Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Syneos Health, Inc. | d874191d8k.htm |

January 15, 2020 J.P. Morgan Healthcare Conference Alistair Macdonald Chief Executive Officer Exhibit 99.1

Forward-Looking Statements and Non-GAAP Financial Measures Forward-Looking Statements Except for historical information, all of the statements, expectations, and assumptions contained in this presentation are forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995, including market opportunities, environmental, social and charitable goals, anticipated financial results for the full year 2020 and full year 2019, our 2020 synergies target, and plans for margin improvement and capital deployment. Actual results might differ materially from those explicit or implicit in the forward-looking statements. Important factors that could cause actual results to differ materially include, but are not limited to: reliance on key personnel; principal investigators and patients; general and international economic, political, and other risks, including currency and stock market fluctuations and the uncertain economic environment; the Company's ability to adequately price its contracts and not overrun cost estimates; any adverse effects from the Company's customer or therapeutic area concentration; the Company's ability to maintain or generate new business awards; the Company's ability to increase its market share, grow its business, and execute its growth strategies; the Company's backlog not being indicative of future revenues and its ability to realize the anticipated future revenue reflected in its backlog; fluctuations in the Company’s operating results and effective income tax rate; risks related to the Company's information systems and cybersecurity; changes and costs of compliance with regulations related to data privacy; risk related to the United Kingdom’s withdrawal from the European Union; risks related to the Company's transfer pricing policies; failure to perform services in accordance with contractual requirements, regulatory requirements and ethical considerations; risks relating to litigation and government investigations; risks associated with the Company's early phase clinical facilities; insurance risk; risks of liability resulting from harm to patients; success of investments in the Company's customers’ business or drugs; foreign currency exchange rate fluctuations; risks associated with the integration of the Company's businesses with the business of inVentiv Health and its operation of the combined business following the closing of the merger with inVentiv Health in 2017; risks related to the Company's income tax expense and tax reform; risks relating to the Company's intellectual property; risks associated with the Company's acquisition strategy; failure to realize the full value of goodwill and intangible assets; restructuring risk; potential violations of anti-corruption and anti-bribery laws; risks related to the Company's dependence on third parties; downgrades of the Company's credit ratings; competition in the biopharmaceutical services industry; changes in outsourcing trends; regulatory risks; trends in the Company's customers’ businesses; the Company's ability to keep pace with rapid technological change; risks related to the Company's indebtedness; fluctuations in the Company's financial results and stock price; and other risk factors set forth in the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2018 and other SEC filings, copies of which are available free of charge on the Company's website at investor.syneoshealth.com. The Company assumes no obligation and does not intend to update these forward-looking statements, except as required by law. Non-GAAP Financial Measures In addition to the financial measures prepared in accordance with U.S. Generally Accepted Accounting Principles ("GAAP"), this presentation contains certain non-GAAP financial measures, including adjusted net income (including adjusted diluted earnings per share), EBITDA, adjusted EBITDA, adjusted EBITDA margin, segment adjusted EBITDA and unallocated corporate and other EBITDA, and non-GAAP effective tax rate. A “non-GAAP financial measure” is generally defined as a numerical measure of a company’s financial performance that excludes or includes amounts from the most directly comparable measure calculated and presented in accordance with GAAP in the statements of operations, balance sheets, or statements of cash flows of the Company. The Company defines adjusted net income (including adjusted diluted earnings per share) as net income (including diluted earnings per share) excluding acquisition-related deferred revenue adjustments; acquisition-related amortization; restructuring and other costs; transaction and integration-related expenses; share-based compensation expense; loss on extinguishment of debt; and other income (expense), net. After giving effect to these items, the Company has also included an adjustment to its income tax rate to reflect the expected long-term income tax rate and estimated impact of the enactment of the Tax Act. EBITDA represents earnings before interest, taxes, depreciation and amortization. The Company defines adjusted EBITDA, both at the company and segment level, as EBITDA, further adjusted to exclude expenses and transactions that the Company believes are not representative of its core operations, namely: acquisition-related deferred revenue adjustments; restructuring and other costs; transaction and integration-related expenses; asset impairment charges; share-based compensation expense; other income (expense), net; and loss on extinguishment of debt. The Company presents EBITDA and adjusted EBITDA because it believes they are useful metrics for investors as they are commonly used by investors, analysts and debt holders to measure the Company's ability to fund capital expenditures and meet working capital requirements. Each of the non-GAAP measures noted above are used by management and the Board to evaluate the Company's core operating results because they exclude certain items whose fluctuations from period-to-period do not necessarily correspond to changes in the core operations of the business. Adjusted net income (including adjusted diluted earnings per share) and adjusted EBITDA are used by management and the Board to assess the performance of the Company's business. Non-GAAP measures have limitations in that they do not reflect all of the amounts associated with the Company's results of operations as determined in accordance with GAAP. Also, other companies might calculate these measures differently. Investors are encouraged to review the reconciliations of the non-GAAP financial measures to their most directly comparable GAAP measures included on slides 20 - 21 in the Appendix of this presentation.
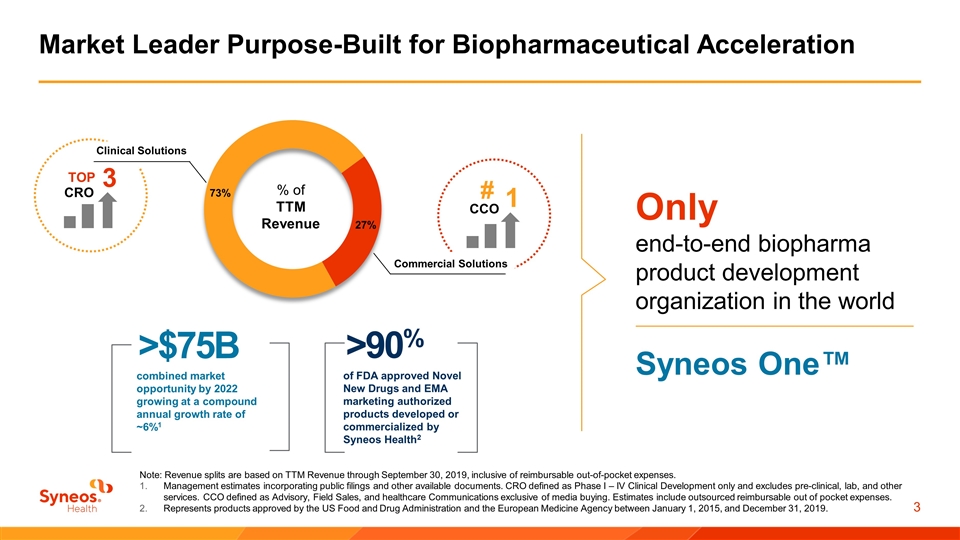
Market Leader Purpose-Built for Biopharmaceutical Acceleration Note: Revenue splits are based on TTM Revenue through September 30, 2019, inclusive of reimbursable out-of-pocket expenses. Management estimates incorporating public filings and other available documents. CRO defined as Phase I – IV Clinical Development only and excludes pre-clinical, lab, and other services. CCO defined as Advisory, Field Sales, and healthcare Communications exclusive of media buying. Estimates include outsourced reimbursable out of pocket expenses. Represents products approved by the US Food and Drug Administration and the European Medicine Agency between January 1, 2015, and December 31, 2019. CCO # 1 CRO TOP 3 Clinical Solutions Commercial Solutions >$75B combined market opportunity by 2022 growing at a compound annual growth rate of ~6%1 of FDA approved Novel New Drugs and EMA marketing authorized products developed or commercialized by Syneos Health2 >90% Only end-to-end biopharma product development organization in the world Syneos One™
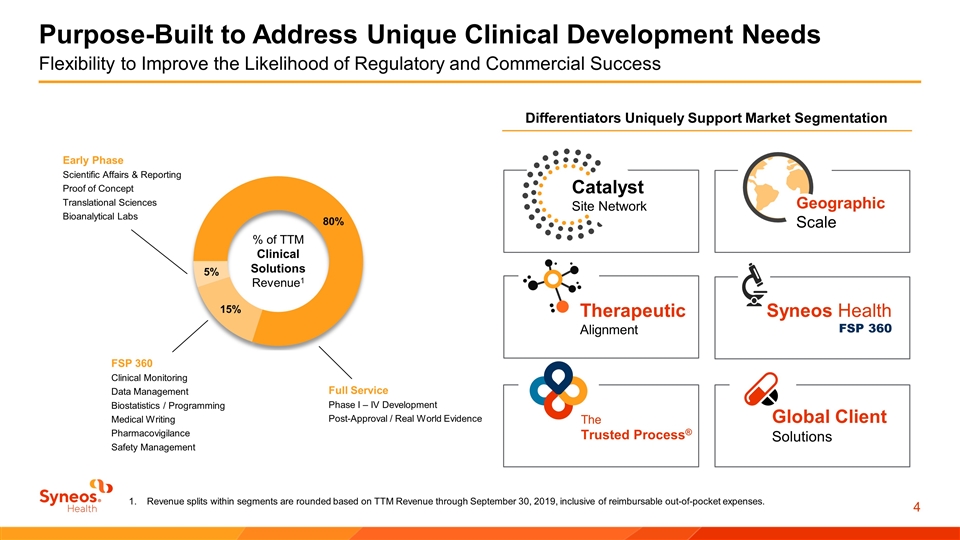
Purpose-Built to Address Unique Clinical Development Needs Flexibility to Improve the Likelihood of Regulatory and Commercial Success Full Service Phase I – IV Development Post-Approval / Real World Evidence Early Phase Scientific Affairs & Reporting Proof of Concept Translational Sciences Bioanalytical Labs FSP 360 Clinical Monitoring Data Management Biostatistics / Programming Medical Writing Pharmacovigilance Safety Management % of TTM Clinical Solutions Revenue1 Revenue splits within segments are rounded based on TTM Revenue through September 30, 2019, inclusive of reimbursable out-of-pocket expenses. Catalyst Site Network Global Client Solutions Syneos Health FSP 360 Geographic Scale Differentiators Uniquely Support Market Segmentation The Trusted Process® Therapeutic Alignment
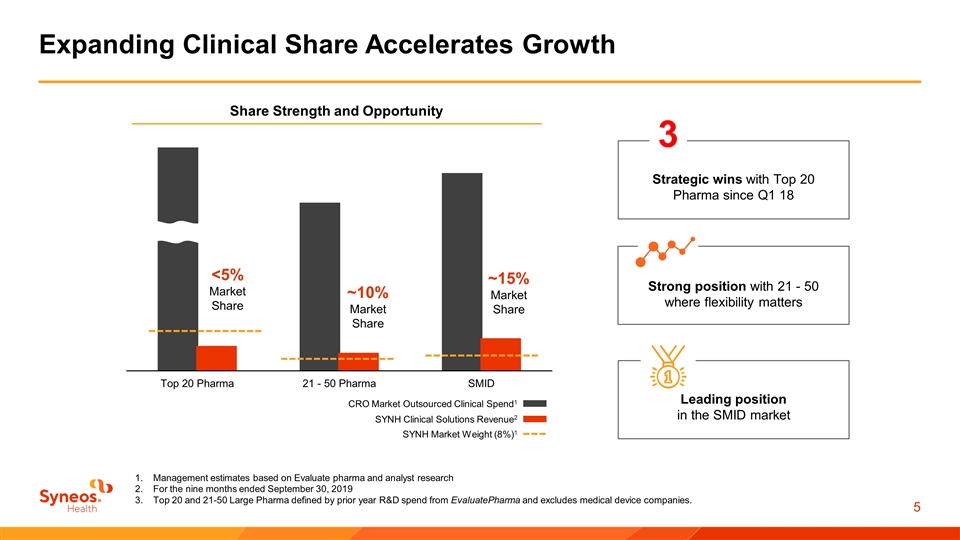
Expanding Clinical Share Accelerates Growth Management estimates based on Evaluate pharma and analyst research For the nine months ended September 30, 2019 Top 20 and 21-50 Large Pharma defined by prior year R&D spend from EvaluatePharma and excludes medical device companies. Leading position in the SMID market Strategic wins with Top 20 Pharma since Q1 18 3 Strong position with 21 - 50 where flexibility matters Share Strength and Opportunity CRO Market Outsourced Clinical Spend1 SYNH Clinical Solutions Revenue2 <5% Market Share ~10% Market Share ~15% Market Share SYNH Market Weight (8%)1
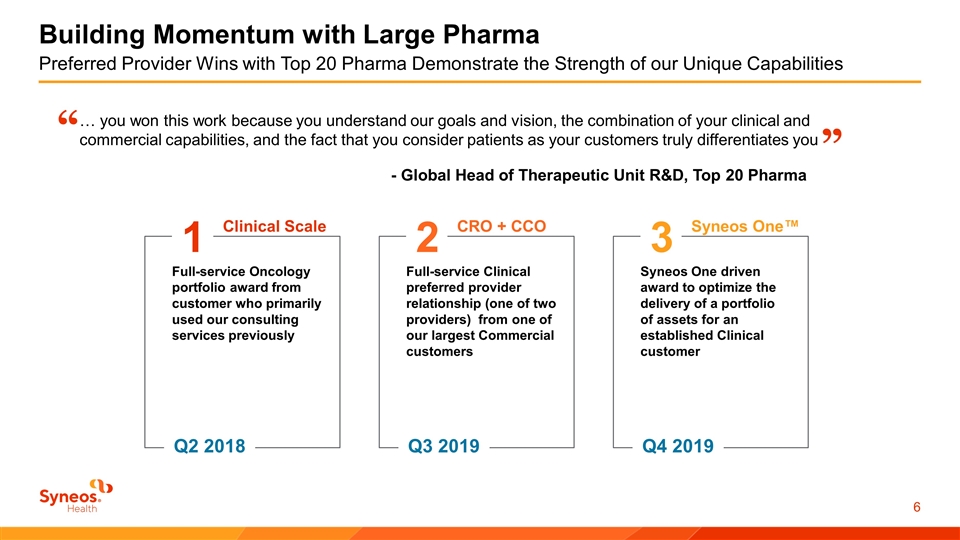
Building Momentum with Large Pharma Preferred Provider Wins with Top 20 Pharma Demonstrate the Strength of our Unique Capabilities Clinical Scale 1 Full-service Oncology portfolio award from customer who primarily used our consulting services previously Q2 2018 CRO + CCO 2 Full-service Clinical preferred provider relationship (one of two providers) from one of our largest Commercial customers Q3 2019 Syneos One™ 3 Syneos One driven award to optimize the delivery of a portfolio of assets for an established Clinical customer Q4 2019 … you won this work because you understand our goals and vision, the combination of your clinical and commercial capabilities, and the fact that you consider patients as your customers truly differentiates you “ ” - Global Head of Therapeutic Unit R&D, Top 20 Pharma
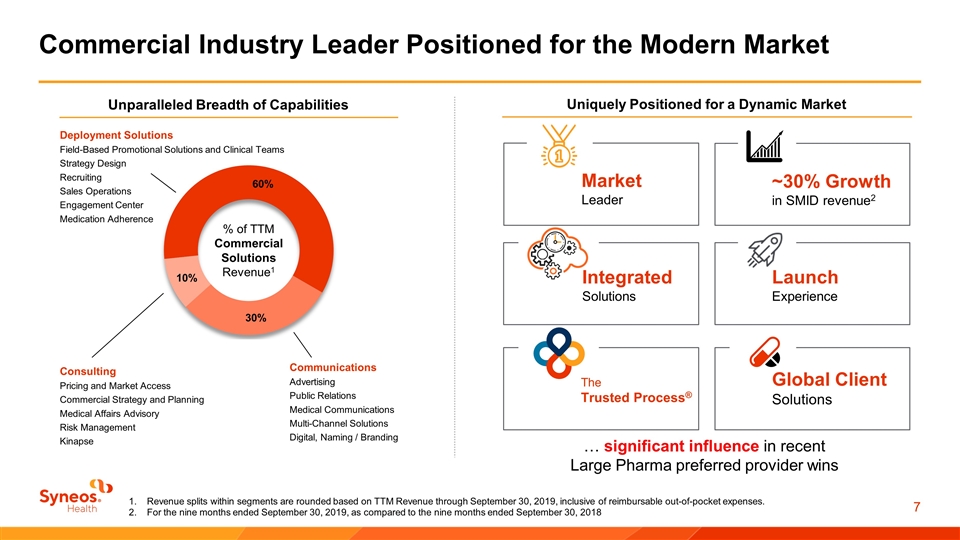
Commercial Industry Leader Positioned for the Modern Market % of TTM Commercial Solutions Revenue1 Deployment Solutions Field-Based Promotional Solutions and Clinical Teams Strategy Design Recruiting Sales Operations Engagement Center Medication Adherence Communications Advertising Public Relations Medical Communications Multi-Channel Solutions Digital, Naming / Branding Consulting Pricing and Market Access Commercial Strategy and Planning Medical Affairs Advisory Risk Management Kinapse Uniquely Positioned for a Dynamic Market Global Client Solutions The Trusted Process® Launch Experience Market Leader Integrated Solutions ~30% Growth in SMID revenue2 Unparalleled Breadth of Capabilities … significant influence in recent Large Pharma preferred provider wins Revenue splits within segments are rounded based on TTM Revenue through September 30, 2019, inclusive of reimbursable out-of-pocket expenses. For the nine months ended September 30, 2019, as compared to the nine months ended September 30, 2018
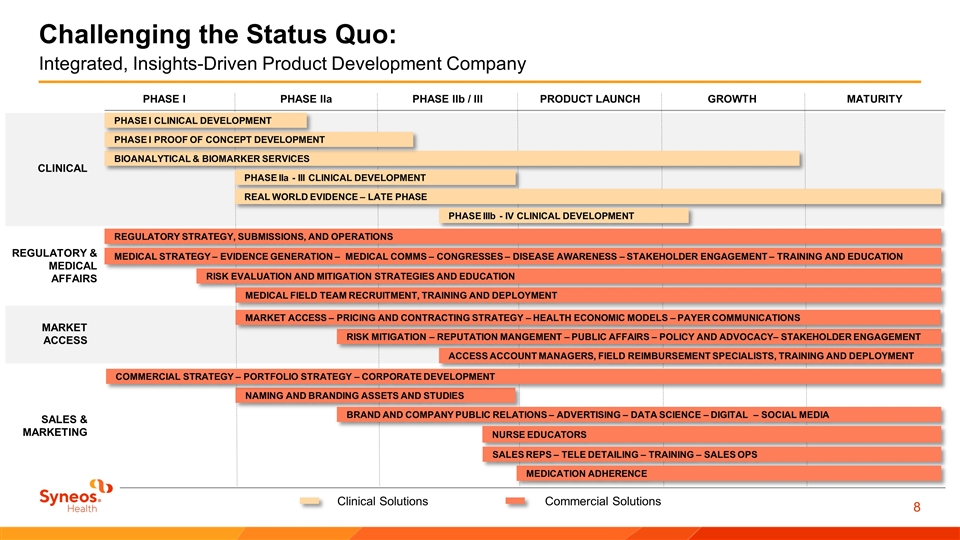
Integrated, Insights-Driven Product Development Company BIOANALYTICAL & BIOMARKER SERVICES PHASE I PROOF OF CONCEPT DEVELOPMENT PHASE I CLINICAL DEVELOPMENT PHASE IIa - III CLINICAL DEVELOPMENT REAL WORLD EVIDENCE – LATE PHASE PHASE IIIb - IV CLINICAL DEVELOPMENT REGULATORY STRATEGY, SUBMISSIONS, AND OPERATIONS NAMING AND BRANDING ASSETS AND STUDIES MEDICAL STRATEGY – EVIDENCE GENERATION – MEDICAL COMMS – CONGRESSES – DISEASE AWARENESS – STAKEHOLDER ENGAGEMENT – TRAINING AND EDUCATION RISK EVALUATION AND MITIGATION STRATEGIES AND EDUCATION BRAND AND COMPANY PUBLIC RELATIONS – ADVERTISING – DATA SCIENCE – DIGITAL – SOCIAL MEDIA RISK MITIGATION – REPUTATION MANGEMENT – PUBLIC AFFAIRS – POLICY AND ADVOCACY– STAKEHOLDER ENGAGEMENT ACCESS ACCOUNT MANAGERS, FIELD REIMBURSEMENT SPECIALISTS, TRAINING AND DEPLOYMENT NURSE EDUCATORS SALES REPS – TELE DETAILING – TRAINING – SALES OPS MEDICATION ADHERENCE MARKET ACCESS – PRICING AND CONTRACTING STRATEGY – HEALTH ECONOMIC MODELS – PAYER COMMUNICATIONS COMMERCIAL STRATEGY – PORTFOLIO STRATEGY – CORPORATE DEVELOPMENT MEDICAL FIELD TEAM RECRUITMENT, TRAINING AND DEPLOYMENT PHASE I PHASE IIa PHASE IIb / III PRODUCT LAUNCH GROWTH MATURITY Clinical Solutions Commercial Solutions Challenging the Status Quo: CLINICAL REGULATORY & MEDICAL AFFAIRS MARKET ACCESS SALES & MARKETING
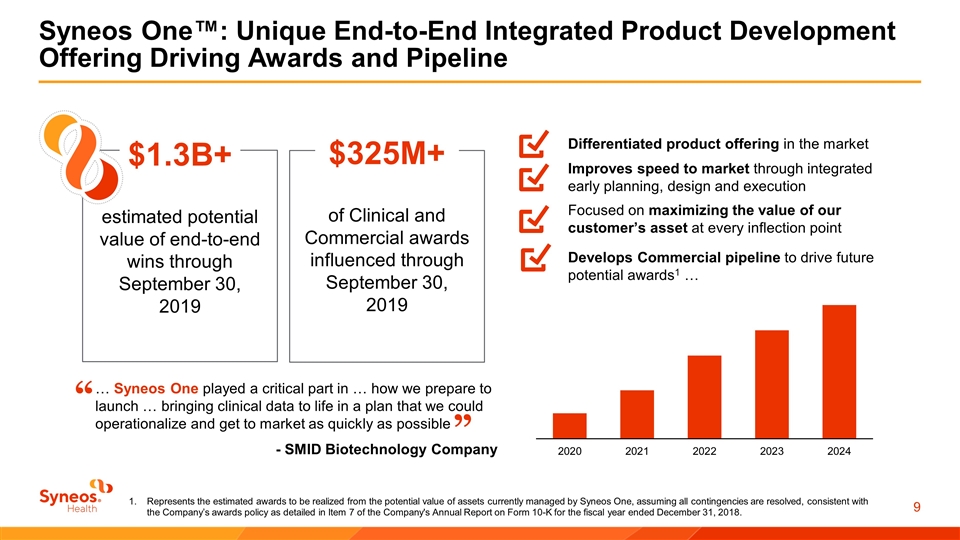
Syneos One™: Unique End-to-End Integrated Product Development Offering Driving Awards and Pipeline $1.3B+ estimated potential value of end-to-end wins through September 30, 2019 $325M+ of Clinical and Commercial awards influenced through September 30, 2019 Differentiated product offering in the market Develops Commercial pipeline to drive future potential awards1 … Improves speed to market through integrated early planning, design and execution Focused on maximizing the value of our customer’s asset at every inflection point … Syneos One played a critical part in … how we prepare to launch … bringing clinical data to life in a plan that we could operationalize and get to market as quickly as possible “ ” - SMID Biotechnology Company Represents the estimated awards to be realized from the potential value of assets currently managed by Syneos One, assuming all contingencies are resolved, consistent with the Company’s awards policy as detailed in Item 7 of the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2018.
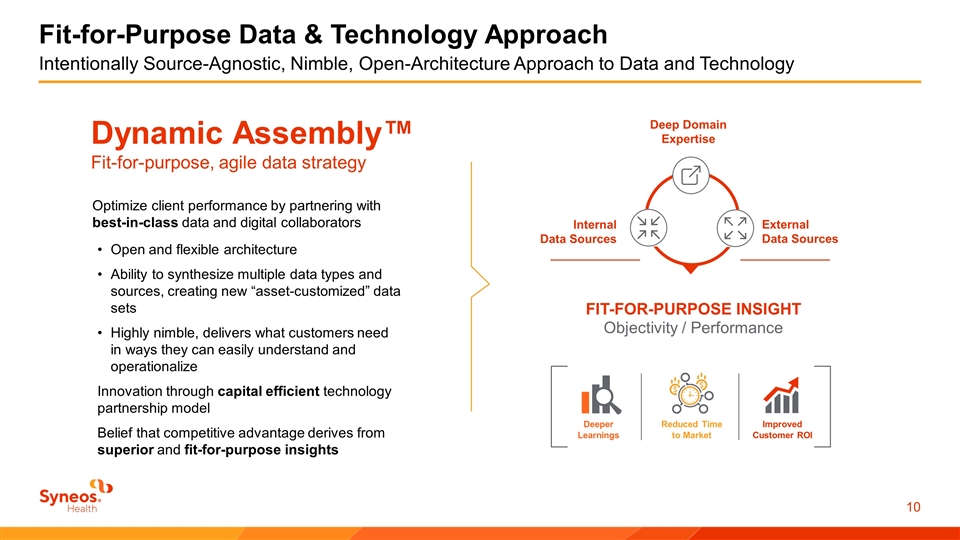
Dynamic Assembly™ Fit-for-purpose, agile data strategy Optimize client performance by partnering with best-in-class data and digital collaborators Open and flexible architecture Ability to synthesize multiple data types and sources, creating new “asset-customized” data sets Highly nimble, delivers what customers need in ways they can easily understand and operationalize Innovation through capital efficient technology partnership model Belief that competitive advantage derives from superior and fit-for-purpose insights Deeper Learnings Reduced Time to Market Improved Customer ROI Internal Data Sources Deep Domain Expertise External Data Sources FIT-FOR-PURPOSE INSIGHT Objectivity / Performance Fit-for-Purpose Data & Technology Approach Intentionally Source-Agnostic, Nimble, Open-Architecture Approach to Data and Technology

Superior Insights and Fit-for-Purpose Technology and Data Best of Breed Providers Strategically Address the Nuances of Unique Customer Engagements Data Technology Therapeutic Expertise … and Global Commercialization Experience
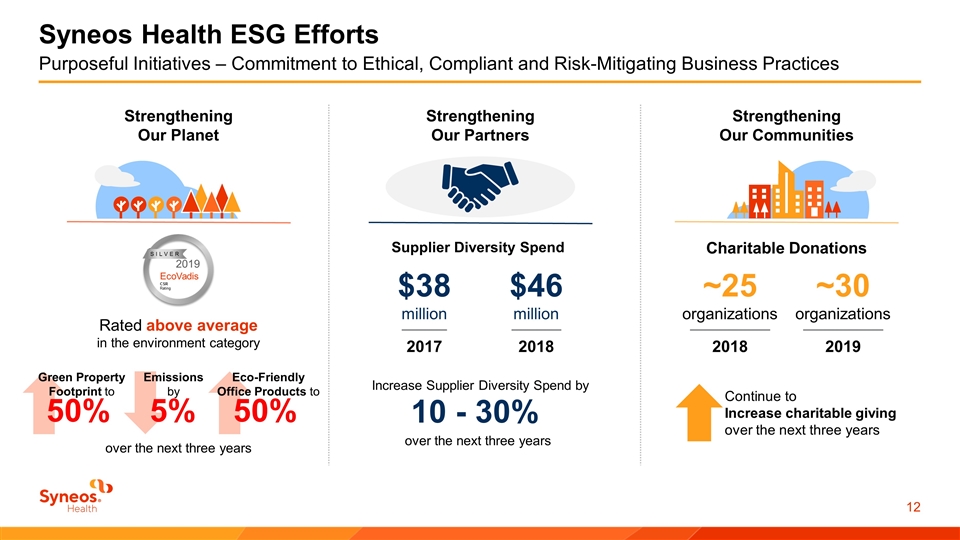
Syneos Health ESG Efforts Purposeful Initiatives – Commitment to Ethical, Compliant and Risk-Mitigating Business Practices Strengthening Our Planet Strengthening Our Partners Strengthening Our Communities Rated above average in the environment category Green Property Footprint to 50% Emissions by 5% Eco-Friendly Office Products to 50% over the next three years Increase Supplier Diversity Spend by 10 - 30% over the next three years Supplier Diversity Spend $38 million $46 million 2017 2018 Continue to Increase charitable giving over the next three years Charitable Donations ~25 organizations ~30 organizations 2018 2019
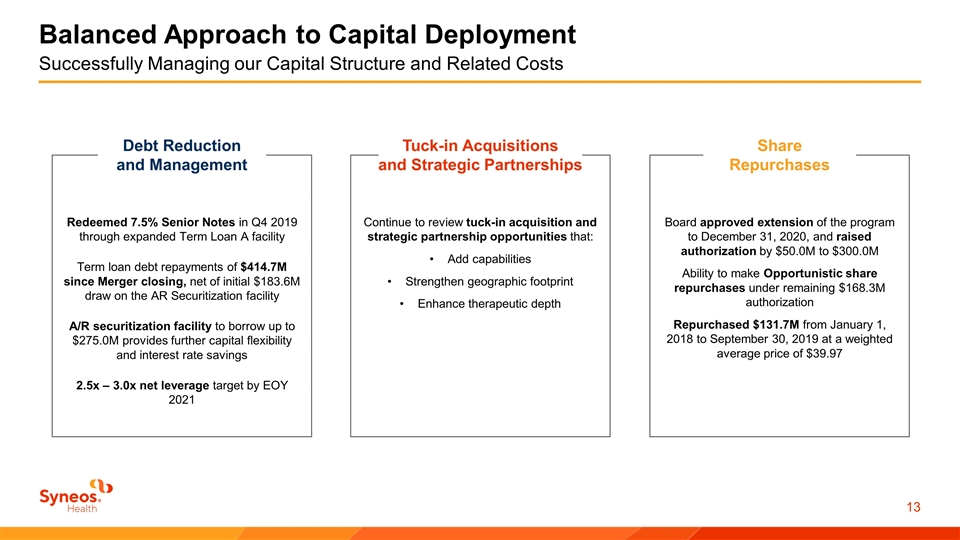
Balanced Approach to Capital Deployment Successfully Managing our Capital Structure and Related Costs Debt Reduction and Management Redeemed 7.5% Senior Notes in Q4 2019 through expanded Term Loan A facility Term loan debt repayments of $414.7M since Merger closing, net of initial $183.6M draw on the AR Securitization facility A/R securitization facility to borrow up to $275.0M provides further capital flexibility and interest rate savings 2.5x – 3.0x net leverage target by EOY 2021 Tuck-in Acquisitions and Strategic Partnerships Continue to review tuck-in acquisition and strategic partnership opportunities that: Add capabilities Strengthen geographic footprint Enhance therapeutic depth Share Repurchases Board approved extension of the program to December 31, 2020, and raised authorization by $50.0M to $300.0M Ability to make Opportunistic share repurchases under remaining $168.3M authorization Repurchased $131.7M from January 1, 2018 to September 30, 2019 at a weighted average price of $39.97
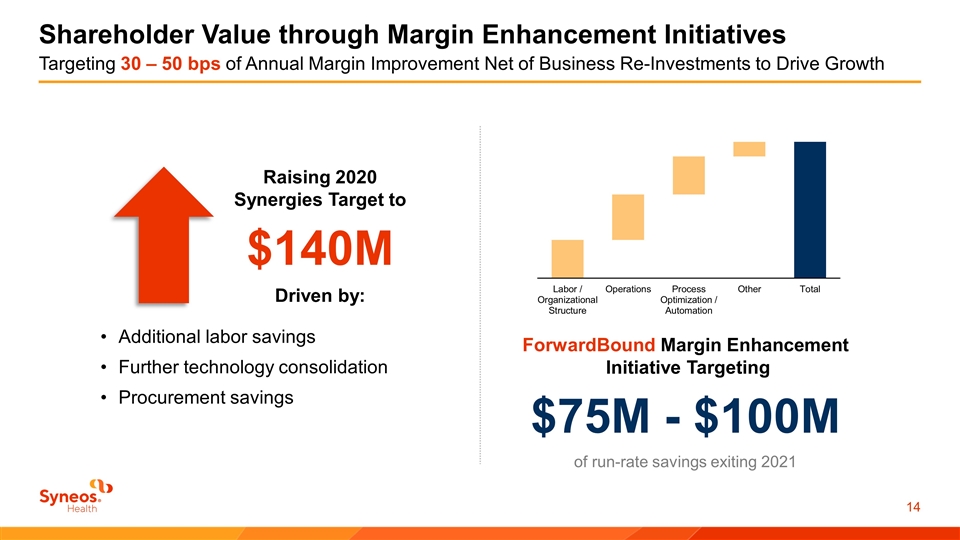
Shareholder Value through Margin Enhancement Initiatives Targeting 30 – 50 bps of Annual Margin Improvement Net of Business Re-Investments to Drive Growth ForwardBound Margin Enhancement Initiative Targeting $75M - $100M of run-rate savings exiting 2021 Additional labor savings Further technology consolidation Procurement savings Raising 2020 Synergies Target to $140M Driven by:
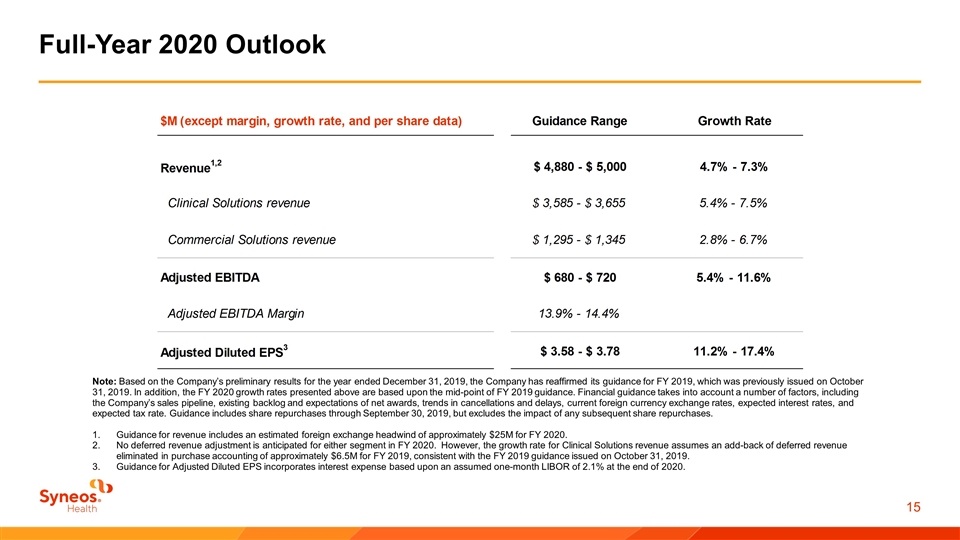
Full-Year 2020 Outlook Note: Based on the Company’s preliminary results for the year ended December 31, 2019, the Company has reaffirmed its guidance for FY 2019, which was previously issued on October 31, 2019. In addition, the FY 2020 growth rates presented above are based upon the mid-point of FY 2019 guidance. Financial guidance takes into account a number of factors, including the Company’s sales pipeline, existing backlog and expectations of net awards, trends in cancellations and delays, current foreign currency exchange rates, expected interest rates, and expected tax rate. Guidance includes share repurchases through September 30, 2019, but excludes the impact of any subsequent share repurchases. Guidance for revenue includes an estimated foreign exchange headwind of approximately $25M for FY 2020. No deferred revenue adjustment is anticipated for either segment in FY 2020. However, the growth rate for Clinical Solutions revenue assumes an add-back of deferred revenue eliminated in purchase accounting of approximately $6.5M for FY 2019, consistent with the FY 2019 guidance issued on October 31, 2019. Guidance for Adjusted Diluted EPS incorporates interest expense based upon an assumed one-month LIBOR of 2.1% at the end of 2020.
Investment Summary: Driving Shareholder Value Comprehensive Product Development solutions Unique Syneos One™ product offering Value creation via Synergies and Margin Enhancement Increasing share with Large Pharma Balanced capital deployment $ $ $ Fit-for-Purpose Insights across Clinical and Commercial


Appendix
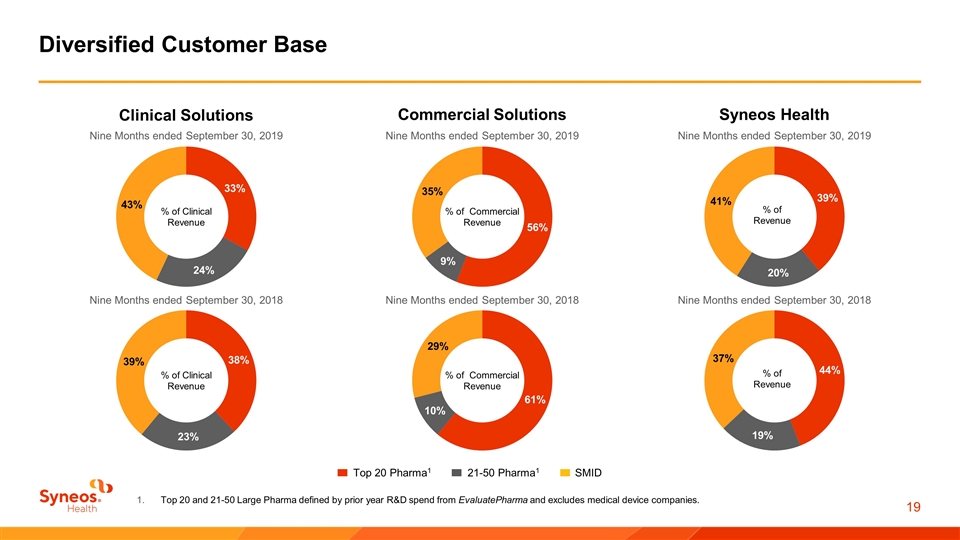
Diversified Customer Base 21-50 Pharma1 Top 20 Pharma1 SMID Top 20 and 21-50 Large Pharma defined by prior year R&D spend from EvaluatePharma and excludes medical device companies. Nine Months ended September 30, 2019 Clinical Solutions % of Clinical Revenue Nine Months ended September 30, 2019 Commercial Solutions % of Commercial Revenue Nine Months ended September 30, 2019 Syneos Health Nine Months ended September 30, 2018 % of Clinical Revenue Nine Months ended September 30, 2018 % of Commercial Revenue Nine Months ended September 30, 2018
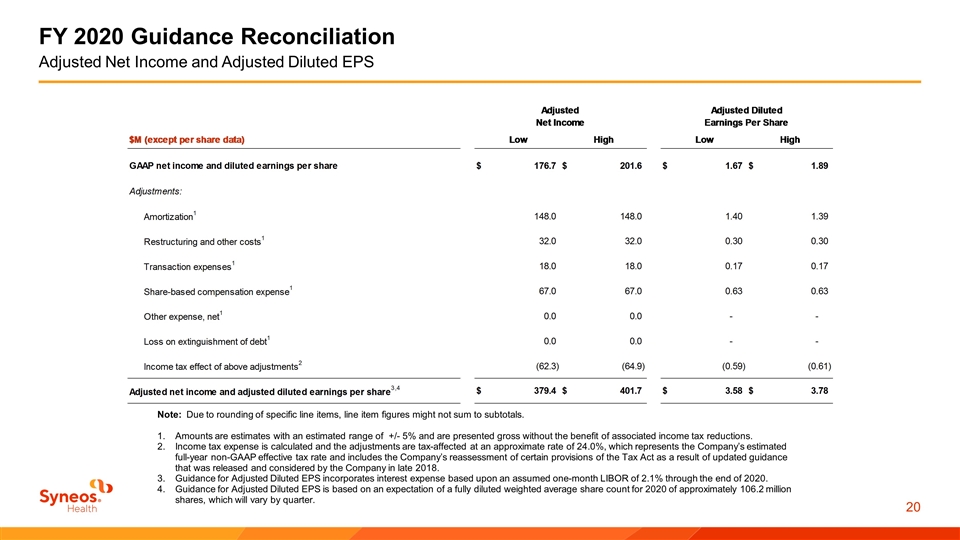
FY 2020 Guidance Reconciliation Adjusted Net Income and Adjusted Diluted EPS Note: Due to rounding of specific line items, line item figures might not sum to subtotals. Amounts are estimates with an estimated range of +/- 5% and are presented gross without the benefit of associated income tax reductions. Income tax expense is calculated and the adjustments are tax-affected at an approximate rate of 24.0%, which represents the Company’s estimated full-year non-GAAP effective tax rate and includes the Company’s reassessment of certain provisions of the Tax Act as a result of updated guidance that was released and considered by the Company in late 2018. Guidance for Adjusted Diluted EPS incorporates interest expense based upon an assumed one-month LIBOR of 2.1% through the end of 2020. Guidance for Adjusted Diluted EPS is based on an expectation of a fully diluted weighted average share count for 2020 of approximately 106.2 million shares, which will vary by quarter.
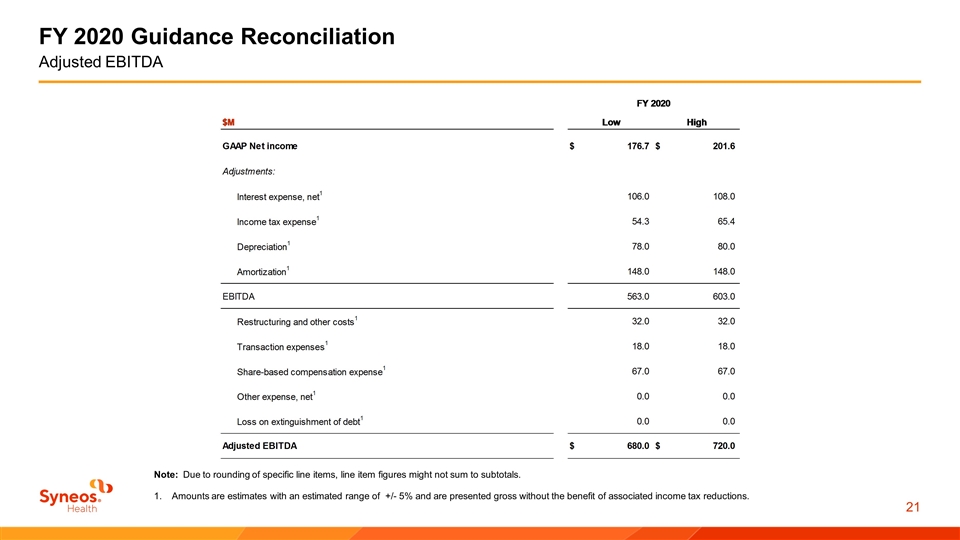
FY 2020 Guidance Reconciliation Adjusted EBITDA Note: Due to rounding of specific line items, line item figures might not sum to subtotals. Amounts are estimates with an estimated range of +/- 5% and are presented gross without the benefit of associated income tax reductions.

