Attached files
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.
20549
FORM 10-K
|
(Mark
One)
|
|
☑
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
|
|
For
the fiscal year ended
September 30, 2019
|
or
|
☐
|
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES
EXCHANGE ACT OF 1934
|
|
For
the transition period
from _______________ to _______________
|
|
Commission
file number:
001-38299
|

|
CBDMD, INC.
|
|
(Exact name of
registrant as specified in its charter)
|
|
North Carolina
|
47-3414576
|
|
(State or other jurisdiction of incorporation or
organization)
|
(I.R.S. Employer Identification No.)
|
8845 Red Oak Blvd, Charlotte, NC 28217
(Address of principal executive offices)(Zip Code)
Registrant's
telephone number, including area code: (704) 445-3060
Securities
registered under Section 12(b) of the Act:
|
Title
of each class
|
Trading
Symbol(s)
|
Name of
each exchange on which registered
|
|
Common stock
|
YCBD
|
NYSE American
|
|
8% Series A Cumulative Convertible Preferred Stock
|
YCBD PR A
|
NYSE American
|
Securities
registered under Section 12(g) of the Act:
|
None
|
|
(Title
of class)
|
Indicate
by check mark if the registrant is a well-known seasoned issuer, as
defined in Rule 405 of the Securities
Act. ☐ Yes ☑ No
Indicate
by check mark if the registrant is not required to file reports
pursuant to Section 13 or Section 15(d) of the
Act. ☐ Yes ☑ No
Indicate
by check mark whether the registrant (1) has filed all reports
required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding 12 months (or for such
shorter period that the registrant was required to file such
reports), and (2) has been subject to such filing requirements for
the past 90 days. ☑
Yes ☐ No
Indicate
by check mark whether the registrant has submitted electronically
every Interactive Data File required to be submitted pursuant to
Rule 405 of Regulation S-T (§232.4.05 of this chapter) during
the preceding 12 months (or for such shorter period that the
registrant was required to submit such
files). ☑ Yes ☐ No
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated filer,
smaller reporting company, or an emerging growth company. See the
definitions of “large accelerated filer,”
“accelerated filer,” “smaller reporting
company,” and “emerging growth company” in Rule
12b-2 of the Exchange Act.
|
Large
accelerated filer
|
☐
|
Accelerated
filer
|
☐
|
|
Non-accelerated
filer
|
☑
|
Smaller
reporting company
|
☑
|
|
Emerging
growth company
|
☑
|
|
|
|
If an emerging growth company, indicate by check mark if the
registrant has elected not to use the extended transition period
for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange
Act.
|
☐
|
Indicate
by check mark whether the registrant is a shell company (as defined
in Rule 12b-2 of the Exchange
Act) ☐ Yes ☑ No
State
the aggregate market value of the voting and non-voting common
equity held by non-affiliates computed by reference to the price at
which the common equity was sold, or the average bid and asked
prices of such common equity, as of the last business day of the
registrant's most recently completed second fiscal quarter.
$42,356,785 on March 31, 2019.
Indicate
the number of shares outstanding of each of the registrant's
classes of common stock, as of the latest practicable date.
27,720,356 shares of common stock are issued and outstanding as of
December 01, 2019.
DOCUMENTS INCORPORATED BY REFERENCE
Part III of this report incorporates by reference certain portions
of the registrant’s definitive Proxy Statement for its 2020
Annual Meeting of Shareholders, which the registrant currently
anticipates will be filed with the SEC on or before January 28,
2020.
TABLE OF CONTENTS
|
|
|
Page No.
|
|
|
|
|
|
|
|
|
|
Business.
|
5
|
|
|
Risk
Factors.
|
12
|
|
|
Unresolved
Staff Comments.
|
23
|
|
|
Properties.
|
23
|
|
|
Legal
Proceedings.
|
23
|
|
|
Mine
Safety Disclosures.
|
23
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Market
for Registrant's Common Equity, Related Stockholder Matters and
Issuer Purchases of Equity Securities.
|
24
|
|
|
Selected
Financial Data.
|
24
|
|
|
Management's
Discussion and Analysis of Financial Condition and Results of
Operations.
|
25
|
|
|
Quantitative
and Qualitative Disclosures About Market Risk.
|
31
|
|
|
Financial
Statements and Supplementary Data.
|
31
|
|
|
Changes
in and Disagreements With Accountants on Accounting and Financial
Disclosure.
|
31
|
|
|
Controls
and Procedures.
|
31
|
|
|
Other
Information.
|
32
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Directors,
Executive Officers and Corporate Governance.
|
33
|
|
|
Executive
Compensation.
|
33
|
|
|
Security
Ownership of Certain Beneficial Owners and Management and Related
Shareholder Matters.
|
33
|
|
|
Certain
Relationships and Related Transactions, and Director
Independence.
|
33
|
|
|
Principal
Accounting Fees and Services.
|
33
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibits,
Financial Statement Schedules.
|
33
|
|
|
Form
10-K Summary
|
33
|
|
|
SIGNATURES
|
|
34
|
Unless
the context otherwise indicates, when used in this report, the
terms the “Company,” “cbdMD, “we,”
“us, “our” and similar terms refer to cbdMD,
Inc., a North Carolina corporation formerly known as Level Brands,
Inc., and our subsidiaries CBD Industries LLC, a North Carolina
limited liability company formerly known as cbdMD LLC, which we
refer to as “CBDI”, Paw CBD, Inc., a recently formed
North Carolina corporation which we refer to as “Paw
CBD”, Beauty and Pinups, LLC, a North Carolina limited
liability company which we refer to as “BPU”, I | M 1,
LLC, a California limited liability company, which we refer to as
“IM1”, Encore Endeavor 1 LLC, a California limited
liability company which we refer to as “EE1,” and Level
H&W, LLC, a North Carolina limited liability company which we
refer to as “Level H&W.” In addition, "fiscal 2018"
refers to the year ended September 30, 2018, “fiscal
2019” refers to the year ended September 30, 2019, and
“fiscal 2020” refers to the year ending September 30,
2020.
We maintain a corporate website at
www.cbdmd.com.
The information contained on our corporate website and our various
social media platforms are not part of this
report.
3
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING
INFORMATION
This
report contains forward-looking statements within the meaning of
Section 27A of the Securities Act of 1933, as amended, or
"Securities Act" and Section 21E of the Securities Exchange Act of
1934, as amended, or “Exchange Act.” These
forward-looking statements are subject to known and unknown risks,
uncertainties and other factors which may cause actual results,
performance or achievements to be materially different from any
future results, performance or achievements expressed or implied by
such forward-looking statements. These forward-looking statements
were based on various factors and were derived utilizing numerous
assumptions and other factors that could cause our actual results
to differ materially from those in the forward-looking statements.
These factors include, but are not limited to:
●
our history of
losses;
●
the impact of
changes in the fair value of our contingent liabilities associated
with the Earnout Shares;
●
the possible need
to raise additional capital in the future;
●
dilution to our
shareholders upon the issuance of the Earnout Shares;
●
changes in state
laws, costs associated with compliance with applicable laws and
potential uncertain changes to regulations impacting our
industry;
●
risks associated
with any future failure to satisfy the NYSE American LLC continued
listing standards;
●
terms of and
provisions of our Series A Convertible Preferred
Stock;
●
risks associated
with developing a liquid market for our Series A Convertible
Preferred Stock and possible future volatility in its trading price
and the trading price of our common stock;
●
risks associated
with our status as an emerging growth company;
●
risks associated
with control by our executive officers, directors and
affiliates;
●
risks associated
with unfavorable research reports;
●
the accounting
treatment of securities we accept as partial compensation for
services;
●
our ability to
liquidate securities we accept as partial compensation for
services;
●
the lack of
long-term contracts for the purchase of our products;
●
our ability to
protect our intellectual property;
●
additional
operational risks associated with our CBD business;
●
dilution to our
shareholders from the issuance of additional shares of common stock
by us, the conversion of shares of our Series A Convertible
Preferred Stock and/or the exercise of outstanding options and
warrants; and
●
risks associated
with our articles of incorporation, bylaws and North Carolina
law.
Most of
these factors are difficult to predict accurately and are generally
beyond our control. You should consider the areas of risk described
in connection with any forward-looking statements that may be made
herein. Readers are cautioned not to place undue reliance on these
forward-looking statements and readers should carefully review this
report and our other filings with the SEC in their entirety. Except
for our ongoing obligations to disclose material information under
the federal securities laws, we undertake no obligation to release
publicly any revisions to any forward-looking statements, to report
events or to report the occurrence of unanticipated events. These
forward-looking statements speak only as of the date of this
report, and you should not rely on these statements without also
considering the risks and uncertainties associated with these
statements and our business.
4
PART I
ITEM
1.
DESCRIPTION
OF BUSINESS.
Our Company
cbdMD
was originally founded in 2015 as an innovative branding and marketing company with a
focus on lifestyle-based brands. In December 2018 pursuant to a
two-step merger (the “Mergers”), we acquired Cure Based
Development LLC ("Cure Based Development”) following the
passage of the United States Agricultural Improvement Act of
2018, commonly known as the “Farm Bill”, which
contained a permanent declassification of cannabidiol (CBD) as a
controlled substance under federal law. As a result of that transaction, we own
and operate the nationally recognized CBD brand cbdMD which now
represents our focus and substantially all of our
revenues.
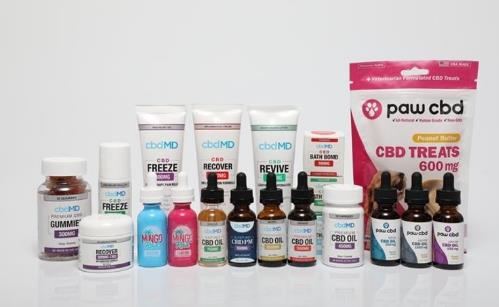
Through
our CBDI subsidiary which succeeded to the business of Cure Based
Development, we produce and distribute
various high-grade, premium CBD products, including tinctures,
capsules, gummies, bath bombs and topical creams. In the third
quarter of fiscal 2019, we launched a line of pet related CBD
products under our Paw CBD brand which includes tinctures, treats,
and balms, with additional products under development. In October
2019, following the initial positive response to the Paw CBD brand
from retailers and consumers, we organized Paw CBD as a separate
wholly-owned subsidiary in an effort to take advantage of its early
mover status in the CBD animal health industry. With over 40
SKU’s of premium pet CBD products for dogs, cats and horses,
we are seeking to grow Paw CBD into a leading brand. We also
anticipate that Paw CBD will also partner with existing pet brands
seeking to leverage our experience in sales, marketing and
distribution in the CBD industry.
We
either manufacture our premium line of products at our Charlotte,
NC facility or work with third party manufacturers. We only
source cannabinoids, including CBD, which are extracted
from non-GMO hemp grown on farms in the United
States. We utilize a manufacturing process which
creates hybrid broad-spectrum concentrations including CBD, other
cannabinoids, and various other compounds, which we believe creates
a superior product, while eliminating tetrahydrocannabinol
(THC) content.
5

Since
December 2018, we have significantly increased the number of locations cbdMD products
are available in, and with the building momentum of retailer
acceptance subsequent to the passage of the Farm Bill, we are
pursuing multiple opportunities to expand our product distribution
as we continue to work to build cbdMD into a top recognized brand
in the industry. We are also utilizing partnerships and
sponsorships with professional athletes as a way to gain brand
recognition. During the third quarter of fiscal 2019 we signed
several significant sponsorships, including, but not limited to,
professional golfer and 12-time PGA Tour winner Bubba Watson, Ice
Cube’s Big 3 basketball league, Bellator MMA, and Nitro
Circus.

Discontinued operations
Historically our operations included our BPU, EE1,
IM1 and Level H&W subsidiaries. These subsidiaries
accounted for our licensing, entertainment, and products segments
prior to fiscal 2019. As revenues from
the operations of these subsidiaries continued to decline in fiscal
2019 from fiscal 2018 levels, and the revenues associated with CBDI
began to represent substantially all of our revenues,
effective September 30, 2019, we discontinued operations of
EE1, IM1, BPU and Level H&W. The
decision to exit these businesses has resulted in these businesses
being accounted for as discontinued operations in our audited
consolidated financial statements appearing later in this
report. Continuing operations discussed below, now reported
as one segment, refer to our CBD business unless otherwise
indicated, and prior periods in such discussion have been restated
to reflect results excluding EE1, IM1, BPU and Level H&W. See
Note 15, “Discontinued Operations”, of the Notes to
consolidated financial statements elsewhere in this report for
additional disclosures related to the
reclassification of prior period amounts related to discontinued
operations to conform to the current period financial statement
presentation.
6
Growth Strategies and Outlook
With
the passage of the Farm Bill in December 2018, which removed hemp from the
Controlled Substances Act, making it no longer an illegal substance under
federal law, an opportunity to strategically position the Company
in an emerging market was presented. With the acquisition of Cure
Based Development described later in this report, we believe we
have established a solid foundation which will allow us to
significantly grow the Company and become a leader in a new
market.
We are
pursuing the following strategies to continue to grow our revenues
and expand our business and operations in fiscal 2020 and
beyond:
●
Increase our base of product offerings:
We currently have a broad offering of CBD products, including
topicals, tinctures, gummies, bath bombs, vape oils, capsules, and
pet products and continue to evaluate additional offerings within
these categories as well as new ways to provide CBD in a manner
that meets consumer demands. To that end we are devoting resources
to ongoing research and development processes with the goal of
expanding our product offerings to meet these expanding consumer
demands;
●
Expand our sales channels: As the
market continues to evolve, we are expanding our sales channels.
During fiscal 2019, we moved from a 100% online sales channel to
working with wholesalers and retail channels. Big box retailers are
beginning to explore CBD products and we believe this will provide
another significant opportunity. In addition, sales channels for
the pet line include expanding to not only retail stores but
veterinary and pet care operations;
●
Expand our recently formed CBD animal health
division: With the formation in October 2019 of Paw CBD as a
separate wholly-owned subsidiary, we have committed resources to
branding and marketing the Paw CBD product line, which we believe
will enable us to more effectively target new sales channels as
well as utilize our marketing efforts in a more defined manner that
we believe will generate other sales opportunities;
●
Expand our sponsorships toward targeted
segments: We have had significant success with attracting
high profile sponsors and influencers and expect to continue to
assess the segments we have covered as well as additional sponsors
for segments we do not cover but we believe could provide
opportunity for growth; and
●
Acquisitions. We may also choose to
further build and maintain our brand portfolio by acquiring
additional brands directly or through joint ventures if
opportunities arise that we believe are in our best interests. As
we are in an emerging market, opportunities could be present as
companies establish strong brands and begin to obtain large market
share. In assessing potential acquisitions or investments, we
expect to utilize our internal resources to primarily evaluate
growth potential, the strength of the target brand, offerings of
the target, as well as possible efficiencies to gain. We believe
that this approach will allow us to effectively screen consumer
brand candidates and strategically evaluate acquisition targets and
efficiently complete due diligence for potential acquisitions. We
are not a party, however at this time, to any agreements or
understandings regarding the acquisition of additional brands or
companies and there are no assurances we will be successful in
expanding our brand portfolio.
Our business
cbdMD
produces and distributes
various high-grade, premium CBD products, including:
●
tinctures;
●
capsules;
●
gummies;
●
bath
bombs;
●
topical
creams; and
●
animal
treats and oils.
7
cbdMD’s
website provides up-to-date information about CBD quality and
current industry events, as well as U.S. based customer service. We
test our hybrid broad-spectrum CBD extractions through independent,
third-party laboratories in an effort to achieve the highest of
standards and we offer a 30-day, money-back guarantee. Our
products are available online at
www.cbdMD.com and over 4,000 non-affiliated
stores.
Sales and marketing
Our
business model is designed first and foremost to build a strong
brand as quickly as possible as we operate in an emerging market.
As CBD is a new market, we believe the cost to acquire customers is
most likely at the lowest point it will be, therefore, as part of
our overall strategy we started with an internet and online
marketing strategy and are investing heavily in brand promotion and
customer acquisition. In addition, we have been executing on our
strategy to build and now expand our wholesale distribution channel
as part of the overall business model.
Sales
of our products mainly come from online sales at our website
www.cbdmd.com
and through our inside sales department which concentrates on
wholesale distributors, who we believe can offer large quantities
of cbdMD products at physical retail locations. cbdMD utilizes a
broad-based marketing strategy across multiple platforms
including:
●
secured
online and print advertising;
●
blog,
web, and visual content;
●
social
media engagement through accounts with large
followings;
●
live
and sponsored events;
●
influencers
and celebrities;
●
affiliate
marketing; and
●
high-quality
brand apparel.
Product manufacturing and distribution
We
either manufacture our premium line of products at our Charlotte,
NC facility or work with third party manufacturers. We only
source cannabinoids, including CBD, which are extracted
from non-GMO hemp grown on farms in the United
States. We utilize a manufacturing process which
creates hybrid broad-spectrum concentrations including CBD, other
cannabinoids, and various other compounds, which we believe creates
a superior product while eliminating tetrahydrocannabinol
(THC) content.
We
currently hold short term supply contracts with preferred
vendors for our critical raw materials. We have
first rights of refusal on purchases so that we can ensure the
availability of such materials as needed. Our preferred
vendors are extractors and brokers who have access to the sources
of the critical raw materials on the spot buy market to meet our
growing demands for raw materials.
We
distribute our products both through our online sales channel as
well as through a number of wholesalers and retailers that resell
our products both in brick and mortar locations as well as via
their online websites.
Research and development
The key
objectives and input points that drive cbdMD’s research and
development process include current product and new product
development activities:
Our
current product improvement efforts include:
●
consumer
feedback analysis;
●
optimization
of its product manufacturing process;
●
sourcing
of reliable, high-quality raw materials while maintaining strong
distributor relationships;
●
lab
testing; and
●
feedback
from panels of product testers.
8
Our new
product development efforts are focused on both near-term and
long-term results for the company:
With a
view toward near-term results, our efforts include:
●
in-depth
market research on current competitor campaigns;
●
website
and product development;
●
sample
size testing and research;
●
implementation
of new product campaigns utilizing social media, digital, affiliate
and email;
●
rapid
product development following testing; and
●
quality
ingredient sourcing.
With a
view towards long-term results, our efforts include:
●
development
of new product lines following initial market
research;
●
use of
current and expected future product trends and research to develop
new product offerings;
●
refinement
of extraction and production methods for product
efficiency;
●
ingredient
research through sustainability testing;
●
manufacturing
process optimization;
●
in-depth
product testing;
●
package
and graphic development; and
●
large
scale product and brand marketing campaigns.
In
fiscal 2019 we introduced CBD PM and a pet line branded under Paw
cbd with over 40 SKU’s.
Intellectual property
We
currently hold fourteen U.S. trademark applications which are held
for current and future product offerings and extended branding
capability, which are set forth below:
|
Mark
|
|
Federal
registration number
|
|
Filing
Date
|
|
Description of
Mark Usage
|
|
CBDMD
Synergy
|
|
87613823
|
|
9/19/2017
|
|
Standard
word mark
|
|
Synergy
CBDMD
|
|
87613850
|
|
9/19/2017
|
|
Standard
word mark
|
|
CBD
Synergy
|
|
87613876
|
|
9/19/2017
|
|
Standard
word mark
|
|
CBD
Paws
|
|
87614081
|
|
9/19/2017
|
|
Standard
word mark
|
|
Hemp
Synergy
|
|
87625996
|
|
9/28/2017
|
|
Standard
word mark
|
|
Mingo
Rad
|
|
88010754
|
|
6/22/2018
|
|
Standard
word mark
|
|
Powered
by Nature. Enhanced by Science
|
|
88363595
|
|
3/29/2019
|
|
Standard
word mark
|
|
cbdMD
|
|
88451429
|
|
5/29/2019
|
|
Stylized
word mark (logo in color)
|
|
cbdMD
Premium CBD Oil
|
|
88451556
|
|
5/29/2019
|
|
Standard
word mark
|
|
CBDMD
Premium
|
|
88451595
|
|
5/29/2019
|
|
Design
mark
|
|
paw
cbd
|
|
88451641
|
|
5/29/2019
|
|
Design
mark
|
|
Paw
cbd
|
|
88451671
|
|
5/29/2019
|
|
Standard
word mark
|
|
Hemplex
Naturals
|
|
88575252
|
|
8/12/2019
|
|
Standard
word mark
|
|
Hemp
MD
|
|
88109782
|
|
9/09/2019
|
|
Standard
word mark
|
In
addition to these trademarks, we currently rely on a combination of
trade secret laws and restrictions on disclosure to protect our
intellectual property rights. Our success depends on the protection
of the proprietary aspects of our product development, formulation
and knowhow, as well as our ability to operate without infringing
on the proprietary rights of others. We also enter into proprietary
information and confidentiality agreements with our employees,
consultants and commercial partners and control access to, and
distribution of, our formulas and other proprietary
information.
9
In
addition to www.cbdmd.com
and www.pawcbd.com,
we own multiple domain names that we may or may not operate in the
future. However, as with phone numbers, we do not have and cannot
acquire any property rights in an Internet address. The regulation
of domain names in the United States and in other countries is
subject to change. Regulatory bodies could establish additional
top-level domains, appoint additional domain name registrars or
modify the requirements for holding domain names. As a result, we
might not be able to maintain our domain names or obtain comparable
domain names, which could harm our business.
Competition
The market for the sale of CBD-based products is
fragmented and intensely competitive. Currently, in the United
States, cbdMD does not believe that there are any businesses that
can demonstrate or claim a dominant market share of the
growing CBD products market.
Our competitors in the retail location sales of CBD-based
products include Green Roads, PlusCBD, and Select CBD, and in the
digital space include Diamond CBD, CBDistillery, and Lazarus
Naturals. We believe we compete based upon the quality of our
products. We expect that the quantity
and composition of the competitive environment will continue to
evolve as the industry matures and new customers enter the
marketplace.
Government regulations
On
December 20, 2018 the President of the United States signed the
Farm Bill into law. Among
other things, this new law changed certain federal authorities
relating to the production and marketing of hemp, defined as
cannabis (Cannabis sativa
L.), and derivatives
of cannabis with extremely low (less than 0.3 percent on a dry
weight basis) concentrations of the psychoactive compound
delta-9-tetrahydrocannabinol (THC). These changes include
removing hemp and derivatives of hemp from the Controlled
Substances Act, which means that it is no longer an illegal
substance under federal law. For the first time since 1937,
industrial hemp has been legalized at the federal level and this
paved the way for the growth of the industry. With the recent
publication of the USDA interim final rule regarding the
Establishment of a Domestic Hemp Production Program on October 31,
2019, hemp can now be grown and processed legally in the United
States, and is legal to transport in interstate commerce. Although
this interim final rule became effective on the date of
publication, it is still subject to comment and there is a
possibility it will be modified from its current
application.
The
Farm Bill recognizes hemp as distinct from its genetic cousin,
marijuana, and specifically industrial hemp has been excluded from
U.S. drug laws. The Farm Bill allows for each individual
state to regulate industrial hemp and industrial hemp based
products or accept the USDA rules. Although no longer a
controlled substance under federal law, cannabinoids derived from
industrial hemp (other than THC) are still subject to a patchwork
of state regulations. We are actively monitoring the
regulations and proposed regulations in each state to ensure our
operations are compliant.
In
conjunction with the enactment of the Farm Bill, the United States
Food and Drug Administration (“FDA”) released a
statement about the status of CBD and the agency’s actions in
the short term with regards to CBD will guide the industry.
The statement noted that
the Farm Bill explicitly preserved the FDA’s authority to
regulate products containing cannabis or cannabis-derived compounds
under the Federal Food, Drug, and Cosmetic Act (FD&C Act) and
Section 351 of the Public Health Service Act. This authority allows
the FDA to continue enforcing the law to protect patients and the
public while also providing potential regulatory pathways for
products containing cannabis and cannabis-derived compounds.
The statement also noted the growing public interest in cannabis
and cannabis-derived products, including CBD, and informed the
public that the FDA will treat products containing cannabis or
cannabis-derived compounds as it does any other FDA-regulated
products — meaning the products will be subject to the same
authorities and requirements as FDA-regulated products containing
any other substance, regardless of the source of the substance,
including whether the substance is derived from a plant that is
classified as hemp under the Farm Bill.
Recently, both the
U.S. House of Representatives (McNerney CA-09) and the Senate
(McConnell R-KY) have passed amendments to the respective
appropriations bills working their way through each chamber, which
would help clear the way for cannabidiol (CBD) to become an
approved dietary ingredient by the FDA through an alternative rule
making process available to the FDA. Although it is uncertain
if either of these amendments will make it to the final federal
appropriation bill, or if the President will ultimately sign the
appropriations bill, this signifies the legislatures clear intent
to pave a pathway for clear and consistent federal regulation for
cannabidiol.
10
As of
the date of this report, and based upon publicly available
information, to our knowledge the FDA has not taken any enforcement
actions against CBD companies. The FDA, however, has sent warning
letters to companies demanding they cease and desist from the
production, distribution, or advertising of CBD products, only
relating to instances that such CBD companies have made misleading
and unapproved label claims. We will continue to monitor the
FDA’s position on CBD.
As a
consumer goods manufacturer, we strive to meet or exceed the FDAs
Good Manufacturing Practice (GMP) guidelines. With our growth and
evolution, challenges could exist and we must continue to review
processes and controls and adapt our day to day GMP policies and
practices as our manufacturing volume increases. We are
dedicated to providing the highest quality CBD consumer goods on
the market and therefore will continue to focus substantial efforts
on GMP compliance. We are currently undergoing third party audit
and consulting to ensure we are using best practices and we intend
to seek multiple third party certifications possibly as early as
the second quarter of 2020 from NSF and the U.S. Hemp
Authority. Additionally, we have secured third party contract
manufacturing from an FDA registered facility which is GMP
certified and subject to continuing independent audit and
certification, to handle our increased manufacturing
needs.
We are
subject to federal and state consumer protection laws, including
laws protecting the privacy of customer non-public information and
the handling of customer complaints and regulations prohibiting
unfair and deceptive trade practices. The growth and demand for
online commerce has and may continue to result in more stringent
consumer protection laws that impose additional compliance burdens
on online companies. These laws may cover issues such as user
privacy, spyware and the tracking of consumer activities, marketing
e-mails and communications, other advertising and promotional
practices, money transfers, pricing, product safety, content and
quality of products and services, taxation, electronic contracts
and other communications and information security.
There
is also great uncertainty over whether or how existing laws
governing issues such as sales and other taxes, auctions, libel and
personal privacy apply to the internet and commercial online
services. These issues may take years to resolve. For example, tax
authorities in a number of states, as well as a Congressional
advisory commission, are currently reviewing the appropriate tax
treatment of companies engaged in online commerce, and new state
tax regulations may subject us to additional state sales and income
taxes. New legislation or regulation, the application of laws and
regulations from jurisdictions whose laws do not currently apply to
our business or the application of existing laws and regulations to
the internet and commercial online services could result in
significant additional taxes or regulatory restrictions on our
business. These taxes or restrictions could have an adverse effect
on our cash flows, results of operations and overall financial
condition. Furthermore, there is a possibility that we may be
subject to significant fines or other payments for any past
failures to comply with these requirements.
Employees
At
December 01, 2019 we had 182 full-time employees. There are no
collective bargaining agreements covering any of our
employees.
Our history
Our company was formed under the laws of the state
of North Carolina in March 2015 under the name Level Beauty Group,
Inc. In November 2016 we changed the name of our parent company to
Level Brands, Inc. In December 2016 we effected a 1:5 reverse stock
split of our outstanding common stock. In December
2018, we completed the Mergers of Cure Based Development into our
newly formed and wholly owned subsidiary cbdMD LLC. As part of the
overall business strategy, in April 2019 we renamed cbdMD LLC to
CBD Industries LLC and renamed Level Brands, Inc. to cbdMD, Inc. In
October 2019 we formed a wholly owned subsidiary Paw CBD, Inc.
which will operate the CBD pet line.
Additional
information on the terms of material acquisitions may be found in
Note 2 to the notes to our consolidated financial statements
appearing elsewhere in this report.
Additional information
We file
annual and quarterly reports on Forms 10-K and 10-Q, current
reports on Form 8-K and other information with the Securities and
Exchange Commission (“SEC” or the
“Commission”). The Commission also maintains an
Internet site at http://www.sec.gov that
contains reports, proxy and information statements, and other
information regarding issuers that file electronically with the
Commission.
Other
information about cbdMD can be found on our website www.cbdmd.com.
Reference in this document to that website address does not
constitute incorporation by reference of the information contained
on the website.
11
ITEM
1.A
RISK
FACTORS.
Investing in our securities involves risks. In addition to the
other information contained in this report, you should carefully
consider the following risks before deciding to purchase our
securities. The occurrence of any of the following risks might
cause you to lose all or a part of your investment. Some statements
in this report, including statements in the following risk factors,
constitute forward-looking statements. Please refer to
“Cautionary Statement Regarding Forward-Looking
Statements” for more information regarding forward-looking
statements.
RISKS RELATED TO OUR OVERALL BUSINESS
We have a history of losses and there are no assurances we will
report profitable operations in future periods.
We
reported net losses to common shareholders of $50,428,226 and
$412,075 for fiscal 2019 and fiscal 2018, respectively. Included in
our net loss for fiscal 2019 is $5,993,773 associated with losses
from our discontinued operations, and an expense reflecting an
increase of $32,461,680 in the non-cash contingent liability
associated with the Earnout Shares (as hereinafter defined)
primarily as a result of the change in the market price of our
common stock . Until such time, if ever, that we are successful in
generating profits which are sufficient to pay our operating
expenses it is likely we will continue to report losses in future
periods. There are no assurances we will generate substantial
revenues from the new businesses or that we will ever generate
sufficient revenues to report profitable operations or a net
profit.
We have a limited operating history in a new market segment that
could impede our ability to achieve our goals for future
performance and brand strategy.
Our
wholly-owned subsidiary, CBDI, succeeded to the operations of Cure
Based Development following the closing of the Mergers in December
2018. We formed this subsidiary in connection with the Mergers and
it had no operating history prior to the Mergers. Cure Based
Development was formed in 2017 and did not begin reporting any
meaningful revenues until mid-2018. We are operating in a market
segment that has experienced significant growth and competition
since the passage of the Farm Bill in December 2018. We are
experiencing all of the challenges of a company with a limited
operating history in its current business seeking to effectively
develop a brand in a market segment with growing competition. These
challenges make it difficult for investors to evaluate our future
business prospects and make decisions based on estimates of our
future performance. To address these risks and uncertainties, we
must do the following:
●
successfully
execute our business strategy to offer the highest quality CBD in
the industry;
●
introduce
new, differentiated botanical products;
●
respond
to competitive business developments;
●
effectively
and efficiently market and sell our line of CBD
products;
●
improve
the distribution of our CBD products; and
●
attract,
integrate, retain and motivate qualified personnel.
During
fiscal 2019 we spent approximately $7,831,000 on brand development
and marketing. In order to continue to develop brand recognition
and grow our sales, we will need to continue to invest in our brand
development and marketing strategies which will require us to raise
additional capital. Even with our focus on brand development in an
effort to increase our sales, our business strategy may not be
successful and we may not successfully address these risks. In the
event that we do not successfully address these risks, our
business, prospects, financial condition and results of operations
may be materially and adversely affected.
12
The impact of changes in the fair value of our contingent
liabilities associated with the Earnout Shares may materially
impact our results of operations in future periods.
As
consideration for the Mergers, we had a contractual obligation,
after approval by our shareholders, to issue 15,250,000 shares of
our common stock (the “Initial Shares”) to the members
of Cure Based Development of which 8,750,000 of the shares will
vest over a five year period and are subject to a voting proxy
agreement, as well as to issue another 15,250,000 shares of our
common stock (the “Earnout Shares”) in the future upon
earnout goals being met within the next five years. Our
shareholders approved the issuance of the both the Initial Shares
and the Earnout Shares at our 2019 annual shareholder meeting and
the Initial Shares were issued to members of Cure Based Development
on April 19, 2019. Under US GAAP we were required to record a
non-cash contingent liability associated with both the Initial
Shares and the Earnout Shares. As of September 30, 2019, the
Initial Shares have been issued and have been reclassified from
contingent liability to additional paid in capital. At September
30, 2019, the total of this contingent liability was $32,461,680
and is reflective of the Earnout Shares. We are obligated to
reassess the obligations associated with the Earnout Shares and, in
the event our estimate of the fair value of the contingent
consideration changes, we will record increases or decreases in the
fair value as an adjustment to earnings, which could have a
material impact on our results of operations, our
shareholders’ equity and the market price of our securities.
In particular, changes in the market price of our common stock,
which is one of the inputs used in determining the amount of the
non-cash contingent liability, will result in increases or
decreases in this liability and positively or negatively impact our
net loss or profit for the period. Investors should not place undue
reliance on the impact of these non-cash changes when evaluating
our results of operations in future periods, as they have no impact
on the operations of the business.
We may require additional capital to finance operations or the
acquisition of additional brands, and if we are unable to raise
such capital on beneficial terms this could restrict our
growth.
We
may, in the future, require additional capital to help fund all or
part of our operation or for potential acquisitions. If, at the
time required, we do not have sufficient cash to finance those
additional capital needs, we will need to raise additional funds
through equity and/or debt financing. We cannot guarantee that, if
and when needed, additional financing will be available to us on
acceptable terms or at all. Further, if additional capital is
needed and is either unavailable or cost prohibitive, our growth
may be limited as we may need to change our business strategy to
slow the rate of our expansion plans. In addition, any additional
financing we undertake could impose additional covenants upon us
that restrict our operating flexibility, and, if we issue equity
securities to raise capital or as acquisition consideration, our
existing shareholders may experience dilution or the new securities
may have rights senior to those of our 8% Series A Cumulative
Convertible Preferred Stock (“Series A Convertible Preferred
Stock”), assuming the holders of the Series A Convertible
Preferred Stock approve the issuance of such senior
securities.
The issuances of the Earnout Shares to the Cure Based Development
members will significantly dilute our existing
shareholders.
Upon
the terms set forth in the Merger Agreement, on the closing date
the members of Cure Based Development received the right to receive
15,250,000 Initial Shares, representing approximately 60% of our
outstanding common stock following such issuance at the time, as
the consideration for the Mergers. The Merger Agreement also
provides that we may issue up to an additional 15,250,000 Earnout
Shares as part of the Mergers consideration upon the satisfaction
of certain aggregate net revenue criteria by cbdMD within 60 months
following the closing date. As of December 1, 2019, there were
27,720,356 shares of our common stock issued and outstanding, which
includes the 15,250,000 Initial Shares which were issued in April
2019. Assuming the possible issuance of an additional 15,250,000
Earnout Shares, but giving effect to no other change to the number
of shares of our common stock issued and outstanding, the members
of Cure Based Development, which includes Mr. R. Scott Coffman, our
co-CEO and member of our board of directors, would own
approximately 64% of our then outstanding shares of common stock.
Therefore, the ownership and voting rights of our existing
shareholders will be proportionally reduced.
13
We may be unable to liquidate securities we accept as partial
compensation under consulting agreements which could adversely
impact our liquidity in future periods.
Our
ability to sell any securities we had accepted as partial
compensation under consulting agreements is dependent upon a number
of factors, including the existence of a liquid market for the
securities and our compliance with the resale provisions of federal
securities laws which require us to hold the shares for at least
six months, among other factors. If we accept securities, we expect
to generally accept securities from issuers who are publicly traded
or who are expecting to become a publicly traded company. There are
no assurances a liquid market will exist in such securities at such
time as we are able to resell the shares, or that the price we may
receive will be commensurate with the value of the services we are
providing. In that event, we would not benefit from the expected
rise in the market price of the securities we own as a result of
our efforts on behalf of the client company. In addition, depending
upon the terms of our business relationship with the issuer of the
securities, it is possible that from time to time we could be in
possession of non-public information regarding the issuer which
could prohibit us from disposing of the shares at a time when it is
advantageous to us to do so. If we are unable to readily liquidate
any securities we accept as compensation, we could be deprived of
the cash value of those services and we would be required to
write-off the carrying value of the securities which could
adversely impact our results of operations in future
periods.
We rely on third-parties to manufacture and to compound some of our
products, we have no control over these manufactures and may not be
able to obtain quality products on a timely basis or in sufficient
quantity.
Some
of our products are manufactured or compounded by unaffiliated
third parties. We do not have any long-term contracts with any of
these third parties, and we expect to compete with other companies
for raw materials, production and import capacity. If we experience
significant increased demand, or need to replace an existing
manufacturer, there can be no assurance that additional
manufacturing capacity will be available when required on terms
that are acceptable to us, or at all, or that any manufacturer or
compounder would allocate sufficient capacity to us in order to
meet our requirements. In addition, even if we are able to expand
existing or find new sources, we may encounter delays in production
and added costs as a result of the time it takes to engage third
parties. Any delays, interruption or increased costs in the
manufacturing or compounding of our products could have an adverse
effect on our ability to meet retail customer and consumer demand
for our products and result in lower revenues and net income both
in the short and long-term.
We face risks associated with the manufacture of our products which
could adversely affect our business and financial
results.
We
are subject to the risks inherent in manufacturing our products,
including industrial accidents, environmental events, strikes and
other labor disputes, disruptions in supply chain or information
systems, loss or impairment of key manufacturing sites or
suppliers, product quality control, safety, increase in commodity
prices and energy costs, licensing requirements and other
regulatory issues, as well as natural disasters and other external
factors over which we have no control. If such an event were to
occur, it could have an adverse effect on our business and
financial results.
We could experience losses related to undisclosed liabilities of
EE1 and I’M1.
Effective September
30, 2019 we discontinued the operations of EE1 and
I’M1. In November 2019 we entered into a Settlement and
Release Agreement with the minority holders of EE1 and I’M1
and other related parties. The terms of this agreement
provided, in part, that the parties agreed to transfer the accounts
receivable of EE1 and the minority interest of both EE1 and IM1 to
us and we agreed to have all rights to certain past contracts or
customers for those entities assigned to the minority
holders. As a result, we are now the sole member of these
entities. While the Settlement and Release Agreement contains
an indemnification provision, if we should discover in the future
that there are undisclosed liabilities in one or both of these
entities, we could be liable for the payment of these undisclosed
liabilities, which could be material , until such time, if ever,
that we were able to recoup any such amounts from the indemnifying
parties.
14
Risks Related to the Regulatory Environment
Changes to state laws pertaining to industrial hemp could slow the
use of industrial hemp which would materially impact our revenues
in future periods.
As of
the date hereof, approximately 40 states authorized industrial hemp
programs pursuant to the Farm Bill. Continued development of the
industrial hemp industry will be dependent upon new legislative
authorization of industrial hemp at the state level, and further
amendment or supplementation of legislation at the federal level.
Any number of events or occurrences could slow or halt progress all
together in this space. While progress within the industrial hemp
industry is currently encouraging, growth is not assured. While
there appears to be ample public support for favorable legislative
action, numerous factors may impact or negatively affect the
legislative process(es) within the various states where we have
business interests. Any one of these factors could slow or halt use
of industrial hemp, which could negatively impact the business up
to possibly causing us to discontinue operations as a
whole.
Costs associated with compliance with numerous laws and regulations
could impact our financial results. In addition, we could become
subject to increased litigation risks associated with the CBD
industry.
The
manufacture, labeling and distribution by us of the CBD products is
regulated by various federal, state and local agencies. These
governmental authorities may commence regulatory or legal
proceedings, which could restrict the permissible scope of our
product claims or the ability to sell products in the future. The
FDA may regulate our products to ensure that the products are not
adulterated or misbranded. We are subject to regulation by the
federal government andother state and local agencies as a result of
our CBD products. The shifting compliance environment and the need
to build and maintain robust systems to comply with different
compliance in multiple jurisdictions increases the possibility that
we may violate one or more of the requirements. If our operations
are found to be in violation of any of such laws or any other
governmental regulations that apply to our company, we may be
subject to penalties, including, without limitation, civil and
criminal penalties, damages, fines, the curtailment or
restructuring of our operations, any of which could adversely
affect the ability to operate our business and our financial
results. Failure to comply with FDA requirements may result in,
among other things, injunctions, product withdrawals, recalls,
product seizures, fines and criminal prosecutions. Our advertising
is subject to regulation by the U.S. Federal Trade Commission, or
FTC, under the Federal Trade Commission Act. Additionally, some
states also permit advertising and labeling laws to be enforced by
attorneys general who may seek relief for consumers, seek
class-action certifications, seek class-wide damages and product
recalls of products sold by us. For example, in November 2019 the
FDA issued warning letters to 15 companies for
illegally selling products containing CBD in ways that violate the
FD&C Act. Notwithstanding that we were not a recipient of a
warning letter, and we believe our products are properly labeled as
required under federal law, cbdMD, along with other of our
competitors who also did not receive FDA warning letters, have been
named in a class action lawsuit which we believe is without
merit. Any actions against our company by governmental
authorities or private litigants could be time consuming, costly to
defend and could have a material adverse effect on our business,
financial condition and results of operations.
Uncertainty caused by potential changes to legal regulations could
impact the use of CBD products.
There
is substantial uncertainty and different interpretations among
federal, state and local regulatory agencies, legislators,
academics and businesses as to the scope of operation of Farm
Bill-compliant hemp programs relative to the emerging regulation of
cannabinoids. These different opinions include, but are not limited
to, the regulation of cannabinoids by the U.S. Drug Enforcement
Administration, or DEA, and/or the FDA and the extent to which
manufacturers of products containing Farm Bill-compliant
cultivators and processorsmay engage in interstate commerce. The
uncertainties cannot be resolved without further federal, and
perhaps even state-level, legislation, regulation or a definitive
judicial interpretation of existing legislation and rules. If these
uncertainties continue, they may have an adverse effect upon the
introduction of our products in different markets.
15
RISKS RELATED TO OWNERSHIP OF OUR SECURITIES
We are subject to the continued
listing standards of the NYSE American and our failure to satisfy
these criteria may result in delisting of our common
stock.
Both
the shares of our common stock and our Series A Convertible
Preferred Stock are listed on the NYSE American. In order to
maintain these listings, we must maintain certain share prices,
financial and share distribution targets, including maintaining a
minimum amount of shareholders’ equity and a minimum number
of public shareholders. In addition to these objective standards,
the NYSE American may delist the securities of any issuer (i) if,
in its opinion, the issuer’s financial condition and/or
operating results appear unsatisfactory; (ii) if it appears that
the extent of public distribution or the aggregate market value of
the security has become so reduced as to make continued listing on
the NYSE American inadvisable; (iii) if the issuer sells or
disposes of principal operating assets or ceases to be an operating
company; (iv) if an issuer fails to comply with the NYSE
American’s listing requirements; (v) if an issuer’s
securities sell at what the NYSE American considers a “low
selling price” and the issuer fails to correct this via a
reverse split of shares after notification by the NYSE American; or
(vi) if any other event occurs or any condition exists which makes
continued listing on the NYSE American, in its opinion,
inadvisable. If the NYSE American delists either our common stock
and/or our Series A Convertible Preferred Stock, investors may face
material adverse consequences, including, but not limited to, a
lack of trading market for our securities, reduced liquidity,
decreased analyst coverage of our securities, and an inability for
us to obtain additional financing to fund our
operations.
The Series A Convertible Preferred Stock ranks junior to all of our
indebtedness and other liabilities and is effectively junior to all
indebtedness and other liabilities of our
subsidiaries.
In the
event of our bankruptcy, liquidation, dissolution or winding-up of
our affairs, our assets will be available to pay obligations on the
Series A Convertible Preferred Stock only after all of our
indebtedness and other liabilities have been paid. The rights of
holders of the Series A Convertible Preferred Stock to participate
in the distribution of our assets will rank junior to the prior
claims of our current and future creditors and any future series or
class of preferred stock we may issue (subject to Series A
Convertible Preferred Stockholder approval) that ranks senior to
the Series A Convertible Preferred Stock. In addition, the Series A
Convertible Preferred Stock effectively ranks junior to all
existing and future indebtedness and other liabilities of our
existing subsidiaries and any future subsidiaries. Our existing
subsidiaries are, and any future subsidiaries would be, separate
legal entities and have no legal obligation to pay any amounts to
us in respect of dividends due on the Series A Convertible
Preferred Stock. If we are forced to liquidate our assets to pay
our creditors, we may not have sufficient assets to pay amounts due
on any or all of the Series A Convertible Preferred Stock then
outstanding.
Future offerings of debt may adversely affect the market price of
the Series A Convertible Preferred Stock.
If we
decide to issue debt securities in the future, it is possible that
these securities will be governed by an indenture or other
instruments containing covenants restricting our operating
flexibility. Additionally, any convertible or exchangeable
securities that we issue in the future may have rights, preferences
and privileges more favorable than those of the Series A
Convertible Preferred Stock and may result in dilution to owners of
the Series A Convertible Preferred Stock. We and, indirectly, our
shareholders, will bear the cost of issuing and servicing such
securities. Because our decision to issue debt securities in any
future offering will depend on market conditions and other factors
beyond our control, we cannot predict or estimate the amount,
timing or nature of our future offerings. The holders of our
securities may bear the risk of our future offerings, potentially
reducing the market price of the Series A Convertible Preferred
Stock and diluting the value of their holdings in us.
We may not be able to pay dividends on the Series A Convertible
Preferred Stock.
Our
ability to pay cash dividends on the Series A Convertible Preferred
Stock requires us to (i) either be able to pay our debts as they
become due in the usual course of business, or (ii) have
total assets that are greater than the sum of our total liabilities
plus the amount that would be needed if we were to be dissolved at
the time of the distribution to satisfy the preferential rights
upon dissolution of shareholders whose preferential rights are
superior to those receiving the distribution. Further,
notwithstanding these factors, we may not have sufficient cash to
pay dividends on the Series A Convertible Preferred Stock. Our
ability to pay dividends may be impaired if any of the risks
described in this report were to occur. Also, payment of our
dividends depends upon our financial condition and other factors as
our board of directors may deem relevant from time to time. We
cannot assure you that our businesses will generate sufficient cash
flow from operations in an amount sufficient to enable us to make
distributions on our common stock and preferred stock, including
the Series A Convertible Preferred Stock, or to fund our other
liquidity needs.
16
Market interest rates may materially and adversely affect the value
of the Series A Convertible Preferred Stock.
One of
the factors that influences the price of the Series A Convertible
Preferred Stock is the dividend yield on the Series A Convertible
Preferred Stock (as a percentage of the market price of the Series
A Convertible Preferred Stock) relative to market interest rates.
Continued increase in market interest rates, which are currently at
low levels relative to historical rates, may lead prospective
purchasers of the Series A Convertible Preferred Stock to expect a
higher dividend yield (and higher interest rates would likely
increase our borrowing costs and potentially decrease funds
available for dividend payments). Thus, higher market interest
rates could cause the market price of the Series A Convertible
Preferred Stock to materially decrease, assuming a market is
established of which there are no assurances.
Holders of the Series A Convertible Preferred Stock may be unable
to use the dividends-received deduction and may not be eligible for
the preferential tax rates applicable to “qualified dividend
income.”
Distributions paid
to corporate U.S. holders of the Series A Convertible Preferred
Stock may be eligible for the dividends-received deduction, and
distributions paid to non-corporate U.S. holders of the Series A
Convertible Preferred Stock may be subject to tax at the
preferential tax rates applicable to “qualified dividend
income,” if we have current or accumulated earnings and
profits, as determined for U.S. federal income tax purposes. We do
not currently have any accumulated earnings and profits.
Additionally, we may not have sufficient current earnings and
profits during future fiscal years for the distributions on the
Series A Convertible Preferred Stock to qualify as dividends for
U.S. federal income tax purposes. If the distributions fail to
qualify as dividends, U.S. holders would be unable to use the
dividends-received deduction and may not be eligible for the
preferential tax rates applicable to “qualified dividend
income.” If any distributions on the Series A Convertible
Preferred Stock with respect to any fiscal year are not eligible
for the dividends-received deduction or preferential tax rates
applicable to “qualified dividend income” because of
insufficient current or accumulated earnings and profits, it is
possible that the market value of the Series A Convertible
Preferred Stock might decline.
The Series A Convertible Preferred Stock represents perpetual
equity interests in us, and investors should not expect us to
redeem or convert the Series A Convertible Preferred Stock on the
date the Series A Convertible Preferred Stock becomes redeemable or
convertible by us or on any particular date
afterwards.
The
Series A Convertible Preferred Stock represents perpetual equity
interests in our Company, and it has no maturity or mandatory
redemption except upon a Change of Control, and is not redeemable
at the option of investors under any other circumstances. A
“Change of Control” will generally be deemed to occur
when, after the original issuance of the Series A Convertible
Preferred Stock, the acquisition by any person, including any
syndicate or group deemed to be a “person” under
Section 13(d)(3) of the Exchange Act, of beneficial ownership,
directly or indirectly, through a purchase, merger or other
acquisition transaction or series of purchases, mergers or other
acquisition transactions which were pre-approved by our board of
directors of our stock entitling that person to exercise more than
50% of the total voting power of all of our stock entitled to vote
generally in the election of the our directors, subject to certain
exclusions. As a result, the Series A Convertible Preferred Stock
will not give rise to a claim for payment of any amount at a
particular date. As a result, holders of the Series A Convertible
Preferred Stock may be required to bear the financial risks of an
investment in the Series A Convertible Preferred Stock for an
indefinite period of time unless the holder chooses to voluntarily
convert the shares of Series A Convertible Preferred Stock into
shares of our common stock.
The Series A Convertible Preferred Stock has not been
rated.
We have
not sought to obtain a rating for the Series A Convertible
Preferred Stock. However, one or more rating agencies may
independently determine to issue such a rating or such a rating, if
issued, may adversely affect the market price of the Series A
Convertible Preferred Stock. In addition, we may elect in the
future to obtain a rating for the Series A Convertible Preferred
Stock, which could adversely affect the market price of the Series
A Convertible Preferred Stock. Ratings only reflect the views of
the rating agency or agencies issuing the ratings and such ratings
could be revised downward, placed on a watch list or withdrawn
entirely at the discretion of the issuing rating agency if in its
judgment circumstances so warrant. Any such downward revision,
placing on a watch list or withdrawal of a rating could have an
adverse effect on the market price of the Series A Convertible
Preferred Stock.
17
Change of Control redemption obligations may make it more difficult
for a party to acquire us or discourage a party from acquiring
us.
The
Change of Control redemption feature of the Series A Convertible
Preferred Stock may have the effect of discouraging a third party
from making an acquisition proposal for our Company or of delaying,
deferring or preventing certain of change of control transactions
under circumstances that otherwise could provide the holders of our
common stock and Series A Convertible Preferred Stock with the
opportunity to realize a premium over the then-current market price
of such stock or that shareholders may otherwise believe is in
their best interests.
If our Series A Convertible Preferred Stock is delisted, the
ability to transfer or sell shares of the Series A Convertible
Preferred Stock may be limited and the market value of the Series A
Convertible Preferred Stock will likely be materially adversely
affected.
The
Series A Convertible Preferred Stock does not contain provisions
that are intended to protect investors if our Series A Convertible
Preferred Stock is delisted from the NYSE American. If our Series A
Convertible Preferred Stock is delisted from the NYSE American,
investors’ ability to transfer or sell shares of the Series A
Convertible Preferred Stock will be limited and the market value of
the Series A Convertible Preferred Stock will likely be materially
adversely affected. Moreover, since the Series A Convertible
Preferred Stock has no stated maturity date, absent a
holder’s voluntary conversion of the Series A Convertible
Preferred Stock investors may be forced to hold shares of the
Series A Convertible Preferred Stock indefinitely while receiving
stated dividends thereon when, as and if authorized by our board of
directors and paid by us with no assurance as to ever receiving the
liquidation value thereof.
A holder of Series A Convertible Preferred Stock has extremely
limited voting rights.
The
voting rights for a holder of Series A Convertible Preferred Stock
are limited. Our shares of common stock are the only class of our
securities that carry full voting rights. Holders of the shares of
Series A Convertible Preferred Stock do not have any voting rights
other than as set forth below in the next two sentences or unless
dividends on the Series A Convertible Preferred Stock are in
arrears for each of 12 or more consecutive monthly periods, in
which case the holders of the Series A Convertible Preferred Stock
will be entitled to vote as a separate class for the election of
two additional directors to serve on the board of directors until
all dividends that are owed have been paid. Holders of shares of
Series A Convertible Preferred Stock, voting as a class, are also
entitled to vote if we should seek to issue or create any class or
series of capital stock ranking senior to the Series A Convertible
Preferred Stock with respect to dividends or distributions, in
which event the consent of holders of at least two thirds of the
then outstanding Series A Convertible Preferred Stock is required.
The consent of the holders of a majority of the Series A
Convertible Preferred Stock, voting as a class, is required if we
were to seek to adopt any amendment to
our articles of incorporation or bylaws that would materially
affect existing terms of the Series A Convertible Preferred Stock,
or increase the number of authorized shares of that series,
other than in connection with the anti-dilution provisions, or if
we seek to create a series or class which ranks pari passu with the
Series A Convertible Preferred Stock. Other than these limited
circumstances and except to the extent required by law, holders of
Series A Convertible Preferred Stock do not have any voting
rights.
We may redeem the Series A Convertible Preferred Stock at our
option, we will be required to redeem the Series A Convertible
Preferred Stock upon a Change of Control and we may convert shares
of Series A Convertible Preferred Stock upon a Market Trigger into
shares of our common stock. In the event of any of these
occurrences, you may not receive dividends that you
anticipate.
On or after October 16, 2023 we
may, at our option, redeem the Series A Convertible Preferred
Stock, in whole or in part, at any time or from time to
time. In addition, upon the
occurrence of a board approved Change of Control, we are required
to redeem any or all of the shares of Series A Convertible
Preferred Stock at a redemption price of $11.00 per share, plus any
accrued but unpaid dividends to, but excluding, the redemption
date. Furthermore, upon a Market Trigger (as that
term is defined in the designations, rights and preferences of the
Series A Convertible Preferred Stock), we may convert all or any
portion of those shares of
Series A Convertible Preferred Stock into shares of our common stock.
We may have an incentive to redeem or convert the Series A
Convertible Preferred Stock voluntarily if market conditions allow
us to issue other preferred stock or debt securities at a rate that
is lower than the dividend rate on the Series A Convertible
Preferred Stock. If we redeem or convert the Series A Convertible
Preferred Stock, then from and after the redemption date or
conversion date, as applicable, dividends will cease to accrue on
shares of Series A Convertible Preferred Stock, the shares of
Series A Convertible Preferred Stock shall no longer be deemed
outstanding and all rights as a holder of those shares will
terminate, including the rights to receive dividend
payments.
18
The liquidation preference of the shares of our Series A
Convertible Preferred Stock would reduce the amount available to
our common shareholders in the event of our liquidation or winding
up.
Holders
of our Series A Convertible Preferred Stock have a liquidation
preference of $10.00 per share in the event of our liquidation or
winding up. This means that those holders are entitled to receive
the liquidation preference before any payment or other distribution of assets to our common
shareholders, and the amount of any such payment or other
distribution will be reduced by that amount.
The issuance of shares upon exercise of our outstanding options and
warrants, or the conversion of the Series A Convertible Preferred
Stock may cause immediate and substantial dilution to our existing
shareholders.
In
addition to our obligation to issue the Earnout Shares if the goals
are met, we presently have options and warrants that if exercised
would result in the issuance of an additional 1,691,178 shares of
our common stock, and our Series A Convertible Preferred Stock is
presently convertible into an additional 833,500 shares of common
stock. The issuance of shares upon exercise of warrants and options
and/or the conversion of shares of our Series A Convertible
Preferred Stock will result in dilution to the interests of other
shareholders.
19
The price of our securities may be volatile, and you could lose all
or part of your investment.
Stock
markets have experienced extreme volatility that has often been
unrelated to the operating performance of particular companies.
These broad market fluctuations may adversely affect the trading
price of our common stock and/or our Series A Convertible Preferred
Stock. In addition, limited trading volume of our stock may
contribute to its future volatility. Price declines in our
securities could result from general market and economic
conditions, some of which are beyond our control, and a variety of
other factors, including any of the risk factors described in this
report. These broad market and industry factors may harm the market
price of our securities, regardless of our operating performance,
and could cause you to lose all or part of your investment in our
securities since you might be unable to sell your shares at or
above the price you paid. Factors that could cause fluctuations in
the market price of our securities include the
following:
●
price and volume fluctuations in the overall stock market from time
to time;
●
changes in operating performance and stock market valuations of
other CBD product companies generally;
●
prevailing
interest rates, increases in which may have an adverse effect on
the market price of the Series A Convertible Preferred
Stock;
●
the
annual yield from distributions on the Series A Convertible
Preferred Stock as compared to yields on other financial
instruments;
●
sales of shares of our securities by us or our
shareholders;
●
failure of securities analysts to initiate or maintain coverage of
us, changes in financial estimates by securities analysts who
follow our company, or our failure to meet these estimates or the
expectations of investors;
●
the financial projections we may provide to the public, any changes
in those projections or our failure to meet those
projections;
●
rumors and market speculation involving us or other companies in
our industry;
●
actual or anticipated changes in our results of operations or
fluctuations in our results of operations;
●
actual or anticipated developments in our business, our
competitors’ businesses or the competitive landscape
generally;
●
litigation, including class action lawsuits, involving us, our
industry or both, or investigations by regulators into our
operations or those of our competitors;
●
developments or disputes concerning our intellectual property or
other proprietary rights;
●
announced or completed acquisitions of businesses or brands by us
or our competitors;
●
new laws or regulations or new interpretations of existing laws or
regulations applicable to our business;
●
changes in accounting standards, policies, guidelines,
interpretations or principles;
●
any significant change in our management; and
●
general economic conditions and slow or negative growth of our
markets.
In
addition, in the past, following periods of volatility in the
overall market and the market price of a particular company’s
securities, securities class action litigation has often been
instituted against these companies. This litigation, if instituted
against us, could result in substantial costs and a diversion of
our management’s attention and resources.
20
We are an “emerging growth company,” and the reduced
reporting requirements applicable to emerging growth companies may
make our common stock less attractive to investors.
We
are an “emerging growth company,” as defined in the
JOBS Act. For as long as we continue to be an emerging growth
company, we may take advantage of exemptions from various reporting
requirements that are applicable to other public companies but not
to “emerging growth companies,” including, but not
limited to:
●
being permitted to provide only two years of audited financial
statements, in addition to any required unaudited interim financial
statements, with correspondingly reduced “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” disclosure;
●
not being required to comply with the auditor attestation
requirements in the assessment of our internal control over
financial reporting under Section 404 of the Sarbanes-Oxley Act of
2002;
●
not being required to comply with any requirement that may be
adopted by the Public Company Accounting Oversight Board regarding
mandatory audit firm rotation or a supplement to the
auditor’s report providing additional information about the
audit and the financial statements;
●
reduced disclosure obligations regarding executive compensation in
our periodic reports and proxy statements; and
●
exemptions from the requirements of holding a nonbinding advisory
vote on executive compensation and shareholder approval of any
golden parachute payments not previously approved.
Investors may find our securities less attractive
if we choose to rely on these exemptions. If some investors find
our securities less attractive as a result of any choices to reduce
future disclosure, there may be a less active trading market for
our common stock and/or our Series A Convertible Preferred Stock
and the price of either or both of these securities may be more
volatile.
Our executive officers, directors and their affiliates may exert
control over us and may exercise influence over matters subject to
shareholder approval.
Our
executive officers and directors, together with their respective
affiliates, beneficially own approximately 54% of our outstanding
common stock as of December 01, 2019. Accordingly, these
shareholders, if they act together, may exercise substantial
influence over matters requiring shareholder approval, including
the election of directors and approval of corporate transactions,
such as a merger. This concentration of ownership could have the
effect of delaying or preventing a change in control or otherwise
discourage a potential acquirer from attempting to obtain control
over us, which in turn could have a material adverse effect on the
market value of our common stock.
If securities or industry analysts do not publish research or
publish unfavorable or inaccurate research about our business, our
securities share prices and trading volumes could
decline.
An
active trading market for our securities will depend, in part, on
the research and reports that securities or industry analysts
publish about us or our business. We may be unable to attract or
sustain coverage by well-regarded securities and industry analysts.
If either none or only a limited number of securities or industry
analysts cover us or our business, or if these securities or
industry analysts are not widely respected within the general
investment community, the trading price for our common stock and/or
our Series A Convertible Preferred Stock would be materially and
negatively impacted. In the event we obtain securities or industry
analyst coverage, if one or more of the analysts who cover us or
our business downgrade our securities or publish inaccurate or
unfavorable research about us or our business, the price of our
securities would likely decline. If one or more of these analysts
cease coverage of us or our business, or fail to publish reports on
us or our business regularly, demand for our securities could
decrease, which might cause the price of our securities and trading
volume to decline.
21
Some provisions of our charter documents and North Carolina law may
have anti-takeover effects that could discourage an acquisition of
us by others, even if an acquisition would be beneficial to our
shareholders and may prevent attempts by our shareholders to
replace or remove our current management.
Provisions
in our articles of incorporation and bylaws, as well as provisions
of North Carolina law, could make it more difficult for a third
party to acquire us or increase the cost of acquiring us, even if
doing so would benefit our shareholders, or remove our current
management. These include provisions that:
●
permit
our board of directors to issue up to 45,000,000 shares of
preferred stock (net of the previous designation in October 2019 of
5,000,000 shares of preferred stock as Series A Convertible
Preferred Stock), with any rights, preferences and privileges as
they may designate;
●
are
part of the rights and preferences of our Series A Convertible
Preferred Stock;
●
provide
that all vacancies on our board of directors, including as a result
of newly created directorships, may, except as otherwise required
by law, be filled by the affirmative vote of a majority of
directors then in office, even if less than a quorum;
and
●
do
not provide for cumulative voting rights, thereby allowing the
holders of a majority of the shares of common stock entitled to
vote in any election of directors to elect all of the directors
standing for election.
These
provisions may frustrate or prevent any attempts by our
shareholders to replace or remove our current management by making
it more difficult for shareholders to replace members of our board
of directors, who are responsible for appointing the members of our
management. In addition, North Carolina has two primary
anti-takeover statutes, the Shareholder Protection Act and the
Control Share Acquisition Act, which govern the shareholder
approval required for certain business combinations. As permitted
by North Carolina law, we have opted out of both these provisions.
Accordingly, we are not subject to any anti-takeover effects of the
North Carolina Shareholder Protection Act or Control Share
Acquisition Act. Any provision of our articles of incorporation,
bylaws or North Carolina law that has the effect of delaying or
deterring a change in control could limit the opportunity for our
shareholders to receive a premium for their shares of common stock,
and could also affect the price that some investors are willing to
pay for our shares of common stock.
We have additional securities available for issuance, which, if
issued, could adversely affect the rights of the holders of our
securities.
Our
articles of incorporation, as amended, authorizes the issuance of
150,000,000 shares of our common stock and 50,000,000 shares of
preferred stock. As of the date hereof, we have designated
5,000,000 shares of the preferred stock as Series A Convertible
Preferred Stock and have issued 500,000 of those shares. In certain
circumstances, the common stock and/or the Series A Convertible
Preferred Stock, as well as the awards available for issuance under
our equity incentive plans, can be issued by our board of
directors, without shareholder approval. Any future issuances of
such stock would further dilute the percentage ownership of us held
by holders of either class of our securities. In addition, the
issuance of certain securities, may be used as an
“anti-takeover” device without further action on the
part of our shareholders, and may adversely affect the holders of
the securities.
22
ITEM 1B.
UNRESOLVED
STAFF COMMENTS.
Not
applicable to a smaller reporting company.
ITEM 2.
DESCRIPTION
OF PROPERTY.
Our headquarters are located in approximately
50,000 square feet in a modern two-story building in Charlotte,
North Carolina which we sub-lease under an agreement through
December 2026. The agreement calls for an annual base
monthly rent of $76,041 for the first year and escalates 3%
annually. We have a manufacturing and warehouse facility in
Charlotte, North Carolina which we lease approximately 40,000
square feet under an agreement through December 2021. The agreement
calls for an annual base monthly rent of $18,700, inclusive of
monthly taxes insurance and common area maintenance
(“TICAM”) for the first year and the rent escalates 3%
annually. We also have an 80,000 square foot warehouse in
Charlotte, North Carolina under an agreement through December 2024.
The agreement calls for an annual base monthly rent of $34,766,
inclusive of monthly TICAM for the first year and the rent
escalates 3% annually.
ITEM 3.
LEGAL
PROCEEDINGS.
In
December 2019, Cynthia Davis (“plaintiffs”) filed a
purported collective and class action lawsuit (the
“Complaint”) in the United States District Court for
the Central District of California against the Company and certain
of the Company’s competitors alleging violations of the
California’s Unfair Competition Law, California’s False
Advertising Law and California’s Consumer Legal Remedies Act,
as well as claims for Breach of Express Warranties, Breach of
Implied Warranty of Merchantability and Declaratory Relief (the
“California Laws”). The plaintiffs allege that the
Company violated the California Laws by unlawfully selling and
marketing mislabeled products which have not been approved by the
FDA. The Complaint was brought as a nationwide “collective
action,” and, alternatively, as a “class action”
under the laws of the State of California. The Company intends to
vigorously defend this action. This case is at an early stage, and
the Company is therefore unable to make a reasonable estimate of
the probable loss or range of losses, if any, that might arise from
this matter.
In
April 2019 CBD Industries LLC sued Majik Medicine, LLC to cancel
their issued trademark for "CBD MD" on multiple grounds.
This matter is currently pending a decision from the USPTO Trial
and Appeal Board (TTAB) on a motion to dismiss by Majik Medicine,
LLC. We believe this motion will be denied or we will be
granted leave to amend the complaint and the matter will
continue.
ITEM 4.
MINE
SAFETY DISCLOSURES.
Not
applicable to our company.
23
PART II
ITEM 5.
MARKET
FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS
AND ISSUER PURCHASES OF EQUITY SECURITIES.
Since
November 17, 2017 our common stock had been listed on the NYSE
American under the symbol "LEVB." With our name change in July 2019
we also changed our symbol and our common stock is now listed under
the symbol “YCBD”.
Our
Series A Convertible Preferred Stock has been listed on the NYSE
American since October 21, 2019 under the symbol “YCBD PR
A.”
As of
December 01, 2019, there were approximately 101 record owners of
our common stock and one record holder of our Series A Convertible
Preferred Stock. These amounts do not
reflect persons or entities that hold our securities in nominee or
“street” name through various brokerage
firms.
Dividend policy
Common Stock
We
do not currently intend to pay dividends on our common stock. The
declaration, amount and payment of any future dividends on shares
of our common stock, if any, is subject to the designations, rights
and preferences of the Series A Convertible Preferred Stock and
will be at the sole discretion of our Board, which may take into
account general and economic conditions, our financial condition
and results of operations, our available cash and current and
anticipated cash needs, capital requirements, contractual, legal,
tax and regulatory restrictions, the implications of the payment of
dividends by us to our shareholders or by our subsidiaries to us,
and any other factors that our Board may deem
relevant.
Series A Convertible Preferred Stock
In October 2019 we closed a firm commitment
underwritten public offering of our Series A Convertible Preferred
Stock. The designations, rights and preferences of our Series A
Convertible Preferred Stock provide that we will pay, when, as and
if declared by our board of directors, monthly cumulative cash
dividends at an annual rate of 8.0%, which is equivalent to $0.80
per annum per share, based on the $10.00 liquidation
preference. Dividends on the Series A Convertible Preferred Stock will
accrue daily and be cumulative from, and including, the first day
of the calendar month in which the shares are issued and will be
payable monthly in arrears on the 15th day of each calendar month.
On November 1, 2019 the Audit Committee of our board of directors
declared a cash dividend of $0.0667 per share of
Series A Convertible Preferred Stock payable on November 15, 2019
to holders of record on November 1, 2019. We expect that our board
of directors will continue to declare and pay monthly cash
dividends on our Series A Convertible Preferred Stock, subject to
the limitations to do so under North Carolina law. See Item 1A.
Risk Factors.
The
total amount of dividends declared and paid were $67,000 and
$33,500, respectively, as of December 01, 2019.
Recent sales of unregistered securities
None,
except as previously reported.
Purchases of equity securities by the issuer and affiliated
purchasers
None.
ITEM 6.
SELECTED
FINANCIAL DATA.
Not
applicable to a smaller reporting company.
24
ITEM 7.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS.
The
following discussion of our financial condition and results of
operations should be read in conjunction with the consolidated
financial statements and the notes to those statements that are
included elsewhere in this report. Our discussion includes
forward-looking statements based upon current expectations that
involve risks and uncertainties, such as our plans, objectives,
expectations and intentions. Actual results and the timing of
events could differ materially from those anticipated in these
forward-looking statements as a result of a number of factors,
including those set forth under the Risk Factors, Cautionary Notice
Regarding Forward-Looking Statements and Business sections in this
report. We use words such as “anticipate,”
“estimate,” “plan,” “project,”
“continuing,” “ongoing,”
“expect,” “believe,” “intend,”
“may,” “will,” “should,”
“could,” and similar expressions to identify
forward-looking statements. Our future operating results, however,
are impossible to predict and no guaranty or warranty is to be
inferred from those forward-looking statements.
Overview
cbdMD
was originally founded in 2015 as an innovative branding and marketing company with a
focus on lifestyle-based brands. In December 2018 we acquired Cure
Based Development following the passage of the Farm Bill
which contained a permanent declassification of cannabidiol (CBD)
as a controlled substance under Federal law. As a result of that transaction, we own
and operate the nationally recognized CBD brand cbdMD which now
represents our focus and substantially all of our
revenues.
Fiscal
2019 was a transformative year for our company. Following the
closing of the Mergers in December 2018, we began a concerted
effort to build our brand recognition, expand the CBD product line,
expand our sales channels, and invest in human and technology
infrastructure to support our current operations and expected
growth. These efforts included:
●
implementation of a
full marketing strategy focused on three key points:
o
Long term brand
building – through partnerships and
athletes/influencers;
o
Short term
conversion/sales – utilizing social media, affiliates, email
marketing, and an integrated website tied to the marketing
strategy; and
o
Increased brand
visibility – through digital content/ads,
podcasts/radio;
●
we expanded our
product offerings and introduced CBDMD PM and a full line of pet
products branded under Paw CBD with over 40
SKU’s;
●
we have built upon
Cure Based Development’s early online sales channels to
expand our online sales through development of our marketing
strategy and have established a growing wholesale and retail
distribution network of over 4,000 locations through our internal
sales team and tradeshow participation;
●
to support our
growing operations, in fiscal 2019, we relocated our corporate
headquarters to a 50,000 square foot facility which allowed us to
concentrate our senior management, sales, marketing and brand
development, finance, technology and administrative personnel in
one location. We also expanded our manufacturing and warehouse
facilities and capability to support production and delivery needs
as part of our growth; and
●
increased our
employee count by 117 primarily in our sales, marketing (social
media, digital and content), technology, laboratory and warehouse
areas.
Our
revenue guidance for fiscal 2019 was $24 million to $26 million.
While our net sales were $23.7 million, we attribute the difference
to our decision to discontinue the operations of our legacy
divisions effective September 30, 2019 and focus all of our time,
attention and resources on our CBD business. As disclosed in our
consolidated financial statements appearing later in this report,
revenues from these discontinued businesses were approximately
$888,000 which, if added to our net sales for fiscal 2019, would
have put us within our expected net sales range for fiscal
2019.
25
Discontinued Operations
Effective September
30, 2019, we discontinued operations of four business subsidiaries:
EE1, IM1, BPU and Level H&W. These subsidiaries accounted for
our licensing, entertainment, and products segments prior to fiscal
2019. Therefore, the results of operations related to these
subsidiaries for the Company are reported as discontinued
operations. Continuing operations discussed below refer to the
Company’s CBD business unless otherwise indicated, and prior
periods in such discussion have been restated to reflect results
excluding EE1, IM1, BPU and Level H&W. See Note 15,
“Discontinued Operations”, of the notes to consolidated
financial statements elsewhere in this report for additional
information. As a result, the discussion below is of the
Company’s continuing operations, which is comprised of the
CBD business and the Company’s corporate office.
Results of Operations
The
following tables provide certain selected consolidated financial
information for the periods presented:
|
|
Fiscal 2019
|
Fiscal 2018
|
Change
|
|
Net
sales
|
$23,595,955
|
$-
|
$23,595,955
|
|
Net
sales related party
|
$55,596
|
459,091
|
$(403,495)
|
|
Total
net sales
|
$23,651,551
|
$459,091
|
$23,192,460
|
|
Costs
of sales
|
$9,136,677
|
$104,010
|
$9,032,667
|
|
Gross
profit as a percentage of net sales
|
61.4%
|
77.3%
|
(15.9)%
|
|
Operating
expenses
|
$29,311,764
|
$2,152,149
|
$27,159,615
|
|
Other
income (expenses)
|
$(32,991,885)
|
$-
|
$(32,991,885)
|
|
Net
income (loss) before taxes
|
$(47,788,776)
|
$(1,797,068)
|
$(45,991,708)
|
|
Net
income (loss) from continuing operations
|
$(45,429,776)
|
$(1,781,068)
|
$(43,648,708)
|
|
Net
income (loss) from discontinued operations, net of
taxes
|
$(5,927,773)
|
$1,843,902
|
$(7,771,675)
|
|
Net
income (loss)
|
$(50,428,226)
|
$(412,075)
|
$(50,016,151)
|
The
following tables provide certain selected unaudited consolidated
financial information for the three months ended September 30, 2019
and 2018:
|
|
September 30,
2019
|
September 30,
2018
|
Change
|
|
Net
sales
|
$9,518,687
|
$-
|
$9,518,687
|
|
Net
sales related party
|
$25,450
|
-
|
$25,450
|
|
Total
net sales
|
$9,544,137
|
$-
|
$9,544,137
|
|
Costs
of sales
|
$4,127,491
|
$17,311
|
$4,110,180
|
|
Gross
profit as a percentage of net sales
|
56.7%
|
0%
|
56.7%
|
|
Operating
expenses
|
$10,266,281
|
$605,718
|
$9,660,563
|
|
Other
income (expenses)
|
$19,060,830
|
$-
|
$19,060,830
|
|
Net
income (loss) before taxes
|
$14,211,194
|
$(623,030)
|
$14,834,224
|
|
Net
income (loss) from continuing operations
|
$14,274,194
|
$(601,030)
|
$14,875,224
|
|
Net
income (loss) from discontinued operations, net of
taxes
|
$(5,927,773)
|
$203,994
|
$(6,131,767)
|
|
Net
income (loss)
|
$7,634,352
|
$(412,075)
|
$8,046,427
|
Sales
We
record product sales primarily through two main delivery channels,
direct to consumers via online capabilities (E-commerce) and direct
to wholesalers utilizing our internal sales team. In addition, we
record revenue upon delivery of services (consulting, marketing and
brand strategy). The following table provides information on the
contribution of net sales by type of sale to our total net
sales.
|
|
Fiscal
2019
|
% of
total
|
Fiscal
2018
|
% of
total
|
|
|
|
|
|
|
|
Wholesale
sales
|
$8,878,901
|
37.5%
|
$-
|
0%
|
|
Consumer
sales
|
14,772,650
|
62.5%
|
-
|
0%
|
|
Service oriented
sales
|
-
|
|
459,091
|
100%
|
|
Total net
sales
|
$23,651,551
|
|
$459,091
|
|
26
The
increase in net sales attributable to our wholesale and consumer
sales are directly related to the Mergers in December
2018.
As
described elsewhere in this report, from time to time we have
accepted equity positions as compensation for our services. The
following table provides information for fiscal 2019 and fiscal
2018 regarding the amount of our total net sales in each of those
periods for which we received an equity position in lieu of
cash.
|
Fiscal
2019
|
Fiscal
2018
|
||
|
Amount
|
% total net
sales
|
Amount
|
% total net
sales
|
|
|
|
|
|
|
$-
|
-%
|
$200,000
|
43.5%
|
While
our management believes this policy could potentially benefit our
company, this practice has had an adverse impact on our cash flow
from operations and holding these securities could subject our
company to additional valuation impacts in future periods as a
result of the need to value these holdings on a quarterly
basis.
Cost of sales
Our cost of sales includes costs associated with
distribution, fill and labor expense, components, manufacturing
overhead, third-party providers, and outbound freight for our
product sales, and includes labor for our service
sales.
The following table provides
information on the cost of sales to our net sales for fiscal 2019
and 2018:
|
|
Fiscal
2019
|
Fiscal
2018
|
change
|
|
|
|
|
|
|
Product
sales
|
$9,083,558
|
$-
|
$9,083,558
|
|
Service related
sales
|
53,119
|
104,010
|
(50,891)
|
|
Total cost of
sales
|
$9,136,677
|
$104,010
|
$9,032,667
|
Operating expenses
Our
principal operating expenses include staff related expense,
advertising (which includes expenses related to industry
distribution and trade shows), sponsorships, affiliate commissions,
merchant fees, travel, rent, professional service fees, and
business insurance expense. Our operating expenses on a
consolidated basis increased 1,262% in fiscal 2019 from fiscal 2018
and is directly related to the Mergers and the significant ramp up
of that business.
The
following table provides information on our approximate operating
expenses for fiscal 2019 and fiscal 2018:
|
|
Fiscal
2019
|
Fiscal
2018
|
change
|
|
|
|
|
|
|
Staff related
expense
|
$8,995,969
|
$1,105,102
|
$7,890,867
|
|
Accounting/legal
expense
|
1,041,267
|
397,630
|
643,637
|
|
Professional
outside services
|
1,559,330
|
336,273
|
1,263,057
|
|
Advertising/marketing/social
media/events/tradeshows
|
5,151,795
|
143,701
|
5,008,094
|
|
Sponsorships
|
2,679,330
|
-
|
2,679,330
|
|
Affiliate
commissions
|
1,627,372
|
-
|
1,627,372
|
|
Merchant
fees
|
1,670,085
|
-
|
1,670,085
|
|
Travel
expense
|
712,811
|
158,357
|
554,453
|
|
Rent
|
672,697
|
213,697
|
459,000
|
|
Business
insurance
|
344,432
|
201,171
|
143,261
|
|
Non-cash stock
compensation
|
2,688,530
|
598,581
|
2,089,949
|
|
Totals
|
$27,183,620
|
$3,154,513
|
$24,029,107
|
27
The increase in staff related expense is a direct
result of the build out of the CBDI team. The increase in
professional outside services is related to the use of outside
agencies and firms to support the growth while we built our
infrastructure. The increase in advertising/marketing,
sponsorships, affiliate commissions, and travel are a result of
execution on the business strategy and building of the CBD brand
while increasing market share. The increase in merchant fees is a
direct result of increased business through our E-commerce site.
For the year ended September 30, 2019, the Company also had
an impairment of $436,578 on intangible assets that no longer have
a useful life or value for the business based on the current
business focus.
Corporate
overhead
Included in our
consolidated operating expenses are expenses associated with our
corporate overhead which are not allocated to the operating
business unit, including (i) staff related expenses; (ii)
accounting and legal expenses; (iii) professional outside services;
(iv) travel and entertainment expenses; (v) rent; (vi) business
insurance; and (vii) non-cash stock compensation
expense.
The
following table provides information on our approximate corporate
overhead for fiscal 2019 and 2018:
|
|
Fiscal
2019
|
Fiscal
2018
|
change
|
|
|
|
|
|
|
Staff related
expense
|
$1,157,054
|
$1,105,102
|
$51,952
|
|
Accounting/legal
expense
|
931,373
|
397,630
|
533,743
|
|
Professional
outside services
|
931,460
|
336,273
|
595,187
|
|
Travel
expense
|
92,593
|
158,357
|
(65,764)
|
|
Rent
|
175,592
|
213,697
|
(38,105)
|
|
Business
insurance
|
262,200
|
201,171
|
61,029
|
|
Non-cash stock
compensation
|
2,688,530
|
598,581
|
2,089,949
|
|
Totals
|
$6,238,802
|
$3,010,811
|
$3,227,992
|
The
overall increase in corporate operating expenses is related to the
maturation of the entire organization and ongoing pubic company
related expenses. Specifically, the accounting/legal expense and
professional outside services increases are related to the Mergers,
two additional registration statement filings and secondary
financings by the company. The increase in non-cash stock
compensation reflects the issuance of options relevant to the
increased employee count.
Other income and other non-operating expenses
Interest income (expense)
Our
interest income (expense) was $75,071 and $0 for fiscal 2019 and
fiscal 2018, respectively.
Contingent liability
As
consideration for the Mergers, under the terms of the Merger
Agreement, we had a contractual obligation to issue 15,250,000
Initial Shares of our common stock, after approval by our
shareholders, to the members of Cure Based Development, issued in
two tranches 6,500,000 shares and 8,750,000 shares, both of which
are subject to leak out provisions, and the 8,750,000 tranche of
shares will also vest over a five year period and are subject to a
voting proxy agreement. The Merger Agreement also provides that an
additional 15,250,000 Earnout Shares of our common stock can be
issued upon the satisfaction of certain aggregate net revenue
criteria by cbdMD within 60 months following the closing date of
the Mergers.
The
Initial Shares and Earnout Shares were approved by our shareholders
and the Initial Shares were issued on April 19, 2019. The Initial
Shares value at April 19, 2019 was $53,215,163, and with the
issuance of the Initial Shares, the contingent liability related to
the Initial Shares was reclassified to shareholders’ equity
by $53,215,163.
28
The
earn-out provision for the Earnout Shares is accounted for and
recorded as a contingent liability with increases in the liability
recorded as non cash other expense and decreases in the liability
recorded as non cash other income. The value of the contingent
liability is $50,600,000 at September 30, 2019, as compared to
$74,353,484 at the merger date, December 20, 2018. During the year
we had an increase in value of $32,461,680 of the continent
liability and a reclassification out of the liability of
$56,215,164 for issuance of the Initial Shares and settlement of
acquired liabilities. The increase of $32,461,680 is recorded as
other expense in our consolidated statement of operations appearing
later in this report. The Company utilizes both a market approach
and a Monte Carlo simulation in valuing the contingent liability
and a key input in both of those methods is the stock price. The
main driver of the increase in the value of the contingent
liability was the increase of the Company’s common stock
price, which was $3.96 at September 30, 2019 as compared to $3.11
on December 20, 2018.
Realized and unrealized gain (loss) on marketable and other
securities
We
value investments in marketable securities at fair value and record
a gain or loss upon sale at each period in realized gain (loss) on
marketable securities. On October 1, 2018, as a result of the
adoption of ASU 2016-01 – Financial Instruments, the Company now
records changes in fair value of equities held as unrealized gains
(losses) on marketable securities in the statement of operations at
each period beginning October 1, 2018 and prior to that recorded
unrealized gain or loss in other comprehensive income (loss). For
fiscal 2019 and 2018 we recorded $(605,276) and $0 of realized and
unrealized gain (loss) on marketable and other securities, which
includes an impairment of $502,560. The discontinuing operations
recorded an realized and unrealized gain (loss) of $(2,337,280)
which is included in the net gain (loss) from discontinued
operations on the statement of operations. For fiscal 2018 we
recorded other comprehensive loss of $(2,512,539).
Liquidity and Capital Resources
We had cash and cash equivalents on hand of
$4,689,966 and working capital of $12,033,157 at September 30, 2019
as compared to cash on hand of $4,282,553 and working capital of
$10,820,192 at September 30, 2018. Our current assets increased
approximately 33.5% at September 30, 2019 from September 30, 2018,
and is primarily attributable to an increase of cash, accounts
receivable, deposits, merchant reserve, inventory, and prepaid
expenses, offset by a decrease in marketable and other securities
and assets from discontinued operations. Our current liabilities
increased approximately 282.5% at September 30, 2019 from September
30, 2018. This increase is primarily attributable to increases in
accounts payable and accrued expenses, offset by decreases in
liabilities from discontinued operations. Both the changes in our
current assets and current liabilities are also reflective of the
change in focus to the CBD business during fiscal
2019.
During
fiscal 2019 we used cash primarily to fund our operations in
addition to increases in our accounts receivable.
We do not have any commitments for capital
expenditures. We have multiple endorsement or sponsorship
agreements for varying time periods up through December 2022 and
provide for financial commitments from the Company based on
performance/participation (see Note 13 Commitments and
Contingencies). We have sufficient working capital to fund
our operations.
Our
goal from a liquidity perspective is to use operating cash flows to
fund day to day operations and we have not met this goal as cash
flow from operations has been a net use of $15,377,467 and
$5,573,094 for fiscal 2019 and fiscal 2018,
respectively.
On
November 16, 2017 we closed an IPO and raised net proceeds of
$10,932,535. On October 2, 2018 we closed a follow-on firm
underwritten public offering of shares of our common stock
resulting in total net proceeds to us of $6,356,998. On May 15,
2019 we closed a follow-on firm underwritten public offering of
shares of our common stock resulting in total net proceeds to us of
$12,650,600. On October 16, 2019 we closed a follow-on firm
underwritten public offering of shares
of our Series A Convertible Preferred Stock resulting in total net
proceeds to us of $4,525,100. We are using the net proceeds
from the offerings for brand development and expansion,
advertising, marketing, and general working capital.
29
Related Parties
As described in Note 9 notes to our consolidated
financial statements appearing elsewhere in this report, we have
engaged in a number of related party transactions. We have
reported transactions with related parties within the consolidated
financial statements as well as within the notes to the
consolidated financial statements. These transactions also are
reported as sales with related parties (see Note 9 Related Party
Transactions in the consolidated financial statements for more
information).
Critical Accounting Policies
The
preparation of financial statements and related disclosures in
conformity with U.S. generally accepted accounting principles
(“US GAAP”) and our discussion and analysis of our
financial condition and operating results require our management to
make judgments, assumptions and estimates that affect the amounts
reported in its consolidated financial statements and accompanying
notes. Note 1, “Organization and Summary of Significant
Accounting Policies,” of the notes to our consolidated
financial statements appearing elsewhere in this report describes
the significant accounting policies and methods used in the
preparation of our consolidated financial statements. Management
bases its estimates on historical experience and on various other
assumptions it believes to be reasonable under the circumstances,
the results of which form the basis for making judgments about the
carrying values of assets and liabilities. Actual results may
differ from these estimates, and such differences may be
material.
We believe that the following critical accounting
policies involve the more significant judgments and estimates used
in the preparation of our consolidated financial statements and are
the most critical to aid you in fully understanding and evaluating
our reported financial results. Management considers these
policies critical because they are both important to the portrayal
of our financial condition and operating results, and they require
management to make judgments and estimates about inherently
uncertain matters.
Inventory
Inventory is stated
at the lower of cost or net realizable value with cost being
determined on a weighted average basis. The cost of inventory
includes product cost, freight-in, and production fill and labor
(portions of which we outsource to third party manufacturers).
Write-offs of potentially slow moving or damaged inventory are
recorded based on management’s analysis of inventory levels,
forecasted future sales volume and pricing and through specific
identification of obsolete or damaged products. We assess inventory
quarterly for slow moving products and potential impairments and at
a minimum perform a physical inventory count annually near fiscal
year end.
Marketable
Securities
Marketable
securities that are equity securities are carried at fair value on
the consolidated balance sheets with changes in fair value recorded
as an unrealized gain or (loss) in the Statements of Operations in
the period of the change. Upon the disposition of a marketable
security, the Company records a realized gain or (loss) on the
Company’s consolidated statements of operations. On
October 1, 2018, as a result of the adoption of ASU 2016-01 –
Financial Instruments, the
Company reclassified $2,512,539 of net unrealized losses on
marketable securities, that were formerly classified as
available-for-sale securities before the adoption of the new
standard, from Accumulated Other Comprehensive Loss to Accumulated
Deficit.
Investment
Other Securities
For
equity investments where the Company neither controls nor has
significant influence over the investee and which are
non-marketable, which is without a readily determinable fair value,
the Company may elect to estimate its fair value at cost less
impairment plus or minus changes resulting from observable price
changes.
Recent Accounting Pronouncements
Please see Note 1–Organization and
Summary of Significant Accounting Policies appearing in the notes to our consolidated
financial statements included in this report for information on
accounting pronouncements.
30
Off Balance Sheet Arrangements
We
have multiple leases for office, warehouse and lab facilities with
varying termination dates up through December 2027. These leases
provide for standard annual increases and are described further in
Note 17 – Leases.
In
addition, we have multiple endorsement or sponsorship agreements
for varying time periods up through December 2022 and provide for
financial commitments from the Company based on
performance/participation (see Note 13 Commitments and
Contingencies).
The
term “off-balance sheet arrangement” generally means
any transaction, agreement or other contractual arrangement to
which an entity unconsolidated with us is a party, under which we
have any obligation arising under a guarantee contract, derivative
instrument or variable interest or a retained or contingent
interest in assets transferred to such entity or similar
arrangement that serves as credit, liquidity or market risk support
for such assets.
ITEM 7A.
QUANTITATIVE
AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not
applicable for a smaller reporting company.
ITEM 8.
FINANCIAL
STATEMENTS AND SUPPLEMENTARY DATA.
Please
see our Financial Statements beginning on page F-1 of this annual
report.
ITEM 9.
CHANGES
IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL
DISCLOSURE.
None.
ITEM 9A.
CONTROLS
AND PROCEDURES.
Evaluation of Disclosure Controls and
Procedures.
We
maintain disclosure controls and procedures that are designed to
ensure that information required to be disclosed in our reports
filed pursuant to the Exchange Act is recorded, processed,
summarized, and reported within the time periods specified in the
Commission’s rules and forms and that such information is
accumulated and communicated to our management, including our
co-Chief Executive Officers and Chief Financial Officer, as
appropriate, to allow for timely decisions regarding required
disclosure. In designing and evaluating the disclosure controls and
procedures, management recognizes that any controls and procedures,
no matter how well designed and operated, can provide only
reasonable assurance of achieving the desired control objectives,
and management is required to apply its judgment in evaluating the
cost-benefit relationship of possible controls and
procedures.
As
required by Exchange Act Rule 13a-15(e), we carried out an
evaluation, under the supervision and with the participation of our
management, including our co-Chief Executive Officers and our Chief
Financial Officer, of the effectiveness of the design and operation
of our disclosure controls and procedures. Based on that
evaluation, our co-Chief Executive Officers and our Chief Financial
Officer concluded that our disclosure controls were effective at
September 30, 2019.
31
Management’s Report on Internal Control
over Financial Reporting.
Our
management is responsible for establishing and maintaining adequate
internal control over financial reporting as defined in Rule
13a-15(f) under the Exchange Act. Our internal control over
financial reporting is designed to provide reasonable assurance
regarding the reliability of financial reporting and the
preparation of financial statements for external purposes in
accordance with generally accepted accounting principles. Our
internal control over financial reporting includes those policies
and procedures that:
●
pertain to the
maintenance of records that, in reasonable detail, accurately and
fairly reflect the transactions and dispositions of our
assets;
●
provide reasonable
assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally
accepted accounting principles, and that our receipts and
expenditures are being made only in accordance with authorizations
of our management and directors; and
●
provide reasonable
assurance regarding prevention or timely detection of unauthorized
acquisition, use or disposition of our assets that could have a
material effect on the financial statements.
As an
emerging growth company experiencing rapid growth, we have worked
diligently to improve processes within the Company which has
created continuous change, specifically including in our IT and
manufacturing environments that increase risk related to
transaction processing which can impact our financial reporting. We
have implemented a significant number of manual compensating
controls to address this risk.
Because
of the inherent limitations, internal control over financial
reporting may not prevent or detect misstatements. Also,
projections of any evaluation of effectiveness to future periods
are subject to the risk that controls may become inadequate because
of changes in conditions, or that the degree of compliance with the
policies or procedures may deteriorate. All internal control
systems, no matter how well designed, have inherent limitations.
Therefore, even those systems determined to be effective can
provide only reasonable assurance with respect to financial
statement preparation and presentation. Because of the inherent
limitations of internal control, there is a risk that material
misstatements may not be prevented or detected on a timely basis by
internal control over financial reporting. However, these inherent
limitations are known features of the financial reporting process.
Therefore, it is possible to design into the process safeguards to
reduce, though not eliminate, this risk.
Our
management, including our co-CEOs and our CFO, assessed the
effectiveness of our internal control over financial reporting as
of September 30, 2019. In making this assessment, our management
used the criteria set forth by the Committee of Sponsoring
Organizations of the 2013 Treadway Commission (“COSO”)
in Internal Control-Integrated
Framework. Based on that evaluation, they concluded that,
during the period covered by this report, such internal controls
and procedures were effective.
Changes in Internal Control over Financial Reporting
There
have been no changes in our internal control over financial
reporting during our last fiscal year that has materially affected,
or is reasonably likely to materially affect, our internal control
over financial reporting.
ITEM 9B.
OTHER
INFORMATION.
None.
32
PART III
ITEM 10.
DIRECTORS,
EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The
information required by this Item will be contained in our proxy
statement for our 2020 Annual Meeting of Shareholders to be filed
on or prior to January 28, 2020 (the “Proxy Statement”)
and is incorporated herein by this reference.
ITEM 11.
EXECUTIVE
COMPENSATION.
The
information required by this item will be contained in our Proxy
Statement and is incorporated herein by this
reference.
ITEM 12.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED
STOCKHOLDER MATTERS.
The
information required by this item will be contained in our Proxy
Statement and is incorporated herein by this
reference.
ITEM 13.
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR
INDEPENDENCE.
The
information required by this item will be contained in our Proxy
Statement and is incorporated herein by this
reference.
ITEM 14.
PRINCIPAL
ACCOUNTING FEES AND SERVICES.
The
information required by this item will be contained in our Proxy
Statement and is incorporated herein by this
reference.
ITEM 15.
EXHIBITS,
FINANCIAL STATEMENT SCHEDULES.
(a)
(1)
Financial
statements.
The
consolidated financial statements and Report of Independent
Registered Accounting Firm are listed in the “Index to
Financial Statements and Schedules” beginning on page
F-1.
(2)
Financial statement schedules
All
schedules for which provision is made in the applicable accounting
regulations of the SEC are either not required under the related
instructions, are not applicable (and therefore have been omitted),
or the required disclosures are contained in the consolidated
financial statements herein.
(3)
Exhibits.
The
exhibits that are required to be filed or incorporated by reference
herein are listed in the Exhibit Index.
ITEM 16.
FORM
10-K SUMMARY.
None
33
SIGNATURES
Pursuant to the
requirements of Section 13 or 15(d) of the Securities Exchange Act
of 1934, the registrant has duly caused this report to be signed on
its behalf by the undersigned, thereunto duly
authorized.
|
|
cbdMD, Inc.
|
|
|
|
|
|
|
|
|
Date: December 18,
2019
|
By:
|
/s/ Martin A.
Sumichrast
|
|
|
|
|
Martin A.
Sumichrast
|
|
|
|
|
Co-Chief
Executive Officer (co-Principal Executive
Officer)
|
|
|
|
cbdMD, Inc.
|
|
|
|
|
|
|
|
|
Date: December 18,
2019
|
By:
|
/s/ Raymond S.
Coffman
|
|
|
|
|
Raymond S.
Coffman
|
|
|
|
|
Co-Chief
Executive Officer (co-Principal
Executive Officer)
|
|
|
|
cbdMD, Inc.
|
|
|
|
|
|
|
|
|
Date: December 18,
2019
|
By:
|
/s/ Mark S.
Elliott
|
|
|
|
|
Mark S.
Elliott
|
|
|
|
|
Chief
Financial Officer, (Principal Accounting and Financial
Officer)
|
|
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each
person whose signature appears below hereby constitutes and
appoints Mark S. Elliott his true and lawful attorney-in-fact and
agent, with full power of substitution and resubstitution, for him
and in his name, place and stead, in any and all capacities, to
sign any and all amendments and supplements to this report, and to
file the same, with all exhibits thereto, and other documents in
connection therewith, with the Securities and Exchange Commission,
and hereby grants to such attorney-in-fact and agent, full power
and authority to do and perform each and every act and thing
requisite and necessary to be done, as fully to all intents and
purposes as he might or could do in person, hereby ratifying and
confirming all that said attorney-in-fact and agent, or his
substitute or substitutes, may lawfully do or cause to be done by
virtue hereof.
Pursuant
to the requirements of the Securities Exchange Act of 1934, this
report has been signed below by the following persons on behalf of
the registrant and in the capacities and on the dates
indicated.
|
Name
|
|
Positions
|
|
Date
|
|
|
|
|
|
|
| /s/ Martin A. Sumichrast |
|
Chairman
of the Board of Directors, Co-Chief Executive
Officer
|
|
|
|
Martin
A. Sumichrast
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
| /s/ Raymond S, Coffman |
|
Co-Chief Executive Officer, Director |
|
|
|
Raymond
S. Coffman
|
|
|
December 18, 2019
|
|
|
|
|
|
|
|
| /s/ Anthony K. Shriver |
|
Director |
|
|
|
Anthony
K. Shriver
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
| /s/ Seymour G. Siegel |
|
Director |
|
|
|
Seymour G. Siegel
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
| /s/ Bakari Sellers |
|
Director |
|
|
|
Bakari Sellers
|
|
|
December 18, 2019
|
|
|
|
|
|
|
|
| /s/ Gregory C. Morris |
|
Director |
|
|
|
Gregory C. Morris
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
|
/s/ Scott Stephen
|
|
Director |
|
|
|
Scott Stephen
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
| /s/ Peter Ghiloni |
|
Director |
|
|
|
Peter Ghiloni
|
|
|
|
December 18, 2019
|
|
|
|
|
|
|
| /s/ William Raines III |
|
Director |
|
|
|
William Raines III
|
|
|
|
December 18, 2019
|
34
EXHIBIT INDEX
|
|
|
|
|
Incorporated by Reference
|
|
Filed or
Furnished
Herewith
|
|||||
|
No.
|
|
Exhibit Description
|
|
Form
|
|
Date Filed
|
|
Number
|
|
|
|
|
|
Form of
Underwriting Agreement dated September 28, 2018 by and between
Level Brands, Inc. and ThinkEquity, a Division of Fordman Financial
Management, Inc.
|
|
S-1
|
|
9/26/18
|
|
1.1
|
|
|
||
|
|
Form of
Underwriting Agreement dated May 13, 2019 by and between cbdMD,
Inc. and ThinkEquity, a Division of Fordman Financial Management,
Inc.
|
|
8-K
|
|
5/14/19
|
|
1.1
|
|
|
||
|
|
Underwriting
Agreement dated October 10, 2019 by and between cbdMD, Inc. and
ThinkEquity, a Division of Fordham Financial Management,
Inc.
|
|
8-K
|
|
10.16
|
|
1.1
|
|
|
||
|
|
Merger
Agreement dated December 3, 2018 by and among Level Brands, Inc.,
AcqCo, LLC, cbdMD LLC and Cure Based Development, LLC
|
|
8-K
|
|
12/4/18
|
|
2.1
|
|
|
||
|
2.2
|
|
Articles
of Merger dated December 20, 2018 as filed with the Secretary of
State of Nevada merging AcqCo, LLC with and into Cure Based
Development, LLC
|
|
10-Q
|
|
2/14/19
|
|
2.2
|
|
|
|
|
|
Articles
of Merger dated December 20, 2018 as filed with the Secretary of
State of North Carolina merging AcqCo, LLC with and into Cure Based
Development, LLC
|
|
10-Q
|
|
2/14/19
|
|
2.3
|
|
|
||
|
|
Articles
of Merger dated December 20, 2018 as filed with the Secretary of
State of Nevada merging Cure Based Development, LLC with an into
cbdMD LLC
|
|
10-Q
|
|
2/14/19
|
|
2.4
|
|
|
||
|
|
Articles
of Merger dated December 20, 2018 as filed with the Secretary of
State of North Carolina merging Cure Based Development, LLC with an
into cbdMD LLC
|
|
10-Q
|
|
2/14/19
|
|
2.5
|
|
|
||
|
|
Articles
of Incorporation
|
|
1-A
|
|
9/18/17
|
|
2.1
|
|
|
||
|
|
Articles
of Amendment to the Articles of Incorporation filed April 22,
2015
|
|
1-A
|
|
9/18/17
|
|
2.2
|
|
|
||
|
|
Articles
of Amendment to the Articles of Incorporation filed June 22,
2015
|
|
1-A
|
|
9/18/17
|
|
2.3
|
|
|
||
|
|
Articles
of Amendment to the Articles of Incorporation filed November 17,
2016
|
|
1-A
|
|
9/18/17
|
|
2.4
|
|
|
||
|
|
Articles
of Amendment to the Articles of Incorporation filed December 5,
2016
|
|
1-A
|
|
9/18/17
|
|
2.5
|
|
|
||
|
|
Bylaws,
as amended
|
|
1-A
|
|
9/18/17
|
|
2.6
|
|
|
||
|
|
Articles
of Amendment to Articles of Incorporation
|
|
8-K
|
|
4/29/19
|
|
3.7
|
|
|
||
|
|
Articles
of Amendment to the Articles of Incorporation including the
Certificate of Designations, Rights and Preferences of the 8%
Series A Cumulative Convertible Preferred Stock filed October 11,
2019
|
|
8-A
|
|
10/11/19
|
|
3.1(f)
|
|
|
||
|
|
Form of placement agent warrant issued in June 2015 private
placement
|
|
1-A
|
|
9/18/17
|
|
3.3
|
|
|
||
|
|
Form of placement agent warrant issued in December 2015 private
placement
|
|
1-A
|
|
9/18/17
|
|
3.4
|
|
|
||
|
|
Form of warrant issued in 8% convertible promissory note
offering
|
|
1-A
|
|
9/18/17
|
|
3.5
|
|
|
||
|
|
Form of selling agents’ warrant issued in November 2017
initial public offering
|
|
1-A/A
|
|
10/12/17
|
|
3.6
|
|
|
||
|
|
Form of common stock certificate of the registrant
|
|
1-A
|
|
9/18/17
|
|
3.7
|
|
|
||
|
|
2015
Equity Compensation Plan
|
|
1-A
|
|
9/18/17
|
|
3.8
|
|
|
||
|
|
Form of
stock option award under 2015 Equity Compensation Plan+
|
|
1-A
|
|
9/18/17
|
|
3.9
|
|
|
||
|
|
Form of
warrant issued to Andre Carthen
|
|
1-A
|
|
9/18/17
|
|
3.10
|
|
|
||
|
|
Form of
warrant issued to Nicholas Walker
|
|
1-A
|
|
9/18/17
|
|
3.11
|
|
|
||
|
|
Form of
representative’s warrant dated November 16, 2018
|
|
S-1
|
|
9/26/18
|
|
4.10
|
|
|
||
|
|
Form of
Representative’s Warrant dated May 15, 2019
|
|
8-K
|
|
5/14/19
|
|
4.1
|
|
|
||
|
|
Form of
Representative’s Warrant dated October 16, 2019
|
|
8-K
|
|
10/16/19
|
|
4.1
|
|
|
||
|
|
Contribution Agreement by and between Beauty & Pin-Ups, Inc.
and Beauty and Pin Ups LLC dated April 13, 2015
|
|
1-A
|
|
9/18/17
|
|
7.1
|
|
|
||
35
|
|
Operating Agreement of Beauty and Pin Ups LLC, as
amended
|
|
1-A
|
|
9/18/17
|
|
6.1
|
|
|
|
|
|
Executive Employment Agreement dated January 1, 2017 by and between
Level Brands, Inc. and Martin A. Sumichrast +
|
|
1-A
|
|
9/18/17
|
|
6.10
|
|
|
|
|
|
Executive Employment Agreement dated January 2, 2017 by and between
Level Brands, Inc. and Mark S. Elliott +
|
|
1-A
|
|
9/18/17
|
|
6.11
|
|
|
|
|
|
Master Advisory and Consulting Agreement dated February 8, 2017 by
and between Level Brands, Inc. and kathy
Ireland®
Worldwide
|
|
1-A
|
|
9/18/17
|
|
6.12
|
|
|
|
|
|
Sublease dated January 1, 2017 by and between Kure Franchise, LLC
and Level Brands, Inc.
|
|
1-A
|
|
9/18/17
|
|
6.16
|
|
|
|
|
|
Wholesale License Agreement dated January 12, 2017 by and
between kathy ireland
®Worldwide and I'M1,
LLC
|
|
1-A
|
|
9/18/17
|
|
6.18
|
|
|
|
|
|
Amended and Restated Limited Liability Company Agreement of I'M1,
LLC effective January 1, 2017
|
|
1-A
|
|
9/18/17
|
|
6.19
|
|
|
|
|
|
Amended and Restated Limited Liability Company Agreement of Encore
Endeavor 1 LLC effective January 1, 2017
|
|
1-A
|
|
9/18/17
|
|
6.20
|
|
|
|
|
|
Amended and Restated Membership Interest Exchange Agreement dated
March 24, 2017, effective January 6, 2017, by and among IM1
Holdings, LLC, I'M1, LLC and Level Brands, Inc.
|
|
1-A
|
|
9/18/17
|
|
7.2
|
|
|
|
|
|
Amended and Restated Membership Interest Exchange Agreement dated
March 24, 2017, effective January 6, 2017, by and among EE1
Holdings, LLC, Encore Endeavor I LLC and Level Brands,
Inc.
|
|
1-A
|
|
9/18/17
|
|
7.3
|
|
|
|
|
|
Form of Indemnification Agreement
|
|
1-A
|
|
9/18/17
|
|
6.21
|
|
|
|
|
|
Amendment to Executive Employment Agreement dated April 1, 2017 by
and between Level Brands, Inc. and Martin A. Sumichrast
+
|
|
1-A
|
|
9/18/17
|
|
6.27
|
|
|
|
|
|
License Agreement dated March 29, 2017 by and among I'M1, LLC, Kure
Corp. and Kure Franchise, LLC
|
|
1-A
|
|
9/18/17
|
|
6.28
|
|
|
|
|
|
Television Series Consulting Agreement dated March 1, 2017 by and
between Multi-Media Productions Inc. and Encore Endeavor 1,
LLC
|
|
1-A
|
|
9/18/17
|
|
6.30
|
|
|
|
|
|
Advisory Agreement dated May 9, 2017 by and between Formula Four
Beverages Inc., I'M1, LLC and Encore Endeavor 1, LLC
|
|
1-A
|
|
9/18/17
|
|
6.31
|
|
|
|
|
|
Membership Interest Sale and Purchase Agreement by and among Priel
Maman, Level Brands, Inc. and Beauty and Pin-Ups, LLC dated April
26, 2017
|
|
1-A
|
|
9/18/17
|
|
6.33
|
|
|
|
|
|
License Agreement dated March 29, 2017 by and between I'M1, LLC and
Andre Phillipe, Inc.
|
|
1-A
|
|
9/18/17
|
|
6.35
|
|
|
|
|
|
Recording Master License Agreement dated May 23, 2017 by and
between McCoo & Davis, Inc. and Encore Endeavor 1
LLC
|
|
1-A
|
|
9/18/17
|
|
6.36
|
|
|
|
|
|
License Agreement dated June 27, 2017 by and between I'M1, LLC and
Loose Leaf Eyewear and Accessories LLC.
|
|
1-A
|
|
9/18/17
|
|
6.42
|
|
|
|
|
|
Advisory Agreement dated August 9, 2017 by and among Damiva Inc.,
I'M1, LLC and Encore Endeavor 1, LLC
|
|
1-A
|
|
9/18/17
|
|
6.43
|
|
|
|
|
|
Representation Agreement dated August 1, 2017 by and among Encore
Endeavor 1 LLC, Romero Britto and Britto Central, Inc.
|
|
1-A
|
|
9/18/17
|
|
6.44
|
|
|
|
|
|
Amended and Restated Representation Agreement dated September 12,
2017 by and among Encore Endeavor 1 LLC, Dada Media, Inc. and David
Tutera
|
|
1-A
|
|
9/18/17
|
|
6.45
|
|
|
|
|
|
Amendment dated September 8, 2017 to Master Advisory and Consulting
Agreement by and between Level Brands, Inc. and kathy
Ireland®
Worldwide
|
|
1-A
|
|
9/18/17
|
|
6.47
|
|
|
|
|
|
Wholesale License Agreement dated September 8, 2017 by and between
Level Brands, Inc. and kathy
ireland®
Worldwide+
|
|
1-A
|
|
9/18/17
|
|
6.48
|
|
|
|
|
|
Wholesale License Agreement dated September 8, 2017 by and between
Level Brands, Inc. and Andre Carthen
|
|
1-A
|
|
9/18/17
|
|
6.49
|
|
|
|
|
|
Wholesale License Agreement dated September 8, 2017 by and between
Level Brands, Inc. and Nicholas Walker
|
|
1-A
|
|
9/18/17
|
|
6.50
|
|
|
|
|
|
Advisory Agreement dated September 1, 2017 by and between Level
Brands, Inc. and Jon Carrasco +
|
|
1-A
|
|
9/18/17
|
|
6.52
|
|
|
36
|
|
Production
Services Agreement dated September 19, 2017 by and between
Multimedia Productions, Inc. and Encore Endeavor 1,
LLC
|
|
1-A/A
|
|
10/12/17
|
|
6.53
|
|
|
|
|
|
License
Agreement dated September 8, 2017 by and between Level Brands, Inc.
and kathy ireland®
Worldwide
|
|
1-A
|
|
9/18/17
|
|
6.54
|
|
|
|
|
|
Advisory
Agreement dated September 22, 2017 by and between SG Blocks, Inc.
and Encore Endeavor 1, LLC
|
|
1-A/A
|
|
10/12/17
|
|
6.55
|
|
|
|
|
|
Form of
Revolving Line of Credit Loan Agreement dated December 12, 2017 by
and between Level Brands, Inc. and Kure Corp.
|
|
8-K
|
|
12/12/17
|
|
10.64
|
|
|
|
|
|
Form of
Security Agreement dated December 12, 2017 by and between Level
Brands, Inc. and Kure Corp.
|
|
8-K
|
|
12/12/17
|
|
10.65
|
|
|
|
|
|
Form of
Promissory Note in the principal amount of $500,000 dated December
12, 2017 due from Kure Corp.
|
|
8-K
|
|
12/12/17
|
|
10.66
|
|
|
|
|
|
Sublease
dated December 21, 2017 by and between Kure Franchise, LLC and
Level Brands, Inc.
|
|
10-K
|
|
12/26/17
|
|
10.66
|
|
|
|
|
|
License
Agreement dated December 30, 2017 by and between Level Brands, Inc.
and Isodiol International, Inc.
|
|
8-K
|
|
1/5/18
|
|
10.67
|
|
|
|
|
|
Advisory
Agreement dated March 8, 2018 by and between Level Brands, Inc. and
Nic Mendoza
|
|
10-Q
|
|
5/15/18
|
|
10.69
|
|
|
|
|
|
Advisory
Agreement dated March 8, 2018 by and between Level Brands, Inc. and
Tommy Meharey
|
|
10-Q
|
|
5/15/18
|
|
10.70
|
|
|
|
|
|
Advisory
Agreement dated March 8, 2018 by and between Level Brands, Inc. and
Stephen Roseberry
|
|
10-Q
|
|
5/15/18
|
|
10.71
|
|
|
|
|
|
Sublease
effective April 11, 2018 by and between 4th Floor Properties
LLC and Level Brands, Inc.
|
|
10-Q
|
|
5/15/18
|
|
10.72
|
|
|
|
|
|
Amendment
to promissory note with Stone Street Partners LLC
|
|
10-Q
|
|
8/14/18
|
|
10.74
|
|
|
|
|
|
License
Agreement dated June 26, 2018 by and between Level Brands, Inc. and
Boston Therapeutics, Inc.
|
|
8-K
|
|
6/27/18
|
|
10.73
|
|
|
|
|
|
First
Amendment to License Agreement dated January 19, 2018 by and
between Level Brands, Inc. and Isodiol International,
Inc.
|
|
8-K
|
|
1/22/18
|
|
10.69
|
|
|
|
|
|
Executive
Employment Agreement dated September 6, 2018 by and between Level
Brands, Inc. and Martin A. Sumichrast+
|
|
8-K
|
|
9/7/18
|
|
10.75
|
|
|
|
|
|
Executive
Employment Agreement dated September 6, 2018 by and between Level
Brands, Inc. and Mark S. Elliott+
|
|
8-K
|
|
9/7/18
|
|
10.76
|
|
|
|
|
|
Secured
Promissory Note dated December 4, 2018 in the principal amount of
$2,000,000 from Cure Based Development LLC
|
|
8-K
|
|
12/4/18
|
|
10.1
|
|
|
|
|
|
Security
Agreement dated December 4, 2018 by and between Level Brands, Inc.
and Cure Based Development, LLC
|
|
8-K
|
|
12/4/18
|
|
10.2
|
|
|
|
|
|
Form of
leak out agreement
|
|
8-K
|
|
12/20/18
|
|
10.1
|
|
|
|
|
|
Form of
voting proxy
|
|
8-K
|
|
12/20/18
|
|
10.2
|
|
|
|
|
|
6%
promissory note dated December 20, 2018 to Edge of Business,
LLC
|
|
8-K
|
|
12/20/18
|
|
10.3
|
|
|
|
|
|
Executive
Employment Agreement dated December 20, 2018 by and between cbdMD
LLC and R. Scott Coffman+
|
|
8-K
|
|
12/20/18
|
|
10.4
|
|
|
|
|
|
Executive
Employment Agreement dated December 20, 2018 by and between cbdMD
LLC and Caryn Dunayer+
|
|
8-K
|
|
12/20/18
|
|
10.5
|
|
|
|
|
|
Mutual
Termination of License Agreement dated January 7, 2019 by and
between Level Brands, Inc. and Isodiol International,
Inc
|
|
8-K
|
|
1/1/19
|
|
10.1
|
|
|
|
|
|
Amendment
to Advisory Agreement dated January 14, 2019 with Maxim Group
LLC
|
|
10-Q
|
|
2/14/19
|
|
10.85
|
|
|
|
|
|
Advisory
Agreement dated January 15, 2019 with Joseph Gunnar
LLC
|
|
10-Q
|
|
2/14/19
|
|
10.86
|
|
|
|
|
|
Amendment
to Wholesale License Agreement dated September 8, 2017 by and
between Level Brands, Inc., and kathy ireland ®
Worldwide
|
|
10-Q
|
|
2/15/19
|
|
10.87
|
|
|
|
|
|
Office
Lease, dated July 11, 2019
|
|
10-Q
|
|
8/14/19
|
|
10.1
|
|
|
|
|
|
Form of
Lock-Up Agreement with the Affiliates
|
|
8-K
|
|
5/14/19
|
|
10.1
|
|
|
|
|
|
Form of
Lock-Up Agreement with the FINRA Member
|
|
8-K
|
|
5/14/19
|
|
10.2
|
|
|
|
|
|
Form of
Lock-Up Agreement
|
|
8-K
|
|
10/16/19
|
|
10.1
|
|
|
|
|
|
Code of
Business Conduct and Ethics
|
|
1-A
|
|
9/18/17
|
|
15.1
|
|
|
|
|
|
Subsidiaries
of the registrant
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Consent
of Cherry Bekaert LLP
|
|
|
|
|
|
|
|
Filed
|
37
|
|
Power
of attorney (included on signature page of this
report)
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification of Co-Chief Executive
Officer
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification of Co-Chief Executive
Officer
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification of Chief Financial
Officer
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Section 1350 Certification of Chief Executive Officer and Chief
Financial Officer
|
|
|
|
|
|
|
|
Filed
|
|
|
|
Audited
financial statements of Cure Based Development, LLC for the period
of August 3, 2017 (inception) through December 31, 2017 and for the
eight months ended August 31, 2018
|
|
8-K
|
|
12/20/18
|
|
99.1
|
|
|
|
|
|
Unaudited
financial statements of Cure Based Development LLC for the nine
months ended September 30, 2018
|
|
8-K
|
|
2/22/19
|
|
99.2
|
|
|
|
|
|
Proforma
Financial statements of Level Brands, Inc for the fiscal year ended
September 30, 2018.
|
|
8-K
|
|
2/22/19
|
|
99.3
|
|
|
|
|
101
INS
|
|
XBRL
Instance Document
|
|
|
|
|
|
|
|
Filed
|
|
101 SCH
|
|
XBRL
Taxonomy Extension Schema
|
|
|
|
|
|
|
|
Filed
|
|
101 CAL
|
|
XBRL
Taxonomy Extension Calculation Linkbase
|
|
|
|
|
|
|
|
Filed
|
|
101
LAB
|
|
XBRL
Taxonomy Extension Label Linkbase
|
|
|
|
|
|
|
|
Filed
|
|
101
PRE
|
|
XBRL
Taxonomy Extension Presentation Linkbase
|
|
|
|
|
|
|
|
Filed
|
|
101
DEF
|
|
XBRL
Taxonomy Extension Definition Linkbase
|
||||||||
|
|
|
|
||||||||
|
+
|
|
Indicated
management contract or compensatory plan.
|
||||||||
38
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the
Shareholders of
cbdMD,
Inc. and subsidiaries
Charlotte,
North Carolina
Opinion on the Financial Statements
We have
audited the accompanying consolidated balance sheets of cbdMD, Inc.
and subsidiaries (the “Company”) as of September 30,
2019 and 2018, and the related consolidated statements of income,
comprehensive income (loss), shareholders’ equity, and cash
flows for each of the years in the two-year period ended September
30, 2019, and the related notes (collectively referred to as the
financial statements). In our opinion, the financial statements
present fairly, in all material respects, the financial position of
the Company as of September 30, 2019 and 2018, and the results of
its operations and its cash flows for each of the years in the
two-year period ended September 30, 2019, in conformity with
accounting principles generally accepted in the United States of
America.
Basis for Opinion
These
financial statements are the responsibility of the Company’s
management. Our responsibility is to express an opinion on the
Company’s financial statements based on our audits. We are a
public accounting firm registered with the Public Company
Accounting Oversight Board (United States) (“PCAOB”)
and are required to be independent with respect to the Company in
accordance with the U.S. federal securities laws and the applicable
rules and regulations of the Securities and Exchange Commission and
the PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB.
Those standards require that we plan and perform the audit to
obtain reasonable assurance about whether the financial statements
are free of material misstatement, whether due to error or fraud.
The Company is not required to have, nor were we engaged to
perform, an audit of its internal control over financial reporting.
As part of our audits, we are required to obtain an understanding
of internal control over financial reporting, but not for the
purpose of expressing an opinion on the effectiveness of the
Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our
audits included performing procedures to assess the risks of
material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting
principles used and significant estimates made by management, as
well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis
for our opinion.
|
/s/
Cherry Bekaert LLP
|
|
|
|
|
|
We have
served as the Company’s auditor since 2016.
|
|
|
|
|
|
Charlotte,
North Carolina
|
|
|
December
18,
2019
|
|
See
Notes to Consolidated Financial Statements
F-1
CBDMD, INC.
CONSOLIDATED BALANCE SHEETS
SEPTEMBER 30, 2019 AND 2018
|
|
2019
|
2018
|
|
Assets
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
Cash
and cash equivalents
|
$4,689,966
|
$4,282,553
|
|
Accounts
receivable
|
1,425,697
|
307,874
|
|
Accounts
receivable - related party
|
-
|
1,537,863
|
|
Accounts
receivable other
|
160,137
|
1,743,874
|
|
Accounts
receivable – discontinued operations
|
1,080,000
|
-
|
|
Marketable
securities
|
198,538
|
1,050,961
|
|
Investment
other securities
|
600,000
|
1,159,112
|
|
Note
receivable
|
-
|
459,000
|
|
Note
receivable – related party
|
-
|
156,147
|
|
Deposits
|
6,850
|
-
|
|
Merchant
reserve
|
519,569
|
-
|
|
Inventory
|
4,301,586
|
123,223
|
|
Inventory
prepaid
|
903,458
|
-
|
|
Deferred
issuance costs
|
93,954
|
28,049
|
|
Prepaid
consulting agreement
|
-
|
200,000
|
|
Prepaid
rent
|
-
|
180,000
|
|
Prepaid
software
|
206,587
|
-
|
|
Prepaid
equipment deposits
|
868,589
|
-
|
|
Prepaid
expenses and other current assets
|
688,104
|
561,491
|
|
Total
current assets
|
15,743,035
|
11,790,147
|
|
|
|
|
|
Other
assets:
|
|
|
|
Property
and equipment, net
|
1,715,557
|
53,480
|
|
Deposits
for facilities
|
754,533
|
-
|
|
Intangible
assets, net
|
21,635,000
|
3,173,985
|
|
Goodwill
|
54,669,997
|
-
|
|
Total
other assets
|
78,775,087
|
3,227,465
|
|
|
|
|
|
Total
assets
|
$94,518,122
|
$15,017,612
|
See
Notes to Consolidated Financial Statements
F-2
CBDMD, INC.
CONSOLIDATED BALANCE SHEETS
SEPTEMBER 30, 2019 AND 2018
(continued)
|
|
2019
|
2018
|
|
Liabilities
and shareholders' equity
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
Accounts
payable
|
$3,021,271
|
$473,717
|
|
Accounts
payable – related party
|
-
|
7,860
|
|
Deferred
revenue
|
-
|
161,458
|
|
Accrued
expenses
|
681,269
|
6,920
|
|
Accrued
expenses – related party
|
-
|
320,000
|
|
Customer
deposit – related party
|
7,339
|
-
|
|
Total
current liabilities
|
3,709,878
|
969,955
|
|
|
|
|
|
Long term
liabilities
|
|
|
|
Long
term liabilities
|
363,960
|
7,502
|
|
Contingent
liability
|
50,600,000
|
-
|
|
Deferred
tax liability
|
2,240,300
|
21,000
|
|
Total long term
liabilities
|
53,204,260
|
28,502
|
|
|
|
|
|
Total
liabilities
|
56,914,138
|
998,457
|
|
|
|
|
|
cbdMD, Inc.
shareholders' equity:
|
|
|
|
Preferred stock,
authorized 50,000,000 shares, $0.001 par value, no shares issued
and outstanding
|
-
|
-
|
|
Common stock,
authorized 150,000,000 shares, $0.001 par value,
|
|
|
|
27,720,356
and 8,123,928 shares issued and outstanding,
respectively
|
27,720
|
8,124
|
|
Additional paid in
capital
|
97,186,524
|
21,781,095
|
|
Accumulated other
comprehensive income (loss)
|
-
|
(2,512,539)
|
|
Accumulated
deficit
|
(59,610,260)
|
(6,669,497)
|
|
Total
cbdMD, Inc. shareholders' equity
|
37,603,984
|
12,607,183
|
|
Non-controlling
interest
|
-
|
1,411,972
|
|
Total
shareholders' equity
|
37,603,984
|
14,019,155
|
|
|
|
|
|
Total
liabilities and shareholders' equity
|
$94,518,122
|
$15,017,612
|
See
Notes to Consolidated Financial Statements
F-3
CBDMD, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
|
|
2019
|
2018
|
|
Sales
|
$28,023,848
|
$-
|
|
Sales
related party
|
55,596
|
459,091
|
|
Total
Gross Sales
|
28,079,444
|
459,091
|
|
Allowances
|
(4,427,893)
|
-
|
|
Net
sales
|
23,595,955
|
-
|
|
Net
sales related party
|
55,596
|
459,091
|
|
Total Net Sales
|
23,651,551
|
459,091
|
|
Costs
of sales
|
9,136,677
|
104,010
|
|
Gross profit
|
14,514,873
|
355,081
|
|
|
|
|
|
Operating
expenses excluding impairment losses
|
28,875,186
|
-
|
|
Impairment
of intangible assets
|
436,578
|
-
|
|
Operating
expenses
|
29,311,764
|
2,152,149
|
|
Income (loss) from
operations
|
(14,796,891)
|
(1,797,068)
|
|
Realized
and unrealized gain (loss) on marketable securities
|
(102,716)
|
-
|
|
Impairment
on investment other securities
|
(502,560)
|
-
|
|
(Increase)
decrease of contingent liability
|
(32,461,680)
|
-
|
|
Interest
income
|
75,071
|
-
|
|
Income (loss) before provision
for income taxes
|
(47,788,776)
|
(1,797,068)
|
|
Provision
for income taxes
|
2,359,000
|
-
|
|
Net Income (loss) from
continuing operations
|
(45,429,776)
|
(1,797,068)
|
|
Net
Income (loss) from discontinued operations, net of tax (Note
15)
|
(5,927,773)
|
1,859,902
|
|
|
|
|
|
Net Income (loss)
|
$(51,357,549)
|
62,834
|
|
Net Income (loss) attributable to non-controlling interest from
discontinued operations (Note 15)
|
(929,323)
|
474,909
|
|
Net Income (loss) attributable to common shareholders
|
$(50,428,226)
|
$(412,075)
|
|
|
|
|
|
Earnings (Loss) per share
|
|
|
|
Continuing
operations
|
$(2.54)
|
$(0.23)
|
|
Discontinued
operations
|
(0.28)
|
0.18
|
|
Basic
earnings per share
|
$(2.82)
|
$(0.05)
|
|
Basic
weighted average number of shares
|
17,887,247
|
7,742,644
|
|
|
|
|
|
Diluted
|
|
|
|
Continuing
operations
|
$-
|
$-
|
|
Discontinued
operations
|
-
|
0.18
|
|
Diluted
earnings per share
|
$-
|
$-
|
|
Diluted
weighted average number of shares
|
17,887,247
|
7,749,942
|
See
Notes to Consolidated Financial Statements
F-4
CBDMD, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
|
|
2019
|
2018
|
|
|
|
|
|
Net
Income (Loss)
|
$(51,357,549)
|
$62,834
|
|
Other
Comprehensive Income:
|
|
|
|
Continued
operations – Net Unrealized Gain (Loss) on Marketable
Securities, net of tax of $0
|
-
|
(130,026)
|
|
Discontinued
operations - Net Unrealized Gain (Loss) on Marketable Securities,
net of tax of $0
|
-
|
(2,382,513)
|
|
Comprehensive Income (Loss)
|
(51,357,549)
|
(2,449,705)
|
|
|
|
|
|
Comprehensive
Income (loss) attributable to non-controlling interest
|
(929,323)
|
474,909
|
|
Comprehensive Income (Loss) attributable to cbdMD, Inc. common
shareholders
|
$(50,428,226)
|
$(2,924,614)
|
See
Notes to Consolidated Financial Statements
F-5
CBDMD, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
|
|
2019
|
2018
|
|
Cash
flows from operating activities:
|
|
|
|
Net income
(loss)
|
$(51,357,549)
|
$62,834
|
|
Adjustments
to reconcile net loss to net
|
|
|
|
cash
used by operating activities:
|
|
|
|
Stock
based compensation
|
2,458,530
|
639,631
|
|
Restricted
stock expense
|
230,000
|
39,100
|
|
Depreciation
and amortization
|
289,574
|
222,546
|
|
Issuance
of stock / warrants for services
|
289,750
|
496,502
|
|
Realized
and unrealized (gain)/loss on marketable securities
|
2,439,996
|
-
|
|
Impairment
on investment other securities
|
502,560
|
-
|
|
Inventory
impairment
|
-
|
262,343
|
|
Impairment
on discontinued operations
|
3,398,438
|
-
|
|
Payment
in-kind interest
|
(30,000)
|
-
|
|
Loss on
sale of property and equipment -discontinued
operations
|
39,013
|
69,311
|
|
Increase/(decrease)
in contingent liability
|
32,461,680
|
-
|
|
Intangible
impairment
|
436,578
|
240,000
|
|
Non-cash
consideration received for services provided
|
(470,000)
|
(3,404,502)
|
|
Changes
in operating assets and liabilities:
|
|
|
|
Accounts
receivable
|
60,155
|
(499,373)
|
|
Accounts
receivable - related party
|
(462,137)
|
(492,577)
|
|
Other
accounts receivable
|
2,737
|
(1,890,434)
|
|
Other
accounts receivable – related party
|
-
|
236,364
|
|
Inventory
|
(3,123,437)
|
255,894
|
|
Note
receivable
|
-
|
(459,000)
|
|
Note
receivable – related party
|
156,147
|
120,228
|
|
Deposits
|
(761,383)
|
-
|
|
Merchant
reserve
|
(93,316)
|
-
|
|
Prepaid
inventory
|
(903,458)
|
-
|
|
Proceeds
from sale of securities
|
410,094
|
-
|
|
Prepaid
consulting agreement
|
-
|
(200,000)
|
|
Prepaid
rent
|
180,000
|
(180,000)
|
|
Prepaid
expenses and other current assets
|
(963,044)
|
(529,335)
|
|
Accounts
payable and accrued expenses
|
2,280,726
|
285,156
|
|
Accounts
payable and accrued expenses – related party
|
(7,502)
|
(951,824)
|
|
Deferred
Revenue/customer deposits
|
(416,619)
|
120,041
|
|
Deferred
tax liability
|
(2,425,000)
|
(16,000)
|
|
Cash
used by operating activities
|
(15,377,467)
|
(5,573,095)
|
|
|
|
|
|
Cash
flows from investing activities:
|
|
|
|
Net
cash used for merger
|
(916,555)
|
-
|
|
-
|
(300,000)
|
|
|
Purchase
of intangible assets
|
(50,000)
|
(360,000)
|
|
Purchase
of property and equipment
|
(1,198,618)
|
(23,559)
|
|
Cash provided by
(used by) investing activities
|
(2,133,850)
|
(683,559)
|
|
|
|
|
See
Notes to Consolidated Financial Statements
F-6
CBDMD, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
(continued)
|
Cash
flows from financing activities:
|
|
|
|
Proceeds
from issuance of common stock
|
19,009,897
|
10,927,535
|
|
Note
payable – related party
|
(764,300)
|
-
|
|
Deferred
issuance costs
|
(326,868)
|
(672,574)
|
|
Cash provided by
financing activities
|
17,918,729
|
10,254,961
|
|
Net (decrease)
increase in cash
|
407,413
|
3,998,307
|
|
Cash and cash
equivalents, beginning of year
|
4,282,553
|
284,246
|
|
Cash
and cash equivalents, end of year
|
$4,689,966
|
$4,282,553
|
Supplemental Disclosures of Cash Flow Information:
|
|
2019
|
2018
|
|
|
|
|
|
Cash Payments
for:
|
|
|
|
Interest
expense
|
$39,295
|
$955
|
|
|
|
|
|
|
|
|
|
Non-cash
financial/investing activities:
|
|
|
|
Equity investment
exchange to be issued in the future, included in Accounts
receivable other
|
$-
|
$160,000
|
|
Stock received for
prior period services, adjusted for other accounts receivable write
down prior to receipt – from discontinued
operations
|
$1,352,000
|
$-
|
|
Warrants issued to
secondary selling agent
|
$309,592
|
171,600
|
|
Adoption of ASU
2016-01
|
$2,512,539
|
$-
|
|
Acquisition of
property and equipment through a finance lease
arrangement
|
$249,100
|
$-
|
See
Notes to Consolidated Financial Statements
F-7
CBDMD, INC.
CONSOLIDATED STATEMENT OF SHAREHOLDERS' (DEFICIT)
EQUITY
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
|
|
Common Stock
|
Additional Paid in
|
Other Comprehensive
|
Accumulated
|
Non-controlling
|
|
|
|
|
Shares
|
Amount
|
Capital
|
Income(loss)
|
Deficit
|
Interest
|
Total
|
|
Balance, September 30, 2018
|
8,123,928
|
8,124
|
21,781,095
|
(2,512,539)
|
(6,669,497)
|
1,411,972
|
14,019,155
|
|
Issuance of
common stock
|
1,971,428
|
1,971
|
6,355,027
|
-
|
-
|
-
|
6,356,998
|
|
Issuance of
options for share based compensation
|
-
|
-
|
143,673
|
-
|
-
|
-
|
143,673
|
|
Issuance of
stock costs
|
-
|
-
|
(205,569)
|
-
|
-
|
-
|
(205,569)
|
|
Adoption of
ASU 2016-01
|
-
|
-
|
-
|
2,512,539
|
(2,512,539)
|
-
|
-
|
|
Net
loss
|
-
|
-
|
-
|
-
|
(2,109,715)
|
(79,149)
|
(2,188,864)
|
|
Balance, December 31, 2018
|
10,095,356
|
10,095
|
28,074,224
|
-
|
(11,291,751)
|
1,332,823
|
18,125,391
|
|
Issuance of
options for share based compensation
|
-
|
-
|
19,475
|
-
|
-
|
-
|
19,475
|
|
Issuance of
stock and warrants for services
|
75,000
|
75
|
289,675
|
-
|
-
|
-
|
289,750
|
|
Net
loss
|
-
|
-
|
-
|
-
|
(31,791,738)
|
(58,536)
|
(31,850,274)
|
|
Balance, March 31, 2019
|
10,170,356
|
10,170
|
28,383,374
|
-
|
(43,083,489)
|
1,274,287
|
(13,415,658)
|
|
Issuance of
common stock for merger
|
15,250,000
|
15,250
|
53,199,913
|
-
|
-
|
-
|
53,215,163
|
|
Issuance of
common stock
|
2,300,000
|
2,300
|
12,650,600
|
-
|
-
|
-
|
12,652,900
|
|
Issuance of
options for share based compensation
|
-
|
-
|
1,859,664
|
-
|
-
|
-
|
1,859,664
|
|
Issuance of
stock costs
|
-
|
-
|
(55,393)
|
-
|
-
|
-
|
(55,393)
|
|
Issuance of
stock and warrants for services
|
|
|
92,000
|
-
|
-
|
-
|
92,000
|
|
Net
loss
|
-
|
-
|
-
|
-
|
(27,699,247)
|
(1,503,707)
|
(29,202,954)
|
|
Balance, June 30, 2019
|
27,720,356
|
27,720
|
96,130,158
|
-
|
(70,782,736)
|
(229,420)
|
25,145,722
|
|
Issuance of
options for share based compensation
|
-
|
-
|
573,718
|
-
|
-
|
-
|
573,718
|
|
Transfer NCI
to APIC (see Note14)
|
-
|
-
|
482,648
|
-
|
-
|
(482,648)
|
-
|
|
Net income
(loss)
|
-
|
-
|
-
|
-
|
11,172,476
|
712,068
|
11,884,544
|
|
Balance, September 30, 2019
|
27,720,356
|
27,720
|
97,186,524
|
-
|
(59,610,260)
|
-
|
37,603,984
|
See
Notes to Consolidated Financial Statements
F-8
CBDMD, INC.
CONSOLIDATED STATEMENT OF SHAREHOLDERS' (DEFICIT)
EQUITY
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
|
|
Common Stock
|
Additional Paid in
|
Other Comprehensive
|
Accumulated
|
Non-controlling
|
|
|
|
|
Shares
|
Amount
|
Capital
|
Income(loss)
|
Deficit
|
Interest
|
Total
|
|
Balance, September 30, 2017
|
5,792,261
|
5,792
|
10,463,480
|
-
|
(6,257,421)
|
937,063
|
5,148,914
|
|
Issuance of
common stock
|
2,000,000
|
2,000
|
9,971,114
|
-
|
-
|
-
|
9,973,114
|
|
Issuance of
options for share based compensation
|
-
|
-
|
17,114
|
-
|
-
|
-
|
17,114
|
|
Issuance of
stock for deferred IPO costs
|
-
|
-
|
171,600
|
-
|
-
|
-
|
171,600
|
|
Issuance of
stock and warrants for services
|
6,667
|
7
|
36,995
|
-
|
-
|
-
|
37,002
|
|
Issuance of
restricted stock for share based compensation
|
-
|
-
|
39,100
|
-
|
-
|
-
|
39,100
|
|
Other
Comprehensive income (loss)
|
-
|
-
|
-
|
33,500
|
-
|
-
|
33,500
|
|
Net
loss
|
-
|
-
|
-
|
-
|
(1,132,928)
|
(131,855)
|
(1,264,783)
|
|
Balance, December 31, 2017
|
7,798,928
|
7,799
|
20,699,403
|
33,500
|
(7,390,349)
|
805,208
|
14,155,561
|
|
Issuance of
common stock
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
|
Issuance of
options for share based compensation
|
-
|
-
|
13,952
|
-
|
-
|
-
|
13,952
|
|
Issuance of
stock and warrants for services
|
235,000
|
235
|
19,765
|
-
|
-
|
-
|
20,000
|
|
Other
Comprehensive income (loss)
|
-
|
-
|
-
|
(630,077)
|
-
|
-
|
(630,077)
|
|
Net
income
|
-
|
-
|
-
|
-
|
1,404,397
|
238,523
|
1,642,920
|
|
Balance, March 31, 2018
|
8,033,928
|
8,034
|
20,733,120
|
(596,577)
|
(5,985,952)
|
1,043,731
|
15,202,356
|
|
Issuance of
options for share based compensation
|
-
|
-
|
355,653
|
-
|
-
|
-
|
355,653
|
|
Issuance of
stock and warrants for services
|
85,000
|
85
|
420,915
|
-
|
-
|
-
|
421,000
|
|
Other
Comprehensive income (loss)
|
-
|
-
|
-
|
(1,326,727)
|
-
|
-
|
(1,326,727)
|
|
Net
income
|
-
|
-
|
-
|
-
|
206,073
|
359,180
|
565,253
|
|
Balance, June 30, 2018
|
8,118,928
|
8,119
|
21,509,688
|
(1,923,304)
|
(5,779,879)
|
1,402,911
|
15,217,535
|
|
Issuance of
options for share based compensation
|
-
|
-
|
252,912
|
-
|
-
|
-
|
252,912
|
|
Issuance of
stock and warrants for services
|
5,000
|
5
|
18,495
|
-
|
-
|
-
|
18,500
|
|
Other
Comprehensive income (loss)
|
-
|
-
|
-
|
(589,235)
|
-
|
-
|
(589,235)
|
|
Net income
(loss)
|
-
|
-
|
-
|
-
|
(889,618)
|
9,061
|
(880,557)
|
|
Balance, September 30, 2018
|
8,123,928
|
8,124
|
21,781,095
|
(2,512,539)
|
(6,669,497)
|
1,411,972
|
14,019,155
|
See
Notes to Consolidated Financial Statements
F-9
CBDMD, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2019 AND 2018
NOTE 1 – ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING
POLICIES
cbdMD,
Inc. ("cbdMD", "we", "us", “our”, "Parent
Company” or the “Company”) is a North Carolina
corporation formed on March 17, 2015 as Level Beauty Group, Inc. In
November 2016 we changed the name of the Company to Level Brands,
Inc. On April 22, 2019, following
approval by our shareholders at the 2019 annual meeting held on
April 19, 2019, we filed Articles of Amendment to our Articles of
Incorporation changing the name of our Company to “cbdMD,
Inc.” effective May 1, 2019. We operate from our
offices located in Charlotte, North Carolina. Our fiscal year end
is established as September 30.
On December 20, 2018 the Company, and its newly organized
wholly-owned subsidiaries AcqCo, LLC and cbdMD LLC, completed a
two-step merger (the “Mergers”) with Cure Based
Development, LLC, a Nevada limited liability company (“Cure
Based Development”). Upon completion of the Mergers, cbdMD
LLC survived and operates the prior business of Cure Based
Development. On April 10, 2019, cbdMD LLC was renamed to CBD
Industries LLC (“CBDI”). As consideration for the
Mergers, the Company had a contractual obligation, after approval
by our shareholders, to issue 15,250,000 shares of our common stock
to the members of Cure Based Development, of which 8,750,000 of the
shares will vest over a five year period and are subject to a
voting proxy agreement, as well as to issue another 15,250,000
shares of our common stock in the future upon earnout goals being
within the next 5 years. The Company’s shareholders approved
the issuance of the 15,250,000 shares of common stock and they were
issued to members of Cure Based Development on April 19, 2019. CBDI
produces and distributes
various high-grade, premium
cannabidiol oil (“CBD”) products under the cbdMD brand.
CBD is a natural substance produced from the hemp plant and the
products manufactured by CBDI are non psychoactive as they do not
contain tetrahydrocannabinol (THC).
Effective
September 30, 2019, the Company abandoned and ceased operations of
four business subsidiaries: Encore Endeavor 1, LLC
(“EE1”), I’M1, LLC (“IM1”), Beauty
and Pin Ups, LLC (“BPU”) and Level H&W, LLC
(“Level H&W”). Therefore, the results of operations
related to these subsidiaries for the Company are reported as
discontinued operations.
Principles of Consolidation
The
consolidated financial statements include the accounts of the
Company and its wholly owned subsidiary CBDI. All material
intercompany transactions and balances have been eliminated in
consolidation.
Reclassifications
Certain
amounts previously presented in the consolidated financial
statements have been reclassified to conform to the current year
presentation. Such reclassifications had no effect on
previously reported net loss, shareholders’ equity or cash
flows.
Use of Estimates
The
preparation of the Company's consolidated financial statements have
been prepared in accordance with accounting principles generally
accepted in the United States (“US GAAP”), and requires
management to make estimates and assumptions that affect amounts of
assets and liabilities and disclosures of contingent assets and
liabilities as of the date of the financial statements and reported
amounts of revenues and expenses during the periods presented.
Estimates and assumptions are reviewed periodically and the effects
of revisions are reflected in the consolidated financial statements
in the period they are determined to be necessary. Significant
estimates made in the accompanying consolidated financial
statements include, but are not limited to, allowances for doubtful
accounts, inventory valuation reserves, expected sales returns and
allowances, certain assumptions related to the valuation of
investments other securities, common stock issued prior to the IPO,
acquired intangible and long-lived assets and the recoverability of
intangible and long-lived assets and income taxes, including
deferred tax valuation allowances and reserves for estimated tax
liabilities, contingent liability and, hence consideration for the
Mergers is a material estimate. Actual results could differ from
these estimates.
F-10
Cash and Cash Equivalents
For
financial statements purposes, the Company considers all highly
liquid investments with a maturity of less than three months when
acquired to be cash equivalents.
Accounts receivable and Accounts receivable other
Accounts
receivable are stated at cost less an allowance for doubtful
accounts, if applicable. Credit can be extended to wholesale and
retail customers after an evaluation of customer’s financial
condition, and generally collateral is not required as a condition
of credit extension. Management’s determination of the
allowance for doubtful accounts is based on an evaluation of the
receivables, past experience, current economic conditions, and
other risks inherent in the receivables portfolio. As of September 30, 2019, we have an allowance for
doubtful accounts of $7,286, and had no allowance at September 30,
2018.
In
addition, the Company has in the past entered into contracts where
a portion of the consideration provided by the customer in exchange
for the Company's services is common stock, options or
warrants (an equity position). In these situations, upon
invoicing the customer for the stock or other instruments, the
Company will record the receivable as accounts receivable other,
and use the value of the stock or other instrument upon invoicing
to determine the value. Where an accounts receivable is settled
with the receipt of the common stock or other instrument, the
common stock or other instrument will be classified as an asset on
the balance sheet as either an investment marketable security (when
the customer is a publicly traded entity) or as an investment other
security (when the customer is a privately held
entity).
Accounts
receivable and accounts receivable other items that involve a
related party are indicated as such on the face of the consolidated
financial statements.
Receivable and Merchant Reserve
The
Company primarily sells its products through the internet and has
an arrangement to process customer payments with third-party
payment processors. The arrangement with the payment processors
requires that the Company pay a fee between 5.95% - 6.95% of the
transaction amounts processed. Pursuant to this agreement, there is
a waiting period between 2 - 14 days prior to reimbursement to the
Company, and as well as a calculated reserve which some payment
processors hold back. Fees and reserves can change periodically
with notice from the processors. At September 30, 2019, the
receivable from payment processors included approximately $227,050
for the waiting period amount and is recorded as accounts
receivable in the accompanying consolidated balance sheet and
$519,569 for the reserve amount for a total receivable of
$746,619.
Marketable Securities
Marketable
securities that are equity securities are carried at fair value on
the consolidated balance sheets with changes in fair value recorded
as an unrealized gain or (loss) in the Statements of Operations in
the period of the change. Upon the disposition of a marketable
security, the Company records a realized gain or (loss) on the
Company’s consolidated statements of operations. On
October 1, 2018, as a result of the adoption of ASU 2016-01 –
Financial Instruments, the
Company reclassified $2,512,539 of net unrealized losses on
marketable securities, that were formerly classified as
available-for-sale securities before the adoption of the new
standard, from Accumulated Other Comprehensive Loss to Accumulated
Deficit.
Investment Other Securities
For
equity investments where the Company neither controls nor has
significant influence over the investee and which are
non-marketable, which is without a readily determinable fair value,
the Company may elect to estimate its fair value at cost less
impairment plus or minus changes resulting from observable price
changes.
Inventory
Inventory
is stated at the lower of cost or net realizable value with cost
being determined on a weighted average basis. The cost of inventory
includes product cost, freight-in, and production fill and labor
(portions of which we outsource to third party manufacturers).
Write-offs of potentially slow moving or damaged inventory are
recorded based on management’s analysis of inventory levels,
forecasted future sales volume and pricing and through specific
identification of obsolete or damaged products. We assess inventory
quarterly for slow moving products and potential impairments and at
a minimum perform a physical inventory count annually near fiscal
year end.
F-11
Customer Deposits
Customer
deposits consist of payments received in advance of revenue
recognition. Revenue is recognized as revenue recognition criteria
are met.
Property and Equipment
Property
and equipment items are stated at cost less accumulated
depreciation. Expenditures for routine maintenance and repairs are
charged to operations as incurred. Depreciation is charged to
expense over the estimated useful lives of the assets using the
straight-line method. Generally, the useful lives are five years
for show booths and equipment, three years for computer, furniture
and equipment, and three years for software. The cost and
accumulated depreciation of property are eliminated from the
accounts upon disposal, and any resulting gain or loss is included
in the consolidated statements of operations for the applicable
period. Long-lived assets held and used by the Company are reviewed
for impairment whenever changes in circumstance indicate the
carrying value of an asset may not be recoverable.
Fair value accounting
The
Company utilizes accounting standards for fair value, which include
the definition of fair value, the framework for measuring fair
value, and disclosures about fair value measurements. Fair value is
a market-based measurement, not an entity-specific measurement.
Therefore, a fair value measurement should be determined based on
the assumptions that market participants would use in pricing the
asset or liability. As a basis for considering market participant
assumptions in fair value measurements, fair value accounting
standards establish a fair value hierarchy that distinguishes
between market participant assumptions based on market data
obtained from sources independent of the reporting entity
(observable inputs that are classified within Levels 1 and 2
of the hierarchy) and the reporting entity’s own assumptions
about market participant assumptions (unobservable inputs
classified within Level 3 of the hierarchy).
Level 1
inputs utilize quoted prices in active markets for identical assets
or liabilities that the Company has the ability to access.
Level 2 inputs are inputs other than quoted prices included in
Level 1 that are directly or indirectly observable for the
asset or liability. Level 2 inputs may include quoted prices
for similar assets and liabilities in active markets, as well as
inputs that are observable for the asset or liability. Level 3
inputs are unobservable inputs for the asset or liability, which
are based on an entity’s own assumptions, as there is little,
if any, observable market activity. In instances where the fair
value measurement is based on inputs from different levels of the
fair value hierarchy, the level in the fair value hierarchy within
which the entire fair value measurement falls is based on the
lowest level input that is significant to the fair value
measurement in its entirety. The Company’s assessment of the
significance of a particular input to the fair value measurement in
its entirety requires judgment and considers factors specific to
the asset or liability.
When
the Company records an investment in marketable securities the
carrying value is assigned at fair value. Any changes in fair
value for marketable securities during a given period will be
recorded as an unrealized gain or loss in the consolidated
statement of operations. For investment other securities without a
readily determinable fair value, the Company may elect to estimate
its fair value at cost less impairment plus or minus changes
resulting from observable price changes.
Goodwill
Goodwill
represents the excess of cost of an acquired business over the fair
value of the identifiable tangible and intangible assets acquired
and liabilities assumed in a business combination. Identifiable
intangible assets acquired in business combinations are recorded
based on their fair values at the date of acquisition. Goodwill is
not subject to amortization but must be evaluated for impairment
annually. The Company tests for goodwill impairment annually or
whenever events occur or circumstances change that would more
likely than not reduce the fair value of a reporting unit below its
carrying amount.
In
performing a goodwill test, the Company performs a qualitative
evaluation and if necessary, a quantitative evaluation. Factors
considered in the qualitative test include specific operating
results as well as new events and circumstances impacting the
operations or cash flows of the business acquired. For the
quantitative test, the Company assesses goodwill for impairment by
comparing the carrying value of the business to the respective fair
value. The Company determines the fair value of its acquired
business using a combination of income-based and market-based
approaches and incorporates assumptions it believes market
participants would utilize. The income-based approach utilizes
discounted cash flows while the market-based approach utilizes
market multiples. These approaches are dependent upon
internally-developed forecasts that are based upon annual budgets
and longer-range strategic plans. The Company uses discount rates
that are commensurate with the risks and uncertainty inherent in
the respective acquired business and in the internally-developed
forecasts.
F-12
Intangible Assets
The
Company's intangible assets consist of trademarks and other
intellectual property, all of which are accounted for in accordance
with ASC Topic 350, Intangibles – Goodwill and Other. The
Company employs the non-amortization approach to account for
purchased intangible assets having indefinite lives. Under the
non-amortization approach, intangible assets having indefinite
lives are not amortized into the results of operations, but instead
are reviewed annually or more frequently if events or changes in
circumstances indicate that the assets might be impaired, to assess
whether their fair value exceeds their carrying value. We perform
an impairment analysis at August 1 annually on the indefinite-lived
intangible assets following the steps laid out in ASC 350-30-35-18.
Our annual impairment analysis includes a qualitative assessment to
determine if it is necessary to perform the quantitative impairment
test. In performing a qualitative assessment, we review events and
circumstances that could affect the significant inputs used to
determine if the fair value is less than the carrying value of the
intangible assets. If a quantitative analysis is necessary, we
would analyze various aspects including revenues from the business,
associated with the intangible assets. In addition, intangible
assets will be tested on an interim basis if an event or
circumstance indicates that it is more likely than not that an
impairment loss has been incurred.
Intangible
assets with finite useful lives are amortized using the
straight-line method over their estimated period of benefit. In
accordance with ASC 360-10-35-21, definite lived intangibles are
reviewed annually or more frequently if events or changes in
circumstances indicate that the assets might be impaired, to assess
whether their fair value exceeds their carrying value.
In
conjunction with any acquisitions, the Company refers to ASC-805 as
amended by Accounting Standards Update (“ASU”) 2017-01
in determining if the Company is acquiring any inputs, processes or
outputs and the impact that such factors would have on the
classification of the acquisition as a business combination or
asset purchase. Additionally, the Company refers to the
aforementioned guidance in reviewing all acquired assets and
assumed liabilities for valuation in a business combination,
including the determination of intangible asset values and
contingent liabilities.
Contingent
liability
A significant component of the purchase price consideration for the
Company’s acquisition of Cure Based Development includes a
fixed number of future shares to be issued as well as a variable
number of future shares to be issued based upon the
post-acquisition entity reaching certain specified future revenue
targets, as further described in Note 8. The Company made a
determination of the fair value of the contingent liabilities as
part of the valuation of the assets acquired and liabilities
assumed in the business combination.
In determining the fair value of the contingent liability, the
Company utilizes level 3 inputs and engages a third party valuation
firm for assistance in the fair value determination.
The Company recognized both the fixed number of shares to be
issued, and the variable number of shares to be potentially issued,
as contingent liabilities on its Consolidated Balance Sheets. These
contingent liabilities were recorded at fair value upon the
acquisition date and are remeasured quarterly based on the
reassessed fair value as of the end of that quarterly reporting
period. Additionally, as the fixed shares were issued on
April 19, 2019, the value of the shares at that time, in the amount
of $53,215,163, was reclassified from contingent liability to
additional paid in capital on the balance sheet.
For the fiscal year ended September 30, 2019, the contingent
liabilities associated with the business combination were increased
by $32,461,680 to reflect their reassessed fair values as of
September 30, 2019. This increase is reflective of a change in
value on the variable number of shares from the merger date of
December 20, 2018. In August 2019, the Company updated the forecasts for performance
of the post-acquisition entity based on current trends and
performance that would impact the estimated likelihood that the
revenue targets disclosed in Note 8 would be met. The primary
catalyst for the $32,461,680 increase in contingent liabilities is
the change in the Company’s share price between December 20,
2018 and September 30, 2019. These increases or reductions to the
contingent liabilities are reflected within Other Expenses on the
consolidated statements of operations.
F-13
Revenue Recognition
The
Company adopted ASC 606, Revenue
from Contracts with Customers using the modified
retrospective method beginning with our quarter ended December 31,
2018. The adoption of the new revenue standards as of October 1,
2018 did not change the Company’s revenue recognition as the
majority of its revenues continue to be recognized when the
customer takes control of its product, the services have been
rendered, or the usage-based royalty has been earned. As the
Company did not identify any accounting changes that impacted the
amount of reported revenues with respect to any of its revenue
streams, no adjustment to retained earnings was required upon
adoption.
Under
ASC 606, the Company recognizes revenues when its customer obtains
control of promised goods or services, in an amount that reflects
the consideration which it expects to receive in exchange for those
goods. The Company recognizes revenues following the five step
model prescribed under ASC 606: (i) identify contract(s) with a
customer; (ii) identify the performance obligations in the
contract; (iii) determine the transaction price; (iv) allocate the
transaction price to the performance obligations in the contract;
and (v) recognize revenues when (or as) we satisfy the performance
obligation.
Performance Obligations
A
performance obligation is a promise in a contract to transfer a
distinct good or service to a customer. The Company has reviewed
its various revenue streams for its existing contracts under the
five-step approach. The Company has entered into various license
agreements that provide revenues based on guarantee minimum royalty
payments with additional royalty revenues based on a percentage of
defined sales. Guaranteed minimum royalty payments (fixed revenue)
are recognized on a straight-line basis over the term of the
contract, as defined in each license agreement. Earned royalties
and earned royalties in excess of the fixed revenue (variable
revenue) are recognized as income during the period corresponding
to the licensee’s sales. Earned royalties in excess of fixed
revenue are only recognized when the Company is reasonably certain
that the guaranteed minimums payments for the period will be
exceeded.
The
below table summarizes amounts related to future performance
obligations under fixed contractual arrangements as of September
30, 2019:
|
|
At September 30,
2019
|
2020 and
thereafter
|
|
|
|
|
|
Future
performance obligations
|
$0
|
$0
|
Allocation of transaction price
In our
current business model we do not have contracts with customers
which have multiple elements as revenue is driven purely by online
product sales or purchase order based product sales. However, at
times in the past, the Company had entered into contracts with
customers wherein there were multiple elements that may have
disparate revenue recognition patterns. In such instances, the
Company must allocate the total transaction price to these various
elements. This is achieved by estimating the standalone selling
price of each element, which is the price at which we sell a
promised good or service separately to a customer.
In
circumstances where we have not historically sold relevant products
or services on a standalone basis, the Company utilizes the most
situationally appropriate method of estimating standalone selling
price. These methods include (i) an adjusted market assessment
approach, wherein we refer to prices from our competitors for
similar goods or serves and adjust those prices as necessary to
reflect our typical costs and margins, (ii) an expected cost plus
margin approach, wherein we forecast the costs that we will incur
in satisfying the identified performance obligation and adding an
appropriate margin to such costs, and (iii) a residual approach,
wherein we adjust the total transaction price to remove all
observable standalone selling prices of other goods or services
included in the contract and allocate the entirety of the remaining
contract amount to the remaining obligation.
F-14
Revenue recognition
The
Company records revenue from the sale of its products when risk of
loss and title to the product are transferred to the customer,
which is upon shipping (and is typically FOB shipping) which is
when our performance obligation is met. Net sales are comprised of
gross revenues less product returns, trade discounts and customer
allowances, which include costs associated with off-invoice
mark-downs and other price reductions, as well as trade promotions.
These incentive costs are recognized at the later of the date on
which the Company recognizes the related revenue or the date on
which the Company offers the incentive. The Company currently
offers a 30 day, money back guarantee.
In
regard to sales for services provided, the Company records revenue
when the customer has accepted services and the Company has a right
to payment. Based on the contracted services, revenue is recognized
when the Company invoices customers for completed services at
agreed upon rates or revenue is recognized over a fixed period of
time during which the service is performed.
Disaggregated Revenue
Our
product revenue is generated primarily through two sales channels,
E-commerce and wholesale channels. We also generate service related
sales, although this type of revenue is not a primary focus. We
believe that these categories appropriately reflect how the nature,
amount, timing and uncertainty of revenue and cash flows are
impacted by economic factors.
A description of our principal revenue generating activities are as
follows:
-
Consumer
sales - consumer products sold through our online and telephonic
channels. Revenue is recognized when control of the merchandise is
transferred to the customer, which generally occurs upon shipment.
Payment is typically due prior to the date of
shipment.
-
Wholesale
sales - products sold to our wholesale customers for subsequent
resale. Revenue is recognized when control of the goods is
transferred to the customer, in accordance with the terms of the
applicable agreement. Payment terms vary and can typically be 30
days from the date control over the product is transferred to the
customer.
-
Service
related sales – services provided to organizations typically
consulting services related to branding, marketing, or advisory.
Revenue is recognized when services are delivered to the customer,
in accordance with the terms of the applicable agreement. Payment
terms vary and typically are based on deliverables and agreed upon
timelines.
The
following table represents a disaggregation of revenue by sales
channel:
|
|
Fiscal
2019
|
% of
total
|
Fiscal
2018
|
% of
total
|
|
Wholesale product
sales
|
$8,878,901
|
37.5%
|
$-
|
0%
|
|
Consumer product
sales
|
14,772,650
|
62.5%
|
-
|
0%
|
|
Service related
sales
|
-
|
-
|
459,091
|
100%
|
|
Total net
sales
|
$23,651,551
|
|
$459,091
|
|
Contract Balances
Contract
assets represent unbilled receivables and are presented within
accounts receivable, net on the condensed consolidated balance
sheets. Contract liabilities represent unearned revenues and are
presented as deferred revenue or customer deposits on the condensed
consolidated balance sheets.
F-15
The
below table summarize the net change in contract assets and
contract liabilities from October 1, 2018 to September 30, 2019, of
which all of these contract liabilities are associated with the
discontinued operations more fully described in Note
15:
|
|
Entertainment
|
Products
|
Licensing
|
Total
|
|
Balance at
September 30, 2018
|
$37,500
|
-
|
$115,625
|
$153,125
|
|
Billed during three
months ended December 31, 2018
|
75,000
|
265,000
|
-
|
340,000
|
|
Earned during three
months ended December 31, 2018
|
(68,750)
|
-
|
(115,625)
|
(184,375)
|
|
Balance at December
31, 2018
|
$43,750
|
$265,000
|
-
|
$308,750
|
|
Amount returned
during three months ended March 31, 2019
|
|
(175,000)
|
|
(175,000)
|
|
Billed during three
months ended March 31, 2019
|
-
|
-
|
10,000
|
10,000
|
|
Earned during three
months ended March 31, 2019
|
(18,750)
|
-
|
(1,667)
|
(20,417)
|
|
Balance at March
31, 2019
|
$25,000
|
$90,000
|
$8,333
|
$123,333
|
|
Billed during three
months ended June 30, 2019
|
-
|
-
|
-
|
-
|
|
Earned during three
months ended June 30, 2019
|
(18,750)
|
(55,596)
|
(1,667)
|
(76,013)
|
|
Balance at June 30,
2019
|
$6,250
|
$34,404
|
$6,666
|
$47,320
|
|
Billed during three
months ended September 30, 2019
|
-
|
-
|
-
|
-
|
|
Earned or written
off during three months ended September 30, 2019
|
(6,250)
|
(34,404)
|
(6,666)
|
(47,320)
|
|
Balance at
September 30, 2019
|
$-
|
$-
|
$-
|
$-
|
Cost of Sales
Our cost of sales includes costs associated with distribution, fill
and labor expense, components, manufacturing overhead, third-party
providers, and outbound freight for our products sales, and
includes labor for our service sales. For our product sales,
cost of sales also includes the cost of refurbishing products
returned by customers that will be offered for resale, if any, and
the cost of inventory write-downs associated with adjustments of
held inventories to their net realizable value. These expenses are
reflected in the Company’s consolidated statements of
operations when the product is sold and net sales revenues are
recognized or, in the case of inventory write-downs, when
circumstances indicate that the carrying value of inventories is in
excess of their net realizable value.
Advertising Costs
The
Company expenses all costs of advertising and related marketing and
promotional costs as incurred. The Company incurred approximately
$5,151,795 and $143,701 in advertising and related marketing and
promotional costs included in operating expenses during the years
ended September 30, 2019 and 2018, respectively.
Shipping and Handling Fees and Costs
All
fees billed to customers for shipping and handling are classified
as a component of sales. All costs associated with shipping and
handling are classified as a component of cost of goods
sold.
F-16
Income Taxes
The
Parent Company is a North Carolina corporation that is treated as a
corporation for federal and state income tax purposes. Prior to
April 2017, BPU was a multi-member limited liability company that
was treated as a partnership for federal and state income tax
purposes. As such, the Parent Company’s partnership share in
the taxable income or loss of BPU was included in the tax return of
the Parent Company. Beginning in April 2017, the Parent Company
acquired the remaining interests in BPU. As a result of the
acquisition, BPU became a disregarded entity for tax purposes and
its entire share of taxable income or loss was included in the tax
return of the Parent Company. CBDI and Level H&W are wholly
owned subsidiaries and are disregarded entities for tax purposes
and their entire share of taxable income or loss is included in the
tax return of the Parent Company. IM1 and EE1 are multi-member
limited liability companies that are treated as partnerships for
federal and state income tax purposes. As such, the Parent
Company’s partnership share in the taxable income or loss of
IM1 and EE1 are included in the tax return of the Parent
Company.
The
Parent Company accounts for income taxes pursuant to the provisions
of the Accounting for Income Taxes topic of the FASB ASC 740 which
requires, among other things, an asset and liability approach to
calculating deferred income taxes. The asset and liability approach
requires the recognition of deferred tax assets and liabilities for
the expected future tax consequences of temporary differences
between the carrying amounts and the tax bases of assets and
liabilities. The Parent Company uses the inside basis approach to
determine deferred tax assets and liabilities associated with its
investment in a consolidated pass-through entity. A valuation
allowance is provided to offset any net deferred tax assets for
which management believes it is more likely than not that the net
deferred asset will not be realized.
US GAAP
requires management to evaluate tax positions taken by the Company
and recognize a tax liability (or asset) if the Company has taken
an uncertain tax position that more likely than not would not be
sustained upon examination by the Internal Revenue Service.
Management has analyzed the tax positions taken by the Company, and
has concluded that as of September 30, 2019 and 2018, there were no
uncertain tax positions taken or expected to be taken that would
require recognition of a liability (or asset) or disclosure in the
consolidated financial statements.
Concentrations
Financial
instruments that potentially expose the Company to concentrations
of credit risk consist primarily of cash and cash equivalents,
accounts receivable, and securities.
The
Company places its cash and cash equivalents on deposit with
financial institutions in the United States. The Federal Deposit
Insurance Corporation (“FDIC”) covers $250,000 for
substantially all depository accounts. The Company from time to
time may have amounts on deposit in excess of the insured limits.
The Company had a $4,097,190 uninsured balance at September 30,
2019 and a $0 uninsured balance at September 30, 2018. Funds which
are not subject to coverage or loss under FDIC were $0 and
$4,003,003 at September 30, 2019 and 2018,
respectively.
Concentration
of credit risk with respect to receivables is principally limited
to trade receivables with corporate customers that meet specific
credit policies. Management considers these customer receivables to
represent normal business risk. The Company did not have any
customers that represented a significant amount of our sales for
the year ended September 30, 2019. We have four customers whose
aggregate accounts receivable balance was approximately 84% of the
combined total accounts receivable and accounts receivable
discontinued operations as of September 30, 2019. The Company had
three customers whose revenue in total represented 75% of the
Company’s net sales for the year ended September 30, 2018,
such customers represented 51%, 10% and 14% of net sales. The
aggregate accounts receivable balance of such customers represented
92% of the Company’s total accounts receivable as of
September 30, 2018.
Stock-Based Compensation
We
account for our stock compensation under ASC -718-10-30
“Compensation - Stock Compensation” using the fair
value based method. Under this method, compensation cost is
measured at the grant date based on the value of the award and is
recognized over the service period, which is usually the vesting
period. This guidance establishes standards for the accounting for
transactions in which an entity exchanges its equity instruments
for goods or services. It also addresses transactions in which an
entity incurs liabilities in exchange for goods or services that
are based on the fair value of the entity's equity instruments or
that may be settled by the issuance of those equity
instruments.
F-17
We use
the Black-Scholes model for measuring the fair value of options and
warrants. The stock based fair value compensation is determined as
of the date of the grant or the date at which the performance of
the services is completed (measurement date) and is recognized over
the vesting periods. Under ASU 2016-09 which amends ASC 718, which
became effective October 1, 2017, we elected to change our
accounting principle to recognize forfeitures when they occur. This
change had no impact on beginning retained earnings as there had
been no forfeitures estimated or incurred in prior
periods.
Earnings (Loss) Per Share
The
Company uses ASC 260-10, “Earnings Per Share” for
calculating the basic and diluted loss per share. The Company
computes basic loss per share by dividing net loss attributable to
common shareholders by the weighted average number of common shares
outstanding. Common equivalent shares are excluded from the
computation of net loss per share if their effect is
anti-dilutive.
At
September 30, 2019 and 2018, 1,643,255 and 781,826 potential
shares, respectively, were excluded from the shares used to
calculate diluted loss per share as their inclusion would reduce
net loss per share.
Deferred IPO or issuance costs
In
following the guidance under ASC 340-10-S99-1, IPO or issuance
costs directly attributable to an offering of equity securities
have been deferred and charged against the gross proceeds of the
offering as a reduction of additional paid-in capital. These costs
include legal fees related to the registration drafting and
counsel, independent audit costs directly related to the
registration and offering, SEC filing and print related costs,
exchange listing costs, and IPO/issuance roadshow related
costs.
New Accounting Standards
In May
2014, August 2015 and May 2016, the FASB issued ASU 2014-09,
Revenue from Contracts with
Customers, and ASU 2015-14 Revenue from Contracts with Customers,
Deferral of the Effective Date, respectively, which
implement ASC Topic 606. ASC Topic 606 outlines a single
comprehensive model for entities to use in accounting for revenue
arising from contracts with customers and supersedes most current
revenue recognition guidance under US GAAP, including
industry-specific guidance. It also requires entities to disclose
both quantitative and qualitative information that enable financial
statements users to understand the nature, amount, timing, and
uncertainty of revenue and cash flows arising from contracts with
customers. Subsequently, the FASB has issued the following
standards related to ASU 2014-09: ASU No. 2016-08,
Revenue from Contracts with
Customers (Topic 606): Principal versus Agent Considerations
(“ASU 2016-08”); ASU No. 2016-10, Revenue from Contracts with Customers
(Topic 606): Identifying
Performance Obligations and Licensing (“ASU
2016-10”); ASU No. 2016-12, Revenue from Contracts with Customers
(Topic 606): Narrow-Scope
Improvements and Practical Expedients (“ASU
2016-12”); and ASU No. 2016-20, Technical Corrections and Improvements to
Topic 606, Revenue from Contracts with Customers (“ASU
2016-20”). The amendments in these ASUs are effective for
annual periods beginning after December 15, 2017, and interim
periods therein. Early adoption is permitted for annual periods
beginning after December 15, 2016. These ASUs may be applied
retrospectively to all prior periods presented, or retrospectively
with a cumulative adjustment to retained earnings in the year of
adoption. The new revenue standards became effective for the
Company on October 1, 2018 and were adopted using the modified
retrospective method. The adoption of the new revenue standards as
of October 1, 2018 did not change the Company’s revenue
recognition as the majority of its revenues continue to be
recognized when the customer takes control of its product, the
services have been rendered, or the royalty has been received. As
the Company did not identify any accounting changes that impacted
the amount of reported revenues with respect to its product
revenues, no adjustment to retained earnings was required upon
adoption.
In
February 2016, the FASB issued ASU 2016-02, Leases. The purpose of ASU
2016-02 is to establish the principles to report transparent and
economically neutral information about the assets and liabilities
that arise from leases. This guidance results in a more faithful
representation of the rights and obligations arising from operating
and capital leases by requiring lessees to recognize the lease
assets and lease liabilities that arise from leases in the
statement of financial position and to disclose qualitative and
quantitative information about lease transactions, such as
information about variable lease payments and options to renew and
terminate leases. ASU 2016-02 is effective for fiscal years and
interim periods beginning after December 15, 2018. The Company does
have a 3 year lease for a manufacturing facility and is assessing
the impact of implementing this guidance on its consolidated
financial position, results of operations and
liquidity.
F-18
In August 2018, the FASB issued ASU No.
2018-13, Fair Value Measurement (Topic
820). The ASU modifies,
removes, and adds several disclosure requirements on fair value
measurements in Topic 820, Fair Value Measurement. The ASU 2018-13
is effective for all entities for fiscal years, and interim periods
within those fiscal years, beginning after December 15, 2019. The
amendments on changes in unrealized gains and losses, the range and
weighted average of significant unobservable inputs used to develop
Level 3 fair value measurements, and the narrative description of
measurement uncertainty should be applied prospectively for only
the most recent interim or annual period presented in the initial
fiscal year of adoption. All other amendments should be applied
retrospectively to all periods presented upon their effective date.
Early adoption is permitted upon issuance of ASU 2018-13. An entity
is permitted to early adopt any removed or modified disclosures
upon issuance of ASU 2018-13 and delay adoption of the additional
disclosures until their effective date. The Company is evaluating
the effect ASU 2018-13 will have on its consolidated financial
statements and disclosures and has not yet determined the effect of
the standard on its ongoing financial reporting at this
time.
NOTE 2 – ACQUISITIONS
On December 20, 2018 (the “Closing”), the Company, and
its newly organized wholly-owned subsidiaries AcqCo, LLC and cbdMD
LLC, both North Carolina limited liability companies, completed a
two-step merger (the “Merger Agreement”) with Cure
Based Development, LLC, a Nevada limited liability company
(“Cure Based Development”). The Merger Agreement
provided that AcqCo LLC merge with and into Cure Based Development
with Cure Based Development as the surviving entity (the
“Merger”), and immediately thereafter Cure Based
Development merged with and into cbdMD LLC with cbdMD LLC as the
surviving entity (the “Secondary Merger” and
collectively with the Merger, the “Mergers”). cbdMD LLC
was renamed on April 10, 2019 to CBD Industries LLC
(“CBDI”) and has continued as a wholly-owned subsidiary
of the Company and maintains the operations of Cure Based
Development pre-closing. As consideration for the Merger, the
Company has a contractual obligation, after approval by our
shareholders, to issue 15,250,000 shares of our common stock to the
members of Cure Based Development, of which 8,750,000 of the shares
will vest over a five year period and are subject to a voting proxy
agreement. The Merger Agreement also provides that an additional
15,250,000 shares of our common stock can be issued upon the
satisfaction of aggregate net revenue criteria by CBDI, within 60
months following the Closing. The net revenue criteria are: $20.0,
$40.0, $80.0 and $160.0 million, in aggregate $300.0 million (See
Note 8 for more information).
The initial 15,250,000 shares were approved by our shareholders and
issued on April 19, 2019.
The
Company owns 100% of the equity interest of CBDI. The valuation and
purchase price allocation for the Mergers has been finalized at
September 30, 2019.
During
the three months ended March 31, 2019, the Company identified
equipment that was improperly excluded from the identified assets
acquired in the Mergers. The fair value of this equipment was
determined to be $114,275. The purchase price allocation was
adjusted by increasing Property and equipment, net and reducing
Goodwill by this amount.
In
preparing the fiscal year end tax provision, the Company decreased
the deferred tax liability by $463,700 and reduced Goodwill by this
amount.
During
the three months ended June 30, 2019, the Company identified
interest overly accrued valued at $10,572 that was in the initial
purchase price allocation. The purchase price allocation was
adjusted by decreasing goodwill and accrued expenses by this
amount.
F-19
The
following table presents the final purchase price
allocation:
|
Consideration
|
$74,353,483
|
|
|
|
|
Assets
acquired:
|
|
|
Cash
and cash equivalents
|
$1,822,331
|
|
Accounts
receivable
|
850,921
|
|
Inventory
|
1,054,926
|
|
Other
current assets
|
38,745
|
|
Property
and equipment, net
|
723,223
|
|
Intangible
assets
|
21,585,000
|
|
Goodwill
|
54,669,997
|
|
Total
assets acquired
|
80,745,143
|
|
|
|
|
Liabilities
assumed:
|
|
|
Accounts
payable
|
257,081
|
|
Notes
payable – related party
|
764,300
|
|
Customer
deposits - related party
|
265,000
|
|
Accrued
expenses
|
460,979
|
|
Deferred
tax liability
|
4,644,300
|
|
Total
Liabilities assumed
|
6,391,660
|
|
|
|
|
Net
Assets Acquired
|
$74,353,483
|
The
goodwill generated from this transaction can be attributed to the
benefits the Company expects to realize from the growth strategies
the acquired Company had developed and the entry into an emerging
market with high growth potential. See Note 8 regarding contingent
liability.
In
connection with the purchase price allocation, the Company recorded
a deferred tax liability of approximately $5,108,000, with a
corresponding increase to goodwill, for the tax effect of the
acquired intangible assets from Cure Base Development. This
liability was recorded as there will be no future tax deductions
related to the acquired intangibles, and we have identified these
as indefinite-lived intangible assets.
The
Company also acquired estimated net operating loss carryforwards of
approximately $1,996,000, Under Internal Revenue Code (IRC) Section
382, the use of net operating loss (“NOL”)
carryforwards may be limited to an annual limit if a change in
ownership of a company occurs.
NOTE 3 – MARKETABLE SECURITIES AND INVESTMENT OTHER
SECURITIES
The
Company may, from time to time, enter into contracts where a
portion of the consideration provided by the customer in exchange
for the Company's services is common stock, options or
warrants (an equity position). In these situations, upon
invoicing the customer for the stock or other instruments, the
Company will record the receivable as accounts receivable other,
and use the value of the stock or other instrument upon invoicing
to determine the value. If there is insufficient data to support
the valuation of the security directly, the company will value it,
and the underlying revenue, on the estimated fair value of the
services provided. Where an accounts receivable other is settled
with the receipt of the common stock or other instrument, the
common stock or other instrument will be classified as an asset on
the balance sheet as either an investment marketable security (when
the customer is a public entity) or as an investment other security
(when the customer is a privately held entity).
F-20
On June
23, 2017, I’M1 and EE1 in aggregate exercised a warrant for
1,600,000 shares of common stock for services delivered to a
customer and accounted for this in Investment other securities. The
common stock was issued to the Company’s subsidiaries
I’M1 and EE1. The customer is a private entity and the stock
was valued at $912,000, which was based on its recent financing in
June 2017 at $0.57 per share. The Company has classified this
common stock as Level 3 for fair value measurement purposes as
there are no observable inputs. In valuing the stock the Company
used the fair value of the services provided, utilizing an analysis
of vendor specific objective evidence of its selling price. In
August 2017, each of I’M1 and EE1 distributed the shares to
its majority owner, cbdMD, and also distributed shares valued at
$223,440 to its non-controlling interests. In August 2017, the
Company also provided referral services for kathy Ireland®
Worldwide and this customer. As compensation the Company received
an additional 200,000 shares of common stock valued at $114,000
using the pricing described above. On December 21, 2017, the
Company purchased 300 shares of preferred stock in a private
offering from this customer for $300,000. The preferred shares are
convertible into common stock at a 20% discount of a defined
subsequent financing, or an IPO offering of a minimum $15 million,
or at a company valuation of $45 million whichever is the least.
The Company has classified this stock as Level 3 for fair value
measurement purposes as there are no observable inputs. In valuing
the stock the Company used the value paid, which was the price
offered to all third party investors. Subsequently, the Company has
recently met with other investors of the customer and has indicated
desire to sell the equity interest of the Company. As of September
30, 2019, based on conversations with other investors, the market
for this equity, and potential selling prices negotiated, the
Company has determined that the value at September 30, 2019 is
$600,000 and an impairment of $502,560 is appropriate for the year
ended September 30, 2019.
On
September 19, 2017, I’M1 and EE1 in aggregate exercised a
warrant for 56,552 shares of common stock for services delivered to
a customer and accounted for this in Investment other securities.
The common stock was issued to the Company’s subsidiaries
I’M1 and EE1. The customer is a private entity and the stock
was valued at $56,552, which was based on all 2017 financing
transactions of the customer set at $1.00 per share, with the most
recent third party transaction in August 2017. The Company has
classified this common stock as Level 3 for fair value measurement
purposes as there are no observable inputs. In valuing the stock
the Company uses factors including financial projections provided
by the issuer and conversations with the issuer management
regarding the Company’s recent results and future plans and
the Company’s financing transactions over the past twelve
months. The Company assessed the investment and a recorded a full
impairment of $56,552 for the year ended September 30, 2019 in the
discontinued operations.
In
December 2017, the Company completed services per an advisory
services agreement with Kure Corp, formerly a related party. As
payment for these services, Kure Corp issued 800,000 shares of its
stock to the Company. The customer was a private entity and the
stock was valued at $400,000, which was based on financing
activities by Kure Corp in September 2017 in which shares were
valued at $0.50 per share. The Company had classified this common
stock, cumulative value of $400,000, as Level 3 for fair value
measurement purposes as there were no observable inputs. In valuing
the stock the Company used factors including information provided
by the issuer regarding their recent results and future plans as
well as their most recent financing transactions. On April 30,
2018, Kure Corp. merged with Isodiol International, Inc. (CSE:
ISOL, OTCQB: ISOLF, FSE:LB6A.F), a Canadian company. Details can be
reviewed in our Form 10-K previously filed. As a result of this
merger we received the first issuance of 380,952 shares from
Isodiol and valued them based on the trading price on April 30,
2018 of $0.63 per share which totaled $240,000. We also removed the
value of the Kure equity of $400,000 from our Level 3 investments
as part of the exchange described above. As the full value of the
Kure equity will not be received until the future issuances based
on earn out goals, we have recorded an accounts receivable other of
$160,000 as of December 31, 2018. On March 31, 2019, Isodiol spun
off Kure to its original shareholders by issuing back all original
Kure stock. As a result of the spin off, the Company will receive
800,000 shares of Kure stock which we valued at the $160,000 and as
Kure is private, the shares will be treated as a Level 3 stock and
will be accounted for as the $160,000 accounts receivable other.
The Company has determined that the 800,000 shares have a fair
market value over $160,000. The Company has assessed the common
stock and determined there was not an indication of an impairment
at September 30, 2019.
On
December 30, 2017 the Company entered into an Agreement with
Isodiol International, Inc. (CSE: ISOL, OTCQB: ISOLF, FSE:LB6A.F),
a Canadian company which is a developer of pharmaceutical grade phytochemical
compounds and a manufacturer and developer of phytoceutical
consumer products. As payment for these services, the
Company has received 1,226,435 shares of Isodiol common stock
between December 31, 2017 and January 2019. The Company also
received 38,095 shares of Isodiol stock upon Isodiol’s
acquisition of Kure Corp, giving the Company a total of 1,264,530
shares. At September 30, 2019, the
Company has 1,042,193 shares valued at
$198,538.
F-21
The
table below summarizes the assets valued at fair value as of
September 30, 2019:
|
|
In Active
Markets for Identical Assets and Liabilities
(Level
1)
|
Significant
Other Observable Inputs
(Level
2)
|
Significant
Unobservable Inputs
(Level
3)
|
Total Fair Value
at September 30, 2019
|
|
|
|
|
|
|
|
Marketable
securities
|
$198,538
|
-
|
$-
|
$198,538
|
|
Investment other
securities
|
-
|
-
|
$600,000
|
$600,000
|
|
|
Level
1
|
Level
2
|
Level
3
|
Total
|
|
Balance at
September 30, 2017
|
$-
|
$-
|
$859,112
|
$859,112
|
|
Receipt of equity
investment upon completion of services
|
$3,004,500
|
$-
|
$400,000
|
$3,404,500
|
|
Purchase of
preferred shares, convertible into common stock
|
$-
|
$-
|
$300,000
|
$300,000
|
|
Exchange of equity
via owner merger into public company
|
$240,000
|
$-
|
$(400,000)
|
$(160,000)
|
|
Change in value of
equities, other comprehensive income
|
$(2,193,539)
|
$-
|
$-
|
$(2,193,539)
|
|
Balance at
September 30, 2018
|
$1,050,961
|
$-
|
$1,159,112
|
$2,210,073
|
|
Receipt of equity
investment upon completion of services
|
$470,000
|
$-
|
$-
|
$470,000
|
|
Transfer from AR
Other
|
$1,500,000
|
$-
|
$-
|
$1,500,000
|
|
Sale of
equities
|
$(382,428)
|
$-
|
$-
|
$(382,428)
|
|
Change in value of
equities,
|
$(2,439,995)
|
$-
|
$(559,112)
|
$(2,999,107)
|
|
Balance at
September 30, 2019
|
$198,538
|
$-
|
$600,000
|
$798,538
|
NOTE 4 –
INVENTORY
Inventory at September 30, 2019 and 2018 consists of the
following:
|
|
September 30,
|
September 30,
|
|
|
2019
|
2018
|
|
Continued
Operations:
|
|
|
|
Finished
goods
|
$3,050,120
|
$-
|
|
Inventory
components
|
1,251,466
|
-
|
|
Inventory
prepaid
|
903,458
|
-
|
|
Total
Continued Operations
|
$5,205,044
|
$-
|
|
Discontinued
Operations:
|
|
|
|
Finished
goods
|
$-
|
$18,531
|
|
Inventory
components
|
-
|
104,692
|
|
Inventory
prepaid
|
-
|
-
|
|
Total
Discontinued Operations
|
$--
|
$123,223
|
|
Total
|
$5,205,044
|
$123,223
|
F-22
The discontinued operations at the year ended September 30, 2019
and 2018 had an impairment on inventory of $139,217 and $262,000,
respectively. Impairment charges were recorded within operating
expenses for the respective periods.
NOTE 5 – PROPERTY AND EQUIPMENT
Major classes of property and equipment at September 30, 2019 and
2018 consist of the following:
|
|
September 30,
|
September 30,
|
|
|
2019
|
2018
|
|
Continued
Operations:
|
|
|
|
Computers,
furniture and equipment
|
$131,077
|
$18,258
|
|
Manufacturing
equipment
|
1,375,986
|
-
|
|
Leasehold
improvements
|
375,954
|
-
|
|
Automobiles
|
24,892
|
-
|
|
Manufactures’
molds and plates
|
-
|
-
|
|
|
1,907,909
|
18,258
|
|
Less
accumulated depreciation
|
(192,352)
|
(13,652)
|
|
Net
property and equipment -continued operations
|
$1,715,557
|
$4,606
|
|
Discontinued
Operations:
|
|
|
|
Computers,
furniture and equipment
|
$-
|
$41,512
|
|
Show
booth and equipment
|
-
|
49,123
|
|
Manufactures’
molds and plates
|
-
|
34,200
|
|
|
-
|
124,835
|
|
Less
accumulated depreciation
|
-
|
(75,961)
|
|
Net
property and equipment -discontinued operations
|
$-
|
$48,874
|
|
Total
Net property and equipment
|
$1,715,557
|
$53,480
|
Depreciation expense for continuing operations related to property
and equipment was $187,987 and $6,235 for the years ended September
30, 2019 and 2018, respectively. Depreciation expense for
discontinued operations related to property and equipment was
$9,861 and $30,009 for the years ended September 30, 2019 and
2018.
NOTE 6 – INTANGIBLE ASSETS
With
the Mergers of Cure Based Development, the Company has made a
strategic shift toward the CBD business and all entities and their
associated intangibles have been assessed during the year ended
September 30, 2019 with that focus and their ability to support
that business line.
On
September 8, 2017, the Company entered into a seven year wholesale
license agreement with Andre Carthen and issued 45,500 shares of
common stock, valued at $179,725. In addition, the Company agreed
to pay $65,000 in cash within 30 days completion of its initial
public offering and also issued warrants to purchase 45,500 shares
of common stock at a strike price of $4.00. The warrants were
valued at $65,338. Under the terms of this nonexclusive agreement,
we have the right to use, assign and sublicense the marks,
intellectual property and other rights in connection with "Chef
Andre," "Andre Carthen," ACafe" or "Fit Chef" and all trade names,
trademarks and service marks related to this intellectual property
for the purpose of entering into sublicense agreements with third
parties for the manufacture, marketing and sale of products
utilizing these marks. In December 2018, the parties amended the
agreement to remove the annual minimum guarantee in return for a
one time payment of $70,000. We are amortizing the capitalized
value of the cash, warrants and common stock over the seven year
term of the agreement. Effective September 30, 2019 it was
determined that this asset was no longer useable for the current
business focus and at September 30, 2019, the Company recorded an
impairment charge for the full carrying value of $296,460 (see
below).
F-23
On
September 8, 2017, the Company entered into a seven year wholesale
license agreement with Nicholas Walker and issued 25,000 shares of
common stock, valued at $98,750. In addition, the Company agreed to
pay $40,000 in cash within 30 days completion of its initial public
offering and also issued warrants to purchase 25,000 shares of
common stock at a strike price of $4.00. The warrants were valued
at $35,900. Under the terms of this nonexclusive agreement, we have
the right to use, assign and sublicense the marks, intellectual
property and other rights in connection with "Jardin," "Nicholas
Walker," "Nicholas Walker Jardin," "Nicholas Walker Garden Party,"
"Cultivated by Nicholas Walker," and "Jardin Du Jour," and all
trade names, trademarks and service marks related to this
intellectual property for the purpose of entering into sublicense
agreements with third parties for the manufacture, marketing and
sale of products utilizing these marks. In December 2018, the
parties amended the agreement to remove the annual minimum
guarantee in return for a one time payment of $10,000. We are
amortizing the capitalized value of the cash, warrants and common
stock over the seven year term of the agreement. Effective
September 30, 2019 it was determined that this asset was no longer
useable for the current business focus and at September 30, 2019,
the Company recorded an impairment charge for the full carrying
value of $140,118 (see below).
On
December 20, 2018, the Company completed the Mergers with Cure
Based Development and acquired certain assets, including the
trademark "cbdMD" and its variants and certain other intellectual
property. The trademark is the cornerstone of this subsidiary and
is key as we create and distribute products and continue to build
this brand. We believe the trademark does not have limits on the
time it will contribute to the generation of cash flows and
therefore we have identified these as indefinite-lived intangible
assets (see Note 2 for more
information).
In September 2019, the Company purchased the rights to the
trademark name HempMD for $50,000. This trademark will be used in
the marketing and branding of certain products to be released under
this brand name. We believe the trademark does not have limits on
the time it will contribute to the generation of cash flows and
therefore we have identified these as indefinite-lived intangible
assets.
Intangible
assets as of September 30, 2019 and 2018 consisted of the
following:
|
|
September
30,
|
September
30,
|
|
|
2019
|
2018
|
|
Trademark related
to cbdMD
|
$21,585,000
|
$-
|
|
Trademark for
HempMD
|
50,000
|
-
|
|
Wholesale license
agreement with Chef Andre Carthen, net
|
-
|
262,077
|
|
Wholesale license
agreement with Nicholas Walker, net
|
-
|
147,620
|
|
Continuing
Operations
|
21,635,000
|
409,697
|
|
|
|
|
|
Trademark and other
intellectual property related to I’M1
|
-
|
971,667
|
|
Trademark and other
intellectual property related to EE1
|
-
|
471,667
|
|
Trademark,
tradename and other intellectual property related to kathy
ireland® Health & Wellness™, net
|
-
|
1,074,194
|
|
Trademark and other
intellectual property related to BPU
|
-
|
246,760
|
|
Discontinued
Operations
|
-
|
2,764,288
|
|
Total
|
$21,635,000
|
$3,173,985
|
The
Company performs an impairment analysis at August 1 annually on the
indefinite-lived intangible assets following the guidance in ASC
350-30-35-18. Our annual impairment analysis includes a qualitative
assessment to determine if it is necessary to perform the
quantitative impairment test. In performing a qualitative
assessment, we review events and circumstances that could affect
the significant inputs used to determine if the fair value is less
than the carrying value of the intangible assets. In addition,
intangible assets will be tested on an interim basis if an event or
circumstance indicates that it is more likely than not that an
impairment loss has been incurred and the Company evaluates the
indefinite-lived intangible assets each reporting period to
determine whether events and circumstances continue to support an
indefinite useful life. The Company performed a qualitative
analysis for the year ended September 30, 2019 and has determined
there has been no impairment. As the business focus of the Company
has shifted to the CBD business, all intangible assets were
assessed to determine how they can support the CBD business –
see Note 15 for impairments related to discontinued
operations.
F-24
The
Company also performs an impairment analysis at August 1 annually
on the definite lived intangible assets following the guidance in
ASC 360-10-35-21. We first assess if there is an indicator of
possible impairment such as change in the use of the asset, market
price changes in the asset, or other events that impact the value
of the asset. If an indicator is present we then perform a
quantitative analysis to determine if the carrying amount of the
asset is recoverable. This is done by comparing the total
undiscounted future cash flows of the long-lived asset to its
carrying amount. If the total undiscounted future cash flows exceed
the carrying amount of the asset, the carrying amount is deemed
recoverable and an impairment is not recorded. If the carrying
amount of a long-lived asset is deemed to be unrecoverable, an
impairment loss needs to be estimated.
As
previously indicated, the Company has made a strategic shift toward
the CBD business and all intangible assets were assessed with that
focus and their ability to support that business line. As such the
definite lived intangible assets, the two wholesale license
agreements with Nicolas Walker and Andre Carthen do not fit into
the strategic direction and the value associated with future cash
flows as it relates to these assets may not be positive. As a
result, the Company recorded an impairment charge during the twelve
months ended September 30, 2019 for the definite lived intangible
assets totaling $436,578 which is representative of $296,460 and
$140,118 for the two wholesale license agreements.
In
order to calculate the impairment loss, the fair value of the asset
must be determined. Fair value referenced here is determined using
the guidance in FASB ASC Topic 820.
NOTE 7 – PRO FORMA FINANCIAL INFORMATION
(UNAUDITED)
The
following unaudited pro-forma data summarizes the results of
operations for the year ended September 30, 2019 and 2018, as if
the Mergers with Cure Based Development had been completed on
October 1, 2017. The pro-forma financial information is presented
for informational purposes only and is not indicative of the
results of operations that would have been achieved if the Mergers
had taken place on October 1, 2017. The pro-forma financial
information represents the continuing operations only.
|
|
Fiscal Year Ended
September 30,
2019
|
Fiscal Year Ended
September 30,
2018
|
|
|
|
|
|
Net
sales
|
$26,734,979
|
$4,355,684
|
|
Operating
income (loss)
|
$(15,997,942)
|
$(2,634,895)
|
|
Net
income (loss)
|
$(51,687,603)
|
$(2,618,895)
|
|
Net
income per share – average weighted shares
|
$(1.86)
|
$(0.11)
|
|
Net
income per share – fully diluted
|
$(1.86)
|
$(0.11)
|
For the
per share calculation prior to April 2019, it is being assumed that
the shares to be issued contractually under the Merger Agreement,
upon shareholder approval, were issued at the beginning of each
period. This would account for an additional 6,500,000 shares
issued directly to the members of Cure Based Development and
another 8,750,000 shares issued which would have a voting proxy and
leak out on voting rights over a 5 year period.
NOTE 8 – CONTINGENT LIABILITY
As consideration for the Mergers, described in Note 2, the Company
has a contractual obligation to issue 15,250,000 shares of our
common stock, after approval by our shareholders, to the members of
Cure Based Development, issued in two tranches 6,500,000 and
8,750,000, both of which are subject to leak out provisions, and
the 8,750,000 tranche of shares will also vest over a five year
period and are subject to a voting proxy agreement. The Merger
Agreement also provides that an additional 15,250,000 shares of our
common stock can be issued upon the satisfaction of certain
aggregate net revenue criteria by cbdMD within 60 months following
the Closing Date (“earn out”).
The
contractual obligations and earn out provision are accounted for as
a contingent liability and fair value is determined using Level 3
inputs, as estimating the fair value of these contingent
liabilities require the use of significant and subjective inputs
that may and are likely to change over the duration of the
liabilities with related changes in internal and external market
factors.
F-25
The
initial two tranches totaling 15,250,000 shares have been valued
using a market approach method and included the use of the
following inputs: share price upon contractual obligation, discount
for lack of marketability to address leak out restrictions, and
probability of shareholder disapproval. In addition, the 8,750,000
shares in the second tranche also included an input for a discount
for lack of voting rights during the vest periods.
The
Merger Agreement also provides that an additional 15,250,000 shares
(Earnout Shares) would be issued as part of the consideration for
the Mergers, upon the satisfaction of certain aggregate net revenue
criteria by cbdMD within 60 months following the Closing Date as
follows, as measured at four intervals (Marking Period): the
completion of 12, 24, 42, and 59 calendar months from the Closing
Date, and based upon the ratios set forth below:
|
Aggregate
Net Revenues
|
|
Shares
Issued / Each $ of Aggregate Net Revenue Ratio
|
|
|
|
|
|
$1 -
$20,000,000
|
|
.190625
|
|
$20,000,001
- $60,000,000
|
|
.0953125
|
|
$60,000,001
- $140,000,000
|
|
.04765625
|
|
$140,000,001
- $300,000,000
|
|
.023828125
|
For
clarification purposes, the Aggregate Net Revenues during a Marking
Period shall be multiplied by the applicable Shares Issued/Each $
of Aggregate Net Revenue Ratio, minus, the number of shares issued
as a result of Aggregate Net Revenues during the prior Marking
Periods.
The initial 15,250,000 shares and Earnout Shares were approved by
our shareholders and the initial shares were issued on April 19,
2019.
The
15,250,000 Earnout Shares which would be issued in the future, upon
the satisfaction of net revenue criteria have been valued using a
Monte Carlo Simulation. Inputs used included: stock price,
volatility, interest rates, revenue projections, and likelihood of
obtaining revenue projections, amongst others.
The
value of the contingent liability was $50,600,000 at September 30,
2019 and represents the Earnout Shares. The initial shares were
issued upon shareholder approval on April 19, 2019 and had a
carrying value of $53,215,163. Additionally, as the 15,250,000
initial shares were issued, the value of the shares in the amount
of $53,215,163 was reclassified from the contingent liability to
additional paid in capital on the balance sheet. The Earnout Shares
were valued at $50,600,000 on September 30, 2019 as compared to
$35,941,000 at December 20, 2018. The increase in value of the
contingent liability of $32,461,680 is recorded in the Statement of
Operations for the year ended September 30, 2019 and represents the
change in value of the earnout shares of $14,659,000 and the
increased value in the previously issued initial shares recorded
until issuance of $17,802,679. The Company utilized both a market
approach and a Monte Carlo simulation in valuing the contingent
liability and a key input in both of those methods is the stock
price. The main driver of the increase in the value of the Earnout
Shares within the contingent liability was the increase of the
Company’s stock price, which was $3.96 at September 30, 2019
as compared to $3.11 on December 20, 2018.
NOTE 9 – RELATED PARTY TRANSACTIONS
On December 11, 2017, the Company entered into a service agreement
with Kure Corp., then a related party, to facilitate the
“Vape Pod” transaction with the modular building
systems vendor, SG Blocks, Inc., which is also a customer of our
company. Under the terms of this agreement we also agreed to
facilitate the introduction to third parties in connection with
Kure Corp.'s initiative to establish Vape Pod's at U.S. military
base retail locations and advising and aid in site selection for
Kure retail stores on military bases and adjoining convenience
stores, gas stations, and other similar retail properties utilizing
Kure Corp.'s retail Vape Pod concept, among other services. As
compensation for this agreement, we were issued 400,000 shares of
Kure Corp.'s common stock which was valued at $200,000 (see Note
3).
In June
2018, per our agreement with kathy ireland® Worldwide, the
company earned a referral fee of $150,000 for facilitating a
business opportunity which led to a new license agreement for kathy
ireland® Worldwide. The Company is to receive 50% of all
royalty revenue earned ongoing via the new business contract. This
agreement has been terminated effective September 30,
2019.
F-26
On December 20, 2018, with the closing of the Merger Agreement with
Cure Based Development, we recognized the following related party
transactions which happened prior to the Mergers:
Cure
Based Development received $90,000 from Verdure Holdings LLC for
future orders of the Company’s products. Verdure Holdings LLC
is an affiliate of the CEO of Cure Based Development. This amount
has been adjusted based on sales to Verdure Holdings subsequent to
the mergers and is recorded as customer deposits - related party on
the accompanying balance sheet of $7,339 and reflects related party
sales for fiscal 2019 on the statement of operations of
$55,596.
Cure
Based Development entered a lease for office space, which also
provides administrative and IT services, from an affiliate of the
CEO of Cure Based Development. The lease was a month to month lease
for $9,166 per month and ended September 2019.
Cure
Based Development leases its manufacturing facility from an entity
partially owned by an individual who now has a contractual right to
receive shares of the Company as part of the Mergers. The current
lease was entered into on December 15, 2018 and ends December 15,
2021 and has been amended at an annual base rent rate of $199,200
allowing for a 3% annual increase. In addition, common area
maintenance rent is set at $25,200 annually.
See Note 15 for related party information on the discontinued
operations.
NOTE 10 – SHAREHOLDERS’ EQUITY
Preferred Stock – We are authorized to issue 50,000,000
shares of preferred stock, par value $0.001 per share. As described
in Note 18, in October 2019, the Company designated 5,000,000 of
these shares as 8% Series A Cumulative Convertible Preferred Stock
and subsequently completed an offering and issued 500,000 shares.
Our 8% Series A Cumulative Convertible Preferred Stock ranks senior
to our common stock for liquidation or dividend provisions and are
entitled to receive cumulative cash dividends at an annual rate of
8% payable monthly in arrears for the prior month.
Common Stock – We are authorized to issue 150,000,000 shares
of common stock, par value $0.001 per share. There were 27,720,356
and 8,123,928 shares of common stock issued and outstanding at
September 30, 2019 and 2018, respectively.
Common stock transactions:
Fiscal 2019:
On
October 2, 2018, the Company completed
a secondary public offering of 1,971,428 shares of its common stock
for aggregate gross proceeds of approximately $6.9 million. The
Company received approximately $6.3 million in net proceeds after
deducting underwriting discounts and commissions and other
estimated offering expenses payable by us. The Company also issued
to the selling agent warrants to purchase in aggregate 51,429
shares of common stock with an exercise price of $4.375. The
warrants were valued at $86,092 and expire on September 28,
2023.
In January 2019, we issued 25,000 shares of our common stock to an
investment banking firm for general financial advisory services.
The shares were valued at $77,250, based on the trading price upon
issuance, and is being amortized and expensed as professional
services over the service period ending December 2019.
In January 2019, we issued 50,000 shares of our common stock to an
investment banking firm for general advisory and investment bank
services. The shares were valued at $212,500, based on the trading
price upon issuance, and is being amortized and expensed as
professional services over the service period ending April
2020.
In April 2019, we issued 15,250,000 shares or our common stock as
consideration for the Mergers with Cure Based Development, of which
8,750,000 of the shares will vest over a five year period and are
subject to a voting proxy agreement.
In May 2019, the Company completed a secondary public offering of
2,300,000 shares of its common stock for aggregate gross proceeds
of $13.8 million. The Company received approximately $12.5 million
in net proceeds after deducting underwriting discounts and
commissions and other estimated offering expenses payable by us.
The Company also issued to the selling agent warrants to purchase
in aggregate 60,000 shares of common stock with an exercise price
of $7.50. The warrants were valued at $223,500 and expire on May
15, 2024.
F-27
Fiscal 2018:
On November 17, 2017, the Company completed an IPO of 2,000,000
shares of its common stock for aggregate gross proceeds of $12.0
million and net proceeds of $10.9 million.
In November 2017, we issued 6,667 shares of our common stock to an
individual as part of a consulting agreement. The shares were
valued at $37,002, based on the trading price upon issuance and
expensed as contract compensation.
In January 2018, we issued 230,000 shares of our common stock,
which were granted as restricted stock awards on October 1, 2016 to
board members. The restricted stock awards vested on January 1,
2018. The shares were valued at fair market value upon issuance at
$195,500 and amortized over the vesting period and expensed as
stock compensation.
In March 2018, we issued 5,000 shares of our common stock to an
investor relations firm for services. The shares were valued at
$20,000, based on the trading price upon issuance, and is being
amortized and expensed as professional services over the service
period ending June 2018.
In May 2018, we issued 60,000 shares of our common stock to an
investment banking firm for general financial advisory and
investment banking services. The shares were valued at $303,000,
based on the trading price upon issuance, and were amortized and
expensed as professional services over the service period ending
April 2019.
In June 2018, we issued 25,000 shares of our common stock to a
broker dealer for business advisory services. The shares were
valued at $118,000, based on the trading price upon issuance, and
are amortized and expensed as professional services over the
service period ending December 2019.
In July 2018 we issued 5,000 shares of our common stock to an
investor relations firm for services. The shares were valued at
$18,500, based on the trading price upon issuance, and were
amortized and expensed as professional services over the service
period ending November 2018.
Stock option transactions:
Fiscal 2019:
In August 2019 we granted per the annual board compensation plan,
20,000 common stock options to one non-management director. The
options vest immediately, have an exercise price of $5.41 per share
and a term of ten years. We have recorded an expense for the
options of $83,920 for the fiscal year ended September 30,
2019.
In May 2019 we granted per the annual board compensation plan, an
aggregate of 120,000 common stock options to six independent
directors. The options vest immediately, have an exercise price of
$5.41 per share and a term of ten years. We have recorded an
expense for the options of $562,440 for the fiscal year ended
September 30, 2019.
In May 2019 we granted an aggregate of 610,000 common stock options
to twelve employees. The options vary in amounts issued and vesting
tiers, which include no vesting with an exercise price of $6.40,
vesting at May 15, 2020 with an exercise price of $7.00, vesting at
May 15, 2021 with an exercise price of $7.50, and vesting at May
15, 2022 with an exercise price of $7.50. The options have a term
of ten years. We have recorded an expense for the options of
$1,642,530 for the fiscal year ended September 30,
2019.
F-28
Fiscal 2018:
On May 14, 2018 we granted an aggregate of 50,000 common stock
options to an employee. The options vested 50% November 14, 2018
and 50% May 14, 2019. The options have an exercise price of $5.27
per share and a term of seven years. We recorded an expense for the
options of $38,950 for the fiscal year ended September 30,
2018.
On May 29, 2018 we granted an aggregate of 150,000 common stock
options to an employee. The options vested 50% immediately and 50%
January 1, 2019. The options have an exercise price of $4.78 per
share and a term of ten years. We recorded an expense for the
options of $302,750 for the fiscal year ended September 30,
2018.
On August 16, 2018 we granted per the annual board compensation
plan, an aggregate of 35,000 common stock options to five
directors. The options vested immediately, have an exercise price
of $3.34 per share and a term of ten years. We recorded an expense
for the options of $80,150 for the fiscal year ended September 30,
2018.
The
expected volatility rate was estimated based on comparison to the
volatility of a peer group of companies in the similar industry.
The expected term used was the full term of the contract for the
issuances. The risk-free interest rate for periods within the
contractual life of the option is based on U.S. Treasury
securities. The pre-vesting forfeiture rate of zero is based upon
the experience of the Company. As required under ASC 718, we will
adjust the estimated forfeiture rate to our actual experience.
Management will continue to assess the assumptions and
methodologies used to calculate estimated fair value of share-based
compensation. Circumstances may change and additional data may
become available over time, which could result in changes to these
assumptions and methodologies, and thereby materially impact our
fair value determination.
The following table summarizes the inputs used for the
Black-Scholes pricing model on the options issued in the years
ended September 30, 2019 and 2018:
|
|
|
2019
|
|
2018
|
|
Exercise price
|
|
$5.41 – $7.50
|
|
$3.34
- $5.27
|
|
Risk free interest rate
|
|
2.41%
- 2.47%
|
|
2.77%
- 2.96%
|
|
Volatility
|
|
89.60% - 90.68%
|
|
57.76%
- 64.74%
|
|
Expected term
|
|
10 years
|
|
7 -
10 years
|
|
Dividend yield
|
|
None
|
|
None
|
Warrant transactions:
Fiscal 2019:
On October 2, 2018 in relation to the secondary offering, we
issued to the selling agent warrants to purchase in aggregate
51,429 shares of common stock with an exercise price of $4.375. The
warrants expire on September 28,
2023.
In May 2019 in relation to the secondary offering, we issued
to the selling agent warrants to purchase in aggregate 60,000
shares of common stock with an exercise price of $7.50. The
warrants expire on May 15, 2024.
Fiscal 2018:
On
November 17, 2017 in relation to the IPO, we issued to the selling
agent warrants to purchase in aggregate 100,000 shares of common
stock with an exercise price of $7.50. The warrants expire on
October 27, 2022. The warrants were valued at $171,600 and recorded
as paid in capital.
F-29
The following table summarizes the inputs used for the
Black-Scholes pricing model on the warrants issued in the years
September 30, 2019 and 2018:
|
|
|
2019
|
|
2018
|
|
Exercise
price
|
|
$4.375
- $7.50
|
|
$7.50
|
|
Risk
free interest rate
|
|
2.15% -
2.90%
|
|
2.06%
|
|
Volatility
|
|
70.61%
- 75.03%
|
|
43.12%
|
|
Expected
term
|
|
5
years
|
|
5
years
|
|
Dividend
yield
|
|
None
|
|
None
|
NOTE 11 – STOCK-BASED COMPENSATION
Equity Compensation Plan – On June 2, 2015, the
Company’s board of directors approved the 2015 Equity
Compensation Plan (“Plan”). The Plan made 1,175,000
common stock shares, either unissued or reacquired by the Company,
available for awards of options, restricted stock, other stock
grants, or any combination thereof. The number of shares of common
stock available for issuance under the Plan shall automatically
increase on the first trading day of January each calendar year
during the term of the Plan, beginning with calendar year 2016, by
an amount equal to one percent (1%) of the total number of shares
of common stock outstanding on the last trading day in December of
the immediately preceding calendar year, but in no event shall any
such annual increase exceed 100,000 shares of common stock. On
April 19, 2019, shareholders approved an amendment to the Plan and
increased the amount of shares available for issuance under the
Plan to 2,000,000 and retained the annual evergreen increase
provision of the Plan.
We account for stock-based compensation using the provisions of ASC
718. ASC 718 codification requires companies to
recognize the fair value of stock-based compensation expense in the
financial statements based on the grant date fair value of the
options. We have only awarded stock options since December 2015.
All options are approved by the Compensation Committee of the board
of directors. Restricted stock awards that vest in accordance with
service conditions are amortized over their applicable vesting
period using the straight-line method. The fair value of our stock
option awards or modifications is estimated at the date of grant
using the Black-Scholes option pricing model.
Eligible recipients include employees, officers, directors and
consultants who are deemed to have rendered or to be able to render
significant services to the Company or its subsidiaries and who are
deemed to have contributed or to have the potential to contribute
to the success of the Company. Options granted generally have a
five to ten year term and have vesting terms that cover one to
three years from the date of grant. Certain of the stock options
granted under the plan have been granted pursuant to various stock
option agreements. Each stock option agreement contains specific
terms.
Stock Options – The Company currently has awards outstanding
with service conditions and graded-vesting features. We recognize
compensation cost on a straight-line basis over the requisite
service period.
The fair value of each time-based award is estimated on the date of
grant using the Black-Scholes option valuation model. Our
weighted-average assumptions used in the Black-Scholes valuation
model for equity awards with time-based vesting provisions granted
during the year.
F-30
The
following table summarizes stock option activity under the
Plan:
|
|
Number
ofshares
|
Weighted-averageexerciseprice
|
Weighted-averageremainingcontractual
term(in years)
|
Aggregateintrinsicvalue
(inthousands)
|
|
Outstanding at
September 30, 2017
|
333,300
|
$5.83
|
|
|
|
Granted
|
235,000
|
4.67
|
|
|
|
Exercised
|
-
|
-
|
|
|
|
Forfeited
|
(98,650)
|
6.38
|
|
|
|
Outstanding at
September 30, 2018
|
469,650
|
5.13
|
|
|
|
Granted
|
750,000
|
6.66
|
|
|
|
Exercised
|
-
|
-
|
|
|
|
Forfeited
|
-
|
-
|
|
|
|
Outstanding at
September 30, 2019
|
1,219,650
|
$6.07
|
8.23
|
$—
|
|
|
|
|
|
|
|
Exercisable at
September 30, 2019
|
809,650
|
$5.49
|
7.52
|
$—
|
We
recognized $2,458,530 and $639,631 of non cash stock option expense
for the years ended September 30, 2019 and 2018, respectively. As
of September 30, 2019, there was approximately $1,556,459 of total
unrecognized compensation cost related to non-vested stock options
which vest over a period of approximately 2.58
years.
Restricted Stock Award transactions:
On
October 1, 2016, the Company issued 230,000 restricted stock awards
in aggregate to board members and the Chairman who is also our
Chief Executive Officer. The restricted stock awards vested January
1, 2018. The stock awards were valued at fair market upon issuance
at $195,500 and amortized over the vesting period.
In May 2019 the Company issued 57,500 restricted stock awards in
aggregate to eleven employees. The restricted stock awards vest
January 1, 2020. The stock awards were valued at fair market upon
issuance at $368,000 and amortized over the vesting
period.
We
recognized $230,000 and $39,100 of stock based compensation expense
for the restricted stock awards or the years ended September 30,
2019 and 2018, respectively.
NOTE 12 – WARRANTS
Transactions
involving our equity-classified warrants are summarized as
follows:
|
|
Number
ofshares
|
Weighted-averageexerciseprice
|
Weighted-
averageremainingcontractualterm
(in years)
|
Aggregateintrinsicvalue
(inthousands)
|
|
Outstanding at
September 30, 2017
|
212,176
|
$6.53
|
|
|
|
Issued
|
100,000
|
7.50
|
|
|
|
Exercised
|
-
|
-
|
|
|
|
Forfeited
|
-
|
-
|
|
|
|
Outstanding at
September 30, 2018
|
312,176
|
6.84
|
|
|
|
Issued
|
111,429
|
6.06
|
|
|
|
Exercised
|
—
|
—
|
|
|
|
Forfeited
|
—
|
—
|
|
|
|
Outstanding at
September 30, 2019
|
423,605
|
$6.64
|
3.03
|
$—
|
|
|
|
|
|
|
|
Exercisable at
September 30, 2019
|
423,605
|
$6.64
|
3.03
|
$—
|
F-31
The
following table summarizes outstanding common stock purchase
warrants as of September 30, 2019:
|
|
Number
ofshares
|
Weighted-averageexerciseprice
|
Expiration
|
|
|
|
|
|
|
Exercisable at
$7.80 per share
|
141,676
|
$7.80
|
September
2021
|
|
Exercisable at
$4.00 per share
|
70,500
|
$4.00
|
September
2022
|
|
Exercisable at
$7.50 per share
|
100,000
|
$7.50
|
October
2022
|
|
Exercisable at
$4.375 per share
|
51,429
|
$4.375
|
September
2023
|
|
Exercisable at
$7.50 per share
|
60,000
|
$7.50
|
May
2024
|
|
|
423,605
|
6.64
|
|
NOTE 13 – COMMITMENTS AND CONTINGENCIES
In May 2019, the Company entered into an endorsement agreement with
a professional athlete. The term of the agreement is through
December 31, 2022 and is tied to performance of the athlete in so
many professional events annually, and also includes promotion of
the Company via social media, wearing of logo during competition,
provide production days for advertising creation and attend meet
and greets. The potential payments, if all services are provided,
in aggregate is $4,900,000 and is paid based on the services above
for the period ending: December 2019 - $400,000, December 2020 -
$800,000, December 2021 - $1,800,000, and December 2022 -
$1,900,000.
In
September 2019, the Company entered into a sponsorship agreement
with Life Time, Inc, an operator of fitness clubs, facilities and
events. The term of the agreement is through December 31, 2022 and
is tied to the Company being the exclusive CBD company and
performance of Life Time Inc. regarding advertisement, marketing
and display within facilities and at identified events. The
potential payments, if all commitments are met, in aggregate is
$4,900,000 and is to be paid for the period ending: December 2019 -
$1,125,555, December 2020 - $1,258,148, December 2021 - $1,258,148
and December 2022 - $1,258,149.
In
October 2019, the Company entered into a sponsorship agreement with
Feld Motor Sports to be an official sponsor of the Monster Energy
Cup events through 2021, the United States AMA Supercross and FIM
World Championship events through 2021, and US Supercross Futures
event through 2021. The sponsorship includes various media,
marketing, and promotion activities. The payments in aggregate are
$1,750,000 and is to be paid for the period ending: December 2019 -
$150,000, December 2020 - $800,000 and December 2021 -
$800,000.
NOTE 14 – LONG TERM LIABILITIES
Our long term liabilities at September 30, 2019 consist of a five
year lease on equipment and deferred rent of $169,494 related to
the seven year lease on our corporate office.
In July
2019, we entered a financing arrangement for $249,100 for a line of
equipment, of which $194,466 is a long term liability at September
30, 2019. Payments are for 60 months and have a financing rate of
7.01 %, which requires a monthly payment of $4,905.
F-32
NOTE 15 – DISCONTINUED OPERATIONS
Effective
September 30, 2019, the Company ceased operations of four business
subsidiaries: EE1, IM1, BPU and Level H&W. These subsidiaries
accounted for our licensing, entertainment, and products segments
prior to fiscal 2019 and the Company has determined that these
business units are not able to provide support or value to the CBD
business, which the Company is now strategically focused
on.
Therefore,
the Company has classified the operating results of these
subsidiaries as discontinued operations, net of tax in the
Consolidated Statements of Operations.
The
following table shows the summary operating results of the
discontinued operations for the years ended:
|
|
September 30,
|
September 30,
|
|
|
2019
|
2018
|
|
|
|
|
|
Sales
|
$888,254
|
$6,453,173
|
|
Sales
related party
|
-
|
1,532,955
|
|
Total
Gross Sales
|
888,254
|
7,986,128
|
|
Allowances
|
(12,129)
|
(25,077)
|
|
Net
sales
|
876,126
|
6,428,096
|
|
Net
sales related party
|
-
|
1,532,955
|
|
Total Net Sales
|
876,126
|
7,961,051
|
|
Costs
of sales
|
604,714
|
2,569,262
|
|
Gross profit
|
271,412
|
5,391,789
|
|
Operating
expenses
|
539,581
|
3,477,622
|
|
Income (loss) from
operations
|
(268,169)
|
1,914,167
|
|
|
|
|
|
Other
income
|
20,000
|
|
|
Realized
and Unrealized gain (loss) on marketable securities
|
(2,337,280)
|
-
|
|
Impairment
on discontinued operations
|
(3,398,450)
|
|
|
Loss
on disposal of property
|
(39,015)
|
(69,310)
|
|
Interest
income (expense)
|
29,141
|
(955)
|
|
Income (loss) before provision
for income taxes
|
(5,993,773)
|
1,843,902
|
|
Provision
for income taxes
|
66,000
|
16,000
|
|
Net Income (loss)
|
(5,927,773)
|
1,859,902
|
|
Net
Income (loss) attributable to non-controlling interest
|
$(929,323)
|
$474,909
|
F-33
The
following table shows the summary assets and liabilities of the
discontinued operations as of September 30, 2019 and
2018.
|
|
2019
|
2018
|
|
Assets
|
|
|
|
|
|
|
|
Current
assets:
|
|
|
|
Cash
and cash equivalents
|
$-
|
$212,614
|
|
Accounts
receivable
|
1,080,000
|
307,874
|
|
Accounts
receivable - related party
|
-
|
1,532,955
|
|
Accounts
receivable other
|
-
|
1,581,000
|
|
Marketable
securities
|
-
|
940,988
|
|
Investment
other securities
|
-
|
56,552
|
|
Note
receivable
|
-
|
459,000
|
|
Note
receivable - related party
|
-
|
156,147
|
|
Inventory
|
-
|
123,223
|
|
Prepaid
expenses and other current assets
|
-
|
210,882
|
|
Total
current assets included as part of discontinued
operations
|
1,080,000
|
5,581,235
|
|
|
|
|
|
Other
assets:
|
|
|
|
Property
and equipment, net
|
-
|
48,874
|
|
Intangible
assets, net
|
-
|
2,764,288
|
|
Total
other assets included as part of discontinued
operations
|
-
|
2,813,162
|
|
|
|
|
|
Total
assets included as part of discontinued operations
|
$1,080,000
|
$8,394,397
|
|
Liabilities
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
Accounts
payable
|
$-
|
$363,042
|
|
Accounts
payable - related party
|
-
|
7,860
|
|
Deferred
revenue
|
-
|
161,458
|
|
Accrued
expenses
|
-
|
6,920
|
|
Accrued
expenses - related party
|
-
|
320,000
|
|
Total
current liabilities included as part of discontinued
operations
|
-
|
859,280
|
|
|
|
|
|
Long term
liabilities
|
|
|
|
Long
term liabilities
|
-
|
7,502
|
|
Deferred
tax liability
|
-
|
-
|
|
Total
long term liabilities included as part of discontinued
operations
|
-
|
7,502
|
|
|
|
|
|
Total
liabilities included as part of discontinued
operations
|
$-
|
$866,782
|
F-34
The
following table shows the significant cash flow items from
discontinued operations for the years ended September
30,:
|
|
2019
|
2018
|
|
Depreciation/
amortization
|
$22,199
|
$30,009
|
|
Realized/unrealized
(gain) loss on securities expenditures
|
$2,337,280
|
$-
|
|
Impairment on
discontinued operations assets
|
$762,629
|
$-
|
|
Impairment on
intangibles
|
$2,635,821
|
$-
|
|
Non cash
consideration received for services
|
$(470,000)
|
$(3,004,502)
|
On June
26, 2018 the Company entered into an Agreement with Boston
Therapeutics, Inc. (OTC: BTHE), a pharmaceutical company focused on
the development, manufacturing and commercialization of novel
compounds to address unmet medical needs in diabetes. The agreement involved a licensing
agreement and required the Company to create IP for a branding /
marketing campaign. As payment for these services, Boston
Therapeutics agreed to pay $850,000, of which $450,000 was issued
as a note due no later than December 31, 2019 and $400,000 to be
paid thru the issuance of BTI common stock based on the trading
price at the agreement date ($0.075). As the stock has not been
issued this is recorded as an other accounts receivable. In June
2019 the Company began the arbitration process to collect amounts
owed and at September 30, 2019 determined the amounts were not
collectible and recorded an impairment for the carrying value of
$53,333 for the other accounts receivable and $450,000 for the note
receivable.
Effective
September 30, 2019, the Company ceased operations of four business
subsidiaries: EE1, IM1, BPU and Level H&W. Level H&W had an
intangible asset with a carrying value of $958,065 which the
Company recorded an impairment charge at September 30, 2019 for the
full value of $958,065. Previously in fiscal 2019, impairments on
intangible assets in the subsidiaries included $971,667 in IM1,
$471,667 in EE1, and $234,422. In addition, for all subsidiaries,
we had an aggregate impairment on discontinued assets: accounts
receivable, note receivable, and investment other security and
property and equipment of $762,629.
At
September 30, 2019, EE1 had an accounts receivable for prior
services delivered to two customers in aggregate of $1,080,000 of
which $1,000,000 was from a related party at the time.
As two
of the subsidiaries, EE1 and IM1, had minority interests
(non-controlling interests) and all parties agreed to transfer the
non- controlling interest to the Company, we have reclassified the
non-controlling interest balance of $(482,648) to additional paid
in capital as of September 30, 2019.
Related party transactions:
In April 2018 through June 2018, EE1 engaged in five
separate statements of work for various marketing campaigns,
production processes, and documentary related services for Sandbox
LLC. Under the terms of the agreements, EE1 earned in aggregate,
$1,200,000 for these statements of work, from Sandbox LLC. Sandbox
LLC is an affiliate of a former member of our board of directors
and was a related party at the time.
In September 2018, B&B Bandwidth purchased products from our
subsidiary BPU for resale. The total purchase was $332,955. B&B
Bandwidth management are affiliates of kathy ireland®
Worldwide.
NOTE 16 – INCOME TAXES
The Company generated operating losses for the years ended
September 30, 2019 and 2018 on which it has recognized a full
valuation allowance. The Company accounts for its state franchise
and minimum taxes as a component of its general and administrative
expenses.
F-35
The following table presents the components of the provision for
income taxes from continuing operations for the periods
presented:
|
|
Years Ended
September 30,
|
|
|
|
2019
|
2018
|
|
Current
|
|
|
|
Federal
|
$—
|
$—
|
|
State
|
—
|
—
|
|
Total
current
|
—
|
—
|
|
Deferred
|
|
|
|
Federal
|
(3,071,000)
|
—
|
|
State
|
712,000
|
—
|
|
Total
deferred
|
(2,359,000)
|
—
|
|
Total
provision
|
$(2,359,000)
|
$—
|
A reconciliation of the federal statutory income tax rate to the
Company's effective income tax rate is as follows:
|
|
Years Ended
September 30,
|
|
|
|
2019
|
2018
|
|
Federal statutory
income tax rate
|
21.0%
|
24.3%
|
|
State income taxes,
net of federal benefit
|
(1.2)
|
49.0
|
|
Permanent
differences
|
(1.0)
|
(384.5)
|
|
Contingent
derivative expense
|
(14.3)
|
-
|
|
Tax impact of
federal tax rate change
|
-
|
1,708.1
|
|
Limitation on net
operating losses
|
-
|
1,065.8
|
|
Tax impact of
non-controlling interest
|
-
|
(246.4)
|
|
Change in valuation
allowance
|
0.4
|
(2,250.5)
|
|
Reclassification
to discontinued operations
|
-
|
(34.2)%
|
|
Provision for
income taxes
|
4.9%
|
-
|
Significant components of the Company's deferred income taxes are
shown below:
|
|
Years Ended
September 30,
|
|
|
|
2019
|
2018
|
|
Deferred tax
assets:
|
|
|
|
Net operating loss
carryforwards
|
$4,933,000
|
$1,396,000
|
|
Capital loss
carryforward
|
485,000
|
0
|
|
Allowance for
doubtful accounts
|
95,000
|
0
|
|
Stock
compensation
|
510,000
|
139,000
|
|
Investments
|
608,000
|
0
|
|
Accrued
expenses
|
66,000
|
0
|
|
Intangibles
|
0
|
42,000
|
|
Capitalized
expenses
|
66,000
|
7,000
|
|
Charitable
contributions
|
41,700
|
26,000
|
|
|
|
|
|
Total deferred tax
assets
|
6,804,700
|
1,610,000
|
|
|
|
|
|
Deferred tax
liabilities:
|
|
|
|
Prepaid
expenses
|
(405,000)
|
(177,000)
|
|
Management
fees
|
0
|
(169,000)
|
|
Intangibles
|
(4,604,000)
|
(21,000)
|
|
Fixed
assets
|
(360,000)
|
(1,000)
|
|
Total deferred tax
liabilities
|
(5,369,000)
|
(368,000)
|
|
Net deferred tax
assets
|
1,435,700
|
1,242,000
|
|
Valuation
allowance
|
(3,676,000)
|
(1,263,000)
|
|
|
|
|
|
Net
deferred tax liability
|
$(2,240,300)
|
$(21,000)
|
F-36
The Company has established a valuation allowance against net
deferred tax assets due to the uncertainty that such assets will be
realized. The deferred tax liabilities that result from indefinite
life intangibles cannot be offset by deferred tax assets. The
Company periodically evaluates the recoverability of the deferred
tax assets. At such time as it is determined that it is more likely
than not that deferred tax assets will be realizable, the valuation
allowance will be reduced.
Under Internal Revenue Code (IRC) Section 382, the use of net
operating loss (“NOL”) carryforwards may be limited if
a change in ownership of a company occurs. During the year ending
September 30, 2018, the company determined that a change of
ownership under IRC Section 382 had occurred during the years
ending September 30, 2017 and 2015. As a result of these ownership
changes, the pre-ownership change NOL carryforwards would be
limited and approximately $2.1 million of such NOLs will expire
before being utilized. Therefore, at September 30, 2018 the Company
reduced the deferred tax asset and related valuation allowance
associated with these NOLs by approximately $0.5 million due to IRC
Section 382. The Company is currently evaluating if any additional
ownership changes occurred that would potentially limit the use of
the NOLs in a future year.
The total valuation allowance increased by $1,359,000 and decreased
by $1,054,000 as of September 30, 2019 and 2018,
respectively.
At September 30, 2019, the Company has utilizable NOL
carryforwards of approximately $21.1 million. Approximately $6.1
million of the federal NOL carryforwards will begin to expire in
2035. The remaining $15.0 million of federal NOL carryforwards will
carryforward indefinitely.
The Company accounts for its state franchise and minimum taxes as a
component of its general and administrative expenses.
On
December 22, 2017, the Tax Cuts and Jobs Act (“TCJA”)
was enacted. As a result of the enactment, the U.S. corporate
tax rate was changed from a progressive bracketed tax rate with the
highest marginal rate of 35% to a flat corporate tax rate of
21%. As the Company has a fiscal year ending September 30, the
blended effective rate is 24.3% for the fiscal year ended September
30, 2018. The Company revalued its deferred tax assets and
liabilities, as well as the valuation allowance, at the date of
enactment and these repricings are reflected gross in the above
presented rate reconciliation. Finally, as the Company
does not own more than 10% of any foreign companies, there is
no impact of IRC Section 965 to the Company as a result of the
TCJA.
The Company files income tax returns in the United States, and
various state jurisdictions. The Company’s policy is to
recognize interest expense and penalties related to income tax
matters as tax expense. At September 30, 2019 and 2018, there
are no unrecognized tax benefits, and there are no significant
accruals for interest related to unrecognized tax benefits or tax
penalties.
NOTE 17 – LEASES
In July 2019, the Company signed a lease for a new corporate office
consisting of approximately 50,000 square feet. The lease is from
August 1, 2019 thru December 31, 2026. The monthly base rent for
the first year is $76,041 and will increase annually by
approximately 3%.
We also
have a lease for a manufacturing and warehouse facility in
Charlotte, North Carolina which we lease approximately 40,000
square feet under an agreement through December 2021. The agreement
calls for an annual base monthly rent of $18,700, inclusive of
monthly taxes insurance and common area maintenance
(“TICAM”) for the first year and the rent escalates 3%
annually. The lease is through a related party, see Note
9.
In
October 2019 we began a lease for a new 80,000 square foot
warehouse in Charlotte, North Carolina under an agreement through
December 2024. The agreement calls for an annual base monthly rent
of $34,766, inclusive of monthly TICAM for the first year and the
rent escalates 3% annually.
F-37
The
future minimum payments under non-cancelable operating leases with
initial remaining terms in excess of one year as of September 30,
2019, are as follows for fiscal years:
|
2020
|
$1,487,207
|
|
2021
|
$1,544,834
|
|
2022
|
$1,409,006
|
|
2023
|
$1,449,260
|
|
2024
|
$1,490,722
|
|
2025
|
$1,533,428
|
|
2026
|
$1,092,297
|
|
2027
|
$374,087
|
NOTE 18 – SUBSEQUENT EVENTS
On
October 16, 2019, the Company completed a secondary public offering
of 500,000 shares of its 8.0% Series A
Cumulative Convertible Preferred Stock for aggregate gross
proceeds of $5,000,000. The Company received approximately $4.5
million in net proceeds after deducting underwriting discounts and
commissions and other estimated offering expenses payable by us.
The Company reviewed ASC 480 – Distinguishing Liabilities
from Equity in order to determine the appropriate accounting
treatment for the preferred stock and determined that the preferred
stock should be treated as equity. Therefore, during the quarter
ended December 31, 2019 the issuance of the shares will be recorded
as equity.
On
November 8, 2019, the Company and the minority holders of EE1 and
IM1, two discontinued subsidiaries, agreed to a settlement and
release related to the two non-operating entities. Effective
September 30, 2019, the parties agreed to transfer the accounts
receivable of EE1 and the minority interest of both EE1 and IM1 to
the Company and the Company agreed to have all rights to certain
past contracts or customers assigned to the minority holders. See
Note 15 for more information.
On
November 19, 2019, the Company entered into a stock sale and
purchase agreement (“Agreement”) with a third party
regarding our ownership of equity in a private entity, which we
received in 2017 as compensation for services performed. The
Agreement provides for the sale to be completed by June 30, 2020 at
a price of $600,000 and required a non-refundable deposit of
$30,000 by the purchaser for the purchase option, which would be
applied to the sale if executed.
On
December 11, 2019, the board of directors authorized and we issued
an aggregate of 280,000 common stock
options to our co-Chief Executive Officers. The options vest 1/3
January 1, 2020, 1/3 January 1, 2021 and 1/3 January 1, 2022 and
have an exercise price of $3.15 per share and a term of five
years.
F-38
