Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - Theravance Biopharma, Inc. | tm207823d1_ex99-2.htm |
| EX-5.1 - EXHIBIT 5.1 - Theravance Biopharma, Inc. | tm207823d1_ex5-1.htm |
| EX-1.1 - EXHIBIT 1.1 - Theravance Biopharma, Inc. | tm207823d1_ex1-1.htm |
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | tm207823-1_8k.htm |
Exhibit 99.1
SUMMARY
Overview
Theravance Biopharma, Inc. ("Theravance Biopharma") is a diversified biopharmaceutical company primarily focused on the discovery, development and commercialization of organ-selective medicines. Our purpose is to create transformational medicines to improve the lives of patients suffering from serious illnesses. Our research is focused in the areas of inflammation and immunology.
In pursuit of our purpose, we apply insights and innovation at each stage of our business and utilize our internal capabilities and those of partners around the world. We apply organ-selective expertise to biologically compelling targets to discover and develop medicines designed to treat underserved localized diseases and to limit systemic exposure, in order to maximize patient benefit and minimize risk. These efforts leverage years of experience in developing lung-selective medicines to treat respiratory disease, including the United States ("US") Food and Drug Administration (the "FDA") approved YUPELRI® (revefenacin) inhalation solution indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease ("COPD"). Our pipeline of internally discovered programs is targeted to address significant patient needs.
We have an economic interest in potential future payments from Glaxo Group or one of its affiliates ("GSK") pursuant to its agreements with Innoviva, Inc. ("Innoviva") relating to certain programs, including TRELEGY ELLIPTA.
Our Programs
The table below summarizes the status of our approved product and our other product candidates in development. The table also includes the status of the respiratory programs in which we have an economic interest and for which GSK is responsible pursuant to agreements between Innoviva and GSK ("GSK-Partnered Respiratory Programs"). These programs consist primarily of the TRELEGY ELLIPTA program. We have an economic interest in these programs through our interest in Theravance Respiratory Company, LLC, a limited liability company managed by Innoviva. The status of all GSK-Partnered Respiratory Programs referenced in this prospectus supplement are based solely upon publicly available information and may not reflect the most recent developments under the programs.
1
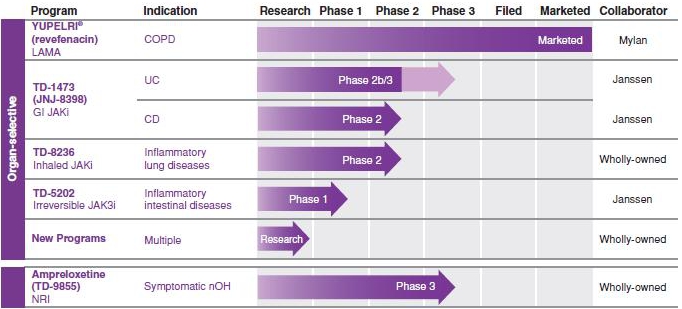
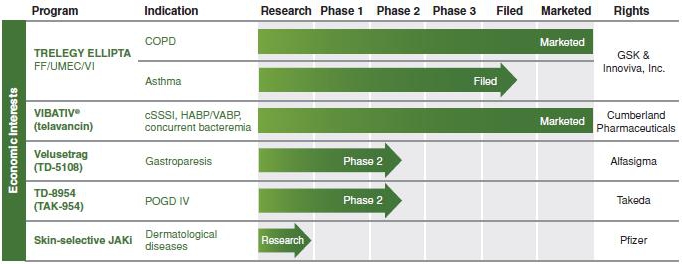
Glossary of Defined Terms used in Table Above:
COPD: Chronic Obstructive Pulmonary Disease;
CD: Crohn's Disease
cSSSI: Complicated Skin and Skin Structure Infections;
FF: Fluticasone Furoate;
HABP/VABP: Hospital-Acquired and Ventilator-Associated Bacterial Pneumonia;
IV: Intravenous;
JAKi: Janus Kinase Inhibitor;
LAMA: Long-Acting Muscarinic Antagonist;
nOH: Neurogenic Orthostatic Hypotension;
NRI: Norepinephrine Reuptake Inhibitor;
POGD: Post-Operative Gastrointestinal Dysfunction;
UC: Ulcerative Colitis;
UMEC: Umeclidinium; and
VI: Vilanterol
2
Recent Developments
Estimates as of December 31, 2019
We are currently finalizing our financial results for the year ended December 31, 2019. The financial results discussed below as of December 31, 2019 are preliminary and subject to completion of financial and operating closing procedures. The results below are not a comprehensive statement of our financial results as of December 31, 2019, and our actual results may differ materially from these amounts following the completion of our financial and operating closing procedures, or as a result of other adjustments or developments that may arise before the results as of December 31, 2019 are finalized. In addition, even if our actual results are consistent with these preliminary results, those results or developments may not be indicative of results or developments in subsequent periods.
We expect to report that our cash, cash equivalents and marketable securities were approximately $285.8 million as of December 31, 2019.
Global License Agreement with Pfizer Inc. for Skin-Selective Pan-JAK Inhibitors
In the fourth quarter of 2019, we entered into a global license agreement with Pfizer Inc. for our preclinical skin-selective, locally-acting pan-JAK inhibitor program. The compounds in this program are designed to be rapidly metabolized, target validated pro-inflammatory pathways, and are specifically designed to possess skin-selective activity with minimal systemic exposure.
Under this agreement, Pfizer has an exclusive license to develop, manufacture and commercialize certain compounds for all uses other than gastrointestinal, ophthalmic and respiratory applications. We received an upfront cash payment of $10.0 million and are eligible to receive up to an additional $240.0 million in development and sales milestone payments from Pfizer. In addition, we will be eligible to receive a tiered marginal royalty on worldwide net sales of any potential products under the license at percentage royalty rates ranging from middle single-digits to low double-digits.
Theravance Respiratory Company, LLC ("TRC")
In January 2020, we were informed by Innoviva that GSK had declined to adopt certain TRELEGY ELLIPTA development and commercialization initiatives proposed by Innoviva. As a result, Innoviva would not continue to withhold any funds that had been reserved for those initiatives, and we subsequently received $15.8 million in a distribution from Innoviva representing our share of the net royalty income payments for the third quarter of 2019 plus the $6.9 million previously withheld, less estimated TRC expenses for the quarter ended December 31, 2019 and estimated expenses through 2020. For additional discussion regarding risks related to royalty distributions by Innoviva and TRC, please see the risk factor entitled "We do not control the commercialization of TRELEGY ELLIPTA and we do not control TRC; accordingly the amount of royalties we receive will depend, among other factors, on GSK's ability to further commercialize TRELEGY ELLIPTA and TRC's decisions concerning use of cash in accordance with the TRC LLC Agreement."
Note Refinancing
We have in the past and are currently engaged in discussions with a limited number of investors to explore alternative financing strategies with respect to the Non-Recourse 2033 Notes. In particular, although we have no definitive agreements with respect to the refinancing of the Non-Recourse 2033 Notes, we are in advanced negotiations and it is possible that we could enter into definitive agreements with new lenders to, among other things, lend us $400.0 million on a non-recourse basis similar to the Non-Recourse 2033 Notes and allow us to redeem the $250.0 million aggregate principal amount of Non-Recourse 2033 Notes. We would expect this new loan to bear interest at a slightly higher rate than the Non-Recourse 2033 Notes and have a term of at least 15 years. We also expect that the primary source of funds to make payments on this new loan will continue to be the 63.75% economic interest of our affiliate in any future royalties due on net sales of the TRELEGY ELLIPTA program. We cannot assure you that we will be successful in negotiating this or any new loan agreement, that we will be able to refinance the Non-Recourse 2033 Notes and secure additional financing, or the timing or terms of any such financing.
3
Corporate Information
Theravance Biopharma was incorporated in the Cayman Islands in July 2013 under the name Theravance Biopharma, Inc. Our corporate address in the Cayman Islands and principal executive office is P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands and the address of our wholly-owned US operating subsidiary Theravance Biopharma US, Inc. is 901 Gateway Boulevard, South San Francisco, California 94080. Our telephone number is (650) 808-6000 and our corporate website address is www.theravance.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only. While Theravance Biopharma is incorporated under Cayman Islands law, Theravance Biopharma became an Irish tax resident effective July 1, 2015. The address of our wholly-owned Irish operating subsidiary, Theravance Biopharma Ireland Limited, is Connaught House, Burlington Road, Dublin 4, Ireland.
4
