Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - HALOZYME THERAPEUTICS, INC. | d662328dex991.htm |
| 8-K - FORM 8-K - HALOZYME THERAPEUTICS, INC. | d662328d8k.htm |

HALO-301 Trial Update November 26, 2018 Exhibit 99.2

Forward-Looking Statements All of the statements in this presentation that are not statements of historical facts constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Examples of such statements include possible activity, benefits and attributes of PEGPH20, future product development and regulatory events and goals, anticipated clinical trial results and strategies, product collaborations, our business intentions and financial estimates and results, including projected revenue amounts. These statements are based upon management’s current plans and expectations and are subject to a number of risks and uncertainties which could cause actual results to differ materially from such statements. A discussion of the risks and uncertainties that can affect these statements is set forth in the Company’s annual and quarterly reports filed from time to time with the Securities and Exchange Commission under the heading “Risk Factors.” The Company disclaims any intention or obligation to revise or update any forward-looking statements, whether as a result of new information, future events, or otherwise. 2
Summary Agreement reached with FDA to change the primary endpoint of HALO-301 to a single primary endpoint, Overall Survival (OS) Company and advisors remain blinded to all efficacy data Additional statistical plan changes: No interim analysis Target number of OS events for final analysis, unchanged at 330, projected to occur August - November 2019 93% power if HR (Hazard Ratio) is 0.67: corresponds to a median OS benefit of 12.7 months versus 8.5 months Observed HR of 0.795 would be statistically significant, with a minimum observable Overall Survival difference of 2.2 months Enrollment will be ~500 patients, assuring a mature data set All patients expected to be followed for at least 8.5 months and only 9% will have less than ~13 months follow-up FDA provided feedback that these details appear acceptable with a final determination pending review of amended protocol and statistical plan to be submitted in December 2018
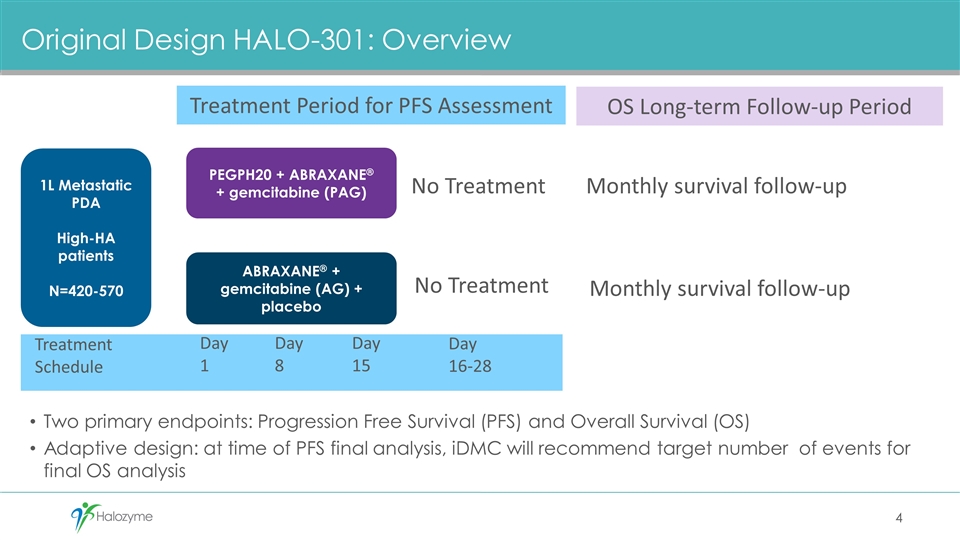
Original Design HALO-301: Overview Two primary endpoints: Progression Free Survival (PFS) and Overall Survival (OS) Adaptive design: at time of PFS final analysis, iDMC will recommend target number of events for final OS analysis PEGPH20 + ABRAXANE® + gemcitabine (PAG) ABRAXANE® + gemcitabine (AG) + placebo 1L Metastatic PDA High-HA patients N=420-570 Treatment Period for PFS Assessment Day 1 Day 8 Day 15 No Treatment No Treatment Day 16-28 Monthly survival follow-up OS Long-term Follow-up Period Treatment Schedule Monthly survival follow-up
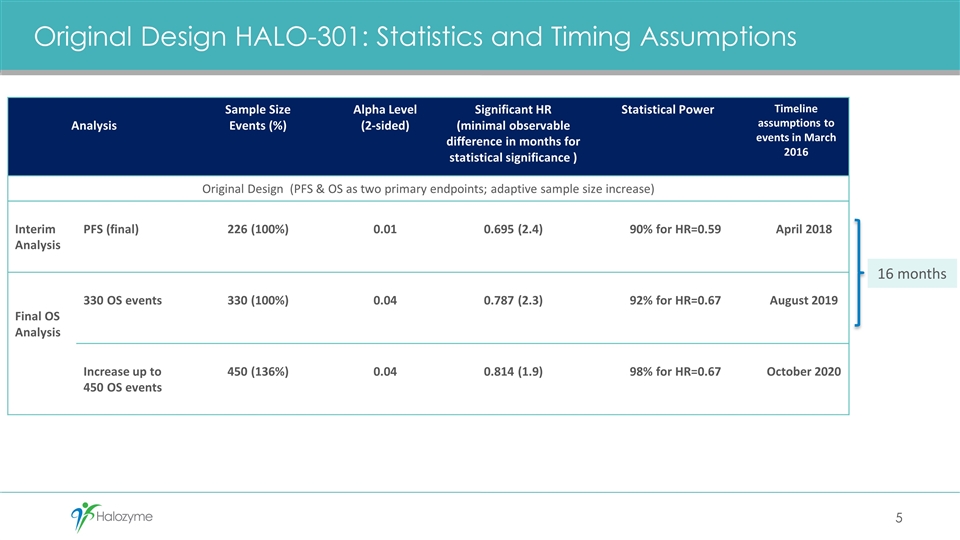
Original Design HALO-301: Statistics and Timing Assumptions Analysis Sample Size Events (%) Alpha Level (2-sided) Significant HR (minimal observable difference in months for statistical significance ) Statistical Power Timeline assumptions to events in March 2016 Original Design (PFS & OS as two primary endpoints; adaptive sample size increase) Interim Analysis PFS (final) 226 (100%) 0.01 0.695 (2.4) 90% for HR=0.59 April 2018 Final OS Analysis 330 OS events 330 (100%) 0.04 0.787 (2.3) 92% for HR=0.67 August 2019 Increase up to 450 OS events 450 (136%) 0.04 0.814 (1.9) 98% for HR=0.67 October 2020 5 16 months
FDA Provided Feedback on Original Design in Type B Meeting March 2015 FDA feedback on PFS as an endpoint with potential to support a marketing application: “FDA did not object to the proposal to retain PFS as a co-primary endpoint. However, the ability of the PFS results to support a marketing application would be dependent upon the magnitude of the treatment effect observed, the toxicity profile of the drug, and the interim overall survival data.” Excerpted from FDA minutes of Type B End Of Phase 2 Meeting March 2015
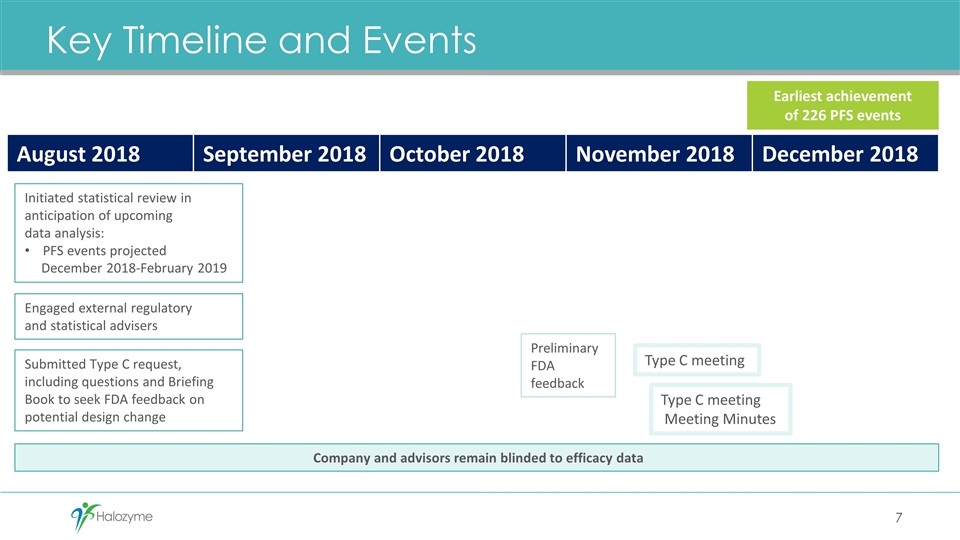
Key Timeline and Events August 2018 September 2018 October 2018 November 2018 December 2018 Initiated statistical review in anticipation of upcoming data analysis: PFS events projected December 2018-February 2019 Engaged external regulatory and statistical advisers Submitted Type C request, including questions and Briefing Book to seek FDA feedback on potential design change Type C meeting Company and advisors remain blinded to efficacy data Preliminary FDA feedback Type C meeting Meeting Minutes Earliest achievement of 226 PFS events
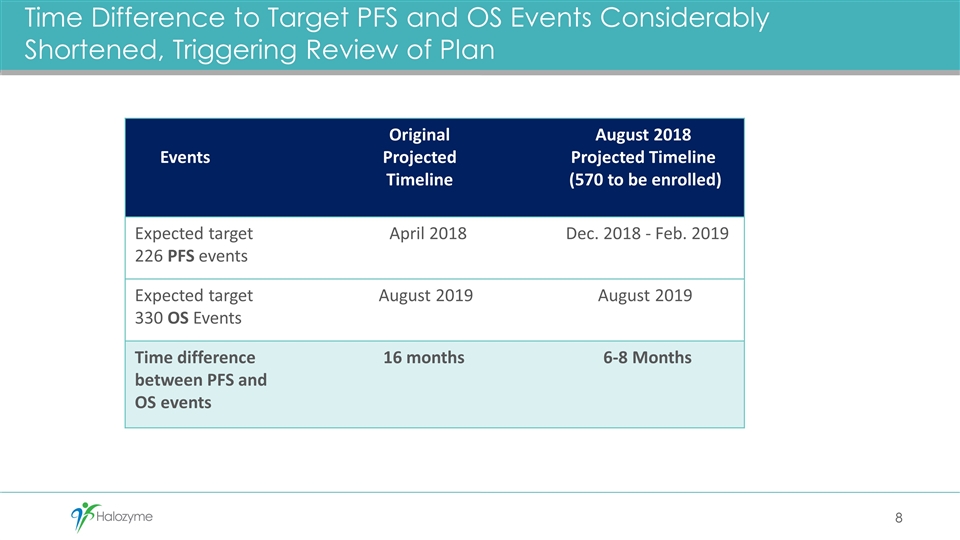
Time Difference to Target PFS and OS Events Considerably Shortened, Triggering Review of Plan Events Original Projected Timeline August 2018 Projected Timeline (570 to be enrolled) Expected target 226 PFS events April 2018 Dec. 2018 - Feb. 2019 Expected target 330 OS Events August 2019 August 2019 Time difference between PFS and OS events 16 months 6-8 Months
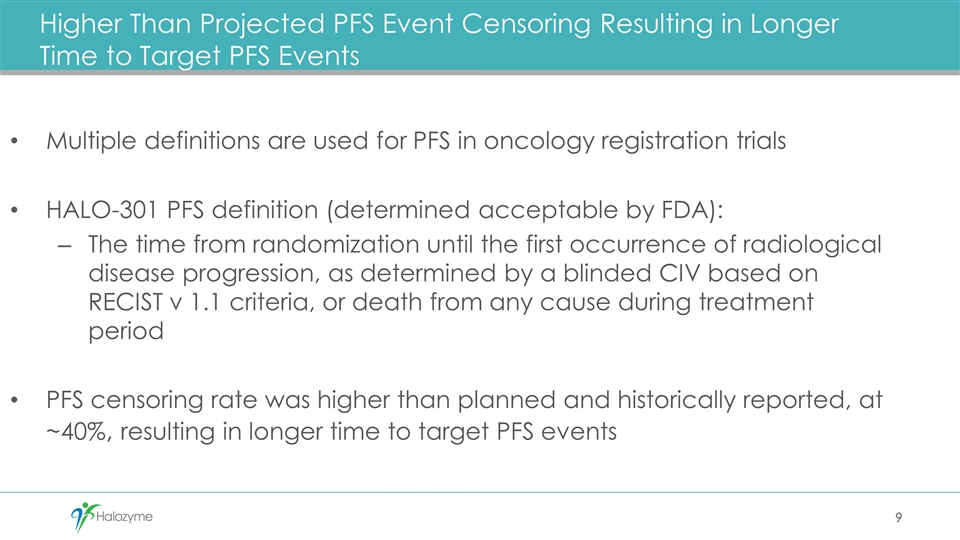
Higher Than Projected PFS Event Censoring Resulting in Longer Time to Target PFS Events Multiple definitions are used for PFS in oncology registration trials HALO-301 PFS definition (determined acceptable by FDA): The time from randomization until the first occurrence of radiological disease progression, as determined by a blinded CIV based on RECIST v 1.1 criteria, or death from any cause during treatment period PFS censoring rate was higher than planned and historically reported, at ~40%, resulting in longer time to target PFS events
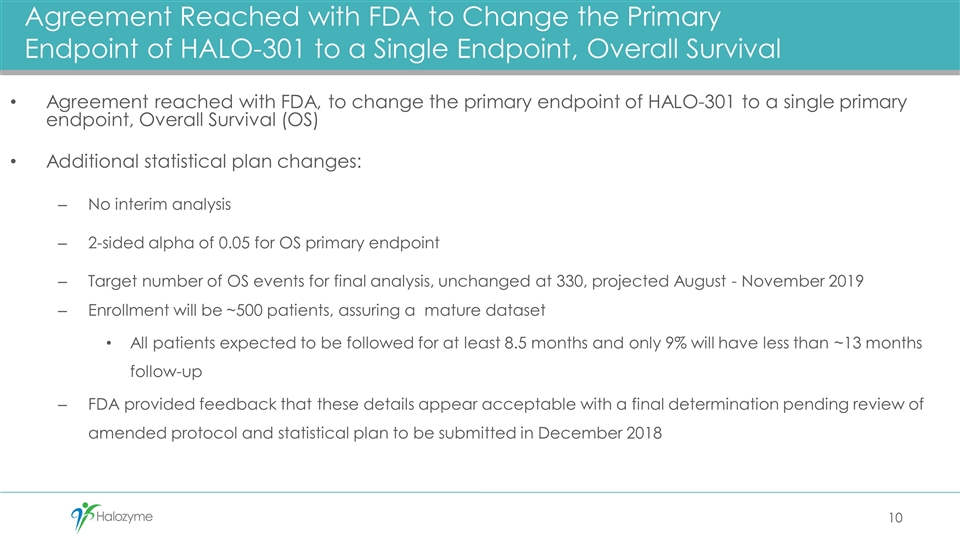
Agreement reached with FDA, to change the primary endpoint of HALO-301 to a single primary endpoint, Overall Survival (OS) Additional statistical plan changes: No interim analysis 2-sided alpha of 0.05 for OS primary endpoint Target number of OS events for final analysis, unchanged at 330, projected August - November 2019 Enrollment will be ~500 patients, assuring a mature dataset All patients expected to be followed for at least 8.5 months and only 9% will have less than ~13 months follow-up FDA provided feedback that these details appear acceptable with a final determination pending review of amended protocol and statistical plan to be submitted in December 2018 Agreement Reached with FDA to Change the Primary Endpoint of HALO-301 to a Single Endpoint, Overall Survival
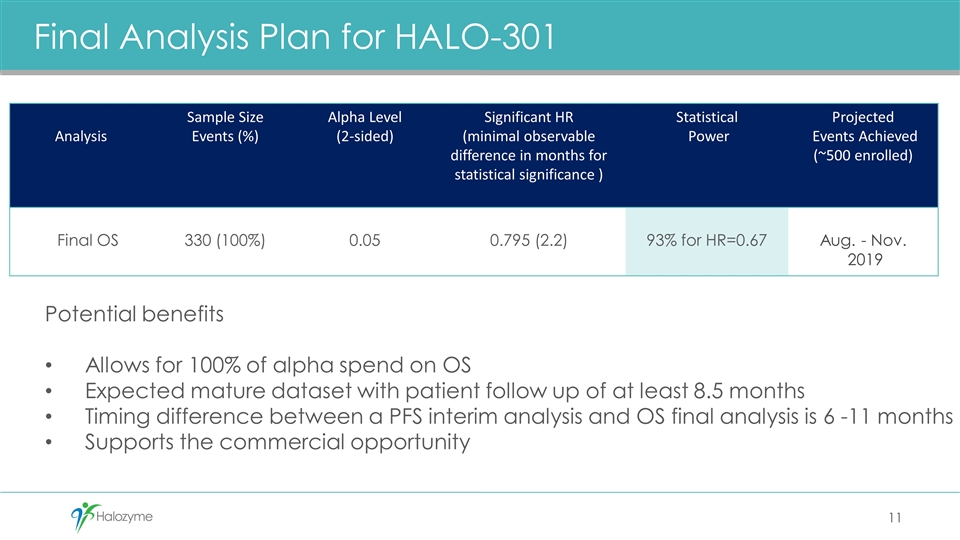
Final Analysis Plan for HALO-301 Analysis Sample Size Events (%) Alpha Level (2-sided) Significant HR (minimal observable difference in months for statistical significance ) Statistical Power Projected Events Achieved (~500 enrolled) Final OS 330 (100%) 0.05 0.795 (2.2) 93% for HR=0.67 Aug. - Nov. 2019 11 Potential benefits Allows for 100% of alpha spend on OS Expected mature dataset with patient follow up of at least 8.5 months Timing difference between a PFS interim analysis and OS final analysis is 6 -11 months Supports the commercial opportunity
Final Analysis Approach: Potential Benefits Full FDA and MAA approval pathway, with Overall Survival endpoint Mature data set, maximizing ability to observe treatment effect High probability of definitive HALO-301data in 2H 2019
