Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - vTv Therapeutics Inc. | vtvt-ex322_10.htm |
| EX-32.1 - EX-32.1 - vTv Therapeutics Inc. | vtvt-ex321_9.htm |
| EX-31.2 - EX-31.2 - vTv Therapeutics Inc. | vtvt-ex312_11.htm |
| EX-31.1 - EX-31.1 - vTv Therapeutics Inc. | vtvt-ex311_6.htm |
| EX-10.2 - EX-10.2 - vTv Therapeutics Inc. | vtvt-ex102_203.htm |
| EX-10.1 - EX-10.1 - vTv Therapeutics Inc. | vtvt-ex101_202.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
|
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2018
Or
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-37524
vTv Therapeutics Inc.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
47-3916571 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
4170 Mendenhall Oaks Pkwy High Point, NC |
|
27265 |
|
(Address of principal executive offices) |
|
(Zip Code) |
(336) 841-0300
(Registrant’s telephone number, including area code)
(Former name, former address and former fiscal year, if changed since last report)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
|
|
|
|
|
|
Non-accelerated filer |
☐ |
Smaller reporting company |
☒ |
|
|
|
|
|
|
Emerging growth company |
☒ |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
|
Class of Stock |
|
Shares Outstanding as of November 8, 2018 |
|
|
|
Class A common stock, par value $0.01 per share |
|
|
17,652,148 |
|
|
Class B common stock, par value $0.01 per share |
|
|
23,094,221 |
|
vTv THERAPEUTICS INC. AND SUBSIDIARIES
INDEX TO FORM 10-Q
FOR THE QUARTER ENDED September 30, 2018
|
|
|
|
|
PAGE |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Item 1. |
|
Condensed Consolidated Balance Sheets as of September 30, 2018 (Unaudited) and December 31, 2017 |
|
4 |
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
7 |
|
|
|
|
|
|
|
|
|
|
|
8 |
|
|
|
|
|
|
|
|
Item 2. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
22 |
|
|
|
|
|
|
|
Item 3. |
|
|
31 |
|
|
|
|
|
|
|
|
Item 4. |
|
|
31 |
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
Item 1. |
|
|
32 |
|
|
|
|
|
|
|
|
Item 1A. |
|
|
32 |
|
|
|
|
|
|
|
|
Item 2. |
|
|
33 |
|
|
|
|
|
|
|
|
Item 3. |
|
|
33 |
|
|
|
|
|
|
|
|
Item 4. |
|
|
33 |
|
|
|
|
|
|
|
|
Item 5. |
|
|
34 |
|
|
|
|
|
|
|
|
Item 6. |
|
|
35 |
|
|
|
|
|
|
|
|
|
|
|
36 |
|
2
PART I – FINANCIAL INFORMATION
The financial statements and other disclosures contained in this report include those of vTv Therapeutics Inc. (“we”, the “Company” or the “Registrant”), which is the registrant, and those of vTv Therapeutics LLC (“vTv LLC”), which is the principal operating subsidiary of the Registrant. Unless the context suggests otherwise, references in this Quarterly Report on Form 10-Q to the “Company”, “we”, “us” and “our” refer to vTv Therapeutics Inc. and its consolidated subsidiaries.
3
Condensed Consolidated Balance Sheets
(in thousands, except number of shares and per share data)
|
|
September 30, |
|
|
December 31, |
|
||
|
|
2018 |
|
|
2017 |
|
||
|
|
(Unaudited) |
|
|
|
|
|
|
|
Assets |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
$ |
3,766 |
|
|
$ |
11,758 |
|
|
Restricted cash and cash equivalents |
|
— |
|
|
|
162 |
|
|
Accounts receivable, net |
|
— |
|
|
|
8,000 |
|
|
Prepaid expenses and other current assets |
|
1,006 |
|
|
|
442 |
|
|
Current deposits |
|
1,124 |
|
|
|
— |
|
|
Total current assets |
|
5,896 |
|
|
|
20,362 |
|
|
Restricted cash and cash equivalents, long-term |
|
2,500 |
|
|
|
2,500 |
|
|
Property and equipment, net |
|
177 |
|
|
|
283 |
|
|
Long-term investments |
|
2,480 |
|
|
|
2,480 |
|
|
Long-term deposits |
|
36 |
|
|
|
2,292 |
|
|
Total assets |
$ |
11,089 |
|
|
$ |
27,917 |
|
|
Liabilities, Redeemable Noncontrolling Interest and Stockholders’ Deficit |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable and accrued expenses |
$ |
8,965 |
|
|
$ |
13,901 |
|
|
Current portion of deferred revenue |
|
6,747 |
|
|
|
8,757 |
|
|
Current portion of notes payable |
|
9,597 |
|
|
|
4,271 |
|
|
Total current liabilities |
|
25,309 |
|
|
|
26,929 |
|
|
Notes payable |
|
8,611 |
|
|
|
15,316 |
|
|
Deferred revenue, net of current portion |
|
595 |
|
|
|
4,497 |
|
|
Warrant liability, related party |
|
382 |
|
|
|
492 |
|
|
Other liabilities |
|
258 |
|
|
|
290 |
|
|
Total liabilities |
|
35,155 |
|
|
|
47,524 |
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
Redeemable noncontrolling interest |
|
19,912 |
|
|
|
131,440 |
|
|
Stockholders’ deficit: |
|
|
|
|
|
|
|
|
Class A Common Stock, $0.01 par value; 100,000,000 shares authorized, 15,772,449 and 9,693,254 shares outstanding as of September 30, 2018 and December 31, 2017, respectively |
|
158 |
|
|
|
97 |
|
|
Class B Common Stock, $0.01 par value; 100,000,000 shares authorized, 23,094,221 and 23,119,246 shares outstanding as of September 30, 2018 and December 31, 2017, respectively |
|
232 |
|
|
|
232 |
|
|
Additional paid-in capital |
|
144,617 |
|
|
|
127,682 |
|
|
Accumulated deficit |
|
(188,985 |
) |
|
|
(279,058 |
) |
|
Total stockholders’ deficit attributable to vTv Therapeutics Inc. |
|
(43,978 |
) |
|
|
(151,047 |
) |
|
Total liabilities, redeemable noncontrolling interest and stockholders’ deficit |
$ |
11,089 |
|
|
$ |
27,917 |
|
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
4
Condensed Consolidated Statements of Operations - Unaudited
(in thousands, except number of shares and per share data)
|
|
Three Months Ended |
|
|
Nine Months Ended |
|
||||||||||
|
|
September 30, |
|
|
September 30, |
|
||||||||||
|
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
||||
|
Revenue |
$ |
3,375 |
|
|
$ |
15 |
|
|
$ |
7,912 |
|
|
$ |
58 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
2,698 |
|
|
|
8,989 |
|
|
|
20,235 |
|
|
|
29,572 |
|
|
General and administrative |
|
2,158 |
|
|
|
2,567 |
|
|
|
7,150 |
|
|
|
8,396 |
|
|
Total operating expenses |
|
4,856 |
|
|
|
11,556 |
|
|
|
27,385 |
|
|
|
37,968 |
|
|
Operating loss |
|
(1,481 |
) |
|
|
(11,541 |
) |
|
|
(19,473 |
) |
|
|
(37,910 |
) |
|
Other income |
|
10 |
|
|
|
— |
|
|
|
46 |
|
|
|
— |
|
|
Other income – related party |
|
319 |
|
|
|
— |
|
|
|
610 |
|
|
|
— |
|
|
Interest income |
|
13 |
|
|
|
35 |
|
|
|
47 |
|
|
|
95 |
|
|
Interest expense |
|
(822 |
) |
|
|
(849 |
) |
|
|
(2,547 |
) |
|
|
(2,240 |
) |
|
Loss before income taxes and noncontrolling interest |
|
(1,961 |
) |
|
|
(12,355 |
) |
|
|
(21,317 |
) |
|
|
(40,055 |
) |
|
Income tax provision |
|
— |
|
|
|
— |
|
|
|
200 |
|
|
|
— |
|
|
Net loss before noncontrolling interest |
|
(1,961 |
) |
|
|
(12,355 |
) |
|
|
(21,517 |
) |
|
|
(40,055 |
) |
|
Less: net loss attributable to noncontrolling interest |
|
(1,165 |
) |
|
|
(8,705 |
) |
|
|
(14,697 |
) |
|
|
(28,222 |
) |
|
Net loss attributable to vTv Therapeutics Inc. |
$ |
(796 |
) |
|
$ |
(3,650 |
) |
|
$ |
(6,820 |
) |
|
$ |
(11,833 |
) |
|
Net loss per share of vTv Therapeutics Inc. Class A Common Stock, basic and diluted |
$ |
(0.06 |
) |
|
$ |
(0.38 |
) |
|
$ |
(0.64 |
) |
|
$ |
(1.22 |
) |
|
Weighted-average number of vTv Therapeutics Inc. Class A Common Stock, basic and diluted |
|
12,305,949 |
|
|
|
9,693,254 |
|
|
|
10,701,599 |
|
|
|
9,693,254 |
|
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
5
Condensed Consolidated Statement of Changes in Redeemable Noncontrolling Interest and Stockholders’ Deficit - Unaudited
(in thousands, except number of shares)
|
|
|
|
|
|
Class A Common Stock |
|
|
Class B Common Stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
|
|
Redeemable Noncontrolling Interest |
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Additional Paid-in Capital |
|
|
Accumulated Deficit |
|
|
Total Stockholders' Deficit |
|
||||||||
|
Balances at December 31, 2017 |
$ |
131,440 |
|
|
|
9,693,254 |
|
|
$ |
97 |
|
|
|
23,119,246 |
|
|
$ |
232 |
|
|
$ |
127,682 |
|
|
$ |
(279,058 |
) |
|
$ |
(151,047 |
) |
|
Net loss |
|
(14,697 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6,820 |
) |
|
|
(6,820 |
) |
|
Cumulative effect of accounting change |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
213 |
|
|
|
213 |
|
|
Share-based compensation |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,345 |
|
|
|
— |
|
|
|
2,345 |
|
|
Exchange of Class B Common Stock for Class A Common Stock |
|
(151 |
) |
|
|
25,025 |
|
|
|
— |
|
|
|
(25,025 |
) |
|
|
— |
|
|
|
151 |
|
|
|
— |
|
|
|
151 |
|
|
Issuance of Class A Common Stock to a related party under the Letter Agreements |
|
— |
|
|
|
6,042,503 |
|
|
|
61 |
|
|
|
— |
|
|
|
— |
|
|
|
14,939 |
|
|
|
— |
|
|
|
15,000 |
|
|
Issuance of Letter Agreement and warrants to purchase Class A Common Stock - related party |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(500 |
) |
|
|
— |
|
|
|
(500 |
) |
|
Vesting of restricted stock units |
|
— |
|
|
|
11,667 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Change in redemption value of noncontrolling interest |
|
(96,680 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
96,680 |
|
|
|
96,680 |
|
|
Balances at September 30, 2018 |
$ |
19,912 |
|
|
|
15,772,449 |
|
|
$ |
158 |
|
|
|
23,094,221 |
|
|
$ |
232 |
|
|
$ |
144,617 |
|
|
$ |
(188,985 |
) |
|
$ |
(43,978 |
) |
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
6
Condensed Consolidated Statements of Cash Flows - Unaudited
(in thousands)
|
|
|
Nine Months Ended September 30, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
Net loss before noncontrolling interest |
|
$ |
(21,517 |
) |
|
$ |
(40,055 |
) |
|
Adjustments to reconcile net loss before noncontrolling interest to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
(Gain) loss on disposal of property and equipment, net |
|
|
(12 |
) |
|
|
(11 |
) |
|
Depreciation expense |
|
|
111 |
|
|
|
152 |
|
|
Share-based compensation expense |
|
|
2,345 |
|
|
|
2,657 |
|
|
Change in fair value of warrants, related party |
|
|
(610 |
) |
|
|
— |
|
|
Amortization of debt discount |
|
|
795 |
|
|
|
753 |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
8,000 |
|
|
|
— |
|
|
Prepaid expenses and other assets |
|
|
(1,475 |
) |
|
|
(113 |
) |
|
Long-term deposits |
|
|
2,256 |
|
|
|
(317 |
) |
|
Accounts payable and accrued expenses |
|
|
(4,936 |
) |
|
|
(1,293 |
) |
|
Deferred revenue |
|
|
(5,912 |
) |
|
|
(21 |
) |
|
Other liabilities |
|
|
(32 |
) |
|
|
19 |
|
|
Net cash used in operating activities |
|
|
(20,987 |
) |
|
|
(38,229 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
Proceeds from sale of assets |
|
|
12 |
|
|
|
32 |
|
|
Purchases of property and equipment |
|
|
(5 |
) |
|
|
(39 |
) |
|
Net cash provided by (used in) investing activities |
|
|
7 |
|
|
|
(7 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
Issuance of Class A Common Stock to a related party under the Letter Agreements |
|
|
15,000 |
|
|
|
— |
|
|
Proceeds from debt issuance |
|
|
500 |
|
|
|
7,500 |
|
|
Repayment of notes payable |
|
|
(2,674 |
) |
|
|
— |
|
|
Net cash provided by financing activities |
|
|
12,826 |
|
|
|
7,500 |
|
|
Net decrease in cash, cash equivalents and restricted cash and cash equivalents |
|
|
(8,154 |
) |
|
|
(30,736 |
) |
|
Total cash, cash equivalents and restricted cash and cash equivalents, beginning of period |
|
|
14,420 |
|
|
|
51,505 |
|
|
Total cash, cash equivalents and restricted cash and cash equivalents, end of period |
|
$ |
6,266 |
|
|
$ |
20,769 |
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash activities: |
|
|
|
|
|
|
|
|
|
Change in redemption value of noncontrolling interest |
|
$ |
(96,680 |
) |
|
$ |
36,349 |
|
|
Exchange of vTv Therapeutics Inc. Class B Common Stock and vTv Therapeutics, LLC member units for vTv Therapeutics Inc. Class A Common Stock |
|
$ |
151 |
|
|
$ |
— |
|
|
Issuance of Letter Agreement and warrants to purchase vTv Therapeutics Inc. Class A Common Stock to a related party |
|
$ |
500 |
|
|
$ |
— |
|
The accompanying notes are an integral part of the unaudited condensed consolidated financial statements.
7
Notes to Condensed Consolidated Financial Statements – Unaudited
(dollar amounts are in thousands, unless otherwise noted)
|
Note 1: |
Description of Business, Basis of Presentation and Going Concern |
Description of Business
vTv Therapeutics Inc. (the “Company,” the “Registrant,” “we” or “us”) was incorporated in the state of Delaware in April 2015. The Company was formed to discover and develop orally administered small molecule drug candidates to fill significant unmet medical needs.
Principles of Consolidation
vTv Therapeutics Inc. is a holding company and its principal asset is a controlling equity interest in vTv Therapeutics LLC (“vTv LLC”), the Company’s principal operating subsidiary, which is a clinical-stage biopharmaceutical company engaged in the discovery and development of orally administered small molecule drug candidates to fill significant unmet medical needs.
The Company has determined that vTv LLC is a variable-interest entity (“VIE”) for accounting purposes and that vTv Therapeutics Inc. is the primary beneficiary of vTv LLC because (through its managing member interest in vTv LLC and the fact that the senior management of vTv Therapeutics Inc. is also the senior management of vTv LLC) it has the power and benefits to direct all of the activities of vTv LLC, which include those that most significantly impact vTv LLC’s economic performance. vTv Therapeutics Inc. has therefore consolidated vTv LLC’s results pursuant to Accounting Standards Codification Topic 810, “Consolidation” in its Condensed Consolidated Financial Statements. As of September 30, 2018, various holders own non-voting interests in vTv LLC, representing a 59.4% economic interest in vTv LLC, effectively restricting vTv Therapeutics Inc.’s interest to 40.6% of vTv LLC’s economic results, subject to increase in the future, should vTv Therapeutics Inc. purchase additional non-voting common units (“vTv Units”) of vTv LLC, or should the holders of vTv Units decide to exchange such units (together with shares of Class B Common Stock) for shares of Class A Common Stock (or cash) pursuant to the Exchange Agreement (as defined in Note 8). vTv Therapeutics Inc. has provided financial and other support to vTv LLC in the form of its purchase of vTv Units with the net proceeds of the Company’s initial public offering (“IPO”) in 2015, its agreeing to be a co-borrower under the Venture Loan and Security Agreement (the “Loan Agreement”) with Horizon Technology Finance Corporation and Silicon Valley Bank (together, the “Lenders”) which was entered into in 2016 and its entrance into the letter agreements, dated as of December 5, 2017 and July 30, 2018, with MacAndrews and Forbes Group LLC (the “Letter Agreements”). vTv Therapeutics Inc. will not be required to provide financial or other support for vTv LLC outside of its obligations pertaining to the Loan Agreement as a co-borrower. However, vTv Therapeutics Inc. will control its business and other activities through its managing member interest in vTv LLC, and its management is the management of vTv LLC. The creditors of vTv LLC do not have any recourse to the general credit of vTv Therapeutics Inc. except as allowed under the provisions of the Loan Agreement. Nevertheless, because vTv Therapeutics Inc. will have no material assets other than its interests in vTv LLC, any financial difficulties at vTv LLC could result in vTv Therapeutics Inc. recognizing a loss.
Going Concern and Liquidity
To date, the Company has not generated any product revenue and has not achieved profitable operations. The continuing development of our drug candidates will require additional financing. From its inception through September 30, 2018, the Company has funded its operations primarily through a combination of private placements of common and preferred equity, research collaboration agreements, upfront and milestone payments for license agreements, debt and equity financings and the completion of its IPO in August 2015. As of September 30, 2018, the Company had an accumulated deficit of $189.0 million and has generated net losses in each year of its existence. As of September 30, 2018, the Company’s liquidity sources included cash and cash equivalents of $3.8 million and $5.0 million of remaining funds available under the Letter Agreements. Management estimates that these sources of funding will allow the Company to continue its operations and activities for a period of less than twelve months from the issuance of these Condensed Consolidated Financial Statements.
Based on the Company’s current operating plan, management believes that its current cash and cash equivalents and the remaining funds available under the Letter Agreements will allow the Company to meet its liquidity requirements into December 2018.
Though the Company’s expected cash needs for the foreseeable future have been significantly reduced with the discontinuation of the STEADFAST and open label extension studies, the Company will require additional financing to continue its operations. The Company is seeking possible additional partnering opportunities for its GKA, GLP-1r and other drug candidates which it believes may provide additional cash for use in its operations and the continuation of clinical trials for its drug candidates. The Company is also
8
pursuing other sources of financing to provide flexibility to its operating plan. The timing and availability of such financing is not yet known. The failure of the STEADFAST Study to meet either co-primary endpoint may make it more difficult for the Company to obtain such financing. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
The Company’s financial statements have been prepared assuming the Company will continue as a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the normal course of business. The Condensed Consolidated Financial Statements do not include adjustments to reflect the possible future effects on the recoverability and classification of recorded assets or the amounts of liabilities that might be necessary should the Company be unable to continue as a going concern.
|
Note 2: |
Summary of Significant Accounting Policies |
Unaudited Interim Financial Information
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The accompanying Condensed Consolidated Balance Sheet as of September 30, 2018, Condensed Consolidated Statements of Operations for the three and nine months ended September 30, 2018 and 2017, Condensed Consolidated Statement of Changes in Redeemable Noncontrolling Interest and Stockholders’ Deficit for the nine months ended September 30, 2018 and Condensed Consolidated Statements of Cash Flows for the nine months ended September 30, 2018 and 2017 are unaudited. These unaudited financial statements have been prepared in accordance with the rules and regulations of the United States Securities and Exchange Commission (“SEC”) for interim financial information. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. These financial statements should be read in conjunction with the audited financial statements and the accompanying notes for the year ended December 31, 2017 contained in the Company’s Annual Report on Form 10-K. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments (consisting of normal recurring adjustments) necessary to state fairly the Company’s financial position as of September 30, 2018, the results of operations for the three and nine months ended September 30, 2018 and 2017 and cash flows for the nine months ended September 30, 2018 and 2017. The December 31, 2017 Condensed Consolidated Balance Sheet included herein was derived from the audited financial statements but does not include all disclosures or notes required by GAAP for complete financial statements.
The financial data and other information disclosed in these notes to the financial statements related to the three and nine months ended September 30, 2018 and 2017 are unaudited. Interim results are not necessarily indicative of results for an entire year.
The Company does not have any components of other comprehensive income recorded within its Condensed Consolidated Financial Statements, and, therefore, does not separately present a statement of comprehensive income in its Condensed Consolidated Financial Statements.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
On an ongoing basis, the Company evaluates its estimates, including those related to the grant date fair value of equity awards, the fair value of warrants to purchase shares of its Class A Common Stock, the fair value of the Class B Common Stock, the useful lives of property and equipment, the fair value of derivative liabilities, and the fair value of the Company’s debt, among others. The Company bases its estimates on historical experience and on various other assumptions that it believes to be reasonable, the results of which form the basis for making judgments about the carrying value of assets and liabilities.
Concentration of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist principally of cash on deposit with multiple financial institutions. The balances of these cash accounts frequently exceed insured limits.
The accounts receivable balance at December 31, 2017 related to an upfront payment received in the first quarter of 2018 pursuant to the Company’s license agreement with Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. (“Huadong”).
Three customers represented 100% of the revenue earned during the three and nine months ended September 30, 2018. One customer represented 100% of the revenue earned during the three and nine months ended September 30, 2017.
9
The Company considers any highly liquid investments with an original maturity of three months or less to be cash and cash equivalents.
Restricted Cash and Cash Equivalents
Restricted cash and cash equivalents, current as of December 31, 2017 was $0.2 million. This amount was received through a research, development and commercialization agreement with JDRF (the “JDRF Agreement”). There were no amounts held as restricted cash and cash equivalents as of September 30, 2018 related to this agreement. Restricted cash and cash equivalents, long-term as of September 30, 2018 and December 31, 2017 was $2.5 million at each date. These amounts relate to the minimum balance that the Company must maintain in a deposit account that is pledged to secure the Loan Agreement and is subject to an account control agreement pursuant to the Loan Agreement.
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported within the Condensed Consolidated Balance Sheets as of September 30, 2018 and December 31, 2017 that sum to the total of the same such amounts shown in the Condensed Consolidated Statements of Cash Flows (in thousands):
|
|
September 30, 2018 |
|
|
December 31, 2017 |
|
||
|
Cash and cash equivalents |
$ |
3,766 |
|
|
$ |
11,758 |
|
|
Restricted cash and cash equivalents |
|
— |
|
|
|
162 |
|
|
Restricted cash and cash equivalents, long-term |
|
2,500 |
|
|
|
2,500 |
|
|
Total cash, cash equivalents and restricted cash and cash equivalents shown in the consolidated statement of cash flows |
$ |
6,266 |
|
|
$ |
14,420 |
|
Investments
In connection with the License Agreement with Reneo Pharmaceuticals, Inc. (“Reneo”) (the “Reneo License Agreement”), the Company received common stock and certain participation rights representing a minority equity interest in Reneo that is classified as a long-term investment in the Company’s Condensed Consolidated Balance Sheets as of September 30, 2018 and December 31, 2017. This investment is accounted for under the cost method because the Company owns less than 20% of the voting equity and does not have the ability to exercise significant influence over Reneo.
On January 1, 2018, the Company adopted ASU No. 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities”. This guidance requires equity investments to be measured at fair value with changes in fair value recognized in net income. Since it does not have a readily determinable market value, the Company has elected to measure its investment in Reneo at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment.
No adjustments have been made to the value of the Company’s investment in Reneo for the three and nine months ended September 30, 2018 either due to impairment or based on observable price changes.
Revenue Recognition
On January 1, 2018, the Company adopted ASC Topic 606, “Revenue From Contracts With Customers” (“ASC Topic 606”), using the modified retrospective method applied to those contracts which were not completed as of the adoption date. Results for reporting periods beginning after January 1, 2018 are presented under ASC Topic 606, while prior period amounts are not adjusted and continue to be reported in accordance with the Company’s historic accounting under ASC Topic 605.
The Company recorded a net reduction to its opening accumulated deficit of $0.2 million as of January 1, 2018 due to the cumulative impact of adopting ASC Topic 606, with the impact primarily related to the recognition of an asset for the incremental costs of obtaining contracts.
The majority of the Company’s revenue results from its license and collaboration agreements associated with the development of investigational drug products. The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties are identified, payment terms are identified, the contract has commercial substance and collectability of consideration is probable. For each contract meeting these criteria, the Company identifies the performance obligations included within the contract. A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. The Company then recognizes revenue under each contract as the related performance obligations are satisfied.
10
The transaction price under the contract is determined based on the value of the consideration expected to be received in exchange for the transferred assets or services. Development, regulatory and sales milestones included in the Company’s collaboration agreements are considered to be variable consideration. The amount of variable consideration expected to be received is included in the transaction price when it becomes probable that the milestone will be met. For contracts with multiple performance obligations, the contract’s transaction price is allocated to each performance obligation using the Company’s best estimate of the standalone selling price of each distinct good or service in the contract. The primary method used to estimate standalone selling price is the expected cost plus margin approach. Revenue is recognized over the related period over which the Company expects the services to be provided using a proportional performance model or a straight-line method of recognition if there is no discernable pattern over which the services will be provided.
Research and Development
Major components of research and development costs include cash and share-based compensation, depreciation expense on research and development property and equipment, costs of preclinical studies, clinical trials and related clinical manufacturing, costs of drug development, costs of materials and supplies, facilities costs, overhead costs, regulatory and compliance costs, and fees paid to consultants and other entities that conduct certain research and development activities on the Company’s behalf. Research and development costs are expensed as incurred.
The Company records accruals based on estimates of the services received, efforts expended and amounts owed pursuant to contracts with numerous contract research organizations. In the normal course of business, the Company contracts with third parties to perform various clinical study activities in the ongoing development of potential products. The financial terms of these agreements are subject to negotiation and variation from contract to contract and may result in uneven payment flows. Payments under the contracts depend on factors such as the achievement of certain events and the completion of portions of the clinical study or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses in its financial statements to the actual services received and efforts expended. As such, expense accruals related to clinical studies are recognized based on the Company’s estimate of the degree of completion of the event or events specified in the specific clinical study.
The Company records nonrefundable advance payments it makes for future research and development activities as prepaid expenses. Prepaid expenses are recognized as expense in the Condensed Consolidated Statements of Operations as the Company receives the related goods or services.
Research and development costs that are reimbursed under a cost-sharing arrangement are reflected as a reduction of research and development expense.
Recently Issued Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue From Contracts With Customers”, that outlines a single comprehensive model for entities to use in accounting for revenue arising from contracts with customers and supersedes most current revenue recognition guidance, including industry-specific guidance. The Company adopted this guidance as of January 1, 2018 using the modified retrospective transition method. See Note 2 – “Revenue Recognition” for further details.
In January 2016, the FASB issued ASU No. 2016-01, “Recognition and Measurement of Financial Assets and Financial Liabilities”, which amends ASC 825-10, “Financial Instruments – Overall”. This ASU amends various aspects of the recognition, measurement, presentation and disclosure of financial instruments. This ASU is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The Company adopted this guidance in the first quarter of fiscal 2018. The Company has elected to use the measurement alternative, defined as cost, less impairments, adjusted by observable price changes. The adoption of this guidance did not have a material impact on the Company’s Condensed Consolidated Financial Statements. See Note 2 – “Investments” for further details.
In May 2017, the FASB issued ASU No. 2017-09, “Compensation-Stock Compensation (Topic 718): Scope of Modification Accounting” (“ASU 2017-09”), which clarifies the changes to terms or conditions of a share-based payment award that require an entity to apply modification accounting. ASU 2017-09 is effective for annual reporting periods, and interim periods therein, beginning after December 15, 2017. The Company adopted this guidance in the first quarter of fiscal 2018. The adoption of this guidance did not have a material impact on the Company's Condensed Consolidated Financial Statements.
11
Recently Issued Accounting Pronouncements Not Yet Adopted
In February 2016, the FASB issued ASU No. 2016-02, “Lease (Topic 842)” (“ASU 2016-02”), which increases transparency and comparability among companies accounting for lease transactions. The most significant change of this update will require the recognition by a lessee of lease assets and liabilities on its balance sheet for operating lease arrangements with lease terms greater than 12 months. This update will require a modified retrospective application which includes a number of optional practical expedients related to the identification and classification of leases commenced before the effective date. This ASU is effective for fiscal years and interim periods within those fiscal years, beginning after December 15, 2018. The adoption of this guidance will result in the recognition of additional assets and liabilities related to the Company’s operating leases within its Condensed Consolidated Balance Sheets.
Note 3:Collaboration Agreements
Reneo License Agreement
On December 21, 2017, the Company entered into the Reneo License Agreement, under which Reneo obtained an exclusive, worldwide, sublicensable license to develop and commercialize the Company’s peroxisome proliferation activated receptor delta (PPAR-δ) agonist program, including the compound HPP593, for therapeutic, prophylactic or diagnostic application in humans. Under the terms of the Reneo License Agreement, Reneo paid the Company an upfront cash payment of $3.0 million. The Company is eligible to receive additional potential development, regulatory and sales-based milestone payments totaling up to $94.5 million. In addition, Reneo is obligated to pay the Company royalty payments at mid-single to low-double digit rates, based on tiers of annual net sales of licensed products. Such royalties will be payable on a licensed product-by-licensed product and country-by-country basis until the latest of expiration of the licensed patents covering a licensed product in a country, expiration of data exclusivity rights for a licensed product in a country or a specified number of years after the first commercial sale of a licensed product in a country. As additional consideration, the Company has also received common stock and certain participation rights representing a minority equity interest in Reneo.
Pursuant to the terms of the Reneo License Agreement, the Company is required to provide technology transfer services for a defined period after the effective date. In accordance with ASC Topic 606, the Company identified all of the performance obligations at the inception of the Reneo License Agreement. The significant obligations were determined to be the license and the technology transfer services. The Company has determined that the license and technology transfer services represent a single performance obligation because they were not capable of being distinct on their own. The transaction price has been fully allocated to this combined performance obligation. The remaining milestone payments that the Company is eligible to receive have not been included in the transaction price as of September 30, 2018, as it is not considered probable that such payments will be received. The unrecognized amount of the transaction price allocated to this performance obligation as of September 30, 2018 was $2.6 million.
The Company determined that there was no discernable pattern in which the technology services would be provided during the transfer services period. As such, the Company determined that the straight-line method would be used to recognize revenue over the transfer service period. The remainder of this performance obligation will be recognized over approximately 8.5 months. For the three and nine months ended September 30, 2018, $0.9 million and $2.7 million of revenue has been recognized related to this combined performance obligation, respectively.
Huadong License Agreement
On December 21, 2017, the Company entered into a License Agreement with Huadong (the “Huadong License Agreement”), under which Huadong obtained an exclusive and sublicensable license to develop and commercialize the Company’s glucagon-like peptide-1 receptor agonist (“GLP-1r”) program, including the compound TTP273, for therapeutic uses in humans or animals, in China and certain other Pacific Rim countries, including Australia and South Korea (collectively, the “Huadong License Territory”). Additionally, under the Huadong License Agreement, the Company obtained a non-exclusive, sublicensable, royalty-free license to develop and commercialize certain Huadong patent rights and know-how related to the Company’s GLP-1r program for therapeutic uses in humans or animals outside of the Huadong License Territory. Under the terms of the Huadong License Agreement, Huadong paid the Company an initial license fee of $8.0 million and is obligated to pay potential development and regulatory milestone payments totaling up to $25.0 million, with an additional potential regulatory milestone of $20.0 million if Huadong receives regulatory approval for a central nervous system indication. In addition, the Company is eligible for an additional $50.0 million in potential sales-based milestones, as well as royalty payments ranging from low-single to low-double digit rates, based on tiered sales of licensed products.
Under the Huadong License Agreement, the Company is also responsible for conducting a Phase 2 multi-region clinical trial (the “Phase 2 MRCT”) including sites in both the United States and Huadong License Territory for the purpose of assessing the safety and efficacy of TTP273 in patients with type 2 diabetes. The Phase 2 MRCT will be designed to satisfy the requirements of the China
12
Food and Drug Administration necessary in order for Huadong to begin a Phase 3 clinical trial in China. The Company will also be responsible for contributing up to $3.0 million in connection with the Phase 2 MRCT.
In accordance with ASC Topic 606, the Company identified all of the performance obligations at the inception of the Huadong License Agreement. The significant performance obligations were determined to be (i) the exclusive license to develop and commercialize the Company’s GLP-1r program, (ii) technology transfer services related to the chemistry and manufacturing know-how for a defined period after the effective date (iii) the obligation to sponsor and conduct the Phase 2 MRCT, (iv) the Company’s obligation to participate on a joint development committee, and (v) other obligations considered to be de minimis in nature.
The transaction price has been allocated to these performance obligations based on their relative standalone selling prices, which were estimated using an expected cost plus margin approach. The remaining milestone payments that the Company is eligible to receive have not been included in the transaction price as of September 30, 2018, as it is not considered probable that such payments will be received.
The Company has determined that the license and technology transfer services related to the chemistry and manufacturing know-how represent a combined performance obligation because they were not capable of being distinct on their own. The Company also determined that there was no discernable pattern in which the technology transfer services would be provided during the transfer service period. As such, the Company determined that the straight-line method would be used to recognize revenue for this performance obligation over the transfer service period. The unrecognized amount of the transaction price allocated to this performance obligation of $3.3 million will be recognized over approximately 8.5 months. For the three and nine months ended September 30, 2018, $1.2 million and $3.5 million of revenue has been recognized related to this combined performance obligation, respectively.
The Company also determined that the obligation to sponsor and conduct a portion of the Phase 2 MRCT should be treated as a separate performance obligation. A portion of the total consideration received under the Huadong License Agreement was allocated to this performance obligation based on its estimated standalone selling price. This amount was deferred as of September 30, 2018 and revenue will be recognized using the proportional performance model over the period during which the Company conducts the Phase 2 MRCT trial. No revenue for this performance obligation has been recognized during the three and nine months ended September 30, 2018.
The Company also determined that the obligation to participate in the joint development committee (the “JDC”) to oversee the development of products and the Phase 2 MRCT in accordance with the development plan should be treated as a separate performance obligation. A portion of the total consideration received under the Huadong License Agreement was allocated to this performance obligation based on its estimated standalone selling price. This amount was deferred as of September 30, 2018 and revenue will be recognized using the proportional performance model over the period of the Company’s participation on the JDC. An immaterial amount of revenue for this performance obligation has been recognized during the three and nine months ended September 30, 2018.
Newsoara License Agreement
On May 31, 2018, the Company entered into a license agreement with Newsoara Biopharma Co., Ltd., (“Newsoara”) (the “Newsoara License Agreement”), under which Newsoara obtained an exclusive and sublicensable license to develop and commercialize the Company’s phosphodiesterase type 4 inhibitors (“PDE4”) program, including the compound HPP737, in China, Hong Kong, Macau, Taiwan and other pacific rim countries (collectively, the “Newsoara License Territory”). Additionally, under the Newsoara License Agreement, the Company obtained a non-exclusive, sublicensable, royalty-free license to develop and commercialize certain Newsoara patent rights and know-how related to the Company’s PDE4 program for therapeutic uses in humans outside of the Newsoara License Territory. Under the terms of the Newsoara License Agreement, Newsoara paid the Company an upfront cash payment of $2.0 million. The Company is eligible to receive additional potential development, regulatory and sales-based milestone payments totaling up to $63.0 million. In addition, Newsoara is obligated to pay the Company royalty payments at high-single to low-double digit rates, based on tiers of annual net sales of licensed products. Such royalties will be payable on a licensed product-by-licensed product and country-by-country basis until the latest of expiration of the licensed patents covering a licensed product in a country, expiration of data exclusivity rights for a licensed product in a country or a specified number of years after the first commercial sale of a licensed product in a country.
Pursuant to the terms of the Newsoara License Agreement, the Company is required to provide technology transfer services for a defined period after the effective date. In accordance with ASC Topic 606, the Company identified all of the performance obligations at the inception of the Newsoara License Agreement. The significant obligations were determined to be the license and the technology transfer services. The Company has determined that the license and technology transfer services represent a single performance obligation because they were not capable of being distinct on their own. The transaction price has been fully allocated to this combined performance obligation. The remaining milestone payments that the Company is eligible to receive have not been included in the transaction price as of September 30, 2018, as it is not considered probable that such payments will be received. The unrecognized amount of the transaction price allocated to this performance obligation as of September 30, 2018 was $0.3 million.
13
The Company determined that there was no discernable pattern in which the technology services would be provided during the transfer services period. As such, the Company determined that the straight-line method would be used to recognize revenue over the transfer service period. The remainder of this performance obligation will be recognized over approximately 0.5 months. For the three and nine months ended September 30, 2018, $1.3 million and $1.7 million of revenue has been recognized related to this combined performance obligation, respectively.
JDRF Agreement
In August 2017, the Company entered into the JDRF Agreement to support the funding of the Simplici-T1 Study, an adaptive Phase 1b/2 study to explore the effects of TTP399 in type 1 diabetics. The Company initiated the Phase 2 portion of this study in the second quarter of 2018. According to the terms of the JDRF Agreement, JDRF will provide research funding of up to $3.0 million based on the achievement of research and development milestones, with the total funding provided by JDRF not to exceed approximately one-half of the total cost of the project. Additionally, the Company has the obligation to make certain milestone payments to JDRF upon the commercialization, licensing, sale or transfer of TTP399 as a treatment for type 1 diabetes.
Payments that the Company receives from JDRF under this agreement will be recorded as restricted cash and current liabilities and recognized as an offset to research and development expense, based on the progress of the project, and only to the extent that the restricted cash is utilized to fund such development activities. As of September 30, 2018, the Company had received funding under this agreement of $0.8 million. Research and development costs were offset by a total of $0.8 million over the course of this agreement. As of September 30, 2018, the Company has recognized restricted cash of an immaterial amount related to this agreement.
Contract Liabilities
Contract liabilities related to the Company’s collaboration agreements consisted of the following (in thousands):
|
|
September 30, 2018 |
|
|
December 31, 2017 |
|
|
Change |
|
|||
|
Deferred revenue |
$ |
6,747 |
|
|
$ |
8,757 |
|
|
$ |
(2,010 |
) |
|
Deferred revenue - net of current portion |
|
595 |
|
|
|
4,497 |
|
|
|
(3,902 |
) |
|
Total contract liabilities |
$ |
7,342 |
|
|
$ |
13,254 |
|
|
$ |
(5,912 |
) |
The change in the Company’s contract liabilities for the nine months ended September 30, 2018 was due to the recognition of revenue based on the estimated performance of services under the related collaboration agreements as well as the recognition of deferred revenue related to the Company’s Newsoara License Agreement. There were no changes in the estimated transaction prices for the related contracts during the three and nine months ended September 30, 2018.
|
Note 4: |
Share-Based Compensation |
During the three and nine months ended September 30, 2018, the Company issued non-qualified stock option awards to certain employees of the Company. These option awards vest ratably over a three-year period and the option awards expire after a term of ten years from the date of grant. As of September 30, 2018, the Company had total unrecognized stock-based compensation expense for its outstanding stock option awards of approximately $1.6 million, which is expected to be recognized over a weighted average period of 1.5 years. The weighted average grant date fair value of option grants during the nine months ended September 30, 2018 and 2017 was $2.28 and $4.17 per option, respectively. The aggregate intrinsic value of the in-the-money awards outstanding at September 30, 2018 was $0.
The Company uses the Black-Scholes option pricing model to calculate the fair value of stock options granted. The fair value of stock options granted was estimated using the following assumptions:
|
|
For the Nine Months Ended September 30, |
|
|||
|
|
2018 |
|
|
2017 |
|
|
Expected volatility |
71.15% - 99.23% |
|
|
76.88% - 85.93% |
|
|
Expected life of option, in years |
5.7 - 6.0 |
|
|
5.8 - 6.0 |
|
|
Risk-free interest rate |
2.69% - 2.84% |
|
|
1.87% - 2.24% |
|
|
Expected dividend yield |
0.00% |
|
|
0.00% |
|
14
The following table summarizes the activity related to the stock option awards for the nine months ended September 30, 2018:
|
|
Number of Shares |
|
|
Weighted- Average Exercise Price |
|
||
|
Awards outstanding at December 31, 2017 |
|
1,960,732 |
|
|
$ |
8.50 |
|
|
Granted |
|
102,750 |
|
|
|
3.25 |
|
|
Forfeited |
|
(280,794 |
) |
|
|
6.17 |
|
|
Awards outstanding at September 30, 2018 |
|
1,782,688 |
|
|
$ |
8.57 |
|
|
Options exercisable at September 30, 2018 |
|
1,173,468 |
|
|
$ |
10.20 |
|
|
Weighted average remaining contractual term |
7.3 Years |
|
|
|
|
|
|
|
Options vested and expected to vest at September 30, 2018 |
|
1,742,506 |
|
|
$ |
8.64 |
|
|
Weighted average remaining contractual term |
7.7 Years |
|
|
|
|
|
|
The following table summarizes the activity related to the RSU awards for the nine months ended September 30, 2018:
|
|
Number of Shares |
|
|
Weighted- Average Grant Date Fair Value |
|
||
|
Awards outstanding at December 31, 2017 |
|
35,000 |
|
|
$ |
5.81 |
|
|
Vested |
|
(11,667 |
) |
|
|
5.81 |
|
|
Awards outstanding at September 30, 2018 |
|
23,333 |
|
|
$ |
5.81 |
|
|
RSUs expected to vest at September 30, 2018 |
|
22,896 |
|
|
$ |
5.81 |
|
As of September 30, 2018, the Company had total unrecognized stock-based compensation expense for its outstanding RSU awards of approximately $0.1 million, which is expected to be recognized over a weighted-average period of 1.4 years. The aggregate intrinsic value of the RSUs outstanding at September 30, 2018 was de minimis.
Compensation expense related to the grants of stock options and RSUs is included in research and development and general and administrative expense as follows (in thousands):
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
||||
|
Research and development |
$ |
247 |
|
|
$ |
405 |
|
|
$ |
871 |
|
|
$ |
1,077 |
|
|
General and administrative |
|
332 |
|
|
|
554 |
|
|
|
1,474 |
|
|
|
1,580 |
|
|
Total share-based compensation expense |
$ |
579 |
|
|
$ |
959 |
|
|
$ |
2,345 |
|
|
$ |
2,657 |
|
Note 5:Notes Payable
Notes payable consist of the following (in thousands):
|
|
September 30, 2018 |
|
|
December 31, 2017 |
|
||
|
Notes payable under the Loan Agreement |
$ |
17,396 |
|
|
$ |
20,000 |
|
|
Short-term financing |
|
431 |
|
|
|
— |
|
|
Accreted final payment (unamortized debt discount) |
|
381 |
|
|
|
(413 |
) |
|
Total notes payable |
|
18,208 |
|
|
|
19,587 |
|
|
Less: Current portion |
|
(9,597 |
) |
|
|
(4,271 |
) |
|
Total notes payable, net of current portion |
$ |
8,611 |
|
|
$ |
15,316 |
|
In October 2016, the Company entered into the Loan Agreement with Horizon Technology Finance Corporation and Silicon Valley Bank, under which the Company and vTv LLC borrowed $20.0 million.
Each loan tranche bears interest at a floating rate equal to 10.5% plus the amount by which the one-month London Interbank Offer Rate (“LIBOR”) exceeds 0.5%.
The Company borrowed the first tranche of $12.5 million upon close of the Loan Agreement in October 2016. The first tranche requires only monthly interest payments until May 1, 2018 followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date on May 1, 2020. In addition, a final payment for the first tranche loan equal to $0.8 million will be due on May 1, 2020, or such earlier date specified in the Loan Agreement. The Company borrowed the second tranche of $7.5 million in March 2017. The second tranche requires only monthly interest payments until October 1, 2018 followed by equal monthly
15
payments of principal plus accrued interest through the scheduled maturity date on October 1, 2020. In addition, a final payment for the second tranche loan equal to $0.5 million will be due on October 1, 2020, or such earlier date specified in the Loan Agreement. The availability of the third tranche of $5.0 million expired unused on June 30, 2017.
If the Company repays all or a portion of the loan prior to the applicable maturity date, it will pay the Lenders a prepayment penalty fee, based on a percentage of the then outstanding principal balance equal to 4.0% during the first 18 months following the funding of the second tranche and 2.0% thereafter.
The Company’s obligations under the Loan Agreement are secured by a first priority security interest in substantially all of its assets. As a result of the termination of the STEADFAST Study, the Company granted the Lenders a first priority security interest in all of the Company’s intellectual property, subject to certain limited exceptions. The Company has agreed not to pledge or otherwise encumber its intellectual property assets, subject to certain exceptions.
The Loan Agreement includes customary affirmative and restrictive covenants, including, but not limited to, restrictions on the payment of dividends or other equity distributions and the incurrence of debt or liens upon the assets of the Company or its subsidiaries. The Loan Agreement does not contain any financial maintenance covenants other than a requirement to maintain a minimum cash balance of not less than $2.5 million in a deposit account pledged to secure the Loan Agreement and subject to an account control agreement. The minimum cash balance covenant was included as part of an amendment to the Loan Agreement in connection with the entry into the Huadong License Agreement in December 2017. The Loan Agreement includes customary events of default, including payment defaults, covenant defaults, and material adverse change default. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5.0% will be applied to the outstanding loan balances, and the Lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement.
In connection with the Loan Agreement, the Company issued to the Lenders warrants to purchase shares of the Company’s Class A Common Stock (the “Warrants”). On October 28, 2016, the Company issued Warrants to purchase 152,580 shares of its Class A Common Stock at a per share exercise price of $6.39 per share, which aggregate exercise price represents 6.0% of the principal amount borrowed under the first tranche of the Loan Agreement and 3.0% of the amount available under the second tranche of the Loan Agreement. On March 24, 2017, in connection with the funding of the second tranche, the Company issued Warrants to purchase 38,006 shares of its Class A Common Stock at a per share exercise price of $5.92 per share, which aggregate exercise price represents 3.0% of the principal amount of the second tranche of the Loan Agreement. In each instance, the Warrants have an exercise price equal to the lower of (a) the volume weighted average price per share of the Company’s Class A Common Stock, as reported on the principal stock exchange on which the Company’s Class A Common Stock is listed, for 10 trading days prior to the issuance of the applicable Warrants or (b) the closing price of a share of the Company’s Class A Common Stock on the trading day prior to the issuance of the applicable Warrants. The Warrants will expire seven years from their date of issuance.
The costs incurred in connection with the Loan Agreement, along with the allocated fair value of the Warrants issued of $0.9 million were treated as a debt discount and are offset against the carrying value of the notes payable in the Company’s Condensed Consolidated Balance Sheet as of September 30, 2018 and December 31, 2017. These costs will be recognized as interest expense over the term of the first tranche using the effective interest method. The final payments for the first and second loan tranches of $0.8 million and $0.5 million, respectively, will be accrued as additional interest expense, using the effective interest method, over the term of the relevant tranche.
|
Note 6: |
Commitments and Contingencies |
Legal Matters
From time to time, the Company is involved in various legal proceedings arising in the normal course of business. If a specific contingent liability is determined to be probable and can be reasonably estimated, the Company accrues and discloses the amount. The Company is not currently a party to any material legal proceedings.
Columbia University Agreement
In May 2015, the Company entered into a worldwide exclusive agreement with Columbia University (“Columbia”) to license certain intellectual property from Columbia. Under the agreement, the Company is obligated to pay to Columbia (1) an annual fee of $0.1 million from 2015 through 2021, (2) a potential regulatory milestone payment of $0.8 million and (3) potential royalty payments at a single digit royalty rate based on net sales of licensed products as defined in the agreement.
16
In February 2007, the Company entered into an Agreement Concerning Glucokinase Activator Project with Novo Nordisk A/S (the “Novo License Agreement”) whereby we obtained an exclusive, worldwide, sublicensable license under certain Novo Nordisk intellectual property rights to discover, develop, manufacture, have manufactured, use and commercialize products for the prevention, treatment, control, mitigation or palliation of human or animal diseases or conditions. As part of this license grant, the Company obtained certain worldwide rights to Novo Nordisk’s GKA program, including rights to preclinical and clinical compounds such as TTP399. Under the terms of the Novo License Agreement, the Company has additional potential developmental and regulatory milestone payments totaling up to $115.0 million for approval of a product. The Company may also be obligated to pay an additional $75.0 million in potential sales-based milestones, as well as royalty payments, at mid-single digit royalty rates, based on tiered sales of commercialized licensed products.
Huadong License Agreement
Under the terms of the Huadong License Agreement, vTv LLC is responsible for sponsoring the Phase 2 MRCT including sites in both the US and the Huadong License Territory for the purpose of assessing the safety and efficacy of TTP273 in patients with type 2 diabetes. vTv LLC will be responsible for contributing up to $3.0 million in connection with the Phase 2 MRCT.
|
Note 7: |
Redeemable Noncontrolling Interest |
The Company is subject to the Exchange Agreement with respect to the vTv Units representing the 59.4% noncontrolling interest in vTv LLC outstanding as of September 30, 2018 (see Note 8). The Exchange Agreement requires the surrender of an equal number of vTv Units and Class B Common Stock for (i) shares of Class A Common Stock on a one-for-one basis or (ii) cash (based on the fair market value of the Class A Common Stock as determined pursuant to the Exchange Agreement), at the Company’s option (as the managing member of vTv LLC), subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications. The exchange value is determined based on a 20-day volume weighted average price of the Class A Common Stock as defined in the Exchange Agreement, subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications.
The redeemable noncontrolling interest is recognized at the higher of (1) its initial fair value plus accumulated earnings/losses associated with the noncontrolling interest or (2) the redemption value as of the balance sheet date. At September 30, 2018 and December 31, 2017, the redeemable noncontrolling interest was recorded based on the redemption value as of the balance sheet date of $19.9 million and $131.4 million, respectively.
|
Note 8: |
Related-Party Transactions |
MacAndrews & Forbes Incorporated
As of September 30, 2018, subsidiaries and affiliates of MacAndrews & Forbes Incorporated (collectively “MacAndrews”) indirectly controlled 23,084,267 shares of the Company’s Class B Common Stock and 8,658,169 shares of the Company’s Class A Common Stock. As a result, MacAndrews’ holdings represent approximately 81.7% of the combined voting power of the Company’s outstanding common stock.
The Company has entered into several agreements with MacAndrews or its affiliates as further detailed below:
Letter Agreements
The Company has entered into the Letter Agreements with MacAndrews. Under the terms of the Letter Agreements, the Company has the right to sell to MacAndrews shares of its Class A Common Stock at a specified price per share, and MacAndrews has the right (exercisable up to three times) to require the Company to sell to it shares of Class A Common Stock at the same price. An aggregate of $10.0 million worth of Class A Common Stock may be sold under each of the Letter Agreements (whether at the Company’s or MacAndrews’ option). In addition, in connection with and as a commitment fee for the entrance into these Letter Agreements, the Company also issued MacAndrews warrants (the “Letter Agreement Warrants”) to purchase additional shares of the Company’s Class A Common Stock.
17
The critical terms of these Letter Agreements are set forth in the table below:
|
|
December 5, 2017 Letter Agreement |
|
|
July 30, 2018 Letter Agreement |
|
||
|
Specified purchase price per share |
$ |
4.38 |
|
|
$ |
1.33 |
|
|
Expiration date of letter agreement |
December 5, 2018 |
|
|
July 30, 2019 |
|
||
|
Shares available to be issued under related warrants |
|
198,267 |
|
|
|
518,654 |
|
|
Exercise price of related warrants |
$ |
5.04 |
|
|
$ |
1.53 |
|
|
Expiration date of related warrants |
December 5, 2024 |
|
|
July 30, 2025 |
|
||
|
Total shares issued as of September 30, 2018 |
|
2,283,105 |
|
|
|
3,759,398 |
|
|
Remaining shares to be issued as of September 30, 2018 |
|
— |
|
|
|
3,759,399 |
|
Exchange Agreement
The Company and MacAndrews are party to an exchange agreement (the “Exchange Agreement”) pursuant to which the vTv Units (along with a corresponding number of shares of the Class B Common Stock) are exchangeable for (i) shares of the Company’s Class A Common Stock on a one-for-one basis or (ii) cash (based on the fair market value of the Class A Common Stock as determined pursuant to the Exchange Agreement), at the Company’s option (as the managing member of vTv LLC), subject to customary conversion rate adjustments for stock splits, stock dividends and reclassifications. Any decision to require an exchange for cash rather than shares of Class A Common Stock will ultimately be determined by the entire board of directors of vTv Therapeutics Inc. (the “Board of Directors”). As of September 30, 2018, MacAndrews had not exchanged any shares under the provisions of this agreement.
Tax Receivable Agreement
The Company and MacAndrews are party to a tax receivable agreement (the “Tax Receivable Agreement”), which provides for the payment by the Company to M&F TTP Holdings Two LLC (“M&F”), as successor in interest to vTv Therapeutics Holdings, LLC (“vTv Therapeutics Holdings”), and M&F TTP Holdings LLC (or certain of its transferees or other assignees) of 85% of the amount of cash savings, if any, in U.S. federal, state and local income tax or franchise tax that the Company actually realizes (or, in some circumstances, the Company is deemed to realize) as a result of (a) the exchange of Class B Common Stock, together with the corresponding number of vTv Units, for shares of the Company’s Class A Common Stock (or for cash), (b) tax benefits related to imputed interest deemed to be paid by the Company as a result of the Tax Receivable Agreement and (c) certain tax benefits attributable to payments under the Tax Receivable Agreement.
As no shares have been exchanged by MacAndrews pursuant to the Exchange Agreement (discussed above), the Company has not recognized any liability nor has it made any payments pursuant to the Tax Receivable Agreement as of September 30, 2018.
Investor Rights Agreement
The Company is party to an investor rights agreement with M&F, as successor in interest to vTv Therapeutics Holdings (the “Investor Rights Agreement”). The Investor Rights Agreement provides M&F with certain demand, shelf and piggyback registration rights with respect to its shares of Class A Common Stock and also provides M&F with certain governance rights, depending on the size of its holdings of Class A Common Stock. Under the Investor Rights Agreement, M&F was initially entitled to nominate a majority of the members of the Board of Directors and designate the members of the committees of the Board of Directors.
|
Note 9: |
Income Taxes |
The Company is subject to U.S. federal income taxes as well as state taxes. The Company recorded an income tax provision of $0.2 million for the nine months ended September 30, 2018 representing foreign withholding taxes incurred in connection with the Newsoara License Agreement. The Company did not record an income tax provision for the three and nine months ended September 30, 2017. Management has evaluated the positive and negative evidence surrounding the realization of its deferred tax assets, including the Company’s history of losses, and under the applicable accounting standards determined that it is more-likely-than-not that the deferred tax assets will not be realized. The difference between the effective tax rate of the Company and the U.S. statutory tax rate of 21% at September 30, 2018 is due to the valuation allowance against the Company’s expected net operating losses.
As discussed in Note 8, the Company is party to a tax receivable agreement with a related party which provides for the payment by the Company to M&F (or certain of its transferees or other assignees) of 85% of the amount of cash savings, if any, in U.S. federal, state and local income tax or franchise tax that the Company actually realizes (or, in some circumstances, the Company is deemed to
18
realize) as a result of certain transactions. As no transactions have occurred which would trigger a liability under this agreement, the Company has not recognized any liability related to this agreement as of September 30, 2018.
On December 22, 2017, the U.S. federal government enacted comprehensive tax reform commonly referred to as the Tax Cuts and Jobs Act (“TCJA”). Under ASC Topic 740, the effects of changes in tax rates and laws are recognized in the period which the new legislation is enacted. Among other things, the TCJA (1) reduces the U.S federal statutory corporate income tax rate from 35% to 21% effective January 1, 2018, (2) eliminates the corporate alternative minimum tax, (3) eliminates the Section 199 deduction, and (4) changes rules related to uses and limitations of net operating loss carryforwards beginning after December 31, 2017.
The SEC staff issued Staff Accounting Bulletin No. 118 (“SAB 118”), which provides guidance on accounting for the tax effects of TCJA. SAB 118 provides a measurement period that should not extend beyond one year from the TCJA enactment date for companies to complete the accounting under ASC Topic 740. To the extent that a company’s accounting for certain income tax effects of the TCJA is incomplete but is able to determine a reasonable estimate, it must record a provisional estimate in the financial statements.
The TCJA reduces the corporate tax rate to 21% effective January 1, 2018. While we are able to make a reasonable estimate of the impact of the reduction in the corporate rate, it may be affected by other analyses related to the TCJA. The Company will continue to assess and refine, as necessary, its accounting for the TCJA as additional guidance and interpretation is provided.
|
Note 10: |
Net Loss per Share |
Basic loss per share is computed by dividing net loss attributable to vTv Therapeutics Inc. by the weighted-average number of shares of Class A Common Stock outstanding during the period. Diluted loss per share is computed giving effect to all potentially dilutive shares. Diluted loss per share for all periods presented is the same as basic loss per share as the inclusion of potentially issuable shares would be antidilutive.
A reconciliation of the numerator and denominator used in the calculation of basic and diluted net loss per share of Class A Common Stock is as follows (in thousands, except share and per share amounts):
|
|
For the Three Months Ended September 30, |
|
|
For the Nine Months Ended September 30, |
|
||||||||||
|
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
||||
|
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
$ |
(1,961 |
) |
|
$ |
(12,355 |
) |
|
$ |
(21,517 |
) |
|
$ |
(40,055 |
) |
|
Less: Net loss attributable to noncontrolling interests |
|
(1,165 |
) |
|
|
(8,705 |
) |
|
|
(14,697 |
) |
|
|
(28,222 |
) |
|
Net loss attributable to vTv Therapeutics Inc., basic and diluted |
$ |
(796 |
) |
|
$ |
(3,650 |
) |
|
$ |
(6,820 |
) |
|
$ |
(11,833 |
) |
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-average vTv Therapeutics Inc. Class A Common Stock, basic and diluted |
|
12,305,949 |
|
|
|
9,693,254 |
|
|
|
10,701,599 |
|
|
|
9,693,254 |
|
|
Net loss per share of vTv Therapeutics Inc. Class A Common Stock, basic and diluted |
$ |
(0.06 |
) |
|
$ |
(0.38 |
) |
|
$ |
(0.64 |
) |
|
$ |
(1.22 |
) |
Potentially dilutive securities not included in the calculation of diluted net loss per share are as follows:
|
|
September 30, 2018 |
|
|
September 30, 2017 |
|
||
|
Class B Common Stock (1) |
|
23,094,221 |
|
|
|
23,119,246 |
|
|
Common stock options granted under the Plan |
|
1,782,688 |
|
|
|
1,952,399 |
|
|
Restricted stock units |
|
23,333 |
|
|
|
35,000 |
|
|
Common stock options granted under Letter Agreement |
|
3,759,399 |
|
|
|
— |
|
|
Common stock warrants |
|
907,507 |
|
|
|
190,586 |
|
|
Total |
|
29,567,148 |
|
|
|
25,297,231 |
|
|
|
(1) |
Shares of Class B Common Stock do not share in the Company’s earnings and are not participating securities. Accordingly, separate presentation of loss per share of Class B Common Stock under the two-class method has not been provided. Each share of Class B Common Stock (together with a corresponding vTv Unit) is exchangeable for one share of Class A Common Stock. |
19
Note 11:Fair Value of Financial Instruments
The carrying amount of certain of the Company’s financial instruments, including cash and cash equivalents, net accounts receivable, accounts payable and other accrued liabilities approximate fair value due to their short-term nature.
The fair value of the Company’s Loan Agreement is considered to approximate its carrying value because it bears interest at a variable interest rate.
The Company measures the value of its investment in Reneo at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment. During the three and nine months ended September 30, 2018, there were no observable price changes in identical or similar investments, nor were there any indications of impairment. As such, the value of the Company’s investment in Reneo was not remeasured.
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The Company evaluates its financial assets and liabilities subject to fair value measurements on a recurring basis to determine the appropriate level in which to classify them for each reporting period. This determination requires significant judgments. The following table summarizes the conclusions reached regarding fair value measurements as of September 30, 2018 and December 31, 2017 (in thousands):
|
|
Balance at September 30, 2018 |
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
||||
|
Warrant liability, related party (1) |
$ |
382 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
382 |
|
|
Total |
$ |
382 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
382 |
|
|
|
Balance at December 31, 2017 |
|
|
Quoted Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
||||
|
$ |
492 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
492 |
|
|
|
Total |
$ |
492 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
492 |
|
|
(1) |
Fair value determined using the Black-Scholes option pricing model. Expected volatility is based on a portfolio of selected stocks of companies believed to have market and economic characteristics similar to its own. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of the valuation. |
|
(2) |
Fair value determined using an option pricing model based on the Company’s current capitalization. Expected volatility is based on a portfolio of selected stocks of companies believed to have market and economic characteristics similar to its own. The risk-free rate is based on the yield of U.S. government securities with the same term as the option as of the valuation date. |
|
|
Changes in Level 3 instruments for the nine months ended September 30, |
|
|||||||||||||||||
|
|
Balance at January 1 |
|
|
Net Change in fair value included in earnings |
|
|
Purchases / Issuance |
|
|
Sales / Repurchases |
|
|
Balance at September 30, |
|
|||||
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability, related party |
|
492 |
|
|
|
(610 |
) |
|
|
500 |
|
|
|
— |
|
|
|
382 |
|
|
Total |
$ |
492 |
|
|
$ |
(610 |
) |
|
$ |
500 |
|
|
$ |
— |
|
|
$ |
382 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2017 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Warrant liability |
$ |
167 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(167 |
) |
|
$ |
— |
|
|
Total |
$ |
167 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
(167 |
) |
|
$ |
— |
|
During the three and nine months ended September 30, 2018, the Company recognized a gain of $0.3 million and $0.6 million, respectively, related to the change in fair value of the Letter Agreement Warrants. This gain was recognized as a component of other
20
income – related party in the Condensed Consolidated Statements of Operations. Significant inputs utilized in the valuation of the Letter Agreement Warrants as of September 30, 2018 were:
|
Expected volatility |
96.05% - 98.58% |
|
Risk-free interest rate |
2.98% - 3.00% |
Changes in the unobservable inputs noted above would impact the amount of the liability for the Letter Agreement Warrants. Increases (decreases) in the estimates of the Company’s annual volatility would increase (decrease) the liability and an increase (decrease) in the annual risk-free rate would increase (decrease) the liability.
Note 12:Subsequent Events
On October 26, 2018, we entered into amendments to the Letter Agreement Warrants with MacAndrews, which removed certain anti-dilution provisions of the Letter Agreement Warrants.
On November 6, 2018, the Company caused MacAndrews to purchase an additional 1,879,699 shares of its Class A Common Stock under the terms of the letter agreement entered into on July 30, 2018 for $2.5 million in cash.
21
As used in this Quarterly Report on Form 10-Q, the “Company”, the “Registrant”, “we” or “us” refer to vTv Therapeutics Inc. and “vTv LLC” refers to vTv Therapeutics LLC. The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this report. In addition to historical financial information, the following discussion contains forward-looking statements that reflect our plans, estimates, assumptions and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this report under “Part II, Other Information—Item 1A, Risk Factors.” Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies and operations, financing plans, potential growth opportunities, potential market opportunities, potential results of our drug development efforts or trials, and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our management’s plans, estimates, assumptions and beliefs only as of the date of this report. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
Overview
We are a clinical-stage biopharmaceutical company engaged in the discovery and development of orally administered small molecule drug candidates to fill significant unmet medical needs. To date, we have primarily focused our efforts on advancing our programs for the treatment of mild Alzheimer’s disease (“AD”) and diabetes.
As discussed further in “Our Alzheimer’s Program – Azeliragon – Phase 3 STEADFAST Study” below, both Parts A and B of our Phase 3 STEADFAST Study of the investigational medication azeliragon (“TTP488”) in people with mild Alzheimer’s disease (the “STEADFAST Study”) failed to meet either of the co-primary efficacy endpoints. We are currently evaluating future development pathways for TTP488 based on the data received from the STEADFAST Study and feedback received from the Food and Drug Administration (“FDA”) and European Medicines Agency (“EMA”).
In addition to the azeliragon program, vTv is developing two compounds for the treatment of diabetes. TTP399 is an orally administered, liver-selective glucokinase activator (“GKA”), for which we have completed our Phase 2b clinical trial in type 2 diabetes (the “AGATA Study”) and are conducting an adaptive Phase 1b/2 clinical trial in type 1 diabetes (the “SimpliciT-1 Study”). TTP273 is an orally administered, non-peptide agonist that targets the glucagon-like peptide-1 receptor (“GLP-1r”), for which we have completed a Phase 2 clinical trial in type 2 diabetes (the “LOGRA Study”), and that is currently being developed by Huadong under the Huadong License Agreement.
vTv also has two programs in various stages of preclinical and clinical development for the treatment of inflammatory disorders that have been out-licensed, either in whole or in part, and one program for which we are seeking a partner to further develop.
We are currently exploring further development options for azeliragon and our other programs whether conducted internally, upon the receipt of additional funding, or through partnerships with other life sciences entities to either finance or perform the required development activities.
22
The following table summarizes our current drug candidates and their respective stages of development:
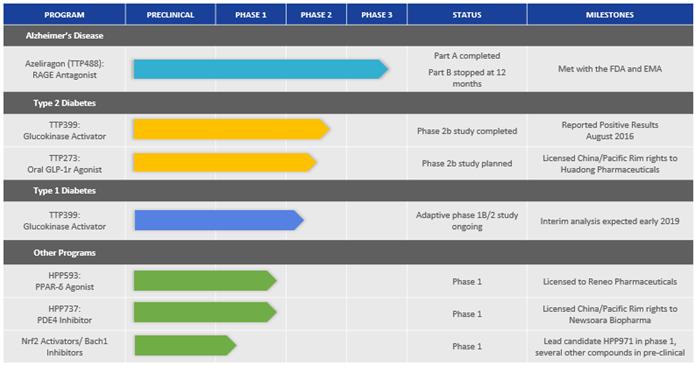
Our Alzheimer’s Program – Azeliragon
Phase 3 STEADFAST Study
We initiated the STEADFAST Study in April 2015 pursuant to a Special Protocol Assessment (“SPA”) with the FDA. The study was conducted in the United States and certain English-speaking foreign countries under a single protocol and was designed to enroll 800 mild AD patients in total, divided equally across two independent 400-patient sub-studies, in which each subject received either a 5 mg/day dose of azeliragon or placebo, randomized on a one-to-one basis, added to the standard of care. In April 2018, we announced that the results from Part A did not meet either co-primary efficacy endpoint. Patients taking azeliragon compared with placebo did not improve in cognitive or functional outcomes as measured by the Alzheimer’s Disease Assessment Scale-cognitive subscale (“ADAS-cog”) and the Clinical Dementia Rating Scale Sum of Boxes (“CDR-sb”). Following the April 2018 announcement of Part A topline results, we discontinued clinical trials involving azeliragon, including Part B of the STEADFAST study and open label extension.
At the time of the closure of Part B, a substantial number of participants completed 12 months of treatment under the study protocol. Based on the subpopulation data analyses from Part A and the prior azeliragon trials, we prepared and submitted a revised Statistical Analysis Plan (“SAP”) to the FDA for Part B that pre-specified a target population for the primary study analysis at 12 months. In June 2018, we announced that the results from Part B did not meet either co-primary efficacy endpoint.
Relying upon the program’s Fast Track Designation status and study results to date, we have pursued discussions with the FDA and EMA to propose a pathway for further clinical development in support of regulatory approval of azeliragon. On July 31, 2018, we submitted a full briefing book to the FDA in support of our request for a Type C meeting seeking development guidance for azeliragon. On September 17, 2018, we received a written response from the FDA to our Type C Meeting Request. In the response, the FDA advised that the efficacy of azeliragon should be demonstrated in at least two adequate and well-controlled trials, unless under the exceptional circumstances in which a single trial might suffice as set forth in the FDA’s guidance document entitled “Providing Clinical Effectiveness for Human Drug and Biological Products”. We met with the Scientific Advice Working Party (“SAWP”) of the EMA on October 30, 2018, to discuss future development requirements in support of approval of azeliragon in the European Union. We expect to receive formal guidance from the SAWP during the fourth quarter of 2018.
Holding Company Structure
vTv Therapeutics Inc. is a holding company, and its principal asset is a controlling equity interest in vTv Therapeutics LLC (“vTv LLC”), the principal operating subsidiary. We have determined that vTv LLC is a variable-interest entity (“VIE”) for accounting purposes and that vTv Therapeutics Inc. is the primary beneficiary of vTv LLC because (through its managing member
23
interest in vTv LLC and the fact that the senior management of vTv Therapeutics Inc. is also the senior management of vTv LLC) it has the power to direct all of the activities of vTv LLC, which include those that most significantly impact vTv LLC’s economic performance. vTv Therapeutics Inc. has therefore consolidated vTv LLC’s results under the VIE accounting model in its consolidated financial statements.
Financial Overview
Revenue
To date, we have not generated any revenue from drug sales. Our revenue has been primarily derived from up-front proceeds and research fees under collaboration and license agreements.
In the future, we may generate revenue from a combination of product sales, license fees, milestone payments and royalties from the sales of products developed under licenses of our intellectual property. We expect that any revenue we generate will fluctuate from quarter to quarter as a result of the timing and amount of license fees, milestone and other payments, and the amount and timing of payments that we receive upon the sale of our products, to the extent any are successfully commercialized. If we fail to complete the development of our drug candidates in a timely manner or obtain regulatory approval for them, our ability to generate future revenue and our results of operations and financial position will be materially adversely affected.
Research and Development Expenses
Since our inception, we have focused our resources on our research and development activities, including conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings for our drug candidates. We recognize research and development expenses as they are incurred. Our direct research and development expenses consist primarily of external costs such as fees paid to investigators, consultants, central laboratories and clinical research organizations (“CRO(s)”) in connection with our clinical trials, and costs related to acquiring and manufacturing clinical trial materials. Our indirect research and development costs consist primarily of cash and share-based compensation costs, the cost of employee benefits, related overhead expenses for personnel in research and development functions and depreciation of leasehold improvements, laboratory equipment and computers. Since we typically use our employee and infrastructure resources across multiple research and development programs such costs are not allocated to the individual projects.
From our inception, including our predecessor companies, through September 30, 2018, we have incurred approximately $562.1 million in research and development expenses.
Our research and development expenses by project for the three and nine months ended September 30, 2018 and 2017 were as follows (in thousands):
|
|
Three Months Ended September 30, |
|
|
Nine Months Ended September 30, |
|
||||||||||
|
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
||||
|
Direct research and development expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Azeliragon |
$ |
720 |
|
|
$ |
6,163 |
|
|
$ |
12,996 |
|
|
$ |
20,948 |
|
|
TTP399 |
|
66 |
|
|
|
60 |
|
|
|
587 |
|
|
|
199 |
|
|
TTP273 |
|
24 |
|
|
|
42 |
|
|
|
58 |
|
|
|
323 |
|
|
Other projects |
|
85 |
|
|
|
290 |
|
|
|
427 |
|
|
|
942 |
|
|
Indirect research and development expense |
|
1,803 |
|
|
|
2,434 |
|
|
|
6,167 |
|
|
|
7,160 |
|
|
Total research and development expense |
$ |
2,698 |
|
|
$ |
8,989 |
|
|
$ |
20,235 |
|
|
$ |
29,572 |
|
We expect to continue to incur research and development expenses as we wind down the STEADFAST Study and its open-label extension and as we further advance the development of our diabetes drug candidates, subject to the availability of additional funding. However, due to the termination of the STEADFAST Study and its open-label extension, we expect our overall research and development expense to decrease substantially. To the extent we initiate further development of azeliragon or our other programs, our expenses, cash needs and operating losses may further increase.
The successful development of our clinical and preclinical drug candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs of the efforts that will be necessary to complete the remainder of the development of any of our clinical or preclinical drug candidates or the period, if any, in which material net cash inflows from these drug candidates may commence. This is due to the numerous risks and uncertainties associated with the development of our drug candidates, including:
|
|
• |
the uncertainty of the scope, rate of progress and expense of our ongoing, as well as any additional, clinical trials and other research and development activities; |
24
|
|
• |
our ability to market, commercialize and achieve market acceptance for any of our drug candidates that we are developing or may develop in the future; |
|
|
• |
future clinical trial results; |
|
|
• |
our ability to enroll patients in our clinical trials; |
|
|
• |
the timing and receipt of regulatory approvals, if any; and |
|
|
• |
the filing, prosecuting, defending and enforcing of patent claims and other intellectual property rights, and the expense of doing so. |
A change in the outcome of any of these variables with respect to the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of clinical development of a drug candidate, or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time with respect to the development of that drug candidate.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries, benefits and related costs for employees in executive, finance, corporate development, human resources and administrative support functions. Other significant general and administrative expenses include accounting and legal services, expenses associated with obtaining and maintaining patents, cost of various consultants, occupancy costs and information systems.
Interest Expense
Interest expense primarily consists of cash and non-cash interest expense related to our Loan Agreement. Cash interest on the Loan Agreement is recognized at a floating interest rate equal to 10.5% plus the amount by which the one-month London Interbank Offer Rate (“LIBOR”) exceeds 0.5%. Non-cash interest expense represents the amortization of the costs incurred in connection with the Loan Agreement, the allocated fair value of the warrants to purchase shares of our Class A Common Stock issued in connection with the Loan Agreement (the “Warrants”) and the accretion of the final interest payments (which will be paid in cash upon loan maturity), all of which are recognized in our Condensed Consolidated Statement of Operations using the effective interest method.
Results of Operations
Comparison of the three months ended September 30, 2018 and 2017
The following table sets forth certain information concerning our results of operations for the periods shown:
|
(dollars in thousands) |
Three Months Ended September 30, |
|
|||||||||
|
Statement of operations data: |
2018 |
|
|
2017 |
|
|
Change |
|
|||
|
Revenue |
$ |
3,375 |
|
|
$ |
15 |
|
|
$ |
3,360 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
2,698 |
|
|
|
8,989 |
|
|
|
(6,291 |
) |
|
General and administrative |
|
2,158 |
|
|
|
2,567 |
|
|
|
(409 |
) |
|
Total operating expenses |
|
4,856 |
|
|
|
11,556 |
|
|
|
(6,700 |
) |
|
Operating loss |
|
(1,481 |
) |
|
|
(11,541 |
) |
|
|
10,060 |
|
|
Interest income |
|
13 |
|
|
|
35 |
|
|
|
(22 |
) |
|
Interest expense |
|
(822 |
) |
|
|
(849 |
) |
|
|
27 |
|
|
Other income, net |
|
329 |
|
|
|
— |
|
|
|
329 |
|
|
Loss before income taxes |
|
(1,961 |
) |
|
|
(12,355 |
) |
|
|
10,394 |
|
|
Income tax provision |
|
— |
|
|
|
— |
|
|
|
— |
|
|
Net loss before noncontrolling interest |
|
(1,961 |
) |
|
|
(12,355 |
) |
|
|
10,394 |
|
|
Less: net loss attributable to noncontrolling interest |
|
(1,165 |
) |
|
|
(8,705 |
) |
|
|
7,540 |
|
|
Net loss attributable to vTv Therapeutics Inc. |
$ |
(796 |
) |
|
$ |
(3,650 |
) |
|
$ |
2,854 |
|
25
Revenue was $3.4 million for the three months ended September 30, 2018 and was insignificant for the three months ended September 30, 2017. The revenue earned during the three months ended September 30, 2018 relates to the Huadong, Reneo and Newsoara License Agreements. We recognize the portion of the consideration received allocated to the license performance obligation for each of these agreements over the requisite knowledge transfer or research service periods in accordance with the applicable accounting guidance. The portion of revenue allocated to the other performance obligations under the license agreements will be recognized as performance occurs.
Research and Development Expenses
Research and development expenses were $2.7 million and $9.0 million for the three months ended September 30, 2018 and 2017, respectively. The decrease in research and development expenses during the period of $6.3 million, or 70.0%, was primarily due to a decrease in clinical trial costs of $5.4 million for azeliragon which was mainly driven by a decrease of $4.6 million related to the termination of our STEADFAST and open-label extension (“OLE”) studies in early April 2018. In addition, we incurred significantly lower costs for compound manufacturing and other adjunct studies for TTP488 in the three months ended September 30, 2018 compared to the three months ended September 30, 2017 due to their completion in early 2018.
General and Administrative Expenses
General and administrative expenses were $2.2 million and $2.6 million for the three months ended September 30, 2018 and 2017, respectively. The decrease in general and administrative expenses during the period of $0.4 million, or 15.9%, was attributable to decreases in the amount of incentive compensation expected to be paid to our employees and decreases in share-based compensation expense recognized in the 2018 period.
Interest Expense
Interest expense was $0.8 million for each of the three months ended September 30, 2018 and 2017. Interest expense relates to the cash and non-cash interest for our Loan Agreement which bears interest at 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%.
Comparison of the nine months ended September 30, 2018 and 2017
The following table sets forth certain information concerning our results of operations for the periods shown:
|
(dollars in thousands) |
Nine Months Ended September 30, |
|
|||||||||
|
Statement of operations data: |
2018 |
|
|
2017 |
|
|
Change |
|
|||
|
Revenue |
$ |
7,912 |
|
|
$ |
58 |
|
|
$ |
7,854 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
20,235 |
|
|
|
29,572 |
|
|
|
(9,337 |
) |
|
General and administrative |
|
7,150 |
|
|
|
8,396 |
|
|
|
(1,246 |
) |
|
Total operating expenses |
|
27,385 |
|
|
|
37,968 |
|
|
|
(10,583 |
) |
|
Operating loss |
|
(19,473 |
) |
|
|
(37,910 |
) |
|
|
18,437 |
|
|
Interest income |
|
47 |
|
|
|
95 |
|
|
|
(48 |
) |
|
Interest expense |
|
(2,547 |
) |
|
|
(2,240 |
) |
|
|
(307 |
) |
|
Other income, net |
|
656 |
|
|
|
— |
|
|
|
656 |
|
|
Loss before income taxes |
|
(21,317 |
) |
|
|
(40,055 |
) |
|
|
18,738 |
|
|
Income tax provision |
|
200 |
|
|
|
— |
|
|
|
200 |
|
|
Net loss before noncontrolling interest |
|
(21,517 |
) |
|
|
(40,055 |
) |
|
|
18,538 |
|
|
Less: net loss attributable to noncontrolling interest |
|
(14,697 |
) |
|
|
(28,222 |
) |
|
|
13,525 |
|
|
Net loss attributable to vTv Therapeutics Inc. |
$ |
(6,820 |
) |
|
$ |
(11,833 |
) |
|
$ |
5,013 |
|
Revenue
Revenue was $7.9 million for the nine months ended September 30, 2018 and was insignificant for the nine months ended September 30, 2017. The revenue earned during the nine months ended September 30, 2018 relates to the Huadong, Reneo and Newsoara License Agreements. We recognize the portion of the consideration received allocated to the license performance obligation for each of these agreements over the requisite knowledge transfer or research service periods in accordance with the
26
applicable accounting guidance. The portion of revenue allocated to the other performance obligations under the license agreements will be recognized as performance occurs.
Research and Development Expenses
Research and development expenses were $20.2 million and $29.6 million for the nine months ended September 30, 2018 and 2017, respectively. The decrease in research and development expenses during the period of $9.3 million, or 31.6%, was primarily due to:
|
|
• |
A decrease in clinical trial costs of $8.0 million for azeliragon which was mainly driven by a decrease of $6.3 million related to the termination of our STEADFAST and OLE studies in early April 2018; |
|
|
• |
Additionally, costs related to other adjunct studies for TTP488 decreased by $1.7 million as such studies were being performed in the 2017 period but were completed prior to the first half of 2018; |
|
|
• |
Further, decreases in compound manufacturing costs for TTP488 of $0.8 million were offset by increases of $0.9 million related to the costs of consultants used to provide analysis of the results of the STEADFAST Study results; |
|
|
• |
Costs related to TTP399 in the nine months ended September 30, 2018 increased $0.4 million from the nine months ended September 30, 2017, due to the initiation of the Simplici-T1 Study in the fourth quarter of 2017. |
General and Administrative Expenses
General and administrative expenses were $7.2 million and $8.4 million for the nine months ended September 30, 2018 and 2017, respectively. The decrease in general and administrative expenses during the period of $1.2 million, or 14.8%, was primarily attributable to decreases in the amount of incentive compensation expected to be paid to our employees and decreases in professional fees incurred in the 2018 period.
Interest Expense
Interest expense was $2.5 million and $2.2 million for the nine months ended September 30, 2018 and 2017, respectively. Interest expense relates to the cash and non-cash interest for our Loan Agreement which bears interest at 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%. The increase in interest expense relates to the increased principal balance outstanding during the 2018 period as the second tranche of the Loan Agreement was funded in March 2017.
Liquidity and Capital Resources
Liquidity and Going Concern
As of September 30, 2018, we have an accumulated deficit of $189.0 million as well as a history of negative cash flows from operating activities. We anticipate that we will continue to incur losses for the foreseeable future as we continue our clinical trials. Further, we expect that we will need additional capital to continue to fund our operations. As of September 30, 2018, our liquidity sources included cash and cash equivalents of $3.8 million and the $5.0 million of remaining funds available under the letter agreements entered into with MacAndrews and Forbes Group LLC (“MacAndrews”) (the “Letter Agreements”). Based on our current operating plan, we believe that our current cash and cash equivalents and remaining funds available under the Letter Agreements will allow us to meet our liquidity requirements into December 2018. These factors raise substantial doubt regarding our ability to continue as a going concern. In addition to available cash and cash equivalents and available funds discussed above, we are seeking possible partnering opportunities for our GKA, GLP-1r and other drug candidates which we believe may provide additional cash for use in our operations and the continuation of the clinical trials for our drug candidates. We are also pursuing other sources of additional financing to provide flexibility to our operating plan. The timing and availability of such additional financing is not yet known and the failure of the STEADFAST Study to meet either of its clinical endpoints may make it more difficult for us to obtain such financing.
Letter Agreements
The Company has entered into the Letter Agreements with MacAndrews. Under the terms of these letter agreements, the Company has the right to sell to MacAndrews shares of its Class A Common Stock at a specified price per share, and MacAndrews has the right (exercisable up to three times) to require the Company to sell to it shares of Class A Common Stock at the same price. An aggregate of $10.0 million worth of Class A Common Stock may be sold under each of the Letter Agreements (whether at the Company’s or MacAndrews’ option). In addition, in connection with the entrance into these Letter Agreements, the Company also
27
issued MacAndrews warrants (the “Letter Agreement Warrants”) to purchase additional shares of the Company’s Class A Common Stock. On October 26, 2018, we entered into amendments to the Letter Agreement Warrants with MacAndrews, which removed certain anti-dilution provisions of the Letter Agreement Warrants.
The critical terms of these Letter Agreements are set forth in the table below:
|
|
December 5, 2017 Letter Agreement |
|
|
July 30, 2018 Letter Agreement |
|
||
|
Specified purchase price per share |
$ |
4.38 |
|
|
$ |
1.33 |
|
|
Expiration date of letter agreement |
December 5, 2018 |
|
|
July 30, 2019 |
|
||
|
Shares available to be issued under related warrants |
|
198,267 |
|
|
|
518,654 |
|
|
Exercise price of related warrants |
$ |
5.04 |
|
|
$ |
1.53 |
|
|
Expiration date of related warrants |
December 5, 2024 |
|
|
July 30, 2025 |
|
||
|
Total shares issued as of September 30, 2018 |
|
2,283,105 |
|
|
|
3,759,398 |
|
|
Remaining shares to be issued as of September 30, 2018 |
|
— |
|
|
|
3,759,399 |
|
Debt Transaction
In October 2016, we and vTv LLC entered into the Loan Agreement, under which we have borrowed $20.0 million. Each loan tranche bears interest at a floating rate equal to 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%.
We borrowed the first tranche of $12.5 million upon the close of the Loan Agreement in October 2016. The first tranche required only monthly interest payments until May 1, 2018, followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date on May 1, 2020. In addition, a final payment for the first tranche loan equal to $0.8 million will be due on May 1, 2020, or such earlier date specified in the Loan Agreement. We borrowed the second tranche of $7.5 million in March 2017. The second tranche requires only monthly interest payments until October 1, 2018, followed by equal monthly payments of principal plus accrued interest through the scheduled maturity date on October 1, 2020. In addition, a final payment for the second tranche loan equal to $0.5 million will be due on October 1, 2020, or such earlier date specified in the Loan Agreement. The availability of the third tranche of $5.0 million expired unused on June 30, 2017.
If we repay all or a portion of the loan prior to the applicable maturity date, we will pay the Lenders a prepayment penalty fee, based on a percentage of the then outstanding principal balance equal to 4.0% during the first 18 months following the funding of the second tranche and 2.0% thereafter.
In connection with the Loan Agreement, we have issued to the Lenders warrants to purchase shares of our Class A Common Stock (the “Warrants”). On October 28, 2016, we issued Warrants to purchase 152,580 shares of our Class A Common Stock at a per share exercise price of $6.39 per share, which aggregate exercise price represents 6.0% of the principal amount borrowed under the first tranche of the Loan Agreement and 3.0% of the amount available under the second tranche of the Loan Agreement. On March 24, 2017, in connection with the funding of the second tranche, we issued Warrants to purchase 38,006 shares of our Class A Common Stock at a per share exercise price of $5.92 per share, which aggregate exercise price represents 3.0% of the principal amount of the second tranche. In each instance, the Warrants have an exercise price equal to the lower of (a) the volume weighted average price per share of our Class A Common Stock, as reported on the principal stock exchange on which our Class A Common Stock is listed, for 10 trading days prior to the issuance of the applicable Warrants or (b) the closing price of a share of our Class A Common Stock on the trading day prior to the issuance of the applicable Warrants. The Warrants will expire seven years from their date of issuance.
The Loan Agreement includes customary affirmative and restrictive covenants, including, but not limited to, restrictions on the payment of dividends or other equity distributions and the incurrence of debt or liens upon the assets of the Company or its subsidiaries. The Loan Agreement does not contain any financial maintenance covenants other than a requirement to maintain a minimum cash balance of not less than $2.5 million in a deposit account pledged to secure the Loan Agreement and subject to an account control agreement. The minimum cash balance covenant was included as part of an amendment to the Loan Agreement in connection with our entry into the Huadong License Agreement in December 2017. The Loan Agreement includes customary events of default, including payment defaults, covenant defaults, and material adverse change default. Upon the occurrence of an event of default and following any applicable cure periods, a default interest rate of an additional 5.0% will be applied to the outstanding loan balances, and the Lenders may declare all outstanding obligations immediately due and payable and take such other actions as set forth in the Loan Agreement. As a result of the termination of the STEADFAST Study, we granted the Lenders a first priority security interest in all of our intellectual property, subject to certain limited exceptions.
28
|
|
|
Nine Months Ended |
|
|||||
|
|
|
September 30, |
|
|||||
|
|
|
2018 |
|
|
2017 |
|
||
|
(dollars in thousands) |
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
$ |
(20,987 |
) |
|
$ |
(38,229 |
) |
|
Net cash provided by (used in) investing activities |
|
|
7 |
|
|
|
(7 |
) |
|
Net cash provided by financing activities |
|
|
12,826 |
|
|
|
7,500 |
|
|
Net decrease in cash and cash equivalents |
|
$ |
(8,154 |
) |
|
$ |
(30,736 |
) |
Operating Activities
For the nine months ended September 30, 2018, our net cash used in operating activities decreased $17.2 million from the nine months ended September 30, 2017. The decreased use of cash was driven primarily by lower expenses incurred in the nine months ended September 30, 2018.
Investing Activities
For the nine months ended September 30, 2018 and 2017, net cash used in investing activities was insignificant.
Financing Activities
For the nine months ended September 30, 2018, net cash provided by financing activities was impacted by the receipt of $15.0 million for the issuance of shares of our Class A Common Stock under the Letter Agreements. Such receipts were offset by $2.7 million of principal payments required by our Loan Agreement. For the nine months ended September 30, 2017, net cash provided by financing activities was $7.5 million, as we borrowed the second tranche under our Loan Agreement.
Future Funding Requirements
To date, we have not generated any revenue from drug product sales. We do not know when, or if, we will generate any revenue from drug product sales. We do not expect to generate revenue from drug sales unless and until we obtain regulatory approval of and commercialize any of our drug candidates. We anticipate that we will need substantial additional funding in connection with our continuing operations.
Based on our current operating plan, we believe that our current cash and cash equivalents and remaining funds available under the Letter Agreements will allow us to meet our liquidity requirements into December 2018. In addition to the available cash and cash equivalents and other sources of liquidity, we are seeking possible additional partnering opportunities for our GKA, GLP-1r and other drug candidates which we believe may provide additional cash for use in our operations and the continuation of the clinical trials for our drug candidates. We may also pursue other sources of financing to provide flexibility to our operating plan. The timing and availability of such financing is not yet known and the failure of the STEADFAST Study to meet either of its co-primary endpoints may make it more difficult for us to obtain such financing. We have based our estimates on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures necessary to complete the development of our drug candidates.
Our future capital requirements will depend on many factors, including:
|
|
• |
the extent of costs associated with the wind-down of the STEADFAST Study and the OLE, as well as work required to complete the analysis of Part B data; |
|
|
• |
the outcome of our discussions with the FDA and EMA regarding the future development of azeliragon and what pathway for further clinical development in support of regulatory approval is determined to be appropriate, if any; |
|
|
• |
the outcome, costs and timing of seeking and obtaining FDA and any other regulatory approvals; |
|
|
• |
the number and characteristics of drug candidates that we pursue, including our drug candidates in preclinical and clinical development; |
|
|
• |
the ability of our drug candidates to progress through clinical development successfully; |
|
|
• |
our need to expand our research and development activities; |
29
|
|
• |
the costs of acquiring, licensing or investing in businesses, products, drug candidates and technologies; |
|
|
• |
our ability to maintain, expand and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
|
|
• |
our need and ability to retain management and scientific and medical personnel; |
|
|
• |
the effect of competing technological and market developments; |
|
|
• |
our need to implement additional internal systems and infrastructure, including financial and reporting systems; |
|
|
• |
the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future; and |
|
|
• |
the amount of any payments we are required to make to M&F TTP Holdings Two LLC in the future under the Tax Receivable Agreement. |
Until such time, if ever, as we can generate substantial revenue from drug sales, we expect to finance our cash needs through a combination of equity offerings, debt financings, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements. We do not currently have any committed external source of funds other than those available through the Letter Agreements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants that will further limit or restrict our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may be required to relinquish valuable rights to our technologies, future revenue streams or drug candidates or grant licenses on terms that may not be favorable to us. If we are unable to obtain additional funding, we could be forced to delay, reduce or eliminate our research and development programs or commercialization efforts, which could adversely affect our business prospects.
Disclosures About Contractual Obligations and Commitments
As of September 30, 2018, there were no material changes to our total contractual cash obligations, as set forth in the contractual obligations and commitments disclosure included in Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” of our Annual Report on Form 10-K for the year ended December 31, 2017.
We enter into contracts in the normal course of business with CROs for clinical trials and clinical supply manufacturing and with vendors for preclinical research studies and other services and products for operating purposes, which generally provide for termination or cancellation within 30 days of notice.
Off-Balance Sheet Arrangements
We have entered into the Letter Agreements with MacAndrews and Forbes Group LLC which, as of September 30, 2018, provide us the right to sell to MacAndrews an additional 3,759,399 shares of our Class A Common Stock at a price equal to $1.33 per share. Further, MacAndrews has the right (exercisable up to three times) to require us to sell to it shares of Class A Common Stock at the same price. As of September 30, 2018 we had received funding of $15.0 million under the Letter Agreements and, in exchange, had issued a total of 6,042,503 shares of our Class A Common Stock.
Discussion of Critical Accounting Policies
For a discussion of our critical accounting policies and estimates, please refer to Part II, Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2017. Significant changes made to our critical accounting policies and estimates in 2018 with respect to our adoption of Accounting Standards Codification Topic 606 “Revenue From Contracts with Customers” are discussed within Note 2 of the Condensed Consolidated Financial Statements included in Item 1 of this Quarterly Report on Form 10-Q.
Forward-Looking Statements
This quarterly report includes certain forward-looking statements within the meaning of the federal securities laws regarding, among other things, our management’s intentions, plans, beliefs, expectations or predictions of future events, which are considered
30
forward-looking statements. You should not place undue reliance on those statements because they are subject to numerous uncertainties and factors relating to our operations and business environment, all of which are difficult to predict and many of which are beyond our control. Forward-looking statements include information concerning our possible or assumed future results of operations, including descriptions of our business strategy. These statements often include words such as “may,” “will,” “should,” “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate” or similar expressions. These statements are based upon assumptions that we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors that we believe are appropriate under the circumstances. As you read this quarterly report, you should understand that these statements are not guarantees of performance or results. They involve known and unknown risks, uncertainties and assumptions, including those described under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K and under Item 1A of Part II of this Quarterly Report on Form 10-Q. Although we believe that these forward-looking statements are based upon reasonable assumptions, you should be aware that many factors, including those described under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K and under Item 1A of Part II of this Quarterly Report on Form 10-Q, could affect our actual financial results or results of operations and could cause actual results to differ materially from those in the forward-looking statements.
Our forward-looking statements made herein are made only as of the date of this quarterly report. We expressly disclaim any intent, obligation or undertaking to update or revise any forward-looking statements made herein to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. All subsequent written and oral forward-looking statements attributable to us or persons acting on our behalf are expressly qualified in their entirety by the cautionary statements contained in this quarterly report.
Effect of Recent Accounting Pronouncements
See discussion of recent accounting pronouncements in Note 2, “Summary of Significant Accounting Policies”, to the Condensed Consolidated Financial Statements in this Form 10-Q.
Interest Rate Risk
Our Loan Agreement bears interest at a floating rate equal to 10.5% plus the amount by which the one-month LIBOR exceeds 0.5%. A one percent increase in the variable rate of interest on the Loan Agreement would increase interest expense by approximately $0.2 million annually based on the amounts currently outstanding. We do not currently hedge our interest rate exposure.
Market Risk
Our exposure to market risk is limited to our cash and cash equivalents, all of which have maturities of one year or less. The goals of our investment strategy are preservation of capital, fulfillment of liquidity needs and fiduciary control of cash and investments. We also seek to maximize income from our investments without assuming significant risk. To achieve our goals, we maintain a portfolio of cash equivalents and investments in a variety of securities that management believes to be of high credit quality. The securities in our investment portfolio are not leveraged and are, due to their short-term nature, subject to minimal interest rate risk. Because of the short-term maturities of our investments, we do not believe that an increase in market rates would have a material negative impact on the value of our investment portfolio.
Foreign Currency Risk
We do not have any material foreign currency exposure.
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, management has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) or 15d-15(e) of the Securities Exchange Act of 1934) as of September 30, 2018. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of September 30, 2018, our disclosure controls and procedures were effective in causing material information relating to us (including our consolidated subsidiaries) to be recorded, processed, summarized and
31
reported by management on a timely basis and to ensure the quality and timeliness of our public disclosures pursuant to SEC disclosure obligations.
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, with the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error and mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of controls.
The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, a control may become inadequate because of changes in conditions or because the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
Changes to Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Website Availability of Reports and other Corporate Governance Information
The Company maintains a comprehensive corporate governance program, including Corporate Governance Guidelines for its Board of Directors, Board Guidelines for Assessing Director Independence and charters for its Audit Committee, Nominating and Corporate Governance Committee and Compensation Committee. The Company maintains a corporate investor relations website, www.vtvtherapeutics.com, where stockholders and other interested persons may review, without charge, among other things, corporate governance materials and certain SEC filings, which are generally available on the same business day as the filing date with the SEC on the SEC’s website http://www.sec.gov.
We are not currently a party to any material legal proceedings.
In addition to the risk factors listed below and other information in this report, investors should carefully consider the risk factors set forth under the heading “Risk Factors” under Item 1A of Part I in our Annual Report on Form 10-K for the year ended December 31, 2017.
We may not be able to continue the development of, obtain regulatory approval for, or successfully commercialize azeliragon.
We have expended considerable resources and efforts on the development of azeliragon. In April 2018, we announced that results from Part A of our Phase 3 STEADFAST Study of the investigational medication azeliragon (TTP488) in people with mild Alzheimer’s disease (the “STEADFAST Study”) did not meet either co-primary efficacy endpoint as required by the Special Protocol Agreement (“SPA”) with the FDA. Following the April 2018 announcement, we discontinued clinical trials involving azeliragon, including Part B and open label extension.
At the time of the closure of Part B, a substantial number of participants completed 12 months of treatment under the study protocol. Based on the subpopulation data analyses from Part A and the prior azeliragon trials, we prepared and submitted a revised Statistical Analysis Plan (“SAP”) to the Food and Drug Administration (“FDA”) for Part B that pre-specified a target population for the primary study analysis at 12 months. In June 2018, we announced that the results from Part B did not meet either co-primary efficacy endpoint.
32
Relying upon the program’s Fast Track Designation status and study results to date, we have pursued discussions with the FDA and European Medicines Agency (“EMA”) to propose a pathway for further clinical development in support of regulatory approval of azeliragon. On July 31, 2018, we submitted a full briefing book to the FDA in support of our request for a Type C meeting seeking development guidance for azeliragon. On September 17, 2018, we received a written response from the FDA to our Type C Meeting Request. In the response, the FDA advised that the efficacy of azeliragon should be demonstrated in at least two adequate and well-controlled trials, unless under the exceptional circumstances in which a single trial might suffice as set forth in the FDA’s guidance document entitled “Providing Clinical Effectiveness for Human Drug and Biological Products”.
The failure of Parts A and B of the STEADFAST Study to meet their co-primary endpoints is expected to delay the potential commercialization of azeliragon and may make such commercialization more difficult or impossible. We will need to commence and complete additional clinical trials that satisfy the specified primary endpoint criteria, manage clinical and manufacturing activities, obtain necessary regulatory approvals from the FDA or other comparable regulatory authorities, and, if approved, successfully market and commercialize azeliragon. If we continue with the development of azeliragon, there is no guarantee that we will be able to successfully complete these steps, and if we do not continue with the development of azeliragon, then we may not be able to continue our business in its current form and will be required to pursue alternative business strategies. As an organization, we have never completed a successful Phase 3 clinical trial or submitted a New Drug Application before, and we may be unsuccessful in doing so for azeliragon.
We require additional funding and there is a substantial doubt about our ability to continue as a going concern; if we fail to raise additional funding we will cease operations and/or seek protection under applicable bankruptcy laws.
We require additional financing in order to continue to fund operations, and there is a substantial doubt about our ability to continue as a going concern. No assurance can be given that we will be successful in obtaining any such financing on acceptable terms, if at all, or if secured, that such financing will provide for funding or payments to us sufficient to continue to fund operations. After giving effect to our existing sources of liquidity, including the Letter Agreements, we believe we have cash sufficient to fund operations only into December 2018. In the absence of the receipt of additional financing prior to such time, we will be required to scale back or terminate operations and/or seek protection under applicable bankruptcy laws.
Our recurring losses, accumulated deficit and our current levels of cash and cash equivalents raise substantial doubt about our ability to continue as a going concern as of the date of this report. If we are unable to continue as a going concern, we may have to liquidate our assets and it is likely that investors will lose all or a significant part of their investments. If we seek additional financing to fund our business activities in the future and there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms or at all, and such additional funding may cause substantial dilution to our existing investors. Further, if adequate funds are not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or development programs.
Our stock price may decline and we may not be able to maintain compliance with NASDAQ listing requirements
Our common stock is listed on The NASDAQ Capital Market, which imposes certain minimum continued listing requirements. If compliance with these requirements is not maintained, NASDAQ may make a determination to delist our common stock.
There were no sales of unregistered equity securities during the three months ended September 30, 2018 that have not previously been included in a Current Report on Form 8-K.
Our ability to pay dividends is restricted by our Loan Agreement. See “Management's Discussion and Analysis of Financial Condition and Results of Operations”.
None.
None.
33
On November 6, 2018, the Company caused MacAndrews to purchase an additional 1,879,699 shares of its Class A Common Stock under the terms of the letter agreement entered into on July 30, 2018 for $2.5 million in cash.
34
|
Exhibit |
|
Description |
|
|
|
|
|
10.1 |
|
|
|
|
|
|
|
10.2 |
|
|
|
|
|
|
|
31.1 |
|
|
|
|
|
|
|
31.2 |
|
|
|
|
|
|
|
32.1 |
|
|
|
|
|
|
|
32.2 |
|
|
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Document |
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase |
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase |
35
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: November 8, 2018
|
|
|
VTV THERAPEUTICS INC. |
|
|
|
(Registrant) |
|
|
|
|
|
By: |
|
/s/ Stephen L. Holcombe |
|
|
|
Stephen L. Holcombe |
|
|
|
President and Chief Executive Officer |
|
|
|
|
|
By: |
|
/s/ Rudy C. Howard |
|
|
|
Rudy C. Howard |
|
|
|
Chief Financial Officer |
36
