintiff filed an amended complaint that asserted the same claims and sought the same relief but included new allegations and named additional officers as defendants. On February 8, 2019, the Company filed a motion to dismiss the amended complaint in its entirety, which the lead plaintiff opposed on April 9, 2019. On May 9, 2019, the Company filed its response and briefing was completed on the motion to dismiss. In response to questions from the Judge, the parties submitted supplemental briefs with regard to the motion to dismiss the amended complaint during the fall of 2019. On February 18, 2020, the motion to dismiss was granted without prejudice; however, the plaintiffs have indicated that they intend to file a new complaint. In connection with the separation of Baudax Bio, Baudax Bio accepted assignment by the Company of all of its obligations in connection with the Securities Litigation and agreed to indemnify the Company for all liabilities related to the Securities Litigation. The Company believes that the lawsuit is without merit and intend to vigorously defend against it if the plaintiffs file a new complaint. The lawsuit is in the early stages and, at this time, no assessment can be made as to its likely outcome or whether the outcome will be material to the Company.
|
|
(b) |
Leases |
The Company is a party to various operating leases in Georgia for office, manufacturing, chemistry, and manufacturing and controls development space. The Company is also a party to leases for office equipment and storage.
The Company determines if an arrangement is a lease at inception. The arrangement is a lease if it conveys the right to the Company to control the use of identified property, plant, or equipment for a period of time in exchange for consideration. Lease terms vary based on the nature of operations, however, all leased facilities are classified as operating leases with remaining lease terms between less than one year and 6 years. Most leases contain specific renewal options where notice to renew must be provided in advance of lease expiration or automatic renewals where no advance notice is required. Periods covered by an option to extend the lease were included in the non-cancellable lease term when exercise of the option was determined to be reasonably certain. Costs determined to be variable and not based on an index or rate were not included in the measurement of operating lease liabilities. As most leases do not provide an implicit rate, the Company's incremental borrowing rate was used to discount its lease liabilities.
The Company’s leases with an initial term of 12 months or less that do not have a purchase option or extension that is reasonably certain to be exercised are not included in the right of use asset or lease liability on the Consolidated Balance Sheets. Lease expense is recognized on a straight-line basis over the lease term.
F-21
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
As of December 31, 2019, undiscounted future lease payments for non-cancellable operating leases, are as follows:
|
|
|
Lease payments |
|
|
|
2020 |
|
$ |
203 |
|
|
2021 |
|
|
165 |
|
|
2022 |
|
|
156 |
|
|
2023 |
|
|
156 |
|
|
2024 |
|
|
155 |
|
|
2025 and thereafter |
|
|
91 |
|
|
Total lease payments |
|
|
926 |
|
|
Less imputed interest |
|
|
(411 |
) |
|
Total operating liabilities |
|
$ |
515 |
|
As of December 31, 2018 under legacy ASC 840 “Leases”, undiscounted future lease payments for non-cancellable operating leases were as follows:
|
|
|
Lease payments |
|
|
|
2019 |
|
$ |
264 |
|
|
2020 |
|
|
199 |
|
|
2021 |
|
|
156 |
|
|
2022 |
|
|
156 |
|
|
2023 |
|
|
156 |
|
|
2024 and thereafter |
|
|
247 |
|
|
Total |
|
$ |
1,178 |
|
For the year ended December 31, 2019, the weighted average remaining lease term was 5 years and the weighted average discount rate was 16%.
The components of the Company’s lease cost were as follows for the year ended December 31, 2019:
|
|
|
|
|
Year Ended December 31, 2019 |
|
|
|
Operating lease cost |
|
|
|
$ |
227 |
|
|
Short-term lease cost |
|
|
|
|
57 |
|
|
Variable lease cost |
|
|
|
|
22 |
|
|
Total lease cost |
|
|
|
$ |
306 |
|
|
|
(c) |
Purchase Commitments |
As of December 31, 2019, the Company had outstanding non-cancelable and cancelable purchase commitments of $5,593 related to inventory, capital expenditures, transition services agreement costs and other goods and services.
|
|
(d) |
Certain Compensation and Employment Agreements |
The Company has entered into employment agreements with certain of its named executive officers. As of December 31, 2019, these employment agreements provided for, among other things, annual base salaries in an aggregate amount of not less than $1,018 from that date through calendar year 2020.
|
(13) |
Capital Structure |
|
|
(a) |
Common Stock |
The Company is authorized to issue 50,000,000 shares of common stock, with a par value of $0.01 per share.
Reflected below are the Company’s capital raises since its initial public offering, or IPO:
F-22
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
On March 12, 2014, the Company completed an IPO, in which the Company sold 4,312,500 shares of common stock at $8.00 per share, resulting in gross proceeds of $34,500. In connection with the IPO, the Company paid $4,244 in underwriting discounts, commissions and offering costs, resulting in net proceeds of $30,256. Also in connection with the IPO, all of the outstanding shares of the Company’s Series A Redeemable Convertible Preferred Stock, including accreted dividends, and Bridge Notes, including accrued interest, were converted into common stock.
On July 7, 2015, the Company closed a private placement with certain accredited investors in which the Company sold 1,379,311 shares of common stock at a price of $11.60 per share, for net proceeds of $14,812. The Company paid the placement agents a fee equal to 6.0% of the aggregate gross proceeds from the private placement, plus reimbursement of certain expenses.
On August 19, 2016, the Company closed an underwritten public offering in which the Company sold 1,986,666 shares of common stock at a price per share of $7.50, for net proceeds of $13,367 after deducting underwriting commissions and offering expenses.
On December 16, 2016, the Company closed an underwritten public offering in which the Company sold 6,670,000 shares of common stock at a price per share of $6.00, for net proceeds of $36,888 after deducting underwriting commissions and offering expenses.
On December 29, 2017, the Company entered into a sales agreement, or the Sales Agreement, with Cowen and Company, LLC, or Cowen, pursuant to which the Company may sell from time to time, at its option, shares of its common stock, $0.01 par value per share, having an aggregate offering price of up to $40,000 through Cowen, as the placement agent. As of December 31, 2019, the Company did not have any sales of common stock under the Sales Agreement.
|
|
(b) |
Common Stock Purchase Agreement |
On March 2, 2018, the Company entered into a common stock purchase agreement, or the 2018 Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth in the 2018 Purchase Agreement, Aspire Capital is committed to purchase, at the Company’s sole election, up to an aggregate of $20,000 of its shares of common stock over the approximately 30-month term of the 2018 Purchase Agreement. On the execution of the 2018 Purchase Agreement, the Company agreed to issue 33,040 shares of common stock to Aspire Capital with a fair value of $357 as consideration for entering into the 2018 Purchase Agreement. As of December 31, 2019, the Company sold 1,950,000 shares of common stock under the 2018 Purchase Agreement for proceeds of $16,999, at an average per share price of $8.72.
On February 19, 2019, the Company entered into a common stock purchase agreement, or the 2019 Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth in the 2019 Purchase Agreement, Aspire Capital is committed to purchase, at the Company’s sole election, up to an aggregate of $20,000 of its shares of common stock over the approximately 30-month term of the Purchase Agreement. On the execution of the 2019 Purchase Agreement, the Company agreed to issue 34,762 shares of common stock to Aspire Capital as consideration for entering into the 2019 Purchase Agreement.
|
|
(c) |
Preferred Stock |
The Company is authorized to issue 10,000,000 shares of preferred stock, with a par value of $0.01 per share. As of December 31, 2019, no preferred stock was issued or outstanding.
|
|
(d) |
Warrants |
As of December 31, 2019, the Company had the following warrant outstanding to purchase shares of the Company’s common stock:
|
Number of Shares |
|
Exercise Price per Share |
|
|
Expiration Date |
|
|
348,664 |
|
$ |
6.84 |
|
|
November 2024 |
The warrant to purchase 348,664 shares relates to Athyrium and is equity classified. During the year ended December 31, 2017, the Company recorded Athyrium equity classified warrants of $1,966, which is the fair value of $2,143, net of the related tax effect of $177.
F-23
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
In November 2019, the warrant to purchase 350,000 shares issued to Alkermes, which was liability classified as it contained a contingent net cash settlement feature, was exercised on a cashless basis, with Alkermes surrendering 165,673 shares to cover the aggregate exercise price, resulting in the issuance of 184,327 shares of common stock based on the closing bid price of the Company’s common stock on November 8, 2019 of $17.45.
For the year ended December 31, 2019, no liability classified warrants remain outstanding. The following table summarizes the fair value and the assumptions used for the Black-Scholes option-pricing model for the liability classified warrants for the year ended December 31, 2018:
|
|
|
December 31, 2018 |
|||
|
Fair value |
|
$ |
1,101 |
|
|
|
Expected dividend yield |
|
|
— |
|
% |
|
Expected volatility |
|
69 |
|
% |
|
|
Risk-free interest rates |
|
2.49 |
|
% |
|
|
Remaining contractual term |
|
3.25 years |
|
|
|
Each of the warrant agreements include usual and customary standard antidilution provisions and the Athyrium agreement contains additional antidilution provisions as well.
|
(14) |
Comprehensive Loss |
The Company’s comprehensive loss is shown on the Consolidated Statements of Operations and Comprehensive Loss for the years ended December 31, 2019, 2018 and 2017 and is comprised of net unrealized gains and losses on the Company’s available-for-sale securities. The total comprehensive loss for the twelve months ended December 31, 2019, 2018 and 2017 was $18,630, $79,722 and $50,083, respectively. There was no tax effect for the twelve months ended December 31, 2019, 2018 or 2017 of other comprehensive loss.
|
(15) |
Stock-Based Compensation |
The Company established the 2008 Stock Option Plan, or the 2008 Plan, which allows for the granting of common stock awards, stock appreciation rights, and incentive and nonqualified stock options to purchase shares of the Company’s common stock to designated employees, non-employee directors, and consultants and advisors. As of December 31, 2019, no stock appreciation rights have been issued. Subsequent to adoption, the 2008 Plan was amended to increase the authorized number of shares available for grant to 444,000 shares of common stock. This plan expired in 2018. In October 2013, the Company established the 2013 Equity Incentive Plan, or the 2013 Plan, which allows for the grant of stock options, stock appreciation rights and stock awards for a total of 600,000 shares of common stock. In June 2015, the Company’s shareholders approved the Amended and Restated Equity Incentive Plan, or the A&R Plan, which amended and restated the 2013 Plan and increased the aggregate amount of shares available for issuance to 2,000,000. In May 2018, the Company’s shareholders approved the 2018 Amended and Restated Equity Incentive Plan, which amended and restated the A&R Plan to increase the aggregate amount of shares available for issuance to 8,119,709. On December 1st of each year, pursuant to the “Evergreen” provision of the A&R Plan, the number of shares available under the plan may be increased by the board of directors by an amount equal to 5% of the outstanding common stock on December 1st of that year. In December 2019, 2018 and 2017, the number of shares available for issuance under the A&R Plan was increased by 1,161,693, 1,082,972 and 956,341, respectively. The total number of shares authorized for issuance under the A&R plan as of December 31, 2019 is 9,281,402.
Stock options are exercisable generally for a period of 10 years from the date of grant and generally vest over four years. As of December 31, 2019, 3,498,500 shares are available for future grants under the A&R Plan.
F-24
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The weighted average grant-date fair value of the options awarded to employees during the years ended December 31, 2019, 2018 and 2017 was $5.72, $5.95 and $5.44, respectively. The fair value of the options was estimated on the date of grant using a Black-Scholes option pricing model with the following assumptions:
|
|
|
December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Range of expected option life |
|
5.5 - 6 years |
|
|
5.5 - 6 years |
|
|
6 years |
|
|||
|
Expected volatility |
|
78.26% - 81.54% |
|
|
73.26% - 82.00% |
|
|
75.10 - 84.71% |
|
|||
|
Risk-free interest rate |
|
1.56 - 2.66% |
|
|
2.32 - 3.03% |
|
|
1.87 - 2.27% |
|
|||
|
Expected dividend yield |
|
|
— |
|
|
|
— |
|
|
|
— |
|
The following table summarizes stock option activity during the years ended December 31, 2019 and 2018:
|
|
|
Number of shares |
|
|
Weighted average exercise price |
|
|
Weighted average remaining contractual life |
||
|
Balance, December 31, 2017 |
|
|
3,594,875 |
|
|
$ |
7.17 |
|
|
7.1 years |
|
Granted |
|
|
949,861 |
|
|
$ |
8.92 |
|
|
|
|
Exercised |
|
|
(355,312 |
) |
|
$ |
5.17 |
|
|
|
|
Expired/forfeited/cancelled |
|
|
(414,359 |
) |
|
$ |
8.81 |
|
|
|
|
Balance, December 31, 2018 |
|
|
3,775,065 |
|
|
$ |
7.62 |
|
|
7.4 years |
|
Granted |
|
|
1,526,679 |
|
|
$ |
8.24 |
|
|
|
|
Exercised |
|
|
(871,790 |
) |
|
$ |
7.01 |
|
|
|
|
Expired/forfeited/cancelled |
|
|
(734,305 |
) |
|
$ |
7.88 |
|
|
|
|
Balance, December 31, 2019 |
|
|
3,695,649 |
|
|
$ |
7.97 |
|
|
7.2 years |
|
Vested |
|
|
2,207,150 |
|
|
$ |
7.74 |
|
|
6.2 years |
|
Vested and expected to vest |
|
|
3,695,649 |
|
|
$ |
7.97 |
|
|
7.2 years |
Included in the table above are 439,490 options outstanding as of December 31, 2019 that were granted outside the plan. The grants were made pursuant to the NASDAQ inducement grant exception in accordance with NASDAQ Listing Rule 5635(c)(4).
The following table summarizes restricted stock units activity during the years ended December 31, 2019 and 2018.
|
|
Number of shares |
|
|
|
Balance, December 31, 2017 |
|
270,593 |
|
|
Granted |
|
1,011,487 |
|
|
Vested and settled |
|
(133,268 |
) |
|
Expired/forfeited/cancelled |
|
(45,416 |
) |
|
Balance, December 31, 2018 |
|
1,103,396 |
|
|
Granted |
|
1,161,836 |
|
|
Vested and settled |
|
(586,685 |
) |
|
Expired/forfeited/cancelled |
|
(481,045 |
) |
|
Balance, December 31, 2019 |
|
1,197,502 |
|
|
Expected to vest |
|
931,302 |
|
Included in the table above are 18,625 time-based RSUs outstanding as of December 31, 2019 that were granted outside the plan. The grants were made pursuant to the NASDAQ inducement grant exception in accordance with NASDAQ Listing Rule 5635(c)(4).
Stock-based compensation expense from continuing operations for the twelve months ended December 31, 2019, 2018 and 2017 was $6,191, $4,279 and $4,178, respectively.
F-25
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
As of December 31, 2019, there was $17,018 of unrecognized compensation expense related to unvested options and time-based RSUs that are expected to vest and will be expensed over a weighted average period of 1.7 years, of which approximately $3,800 relates to Baudax Bio employees and will be recorded in Baudax Bio in future periods. As of December 31, 2019, there was $2,127 of unrecognized compensation expense related to unvested performance-based RSUs that will be expensed if the performance criteria are met.
In January 2020, the Company cancelled 251,200 performance-based RSUs as the performance criteria was based on Acute Care business related goals.
The aggregate intrinsic value represents the total amount by which the fair value of the common stock subject to options exceeds the exercise price of the related options. As of December 31, 2019, the aggregate intrinsic value of the vested and unvested options was $23,368 and $14,932, respectively.
|
(16) |
Revenue Recognition |
Effective January 1, 2018, the Company adopted ASU 2014-09 using the modified retrospective method applied to contracts existing as of January 1, 2018. See Note 2 for additional information on the Company’s revenue recognition policies.
The Company uses the practical expedient to not account for significant financing components because the period between recognition and collection does not exceed one year in any contract.
Contract assets represent revenue recognized for performance obligations completed before an unconditional right to payment exists, and therefore invoicing or associated reporting from the customer regarding the computation of the net product sales has not yet occurred. Contract assets were $8,851 and $5,201 at December 31, 2019 and December 31, 2018, respectively. Generally, the contract assets balance is impacted by the recognition of additional contract assets, offset by amounts invoiced to customers or actual net product sale amounts reported by the commercial partner for the period. For the years ended December 31, 2019, actual net product sale amounts reported by the Company’s commercial partners exceeded estimates of royalty amounts attributed to manufactured product shipped as of December 31, 2018 for the related arrangements by approximately $2,083.
The following table presents changes in the Company’s contract assets for the twelve months ended December 31, 2019:
|
Contract asset, beginning of year |
|
$ |
5,201 |
|
|
Change in estimate arising from changes in transaction price |
|
|
2,083 |
|
|
Reclassification of contract asset to receivables, as the result of rights to consideration becoming unconditional |
|
|
(7,284 |
) |
|
Contract assets recognized |
|
|
8,851 |
|
|
Contract asset, end of period |
|
$ |
8,851 |
|
The following table disaggregates revenue by timing of revenue recognition:
|
|
|
Twelve Months Ended December 31, 2019 |
|
|||||||||
|
|
|
Point in time |
|
|
Over time |
|
|
Total |
|
|||
|
Revenue |
|
$ |
96,346 |
|
|
$ |
2,873 |
|
|
$ |
99,219 |
|
|
|
|
|
|
|||||||||
|
|
|
Twelve Months Ended December 31, 2018 |
|
|||||||||
|
|
|
Point in time |
|
|
Over time |
|
|
Total |
|
|||
|
Revenue |
|
$ |
76,270 |
|
|
$ |
1,077 |
|
|
$ |
77,347 |
|
Adoption of ASU 2014-09 did not require capitalization of any costs to obtain or fulfill contracts. In general, the Company’s payment terms for manufacturing revenue and research and development services is 30 days. Royalty revenue is recorded to
F-26
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
accounts receivable in the quarter that the product is sold by the commercial partner upon reporting from the commercial partner and payment terms are generally 45 days after quarter end. Based on the adoption of ASU 2014-09, the timing difference between recognition of certain royalty revenues as a contract asset and cash receipt is increased by an estimated 90 days.
|
(17) |
Income Taxes |
The components of income from continuing operations before income tax are as follows:
|
|
|
December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Domestic |
|
$ |
4,625 |
|
|
$ |
4,368 |
|
|
$ |
600 |
|
The components of the income tax provision (benefit) from continuing operations are as follows:
|
|
|
December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
— |
|
|
$ |
(130 |
) |
|
$ |
(190 |
) |
|
State and local |
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
|
$ |
— |
|
|
|
(129 |
) |
|
|
(190 |
) |
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
1,368 |
|
|
$ |
124 |
|
|
$ |
7,769 |
|
|
State and local |
|
|
(356 |
) |
|
|
(1,327 |
) |
|
|
(262 |
) |
|
|
|
|
1,012 |
|
|
|
(1,203 |
) |
|
|
7,507 |
|
|
Change in valuation allowance |
|
|
(1,012 |
) |
|
|
18,768 |
|
|
|
— |
|
|
Total income tax provision from continuing operations |
|
$ |
— |
|
|
$ |
17,436 |
|
|
$ |
7,317 |
|
A reconciliation of the statutory U.S. federal income tax rate to the Company’s effective tax rate from continuing operations is as follows:
|
|
|
Year ended December 31, |
|
|||||||||
|
|
|
2019 |
|
|
2018 |
|
|
2017 |
|
|||
|
U.S. federal statutory income tax rate |
|
|
21.0 |
% |
|
|
21.0 |
% |
|
|
34.0 |
% |
|
State taxes, net of federal benefit |
|
|
(7.7 |
)% |
|
|
(30.4 |
)% |
|
|
(43.7 |
)% |
|
Nondeductible expenses |
|
|
11.4 |
% |
|
|
0.3 |
% |
|
|
(21.0 |
)% |
|
Research and development credits |
|
|
(2.8 |
)% |
|
|
(21.0 |
)% |
|
|
(81.5 |
)% |
|
Change in federal tax rate |
|
|
— |
|
|
|
(1.4 |
)% |
|
|
1315.6 |
% |
|
Change in valuation allowance |
|
|
(21.9 |
)% |
|
|
429.7 |
% |
|
|
— |
|
|
Other |
|
|
— |
|
|
|
1.0 |
% |
|
|
16.8 |
% |
|
Effective income tax rate |
|
|
— |
|
|
|
399.2 |
% |
|
|
1220.2 |
% |
F-27
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
The tax effects of temporary differences that gave rise to significant portions of the deferred tax assets were as follows:
|
|
|
December 31, |
|
|||||
|
|
|
2019 |
|
|
2018 |
|
||
|
Net operating loss carryforwards |
|
$ |
35,052 |
|
|
$ |
17,923 |
|
|
Research and development credits |
|
|
4,443 |
|
|
|
4,307 |
|
|
Capitalized start-up costs |
|
|
1,588 |
|
|
|
1,489 |
|
|
Intangibles |
|
|
66 |
|
|
|
3,194 |
|
|
Contingent consideration |
|
|
— |
|
|
|
9,816 |
|
|
Stock-based compensation |
|
|
4,441 |
|
|
|
4,797 |
|
|
Operating lease liability |
|
|
147 |
|
|
|
— |
|
|
Interest expense |
|
|
6,966 |
|
|
|
1,370 |
|
|
Other temporary differences |
|
|
(1,918 |
) |
|
|
288 |
|
|
Gross deferred tax asset |
|
|
50,785 |
|
|
|
43,184 |
|
|
Valuation allowance |
|
|
(45,214 |
) |
|
|
(40,417 |
) |
|
Net deferred tax asset |
|
|
5,571 |
|
|
|
2,767 |
|
|
Deferred tax liability |
|
|
(5,571 |
) |
|
|
(2,767 |
) |
|
Net deferred taxes |
|
$ |
— |
|
|
$ |
— |
|
During the year ended December 31, 2019, the Company made an election to treat its Irish subsidiary as a disregarded entity for U.S federal income tax purposes, which resulted in a worthless stock and bad debt deduction of approximately $97.0 million for U.S. federal income tax purposes. There was no impact on the consolidated financial statements for this benefit as a result of the full valuation allowance against deferred tax assets.
As a result of the Separation (see Note 4), certain deferred tax assets and liabilities that existed as of December 31, 2018 were attributable to Baudax subsequent to the transaction. As of December 31, 2019, deferred tax assets represent the deferred taxes attributable to the Company following the Separation. There was no impact on the consolidated financial statements for these adjustments to deferred tax assets as a result of the Separation due to the full valuation allowance against deferred tax assets.
In assessing the realizability of the net deferred tax asset, the Company considers all relevant positive and negative evidence in determining whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The realization of the gross deferred tax assets is dependent on several factors, including the generation of sufficient taxable income prior to the expiration of the net operating loss carryforwards.
In 2018, the Company recorded a valuation allowance against its U.S. and state deferred tax assets based on the available positive and negative evidence available. An important aspect of objective negative evidence evaluated was the Company’s historical operating results over the prior three-year period. The Company maintains the valuation allowance as of December 31, 2019 as a result of historical losses, inclusive of discontinued operations, during the most recent three year period. The Company will re-evaluate the need for a valuation allowance in future periods based on its operating results as a standalone entity.
The following table summarizes carryforwards of Federal net operating losses and tax credits as of December 31, 2019:
|
|
|
Amount |
|
|
Expiration |
|
|
Federal net operating losses - 2008 to 2017 |
|
$ |
8,200 |
|
|
2028 – 2038 |
|
Federal net operating losses - 2018 to 2019 |
|
$ |
113,417 |
|
|
No expiration |
|
State net operating losses |
|
$ |
128,095 |
|
|
2028 – 2039 |
|
Federal and state research and development credits |
|
$ |
4,443 |
|
|
2028 – 2038 |
Under the Tax Reform Act of 1986, as amended (the “Act”), the utilization of a corporation’s net operating loss and research and development tax credit carryforwards is limited following a greater than 50% change in ownership during a three-year period. Any unused annual limitation may be carried forward to future years for the balance of the carryforward period. The Company has done an analysis to determine whether or not ownership changes, as defined by the Act, have occurred since inception. The Company determined that it experienced ownership changes, as defined by the Act, during the 2008, 2014 and 2016 tax years as a result of past financings; accordingly, the Company’s ability to utilize the aforementioned carryforwards will be limited. In addition, state net operating loss carryforwards may be further limited, including in Pennsylvania, which has a
F-28
RECRO PHARMA, INC. AND SUBSIDIARIES
Notes to the Consolidated Financial Statements
(amounts in thousands, except share and per share data)
limitation of 30%, 35% or 40% of taxable income after modifications and apportionment on state net operating losses utilized in any one year during tax years beginning during 2017, 2018 or 2019 going forward, respectively.
The Company will recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2019, the Company had no accrued interest or penalties related to uncertain tax positions and no amounts have been recognized in the Company’s statements of operations. Due to net operating loss and tax credit carry forwards that remain unutilized, income tax returns for tax years from inception through 2016 remain subject to examination by the taxing jurisdictions.
On December 22, 2017, the Tax Cuts and Jobs Act (H.R. 1) (the “Tax Act”) was signed into law. The Tax Act contains significant changes to corporate taxation, including (i) the reduction of the corporate income tax rate to 21%, (ii) the acceleration of expensing for certain business assets, (iii) the one-time transition tax related to the transition of U.S. international tax from a worldwide tax system to a territorial tax system, (iv) additional limitations on the deductibility of interest expense, and (v) expanded limitations on executive compensation. The most significant impacts on the Company are as follows:
|
|
• |
The Company remeasured its existing U.S. federal deferred tax assets and liabilities at the rate that the Company expects to be in effect when those deferred taxes will be realized, which is now 21%. In 2017, the Company recognized a one-time net expense from the deferred tax remeasurement of approximately $7,900. |
|
|
• |
The Company will be able to claim an immediate deduction for investments in qualified fixed assets acquired and placed in service beginning September 27, 2017 through 2022. This provision phases out through 2026. |
|
|
• |
Given our taxable losses in the U.S., we will be limited in our ability to deduct interest expense, and any disallowed interest expense for 2018 and tax years following will result in an indefinite carry forward until such time as we meet the taxable income thresholds required to deduct interest expense. |
Pursuant to the Securities and Exchange Commission Staff Accounting Bulletin No. 118, Income Tax Accounting Implications of the Tax Act ("SAB 118"), the SEC gave issuers a one year measurement period to finalize accounting adjustments related to the act. For the year-ended December 31, 2017, the Company disclosed it was unable to determine a reasonable estimate of the decrease to its stock compensation deferred tax asset, if any, under the Tax Act due to expanded limitations on the deductibility of executive compensation. The Company has subsequently determined there were no material changes required related to any provisional amounts recorded and the measurement period under SAB 118 has closed.
|
(18) |
Related Party Transactions |
Baudax Bio became a related party to the Company following the Separation. As part of the Separation, the Company entered into a transition services agreement with Baudax Bio. Under the transition services agreement, Baudax Bio provides certain services to the Company, each related to corporate functions, and are charged to the Company. Additionally, the Company may incur expenses that are directly related to Baudax Bio after the Separation, which are billed to Baudax Bio. For the year ended December 31, 2019, for periods subsequent to the Separation, the Company recorded expense of $206 related to the transition services agreement, which is recorded as an increase in selling, general and administrative expenses. The Company recorded a net payable of $273 for such activities and other activity with Baudax Bio as of December 31, 2019.
(19) Retirement Plan
The Company has a voluntary 401(k) Savings Plan (the 401(k) Plan) in which all employees are eligible to participate. The Company’s policy is to match 100% of the employee contributions up to a maximum of 5% of employee compensation. Total Company contributions to the 401(k) Plan for the year ended December 31, 2019, 2018 and 2017 were $926, $860 and $849, respectively.
F-29
